Determination of humectants in whole tobacco: T-304
1 Scope of application
1.1
Applicable to the determination of the amount of glycerol, propylene glycol, and triethylene glycol which may be added to whole tobacco as humectants.
1.2
This method is not designed to measure trace quantities of contamination from external sources and does not distinguish between the amount of humectant added and any potential naturally occurring humectants.
2 Normative references
2.1
Health Canada Official Method T-115. Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 2016.
2.2
Health Canada Official Method T-402. Preparation of Sample for Testing of Cigarettes, Tobacco Sticks, Cigarette Tobacco, Cigars, Little Cigars, Kreteks, Bidis, Leaf, Pipe and Smokeless Tobacco, 2016.
2.3
International Organization for Standardization, ISO 8243 Cigarettes - Sampling. 2013.
2.4
International Organization for Standardization, ISO 15592-1 Fine-Cut tobacco and smoking articles made from it - Methods of sampling, conditioning and analysis - Part 1: Sampling. 2001.
2.5
AOAC INTERNATIONAL, AOAC Official Method 971.02 Glycerol, Propylene Glycol, and Triethylene Glycol in Cased Cigarette Cut Filler and Ground Tobacco, Gas Chromatographic Method. Official Methods of Analysis of AOAC INTERNATIONAL, 20th Ed., 2016
2.6
AOAC INTERNATIONAL, AOAC Official Method 968.03 Menthol in Cigarette Filler, Gas Chromatographic Method. Official Methods of Analysis of AOAC INTERNATIONAL, 20th Ed., 2016.
3 Definitions
3.1
Refer to T-301 for definitions of terms used in this document.
4 Method summary
4.1
This method is a gas chromatographic (GC) method using a megabore fused silica column and a flame ionization detector (FID). Tobacco from a freshly opened source (package or tin of fine cut tobacco) is extracted with a methanol based extraction solvent on a mechanical shaker. The sample is placed in the dark and allowed to sit until the supernatant is clear. A portion of the supernatant is transferred to an autosampler vial and the humectant content evaluated by gas chromatography (GC).
4.2
The humectants are analyzed on a megabore fused silica column, which has a polyethylene glycol (PEG) stationary phase. Quantification is achieved using an internal standard calibration by comparing the FID response of the analytes in the samples against a multi-point calibration of the corresponding humectant in the standards.
4.3
In order to accurately compare the humectant concentration of one brand to another, a moisture analysis is done and the humectant concentration on a 'dry matter' basis is determined.
Warning: The testing and evaluation of certain products against this test method may require the use of materials and/or equipment that are potentially hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with all existing applicable regulatory requirements prior to its use.
5 Apparatus and equipment
5.1
Erlenmeyer flasks with stoppers, 125 mL or equivalent.
5.2
Volumetric flasks 10, 100, 2000 mL.
5.3
Pipette, 20 mL.
5.4
Volumetric pipettes, various sizes.
5.5
Bottletop dispenser, 10-50 mL or equivalent.
5.6
Gas chromatograph with flame ionization detector (FID).
5.7
Autosampler.
5.8
Autosampler vials with screw caps and Teflon-lined septa.
5.9
DB-Wax fused silica column 15 m × 0.53 mm × 1 µm or equivalent.
5.10
Mechanical wrist-action shaker.
5.11
Disposable transfer pipettes.
5.12
Analytical balance measuring to at least 4 decimal places.
6 Reagents and supplies
6.1
All reagents shall be at least analytical reagent grade.
Note: Wherever possible, reagents are identified by their Chemical Abstract Service [CAS] registry numbers in square brackets.
6.2
1,3-Butanediol - [107-88-0] (used as an internal standard).
6.3
Glycerol - [56-81-5].
6.4
Methanol - [67-56-1] Distilled-in-Glass.
6.5
Propylene Glycol - [57-55-6].
6.6
Triethylene Glycol - [112-27-6].
7 Preparation of glassware
7.1
Clean and dry glassware in a manner to ensure that contamination from residues on glassware does not occur.
8 Preparation of solutions
8.1
Extraction Solution (Concentration approximately 2 mg/mL 1,3-butanediol)
8.1.1
Weigh 20 g (± 0.05 g) of 1,3-butanediol into a 100 mL volumetric flask, accurately record, and make to volume with methanol.
8.1.2
Label as '1,3-butanediol primary stock'.
8.1.3
Pipette 20 mL of primary stock to a 2 L volumetric flask and make to volume with methanol. Mix well.
9 Preparation of standards
9.1
Glycerol Primary Standard (Approximately 100 mg/mL)
9.1.1
Weigh 10 g (± 0.05 g) of glycerol into a 100 mL volumetric flask, accurately record, and make to volume with extraction solution.
9.2
Propylene Glycol Primary Standard (Approximately 50 mg/mL)
9.2.1
Weigh 5 g (± 0.05 g) of propylene glycol into a 100 mL volumetric flask, accurately record, and make to volume with extraction solution.
9.3
Triethylene Glycol Primary Standard (Approximately 50 mg/mL)
9.3.1
Weigh 5 g (± 0.05 g) of triethylene glycol into a 100 mL volumetric flask, accurately record, and make to volume with extraction solution.
9.4
Mixed Secondary Standard (Std. 4 of working standards)
9.4.1
Pipette a 4 mL aliquot of each of the prepared primary stocks into a 100 mL volumetric flask.
9.4.2
Make to volume with extraction solution.
9.5
Working Standards
9.5.1
All standards are made in volumetric flasks using the dilutions described:
| Standards No. |
Volume of Mixed Stock (mL) |
Final Volume(mL) | Glycerol [mg/mL] |
Propylene Glycol [mg/mL] |
Triethylene Glycol [mg/mL] |
|---|---|---|---|---|---|
| 1 | 10 | 10 | 4.00 | 2.00 | 2.00 |
| 2 | 6 | 10 | 2.40 | 1.20 | 1.20 |
| 3 | 3 | 10 | 1.20 | 0.60 | 0.60 |
| 4 | 1.5 | 10 | 0.60 | 0.30 | 0.30 |
| 5 | 0.5 | 10 | 0.20 | 0.10 | 0.10 |
Note: Volumetric flasks are made to volume with extraction solution (which contains the internal standard).
Note: Additional standards may have to be prepared to cover the range of anticipated responses for test samples.
10 Sampling
10.1
The sampling of cigarettes for the purpose of testing shall be in accord with ISO 8243.
10.2
The sampling of kreteks, little cigars, bidis, tobacco sticks for the purpose of testing shall be in accord with ISO 8243, but modified such that the term "cigarette" is substituted with "kreteks", "little cigars", "bidis" or "tobacco sticks", whereby the term "carton" is equivalent to 200 units.
10.3
The sampling of cigars for the purpose of testing shall be in accord ISO 8243, but modified such that the term "cigarette" is substituted with "cigar", whereby 200 units of cigarette is equivalent to 200 grams of cigar.
10.4
The sampling of cigarette tobacco for the purpose of testing shall be in accord with ISO 15592-1.
10.5
The sampling of leaf tobacco, pipe tobacco or smokeless tobacco shall be in accord with ISO 15592-1 but modified such that the term "fine-cut" is substituted with "leaf tobacco", "pipe tobacco" or "smokeless tobacco".
11 Tobacco product preparation
11.1
The preparation of tobacco products for the purpose of testing shall be as specified in T-402.
12 Sample preparation
12.1
Extraction of Sample
12.1.1
Weigh 2 g of test sample into a 125 mL Erlenmeyer flask and record the weight accurately.
12.1.2
Add 25 mL of the extraction solution to the sample.
12.1.3
Stopper the flasks and place on a wrist-action shaker for 60 minutes.
12.1.4
Remove the samples from the shaker, swirling the flask to get all of the tobacco into the solvent.
12.1.5
Allow the samples to sit for 30 minutes until the supernatant is clear.
12.1.6
Transfer the supernatant to an autosampler vial and analyze on the GC.
13 Sample analysis
13.1
Gas Chromatograph Conditions
- Injector:
- Split
- Column:
- DB-WAX, 15 m × 0.53 mm × 1.0 µm
- Detector:
- Flame Ionization (FID)
- Carrier:
- Helium
Note: Set detector gas flows as per manufacturer's specifications for H2 and X-Dry air.
13.1.1
Temperature Program
- Injector:
- 220 °C
- Detector:
- 260 °C
- Start Temperature:
- 120 °C, hold for 2 minutes
- Rate:
- 15 °C/minute to 180 °C, hold for 4 minutes
Total Run Time: 10.00 minutes
Note: Adjustment to the operating conditions may be required, depending on instrument and column conditions as well as resolution of the analyte peak.
13.1.2
Autosampler Conditions
Injection volume: 1.0 µL
Note: The first standard should be injected a minimum of three times initially to recondition the column.
13.1.3
The DB-WAX column has a polyethylene glycol stationary phase ideal for the separation of glycols. Tailing effects, however, may be very severe in the case of glycerol if the incorrect chromatographic conditions are used. Tailing effects may also be caused by solvent effect and/or reactivity in the injector.
Note: Methanol is generally a poor solvent choice for injecting onto the GC because it causes an enormous amount of tailing. This effect is minimized by having a large split ratio, a very high linear velocity, a thick stationary phase, and deactivated glass injection liners.
Note: Reactivity in the injector is minimized by using deactivated glass inserts. It is necessary that the injection liner be changed between each set of samples (roughly 40 'true' samples) since the injected solution is quite dirty and creates active sites on the liner after repeated injections of sample.
14 Calculations
14.1
All results are expressed on an ‘as received’ basis. These may be expressed on a ‘dry matter’ basis using the appropriate moisture result.
Analytical Result:
Analyte [mg/g] = (AreaAnalyte Sample / AreaISTD Sample) × RF (mg/mL) × (Multiplier (mL) / Divisor (g)) where RF is determined from the calibration curve.
This figure shows the conversion of analyte % (as is) to a dry matter basis: where the percent moisture is determined from the same sample as received for humectant analysis.

Figure 14.1-a: Text description
Analyte (%)as is = Result (in mg/g)
divided by
10
Conversion of analyte % (as is) to a dry matter basis:
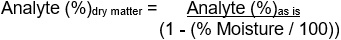
Figure 14.1-b: Text description
Analyte (%)dry matter = Analyte (%)as is
divided by
(1 - (% Moisture / 100))
where the percent moisture is determined from the same sample as received for humectant analysis.
15 Quality control
15.1
For a typical chromatogram, see appendix 1.
15.2
Typical Control Parameters
Note: If the control measurements are outside the tolerance limits of the expected values, appropriate investigation and action must be taken.
15.2.1
Laboratory Reagent Blank (LRB)
To detect potential contamination during the sample preparation and analysis processes, include a laboratory reagent blank (LRB). The LRB consists of all reagents and materials used in performing the analysis on test samples and is analyzed as a test sample.
15.2.2
Laboratory Fortified Matrix (LFM)
To detect potential matrix interferences, include a laboratory fortified matrix (LFM). During the sample preparation and/or analysis processes, divide a test sample and fortify an aliquot with at least one of the analytes of interest in known concentration. The level of fortification should reflect the range of typical results for that sample. The LFM is then analyzed as a test sample.
15.2.3
Laboratory Control Sample
To assess the overall performance of an analysis, a control sample is analyzed. The results of the control sample should be compared, using appropriate statistical techniques, to ‘expected values’ generated by the laboratory or, if none exist, to values found in literature. This provides information to the laboratory, on test accuracy and precision.
15.2.4
Standard as Sample
To assess the stability of the analytical system, a standard is analyzed as a sample. The results of this standard should be compared, using appropriate statistical techniques, to expected concentrations.
15.3
Recoveries and Levels of Contamination
15.3.1
Typical LRB values are small as shown in the table below:
| LRB (mg/g) |
|
|---|---|
| Propylene Glycol | 0.000 ± 0.000 |
| Glycerol | 0.002 ± 0.003 |
| Triethylene Glycol | 0.000 ± 0.001 |
15.3.2
Typical LFB recoveries fall in the range 85-115 % recovery.
15.3.3
Typical LFM recoveries fall in the range 90-110 % recovery.
15.4
Limit of Quantification (LOQ) and Limit of Detection (LOD)
15.4.1
The LOQ can be determined as the lowest standard times the dilution volume divided by the sample weight used in the preparation of the calibration curve (excluding a blank).
Example: Glycerol LOQ = 0.2 mg/mL × 25 mL / 2g
See 15.4.3 for typical values.
15.4.2
The Limit of Detection is 0.3 × LOQ.
15.4.3
Typical values for humectants:
| N/A | LOQ (mg/g) |
LOD (mg/g) |
|---|---|---|
| Propylene Glycol | 1.25 | 0.375 |
| Glycerol | 2.50 | 0.750 |
| Triethylene Glycol | 1.25 | 0.375 |
15.5
Stability of Reagents and Samples
15.5.1
Stock solutions are stable for at least one month if stored at 4 °C.
15.5.2
All samples should be analyzed within one week after extraction.
Appendix 1:
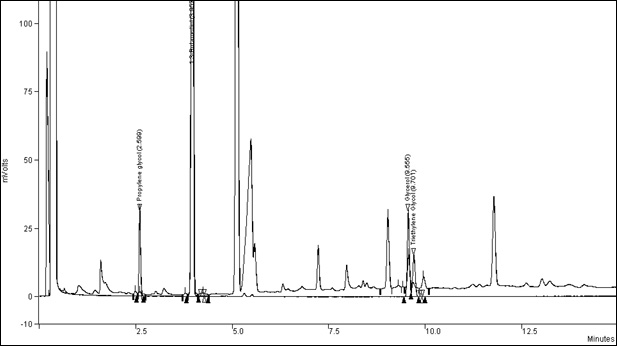
Top curve is a Canadian Monitor 7 tobacco and bottom is a standard.
Figure 1. Typical Chromatogram of Humectants: Text description
This figure includes the example chromatogram of Canadian Monitor 7 tobacco over a standard.
Typical retention times are:
- Propylene Glycol:
- 2.599 min
- 1,3-Butanediol:
- 3.957 min
- Glycerol:
- 9.555 min
- Triethylene Glycol:
- 9.701 min