Health Canada’s Open Science Action Plan
Download in PDF format
(963 KB / KB, 32 pages)
Organization: Health Canada
Date published: June 2021
June 21, 2021
Table of Contents
- Executive Summary
- 1. Government of Canada Open Science Context
- 2. Chief Science Advisor of Canada's Roadmap for Open Science
- 3. Engaging Health Canada employees
- 4. Health Canada's Open Science Action Plan
- 5. Glossary
Executive Summary
The Government of Canada's efforts to support open science have evolved over recent years with the aim to accelerate scientific research by allowing for a greater access to federal led science or research. Groundwork began in 2012 through the National Action Plan on Open Government (NAP).
In response to the fourth National Action Plan on Open Government (2018-20), the Chief Science Advisor released the Roadmap for Open Science. The Roadmap sets a common framework for federal science based on the principles of people, transparency, inclusiveness, collaboration and sustainability. These principles aim to promote a federal science culture that is "Open by Design and by Default," meaning open science is an integral part of the scientific process.
The Roadmap also includes five recommendations for federal science-based departments and agencies (SBDAs) to support its open science objectives, namely:
- Engage their departmental scientific community to identify challenges and opportunities on open science;
- Develop an Open Science Action Plan describing how they intend to meet the recommendations set out in the Roadmap;
- Make all new scientific articles published in scholarly journals open access without an embargo period;
- Develop strategies to ensure that scientific data is FAIR (Findable, Accessible, Interoperable, and Reusable); and
- Appoint a Chief Scientific Data Officer.
Health Canada has already made significant progress in addressing the recommendations of the Roadmap,by:
- Appointing Dr. Raman Srivastava as Chief Scientific Data Officer;
- Conducting internal consultations on Open Science; and
- Developing an Open Science Action Plan that aims to support the advancement of the Roadmap's objectives within Health Canada.
Summary of Health Canada's Open Science Action Plan
Action Item #1 – Supporting "Open by Design and by Default"
- The Open Science Steering Committee, with support from the DSA, will work to develop a department-wide approach to help improve awareness and understanding of open science; and
- Health Canada will develop promotional material to raise awareness of the "Open by Design and by Default" approach to conducting science.
Action Item #2 – Supporting Open Access Publications
Health Canada will:
- Work with science branches to develop awareness of Open Access commitments;
- Develop guidance tools to support its research community navigate open access publications; and
- Ensure that its publication policies support Open Access publishing.
Action Item #3 – Supporting the Official Languages Act (OLA)
- Health Canada, in collaboration with other SBDAs, will consult with the Office of the Chief Science Advisor (OCSA) and the Treasury Board Secretariat (TBS), on possible approaches to the OLA directive. For example, the National Research Council's Publication Archive only posts journal articles in their original language, but offers on-demand translation of abstracts; and
- Health Canada will revise policies on the Official Languages Act with respect to open access of its scientific publications.
Action Item #4 – Addressing the Cost Burdens of Open Access
- Health Canada will complete a cost analysis to fully understand the financial implications of open access, for example:
- exploring the feasibility of moving the cost burden of open access publishing from individual project budgets to a centralized cost center; and
- the current $5,000 limit on departmental credit cards creates an administrative burden for scientists who often have to pay open access journal fees in excess of that limit.
Action Item #5 – Supporting IM/IT Infrastructure for Open Access
- Health Canada, in collaboration with Innovation, Science and Economic Development Canada (ISED), will launch its newly developed internal scientific publications database platform. The new publications management system will replace the existing publications database and will be interoperable with a future federal scientific publications repository.
- Health Canada is also collaborating with the federal Open Science Steering Committee (consisting of the Chief Science Advisor, the Chief Information Officer of the federal government, the President of Shared Services Canada, and TBS) for the creation of an open access repository for federal science publications. The Committee will be conducting a feasibility assessment of this repository.
Action Item #6 – Framework for Exclusion Criteria for Open Access
- Health Canada will review the Framework in the context of departmental data requirements and mandates and, if necessary, work with the science branches to establish department-specific exclusions.
Action Item #7 – Developing Strategies for FAIR Data Principles
Under the leadership of the Chief Scientific Data Officer, Health Canada will develop strategies that include the following activities:
- Engage science branches to assess their common and unique digital and data infrastructure needs and assess the current gaps and opportunities;
- Establish a common set of departmental scientific data needs and support departmental data tools;
- Continue to leverage and integrate departmental architecture into the design of digital data systems; and
- Support science branches in adopting the use of Data Management Plans (DMPs) as an integral part of project planning.
Action Item #8 – Measuring Progress of the Action Plan
- Health Canada's Open Science Steering Committee will provide oversight of the implementation of the Open Science Action Plan. This Committee will report to the Director General (DG)-Science Committee on a bi-annual basis as the Action Plan is being implemented, then on a yearly basis after 2025; and
- As an active member of the Open Science Metrics Working Group, which is responsible for developing the performance indicators and open science annual report, Health Canada will collaborate to identify key metrics to measure the progress of SBDAs in meeting the objectives of the Roadmap.
1. Government of Canada Open Science Context
Open Science is a concept that embraces all forms of science communications including scientific publications and data, as well as access to and use of materials, equipment and software. The benefits of open science include:
- Ensuring accountability;
- Increasing reproducibility and rigour of research;
- Fostering a culture of open engagement and collaboration;
- Reducing duplication;
- Creating opportunities for impact;
- Leveraging diversity and inclusion;
- Accelerating knowledge transfer; and
- Building synergies with open science movements.
The Government of Canada's efforts to support open science have evolved over recent years with the aim to accelerate scientific research by allowing for a greater access to federal science. Groundwork began in 2012 through the National Action Plan on Open Government (NAP). The 3rd NAP (2016-18) created the position of a Chief Science Advisor of Canada (CSA) and a mandate for Environment and Climate Change Canada (ECCC) to develop metrics that track the progress of science based departments and agencies (SBDAs) on open science. In response to the 4th NAP on Open Government (2018-20), the CSA unveiled a Roadmap for Open Science on February 26, 2020. The Roadmap aims to promote greater openness in federal science and sets expectations for SBDAs to monitor and report on open science.
At Health Canada, policy experts work with scientists and researchers to adapt regulatory frameworks to changing environments and emerging technological and health innovations. To this end, Health Canada developed a Framework for Science and Research Excellence to help guide its focus towards key elements of the science and research enterprise. The Framework provides a clear, structured approach for thinking and talking about science, which includes best practices that will support an Open Science approach throughout the department.
2. Chief Science Advisor of Canada's Roadmap for Open Science
The Chief Science Advisor's (CSA) Roadmap for Open Science sets a common framework for federal science. The Roadmap rests on the five foundational principles of people, transparency, inclusiveness, collaboration and sustainability. These principles aim to promote a federal science culture that is "Open by Design and by Default," meaning open science is an integral part of the scientific process. The Roadmap also sets out the following key objectives for SBDAs:
- Engage the departmental scientific community to identify challenges and opportunities for open science by February 2021;
- Develop an Open Science Action Plan (draft due March 2021, final due June 2021). The action plan must describe how SBDAs intend to meet the recommendations set out in the Roadmap:
- Make all new science articles published in scholarly journals open access without an embargo period by 2022, and all new science publications open access by 2023;
- Develop strategies to ensure that departmental scientific data is FAIR (Findable, Accessible, Interoperable, and Reusable) by 2023, with full implementation by 2025;
- Appoint a Chief Scientific Data Officer (CSDO), to promote coordination between Open Data, Open Science, and Science Data Management by January 2021; and
- Develop criteria the department will use to measure its progress in meeting the Action Plan's objectives.
3. Engaging Health Canada employees
Internal consultations with scientists and researchers at Health Canada identified challenges and opportunities for federal open science, which informed the development of this Open Science Action Plan. Given that open science at Health Canada encompasses an array of science and research activities, including providing access to peer-reviewed publications, the release of datasets, and public engagements, all staff were invited to share their perspectives on open science through an online survey.
Conducted in the Fall of 2020, the Health Canada Open Science Survey focused on four key areas of interest to open science:
- Knowledge of open science and the Open Science Roadmap at Health Canada;
- Experience with open access publishing of research;
- Communicating science to the public; and
- Experience with open data in scientific activities.
The survey indicated that respondents were generally aware of open science and are supportive of making federal science more open and accessible. However, over a third (36%) of respondents reported being unaware of open science prior to the survey.
Though many respondents (59%) indicated having published their scientific research in open access journals, many saw the cost of open access publishing as a challenge. In some cases, budget constraints limited the choice of journals in which they wished to publish (publisher fees for open access at time of publication are $3,700 - $6,200).
In terms of communicating HC science to the Canadian public, almost half of respondents (43%) reported having done so. The main challenges scientists faced when communicating their research to the public were a lack of communications training, multi-layered approval processes, and limitations due to confidential business information.
The survey results showed several gaps in knowledge and experience with respect to open data. For example, only 19% of respondents reported having made their research data open, with the majority (61%) reporting that they do not publish datasets on the Federal Open Data Portal. Over a third of respondents (36%) were either unaware of the Open Data Portal, or did not know how to post their data. Looking to the future of open data needs, 61% felt that they did not have access to the necessary infrastructure.
Similarly, respondents anticipated they would face challenges in making their scientific information FAIR (Findable, Accessible, Interoperable, and Reusable) in the future. In particular, respondents felt that government websites are difficult to navigate, making the information hard to find. Other challenges identified include the time lag due to multi-level approval processes that affect the timely accessibility of research information, and limitations imposed by confidential business information.
4. Health Canada's Open Science Action Plan
Health Canada has been actively engaged in federal open science since 2012. Key initiatives include providing open access to peer-reviewed scientific publications and plain language summaries, the release of open datasets, and public engagement activities involving our scientists and researchers.
Scientific Articles and Publications
Open Access policies and instruments:
Health Canada does not have an open science policy. However, branches that publish scientific information refer to the Health Canada-Public Health Agency of Canada Scientific Integrity Policy (SIP) (2018) and the Health Canada Scientific Publications Policy (2012) for guidance. Both these policies support openness of science publications. For example, both encourage the sharing of scientific knowledge, and outline responsibilities ensuring public accessibility to scientific research in a timely manner.
The Healthy Environments and Consumer Safety Branch (HECSB) has also developed an Open Science Strategy (2020) that serves as a best-practices guide to support the advancement of open science and research within the branch.
Publication of Scientific Information:
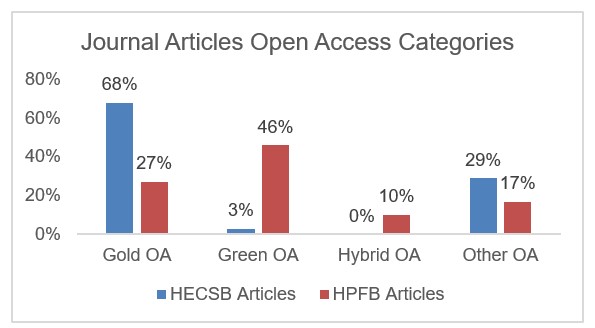
HECSB: based on 279 articles published 2017-2018.
HPFB: Based on 452 articles published 2015-2020.
* PMRA seldom publishes articles.
Figure 1 - Text Equivalent
| Proportion of Articles (in percentage) | Gold Open Access | Green Open Access | Hybrid Open Access | Other |
|---|---|---|---|---|
| HECSB | 68% | 3% | 0% | 29% |
| HPFB | 27% | 56% | 10% | 17% |
HC averaged 258 scientific journal articles per year, over the last 10 years. While peer-reviewed journals are the most commonly used format for scientists to report new research findings, HC researchers also publish in conference proceedings, books/book chapters, international reports, Organisation for Economic Co-operation and Development publication series, and extended abstracts.
The main branches that publish scientific information are HECSB and the Health Products and Food Branch (HPFB). As indicated in the chart, the majority of journal articles are published as open accessFootnote 1
With respect to other scientific publication formats, at this time HC does not systematically track open access information.
Examples of HC scientific publications released as open access:
- In 2017, HECSB researchers published a peer-reviewed publication in the Lancet (open access) titled "Living near major roads and the incidence of dementia, Parkinson's disease, and multiple sclerosis", a population-based study, which was ranked among the top 100 articles around the world by Altmetric.
- Cooper M, Randall Simpson J, Klutka R. "Development and validation of a sodium AnaLysis tool (SALT)." Nutrition Journal. 2020; 19:55.
- Alomaim H, Griffin P, Swist E, Plouffe LJ, Vandeloo M, Demonty I, Kumar A, Bertinato J. "Dietary calcium affects body composition and lipid metabolism in rats." I. 2019; 14(1): e0210760.
Cost Associated with Open Access:
There are no consistent internal numbers in place to track open access costs. However, based on an average of 258 articles published per year, over the last 10 years, full implementation costs of Open Access could be as high as $1.29M for the department (based on average open access fees of $5,000 per article). These numbers are expected to increase.
Open Data
Datasets Published over the Past Five years
Over the past five years, Health Canada has released over 160 datasets on the Treasury Board Secretariat Open Data Portal. At present, solid figures needed to calculate the percentage of datasets released as open data are a challenge to provide, in part due to the stage of open data maturity across the Department. Given that a significant amount of Health Canada's data holdings are not eligible for release (due to confidentiality restrictions), it is estimated that between 5% to 10% are released as open data.
Examples of Health Canada datasets released on the open data portal:
- The Canadian Laboratory Information Network (CANLINE):
CANLINE is the Food Directorate's (Health Canada) searchable database for chemical, nutritional, and microbiological laboratory surveillance data. This includes data from Canada's Total Diet Study and other targeted surveillance data on contaminants in food. CANLINE data can be searched by sample characteristics, food category, contaminant, pathogen, nutrient, geographical details, and date, or combinations of the above. Search results can be exported in various formats. - Edmonton Indoor Air Quality Study (2010): Volatile Organic Compounds (VOC) Data Summary:
a summary of 24-hour VOC statistics (per season) obtained as part of a residential indoor air quality study in Edmonton, conducted by Health Canada in 2010, and is intended to provide relevant Canadian information on exposure to VOCs found indoors and outdoors within non-smoking residences. VOCs are emitted as gases from certain solids or liquids, and include a variety of chemicals, some of which may have short- and long-term adverse health effects. - The Canadian Radiological Monitoring Network (CRMN) – Tritium in Atmospheric Water Vapour: data on tritium content in atmospheric water vapor sampled from radiation monitoring stations in Ontario, Quebec and New Brunswick. By continually monitoring radiation levels nationwide, this data helps to inform Health Canada in protecting the health of Canadians.
- Canadian Health Measures Survey (CHMS) Biomonitoring Data: 2007-2017:
Ongoing national direct health measures survey called the Canadian Health Measures Survey (CHMS), which collects human biomonitoring data for environmental chemicals, in two-year cycles and used to estimate exposure to environmental chemicals by measuring the chemical, its metabolites, or reaction products in biological specimens (i.e. in Canadians).
Policies on FAIR Data Practices and the Release of Open Datasets:
FAIR data principles are a component of open data. The guidance for the department regarding FAIR data principles can be found within the Open Government Guidebook published by TBS. Under this Guidebook, all Health Canada datasets (eligible for open access) are released taking into account the FAIR data principles as follows:
- F – Mandatory metadata ensures that datasets are findable, e.g. Key search words are uploaded with the data to make it more findable.
- A – Dataset content must be accessible, in compliance with the Standard on Web Accessibility - Canada.ca.
- I – The interoperability of datasets must adhere to the international ISO standards, e.g. HC uses standards (ISO – date code), controlled vocabs, common (open) format.
- R – The Open Government License allows for the reuse of data. Metadata and data should also be well described so that it can be replicated and/or combined in different settings.
Health Canada's Open Science Action Plan
Health Canada's Open Science Action Plan builds on the knowledge and experiences gained from these initiatives with an approach that outlines how it will address the recommendations put forth in the CSA's Roadmap for Open Science. Specifically, the Plan articulates a forward approach to making Health Canada science "Open by Design and by Default" through the following:
- Making Health Canada articles and publications openly accessible;
- Developing FAIR data strategies; and
- Establishing a Framework for open access exclusions.
4.1. Making Health Canada science "Open by Design and by Default"
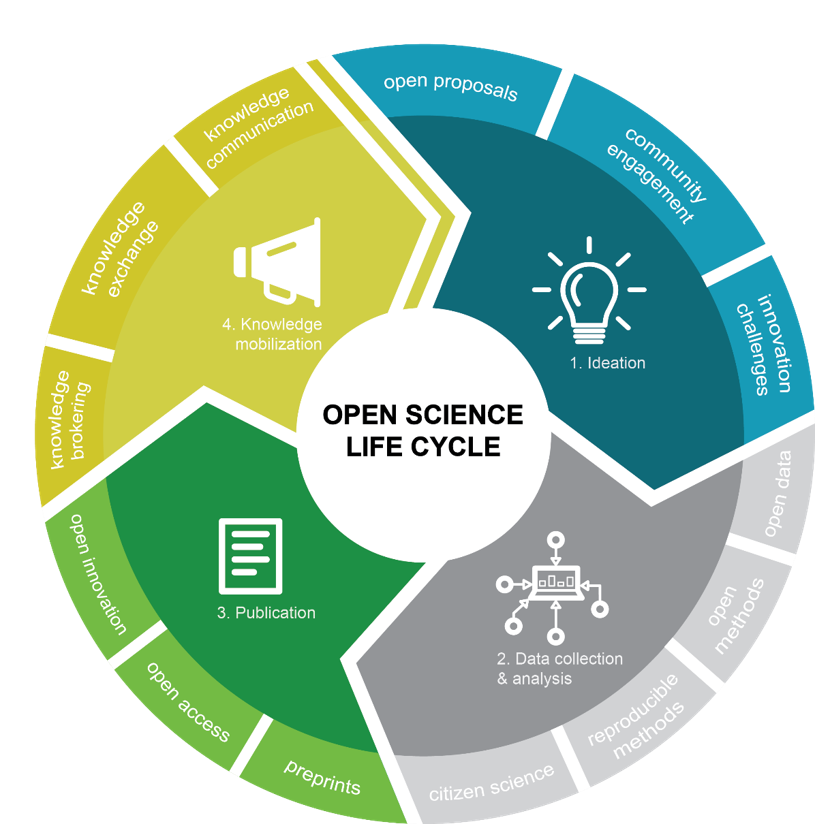
Courtesy of Environment and Climate Change Canada
Figure 2 - Text Equivalent
A circle diagram depicting four stages of the Open Science life cycle. Each stage has supporting elements:
- Ideation
- Open proposals
- Community engagement
- Innovation challenges
- Data Collection and Analysis
- Open data
- Open methods
- Reproducible methods
- Citizen science
- Publication
- Preprints
- Open access
- Open innovation
- Knowledge Mobilization
- Knowledge brokering
- Knowledge exchange
- Knowledge communication
Open Science is the practice of making scientific inputs, outputs and processes freely available to all, with minimal restrictions. Encouraging the release of scientific information contributes to the growth and sharing of knowledge, a critical component of the scientific process. The sharing of data and results also improves transparency and accountability for the use of public funds and promotes greater public trust in federally funded research. A key principle of open science is "Open by Default." This means that government scientific information is presumed to be open to the public unless there is a compelling reason for them to be not open or accessible (such as privacy, confidential business information, or national security).
"Open by Design", however, seeks to develop an open science landscape that promotes well-considered, systematic and safe release of information. "Open by Design and by Default", therefore, is about thinking of scientific information as being open throughout its lifecycle: from ideas; to research; to publication.
At Health Canada, science branches endeavor to foster an organizational culture that promotes an "Open by Design and by Default" approach to science. It harnesses collaborative relationships with partners and networks, and supports knowledge transfer and exchange on science issues of value to Canadians.
Action Item #1 – Supporting "Open by Design and by Default"
- The Open Science Steering Committee, with support from the DSA, will work to develop a department-wide approach to help improve awareness and understanding of open science; and
- Health Canada will develop promotional material to raise awareness of the "Open by Design and by Default" approach to conducting science.
4.2. Making federal science articles and publications openly accessible
Open access (OA) supports the goals of open science by providing free peer-reviewed scientific publications online at no cost, immediately upon publishing (no publisher delay or embargo period), and with limited restrictions regarding reuse. OA of federal science helps foster important collaboration among the wider scientific community, such as with policy makers and the public at large, promoting greater societal advancements through barrier-free access to cutting-edge research. A prime example of this was the knowledge mobilization by the research community in the global fight against COVID-19. Publishers, such as the Journal of Medical Internet Research (JMIR), voluntarily agreed to make their COVID-19 and coronavirus-related publications immediately accessible to support the ongoing public health emergency response efforts. This enhanced the ability of researchers and other stakeholders to access, re-use all published articles on COVID-19, and identify critical trends and relevant information.
There are two primary vehicles for achieving OA: Open Access Journals (Gold OA), and Open Access Repositories (Green OA). Open Access Journals offer an alternative to traditional subscription-based journals by providing free access to the peer-reviewed articles. Gold OA journals make the final version of an article freely and permanently accessible, immediately at publication. Gold OA articles can be published either in fully OA journals or in hybrid journals, which are subscription-based journals that offer an OA option for a fee, paid by the author, to lift any embargo periods. For example, articles in Nature journals are free to access, download, share and re-use, but the journal charges authors an $11,000 fee to make their articles open access.
Green OA, also referred to as self-archiving, is the practice of placing a version of an author's manuscript into an online repository, making it freely accessible for everyone. OA repositories include preprints and postprints of journal articles, but also other forms of scientific publications such as scientific reports, monographs, conference proceedings, conference papers, and technical scientific products, that have been peer-reviewed.
To support the goal of achieving OA for all federal science, the Roadmap sets out two objectives for SBDAs:
- All new federal science journal articles must be openly accessible by January 2022; and
- All new federal science publications (data, maps, posters, videos, etc.) must be openly accessible by January 2023.
In developing options to address these two objectives, Health Canada has considered feedback from its Open Science Survey and is collaborating with the federal science community on approaches to common elements of respective action plans.
Action Item #2 – Supporting Open Access Publications
Health Canada will:
- Work with science branches to develop awareness of Open Access commitments;
- Develop guidance tools to support its research community navigate open access publications; and
- Ensure that its publication policies support Open Access publishing.
The Official Languages Act (OLA): The OLA requires federal departments to communicate with the public in both official languages. This implies that all peer-reviewed scientific outputs, journal articles and publications, would need to be translated (into French or English) at the time of publication. OA by default would significantly increase the amount of scientific information requiring translation. Though this would lead to a substantial increase in translation budgets, the main challenges lie in human resource issues:
- Health Canada scientists are highly specialized in their respective fields of research. Translating this type of information would require equally specialized translators to capture the technical and scientific nuances of each field;
- Verification of translated material would require authors to work with translators to ensure quality control of translations. Health Canada scientists are not necessarily proficient in both official languages; and
- Given the complexity and length of some journal articles (which often include detailed charts, graphics, maps), such translation requirements could pose significant delays in getting federal science out to the public – which goes against the intent of open access.
Action Item #3 – Supporting the Official Languages Act (OLA)
- Health Canada, in collaboration with other SBDAs, will consult with the Office of the Chief Science Advisor (OCSA) and the Treasury Board Secretariat (TBS), on possible approaches to the OLA directive. For example, the National Research Council's Publication Archive only posts journal articles in their original language, but offers on-demand translation of abstracts; and
- Health Canada will revise policies on the Official Languages Act with respect to open access of its scientific publications.
Cost burdens of OA hybrid articles: The costs associated with making science articles openly accessible at the time of publication are challenging. Based on a metrics exercise conducted in 2017 by Environment and Climate Change Canada Science-Metrix Bibliometric Assessment, Health Canada published an average of 258 scientific journal articles per year over the last 10 years. This aligns with the productivity of SBDAs. Health Canada's Open Science Survey clearly demonstrated that OA costs are a burden to already tight project budgets, as publishing costs usually fall under the responsibility of researchers. The survey also brought to light an unforeseen consequence of OA: some of the specialized journals have significantly higher OA fees, which can make them out of reach for Health Canada scientists. In some cases, scientists have had to forgo publishing in the lead journal of their field, negatively impacting the visibility and influence of federal science. Currently, the spending limit of $5,000 on staff business credit cards also creates administrative burdens for researchers as open access publishing fees often exceed $5,000 and programs are directed to take out sole source contracts.
OA budgets will also have to take into consideration costs of additional human resource capacity needs to respond to OA requirements and translation costs for datasets accompanying OA journals.
Action Item #4 – Addressing the Cost Burdens of Open Access
- Health Canada will complete a cost analysis to fully understand the financial implications of open access, for example:
- exploring the feasibility of moving the cost burden of open access publishing from individual project budgets to a centralized cost center; and
- the current $5,000 limit on departmental credit cards creates an administrative burden for scientists who often have to pay open access journal fees in excess of that limit.
IM/IT infrastructure needs: In order to support OA, an electronic platform for posting federal scientific publications directly to the public will be needed. This platform would increase findability of federal science publications, and be recognized as a credible source of scientific information, but would also need to be compliant with any federal requirements (i.e. crown copyright, official languages requirements, user accessibility and privacy laws). In addition to technical considerations, future platforms will need to be user friendly and limit administrative burdens on scientists, given that OA rests on the principles of accessibility and timely access to scientific information.
Currently, Health Canada has a scientific publications tracking system through Lotus Notes. While this system provides employees, who author and co-author scientific research documents, with a tool to track reviews, approvals, and submissions of their scientific publications, it lacks the functions necessary to support open access.
Action Item #5 – Supporting IM/IT Infrastructure for Open Access
- Health Canada, in collaboration with Innovation, Science and Economic Development Canada (ISED), will launch its newly developed internal scientific publications database platform. The new publications management system will replace the existing publications database and will be interoperable with a future federal scientific publications repository.
- Health Canada is also collaborating with the federal Open Science Steering Committee (consisting of the Chief Science Advisor, the Chief Information Officer of the federal government, the President of Shared Services Canada, and TBS) for the creation of an open access repository for federal science publications. The Committee will be conducting a feasibility assessment of this repository.
4.3. Framework for exclusion criteria of Open Access
Under Open Science, all data resources of business value held by the Government of Canada departments and agencies are to be open by default and released as open data unless subject to valid exceptions. To support SBDAs and encourage a whole-of-government approach, the Chief Science Advisor (CSA) is developing a framework to identify criteria for restricting access to federal scientific research outputs. However, the decision to withhold certain information ultimately lies with the respective organizations.
The draft framework articulates the considerations related to privacy, confidential business information, national security, commitments to Indigenous peoples, and to the public interest, when defining appropriate access to research outputs.
- The first consideration is the application of the Privacy Act to scientific research outputs. For example, personal information under the control of a government institution cannot be released without explicit consent of the individual except in limited circumstances.
- Second, the framework seeks to determine the right degree of openness while balancing risks and benefits.
The management of "Open by Default" principles becomes a question of finding the right degree of openness if there are limitations on what information can be released. In assessing the release of scientific research outputs, the value and impact of the information must be determined, and whether degrees of openness could be considered in the case of sensitive information.
One notable example for limiting access to information is research outputs that contain recorded information about an identifiable individual. This includes the situation where there is a serious possibility that an individual could be identified from information, alone or in combination with other available information (such as DNA from test results). Canada has strict policies that protect an individual's health record. Respect for personal privacy is also a core principle of research ethics.
Other types of scientific information Health Canada would not make open access include its regulatory assessments and scientific data it uses for research and surveillance purposes that it does not own, but obtains under third-party agreements.
The Framework encourages decision making at the design stage of any science experiment or project, thereby operationalizing "Open by Design and by Default" for all scientific research outputs. For example, initial designs should consider how much work will be needed to make the scientific research outputs accessible (e.g. the availability of metadata, the cost of publishing) and whether a partial or targeted release of the information would meet the spirit and intent of "Open by Design and by Default." Any information release should consider whether there is value and utility of "intermediate" products such as raw data or intermediate project reports or preprints, especially if the volume or preparation burden for release is operationally excessive.
Action Item #6 – Framework for Exclusion Criteria for Open Access
- Health Canada will review the Framework in the context of departmental data requirements and mandates and, if necessary, work with the science branches to establish department-specific exclusions.
4.4. Chief Scientific Data Officer
The Roadmap for Open Science recommends that SBDAs appoint a Chief Scientific Data Officer (CSDO) to support the integration of scientific and research data in their respective departmental data strategies. The purpose of designating a CSDO is to ensure alignment between the data strategy and the open science action plan in Health Canada.
Health Canada appointed Dr. Raman Srivastava as both Chief Data Officer (CDO) and Chief Scientific Data Officer (CSDO) in January 2021. Given his responsibilities in leading Health Canada's Data Strategy, he is well positioned to provide strategic coordination between open data, open science, and science data management. Specifically, Dr. Srivastava will support the alignment and integration of Health Canada's Open Science Action Plan with that of its Data Strategy with a focus on:
- People and Culture (improving data literacy);
- Key Role of Governance in Process and Projects (shifting from ownership to stewardship); and
- Environment and Digital Infrastructure (ensuring analysts have the tools and technology to focus on data as an asset).
Dr. Srivastava has also been consulted in the drafting of this action plan to ensure alignment with the Data Strategy, particularly in terms of developing FAIR data principles and determining strategies on how we can collectively reduce barriers to and build on our effort to promote open data and open science.
The CSDO will work with partners across the department to further promote open data and open science by:
- Creating a Departmental Data inventory (understanding the scope of our data holdings);
- Developing FAIR data principles (Findable, Accessible, Interoperable and Reusable) in support of the Government of Canada's Roadmap for Open Science;
- Investigating, adopting and promoting tools that anonymize datasets (similar to Statistics Canada's Public Use Microdata Files);
- Linking Departmental privacy and ethics programs with HC's Data Strategy to address barriers at the outset;
- Incorporating plans to reduce barriers to open data into the Data Management Plan process at the outset;
- Working with program areas to promote data literacy and "Open by Default" through training programs; and
- Partnership and coordination with Information Management Services Directorate on open data by establishing linkages to the HC's Data Strategy, Chief Data Office, Open Science Action Plan, and interdepartmental networks.
4.4.1. FAIR data principles
Sound data management will serve Canadian research excellence, support discovery and fuel innovation. While Health Canada is already making significant data investments, there is more to do from a scientific data management perspective. One example is building a research system that reflects the FAIR principles—that data should be findable, accessible, interoperable and reusable. Together these principles will increase the value of the scientific data generated. Adopting scientific data standards across the department will make it easier to share and reuse data. This includes data management planning, institutional support for research data management, and data deposit and access.
Health Canada's Data Strategy lays out the groundwork for developing FAIR data standards by establishing strong data management practices. For example:
- Developing a common understanding of data;
- Collaborating with partners to access and reuse and share data with Canadians;
- Leveraging its talent and capacity to manage, use, and understand open data;
- Establishing governance at the right levels to ensure that data is managed as a strategic asset; and
- Leveraging technology and infrastructure needed to turn data and analysis into action.
In accordance with the Roadmap, Health Canada will develop strategies and tools to implement FAIR data principles to ensure interoperability of its scientific and research data and metadata by January 2023, with a phased plan for full implementation by January 2025.
Action Item #7 – Developing Strategies for FAIR Data Principles
Under the leadership of the Chief Scientific Data Officer, Health Canada will develop strategies that include the following activities:
- Engage science branches to assess their common and unique digital and data infrastructure needs and assess the current gaps and opportunities;
- Establish a common set of departmental scientific data needs and support departmental data tools;
- Continue to leverage and integrate departmental architecture into the design of digital data systems; and
- Support science branches in adopting the use of Data Management Plans (DMPs) as an integral part of project planning.
4.5. Performance measurement of Health Canada's Action Plan
The Government of Canada publishes an annual report measuring the progress of its open science commitments. The report uses a combination of core metrics designed to capture general progress across federal science and supplemental metrics to highlight individual SBDA efforts. Core metrics outlined in the Open Science Annual Report have included:
- Open Access Publications;
- Open Scientific Data; and
- Open Science Public Engagement.
One of the foundational principles of the Roadmap is inclusiveness, which encourages SBDAs to achieve inclusive and diverse approaches to open science. Moving forward, baseline performance indicators will need to be established to measure the effectiveness of federal open science initiatives, and their impact on various stakeholders.
Action Item #8 – Measuring Progress of the Action Plan
- Health Canada's Open Science Steering Committee will provide oversight of the implementation of the Open Science Action Plan. This Committee will report to the Director General (DG)-Science Committee on a bi-annual basis as the Action Plan is being implemented, then on a yearly basis after 2025; and
- As an active member of the Open Science Metrics Working Group, which is responsible for developing the performance indicators and open science annual report, Health Canada will collaborate to identify key metrics to measure the progress of SBDAs in meeting the objectives of the Roadmap.
5. Implementing the Action Plan
To support and promote its Open Science Action Plan, Health Canada has established an Open Science Steering Committee. This Steering Committee is comprised of key players able to advance the Plan's priorities, including science-branch leads from the Strategic Policy Branch, Healthy Environments and Consumer Safety Branch, Health Products and Food Branch, the Pest Management Regulatory Agency and the Office of the Chief Scientific Data Officer, and members of the Science and Research Integration Network.
Reporting to Health Canada's DG-Science Committee, the Steering Committee aims to:
- Propose solutions to implement the HC Open Science Action Plan;
- Provide recommendations for a department-wide approach to support the Action Plan's action items; and
- Develop options for measurable, results-focused deliverables and approaches to promote open science.
6. Glossary
- Canada's 4th National Action Plan (NAP) on Open Government:
- In the context ofOpen government, which is an approach to governance that focuses on transparency, accountability, and citizen participation, the National Action Plan on Open Government outlines how the Federal Government will improve financial transparency, upgrade digital services, and keep making government more open for Canadians.
- Chief Science Advisor of Canada (CSA):
- The CSA provides advice on the development and implementation of guidelines to ensure that government science is fully available to the public, and that federal scientists are able to speak freely about their work. The CSA also advises on creating and implementing processes to ensure that scientific analyses are considered when the Government makes decisions, and to improve the existing science advisory function within the federal government.
- Departmental Science Advisor (DSA):
- Reporting directly to the Deputy Minister of Health, the DSA plays an important role in supporting high quality scientific research within Health Canada. The DSA also supports the vital work of researchers by strengthening the linkages between science and policy decisions and improving collaboration across sectors and partners.
- FAIR data principles:
- "FAIR" research data should be Findable, Accessible, Interoperable, and Reusable. FAIR data principles are a fundamental part of open science, and describe some of the central guidelines to good data management and open access to research data.
- Federal science articles:
- Scholarly articles authored or co-authored by federal scientists(s) in peer-reviewed academic journals.
- Federal science publications:
- Scientific communications that scientists and researchers use to share their work. These include research or scientific reports, monographs, edited books, book chapters, conference proceedings, conference papers, posters, plain language summaries and technical scientific products.
- Federal scientific and research data:
- Data that include, but are not limited to, observational, monitoring, operational, modelling and simulation, risk-assessment, survey and surveillance, research and development and technology innovation data, that are funded by federal budgets.
- Gold Open Access:
- Authors publish peer-reviewed articles in fully open access journals, which are accessed via the journal or publisher's website (e.g., journals in PLoS One Open access fees are paid by the author(s), usually through grants or via their institution).
- Green Open Access:
- Authors publish peer-reviewed articles in a journal and are allowed to deposit a version of their work (either pre or post publication) in a repository which becomes freely available sometime after an embargo period.
- Hybrid Open Access:
- Subscription-based journals that make individual articles fully open access at time of publication for a fee.
- Open Science:
- The practice of making scientific inputs, outputs and processes freely available to all with minimal restrictions. Scientific research outputs include peer-reviewed science articles and publications, scientific and research data, and public contribution about science. Open Science is enabled by people, technology and infrastructure. It is practiced with full respect to privacy, security, ethical considerations and appropriate intellectual property protection.
- Open Access:
- Open access publishing and archiving makes it easier to circulate research results more widely. Open access enables scientists and researchers to make their publications freely available to the domestic and international research community and to the public at large, thereby enhancing the use, application and impact of scientific information.
- Open Data:
- Open data is defined as structured data that is machine-readable, freely shared, used and built on without restrictions.
- Peer Review:
- A process by which a research article is evaluated by content experts from the scientific community before publication.
- Preprint:
- A manuscript draft that has not yet been subject to formal peer review, distributed to receive early feedback on research from peers.
- Repository:
- Repository is defined as the infrastructure and corresponding service that allows for the persistent, efficient and sustainable storage of digital objects (such as documents, data and code).
- Roadmap for Open Science:
- Published by the Chief Science Advisor of Canada, it outlines the recommended next steps to make federal science open to all, while respecting privacy, security, ethical considerations and appropriate intellectual property protection. The Roadmap for Open Science provides overarching principles and recommendations to guide Open Science activities in Canada. The recommendations are intended for science and research funded by federal government departments and agencies.
- SBDAs:
- Science Based Departments and Agencies.
Footnotes
- Footnote 1
-
Gold OA: Fully open access journals. Green OA: Pre or post publications deposited in a repository, which becomes freely available after an embargo period. Hybrid OA: Subscription-based journals that make individual articles fully open access at time of publication for a fee. Other OA: HECSB: Not OA, HPFB: Bronze OA - Publisher has chosen to provide temporary or permanent free access.
