Draft assessment - Terpenes and terpenoids - Tricyclic sesquiterpenes and triterpenoids
Official title: Draft Assessment - Terpenes and Terpenoids - Tricyclic Sesquiterpenes and Triterpenoids
Environment and Climate Change Canada
Health Canada
March 2025
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted an assessment of 14 substances hereinafter referred to as the Tricyclic Sesquiterpenes and Triterpenoids Group. The Chemical Abstracts Service Registry Numbers (CAS RNsFootnote 1), the subgroups, Domestic Substances List (DSL) names, and common names used in this assessment are listed in the table below.
| CAS RN | Subgroup | DSL name | Common name |
|---|---|---|---|
| 469-61-4 | Tricyclic Sesquiterpene subgroup 1 | 1H-3a,7-Methanoazulene, 2,3,4,7,8,8a-hexahydro-3,6,8,8-tetramethyl-, [3R-(3α,3aβ,7β,8aα)]- | Alpha-cedrene |
| 470-40-6 | Tricyclic Sesquiterpene subgroup 1 | Cyclopropa[d]naphthalene, 1,1a,4,4a,5,6,7,8-octahydro-2,4a,8,8-tetramethyl-, [1aS-(1aα,4aβ,8aR)]- | Thujopsene |
| 489-40-7 | Tricyclic Sesquiterpene subgroup 1 | 1H-Cycloprop[e]azulene, 1a,2,3,4,4a,5,6,7b-octahydro-1,1,4,7-tetramethyl-, [1aR-(1aα,4α,4aβ,7bα)]- | Alpha-gurjunene |
| 514-51-2 | Tricyclic Sesquiterpene subgroup 1 | 4,7-Methanoazulene, 1,2,3,4,5,6,7,8-octahydro-1,4,9,9-tetramethyl-, [1S-(1α,4α,7α)]- | Beta-patchoulene |
| 546-28-1 | Tricyclic Sesquiterpene subgroup 1 | 1H-3a,7-Methanoazulene, octahydro-3,8,8-trimethyl-6-methylene-, [3R-(3α,3aβ,7β,8aα)]- | Beta-cedrene |
| 8000-27-9a | Tricyclic Sesquiterpene subgroup 1 | Oils, cedarwood | Cedarwood oil |
| 68608-32-2a | Tricyclic Sesquiterpene subgroup 1 | Terpenes and Terpenoids, cedarwood-oil | T&T cedarwood oil |
| 68990-83-0a | Tricyclic Sesquiterpene subgroup 1 | Oils, cedarwood, Texan | Texan cedarwood oil |
| 59056-62-1 | Individual (Tricyclic Sesquiterpene) | 2,3b-Methano-3bH-cyclopenta[1,3]cyclopropa[1,2]benzene-4-methanol, octahydro-7,7,8,8-tetramethyl-, acetate | Amboryl acetate |
| 471-53-4 | Triterpenoid subgroup 2 | Olean-12-en-29-oic acid, 3-hydroxy-11-oxo-, (3β,20β)- | Enoxolone |
| 4572-09-2a | Triterpenoid subgroup 2 | Olean-12-en-29-oic acid, 3-hydroxy-11-oxo-, (3β,20β)-, compd. with (2,5-dioxo-4-imidazolidinyl)urea (1:1) | Allantoin glycyrrhetinic acid |
| 8031-03-6a | Individual (Triterpenoid) | Oils, mimosa | Mimosa oil |
| 84082-54-2a | Individual (Triterpenoid) | Ivy, Hedera helix, ext. | Ivy extract |
| 90045-38-8a | Individual (Triterpenoid) | Ginseng, Panax quinquefolium, ext. | American ginseng extract |
This CAS RN is a substance of unknown or variable composition, complex reaction products, or biological materials.
All of the substances in the Tricyclic Sesquiterpenes and Triterpenoids Group have been included in surveys issued pursuant to section 71 of CEPA, with thujopsene, alpha-gurjunene, beta-patchoulene, beta-cedrene, T&T cedarwood oil, amboryl acetate, enoxolone, allantoin glycyrrhetinic acid, mimosa oil, ivy extract, and American ginseng extract not reported as being manufactured or imported above the reporting threshold of 100 kg in 2011. Alpha-cedrene and cedarwood oil are reported as being imported into Canada at quantities of between 100 kg and 1000 kg; however, there were no reports of manufacture above the reporting threshold of 100 kg in 2011. Texan cedarwood oil is reported as being manufactured and imported into Canada at quantities of 277 kg and 200 kg, respectively, in 2011. The substances in the Tricyclic Sesquiterpenes and Triterpenoids Group are generally used as fragrances in cosmetics, natural health products (NHPs), non-prescription drugs (NPDs), cleaning products, and air fresheners. Some of them are also present in pest control products as formulants, and cedarwood oil is an active ingredient used only to manufacture pest control products intended for export out of Canada. In addition, some of them occur naturally in foods and are potentially used as food flavouring agents.
The ecological risks of the substances in the Tricyclic Sesquiterpenes and Triterpenoids Group were characterized using the ecological risk classification of organic substances (ERC) approach, which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate, or high level of potential concern for substances on the basis of their hazard and exposure profiles. Considering the outcome of the ERC analysis, the substances in the Tricyclic Sesquiterpenes and Triterpenoids Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft assessment, there is low risk of harm to the environment from the 14 substances in the Tricyclic Sesquiterpenes and Triterpenoids Group. It is proposed to conclude that the 14 substances in the Tricyclic Sesquiterpenes and Triterpenoids Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the human health risk assessment, 10 of the substances in this group have been addressed under two subgroups owing to similarities in their chemical structure, properties, and/or toxicity, while the remaining 4 substances were addressed individually. An impact on human health from exposure to these substances from environmental media is not expected due to the low quantities reported in response to a survey issued pursuant to section 71 of CEPA or to the estimated exposures from environmental monitoring and modelling. Where applicable, exposures were characterized from the use of cosmetics, NPDs, NHPs, from possible use as food flavouring agents, cleaning products, air fresheners, and do-it-yourself (DIY) products containing the tricyclic sesquiterpenes and triterpenoids.
For Tricyclic Sesquiterpene subgroup 1 (alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, beta-cedrene, cedarwood oil, T&T cedarwood oil, and Texan cedarwood oil), hazard information for cedarwood oil was used to inform the human health risk assessment. Thujopsene and alpha- and beta-cedrene are identified as major components of cedarwood oil, T&T cedarwood oil, and Texan cedarwood oil. For the dermal route, the critical effect level was based on systemic effects (decreased thymus weights), whereas for the oral route and inhalation, it was based on thyroid hormone changes.
The margins of exposure (MOEs) for cedarwood oil from dermal exposure to massage oil, fragrance, deodorant/antiperspirant (solid), moisturizer (body and face), conditioner (leave-on), body exfoliant (children who are 14 to 18 years old), aftershave (face), after hair removal product (body), antiseptic skin cleanser (spray) (NHP) (children who are 2 to 8 years old, for situations of public health concern resulting in increased use), counterirritant (spray) (NHP) (children who are 9 to 18 years old), and irritation relief balm (NHP) are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk. The MOEs for cedarwood oil from inhalation exposure to fragrance (children who are 2 to 3 years old) are also considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk.
Furthermore, the MOEs for daily dermal exposure from the use of cedarwood oil in DIY aroma diffuser/air freshener, DIY massage oil, DIY body moisturizer, and DIY facial steamer/mist are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk. The MOEs for daily inhalation exposure from the use of cedarwood oil in DIY aroma diffuser/air freshener and DIY facial steamer/mist are also considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk.
There were no identified sources of exposure for the general population from thujopsene, alpha-gurjunene, beta-patchoulene, and beta-cedrene, and a qualitative approach to risk characterization was taken.
The MOE for T&T cedarwood oil from foods, which is based on its potential use as a food flavouring agent, is considered adequate to address uncertainties in the health effects and exposure data. There were no other identified sources of exposure for the general population from the substance; as a result, T&T cedarwood oil is considered to be of low concern for human health at current levels of exposure.
The MOEs for Texan cedarwood oil from daily dermal exposure to massage oil, fragrance, deodorant/antiperspirant (solid), and moisturizer (body and face) are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
In addition, the dermal exposure to Texan cedarwood oil in DIY aroma diffuser/air freshener, DIY massage oil, DIY body moisturizer, and DIY facial steamer/mist are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk. The MOEs for Texan cedarwood oil from daily inhalation exposure to DIY aroma diffuser/air freshener and DIY facial steamer/mist are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
For amboryl acetate, health effects of concern were not identified, and there were no identified sources of exposure to the general population; as a result, amboryl acetate is considered to be of low concern for human health at current levels of exposure.
Triterpenoid subgroup 2 consists of enoxolone and allantoin glycyrrhetinic acid. The critical health effect identified for enoxolone was developmental neurotoxicity. The MOEs for enoxolone from face moisturizer, body moisturizer (spray and lotion), permanent hair dye, sunscreen (cream) (NHP and NPD), analgesic patch (NHP) (children who are 13 years old and under), acne therapy (cream) (NHP), medicated skin care product (cream) (NHP), and licorice tea and black licorice candy are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk. For allantoin glycyrrhetinic acid, there were no available empirical data. There were no identified sources of exposure of the general population to allantoin glycyrrhetinic acid; as a result, allantoin glycyrrhetinic acid is considered to be of low concern for human health at current levels of exposure.
For mimosa oil, the risk characterization of the main components, lupenone and lupeol, have been considered using health effects information on the analogue enoxolone. The critical health effect identified was developmental neurotoxicity. The MOEs for mimosa oil from fragrance (roll-on and spray), body moisturizer, face moisturizer, massage oil (children who are 1 year old and under), massage bar, sunless tanning product, facial makeup (liquid foundation), lipstick (children who are 8 years old and under), and sunscreen (lotion) (NHP) (children who are 3 years old and under and 14 to 18 years old) are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk. Furthermore, the MOEs between the critical effect level and the estimates of daily exposure from the use of mimosa oil in DIY aroma diffuser/air freshener, DIY massage oil, and DIY body moisturizer are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk.
For ivy extract, the risk characterization of the main components, hederacoside C, hederagenin, and alpha-hederin, have been considered using health effects information on the analogue enoxolone. The critical health effect identified was developmental neurotoxicity. The MOEs for ivy extract from massage oil, body moisturizer, face moisturizer, facial makeup fixer (spray), body exfoliant, and hair conditioner (leave-on) are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk. Furthermore, the MOEs between the critical effect level and the estimates of daily exposure from the use of ivy extract in DIY massage oil and DIY body moisturizer are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk.
For American ginseng extract, a read-across analogue, Asian ginseng (Panax ginseng), was used to inform the health effects assessment. Health effects of concern were not identified for American ginseng extract; as a result, American ginseng extract is considered to be of low concern for human health at current levels of exposure.
The human health assessment for each substance took into consideration those groups of individuals within the population in Canada who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. Certain subpopulations are routinely considered throughout the assessment process, such as infants, children, and people of reproductive age. For instance, age-specific exposures are routinely estimated, and developmental and reproductive toxicity studies are evaluated for potential adverse health effects. These subpopulations with potential for higher exposure and those who may be more susceptible were taken into account in the human health risk assessment outcomes.
Considering all the information presented in this draft assessment, it is proposed to conclude that cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, and ivy extract meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health. Considering all the information presented in this draft assessment, it is proposed to conclude that alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, beta-cedrene, T&T cedarwood oil, amboryl acetate, allantoin glycyrrhetinic acid, and American ginseng extract do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, and ivy extract meet one or more of the criteria set out in section 64 of CEPA and that alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, beta-cedrene, T&T cedarwood oil, amboryl acetate, allantoin glycyrrhetinic acid, and American ginseng extract do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted an assessment on 14 of 76 substances referred to collectively under the Chemical Management Plan (CMP) as the Terpenes and Terpenoids Group to determine whether these substances present or may present a risk to the environment or to human health. The substances in this group were identified as priorities for assessment as they met categorization criteria as described in the ECCC, HC (modified 2017) or were prioritized through other mechanisms (ECCC, HC [modified 2017]).
Of the other substances in the Terpenes and Terpenoids Group, 46 have been assessed in terms of risk to the environment and human health, and the decisions for these substances are provided in separate reports.Footnote 2 Decisions on the remaining substances will be communicated in separate assessments.
The 14 substances addressed in this draft assessment will hereinafter be referred to as the Tricyclic Sesquiterpenes and Triterpenoids Group. Some substances are assessed in subgroups owing to similarities in chemical structure, properties, and/or toxicity. Given the potential for these substances to be used in similar ways and applications, the potential for risk to human health is assessed using similar exposure assumptions across the assessment.
The ecological risks of the substances in the Tricyclic Sesquiterpenes and Triterpenoids Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as either warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
Some substances in the Tricyclic Sesquiterpenes and Triterpenoids Group or read-across analogues currently being evaluated have been reviewed by the United States Environmental Protection Agency (US EPA), European Chemicals Agency (ECHA), European Food Safety Authority (EFSA), Joint (Food and Agriculture Organization/World Health Organization [FAO/WHO]) FAO/WHO Expert Committee on Food Additives (JECFA), European Scientific Committee on Consumer Safety (SCCS), French Agency for Food, Environmental and Occupational Health & Safety (ANSES), Norwegian Scientific Committee for Food and Environment (VKM), and World Health Organization (WHO). Reviews conducted by these institutions were used to inform the human health effects characterization in this assessment.
This draft assessment includes the consideration of information on chemical properties, environmental fate, hazards, uses, and exposures, including additional information submitted by stakeholders. Relevant data were identified up to December 2021. Empirical data from key studies as well as some results from models were used to reach proposed conclusions.
Alpha-cedrene and alpha-gurjunene have been identified in vaping products, also known as electronic cigarettes (US EPA 2019). The assessment of risk to the general population from this use, including risk relative to that associated with conventional cigarettes, and possible options to mitigate risk associated with these products are being addressed through a separate legislative framework (HC [modified 2020]).
This draft assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external peer review and/or consultation. Comments on the technical portions relevant to human health were received from Jennifer Flippin, Theresa Lopez, and Joan Garey, all affiliates of TetraTech Inc. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
Assessments focus on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by considering scientific information, including information, if available, on subpopulations who may have greater susceptibility or greater exposure, vulnerable environments and cumulative effectsFootnote 3, and by incorporating a weight-of-evidence approach and precautionFootnote 4. This draft assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RNsFootnote 5), Domestic Substances List (DSL) names, and common names, as well as the chemical structure or representative chemical name(s), structure(s), and their range of concentration(s) in the essential oil and molecular formula for the discrete substances and representative substances for Unknown or Variable composition Complex reaction products or Biological materials (UVCBs) for the substances in the Tricyclic Sesquiterpenes and Triterpenoids Group are presented in Table 2-1 and Table 2-2. UVCBs are derived from natural sources or complex reactions. A UVCB is not an intentional mixture of discrete substances and is considered a single substance. The complexity and variability of their compositions can make them difficult to fully and consistently characterize. The substances in this assessment have been divided into 2 subgroups according to their chemical structure, properties, and/or toxicity, and into 4 individual substances.
Terpenes are simple hydrocarbons consisting of repeating five-carbon isoprene units (Figure 2-1). Terpenoids are a modified class of terpenes with different functional groups and an oxidized methyl group moved at various positions. Both terpenes and terpenoids are classified according to the number of isoprene units they contain (Caputi and Aprea 2011; Perveen 2018). Monoterpenes contain 2 isoprene units. The prefixes di-, tri-, or tetra- refer to two, three, and 4 monoterpene units, respectively. Furthermore, sesquiterpenes and sesterterpenes contain 3 and 5 isoprene units, respectively.
Figure 2-1. General structure of isoprene unit

Long description
General structure of isoprene unit (2-methyl-1,3-butadiene)
These substances are the components of essential oils found in a wide variety of plants. Essential oils are mixtures of volatile, organic compounds originating from a single botanical source and contribute to the flavour and fragrance of a plant. These plant-derived essential oils have many components, which can be extracted from different parts of the plant (for example, leaves, seeds, stems, flowers, roots, fruits, woods, barks, grass, gum, tree blossoms, bulbs, and/or flower buds) (Tisserand and Young 2014). In addition, the concentration of these major components can be affected by different factors such as plant origin, species, temperature, soil, and geography; thus, essential oils extracted from plants of the same genus and species can be chemically different even though their origin is the same.
| Subgroupa | CAS RN | DSL name (common name) |
Chemical structure or representative chemical name(s), structure(s), and their range of concentration(s) in the essential oil and molecular formula |
|---|---|---|---|
| 1 | 469-61-4 | 1H-3a,7-Methanoazulene, 2,3,4,7,8,8a-hexahydro-3,6,8,8-tetramethyl-, [3R-(3α,3aβ,7β,8aα)]- (alpha-cedrene) |
![C[C@@H]1CC[C@@H]2[C@]13CC=C([C@H](C3)C2(C)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-1.jpg) C15H24 |
| 1 | 470-40-6 | Cyclopropa[d]naphthalene, 1,1a,4,4a,5,6,7,8-octahydro-2,4a,8,8-tetramethyl-, [1aS-(1aα,4aβ,8aR)]- (thujopsene) |
![CC1=CC[C@@]2(CCCC([C@]23[C@H]1C3)(C)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-2.jpg) C15H24 |
| 1 | 489-40-7 | 1H-Cycloprop[e]azulene, 1a,2,3,4,4a,5,6,7b-octahydro-1,1,4,7-tetramethyl-, [1aR-(1aα,4α,4aβ,7bα)]- (alpha-gurjunene) |
![C[C@@H]1CC[C@@H]2[C@@H](C2(C)C)C3=C(CCC13)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-3.jpg) C15H24 |
| 1 | 514-51-2 | 4,7-Methanoazulene, 1,2,3,4,5,6,7,8-octahydro-1,4,9,9-tetramethyl-, [1S-(1α,4α,7α)]- (beta-patchoulene) |
![C[C@H]1CCC2=C1C[C@H]3CC[C@@]2(C3(C)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-4.jpg) C15H24 |
| 1 | 546-28-1 | 1H-3a,7-Methanoazulene, octahydro-3,8,8-trimethyl-6-methylene-, [3R-(3α,3aβ,7β,8aα)]- (beta-cedrene) |
![C[C@@H]1CC[C@@H]2[C@]13CCC(=C)[C@H](C3)C2(C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-5.jpg) C15H24 |
| 1 | 8000-27-9 | Oils, cedarwoodb (cedarwood oil) |
|
| 1 | 68608-32-2 | Terpenes and Terpenoids, cedarwood oilb (T&T cedarwood oil) |
|
| 1 | 68990-83-0 | Oils, cedarwood, Texanb (Texan cedarwood oil) |
|
| Individual | 59056-62-1 | 2,3b-Methano-3bH-cyclopenta[1,3]cyclopropa[1,2]benzene-4-methanol, octahydro-7,7,8,8-tetramethyl-, acetate (amboryl acetate) | 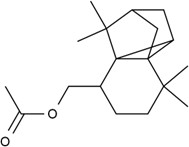 C18H28O2 |
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List.
a The Tricyclic Sesquiterpenes Group was assessed under one subgroup and one individual substance. Tricyclic Sesquiterpene subgroup 1 includes alpha-cedrene, beta-cedrene, and thujopsene as discrete substances and main components of cedarwood oil, Texan cedarwood, and T&T cedarwood oil, as well as alpha-gurjunene and beta-patchoulene on the basis of the read-across approach with alpha-cedrene. Amboryl acetate was assessed individually for the purposes of the human health risk characterization.
b This substance is a UVCB.
c Concentration range of the main component(s) for cedarwood oil (Juniperus virginiana) and T&T cedarwood oil captured from Du et al. (2011), National Toxicology Program (NTP) (2016), and Tisserand and Young (2014).
d Concentration range of the main component(s) for cedarwood oil (Cedrus atlantica) and T&T cedarwood oil captured from Chalchat et al. (1994), Aberchane et al. (2004), Satrani et al. (2006), Tisserand and Young (2014), Zrira and Ghanmi (2016), and Uehara et al. (2017).
e Concentration range of the main component(s) for Texan cedarwood oil captured from Kamatou et al. (2010), Tisserand and Young (2014), and Surburg and Panten (2016).
| Subgroupa | CAS RN | DSL name (common name) |
Chemical structure or representative chemical name(s), structure(s), and their range of concentration(s) in the essential oil and molecular formula |
|---|---|---|---|
| 2 | 471-53-4 | Olean-12-en-29-oic acid, 3-hydroxy-11-oxo-, (3β,20β)- (enoxolone) |
![C[C@]12CC[C@](C[C@H]1C3=CC(=O)[C@@H]4[C@]5(CC[C@@H](C([C@@H]5CC[C@]4([C@@]3(CC2)C)C)(C)C)O)C)(C)C(=O)O](/content/dam/eccc/images/pded/terpenes-group-4/Table2-2-1.jpg) C30H46O4 |
| 2 | 4572-09-2 | Olean-12-en-29-oic acid, 3-hydroxy-11-oxo-, (3β,20β)-, compd. with (2,5-dioxo-4-imidazolidinyl)urea (1:1)b (allantoin glycyrrhetinic acid) |
|
| Individual | 8031-03-6 | Oils, mimosab (mimosa oil) |
|
| Individual | 84082-54-2 | Ivy, Hedera helix, ext.b (Ivy extract) |
|
| Individual | 90045-38-8 | Ginseng, Panax quinquefolium, ext.b (American ginseng extract) |
![CC(=CCCC(C)([C@H]1CC[C@@]2(C1CC[C@H]3[C@]2(CC[C@@H]4[C@@]3(CC[C@@H](C4(C)C)O)C)C)C)O)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-2-10.jpg) Ginsenosides 70% to 90%e C54H92O23 |
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List.
a The Triterpenoids Group was assessed under one subgroup and 3 individual substances. For the Triterpenoid subgroup 2, hazard information for enoxolone was used to inform the human health risk characterization. Enoxolone is a discrete substance in the Triterpenoid subgroup 2 and is the main component of allantoin glycyrrhetinic acid. Mimosa oil, ivy extract, and American ginseng extract were assessed individually..
b This substance is a UVCB..
c Concentration range of the main component(s) for mimosa oil captured from Perriot et al. (2010) and Tisserand and Young (2014)..
d Concentration range of the main component(s) for ivy extract captured from Lutsenko et al. (2010), Havlíková et al. (2015), Yu et al. (2015), and Bezruk et al. (2020)..
e Concentration range of the main component(s) for American ginseng extract captured from Assinewe et al. (2003), Wang et al. (2015), and Han et al. (2016). The highest percentage components are the saponin triterpenes ginsenosides (six isoprene units or 3 monoterpene units) and ginsenosides (G)-Rb1, G-Rb2, G-Rc, G-Rg1, G-Re, G-Rd, G-Rg3, G-Rh2, G-Rg2, and G-Rh1.
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship ([Q]SAR) models, where appropriate, have been used to inform the ecological and human health assessments. The analogues that were selected were structurally similar and/or functionally similar to substances within this group (similar physical-chemical properties, toxicokinetics) and had relevant empirical data that could be used to read across to substances with limited empirical data. Analogue selection was based on analysis carried out using the Organisation for Economic Co-operation and Development (OECD) (quantitative) structure-activity relationship [(Q)SAR] toolbox version 4.2 (OECD QSAR Toolbox 2019). The applicability of (Q)SAR models was determined on a case-by-case basis. Details of the read-across data and (Q)SAR models chosen to inform the human health assessments of Triterpenoid subgroup 2 and American ginseng extract are further discussed in the relevant sections of this assessment. A list of the various analogues used to inform the human health risk assessment is presented in Table 2-3, along with an indication of the read-across data available for different parameters.
| Subgroup or substance being assessed | CAS RN for analogue | Common name | Chemical structure, molecular formula and SMILES | Molecular weight (g/mol) |
|---|---|---|---|---|
| Triterpenoid subgroup 2 | 1405-86-3 | Glycyrrhizic acid | ![[H][C@@]12C[C@](C)(CC[C@]1(C)CC[C@]1(C)C2=CC(=O)[C@]2([H])[C@@]3(C)CC[C@H](O[C@H]4O[C@@H]([C@@H](O)[C@H](O)[C@H]4O[C@@H]4O[C@@H]([C@@H](O)[C@H](O)[C@H]4O)C(O)=O)C(O)=O)C(C)(C)[C@]3([H])CC[C@@]12C)C(O)=O](/content/dam/eccc/images/pded/terpenes-group-4/Table2-3-1.jpg) C42H62O16 [H][C@@]12C[C@](C)(CC[C@]1(C)CC[C@]1(C)C2=CC(=O)[C@]2([H])[C@@]3(C)CC[C@H](O[C@H]4O[C@@H]([C@@H](O)[C@H](O)[C@H]4O[C@@H]4O[C@@H]([C@@H](O)[C@H](O)[C@H]4O)C(O)=O)C(O)=O)C(C)(C)[C@]3([H])CC[C@@]12C)C(O)=O |
822.9 |
| American ginseng extract | 50647-08-0 | Panax ginseng | 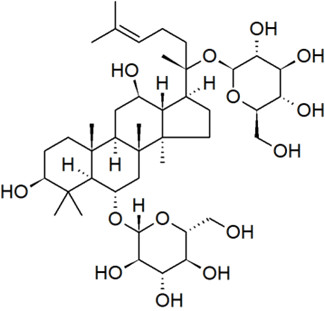 C42H66O17 UVCB, no SMILES |
843 |
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number
3. Physical and chemical properties
A summary of physical and chemical property data on the substances in the Tricyclic Sesquiterpenes and Triterpenoids Group are presented in Table 3-1, with the range of values indicated for each property. Where experimental information was limited or not available for a property, data from analogues were used for read-across and/or (Q)SAR models were used to generate predicted values for the substance. Additional physical and chemical properties are reported in ECCC (2016b).
| Substance(s) | Representative constituent(s)’ common name (CAS RN) | Molecular weight (g/mol)a | Water solubility (mg/L)a | Vapour pressure (Pa)a | Log Kowa |
|---|---|---|---|---|---|
| Alpha-cedrene | N/A | 204.35 | 0.1504M | 0.0184M | 5.74M |
| Thujopsene | N/A | 204.35 | 0.07152M | 9.37M | 6.12M |
| Alpha-gurjunene | N/A | 204.35 | 0.0638M | 2.72M | 6.18M |
| Beta-patchoulene | N/A | 204.35 | 0.1165M | 3.25M | 5.87M |
| Beta-cedrene | N/A | 204.35 | 0.1289M | 6.08M | 5.82M |
Cedarwood oil T&T cedarwood oil |
Alpha-cedrene (469-61-4) | 204.35 | 0.1504M | 0.0184M | 5.74M |
Cedarwood oil T&T cedarwood oil |
Thujopsene (470-40-6) | 204.35 | 0.07152M | 9.37M | 6.12M |
Cedarwood oil T&T cedarwood oil |
Cedrol (77-53-2) | 222.37 | 21.88M | 0.019M | 4.33M |
Cedarwood oil T&T cedarwood oil |
Beta-cedrene (546-28-1) | 204.35 | 0.1289M | 6.08M | 5.82M |
Cedarwood oil T&T cedarwood oil |
Alpha-himachalene (3853-83-6) | 204.19 | 0.05011M | 4.11M | 6.30M |
Cedarwood oil T&T cedarwood oil |
Beta-himachalene (1461-03-6) | 204.19 | 0.04532M | 1.47M | 6.35M |
Cedarwood oil T&T cedarwood oil |
Gamma-himachalene (53111-25-4) | 204.19 | 0.05849M | 3.23M | 6.22M |
Cedarwood oil T&T cedarwood oil |
Alpha-atlantone (108645-54-1) | 218.34 | 1.078M | 0.43M | 5.28M |
| Texan cedarwood oil | Thujopsene (470-40-6) | 204.35 | 0.07152M | 9.37M | 6.12M |
| Texan cedarwood oil | Alpha-cedrene (469-61-4) | 204.35 | 0.1504M | 0.0184M | 5.74M |
| Texan cedarwood oil | Cedrol (77-53-2) | 222.37 | 21.88M | 0.017M | 4.33M |
| Amboryl acetate | N/A | 276.41 | 0.278M | 0.033M | 5.60M |
| Enoxolone | N/A | 470.684 | 0.0114M | 3.88×10-13M | 6.90M |
| Allantoin glycyrrhetinic acid | Enoxolone (471-53-4) | 470.684 | 0.0114M | 3.88×10-13M | 6.90M |
| Allantoin glycyrrhetinic acid | Allantoin (97-59-6) | 158.12 | 1×106M | 1.74×10-7M | -3.14M |
| Mimosa oil | Lupenone (1617-70-5) | 424.70 | 7.64×10-5M | 3.88×10-6M | 8.72M |
| Mimosa oil | Lupeol (545-47-1) | 426.72 | 8.783×10-5M | 6.7×10-9M | 9.23M |
| Ivy extract | Hederacoside C (14216-03-6) | 1221.41 | 0.103M | 0M | -1.20M |
| Ivy extract | Hederagenin (465-99-6) | 472.71 | 3.4×10-3M | 2.07×10-13M | 6.90M |
| Ivy extract | Alpha-hederin (27013-91-8) | 750.98 | 6.58×10-3M | 8.88×10-27M | 4.43M |
| American ginseng extract | Ginsenoside-Rb1 (41753-43-9) | 1109.30 | 0.551M | 0M | -1.14M |
| American ginseng extract | Ginsenoside-Rb2 (11021-13-9) | 1079.27 | 0.918M | 0M | -1.16M |
| American ginseng extract | Ginsenoside-Rc (11021-14-0) | 1079.27 | 0.364M | 0M | -0.69M |
| American ginseng extract | Ginsenoside-Rg1 (22427-39-0) | 801.01 | 0.535M | 0M | 1.33M |
| American ginseng extract | Ginsenoside-Re (52286-59-6) | 947.15 | 0.496M | 0M | 0.21M |
| American ginseng extract | Ginsenoside-Rd (52705-93-8) | 947.15 | 0.053M | 0M | 1.35M |
| American ginseng extract | Ginsenoside-Rg3 (14197-60-5) | 785.01 | 0.008M | 0M | 3.36M |
| American ginseng extract | Ginsenoside-Rh2 (78214-33-2) | 622.87 | 0.005M | 0M | 5.15M |
| American ginseng extract | Ginsenoside-Rg2 (52286-74-5) | 785.01 | 0.071M | 0M | 2.49M |
| American ginseng extract | Ginsenoside-Rh1 (63223-86-9) | 638.89 | 0.073M | 0M | 3.61M |
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number; N/A, not applicable; Kow, octanol-water partition coefficient; (M), modelled
a US EPA (2012a)
4. Sources and uses
All of the substances in the Tricyclic Sesquiterpenes and Triterpenoids Group have been included in a survey issued pursuant to section 71 of CEPA (Canada 2012). Table 4-1 presents a summary of the information reported on the total manufacture and total import quantities of the Tricyclic Sesquiterpenes and Triterpenoids Group (Environment Canada 2013).
| Common name | Total manufacturea (kg) | Total importsa (kg) | Reporting year |
|---|---|---|---|
| Alpha-cedrene | NR | 100 to 1000 | 2011 |
| Thujopsene | NR | NR | 2011 |
| Alpha-gurjunene | NR | NR | 2011 |
| Beta-patchoulene | NR | NR | 2011 |
| Beta-cedrene | NR | NR | 2011 |
| Cedarwood oil | NR | 100 to 1000 | 2011 |
| T&T cedarwood oil | NR | NR | 2011 |
| Texan cedarwood oil | 277 | 200 | 2011 |
| Amboryl acetate | NR | NR | 2011 |
| Enoxolone | NR | NR | 2011 |
| Allantoin glycyrrhetinic acid | NR | NR | 2011 |
| Mimosa oil | NR | NR | 2011 |
| Ivy extract | NR | NR | 2011 |
| American ginseng extract | NR | NR | 2011 |
Abbreviation: NR, no reports above the reporting threshold of 100 kg.
a Values reflect quantities reported in response to a CEPA section 71 survey (Canada 2012). See survey for specific inclusions and exclusions (Schedules 2 and 3).
Information submitted in response to a CEPA section 71 survey indicated uses of Texan cedarwood oil as an odour agent in cleaning and furnishing care, laundry and dishwashing, personal care,Footnote 6 air care, apparel and footwear care, pet care, automotive care, and lubricants and greases (Environment Canada 2013). Information submitted in response to a CEPA section 71 survey indicated uses of alpha-cedrene in personal care, cleaning and furnishing care, and air care (Environment Canada 2013). In addition, information submitted in response to a CEPA section 71 survey indicated uses of thujopsene, alpha-gurjunene, beta-patchoulene, amboryl acetate, beta-cedrene, cedarwood oil, and mimosa oil in personal care products (Environment Canada 2013).
Additional uses for Tricyclic Sesquiterpene subgroup 1, amboryl acetate, Triterpenoid subgroup 2, mimosa oil, ivy extract, and American ginseng extract are outlined in Table 4-2.
| Use | Food additivea | Incidental additivea,b | Food packaging materialsa | Drugc | NHPd | Cosmetice | PCPf |
|---|---|---|---|---|---|---|---|
| Alpha-cedrene | N | N | N | N | N | Y | Y (F) |
| Thujopsene | N | N | N | N | N | N | N |
| Alpha-gurjunene | N | N | N | N | N | N | N |
| Beta-patchoulene | N | N | N | N | N | N | Y (F) |
| Beta-cedrene | N | N | N | N | N | N | N |
| Cedarwood oil | N | Y | N | N | Y (MI, NMI) | Y | Y (A, F) |
| T&T cedarwood oil | N | N | N | N | N | N | Y (F) |
| Texan cedarwood oil | N | Y | N | N | Y (NMI) | Y | Y (F) |
| Amboryl acetate | N | N | N | N | N | N | N |
| Enoxolone | N | N | N | Y (NMI) | Y (MI, NMI) | Y | N |
| Allantoin glycyrrhetinic acid | N | N | N | N | N | N | N |
| Mimosa oil | N | N | N | N | Y (NMI) | Y | N |
| Ivy extract | N | N | N | N | Y (MI, NMI) | Y | N |
| American ginseng extract | N | N | N | Y (MI, NMI) | Y (MI, NMI) | Y | N |
Abbreviations: A, active ingredient; F, formulant; MI, medicinal ingredient; NHP, natural health product; NMI, non-medicinal ingredient; PCP, pest control product; Y, use was reported for this substance; N, use was not reported for this substance.
a Personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced.
b While not defined under the Food and Drugs Act, incidental additives may be regarded, for administrative purposes, as those substances which are used in food processing plants and which may potentially become adventitious residues in foods (for example, cleaners, sanitizers).
c Listed in the Drug Product Database as being present as a medicinal or non-medicinal ingredient in disinfectant, human, or veterinary drug products in Canada. Personal communication, emails from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced.
d Listed in the Licensed Natural Health Products Database as being present as a medicinal or non-medicinal ingredient in NHPs in Canada. Personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced.
e Notified to be present in cosmetics on the basis of notifications submitted under the Cosmetic Regulations to Health Canada. Personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2020; unreferenced.
f Active ingredient or formulant in pest control products registered in Canada. Personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2015 and 2020; unreferenced.
Notifications submitted under the Cosmetic Regulations to Health Canada for the Tricyclic Sesquiterpenes and Triterpenoids Group, the Licensed Natural Health Products Database (LNHPD [modified 2024]), the Drug Product Database (DPD [modified 2021]), publicly available databases and websites (for example, Consumer Product Information Database [CPID], c2021), and safety and technical datasheets were used to identify products where there is the potential for exposure. These products and their associated exposures are presented below.
Do-it-yourself (DIY) products
Certain terpene and terpenoid substances that have aromatic properties within the Tricyclic Sesquiterpenes and Triterpenoids Group are currently available on the Canadian market at a concentration of up to 100%. It is possible that these undiluted substances are purchased and used by consumers to make DIY products. DIY products that may result in high consumer exposures include aroma diffuser/air freshener, massage oil, bath oil, body moisturizer, facial steamer/mist, and liquid floor cleaner. Consequently, uses of undiluted substances to make these DIY products are evaluated in this assessment. Parameters for estimating dermal and inhalation exposures to DIY products are available in Appendix B.
Tricyclic Sesquiterpene subgroup 1 (alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, beta-cedrene, cedarwood oil, T&T cedarwood oil, and Texan cedarwood oil)
There are 5 discrete substances (alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, and beta-cedrene) and 3 UVCBs (cedarwood oil, T&T cedarwood oil, and Texan cedarwood oil) in Tricyclic Sesquiterpene subgroup 1.
Alpha-cedrene is a naturally occurring substance obtained by fractional distillation of cedarwood oil or from cedrol by dehydration (Opdyke 1978). It is a component of cedarwood, sage, costus, lavender, and other oils (Opdyke 1978). Alpha-cedrene has been detected at 21.1% to 38% in cedarwood oil and T&T cedarwood oil and at 15% to 30.7% in Texan cedarwood oil.
Thujopsene is a naturally occurring substance reported to be a constituent of the wood oil and heartwood of trees from the order Cupressales (Dauben 1963). Thujopsene has been detected at ~27.6% in cedarwood oil and T&T cedarwood oil and at 35% to 60.4% in Texan cedarwood oil.
Alpha-gurjunene is a naturally occurring substance in essential oils including cedarwood, eucalyptus, labdanum, laurel leaf, lemon verbena, pepper tree berry, sage, and tea tree (Goodscents 2017).
Beta-patchoulene is a naturally occurring fragrant substance identified as a component of several essential oils including patchouli (Zhang 2016), basil, and tagete (Goodscents 2017).
Beta-cedrene is a naturally occurring substance and is a component of cedarwood oil. Beta-cedrene has been detected at 8.2% to 9.2% in cedarwood oil and T&T cedarwood oil, respectively.
Cedarwood oil is a naturally occurring UVCB substance extracted from Juniperus virginiana, Cupressaceae (CIR 2001; NTP 2016). These trees are grown in parts of Europe, Asia, and North America (CIR 2001; Catlin 2016). Alternatively, the CAS RN for cedarwood oil (8000-27-9) and “cedarwood essential oil” are associated with Cedrus atlantica oil, which is extracted from Cedrus atlantica, Pinaceae (CosIng 2021) and may contain different components than Juniperus virginiana, Cupressaceae, such as alpha-, beta-, and gamma-himachalene, and alpha-atlantone. T&T cedarwood is a naturally occurring UVCB substance composed of the terpenes fraction extracted from cedarwood (Burdock 2010). Texan cedarwood oil is a naturally occurring UVCB substance extracted from Juniperus mexicana or Juniperus ashei, both from the Cupressaceae family (NTP 2016). Cupressus funebris (commonly known as weeping cypress) is also regarded as a potential botanical source of cedarwood oil (Carroll et al. 2011).
Components of cedarwood oil are frequently isolated and acetylated (cedryl acetate, cedryl methylether) and used in cosmetics such as perfumes, lotions, and soaps (Catlin 2016). Cedarwood oil is also used in aromatherapy and homeopathic medicine (NTP 2016).
Alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, and beta-cedrene are naturally occurring in various essential oils or from plants. Alpha-cedrene, cedarwood oil, and Texan cedarwood oil are all notified under the Cosmetic Regulations to Health Canada and are identified in various rinse-off and leave-on cosmetics, including fragrance products, moisturizers, bath products, cleansers, massage products, shaving products, hair care products, and antiperspirants/deodorants.
On the basis of notifications submitted under the Cosmetic Regulations to Health Canada, alpha-cedrene is used in a limited number of cosmetics in Canada, with all of the products having a concentration of less than or equal to 1% (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced).
On the basis of notifications submitted under the Cosmetic Regulations to Health Canada, cedarwood oilFootnote 7 is used in 1900 cosmetics in Canada, with the majority (more than 90%) of the products having a concentration of less than or equal to 3% (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced).
On the basis of notifications submitted under the Cosmetic Regulations to Health Canada, Texan cedarwood oilFootnote 8 is used in over 470 cosmetics in Canada, with the majority (more than 90%) of the products having a concentration of less than or equal to 3% (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced).
There are also natural health products (NHPs) that contain cedarwood oilFootnote 9 as a medicinal or non-medicinal ingredient (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced). Preparations of Cedrus atlantica and Juniperus virginiana that are listed with a medicinal or non-medicinal role in the Natural Health Products Ingredients Database (NHPID) include Cedrus atlantica, Atlas cedarwood essential oil, Cedrus atlantica bark extract, Cedrus atlantica bark oil, and Cedrus atlantica wood oil, as well as Juniperus virginiana and Juniperus virginiana essential oil (NHPID [modified] 2024). There are also NHPs that contain Texan cedarwood oilFootnote 10 as a non-medicinal ingredient (NMI) (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced). Juniperus mexicana, as a taxonomical synonym of Juniperus ashei, is listed with a medicinal role in the NHPID (NHPID [modified] 2024).
Alpha-cedrene, cedarwood oil, and Texan cedarwood oil are used as fragrances in products including all-purpose cleaners, dish care products, laundry care products, and air freshener products. Alpha-gurjunene and beta-patchoulene are also listed by the International Fragrance Association (IFRA) as fragrance ingredients used in consumer goods (IFRA Standards Library 2021).
In Canada, alpha-cedrene, beta-patchoulene, cedarwood oil, Texan cedarwood oil, and T&T cedarwood oil are reported to be used as formulants in pest control products (personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021, 2023; unreferenced). Cedarwood oil is reported to be used as an active ingredient under the Pest Management Regulatory Agency’s Importation for Manufacturing and Export Program. As such, it is currently used only in the manufacture of pest control products intended for export from Canada (personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021, 2023; unreferenced).
In Europe, alpha-gurjunene and beta-patchoulene are reported as being used in cosmetics for their perfuming function (CosIng 2021). Juniperus virginiana oil is reported as being used in cosmetics for its fragrance and tonic functions (CosIng 2021). Cedarwood oil, which is associated with the name Cedrus atlantica bark oil, is also reported as being used in cosmetics for its fragrance, perfuming, and skin-conditioning functions (CosIng 2021). Juniperus mexicana oil is reported as being used in cosmetics for its fragrance function (CosIng 2021).
Thujopsene, as a component of lovage root extract, has been used to flavour tobacco products (personal communication, email from the Tobacco Control Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
Alpha-gurjunene has been identified in vaping products as a fragrance agent (personal communication, email from the Tobacco Control Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced). Alpha-cedrene and alpha-gurjunene have been identified in vaping products, also known as electronic cigarettes (US EPA 2019).
No definitive information is available concerning the potential use of T&T cedarwood oil as a flavouring agent in foods sold in Canada. However, since T&T cedarwood oil is identified as a food flavouring agent internationally, it is possible that this substance is present as a flavouring agent in foods sold in Canada.
T&T cedarwood oil (cedarwood oil terpenes) is permitted in the United States (US) as a synthetic flavouring substance or adjuvant that may be safely used in foods in accordance with good manufacturing practice (GMP) (Burdock 2010; US CFR 2021a).
No definitive information is available concerning the potential uses of alpha-gurjunene and beta-patchoulene in cosmetics, NHPs, food flavouring agents and food packaging, and other products available to the general population in Canada.
Amboryl acetate
Amboryl acetate does not occur naturally in the environment. It is a chemical synthesized from isolongifolene, polyformaldehyde, and acetic acid (Zhao and Li 1998; Sell 2003; Kirk-Othmer 2012).
No definitive information is available concerning the potential use of amboryl acetate in cosmetics, NHPs, foods and food packaging, and other products available to the general population in Canada.
In Europe, amboryl acetate is reported as being used in cosmetics with a perfuming function (CosIng 2021). It is also listed by IFRA as a fragrance ingredient used in consumer goods (IFRA Standards Library 2021).
Triterpenoid subgroup 2 (enoxolone and allantoin glycyrrhetinic acid)
There is one discrete substance (enoxolone, also called glycyrrhetinic acid) and one UVCB (allantoin glycyrrhetinic acid) in Triterpenoid subgroup 2. Enoxolone is either derived from glycyrrhizic acid or isolated from the shredded roots of Glycyrrhiza glabra (CIR 2007). Commonly known as licorice, Glycyrrhiza glabra is an herb native to central and southwestern Asia and the Mediterranean region (Isbrucker and Burdock 2006; VKM 2018). Fresh licorice roots typically contain 3% to 5% glycyrrhizic acid (Isbrucker and Burdock 2006), while dried licorice root extracts contain between 4% and 25% glycyrrhizic acid (WHO 2005; VKM 2018). Glycyrrhizic acid is composed of enoxolone (the aglycone of glycyrrhizic acid) and a disaccharide of glucuronic acid (Isbrucker and Burdock 2006). Glycyrrhizic acid is poorly absorbed from the gastrointestinal tract and is hydrolysed to enoxolone by intestinal microflora in the gastrointestinal tract (WHO 2005).
Allantoin glycyrrhetinic acid is a white-yellowish powder that is a derivative of allantoin and enoxolone in molar ratios of 1:1, 1:2, or 1:3 (Becker et al. 2010). Generally, enoxolone is used as a skin-conditioning agent in cosmetics (CIR 2007). Allantoin glycyrrhetinic acid is also used as a skin-conditioning agent (EWG Skin Deep 2021).
Enoxolone is used in a number of products available to consumers such as antiperspirant/deodorant, makeup products, massage products, skin and hair care products (lotions/cleansers), and toothpaste. On the basis of notifications submitted under the Cosmetic Regulations to Health Canada, enoxolone is used in more than 160 cosmetics in Canada, with the majority of the products (more than 90%) having a concentration of less than or equal to 1% (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced). There also are NHPs that contain enoxoloneFootnote 11 as a medicinal or non-medicinal ingredient (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced), as well as a NMI in non-prescription drugs (NPDs) (personal communication, emails from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced). Enoxolone is listed as glycyrrhetinic acid with a medicinal and non-medicinal role in the NHPID (NHPID [modified] 2024).
In Europe, enoxolone is reported as being used in cosmetics for its skin-conditioning function, and allantoin glycyrrhetinic acid is reported as being used in cosmetics for its skin protecting and soothing functions (CosIng 2021). Information from the EWG’s Skin Deep Cosmetics Database website also suggests the use of allantoin glycyrrhetinic acid in skin care products (moisturizers) (EWG Skin Deep 2021).
In the US, enoxolone is not identified as a food ingredient; however, licorice root and its derivatives are Generally Recognized as Safe (GRAS) as food ingredients for the functional uses of flavour enhancer, flavouring agent, and surface-active agent (US CFR 2021b).
No definitive information is available concerning the potential use of allantoin glycyrrhetinic acid in cosmetics, NHPs, foods and food packaging, and other products available to the general population in Canada. Allantoin glycyrrhetinic acid was reported to be used in 6 cosmetics, including shaving cream and skin care products (Becker et al. 2010).
Mimosa oil
Mimosa oil is a naturally occurring UVCB substance obtained from the Acacia decurrens plant, which is native to Australia and is also grown in France and Italy (Burdock 2010).
Mimosa oil is used in a number of products available to consumers such as skin and hair care products (lotions/cleansers), fragrances, and massage products. Based on notifications submitted under the Cosmetic Regulations to Health Canada, mimosa oilFootnote 12 is used in more than 740 cosmetics in Canada, with the majority of the products (more than 90%) having a concentration of less than or equal to 3% (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2022; unreferenced). There also are NHPs that contain mimosa oilFootnote 13 as a NMI (personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced). Preparations of Acacia decurrens that are listed with a non-medicinal role in the NHPID include Acacia dealbata flower/stem extract, Acacia decurrens flower extract, Acacia decurrens flower wax, and Acacia decurrens/jojoba/sunflower seed wax/polyglyceryl-3 esters (NHPID [modified 2024]).
Mimosa oil is listed as a fragrance ingredient used in consumer goods by the IFRA Standards Library (2021). Information from the EWG’s Skin Deep Cosmetics Database website also suggests use of mimosa oil in skin care products (EWG Skin Deep 2021).
In the US, mimosa oil is reported to be used as a food flavouring agent in alcoholic and non-alcoholic beverages, baked goods, candy, frozen dairy, and gelatins/puddings (Burdock 2010).
Extracts, oils, absolutes, and other derivatives of mimosa flowers (Acacia decurrens var. dealbata) may be safely used in foods in the US as natural flavouring substances and natural adjuvants in accordance with the principles of GMP (US CFR 2021c). Mimosa oil (FEMA number 2755) has been determined to be GRAS by the Flavor & Extract Manufacturers Association (FEMA) Expert Panel (Hall and Oser 1965) and is listed in the FEMA Flavor Library (FEMA c2021).
No definitive information is available concerning the potential use of mimosa oil in foods and food packaging, and other products available to the general population in Canada.
Ivy extract
Ivy extract is a naturally occurring UVCB substance obtained from the Hedera helix plant, which is native to Europe and is grown in North America and Asia (Baharara et al. 2021). Ivy has a long history of use in traditional herbal medicine (Cwientzek et al. 2011; Mendel et al. 2011).
Ivy extract is used in a number of products available to consumers, such as moisturizers, bath products, cleansers, hair care products, makeup products, and massage products. On the basis of notifications submitted under the Cosmetic Regulations to Health Canada, ivy extractFootnote 14 is used in more than 390 cosmetics in Canada, with the majority of the products (more than 90%) having a concentration of less than or equal to 1% (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced). There are also NHPs that contain ivy extractFootnote 15 as a medicinal or non-medicinal ingredient (personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced). Preparations of Hedera helix that are listed with a medicinal or non-medicinal role in the NHPID include Hedera helix and Hedera helix (Ivy) extract (NHPID [modified 2024]).
In Europe, ivy extract is reported as being used in cosmetics for its anti-dandruff, hair conditioning, anti-caking, anti-microbial, astringent, skin conditioning, soothing, and tonic functions (CosIng 2021). Information from the EWG’s Skin Deep Cosmetics Database website also suggests use of ivy extract in skin and hair care products (cleansers/lotions/sunscreens) (EWG Skin Deep 2021).
American ginseng extract
American ginseng extract is a naturally occurring UVCB substance that is typically obtained by solvent extraction of Panax quinquefolium, which is a perennial herb indigenous to North America (Kitts and Hu 2000; CIR 2012). Ginseng is widely used in traditional medicine as an herbal remedy and is also used in cosmetics (Kitts and Hu 2000; CIR 2012). While various plant parts are used to obtain ginseng extract, the majority of uses involve ginseng extract derived from the root (CIR 2012).
American ginseng extract is used in a number of products available to consumers such as skin and hair care products (lotions, cleanser, shampoos, and conditioners) as well as in makeup, mouthwash, toothpaste, fragrance, and massage products. On the basis of notifications submitted under the Cosmetic Regulations to Health Canada, American ginseng extractFootnote 16 is used in over 190 cosmetics in Canada, with the majority of the products (more than 80%) having a concentration of less than or equal to 1% (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced).
There are also NHPs that contain American ginseng extractFootnote 17 as a medicinal or non-medicinal ingredient (personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced). Preparations of Panax quinquefolius that are listed with a medicinal or non-medicinal role in the NHPID include Panax quinquefolius, Panax quinquefolius root extract, and radix panacis quinquefolii (NHPID [modified 2024]).
No definitive information is available concerning the potential uses of American ginseng extract in foods and food packaging, or other products available to the general population in Canada. Ginseng (Panax quinquefolius or Panax ginseng; CAS RN 84650-12-4) had an annual world production of 22 154 000 lbs (10 049 000 kg) in 1993 (Burdock 2010). Ginseng is known to be consumed as herbal teas and in chewing gums and candies (Burdock 2010).
In addition, American ginseng extract may be present in hair dye kits (CPID c2021). Similarly, information from the EWG’s Skin Deep Cosmetics Database website suggests use of American ginseng extract in skin and hair care products (cleansers, lotions, sunscreens) (EWG Skin Deep 2021). In Europe, American ginseng extract (Panax quinquefolius root extract) is reported as being used in cosmetics for its astringent function (CosIng 2021).
5. Environmental fate and behaviour
5.1 Environmental persistence and potential for bioaccumulation
According to models used in ERC (ECCC 2016b), alpha-cedrene, beta-cedrene, cedarwood oil, and Texan cedarwood oil are expected to persist in sediment but are not expected to persist in air, water, or soil. The half-lives of thujopsene, alpha-gurjunene, enoxolone, allantoin glycyrrhetinic acid, mimosa oil, ivy extract, and American ginseng extract indicate that these substances are expected to persist in water, sediment, and soil but are not expected to persist in air. Beta-patchoulene, T&T cedarwood oil, and amboryl acetate are not expected to persist in air, water, sediment, or soil.
Given their high log Kow and high bioconcentration factors (ECCC 2016b), alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, beta-cedrene, cedarwood oil, T&T cedarwood oil, and Texan cedarwood oil are expected to significantly bioaccumulate in organisms. Although the log Kow values for amboryl acetate, enoxolone, and allantoin glycyrrhetinic acid are high, the bioconcentration factors for these substances are low (456.6, 757.7, and 840.4 L/kg, respectively). In addition, although the log Kow values for American ginseng extract range from low to high (see section 3), the bioconcentration factor for this substance is also low (93.0 L/kg). As a result, amboryl acetate, enoxolone, allantoin glycyrrhetinic acid, and American ginseng extract are not expected to significantly bioaccumulate in organisms (ECCC 2016b).
Although most of the bioconcentration factor (BCF) data generated from ECCC (2016b) had applied the CATALOGIC model on suitable representative structures, mimosa oil and ivy extract showed results that were outside of the model’s domain. Therefore, the Arnot-Gobas model (Arnot et al. 2010) was applied to the components of the UVCBs, which indicate a low bioaccumulation potential for both substances. The BCFs values for the individual components were low, ranging from 36 L/kg to 212 L/kg and from 262 L/kg to 1820 L/kg for mimosa oil and ivy extract, respectively. There were also no empirical data available from the literature on the UVCB’s components. In addition, it should be noted that there is reduced bioavailability in water for chemicals with a high log Kow (that is, some of the components of mimosa oil and ivy extract), which can reduce chemical absorption efficiency in fish gills (Arnot et al. 2010). For substances with a log Kow greater than 7, chemical absorption efficiency declines as diffusion becomes controlled by dissolution in aqueous layers (Arnot et al. 2010).
Therefore, it is expected that alpha-gurjunene, beta-cedrene, cedarwood oil, and Texan cedarwood oil will persist in the environment and bioaccumulate in organisms. Alpha-cedrene and thujopsene, which are main components of cedarwood oil (representing 21.1% to 38%, and 27.6% of its composition, respectively) and Texan cedarwood oil (representing 35% to 60.4% and 15% to 30.7% of its composition, respectively), are also expected to persist in the environment and to have a high bioaccumulation potential.
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risks of the substances in the Tricyclic Sesquiterpenes and Triterpenoids Group were characterized using the ERC approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a). It is also noted that since American ginseng extract is a UVCB substance and could not be suitably represented by a single chemical structure, a manual judgment-based approach to its classification was used.
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (for example, OECD QSAR Toolbox 2014), from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (Q)SAR or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (for example, classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of American ginseng extract, hazard and exposure could not be fully profiled because of the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed by examining the UVCB constituents, analyzing information submitted in response to a CEPA section 71 survey, making decisions on the basis of consideration of similar substances, and/or application of expert judgment.
A risk matrix was used to assign a low, moderate, or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. The ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (that is, in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
The ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes 2 of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (that is, mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity, and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity and may not reflect future trends.
In addition, it should be noted that in this assessment, evaluation of the potential to cause ecological harm considered each substance individually. If exposure to multiple substances occurs simultaneously, this could result in cumulative effects on organisms and potentially present a higher risk. The potential for cumulative effects and how they may manifest in the environment were not further investigated due to the low ecological risk classification of these substances considering both ecological exposure and hazard under the ERC approach.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Tricyclic Sesquiterpenes and Triterpenoids Group and the hazard, exposure, and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the 14 substances in the Tricyclic Sesquiterpenes and Triterpenoids Group are summarized in Table 6-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Alpha-cedrene | low | low | low |
| Thujopsene | high | low | low |
| Alpha-gurjunene | high | low | low |
| Beta-patchoulene | low | low | low |
| Beta-cedrene | low | low | low |
| Cedarwood oil | low | low | low |
| T&T cedarwood oil | low | low | low |
| Texan cedarwood oil | low | low | low |
| Amboryl acetate | moderate | low | low |
| Enoxolone | high | low | low |
| Allantoin glycyrrhetinic acid | high | low | low |
| Mimosa oil | low | moderate | low |
| Ivy extract | low | low | low |
| American ginseng extract | high | low | low |
On the basis of both low hazard and low exposure classifications, according to information considered under the ERC, alpha-cedrene, beta-patchoulene, beta-cedrene, cedarwood oil, T&T cedarwood oil, Texan cedarwood oil, and ivy extract were classified as having a low potential for ecological risk. It is unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under the ERC, thujopsene, alpha-gurjunene, enoxolone, allantoin glycyrrhetinic acid, and American ginseng extract were classified as having a low exposure potential. Thujopsene, alpha-gurjunene, enoxolone, and allantoin glycyrrhetinic acid were classified as having a high hazard potential on the basis of their high potential to cause adverse effects in aquatic food webs. Enoxolone was also classified as having a high hazard potential on the basis of the agreement between its reactive mode of action and elevated ecotoxicity ratio, both of which suggest that this chemical is likely of high potency, and structural alerts from OECD QSAR Toolbox (2014), which identified this substance as being a potential endocrine receptor binder. American ginseng extract was classified as having a high hazard potential through a conservative manual classification that was applied due to uncertainties in the model outcomes for this substance. These 5 substances were classified as having a moderate potential for ecological risk; however, the risk classifications were decreased to low following the adjustment of risk classification based on current use quantities (see section 7.1.1 of the ERC approach document [ECCC 2016a]). The potential effects and how they may manifest in the environment were not further investigated due to the low exposure potential of these substances. It is unlikely that thujopsene, alpha-gurjunene, enoxolone, allantoin glycyrrhetinic acid, and American ginseng extract are resulting in concerns for the environment in Canada.
According to information considered under the ERC, amboryl acetate was classified as having a low exposure potential. This substance was classified as having a moderate hazard potential on the basis of its moderate potential to cause adverse effects in aquatic food webs. Amboryl acetate was classified as having a low potential for ecological risk. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure potential of this substance. It is unlikely that this substance is resulting in concerns for the environment in Canada.
According to information considered under the ERC, mimosa oil was classified as having a moderate exposure potential on the basis of a long overall persistence (that is, the sum of chemical half-lives in all media weighted by the mass fraction of the chemical in the medium) and a moderate reported use quantity according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). Mimosa oil was classified as having a low hazard potential and low potential for ecological risk. Although the current use patterns result in a moderate exposure potential, considering the low hazard potential, mimosa oil is unlikely to be resulting in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Tricyclic Sesquiterpene subgroup 1 (alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, beta-cedrene, cedarwood oil, T&T cedarwood oil, and Texan cedarwood oil)
7.1.1 Exposure assessment
Environmental media
Given the low manufacture and import quantity values (<1000 kg/substance) of the substances submitted in response to a CEPA section 71 survey (Environment Canada 2013), significant exposure to alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, beta-cedrene, cedarwood oil, T&T cedarwood oil, and Texan cedarwood oil from environmental media is not expected in Canada. No reports of monitoring for alpha-cedrene, alpha-gurjunene, beta-patchoulene, cedarwood oil, T&T cedarwood oil, and Texan cedarwood oil in environmental media in Canada or elsewhere were identified.
In the US, thujopsene has been measured in indoor air in 9 homes at average concentrations of 5.3 µg/m3 (range of 2.9 to 8.8 µg/m3) and 5.9 µg/m3 (range of 3.2 to 9.8 µg/m3) in the basement and on the main floor, respectively (Ryan and Beaucham 2013). Alpha-cedrene and beta-cedrene have been measured in indoor air in China in 20 homes, and levels were reported to be below the detection limit (BDL to 13 µg/m3 and BDL to 12.9 µg/m3, respectively; Norris et al. 2019).
Food
Cedarwood oil and Texan cedarwood oil are identified as incidental additives as components in food-contact surface cleaners, dish detergents, hand cleaners, and degreaser cleaners (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
T&T cedarwood oil is identified as a food flavouring agent internationally; as such, it is possible that this substance is present as a flavouring agent in foods sold in Canada. The Fenaroli’s Handbook of Flavor Ingredients reports the estimated per capita (“individual”) intake of T&T cedarwood oil from its use as a food flavouring agent to be 1.412 × 10-1 μg/kg bw/day for the US population on the basis of production volumes reported by the food industry (Burdock 2010). In the absence of data on the actual use, if any, of T&T cedarwood oil as a flavouring agent in foods sold in Canada, the per capita intake estimate for the US population (Burdock 2010) is an acceptable estimate of possible Canadian dietary exposure for the general population 1 year of age and older to this substance from its potential use as a food flavouring agent (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
Exposure from natural occurrence in foods
Alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, and beta-cedrene are reported to occur naturally at low levels in a small number of foods and essential oils (VCF 2021). There is expected to be limited, if any, dietary exposure to these substances from their natural presence in foods and in the plant materials used to obtain the essential oils.
Products available to consumers
Alpha-cedrene
Alpha-cedrene is used in products available to consumers, such as fragrances at 1%, shaving products at 0.1%, and air freshener products (for example, plug-in and car vent clip) at 1% (SDS Search Tool 2019). Alpha-cedrene is also used in leather maintenance spray, and exposure was quantified at an assumed maximum concentration of 5%. In addition, alpha-cedrene is identified in toilet or urinal cleaning or deodorizing product (automatic toilet bowl cleaner), and exposure was quantified at an assumed maximum concentration of 10% (ACI 2021).
Cedarwood oil
To evaluate the potential for exposure to cedarwood oil from cosmetics and NHPs applied by the dermal and inhalation routes, sentinel scenarios were selected on the basis of a combination of use frequencies and reported concentrations of cedarwood oil in these products. These selected sentinel scenarios represented the highest exposures, relative to other dermally applied cosmetics, as well as NHPs where cedarwood oil is used as a NMI, on the basis of identified products reported to contain the substance. Exposure to cedarwood oil from the use of massage oil, fragrance, deodorant/antiperspirant (solid), body and face moisturizer, conditioner (leave-on), shampoo, hair perm/straighteners, bath product (salt, oil), body cleanser (solid and liquid), body exfoliant, facial makeup (liquid foundation), shaving cream (face and body), aftershave (face), after hair removal product (body), body adhesive, antiseptic skin cleanser (spray) (NHP), counterirritant (spray) (NHP), irritation relief balm (NHP), aromatherapy – liquid for steam bowl inhalation (NHP), aromatherapy – liquid for diffuser inhalation (NHP), aromatherapy – respiratory spray (NHP), and acne therapy (gel) (NHP) were considered to be the sentinel scenarios for dermal applications (Table 7-1 and Table 7-2).
Exposure to cedarwood oil by the oral route from the use of lip balm, mouthwash, and toothpaste were also assessed (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced).
Information from the American Cleaning Institute (ACI) website indicates potential use of cedarwood oil as a fragrance in all-purpose cleaners at 0.01% to 10%, dish care products at 0.01% to 1%, and laundry care products at 0.01% to 10% (ACI 2021). Where concentration information was unavailable, assumed maximum concentrations of 10% in toilet or urinal cleaning or deodorizing product were used to derive the exposure estimates (ACI 2021). In addition, cedarwood oil has been identified in various air freshener products (for example, candle, spray, liquid plug-in, and solid gel). Exposure was quantified from plug-in air freshener product at an assumed maximum concentration of 5%.
Texan cedarwood oil
Texan cedarwood oil is used in products available to consumers. Exposure to Texan cedarwood oil from the use of a massage oil, fragrance, deodorant/antiperspirant (solid), moisturizer (body, face), shampoo, temporary hair dye, hairspray, bath product (salt, foam/bubbles), body cleanser (solid), body exfoliant, facial makeup (liquid foundation), and shaving cream (body) were considered to be the sentinel scenarios for cosmetics (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced). Texan cedarwood oil is used in cleaning products such as laundry detergent, and potential exposure to Texan cedarwood oil from its use in liquid laundry detergent was quantified by the inhalation and dermal routes. Texan cedarwood oil has also been identified in air freshener products (for example, wax melt, solid gel), and exposure was quantified from air freshener wax melt. Texan cedarwood oil is also identified in toilet or urinal cleaning or deodorizing product, and exposure was quantified at a concentration of 1%.
Other Tricyclic Sesquiterpene subgroup 1 substances (thujopsene, alpha-gurjunene, beta-patchoulene beta-cedrene, and T&T cedarwood oil)
There were no identified products containing thujopsene, alpha-gurjunene, beta-patchoulene, and beta-cedrene available to consumers in Canada; therefore, human exposure is considered to be minimal. As mentioned, T&T cedarwood oil is identified as a food flavouring agent internationally; and as such, it is possible that this substance is present as a flavouring agent in foods sold in Canada. There were no identified products containing T&T cedarwood oil available to consumers in Canada. Therefore, human exposure from this substance is considered to be minimal.
DIY
For the use of 100% cedarwood oil and Texan cedarwood oil in DIY products, the highest daily exposures expected to occur from the use of the oil were in aroma diffuser/air freshener, massage oil, body moisturizer, bath oil, and facial steamer/mist and as a liquid floor cleaner. Although the upper concentration reported for cedarwood oil and Texan cedarwood oil used in massage oil is assumed to be 100%, massage oils are typically diluted prior to use. It is reported that essential oils and extracts used in body products are typically diluted to concentrations of between 1 and 4% (Tisserand Institute 2021). On the basis of this information, the maximum concentration of cedarwood oil or Texan cedarwood oil in DIY body moisturizer was assumed to be 3% (RIVM 2006).
Dermal absorption
To estimate of the amount of product available for inhalation exposure after dermal exposure to the Tricyclic Sesquiterpene subgroup 1, a dermal absorption value of 25% was used for all Tricyclic Sesquiterpene subgroup 1 substances on the basis of available information and the following considerations:
- Alpha-cedrene (204.35 g/mol), a representative Tricyclic Sesquiterpene subgroup 1 substance, has a similar molecular weight to acetyl cedrene (246.39 g/mol) and 1-(1,2,3,4,5,6,7,8-octahydro-2,3,8,8-tetramethyl-2-naphthalenyl) ethanone (OTNE; 234.38 g/mol) and also has a moderate vapour pressure. All 3 substances (acetyl cedrene, OTNE, and alpha-cedrene) have low water solubility (0.01 mg/L to 10 mg/L) and moderate log Kow (3-6) values. This would suggest that the dermal absorption potential of alpha-cedrene would be similar to that of these substances.
- A maximum dermal absorption value of 25% was considered on the basis of an in vitro human dermal absorption study for analogue substances, acetyl cedrene, and OTNE (Belsito et al. 2013). In this study, dermal absorption rates of 11% and 15% were noted for acetyl cedrene and OTNE after 48 hours of exposure to 1% w/v of the substances in ethanol. However, the overall recovery was less than 70% for these substances. Due to limited study details and recovery, the maximum dermal absorption rate was conservatively adjusted.
Inhalation exposure
The Tricyclic Sesquiterpene subgroup 1 substances have vapour pressures ranging from 0.0184 Pa to 9.37 Pa and are considered to be volatile. Therefore, exposure by the inhalation route was also quantified for the sentinel scenarios. To account for the amount of product absorbed by the dermal route, the product amount available for inhalation was adjusted by 75%. For body lotion, since the product amount for inhalation was adjusted for the exposed surface area and since this value was less than 75% of the product amount, no further adjustment was made to the product amount.
Exposure estimates for the lowest and highest exposed age groups from cosmetics that contain Tricyclic Sesquiterpene subgroup 1 substances are summarized in Table 7-1. Estimated dermal, inhalation, and oral exposures to Tricyclic Sesquiterpene subgroup 1 substances from the use of NHPs and other products are presented in Table 7-2 and Table 7-3, respectively. In addition, exposure estimates for the lowest and highest exposed age groups from DIY products were calculated and are summarized in Table 7-4.
| Product scenario | % in product | Dermala (mg/kg bw/day) | Inhalationa (mg/kg bw/day)a | Orala (mg/kg bw/day) |
|---|---|---|---|---|
| Massage oilb (cedarwood oil, Texan cedarwood oil) | 3 | 1.30 (adults) to 8.57 (0 to 5 months) | 6.12 × 10-2 (adults) to 1.24 × 10-1 (1 year) | Not applicable |
| Fragrance (cedarwood oil) | 100 | 6.33 (14 to 18 years) to 18.70 (2 to 3 years) |
1.50 × 10-1 (adults) to 2.63 × 10-1 (2 to 3 years) | Not applicable |
| Fragrance (Texan cedarwood oil) | 30 | 1.93 (adults) to 5.61 (2 to 3 years) | 4.51 × 10-2 (adults) to 8.19 × 10-2 (2 to 3 years) | Not applicable |
| Fragrance (alpha-cedrene) | 1 | 0.06 (14 to 18 years) to 0.19 (2 to 3 years) | 3.62 × 10-4 (adults) to 7.40 x 10-4 (2 to 3 years) | Not applicable |
| Deodorant/antiperspirant (solid) (cedarwood oil) | 42 | 4.40 (9 to 13 years) to 7.45 (14 to 18 years) | 6.62 × 10-2 (9 to 13 years) to 1.26 × 10-1 (14 to 18 years) | Not applicable |
| Deodorant/antiperspirant (solid) (Texan cedarwood oil) | 10 | 1.05 (9 to 13 years) to 1.77 (14 to 18 years) | 1.56 × 10-2 (9 to 13 years) to 3.08 × 10-2 (14 to 18 years) | Not applicable |
| Body moisturizer (cedarwood oil, Texan cedarwood oil) | 10 | 13.51 (adults) to 31.75 (0 to 5 months) | 7.63 × 10-2 (0 to 5 months) to 1.69 × 10-1 (14 to 18 years) | Not applicable |
| Face moisturizer (cedarwood oil) | 10 | 2.42 (14 to 18 years) to 4.05 (adults) | 9.27 × 10-2 (9 to 13 years) to 1.57 × 10-1 (adults) | Not applicable |
| Face moisturizer (Texan cedarwood oil) | 5 | 1.21 (14 to 18 years) to 2.03 (adults) | 6.29 × 10-2 (9 to 13 years) to 1.04 × 10-1 (adults) | Not applicable |
| Conditioner (leave-on) (cedarwood oil) | 14 | 2.26 (14 to 18 years) to 4.85 (2 to 3 years) | 9.23 × 10-2 (14 to 18 years) to 1.35 ×10-1 (4 to 8 years) | Not applicable |
| Shampoo (cedarwood oil, Texan cedarwood oil) | 5 | 8.39 × 10-2 (14 to 18 years) to 3.10 × 10-1 (0 to 5 months) | 1.47 × 10-4 (2 to 3 years) to 2.78 × 10-4 (1 year) | Not applicable |
| Hair perm/straighteners (cedarwood oil) | 0.1 | 0.11 (adults) to 0.29 (4 to 8 years) | 1.60 × 10-5 (14 to 18 years) to 2.51 ×10-5 (4 to 8 years) | Not applicable |
| Temporary hair dye (Texan cedarwood oil) | 0.1 | 4.73 × 10-2 (adults) to 1.52 × 10-1 (4 to 8 years) | 7.55 × 10-4 (adults) to 1.79 × 10-3 (4 to 8 years) | Not applicable |
| Hairspray (Texan cedarwood oil) | 5 | 0.16 (14 to 18 years) to 0.43 (4 to 8 years) | 9.79 × 10-4 (adults) to 2.32 × 10-3 (4 to 8 years) | Not applicable |
| Bath product (salt) (cedarwood oil, Texan cedarwood oil) |
10 | 1.09 × 10-2 (adults) to 1.39 × 10-2 (9 to 13 years) | 3.21 × 10-2 (9 to 13 years) to 4.72 × 10-2 (adults) | Not applicable |
| Bath product (foam/bubbles) (Texan cedarwood oil) | 5 | 9.32 × 10-3 (9 to 13 years) to 2.99 × 10-2 (14 to 18 years) | 1.85 × 10-5 (0 to 5 months) to 1.04 × 10-3 (adults) | Not applicable |
| Bath product (cedarwood oil) | 3.5 | 6.53 × 10-3 (9 to 13 years) to 2.09 × 10-2 (14 to 18 years) | 4.05 × 10-4 (0 to 5 months) to 2.24 × 10-2 (14 to 18 years) | Not applicable |
| Body cleanser (solid) (cedarwood oil, Texan cedarwood oil) | 100 | 0.18 (adults) to 0.31 (0 to 5 months) | 8.08 × 10-3 (0 to 5 months) to 2.14 × 10-2 (14 to 18 years) | Not applicable |
| Body cleanser (liquid) (cedarwood oil) | 3 | 0.06 (adults) to 0.26 (0 to 5 months) | 6.61 × 10-4 (0 to 5 months) to 1.54 × 10-3 (14 to 18 years) | Not applicable |
| Body exfoliant (Texan cedarwood oil) | 1 | 0.14 (adults) to 0.16 (14 to 18 years) | 2.93 × 10-3 (adults) to 3.69 × 10-3 (14 to 18 year) | Not applicable |
| Body exfoliant (cedarwood oil) | 3 | 0.41 (adult) to 0.48 (14 to 18 years) | 1.06 × 10-3 (adults) to 1.28 × 10-3 (14 to 18 year) | Not applicable |
| Facial makeup (liquid foundation) (cedarwood oil, Texan cedarwood oil) | 0.3 | 1.98 × 10-2 (14 to 18 years) to 4.43 × 10-2 (4 to 8 years) | 8.21 × 10-4 (14 to 18 years) to 1.25 × 10-3 (4 to 8 years) | Not applicable |
| Lip balm (cedarwood oil) | 3 | Not applicable | Not applicable | 1.78 × 10-2 (adults) to 4.4 × 10-2 (2 to 3 years) |
| Mouthwash (cedarwood oil) | 0.1 | Not applicable | Not applicable | 2.02 × 10-2 (9 to 13 years) to 4.4 × 10-2 (2 to 3 years) |
| Toothpaste (cedarwood oil) | 0.5 | Not applicable | Not applicable | 1.35 × 10-2 (adults) to 2.03 × 10-1 (2 to 3 years) |
| Shaving cream (face) (cedarwood oil) | 5 | 4.59 × 10-2 (adults) to 7.62 × 10-2 (9 to 13 years) | 3.47 × 10-4 (14 to 18 years) to 4.25 × 10-4 (adults) | Not applicable |
| Shaving cream (body) (Texan cedarwood oil) | 10 | 0.17 (adults) to 0.24 (9 to 13 years) | 2.06 × 10-2 (adult) to 2.45 × 10-2 (9 to 13 years) | Not applicable |
| Shaving cream (body) (cedarwood oil) | 6.66 | 0.11 (adults) to 0.16 (9 to 13 years) | 1.49 × 10-2 (adults) to 1.76 × 10-2 (9 to 13 years) | Not applicable |
| Shaving cream (body) (alpha-cedrene) | 0.1 | 1.72 × 10-3 (adults) to 2.36 × 10-3 (9 to 13 years) | 1.42 × 10-6 (adults) to 1.69 × 10-6 (14 to 18 years) | Not applicable |
| Aftershave (face) (cedarwood oil) | 5 | 1.08 (adults) to 1.99 (9 to 13 years) | 5.71 × 10-2 (adults) to 9.60 × 10-2 (9 to 13 years) | Not applicable |
| After hair removal product (body) (cedarwood oil) | 3 | 2.88 (adults) to 3.93 (9 to 13 years) | 4.69 × 10-2 (adults) to 5.96 × 10-2 (9 to 13 years) | Not applicable |
| Body adhesive (cedarwood oil) | 0.1 | 0.14 (adults) to 0.16 (14 to 18 years) | 5.31 × 10-3 (adults) to 6.67 × 10-4 (14 to 18 years) | Not applicable |
a See Appendix A for calculation details.
b Although the upper concentration reported for massage oil containing cedarwood oil was 100%, massage oils are typically diluted prior to use. Thus, the maximum concentration of cedarwood oil in a massage oil was assumed to be 3% (RIVM 2006).
| Product scenario | % in product | Dermala (mg/kg bw/day) | Inhalationa (mg/kg bw/day) |
|---|---|---|---|
| Antiseptic skin cleanser (spray) (NHP) (cedarwood oil) | 0.05 | 1.44 × 10-2 (14 to 18 years) to 4.25 x10-2 (2 to 3 years) | 1.62 × 10-5 (14 to 18 years) to 3.05 × 10-5 (4 to 8 years) |
| Antiseptic skin cleanser (spray) (NHP)b (cedarwood oil) | 0.05 | 0.22 (adults) to 1.06 (2 to 3 years) | 2.30 × 10-4 (adults) to 6.92 × 10-4 (2 to 3 years) |
| Counterirritant (spray) (NHP) (cedarwood oil) | 2 | 0.42 (adults) to 0.74 (9 to 13 years) | 9.35 × 10-4 (adults) to 1.52 × 10-3 (9 to 13 years) |
| Irritation relief balm (NHP) (cedarwood oil) | 1.21 | 0.90 (adults) to 3.03 (1 year) | 1.45 × 10-2 (adults) to 3.43 × 10-2 (4 to 8 years) |
| Aromatherapy – Liquid for steam bowl inhalation (NHP) (cedarwood oil) | 5 | Not applicable | 1.49 × 10-3 (adults) |
| Aromatherapy – Liquid for diffuser inhalation (NHP) (cedarwood oil) | 5 | Not applicable | 5.10 × 10-2 (adults) |
| Aromatherapy – Respiratory spray (NHP) (cedarwood oil) | 0.68 | Not applicable | 3.19 × 10-4 (adults) |
| Acne therapy (gel) (NHP) (cedarwood oil) | 0.02 | 1.22 × 10-2 (adults) to 1.57 × 10-2 (9 to 13 years) | 4.69 × 10-4 (adults) to 5.64 × 10-4 (14 to 18 years) |
Abbreviation: NHP, natural health product
a See Appendix A for calculation details.
b For situations of public health concern, the use of antiseptic skin cleanser among the general population may increase up to 25 uses per day (personal use by adults, increased use by children in schools and childcare facilities) (RIVM 2021a).
| Product scenario | % in product | Dermala (mg/kg bw/day) | Inhalationa (mg/kg bw/day) | Orala (mg/kg bw/day) |
|---|---|---|---|---|
| Air freshener (plug-in) (alpha-cedrene) | 1 | 7.20 × 10-2 (adults) | 4.69 × 10-3 (adults) to 1.67 × 10-2 (1 year) | Not applicable |
| Air freshener (wax melt) (Texan cedarwood oil) | 1 | 3.80 × 10-2 (adults) | 6.94 × 10-3 (adults) to 2.47 × 10-2 (1 year) | Not applicable |
| Liquid laundry detergent (handwash)b (Texan cedarwood oil) | 1 | 1.10 × 10-1 (adults) | 7.30 × 10-5 (adults) | Not applicable |
| Liquid laundry detergent (machine wash)c (Texan cedarwood oil) | 1 | 7.42 × 10-2 (adults) | 6.13 × 10-6 (adults) | Not applicable |
| Liquid laundry detergentd (Texan cedarwood oil) | 1 | 2.74 × 10-3 (1 year) | Not applicable | 7.64 × 10-7 (1 year) |
| Leather maintenance spray (alpha-cedrene) | 5 | 3.97 × 10-1 (adults) | 2.86 × 10-3 (adults) | Not applicable |
| Toilet or urinal cleaning or deodorizing product (automatic toilet bowl cleaner) (alpha-cedrene) | 10 | Negligible | 2.02 × 10-1 (2 to 3 years) | Not applicable |
| Toilet or urinal cleaning or deodorizing product (application) (Texan cedarwood oil) | 1 | 5.22 × 10-2 (adults) | 5.92 × 10-6 (adults) | Not applicable |
a See Appendix A for calculation details.
b Estimated exposure from mixing, loading, handwashing, and hanging laundry.
c Estimated exposure from mixing, loading, and hanging laundry.
d Estimated exposure through migration from washed textiles for 1 year.
| Product scenario | % in product | Dermala (mg/kg bw/day) | Inhalationa (mg/kg bw/day) | Orala (mg/kg bw/day) |
|---|---|---|---|---|
| Aroma diffuser/air freshener (inhalation and refill) | 100 | 1.29 (adults) to 2.27 (9 to 13 years) | 0.55 (adults) to 0.89 (9 to 13 years) | Not applicable |
| Aroma diffuser/air freshener (bystander exposure) | 100 | Not applicable | 1.30 (4 to 8 years) to 1.96 (1 year) | Not applicable |
| Massage oil | 3 | 1.30 (adult) to 8.57 (0 to 5 months) | 6.12 × 10-2 (adults) to 1.24 × 10-1 (1 year) | Not applicable |
| Body moisturizer | 3 | 4.05 (adults) to 9.52 (0 to 5 months) | 2.35 × 10-2 (0 to 5 months) to 5.13 × 10-2 (14 to 18 years) | Not applicable |
| Bath oil | 100 | 9.40 × 10-3 (adults) to 1.20 × 10-2 (9 to 13 years) | 4.16 x 10-6 (9 to 13 years) to 8.86 × 10-3 (adults) | Not applicable |
| Facial steamer/mistb (application) | 100 | 1.42 (adults) to 4.57 (4 to 8 years) | 0.43 (adults) to 1.03 (4 to 8 years) | Not applicable |
| Facial steamer/mistb (bystander exposure) | 100 | Not applicable | 4.61 × 10-1 (1 year) | Not applicable |
| Liquid floor cleanerc | 100 | 9.17 × 10-2 (adults) | 2.24 × 10-4 (adults) | Not applicable |
| Post-application exposure to cleaned floorsd | 100 | 3.48 × 10-2 (1 year) | 8.00 × 10-4 (1 year) | 2.61 × 10-3 (1 year) |
a See Appendix B for calculation details.
b After a total of 4 hours of exposure; once the device is turned off after 20 minutes of use, it is assumed that the person remains in the room for 3 hours and 40 minutes.
c Estimated exposure from mix/load and application.
d Estimated exposure through migration from cleaned floors for 1 year.
7.1.2 Health effects assessment
There is no empirical information or international assessment for alpha- and beta-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, Texan cedarwood oil and T&T cedarwood oil.
Alpha- and beta-cedrene are organic compounds classified as tricyclic sesquiterpenes, alkenes, and isomers of each other (91.77% of structural similarity on the OECD QSAR Toolbox (2019)) and share similar physical-chemical properties.
In the OECD QSAR Toolbox (2019), thujopsene, alpha-cedrene, alpha-gurjunene, and beta-patchoulene had similar structures and metabolites identified by the in vivo rat metabolism simulator, and all 4 substances are tricyclic sesquiterpenes with cycloalkane and unsaturated carbocyclic fragment. Most of the OECD QSAR Toolbox (2019) flags (for example, DNA binding or estrogen-receptor binding, except for protein binding glutathione [moderate reactivity], toxic hazard classification from Cramer [low Class1], rainbow trout estrogen receptor expert system –US EPA [multicyclic hydrocarbons]) were negative for all 4 substances. Alpha-cedrene was determined to be the most suitable analogue for thujopsene, alpha-gurjunene, and beta-patchoulene.
Since thujopsene and alpha- and beta-cedrene are identified as major components of T&T cedarwood, Texan cedarwood, and cedarwood oils, the data on cedarwood oil was used to inform the health effects assessment of the whole subgroup.
In a combined repeated-dose study with reproductive and developmental toxicity screening conducted according to OECD Test Guidelines 422, male and female Wistar Han rats (10/dose/sex) were orally administered 0, 750 ppm (62 and 104 mg/kg bw/day, respectively), 1500 ppm (120 mg/kg bw/day and 207 mg/kg bw/day, respectively), or 5000 ppm (381 mg/kg bw/day and 560 mg/kg bw/day, respectively) cedarwood oil by diet for 28 days in males and for 49 to 62 days in females (two weeks before mating, during mating and gestation, and up to 13 post-natal days [PNDs]). 5 males and 5 females in the control and highest dose groups had a recovery period of 14 days (ECHA 2018). Animals of both sexes showed a decrease in food consumption from pre-mating (-15%), whereas only females showed a decrease through gestation and lactation (-25% and -30%, respectively) at the 560 mg/kg bw/day dose. The body weight gain for males and females was dose-dependently reduced (values are not given by authors) and was significant at the highest doses of 381 mg/kg bw/day and 560 mg/kg bw/day for males and females, respectively. However, this effect was not considered to be adverse since it never exceeded 10%. Nonetheless, the body weights of animals, males and females, treated with 381 mg/kg bw/day and 560 mg/kg bw/day, respectively, were still significantly lower after the recovery period. Plasma levels of alkaline phosphatase activity increased in both sexes, while alanine aminotransferase activity, bilirubin, and cholesterol increased in females only at all doses tested. The magnitude of these changes are not presented in the dossier and are considered non-adverse by the authors in the absence of inflammatory hepatic changes. An increase in liver weights (absolute and relative) and a decrease of the thymus weight (absolute) in females at 560 mg/kg bw/day were observed. After the treatment-free recovery period, the liver and thymus weights of the 560 mg/kg bw/day dosed females were comparable to those of the control females. A hepatocellular hypertrophy (centrilobular) was observed at 207 mg/kg bw/day and above in females, but the animals showed complete recovery after a treatment-free period of 19 days. In the highest dosed group, the serum levels of thyroid hormone T4 in both sexes of the F0 generation were decreased at all doses in a dose-related manner (-32%, -43%, and -59% in males and -19%, -28%, and -53% in females at 62 and 104,120 and 207, and 381 and 560 mg/kg bw/day, respectively). A full recovery was seen in males at the 381 mg/kg bw/day dose after a treatment-free period of 14 days (not determined in females). No changes in thyroid gland were observed. Some organ weight differences were statistically significant (in males) when compared with the control group (that is, brain, heart, epididymides, and kidney), but no further details were provided. Pups in the 560 mg/kg bw/day group showed a decrease in mean body weights on PND 1 (4%), PND 4 (8%), PND 7 (11%), and PND 13 (17%), without any modification to maternal care or clinical signs observed by the authors of the study. No treatment-related changes were noted in any of the other developmental parameters, also no effects were observed in the serum level of thyroid hormone T4 in PND 13-15 pups. A lowest-observed-adverse-effect level (LOAEL) of 750 ppm (corresponding to 62 mg/kg bw/day for males) was determined on the basis of a dose-response decrease of thyroid hormone T4 levels in male (after 28 days of exposure) and female (after 62 days of exposure) rats in this health effects assessment.
In a repeated dermal exposure study, F344/N male and female rats (10/sex/dose) were dermally treated with 0 (vehicle only), 31.25, 62.5, 125, 250, or 500 mg/kg bw/day cedarwood oil dissolved in 95% aqueous ethanol applied to a shaven area on the back below the scapula for 5 days a week over 3 months; an additional group of 10 males and 10 females were untreated (NTP 2016). Formulations were administered at a volume of 0.5 mL/kg bw/day. Except for 2 males in the 500 mg/kg bw/day group that were removed from the study due to severe skin lesions, all rats survived to the end of the study. Mean body weights and body weight gains of males and females in the 250 mg/kg bw/day and 500 mg/kg bw/day groups were significantly lower. Body weight gain of males in the 125 mg/kg bw/day group was also significantly lower. The main clinical observation was on the skin at the site of application, where irritation, thickening, and ulcerations were observed at doses of 125 mg/kg bw/day and above in males and at doses of 62.5 mg/kg bw/day and above in females. Liver weight increased in males at the 2 highest doses and in females at the highest dose. The absolute thymus weight was reduced at the 125 mg/kg bw/day and 500 mg/kg bw/day doses in males, while the absolute and relative thymus weights decreased at the 500 mg/kg bw/day dose in females. In addition, numerous non-neoplastic lesions at the site of application such as epidermis hyperplasia and hyperkeratosis, chronic inflammation, and hyperplasia of the hair follicle and sebaceous gland were seen in females at all doses and in males at doses of 62.5 mg/kg bw/day and above. The presence of bone marrow hyperplasia was significantly higher in males and females at 500 mg/kg bw/day. A significant increase of total white blood cells and neutrophil counts was observed in females at the 250 mg/kg bw/day dose and in both sexes at the 500 mg/kg bw/day dose. This is most likely due to an immune response, as indicated by the presence of ulcerations and chronic inflammation at the application site for the corresponding doses. The no-observed-adverse-effect level (NOAEL) for this study is 62.5 mg/kg bw/day based on the decrease in thymus weight in males at the 125 mg/kg bw/day dose, the decrease in body weight in males and females at the 250 mg/kg bw/day and 500 mg/kg bw/day doses, and the increase in liver weight in males at the 250 mg/kg bw/day and 500 mg/kg bw/day doses and in females at the 500 mg/kg bw/day dose.
In a similar study, B6C3F1 mice (10/sex/dose) were dermally treated with 0 (vehicle only), 125, 250, 500, 1000, or 2000 mg/kg bw/day cedarwood oil for 5 days a week over 3 months (NTP 2016). Formulations were administered at a volume of 2.0 mL/kg bw in 95% aqueous ethanol. The study revealed severe irritation, lesions, and ulcerations of the skin at the site of application, to the point where all mice receiving the highest dose were euthanized prematurely; one male and one female from the 1000 mg/kg bw/day dose group and one male from the 250 mg/kg bw/day dose group were also euthanized. The body weights of 500mg/kg bw/day and 1000 mg/kg bw/day males and 250 mg/kg bw/day females and above were significantly reduced. The absolute liver weights of 1000 mg/kg bw/day males and females and relative liver weights of all dose groups of males and females were significantly higher. The absolute thymus weight of the 500mg/kg bw/day and 1000 mg/kg bw/day doses for males and at the 250 mg/kg bw/day dose and above for females were significantly decreased. The absolute kidney weights of 1000 mg/kg bw/day females and relative kidney weights of 250 mg/kg bw/day and above females were significantly higher. In addition, numerous non-neoplastic lesions at the site of application such as epidermis hyperplasia and hyperkeratosis, chronic inflammation, and hyperplasia of the hair follicle and sebaceous gland were seen in males and females at all doses. Increases in hepatocyte depletion of glycogen were present in the liver at 250 mg/kg bw/day and above in both sexes. Non-neoplastic lesions of lymphoid hyperplasia in mandibular lymph nodes were present at all doses as a secondary effect to lesions of the epidermis. Incidences of myeloid cell hyperplasia in the bone marrow and hematopoietic cell proliferation in the spleen increased at all doses in both sexes. Males showed a significant increase of thymus atrophy at 500 mg/kg bw/day. Hematology effects included increased leukon (white blood cells and differential counts) in females at 500 mg/kg bw/day and treatment-related decreases in erythron (that is, hematocrit, hemoglobin, and erythrocyte counts) in males at 250 mg/kg bw/day or greater and in females at 500 mg/kg bw/day and 1000 mg/kg bw/day. A NOAEL of 125 mg/kg bw/day was selected on the basis of the decrease in thymus weight in females at doses of 250 mg/kg bw/day or higher.
On the basis of the decrease in thymus weight in mice and rats in both sexes, a NOAEL of 62.5 mg/kg bw/day is determined in this health effects assessment for systemic effects.
Cedarwood oil was not genotoxic in bacterial reverse mutation assay using Salmonella typhimurium strains TA98, TA100, TA1535, and TA1537, in the in vitro mammalian cell gene mutation tests using the thymidine kinase gene (ECHA 2018), or in the in vivo micronucleus assay in mice (NTP 2016).
The health effects information available for the other main component of cedarwood oil, cedrol (12.3% to 22.2%), as well as alpha- (5.7% to 16.9%), beta- (14.1% to 46%), and gamma-himachalene (4.8% to 9.7%) and alpha-atlantone (5.2% to 31.9%), were also considered.
Cedrol
International and empirical data on cedrol are limited. On the basis of the only short-term study available and a group read-across, EFSA and the Research Institute for Fragrance Materials concluded that there is no safety concern for cedrol (Bhatia et al. 2008; EFSA 2015).
In a study of chemotherapy against brain tumour, authors of the study treated F344 female rats and Foxn1 nu/nu female mice with 0, 75 mg/kg bw/day or 150 mg/kg bw/day cedrol by subcutaneous injection every 2 days for 10 days (rats) or 20 days (mice) (Chang et al. 2020). Cedrol was able to suppress brain tumour growth in both species by targeting the androgen receptor (AR) and reducing the dihydrotestosterone (DHT)-mediated AR nuclear translocation, downstream gene KLK3/TMPRSS2 expression, and cell proliferation. The results suggest that cedrol may act like an endocrine disruptor.
In a short-term study, Sprague-Dawley male and female rats (10/sex/dose) were orally administered 0 mg/kg bw/day or 8.4 mg/kg bw/day cedrol by gavage in 1% carboxymethycellulose in distilled water, 7 days per week for 4 weeks (Bhatia et al. 2008). No clinical effects were observed in all animals. However, a significant decrease in absolute brain weight and brain-to-body weight and in ovary-to-body weight was observed in treated female rats only. The authors of the study did not consider these effects to be adverse because they were not consistent between sexes and correlative clinical changes were absent. No further details are provided. However, on the basis of the cedrol’s capacity to reduce DHT action on AR in females in the previous study, these effects are not discarded in this health effects assessment, and cedrol is considered to be a potential anti-androgenic substance in female rodents.
Cedrol was found to be negative in a reverse mutation assay in multiple Salmonella typhimurium strains, with and without metabolic activation (Marnette et al. 2014).
Alpha-, beta-, and gamma-himachalene
There are no empirical data on alpha-, beta-, and gamma-himachalene. Because neither beta-himachalene, gamma-himachalene, nor alpha-atlantone were included in the OECD QSAR Toolbox (2019), a read-across approach was not possible on these compounds. A read-across approach was taken on alpha-himachalene, alpha-cedrene, and thujopsene. All 3 were found to be analogues of each other and share similar physical-chemical properties, molecular weight, volatility, and Log kow. All 3 substances are lipophilic sesquiterpenes and multi-cyclic hydrocarbons with cycloalkanes. On the basis of this information, they were assessed all together using the health effects data on cedarwood oil.
7.1.3 Characterization of risk to human health
For the dermal route, the critical effect level identified for the Tricyclic Sesquiterpene subgroup 1 substances was a dermal NOAEL of 62.5 mg/kg bw/day from cedarwood oil. This is based on a decrease in thymus weight after a 90-day exposure period in male rats at 125 mg/kg bw/day and 500 mg/kg bw/day and in female rats at 500 mg/kg bw/day. In mice, the same effects were seen in females at doses of 250 mg/kg bw/day or higher and in male mice at 500 mg/kg bw/day (NTP 2016). This systemic effect on the thymus is supported by a decrease of thymus weight in female rats at a dose of 560 mg/kg bw/day cedarwood oil by the oral route after 69 days of exposure (ECHA 2018).
For the oral and inhalation routes, a LOAEL of 62 mg/kg bw/day (lowest dose tested in males) of cedarwood oil from the one-generation study was used to characterize risk on the basis of the critical health effect of dose-response decrease of thyroid hormone T4 in male and female rats (28-day and 69-day exposures, respectively) (ECHA 2018). The LOAEL in males is considered protective for females since the same adverse effects were observed in female rats at 104 mg/kg bw/day. In addition, because there is no assessment of the effect of cedarwood oil on the risk of developmental neurotoxicity, the LOAEL of 62 mg/kg bw/day cedarwood oil in male rats will be used to support this endpoint. This point of departure (POD) was considered relevant for all age groups independently of the composition and the species of the cedarwood used to extract the oil. This is considered protective for both short- and long-term systemic effects as well as for localized effects during the exposure in question.
Daily exposure estimates to Tricyclic Sesquiterpene subgroup 1 substances for the highest and lowest exposure age groups and resulting MOEs are summarized in Table 7-5 and Table 7-6 for cosmetics and food flavouring, Table 7-7 and Table 7-8 for NHPs, and Table 7-9 and Table 7-10 for other products, respectively. Daily exposure estimates to the Tricyclic Sesquiterpene subgroup 1 substances and resulting MOEs from DIY products are summarized in Table 7-11 and Table 7-12.
| Product scenario | % in product | Dermal exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|
| Massage oil (cedarwood oil, Texan cedarwood oil) (all) | 3 | 1.30 (adults) to 8.57 (0 to 5 months) | 5 (0 to 5 months) to 34 (adults) |
| Fragrance (cedarwood oil) (2 to 3 years to adults) | 100 | 6.33 (14 to 18 years) to 18.70 (2 to 3 years) | 2 (2 to 3 years) to 7 (14 to 18 years) |
| Fragrance (Texan cedarwood oil) (2 to 3 years to adults) | 30 | 1.93 (adults) to 5.61 (2 to 3 years) | 8 (2 to 3 years) to 23 (adults) |
| Fragrance (alpha-cedrene) (2 to 3 years to adults) | 1 | 0.06 (14 to 18 years) to 0.19 (2 to 3 years) | 239 (2 to 3 years) to 704 (14 to 18 years) |
| Deodorant/antiperspirant (solid) (cedarwood oil) (9 to 13 years to adults) | 42 | 4.40 (9 to 13 years) to 7.45 (14 to 18 years) | 6 (14 to 18 years) to 10 (9 to 13 years) |
| Deodorant/antiperspirant (solid) (Texan cedarwood oil) (9 to 13 years to adults) | 10 | 1.05 (9 to 13 years) to 1.77 (14 to 18 years) | 25 (14 to 18 years) to 43 (9 to 13 years) |
| Body moisturizer (cedarwood oil, Texan cedarwood oil) (all) | 10 | 13.51 (adults) to 31.75 (0 to 5 months) | 1 (0 to 5 months) to 3 (adults) |
| Face moisturizer (cedarwood oil) (9 to 13 years to adults) | 10 | 2.42 (14 to 18 years) to 4.05 (adults) | 11 (adults) to 18 (14 to 18 years) |
| Face moisturizer (Texan cedarwood oil) (9 to 13 years to adults) | 5 | 1.21 (14 to 18 years) to 2.03 (adults) | 22 (adults) to 37 (14 to 18 years) |
| Conditioner (leave-on) (cedarwood oil) (2 to 3 years to adults) | 14 | 2.26 (14 to 18 years) to 4.85 (2 to 3 years) | 9 (2 to 3 years) to 20 (14 to 18 years) |
| Shampoo (cedarwood oil, Texan cedarwood oil) (all) | 5 | 8.39 × 10-2 (14 to 18 years) to 3.10 × 10-1 (0 to 5 months) | 144 (0 to 5 months) to 532 (14 to 18 years) |
| Hair perm/straighteners (cedarwood oil) (4 to 8 years to adults) | 0.1 | 0.11 (adults) to 0.29 (4 to 8 years) | 156 (4 to 8 years) to 413 (adults) |
| Temporary hair dye (Texan cedarwood) (4 to 8 years to adults) | 0.1 | 4.73 × 10-2 (adults) to 1.52 × 10-1 (4 to 8 years) | 293 (4 to 8 years) to 944 (adults) |
| Hairspray (Texan cedarwood oil) (4 to 8 years to adults) | 5 | 0.16 (14 to 18 years) to 0.43 (4 to 8 years) | 105 (4 to 8 years) to 283 (14 to 18 years) |
| Bath product (salt) (cedarwood oil, Texan cedarwood oil) (9 to 13 years to adults) | 10 | 1.09 × 10-2 (adults) to 1.39 × 10-2 (9 to 13 years) | 3 217 (9 to 13 years) to 4 098 (adults) |
| Bath product (foam/bubbles) (Texan cedarwood oil) (all) | 5 | 9.32 × 10-3 (9 to 13 years) to 2.99 × 10-2 (14 to 18 years) | 1 495 (14 to 18 years) to 4 788 (9 to 13 years) |
| Bath product (cedarwood oil) | 3.5 | 6.53 × 10-3 (9 to 13 years) to 2.09 × 10-2 (14 to 18 years) | 2 135 (14 to 18 years) to 6 840 (9 to 13 years) |
| Body cleanser (solid) (cedarwood, Texan cedarwood oil) (all) | 100 | 0.18 (adults) to 0.31 (0 to 5 months) | 142 (0 to 5 months) to 250 (adults) |
| Body cleanser (liquid) (cedarwood oil) (all) | 3 | 0.06 (adults) to 0.26 (0 to 5 months) | 174 (0 to 5 months) to 715 (adults) |
| Body exfoliant (Texan cedarwood oil) (14 to 18 years to adults) | 1 | 0.14 (adults) to 0.16 (14 to 18 years) | 277 (14 to 18 years) to 330 (adults) |
| Body exfoliant (cedarwood oil) (14 to 18 years to adults) | 3 | 0.41 (adult) to 0.48 (14 to 18 years) | 92 (14 to 18 years) to 110 (adults) |
| Facial makeup (liquid foundation) (cedarwood oil, Texan cedarwood oil) (4 to 8 years to adults) | 0.3 | 1.98 × 10-2 (14 to 18 years) to 4.43 × 10-2 (4 to 8 years) | 1 007 (4 to 8 years) to 2 250 (14 to 18 years) |
| Shaving cream (face) (cedarwood oil) (9 to 13 years to adults) | 5 | 4.59 × 10-2 (adults) to 7.62 × 10-2 (9 to 13 years) | 586 (9 to 13 years) to 972 (adults) |
| Shaving cream (body) (Texan cedarwood oil) (9 to 13 years to adults) | 10 | 0.17 (adults) to 0.24 (9 to 13 years) | 189 (9 to 13 years) to 260 (adults) |
| Shaving cream (body) (cedarwood oil) (9 to 13 years to adults) | 6.66 | 0.11 (adults) to 0.16 (9 to 13 years) | 284 (9 to 13 years) to 391 (adults) |
| Shaving cream (body) (alpha-cedrene) (9 to 13 years to adults) | 0.1 | 1.72 × 10-3 (adults) to 2.36 × 10-3 (9 to 13 years) | 18 930 (9 to 13 years) to 25 999 (adults) |
| Aftershave (face) (cedarwood oil) (9 to 13 years to adults) | 5 | 1.08 (adults) to 1.99 (9 to 13 years) | 22 (9 to 13 years) to 41 (adults) |
| After hair removal product (body) (cedarwood oil) (9 to 13 years to adults) | 3 | 2.88 (adults) to 3.93 (9 to 13 years) | 11 (9 to 13 years) to 16 (adults) |
| Body adhesive (cedarwood oil) (14 to 18 years to adults) | 0.1 | 0.14 (adults) to 0.16 (14 to 18 years) | 277 (14 to 18 years) to 330 (adults) |
Abbreviation: MOE, margin of exposure.
a Using a dermal NOAEL of 44.64 mg/kg bw/day (62.5 mg/kg bw/day, adjusted for 5 days/week), which is based on a decreased thymus weight at higher doses.
b Target MOE = 100 (10x for interspecies extrapolation × 10x for intraspecies variation). Bolded MOEs are considered potentially inadequate.
| Product scenario | % in product | Route(s) of exposure | Exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|---|
| Food flavouring agent (T&T cedarwood oil) (1 year and older) | - | Oral | 1.41 × 10-4 (1 year and older) | 439 093 (1 year and older) |
| Massage oil (cedarwood oil, Texan cedarwood oil) (all) | 3 | Inhalation | 6.12 × 10-2 (adults) to 1.24 × 10-1 (1 year) | 501 (1 year) to 1 013 (adults) |
| Fragrance (cedarwood oil) (2 to 3 years to adults) | 100 | Inhalation | 1.50 × 10-1 (adults) to 2.63 × 10-1 (2 to 3 years) | 236 (2 to 3 years) to 414 (adults) |
| Fragrance (Texan cedarwood oil) (2 to 3 years to adults) | 30 | Inhalation | 4.51 × 10-2 (adults) to 8.19 × 10-2 (2 to 3 years) | 757 (2 to 3 years) to 1 375 (adults) |
| Fragrance (alpha-cedrene) (2 to 3 years to adults) | 1 | Inhalation | 3.62 × 10-4 (adults) to 7.40 × 10-4 (2 to 3 years) | 83 736 (2 to 3 years) to 171 399 (adults) |
| Deodorant/antiperspirant (solid) (cedarwood oil) (9 to 13 years to adults) | 42 | Inhalation | 6.62 × 10-2 (9 to 13 years) to 1.26 × 10-1 (14 to 18 years) | 355 (14 to 18 years) to 674 (9 to 13 years) |
| Deodorant/antiperspirant (solid) (Texan cedarwood oil) (9 to 13 years to adults) | 10 | Inhalation | 1.56 × 10-2 (9 to 13 years) to 3.08 × 10-2 (14 to 18 years) | 1 451 (14 to 18 years) to 2 870 (9 to 13 years) |
| Body moisturizer (cedarwood oil, Texan cedarwood oil) (all) | 10 | Inhalation | 7.63 × 10-2 (0 to 5 months) to 1.69 × 10-1 (14 to 18 years) | 366 (14 to 18 years) to 812 (0 to 5 months) |
| Face moisturizer (cedarwood oil) (9 to 13 years to adults) | 10 | Inhalation | 9.27 × 10-2 (9 to 13 years) to 1.57 × 10-1 (adults) | 395 (adults) to 669 (9 to 13 years) |
| Face moisturizer (Texan cedarwood oil) (9 to 13 years to adults) | 5 | Inhalation | 6.29 × 10-2 (9 to 13 years) to 1.04 × 10-1 (adults) | 596 (adults) to 986 (9 to 13 years) |
| Conditioner (leave-on) (cedarwood oil) (2 to 3 years to adults) | 14 | Inhalation | 9.23 × 10-2 (14 to 18 years) to 1.35 × 10-1 (4 to 8 years) | 459 (4 to 8 years) to 672 (14 to 18 years) |
| Shampoo (cedarwood oil, Texan cedarwood oil) (all) | 5 | Inhalation | 1.47 × 10-4 (2 to 3 years) to 2.78 × 10-4 (1 year) | 223 200 (1 year) to 421 928 (2 to 3 years) |
| Hair perm/straighteners (cedarwood oil) (4 to 8 years to adults) | 0.1 | Inhalation | 1.60 × 10-5 (14 to 18 years) to 2.51 × 10-5 (4 to 8 years) | 1 780 000 (4 to 8 years) to 2 790 000 (adults) |
| Temporary hair dye (Texan cedarwood) (4 to 8 years to adults) | 0.1 | Inhalation | 7.55 × 10-4 (adults) to 1.79 × 10-3 (4 to 8 years) | 34 721 (4 to 8 years) to 82 119 (adults) |
| Hairspray (Texan cedarwood oil) (4 to 8 years to adults) | 5 | Inhalation | 9.79 × 10-4 (adults) to 2.32 × 10-3 (4 to 8 years) | 26 764 (4 to 8 years) to 63 300 (adults) |
| Bath product (salt) (cedarwood oil, Texan cedarwood oil) (9 to 13 years to adults) | 10 | Inhalation | 3.56 × 10-2 (9 to 13 years) to 4.99 × 10-2 (adults) | 1 242 (adults) to 1 741 (9 to 13 years) |
| Bath product (foam/bubbles) (Texan cedarwood oil) (all) | 5 | Inhalation | 2.54 × 10-3 (9 to 13 years) to 8.56 × 10-3 (14 to 18 years) | 7 243 (14 to 18 years) to 24 458 (0 to 5 months) |
| Bath product (cedarwood oil) (all) | 3.5 | Inhalation | 4.05 × 10-4 (0 to 5 months) to 2.24 × 10-2 (14 to 18 years) | 1 997 (14 to 18 years) to 110 314 (0 to 5 months) |
| Body cleanser (solid) (cedarwood, Texan cedarwood oil) (all) | 100 | Inhalation | 8.08 × 10-3 (0 to 5 months) to 2.14 × 10-2 (14 to 18 years) | 2 089 (14 to 18 years) to 5 528 (0 to 5 months) |
| Body cleanser (liquid) (cedarwood oil) (all) | 3 | Inhalation | 6.61 × 10-4 (0 to 5 months) to 1.54 × 10-3 (14 to 18 years) | 29 013 (14 to 18 years) to 67 568 (0 to 5 months) |
| Body exfoliant (Texan cedarwood oil) (14 to 18 years to adults) | 1 | Inhalation | 2.93 × 10-3 (adults) to 3.69 × 10-3 (14 to 18 year) | 12 000 (14 to 18 years) to 15 000 (adults) |
| Body exfoliant (cedarwood oil) (14 to 18 years to adults) | 3 | Inhalation | 1.06 × 10-3 (adults) to 1.28 × 10-3 (14 to 18 year) | 34 000 (14 to 18 years) to 42 000 (adults) |
| Facial makeup (liquid foundation) (cedarwood oil, Texan cedarwood oil) (4 to 8 years to adults) | 0.3 | Inhalation | 8.21 × 10-4 (14 to 18 years) to 1.25 × 10-3 (4 to 8 years) | 49 400 (4 to 8 years) to 75 550 (14 to 18 years) |
| Lip balm (cedarwood oil) (2 to 3 years to adults) | 3 | Oral | 1.78 × 10-2 (adults) to 4.40 × 10-2 (2 to 3 years) | 1 409 (2 to 3 years) to 3 476 (adults) |
| Mouthwash (cedarwood oil) (4 to 8 years to adults) | 0.1 | Oral | 2.02 × 10-2 (9 to 13 years) to 3.7 × 10-2 (4 to 8 years) | 1 678 (4 to 8 years) to 3 064 (9 to 13 years) |
| Toothpaste (cedarwood oil) (2 to 3 years to adults) | 0.5 | Oral | 1.35 × 10-2 (adults) to 2.03 × 10-1 (2 to 3 years) | 305 (2 to 3 years) to 45 888 (adults) |
| Shaving cream (face) (cedarwood oil) (9 to 13 years to adults) | 5 | Inhalation | 3.47 × 10-4 (14 to 18 years) to 4.25 × 10-4 (adults) | 105 014 (adults) to 128 551 (14 to 18 years) |
| Shaving cream (body) (Texan cedarwood oil) (9 to 13 years to adults) | 10 | Inhalation | 2.06 × 10-2 (adult) to 2.45 × 10-2 (9 to 13 years) | 1 824 (9 to 13 years) to 2 165 (adults) |
| Shaving cream (body) (cedarwood oil) (9 to 13 years to adults) | 6.66 | Inhalation | 1.49 × 10-2 (adults) to 1.76 × 10-2 (9 to 13 years) | 2 539 (9 to 13 years) to 3 000 (adults) |
| Shaving cream (body) (alpha-cedrene) (9 to 13 years to adults) | 0.1 | Inhalation | 1.42 × 10-6 (adults) to 1.69 × 10-6 (14 to 18 years) | 36 702 980 (9 to 13 years) to 43 535 435 (adults) |
| Aftershave (face) (cedarwood oil) (9 to 13 years to adults) | 5 | Inhalation | 5.71 × 10-2 (adults) to 9.60 × 10-2 (9 to 13 years) | 646 (9 to 13 years) to 1 085 (14 to 18 years) |
| After hair removal product (body) (cedarwood oil) (9 to 13 years to adults) | 3 | Inhalation | 4.69 × 10-2 (adults) to 5.96 × 10-2 (9 to 13 years) | 1 041 (9 to 13 years) to 1 321 (adults) |
| Body adhesive (cedarwood oil) (14 to 18 years to adults) | 0.1 | Inhalation | 5.31 × 10-3 (adults) to 6.67 × 10-4 (14 to 18 years) | 11 686 (adults) to 92 985 (14 to 18 years) |
Abbreviation: MOE, margin of exposure.
a Using an oral LOAEL of 62 mg/kg bw/day, which is based on a decreased level of thyroid hormone T4 in the F0 generation.
b Target MOE = 300 (10x for interspecies extrapolation × 10x for intraspecies variation × 3x for use of a LOAEL). Bolded MOEs are considered potentially inadequate.
| Product scenario | % in product | Dermal exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|
| Antiseptic skin cleanser (spray) (NHP) (cedarwood oil) (2 to 3 years to adults) | 0.05 | 1.44 × 10-2 (14 to 18 years) to 4.25 × 10-2 (2 to 3 years) | 1050 (2 to 3 years) to 3100 (14 to 18 years) |
| Antiseptic skin cleanser (spray) (NHP)3 (cedarwood oil) (2 to 3 years to adults) | 0.05 | 0.22 (adults) to 1.06 (2 to 3 years) | 42 (2 to 3 years) to 207 (adults) |
| Counterirritant (spray) (NHP) (cedarwood oil) (9 to 13 years to adults) | 2 | 0.42 (adults) to 0.74 (9 to 13 years) | 60 (9 to 13 years) to 106 (adults) |
| Irritation relief balm (NHP) (cedarwood oil) (1 year to adults) | 1.21 | 0.90 (adults) to 3.03 (1 year) | 15 (1 year) to 50 (adults) |
| Aromatherapy – Liquid for steam bowl inhalation (NHP) (cedarwood oil) (adults) | 5 | Not applicable | Not applicable |
| Aromatherapy – Liquid for diffuser inhalation (NHP) (cedarwood oil) (adults) | 5 | Not applicable | Not applicable |
| Aromatherapy – Respiratory spray (NHP) (cedarwood oil) (adults) | 0.68 | Not applicable | Not applicable |
| Acne therapy (gel) (NHP) (cedarwood oil) (9 to 13 years to adults) | 0.02 | 1.22 × 10-2 (adults) to 1.57 × 10-2 (9 to 13 years) | 2841 (9 to 13 years) to 3671 (adults) |
Abbreviations: MOE, margin of exposure; NHP, natural health product
a Using a dermal NOAEL of 44.64 mg/kg bw/day (62.5 mg/kg bw/day adjusted for 5 days/week), which is based on a decreased thymus weight at higher doses.
b Target MOE = 100 (10x for interspecies extrapolation × 10x for intraspecies variation). Bolded MOEs are considered potentially inadequate.
c For situations of public health concern, the use of antiseptic skin cleanser among the general population may increase up to 25 uses per day (personal use by adults, increased use by children in schools and childcare facilities) (RIVM 2021a).
| Product scenario | % in product | Inhalation exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|
| Antiseptic skin cleanser (spray) (NHP) (cedarwood oil) (2 to 3 years to adults) | 0.05 | 1.62 × 10-5 (14 to 18 years) to 3.05 × 10-5 (4 to 8 years) | 2 032 908 (4 to 8 years) to 3 825 669 (14 to 18 years) |
| Antiseptic skin cleanser (spray) (NHP)c (cedarwood oil) (2 to 3 years to adults) | 0.05 | 2.30 × 10-4 (adults) to 6.92 × 10-4 (2 to 3 years) | 89 579 (2 to 3 years) to 269 250 (adults) |
| Counterirritant (spray) (NHP) (cedarwood oil) (9 to 13 years to adults) | 2 | 9.35 × 10-4 (adults) to 1.52 × 10-3 (9 to 13 years) | 40 874 (9 to 13 years) to 66 293(adults) |
| Irritation relief balm (NHP) (cedarwood oil) (1 year to adults) | 1.21 | 1.45 × 10-2 (adults) to 3.43 x10-2 (4 to 8 years) | 1 809 (4 to 8 years) to 4279 (adults) |
| Aromatherapy – Liquid for steam bowl inhalation (NHP) (cedarwood oil) (adults) | 5 | 1.49 × 10-3 (adults) | 41 670 (adults) |
| Aromatherapy – Liquid for diffuser inhalation (NHP) (cedarwood oil) (adults) | 5 | 5.10 × 10-2 (adults) | 1 215 (adults) |
| Aromatherapy – Respiratory spray (NHP) (cedarwood oil) (adults) | 0.68 | 3.19 × 10-4 (adults) | 194 458 (adults) |
| Acne therapy (gel) (NHP) (cedarwood oil) (9 to 13 years to adults) | 0.02 | 4.69 × 10-4 (adults) to 5.64 x 10-4 (9 to 13 years) | 109 891 (9 to 13 years) to 132 105 (adults) |
Abbreviations: MOE, margin of exposure; NHP, natural health product
a Using a LOAEL of 62 mg/kg bw/day, which is based on a decreased level of thyroid hormone T4 in the F0 generation .
b Target MOE = 300 (10x for interspecies extrapolation × 10x for intraspecies variation × 3x for use of a LOAEL). Bolded MOEs are considered potentially inadequate.
c For situations of public health concern, the use of antiseptic skin cleanser among the general population may increase up to 25 uses per day (personal use by adults, increased use by children in schools and childcare facilities) (RIVM 2021a).
| Product scenario | % in product | Dermal exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|
| Air freshener (plug-in) (alpha-cedrene) (adults) | 1 | 7.20 × 10-2 (adults only) | 620 (adults only) |
| Air freshener (wax melt) (Texan cedarwood oil) (adults) | 1 | 3.80 × 10-2 (adults only) | 1 175 (adults only) |
| Liquid laundry detergent (handwash) (Texan cedarwood oil) (adults) | 1 | 0.11 (adults) | 412 (adults) |
| Liquid laundry detergent (machine wash) (Texan cedarwood oil) (adults) | 1 | 7.42 × 10-2 (adults) | 602 (adults) |
| Liquid laundry detergent (Texan cedarwood oil) (1 year) | 1 | 2.74 × 10-3 (1 year) | 16 297 (1 year) |
| Leather maintenance spray (alpha-cedrene) (adults) | 5 | 3.97 × 10-1 (adults) | 112 (adults) |
| Toilet or urinal cleaning or deodorizing product (Automatic toilet bowl cleaner) (alpha-cedrene) (2 to 3 years) | 10 | Negligible | Not applicable |
| Toilet or urinal cleaning or deodorizing product (application) (Texan cedarwood oil) (adults) | 1 | 5.22 × 10-2 (adults) | 856 (adults) |
Abbreviation: MOE, margin of exposure.
a Using a dermal NOAEL of 44.64 mg/kg bw/day (62.5 mg/kg bw/day adjusted for 5 days/week), which is based on a decreased thymus weight at higher doses.
b Target MOE = 100 (10x for interspecies extrapolation × 10x for intraspecies variation). Bolded MOEs are considered potentially inadequate.
| Product scenario | % in product | Route(s) of exposure | Exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|---|
| Air freshener (plug-in) (alpha-cedrene) (all) | 1 | Inhalation | 4.69 × 10-3 (adults) to 1.67 × 10-2 (1 year) | 3 707 (1 year) to 13 210 (adults) |
| Air freshener (wax melt) (Texan cedarwood oil) (all) | 1 | Inhalation | 6.94 × 10-3 (adults) to 2.47 × 10-2 (1 year) | 2 507 (1 year) to 8 937 (adults) |
| Liquid laundry detergent (handwash) (Texan cedarwood oil) (adults) | 1 | Inhalation | 7.30 × 10-5 (adults) | 849 438 (adults) |
| Liquid laundry detergent (machine wash) (Texan cedarwood oil) (adults) | 1 | Inhalation | 6.13 × 10-6 (adults) | 10 108 730 (adults) |
| Liquid laundry detergent (Texan cedarwood oil) (1 year, incidental exposure) | 1 | Oral | 7.64 × 10-7 (1 year) | 81 142 255 (1 year) |
| Leather maintenance spray (alpha-cedrene) | 5 | Inhalation | 2.86 × 10-3 (adults) | 21 703 (adults) |
| Toilet or urinal cleaning or deodorizing product (Automatic toilet bowl cleaner) (alpha-cedrene) | 10 | Inhalation | 2.02 × 10-1 (2 to 3 years) | 306 (2 to 3 years) |
| Toilet or urinal cleaning or deodorizing product (application) (Texan cedarwood oil) | 1 | Inhalation | 5.92 × 10-6 (adults) | 10 477 278 (adults) |
Abbreviation: MOE, margin of exposure.
a Using a LOAEL of 62 mg/kg bw/day, which is based on a decreased level of thyroid hormone T4 in the F0 generation.
b Target MOE = 300 (10x for interspecies extrapolation × 10x for intraspecies variation × 3x for use of a LOAEL). Bolded MOEs are considered potentially inadequate.
| Product scenario | % in product | Dermal exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|
| Aroma diffuser/air freshener (refill) (9 to 13 years to adults) | 100 | 1.29 (adults) to 2.27 (9 to 13 years) | 20 (9 to 13 years) to 35 (adults) |
| Massage oil (all) | 3 | 1.30 (adult) to 8.57 (0 to 5 months) | 5 (0 to 5 months) to 34 (adults) |
| Body moisturizer (all) | 3 | 4.05 (adults) to 9.52 (0 to 5 months) | 5 (0 to 5 months) to 11 (adults) |
| Bath oil (9 to 13 years to adults) | 100 | 9.40 × 10-3 (adults) to 1.20 × 10-2 (9 to 13 years) | 3722 (9 to 13 years) to 4751 (adults) |
| Facial steamer/mist (application) (4 to 8 years to adults) | 100 | 1.42 (adults) to 4.57 (4 to 8 years) | 10 (4 to 8 years) to 31 (adults) |
| Liquid floor cleaner (adults) | 100 | 9.17 × 10-2 (adults) | 487 (adults) |
| Post-application exposure to cleaned floors (1 year) | 100 | 3.48 × 10-2 (1 year) | 1282 (1 year) |
Abbreviation: MOE, margin of exposure.
a Using a dermal NOAEL of 44.64 mg/kg bw/day (62.5 mg/kg bw/day adjusted for 5 days/week), which is based on a decreased thymus weight at higher doses.
b Target MOE = 100 (10x for interspecies extrapolation × 10x for intraspecies variation). Bolded MOEs are considered potentially inadequate.
| Product scenario | % in product | Route(s) of exposure | Exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|---|
| Aroma diffuser/air freshener (inhalation) (9 to 13 years to adults) | 100 | Inhalation | 5.5 × 10-1 (adults) to 8.9 × 10-1 (9 to 13 years) |
69 (9 to 13 years) to 113 (adults) |
| Aroma diffuser/air freshener (bystander exposure) (4 to 8 years and younger) | 100 | Inhalation | 1.30 (4 to 8 years) to 1.96 (1 year) | 32 (1 year) to 48 (4 to 8 years) |
| Massage oil (all) | 3 | Inhalation | 6.12 × 10-2 (adults) to 1.24 × 10-1 (1 year) | 501 (1 year) to 1 013 (adults) |
| Body moisturizer (all) | 3 | Inhalation | 2.35 × 10-2 (4 to 8 years) to 5.13 × 10-2 (0 to 5 months) | 1 209 (14 to 18 years) to 2639 (0 to 5 months) |
| Bath oil (9 to 13 years to adults) | 100 | Inhalation | 4.16 × 10-6 (9 to 13 years) to 8.86 × 10-3 (adults) | 5 039 107 (adults) to 10 737 523 (9 to 13 years) |
| Facial steamer/mist (application) (4 to 8 years to adults) | 100 | Inhalation | 4.27 × 10-1 (adults) to 1.03 (4 to 8 years) | 60 (4 to 8 years) to 145 (adults) |
| Facial steamer/mist (bystander exposure) (1 year) | 100 | Inhalation | 4.61 × 10-1 (1 year) | 134 (1 year) |
| Liquid floor cleaner (adults) | 100 | Inhalation | 2.24 × 10-4 (adults) | 276 219 (adults) |
| Post-application exposure to cleaned floors (1 year) | 100 | Inhalation | 8.00 × 10-4 (1 year) | 77 500 (1 year) |
| Post application hand-to-mouth exposure from clean floors (1 year) | 100 | Oral | 2.61 × 10-3 (1 year) | 23 731 (1 year) |
Abbreviation: MOE, margin of exposure.
a Using a LOAEL of 62 mg/kg bw/day, which is based on decreased levels of thyroid hormone T4 in the F0 generation.
b Target MOE = 300 (10x for interspecies extrapolation × 10x for intraspecies variation × 3x for use of a LOAEL). Bolded MOEs are considered potentially inadequate.
The MOEs between the critical effect level and the estimates of daily dermal exposure from the use of cedarwood oil in massage oil, fragrance, deodorant/antiperspirant (solid), moisturizer (body and face), conditioner (leave-on), body exfoliant (14 to 18 years), aftershave (face), after hair removal product (body), antiseptic skin cleanser (spray) (NHP) (2 to 8 years, for situations of public health concern resulting in increased use), counterirritant (spray) (NHP) (9 18 years), and irritation relief balm (NHP) are below the target MOE of 100. They are therefore considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
The MOE between the critical effect level and the estimates of daily inhalation exposure from the use of cedarwood oil in fragrance (2 to 3 years) is below the target MOE of 300 and is considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
In addition, the MOEs between the critical effect level and the estimates of daily dermal exposure from the use of cedarwood oil in DIY aroma diffuser/air freshener, DIY massage oil, DIY body moisturizer, and DIY facial steamer/mist are below the target MOE of 100 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
The MOEs between the critical effect level and the estimates of daily inhalation exposure from the use of cedarwood oil in DIY aroma diffuser/air freshener and DIY facial steamer/mist are below the target MOE of 300 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
The MOEs between the critical effect level and the estimates of daily dermal exposure from the use of Texan cedarwood oil in massage oil, fragrance, deodorant/antiperspirant (solid), and moisturizer (body and face) are below the target MOE of 100 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
In addition, the MOEs between the critical effect level and the estimates of daily dermal exposure from the use of Texan cedarwood oil in DIY aroma diffuser/air freshener, DIY massage oil, DIY body moisturizer, and DIY facial steamer/mist are below the target MOE of 100 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
The MOEs between the critical effect level and the estimates of daily inhalation exposure from the use of Texan cedarwood oil in DIY aroma diffuser/air freshener and DIY facial steamer/mist are below the target MOE of 300 and are considered inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
Since there were no identified sources of significant exposure to the general population for T&T cedarwood oil, a qualitative approach to risk characterization was taken, and the risk to human health from T&T cedarwood oil was considered to be low. Similarly, no products containing thujopsene, alpha-gurjunene, beta-patchoulene, or beta-cedrene were identified, and therefore, exposure to the general population was considered to be minimal. Thus, a qualitative approach to risk characterization was taken, and the risk to human health from thujopsene, alpha-gurjunene, beta-patchoulene, and beta-cedrene was considered to be low.
7.1.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| The concentration of the main components in essential oils differs depending on the plant origin, species, temperature, soil, and geography. Therefore, the composition of essential oils in products available to consumers is variable, which represents an uncertainty in the assessment. | +/- |
| There is uncertainty in extrapolating the dermal absorption data from acetyl cedrene and OTNE to cedarwood oil. | +/- |
| The potential use of more than one product containing the same substance by a single person in a day (that is, aggregate exposure) was not considered. This can potentially result in underestimates of exposure for some individuals. | - |
| Cedarwood oil may be used in antiseptic skin cleanser. There is uncertainty regarding the duration of increased antiseptic skin cleanser use that may occur in a situation of public health concern. | +/- |
| There are no hazard data available for alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, or beta-cedrene. The read-across analogue and member of the Tricyclic Sesquiterpene subgroup 1, cedarwood oil, was used to inform the human health risk assessment . | +/- |
+, uncertainty with potential to cause overestimation of exposure/risk; -, uncertainty with potential to cause underestimation of exposure/risk; +/-, unknown potential to cause over- or underestimation of risk.
7.2 Amboryl acetate
7.2.1 Exposure assessment
Amboryl acetate was not reported to be manufactured or imported in quantities greater than the reporting threshold of 100 kg, according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). No reports of monitoring for amboryl acetate in environmental media in Canada or elsewhere were identified. Considering the low quantities of amboryl acetate in Canada, exposure to this substance from environmental media is not expected.
Information obtained pursuant to section 71 of CEPA reported uses of amboryl acetate in personal care products; however, exposure from these uses is minimal, considering the low quantities reported (Environment Canada 2013).
In Canada, there is no expected exposure to amboryl acetate in cosmetics, NHPs, foods and food packaging, or other products available to the general population (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced; personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced; personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
7.2.2 Health effects assessment
Amboryl acetate is assessed individually because it was not identified as an analogue of any of the substances in this assessment.
Amboryl acetate was not identified as posing a high hazard to human health on the basis of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity, or reproductive toxicity. It is also not on ECHA’s Candidate List of Substances of Very High Concern for Authorisation (ECHA 2019). Further investigation of health effects is not warranted at this time, given that exposure of the general Canadian population to amboryl acetate is not expected.
7.2.3 Characterization of risk to human health
Release of amboryl acetate is not expected, exposure to the general population is not expected, and exposure to the environment is expected to be limited. Therefore, the potential to cause harm to the environment or to human health for the general population of Canada is expected to be low.
7.3 Triterpenoid subgroup 2 (enoxolone and allantoin glycyrrhetinic acid)
7.3.1 Exposure assessment
Glycyrrhizic acid is isolated from the dried roots of Glycyrrhiza glabra, also known as licorice, an herb native to central and southwestern Asia and the Mediterranean region. Glycyrrhizic acid is a conjugate of enoxolone and a disaccharide of glucuronic acid. In humans and rats, enoxolone is the main metabolite of glycyrrhizic acid (Isbrucker and Burdock 2006; VKM 2018). Both substances have similar synonyms such as glycyrrhizin; consequently, a consumer may misidentify one for the other.
Environmental media
On the basis of the low manufacture and import quantity (<100 kg) of the substances reported in response to a CEPA section 71 survey (Environment Canada 2013), exposure to enoxolone and allantoin glycyrrhetinic acid from environmental media is not expected. No reports of monitoring for enoxolone and allantoin glycyrrhetinic acid in environmental media in Canada or elsewhere were identified.
Food
No definitive information is available concerning the potential use of enoxolone and allantoin glycyrrhetinic acid as flavouring agents in foods sold in Canada. No definitive information is available concerning the natural occurrence of enoxolone and allantoin glycyrrhetinic acid in foods. Direct exposure to enoxolone from the diet is expected to be very low, if any. Endogenous exposure is expected from the natural presence of glycyrrhizic acid in foods, which in turn, is hydrolyzed to enoxolone by gut microflora (WHO 2005; VKM 2018).
No Canadian consumption data suitable for licorice tea and black licorice candy were identified. In the Dutch National Food Consumption Survey, a mean daily consumption of 13 g licorice was reported (Hulshof and Kistemaker 1994). In 19 samples of licorice candies on the Dutch market, glycyrrhizic acid content ranged from 0.03% to 0.51% (equivalent to 0.017% to 0.29% of enoxolone) (Maas 2000). In a recent study, a mean glycyrrhizic acid concentration of 0.1% (equivalent to 0.057% enoxolone) was reported in 137 samples of licorice candies on the Danish market (Ballin et al. 2023). In brewed teas, a mean glycyrrhizic acid concentration of 114 mg/L (equivalent to 65 mg/L of enoxolone) was reported (Ballin et al. 2023).
The per capita (“individual”) intake from food uses of licorice root, licorice root extract powder, licorice root extract, and ammoniated glycyrrhizin is estimated to be 1.63 mg/day (0.027 mg/kg bw/day, equivalent to 0.015 mg/kg bw/day of enoxolone, assuming complete hydrolysis of glycyrrhizin in the gut) for the US population on the basis of production volumes reported by the food industry (Isbrucker and Burdock 2006). In the absence of data on the actual use, if any, of licorice root, licorice root extract powder, licorice root extract and ammoniated glycyrrhizin in foods sold in Canada, the per capita intake for the US population is an acceptable estimate of potential average daily lifetime dietary exposure to glycyrrhizic acid for the Canadian general population 1 year of age and older. The per capita intake assumes that consumption of these licorice ingredients is evenly distributed throughout the population, thus potentially underestimating exposure for consumers who may seek out licorice-containing foods and potentially overestimating exposure for individuals who do not like licorice flavour. It is possible that acute exposures in some consumers with a penchant for licorice flavour may well exceed the estimated per capita intake, even though excess intake of licorice-containing foods is not expected to occur on a daily basis for the majority of consumers. To assess enoxolone exposure for consumers who regularly consume licorice-containing foods, exposure estimates from the regular consumption of licorice tea and the short-term, high-level consumption of black licorice candy were considered. The highest exposure to enoxolone from licorice tea containing a mean glycyrrhizic acid concentration of 114 mg/L and a mean glycyrrhizic acid concentration of 0.1% in black licorice candy were considered (Ballin et al. 2023).
Products available to consumers
Enoxolone is present in products available to consumers. To evaluate the potential for exposure to enoxolone from cosmetics, NHPs and NPDs applied by the dermal route, sentinel scenarios were selected on the basis of a combination of use frequencies and reported concentrations of enoxolone in these products. These scenarios represented the highest exposures, relative to other dermally applied cosmetics, as well as NHPs and NPDs where enoxolone is used as a NMI, on the basis of identified products reported to contain this substance. Exposure to enoxolone from the use of facial makeup (liquid foundation), face moisturizer (cosmetic and NHP), body moisturizer (lotion and spray), bath product (foam/bubbles), cleanser (face), makeup remover (face and eye), shampoo, conditioner (rinse-off), deodorant/antiperspirant (solid), shaving cream (face), hairspray (pump), permanent hair dye, sunscreen (cream) (NHP and NPD), analgesic patch (NHP), acne therapy (cream) (NHP), and medicated skin care product (cream) (NHP) were considered to be the sentinel scenarios for dermal applications.
To estimate systemic exposure derived from dermal exposure to the Triterpenoid subgroup 2 substances, a dermal absorption value of 25% was used on the basis of the following consideration:
- Enoxolone, a representative Triterpenoid subgroup 2 substance, has a molecular weight of 470 g/mol and log Kow of >6. A value of 10% dermal absorption is considered (SCCS 2010) for a substance with a molecular weight of >500 g/mol and log Kow of >4. As the molecular weight of enoxolone is below 500 g/mol, a default dermal absorption rate value was conservatively adjusted.
The dermal route of exposure is expected to be the predominant route of exposure from products available to consumers. Owing to the low vapour pressure of Triterpenoid subgroup 2 substances (that is, 3.88 × 10-13 Pa) and the identified uses, the inhalation route is not expected to be a significant route of exposure, except for body moisturizer (spray), which has a reported upper concentration of 3%, and hairspray (pump), which has a reported upper concentration of 0.1%, where inhalation exposure to droplets of the product during application were quantified (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
Enoxolone is also present in toothpaste (cosmetic and NHP) and lip balm (NHP). Exposures by the oral route from using toothpaste (cosmetic), toothpaste (NHP), and lip balm were quantified at an upper concentration of 0.1%, 0.05%, and 1%, respectively (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, and the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced).
Products available to consumers containing allantoin glycyrrhetinic acid in Canada were not identified.
Given the number of products containing enoxolone that are present in the Canadian market, it is possible that exposure from several different types of products (for example, cosmetics, NHPs, and NPDs) and from different routes of exposure may occur on the same day (that is, aggregate exposure). Exposures are expected to occur primarily by the dermal or oral routes of exposure.
Combined systemic exposures from products available to consumers are summarized in Table 7-14.
| Product scenario | % in product | Route(s) of exposure | Systemic exposure range (mg/kg bw/day)a |
|---|---|---|---|
| Facial makeup (liquid foundation) | 0.1 | Dermal | 1.65 × 10-3 (14 to 18 years) to 3.70 × 10-3 (4 to 8 years) |
| Face moisturizer | 30 | Dermal | 1.81 (14 to 18 years) to 3.04 (adults) |
| Body moisturizer (spray) | 3 | Dermal and inhalation | 0.45 (adults) to 2.53 (0 to 5 months) |
| Body moisturizer (lotion) | 3 | Dermal | 1.01 (adults) to 2.38 (0 to 5 months) |
| Lip balm | 1 | Oral | 5.95 × 10-3 (adults) to 8.87 × 10-3 (14 to 18 years) |
| Bath product (foam/bubbles) | 3 | Dermal | 1.40 × 10-3 (9 to 13 years) to 4.48 × 10-3 (14 to 18 years) |
| Facial cleanser | 10 | Dermal | 1.60 × 10-2 (14 to 18 years) to 2.21 × 10-2 (9 to 13 years) |
| Facial makeup remover (lotion) | 0.1 | Dermal | 1.48 × 10-3 (adults) to 2.37 × 10-3 (4 to 8 years) |
| Shampoo | 0.3 | Dermal | 1.26 × 10-3 (14 to 18 years) to 4.64 × 10-3 (0 to 5 months) |
| Conditioner (rinse-off) | 0.3 | Dermal | 1.21 × 10-3 (14 to 18 years) to 2.60 × 10-3 (2–3 years) |
| Deodorant/antiperspirant (solid) | 0.1 | Dermal | 6.18 × 10-4 (9 to 13 years) to 1.35 × 10-3 (adults) |
| Shaving cream (face) | 0.1 | Dermal | 2.30 × 10-4 (adults) to 3.81 × 10-4 (9 to 13 years) |
| Hairspray (pump) | 0.1 | Dermal and inhalation | 4.08 × 10-4 (14 to 18 years) to 1.59 × 10-3 (adults) |
| Permanent hair dye | 0.1 | Dermal | 4.48 × 10-2 (adults) to 5.35 × 10-2 (14 to 18 years) |
| Sunscreen (cream) (NHP) | 0.5 | Dermal | 4.30 × 10-1 (adults) |
| Sunscreen (cream) (NPD) | 0.5 | Dermal | 2.63 × 10-1 (9 to 13 years) to 1.19 (6 to 11 months) |
| Analgesic patch (NHP) | 0.18 | Dermal | 1.10 × 10-2 (adults) to 1.16 × 10-1 (2 to 3 years) |
| Acne therapy (cream) (NHP) | 0.3 | Dermal | 4.56 × 10-2 (adults) to 5.89 × 10-2 (9 to 13 years) |
| Medicated skin care product (cream) (NHP) | 0.2 | Dermal | 5.16 × 10-2 (14 to 18 years) to 1.52 × 10-1 (1 year) |
| Sunscreen (lotion) (NHP) | 0.1 | Dermal | 1.01 × 10-2 (adults) |
| Toothpaste | 0.1 | Oral | 2.70 × 10-3 (adults) |
| Toothpaste (NHP) | 0.05 | Oral | 1.35 × 10-3 (adults) to 2.03 × 10-2 (2 to 3 years) |
Abbreviations: NHP, natural health product; NPD, non-prescription drug.
a See Appendix A for calculation details. Dermal absorption was assumed to be 25%, except for the medicated skin care product (cream) (NHP) scenario, where dermal absorption was assumed to be 100%.
7.3.2 Health effects assessment
There are no empirical data or international assessments on allantoin glycyrrhetinic acid. There are limited empirical data on enoxolone.
In rats, enoxolone is able to cross the placenta barrier and can be detected in the fetus (Isbrucker and Burdock 2006). In an in vivo rat study, enoxolone inhibited the metabolism of cortisone and cortisol by inhibiting 11β-hydroxysteroid dehydrogenase (11β-HSD) (Lin et al. 2012). A human ex vivo study showed that the enzymatic activity of 11β-HSD in human placenta cells was inhibited (Blum et al. 1995; Benediktsson et al. 1997), which can be accompanied by an increase of cortisol levels in umbilical cord blood and a decrease of fetal weights in preeclamptic patients (McCalla et al. 1998).
Pregnant Wistar rats (6/dose) were fed 0, 10, 100, or 1000 mg/kg bw/day enoxolone in the diet from gestational day (GD) 13 until term (Hundertmark et al. 2002). Fetuses were analyzed at GDs 17, 19, and 21 and at PND 1. No maternal toxicity was observed. A reduction of pulmonary 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) activity in cultured fetal lung cells from the high-dose group of enoxolone-treated dams impaired fetal lung maturation. Cultured lung cells from fetuses in the high-dose group of enoxolone-treated dams had lower surfactant protein-A (mRNA and protein) levels. Histopathology from high-dose fetuses showed abnormal alveolar wall. Other doses were not tested. A dose-dependent decrease in amniotic fluid lecithin/sphingomyelin ratio (L/S ratio) was observed. The L/S ratio is a traditional standard test that was developed in the 1970s to help clinicians assess fetal lung maturation and the risk of the neonate developing respiratory distress syndrome in pregnant people (Gluck et al. 1971).
Adult Wistar males (10/dose) were administered 0 or 4.5 mg/kg bw/day enoxolone or glycyrrhizic acid for 20 days by gavage (Sakamoto and Wakabayashi 1988). After administration, their testes were removed, and the capacity of the Leydig cells to produce testosterone was tested in vitro. Glycyrrhizic acid and enoxolone caused a significant decrease in testosterone production, with an accumulation of 17α-hydroxyprogesterone. Glycyrrhizic acid and enoxolone are able to inhibit the conversion of androstenedione to testosterone by inhibiting the 17β-hydroxysteroid dehydrogenase. Enoxolone is classified as a potential endocrine disruptor in this assessment.
Adult women and men volunteers (15/sex/dose) with a mean age of 35 years and 33.4 years, respectively, consumed 100 g of licorice containing 150 mg enoxolone daily for 9 weeks (equivalent to 1.7 mg/kg bw/day enoxolone for men and 2.1 mg/kg bw/day enoxolone for women), with a 4-week period of no licorice consumption (Sigurjonsdottir et al. 2006). A significant decrease in serum dehydroepiandrosterone was observed after 4 weeks of licorice consumption in men only; however, levels returned to normal after the 4-week period of cessation. Nevertheless, levels of testosterone and serum dehydroepiandrosterone were significantly higher after the 4-week cessation in women only. Enoxolone seems to disrupt androgen serum levels in humans, with different effects between sexes.
In an in vivo zebrafish toxicity assay model, fish were exposed to between 1 µM and 5 µM of enoxolone. A dose-dependant decrease in the survival rate of embryos was observed and reached 60% at the highest dose after 96 hours. The hatching rate decreased significantly at all doses after 48 hours post fertilization (hpf). The hatching rate declined significantly at all doses after 72 hpf and reached 57.8% at the highest dose. The authors of the study observed a significant decrease of heart rate in the zebrafish embryos exposed to the 3 highest doses. The 2 highest doses significantly reduced the length of animals (Zhong et al. 2022). This study supports the risk that enoxolone may impair fetal development.
Because the empirical data on enoxolone are limited, hazard information on the precursor, glycyrrhizic acid, was used to inform the health effects assessment.
In the OECD QSAR Toolbox (2019), enoxolone was proposed as the main metabolite of glycyrrhizic acid by the in vivo rat metabolism simulator, and both substances are pentacyclic triterpenoids with cycloketone and carboxylic acid. Both substances share alerts on protein binding potency human Cell Line Activation Test (h-CLAT), keratinocyte gene expression, in vitro and in vivo mutagenicity, and carcinogenicity and are not bioavailable.
The JECFA confirmed that consumption of glycyrrhizic acid should not exceed 100 mg/day (about 2 mg/kg bw/day) and recognized that adverse effects may occur below this limit for vulnerable populations (for example, pregnant and breastfeeding people and children). However, the JECFA was not able to establish an acceptable daily intake (ADI) for vulnerable populations because of the uncertainty of the database (WHO 2009). The Nordic Council of Ministers (NCM) concluded that it was not possible to determine a safety limit on the basis of adverse effects produced such as hypermineralocorticoidism, which results in sodium retention and potassium loss, oedema, increased blood pressure, and depression of the renin-angiotensin-aldosterone system, with great interindividual variation (Nordic Council of Ministers 1993). The NCM estimated a provisional LOAEL of approximately 100 mg/day for the short-term (>1 to 2 weeks) intake of glycyrrhizic acid in sensitive adults (for example, those with pre-existing medical conditions) based on clinical studies including case reports (Nordic Council of Ministers 1993). The European Medicines Agency (EMA) determined a safety dose of between 80 mg/day and 100 mg/day of glycyrrhizic acid for short-term use only on the basis of hypokalemia and hypertension following chronic use at the highest doses (EMA 2013). The EMA stated that this safety dose was not recommended for pregnant and breastfeeding people, children, and adolescents (˂18 years). The United States Food and Drug Administration (US FDA) warned consumers that black licorice contains glycyrrhizin, which can cause diminished potassium levels in the body, abnormal heart rhythms, high blood pressure, edema (swelling), lethargy, and congestive heart failure (US FDA 2017). The US FDA advised against eating large amounts of black licorice at one time and to refrain from further ingestions should any of the previously cited symptoms appear. According to the study carried out by ANSES and the French poison control centres over the period of 2012 to 2021, ANSES recommends consuming no more than 10 mg/day glycyrrhizin, consuming glycyrrhizin only occasionally, and avoiding consumption from multiple sources of intake (for example, food, medication) (ANSES 2022).
On the basis of available information, the absorption, distribution, metabolism, and excretion of glycyrrhizin appear to be relatively similar between humans and rats (VKM 2018). Glycyrrhizic acid is poorly absorbed from the gastrointestinal tract; instead, it is hydrolyzed by intestinal bacteria to enoxolone, which is rapidly absorbed by the gut. The absorption of enoxolone by the human gut is complete, regardless of whether the enoxolone is formed by hydrolysis of the glycyrrhizic acid or is initially present as the glycoside or aglycone in a food matrix (WHO 2009). Enoxolone is conjugated in the liver before being excreted in the bile; the bacteria in the gut hydrolyze the new metabolites, leading to enterohepatic recycling in rats and, presumably, in humans as well (WHO 2009; EMA 2013; VKM 2018). As a result of the enterohepatic recycling, the complete elimination of enoxolone may take several days, with the potential for accumulation increasing with long-term exposure (Isbrucker and Burdock 2006).
In a short-term study, male and female Wistar rats (unknown numbers) were orally administered 0, 310, 630, 1250, or 2500 mg/kg bw/day of licorice containing 53% glycyrrhizin, that is, glycyrrhizic acid (equivalent to 0, 164.3, 333.9, 662.5, or 1325 mg/kg bw/day) by gavage for 90 days (Isbrucker and Burdock 2006). Body weight in both sexes decreased at the highest dose. At 1250 mg/kg bw/day and above, hematological evaluation revealed a significant decrease in red blood cell counts and in hematocrit in males but not in females. Males administered the highest dose showed a significantly elevated neutrophil count and a lower lymphocyte count. The serum biochemistry showed significantly elevated levels of total protein, albumin, aspartate transaminase, and alanine aminotransferase in male rats but was significantly decreased in females at 1250 mg/kg bw/day and above. Serum cholesterol also decreased in both male and female rats at all doses. The liver and kidney weights increased in the 1250 mg/kg bw/day and 2500 mg/kg bw/day groups, males and females, but there were no histopathological changes in these organs. At the highest dose, histopathology showed an atrophy of the thymus medulla, along with some lymphofollicular formations and an atrophy and catarrh of the stomach mucosa.
In an epidemiological pilot study, 16 healthy volunteers (eight women and 8 men per dose) aged 19 to 30 years, with a body weight range of 58 kg to 71 kg for women and 56 kg to 91 kg for men, were orally administered 0, 400, or 800 mg/day of glycyrrhizic acid (equivalent to 0, 6.6, or 13.3 mg/kg bw/day as determined by the authors) in capsules for 4 weeks, followed by a two-week recovery period (van Gelderen et al. 2000). All volunteers showed edema after a week of ingestion and an increase in body weight (up to 6 kg for some). The serum potassium concentration decreased in all volunteers for both doses and sexes, especially in women. A considerable decrease was reported in the aldosterone concentration and plasma renin activity for both doses and sexes, an effect that was, again, more marked in women. The symptoms observed were similar to the state of apparent mineralocorticoid excess.
In an epidemiological study, 39 healthy female volunteers aged 19 to 40 years with a body weight range of 55 kg to 83 kg were orally administered 0, 1, 2, or 4 mg/kg bw/day glycyrrhizic acid in capsules for 6 weeks, followed by a two-week recovery period (van Gelderen et al. 2000). The experiment was performed according to a randomized double-blind treatment scheme. The volunteers were asked to fill in a dietary questionnaire for 3 days every fortnight, and their physical condition was evaluated weekly. No change in body weight was observed. Authors of the study observed a dose-related increase in headache, nausea, and vomiting, an effect that was significant at 4 mg/kg bw/day. An increase in defecation pattern, swollen face, and tingling in arms and legs was observed in the 4 mg/kg bw/day group. While the aldosterone concentration in serum was significantly lower in the 4 mg/kg bw/day group, no significant differences were observed in the 1 mg/kg bw/day and 2 mg/kg bw/day groups. Plasma renin activity decreased at all doses but was significant in the 4 mg/kg bw/day group only. The systolic and diastolic blood pressure increased at 2 mg/kg bw/day and was significantly higher at 4 mg/kg bw/day.
In a cross-sectional study, the effect of maternal consumption of glycyrrhizin (glycyrrhizic acid) was studied in a sample of 1049 Finnish women and their healthy infants (Strandberg et al. 2001). Glycyrrhizin intake was calculated using detailed questionnaires on licorice consumption, which were answered by the mothers while they were in the maternity ward. Weekly glycyrrhizin intake was calculated from the reported quantity (in grams) and frequency (never, seldom, weekly, daily) of licorice consumption (as brand names). A list of all brands of licorice-containing confectionery sold in Finland, which was based on a report prepared by the National Food Administration in 1993 and updated with information from manufacturers, was used. Glycyrrhizin intake was analyzed both as a continuous variable and as a categorical variable grouped into 3 levels: low (≤250 mg/week; n = 751), moderate (250 mg/week to 499 mg/week; n = 145), and heavy (≥500 mg/week; n = 110), which comprised 75%, 14%, and 11% of the births, respectively. All data on gestation and infants were obtained from hospital records. No change in infant body weight was observed, but the heavy-dose group was born significantly earlier (before 38 weeks).
In a second cross-sectional study, the same author examined whether this association applied to preterm births (delivery before 37 weeks). A sample of 95 Finnish women who delivered preterm infants was compared with a sample of 107 women who delivered their babies after 38 weeks (Strandberg 2002). Glycyrrhizin intake was calculated using an approach similar to that in Strandberg et al. (2001). Glycyrrhizin exposure was grouped into 3 levels: low (≤250 mg/week), moderate (250 mg/week to 499 mg/week), and heavy (≥500 mg/week). The heavy consumption was associated with a more than two-fold increased risk of preterm birth (˂37 weeks) and three-fold increased risk of early preterm birth (˂34 weeks) for the fully adjusted model (mother’s age, sex, parity, and smoking). The authors concluded that the heavy glycyrrhizin exposure was associated with preterm delivery via an increased cortisol level in the placenta.
Using the same cohort from the 2 previous studies, the cognitive performance of children prenatally exposed to glycyrrhizin was assessed (Räikkönen et al. 2009). The authors of the study performed tests from the Wechsler Intelligence Scale for Children III and the Children’s Developmental Neuropsychological Assessment on 371 healthy children with a mean age of 8.1 years. The Beery Developmental Test of Visual-Motor Integration was also performed on the same children to observe cognitive performance. Finally, authors conducted the Child Behavior Checklist to detect psychiatric symptoms in these children. Occupational status, educational level, maternal health, smoking habits, alcohol consumption, and stress did not differ between groups. Children exposed to heavy levels (≥500 mg/week) of glycyrrhizin showed significant decrements in verbal and visuospatial abilities and in narrative memory, and significant increases in externalizing symptoms as well as in attention, rule-breaking, and aggression problems. The maternal glycyrrhizin consumption and the effects on cognitive performance showed a linear association. However, the children’s own licorice intake was not reported by the authors of the study. The authors concluded that a potential prenatal overexposure to glucocorticoids may have led to the adverse effects mentioned.
The same authors measured levels of diurnal salivary cortisol in the same children, who had a mean age of 8.1 years (range of 7.4 to 8.8), during the Trier Social Stress Test for Children (Räikkönen et al. 2010). Children prenatally exposed to heavy levels of glycyrrhizin (that is, ≥500 mg/week) showed a significantly higher level of salivary cortisol than the zero-low exposure group (that is, 0 to 250 mg/week). These effects appeared to be dose-related. The authors concluded that a prenatal exposure to heavy levels of glycyrrhizin could change the hypothalamic-pituitary-adrenocortical axis function in children.
In the same children, but who now had a mean age of 12.5 years (n = 328), the same authors measured pubertal maturation (for example, height, weight, body mass index for age, difference between current and expected adult height, Tanner staging, and score on the Pubertal Development Scale), neuroendocrine function (for example, diurnal salivary cortisol and dexamethasone suppression), cognition (for example, neuropsychological tests), and psychiatric problems (for example, using the Child Behavior Checklist) (Räikkönen et al. 2017). The adolescents’ own licorice consumption (never, less than once a week, once a week, 2 to 4 days a week, daily, no answer) was reported in this study and used as a potential confounder. Girls prenatally exposed to heavy consumption of glycyrrhizin (that is, ≥500 mg/week) were significantly taller, had a higher body mass index, and had a more advanced pubertal development. Similar findings were not seen in boys. Girls and boys prenatally exposed to heavy consumption of glycyrrhizin (that is, ≥500 mg/week) scored significantly lower on intelligence quotient tests, had poorer memory, and were 3.3 times more likely to have attention deficit/hyperactivity disorder problems. No differences in cortisol levels were found at this age. The authors concluded on a LOAEL of ≥500 mg/week (equivalent to 0.91 mg/kg bw/day on the basis of the lowest dose intake estimated by the authors inducing adverse effects), which is based on the developmental neurotoxicity.
Glycyrrhizic acid was not genotoxic in Ames assay with Salmonella typhimurium TA98 with or without metabolic activation and in COMET assay in male mice in various organs (Isbrucker and Burdock 2006).
7.3.3 Characterization of risk to human health
The critical effect level identified for Triterpenoid subgroup 2 was an oral developmental neurotoxicity LOAEL of ≥500 mg/week (equivalent to 0.91 mg/kg bw/day on the basis of the lowest dose intake estimated by the authors inducing adverse effects) glycyrrhizin in children prenatally exposed via maternal consumption during pregnancy (Räikkönen et al. 2009; Räikkönen et al. 2017). A decrease in verbal and visuospatial abilities and in narrative memory, increases in externalizing symptoms, and attention, rule-breaking, and aggression problems were observed at 8.1 years. At 12.5 years, the same children had lower scores on intelligence quotient tests, poorer memory and a higher incidence of attention deficit/hyperactivity disorder problems. Developmental neurotoxicity effects are supported by the ability of enoxolone to disrupt placental 11β-HSD enzyme to inactive cortisol before it reaches the fetus, leading to higher levels of fetal cortisol exposure (Blum et al. 1995; Benediktsson et al. 1997). In addition, there is a risk of increased blood pressure and blood potassium levels, thereby resulting in potential maternal arrhythmias and potential life-threatening effects on the fetus. Considering the severity of the developmental effects and the uncertainty associated with the neurological developmental and endocrine disruptor effects of enoxolone in fetuses and children, this POD was considered relevant for people of childbearing age, pregnant and breastfeeding people, fetuses, and children.
The developmental critical effect is also supported by an oral LOAEL of 10 mg/kg bw/day enoxolone (lowest dose tested) on the basis of a reduction of pulmonary 11β-HSD1 activity in all enoxolone-treated fetuses, impaired fetal lung maturation in rats, and lower surfactant protein-A (mRNA and protein) levels. In addition, a dose-dependent decrease in amniotic fluid lecithin/sphingomyelin ratio was observed in rat fetuses without maternal toxicity, which is a marker of the risk of the neonate developing respiratory distress syndrome (Gluck et al.1971; Hundertmark et al. 2002).
Daily systemic exposure estimates to enoxolone for the highest and lowest exposed age groups and resulting MOEs from cosmetics NHPs, and foods are summarized in Table 7-15.
| Product scenario | % in product | Route(s) of exposure | Systemic exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|---|
| Facial makeup (liquid foundation) (4 to 8 years to adults) | 0.1 | Dermal | 1.65 × 10-3 (14 to 18 years) to 3.70 × 10-3 (4 to 8 years) | 246 (4 to 8 years) to 550 (14 to 18 years) |
| Face moisturizer (9 to 13 years to adults) | 30 | Dermal | 1.81 (14 to 18 years) to 3.04 (adults) | 0.30 (adults) to 0.50 (14 to 18 years) |
| Body moisturizer (spray) (all) | 3 | Dermal and inhalation | 0.45 (adults) to 2.53 (0 to 5 months) | 2.03 (0 to 5 months) to 0.36 (adults) |
| Body moisturizer (lotion) (all) | 3 | Dermal | 1.01 (adults) to 2.38 (0 to 5 months) | 0.38 (0 to 5 months) to 0.90 (adults) |
| Lip balm (9 to 13 years to adults) | 1 | Oral | 5.95 × 10-3 (adults) to 8.87 × 10-3 (14 to 18 years) | 103 (14 to 18 years) to 153 (adults) |
| Bath product (foam/bubbles) (all) | 3 | Dermal | 1.40 × 10-3 (9 to 13 years) to 4.48 × 10-3 (14 to 18 years) | 203 (14 to 18 years) to 651 (9 to 13 years) |
| Facial cleanser (9 to 13 years to adults) | 10 | Dermal | 1.60 × 10-2 (14 to 18 years) to 2.21 × 10-2 (9 to 13 years) | 41 (9 to 13 years) to 57 (14 to 18 years) |
| Facial makeup remover (lotion) (4 to 8 years to adults) | 0.1 | Dermal | 1.48 × 10-3 (adults) to 2.37 × 10-3 (4 to 8 years) | 384 (4 to 8 years) to 613 (adults) |
| Shampoo (all) | 0.3 | Dermal | 1.26 × 10-3 (14 to 18 years) to 4.64 × 10-3 (0 to 5 months) | 196 (0 to 5 months) to 723 (14 to 18 years) |
| Conditioner (rinse-off) (2 to 3 years to adults) | 0.3 | Dermal | 1.21 × 10-3 (14 to 18 years) to 2.60 × 10-3 (2 to 3 years) | 350 (2 to 3 years) to 752 (14 to 18 years) |
| Deodorant/antiperspirant (solid) (9 to 13 years to adults) | 0.1 | Dermal | 6.18 × 10-4 (9 to 13 years) to 1.35 × 10-3 (adults) | 672 (adults) to 1 473 (9 to 13 years) |
| Shaving cream (face) (9 to 13 years to adults) | 0.1 | Dermal | 2.30 × 10-4 (adults) to 3.81 × 10-4 (9 to 13 years) | 2 389 (9 to 13 years) to 3961 (adults) |
| Hairspray (pump) (4 to 8 years to adults) | 0.1 | Dermal and inhalation | 4.08 × 10-4 (14 to 18 years) to 1.59 × 10-3 (adults) | 571 (adults) to 2 232 (14 to 18 years) |
| Permanent hair dye (14 to 18 years to adults) | 0.1 | Dermal | 4.48 × 10-2 (adults) to 5.35 × 10-2 (14 to 18 years) | 17 (14 to 18 years) to 20 (adults) |
| Sunscreen (cream) (NHP) (adults) | 0.5 | Dermal | 4.30 × 10-1 (adults) | 2.11 (adults) |
| Sunscreen (cream) (NPD) (6 to 11 months to adults) | 0.5 | Dermal | 2.63 × 10-1 (9 to 13 years) to 1.19 (6 to 11 months) | 0.77 (6 to 11 months) to 3.47 (9 to 13 years) |
| Analgesic patch (NHP) (2 to 3 years to adults) | 0.18 | Dermal | 1.10 × 10-2 (adults) to 1.16 × 10-1 (2 to 3 years) | 8 (2 to 3 years) to 83 (adults) |
| Acne therapy (cream) (NHP) (9 to 13 years to adults) | 0.3 | Dermal | 4.56 × 10-2 (adults) to 5.89 × 10-2 (9 to 13 years) | 15 (9 to 13 years) to 20 (adults) |
| Medicated skin care product (cream) (NHP) (6 to 11 months to adults) | 0.2 | Dermal | 5.16 × 10-2 (14 to 18 years) to 1.52 × 10-1 (1 year) | 6 (1 year) to 18 (14 to 18 years) |
| Sunscreen (lotion) (NHP) (adults) | 0.1 | Dermal | 1.01 × 10-2 (adults) | 90 (adults) |
| Toothpaste (adults) | 0.1 | Oral | 2.70 × 10-3 (adults) | 34 (adults) |
| Toothpaste (NHP) (2 to 3 years to adults) | 0.05 | Oral | 1.35 × 10-3 (adults) to 2.03 × 10-2 (2 to 3 years) | 45 (2 to 3 years) to 673 (adults) |
| Dietary intake (licorice as glycyrrhizin) (1 year and older) | Various | Oral | 1.55 × 10-2 (1 year and older) | 59 (1 year and older) |
| Regular consumption of licorice tea (adults) | Various | Oral | 2.09 × 10-1 (adults) | 4 (adults) |
| Short-term, high-level consumption of black licorice candy (adults) | Various | Oral | 6.00 × 10-1 (adults) | 1 (adults) |
Abbreviations: MOE, margin of exposure; NHP, natural health product; NPD, non-prescription drug.
a MOE was calculated using the critical effect level (that is, a LOAEL of 0.91 mg/kg bw/day), which is based on developmental neurotoxicity.
b Target MOE = 30 (10x for intraspecies variation × 3x for use of a LOAEL). Bolded MOEs are considered potentially inadequate.
The MOEs between the critical effect level and the estimates of daily exposure from the use of enoxolone in face moisturizer, body moisturizer (spray and lotion), permanent hair dye, sunscreen (cream) (NHP and NPD), analgesic patch (NHP) for 13 years and under, acne therapy (cream) (NHP), medicated skin care product (cream) (NHP), regular consumption of licorice tea, and short-term, high exposure from consumption of black licorice candy are below the target MOE of 30 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk. Given the number of products containing enoxolone available on the Canadian market, it is possible that exposure from several different types of products may occur on the same day (that is, aggregate exposure), in which case the MOE would be even lower.
Since there were no identified sources of exposure to the general population for allantoin glycyrrhetinic acid, a qualitative approach to risk characterization was taken, and the risk to human health from this substance was considered to be low.
7.3.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| There were no dermal absorption studies available for substances in Triterpenoid subgroup 2. | +/- |
| The potential use of more than one product by a single person in a day (that is, aggregate exposure) was not considered. This may potentially underestimate exposure to some individuals. | - |
| The per capita intake of licorice-containing foods assumes that consumption of such foods is evenly distributed throughout the population. This may potentially underestimate exposure to individuals with a penchant for licorice flavour and overestimate exposure for individuals who do not like licorice flavour. | +/- |
| In the absence of data on the consumption of black licorice candy in Canada, a serving size of 80 g candy based on the reference amount for candies in Health Canada’s Table of Reference Amounts for Food (Health Canada 2022) was conservatively assumed to represent short-term high-level consumption of black licorice candy. Additionally, some licorice flavoured foods may contain non-licorice containing flavourings (for example, anethole, anise) as alternatives to licorice-based flavourings. As such, exposure may potentially be overestimated for some black licorice candy consumers. | + |
| There are limited hazard data on enoxolone and no hazard data on allantoin glycyrrhetinic acid. The read-across analogue, glycyrrhizin, was therefore used to inform the human health risk assessment. | +/- |
| There are a lack of data on the impact of enoxolone on reproductive and developmental toxicity as well as on developmental neurotoxicity. | - |
| There were no suitable studies for the dermal or inhalation routes of exposure; therefore, route-to-route extrapolation for enoxolone was carried out for dermal and inhalation scenarios by comparing with an effect level from an oral study. | +/- |
+, uncertainty with potential to cause overestimation of exposure/risk; -, uncertainty with potential to cause underestimation of exposure/risk; +/-, unknown potential to cause over- or underestimation of risk.
7.4 Mimosa oil
7.4.1 Exposure assessment
Environmental media
On the basis of the low manufacture and import quantity (<100 kg) of the substance reported in response to a CEPA section 71 survey (Environment Canada 2013), exposure to mimosa oil from environmental media is not expected. No reports of monitoring for mimosa oil in environmental media in Canada or elsewhere were identified.
Food
Exposure from use as food flavouring agent
No definitive information is available concerning the potential use of mimosa oil in foods sold in Canada. However, since mimosa oil is identified as a food flavouring agent internationally, it is possible that this substance is present as a flavouring agent in foods sold in Canada.
The Fenaroli’s Handbook of Flavor Ingredients reports per capita (“individual”) estimated intake of mimosa oil (listed as mimosa absolute) from food flavouring use to be 3.8 × 10‑2 µg/kg bw/day for the US population on the basis of production volumes reported by the food industry (Burdock 2010). In the absence of data on the actual use, if any, of mimosa oil, as a flavouring agent in foods sold in Canada, the per capita intake estimate reported for the US population (Burdock 2010) serves as an acceptable estimate of possible Canadian dietary exposure for the general population 1 year of age and older to this substance from its use as a food flavouring agent (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
Exposure from natural occurrence in foods
No definitive information is available concerning the natural occurrence of mimosa oil in foods. Mimosa oil is obtained from the plant material of the named plant source (Burdock 2010). There is expected to be little, if any, dietary exposure to this substance from its natural presence in foods.
Products available to consumers
Mimosa oil is present in products available to consumers. To evaluate the potential for exposure to mimosa oil from cosmetics and NHPs applied by the dermal route, sentinel scenarios were selected on the basis of a combination of use frequencies and reported concentrations of mimosa oil in these products. These scenarios represented the highest exposures, relative to other dermally applied cosmetics, as well as NHPs where mimosa oil is used as a NMI, on the basis of identified products reported to contain this substance. Exposure to mimosa oil from the use of body fragrance (roll-on and spray), moisturizer (body, face, eye), massage product (oil and bar), deodorant/antiperspirant (solid), sunless tanning product, facial makeup (liquid foundation, eye shadow, blush), cleanser (body and face), exfoliant (body and face), cleanser (2-in-1 body wash/shampoo), bath product (foam/bubbles), facial makeup remover, hair styling product (gel/wax/putty, hairspray, mousse), shampoo, conditioner, and sunscreen (lotion) (NHP) were considered to be the sentinel scenarios for dermal applications.
For the use of 100% mimosa oil in DIY products, the highest daily exposures were expected to occur from the use of the oil in aroma diffuser/air freshener, massage oil, body moisturizer, and as a facial steamer/mist. Although the upper concentration reported for mimosa oil used in massage oil is assumed to be 100%, massage oils are typically diluted prior to use. Thus, the maximum concentration of mimosa oil in massage oil was assumed to be 3% (RIVM 2006). It is reported that essential oils and extracts used in body products are typically diluted to concentrations of between 1% and 4% (Tisserand Institute 2021). On the basis of this information, the maximum concentration of mimosa oil in DIY body moisturizer was assumed to be 3%.
No dermal absorption studies were identified for mimosa oil. Lupenone and lupeol, which are components of mimosa oil, have molecular weights of 424.70 g/mol and 426.72 g/mol, respectively, and log Kow values of >6 (Table 3-1). Since the physical-chemical properties of the main components of mimosa oil and other triterpenoids in this assessment are similar and no substance-specific dermal absorption data are available, a group approach was taken, whereby a single dermal absorption value was used to represent the dermal absorption of all the triterpenoids. On the basis of the available information, a dermal absorption of 25% was identified for both occluded and non-occluded skin sites. Further information on the dermal absorption is provided in section 7.3.1 on the exposure assessment for the Triterpenoid subgroup 2 substances.
Mimosa oil is also present in lipstick and sunscreen (lip balm) (NHP). Exposures by the oral route from the use of lipstick and sunscreen (lip balm) (NHP) were quantified at upper concentrations of 10 and 3.2%, respectively (personal communication, emails from the Consumer and Hazardous Products Safety Directorate and the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, 2021; unreferenced).
The dermal route of exposure is expected to be the predominant route of exposure from products available to consumers. Owing to the negligible vapour pressure of the main components of mimosa oil and its identified uses, the inhalation route is not expected to be a significant route of exposure. Inhalation exposure from body fragrance and hairspray were taken into account in order to estimate the systemic exposure (sum of inhalation and dermal) from these products.
Exposure estimates for the lowest and highest exposed age groups from products available to consumers are summarized in Table 7-17. Daily exposure estimates from DIY products containing mimosa oil are summarized in Table 7-18.
| Product scenario | % in product | Route(s) of exposure | Systemic exposure range (mg/kg bw/day)a |
|---|---|---|---|
| Fragrance (roll-on) | 10 | Dermal and inhalation | 5.59 × 10-2 (14 to 18 years) to 1.65 × 10-1 (2 to 3 years) |
| Fragrance (spray) | 3 | Dermal and inhalation | 4.84 × 10-2 (14 to 18 years) to 1.42 × 10-1 (2 to 3 years) |
| Body moisturizer | 10 | Dermal | 1.01 (adults) to 2.38 (0 to 5 months) |
| Face moisturizer | 10 | Dermal | 1.81 × 10-1 (14 to 18 years) to 3.04 × 10-1 (adults) |
| Eye moisturizer | 3 | Dermal | 1.52 × 10-2 (adults) to 1.81 × 10-2 (14 to 18 years) |
| Massage oil | 0.3 | Dermal | 9.73 × 10-3 (adults) to 6.43 × 10-2 (0 to 5 months) |
| Massage bar | 1 | Dermal | 3.24 × 10-2 (adults) |
| Deodorant/antiperspirant (solid) | 0.063 | Dermal | 4.95 × 10-4 (9 to 13 years) to 8.38 × 10-4 (14 to 18 years) |
| Sunless tanning product | 10 | Dermal | 1.58 (9 to 13 years) to 3.08 (14 to 18 years) |
| Facial makeup (liquid foundation) | 10 | Dermal | 4.96 × 10-2 (14 to 18 years) to 1.11 × 10-1 (4 to 8 years) |
| Eye shadow | 1 | Dermal | 1.09 × 10-4 (14 to 18 years) to 2.93 × 10-4 (4 to 8 years) |
| Blush | 1 | Dermal | 1.32 × 10-4 (adults) to 4.57 × 10-4 (4 to 8 years) |
| Lipstick/lip balm | 10 | Oral | 1.78 × 10-2 (adults) to 4.40 × 10-2 (2 to 3 years) |
| Body cleanser (liquid) | 0.3 | Dermal | 4.68 × 10-4 (adults) to 1.93 × 10-3 (0 to 5 months) |
| Facial cleanser | 1 | Dermal | 4.79 × 10-4 (14 to 18 years) to 6.64 × 10-4 (9 to 13 years) |
| Body exfoliant | 0.3 | Dermal | 3.39 × 10-3 (adults) to 6.01 × 10-3 (14 to 18 years) |
| Face exfoliant | 1 | Dermal | 3.14 × 10-3 (adults) to 3.75 × 10-3 (14 to 18 years) |
| Cleanser (2-in-1 body wash/shampoo) | 1 | Dermal | 3.00 × 10-3 (2 to 3 years) to 7.14 × 10-3 (0 to 5 months) |
| Bath product (foam/bubbles) | 1 | Dermal | 1.40 × 10-4 (9 to 13 years) to 4.48 × 10-4 (14 to 18 years) |
| Facial makeup remover | 0.1 | Dermal | 2.65 × 10-4 (adults) to 7.11 × 10-4 (4 to 8 years) |
| Hair styling product (gel/wax/putty) | 1 | Dermal | 3.75 × 10-3 (adults) to 1.40 × 10-2 (2 to 3 years) |
| Hairspray | 0.1 | Dermal and inhalation | 2.37 × 10-4 (14 to 18 years) to 6.38 × 10-4 (4 to 8 years) |
| Hair styling product (mousse) | 0.1 | Dermal | 7.80 × 10-4 (adults) to 2.05 × 10-3 (2 to 3 years) |
| Shampoo | 0.1 | Dermal | 1.26 × 10-4 (14 to 18 years) to 4.64 × 10-4 (0 to 5 months) |
| Conditioner (rinse-off) | 0.1 | Dermal | 1.21 × 10-4 (14 to 18 years) to 2.60 × 10-4 (2 to 3 years) |
| Sunscreen (lotion) (NHP) | 0.105 | Dermal | 1.65 × 10-2 (9 to 13 years) to 7.48 × 10-2 (6 to 11 months) |
| Sunscreen (lip balm) (NHP) | 3.2 | Oral | 5.71 × 10-3 (adults) to 2.32 × 10-2 (6 to 11 months) |
Abbreviation: NHP, natural health product
a Exposure estimates were adjusted by 30% for the maximum amount of main components in mimosa oil. Dermal absorption was assumed to be 25%. See Appendix A for calculation details.
| Product scenario | % in product | Route(s) of exposure | Systemic exposure range (mg/kg bw/day)a |
|---|---|---|---|
| Aroma diffuser/air freshener (inhalation and refill) | 100 | Dermal and inhalation | 0.70 (adult) to 1.21 (9 to 13 years) |
| Aroma diffuser/air freshener (bystander exposure) | 100 | Inhalation | 0.39 (4 to 8 years) to 0.59 (1 year) |
| Massage oil | 3 | Dermal | 9.73 × 10-2 (adults) to 6.43 × 10-1 (0 to 5 months) |
| Body moisturizer | 3 | Dermal | 0.30 (adults) to 0.71 (0 to 5 months) |
| Facial steamer/mist (application) | 100 | Dermal and inhalation | 3.39 × 10-9 (adults) to 1.03 × 10-8 (4 to 8 years) |
| Facial steamer/mist (bystander exposure) | 100 | Inhalation | 3.96 × 10-9 (1 year) |
a Exposure estimates were adjusted by 30% for the maximum amount of main components in mimosa oil. Dermal absorption was assumed to be 25%. See Appendix B for calculation details.
7.4.2 Health effects assessment
There are no empirical data or international assessments for mimosa oil.
In order to inform the health effects assessment, the hazard information available for the main components of mimosa oil, lupenone (20%) and lupeol (7.8%), have been considered.
Lupenone and lupeol
There are no empirical data or international assessments on lupenone nor is it available in the OECD QSAR Toolbox (2019). Structurally, the only difference between lupenone and lupeol is the presence of a ketone on lupenone, whereas a hydroxyl group is located on lupeol. Therefore, data on lupeol are used to assess lupenone. In an in silico study, lupeol and several analogues such as lupenone and lupeol acetate showed a strong affinity for targeting and binding nuclear receptors involved in mammalian fertility, such as aryl hydrocarbon receptor and progesterone receptors (Ruiz-Rodríguez et al. 2017).
Lupeol showed anti-androgenic effects by decreasing the testicular cell proliferation and testosterone biosynthesis in vitro in rat testicular cells (Ogunwole et al. 2019) and by inhibiting the transcriptional activity of androgenic receptors in normal prostate epithelial cells (Siddique et al. 2011).
In an in vitro study, lupeol was able to diminish the hyperactivation of capacitated sperm, which is mandatory for the fertilization of an oocyte, by inhibiting the progesterone pathway (Mannowetz et al. 2017).
On the basis of the literature (Siddique et al. 2011; Ruiz-Rodríguez et al. 2017; Ogunwole et al. 2019), lupeol and lupenone are determined to be potential endocrine disruptors.
Due to the limited health effects data for lupenone and lupeol, a read-across approach was used. The most adequate analogue containing empirical data provided by the OECD QSAR Toolbox (2019) was enoxolone, which shares the same alert on the estrogen-receptor binding (strong binder). All 3 substances are pentacyclic triterpenoids with cycloalkane, alcohol groups, and fused saturated carbocycles. The analogue was selected on the basis of its shared endocrine disruptive properties with the target substances and is therefore protective of vulnerable populations such as children and pregnant people.
In this health effects assessment, the identified endpoint for enoxolone was an oral developmental neurotoxicity LOAEL of 0.91 mg/kg bw/day glycyrrhizin in children prenatally exposed via maternal consumption during pregnancy (Räikkönen et al. 2009; Räikkönen et al. 2017). Further information on the health effects assessment of enoxolone is provided in section 7.3.2.
7.4.3 Characterization of risk to human health
Due to limited hazard data available on mimosa oil and its main components (lupenone and lupeol), health effects information on the analogue enoxolone was used to characterize the risk to human health.
The critical effect level identified for enoxolone was an oral developmental neurotoxicity LOAEL of ≥500 mg/week (equivalent to 0.91 mg/kg bw/day on the basis of the lowest dose intake estimated by the authors inducing adverse effects) glycyrrhizin in children prenatally exposed via maternal consumption during pregnancy (Räikkönen et al. 2009; Räikkönen et al. 2017). A decrease in verbal and visuospatial abilities and in narrative memory, increases in externalizing symptoms, and attention, rule-breaking, and aggression problems were observed at 8.1 years. At 12.5 years, the same children had lower scores on intelligence quotient tests, poorer memory, and a higher incidence of attention deficit/hyperactivity disorder problems. Developmental neurotoxicity effects are supported by the ability of enoxolone to disrupt placental 11β-HSD enzyme to inactive cortisol before it reaches the fetus, leading to higher levels of fetal cortisol exposure (Blum et al. 1995; Benediktsson et al. 1997). In addition, on the basis of the literature (Siddique et al. 2011; Ruiz-Rodríguez et al. 2017; Ogunwole et al. 2019), lupeol and lupenone (the main components of mimosa oil) are determined to be potential endocrine disruptors. Considering the severity of the developmental effects and the uncertainty associated with the neurological developmental and endocrine disruptor effects of mimosa oil in fetuses and children, this POD was considered relevant for people of childbearing age, pregnant and breastfeeding people, fetuses, and children.
This POD was considered relevant for all age groups and durations of exposure.
Taking into consideration the reported concentrations of lupenone (20%) and lupeol (7.8%), it was assumed that the sum of all main components in mimosa oil was 30%. Exposure estimates calculated for mimosa oil were adjusted by 30%.
Daily systemic exposure estimates of mimosa oil for the highest and lowest exposure age groups and the resulting MOEs from food flavouring agent, cosmetics, and NHPs are summarized in Table 7-19. Systemic daily exposure estimates of mimosa oil and the resulting MOEs from DIY products containing mimosa oil are summarized in Table 7-20.
| Product scenario | % in product | Route(s) of exposure | Systemic exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|---|
| Fragrance (roll-on) (2 to 3 years to adults) | 10 | Dermal and inhalation | 5.59 × 10-2 (14 to 18 years) to 1.65 × 10-1 (2 to 3 years) | 6 (2 to 3 years) to 16 (14 to 18 years) |
| Fragrance (spray) (2 to 3 years to adults) | 3 | Dermal and inhalation | 4.84 × 10-2 (14 to 18 years) to 1.42 × 10-1 (2 to 3 years) | 6 (2 to 3 years) to 19 (14 to 18 years) |
| Body moisturizer (all) | 10 | Dermal | 1.01 (adults) to 2.38 (0 to 5 months) | 0.38 (0 to 5 months) to 0.90 (adults) |
| Face moisturizer (9 to 13 years to adults) | 10 | Dermal | 1.81 × 10-1 (14 to 18 years) to 3.04 × 10-1 (adults) | 3 (adults) to 5 (14 to 18 years) |
| Eye moisturizer (14 to 18 years to adults) | 3 | Dermal | 1.52 × 10-2 (adults) to 1.81 × 10-2 (14 to 18 years) | 50 (14 to 18 years) to 60 (adults) |
| Massage oil (all) | 0.3 | Dermal | 9.73 × 10-3 (adults) to 6.43 × 10-2 (0 to 5 months) | 14 (0 to 5 months) to 94 (adults) |
| Massage bar (adults) | 1 | Dermal | 3.24 × 10-2 (adults) | 28 (adults) |
| Deodorant/antiperspirant (solid) (9 to 13 years to adults) | 0.063 | Dermal | 4.95 × 10-4 (9 to 13 years) to 8.38 × 10-4 (14 to 18 years) | 1 086 (14 to 18 years) to 1 838 (9 to 13 years) |
| Sunless tanning product (9 to 13 years to adults) | 10 | Dermal | 1.58 (9 to 13 years) to 3.08 (14 to 18 years) | 0.30 (14 to 18 years) to 0.58 (9 to 13 years) |
| Facial makeup (liquid foundation) (4 to 8 years to adults) | 10 | Dermal | 4.96 × 10-2 (14 to 18 years) to 1.11 × 10-1 (4 to 8 years) | 8 (4 to 8 years) to 18 (14 to 18 years) |
| Eye shadow (4 to 8 years to adults) | 1 | Dermal | 1.09 × 10-4 (14 to 18 years) to 2.93 × 10-4 (4 to 8 years) | 3 101 (4 to 8 years) to 8 359 (14 to 18 years) |
| Blush (4 to 8 years to adults) | 1 | Dermal | 1.32 × 10-4 (adults) to 4.57 × 10-4 (4 to 8 years) | 1 993 (4 to 8 years) to 6 907 (adults) |
| Lipstick (2 to 3 years to adults) | 10 | Oral | 1.78 × 10-2 (adults) to 4.40 × 10-2 (2 to 3 years) | 21 (2 to 3 years) to 51 (adults) |
| Body cleanser (liquid) (all) | 0.3 | Dermal | 4.68 × 10-4 (adults) to 1.93 × 10-3 (0 to 5 months) | 472 (0 to 5 months) to 1 943 (adults) |
| Facial cleanser (9 to 13 years to adults) | 1 | Dermal | 4.79 × 10-4 (14 to 18 years) to 6.64 × 10-4 (9 to 13 years) | 1 370 (9 to 13 years) to 1 900 (14 to 18 years) |
| Body exfoliant (14 to 18 years to adults) | 0.3 | Dermal | 3.39 × 10-3 (adults) to 6.01 × 10-3 (14 to 18 years) | 152 (14 to 18 years) to 268 (adults) |
| Face exfoliant (14 to 18 years to adults) | 1 | Dermal | 3.14 × 10-3 (adults) to 3.75 × 10-3 (14 to 18 years) | 243 (14 to 18 years) to 290 (adults) |
| Cleanser (2-in-1 body wash/shampoo) (all) | 1 | Dermal | 3.00 × 10-3 (2 to 3 years) to 7.14 × 10-3 (0 to 5 months) | 127 (0 to 5 months) to 303 (2 to 3 years) |
| Bath product (foam/bubbles) (all) | 1 | Dermal | 1.40 × 10-4 (9 to 13 years) to 4.48 × 10-4 (14 to 18 years) | 2 031 (14 to 18 years) to 6 507 (9 to 13 years) |
| Facial makeup remover (4 to 8 years to adults) | 0.1 | Dermal | 2.65 × 10-4 (adults) to 7.11 × 10-4 (4 to 8 years) | 1 280 (4 to 8 years) to 3 436 (adults) |
| Hair styling product (gel/wax/putty) (2 to 3 years to adults) | 1 | Dermal | 3.75 × 10-3 (adults) to 1.40 × 10-2 (2 to 3 years) | 65 (2 to 3 years) to 243 (adults) |
| Hairspray (4 to 8 years to adults) | 0.1 | Dermal and inhalation | 2.37 × 10-4 (14 to 18 years) to 6.38 × 10-4 (4 to 8 years) | 1 427 (4 to 8 years) to 3 846 (14 to 18 years) |
| Hair styling product (mousse) (2–3 years to adults) | 0.1 | Dermal | 7.80 × 10-4 (adults) to 2.05 × 10-3 (2–3 years) | 443 (2 to 3 years) to 1166 (adults) |
| Shampoo (all) | 0.1 | Dermal | 1.26 × 10-4 (14 to 18 years) to 4.64 × 10-4 (0 to 5 months) | 1 960 (0 to 5 months) to 7 233 (14 to 18 years) |
| Conditioner (rinse-off) (2 to 3 years to adults) | 0.1 | Dermal | 1.21 × 10-4 (14 to 18 years) to 2.60 × 10-4 (2 to 3 years) | 3 500 (2 to 3 years) to 7 523 (14 to 18 years) |
| Sunscreen (lotion) (NHP) (6 to 11 months to adults) | 0.105 | Dermal | 1.65 × 10-2 (9 to 13 years) to 7.48 × 10-2 (6 to 11 months) | 12 (6 to 11 months) to 55 (9 to 13 years) |
| Sunscreen (lip balm) (NHP) (6 to 11 months to adults) | 3.2 | Oral | 5.71 × 10-3 (adults) to 2.32 × 10-2 (6 to 11 months) | 39 (6 to 11 months) to 159 (adults) |
| Food flavouring agent (1 year and older) | - | Oral | 1.14 × 10-5 (1 year and older) | 79 552 (1 year and older) |
Abbreviations: MOE, margin of exposure; NHP, natural health product.
a MOE was calculated using the critical effect level (that is, a LOAEL of 0.91 mg/kg bw/day) for the read-across analogue enoxolone, which is based on developmental neurotoxicity.
b Target MOE = 30 (10x for intraspecies variation × 3x for use of a LOAEL). Bolded MOEs are considered potentially inadequate.
| Product scenario | % in product | Route(s) of exposure | Systemic exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|---|
| Aroma diffuser/air freshener (inhalation and refill) (9 years and older) | 100 | Dermal and inhalation | 0.70 (adults) to 1.21 (9 to 13 years) | 0.75 (9 to 13 years) to 1.30 (adults) |
| Aroma diffuser/air freshener (bystander exposure) (younger than 9 years) | 100 | Inhalation | 0.39 (4 to 8 years) to 0.59 (1 year) | 1.54 (1 year) to 2.33 (4 to 8 years) |
| Massage oil (all) | 3 | Dermal | 9.73 × 10-2 (adults) to 6.43 x 10-1 (0-5 months) | 1 (0 to 5 months) to 9 (adults) |
| Body moisturizer (all) | 3 | Dermal | 0.30 (adults) to 0.71 (0 to 5 months) | 1.27 (0 to 5 months) to 2.99 (adults) |
| Facial steamer/mist (application) (4 to 8 years to adults) | 100 | Dermal and inhalation | 3.39 × 10-9 (adults) to 1.03 × 10-8 (4 to 8 years) | 8.85 × 107 (4 to 8 years) to 2.69 × 108 (adults) |
| Facial steamer/mist (bystander exposure) (1 year) | 100 | Inhalation | 3.96 × 10-9 (1 year) | 2.30 × 108 (1 year) |
Abbreviation: MOE, margin of exposure.
a MOE was calculated using the critical effect level (that is, a LOAEL of 0.91 mg/kg bw/day) for the read-across analogue enoxolone, which is based on developmental neurotoxicity.
b Target MOE = 30 (10x for intraspecies variation × 3x for use of a LOAEL). Bolded MOEs are considered potentially inadequate.
The MOEs between the critical effect level and the estimates of daily exposure from the use of mimosa oil in fragrance (roll-on and spray), body moisturizer, face moisturizer, massage oil for 1 year and under, massage bar, sunless tanning product, facial makeup (liquid foundation), lipstick for 8 years and under, and sunscreen (lotion) (NHP) for 3 years and under and 14 to 18 years are below the target MOE of 30. They are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
In addition, the MOEs between the critical effect level and the estimates of daily exposure from the use of mimosa oil in DIY aroma diffuser/air freshener, DIY massage oil, and DIY body moisturizer are below the target MOE of 30 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
7.4.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| The concentration of the main components in essential oils differs depending on the plant origin, species, temperature, soil, and geography. Therefore, the composition of essential oils in products available to consumers varies, which represents an uncertainty in the assessment. | +/- |
| There is uncertainty associated with the dermal absorption factor used for mimosa oil when enoxolone is considered as a read-across analogue. | +/- |
| The potential use of more than one product containing the same substance by a single person in a day (that is, aggregate exposure) was not considered. This may potentially underestimate exposure for some individuals. | - |
| There are no hazard data on mimosa oil and limited data on lupeol and lupenone. The read-across analogue, enoxolone, was used to inform the human health risk assessment. | +/- |
| There are a lack of data on the impact of mimosa oil and its main components on reproductive and developmental toxicity and developmental neurotoxicity. | +/- |
| Route-to-route extrapolation for mimosa oil was carried out for dermal and inhalation exposure scenarios using a critical effect level from an oral toxicity study. | +/- |
+, uncertainty with potential to cause overestimation of exposure/risk; -, uncertainty with potential to cause underestimation of exposure risk; +/-, unknown potential to cause over- or underestimation of risk
7.5 Ivy extract
7.5.1 Exposure assessment
Environmental media and food
On the basis of the low manufacture and import quantity (<100 kg) of the substance reported in response to a CEPA section 71 survey (Environment Canada 2013), exposure to ivy extract from environmental media is not expected. No reports of monitoring for ivy extract in environmental media in Canada or elsewhere were identified. In Canada, there is no expected exposure of the general population to ivy extract in food (foods or food flavouring agent) and food packaging.
Products available to consumers
Ivy extract is present in products available to consumers. To evaluate the potential for exposure to ivy extract from cosmetics and NHPs applied by the dermal route, sentinel scenarios were selected on the basis of a combination of use frequencies and reported concentrations of ivy extract in these products. These scenarios represented the highest exposures, relative to other dermally applied cosmetics, as well as NHPs where ivy extract is used as a NMI, on the basis of identified products reported to contain the substance. Exposures to ivy extract from the use of a cleanser (face, body), massage oil, moisturizer (body, face, eye), facial makeup (liquid foundation), facial makeup fixer (spray), exfoliant (face, body), facial makeup remover, bath product (foam/bubbles and salt), conditioner (leave-on and rinse-off), shampoo, hair colour (temporary and permanent), hair styling products (gel, mousse, hairspray), body adhesive, and sunscreen (powder) (NHP) were considered to be the sentinel scenarios for dermal applications.
The use of 100% ivy extract in DIY products such as massage oil and body moisturizer was assessed. Although the upper concentration reported for ivy extract used in massage oil is assumed to be 100%, massage oils are typically diluted prior to use. Therefore, the maximum concentration of ivy extract in massage oil was assumed to be 3% (RIVM 2006). It is reported that essential oils and extracts used in body products are typically diluted to concentrations of between 1% and 4% (Tisserand Institute 2021). On the basis of this information, the maximum concentration of ivy extract in DIY body moisturizer was assumed to be 3%.
No dermal absorption studies were identified for ivy extract. Steroid-based compounds (hederacoside C, hederagenin, and alpha-hederin), which are components of ivy extract, have molecular weights of 1221, 472, and 750 g/mol, respectively, and log Kow values of >4, with the exception of hederacoside C (Table 3-1). Since the physical-chemical properties of the main components of ivy extract and other triterpenoids in this assessment are similar and there are no substance-specific dermal absorption data, a group approach was taken, whereby a single dermal absorption value was used to represent the dermal absorption of all the triterpenoids. On the basis of the available information, a dermal absorption of 25% was identified for both occluded and non-occluded skin sites. Further information on the dermal absorption is provided in section 7.3.1 on the exposure assessment for the Triterpenoid subgroup 2 substances.
The dermal route of exposure is expected to be the predominant route of exposure from products available to consumers. Owing to the negligible vapour pressure of the main components of ivy extract and its identified uses, the inhalation route is not expected to be a significant route of exposure. Inhalation exposures from facial makeup spray, hairspray, and sunscreen powder (NHP) were taken into account in order to estimate the systemic exposure (sum of inhalation and dermal) from these products.
Exposure estimates for the lowest and highest exposed age groups from products available to consumers are summarized in Table 7-22. Daily exposure estimates from DIY products containing ivy extract are summarized in Table 7-23.
| Product scenario | % in product | Route(s) of exposure | Systemic exposure range (mg/kg bw/day)a |
|---|---|---|---|
| Facial cleanser | 3 | Dermal | 2.40 × 10-3 (14 to 18 years) to 3.32 × 10-3 (9 to 13 years) |
| Massage oil | 3 | Dermal | 0.16 (adults) to 1.07 (0 to 5 months) |
| Body moisturizer | 5 | Dermal | 0.84 (adults) to 1.98 (0 to 5 months) |
| Face moisturizer | 3 | Dermal | 0.09 (14 to 18 years) to 0.15 (adults) |
| Eye moisturizer | 1 | Dermal | 1.01 × 10-2 (14 to 18 years) to 1.69 × 10-2 (adults) |
| Facial makeup fixer (spray) | 6 | Dermal and inhalation | 1.59 × 10-1 (adults) to 4.24 × 10-1 (4 to 8 years) |
| Facial makeup (liquid foundation) | 0.1 | Dermal | 8.27 × 10-3 (14 to 18 years) to 1.85 × 10-2 (4 to 8 years) |
| Body cleanser (liquid) | 0.1 | Dermal | 2.60 × 10-4 (adults) to 1.07 × 10-3 (0 to 5 months) |
| Face exfoliant | 1 | Dermal | 5.25 × 10-3 (adults) to 6.27 × 10-3 (14 to 18 years) |
| Facial makeup remover | 0.2 | Dermal | 1.31 × 10-3 (9 to 13 years) to 3.28 × 10-3 (3 years) |
| Body exfoliant | 30 | Dermal | 0.56 (adults) to 0.67 (14 to 18 years) |
| Bath product (foam/bubbles) | 0.3 | Dermal | 6.99 × 10-5 (9 to 13 years) to 2.24 × 10-4 (14 to 18 years) |
| Bath product (salt) | 3 | Dermal | 1.41 × 10-3 (adults) to 1.80 × 10-3 (9 to 13 years) |
| Conditioner (rinse-off) | 1 | Dermal | 2.02 × 10-3 (14 to 18 years) to 4.33 × 10-3 (2 to 3 years) |
| Shampoo | 3 | Dermal | 6.29 × 10-3 (14 to 18 years) to 2.32 × 10-2 (0 to 5 months) |
| Temporary hair dye | 0.1 | Dermal | 5.91 × 10-3 (adults) to 1.90 × 10-2 (4 to 8 years) |
| Permanent hair dye | 0.1 | Dermal | 2.24 × 10-2 (adults) to 2.67 × 10-2 (14 to 18 years) |
| Hair conditioner (leave-on) | 3 | Dermal | 6.05 × 10-2 (14 to 18 years) to 1.30 × 10-1 (2 to 3 years) |
| Hair styling product (gel) | 0.5 | Dermal | 3.13 × 10-3 (adults) to 1.17 × 10-2 (2 to 3 years) |
| Hair styling product (mousse) | 0.1 | Dermal | 1.30 × 10-3 (adults) to 4.75 × 10-3 (2 to 3 years) |
| Hairspray | 0.1 | Dermal and inhalation | 1.38 × 10-3 (14 to 18 years) to 5.63 × 10-3 (adults) |
| Body adhesive | 0.1 | Dermal | 3.32 × 10-3 (adults) to 4.27 × 10-3 (14 to 18 years) |
| Sunscreen (powder) (NHP) | 0.1 | Dermal and inhalation | 1.35 × 10-4 (adults) |
Abbreviation: NHP, natural health product.
a See Appendix A for calculation details. Exposure estimates were adjusted by 50% for the maximum amount of main components in ivy extract that metabolize to alpha-hederin. Dermal absorption was assumed to be 25%.
| Product scenario | % in product | Route(s) of exposure | Systemic exposure range (mg/kg bw/day)a |
|---|---|---|---|
| Massage oil | 3 | Dermal | 1.62 × 10-1 (adults) to 1.07 (0 to 5 months) |
| Body moisturizer | 3 | Dermal | 0.51 (adults) to 1.19 (0 to 5 months) |
a See Appendix B for calculation details. Exposure estimates were adjusted by 50% for the maximum amount of main components in ivy extract that metabolize to alpha-hederin. Dermal absorption was assumed to be 25%.
7.5.2 Health effects assessment
There are limited international assessments available on ivy extract. The EMA published an assessment report on ivy extract, and its use as an herbal medicinal product for the respiratory system has been considered (EMA 2017). The use of ivy extract by pregnant and breastfeeding people is not recommended by the EMA due to a lack of data and the presence of alpha-hederin as a main component. Alpha-hederin has been shown to disturb maternal zinc distribution and induce adverse developmental outcomes in rats (EMA 2017).
There are limited empirical data for ivy extract. There are no genotoxicity, carcinogenicity, or reproductive and developmental toxicity studies available in the database.
In rats, the main component of ivy extract, alpha-hederin, was still detected in the blood 3 hours after a single oral exposure of 1000 mg/kg ivy extract. However, lower dosages could not be analyzed because the limit of detection of alpha-hederin showed potential poor absorption and rapid elimination in rats (EMA 2017). Other main components, such as hederacoside C, are metabolized to alpha-hederin in the stomach (EMA 2017).
Only one repeated-dose study is available for ivy extract, as a summary in EMA (2017). Daily oral exposure of rats (number and strain unknown) to ivy extract at 0, 1500, or 4000 mg/kg bw/day for 100 days did not cause any toxic effects. Hematological and biochemical parameters, histological findings, and kidney and liver weights were normal compared with those of the control animals (EMA 2017).
Because of the popularity of ivy extract in natural medicine, several clinical pharmacology studies are available.
In one study, 268 children aged 0 to 12 years were treated orally for 14 days with 0 mg/kg bw/day or between 6.6 mg/kg bw/day and 11 mg/kg bw/day ivy extract in the form of a syrup or drops for cough and bronchitis (Schmidt et al. 2012). The adverse effects reported were diarrhea, nausea, vomiting, and dermatitis, which disappeared when the treatment stopped.
In another study, both men (124) and women (206) with bronchitis were orally given tablets containing 25 mg ivy extract twice per day for a median duration of 8 days, before being evaluated by means of a questionnaire to assess the tolerability of the treatment. The results showed a good-to-very-good tolerability of the treatment. The authors did not measure any parameters or provide any other details on the medical condition of the participants (Stauss-Grabo et al. 2011).
A large study was conducted on 9657 patients (188 aged under 1 year, 2822 aged between 1 and 5 years, 1843 aged between 6 and 12 years, and 4804 aged above 12 years), who were treated orally with 52.2 mg to 157.5 mg (equivalent to between 2.3 mg/kg bw/day and 5.77 mg/kg bw/day; no control group) ivy extract in a syrup (Fazio et al. 2009). The principle adverse effects noted by the authors were gastrointestinal disorders, diarrhea, abdominal and epigastric pain, nausea, and vomiting (which disappeared when treatment stopped).
To inform the health effects assessment, the hazard information available for the main components of ivy extract, hederacoside C (1.7% to 20%), hederagenin (7.4% to 18.4%), and alpha-hederin (0.8% to 2.3%), has been considered.
Hederacoside C, hederagenin, and alpha-hederin
There are no empirical data on hederacoside C and limited data on hederagenin and alpha-hederin.
An in vitro study showed that hederagenin was able to inhibit the mouse enzyme 11β-HSD1 (Yan et al. 2021).
Alpha-hederin showed no mutagenicity potential in Ames assay using Salmonella typhimurium, with and without metabolic activation (EMA 2017).
Two developmental toxicity studies showed a decrease in fetal weights and an increase in fetal abnormalities such as delayed skeletal ossification, enephalocele or umbilical hernia and resorption in pregnant rats treated with 0 µg/kg bw/day to 225 µg/kg bw/day at GD 11 or from GDs 6 to 15 via a subcutaneous injection (Daston et al. 1994; Duffy et al. 1997). The authors of the studies showed that these developmental effects were induced by the fact that alpha-hederin increased the levels of maternal hepatic metallothionein, which resulted in reduced levels of Fe and Zn in maternal plasma.
Due to the limited health effects data available for hederacoside C, hederagenin, and alpha-hederin, a read-across approach was used. The most adequate analogue containing empirical data provided by the OECD QSAR Toolbox (2019) was enoxolone, which shares similar structures and metabolites, as identified by the in vivo rat metabolism simulator, with hederagenin and alpha-hederin. It also shares with hederagenin the same alert on the estrogen receptor binding (strong binder). All 4 substances are pentacyclic triterpenoids with cycloalkane, alcohol groups, and fused saturated carbocycles. In addition, enoxolone and hederagenin are able to inhibit 11β-HSD in rodents. Hederagenin C is not available on the OECD QSAR Toolbox (2019). The analogue was selected on the basis of its shared capacity to interfere with fetal development and is therefore protective of vulnerable populations such as children and pregnant people.
In this health effects assessment, the identified endpoint was an oral developmental neurotoxicity LOAEL of 0.91 mg/kg bw/day in children prenatally exposed via maternal consumption during pregnancy (Räikkönen et al. 2009; Räikkönen et al. 2017). Further information on the health effects assessment of enoxolone is provided in section 7.3.2.
7.5.3 Characterization of risk to human health
Because of the limitation of the hazard data available on ivy extract and its main components (hederacoside C, hederagenin, and alpha-hederin), health effects information on the analogue enoxolone was used to characterize the risk to human health.
The critical effect level identified for enoxolone was an oral developmental neurotoxicity LOAEL of ≥500 mg/week (equivalent to 0.91 mg/kg bw/day on the basis of the lowest dose intake estimated by the authors inducing adverse effects) glycyrrhizin in children prenatally exposed via maternal consumption during pregnancy (Räikkönen et al. 2009; Räikkönen et al. 2017). A decrease in verbal and visuospatial abilities and in narrative memory, increases in externalizing symptoms, and attention, rule-breaking, and aggression problems were observed at 8.1 years. At 12.5 years, the same children had lower scores on intelligence quotient tests, poorer memory, and a higher incidence of attention deficit/hyperactivity disorder problems. Developmental neurotoxicity effects are supported by the ability of enoxolone to disrupt placental 11β-HSD enzyme to inactive cortisol before it reaches the fetus, leading to higher levels of fetal cortisol exposure (Blum et al. 1995; Benediktsson et al. 1997). Considering the severity of the developmental adverse effects and the uncertainty associated with the neurological developmental and endocrine disruptor effects of ivy extract in fetuses and children, this POD was considered relevant for people of child-bearing age, pregnant and breastfeeding people, fetuses, and children.
Considering the reported concentrations of hederacoside C (1.7% to 20%), hederagenin (7.4% to 18.4%), and alpha-hederin (0.8% to 2.3%), as well as the fact that hederacoside C is metabolized to alpha-hederin in the stomach, it was assumed that the sum of all main components in ivy extract was 50%. Calculated exposure estimates for ivy extract were adjusted by 50%.
Daily exposure estimates for the highest and lowest exposed age groups and resulting MOEs are summarized in Table 7-24 for products available to consumers. In addition, daily estimates for the highest and lowest age groups and resulting MOEs are summarized in Table 7-25 for DIY products.
| Product scenario | % in product | Route(s) of exposure | Systemic exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|---|
| Facial cleanser (9 to 13 years to adults) | 3 | Dermal | 2.40 × 10 -3 (14 to 18 years) to 3.32 × 10 -3 (9 to 13 years) | 274 (9 to 13 years) to 380 (14 to 18 years) |
| Massage oil (all) | 3 | Dermal | 0.16 (adults) to 1.07 (0 to 5 months) | 0.85 (0 to 5 months) to 5.61 (adults) |
| Body moisturizer (all) | 5 | Dermal | 0.84 (adults) to 1.98 (0 to 5 months) | 0.46 (0 to 5 months) to |
| Face moisturizer (9 to 13 years to adults) | 3 | Dermal | 9.07 × 10-2 (14 to 18 years) to 1.52 ×10-1 (adults) | 6 (adults) to 10 (14 to 18 years) |
| Eye moisturizer (14 to 18 years to adults) | 1 | Dermal | 1.01 × 10-2 (14 to 18 years) to 1.69 × 10-2 (adults) | 54 (adults) to 90 (14 to 18 years) |
| Facial makeup fixer (spray) (4 to 8 years to adults) | 6 | Dermal and inhalation | 1.59 × 10-1 (adults) to 4.24 × 10-1 (4 to 8 years) | 2 (4 to 8 years) to 6 (adults) |
| Facial makeup (liquid foundation) (4 to 8 years to adults) | 0.1 | Dermal | 8.27 × 10-3 (14 to 18 years) to 1.85 × 10-2 (4 to 8 years) | 49 (4 to 8 years) to 110 (14 to 18 years) |
| Body cleanser (liquid) (all) | 0.1 | Dermal | 2.60 × 10-4 (adults) to 1.07 × 10-3 (0 to 5 months) | 849 (0 to 5 months) to 3 498 (adults) |
| Face exfoliant (14 to 18 years to adults) | 1 | Dermal | 5.25 × 10-3 (adults) to 6.27 × 10-3 (14 to 18 years) | 145 (14 to 18 years) to 173 (adults) |
| Facial makeup remover (3 years to adults) | 0.2 | Dermal | 1.31 × 10-3 (9 to 13 years) to 3.28 × 10-3 (3 years) | 278 (3 years) to 695 (9 to 13 years) |
| Body exfoliant (14 to 18 years to adults) | 30 | Dermal | 0.56 (adults) to 0.67 (14 to 18 years) | 1.37 (14 to 18 years) to 1.63 (adults) |
| Bath product (foam/bubbles) (all) | 0.3 | Dermal | 6.99 × 10-5 (9 to 13 years) to 2.24 × 10-4 (14 to 18 years) | 4 062 (14 to 18 years) to 13 014 (9 to 13 years) |
| Bath product (salt) (9 to 13 years to adults) | 3 | Dermal | 1.41 × 10-3 (adults) to 1.80 × 10-3 (9 to 13 years) | 507 (9 to 13 years) to 647 (adults) |
| Conditioner (rinse-off) (2 to 3 years to adults) | 1 | Dermal | 2.02 × 10-3 (14 to 18 years) to 4.33 × 10-3 (2 to 3 years) | 210 (2 to 3 years) to 451 (14 to 18 years) |
| Shampoo (all) | 3 | Dermal | 6.29 × 10-3 (14 to 18 years) to 2.32 × 10-2 (0 to 5 months) | 39 (0 to 5 months) to 145 (14 to 18 years) |
| Temporary hair dye (4 to 8 years to adults) | 0.1 | Dermal | 5.91 × 10-3 (adults) to 1.90 × 10-2 (4 to 8 years) | 48 (4 to 8 years) to 154 (adults) |
| Permanent hair dye (14 to 18 years to adults) | 0.1 | Dermal | 2.24 × 10-2 (adults) to 2.67 × 10-2 (14 to 18 years) | 34 (14 to 18 years) to 41 (adults) |
| Hair conditioner (leave-on) (4 to 8 years to adults) | 3 | Dermal | 6.05 × 10-2 (14 to 18 years) to 1.30 × 10-1 (2 to 3 years) | 7 (2 to 3 years) to 15 (14 to 18 years) |
| Hair styling product (gel) (2 to 3 years to adults) | 0.5 | Dermal | 3.13 × 10-3 (adults) to 1.17 × 10-2 (2 to 3 years) | 78 (2 to 3 years) to 291 (adults) |
| Hair styling product (mousse) (2 to 3 years to adults) | 0.1 | Dermal | 1.30 × 10-3 (adults) to 4.75 × 10-3 (2 to 3 years) | 192 (2 to 3 years) to 700 (adults) |
| Hairspray (4 to 8 years to adults) | 0.1 | Dermal and inhalation | 1.38 × 10-3 (14 to 18 years) to 5.63 × 10-3 (adults) | 162 (adults) to 658 (14 to 18 years) |
| Body adhesive (14 to 18 years to adults) | 0.1 | Dermal | 3.32 × 10-3 (adults) to 4.27 × 10-3 (14 to 18 years) | 213 (14 to 18 years) to 274 (adults) |
| Sunscreen (powder) (NHP) (adults) | 0.1 | Dermal and inhalation | 1.35 × 10-4 (adults) | 6 749 (adults) |
Abbreviations: MOE, margin of exposure; NHP, natural health product.
a MOE was calculated using the critical effect level (that is, a LOAEL of 0.91 mg/kg bw/day) for the read-across analogue enoxolone, which was based on developmental neurotoxicity.
b Target MOE = 30 (10x for intraspecies variation × 3x for use of a LOAEL). Bolded MOEs are considered potentially inadequate.
| Product scenario | % in product | Route(s) of exposure | Systemic exposure (mg/kg bw/day) | MOEa, b |
|---|---|---|---|---|
| Massage oil (all) | 3 | Dermal | 0.16 (adults) to 1.07 (0 to 5 months) | 0.85 (0 to 5 months) to 5.61 (adults) |
| Body moisturizer (all) | 3 | Dermal | 0.51 (adults) to 1.19 (0 to 5 months) | 0.76 (0 to 5 months) to 1.80 (adults) |
Abbreviation: MOE, margin of exposure.
a MOE was calculated using the critical effect level (that is, a LOAEL of 0.91 mg/kg bw/day), which was based on developmental neurotoxicity.
b Target MOE = 30 (10x for intraspecies variation × 3x for use of a LOAEL). Bolded MOEs are considered potentially inadequate.
The MOEs between the critical effect level and the estimates of daily exposure from the use of ivy extract in massage oil, body moisturizer, face moisturizer, facial makeup fixer (spray), body exfoliant, and hair conditioner (leave-on) are below the target MOE of 30 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
In addition, the MOEs between the critical effect level and the estimates of daily exposure from the use of ivy extract in DIY massage oil and DIY body moisturizer are below the target MOE of 30 and are considered potentially inadequate to address uncertainties in the health effects and exposure data used to characterize risk.
7.5.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| The concentration of the main components in extracts differs depending on the plant origin, species, temperature, soil, and geography. Therefore, the composition of extract present in products available to consumers is variable, which represents an uncertainty in the assessment. | +/- |
| There is uncertainty associated with the dermal absorption factor used for ivy extract when enoxolone is considered as a read-across analogue. | +/- |
| The potential use of more than one product containing the same substance by a single person in a day (that is, aggregate exposure) was not considered. This may potentially underestimate exposure to some individuals. | - |
| There are limited hazard data on ivy extract and no data on hederacoside C, hederagenin, or alpha-hederin. The read-across analogue, enoxolone, was used to inform the human health risk assessment. | +/- |
| There are a lack of data on the impact of ivy extract and its main components on reproductive and developmental toxicity and developmental neurotoxicity. | +/- |
| Route-to-route extrapolation for ivy extract was carried out for dermal and inhalation exposure scenarios using a critical effect level from an oral toxicity study. | +/- |
7.6 American ginseng extract
7.6.1 Exposure assessment
Environmental media
On the basis of the low manufacture and import quantity (<100 kg) of the substance reported in response to a CEPA section 71 survey (Environment Canada 2013), exposure to American ginseng extract from environmental medial is not expected.
Food
In Canada, there is no expected exposure of the general population to American ginseng extract from its use as a food flavouring agent and in food packaging. American ginseng extract is obtained from the plant material of the named plant source (Burdock 2010). There is expected to be little, if any, dietary exposure to this substance from its natural presence in foods (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
Products available to consumers
American ginseng extract is present in products available to consumers. Exposure to American ginseng extract via the oral and dermal routes may result from the use of NHPs, as well as from the use of cosmetics (for example, mouthwash, toothpaste, fragrance, makeup, moisturizers, cleansers, and hair care products). As American ginseng extract is considered to be of low hazard potential (more details provided in section 7.6.2 below), quantitative estimates of these potential exposures were not derived.
7.6.2 Health effects assessment
There are no international assessment or empirical data on American ginseng extract. Because the empirical data on American ginseng extract are limited, a read-across approach was taken, and hazard information on the analogue, Asian ginseng (Panax ginseng), was used to inform the health effects assessment. Like American ginseng, Asian ginseng contains a similar composition of hundreds of ginsenosides that act as the major biologically active components (CIR 2012; Chen et al. 2019), particularly in the main root, fine root, and rhizome (Chen et al. 2020). In addition, the term Panax ginseng seems to be used interchangeably for both types of ginseng (Burdock 2010; CIR 2012).
The EMA conducted a risk assessment on the health and safety effects of Panax ginseng and determined that a dose range of 1000 to 3000 mg/day for 3 months is safe for human consumption (EMA 2014).
Several short-term exposure (15 to 25 days) and long-term (90 days) studies in rodents and dogs orally administered 1.5 mg/kg bw/day to 100 mg/kg bw/day Panax ginseng by gavage or drinking water did not show any adverse effects (CIR 2012).
The NTP has also conducted toxicological and carcinogenic studies on Panax ginseng. In one study, the authors concluded that there were no adverse effects seen in B6C3F1 mice or F344/N rats that were administered Panax ginseng at doses of up to 5000 mg/kg bw/day by gavage for 5 days a week for 3 months (NTP 2011).
In a carcinogenic study, B6C3F1 mice or F344/N rats were administered 0 to 5000 mg/kg bw/day Panax ginseng by gavage, 5 days a week for 2 years (NTP 2011). The authors of the study observed intermittent diarrhea and the presence of inflammation in the respiratory epithelium in female rats at 5000 mg/kg bw/day and a significantly negative trend in the incidence of fibro adenoma in the mammary gland of female rats. In mice, intermittent diarrhea was observed in males at 5000 mg/kg bw/day. Overall, no adverse effects or carcinogenic effects of Panax ginseng were observed at doses of up to 5000 mg/kg bw/day.
Panax ginseng was not genotoxic in micronucleus assay with B6C3F1 mice or in Ames test with Salmonella typhimurium strains TA97, TA98, TA100, TA102, TA104, and TA1535, with and without metabolic activation (NTP 2011).
In 2 male reproductive studies, Wistar male rats were administered 0 mg/kg bw/day or 500 mg/kg bw/day Panax ginseng by gavage for 28 or 50 days (Akram et al. 2012; Hosseini et al. 2018). No adverse or reproductive effects were observed by the authors of the study.
In a developmental study, pregnant Wistar dams (10/dose) were administered 0 or 20 mg/kg bw/day Panax ginseng by gavage from GD 6 to 15 (Elsaieed and Nada 2002). No adverse effects or developmental effects were observed by the authors of the study.
In a two-generation reproductive toxicity study, Sprague-Dawley male and female rats (15/dose/sex) were fed 0, 1.5, 5, or 15 mg/kg bw/day Panax ginseng 3 weeks before mating and during mating; females were also exposed during gestation and lactation. At weaning, F1 offspring were started on the treatment until mating at PND 90. The F2 offspring were raised to PND 21 (Hess et al. 1982). No adverse effects or reproductive and developmental effects were observed by the authors of the study.
In several clinical studies, volunteers were administered between 105 mg and 4500 mg of Panax ginseng extract daily for a few days to up to 9 months (Coon and Ernst 2002). No adverse effects related to Panax ginseng consumption were observed in volunteers.
On the basis of the available information, no health effects of concern are identified for Panax ginseng or American ginseng.
7.6.3 Characterization of risk to human health
On the basis of the available information, health effects of concern were not identified for American ginseng extract. For this reason, PODs were not defined, and a qualitative approach to risk characterization was taken. Exposure of the general population to American ginseng extract is therefore considered to be of low risk to human health.
8. Consideration of subpopulations who may have greater susceptibility or exposure
The human health assessment for each substance takes into consideration those groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. Certain subpopulations are routinely considered throughout the assessment process, such as infants, children, and people of reproductive age. For instance, age-specific exposures are routinely estimated, and developmental and reproductive studies are evaluated for potential adverse health effects. Those subpopulations with the potential for higher exposure and those who may be more susceptible were taken into account in the human health risk assessment outcomes.
9. Conclusion
Considering all available lines of evidence presented in this draft assessment, there is low risk of harm to the environment from the 14 substances in the Tricyclic Sesquiterpenes and Triterpenoids Group. It is proposed to conclude that the 14 substances in the Tricyclic Sesquiterpenes and Triterpenoids Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this draft assessment, it is proposed to conclude that cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, and ivy extract meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health. Considering all the information presented in this draft assessment, it is proposed to conclude that alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, beta-cedrene, T&T cedarwood oil, amboryl acetate, allantoin glycyrrhetinic acid, and American ginseng extract do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, and ivy extract meet one or more of the criteria set out in section 64 of CEPA and that alpha-cedrene, thujopsene, alpha-gurjunene, beta-patchoulene, beta-cedrene, T&T cedarwood oil, amboryl acetate, allantoin glycyrrhetinic acid, and American ginseng extract do not meet any of the criteria set out in section 64 of CEPA.
References
[ACI] American Cleaning Institute [database]. 2021. Washington (DC): ACI. [accessed 2021 Jun].
Aberchane M, Fechtal M, Chaouch A. 2004. Analysis of Moroccan atlas cedarwood oil (Cedrus atlantica Manetti). J Essent Oil Res. 16(6):542-547. DOI: 10.1080/10412905.2004.9698793.
Akram H, Ghaderi Pakdel F, Ahmadi A, Zare S. 2012. Beneficial effects of American ginseng on epididymal sperm analyses in cyclophosphamide treated rats. Cell J. 14(2):116-121.
[ANSES] L’agence national de sécurité sanitaire de l’alimentation, de l’environnement et du travail. 2022. Effets indésirables induits par la réglisse consommée dans le cadre alimentaire Étude des cas enregistrés par les centres antipoison (de janvier 2012 à décembre 2021- Rapport d’étude de toxicovigilance). Réglis–e - 2022-AUTO-0077. Maison-Alford (FR): ANSES. 26 p. Available only in French.
Arnot JA, Arnot MI, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P. 2010. Molecular size cutoff criteria for screening bioaccumulation potential: fact or fiction? Integr Environ Assess Manag. 6(2):210-224.
Assinewe VA, Baum BR, Gagnon D, Arnason JT. 2003. Phytochemistry of wild populations of Panax quinquefolius L. (North American ginseng). J Agric Food Chem. 51(16):4549-4553. DOI: 10.1021/jf030042h.
Baharara H, Moghadam AT, Sahebkar A, Emami SA, Tayebi T, Mohammadpour AH. 2021. The effects of ivy (Hedera helix) on respiratory problems and cough in humans: a review. Adv Exp Med Biol. 1328:361-376.
Ballin NZ, Larsen DM, Jensen ST, Andersen LB, Olesen PT. 2023. Glycyrrhizinic acid in licorice products on the Danish market. Food Control. 143:109322.DOI: 10.1016/j.foodcont.2022.109322.
Bansaghi S, Soule H, Guitart C, Pittet D, Haidegger T. 2020. Critical reliability issues of common type alcohol-based handrub dispensers. Antimicrob Resist Infect Control. 9(1):90.
Becker LC, Bergfeld WF, Belsito DV, Klaassen CD, Marks Jr JG, Shank RC, Slaga TJ, Snyder PW, Andersen FA. 2010. Final report of the safety assessment of allantoin and its related complexes. Int J Toxicol. 29(3 Suppl):84S-97S.DOI: 10.1177/1091581810362805.
Becker LC, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG, Shank RC, Slaga TJ, Snyder PW, et al. 2015. Safety assessment of panax spp root-derived ingredients as used in cosmetics. Int J Toxicol. 34(3 Suppl):5S-42S.DOI: 10.1177/1091581815610508.
Belsito D, Bickers D, Bruze MLU, Calow P, Dagli ML, Fryer AD, Greim H, Miyachi Y, Saurat JH, Sipes IG. 2013. A toxicological and dermatological assessment of alkyl cyclic ketones when used as fragrance ingredients. The RIFM expert panel. Food Chem Toxicol. 62(1 Suppl):S1-44.
Benediktsson R, Calder AA, Edwards CRW, Seckl JR. 1997. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf). 46(2):161-166. DOI: 10.1046/j.1365-2265.1997.1230939.x.
Bezruk I, Kotvitska A, Korzh I, Materiienko A, Gubar S, Budanova L, Ivanauskas L, Vyshnevsky I, Georgiyants V. 2020. combined approach to the choice of chromatographic methods for routine determination of hederacoside C in ivy leaf extracts, capsules, and syrup. Sci Pharm. 88(2):24. DOI: 10.3390/scipharm88020024.
Bhatia SP, McGinty D, Letizia CS, Api AM. 2008. Fragrance material review on cedrol. Food Chem Toxicol. 46(11):S100-S102. DOI: 10.1016/j.fct.2008.06.038.
Blum S, Bühler H, Lichtenstein I, Siebe H. 1995. Characterization of human placental 11β-hydroxysteroid dehydrogenase, a key enzyme of corticosteroid metabolism. Cell Physiol Biochem. 5(3):167-175. DOI: 10.1159/000154751.
Bremmer HF, Prud’homme de Lodder LCH, van Engelen JGM. 2006. Cosmetics fact sheet. RIVM report 320104001/2006.
Bucks DAW, Guy RH, Maibach HI. 1989. Percutaneous penetration and mass balance accountability: technique and implications for dermatology. J Toxicol Cutan Ocul Toxicol. 8(4):439-451. DOI: 10.3109/15569528909062949.
Burdock GA. 2010. Fenaroli’s handbook of flavor ingredients.. 6th ed. Boca Raton (FL:): CRC press.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, supplement.
Canada. 1978. Food and Drug Regulations. C.R.C., c.870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette, Part III, vol. 22, no. 3.
Caputi L, Aprea E. 2011. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat Food Nutr Agric. 3(1):9-16.
Carroll JF, Tabanca N, Kramer M, Elejalde NM, Wedge DE, Bernier UR, Coy M, Becnel JJ, Demirci B, Başer KHC, et al. 2011. Essential oils of Cupressus funebris, Juniperus communis, and J. chinensis (Cupressaceae) as repellents against ticks (Acari: Ixodidae) and mosquitoes (Diptera: Culicidae) and as toxicants against mosquitoes. J Vector Ecol. 36(2):258-268. DOI: 10.1111/j.1948-7134.2011.00166.x.
Catlin NR, Herbert R, Janardhan K, Hejtmancik MR, Fomby LM, Vallant M, Kissling GE, DeVito MJ. 2016. Dose-response assessment of the dermal toxicity of Virginia cedarwood oil in F344/N rats and B6C3F1/N mice. Food Chem Toxicol. 98:159-168. DOI: 10.1016/j.fct.2016.10.016.
Chalchat JC, Garry RP, Miehet A, Benjilali B. 1994. Essential oil components in sawdust of Cedrus atlantica from Morocco. J Essent Oil Res. 6(3):323-325. DOI: 10.1080/10412905.1994.9698386.
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1983. Summary for the Results of Surveys of the amount and Frequency of use of cosmetic products by Women. Washington (DC): Environ Corporation. Report Prepared by Pitkin B, Rodericks JV, Turnbull D.
Chang KF, Huang XF, Chang JT, Huang YC, Weng JC, Tsai NM. 2020. Cedrol suppresses glioblastoma progression by triggering DNA damage and blocking nuclear translocation of the androgen receptor. Cancer Lett. 495:180-190. DOI: 10.1016/j.canlet.2020.09.007.
Chen W, Balan P, Popovich DG. 2019. Comparison of the ginsenoside composition of Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolius L.) and their transformation pathways. In: Atta-ur-Rahman FRS, editor. Studies in natural products chemistry. Vol. 63. Amsterdam (NL): Elsevier. p. 161-195. [accessed 2021 Nov 16].
Chen W, Balan P, Popovich DG. 2020. Comparison of ginsenoside components of various tissues of New Zealand forest-grown Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolium L.). Biomolecules. 10(3):372. DOI: 10.3390/biom10030372.
Coon JT, Ernst E. 2002. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 25(5):323-344.DOI: 10.2165/00002018-200225050-00003.
[CIR] Cosmetic Ingredient Review. 2001. Final report on the safety assessment of Juniperus communis extract, Juniperus oxycedrus extract, Juniperus oxycedrus tar, Juniperus phoenicea extract, and Juniperus virginiana extract. Int J Toxicol. 20(2):41-56. DOI: 10.1080/10915810160233758.
[CIR] Cosmetic Ingredient Review. 2007. Final report on the safety assessment of glycyrrhetinic acid, potassium glycyrrhetinate, disodium succinoyl glycyrrhetinate, glyceryl glycyrrhetinate, glycyrrhetinyl stearate, stearyl glycyrrhetinate, glycyrrhizic acid, ammonium glycyrrhizate, dipotassium glycyrrhizate, disodium glycyrrhizate, trisodium glycyrrhizate, methyl glycyrrhizate, and potassium glycyrrhizinate. Int J Toxicol. 26(2 Suppl):79-112. DOI: 10.1080/10915810701351228.
[CIR] Cosmetic Ingredient Review. 2012. Safety assessment of panax spp. root-derived ingredients as used in cosmetics. Washington (DC): CIR.
[CosIng] European Commission Database for Information on Cosmetic Substances and Ingredients [database]. 2021. [modified 2021 Dec 7]. Brussels (BE): European Commission Cosmetics Directive. [accessed 2021 Jul].
[CPID] Consumer Product Information [database]. c2021. McLean (VA): Delima Associates. [accessed 2021 Jul].
Cuzzolin L, Francini-Pesenti F, Verlato G, Joppi M, Baldelli P, Benoni G. 2010. Use of herbal products among 392 Italian pregnant women: focus on pregnancy outcome. Pharmacoepidemiol Drug Saf. 19(11):1151-1158.
Cwientzek U, Ottillinger B, Arenberger P. 2011. Acute bronchitis therapy with ivy leaves extracts in a two-arm study. A double-blind, randomised study vs. an other ivy leaves extract. Phytomedicine. 18(13):1105-1109. DOI: 10.1016/j.phymed.2011.06.014.
Daston GP, Overmann GJ, Baines D, Taubeneck MW, Lehman-McKeeman LD, Rogers JM, Keen CL. 1994. Altered Zn status by alpha-hederin in the pregnant rat and its relationship to adverse developmental outcome. Reprod Toxicol. 8(1):15-24. DOI: 10.1016/0890-6238(94)90063-9.
Dauben WG, Ashcraft AC. 1963. The total synthesis of (±)-Thujopsene. J Am Chem Soc. 85(22):3673-3676. DOI: 10.1021/ja00905a032.
[DPD] Drug Product Database [database]. [modified 2021 Nov 3]. Ottawa (ON): Government of Canada. [accessed 2021 Jul].
Du T, Shupe TF, Hse CY. 2011. Antifungal activities of 3 supercritical fluid extracted cedar oils. Holzforschung. 65(2). [accessed 2021 Sep 8].DOI: 10.1515/hf.2011.005.
Duffy JY, Baines D, Overmann GJ, Keen CL, Daston GP. 1997. Repeated administration of alpha-hederin results in alterations in maternal zinc status and adverse developmental outcome in the rat. Teratology. 56(5):327-334. DOI: 10.1002/(SICI)1096-9926(199711)56:5<327::AID-TERA6>3.0.CO;2-U.
[EC] European Commission. 2003. Technical Guidance Document on risk assessment, Part I [PDF]. 1998. Ispra (IT): Institute for Health and Consumer Protection, European Chemicals Bureau.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization of chemical substances. Ottawa (ON): Government of Canada. [accessed 2023 Nov].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018a. Rapid screening of substances with limited general population exposure. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018b. Screening assessment: substances identified as being of low concern using the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018c. Draft screening assessment: talc [PDF]. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. 2015. Biocides human health exposure methodology. 1st ed. Helsinki (FI): ECHA.
[ECHA] European Chemicals Agency. 2018. Registration dossier: Juniper, Juniperus virginiana, ext. Helsinki (FI): ECHA.
[ECHA] European Chemicals Agency. 2019. Candidate list of substances of very high concern for authorisation. Helsinki (FI): ECHA. [accessed 2021 Dec 10].
[EFSA] European Food Safety Authority. 2011. Scientific opinion on flavouring group evaluation 25, revision 2 (FGE.25Rev2): aliphatic and aromatic hydrocarbons from chemical group 31. EFSA J. 9(6):2177. [accessed 2022 Feb 7]. DOI: 10.2903/j.efsa.2011.2177.
[EFSA] European Food Safety Authority. 2015. Scientific opinion on flavouring group evaluation 18, revision 3 (FGE.18Rev3): aliphatic, alicyclic and aromatic saturated and unsaturated tertiary alcohols, aromatic tertiary alcohols and their esters from chemical groups 6 and 8. EFSA J. 13(5):4118.
Elsaieed EM, Nada SA. 2002. Teratogenicity of hexavalent chromium in rats and the beneficial role of ginseng. Bull Environ Contam Toxicol. 68(3):361-368. DOI: 10.1007/s001280262.
[EMA] European Medicines Agency. 2013. Assessment report on Glycyrrhiza glabra L. and/or Glycyrrhiza inflata Bat. and/or Glycyrrhiza uralensis Fisch., radix [PDF]. London (GB): European Medicines Agency. EMA/HMPC/571122/2010.
[EMA] European Medicines Agency. 2014. Assessment report on Panax ginseng C.A. Meyer, radix [PDF]. London (GB): European Medicines Agency. EMA/HMPC/321232/2012.
[EMA] European Medicines Agency. 2017. Assessment report on Hedera helix L., folium Final. London (GB): European Medicines Agency.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2014. Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA 1999). Ottawa (ON): Government of Canada.
[EWG Skin Deep] EWG’s Skin Deep Cosmetics [database]. 2021. [accessed 2021Jul].
Fazio S, Pouso J, Dolinsky D, Fernandez A, Hernandez M, Clavier G, Hecker M. 2009. Tolerance, safety and efficacy of Hedera helix extract in inflammatory bronchial diseases under clinical practice conditions: a prospective, open, multicentre postmarketing study in 9657 patients. Phytomedicine. 16(1):17-24.DOI: 10.1016/j.phymed.2006.05.003.
Feldmann RJ, Maibach HI. 1969. Percutaneous penetration of steroids in man. J Invest Dermatol. 52(1):89-94. DOI: 10.1038/jid.1969.12.
[FEMA] Flavor and Extract Manufacturers Association. Flavor Ingredient Library [database]. c2021. [accessed 2021 Jul].
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: frequency data. Food Chem Toxicol. 78:159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, et al. 2016. Consumption of cosmetic products by the French population. Second part: amount data. Food Chem Toxicol. 90:130-141.
Garcia-Hidalgo E, von Goetz N, Siegrist M, Hungerbühler K. 2017. Use-patterns of personal care and household cleaning products in Switzerland. Food Chem Toxicol. 99:24-39.
Gluck L, Kulovich MV, Borer RC, Brenner PH, Anderson GG, Spllacy WN. 1971. Diagnosis of respiratory distress syndrome by amniocentesis. Am J Obstet Gynecol. 109(3):440-445.
Gomez-Berrada MP, Gautier F, Parent-Massin D, Ferret PJ. 2013. Retrospective exposure data for baby and children care products: an analysis of 48 clinical studies. Food Chem Toxicol. 57:185-194.
[Goodscents] The Good Scents Company [database]. c2017. [accessed 2017 Jul].
Hall RL, Oser BL. 1965. Recent progress in the consideration of flavoring Ingredients the Food Additives Amendment. Ill. GRAS Substances [PDF]. Food Technol. 19:151-197.
Hall B, Tozer S, Safford B, Coroama M, Steiling W, Leneveu-Duchemin MC, McNamara C, Gibney M. 2007. European consumer exposure to cosmetic products, a framework for conducting population exposure assessments. Food Chem Toxicol. 45(11):2097-2108.
Han L, Li Z, Zheng Q, Liu JP, Li PY. 2016. A new triterpenoid compound from stems and leaves of American ginseng. Nat Prod Res. 30(1):13-19. DOI: 10.1080/14786419.2015.1030403.
Havlíková L, Macáková K, Opletal L, Solich P. 2015. Rapid determination of α-Hederin and hederacoside C in extracts of Hedera helix leaves available in the Czech Republic and Poland. Nat Prod Commun. 10(9):1529-1531. DOI: 10.1177/1934578X1501000910.
Health Canada. 2015. Aromatherapy — essential oils. Ottawa (ON): Government of Canada.
Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
Health Canada. 2019. Self-care products and Health Canada. Ottawa (ON): Government of Canada.
Health Canada. 2020. [modified 2020 Jul 14]. Vaping product regulations. Ottawa (ON): Government of Canada. [accessed 2021 Apr 22].
Health Canada. 2021. Canadian exposure factors used in human health risk assessment. Ottawa (ON): Government of Canada.
Health Canada. 2022. Nutrition Labelling - Table of Reference Amounts for Food. Ottawa (ON): Government of Canada.
Heck JD, Vollmuth TA, Cifone MA, Jagannath DR, Myhr B, Curren RD. 1989. An evaluation of food flavoring ingredients in a genetic toxicity screening battery. Toxicologist. 9:257-272.
Hess FG, Parent RA, Cox GE, Stevens KR, Becci PJ. 1982. Reproduction study in rats of ginseng extract G115. Food Chem Toxicol. 20(2):189-192. DOI: 10.1016/S0278-6915(82)80246-9.
Hosseini A, Zare S, Borzouei Z, Ghaderi Pakdel F. 2018. Cyclophosphamide-induced testicular toxicity ameliorate by American ginseng treatment: an experimental study. Int J Reprod Biomed. 16(11):711-718.
Hulshof KFAM, Kistemaker C. 1994. The consumption of licorice and the intake of ammonium chloride among Dutch population groups (second Dutch National Food Consumption Survey). TNO-report V 94.298.
Hundertmark S, Dill A, Bühler H, Stevens P, Looman K, Ragosch V, Seckl JR, Lipka C. 2002. 11beta-hydroxysteroid dehydrogenase type 1: a new regulator of fetal lung maturation. Horm Metab Res. 34(10):537-544. DOI: 10.1055/s-2002-35424.
[IFRA] International Fragrance Association Standards Library [database]. 2021. Geneva (CH): IFRA. [accessed 2021 Jul].
[IQWiG] Institute for Quality and Efficiency in Health Care. 2017. Eczema: steroids and other topical medications. Berlin (DE): IQWiG.
Isbrucker RA, Burdock GA. 2006. Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 46(3):167-192. DOI: 10.1016/j.yrtph.2006.06.002.
Kamatou GPP, Viljoen AM, Özek T, Başer KHC. 2010. Chemical composition of the wood and leaf oils from the “Clanwilliam Cedar” (Widdringtonia cedarbergensis J.A. Marsh): a critically endangered species. South Afr J Bot. 76(4):652-654. DOI: 10.1016/j.sajb.2010.04.002.
Kampf G, Ruselack S, Eggerstedt S, Nowak N, Bashir M. 2013. Less and less–influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 13:472.
Kirk-Othmer. 2012. Kirk-Othmer Chemical Technology of Cosmetics. 1st ed. Hoboken, N.J., Somerset: Wiley, John Wiley & Sons, Incorporated Hoboken, N.J., Somerset.
Kitts D, Hu C. 2000. Efficacy and safety of ginseng. Public Health Nutr. 3(4a):473-485. DOI: 10.1017/S1368980000000550.
Lin D, Sun W, Wang Z, Chen LG, Chen XL, Wang SH, Li WS, Ge RS, Hu GX. 2012. The effect of glycyrrhetinic acid on pharmacokinetics of cortisone and its metabolite cortisol in rats. J Biomed Biotechnol. 2012:1-5. DOI: 10.1155/2012/856324.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2024 Apr 10]. Ottawa (ON): Government of Canada. [accessed 2024 Jun].
Loretz LJ, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, et al. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43(2):279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, et al. 2006. Exposure data for personal care products: hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44(12):2008-2018.
Loretz LJ, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, et al. 2008. Exposure data for cosmetic products: facial cleaner, hair conditioner, and eye shadow. Food Chem Toxicol. 46(5):1516-1524.
Lutsenko Y, Bylka W, Matlawska I, Darmohray R. 2010. Hedera helix as a medicinal plant. Herba Pol. 56(1):83-96.
Maas P. 2000. Zoethout in levensmiddelen: onderzoek naar het glycyrrhizine gehalte van thee, kruidenmengsels, dranken en drop [Liquorice root in food stuffs: survey of the glycyrrhizin content of tea, herbal mixtures, alcoholic drinks and liquorice]. De Ware(n)Chemicus. 30:10. Available only in Dutch.
Macinga DR, Edmonds SL, Campbell E, Shumaker BS, Arbogast, JW. 2013. Efficacy of novel alcohol-based hand rub products at typical in-use volumes. Infect Control Hosp Epidemiol. 34(3):299-301.
Mannowetz N, Miller MR, Lishko PV. 2017. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc Natl Acad Sci. 114(22):5743-5748. DOI: 10.1073/pnas.1700367114.
Marnette LJ, Cohen SM, Fukushima S, Gooderham NJ, Hecht SS, Rietjens IMCM, Smith RL, Adams TB, Bastaki M, Harman CL, et al. 2014. GRASr2 evaluation of aliphatic acyclic and alicyclic terpenoid tertiary alcohols and structurally related substances used as flavoring ingredients. J Food Sci. 79(4):R428-R441.
McCalla CO, Nacharaju VL, Muneyyirci-Delale O, Glasgow S, Feldman JG. 1998. Placental 11 beta-hydroxysteroid dehydrogenase activity in normotensive and pre-eclamptic pregnancies. Steroids. 63(10):511-515. DOI: 10.1016/S0039-128X(98)00056-7.
Mendel M, Chłopecka M, Dziekan N, Wiechetek M. 2011. The effect of the whole extract of common ivy (Hedera helix) leaves and selected active substances on the motoric activity of rat isolated stomach strips. J Ethnopharmacol. 134(3):796-802. DOI: 10.1016/j.jep.2011.01.036.
[NHPID] [Natural Health Products Ingredients Database] [database]. [modified 2024 Mar 19]. Ottawa (ON): Government of Canada. [accessed 2024 Jun].
Nordic Council of Ministers. 1993. Adverse health effects of glycyrrhizic acid in licorice: a risk assessment. Copenhagen (DK): Nordiske Seminar-og Arbejdsrapporter.
Norris C, Fang L, Barkjohn KK, Carlson D, Zhang Y, Mo J, Li Z, Zhang J, Cui X, Schauer JJ, et al. 2019. Sources of volatile organic compounds in suburban homes in Shanghai, China, and the impact of air filtration on compound concentrations. Chemosphere. 231:256-268. DOI: 10.1016/j.chemosphere.2019.05.059.
[NTP] National Toxicology Program. 2002. NTP technical report on the toxicology and carcinogenesis studies of cedarwood oil (CAS No. 8000-27-9). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. [accessed 2021 Jul].
[NTP] National Toxicology Program. 2011. Toxicology and carcinogenesis studies of ginseng (CAS No. 50647-08-0) in F344/N rats and B6C3F1 mice (gavage studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program. 2016. NTP technical report on the toxicity studies of cedarwood oil (virginia) (casrn 8000-27-9) administered dermally to F344/N rats and B6C3F1/N mice. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. NTP TOX 86.
O.Berk Leaders in Packing Solutions. O.Berk online product catalog – fine mist sprayers. [accessed 2018 Sept].
OECD QSAR Toolbox [read-across tool]. 2014. Ver. 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
OECD QSAR Toolbox [read-across tool]. 2019. Version 4.2. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Ogunwole E, Kunle-Alabi O, Akindele O, Raji Y. 2019. Adverse effects of saccharum officinarum molasses on rat testicular cells. Adv Toxicol Toxic Eff. 3(1):001-008. DOI: 10.17352/atte.000003.
Opdyke D. 1978. Fragrance raw materials monographs: alpha-Cedrene. Food Cosmet Toxicol. 16(5-6):679-680.
Perriot R, Breme K, Meierhenrich UJ, Carenini E, Ferrando G, Baldovini N. 2010. Chemical composition of French mimosa absolute oil. J Agric Food Chem. 58(3):1844-1849. DOI: 10.1021/jf903264n.
Perveen S. 2018. Introductory chapter: terpenes and terpenoids. In: Perveen S, Al-Taweel A, editors. Terpenes and terpenoids. London (GB): IntechOpen. [accessed 2019 Dec 18].
Räikkönen K, Pesonen A-K, Heinonen K, Lahti J, Komsi N, Eriksson JG, Seckl JR, Jarvenpaa AL, Strandberg TE. 2009. Maternal licorice consumption and detrimental cognitive and psychiatric outcomes in children. Am J Epidemiol. 170(9):1137-1146. DOI: 10.1093/aje/kwp272.
Räikkönen K, Seckl JR, Heinonen K, Pyhälä R, Feldt K, Jones A, Pesonen AK, Phillips DIW, Lahti J, Järvenpää AL, et al. 2010. Maternal prenatal licorice consumption alters hypothalamic–pituitary–adrenocortical axis function in children. Psychoneuroendocrinology. 35(10):1587-1593. DOI: 10.1016/j.psyneuen.2010.04.010.
Räikkönen K, Martikainen S, Pesonen AK, Lahti J, Heinonen K, Pyhälä R, Lahti M, Tuovinen S, Wehkalampi K, Sammallahti S, et al. 2017. Maternal licorice consumption during pregnancy and pubertal, cognitive, and psychiatric outcomes in children. Am J Epidemiol. 185(5):317-328. DOI: 10.1093/aje/kww172.
Ramirez-Martinez A, Granda-Torres P, Wesolek N, Ficheux AS, Roudot AC. 2015. Exposure of hairdressers to the main cosmetics used in hairdressing salons in France: a preliminary study. Arch Environ Occup Health. 71(5):247-258.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006. Cosmetics fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No.: 320104001/2006.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2018. ConsExpo. 2016. ConsExpo Web Consumer Exposure Models. Bilthoven (NL): RIVM
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2018. Cleaning products factsheet: default parameters for estimating consumer exposure – updated version 2018 [PDF]. Bilthoven (NL): RIVM. Report No. 2016-0179. Contains erratum d.d. 13-9-2018.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2021a. Health risk assessment when using ethanol-containing hand gel. Bilthoven (NL): RIVM. Report No.: 2021-0026.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2021b. Air fresheners fact sheet. Bilthoven (NL): RIVM. Report No.: 2021-0233.
Roskos KV, Maibach HI, Guy RH. 1989. The effect of aging on percutaneous absorption in man. J Pharmacokinet Biopharm. 17(6):617-630. DOI: 10.1007/BF01062121.
Ruiz-Rodríguez MA, Vedani A, Flores-Mireles AL, Cháirez-Ramírez MH, Gallegos-Infante JA, González-Laredo RF. 2017. In silico prediction of the toxic potential of lupeol. Chem Res Toxicol. 30(8):1562-1571. DOI: 10.1021/acs.chemrestox.7b00070.
Ryan TJ, Beaucham C. 2013. Dominant microbial volatile organic compounds in 23 US homes. Chemosphere. 90(3):977-985.
Sakamoto K, Wakabayashi K. 1988. Inhibitory effect of glycyrrhetinic acid on testosterone production in rat gonads. Endocrinol Jpn. 35(2):333-342.
Satrani B, Aberchane M, Farah A, Chaouch A, Talbi M. 2006. Composition chimique et activité antimicrobienne des huiles essentielles extraites par hydrodistillation fractionnée du bois de Cedrus atlantica Manetti. Acta Bot Gallica. 153(1):97-104. DOI: 10.1080/12538078.2006.10515524.
[SCCS] Scientific Committee on Consumer Safety. 2010. Basic criteria for the in vitro assessment of dermal absorption of cosmetic ingredients adopted by the SCCS at its 7th plenary meeting of 22 June 2010. Brussels (BE): European Commission, Health & Consumers.
[SCCS] Scientific Committee on Consumer Safety. 2015. The SCCS notes of guidance for the testing of cosmetic ingredients and their safety evaluation.. 9th revision [PDF]. Brussels (BE): European Commission. [revised 2016 Apr 25; accessed 2020 Apr]. Report No. SCCS/1564/15.
Schmidt M, Thomsen M, Schmidt U. 2012. Suitability of ivy extract for the treatment of paediatric cough: ivy extract in the treatment of paediatric cough. Phytother Res. 26(12):1942-1947. DOI: 10.1002/ptr.4671.
SDS Search Tool [database]. 2019. Ottawa (ON): Government of Canada. [updated 2019 Mar 1; accessed 2021 Aug 10]. [restricted access].
Sell CS. 2003. A fragrant introduction to terpenoid chemistry. Cambridge: Royal Society of Chemistry Camb://site.ebrary.com/id/10621154.
Siddique HR, Mishra SK, Karnes RJ, Saleem M. 2011. Lupeol, a novel androgen receptor inhibitor: implications in prostate cancer therapy. Clin Cancer Res. 17(16):5379-5391. DOI: 10.1158/1078-0432.CCR-11-0916.
Sigurjonsdottir HA, Axelson M, Johannsson G, Manhem K, Nystrom E, Wallerstedt S. 2006. Liquorice in moderate doses does not affect sex steroid hormones of biological importance although the effect differs between the genders. Horm Res. 65(2):106-110. DOI: 10.1159/000091302.
Statistics Canada. 2017. Custom tabulation of grooming products data from the Canadian Health Measures Survey Cycle 2 (2010-2011). [restricted access]. Prepared for Existing Substances Risk Assessment Bureau, Health Canada by Statistics Canada.
Stauss-Grabo M, Atiye S, Warnke A, Wedemeyer RS, Donath F, Blume HH. 2011. Observational study on the tolerability and safety of film-coated tablets containing ivy extract (Prospan® Cough Tablets) in the treatment of colds accompanied by coughing. Phytomedicine. 18(6):433-436. DOI: 10.1016/j.phymed.2010.11.009.
Strandberg TE, Jarvenpaa AL, Vanhanen H, McKeigue PM. 2001. Birth outcome in relation to licorice consumption during pregnancy. Am J Epidemiol. 153(11):1085-1088s://doi.org/10.1093/aje/153.11.1085.
Strandberg TE. 2002. Preterm birth and licorice consumption during pregnancy. Am J Epidemiol. 156(9):803-805. DOI: 10.1093/aje/kwf130.
Strittholt CA, McMillan DA, He T, Baker RA, Barker ML. 2016. A randomized clinical study to assess ingestion of dentrifrice by children. Regul Toxicol Pharmacol. 75:66-71.
Surburg H, Panten J. 2016. Common fragrance and flavor materials: preparation, properties and uses. 6th ed. Weinheim (DE): Wiley-VCH Verlag GmbH & Co. KGaA.
Tisserand Institute. 2021. How to use essential oils safely. [accessed 2021 Jul].
Tisserand R, Young R. 2014. Essential oil safety: a guide for health care professionals. 2nd ed. London (GB): Churchill Livingstone.
Uehara A, Tommis B, Belhassen E, Satrani B, Ghanmi M, Baldovini N. 2017. Odor-active constituents of Cedrus atlantica wood essential oil. Phytochemistry. 144:208-215.
[US CFR] US Code of Federal Regulations. 2021a. Title 21, vol. 3, c. I, part 172.515: synthetic flavoring substances and adjuvants [PDF]. Washington (DC): National Archives and Records Administration’s Office of the Federal Register (OFR); Government Publishing Office. [accessed 2021 Aug].
[US CFR] US Code of Federal Regulations. 2021b. Title 21, part 184.1408: direct food substances affirmed as generally recognized as safe [PDF]. Washington (DC): National Archives and Records Administration’s Office of the Federal Register (OFR); Government Publishing Office. [accessed 2021 Aug].
[US CFR] US Code of Federal Regulations. 2021c. Title 21, vol. 3, part 172.510: natural flavoring substances and natural substances used in conjunction with flavors. Washington (DC): National Archives and Records Administration’s Office of the Federal Register (OFR); Government Publishing Office. [accessed 2021 Aug].
[US EPA] United States Environmental Protection Agency. 2012a. EPI Suite [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[US EPA] United States Environmental Protection Agency. 2012b. Standard operating procedures for residential pesticide exposure assessment [PDF]. Washington (DC): Health Effects Division, Office of Pesticide Programs, Office of Chemical Safety and Pollution Prevention.
[US EPA] United States Environmental Protection Agency. 2019. The Chemistry Dashboard. LIST: terpenes in vape. Research Triangle Park (NC): US EPA, Chemical Safety for Sustainability Research Program.
[US FDA] United States Food and Drug Administration. 2017. Black licorice: trick or treat? [updated 2017 Oct 30].
van Gelderen CEM, Bijlsma JA, van Dokkum W, Savelkoull TJF. 2000. Glycyrrhizic acid: the assessment of a no effect level. Hum Exp Toxicol. 19(8):434-439. DOI: 10.1191/096032700682694251.
[VCF] Volatile Compounds in Food [database]. 2021. Ver. 16.7. Van Dongen WD, Donders JJH, editors. . – Reeuwijk (NL): BeWiDo B.V., 1963-2021.
[VKM] Vitenskapskomiteen for mat og milijo [Norwegian Scientific Committee for Food and Environment]. 2018. Hazard assessment of glycyrrhizic acid from licorice. Oslo (NO): VKM. VKM Report 2019: 09.
Wang Y, Choi HK, Brinckmann JA, Jiang X, Huang L. 2015. Chemical analysis of Panax quinquefolius (North American ginseng): a review. J Chromatogr A. 1426:1-15. DOI: 10.1016/j.chroma.2015.11.012.
Wester RC, Bucks DAW, Maibach HI. 1983. In vivo percutaneous absorption of hydrocortisone in psoriatic patients and normal volunteers. J Am Acad Dermatol. 8(5):645-647. DOI: 10.1016/S0190-9622(83)70072-1.
[WHO] World Health Organization. 2005. Evaluation of certain food additives. Sixty-third report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Geneva (CH): World Health Organization. WHO Technical Report Series: 928.
[WHO] World Health Organization JECFA. 2009. Safety evaluation of certain food additives: Rome, Italy, from –17 - 26 June 2008. Geneva (CH): World Health Organization. WHO food additives series: 60.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48(11):3109-3119.
Yan H, Li X, Ni W, Zhao Q, Leng Y, Liu H. 2021. Phytochemicals from the leaves of cyclocarya paliurus and their 11 β ‐hsd1 enzyme inhibitory effects. Chem Biodivers. 18(1): e2000772. [accessed 2021 Oct 27]. DOI: 10.1002/cbdv.202000772.
Yu M, Shin YJ, Kim N, Yoo G, Park S, Kim SH. 2015. Determination of saponins and flavonoids in ivy leaf extracts using HPLC-DAD. J Chromatogr Sci. 53(4):478-483. DOI: 10.1093/chromsci/bmu068.
Zhang Z, Chen X, Chen H, Wang L, Liang J, Luo D, Liu Y, Yang H, Li Y, Xie J, et al. 2016. Anti-inflammatory activity of β-patchoulene isolated from patchouli oil in mice. Eur J Pharmacol. 781:229-238.DOI: 10.1016/j.ejphar.2016.04.028.
Zhao L, Li X. 1998. Zhao, L., & Li, X. (1998). Synthesis of amboryl acetate-A woody perfumery. Jiangsu Institute of Petrochemical Technology, 2 Abstract.
Zhong Y, Ding Y, Xiao D, Hu D, Li Y. 2022. New 18β-glycyrrhetinic acid-emodin esters synthetized by a one-step innovative route, its structural characterization, and in vivo toxicity assessed on zebrafish models. Chem Biodivers. 19(4):e202100928. DOI: 10.1002/cbdv.202100928.
Zrira S, Ghanmi M. 2016. Chemical composition and antibacterial activity of the essential of Cedrus atlantica (cedarwood oil). J Essent Oil-Bear Plants. 19(5):1267-1272. DOI: 10.1080/0972060X.2015.1137499.
Appendix A. Parameters for estimating oral, dermal, and inhalation exposures to products available to consumers
Exposure to products available to consumers was estimated using ConsExpo Web (2016). Exposure estimates were calculated using default body weights and inhalation rates of 74 kg/15.1 m3/day, 62 kg/15.9 m3/day, 42 kg/13.9 m3/day, 23 kg/11.1 m3/day, 15 kg/9.2 m3/day, 11 kg/8.0 m3/day, 9.1 kg/5.4 m3/day, and 6.3 kg/3.7 m3/day for adults (19 years and older), 14 to 18 years, 9 to 13 years, 4 to 8 years, 2 to 3 years, 1 year, 6 to 11 months, and 0 to 5 months, respectively (Health Canada 2021).
A dermal absorption factor of 25% was used for the Tricyclic Sesquiterpene subgroup 1 substances, as well as for all triterpenoids, including enoxolone, mimosa oil, and ivy extract, unless indicated otherwise. Calculated exposure estimates for mimosa oil and ivy extract were adjusted by 30% and 50% for the maximum amount of lupeol-like compounds (lupenone and lupeol) in mimosa oil and steroid-based compounds (hederacoside C, hederagenin, and alpha-hederin) in ivy extract, respectively.
Dermal exposure (mg/kg bw/day) was calculated using the following formula, unless indicated otherwise: [mean product (g/application) * mean daily frequency * product concentration * retention factor * conversion factor (1000 mg/g)] ÷ body weight (kg). Systemic exposure derived from dermal exposure (mg/kg bw/day) was calculated using the following formula: dermal exposure * dermal absorption.
Inhalation exposure (mg/kg bw/day) was calculated using the following formula, unless indicated otherwise: [air concentration (mg/m3) (24 hrs time-weighted average) * inhalation rate (m3/day)] ÷ body weight (kg).
Oral exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) × mean daily frequency × product concentration × conversion factor (1000 mg/g)] ÷ body weight (kg).
Combined exposure (mg/kg bw/day) was calculated using the following formula: dermal systemic exposure (mg/kg bw/day) + inhalation exposure (mg/kg bw/day) + oral exposure (mg/kg bw/day).
| Exposure scenario | Assumptions |
|---|---|
| Acne therapy (gel/cream) (cedarwood oil, enoxolone) (NHP) | Concentration: 0.02% cedarwood oil (reported as Cedrus atlantica bark extract), 0.3% enoxolone (reported as glycyearrhetinic acid) Product amount: 1.5 g (adults, 14 to 18 years), 1.1 g (9 to 13 years) Inhalation exposure for enoxolone was not quantified since the substance was not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode.Product amount: As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance. |
| After hair removal product (body) (cedarwood oil) | Concentration: 3% cedarwood oil Product amount: 7.10 g (adults, 14 to 18 years) (Ficheux et al. 2016), 5.50 g (9 to 13 years) (SA adjustment) Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Aftershave (face) (cedarwood oil) | Concentration: 5% cedarwood oil Product amount: 2.4 g (adults, 14 to 18 years) (Ficheux et al. 2016), 2.3 g (9 to 13 years) (SA adjustment) Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Analgesic patch (enoxolone) (NHP) | Concentration: 0.18% enoxolone (reported as glycyearrhetinic acid) Product amount: 0.453 g (adults), 0.716 g (14 to 18 years), 0.757 g (9 to 13 years), 0.868 g (4 to 8 years), 0.963 g (2 to 3 years). Inhalation exposure for enoxolone was not quantified since the substance was not considered volatile. |
| Antiseptic skin cleanser (spray) (cedarwood oil) (NHP) | Concentration: 0.05% cedarwood oil (spray) (reported as Cedrus atlantica wood essential oil) Product amount: 1.5 g (all age groups, Kampf et al. 2013; Macinga et al. 2013; Bansaghi et al. 2020) Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant rate release area mode: |
| Bath oil (cedarwood oil) | Concentration: 10% cedarwood oil Product amount (adjusted to account for the dilution): 0.0219 g (adults); 0.0206 g (14 to 18 years); 0.0159 g (9 to 13 years) (CFTA 1983) Frequency: The mean frequency was assumed to be 1. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Bath product (foam/bubbles) (cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, ivy extract) | Concentration: 3.5% cedarwood oil, 5% Texan cedarwood oil, 3% enoxolone, 1% mimosa oil, 0.3% ivy extract Product amount (adjusted to account for the dilution): 0.0394 g (adults), 0.0370 g (14 to 18 years) (Ficheux et al. 2016); 0.0078 g (9 to 13 years), 0.0051 g (4 to 8 years), 0.0055 g (2 to 3 years), 0.0041 g (1 year), 0.0034 g (6 to 11 months), 0.0026 g (0 to 5 months) (Garcia-Hidalgo et al. 2017) Inhalation exposures for enoxolone, mimosa oil, and ivy extract were not quantified since the substances were not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Bath product (salt) (cedarwood oil, Texan cedarwood oil, ivy extract) | Concentration: 10% cedarwood, 10% Texan cedarwood oil, 3% ivy extract. Product amount: 0.0278 g (adults), 0.0261 g (14 to 18 years), 0.0201 g (9 to 13 years) (Ficheux et al. 2016) Inhalation exposure for ivy extract was not quantified since the substance was not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Blush (mimosa oil) | Concentration: 1% mimosa oil Product amount: 0.013 g (adults,14 to 18 years and 9 to 13 years) (Ficheux et al. 2016); 0.014 (4 to 8 years) (SA adjustment) Inhalation exposure for mimosa oil was not quantified since the substance was not considered volatile. |
| Body adhesive (cedarwood oil, ivy extract) | Concentration: 0.1% cedarwood oil, 0.1% ivy extract Product amount: 10 g (adults, 14 to 18 years) (Ficheux et al. 2016) for cedarwood oil; 1.97 g (adults), 2.12 g (14 to 18 years) for ivy extract (product information). Inhalation exposure for ivy extract was not quantified since the substance was not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Body cleanser (liquid) (cedarwood oil, mimosa oil, ivy extract) | Concentration: 3% cedarwood oil, 0.3% mimosa oil, 0.1% ivy extract Product amount: 11 g (adults) (Loretz et al. 2006); 11 g (14 to 18 years) (Ficheux et al. 2016); 10.9 g (9 to 13 years, 4 to 8 years) (Garcia-Hidalgo et al. 2017); 6.7 g (2 to 3 years) (Garcia-Hidalgo et al. 2017); 5.4 g (1 year) (Ficheux et al. 2016); 4.9 g (6 to 11 months) (Gomez-Berrada et al. 2013); 4.5 g (0 to 5 months) (Gomez-Berrada et al. 2013) Inhalation exposures for ivy extract and mimosa oil were not quantified since the substances were not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Body cleanser (solid) (cedarwood oil, Texan cedarwood oil) | Concentration: 100% cedarwood oil, 100% Texan cedarwood oil Product amount: 1.1 g (adults), 1.1 g (14 to 18 years) (Ficheux et al. 2016); 0.82 g (9 to 13 years), 0.53 g (4 to 8 years), 0.38 g (2 to 3 years), 0.29 g (1 year), 0.24 g (6 to 11 months), 0.18 g (0 to 5 months) (SA adjustment) Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Body exfoliant (cedarwood oil, Texan cedarwood oil, mimosa oil, ivy extract) | Concentration: 3% cedarwood oil, 1% Texan cedarwood oil, 0.3% mimosa oil, 30% ivy extract Product amount: 10 g (adult), 10 g (14 to 18 years) (Ficheux et al. 2016) Inhalation exposures for mimosa oil and ivy extract were not quantified since the substances were not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–instantaneous release mode: |
| Body moisturizer (cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, ivy extract) | Concentration: 10% cedarwood oil, 10% Texan cedarwood oil, 3% enoxolone, 10% mimosa oil, 5% ivy extract Product amount: 10 g (adults, 14 to 18 years) (Ficheux et al. 2016); 7.7 g (9 to 13 years), 5 g (4 to 8 years) (SA adjustment); 4.1 g (2 to 3 years) (Ficheux et al. 2016); 3.1 g (1 year), 2.5 g (6 to 11 months), 2 g (0 to 5 months) (SA adjustment) Inhalation exposures for enoxolone, mimosa oil, and ivy extract were not quantified since the substances were not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Body moisturizer (spray) (enoxolone) | Concentration: 3% enoxolone Product amount: 5.2 g (adults, 14 to 18 years), 3.1 g (9 to 13 years, 4 to 8 years), .2.5 g (2 to 3 years, 1 year, 6 to 11 months, 0 to 5 months) (Ficheux et al. 2016). Air concentrations were modelled using the ConsExpo exposure to spray–instantaneous release mode: Exposure duration: 20 min |
| Cleanser (2-in-1 body wash/shampoo) (mimosa oil) | Concentration: 1% mimosa oil Product amount: 34.18g (adults), 31.75 g (14 to 18 years), 24.62 g (9 to 13 years), 16.22 g (4 to 8 years) (SA adjustment), 6 g (2 to 3 year, 1 year, 6 to 11 months, 0-5 months) (Gomez-Berrada et al. 2013) Inhalation exposure for mimosa oil was not quantified since the substance was not considered volatile. |
| Conditioner (leave-on) (cedarwood oil, ivy extract) | Concentration: 14% cedarwood oil, 3% ivy extract Product amount: 13.1 g (adults); 10 g (14 to 18 years); 7.8 g (9 to 13 years and 4 to 8 years); 5.2 g (2 to 3 years) (Ficheux et al. 2016; Garcia-Hidalgo et al. 2017) Inhalation exposure for ivy extract was not quantified since the substance was not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Conditioner (rinse-off) (cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, ivy extract) | Concentration: 5% cedarwood oil, 5% Texan cedarwood oil, 0.3% enoxolone, 0.1% mimosa oil, 1% ivy extract Product amount: 13.1 g (adults) (Loretz et al. 2008); 10 g (14 to 18 years) (Ficheux et al. 2016); 7.8 g (9 to 13 years and 4 to 8 years) (Ficheux et al. 2016); 5.2 g (2 to 3 years) (Garcia-Hidalgo et al. 2017) Frequency: 1.1 (adults) (Loretz et al. 2008). For all other subpopulations, the mean frequency was assumed to be 1. Inhalation exposures for enoxolone, mimosa oil, and ivy extract were not quantified since the substances were not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Deodorant/antiperspirant (solid) (cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil) | Concentration: 42% cedarwood oil, 10% Texan cedarwood oil, 0.1% enoxolone, 0.063% mimosa oil Product amount: 1 g (adults and 14 to 18 years); 0.4 g (9 to 13 years) (Ficheux et al. 2016) Inhalation exposures for enoxolone and mimosa oil were not quantified since the substances were not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Irritation relief balm (cedarwood oil) (NHP) | Concentration: 1.21% cedarwood oil (reported as Cedrus atlantica wood essential oil) Product amount: 5.5 g (adults, 14 to 18 years, 9 to 13 years, 4 to 8 years), 2.75 g (2 to 3 years, 1 year) (IQWiG 2017) Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation – constant rate release area mode: |
| Eye makeup remover (enoxolone) | Concentration: 1% enoxolone Product amount: 0.5 g (EC 2003; Bremmer et al. 2006) Inhalation exposure for enoxolone was not quantified since the substance was not considered volatile. |
| Eye moisturizer (mimosa oil, ivy extract) | Concentration: 3% mimosa oil, 1% ivy extract Product amount: 0.5 g (adults, 14 to 18 years). Mean product amount is based on EC 2003 and RIVM 2006 for eye makeup remover. Eye makeup remover was used as a surrogate for eye moisturizer. Inhalation exposures for mimosa oil and ivy extract were not quantified since the substances were not considered volatile. |
| Eye shadow (mimosa oil) | Concentration: 1% mimosa oil Product amount: 0.09 g (Ficheux et al. 2016) Inhalation exposure for mimosa oil was not quantified since the substance was not considered volatile. |
| Face exfoliant (mimosa oil, ivy extract) | Concentration: 1% mimosa oil, 1% ivy extract Product amount: 3.1 g (adults, 14 to 18 years, adults) (Ficheux et al. 2016) Inhalation exposures for mimosa oil and ivy extract were not quantified since the substances were not considered volatile. |
| Sunscreen (lotion) (enoxolone) (NHP) | Concentration: 0.1% enoxolone (reported as glycyearrhetinic acid) Product amount: 1.5 g (adults) (Ficheux et al. 2016) Inhalation exposure for enoxolone was not quantified since the substance was not considered volatile. |
| Face moisturizer (cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, ivy extract) | Concentration: 10% cedarwood oil, 5% Texan cedarwood oil, 30% enoxolone, 10% mimosa oil, 3% ivy extract. Product amount: 1.5 g (adults and 14 to 18years); 1.1 g (9 to 13 years) Inhalation exposures for enoxolone, mimosa oil, and ivy extract were not quantified since the substances were not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Facial cleanser (cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, ivy extract) | Concentration: 10% cedarwood oil, 10% Texan cedarwood oil, 10% enoxolone, 1% mimosa oil, 3% ivy extract. Product amount: 3.3 g (adults, 14 to 18 years) (Ficheux et al. 2016); 3.1 g (9 to 13 years) (SA adjustment) Inhalation exposures for enoxolone, mimosa oil, and ivy extract were not quantified since the substances were not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Facial makeup (liquid foundation) [cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, ivy extract] | Concentration: 0.3% cedarwood oil, 0.3% Texan cedarwood oil, 0.1% enoxolone, 10% mimosa oil, 0.1% ivy extract. Product amount: 0.54 g (adults) (Loretz.et al. 2016); 0.41 g (14 to 18 years) (Ficheux et al. 2016); 0.39 g (9 to 13 years) (SA adjustment); 0.34 g (4 to 8 years) (SA adjustment). Inhalation exposures for enoxolone, mimosa oil, and ivy extract were not quantified since the substances were not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Facial makeup (spray) (ivy extract) | Concentration: 6% ivy extract Product amount: Calculated using the upper range of volume per spray of 0.16 mL and the assumption that the maximum number of sprays per day would be 8. Mean product amount (g/day) = 0.16 mL/spray (O.Berg Product Catalog) * 8 sprays/day (manufacturer instructions) * density (g/mL) = 1.28 g/day (adults, 14 to 18 years, 9 to 13 years, 4 to 8 years) Inhalation exposure to the vapour from spray was quantified using the exposure to spray–spraying mode and the following parameters: |
| Facial makeup remover (enoxolone, mimosa oil, ivy extract) | Concentration: 0.1% enoxolone, 0.1% mimosa oil, 0.2% ivy extract Product amount: 4.4 g (adults, 14 to 18 years), 2.2 g (9 to 13 years, 4 to 8 years) (Ficheux et al. 2016); 2.0 g (3 years) (SA adjustment) Frequency: 1 (adults, 14 to 18 years, 9 to 13 years) (Ficheux et al. 2015). For 4 to 8 years and 3 years, the mean frequency was assumed to be 1. Inhalation exposure for enoxolone, mimosa oil, and ivy extract were not quantified since the substances were not considered volatile. |
| Fragrance (spray) (alpha-cedrene, cedarwood oil, Texan cedarwood oil, mimosa oil) | Concentration: 1% alpha-cedrene, 100% cedarwood oil, 30% Texan cedarwood oil, 3% mimosa oil Product amount: Based on eau de toilette 0.33 g (adults, 14 to 18 years, 9 to 13 years, 4 to 8 years, 2 to 3 years) (Loretz et al. 2006) Product amount: As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance and the daily frequency. Exposure and emission duration: 14 hrs (adults), 17 hrs (14 to 18 years, 9 to 13 years) Inhalation TWA daily calculated using the following formula: [((inhalation spray (mg/m3) * time (0.0833 hrs)) + (inhalation evaporation (mg/m3) * time (23.92 hrs))]/(total time (24 hrs) |
| Fragrance (roll-on) (mimosa oil) | Concentration: 10% mimosa oil Product amount: Based on eau de toilette 0.33 g (adults, 14 to 18 years, 9 to 13 years, 4 to 8 years, 2 to 3 years) (Loretz et al. 2006) Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: Product amount: As above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance and the daily frequency. Exposure and emission duration: 24 hrs |
| Hair perm/straighteners (cedarwood oil) | Concentration: 0.1% cedarwood oil Product amount: 80 g (adults, 14 to 18 years) (Bremmer et al. 2006); 76 g (9 to 13 years) (SA adjustment), 66 g (4 to 8 years) (SA adjustment) Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Hairspray (aerosol) (Texan cedarwood oil, mimosa oil, ivy extract) | Concentration: 5% Texan cedarwood, 0.1% mimosa oil, 0.1% ivy extract Product amount: 2.6 g (adults) (Loretz et al. 2008); 2.3 g (14 to 18 years, 9 to 13 years, and 4 to 8 years) (Ficheux et al. 2016) Air concentrations were modelled using the ConsExpo exposure to spray–spraying mode (spraying towards exposed person) and the following parameters: |
| Hair styling product (gel/wax/putty) (mimosa oil, ivy extract) | Concentration: 1% mimosa oil, 0.5% ivy extract Product amount: 3.7 g (adults and 14 to 18years) (Ficheux et al. 2016); 3.5 g (9 to 13 years); 3.1 g (4 to 8 years); 2.8 g (2 to 3 years) (SA adjustment) Inhalation exposures for mimosa oil and ivy extract were not quantified since the substances were not considered volatile. |
| Hair styling product (mousse) (mimosa oil, ivy extract) | Concentration: 0.1% mimosa oil, 0.1% ivy extract Product amount: 7.7 g (adults, 14 to 18 years) (Ficheux et al. 2016); 7.3 g (9 to 13 years); 6.3 g (4 to 8 years); 5.7 g (2 to 3 years) (SA adjustment) Inhalation exposures for mimosa oil and ivy extract were not quantified since the substances were not considered volatile. |
| Hairspray (pump) (enoxolone) | Concentration: 0.1% enoxolone Product amount: 3.6 g (adult), 1.1g (4 to 18 years) Air concentrations were modelled using the ConsExpo exposure to spray–spraying mode (spraying towards person): |
| Medicated skin care product (cream) (enoxolone) (NHP) | Concentration: 0.2% enoxolone (reported as glycyearrhetinic acid) Product amount: 1.6 g (adults, 14 to 18 years), 1.2 g (9 to 13 years, 4 to 8 years) (Ficheux et al. 2016); 0.87 g (2 to 3 years), 0.837 g (1 year), 0.67 g (6 to 11 months) Frequency: 2 (adults) (Loretz et al. 2015); 1 (14 to 18 years, 9 to 13 years, 4 to 8 years) (Wu et al. 2010; Ficheux et al. 2015); a frequency of 1 was assumed for the 6 months to 3 years age brackets. It was assumed that medicated skin care cream is used on damaged skin; therefore, for this scenario, 100% dermal absorption is also assumed. Inhalation exposure for enoxolone was not quantified since the substance was not considered volatile. |
| Lipstick and lip balm (cedarwood oil, mimosa oil) | Concentration: 3% cedarwood oil, 10% mimosa oil Product amount: 0.022 g (Ficheux et al. 2016) |
| Lip balm (enoxolone) | Concentration: 1% enoxolone Product amount: 0.022 g (Ficheux et al. 2016) |
| Sunscreen (lip balm) (mimosa oil) (NHP) | Concentration: 3.2% mimosa oil (reported as Acacia decurrens/jojoba/sunflower seed wax/polyglyceryl-3 esters) Product amount: 0.022 g (Ficheux et al. 2016) |
| Aromatherapy – Liquid for diffuser inhalation (NHP) (cedarwood oil) | Concentration: 5% cedarwood oil (reported as Cedrus atlantica bark oil) Product amount: 1.7136 g (4–12 drops per use, 1–3 times/day; volume per drop: 0.05 mL; 0.05 mL * 12 drops * density * 3 times/day) Air concentrations were modelled using the ConsExpo exposure to vapour–constant rate mode: |
| Aromatherapy – Liquid for steam bowl inhalation (NHP) (cedarwood oil) | Concentration: 5% cedarwood oil (reported as Cedrus atlantica bark oil) Product amount: 0.5712 g (4–12 drops per use, 1–3 times/day; volume per drop: 0.05 mL; 0.05 mL * 12 drops * density * 1 application/day) Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant rate release area mode: |
| Massage bar (mimosa oil) | Concentration: 1 % mimosa oil Product amount: 3.2 g (adults, subpopulation selected on the basis of product information). (Ficheux et al. 2016). Massage oil was used as a surrogate for massage bar. Inhalation exposure for mimosa oil was not quantified since the substance was not considered volatile. |
| Massage oil (cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, ivy extract) | Concentration: 3% cedarwood oil, 3% Texan cedarwood oil, 0.3% mimosa oil, 3% ivy extract Product amount: 3.2 g (adults); 2.9 g (14 to 18 years); 2.3 g (9 to 13 years); 1.9 g (4 to 8 years); 1.8 g (2 to 3 years, 1 year, 6 to 11 months, 0 to 5 months) (Ficheux et al. 2016) Inhalation exposures for mimosa oil and ivy extract were not quantified since the substances were not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Sunscreen (powder) (ivy extract) (NHP) | Concentration: 0.1% ivy extract [reported as Hedera helix (Ivy) extract] Product amount: 0.073 g (adults) (Ficheux et al. 2016) Air concentration: 1.36 mg/m3 (ECCC, HC 2018c) Inhalation exposure (mg/kg bw/day) = air concentration (mg/m3) * concentration in product * exposure time (hr/day) * inhalation rate (m3/day) / body weight (kg) |
| Mouthwash (cedarwood oil) | Concentration: 0.1% cedarwood oil Product amount: 1.7 g (adults and 14 to 18years) (SCCS 2015; Ficheux et al. 2016); 1 g (9 to 13 and 4 to 8 years) (products labels; SCCS 2015) |
| Counterirritant (spray) (NHP) (cedarwood oil) | Concentration: 2% cedarwood oil (reported as cedarwood essential oil) Product amount: 0.46 g [0.16 mL/spray (O.Berg Product Catalog) * 3 sprays * density] Air concentrations were modelled using the ConsExpo exposure to spray–instantaneous release mode: |
| Permanent hair dye (enoxolone, ivy extract) | Concentration: 0.1% enoxolone, 0.1% ivy extract Product amount: 132.6 g (adults, 14 to 18 years) (Ramirez-Martinez et al. 2015) Inhalation exposures for enoxolone and ivy extract were not quantified since the substances were not considered volatile. |
| Aromatherapy – respiratory spray (NHP) (cedarwood oil) | Concentration: 0.68% cedarwood oil (reported as Cedrus atlantica wood essential oil) Product amount: 0.46 g [0.16 mL/spray (O.Berg Product Catalog) * 3 sprays * density] Air concentrations were modelled using the ConsExpo exposure to spray–instantaneous release mode: |
| Shampoo (cedarwood oil, Texan cedarwood oil, enoxolone, mimosa oil, ivy extract) | Concentration: 5% cedarwood oil, 5% Texan cedarwood oil, 0.3% enoxolone, 0.1% mimosa oil, 3% ivy extract Product amount: 11.8 g (adults) (Loretz et al. 2008); 10.4 g (14 to 18 years), 7.5 g (9 to 13 years) (Ficheux et al. 2016); 9.7 g (4 to 8 years), 7.9 g (2 to 3 years), 6.1 g (1 year), 5.6 g (6 to 11 months), 3.9 g (0 to 5 months) (Gomez-Berrada et al. 2013) Inhalation exposures for enoxolone, mimosa oil, and ivy extract were not quantified since the substances were not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Shaving cream (body) (alpha-cedrene, cedarwood oil, Texan cedarwood oil) | Concentration: 0.1% alpha-cedrene, 6.66% cedarwood oil, 10% Texan cedarwood oil Product amount: 12.7 g (14 years to adults) (Ficheux et al. 2016), 9.9 g (9 to 13 years) (SA adjustment) Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Shaving cream (face) (cedarwood oil, enoxolone) | Concentration: 5% cedarwood oil, 0.1% enoxolone Product amount: 6.8 g (14 years to adults) (Ficheux et al. 2016), 6.4 g (9 to 13 years) (SA adjustment) Inhalation exposure for enoxolone was not quantified since the substance was not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Sunless tanning product, body (mimosa oil) | Concentration: 10% mimosa oil Product amount: 18.2 g (14 years to adults) (Ficheux et al. 2016) Inhalation exposure for mimosa oil was not quantified since the substance was not considered volatile. |
| Sunscreen (cream/lotion) (enoxolone, mimosa oil) (NHP and NPD) | Concentration: 0.5% enoxolone (reported as glycyearrhetinic acid) (NHPa), and 0.5% (NPD), 0.105% mimosa oil (reported Acacia decurrens flower wax) Product amount: 18.2 g (adults, 14 to 18 years), 6.3 g (9 to 13 years, 4 to 8 years), 5.4 g (2 to 3 years, 1 year, 6 to 11 months) (Ficheux et al. 2016) Inhalation exposures for enoxolone and mimosa oil were not quantified since the substances were not considered volatile. |
| Temporary hair dye (Texan cedarwood oil, ivy extract) | Concentration: 0.1% Texan cedarwood oil, 0.1% ivy extract Product amount: 35 g (adults, 14 to 18 years, 9 to 13 years, 4 to 8 years) (SCCS 2015) Inhalation exposure for ivy extract was not quantified since the substance was not considered volatile. Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| Toothpaste (cedarwood oil, enoxolone (cosmetic and NHP) | Concentration: 0.5% cedarwood oil, 0.1% (cosmetic) and 0.05% (NHP) enoxolone Product amount: 0.08 g (adults and 14 to 18 years); 0.14 g (9 to 13 years) (SCCS 2015; Ficheux et al. 2016); 0.21 g (4 to 8 years and 2 to 3 years) (Strittholt et al. 2016) Frequency: 2.5 (adults); 2.6 (14 to 18 years, 9 to 13 years); 2.9 (4 to 8 years, 2 to 3 years) (Garcia-Hidalgo et al. 2017) Only adults are identified as users of cosmetic toothpaste containing enoxolone. |
| Exposure scenario | Assumptions |
|---|---|
| Air freshener (plug-in) (alpha-cedrene) | Concentration: 1% alpha-cedrene Air concentrations were modelled using the ConsExpo exposure to vapour–constant rate mode: Inhalation exposure (mg/kg bw/day) = [air concentration (mg/m3) (24 hrs time-weighted average) * inhalation rate (m3/day)] ÷ body weight (kg) Dermal product amount (adults only): 0.53 g (RIVM 2021b) |
| Air freshener (wax melt) (Texan cedarwood oil) | Concentration: 1% Texan cedarwood oil Dermal exposure was calculated using the ConsExpo-direct product contact-instant application model: Air concentrations were modelled using the ConsExpo exposure to vapour-constant rate mode and the following parameters: |
| Leather maintenance spray (alpha-cedrene) | Concentration: 5% alpha-cedrene Product amount: 138 g (adults) (according to the RIVM, 2018, leather maintenance spray, trigger spray; the contact rate is 46 mg/min, and we have a release duration of 3 min, which gives a product amount of 138 mg) Air concentrations were modelled using the ConsExpo exposure to spray-instantaneous release mode (Ieather spraying volatile substances) and the following parameters: |
| Liquid laundry detergent (mixing and loading a liquid for hand-washing, hanging machine-washed clothes) (adults) (Texan cedarwood oil) | Concentration: 1% Texan cedarwood oil Mixing and loading (dermal): Washing (dermal): Hanging (dermal): Total dermal exposure (mg/kg bw/day) = [dermal (mixing/loading) (mg) + dermal (washing) (mg) + dermal (hanging) (mg)] ÷ body weight (kg) Mixing and loading (inhalation): Washing (inhalation): Hanging (inhalation): Air concentration (24 hrs TWA) = {[mean air concentration – mixing and loading (mg/m3) * time (0.75 min)] + [mean air concentration – washing (mg/m3) * time (10 min)] + [mean air concentration – hanging (mg/m3) * time (240 min)]} ÷ {[total time (250.75 min)] * [250.75 min * (24 hrs/60 min)]} |
| Liquid laundry detergent (migration from washed clothes) (1 year) (Texan cedarwood oil) | Concentration: 1% Texan cedarwood oil Migration from machine-washed clothes (dermal): Weight fraction of product on textile: 7.6 × 10-4 (regular liquid) (RIVM 2018, Table 6.7), defaults for leachable fraction – 3.8 g residual from 150 g product in 5000 g textiles Incidental oral (mouthing of washed textiles) (1 year): Surface residue (mg/cm2): See calculation above Combined exposure (1 year) = Dermal migration from washed clothing + Incidental oral from mouthing washed clothing or textiles |
| Liquid laundry detergent (mixing and loading a liquid for machine washing, hanging machine-washed clothes) (adults) (Texan cedarwood oil) | Concentration: 1% Texan cedarwood oil Mixing and loading (dermal): Hanging laundry (dermal): Mixing and loading (inhalation): Hanging (inhalation): Air concentration (24 hrs TWA) = {[mean air concentration – mixing and loading (mg/m3) * time (0.75 min)] + [mean air concentration – hanging (mg/m3) * time (240 min)]} / {[total time (240.75 min)] * (240.75 min) (24 hrs/60 min)} Total dermal exposure = dermal mixing and loading + dermal hanging washed clothing |
| Toilet or urinal cleaning or deodorizing product (automatic toilet bowl cleaner) (alpha-cedrene) | Concentration: 10% alpha-cedrene Application (dermal): Application (inhalation): |
| Toilet or urinal cleaning or deodorizing product (Texan cedarwood oil) | Concentration: 1% Texan cedarwood oil Application (dermal): Application (inhalation): |
| Exposure scenario | Assumptions |
|---|---|
| Black licorice candy (enoxolone) | Level of glycyrrhizinic acid in candy: 0.1% (Ballin et al. 2023) Black licorice candy consumption: 80 g/serving/daya |
| Licorice tea (enoxolone) | Level of glycyrrhizinic acid in tea: 114 mg/L (Ballin et al. 2023) 1 cup: 237 mL |
a The Table of Reference Amounts for Food (Health Canada 2022) reference amount for candies is 40 g, however, the serving size may range from 50% to 200% or more of the reference amount.
Appendix B. Parameters for estimating oral, dermal, and inhalation exposures to DIY products
Exposure to products available to consumers was estimated using ConsExpo Web (2016). Exposure estimates were calculated using default body weights and inhalation rates of 74 kg/15.1 m3/day, 62 kg/15.9 m3/day, 42 kg/13.9 m3/day, 23 kg/11.1 m3/day, 15 kg/9.2 m3/day, 11 kg/8.0 m3/day, 9.1 kg/5.4 m3/day, and 6.3 kg/3.7 m3/day for adults (19 years and older), 14 to 18 years, 9 to 13 years, 4 to 8 years, 2 to 3 years, 1 year, 6 to 11 months, and 0 to 5 months, respectively (Health Canada 2021).
A dermal absorption factor of 25% was used for the Tricyclic Sesquiterpene subgroup 1 substances, as well as for all triterpenoids, including mimosa oil and ivy extract. Calculated exposure estimates for mimosa oil and ivy extract were adjusted by 30% and 50% for the maximum amount of lupeol-like compounds (lupenone and lupeol) in mimosa oil and steroid-based compounds (hederacoside C, hederagenin, and alpha-hederin) in ivy extract, respectively.
Dermal exposure (mg/kg bw/day) was calculated using the following formula, unless indicated otherwise: [mean product (g/application) * mean daily frequency * product concentration * retention factor * conversion factor (1000 mg/g)] ÷ body weight (kg). Systemic exposure derived from dermal exposure (mg/kg bw/day) was calculated using the following formula: dermal exposure * dermal absorption.
Inhalation exposure (mg/kg bw/day) was calculated using the following formula, unless indicated otherwise: [air concentration (mg/m3) (24 hrs time-weighted average) * inhalation rate (m3/day)] ÷ body weight (kg).
Oral exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * conversion factor (1000 mg/g)] ÷ body weight (kg).
Combined exposure (mg/kg bw/day) was calculated using the following formula: dermal systemic exposure (mg/kg bw/day) + inhalation exposure (mg/kg bw/day) + oral exposure (mg/kg bw/day).
| Exposure scenario | Assumptions |
|---|---|
| DIY aroma diffuser / air freshener (cedarwood oil, Texan cedarwood oil, mimosa oil) | Concentration: 100% cedarwood oil, 100% Texan cedarwood oil, 100% mimosa oil Product amount for the dermal exposure: Air concentrations modelled using an exposure to vapour–constant rate mode: Inhalation exposure (mg/kg bw/day) = [air concentration (mg/m3) (24 hrs time-weighted average) * inhalation rate (m3/day) * frequency (365 times/365 (year))] ÷ body weight |
| DIY massage oil (cedarwood oil, Texan cedarwood oil, mimosa oil, ivy extract) | Concentration: 3% cedarwood oil, 3% Texan cedarwood oil, 3% mimosa oil, 3% ivy extract. Product amount: 3.2 g (adults); 2.9 g (14 to 18 years); 2.3 g (9 to 13 years); 1.9 g (4 to 8 years); 1.8 g (2 to 3 years, 1 year, 6 to 11 months, 0 to 5 months) (Ficheux et al. 2016) Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| DIY bath product (cedarwood oil, Texan cedarwood oil) | Concentration: 100% cedarwood oil, 100% Texan cedarwood oil. Product amount: based on approximately 10 drops of pure essential oil being added to a bath filled with 120 L water (one drop equivalent to 0.05 mL), multiplied by the oil density (RIVM 2006). |
| DIY body moisturizer (cedarwood oil, Texan cedarwood oil, mimosa oil, ivy extract) | Concentration: 3% cedarwood oil, 3% Texan cedarwood oil, 3% mimosa oil, 3% ivy extract Product amount: 10 g (adults and 14 to 18 years); 7.7 g (9 to 13 years); 5 g (4 to 8 years); 4.1 g (2 to 3 years); 3.1 g (1 year); 2.5 g (6 to 11 months); 2 g (0 to 5 months) (Ficheux et al. 2016) Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area mode: |
| DIY facial steamer/mist (cedarwood oil, Texan cedarwood oil, mimosa oil) | Concentration: 100% cedarwood oil, 100% Texan cedarwood oil, 100% mimosa oil Product amount: Based on approximately 10 drops of essential oil being added to the stationary device (one drop equivalent to 0.05 mL) * density (RIVM 2006; Health Canada 2015). Air concentrations were modelled using the ConsExpo exposure to vapour–constant rate mode: It is assumed that 50% of the mean event concentration will be inhaled and 50% of the mean event concentration will be exposed dermally. For inhalation exposure after 20 minutes of facial steaming, it is assumed that a person will remain in a 20 m3 room for 3 hours and 40 minutes. It is assumed that a one-year-old bystander will be present in the room for 4 hours. Inhalation exposure for 20 minutes (mg/kg bw/day) = {mean event concentration (mg/m3) * 0.5 * [inhalation rate (m3/day) * exposure time (20 minutes) ÷ (60 * 24)] * room volume} ÷ body weight Dermal product amount: mean event concentration (mg/m3) * room volume (1 m3) * 0.5 Secondary inhalation exposure for 3 hours and 40 minutes: Total amount inhaled in 20 minutes (mg) = mean event concentration (mg/m3) * 0.5 * air inhaled in 20 min (m3) Inhalation exposure (mg/kg bw/day) for bystanders (one-year-old only) = product amount in air after 20 minutes spread in the 20 m3 room (mg/m3) * inhalation rate (m3/day) * [220 min ÷ (60 min/hr * 24 hr/day)] ÷ body weight |
| DIY liquid floor cleaner (application) (adults) (cedarwood oil, Texan cedarwood oil) | Concentration: 100% cedarwood oil, 100% Texan cedarwood oil Product amount: 25 drops of essential oils in 3.8 L of water Application: Application (inhalation): |
| DIY liquid floor cleaner (exposure from contacting cleaned floor) (one year) (cedarwood oil, Texan cedarwood oil) | Concentration: 100% cedarwood oil, 100% Texan cedarwood oil Dermal exposure (mg/kg bw/day) = [deposited residue (mg/cm2) * fraction available for transfer (%) * transfer coefficient (cm2/hr) * exposure time (hrs) * dermal absorption (%)] ÷ body weight (kg) Deposited residue (mg/cm2): 4 mg/cm2(40 mL of water covered 1 m2 of floor * density of water (RIVM 2018) Transfer coefficient: 1927 cm2/hr (adult transfer coefficient [6800 cm2/hr] adjusted for the body surface area of a 1 to 2 year old, that is, 0.28 [5300 cm2/18700 cm2]) (Health Canada 2021). Fraction available for transfer: 8% Exposure time: 2 hr (time spent in kitchens and bathrooms) Inhalation exposure (mg/kg bw/day) = [air concentration (mg/m3) (24 hrs time-weighted average) * inhalation rate (m3/day)] ÷ body weight (kg) Incidental oral exposure (mg/day) = [HR (mg/cm2) * (FM * SAH (cm2)) * (ET * N_Replen) * (1 − (1 − SE)Freq_HtM/N_Replen)] Combined exposure = dermal + inhalation + incidental oral |

![C[C@@H]1CC[C@@H]2[C@]13CC=C([C@H](C3)C2(C)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-6.jpg)
![CC1=CC[C@@]2(CCCC([C@]23[C@H]1C3)(C)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-7.jpg)
![C[C@@H]1CC[C@@H]2[C@]13CCC(=C)[C@H](C3)C2(C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-9.jpg)
![CC1=C[C@H]2[C@@H](CC1)C(=C)CCCC2(C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-10.jpg)
![CC1=C[C@H]2C(=C(CCCC2(C)C)C)CC1](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-11.jpg)
![CC1=C[C@H]2[C@@H](CC1)C(=CCCC2(C)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-12.jpg)
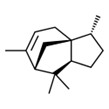
![CC1=CC[C@@]2(CCCC([C@]23[C@H]1C3)(C)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-15.jpg)
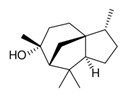
![C[C@@H]1CC[C@@H]2[C@]13CCC(=C)[C@H](C3)C2(C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-17.jpg)
![CC1=C[C@H]2[C@@H](CC1)C(=C)CCCC2(C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-18.jpg)
![CC1=C[C@H]2C(=C(CCCC2(C)C)C)CC1](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-19.jpg)
![CC1=C[C@H]2[C@@H](CC1)C(=CCCC2(C)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-20.jpg)
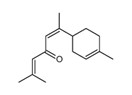
![CC1=CC[C@@]2(CCCC([C@]23[C@H]1C3)(C)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-22.jpg)
![C[C@@H]1CC[C@@H]2[C@]13CC=C([C@H](C3)C2(C)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-1-23.jpg)
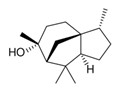
![C[C@]12CC[C@](C[C@H]1C3=CC(=O)[C@@H]4[C@]5(CC[C@@H](C([C@@H]5CC[C@]4([C@@]3(CC2)C)C)(C)C)O)C)(C)C(=O)O](/content/dam/eccc/images/pded/terpenes-group-4/Table2-2-2.jpg)
![CC(=C)[C@@H]1CC[C@]2([C@H]1[C@H]3CC[C@@H]4[C@]5(CCC(=O)C([C@@H]5CC[C@]4([C@@]3(CC2)C)C)(C)C)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-2-4.jpg)
![CC(=C)[C@@H]1CC[C@]2([C@H]1[C@H]3CC[C@@H]4[C@]5(CC[C@@H](C([C@@H]5CC[C@]4([C@@]3(CC2)C)C)(C)C)O)C)C](/content/dam/eccc/images/pded/terpenes-group-4/Table2-2-5.jpg)
![C[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1)O[C@@H]2[C@H](O[C@H]([C@@H]([C@H]2O)O)OC[C@@H]3[C@H]([C@@H]([C@H]([C@@H](O3)OC(=O)](/content/dam/eccc/images/pded/terpenes-group-4/Table2-2-7.jpg)
![C[C@]12CC[C@@H]([C@@]([C@@H]1CC[C@@]3([C@@H]2CC=C4[C@]3(CC[C@@]5([C@H]4CC(CC5)(C)C)C(=O)O)C)C)(C)CO)O](/content/dam/eccc/images/pded/terpenes-group-4/Table2-2-8.jpg)
![C[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1)O[C@@H]2[C@H]([C@H](CO[C@H]2O[C@H]3CC[C@]4([C@H]([C@]3(C)CO)](/content/dam/eccc/images/pded/terpenes-group-4/Table2-2-9.jpg)