Determination of eugenol in whole tobacco: T-314
1 Scope of application
1.1
This method is to be used for routine analysis of eugenol without the need for derivatization. It is applicable to whole tobacco in tobacco products by reversed phase High Performance Liquid Chromatography (HPLC) with UV detection.
2 Normative references
2.1
Health Canada Official Method T-115. Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 2016.
2.2
Health Canada Official Method T-402. Preparation of Sample for Testing of Cigarettes, Tobacco Sticks, Cigarette Tobacco, Cigars, Little Cigars, Kreteks, Bidis, Leaf, Pipe and Smokeless Tobacco, 2016.
2.3
International Organization for Standardization, ISO 8243 Cigarettes - Sampling. 2013.
2.4
International Organization for Standardization, ISO 15592-1 Fine-Cut tobacco and smoking articles made from it - Methods of sampling, conditioning and analysis - Part 1: Sampling. 2001.
3 Definitions
3.1
Refer to T-301 for definitions of terms used in this document.
4 Method summary
4.1
The sample is accurately weighed into a culture tube equipped with a screw cap and ethanol is added.
4.2
The tube is sealed and the tobacco is extracted.
4.3
An aliquot is syringe-filtered into an amber autosampler vial and analyzed by High Performance Liquid Chromatograph (HPLC) with UV detection.
4.4
Eugenol in whole tobacco is quantified by external standard calibration procedures where the relative response of the samples is compared against a multi-point calibration.
Warning: The testing and evaluation of certain products against this test method may require the use of materials and/or equipment that are potentially hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with all existing applicable regulatory requirements prior to its use.
5 Apparatus and equipment
5.1
Batch processor, Robot Coupe RS1 2V or equivalent.
5.2
Sonicator.
5.3
Bottletop dispenser, 10-50 mL.
5.4
Analytical balance measuring to at least 4 decimal places.
5.5
Centrifuge.
5.6
Vortex.
5.7
Volumetric flasks, 10 and 100 mL, amber Class A.
5.8
Glass graduated measuring cylinder, 50 mL.
5.9
Screw-top glass culture/centrifuge tubes, 200 × 25 mm.
5.10
White polypropylene caps without liners.
5.11
Micropipettes, 10-5000 μL or equivalent.
5.12
Pipettes, Class A, 2 mL, 5 mL, 50 mL.
5.13
Syringe filter, 0.45 μm PVDF.
5.14
Disposable 5 mL syringes.
5.15
Disposable glass Pasteur pipettes.
5.16
Glass filtering funnel.
5.17
Parafilm® or equivalent.
5.18
Autosampler vials with screw caps and Teflon-lined septa.
5.19
Shaker bath.
5.20
High Performance Liquid Chromatography System with:
5.20.1
UV detector.
5.20.2
RP 18e column or equivalent.
5.20.3
Disposable guard column.
6 Reagents and supplies
6.1
All reagents shall be at least analytical reagent grade.
Note: Wherever possible, reagents are identified by their Chemical Abstract Service [CAS] registry numbers in square brackets.
6.2
Ethanol - [67-17-5] HPLC grade.
6.3
Eugenol - [97-53-0] > 99 % purity.
6.4
Helium - [7440-59-7] (UHP).
6.5
Isopropanol - [67-63-0].
6.6
Methanol - [67-56-1].
6.7
Water, Type I (as outlined in ASTM D1193, Table 1: Processes for Reagent Water Production, Note A).
7 Preparation of glassware
7.1
Clean and dry glassware in a manner to ensure that contamination from residues on glassware does not occur.
8 Preparation of standards
8.1
Prepare a primary eugenol stock solution (2.0 mg/ml) by accurately weighing 200 mg of pure eugenol into a 100 mL volumetric flask and diluting to the mark with ethanol.
8.2
Prepare working standards in the range (2-1000 μg/ml) from the primary eugenol stock solution by dilution (0.01-5000 μL) to 10 mL with ethanol. (See appendix 1, table 1.)
Note: Additional standards may have to be prepared to cover the range of anticipated responses for test samples.
8.3
Transfer to amber autosampler vials. Rinse vials first and then fill to minimize head space.
8.4
Store vials at 4 °C, protected from light, until analyzed.
8.5
Prepare eugenol calibration standards fresh every 5 days.
9 Sampling
9.1
The sampling of cigarettes for the purpose of testing shall be in accord with ISO 8243.
9.2
The sampling of kreteks, little cigar, bidis, tobacco sticks for the purpose of testing shall be in accord with ISO 8243, but modified such that the term "cigarette" is substituted with "kreteks", "little cigars", "bidis" or "tobacco sticks", whereby the term "carton" is equivalent to 200 units.
9.3
The sampling of cigars for the purpose of testing shall be in accord ISO 8243, but modified such that the term "cigarette" is substituted with "cigar", whereby 200 units of cigarette is equivalent to 200 grams of cigar.
9.4
The sampling of cigarette tobacco for the purpose of testing shall be in accord with ISO 15592-1.
9.5
The sampling of leaf tobacco, pipe tobacco or smokeless tobacco shall be in accord with ISO 15592-1 but modified such that the term "fine-cut" is substituted with "leaf tobacco", "pipe tobacco" or "smokeless tobacco".
10 Tobacco product preparation
10.1
The preparation of tobacco products for the purpose of testing shall be as specified in T-402.
Note: Samples are to be tested within 24 hours of product preparation to minimize the potential loss of eugenol.
11 Sample preparation
11.1
Extraction of Whole Tobacco
11.1.1
Before testing, grind the test sample to pass through a 20 mesh screen (20 sections per square inch).
Note: Since samples containing a high moisture content cannot be ground in this manner, extraction may start at 11.1.2.
11.1.2
Accurately weigh 2 g of the test sample into a screw cap culture tube.
11.1.3
Add 50 mL of ethanol to the sample. Screw the cap on and seal the cap with Parafilm®.
11.1.4
Extract in a shaker bath for 2 hours at 50 °C.
11.1.5
Allow the sample to cool to room temperature, then filter an aliquot into an amber autosampler vial (in duplicate) using a disposable syringe filter attached to a disposable syringe. Cap and store at 4 °C.
Note: It may be necessary to centrifuge at about 1200 rpm for 10 minutes to compress the whole tobacco before attempting to filter.
12 Sample analysis
12.1
Chromatographic Conditions (Reversed Phase Analysis)
- Column Temp.:
- 30 °C
- Mobile Phase:
- Solvent A: Methanol: Type 1 water (80:20) filter and degas. (UHP helium sparged)
- Sample Wash:
- Solvent A
- UV Wavelength:
- 280 nm
- Mode:
- Isocratic
- Flow rate:
- 0.7 mL/minute
| Time (min) |
Composition | ||
|---|---|---|---|
| A (%) |
B (%) |
C (%) |
|
| 0.0 | 100 | 0 | 0 |
| 20.0 | 100 | 0 | 0 |
| Method End Action | 100 | 0 | 0 |
Equilibration Time: 10 minutes
Note: Adjustments to the mobile phase may be required, depending on instrument and column conditions as well as the resolution of the analyte peak.
Note: These settings are detector dependent and may have to be modified in order to achieve a linear response over the range of concentrations for the analyte of interest.
12.2
Sample Analysis
12.2.1
Inject 20 μL of each sample vial onto the HPLC column and analyze. The elution pattern should be similar to appendix 2, figure 1 and figure 2.
13 Calculations
13.1
Construct a Calibration Curve
13.1.1
Prepare a calibration curve by plotting the concentration of eugenol versus the peak area. Determine the response factor from the calibration curve.
13.2
Sample Quantification
13.2.1
Quantify the amount of eugenol in tobacco samples by the external standard method.
13.2.2
Identify peaks by comparing retention times with standards, and the spiking of whole tobacco samples. (LFM)
13.3
Determination of Eugenol Content in μg/g

Figure 13-3: Text description
Eugenol [μg/g] =
Peak area
divided by
Resp. Factor
×
mL of Solution
divided by
Wt. (g) of tobacco
13.3.1
Enter the correct multiplier (overall volume of the original sample in mL) and divisor (the original sample weight in g) and the concentration of eugenol is automatically calculated in μg/g.
13.3.2
To convert this concentration to a percentage, divide the μg/g result by 10 000.
13.3.3
All results are expressed on an 'as analyzed' basis. These may be expressed on a 'dry matter' basis using the appropriate moisture result.
14 Quality control
14.1
For typical chromatograms, see appendix 2, figure 1, figure 2 and figure 3.
14.2
Typical Control Parameters
Note: If the control measurements are outside the tolerance limits of the expected values, appropriate investigation and action must be taken.
14.2.1
Laboratory Reagent Blank (LRB)
To detect potential contamination during the sample preparation and analysis processes, include a laboratory reagent blank (LRB). The LRB consists of all reagents and materials used in performing the analysis on test samples and is analyzed as a test sample.
14.2.2
Laboratory Fortified Blank (LFB)
To detect potential loss of analyte during the sample preparation and analysis processes, include a laboratory fortified blank (LFB). The LFB consists of all reagents and materials used in performing the analysis on test samples plus fortification with a known concentration of at least one of the analytes of interest. The level of fortification should reflect the range of typical results for that sample. The LFB is then analyzed as a test sample.
14.2.3
Laboratory Fortified Matrix (LFM)
To detect potential matrix interferences, include a laboratory fortified matrix (LFM). During the sample preparation and/or analysis processes, divide a test sample and fortify an aliquot with at least one of the analytes of interest in known concentration. The level of fortification should reflect the range of typical results for that sample. The LFM is then analyzed as a test sample.
14.2.4
Laboratory Control Sample
To assess the overall performance of an analysis, a control sample is analyzed. The results of the control sample should be compared, using appropriate statistical techniques, to 'expected values' generated by the laboratory or, if none exist, to values found in literature. This provides information to the laboratory, on test accuracy and precision.
14.2.5
Standard as Sample
To assess the stability of the analytical system, a standard is analyzed as a sample. The results of this standard should be compared, using appropriate statistical techniques, to expected concentrations.
14.3
Recoveries and Levels of Contamination
14.3.1
Typically, the LRB is less than the LOD.
14.3.2
Typical LFB recoveries range between 90-100 % recovery.
14.3.3
Typical LFM recoveries range between 85-105 % recovery.
14.4
Limit of Detection (LOD) and Limit of Quantification (LOQ)
14.4.1
The LOD can be determined as 3 times the standard deviation of results obtained by analyzing the lowest standard level a minimum of 10 times over several days.
14.4.1.1
A typical value for LOD is 0.81 μg/g (as received).
14.4.2
The LOQ can be determined as 10 times the standard deviation of results obtained by analyzing the lowest standard level a minimum of 10 times over several days.
14.4.2.1
A typical value for LOQ is 2.69 μg/g (as received).
14.5
Stability of Reagents and Supplies
14.5.1
Prepare all primary stock eugenol standards fresh weekly.
14.5.2
Prepare all work standards and extraction solvents fresh weekly.
14.5.3
Analyze all samples within 24 hours of extraction.
15 References
15.1
Fischer, I. U. and Dengler, H. J. 1990. Sensitive high performance liquid chromatographic assay for the determination of eugenol in body fluids. Journal of Chromatography. 525: 369-377.
15.2
Myint, S. et al. 1995. Separation and identification of eugenol in ethanol extract of cloves by reversed-phase high performance liquid chromatography. Journal of American Oil Chemist. Society. 72: 1231-1233.
15.3
Smith, R. M. and Beck, S. 1984. High performance liquid chromatographic analysis of eugenol in pimento using ultraviolet and electrochemical detection. Journal of Chromatography. 291: 424-427.
15.4
ASTM International, ASTM Standard D1193-06(2011). Standard Specifications for Reagent Water.
Appendix 1
Table 1. Eugenol Calibration Standards
| Standard No |
Volume of Primary Eugenol (mL) |
Final Volume (mL) |
Eugenol [μg/mL] |
|---|---|---|---|
| 1 | 5.0 | 10 | 1000 |
| 2 | 2.5 | 10 | 500 |
| 3 | 1.0 | 10 | 200 |
| 4 | 0.500 | 10 | 100 |
| 5 | 0.100 | 10 | 20.0 |
| 6 | 0.010 | 10 | 2.0 |
Note: The concentration of Eugenol will vary depending on the exact concentration of primary stock prepared.
Note: Additional standards may have to be prepared to cover the range of anticipated responses for test samples.
Appendix 2

Figure 1. Typical HPLC Chromatogram of Tobacco Sample Analyzed for Sorbic Acid: Text description
This figure is a chromatogram result of a whole tobacco sample and calibration standard. The eugenol peak is at retention time of 4.672 minutes.
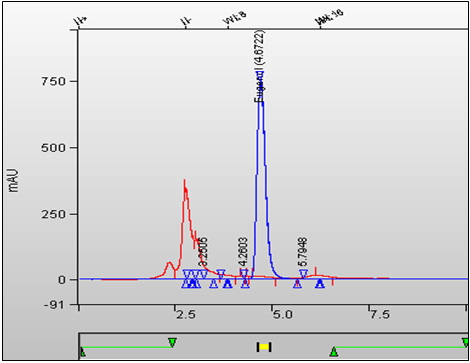
Figure 2. Typical HPLC Chromatogram of Tobacco Sample Analyzed for Sorbic Acid: Text description
This figure is an overlay chromatogram result of a whole tobacco (1R4F cigarette type) and calibration standard. The eugenol peak is absent here at 4.672 minutes in the sample.
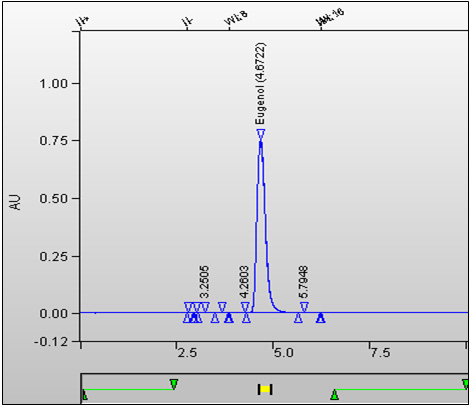
Figure 3. Typical HPLC Chromatogram of Tobacco Sample Analyzed for Sorbic Acid: Text description
This figure displays a chromatogram of eugenol calibration standard. The retention time is 4.672 minutes.