Determination of tobacco specific nitrosamines in whole tobacco by LC-MS/MS: T-309B
1 Scope of applications
1.1
This method is suitable for the quantitative determination of 4 tobacco specific N-nitrosamines (TSNA) in whole tobacco and tobacco products:
N-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), N-nitrosoanatabine (NAT) and N-nitrosoanabasine (NAB) using Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS).
2 Normative references
2.1
Health Canada Official Method T-402. Preparation of Sample for Testing of Cigarettes, Tobacco Sticks, Cigarette Tobacco, Cigars, Little Cigars, Kreteks, Bidis, Leaf, Pipe and Smokeless Tobacco, 2016.
2.2
Health Canada Official Method T-115. Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 2016.
2.3
International Organization for Standardization, ISO 8243 Cigarettes-Sampling. 2013
2.4
International Organization for Standardization, ISO 15592-1 Fine-Cut tobacco and smoking articles made from it - Methods of sampling, conditioning and analysis - Part 1: Sampling. 2001.
2.5
AOAC INTERNATIONAL, AOAC Official Method 966.02 Moisture in Tobacco, Gravimetric Method. Official Methods of Analysis of AOAC INTERNATIONAL, 20th Ed., 2016.
3 Definitions
3.1
Refer to T-115 for definitions of terms used in this document.
4 Method summary
4.1
An aliquot of an internal standard solution (e.g. 300 µL), containing four deuterium labeled TSNA analogues (nominally 5000 ng/mL for NNN-d4, NAT-d4, NNK-d4 and 2000 ng/mL for NAB-d4), is spiked onto the tobacco product to be tested (e.g. 0.75 g).
4.2
TSNAs are extracted from the tobacco product using an aqueous ammonium acetate solution on a wrist action shaker.
4.3
The extract is then filtered and subject to high performance liquid chromatography tandem mass spectrometry (LC-MSMS) using electrospray ionization (ESI) in the positive mode. The triple-quadrupole mass analyzer, operating in multiple-reaction-monitoring (MRM) mode, allows for the mass-specific determination of target analytes by monitoring specific parent/daughter fragmentation patterns (MRM transitions).
4.4
Two mass transition pairs for each analyte can be used for quantification and assist with analyte confirmation. The most intensive pairs are normally used for quantification with the less intense transition pairs used as qualifiers for further compound confirmation.
Warning: The testing and evaluation of certain products against this test method may require the use of materials and/or equipment that are potentially hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with all existing applicable regulatory requirements prior to its use.
Warning: All 4 TSNAs are carcinogenic in several species of laboratory animals. Extreme care should be taken in handling these compounds (see guidelines for Laboratory Use of Chemical Carcinogens [16.7]).
All 4 TSNAs are sensitive to UV light. All preparation, and testing should be conducted in a UV sensitive environment.
5 Apparatus and equipment
5.1
Equipment needed to prepare tobacco for testing as specified in T-402
5.2
Analytical balance measuring to at least 4 decimal places
5.3
Burrell wrist action shaker, or equivalent
5.4
Non-ultra violet (non-UV) lighting, or equivalent
5.5
Gloves (as part of personal protective equipment – PPE)
5.6
100 mL capacity Erlenmeyer flasks (glass) or equivalent extraction vessel.
5.7
25 mm × 0.45 µm GD/X Nylon syringe filters (Whatman©6870-2504) or equivalent
5.8
5cc disposable syringe
5.9
1.5 mL capacity, amber screw top auto-sampler vials, or equivalent
5.10
Blue, Teflon-lined septa, or equivalent
5.11
Volumetric flasks: 10 mL, 50 mL, 100 mL, 200 mL, and 2 L
5.12
Disposable glass transfer pipettes
5.13
Glass graduated cylinders: 25 mL and 2000 mL
5.14
47 mm × 0.45 µm Nylon membrane filters, or equivalent
5.15
Volumetric pipette(s), or equivalent: 2 mL
5.16
Volumetric micropipettes, or equivalent: range 100 µL to 1000 µL
5.17
LC-MS/MS system- Liquid chromatographic system equipped with an autosampler, binary pump and column oven. Triple quadrupole mass spectrometer with an electrospray ionization (ESI) source. The system shall be equipped with a computerized control and data acquisition and processing system. The system must be able to obtain data under multiple reaction monitoring (MRM) detection mode.
5.18
Analytical column: Example Agilent Zorbax Eclipse XDB-C18 (narrow-bore, 2.1 × 150 mm, 3.5 µm particle size), or equivalent.
5.19
Guard column: Example OPTI-GUARD RP C18 guard column (1 mm, Violet) or equivalent
6 Reagents and supplies
6.1
All reagents shall be at least analytical reagent grade.
Note: Wherever possible, reagents are identified by their Chemical Abstract Service [CAS] registry numbers in square brackets.
6.2
N-Nitrosonornicotine (NNN) ≥ 98%
6.3
Deuterium-labeled N-Nitrosonornicotine (NNN-d4) ≥ 98%
6.4
N-Nitrosoanatabine (NAT) ≥ 98%
6.5
Deuterium-labeled N-Nitrosoanatabine (NAT-d4) ≥ 98%
6.6
N-Nitrosoanabasine (NAB) ≥ 98%
6.7
Deuterium-labeled N-Nitrosoanabasine (NAB-d4) ≥ 98%
6.8
4-(methylnitrosamino)-1-(3-pyridyl) -1- butanone (NNK) ≥ 98%
6.9
Deuterium-labeled 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK-d4) ≥ 98%
6.10
Ammonium acetate - [631-61-8]
6.11
Glacial acetic acid – [64-19-7]
6.12
Acetonitrile – [75-05-8] HPLC grade
6.13
Methanol - [67-56-1] HPLC grade
6.14
Water, Type I (as outlined in ASTM D1193, Table 1: Processes for Reagent Water Production, Note A)
7 Preparation of glassware
7.1
Clean and dry glassware in a manner to ensure that contamination from residues on glassware does not occur.
8 Preparation of solutions
8.1
Preparation of 100 mM Ammonium Acetate - Extraction Solution
8.1.1
Weigh 7.70 g of solid ammonium acetate into a 1 L volumetric flask.
8.1.2
Dissolve ammonium acetate with Type I water and make to volume.
8.1.3
Filter the buffer solution through a 0.45 µm Nylon membrane filter (or equivalent).
8.2
Preparation of 0.1% Acetic Acid in Methanol (v/v) (Mobile Phase Solution)
8.2.1
Transfer approximately 1400 mL of methanol into a 2 L volumetric flask.
8.2.2
Pipette 2 mL of glacial acetic acid into the flask.
8.2.3
Mix and make to volume with methanol.
8.2.4
Filter the solution through a 0.45 µm Nylon membrane filter or equivalent.
8.3
Preparation of 0.1% Acetic Acid in Water (v/v) (Mobile Phase Solution)
8.3.1
Transfer approximately 1400 mL of Type I water into a 2 L volumetric flask.
8.3.2
Pipette 2 mL of glacial acetic acid into the flask.
8.3.3
Mix and make to volume with Type I water.
8.3.4
Filter the solution through a 0.45 µm Nylon membrane filter, or equivalent.
9 Preparation of standards
9.1
Preparation of Internal Standard (ISTD) Stock Solutions
9.1.1
Prepare separate Internal Standard (ISTD) Primary stock solutions of approximately 1 mg/mL in acetonitrile by measuring 10 mg of each of the isotopically labeled TSNA into individual 10 mL volumetric flask using an analytical balance (record the weight to the nearest 0.1 mg) and make to volume with acetonitrile.
9.1.2
Prepare a combined ISTD Spiking Solution by transferring 0.5 mL of each ISTD primary stock solution of NNN-d4, NNK-d4, NAT-d4 and 0.2 mL of ISTD primary stock of NAB-d4 into a 100 mL volumetric flask and make to volume with acetonitrile.
9.1.3
Internal Standard (ISTD) Stock Solutions may be stored for up to 12 months if stored at -20 °C (± 5 °C) and protected from light.
Note: All standard solutions must be evaluated after more than 12 months of storage to ensure the integrity of the solutions.
9.2
Preparation of TSNA Stock Solutions
9.2.1
Prepare independent Primary Stock solutions of 1 mg/mL for NNN, NNK, NAT and 0.5 mg/mL for NAB by measuring 10 mg of NNN, NNK and NAT and 5 mg of NAB into individual 10 mL volumetric flask using an analytical balance (record the weight to the nearest 0.1 mg) and make to volume with acetonitrile.
9.2.2
Prepare a combined TSNA stock solution (Secondary Stock 1) of approximately 40 µg/mL for NNN, NAT and NNK, and approximately 10 µg/mL NAB in acetonitrile.
9.2.3
Prepare a diluted TSNA stock solution (Secondary Stock 2) of approximately 400 ng/mL for NNN, NAT and NNK, and approximately 100 ng/mL NAB in 30% acetonitrile / 70% Type I water.
9.3
Preparation of Working Standard Solutions (Calibration Standards)
9.3.1
Prepare Working Standard Solutions by transferring the prescribed volume of TSNA Secondary Stock 2 solution into 9 separate 10 mL volumetric flasks (see Table 1 for details).
9.3.2
Add 100 µL of the combined ISTD spiking solution into each flask.
9.3.3
Add the prescribed volume of acetonitrile to each volumetric flask.
9.3.4
Make up to volume (in 10 mL volumetric flask) with 100 mM ammonium acetate solution.
Table 1: Preparation of TSNA working Standards (Final volume 10 mL for each level)
| Working Std. Level | Volume of TSNA Secondary Stock 2 (μL) |
Volume of Acetonitrile (μL) |
|---|---|---|
| S0 | 0 | 1000 |
| Lowest std | 10 | 1000 |
| S1 | 20 | 1000 |
| S2 | 100 | 1000 |
| S3 | 200 | 1000 |
| S4 | 500 | 800 |
| S5 | 1000 | 700 |
| S6 | 2500 | 250 |
| S7 | 5000 | 0 |
9.3.5
Working standard solutions, stored at 4 °C (± 1 °C) should be stable for 6 months when protected from light.
Note: The calibration solutions range from 0.1 to 220 ng/mL dependent on the analyte (see appendix 1 for details). The lowest standard can be used in the determination of the limit of detection (LOD) and the limit of quantification (LOQ).
Note: Additional standards may need to be prepared to cover the range of anticipated responses for test samples.
Note: All standard solutions may be stored for up to 6 months if refrigerated at 4 °C (± 1 °C) or 12 months if kept at -20 °C (± 5 °C) and if protected from light.
10 Sampling
10.1
The sampling of cigarettes for the purpose of testing shall be in accord with ISO 8243.
10.2
The sampling of kreteks,"little cigars" bidis, tobacco sticks for the purpose of testing shall be in accord with ISO 8243, but modified such that the term "cigarette" is substituted with "kreteks", "little cigars", "bidis" or "tobacco sticks", whereby the term "carton" is equivalent to 200 units.
10.3
The sampling of cigars for the purpose of testing shall be in accord ISO 8243, but modified such that the term "cigarette" is substituted with "cigar", whereby 200 units of cigarette is equivalent to 200 grams of cigar.
10.4
The sampling of cigarette tobacco for the purpose of testing shall be in accord with ISO 15592-1.
10.5
The sampling of leaf tobacco, pipe tobacco or smokeless tobacco shall be in accord with ISO 15592-1 but modified such that the term "fine-cut" is substituted with "leaf tobacco", "pipe tobacco" or "smokeless tobacco".
11 Tobacco product preparation
11.1
Preparation of Test Sample
11.1.1
Remove product from its packaging to make a composite sample.
11.1.2
The preparation of tobacco products for the purpose of testing shall be as specified in T-402.
Note: A separate 5.000 g portion (per replicate) of the test sample is to be analyzed for moisture in order to present results on a "dry matter" basis (if necessary).
12 Sample preparation
12.1
Extraction of Tobacco and Tobacco Products
12.1.1
Use only non-UV lighting in the room(s) in which this analysis is conducted.
12.1.2
Using an analytical balance, weigh ~ 0.750 g (note the exact weight to 3 decimals) of the test sample into a 100 mL capacity extraction vessel (glass).
12.1.3
Add 300 µL of Internal Standard (IS) spiking solution onto the sample.
12.1.4
Add 30 mL of 100 mM ammonium acetate solution to the test sample in the extraction vessel.
12.1.5
Place the test sample on a wrist-action shaker for 30 minutes to extract the TSNA.
12.2
Sample Clean-up
12.2.1
Syringe filter the extract into two amber auto-sampler vials (allows for a back-up sample).
12.2.2
Store the vials at 4 °C (± 1 °C) until analyzed.
Note: Samples are stable under these storage conditions for a maximum of 6 days.
13 Sample analysis
13.1
Operating conditions of the HPLC (Example):
13.1.1
Column: Agilent Zorbax Eclipse XDB-C18 (2.1 × 150 mm. 3.5 μm particle size)
13.1.2
Column oven: 40 °C
13.1.3
Mobile phase:
- A: 0.1% acetic acid in H2O
- B: 0.1% acetic acid in MeOH
13.1.4
Gradient Mobile phase: Example
| N/A | Time (min) |
Mobile phase Composition | Flow rate (µL/min) |
|
|---|---|---|---|---|
| A (%) | B (%) | |||
| Equilibrium | 6 | 50 | 50 | 200 |
| 3 | 10 | 90 | 200 | |
| 4 | 0 | 100 | 200 | |
| 5 | 0 | 100 | 200 | |
| 5.5 | 50 | 50 | 200 | |
| 6 | 50 | 50 | 200 | |
Note: Adjustment to the operating conditions may be required, depending on instrument and column conditions as well as resolution of the analyte peak.
13.1.5
Injection volume: 5 µL
13.1.6
Sample wash: Methanol
13.1.7
Total analysis time: 12 min (including column equilibration)
13.2
Operating conditions for ESI interface (Example):
13.2.1
Ionization / Mode: ESI+/MRM
13.2.2
IonSpray Voltage: 1500 V
13.2.3
TurboIon Spray Temp: 450 °C
13.2.4
Curtain Gas Type: Nitrogen Setting: 10
13.2.5
CID Gas Type: Nitrogen Setting: 10
13.2.6
Nebulizing Gas Type: Nitrogen Setting: 10
13.3
Operating conditions for mass spectrometer Analyzer (Example):
| Analyte | Quad1 (m/z) |
Quad3 (m/z) |
|---|---|---|
| NNN1 | 178.2 | 148 |
| NNN2 | 178.2 | 120 |
| NNN-d4 | 182.2 | 152 |
| NNK1 | 208.2 | 122 |
| NNK2 | 208.2 | 106 |
| NNK-d4 | 212.2 | 126 |
| NAT1 | 190.1 | 160 |
| NAT2 | 190.1 | 106 |
| NAT-d4 | 194.1 | 164 |
| NAB1 | 192.2 | 162 |
| NAB2 | 192.2 | 133 |
| NAB-d4 | 196.2 | 166 |
13.4
LC-MS/MS Calibration:
13.4.1
Inject each standard solution using optimized conditions and determine peak areas for each of the primary mass transitions and corresponding IS (deuterated analogue) transition.
13.4.2
Determine the corresponding response ratio of analyte to internal standard to build a calibration (response) curve.
13.5
TSNA Determination:
13.5.1
Inject each test sample extract using optimised conditions and determine peak areas for each of the primary mass transitions and corresponding IS (deuterated analogue) transition.
14 Calculations
14.1
Obtain the content, M (ng/g), of a given TSNA from the formula:
- M (ng/g) = CVs/N
- where:
- C = analytical concentration determined by ISTD calibration of given TSNA.
- Vs = extract volume (e.g 30mL).
- N = the weight (in g) of tobacco extracted (e.g. 0.75 g).
14.2
In order to convert the result to ng/g on a 'dry matter basis,' correct for moisture by using the following formula:
M (ng/g) dry matter = M (ng/g) as is / (1 - (% Moisture/100))
where the % moisture is determined from the same sample 'as received' by AOAC Official Method 966.02.
15 Quality control
15.1
For a typical chromatogram, see appendix 2.
15.2
Typical Control Parameters
Note: If the control measurements are outside the tolerance limits of the expected values, appropriate investigation and action must be taken.
15.2.1
Laboratory Reagent Blank (LRB)
To detect potential contamination during the sample preparation and analysis processes, include a laboratory reagent blank (LRB). The LRB consists of all reagents and materials used in performing the analysis on test samples and is analyzed as a test sample.
15.2.2
Laboratory Fortified Blank (LFB)
To detect potential loss of analyte during the sample preparation and analysis processes, include a laboratory fortified blank (LFB). The LFB consists of all reagents and materials used in performing the analysis on test samples plus fortification with a known concentration of at least one of the analytes of interest. The level of fortification should reflect the range of typical results for that sample. The LFB is then analyzed as a test sample.
15.2.3
Laboratory Fortified Matrix (LFM)
To detect potential matrix interferences, include a laboratory fortified matrix (LFM). During the sample preparation and/or analysis processes, divide a test sample and fortify an aliquot with at least one of the analytes of interest in known concentration. The level of fortification should reflect the range of typical results for that sample. The LFM is then analyzed as a test sample.
15.2.4
Laboratory Control Sample
To assess the overall performance of an analysis, a control sample is analyzed. The results of the control sample should be compared, using appropriate statistical techniques, to 'expected values' generated by the laboratory or, if none exist, to values found in literature. This provides information to the laboratory, on test accuracy and precision.
15.2.5
Standard as Sample
To assess the stability of the analytical system, a standard is analyzed as a sample. The results of this standard should be compared, using appropriate statistical techniques, to expected concentrations.
15.3
Recoveries and Levels of Contamination
15.3.1
A typical LRB should be less than the LOD.
15.3.2
Typical LFB and LFM recoveries fall in the range 85-115% recovery.
15.4
Limit of Detection (LOD) and Limit of Quantification (LOQ)
15.4.1
The LOD can be determined as 3 times the standard deviation of results obtained by analyzing the lowest standard level a minimum of 10 times over several days. See Table 2 for typical values.
15.4.2
The LOQ can be determined as 10 times the standard deviation of results obtained by analyzing the lowest standard level a minimum of 10 times over several days. See Table 2 for typical values.
Table 2: Typical Values for Limit of Detection (LOD) and Limit of Quantification (LOQ)
| Compounds | Instrument | Whole tobacco | ||
|---|---|---|---|---|
| LOD (ng/mL) |
LOQ (ng/mL) |
LOD (ng/mL) |
LOQ (ng/mL) |
|
| NNN | 0.120 | 0.400 | 4.80 | 16.00 |
| NAT | 0.120 | 0.400 | 4.80 | 16.00 |
| NAB | 0.030 | 0.100 | 1.20 | 4.00 |
| NNK | 0.120 | 0.400 | 4.80 | 16.00 |
Note: In the above calculations the volume of extraction solution is 30 mL and the weight of tobacco extracted is 0.75 g.
The LOD and LOQ expressed in this table do not account for signal suppression.
Note: For certain test samples, the LOD/LOQ may also be affected by the sample matrix and may significantly reduce the analyte signal compared to that in the standards solution due to ion suppression. Therefore, the sample matrix can influence the LOD and LOQ level such that it may need to be determined using the Signal to Noise Ratio: S/N = 3 for LOD and S/N = 10 for LOQ.
15.5
Stability of Reagents and Samples
15.5.1
All standard solutions may be stored for up to 6 months if refrigerated at 4 °C (± 1 °C) or 12 months if kept at -20 °C (± 5 °C) and if protected from light.
Note: To minimize potential evaporation, ISTD secondary stock solution should be kept in small portions in separate screw cap scintillation vials.
15.5.2
All samples are stable for a maximum of 6 days if refrigerated at 4 °C (± 1 °C) or for a maximum of 2 months if kept at -20 °C (± 5 °C) and if stored in the dark.
16 References
16.1
Wu, W.; Ashley, D. L.; Watson, C. H. Anal. Chem. 2003, 75, 4827-4832.
16.2
Wagner, K. A.; Finkel, N. H.; Fossett, J. E.; Gillman, I. G. Anal. Chem. 2005, 77, 1001-1006.
16.3
Lee, J-M.; Shin, J-W.; Oh, I-H.; Lee U-C.; Rhee M-S. 2004 CORESTA Congress Kyoto. Paper SS20; full text available on CORESTA CD-ROM Vol. 22; abstract available on the Internet at http://www.coresta.org/Past_Abstracts/Kyoto2004-SmokeTech.pdf (PDF format) (accessed December 29, 2006).
16.4
Chwojdak, C. A.; Self, D. A.; Wheeler, H. R. A Collaborative, Harmonized LC-MS/MS Method for the Determination of Tobacco Specific Nitrosamines (TSNA) in Tobacco and Tobacco Related Materials. 61st Tobacco Science Research Conference, Charlotte, NC. USA. September 24, 2007.
16.5
Risner, C. H. and Wendelboe, F. N. 1994. Quantification of tobacco specific nitrosamines in tobacco. Tob. Sci. 38: 1-6.
16.6
United States. Protocol to Measure the Quantity of Nicotine Contained in Smokeless Tobacco Products Manufactured, Imported, or Packaged in the United States. Federal Registrar. 62: 85. 1997.
16.7
NIH Guidelines for the Laboratory Use of Chemical Carcinogens; NIH Publication 81-2385, 1981.
16.8
ASTM International, ASTM Standard D1193-06(2011). Standard Specifications for Reagent Water.
Appendix 1: Target concentrations of working standard and internal standard solutions
| Running Standard ID |
NNN (ng/mL) |
NNK (ng/mL) |
NAT (ng/mL) |
NAB (ng/mL) |
|---|---|---|---|---|
| 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Lowest std | 0.4 | 0.4 | 0.4 | 0.1 |
| 1 | 0.8 | 0.8 | 0.8 | 0.2 |
| 2 | 4.0 | 4.0 | 4.0 | 1.0 |
| 3 | 8.0 | 8.0 | 8.0 | 2.0 |
| 4 | 20.0 | 20.0 | 20.0 | 5.0 |
| 5 | 40.0 | 40.0 | 40.0 | 10.0 |
| 6 | 100.0 | 100.0 | 100.0 | 25.0 |
| 7 | 200.0 | 200.0 | 200.0 | 50.0 |
| Internal Standard | NNN-d4 | NNK-d4 | NAT-d4 | NAB-d4 |
| Conc. (ng/mL) | 50 | 50 | 50 | 20 |
Appendix 2: Typical chromatogram of a standard
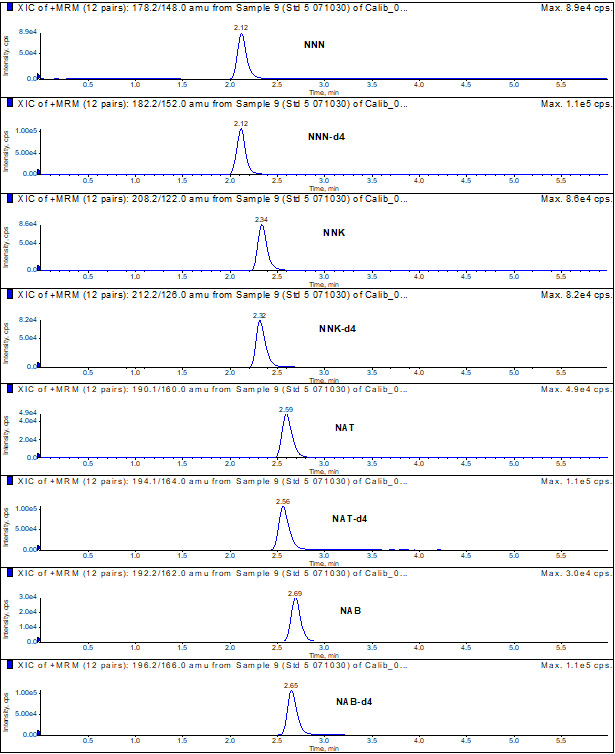
Typical chromatogram of a standard: Text description
This figure shows the typical chromatogram of the standard solutions. The retention times are given for the tobacco specific nitrosamines: NNN (2.12), NNN-d4 (2.12), NNK (2.34), NNK-d4 (2.32), NAT (2.59), NAT-d4 (2.56), NAB (2.69), NAB-d4 (2.65).