2021-22 Departmental Results Report
ISSN: 2561-0732
Patented Medicine Prices Review Board
The Honourable Jean-Yves Duclos
Minister of Health
Table of contents
From the Acting Chairperson
I am pleased to present the 2021-22 Departmental Results Report for the Patented Medicine Prices Review Board (PMPRB).
The PMPRB is an independent quasi-judicial body established by Parliament in 1987 under the Patent Act (the “Act”). The PMPRB is a consumer protection agency with a dual regulatory and reporting mandate. Its regulatory mandate is to ensure that the prices of patented medicines sold in Canada are not excessive. Its reporting mandate is to provide stakeholders with pharmaceutical trends information to help them make informed choices.
For the PMPRB, year two of the COVID-19 pandemic was very much a repeat performance of year one. Like most other government departments, our focus was on supporting the mental and physical health of staff in their continuing efforts to deliver on the PMPRB’s reporting and regulatory mandate in a fully remote working environment. Similarly, pre-existing policy priorities, such as reform and modernization of the PMPRB’s decades old regulatory framework, continued to take a backseat to a whole of government effort to mitigate the economic and social impact of the pandemic on Canadians.
The upshot of a sustained pause in the PMPRB’s policy development activities is that it afforded staff ample time to update and perfect the changes in our operating systems necessary to give effect to the eventual coming into force of the amendments to the Patented Medicines Regulations which is slated for later this year. In particular, the PMPRB made a number of significant improvements to our online filing system, our standard operating procedures and our internal data management systems. Staff also made good use of this additional time to refine and quantify the benchmarks the PMPRB will use in its Guidelines Monitoring and Evaluation Plan (GMEP). The purpose of the GMEP is to enable the PMPRB to gauge the impact of the regulatory reforms on various key indicators and ensure that they are meeting the government’s underlying policy objectives.
As a member of the Health Portfolio, the PMPRB plays a role in advancing the broader objective of improving the health of Canadians through a responsible, accessible, and sustainable health system. The PMPRB continues to work closely with our federal, provincial, and territorial (F/P/T) health partners to align and optimize our respective processes. Likewise, under our National Prescription Drug Utilization Information System (NPDUIS) reporting mandate established by the Minister under s. 90 of the Act, we provided analytical support and expertise to our health partners, as appropriate, in our collective efforts to advance policy work on pan-Canadian initiatives to improve the pricing and reimbursement of pharmaceuticals in Canada, including the potential establishment of a Canadian drug agency, a national formulary and a rare disease drug strategy.
Finally, 2021-22 was a year of significant change in senior leadership, with Dr. Mitchell Levine’s five-year term as the PMPRB’s Chairperson coming to an end in November 2021. Although a permanent replacement has yet to be named, I and my fellow Board members look forward to turning the page on the multiyear effort to modernize our regulatory framework and working with our stakeholders and partners to support the government’s ambitious suite of priorities in pharmaceutical policy under new leadership.
Mélanie Bourassa Forcier
Acting Chairperson
Results at a glance
Priority 1 – Implement new pricing framework and begin evaluating its impact
The PMPRB continued to prepare for the implementation of the Health Canada-sponsored amendments to the Patented Medicines Regulations, which came into force in July 2022. In July 2021, the PMPRB held a Notice and Comment consultation on proposed changes to the new 2020 Guidelines. These changes included revising the definition of Gap medicines and the international price tests for Grandfathered medicines. Although the Board ultimately decided not to proceed with the proposed changes until such time as there is clarity on the fate of the regulatory amendments, changes of this kind may still be necessary at a later date.
The PMPRB also further developed its Guidelines Monitoring and Evaluation Plan (GMEP) to monitor and analyze trends in the pharmaceutical market before and after the implementation of the PMPRB’s new regulations and Guidelines to assess whether they are working as intended and to inform the need for any future adjustments. In the spring of 2021, the PMPRB invited stakeholders to help with the development of this plan by providing comments on the proposed draft GMEP and participating in a public webinar.
Priority 2 –Support the Government’s high-level priorities for the future of pharmaceutical management in Canada.
The PMPRB continued to work with F/P/T health partners to align and optimize their respective processes in the context of the new framework and other recent or ongoing reforms that impact pricing and reimbursement.
In the past year, the PMPRB also continued to provide analytical support and expertise to health partners, as appropriate, in efforts to advance policy work relating to the foundational elements of national pharmacare and other pan-Canadian initiatives to improve the pricing and reimbursement of pharmaceuticals in Canada.
Furthermore, the PMPRB continued to leverage its resources and expertise to optimize its ability to protect consumers from excessive prices and maximize its value proposition to its F/P/T health partners and the health system as a whole.
For more information on the PMPRB’s plans, priorities and results achieved, see the “Results: what we achieved” section of this report.
Results: what we achieved
Core responsibility
Regulate Patented Medicine Prices
Description
The Patented Medicine Prices Review Board (PMPRB) regulates the prices of patented medicines by setting non-excessive price ceilings and taking enforcement action before the Board in the event of non-compliance.Results
In response to a third delay in the implementation of the amended Patented Medicine Regulations (Regulations)to January 1, 2022, the PMPRB issued a Notice and Comment period on proposed changes to their revised Guidelines, which give effect to the Regulations. The proposals addressed the definition of Gap medicines, the references to the comparator countries and the international price tests for Grandfathered medicines and their line extensions. With consideration to stakeholder feedback, the Board ultimately decided not to proceed with the proposed changes. However, as the coming-into-force of the Regulations was postponed to July 1, 2022, additional revisions to the Guidelines have since been made necessary to support the new framework and timeline.
The PMPRB faced legal challenges related to the reform of the Regulations and had relevant matters before the Federal Court, Federal Court of Appeal, and the Quebec Court of Appeal in 2021-22, for which it provided expertise and legal support to counsel for the Attorney General in their defence of the reforms. A total of 128 Access to Information (ATI) applications were also submitted to the PMPRB this fiscal year seeking information on the reform process and departmental consultation strategies, presenting a significant increase over the 47 requests received the year before.
In February 2022, the Quebec Court of Appeal issued its decision in respect of the Merck challenge to the constitutionality of the amended Patented Medicines Regulations. The new basket of comparator countries was upheld but new section 85(1) excessive pricing factors and amendments relating to the calculation of net prices, subsection 3(4) of the amended Regulations, were deemed unconstitutional.
In September 2021, the Attorney General sought leave to appeal a decision of the Federal Court of Appeal that remitted the matter of Alexion’s Soliris (eculizumab) to the Patented Medicine Prices Review Board for redetermination. In March 2022, the Supreme Court of Canada dismissed the Attorney General of Canada’s application for leave to appeal. The decision from the Federal Court of Appeal found the Board’s reasoning to be insufficiently supported in its determination that Soliris was sold at an excessive price. Public hearings on the matter started in June 2022.
The PMPRB saw a decrease in the rate of compliance with its Guidelines this fiscal year, from 86.3% in 2020-21 to 84.6% in 2021-22, well below the 95% target. In recent years, patentees have been more apt to challenge the price ceilings applied under the PMPRB’s pricing Guidelines to the latest generation of very high-cost medicines that are coming to dominate the market. In 2021-22, the PMPRB accepted three Voluntary Compliance Undertakings (VCU), which resulted in a price reduction for seven DINs,Footnote i and recovered $38,309.14 in excess revenues through payments to the Government of Canada.
Under its Pharmaceutical Trends Program, the PMPRB published four analytical studies on Canada’s pharmaceutical market in 2021-22, exploring public drug plan expenditures, formulary alignment and spending on expensive drugs for rare diseases (EDRDs), and delivered oral and poster presentations at five academic and scientific conferences. These studies have served to support informed decision-making on drug pricing in Canadian and international markets and have provided evidence-based context for the regulatory reform.
The introduction of new exceptionally high-priced patented medicines has been a major driver of sales growth in recent years. High-cost medicines now account for more than 50 percent of all patented medicine sales in Canada, despite being used by less than one percent of Canadians.Gender-based analysis plus
The PMPRB recognizes that sex and gender differences, race, ethnicity, age and mental or physical disability are factors to consider in the accessibility, affordability and appropriate use of prescription medicines and medical devices. Differences in sex and gender+ roles, income and utilization of health care services can affect access to medicines and health insurance, as well as prescribing patterns and medicine use, and may have important repercussions for health and well-being.
Since the price of a patented medicine does not vary for the sex or gender+ of the user, the PMPRB’s price review process does not take explicit account of the diversity of user groups or their economic situation. Lower patented medicine prices, and associated savings for all payers, will benefit all populations directly through lower out-of-pocket costs and indirectly through health system reinvestments and improved access to better care.
Experimentation
The PMPRB did not engage in any experimentation in 2021-22. The primary focus of the organization continues to be the reform and modernization of its regulatory framework. To that end, it has conducted extensive and far-reaching public consultations. As the PMPRB switches gears from policy development of the new framework to its implementation, we will be monitoring and assessing the impacts of the Guidelines and the effects of the new legal regime both on the external operating context and on internal PMPRB operations. The PMPRB lacks the human or financial resources at this time to take on any additional experimental initiatives.
Key risks
The PMPRB has taken action to mitigate five potential risks to the achievement of results for its Core Responsibility. First, to prepare for the possibility that the new Regulations will be further delayed because of the COVID-19 pandemic, the PMPRB has continued to conduct price reviews under the old Guidelines and Regulations to achieve the best possible result for Canadians and has focused its enforcement resources on cases that are most relevant to consumers. In addition, it has worked internally to prepare its technical and procedural systems to respond to the demands of the revised reporting requirements when they come into force.
Second, to avoid confusion for patentees about what data to file and how and when that data will be used for ceiling price calculation and compliance purposes under the new regulatory requirements, the PMPRB has implemented an outreach strategy so that patentees understand the new Guidelines and has developed tools to help them comply.
Third, in the case of unintended consequences of the new Regulations on patient access to innovative new medicines in Canada, the PMPRB has developed a Guideline Monitoring and Evaluation Plan (GMEP) in consultation with stakeholders to review the effects on price, access and research and development in Canada so that corrective action can be taken quickly if warranted.
Fourth, the PMPRB is making use of additional funding received through Budget 2017 to prepare for the possible need to contend with contested pricing matters as voluntary compliance with the PMPRB’s non-binding Guidelines may initially decline after implementation. The PMPRB’s own dedicated hearing facilities will ensure that it has the capacity to accommodate multiple parallel hearings, if necessary.
Finally, the PMPRB is working with the federal Attorney General to ensure that legal challenges to the new Regulations and Guidelines are properly defended, and the risk of an adverse judgement(s) is mitigated.
Results achieved
The following table shows, for Regulate Patented Medicine Prices, the results achieved, the performance indicators, the targets and the target dates for 2021–22, and the actual results for the three most recent fiscal years for which actual results are available.
| Departmental results | Performance indicators | Target | Date to achieve target | 2019–20 actual results | 2020–21 actual results | 2021–22 actual results |
|---|---|---|---|---|---|---|
Affordable patented medicine prices |
% of patented medicine prices in Canada are below the median of the PMPRB’s comparator countries |
50%(a) |
March 31, 2022 |
56.9% |
58.2% |
59.5%(b) |
% of patented medicine prices in Canada within the thresholds set out in the Guidelines |
95%(c) |
March 31, 2022 |
88.4%(d) |
86.3% |
84.6%(e) |
|
(a) Operating under the premise that the PMPRB would continue to conduct its price reviews without significant changes in its regulatory framework, the PMPRB established a target of 50% of patented medicine prices being below the median price, primarily as a result of voluntary compliance with the non-binding Guidelines by patentees. Analysis in the PMPRB’s 2015 Annual Report indicated that the percentage of patented medicines priced below the median price of the PMPRB’s comparator countries was 51.8%, a decline from the previous two years. Based on these factors, it was determined that 50% would be a reasonable target. (b) The 59.5% of patented medicine prices in Canada reported as being below the median international price includes a significant number of patented medicines being sold in fewer than five countries and therefore are not being compared to the actual median international price. Of the 1,158 patented medicines sold in Canada in 2021, only 700 were sold in five or more countries. Of this 700, only 343 patented medicines (49.0%) had a Canadian price below the median price. This is a significant difference from the reported 59.5%, in part because the median international price for medicines with fewer than five comparator countries are typically heavily skewed towards prices in the United States. (c) This percentage, based on the number of price reviews completed by March 31 of the fiscal year referred to, is calculated as follows: the sum of the number of price reviews found to be within the Guidelines, plus the number of price reviews which did not trigger an investigation, plus the number of Voluntary Compliance Undertakings; divided by the number of patented medicines for which the price review was completed at March 31 of the fiscal year. (d) Because of an adjustment to the calculation of the denominator, this number does not match the number reported in the 2021-22 Departmental Plan (85.9%). (e) As of March 31, 2022, 44 patented medicines were still under review, and 169 were under investigation, four were the subject of a hearing and one was subject to a Stay Order. |
||||||
Financial, human resources and performance information for the PMPRB’s Program Inventory is available in GC InfoBase.Footnote ii
Budgetary financial resources (dollars)
The following table shows, for Regulate Patented Medicine Prices, budgetary spending for 2021–22, as well as actual spending for that year.
| 2021–22 Main Estimates |
2021–22 planned spending |
2021–22 total authorities available for use |
2021–22 actual spending (authorities used) |
2021–22 difference (actual spending minus planned spending) |
|---|---|---|---|---|
15,805,187 |
15,805,187 |
15,718,357 |
8,999,721 |
(6,805,466) |
Financial, human resources and performance information for the PMPRB’s Program Inventory is available in GC InfoBase.Footnote iii
Human resources (full-time equivalents)
The following table shows, in full‑time equivalents, the human resources the department needed to fulfill this core responsibility for 2021–22.
| 2021–22 planned full-time equivalents |
2021–22 actual full-time equivalents |
2021–22 difference (actual full-time equivalents minus planned full‑time equivalents) |
|---|---|---|
| 61 | 55 | (6)(a) |
(a) Staff departures, difficulties in hiring replacements, and the postponement of some intended staffing actions due to the delays in the regulatory reform process contributed to this difference. |
||
Financial, human resources and performance information for the PMPRB’s Program Inventory is available in GC InfoBase.Footnote iv
Internal Services
Description
Internal services are those groups of related activities and resources that the federal government considers to be services in support of programs and/or required to meet corporate obligations of an organization. Internal services refers to the activities and resources of the 10 distinct service categories that support program delivery in the organization, regardless of the internal services delivery model in a department. The 10 service categories are:
- acquisition management services
- communication services
- financial management services
- human resources management services
- information management services
- information technology services
- legal services
- material management services
- management and oversight services
- real property management services
Results:
In 2021-22, the PMPRB responded to increased internal demands related to the upcoming implementation of the revised Patented Medicine Regulations and took important steps towards solidifying its position as an employer of choice through engagement with employment equity and wellness initiatives.
IT infrastructure has been put in place to facilitate the adoption of a new online filing tool launched in January 2022 and the continued remote work of all PMPRB Staff. The PMPRB is scheduled to make in-person use of its new dedicated hearing room facilities in June 2022.
The PMPRB’s Compliance Information Management System (“CIMS”) is a web-based application used to review and analyze data filed by patentees and assess patentees’ compliance. Several enhancements to CIMS are underway to allow the system to accept and process the additional information patentees must provide under the new Regulations and Guidelines. While modernization of the online filling tool is largely complete, updating price tests for new medicine reviews and upgrading several data management components of the application is ongoing.
The PMPRB also launched a reconstructed version of its internal information management system in 2021-22. The Records and Information Management System (“RIMS”) stores all PMPRB documents in digital form throughout their lifecycle. Recent updates ensure that the PMPRB continues to remain compliant with information management policies and the system is accessible and efficient.
As part of the Clerk of the Privy Council’s call to action on anti-racism, equity and inclusion, the PMPRB is on the Mentorship Plus Interdepartmental Working group for Small Departments and Agencies and has taken significant steps to implement this initiative over the past fiscal year. Mentorship Plus is a new initiative co‑developed by members of employment equity (EE) and equity‑seeking groups to better support leadership development, with specific emphasis on supporting members of underrepresented groups who aspire to leadership and executive positions. The PMPRB launched the Sponsorship component of this initiative in 2021-22, with the Mentorship component set for release in May 2022.
In compliance with Indigenous Services Canada requirements, the PMPRB formulated and implemented a strategy to increase procurement purchases from Indigenous businesses and suppliers to at least 5% of goods and services funds spent from 2022-23 onward.
As reforms to the PMPRB’s regulatory framework are ongoing, a review of the Departmental Results Framework (DRF) has not been initiated. Performance indicators will be reassessed following the coming-into-force of the amended Regulations.
Budgetary financial resources (dollars)
The following table shows, for internal services, budgetary spending for 2021–22, as well as spending for that year.
| 2021–22 Main Estimates |
2021–22 planned spending |
2021–22 total authorities available for use |
2021–22 actual spending (authorities used) |
2021–22 difference (actual spending minus planned spending) |
|---|---|---|---|---|
3,087,135 |
3,087,135 |
4,122,282 |
3,339,688 |
252,553 |
Human resources (full-time equivalents)
The following table shows, in full‑time equivalents, the human resources the department needed to carry out its internal services for 2021–22.
| 2021–22 planned full-time equivalents |
2021–22 actual full-time equivalents |
2021–22 difference (actual full-time equivalents minus planned full‑time equivalents) |
|---|---|---|
24 |
23 |
(1) |
Spending and human resources
Spending
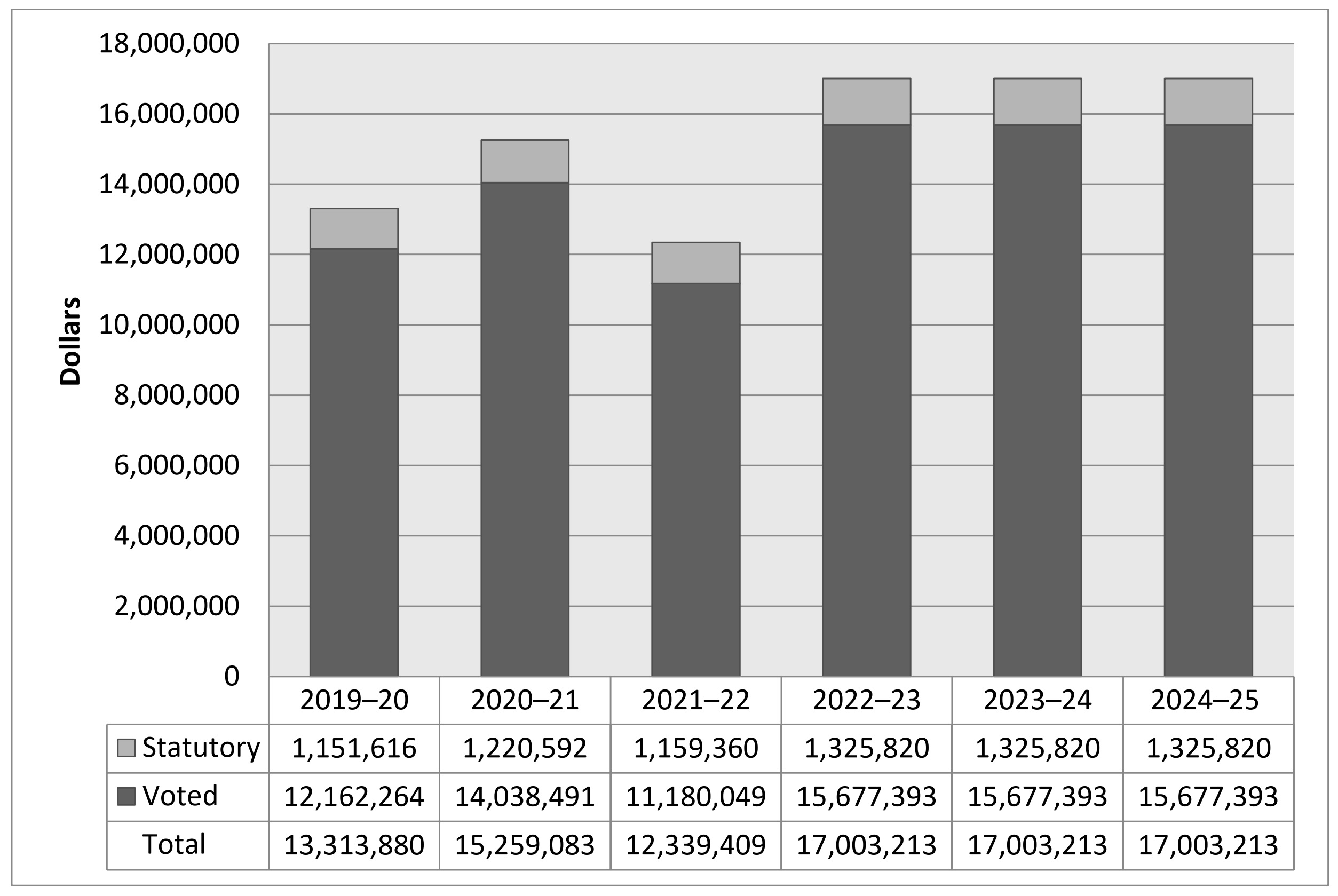
Spending 2019–20 to 2024–25
The following graph presents planned (voted and statutory spending) over time.
Long description
| 2019-20 | 2020-21 | 2021-22 | 2022-23 | 2023-24 | |
|---|---|---|---|---|---|
Voted |
12,162,264 |
14,038,491 |
11,180,049 |
15,677,393 |
15,677,393 |
Statutory |
1,151,616 |
1,220,592 |
1,159,360 |
1,325,820 |
1,325,820 |
Total |
13,313,880 |
15,259,083 |
12,339,409 |
17,003,213 |
17,003,213 |
As announced in Budget 2017, the PMPRB received additional funding for future years; $3,849,215 in 2018-19, $5,694,677 in 2019-20, $6,671,853 in 2020-21, $7,668,725 in 2021-22 and $5,680,633 in 2022-23 and ongoing, including Employee Benefits Payments (EBP) and increased funding for the Special Purpose Allotment (SPA).
Voted spending in 2020-21 was higher than voted spending in 2019-20 largely due to hearing costs in the matter of the price of “Procysbi” by Horizon Therapeutics Canada, as well as the construction costs for the PMPRB’s dedicated hearing facilities. As these were not factors in the following year, spending was lower in 2021-22.
For purposes of forecasting Planned Spending for 2022-23 and future years, it is necessary to assume that the entire SPA funding for hearings will be spent because these expenditures are dependent on the number of hearings, and the length and complexity of the hearings held, which are difficult to predict. The amount of the SPA for 2022-23 and beyond is $4,463,361.
Budgetary performance summary for core responsibilities and internal services (dollars)
The “Budgetary performance summary for core responsibilities and internal services” table presents the budgetary financial resources allocated for the PMPRB’s core responsibilities and for internal services.
| Core responsibilities and internal services | 2021–22 Main Estimates |
2021–22 planned spending |
2022–23 planned spending |
2023–24 planned spending |
2021–22 total authorities available for use |
2019–20 actual spending (authorities used) | 2020–21 actual spending (authorities used) | 2021–22 actual spending (authorities used) |
|---|---|---|---|---|---|---|---|---|
Regulate patented medicine prices |
15,805,187 |
15,805,187 |
13,870,473 |
13,870,473 |
15,178,357 |
9,336,597 |
10,858,873 |
8,999,721 |
Subtotal |
15,805,187 |
15,805,187 |
13,870,473 |
13,870,473 |
15,178,357 |
9,336,597 |
10,858,873 |
8,999,721 |
Internal services |
3,087,135 |
3,087,135 |
3,132,740 |
3,132,740 |
4,122,282 |
3,977,283 |
4,400,210 |
3,339,688 |
Total |
18,892,322 |
18,892,322 |
17,003,213 |
17,003,213 |
19,300,639 |
13,313,880 |
15,259,083 |
12,339,409 |
Planned spending in 2021-22 was higher than Actual spending largely due to a lapse of funding for the Special Purpose Allotment (SPA) to conduct Public Hearings. The SPA can only be used to cover the costs of public hearings, such as external legal counsel and expert witnesses, etc. For purposes of forecasting Planned Spending, it is necessary to assume that the entire SPA funding will be spent because these expenditures are dependent on the number of hearings, and the length and complexity of the hearings held, which are difficult to predict. In 2021-22, the SPA was $6,206,486 and the PMPRB only spent $645,553, a difference of $5,560,933. Any unspent amount is returned to the Consolidated Revenue Fund.
Planned spending in 2022-23 and 2023-24 is lower than planned spending in 2021-22 because of a reduction in funding of approximately $2 million, as set out in Budget 2017.
Human resources
The “Human resources summary for core responsibilities and internal services” table presents the full-time equivalents (FTEs) allocated to each of the PMPRB’s core responsibilities and to internal services.
Human resources summary for core responsibilities and internal services
| Core responsibilities and internal services | 2019–20 actual full‑time equivalents | 2020–21 actual full‑time equivalents | 2021–22 planned full-time equivalents |
2021–22 actual full‑time equivalents | 2022–23 planned full‑time equivalents | 2023–24 planned full‑time equivalents |
|---|---|---|---|---|---|---|
Regulate patented medicine prices |
58 |
57 |
61 |
55(a) |
60 |
60 |
Subtotal |
58 |
57 |
61 |
55 |
60 |
60 |
Internal services |
21 |
23 |
24 |
23 |
24 |
24 |
Total |
79 |
80 |
85 |
78 |
84 |
84 |
(a) Staff departures, difficulties in hiring replacements, and the postponement of some intended staffing actions due to the delays in the regulatory reform process contributed to this difference. |
||||||
Estimates by vote
For information on the PMPRB’s organizational voted and statutory expenditures, consult the Public Accounts of Canada 2021.Footnote v
Government of Canada spending and activities
Information on the alignment of the PMPRB’s spending with Government of Canada’s spending and activities is available in GC InfoBase.Footnote vi
Financial statements and financial statements highlights
Financial statements
The PMPRB’s financial statements (unaudited) for the year ended March 31, 2022, are available on the departmental website.Footnote vii
Financial statement highlights
Condensed Statement of Operations (unaudited) for the year ended March 31, 2022 (dollars)
| Financial information | 2021–22 planned results |
2021–22 actual results |
2020–21 actual results |
Difference (2021–22 actual results minus 2021–22 planned results) |
Difference (2021–22 actual results minus 2020–21 actual results) |
|---|---|---|---|---|---|
Total expenses |
20,583,155 |
13,884,528 |
17,106,372 |
(6,698,627) |
(3,221,844) |
Total revenues |
– |
527 |
3,689 |
527 |
(3,162) |
Net cost of operations before government funding and transfers |
20,583,155 |
13,884,001 |
17,102,683 |
(6,699,154) |
(3,218,682) |
Condensed Statement of Financial Position (unaudited) as of March 31, 2022 (dollars)
| Financial information | 2021–22 | 2020–21 | Difference (2021–22 minus 2020–21) |
|---|---|---|---|
Total net liabilities |
1,867,625 |
2,786,048 |
(918,423) |
Total net financial assets |
1,159,848 |
1,982,159 |
(822,311) |
Departmental net debt |
914,712 |
1,026,390 |
(111,678) |
Total non-financial assets |
45,968 |
93,060 |
(47,092) |
Departmental net financial position |
(868,744) |
(933,330) |
64,586 |
The 2021–22 planned results information is provided in the PMPRB’s Future-Oriented Statement of Operations and Notes 2021–22.Footnote viii
Corporate information
Organizational profile
Appropriate minister[s]: The Honourable Jean-Yves Duclos
Institutional head: Mélanie Bourassa Forcier, Acting Chairperson
Ministerial portfolio: Health Canada
Enabling instrument[s]: Patent ActFootnote ixand Patented Medicines RegulationsFootnote x
Year of incorporation / commencement: 1987
Other: The Minister of Health is responsible for the pharmaceutical provisions of the Patent Act set out in sections 79 to 103. Although the PMPRB is part of the Health Portfolio, because of its quasi-judicial responsibilities the PMPRB carries out its mandate at arm’s length from the Minister. It also operates independently of Health Canada, which approves drugs for safety, efficacy and quality; other Health Portfolio members, such as the Public Health Agency of Canada, the Canadian Institutes of Health Research and the Canadian Food Inspection Agency; and federal, provincial and territorial (F/P/T) public drug plans, which approve the listing of drugs for their respective formularies for reimbursement purposes; and the Common Drug Review, administered by the Canadian Agency for Drugs and Technologies in Health (CADTH), which recommends drugs that should qualify for reimbursement purposes by participating public drug plans.
Raison d’être, mandate, and role: who we are and what we do
“Raison d’être, mandate and role: who we are and what we do” is available on the PMPRB’s websiteFootnote xi.
For more information on the department’s organizational mandate letter commitments, see the Minister’s mandate letterFootnote xii.
Operating context
Information on the operating contextFootnote xiii is available on the PMPRB’s website.
Reporting framework
The PMPRB’s Departmental Results Framework and Program Inventory of record for 2021–22 are shown below.
| Departmental Results Framework | Core Responsibility: Regulate Patented Medicine Prices | Internal Services | |
| Departmental Result: Affordable drug medicine prices | Indicator 1: % of patented drug prices in Canada are below the median price of the PMPRB’s comparator countries | ||
| Indicator 2: % of patented drug prices in Canada within the threshold set out in the Guidelines | |||
| Program Inventory | Patented Medicine Price Regulation Program | ||
| Pharmaceutical Trends Program | |||
Supporting information on the program inventory
Financial, human resources and performance information for the PMPRB’s Program Inventory is available in GC InfoBase.Footnote xiv
Supplementary information tables
The following supplementary information tables are available on the PMPRB’s website:
- Departmental Sustainable Development Strategy/Reporting on Green ProcurementFootnote xv
- Gender-based analysis plusFootnote xvi
Federal tax expenditures
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals, and credits. The Department of Finance Canada publishes cost estimates and projections for these measures each year in the Report on Federal Tax Expenditures.Footnote xvii This report also provides detailed background information on tax expenditures, including descriptions, objectives, historical information and references to related federal spending programs as well as evaluations and GBA Plus of tax expenditures.
Organizational contact information
Mailing address:
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario K1P 1C1
Telephone: 1-877-861-2350
TTY: (613) 288-9654
Fax: (613) 288-9643
Email: PMPRB.Information-Renseignements.CEPMB@pmprb-cepmb.gc.ca
Website(s): https://www.canada.ca/en/patented-medicine-prices-review.htmlFootnote xviii
Appendix: definitions
appropriation (crédit)
Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
budgetary expenditures (dépenses budgétaires)
Operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to Crown corporations.
core responsibility (responsabilité essentielle)
An enduring function or role performed by a department. The intentions of the department with respect to a core responsibility are reflected in one or more related departmental results that the department seeks to contribute to or influence.
Departmental Plan (plan ministériel)
A report on the plans and expected performance of an appropriated department over a 3‑year period. Departmental Plans are usually tabled in Parliament each spring.
departmental priority (priorité)
A plan or project that a department has chosen to focus and report on during the planning period. Priorities represent the things that are most important or what must be done first to support the achievement of the desired departmental results.
departmental result (résultat ministériel)
A consequence or outcome that a department seeks to achieve. A departmental result is often outside departments’ immediate control, but it should be influenced by program-level outcomes.
departmental result indicator (indicateur de résultat ministériel)
A quantitative measure of progress on a departmental result.
departmental results framework (cadre ministériel des résultats)
A framework that connects the department’s core responsibilities to its departmental results and departmental result indicators.
Departmental Results Report (rapport sur les résultats ministériels)
A report on a department’s actual accomplishments against the plans, priorities and expected results set out in the corresponding Departmental Plan.
experimentation (expérimentation)
The conducting of activities that seek to first explore, then test and compare the effects and impacts of policies and interventions in order to inform evidence-based decision-making, and improve outcomes for Canadians, by learning what works, for whom and in what circumstances. Experimentation is related to, but distinct from innovation (the trying of new things), because it involves a rigorous comparison of results. For example, using a new website to communicate with Canadians can be an innovation; systematically testing the new website against existing outreach tools or an old website to see which one leads to more engagement, is experimentation.
full‑time equivalent (équivalent temps plein)
A measure of the extent to which an employee represents a full person‑year charge against a departmental budget. For a particular position, the full‑time equivalent figure is the ratio of number of hours the person actually works divided by the standard number of hours set out in the person’s collective agreement.
gender-based analysis plus (GBA Plus) (analyse comparative entre les sexes plus [ACS Plus])
An analytical tool used to support the development of responsive and inclusive policies, programs and other initiatives; and understand how factors such as sex, race, national and ethnic origin, Indigenous origin or identity, age, sexual orientation, socio-economic conditions, geography, culture and disability, impact experiences and outcomes, and can affect access to and experience of government programs.
government-wide priorities (priorités pangouvernementales)
For the purpose of the 2021–22 Departmental Results Report, government-wide priorities refers to those high-level themes outlining the government’s agenda in the 2020 Speech from the Throne, namely: Protecting Canadians from COVID-19; Helping Canadians through the pandemic; Building back better – a resiliency agenda for the middle class; The Canada we’re fighting for.
horizontal initiative (initiative horizontale)
An initiative where two or more federal organizations are given funding to pursue a shared outcome, often linked to a government priority.
non‑budgetary expenditures (dépenses non budgétaires)
Net outlays and receipts related to loans, investments and advances, which change the composition of the financial assets of the Government of Canada.
performance ( rendement)
What an organization did with its resources to achieve its results, how well those results compare to what the organization intended to achieve, and how well lessons learned have been identified.
performance indicator (indicateur de rendement)
A qualitative or quantitative means of measuring an output or outcome, with the intention of gauging the performance of an organization, program, policy or initiative respecting expected results.
performance reporting (production de rapports sur le rendement)
The process of communicating evidence‑based performance information. Performance reporting supports decision making, accountability and transparency.
plan (plan)
The articulation of strategic choices, which provides information on how an organization intends to achieve its priorities and associated results. Generally, a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead to the expected result.
planned spending (dépenses prévues)
For Departmental Plans and Departmental Results Reports, planned spending refers to those amounts presented in Main Estimates.
A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their Departmental Plans and Departmental Results Reports.
program (programme)
Individual or groups of services, activities or combinations thereof that are managed together within the department and focus on a specific set of outputs, outcomes or service levels.
program inventory (répertoire des programmes)
Identifies all the department’s programs and describes how resources are organized to contribute to the department’s core responsibilities and results.
result (résultat)
A consequence attributed, in part, to an organization, policy, program or initiative. Results are not within the control of a single organization, policy, program or initiative; instead they are within the area of the organization’s influence.
statutory expenditures (dépenses législatives)
Expenditures that Parliament has approved through legislation other than appropriation acts. The legislation sets out the purpose of the expenditures and the terms and conditions under which they may be made.
target (cible)
A measurable performance or success level that an organization, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
voted expenditures (dépenses votées)
Expenditures that Parliament approves annually through an appropriation act. The vote wording becomes the governing conditions under which these expenditures may be made.