Chemicals Management Plan (CMP) Science Committee Objectives Paper Meeting no. 5; 16-17 November 2016 Integrating New Approach Methodologies within the CMP: Identifying Priorities for Risk Assessment, Existing Substances Risk Assessment Program
Table of Contents
List of Tables and Figures
- Figure 3-1. Criteria used for categorization of the domestic substances list
- Figure 3-2. Mechanisms to identify priorities
- Table 4-1. Available (Q)SAR Models Used for Bacterial Mutagenicity Assessment
- Figure 4-1: Determining the level of priority or concern based on the distance (i.e., the BER) between the bioactivity (converted to a dose) and the estimated exposure
- Figure B-1. General conceptual framework of the RISK21 approach
- Figure B-2. EPA Next Gen framework for risk science
- Figure B-3. Evaluation roadmap for safety assessment of food and ingredients
- Figure B-4a. Flowchart outlining Tier 1 in the proposed framework
- Figure B-4b. Flowchart outlining Tier 2 in the proposed framework
1.0 Meeting Objectives
Health Canada (HC) and Environment and Climate Change Canada (ECCC) are developing a roadmap for integrating new approach methodologies (NAM)Footnote1 with traditional risk assessment. It is expected that input from the Chemicals Management Plan (CMP) Science Committee will be sought at various stages. The roadmap is anticipated to cover multiple aspects of chemical risk assessment including priority-setting, hazard characterization, exposure characterization, and risk characterization. The development of the roadmap will occur in stages and will contribute to both short-term (i.e., CMP3) and longer-term (post-2020) initiatives.
This meeting focuses on new and emerging tools and methodologies with application in identifying risk assessment priorities. The objectives for this meeting are twofold: (1) to seek general input on developing a roadmap for integrating NAM as part of the risk assessment paradigm; and (2) to seek specific input on how to enhance current priority-setting approaches through the incorporation of NAM.
The CMP Science Committee is requested to consider the Charge Questions interspersed throughout this Objectives Paper in the context of the existing substances risk assessment program of the Canadian Environmental Protection Act, 1999 (CEPA).
2.0 Towards a Roadmap for Integrating NAM into the Risk Assessment Program
It has been close to 10 years since the publication of the National Research Council's seminal report on toxicity testing in the 21st century (NRC 2007), and we are now beginning to see the impact of advances in the biological sciences and technologies by way of high-throughput screening (HTS) assays, omics data, and in silico methods in assessing the potential risk to human health from chemicals. Furthermore, the need for developing testing strategies for environmental risk assessment was identified by the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) (ECETOC 2007). These testing strategies include the use of thresholds of toxicological concern (TTCs), validated (quantitative) structure-activity relationships ((Q)SARs), read-across methods, in vitro test protocols, and prioritization of non-vertebrate ecotoxicity tests. In addition, ECETOC (2007) recognized the use of mode of action (MOA) information for specific acting chemicals and the need for addressing data gaps.
Since the publication of the early foundational reports, international interest in using emerging technologies for regulatory risk assessment has advanced significantly. Notably, by request of the Minister of Health (on behalf of the Pest Management Regulatory Agency), the Council of Canadian Academies (CCA) published a report in 2012 titled, "Integrating Emerging Technologies into Chemical Safety Assessment". The expert panel concluded that available evidence suggested the current challenge of lack of toxicity data for many industrial chemicals could be met by embracing an integrated approach to testing and assessment (IATA) of chemicals (CCA 2012).
Some of the emerging approaches to toxicity testing and risk assessment are, in fact, beginning to be applied in practice as illustrated by prototypical case studies (Karmaus et al. 2016; Pham et al. 2016; Shah and Greene 2014), while others are being integrated with existing tools (e.g., physiologically based pharmacokinetic modelling, benchmark dose modelling, categorical regression, and the Organisation for Economic Co-operation and Development [OECD] QSAR toolbox) to enhance and strengthen risk assessment practices. Taking a case study approach to engage the international audience in attendance, a recent European Chemicals Agency (ECHA) topical scientific workshop further addressed the use of data and information from NAM for a number of regulatory uses, including screening and prioritization, read-across techniques, and future risk assessment activities (see ECHA 2016 for main outcomes and conclusions of the workshop). Interestingly, it was acknowledged that there is a need for a better understanding of the taxonomy of methods that could be applied, and that the flexible and innovative use of new approaches may drive change in future regulatory assessment practices (ECHA 2016).
The planned roadmap is envisioned as a strategy that maps short-term and longer-term program objectives with specific existing and emerging NAM tools/applications. The roadmap would outline available or emerging NAM tools and illustrate their respective scientifically sound use in specific decision contexts related to priority setting and risk assessment. The roadmap will guide our efforts to modernize the risk assessment program and facilitate acceptance for the use of emerging technologies in future priority-setting and risk assessment practices, and ultimately strengthen our overall priority-setting and risk assessment regimes. The process of developing the roadmap would also support the identification of knowledge gaps where further research or expertise is needed. Due to the rapid advancement of NAM, the roadmap would be"evergreen" and incorporate additional tools as they are developed.
The Government of Canada has outlined methodology for setting priorities and conducting risk assessment while implementing the existing substances risk assessment program of CEPA. These include the following:
- Initial prioritization process used in 2006 to sort through or "categorize" all 23,000 chemical substances on the domestic substances list (DSL) to identify substances that required further attention under the CMPFootnote2;
- Ongoing process for the identification of risk assessment priorities (IRAP)Footnote3, which is used to update our priorities for risk assessment on an ongoing basis (i.e., post-2006 process to identify "new" priorities); and
- Approaches for conducting risk assessments under the CMP (Risk Assessment Toolbox)Footnote4.
At present, these do not substantively incorporate NAM. However, conceptualized strategies (see Section 2.1) have been described by others, which elaborate on the potential utility of NAM for priority setting and risk assessment. Examples of these strategies, including the US Environmental Protection Agency's (EPA) Next Generation Compliance (Next Gen) project and the Risk Assessment in the 21st Century (RISK21) Project, are summarized in Section 2.1. These conceptual strategies are useful for presenting what types of NAM could be integrated into the IRAP process or the Risk Assessment Toolbox, and are used to support decisions related to risk assessment. However, HC and ECCC are ultimately looking to develop a roadmap that consists of specific and practical applications that can be integrated into the above methodologies for priority setting and risk characterization. An example of a specific tool, namely, a data-driven framework for toxicity testing (Thomas et al. 2013), is presented in Section 2.2. This tool illustrates the types of specific and practical applications that HC and ECCC envision for the roadmap as we work towards our goals of integrating NAM that are fit for purpose for priority setting and risk assessment under CEPA.
2.1 Conceptual Strategies for Incorporating NAM for Priority-Setting/Risk Assessment
Health and Environmental Sciences Institute's International Life Sciences Institute RISK21 Strategy
The RISK21 strategy was developed by the Health and Environment Science Institute (HESI) within the International Life Science Institute (ILSI) through collaboration with scientists from multiple countries, representing governments, academic institutions, non-governmental organizations, as well as industryFootnote5 (Embry et al. 2014). The integrated evaluation strategy outlines considerations that could be used for decision making at various stages in risk assessment (i.e., from prioritization through to quantitative risk assessment). The process includes problem formulation, as well as exposure and toxicity considerations (and their intersection) in order to assess whether additional refinements are needed for a given risk assessment decision (Embry et al. 2014). The exposure-driven risk assessment strategy uses the concept of tiered information sources for both exposure and hazard, with increasing complexity and resources necessary for higher-tiered assessments. The strategy encourages the use of the appropriate tier that allows for the most efficient use of resources in order to arrive at a decision that provides the necessary level of precision for the given decision context (Embry et al. 2014). Embry and colleagues identify Tier 0 through to Tier 3 for hazard characterization, where the TTCs and the (Q)SARs are considered for Tier 0; in vitro assays with appropriate in vitro-to-in vivo (IVIVE) extrapolation models for are considered for Tier 1; and in vivo apical endpoints and MOA analysis are considered as information sources for Tiers 2 and 3, respectively.
Similarly, for exposure characterization, tiered approaches increase in complexity and resource requirements. Examples of Tier 0 approaches included conservative estimates based on limited information (such as physiochemical properties and uses). Exposure approaches for Tiers 1, 2, and 3 include deterministic, probabilistic, and biomonitoring, respectively (Embry et al. 2014). The strategy is reproduced in Figure B-1 in Appendix B. Of note, this fit-for-purpose philosophy is also reflected in the CMP Risk Assessment Toolbox (GoC 2016).
US EPA Next Gen Project: A Framework for the Next Generation of Risk Science
In 2011, the US EPA began work on the Next Gen project, a framework to modernize and develop a risk science paradigm that would incorporate the recent advancements in toxicological and exposure methodology (US EPA 2014). The framework addresses problem formulation, risk assessment, and risk management. Under the risk assessment phase, hazard identification, dose-response assessment, and exposure assessment methods are proposed that make use of new scientific tools and technologies. These include HTS assays and computational methods in biology and toxicology (for hazard identification and dose-response assessment); IVIVE extrapolation methods (for calibration of in vitro and human dosimetry); molecular and genetic epidemiology (to identify toxicity pathway perturbations in population-based studies); and high-performance mass spectrometry (to generate human exposure data for assessing risk) (Krewski et al. 2014). This proposed strategy is reproduced in Figure B-2 in Appendix B.
New Methodologies in Strategies for Safety Assessment of Foods and Food Ingredients
A strategy for considering new methodologies in the assessment of novel foods, food ingredients, and mixtures has been proposed by various scientists from academic and industry groups in Europe. The strategy includes the use of (Q)SAR, TTCs, in vitro assays,and IVIVE models, as well as considering integrated testing strategies based on adverse outcome pathways (Blaauboer et al. 2016). This strategy is reproduced in Figure B-3 in Appendix B.
2.2 Example of a Specific NAM-based Tool
21st Century Data-Driven Framework for Tiered Assessment
A data-driven framework for toxicity testing, led by The Hamner Institutes for Health Sciences, with input from various experts (including researchers from HC), was developed (Thomas et al. 2013). This work incorporated NAM in a tiered approach, with the potential for broad international application across multiple regulatory agencies. The first tier of the proposed framework incorporates the use of high-throughput in vitro assays to assign chemicals into selective and non-selective MOAs. For both cases, in vitro assay concentrations (i.e.,in vitro bioactivity) are converted to applied doses through IVIVE. High-throughput exposure models are then used to estimate human exposure, and a margin of exposure (MOE)-type metric is derived by comparing these estimates with the in vitro bioactivity converted to an applied dose (Thomas et al. 2013). Although the term MOE is used in the publication, in order to distinguish this type of calculation from a traditional calculation of margin of exposure (which generally implies adversity), it is frequently referred to as the bioactivity-to-exposure ratio (BER). If this BER is above a certain established cut-off, no further testing is required and a reference value (e.g., reference dose) can be established based on the Tier 1 testing strategy. If the BER is less than the cut-off, Tier 2 testing is undertaken. Tier 2 testing consists of short-term in vivotranscriptomic studies for non-selective chemicals and targeted in vivo studies to confirm MOAs for selective chemicals. Similarly, MOEs are derived and compared against an established cut-off value; if below this value the substance proceeds to Tier 3-type testing, which consists of standard guideline toxicity studies (Thomas et al. 2013). The flowchart for this framework is reproduced in Figures B-4a and B-4b in Appendix B.
The US EPA's National Center for Computational Toxicology continues to advance the development of a multi-dimensional tiered strategy for NAM application, which includes the use of high-throughput transcriptomics assays that have metabolic competence as an early screening method.
Charge Question 1: Does the CMP Science Committee have input for HC and ECCC as they move forward with developing a roadmap for new approach methodologies and risk assessment modernization?
3.0 Identification of Risk Assessment Priorities
As noted, a second objective for this meeting is to examine the Government of Canada's current methods used to identify priorities for risk assessment (Section 3.0) and identify NAM-based tools that can be used to support, expand, and improve upon the current practices for priority setting.
3.1 Historical and Current Process
The core of the risk assessment work currently being conducted on existing substances under the CMP is comprised of the approximately 4,300 substances identified during Categorization. The Categorization process was completed in 2006, and was based on information available at that time. Figure 3-1 shows the criteria used by HC and ECCC to identify priorities during categorization of the DSL.
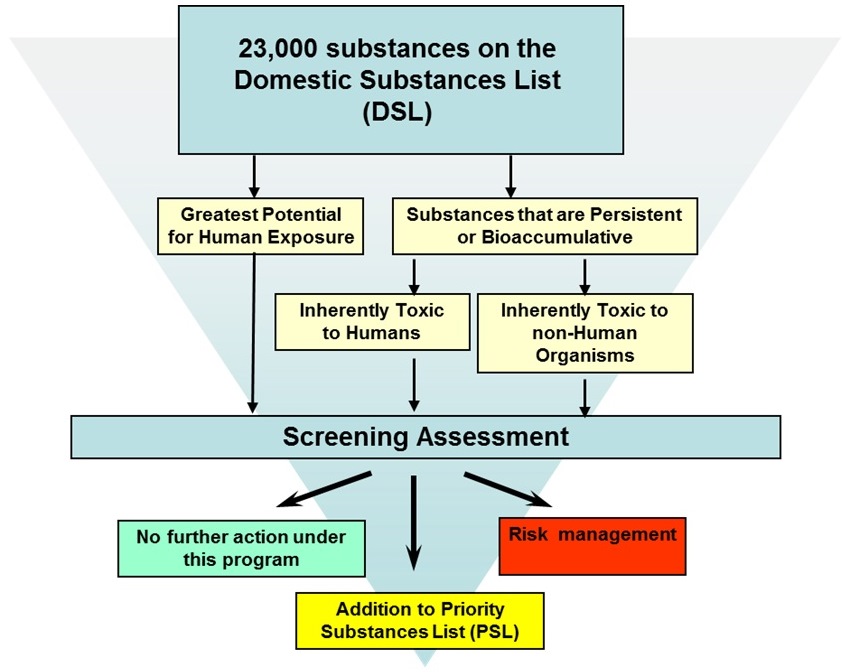
Long description for figure 3-1
Categorization of substances on the Domestic Substances List (DSL) involved the evaluation of these substances by both Health Canada and Environment and Climate Change Canada. These two departments used different criteria to identify substances on the DSL that require further attention by the Government of Canada.
Environment and Climate Change Canada was responsible for looking at all DSL substances, and determined which ones are persistent or bioaccumulative, according to the Persistence and Bioaccumulation Regulations. Substances that were persistent or bioaccumulative and which Environment and Climate Change Canada determined are also inherently toxic to non-human organisms were categorized as requiring further attention through a screening assessment.
Health Canada also looked at these persistent or bioaccumulative substances, and ones that it found are inherently toxic to humans were categorized as requiring further attention through a screening assessment. Health Canada was also responsible for identifying substances on the DSL that present the greatest potential for human exposure, and these substances were also added to the list of categorized substances requiring further attention through a screening assessment.
Once the screening assessment is completed, there are three possible outcomes: 1) no further action under the program; 2) addition to the Priority Substances List; 3) risk management.
In addition to those criteria, HC also identified as priorities any substances that were identified as posing a high hazard to human health (i.e., classified by another agency on the basis of carcinogenicity, mutagenicity, developmental toxicity, or reproductive toxicity, as identified by HC's Simple Hazard Tool). More details on the approaches used can be found on HC's and ECCC's Web sitesFootnote6.
In the fall of 2009, a report on toxic substances prepared by the Office of the Auditor General (OAG) highlighted the need for the program to keep current. The OAG report indicated that "Given that scientific information and research is not static, it is important for Environment Canada and Health Canada to keep up to date with new information regarding toxic substances, such as hazard and routes of exposure" (OAG 2009). In 2011, the CMP Phase 1 Evaluation Report provided further direction:"Develop and implement a formal process and/or criteria for prompting reassessments of substances when new information becomes available" (Health Canada 2011). In response to these reports, in 2014, ECCC and HC published the report, "Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of CEPA" (IRAP), which outlined enhancements in the way new information was acquired, evaluated, and incorporated into forward planning; and formalized the approach to identify risk assessment priorities. The IRAP approach has been applied for two consecutive years, once in 2015 and once in 2016; the approach and results of the 2015 review are available on the Chemical Substances websiteFootnote7.
The IRAP process is divided into three steps: Acquisition, Evaluation, and Action. Each step is explained below.
Acquisition
Figure 3-2 shows the mechanisms for collecting information currently considered in the IRAP approach. Data is collected on an ongoing basis and the review process considers all information available for a given substance to use as the basis for a recommendation (i.e., risk assessment, further data collection/generation, or no further work at this time). The IRAP approach is not prescriptive in the data sources used, and it is anticipated that the data sources considered in future review cycles will be expanded as appropriate. However, due to challenges with staying abreast of new information for large inventories of substances such as the DSL, the data sources currently included in the process are limited to those that are easily accessible and are typically compilations of international decisions, classifications or other tabulated data. This limits the amount and type of emerging science that can be identified through the current process as it is often available in the open literature and not in a database or Microsoft Excel format (e.g., with Chemical Abstracts Service Registry Numbers (CAS RN) and results tabulated). Substances that have been identified for no further work in one review cycle would be considered in a subsequent review cycle should new information become available.
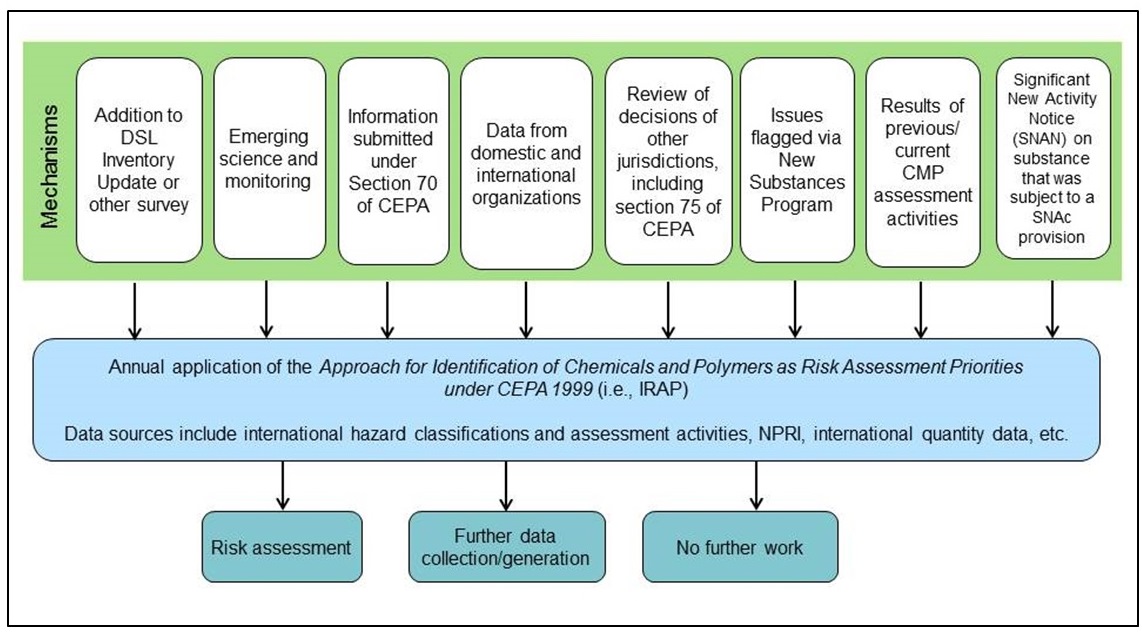
Long description for figure 3-2
Eight mechanisms used to identify priority substances. The mechanisms will lead to risk assessment, further data collection/generation or no further work.
(Abbreviations: NPRI, National Pollutant Release Inventory; SNAc, Significant New Activity provision.)
DSL Inventory Update or other survey:Information-gathering initiatives, such as surveys issued under section 71 of CEPA where Canadian quantities and uses are reported, identify substances with high exposure potential or changing commercial status. This information can be compared with hazard flags to identify substances of potential concern.
Emerging science and monitoring: Program scientists identify emerging science that suggests that a substance is of concern. Monitoring conducted under the CMP is a source of Canadian exposure data used to identify substances measured in humans or the environment. Consideration of NAM data would fit under this mechanism.
Section 70: This section of CEPA requires stakeholders to provide information to ECCC when the results reasonably support the conclusion that the substance is toxic or is capable of becoming toxic, thereby identifying potentially hazardous substances.
Data from domestic and international organizations: A number of domestic and international data sources are included in the review to identify hazard and/or exposure flags. For example, substances with increasing releases to the environment can be identified using data from the ECCC's National Pollutant Release Inventory list. International hazard identification activity is also tracked to see which substances are being prioritized for assessment elsewhere and which substances have increased import/manufacture. To date, specific sources of information used in the IRAP review include:
- Hazard classifications from international agencies (e.g., World Health Organization's International Agency for Research on Cancer (IARC))
- Classifications from the Globally Harmonized System (GHS) of Classification and Labelling of Chemicals (for example, from ECHA's harmonised classification and labelling information)
- International lists of restricted and/or prohibited substances, or other international priorities (e.g., substances of very high concern (SVHCs) from the ECHA Candidate List)
- Notifications to HC concerning substances used in cosmetics
- Non-confidential data reported under the US EPA Chemical Data Reporting (CDR) Rule
- Biomonitoring, environmental monitoring, and surveillance data
Review of decisions of other jurisdictions, including section 75: Regulatory decisions taken in other jurisdictions are also used to flag substances of potential concern or review under section 75.
Issues flagged via ECCC's New Substances program: The New Substances program receives studies from New Substances Notifications that may flag concerns for other similar substances that are in commerce in Canada. This data on analogues can also feed into the identification of future risk assessment priorities.
Results of previous CMP assessment activities:Data used in previous or ongoing CMP assessments that show high hazard may be relevant for other substances not currently identified as priorities for assessment (e.g., analogues).
Significant New Activity Notice: When a Significant New Activity (SNAc) Notice is received on a substance with a significant new activity provision, an assessment is triggered.
Evaluation: Staff at ECCC and HC conduct a periodic analysis of the information that has been acquired. A series of factors are considered and weighed, and judgments are made on the relative importance of different flags. Evaluation can be complex because substances will have entirely different types of information available, and prior activities on a substance are also taken into account. Prioritization decisions are guided by a set of principles and considerations as outlined in the IRAP Approach document (ECCC 2014).
Action: If a substance is identified as a candidate for further work, the following actions may be taken:
- Risk assessment
- The substance may be added to the current risk assessment workplan (for example, similarity to substances in a group already scheduled, opportunity to collaborate with others, urgency) or may be scheduled for future risk assessment activity.
- Further data collection/generation
- The substance may be included in future data collection activities if additional information, alone or in conjunction with the aforementioned actions, would be beneficial to determining the appropriate next step.
- Additionally, internal or external partners may be engaged to collect or generate additional information (including research, monitoring, and/or surveillance).
- No further work at this time
- The hazard and/or exposure flags may not justify further work at this time. These substances could be flagged in future IRAP cycles should new information become available.
Generally, for a substance to be identified as a priority for risk assessment, the process would identify flags for risk - that is, the presence of flags for both hazard and exposure in Canada. If only hazard flags are identified (or if only international exposure data is available), the proposed action would typically be to confirm Canadian exposure through further information gathering. If the results of this information gathering indicate that there is a significant potential for exposure, the substance would then be flagged for risk assessment in the following IRAP cycle.
3.2 Outcomes
The 2015 review identified 28 substances as candidates for risk assessment and 194 substances for further information gathering.
Candidates for risk assessment were identified based on having strong indicators for both human or ecological hazard and Canadian exposure. These substances have been recommended for addition to existing CMP risk assessment plans, and in most cases, would augment groups of substances that were already identified as priorities in CMP3.
Candidates for data gathering were identified based on their hazard and international exposure indicators. They require further data gathering to determine whether they are priorities for risk assessment. Generally, this is because the Canadian commercial status is uncertain. There are a variety of options available to address the data needs including, but not limited to, their addition to future section 71 surveys such as Inventory Updates or targeted surveys, and addition to research and monitoring plans.
Historically, substances have been prioritized with an ecological concern when there is evidence of inherent toxicity and persistence and/or bioaccumulation. However, in the 2016 IRAP review, NAM data were used as an additional line of evidence to identify substances of potential ecological concern. These data was used to flag substances for further data gathering where there was potentially high potency and potentially high use quantity, regardless of any evidence of persistence or bioaccumulation. More specifically, substances with a mechanistic alert flag, experimental data indicating high toxicity and internationally high use quantities were prioritized for data gathering. Although NAM data has been used within IRAP as one line of evidence to identify substances of potential ecological concern, it alone has not been used within this approach to identify substances for risk assessment.
3.3 Lessons Learned
The IRAP process continues to be adapted as experience is gained throughout the reviews. The data sources used in the process are being monitored on an ongoing basis for updates, and the list of sources is expanded as new sources are identified. Due to the labour-intensive nature of this exercise, the most practical data sources are compilations or databases of international decisions, classifications, or other tabulated data (e.g., US CDR or Canadian section 71 quantity data). As a result, it has proven difficult to identify new priorities based on data published in literature. To date, we have not developed a feasible process for reviewing individual scientific publications. We are largely reliant upon other jurisdictions/organizations to review the literature and add to the compilations or databases that we are already referencing, or to identify new substances as priorities in their jurisdiction. Delineating a process by which we could identify and use emerging tools and technologies to collect and collate individual pieces of information would be beneficial to Canada in order to identify priorities for assessment.
In addition, although emerging science has become available to us in the form of databases (e.g., results of HTS testing), which can be incorporated into the IRAP process, there is a need to seek input on how best to interpret such results and apply them to identify new priorities for risk assessment.
While hazard data is universal (i.e., toxicity is not typically dependent on location), exposure can vary between countries. Although the IRAP process attempts to identify exposure flags for the substances, it has been difficult to identify useful exposure information without further data gathering (e.g., conducting a survey of use in Canada). Approaches for identifying exposure information in Canada, or a surrogate for that data, would be helpful in making better recommendations for risk assessment.
4.0 Priority-Setting Moving Forward
After completing the first two cycles of the IRAP process, refinements are being considered to address some of the lessons learned and to improve the overall process. Refinements being considered include: (1) systematic computational approaches to mitigate the labour-intensive nature of the activity and, potentially, incorporate a systematic approach of ranking hazard, exposure and risk; and (2) integration of NAM (from both an exposure and hazard perspective) to better address chemicals that lack traditional data sources and to harness/make use of emerging science.
4.1 Systematic Computational Approaches
In order to improve on the efficiency and effectiveness of future IRAP cycles, a more systematic approach, with computational underpinnings, is sought. Collecting information from new and emerging data sources could be facilitated using computational data collection techniques. From this, a ranking approach could conceivably be applied to identify priorities for assessment without relying as heavily on other jurisdictions/organizations.
In the past, systematic schemes have been developed for use in the existing substances risk assessment program to support prioritization, including: (1) ecological (Robinson et al. 2004) and human health criteria outlined for the DSL categorization process (i.e., SimHaz and ComHaz (simple and complex hazard tools)) (Hughes, Paterson and Meek 2009); and (2) ecological criteria outlined in the ECCC Ecological Risk Classification of Organic Substance Science Approach Document (ECCC 2016). Another illustration is the priority-setting exercise that informed the US EPA 2014 Toxic Substances Control Act Work Plan (US EPA 2012).
Specifically, one element of this systematic scheme involved assigning a score for each chemical based on its hazard, exposure, and potential for persistence and/or bioaccumulation. The overall approach is well documented and the individual prioritization decisions are transparently described through the use of normalized scores.
As noted above, the current process used to identify new risk assessment priorities within the existing substances risk assessment program has been performed manually, and is labour intensive. Moving forward, we are seeking a more efficient and effective process using systematic approaches. Some work has been explored by the departments to incorporate automated data scrapping techniques and other computational tools (e.g., scripts that process the data and calculate risk metrics) to facilitate the prioritization process. It is also envisioned that NAM-based approaches could be integrated into these systematic approaches.
Charge Question 2: Does the CMP Science Committee have input for HC and ECCC for using systematic approaches within IRAP? Are there specific computational approaches that the Committee is aware of that could be used to lessen the resources required to complete IRAP?
4.2 NAM to Help Facilitate Prioritization
As noted earlier, a key driver for the development of the IRAP approach was to formalize the program's approach for IRAP. Incorporation of NAM into the IRAP process will allow for improvements to the current methods for identifying hazard and exposure flags. Typically, the generation of NAM information is less resource intensive than typical in vivo guideline studies, and as such is being generated at an accelerated pace by a variety of organizations worldwide. Because many of the existing substances in Canadian commerce have limited traditional data, NAM provides the opportunity to identify priorities in the absence of traditional data. Outlined below is an introduction to some approaches being considered moving forward for the identification of new priorities for risk assessment (associated reading materials are also provided). The overview serves as a primer on the current state of the Government of Canada knowledge and experience to date in the area of NAM. The Committee is tasked with providing input on the included examples; however, the Committee is also requested to expand the scope of possibilities by identifying new approaches, methodologies, and considerations to improve the current priority-setting process.
Exposure: As noted earlier, a limitation of the current priority-setting process is the availability of tools that would facilitate the process of identifying exposure flags. Additional new screening or testing methods would be beneficial for identifying new risk assessment priorities (e.g., target, suspect and/or non-target screening methods). Non-target screening approaches present another option for IRAP and/or for targeting future research or monitoring activities. Target screening (semi-quantitative or qualitative screening) for known compounds with reference standards has been used to obtain a quick overview of a large number of known contaminants (often in food monitoring and residue analysis) to readily distinguish positive from negative findings below a certain detection limit. Where a chemical is suspected to be present, this screening has also been used to confirm the presence of such (e.g., suspected degradation products).
During non-target screening, no information on a given substance present in a sample is available; rather, the information is derived solely from the chromatograms and mass spectra. These new screening approaches have already been investigated using environmental samples (e.g., drinking and wastewater, dust) (European Commission 2013; Ferrero et al. 2015; Krauss, Singer and Hollender 2010; Miljo-Direktoratet 2013; Rager et al. 2016) and more recently, using human samples (Plassmann et al. 2016). However, these methods also present labour-intensive data evaluation processes, the need for further validation, and other strategies to narrow the thousands of peaks identified in a single sample.
Other new approach methods for exposure may include the development of exposure ranges for a given sub-population for common exposure scenarios (e.g., drinking water, cosmetics, food additive, paints), investigation of other exposure models, and integration of data from various exposure databases. This includes modelling exposures against a range of possible formulations or concentrations based on historic uses or known compositional data, similar to work undertaken by Isaacs and colleagues(2016) for personal care products, and exposure databases such as the Chemical Product Categories Database (Dionisio et al. 2015). These approaches are particularly useful when information on the concentration of a substance for a given use is unknown but found in other jurisdictions. It may also include the ability to compare model outputs against known exposures using human biomonitoring data for aggregated exposures to help prioritize types of exposures, similar to work by the US EPA's high-throughput exposure forecast (ExpoCast™) computer model project and/or investigating other modelling approaches (e.g., Stochastic Human Exposure and Dose Simulation [SHEDS]-Lite exposure model) (US EPA 2016). Lastly, the use of these ‘high-throughput exposure assessment' outcomes could be further explored, including the comparison of outputs to hazard HTS in order to prioritize substances for further risk assessment as part of the IRAP process.
Charge Question 3: Does the CMP Science Committee have suggestions for NAM-based tools that can be considered for further exploration for estimating human and ecological exposure for the purposes of prioritization (IRAP)?
Hazard
In silico models (e.g., [(Q)SAR): In silico models are also a tool that can be integrated into the IRAP process. In silico models currently inform how ecological priorities are selected under IRAP. The identification of mechanistic alerts and high-potency profiling were conducted by ECCC using chemical profiling software (called the ECCC Chemical Profiler). The ECCC Chemical Profiler is a screening tool for persistent, bioaccumulating and toxic (PBT) substances and mechanistic toxicity. It uses Simplified Molecular-Input Line-Entry System input to generate a profile for a chemical based on:
- structural alerts for mechanisms of toxicity,
- (Q)SAR models, and
- available experimental data for selected endpoints.
Many of the relevant outputs from the OECD QSAR Toolbox (2015) and the Laboratory of Mathematical Chemistry's OASIS-CATALOGIC (2014) and OASIS-TIMES (2015) models are incorporated into the ECCC Chemical Profiler software. Empirical data are collected from databases available in the OECD QSAR Toolbox, as well as some custom databases from other sources. The 2016 IRAP review cycle considered the following mechanism-based structural alerts from the Chemical Profiler: protein and DNA binding, aryl hydrocarbon receptor binding, and estrogen receptor and androgen receptor binding affinity. The US EPA's Toxicity Forecaster (ToxCast) data were not used to inform identification of priorities for ecological concern given the difficulty with extrapolation to adverse outcomes.
Although not currently used for identification of human health hazard flags in the IRAP process, HC has extensive experience applying (Q)SAR models as supporting information in human health risk assessments under the CMP.
One proposed method for using (Q)SAR models would be to implement the recently developed International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) M7 guideline for Assessment of DNA Reactive Impurities in Pharmaceuticals (FDA 2015) into the IRAP process. The guideline recommends using two complementary (Q)SAR methodologies to predict the bacterial mutagenicity of an impurity. According to the guideline, one (Q)SAR methodology should be expert rule-based, and the second methodology should be statistical-based. The absence of structural alerts from these two complementary (Q)SAR methodologies is sufficient to conclude that the impurity is of no mutagenic concern, and no further testing is recommended (FDA 2015). HC and ECCC recently published a new methodology based on a chemical space approach that uses large, international toxicological databases to improve confidence in (Q)SAR model predictions (Kulkarni, Barton-Maclaren, and Benfenati 2016). Earlier, we developed a (Q)SAR consensus approach that integrates predictions from a series of (Q)SAR models--each based on a unique predictive algorithm--to arrive at a reliable prediction of mutagenic potential of a chemical (Kulkarni and Barton-Maclaren 2014). As part of our international collaboration, we also built new (Q)SAR models designed for specific classes of organic compounds and with higher predictivity for Ames mutagenicity (Manganelli et al. 2016). Such approaches could be used to examine mutagenic-related hazard flags, and subsequently prioritize these substances for either additional data gathering or as candidates for risk assessment. We recognize that model validation is an important step in the application of any new (or existing) predictive tool; thus, we performed validation exercises in all of the new approaches or models that we have developed. Software that uses both types of modelling approaches are currently used within the program, and examples are provided in Table 4-1. All models listed comply with the OECD (Q)SAR validation principles (OECD 2014).
| Model Name | Methodology Type | Reference |
|---|---|---|
| Derek Nexus (v4.1.0) Sub-Model: Mutagenicity in vitro (bacterium) |
Expert rule-based | Lhasa Limited 2014 |
| Toxtree (v2.6.13) Sub-Model: In vitro Mutagenicity (Ames test) by interstrain Istituto superior di Sanità (ISS) |
Expert rule-based | Ideaconsult Ltd. 2015 |
| Leadscope Model Applier (v2.1) Sub-Model: Genotoxic Expert Alerts Suite (bacterial mutagenicity) |
Expert rule-based | Leadscope 2013 |
| Leadscope Model Applier (v2.1) Sub-Model: Microbial In vitro Gene Mutation (salmonella) |
Statistical-based | Leadscope 2013 |
| OASIS-TIMES (v2.27.19) Sub-Model: Ames Mutagenicity S9-Activated |
Hybrid (expert rule-based + statistical-based structure feature recognition) coupled with a metabolic simulator | Laboratory of Mathematical Chemistry 2015 |
| MultiCASE CASEUltra (salmonella mutagenicity mixed-strain model) |
Statistical-based | MultiCASE Inc. 2015 |
| MultiCASE CASEUltra (expert rules for bacterial mutagenicity) |
Expert rule-based | MultiCASE Inc. 2015 |
| ACD/Percepta (salmonella Ames model) |
Statistical-based | Advanced Chemistry Development, Inc. 2016 |
| CAESAR (salmonella Ames model) |
Statistical-based | VEGA 2015 |
The examples given in Table 4-1 focus on mutagenicity prediction; however, for other endpoints of interest for priority setting, there are other models available. Rybacka and colleagues (2014) evaluated the utility of freely available and commercial predictive models to prioritise toxicity testing of low-volume industrial chemicals under ECHA's European Regulation on Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) program. The endpoints related to classification and labelling are discussed, including carcinogenicity, mutagenicity, and reproductive toxicity. During their validation analysis, reliable predictions were obtained for carcinogenicity; however, other endpoints (such as reproductive toxicity) were determined to be less reliable. Overall, more guidance for submitters was necessary in order to incorporate the tools for REACH (Rybacka, Rudén, and Andersson 2014). As part of our collaborative endeavours, we have also worked on developing approaches to build confidence in the use of predictive tools for complex endpoints, such as developmental toxicity (Marzo et al. 2016).
High-throughput in vitro assays:High-throughput in vitro assays are used to rapidly generate concentration-response curves for a range of biological activity, across a broad range of concentrations, for large numbers of compounds. An example of such an initiative to generate this type of data is the US EPA ToxCast program. To date, the combined efforts of ToxCast and the Toxicology in the 21st Century (Tox21) consortiumFootnote8 have tested over 8000 chemicals.
Perhaps one of the most developed approaches for applying the high-throughput in vitro assays to inform a regulatory program is the recent development of the ToxCast-based Estrogen Receptor (ER) Bioactivity model for use in the US EPA Endocrine Disruptor Screening Program (EDSP). Briefly, the results from the 18 ToxCast ER assays are integrated into a computational model that can discriminate bioactivity from assay-specific interference and responses related to cytotoxicity (Browne et al. 2015). The model provides a score, ranging from 0 (inactive substances) to 1 (bioactivity for 17β-estradiol). A score of 0 is considered "inactive"; 0-0.1, "inconclusive"; and greater than or equal to 0.1, "active". The ER Bioactivity model results for tested chemicals are available onlineFootnote9. This model was evaluated against a set of reference chemicals for which guideline-type studies were available for the in vivo uterotrophic assay, and it was shown to be highly predictive (Browne et al. 2015). After discussion at various scientific advisory panel meetings, the EPA"intends a future recipient of an EDSP test order to be able to satisfy the screening requirement for ER, ERTA [estrogen receptor transactivation], and uterotrophic in one of three ways: (1) cite existing ToxCast 'ER Model' for bioactivity data as 'other scientifically relevant information' (where available); (2) generate new data relying on the 18 ER high-throughput assays and the ToxCast 'ER Model' for bioactivity; or (3) generate their own data using the current Tier 1 ER binding, ERTA, and uterotrophic assays" (US EPA 2015). Therefore, the intent is to accept the ER Model as an alternative for select Tier 1 assays. Development continues on other ToxCast-based models, including the androgen receptor (AR), steroidogenesis (STR), and thyroid (THY) bioactivity models for the EDSP program (US EPA 2015).
Exploratory work has also been conducted on developing a ToxCast bioactivity-based model for predicting hepatotoxicity. Liu and colleagues (2015) examined ToxCast in vitro bioactivity and molecular structure along with other descriptors to examine associations with in vivohepatotoxicity in order to develop a model to predict chronic hepatotoxicity in rats.
Health Canada is considering incorporation of the ER Model results as a hazard flag, and if significant exposure flags are also present, the substances could potentially be considered as a candidate for additional data gathering or risk assessment. As other models based on in vitro bioactivity become available, they could also be incorporated within the IRAP process.
ECCC is also interested in scoping the applicability of HTS data and results from the EPA ER model to inform ecological assessment initiatives. While it is recognized that most data from the US EPA ToxCast and the Tox21 programs are based on human health lineages, using all available information (including mammalian data) may facilitate the discovery and understanding of mechanisms of action of ecological relevance, thereby targeting the most appropriate organisms for testing (ECETOC 2007; Worth et al. 2014).
A number of NAM-based approaches and tools have been summarized by the European Commission in a recent report on alternative methods in regulatory toxicology (Worth et al. 2014). Many NAM described therein have shared human health and ecological applications; however, to date, they been primarily developed from a human health perspective. There is an opportunity to build on the current work and consider how these NAM can be used to inform, improve, and accelerate ecological prioritization and risk assessment goals over the short term (i.e., CMP3) and longer term (post-2020).
Two promising NAM from an ecological perspective include the TTCs and the Adverse Outcome Pathway (AOP) framework. The TTC approach is based on the premise that there is an exposure limit for chemicals below which no significant risk to the environment is expected. These thresholds can be set using different data sets, and there may be application for in vitro screening data sets to be useful in this regard. The AOP framework imparts biological understanding and relevance to mechanistic endpoints that are usually measured at the gene or cell levels. Essentially, AOPs construct a plausible linkage between a molecular-initiating event and an adverse outcome at a biological level of organization relevant to risk assessment.
Work on the suitability of these NAM-based approaches to the ecological context is ongoing through the OECD and other bodies. The Committee envisions that (Q)SARs and in vitro screens will become a fundamental part of the approaches, particularly as there is an increasing focus on considering "mechanisms of action" in hazard assessments. Consequently, the ability to reliably make IVIVE models to ecological species (e.g., fish) is an important component in any ecological prioritization or assessment roadmap that incorporates in vitro data.
Charge Question 4a: Does the CMP Science Committee have suggestions for NAM-based tools that can be considered for hazard identification for prioritization (IRAP)? How might these NAM be incorporated in the decision points of the IRAP process?
Charge Question 4b: Given that biological pathways are often conserved across species, does the CMP Science Committee have input on how human health-based NAM can inform ecological IRAP approaches and vice versa?
Charge Question 4c: Considering the hazard NAM identified by HC and ECCC and those identified by the Science Committee (Question 4a), what near-term opportunities and challenges are associated with the implementation of NAM for the identification of new priorities for risk assessment?
Risk-based Ranking
Using in vitro assays and in silico tools together as a method for identifying hazard flags could also be an important approach for refining the IRAP process. However, the presence or absence of an in vitro hazard flag alone does not provide a complete picture for risk-based prioritization. The in vitro assays, when examined alone, do not provide an indication of what exposures would be necessary to induce the change in observed bioactivity. Likewise, with the mentioned (Q)SAR models, these alone do not provide information on dose response for the respective hazard flag.
One promising risk-based metric would be the BER approach (as described in Section 2.2) that uses in vitro bioactivity extrapolated to an applied dose through IVIVE models and compares it against an exposure estimate. For the purposes of IRAP, if the required data is available for deriving a BER, then the magnitude could be used as method for prioritizing substances for either additional data gathering or candidates for higher tier risk assessment. In Figure 4-1, the distance between bioactivity and the estimated exposure (i.e., the BER) dictates if the chemical is a priority for further work. It is also envisioned that where the BER exceeds a large cut-off (suggesting low hazard potential and low potential for exposure), the approach could serve as a broad-based assessment approach consistent with a Type 2 approach within the CMP Risk Assessment Toolbox. This low-tier assessment approach is being explored as an element of the broader risk assessment roadmap.
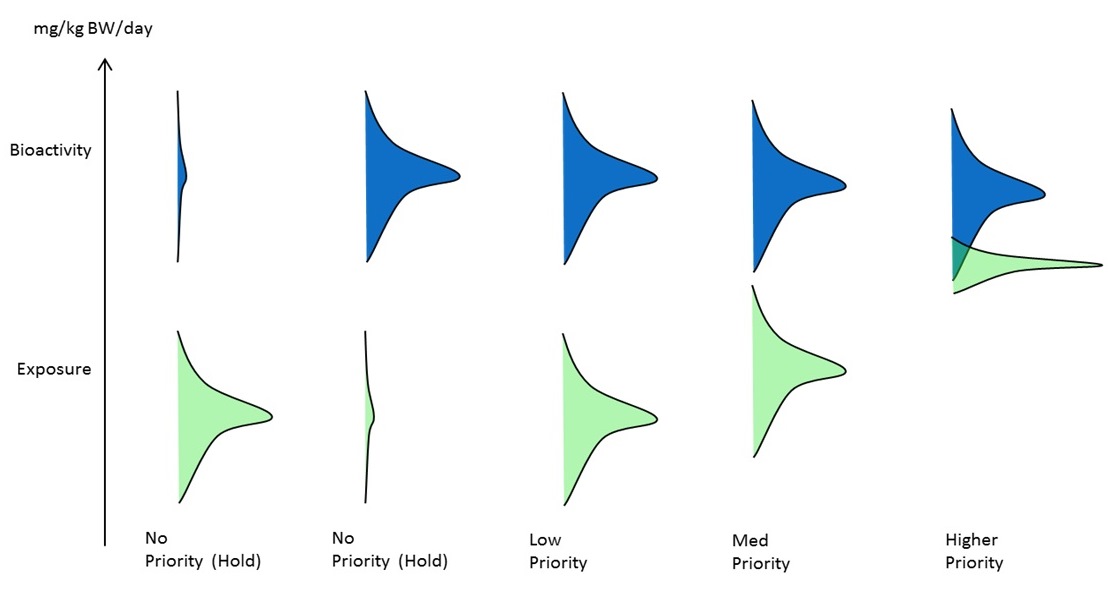
Long description for figure 4-1
The level of priority or concern of a substance can be determined using a bioactivity exposure ratio (BER) approach. The approach uses in vitro bioactivity extrapolated to an applied dose through in vitro to in vivo extrapolation and compares it against an exposure estimate. The relationship between the bioactivity converted to a dose (mg/kg BW/day) and exposure dictates if the substance is a priority.
As the distance between the bioactivity and estimated exposure decreases, the substance becomes higher priority. Substances for which either the bioactivity or estimated exposure is low, are not considered a priority.
(Source: adapted personal communication from D. Dix, US EPA, October 2014, unreferenced.)
Charge Question 5a: Does the CMP Science Committee have suggestions for NAM-based tools that can be considered for developing risk metrics for prioritization (IRAP)? How might these NAM be incorporated in the decision points of the IRAP process?
Charge Question 5b: Considering the risk metric NAM identified by the departments and those identified by the Science Committee (Question 5a), what near-term opportunities and challenges are associated with the implementation of NAM for the identification of new priorities for risk assessment?
References
Advanced Chemistry Development, Inc. ACD/Labs Percepta Platform [prediction module]-latest version v2016.1. 2016. Toronto (ON): Advanced Chemistry Development, Inc.
Blaauboer BJ, Boobis AR, Bradford B, Cockburn A, Constable A, Daneshian M, Edwards G, Garthoff JA, Jeffery B, Krul C, Schuermans J. 2016. Considering new methodologies in strategies for safety assessment of foods and food ingredients. Food Chem Toxicol 91:19-35.
Browne P, Judson RS, Casey WM, Kleinstreuer NC, Thomas RS. 2015. Screening chemicals for estrogen receptor bioactivity using a computational model. Environ Sci Technol 49(14):8804-8014.
[CCA] Council of Canadian Academies. 2012. Integrating emerging technologies into chemical safety assessment: the expert panel in the integrated testing of pesticides.
Dionisio KL, Frame AM, Goldsmith MR, Wambaugh JF, Liddell A, Cathey T, Smith D, Vail J, Ernstoff AS, Fantke P, Jollie, Ol, Judston RS. 2015. Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Environment Toxicol Rep 2:228-237.
[ECCC] Environment and Climate Change Canada. 2014. Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA).
[ECCC] Environment and Climate Change Canada. 2016. Ecological risk classification of organic substances.
[ECETOC] European Centre for Ecotoxicology and Toxicology of Chemicals. 2007. Intelligent testing strategies in ecotoxicology: mode of action approach for specifically acting chemicals. Brussels, Belgium: ECETOC.
[ECHA] European Chemicals Agency. 2016. New approach methodologies in regulatory science: proceedings of a scientific workshop. Helsinki, Finland, 19-20 April 2016.
Embry MR, Bachman AN, Bell DR, Boobis AR, Cohen SM, Dellarco M, Dewhurst IC, Doerrer NG, Hines RN, Moretto A, Pastoor TP, Phillips RD, Rowlands JC, Tanir JY, Wolf DC, Doe JE. 2014. Risk assessment in the 21st century: roadmap and matrix. Crit Rev Toxicol 44(S3):6-16.
European Commission. 2013. State-of-the-art of screening methods for the rapid identification of chemicals in drinking water. EUR 26155 EN. June 2013.
[FDA] US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. 2015. M7 assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk: guidance for industry.
Ferrero PG, Schymanski EL, Bletsou AA, Aalizadeh R, Hollender J, Thomaidis NS. 2015. Extended suspect and non-target strategies to characterize emerging polar organic contaminants in raw wastewater with LC-HRMS/MS. Environ Sci Technol 49(20):12333-12341. DOI: 10.1021/acs.est.5b03454.
[GoC] Government of Canada Chemicals Substances. [modified 2016 May 30]. The risk assessment toolbox. Ottawa (ON): Government of Canada. [accessed 2016 Oct 7].
Health Canada. 2011. Chemicals Management Plan (CMP) horizontal evaluation: final report. Ottawa (ON): Health Canada. [accessed 2016 Oct 7].
Hughes K, Paterson J, Meek ME. 2009. Tools for the prioritization of substances on the Domestic Substances List in Canada on the basis of hazard. Regulatory Toxicology and Pharmacology 55:382-393.
Ideaconsult Ltd. 2015. Toxtree (Toxic Hazard Estimation by decision tree approach) [prediction module v2.6.13]. Sofia (BG): Ideaconsult Ltd.
Isaacs KK, Goldsmith M, Egeghy P, Phillips K, Brooks R, Hong T, Wambaugh, JF. 2016. Characterization and prediction of chemical functions and weight fractions in consumer products. Toxicology Reports 3: 723-732.
Karmaus AL, Filer DL, Martin MT, Houck KA. 2016. Evaluation of food-relevant chemicals in the ToxCast high-throughput screening program. Food Chem Toxicol 92:188-196.
Krauss M, Singer H, Hollender J. 2010. LC-high resolution MS in environmental analysis: from target screening to the identification of unknowns. Anal Bioanal Chem 397: 943. doi:10.1007/s00216-010-3608-9.
Krewski D, Westphal M, Andersen ME, Paoli GM, Chiu WA, Al-Zoughool M, Croteau MC, Burgoon LD, Cote I. 2014. A framework for the next generation of risk science. Environ Health Perspect 122(8):796-805.
Kulkarni SA, Barton-Maclaren TS. 2014. Performance of (Q)SAR models for predicting Ames mutagenicity of aryl azo and benzidine based compounds. J Env Sci Health, Part C: Env Car Eco Rev 32(1):46-82.
Kulkarni SA, Barton-Maclaren TS, Benfenati E. 2016. Improving the confidence in (Q)SAR predictions under the Canada's Chemicals Management Plan-a chemical space approach (restricted access). SAR QSAR Environ Res 27 (in press).
Leadscope. 2013. Model Applier[prediction module v2.1]. Columbus, OH: Leadscope.
Lhasa Limited. Derek Nexus [toxicity prediction module]. 2014. v4.1.0. Leeds (UK): Lhasa Limited.
Liu J, Mansouri K, Judson RS, Martin MT, Hong H, Chen M, Xu X, Thomas RS, Shah I. 2015. Predicting hepatotoxicity using ToxCast in vitro bioactivity and chemical structure. Chem Res Toxicol 28(4):738-51.
Manganelli S, Benfenati E, Manganaro A, Kulkarni S, Barton-Maclaren TS, Honma M. 2016. New quantitative structure-activity relationship models improve predictability of Ames mutagenicity for aromatic azo compounds. Toxicol Sci 153(2):316-326.
Marzo M, Roncaglioni A, Manganaro A, Benfenati E, Kulkarni S, Barton-Maclaren TS, Wu S, Lester C. 2016. Integrating in silico models to enhance predictivity for developmental toxicity (restricted access). Toxicology 370:127-137.
Miljo-Direktoratet. 2013. Non-target screening--a powerful tool for selecting environmental pollutants. M-27/2013.
MultiCASE Inc. 2015. CASEUltra [prediction module for toxicity and bioactivity of chemicals v.1.4].
[NRC] National Research Council of the National Academies of Sciences, Engineering, and Medicine. 2007. Toxicity testing in the 21st century: a vision and a strategy. Washington (DC): The National Academies Press.
[OAG] Office of the Auditor General of Canada. 2009. 2009 fall report of the Commissioner of the Environment and Sustainable Development.
[OASIS-CATALOGIC] Laboratory of Mathematical Chemistry. 2014. [Environmental fate and ecotoxicity model v.5.11.15]. Bourgas, BG: Laboratory of Mathematical Chemistry.
[OASIS-TIMES] Laboratory of Mathematical Chemistry. 2015. TIssue MEtabolism Simulator [prediction module v.2.27.19]. Bourgas, BG: Laboratory of Mathematical Chemistry.
[OECD] Organisation for Economic Co-operation and Development. 2014. Guidance document on the validation of (quantitative)structure-activity relationships [(Q)SAR] models. Paris, France: OECD, Environment Directorate.
OECD QSAR Toolbox. 2015. [v3.2]. Available from: http://www.oecd.org/chemicalsafety/risk-assessment/theoecdqsartoolbox.htm.
Pham N, Iyer S, Hackett E, Lock BH, Sandy M, Zeise L, Marty M. 2016. Using ToxCast to explore chemical activities and hazard traits: a case study with ortho-phthalates. Toxicological Sciences 151(2):286-301. doi:10.1093/toxsci/kfw049.
Plassmann MM, Tengstrand E, Aberg KM, Benskin JP. 2016. Non-target time trend screening: a data reduction strategy for detecting emerging contaminants in biological samples. Anal Bioanal Chem 408:4203-4208.
Rager JE, Strynar MJ, Liang S, McMahen RL, Richard AM, Grulke CM, Wambaugh JF, Isaacs KK, Judson R, Williams AJ, Sobus JR. 2016. Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring. Environment International 88:269-280.
Robinson P, MacDonald D, Davidson N, Okonski A, Sene A. 2004. Use of quantitative structure activity relationships (QSARs) in the categorization of discrete organic substances on Canada's Domestic Substances List (DSL). International Society for Environmental Information Sciences. 2: 122-130.
Rybacka A, Rudén C, Andersson PL. 2014. On the use of in silico tools for prioritising toxicity testing of the low-volume industrial chemicals in REACH. Basic Clin Pharmacol Toxicol 115(1):77-87.
Shah F, Greene N. 2014. Analysis of Pfizer compounds in EPA's ToxCast chemicals-assay space. Chemical Research in Toxicology 27(1):86-98.
Thomas RS, Philbert MA, Auerbach SS, Wetmore BA, Devito MJ, Cote I, Rowlands JC, Whelan MP, Hays SM, Andersen ME, Meek MEB, Reiter LW, Lambert JC, Clewell HJ, Stephens ML, Zhao QJ, Wesselkamper SC, Flowers L, Carney EW, Pastoor TP, Petersen DD, Yauk CL, Nong A. 2013. Incorporating new technologies into toxicity testing and risk assessment: moving from 21st century vision to a data-driven framework. Toxicol Sci 136(1):4-18.
US EPA. 2012. TSCA work plan chemicals: methods document. Environmental Protection Agency, Office of Pollution Prevention and Toxics, February 2012.
US EPA. 2014. Next Generation Risk Assessment: Incorporation of Recent Advances in Molecular, Computational, and Systems Biology (Final Report). Environmental Protection Agency, EPA/600/R-14/004.
US EPA. 2016. Rapid chemical exposure and dose research: evaluating high-throughput exposure predictions.
VEGA. 2015. CAESAR [prediction platform]. Milano, Italy: Instituto di Ricerche Farmacologiche Mario Negri Milano.
Worth A, Barroso J, Bremer S, Burton J, Casati S, Coecke S, Corvi R, Desprez B, Dumont C, Gouliarmou V, Goumenou M, Gräpel R, Griesinger C, Halder M, Janusch Roi A, Kienzler A, Madia F, Munn S, Nepelska M, Paini A, Price A, Prieto P, Rolaki A, Schäffer M, Triebe J, Whelan M, Wittwehr C, Zuang V. 2014. Alternative methods for regulatory toxicology-a state-of-the-art review. Ispra (IT): European Commission Joint Research Centre Report EUR 26797 EN.
Appendices
Appendix A: Key Reading Material
Current IRAP Process
- [ECCC] Environment and Climate Change Canada and Health Canada. 2014. Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA).
- [ECCC] Environment and Climate Change Canada and Health Canada. 2015. Identification of risk assessment priorities: results of the 2015 review.
Workshop Summary
- European Chemicals Agency. 2016. New approach methodologies in regulatory science: proceedings of a scientific workshop. Helsinki, Finland, 19-20 April 2016.
Examples of Strategies for Integrating of NAMs for Priority-setting and Risk Assessment
- Krewski D, Westphal M, Andersen ME, Paoli GM, Chiu WA, Al-Zoughool M, Croteau MC, Burgoon LD, Cote I. 2014. A framework for the next generation of risk science. Environ Health Perspect 122(8):796-805.
- Embry MR, Bachman AN, Bell DR, Boobis AR, Cohen SM, Dellarco M, Dewhurst IC, Doerrer NG, Hines RN, Moretto A, Pastoor TP, Phillips RD, Rowlands JC, Tanir JY, Wolf DC, Doe JE. 2014. Risk assessment in the 21st century: roadmap and matrix. Crit Rev Toxicol 44(S3):6-16.
- Blaauboer BJ, Boobis AR, Bradford B, Cockburn A, Constable A, Daneshian M, Edwards G, Garthoff JA, Jeffery B, Krul C, Schuermans J. 2016. Considering new methodologies in strategies for safety assessment of foods and food ingredients. Food Chem Toxicol 91:19-35.
Examples of Specific NAM-based Tools
- Thomas RS, Philbert MA, Auerbach SS, Wetmore BA, Devito MJ, Cote I, Rowlands JC, Whelan MP, Hays SM, Andersen ME, Meek MEB, Reiter LW, Lambert JC, Clewell HJ, Stephens ML, Zhao QJ, Wesselkamper SC, Flowers L, Carney EW, Pastoor TP, Petersen DD, Yauk CL, Nong A. 2013. Incorporating new technologies into toxicity testing and risk assessment: moving from 21st century vision to a data-driven framework. Toxicol Sci 136(1):4-18.
- Browne P, Judson RS, Casey WM, Kleinstreuer NC, Thomas RS. 2015. Screening chemicals for estrogen receptor bioactivity using a computational model. Environ Sci Technol 49(14):8804-14.
- Liu J, Mansouri K, Judson RS, Martin MT, Hong H, Chen M, Xu X, Thomas RS, Shah I. 2015. Predicting hepatotoxicity using ToxCast in vitro bioactivity and chemical structure. Chem Res Toxicol 28(4):738-51.
- [FDA] US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. 2015. M7 assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk: guidance for industry.
Appendix B: Illustrations of Existing Frameworks/Strategies
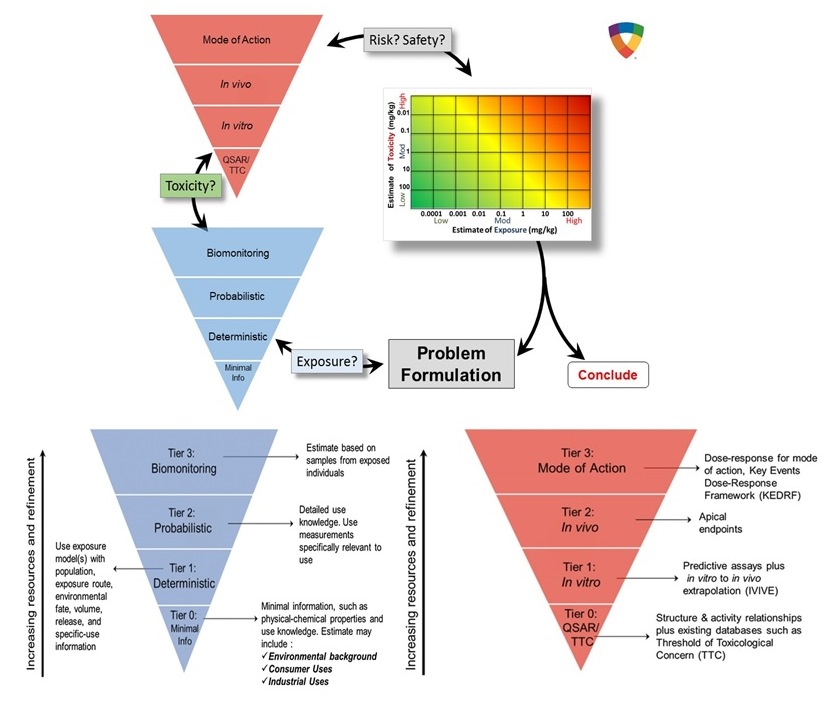
Long description for figure B-1
The RISK21 strategy approach framework outlines considerations that could be used for decision making at various stages in risk assessment (meaning from prioritization through to quantitative risk assessment). The exposure-driven risk assessment strategy uses the concept of tiered information sources for both exposure and hazard with increasing complexity and resources necessary for higher tiered assessments. The process includes problem formulation, exposure and toxicity considerations to help obtain a risk assessment decision.
The strategy encourages the use of the appropriate tier to allow for the most efficient use of resources to arrive at a decision that provides the necessary level of precision for the given decision context. An inverted pyramid depicts the increase in complexity and resource requirements with each tier (tier 0 to tier 3). This applies to both hazard and exposure characterization.
For hazard characterization, the threshold of toxicological concern (TTC) and the Quantitative Structure Activity Relationships (QSAR) are considered for Tier 0, in vitro assays with appropriate in vitro to in vivo extrapolation (IVIVE) for Tier 1 and in vivo apical endpoints and mode of action analysis are considered Tier 2 and 3 information sources, respectively.
For exposure characterization, Tier 0 approaches include conservative estimates based on limited information such as physiochemical properties and uses. Tier 1, 2 and 3 exposure approaches include deterministic, probabilistic and biomonitoring, respectively.
(Source: reproduced from Embry et al. 2014).
Long description for figure B-2
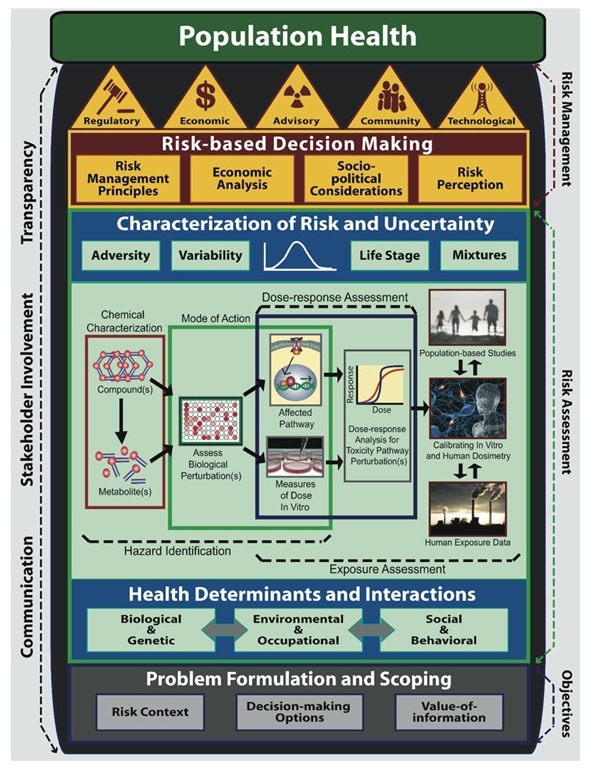
The NextGen framework modernizes and develops a risk science paradigm that would incorporate the recent advancements in toxicological and exposure methodology. The framework addresses problem formulation, risk assessment and risk management.
The problem formulation phase includes determining the risk context, options available for decision making and the value of information.
In the risk assessment phase, hazard identification, dose-response assessment, and exposure assessment methods that make use of new scientific tools and technologies are proposed. These include high-throughput screening assays and computational methods in biology and toxicology for hazard identification and dose-response assessment; in vitro to in vivo extrapolation methods for calibration of in vitro and human dosimetry; molecular and genetic epidemiology to identify toxicity pathway perturbations in population-based studies; and high-performance mass spectrometry to generate human exposure data, to assess risk. Risk and uncertainty are characterized, and a risk-based decision is made.
Risk management principles, economic analysis, risk perception and socio-political considerations inform risk management actions. The framework depicts communication, stakeholder involvement and transparency contributing to all phases of risk-based science.
(Source: reproduced from Krewski et al. 2014).
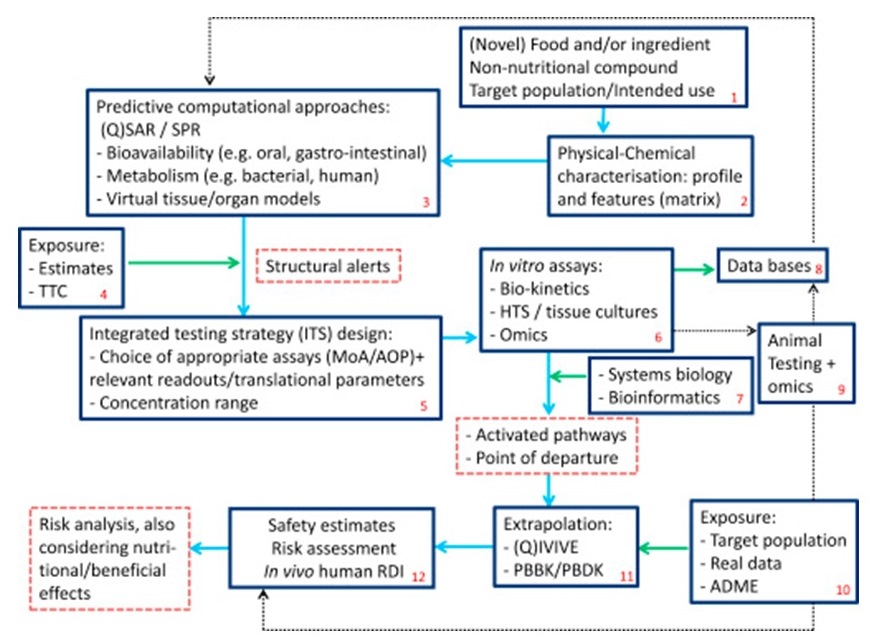
Long description for figure B-3
A roadmap describes a strategy for considering new methodologies in the assessment of novel foods, food ingredients and mixtures. The strategy includes the use of Quantitative Structure Activity Relationships (Q)SAR, threshold of toxicological concern (TTC), in vitro assays, in vitro to in vivo extrapolation (IVIVE) and considerations of integrated testing strategies based on adverse outcome pathways.
The main stream in the evaluation is comprised of seven boxes, additional boxes represent information related to exposure, and at various points, the outcome of a previous box is provided. Feedback routes in the roadmap exist to allow for additional information to (re)consider the next steps. The flow of the evaluation presented in the roadmap is as follows:
- (Novel) Food and/or ingredient Non-nutritional compound Target population/intended use
- Physical-Chemical characterization: profile and features (matrix)
- Predictive computational approaches:
- (Q)SAR/ surface plasmon resonance (SPR)
- Bioavailability (for example, oral, gastro-intestinal)
- Metabolism (for example, bacterial, human)
- Virtual tissue/organ models
- Exposure Estimates/TTC
Structural alerts outcome - Integrated testing strategy (ITS) design:
- Choice of appropriate assays (Mode of Action (MoA)/Adverse Outcome Pathway (AOP)) + relevant readouts/translational parameters
- Concentration range
- In vitro assays:
- Bio-kinetics
- HTS/Tissue cultures
- Omics
- Systems biology and bioinformatics (exposure related)
- Databases (exposure related)
- Animal testing and omics (exposure related)
(Activated pathways and Point of Departure outcome) - Exposure-related:
- Target population
- Real data
- absorption, distribution, metabolism, and excretion (ADME)
- Extrapolation:
- (Q)IVIVE
- Process-based domain knowledge (PBDK)/ , physiologically based pharmacokinetic (PBBK)
- Safety estimates Risk assessment in vivo human recommended dietary intake (RDI)
(Risk analysis, also considering nutritional/beneficial effects outcome)
Numbers represent the flow; solid blocks with blue arrows represent the main stream. The information provided by the blocks with green arrows is related to exposure. The dotted blocks are "outcomes" of the previous blocks; dotted lines are feedback routes, and may provide additional information to (re)consider next steps.
(Source: reproduced [open access] from Blaauboer et al. 2016).
Abbreviations: ADME, absorption, distribution, metabolism, and excretion; AOP, adverse outcome pathway; HTS, high-throughput screening; MoA, mode of action; PBDK, process-based domain knowledge; PBBK, physiologically based pharmacokinetic (model); (Q)IVIVE, quantitative/qualitative in vitro-to-in vivo (extrapolation models); (Q)SAR, quantitative/qualitative structure-activity relationship; RDI, recommended dietary intake; SPR, surface plasmon resonance (biosensors); TTC, threshold of toxicological concern.
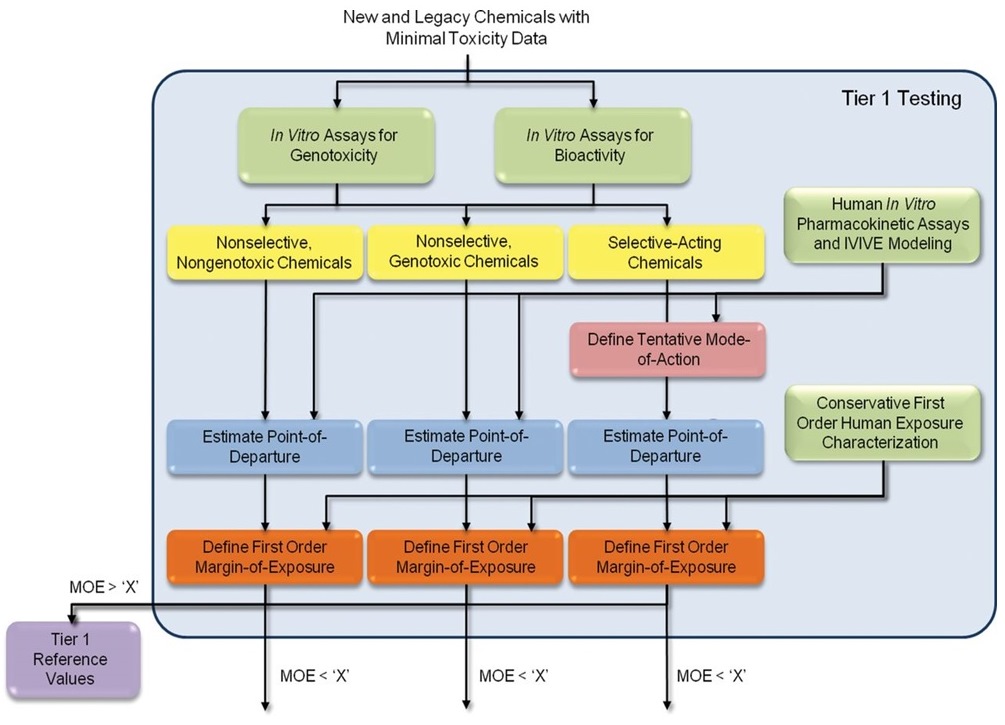
Long description for figure B-4a
A flowchart outlining the Tier 1 testing in the proposed 21st Century Data-Driven framework. The first row of boxes illustrates the Tier 1 data package that includes experimental data and computational modeling results which serve as inputs into the framework. The next step separates the substances into chemical categories determined by the in vitro genotoxicity assays and the high-throughput in vitro screening assays. The categories are (1) nonselective, nongenotoxic chemicals, (2) nonselective, genotoxic chemicals, and (3) selective-acting chemicals. For the selective-acting chemicals, the tentative mode of action is determined based on which high-throughput in vitro assays were selectively activated or inhibited. The point of departure and margin of exposure (MOE) using additional pharmacokinetic and exposure information, respectively, are estimated. For those chemicals with a MOE greater than a defined cut-off, no further testing is performed, and Tier 1 reference values are published. Chemicals with a MOE less than the cut-off are advanced to Tier 2.
Green boxes illustrate Tier 1 data package that includes experimental data and computational modelling results that serve as inputs into the framework. Yellow boxes are separate chemical categories determined by the in vitro genotoxicity assays and the high-throughput in vitro screening assays. For the selective chemicals, the red box represents the determination of the tentative MOA based on which high-throughput in vitro assays were selectively activated or inhibited. Blue and orange boxes represent the estimation of the point of departure and MOE using additional pharmacokinetic and exposure information, respectively. For those chemicals with an MOE greater than a defined cut-off, no further testing is performed, and Tier 1 reference values are published. Chemicals with an MOE less than the cut-off advance to Tier 2.
(Source: reproduced [open access] from Thomas et al. 2013).
Abbreviations: IVIVE, in vitro-to-in vivo; MOE, margin of exposure.
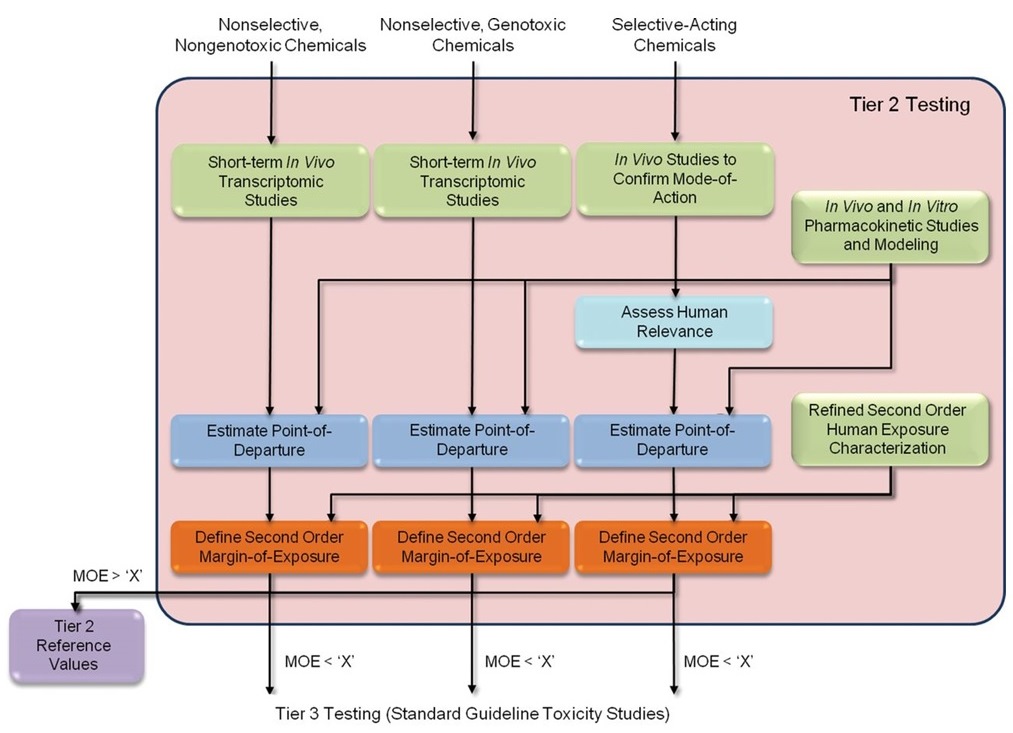
Long description for figure B-4b
A flowchart outlining the Tier 2 testing in the proposed 21st Century Data-Driven framework. The Tier 2 data package includes experimental data and computational modeling results that serve as inputs into the framework. The chemical categories determined by the in vitro genotoxicity assays and the high-throughput in vitro screening assays are retained from Tier 1 ((1) nonselective, nongenotoxic chemicals, (2) nonselective, genotoxic chemicals, and (3) selective-acting chemicals). For the selective chemicals, the human relevance of the mode of action is determined. The point of departure and margin of exposure (MOE) using expanded pharmacokinetic and exposure information, respectively, are estimated. For those chemicals with a MOE greater than a defined cut-off, no further testing is performed, and Tier 2 reference values are published. Chemicals with a MOE less than the cut-off are advanced to Tier 3. Tier 3 consists of standard guideline toxicity studies.
Green boxes illustrate the Tier 2 data package that includes experimental data and computational modelling results that serve as inputs into the framework. The chemical categories determined by the in vitro genotoxicity assays and the high-throughput in vitro screening assays are retained from Tier 1. For the selective chemicals, the light blue box represents the determination of the human relevance of the MOA. The blue and orange boxes represent the estimation of the point of departure and MOE using expanded pharmacokinetic and exposure information, respectively. For those chemicals with an MOE greater than a defined cut-off, no further testing is performed, and Tier 2 reference values are published. Chemicals with an MOE less than the cutoff are advanced to Tier 3.
(Source: reproduced [open access] from Thomas et al. 2013).
Abbreviation: MOE, margin of exposure.