Appendices of the Screening Assessment
Aromatic Azo and Benzidine-based Substance Grouping
Certain Monoazo Pigments
Environment and Climate Change Canada
Health Canada
May 2016
Table of Contents
- Appendix A: Chemical Identity, Structures, Molecular Formulas and Molecular Weights of the 33 Monoazo Pigments, Organized by Subset
- Appendix B: Experimental Physical and Chemical Properties (at Room Temperature when Applicable) of Monoazo Pigments and their Analogues
- Appendix C: Experimental Data on Biodegradation of Monoazo Pigments and their Analogues
- Appendix D: Experimental Aquatic Toxicity Data for the 33 Monoazo Pigments and their Analogues
- Appendix E: Ecological Exposure Calculations for Monoazo Pigments
- Appendix F: Exposure Estimates from Use of Cosmetics and Personal Care Products
- Appendix G: Exposure Estimates from Use of Paint
- Appendix H: Oral Exposure Estimates to PR5 and PO5 from Incidental Ingestion of Paper (Toddlers)
- Appendix I: Exposure to of Pigment in Permanent Tattoo Ink (Adult) for PR4, PR112 and PY3
- Appendix J: Benchmark Dose Calculations for PR3, PO5, PR4 and PR53:1
- Back to the Screening Assessment
Appendix A: Chemical Identity, Structures, Molecular Formulas and Molecular Weights of the 33 Monoazo Pigments, Organized by Subset
| Subset | Chemical Abstracts Service Registry Number (CAS RN) | DSL name (English) | C.I. name (C.I. number) |
Substance identifier | Chemical structure, molecular formula (molecular weight) |
|---|---|---|---|---|---|
| Monoazo yellow pigments | 2512-29-0 | Butanamide, 2-[(4-methyl-2-nitrophenyl)azo]-3-oxo-N-phenyl- | Pigment Yellow 1 (C.I. 11680) |
PY1 |
C17H16N4O4 |
| Monoazo yellow pigments | 6486-23-3 | Butanamide, 2-[(4-chloro-2-nitrophenyl)azo]-N-(2-chlorophenyl)-3-oxo- | Pigment Yellow 3 (C.I. 11710) |
PY3 |
C16H12Cl2N4O4 |
| Monoazo yellow pigments | 13515-40-7 | Butanamide, 2-[(4-chloro-2-nitrophenyl)azo]-N-(2-methoxyphenyl)-3-oxo- | Pigment Yellow 73 (C.I. 11738) |
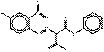
PY73 | 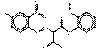 C17H15ClN4O5 (391 g/mol) |
| β-Naphthol pigments | 2425-85-6 | 2-Naphthalenol, 1-[(4-methyl-2-nitrophenyl)azo]- | Pigment Red 3 (C.I. 12120) |
PR3 | C17H13N3O3 (307 g/mol) |
| β-Naphthol pigments | 2814-77-9 | 2-Naphthalenol, 1-[(2-chloro-4-nitrophenyl)azo]- | Pigment Red 4 (C.I. 12085) |
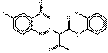
PR4 | 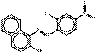 C16H10ClN3O3 (328 g/mol) |
| β-Naphthol pigments | 6410-13-5 | 2-Naphthalenol, 1-[(4-chloro-2-nitrophenyl)azo]- | Pigment Red 6 (C.I. 12090) |
PR6 | C16H10ClN3O3 (328 g/mol) |
| β-Naphthol pigments | 6410-09-9 | 2-Naphthalenol, 1-[(2-nitrophenyl)azo]- | Pigment Orange 2 (C.I. 12060) |
PO2 | C16H11N3O3 (293 g/mol) |
| β-Naphthol pigments | 3468-63-1 | 2-Naphthalenol, 1-[(2,4-dinitrophenyl)azo]- | Pigment Orange 5 (C.I. 12075) |
PO5 | 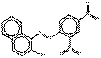 C16H10N4O5 (338 g/mol) |
| β-Naphthol pigments | 49744-28-7 | 2-Naphthalenol, 1-[(4-methoxy-2-nitrophenyl)azo]- | Not available | NONPA | C17H13N3O4 (323 g/mol) |
| β-Naphthol pigment lakes | 1103-38-4 | 1-Naphthalenesulfonic acid, 2-[(2-hydroxy-1-naphthalenyl)azo]-, barium salt (2:1) | Pigment Red 49:1 (C.I. 15630:1) |
PR49:1 | 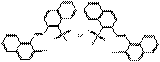 C40H26N4O8S2Ba (892 g/mol) |
| β-Naphthol pigment lakes | 6372-81-2 | Benzoic acid, 2-[(2-hydroxy-1-naphthalenyl)azo]-, barium salt (2:1) | Pigment Red 50:1 (C.I. 15500:1) |
PR50:1 | 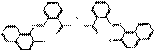 C34H22N4O6Ba (720 g/mol) |
| β-Naphthol pigment lakes | 5160-02-1 | Benzenesulfonic acid, 5-chloro-2-[(2-hydroxy-1-naphthalenyl)azo]-4-methyl-, barium salt (2:1) | Pigment Red 53:1 (C.I. 15585:1) |
PR53:1 | 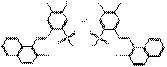 C34H24Cl2N4O8S2Ba (889 g/mol) |
| Naphthol AS pigments | 6410-41-9 | 2-Naphthalenecarboxamide, N-(5-chloro-2,4-dimethoxyphenyl)-4-[[5-[(diethylamino)sulfonyl]-2-methoxyphenyl]azo]-3-hydroxy- | Pigment Red 5 (C.I. 12490) |
PR5 | 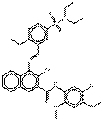 C30H31ClN4O7S (627 g/mol) |
| Naphthol AS pigments | 6535-46-2 | 2-Naphthalenecarboxamide, 3-hydroxy-N-(2-methylphenyl)-4-[(2,4,5-trichlorophenyl)azo]- | Pigment Red 112 (C.I. 12370) |
PR112 | 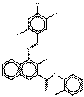 C24H16Cl3N3O2 (485 g/mol) |
| Naphthol AS pigments | 2786-76-7 | 2-Naphthalenecarboxamide, 4-[[4-(aminocarbonyl)phenyl]azo]-N-(2-ethoxyphenyl)-3-hydroxy- | Pigment Red 170 (C.I. 12475) |
PR170 | 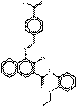 C26H22N4O4 (455 g/mol) |
| Naphthol AS pigments | 59487-23-9 | 2-Naphthalenecarboxamide, 4-[[5-[[[4-(aminocarbonyl)phenyl]amino]carbonyl]-2-methoxyphenyl]azo]-N-(5-chloro-2,4-dimethoxyphenyl)-3-hydroxy- | Pigment Red 187 (C.I. 12486) |
PR187 | 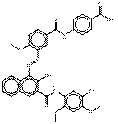 C34H28ClN5O7 (654 g/mol) |
| Naphthol AS pigments | 36968-27-1 | 2-Naphthalenecarboxamide, 4-[[4-(aminocarbonyl)phenyl]azo]-3-hydroxy-N-(2-methoxyphenyl)- | Pigment Red 266 (C.I. 12474) |
PR266 | 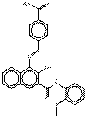 C25H20N4O4 (441 g/mol) |
| Naphthol AS pigments | 16403-84-2 | 2-Naphthalenecarboxamide, 4-[[5-(aminocarbonyl)-2-methylphenyl]azo]-3-hydroxy-N-phenyl- | Pigment Red 268 (C.I. 12316) |
PR268 | 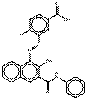 C25H20N4O3 (425 g/mol) |
| Naphthol AS pigments | 12236-64-5 | 2-Naphthalenecarboxamide, N-[4-(acetylamino)phenyl]-4-[[5-(aminocarbonyl)-2-chlorophenyl]azo]-3-hydroxy- | Pigment Orange 38 (C.I. 12367) |
PO38 | 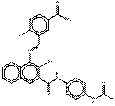 C26H20ClN5O4 (502 g/mol) |
| Naphthol AS pigments | 94199-57-2 | 2-Naphthalenecarboxamide, N-(2-ethoxyphenyl)-3-hydroxy-4-[(2-nitrophenyl)azo]- | Not available | NAPNPA | 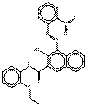 C25H20N4O5 (457 g/mol) |
| Naphthol AS pigments | 85005-63-6 | 2-Naphthalenecarboxamide, 4-[(2,4-dinitrophenyl)azo]-3-hydroxy-N-phenyl- | Not available | NANPAP | 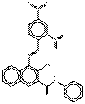 C23H15N5O6 (457 g/mol) |
| Naphthol AS pigments | 13824-00-5 | 2-Naphthalenecarboxamide, 3-hydroxy-N-(4-methoxyphenyl)-4-[(4-methylphenyl)azo]- | Not available | NAPMPA | 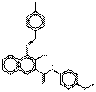 C25H21N3O3 (412 g/mol) |
| Naphthol AS pigments | 17947-32-9 | 2-Naphthalenecarboxamide, 3-hydroxy-N-(4-methoxyphenyl)-4-(phenylazo)- | Not available | NAPPA | 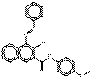 C24H19N3O3 (397 g/mol) |
| BONA pigment lakes | 7023-61-2 | 2-Naphthalenecarboxylic acid, 4-[(5-chloro-4-methyl-2-sulfophenyl)azo]-3-hydroxy-, calcium salt (1:1) | Pigment Red 48:2 (C.I. 15865:2) |
PR48:2 | 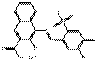 C18H11ClN2O6SCa (459 g/mol) |
| BONA pigment lakes | 71832-83-2 | 2-Naphthalenecarboxylic acid, 4-[(5-chloro-4-methyl-2-sulfophenyl)azo]-3-hydroxy-, magnesium salt (1:1) | Pigment Red 48:5 (C.I. 15865:5) |
PR48:5 | 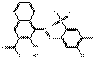 C18H11ClN2O6SMg (443 g/mol) |
| BONA pigment lakes | 17852-99-2 | 2-Naphthalenecarboxylic acid, 4-[(4-chloro-5-methyl-2-sulfophenyl)azo]-3-hydroxy-, calcium salt (1:1) | Pigment Red 52:1 (C.I. 15860:1) |
PR52:1 | 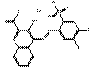 C18H11ClN2O6SCa (459 g/mol) |
| BONA pigment lakes | 6417-83-0 | 2-Naphthalenecarboxylic acid, 3-hydroxy-4-[(1-sulfo-2-naphthalenyl)azo]-, calcium salt (1:1) | Pigment Red 63:1 (C.I. 15880:1) |
PR63:1 | 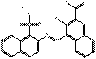 C21H12N2O6SCa (461 g/mol) |
| Benzimidazolone pigments | 12236-62-3 | Butanamide, 2-[(4-chloro-2-nitrophenyl)azo]-N-(2,3-dihydro-2-oxo-1H-benzimidazol-5-yl)-3-oxo- | Pigment Orange 36 (C.I. 11780) |
PO36 | 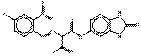 C17H13ClN6O5 (417 g/mol) |
| Naphthol AS pigment lakes | 43035-18-3 | Benzenesulfonic acid, 4-[[3-[[2-hydroxy-3-[[(4-methoxyphenyl)amino]carbonyl]-1-naphthalenyl]azo]-4-methylbenzoyl]amino]-, calcium salt (2:1) | Pigment Red 247:1 (C.I. 15915) |
PR247:1 | 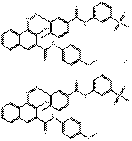 C64H50N8O14S2Ca (1259 g/mol) |
| Pyrazoloquinazolone pigments | 74336-60-0 | 9,10-Anthracenedione, 1-[(5,7-dichloro-1,9-dihydro-2-methyl-9-oxopyrazolo[5,1-b]quinazolin-3-yl)azo]- | Pigment Red 251 (C.I. 12925) |
PR251 | 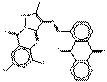 C25H13Cl2N5O3 (502 g/mol) |
| BONA pigment lakes (individual) | 12238-31-2 | Pigment Red 52:2 | Pigment Red 52:2 (C.I. 15860:2) |
PR52:2 | 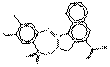 C18H11ClMnN2O6S (474 g/mol) |
| Monoazo yellow pigments (individual) |
6407-74-5 | 3H-Pyrazol-3-one, 4-[(2-chlorophenyl)azo]-2,4-dihydro-5-methyl-2-phenyl- | Pigment Yellow 60 (C.I. 12705) |
PY60 | C16H13ClN4O (313 g/mol) |
| Other pigments (individual) | 83249-60-9 | 1-Naphthalenesulfonic acid, 2-[(2-hydroxy-6-sulfo-1-naphthalenyl)azo]-, calcium salt (1:1) | Not available | NSNAC | 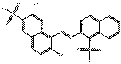 C20H12N2O7S2Ca (497 g/mol) |
Appendix B: Experimental Physical and Chemical Properties (at Room Temperature when Applicable) of Monoazo Pigments and their Analogues
| Subset | Substance | Property (acronym) | Value | Reference |
|---|---|---|---|---|
| Monoazo yellow pigments | PY1 | Decomposition temperature (DT), °C | 249 | Study Submission 2012a |
| Monoazo yellow pigments | PY1 | Water solubility (WS; Sw), µg/L | 0.23 | Study Submission 2012j |
| Monoazo yellow pigments | PY1 | Water solubility (WS; Sw), µg/L | less than 20 | Study Submission 2012c |
| Monoazo yellow pigments | PY1 | Solubility in n-octanol (Soct), µg/L | 9530 | Study Submission 2012k |
| Monoazo yellow pigments | PY1 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 4.62 | Study Submission 2012j, k |
| Monoazo yellow pigments | PY1 | The quotient logarithm of the organic carbon–water partition coefficient (Koc), dimensionless |
5.5 | Study Submission 2012q |
| Monoazo yellow pigments | PY3 | Water solubility (WS; Sw), µg/L | 7.5 | Study Submission 2012m |
| Monoazo yellow pigments | PY3 | Solubility in n-octanol (Soct), µg/L | 5960 | Study Submission 2012m |
| Monoazo yellow pigments | PY3 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 2.90 | Study Submission 2012l |
| Monoazo yellow pigments | PY74* | Melting point (MP), °C | No melting point (endothermic effect at 260–290°C followed by a spontaneous exothermal decomposition at 290–390°C) | ECHA 2012 |
| Monoazo yellow pigments | PY74* | Decomposition temperature (DT), °C | 290 | ECHA 2012 |
| Monoazo yellow pigments | PY74* | Particle size distribution: mass median diameter (D50), µm | 1.96 | ECHA 2012 |
| Monoazo yellow pigments | PY74* | Density, g/cm3 | 1.43 | ECHA 2012 |
| Monoazo yellow pigments | PY74* | Water solubility (WS; Sw), µg/L | 7.6 | ECHA 2012 |
| Monoazo yellow pigments | PY74* | Solubility in n-octanol (Soct), µg/L | 740 | ECHA 2012 |
| Monoazo yellow pigments | PY74* | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 2.0 | ECHA 2012 |
| BONA pigment lakes | PR48:2 | Melting point (MP), °C | No melting point (evaporation and decomposition before melting) | ECHA 2012 |
| BONA pigment lakes | PR48:2 | Decomposition temperature (DT), °C | 350°C | ECHA 2012 |
| BONA pigment lakes | PR48:2 | Particle size distribution: mass median diameter (D50), µm | 2.15; 6.61; 10.44 | ECHA 2012 |
| BONA pigment lakes | PR48:2 | Water solubility (WS; Sw), µg/L | 250–280 | ECHA 2012 |
| BONA pigment lakes | PR48:2 | Solubility in n-octanol (Soct), µg/L | 43–52 | ECHA 2012 |
| BONA pigment lakes | PR48:2 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | −, di | ECHA 2012 |
| BONA pigment lakes | PR48:1* | Melting point (MP), °C | No melting point: evaporation (75–165°C) and decomposition (starts at 370°C) before melting | ECHA 2012 |
| BONA pigment lakes | PR48:1* | Decomposition temperature (DT), °C | 370 | ECHA 2012 |
| BONA pigment lakes | PR48:1* | Particle size distribution: mass median diameter (D50), µm | 17.05 | ECHA 2012 |
| BONA pigment lakes | PR48:1* | Density, g/cm3 | 1.20 | ECHA 2012 |
| BONA pigment lakes | PR48:1* | Water solubility (WS; Sw), µg/L | less than 25 µg/L | ECHA 2012 |
| BONA pigment lakes | PR48:1* | Solubility in n-octanol (Soct), µg/L | 31–33 | ECHA 2012 |
| BONA pigment lakes | PR48:1* | Other solubilities | Soluble in DMF, DMSO, NMP, methanol; not soluble in acetone, 1,4-dioxane, acetonitrile, ethanol | ECHA 2012 |
| BONA pigment lakes | PR48:1* | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | greater than 0.11 | ECHA 2012 |
| BONA pigment lakes | PR48:3* | Melting point (MP), °C | No melting point (evaporation and decomposition before melting) | ECHA 2012 |
| BONA pigment lakes | PR48:3* | Decomposition temperature (DT), °C | 350 | ECHA 2012 |
| BONA pigment lakes | PR48:3* | Particle size distribution: mass median diameter (D50), µm | 10.06 | ECHA 2012 |
| BONA pigment lakes | PR48:3* | Water solubility (WS; Sw), µg/L | 100–120 | ECHA 2012 |
| BONA pigment lakes | PR48:3* | Solubility in n-octanol (Soct), µg/L | 60–70 | ECHA 2012 |
| BONA pigment lakes | PR48:3* | Other solubilities | Soluble in DMF, DMSO, NMP, methanol; not soluble in acetone, 1,4-dioxane, acetonitrile, ethanol | ECHA 2012 |
| BONA pigment lakes | PR48:3* | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | −, di | ECHA 2012 |
| BONA pigment lakes | PR57:1* | Melting point (MP), °C | No melting point (evaporation and decomposition before melting) | ECHA 2012 |
| BONA pigment lakes | PR57:1* | Melting point (MP), °C | 357.5 | CPMA 2006b |
| BONA pigment lakes | PR57:1* | Decomposition temperature (DT), °C | 310 | ECHA 2012 |
| BONA pigment lakes | PR57:1* | Particle size distribution: mass median diameter (D50), µm | 1.69; 4.08 | ECHA 2012 |
| BONA pigment lakes | PR57:1* | Water solubility (WS; Sw), µg/L | 500–580; 1200–1300 | ECHA 2012 |
| BONA pigment lakes | PR57:1* | Water solubility (WS; Sw), µg/L | 8900 | MITI 1992 |
| BONA pigment lakes | PR57:1* | Solubility in n-octanol (Soct), µg/L | 4600–4900; 5100–6000 | ECHA 2012 |
| BONA pigment lakes | PR57:1* | Other solubilities | Soluble in DMF, DMSO, NMP, methanol; not soluble in acetone, 1,4-dioxane, acetonitrile, ethanol | ECHA 2012 |
| BONA pigment lakes | PR57:1* | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 0.65; 0.94 | ECHA 2012 |
| β-Naphthol pigments | PR3 | Melting point (MP), °C | 276 | Øllgaard et al. 1998 |
| β-Naphthol pigments | PR3 | Melting point (MP), °C | 270–272 | Green 1990 |
| β-Naphthol pigments | PR3 | Average particle size, µm | 0.26–0.37 | Clariant 2007 |
| β-Naphthol pigments | PR3 | Average particle size, µm | 0.26–0.53 | NPIRI 2000 |
| β-Naphthol pigments | PR3 | Density, g/cm3 | 1.37–1.50 | Stubbs 1973 |
| β-Naphthol pigments | PR3 | Solubility in n-octanol (Soct), µg/L | 17 900 | Study Submission 2007f |
| β-Naphthol pigments | PR3 | Solubility in n-octanol (Soct), µg/L | 17 000 | Anliker and Moser 1987 |
| β-Naphthol pigments | PR3 | Water solubility (WS; Sw), µg/L | 3.3 | Study Submission 2007g |
| β-Naphthol pigments | PR3 | Water solubility (WS; Sw), µg/L | 800 | Stubbs 1973; Green 1990 |
| β-Naphthol pigments | PR3 | Other solubilities | Soluble in ethanol (0.7 g/L), ethylene glycol methyl ether (0.9 g/L), acetone, benzene; very soluble in mineral spirits, aromatic hydrocarbons, plasticizers | Stubbs 1973; Green 1990 |
| β-Naphthol pigments | PR3 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 3.73 | Study Submission 2007f, g |
| β-Naphthol pigments | PR4 | Melting point (MP), °C | 276 | NPIRI 2000 |
| β-Naphthol pigments | PR4 | Average particle size, µm | 0.27 | Clariant 2007 |
| β-Naphthol pigments | PR4 | Average particle size, µm | 0.24 | NPIRI 2000 |
| β-Naphthol pigments | PR4 | Water solubility (WS; Sw), µg/L | 3.3 | Study Submission 2007h |
| β-Naphthol pigments | PR4 | Solubility in n-octanol (Soct), µg/L | 9400 | Study Submission 2007i |
| β-Naphthol pigments | PR4 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 3.45 | Study Submission 2007h, i |
| β-Naphthol pigments | PO2 | Melting point (MP), °C | 212 | NPIRI 2000 |
| β-Naphthol pigments | PO5 | Melting point (MP), °C | 302 | NPIRI 2000 |
| β-Naphthol pigments | PO5 | Average particle size, µm | 0.29 | Clariant 2007 |
| β-Naphthol pigments | PO5 | Average particle size, µm | 0.32–0.37 | NPIRI 2000 |
| β-Naphthol pigments | PO5 | Water solubility (WS; Sw), µg/L | 6.8 | Study Submission 2007j |
| β-Naphthol pigments | PO5 | Solubility in n-octanol (Soct), µg/L | 1760 | Study Submission 2007j |
| β-Naphthol pigments | PO5 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 2.41 | Study Submission 2007j |
| Naphthol AS pigments | PR5 | Melting point (MP), °C | 306 | NPIRI 2000 |
| Naphthol AS pigments | PR5 | Average particle size, µm | 0.1 | NPIRI 2000 |
| Naphthol AS pigments | PR5 | Density, g/cm3 | 1.40–1.44 | NPIRI 2000 |
| Naphthol AS pigments | PR5 | Water solubility (WS; Sw), µg/L | 7.8 | Study Submission 2007k |
| Naphthol AS pigments | PR5 | Solubility in n-octanol (Soct), µg/L | 133 | Study Submission 2007l |
| Naphthol AS pigments | PR5 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 1.23 | Study Submission 2007k, l |
| Naphthol AS pigments | PR112 | Melting point (MP), °C | No melting point (endothermic effect at 260–270°C followed by a spontaneous exothermal decomposition at 270–290°C) | ECHA 2012 |
| Naphthol AS pigments | PR112 | Decomposition temperature (DT), °C | 270 | ECHA 2012 |
| Naphthol AS pigments | PR112 | Particle size distribution: mass median diameter (D50), µm | 4.56 | ECHA 2012 |
| Naphthol AS pigments | PR112 | Density, g/cm3 | 1.48 | ECHA 2012 |
| Naphthol AS pigments | PR112 | Water solubility (WS; Sw), µg/L | 9.8 | ECHA 2012 |
| Naphthol AS pigments | PR112 | Solubility in n-octanol (Soct), µg/L | 3310 | ECHA 2012 |
| Naphthol AS pigments | PR112 | Solubility in n-octanol (Soct), µg/L | 7800 | Anliker and Moser 1987 |
| Naphthol AS pigments | PR112 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 2.53 | ECHA 2012 |
| Naphthol AS pigments | PR266 | Water solubility (WS; Sw), µg/L | 3.0 | Study Submission 2012n |
| Naphthol AS pigments | PR266 | Solubility in n-octanol (Soct), µg/L | 160 | Study Submission 2012m |
| Naphthol AS pigments | PR266 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 1.73 | Study Submission 2012m |
| Naphthol AS pigments | PR187 | Average particle size, µm | 0.11 | Clariant 2007 |
| Naphthol AS pigments | PR187 | Water solubility (WS; Sw), µg/L | 8.9 | Study Submission 2012m |
| Naphthol AS pigments | PR187 | Solubility in n-octanol (Soct), µg/L | 22.1 | Study Submission 2012m |
| Naphthol AS pigments | PR187 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 0.4 | Study Submission 2012m |
| Naphthol AS pigments | PO38 | Average particle size, µm | 0.17 | Clariant 2007 |
| Naphthol AS pigments | PO38 | Density, g/cm3 | 1.46 | Clariant 2007 |
| Naphthol AS pigments | PO38 | Water solubility (WS; Sw), µg/L | 24.9 | Study Submission 2007m |
| Naphthol AS pigments | PO38 | Solubility in n-octanol (Soct), µg/L | 155 | Study Submission 2007m |
| Naphthol AS pigments | PO38 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 0.79 | Study Submission 2007m |
| Naphthol AS pigments | PR2* | Water solubility (WS; Sw), µg/L | 5.4 | Study Submission 2007p |
| Naphthol AS pigments | PR2* | Solubility in n-octanol (Soct), µg/L | 8630 | Study Submission 2007p |
| Naphthol AS pigments | PR2* | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 3.2 | Study Submission 2007p) |
| Naphthol AS pigments | PR146* | Average particle size, µm | 0.11 | NPIRI 2000 |
| Naphthol AS pigments | PR146* | Water solubility (WS; Sw), µg/L | 8.7 | Study Submission 2007q |
| Naphthol AS pigments | PR146* | Solubility in n-octanol (Soct), µg/L | 100 | Study Submission 2007q |
| Naphthol AS pigments | PR146* | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 1.1 | Study Submission 2007q |
| Naphthol AS pigments | PR253* | Water solubility (WS; Sw), µg/L | 8 | Study Submission 2007m |
| Naphthol AS pigments | PR253* | Solubility in n-octanol (Soct), µg/L | 202 | Study Submission 2007m |
| Naphthol AS pigments | PR253* | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 1.40 | Study Submission 2007m |
| β-Naphthol pigment lakes | PR53:1 | Melting point (MP), °C | greater than 330 | European Commission ©2000a |
| β-Naphthol pigment lakes | PR53:1 | Melting point (MP), °C | 330 | CPMA 2006a |
| β-Naphthol pigment lakes | PR53:1 | Melting point (MP), °C | Melting under decomposition at 330°C | OECD 1999a,b |
| β-Naphthol pigment lakes | PR53:1 | Decomposition temperature (DT), °C | 343–345 | NTP 1982 |
| β-Naphthol pigment lakes | PR53:1 | Density, g/cm3 | 1.5 | European Commission ©2000a |
| β-Naphthol pigment lakes | PR53:1 | Water solubility (WS; Sw), µg/L | 1300; 3400 | European Commission ©2000a |
| β-Naphthol pigment lakes | PR53:1 | Water solubility (WS; Sw), µg/L | 2000 | OECD 1999a,b |
| β-Naphthol pigment lakes | PR53:1 | Water solubility (WS; Sw), µg/L | 2200 | European Commission ©2000a |
| β-Naphthol pigment lakes | PR53:1 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | −, di | OECD 1999a,b |
| Benzimidazolone pigments (individual) | PO36 | Water solubility (WS; Sw), µg/L | 14 | Study Submission 2012p |
| Benzimidazolone pigments (individual) | PO36 | Water solubility (WS; Sw), µg/L | less than 20.6 | Study Submission 2012o |
| Benzimidazolone pigments (individual) | PO36 | Solubility in n-octanol (Soct), µg/L | 86.1 | Study Submission 2012n |
| Benzimidazolone pigments (individual) | PO36 | Solubility in n-octanol (Soct), µg/L | greater than 137 | Study Submission 2012o |
| Benzimidazolone pigments (individual) | PO36 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 0.8 | Study Submission 2012n, o |
| Naphthol AS pigment lakes (individual) | PR247:1 | Average particle size, µm | 0.18 | Clariant 2007 |
| Naphthol AS pigment lakes (individual) | PR247:1 | Water solubility (WS; Sw), µg/L | 112 | Study Submission 2007m |
| Naphthol AS pigment lakes (individual) | PR247:1 | Solubility in n-octanol (Soct), µg/L | 178 | Study Submission 2007m |
| Naphthol AS pigment lakes (individual) | PR247:1 | The quotient logarithm of the molar solute concentrations in octanol and water [calculated as log (Soct/Sw), dimensionless] | 0.2 | Study Submission 2007m |
Appendix C: Experimental Data on Biodegradation of Monoazo Pigments and their Analogues
| Subset (substance) | Biodegradation value (%) | Test duration (days) | Details | Reference |
|---|---|---|---|---|
| Monoazo yellow pigments (PY1) | 14 | 28 | Inherent biodegradability; purity = 99.7% | Study Submission 2012a |
| BONA pigment lakes (PR48:2) | 0 | 28 | Ready biodegradability | ECHA 2012 |
| BONA pigment lakes (PR57:1*) | 0 | 28 | Ready biodegradability; HPLC analysis | MITI 1992 |
| BONA pigment lakes (PR57:1*) | 9; 12.9 | 28 | Ready biodegradability; BOD analysis | MITI 1992 |
| β-Naphthol pigment lakes (PR53:1) | 0 | 14 | Ready biodegradability | MITI 1992 |
| β-Naphthol pigment lakes (PR53:1) | 33 | 21 | Inherent biodegradability; 33% eliminated after 21 days; in Zahn Wellens test, 10% of elimination due to adsorption onto the sludge | European Commission ©2000 |
Appendix D: Experimental Aquatic Toxicity Data for the 33 Monoazo Pigments and their Analogues
| Substance (subset) | Test type (duration) | Organism | Endpoint, value | Details | Reference |
|---|---|---|---|---|---|
| PY1 (monoazo yellow pigments) | Acute (96 h) |
Zebrafish (Brachydanio rerio) | LC0 = 1 mg/L; LC50 greater than 1 mg/L | Semi-static. OECD TG 203. Saturated solution (1 mg/L; purity 99.7%). Undissolved particles removed by membrane filtration (0.45 µm). Measured concentrations less than LOQ. | Study Submission 2012b |
| PY1 (monoazo yellow pigments) | Chronic (21 days) |
Water flea (Daphnia magna) | NOEC = 1 mg/L | Semi-static. OECD TG 211 (reproduction). Saturated solution (1 mg/L; purity 99.7%). Undissolved particles removed by membrane filtration (0.45 µm). Effects: reproduction rate; appearance of first brood; number of broods; stillborn juveniles and aborted eggs; adult mortality; body weight and length. No biological and/or statistical significances between the control and 1 mg/L. Measured concentrations less than LOQ. | Study Submission 2012c |
| PY1 (monoazo yellow pigments) | Chronic (72 h) |
Alga Desmodesmus subspicatus | NOEC = 1 mg/L | Static. OECD TG 201. Saturated solution (1 mg/L; purity 99.7%). Undissolved particles removed by membrane filtration (0.45 µm). Effects: growth rate and yield. No biological and/or statistical significance between control and 1 mg/L. Measured concentrations less than LOQ. | Study Submission 2012d |
| PY3 (monoazo yellow pigments) | Acute (48 h) |
Water flea (Daphnia magna) | No effects at 100 mg/L | Static. OECD TG 202. WS less than 1 mg/L reported. Saturated solution (100 mg/L; purity 99.8%). Undissolved particles removed by membrane filtration (0.45 µm). No biological and/or statistical significance in immobilization between control and 100 mg/L. | Study Submission 2012e |
| PO36 (benzimidazolone pigments) | Acute (96 h) |
Zebrafish (Brachydanio rerio) | LC0 = 1 mg/L; LC50 greater than 1 mg/L | Semi-static. OECD TG 203. Saturated solution (1 mg/L; purity 99.5%). Undissolved particles removed by membrane filtration (0.45 µm). Measured concentrations less than LOQ. | Study Submission 2012f |
| PO36 (benzimidazolone pigments) | Chronic (21 days) |
Water flea (Daphnia magna) | NOEC = 1 mg/L | Semi-static. OECD TG 211. Saturated solution (1 mg/L; purity 99.5%). Undissolved particles removed by membrane filtration (0.45 µm). Effects: reproduction rate; appearance of first brood; number of broods; mortality; body weight/length; other. No biological and/or statistical significance between the control and 1 mg/L. Measured concentrations less than LOQ. | Study Submission 2012g |
| PR53:1 (β-naphthol pigment lakes) | Acute (96 h) |
Zebrafish (Brachydanio rerio) | LC50 greater than 500 mg/L | Static. Groups of 10 fish were exposed to five nominal concentrations (I 7.1–180 mg/L), DMSO control (0.5 mg/L) and laboratory water control. | CPMA 2006a |
| PR53:1 (β-naphthol pigment lakes) | Acute (48 h) |
Medaka (Oryzias latipes) | LC50 greater than 500 mg/L | Semi-static system. | CPMA 2006a |
| PR53:1 (β-naphthol pigment lakes) | Acute (48 h) |
Medaka (Oryzias latipes) | LC50 greater than 420 mg/L | Semi-static system. | MITI 1992 |
| PR53:1 (β-naphthol pigment lakes) | Acute (48 h) |
Water flea (Daphnia magna) | EC0 greater than 2.2 mg/L | Saturated solution of the test substance (purity 98.1%). | European Commission ©2000a |
| PR53:1 (β-naphthol pigment lakes) | Acute (48 h) |
Water flea (Daphnia magna) | EC0 greater than 3.8 mg/L | Saturated solution of the test substance (purity 98.1%). | European Commission ©2000a |
| PR3 (β-naphthol pigments) | Acute (48 h) |
Water flea (Daphnia magna) | EC0 = 0.9 mg/L | Static. 20 daphnids; saturated solution (shaking stock solution for 24 h and removing undissolved particles by centrifugation) and a control. DOC was 0.6 mg/L at the start and end of the test, which was estimated to be 0.9 mg/L of the pigment concentration. No biologically significant effects (immobilization) at saturation. | Study Submission 2007n |
| PO5 (β-naphthol pigments) | Acute (48 h) |
Water flea (Daphnia magna) | EC0 = 1.6 mg/L | Static. Saturation achieved by shaking the stock solution for 24 h and removing undissolved particles by 0.45 μm membrane filtration. The DOC was measured and was found to correspond to a concentration of 1.6 mg/L of pigment. No biologically significant effects (immobilization) at saturation. | Study Submission 2007o |
| PR112 (naphthol AS pigments) | Chronic (21 days) |
Water flea (Daphnia magna) | NOEC = 1 mg/L | Semi-static. OECD TG 211. Saturated solution: 1 mg/L (shaking at 20 rpm for 48 h; undissolved particles removed by membrane filtration, 0.45 µm). Loading: saturated solution as limit concentration. Effects: adult mortality (daily); number of juveniles (daily); stillborn juveniles and aborted eggs (daily); intrinsic rate of natural increase (test end); growth – total length and dry weight (test end). No biologically or statistically significant effects at 1 mg/L. | ECHA 2012 |
| PR112 (naphthol AS pigments) | Chronic (72 h) |
Alga Desmodesmus subspicatus | NOEC = 1 mg/L (rate-related inhibition; inhibition of yield) | Static. OECD TG 201. Saturated solution (dispersion of 1 mg/L; shaken at 20 rpm for 48 h; membrane filtration, 0.45 µm; no measured concentrations). Differential loading: saturated solution as limit concentration. Effects: rate-related inhibition; inhibition of yield. No inhibition after 72 h at the saturated solution (1 mg/L). | ECHA 2012 |
| PR2* (naphthol AS pigments) | Acute (48 h); static | Water flea (Daphnia magna) | EC50 greater than 100 mg/L | Daphnids exposed to a saturated solution of 100 mg/L (shaking at 20 rpm for 24 h; undissolved particles removed by filtration on 0.45 μm membrane). No measured concentrations. No biologically significant effects (immobilization) observed at saturation. | Study Submission 2007a |
| PR253* (naphthol AS pigments) | Acute (96 h) |
Common carp (Cyprinus carpio) | LC50 = 172 mg/L | Static. Six test concentrations (not measured). No toxic responses were recorded up to a concentration of 90 mg/L. | Study Submission 2007a |
| PR253* (naphthol AS pigments) | Acute (24 h) |
Water flea (Daphnia magna) | EC50 = 990.7 mg/L | Static. Seven test concentrations (not measured). 10% immobilization observed up to a concentration of 500 mg/L. | Study Submission 2007a |
| PR146* (naphthol AS pigments) | Acute (48 h) |
Water flea (Daphnia magna) | EC50 greater than 100 mg/L | Static. Daphnids exposed to a saturated solution of 100 mg/L (shaking at 20 rpm for 24 h; undissolved particles removed by filtration on 0.45 μm membrane). No measured concentrations. No biologically significant effects (immobilization) observed at saturation. | Study Submission 2007d |
| PR57:1* (BONA pigment lakes) | Acute (96 h) |
Medaka (Oryzias latipes) | LC50 = 33 mg/L | Semi-static. Groups of 10 fish were exposed to five nominal concentrations (7.1–180 mg/L), DMSO control (0.5 mg/L) and laboratory water control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Acute (72 h) |
Medaka (Oryzias latipes) | LC50 = 44 mg/L | Semi-static. Groups of 10 fish were exposed to five nominal concentrations (7.1–180 mg/L), DMSO control (0.5 mg/L) and laboratory water control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Acute (48 h) |
Medaka (Oryzias latipes) | LC50 = 98 mg/L | Semi-static. Groups of 10 fish were exposed to five nominal concentrations (7.1–180 mg/L), DMSO control (0.5 mg/L) and laboratory water control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Acute (24 h) |
Medaka (Oryzias latipes) | LC50 = 170 mg/L | Semi-static. Groups of 10 fish were exposed to five nominal concentrations (7.1–180 mg/L), DMSO control (0.5 mg/L) and laboratory water control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Acute (48 h) |
Medaka (Oryzias latipes) | LC50 = 50 mg/L | Flow-through system. | MITI 1992 |
| PR57:1* (BONA pigment lakes) | Acute (24 h) |
Water flea (Daphnia magna) | EC50 = 280 mg/L | Static. OECD TG 202. Five nominal concentrations (90–940 mg/L; purity 87%), control of DMSO-to-HCO-40 = 9:1 (100 mg/l) and laboratory water control. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Chronic (72 h) |
Alga Selenastrum capricornutum | NOEC = 5.8 mg/L | Static. Effects: biomass. EC50 calculated from 13 nominal concentrations (1–1000 mg/L) and control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Chronic (72 h) |
Alga Selenastrum capricornutum | LC50 = 190 mg/L | Static. Effects: biomass. EC50 calculated from 13 nominal concentrations (1–1000 mg/L) and control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Acute (24 h) |
Water flea (Daphnia magna) | LC50 = 210 mg/L | Semi-static. Effects: mortality. Daphnids exposed to five nominal concentrations (3–300 mg/L), control of DMSO-to-HCO-40 = 9:1 (100 mg/l) and laboratory water control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Acute (48 h) |
Water flea (Daphnia magna) | LC50 = 43 mg/L | Semi-static. Effects: mortality. Daphnids exposed to five nominal concentrations (3–300 mg/L), control of DMSO-to-HCO-40 = 9:1 (100 mg/l) and laboratory water control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Acute (96 h) |
Water flea (Daphnia magna) | LC50 = 18 mg/L | Semi-static. Effects: mortality. Daphnids exposed to five nominal concentrations (3–300 mg/L), control of DMSO-to-HCO-40 = 9:1 (100 mg/l) and laboratory water control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Acute (7 days) |
Water flea (Daphnia magna) | LC50 = 13 mg/L | Semi-static. Effects: mortality. Daphnids exposed to five nominal concentrations (3–300 mg/L), control of DMSO-to-HCO-40 = 9:1 (100 mg/l) and laboratory water control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Chronic (14 days) |
LC50 = 10 mg/L | Water flea (Daphnia magna) | Semi-static. Effects: mortality. Daphnids exposed to five nominal concentrations (3–300 mg/L), control of DMSO-to-HCO-40 = 9:1 (100 mg/l) and laboratory water control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Chronic (21 days) |
LC50 = 9.7 mg/L | Water flea (Daphnia magna) | Semi-static. Effects: mortality. Daphnids exposed to five nominal concentrations (3–300 mg/L), control of DMSO-to-HCO-40 = 9:1 (100 mg/l) and laboratory water control. Purity of the pigment: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Chronic (14 days) |
Water flea (Daphnia magna) | EC50 = 4.4 mg/L | Semi-static. Effects: reproduction. Daphnids exposed to five nominal concentrations (3–300 mg/L), control of DMSO-to-HCO-40 = 9:1 (100 mg/l) and laboratory water control. Purity: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Chronic (21 days) |
Water flea (Daphnia magna) | EC50 = 9.1 mg/L | Semi-static. Effects: reproduction. Daphnids exposed to five nominal concentrations (3–300 mg/L), control of DMSO-to-HCO-40 = 9:1 (100 mg/l) and laboratory water control. Purity: 87%. | EA Japan 1992 |
| PR57:1* (BONA pigment lakes) | Chronic (21 days) |
Water flea (Daphnia magna) | NOEC = 3 mg/L | Semi-static. Effects: reproduction. Daphnids exposed to five nominal concentrations (3–300 mg/L), control of DMSO-to-HCO-40 = 9:1 (100 mg/l) and laboratory water control. Purity: 87%. | EA Japan 1992 |
Appendix E: Ecological Exposure Calculations for Monoazo Pigments
A5.1 Aquatic Exposure Calculations for Pigment Manufacture
A large pigment manufacturing facility in Canada was selected for estimating the level of aquatic exposure to the Monoazo Pigments. The estimate was derived as follows.
The daily production of the Monoazo Pigments was an essential parameter for the aquatic exposure calculations, but was unknown. This parameter was estimated based on the reported total quantity of organic pigments manufactured in Canada, 3 000 000 kg/year (Linak et al. 2011), and the number of annual production days, 300 days/year, derived for the manufactured quantity of 3 000 000 kg/year according to the European Commission’s Technical Guidance Document on Risk Assessment (European Commission 2003):
Daily production of Monoazo Pigments
= Annual quantity of organic pigments manufactured / Annual production days
= 3 000 000 kg/year / 300 days/year
= 10 000 kg/day
Environment Canada conducted a number of site visits to pigment manufacturing facilities in 2010 (Environment Canada 2010) and learned that the pigment production equipment was cleaned with water on a regular basis. The wastewater generated was subsequently treated for solids removal by an on-site wastewater treatment system prior to discharge to the sewer system. Environment Canada also conducted various site visits to other types of facilities, such as latex paint formulation (Environment Canada 2013a), powder coatings manufacturing (Environment Canada 2013b), cosmetics manufacturing (Environment Canada and Health Canada 2010) and tanktruck cleaning facilities (Environment Canada 2009b). All these site visits, including those for pigment manufacture, showed that the products lost to wastewater from equipment cleaning varied in a wide range, but did not exceed 10% of the total production or the equipment holding volume. This 10% value was used as a conservative estimate for the emission factor to wastewater from the cleaning of the pigment production equipment.
Emission factor to wastewater from equipment cleaning = 10%
The daily release of the Monoazo Pigments to wastewater was then estimated by multiplying the daily production by the emission factor:
Daily release of Monoazo Pigments to wastewater
= Daily production of Monoazo Pigments × Emission factor to wastewater from equipment cleaning
= 10 000 kg/day × 10%
= 1000 kg/day
It was reported that on-site industrial primary wastewater treatment was capable of removing 90% of pigments with water solubility below 1 mg/L (OECD 2009, p. 58). Since all the Monoazo Pigments have water solubility well below 1 mg/L and the on-site wastewater treatment of the facility evaluated was expected to have at least primary or equivalent removal, according to the site visits to pigment manufacturing facilities (Environment Canada 2010), the removal of 90% was used for on-site wastewater treatment.
On-site wastewater treatment removal = 90%
The daily release of the Monoazo Pigments to the sewer system was then estimated:
Daily release of Monoazo Pigments to sewer system
= Daily release of Monoazo Pigments to wastewater × (1 − On-site wastewater treatment removal)
= 1000 kg/day × (1 − 0.9)
= 100 kg/day
The treated wastewater discharged from the facility was further treated by an off-site wastewater treatment system. This system had a flow rate of 334 900 000 L/day. The concentration of the Monoazo Pigments in the influent was estimated by dividing the daily release to the sewer system by the flow:
Concentration of Monoazo Pigments in influent
= Daily release of Monoazo Pigments to sewer system / Flow rate of off-site wastewater treatment system
= 100 kg/day / 334 900 000 L/day
= 298.6 × 10−9 kg/L
= 298.6 μg/L
The removal of Monoazo Pigments by the off-site wastewater treatment system was estimated by models. The system was a secondary type, and its removal was estimated by ASTreat (2006) to be in the range of 0.5–49.2%, based on the log Kowrange of −0.75 to 4.62 for the Monoazo Pigments. In this estimate, the Monoazo Pigments were considered to be non-volatile substances and were assumed not to biodegrade, due to a lack of biodegradation data. The removal estimated was therefore a result of sludge sorption only. The lower-end value was used in order to derive a conservative aquatic PEC.
Off-site wastewater treatment removal = 0.5%
The concentration of the Monoazo Pigments in the effluent was then calculated:
Concentration of Monoazo Pigments in effluent
= Concentration of Monoazo Pigments in influent × (1 − Off-site wastewater treatment removal)
= 298.6 μg/L × (1 − 0.005)
= 297 μg/L
The receiving water for the off-site wastewater treatment system was a large lake, and the dilution factor at the discharge point was assumed to be 10-fold. Thus, the concentration of the Monoazo Pigments in receiving water near the discharge point, or the aquatic PEC, was estimated:
Aquatic PEC
= Concentration of Monoazo Pigments in effluent / Receiving water dilution factor
= 297 μg/L / 10
= 29.7 μg/L
This aquatic PEC for pigment manufacture is presented in Table 14.
A5.2 Aquatic Exposure Calculations for Paint and Coating Formulation
Seventy-five paint and coating formulation facilities were identified as industrial users of the Monoazo Pigments from CEPA 1999 section 71 surveys (Canada 2006, 2007, 2008, 2011), and eight of them were determined to be the largest users. On average, these eight facilities used 5000–10 000 kg/year per pigment for one or more Monoazo Pigments. The eight facilities were therefore selected to determine the level of aquatic exposure for the paint/coating formulation sector. In comparison, the other facilities used quantities below 5000 kg/year per pigment on average. Less than five of these other facilities were initially identified among the largest users based on the above-mentioned CEPA 1999 section 71 surveys, but each of these facilities was found to use less than 5000 kg/year for all Monoazo Pigments combined through follow-up questions (2013 emails from facilities to Environment Canada; unreferenced).
Pigments can be released to the aquatic environment through wastewater treatment when the formulation equipment is cleaned with water. The cleaning is expected for water-based paints and coatings. The exposure calculations presented below were therefore based on data relating to water-based paints and coatings.
The size of a paint/coating production batch was found to range from 1000 to 19 000 kg, according to an analysis of industry data (Environment Canada 2012). Since the amount released from equipment cleaning is proportional to the batch size, the largest batch size (19 000 kg) was used in order to derive a conservative PEC.
Maximum batch size = 19 000 kg
The amount of residue lost to equipment cleaning was also obtained from an analysis of industry data. This amount, commonly expressed as a percentage of a batch size and referred to as an emission factor to wastewater, was 0.3% (Environment Canada 2012) and was used for the paint/coating formulation scenario.
Emission factor to wastewater = 0.3%
The content of pigments in water-based paints and coatings ranged from 15% to 45%, according to a number of site visits to paint/coating formulation facilities (Crechem Technologies 2003), and 2–30% from a more recent site visit (Environment Canada 2013c). The highest content (45%) was selected as the worst-case scenario.
Content of pigments in paints/coatings = 45%
An analysis of industry data showed that the cleaning of coating formulation equipment was completed within 1 day (Environment Canada 2012). The maximum amount of pigments released to on-site raw wastewater was then estimated:
Maximum daily amount released to on-site raw wastewater
= Maximum batch size × Maximum content of pigments in paint/coatings × Emission factor to wastewater
= 19 000 kg × 45% × 0.3% = 25.7 kg/day
Site visits to paint/coating formulation facilities indicated that on-site wastewater treatment for solids removal was common for large facilities (Crechem Technologies 2003). The site visits also indicated that the treated wastewater from the on-site treatment was treated further by off-site wastewater treatment before reaching the aquatic environment. Both on-site and off-site wastewater treatment were therefore assumed in the exposure calculations.
Pigments with water solubility under 1 mg/L are expected to be 90% removed via primary sludge (OECD 2009). Since all the Monoazo Pigments have water solubility well below 1 mg/L and on-site wastewater treatment systems are expected to have at least primary or equivalent removal, the removal efficiency can be expected to be 90% for on-site treatment.
On-site wastewater treatment removal = 90%
The maximum amount released to the sewer system after on-site wastewater treatment was then estimated:
Maximum amount released to sewer system
= Maximum daily amount released to on-site raw wastewater × (1 − On-site wastewater treatment removal)
= 25.7 kg/day × (1 − 0.9)
= 2.57 kg/day
The calculations below depend upon the location of each facility. For a facility at Site C-2, the wastewater flow rate of the off-site wastewater treatment system was 14 700 000 L/day. The concentration of pigments in the influent was then estimated:
Concentration of pigments in influent
= Maximum amount released to sewer system / Flow rate of off-site wastewater treatment system
= 2.57 kg/day / 14 700 000 L/day
= 1.75 × 10−7 kg/L = 175 μg/L
The removal efficiencies of the off-site wastewater treatment systems at the eight paint/coating formulation sites were estimated by models. The treatment systems used at these sites were all secondary, and their removal efficiencies were estimated by ASTreat (2006). The Monoazo Pigments were considered to be non-volatile and were assumed not to biodegrade through wastewater treatment, due to a lack of biodegradation data. The removal efficiencies estimated were therefore a result of sludge sorption only. These estimates were in the range of 0.5–49.2% for secondary systems, based on the log Kow range of −0.75 to 4.62. The lower-end value was used in order to derive conservative aquatic PECs.
Off-site wastewater treatment removal = 0.5%
For Site C-2, the concentration of pigments in the effluent was estimated:
Concentration of pigments in effluent
= Concentration of pigments in influent × (1 − Off-site wastewater treatment removal)
= 175 μg/L × (1 − 0.005) = 174 μg/L
The aquatic PEC was estimated by dividing the effluent concentration by an appropriate dilution factor of the receiving water. Since the aquatic PEC is determined near the discharge point, the receiving water dilution factor selected should be applicable to this requirement. The full dilution potential of a river based on its 10th percentile flow rate is considered appropriate if the dilution factor is between 1 and 10. Otherwise, a 10-fold dilution is assumed for both large rivers and still waters. The receiving water for the wastewater treatment system at Site C-2 is a river with a 10th percentile flow rate of 98 120 000 L/day. Thus, the dilution factor was calculated as:
Receiving water dilution factor = 10th percentile flow rate / Flow rate of off-site wastewater treatment system
= 98 120 000 L/day / 14 700 000 L/day
= 6.7
This dilution factor was used in the calculation for the aquatic PEC at Site C-2:
Aquatic PEC =
Concentration of pigments in effluent / Receiving water dilution factor
= 174 μg/L / 6.7 = 25.9 μg/L
The aquatic PEC results for the eight paint/coating formulation facilities are summarized in Table 14.
A5.3 Aquatic Exposure Calculations for Deinking Operations
Seventeen facilities were identified as performing recycled paper deinking operations from the Pulp and Paper Canada Directory (2013), the Lock-wood Post Directory (2011) and FisherSolve™ Platform (2013). Out of this total, 13 facilities were found to have sufficient information for aquatic exposure calculations. The 13 facilities were judged to be a good representation of the Canadian deinking sector.
The aquatic PEC was estimated for each of the 13 facilities. These facilities generated and treated their respective wastewater on site and subsequently discharged the treated wastewater directly to the receiving water. The aquatic PEC for each facility was estimated based on the quantity of the Monoazo Pigments entering the facility, the emission factor to wastewater, the wastewater volume, the removal efficiency of the on-site wastewater treatment and the dilution of the receiving water.
A detailed explanation of the aquatic PEC calculations for the Monoazo Pigments is provided below using Site D-1 as an example.
In Canada, the quantity of paper recycled was 4 170 000 tonnes, or 69% of the waste paper generated in 2010, according to the Pulp and Paper Products Council (2012 e-mail from Marketing Strategy and Sustainability Consulting, Kitchener, Ontario, to Ecological Assessment Division, Environment Canada; unreferenced). These figures translated into 6 043 000 tonnes of waste paper generated in 2010 in Canada:
Quantity of waste paper generated: = 4 170 000 tonnes / 69% = 6 043 000 tonnes/year
The average content of the Monoazo Pigments in printed paper was estimated by dividing the total quantity of the Monoazo Pigments used for printing by the quantity of waste paper generated. The total quantity of the Monoazo Pigments used for printing was in the range of 10 000–100 000 kg/year, as per CEPA 1999 section 71 surveys (Canada 2006, 2007, 2008, 2011). The higher-end value of this range was used to derive a conservative average content of Monoazo Pigments in waste paper:
Average content of pigments in paper: : (100 000 kg/year) / 6 043 000 tonnes/year = 0.0165 kg/tonne
This average content was used to estimate the amount of the pigments entering a given deinking facility based on its deinking capacity. For example, the deinking capacity of the facility at Site D-1 was 71 375 tonnes/year (FisherSolve™ Platform 2013). The annual input of the pigments into the facility was then estimated:
Annual input of pigments = 0.0165 kg/tonne × 71 375 tonnes/year = 1178 kg/year
The facility at Site D-1 operates 350 days/year on a continuous basis (FisherSolve™ Platform 2013). The daily input of the pigments into the facility at Site D-1 was estimated:
Daily input of pigments: (1178 kg/year) / 350 days/year = 3.37 kg/day
A literature search was conducted to find emission factors to wastewater from deinking operations. Few data were found. An industry association was then contacted, and it provided an estimate of 20% for the proportion of pigments entering wastewater from deinking (2013 telephone discussions between a pulp and paper expert from the industry association and Environment Canada; unreferenced). This estimate was used in further calculations.
Emission factor to wastewater = 20%
The daily quantity of the Monoazo Pigments emitted to raw wastewater from the facility at Site D-1 was estimated based on the 20% emission factor and the daily input of Monoazo Pigments into the facility:
Daily emission of pigments to raw wastewater = 3.37 kg/day × 20% = 0.67 kg/day
The concentration of Monoazo Pigments in raw wastewater was estimated by dividing the daily emission by the daily flow rate of the wastewater generated. For Site at D-1, the daily flow rate of the wastewater generated was 40 000 000 L/day (FisherSolve™ Platform 2013). The concentration of Monoazo Pigments in raw wastewater was then calculated:
Concentration of pigments in raw wastewater = (0.67 kg/day × 109 µg/kg) / 40 000 000 L/day = 16.8 μg/L
where 109 μg/kg is a conversion factor.
Pigments with water solubility below 1 mg/L are expected to be 90% removed via primary sludge (OECD 2009). Since all the Monoazo Pigments have water solubility well below 1 mg/L and the wastewater generated from deinking facilities in Canada is subject to secondary treatment, the reduction in the concentration of the Monoazo Pigments would be at least 90% through wastewater treatment.
For the facility at Site D-1, the maximum concentration of the Monoazo Pigments in treated wastewater was estimated:
Maximum concentration of pigments in treated wastewater
= 16.8 μg/L × (1 − 0.9) = 1.7 μg/L
The receiving water for the facility at Site D-1 is a river with a 10th percentile flow of 950 400 000 L/day. The full dilution capacity of the receiving water was estimated as the ratio of the 10th percentile flow to the daily wastewater flow:
Receiving water full dilution capacity =(950 400 000 L/day) / 40 000 000 L/day = 24
In estimating the concentration of a chemical in receiving water, an appropriate dilution factor should be used to properly characterize the concentration near the discharge point. For the purpose of this risk assessment, 10-fold dilution was chosen to account for limited dilution near the discharge point when the full dilution capacity was over 10. For the facility at Site D-1, the concentration of the Monoazo Pigments in receiving water near the discharge point, or aquatic PEC, was therefore estimated:
Aquatic PEC = 1.7 µg/L / 10 = 0.17 μg/L
The aquatic PEC results for all the deinking sites are summarized in Table 14.
A5.4 Sediment Exposure Calculations
An equilibrium sediment–water partition approach described by the European Chemicals Agency (ECHA 2010) was used to estimate the concentration of the Monoazo Pigments in sediment. This approach assumes that the concentration in bottom sediment is in equilibrium with the concentration in the overlying water. At equilibrium, the PEC in bottom sediment can linearly correlate with the concentration in the aqueous phase of the overlying water as follows:
Sediment PEC = KswCw
where:
- K sw:
- sediment–water partition coefficient (L/kg)
- C w:
- chemical concentration in aqueous phase (mg/L)
The sediment–water partition coefficient (Ksw, L/kg) can be estimated from the organic carbon (OC) fraction of the sediment (Foc, kg OC/kg), the sorptive capacity of the sediment’s OC (Aoc, L/kg OC) and the substance’s octanol–water partition coefficient (Kow, unitless) (Gobas 2010):
Ksw = FocAocKow
The sediment PEC can then be calculated from the equation:
Sediment PEC = FocAocKowCw
The concentration in the aqueous phase (Cw, mg/L) can be estimated from the aquatic PEC (mg/L). There are three distinctive phases in the water column: aqueous phase, particulate suspended sediment and dissolved suspended sediment (Gobas 2007). Accordingly, the total concentration in the water column, or the aquatic PEC (mg/L), can be expressed as a sum of the concentrations in the aqueous phase (Cw, mg/L), particulate suspended sediment (Cps, mg/L) and dissolved suspended sediment (Cds, mg/L):
Aquatic PEC = Cw + Cps + Cds
When the OC phase in particulate or dissolved suspended sediment is the phase of sorption for a substance, the above equation can be converted to an expression for estimating the ratio of the aquatic PEC (mg/L) to the concentration in the aqueous phase (Cw, mg/L) (Gobas 2007):
Aquatic PEC/Cw = 1 + (XpsFpocApoc + XdsFdocAdoc)Kow
where:
- A poc:
- sorptive capacity of particulate OC relative to octanol (L/kg OC)
- A doc:
- sorptive capacity of dissolved OC relative to octanol (L/kg OC)
- F poc:
- OC fraction of particulate suspended sediment (kg OC/kg)
- F doc:
- OC fraction of dissolved suspended sediment (kg OC/kg)
- K ow:
- octanol–water partition coefficient (unitless)
- X ps:
- content of particulate suspended sediment in water column (kg/L)
- X ds:
- content of dissolved suspended sediment in water column (kg/L)
In Canada, the middle level for the content of particulate suspended sediment in the water column (Xps) was 47 mg/L (Environment Canada 2013d). This value was used in the derivation of the sediment PECs at the sites evaluated.
Xps = 47 mg/L = 4.7 × 10−5 kg/L
According to Gobas (2010), the OC fraction of particulate suspended sediment varied from 0.1 to 0.2 kg OC/kg sediment. The lower end of this range was used in order to derive conservative sediment PECs.
Fpoc = 0.1 kg OC/kg
Karickhoff (1981) proposed a value of 0.41 L/kg OC for the sorptive capacity of sediment’s OC based on a set of 17 sediment and soil samples and various hydrophobic non-polar organic compounds. This value was used for the sorptive capacity of particulate OC, Apoc.
Apoc = 0.41 L/kg OC
In Canada, the dissolved OC content in the water column averaged 2.7 mg OC/L (Environment Canada 2013d). This value was used in the derivation of the sediment PECs at the sites evaluated. Note that this OC content equals the product of the content of dissolved suspended sediment (Xds, mg/L) and its OC fraction (Fdoc, kg OC/kg):
XdsFdoc = 2.7 mg OC/L = 2.7 × 10−6 kg OC/L
Gobas (2007) provided an estimate of 0.08 L/kg OC for the sorptive capacity of dissolved OC, Adoc. This estimate was used.
Adoc = 0.08 L/kg OC
The octanol–water partition coefficient (Kow) exhibits a significant influence on the sediment PEC. According to the following two equations, described previously:
Sediment PEC = FocAocKowCw
Aquatic PEC/Cw = 1 + (XpsFpocApoc + XdsFdocAdoc)Kow
the dependence of the sediment PEC on Kow is given as:
Sediment PEC = Aquatic PEC × FocAoc/(1/Kow + XpsFpocApoc + XdsFdocAdoc)
This dependence reveals that the sediment PEC approaches zero for water-soluble substances with a low Kow and approaches a maximum constant concentration for highly hydrophobic substances with a high Kow. In other words, the sediment PEC increases with Kow. The log Kow values for the Monoazo Pigments were in the range of −0.75 to 4.62. The higher-end value of this range was used to derive conservative sediment PECs.
log Kow = 4.62, or Kow = 41 687
The ratio of the aquatic PEC to the concentration in the aqueous phase (Cw) was calculated:
Aquatic PEC/Cw = 1 + (XpsFpocApoc + XdsFdocAdoc)Kow
= 1 + (4.7 × 10−5 kg/L × 0.1 kg OC/kg × 0.41 L/kg OC + 2.7 × 10−6 kg OC/L × 0.08 L/kg OC) × 41 687
= 1 + 2.14 × 10−6 × 41 687
= 1 + 0.089 = 1.089
As an example, the aquatic PEC at Site C-2 for the formulation of paints and coatings was estimated as 25.9 µg/L. The concentration in the aqueous phase (Cw) at this site was then calculated from the ratio of the aquatic PEC to Cw:
Cw = Aquatic PEC/1.089 = 25.9 µg/L/1.089 = 23.8 µg/L
Gobas (2010) suggested a default value of 0.01–0.03 OC/kg for the OC fraction of bottom sediment in rivers. The higher end of this range was selected as a standard for the sediment PECs derived.
Foc = 0.03 kg OC/kg
As for particulate suspended sediment, the sorptive capacity of bottom sediment’s OC was taken as 0.41 L/kg OC, based on the work from Karickhoff (1981).
Aoc = 0.41 L/kg OC
The sediment PEC at Site C-2 was then estimated from the above values:
Sediment PEC = FocAocKowCw
= 0.03 kg OC/kg × 0.41 L/kgOC × 41 687 × 23.8 µg/L
= 512.8 L/kg × 23.8 µg/L
= 12 205 µg/kg
= 12.2 mg/kg
The sediment PECs for all the sites were estimated according to the above method and are summarized in Table 14.
A5.5 Soil Exposure Calculations
The deinking sector was selected to derive a conservative estimate of the concentration of Monoazo Pigments in soil. Among the major release sectors (pigment manufacture, paint/coating formulation and deinking) considered for exposure calculations, the deinking sector represents the highest release to sludge. When this sludge is converted to biosolids and subsequently applied to land, the entire quantity of the Monoazo Pigments removed from deinking can end up in soil, while the other two sectors release only a small percentage to soil through biosolids generated from off-site wastewater treatment systems. The deinking sector was therefore expected to result in the highest concentrations of Monoazo Pigments in soil.
The concentration of Monoazo Pigments in soil was estimated under a conservative scenario. In this scenario, it was assumed that the pigment-containing biosolids generated from the deinking sector were applied onto agricultural land at an average rate of 28 wet tonnes/ha, as reported in Quebec (Hébert and Chaker 2011), over a substantial number of years (10 years). It was also assumed that the pigments were accumulated in soil and did not incur any degradation, volatilization, soil runoff or leaching losses. Detailed calculations are presented below.
The total annual quantity of the Monoazo Pigments used for printing was in the range of 10 000–100 000 kg/year, as per CEPA 1999 section 71 surveys (Canada 2006, 2007, 2008, 2011). The higher-end value of this range was used to derive a conservative soil exposure estimate.
Total annual quantity of Monoazo Pigments = 100 000 kg/year
According to the Pulp and Paper Products Council (2012 e-mail from Marketing Strategy and Sustainability Consulting, Kitchener, Ontario, to Ecological Assessment Division, Environment Canada; unreferenced), in Canada, the paper recycling rate in 2010 was 69%. Based on this rate, the quantity of the Monoazo Pigments in recycled paper was estimated:
Quantity of pigments in recycled paper = 100 000 kg/year × 69% = 69 000 kg/year
As a conservative estimate, it was assumed that the entire quantity of the Monoazo Pigments in recycled paper ended up in sludge from deinking mills.
Quantity of pigments in sludge = 69 000 kg/year
Newsprint mills with deinking operations reported an average sludge production rate of 835 oven-dry tonnes/day, according to a contract study commissioned by Environment Canada (2009a). This daily rate translates to an annual rate of 292 250 tonnes/year, assuming that newsprint mills operate 350 days/year.
Annual sludge production from deinking mills = 292 250 tonnes/year
The concentration of the Monoazo Pigments in the sludge from deinking mills was estimated by dividing the quantity of the pigments in the sludge by the sludge quantity:
Concentration of pigments in sludge = (69 000 kg/year) / 292 250 tonnes/year = 0.236 kg/tonne = 236 mg/kg
The land application rate of pulp and paper mill sludge in Quebec was used. The average rate was reported to be 28 wet tonnes/ha (Hébert and Chaker 2011). Assuming an average of 40% solids (Environment Canada 2009a), this translates to an average land application rate of approximately 11 dry tonnes/ha. Since 1 ha = 10 000 m2, the annual land application rate would be:
Annual land application rate = 11 tonnes/ha per year = 1.1 kg/m2 per year
The European Chemicals Agency (ECHA 2010) suggested using 10 consecutive years as a length of accumulation in evaluating soil concentrations resulting from biosolids application. The quantity of the Monoazo Pigments received per square metre of the amended soil during this 10-year period would be:
Quantity of pigments per square metre of soil
= Annual land application rate × 10 years × Concentration of pigments in sludge
= 1.1 kg/m2 per year × 10 years × 236 mg/kg
= 2596 mg/m2
The European Chemicals Agency (ECHA 2010) also suggested using 20 cm (i.e., 0.2 m) as the ploughing depth in determining a mixing layer. Using a dry-soil density of 1200 kg/m3 (Williams 1999), the mass of the top 20-cm soil layer per square metre was estimated:
Mass of ploughing layer per square metre = 1200 kg/m3 × 1 m2 × 0.2 m = 240 kg/m2
The soil PEC was determined by dividing the quantity of the pigments upon 10-year land application by the mass of ploughing layer soil per square metre:
Soil PEC = (2596 mg/m2) / 240 kg/m2 = 10.8 mg/kg
Appendix F: Exposure Estimates from Use of Cosmetics
| Substance | Product scenario (adult unless otherwise indicated) | Concentration range (w/w %) | Per event exposureFootnote Appendix F Table 1 [a](µg/kg-bw) | Daily exposure[a] (µg/kg-bw per day) |
|---|---|---|---|---|
| PY1 | Body cream, lotion, moisturizer | 1–3 | 37-112 | 41-123 |
| PY1 | Facial makeup | 0.3–1 | 1.4-4.6 | 1.7-5.6 |
| PY1 | Spray perfume/fragrance | less than 0.1 | less than 0.3 | 0–0.48 |
| PY1 | Soap liquid: showering | less than 1 | less than 0.24 | 0–0.24 |
| PY1 | Hair conditioner | less than 0.1 | less than 0.12 | 0–0.12 |
| PY1 | Shaving cream for men’s face | less than 0.1 | less than 0.06 | 0–0.06 |
| PY1 | Soap solid: washing hands | less than 0.1 | less than 0.01 | less than 0.01 |
| PY1 | Bath products: oil | 1–3 | less than 0.06 | N/A |
| PY1 | Nail polish | 3–10 | 1.3-4.3 | N/A |
| PY1 | Bath salts | less than 3 | less than 0.09 | N/A |
| PY3 | Face mask/pack | 0.3–1 | 5.1–16.9 | 1.5-4.9 |
| PY3 | Spray perfume/fragrance | less than 0.1 | less than 0.3 | 0–0.5 |
| PY3 | Soap liquid: showering | less than 1 | less than 0.24 | 0–0.24 |
| PY3 | Hair conditioner | less than 0.1 | less than 0.12 | 0–0.12 |
| PY3 | Shaving cream for men’s face | less than 0.1 | less than 0.06 | 0–0.06 |
| PY3 | Nail polish | less than 0.1 | less than 0.06 | N/A |
| PY3 | Bath salts | less than 0.1 | less than 0.003 | N/A |
| PY73 | Nail polish | less than 0.1 | less than 0.042 | N/A |
| PR4 | Face mask/pack | less than 1 | less than 16.9 | 0–4.9 |
| PR4 | Body cream, lotion, moisturizer | less than 0.1 | less than 3.72 | 0–4.08 |
| PR4 | Facial makeup (blush) | 3–10 | 0.9-3 | 1.08-3.72 |
| PR4 | Antiwrinkle preparation (former use) | 0.3–1 | 3.1-10.1 | 5.5-18.3 |
| PR4 | Eyeliner | 3–10 | 0.06-0.12 | 0–0.06 |
| PR4 | Eye shadow | 1–3 | 0.06–0.2 | 0.12–0.3 |
| PR4 | Soap liquid: showering | less than 1 | less than 0.2 | 0–0.2 |
| PR4 | Hair conditioner | less than 0.1 | less than 0.12 | 0–0.12 |
| PR4 | Bath products: oil | 1–3 | less than 0.06 | N/A |
| PR4 | Nail polish | less than 30 | less than 12.7 | N/A |
| PR4 | Essential oil: massage | less than 0.1 | less than 6.8 | N/A |
| PR4 | Bath salts | 0.1–3 | less than 0.09 | N/A |
| PR4 | Depilatory cream | less than 0.1 | less than 0.5 | N/A |
| PR49:1 | Hair dye – non-spray/wash-in; semi-permanent | 0.3–1 | 0.5–1.5 | N/A |
| PR53:1 | Mascara | less than 0.1 | less than 0.01 | less than 0.01 |
| PR53:1 | Hair dye – non-spray/wash-in; semi-permanent | 0.3–1 | 1.5–4.9 | 0.2–0.7 |
| PR112 | Eyeshadow | 0.1–0.3 | 0.003–0.02 | 0.01–0.03 |
| PR112 | Soap liquid: showering | less than 0.1 | less than 0.02 | less than 0.02 |
| PR112 | Nail polish | less than 0.1 | less than 0.04 | N/A |
| PR63:1 | Nail polish | 10–30 | 0.71–2.12 | N/A |
| Product scenario | Exposure factorsFootnote Appendix F Table 2[a] |
|---|---|
| Body cream, lotion, moisturizer | Exposure frequency: 1.1/day (Loretz et al. 2005) Product amount: 4.4 g/application (Loretz et al. 2005) Overall retention factor: 1 (Cadby et al. 2002; Wormuth et al. 2005; SCCP 2006; NICNAS 2009; SDA 2010a, b) Surface area: 16 925 cm2 |
| Bath products: oil , bath products: salts | Exposure frequency: 0.285/day Product amount: 25 g/application Overall retention factor: 2.08 × 10−7 (dilution factor from RIVM 2006a; assumed retention factor after rinse-off is 0.001, based on professional judgement) |
| Hair spray | Exposure frequency: 1.51/day (Loretz et al. 2006) Product amount: 3.64 g/application (Loretz et al. 2006) Overall retention factor: 0.09 |
| Soap liquid: showering | Exposure frequency: 0.901/day Product amount: 8.7 g/application Overall retention factor: 0.0033 (SDA 2010a) |
| Hair gel | Exposure frequency: 0.586/day Product amount: 1.9 g/application Overall retention factor: 0.1 (SCCP 2006) |
| Hair conditioner | Exposure frequency: 1.1/day (Loretz et al. 2008) Product amount: 13.1 g/application (Loretz et al. 2008) Overall retention factor: 0.01 (Wormuth et al. 2005; SCCP 2006; SDA 2010b) |
| Spray perfume | Exposure frequency: 1.7/day (Loretz et al. 2006) Product amount: 0.33 g/application (Loretz et al. 2006) Overall retention factor: 1 (Wormuth et al. 2005; SDA 2010a, b) |
| Soap solid: washing hands | Exposure frequency: 6/day Product amount: 2.4 g/application Overall retention factor: 0.0033 (Cadby et al. 2002; RIVM 2006a; SDA 2010a) |
| Hair dye | Exposure frequency: 0.02/day (personal communication: data tables compiled by Statistics Canada on CHMS Cycle 1 Survey on use of grooming products by Canadians (2007-2009) for Existing Substances Risk Assessment Bureau, March 2012, unreferenced) Product amount: 100 g/application |
| Hair dye spray – temporary (child) | Child body weight (5–11 years): 31 kg (Health Canada 1998) Exposure frequency: 0.016/day (i.e., 6/year) |
| Nail polish | Exposure frequency: 0.43/year Product amount upon skin: 0.05 g/application (adult ) |
| Facial makeup (including blush) | Exposure frequency: 1.24/day (Loretz 2006) Product amount: 0.54 g/application (Loretz 2006) Product amount (blush): 0.54 g/application (Loretz 2006) × 160 cm2 (RIVM 2006a) / 637 cm2 (Health Canada 1998) = 0.14 g/application |
| Shaving cream for men’s face | Exposure frequency: 1/day (European Commission 2003) Product amount: 4 g/application (SDA 2010a) |
| Face mask/pack | Exposure frequency: 2/week Product amount: 20 g/application |
| Mascara | Exposure frequency: 0.67/day (Wu et al. 2010) Product amount: 0.025 g/application |
| Eyeshadow | Exposure frequency: 1.2/day (Loretz et al. 2010) Product amount: 0.009 g/application (Loretz et al. 2010) |
| Eyeliner | Exposure frequency: 0.68/day (Wu et al. 2010) Product amount: 0.005 g/application (CTFA 1983b) |
| Antiwrinkle preparation | Exposure frequency: 1.80/day (Loretz et al. 2005) Product amount: 1.2 g/application (Loretz et al. 2005) Used body cream, lotion, moisturizer scenario |
| Depilatory cream | Exposure frequency: 0.0466/day Product amount: 5.5 g/application Overall retention factor: 0.1 (US EPA 2011) |
| Substance | Product scenario (adult unless otherwise indicated) | Concentration range (w/w %) | Per event exposure (µg/kg-bw) | Daily exposure (µg/kg-bw per day) |
|---|---|---|---|---|
| PY1 | Spray perfume/fragranceFootnote Appendix F Table 3 [a] | less than 0.1 | less than 0.1 | N/A |
| PY3 | Spray perfume/fragrance[a] | less than 0.1 | less than 0.1 | N/A |
| PR4 | Lipstick | less than 3 | less than 4.2 | less than 10.2 |
| PR53:1 | Lipstick/lip balm | less than 0.3 | less than 0.4 | less than 1.0 |
| PR53:1 | Lipstick/lip balm (toddler) | less than 0.3 | less than 1.9 | less than 1.1 |
| PR112 | Lipstick/lip balm | 0.1–0.3 | 0.1–0.4 | 0.3–1.0 |
| PR112 | Lipstick/lip balm (toddler) | 0.1–0.3 | 0.6–1.9 | 0.4–1.1 |
| Substance | Product scenario (adult unless otherwise indicated) | Concentration range (w/w %) | Per event exposure (µg/kg-bw) | Daily exposure (µg/kg-bw per day) |
|---|---|---|---|---|
| PY1 | Spray perfume/fragrance | less than 0.1 | less than 0.2 | less than 0.1 |
| PY3 | Spray perfume/fragrance | less than 0.1 | less than 0.2 | less than 0.1 |
| Product | Routes | Exposure factors |
|---|---|---|
| Lipstick | Oral | Exposure frequency: 2.4/day (Loretz et al. 2005) Product amount: 0.01 g/application (Loretz et al. 2005) Overall retention factor: 1 (SCCP 2006; SDA 2010a, b) Adult body weight: 70.9 kg (Health Canada 1998) Concentration: various, as listed in Table A6-3 (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, [Health Canada], dated 2011-2014; unreferenced) |
| Spray perfume/ fragrance | Inhalation and oral non-respirable route | ConsExpo v4.1: “Exposure, spray model” Exposure frequency: 1.7/day (Loretz et al. 2006) Product amount: 0.33 g/application (Loretz et al. 2006) Overall retention factor: 1 (Wormuth et al. 2005; SDA 2010a, b) Adult body weight: 70.9 kg (Health Canada 1998) Concentration: various, as listed in Tables A6-3 and A6-4 ( 2011 and 2013 emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced) Spray duration: 0.08 min Exposure duration: 5 min Room volume: 10 m3 Room height: 2.5 m Ventilation rate: 2/h Cloud volume: 0.0625 m3 Mean mass generation rate: 0.1 g/s (RIVM 2010) Airborne fraction: 0.2 g/g Weight fraction non-volatile: 0.05 g/g Density non-volatile: 1.5 g/cm3 Initial particle distribution median diameter (CV): 2.7 µm (0.73) (RIVM 2010) Inhalation cut-off diameter: 15 µm |
| Monoazo pigment subset | Substance | Potential tattoo use in Canada | Potential tattoo use in Europe |
|---|---|---|---|
| β-Naphthol pigments | PR3 | Hauri (2010b) | |
| β-Naphthol pigments | PR4 | Health Canada (2011, 2013)Footnote Appendix F Table 6 [a] | Hauri (2010b) Hauri (2011b) NVWA (2008) |
| β-Naphthol pigments | PO5 | Danish EPA (2012) Hauri (2010c) |
|
| β-Naphthol pigment lakes | PR49:1 | Hauri (2010c) | |
| β-Naphthol pigment lakes | PR53:1 | Hauri (2010c) | |
| BONA pigment lakes | PR63:1 | Starbrite (2013) | Danish EPA (2012) |
| Monoazo yellow pigments | PY1 | Danish EPA (2012) De Cuyper and D’hollander (2010) Hauri (2010b) Hauri (2011b) Høgsberg et al. (2010) NVWA (2008) |
|
| Monoazo yellow pigments | PY3 | Health Canada (2011, 2013)[a] SkinCandy (2013) |
Hauri (2011b) Høgsberg et al. (2010) NVWA (2008) |
| Naphthol AS pigments | PR5 | Bäumler et al. (2000) Danish EPA (2012) De Cuyper and D’hollander (2010) Hauri (2010b) Hauri (2011b) NVWA (2008) |
|
| Naphthol AS pigments | PR112 | Health Canada (2011, 2013)[a] | Bäumler et al. (2000) Hauri (2010c) Hauri (2011b) Høgsberg et al. (2010) NVWA (2008) |
| Naphthol AS pigments | PR170 | SkinCandy (2013) | Bäumler et al. (2000) Danish EPA (2012) De Cuyper and D’hollander (2010) Hauri (2010c) Hauri (2011b) Høgsberg et al. (2010) NVWA (2008) |
| Benz-imidazolone pigments | PO36 | Hauri (2010b) |
Appendix G: Exposure Estimates from Use of Monoazo Pigments in Paint Products
| Monoazo pigment subset | Substance | Maximum expected pigment content (%)Footnote Appendix G Table 1 [a] | Oral exposure estimate (mg/kg-bw per event) | Dermal applied exposure estimate (mg/kg-bw per event)Footnote Appendix G Table 1 [b] |
|---|---|---|---|---|
| β-Naphthol pigments | PR4 | 1.75 | 0.452 | 0.12906 |
| β-Naphthol pigments | PO5 | 0.57 | 0.147 | 0.04206 |
| β-Naphthol pigment lakes | PR49:1 | 2 | 0.516 | 0.02458 |
| BONA pigment lakes | PR48:2 | 0.7 | 0.181 | 0.00860 |
| BONA pigment lakes | PR63:1 | 3 | 0.774 | 0.03687 |
| Monoazo yellow pigments | PY1 | 0.8 | 0.206 | 0.05898 |
| Monoazo yellow pigments | PY3 | 1.3 | 0.335 | 0.06959 |
| Monoazo yellow pigments | PY73 | 1 | 0.258 | 0.07374 |
| Naphthol AS pigments | PR5 | 5 | 1.290 | 0.36870 |
| Naphthol AS pigments | PR112 | 1.1 | 0.284 | 0.08112 |
| Naphthol AS pigments | PR170 | 1.3 | 0.335 | 0.09588 |
| Naphthol AS pigments | PO38 | 3 | 0.774 | 0.03687 |
| Substance | Maximum expected pigment content (%)Footnote Appendix G Table 2 [a] | Dermal applied exposure estimate (mg/kg-bw per event)Footnote Appendix G Table 2 [b] |
|---|---|---|
| PY3, PY73, PR112 | 15 | 0.813 |
| PR49:1, PR53:1, PR63:1 | 15 | 0.135 |
| PY1 | 0.3–1.0 | 0.016 – 0.054 |
| PR4 | 0.1–1.0 | 0.005 – 0.054 |
| Product scenario | Exposure factorsFootnote Appendix G Table 3[a] |
|---|---|
| Finger paint | Oral exposure (Used chalk scenario) Oral exposure estimate (per event) Dermal exposure (adapted from LGC 2000): Dermal exposure estimate (per event) |
| Face paint (child) | Dermal exposure Dermal exposure estimate (per event) |
| Monoazo pigment subset | Substance | Clariant 2011Footnote Appendix G Table 4[a] | Hansen 2008 | Hauri 2009b | Mont Marte 2009a, 2009b |
|---|---|---|---|---|---|
| beta-naphthol | PR3 | aquarelle & gouache paint, pencil, chalk |
poster paint | ||
| beta-naphthol | PR4 | oil paint, aquarelle & gouache paint, chalk |
|||
| beta-naphthol | PO5 | aquarelle & gouache paint, acrylic paint, wax crayon, chalk |
|||
| beta-naphthol lake | PR53:1 | pencil, chalk |
play dough | ||
| BONA | PR63:1 | acrylic paint, watercolour paint |
|||
| monoazo yellow | PY1 | oil paint, aquarelle & gouache paint, acrylic paint, pencil, wax crayon, chalk |
play dough | acrylic paint, watercolour paint |
|
| monoazo yellow | PY3 | oil paint, aquarelle & gouache paint, acrylic paint, pencil, wax crayon, chalk |
acrylic paint, watercolour paint |
||
| Naphthol AS | PR5 | oil paint, aquarelle & gouache paint, acrylic paint, pencil, chalk |
play dough | ||
| Naphthol AS | PR112 | oil paint, aquarelle & gouache paint, acrylic paint, pencil, wax crayon, chalk |
play dough | ||
| Naphthol AS | PR170 | oil paint, aquarelle & gouache paint, acrylic paint, pencil |
|||
| other | PO36 | oil paint, aquarelle & gouache paint, pencil, wax crayon |
| Substance | Maximum expected pigment content (%)Footnote Appendix G Table 5 [a] | wall painting with airless sprayerFootnote Appendix G Table 5 [b] (mg/kg-bw per event) |
sanding paintb (mg/kg-bw per event) |
|---|---|---|---|
| β-napthhol pigments PR3, PR4, PO5, NONPA; β-napthhol pigment lakes PR49:1, PR53:1; BONA pigment lakes PR48:2, PR52:1, PR52:2, PR63:1; monoazo yellow pigments PY1, PY3, PY73; napthol AS pigments PR5, PR112, PR170, PR266; other PO36 |
5 | 0.000367 | 0.00055 |
Appendix H: Estimate of Short-Term Exposure to Monoazo Pigment in Permanent Tattoo Ink (Adult)
Exposure factors were derived based on a study that examined the loss of a monoazo tattoo pigment from mouse skin in vivodue to biological dissemination and photochemical decomposition (Engel et al. 2009). While this study was specifically on Pigment Red 22 (PR22), a generic approach is taken as a conservative approach to estimate exposure to any azo pigment in tattoo ink. In Engel et al. (2009), 19 hairless female SKH-1 mice, divided into four groups, were tattooed on their dorsa with PR22. Exposure to normal ambient light for 32 days after 10 days of recovery following the initial injection (total of 42 days) resulted in a 32% reduction of PR22 in skin. The loss percentage was considered predominantly attributable to biological dissemination of the tattoo pigment into the lymphatic system. A separate group of mice exposed to simulated solar radiation instead of normal ambient light resulted in a 60% reduction in the initial skin pigment concentration. The fraction of photodecomposed Pigment Red 22 that resulted in the formation of aromatic amines is unknown for simulated solar radiation. Therefore, this exposure scenario focuses on systemic exposures of the intact pigment only.
Exposure scenario (Danish EPA 2012 unless specified otherwise)
Route of exposure: Injection into the dermis
Average skin concentration: 2.53 mg pigment/cm2 ex vivo human or pig skin (Engel et al. 2008)
Realistic worst-case skin concentration: 9.42 mg pigment/cm2
Skin area covered (average): 430 cm2
Skin area covered (whole back): 1090 cm2
Amount of azo pigment in tattoo potentially available for absorption:
AV (average): 1.09 g
AW (whole back): 10.3 g
BW (adult body weight): 70.9 kg-bw (Health Canada 1998)
FP: Fraction of intact pigment in dermis that is mobilized into the lymphatic system:32% over 42 days (Engel et al. 2009)
Exposure to pigment = [(AV – AW) × FP] ÷ (BW × Length of study)
= [(1.09 – 10.3 g) × 0.32] ÷ [70.9 kg-bw × 42 days]
= 0.12–1.1 mg/kg-bw per day
Therefore, the short-term systemic daily exposure to monoazo pigments in tattooed individuals is assumed to be 0.12 mg/kg-bw per day on average and 1.1 mg/kg-bw per day as a conservative estimate.
Appendix I: Benchmark Dose Calculations for PR3, PR4 and PR53:1
| Substance | Sex/species (strain) | Tumour location | Tumour type | BMD10 (mg/kg-bw per day) | BMDL10 (mg/kg-bw per day) |
Reference |
|---|---|---|---|---|---|---|
| PR3 | FR (F344) | Liver | Adenoma | 1096.05 | 910.83 | NTP 1992 |
| PR4 | FR (Wistar) | Liver | Neoplastic nodules and cholangioma total | 88.42 | 41.38 | Kupradinun et al. 2002 |
| PR53:1 | MR (F344) | Liver | Neoplastic nodules | 70.64 | 44.09 | NTP 1982 |
| Male F344 rats | Incidence of malignant tumours 0 ppm |
Incidence of malignant tumours 6000 ppm |
Incidence of malignant tumours 12 500 ppm |
Incidence of malignant tumours 25 000 ppm |
|---|---|---|---|---|
| Equivalent dose (mg/kg-bw per day) | 0 | 272 | 568 | 1181 |
| Liver eosinophilic foci | 6/50 | 37/50 | 36/50 | 41/50 |
| Liver mixed cell foci | 2/50 | 24/50 | 21/50 | 15/50Footnote Appendix I Table 2A[a] |
| Liver cystic degeneration | 9/50 | 36/50 | 40/50 | 36/50[a] |
| Liver multifocal angiectasis | 3/50 | 20/50 | 21/50 | 29/50 |
| Female F344 rats | Incidence of malignant tumours 0 ppm |
Incidence of malignant tumours 6000 ppm |
Incidence of malignant tumours 12 500 ppm |
Incidence of malignant tumours 25 000 ppm |
|---|---|---|---|---|
| Equivalent dose (mg/kg-bw per day) ( | 0 | 321 | 682 | 1389 |
| Liver adenoma | 0/50 | 0/50 | 1/50 | 10/50 |
| Tumours/effects | Model name | No. of groups | AIC | P-value | SRI | BMR | BMD10 (mg/kg-bw per day) | BMDL10(mg/kg-bw per day) |
|---|---|---|---|---|---|---|---|---|
| Liver eosinophilic foci | LogLogistic | 4 | 209.11 | 0.096 | −.0961 | 0.1 | 22.30 | 15.40 |
| Liver mixed cell foci | LogLogistic | 3 | 163.48 | 0.019 | −.0198 | 0.1 | 60.62 | 41.84 |
| Liver cystic degeneration | LogLogistic | 3 | 160.74 | 0.607 | −.607 | 0.1 | 17.66 | 11.25 |
| Liver multifocal angiectasis | LogLogistic | 4 | 232.8 | 0.242 | −.242g | 0.1 | 88.61 | 63.12 |
| Tumours/effects | Model name | No. of groups | AIC | P-value | SRI | BMR | BMD10 (mg/kg-bw per day) | BMDL10(mg/kg-bw per day) |
|---|---|---|---|---|---|---|---|---|
| Liver adenoma | Multistage-cancer | 4 | 62.18 | 0.978 | 0.125 | 0.1 | 1096.05 | 910.828 |
| Incidence of tumours | 0 ppm | 1000 ppm | 2000 ppm |
|---|---|---|---|
| Equivalent dose for female rats (mg/kg-bw per day) (Health Canada conversion) | 0 | 50 | 100 |
| Liver nodules and cholangioma total | 3/50 | 6/47 | 8/50 |
| Tumours | Model name | No. of groups | AIC | P-value | SRI | BMR | BMD10 (mg/kg-bw per day) | BMDL10 (mg/kg-bw per day) |
|---|---|---|---|---|---|---|---|---|
| Liver nodules and cholangioma total | LogLogistic | 3 | 106.63 | 0.796 | −.7963 | 0.1 | 88.42 | 41.38 |
| Incidence of tumours | 0 ppm | 1000 ppm | 3000 ppm |
|---|---|---|---|
| Equivalent dose for male rats (mg/kg-bw per day) (Health Canada conversion) | 0 | 50 | 150 |
| Liver neoplastic nodules | 0/50 | 6/50 | 7/49 |
| Tumours | Model name | No. of groups | AIC | P-value | SRI | BMR | BMD10 (mg/kg-bw per day) | BMDL10 (mg/kg-bw per day) |
|---|---|---|---|---|---|---|---|---|
| Liver adenomas | LogLogistic | 3 | 81.0622 | 0.3054 | 1.281 | 0.1 | 70.6374 | 44.0888 |
Appendix J. Monoazo Pigments With Effects of Concern
Some of the Monoazo Pigments in this assessment have effects of concern based on potential carcinogenicity. The details for supporting the potential carcinogenicity for these substances are outlined in section 7.2 Health Effects Assessment (see specific sub-sections), and generally based on one or more of the following lines of evidence:
- Classifications by national or international agencies for carcinogenicity (may be a group classification).
- Evidence of carcinogenicity in animal studies and/or human epidemiology based on the specific substance.
- Potential to release one or more of the EU22 aromatic amines by azo bond cleavage.
- Read-across to related substances for which one or more of the above lines of evidence apply.
| Substance Name/ acronym and CAS RN | Classification for carcinogenicityFootnote Appendix J Table 1 [a] | Evidence of carcinogenicity from animal studies and/or human epidemiology | Release of EU22 aromatic amine by azo bond cleavage | Read-across |
|---|---|---|---|---|
| PO5 3468-63-1 |
x (Hart et al. 1986, US FDA 1986) |
|||
| PR4 2814-77-9 |
read-across to PR3Footnote Appendix J Table 1 [b] and PO5 (β-Naphthol pigments, see Section 7.2.1) |
|||
| PO2 6410-09-9 |
read-across to PR3[b] and PO5 (β-Naphthol pigments, see Section 7.2.1) |
|||
| PR6 6410-13-5 |
read-across to PR3[b] and PO5 (β-Naphthol pigments, see Section 7.2.1) |
|||
| NONPA 49744-28-7 |
read-across to PR3[b] and PO5 (β-Naphthol pigments, see Section 7.2.1) |
|||
| PR53:1 5160-02-1 |
x (NTP 1982) |