Reference method for measuring asbestos emissions from asbestos mining and milling operations: part III
Part II - Analysis - Method A-1
Analysis of Asbestos Samples From Mining and Milling Operations by Optical Phase-Contrast Microscopy
- A-1.1 Scope
- A-1.2 Apparatus
- A-1.3 Reagents
- A-1.4 Procedure
- A-1.5 Calculations
A-1.1 Scope
The equipment and procedures necessary for mounting, sizing, and counting asbestos fibres obtained by standard sampling techniques are described. The method applies to samples collected on membrane filters and considers only those asbestos fibres greater than 5 μm in length with an aspect ratio of at least 3:1.
A-1.2 Apparatus
A-1.2.1 Filter-Mounting Equipment
Filter samples should be mounted in a clean environment to avoid contamination. The following items will facilitate the mounting of the sample.
Microscope Slides. Glass slides 2.5 × 7.5 cm are used. Those with a frosted end are preferable so that sample data can be easily recorded.
Cover Slips. Cover slips of a size sufficient to cover the filter wedge are required. A No. 1 ½ (.17 mm) cover slip is generally used as most objectives are optically corrected for this thickness.
Scalpel. A scalpel is required to cleanly and easily section the filter sample.
Tweezers. A pair of tweezers is required to remove the filter sample from its holder and to place the sector to be analysed on the glass slide.
Lens Tissue. Lens tissue (lint-free) is used to clean all mounting equipment and slides prior to use.
Glass Rod. A glass rod is required to spread the mounting solution on the slide.
Wheaton Balsam Bottle. This bottle is used to hold the mounting solution and has a special top which helps prevent contamination of the solution. A glass rod is included for dispensing the solution.
A-1.2.2 Optical Equipment
A light-field microscope fitted with phase-contrast accessories and capable of achieving a magnification of 400X is required. The following parts and accessories must be available with this unit.
- 10X Huygenian eye-piece;
- Koehler illumination;
- Mechanical stage;
- Abbe or Zernike condensor fitted with a phase ring with a numerical aperture (N.A.) equal to or greater than the N.A. of the objective;
- 40X - 45X positive phase-contrast achromatic objective (N.A. 0.65-0.75);
- Phase ring-centering telescope;
- Porton reticle;
- Stage micrometer (2 mm divided into units of 0.01 mm).
A-1.3 Reagents
A-1.3.1 Mounting Solution
Reagent-grade dimethyl phthalate and diethyl oxalate which are free of particles and colour are required. Filter material identical to that used in sampling is also required.
A-1.4 Procedure
A-1.4.1 Preparation of Mounting Solution
Prepare a 1:1 solution of dimethyl phthalate in diethyl oxalate by mixing together equal volumes of these reagents For each millilitre of this solution add 0.05g of filter medium and stir until the filter dissolves. This mounting solution is stable for up to six months but only a small amount should be prepared at any time since very little is required to mount each sample. About 300 samples can be prepared from 20 ml of mounting solution.
A-1.4.2 Sample Mounting
The exposed filter is carefully sliced into the appropriate number of sectors for analysis of the sample. (Refer to sampling methods S-1 and S-2 for number of sectors per filter sample which must be analysed.) Each sector is a 45° wedge of the filter sample.
With the glass rod supplied with the Wheaton Balsam bottle apply a small drop of mounting solution to a cleaned glass slide. It is important to avoid using an excessive amount of solution in order to prevent migration of the asbestos particles. To lessen this problem, it is useful to prepare a template on which a 45° wedge in the shape of a sample sector has been etched (Figure A-1-1). The glass slide is placed over this template so that the wedge appears in the centre of the slide. A drop of mounting solution is then placed in the centre of the wedge. The size of the drop is correct when, on settling, it touches or slightly overlaps the edges of the wedge.
With a clean glass rod, spread the spot of mounting solution so that it coincides with the shape of the filter wedge.
Using tweezers, grasp the sector to be mounted by its unexposed outer edge and place the sector, exposed side up, on the mounting medium.
Carefully place a clean cover slip over the filter sector. Do not reposition the cover slip once contact has been made.
Label the slide with a sample number and mounting date.
The sample should become transparent within 15 min but approximately 1 h should be allowed for all graininess to disappear before the slide is counted.
If the edge of the cover glass is not made air-tight to form a 'sealed' mount, the slide should be counted within three days. A sample with a sealed mount must be counted within 25 days but preferably should be counted as early as possible.
Figure A-1-1: Filter-Mounting Template

A-1.4.3 Optical System - Adjustment and Calibration
A-1.4.3.1 Microscope Adjustment
The microscope is adjusted, according to manufacturer's instructions, to produce positive phase-contrast images using Koehler illumination. The following general guidelines must be observed.
- The light source must be in focus and centered on the condensor iris or annular diaphragm.
- The object for examination must be in focus.
- The illuminator field iris must be in focus, centered on the sample, and opened only to the point where the field of view is illuminated.
- The phase rings (annular diaphragm and phase-shifting elements) must be concentric.
A-1.4.3.2 Calibration of Porton Reticle
The porton reticle is a glass plate inscribed with a series of circles and rectangles (Figure A-1-2). This reticle is used to determine fibre length and also to define a field area in which the asbestos fibres can be counted. The fibre lengths are determined by comparison with the circles on the reticle. The square on the left of the reticle, which is divided into six rectangles, is the counting field area (f.a.).
The porton reticle rests on the field-limiting diaphragm in the Huygenian eyepiece, and remains in sharp focus, superimposed on the microscope's field of view.
In order to evaluate the asbestos fibre concentration of the sample, the reticle must be properly calibrated. The calibration of the reticle will vary with the specific eyepiece-objective-reticle combination used. Should any of these be changed (i.e. disassembly, replacement, zoom adjust, etc.) the combination must be recalibrated. The stage micrometer is used to calibrate the porton reticle as follows.
- Clip the stage micrometer onto the mechanical stage;
- Focus on the micrometer scale at the 40X - 45X objective magnification;
- Align the left boundar y of the large rectangle on the reticle with the first scale division of the micrometer;
- Determine the length of the large rectangle in millimetres by counting the number of scale divisions of the micrometer along this length.
When the length of the large rectangle has been determined, the field area (f.a.) and circle diameters (Dn) can be calculated from the following relationships:
- The length of the large rectangle is defined as 200L units.
- The width of the large rectangle is 100L units.
- Thus the area of the counting field (f.a.) is (100L)2.
- The diameters of the circles are given by the equation
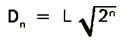 where
where- n = circle number
Figure A-1-2: Porton Reticle


A-1.4.4 Counting and Sizing
- For each field investigated, count only those fibres >5 μm in length with an aspect ratio of at least 3:1.
- Count any fibre >5 μm if it is entirely within the field area.
- If some portion of the fibre extends out of the field area then only count that fibre if it crosses one or both of adjacent preselected sides. It is convenient to use the left and bottom sides of the field area as reference sides.
- Count all fibres >5 μm present in a total of 100 fields. Count 25 fields in the inner area (Ai) of the wedge and 75 fields in the outer area (Ao).
- Select the counting fields without looking into the eyepiece.
- Select the fields in a straight line running from the tip of the wedge (centre of filter) to the centre of the arc side (circumference of the filter). If further counts are required to achieve the necessary 100 fields, select them along straight lines parallel to and slightly above or below the original counting line.
- Do not count a field containing more than 20 fibres unless there is very little fibre overlap and few background particulates.
- When an agglomerate of particulates covers 1/6 or more of the field area, reject the field and count another
- Size and count only free fibres which are unattached and have both ends visible
- When counting a field, adjust the fine focus control so that any fibres embedded in the filter matrix are not missed
- Count at least one unexposed blank filter, treated in the same manner as the samples except that no air is drawn through it, in order to obtain the background fibre loading for the samples.
- Record the fibre counts for each sample on the appropriate form, Figure A-1-3.
Figure A-1-3: Asbestos Fibre Counting Report Form

A-1.5 Calculations.
A-1.5.1 Standard Sample Volume
A-1.5.1.1 Stack Samples
Equation S-1-1 in Part I is used to determine the standard sample volume at reference conditions expressed in cubic feet. Using Equation A-1-1, convert this value to the standard sample volume at reference conditions expressed in cubic centimeters.
Equation A-1-1

- Vs = sample volume corrected to reference conditions (760 mm Hg, 298°K), cc
- (Vm)ref = sample volume corrected to reference conditions (29.92 in. Hg, 537°R), ft3
- 28.32 × 10-3 = conversion factor, cc/ft3
A-1.5.1.2 Baghouse Samples
Use Equation A-1-2 to determine the sample volume corrected to standard conditions of 760 mm Hg and 298°K.
Equation A-1-2

- Vs = sample volume corrected to reference conditions, cc
- Va = sample volume measured during sampling, cc
- Tref = absolute temperature at reference conditions, 298°K
- Ta = absolute temperature during sampling, °K
- Pref = absolute pressure at reference conditions, 760 mm Hg
- Pa = absolute pressure during sampling, mm Hg
A-1.5.2 Concentration of Asbestos Fibres
Use Equation A-1-3 to determine the concentration of asbestos fibres in the sample.
Equation A-1-3

- C = asbestos fibre concentration fibres, cc
- S = average asbestos fibre count of sample, fibres/field
- B = average asbestos fibre count of blanks, fibres/field
- A = effective filtering area of filter sample, mm2
- f.a. = field area of counting field, mm2
- Vs = sample volume corrected to reference conditions, cc