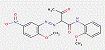Screening Assessment
Aromatic Azo and Benzidine-based Substance Grouping
Certain Monoazo Pigments
Environment and Climate Change Canada
Health Canada
May 2016
Table of Contents
- Synopsis
- 1. Introduction
- 2. Identity of Substances.
- 2.1 Monoazo Yellow Pigments (3 Substances: PY1, PY3, PY73)
- 2.2 β-Naphthol Pigments (6 Substances: PR3, PR4, PR6, PO2, PO5, NONPA)
- 2.3 β-Naphthol Pigment Lakes (3 Substances: PR49:1, PR50:1, PR53:1)
- 2.4 BONA Pigment Lakes (4 Substances: PR48:2, PR48:5, PR52:1, PR63:1)
- 2.5 Naphthol AS Pigments (or “Naphthol Reds”) (11 Substances: PR5, PR112, PR170, PR187, PR266, PR268, PO38, NAPNPA, NANPAP, NAPMPA, NAPPA), Lakes (PR247:1)
- 2.6 Naphthol AS Pigment Lakes (PR247:1)
- 2.7 Benzimidazolone Pigments (PO36)
- 2.8 Pyrazoloquinazolone Pigments (PR251)
- 2.9 Selection of Analogues and Use of (Q)SAR Models
- 3. Physical and Chemical Properties
- 4. Sources and Uses
- 5. Environmental Fate and Behavior
- 6. Potential to Cause Ecological Harm
- 7. Potential to Cause Harm to Human Health
- 8. Conclusion
- References
- Appendices
List of Tables
- Table 2-1: Identity of the 33 monoazo pigments
- Table 2-2: Structural analogues for the given subsets used in read-across of physical-chemical properties and endpoints in the ecological and/or human health effects assessment
- Table 3-1: Summary of experimental data on physical-chemical properties (at room temperature) of Monoazo Pigments and their analogues
- Table 4-1: 21 Monoazo Pigments that have been identified with an annual import or manufacturing quantity above the 100 kg/year reporting threshold in Canada in a section 71 survey since 2005
- Table 4-2: Summary of use information for Monoazo Pigments used in food packaging in Canada
- Table 4-3: List of pest control products containing Monoazo Pigments as formulants
- Table 5-1: Summary of biodegradation data on Monoazo Pigments and their analogues
- Table 5-2: Calculated molecular diameters of some of the 33 Monoazo Pigments
- Table 5-3: Experimental bioconcentration factor (BCF) data for Monoazo Pigments and analogues in studies with common carp (Cyprinus carpio)
- Table 6-1: Summary of empirical data on aquatic toxicity of Monoazo Pigments and their analogues
- Table 6-2: Empirical data for soil toxicity of some Monoazo Pigments
- Table 6-3: Aquatic critical toxicity values (CTVs) and Predicted no-effect concentrations (PNEC) values for the subgroup of 33 Monoazo Pigments
- Table 6-4: Releases of Monoazo Pigments to wastewater from various sectors in Canada
- Table 7-1: Overview of the exposure potential and health effects data availability for the substances considered in assessment of subsets of the Monoazo Pigments
- Table 7-2: Summary of estimates of oral and dermal exposure to β-naphthol pigments via use of finger paint, face paint, face mask and lipstick
- Table 7-3: Summary of oral and dermal estimates of exposure to β-naphthol pigment lakes via use of finger paint and face paint
- Table 7-4: Summary of oral and dermal estimates of exposure to BONA pigment lakes via use of finger paint and face paint
- Table 7-5: Summary of oral and dermal estimates of exposure to monoazo yellow pigments via use of face paint and finger paint
- Table 7-6: Summary of oral and dermal estimates of exposure to naphthol AS pigments via use of face paint and finger paint
- Table 7-7: β-Naphthol pigments and their potential azo bond cleavage products
- Table 7-8: Summary of critical effect levels for the β-naphthol pigments
- Table 7-9: β-Naphthol pigment lakes and potential azo bond cleavage products
- Table 7-10: Summary of critical effect levels for the β-naphthol pigment lakes
- Table 7-11: BONA pigment lakes and potential azo bond cleavage products
- Table 7-12: Summary of critical effect levels for the BONA pigment lakesab
- Table 7-13: Monoazo yellow pigments and analogues and potential azo bond cleavage products
- Table 7-14: Summary of available critical effect levels for the monoazo yellow pigments subset
- Table 7-15: Summary of critical effect levels for the naphthol AS pigments
- Table 7-16: Margins of exposure for the β-naphthol pigment PR4 in finger paint, lipstick, and face mask
- Table 7-17: Margins of exposure for the β-naphthol pigment PO5 in finger paint (child)
- Table 7-18: Margins of exposure for the β-naphthol pigment lakes in finger paint, face paint, and lipstick/lip balm
- Table 7-19: Margins of exposure for the BONA pigment lakes in finger paint and face paint
- Table 7-20: Margins of exposure for the monoazo yellow pigments in finger paint and face paint
- Table 7-21: Margins of exposure for the naphthol AS pigments in finger paint and face paint
Synopsis
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a Screening Assessment on 33 Monoazo Pigments. These substances constitute a subgroup of the Aromatic Azo and Benzidine-based Substance Grouping being assessed as part of the Substance Groupings Initiative of the Government of Canada’s Chemicals Management Plan (CMP) based on structural similarity and applications. Substances in this Grouping were identified as priorities for assessment as they met the categorization criteria under subsection 73(1) of CEPA 1999 and/or were considered as a priority based on other human health concerns.
The Chemical Abstracts Service Registry Number (CAS RN)Footnote[1] , Domestic Substances List (DSL) name, Colour Index (C.I) name or acronym of the 33 substances are presented in the following table.
| CAS RN | DSL name | Colour Index name (Colour Index number) | Chemical acronym |
|---|---|---|---|
| 1103-38-4 | 1-Naphthalenesulfonic acid, 2-[(2-hydroxy-1-naphthalenyl)azo]-, barium salt (2:1) | Pigment Red 49:1 (C.I. 15630:1) |
PR49:1 |
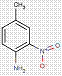
| 2425-85-6Footnote Table 0[a] | 2-Naphthalenol, 1-[(4-methyl-2-nitrophenyl)azo]- | Pigment Red 3 (C.I. 12120) |
PR3 |
| 2512-29-0Footnote Table 0[b] | Butanamide, 2-[(4-methyl-2-nitrophenyl)azo]-3-oxo-N-phenyl- | Pigment Yellow 1 (C.I. 11680) |
PY1 |
| 2786-76-7 | 2-Naphthalenecarboxamide, 4-[[4-(aminocarbonyl)phenyl]azo]-N-(2-ethoxyphenyl)-3-hydroxy- | Pigment Red 170 (C.I. 12475) |
PR170 |
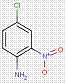
| 2814-77-9[a] | 2-Naphthalenol, 1-[(2-chloro-4-nitrophenyl)azo]- | Pigment Red 4 (C.I. 12085) |
PR4 |
| 3468-63-1[a] | 2-Naphthalenol, 1-[(2,4-dinitrophenyl)azo]- | Pigment Orange 5 (C.I. 12075) |
PO5 |
| 5160-02-1 | Benzenesulfonic acid, 5-chloro-2-[(2-hydroxy-1-naphthalenyl)azo]-4-methyl-, barium salt (2:1) | Pigment Red 53:1 (C.I. 15585:1) |
PR53:1 |
| 6372-81-2 | Benzoic acid, 2-[(2-hydroxy-1-naphthalenyl)azo]-, barium salt (2:1) | Pigment Red 50:1 (C.I. 15500:1) |
PR50:1 |
| 6407-74-5[a] | 3H-Pyrazol-3-one, 4-[(2-chlorophenyl)azo]-2,4-dihydro-5-methyl-2-phenyl- | Pigment Yellow 60 (C.I. 12705) |
PY60 |
| 6410-09-9[a] | 2-Naphthalenol, 1-[(2-nitrophenyl)azo]- | Pigment Orange 2 (C.I. 12060) |
PO2 |
| 6410-13-5[a] | 2-Naphthalenol, 1-[(4-chloro-2-nitrophenyl)azo]- | Pigment Red 6 (C.I. 12090) |
PR6 |
| 6410-41-9[a] | 2-Naphthalenecarboxamide, N-(5-chloro-2,4-dimethoxyphenyl)-4-[[5-[(diethylamino)sulfonyl]-2-methoxyphenyl]azo]-3-hydroxy- | Pigment Red 5 (C.I. 12490) |
PR5 |
| 6417-83-0[b] | 2-Naphthalenecarboxylic acid, 3-hydroxy-4-[(1-sulfo-2-naphthalenyl)azo]-, calcium salt (1:1) | Pigment Red 63:1 (C.I. 15880:1) |
PR63:1 |
| 6486-23-3[b] | Butanamide, 2-[(4-chloro-2-nitrophenyl)azo]-N-(2-chlorophenyl)-3-oxo- | Pigment Yellow 3 (C.I. 11710) |
PY3 |
| 6535-46-2[b] | 2-Naphthalenecarboxamide, 3-hydroxy-N-(2-methylphenyl)-4-[(2,4,5-trichlorophenyl)azo]- | Pigment Red 112 (C.I. 12370) |
PR112 |
| 7023-61-2[b] | 2-Naphthalenecarboxylic acid, 4-[(5-chloro-4-methyl-2-sulfophenyl)azo]-3-hydroxy-, calcium salt (1:1) | Pigment Red 48:2 (C.I. 15865:2) |
PR48:2 |
| 12236-62-3[b] | Butanamide, 2-[(4-chloro-2-nitrophenyl)azo]-N-(2,3-dihydro-2-oxo-1H-benzimidazol-5-yl)-3-oxo- | Pigment Orange 36 (C.I. 11780) |
PO36 |
| 12236-64-5[a] | 2-Naphthalenecarboxamide, N-[4-(acetylamino)phenyl]-4-[[5-(aminocarbonyl)-2-chlorophenyl]azo]-3-hydroxy- | Pigment Orange 38 (C.I. 12367) |
PO38 |
| 12238-31-2 | Pigment Red 52:2 | Pigment Red 52:2 (C.I. 15860:2) |
PR52:2 |
| 13515-40-7[b] | Butanamide, 2-[(4-chloro-2-nitrophenyl)azo]-N-(2-methoxyphenyl)-3-oxo- | Pigment Yellow 73 (C.I. 11738) |
PY73 |
| 13824-00-5 | 2-Naphthalenecarboxamide, 3-hydroxy-N-(4-methoxyphenyl)-4-[(4-methylphenyl)azo]- | Not available | NAPMPA |
| 16403-84-2 | 2-Naphthalenecarboxamide, 4-[[5-(aminocarbonyl)-2-methylphenyl]azo]-3-hydroxy-N-phenyl- | Pigment Red 268 (C.I. 12316) |
PR268 |
| 17852-99-2[b] | 2-Naphthalenecarboxylic acid, 4-[(4-chloro-5-methyl-2-sulfophenyl)azo]-3-hydroxy-, calcium salt (1:1) | Pigment Red 52:1 (C.I. 15860:1) |
PR52:1 |
| 17947-32-9 | 2-Naphthalenecarboxamide, 3-hydroxy-N-(4-methoxyphenyl)-4-(phenylazo)- | Not available | NAPPA |
| 36968-27-1 | 2-Naphthalenecarboxamide, 4-[[4-(aminocarbonyl)phenyl]azo]-3-hydroxy-N-(2-methoxyphenyl)- | Pigment Red 266 (C.I. 12474) |
PR266 |
| 43035-18-3[a] | Benzenesulfonic acid, 4-[[3-[[2-hydroxy-3-[[(4-methoxyphenyl)amino]carbonyl]-1-naphthalenyl]azo]-4-methylbenzoyl]amino]-, calcium salt (2:1) | Pigment Red 247:1 (C.I. 15915) |
PR247:1 |
| 49744-28-7 | 2-Naphthalenol, 1-[(4-methoxy-2-nitrophenyl)azo]- | Not available | NONPA |
| 59487-23-9[a] | 2-Naphthalenecarboxamide, 4-[[5-[[[4-(aminocarbonyl)phenyl]amino]carbonyl]-2-methoxyphenyl]azo]-N-(5-chloro-2,4-dimethoxyphenyl)-3-hydroxy- | Pigment Red 187 (C.I. 12486) |
PR187 |
| 71832-83-2 | 2-Naphthalenecarboxylic acid, 4-[(5-chloro-4-methyl-2-sulfophenyl)azo]-3-hydroxy-, magnesium salt (1:1) | Pigment Red 48:5 (C.I. 15865:5) |
PR48:5 |
| 74336-60-0[a] | 9,10-Anthracenedione, 1-[(5,7-dichloro-1,9-dihydro-2-methyl-9-oxopyrazolo[5,1-b]quinazolin-3-yl)azo]- | Pigment Red 251 (C.I. 12925) |
PR251 |
| 83249-60-9 | 1-Naphthalenesulfonic acid, 2-[(2-hydroxy-6-sulfo-1-naphthalenyl)azo]-, calcium salt (1:1) | Not available | NSNAC |
| 85005-63-6 | 2-Naphthalenecarboxamide, 4-[(2,4-dinitrophenyl)azo]-3-hydroxy-N-phenyl- | Not available | NANPAP |
| 94199-57-2 | 2-Naphthalenecarboxamide, N-(2-ethoxyphenyl)-3-hydroxy-4-[(2-nitrophenyl)azo]- | Not available | NAPNPA |
Assessments to determine whether 11 of the Monoazo Pigments (PR3, PR4, PR5, PR6, PR187, PR247:1, PR251, PO2, PO5, PO38 and PY60) met one or more criteria under section 64 of CEPA 1999 were previously conducted under the Challenge Initiative of the CMP. Among them, one substance (Pigment Red 3) was concluded to meet the criteria as set out in paragraph 64(c) of CEPA 1999. As outlined in the Notice of Intent for the Aromatic Azo and Benzidine-based Substance GroupingFootnote[2], it was recognized that assessments and conclusions pertaining to some of the substances in the grouping may be subsequently updated as part of the current subgroup assessment. Specifically, significant new information has been identified to inform the ecological assessment of the Monoazo Pigments subgroup and the assessments for the 11 substances have been updated accordingly. Similarly, significant new information pertaining to human health has been identified for 10 of the 11 substances, excluding Pigment Red 3, therefore the human health risk assessment for these 10 substances has been updated.
These 33 Monoazo Pigments are not expected to be naturally occurring in the environment. Twenty-one of the 33 Monoazo Pigments are reported as being either manufactured and/or imported into Canada at levels above the reporting threshold of 100 kg/year. Some of the 33 Monoazo Pigments were also identified as being used in products available to consumers. No measured concentrations in the Canadian environment have been identified for any of these substances.
Environment
The Monoazo Pigments exist principally as particles in the sub- or low-micron range, and the pigment powder is typically composed of primary particles (i.e. the crystal lattice of a pigment), aggregates, and agglomerates. These 33 Monoazo Pigments have very low solubility in water (sub- to low-microgram per litre) and low solubility in octanol (below 20 mg/L); because of this, it is proposed that a quotient logarithm of the molar solute concentrations in octanol and water would reasonably represent the octanol-water partition coefficient for these pigments. Physical-chemical properties and the particulate nature of these substances suggest that soil and sediments are expected to be the two major environmental media of concern for the Monoazo Pigments.
Experimental data indicate that under aerobic conditions, Monoazo Pigments are expected to be persistent in water, soil, and sediments. Bioavailability of Monoazo Pigments is expected to be low based on particulate character of these substances and low solubility in water. As a result, a potential to bioaccumulate in pelagic organisms is expected to be low, which is confirmed by the results of bioconcentration studies.
Due to limited bioavailability of Monoazo Pigments, in chronic soil toxicity studies, no effects were found at the concentration of 1 000 mg/kg soil (dry weight). These pigments also showed “no effect at saturation” in acute and chronic aquatic ecotoxicological studies where solvents were not used. The results of these studies allowed for a conclusion that Monoazo Pigments are not expected to be harmful to aquatic and soil-dwelling organisms at low (environmentally relevant) concentrations.
To evaluate potential exposures to Monoazo Pigments in the environment, environmental concentrations (PECs) were estimated; the industrial release scenario was chosen to evaluate the potential exposure to these substances. Predicted no-effect concentrations (PNECs) for water and soil were calculated based on the experimental critical toxicity values. Calculated risk quotients (PEC/PNEC) were lower than one, indicating that harm to organisms in water and soil is not expected.
Considering all available lines of evidence presented in this Screening Assessment, there is low risk of harm to organisms and the broader integrity of the environment from the 33 Monoazo Pigments evaluated in this assessment. It is concluded that these Monoazo Pigments do not meet criteria under paragraph 64(a) or 64(b) of CEPA 1999, as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Human Health
With respect to human health, this Screening Assessment addresses 32 of 33 substances in the Monoazo Pigments subgroup, including substances previously assessed for which significant new information has become available. The remaining substance, Pigment Red 3, was previously assessed and concluded on under the Challenge Initiative of the CMP. As significant new information relevant to the health assessment was not identified for Pigment Red 3, the previous conclusion on human health for this substance has not been updated. However, Pigment Red 3 was considered to support a read-across approach for the β-naphthol pigments subset in the health assessment.
For the health assessment, most of the substances were evaluated as part of structurally-related subsets; β-naphthol pigments (PO2, PO5, PR4, PR6 and NONPA), β-naphthol pigment lakes (PR49:1, PR50:1 and PR53:1), BONA pigment lakes (PR48:2, PR48:5, PR52:1, PR52:2 and PR63:1), monoazo yellow pigments (PY1, PY3 and PY73), or napththol AS pigments (NANPAP, NAPMPA, NAPNPA, NAPPA, PO38, PR5, PR112, PR170, PR187, PR266 and PR268). The remaining five substances (NSNAC, PO36, PR247:1, PR251 and PY60) were evaluated as individual substances.
A range of data availability was identified across the subsets; while a number of health effects studies were identified for the β-naphthol pigment subset, β-naphthol pigment lake subset and BONA pigment lake subset, limited health effects studies were identified for monoazo yellow pigment subset and naphthol AS pigment subset. No studies were identified for the other individual Monoazo Pigments in this assessment.
The β-naphthol pigments and β-naphthol pigment lakes exhibited similar toxicity in repeated-dose animal studies with target organs including the hematopoetic system, liver and kidney. While the β-naphthol pigments demonstrated mutagenic potential, the β-naphthol pigment lakes were predominantly negative in genotoxicity assays. Evidence for carcinogenicity was observed for both the β-naphthol pigment subset (liver tumours) and the β-naphthol pigment lake subset (liver and spleen tumours). In repeated-dose animal studies, the kidney was identified as the primary target organ for the BONA pigment lakes while these substances did not generally show the same hemolysis and liver toxicity observed for the β-naphthol pigments and β-naphthol pigment lakes. The BONA pigment lakes were generally negative in genotoxicity assays and, based on results from studies with the analogue PR57:1 did not exhibit carcinogenic potential. The available short-term toxicity data indicate a low hazard potential for the monoazo yellow pigment subset and naphthol AS pigment subset. For the five substances considered as individuals (NSNAC, PO36, PR247:1, PR251 and PY60), only limited empirical data were identified; hence their critical health effects cannot be conclusively determined.
Exposure to the 32 Monoazo Pigments via environmental media is not expected to be a significant source of exposure to the general population of Canada, therefore the risk to human health is considered to be low from environmental media.
For 19 Monoazo Pigments (NONPA, PO5, PO36, PO38, PR4, PR5, PR48:2, PR49:1, PR52:1, PR52:2, PR53:1, PR63:1, PR112, PR170, PR187, PR266, PY1, PY3 and PY73), they were identified to be used in certain products available to consumers in the Canadian marketplace (e.g. face paint, finger paint, face mask, lipstick, and natural health products) and the exposure to these substances for the general population of Canada has been characterized. Margins between the estimates of exposures and critical effect levels from animal studies were considered adequate to address uncertainties in the exposure and health effects databases.
For two Monoazo Pigments, (PR247:1 and PR268), limited uses in Canada were identified, however exposure for the general population of Canada is not expected from these uses, therefore the risk to human health is not expected.
For the other 11 Monoazo Pigments (NANPAP, NAPMPA, NAPNPA, NAPPA, NSNAC, PO2, PR6, PR48:5, PR50:1, PR251 and PY60), no uses of these substances in products in the Canadian marketplace were identified. Therefore, based on available information for exposure in Canada, the risk to human health is not expected for these 13 Monoazo Pigments.
Some of the Monoazo Pigments in this assessment have effects of concern based on potential carcinogenicity. While available information does not indicate a risk to human health for Canadians at current levels of exposure, there may be a concern if exposures were to increase.
Based on the information presented in this Screening Assessment, it is concluded that the 32 Monoazo Pigments evaluated in this assessment for human health do not meet the criteria under paragraph 64(c) of CEPA 1999 as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health. In addition, there are no updates to the conclusion made with respect to paragraph 64(c) for Pigment Red 3, previously assessed by the Government of Canada under the Challenge Initiative of the CMP.
Overall Conclusion
It is concluded that 32 Monoazo Pigments evaluated in this assessment do not meet any of the criteria set out in section 64 of CEPA 1999.
The conclusion previously made under the Challenge Initiative that Pigment Red 3 meets the criteria set out in paragraph 64(c) of CEPA 1999 remains unchanged.
1. Introduction
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999), the Minister of the Environment and the Minister of Health conduct screening assessments of substances to determine whether these substances present or may present a risk to the environment or to human health.
The Substance Grouping Initiative is a key element of the Government of Canada’s Chemicals Management Plan (CMP). The Aromatic Azo and Benzidine-based Substance Grouping consists of 358 substances that were identified as priorities for assessment as they met the categorization criteria under section 73 of CEPA 1999 and/or were considered as a priority based on human health concerns (Environment Canada and Health Canada 2007). Some substances within this Substance Grouping have been identified by other jurisdictions as a concern due to the potential cleavage of the azo bonds, which can lead to the release of aromatic amines that are known or likely to be carcinogenic.
While many of these substances have common structural features and similar functional uses as dyes or pigments in multiple sectors, diversity within the substance group has been taken into account through the establishment of subgroups. Subgrouping based on structural similarities, physical and chemical properties, and common functional uses and applications accounts for variability within this substance grouping and allows for subgroup-specific approaches in the conduct of screening assessments. This Screening Assessment considers substances that belong to the Monoazo Pigments subgroup. Consideration of azo bond cleavage products (aromatic amines) is a key element of human health assessment in each subgroup. Some aromatic amines, commonly referred to as EU22 aromatic aminesFootnote[3] , as well as associated azo dyes are restricted in other countries (EU 2006). Information on the subgrouping approach for the Aromatic Azo and Benzidine-based Substance Grouping under Canada’s CMP, as well as additional background information and regulatory context, is provided in a separate document prepared by the Government of Canada (Environment Canada, Health Canada 2013b).
The 33 substances considered in this Screening Assessment (Table 2-1) constitute a subgroup of the Monoazo Pigments. Eleven substances in this subgroup have been previously assessed by the Government of Canada under the Challenge Initiative of the CMP. Among these, Pigment Red 3 (Chemical Abstracts Service Registry Number [CAS RN] 2425-85-6) was previously assessed and concluded to meet paragraph 64(c) under CEPA 1999 (Environment Canada, Health Canada 2009b). No significant new information was identified with respect to human health risk characterization for Pigment Red 3, therefore the previous conclusion on human health has not been updated for this substance in this Screening Assessment. However, Pigment Red 3 is used for read-across purposes in the human health assessment of the other 32 Monoazo Pigments. In contrast, Pigment Red 3 was assessed as part of the ecological risk characterization for all 33 Monoazo Pigments in this Screening Assessment. Similarly, two of the substances considered in this Screening Assessment, NANPAP (CAS RN 85005-63-6) and NAPNPA (CAS RN 94199-57-2), were previously included as part of a screening assessment, in April 2008, of 145 persistent, bioaccumulative, and inherently toxic (PBiT) substances that were considered not to be in commerce. These two substances are considered in this Screening Assessment of Certain Monoazo Pigments because significant new information has been identified since the previous assessment of 145 PBiT substances.
Screening assessments focus on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA 1999, by examining scientific information to develop conclusions by incorporating a weight of evidence approach and precaution.Footnote[4]
This Screening Assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to May 2014. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
The Screening Assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
The Screening Assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review and consultation. Comments on the technical portions relevant to the environment were received from Dr. Harold Freeman (North Carolina State University, USA) and Dr. Gisela Umbuzeiro (University of Campinas, Brazil). Comments on the technical portions relevant to human health were received from Dr. Harold Freeman (North Carolina State University, USA), Dr. David Josephy (University of Guelph, Canada), Dr. Michael Bird (University of Ottawa, Canada), and Dr. Kannan Krishnan (Université du Montréal, Canada). Additionally, the draft of this Screening Assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the Screening Assessment remain the responsibility of Health Canada and Environment Canada.
The critical information and considerations upon which the Screening Assessment is based are given below.
2. Identity of Substances
This Screening Assessment focuses on 33 substances that belong to the subgroup of Monoazo Pigments that is part of the Aromatic Azo and Benzidine-based Substance Grouping. The identities of the individual substances in this Screening Assessment are presented in Table 2-1. The CAS RNs, Domestic Substances List (DSL) names, Colour Index (C.I.) generic names, C.I. constitution numbers and chemical acronyms are presented in Table 2-1. Chemical acronyms are derived from the C.I. generic names when available; otherwise, they are based on the DSL names. A list of additional chemical names (e.g., trade names) is available from the National Chemical Inventories (NCI 2007).
| CAS RN | DSL name | C.I. generic name(C.I. number) | Chemical acronym |
|---|---|---|---|
| 1103-38-4 | 1-Naphthalenesulfonic acid, 2-[(2-hydroxy-1-naphthalenyl)azo]-, barium salt (2:1) | Pigment Red 49:1 (C.I. 15630:1) |
PR49:1 |
| 2425-85-6Footnote Table 2-1[a] | 2-Naphthalenol, 1-[(4-methyl-2-nitrophenyl)azo]- | Pigment Red 3 (C.I. 12120) |
PR3 |
| 2512-29-0 | Butanamide, 2-[(4-methyl-2-nitrophenyl)azo]-3-oxo-N-phenyl- | Pigment Yellow 1 (C.I. 11680) |
PY1 |
| 2786-76-7 | 2-Naphthalenecarboxamide, 4-[[4-(aminocarbonyl)phenyl]azo]-N-(2-ethoxyphenyl)-3-hydroxy- | Pigment Red 170 (C.I. 12475) |
PR170 |
| 2814-77-9Footnote Table 2-1[b] | 2-Naphthalenol, 1-[(2-chloro-4-nitrophenyl)azo]- | Pigment Red 4 (C.I. 12085) |
PR4 |
| 3468-63-1[b] | 2-Naphthalenol, 1-[(2,4-dinitrophenyl)azo]- | Pigment Orange 5 (C.I. 12075) |
PO5 |
| 5160-02-1 | Benzenesulfonic acid, 5-chloro-2-[(2-hydroxy-1-naphthalenyl)azo]-4-methyl-, barium salt (2:1) | Pigment Red 53:1 (C.I. 15585:1) |
PR53:1 |
| 6372-81-2 | Benzoic acid, 2-[(2-hydroxy-1-naphthalenyl)azo]-, barium salt (2:1) | Pigment Red 50:1 (C.I. 15500:1) |
PR50:1 |
| 6407-74-5[b] | 3H-Pyrazol-3-one, 4-[(2-chlorophenyl)azo]-2,4-dihydro-5-methyl-2-phenyl- | Pigment Yellow 60 (C.I. 12705) |
PY60 |
| 6410-09-9[b] | 2-Naphthalenol, 1-[(2-nitrophenyl)azo]- | Pigment Orange 2 (C.I. 12060) |
PO2 |
| 6410-13-5[b] | 2-Naphthalenol, 1-[(4-chloro-2-nitrophenyl)azo]- | Pigment Red 6 (C.I. 12090) |
PR6 |
| 6410-41-9[b] | 2-Naphthalenecarboxamide, N-(5-chloro-2,4-dimethoxyphenyl)-4-[[5-[(diethylamino)sulfonyl]-2-methoxyphenyl]azo]-3-hydroxy- | Pigment Red 5 (C.I. 12490) |
PR5 |
| 6417-83-0 | 2-Naphthalenecarboxylic acid, 3-hydroxy-4-[(1-sulfo-2-naphthalenyl)azo]-, calcium salt (1:1) | Pigment Red 63:1 (C.I. 15880:1) |
PR63:1 |
| 6486-23-3 | Butanamide, 2-[(4-chloro-2-nitrophenyl)azo]-N-(2-chlorophenyl)-3-oxo- | Pigment Yellow 3 (C.I. 11710) |
PY3 |
| 6535-46-2 | 2-Naphthalenecarboxamide, 3-hydroxy-N-(2-methylphenyl)-4-[(2,4,5-trichlorophenyl)azo]- | Pigment Red 112 (C.I. 12370) |
PY112 |
| 7023-61-2 | 2-Naphthalenecarboxylic acid, 4-[(5-chloro-4-methyl-2-sulfophenyl)azo]-3-hydroxy-, calcium salt (1:1) | Pigment Red 48:2 (C.I. 15865:2) |
PR48:2 |
| 12236-62-3 | Butanamide, 2-[(4-chloro-2-nitrophenyl)azo]-N-(2,3-dihydro-2-oxo-1H-benzimidazol-5-yl)-3-oxo- | Pigment Orange 36 (C.I. 11780) |
PO36 |
| 12236-64-5[b] | 2-Naphthalenecarboxamide, N-[4-(acetylamino)phenyl]-4-[[5-(aminocarbonyl)-2-chlorophenyl]azo]-3-hydroxy- | Pigment Orange 38 (C.I. 12367) |
PO38 |
| 12238-31-2 | Pigment Red 52:2 | Pigment Red 52:2 (C.I. 15860:2) |
PR52:2 |
| 13515-40-7 | Butanamide, 2-[(4-chloro-2-nitrophenyl)azo]-N-(2-methoxyphenyl)-3-oxo- | Pigment Yellow 73 (C.I. 11738) |
PY73 |
| 13824-00-5 | 2-Naphthalenecarboxamide, 3-hydroxy-N-(4-methoxyphenyl)-4-[(4-methylphenyl)azo]- | Not available | NAPMPA |
| 16403-84-2 | 2-Naphthalenecarboxamide, 4-[[5-(aminocarbonyl)-2-methylphenyl]azo]-3-hydroxy-N-phenyl- | Pigment Red 268 (C.I. 12316) |
PR268 |
| 17852-99-2 | 2-Naphthalenecarboxylic acid, 4-[(4-chloro-5-methyl-2-sulfophenyl)azo]-3-hydroxy-, calcium salt (1:1) | Pigment Red 52:1 (C.I. 15860:1) |
PR52:1 |
| 17947-32-9 | 2-Naphthalenecarboxamide, 3-hydroxy-N-(4-methoxyphenyl)-4-(phenylazo)- | Not available | NAPPA |
| 36968-27-1 | 2-Naphthalenecarboxamide, 4-[[4-(aminocarbonyl)phenyl]azo]-3-hydroxy-N-(2-methoxyphenyl)- | Pigment Red 266 (C.I. 12474) |
PR266 |
| 43035-18-3[b] | Benzenesulfonic acid, 4-[[3-[[2-hydroxy-3-[[(4-methoxyphenyl)amino]carbonyl]-1-naphthalenyl]azo]-4-methylbenzoyl]amino]-, calcium salt (2:1) | Pigment Red 247:1 (C.I. 15915) |
PR247:1 |
| 49744-28-7 | 2-Naphthalenol, 1-[(4-methoxy-2-nitrophenyl)azo]- | Not available | NONPA |
| 59487-23-9[b] | 2-Naphthalenecarboxamide, 4-[[5-[[[4-(aminocarbonyl)phenyl]amino]carbonyl]-2-methoxyphenyl]azo]-N-(5-chloro-2,4-dimethoxyphenyl)-3-hydroxy- | Pigment Red 187 (C.I. 12486) |
PR187 |
| 71832-83-2 | 2-Naphthalenecarboxylic acid, 4-[(5-chloro-4-methyl-2-sulfophenyl)azo]-3-hydroxy-, magnesium salt (1:1) | Pigment Red 48:5 (C.I. 15865:5) |
PR48:5 |
| 74336-60-0[b] | 9,10-Anthracenedione, 1-[(5,7-dichloro-1,9-dihydro-2-methyl-9-oxopyrazolo[5,1-b]quinazolin-3-yl)azo]- | Pigment Red 251 (C.I. 12925) |
PR251 |
| 83249-60-9 | 1-Naphthalenesulfonic acid, 2-[(2-hydroxy-6-sulfo-1-naphthalenyl)azo]-, calcium salt (1:1) | Not available | NSNAC |
| 85005-63-6Footnote Table 2-1[c] | 2-Naphthalenecarboxamide, 4-[(2,4-dinitrophenyl)azo]-3-hydroxy-N-phenyl- | Not available | NANPAP |
| 94199-57-2[c] | 2-Naphthalenecarboxamide, N-(2-ethoxyphenyl)-3-hydroxy-4-[(2-nitrophenyl)azo]- | Not available | NAPNPA |
The subgroup of 33 Monoazo Pigments was further divided into smaller, structurally related “subsets” for the purposes of read-across (OECD 2007). Each of the Monoazo Pigment subsets is based on well-defined synthetic organic pigment classes that are defined by related structures, including common coupling components, similar physical-chemical properties and application classes. This approach is based on stakeholder input on grouping of azo substances (Environment Canada and Health Canada 2012) and is consistent with general principles for building categories, as outlined in Organisation for Economic Co-operation and Development (OECD) guidance documents (OECD 2007). The Monoazo Pigment subsets considered in this assessment are described below.
2.1 Monoazo Yellow Pigments (3 Substances: PY1, PY3, PY73)
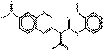
This subset is defined by the following structure with a common acetoacetanilide coupling component, where R represents substituents such as CH3, OCH3, NO2, Cl and other moieties (Herbst and Hunger 2004):

PY60 is typically included with monoazo yellow pigments according to the industry classification (Herbst and Hunger 2004). However, it is not considered as a close structural analogue to the other members of the monoazo yellow pigments subset as represented by the above-mentioned structure. Therefore, PY60 is not included in the monoazo yellow pigments subset in this Screening Assessment and is instead treated as an individual pigment.
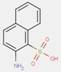
2.2 β-Naphthol Pigments (6 Substances: PR3, PR4, PR6, PO2, PO5, NONPA)
The β-naphthol pigments subset is defined by the following general chemical structure with a common β-naphthol coupling component, where R1 and R2 represent substituents such as CH3, OCH3, NO2, Cl and other moieties (Herbst and Hunger 2004):

2.3 β-Naphthol Pigment Lakes (3 Substances: PR49:1, PR50:1, PR53:1)
Lake pigments are manufactured by precipitating water-soluble dyes on inert binders, usually salts of calcium, magnesium, barium or strontium (e.g., BaSO4). The β-naphthol pigment lakes are characterized by the general structure below, including the same common β-naphthol coupling component as for the β-naphthol pigments subset. However, in contrast, this subset also contains ionizable groups (e.g., –SO3−, –COO−) on the ring(s) opposite the β-naphthol moiety, with additional substitutions, where A typically stands for a benzene or a naphthalene ring, R represents CH3, C2H5 or COOM, n is a number from 0 to 2 and M is usually an alkaline earth metal (Herbst and Hunger 2004):

2.4 BONA Pigment LakesFootnote[5] (4 Substances: PR48:2, PR48:5, PR52:1, PR63:1)
The BONA pigment lakes subset is characterized by the following general chemical structure including a common BONA coupling component, where RD usually stands for H, Cl or CH3 and M is usually an alkaline earth metal (Herbst and Hunger 2004):

PR52:2, belonging to the BONA pigment lakes class according to the industry classification, was not considered as a close structural analogue to the other members of this subset having the general chemical structure shown above. Based on the chemical name of this substance and its structure presented in the National Chemical Inventories, this substance is a manganese complex (having manganese–nitrogen and manganese–oxygen bonds) and is not considered part of this subset for most of this assessment, except in the human health effects characterization section. Given the limited health effects data available for this substance, read-across was considered for human health effects within BONA pigment lake subset.

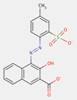
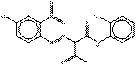
2.5 Naphthol AS Pigments (or “Naphthol Reds”) (11 Substances: PR5, PR112, PR170, PR187, PR266, PR268, PO38, NAPNPA, NANPAP, NAPMPA, NAPP NAPPA), Lakes (PR247:1)
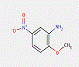
The naphthol AS pigments subset is defined by the following general chemical structure including a common coupling component based on derivatives of the naphthol AS moiety, where RK represents substituents such as CH3, OCH3, NO2, Cl and others, and RDrepresents RK, COOCH3, CONHC6H5, or SO2N(C2H5)2, and m and n are numbers between 0 and 3 (Herbst and Hunger 2004):

There is only one substance identified for each of the following three subsets.
2.6 Naphthol AS Pigment Lakes (PR247:1)
There is no common structural principle to these pigments beyond the basic naphthol AS pigment skeleton. However, Herbst and Hunger (2004) indicated some general structure that can represent a variety of representatives of pigments in this class (where RD2 usually stands for CH3 and/or SO3–; RD4 for H and/or CH3; RD5 for H, Cl or CONHC6H4SO3–; RK2 for H and/or OCH3; RK4 for H, SO3– or OCH3; and M for barium or calcium):

2.7 Benzimidazolone Pigments (PO36)
Orange and yellow benzimidazolone pigments are derived from the following general structure, where RD usually stands for Cl, Br, CH3, NO2, OCH3, COOH, CONH2 and other functional groups (Herbst and Hunger 2004):

2.8 Pyrazoloquinazolone Pigments (PR251)
PR251 is obtained by coupling 1-aminoanthraquinone (1) onto pyrazolo-(5,1b)-quinazolone, with the general structure (2):
(1)

(2)

As a result, the following general structure can represent pyrazoloquinazolone pigments, where R1 usually represents Cl and R2 usually represents CH3:

No analogues with available experimental data have been identified for these substances. In addition, one substance (CAS RN 83249-60-9) that is not a close structural analogue to any other substances within the group of 33 pigments (and which has neither a C.I. name nor a C.I. number) was also not part of a subset.
Further information on the chemical structures, molecular formulas and molecular weights of all 33 Monoazo Pigments is presented in Appendix 1.
2.9 Selection of Analogues and Use of (Q)SAR Models
Guidance on the use of read-across approaches has been prepared by various organizations, such as the Organisation for Economic Co-operation and Development (OECD 2014). It has been applied in various regulatory programs, including the European Union’s (EU) Existing Substances Programme. The general method for analogue selection and the use of (quantitative) structure–activity relationship ((Q)SAR) models is provided in Environment Canada and Health Canada (2013a). For characterization of human health effects, the basis for the use of analogues and/or (Q)SAR modelling data is documented in the Health Effects Assessment section of this report.
Analogues used to inform the ecological assessment were selected based on the availability of relevant empirical data pertaining to physical-chemical properties, persistence, bioaccumulation and ecotoxicity. Such data were used as read-across data for those Monoazo Pigments that lacked empirical data, where appropriate, or to support the weight of evidence of existing empirical information. Although analogue data are used preferentially to fill data gaps for the substances in this assessment, the applicability of (Q)SAR models to the Monoazo Pigments is determined on a case-by-case basis.
All Monoazo Pigments within the given subsets (including identified analogues) are considered to have reasonably similar physical-chemical properties, environmental fate, bioavailability and ecological effects. Analogues for a given subset were selected that generally met the structural applicability domain for the subset (see previous section) and that had empirical data applicable for read-across purposes. In this assessment report, the analogues are presented with an asterisk (e.g., PY74*) as shown on Table 2-2. Additional considerations for use of these analogues in terms of absorption, potential for azo bond cleavage and mammalian toxicity are discussed in the Human Health Effects Assessment section. The physical-chemical properties of the Monoazo Pigments subsets, including the selected analogues, are discussed in the following section.
| Subset | Substance (CAS RN) | C.I. generic name (C.I. number) |
Chemical structure, molecular formula (molecular weight) |
Read-across usage |
|---|---|---|---|---|
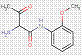
| β-Naphthol pigments | Para Red* (6410-10-2) |
Pigment Red 1* (C.I. 12070) |
 C16H11N3O3 (293 g/mol) |
human health effects |
| Monoazo yellow pigments | PY74* (6358-31-2) |
Pigment Yellow 74* (C.I. 11741) |
C18H18N4O6 (386 g/mol) |
Physical-chemical properties, ecological effects, human health effects |
| Monoazo yellow pigments | 80675-49-6* (80675-49-6) |
N/A (no C.I. name) |  C18H18N4O6 (386 g/mol) |
Human health effects |
| BONA pigment lakes | PR48:1* (7585-41-3) |
Pigment Red 48:1* (C.I. 15865:1) |
 C18H11ClN2O6SBa (556 g/mol) |
Physical-chemical properties |
| BONA pigment lakes | PR48:3* (15782-05-5) |
Pigment Red 48:3* (C.I. 15865:3) |
C18H11ClN2O6SSr (506 g/mol) |
Physical-chemical properties |
| BONA pigment lakes | PR57:1* (5281-04-9) |
Pigment Red 57:1* (C.I. 15850:1) |
 C18H12N2O6SCa (424 g/mol) |
Physical-chemical properties, persistence and bioaccumulation potential, ecological effects, human health effects |
| Naphthol AS pigments | PR2* (6041-94-7) |
Pigment Red 2* (C.I. 12310) |
 C23H15Cl2N3O2 (436 g/mol) |
Physical-chemical properties, ecological effects |
| Naphthol AS pigments | PR22* (6448-95-9) |
Pigment Red 22* (C.I. 12315) |
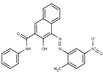 C24H18N4O4 (426 g/mol) |
Human health effects |
| Naphthol AS pigments | PR23* (6471-49-4) |
Pigment Red 23* (C.I. 12355) |
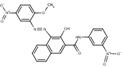 C24H17N5O7 (487 g/mol) |
Human health effects |
| Naphthol AS pigments | PR146* (5280-68-2) |
Pigment Red 146* (C.I. 12485) |
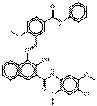 C33H27ClN4O6 (611 g/mol) |
Physical-chemical properties, ecological effects |
| Naphthol AS pigments | PR253* (85776-13-2) |
Pigment Red 253* (C.I. 12375) |
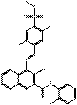 C25H20Cl2N4O4S (543 g/mol) |
Physical-chemical properties, ecological effects |
3. Physical and Chemical Properties
A summary of experimental data for the specific physical and chemical properties that play a critical role in determining the environmental fate and biological effects of Monoazo Pigments is presented in Table 3-1. Detailed substance-specific information (with references) on these pigments and their analogues can be found in Appendix 2 of this report. Importantly, the properties of pigments depend strongly on the manner in which they have been prepared by the manufacturers.
Experimental data on vapour pressure and Henry’s Law constant are not available for most of the pigments. However, since many Monoazo Pigments are similar in molecular size and complexity to disperse dyes, they can be expected to have vapour pressures in the same range as values reported for disperse dyes (i.e., 10−11 to 10−9 Pa; Baughman and Perenich 1988). Similarly, all Monoazo Pigments are expected to have very low Henry’s Law constants. Therefore, a scenario involving exposure to Monoazo Pigments in air is expected to be environmentally irrelevant for this group of substances. However, airborne exposure to Monoazo Pigments as dusts or particulates may be possible, especially from some products for which inhalation exposure may be relevant (e.g., spray paints).
The β-naphthol pigment lakes and BONA pigment lakes contain ionizable sulfonate and/or carboxylate groups; therefore, dissociation of the metal counter-ion may be possible. However, based on empirical evidence of low toxicity from ecotoxicity studies, any dissociation is expected to be limited and is considered less relevant to the environmental fate and ecotoxicity of these pigments. In contrast, based on observed toxicity following oral exposure in rodent studies, it is clear that some degree of dissociation and/or bioavailability of the pigment lakes must occur in the gastrointestinal tract (see the Health Effects Assessment section for more discussion). The other Monoazo Pigments subsets do not contain ionizable groups, and therefore discussion of dissociation is not relevant for these subsets. Also, Monoazo Pigments decompose before boiling, so a boiling point is not applicable for these substances.
| Subset | Substance | Property (acronym) and units | Range or values |
|---|---|---|---|
| Monoazo yellow pigments | PY1; PY3; PY74* | Melting point (MP), °C | No melting point (endothermic effect in the temperature range 260–290°C directly followed by spontaneous exothermal decomposition in the temperature range 290–390°C) |
| Monoazo yellow pigments | PY1; PY3; PY74* | Decomposition temperature (DT), °C | 249; 290 |
| Monoazo yellow pigments | PY1; PY3; PY74* | Particle size distribution: mass median diameter (D50), µm | 1.96 |
| Monoazo yellow pigments | PY1; PY3; PY74* | Density, g/cm3 | 1.43 |
| Monoazo yellow pigments | PY1; PY3; PY74* | Water solubility (WS, Sw), µg/L | 0.23; 7.5–7.6 |
| Monoazo yellow pigments | PY1; PY3; PY74* | Solubility in n-octanol (Soct), µg/L | 740; 5960–9530 |
| Monoazo yellow pigments | PY1; PY3; PY74* | The quotient logarithm of the molar solute concentrations in n-octanol and water (log (Soct/Sw)), dimensionless | 2.0; 2.9; 4.6 |
| Monoazo yellow pigments | PY1; PY3; PY74* | The quotient logarithm of the organic carbon–water partition coefficient (Koc), dimensionless |
5.5 |
| BONA pigment lakes | PR48:2; PR48:1*; PR48:3*; PR57:1* | Melting point (MP), °C | No melting point (the substance evaporates and decomposes before melting); 357.5°C |
| BONA pigment lakes | PR48:2; PR48:1*; PR48:3*; PR57:1* | Decomposition temperature (DT), °C | 310–370 |
| BONA pigment lakes | PR48:2; PR48:1*; PR48:3*; PR57:1* | Particle size distribution: mass median diameter (D50), µm | 1.7–17.1 |
| BONA pigment lakes | PR48:2; PR48:1*; PR48:3*; PR57:1* | Density, g/cm3 | 1.20 |
| BONA pigment lakes | PR48:2; PR48:1*; PR48:3*; PR57:1* | Water solubility (WS, Sw), µg/L | less than 25; 100–280; 500–1300; 8900 |
| BONA pigment lakes | PR48:2; PR48:1*; PR48:3*; PR57:1* | Solubility in n-octanol (Soct), µg/L | 31–70; 4600–6000 |
| BONA pigment lakes | PR48:2; PR48:1*; PR48:3*; PR57:1* | The quotient logarithm of the molar solute concentrations in n-octanol and water (log (Soct/Sw)), dimensionless | −), dimension |
| β-Naphthol pigments | PR3; PR4; PO2; PO5 | Melting point (MP), °C | 212; 270–302 |
| β-Naphthol pigments | PR3; PR4; PO2; PO5 | Average particle size, µm | 0.26–0.37 |
| β-Naphthol pigments | PR3; PR4; PO2; PO5 | Density, g/cm3 | 1.37–1.50 |
| β-Naphthol pigments | PR3; PR4; PO2; PO5 | Water solubility (WS, Sw), µg/L | 3.3–6.8; 800 |
| β-Naphthol pigments | PR3; PR4; PO2; PO5 | Solubility in n-octanol (Soct), µg/L | 1760; 9400–17 900 |
| β-Naphthol pigments | PR3; PR4; PO2; PO5 | The quotient logarithm of the molar solute concentrations in n-octanol and water (log (Soct/Sw), dimensionless | 2.4; 3.5–3.7 |
| Naphthol AS pigments | PR5; PR112; PR187; PR266 PO38; PR2*; PR146*; PR253* | Melting point (MP), °C | No melting point (endothermic effect in the temperature range 260–270°C directly followed by spontaneous exothermal decomposition in the temperature range 270–290°C); 306°C |
| Naphthol AS pigments | PR5; PR112; PR187; PR266 PO38; PR2*; PR146*; PR253* | Decomposition temperature (DT), °C | 270 |
| Naphthol AS pigments | PR5; PR112; PR187; PR266 PO38; PR2*; PR146*; PR253* | Average particle size, µm | 0.10–0.17 |
| Naphthol AS pigments | PR5; PR112; PR187; PR266 PO38; PR2*; PR146*; PR253* | Particle size distribution: mass median diameter (D50), µm | 4.56 |
| Naphthol AS pigments | PR5; PR112; PR187; PR266 PO38; PR2*; PR146*; PR253* | Density, g/cm3 | 1.40–1.48 |
| Naphthol AS pigments | PR5; PR112; PR187; PR266 PO38; PR2*; PR146*; PR253* | Water solubility (WS, Sw), µg/L | 3.0–24.9 |
| Naphthol AS pigments | PR5; PR112; PR187; PR266 PO38; PR2*; PR146*; PR253* | Solubility in n-octanol (Soct), µg/L | 22.1; 100–202; 3310–8630 |
| Naphthol AS pigments | PR5; PR112; PR187; PR266 PO38; PR2*; PR146*; PR253* | The quotient logarithm of the molar solute concentrations in n-octanol and water (log (Soct/Sw)), dimensionless | 0.4–1.1; 1.2–1.7; 2.5–3.2 |
| β-Naphthol pigment lakes | PR53:1 | Melting point (MP), °C | Melting under decomposition at 330°C |
| β-Naphthol pigment lakes | PR53:1 | Decomposition temperature (DT), °C | 343–345 |
| β-Naphthol pigment lakes | PR53:1 | Density, g/cm3 | 1.5 |
| β-Naphthol pigment lakes | PR53:1 | Water solubility (WS, Sw), µg/L | 1300–3400 |
| β-Naphthol pigment lakes | PR53:1 | The quotient logarithm of the molar solute concentrations in n-octanol and water (log (Soct/Sw)), dimensionless | −), d |
| Benzimidazolone pigments | PO36 | Water solubility (WS, Sw), µg/L | 14; less than 20.6 |
| Benzimidazolone pigments | PO36 | Solubility in n-octanol (Soct), µg/L | 86.1; greater than 137 |
| Benzimidazolone pigments | PO36 | The quotient logarithm of the molar solute concentrations in n-octanol and water (log (Soct/Sw)), dimensionless | 0.8 |
| Naphthol AS pigment lakes | PR247:1 | Average particle size, µm | 0.18 |
| Naphthol AS pigment lakes | PR247:1 | Water solubility (WS, Sw), µg/L | 112 |
| Naphthol AS pigment lakes | PR247:1 | Solubility in n-octanol (Soct), µg/L | 178 |
| Naphthol AS pigment lakes | PR247:1 | The quotient logarithm of the molar solute concentrations in n-octanol and water (log (Soct/Sw)), dimensionless | 0.2 |
3.1 Particle Size Distribution and Density
The majority of organic pigments generally do not exist as individual molecules but are principally particles in the sub- or low-micrometre size range. The pigment powder is typically composed of primary particles (i.e., the crystal lattice of a pigment), aggregates and agglomerates. Manufacturers usually provide the physical specifications of their pigments, which include the particle size distribution (mass median diameter, D50) or the average particle size of the pigment powder. Users can thereby determine which pigment is the most appropriate to colour their products, since performance is chiefly controlled by the particle size distribution (Herbst and Hunger 2004).
In terms of the particle size distribution, reported data on mass median diameter (D50) for these substances were taken from the Registration, Evaluation, Authorisation and Restriction of Chemical Substances (REACH) registration dossiers for these substances, available from the European Chemicals Agency (ECHA).Footnote[6] The particle size distribution data, presented in Table 3-1, indicate that for this group of pigments, the D50 values vary within the range of 1.7–17.1 µm (i.e., 50% of the total mass of particles are smaller than 1.7–17.1 µm).
In terms other than mass-dependent particle size distribution, some authors have reported particle sizes for monoazo pigments to be very small, often below 1 µm. Data presented in Table 3-1 indicate that naphthol AS pigments and naphthol AS pigment lakes have average particle sizes of only 0.10–0.18 µm (i.e., 100–180 nm). The particles of the β-naphthol pigments are a bit larger, but their average size is still below 1 µm (0.26–0.37 µm).
Bäumler et al. (2000) also reported particle sizes of azo pigments in tattoo inks, ranging from only 20 to 900 nm. In another study, Høgsberg et al. (2011) demonstrated tattoo inks containing red and yellow azo pigments of the monoazo class and disazo dichlorobenzidine-based pyrazolone class exhibiting particle size ranges from less than 100 to 1 000 nm. Therefore, it should be considered that Monoazo Pigments have a broad range of particle sizes, (Canada 2007; Health Canada 2011a). that may be a factor in potential uptake and absorption of the insoluble particulate form (which is discussed further in the respective sections on ecological and human health assessment).
The density of Monoazo Pigments varies within a relatively narrow range, from 1.2 to 1.5 g/cm3, which is higher than the density of water. Therefore, when released to water, the Monoazo Pigments, being relatively heavy particles, are expected to precipitate and reside in sediments.
3.2 Melting and Decomposition Temperatures
Results indicate that for many Monoazo Pigments, melting points are very close to the decomposition temperatures; in some studies, the substances evaporated and decomposed before melting. In some tests, melting points are reported, but data variation is very significant (e.g., from 212 to 270–302ºC for a subset of β-naphthol pigments) (Table 3-1). For some substances, melting points could not be reported because of the occurrence of an endothermic effect (at 260–290°C), followed by spontaneous exothermal decomposition (at 270–390°C) (see Appendix 2 for more details).
Data indicate that compared with the pigments, the decomposition temperatures of the pigment lakes are higher. For example, decomposition temperatures of monoazo yellow pigments and naphthol AS pigments vary within the range of 249–290ºC, compared with 310–370ºC for BONA pigment lakes and β-naphthol pigment lakes. Therefore, it may be concluded that within this subgroup of 33 substances, the thermostability of monoazo pigment lakes is greater than that of non-lake pigments.
3.3 Solubility in Water and n-Octanol
Overall, Monoazo Pigments in this Screening Assessment are characterized by low water solubility. At the same time, some substances are significantly less soluble in water than others (Table 3-1; Appendix 2). Within some subsets of pigments (e.g., BONA pigment lakes), variation in water solubility values is significant.
The low solubility of organic pigments is a result of the inherent design of colourants, which have strong interactive forces between molecules, achieved by the introduction of substituents like –CONH– in the molecule (Lincke 2003; Herbst and Hunger 2004). The resulting intermolecular bonding, in turn, generates a crystal structure that lends stability to the organic pigments (Lincke 2003). Panina (2009) emphasized that due to their molecular structure features, organic pigments tend to form highly crystalline solids; very typical structural motifs are π-π stacking of conjugated rings and intermolecular hydrogen bonds C=O…H–N. Such strong intermolecular interactions inside the crystal structure lead to a high lattice energy and, often as a consequence, a very low solubility. (It should, however, also be mentioned that all azo pigments exist in crystal form as solid particulate with hydrogen bonding, yet there might be substantial differences in solubility in water and octanol between diarylide and some monoazo pigments; therefore, some major differences in apparent stability of the crystal also have to be taken into account. Such differences in water and octanol solubilities have been observed for azo pigments of different structural classes [e.g., Anliker and Moser 1987; Environment Canada and Health Canada 2013b]).
Importantly, the non-lake pigments in this subgroup of 33 substances are less soluble in water than the pigment lakes. For example, the subsets of monoazo yellow pigments, β-naphthol pigments, naphthol AS pigments and benzimidazolone pigments are characterized by very low water solubility values of 0.2–25 µg/L (with only one outlier result of 800 µg/L), while BONA pigment lakes, β-naphthol pigment lakes and naphthol AS pigment lakes are, in general, slightly more water-soluble (100–8 900 µg/L; see Table 3-1 and Appendix 2).
Overall, the solubility of both non-lake pigments and pigment lakes in n-octanol is relatively higher than their water solubility, with octanol solubility values reaching the milligram per litre range (Table 3-1). For this parameter, similar to some water solubility values, data variation within the subsets may also be very high (see Table 3-1 and Appendix 2). These significant variations of water and octanol solubility values may be explained by the different purities of the pigments tested (e.g., pure pigment vs. final products). For example, the presence of additives such as dispersing agents in a given commercial pigment product will impact the apparent solubility. Other factors, such as solubility testing method as well as test conditions (e.g., pH), can also contribute to the high variability.
In the case of the pigment lake subsets, the effect of the different counter-ions may contribute to the variability of solubility values. Some subsets of pigment lakes are the salts of different metals--for example, BONA pigment lakes are the salts of calcium, barium and strontium, and different solubility values may, to some extent, reflect the properties of the counter-ions (Ca2+, Ba2+, Sr2+).
3.4 Octanol–Water and Organic Carbon–Water Partition Coefficients
No reliable experimental data on octanol–water partition coefficient (Kow) are generally available for the Monoazo Pigments. Modelled data cannot be considered reliable; for example, the Kow values derived from fragment-based models such as KOWWIN (2010) often overestimate the actual Kow of sparingly soluble substances such as pigments. At the Environment Canada–sponsored Quantitative Structure–Activity Relationship (QSAR) Workshop in 1999, invited modelling experts identified many structural classes of pigments and dyes as “difficult to model” using most QSARs (Environment Canada 2000). The physical and chemical properties of many of the structural classes of pigments and dyes are often not amenable to model prediction because they are typically considered “out of the model domain of applicability” (e.g., structural and/or property parameter domains).
According to Guidance on Information Requirements and Chemical Safety Assessment (ECHA 2008), in order to overcome the difficulties in measuring the Kow, the solubility in n-octanol and the solubility in water may be determined in separate tests. With these solubilities, the quotient logarithm of solubilities in n-octanol and in water (log (Soct/Sw)) can be calculated. Although ECHA (2008) admits that this quotient is not identical to log Kow, as the latter is related to the partitioning of the substance in water-saturated n-octanol and n-octanol-saturated water, it is recommended that this method be considered for sparingly soluble substances. Therefore, it is considered that a log (Soct/Sw) parameter would reasonably represent the octanol–water partition coefficient (Kow) for organic pigments. This approach has been used in previous screening assessments on pigments (e.g., Environment Canada and Health Canada 2009a, b) and is also used in this report.
Within the entire group of 33 Monoazo Pigments, the log (Soct/Sw) values, based on experimental solubility values in water and in n-octanol, vary in a wide range, from less than or equal to 0.2 to 4.6 (see Table 3-1 and Appendix 2). Data comparison between the subsets of substances indicates that, overall, the log (Soct/Sw) values of pigment lakes are very low; for example, four of five pigment lakes have log (Soct/Sw) values of less than or equal to 0.2, and only one pigment lake (PR57:1*) has higher organic carbon–water partition coefficient values of 0.65–0.94 (see Appendix 2). In contrast, monoazo yellow pigments have log (Soct/Sw) values of 2.0–4.6, and β-naphthol pigments, 2.4–3.7 (Table 3-1; Appendix 2).
Similar to melting points, data variability for a log (Soct/Sw) parameter might also be significant; for example, within the group of naphthol AS pigments, the log (Soct/Sw) values vary from 0.4 to 3.2 (Table 3-1; Appendix 1).
Only one experimental study on organic carbon–water partition coefficients (Koc) is available, indicating that PY1 is characterized by a quite high log Koc value of 5.5.
Since octanol–water (Kow) and organic carbon–water (Koc) partition coefficients are important parameters in terms of bioaccumulation of the substances, they will be discussed in more detail in the Potential for Bioaccumulation section of this report.
3.5 Calculated Cross-sectional Diameters
The particulate nature and low water solubility of organic pigments lead to their very limited bioavailability. At the same time, the water-soluble fraction of a pigment, even if it is a very small proportion of the substance, may theoretically pass through biological membranes. Permeability across most biological membranes depends on a variety of factors, one of them being the molecular diameter of the substance.
In terms of cross-sectional diameters of molecules of Monoazo Pigments, they have average effective diameters (Deff) of 0.9–1.2 nm; average maximum diameters (Dmax) vary within the range 1.4–1.8 nm, and average minimum diameters (Dmin) are 0.8 nm or less (see Table 5-2 in the section, 5.2 Potential for Bioaccumulation). Since these parameters are important in terms of the permeation of substances through biological membranes and the process of bioaccumulation, more detailed discussion on the cross-sectional diameters of these pigments is presented in the Potential for Bioaccumulation section of this report.
3.6 Impurities
Some substances, such as resins, rosins, aliphatic amines and other compounds, such as surfactants, dispersing agents and coupling agents, are common additives used in pigment preparations, depending on the application of the pigments. It is impossible to remove such impurities by pigment filtration and intensive washing, and even the effect of hot extraction procedures tends to be slow and unsatisfactory (Herbst and Hunger 2004). It is possible that certain amounts of these substances were present in some pigments that were tested. If so, this could cause data variability and inconsistency between studies (e.g., biodegradability or water solubility studies).
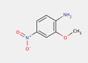
In most cases, the impurities in the pigments are not specified. However, for one substance from this subgroup of 33 Monoazo Pigments, namely PR112, information on purity and impurities is available. According to ECHA (2012), purities of 96–100% and 90–100% for this pigment are reported, and an impurity component is the substance 3-hydroxy-2′-methyl-2-naphthanilide (CAS RN 135-61-5), having the structure:

There is some uncertainty as to whether the purity data reported in different sources of information fully represent the range of available grades of Monoazo Pigments used in products in Canada. It is therefore possible that lower-quality grades may result in exposure to these and other potential impurities at higher values than reported here.
4. Sources and Uses
4.1 Sources
All 33 Monoazo Pigments are anthropogenically produced; they are not expected to occur naturally in the environment.
In recent years, the 33 Monoazo Pigments have been included in industry surveys issued pursuant to section 71 of CEPA 1999. These surveys aimed to collect information on manufacturing and import activities in Canada based on a 100 kg/year reporting threshold. Sixteen substances were surveyed for the reporting year 2005 (Canada 2006), and 11 of these substances were resurveyed as part of Canada’s Challenge Initiative for the reporting years of 2006 and 2007 (Canada 2007, 2008). Twenty substances were included in a survey conducted for the 2010 calendar year that focused on the Aromatic Azo and Benzidine-based Substance Grouping (Canada 2011).
The results of the surveys showed that 21 Monoazo Pigments are imported or manufactured in quantities greater than 100 kg/year in Canada. Among them, 14 Monoazo Pigments were identified with manufacture or import activity in the calendar year 2010 (Canada 2011), and 7 pigments were identified from other recent surveys (Environment Canada 2006, 2007b, 2008), indicating that the total manufacture and import quantity for these substances is in the range of 100 000–1 000 000 kg/year. These activities were reported in the following sectors, listed from highest to lowest volume: Paints and Coatings; Basic Chemical Manufacture; Ink, Toner, and Colourants; Plastic and Rubber; Agricultural; Food Packaging; Textile and Leather; Building Materials; Adhesives and Sealants; and Cleaning and Furniture (Environment Canada 2006, 2007b, 2008, 2012).
| Monoazo Pigment subset | Substance | Year 2005 (Canada 2006) |
Years 2006–2007 (Canada 2007a,2007b) |
Year 2010 (Canada 2011) |
|---|---|---|---|---|
| β-Naphthol pigments | PR3 | X | ||
| β-Naphthol pigments | PR4 | X | X | |
| β-Naphthol pigments | PO5 | X | X | |
| β-Naphthol pigments | NONPA | X | ||
| β-Naphthol pigment lakes | PR49:1 | X | ||
| β-Naphthol pigment lakes | PR53:1 | X | ||
| BONA pigment lakes | PR48:2 | X | ||
| BONA pigment lakes | PR52:1 | X | ||
| BONA pigment lakes | PR52:2 | X | ||
| BONA pigment lakes | PR63:1 | X | ||
| Monoazo yellow pigments | PY1 | X | ||
| Monoazo yellow pigments | PY3 | X | ||
| Monoazo yellow pigments | PY73 | X | ||
| Naphthol AS pigments | PR5 | X | ||
| Naphthol AS pigments | PR112 | X | ||
| Naphthol AS pigments | PR170 | X | ||
| Naphthol AS pigments | PR187 | X | X | |
| Naphthol AS pigments | PR266 | X | ||
| Naphthol AS pigments | PO38 | X | X | |
| Benz-imidazolone pigments | PO36 | X | ||
| Naphthol AS pigment lakes | PR247:1 | X | X |
4.2 Uses
Monoazo Pigments are used in a wide variety of sectors, identified in the previous section. These uses, as well as uses identified outside of section 71 of CEPA 1999, include the following: cosmetics, paints and coatings, textile and leather, food packaging, colouring agents in drugs and natural health products (NHPs), formulants in pest control products, military applications, and in tattoo inks. These uses are discussed further below.
Cosmetics
Based on notifications submitted under the Cosmetic Regulations to Health Canada, PY1, PY3, PY73, PR4, PR49:1, PR53:1, PR63:1 and PR112 are used in certain cosmetic products in Canada (2011 and 2013 emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced ). Cosmetic products containing these substances include facial makeup; body cream, lotion or moisturizers; hair conditioners; hair dyes; fragrances; shaving cream; soaps; bath products; nail polish; face paints; and lipstick (refer to Appendix F for more details).
PR3 and PO5 are included on the List of Prohibited and Restricted Cosmetic Ingredients (more commonly referred to as the Cosmetic Ingredient Hotlist or simply the Hotlist), an administrative tool that Health Canada uses to communicate to manufacturers and others that products containing certain substances are unlikely to be classified as a cosmetic under the Food and Drugs Act (FDA), and in addition, that certain substances, when present in a cosmetic, may contravene the general prohibition found in section 16 of the Food and Drugs Act or a provision of the Cosmetic Regulations (Health Canada 2011b).
Paints and Coatings
Nineteen Monoazo Pigments (β-napthhol pigments PR3, PR4, PO5, NONPA; β-napthhol pigment lakes PR49:1, PR53:1; BONA pigment lakes PR48:2, PR52:1, PR52:2, PR63:1; monoazo yellow pigments PY1, PY3, PY73; napthol AS pigments PO38, PR5, PR112, PR170, PR266; other PO36) were identified for use in paints and coatings in Canada based on the information submitted pursuant to section 71 (Environment Canada 2006, 2007b, 2008, 2012). Based on the information submitted pursuant to section 71, a number of Monoazo Pigments used in paints and coatings were also identified to be used in a product or manufactured item intended for use by or for children although specific product information was not provided (Environment Canada 2012). However, while limited details were available on the specific products for the paints and coatings uses submitted under the section 71 notifications, other information sources were also considered to determine reasonably foreseeable products nd concentrations for these substances. Rather than an exhaustive list of all potential products associated with use in paints and coatings, this section focusses on the uses most relevant for children (finger paints, other arts and crafts materials including poster paints, face paints, paints in children’s toys) and additional uses for the general population (spray paint) (see Appendix G)
- Finger paints: The use in finger paints in the United States was identified for some substances in the subsets of β-napthhol pigments (PR4, PO5), β-napthhol pigment lakes (PR49:1), BONA pigment lakes (PR48:2), monoazo yellow pigments (PY1, PY3, PY73), and Naphthol AS pigments (PR5, PR112, PR170) (personal communication, email from Duke University Toxicology Program to the Existing Substances Risk Assessment Bureau [Health Canada], dated 2013; unreferenced). Recent product testing in Europe also identified PY1, PY3, PR4, PO5, PR5 and PR112 in finger paints (Hauri 2006, 2008, 2009a, 2010a, 2011a). The European Standard EN71-7 (Safety of Toys, Part 7: Finger paints – requirements and test methods) also lists PY1, PY3, PR5, PR170, PO38, PR48:2 and PR63:1 as colourants allowed for use in finger paints in the European Union (EU) (BS 2002). Therefore, based on the information above, these monoazo pigments are considered to have reasonable foreseeable use in finger paints in the Canadian market (Appendix G, Table G-1).
- Face paints: Based on notifications submitted under the Cosmetic Regulations to Health Canada, PR4 and PY1 are used as ingredient in face paint products (body makeup) in Canada (personal communication, emails from the Consumer Product Safety Directorate [Health Canada] to the Existing Substances Risk Assessment Bureau [Health Canada], dated 2011 and 2013; unreferenced). However, since face paint is considered as a cosmetic covered under the Cosmetic Regulations, it is assumed that other monoazo pigments notified for uses in cosmetics may also have reasonable foreseeable uses in face paints in Canada (see Cosmetics section above: PY1, PY3, PY73, PR4, PR49:1, PR53:1, PR63:1 and PR112). In addition to the concentration ranges for PR4 and PY1 in face paints notified under the Cosmetic Regulations to Health Canada, evidence from international material safety data sheets (MSDS) indicates that pigments in general are found in face and body paints at a concentration range of 1–15% (Derivan 2012; Mont Marte International Ltd. 2012a). (Appendix G, Table G-2)
- Other arts and crafts materials: In addition to finger paints above, the available information indicates uses of monoazo pigments in various arts and crafts materials. Product testing by the Danish EPA found PR3 in acrylic poster paints a concentration of 10% (Hansen et al. 2008) while evidence from material safety data sheets (MSDS) indicates that PR63:1, PY1 and PY3 are also used in children’s acrylic and water colour paint products (Mont Marte International Ltd. 2009a, 2009b). Another source indicated several of the monoazo pigments to be “recommended” and/or “suitable” for application in art, creative and school materials including oil paints, aquarelle and gouache paints, acrylic paints, pencil, wax crayons and chalk (Clariant 2011). Product testing testing in Switzerland also found PY1, PY112, PR5 and PR53 in children’s paly dough (Hauri 2009b). Collectively, the above information supports the reasonable foreseeable use of monoazo pigments in these types of arts and crafts materials (see Appendix G, Table G-4)
- Paints in children’s toys: Several sources indicate the potential use of other Monoazo Pigments in toy paints. PR49:1 is listed as being used in toys in Hawley’s Condensed Chemical Dictionary (Lewis 2007), and the supplier of this substance stated that its organic pigments are widely used, including in toys (LookChem 2008). An industry MSDS indicates the use of β-naphthol pigment lakes for paints on toys (Siegwerk 2012). A North American pigment industry monograph also indicates several Monoazo Pigments that may be used for paints on toys including PR3, PO5 and PR112 (Clariant 2013).
- Spray Paint: For the purposes of this Screening Assessment, it is assumed that all nineteen Monoazo Pigments identified for use in paints and coatings in Canada based on the information submitted pursuant to section 71 may be used in spray paints. Generic concentrations of azo pigments in paints and coatings have been reported to range from 3% to 60% (IARC 2010b), however the actual pigment concentration is expected to be dependent on the specific paint and coating use. Available information indicates that some Monoazo Pigments (PR170 and PY73) are used in spray paint at a concentration of 5% (Household Products Database 1993–; Rust-Oleum Corp. 2006).
Food Packaging
In Canada, food colouring agents are regulated as food additives under the Food and Drug Regulations (Canada [1978]). Colours that are permitted for use in food are included in the List of Permitted Colouring Agents, incorporated by reference in the Marketing Authorization for Food Additives that May be Used As Colouring Agents, issued under the authority of the Food and Drugs Act (Canada 1985). None of the monoazo pigments in this Screening Assessment are included on the List of Permitted Colouring Agents as a permitted food colouring agent.
Eight substances (PO5, PO38, PR53:1, PR112, PR170, PR187, PR266 and PR268) in this Screening Assessment were identified for use in food packaging materials in Canada (personal communication, email from the Food Directorate [Health Canada] to the Risk Management Bureau [Health Canada], dated 2011; unreferenced). A summary of the uses of Monoazo Pigments in food packaging is given in Table 4-2.
| Substance | Use information |
|---|---|
| PO5 | Used in the manufacture of printing inks and coatings with no direct food contact. No exposure is expected. |
| PO38 | Used in one ink system for food packaging materials with no food contact. No exposure is expected. |
| PR187 | Component in polyolefin base colour concentrates, which may be used in the manufacture of containers, closures, films and resins and have direct contact with all types of food, excluding milk. The exposure is not expected to be significant. |
| PR170 | Generally used in inks with no direct food contact. One application with minimal direct food contact for use in the formulation of plastic casing for packaging sausages. The exposure is not expected to be significant. |
| PR53:1 | Component of inks with no direct food contact and colour concentrates with few applications in direct contact with food. The exposure is not expected to be significant. |
| PR112 | Component of inks and paints with no direct food contact. No exposure is expected. |
| PR268 | Component of inks with no direct food contact. No exposure is expected. |
| PR266 | Component of inks with no direct food contact. No exposure is expected. |
Colouring Agents in Drugs and Natural Health Products (NHPs)
Colouring agents permitted to be used in drugs in Canada are regulated under Part C, Division 1, of the Food and Drug Regulations (Canada [1978]). PR4 (identified as FLAMING RED (D & C Red No. 36; C.I. No. 12085) is listed in the Food and Drug Regulations as a colouring agent permitted in drugs for internal and external use; and PR63:1 (identified as DEEP MAROON (D&C Red No. 34; C.I. No. 15880:1) is listed in the Food and Drug Regulations as a colouring agent permitted in drugs for external use. None of the Monoazo Pigments in this Screening Assessment were identified as being present in human pharmaceuticals, veterinary drugs or biologics in Canada (DPD 2010; personal communication, email from the Therapeutic Products Directorate [Health Canada] to the Risk Management Bureau [Health Canada], dated 2011, unreferenced; personal communication, email from the Biologics and Genetic Therapies Directorate [Health Canada] to the Risk Management Bureau [Health Canada], dated 2011, unreferenced).
PR5, PR63:1 (listed as D&C Red No. 34) and PR4 (listed as D&C Red No. 36 or Flaming Red) are listed in the Natural Health Products Ingredients Database (NHPID) with a non-medicinal ingredient role in natural health products as colour additives (NHPID 2011). The NHPID listing for PR4 also specifies that it is permitted for use in oral products, up to 1.0 mg/day, unless additional evidence for safety is submitted; while PR5 and PR63:1 are listed as for topical use (NHPID 2011). PR4 and PR63:1 are listed in the Licensed Natural Health Products Database (LNHPD) as being present as non-medicinal ingredients in currently licensed natural health products. PR5 was not found in any currently licensed natural health products (LNHPD 2008).
Formulants in Pest Control Products
Nine Monoazo Pigments are used as formulants in pest control products registered in Canada under the Pest Control Products Act (XXXX) a CEPA equivalent legislation (personal communication, emails from the Pest Management Regulatory Agency [Health Canada] to the Risk Management Bureau [Health Canada], dated 2011 and 2013; unreferenced), as shown in Table 4-3 are not further considered in this assessments.
| Substance | Use | Concentration (%) |
|---|---|---|
| PR3 | Antifouling paint | 0.965–3.39 |
| PR63:1 | Fungicides for plant disease control | 0.027–0.34 |
| PR112 | Antifouling paint | 1.692 |
| PR48:2 | Fungicides, seed treatments, insecticides, rodenticides | 0.015–10.33 |
| PO36 | Antifouling paint, wood preservatives | 1.25–1.47 |
| PY73 | Antifouling paint, fly baits, wood preservatives | 0.0746–1.25 |
| PR247:1 | Flea and tick collars | 0.3 |
| NONPA | Antifouling paint | 0.018 |
| PR187 | Antifouling paint | 1.9–3.9 |
Tattoo Inks
Basedon notifications submitted under the Cosmetic Regulations to Health Canada,PR4, PR112 and PY3 are used in permanent tattoo inks (personal communication, email from the Consumer Product Safety Directorate [Health Canada] to the Existing Substances Risk Assessment Bureau [Health Canada], dated 2011 and 2013; unreferenced). The monoazo pigments PR63:1, PR170, and PY3 are also listed as ingredients in the MSDS sheets of two brands of tattoo inks available internationally including in Canada (SkinCandy 2013a, 2013b; Starbrite 2013). The Color Pigment Manufacturers Association (CPMA), representing importers and manufacturers of azo pigments in Canada, have indicated that in Canada, their members do not knowingly supply these substances for use in tattoo inks (CPMA 2013).
In studies from Europe, the uses in tattoo inks of several monoazo pigments from this assessment (PR3, PR4, PR5, PR49:1, PR53:1, PR63:1, PR112, PR170, PO5, PO36, PY1, PY3) have also been reported (Bäumler et al. 2000; NVWA 2008; De Cuyper and D’hollander 2010; Hauri 2010b, 2010c, 2011b; Høgsberg et al. 2010; Danish EPA 2012) (see Appendix 6, Table F-6).
Other Uses
Five Monoazo Pigments (PY3, PR4, PR112, PR187, and PO5 are identified as being used in textiles and leather, based on a recent section 71 survey (Environment Canada 2012a).
Two Monoazo Pigments were identified for use in military applications in Canada: PR49:1 is used as part of a corrosion prevention compound, and PR170 is used in an enamel for resistance to fire, corrosion and dirt (2011 email from the Department of National Defence to the Risk Management Bureau, Health Canada; unreferenced).
NONPA was identified as being used in Canada based on information submitted by the Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers (ETAD) (2010 email from ETAD to Environment Canada; unreferenced). The end use product was not confirmed.
5. Environmental Fate and Behavior
The environmental fate and behavior of chemicals describes the processes by which they move and are transformed in the environment. Environmental fate processes that are usually addressed include persistence of the substances in environmental compartments, their degradation, their distribution among media, their migration in groundwater, their removal from effluents by standard wastewater treatment methods and their bioaccumulation in organisms.
However, the combination of variability between chemicals and between environments creates such complexity that it is challenging to readily survey the set of properties of the chemical and forecast how a specific chemical is likely to behave (Mackay et al. 2001). While certain attributes of chemicals in the environment (e.g., concentrations) can be measured directly, other attributes (e.g., evaporation rates or distance travelled) cannot be measured directly and can only be estimated using models. However, present models do not always satisfactorily address some chemicals, such as pigments (Mackay et al. 2009).
It must also be emphasized that the majority of organic pigments generally do not exist as individual molecules but are principally particles in the sub- or low-micrometre size range. The pigment powder is typically composed of primary particles (i.e., the crystal lattice of a pigment), aggregates and agglomerates.
Therefore, taking all this information into account, it was proposed not to use fugacity modelling for describing the distribution of pigments between environmental compartments. It was also believed that reasonably reliable conclusions on the environmental fate of these pigments could be made taking into consideration available experimental information on the physical-chemical properties of Monoazo Pigments.
As has already been mentioned, Monoazo Pigments have very low water solubility, in the sub- or low-microgram per litre range (see Table 3-1). Also taking into account that these pigments are principally particles in the sub- or low-micrometre size range, it may be supposed that when released into water, these substances would be mostly present as particles or adsorbed to other suspended solids and therefore would eventually sink to bed sediments.
Direct releases of Monoazo Pigments to air are not expected to be significant; however, even when such releases occur, these substances are not expected to reside in this environmental compartment. Indeed, even in a theoretical worst-case scenario (i.e., if pigments are released as molecules, not as particles), the pigments can be expected to have very low vapour pressures. While experimental data on vapour pressure are not available for most of the pigments, these substances can be expected to have vapour pressures in the range typical of the structurally similar disperse dyes (i.e., from 10−11 to 10−9Pa, as indicated by Baughman and Perenich 1988).
Another reason for volatilization to be unlikely for the uncharged pigments is that the escaping tendency or fugacity that drives volatilization is also the driving force for both sorption and bioconcentration (Baughman and Perenich 1988).
The particulate character of Monoazo Pigments should have a key influence on their fate in the environment; together with their density (higher than the density of water), their high chemical stability and their extremely low aqueous solubility, this suggests that Monoazo Pigments will partition by gravity to sediments if released to surface waters and will tend to remain in soils if released to terrestrial environments.
Therefore, for this subgroup of Monoazo Pigments, soil and sediments are expected to be the two major environmental media of concern.
Because of their low solubility in water, Monoazo Pigments may be considered not available for uptake by organisms and not available for biodegradation. In addition, when incorporated into the materials they are destined to colour, these pigments are probably no longer in a form easily available to biota (Øllgaard et al. 1998; Herbst and Hunger 2004).
It should also be noted that, based on the information on the physical-chemical properties and uses of Monoazo Pigments, air emissions of these substances are not expected to occur. Therefore, long-range atmospheric transport potentials of these substances from their emission sources were not calculated.
5.1 Persistence and Bioaccumulation Potential
5.1.1 Environmental Persistence
In order to evaluate the environmental persistence of the substances in the Monoazo Pigments subgroup, empirical data, modelled data and information available for structural analogues were considered.
It was expected that the characteristics imparted to pigments would result in Monoazo Pigments being persistent in the environment. For example, the Color Pigments Manufacturers Association (CPMA 2003) has indicated that pigments are designed to be durable or persistent in the environment in order to provide colour to finished products (e.g., coatings, inks, paints and others).
5.1.2 Biodegradation in the Aquatic Environment
The results of several biodegradation studies are available for some of the substances in this subgroup of Monoazo Pigments. These are summarized in Table 5-1 (see Appendix 3 for the detailed, substance-specific information).
The results of five studies (Appendix 3) indicate that the biodegradation values of monoazo yellow pigments and BONA pigment lakes are quite low--from 0% to 14%. The results of the biodegradation studies on PR53:1, representing the subset of β-naphthol pigment lakes, are somewhat contradictory--0% and 33% (Table 5-1; Appendix 3).
| Subset (substance) | Biodegradation value (%) | Test duration (days) | Type of biodegradability | Reference |
|---|---|---|---|---|
| Monoazo yellow pigments (PY1) | 14 | 28 | Inherent | Study Submission 2012a |
| BONA pigment lakes (PR48:2; PR57:1*) | 0–12.9 | 28 | Ready | MITI 1992; ECHA 2012 |
| β-Naphthol pigment lakes (PR53:1) | 0; 33 | 14; 21 | Ready (0); inherent (33) |
MITI 1992; European Commission ©2000a |
The relatively high biodegradation values (12.9%; 14%; 33%) could probably be attributed to the biodegradation of organic impurities or additives in the pigment rather than to the pigment component itself. Indeed, resins, rosins, aliphatic amines and other compounds, such as surfactants, dispersing agents and coupling agents, are common additives used in pigment preparations, depending on the application of the pigments (Herbst and Hunger 2004). Importantly, azo pigments are very difficult to purify; in particular, it is almost impossible to remove some impurities by pigment filtration and intensive washing, and even the effect of hot extraction procedures tends to be slow and unsatisfactory. Considerable amounts of such soluble species may remain within the pigment even after hours of refluxing and repeated filtration with freshly distilled water (Herbst and Hunger 2004). In most cases, the impurities in the pigments are not specified; however, for PR112, the substance 3-hydroxy-2′-methyl-2-naphthanilide is reported as an impurity component, while the purity of the pigment is reported as 90–100% (ECHA 2012).
Therefore, biodegradation of impurities/additives--not the pigment components--in the biodegradability studies could result in higher than expected biodegradation values.
However, the purity of the pigments may not be the only reason for the relatively high biodegradation values in some studies. For example, the two highest biodegradation values (14% and 33%; see Table 5-1) are reported in the inherent biodegradation studies, while ready biodegradability protocols were used in all other tests (lower biodegradation values). Therefore, the differences in the test procedures of these two types of biodegradability tests may explain the differences in the data obtained.
Indeed, the ready biodegradability tests are stringent screening tests, conducted under aerobic conditions, in which the inoculum should not have been pre-adapted to degradation of the test substance by previous exposure to the test substance or structurally related chemicals. On the contrary, inherent biodegradability tests allow prolonged exposure of microorganisms to the test substance and a low ratio of test substance to biomass, which offers a better chance to obtain a positive result compared with tests for ready biodegradability. Some of these tests may be conducted using microorganisms that have previously been exposed to the test substance, which frequently results in adaptation, leading to a significant increase in the degradation rate (OECD 2005). As a result, biodegradation values from inherent biodegradability studies are usually higher (and sometimes much higher) than values from ready biodegradation tests.
Therefore, it may be supposed that in the inherent biodegradation studies (i.e., under the most favourable conditions) with the Monoazo Pigments, organic impurities or additives may degrade, resulting in higher than expected biodegradation values, which do not reflect the “actual” degradability of the pigment component in the natural environment.
Adsorption of pigments on sludge (used as inoculum, i.e., the source of microorganisms in the test) could be another explanation for the relatively high biodegradation value of 33%, not typical for the pure pigments. Indeed, the study with PR53:1 contained the important statement that in the test, 10% of dissolved organic carbon (DOC) elimination occurred due to adsorption onto sludge (see Appendix 3 for more details).
Therefore, the presence of organic impurities/additives and their further biodegradation, the type of biodegradation test (i.e., inherent vs. ready biodegradability) and the adsorption of DOC on the inoculum (sludge) would be the major factors explaining the higher than expected biodegradation values in some biodegradation tests with the Monoazo Pigments in the water compartment. As a consequence, such studies are not considered as key studies, and their results were not used in the risk assessment of Monoazo Pigments.
Results of biodegradability tests are not available for all of the subsets of pigments (or individual pigments) in the subgroup of 33 Monoazo Pigments. However, considering that the results of ready biodegradability studies with the pure pigments are very low (usually less than 10–13%, and often 0%) for three subsets of this Monoazo Pigments subgroup (see Table 5-1 and Appendix 3), as well as for a group of diarylide yellow pigments that have been previously assessed by Environment Canada and Health Canada (2014c), it may be assumed that all Monoazo Pigments are not readily biodegradable.
Thus, considering all this information, it may be concluded that under aerobic conditions, Monoazo Pigments are expected to be not readily biodegradable in water..
The environmental persistence of Monoazo Pigments in anoxic environments is an important area of uncertainty because of a lack of data for the pigments. While some azo dyes are reported to be biodegradable in anoxic waters via anaerobic reduction of the azo bond (–N=N–), which results in potentially harmful aromatic amines (Øllgaard et al. 1998), almost no documentation has been found regarding the potential for the anaerobic degradation of azo pigments in aqueous environments. In principle, the pigment crystals would have to dissolve first, which would release their constituent molecules to the aqueous medium and make the azo bonds available for biotic reduction (Øllgaard et al. 1998). However, it may be expected that only a small proportion of the Monoazo Pigments may be reduced in this manner, given their unique physical state--pigments are typically composed of primary particles (i.e., the crystal lattice of a pigment), as well as aggregates and agglomerates--along with their low water solubility, which would limit the availability of the molecules for biotic reduction.
5.1.3 Biodegradation in Soil and Sediments
No studies on the biodegradation of Monoazo Pigments in soil and sediments have been identified. However, approximate soil and sediment half-lives can be derived using the recommended values for water multiplied by scaling factors. Scaling factors are numbers that, when multiplied by a degradation rate constant or half-life for one set of environmental or test conditions, yield a rate for a second, different set of conditions (US EPA 2000).
Boethling et al. (1995) collected measured half-life data for a wide variety of chemicals that had been tested in both soil and water samples collected from the environment and then calculated mean ratios of half-life in water to half-life in aerobic surface soil for 20 chemicals. It was suggested that for screening purposes, it is valid to assume that biodegradation in aerobic surface water is about as fast as degradation in aerobic surface soil and that sediment half-lives may be assumed to be 3–4 times longer (US EPA 2000).
Therefore, in terms of biodegradation half-life, using a water-to-soil-to-sediment extrapolation ratio of 1:1:4 (Boethling et al. 1995) and the ultimate biodegradation half-life in water of greater than or equal to 182 days, based on experimental biodegradation values of 0–13% (for pure Monoazo Pigments).
In anaerobic sediment conditions, solubility-limited azo reduction may occur. However, given the unique physical-chemical characteristics of Monoazo Pigments (particulate nature, low water solubility), it is expected that only a very small proportion of these pigments may be available to microorganisms for biotic reduction.
Therefore, taking into account that Monoazo Pigments generally do not exist as individual molecules but are principally particles in the sub- or low-micrometre size range and that the water solubility of these pigments is low (sub- to low micrograms per litre), the bioavailability of these substances to microorganisms for biotransformation (biotic reduction) is expected to be very limited. This is confirmed by the results of multiple ready biodegradability studies with the pigments from the three subsets of this Monoazo Pigments subgroup (see Table 5-1 and Appendix 3), as well as substances representing a group of diarylide yellow pigments (see Environment Canada and Health Canada 2014c). In soil and sediments, these substances are also expected to be not readily biodegradable.
5.1.4 Abiotic Degradation
No experimental data have been found for the photodegradation of Monoazo Pigments in air. The predictions using the AOPWIN model from EPISuite v.4.10 (AOPWIN 2010) indicate that the calculated half-lives (indirect reaction with hydroxyl radicals based on a 12-hour day) of most pigments were relatively short, only 0.6–11.5 hours; only three substances (PY3, PR52:2 and NSNAC) have slightly longer half-lives of 24–26 hours.
Monoazo Pigments are expected to be hydrolytically stable. For example, in a study on PR57:1* (analogue, representing the subset of BONA pigment lakes), the pigment was not hydrolyzed at pH 4, 7 and 9 (MITI 1992).
5.1.5 Summary of Persistence in the Environment
Based on empirical data (Table 5-1 and Appendix 3) and the above-mentioned considerations, it is expected that the 33 Monoazo Pigments are persistent in water, soil and sediment, but not in air (due to reactions with hydroxyl radicals).
The particulate character and unique physical-chemical properties of Monoazo Pigments (low solubility, chemical and hydrolytic stability, low ready biodegradability) suggest that they will partition to sediments (if released to surface waters), and will have limited bioavailability. As a result, their bioaccumulation in aquatic organisms and aquatic toxicity are expected to be relatively low.
5.2 Potential for Bioaccumulation
In order to evaluate the bioaccumulation potential of substances in the subgroup of Monoazo Pigments, only empirical data were considered, given that the lack of structural coverage of pigments in current bioaccumulation models results in a high level of uncertainty associated with model predictions. A weight of evidence approach, involving the intrinsic properties of the substances, and the available empirical bioaccumulation data were used to determine the overall bioaccumulation potential of this pigment subgroup.
5.2.1 Physical and Chemical Factors
5.2.1.1 Particulate Nature of Pigments
Monoazo Pigments exist principally as particles in the sub- or low-micrometre size range, and the pigment powder is typically composed of primary particles (i.e., the crystal lattice of a pigment), aggregates and agglomerates. This suggests limited bioavailability of these substances to organisms.
5.2.1.2 Water Solubility
The water solubility of Monoazo Pigments is very low, only sub- to low micrograms per litre. Therefore, the bioavailability of these substances to organisms is expected to be very limited.
5.2.1.3 Octanol–Water and Organic Carbon–Water Partition Coefficients
As indicated in the Physical and Chemical Properties section of this report, a Soct/Sw parameter would more or less reliably represent the octanol–water partition coefficient for organic pigments.
For all subsets of pigment lakes (i.e., BONA pigment lakes, β-naphthol pigment lakes and naphthol AS pigment lakes) and the only benzimidazolone pigment, PO36, representing 9 out of the 33 substances, the log (Soct/Sw) values, based on experimental solubility values in water and in n-octanol, vary within a reasonably narrow range of −0.75 to 0.94 (see Table 3-1 and Appendix 2). These values are significantly lower than the criterion of Kow for bioaccumulation (log Kow greater than or equal to 5) when neither the bioaccumulation factor (BAF) nor the bioconcentration factor (BCF) of the substance can be determined in accordance with a method referred to in section 5 of the Persistence and Bioaccumulation Regulations (Canada 2000). Therefore, based on the experimental log (Soct/Sw) values for these nine substances, they have a low potential to bioaccumulate in organisms.
In contrast to the pigment lakes, non-lake pigments have, overall, higher log (Soct/Sw) values, which, at the same time, are still lower than the above-mentioned criterion of Kow for bioaccumulation (log Kowgreater than or equal to 5). For example, PR5, PR187, PR266 and PO38, representing the subset of naphthol AS pigments, have log (Soct/Sw) values of 0.4–1.7, while log (Soct/Sw) values for PO5, PR112 and PY3, representing three different subsets of the 33 Monoazo Pigments, are a bit higher, from 2.4 to 2.9 (Appendix 2).
Only three pigments have relatively high values of the quotient logarithm of the molar solute concentrations in n-octanol and water: PR3 and PR4 (subset of β-naphthol pigments), having log (Soct/Sw) values of 3.5–3.7, and PY1 (subset of monoazo yellow pigments), characterized by quite a high log (Soct/Sw) value of 4.6 (Appendix 2). Even so, these results are lower than the criterion of Kowfor bioaccumulation (log Kow greater than or equal to 5).
Only one experimental study on organic carbon–water partition coefficients (Koc) is available, indicating that PY1 (subset of monoazo yellow pigments) is characterized by a quite high log Koc value of 5.5 (see Table 3-1). According to the results of the study, the calculated log Koc value is outside the calibrated range, which means that the result can be regarded only as an order of magnitude value instead of an absolute value. Even so, considering the order of magnitude range, both values (i.e., log (Soct/Sw) = 4.6 and log Koc = 5.5) are in good agreement, showing that both partition coefficients are close to the criterion of Kowfor bioaccumulation (log Kow greater than or equal to 5) when neither the BAF nor the BCF of the substance can be determined.
Therefore, based on the experimental log (Soct/Sw) values, it is expected that all Monoazo Pigments except PY1 (subset of monoazo yellow pigments) have a low potential to bioaccumulate in organisms.
5.2.1.4 Molecular Size and Cross-sectional Diameters
In terms of bioaccumulation, it is also useful to consider molecular size and cross-sectional diameters, which are commonly used by international jurisdictions in the weight of evidence for conclusions on bioaccumulation potential. For example, ECHA (2008), describing “Indicators for limited bioaccumulation,” showed that some additional indicators of low bioaccumulation potential, in particular, an average Dmax of greater than 1.7 nm, might be applicable for substances with low solubility in n-octanol and water.
Investigations relating fish BCF data and molecular size parameters (Dimitrov et al. 2002, 2005) suggest that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing Dmax: the probability decreases appreciably when Dmax is greater than ~1.5 nm, and much more so when Dmax is greater than 1.7 nm. Sakuratani et al. (2008) also investigated the effect of cross-sectional diameter on passive diffusion in a BCF test set of about 1 200 new and existing chemicals. They observed that substances that do not have a very high bioconcentration potential (i.e., BCF less than 5 000) often have a Dmax of greater than 2.0 nm and a Deffof greater than 1.1 nm. Anliker et al. (1988) proposed that a second largest cross-sectional diameter of more than 1.05 nm with a molecular weight of greater than 450 g/mol would suggest a lack of bioconcentration for organic colourants. Therefore, Deffvalues of greater than 1.05–1.1 nm and Dmax values of greater than 1.5–1.7 nm can be considered indicators for the potential for a reduced rate of uptake from water. A reduced rate of uptake allows other internal elimination processes, such as metabolism and fecal egestion, to reduce the overall burden of chemical in the tissues of organisms, reducing bioaccumulation on a whole-body basis.
It is acknowledged that synthetic organic pigments have a particulate nature; in general, they are characterized by very low water solubility and therefore by very limited bioavailability. At the same time, the water-soluble fraction of a pigment, even though it constitutes a very small proportion of the substance, may theoretically pass through biological membranes. Therefore, molecular diameters of pigments can be related to their permeability across most biological membranes.
Table 5-2 presents estimates of average Deff, Dmax and Dmin of some Monoazo Pigments, calculated by the BCFmax model with mitigating factors (Dimitrov et al. 2005). The calculations of cross-sectional diameters of Monoazo Pigments include the analysis of up to 30 conformers.
| Subset (number of pigments) | Average Deff (nm) | Average Dmax (nm) | Average Dmin (nm) |
|---|---|---|---|
| Monoazo yellow pigments (n = 3) | 1.0 | 1.5 | 0.7 |
| β-Naphthol pigments (n = 6) | 0.9 | 1.4 | 0.6 |
| Naphthol AS pigments (n = 11) | 1.2 | 1.8 | 0.8 |
| Benzimidazolone pigments (n = 1) | 1.1 | 1.6 | 0.7 |
| BONA pigment lakes (n = 1) | 1.1 | 1.5 | 0.7 |
| Pyrazoloquinazolone pigments (n = 1) | 1.1 | 1.8 | 0.7 |
Data show that cross-sectional diameters of molecules of naphthol AS pigments, BONA pigment lakes, benzimidazolone pigments and pyrazoloquinazolone pigments have average Deff of greater than 1.05 nm. Average Dmax of all subsets of pigments except β-naphthol pigments are greater than or equal to 1.5 nm, while naphthol AS pigments and pyrazoloquinazolone pigments have Dmax of greater than 1.7 nm (Table 5-2). Therefore, from the cross-sectional diameter viewpoint, it can be supposed that monoazo yellow pigments (including PY1, which is characterized by high log (Soct/Sw) and log Koc values) and β-naphthol pigments, whose diameter values do not exceed the threshold values recommended by Dimitrov et al. (2002, 2005) and Sakuratani et al. (2008), will not likely experience restricted uptake from steric effects at the gill surface of fish.
It should, however, be noted that according to Arnot et al. (2010), there are some uncertainties associated with the thresholds proposed by Dimitrov et al. (2002, 2005) and Sakuratani et al. (2008), since the bioaccumulation studies used to derive them were not always critically evaluated. Arnot et al. (2010) pointed out that molecular size influences solubility and diffusivity in water and organic phases (membranes), and larger molecules may have slower uptake rates. However, these same kinetic constraints apply to diffusive routes of chemical elimination (i.e., slow uptake = slow elimination). Thus, significant bioaccumulation potential may remain for substances that are subject to slow absorption processes, if the substances are slowly biotransformed or slowly eliminated by other processes. However, if the rate of gill uptake is sufficiently mitigated by steric hindrance to the point that the rate of elimination exceeds uptake, bioconcentration will be lowered.
Other physiological parameters and processes such as metabolism are important to consider, along with information on Kow(indeed, the substance may be characterized by a high Kow and, at the same time, can be quickly metabolized/biotransformed in the organism). Therefore, Kow data ideally have to be considered along with other information related to the bioaccumulation of these substances.
5.2.2 Bioconcentration Factor (BCF)
For the subgroup of Monoazo Pigments, two experimental BCF studies have been identified. Results of these studies are presented in Table 5-3.
| Substance | Subset | BCF (L/kg) | Test conditions | Reference |
|---|---|---|---|---|
| PR53:1 | β-Naphthol pigment lakes | 0.9–1.8 (at 700 µg/L) | Test duration: 6 weeks; lipid content in fish: 3.4% |
MITI 1992 |
| PR53:1 | β-Naphthol pigment lakes | 8.5–15 (at 70 µg/L) | Test duration: 6 weeks; lipid content in fish: 3.4% |
MITI 1992 |
| PR57:1* | BONA pigment lakes | less than 0.7–1.8 (at 300 µg/L) | Test duration: 6 weeks; lipid content in fish: 3.7% |
MITI 1992 |
| PR57:1* | BONA pigment lakes | less than 6.9 (at 30 µg/L) | Test duration: 6 weeks; lipid content in fish: 3.7% |
MITI 1992 |
Data show that for the pigments PR53:1 and PR57:1*, representing the subsets of β-naphthol pigment lakes and BONA pigment lakes, respectively, all available BCF values do not exceed 15 L/kg in common carp, indicating that these two subsets of Monoazo Pigments have a low potential to bioconcentrate in fish from water. These data are in good agreement with the above-mentioned log (Soct/Sw) values, which are very low for these subsets of pigments.
No experimental data on BCFs for other subsets of the 33 Monoazo Pigments (or their analogues) in aquatic organisms have been identified. At the same time, BCF data available for other groups (classes) of pigments suggest that those pigments also have a low potential to bioconcentrate in fish from water (in fact, no experimental studies with high BCF values--for any pigment--have been located). For example, the results of several BCF studies on diarylide yellow pigments indicate that all available BCF values do not exceed 6.2 L/kg in common carp, which confirms that diarylide yellow pigments have a low potential to bioconcentrate in fish from water (for more details, see Environment Canada and Health Canada 2013d).
ECHA (2008) presented a weight of evidence approach for one of the diarylide yellow pigments--Pigment Yellow 12. Based on low solubility in n-octanol and low log (Soct/Sw), as well as on pharmacokinetic data (14C pharmacokinetic rat study showing no uptake from feed and complete excretion of Pigment Yellow 12 through feces), it was concluded that Pigment Yellow 12 is not a bioaccumulative substance (ECHA 2008).
In the scientific literature and recommendations from international jurisdictions, there are some other data regarding the bioaccumulation of pigments. For example, Anliker and Moser (1987) studied the limits of bioconcentration of azo pigments in fish and their relation to the partition coefficient and solubility in water and n-octanol. Despite a high calculated log Kow for two pigments, the experimentally determined log BCFs were low. The explanation for this apparent inconsistency is the very limited fat (lipid) storage potential of these pigments, as indicated by their low solubility in n-octanol ( less than 1 and less than 0.1 mg/L) and their large molecular size (cross-sectional diameters of 0.97 and 1.68 nm).
In another study, Anliker et al. (1988) assessed different dyes and pigments, including two organic pigments, for which the experimental BCFs in fish were known (16 halogenated aromatic hydrocarbons were included for comparison). None of the disperse dyestuffs, even the highly lipophilic colourants with log Kow greater than 3, accumulated significantly in fish. The authors indicated that the large molecular size of colourants prevents their effective permeation through biological membranes (i.e., gill tissues) and thus limits their uptake during the period of exposure.
Therefore, the experimental results of BCF and pharmacokinetic studies indicate that pigments have limited potential for bioaccumulation in organisms.
5.2.3 Considerations on Other Possible Mechanisms of Pigment Uptake and Bioaccumulation
Another aspect of the bioavailability and bioaccumulation of pigments in non-human organisms may also be considered. Many pigments have a particle size in the nanometre to micrometre range, therefore potentially falling partially within the nanoscale size range (i.e., 1–100 nm) (Canada 2007; Health Canada 2011a). Lynch et al. (2006), Rothen-Rutishauser et al. (2006), Smart et al. (2006) and others indicate that nanoparticles can be taken up by different types of mammalian cells and are able to cross the cell membrane and become internalized. Importantly, the interaction of nanoparticles with the cells and their uptake are size dependent (Limbach et al. 2005; Chithrani et al. 2006) and shape dependent (Pal et al. 2007), and uptake occurs via endocytosis or by phagocytosis in specialized cells.
Passive diffusion is considered the predominant mechanism for the transport of substances across epithelia for most pharmaceuticals and environmental organic contaminants, although facilitated, active, paracellular and phagocytosis (pinocytosis and endocytosis) transport mechanisms can be important for certain substances (DeVito 2000). Passive diffusion rather than a facilitated process controls the absorption of hydrophobic persistent organic pollutants (Kelly et al. 2004).
At the same time, bioaccumulation studies, where endocytosis or phagocytosis mechanisms would be reliably confirmed, could not be identified for the Monoazo Pigments. If these mechanisms were typical (or significant) for pigments, the results of bioaccumulation studies, namely high (or relatively high) BCF values, would reflect the existence of this phenomenon in terms of the pigments’ uptake. However, all available experimental BCF values show that some Monoazo Pigments--in particular, β-naphthol pigment lakes and BONA pigment lakes--as well as diarylide yellow pigments (see Environment Canada and Health Canada 2013d) have a very low potential to bioconcentrate in fish from water.
The results of ecotoxicological studies, which are discussed in the next section of this report, indicate that aqueous dispersions of high-purity Monoazo Pigments do not cause noticeable biological effects. This indicates that the bioavailability of these substances is limited; most likely, phagocytosis does not play a significant role in the uptake of Monoazo Pigments.
5.2.4 Summary of Bioaccumulation Potential
Based on the consistency of various lines of evidence, including low experimental log (Soct/Sw) values ( less than 3) for most Monoazo Pigments (for which experimental solubility data are available), average Deff values of greater than or equal to 1.1 for some pigments and very low experimental BCF values ( less than or equal to 15 L/kg), in general, the substances in the Monoazo Pigments subgroup are not expected to bioaccumulate in aquatic organisms. Importantly, no studies showing high BCF values for any pigments have been identified to date.
At the same time, based on high log (Soct/Sw) and log Koc values (4.6 and 5.5, respectively), PY1 may theoretically be expected to have relatively high bioaccumulation potential; however, the measurement of these properties is itself uncertain, and extrapolation from partitioning properties to a bioaccumulation result within an organism without consideration of the organism’s physiology is also highly uncertain.
Therefore, based on the particulate nature of Monoazo Pigments, their low water solubility, their generally low log (Soct/Sw) values, the relatively high cross-sectional diameters of many of the pigments and the very low experimental BCF values for some of them, it may be expected that Monoazo Pigments have low potential for bioaccumulation in aquatic organisms.
It may also be mentioned that the low potential of Monoazo Pigments to bioaccumulate in fish due to their low bioavailability indicates that low toxicity is expected for this group of substances. This is confirmed by the ecotoxicity data (see next section) and by the lack of significant adverse effects observed in any of the bioconcentration tests.
6. Potential to Cause Ecological Harm
6.1 Ecological Effects Assessment
In order to provide the best possible weight of evidence for assessing the ecological effects of substances in the Monoazo Pigments subgroup, only empirical data were considered, given the high level of uncertainty associated with modelling the ecotoxicity of this substance subgroup.
6.1.1 Aquatic Environment
Both acute and chronic aquatic toxicity studies are available for a group of Monoazo Pigments. Table 6-1 presents a summary of available empirical ecotoxicity data for this group of substances and their structural analogues, while Appendix 4 contains more detailed, substance-specific information on the particular studies. The studies have been conducted with different test systems (static, semi-static and flow-through) at different test durations (acute and chronic tests) using different organisms: fish (zebrafish Brachydanio rerio; medaka Oryzias latipes; common carp Cyprinus carpio), water flea (Daphnia magna) and algae (Desmodesmus subspicatus and Pseudokirchneriella subcapitata, formerly known as Selenastrum capricornutum).
| Subset (substance) | Test type (duration) | Organism | Endpoint, value | Details | Reference |
|---|---|---|---|---|---|
| Monoazo yellow pigments (PY1; PY3) | Acute (96 h) |
Zebrafish (B. rerio) | LC0 = 1 mg/L; LC50 greater than 1 mg/L | Semi-static. Saturated solution of pigment (1 mg/L; purity 99.7%). | Study Submission 2012b |
| Monoazo yellow pigments (PY1; PY3) | Chronic (21 days) | Water flea (D. magna) | NOEC = 1 mg/L | Semi-static. Saturated solution (1 mg/L; purity 99.7%). Effects: mortality; reproduction rate; body weight and length; appearance of first brood; number of broods; other. | Study Submission 2012c |
| Monoazo yellow pigments (PY1; PY3) | Chronic (72 h) | Alga D. subspicatus |
NOEC = 1 mg/L | Static. Saturated solution (1 mg/L; purity 99.7%). Effects: growth rate/yield. | Study Submission 2012d |
| Monoazo yellow pigments (PY1; PY3) | Acute (48 h) |
Water flea (D. magna) | No effects at 100 mg/L | Static. Saturated solution (100 mg/L; purity 99.8%). Effects: immobilization. | Study Submission 2012e |
| Benzimidazolone pigments (PO36) | Acute (96 h) |
Zebrafish (B. rerio) | LC0 = 1 mg/L; LC50 greater than 1 mg/L | Semi-static. Saturated solution of 1 mg/L of pigment (purity 99.5%). | Study Submission 2012f |
| Benzimidazolone pigments (PO36) | Chronic (21 days) | Water flea (D. magna) | NOEC = 1 mg/L | Semi-static. Saturated solution (1 mg/L; purity 99.5%). Effects: adult body weight/length; reproduction rate; appearance of first brood; number of broods. | Study Submission 2012g |
| β-Naphthol pigment lakes (PR53:1) | Acute (96 h) |
Zebrafish (B. rerio) | LC50 greater than 500 mg/L | Static. Five nominal concentrations (7–180 mg/L), DMSO control (0.5 mg/L) and laboratory water control. | CPMA 2006a |
| β-Naphthol pigment lakes (PR53:1) | Acute (48 h) |
Medaka (O. latipes) | LC50 greater than 420 mg/L; LC50 greater than 500 mg/L |
Semi-static. | CPMA 2006a |
| β-Naphthol pigment lakes (PR53:1) | Acute (48 h) |
Water flea (D. magna) | No effects at saturation (EC0 = 2.2 mg/l; EC0 = 3.4 mg/l) |
Saturated solution. Pigment purity: 98.1%. No adverse effects at saturation (at 2.2 and 3.4 mg/L). | European Commission ©2000a |
| β-Naphthol pigments (PR3) | Acute (48 h) |
Water flea (D. magna) | No effects at saturation (at 0.9 and 1.6 mg/L) | Static. Saturated solution; pigment concentration measured by DOC. No adverse effects (immobilization) at saturation. | Study Submission 2007e |
| Naphthol AS pigments (PR112; PR2*; PR253*; PR146*) | Chronic (21 days) | Water flea (D. magna) | NOEC = 1 mg/L | Semi-static. Saturated solution (1 mg/L). Effects: mortality; number of juveniles; appearance of first brood; intrinsic rate of natural increase; growth. | ECHA 2012 |
| Naphthol AS pigments (PR112; PR2*; PR253*; PR146*) | Acute (72 h) |
Alga D. subspicatus | NOEC = 1 mg/L | Static. 1 mg/L saturated solution. Effects: rate-related inhibition; inhibition of yield. | ECHA 2012 |
| Naphthol AS pigments (PR112; PR2*; PR253*; PR146*) | Acute (48 h) |
Water flea (D. magna) | EC50 greater than 100 mg/L | Static. Saturated solution (100 mg/L). No significant effects (immobilization) observed at saturation. | Study Submission 2007a |
| Naphthol AS pigments (PR112; PR2*; PR253*; PR146*) | Acute (96 h) |
Common carp (C. carpio) | LC50 = 172 mg/L | Static. Six test concentrations (not measured). No toxic responses recorded up to a concentration of 90 mg/L. | Study Submission 2007b |
| Naphthol AS pigments (PR112; PR2*; PR253*; PR146*) | Acute (24 h) |
Water flea (D. magna) | EC50 = 990.7 mg/L | Static. Seven concentrations; 10% immobilization observed up to 500 mg/L. | Study Submission 2007c |
| Naphthol AS pigments (PR112; PR2*; PR253*; PR146*) | Acute (48 h) |
Water flea (D. magna) | EC50 greater than 100 mg/L | Static. Saturated solution (100 mg/L). No significant effects (immobilization) observed at saturation. | Study Submission 2007d |
| BONA pigment lakes (PR57:1*) | Acute (24–96 h) |
Medaka (O. latipes) | LC50 values: 33; 44; 50; 98; and 170 mg/L | Semi-static and flow-through. Five nominal concentrations (7–180 mg/L; purity 87%), DMSO control (0.5 mg/L) and laboratory water control. | EA Japan 1992 |
| BONA pigment lakes (PR57:1*) | Acute (24 h) |
Water flea (D. magna) | EC50 = 280 mg/L | Static. Five nominal concentrations (90–940 mg/L; purity 87%), control of DMSO to HCO-40 = 9:1 (100 mg/L) and laboratory water control. | EA Japan 1992 |
| BONA pigment lakes (PR57:1*) | Acute (24 h – 7 days) |
Water flea (D. magna) | LC50 = 13; 18; 43; 210 mg/L | Semi-static. Five nominal concentrations (3–300 mg/L; purity 87%), control of DMSO to HCO-40 = 9:1 (100 mg/L) and laboratory water control. Effects: mortality, immobilization. | EA Japan 1992 |
| BONA pigment lakes (PR57:1*) | Chronic (14–21 days) | Water flea (D. magna) | LC50 = 9.7; 10 mg/L | Semi-static. Five nominal concentrations (3–300 mg/L; purity 87%), control of DMSO to HCO-40 = 9:1 (100 mg/L) and laboratory water control. Effects: mortality, immobilization. | EA Japan 1992 |
| BONA pigment lakes (PR57:1*) | Chronic (14–21 days) | Water flea (D. magna) | EC50 = 4.4; 9.1 mg/L; NOEC = 3 mg/L | Semi-static. Five nominal concentrations (3–300 mg/L; purity 87%), control of DMSO to HCO-40 = 9:1 (100 mg/L) and laboratory water control. Effects: mortality, immobilization. | EA Japan 1992 |
| BONA pigment lakes (PR57:1*) | Chronic (72 h) | Alga (P. subcapitata) | NOEC = 5.8 mg/L; LC50 = 190 mg/L | Static. EC50 from 13 nominal concentrations (1–1000 mg/L; purity 87%) and control. Effects: biomass. | EA Japan 1992 |
Importantly, the results of all these studies are based on loading rates, and no studies with nominal concentrations or measured concentrations have been identified. For aquatic tests, the nominal concentration is the concentration that would exist if all test material added to the test solution were completely dissolved and did not dissipate (US EPA 1996). Although many of the studies shown in Table 6-1 refer to “nominal concentrations,” they appear to be using this terminology as a synonym for “loading rate” or “non-measured concentration,” which, strictly speaking, is not necessarily the same as nominal concentration. For example, in studies where solvents or dispersants were not applied, the reported toxicity values were several orders of magnitude above the water solubility limits of the tested pigments (e.g., EC50 of 990 mg/L vs. water solubility of 0.008 mg/L for PR253*; see Appendices 4 and 2, respectively), suggesting that the test solution was not completely dissolved and that these were, in fact, loading rates rather than “traditional” nominal concentrations.
Consequently, in studies where the loading rates exponentially exceeded the water solubility limits of the pigments (when solvents/dispersants were not applied), the endpoints probably should have been more accurately reported as EL50(loading rate causing adverse effect in 50% of exposed organisms) instead of EC50 (concentration causing adverse effect in 50% of exposed organisms), LL50 (loading rate causing mortality of 50% of exposed organisms) instead of LC50(concentration causing mortality of 50% of exposed organisms) and NOELR (no-observed-effect loading rate) instead of NOEC (no-observed-effect concentration). However, there appear to be differing opinions in the literature. The Guidance Document on Aquatic Toxicity Testing of Difficult Substances and Mixtures (OECD 2000), in which the water accommodated fractions (WAF) protocol is discussed, indicates that “WAFs may be thus considered analogous to the term “nominal concentration” used for typical test substances, with all the limitations inherent to that term.” It is further stated that “LL50 or EL50 values are comparable to LC50 or EC50 values determined for pure substances tested within their solubility range.”
Another problem with the tests in which the WAF protocol is applied is whether only water-soluble fractions of pigments are indeed present in test water after undissolved particles are removed using membrane filtration. In some studies, despite the use of membrane filtration, pigment particles are probably still present in the test water. In some tests, there are statements that undissolved particles were removed by membrane filtration using filters with a pore size of 0.45 µm. However, some pigments have average particle sizes much less than 0.45 µm. For example, in the Challenge assessment on PR5 (see Environment Canada and Health Canada 2009c), the study on PR146* (analogue for PR5) is discussed, and it is mentioned that particulate matter of PR146* may pass through the filter because the mean particle size of PR146* is only 110 nm, whereas the pore size of the filter used was 0.45 µm (i.e., 450 nm). It is understood that a 0.45 µm filter actually means that 0.45 µm is the size of the biggest pore; however, since the pigment particles are significantly smaller than the 0.45 µm (450 nm) pore size, there is a high possibility that undissolved pigment particles can be present in the test water.
In aquatic tests where the concentrations are exponentially higher than the water solubility limits for pigments, a significant amount of uncertainty exists regarding the actual exposure concentration in the test water. Therefore, in all aquatic tests with the Monoazo Pigments where solvents/dispersants were not applied (see Table 6-1 and Appendix 4) and where the toxicity values were far above the water solubility values of the substances, the results are interpreted here as “not toxic at saturation” or “no effect at saturation” (i.e., at water solubility limits).
It must also be mentioned that in aquatic tests where solvents or dispersants were used, very significant biological effects (such as EC50 or LC50) have been reported, and some toxicity values are at relatively low concentrations (e.g., EC50 of 4.4 mg/L or LC50 of 9.7 mg/L for PR57:1*; see Table 6-1 and Appendix 4), which, according to some ecotoxicity classification schemes, indicates that the tested pigments can be considered as moderately toxic (i.e., EC50 or LC50 values from 1 to 100 mg/L). Unfortunately, similar to the above-mentioned studies without solvents, no measured concentrations were reported in these studies.
There are different reasons for such pronounced biological effects in the studies with solvents or dispersants. One is that Monoazo Pigments might be potentially moderately toxic substances; that is, when their solubility limits are substantially increased to certain levels (by using solvents and/or dispersants), these substances can cause adverse effects.
Herbst and Hunger (2004) indicated that although, according to definition, the ideal pigment is practically insoluble in its medium of application, organic pigments may, in reality, deviate more or less from this postulate of insolubility. Since a pigment that is to a certain extent soluble in its carrier is expected to perform poorly and may even recrystallize, bleed or bloom, it is important to prevent pigment dissolution. There are even certain accepted tests used to determine the extent to which a given organic pigment tolerates solvents (Herbst and Hunger 2004).
Therefore, it is possible that the solubility of pigments in aquatic toxicity tests using dispersants and solvents (see Table 6-1 and Appendix 4) may have increased significantly (e.g. from the low microgram per litre range to the low milligram per litre range).
It has also been expressed that even non-toxic dispersants may have a pronounced effect on the physical form of the hydrophobic test substances in the test medium and, consequently, may influence their bioavailability (Rufli et al. 1998). Thus, results from a test involving a dispersant may be specific for a defined substance–dispersant system, and it may be difficult to extrapolate to other exposure conditions. Rufli et al. (1998) believed that controls containing dispersant can only identify dispersant-related effects, but not dispersant–substance interactions.
In addition, test concentrations far above the water solubility of the test substance can contain more soluble impurities whose effects might also confuse the interpretation of true substance toxicity (Weyman et al. 2012). Indeed, the substance PR57:1*, characterized by quite low acute toxicity values (low milligrams per litre), had a very low purity of only 87% (see Table 6-1 and Appendix 4). Therefore, impurities whose proportion in the pigment was quite significant (13%), dissolved in the solvent that was used in the tests, could cause significant adverse biological effects.
Weyman et al. (2012) also indicated that when solvent is used, but the tested substance is not completely dissolved, undissolved material present in the test medium has the potential to exert adverse (physical) effects on test organisms, such as blocking of fish gill membranes, encapsulation/entrapment of daphnids, or the reduction of light intensity (and, therefore, inhibition of photosynthesis rates) in algal tests.
Regarding the low milligram per litre experimental toxicity values in aquatic tests where solvents and dispersants are used to enhance the apparent water solubility above the maximum thermodynamic equilibrium solubility in water, it should be emphasized that it is unlikely that such high concentrations (in molecular form) of Monoazo Pigments would be reached in the Canadian environment. It is acknowledged that water solubility values will rarely be identical in the aquatic environment and in laboratory studies. Indeed, laboratory tests are conducted under conditions that often do not take into account the various co-solvents that exist in the near-field environment, which may ultimately affect the solubility and bioavailability of a substance close to the emission source. Temperature, pressure and surfactants (which may be present in aquatic environments) are other important factors that may affect the solubility of the chemicals in the environment. At the same time, water solubility enhancement in the environment is not likely to be as high as 3–6 orders of magnitude over solubility limits measured under laboratory conditions.
Accordingly, studies without strong solvents or dispersants are regarded as more environmentally relevant and thus allow for a higher strength of inference on the toxicity potential of pigments in the aquatic environment. In these studies, no effects at saturation occurred due to the low bioavailability of Monoazo Pigments in water and tissues. Therefore, it is unlikely that Monoazo Pigments will be harmful to aquatic organisms under realistic environmental conditions due to the limited bioavailability of these substances.
Finally, it should be noted that for the risk assessment of substances, chronic studies are often preferred over acute studies, because the absence of effects in short-term studies does not necessarily mean that the substance would not be toxic during long-term exposure. For the subgroup of 33 Monoazo Pigments, three no-solvent chronic studies with Daphnia magna are available (see Appendix 4). In these studies, multiple endpoints have been studied, including (but not limited to) reproduction, mortality and immobilization of organisms, as well as body weight and body length. Reported NOEC (NOELR) values were 1 mg/L. In addition, three chronic studies with algae are also available. In two chronic studies, NOEC (NOELR) values were 1 mg/L, and in another one, 5.8 mg/L. In all cases, the results may be interpreted as “no effects at saturation.”
Therefore, the available information suggests that “no effects at saturation” situations in both acute and chronic studies occurred due to the low bioavailability of the pigments. Lack of observed adverse effects in water saturated with the pigments has been recorded consistently in many tests and agrees with the results of little to no bioconcentration. These results suggest that critical internal toxicity thresholds are not achieved at and above maximum thermodynamic equilibrium concentrations in water.
Unfortunately, no data are available to address dietary exposures in aquatic organisms (i.e., via the food web), so the potential for effects from this exposure route remain uncertain.
6.1.2 Soil and Sediments
In order to link toxicity with the potential fate of the 33 Monoazo Pigments in the environment, empirical data for soil were also considered. This environmental compartment is critical because, when emitted to soil (e.g., via biosolids application), the substances from this subgroup are expected to reside almost solely in soils (i.e., little degradation or advection).
Three studies on soil toxicity are available for the subsets of monoazo yellow pigments and naphthol AS pigments (Table 6-2). Data show that at a loading rate of 1 000 mg/kg of soil, no adverse effects (e.g., mortality, reproduction and biomass of organisms for earthworms; shoot height/weight, emergence rate and visual phytotoxic effects for plants) were observed in chronic toxicity studies.
| Subset (substance) | Test type | Organism | Endpoint, value | Details | Reference |
|---|---|---|---|---|---|
| Monoazo yellow pigments (PY1) | Chronic (56 days) |
Earthworm (Eisenia fetida) | NOEC = 1000 mg/kg soil (dry weight) | OECD Test Guideline 222. High-purity pigment (99.7%). Effects: mortality; reproduction; body weight. | Study Submission 2012b |
| Monoazo yellow pigments (PY1) | Chronic (21 days) | Oat (Avena sativa); rape (Brassica napus); soybean (Glycine max) | NOEC = 1000 mg/kg soil (dry weight) | OECD Test Guideline 208. High-purity pigment (99.7%). Effects: shoot height and weight; emergence rate. | Study Submission 2012i |
| Naphthol AS pigments (PR112) | Chronic (21 days) | Oat (Avena sativa); rape (Brassica napus); soybean (Glycine max) | NOEC = 1000 mg/kg soil (dry weight) | OECD Test Guideline 208. Natural soil. Effects: number of emerged seedlings and dead plants; visual phytotoxic effects. | ECHA 2012 |
Therefore, based on these data, it may be concluded that at low concentrations, Monoazo Pigments are not expected to be harmful to soil-dwelling organisms. This most likely can be explained by the low bioavailability of these substances in the various phases that comprise bulk soil (solids, water and air).
Although these dense water-insoluble pigments are expected to reside predominantly in sediments, no sediment toxicity studies are available for this subgroup of 33 Monoazo Pigments. Therefore, conclusions on sediment toxicity could not be made.
6.1.3 Derivation of Predicted No-Effect Concentrations (PNECs)
6.1.3.1 Aquatic PNEC Values
Multiple experimental toxicity data from aquatic acute and chronic ecotoxicity studies are available for Monoazo Pigments (see Table 6-2 and Appendix 4). The range of empirical acute toxicity values from these studies is quite significant: from 4.4 mg/L to almost 1 000 mg/L. It has already been noted that in most studies, loading rates were reported, and very often the acute toxicity data were expressed as ranges, not as definitive values (e.g., LC50 greater than 100 mg/L). For chronic studies, NOEC values were reported; in most cases, the reported value was 1 mg/L.
For deriving PNEC values for different subsets of Monoazo Pigments, critical toxicity values (CTVs) had to be selected. Aquatic PNEC values were then calculated as follows:
PNEC (mg/L) = CTV / AF
where AF is an assessment factor.
Table 6-3 contains the rationale for selecting the CTVs as well as the CTVs themselves, assessment factors and derived PNEC values. The following principles were used when choosing an assessment factor. For the same substance (or subset of substances), chronic studies were preferred over acute studies. An assessment factor of 10 was applied for chronic studies where relatively low (e.g., 1 mg/L) toxicity values (e.g., a NOEC) were reported. Since no biologically or statistically significant effects (including sensitive endpoints, such as reproduction rate) were found (compared with controls) for some substances in both acute and chronic studies with invertebrates, an assessment factor of 10 was used, even in acute studies. When “no effects at saturation” were reported, an assessment factor of 10 was used. In the acute study with fish (subset of BONA pigment lakes), an assessment factor of 100 was applied.
For some pigment subsets or individual pigments, PNEC values could not be derived due to the absence of experimental toxicity values. For these substances, the lowest available PNEC value from the entire group of 33 pigments was chosen as a surrogate (in Table 6-3, these PNEC values are marked with an asterisk).
| Subset | Aquatic CTV | Assessment factor | Aquatic PNECFootnote Table 6-3[a] |
|---|---|---|---|
| Monoazo yellow pigments | 1 mg/L (lowest NOEC value from a chronic test within the subset) | 10 | 0.1 mg/L |
| BONA pigment lakes | Fish: 33 mg/L (lowest acute toxicity value); invertebrates: 3 mg/L (NOEC from a chronic study); algae: 5.8 mg/L (NOEC from a chronic study) | Fish: 100; invertebrates and algae:10 | Fish: 0.33 mg/L; invertebrates: 0.3 mg/L; algae: 0.58 mg/L. Final PNEC: 0.3 mg/L (lowest from all organisms tested) |
| β-Naphthol pigment lakes | 2.2 mg/L (lowest EC0 value from acute test) | 10 | 0.22 mg/L |
| β-Naphthol pigments | 0.9 mg/L (lowest acute toxicity value within the subset) | 10 | 0.09 mg/L |
| Naphthol AS pigments | 1 mg/L (lowest NOEC value from a chronic test) | 10 | 0.1 mg/L |
| Benzimidazolone pigments | 1 mg/L (NOEC value from chronic test) | 10 | 0.1 mg/L |
| BONA pigment lakes – individual | Not available | Not available | 0.09 mg/L* |
| Naphthol AS pigment lakes | Not available | Not available | 0.09 mg/L* |
| Pyrazoloquinazolone pigments | Not available | Not available | 0.09 mg/L* |
| Monoazo yellow pigments – individual | Not available | Not available | 0.09 mg/L* |
| Other pigments – individual (NSNAC) | Not available | Not available | 0.09 mg/L* |
6.1.3.2 Soil and Sediment PNEC Values
Three studies on soil toxicity are available for the subgroup of 33 Monoazo Pigments (see Table 6-2). These studies have been considered as key, high-quality studies, and the toxicity value (NOEC) of 1 000 mg/kg soil (dry weight) was selected as the CTV.
Taking into account that in the two studies with plants, CTVs were based on NOEC values in long-term studies, where no lethal or sub-lethal adverse effects (or visual phytotoxic effects in the tests with different plants) were found at the loading concentration of 1 000 mg/kg soil, an assessment factor of 10 was used to account for interspecies and intra-species variability in sensitivity only.
One soil toxicity study was conducted with earthworms, which are excellent model organisms in ecotoxicity studies due to their exposure to soil contaminants via both ingestion and passive absorption through the skin. Since no adverse biological effects (including sensitive endpoints such as reproduction) were observed in this long-term study in a sensitive species, it was thought that an assessment factor of 100 would be overly conservative for calculation of a PNEC value, so an assessment factor of 10 was applied.
The resulting PNEC value for soil can therefore be calculated as:
Soil PNEC = (1000 mg/kg dry weight) / 10 = 100 mg/kg soil (dry weight)
As no sediment toxicity studies were located for the subgroup of 33 Monoazo Pigments, PNEC values for sediments, based on experimental CTVs, could not be derived.
6.1.4 Ecological Effects Summary
Based on various lines of evidence involving empirical ecotoxicity data in various environmental compartments (water and soil), it may be concluded that the 33 Monoazo Pigments are not expected to cause harm to aquatic or soil-dwelling organisms at low concentrations. Empirical data allowed derivation of PNEC values for water and soil for further characterization of the ecological risk (i.e., PEC vs. PNEC values) of Monoazo Pigments in different environmental compartments. No conclusion on the ecological effects of the 33 Monoazo Pigments on sediment-dwelling organisms can be made due to the lack of ecotoxicological data.
6.2 Ecological Exposure Assessment
6.2.1 Measured Environmental Concentrations
6.2.1.1 Canada
No data on measured environmental concentrations (in water, soils or sediments) of the 33 Monoazo Pigments in Canada have been identified, and environmental concentrations were therefore estimated from available information.
6.2.1.2 Other Countries
During 2007–2008, the Swedish Environmental Protection Agency developed analytical methods and performed a “screening study” of some pigments (Lilja et al. 2008). In this monitoring study, four monoazo pigments (PY1, PR53:1, PR170 and PO5) were analyzed in various environmental samples. The results of this study are summarized below.
6.2.1.2.1 Background Areas
None of the four pigments was found in soil, surface water, sediment or fish samples from the background areas (i.e., concentrations of all four were below their detection limits).
6.2.1.2.2 Diffuse Sources
PY1, PR53:1 and PR170 were detected in sludge from wastewater treatment plants. The highest concentrations were 100 000 µg/kg dry weight (dw) and 88 000 µg/kg dw (PR170, two consecutive months at a wastewater treatment plant), which were approximately 1 000 times higher than the levels at other wastewater treatment plants. Levels of PY1 reached approximately 70–360 µg/kg dw (three sites; data are extrapolated from diagrams), while concentrations of PR53:1 reached approximately 50–200 µg/kg dw (four sites; data are extrapolated from diagrams).
All four pigments were found in wastewater treatment plant influent. PY1 was found at three wastewater treatment plants at the highest concentrations of 0.13–0.38 µg/L. Concentrations of PR53:1 were 0.007–0.027 µg/L (four STPs), and PR170, 0.068–0.090 µg/L (three STPs), whereas PO5 was found at concentrations of 0.027–0.067 µg/L. Importantly, for PY1, PR53:1 and PR170, 100% of the content in the influent was found in the particulate phase. For PO5, a small fraction, 7.5% of the content, was found in the water phase.
Concentrations of pigments in effluent are lower than in influent: PY1, less than 0.013 µg/L; PR53:1 and PO5, less than 0.003 µg/L; and PR170, less than 0.053 µg/L. The authors suggested that the lack of pigments in effluent indicates that removal of these pigments takes place during the wastewater treatment process.
None of the pigments was detected in the surface water samples, but both PR53:1 and PR170 were found in the sediment samples. Interestingly, the concentrations were slightly higher upstream compared with downstream of the effluent outlet (3.9 and 1.4 µg/kg dw, respectively, for PR53:1, and 96 and 65 µg/kg dw, respectively, for PR170), which suggests that the pigments do not reachthe receiving watervia only discharges from wastewater treatment plants.
6.2.1.2.3 Point Sources
All four pigments were detected in the sample of effluent from one public laundry at the following concentrations: 0.19 µg/L (PY1), 0.085 µg/L (PR53:1), 1 µg/L (PR170) and 0.098 µg/L (PO5). This sample was taken before treatment at a wastewater treatment plant. In effluent from a second laundry, sampled after passage through a municipal wastewater treatment plant, only PR53:1 was found (at 0.013 µg/L). Interestingly, most of the PR53:1 detected in these two samples was found in the water phase (85% and 100%, respectively). This is opposite to the distribution in the wastewater treatment plant influent , where 100% of the pigment was found in the particulate phase, but relatively similar to effluent water from a wastewater treatment plant, where 38% was found in the water phase. The other pigments were detected only in the particulate phase of the laundry effluent samples.
All four pigments were found in process water from the deinking operations of recycled paper at a paper mill at the following concentrations: 27–61 µg/L (PY1), 160–450 µg/L (PR53:1), 6–9 µg/L (PT170) and 3–10 µg/L (PO5).
High levels of all four pigments were also found in sludge from the deinking operations of recycled paper at a paper mill: 370–800 µg/kg dw (PY1), 1 100–4 200 µg/kg dw (PR53:1), 420–1 600 µg/kg dw (PT170) and up to 650 µg/kg dw (PO5).
Since spring preparation of boats, including sanding, painting and polishing, often takes place in the open air, resulting in the spread of dust containing pigments, a soil sample from a marina was analyzed. Concentrations of 0.13 µg/kg dw and 0.007 µg/kg dw were found for PR170 and PO5, respectively, while concentrations of PR53:1 and PY1 were below the detection limits.
6.2.1.2.4 Urban Environment
In surface waters, only PR53:1 was found (0.005 µg/L; one sample).
All four pigments were found in stormwaters from the city of Stockholm. Concentrations were below the detection limits in one sample , whereas high concentrations (in the same range as or even higher than in wastewater treatment plant influent ) were found at two other sites, where 100% of the detected pigments were found in the particulate matter of stormwater. Levels of PY1 reached 0.75 µg/L (one sample), PR53:1, 0.21 and 0.37 µg/L (two samples), and PR170, 0.39 and 2.8 µg/L (two samples), while the levels of PO5 were around 0.06 µg/L (two samples).
In sediments, PR53:1 was found in three samples (5.9–13 µg/kg dw), while PR170 was detected in two samples (190 and 330 µg/kg dw).
None of the four pigments was found in fish samples.
Considering all this information, the authors concluded that the presence of the pigments in wastewater treatment plant sludge and waters, effluent from public laundries, stormwater, sediments, surface water and soil indicates that diffuse emissions of the pigments occur. Importantly, the authors also indicated that since none of the pigments could be detected in the samples from background areas, the emissions in and transport to rural areas seem to be minor.
Elsewhere, PR53:1 was also detected in a sample of a wastewater treatment plant effluent at a deinking plant in Germany (OECD 1999). The arithmetic mean concentration (three runs) was 3.4 μg/l; using a dilution factor of 30, a PEClocal value of 0.11 μg/l was derived.
6.2.2 Releases to the Environment
Since no data on measured environmental concentrations of the 33 Monoazo Pigments in Canada have been identified, environmental concentrations were estimated from available information.
Anthropogenic releases of a substance to the environment depend upon various losses that occur during the manufacture, industrial, consumer and commercialFootnote[7] use and disposal of a substance. In order to estimate releases to the environment occurring at different stages of the life cycle of Monoazo Pigments, Environment Canada compiled information on the relevant sectors and product lines as well as emission factorsFootnote[8] to wastewater, land and air at different life cycle stages in order to identify the life cycle stages that are the largest contributors to environmental concentrations. Recycling activities and transfer to waste disposal sites (landfill, incineration) were also considered. However, releases to the environment from disposal were not quantitatively accounted for unless reliable specific information on the rate of (or potential for) release from landfills and incinerators was available.
Factors relevant to the life cycle of these substances have been considered, uncertainties have been recognized and assumptions have been made, subject to the availability of information. Exposure scenarios for the uses and media of concern have been developed, including the determination of applicable predicted environmental concentrations (PECs). Further, risk quotient (RQ) analyses compare the estimated or measured environmental concentrations with the appropriate PNEC values in order to evaluate potential risks.
6.2.3 Identification of the Most Important Exposure Scenarios in Canada
Exposure characterization focuses on the most important exposure scenarios. These scenarios represent major environmental releases and relatively high levels of exposure. In general, the magnitude of release is a direct function of the quantity of a substance manufactured or used and its applicable emission factors. In cases where industrial releases are similar in quantity to consumer and/or commercial releases, the former normally result in higher levels of environmental exposure than the latter. This is because industrial releases are concentrated at a limited number of sites, while consumer and/or commercial releases are dispersed across the country.
Three scenarios were identified as potentially having the highest environmental releases from the sectors identified from the CEPA 1999 section 71 surveys (see Sources section of this report). These scenarios were pigment manufacture, paint/coating formulation and deinking, as summarized in Table 6-4. The quantity of the Monoazo Pigments manufactured in Canada accounted for more than 40% of the net Monoazo Pigments in Canadian commerce. The site visits conducted by Environment Canada in 2010 found that the emission factors to wastewater at pigment manufacturing facilities were high, at more than 1% (Environment Canada 2010). The 40% weight for the manufacturing sector and the more than 1% emission factor translated into a relatively high release to wastewater, at more than 0.4% of the net Monoazo Pigments in Canadian commerce. Pigment manufacture was therefore selected as the first important exposure scenario.
| Sector | Sector weight as % of net 33 Monoazo Pigments | Emission factor to wastewater (%) | Release to wastewater as % of net 33 Monoazo Pigments |
|---|---|---|---|
| Pigment manufacture | 40 | greater than 1 | greater than 0.4 (high) |
| Paint/coating formulation | 65 | 0.3 | 0.2 (high) |
| Ink/toner formulation | 12 | 0.1 | 0.01 (low) |
| Deinking | 8.3 | 20 | 1.7 (high) |
| Printing | 12 | 0.036 | 0.004 (low) |
| Plastics and rubber | 11 | 0.05 | 0.006 (low) |
| Agricultural products | 4 | 2 | 0.08 (low) |
| Others | 1 | 2 | 0.02 (low) |
The quantity of Monoazo Pigments used in paints and coatings was the highest of all uses. This use amounted to 65% of the net Monoazo Pigments in Canadian commerce. Paint/coating formulation was identified as the major industrial activity from the survey data. The emission factor to wastewater from paint/coating formulation was found to be moderate, at 0.3% (Environment Canada 2012b). When the moderate emission factor was combined with the large quantity of Monoazo Pigments used in paint/coating formulation, the environmental releases were expected to be significant. In other words, the 65% weight for the paint/coating formulation sector and the 0.3% emission factor translated into a significant release to wastewater, at 0.2% of the net Monoazo Pigments in Canadian commerce. For this reason, paint/coating formulation was selected as the second important exposure scenario. The consumer and/or commercial uses of paints and coatings were not considered as important as the industrial formulation activity on a per site basis due to the dispersive nature of the releases from consumer and/or commercial use. For this reason, consumer and/or commercial uses were not selected for quantitative exposure analysis.
For the ink/toner formulation sector, the emission factor to wastewater was unknown, while the quantity used in the sector was moderately high, at 12% of the net Monoazo Pigments in Canadian commerce. Companies indicated that there was no release to wastewater for Monoazo Pigments from its formulation operations (2013 email to Environment Canada; unreferenced), while no release data were available for other ink/toner formulators. Due to the lack of these data for ink/toner formulation as a whole, the emission factor of 0.1% for the emission of pigments from ink formulation to wastewater reported by US EPA (2012a) was used to estimate the potential release of the Monoazo Pigments. This potential release was determined to be relatively low, at 0.01% of the net Monoazo Pigments in Canadian commerce, based on the 12% sector weight and the 0.1% emission factor. Ink/toner formulation was therefore not considered to be an important exposure scenario and was excluded from quantitative exposure analysis.
There are, however, two downstream sectors of ink/toner formulation: printing and recycled paper deinking. The Monoazo Pigments identified for use in printing inks were used for printing on both paper and plastic film, according to information provided by industry (2013 emails from industry to Environment Canada; unreferenced). It was therefore induced that the entire quantity of the Monoazo Pigments formulated into inks and toners ended up on printed paper and plastic film. Although the proportions of these pigments between the two substrates were unknown, a substantial quantity was expected for the paper substrate. Considering this and a large recycled portion of printed paper, it was expected that a significant quantity of the ink/toner-destined Monoazo Pigments was subject to deinking. Furthermore, the emission factor to wastewater from deinking was estimated to be high, at 20% (2013 telephone discussions between a pulp and paper expert of an industry association and Environment Canada; unreferenced). The 12% sector weight for the ink/toner formulation yielded an 8.3% sector weight for deinking when the ink/toner-destined Monoazo Pigments were conservatively assumed to end up on paper, and paper recycling in Canada was at 69% in 2010 (2012 e-mail from Marketing Strategy and Sustainability Consulting, Kitchener, Ontario, to Ecological Assessment Division, Environment Canada; unreferenced). The 8.3% sector weight and the 20% emission factor translated into a significant release to wastewater, at 1.7% of the net Monoazo Pigments in Canadian commerce. The deinking operations scenario was therefore selected as the third important exposure scenario.
Compared with the deinking scenario, the printing scenario had a much lower release to wastewater. According to US EPA (1995), lithography, gravure, flexography, letterpress and screen were five basic technologies used for printing, and only screen printing generated wastewater and released it to the sewer system. The source of this wastewater was cleaning operations for screen reclamation. Thus, the emission factor to wastewater from screen printing was used to represent the worst-case aquatic release for the printing scenario.
The emission factor to wastewater from screen printing was estimated at 0.036% as follows. The amount of residual ink left on a screen after a printing job was determined to be 0.039 kg for an average screen size of 0.0137 m2, based on an amount of ink remover used of 0.227 kg (US EPA 1994b) and a ratio of ink to ink remover of 1:5.8 (US EPA 1998) (0.227 kg × 1/5.8 = 0.039 kg). The maximum amount of residual ink ending up in wastewater from cleaning operations was therefore 0.039 kg. In contrast, the volume of ink required for a printing job was calculated to be 102 L for the same screen size, according to a method described by Screen Web (2000). This volume was converted to 109 kg for a screen printing ink density of 1.07 kg/L obtained from 3M (2007) (102 L × 1.07 kg/L = 109 kg). The maximum amount of residual ink ending up in wastewater was therefore 0.036% of the amount of ink used (0.039 kg/109 kg × 100% = 0.036%). This percentage was used as an estimate for the emission factor to wastewater from screen printing or a conservative estimate for the printing scenario.
The 0.036% emission factor combined with the 12% sector weight for printing yielded a relatively low release to wastewater, at 0.004% of the net Monoazo Pigments in Canadian commerce. For this reason, the printing scenario was not selected for quantitative exposure analysis.
The quantities of the Monoazo Pigments reported for all other uses were 16% of the total quantity used in Canada; these uses included “Plastics and Rubber” at 11%, “Agricultural Products” at 4% and all remaining categories at 1%. The emission factors to wastewater associated with these other uses were 0.05% for the plastics and rubber manufacturing industry and 2% for the agricultural industry and the remaining categories (European Commission 2003). These sector weights and emission factors translated into relatively low releases to wastewater, at 0.006% of the net Monoazo Pigments in Canadian commerce for plastics and rubbers, 0.08% for agricultural products and 0.02% for all remaining categories. Compared with the three important scenarios identified above, these other uses had much lower releases to wastewater and were therefore not selected for quantitative exposure analyses.
The deinking sector represented an important exposure scenario not only for water, but also for soil. In the soil exposure analysis conducted for this sector, it was assumed that all pigments contained in recycled paper were removed from deinking operations and captured in sludge. As a result, the quantity entering soil via sludge land application represented 8.3% of the net Monoazo Pigments in Canadian commerce, since the sector weight for deinking was conservatively estimated at 8.3%. In comparison, agricultural products accounted for much less, at 4%, and their contribution to soil exposure would be limited to 4%. The deinking scenario was therefore conservative enough for a screening-level soil exposure analysis, and a soil exposure analysis for the use of agricultural products was not pursued.
6.2.4 Estimated Predicted Environmental Concentrations (PECs)
Exposure to the Monoazo Pigments was quantified in the form of PECs for each of the three important exposure scenarios identified. These concentrations were based on available information on the quantities of the Monoazo Pigments, sector-specific emission factors, the characteristics of the wastewater treatment systems involved and the characteristics of the receiving environment.
PECs were estimated for the aquatic, sediment and soil compartments. This was because the Monoazo Pigments were primarily present in these compartments as a result of their release to receiving water from wastewater treatment systems, their partitioning from water to sediment and the land application of wastewater sludge. Aquatic and sediment PECs were estimated for each of the three scenarios, while one conservative soil PEC was provided for the deinking scenario only, as it was the largest contributor to soil exposure. This soil PEC was estimated as 10.8 mg/kg and is expected to represent an upper limit for all other scenarios. A discussion of these PECs is provided below. Detailed calculations can be found in Appendix 5.
6.2.4.1 Aquatic PECs from Pigment Manufacture
Fewer than five pigment manufacturers were identified from CEPA 1999 section 71 surveys (Canada 2006, 2007, 2008, 2011). One large pigment manufacturing facility was selected to estimate the level of aquatic exposure for the pigment manufacture scenario.
The Monoazo Pigments were anticipated to enter the aquatic environment through wastewater treatment systems. Environment Canada learned from a number of site visits in 2010 that pigment production equipment was cleaned with water on a regular basis (Environment Canada 2010). The wastewater generated was subsequently treated on site for solids removal and then discharged to the sewer system. Any residual pigments following the on-site and off-site treatment were therefore expected to be released to receiving water in the effluent fromthe wastewater treatment system.
The aquatic PEC was estimated from a number of parameters based on the above understanding of the aquatic release of the Monoazo Pigments from the pigment manufacturing facility. These parameters included the daily quantity of the Monoazo Pigments produced, their emission factor to wastewater, the removal efficiencies of both on-site and off-site wastewater treatment systems, the flow of the off-site wastewater treatment system and the dilution of the receiving water. The aquatic PEC of the Monoazo Pigments derived was 29.7 μg/L.
The aquatic PEC derived was considered conservative. Firstly, the emission factor to wastewater was assumed to be 10% of the quantity of the Monoazo Pigments produced. Environment Canada learned that the products lost during equipment cleaning varied over a wide range but did not exceed 10% of production or equipment holding volume, based on various site visits, including those to a pigment manufacturing facility (Environment Canada 2010), latex paint formulation facility (Environment Canada 2013a), powder coatings manufacturing facility (Environment Canada 2013b), cosmetics manufacturing facility (Environment Canada and Health Canada 2010) and tank truck cleaning facility (Environment Canada 2009b). This 10% value was used as the highest emission factor to wastewater from the cleaning of the pigment production equipment. Secondly, the estimated removal of the off-site wastewater treatment system varied from 0.5% to 49.2% due to the variability in the hydrophobicity of the Monoazo Pigments. The lowest value, 0.5%, was used in the calculations, so that the estimated aqueous release of the Monoazo Pigments from the off-site wastewater treatment system represented the maximum quantity entering the aquatic compartment. The combination of the highest emission factor with the lowest wastewater treatment removal was expected to yield a conservative estimate of the aquatic PEC.
6.2.4.2 Aquatic PECs from Paint and Coating Formulation
Seventy-five paint and coating formulation facilities were identified as industrial users of the Monoazo Pigments from CEPA 1999 section 71 surveys (Canada 2006, 2007, 2008, 2011), and eight of them were determined to be the largest users. On average, these eight facilities used 5 000–10 000 kg/year per pigment for one or more Monoazo Pigments. The eight facilities were therefore selected to determine the level of aquatic exposure for the paint and coating formulation sector. In comparison, the other facilities used quantities below 5 000 kg/year per pigment on average. Less than five of these other facilities were initially identified among the largest users based on the CEPA 1999 section 71 surveys (Canada 2006, 2007, 2008, 2011), but follow-up questions (2013 emails from facilities to Environment Canada; unreferenced) showed each of these facilities to use less than 5 000 kg/year for all Monoazo Pigments combined.
One or more Monoazo Pigments were anticipated to enter the aquatic environment through wastewater treatment systems. A number of site visits to paint and coating formulation facilities revealed that wastewater was generated from equipment cleaning and discharged to the sewer system following on-site solids removal (Crechem Technologies 2005). It was therefore assumed that wastewater was generated from each of the eight facilities selected as a result of formulation equipment cleaning. The wastewater was first treated on site for solids removal and further treated by an off-site wastewater treatment system. The treated wastewater was finally discharged to a water body.
The aquatic PEC for each facility was estimated from a number of parameters. These parameters included formulation batch size, pigment content in paints and coatings, emission factor to wastewater, removal efficiencies of both on-site and off-site wastewater treatment systems, off-site wastewater treatment flow and receiving water dilution factor. The aquatic PECs derived for the eight facilities were in the range of 0.51–25.9 μg/L.
The aquatic PECs derived for the eight facilities were all conservative estimates. In these estimates, the largest formulation batch size, at 19 000 kg of paints and coatings, was found from an analysis of industry data (Environment Canada 2012) and used to determine the loss of the Monoazo Pigments to wastewater from the cleaning of the formulation equipment. Since the range of the batch size spanned over more than one order of magnitude, from 1 000 kg to 19 000 kg, according to the analysis of industry data, the loss to wastewater determined represented an upper limit. The level of aquatic exposure resulting from the formulation of paints and coating was therefore not expected to exceed the PECs derived.
6.2.4.3 Aquatic PECs from Deinking Operations
Seventeen facilities were identified as sites that perform recycled paper deinking operations from three reference sources: the Pulp and Paper Canada Directory (2013), the Lock-wood Post Directory (2011) and FisherSolve™ Platform (2013). Of these 17 facilities, sufficient information to permit aquatic exposure analyses was available for only 13 facilities. Nevertheless, these 13 facilities were judged to be a good representation of the Canadian deinking sector.
Aquatic PECs were estimated for the 13 recycled paper deinking facilities. These facilities generated and treated their respective wastewaters on site and subsequently discharged them directly to the receiving water. The parameters used in the derivation of the PECs included the total quantity of the Monoazo Pigments used for printing, the quantity of the Monoazo Pigments entering each facility, the emission factor to wastewater, wastewater volume, on site wastewater treatment removal efficiency and receiving water dilution factor. The aquatic PECs derived were in the range of 0.09–7.67 μg/L . They were considered conservative, since the total quantity of the Monoazo Pigments used for printing was assumed to end up on paper only, without correction for the quantity used for plastic film.
6.2.4.4 Sediment PECs from Pigment Manufacture, Paint and Coating Formulation, and Deinking Operations
An equilibrium sediment–water partition approach described by ECHA (2010) was used to estimate the PECs of the Monoazo Pigments in sediment. This approach assumes that the concentration in bottom sediment is in equilibrium with the concentration in the overlying water. According to Gobas (2007), the concentration in the overlying water should pertain to the concentration in the aqueous phase and should not include quantities adsorbed to suspended sediment. The concentration in the aqueous phase can be estimated from the aquatic PEC, which is the total concentration in water. Thus, the sediment PECs for the sites of pigment manufacture, paint/coating formulation and deinking were derived from their respective aquatic PECs and standardized to an organic carbon (OC) content of 3% for bottom sediment. ,
The sediment PECs were conservative, because the concentrations in the aqueous phase were conservatively estimated. In the water column, the total concentration of the Monoazo Pigments or the aquatic PEC at a given site was a fixed value. It was split between the aqueous phase and the solids phase (suspended sediment). The concentration in the solids phase depended upon the OC content of the solids phase, and the latter varied from a low value of 0.1 kg OC/kg to a high value of 0.2 kg OC/kg (Gobas 2010). By selecting the low value of 0.1 kg OC/kg, the minimum concentration in the solids phase resulted, thereby yielding the maximum concentration in the aqueous phase. This maximum concentration then resulted in the maximum sediment PEC value at each site.
6.2.4.5 Soil PEC from Pigment Manufacture, Paint and Coating Formulation, and Deinking Operations
Soil exposure to the Monoazo Pigments was estimated under a conservative scenario. This scenario was represented by the recycled paper deinking scenario. The deinking scenario yielded the highest release to soil through sludge land application, since the entire quantity of the Monoazo Pigments used for printing was assumed to end up in sludge. In addition, the land application was assumed to occur at an average rate without any degradation, volatilization, soil runoff or leaching losses over a long period. This conservative scenario yielded a soil PEC of 10.8 mg/kg, which is expected to represent an upper limit for all other scenarios (see Appendix 5).
6.2.5 Predicted Environmental Concentrations (PECs) versus Measured Environmental Concentrations (MECs)
Measured environmental concentrations (MECs) of monoazo pigments in Sweden were compared with the PECs derived for Canada. Direct comparisons of the Canadian PECs with MECs from other countries must be treated with caution, given that there are various factors that could account for differences, such as differences in the use patterns of the substances, use volumes, conditions in the receiving environments, industrial process operations, mitigation measures, treatment methods at wastewater treatment systems, etc. However, many similarities can be drawn between Canada and Sweden. In both countries, monoazo pigments are used for relatively similar purposes. The volumes of the four monoazo pigments covered in the Swedish study (PY1, PR53:1, PR170 and PO5) are also similar: in Sweden, 122–180 t/year (2001–2005); in Canada, within 100–200 t/year (recent surveys). In addition, both Sweden and Canada are northern countries with similar climates and environmental conditions; therefore, certain similarities in the environmental fates and biological effects of substances can be expected. Therefore, in this case, some qualitative analysis of the data from Sweden was deemed appropriate as an additional line of evidence. As described below, the Swedish monitoring data provide support that the PEC values derived for certain Monoazo Pigments for the Canadian environment are not underestimated.
The MEC (Sweden) and PEC (Canada) results indicate that all sediment PEC values calculated for the Canadian environment are higher than the measured concentrations of some monoazo pigments in sediments reported by Lilja et al. (2008) in Sweden: 0.04–14 mg/kg estimated in Canada compared with 0.001 4–0.096 mg/kg (diffuse sources) and 0.005 9–0.330 mg/kg (urban environment) measured in Sweden. In background areas in Sweden, measured concentrations of monoazo pigments in sediments were below the detection limits (i.e., less than 0.000 7–0.077 μg/kg).
For the water compartment, aquatic PEC values for Canada vary from 0.1–8 μg/L (deinking operations scenario) to 30 μg/L (pigment manufacture scenario), whereas measured concentrations of the four monoazo pigments in surface water in both background and diffuse source areas in Sweden were below the detection limits (i.e., less than 0.001 1–0.015 μg/L for PY1, PR53:1 and PO5 and less than 0.048–0.085 μg/L for PR170).
In terms of the soil compartment, the PEC value derived for Canadian soils was 10.8 mg/kg, whereas the highest measured concentration of one of the monoazo pigments (PR170) in Sweden was remarkably lower, only 0.13 μg/kg, while concentrations of the other pigments (PY1, PR53:1 and PO5) were below the detection limits.
In summary, data indicate that MECs of some monoazo pigments in Sweden are lower (and sometimes significantly lower) than PECs derived for the Canadian environment. Given similar overall expected use patterns, this information provides indirect confirmation that the PEC values derived for Monoazo Pigments in the Canadian environment should represent conservative scenarios and will result in risk quotients that can be considered protective.
6.3 Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight of evidence approach and using precaution as required under CEPA 1999. Lines of evidence considered include information on physical and chemical properties, environmental fate, ecotoxicity and sources of the substances, as well as results from conservative risk quotient analyses, which are outlined below.
The Monoazo Pigments are substances that have a particulate nature, have very low solubility, are chemically and hydrolytically stable and are not readily biodegradable. They are expected to partition by gravity to suspended solids and bed sediments if released to surface waters. When considering potential releases to soil, it is expected that the physical and chemical properties of these pigments will be a driving force behind their ultimate partitioning to soil particles.
Empirical data indicate that the 33 Monoazo Pigments are expected to be persistent in water, soil and sediment but not in air (due to reactions with hydroxyl radicals). In anaerobic sediment conditions, solubility-limited azo reduction may occur. However, given the unique physical-chemical characteristics of Monoazo Pigments (particulate nature, very low water solubility), it is expected that only a very small proportion of these pigments will be available to microorganisms for biotic reduction.
Based on the particulate nature of Monoazo Pigments, their very low water solubility, their generally low log (Soct/Sw) values, the relatively high cross-sectional diameters of many of these pigments, and the very low experimental BCF values, available for some of the pigments, it is expected that the group of 33 Monoazo Pigments will not bioaccumulate in organisms.
Available acute and chronic aquatic toxicity studies showed no adverse effects “at saturation” (i.e., at water solubility limits) due to the low bioavailability of the pigments. The lack of observed adverse effects in water saturated with the pigments agrees with the findings of little to no bioconcentration of these substances in aquatic organisms. These results suggest that critical internal toxicity thresholds are not achieved at and above maximum thermodynamic equilibrium concentrations in water.
Chronic soil toxicity studies show no adverse effects (e.g., mortality, reproduction and biomass of organisms for earthworms; shoot height/weight, emergence rate and visual phytotoxic effects for plants) at a loading rate of 1 000 mg/kg of soil.
It may be concluded that at low concentrations, Monoazo Pigments are not expected to be harmful to aquatic or soil-dwelling organisms.
For deriving PNEC values in water and soil, most of the CTVs have been chosen from chronic studies; when multiple studies were available, the lowest values were used. Assessment factors were also applied.
To assess the risk associated with the 33 Monoazo Pigments, the most important exposure scenarios were identified based on the manufacture, import and use of these substances in Canada. Three aquatic exposure scenarios (pigment manufacture, paint/coating formulation and deinking operations) as well as sediment and soil exposure scenarios were considered. The potential releases of the Monoazo Pigments to the aquatic and terrestrial environment were also estimated.
The PEC value for aquatic exposure from pigment manufacture was 29.7 μg/L. For the other two aquatic exposure scenarios--from paint and coating formulation and from deinking operations (based on a variety of industrial sites)--the PEC values ranged from 0.51 to 25.9 μg/L and from 0.09 to 7.67 μg/L, respectively.
A risk quotient analysis was conducted by comparing these PEC values with the aquatic PNEC values (which ranged from 90 to 300 μg/L for different subsets of the Monoazo Pigments). This analysis indicates that even if a conservative approach is used--i.e., when the highest aquatic PEC values representing the three exposure scenarios (29.7 μg/L, 25.9 μg/L, and 7.67 μg/L) are compared with the lowest aquatic PNEC value (90 μg/L)--the resulting risk quotients are in the range of 0.085–0.33. These calculated conservative (i.e., the highest possible) risk quotients (for each of the three exposure scenarios based on the available information) are below 1, which indicates that harm to aquatic organisms is not expected.
A risk quotient analysis was also conducted for the soil compartment. Comparing a conservative soil PEC value of 10.8 mg/kg with the soil PNEC value of 100 mg/kg (dry weight), the derived risk quotient is only 0.1, which indicates that harm to soil-dwelling organisms is not expected.
Although PEC values (ranging from 0.04 to14.0 mg/kg for various industrial sites and different types of exposure) are available for sediments, a risk quotient analysis was not conducted for this environmental compartment, because no sediment toxicity studies were located for the group of 33 Monoazo Pigments. Therefore, PNEC values for sediments, based on experimental CTVs, could not be derived. Monoazo Pigments may be hazardous to sensitive sediment-dwelling organisms at low concentrations in sediment. There is also the potential for the breakdown products of some of these substances to be genotoxic or carcinogenic to aquatic organisms.
Considering all available lines of evidence with respect to the physical-chemical properties, persistence, potential bioaccumulation, ecotoxicity, industrial uses and potential releases of these substances, it is expected that the 33 Monoazo Pigments have low potential to cause ecological harm in Canada.
6.3.1 Uncertainties in the Evaluation of Ecological Risk
There were some uncertainties associated with the assessment of this group of 33 Monoazo Pigments. For example, experimental concentrations reported in aquatic toxicity tests may be uncertain due to the use of dispersants or solvents to increase the solubility of pigments in test water. Some acute experimental studies using dispersants/solvents indicated pronounced biological effects at relatively low concentrations (e.g., low milligrams per litre), which may be attributed to toxic effects caused by impurities contained in the tested low-purity (e.g., 87% in some studies) pigments, different additives contained in the pigment products and dispersant–pigment (or solvent–pigment) interactions.
There may also be uncertainty associated with aquatic toxicity studies that did not use solvents, since only loading rates were reported, and no information on measured concentrations was provided. In some tests, it was indicated that undissolved pigment particles were removed by membrane filtration using filters with a pore size of 0.45 µm. However, some pigments have average particle sizes much less than 0.45 µm. Therefore, pigment particles could easily pass through the filter. Therefore, membrane filtration in some studies does not necessarily mean that only water-soluble fractions of pigment are present in the test water.
There is also uncertainty associated with the absence of sediment toxicity studies, which does not allow the derivation of PNEC values for sediments and the calculation of a risk quotient for the sediment exposure scenario. This information is relevant, as sediment and soil represent the environmental media in which Monoazo Pigments are expected to reside.
The purity of Monoazo Pigments is a source of uncertainty, as many types of substances (e.g., resins, rosins, different surfactants, dispersing agents, coupling agents) are used in pigment preparation. It is impossible to completely remove such impurities, which can change some properties of the pigments.
Another area of uncertainty relates to the degradation of Monoazo Pigments. Due to a lack of experimental data, there is uncertainty as to the rate and extent to which degradation of Monoazo Pigments occurs in anaerobic environments and whether those degradation products (e.g., aromatic amines) could ever become biologically available. It is expected that the unique physical state of Monoazo Pigments (particles) along with their quite low water solubility would limit the availability of the molecules for biotic reduction, so the formation of degradation products will be limited.
In terms of bioavailability and bioaccumulation of pigments from an ecotoxicological perspective, there is also some uncertainty. Since many pigments have particle sizes in the nanometre to micrometre range, they fall partially in the “nanoparticle domain.” Some nanoparticles can be taken up by different types of cells and are able to cross the cell membrane and become internalized. Importantly, the interaction of nanoparticles with the cells and their uptake can occur by, for example, endocytosis or by phagocytosis in specialized cells. It should be noted, however, that no bioaccumulation studies with Monoazo Pigments (or other classes of organic pigments) where these mechanisms could be reliably confirmed have been identified.
One more area of uncertainty is the use of partition coefficients to predict the bioaccumulation potential of pigments when BAF/BCF data are not available. For example, based on high log (Soct/Sw) and log Koc values (4.6 and 5.5, respectively), PY1 may theoretically be expected to have relatively high bioaccumulation potential; however, the measurement of these properties is itself uncertain, and extrapolation from partitioning properties to a bioaccumulation result within an organism without consideration of the organism’s physiology is also highly uncertain.
Another area of uncertainty is data extrapolation across the subsets of Monoazo Pigments, when the substances from different subsets (pigment classes) could not always be considered close structural analogues.
Finally, uncertainties are also associated with the lack of information on environmental concentrations of Monoazo Pigments in Canada. It is also recognized that releases from waste disposal sites are possible, although difficult to quantify due to the lack of data, and would contribute to overall environmental concentrations. However, it is anticipated that the proportions of these substances released to the various environmental media would not be significantly higher than those estimated here, given that conservative assumptions were used in the exposure analysis.
7. Potential to Cause Harm to Human Health
The potential to cause harm to human health for the Monoazo Pigments Subgroup is characterized for each of the subsets as summarized in Table 7-1 with the focus on substances to which the exposure for the general population of Canada is expected. As Monoazo Pigments are generally used in a variety of products available to consumers, exposure assessment focuses on the products that lead to the most conservative estimates of exposure for each route (oral, dermal and inhalation). Additional information on the health effects from structurally-related substances within a given subset (denoted by “*” in Table 7-1 below) was used for read-across in some cases.
| Monoazo Pigment subset | SubstanceFootnote Table 7-1 [a] | Exposure potential for the general population of CanadaFootnote Table 7-1 [b] | Health effects data availabilityFootnote Table 7-1[c] |
|---|---|---|---|
| β-Naphthol pigments | PR4 | Yes | Short-term toxicity; subchronic toxicity; chronic toxicity/ carcinogenicity; genotoxicity |
| β-Naphthol pigments | PO5 | Yes | Short-term toxicity; subchronic toxicity; chronic toxicity/ carcinogenicity; genotoxicity; absorption (in vitro dermal); microbial azo reduction (other) |
| β-Naphthol pigments | NONPA | Yes | - |
| β-Naphthol pigments | PR6 | No | - |
| β-Naphthol pigments | PO2 | No | genotoxicity |
| β-Naphthol pigments | PR3Footnote Table 7-1 [*] | N/A (read-across) | Short-term toxicity; subchronic toxicity; chronic toxicity/ carcinogenicity; genotoxicity; absorption/metabolism (oral); microbial azo reduction (fecal) |
| β-Naphthol pigments | Para Red[*] | N/A (read-across) | genotoxicity; metabolism (oral); microbial azo reduction (fecal) |
| β-Naphthol pigment lakes | PR49:1 | Yes | - |
| β-Naphthol pigment lakes | PR53 :1 | Yes | Short-term toxicity; subchronic toxicity; chronic toxicity/ carcinogenicity; genotoxicity; absorption/metabolism (oral); absorption (in vitrodermal); microbial azo reduction (fecal) |
| β-Naphthol pigment lakes | PR50:1 | No | genotoxicity; microbial azo reduction (fecal) |
| β-Naphthol pigment lakes | PR49[*] | N/A (read-across) | Short-term toxicity; subchronic toxicity; chronic toxicity/ carcinogenicity; genotoxicity |
| BONA pigment lakes | PR48:2 | Yes | Short-term toxicity; genotoxicity |
| BONA pigment lakes | PR63:1 | Yes | Subchronic toxicity; genotoxicity; microbial azo reduction (fecal, skin) |
| BONA pigment lakes | PR52:1 | Yes | - |
| BONA pigment lakes | PR52:2 | Yes | - |
| BONA pigment lakes | PR48:5 | No | - |
| BONA pigment lakes | PR57:1[*] | N/A (read-across) | Short-term toxicity; subchronic toxicity; chronic toxicity/ carcinogenicity; genotoxicity; absorption (in vitro dermal) |
| Monoazo yellow pigments | PY1 | Yes | Short-term toxicity; genotoxicity |
| Monoazo yellow pigments | PY3 | Yes | Short-term toxicity; genotoxicity |
| Monoazo yellow pigments | PY73 | Yes | genotoxicity;microbial azo reduction (fecal) |
| Monoazo yellow pigments | PY74[*] | N/A (read-across) | Short-term toxicity; subchronic toxicity; genotoxicity; absorption/ metabolism (oral); microbial azo reduction (other) |
| Monoazo yellow pigments | CAS RN 80675-49-6[*] | N/A (read-across) | Short-term toxicity; genotoxicity |
| Naphthol AS pigments | PR5 | Yes | - |
| Naphthol AS pigments | PR112 | Yes | Short-term toxicity; genotoxicity; microbial azo reduction (other) |
| Naphthol AS pigments | PR170 | Yes | Short-term toxicity; genotoxicity |
| Naphthol AS pigments | PR187 | Yes | - |
| Naphthol AS pigments | PR266 | Yes | - |
| Naphthol AS pigments | PR268 | NoFootnote Table 7-1 [d] | genotoxicity; microbial azo reduction (fecal) |
| Naphthol AS pigments | PO38 | Yes | - |
| Naphthol AS pigments | NAPNPA | No | - |
| Naphthol AS pigments | NANPAP | No | - |
| Naphthol AS pigments | NAPMPA | No | - |
| Naphthol AS pigments | NAPPA | No | - |
| Others | PR247:1 | NoFootnote Table 7-1 [e] | - |
| Others | PO36 | Yes | - |
| Others | PR251 | No | - |
| Others | PY60 | No | - |
| Others | NSNAC | No | - |
7.1 Exposure Assessment
7.1.1 Environmental Media and Food
Empirical data on concentrations of the 32 Monoazo Pigments in environmental media in Canada or elsewhere were not identified.
Due to the very low vapour pressures of these substances, inhalation of the volatile fraction via air is not expected to be a significant route of exposure (refer to Environmental Fate section). Similarly, due to the very low volatility and water solubility of Monoazo Pigments, these substances are expected to be adsorbed onto soil and sediments when released to water. In addition, the soil PECs values were derived from biosolids land application considering that Monoazo Pigments may be present in wastewater biosolids after wastewater treatment. However, exposure of the general population to Monoazo Pigments through environmental media is not expected to be significant.
A number of Monoazo Pigments are used in food packaging and as a result may potentially be present in food (see Table 4-2 of the Uses section). However, based on the minimal potential for direct food contact, exposure from this use is either not expected or is not considered to be significant .
7.1.2 Products
Among the substances considered in this assessment, potential exposure of the general population from use of products was identified for 19 substances from various subsets of β-naphthol pigments (PR4, PO5, NONPA), β-naphthol pigment lakes (PR49:1, PR53:1), BONA pigment lakes (PR48:2, PR63:1, PR52:1, PR52:2), monoazo yellow pigments (PY1, PY3, PY73), naphthol AS pigments (PR5, PR112, PR170, PR187, PR266, PO38) and other (PO36).
A variety of exposure scenarios were considered, based on the expected uses of Monoazo Pigments, including finger paint and cosmetic products such as face paint. Both finger paints and face paints were considered as sentinel products for per event oral and dermal exposure based on uses identified in the paints and coatings sector (see Uses section). In addition, face mask, body cream, lotion or moisturizer and lipstick were identified as sentinel products for the cosmetics uses. Exposure estimates were derived based on either substance specific information or generic estimates (refer to Appendices –F and G for details).
For incidental mouthing of certain products by a child, finger painting is considered to result in higher exposure compared with face paint, due to the higher likelihood of ingestion during the activity. For dermal exposure from finger painting and face painting, the pigment content in paint determines which activity results in higher exposure estimates (see Appendix 7). While some face paints are notified to Heath Canada (Health Canada, 2011c) at a range of 0.3–10%, a concentration range of 10–15% (Derivan 2012) was used as a generic default for pigments in face paints where Canadian data was unavailable. For finger paints, substance-specific concentrations were used when available which generally did not exceed concentrations of 5% (personal communication, email from Duke University Toxicology Program to the Existing Substances Risk Assessment Bureau [Health Canada], dated 2013; unreferenced). Otherwise, a concentration range of 1–3% (Delta Creative 2008) was used for finger paints where substance-specific information was not available. Since the concentration of 15% pigment in face paints exceeds that of finger paints, only the face paint scenario was used to cover off the most conservative short-term dermal exposure estimate.
Estimates resulting in the highest exposure across the identified products are summarized for each route of exposure in the following sections for each subset of Monoazo Pigments. Details of estimated exposure are available in Appendices –F and G.
β-Naphthol Pigments (PR4, PO5 and NONPA)
The oral and dermal exposure to the β-naphthol pigments is shown in Table 7-2. The oral per event exposure from the use of finger paint estimated for β-naphthol pigments range from 0.15 to 0.45 mg/kg-bw per event. The oral daily exposure estimate of 0.0102 mg/PR4 /kg-bw per day was based on 3% (w/w) PR4 lipstick (personal communication, emails from the Consumer Product Safety Directorate [Health Canada] to the Existing Substances Risk Assessment Bureau [Health Canada], dated 2013-2014; unreferenced) The per event dermal exposure to PR4 was estimated to be up to 0.054 mg/kg-bw in children’s face paint and up to 0.136 mg/kg-bw per day in finger paints, while the dermal daily exposure estimate to PR4 in adult face mask is 0.005 mg/kg-bw per day. For inhalation exposure to PR4, PO5 and NONPA from wall painting with an airless sprayer and sanding paint, a weight fraction of 5% pigment in the paint was used (Household Products Database 1993– ; Rust-Oleum Corp. 2006). The per event exposure was estimated to be 0.00037 and 0.00055 mg/kg-bw respectively from these uses.
| Product (age group) | Substance | Concen-tration range (% w/w) | Oral per event (mg/kg-bw) | Oral daily (mg/kg-bw per day) | Dermal per event (mg/kg-bw)Footnote Table 7-2[d] | Dermal daily (mg/kg-bw per day)[d] |
|---|---|---|---|---|---|---|
| Finger paint (child) | PO5 | 0.57Footnote Table 7-2 [a] | 0.15 | - | 0.042 | - |
| Finger paint (child) | PR4 | 1.75[a] | 0.45 | - | 0.129 | - |
| Face paint (child) | PR4 | 0.1–1Footnote Table 7-2[b] | 0.0136 – 0.136 | - | 0.0054–0.054 | - |
| Face mask (adult) | PR4 | less than 1Footnote Table 7-2[c] | - | - | less than 0.017 | less than 0.0049 |
| Lipstick (adult) | PR4 | less than 3[c] | - | 0.0102 | - | - |
| natural health products (child) | PR4 | N/AFootnote Table 7-2 [e] | - | 0.032 – 0.065f | - | - |
| natural health products (adolescent) | PR4 | N/A[e] | - | 0.017Footnote Table 7-2[f] | - | - |
| natural health products (adult) | PR4 | N/A[e] | - | 0.014[f] | - | - |
PR4 is identified as a non-medicinal ingredient under the name D&C Red No. 36 in several types of licensed natural health products intended to be taken by the oral route and is permitted for oral use up to 1.0 mg/day (LNHPD 2008; NHPID 2011). Based on the use of PR4 in multi-vitamin/mineral supplements for which daily exposure would be expected, oral exposure to PR4 from this use corresponds to conservative exposure estimates of 0.014, 0.017, and 0.032-0.065 mg/kg-bw per day for adults, adolescents, and children respectively(Table 7-2).
β-Naphthol Pigment Lakes (PR49:1 and PR53:1)
The oral and dermal exposure to the β-naphthol pigment lakes is shown in Table 7-3. For PR49:1 and PR53:1, finger paint and face paint were identified as sentinel exposure scenarios. The oral exposure estimate from β-naphthol pigment lakes is 0.52 mg/kg-bw per event from finger paint. The dermal exposure estimate from β-naphthol pigment lakes is 0.14 mg/kg-bw per event for PR53:1 in children’s face paint. For inhalation exposure to PR53:1 from wall painting with an airless sprayer and sanding paint, a weight fraction of 5% pigment in the paint was used (Household Products Database 1993– ; Rust-Oleum Corp. 2006). The per event exposure was estimated to be 0.00037 and 0.00055 mg/kg-bw respectively from these uses.
| Product (age group) | Substance | Concentration range (% w/w) | Oral per event (mg/kg-bw) | Dermal per event Footnote Table 7-3[c] (mg/kg-bw) |
|---|---|---|---|---|
| Finger paint (child) | PR49:1 | 2Footnote Table 7-3 [a] | 0.52 | - |
| Face paint (child) |
PR53:1 | 10–15Footnote Table 7-3[b] | - | 0.09–0.14 |
BONA Pigment lakes (PR48:2, PR52:1, PR52:2 and PR63:1)
The oral and dermal exposure to the BONA pigment lakes is shown in Table 7-4. The oral exposure estimate for BONA pigment lakes is 0.8 mg/kg-bw per event for PR63:1 in finger paint. The dermal exposure estimate is 0.14 mg/kg-bw per event for PR48:2, PR52:2 and PR63:1 in children’s face paint. For inhalation exposure to PR48:2, PR52:1 and PR63:1 from wall painting with an airless sprayer and sanding paint, a weight fraction of 5% pigment in the paint was used (Household Products Database 1993– ; Rust-Oleum Corp. 2006). The per event exposure was estimated to be 0.00037 and 0.00055 mg/kg-bw respectively from these uses.
| Product (age group) | Substances | Concentration range (% w/w) | Oral per event (mg/kg-bw) | Dermal per event Footnote Table 7-4[c](mg/kg-bw) |
|---|---|---|---|---|
| Finger paint (child) | PR48:2, PR52:2, PR63:1 |
0.7–3Footnote Table 7-4[a] | 0.2–0.8 | - |
| Face paint (child) | PR48:2, PR52:2, PR63:1 |
10–15Footnote Table 7-4[b] | - | 0.09–0.14 |
Monoazo Yellow Pigments (PY1, PY3 and PY73)
The oral and dermal exposure to the monoazo yellow pigments is shown in Table 7-5. The oral exposure estimate for monoazo yellow pigments range from 0.21 to 0.34 mg/kg-bw per event from finger paint. The dermal exposure estimate is 0.81 mg/kg-bw per event for children’s face paint. For inhalation exposure to PY1, PY3, and PY73 from wall painting with an airless sprayer and sanding paint, a weight fraction of 5% pigment in the paint was used (Household Products Database 1993– ; Rust-Oleum Corp. 2006). The per event exposure was estimated to be 0.00037 and 0.00055 mg/kg-bw respectively from these uses.
| Product (age group) | Substance | Concentration range (% w/w) | Oral per event (mg/kg-bw) | DermalFootnote Table 7-5 [c] per event (mg/kg-bw) |
|---|---|---|---|---|
| Face paint (child) |
PY1, PY3, PY73 | 0.3–15Footnote Table 7-5[a] | - | 0.016–0.81 |
| Finger paint (child) |
PY1, PY3, PY73 | 0.8–1.3Footnote Table 7-5[b] | 0.21–0.34 | - |
Naphthol AS Pigments (PR5, PR112, PR170, PR187, PR266 and PO38)
The oral and dermal exposure to the Naphthol AS pigments is shown in Table 7-6. The oral exposure estimate for naphthol AS pigments range from 1.4 to 6.1 mg/kg-bw per event for PR5, PR112 and PR170 in finger paint. The dermal exposure estimate is 0.81 mg/kg-bw per event for PR5, PR112, PR170, PR266 and PO38 in children’s face paint. For inhalation exposure to PR112, PR170 and PR266 from wall painting with an airless sprayer and sanding paint, a weight fraction of 5% pigment in the paint was used (Household Products Database 1993– ; Rust-Oleum Corp. 2006). The per event exposure was estimated to be 0.00037 and 0.00055 mg/kg-bw respectively from these uses.
| Product (age group) | Substance | Concentration range (% w/w) | Oral per event (mg/kg-bw) | Dermal per eventFootnote Table 7-6[c] (mg/kg-bw) |
|---|---|---|---|---|
| Face paint (child) |
PR5, PR112, PR170, PR266, PO38 | 10–15Footnote Table 7-6[a] | - | 0.54–0.81 |
| Finger paint (child) |
PR5, PR112, PR170 | 1.1–5Footnote Table 7-6[b] | 0.28 – 1.29 | - |
PO36 (Benzimidazolone Pigment)
For PO36, exposure was determined for the following sentinel products: face paints, and spray paint. The dermal exposure estimate is 0.81 mg/kg-bw per event in children’s face paint. A dermal absorption of 6% was applied to the dermal exposure estimates (refer to the Health Effects Assessment section for more details). For inhalation exposure to PO36 from wall painting with an airless sprayer and sanding paint, a weight fraction of 5% pigment in the paint was used (Household Products Database 1993– ; Rust-Oleum Corp. 2006). The per event exposure was estimated to be 0.00037 and 0.00055 mg/kg-bw respectively from these uses.
Exposure to Monoazo Pigments in Tattoo Inks
PR4, PR112 and PY3 were identified as being present in permanent tattoo inks in Canada (personal communication, email from the Consumer Product Safety Directorate [Health Canada] to the Existing Substances Risk Assessment Bureau [Health Canada], dated 2011 and 2013; unreferenced). In addition, PR63:1, PR170, and PY3 are also listed as ingredients in the MSDS sheets of two brands of tattoo inks available internationally including in Canada (SkinCandy 2013a, 2013b; Starbrite 2013). Therefore, the exposure potential to monoazo pigments from use in tattoos is considered.
Permanent tattoos are a potential source of exposure, as they are injected into the dermis, below the epidermal–dermal junction at a depth of 1–2 mm (Lea and Pawlowski 1987; Sperry 1992). Therefore, in contrast to dermal exposures to monoazo pigments, where dermal absorption is expected to be lower (estimated 6% for monoazo pigments and 1% for pigment lakes), intradermal injection of tattoo ink is considered to be a route of systemic exposure to these substances.
After injection of tattoo ink into the dermis, the fate of the pigment particles is expected to follow one of three paths (Danish EPA 2012). First, injected pigment may migrate upward through the individual needle tracts into the epidermis where it is sloughed off (epidermal removal)(Lea and Pawlowski 1987). Second, removal of the injected pigment can occur over the short-term (first few weeks after injection) by macrophages into the lymphatic system due to the dermal inflammatory response (Sperry 1992). Finally, the injected tattoo pigment which is retained in the dermis forms the stable tattoo by which the pigment is sequestered into secondary lysosomes after being engulfed by dermal fibroblasts and macrophages (Bäumler et al. 2000). The fractions of injected pigment making up the short-term removal and the stable tattoo are together considered potential sources of systemic exposure from a single tattoo.
For the fraction of the tattoo subject to lymphatic removal over the short-term, a short-term exposure estimate for the monoazo pigments in tattoo inks was based mainly on the results of Engel et al. (2009), an in vivo study of mice that were injected with monoazo Pigment Red 22 into their dermis. The short-term daily systemic exposure was estimated to range from 0.12 mg/kg-bw per day - 1.1 mg/kg-bw per day for an adult (refer to Appendix H for details).
For the fraction of injected tattoo pigment forming the stable tattoo, the long-term in vivo fate of this injected material is largely unknown. While fading of tattoos over time is known to occur (Lehner et al. 2011), several mechanisms may be responsible including: ongoing phagocytosis and translocation via the lymphatic system (Gopee et al. 2005, Jemec 2010), photodegradation of the pigment at the tattoo site (Doll et al. 2008, Kuramoto et al. 1996, Engel et al. 2007, 2009; Cui et al. 2004; Vasold et al. 2004; Bäumler et al. 2000, 2004; Hauri 2013), in vivo metabolism, and removal via venous drainage (Danish EPA 2012). The Danish EPA (2012) stated that regarding tattoo exposure, “the current knowledge is considered as being insufficient for a valid quantitative exposure assessment”. Accordingly, an estimate of long-term systemic exposure from monoazo pigments in permanent tattoo inks has not been derived.
7.1.3 Uncertainty In Exposure Estimates
Uncertainty is recognized pertaining to the specific types of paint products that contain some of the Monoazo Pigments presented in this assessment. As a result, because conservative inputs were selected in deriving exposure estimates, the exposure estimates for finger paint, face paint and spray paint have varying levels of uncertainty including the lack of specific pigment content information for the respective types of paint. Where data was available from North American sources, exposure estimates were based on substance-specific concentrations. However, where this information was unavailable, exposure estimates incorporated generic pigment content obtained from non-North American sources. There is uncertainty with respect to the use of generic assumptions for pigment content in finger paints, face paints and spray paints.
7.2 Health Effects Assessment
The health effects assessment of Monoazo Pigments focuses on those substances for which there is current information indicating potential exposure to the general Canadian population (refer to the Exposure Assessment section). The health effects for the various monoazo pigments subsets are presented in Table 7-1 in descending order of confidence (highest to lowest) based on the availability and quality of the health effects data identified.
A key consideration in characterizing the potential hazard of Aromatic Azo and Benzidine-based Substances is the potential generation of aromatic amine metabolites following reduction of the azo bond under anaerobic conditions. While biological azo bond cleavage is generally considered an important metabolic reaction for more soluble azo substances, it is not applicable to the same magnitude to poorly soluble azo pigments such as diarylide pigments, for which the available data from experimental animal studies indicate low to negligible bioavailability and corresponding limited systemic toxicity (Golka et al. 2004; Environment Canada and Health Canada 2013d). In the case of Monoazo Pigments however, the observation of toxicity following oral exposure in several animal studies indicates that some of the monoazo pigments considered in this assessment are sufficiently bioavailable by the oral route. For these substances they are likely to undergo azo bond cleavage in the gastrointestinal tract to some extent to aromatic amine metabolites that may be further converted to reactive electrophilic intermediates through metabolic activation. However, due to the degree of lipophilicity of some of the monoazo pigments (e.g. β-naphthol pigments), these substances may also be absorbed as the parent pigment with azo bond intact. Therefore, another activation pathway of the absorbed parent pigment (oxidative cleavage of the azo bond to which releases benzenediazonium and other reactive electrophilic metabolites) may also potentially exist for some of these substances (Moller and Wallin 2000). General considerations for absorption/metabolism and read-across for health effects are outlined for each subset.
The similarity in physical chemical properties and potential health effects within subsets is considered to be primarily due to the common coupling component for each respective subset while differences between substances in a subset is expected to be due to the unique chemical structures for each substance (e.g., PR4 and PO5 share the 1-amino-2-naphthol coupling component in common to all β-naphthol pigments, however the azo bond is to 2-chloro-4-methylaniline for PR4 and to 2,4-dinitroaniline for PO5). The various monoazo pigment subsets in this Screening Assessment are further subdivided between the uncharged subsets (β-naphthol pigments, Naphthol AS pigments, monoazo yellow pigments) and those pigment “lake” subsets containing ionizable groups (β-naphthol pigment lakes, BONA pigment lakes) Due to the presence of ionizable groups (e.g. –SO3−, –COO−) and dissociation potential, the pigment lake subsets are considered separately from the other non-laked pigment subsets with respect to oral and dermal bioavailability routes.
For the pigment lake subsets (β-naphthol pigment lakes and BONA pigment lakes), a reasonably high degree of oral bioavailability and azo bond cleavage potential is indicated based on an oral absorption/metabolism study on PR53:1 (a β-naphthol pigment lake), together with several in vitro anaerobic fecal incubation studies on PR53:1 and PR50:1 (β-naphthol pigment lakes) as well as PR63:1 (a BONA pigment lake). In contrast, for the pigment subsets, only limited empirical bioavailability data were identified. Based on the collective data for the non-lake Monoazo Pigments, some degree of oral bioavailability of the parent pigment and/or potential azo bond cleavage metabolites is indicated for the β-naphthol pigments subset, while a relatively lower anticipated bioavailability is assumed for the monoazo yellow pigments and naphthol AS pigments subsets by virtue of the lower oral toxicity observed for the latter two subsets.
Absorption for these substances following dermal exposure is anticipated to be much lower than that following oral exposure. Based on available data (Platzek 1999; Stingley et al. 2010; BRI 2012) and taking into consideration the potential for azo bond cleavage by skin bacteria, a dermal absorption value of 1% is considered appropriate for the two pigment lake subsets (i.e. BONA pigment lakes, β-naphthol pigment lakes). Overall the pigments are considered to have higher dermal absorption than the lake pigments, though for both, absorption is still considered to be considerably lower than gastrointestinal (oral) absorption. Based on the available dermal absorption data for PO5 (a β-naphthol pigment) and Solvent Red 23 (a disazo dye containing the lipophilic β-naphthol moiety, with a molecular weight similar to that of PO5 but with greater lipophilicity), a conservative value of 6% dermal absorption is considered for the non-lake pigment subsets.
In the following sections, a health effects assessment that contains a summary of critical health effect levels is presented for each subset.
7.2.1 β-Naphthol Pigments (PR4, PO5, NONPA, PR6, PO2)
Health effects information is available primarily for two of the five β-naphthol pigments (PR4 and PO5) in this subset. Repeated-dose animal studies are also available for PR3, however this substance was previously assessed under the Challenge Initiative (Environment Canada, Health Canada 2009b) and is used for read-across purposes only. All six substances are structurally related by containing a nitro-substituted aniline, which is azo coupled to a β-naphthol moiety (Table 7-7). Reductive cleavage of the azo bond is expected to generate the respective nitro-substituted aniline (Table 7-7) along with the 1-amino-2-naphthol moiety below (CAS RN 2834-92-6, MW 159.2 g/mol). Since 1-amino-2-naphthol is a common structural feature and potential azo cleavage product for all the β-naphthol pigments it is shown below, while the substance-specific parent structures and corresponding azo cleavage metabolites are shown in Table 7-7:

| Parent β-naphthol pigment chemical identityFootnote Table 7-7 [a] and structure | Potential azo bond cleavage productsFootnote Table 7-7 [b] chemical identity and structure |
|---|---|
| PR3 CAS RN 2425-85-6 (D&C Red No. 35) MW 307.3 g/mol 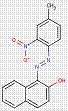 |
2-Nitro-4-methylaniline CAS RN 89-62-3 MW 152.2 g/mol 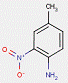 |
| PR4 CAS RN 2814-77-9 (D&C Red No. 36) MW 327.7 g/mol  |
2-Chloro-4-nitroaniline CAS RN 121-87-9 MW 172.6 g/mol  |
| PO5 CAS RN 3468-63-1 (D&C Orange No. 17) MW 338.3 g/mol 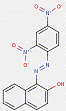 |
2,4-Dinitroaniline CAS RN 97-02-4 MW 183.1 g/mol 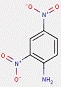 |
| NONPA CAS RN 49744-28-7 MW 323.3 g/mol 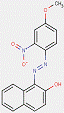 |
2-Nitro-4-methoxyaniline CAS RN 96-96-8 MW 168.2 g/mol  |
| PR6 CAS RN 6410-13-5 MW 327.7 g/mol  |
2-Nitro-4-chloroaniline CAS RN 89-63-4 MW 172.6g/mol 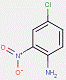 |
| PO2 CAS RN 6410-09-9 MW 293.3 g/mol 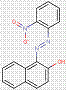 |
2-Nitroaniline CAS RN 88-74-4 MW 138.1 g/mol  |
Para Red*
|
4-Nitroaniline
|
Absorption and Azo Bond Cleavage Potential
For oral absorption/metabolism, one study on PR3 was identified, in which male F344 rats (three per sample period) received a single oral PR3 dose of 11.8 mg/kg-bw by gavage, with gut contents, feces, urine, blood and other tissues sampled at 1, 4, 24 and 48 hours post-dosing (El Dareer et al. 1984). It should be noted that PR3 dose was not radiolabelled in this study and the recovery analysis in feces, urine and tissues only measured unmodified parent PR3. Consequently, total recovery was based only on unmodified parent PR3 and did not include PR3 metabolites or potential azo cleavage products. Recovery of unmodified PR3 from feces and all sampled tissues was incomplete; 50.0% (± 10.1%) and 72.4% (± 10.1%) of the administered PR3 dose were recovered at the 24- and 48-hour sampling times respectively.
The majority of the PR3 dose was recovered in the gut contents and feces, with small amounts ( less than 1%) detected in the 24- and 48-hour urine samples. Limited amounts of PR3 ( less than 2%) were also detected in “tissues directly in contact with the compounds,” while no PR3 was detected in other sampled tissues (plasma, whole blood, liver, kidneys, lung) at a reported dose of 10 times higher (data not presented in the study). Although the study was limited (i.e. PR3 conjugates and azo cleavage metabolites not included in measurement, large standard deviations in recovery of PR3), some information on oral absorption of PR3 can be interpreted. First, the presence of detectable levels of unmodified PR3 in the urine, although low ( less than 1%) provides evidence for absorption of the intact parent PR3. This observation is consistent with more lipophilic azo β-naphthol substances such as Sudan I which was excreted unmodified in small amounts in the urine of rats and rabbits following oral exposure while much larger fraction of the dose was excreted as Sudan I conjugates and azo cleavage metabolites (Environment Canada, Health Canada 2014a). The presence of unmodified PR3 in the urine of rats in this study together with the incomplete recovery of the administered PR3 dose suggests that some fraction of the PR3 dose was either absorbed intact and subsequently metabolized in vivo and/or underwent azo cleavage to aromatic amines by intestinal microflora in the gastrointestinal tract (NTP 1992).
Information on in vivo metabolism and microbial azo reduction is available for the structurally-related substance Para Red* and is used for read-across to the other β-naphthol pigments in this subset. Para Red* is a β-naphthol pigment with the name C.I. Pigment Red 1 (C.I. 12070) (Herbst and Hunger 2004) and is a positional isomer of PO2 with the mononitro-substitution in para- vs ortho- position respectively (Table 7-7). In a study using F344 rats (8 males/group), “free amines” (specific chemical identity not investigated) were detected in the feces following a single oral dose of 15mg Para Red* (Goldin and Gorbach 1984). Additional Para Red* treatment groups were also exposed to antibiotics to reduce the level of azo reductase activity of the intestinal microflora and subsequently lower amounts of total amines were detected in the feces in these groups. Although this study did not distinguish which “free amines” were detected, the authors interpreted the results as evidence for azo cleavage of Para Red* and that the antibiotics reduced the level of azo cleavage of this substance (Goldin and Gorbach 1984). In two separate in vitro studies using a human fecal suspension or with cultured human intestinal bacteria, Para Red* was also found to undergo near complete azo cleavage to the predicted metabolite 4-nitroaniline after 1-2 days of incubation under anaerobic conditions (Xu et al. 2007; Xu et al. 2010)
Further support for the microbial azo bond cleavage potential of β-naphthol pigments comes from a study on PO5 with the environmental bacterium Shewanella strain J18 143 under anaerobic conditions (Pearce et al. 2008). Azo bond cleavage was measured as a reduction in the PO5 absorption by ultraviolet–visible spectroscopy. This study also demonstrated that the reduction was efficient for the pigment dispersion form, while it was much more limited for the pigment powder, in part due to the lower solubility in the culture medium. An in vitroincubation of PR3 in culture medium with a human fecal homogenate showed similar results due to its low solubility (BRI 2013).
The above studies, together with the empirical observations of toxicological effects in animals dosed orally with PR3, PR4 and PO5 (refer to the following Health Effects section), suggest that some amounts of these β-naphthol pigments and/or their azo cleavage metabolites are absorbed at levels sufficient to elicit biological responses. However, a quantitative estimate of absolute oral absorption for the β-naphthol pigments and relative fraction of moiety absorbed (parent pigment vs azo cleavage products) cannot be determined due to the limited data available.
For dermal absorption, one in vitro dermal penetration study identified for PO5 reported that most of the PO5 was recovered from skin washes, while some radioactivity was found in all skin preparations. The daily absorption of the dose applied to skin was estimated to be 0.005% for creams and oil formulations and 0.001% for talc; however, these estimates were based only on concentrations in the receptor fluid (Hart et al. 1986; US FDA 1986; BG Chemie 2000a). From a more recent in vitro study with a lipophilic azo solvent dye, Solvent Red 23, similar recovery was obtained in the receptor fluid when it was applied to human skin in a hydrophobic suntan oil formulation; up to 6% of the applied dose was found in the human skin epidermis (excluding stratum corneum) and dermis (Yourick et al. 2007). β-Naphthol pigments and Solvent Red 23 have similar molecular weights (approximately 300–350 g/mol) and both contain the lipophilic β-naphthol moiety, and exhibit extremely low water solubility (3.3-6.8 µg/L for β-naphthol pigments (Appendix B), 13.7 µg/L for Solvent Red 23 (Environment Canada, Health Canada 2014a)), although Solvent Red 23 is much more lipophilic than the β-naphthol pigments (estimated log Kow greater than 7 for Solvent Red 23 vs. log (Soct/Sw) of 2.4–3.7 for β-naphthol pigments). The absolute lipid solubility of Solvent Red 23 (“soluble” in oils; IARC 1975) is far greater than that of the β-naphthol pigments (1.8–18 mg/L in octanol); therefore, the fraction dissolved in a hydrophobic formulation available for dermal absorption would be much lower for the β-naphthol pigments than for Solvent Red 23. Nonetheless, the β-naphthol pigments are considered to have some degree of lipid solubility, leading to potential dermal absorption. Consequently, application of 6% dermal absorption from Solvent Red 23, based on the recovery from skin layers and receptor fluid, is considered as appropriate.
Health Effects
A summary of critical effect levels for the β-naphthol pigments is presented in Table 7-8. The available toxicity data have largely been reviewed in previous evaluations of PR3 (NTP 1992; Environment Canada, Health Canada 2009b), PR4 (US FDA 1988) and PO5 (Hart et al. 1986; US FDA 1986; SCC1993; BG Chemie 2000a). Genotoxicity data on these three substances were also reviewed by Moller and Wallin (2000). For PR3, a point of departure for carcinogenicity has been derived since the previous evaluation by Environment Canada, Health Canada (2009b) (refer to Appendix I for details of benchmark dose (BMD) calculation for PR3).
| Substance | Short-term LOAEL/ NOAEL (mg/kg-bw per day) | Subchronic LOAEL/ NOAEL (mg/kg-bw per day) |
Chronic LOAEL/ NOAEL (mg/kg-bw per day) |
Cancer Point of Departure (mg/kg-bw per day) |
|---|---|---|---|---|
| PR3 | LOAEL = 541–542(MR, FR) ↑ liver wt ↑ ALT ↑ SDH ↓ Hb ↓ hematocrit (NTP 1992) |
LOAEL = 125–183(MR) ↑ liver wt, histopathology of spleen + bone marrow ↓ hematocrit ↑ reticulocytes ↑ serum creatinine ↑ urine bilirubin (Graham and Davis 1968; NTP 1992) |
LOAEL = 272 (MR) BMDL10 = 11–15 (MR) |
BMDL10 = 911 0/50 (0%) 321 mg/kg-bw per day 1/50 (2%) 682 mg/kg-bw per day 10/50Footnote Table 7-8 [b] (20%) 1389 mg/kg-bw per day |
| PR4 | Read-across from PR3 and PO5 | Read-across from PR3 and PO5 | LOAEL = 50 NOAEL = 12.5 (MR/FR) Histopathological changes in spleen, hemolytic anemia (US FDA 1988) |
Read-across from PR3 and PO5 Supporting data from PR4: liver neoplastic nodules and cystic cholangioma in female rats (Kupradinun et al. 2002) |
| PO5 | LOAEL = 100 (MR/FR) ↓ Hb and RBCs ↓ body wt gain (Hoechst 1973; BG Chemie 2000a) |
LOAEL = 7.5 (dog) ↑ urine bilirubin (Hazleton 1964a; SCC 1993; BG Chemie 2000a) |
LOAEL = 50(MR/FR) ↑ liver wt ↓ hematocrit + Hb + RBCs ↑ urine bilirubin (Hazleton 1964b; Biodynamics 1982) |
tumorigenic dose = 500 (FR) 3/52 (6%) control 21/59Footnote Table 7-8 [c],Footnote Table 7-8 [d] (36%) 500 mg/kg-bw per day (10,000ppm diet) (Biodynamics 1982; BG Chemie 2000a; Hart et al. 1986; US FDA 1986) |
Genotoxicity
In vitro genotoxicity assays were identified for PR3, PR4,PO2, PO5, and Para Red*, while some in vivo assays were also identified for PR3 and PO5 (BG Chemie 2000a; Moller and Wallin 2000; Environment Canada, Health Canada 2009b).
For Salmonella in the standard Ames assay, the mutagenic potency appears to be strongest for PO5, followed by PR4, then PR3, which demonstrated a weakly positive response in one study. PO5 was reported to be consistently positive in strain TA98, with a much stronger response without metabolic S9 activation, compared to with S9. Positive results were also observed for strains TA100, TA1537 and TA1538, but results varied among the different studies (BG Chemie 2000a; Moller and Wallin 2000). PR4 demonstrated a mutagenic response, the strongest of which was also in TA98, but generally only after S9 activation; responses in other strains were mixed or negative (Moller and Wallin 2000). PR4 was also positive in TA98 with and without S9 when the flavin mononucleotide (FMN) Prival modification was included in the assay (ILS 2011). PR3 appeared to be much less mutagenic than either PR4 or PO5, with only a weak positive response observed in TA98 and TA100 with metabolic S9 from hamster rather than rat (Mortelmans et al. 1986) and negative in other strains with or without S9 in other studies (Moller and Wallin 2000; Environment Canada, Health Canada 2009b). PO2 was only tested in one study in which it was reported to be positive for mutagenicity in TA98 without S9 activation (Matsushima et al.1978). The positional isomer of PO2, Para Red* was also positive for mutagenicity in strains TA98 and TA1538 only with S9 activation, and was negative in TA1535 with and without activation (Milvy and Kay 1978).
In a more recent study, Para Red* and a more lipophilic β-naphthol azo dye Sudan I were tested for their comparative genotoxic potential following oxidative metabolism in human B lymphoblastoid immortal cell lines AHH-1 and MCL-5, designed to express high levels of P450 activity (Johnson et al. 2010). The AHH-1 cell line expresses inducible CYP1A1 while the cell line MCL-5 cell exhibits activity for additional five other CYP enzymes along wth higher levels of CYP1A1 compared to AHH-1. Since Sudan I is known to be activated to benzenediazonium and other DNA-reactive electrophiles following microsomal and peroxidase mediated oxidation (Stiborova et al. 2009), dose-response analysis of its genotoxicity following oxidation was investigated in AHH-1 and MCL-5 cells while Para Red* was also included to determine if the common β-napthol azo structure conferred a genotoxic potential similar to Sudan I (Johnson et al. 2010). In this study, Para Red* induced forward mutations in vitro at the hprtlocus of both AHH-1 and MCL-5 with higher mutagenicity observed in the MCL-5 line correlating to relatively higher levels of of P450 activity expressed in MCL-5 cells (Johnson et al. 2010). A similar correlation between the micronuclei and P450 activity was observed for Para Red* with a greater response seen in the MCL-5 cells due to higher P450 activity. In this study the dose-response for mutagenicity and mincronuclei were identical for Para Red* and Sudan I, however with a lower relative potency for Para Red*. This study provides evidence that a β-naphthol monoazo pigment, Para Red*, with nitro-substitution on benzene ring and lower water solubility and lipophilicity, nonetheless exhibited similar genotoxic potential comparable to the more lipophilic Sudan I. Based on these observations, the study authors proposed that like Sudan I, Para Red* was similarly oxidized to benzenediazonium ion and/or other DNA reactive electrophiles following oxidation by microsomal enzymes. Similarly, other studies have also demonstrated that oxidation of Sudan I and another low solubility β-naphthol azo dye NDY3Footnote[9] by peroxidases also form reactive electrophilic metabolites via a similar pathway (Stiborova et al. 2009; Spadaro and Renganathan 1994). Although data is limited, the information above suggests that besides Sudan I, other related β-naphthol azo substances may also be similarly oxidized as the parent azo structure to reactive metabolites (Moller and Wallin 2000). However, it is uncertain the degree to which this potential mechanism occurs for the β-naphthol pigments considered in this assessment and whether it contributes to the toxicity and genotoxicity observed for these substances.
Other evidence of genotoxicity of the β-naphthol pigments comes from the comet assay in vitro (PO5) and in vivo(PR3). PO5 was positive in the comet assay in exposed rat hepatocytes (Moller et al. 1998), while oral exposure of male ddY mice to PR3 showed increased deoxyribonucleic acid (DNA) damage in colon tissue 24 hours after exposure (Tsuda et al. 2000), thus indicating a potential for DNA damage by these substances. Conversely, all other in vitro genotoxicity tests were negative for PR3 and PO5. Mutagenicity in in vitromammalian cells was tested only for PO5, which showed a negative response in mouse lymphoma L5178Y cells and Chinese hamster V79 cells (BG Chemie 2000a). No increase in chromosomal aberrations was reported from studies on Chinese hamster cells in vitrofor PR3 (Chinese hamster ovary [CHO] cells; Environment Canada, Health Canada 2009b) or PO5 (V79 cells; BG Chemie 2000a), and PR3 was also negative for sister chromatid exchanges (SCEs) in CHO cells (Environment Canada, Health Canada 2009b). PO5 was also reported as negative in the unscheduled DNA synthesis (UDS) assay in primary rat hepatocytes (BG Chemie 2000a). The only identified in vivo genotoxicity studies were negative for chromosomal aberrations in bone marrow of Chinese hamsters exposed to PO5 by gavage (Hoechst 1990; BG Chemie 2000a) or for micronuclei in the bone marrow or spleen of mice following intraperitoneal injection (Baranski et al. 1992; Environment Canada, Health Canada 2009b).
Data on several β-naphthol pigments (PR3*, Para Red*, PR4, PO5, PO2) indicate some genotoxic potential via mutagenicity and DNA damage although a range of potency was observed across the tested substances (e.g. qualitative ranking of mutagenic potency in Salmonella was highest for PO5, intermediate for PR4, and weak for PR3). No genotoxicity studies were identified for two β-naphthol pigments in this subset (NONPA, PR6). However, there is some evidence from a comparative study on Para Red* and Sudan I (Johnson et al. 2010) that both of these β-naphthol containing azo substances are activated via a similar pathway, via formation of reactive metabolites after microsomal oxidation. If true, this activation pathway for mutagenicity may apply to other β-naphthol pigments in this subset. This is consistent with the opinions by the European Food Safety Authority (EFSA) and the UK Committee on Toxicology (UK COT), that based on structural similarities to Sudan I and in the absence of data, Para Red* was assumed to be potentially genotoxic (EFSA 2005; UK COT 2005). Overall, there appears to be some evidence of mutagenic potential for the β-naphthol pigments.
Carcinogenicity
Carcinogenic effects were observed in chronic rodent studies for PR3 (NTP 1992) and PO5 (Hart et al. 1986; US FDA 1986; BG Chemie 2000a) with an increase in liver tumours in female rats as the common effect seen for both these substances. In addition to liver tumours in female rats, additional tumours were reported to be related to exposure to both these substances including a higher incidence of tumours of the adrenal medulla in male rats and renal tubule adenomas in male mice for PR3 (NTP 1992) and an increased incidence of hepatocellular adenoma and carcinomas of male CD-1 mice exposed to PO5 (Hart et al. 1986; US FDA 1986; BG Chemie 2000a). The details of observations for neoplastic effects from PR4 and PO5 can be found in other references, however, since the liver tumours in female rats were the consistent observation between PR3 and PO5, this effect is discussed in more detail (below and Table 7-8) along with supporting evidence of a similar effect in female rats exposed to PR4.
PR3 was tested in a chronic feeding study in F344 rats (NTP 1992) and the results from this study were previously described in the screening assessment for this substance as part of the Challenge Initiative (Environment Canada, Health Canada 2009b). A statistically significant increase in hepatocellular adenomas was observed in the high dose group females compared to concurrent controls (Table 7-8). Other non-neoplastic effects in the liver of exposed females were also observed starting at the lowest test dose including histopathologic changes (eosinophilic foci, mixed-cell foci) and increased liver weights (NTP 1992; Environment Canada, Health Canada 2009b) supporting the liver as a target organ for PR3 in this study. Hepatocellular adenoma was reported as an uncommon tumour type in historical control female rats of this strain and therefore the increased incidence observed in exposed females was considered by the NTP to be attributed to PR3 exposure (NTP 1992). A cancer point of departure (BMDL10 = 911mg/kg-bw per day) for PR3 was derived for the hepatocellular adenoma data (Table 7-8, details provided in Appendix I). Additional effects observed in female and male rats from this study are provided elsewhere (NTP 1992; Environment Canada, Health Canada 2009b).
For PO5, several unpublished chronic feeding studies in rats and mice were reported in secondary sources (Hart et al. 1986; US FDA 1986; SCC1993; BG Chemie 2000a). The critical study was in rats (Charles River albino (CD) strain, 70/sex/dose) exposed through the dams during gestation and lactation and then via feed until study termination at 30 months or 130 weeks (Biodynamics 1982; BG Chemie 2000a). A statistically significant increase in combined incidence of hepatocellular adenomas and carcinomas was observed in the high dose females (10,000ppm or 500mg/kg-bw per day) compared to the concurrent control group (Table 7-8). Similar to the chronic PR3 study, additional non-neoplastic effects in the high-dose females exposed to PO5 included increased liver weights and histopathological changes in the liver also at the high dose (focal vacuolation, subacute-nonsuppurative hepatitis, zonal necrosis/degeneration). Besides increased liver weights which were also observed at the next lower dose (1000ppm or 50mg/kg-bw per day), limited details were provided in the secondary source for the incidence or tumours or non-neoplastic effects in the liver at the lower doses in this unpublished study (BG Chemie 2000a). Other details on effects from the chronic PO5 studies are provided in the secondary sources (Hart et al. 1986; US FDA 1986; SCC 1993; BG Chemie 2000a).
For PR4, a secondary source reported that several unpublished chronic oral studies on PR4 had been conducted in the rat and mouse (US FDA 1988). However, these original data were not available, and only very limited details were provided in the secondary source for the non-neoplastic and carcinogenic effects in the liver for these studies. Therefore, for comparison to the female rat liver carcinogenicity observed for PR3 and PO5, in the absence of details for the unpublished PR4 chronic rat studies, a more recent published chronic rat study for PR4 is also considered here (Kupradinun et al. 2002). Briefly, this study exposed Wistar rats (50/sex/dose) to PR4 in the feed (control, 1000ppm or 50mg/kg-bw per day, 2000ppm or 100mg/kg-bw per day) for 78 weeks with the terminal sacrifice at 98 weeks. A weakly dose-dependent increased incidence in liver tumours (combined incidence of benign tumours: hyperplastic nodules and cystic cholangiomas) in the female rats (control 3/50 or 6.0%, 1000ppm 6/47 or 12.8%, 2000ppm 8/50 or 16.0%, p=0.059, Cochrane-Armitage Trend Test) was reported to be related to PR4 exposure (Kupradinun et al. 2002). No other details on liver effects (clinical chemistry, liver weights, non-neoplastic histopathology) were provided in this study.
While the Kupradinun et al. study demonstrated some potential for carcinogenicity of PR4 in female rat liver at doses much lower than the doses tested for either PR3 or PO5, the tumour response above the concurrent control group seen for PR4 was relatively weaker than that observed for either PR3 or PO5 (Table 7-8). The exposure duration for PR4 in Kupradinun et al. (2002) was shorter (78 weeks) compared to the studies conducted with either PR3 (102 weeks, NTP 1992) or PO5 (130 weeks, Biodynamics 1982). Therefore, although the terminal necropsy for PR4 occurred at 98 weeks in Kupradinun et al. (2002), the relatively shorter exposure and termination periods in this study may have resulted in a tumour incidence for PR4 if the tumours were late developing. Besides the shorter exposure duration, other limitations of the Kupradinun et al. study included small dose spacing between the high and low doses, limited reporting of non-cancer effects in the liver or other tissues, and lack of historical liver tumour incidence for female Wistar rats from this laboratoryFootnote[10]. However, based on consideration of read-across of observed liver tumours in female rats for PR3 and PO5, similar expected active metabolites for PR4, PR3 and PO5 (i.e., nitroaniline derivatives and 1-amino-2-naphthol, Table 7-7) and the supporting evidence of a tumour response for PR4 in the liver of female rats from Kupradinun et al. (2002), it is considered that PR4 may also pose a carcinogenic potential similar to PR3 and PO5.
The overall evidence for PR3, PR4 and PO5, principally from observed liver tumours in female rats, indicates a carcinogenic potential for these substances. No empirical cancer studies were identified for the other β-naphthol pigments (NONPA, PO2, PR6, Para Red*). The potential for read-across on carcinogenicity to the untested β-naphthol pigments is discussed in a following section (Section 7.2.1 Summary and Considerations for Read-Across).
Other Repeated-dose Toxicity Studies
For PR3, PR4 and PO5, similar non-cancer effects were noted, the most common being hemolysis, red blood cell regeneration, and increased weights of and histopathological changes in the spleen and liver in short-term, subchronic and chronic studies (Table 7-8). For PR3, the hemolysis observed in the hematology findings (decreases in hematocrit, hemoglobin and/or red blood cells, increased reticulocytes) was observed in rats and/or mice following 2-week and 90 day exposure durations and at the 15-month interim sacrifice for the chronic portion of these studies (NTP 1992). These hemolytic effects were typically associated with corresponding histopathologic changes in the spleen (increased hematopoietic cell proliferation and pigment/hemosiderin) in the short-term and sub-chronic studies, however this effect was no longer clearly observed in the chronic portion of the study. Histopathologic changes in the liver were also observed in the rat following PR3 exposure with increased liver weight at all exposure durations, clinical chemistry findings after 2 weeks (increased ALT and SDH), and histopathologic liver changes in the interim and terminal sacrifice of the chronic study (focal cellular changes, cystic degeneration). While the liver toxicity was observed in both sexes of rats exposed to PR3, only the females demonstrated exposure-related increase in hepatocellular adenomas (NTP 1992, see previous section). For PO5, while the original toxicity studies were unpublished, details from secondary sources indicated similar observations of hemolysis and associated regenerative responses in the spleen, along with increased weights and histopathologic changes in the liver observed in mice, rats and dogs (BG Chemie 2000a). For PR4, all available data on non-neoplastic effects was unpublished and came from limited reporting in a secondary source (US EPA 1988). However, the available information indicated hemolysis and associated histopathologic changes in the rat spleen (parenchymal fibrosis, capsular thickening, capsular fibrous tags, extramedullary hematopoiesis, pigmentation) was also observed for this substance (Table 7-8) while observations of non-neoplastic effects in the liver were not reported in the secondary sources.
Additional effects in the kidney in both rats and mice were also noted for PR3, including increased incidence and severity of chronic progressive nephropathy, hyperplasia of tubules and renal papillary transitional cell epithelium, in addition to renal tubule cytomegaly and adenomas in male mice (NTP 1992). Since the kidney was not clearly identified as a target organ for toxicity in studies on the other β-naphthol pigment PO5 or PR4, it is uncertain whether this effect is substance specific or may be expected following exposure to the other β-napthol pigments.
No repeated-dose toxicity studies were identified for the other β-naphthol pigments (NONPA, PO2, PR6, Para Red*)
Summary and Considerations for Read-Across
Toxicity data were not identified for all the substances in the β-naphthol pigment subset, therefore a read-across approach is used to inform the health effects for substances in this subset based on the following lines of evidence.
Based on the available data for oral absorption (PR3), microbial azo reduction (Para Red*, PO5), and similar toxicity in animal studies for the β-naphthol pigments tested (i.e. PR3, PR4, PO5), it is considered that some amount of these substances are systemically absorbed by the oral route either as the parent β-naphthol pigment and/or azo cleavage metabolites (1-amino-2-naphthol, various nitroanilines). Since the other members of the β-naphthol pigment subset share the same parent β-naphthol azo structure, similarity of the azo cleavage metabolites, and are expected to have similar physical-chemical properties including water and octanol solubility, it is considered that comparable oral bioavailability can be expected for all substances in this subset.
While limited mechanistic data is available for these substances, based on the existing body of literature on health effects of azo substances in general, it is understood that the observed toxicity for azo substances is considered due to the aromatic amine metabolites following azo cleavage (prior to absorption or in vivo following absorption) and/or by activation of the absorbed parent azo substance (Environment Canada, Health Canada 2013b). For other azo substances sharing the same β-naphthol azo structure as the β-naphthol pigments, it is known that activation of the parent azo may contribute as much or more to the toxicity than their corresponding aromatic amine metabolites. For example, the principle adverse effect of Sudan I in rodents is a dose-dependent increase of hepatic tumours in the livers of rats following chronic oral exposure at relatively low doses (Environment Canada, Health Canada 2014a), an effect not observed for aniline, one of the dominant azo cleavage metabolites of Sudan I. Since Sudan I is known to be absorbed in part as the parent structure with intact azo bond (Environment Canada, Health Canada 2014a), and since electrophilic metabolites of Sudan I parent structure following enzymatic oxidation have been demonstrated to cause DNA adducts of rat livers in vivo(Stiborova et al. 2009), it is considered that the activated parent structure of Sudan I contributes to the liver tumours observed in rodents. A similar observation is observed in genotoxicity studies of Sudan I, with observed in vitro mutagenicity of Sudan I in the Ames test being eliminated under reductive test conditions (Environment Canada, Health Canada 2014a), indicating that the parent Sudan I, and not its aromatic amine metabolites are mutagenic in this assay. This is supported in part by a mutagenicity study of both Sudan I and Para Red* which showed elevated mutagenicity conditions favoring increased oxidation by CYP1A1 and other P450 isozymes (Johnson et la. 2010), rather than reductive azo cleavage, and therefore it is the oxidation of the parent structures that is responsible for the observed mutagenicity of Sudan I and Para Red* in these studies. Therefore, with the premise that the toxicity of a β-naphthol azo substance may be due to both the parent and/or the aromatic amine metabolites, and since the β-naphthol pigments are also expected to be absorbed in part as the parent pigment as well as the azo cleavage metabolites, both the parent structures and metabolites are considered to help inform the read-across within the β-naphthol pigments subset.
All parent β-naphthol pigment structures share the common 1-amino-2-naphthol (β-naphthol) joined to aniline by an azo bond, and with at least one nitro-substituent on the benzene ring of aniline in ortho- and/or para- position as well as additional substitutions (methyl-, chloro-, methoxy-) in some cases (Herbst and Hunger 2004, Table 7-7). In the case of the specific β-naphthol pigments tested in chronic animal studies, substances with nitro in both ortho- (PR3, PO5) and para- (PR4, PO5) positions have shown similar toxicity including damage to erythrocytes, as well as toxicity in spleen and liver, and hepatic tumours (see sections above and Table 7-8). Therefore while some apparent differences in potency were observed in these studies (e.g. the point of departure for liver tumours was lower for PO5 compared to PR3), the tested substances appeared to qualitatively demonstrate similar toxicity irrespective of the position of nitro substitution. Similarly, the presence of additional electron withdrawing (chloro- in PR4) or electron donating (methyl- in PR3) substitutions, did not appear to dramatically alter the types of effects observed in animal studies for these substances. It is of note that another β-naphthol azo substance, Sudan I (no substitutions on benzene ring of aniline) was also associated with liver toxicity and increased hepatic tumours in oral exposed rats (Environment Canada, Health Canada 2014a) similar that observed for the β-naphthol pigments PR3 and PO5. The structural similarity of β-naphthol pigments with Sudan I was in fact the basis for the opinions of EFSA and the UK COT to assume that Para Red* may be potentially genotoxic and carcinogenic (EFSA 2005; UK COT 2005). Therefore, despite the differences in position and substitution of nitro- and other substitutions in the β-naphthol pigment subset (Table 7-7), there is nothing obviously apparent in the structures of these substances which would lead to the expectation of qualitative difference in potential toxicity across these substances. However different toxicological potencies would be expected as was observed for the available data on PR3, PR4 and PO5.
This assertion of read-across for the β-naphthol pigments is supported by the available data on toxicity of the corresponding aromatic amine metabolites of the β-naphthol pigments: 1-amino-2-naphthol, and the various nitroaniline derivatives (Table 7-7). The health effects for these azo cleavage metabolites is discussed below as it relates to read-across within the β-naphthol pigments subset.
The aromatic amine 1-amino-2-naphthol is common to all the β-napthol pigments, as well as a common moiety in the β-napthol pigment lakes, Sudan dyes, and some soluble azo dyes such as Acid Orange II and has been detected as a metabolite following oral exposure to those substances (Environment Canada, Health Canada 2014a; SCCS 2011). Although no adequate repeated-dose toxicity studies were identified for 1-amino-2-naphthol, this substance formed covalently bound adducts in erythrocytes of rats in vivo and was considered to be the metabolite most likely responsible for the hemolysis, spleen and liver toxicity observed for the β-napthol pigment lakes (section 7.2.2) and Acid Orange II (SCCS 2011), since the other sulfonated aromatic amines released from these substances are not associated with this type of toxicity. Significantly stimulated liver regeneration in partially hepatectomized rats was also observed following dietary exposure to 1-amino-2-naphthol (Gershbein 1982). 1-Amino-2-naphthol was also shown to generate reactive oxygen species (Nakayama et al. 1983) and be cytotoxic to bacteria (Pan et al. 2012). 1-Amino-2-naphthol may be oxidized to a N-hydroxylamine and subsequent nitroso, but this substance may also be converted to an intermediate 1,2-naphthoquinoneimine and then rapidly hydrolyzed to 1,2-naphthoquinone (Gregory 1986). The formation of naphthoquinone/ quinoneimines is well characterized for many substances containing aromatic amine and aromatic amide structures and is speculated to be another important mode of action for the observed toxicity of these types of substances via generation of reactive oxygen species (ROS) and/or as reactive electrophiles (Kumagai et al. 2012; Skipper et al. 2010). Since exposure to any of the β-naphthol pigments would result in release of some amount of 1-amino-2-naphthol, this moiety could contribute in part to potential health effects for all the β-naphthol pigments providing further support for read-across among these substances.
Besides 1-amino-2-naphthol, the other azo cleavage metabolites of β-naphthol pigments are all nitroanilines or derivatives with additional methyl, chloro, or methoxy substitutions (Table 7-7). Qualitatively similar types of health effects have been observed for these nitroanilines where empirical data are available with effects frequently observed in erythrocytes/spleen, liver and kidney. For 2-nitro-4-methylaniline (from PR3), a 28-day oral study in rats reported evidence of hemolysis, increased liver and kidney weights, and clinical chemistry revealed increased serum urea (males) and serum GGT (females) at levels of 200 and 1000 mg/kg-bw per day in females and males respectively (BG Chemi 2000b). Genotoxicity for this substance displayed mixed mutagenicity in vitro (positive in mouse lymphoma cells, mixed in Salmonella, negative hamster V79 cells), and was negative for unscheduled DNA synthesis in vitro and micronuclei in mice in vivo. For 2-nitro-4-chloroaniline (from PR6), unpublished short-term and subchronic oral studies by the NTP in rats and mice were reported in a secondary source (BUA 2004). In rats, 2-nitro-4-chloroaniline demonstrated target organs effects in the spleen (hemosiderosis), erythrocytes (hemolysis), liver (increased weights), and kidneys (increased weight) with lowest effects observed as low as 50 and 100 mg/kg-bw per day in females and males respectively. Short-term toxicity studies in rats from Japan (CHRIP ©2008a; Sakuratani et al. 2013) also reported effects in liver (increased weight, focal inflammation) and spleen (extramedullary hematopoiesis) confirming the NTP results in rats. In mice, liver appeared to be the main target organ (increased weight, serum liver enzymes, centrilobular hepatic hypertrophy, multinucleated hepatocytes) with mild hemosiderosis in spleen also observed; effects were observed as low as 75 and 300 mg/kg-bw per day in females and male mice respectively (BUA 2004). Both male rats and mice showed some reproductive toxicity in these studies with reduced sperm motility in male mice and decreased weights of testes and epididimyis in male rats (BUA 2002). 2-Nitro-4-chloroaniline was predominantly positive for mutagenicity in Salmonella, demonstrted clastogenicity in mammalian cells in vitro (SCEs, CAs), while negative for micronucleus assay in mice in vivo (BUA 2004). For 2-nitroaniline (from PO2), studies in rats showed increased methemoglobin from 28-day inhalation exposure (US EPA 2009), however limited toxicity other than decreased body weights was observed during short-term and sub-chronic oral studies; genotoxicity data were larger negative for 2-nitroaniline (OECD 2001). However, the US EPA considered effects observed in 4-nitroaniline (from Para Red*) to conservatively apply in read-across to 2-nitroaniline (US EPA 2009). Toxicity studies on 4-Nitroaniline (from Para-Red*) reported methemoglobin, spleen pigmentation, liver hemosiderin, bone marrow hyperplasia, extra-medullary hematopoiesis in spleen and liver, and increased weights of spleen and liver (Environment Canada, Health Canada 2014b). The NTP reported “equivocal” evidence for carcinogenicity of 4-nitroaniline in rats based on increased incidence of liver hemangiosarcoma and the combined incidence of hemangioma and hemangiosarcoma at all sites, while evidence for genotoxicity was largely negative for this substance (Environment Canada, Health Canada 2014b). For 2-chloro-4-nitroaniline (from PR4), available toxicity studies were summarized from an industry submitted IUCLID dataset which reported acute exposure increased methemoglobin in rats following acute exposure, while dietary exposure to rats for 118 days was reported to be associated with degenerative changes in the spleen, kidney, liver and testes with effects starting at 300 mg/kg-bw per day (European Commission ©2000b). This substance was reported to be positive for mutagenicity in Salmonella and E.coli, but negative for mutagenicity in hamster V79 cells, negative for unscheduled DNA synthesis in vitro, and did not increase micronuclei in hamsters in vivo (European Commission ©2000b). For 2,4-dinitroaniline (from PO5), an industry submitted IUCLID dataset reported anemia and extramedullary hematopoiesis starting at 20 mg/kg-bw per day and testicular effects at higher doses in a 28-day oral rat study (European Commission ©2000c). This substance was reported to be mostly positive for in vitro mutagenicity in Salmonella (European Commission ©2000c; Assman et al. 1997) and also positive for chromosome aberrations in human lymphocytes (Huang et al. 1995), but negative for other in vitro and in vivo clastogenicity and mutagenicity assays (European Commission ©2000c). For 2-nitro-4-methoxyaniline (from NONPA), no animal toxicity data were identified, however repeated-dose studies were available for two nitro-methoxyaniline isomers 2-methoxy-4-nitroaniline (CAS RN 97-52-9) and 2-methoxy-5-nitroaniline (CAS RN 99-59-2) (NTP 2006). For 2-methoxy-4-nitroaniline, hemolysis, increased reticulocytes, extramedullary hematopoiesis, increased spleen & liver weights, increased serum liver enzymes were reported at 300mg/kg-bw per day in a 28-day oral study in rats (NTP 2006). For 2-methoxy-5-nitroaniline, chronic dietary exposure resulted in increased tumours in male rats (skin, Zymbal gland), female rats (Zymbal gland, clitoral gland), and in both sexes of mice (liver). Both methoxy-nitroaniline isomers were positive for mutagenicity in Salmonella (NTP 2006). It is uncertain if 2-nitro-4-methoxyaniline (from NONPA) which is untested would display similar toxicity to these two isomers.
An adverse outcome pathway (AOP) for erythrocyte/splenic toxicity and liver toxicity for a nitroaromatics category has been developed including nitroanilines in its structural (Sakuratani et al. 2013). Both AOPs for hemolysis and liver toxicity are based on proposed on oxidation of aromatic amine group or reduction of the nitro group to a hydroxylamine which can be further metabolized to the electrophilic nitroso group and/or nitrenium ion resulting in protein and DNA binding respectively (Sakuratani et al. 2013; Neumann 2005), while ring hydroxylation and subsequent formation of a reactive quinone-imine has also been proposed as an additional mechanism for protein binding nitroanilines (Matthews et al. 2012). Therefore, the qualitatively similar empirical toxicity data on the nitroanilines expected to be released from azo cleavage of the β-naphthol pigments is substantiated mechanistically by the common activation pathway for these substances. Similar tissue pharmacokinetics and metabolism following oral exposure has also been observed for several of these nitroanilines (Chopade and Matthews 1983; Chopade and Matthews 1984; Matthews et al. 1986; Mathews et al. 2012). As such, read-across for potential health effects of the β-naphthol pigments subset is supported by the qualitatively similar toxicity,pharmacokinetics, and common AOP expected from the various nitroaniline metabolites of the β-naphthol pigments.
In summary, based on the available health effects data for some of these substances (PR3, PR4, and PO5), qualitatively similar toxicity was observed in animals primarily manifesting as hemolysis and organ toxicity in spleen and liver (Table 7-8). There was also an increased incidence of liver tumours in female rats from studies on PR3 (NTP 1992) and PO5 (Hart et al. 1986; US FDA 1986) with supporting data from PR4 (Kupradinun et al. 2002). The another β-naphthol pigment Para Red* was assumed to have potential carcinogenicity based on structural similarity to the β-naphthol azo dye Sudan I (EFSA 2005; UK COT 2005) which was also associated with increased liver tumours in rats (Environment Canada, Health Canada 2014a). Despite limited data, there was also some demonstration of mutagenic potential for the β-naphthol pigments tested (PR3, PR4, PO5, PO2, Para Red*) although with varying potency. No repeated-dose toxicity studies were identified for the other β-naphthol pigments considered here (NONPA, PO2, PR6). However, based on comparable physical-chemical properties, structural similarity of the β-naphthol pigments, azo bond cleavage potential, similar expected toxicokinetics and toxicodynamics, and common health effects for expected metabolites (summarized above), all the β-naphthol pigments in this subset are expected to have a qualitatively similar toxicity including potential carcinogenicity (Table 7-8).
7.2.2 β-Naphthol Pigment Lakes (PR53:1, PR49:1 and PR50:1)
All substances in the β-naphthol pigment lakes subset have the same β-naphthol moiety as found in the β-naphthol pigments subset. However, the key difference with the β-naphthol pigment lakes is that they are all barium salts (pigment lakes) of either sulfo- or carboxyl-substituted anilines (PR50:1, PR53:1) or naphthylamine (PR49:1) (Table 7-9). Since 1-amino-2-naphthol is a common structural feature and potential azo cleavage product for all the β-naphthol pigment lakes, its structure is shown below, while the substance specific parent structures and corresponding azo cleavage metabolites are shown in Table 7-9:

| Parent β-naphthol pigment lake chemical identityFootnote Table 7-9 [a] and structure | Potential azo bond cleavage productsFootnote Table 7-9 [b] chemical identity and structure |
|---|---|
| PR53:1 CAS RN 5160-02-1 (D&C Red No. 9) 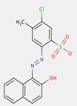 |
Red Lake C amine CAS RN 88-53-9 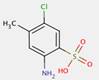 |
| R49:1 CAS RN 1103-38-4 (D&C Red No. 12)  |
Tobias acid CAS RN 81-06-1 (2-amino-1-naphthalene sulfonic acid) 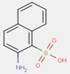 |
| PR50:1 CAS RN 6372-81-2 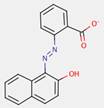 |
Anthranilic acid CAS RN 118-92-3 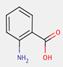 |
Absorption and Azo Bond Cleavage Potential
An in vivo absorption/metabolism study was identified for PR53:1. In this study, groups of four male F344 rats were fed 14C-labelled PR53:1 (called “Red No. 9”) for 24 hours in the diet at a concentration of 3 000 parts per million (ppm) (equivalent to a dose of 150 mg/kg-bw) followed by a 24-hour recovery period (Chadwick et al. 1984). The PR53:1 was labelled on either the benzene or the naphthol ring in order to distinguish between two azo bond cleavage products on either side of the azo bond, 14C-benzene for Red Lake C amine and 14C-naphthol for 1-amino-2-naphthol. Urine, feces and blood samples were taken during the 0- to 48-hour study period. Substantial metabolism was noted, as the majority of the applied dose was recovered in urine as Red Lake C amine (35%) and 1-amino-2-naphthol (54%), while only a small fraction of the dose ( less than 1%) was recovered as unchanged PR53:1. Very little accumulation of Red Lake C amine was observed in blood erythrocytes, which is supported by an earlier 10-day dietary absorption study reported by the same authors while the data was not presented (Chadwick et al. 1984). However, 1-amino-2-naphthol was detected in blood samples at 24 and 48 hours at 2% of the administered dose, a quarter of which was covalently bound to the erythrocytes. Overall, the results of this study suggest that a large fraction of PR53:1 and/or its azo bond cleavage products is absorbed by the oral route, with substantial azo bond cleavage occurring either by bacteria of the gastrointestinal tract or by tissue enzymes in vivo. The lack of full recovery also suggests possible distribution to other tissues that were not analyzed, and therefore the ultimate oral absorption as either the parent or azo bond cleavage products is likely greater than indicated in the study. It is expected that a similar potential for oral absorption also applies to the other two β-naphthol pigment lakes.
In an in vitro study, substantial (~99%) azo bond cleavage of PR53:1 was observed following an overnight incubation with a rat fecal preparation (Dillon et al. 1994, while another study demonstrated gradual but consistent azo bond cleavage of PR50:1 in a 24-hour anaerobic culture with a human fecal homogenate (BRI 2013). These in vitro studies support the above oral study suggesting that azo bond cleavage of the β-naphthol pigment lakes can occur, and likely occurs, in the mammalian gastrointestinal tract, based on the similar structures and physical-chemical properties among these substances.
With respect to the Ba2+ counter-ion, which is expected to dissociate upon absorption and/or intestinal azo cleavage of the organic component, a wide range of oral absorption of Ba2+ has been reported in experimental animals (7–85% for rodents and dogs) with greater absorption in younger animals (SCHER 2012, CCME 2013). The gastrointestinal absorption of Ba2+ ion in humans has been estimated to be 20% in adults, 30% in children (1–15 years old) and 60% in infants (SCHER 2012, CCME 2013); therefore, oral exposure to β-naphthol pigment lakes is expected to result in some bioavailable fraction of the dissociated Ba2+ ion. The instability of β-naphthol pigment lakes under simulated gastric conditions is suggested in the paints and coatings literature (Lewis 1995), which includes caution with respect to applications of these colourants in coatings that could result in oral exposure, such as toys, due to potential release of the Ba2+ ion (Siegwerk 2012). Other azo pigment Ba2+ lakes coupled to β-oxynaphthoic acid (BONA) have also been shown to be capable of releasing Ba2+ ions under simulated gastric conditions (Rastogi and Pritzle 1998). The dissociated organic azo moieties of the β-naphthol pigment lakes following oral absorption are likely more soluble in gastrointestinal tract contents than expected from the measured water and octanol solubilities for the parent β-naphthol pigment lakes. Therefore, the dissociated β-naphthol pigment lakes from the oral route are more analogous to their respective soluble salts/acid dyes. The same assumption was applied to PR53:1 and its corresponding soluble sodium salt (D&C Red No. 8, CAS RN 1658-56-6) by the US Food and Drug Administration (FDA) to consider that oral exposure to either salt would be essentially equivalent in the acidic conditions of the stomach, and therefore the two salts could be expected to have similar toxicities (Hart et al. 1986).
The only available dermal absorption study identified was an in vitro skin penetration study using human skin, reporting a maximum of 0.06% of 14C-benzene-labelled PR53:1 detected in the receptor fluid (Franz 1983). Due to the paucity of dermal absorption data, in vitro data for a BONA pigment lake analogues, PR57:1* (Ca2+ salt) and PR57* (Na+ salt), are considered for this subset. Therefore, a conservative estimate of 1% dermal absorption is applied for the β-naphthol pigment lakes (refer to the section on BONA pigment lakes for details). Both the β-naphthol pigment lakes and BONA pigment lakes exhibit close structural similarity, with common naphthol and benzene rings opposite the azo bond. Both types of pigment lakes are insoluble salts of ionizable sulfonate and/or carboxyl groups, the difference being that the BONA pigment lakes also contain a carboxyl group on C3 of the 1-amino-2-naphthol moiety and are therefore 1:1 salts of divalent cations, compared with 2:1 for the β-naphthol pigment lakes.
Collectively, the β-naphthol pigment lakes are considered to be bioavailable by the oral route and to undergo metabolism, including azo bond cleavage, in the gastrointestinal tract, with some degree of dissociation of the Ba2+ ion expected to occur. Therefore, the health effects data from related soluble salts of β-naphthol pigment lakes were considered for read-across of this subset.
Health Effects
Data for all study durations were identified for PR53:1, while only repeated-dose toxicity data for subchronic and chronic durations were identified for PR49 the Na+ analogue of PR49:1. Read-across for PR49:1, PR50:1 and other β-naphthol pigment lakes is based on the range of critical effects and associated lowest-observed-adverse-effect levels (LOAELs)/no-observed-adverse-effect levels (NOAELs) from PR53:1 and PR49* (sodium salt). A summary of the critical effect levels for the β-naphthol pigment lakes is presented in Table 7-10.
| Substance | Short-term LOAEL/ NOAEL (mg/kg-bw per day) |
Subchronic LOAEL/ NOAEL (mg/kg-bw per day) |
Chronic LOAEL/NOAEL (mg/kg-bw per day) |
Cancer Point of Departure (mg/kg-bw per day) |
|---|---|---|---|---|
| PR53:1 | LOAEL = 300 (MR/FR) Gross pathological changes in spleen (enlarged, dark red), liver and kidney (dark red) (NTP 1982) |
LOAEL = 30 LOAEL = 125–150 (MR/FR) |
LOAEL = 50 (MR/FR) Histopathological changes in liver (basophilic cytoplasm) and spleen (hemorrhage, fibrosis) (NTP 1982) |
BMDL10 = 44 (MR) 6/50Footnote Table 7-10 [c] (12%) 50 mg/kg-bw per day 7/49[c] (14%) 150 mg/kg-bw per day
|
| PR49:1 | Read-across from PR53:1 | Read-across from PR53:1 and PR49* | Read-across from PR53:1 and PR49* | N/AFootnote Table 7-10 [d] (no carcinogenic effects reported in studies on PR49*) |
| PR49* (Na+ salt) | Read-across from PR53:1 |
LOAEL = 125 (MR/FR) |
LOAEL = 125 LOAEL = 25 |
N/A[d] (no carcinogenic effects reported in studies on PR49*) |
PR53:1
The health effects characterization for PR53:1 is based primarily on data summarized in secondary reviews (Hart et al. 1986; LSRO-FASEB 1984; IARC 1993; OECD 1999a, b; SCC 2000). For genotoxicity potential, a review by BIBRA (1989) was also considered and is briefly summarized below.
Genotoxicity
PR53:1 was generally reported as negative in most in vivo and in vitro studies, with some weak and/or equivocal responses reported in a few in vitro tests (Salmonella mutagenicity following incubation with a fecal preparation, in vitro chromosomal aberrations, in vitro cell transformation assay). The other positive response was observed for in vivo SCEs in bone marrow of mice.
Results from the standard Ames assay in Salmonella in the identified studies were almost entirely negative (Brown et al. 1979; Muzzall and Cook 1979; Miyagoshi et al. 1983; Longstaff et al. 1984; BUA 1993; Zeiger et al. 1988; Dillon et al. 1994), and weakly positive or equivocal responses were reported in strains TA97 and TA98 only at doses resulting in precipitation of the test material (Zeiger et al. 1988). A reverse mutation assay in Escherichia coli was also reported as negative (Hoechst AG 1985). In Prival modified Ames assays with FMN where azo bond reduction is facilitated, PR53:1 was also reported as negative in TA98, TA100, TA1535 and TA1537 (BUA 1993; Dillon et al. 1994) and either negative or weakly positive/equivocal in Salmonellastrain TA100 (Dillon et al. 1994). However, positive and weakly positive responses were observed in TA100 and TA98, respectively, after overnight anaerobic incubation of PR53:1 with a rat fecal preparation. The study authors speculated that previous negative results from FMN modified Ames tests were likely due to insufficient azo bond cleavage and that PR53:1 does possess some mutagenic potential in Salmonella based on the mutagenicity of one or more of its azo bond cleavage products (Dillon et al. 1994).
In vitro genotoxicity tests in mammalian cells were also primarily negative, including the tk locus mutation assay in mouse lymphoma L5178Y cells (Myhr and Caspary 1990), chromosomal aberrations and SCEs in CHO cells (Ivett et al. 1989) and UDS in primary rat hepatocytes (Kornbrust and Barfnecht 1985; Williams et al. 1989). Results for chromosomal aberrations in Chinese hamster lung (V79) cells (BUA 1993) were reported as positive (SCC 2000) and negative (OECD 1999b) in secondary sources; however, insufficient details were provided to allow an evaluation of this study. PR53:1 was also reported as positive in a cell transformation assay using Balb/c 3T3 cells (Tennant et al. 1986) and BHK21/C13 cells (Longstaff et al. 1984); however, both studies were considered by other reviews as being either too insufficiently reported (SCC 2000) or too unreliable (OECD 1999a) to evaluate.
For in vivo genotoxicity studies, following intraperitoneal injection of PR53:1 at doses of 500–2000 mg/kg-bw, no statistically significant increase in UDS was observed in rat hepatocytes or in micronuclei of the bone marrow (Westmoreland and Gatehouse 1992). This result confirms an earlier negative result for UDS in rat hepatocytes at a lower dose of 500 mg/kg-bw (Kornbrust and Barfnecht 1985). A negative result was reported for chromosomal aberrations in bone marrow following intraperitoneal injection (1250–5000 mg/kg-bw) in mice, whereas positive results for SCEs in bone marrow were reported from the same study (NTP 2013). PR53:1 was also reported as negative for mutagenicity in Drosophila (Foureman et al. 1994).
Carcinogenicity
The carcinogenicity of PR53:1 was tested in several chronic studies in rats and mice (NTP 1982; CTFA 1982; Davis and Fitzhugh 1962; OECD 1999a), however the studies from the US National Toxicology Program (NTP) are the most recent with details available in published reports, therefore these studies are summarized here. Briefly, the chronic assay by the NTP (1982) included 2yr exposure of PR53:1 in the diet to F344 rats (50/sex/dose, control, 1000ppm or 50mg/kg-bw per day, 3000ppm or 150mg/kg-bw per day) and B6C3F1 mice (50/sex/dose, 1000ppm or 130mg/kg-bw per day, 2000ppm or 260mg/kg-bw per day)Footnote[11].
An increased incidence of spleen sarcomas were observed only in high-dose male F344 rats (primarily fibrosarcomas and osteosarcomas: 0/50 control, 0/50 at 50mg/kg-bw/day, 26/48 (54%) at 150mg/kg-bw per day). Spleen tumours were considered uncommon in F344 rats with a very low historical control incidence reported in the NTP program at the time of this study (3/2960 or 0.1% splenic fibrosarcomas reported in NTP 1982). Based on the malignancy of the splenic sarcomas and their tendency to metastasize to extra-splenic tissues, combined with the extensive non-cancer spleen toxicity observed in all repeated-dose studies, the NTP considered the association of PR53:1 exposure with the spleen sarcomas to be unequivocal and “positive” evidence for the carcinogenicity of PR53:1 (NTP 1982). Similar types of spleen tumours were observed in Sprague-Dawley rats in the high-dose group (10 000 ppm = 500 mg/kg-bw per day) of another chronic dietary study with PR53:1 (4/70 males, 1/70 females, 0/70 controls of either sex); the tumours were considered as likely exposure related, although their incidence was not statistically significant (OECD 1999a). This additional study is therefore considered as supportive evidence of the carcinogenicity of PR53:1 in the male rat spleen by the oral route. However, no spleen tumours were observed in the exposed female F344 rats from the NTP study (NTP 1982), and other chronic oral studies in mice (CTFA 1982; NTP 1982) and Osborne-Mendel rats (Davis and Fitzhugh 1962) did not demonstrate an observable tumorigenic response to PR53:1 exposure. The reason for the apparent sex and species sensitivity of this tumour type is not known.
In the chronic oral NTP studies in mice and rats, the incidence of hepatic neoplastic nodules observed in the liver of male F344 rats was statistically significant at both test doses (0/50, 6/50 p = 0.013, and 7/49 p = 0.006, for control, 50 and 150 mg/kg-bw per day, respectively) and considered as “positive” evidence for carcinogenicity. These effects were also associated with non-neoplastic liver effects in male rats (basophilic cytoplasm changes, centrilobular necrosis), while a statistically significant trend (p = 0.039) for neoplastic nodules of the liver (1/50, 1/50 and 5/50 for control, 50, 150 mg/kg-bw per day groups, respectively) was also observed for female rats and considered “equivocal” evidence for carcinogenicity (NTP 1982). A secondary source reported a follow-up micro-slide analysis by the US FDA that suggested the revised incidence of neoplastic liver nodules was below statistical significance in both male and female rats in the NTP study (cited in LSRO-FASEB 1984; BIBRA 1989). However, in the absence of the primary source for the FDA analysis and limited details provided in the secondary source, neoplastic nodules observed are considered as indicative of a carcinogenic effect of PR53:1 exposure as per conclusions by the NTP (1982).. In the mouse study, a statistically significant increase in hepatocellular carcinomas of male mice was also reported in the NTP study (4/50, 9/50 and 11/50 for control, low dose and high dose, respectively); however, the incidences were within or below the range of historical controls for this laboratory (65/297) and were not considered exposure related by the NTP. The other available chronic studies in mice and rats did not report an increased incidence of exposure-related liver tumours (NTP 1982).
A lack of carcinogenicity of PR53:1 by the dermal exposure route was reported based on results from two skin painting studies in mice (Carson 1984a; Hart et al. 1986). In one study, ICR Swiss Webster mice (50 of each sex in the treatment and control groups) were exposed on the clipped dorsal skin (6 cm2) twice weekly for 18 months to either water vehicle control or 1 mg of PR53:1 in 0.1 mL water (approximately 33 mg/kg-bw per application doseFootnote[12]). Histological examination did not reveal any obvious differences in tumours of the skin or other tissues between the control and PR53:1 exposure groups (Carson 1984a; IARC 1993). Limited details of another skin painting study on CF-1 Carworth mice also reported no carcinogenic effects from weekly exposure to a 0.1 mL solution of 1% PR53:1 for 26 months (as cited in Hart et al. 1986). Although these studies were rather limited (i.e., single dose tested, dose lower than oral LOAELs), the data provide some evidence of a lack of carcinogenic potential of PR53:1 directly applied to the skin of mice for the given test doses. Due to the relatively low solubility of PR53:1 in the vehicle in these studies (water), its limited dermal penetration observed in vitro (1%) and the low dose tested relative to the oral LOAELs for mice (external applied dose of 33 mg/kg-bw twice weekly vs. daily oral LOAEL in mice of greater than or equal to 130 mg/kg-bw per day), the systemic dose from these dermal studies is expected to be negligible, which helps to explain the observed lack of carcinogenicity.
A clear statistically significant decrease in the incidence of lymphocytic leukemia in both sexes of rats and a decrease of tumours in the preputial gland of males were observed in the chronic NTP study (NTP 1982). A similar decrease in lymphocytic leukemia was observed in other chronic NTP studies of azo dyes that are lipophilic (Sudan I, Solvent Yellow 77) or hydrophilic (Acid Red 14, Acid Orange 10) (Haseman and Johnson 1996). While the majority of tested NTP substances that reduced the incidence of leukemia also showed toxicity to the spleen, this was not observed in all cases, including with the water-soluble azo dyes above. The mechanism of the apparent reduction of spontaneous leukemia incidence in the F344 rat by these substances is unclear.
Other Repeated-dose Toxicity Studies
The repeated-dose oral toxicity studies available for PR53:1 demonstrate some common non-cancer effects, with the spleen, blood, liver and kidney as the primary target organs or tissues in the tested species in the following general order of sensitivity: dog greater than rat, greater than mouse. The most consistent reported observations across the studies involved spleen toxicity (i.e., congestion, hemorrhage, fibrosis, hyperplasia, hemosiderosis, fatty changes) and were observed in both sexes of several strains of rat from short-term, subchronic and chronic studies (Davis and Fitzhugh 1962; CTFA 1982; NTP 1982). Splenic toxicity was also observed in studies in dogs (CTFA 1983a) as well as in short-term and subchronic studies in mice (NTP 1982). In the chronic mouse studies that did not report splenic toxicity, the maximum tolerated dose was likely not reached, and therefore test doses may have been insufficient to elicit this effect, supporting the lower sensitivity of mice compared with rats and dogs for this effect (NTP 1982). Overall, spleen toxicity was observed at LOAELs of 30 mg/kg-bw per day in the 2-year dog study (CFTA 1983), 50 mg/kg-bw per day in the chronic rat study (NTP 1982) and 163 mg/kg-bw per day in the 13-week mouse study (NTP 1982). The spleen toxicity is likely associated with, or secondary to, splenic clearance following primary toxicity on the red blood cells; this was similarly reported following exposure of dogs, rats and mice to aniline. Doses similar to those at which spleen toxicity was observed included effects in red blood cells (anemia, decreased hemoglobin and red blood cell volume, Heinz bodies, increased reticulocytes) as well as hemosiderosis/pigmentation in liver and kidneys, which are indicative of increased red blood cell destruction following primary red blood cell toxicity resulting from exposure to PR53:1 or its azo bond cleavage products. The association of toxicity in the spleen with toxicity in red blood cells observed in this study has also been reported for several other aromatic amines (aniline, p-chloroaniline, o-toluidine) (NTP 1982; Goodman et al. 1984; Weinburger et al. 1985).
Other common non-cancer observations involved effects in the liver and kidney. Liver toxicity in rats (Davis and Fitzhugh 1962; CTFA 1982; NTP 1982) and dogs (CTFA 1983a) involved increased liver size/weight and hemosiderosis as well as additional effects (basophilic cytoplasm changes and centrilobular necrosis) in male rats (NTP 1982). Observations in the kidneys included increased pigmentation in both sexes of rats following short-term, subchronic and chronic exposure and kidney tubule regeneration in female rats after chronic exposure (NTP 1982). Other less common effects were reported in studies in rats by the NTP (testes tubule degeneration, dilatation of mammary acini, hyperplasia of bronchiolar lymph nodes; NTP 1982) and the US FDA (bone marrow hyperplasia; Davis and Fitzhugh 1962) and a study in mice (chronic inflammation of the stomach; CTFA 1982).
PR49:1Footnote[13]
There were no repeated-dose toxicity or absorption/metabolism studies identified for PR49:1. However, based largely on similar assumptions as for PR53:1, it is considered that some degree of bioavailability and dissociation of PR49:1 occurs following oral exposure. Therefore, as dissociation of the Ba2+ ion and organic azo anion is likely to occur, oral toxicity studies on the sodium salt, PR49* (D&C Red No. 10), are considered here to inform the oral toxicity of PR49:1. Similar assumptions for the dermal route of exposure are also considered to apply to PR49:1.
Genotoxicity
Several salts of PR49, including the barium salt PR49:1, have been reported as negative in the standard Ames assay (reviewed in Combes and Haveland-Smith 1982), whereas PR49* (sodium salt) was reported as positive in Salmonella strain TA1538 with and without S9 (Chung et al. 1981). PR49* was also reported to be negative in a Prival modified Ames assay in strain TA98 (Zhou et al. 1987). The sodium salt would be expected to be more soluble and available for facilitated azo cleavage in this assay; therefore, a negative response suggests that the azo cleavage products were not mutagenic in strain TA98 under the test conditions. PR49:1 was also reported as inconclusive and negative for mutagenicity without and with S9 activation, respectively, in the mouse lymphoma assay (Seifried et al. 2006). No other genotoxicity data were identified for PR49:1 or its related salts; however, the available in vitro data do not suggest a strong mutagenic potential.
Carcinogenicity
In a chronic skin painting study, ICR Swiss Webster mice (50 of each sex in exposure group and 25 of each sex in control group) were exposed on the clipped dorsal skin (6 cm2) twice weekly for 18 months to either water vehicle control or 1 mg “Red No. 10” (D&C Red No. 10 or PR49* sodium salt) in 0.1 mL water (approximately 33 mg/kg-bwFootnote[14] per application dose). Histological examination did not reveal any obvious differences in tumours of the skin or other tissues between the control and the exposure groups (Carson 1984). As in the case of PR53:1, these data provide some evidence for a lack of carcinogenic potential of PR49* sodium salt when applied directly to the skin of mice for the given test doses. However, due to the limited dermal penetration expected for the large soluble ionized substance as well as the low dose tested relative to the oral LOAELs for rats and dogs (external dermal dose of 33 mg/kg-bw biweekly vs. daily oral LOAEL in rats and dogs greater than or equal to 25–30 mg/kg-bw per day with significant expected absorption), the systemic dose from these dermal studies is expected to be negligible, which helps to explain the lack of carcinogenicity observed.
Other Repeated-dose Toxicity Studies
The results of a 20-week subchronic feeding study conducted by the US FDA, in which five Osborne-Mendel rats of each sex were exposed to a dietary concentration of 0%, 0.25%, 0.5%, 1% or 2% (equivalent to doses of 0, 125, 250, 500 and 1 000 mg/kg-bw per day) of PR49*, indicated no changes in mortality, growth or other gross signs of toxicity. However, a slight effect on hematological parameters (decreased hemoglobin and hematocrit, increased circulating blood normoblasts) as well as moderate splenomegaly were observed in the test groups compared with the control group, suggesting a subchronic LOAEL of 125 mg/kg-bw per day, the lowest dose tested (Davis and Fitzhugh 1963). In the chronic 2-year feeding study also conducted by the US FDA, 25 rats of each sex were exposed to a dietary concentration of 0%, 0.01%, 0.05%, 0.25% or 1% (equivalent to doses of 0, 5, 25, 125 and 500 mg/kg-bw per day) for 103 weeks. The only exposure-related effects reported were a statistically significant enlargement of the spleen in the 0.25% and 1% groups (125 and 500 mg/kg-bw per day) with associated splenic hemosiderosis and erythropoiesis confirmed by histology, while the spleens of the 0.05% group (25 mg/kg-bw per day) were not histologically different from those of the control group. Moderate bone marrow hyperplasia was also observed at 0.05% and above ( greater than or equal to 25 mg/kg-bw per day). Blood samples throughout the study period from five rats of each sex in each group showed no apparent effects on hemoglobin, hematocrit or white cell counts; however, test groups did show “polychromasia, target cells, and occasional normoblasts in peripheral blood.” No other obvious differences in effects such as organ weights or tumour incidences were reported between the exposure and control groups (Davis and Fitzhugh 1963). The chronic LOAELs are considered to be 125 mg/kg-bw per day for the spleen effects (NOAEL = 25 mg/kg-bw per day) and 25 mg/kg-bw per day for bone marrow hyperplasia (NOAEL = 5 mg/kg-bw per day).
In a dietary study in Beagle dogs (six of each sex per group) fed PR49* at a concentration of 0%, 0.015%, 0.1% or 5% (equivalent to doses of 0, 4.5, 30 and 150 mg/kg-bw per day) for 2 years, no exposure-related changes in body weight, feed consumption or behaviour were reported. Hematological parameters were reportedly affected in dogs fed at and above 0.1% (30 mg/kg-bw per day), with decreases in hematocrit, hemoglobin and erythrocyte counts, which increased in severity at the 5% dose. Urine bilirubin was also increased at and above 0.1%. A dose-dependent increased incidence of splenomegaly was shown at and above 0.1%, while liver weights increased in the 5% group. Evidence of red blood cell destruction was reported at and above 0.1%, based on histological findings of increased hemosiderosis and erythropoiesis of the spleen, as well as pigmentation of the liver, bone marrow and renal tubules. Severe acute hyperemia was found in tissue sections of all dogs in the 5% group. A more limited 90-day subchronic study (one Beagle of each sex per group) in the same dose range also demonstrated some evidence of effects on the blood (bilirubinuria, splenomegaly, hemosiderosis of spleen/liver/kidney, increased nucleated red blood cells) and supports the findings from the chronic study (US FDA 1972). Based on these data, the chronic LOAEL is considered to be 30 mg/kg-bw per day, with a NOAEL of 4.5 mg/kg-bw per day.
PR50:1
No empirical repeated-dose toxicity studies were identified for PR50:1. Consistent with azo bond cleavage data for PR53:1, PR50:1 showed gradual but consistent azo bond cleavage over the 24-hour incubation by a human fecal homogenate under anaerobic conditions (BRI 2013). Also consistent with PR53:1 (Dillon et al. 1994), PR50:1 was negative for mutagenicity in Salmonella strains TA98 and TA100 with S9 activation and the FMN Prival modification (ILS 2011). However, Dillon et al. (1994) suggested that FMN alone may be insufficient to promote azo bond cleavage for PR53:1, as it showed a mutagenic response in TA98 and TA100 after overnight anaerobic incubation of PR53:1 with a rat fecal preparation. Therefore, it is uncertain whether the negative modified Ames test results for PR50:1 are because the metabolites are not mutagenic or due to insufficient azo bond cleavage by the FMN modification.
Summary and Considerations for Read-Across
Limited mechanistic data are available for the substances in the β-naphthol pigment lakes subset. However, based on similar physical-chemical properties, structures and expected azo bond cleavage metabolites, read-across for health effects within this subset is considered reasonable. Since the β-naphthol pigment lakes are expected to undergo dissociation of the Ba2+ion and also azo cleavage to some degree, the health effects of the potential azo cleavage metabolites are also considered here.
Besides 1-amino-2-naphthol which is common to all the β-naphthol pigment lakes (potential health effects of 1-amino-2-naphthol are discussed in Section 7.2.1, Summary and Considerations for Read-Across), the other corresponding metabolites are all sulfo- or carboxyl-substituted anilines (PR50:1, PR53:1) or naphthylamine (PR49:1) (Table 7-9) along with Ba2+ ion. The potential contribution of Ba2+ion and the other metabolites of the observed toxicity of their parent β-naphthol pigment lakes is based on a comparison to data on these metabolites focussing only on the most common critical effects for the β-naphthol pigment lakes from Table 7-10, namely hemolysis and corresponding effects in spleen (splenomegaly, increased weight, hemosiderosis, degenerative changes) as well as liver toxicity (for PR53:1).
The toxicity of Ba2+ ion has been summarized in several recent reviews indicating the kidney to be the target organ in both mice, rats, and guinea pigs with evidence of pigmentation, increased kidney weights, and nephropathy (SCHER 2012; CCME 2013). In the available studies on Ba2+ ion, no clear exposure-related effects were apparent in hematology parameters or in either the spleen or liver (organ weight, histopathology) at doses much higher than where hemolysis and spleen/liver toxicity was observed for PR53:1 studies. For example, clear effects were observed in the spleen of both rats and mice in the 13wk study of PR53:1 at doses of 150 and 163 mg/kg/d respectively, corresponding to equivalent doses of Ba2+ ion to be 23 and 25 mg/kg-bw per day for rat and mice (assuming 100% of PR53:1 dissociates to release free Ba2+ ion). No similar effects were observed in the subchronic component of a barium chloride drinking water study t (NTP 1994) at Ba2+ ion doses 10-20x higher in rat and mouse respectively (200mg Ba2+ ion /kg-bw per day in rat, 495mg Ba2+ ion /kg-bw per day mouse). Therefore any PR53:1 related effects in the blood or spleen seem unrelated to the contribution of Ba2+ ion. Similar analysis supports the conclusion that Ba2+ ion is unlikely to be contributing to the PR53:1 related liver lesions. Therefore, these observed effects in PR53:1 are likely due to the organic moiety of this substance as either the parent azo dye and/or the azo cleavage products. Since animal studies of PR49* (Na+ salt) did not include exposure to Ba2+ ion, the effects observed in these studies (e.g. hemolysis, splenomegaly, hemosiderosis, bone marrow hyperplasia, urine bilirubin) support that the common organic moieties of both PR53:1 and PR49* are responsible for the effects observed in erythrocytes, spleen and liver for these substances.
The moiety 1-amino-2-naphthol is common to both PR53:1, PR49:1, and PR50:1 would be expected to be contributing similarly to the toxicity of both substances. Therefore the potential toxicity of the unique azo cleavage metabolites of these substances are considered first. For both these substances, the unique azo cleavage metabolites contain solubilizing groups by virtue of either sulfonate substitution (Red Lake C amine from PR53:1, or Tobias acid from PR49:1) or carboxyl substitution (anthranilic acid from PR50:1) (see Table 7-9).
For Red Lake C amine, a 28day oral study in rats did not report any exposure related changes in hematology, clinical chemistry, organ weights, or in gross/microscopic changes in tissues at doses up to 1000mg/kg-bw per day (Environment Canada, Health Canada 2014b). In comparison, the short-term component of the NTP study on PR53:1 in rats reported grossly observable changes in the spleen (2-5x size increase, dark red) as well as the liver and kidneys (dark red /reddish tan colour) at a dose of 300mg-kg-bw per day, corresponding to a maximum theoretical dose of 148mg/kg-bw per day Red Lake C amineFootnote[15]. Since Red Lake C Amine did not show any toxic effects at the highest tested dose of 1000mg/kg-bw per day in a longer study (28day vs 14day for short-term component of NTP study on PR53:1), it is unlikely that the much lower corresponding dose of Red Lake C Amine was responsible for the PR53:1-related changes in the spleen. For Tobias acid (from PR49:1 and PR49*), a similar lack of clear toxicity was observed at orally dosed rats up to 1000mg/kg-bw per day in a reproductive/ developmental toxicity screening study (exposure duration 49 and 39 days for males and females respectively) although a decrease in liver weights was reported at the high dose (JECDB 2013a). A chronic dietary study in mice for Tobias acid did not report any changes in mortality, survival or in tumour incidence at doses up to 1300 mg/kg-bw per day, however other effects were not reported (Della Porta and Dragani 1982). ). In comparison to the toxicity of PR49:1, studies on the sodium salt PR49* indicated effects in rats as low as 25-125mg/kg/d (bone marrow hyperplasia, hematologic changes indicating RBC destruction, splenomegaly) which correspond to maximum theoretical Tobias acid doses of approximately 14-70mg/kg/dFootnote[16]. Since these types of effects were not observed in the short-term study of Tobias acid in rats at much higher doses (up to 1000 mg/kg-bw per day, JECDB 2013a) it is unlikely that the Tobias acid moiety had contributed to the hemolysis and regenerative response observed in the PR49* studies. The low toxicity observed for both Red Lake C amine (from PR53:1) and Tobias acid (from PR49:1 and PR49*) are consistent with general observations for sulfonated amines as a class that are not expected to have carcinogenic potential and would generally be associated with lower systemic toxicity (Jung et al. 1992).
For PR50:1, the unique azo cleavage product is anthranilic acid (Table 7-9) which is found endogenously from metabolism of tryptophan and for which low toxicity is inferred from chronic dietary study in rats and mice on anthranilic acid that did not demonstrate exposure related increases in survival or tumour incidence at dietary concentrations up to 30,000ppm in rat (equivalent to 1500 mg/kg-bw per day) and 50,000ppm in mice (equivalent to 6500 mg/kg-bw per day)Footnote[17] while some slight increase in hematopoiesis was observed in both male and female rats (NCI 1978). Therefore, any exposure to PR50:1, would not expect to have significant toxicity contributed by the anthranilic acid moiety following azo cleavage.
Based on the data identified for the Ba2+ ion and the azo bond cleavage products of PR53:1 (Red Lake C amine) and PR49:1 (Tobias acid), there is little evidence that these substances contributed significantly to the target organ toxicity observed for PR53:1 and PR49:1 (data based on PR49*) -- namely, evidence for hemolytic anemia and histopathological changes in the spleen (and liver for PR53:1). However, similar toxicities for both PR53:1 and PR49:1 (tested as PR49*) were observed at reasonably similar doses, suggesting that the metabolite common to both of these substances, 1-amino-2-naphthol, is responsible for these effects. Although no adequate repeated-dose toxicity studies were identified for 1-amino-2-naphthol, mechanistic data suggest that while it may be theoretically activated to an N-hydroxylamine, but more likely to a reactive naphthoquinoneimine, supporting the role 1-amino-2-naphthol plays in the observed toxicity of PR53:1 and PR49:1 (tested as PR49*). The formation of naphthoquinone/ quinoneimines is well characterized for many substances based on an aromatic amine and aromatic amide structure and is speculated to be another important mode of action for the observed toxicity of these substances (Skipper et al. 2010). Other substances that could generate 1-amino-2-naphthol have demonstrated similar target organ toxicity in blood, spleen and liver, including the Sudan-type dyes, β-naphthol pigments (Section 7.2.1) and water soluble acid dyes such as Acid Orange II (CAS RN 633-96-5). In the case of Acid Orange II, potent hemolytic effects were demonstrated by the oral route along with evidence of substantial azo bond cleavage to sulfanilic acid and 1-amino-2-naphthol (SCCS 2011). Based on a lack of blood/spleen/liver toxicity expected for sulfanilic acid (Jung et al. 1992), the target organ toxicity observed in Acid Orange II also points to the contribution from 1-amino-2-naphthol. Also, the observation of covalent binding of the 14C-naphthol label to red blood cells in the oral absorption/metabolism study for PR53:1 (see Section 7.2.2 Absorption and Azo Bond Cleavage Potential) provides evidence that the hemolytic effects of PR53:1 are most likely due to 1-amino-2-naphthol.
In summary, the available health effects data for some of these substances (PR53:1, PR49*), qualitatively similar toxicity was observed in animals primarily manifesting as hemolysis and associated effects in the spleen (Table 7-10). These substances were largely negative in the available genotoxicity studies. There was an increased incidence of spleen tumours in male rats from studies on PR53:1, which similar to that observed for aniline (Environment Canada, Health Canada 2011) and in the absence of strong genotoxicity of PR53:1 is considered to be secondary to the non-neoplastic splenic toxicity (Westmoreland and Gatehouse 1992; Weinburger et al. 1985). Since hemolysis and spleen toxicity was also observed for PR49*, it is reasonable to assume a similar potential for spleen tumours in PR49:1 and the other β-naphthol pigment lakes if tested under the same conditions as for PR53:1. The more significant liver toxicity observed for PR53:1 was not replicated to the same degree in the studies on PR49* and no liver tumours were reported in chronic studies of PR49*, therefore it is uncertain whether the liver tumours seen in rats for PR53:1 are a universal effect applicable to the other -naphthol pigment lakes. No repeated-dose toxicity studies were identified for the other β-naphthol pigment lake, PR50:1, however the potential toxicity contributed by the anthranilic acid moiety following azo cleavage would be low. However, based on comparable physical-chemical properties, structural similarity of the β-naphthol pigment lakes, azo bond cleavage potential, similar expected toxicokinetics and toxicodynamics, and common health effects for expected metabolite (with toxicity to erythrocytes conferred by the common 1-amino-2-naphthol moiety), all the β-naphthol pigment lakes in this subset are expected to have a qualitatively similar toxicological potential. Therefore, in the absence of health effects data for all substances in this subset, a conservative read-across is considered with the critical health effects identified for these substances (Table 7-10) applying to all substances in this subset, excluding liver carcinogenicity for PR53:1. There is uncertainty whether the liver tumours observed for PR53:1 can be extrapolated to the other β -naphthol pigment lakes.
7.2.3 BONA Pigment Lakes (PR48:2, PR63:1, PR52:1, PR52:2 and PR48:5)
The BONA pigment lakes subset consists of PR63:1 (Ca2+), PR48-based pigment lakes (PR48:2 Ca2+, PR48:5 Mg2+) and PR52-based pigment lakes (PR52:1 Ca2+, PR52:2 Mn2+); the free acid forms are presented in Table 7-11. Although PR52:2 was not considered as part of this subset in the previous sections, it is included here to inform the human health effects characterization of this subset, given the limited data available for the entire subset. Similarly, PR57:1* is included as an analogue for this subset, and any health effects data identified for other related salts of PR48, PR52, PR63 and PR57* (D&C Red No. 6, Lithol Rubine B) are also included. All the BONA pigment lakes substances are relatively low solubility metal salts of an o-sulfonate group on the phenyl (PR48-based, PR52-based) or naphthyl ring (PR63:1) opposite a common azo bonded BONA moiety. Azo bond cleavage of all these substances would yield the common 1-amino derivative of BONA, 1-amino-2-naphthol-3-carboxylic acid (abbreviated 1-amino-2-naphthol-3CA).
The BONA pigment lakes and analogue all share common structural features, where o-sulfonated aniline derivatives (PR48-based, PR52-based, PR57*) or sulfonated amino-naphthalene (i.e. Tobias acid in PR63:1) is azo coupled to a BONA moiety. They share common physical-chemical properties and solubilities and therefore are expected to behave similarly following oral and dermal exposure in terms of absorption and potential for azo bond cleavage. Azo bond cleavage would result in similar o-sulfo-substituted aniline derivatives or Tobias acid along with the common moiety, 1-amino-2-naphthol-3-carboxylic acid (1-amino-2-naphthol-3CA, CAS RN 13065-86-6). CA. Since 1-amino-2-naphthol-3CA is a common structural feature and potential azo cleavage product for all the BONA pigment lakes, its structure is shown below, while the substance specific parent structures and corresponding azo cleavage metabolites are shown in Table 7-11:

| Parent BONA pigment lake chemical identityFootnote Table 7-11 [a] and structure | Potential azo bond cleavage productsFootnote Table 7-11 [b] chemical identity and structure |
|---|---|
PR48:2 PR48:5
|
Red 2B acid
|
PR52:1 PR52:2
|
Red Lake C amine
|
PR63:1
|
Tobias acid
|
PR57:1* PR57*
|
Red 4B acid
|
Absorption and Azo Bond Cleavage Potential
Based on the limited data on oral metabolism and bioavailability, the BONA pigment lakes are expected to behave similarly to the β-naphthol pigment lakes in terms of their oral and dermal bioavailability due to their similar physical and chemical properties. Therefore, some degree of oral bioavailability is expected, such as dissociation of the counter-ion (Ca2+, Mg2+, Mn2+) and absorption of the parent free acid component of the BONA pigment molecule and/or the azo bond cleavage products. PR63:1 is expected to go through substantial azo bond cleavage, as indicated by an in vitro study demonstrating rapid and extensive azo bond cleavage with a half-life of 0.54 hour under anaerobic conditions in a human fecal homogenate (BRI 2013). Therefore, PR63:1 and the other BONA pigment lakes are expected to go through azo bond cleavage following oral exposure, with subsequent absorption of the metabolites. Other clear evidence of oral bioavailability of the BONA pigment lakes comes from the empirical observation of toxicological effects observed in experimental animal studies following oral exposure to these substances, clearly indicating absorption of the parent pigments or their metabolites. Although no precise value for oral absorption can be determined, a conservative estimate of 100% oral absorption of either the parent azo structure or azo cleavage metabolites is assumed, in line with the substantial oral absorption observed for the related β-naphthol pigment lakes ( greater than 35–54% from 14C-labelled urinary metabolites following oral exposure to PR53:1).
In vitro dermal absorption studies for PR57* used porcine skin from the back and trunk or ear (SCCS 2012) The former study did not detect any PR57* in a 72-hour sample from receptor fluid or skin layers and reported less than 0.07% estimated dermal absorption based on the limits of detection (high-performance liquid chromatography, limits of detection of 9–21 ng/mL). The latter study reported that 0.091% of the applied dose (presumably receptor fluid and skin layers) and greater than 99% of the test article were recovered in the skin wash. Although limitations were noted for both studies, an overall low dermal absorption of PR57* is evident. Given that the water solubility of PR57:1* (Ca2+ salt) is approximately three orders of magnitude lower than that of the PR57* (Na+ salt) tested in these studies, dermal absorption of PR57:1* is considered correspondingly lower than that of PR57*, generally in line with the low dermal absorption data for the β-naphthol pigment lakes (0.06%) presented in the previous section. However, the available in vitrodermal absorption studies may not account for the microbial activity of the normal flora of the skin which has been reported to cleave the azo bond (Stingley et al. 2010, Platzek 1999). An in vitro incubation of PR63:1 with the dermal Staphylococcus and Micrococcus sp. bacteria demonstrated azo cleavage occurred for this substance (BRI 2012). Based on the available studies, dermal absorption of 1% is considered a conservative estimate for the BONA pigment lakes accounting for the potential azo bond cleavage occurring on the skin.
Health Effects
The selected lowest critical effect levels among the available toxicity data are presented in Table 7-12. For short-term toxicity, the range of LOAELs for PR48:2 and PR57:1* are used for read-across to the other BONA pigment lakes. No critical effect levels from subchronic studies are selected due to low confidence in the available data set. For chronic studies, data for the BONA pigment lakes come directly via read-across from PR57:1*. While PR52:2 is considered to be an organometallic substance, a toxicity profile similar to those of the other BONA pigment lakes is assumed as a conservative approach.
Due to the limited health effects database for the BONA pigment lakes, data for repeated-dose toxicity and other effects are largely based on PR57:1*. In addition, the health effects associated with the potential azo bond cleavage products of PR48, PR52 and PR63 are also considered to inform the health effects of the corresponding parent substances. A comparison of some of the BONA pigment lakes with their corresponding homologue β-naphthol pigment lakes is also considered, since differences in toxicity may be explained in part by the 3-carboxyl group of 1-amino-2-naphthol-3CA, which differentiates 1-amino-2-naphthol-3CA from the 1-amino-2-naphthol moiety (e.g., PR63:1 vs. PR49:1).
PR57:1* has an o-sulfonate substituent on the phenyl ring opposite the azo bonded BONA moiety. The physical-chemical properties of all five BONA pigment lakes are similar; therefore, all could potentially generate similar azo bond cleavage products with a common 1-amino-2-naphthol-3CA moiety. Therefore, the available toxicity data for PR57:1* are considered applicable for read-across to the other BONA pigment lakes in this subset. PR57:1* and PR57* have been well studied, with many data generated to support approved uses as a cosmetic colourant in the USA and Europe as well as a limited use in Europe as a food additive in edible cheese rinds as the additive “E 180.” PR57:1* and PR57* are considered together in line with the assumption of toxicological equivalence for both salts via oral exposure (US FDA 1982), and a summary of the available toxicity data as cited from secondary sources is presented below (US FDA 1982; JECFA 1986; BIBRA 1993a, b; OECD 1994; EFSA 2010; SCCS 2012). For the two PR48-based pigment lakes (PR48:2 Ca2+, PR48:5 Mg2+), the available toxicity data include a review by BIBRA (1997a) and a reproductive/developmental screening study in rats (CHRIP ©2008b). For PR63:1, no publically available review of the health effects data of this substance was identified. While a safety evaluation by the US FDA was conducted on PR63:1 under the name D&C Red No. 34 for approved use as a colourant in externally applied drugs and cosmetics (US FDA 1976), the details of this evaluation are not publically available. Therefore, the available published toxicity data on PR63:1 are summarized. No toxicity data were identified for the free acid or any salts of PR52.
| Substance | Short-term LOAEL/NOAEL (mg/kg-bw per day) |
Subchronic LOAEL/ NOAEL (mg/kg-bw per day) |
Chronic LOAEL/ NOAEL (mg/kg-bw per day) |
|---|---|---|---|
| PR48:2 | LOAEL = 200 (FR) LOAEL = 1000 (MR) Kidney (degeneration/ necrosis of proximal tubules in FR, increased kidney wt and tubule basophilic changes in MR (CHRIP ©2008b) |
No suitable data | Read-across from PR57:1* |
| PR52:1; PR52:2 | Read-across from PR48:2 and PR57:1* | No suitable data | Read-across from PR57:1* |
| PR63:1 | Read-across from PR48:2 and PR57:1* | NOAEL = 2500 (MR/FR) (Hansen et al. 1960)Footnote Table 7-12 [c] |
Read-across from PR57:1* |
| PR57:1* | LOAEL = 100 (FR) Kidney (necrosis, regeneration, foamy cells in tubular epithelium) (FDSC 1993) |
NOAEL = 1000 (MR/FR) (Hansen et al. 1958)[c] |
LOAEL = 150 NOAEL = 25 (MR) Kidney (increased severity of chronic progressive nephrosis in MR and FR) (IRDC 1981) |
Carcinogenicity and Genotoxicity
No empirical data on carcinogenicity were identified for the BONA pigment lakes considered in this assessment (PR63:1, PR48-based pigment lakes PR48:2 and PR48:5, PR52-based pigment lakes PR52:1 and PR52:2) however chronic toxicity data was available for another BONA pigment lakes, PR57:1* and its sodium salt PR57*. The chronic dietary studies on PR57* in mice and rats and a lifetime skin painting study on PR57:1* in mice were also considered to provide evidence for the lack of carcinogenicity of these substances by the oral and dermal exposure routes (US FDA 1982, described in section below, Other Repeated-dose Toxicity Studies). Based on the available data, PR57:1* and PR57* are not considered to present a high potential for either carcinogenicity.
The available genotoxicity for the BONA pigment lakes are summarized below. Negative genotoxic responses in the standard Ames assay with and without S9 activation were reported for “Red 2B, CI 15865” (presumably PR48) in TA1535 and TA1537 (Milvy and Kay 1978) and for PR48:4 (Mn2+ salt) in TA98, TA100, TA1535 and TA1537 (ETAD 1988). PR63:1 was reported as negative in both the standard Ames assay in Salmonella strains TA98 and TA100, with or without S9 activation (Miyagoshi et al. 1983). PR63:1 was also reported to be negative and weakly positive in a Prival modified Ames assay with FMN supplement in TA98 and TA100, respectively (ILS 2011). Both PR57:1* and PR57* were negative for several genotoxicity endpoints, including in vitro mutagenicity in bacteria and mammalian cells, in vitro chromosomal aberrations in mammalian cells and in vivo micronucleus assay in mice (SCCS 2012).
Other Repeated-dose Toxicity Studies
The critical health effect for PR48:2 is based on a Japanese study conducted according to OECD Test Guideline 422 under good laboratory practice (GLP) conditions in which groups of rats (Crl: CD (SD) strain, 12 of each sex per group) were exposed by daily oral gavage to PR48:2 at a dose of 0, 40, 200 or 1 000 mg/kg-bw per day for 42 days (14 days premating to day 4 of lactation). Sacrifice was at day 43 (main group) or following a 14-day recovery period in a satellite group (five of each sex for control and high-dose groups), while F1 offspring were sacrificed 4 days after birth. Increased degeneration or necrosis of the proximal tubular epithelium in kidney was observed at 200 mg/kg-bw per day. However, the effect was reported to have disappeared following the 14-day recovery period (CHRIP ©2008b). Based on a single oral gavage screening reproductive/developmental toxicity study in rats from the same report, the target organ for PR48:2 toxicity appears to be the kidney (CHRIP ©2008b).
The critical health effect for PR57:1* is considered to be non-cancer changes in the kidney, with a short-term/subchronic LOAEL of 100 mg/kg-bw per day (the lowest dose tested) based on kidney lesions in female rats (FDSC 1993) and a chronic LOAEL of 150 mg/kg-bw per day (NOAEL of 25 mg/kg-bw per day) based on an increased incidence of spontaneous kidney disease (chronic progressive nephrosis) in male rats (IRDC 1981; US FDA 1982).
The kidney appeared to be the main target organ of PR57:1* toxicity in both sexes of rats, showing consistent dose-dependent increased incidence and severity of various lesions in the kidney tubules. The most recent study on PR57:1* among those identified was conducted in 1993 by the Japanese Food & Drug Safety Center (Hatano Research Institue, Japan) according to OECD Test Guideline 422 under GLP conditions and is considered the highest quality study available for PR57:1* or PR57* (FDSC 1993; OECD 1994; EFSA 2010; SCCS 2012;). In this study, male and female rats (13 of each sex per dose group, Crj:CD (Sprague-Dawley) strain) were exposed by oral gavage to a dose of 0, 100, 300 or 1000 mg/kg-bw per day (in 5% gum arabic solution) for 42 days in males or approximately 39 days in females (14 days premating, 22 days of gestation, 3 days of lactation). No exposure-related changes in survival, body weight, feed consumption or hematological parameters were observed in males or females. No exposure-related changes in the reproductive parameters or developmental toxicity endpoints were observed. Similar histopathological findings on the kidney tubules were also observed in males in the 14-day range-finding study, as well as increased kidney weights in high-dose males in the main study, supporting the kidney as a target organ in this study. The LOAELs for this study were considered to be 100 mg/kg-bw per day for female rats and 300 mg/kg-bw per day in male rats (FDSC 1993; OECD 1994). Other older repeated-dose toxicity studies for PR57:1* or PR57* also support the kidney as the target organ, although it has been noted that lower, or lack of, apparent potency for kidney and other effects in several short-term and subchronic dietary studies may have been due to the lower expected peak systemic dose compared with the gavage studies (Leist 1982; FDSC 1993).
Several reviews of the above studies on PR57:1* considered the available toxicity data insufficient to derive a definitive NOAEL for establishing guideline levels (JECFA 1986; EFSA 2010), although 100 mg/kg-bw per day was considered to be close, based on the available data set (EFSA 2010). The NOAEL of 15 mg/kg-bw per day from the three-generation study in rats (Weil and Carpenter 1973) was considered by the US FDA (1982) as the basis for derivation of an acceptable daily intake.
A brief meeting abstract summarizes the results of a subchronic oral toxicity study conducted by the US FDA in which rats (10 of each sex per group, strain not provided) were exposed to dietary PR63:1 concentrations of 0%, 0.25%, 0.5%, 1%, 2% or 5% (equivalent to doses of 0, 125, 250, 500, 1 000 and 2 500 mg/kg-bw per day) for 90 days (Hansen et al. 1960). No exposure-related changes were observed in body weight, feed intake, blood counts or terminal organ weights. Therefore, the highest tested dose of 2 500 mg/kg-bw per day (5%) was considered the NOAEL for this study (Hansen et al. 1960).
The dermal toxicity of BONA pigment lakes was investigated in a chronic skin painting study in mice. Briefly, ICR Swiss Webster mice (50 of each sex in exposure group and 25 of each sex in control group) were exposed on the clipped dorsal skin (6 cm2) twice weekly for 18 months to either water vehicle control or PR63:1 (as D&C Red No.34) and PR57:1* (as D&C Red No.7) at 0.1 mg/mL in water (per application dose of approximately 33 mg/kg-bwFootnote[18]). No exposure-related changes in body weight, survival, behaviour or gross pathology were reported. Histological examination did not reveal any obvious differences in tumours of the skin or other tissues between the control and the exposure groups (Carson 1984a, b; IARC 1993). Based on the limited dermal absorption expected, the predicted systemic dose from this application would be low and insufficient to cause any systemic effects. Therefore, the study design and low doses selected limit the interpretation of the results from this study.
Summary and Considerations for Read-across
Based on comparable physical-chemical properties, structural similarity of the BONA pigment lakes, azo bond cleavage potential, similar expected toxicokinetics and toxicodynamics, and common azo cleavage metabolites, all the BONA pigment lakes in this subset are expected to have a qualitatively similar toxicity. The primary basis for read-across of health effects for the BONA pigment lakes is based on available short-term toxicity data for PR48:2 as well as shor-term, sub-chronic and chronic data for the analogue BONA pigment lakes PR57* and PR57:1*. This and additional information on the azo cleavage metabolites are provided in the sections below.
Overall, based primarily on read-across for the health effects data on PR57:1*, the BONA pigment lakes are expected to have a low potential for carcinogenicity and genotoxicity. Therefore, non-cancer health effects are considered to be the endpoints of concern. Based on read-across for PR48:2 and PR57:1*, the critical short-term/sub-chronic health effect levels are based on LOAELs of 100–200 mg/kg-bw per day (range of LOAELs for PR57:1* and PR48:2) for kidney lesions in female rats while a chronic LOAEL of 150 mg/kg-bw per day (NOAEL = 25 mg/kg-bw per day) is based on increased incidence of spontaneous kidney disease (chronic progressive nephrosis) in male rats (also at higher doses in female rats) exposed to PR57:1* (IRDC 1981; US FDA 1982). Since the molecular weights of PR57:1* and the evaluated BONA pigments are very close in range (425 g/mol vs. 443–461 g/mol, respectively), molar equivalent doses have not been derived for each substance.
Similar kidney effects were observed for PR48:2 and PR57:1* under the same experimental study protocol in rats in terms of lesions (degeneration/necrosis of renal tubule cells) and potency range: LOAEL of 200 mg/kg-bw per day for PR48:2 (CHRIP ©2008b) compared with a LOAEL of 100 mg/kg-bw per day for PR57:1* (FDSC 1993). In other short-term and chronic studies on PR57:1*, the kidney was also identified as the target organ for toxicity. Kidney tubule toxicity was also observed in short-term oral studies in rats for other BONA pigment lake analogues, including CAS RN 17841-23-2 (Sun Chemical 2004). However, in these latter studies, the male rats appeared to be more sensitive than the females to the kidney toxicity; this is more typical of kidney toxicity in general, as evidenced by observations of chronic progressive nephrosis in the male rat (Hard and Khan 2004). The greater apparent sensitivity of female rats in the reproductive/ developmental toxicity screening studies (OECD Test Guideline 422) on PR48:2 and PR57:1* may have been due to the pregnant status of the females in these studies, however there is uncertainty regarding the reason for observed sensitivity in pregnant females. However, the collective information on the available BONA pigment lakes strongly suggests the kidney as a target organ for the toxicity of BONA pigment lakes.
Based on the assumption that exposure to the BONA pigment lakes will undergo azo bond cleavage to some degree, the toxicity of the potential azo bond cleavage products (Table 7-11) of the BONA pigment lakes are also considered here.
While no toxicity data were identified for the common metabolite 1-amino-2-naphthol-3CA which is shared by all the BONA pigment lakes (Table 7-11), some health effects data were available for Red 2B acid (from PR48:2, PR48:5), Red Lake C amine (from PR52:1 and PR52:2), Tobias Acid (PR63:1), and Red 4B acid (from the analogue PR57:1*).
The toxicity of Red 4B acid (from analogue PR57:1*) has been studied in the reproductive/ developmental toxicity screening study (OECD TG 422) in rats using the same protocol as above for PR57:1 with no exposure-related effects in the F0 males or females, on reproductive parameters or on developmental toxicity endpoints observed up to the highest tested dose of 1000mg/kg-bw per day (JECDB 2013b). In comparison, a study with the parent azo PR57:1* using the same design (OECD TG 422) reported degeneration/necrosis of renal tubule cells at a much lower dose of 100mg/kg-bw per day. Given the differential responses for Red 4B acid and the parent azo PR57:1* in the type of study, any Red 4B acid potentially generated from the parent PR57:1* would not be expected to contributing significantly to the observed toxicity of PR57:1*. Therefore, the toxicity of PR57:1* may be either due to activation of the parent PR57:1* itself or from the other azo bond cleavage product, 1-amino-2-naphthol-3CA. While no toxicity data were available for 1-amino-2-naphthol-3CA, given that PR57:1* is likely to undergo some degree of azo bond cleavage following oral exposure, it is likely that 1-amino-2-naphthol-3CA contributes to some extent to the observed oral toxicity of PR57:1*. With respect to genotoxicity, Red 4B acid was reported to be negative for in vitro mutagenicity in Salmonella , E. coli, and in mouse lymphoma cells and this substance was also negative for UDS in primary rat hepatocytes (CCRIS 2012). Overall, the metabolite Red 4B acid is expected to pose a low potential for systemic toxicity and genotoxicity. There were no repeated-dose studies identified for Red 2B acid (from PR48:2 and PR48:5) However a 28day oral study in rats for the positional isomer Red Lake C amine (from PR52:1 and PR52:2, and also the β-pigment lake PR53:1) indicated no exposure-related effects in body weight, feed consumption, hematological or clinical chemistry parameters, organ weights, or gross and/or microscopic abnormalities up to the highest tested dose of 1000mg/kg-bw per day (Environment Canada, Health Canada 2014b). This low systemic toxicity of Red Lake C amine is consistent with that for Red 4B acid (previous paragraph) and other sulfonated aromatic amines (Jung et al. 1992) and likely applies also to Red 2B acid. The toxicity of Tobias acid (from PR63:1) was described in a previous section (see Section 7.2.2 Summary and Considerations for Read-Across) as this substance is also a metabolite of the β-Naphthol pigment lakes PR49:1* and PR49*. In a reproductive/ developmental toxicity screening study (exposure duration 49 and 39 days for males and females respectively) exposure of rats to Tobias acid resulted in a similar lack of clear toxicity observed up to the highest tested dose of 1000mg/kg-bw per day, although a decrease in liver weights was reported at the high dose (JECDB 2013a).
Therefore, the available information in the paragraph above indicates a limited systemic toxicity from the most of the expected azo cleavage metabolites of the BONA pigment lakes. While no health effects data were identified for 1-amino-2-naphthol-3CA, the azo bond cleavage product appears likely to contribute in some degree to the observed toxicity of the BONA pigment lakes, including PR57:1* . Since the other potential azo bond cleavage products, o-sulfo-substituted aniline derivatives (PR48, PR52, PR57*) and Tobias acid (PR63), indicate low hazard potential, it is reasonable to expect that the common metabolite 1-amino-2-naphthol-3CA could be responsible for the effects observed for PR48:2 and PR57:1* and could contribute similarly to the toxicity of the other BONA pigment lakes in this subset.
Overall, although data is limited, a read-across for the health effects of PR48:2 and PR57:1* (Table 7-12) are considered to apply to all substances in the BONA pigment lakes subset.
7.2.4 Monoazo Yellow Pigments (PY1, PY3, PY73)
PY1, PY3 and PY73 do not have robust data sets for human health effects. The limited data identified were genotoxicity studies, including Prival modified Ames assays for all three substances, as well as short-term oral toxicity studies on PY1 and PY3. No chronic toxicity studies were identified for any of these substances, and the only repeated-dose toxicity studies identified were for PY73. Two analogues, PY74* and CAS RN 80675-49-6*, were considered to inform the hazard potential of PY1, PY3 and PY73. These five substances, together with their potential azo bond cleavage products, are presented in Table 7-13.
Absorption and Azo Bond Cleavage Potential
Despite similar molecular weights and solubilities in water and octanol, monoazo yellow pigments appear less bioavailable than β-naphthol pigments following oral exposure. In the oral toxicokinetics study of PY74* and PR3 (a β-naphthol pigment) in rats, a single oral gavage dose of PY74* in corn oil of 12.6 mg/kg-bw was given to male F344 rats (n = 12), with pooled tissues (gut contents, feces, plasma, whole blood, liver, kidneys, lungs and urine) from three rats sampled at 1, 4, 24 and 48 hours after dosing (El Dareer et al. 1984). No PY74* was detected, except in the intestinal tissues directly in contact with the gut contents and in the urine (less than 1%) at 24 and 48 hours. By 48 hours, up to 85.9% of the administered PY74* was recovered in the feces, with 2.1% in the gut contents, giving a total recovery of 88.2% (standard deviation ± 21.7%). The metabolites of PY74* were not investigated. Despite the large variability in recovery noted in this study, relatively lower amounts of PR3 were recovered, indicating relatively greater absorption and/or azo bond cleavage of this substance. Therefore, a limited potential for absorption and/or azo bond cleavage of PY74* is considered, at least relatively lower than that for PR3.
A similar comparison is observed from the results of a microbial azo degradation study (Pearce et al. 2008). In this study, PO5 (a β-naphthol pigment) demonstrated much greater relative potential for azo bond reduction by Shewanella strain J18 143 compared with PY74*. For commercial pigments containing dispersions, there was greater than 80% reduction in absorbance of PO5 after 160 hours of incubation, compared with roughly 10% reduction for PY74*. After 20 hours, PO5 showed greater than 20% reduction in absorbance, while less than 5% of PY74* was absorbed. It was suggested that relatively higher resistance to organic solvents and less microbial azo reduction of PY74* were due to its crystal structure being stabilized by both hydrogen bonding and van der Waals forces, while only van der Waals forces are present in β-naphthol pigments.
| Parent monoazo yellow pigment | Potential azo bond cleavage products |
|---|---|
PY1
|
2-Nitro-4-methylaniline
Amine of acetoacetanilide
|
PY3
|
2-Nitro-4-chloroaniline
Amine of chloracetoacetanilide
|
PY73
|
2-Nitro-4-chloroaniline
Amine of acetoacet-o-anisidide
|
PY74*
|
2-Methoxy-4-nitroaniline
Amine of acetoacet-o-anisidide
|
AS RN 80675-49-6*
|
2-Methoxy-5-nitroaniline
(no name or CAS RN)
|
Reductive cleavage of the azo bond would theoretically produce the respective nitroaniline derivative and an amine of the acetoacetanilide derivative.
Collectively, the above studies indicate that the expected amount and rate of azo bond cleavage would be low and relatively much less for PY74* than for the β-naphthol pigments such as PR3 and PO5. Therefore, an overall lower oral bioavailability and azo bond cleavage potential for monoazo yellow pigments are expected relative to the β-naphthol pigments.
Health Effects
Although the analogues provide some additional information on short-term and subchronic toxicity, information on all relevant endpoints, particularly from chronic toxicity studies, was not available. As a conservative approach, the health effects of the potential azo bond cleavage products are also considered, although no chronic toxicity studies were identified for them either. While chronic toxicity studies are generally lacking, it is considered that potential chronic health effects may be qualitatively similar to those of the other nitroaniline-based Monoazo Pigments (i.e., the β-naphthol pigments subset), with an assumption of lower relative potency, considering the following factors.
The assumption of similarity in chronic health effects between monoazo yellow pigments and β-naphthol pigments is based primarily on the fact that these two subsets would release similar nitroaniline derivatives following azo bond cleavage, and these derivatives are at least partially responsible for the chronic toxicity of the parent substances. As an example, both PY1 and PR3 can potentially release 2-nitro-4-methylaniline and therefore are homologues of each other for this metabolite. The nitroaniline derivative metabolites formed from both of these subsets would be expected to exhibit similar genotoxicity, target organ toxicity (hemolysis, and histopathology of spleen and livers) and potency levels that are characterized by the more general category of nitroaromatics (Sakuratani et al. 2013). These types of effects are also observed in chronic toxicity studies for the β-naphthol pigments, where the nitroaniline metabolites may be responsible for the observed effects.
The lower relative potency assumed for the chronic toxicity of the monoazo yellow pigments is based on the lower apparent bioavailability and/or azo bond cleavage following oral exposure, as described in the previous section, as well as the lower relative short-term toxicity compared with the β-naphthol pigments. The available short-term toxicity studies on PY1 and PY3 in rats do not suggest a strong potential for toxicity. While the 30-day gavage study with PY1 suggested some possible exposure-related effects (increased liver weight in females and some indication of hemolysis in males) at 1 000 mg/kg-bw per day (Hoechst 1979), the short-term feeding study with PY3 did not indicate any response at the highest tested feed concentration of 1 250 mg/kg-bw per day (Study Submission 2012r). Such limited toxicity is also supported by the analogues; for example, PY74* demonstrates possible exposure-related changes (increased liver weight and evidence for hemolysis) only at the highest tested dose of 1 000 mg/kg-bw per day in a 90-day gavage study in rats (ECHA 2012), similar to the study on PY1 (Hoechst 1979). However, similar to the lack of effects from the short-term feeding study on PY3, no effects were also observed in rats at 1 126–1 160 mg/kg-bw per day (the highest dose tested) in a 28-day feeding study on another monoazo yellow pigment analogue CAS RN 80675-49-6* (ECHA 2012). The apparent exposure-related effects observed for PY1 and PY74* at lower doses may be due to the higher expected peak plasma concentrations from the bolus delivery of test substance from gavage route compared to the lack of effects seen in the feeding studies on PY3 and CAS RN 80675-49-6*. In comparison, the short-term oral LOAELs in rats for the β-naphthol pigments range from 100 to 542 mg/kg-bw per day for PO5 (32 days) and PR3 (14 days), as previously discussed. Therefore, the potency of the monoazo yellow pigments is considered to be lower than that of the β-naphthol pigments. The apparent relative potency difference for short-term toxicity is considered even lower as the observed effects for PY1 and PY74* were not adverse compared with the more pronounced effects for PO5 and PR3. In addition, since the studies for PY1 and PY74*were gavage versus feeding studies for PR3 and PO5, the monoazo yellow pigments are expected to exhibit even lower relative toxicity from feeding studies (as seen for NOAELs at highest tested feed doses for PY3 and CAS RN 80675-49-6*). Overall, despite the lack of clear toxicological effects for the monoazo yellow pigments in short-term studies, the potency of the monoazo yellow pigments is considered lower than that of the β-naphthol pigments; this relative potency is also considered to apply for the potential chronic toxicity of the monoazo yellow pigments.
Critical effect levels for short-term toxicity of the monoazo yellow pigments are summarized in Table 7-14.
| Substance | Short-term LOEL/ NOAEL(mg/kg-bw per day) |
|---|---|
| PY1 | LOEL = 1 000 (FR; increased liver wt; Hoechst 1979) |
| PY3 | NOAEL = 1 250 (MR/FR; Study Submission 2012r) |
| PY73 | Read-across from PY1 and PY3 |
7.2.5 Naphthol AS Pigments (PR5, PR112, PR170, PR187, PR266, PR268 and PO38)
Among substances in the naphthol AS pigments subset, seven substances are expected to have current exposure in Canada. These substances all contain the same core structure of an aniline ring that is azo coupled to the naphthol AS moiety; however, there is much variability among the substitutions on both sides of the azo bond, making this subset more heterogeneous than previously discussed subsets. Unlike the monoazo yellow pigments subset, the potential azo bond cleavage of these substances would generate very different aromatic amine metabolites, all of which could have differing toxicities. The physical-chemical properties of the naphthol AS pigments are also more variable, presumably due to the structural differences among substances in this subset. For example, while the available data indicate a narrow range of water solubility across the naphthol AS pigments subset (3–24.9 µg/L), the octanol solubility ranges more than 2 orders of magnitude (e.g. 22.1 µg/L for PR187 compared with 7800 µg/L for PR112). Such differences in octanol solubility may result in variability in the potential bioavailability among the naphthol AS pigments. Given the paucity of toxicity data available and noting the higher uncertainties associated with the greater structural variability of the naphthol AS pigments, a broader read-across is considered for the hazard characterization, primarily using PR22* and PR23* as the analogues, but also beyond this subset. Both PR22* and PR23* contain nitroaniline moieties that would be released upon azo bond cleavage. However, substituted nitroanilines may not be as applicable for read-across to the other naphthol AS pigments that do not contain nitroaniline moieties.
Absorption and Azo Bond Cleavage Potential
The only data identified for azo reduction potential were for PR112. The potential for azo bond cleavage of PR112 was studied in an anaerobic incubation with the bacterium Shewanellastrain J18 142 (Pearce et al. 2008). While some low levels of azo reduction were observed for PR112, the study demonstrated a much more limited potential for azo reduction of PR112 compared with the β-naphthol pigment PO5, which underwent substantial azo reductive cleavage over the study period.
Limited amounts of PR23* were metabolized to 5-nitro-o-anisidine, suggesting limited bioavailability (NTP 1978). In addition, an oral toxicokinetics study of PR23* in rats indicated rather limited absorption and/or intestinal azo bond cleavage of PR23* in comparison with the β-naphthol pigment PR3, despite the large standard deviations on the recovered PR23* (El Dareer et al. 1984).
Health Effects
Health effects data were identified for PR112 (short-term toxicity, genotoxicity, microbial azo reduction), PR170 (short-term toxicity, genotoxicity) and PR268 (genotoxicity, microbial azo reduction).
Among several genotoxicity studies identified for PR112, earlier studies on PR112 reported positive mutagenicity in the standard Ames assay and Chinese hamster V79 cells; however, these results were either proved to be due to impurities or not repeatable (NTIS 1986; BIBRA 1997b). Negative results were obtained from another standard Ames assay (ETAD 1988) and more recent studies with the FMN Prival modification that were compliant with GLP, as well as for mutagenicity and clastogenicity in Chinese hamster V79 cells (ECHA 2012). PR170 also indicated a lack of mutagenicity in the standard Ames assay (Study Submission 2012s); as well, negative results for mutagenicity and chromosomal aberrations was observed in Chinese hamster V79 cells (Study Submission 2012t, u). PR268 was negative for mutagenicity in Salmonella strains TA98 and TA100 with hamster liver S9 and with the FMN Prival modification (ILS 2011).
An in vitro study on PR22* with Chinese hamster lung cells showed no increase in chromosomal aberrations or polyploidy with or without rat liver S9 (JECDB 2013c). However, mixed mutagenicity results were obtained in the standard Ames assay with both positive (TA98, TA100, TA1537, ±S9; E. coli WP2 uvrA +S9) and negative results (TA1535 ± S9; E. coli WP2 uvrA −S9). The positive responses are consistent with those for other Monoazo Pigments containing nitro substitutions (β-naphthol pigments), which may indicate that the pigment is biologically available to the bacteria either as the parent molecule or after azo reduction to the nitroaniline. In either case, these results suggest that PR22* was sufficiently soluble or bioavailable to elicit a positive response in this study, supported by the release of 5-nitro-o-toluidine at levels greater than 30 ppm following chemical reduction from PR22* and the related naphthol AS pigment, PR8 (ETAD 2008). Overall, the data on PR22* indicate some potential for toxicity and genotoxicity in vitro, which suggests at least a limited bioavailability of PR22* or its azo bond cleavage metabolites in these studies.
PR23* has been tested in a chronic feeding study in rats and mice (NTP 1992). The short-term and subchronic stages of this study indicated hemolysis and liver weight increases at a feed concentration of 50 000 ppm in rats (dose equivalent to 2 500 mg/kg-bw per day). The study also demonstrated a low carcinogenic potency for PR23*, with equivocal evidence for renal tumours and tubule hyperplasia at a feed concentration of 50 000 ppm in rats (dose equivalent to 2 500 mg/kg-bw per day). Since the potential azo bond cleavage product, 5-nitro-o-anisidine, was itself positive for carcinogenicity at numerous sites in rats at doses as low as 200–400 mg/kg-bw per day (NTP 1978), the overall lower potency and weaker response of PR23* suggests that limited amounts of PR23* were cleaved at the azo bond to produce 5-nitro-o-anisidine; this suggests limited bioavailability of the toxic metabolite, as discussed in the previous section.
A recent short-term toxicity study in rats conducted according to OECD Test Guideline 407 was reported in the REACH dossier for PR112 (ECHA 2012). In this study, Wistar rats (five of each sex per group, Crl:Wi(Han) strain) were exposed for 28 days to a daily gavage of PR112 in propylene glycol vehicle at a dose of 0, 100, 300 or 1 000 mg/kg-bw per day. No toxicologically significant effects were reported in any parameter measured (clinical signs, body and organ weights, hematology, urine analysis, clinical chemistry, gross pathology, histopathology), and the highest tested dose of 1 000 mg/kg-bw per day was considered to be the NOAEL by the study authors. Some hematological changes were observed, including increased reticulocytes (males, 1 000 mg/kg-bw per day), increased methemoglobin levels (females greater than or equal to 100 mg/kg-bw per day) and lower mean corpuscular volume (females greater than or equal to 100 mg/kg-bw per day), although they are not considered toxicologically significant by the study authors, given that these values were within the normal ranges (ECHA 2012).
2,4,5-Trichloroaniline (CAS RN 636-30-6), a potential azo bond cleavage product of PR112, has been observed to cause methemoglobinemia and Heinz bodies following acute exposure, while short-term oral studies in rats also caused hematological changes consistent with hemolysis and increased liver weights at doses as low as 40 mg/kg-bw per day (BUA 2001). Although the increased reticulocytes and methemoglobin observed for PR112 are consistent with the effects of 2,4,5-trichloroaniline, the observation of lower increases in mean cell volume is inconsistent with a hemolytic effect in this study. Given the uncertainty around whether the increased reticulocytes and methemoglobin observed in the REACH dossier study are related to PR112 exposure, a no-observed-adverse-effect level (NOAEL) of 1 000 mg/kg-bw per day is considered for this assessment. Another short-term study in rats indicated an absence of effects on behaviour, urine parameters, hematology or gross or microscopic pathology following 28 gavage doses of PR112 at 500 mg/kg-bw over a 43-day period (SCC 1988; BIBRA 1997b). A local lymph node assay in mice was also reported as negative for PR112 (ECHA 2012).
Overall, the available repeated-dose toxicity studies on PR112 do not demonstrate evidence for clear adverse effects at doses up to 1 000 mg/kg-bw per day, and an absence of positive response in in vitro assays has also indicated a lack of genotoxic potential for this substance.
An unpublished short-term oral toxicity study in which Wistar rats (five of each sex per group) were dosed with 0, 500, 2 500 or 12 500 ppm of PR170 in the feed following OECD Test Guideline 407 for 28 days (Study Submission 2012v) showed no apparent exposure-related dose-dependent changes in hematology, urine analysis, clinical chemistry or tissue pathology. The highest exposure group of 12 500 ppm (1 172 and 1 193 mg/kg-bw day for males and females, respectively) is considered as the NOAEL in this study.
In a short-term reproductive/developmental toxicity screening study (OECD Test Guideline 422) in which rats (12 of each sex per group) were exposed to PR22* by gavage for 37 days in males and approximately 40 days in females (14 days premating, 23 days of gestation to day 4 of lactation) to a dose of 0, 100, 300 or 1 000 mg/kg-bw per day (JECDB 2013c), no exposure-related changes were reported in reproductive or developmental parameters, nor were any consistent dose-dependent effects observed in hematology, urinary parameters, clinical chemistry or findings from gross pathology and histopathology. However, a dose-dependent increase in liver weights was observed in both males and females, which reached statistical significance at 1 000 mg/kg-bw per day. The middle dose of 300 mg/kg-bw per day was considered as the no-observed-effect level (NOEL) for this study (JECDB 2013c).
In the absence of empirical health effects data, read-across is applied across this subset including those Naphthol AS pigments that do not share the same metabolite (5-nitro-o-anisidine) as PR23*. Overall read-across within this subset indicates a low toxicity from short-term studies; 1 000 mg/kg-bw per day is considered as a short-term NOAEL for the entire subset based on effects observed for PR22*, taking into account the results from PR112 (NOAEL of 1000 mg/kg-bw per day), PR170 (NOAEL of 1172 mg/kg-bw per day) and PR23* (short-term LOAEL of 2 500 mg/kg-bw per day). Summary of critical effect levels are presented in Table 7-15.
| Substance | Short-term NOAEL (mg/kg-bw per day) |
|---|---|
| PR112 | NOAEL = 1000(MR/FR) (ECHA 2012) |
| PR170 | NOAEL = 1172/1193(MR/FR) (Study Submission 2012v) |
| PR5; PR187; PR266; PR268; PO38; NAPNPA; NANPAP; NAPMPA; NAPPA | Read-across from PR22*, PR112 and PR170 |
The only chronic toxicity study was for the analogue PR23*, which showed low carcinogenic potency at doses as low as 2 500 mg/kg-bw per day in rats.
Although PR22* and PR23* contain nitroaniline moieties, these analogues also demonstrated a low potency for effects in short-term studies in rats, with LOELs of 1 000 mg/kg-bw per day for PR22* (increased liver weight) and 2 500 mg/kg-bw per day for PR23* (liver weight, evidence of hemolysis). The chronic toxicity study for PR23* also indicates a lower carcinogenic potency at the high dose of 2 500 mg’ kg bw per day, than would be expected from its metabolite (5-nitro-o-anisidine), suggesting a low bioavailability of the parent PR23* to degradation pathways. The available data on microbial azo reduction for PR112 (Pearce et al. 2008) and oral toxicokinetics in the rat for PR23* (El Dareer et al. 1984) also indicate a low overall potential for oral absorption and/or microbial azo reduction relative to β-naphthol pigments also investigated in the same studies. Overall, these Napthhol AS pigments are considered to exhibit similar qualitative short-term and chronic toxicity as for the β-naphthol pigments with a lower relative potency based on the comparison of available short-term toxicity data.
7.2.6 Other monoazo pigments (PO36, PR247:1, PR251, PY60, NSNAC)
No susbstance specific toxicity data were identified for the other monoazo pigments not otherwise covered in one of the pigment subsets from previous sections. Among these substances, evidence for exposure was only identified for PO36.
PO36
No relevant health effects data were identified for PO36. However, since PO36 can potentially generate a nitroaniline from azo bond cleavage similar to those for the β-naphthol pigments subset, a conservative supposition is made that similar qualitative toxicity as for the β-naphthol pigments may occur from short-term and chronic exposures to PO36. Based principally on the overall 10–100 times lower octanol solubility of PO36 (86.1 to greater than 137 µg/L) compared with the β-naphthol pigments (1760–17900 µg/L), the bioavailability of PO36 is also considered to be lower in terms of potency for both short-term and chronic effects.
PR247:1, PR251, PY60, NSNAC
The available information did not identify sources of exposure to these substances, therefore as no exposure is expected, only a limited hazard characterization was conducted on these substances. No health effects data were identified for PR247:1, PR251, PY60, or NSNAC and no close data-rich analogues were identified for use in read-across. Other than for PR247:1, no empirical data on the most relevant physical chemical properties (i.e. solubility in water and/or octanol) were identified to help infer bioavailability and the potential for these substances to undergo cleavage. However, it should be noted that two of these substances contain moieties for which some health effects may be expected.
Theoretical azo cleavage of PR251 would generate 1-aminoanthraquinone (CAS RN 82-45-1) which has shown effects in spleen, kidney repeat-dose studies and in nursing behaviour in a reproductive study (OECD 1996). Several related aminoanthraquinones have shown positive evidence of carcinogenicity in rodents in chronic studies although clear read-across of this effect to 1-aminoanthraquinone is uncertain (Sendelbach 1989).
Theoretical azo bond cleavage of PY60 would release 2-chloroaniline (CAS RN 95-51-2), for which toxicity to red blood cells and spleen, typical of other chloroanilines, has been observed in rats (Environment Canada, Health Canada 2014b). No health effects data were identified for the potential azo cleavage metabolites of PR247:1 or NSNAC. However, both of the aminonaphthol moieties on each side of the azo bond of NSNAC are sulfonated and one of the Naphthol AS derivative moieties in PR247:1 is sulfonated which would suggest increased bioavailability by the oral route for these substances (by analogy to the β-naphthol pigment lake and BONA pigment lake subsets evaluated in this assessment). However, these sulfonated amines would not be expected to have carcinogenic potential and would generally be associated with lower systemic toxicity (Jung et al. 1992). There were no health effects data identified for the remaining unsulfonated Naphthol AS derivative moiety in PR247:1, therefore the potential toxicity associated with this structure is uncertain.
While there may be health effects associated with the theoretical azo cleavage metabolites of some of these substances (1-aminoanthraquinone from PR251, and 2-chloraniline from PY60), there is high uncertainty regarding potential release of these metabolites from the parent pigments. Therefore, in the absence of data on these substances the critical health effects cannot be conclusively determined.
7.2.7 Uncertainty in Toxicological Data
Recognizing the inadequacy of the data set to varying degrees, depending on the subset, some general assumptions for predicting hazard potential have been applied to all the Monoazo Pigment subsets. The primary assumption is that the toxicity of substances and analogues within a subset is due to the potential aromatic amine metabolites, which for a given Monoazo Pigment subset may be a common metabolite (e.g., 1-amino-2-naphthol is a common potential metabolite for all substances in the β-naphthol pigments and β-naphthol pigment lakes subsets). In other cases, very closely related metabolites may be expected for a given subset (e.g., substituted nitroaniline derivatives for substances in the β-naphthol pigments subset). It is expected that systemic exposure to these common and/or very closely related aromatic amine metabolites would lead to similar health effects following exposure to the parent substances. This read-across approach has also been extended to those Monoazo Pigment subsets for which health effects data are more limited (e.g., monoazo yellow pigments) and those that are inherently more diverse in chemical structure and physical-chemical properties (e.g. naphthol AS pigments subset). In addition, since no chronic studies were identified for substances or analogues of the monoazo yellow pigments and naphthol AS pigments subsets, read-across from the β-naphthol pigments subset has been applied as a conservative worst-case scenario for chronic health effects. Overall, while the application of a read-across approach for the hazard potential within Monoazo Pigment subsets is considered a logical approach, it should be noted that the read-across uncertainty is higher for those Monozo Pigment subsets with a lack of, or very limited, toxicity data and more heterogeneous structures and physical-chemical properties. These factors lead to a graded confidence level in the health effects assessment of the different Monoazo Pigment subsets. The subsets ranked in order of highest to lowest confidence are: β-naphthol pigments greater than β-naphthol pigment lakes greater than BONA pigment lakes greater than monoazo yellow pigments greater than naphthol AS pigments greater than individual pigments. This order was followed in the discussion of the available health effects data specifically for those substances for which there is current known consumer exposure in Canada (see the Exposure Assessment section)
Monoazo Pigments in the β-naphthol pigments subset have relatively low water solubility values compared with either the very water soluble azo dyes or the lipid-soluble azo solvent dyes. Therefore, there is some uncertainty regarding the bioavailability and absorption of the parent Monoazo Pigments and/or their azo bond cleavage metabolites, which is taken into consideration in the health effects assessment. There is uncertainty regarding the degree of absorption of the β-naphthol pigment lakes following oral exposure; however, it is expected that oral absorption is substantial ( greater than 35–54% from 14C-labelled urinary metabolites following oral exposure to PR53:1).
While it seems clear that some oral absorption, intestinal azo bond cleavage and dissociation are expected following oral exposure to the β-naphthol pigment lakes, uncertainty is recognized for extrapolation to the dermal route of exposure. There is also uncertainty regarding the specific contributing factors responsible for the apparent oral bioavailability of the β-naphthol pigment lakes. For BONA pigment lakes, uncertainty is also recognized regarding the absolute value of oral absorption; however, it is expected that substantial absorption and azo bond cleavage are likely to occur following oral exposure.
Dermal absorption values of 6% for pigments and 1% for pigment lakes are applied across the assessment; these are considered conservative estimates based on data from a substance of much higher lipophilicity (Solvent Red 23) and data from the BONA pigment lakes analogue, PR57*, respectively. These dermal absorption values account for the lack of microbial activity of the normal flora of the skin in in vitro dermal absorption studies (Platzek 1999; Stingley et al. 2010) while observing azo bond cleavage in an in vitro incubation of PR63:1 with the dermal Staphylococcus and Micrococcus sp. bacteria (BRI 2012).
For naphthol AS pigments, due to the limited available toxicity data, the structural variability within the subset adds uncertainty regarding read-across within this subset for potential health effects. For example, since the potential toxicity of azo substances may be attributed in part to their respective aromatic amine metabolites, differences in substitutions on the aromatic amine moiety of the naphthol AS pigments may affect their metabolism and hence the relative toxicity of these substances.
For BONA pigment lakes, the two subchronic toxicity studies reported for PR63:1 and PR57:1* are of lower confidence due to the limited details provided and the reported effects at much lower levels in higher-quality short-term toxicity studies for PR48:2 and PR57:1*.
For the naphthol AS pigment subset, due to variability in substitutions of the aniline ring of the naphthol AS coupling component, there is much uncertainty regarding the bioavailability and toxicity and their theoretical azo bond cleavage metabolites of pigments in this subset. Uncertainty is recognized for health effects read-across from PR22* and PR23* to the other substances lacking nitroaniline moieties. There is also high uncertainty regarding the potential chronic effects of the naphthol AS pigments. The only chronic study was for the analogue PR23*, which showed low carcinogenic potency at doses as low as 2 500 mg/kg-bw per day in rats. A conservative assumption is applied that the naphthol AS pigments may exhibit chronic toxicity qualitatively similar to that for the β-naphthol pigments (PR3, PR4, PO5), but the overall lower relative potency from short-term toxicity data on the naphthol AS pigments is supported by limited evidence for lower relative bioavailability and azo bond cleavage potential for the naphthol AS pigments.
For PO36, the assumption of similar qualitative toxicity between PO36 and the β-naphthol pigments (PR3, PR4, PO5) is based on the assumption that these substances could generate similar nitroaniline derivatives. Unlike the monoazo yellow pigments, however, which also could generate nitroaniline derivatives, there are no toxicity studies on PO36 to make the assumption of overall lower potency of PO36. High uncertainty is recognized in assuming that the lower relative octanol solubility would result in lower relative bioavailability of PO36 and basing data read-across solely on this assumption.
7.3 Risk Characterization
The focus of the human health risk characterization was on those Monoazo Pigments to which exposure of the general population of Canada may be expected.
For 11 of the monoazo pigments considered in this assessment (PR6, PO2, PR50:1, PR48:5, NAPNPA, NANPAP, NAPMPA, NAPPA, PR251, PY60 and NSNAC) whose presence in the Canadian marketplace has not been reported, exposure of the general population of Canada is not expected and therefore these substances are not considered in the risk characterization sections below. While limited use in Canada has been identified for PR247:1 and PR268 (see Section 4. Sources and Uses), exposure for the general population to these substances is not expected to be significant and therefore these substances are also not included in the sections below.
Conversely, for 19 of the monoazo pigments in this assessment whose presence in certain products available in Canadian commerce is indicated (PR49:1, PY1, PR170, PR53:1, PR63:1, PY3, PR112, PO36, PR52:2, PY73, PR266, NONPA, PR48:2, PR52:1, PR4, PO5, PO38, PR187 and PR5.), exposures to these substances have been estimated for the general population of Canada for relevant exposure routes and durations. Exposure to these substances through environmental media is not expected to be a significant source of exposure, therefore risk for the general population of Canada from environmental media is expected to be low. Risk from exposure to specific products for the various Monoazo Pigment subsets is characterized for each subset.
7.3.1 β-Naphthol Pigments
Exposure to β-naphthol pigments in various products available to consumers, including finger paint, face paint, and lipstick was estimated. Estimates of risk associated with exposure scenarios for PR4 and PO5 are presented in Tables 7-16 and 7-17, respectively.
The risk for per event oral exposure to PR4 and PO5 from hand-to-mouth activity during finger painting by children was estimated by comparing estimates of exposure to effect levels. Given the intermittent nature of the finger paint scenario, it was considered that continuous daily exposure was unlikely and therefore margins of exposure may be more appropriate for acute versus short-term effect levels, however both risk estimates are presented in Tables 7-16 and 7-17. For PR4, read-across to health effects to other β-naphthol pigments PO5 and PR3 was used in the absence of short-term or acute toxicity data for PR4 (Table 7-16). Based on short-term effect levels, margins of exposure (MOEs) for PR4 range from 220 – 1200 (Table 7-16) and the MOE was 670 for PO5 (Table 7-17). However, based on comparison to an acute LOAEL of 100mg/kg-bw per day (clinical signs from single dose oral study in rats, BG Chemie 2000a) the MOEs for were much higher and range from 4780 to 14330 for PR4 and PO5 respectively. Taking into consideration the intermittent nature of the exposures for oral exposure to finger paint in children, and that continuous daily exposure would be unlikely to occur, the MOEs for the acute effect level may be more appropriate. Therefore, the range of MOEs presented above including those for the acute LOAEL are considered adequate to address uncertainties in the exposure and health effects databases for this exposure scenario.
The risk from per event dermal exposure to PR4 from finger painting in children was estimated by comparing estimates of exposure with effect levels for PR3 and PO5 from short-term and acute oral studies (described in paragraph above, Table 7-16). Based on the short-term oral effect levels, dermal MOEs for PR4 range from 780 – 4200. In contrast, based on an acute oral LOAEL, the dermal MOE for PR4 was 16670. For per event dermal exposure to PO5 from finger painting in children (Table 7-17), the MOEs based on short-term LOAEL and acute LOAEL were 2380 and 51190 respectively. Considering the intermittent nature of the finger paint exposure scenario, and that per event dermal MOEs based on acute effect levels may be more appropriate, and that the dermal exposure is being compared to effect levels from oral studies, the MOEs are considered adequate to address uncertainties in the exposure and health effects databases for this exposure scenario.
| Exposure duration and route | Product (age group) | Exposure estimates (mg/kg-bw per day) |
Critical effect levels (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|
| Per event oral | Finger paint (child) | 0.45 | Short-term (14–32 days) acute (single dose) LOAEL = 2150Footnote Table 7-16 [b] |
220 - 1200 4780 |
| Per event dermal | Finger paint (child) | 0.129 | Short-term (14–32 days) acute (single dose) LOAEL = 2150[b] |
780 – 4200 16670 |
| Daily oral | Lipstick (adult) |
0.0102 | Chronic non-cancer NOAEL =12.5 |
1230 |
| Daily oral | Lipstick (adult) |
0.0102 | Liver tumour (range of points of departure) 500 – 911Footnote Table 7-16 [d] |
49000–89300 |
| Daily oral | natural health products (child) | 0.032 – 0.065 | Chronic non-cancer NOAEL =12.5 |
190 – 390 |
| Daily oral | natural health products (child) | 0.032 – 0.065 | Liver tumour (range of points of departure) 500 – 911[d] |
7700 – 28500 |
| Daily oral | natural health products (adolescent) | 0.017 | Chronic non-cancer NOAEL =12.5 |
740 |
| Daily oral | natural health products (adolescent) | 0.017 | Liver tumour (range of points of departure) 500 – 911[d] |
29400 – 53600 |
| Daily oral | natural health products (adult) | 0.014 | Chronic non-cancer NOAEL =12.5 |
890 |
| Daily oral | natural health products (adult) | 0.014 | Liver tumour (range of points of departure) 500 – 911[d] |
35700 – 65100 |
| Daily dermal | Face mask (adult) | 0.0049Footnote Table 7-16 [c] | Chronic non-cancer NOAEL =12.5 |
25500 |
| Daily dermal | Face mask (adult) | 0.0049[c] | Liver tumour (range of points of departure) 500 – 911[d] |
102000–186000 |
| Exposure duration and route | Exposure estimates (mg/kg-bw per day) |
Critical effect levels (mg/kg-bw per day) |
MOEs |
|---|---|---|---|
| Per event oral | 0.15 | Short-term (32 days) acute (single dose) LOAEL = 2150 |
670 14330 |
| Per event dermal | 0.042Footnote Table 7-17 [a] | Short-term (32 days) acute (single dose) LOAEL = 2150 |
2380 51190 |
The risk from daily oral exposure to PR4 in lipstick was estimated by comparing estimates of exposure with chronic effect levels (Table 7-16). For carcinogenicity, read-across cancer points of departure from other β-naphthol pigments PO5 and PR3 were used in the absence of adequate data for PR4. For non-cancer effects of PR4 (chronic NOAEL=12.5 mg/kg-bw per day), the MOE was 1230. For carcinogenicity, the MOEs range from 49000 – 89300 based on points of departure for liver tumours observed in female rats for PO5 (tumourigenic dose = 500 mg/kg-bw per day, 26-30% incidence above control) and PR3 (BMDL10 = 911 mg/kg-bw per day). Given that the exposure estimate was based on the upper end of PR4 concentration reported for lipstick products currently used in Canada (3%), and that the majority of lipsticks in the Canadian market containing PR4 would be at concentrations less than 3%, these MOEs are considered adequate to address uncertainties in the health effects and exposure databases for this exposure scenario.
The risk from daily oral exposure to PR4 in natural health products was derived by comparing estimates of exposure based on the permitted maximum limit of 1.0 mg/day (NHPID 2011) with the chronic effect levels (Table 7-16). For non-cancer effects of PR4 (chronic NOAEL= 12.5 mg/kg-bw per day), the MOEs range from 190 - 890 dependant on the age group and are considered adequate to address uncertainties in the health effects and exposure databases for this exposure scenario. For carcinogenicity, read-across points of departure from the other β-naphthol pigments PO5 and PR3 were used in the absence of adequate data for PR4 (liver tumours in female rats; PO5, tumourigenic dose = 500 mg/kg-bw per day, 26-30% incidence above control; PR3, BMDL10 = 911 mg/kg-bw per day). The MOEs for carcinogenicity range from 7700 – 65100, dependent on the age group and the cancer point of departure. While the draft assessment presented the lower end of the range of MOEs as potentially inadequate, the lower end of MOEs is based on application of several conservative assumptions (e.g. assumption of continuous exposure to PR4 in natural health products over a lifetime, PR4 being present at the permitted maximum limit of 1.0 mg/day , and equal carcinogenic potency to the more potent analogue PO5). In addition, averaging the age-group specific exposures over a lifetime would result in an overall lower lifetime average daily dose and a range of MOEs that are correspondingly higher (Health Canada 2013; US EPA 2005). Further, the intended sub-population for the NHP use in children (i.e. multivitaminFootnote[19]) is for ages 4-8yrs, therefore the body weight of 15.5kg used to represent 4yrs age (see Table 7-2) and corresponding lower range of MOEs for children are considered to be conservative. Therefore, in light of the conservative assumptions above, the margins between the estimated exposure to PR4 from use in oral natural health products and the critical effect levels from animal studies were considered adequate to address uncertainties in the exposure and health effects databases.
For risk from daily dermal exposure to PR4 in face masks, a comparison of estimates for dermal exposure with a chronic non-cancer NOAEL of 12.5 mg/kg-bw per day results in an MOE of 2550. For carcinogenicity, the MOEs range from 102000 - 186000 based on points of departure for liver tumours observed in female rats for PO5 (tumourigenic dose = 500 mg/kg-bw per day, 26-30% incidence above control) and PR3 (BMDL10 = 911 mg/kg-bw per day). These MOEs are considered adequate to address uncertainties in the health effects and exposure databases for this exposure scenario.
Overall, the derived MOEs for the exposure scenarios in Tables 7-16 and 7-17 are considered adequate to address uncertainties in the health effects and exposure databases.
The exposure scenarios characterized in Tables 7-16 and Table 7-17 above are considered to represent sentinel product exposures to these substances, and therefore the exposure and risk from the other potential product uses identified for these substances (see 4.2 Uses) are expected to be lower than presented here.
Based on read-across from PR3, PR4 and PO5, the other substances in the β-naphthol pigments subset (PR6, PO2 and NONPA) are also expected to have similar human health effects including potential carcinogenicity.
7.3.2 β-Naphthol Pigment Lakes (PR49:1, PR53:1)
Exposure to β-naphthol pigment lakes was characterized from use of various products available to consumers including finger paints, face paints and lipstick, that are expected to result in highest exposure among the products identified. Comparison of exposure estimates from use of each product identified with an appropriate critical health effect level results in MOEs that are considered adequate to address uncertainties in the health effects and exposure databases (refer to Table 7-18).
The critical effect level for derivation of the short-term oral and dermal MOEs was based on gross pathological changes in the spleen, liver and kidney of rats from a 14-day oral study on PR53:1 (NTP 1982) with effects observed at the lowest dose tested (LOAEL of 300 mg/kg-bw per day). Comparison of the short-term oral LOAEL with per event oral exposure to PR49:1 from use in finger paints (child) results in an MOE of 580. Comparison with per event dermal exposure estimates from use of PR49:1 and PR53:1 in face paints results in an MOE of 2200.
The critical effect levels for chronic non-cancer (LOAEL = 50 mg/kg-bw per day) and carcinogenic effects (liver tumour BMDL10 = 44 mg/kg-bw per day) for derivation of MOEs were based on a chronic feeding study of PR53:1 in the rat (NTP 1982). The daily oral exposure of PR53:1 through use of lipstick resulted in MOEs of greater than or equal to 44 000.
Overall, the derived MOEs for the product scenarios in Table 7-18 are considered adequate to address uncertainties in the health effects and exposure databases. The exposure scenarios characterized here are considered to represent sentinel cproducts for these substances, and therefore the exposure and risk from the other potential product uses identified for these substances (see 4.2 Uses) are expected to be lower than presented here.
| Substance | Exposure duration and route | Product (age group) | Exposure estimates (mg/kg-bw per event/ per day) | Critical effect levels (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|---|
| PR49:1 | Per event oral | Finger paint (child) | 0.52 | Short-term LOAEL (14 days) = 300 | 580 |
| PR49:1 PR53:1 |
Per event dermalFootnote Table 7-18 [a] | Face paint (child) | 0.135 | Short-term LOAEL (14 days) = 300 | 2220 |
| PR53:1 | Daily oral | Lipstick/lip balm (adult) | 0.001 | Chronic non-cancer LOAEL = 50 | 50 000 |
| PR53:1 | Daily oral | Lipstick/lip balm (adult) | 0.001 | Liver tumours BMDL10 = 44 |
44 000 |
7.3.3 BONA Pigment Lakes (PR48:2 and PR63:1)
Exposure to BONA pigment lakes was estimated from use of various products available to consumers including finger paint and face paint (Table 7-19). For risk from per event exposure, a short-term LOAEL of 200 mg/kg-bw per day was considered to represent the critical effect level for both PR48:2 and PR63:1 based on a repeated-dose study on PR48:2 (42-day gavage, kidney tubule histopathological changes). Comparison of per event oral exposure to finger paints and per event dermal exposure from finger paints and/or face paints resulted in oral MOEs ranging from 250 to 1100. There is indication of reversibility of the kidney toxicity from the critical short-term animal study on PR48:2 and for another related BONA pigment lake (PR57:1*). In addition, the study duration of 42 days is much longer than the expected duration of exposure to both finger paint (2–3 times per week) and face paint (6 times per year). As a result, the range of oral MOEs is considered to be conservative. For per event dermal exposure to finger paints and face paints resulted in dermal MOEs ranging from 1480 to 22200.
Overall, the derived MOEs for the product scenarios in Table 7-19 are considered adequate to address uncertainties in the health effects and exposure databases. The exposure scenarios characterized here are considered to represent sentinel products for these substances, and therefore the exposure and risk from the other potential product uses identified for these substances (see 4.2 Uses) are expected to be lower than presented here.
| Substance | Exposure duration and route | Product (age group) | Exposure estimates (mg/kg-bw per event) | Critical effect levels (mg/kg-bw per day)d | MOEs |
|---|---|---|---|---|---|
| PR48:2 PR63:1 |
Per event oral | Finger paint (child) | 0.18–0.77 | Short-term (42 days) LOAEL = 200 |
250–1110 |
| PR48:2 PR63:1 |
Per event dermal | Finger paint (child) | 0.009–0.037Footnote Table 7-19 [a] | Short-term (42 days) LOAEL = 200 |
5400 – 22220 |
| PR63:1 | Per event dermal | Face paint (child) | 0.135[a] | Short-term (42 days) LOAEL = 200 |
1480 |
7.3.4 Monoazo Yellow Pigments (PY1, PY3, PY73)
Exposure to monoazo yellow pigments was estimated in various products available to consumers including finger paints and face paints (Table 7-20). Daily exposure from use of body cream, lotion and moisturizer containing PY1 has also been estimated (see Appendix F, Table F-1), however, no chronic toxicity studies were identified for any of the substances from the monoazo yellow pigments subset. Despite the lack of clear toxicological effects for the monoazo yellow pigments from shorter-term studies, the potency of the monoazo yellow pigments is considered lower than that of the β-naphthol pigments based on the comparison of short-term studies. Since the available data for β-naphthol pigments do not indicate health concerns from chronic dermal exposure from cosmetics (Table 7-16), the potential risk for the general population of Canada from exposure to PY1 from use of body cream, lotion and moisturizer is not expected to be high.
Overall, the derived MOEs for the product scenarios in Table 7-20 are considered adequate to address uncertainties in the health effects and exposure databases. The exposure scenarios characterized here are considered to represent sentinel products for these substances, and therefore the exposure and risk from the other potential product uses identified for these substances (see 4.2 Uses) are expected to be lower than presented here.
| Substance | Exposure duration and route | Product (age group) | Exposure estimates (mg/kg-bw per event) | Critical effect levels (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|---|
| PY1 PY3 PY73 |
Per event oral | Finger paint (child) | 0.206–0.335 | Short-term (28 days) NOAEL = 1250 |
2990–4850 3730–6070 |
| PY1 PY3 PY73 |
Per event dermal | Face paint (child) | 0.016–0.813Footnote Table 7-20 [a] | Short-term (28 days) NOAEL = 1250 |
1230–61500 1540–76880 |
7.3.5 Naphthol AS Pigments
Exposure to naphthol AS pigments was estimated in various products available to consumers including finger paints and face paints (Table 7-21). Daily dermal and oral exposure from cosmetics containing PR112 has also been estimated (see Appendix F, Tables F-1 and F-3), however, no chronic toxicity studies were identified for any of the substances from the naphthol AS pigments subset. Despite the lack of clear toxicological effects for the naphthol AS pigments from shorter-term studies, the potency of the naphthol AS pigments is considered lower than that of the β-naphthol pigments based on the comparison of short-term studies. Since the available data for β-naphthol pigments do not indicate health concerns from chronic dermal or oral exposure from cosmetics (Table 7-16), the potential risk for the general population of Canada from exposure to PR112 from use in cosmetics is not expected to be high.
Overall, the derived MOEs for the product scenarios in Table 7-21 are considered adequate to address uncertainties in the health effects and exposure databases. The exposure scenarios characterized here are considered to represent sentinel products for these substances, and therefore the exposure and risk from the other potential product uses identified for these substances (see 4.2 Uses) are expected to be lower than presented here.
| Substance | Exposure duration and route | Product (age group) | Exposure estimates (mg/kg-bw per day) |
Critical effect levels (mg/kg-bw per day)d | MOEs |
|---|---|---|---|---|---|
| PR5 PR112 PR170 PO38 |
Short-term oral | Finger paint (child) | 0.284–1.290 | Short-term (28 days) NOAEL = 1000 - 1172 |
780–4130 |
| PR112 | Short-term dermal | Face paint (child) | 0.813Footnote Table 7-21 [a] | Short-term (28 days) NOAEL = 1000 |
1230–1440 |
7.3.6 PO36 (Benzimidazolone Pigment)
Exposure to PO36 from use in paints and coatings including arts and crafts materials is expected (Appendix G, Table G-4). However, no exposure or estimates for these specific uses were determined as there were no relevant health effects data identified for this substance. However, while no specific MOEs were derived for PO36, the estimated risks from products are expected to be similar to or lower than those already presented in previous sections for other Monoazo Pigment subsets.
7.3.7 Exposures to Monoazo Pigments Not Otherwise Characterized
Potential dermal and oral exposure from use of 19 of the monoazo pigments in paints and coatings such as uses in arts and crafts materials (Appendix G, Table G-4) are expected to be covered off by the risk characterization for the sentinel exposures from finger paints and face paints. The risk from exposure to finger paints and face paints do not indicate a high concern at current levels of exposure,therefore, the expected risk from the dermal and oral exposure tothe monoazo pigments from other paints and coatings uses are also considered to not indicate a high concern. For inhalation exposure from paints and coatings uses such as spray paint and sanding paint, exposure estimates for these uses (Appenxic G, Table G-5), indicate low exposure (0.000367 – 0.00055 mg/kg-bw per event). As these exposure estimates are well below the per event exposures for finger paint and face paint, the risk from these inhalation exposures are considered to be low.
7.3.8 Uncertainties with Evaluation of Risk to Human Health
The lack of or limited health effects data for all relevant routes and durations of exposure are an uncertainty. Accordingly, route-to-route and duration-to-duration extrapolation was required in some cases introducing uncertainty to the derived risk estimates. For example, points of departure for health effects by the oral route were used to derive MOEs for dermal exposures (e.g. face paint, body cream, etc.), however since the dermal absorption factors applied to the exposure estimate were considered conservative, the resulting MOEs are also considered conservative. Also, the exposure duration and frequencies for several product scenarios such as finger paints (2-3 times per week) and face paints (6 times per year) did not specifically match those of the toxicology point of departure which often were based on animal studies of longer duration ( greater than or equal to 14 days) and continuous exposure. Therefore, the calculated MOEs for exposure scenarios involving duration-to-duration extrapolations are also considered conservative.
Based on available information, some of these monoazo pigments are listed as ingredients in tattoo inks available to consumers in Canada. However, the current knowledge on chronic systemic exposure from injected tattoo colourants is considered insufficient for a valid quantitative exposure assessment (Danish EPA 2012). While the health effects for several of these monoazo pigments are recognized, including potential carcinogenicity (e.g. PR3, PR4, PO5 and other β-naphthol pigments; PR53:1), due to the high uncertainty with respect to the exposure characterization from tattoo use, the risk from tattoo use has not been characterized in this screening assessment and remains an uncertainty.
While the exposure to PR4 from use in oral natural health products does not indicate a high concern for risk to human health, the health effects of PR4 including potential carcinogenicity are still recognized. Further information regarding the actual concentration or quantity of PR4 in NHPs, the number of Canadians consuming oral natural health products containing PR4 at maximum permitted levels, the extent to which the directions of use for the products are followed, and the exposure levels of PR4 from other sources in these individuals would aid in reducing the uncertainty in the exposure characterization for this subpopulation.
7.3.9 Monoazo Pigments With Effects of Concern
Overall, human health risk from the substances in this assessment is low based on the current levels of exposure. However, some of the 32 monoazo pigments evaluated in this assessment for human health have effects of concern based on potential carcinogenicity. A list of these substances are shown in Appendix J.
8. Conclusion
Considering all available lines of evidence presented in this Screening Assessment, there is low risk of harm to organisms and the broader integrity of the environment from the 33 monoazo pigments evaluated in this assessment. It is concluded that these monoazo pigments do not meet criteria under paragraph 64(a) or 64(b) of CEPA 1999, as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based upon comparison between the estimates of short-term and chronic exposures from specifically identified products and critical effect levels from animal studies, it is concluded that the following 19 Monoazo Pigments are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health: PR4, PO5, PR49:1, PR53:1, PR48:2, PR63:1, PR52:1, PR52:2, PY1, PY3, PY73, PR5, NONPA, PR187, PR112, PR170, PR266, PO38 and PO36. For the remaining 13 substances (PR6, PO2, PR50:1, PR48:5, NAPNPA, NANPAP, NAPMPA, NAPPA, PR251, PR268, PR 247:1, PY60 and NSNAC), available information did not identify sources of current exposure of the general population of Canada and therefore the risk to human health for these 13 monoazo pigments is considered to be low. Based on the information presented in this Screening Assessment, it is concluded that the above 32 Monoazo Pigments evaluated in this assessment for human health do not meet the criteria under paragraph 64(c) of CEPA 1999 as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health. In addition, there are no updates to the conclusion made with respect to paragraph 64(c) for Pigment Red 3, previously assessed by the Government of Canada under the Challenge Initiative of the CMP.
It is concluded that the 32 monoazo pigments evaluated in this assessment do not meet any of the criteria set out in section 64 of CEPA 1999.
The conclusion previously made under the Challenge Initiative that Pigment Red 3 meets the criteria set out in paragraph 64(c) of CEPA 1999 remains unchanged.
References
3M. 2007. Material Safety Data Sheet: 3M Screen Printing Ink 1918 Yellow Shade Red [Internet]. St. Paul (MN): 3M. [cited 2013 Apr 18]. Available from: http://solutions.3mcanada.ca/wps/portal/3M/en_CA/WW2/Country
Anliker R, Moser P. 1987. The limits of bioaccumulation of organic pigments in fish: their relation to the partition coefficient and the solubility in water and octanol. Ecotoxicol Environ Saf 13:43–52.
Anliker R, Moser P, Poppinger D. 1988. Bioaccumulation of dyestuffs and organic pigments in fish. Relationships to hydrophobicity and steric factors. Chemosphere 17:1631–1644.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2010. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/opptintr/exposure/pubs/episuite.htm
Arnot JA, Arnot MI, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P. 2010. Molecular size cutoff criteria for screening bioaccumulation potential: fact or fiction? Integr Environ Assess Manag 6(2):210–224.
Assmann N, Emmrich M, Kampf G, Kaiser M. 1997. Genotoxic activity of important nitrobenzenes and nitroanilines in the Ames test and their structure-activity relationship. Mutat Res. 395(2-3):139-44.
ASTreat [sewage treatment plant removal model]. 2006. Version 1.0. Cincinnati (OH): Procter & Gamble Company, P.O. Box 538707, Cincinnati, OH 45253-8707, US.
Baranski B, Przybojewska B, Spiechowicz E, Wyszynska K, Zimnicki J. 1992. Identification of potential carcinogenic dyes and intermediates on the basis of their genotoxicity (in Polish). Med Pr 43:469-477.
BASF. 2008. Pigmosol Brochure. Available at: http://www.basf.com.mx/specialty_colorants/pdfs/pigmosol_brochure.pdf
Baughman GL, Perenich TA. 1988. Investigating the fate of dyes in the environment. Am Dyest Rep 7(2):19–20, 22, 47–48. [cited in Øllgaard et al. 1998].
Bäumler W, Vasold R, König B, Landthaler M. 2004. Q-switched laser and tattoo pigments, a chemical analysis of laser induced decomposition compounds. Toxicologist 78(Suppl 1):1079.
Bäumler W, Eibler ET, Hohenleutner U, Sens B, Sauer J, Landthaler M. 2000. Q-Switch laser and tattoo pigments: first results of the chemical and photophysical analysis of 41 compounds. Laser Surg Med 26:13–21.
[BG Chemie] Berufsgenossenschaft der chemischen Industrie. 2000a. Toxicological Evaluation No.223: 1-1(2,4-Dinitro-phenylazo)-2-naphthol. CAS No. 3468-63-1. Heidelberg, Germany. Available at: http://www.bgrci.de/fileadmin/BGRCI/Downloads/DL_Praevention/Fachwissen/Gefahrstoffe/TOXIKOLOGISCHE_BEWERTUNGEN/Bewertungen/ToxBew223-E.pdf
[BG Chemie] Berufsgenossenschaft der chemischen Industrie. 2000b. Toxicological Evaluation No.118:2-Nitro-4-methylaniline. CAS No.89-62-3. Heidelberg, Germany. Available at: https://www.bgrci.de/fileadmin/BGRCI/Downloads/DL_Praevention/Fachwissen/Gefahrstoffe/TOXIKOLOGISCHE_BEWERTUNGEN/Bewertungen/ToxBew118-E.pdf
[BIBRA] British Industrial Biological Research Association. 1989. Toxicity profile: D&C Red 9. Surrey (GB): BIBRA Information Services Ltd. Available from: www.bibra-information.co.uk/profile-258.html
[BIBRA] British Industrial Biological Research Association. 1993a. Toxicity profile: Lithol rubine BK. Surrey (GB): BIBRA Information Services Ltd. Available from: www.bibra-information.co.uk/profile-213.html
[BIBRA] British Industrial Biological Research Association. 1993b. Toxicity profile: Lithol rubine B Surrey (GB): BIBRA Information Services Ltd. Available from: www.bibra-information.co.uk/profile-388.html?
[BIBRA] British Industrial Biological Research Association. 1997a. Toxicity profile: Pigment Red 48, 48:1, 48:2, 48:3, 48:4 Surrey (GB): BIBRA Information Services Ltd. Available from: www.bibra-information.co.uk/profile-144.html
[BIBRA] British Industrial Biological Research Association. 1997b. Toxicity profile: Pigment Red 112. Surrey (GB): BIBRA Information Services Ltd. Available from: www.bibra-information.co.uk/profile-448.html
Biodynamics 1982. A long-term and toxicity/carcinogenicity study of 1% D&C Orange No.17 in rats. CTFA Color Additive Master File No.9, Entry 512. [cited in BG Chemie 2000a].
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolation in biodegradability assessment. Chemosphere 30:741–752.
[BRI] Biopharmaceutical Research Inc. 2012. Testing the metabolism potential of selected aromatic azo and benzidine dyes in vitro in two representative human skin bacterial cultures under aerobic conditions. Unpublished report prepared for Health Canada. Vancouver (BC): BRI. Study No. HEA-2011-002.
[BRI] Biopharmaceutical Research Inc. 2013. Determination of the metabolic reduction potential of selected aromatic azo- and benzidine-based substances: in vitro anaerobic fecal bacterial cultures of the human gastrointestinal tract. Unpublished report prepared for Health Canada. Vancouver (BC): BRI. Study Nos. HEA-2012-001 and HEA-2012-002.
Brown JP, Dietrich PS, Bakner CM. 1979 Mutagenicity testing of some drug and cosmetic dye lakes with the Salmonella/mammalian microsome assay. Mutat Res 66(2):181–186.
[BS] British Standards. 2002. BS EN 71-7:2002: Safety of toys. Part 7: Finger paints--requirements and test methods. London (GB): British Standards Institution. Available from: www.ee-techs.com/test/en71-7.pdf
[BUA] GdCh-Advisory Committee on Existing Chemicals. 1993. Pigment Red 53:1. BUA Report 124 (August 1993). Available at: http://www.hirzel.de/bua-report/
[BUA] GdCh-Advisory Committee on Existing Chemicals. 2001. 2,4,5-Trichloroaniline. BUA Report 227 (June 2001). Available at: http://www.hirzel.de/bua-report/
[BUA] GdCh-Advisory Committee on Existing Chemicals. 2004. 4-Chloro-2-nitroaniline. BUA Report 235 (February 2002). Available at: http://www.hirzel.de/bua-report/
Cadby PA, Troy WR, Vey MGH. 2002. Consumer exposure to fragrance ingredients: providing estimates for safety evaluation. Regul Toxicol Pharmacol 36:246–252.
CAM Supply Inc. 2005. Material Safety Data Sheet: Colour Dispersion 13--Deep Turquoise. Hemet (CA): CAM Supply Inc. [cited 2013 Jan 21. Available from: www.camtattoo.com/msds/Deep_turquoise.pdf
Canada. [1978]. Food and Drug Regulations, C.R.C., c. 870. Available from: www.canlii.org/en/ca/laws/regu/crc-c-870/latest/crc-c-870.html
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. Canada Gazette, Part III, vol. 22, no. 3. Available from: http://publications.gc.ca/gazette/archives/p3/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 29 March 2000, SOR/2000-107. Available from: http://publications.gc.ca/gazette/archives/p2/2000/2000-03-29/pdf/g2-13407.pdf
Canada, Dept. of the Environment. 2006. Canadian Environmental Protection Act, 1999 : Notice with respect to selected substances identified as priority for action. Canada Gazette, Part I, vol. 140, no. 9, p. 435–459. Available from: http://publications.gc.ca/gazette/archives/p1/2006/2006-03-04/pdf/g1-14009.pdf
Canada, Dept. of the Environment. 2007a. Canadian Environmental Protection Act, 1999: Notice of first release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, vol 141, no. 5. p. 164 - 165. Available from: http://publications.gc.ca/gazette/archives/p1/2007/2007-02-03/pdf/g1-14105.pdf
Canada, Dept. of the Environment. 2007b. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 3 Challenge substances. Canada Gazette, Part I, vol 141, no. 33. p. 2378. Available from: http://publications.gc.ca/gazette/archives/p1/2007/2007-08-18/pdf/g1-14133.pdf
Canada, Dept. of the Environment, Dept. of Health. 2007c. Proposed regulatory framework for nanomaterials under the Canadian Environmental Protection Act, 1999. Available from: www.ec.gc.ca/subsnouvelles-newsubs/default.asp?lang=En&n=FD117B60-1
Canada. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Canada Gazette, Part I, vol. 143, no. 40. Available from: www.gazette.gc.ca/rp-pr/p1/2009/2009-10-03/html/notice-avis-eng.html#d101
Canada, Dept. of the Environment. 2011. Canadian Environmental Protection Act, 1999: Notice with respect to certain aromatic amines and aromatic azo- and benzidine-based substances. Canada Gazette, Part I, vol. 145, no. 51, supplement. Available from: www.gazette.gc.ca/rp-pr/p1/2011/2011-12-17/pdf/g1-14551.pdf
Carson, S. 1984a. Skin Painting Studies in Mice with 14 Fd&c and D&c Colors: Fd&c Blue no. 1, Red No. 3, and Yellow No. 5, D&C Red No. 7, Red No. 9, Red No. 10, Red No. 19, Red No. 21, Red No. 27, Red No. 31, Red No. 36, Orange No. 5, Orange No. 10, and Orange No. 17. J. Toxico1.-Cut. & Ocular Toxicol. 3(4), 357-370.
Carson, S. 1984b. Skin Painting Studies in Mice on 11 Fd&C and D&C Colors: Fd&C Green no. 3, Red No. 2, Red No. 4, Yellow No. 6, and External D&C No. 7, D&C Orange No. 4, Violet No. 2, Red No. 17, Red No. 34, and Yellow No. 7. J. Toxicol.-Cut. & Ocular Toxicol. 3(3), 309-331.
[CCME] Canadian Council of Ministers of the Environment. 2013. Canadian Soil Quality Guidelines for Barium: Protection of Human Health Scientific Criteria Document. ISBN 978-1-896997- 97-1 PDF. Available at: http://www.ccme.ca/assets/pdf/pn_1493_basqg_scd_prob_1.0.pdf
[CCRIS] Chemical Carcinogenesis Research Information System [database on the Internet]. 2012. Data on: 4-Chloro-6-amino-m-toluenesulfonic acid (88-51-7), p-toliudine-m-sulfonic acid (88-44-8). Bethesda (MD): National Library of Medicine (US). [cited 2012. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?CCRIS
Chadwick M, Hayes D, Mazrimas MJ. 1984. Absorption, distribution, metabolism and excretion of Red No. 9. Report from Arthur D. Little to the Cosmetic, Toiletry and Fragrance Association [cited in Hart et al. 1986].
Chithrani BD, Ghazani AA, Chan WCW. 2006. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6:662–668.
Chopade HM, Matthews HB. 1983. Disposition and metabolism of 4-chloro-2-nitroaniline in the male F344 rat. J Toxicol Environ Health. 12(2-3):267-82..
Chopade HM, Matthews HB. 1984. Disposition and metabolism of p-nitroaniline in the male F-344 rat. Fundam Appl Toxicol. 4(3 Pt 1):485-93.
[CHRIP] Chemical Risk Information Platform [database on the Internet]. ©2008a. Combined repeated dose and reproductive/developmental toxicity screening test of 4-chloro-2-nitroaniline by oral administration in rats (OECD TG422). [cited January 2013]. Available from: http://www.safe.nite.go.jp/japan/sougou/data/pdf/meti/sheet/05sheet_89634_422e.pdf
[CHRIP] Chemical Risk Information Platform [database on the Internet]. ©2008b. Combined repeated dose and reproductive/developmental toxicity screening test of Pigment Red 48:2 by oral administration in rats (OECD TG422). [cited January 2013]. Available from: www.safe.nite.go.jp/japan/sougou/view/ComprehensiveInfoDisplay_jp.faces
Chung K-T, Fulk GE, Andrews AW. 1981. Mutagenicity testing of some commonly used dyes. Appl Environ Microbiol 45(4):641–648.
Clariant. 2007. Colorants for the paint industry. [cited 2007 Jun 3]. Muttenz (CH): Clariant International Ltd. Available from: www.pigments.clariant.com/C12576850036A6E9/98D3F2AC436C5075C125786B0026B90B/$FILE/DP8523ED_Feb2011.pdf
Clariant. 2011. Creative colors: solutions to color up your working sphere. Muttenz (CH): Clariant International Ltd. Available from: www.pigments.clariant.com/C12576850036A6E9/1DDFD805A4223FA5C12578DC0027084D/$FILE/DP5029E%207.11.pdf
Clariant 2013. Colorants for coatings are our playground. [cited 2013 Mar 10]. Muttenz (CH): Clariant International Ltd. Available from: www.clariant.com/C125720D002B963C/news/BE00D1FA07C995A5C125742700437CFC/$File/DD1009E_0308_FL_ColorantsforCoatingsareourPlayground.pdf
Combes RD, Haveland-Smith RB. 1982. A review of the genotoxicity of food, drug and cosmetic colours and other azo, triphenylmethane and xanthene dyes. Mutat Res 98(2):101–248.
[ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment). Available from: www.rivm.nl/en/healthanddisease/productsafety/ConsExpo.jsp#tcm:13-42840
[CPMA] Color Pigments Manufacturers Association, Inc. 2003. Comments of the Color Pigments Manufacturers Association, Inc. on the draft guidance manual for the categorization of organic and inorganic substances on Canada’s Domestic Substances List (‘DSL’) and Environment Canada’s computer generated estimates and empirical data on approximately 12,000 discrete organic chemicals on the DSL. Gatineau (QC): Environment Canada, Existing Substances Division. Available upon request.
[CPMA] Color Pigments Manufacturers Association, Inc. 2006a. High Production Volume (HPV) Challenge Program: Test plan for C.I. Pigment Red 49 (barium) (CAS No.:1103-38-4). Alexandria (VA): CPMA, Monoazo and Related Pigments Committee. Available from: www.epa.gov/hpv/pubs/summaries/cired49/c16299.pdf
[CPMA] Color Pigments Manufacturers Association, Inc. 2006b. High Production Volume (HPV) Challenge Program: Test plan for C.I. Pigment Red 48 (calcium) (CAS No.: 7023612) and C.I. Pigment Red 48 (barium) (CAS No.: 758413) and C.I. Pigment Red 52 (calcium) (CAS No.: 17852992). Alexandria (VA): CPMA, Monoazo and Related Pigments Committee. Available from: www.epa.gov/hpv/pubs/summaries/cird4852/c16302tp.pdf
Crechem Technologies. 2003. Release scenarios for Canadian coating facilities. Prepared for Environment Canada, Gatineau, Quebec. Ottawa (ON): Crechem Technologies Inc.
Crechem Technologies. 2005. Fate of wastewater sludge. Prepared for Existing Substances Division, Environment Canada, Gatineau, Quebec. Ottawa (ON): Crechem Technologies Inc.
[CTFA] Cosmetic, Toiletry & Fragrance Association. 1982. Submission by the CTFA: 30-Month Chronic Toxicology and Potential Carcinogenicity Study in Rats with in Utero and Lifetime Exposure to D&C Red No.9 in the Diet. Final Report from Litton Bionetics, July 1982. [cited in Hart et al. 1986; SCC 2000].
[CTFA] Cosmetic, Toiletry & Fragrance Association. 1983a. Final Review and Analysis of Scientific Studies and Risk Assessments Supoorting the Safety of D&C Red No.9. CFTA, Washington DC. [cited in LSRO-FASEB 1984; BIBRA 1989].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1983b. Summary for the Results of Surveys of the amount and Frequency of use of cosmetic products by Women. Report Prepared by Pitkin B, Rodericks JV, Turnbull D. Environ Corporation 1850 K Street, N.W, Washington, DC.
Cui Y, Spann AP, Couch LH, Gopee NV, Evans FE, Churchwell MI, Williams LD, Doerge DR, Howard PC. 2004. Photodecomposition of Pigment Yellow 74, a pigment used in tattoo inks. Photochem Photobiol 80(2):175–184.
[Danish EPA] Danish Environmental Protection Agency. 2012. Chemical substances in tattoo ink. (Survey of Chemical Substances in Consumer Products, No. 116). Copenhagen (DK): Danish Ministry of the Environment, Environmental Protection Agency. Available from: www2.mst.dk/Udgiv/publications/2012/03/978-87-92779-87-8.pdf
Davis KJ, Fitzhugh OG. 1962. Pathologic changes noted in rats fed D&C Red No. 9 for two years. Toxicol Appl Pharmacol 4(2):200–205.
Davis KJ, Fitzhugh OG. 1963. Pathologic changes noted in rats fed D&C Red No. 10 (monosodium salt of 2-(2-hydroxy-1-naphthylazo)-1-naphthalenesulfonic acid) for two years. Toxicol Appl Pharmacol 5:728–734.
De Cuyper C, D’hollander D. 2010. Materials used in body art. In: de Cuyper C, Pérez-Cotapos ML, editors. Dermatologic complications with body art: tattoos, piercings and permanent make-up. Berlin (DE): Springer-Verlag; p. 13–28. Available from: www.springer.com/medicine/dermatology/book/978-3-642-03291-2
Della Porta G, Dragani TA. 1982. Non-carcinogenicity in mice of a sulfonic acid derivative of 2-naphthylamine. Carcinogenesis. 3(6):647-9.
Delta Creative. 2008. Material Safety Data Sheet (MSDS): Finger Paint. City of Industry (CA): Delta Creative, Inc. [cited 2013 May 6]. Available from: www.deltacrafts.com/Uploads/media/documents/MSDS%20OF%20Finger%20paint.pdf
Derivan. 2012. Material Safety Data Sheet (MSDS): Derivan Face and Body Paint. Rhodes (AU): Derivan Pty. Ltd. [cited 2013 May 6]. Available from: www.derivan.com.au/assets/msds/Derivan-Face-and-Body-Paint-MSDS.pdf
DeVito SC. 2000. Absorption through cellular membranes. In: Boethling RS, Mackay D, editors. Handbook of property estimation methods for chemicals: environmental and health sciences. Boca Raton (FL): CRC Press.
Dillon D, Combes R, Zeiger E. 1994. Activation by caecal reduction of the azo dye D and C Red No. 9 to a bacterial mutagen. Mutagenesis 9(4):295–299.
Dimitrov S, Dimitrova N, Walker J, Veith G, Mekenyan O. 2002. Predicting bioconcentration potential of highly hydrophobic chemicals. Effect of molecular size. Pure Appl Chem 74(10):1823–1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
Doll P, Shi F, Kelly S, Wnek W. 1998. The problem of catalytic fading with ink-jet inks. NIP14: International Conference on Digital Printing Technologies. Toronto, Ontario, Canada; October 1998; p. 118-121. Available at: http://www.americaninkjet.com/images/Problem_of_Catalytic_Fading.pdf
[DPD] Drug Product Database [database on the Internet]. 2010. Ottawa (ON): Health Canada. [cited 2012 ]. Available from: www.hc-sc.gc.ca/dhp-mps/prodpharma/databasdon/index-eng.php
[EA Japan] Environment Agency Japan. 1992. Investigation on the ecotoxicological effects of OECD high production volume chemicals. Tokyo (JP): Environment Agency, Environmental Health Department, Office of Health Studies. [cited in OECD 2009b].
[ECHA] European Chemicals Agency. 2008. Guidance on information requirements and chemical safety assessment. Chapter R.11: PBT assessment. [Guidance for the implementation of REACH]. Helsinki (FI): ECHA; 97 p.
[ECHA] European Chemicals Agency. 2010. Guidance on information requirements and chemical safety assessment. Chapter R.16: Environmental exposure estimation, Version 2. Helsinki (FI): ECHA.
[ECHA] European Chemicals Agency. 2012. Registered substances database. CAS RN: 1103-38-4, 2425-85-6, 2512-29-0, 2786-76-7, 2814-77-9, 3468-63-1, 5160-02-1, 6372-81-2, 6407-74-5, 6410-09-9, 6410-09-9, 6410-13-5, 6410-41-9, 6417-83-0, 6486-23-3, 6535-46-2, 7023-61-2, 12236-62-3, 12236-64-5, 12238-31-2, 13515-40-7, 13824-00-5, 16403-84-2, 17852-99-2, 17947-32-9, 36968-27-1, 43035-18-3, 49744-28-7, 59487-23-9, 71832-83-2, 74336-60-0, 80675-49-6, 83249-60-9, 85005-63-6 and 94199-57-2. Helsinki (FI): ECHA. [updated 2012 Nov 27; cited 2012 Jan–May 2013]. Available from: http://echa.europa.eu/web/guest/information-on-chemicals/registered-substances
[EFSA] European Food Safety Authority, EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). 2010. Scientific Opinion on the re-evaluation of Litholrubine BK (E 180) as a food additive. EFSA J 8(5):1586; 26 p. Available from: www.efsa.europa.eu/en/efsajournal/doc/1586.pdf
EI Dareer SM, Tillery KF, Hill DL. 1984. Investigations on the disposition of oral doses of some water-insoluble pigments. Bull. Envir. Contam. Toxicol. 32:171-174.
Engel E, Spannberger A, Vasold R, König B, Landthaler M, Bäumler W. 2007. Photochemical cleavage of a tattoo pigment by UVB radiation or natural sunlight. J Dtsch Dermatol Ges 5(7):583–589.
Engel E, Santarelli F, Vasold R, Maisch T, Ulrich H, Prantl L, Konig B, Landthaler M, Baumler W. 2008. Modern tattoos cause high concentrations of hazardous pigments in skin. Contact Dermatitis 58:228–233.
Engel E, Vasold R, Santarelli F, Maisch T, Gopee NV, Howard PC, Landthaler M, Baumler W. 2009. Tattooing of skin results in transportation and light-induced decomposition of tattoo pigments--a first quantification in vivo using a mouse model. Exp Dermatol 19:54–60.
Environment Canada. 2000. Environmental categorization for persistence, bioaccumulation and inherent toxicity of substances on the Domestic Substances List using QSARs. Final report. Gatineau (QC): Environment Canada, Chemicals Evaluation Division.
Environment Canada. 2006. Data for selected substances collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to selected substances identified as priority for action. Data prepared by: Environment Canada, Health Canada, Existing Substances Program.
Environment Canada. 2007b. Data for Batch 1 substances collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances identified in the Challenge, published in the December 9, 2006, Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2008. Data for Batch 3 substances collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to Batch 3 Challenge substances. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2009a. Generation and management of effluent treatment and deinking residues from the pulp and paper industry. Prepared for Environmental Steward Branch, Environment Canada, Gatineau, Quebec.
Environment Canada. 2009b. A site visit to a tanktruck cleaning facility on October 15, 2009. [Internal document]. Gatineau (QC): Environment Canada.
Environment Canada. 2010. Site visits to pigment manufacturing facilities. [Presentation for Ecological Assessment Division, Environment Canada, Gatineau, Quebec].
Environment Canada. 2012a. Data for certain aromatic amines and aromatic azo- and benzidine-based substances collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain aromatic amines and aromatic azo- and benzidine-based substances. Data compiled by: Environment Canada, Program Development and Engagement Division.
Environment Canada. 2012b. An analysis of exposure data based on USEPA Premanufacture Notices. [Internal document]. Gatineau (QC): Environment Canada.
Environment Canada. 2013a. Report on a site visit to a latex paint formulation facility, January 15, 2013. Gatineau (QC): Environment Canada.
Environment Canada. 2013b. Report on a site visit to a powder coatings manufacture facility, February 6, 2013. Gatineau (QC): Environment Canada.
Environment Canada. 2013c. Report on a site visit to a water-based paint formulation facility in Montreal, Quebec, January 15, 2013. Gatineau (QC): Environment Canada.
Environment Canada. 2013d. Internal database of Canadian surface water sediments. Environment Canada, Ecological Assessment Division, Gatineau, Quebec.
Environment Canada, Health Canada. 2007. Chemical substances: Categorization [Internet]. Ottawa (ON): Government of Canada. [updated 2007 April 20; cited 2014 June 10]. Available from: http://www.chemicalsubstanceschimiques.gc.ca/approach-approche/categor-eng.php
Environment Canada, Health Canada. 2009a. Screening assessment for the Challenge: 2-Naphthalenol, 1-[(2-nitrophenyl)azo]- (Pigment Orange 2): Chemical Abstracts Service Registry Number 6410-09-9 [Internet]. Ottawa (ON): Environment Canada, Health Canada. Available from: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&xml=84D0C842-1635-E36E-9E56-56338A17EDDC
Environment Canada, Health Canada. 2009b. Screening assessment for the Challenge: 2-Naphthalenol, 1-[(4-methyl-2-nitrophenyl)azo]- (Pigment Red 3): Chemical Abstracts Service Registry Number 2425-85-6 [Internet]. Ottawa (ON): Environment Canada, Health Canada. Available from: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&xml=3D4876AF-C407-BF92-EE26-8000E99F867D
Environment Canada, Health Canada. 2009c. Screening assessment for the Challenge: 2-Naphthalenecarboxamide, N-(5-chloro-2,4-dimethoxyphenyl)-4-[[5-[(diethylamino)sulfonyl]-2- methoxyphenyl]azo]-3-hydroxy- (Pigment Red 5): Chemical Abstract Services Registry Number 6410-41-9 [Internet]. Ottawa (ON): Environment Canada, Health Canada. Available from: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&xml=FE00F8D1-4016-80F1-AF8D-96D3B4312EDC
Environment Canada, Health Canada. 2010. A site visit to a cosmetic manufacture facility, June 1, 2010. [Internal document].
Environment Canada, Health Canada. 2011. Follow-up Report on a PSL Substance for Aniline. Chemical Abstract Registry Number 62-53-3. Health Canada, December 2011. Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=CCDA73CC-1
Environment Canada, Health Canada. 2012. Multi-stakeholder technical consultation: proposed subgrouping of aromatic azo- and benzidine-based substances. Summary report. Ottawa (ON): Environment Canada, Health Canada. Available from: www.ec.gc.ca/ese-ees/default.asp?lang=En&n=01656691-1
Environment Canada, Health Canada 2013a. Supporting document for the draft screening assessment. Aromatic Azo and Benzidine-Based Substance Grouping. Certain Monoazo Pigments. Available upon request.
Environment Canada, Health Canada. 2013b. The Chemicals Management Plan Substance Grouping Initiative. Subgrouping Approach and Background Information for the Screening Assessment of Aromatic Azo and Benzidine-based Substances. May 2013. Environment Canada, Health Canada. Available upon request.
Environment Canada, Health Canada. 2013c. Screening Assessment for the Challenge. Carbon Black. Chemical Abstracts Service Registry Number 1333-86-4. Ottawa (ON): Environment Canada, Health Canada. Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=2cf34283-1#a14
Environment Canada, Health Canada. 2014a. Certain Azo Solvent Dyes. Screening Assessment. Aromatic Azo and Benzidine-based Substance Grouping. Ottawa (ON): Environment Canada, Health Canada.
Environment Canada, Health Canada. 2014b. Certain Aromatic Amines. Draft Screening Assessment. Aromatic Azo and Benzidine-based Substance Grouping. Ottawa (ON): Environment Canada, Health Canada. Available at: http://www.ec.gc.ca/ese-ees/C58F84D0-9924-43C2-A967-E4FC58242C79/DSAR_Groupings_Azo-Pkg5-Aromatic%20Amines_EN.pdf
Environment Canada, Health Canada. 2014c. Certain Diarylide Yellow Pigments. Screening assessment. Aromatic Azo and Benzidine-based Substance Grouping. Ottawa (ON): Environment Canada, Health Canada.
Environment Canada, Health Canada. 2014d. Rapid Screening of Substances from Phase One of the Domestic Substances List Inventory Update. March 2014. Environment Canada, Health Canada. Available from: http://www.ec.gc.ca/ese-ees/7340E1B7-1809-4564-8C49-F05875D511CB/FSAR_RSII_EN.pdf
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1988. Executive Summary on the ETAD Project T 2015 Toxicological Testing of Major Colorants, With Attachments and Cover Letter Dated June 3, 1988; 04/15/88; EPA Doc No. FYI-AX-0688-0621; Fiche No. OTS0000621. Available at: http://www.ntis.gov/search/product.aspx?abbr=OTS0000621
[EFSA] European Food Safety Authority. 2005. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) to review the toxicology of a number of dyes illegally present in food in the EU. EFSA Journal 263:1-71. Available at: http://www.efsa.europa.eu/en/efsajournal/pub/263.htm
[ETAD] The Ecological and Toxicological Association of Dyes and Organic Pigment Manufacturers. 2008. The restrictions on the marketing and use of azo colourants according to the European legislation following the Directive 2002/61/EC (19th Amendment of Council Directive 76/769/EEC). (ETAD Information Notice No. 6). Available at: http://www.etad.com/documents/Downloads/publications/notice_no_6_rev_2008.pdf.
European Commission. ©2000a. IUCLID Dataset, barium bis [2-chloro-5-[(2-hydroxy-1-naphthyl)azo]toluene-4-sulphonate], CAS No. 5160-02-1 [Internet]. Year 2000 CD-ROM edition. [place unknown]: European Chemicals Agency, European Commission. [created 2000 Feb 18; cited 2013 Jan 31]. Available from: http://esis.jrc.ec.europa.eu/index.php?PGM=dat
European Commission. ©2000b. IUCLID Dataset, 2-chloro-4-nitroaniline, CAS No. 121-87-9 [Internet]. Year 2000 CD-ROM edition. [place unknown]: European Chemicals Agency, European Commission. [created 2000 Feb 18; cited 2014 Aug 18]. Available from: http://esis.jrc.ec.europa.eu/index.php?PGM=dat
European Commission. ©2000c. IUCLID Dataset, 2,4-dinitroaniline, CAS No. 97-02-9 [Internet]. Year 2000 CD-ROM edition. [place unknown]: European Chemicals Agency, European Commission. [created 2000 Feb 19; cited 2014 Aug 18]. Available from: http://esis.jrc.ec.europa.eu/index.php?PGM=dat
European Commission. 2003. Technical guidance document on risk assessment in support of Commission Directive 93/67/EEC on risk assessment for new notified substances; Commission Regulation (EC) No 1488/94 on risk assessment for existing substances; Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market. Part II. Environmental risk assessment. European Commission, Joint Research Centre; 328 p. Available from: http://ihcp.jrc.ec.europa.eu/our_activities/public-health/risk_assessment_of_Biocides/doc/tgd/tgdpart2_2ed.pdf
EU. 2006. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemical Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC [Internet]. Off J Eur Union L 396:1–849. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=oj:l:2006:396:0001:0849:en:pdf
Face Paints Direct 2013. Ingredients in Common Face Paints. [Internet] 2013. Face Paints Direct. [cited 2013 Feb 12]. Available from: http://www.facepaintsdirect.co.uk/ingredients.html
[FDSC] Food and Drug Safety Center. 1993. D&C Red No.7. Food and Drug Safety Center, Hatano Research Institute, 729-5 Ochiai, Hadano-shi, Kanagawa 257, Japan. Available from: http://dra4.nihs.go.jp/mhlw_data/home/pdf/PDF5281-04-9d.pdf
FisherSolve™ Platform [database on the Internet]. 2013. South Norwalk (CT): Fisher International. [cited 2012 Nov]. Available from: www.fisheri.com/products/index.php?sid=48
Foureman P, Mason JM, Valencia R, Zimmering S. 1994. Chemical mutagenesis testing in Drosophila. X. Results of 70 coded chemicals tested for the National Toxicology Program. Environ Mol Mutagen 23:208–227.
Franz TJ. 1983a. Percutaneous absorption of D&C Orange No. 17 through human skin in vitro. University of Washington, March 28, 1983. Submission by the Cosmetic, Toiletry, and Fragrance Association [cited in Hart et al. 1986].
Franz TJ. 1983b. Percutaneous absorption of D&C Red No. 9 through human skin in vitro. University of Washington, August 8, 1983. Submission by the Cosmetic, Toiletry, and Fragrance Association. [cited in Hart et al. 1986].
Gershbein LL. 1982. Action of dyes and indicators on rat-liver regeneration. Food Chem Toxicol 20:1–8.
Gobas F. 2007. Development and review of a generic water–sediment modeling framework for organic chemicals. Report prepared for Environment Canada. Burnaby (BC): Simon Fraser University, Faculty of Environment. March 26, 2007.
Gobas F. 2010. Comments on approach to sediment exposure approach. Report prepared for Environment Canada. Burnaby (BC): Simon Fraser University, Faculty of Environment. March 25, 2010.
Goldin BR and Gorbach SL. 1984. Alterations of the intestinal microflora by diet, oral antibiotics, and Lactobacillus: decreased production of free amines from aromatic nitro compounds, azo dyes, and glucuronides. JNCI. 73(3):689-695.
Golka K, Kopps S, Myslak ZW. 2004. Carcinogenicity of azo colorants: influence of solubility and bioavailability. Toxicol Lett 151(1):203–210.
Goodman DG, Ward JM, Reichardt WD. 1984. Splenic fibrosis and sarcomas in F344 rats fed diets containing aniline hydrochloride, p-chloroaniline, azobenzene, o-toluidine hydrochloride, 4,4?-sulfonyldianiline, or D & C Red No. 9. J Natl Cancer Inst 73(1):265–273.
Gopee NV, Yanyan C, Olson G, Warbritton AR, Miller BJ, Couch LH, Wamer WG, Howard PC. 2005. Response of mouse skin to tattooing: use of SKH-1 mice as a surrogate model for human tattooing. Toxicol Appl Pharmacol 209:145–158.
Graham SL, Davis KJ. 1968. Subacute toxicity of toluidine red. Toxicol Appl Pharmacol 13(3):388–391.
Green EJ. 1990. The Sigma-Aldrich handbook of stains, dyes and indicators. Milwaukee (WI): Aldrich Chemical Co.; p. 716. [cited in IARC 1993].
Gregory P. 1986. Azo Dyes: Structure – Carcinogenicity Relationships. Dyes and Pigments. 7:45-56.
Hansen W H, Fitzhugh OG, and Williams MW. 1958. Subacute Oral Toxicity of Nine D&C Coal-Tar Colors. J. Pharmacol. Exp. Therap. 122: 29A.
Hansen WH, Wilson DC, and Fitzhugh OG . 1960. Subacute oral toxicity of ten D&C coal-tar colors. Fed. Proc. 19, 390.
Hansen PL, Tønning K, Malmgren-Hansen B, Jacobsen E. 2008. Survey and health assessment of chemical subtances in hobby products for children. (Survey of Chemical Substances in Consumer Products, No. 93). Copenhagen (DK): Danish Ministry of the Environment, Environmental Protection Agency. [cited 2013 Feb 19]. Available from: www2.mst.dk/udgiv/publications/2008/978-87-7052-763-7/pdf/978-87-7052-764-4.pdf
Hard GC and Khan KN. 2004. A Contemporary Overview of Chronic Progressive Nephropathy in the Laboratory Rat, and Its Signi?cance for Human Risk Assessment. Toxicologic Pathology. 32:171–180.
Hart RW, Freni SC, Gaylor DW, Gillette JR, Lowry LK, Ward JM, Weisburger EK, LePore P, Turtur A. 1986. Final report of the Color Additive Scientific Review Panel. FDA Color Report. Risk Anal 6(2):117–154.
Haseman JK, Johnson FM. 1996. Analysis of National Toxicology Program rodent bioassay data for anticarcinogenic effects. Mutat Res 350(1):131–141.
Hauri U. 2006. [Finger paint / preservatives, primary aromatic amines, bitter substances, dyes, pH and declaration. Joint campaign of Basel-Stadt (specialist laboratory) and Aargau. Report No. 67]. Basel (CH): Kantonales Laboratorium. [in German]. Available from: www.kantonslabor-bs.ch/kl/infos/berichte.cfm?sprache=de&site=21&year=2006&subkat=2&detail=Y0E9V99T
Hauri U. 2008. [Finger paint / preservatives, primary aromatic amines, bitter substances, colorants, phthalates, nitrosamines, pH and declaration. Joint campaign of Basel-Stadt (specialist laboratory) and Aargau. Report No. 22]. Basel (CH): Kantonales Laboratorium. [in German]. Available from: www.kantonslabor-bs.ch/kl/infos/berichte.cfm?sprache=de&site=21&year=2008&subkat=2&detail=5F38NZ9T
Hauri U. 2009a. [Finger paint / preservatives, primary aromatic amines, bitter substances, colorants, phthalates, nitrosamines and declaration. Joint campaign of Basel-Stadt (specialist laboratory) and Aargau. Report No. 49]. Basel (CH): Kantonales Laboratorium. [in German]. Available from: www.kantonslabor-bs.ch/kl/infos/berichte.cfm?sprache=de&site=21&year=2009&subkat=2&detail=7ZW62Z7N
Hauri U. 2009b. [Game kneading / preservatives, primary aromatic amines, bitter substances, colorants, phthalates, nitrosamines and declaration. Report No. 44]. Basel (CH): Kantonales Laboratorium. [in German]. Available from: www.kantonslabor-bs.ch/kl/infos/berichte.cfm?sprache=de&site=21&year=2009&subkat=2&detail=7WVJ92SK
Hauri U. 2010a. [Finger paint / preservatives, bitter substances, dyes, nitrosamines. Report No. 35]. Basel (CH): Kantonales Laboratorium. [in German]. Available from: www.kantonslabor-bs.ch/kl/infos/berichte.cfm?sprache=de&site=21&year=2010&subkat=2&detail=46K7Z36C
Hauri U. 2010b. [Permanent make up / preservatives, pigments, aromatic amines, N-nitrosamines. Joint campaign by the cantons of Aargau, Basel-Land and Basel-Stadt (specialist laboratory). Report No. 7]. Basel (CH): Kantonales Laboratorium. [in German]. Available from: www.kantonslabor-bs.ch/kl/infos/berichte.cfm?sprache=de&site=21&year=2010&subkat=2&detail=GQ8R7983
Hauri U. 2010c. [Chinese tattoo inks / preservatives, pigments, N-nitrosamines. Report No. 42]. Basel (CH): Kantonales Laboratorium. [in German]. Available from: www.kantonslabor-bs.ch/kl/infos/berichte.cfm?sprache=de&site=21&year=2010&subkat=2&detail=2D9V242R
Hauri U. 2011a. [Finger paint / preservatives, bitter substances, dyes, nitrosamines. Joint campaign by the cantons of Basel City (specialist laboratory) and Aargau]. Basel (CH): Kantonales Laboratorium. [in German]. Available from: www.kantonslabor-bs.ch/files/berichte/JB_Fingerfarben_2011_korr.pdf
Hauri U. 2011b. [Inks for tattoos and PMU (permanent make-up) / Organic pigments, preservatives and impurities such as primary aromatic amines and nitrosamines. Joint campaign by the Swiss Association of Cantonal Chemists (Verband der Kantonschemiker der Schweiz – VKCS) with financial support from the FOPH (SwissFederal Office of Public Health), laboratory in charge: Basel City]. Basel (CH): Kantonales Laboratorium. [in German]. Available from: www.kantonslabor-bs.ch/files/berichte/JB_Tattoo_PMU_2011.pdf
Hauri U. 2013. Pigments, Preservatives and Impurities in Tattoo Inks. Presenation at the First International Conference on Tattoo Safety. BfR-Symposium, Berlin, June 6 – 7, 2013. Available at: http://www.bfr.bund.de/cm/343/pigments-preservatives-and-impurities-in-tattoo-inks.pdf
Hazleton Laboratories. 1964a. Final Report: two-year feeding study – dogs. CTFA Color Additive Master File No.9, Entry 61. [cited in BG Chemie 2000a]
Hazleton Laboratories. 1964b. Final Report: two-year feeding study – dogs. CTFA Color Additive Master File No.9, Entry 61. [cited in BG Chemie 2000a]
Health Canada. 1994. Human health risk assessment for priority substances. Ottawa (ON): Health Canada. Available from: www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/contaminants/approach/approach-eng.pdf
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2011a. Policy statement on Health Canada’s working definition for nanomaterial. Ottawa (ON): Health Canada. Available from: www.hc-sc.gc.ca/sr-sr/pubs/nano/pol-eng.php
Health Canada. 2011b. The cosmetic ingredient hotlist – September 2011 [Internet]. Ottawa (ON): Health Canada, Consumer Product Safety. [cited 2012 Sep]. Available from: www.hc-sc.gc.ca/cps-spc/cosmet-person/indust/hot-list-critique/hotlist-liste-eng.php
Health Canada. 2012. List of Permitted Colouring Agents (Lists of Permitted Food Additives). Ottawa (ON): Health Canada. Available from: www.hc-sc.gc.ca/fn-an/securit/addit/list/3-colour-color-eng.php
Health Canada. 2013. Interim Guidance on Human Health Risk Assessment for Short-Term Exposure to Carcinogens at Contaminated Sites. Federal Contaminated Sites Risk Assessment in Canada. Ottawa (ON): Health Canada. Available at: www.hc-sc.gc.ca/ewh-semt/contamsite/index-eng.php
Hébert M, Chaker B. 2011. Bilan 2010 du recyclage des matières résiduelles fertilisantes. Québec Ministère du Développement durable, de l'Environnement et des Parcs, Direction des matières résiduelles et des lieux contaminés, Québec, QC. 24 pp.
Herbst W, Hunger K. 2004. Industrial organic pigments. 3rd ed. Weinheim (DE): Wiley-VCH, Verlag GmbH & Co. KGaA; 660 p.
Hoechst AG. 1973. Pharma Forschung Toxikologie Hansaorange CM 70429 – 32-Tage-Fütterungsversuch. Unpublished Report No. 0128/73 [cited in BG Chemie 2000a]
Hoechst AG. 1979. Pigment-Yellow Nr.1. Hansa-Gelb G. 30-Tag-oral-Test an SPF-Wistar-Ratten. ETAD Projeckt T 2005 / T2014. Bericht Nr. 710/79. Unpublisheddata submitted by ETAD to ITC, April 14, 1982. [cited in NTIS/OTS0001185. Initial Submission: Letter from Etad to USEPA Regarding Aminoanthraquinones with Attachments, dated 02/07/86.] Available at: http://www.ntis.gov/search/product.aspx?ABBR=OTS0001185
Hoecht AG. 1990. Evaluation of Hansa-Rot GG in the in vivo cytogenetic test in bone marrow cells of the Chinese hamster – chromosome analysis. Hoecht AG, Pharma Research Toxicology and Pathology. Unpublished Report No. 90.0741. [cited in BG Chemie 2000a]
Høgsberg T, Loeschner K, Löf D, Serup J. 2011. Tattoo inks in general usage contain nanoparticles. Br J Dermatol 165:1210–1218.
Household Products Database [database on the Internet]. 1993– . Bethesda (MD): National Library of Medicine (US). [cited 2012 Dec 12]. Available from: www.householdproducts.nlm.nih.gov/
Huang Q, Wang L, Han S. 1995. The genotoxicity of substituted nitrobenzenes and the quantitative structure-activity relationship studies. Chemosphere. 30(5):915-23.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1975. IARC Monographs on the Evaluation of Carcinogenic Risks to Man: Some aromatic azo compounds, Volume 8. IARC Working Group on the Evaluatation of the Risk of Chemicals to Man which met in Lyon, 26 November - 2 December 1974. Summary available at: http://monographs.iarc.fr/ENG/Monographs/vol8/volume8.pdf
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1993. D&C Red No. 9 (CI Pigment Red 53:1). IARC Monogr Eval Carcinog Risks Hum 57:203–212. Available from: http://monographs.iarc.fr/ENG/Monographs/vol57/mono57-15.pdf
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2010b. Painting, firefighting, and shiftwork. IARC Monogr Eval Carcinog Risks Hum 98. Available from: http://monographs.iarc.fr/ENG/Monographs/vol98/mono98.pdf
[ILS] Integrated Laboratory Systems, Inc. 2011. Assessment of the mutagenicity of twenty aromatic azo substances in the Ames mutagenicity assay using the Prival modification with and without FMN. Unpublished report. Research Triangle Park (NC). ILS Project Study No. C191-006.
[IRDC] International Research and Development Corporation .1981. D&C Red No. 6. Long-term feeding study in rats exposed in utero. Unpublished report No. 355-014 from International Research and Development Corporation. [cited (as 1981b) in US FDA 1982]
Ivett JL, Brown BM, Rodgers C, Anderson BE, Resnick MA, Zeiger E. 1989. Chromosomal aberrations and sister chromatid exchange tests in Chinese hamster ovary cells in vitro. IV. Results with 15 chemicals. Environ Mol Mutagen 14:165–187.
[JECDB] Japanese Existing Chemicals Database. 2013a. 2-Amino-1-naphthalenesulfonic acid ( 81-16-3 ). Available from: http://dra4.nihs.go.jp/mhlw_data/home/file/file81-16-3.html
[JECDB] Japanese Existing Chemicals Database. 2013b. 2-Amino-5-methylbenzenesulfonic acid ( 88-44-8). Available from: http://dra4.nihs.go.jp/mhlw_data/home/file/file88-44-8.html
[JECDB] Japanese Existing Chemicals Database. 2013c. C.I. Pigment Red 22 ( 6448-95-9 ). Available at: http://dra4.nihs.go.jp/mhlw_data/home/file/file6448-95-9.html
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1986. Lithol rubine BK (WHO Food Additive Series 21). Available at: http://www.inchem.org/documents/jecfa/jecmono/v21je09.htm
Jemec GB. 2010. Comment on: Tattooing of skin results in transportation and light-induced decomposition of tattoo pigments. Exp Dermatol. 19(1):61-2.
Johnson GE, Quick EL, Parry EM, Parry JM. 2010. Metabolic influences for mutation induction curves after exposure to Sudan-1 and Para Red. Mutagenesis 25:327–333.
Jung R, Steinle D, Anliker R. 1992. A compilation of genotoxicity and carcinogenicity data on aromatic aminosulphonic acids. Food Chem Toxicol. 30(7):635–60.
Karickhoff S. 1981. Semi-empirical estimation of sorption of hydrophobic pollutants on natural sediments and soils. Chemosphere 10:833–846.
Kelly BC, Gobas FAPC, McLachlan MS. 2004. Intestinal absorption and biomagnification of organic contaminants in fish, wildlife, and humans. Environ Toxicol Chem 23:2324–2336.
Kornbrust D, Barfnecht T. 1985. Testing of 24 food, drug, cosmetic, and fabric dyes in the in vitro and the in vivo/in vitro rat hepatocyte primary culture/DNA repair assays. Environ Mutagen 7:101–120.
[KOWWIN] Octanol–Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2010. Version 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2013 Apr 16]. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Kumagai Y, Shinkai Y, Miura T, Cho AK. 2012. The chemical biology of naphthoquinones and its environmental implications. Annu Rev Pharmacol Toxicol. 52:221-47
Kupradinun P, Rienkijakarn M, Tanyakaset M, Tepsuwan A, Kusamran WR. 2002. Carcinogenicity testing of the cosmetic dye: D&C Red No. 36. Asian Pac J Cancer Prev 3(1):55–60.
Kuramoto N. 1996. The photodegradation of synthetic colorants. pp196-253. In. Physico-Chemical Principles of Color Chemistry. (A. T. Peters, H. S. Freeman Editors). Advances in Color Chemistry Series. Volume 4. Springer Netherlands.
Lea PJ, Pawlowski A. 1987. Human tattoo: electronic microscopic assessment of epidermis, epidermal–dermal junction, and dermis. Int J Dermatol 26(7):453–458.
Leist KH. 1982. Subacute toxicity studies of selected organic colorants. Ecotoxicology and Environmental Safety. 6:457-463.
Lewis PA. 1995. Colored organic pigments. In: Koleske JV, editor. Paint and coating testing manual: fourteenth edition of the Gardner-Sward handbook. West Conshohocken (PA): ASTM International.
Lewis RJ Sr. 2007. Hawley’s condensed chemical dictionary. 15th ed. Wiley-Interscience; John Wiley & Sons, Inc.
Lilja K, Kaj L, Remberger M, Brorström-Lundén E, Leknes H, Schlabach M. 2008. Results from the Swedish National Screening Programme 2007: Subreport 3: pigments. Stockholm (SE): Swedish Environmental Research Institute (IVL) (sponsored by the Swedish Environmental Protection Agency). IVL Report No.: B1811. Available from: www.ivl.se
Limbach LK, Li Y, Grass RN, Brunner TJ, Hintermann MA, Muller M, Gunther D, Stark WJ. 2005. Oxide nanoparticle uptake in human lung fibroblasts: effects of particle size, agglomeration, and diffusion at low concentrations. Environ Sci Technol 39:9370–9376.
Linak E, Kälin T, Inoguchi Y. 2011. CEH marketing research report: organic color pigments [Internet]. SRI Consulting (SRIC); 104 p. Report No.: 575.7000. [cited 2013 Oct]. Available from: http://chemical.ihs.com/CEH/Public/Reports/575.7000
Lincke G. 2003. Molecular stacks as a common characteristic in the crystal lattice of organic pigment dyes. A contribution to the “soluble–insoluble” dichotomy of dyes and pigments from the technological point of view. Dyes Pigments 59:1–24.
[LNHPD] Licensed Natural Health Products Database [database on the Internet]. 2008. Version 1.0. Ottawa (ON): Health Canada. [cited 2014]. Available from: webprod3.hc-sc.gc.ca/lnhpd-bdpsnh/index-eng.jsp
Lockwood-Post Directory 2011, Global Edition. Paperloop, Incorporated, 2011
Longstaff E, McGregor DB, Harris WJ, Robertson JA, Poole A. 1984. A comparioson of the predictive values of the Salmonella/microsome mutation and BHK21 cell transformation assays in relation to dyestuffs and similar materials. Dyes Pigments 5:65–82.
LookChem 2008. Hot product list [Internet]. Zhejiang (CN): LookChem. [cited 2012 Nov 14]. Available from: www.lookchem.com/
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol 43:279–291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, Re T, Renskers K, Scrafford C, Vater S. 2006. Exposure data for personal care products: hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol 44: 2008–2018.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol 46:1516–1524.
[LSRO-FASEB] Life Sciences Research Office – Federation of American Societies for Experimental Biology. 1984. Interim scientific report on evaluation of the evidence of carcinogenicity and genotoxicity of drugs and cosmetic ingredients. Bethesda (MD): FASEB. NTIS Report No.: PB85-122513. Available from: www.ntis.gov/search/product.aspx?ABBR=PB85122513
Lynch I, Dawson KA, Linse S. 2006. Detecting cryptic epitopes created by nanoparticles. Sci STKE 2006(327):pe14.
Mackay D, Di Guardo A, Paterson S, Kicsi G, Cowan CE. 1996a. Assessing the fate of new and existing chemicals: A five-stage process. Environmental Toxicology and Chemistry 15(9): 1618-1626.
Mackay D, Webster E, Cousins I, Cahill T, Foster K, Gouin T. 2001. An introduction to multimedia models. Final report prepared as a background paper for OECD workshop (Ottawa, October 2001). Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry; 30 p. CEMC Report No.: 200102.
Mackay D, Arnot JA, Webster E, Reid L. 2009. The evolution and future of environmental fugacity models. In: Devillers J, editor. Ecotoxicology modeling. Emerging topics in ecotoxicology: principles, approaches and perspectives 2. Springer Science+Business Media, LLC; p. 355–375.
Matsushima T, Teichmann B, Sawamura M, Sugimura T. 1978. Mutagenicity of azo-compounds : improved methods for detecting their mutagenicity by the Salmonella mutation test. Mutat Res 54:220-221.
Mathews JM, Zhan Q, Etheridge AS, Patel PR, Black SR, Banks TT, Fennell TR, Snyder RW, Burgess JP, Warren SD, Surh I, Waidyanatha S. 2012. Metabolism and disposition of 2-methoxy-4-nitroaniline in male and female Harlan Sprague Dawley rats and B6C3F1/N mice. Xenobiotica42(12):1213-24.
Matthews HB, Chopade HM, Smith RW, Burka LT. 1986. Disposition of 2,4-dinitroaniline in the male F-344 rat. Xenobiotica. 16(1):1-10.
Milvy, P. and Kay, K. 1978. Mutagenicity of 19 major graphic arts and printing dyes. Journal of Toxicology and Environmental Health. 4:31-36.
[MITI] Ministry of International Trade & Industry (JP), Basic Industries Bureau, Chemical Products Safety Division. 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (JP): Japan Chemical Industry Ecology-Toxicology & Information Centre. [cited in CPMA 2006b; OECD 2009b].
Miyagoshi M, Hayakawa Y, Nagayama T. 1983. [Studies on the mutagenicity of cosmetic azo-dyes]. Eisei Kagaku 29(4):212–220. [in Japanese].
Møller P, Wallin H, Grunnet N, Risom L, Knudsen LE. 1988. DNA damage in isolated rat hepatocytes exposed to C.I. pigment orange 5 and C.I. pigment yellow 12 by the alkaline comet assay. Teratog Carcinog Mutagen. 18(1):9-16.
Møller P, Wallin H. 2000. Genotoxic hazards of azo pigments and other colorants related to 1-phenylazo-2-hydroxynaphthalene. Mutat Res 462: 13-30.
Mont Marte International Ltd. 2009a. Mont Marte Acrylic Paint 12 PCE. Material Safety Data Sheet. Mont Marte International. [cited 2014 July 4]. Available from: https://www.schoolartsupplies.com.au/images/stories/MSDS_-_MONT_MARTE_ACRYLIC_PAINT_12_PCE_ALL_COLOURS_EXCEPT_BLACK_AND_RAW_UMBER_-_MCG0037.pdf
Mont Marte International Ltd. 2009b. Mont Marte Watercolour Paint 12 PCE. Material Safety Data Sheet. Mont Marte International. [cited 2014 July 4]. Available from: https://www.schoolartsupplies.com.au/images/stories/MSDS_-_MONT_MARTE_WATERCOLOUR_PAINT_12_PCE_ALL_COLOURS_EXCEPT_IVORY_BLACK_-_MCG0038.pdf
Mont Marte International Ltd. 2012. Mont Marte Body and Face Art Paint Safety Data Sheet. Mont Marte International. [ cited 2013 March 20 ]. Available from: http://montmarte.net/image/data/MSDS/MONT%20MARTE%20BODY%20AND%20FACE%20ART%20PAINT%20(EU)%20-%20MBRT2001-3.pdf
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E. 1986. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen 8: 1-119.
Muzzall JM, Cook WL. 1979. Mutagenicity test of dyes used in cosmetics with the Salmonella/mammalian-microsome test. Mutat Res 67:1–8.
Myhr BC, Caspary WJ. 1991. Chemical mutagenesis at the thymidine kinase locus in L5178Y mouse lymphoma cells: results for 31 coded compounds in the National Toxicology Program. Environ Mol Mutagen. 1991;18(1):51-83.
Nakayama T, Kimura T, Kodama M, Nagata C. 1983. Generation of hydrogen peroxide and superoxide anion from active metabolites of naphthylamines and aminoazo dyes: its possible role in carcinogenesis. Carcinogenesis 4:765–769.
[NCI] National Cancer Institute. 1978. Bioassay of Anthranilic Acid for Possible Carcinogenicity (CAS No. 118-92-3). Technical Report Series No.36. Available at: http://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr036.pdf
[NCI] National Chemical Inventories [database on a CD-ROM]. 2012. Issue 2. Columbus (OH): American Chemical Society, Chemical Abstracts Service. Available from: www.cas.org/products/other-cas-products/nci-on-cd
Neumann HG. 2005. Monocyclic aromatic amino and nitro compounds: toxicity, genotoxicity and carcinogenicity, classification in a carcinogen category. The MAK-Collection Part I: MAK Value Documentations, Vol. 21. DFG, Deutsche Forschungsgemeinschaft. Available at: http://onlinelibrary.wiley.com/doi/10.1002/3527600418.mb0maryvere0021/pdf
[NHPID] Natural Health Products Ingredients Database [database on the Internet]. 2011. Version 2.1. Ottawa (ON): Health Canada. [cited 2012 Feb 15]. Available from: webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme (AU). 2009. Triclosan [Internet]. Sydney (AU): Australian Department of Health and Ageing, NICNAS. Priority Existing Chemical Assessment Report No.: 30. Available from: www.nicnas.gov.au/publications/car/pec/pec30/pec_30_full_report_pdf.pdf
[NPC] National Paint & Coatings Association. 2004. Exposure to crystalline silica and estimation of the associated human health risks from painting and sanding interior flat latex paint. Final report. Prepared for National Paint & Coatings Association, Inc. Seattle (WA): Radian International; 48 p. [prepared 2000 Sep 11; errata corrected 2004 Jul 26].
[NPIRI] National Printing Ink Research Institute. 2000a. Raw materials data handbook: a reference guide to regulatory data and technical performance properties, vol. 4. Pigments. 2nd ed. Woodbridge (NJ): NPIRI; p. 62. [cited in Environment Canada and Health Canada 2009b].
[NTIS] National Technical Information Service (US). 1986. Genotoxicity testing with C.I. Pigment Red 112 with Cover Letter Dated 082686 (Company Sanitized). FY-OTS-0986-0511S. NTIS/OTS0000511-1. Available from: http://www.ntis.gov/search/product.aspx?abbr=OTS00005110
[NTP] National Toxicology Program (US). 1978. Toxicology and Carcinogenesis Studies of C.I. Pigment Red 23 (CAS No.6471-49-4) in F344 Rats and B6C3F1 Mice (Feed Studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Technical Report Series No. 411. Available at: http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr411.pdf
[NTP] National Toxicology Program (US). 1982. Carcinogenesis bioassay of D & C Red No. 9 (CAS No. 5160-02-1) in F344 rats and B6C3F1 mice (feed study). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Technical Report Series No.: 225. Available from: http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr225.pdf
[NTP] National Toxicology Program (US). 1994. NTP Toxicology and Carcinogenesis Studies of Barium Chloride Dihydrate (CAS No. 10326-27-9) in F344/N Rats and B6C3F1 Mice (Drinking Water Studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Technical Report Series No. 432. Available from: http://ntp.niehs.nih.gov/results/pubs/longterm/reports/longterm/tr400499/abstracts/tr432/index.html
[NTP] National Toxicology Program (US). 2006. 2-Methoxy-4-Nitroaniline (CAS RN 97-52-9). Prepared for NCI to support chemical nomination by Technical Resources International, Inc. under contract no. N02-CB-07007 (9/05; 3/06). Available at: http://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/97-52-9_508.pdf
[NTP] National Toxicology Program (US). 2013. Rodent Bone Marrow Cytogenetics - Sister Chromatid Exchange. Study Number 832755. CASRN 5160-02-9, D&C Red No. 9. (test report from database, extracted Jan 2013). Available at: http://tools.niehs.nih.gov/ntp_tox/index.cfm?fuseaction=invivosc.scsummary&study_no=832755&cas_no=5160-02-1&endpointlist=SC
[NVWA] Nederlandse Voedsel en Warenautoriteit. 2008 [Monitoring and Compliance of Permanent Make-Up and Tattoo Dyes (Review 2004-2007)]. Nederlandse Voedsel en Warenautoriteit (Netherlands Food and Consumer Product Safety Authority)/ [in Dutch]. Available from: https://www.vwa.nl/txmpub/files/?p_file_id=28126
[OECD] Organisation for Economic Co-operation and Development. 1994. SIDS Initial Assessment Report: 2-Naphthalenecarboxylic acid, 3-hydroxy-4-[(4- methyl-2-sulfophenyl)azo]-, calcium salt (D & C Red No. 7). CAS RN 5281-04-9. SIDS Initial Assessment Meeting 2; 4-6 July 1994. Available from: http://www.inchem.org/documents/sids/sids/5281049.pdf
[OECD] Organisation for Economic Co-operation and Development. 1996. SIDS Initial Assessment Report: 1-aminoanthraquinone (CAS RN 82-45-1). Available at: http://www.inchem.org/documents/sids/sids/82451.pdf
[OECD] Organisation for Economic Co-operation and Development. 1999a. SIDS Initial Assessment Report: Pigment Red 53:1; CAS RN 5160-02-1. SIDS Initial Assessment Meeting 9; June–July 1999. Available from: www.chem.unep.ch/irptc/sids/oecdsids/5160021.pdf
[OECD] Organisation for Economic Co-operation and Development. 1999b. Screening Information Dataset: Pigment Red 53:1; CAS RN 5160-02-1. Available from: http://webnet.oecd.org/hpv/ui/handler.axd?id=B906455B-1CF2-48E8-B3E8-DCE481D4C09A
[OECD] Organisation for Economic Co-operation and Development. 2000. Guidance document on aquatic toxicity testing of difficult substances and mixtures. (OECD Series on Testing and Assessment No. 23). Paris (FR): OECD Environmental Health and Safety Publications Series on Testing and Assessment, No. 23. Report No.: ENV/JM/MONO(2000)6. Available from: www.oecd-ilibrary.org/docserver/download/9750231e.pdf?expires=1371748494&id=id&accname=guest&checksum=F77793DC9424557B142CC94AF4A2E7A4
[OECD] Organisation for Economic Co-operation and Development. 2001. SIDS Initial Assessment Report: 2-Nitroaniline (CAS RN 88-74-4). SIDS Initial Assessment Meeting 13; Nov 2001. Available from: http://www.chem.unep.ch/irptc/sids/OECDSIDS/NITROANILINE.pdf
[OECD] Organisation for Economic Co-operation and Development. 2005. OECD guidelines for testing of chemicals. Annex 1. Proposal for revised introduction to the OECD guidelines for testing of chemicals, section 3. Part 1: Principles and strategies related to the testing of degradation of organic chemicals. Paris (FR): OECD.
[OECD] Organisation for Economic Co-operation and Development. 2007. Guidance on grouping of chemicals. (OECD Series on Testing and Assessment No. 80). Paris (FR): OECD. Report No.: ENV/JM/MONO(2007)28. Available from: http://search.oecd.org/officialdocuments/displaydocumentpdf/?doclanguage=en&cote=env/jm/mono(2007)28
[OECD] Organisation for Economic Co-operation and Development. 2009a. Emission scenario documents on pulp, paper and board industry. OECD series on Emission Scenario Documents, No. 23. Organisation for Economic Co-operation and Development, July 8, 2009.
[OECD] Organisation for Economic Co-operation and Development. 2009b. SIDS Initial Assessment Report: 2-Naphthalenecarboxylic acid, 3-hydroxy-4-[(4-methyl-2- sulfophenyl)azo]-, calcium salt (D & C Red No. 7); CAS RN 5281-04-9. SIDS Initial Assessment Meeting 2; July 1994. Available from: www.inchem.org/documents/sids/sids/5281049.pdf
[OECD] Organisation for Economic Co-operation and Development. 2014. Guidance on Grouping of Chemicals. Second Edition. Series on Testing & Assessment No. 194. Available at: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2014)4&doclanguage=en
Øllgaard H, Frost L, Galster J, Hansen OC. 1998. Survey of azo-colorants in Denmark: consumption, use, health and environmental aspects. Copenhagen (DK): Ministry of Environment and Energy, Danish Environmental Protection Agency. Available from: www2.mst.dk/udgiv/publications/1999/87-7909-548-8/pdf/87-7909-546-1.pdf
Pal S, Tak YK, Song JM. 2007. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73:1712–1720.
Pan H, Feng J, He G, Cerniglia C, Chen H. 2012. Evaluation of impact of exposure of Sudan azo dyes and their metabolites on human intestinal bacteria. Anaerobe 18:445–453.
Panina N. 2009. Crystal structure and morphology prediction of organic pigments. Nijmegen (NL): Radboud University Nijmegen, Radboud Repository. Available from: http://repository.ubn.ru.nl/handle/2066/75422
Pearce CI, Guthrie JT, Lloyd JR. 2008. Reduction of pigment dispersions by Shewanelle strain J18 143. Dyes Pigments 76:696–705.
Platzek T, Lang C, Grohmann G, Gi US, Baltes W. 1999. Formation of a carcinogenic aromatic amine from an azo dye by human skin bacteria in vitro. Hum Exp Toxicol 18(9):552–559.
Pulp and Paper Canada Annual Mill Directory. 2013. Pulp and Paper Canada, © 2013 Business Information Group Magazines LP [division of Glacier BIG Holding Co. Ltd.]. Electronic version available from: http://www.ppcmilldirectory.com
Rastogi SC, Pritzl G. 1998. Red lipstick: a source of barium to humans and the environment. Bull Environ Contam Toxicol. 60(4):507-10.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2006a. Cosmetics fact sheet: to assess the risks for the consumer. Updated version for ConsExpo 4. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). RIVM Report No: 320104001/2006. Available from: www.rivm.nl/bibliotheek/rapporten/320104001.pdf
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2006b. Cleaning products factsheet: to assess the risks for the consumer. Updated version for ConsExpo 4. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). RIVM Report No.: 320104003/2006. Available from: www.rivm.nl/bibliotheek/rapporten/320104003.pdf
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2008. Chemicals in Toys: A general methodology for assessment of chemical safety of toys with a focus on elements. RIVM report 320003001/2008. The Netherlands: The National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu). Available from: http://www.rivm.nl/bibliotheek/rapporten/320003001.pdf
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2010. New default values for the spray model [Internet]. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). [cited 2012 Jul 27]. Available from: www.rivm.nl/dsresource?type=pdf&objectid=rivmp:60686&versionid=&subobjectname=
Rothen-Rutishauser BM, Schürch S, Haenni B, Kapp N, Gehr P. 2006. Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscope techniques. Environ Sci Technol 40:4353–4359.
Rufli H, Fisk PR, Girling AE, King JM, Länge R, Lejeune X, Stelter N, Stevens C, Suteau P, Tapp J, Thus J, Versteeg DJ, Niessen HJ. 1998. Aquatic toxicity testing of sparingly soluble, volatile, and unstable substances and interpretation and use of data. Ecotoxicol Environ Saf 39(2):72–77.
Rust-Oleum Corp. 2006. Material Safety Data Sheet (MSDS): N Industrial Choice Aerosols. [cited 2013 Jan 24]. Vernon Hills (IL): Rust-Oleum Corporation. Available from: www.msdsxchange.com/english/show_msds.cfm?paramid1=3316447
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol 29(1):89?92
Sakuratani Y, Zhang HQ, Nishikawa S, Yamazaki K, Yamada T, Yamada J, Hayashi M. 2013. Categorization of nitrobenzenes for repeated dose toxicity based on adverse outcome pathways. SAR QSAR Environ Res. 24(1):35-46.
[SCC] Scientific Committee on Cosmetology. 1988. CI 12370. Report by the Scientific Committee on Cosmetology Concerning Certain Colouring Agents (Opinion expressed on 1 July, 1986). Reports of the Scientific Committee on Cosmetology (seventh series). Commission of the European Communities. Available at: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/scc_o_7.pdf
[SCC] Scientific Committee on Cosmetology. 1993. CI 12075. Reports of the Scientific Committee on Cosmetology Concerning the Use of Certain Colourants in Cosmetic Products (45th meeting of SCC - October 10-11, 1990). In: Opinion of the Scientific Committee on Cosmetology (11/86 - 10/90). 8th Series Part 2. European Commission. Available at: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/scc_o_8b.pdf
[SCC] Scientific Committee on Cosmetology. 2000. CI 15585: 1-(4-chloro-o-sulpho-5-tolylazo)-2-naphthol. Opinions adopted during the 49th plenary meeting of 10 February 1992. In: Reports of the Scientific Committee on Cosmetology (Ninth Series). Luxembourg: European Commission; p. 137–141. Available at: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/scc_o_9.pdf
[SCCS] Scientific Committee on Consumer Safety. 2011. Opinion on Acid Orange 7. COLIPA n° C15. Scientific Committee on Consumer Safety. Available at: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_057.pdf
[SCCS] Scientific Committee on Consumer Safety. 2012. Opinion on Pigment Red 57. COLIPA n° C181. Scientific Committee on Consumer Safety. Available at: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_112.pdf
[SCHER] Scientific Committee on Health and Environmental Risks. 2012. Assessment of the tolerable daily intake of barium. Brussels (BE): European Commission; 13 p. Available from: http://ec.europa.eu/health/scientific_committees/environmental_risks/docs/scher_o_161.pdf
Scott B, Moore L. 2000. Assessment of the risks to human health posed by azo colourants in toys, writing inks and paper products, and analysis of the advantages and drawbacks of restrictions on their marketing and use. Final Report. Laboratory of the Government Chemist (LGC).
Screen Web [online companion to Screen Printing magazine]. 2000. How much ink do you need? Skokie (IL): ST Media Group International. [cited 2013 Apr 18]. Available from: www.screenweb.com/content/how-much-ink-do-you-need?page=0%2C3
[SDA] Soap and Detergent Association. 2010a. Appendix II-A-1: Dermal exposure parameters to estimate screening exposures to consumer products--North America. In: Consumer product ingredient safety: exposure and risk screening methods for consumer product ingredients. 2nd ed. Washington (DC): SDA.
[SDA] Soap and Detergent Association. 2010b. Appendix II-A-2: Dermal exposure parameters to estimate screening exposures to consumer products--Europe. In: Consumer product ingredient safety: exposure and risk screening methods for consumer product ingredients. 2nd ed. Washington (DC): SDA.
Seifried HE, Seifried RM, Clarke JJ, Junghans TB, San RH. 2006. A compilation of two decades of mutagenicity test results with the Ames Salmonella typhimurium and L5178Y mouse lymphoma cell mutation assays. Chem Res Toxicol 19(5):627–644.
Sendelbach LE. 1989. A review of the toxicity and carcinogenicity of anthraquinone derivatives. Toxicology. 57(3): 227–240.
[Siegwerk] Siegwerk Druckfarben AG & Co. KGaA. 2012. Toy Regulations Global V1.3: Declaration on the suitability for use of Siegwerk products for application on toys which can be sucked, licked or swallowed. Siegburg (DE): Siegwerk. [cited 2013 Feb 14]. Available from: www.siegwerk.com/no_cache/en/customer-segments/product-safety/packaging-safety.html?np_downloads%5Baction%5D=download&np_downloads%5Buid%5D=134
SkinCandy. 2013a. Material Safety Data Sheets (various). Tattoo inks with ingredients listing Pigment Red 170 (Ass, Blacklight Red, Bloodline Flame Red, Bloodline Sunset Orange, Blood Orange, Crimson Blood, Knucklehead Red, Marz Orange, McLovin, Red, Red Grey, Red Hot, Sweet 16, Tangerine, Tokyo Red ) [Accessed December, 2013] Available from: http://skincandytattoosupply.com
SkinCandy. 2013b. Material Safety Data Sheets (various). Tattoo inks with ingredients listing Pigment Yellow 3 (Blacklight Yellow, Bloodline Clover Green, Canary Yellow, Candy Lime, Citron, Emerald Green, Lemon Glow, Lightning Lime, Mantis, Mushroom, OKR, Puce, Sour Apple Green, Tusk) [Accessed December, 2013] Available from: http://skincandytattoosupply.com
Skipper PL, Kim MY, Sun HL, Wogan GN, Tannenbaum SR.Monocyclic aromatic amines as potential human carcinogens: old is new again. Carcinogenesis. 31(1):50-8.
Smart SK, Cassady AI, Lu GQ, Martin DJ. 2006. The biocompatibility of carbon nanotubes. Carbon 44:1034–1047.
Spadaro JT and Renganathan V. 1994. Peroxidase-catalyzed oxidation of azo dyes: mechanism of disperse Yellow 3 degradation. Arch Biochem Biophys. 312(1):301-7.
Sperry K. 1992. Tattoos and tattooing. Part II: Gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 13(1):7–17.
Starbrite. 2013. Material Safety Data Sheets (various). Tattoo inks with ingredients listing Pigment Red 63:1 (Deep Maroon) [Accessed December, 2013] Available from: http://www.painfulpleasures.com/body_jewelry/gallery/Tattoo/tattoo_ink/starbrite_tattoo_ink/Starbright_MSDS_FullChart.pdf
Stiborová M, Martínek V, Semanská M, Hodek P, Dracínský M, Cvacka J, Schmeiser HH, Frei E. 2009. Oxidation of the carcinogenic non-aminoazo dye 1-phenylazo-2-hydroxy-naphthalene (Sudan I) by cytochromes P450 and peroxidases: a comparative study. Interdiscip Toxicol 2:195–200.
Stingley RL, Zou W, Heinze TM, Chen H, Cerniglia CE. 2010. Metabolism of azo dyes by human skin microbiota. J Med Microbiol 59(Pt.1):108–114.
Stubbs DH. 1973. Toluidine, para and chlornitraniline reds. In: Patton TC, editor. Pigment handbook, vol. 1. Properties and economics. New York (NY): John Wiley & Sons; p. 461–472.
Study Submission. 2007a. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Challenge006.
Study Submission. 2007b. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Challenge009.
Study Submission. 2007c. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Challenge010.
Study Submission. 2007d. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Challenge007.
Study Submission. 2007e. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Challenge020.
Study Submission. 2007f. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Submission012.
Study Submission. 2007g. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Submission011.
Study Submission. 2007h. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Submission013.
Study Submission. 2007i. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Submission014.
Study Submission. 2007j. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Submission018.
Study Submission. 2007k. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Challenge019.
Study Submission. 2007l. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Challenge020.
Study Submission. 2007m. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Challenge004.
Study Submission. 2007n. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Challenge017.
Study Submission 2007o. Unpublished confidential study submitted to Environment Canada, Existing Substances Division, under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary Identification No. 13365Challenge015.
Study Submission. 2007p. Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary, Identification No.: 13365Challenge004.
Study Submission. 2007q. Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. Robust Study Summary, Identification No.: 13365Challenge005.
Study Submission. 2012a. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID34].
Study Submission. 2012b. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID35].
Study Submission. 2012c. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID36].
Study Submission. 2012d. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID37].
Study Submission. 2012e. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID69].
Study Submission. 2012f. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID15].
Study Submission. 2012g. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID17].
Study Submission. 2012h. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID39].
Study Submission. 2012i. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID40].
Study Submission. 2012j. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID31].
Study Submission. 2012k. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID72].
Study Submission. 2012l. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID32].
Study Submission. 2012m. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID62].
Study Submission. 2012n. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID11].
Study Submission. 2012o. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID12].
Study Submission. 2012p. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID13].
Study Submission. 2012q. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID44].
Study Submission. 2012r. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID67].
Study Submission. 2012s. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID48].
Study Submission. 2012t. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID53].
Study Submission. 2012u. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID54].
Study Submission. 2012v. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division. [DocID51].
Sun Chemical 2004. Subacute 28-day oral toxicity with Pigment Additive 007 by daily gavage in the rat, followed by a 14-day recovery period. Notox Project 374704; Notox Substance 124983. [Report submitted to the US Environmental Protection Agency]. Cincinnati (OH): Sun Chemical Corporation. Available from: www.epa.gov/oppt/tsca8e/pubs/8ehq/2004/mar04/8ehq_0304_15525a.pdf
Tennant RW, Stasiewicz S, Spalding JW. 1986. Comparison of multiple parameters of rodent carcinogenicity and in vitro genetic toxicity. Environ Mutagen 8:205–227.
Tennekes H, Kaufmann W, Dammann M, van Ravenzwaay B. 2004. The stability of historical control data for common neoplasms in laboratory rats and the implications for carcinogenic risk assessment. Regul Toxicol Pharmacol. 40(3):293-304.
[Therapeutic Guidelines] Therapeutic Guidelines Limited. 2008. Lund and Browder chart for calculating the percentage of total body surface area burnt (Figure 14.19). Published in eTG complete, March 2008. [Internet]. Available upon request.
Tsuda S, Matsusaka N, Madarame H, Ueno S, Susa N, Ishida K, Kawamura N, Sekihashi K, Sasaki YF. 2000. The comet assay in eight mouse organs: results with 24 azo compounds. Mutat Res 465:11–26.
[UK COT] United Kingdom Committee on Toxicity. 2005. Urgent advice provided by COT: Mutagenicity of para red (p12). Joint statements of the COT with the CPM and COC: Para red risk assessment (p72). Committees on: Toxicity, Mutagenicity, Carcinogenicity of Chemicals in Food, Consumer Products and the Environment. Annual Report 2005. Available at: http://cot.food.gov.uk/pdfs/cotannualreportweb2005.pdf
[US EPA] US Environmental Protection Agency. 1994b. Draft cleaner technologies substitutes assessment (CTSA): screen reclamation. Chapter 3: Background information on methodologies used in screen reclamation risk, performance and cost evaluation. Washington (DC): US EPA, Office of Pollution Prevention and Toxics. Report No.: EPA 744R-94-005a.
[US EPA] US Environmental Protection Agency. 1995. EPA office of compliance sector notebook project--profile of the printing and publishing industry. Washington (DC): US EPA, Office of Enforcement and Compliance Assurance.
[US EPA] US Environmental Protection Agency. 1996. Ecological effects test guidelines. OPPTS 850.1000--Special considerations for conducting aquatic laboratory studies. [Public draft]. Washington (DC): US EPA, Office of Prevention, Pesticides and Toxic Substances. Report No.: EPA 712-C-96-113.
[US EPA] US Environmental Protection Agency. 1998. Multimedia compliance/pollution prevention assessment guidance for screen printing facilities. Washington (DC): US EPA, Office of Enforcement and Compliance Assurance.
[US EPA] US Environmental Protection Agency. 2000. Interim guidance for using ready and inherent biodegradability tests to derive input data for multimedia models and wastewater treatment plants (WWT) models (9/1/2000) [Internet]. [cited 2012-2013]. Available from: www.epa.gov/oppt/exposure/pubs/halflife.htm
[US EPA] US Environmental Protection Agency. 2005. Guidelines for carcinogen risk assessment. Risk Assessment Forum. Washington, DC. EPA/630/P-03/001F. Available at: http://www.epa.gov/raf/publications/pdfs/CANCER_GUIDELINES_FINAL_3-25-05.PDF
[US EPA] US Environmental Protection Agency. 2009. Mononitroanilines Category (2-Nitrobenzeneamine CAS RN 88-74-4, 4-Nitrobenzeneamine CAS RN 100-01-6). Screening-Level Hazard Characterization. US Environmental Protection Agency. September 2009. Available at: http://www.epa.gov/hpvis/hazchar/Category_Mononitroanilines_Sept2009.pdf
[US EPA] US Environmental Protection Agency. 2011. Exposure Factors Handbook: 2011 Edition. Washington (DC): National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency. http://www.epa.gov/ncea/efh/pdfs/efh-frontmatter.pdf
[US EPA] US Environmental Protection Agency. 2012a. AP42--Compilation of air pollutant emission factors, vol. I. Stationary point and area sources. Chapter 6: Organic chemical process industry; Section 6.7: Printing inks. Washington (DC): US EPA. [cited 2012 Dec 19]. Available from: www.epa.gov/ttnchie1/ap42
[US FDA] US Food and Drug Administration. 1976. Listing of D&C Red No.34 for Use in Externally Applied Drugs and Cosmetics. Federal Register. 47: 57681-57691.
[US FDA] US Food and Drug Administration. 1982. D&C Red No. 6 and D&C Red No.7 [Docket No. 82N-0378]. Federal Register. 47: 57681-57691.
[US FDA] United States Food and Drug Administration. 1986. Listing of D&C Orange No. 17 for use in externally applied drugs and cosmetics. Federal Register. 51: 28331-28346.
[US FDA] US Food and Drug Administration. 1988. Comparative evaluation for additional safety considerations, D&C Red No. 36. Memorandum. Silver Spring (MD): US Department of Health and Human Services, Food and Drug Administration.
Vasold R, Naarmann N, Ulrich H, Fischer D, König B, Landthaler M, Bäumler W. 2004. Tattoo pigments are cleaved by laser light--the chemical analysis in vitro provide evidence for hazardous compounds. Photochem Photobiol 80(2):185–190.
Weil CS, Carpenter C. 1973. Results of inclusion in the diet of rats for three generations. Unpublished report from the Mellon Institute, submitted to the Inter-Industry Color Committee by the Cosmetic, Toiletry and Fragrance Association, Inc. [cited in EFSA 2010].
Weinburger MA, Albert RH, Montgomery SB. 1985. Splenotoxicity associated with splenic sarcomas in rats fed high doses of D & C Red No. 9 or aniline hydrochloride. J Natl Cancer Inst 75(4):681–690.
Westmoreland C, Gatehouse D. 1992. D and C Red No. 9: genotoxic or non-genotoxic carcinogen? Mutat Res 281:163–167.
Weyman GS, Rufli H, Weltje L, Salinas ER, Hamitou M. 2012. Aquatic toxicity tests with substances that are poorly soluble in water and consequences for environmental risk assessment. Environ Toxicol Chem 31(7):1662–1669.
Williams GM, Mori H, McQueen CA. 1989. Stucture–activity relationships in the rat hepatocyte DNA-repair test for 300 chemicals. Mutat Res 221:263–286.
Williams JH. 1999. Regulations on additions of sludge-borne metals to soil and their adaptation to local conditions. In: L’Hermite P, editor. Treatment and use of sewage sludge and liquid agricultural wastes. London (GB): Commission of the European Communities; Elsevier Applied Science; p. 243–250.
Wormuth M, Scheringer M, Hungerbühler K. 2005. Linking the use of scented consumer products to consumer exposure to polycyclic musk fragrances. J Ind Ecol 9(1–2):237–258.
Wu X, Bennett DH, Ritz B, Gassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food and Chemical Toxicology 48: 3109-3119.
Xu H, Heinze TM, Chen S, Cerniglia CE, Chen H. 2007. Anaerobic metabolism of 1-amino-2-naphthol-based azo dyes (Sudan dyes) by human intestinal microflora. Appl Environ Microbiol 73:7759–7762.
Xu H, Heinze TM, Paine DD, Cerniglia CE, Chen H. 2010. Sudan azo dyes and para red degradation by prevalent bacteria of the human gastrointestinal tract. Anaerobe 16:114–119.
Yourick JJ, Sasik CT and Bronaugh RL. 2007. In vitro dermal absorption and metabolism of D&C red no. 17 in human and porcine skin. J Cosmet Sci 58:255-266.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. 1988. Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environ Mol Mutagen 11(12):1–158.
Zeilmaker MJ, Kroese ED, van Haperen P, van Veen MP, Bremmer HJ, van Kranen HJ, Wouters MFA, Janus JA. 1999. Cancer risk assessment of azo dyes and aromatic amines from garment and footwear. Bilthoven (NL): Rijkinstituut voor Volkgezondheid en Milieu (National Institute of Public Health and the Environment). RIVM Report No.: 601503 014. Available from: www.rivm.nl/bibliotheek/rapporten/601503014.html
Zeilmaker MJ, van Kranen HJ, van Veen MP, Janus JA. 2000. Cancer risk assessment of azo dyes and aromatic amines from tattoo bands, folders of paper, toys, bed clothes, watch straps and ink. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute of Public Health and the Environment); 45 p. RIVM Report No.: 601503 019. Available from: www.rivm.nl/bibliotheek/rapporten/601503019.html
Zhou Y, You X, Ye X. 1987. Mutagenicity of benzidines and their congener derivative dyes. Huanjing Kexue 8(2):31–34.
Appendices
- Appendix A: Chemical Identity, Structures, Molecular Formulas and Molecular Weights of the 33 Monoazo Pigments, Organized by Subset
- Appendix B: Experimental Physical and Chemical Properties (at Room Temperature when Applicable) of Monoazo Pigments and their Analogues
- Appendix C: Experimental Data on Biodegradation of Monoazo Pigments and their Analogues
- Appendix D: Experimental Aquatic Toxicity Data for the 33 Monoazo Pigments and their Analogues
- Appendix E: Ecological Exposure Calculations for Monoazo Pigments
- Appendix F: Exposure Estimates from Use of Cosmetics and Personal Care Products
- Appendix G: Exposure Estimates from Use of Paint
- Appendix H: Oral Exposure Estimates to PR5 and PO5 from Incidental Ingestion of Paper (Toddlers)
- Appendix I: Exposure to of Pigment in Permanent Tattoo Ink (Adult) for PR4, PR112 and PY3
- Appendix J: Benchmark Dose Calculations for PR3, PO5, PR4 and PR53:1