Overview of ecological assessment of substances
Official title: Overview of the ecological assessment of substances under the Canadian Environmental Protection Act, 1999
June 2007
List of acronyms
- ASTM
- American Society for Testing and Materials
- CAS
- Chemical Abstracts Service
- CEPA
- Canadian Environmental Protection Act, 1999
- CTV
- critical toxicity value
- DSL
- Domestic Substances List
- EC50
- median effective concentration
- IUPAC
- International Union of Pure and Applied Chemistry
- LC50
- median lethal concentration
- LD50
- median lethal dose
- LOAEC
- lowest-observed-adverse-effect concentration
- LOEC
- lowest-observed-effect concentration
- LOAEL
- lowest-observed-adverse-effect level
- LOEL
- lowest-observed-effect level
- NOAEC
- no-observed-adverse-effect concentration
- NOEC
- no-observed-effect concentration
- NOAEL
- no-observed-adverse-effect level
- NOEL
- no-observed-effect level
- OECD
- Organisation for Economic Co-operation and Development
- PEC
- predicted environmental concentration
- PNEC
- predicted no-effect concentration
- PSL
- Priority Substances List
- QSAR
- quantitative structure-activity relationship
1. Introduction
This document describes the process by which substancesFootnote 1 (excluding products of biotechnology), are assessed for their current and potential ecological risks in Canada. It outlines the general approach followed by the Existing Substances Division and the New Substances Division of Environment Canada in the preparation of ecological assessments of substances that are, or could be, manufactured in Canada, imported into Canada, or released into the Canadian environment. This overview reflects generic approaches taken for substances and situations; in practice, therefore, some deviations in the approaches may occur, depending on the circumstances. The manner in which the information is presented generally follows the order in which the various steps are typically addressed in the ecological assessment process.
Environment Canada is responsible for carrying out the ecological assessments of substances as part of joint ecological and human health assessment programs that it administers in cooperation with Health Canada. The Existing Substances program assesses "existing" substances, which are those that have been or are currently used commercially (as listed on the Domestic Substances List [DSL]), or that are released into the Canadian environment on their own or as an effluent, mixture or contaminant. The New Substances program is responsible for assessing substances that are proposed for introduction into Canadian commerce and are considered to be "new" to Canada. A substance not on the DSL is considered to be "new" to Canada. All "new" substances must undergo an ecological and human health assessment before they can be brought into the Canadian market.
Although there are some differences between the Existing Substances and New Substances programs and the mandates under which they operate, decisions must be made under both programs as to whether a substance is, or is capable of, being toxic as defined in CEPA (see Figure 1). As a result, the underlying principles and methods used for conducting ecological assessments of existing and new substances are the same. For further information on these two programs, please visit the managing substances in the environment section. Issues related to risk management are not discussed in this document (see management of toxic substances for this information).
Figure 1: Ecological assessment of substances conducted by the New and Existing Substances programs under CEPA
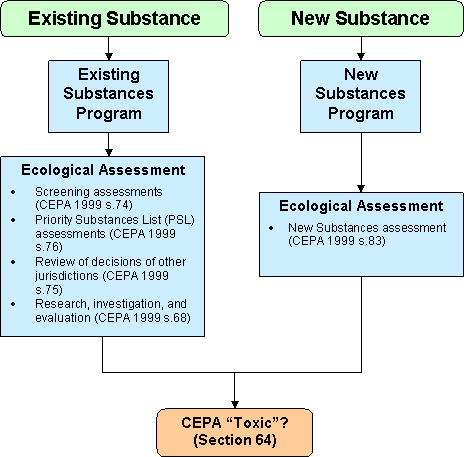
Long description of figure 1
This is a flow chart describing the ecological assessment of substances conducted by the New and Existing Substances programs under CEPA.
Existing Substances program is responsible for:
- screening assessments (under section 74 of CEPA)
- Priority Substances List assessments (under s. 76 of CEPA)
- review of decisions of other jurisdications (under s. 75 of CEPA)
- research, investigation and evaluation (under s. 68 of CEPA)
- new substances assessment (under s. 83 of CEPA)
1.1 Objective of an ecological assessment
An ecological assessment leads to an understanding of the actual or potential risks of a substance and recognition of the associated uncertainties. As a result of this understanding, a decision is made as to whether or not the substance meets or is capable of meeting the criteria set out under section 64 of CEPA. Under section 64, a substance is "toxic"
" ... if it is entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity;constitute or may constitute a danger to the environment on which life depends; orconstitute or may constitute a danger in Canada to human life or health."
Ecological assessments lead to a determination as to whether or not a substance meets the criteria set out in paragraphs 64(a) and (b), whereas human health assessments lead to a determination as to whether or not the criteria in paragraph 64(c) are met.
In the course of an ecological assessment, several steps are carried out:
- the ways in which a substance may enter the environment, what happens to the substance in the environment, and how non-human organisms may be exposed to the substance are determined (characterization of entry, fate, and exposure)
- the potential effects of the substance on the environment or its biological diversity are identified (effects characterization, also known as hazard characterization)
- the ecological risk associated with the substance is characterized by integrating information on its effects and the potential for exposure in Canada (risk characterization)
- the uncertainty associated with the conclusions is outlined (uncertainty analysis)
1.2 Use of a weight-of-evidence approach and the precautionary principle
Assessments conducted for new and existing substances apply the weight-of-evidence approach and precautionary principle. Specifically in CEPA, under section 76.1, it states that the Ministers of the Environment and of Health must apply a weight-of-evidence approach and the precautionary principle when conducting and interpreting the results of screening assessments, reviews of decisions of other jurisdictions, or assessments of substances on the Priority Substances List (PSL).
The weight-of-evidence approach involves the use of several component lines of evidence to make decisions in all phases of an assessment, including risk characterization. These lines of evidence can include risk quotients, results of probabilistic analyses, evidence of harm in the field, evidence of persistence and potential for bioaccumulation, and high or increasing levels of exposure. Use of all the available evidence permits better characterization of uncertainties and can help reduce the overall uncertainties associated with decisions. Rather than reliance on a single approach to ecological assessment, separate lines of evidence are examined, organized in a logical fashion, and used to reach conclusions. The use of a weight of evidence is aimed at bolstering the scientific rigour of an ecological assessment.
CEPA specifically addresses the importance of applying the precautionary principle to the assessment and management of substances. In the preamble to the act and in the introduction under Administrative Duties of the Government of Canada, it is stated that: "where there are threats of serious or irreversible damage, lack of full scientific certainty shall not be used as a reason for postponing cost-effective measures to prevent environmental degradation."
2. Framework for ecological assessment of substances
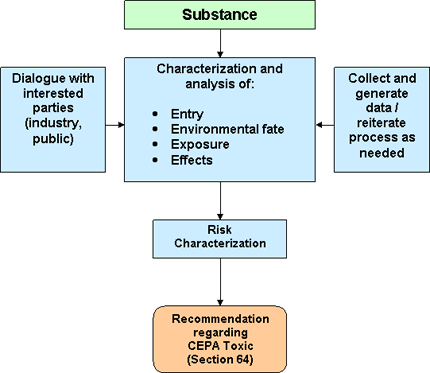
Ecological assessment under CEPA includes several components (Figure 2):
- Characterization of environmental entry, fate, and exposure - Information is gathered on the ways in which a substance enters and behaves in the environment and on how non-human organisms can be exposed to the substance.
- Characterization of ecological effects - The potential for the substance to cause adverse ecological effects is evaluated.
- Risk characterization - The potential for ecological risk of a substance is determined using multiple lines of evidence gathered during the assessment.
Data are collected throughout the ecological assessment process, as needed, to support the assessment. Dialogue with interested parties (for example, regulatory managers and members of industry and the public) is also conducted as needed throughout the ecological assessment and may result in additional considerations or new data.
Figure 2: Framework for conducting ecological assessments of substances under CEPA
3. Information collection and evaluation
At the beginning of an ecological assessment, data are obtained from a variety of sources, evaluated, and selected. Data collection or generation can also occur throughout the assessment process, depending on information needs identified during any of the phases.
3.1 Types of information used in an assessment
Various types of information are used to carry out an ecological assessment of a substance. These include chemical identity information, physical-chemical properties, industrial uses and environmental releases, degradation of the substance in the environment, the potential for exposure to the substance in the environment, and the potential ecotoxicity of the substance.
3.1.1 Chemical identity
The following chemical identity information is often collected for a substance:
- chemical name, using Chemical Abstracts Service (CAS) or International Union of Pure and Applied Chemistry (IUPAC) nomenclature rules
- trade names of substance and chemical name synonyms
- CAS registry number
- structural formula
- technical grade purity
- fraction (by weight) of known impurities and additives
3.1.2 Physical-chemical property data
Data on a substance's physical and chemical properties are gathered in order to understand the fate and behaviour of the substance once it is released to the environment. These data may be used as input parameters in environmental fate models, as discussed below.
Information on the following physical-chemical properties is often collected for a substance:
- molecular mass
- oxidation state, valence
- absorption spectrum (infrared, ultraviolet, mass, or nuclear magnetic resonance)
- melting/decomposition point
- water solubility
- boiling point
- density
- vapour pressure
- dissociation constant (pKa)
- thermodynamic formation constants for ion pairs
- soil adsorption-desorption data
- Henry's law constant
- octanol-water partition coefficient (Kow)
- bioconcentration factor (BCF), bioaccumulation factor (BAF), and/or biomagnification factor (BMF)
- biodegradation information
- hydrolysis rates and products
- photodegradation information
3.1.3 Information for exposure characterization
In order to characterize exposure, information is gathered or generated to determine or estimate how the substance has entered or may enter the environment during its life cycle, what quantity of a substance is entering the environment, and what concentrations of a substance will be found in various environmental compartments.
To understand how a substance has entered or may enter the environment or to estimate the substance's distribution and concentrations in various media, the following information may be collected or generated:
- sources of the substance (including human activities and possibly natural processes)
- use patterns (type of use, quantity, and distribution in Canada)
- frequency and duration of releases (continuous or batch releases)
- monitoring data (for chemicals already in the environment)
- quantity of substance released (or release rates) during the life cycle of the substance
- environmental partitioning
- pathways of exposure
- chemistry of the receiving environment
- persistence and bioaccumulation potential
- natural background concentrations
3.1.4 Information for effects characterization
To characterize potential ecological effects, information is collected or generated on the substance's potential biotic and abiotic effects in various environmental compartments or media (for example, water, soil, sediment, air). Types of information that may be obtained include acute and chronic toxicity values and abiotic endpoints (for example, ozone depletion, effects on soil structure, seasonal mixing in lakes).
Toxicity information preferably includes data from a wide range of food-chain levels to help determine which populations, communities, and ecosystem processes may be particularly susceptible to adverse effects, as well as to determine the types and potential magnitude of these effects.
3.2 Evaluation of information
Once information has been gathered for an assessment, the quality of the key experimental or field data and the accuracy of model estimates are evaluated to determine their acceptability for use in an assessment.
3.2.1 Data quality and reliability
Experimental data
Experimental data are evaluated for quality based on whether or not they have been obtained according to an accepted testing or measurement protocol (for example, Organisation for Economic Co-operation and Development [OECD] Test Guidelines, American Society for Testing and Materials [ASTM] standards). Studies are also evaluated according to quality criteria developed by Environment Canada and the OECD. Studies that are found to be of unacceptable quality are not used in an assessment.
Close analogues
When experimental data are not available for the substance being assessed, or to supplement limited data on the substance, experimental data from a close chemical analogue may be used (the "read-across" approach). In general, a close analogue should preferably contain most, if not all, of the same structural features as the substance being assessed and should have similar physical-chemical properties (for example, water solubility). The more an analogue resembles the substance under assessment, the higher the confidence associated with its use in an assessment.
Model predictions
Experimental and analogue data are often not available for a specific chemical property or toxicity endpoint for a substance, and therefore predictive models may be used to obtain data. For example, quantitative structure-activity relationship (QSAR) models can be used as part of the weight of evidence to predict aquatic toxicity values when experimental data are limited or do not exist.
Determining the quality and reliability of QSAR model predictions for a substance under assessment requires experience with how a model performs with various classes of compounds. Predicted values can be verified for their reliability by evaluating whether the model is suitable for the type of substance being assessed.
3.2.2 Expert judgment
Expert judgment-based on the accumulated knowledge and experience of evaluators and recognized experts in a field of science-is an important component of assessments. Expert judgment usually involves making assumptions based on comparable experiences or extrapolations (for example, from substances of similar chemical structure or composition). Expert judgment may be used throughout the ecological assessment process by the assessment team and can be particularly useful when no experimental data are available for a substance and predicted data cannot be generated. Expert judgment is particularly important when examining experimental data for validity and reliability, selecting appropriate models, identifying closest analogues, and developing a best estimate for a toxicity endpoint.
3.2.3 Data preference
Often a combination of different types of data will be gathered to create a weight of evidence for an endpoint in an assessment. Generally, experimental data are preferred over predicted data from models; however, both could be used as lines of evidence. The following guidelines describe the usual order of data preference for a particular endpoint:
- acceptable experimental data for the substance
- acceptable experimental data from a close structural analogue of the substance being assessed
- reliable model prediction (that is, based on good chemical structural coverage)
- expert judgment
The order listed above is meant as a general guide. In fact, all forms of data are considered in a weight-of-evidence approach. At times, data availability, expert judgment, and simple practicality may result in deviation from this order.
4. Characterization of entry, fate, and exposure
During this phase of the assessment, information on how a substance enters the environment is integrated with information on its fate in the environment in order to establish the degree of contact that is occurring, or may occur, between an ecological receptor and the substance. The main steps are:
- entry characterization - to understand if, how, and in what quantities a substance may enter the environment throughout its life cycle (from manufacture or importation through to disposal)
- characterization of environmental fate and distribution - to determine a substance's fate in different environmental compartments and to understand how an organism comes into contact with a substance entering a particular medium
- quantification of exposure - to estimate potential quantities in the environment and to determine predicted environmental concentrations (PECs) or exposure distributions for relevant environmental compartments (for example, air, water, soil, sediment, terrestrial wildlife)
4.1 Entry characterization
Entry characterization involves identifying where and how a substance may enter or has entered the environment (for example, through natural occurrence, via industrial processes, or in consumer products) and the characterization of releases from these processes (for example, quantities, frequency, and duration). This information is critical for determining the relative significance of a source of release and the scale (in terms of both time and space) of potential exposures. Understanding where a substance enters the environment (for example, whether it is released to water or to air) is also essential for determining its fate in the environment.
4.1.1 Identification of sources
A substance can originate from natural sources in the environment or as a result of human activities. Regarding human sources, there is a need to consider both domestic and potential transboundary sources. Sources may also be indirect. For example, substances may be the products of the degradation of other substances, as a result of biotic and/or abiotic processes. Some of the typical sources of substances entering the Canadian environment include:
Natural sources
- volcanic emissions
- sea spray
- fires
- weathering of rock
- natural atmospheric fallout
- volatile compounds produced by plants or microbial activity
Human activity sources
- manufacturing
- processing and formulation
- transportation, distribution, and storage
- end use (for example, industrial/professional use, consumer use, service life of products)
- disposal (including recycling)
Other sources
- short- and long-range transport (transboundary origin)
- indirect sources (for example, transformation and degradation products)
4.1.2 Use pattern analysis
The majority of substances assessed under CEPA originate from human activity associated with industrial and commercial operations and consumer uses throughout Canada. Understanding the use patterns for a substance is important in determining the points of entry of the substance into the environment. For example, if a substance is used in a personal care product, entry into the environment will be widely dispersed across Canada, largely as a result of down-the-drain releases. Also, a substance that has been designed for a specific use may be associated with certain manufacturing locations, enabling characterization of the releases for these locations.
The objectives of use pattern analysis for a substance are to determine:
- current and potential uses in Canada (for example, in consumer products and/or industrial applications)
- amounts in Canadian commerce (that is, amounts manufactured, imported, and exported)
- spatial distribution of use across Canada
- temporal use patterns (for example, substances may be used seasonally)
4.1.3 Release characterization
Releases to the environment can be characterized using information on the life cycle of the substance, that is, during manufacture, processing (formulation, blending), distribution, application (for example, consumer product), and disposal (for example, landfill, incineration, and recycling).
For each point of entry, release characterization may involve:
- identifying environmental media to which the substance is or may be released (for example, surface water, soil, air, sludge)
- identifying the frequency and duration of releases (for example, continuous versus event-based)
- quantifying the release rates (for example, amount per day or per hour)
- determining if mitigation measures are in place to limit releases (for example, wastewater treatment plants, containment ponds, special disposal practices)
In the absence of information on actual releases of a substance, release rates may be estimated using standard release scenarios that have been developed by and that are widely used in other jurisdictions (for example, the U.S. Environmental Protection Agency and the OECD).
4.2 Environmental fate and distribution
The objective of examining a substance's fate and distribution is to identify where it will end up once it enters the environment and how long it will reside there. Understanding a substance's fate and distribution helps determine which environmental compartments should be included in an ecological assessment (for example, air, water, soil, sediment) and provides information for determining which organisms will come into contact with the substance and how long they will be exposed to it. Fate and distribution analysis involves examining how a substance partitions into various environmental compartments according to its physical and chemical properties (for example, volatility, water solubility, adsorption potential), as well as how it is emitted into the environment (for example, via water or air). The scope of fate and distribution analysis may vary from local considerations to regional or national considerations.
Measured environmental concentrations are not available for most substances assessed under CEPA; consequently, environmental distribution based on chemical fate is most often estimated. Fate analysis may involve a simple qualitative approach using the substance's physical and chemical properties or a more detailed quantitative analysis using fate models.
Figure 3 provides an example of how substances can enter the environment and be distributed as a result of human activity.
Figure 3: Example of substance entry into the environment resulting from human activity
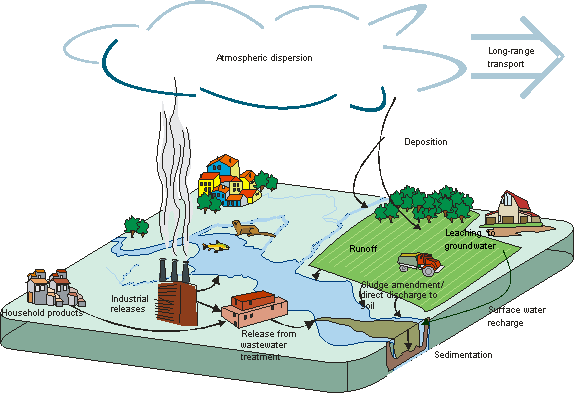
4.2.1 The importance of persistence
Environmental persistence is a measure of the length of time it takes for a substance to break down in the environment. Various processes, both abiotic (for example, hydrolysis) and biotic (for example, biodegradation), can cause substances to undergo irreversible reactions and degrade in the environment. Some substances (for example, elements of the Periodic Table) do not degrade and can be considered infinitely persistent. The persistence of other (organic) substances in the environment depends on their inherent properties and the nature of the surrounding environment. Measures of environmental persistence are often cited using the half-life (the time it takes for half of the quantity of a substance to degrade) in a particular medium.
Persistence is an important concept in fate and environmental distribution. It influences where a substance will ultimately reside in the environment (for example, soil versus air), and the quantity of a substance that will reside in an environmental compartment at any point in time. For example, if a substance is released to water and the fate of the substance suggests that equal amounts will end up in water and sediment, but the substance degrades more rapidly in water than in sediment, a greater quantity of the substance will be found in the sediments over time. Another example concerns a substance that is expected to be found in air and is persistent in air; it may undergo long-range transport in the atmosphere to distant areas, where it may be deposited in precipitation. Accordingly, the scope of an assessment may include not only the local receiving water body but also areas influenced by atmospheric deposition.Footnote 2
When degradation products are identified, they may be assessed along with the parent compound or in a separate assessment. The decision to assess a degradation product depends in part on the likelihood that the parent substance will degrade and on the potential hazard of the degradation product to the environment.
4.2.2 The importance of bioaccumulation
The tendency of a substance to be absorbed from an environmental medium (for example, water, soil) or prey item into the tissues of an organism where it may accumulate is often generally described by the term bioaccumulation. This concept can in fact be measured in different ways depending on the uptake processes considered:
- Bioconcentration occurs in an organism when the rate of uptake of a substance via the skin or respiratory surface (for example, gills) exceeds the rate by which the organism can eliminate it (for example, via metabolism or via excretion). It is usually measured under laboratory conditions and accounts for uptake only from the exposure medium (typically water). The extent of bioconcentration is usually expressed in the form of a bioconcentration factor (BCF), which is the ratio of the chemical concentration in the organism to that in the water.
- Biomagnification refers to the increase in concentration of a substance in an organism with each successive step in the food web. This results in contaminant concentrations in predators that are greater than those in their prey. It can be measured under laboratory and field conditions and accounts for uptake via the diet. The extent of chemical biomagnification may, for example, be expressed in the form of a biomagnification factor (BMF), which is the ratio of the chemical concentration in the predator to that in its food.
- Bioaccumulation occurs when the rate of uptake of a substance from all routes of exposure (for example, diet, skin, respiratory surface, inhalation) exceeds the rate by which an organism can eliminate it (for example, via metabolism or excretion). It is usually measured in water under field conditions. The extent of chemical bioaccumulation is usually expressed in the form of a bioaccumulation factor (BAF), which is the ratio of the chemical concentration in the organism resulting from the various routes of exposure to that in the water.
Bioaccumulation is an important concept for understanding if a substance may be transferred and distributed in the food webs of aquatic and terrestrial organisms and thus understanding the potential for exposure of top predator species to the substance.
Understanding the bioaccumulation of a substance is also very important for determining its potential to cause direct adverse effects in organisms. In general, a substance that is not metabolized or broken down readily by an organism (and thus bioaccumulates) will have a greater potential to cause adverse effects than a substance that can be readily eliminated from the tissues of an organism (assuming that the breakdown products of the substance are non-toxic). This is because the residence time of a non-metabolized substance in an organism is greater than that of a metabolized substance, and so the tissue concentration will slowly build up over time, creating a higher potential for internal damage to an organism.
4.3 Quantifying exposure
The main objective of quantifying exposure is to determine the concentrations of the substance in the media in which it is expected to reside following release to the environment. Concentrations are estimated on a local or regional scale depending on the substance's environmental fate and the nature of the release. Different procedures for quantifying exposure may be used depending on the information available for a substance. When they are available, measured data (that is, from monitoring studies in Canada) may be used to quantify exposure concentrations. Data from monitoring studies in other countries may also be considered, when appropriate, as part of the weight of evidence. Exposure concentrations can also be calculated at the local scale using models based on generic environments to which site-specific information may be incorporated. These exposure concentrations can contribute to the weight of evidence in an assessment, be used as PEC values, or be used to derive an environmental exposure distribution (using probabilistic methods).
For naturally occurring substances, the environmental background levels and tolerance of native organisms to elevated levels of these substances are taken into account in the assessment.
5. Effects characterization
The overall objective of this phase is to characterize the type and magnitude of adverse ecological effects, direct or indirect, that could occur following exposure to a substance (or a degradation product of the substance) in the environment. During ecological assessment under CEPA, effects on both biotic (living) and abiotic (non-living) components of the environment are considered.
5.1 Biotic effects
When characterizing biotic effects, a major objective is to determine or estimate the threshold concentration of a substance that is expected to be associated with adverse effects in sensitive organisms in the Canadian environment. It is also important to determine how the effects vary with exposure. This is usually accomplished by evaluating information from toxicity tests measuring the effects of the substance on one or more species. Ideally, toxicity information consists of data from different species that cover a range of food-chain levels. This type of information can be used to determine which species within a community or ecosystem may be particularly sensitive to the substance.
When sufficient relevant and reliable toxicity data are available for a given route of exposure, a species sensitivity distribution or concentration-effects curve can be constructed to determine an effects threshold or to determine how the magnitude of effects changes with the level of exposure.
Typically, measurements of biotic effects on single species are used to predict effects on populations in the environment. Since effects on populations are rarely measured directly, effects on individual organisms, measured in laboratory tests, are typically considered instead.
Most toxicity data have been derived from short-term or acute exposure studies (that is, 24-96 hours) using relatively high exposure concentrations and severe measures of organism response (for example, mortality). Most toxicity models (for example, QSARs) also report acute lethal effect concentrations. If short-term exposures are of concern (that is, a substance is not persistent and release is intermittent), the use of acute toxicity studies may be preferable over longer-term chronic studies. Often, however, chronic toxicity is a major concern, either because the substance is persistent or because it is released continually so that exposure is long term.
Typical endpoints for acute toxicity studies include:
- LC50 (the median lethal concentration, that is, the concentration lethal to 50% of test organisms, often measured over 24, 48, or 96 hours)
- EC50 (the median effective concentration, that is, for effects other than death)
- LD50 (the median lethal dose)
Chronic sublethal studies, while generally less available, provide a more sensitive measure of effects. This information can be used to determine a threshold concentration that should not adversely affect similar, sensitive organisms exposed over the long term in the Canadian environment. When long-term or continuous exposures are a concern, the following types of toxicity endpoints are often used:
- LC25 or EC25 (the concentrations causing death or an effect in 25% of test organisms, respectively)
- LO(A)EL or LO(A)EC (lowest-observed-[adverse-]effect level or concentration)
- NO(A)EL or NO(A)EC (no-observed-[adverse-]effect level or concentration)
5.1.1 Selection of CTVs
A critical toxicity value (CTV) is the lowest concentration of a substance, from the acceptable available data, at which an adverse effect was observed in the most sensitive species. The selection of toxicological endpoints is aimed at maintaining the survival and reproduction of populations that are expected to be exposed to a substance.
When selecting a CTV, long-term (chronic) toxicity data are generally preferred over short-term (acute) data, since environmental exposure is typically continuous or long term. Acceptable measured data and predicted data may both be considered in a weight-of-evidence approach.
5.1.2 Derivation of PNECs
The Predicted No-Effect Concentration (PNEC) is derived from the CTV and represents the concentration of a substance in the environment that is not expected to induce any adverse effects in a population typically following chronic or long-term exposure. PNECs are calculated by dividing the CTV by an appropriate assessment factor. The size of the assessment factor applied is reflective of the uncertainty in the available data and the level of extrapolation needed. In practice, assessment factors vary in magnitude and are used to account for things such as:
- extrapolation from acute to chronic effects
- extrapolation from single-species laboratory tests to ecosystem impacts
- increased potential for effects due to the presence of other substances
- the quality of data available (measured and estimated)
- the quantity of data available (measured and estimated)
- representativeness of species and tests
- variations in sensitivity between species or between individuals within a species
- mode of toxic action (substances having a specific [non-narcotic] mode of toxic action may require a higher factor)
Tenfold assessment factors are used to account for various sources of uncertainty, and the total assessment factor, derived in a multiplicative approach, is generally not greater than 1000. The 10-fold approach is used by many regulatory agencies for deriving threshold effect concentrations for various media. In general, if only a single toxicity value is available, there is a large uncertainty about the relevance of this value to other organisms, and a large assessment factor (for example, 1000) is applied to cover the range of possible sensitivities among organisms in the environment. However, a smaller assessment factor may be used if, for example, there are more data available.
5.1.3 Effects from substances in food webs
Wildlife predators need to be among the species considered when characterizing effects of substances that do not rapidly degrade and are transferred in food webs as a result of bioaccumulation. For persistent and bioaccumulative substances in particular, exposure of predatory wildlife may be higher than that of any other members of the food web. For some substances, measured effects in wildlife predators (for example, mink, eagle, fox) are available from field studies, and this information can be used directly for effects characterization. In most cases, however, results from repeated oral dose toxicity studies with rodents (mice, rats) are used and extrapolated to wildlife species (in much the same way as in human toxicity assessments) in order to examine the potential effects of a substance in wildlife predators.
5.1.4 Species sensitivity distributions and concentration-effect curves
A species sensitivity distribution, which is a statistically derived distribution of measures of adverse effects (for example, NOECs), may also be used to derive a PNEC. A species sensitivity distribution provides a means of estimating the fraction of species that are potentially affected at given concentrations of a substance. Species sensitivity distributions can be used in probabilistic assessments.
A concentration, effect curve, which illustrates the relationship between a level of adverse effect (for example, percentage mortality) in a particular species and various exposure concentrations, can also be used in probabilistic assessments.
5.2 Abiotic effects
Abiotic effects are effects that impact non-living components of the environment (the environment on which life depends). The release of a chemical substance may, depending on its physical and chemical properties, have impacts that result in a change to the environment, which, in turn, may adversely impact the ability of organisms to inhabit the environment. Adverse abiotic impacts can include chemical oxygen depletion or pH changes in water, chemical-mediated compaction or pH changes in soil, and ozone depletion.
Characterization of abiotic effects varies between substances, depending on their physical and chemical properties. This can involve examining the potential reactions and interactions of the substance in the environment.
There are various ongoing domestic and international activities and approaches to the management of substances that have the potential for atmospheric abiotic effects, such as significant contributions to climate change, ozone depletion, and smog formation. These include, for example, the Montreal Protocol on Ozone Depleting Substances and the Intergovernmental Panel on Climate Change. Information on atmospheric abiotic effects is considered as part of ecological assessments on a case-by-case basis when evidence suggests there is a potential concern (for example, hydrofluorocarbons).
6. Risk characterization
Risk characterization involves using a combination of qualitative and quantitative approaches to understand and describe the risk a substance poses to the environment. The various lines of evidence explored during the assessment are considered, in a weight-of-evidence approach, to evaluate the potential for harmful effects of a substance in the Canadian environment.
6.1 Lines of evidence
The various lines of evidence that may be considered are discussed briefly below.
Evidence that a substance is both persistent and bioaccumulative (or a precursor of such a substance)
The Ministers of the Environment and of Health consider that evidence that a substance is both persistent and bioaccumulative (according to the Persistence and Bioaccumulation Regulations under CEPA), when combined with evidence of toxicity and release or expected release into the environment, is sufficient to conclude that the substance can lead to harmful ecological impacts.
Although it is not possible, using current science, to accurately predict the long-term ecological effects of persistent, bioaccumulative substances, they are generally acknowledged to have the potential to cause serious and possibly irreversible impacts. Persistent substances remain in the environment for long periods of time, increasing the probability and the duration of exposure. Persistent substances that are subject to long-range transport are of particular concern because they can result in low-level, regional contamination. Releases of extremely small amounts of persistent and bioaccumulative substances may lead to relatively high concentrations in organisms over wide areas. Bioaccumulative and persistent substances may also biomagnify through the food chain, resulting in especially high internal exposures for top predators. Because they are widespread, several different persistent and bioaccumulative substances may be present simultaneously in the tissues of organisms, increasing the likelihood and potential severity of harm.
Evidence that a substance is a precursor of a substance on the List of Toxic Substances
Evidence that a substance is a precursor of a substance on the List of Toxic Substances (in Schedule 1 of CEPA) is a strong indication that the substance is "capable of becoming toxic," that is, that it may ultimately harm the environment, even if the untransformed parent compound does not cause direct harm on its own.
There are particular concerns about precursors of substances that are on the List of Toxic Substances and are candidates for virtual elimination. Evidence that a substance is a precursor of a substance that is on the List of Toxic Substances and is a candidate for virtual elimination indicates that release of the precursor into the environment can lead to harmful ecological impacts. This indicates that the precursor meets the criteria in paragraph 64(a) of CEPA.
Field evidence that a substance has caused or is causing environmental harm
Field evidence can provide a strong indication that a substance meets the criteria in paragraph 64(a) of CEPA. However, one of the main objectives of the act is to prevent harm from occurring, rather than reacting after harm has occurred. Pollution prevention cannot occur if ecological assessments rely solely on this line of evidence, which documents environmental harm that has already occurred, although action could be taken to prevent further damage.
Evidence that a substance is causing environmental harm in another country should also be considered, particularly if the environmental conditions in that country are similar to those in Canada.
Results of a quantitative comparison of exposure and effects data
Exposure and effects data can be compared in a number of ways. Possibly the simplest way is to derive a risk quotient (the ratio of the PEC and the PNEC). This is called a deterministic approach, as it uses only single data points for PEC and PNEC to derive a risk conclusion.
The use of the risk quotient method is a common approach in ecological assessment and typically provides an important component of the weight of evidence. A risk quotient is calculated for each assessment endpoint using the following formula:
where:

RQ = risk quotient
PEC = predicted environmental concentration (derived during exposure characterization)
PNEC = predicted no-effect concentration (derived during effects characterization)
The risk quotient approach for characterization of ecological risk usually involves the use of reasonable worst-case PEC and PNEC values and provides a conservative estimate of risk.
A risk quotient greater than or equal to 1 suggests that a substance may cause harm, whereas a risk quotient less than 1 suggests that a substance is unlikely to cause harm unless environmental concentrations increase. Care is taken to ensure that the assumptions used to obtain these risk quotients are realistic.
If a risk quotient analysis based on total concentrations of a substance does not suggest a risk, then the assessment can be concluded without correcting for bioavailability. If, however, a risk quotient based on total PEC and PNEC suggests a likelihood of harmful effects, then the characterization of risk may be refined based on consideration of bioavailability of the substance (which is often dependent on the substance's solubility).
If data permit, a probabilistic risk assessment may be performed. This type of assessment involves analyzing distributions of exposure and/or effects and presenting the results of the analyses in a variety of ways. It allows an in-depth consideration of sources of variability and uncertainty in the risk analysis.
In its simplest form, a probabilistic risk assessment may involve estimating the probability of exceeding the PNEC derived in the assessment by comparing an exposure distribution with a single PNEC value. However, probabilistic assessments may be more complex, using joint probability curves to compare exposure concentration distributions with distributions of adverse effects (for example, species sensitivity distributions or concentration-response curves for sensitive organisms) in order to estimate the probabilities of differing magnitudes of adverse effects.
Evidence that a substance has the potential to harm organisms at relatively low concentrations
Substances that have the potential to harm organisms at relatively low concentrations and/or that have specific modes of toxic action (for example, substances that are carcinogens or endocrine modulators) are of particular concern. Such substances may occur at harmful concentrations in the environment, but exposure to them may be undetected or may not be suspected if it results from low-level but continuous releases.
Evidence that a substance is widespread in the environment and/or that concentrations have been increasing over time
Evidence from monitoring studies indicating that a human-made substance is widespread in the environment and/or that concentrations have been increasing over time may indicate an elevated exposure potential and/or an increasing potential to cause harm.
It may be possible to incorporate trend information in a quantitative comparison of exposure and effects data. For example, it may be possible to predict what exposure concentrations would be, for example, in five years if a trend of increasing exposure continues. These predicted exposure values could be used in a risk quotient analysis or could be incorporated into a probabilistic assessment (see above).
Evidence that a substance is used in Canada in moderate to large quantities
Evidence that a substance is used in Canada in moderate to large quantities, in a variety of locations, and/or that use quantities are increasing may also be taken as an indicator of significant potential for exposure.
Evidence that a substance is likely to affect an endangered or threatened species in Canada or that a substance may be present in a protected or particularly sensitive area at or near a level that could have adverse environmental effects
Endangered and threatened species are apt to be susceptible to any additional environmental stressors. It is therefore important to take into account the likelihood that a substance would affect such species, even if exposure is localized. Similarly, the likelihood of a substance being present or becoming present in a protected or particularly sensitive area should be given close consideration. Ecological communities in such areas might be disrupted by additional stressors.
Restrictions in other jurisdictions
The determination that a substance has been or is being banned or severely restricted in another jurisdiction is an indication that a substance may meet the criteria in section 64 of CEPA. This is particularly true if the substance's use pattern and environmental releases and the environmental conditions, such as climate, types of biota, etc., in the other jurisdiction are similar to those in Canada.
6.2 Dealing with uncertainty
Sources of uncertainty in the ecological assessment are related to information gaps and conflicting data pertaining to entry, exposure, and effects for each assessment endpoint. Within the ecological assessment, key areas of uncertainty are considered and may be discussed in detail when arriving at a final conclusion.
7. Conclusion of the assessment
Using a weight-of-evidence approach, all of the available information for a substance is evaluated and uncertainties are considered in order to characterize the ecological risks posed by the substance. As such, the ecological assessment and its conclusions regarding the ecological risks posed by the substance provide the scientific foundation for recommending whether or not the substance meets the criteria set out in section 64 of CEPA.