Guide to understanding the Canadian Environmental Protection Act: chapter 5
5. Existing substances
The Canadian Environmental Protection Act, 1999 (CEPA 1999) includes specific requirements for the assessment and management of substances currently existing in commerce or being released to the environment in Canada. The Minister of the Environment and the Minister of Health jointly administer this part of the act.
5.1 What are existing substances?
What is the Domestic Substances List?
The Domestic Substances List includes substances that were, between January 1, 1984, and December 31, 1986, in commercial use in Canada, or were used for commercial manufacturing purposes, or were manufactured in or imported into Canada in a quantity of 100 kg or more in any one calendar year. The list is regularly amended to include additional substances that have been assessed under the act and allowed into Canada. The Domestic Substances List currently contains approximately 23 000 substances from the original list along with an additional 1954 substances that have been added to the list following assessments of new substances.
There are currently about 23 000 substances, which can be manufactured in, imported into, or used in Canada on a commercial scale, that have not been assessed for the risks they pose to the environment or human health. These substances comprise the Domestic Substances List. Substances not on this list are considered to be new to Canada. A substance as defined under CEPA 1999 includes any distinguishable kind of organic or inorganic matter, whether animate or inanimate that is capable of being released as a single substance, an effluent, emission, waste or a mixture into the Canadian environment.
CEPA 1999 introduced more processes for assessing these substances to determine if they are toxic according to CEPA 1999. The three key assessment processes are:
- categorization and screening assessment of the Domestic Substances List
- assessment of the Priority Substances List and
- review of other jurisdictions' decisions
Other assessments may be triggered by information provided by other programs, industry and scientific research.
5.2 How are the risks assessed
5.2.1 What are risk assessments?
Risk assessments done under CEPA 1999 consider impacts on human and non-human organisms and the physical environment. These assessments consider not only the hazard posed by a substance, but the exposure or likelihood that a person, organism or the environment will come in contact with that substance. The exposure or potential for exposure of a substance depends on the amount of substance released into the environment and its fate. The conclusion of the assessment is based on the application of the precautionary principle and a weight of evidence approach.
5.2.2 What are categorization and screening assessments?
What is toxic under CEPA 1999?
Determining a substance to be toxic under CEPA 1999 is a function of its release or possible release into the environment, the resulting concentrations in environmental media and its inherent toxicity. Section 64 of CEPA 1999 defines a substance as toxic "if it is entering or may enter the environment in a quantity or concentration or under conditions that:
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health."
Under CEPA 1999, all 23 000 substances on the Domestic Substances List that have not been subject to notification and assessment as new substances must be "categorized" by September 13, 2006, along with all living substances added to the list. Categorization is essentially an initial priority setting mechanism, which involves the systematic identification of substances on the Domestic Substances List that meet the following criteria:
- are inherently toxic (cause toxic effects) to humans or non-human organisms and display either the characteristics of persistence (take a long time to break down) or bioaccumulation (collect in living organisms and end up in the food chain); or
- may present to individuals in Canada the greatest potential for exposure.
Substances that meet the specified criteria will undergo a screening level risk assessment. A screening assessment involves an analysis of a substance to determine whether the substance is toxic or capable of becoming toxic as defined in CEPA 1999.
Figure 2: Categorization and screening process
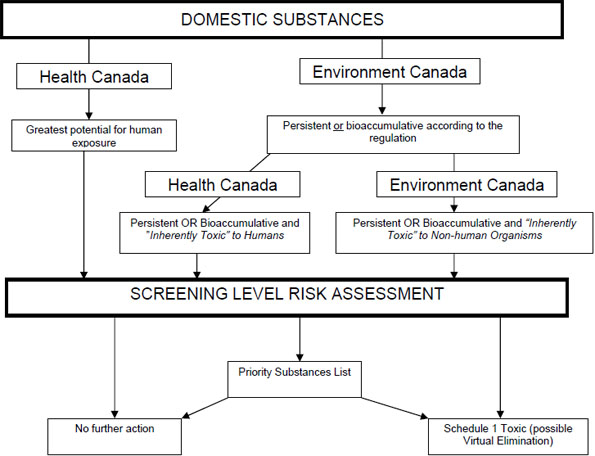
Long description of figure 2
Activities related to the categorization of the Domestic Substances List are conducted by Environment Canada and Health Canada. These activities are focused on determining which substances meet the criteria set out in the legislation, which are the greatest potential for human exposure, persistence, bioaccumulation and inherent toxicity to human and non-human organisms. Substances meeting these criteria must undergo a screening level risk assessment. Following this screening assessment, the substance may be added to the Priority Substances List for additional assessment work or if it is toxic it can be recommended for addition to Schedule 1 under the act. Alternatively the substance can be designated for no further action. The latter two measures, addition to Schedule 1 or no further action, are available upon completion of a Priority Substances List assessment.
5.2.3 What is the Priority Substances List?
Priority Substances List
The first Priority Substances List was established in 1989. Out of the 44 chemicals on the first list, 25 were declared to be toxic under the previous CEPA. The second Priority Substances List of 25 more substances was published in 1995. Out of the 23 assessments published, 18 substances were deemed to be toxic The Ministers of the Environment and Health suspended the assessment period for the other two substances (aluminum compounds and ethylene glycol) in order for Health Canada to collect new or additional information required to assess whether the substances are toxic or capable of becoming toxic.
The CEPA 1999 Priority Substances List continues to be the method used to focus on those chemicals and other substances that require investigation on a priority and in-depth basis to determine if they are toxic under the act. Substances can be added to the Priority Substances List when a more comprehensive assessment is required following a screening assessment or review of another jurisdiction's decision. Also, any person may ask the Minister to add a substance to that list. CEPA 1999 requires that the substance be assessed within five years from the date the substance is added to the list.
5.2.4 What is the review of decisions of other jurisdictions?
CEPA 1999 calls for cooperating and developing procedures for exchanging information on substances with other governments in Canada and member states of the Organization for Economic Co-operation and Development. When the Minister of the Environment receives information that another government has prohibited or substantially restricted a substance for environmental or health reasons, the Ministers of the Environment and Health are obliged to review the decision. The review determines whether the substance is toxic or capable of becoming toxic in the Canadian environment. In this way, Canada will benefit from a streamlined decision-making process through the sharing of scientific data, the capacity of other governments and efforts by others to develop risk management measures.
5.2.5 What are the outcomes of a risk assessment or review of a decision by another jurisdiction
What is the List of Toxic Substances?
Substances that meet the definition of toxic under CEPA 1999 can be placed on Schedule 1 of the act, the List of Toxic Substances. This does not control the substance but allows the government to proceed with regulations, pollution prevention plans or environmental emergency plans.
Under CEPA 1999, once the Ministers have conducted a risk assessment of an existing substance under the Priority Substances List, a screening level risk assessment or a review of a decision by another jurisdiction, they must propose one of three measures:
- They may add the substance to the Priority Substances List. Typically, they will do this if they decide that there is a need for a more comprehensive risk assessment.
- They may recommend that the Governor in Council (the federal Cabinet) add the substance to the List of Toxic Substances (Schedule 1) and, if applicable, to the Virtual Elimination List. They will typically add the substance to Schedule 1 if they determine that the substance meets the criteria for "toxic" under the Act and that regulatory or pollution prevention or environmental emergency planning risk management measures should be taken under CEPA 1999.
- They may propose no further action under CEPA 1999. They will typically do this if they determine that the substance is not "toxic." They also may propose no further action under CEPA 1999 if they determine that the substance is toxic but that actions being taken or about to be taken under other federal acts or by provincial, territorial or Aboriginal governments are sufficient to manage the risks in a timely manner.
5.2.6 What are the other triggers for risk assessment?
Other assessments may be triggered by information provided by other programs, industry and scientific research. Substances can be added to the List of Toxic Substances based on any assessment process that satisfies the Ministers that a substance is toxic, without having gone through one of the three types of CEPA 1999 assessments already discussed. Any other type of assessment can be used that satisfies the Governor in Council, on the recommendation of the Ministers of Environment and Health, that a substance is toxic. The other types of assessments that have been used in the past to add a substance to the list were based on collaborative efforts nationally or internationally.
CEPA 1999 allows the government to require persons to submit information on substances where a significant new activity for a substance has been identified. A significant new activity is an alternative use of the substance or other activity that results or may result in:
- a significantly greater quantity or concentration of the substance in the environment or
- a significantly different manner or circumstances of exposure of the environment to the substance
Significant new activities can apply to existing substances on the Domestic Substances List or new substances. The government assesses the new information on the substance to determine if it is toxic in relation to the significant new activity (see Section 6.4).
CEPA 1999 requires that persons who obtain new information on a substance that indicates it might be toxic must submit this information to the government.
5.2.7 What are the opportunities for public participation?
Summaries of the assessment conclusions and the proposed measure (no further action, addition to the Priority Substances List, or addition to the List of Toxic Substances) are published in the Canada Gazette, Part I for a 60-day public comment period. Interested parties may bring forward additional scientific evidence to support or refute the Ministers' decision or file a notice of objection requesting that a Board of Review be established (see section 18.3 for more information). Depending on the nature of the comments received, the Minister of the Environment then determines if further discussions or a Board of Review are warranted.
After taking into account any information provided during this 60-day period, the Ministers publish their final decision in the Canada Gazette, Part I. The Gazette notices are published on the Canadian Environmental Protection Act Registry.
5.3 How are the risks managed?
5.3.1 What risk management measures are available?
Examples of risk management measures under CEPA 1999 for existing substances include regulations, pollution prevention plans, environmental emergency plans, guidelines, codes of practice and administrative agreements. These measures may target any aspect of the substance's life cycle, from the research and development stage through manufacture, use, storage, transport and ultimate disposal. Risk management measures for toxic substances are developed through the toxics management process. For regulations, pollution prevention plans or environmental emergency plans the substance must be on the List of Toxic Substances or in the case of environmental emergency plans be, at least, recommended for addition to the List.
CEPA 1999 provides the authority for various risk management measures:
- regulations impose restrictions on an activity related to a substance, or set limits on the concentrations of a substance that can be used, released to the environment or be present in a product
- pollution prevention plans require the preparation and implementation of a plan outlining actions to prevent or minimize the creation or release of pollutants and waste
- environmental emergency plans require persons to prepare and implement a plan regarding the prevention of, preparedness for, response to, and recovery from an environmental emergency
- environmental quality objectives recommend qualitative or quantitative goals or purposes for pollution prevention or control of toxic substances. They often recommend ambient environmental quality targets or maximum acceptable levels.
- environmental codes of practice recommend procedures, practices, or quantities of releases relating to facilities and activities during any phase of development of and operation involving a substance, and any subsequent monitoring activities
- environmental quality guidelines can be developed to recommend a concentration for toxic substances in surface water, agricultural water, soil, sediment, and human and animal tissue. Guidelines may also be developed to prevent, prepare for, or respond to an environmental emergency or to restore environmental quality.
- environmental release guidelines include standards expressed as concentrations or quantities, for the release of substances into the environment from facilities or activities
- agreements respecting environmental data and research are usually cooperative arrangements with a provincial, territorial, aboriginal or foreign government or any person respecting the creation, operation, and maintenance of a system for monitoring environmental quality
- administrative agreements are usually work-sharing arrangements between the federal government and provincial, territorial, or aboriginal governments or aboriginal peoples respecting the administration of CEPA 1999
In developing risk management measures, the government must give priority to pollution prevention actions. When substances are inherently toxic to humans or non-human organisms, persistent, bioaccumulative, and present in the environment primarily as a result of human activity but are not naturally occurring radionuclides or naturally occurring inorganic substances, then they must be recommended for addition to the List of Toxic Substances. They are also proposed for virtual elimination of releases to the environment and added to the Virtual Elimination List. Virtual elimination is the reduction of releases to the environment of a substance to a level below which its release cannot be accurately measured (the level of quantification).
Toxics management process
Environment Canada is committed to considering the full range of potential preventive and control measures and recognizing other governments' roles when developing strategies to manage toxic substances under CEPA 1999. The National Advisory Committee of CEPA 1999 plays a key role in advising the federal government on activities under the act and on cooperative, coordinated approaches to the management of toxic substances.
Risk management measures for toxic substances are developed through the toxics management process. This process allows the federal government to meet the obligations set out in CEPA 1999 and ensures that stakeholder consultations are effective. Central to the toxics management process is the development of a risk management strategy. The risk management strategy, which can vary in format, outlines the proposed approach for managing the risks to the environment and human health for a particular toxic substance.
In developing the risk management strategy, Environment Canada and Health Canada identify the sources that pose the greatest risk to the environment and human health, guided by the science in the risk assessment. A risk management objective is then identified for these sources. This objective is usually based on results achieved from the best available processes, products, or techniques used by the sector or, in some cases, environmental quality objectives.
Once an objective has been set, the management measures that could achieve the risk management objective for each source are selected. All available tools, including existing management initiatives, are initially considered. These include instruments under CEPA 1999 as well as other risk management tools that are outside of CEPA 1999, including the regulatory provisions of other governments and voluntary approaches. The suite of tools can comprise a combination of measures representing the most feasible options for managing the substance. For a toxic substance that is subject to the time-clock provisions, at least one of the risk management measures must be a CEPA 1999 instrument. For example, there may be cases in which a new regulation or pollution prevention plan under CEPA 1999 would be the best option for addressing risks posed by one source and would satisfy the time clock requirements of CEPA 1999, while provinces, territories or aboriginal governments may be better situated to address another source, and an existing voluntary agreement may sufficiently address yet another source.
5.3.2 What are CEPA 1999's time-clock provisions?
For a substance found to be toxic through a Priority Substances List assessment, a screening assessment, or the review of a decision by another jurisdiction, and when that substance has been proposed for addition to the List of Toxic Substances, a proposed regulation or instrument establishing "preventive or control actions" for managing the substance must be developed with 24 months. The proposal is published in the Canada Gazette, Part I, for a 60-day comment period. Once proposed, the Ministers have a further 18 months to finalize the regulation or instrument. The Gazette notices are also published on the CEPA Registry.
For a risk management instrument to satisfy CEPA 1999's requirements, it must not simply be made under a provision of CEPA 1999 but must also pass the "legal test" of establishing preventive or control actions that reduce or eliminate the risks to the environment or human health. Each instrument is assessed on a case-by-case basis to determine whether this requirement is met.
The time clock provisions do not apply to substances added to the List of Toxic Substances on the basis of assessments that are not the formal CEPA 1999 assessments [that is, through assessments other than Priority Substances List assessment, a screening assessment, or the review of a decision by another jurisdiction). However, all of the risk management processes, tools and instruments available to the government for toxic substances described above are also available when substances are listed in this manner.
5.3.3 What are the opportunities for public participation?
Within the Toxics Management Process, the government may hold preliminary consultations with the most affected stakeholders during the development of the risk management strategy.
CEPA 1999 also provides formal opportunities for public participation during the risk management stage. Proposed instruments are published in the Canada Gazette, Part I for a 60-day comment period and on the CEPA Registry. Interested parties can provide comments on the proposed regulation or instrument or file a notice of objection requesting that a Board of Review be established. A Board of Review inquires into the nature and extent of the danger posed by the substance that is the subject of the order or the proposed instrument or regulation (see section 18.3 for more information). Depending on the nature of the comments received, the Minister of the Environment then determines if further discussions or a Board of Review are warranted.
After taking into account any information provided during this 60-day period, the Ministers publish the final instrument in the Canada Gazette, Part I or II depending on whether the measure consists of a regulation or other instrument, as well as on the CEPA Registry.
5.4 How are exports of substances managed?
CEPA 1999 provides the authority to establish an Export Control List (Schedule 3 of the act) containing substances whose export is controlled because their use in Canada is prohibited or severely restricted or because Canada has accepted, through an international agreement, to control their export. Prohibited substances can be exported only if they are to be destroyed or if the export is in compliance with regulations. Regulations can be made addressing:
- prohibitions on export
- conditions under which an export may be made
- the type of information to be provided to the Minister with respect to the export and
- the type of information to accompany an export and to be kept by the exporter
Details concerning these exports are made public through the CEPA Registry. These provisions of CEPA 1999 allow the federal government to ratify the Rotterdam Convention on Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade.