Draft screening assessment - Alkanolamines and Fatty Alkanolamides Group
Official title : Draft screening assessment - Alkanolamines and Fatty Alkanolamides group
Environment and Climate Change Canada
Health Canada
July 2020
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 11 substances referred to collectively under the Chemicals Management Plan as the Alkanolamines and Fatty Alkanolamides Group. Substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. One additional substance (CAS RN 85204-21-3) was included in this assessment because it was determined to be a priority as a result of the approach described for Identification of Risk Assessment Priorities (IRAP). Their Chemical Abstracts Service Registry Numbers (CAS RNsFootnote 1 ), Domestic Substances List (DSL) names and abbreviations are listed in the table below. Eight substances in this screening assessment were originally referred to as the Alkanolamines Group under the Chemical Management Plan, while the three fatty alkanolamides (CAS RNs 120-40-1, 142-78-9, 68603-42-9) were moved from the Fatty Amides Group as they potentially contain residual alkanolamines (i.e., CAS RNs 141-43-5 or 111-42-2).
| CAS RN | Sub-group | DSL name | Abbreviation |
|---|---|---|---|
| 141-43-5 | 1 | Ethanol, 2-amino- | MEA |
| 100-37-8 | 1 | Ethanol, 2-(diethylamino)- | DEEA |
| 142-78-9 | 1 | Dodecanamide, N-(2-hydroxyethyl)- | LME |
| 111-42-2 | 2A | Ethanol, 2,2′-iminobis- | DEA |
| 120-40-1 | 2A | Dodecanamide, N,N-bis(2-hydroxyethyl)- | LDE |
| 68603-42-9a | 2A | Amides, coco, N,N-bis(hydroxyethyl) | CDE |
| 61791-31-9a | 2B | Ethanol, 2,2′-iminobis-, N-coco alkyl derivs. | CADEA |
| 61791-44-4a | 2B | Ethanol, 2,2′-iminobis-, N-tallow alkyl derivs. | TADEA |
| 102-71-6 | 3 | Ethanol, 2,2′,2″-nitrilotris- | TEA |
| 122-20-3b | 3 | 2-Propanol, 1,1′,1″-nitrilotris- | TIPA |
| 85204-21-3 a,c | 3 | 2-Butenoic acid, 4-[(2-ethylhexyl)amino]-4-oxo-, (Z)-, compd. with 2,2′,2″-nitrilotris[ethanol] (1:1) | BATEA |
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
b This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
c This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority as a result of the approach described for Identification of Risk Assessment Priorities (IRAP).
MEA is produced endogenously in humans, animals and plants. DEA can also be isolated from plants, but MEA is the only member of the group that occurs naturally in food items. Substances in the Alkanolamines and Fatty Alkanolamides Group, except MEA and BATEA, were included in a survey issued pursuant to section 71 of CEPA. Only DEA (100 000 to 1 000 000 kg), CDE (1 000 000 to 10 000 000 kg), TADEA (1 000 000 to 10 000 000 kg) and TEA (10 000 to 100 000 kg) were reported to be manufactured in Canada in 2011 above the reporting threshold of 100 kg. Canadian manufacture quantities are not available for MEA. In the same year, all reported substances in the Alkanolamines and Fatty Alkanolamides Group were imported into Canada in 2011 above the reporting threshold of 100 kg, ranging from 10 000 to 100 000 kg (for CADEA and LME) and from 1 000 000 to 10 000 000 kg (for CDE). According to the Canadian International Merchandise Trade Database, total imports of MEA into Canada between 2014 and 2017 ranged from 23 806 266 kg (2015) to 28 829 405 kg (2017). Although Canadian manufacturing and import quantities are not available for BATEA, BATEA was not identified in products available to consumers.
Substances in the Alkanolamines and Fatty Alkanolamides Group have been reported to be used in a range of industrial and consumer applications as antistatic agents, corrosion inhibitors, emulsifiers, foam stabilizers, chemical intermediates, pH adjusters, surfactants and viscosity modifiers. Uses of BATEA were not identified for the general population in Canada, but the other substances in the Alkanolamines and Fatty Alkanolamides Group may be present in food (MEA) or may be used in food packaging materials (MEA, DEEA, DEA, LDE, CDE, CADEA, TADEA, TEA, TIPA), cosmetics (MEA, LME, LDE, CDE, TEA, TIPA), drugs (MEA, DEA, CDE, and TEA), natural and non-prescription health products (MEA, DEA, LDE and CDE, TEA, TIPA), various household cleaners (MEA, DEA, CDE, CADEA, TEA, LME), and other products available to consumers.
The ecological risks of the substances in the Alkanolamines and Fatty Alkanolamides Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, the 11 substances in the Alkanolamines and Fatty Alkanolamides Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from MEA, DEEA, LME, DEA, LDE, CDE, CADEA, TADEA, TEA, TIPA and BATEA. It is proposed to conclude that the 11 substances in the Alkanolamines and Fatty Alkanolamides Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
With respect to human health, the substances in this screening assessment have been divided into three subgroups (monohydroxyl, dihydroxyl and trihydroxyl compounds) based on the number of alkanol moieties attached to the nitrogen atom in an amino or amide group. Potential exposure of the general population of Canada to substances in this group can occur through air and drinking water, as well as from use of products available to consumers.
In laboratory studies, the monohydroxyl substance MEA affected reproductive parameters and the larynx. A comparison of levels of MEA to which the general population may be exposed through its natural occurrence in food and through its use in all-purpose cleaner sprays with levels associated with critical effects resulted in margins of exposure that are considered adequate to account for uncertainties in the health effects and exposure databases. DEEA was shown to have effects on the liver and on body weight. A comparison of levels to which the general population may be exposed through its use in floor polish/wax with levels associated with critical effects resulted in margins that are considered adequate to account for uncertainties in the health effects and exposure databases. LME is considered to be of low concern for human health based on consideration of health effects information from structurally similar substances and risk to human health is therefore considered to be low.
DEA, LDE and CDE are dihydroxyl compounds. LDE and CDE have the potential to contain residual DEA. The International Agency for Research on Cancer has classified DEA and CDE as possibly carcinogenic to humans, but it has not assessed LDE. In laboratory studies, there were increased incidences of liver tumours with DEA, as well as with LDE or CDE due to residual DEA. Non-cancer kidney and liver effects were also observed with DEA, LDE and CDE, with additional non-cancer effects in the blood with DEA. For DEA, LDE, or CDE, a comparison of levels to which the general population may be exposed through drinking water (DEA, CDE) or potential use in food packaging materials (LDE only), and through the use of products available to consumers (including DEA in wall paint and dishwashing liquid, LDE in body soap and CDE in shampoo) with critical effect levels resulted in margins of exposure that are considered adequate to account for uncertainties in the health effects and exposure databases. A DEA-based cancer risk assessment was conducted in this document. Margins between levels of exposure of the general population from daily exposures to DEA and cancer effects were considered adequate. The margins were also expected to be adequate for daily exposures to LDE or CDE for cancer effects, given the relatively lower amount of DEA expected in LDE or CDE.
CADEA and TADEA are dihydroxyl compounds that are fatty acid diethanolamines. In laboratory studies, CADEA affected reproductive parameters. Comparison of CADEA levels to which the general population may be exposed through its potential use in food packaging materials and through the use of products available to consumers with critical effect levels resulted in margins of exposure that are considered adequate to account for uncertainties in the health effects and exposure databases. In laboratory studies, TADEA affected body weights. A comparison of TADEA levels to which the general population may be exposed through its potential use in food packaging materials and through the use of products available to consumers with critical effect levels resulted in margins of exposure that are considered adequate to account for uncertainties in the health effects and exposure databases.
TEA, TIPA and BATEA are trihydroxyl compounds. In laboratory studies, TEA caused liver tumours and affected reproductive parameters. Comparison of the levels to which the general population may be exposed through non-fluoridated toothpaste and through the use of products available to consumers with critical effect levels resulted in margins of exposure that are considered adequate to account for uncertainties in the health effects and exposure databases for cancer and non-cancer effects. No health effects have been reported in laboratory studies with TIPA, and risk to the general population is therefore considered to be low. BATEA was not identified as posing a high hazard to human health on the basis of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. Levels of BATEA in environmental media are considered minimal based on expected limited use in Canadaand BATEA was not identified in products available to consumers.
It is proposed to conclude that MEA, DEEA, LME, DEA, LDE, CDE, CADEA, TADEA, TEA, TIPA and BATEA do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is proposed to conclude that the 11 substances in the Alkanolamines and Fatty Alkanolamides Group do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 11 substances referred to collectively under the Chemicals Management Plan as the Alkanolamines and Fatty Alkanolamides Group to determine whether they present or may present a risk to the environment or to human health. The substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]). One additional substance was included in this assessment because it was determined to be a priority as a result of the approach described for Identification of Risk Assessment Priorities (IRAP).
The ecological risks of the substances in the Alkanolamines and Fatty Alkanolamides Group were characterized using the ecological risk classification (ERC) of organic substances (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and it considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
Some substances in the Alkanolamines and Fatty Alkanolamides Group currently being evaluated have been reviewed internationally through the Organisation for Economic Cooperation and Development (OECD) Cooperative Chemicals Assessment Programme, and a Screening Information Data Set (SIDS) and SIDS Initial Assessment Reports (SIARs) are available. These assessments undergo rigorous review and endorsement by international governmental authorities. Health Canada and Environment and Climate Change Canada are active participants in this process and consider these assessments to be reliable. OECD SIARs were used to inform the health effects characterization in this screening assessment. In addition, the health effects of substances in the Alkanolamines and Fatty Alkanolamides Group have been reviewed by the Australian National Industrial Chemicals Notification and Assessment Scheme (NICNAS), the European Commission (EC), the United States Environmental Protection Agency (US EPA), the International Agency for Research on Cancer (IARC) and the US National Toxicology Program (NTP). Reviews conducted by these institutions are also used to inform the health effects characterization in this screening assessment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to June 2018. Empirical data from key studies as well as some results from models were used to reach proposed conclusions.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external peer review. Comments on the technical portions relevant to human health were provided by Dr. Susan Griffin, Dr. Andrew Maier, and Dr. Pamela Williams, from Risk Science Center, University of Cincinnati, USA. The ecological portion of this assessment is based on the ERC document (published July 30, 2016Footnote 2 ), which was subject to an external review and 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 3 This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Number (CAS RNs)Footnote 4 , Domestic Substances List (DSL) names, common names, and abbreviations for the 11 individual substances in the Alkanolamines and Fatty Alkanolamides Group are presented in Table 2-1.
With respect to human health, the substances were divided into three subgroups based on the number of alkanol moieties attached to the amino or amide groups (monohydroxyl, dihydroxyl and trihydroxyl compounds). The number of alkanol moieties present influences their potential toxicity through the perturbance of choline homeostasis (Leung et al. 2005). Subgroup 2 was further divided into two groups based on whether DEA may be present, which also affects choline homeostasis (Leung et al. 2005). Subgroup 2A includes DEA, LDE and CDE. LDE is a reaction product with lauric acid and DEA (Johansson 2001). CDE, a UVCB, is a condensation product of coconut oilFootnote 5 and DEA (Johansson 2001). Since LDE and CDE are condensation reaction products from their corresponding fatty acid and DEA (Johansson 2001), they are expected to contain residual DEA from manufacturing (CIR 2013b). Subgroup 2B includes CADEA and TADEA, UVCBs, which are fatty acid diethanolamines produced by the reaction of ethylene oxide with alkylamines (Frauenkron et al. 2012) and so do not have residual DEA.
| Sub-group | CAS RN (abbreviation) | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|---|
| 1 | 141-43-5 (MEA) | Ethanol, 2-amino- (Monoethanolamine) | 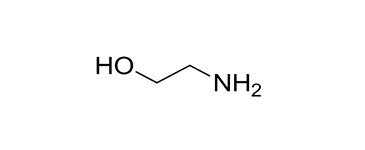 C2H7NO C2H7NO | 61.08 |
| 1 | 100-37-8 (DEEA) | Ethanol, 2-(diethylamino)- (Diethylethanolamine) | 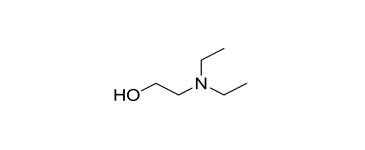 C6H15NO C6H15NO | 117.19 |
| 1 | 142-78-9 (LME) | Dodecanamide,N-(2-hydroxyethyl)- (Lauric monoethanolamide) | 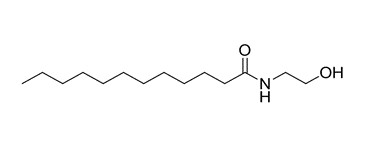 C14H29NO2 C14H29NO2 | 243.39 |
| 2A | 111-42-2 (DEA) | Ethanol, 2,2′-iminobis-(Diethanolamine) | 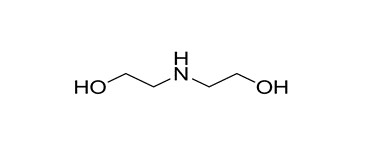 C4H11NO2 C4H11NO2 | 105.14 |
| 2A | 120-40-1 (LDE) | Dodecanamide, N,N-bis(2-hydroxyethyl)-(Lauric diethanolamide) | 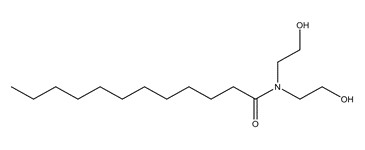 C16H33NO3 C16H33NO3 | 287.44 |
| 2A | 68603-42-9a (CDE) | Amides, coco, N,N-bis(hydroxyethyl)(Coconut diethanolamide) | 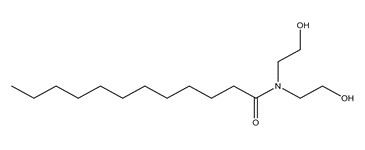 C16H33NO3 C16H33NO3 | 287.44 |
| 2B | 61791-31-9b (CADEA) | Ethanol, 2,2′-iminobis-, N-coco alkyl derivs.(DEA N-coco alkyl derivatives) | 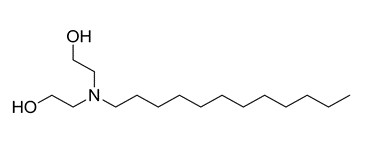 C16H35NO2 C16H35NO2 | 273 |
| 2B | 61791-44-4c (TADEA) | Ethanol, 2,2′-iminobis-, N-tallow alkyl derivs.(DEA N-tallow alkyl derivatives) | 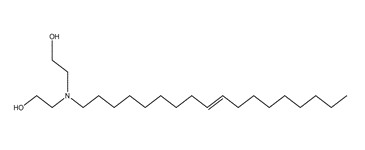 C22H45NO2 C22H45NO2 | 356 |
| 3 | 102-71-6 (TEA) | Ethanol, 2,2′,2″-nitrilotris- (Triethanolamine) | 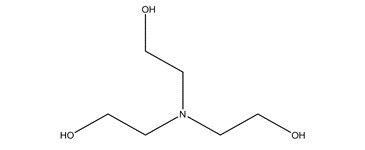 C6H15NO3 C6H15NO3 | 149.19 |
| 3 | 122-20-3 (TIPA) | 2-Propanol, 1,1′,1″-nitrilotris- (Triisopropanolamine) | 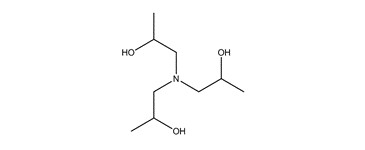 C9H21NO3 C9H21NO3 | 191.27 |
| 3 | 85204-21-3d (BATEA) | 2-Butenoic acid, 4-[(2-ethylhexyl)amino]-4-oxo-, (Z)-, compd. with 2,2′,2″-nitrilotris[ethanol] (1:1) (N-(2-Ethylhexyl)maleamic acid triethanolamine salt) | 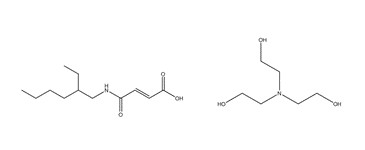 C12H21NO3 C6H15NO3Combined: C18H36N2O6 C12H21NO3 C6H15NO3Combined: C18H36N2O6 | 376.49 |
a UVCB. Representative/typical structure (C12), variable chain length from C8 to C18 ;with 0-2 degrees of unsaturation; US EPA 2010a.
b UVCB. Representative/typical structure (C12), variable chain length from C6 to C18 with 0-2 degrees of unsaturation; US EPA 2010b
c UVCB. Representative/typical structure (C18), variable chain length from C14 to C18; with 0-3 degrees of unsaturation; US EPA 2010b
d As a complex reaction product, BATEA is a UVCB with a defined molecular weight.
2.1 Selection of analogues
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, has been used to inform the human health assessments. The analogues selected were structurally similar to substances within this group (similar physical-chemical properties, toxicokinetics) and had relevant empirical data that could be used to read-across to substances with limited empirical data. The applicability of (Q)SAR models was determined on a case-by-case basis. Details of the read-across data and (Q)SAR models used to inform the human health assessments of the Alkanolamines and Fatty Alkanolamides Group are further discussed in the relevant sections of this report. Analogues which informed this assessment are presented in Table 2-2.
| CAS RN (abbreviation) | DSL or other name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 68140-00-1 (CME) a | Coconut acid monoethanolamide (Cocomide MEA) | 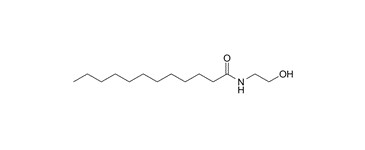 (main component of CME) (main component of CME) | UVCB (C8-18) |
| 111-57-9 (SME) | Stearoyl monoethaolamide (Stearamide MEA) | 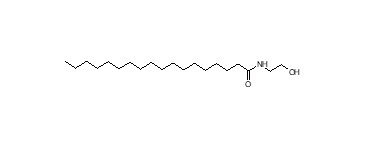 | 328 |
| 111-05-7 (OMIPA) | 9-Octadecenamide, N-(2-hydroxypropyl)-, (Z)- (Oleic acid monoisopropanolamide) | 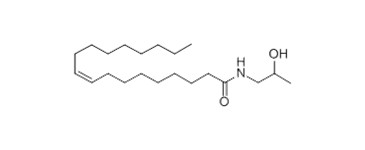 C21H41NO2 C21H41NO2 | 339.56 |
| 93-83-4 (ODE) | (9Z)-N,N-bis(2-hydroxyethyl)octadec-9-enamide (Oleic acid diethanolamide) | 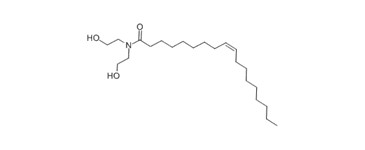 C22-H43-N-O3 C22-H43-N-O3 | 387.6 |
a UVCB. Representative/typical structure (C12), variable chain length from C8 to C18; ;, with 0-2 degrees of unsaturation; US EPA 2010a.
The health effects of LME were characterized on the basis of the analogues coconut acid monoethanolamide (CME)—of which LME is a main component—stearoyl monoethanolamide (SME), and oleic acid monoisopropanolamide (OMIPA) (Appendix D, Table D-1). The health effects of CADEA and TADEA were considered collectively. The contribution of DEA to the toxicity of LDE and CDE was informed by ODE since LDE, CDE, and ODE are structural analogues that differ in the amount of DEA (Appendix A, Table A-2). Details of the read-across data and (Q)SAR models that were selected to inform the human health assessments are further discussed in the relevant sections of this report.
3. Physical and chemical properties
A summary of the key physical and chemical properties of the substances in the Alkanolamines and Fatty Alkanolamides Group are presented in Table 3-1, Table 3-2 and Table 3-3. When experimental information was limited, (Q)SAR models were used to generate predicted values for the substance. Data from analogues were used for read-across. Additional physical and chemical properties are presented in ECCC (2016b).
| Abbreviation | Water solubility (mg/L) | log Kow | Vapour pressure (mm Hg) | pKa |
|---|---|---|---|---|
| MEA | 1.00E+06a | -2.3a | 0.404b | 9.21a |
| DEEA | 1.00E+06b | 0.21c | 1.4b | 10.1c |
| LME | 43.9b,pred | 3.24b,pred | 6.57E-09b,pred | NA |
Abbreviations: Kow, octanol–water partition coefficient; NA, not available; pred, predicted.
a ECHA c2007-2017a.
b ChemIDplus1993- .
c ECHA c2011-2017.
| Abbreviation | Water solubility (mg/L) | log Kow | Vapour pressure (mm Hg) | pKa |
|---|---|---|---|---|
| DEA | 1.00E+06a | -2.46a | 2.80E-04b | 8.99a |
| LDE | 226b,pred | 2.89b,pred | 6.70E-09b,pred | NA |
| CDE | 226c,pred | 2.89c,pred | 6.70E-09c,pred | NA |
| CADEA | 83.13d,pred | 3.90d,pred | 1.76E-08d,pred | 6.2e,pred |
| TADEA | 0.126d,pred | 6.63d,pred | 3.25E-11d,pred | 8.0e,pred |
Abbreviations: Kow, octanol–water partition coefficient; NA, not available; pred, predicted.
a ECHA c2007-2017b.
b ChemIDplus 1993- .
c UVCB, properties based on LDE (C12) as typical homologue in mixture (ChemIDplus 1993- ).
d UVCB, predicted based on representative structure in Table 2-1 for this substance (EPI Suite c.2000-2012)
e US EPA 2010b
| Abbreviation | Water solubility (mg/L) | log Kow | Vapour pressure (mm Hg) | pKa |
|---|---|---|---|---|
| TEA | 1.00E+06a | -1a | 3.59E-06a | 7.86a |
| TIPA | 8.20E+05a | -0.15c | 7.5E-05c | 8.06a |
| BATEA | 1.00E+06b | 3.22b,pred | 0.45b | NA |
Abbreviations: Kow, octanol–water partition coefficient; NA, not available; pred, predicted.
a ChemIDplus 1993- .
b ECHA [modified 2017].
c ECHA c2013-2018a.
4. Sources and uses
MEA occurs naturally in foods and tobacco and may be produced by abiotic and biotic processes (Frauenkron et al. 2012; Simoneit et al. 2000). DEA can be isolated from some plants (Brown and Gray 1986).
All of the substances in the Alkanolamines and Fatty Alkanolamides Group except MEA and BATEA were included in a survey issued pursuant to section 71 of CEPA (Canada 2012). According to the Canadian International Merchandise Trade Database (CIMT), annual average world imports of MEA into Canada from 2014 to 2017 were approximately 26 million kg and ranged from 23 806 266 kg (2015) to 28 829 405 kg (2017) (Statistics Canada [modified 2017]). Table 4-1 presents a summary of the reported total manufacture and import quantities for the nine substances surveyed under Section 71 of CEPA (Environment Canada 2013) and import quantities for MEA according to the CIMT (Statistics Canada [modified 2017]).
| Subgroup | Abbreviation | Total manufacturea (kg) | Total importsa (kg) |
|---|---|---|---|
1 |
MEA |
NA |
23 806 266 – 28 829 405b |
1 |
DEEA |
0c |
347 147 |
1 |
LME |
0c |
10 000 - 100 000 |
2A |
DEA |
100 000 - 1 000 000 |
3 331 373 |
2A |
LDE |
0c |
69 543 |
2A |
CDE |
1 000 000 - 10 000 000 |
1 000 000 - 10 000 000 |
2B |
CADEA |
0c |
10 000 - 100 000 |
2B |
TADEA |
1 000 000 - 10 000 000 |
100 000 - 1 000 000 |
3 |
TEA |
10 000 - 100 000 |
4 595 027 |
3 |
TIPA |
0c |
100 000 - 1 000 000 |
3 |
BATEA |
NA |
NA |
Abbreviations: NA, not surveyed pursuant to Section 71 of CEPA.
aInformation reported in response to a Section 71 survey under CEPA (Environment Canada 2013) except for MEA and BATEA which were not surveyed. See surveys for specific inclusions and exclusions (schedules 2 and 3).
b Annual Canadian import data for “monoethanolamine and its salts” for 2014-2017 from the Canadian International Merchandise Trade database (Statistics Canada [modified 2017]).
c Value reported in response to a section 71 survey under CEPA was 0 kg (Environment Canada 2013).
Table 4-2 presents a summary of the major uses of the nine substances based on information submitted pursuant to section 71 of CEPA (Environment Canada 2013) and of MEA based on information submitted under section 70 of CEPA (ECCC 2017). No uses were submitted for BATEA `under section 70 of CEPA (ECCC 2017) nor were identified elsewhere. Table 4-3 presents additional uses identified in Canada.
| Usea | Subgroup 1 | Subgroup 2A/B | Subgroup 3 |
|---|---|---|---|
Adhesives and sealants |
N |
DEA |
TEA |
Anti-Freeze and de-icing |
N |
N |
TEA |
Apparel and footwear care |
N |
N |
TEA |
Automotive care |
MEAb |
N |
TEA |
Automotive, aircraft and transportation |
N |
DEA |
TEA |
Building or construction materials not otherwise covered in this table |
N |
DEA, CADEA, TADEA, LDE, CDE |
TEA, TIPA |
Cleaning and furnishing care |
N |
DEA, CADEA, TADEA, CDE |
TEA, TIPA |
Drugs |
N |
CDE |
TEA |
Fabric, textile and leather articles not otherwise covered in this table |
N |
DEA, CADEA, TADEA, CDE |
TEA |
Floor coverings |
N |
N |
TIPA |
Food Packaging |
N |
DEA, LDE |
TEA |
Furniture and furnishings not otherwise covered in this table |
N |
N |
TIPA |
Ink, toner and colourants |
N |
DEA |
TEA |
Laundry and dishwashing |
MEAb, LME |
DEA, CDE, TADEA |
TEA |
Lubricants and greases |
N |
DEA, CADEA, TADEA, LDE |
TEA, TIPA |
Metal materials not otherwise covered in this table |
DEEA |
DEA |
TEA |
Natural health |
N |
N |
TEA |
Oil and natural gas extraction |
N |
DEA, CADEA, TADEA |
TEA, TIPA |
Corrosion inhibitor |
N |
N |
TEA |
Paints and coatings |
DEEA |
DEA, CADEA, TADEA |
TEA |
Paper products, mixtures or manufactured items |
MEAb |
DEA |
TEA |
Personal care productsc |
MEAb, DEEA, LME |
DEA, LDE, CDE |
TEA, TIPA |
Plastic and rubber materials not otherwise covered in this table |
N |
DEA, CADEA, LDE |
TEA, TIPA |
Water treatment |
DEEA |
DEA, CADEA, TADEA |
TEA |
Abbreviations: N, use was not reported for any substance in the sub-group.
a Uses reported in response to a section 71 survey under CEPA (Environment Canada 2013) unless otherwise indicated. See surveys for specific inclusions and exclusions (schedules 2 and 3);
b Not surveyed pursuant to a section 71 survey. Uses reported in a submission under section 70 of CEPA (ECCC 2017).
c For the purpose of this document, a personal care product is a product that is generally recognized by the public for use in daily cleansing or grooming. Depending on how the product is represented for sale and its composition, personal care products may fall into one of three regulatory categories in Canada: cosmetics, drugs or natural health products.
| Use | Subgroup 1 | Subgroup 2A/B | Subgroup 3 |
|---|---|---|---|
| Food additivea | N | N | N |
| Food packaging materialsa | MEA, DEEA | DEA, CADEA, TADEA, LDE, CDE | TEA, TIPA |
| Incidental additivea | MEA, DEEA, LME | DEA, CADEA, TADEA, LDE, CDE | TEA |
| Internal Drug Product Database as medicinal or non-medicinal ingredients in final Pharmaceutical, Disinfectant or Veterinary drug products in Canadab | MEA | DEA, CDE | TEA |
| Natural Health Products Ingredients Databasec | MEA | DEA, LDE, CDE | TEA, TIPA |
| Licensed Natural Health Products Database as medicinal or non-medicinal ingredients in natural health products in Canadac | N | DEA, LDE, CDE | TEA, TIPA |
| List of Prohibited and Restricted Cosmetic Ingredientsd | N | DEA | N |
| Notified to be present in cosmetics, based on notifications submitted under the Cosmetic Regulations to Health Canadae | MEA, LME | LDE, CDE | TEA, TIPA |
| Formulant in pest control products registered in Canadaf | MEA, LME | DEA, CADEA, TADEA, LDE, CDE | TEA, TIPA |
Abbreviations: N, use was not reported for any substance in the sub-group.
a Personal communication, e-mails from Food Directorate (FD), Health Canada (HC), to Existing Substances Risk Assessment Bureau (ESRAB), Health Canada (HC), dates ranging from September 2015 to October 2018; unreferenced.
b DEA is only in veterinary drugs. Personal communication, e-mails from Therapeutic Products Directorate (TPD), HC, to ESRAB, HC, dated September 2015 and August 2017; unreferenced.
c Personal communication, e-mails from Natural and Non-prescription Health Products Directorate (NNHPD), HC, to ESRAB, HC, dated September 2015 and August 2017; unreferenced.
d Health Canada [modified 2015].
e Personal communication, e-mails from Consumer and Hazardous Products Safety Directorate (CHPSD), HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced.
f Personal communication, e-mails from Pest Management Regulatory Agency (PMRA), HC, to ESRAB, HC, dated June 2015,August 2017, and March 2020; unreferenced.
DEA is listed under “Dialkanolamines, secondary” on the List of Prohibited Cosmetic Ingredients (HC [amended 2018]). However, DEA may be present as a residual in products with CDE and/or LDE, from the reaction of DEA with the corresponding fatty acid(s) during chemical manufacturing of the amides (personal communication, Consumer and Hazardous Products Safety Directorate, HC, to Existing Substances Risk Assessment Bureau, HC, dated March 2017; unreferenced). DEA may therefore be present as a residual in products, including cosmetics, available to consumers in Canada containing LDE and/or CDE.
According to publicly available product material safety data sheets (MSDSs), TADEA has been identified in an automotive transmission fluid available to consumers in Canada (MSDS 2015i) and CDE has been identified in automotive and marine washes (MSDS 2009; 2010a). In addition, TEA has been identified in a wide range of household, automotive and marine cleaners (e.g., dish, laundry, oven, all-purpose, strippers and degreasers, upholstery), and in some coolant additives, printer inks and waterproof adhesives/epoxys available to consumers in Canada (MSDS 2006; 2007g,h; 2008f; 2010b,c; 2011a,b; 2012a,b,c,d,e,f; 2013j; 2015e,j,k,l,m).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the substances in the Alkanolamines and Fatty Alkanolamides Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. Since CDE, CADEA, TADEA and BATEA are UVCB substances and could not be suitably represented by single chemical structures, a manual judgement-based approach to classification was used. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2016), and from responses to surveys issued pursuant to Section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of CDE, CADEA, TADEA and BATEA, hazard and exposure could not be fully profiled due to the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed by examining the UVCB constituents and Inventory Update information (Environment Canada 2013) and making decisions on the basis of consideration of similar substances and application of expert judgement.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios designed to be protective of the environment to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2016). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics, such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure, as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Alkanolamines and Fatty Alkanolamides Group are presented in ECCC (2016b), together with the hazard, exposure and risk classification results.
The hazard and exposure classifications for the substances in the Alkanolamines and Fatty Alkanolamides Group are summarized in Table 5-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| MEA | low | low | low |
| DEEA | low | low | low |
| LME | low | low | low |
| DEA | low | low | low |
| LDE | low | low | low |
| CDE | low | low | low |
| CADEA | low | low | low |
| TADEA | low | low | low |
| TEA | low | low | low |
| TIPA | low | low | low |
| BATEA | low | low | low |
On the basis of the low hazard and low exposure classifications according to information considered under ERC, MEA, DEEA, LME, DEA, LDE, CDE, CADEA, TADEA, TEA, TIPA and BATEA were classified as having a low potential for ecological risk. It is therefore unlikely that these substances are resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Assessment of subgroup 1 (MEA, DEEA, LME)
6.1.1 Exposure assessment of subgroup 1 (MEA, DEEA, and LME)
Environmental media
On the basis of their measured pKa values, MEA and DEEA are expected to exist mostly as cations under environmental conditions with potential to strongly adsorb to clays, organic carbon, particulates and aerosols through ionic interactions. LME is an amphiphilic, neutral substance with a very low vapour pressure and moderate water solubility, based on model predictions.
No reports of monitoring for DEEA or LME in environmental media in Canada or elsewhere were identified and no reports of MEAmonitoring for MEA in Canada were identified. However, globally, MEA has been identified in ambient air in aerosols (Miyazaki et al. 2009a; Mader et al. 2004; Yang et al. 2005; Miyazaki et al. 2009b; Huang et al. 2016; Gorzelska and Galloway 1990), PM2.5 (Zhang and Anastasio 2003; Yang et al. 2004) and fog waters (Zhang and Anastasio 2003). DEEA . MEAIt was detected in 7 of 64 groundwater samples measured from a decommissioned sour gas plant in southern Alberta (range of below detection limit to 18,935 mg/L; mean of 0.24 mg/L; Mrklas et al. 2006). .
Given their ionic nature under environmentally relevant conditions, MEA and DEEA are not within the domain of applicability of fugacity models traditionally used for estimating exposure of substances from environmental media. However, on the basis of physical chemistry data and fugacity modelling for the uncharged species (see Table B-1, Appendix B) human intakes of MEA, DEEA and LME from air and soil are expected to be negligible (less than 2.5 ng/kg bw/day). Drinking water estimates were generated for DEEA and LME using the down-the-drain consumer use scenario in the EAU Drinking Water Spreadsheets (Health Canada 2015a) and quantities reported in Canadian commerce in 2011 for DEEA and LME (Environment Canada 2013). Details of model parameters for estimating concentrations of DEEA and LME in drinking water can be found in Table B-4 (Appendix B). The maximum 50th percentile surface water concentrations of DEEA and LME among the 10 receiving water bodies modelled are 1.19 and 2.72 µg/L, respectively. The resulting intake estimates from drinking water for formula-fed infantsFootnote 6 are 0.00013 mg/kg bw/day for DEEA and 0.00029 mg/kg bw/day for LME.
Food and beverages
MEA
The estimated exposure of MEA from its potential use as a component in food packaging material is 0.303 µg/kg bw/day, while exposure from its use as a component in incidental additives is expected to be negligible (personal communication, e-mail from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated April 2018; unreferenced).
Details of methods, food occurrence data and estimated dietary exposure to MEA from its natural presence in foods for all age categories can be found in Appendix C. Mean and 90th percentile estimates of exposure to MEA ranged from 70 to 200 µg/kg bw/day and from 170 to 470 µg/kg bw/day, respectively (see Table C-2). At the 90th percentile, the highest estimated dietary exposure to MEA from foods, on a body weight basis, was for children aged 1 to 8 years, which was 470 µg/kg bw/day (personal communication, e-mail from Food Directorate, HC, to Existing Substances Risk Assessment Bureau, HC, dated April 2018; unreferenced).
Exposure to MEA from food packaging materials and incidental additive uses are considered minimal relative to exposure to MEA that is naturally present in food (personal communication, e-mail from Food Directorate, HC, to Existing Substances Risk Assessment Bureau, HC, dated March 2018; unreferenced).
DEEA and LME
In Canada, no reports of DEEA or LME were identified in foods. LME is not used in food packaging, while DEEA may be used in certain food packaging materials, but with no potential for direct food contact (personal communication, e-mails from Food Directorate, HC, to Existing Substances Risk Assessment Bureau, HC, dated January 2016 and August 2017; unreferenced). DEEA and LME may be used as components in incidental additives used in food processing establishments (personal communication, e-mail from Food Directorate, HC, to Existing Substances Risk Assessment Bureau, HC, dated August 2017; unreferenced).
Products available to consumers
Potential exposure from use of products available to consumers was estimated. Details are presented in Appendix D. Estimates for uses that result in the highest level of potential dermal or inhalation exposure (referred to as sentinel scenarios) are presented in Tables 6-1 and 6-2, respectively. Additional potential use scenarios for MEA (topical drugs and NHPs, dish soap, laundry soap and floor cleaners) and LME (shampoo, dish soap) were considered, but resulted in lower exposures than those presented in Tables 6-1 and 6-2.
Dermal absorption
In an in vitro dermal absorption study, the dermal absorption of both undiluted MEA and MEA in aqueous solution (22% w/w) was determined in mouse, rat, rabbit and human skin at a target dose of 4 mg/cm2 following 6 hours of exposure (Sun 1996). Without accounting for skin bound residues, the percent absorbed (%) for undiluted and 22% MEA was 17% and 25% in mouse, 9% and 2% in rabbit, 6% and 1% in rat, and 0.6% and 1% in human skin. Since the MEA dermal absorption information was limited (only determined up to 6 hours; no measurement of skin bound residues) and there was no dermal absorption information for DEEA and LME, dermal absorption for these substances was conservatively considered to be 100%.
| Substance | Product scenarioa,b | Concentration (%) | Per event systemic exposureb (mg/kg bw) | Daily systemic exposureb (mg/kg bw/ day)c |
|---|---|---|---|---|
| MEA | Body soap liquid (infant) | 3 | 0.18 | 0.16 |
| MEA | Body soap liquid | 3 | 0.047 | 0.065 |
| MEA | Hair shampoo (child) | 10 | 0.38 | 0.42 |
| MEA | Hair shampoo | 10 | 0.17 | 0.18 |
| MEA | Hair dye – permanent | 30 | 42 | N/A |
| MEA | All-purpose spray cleaner | 1 | 0.044 | 0.044 |
| MEA | Oven cleaner | 2.4 | 0.20 | N/A |
| DEEA | Floor polish/wax | 1 | 0.078 | N/A |
| LME | Body soap liquid (infant) | 3 | 0.18 | 0.16 |
| LME | Body soap liquid | 3 | 0.047 | 0.065 |
Abbreviation: N/A, not applicable.
a Represents direct exposures to adults, except where noted otherwise.
b Direct exposures from use of products by adults were evaluated, except where noted otherwise. For MEA, DEEA and LME, potential exposure via the dermal route was estimated on the basis of 100% of dermal absorption.
c These values take into account the assumed daily frequency of use, so for ConsExpo estimates the year averaged daily exposure value was used. See Appendix D for more detail on models and parameters used.
| Substance | Product scenarioa | Concentration (%) | 6-hour TWA air concentration (mg/m3) | Mean air concentration on day of exposure (mg/m3) |
|---|---|---|---|---|
| MEA | All-purpose spray cleaner | 1 | 0.083 | 0.021 |
| MEA | Oven cleaner – cleaning | 2.4 | 0.20 | N/A |
| DEEA | Floor polish/wax | 1 | 1.84 | N/A |
Abbreviation: N/A, Not applicable due to intermittent use; TWA, time-weighted average.
6.1.2 Health effects assessment of subgroup 1 (MEA, DEEA, and LME)
MEA
The health effects of MEA have been assessed by the European Commission (EC 2016) and by the Cosmetic Ingredient Review Expert Panel (CIR 2015a). The evaluation report from EC was used to inform the health effects characterization in this screening assessment. A literature search was conducted from September 2015 to December 2017. No significant new studies were identified that would impact the hazard and risk characterization.
Repeated-dose toxicity: In a 4-week inhalation study, Wistar rats (5/sex/dose) inhaled MEA aerosol at 0, 10, 50 or 150 mg/m3 (6 hours per day, 5 days per week, nose-only) (EC 2016). Local effects, including inflammation, hyperplasia, and epithelial necrosis of the larynx, were observed at 150 mg/m3. Animals at 50 mg/m3 were reported to have submucosal inflammation and squamous metaplasia in the larynx, which were reversible and were considered as adaptive responses (EC 2016). EC identified the systemic NOAEC as 150 mg/m3 and the local NOAEC as 10 mg/m3. In this assessment, the local NOAEC was considered to be 50 mg/m3 based on irreversible necrotic effects in the larynx at 150 mg/m3.
Reproductive and developmental toxicity: In a two-generation reproductive toxicity study, Wistar rats (25/sex/dose) received MEA hydrochloride (HCl) in the diet at dose levels of 0, 100, 300 or 1000 mg/kg bw/day (equivalent to 0, 58, 178, 580 mg/kg bw/day of MEA). Similar health effects are expected for MEA HCl and MEA through oral exposure, as they convert in the stomach to the same dominant cation (EC 2016). At the MEA LOAEL of 580 mg/kg bw/day, there were statistically significant decreased absolute and relative weights of epididymides and cauda epididymides in both F0 and F1 parental males, and a statistically significant decreased number of implantation sites, increased post-implantation losses in both F0 and F1 generations, and decreased litter sizes (F1, F2). The MEA NOAEL was identified by EC to be 178 mg/kg bw/day on the basis of reproductive effects (EC 2016).
No developmental toxicity was observed up to 450 or 225 mg/kg bw/day aqueous MEA in an oral or dermal developmental study in rats, respectively, in the presence of decreased maternal weight gain in both studies and dermal irritation (including erythema, necrosis, scabs) in the latter (Liberacki et al. 1996; Hellwig and Liberacki 1997; EC 2016).
Genotoxicity and carcinogenicity: MEA was not genotoxic in vitro or in vivo (JETOC 1996; Dean et al. 1985, as cited in EC 2016).
Carcinogenicity studies for MEA are not available. However, MEA does not affect the biosynthesis of phosphatidylcholine (Zha et al. 1992) and so is not expected to increase tumour development by disrupting choline homeostasis (Kirman et al. 2016).
DEEA
The health effects of DEEA have been characterized by OECD (2002). A literature search was conducted from October 2001 to December 2017. No significant new studies were identified that would impact the hazard and risk characterization.
Repeated-dose toxicity: In a 14-week inhalation study, F344 rats (20/sex/dose) were exposed to 0, 11, 25, or 76 ppm (equivalent to 0, 53, 120 or 365 mg/m3) of DEEA (whole-body exposure, 6 hours/day, 5 days/week) (Hinz et al.1992, as cited in OECD 2002). No neurobehavioural effects were observed in a functional observational battery, nor were there adverse changes in biochemistry/urinalysis or histopathology. OECD considered histological changes indicative of respiratory irritation (such as increased incidences and severity of focal hyperplasia, squamous metaplasia of the respiratory epithelium, infiltration of inflammatory cells in the nasal mucosa) at 120 mg/m3 and above to be adaptive responses. The NOAEL was identified as the highest dose at 365 mg/m3.
Developmental toxicity: In an inhalation developmental study, pregnant Sprague Dawley (SD) rats (25/group) were exposed to 0, 33, 66 and 100 ppm (0, 158, 316, 480 mg/m3) of DEEA (whole-body exposure, 6 hours/day) on gestational days (GDs) 6 to 15 (Leung and Murphy 1998, as cited in OECD 2002). The maternal NOAEL was determined to be 316 mg/m3 on the basis of maternal toxicity at 480 mg/m3, including dry rales, reduced body weight (6%) on GD 15 and reduced body weight gain (52%) from GDs 12 to 15. No development toxicity was observed.
In an oral developmental study, pregnant New Zealand White rabbits (25/group) were administered DEEA in 0.5% carboxymethylcellulose suspension by gavage at 0, 15, 50 and 150 mg/kg bw/day from GDs 6 to 28 (ECHA c2011-2017). The experiment was terminated on GD 29. At 150 mg/kg bw/day, liver toxicity was observed in dams (increased absolute and relative liver weights, increased aspartate aminotransferase and alkaline phosphatase activities, and increased triglyceride and inorganic phosphate levels). The maternal NOAEL was 50 mg/kg bw/day on the basis of liver effects at 150 mg/kg bw/day. No developmental toxicity was observed.
Genotoxicity and carcinogenicity: DEEA was not mutagenic in vitro or clastogenic in vivo (OECD 2002).
No increased tumour incidences or other adverse effects were observed in a 2-year oral rat carcinogenicity study that had methodological limitations (low animal number, inadequate high dose of 50 mg/kg bw/day progressively increased to 400 mg/kg bw/day) (OECD 2002).
LME
The hazard for LME was characterized by the US EPA, as part of the fatty nitrogen derived amides assessment (US EPA 2010a). As a screening-level hazard characterization, no points of departure or analogues were identified. The CIR Expert Panel also described the health effects of LME as part of an ethanolamides assessment (CIR 2015b). A literature search was conducted from September 2009 to December 2017. No significant new studies were identified that would impact the hazard and risk characterization.
Empirical toxicity data for LME is limited to a negative in vitro bacterial mutation assay (Zeiger 1987). In current assessment, the health effects of LME were characterized by consideration of health effects associated with the analogues coconut acid monoethanolamide (CME), of which LME is a main component, stearoyl monoethanolamide (SME), and oleic acid monoisopropanolamide (OMIPA) (Appendix A, Table A-1). These analogues are similar to LME with respect to chemical structure (fatty amides with one hydroxyl molecule), physical-chemical properties (highly lipophilic), and toxicokinetics (hydrolysis yields the corresponding fatty acids and monoalkanolamine) (RSI 2017).
Toxicokinetics: The N-substituted primary amides, such as N-acylethanolamide, are hydrolyzed by fatty acid amide hydrolase, and hydrolysis yields the corresponding fatty acid and MEA (Thabuis et al. 2008). LME is expected to metabolize to MEA and lauric acid.
Repeated-dose toxicity: In a 4-week gavage study, Wistar rats (10/sex/dose) were administered 0, 70, 250 or 750 mg/kg bw/day of CME, 5 days per week (CIR 2015b). No treatment-related adverse effects were observed up to the NOAEL of 750 mg/kg bw/day, the highest dose tested.
In a 4-week dermal toxicity study in rabbits, no observed adverse effects were observed when 2000 mg/kg bw SME (10% aqueous solution) was applied to intact or abraded skin (CIR 2015b).
Reproductive/developmental toxicity: In a combined repeated dose toxicity study and reproductive/developmental toxicity screening test, SD rats were exposed by gavage to 0, 100, 300, or 1000 mg/kg bw/day OMIPA starting 2 weeks prior to mating, through mating, pregnancy, and lactation up to postnatal day 5. No systemic, reproductive, or developmental toxicity was observed up to 1000 mg/kg bw/day, the highest dose tested (ECHA c2013-2017).
Genotoxicity and carcinogenicity: LME was not mutagenic in vitro in a bacterial mutation assay, with or without metabolic activation (Zeiger et al. 1987). OMIPA was similarly negative in vitro, in a bacterial mutation assay and in a micronucleus assay, with or without metabolic activation (ECHA c2013-2017).
Carcinogenicity data for LME or its analogues (OMIPA, CME, SME) are not available.
6.1.3 Risk characterization of subgroup 1 (MEA, DEEA, and LME)
MEA
Table 6-3 provides relevant exposure estimates and critical health effect levels as well as resultant margins of exposure (MOEs) for the characterization of risk to human health from exposure to MEA.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from food and beverages (toddlers) | 0.47 mg/kg bw/day | NOAEL=178 mg/kg bw/day | Reproductive effects at 580 mg/kg bw/day in a two-generation reproductive toxicity study in rats.a | 379 |
| Per event inhalation exposure to oven cleaner | 0.20 mg/m3 | NOAEC=50 mg/m3 | Effects in the larynx, including inflammation, hyperplasia, and necrosis were observed at 150 mg/m3 in a 4-week inhalation study in rats. | 251 |
| Daily inhalation exposure to all-purpose cleaning sprays | 0.021 mg/m3/day | NOAEC=8.9 mg/m3/dayb | Effects in the larynx, including inflammation, hyperplasia, and necrosis were observed at 150 mg/m3 in a 4-week inhalation study in rats. | 430 |
Abbreviations: NOAEC, no observed adverse effect concentration; NOAEL, no observed adverse effect level.
a Effects include decreased epididymides and cauda epididymides weights (F0 and F1 parental males), decreased number of implantation sites in both F0 and F1 generations, associated with increased post-implantation loss, decreased litter sizes (F1, F2).
b Converted to a continuous exposure scenario from the NOAEC of 50 mg/m3 for 6 hours per day, 5 days per week, =50 mg/m3 x (6/24) x (5/7)= 8.9 mg/m3 .
The margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases for MEA.
A risk to human health via rinse-off dermal exposure scenarios with MEA was considered low, given that in a developmental dermal study in rats (Liberacki et al. 1996), there was no developmental toxicity and decreased maternal body weight gain was considered likely to be secondary to dermal irritation.
DEEA
Table 6-4 provides relevant exposure estimates and critical effect levels as well as resultant margins of exposure for the characterization of risk to human health from exposure to DEEA.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from drinking water (infants, formula fed) | 0.00013 mg/kg bw/day | NOAEL=50 mg/kg bw/day | Maternal liver toxicity (increased liver weights, AST and ALP) at 150 mg/kg bw/day in an oral developmental study in rabbits. | 385000 |
| Per event dermal exposure from the application of floor polish/wax (adults) | 0.078 mg/kg bw | NOAEL=50 mg/kg bw (route to route extrapolation) | Maternal liver toxicity (increased liver weights, AST and ALP) at 150 mg/kg bw/day in an oral developmental study in rabbits. | 640 |
| Per event inhalation exposure from the application of floor polish/wax | 1.84 mg/m3 | NOAEC=316 mg/m3 | Reduced maternal body weight and body weight gain at 480 mg/m3 in an inhalation developmental study in rats. | 170 |
Abbreviations: AST, aspartate aminotransferase; ALP, alkaline phosphatase activities; NOAEC, no observed adverse effect concentration; NOAEL, no observed adverse effect level.
The margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
LME
Table 6-5 provides relevant exposure estimates and critical effect levels, as well as resultant margins of exposure, for the characterization of risk to human health from exposure to LME.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from drinking water (infants, formula fed) | 0.00029 mg/kg bw/day | NOAEL=1000 mg/kg bw/day (HDT) | No adverse effects observed in an oral combined repeated-dose and reproductive/developmental toxicity screening study in rats exposed to OMIPA. | 3400000 |
| Daily dermal exposure from body soap liquid (infants) | 0.16 mg/kg bw/day | NOAEL=2000 mg/kg bw/day (HDT) | No adverse effects observed in a 4-week dermal study in rabbits exposed to SME. | 12500 |
| Per event dermal exposure from body soap liquid (infants) | 0.18 mg/kg bw | NOAEL=2000 mg/kg bw (HDT) | No adverse effects observed in a 4-week dermal study in rabbits exposed to SME. | 11000 |
Abbreviations: HDT, highest dose tested; NOAEL, no observed adverse effect level.
The margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
6.1.4 Uncertainties in the evaluation of risk to human health for subgroup 1 (MEA, DEEA, and LME)
The key sources of uncertainty are presented in Table 6-6.
| Key sources of uncertainty | Impact |
|---|---|
| No recent total Canadian manufacture volume data for MEA. | +/- |
| No Canadian data for MEA, DEEA and LME in environmental media. | +/- |
| Only one study was identified reporting concentrations of MEA in citrus juice, and this commodity contributed most significantly to dietary MEA exposure in children as well as in all consumer age groups, combined. | +/- |
| The highest reported arithmetic mean MEA concentration for a given food type was applied to an entire food or beverage category. | + |
| No long-term inhalation study for MEA. | +/- |
| No repeated dose dermal study for DEEA. | +/- |
| No repeated dose, carcinogenicity, or reproductive/developmental toxicity studies via dermal and oral routes of exposure for LME. | +/- |
+ = uncertainty with potential to cause over-estimation of risk; +/- = unknown potential to cause over- or under-estimation of risk.
6.2 Assessment of the subgroup 2 (DEA, LDE, CDE, CADEA and TADEA)
6.2.1 Exposure assessment of subgroup 2 (DEA, LDE, CDE, CADEA and TADEA)
Environmental media
No reports of monitoring for DEA, CADEA or TADEA in environmental media were identified and no reports of monitoring for LDE or CDE were identified for air or soil in Canada or elsewhere. LDE has been detected in indoor dust in a Norwegian study (Pedersen et al. 2002). Although measured concentrations were not identified for air and water, DEA is used in the purification of natural, refinery and synthetic gases and is reportable in Canada to the National Pollutant Release Inventory (NPRI). Releases to water of 340 kg/year (0.34 tonnes/year) from one petroleum refinery and to air from two other refineries at 1 100 and 4 600 kg/year (1.1 and 4.6 tonnes/year), respectively, have been reported (NPRI 2015).
Given their ionic nature under environmentally relevant conditions (pH 6 to 8), DEA and TADEA are not within the domain of applicability of fugacity models traditionally used for estimating exposure of substances from environmental media. However, on the basis of physical chemistry data, fugacity modelling for the uncharged species (see Table B-2, Appendix B) and limited measured concentrations in environmental media reported in the literature, intakes of DEA, LDE, CDE, CADEA and TADEA from ambient/indoor air and soil are expected to be negligible.
Estimates of DEA, LDE, CDE, CADEA and TADEA concentrations in drinking water were generated using the down-the-drain consumer use scenario in the EAU Drinking Water Spreadsheets (Health Canada 2015a) and quantities reported in Canadian commerce in 2011 (Environment Canada 2013). Details of model parameters for estimating concentrations of DEA, LDE, CDE, CADEA and TADEA in drinking water can be found in Table B-4 (Appendix B). The maximum 50th percentile surface water concentrations of DEA, LDE, CDE, CADEA and TADEA among the 10 receiving water bodies modelled are 7.3, 0.24, 68, 0.34 and 2.6 µg/L, respectively.
The resulting intake estimates from drinking water for formula-fed infants are 0.00078 mg/kg bw/day for DEA, 2.5 x 10-5 mg/kg/day for LDE, 7.3 x 10-3 mg/kg bw/day for CDE, 3.6 x 10-5 mg/kg bw/day for CADEA and 2.8 x 10-4 mg/kg bw/day for TADEA.
Food
No reports of DEA, LDE, CDE, CADEA or TADEA were identified in food monitoring studies. CDE was identified in 1 of 3 commercial mussels sampled in Denmark, but only a relative concentration (to total “volatile components”) was provided (Rasmussen et al. 1993). The authors concluded that its presence in the mussel tissue was presumably as an environmental contaminant sequestered from the water.
In Canada, DEA, LDE, CADEA and TADEA may be used in certain food packaging materials with potential for direct food contact. CDE may also be used in certain food packaging materials, but with no potential for direct food contact (personal communication, e-mails from Food Directorate, HC, to Existing Substances Risk Assessment Bureau, HC, dates ranging from September 2015 to March 2018; unreferenced).
The estimated conservative intakes of DEA, LDE, CADEA and TADEA from food packaging are 0.00016, 0.00023, 0.00082 and 0.00882 mg/kg bw/day, respectively (personal communication, e-mails from Food Directorate, HC, to Existing Substances Risk Assessment Bureau, HC, dates ranging from September 2015 to July 2018; unreferenced).
In Canada, DEA, LDE, CADEA and TADEA may be used as components of incidental additives in products used in food processing plants with no potential for direct food contact. CDE has been identified for use as a component of incidental additives in products used in food processing plants, specifically as a component in cleaners, lubricants used on non-food contact surfaces and in sanitizers without a potable water rinse with potential for direct food contact. However, exposure is expected to be negligible (personal communication, e-mails from Food Directorate, HC, to Existing Substances Risk Assessment Bureau, HC, dates ranging from September 2015 to March 2018; unreferenced).
Products available to consumers
Potential exposure from use of products available to consumers was estimated. Details are presented in Appendix D. Estimates for uses that result in the highest level of potential dermal or inhalation exposure (referred to as sentinel scenarios) are presented in Tables 6-7 and 6-8, respectively. Additional potential use scenarios for DEA, LDE and CDE were considered, but resulted in lower exposures than those presented in Tables 6-7 and 6-8. This includes DEA in laundry soap, as a formulant in insect repellants, child insect bite treatments, automotive care products, car and boat washes, topical NHPs and veterinary drugs. This also includes LDE and CDE in antistatic agents, emulsifiers, surfactants, foam boosters/stabilizers and viscosity controlling agents, hair spray, make-up removers, topical NHPs, dish and laundry liquids, all-purpose cleaners, floor cleaners and strippers, and automotive and marine washes.
Dermal absorption
Several dermal absorption studies with DEA relevant to the human cosmetic exposure scenarios of interest were conducted. An in vitro human skin absorption study showed, only a small fraction of DEA penetrated into human skin from cosmetic formulations with DEA (Kraeling et al. 2004). In this study, [14C]-DEA was added to the tested commercial products and applied to excised human skin in flow-through diffusion cells. The products applied were shampoo (0.092% or 0.28% DEA), hair dye (0.61% DEA), and body lotion (0.02% DEA). They remained on the skin for 5 minutes, 30 minutes and 24 hours, respectively. Dermal absorption was estimated to be 0.08%, 0.09% and 0.9% for shampoo, hair dye, and body lotion, respectively, based on the amount that was absorbed into the receptor fluid after 24 hours. In a second part of the same study by Kraeling et al. (2004), body lotion (0.02% DEA) was applied for 3 consecutive days (washed and reapplied every 24 hours). Penetration was 0.5% in 24 hours and increased to 0.8% on day 2 and to 0.9% on day 3.This suggested that dermal absorption increased with repeated dosing. The skin-bound residues were not expected to be systemically available since after 72 hours of daily repeated daily doses DEA accumulated in the skin with little diffusing out into the receptor fluid. While there were slight differences between the two parts of the study, the highest value of 1% (rounded up from 0.9%) dermal absorption for DEA (in body lotion) was used for dermal exposure scenarios in humans.
To determine the amount of residual DEA that was dermally absorbed from cosmetic products with CDE, LDE, and TEA, seven cosmetic formulations were applied under in-use conditions to human skin in an in vitro dermal absorption study (and examined after 24 and 48 hours (Brain et al. 2004). This includes two shampoo formulations with CDE (4.02% CDE, 0.98% DEA and alternative surfactants, 1:10 dilution, rinsed off after 10-minute skin exposure) and LDE (4.75% LDE, 0.25% DEA, 1:10 dilution, rinsed off after 10-minute skin exposure), as well as a bubble bath with LDE (4.75% LDE, 0.25% DEA, 1:300 dilution, blotted off with filter paper after 30-min skin exposure) and a leave-on emulsion formulation with TEA (1.99% TEA, 0.008% DEA, 48-hour skin exposure). The dermal absorption of DEA (% of applied dose) was 0.02% (48 hours) or 0.03% (24 hours) respectively for two CDE shampoos; 0.01% (24 hours) and 0.03% (24 hours) for two LDE shampoos; 0.5% (24 hours) for one LDE bubble-bath in 24 hours, and 0.6% (48 hours) for a leave-on emulsion TEA product (Brain et al. 2004). Use of 1% dermal absorption for DEA accounts for the ranges of dermal absorption values of DEA through human skin in vitro from cosmetic formulations with CDE, LDE, or TEA (0.6% or less) (Brain et al. 2004).
For estimation of systemic exposure from potential dermal exposure to LDE and CDE, a dermal absorption of 1% (rounded up from 0.48%) was used on the basis of an in vitro human skin absorption study conducted by Charles River in 2019 [personal communication, preliminary results from Charles River Laboratories Edinburgh Ltd to the Environmental Health Science Research Bureau (EHSRB), HC, Nov 12, 2019; unreferenced]. Since it was not possible to radiolabel the CDE mixture, the dermal absorption of CDE in humans was determined by its largest component LDE (Charles River Laboratories Edinburgh Ltd. 2020). Since the average molecular size of CDE is larger than the molecular size of LDE, it is expected that less CDE would be absorbed than LDE through dermal exposure.
In this in vitro human dermal absorption study, [14C]-LDE was added to liquid soap at concentrations of 2% or 20% and applied to excised human skin in flow-through diffusion cells, where the test samples remained on the skin for 1 hour. The dermal absorption values not including and including the skin bound residues at 24 hours were 0.07% and 0.48% respectively for 2% CDE (mass balance recovery of 99.3%), and 0.14% and 0.24% respectively for 20% CDE (mass balance recovery of 102.8%) (Charles River Laboratories Edinburgh Ltd. 2020). Since it was unclear whether skin bound residuals were available for systemic absorption, the upper bound dermal absorption including skin bound residues, 1% (rounded up from 0.48%), was applied to external dermal exposure estimates when determining systemic exposure for LDE and CDE.
| Substance | Product scenarioa | Concentration (%) | Per event exposure (mg/kg bw) | Daily exposure (mg/kg bw/ day)b |
|---|---|---|---|---|
| DEA | Dishwashing liquid – handwashing | 5 | 0.0002c | 0.00026c |
| DEA | Latex wall paint – from colourant diluted in base | 1d | 0.0051c | N/A |
| LDE | Body soap liquid (infant) | 10 | 0.0061c | 0.0052c |
| LDE | Body soap liquid | 10 | 0.0016c | 0.0022c |
| CDE | All-purpose cleaning liquid | 2 | 0.00088c | 0.00088c |
| CDE | Face mask/pack | 10 | 0.028c | N/A |
| CDE | Body soap liquid (infant) | 10 | 0.0061c | 0.0052c |
| CDE | Body soap liquid | 10 | 0.0016c | 0.0022c |
| CDE | Hair shampoo (child) | 30 | 0.011c | 0.013c |
| CDE | Hair shampoo | 30 | 0.005c | 0.005c |
| CADEA | Body soap liquid (child) | 5 | 0.095 | 0.089 |
| CADEA | Body soap liquid | 5 | 0.078 | 0.11 |
| CADEA | Hair shampoo | 5 | 0.083 | 0.092 |
| TADEA | Transmission fluid | 25 | 0.072 | N/A |
Abbreviation: N/A, Not applicable due to intermittent use.
a Represents direct exposures to adults, except where noted otherwise
b These values take into account the assumed daily frequency of use, so for ConsExpo estimates the year averaged daily exposure value was used. See appendix D for more detail on models and parameters used
c Systemic exposure through dermal route, based on 1% dermal absorption
d Concentration in colour concentrate
| Substance | Product scenarioa | Concentration (%) | 6-hour TWA air concentration (mg/m3) | Mean air concentration on day of exposure (mg/m3) |
|---|---|---|---|---|
| DEA | Pre-moistened wet tissues - all-purpose cleaning (exposure to vapour) | 1 | 0.0012 | 0.00029 |
| DEA | Wall paint (exposure to vapour) | 1b | 0.0034 | 0.000085 |
| LDE | Hair spray | 1 | 0.00018 | 0.0014 |
Abbreviation: TWA, time-weighted average
a Represents direct exposures to adults.
b Concentration in colour concentrate.
Given the large number (approximately 1200) and variety of cosmetic products applied to the skin reported to contain CDE, an estimate of aggregate exposure to CDE from multiple dermally-applied products was considered, taking into consideration the Scientific Committee on Consumer Safety (SCCS) Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation document (SCCS 2015). Daily aggregate exposure estimates for CDE in cosmetics are presented in Table 6-9.
| Product | Concentrationa (%) | Daily exposureb – Teen (mg/kg bw/day) | Daily exposureb – Adult (mg/kg bw/day) |
|---|---|---|---|
| Body soap liquid | 10 | 0.0019 | 0.0022 |
| Facial cleanser | 30 | 0.00092 | 0.0018 |
| Hair shampoo | 30 | 0.0066 | 0.0055 |
| Total combined exposure (mg/kg bw/day)a | - | 0.0094 | 0.0095 |
a Personal communication, e-mails from Consumer and Hazardous Products Safety Directorate (CHPSD), HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced
b Systemic exposure through dermal route, assuming 1% dermal absorption
DEA has been classified as possibly carcinogenic to humans (IARC 2013a). In order to estimate the potential cancer risk from exposure to DEA, lifetime average daily doses were calculated for daily exposure from drinking water (0.00018 mg/kg bw/day), daily use of manual dishwashing liquids (0.000093 mg/kg bw/day) and daily use of wet cleaning wipes (0.000048 mg/kg bw/day) (see Appendix D).
6.2.2 Health effects assessment of subgroup 2 (DEA, LDE, CDE, CADEA and TADEA)
Subgroup 2A (DEA, LDE, CDE)
Hazard characterizations have been conducted for DEA by OECD (2007a, 2009). and for fatty nitrogen derived amides (including CDE and LDE) by the US EPA (2010a). IARC has classified DEA (IARC 2013a) and CDE (IARC 2013b) as possibly carcinogenic to humans (Group 2B) based on sufficient evidence in experimental animals for carcinogenicity, but not on human data. The CIR expert panel published a recent version of their final report of the safety assessment for DEA (CIR 2017), as well as CDE and LDE (CIR 2013b). The OECD and IARC reports were used to inform the health effects assessment of subgroup 2. The toxicological data of LDE and CDE were used for read-across collectively. A literature search was conducted from April 2006 to December 2017. A pooled dose-response analysis for DEA-induced carcinogenicity was published by Kirman et al. 2016 and was considered in the hazard and risk characterization in this assessment. In addition, a significant new dermal absorption study, summarized in section 6.2.1, informed this assessment (Charles River Laboratories Edinburgh Ltd. 2020).)
CDE, LDE and their structural analogue ODE are condensation products from a corresponding fatty acid and DEA, so they contain unreacted residual DEA at various concentrations (such as 18.2%, 0.83%, and 0.19%, respectively, in NTP 1999b, 1999c, 2001). In dermal carcinogenicity studies in rodents, DEA and residual DEA-containing chemicals (LDE, CDE and ODE) were used to consolidate a dose-response analysis of the carcinogenicity of residual DEA (similar to Kirman et al. 2016). In addition, a dermal repeated dose toxicity study with ODE was used to inform the contribution of residual DEA to the toxicity of LDE and CDE.
While dermal carcinogenicity studies in animals were available for DEA, LDE, and CDE administered in ethanol, direct comparison of critical effect levels from these studies to dermal exposures from product formulation use in humans was expected to overestimate the risk, because it would not take into account either (1) the interspecies differences in dermal absorption with the same vehicle (e.g., Sun et al. 2008) or (2) the relatively higher dermal absorption in the animal studies in which higher doses were applied in ethanol (Mathews et al. 1997). As there were dermal absorption studies with the cosmetic formulations of interest applicable to humans, as well as dermal absorption studies with ethanol vehicles in the same animal species and strains as used in the toxicity studies of interest, it was possible to refine both the exposure and hazard components of the risk characterization with species,- substance- and vehicle-specific dermal absorption representative of each exposure scenario.
Toxicokinetics: Lower relative human dermal absorption of DEA was illustrated in vitro following 6-hour exposure to 37% aqueous DEA. Dermal absorption of aqueous DEA through full thickness skin was lowest (0.2%) in humans, relative to mice (7%), rats (0.6%), and rabbits (3%) (Sun et al. 2008). Mathews et al. (1997) examined dermal absorption of [14C]-DEA in ethanol in male F344 rats and male B63CF1 mice, which is the same vehicle and similar strains as in key dermal toxicity studies (to be discussed in the next sections). Following 48 hours of dermal application of DEA in 95% ethanol solution, 2%, 6% and 12% of administered doses (2.1, 7.6 and 27.6 mg/kg bw DEA, respectively, excluding skin bound residuals) were absorbed in rats, and 23%, 31% and 57% of administered doses (8, 23 and 81 mg/kg bw DEA, respectively, excluding skin bound residuals) were absorbed in mice. The dermal absorption increased with doses applied in rats and mice. Kirman et al. 2016 used this data to estimate dermal absorption in rats at 48 hours.
Appendix E provides the dermal doses in rodents that resulted in a health effect of interest (i.e., critical effect levels) converted to a continuous 24-hour exposure value (i.e., external critical effect levels), as well as corresponding dermal absorption values used in the extrapolation to internal dose. The percent dermal absorption values in Mathews et al. (1997) were determined after 48 hours of exposure, but a dermal absorption value based on 24 hours of exposure was required to convert a 24-hour critical effect level (i.e., external critical effect level in Appendix E) to an internal dose (i.e., internal critical effect level in Appendix E). In an in vitro human skin absorption study with repeated doses of lotion formulation with 0.02% DEA, penetration of applied doses was 0.5% in 24 hours and increased to 0.8% on day 2 and to 0.9% on day 3, suggesting that more than half of the DEA dose was absorbed in the first 24 hours (Kraeling et al. 2004). Although there may be interspecies differences, it was considered reasonable to assume that the percent dermal absorption at 24 hours was half of the dermal absorption after 48 hours in mice. Using half rather than a higher percentage of dermal absorption after a 24-hour exposure is considered conservative (since it results in a lower point of departure).
For the determination of toxicokinetics of DEA by oral administration, rats were administered [14C]-DEA at 7 mg/kg bw/day for up to 8 weeks; 57% of total [14C]-DEA was found in tissues and 24% was found in excreta. This suggests almost complete absorption and high bioavailability through oral administration (Mathews et al. 1997).
Similar to DEA, the dermal absorption of LDE in liquid soap was 1% in humans (Charles River Edinburgh Ltd. 2020), which was lower than that in rodents. Following the dermal application of [14C]-LDE in 95% ethanol in mice (50 to 800 mg/kg bw) and in rats (25 or 400 mg/kg bw) for 72 hours, 21% to 26% of the applied radioactivity (excluding skin bound residuals) was absorbed in mice and rats, respectively, and absorption was similar for all the doses in mice or rats (Mathews et al. 1996).
LDE was rapidly cleared from all tissues except adipose tissue and was primarily excreted as polar metabolites in urine (80% to 90%) following oral administration in rats (Mathews et al. 1996). It was readily hydrolyzed on the fatty acid moiety but was resistant to hydrolysis on the amide group in rats and mice (Mathews et al. 1996). Consequently, DEA is not expected to be a metabolite of LDE or CDE. CDE is expected to undergo similar metabolism as LDE.
Repeated-dose studies: Repeated-dose studies in rodents were conducted via oral (drinking water), dermal, and inhalation routes.
In 2-week and 13-week oral studies in rats, animals exposed to DEA in drinking water exhibited hematological effects (normochromic microcytic anemia) and renal effects (increases in absolute and relative kidney weights) (NTP 1992). The LOAEL was determined to be 79 mg/kg bw/day (lowest tested dose, 2-week oral study) and 25/14 mg/kg bw/day in males/females (lowest dose tested, 13-week oral study) on the basis of hematological and renal effects. In 2-week and 13-week oral studies in mice, the DEA in drinking water resulted in liver effects (increased liver weights and histological changes) in addition to the renal effects (NTP 1992).
In 2-week and 13-week dermal studies in rats or mice, there was skin irritation (including ulceration, acanthosis) (NTP 1992). In rats, dermal application of DEA resulted in hematological effects (normochromic microcytic anemia) and renal effects (increases in relative and absolute kidney weights, urea nitrogen level, urinary lactate dehydrogenase activity and the incidence of tubular epithelial necrosis; while in mice, DEA-treatment related liver effects (increased liver weight and histological changes, 2-week and 13-week studies) and renal effects (increased kidney weights, 13-week study) were observed (NTP 1992). The LOAEL of 125 mg/kg bw/day (the lowest dose tested, 2-week dermal study in mice) on the basis of increases in relative and absolute kidney weights and the LOAEL of 32 mg/kg bw/day (the lowest dose tested, 13-week dermal study in mice) on the basis of hematological and renal effects were identified.
In a 2-week inhalation study, the NOAEC was determined to be 200 mg/m3, based on mild systemic effects including decreased body weight and increased liver weight at 400 mg/m3 (BASF 1993a, as cited in OECD 2008). In 3-month inhalation studies, nose-only exposure of rats to DEA aerosols resulted in normochromic anemia, liver effects (increased liver weight, elevated alkaline phosphate activities in serum), renal effects (increased kidney weight and histological changes), and upper respiratory tract irritation. The effects were transient and considered adaptive (BASF AG 2002, as cited in OECD 2008; Gamer 2008). OECD (2007a) determined the NOAEC for systemic effects to be 15 mg/m³ and the NOAEC for upper respiratory tract irritation to be 3 mg/m³.
In a 13-week oral study in rats receiving LDE (Guant et al. 1967), adverse effects on kidneys and hematology were reported. In this study, rats (SPF, 15/sec/dose) were fed a diet containing 0, 0.1, 0.5, 1.0, 2.0% LDE (with 5.6% residual DEA). Significantly reduced relative and absolute kidney weights were observed in females at 0.5% and above. Reduction in haemoglobin level, haematocrit and red blood cell count were observed in females in 1% and 2% groups, less pronounced effects were observed in males. The NOAEL and LOAEL were identified to be 0.1% (equivalent to 50 mg/kg bw/day) and 0.5% (equivalent to 250 mg/kg bw/day), respectively, on the basis of increased kidney weights and anemia.
In a 14-week dermal study, the skin of B6C3F1 mice (10/sex/dose) was exposed to LDE (0.83% residual DEA) in ethanol at doses of 0, 50,100, 200, 400 or 800 mg/kg bw/day (NTP 1999b). There were no treatment-related effects on body weights or hematology. Increased relative and absolute kidney weights were observed in males receiving 100 mg/kg bw/day and above. In females, liver weight was significantly higher in animals that received 200 mg/kg bw/day or greater as compared to vehicle controls. Increased skin lesions at the site of application, including epidermal and sebaceous gland hyperplasia, chronic inflammation, parakeratosis, and ulcer, were observed in males and females receiving 200 mg/kg bw/day or greater. The NOAEL was identified to be 50 mg/kg bw/day on the basis of increased kidney weights. In a 14-week dermal study in rats, the NOAEL was found to be 100 mg/kg bw/day on the basis of decreased final body weights and body weight gains in males and increased kidney weights in females, at 200 mg/kg bw/day (NTP 1999b).
For CDE, in a 14-week dermal study, F344/N rats (10/sex/dose) were given CDE (contained 18.2% residual DEA) in ethanol by dermal application at 0, 25, 50, 100, 200, or 400 mg/kg bw/day, 5 days per week (NTP 2001). In females, absolute and relative kidney weights increased significantly at 50 mg/kg bw/day and above, with a higher incidence of renal tubule regeneration at 100 mg/kg bw/day and increased severity at 200 mg/kg bw/day and above. At 200 mg/kg bw/day and above, there was anemia (decreased RBC counts, haemoglobin concentration, and hematocrit) in females and decreased body weight and body weight gain in both sexes. At 400 mg/kg bw/day in males, there was increased liver weight. Skin irritation (including epidermal and sebaceous gland hyperplasia, inflammation, parakeratosis, and ulcers) at the site of the application was observed at 100 mg/kg bw/day and greater. The NOAEL was identified to be 50 mg/kg bw/day on the basis of increased kidney weight associated with histopathological changes at 100 mg/kg bw/day in females.
Since CDE and LDE in the repeated-dose dermal studies described above contained residual DEA and since similar adverse effects on the kidney were observed in animals treated with DEA, it was unclear whether the observed adverse effects could be attributed to DEA and/or to CDE or LDE. A 13-week dermal study with ODE, a structural analogue of CDE and LDE, with minimal DEA impurity (0.19%) NTP (1999c), was identified. In this study, the skin of B6C3F1 mice (10/sex/dose) was exposed to 0, 50,100, 200, 400 or 800 mg/kg/day ODE in ethanol. Increased relative and absolute kidney (males only) and liver weights (both sexes) were observed at 50 mg/kg bw/day and above. At higher doses, there was decreased body weight gain (at 400 mg/kg bw/day in females and at 800 mg/kg bw/day in both sexes). There were no adverse effects reported through evaluation of hematology, clinical biochemistry or histopathology. Skin lesions (including epidermal hyperplasia, parakeratosis, inflammation, sebaceous gland hypertrophy and ulcer) at the site of application were observed in all treated mice and the severity of these lesions generally increased with increasing dose. The LOAEL was considered to be 50 mg/kg bw/day (lowest dose tested). Since kidney effects were observed with a lower dose of ODE than LDE or CDE in these dermal toxicity studies in rodents, and given the relatively lower amount DEA residual in ODE, residual DEA is unlikely to be solely responsible for the kidney effects observed in the studies with LDE or CDE.
Reproductive and developmental toxicity: In an oral developmental study in rats (Price et al. 2005, as cited in OECD 2008), administration of DEA by gavage caused maternal toxicity, including increased mortality and reduced body weight/body weight gain and food consumption at 200 mg/kg bw/day. Developmental toxicity was also observed at the same dose, as indicated by reduced pup body weight gain (including in the early postnatal period). The developmental NOAEL was identified to be 50 mg/kg bw/day on the basis of increased postnatal mortality at 125 mg/kg bw/day in the absence of maternal toxicity (OECD 2007a). An inhalation developmental study on a group of rats exposed to DEA aerosol (nose-only) revealed maternal toxicity as observed vaginal hemorrhage on GD 14 at 200 mg/m3, but no developmental toxicity (BASF 1993b, as cited in OECD 2008). The maternal NOAEC was identified to be 50 mg/m3. A dermal developmental study on a group of rabbits exposed to DEA in aqueous solution showed maternal toxicity (reduced body weight gain) at 100 mg/kg bw/day and above, but no indications of developmental toxicity (CIR 2017).
A developmental study following OECD TG 414 was conducted for CDE (Pitterman 1994). Pregnant female rats (SD) received CDE (no information on the composition of the residual DEA in the test substance) in peanut oil by oral gavage at doses of 0, 100, 300 or 1000 mg/kg bw/day from GD 6 to 15. Except for salivation observed across all treatment groups, no other systemic maternal toxicity was observed. The treatments did not result in developmental effects at dose levels up to 1000 mg/kg/day.
Genotoxicity and carcinogenicity: Available information indicates that DEA, CDE, and LDE are not genotoxic. DEA, CDE and LDE, with or without S9 metabolic activation, were not mutagenic in Ames tests (Dean et al. 1985; NTP 2001) or in a mouse lymphoma assay (NTP 1992, 1999a,b, 2001). DEA and CDE were not clastogenic in chromosomal aberration tests and did not increase the frequencies of sister chromatid exchange or chromosome aberrations in cultured Chinese hamster ovary cells (NTP 2001). In Chinese hamster ovary cells, LDE increased frequencies of sister chromatid exchanges, but did not have an effect on chromosomal aberrations, with or without metabolic activations (NTP 1999c). In micronucleus tests in vivo, B6C3F1 mice were treated dermally with DEA (up to the equivalent of 1250 mg/kg bw/day) or LDE (up to 800 mg/kg bw/day) for 13 weeks with no increase in micronuclei observed (NTP 1992, 1999c). In contrast, CDE increased the micronucleus frequency of peripheral normochromatic erythrocytes at the highest dose tested (800 mg/kg) in male and female mice (NTP 2001).
DEA was found to be carcinogenic to mice via the dermal route of exposure (NTP 1999a). In B6C3F1 mice (50/sex/dose), animals in each individual cage were dermally exposed to DEA in ethanol solution at concentrations of 0, 40, 80 or 160 mg/kg bw/day, 5 days a week for 103 weeks (NTP 1999a). Treatment-related non-cancer effects at the site of application, including hyperkeratosis and acanthosis, were observed. Survival of dosed females, but not males, was significantly less than that of their relative vehicle control groups. The mean body weights in females of dosed groups were less than those of the vehicle controls during the second year of the study. The treatment increased liver tumours in both male and female mice, compared to controls. The incidence of hepatocellular tumours (combined adenomas, carcinomas and hepatoblastomas) in both sexes combined were 72%, 97%, 100%, and 99% at 0, 40, 80 or 160 mg/kg bw/day, respectively (Appendix F, Table F-1). Due to the high tumour incidence, a does-response assessment could not be determined. DEA treatment also resulted in an increased incidence of kidney tumours in male mice, as observed incidence rates were 3/50 (6%), 7/50 (14%), 8/50 (16%) and 9/50 (18%) in groups administered doses of 0, 40, 80 and 160 mg/kg bw/day, respectively.
In contrast, DEA was not carcinogenic in a similar study in rats via the dermal route of exposure (NTP 1999a). F344/N rats (50/sex/dose) were dermally exposed to DEA in ethanol solution at concentrations of 0, 8 (female only), 16, 32 and 64 (male only) mg/kg bw/day, 5 days a week for 103 weeks. Similar skin irritation effects as seen in mice were noted in rats. DEA treatment decreased body weights of males in the 64 mg/kg bw/day group compared with the control group starting from week 8. No increase in tumour incidence was observed.
In a 2-year dermal cancer study with CDE limited by two dose groups, the skin of B6C3F1 mice (50/sex/dose) and F344/N rats (50/sex/dose) was exposed to CDE (containing 18.2% residual DEA) in 95% ethanol at doses of 0, 100 or 200 mg/kg bw/day, 5 days a week (NTP 2001). In mice, CDE affected the skin at the site of application (epidermal and sebaceous hyperplasia, hyperkeratosis), and increased liver neoplasms (hepatocellular adenoma, hepatocellular carcinoma, and hepatoblastoma) in both sexes (Appendix F, Table F-1), decreased body weight in females at 100 mg/kg bw/day and above, and increased kidney tumours in males at 200 mg/kg bw/day were observed. In rats, similar skin irritation effects as in mice were observed at the site of application and in female rats there was significantly increased renal tubular hyperplasia at 100 mg/kg bw/day, but no carcinogenicity was reported.
In a 2-year dermal cancer study with LDE limited by two dose groups, the skin of B6C3F1 mice (50/sex/dose) and F344/N rats (50/sex/group) was exposed to LDE (containing 0.83% residual DEA) in 95% ethanol at doses of 0, 100, or 200 mg/kg bw/day, 5 days a week (NTP 1999b). In mice, LDE affected the skin and increased liver tumours at 100 mg/kg bw/day and above (Appendix F, Table F-1), decreased body weight in females was observed at 200 mg/kg bw/day, but no increase in kidney tumours was observed. In rats, LDE had skin effects as well, but no carcinogenic effects were observed.
Data on ODE (a structural analogue of CDE or LDE with a low amount of residual DEA), was used to derive a pooled dose-response analysis of residual DEA containing chemicals (Appendix F). In a 2-year dermal cancer study with ODE limited by two dose groups, the skin of B6C3F1 mice (50/sex/dose) and F344/N rats (50/sex/dose) was exposed to ODE (0.19% residual DEA) in 95% ethanol at doses of 0, 15, or 30 mg/kg bw/day, 5 days a week (NTP 1999c). ODE at 15 and 30 mg/kg bw/day affected the skin, but had no carcinogenic effects, in both mice and rats.
A dose-response assessment of liver tumours could not be determined using the individual dermal study in mice exposed to DEA, or substances with DEA (CDE, LDE or ODE). However, these four independent dermal cancer studies in mice were conducted in the same laboratory under similar conditions (NTP 1999a, b, c and 2001). The development of tumours was associated with the concentration of residual DEA in these studies with CDE and LDE (NTP 1999b, 2001), while IARC (2013b) stated that the tumour response in mice exposed to CDE appears to be due to the presence of residual DEA in the solution tested.
Kirman et al. (2016) combined the data of these independent dermal cancer studies and conducted a benchmark dose (BMD) modelling analysis to consolidate the relationship of DEA to the incidences of liver tumours. In that study, the dose of each substance administered was multiplied by the proportion of residual DEA in each test substance to determine the amount of DEA administered (Appendix F, Table F-1). Kirman et al. then multiplied each administered DEA dose by a dermal absorption fraction at 48 hours based on Mathews et al. (1997) to derive absorbed doses of residual DEA. This was used to determine the dose corresponding to a 10% increase in extra risk (ED10) of combined liver tumours in mice and its 95% lower limit (LED10), which were 0.49 and 0.39 mg/kg bw/day, respectively (equivalent to BMD10 and BMDL10). However, in their analysis, uncertainty was introduced in extrapolating internal doses from externally administered doses with different dermal absorption values based on 48-hour, rather than 24-hour, exposures.
In this assessment, the data of four independent cancer studies were combined based on an approach adapted from Kirman et al. (2016) (Appendix F, Table F-1), but the BMD modelling was conducted for the external DEA administered dose, rather than the internal DEA absorbed dose. This was conducted using the US EPA BMDS (version 2.5) multistage cancer model (Appendix F, Figure F-1). The results showed that the BMD10 and BMDL10 of external DEA administered that increased liver tumours in both sexes of mice were 1.84 mg/kg bw/day and 1.46 mg/kg bw/day, respectively (Appendix F, Figure F-1). This was converted to a continuous 24-hour exposure of 1.04 mg/kg bw/day and based on an estimated 13.4% dermal absorption value, converted to an internal BMDL10 of 0.14 mg/kg bw/day (Appendix F).
Mode of action: The evidence supporting a non-genotoxic mode of action (MOA) for DEA-induced liver carcinogenicity has been thoroughly described relative to the established MOA framework (Leung et al. 2005; Kirman et al. 2016), and supported by IARC 2013a. DEA is a structural analogue to choline, and thus it likely inhibits choline transportation, which likely results in choline deficiency (Leung et al. 2005; Kirman et al. 2016). It subsequently reduces the availability of S-adenosylmethionine, the source of the methyl group in DNA methylation, leading to hypomethylation of DNA and aberrant gene expression that may be involved in carcinogenesis (Leung et al. 2005; Kirman et al. 2016). DEA-induced choline deficiency is, therefore, a plausible MOA for the carcinogenicity of DEA in rodents (Leung et al. 2005; Kirman et al. 2016). Mice may be more susceptible than rats, as DEA-induced liver tumours were only observed in B6C3F1 mice, but not in F344/N rats, as evidenced by the NTP (1999a) study. Similarly, DEA (160 mg/kg bw/day for 4 weeks) administered to a different mouse strain (C57BL/6) did not show a decrease in DNA methylation potential despite a decrease in choline (Lehman-McKeeman et al. 2002).
Normal human diets provide sufficient choline to sustain healthy organ function (Zeisel et al. 1994), while rodents oxidize choline more rapidly than humans (Sidransky and Farber 1960) and have a higher dietary choline requirement. The difference between rodents and humans in terms of the effects of DEA on the induction of DNA synthesis, an important component of the carcinogenesis, has also been studied in vitro (Kamendulis and Klaunig 2005). DNA synthesis was increased in mouse or rat hepatocytes following treatment with 10 μg /ml DEA, but was not affected in human hepatocytes treated with up to 750 μg/ml of DEA, suggesting at least 75-fold lower sensitivity in humans than rodents. However, choline is an essential nutrient for humans. Data from the 2003-2004 National Health and Nutrition Examination Survey (NHANES), showed that 90% of the US population did not meet the recommended adequate intake for choline (425 mg/day for men, 550 mg/kg for women) (Zeisel and da Costa 2009). Although humans are less sensitive to choline deficiency than rodents, the human relevance of tumours induced via this MOA could not be excluded, especially for subgroups that are highly susceptible to dietary choline deficiency (IARC 2013a).
Although it is known that DEA may undergo nitrosation to form NDELA, a possible carcinogen to humans (Group 2B) (IARC 2000a), NDELA formation in vivo at tumorigenic dosages of DEA was not observed, suggesting that NDELA formation is not relevant to the mechanism of DEA-induced carcinogenicity (Stott et al. 2000a).
Subgroup 2B (TADEA, CADEA)
TADEA and CADEA, as the members of the family of polyethylene glycols cocamine and related ingredients, have been assessed by the CIR Expert Panel (CIR 2015c). Given their similarity in structure and physical-chemical properties of TADEA and CADEA, the health effects of CADEA and TADEA were considered collectively.
Repeated-dose toxicity: In a 13-week oral repeated-dose study, Wistar rats (25/sex/dose, except the highest dose with 10/sex) were fed diets containing TADEA dissolved in corn oil at concentrations of 0, 170, 500, 1500 or 4500 ppm (around 0,15, 50,150 and 450 mg/kg bw/day) (Goater et al. 1965, as cited in CIR 2015c). Body weights and body weight gains were reduced at the highest dose of 450 mg/kg bw/day. There were no effects on hematological parameters or organ weights. In the histopathological examination, histiocytosis was noted in the mesenteric lymph nodes in rats at 150 and 450 mg/kg bw/day (CIR 2015c). This observation may indicate that the test substance was ingested by intestinal macrophages that migrated and aggregated to abdominal lymph nodes. This is not considered to be an adverse effect (Greaves 2011). The NOAEL was identified to be 150 mg/kg bw/day on the basis of effects on body weight parameters in rats at 450 mg/kg bw/day.
In a 4-week dermal repeated-dose study, NZW rabbits (5/sex/dose) were administered TADEA dermally at doses of 0 or 40 mg/kg bw/day on abraded skin, 5 days per week (US EPA 2010b). Dermal irritation (including moderate to severe erythema and edema, slight to marked desquamation, moderate coriaceousness, and slight to severe fissuring of the exposure sites) appeared most severe at week 2. The NOAEL was 40 mg/kg bw/day, as no treatment-related systemic effects were observed at the only dose tested.
Reproductive and developmental toxicity: In an oral reproductive and developmental screening study based on OECD TG 422 (ECHA c2013-2018c), Wistar rats (10/sex/dose) received CADEA by gavage at concentrations of 0, 10, 30 and 125 mg/kg bw/day from 2 weeks prior to mating, during mating, throughout gestation and early lactation, for up to 45 consecutive days. No adverse effects on body weight were noted for females in any dose groups during the pre-mating phase and gestation, but lower body weight gain was evident for females treated with 125 mg/kg/day during lactation when compared to controls. No treatment-related effects were evident in the weekly behavioural assessments for sensory reactivity, grip strength or motor activity. No significant effects were detected in the examination of hematology or clinical biochemistry. In the highest dose group (125 mg/kg bw/day), there were lower numbers of corpora luteal and implantation sites, and higher post-implantation loss, resulting in lower litter sizes. The NOAEL for developmental toxicity was considered to be 30 mg/kg bw/day.
Genotoxicity: TADEA was not genotoxic in vitro, nor was it genotoxic in vivo. In vitro TADEA was not mutagenic in a bacterial mutation assay (Haworth 1981, as cited in CIR 2015c) or in a mouse lymphoma assay (Kirby 1980, as cited CIR in 2015c), with and without metabolic activation. In an in vitro chromosome aberration test, the test was negative in the absence of metabolic activity, but positive with metabolic activation (Thiagar 1982, as cited in CIR 2015c). TADEA did not induce unscheduled DNA synthesis in freshly prepared primary cultures of rat hepatocytes (Coppinger 1983, as cited in CIA 2015c). In vivo, a micronuclei test was negative in mice administered a single dose (10860 mg/kg bw) of TADEA by oral gavage (Allen et al. 1984, as cited in CIA 2015c). No chromosomal aberrations were induced in rat bone marrow at any treatment dose in rats administered TADEA (39, 130 or 390 mg/kg bw) by gavage (Esher 1982, as cited in CIR 2015c).
No carcinogenicity data are available for CADEA or TADEA.
6.2.3 Risk characterization of subgroup 2 (DEA, LDE, CDE, CADEA and TADEA)
DEA
Comparison of an external dermal critical effect level in rodents to an external human dermal exposure value may result in an overestimation of the risk to human health due to relatively lower human dermal absorption of DEA (Appendix E, Table E-2 and E-3). For this reason, for dermal DEA exposure scenarios, the internal critical effect levels in rodents were compared to internal dermal exposure values in humans. The latter were determined by applying 0.9% (Kraeling et al. 2004) to external dermal exposure scenarios, as discussed previously. The dermal absorption at 48 hours most relevant to the applied dose in rodents was determined (Mathews et al. 1997; used by Kirman et al. 2016 to derive a linear regression equation) and then halved for an estimation at 24 hours (based on Kraeling et al. 2004, as previously discussed) whichwas applied to the external critical effect level (adjusted for continuous 24-hour exposure, if necessary) to determine the critical internal effect level (see Appendix F).
Non-cancer endpoints: Relevant exposure estimates and critical effect (non-cancer endpoints) levels, as well as resultant margins of exposure, for the characterization of risk to human health from exposure to DEA are listed in in Table 6-10.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from drinking water (infant, formula fed) | 0.00078 mg/kg bw/day | LOAEL= 14 mg/kg bw/day (LDT) (females) | Hematological and renal effects in a 13-week oral study in rats | 18000 |
| Daily dermal exposure to dishwashing liquid (adults) | 0.00026 mg/kg bw/day (internal) |
LOAEL=1.4 a mg/kg bw/day (LDT) | Hematological and renal effects in a 13-week dermal study in rats | 8000 |
| Per event dermal exposure to wall paints (adults) | 0.0051 mg/kg bw (internal) | LOAEL=36.3 b mg/kg bw (LDT) | Increased relative and absolute kidney weights in a 2-week dermal study in rats. | 8400 |
| Daily inhalation exposure from pre-moistened all-purpose cleaner tissues | 0.00029 mg/m3/day | NOAEC= 2.68c mg/m3/day | Systemic effects on kidney and liver in rats at 150 mg/m3 in a 3-month inhalation study | 9116 |
| Per event inhalation exposure to wall paint | 0.0034 mg/m3 | NOAEC = 50 mg/m3 | Maternal toxicity (vaginal hemorrhage) at 200 mg/m3 in an inhalation developmental toxicity study | 14620 |
Abbreviations: LDT, the lowest dose tested; LOAEL, low observed adverse effect level; NOAEC, no-observed-adverse-effect-concentration.
a The dermal LOAEL of 32 mg/kg bw/day was adjusted for continuous exposure (32 x 5/7) and extrapolated to an internal effect level of 1.4 mg/kg bw/day, [(=32 X (5/7) X 6%], where 6% is the estimated dermal absorption in rats for the dermal absorption value of 32 mg/kg bw/day in 24 hours (Appendix E).
b The total dermal absorption value of 62% was derived on the basis of a linear regression of absorption values as a function of dose [y=0.460x+4.14, established by Kirman et al. 2016, where x is the dose (125 mg/kg bw) and Y is the total percent dermal absorption (62%) in 48 hours]. To account for the maximum skin bound residuals of 5% (Mathews et al. 1977), the dermal absorption excluding skin bound residuals at 57% (62% - 5%) in 48 hours was estimated. The dermal absorption of 29% (= 57% X 1/2) in 24 hours was assumed. Therefore, the external critical effect level of 125 mg/kg bw/day was extrapolated to the internal effect level of 36.3 mg/kg be/day (= 125 mg/kg bw/day X 29%).
c The NOAEC of 15 mg/m3 (6 hours/day, 5 days/week) was converted to a continuous NOAEC (24 hours/day, 7 day/week) to 2.68 mg/m3 [=15 x (6/24)x (5/7)].
The margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
LDE
Table 6-11 provides relevant exposure estimates and critical effect (non-cancer end points) levels, as well as resultant margins of exposure, for the characterization of risk to human health from exposure to LDE.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from food packaging (adults) | 0.00023 mg/kg bw/day | NOAEL=50mg/kg bw/day | Increased kidney weights and anemia at 250 mg/kg bw/day in a 13-week oral study in rats | 217390 |
| Daily dermal exposure to body soap liquid (adults) | 0.0022 mg/kg/bw/day a | NOAEL=3.5 mg/kg/day b | Increased kidney weights at 100 mg/kg bw/day in a 14-week dermal study in rats | 1590 |
| Daily dermal exposure to body soap liquid (infants) | 0.0052 mg/kg bw/day a | NOAEL=3.5 mg/kg/day b | Increased kidney weights at 100 mg/kg bw/day in a 14-week dermal study in rats | 670 |
Abbreviation: NOAEL, no observed adverse effect level.
a The internal doses of dermal exposure in humans were estimated by the application of the upper bound of dermal delivery, 1%, to external dermal exposure estimates.
b The dermal NOAEL of 50 mg/kg bw/day was extrapolated to the internal effect level of 3.5 mg/kg bw/day (=50 mg/kg bw/day x 7%), where 7% [= 21% x (24 hours /72 hours)] is the estimated dermal absorption in rats in 24 hours by assuming dermal absorption rate was constant over 72 hours.
The calculated margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
CDE
Table 6-12 provides relevant exposure estimates and critical effect (non-cancer end points) levels, as well as resultant margins of exposure, for the characterization of risk to human health from exposure to CDE.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from drinking water (infant formula fed) | 0.0073 mg/kg bw/day | NOAEL=50mg/kg bw/day) | Increased kidney weights and anemia at 250 mg/kg bw/day LDE (analogue) in a 13-week oral study in rats | 6800 |
| Daily dermal exposure to hair shampoo (child) | 0.013 mg/kg bw/daymg/kg bw/daya | NOAEL=3.5 mg/kg bw/dayb | Increased kidney weight associated with histopathological changes at 100 mg/kg bw/day in a 14-week dermal study in female rats | 270 |
| Daily dermal combined exposure from cosmetics (adults)c | 0.0095 mg/kg bw/daymg/kg bw/daya | NOAEL=3.5 mg/kg bw/dayb | Increased kidney weight associated with histopathological changes at 100 mg/kg bw/day in a 14-week dermal study in female rats | 370 |
Abbreviations: NOAEL, no observed adverse effect level.
a The internal doses of dermal exposure in humans were estimated by the application of the upper bound of dermal delivery, 1%, to external dermal exposure estimates.
b The dermal NOAEL of 50 mg/kg bw/day was extrapolated to the internal effect level of 3.5 mg/kg bw/day, [(=50 x 7 %], where 7% [= 21% x (24 hours/72 hours)] is the estimated dermal absorption in rats in 24 hours by assuming dermal absorption rate was constant over 72 hours.
c Represents the highest combined exposure estimate among the different age groups (teenagers and adults) from cosmetics (liquid body soap, facial cleansers and hair shampoo).
The calculated margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
Cancer effect: Relevant exposure estimates and critical effect levels, as well as resultant margins of exposure, for the characterization of risk to human health from exposure to DEA, are listed in Table 6-13.
IARC (IARC 2013b) classified CDE as possibly carcinogenic to humans (Group 2B). However, the carcinogenic effects were caused by the impurity of residual DEA in CDE products (IARC 2013b).
The cancer risk characterization of DEA involved residual DEA in LDE/CDE products.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Lifetime daily oral exposure from drinking water | 0.00018 mg/kg bw/day (LADD) | BMDL10 = 0.12 mg/kg bw/day a (route-to-route extrapolation) | Liver tumours in 2-year dermal cancer studies in mice exposed to DEA or DEA containing substances | 660 |
| Lifetime daily dermal exposure from dishwashing liquid | 0.00018 mg/kg bw/day (LADD) | BMDL10 = 0.12 mg/kg bw/day a | Liver tumours in 2-year dermal cancer studies in mice exposed to DEA or DEA containing substances | 660 |
| Lifetime daily inhalation exposure from wet all-purpose cleaner tissues | 0.000048 mg/kg bw/day (LADD) | BMDL10 = 0.12 mg/kg bw/day a (Route-to-route extrapolation) | Liver tumours in 2-year dermal cancer studies in mice exposed to DEA or DEA containing substances | 2500 |
Abbreviations: BMDL, the 95% lower confidence limit of benchmark dose; LADD, lifetime average daily dose.
a The external dermal BMDL10 of 1.04 mg/kg bw/day was converted to an internal dose (0.12 mg/kg bw/day = 1.04 mg/kg bw/day X 12%) assuming a dermal absorption of 12% (see Appendix E for details).
Daily ingestion of DEA in drinking water or from use of products used by consumers (such as dishwashing liquid and wet all-purpose cleaner tissues) resulted in MOEs of 660 and above.
In this risk characterization, the following information was considered: (1) a non-genotoxic MOA; (2) interspecies differences in toxicokinetics (reduced dermal absorption in humans relative to rodents); and (3) interspecies differences in toxicodynamics (oxidization of choline more rapidly in rodents than humans, DEA-induced DNA synthesis at least 75-fold less sensitive in human than rodents). Therefore, the calculated MOEs are considered adequate to address uncertainties in the human health effects and exposure databases related to the cancer endpoint.
While exposures of the general population to DEA, LDE and CDE are not of concern of at current levels, these substances are considered to have a health effect of concern on the basis of their potential carcinogenicity. Therefore, there may be a concern for human health if exposures were to increase.
CADEA
Table 6-14 provides relevant exposure estimates and critical effect levels, as well as resultant margins of exposure, for the characterization of risk to human health from exposure to CADEA.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from food packaging (adult) | 0.00082 mg/kg bw/day | NOAEL=30 mg/kg bw/day | Decreased numbers of corpora luteal and implantation sites, and higher post-implantation loss, at 125 mg/kg bw/day CADEA, in an oral reproductive/developmental study in rats | 37000 |
| Daily dermal exposure from body soap liquid (child) | 0.089 mg/kg bw/day | 40 mg/kg bw/day | No systemic effects observed in a 4-week dermal repeated-dose study in rabbits administered TADEA at the only dose tested. | 450 |
| Daily dermal exposure to body soap liquid (adult) | 0.11 mg/kg bw/day | 40 mg/kg bw/day | No systemic effects observed in a 4-week dermal repeated-dose study in rabbits administered TADEA at the only dose tested. | 360 |
Abbreviation: NOAEL, no observed adverse effect level.
The margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
TADEA
Relevant exposure estimates and critical effect levels, as well as resultant margins of exposure, for the characterization of risk to human health from exposure to TADEA, are listed in Table 6-15.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from food packaging (adults) | 0.0088 mg/kg bw/day | NOAEL=30 mg/kg bw/day (Read-across) | Decreased numbers of corpora luteal and implantation sites, and higher post implantation loss at 125 mg/kg bw/day CADEA, in an oral reproductive/developmental study in rats | 3400 |
| Per event dermal exposure to transmission fluid (adults) | 0.072 mg/kg bw/day | 40 mg/kg bw/day | No systemic effects in rabbits administered TADEA at the only dose tested in a 4-week dermal repeated-dose study. | 560 |
Abbreviation: NOAEL, no observed adverse effect level.
The margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
6.2.4 Uncertainties in evaluation of risk to human health for subgroup 2 (DEA, LDE CDE, TADEA and CADEA)
The key sources of uncertainty are presented in Table 6-16 below.
| Key sources of uncertainty | Impact |
|---|---|
| Concentration of residual DEA in CDE and LDE | +/- |
| Lack of Canadian data in environmental media. | +/- |
| There are no cancer studies for DEA by oral or inhalation route of exposure. | +/- |
| For CDE and CADEA, there is no long-term repeated-dose study by the oral route of exposure. | +/- |
+/- = unknown potential to cause over or under estimation of risk.
6.3 Assessment of subgroup 3 (TEA, TIPA and BATEA)
6.3.1 Exposure assessment of subgroup 3 (TEA, TIPA and BATEA)
Environmental media
No reports of monitoring of TEA, TIPA or BATEA were identified for environmental media in Canada or elsewhere.
Given their ionic nature under environmentally relevant conditions, TEA and TIPA are not within the domain of applicability of fugacity models traditionally used for estimating exposure to substances from environmental media. However, on the basis of physical chemistry data and fugacity modelling for the uncharged species (see Table B-3, Appendix B), human intakes of TEA and TIPA from ambient/indoor air and soil are expected to be negligible.
TEA and TIPA drinking water estimates were generated using the down-the-drain consumer use scenario in the EAU Drinking Water Spreadsheets (Health Canada 2015a) and quantities reported in Canadian commerce in 2011 (Environment Canada 2013). Details of model parameters for estimating concentrations of TEA and TIPA in drinking water can be found in Table A-4 (Appendix B). The maximum 50th percentile surface water concentrations of TEA and TIPA among the 10 receiving water bodies modelled are 11.9 and 8.87 µg/L, respectively. The resulting intake estimates from drinking water for formula-fed infants are 0.0013 mg/kg bw/day for TEA and 0.00092 mg/kg bw/day for TIPA.
For BATEA exposures are considered minimal.
Food
No reports of TEA, TIPA or BATEA were identified in food monitoring studies. In Canada TEA and TIPA may be used in certain food packaging materials with potential for direct food contact (personal communication, e-mail from Food Directorate, HC, to Existing Substances Risk Assessment Bureau, HC, dates ranging from September 2015 to March 2018; unreferenced).
The estimated conservative intakes of TEA and TIPA from food packaging are 0.0329 and 0.0067 mg/kg bw/day, respectively (personal communication, e-mail from Food Directorate, HC, to Existing Substances Risk Assessment Bureau, HC, dates ranging from September 2015 to March 2018; unreferenced).
In Canada TEA may be used as a component of incidental additives in products used in food processing plants with no potential for direct food contact (personal communication, e-mail from Food Directorate, HC, to Existing Substances Risk Assessment Bureau, HC, dated August 2017; unreferenced).
Products available to consumers
Potential exposure from use of products available to consumers was estimated. Details are presented in Appendix D. Estimates for uses that result in the highest level of potential oral, dermal exposure or inhalation exposure (referred to as sentinel scenarios) are presented in Tables 6-17 and 6-18, respectively. Additional potential use scenarios for TEA (make-up, styling products, hair spray, shampoos and conditioners, automotive and marine cleaners, coolant additives, printer inks and waterproof adhesives/epoxys, drugs and NHPs) and for TIPA (shampoo, NHPs) were considered, but resulted in lower exposures than those presented in Tables 6-17 and 6-18. BATEA was not identified in any products available to consumers.
Dermal absorption
The dermal absorption of TEA in humans was measured using in vitro diffusion cell techniques (Kraeling and Bronaugh 2003). The percent of TEA absorbed into receptor fluids was determined by adding [14C]-TEA in the formulation as an emulsifying agent to simulate cosmetic exposure. The study showed that the ratio of absorbed TEA into receptor fluid relative to the applied dose was 1% (rounded up from 0.43%) in the formulation with 1% or 5% TEA at the end of the 24 hour study. Following the application of 1% and 5% TEA formulation for 24 hours, the amounts of absorbed TEA in receptor fluid from 24 hours to 72 hours were not significant increased even though substantial amount of TEA (6.9% to 9.6%) remained in the skin at the end of study, suggesting the skin bound residuals of TEA was not bioavailable for systemic absorption (Kraeling and Bronaugh 2003). Consequently a dermal absorption value of 1% was used for estimating human dermal exposure to TEA.
| Sub-stance | Product scenarioa | Concentration (%) | Route of exposure | Per event exposure(mg/kg bw) | Daily exposure (mg/kg bw/day) |
|---|---|---|---|---|---|
| TEA | Toothpaste, non-fluoridated (toddler) | 3 | Oral | 0.41 | 0.57 |
| TEA | Toothpaste, whitening | 10 | Oral | 0.11 | 0.23 |
| TEA | All-purpose cleaning spray, dermal | 10 | Dermal | 0.0044b | 0.0044b |
| TEA | Body moisturizer/lotion (infant) | 10 | Dermal | 0.19b | 0.32b |
| TEA | Body moisturizer/lotion | 10 | Dermal | 0.062b | 0.068b |
| TEA | Sunscreen | 10 | Dermal | 0.26b | 0.36b |
| TEA | Sunscreen (toddlers) | 10 | Dermal | 0.35b | 0.56b |
| TIPA | Hair gel/wax/putty (children) | 3 | Dermal | 0.17 | 0.095 |
| TIPA | Hair gel/wax/putty | 3 | Dermal | 0.08 | 0.044 |
| TIPA (Acute Only) | BBQ cleaner spray – cleaning | 5 | Dermal | 0.42 | N/A |
Abbreviation: N/A; Not applicable
a Represents direct exposures to adults, except where noted otherwise.
b Systemic exposure through dermal route, assuming 1% dermal absorption.
| Substance | Product scenario | Concentration (%) | 6-hour TWA air concentration (mg/m3) | Daily exposure (mg/m3) |
|---|---|---|---|---|
| TEA | All-purpose cleaning spray | 10 | 0.83 | 0.21 |
Abbreviation: TWA, time-weighted average
Given the large number (approximately 8000) and variety of cosmetic products applied to the skin reported to contain TEA, an estimate of aggregate exposure to TEA from multiple products was considered, taking into consideration the SCCS Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation (SCCS 2016). Daily aggregate exposure estimates for TEA in cosmetics are presented in Table 6-19.
| Product | Concentrationa (%) | Daily exposure – teen (mg/kg bw/d)b | Daily exposure – adult (mg/kg bw/d)b |
|---|---|---|---|
| Body moisturizer (cream/lotion/Gel) | 10 | 0.064 | 0.068 |
| Face moisturizer (cream/lotion/gel) | 10 | 0.0.036 | 0.030 |
| Body soap (liquid) | 10 | 0.0019 | 0.0022 |
| Hair shampoo | 10 | 0.0022 | 0.0018 |
| Hair conditioner (leave-in) | 3 | 0.0073 | 0.0061 |
| Hair gel/wax/putty | 10 | 0.0032 | 0.0027 |
| Eye shadow | 10 | 0.0018 | 0.0015 |
| Total combined exposure (mg/kg bw/day)a | - | 0.12 | 0.11 |
a Based on notifications submitted under the Cosmetic Regulations to Health Canada.
b Systemic exposure through dermal route, assuming 1% dermal absorption.
TEA is associated with a potential cancer endpoint (details are provided in the health effects section). In order to estimate the potential cancer risk from exposure to TEA, lifetime average daily doses were calculated for daily exposure from non-fluoridated toothpaste (0.12 mg/kg bw/day), daily use of body moisturizer (0.077 mg/kg bw/day), aggregate use of cosmetics and cleaners (0.094 mg/kg bw/day), and daily use of all-purpose cleaning sprays (0.034 mg/kg bw/day) (see Appendix D).
6.3.2 Health effects assessment of subgroup 3 (TEA, TIPA and BATEA)
TEA
TEA is not classifiable as to its carcinogenicity to humans (Group 3) (IARC 2000b). However, a US NTP (2004) study, conducted after the IARC (2000b) review, demonstrated some evidence of carcinogenic activity in female mice (NTP 2004). The CIR Expert panel reviewed the health effects of TEA (CIR 2013a).
Toxicokinetics: Similar to DEA, the dermal absorption of TEA was lower in humans (0.43%, 24 hours) (Kraeling and Bronaugh 2003) than in rodents. Excluding skin bound residuals, TEA was absorbed extensively in mice (82%) and rats (71%) following 24 or 48 hours of dermal application at the tested dose levels (1000 mg/kg bw in acetone and 2000 mg/kg bw neat or in water) regardless of the vehicle used (Stott et al. 2000b).
Repeated-dose toxicity study: In a 4-week inhalation study, Wistar rats (10/sex/group) were exposed to TEA by inhalation (nose-only) at concentrations of 0, 20, 100 or 500 mg/m3, 6 hours per day, 5 days per week (Gamer 2008). Submucosal inflammation of the larynx observed in males in all treated groups and females at 100 mg/m3 and above was considered an adaptive response. In the absence of any other effect, the NOAEL was considered to be 500 mg/m3 (equivalent to 89 mg/m3 for continuous exposure), the highest concentration tested. This NOAEL is consistent with an unpublished 2-week (12-day) inhalation study in F344 rats (5/sex/group) exposed to higher concentrations (0, 125, 250, 500, 1000, 2000 mg/m3, 6 hours/day), in which mild acute inflammation of the laryngeal submucosa was observed at 1000 mg/m3 and above (unpublished NTP study, cited in Gamer et al. 2008).
In a 13-week dermal study, F344/N rats (10/sex/dose) were exposed to TEA via dermal application (residual MEA and DEA each less than 0.4%) at concentrations of 0, 125, 250, 500 or 1000 mg/kg bw/day in acetone or 2000 mg/kg bw/day (neat) (5 days per week) (NTP 1999d). There was local dermal irritation in both sexes at 1000 mg/kg bw/day and above. Body weight was decreased in females at 1000 mg/kg bw/day and above, and in males at 2000 mg/kg bw/day. Increased absolute and relative kidney weights were noted in both sexes at 500 mg/kg bw/day and above. The NOAEL was identified to be 250 mg/kg bw/day. B6C3F1 mice (10/sex/dose) were exposed to 0, 250, 500, 1000 or 2000 mg/kg bw/day of TEA in acetone or 4000 mg/kg of neat TEA by dermal application for 13 weeks (NTP 1999d). At 4000 mg/kg bw/day, there was increased absolute and relative kidney weights and local dermal irritation (including scaliness and discoloration) in both sexes. The NOAEL was 2000 mg/kg bw/day.
Reproductive and developmental toxicity: In a reproductive and developmental screening test, Wistar rats (10/sex/dose) were given TEA dissolved in water by gavage at the dose levels of 0, 100, 300 or 1000 mg/kg bw/day for 2 weeks prior to mating, during mating and through gestation and lactation up to postnatal day 4 (BASF 2010). Body weight gain was decreased in females at the highest dose group (1000 mg/kg bw/day) during pregnancy and postnatal days 1 to 4. A slight increase in postimplantation loss and related reduced litter size were reported at 1000 mg/kg bw/day. The maternal NOAEL was considered to be 300 mg/kg bw/day.
Genotoxicity and carcinogenicity: No genotoxicity potential was reported for TEA in vitro or in vivo. TEA was not mutagenic in bacterial mutation assays with or without metabolic activation (Inoue et al. 1982; Dean et al.1985; Mortelmans et al. 1986), in a gene conversion assay in yeast (Dean et al. 1985), nor was it clastogenic in a sister chromatid exchange assay in Chinese hamster ovary cells with or without metabolic activation (Galloway et al.1987, Inoue et al. 1982) or in in cultured rat liver cells (Dean et al. 1985). A transformation assay using hamster embryo cells treated with TEA was negative (Inoue et al.1982). The micronucleus test was negative as conducted in male and female mice following 13 weeks of dermal application of 1000 to 4000 mg/kg bw/day TEA (NTP 2004).
In a 2-year oral cancer study, F344 rats (50/sex/dose) were administered TEA in drinking water at concentrations of 0%, 1% or 2% (equivalent to 0, 1400 or 2800 mg/kg bw/day). Throughout the experiment, there was decreased body weight in both sexes and mortality in females (at about week 60) at 1400 mg/kg bw/day and above. Administration of TEA was stopped in both female groups due to increased deaths and restarted using half of each dosing concentration. At 700 mg/kg bw/day and above, increased absolute and relative kidney weights associated with increased non-neoplastic lesions (such as chronic nephropathy, mineralization of the papilla, pyelonephritis) were observed. No treatment-related increase in the incidence of tumours was observed (Maekawa et al. 1986).
The US NTP conducted 2-year dermal cancer studies in B6C3F1 mice and F344/N rats. There was an apparent association of TEA treatment with hepatocellular tumours in both sexes and hepatoblastomas in male mice, but no carcinogenic effects of TEA were observed in rats (NTP 1999d). However, mice used in the study were chronically infected with Helicobacter hepaticus, an organism that is known to induce hepatitis. The interpretation of any relationship between TEA and liver neoplasms was thus inconclusive (IARC 2000b).
In a second 2-year dermal cancer study, B6C3F1 mice (50/sex/dose) received TEA (containing 0.49% DEA) in acetone at concentrations ranging from 0 to 2000 mg/kg bw/day by dermal application, 5 days per week (NTP 2004). Dose-dependent skin irritation occurred at the site of application. There was a decrease in body weight of males at 2000 mg/kg bw/day from week 17 to 37 and at the end of the study. As in the first study (NTP 1999d), TEA increased the incidence of hepatocellular neoplasms in female mice, but not in male mice. The frequency of hepatocellular tumours (adenoma or carcinoma) in females was 12/50 (24%), 23/50 (46%), 24/50 (48%) and 34/50 (68%) for 0, 100, 300, or 1000 mg/kg bw/day, respectively (NTP 2004). Using a multistage-cancer model, the BMD10 and BMDL10 were determined to be 60 and 42 mg/kg bw/day, respectively, on the basis of the increased liver tumours in female mice treated with TEA (details are provided in Appendix F, Figure F-2).
MOA: Since DEA and TEA are structural analogues of choline, and choline depletion is a likely MOA for the carcinogenicity of DEA, the potential for TEA to cause choline depletion in tumorigenesis was also investigated (Stott et al. 2004). In a 3-week dermal study investigating the potential MOA, rodents received TEA in acetone dermally (female B6C3F1 mice and TEA with 0.04% residual DEA in the first trial; female B6C3F1 mice, CDF rats, and TEA with 0.45% residual DEA in the second trial), 5 days a week for 3 weeks. TEA treatment resulted in significant decreases in the levels of choline metabolites (phosphocholine and betaine) in mice treated with TEA containing minimal residual DEA (0.04%) or as high as 0.45% residual DEA. No changes in choline metabolites were observed in rats, which is consistent with no development of tumours in rats exposed to TEA. Like DEA, TEA inhibited the uptake of [3H]choline dose-dependently in Chinese hamster ovary cells, but this effect was less potent for TEA in comparison to DEA (Stott et al. 2004). Stott et al. (2004) concluded that TEA might cause liver tumours through a choline depletion MOA. Although residual DEA in TEA may contribute to choline depletion, the TEA itself could also potentially result in choline depletion (Stott et al. 2004). As a choline-deficiency MOA cannot be excluded in humans, the carcinogenicity of TEA was considered to be relevant to humans.
TIPA
TIPA was assessed as a part of the isopropanolamines category in the SIDS Initial Assessment Report (OECD 2009). A literature search was conducted from October 2008 to December 2017. No significant new studies were identified that were considered to impact the hazard and risk characterization.
Repeated-dose toxicity: In an oral 14-week (100 days) study, dogs (4/sex/dose) were fed a diet containing TIPA at concentrations of 0, 500, 2000 or 7500 ppm (equivalent to 0, 16.8, 71.2 or 272 mg/kg bw/day, respectively) (OECD 2009). Treatment had no effects on food consumption or body weights. No treatment-related effects were reported through the evaluation of organ weights, hematology, urinalysis, biochemistry and histopathology. The NOAEL was considered to be 272 mg/kg bw/day.
In a dermal 4-week repeated-dose study, Fischer 344 rats (5/sex/dose) were exposed to TIPA dermally at dose levels of 0, 300, 1000 or 3000 mg/kg bw/day, 5 days/week (OECD 2009). Patches were applied to the back of each rat and covered with non-absorbent cotton. A dose-dependent increased incidence of skin irritation at the site of application, including slight erythema and scabs, was noted. No systemic toxicity was reported in treated animals. The NOAEL for systemic toxicity was considered to be 3000 mg/kg bw/day, the highest dose tested (OECD 2009).
Reproductive and developmental toxicity: Reproductive and developmental toxicity of TIPA was examined in a one-generation reproductive study (ECHA c2013-2018a). SD rats (25/sex/dose) were fed diets containing TIPA at 0, 500, 2000 or 7500 ppm (equivalent to 40, 200 or 700 mg/kg bw/day, respectively) for 5 weeks prior to mating and during periods of mating, gestation, lactation and weaning. The offspring (20 selected F1 rats/sex/group) were fed the same diets containing TIPA (equivalent to 0/0, 39.7/43.7, 160/182 or 609/700 mg/kg bw/day for male/female offspring respectively) for 90 days after weaning. No treatment-related effects were observed, and the NOAEL was considered to be 609/700 mg/kg bw/day (males/females), the highest dose tested.
Genotoxicity and carcinogenicity: The substance showed negative genotoxicity in in vitro and in vivo assays. It was negative in a bacterial mutagenicity study using Salmonella typhimurium (TA 1535, TA 1537, TA 98 and TA 100) with or without metabolic activation (OECD 2009). TIPA was negative in an in vitro mammalian cell gene mutation assay (Chinese hamster ovary cells) and two chromosomal aberration assays (Chinese hamster ovary cells or rat lymphocytes) with or without metabolic activation. In an in vivo micronucleus assay conducted according to OECD TG 474, NMRI mice (5/sex/group) received a single administration of TIPA by gavage at concentrations of 500, 1000 or 2000 mg/kg bw (OECD 2009). TIPA did not lead to an increase in the rate of micronuclei in polychromatic erythrocytes in bone marrow following 24- or 48-hour treatments at any dose level (OECD 2009).
Male Wistar rats (21 to 28/dose) received 2% TIPA in the diet for 104 weeks, and no hyperplasia and/or pre-neoplastic lesions in livers were reported. The limitations of this carcinogenicity study included the use of male rats only, the evaluation of a single dose and the examination of the liver only (ECHA 2013-2018a).
BATEA
Toxicity information was not available for this substance. BATEA was not identified as posing a high hazard to human health on the basis of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity, or reproductive toxicity. It is also not on the European Chemicals Agency’s Candidate List of Substances of Very High Concern for Authorisation (ECHA [modified 2017]). Further investigation of the health effects is not warranted at this time given the low expected exposure of the general Canadian population to BATEA.
6.3.3 Risk characterization of subgroup 3 (TEA, TIPA and BATEA)
TEA
Non-cancer effects: Table 6-20 provides relevant exposure estimates and critical effect levels, as well as resultant margins of exposure, for the characterization of risk to human health from exposure to TEA.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from non-fluoridated toothpaste (toddlers) | 0.57 mg/kg bw/day | NOAEL = 300 mg/kg bw/day | Decreased maternal body weight gain at 1000 mg/kg bw/day in an oral reproductive and developmental screening test in rats | 526 |
| Daily dermal exposure from body moisturizer (infants) | 0.32 mg/kg bw/day | NOAEL = 177.5 mg/kg bw/daya (internal dose) | Increased kidney weights at 500 mg/kg bw/day in rats in a 13-week dermal study | 537 |
| Daily dermal combined exposure from cosmetics (teenagers and adults)c | 0.15 mg/kg bw/day | NOAEL = 220 mg/kg bw/daya (internal dose) | Increased kidney weights at 500 mg/kg bw/day in rats in a 13-week dermal study | 1496 |
| Daily inhalation from all-purpose cleaning sprays | 0.21 mg/m3/day | NOAEC = 89.3 mg/m3b | No systemic effects observed at the highest dose tested in a 28-day inhalation study in rats | 431 |
| Per event inhalation exposure from all-purpose cleaning sprays | 0.83 mg/m3 (TWA-6h) | NOAEC = 500 mg/m3 | No systemic effects observed at the highest dose tested dose in a 28-day inhalation study in rats | 604 |
Abbreviations: NOAEC, no observed adverse effect concentration; NOAEL, no observed adverse effect level.
a The NOAEL of 250 (mg/ kg bw/day) was extrapolated to and the internal POD of 177.5 mg/kg bw/day based on a dermal absorption of 71% of TEA in rats in 24 hours.
b The NOAEC of 500 mg/m3, based on intermittent exposure of animals (for 6 hours per day, 5 days per week), was adjusted for continuous exposure (24 hours/d, 7 days/week) with the following equation 500 mg/m3 x (6/24) x (5/7)=89.3 mg/m3.
c Represents the highest combined exposure estimate among the different age groups (teenagers and adults) expected to potentially use multiple cosmetics (body moisturizer, face moisturizer, liquid body soap, hair shampoo, hair conditioner, hair gel/wax/putty, eye shadow).
In order to take into consideration the significant difference in dermal absorption of TEA between humans and rodents, the human health risk from dermal exposure to TEA was characterized by comparing an adjusted internal critical effect level derived from animal studies to an estimated internal dermal dose in humans, using dermal absorption values of 1% in humans and 71% in rats.
The MOE of 550 was determined for daily dermal exposure from body moisturizer in infants at 0.32 mg/kg bw/day (internal) compared with the internal NOAEL of 177.5 mg/kg bw/day. The combined (aggregate) dermal exposure to multiple cosmetics in different age groups was estimated, including exposure to TEA in body moisturizer, face moisturizer, body soap liquid, hair shampoo, hair conditioner (leave-in), hair gel/wax putty, and eye shadow. The maximum combined exposure estimate (internal dermal dose) was 0.12 mg/kg bw/day in teenagers, which resulted in an MOE of 1480 when compared to the NOAEL of 177.5 mg/kg bw/day. The calculated MOE is considered adequate to address uncertainties in the health effects and exposure databases.
The daily inhalation exposure from use of all-purpose cleaning sprays was estimated to be 0.207 mg/kg bw/day, and the NOAEC of 89.3 mg/m3/day was identified, as no systemic toxicity was observed at the highest tested dose in a 28-day inhalation study, resulting in an MOE of 430. For acute inhalation exposure scenarios, the exposure estimate of 0.83 mg/m3 (TWA 6h) from the use of all-purpose sprays was compared to the NOAEL of 500 mg/m3 (6 hours/day, 12 days), resulting in an MOE of 600.
The margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
Cancer effect: Table 6-21 provides relevant exposure estimates and critical effect levels, as well as resultant margins of exposure, for the characterization of risk to human health from exposure to TEA.
| Exposure scenario | Systemic exposure | Critical effect levela | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Lifetime daily oral exposure from non-fluoridated toothpaste | 0.12 mg/kg bw/day (internal dose, LADD)b | BMDL10 = 21.3 mg/kg bw/day (internal) | Increased liver tumours in a 2-year dermal cancer study in female mice | 180 |
| Lifetime daily dermal exposure from body moisturizer | 0.077 mg/kg bw/day (internal dose, LADD) | BMDL10 = 21.3 mg/kg bw/day (internal) | Increased liver tumours in a 2-year dermal cancer study in female mice | 280 |
| Combined lifetime daily dermal exposure from cosmetics and cleaners | 0.094 mg/kg bw/day (internal dose, LADD) | BMDL10 = 21.3 mg/kg bw/day (internal) | Increased liver tumours in a 2-year dermal cancer study in female mice | 230 |
| Lifetime daily inhalation exposure from all-purpose cleaning sprays | 0.034 mg/kg bw/day (internal dose, LADD)b | BMDL10 = 21.3 mg/kg bw/day (internal) | Increased liver tumours in a 2-year dermal cancer study in female mice | 630 |
Abbreviations: BMDL, the 95% lower confidence limit of benchmark dose; LADD, lifetime average daily dose.
a The BMDL10 of 42 mg/kg bw/day (5 days per week) is equivalent to a continuous exposure BMDL10 of 30 mg/kg bw/day (7 days per week). An internal BMDL10 of 21.3 was derived using a dermal absorption of 71% in mice (Stott et al. 2000b).
b Assumes 100% absorption through oral intake or inhalation.
By comparing the LADD exposure estimates from oral exposure to non-fluoridated toothpaste, dermal exposure to body moisturizer or combined use of cosmetics and cleaners and inhalation exposure to all-purpose cleaning spray with an increased risk of liver tumours in female mice (the BMDL10 of 21.3 mg/kg bw/day) respectively, the MOEs were 180 and above.
The MOEs are considered adequate to address uncertainties in the health effects and exposure databases on the basis of the following reasons: (1) the non-genotoxic carcinogenic MOA for TEA through choline depletion; (2) the low potency of TEA as a liver carcinogen, as evidenced by no induction of liver tumours in rats and induction in only 1 sex (females) in mice; (3) the MOA is less relevant to humans, due to interspecies differences in metabolism (see section 6.2.2, mode of action); (4) the conservatively estimated LADDs due to the assumptions of that these products (non-fluoridated toothpaste, body moisturizer, dishwashing soap) are used every day for a lifetime.
While the exposures of the general population to TEA are not of concern at current levels, TEA is considered to have a health effect of concern because of its potential carcinogenicity. Therefore, there may be a concern for human health if exposures were to increase.
TIPA
Table 6-22 provides relevant exposure estimates and critical effect levels, as well as resultant margins of exposure, for the characterization of risk to human health from exposure to TIPA.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from food packaging (adults) | 0.0067 mg/kg bw/day | NOAEL = 700 mg/kg bw/day | No adverse effects were noted at the highest dose group in an oral one generation reproductive and developmental study in rats | 104000 |
| Daily dermal exposure to hair gel/wax/putty (children) | 0.095 mg/kg bw/day | NOAEL = 3000 mg/kg bw/day | No adverse effect were observed in a dermal 28-day repeated-dose study in rats | 31600 |
| Per event dermal exposure to hair gel/wax/putty (children) | 0.17 mg/kg bw/day | NOAEL = 3000 mg/kg bw/day | No adverse effects were observed in a dermal 28-day repeated-dose study in rats | 17600 |
| Per event dermal exposure from BBQ cleaner spray - cleaning | 0.423 mg/kg bw/day | NOAEL = 3000 mg/kg bw/day | No adverse effects were observed in a dermal 28-day repeated-dose study in rats | 7090 |
Abbreviations: NOAEL, no observed adverse effect level.
The margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
6.3.4 Uncertainties in evaluation of risk to human health for subgroup 3 (TEA, TIPA and BATEA)
The key sources of uncertainty are presented in Table 6-23 below. Confidence is high that use of maximum concentrations and the high end of the range of product amounts contained in consumer products is a conservative estimate of general population exposures.
| Key sources of uncertainty | Impact |
|---|---|
| No recent total Canadian manufacture or import volume data for BATEA | +/- |
| No measured Canadian data in environmental media. | +/- |
| There are no cancer studies for TEA by the oral or inhalation route of exposure. | +/- |
+/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm the environment from MEA, DEEA, LME, DEA, LDE, CDE, CADEA, TADEA, TEA, TIPA and BATEA. It is proposed to conclude that the 11 substances in the Alkanolamines and Fatty Alkanolamides Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that MEA, DEEA, LME, DEA, LDE, CDE, CADEA, TADEA, TEA, TIPA and BATEA do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is proposed to conclude that the 11 substances in the Alkanolamines and Fatty Alkanolamides Group do not meet any of the criteria set out in section 64 of CEPA.
References
[ACI] American Cleaning Institute. 2010. Consumer product ingredient safety: Exposure and Risk Screening Methods for Consumer Product Ingredients, 2nd edition. Appendix II-A-1 & Appendix II-B-1: Dermal exposure parameters to estimate screening exposures to consumer products – North America. Washington (DC): American Cleaning Institute.
Allen JA, Proudlock RJ, McCaffrey K. 1984. Micronucleus Test on E-2352.01 (ECM BTS 902/01) Tamet. Unpublished Report No. P+G 1114/84560; for Procter and Gamble N.V., Stroombeek-Bever, Belgium; from Huntingdon Research Centre plc, Huntingdon, England. BASF AG. 1993a. Product Safety, Report - Study on the inhalation toxicity including neurotoxicological examination of Diethanolamine as a liquid aerosol in rats, 14-day test. BASF Project No. 36I0233/90009, BG No. 158, Experimental Toxicology and Ecology, BASF AG, Ludwigshafen, Germany, unpublished report, February 25, 1993.
BASF AG. 1993b. Product Safety, Study of the prenatal inhalation toxicity of Diethanolamine in rats after inhalation. BASF Project No. 31R0233/90010, Experimental Toxicology and Ecology, BASF AG, Ludwigshafen, Germany, unpublished report, July 8, 1993.
BASF AG. 2002. Product Safety, Diethanolamine – Subchronic inhalation toxicity study in Wistar rats, liquid aerosol/vapor exposure, study focus on irritation of upper respiratory tract. BASF Project No. 51I0299/99125, Experimental Toxicology and Ecology, BASF AG, Ludwigshafen, Germany, unpublished report, April 2, 2002.
BASF AG. 2010. Product Safety, Results of a production/developmental toxicity screening test in Wistar rats with 2,2′,2″-Nitrioethanol (Triethanolamine) (CAS No. 102-71-6). BASF SE, Ludwigshafen, Germany, unpublished report, August 26, 2010.
Brain KR, Walters KA, Green DM, Brain S, Loretz LJ, Sharma RK, Dressler WE. 2005. Percutaneous penetration of diethanolamine through human skin in vitro: application from cosmetic vehicles. Food Chem Toxicol. 43(5):681-90.
Brown, LSR., Gray, DO. 1986. Diethanolamine, a secondary amine from the compositae. Journal of Natural Products. 49(5): 910-912.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Cerrillo I, Fernández-Pachón MS, Collado-González J, Escudero-López B, Berná G, Herrero-Martín G, Martin F, Ferreres F, Gil-Izquierdo A. 2015. Effect of fermentation and subsequent pasteurization processes on amino acids composition of orange juice. Plant Foods Hum Nutr. 70(2):153-159.
Charles River Laboratories Edinburgh Ltd. 2020. In vitro percutaneous absorption of radiolabelled lauramide DEA and radiolabelled triethyl phosphate (TEP) at 2 dose levels through human skin (OECD 428 and SCCS): 24 h design. Study No. 784630. CRL Edinburgh Ltd, UK, Unpublished report, February 13, 2020.
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Ver. 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [accessed 2018 Jan 31].
[CIR] Cosmetic Ingredient Review Expert Panel. 2013a. Fiume MM, Heldreth B, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler D, Marks JG, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Safety assessment of triethanolamine and triethanolamine-containing ingredients as used in cosmetics. Int J Toxicol. 32 (3 Suppl):59S-83S.
[CIR] Cosmetic Ingredient Review Expert Panel. 2013b. Fiume MM, Heldreth B, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Safety assessment of diethanolamides as used in cosmetics. Int J Toxicol. 32(3 Suppl):36S-58S.
[CIR] Cosmetic Ingredient Review Expert Panel. 2015a. Fiume MM, Heldreth BA, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Safety Assessment of Ethanolamine and Ethanolamine Salts as Used in Cosmetics. Int J Toxicol. 34(2 Suppl):84S-98S.
[CIR] Cosmetic Ingredient Review Expert Panel. 2015b. Fiume MM, Heldreth BA, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Safety Assessment of Ethanolamides as Used in Cosmetics. Int J Toxicol. 34(1 Suppl):18S-34S.
[CIR] Cosmetic Ingredient Review Expert Panel. 2015c. Safety Assessment of PEGs Cocamine and Related Ingredients as Used in Cosmetics. Final amended report. [Released data: 2015 July 10; accessed 2017 Dec 15].
[CIR] Cosmetic Ingredient Review Expert Panel. 2017. Fiume MM, Heldreth B, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. 2017. Safety Assessment of Diethanolamine and Its Salts as Used in Cosmetics. Int J Toxicol. 36(5 Suppl 2):89S-110S.
ConsExpo Web [Consumer Exposure Web Model]. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Coppinger WJ. 1983. Unscheduled DNA Synthesis Assay in Primary Cultures of Rat Hepatocytes. Report No. M0021, The Procter & Gamble Company, BTF – Miami Valley Laboratories, Cincinnati, OH, USA.
Dean BJ, Brooks TM, Hodson-Walker G, Hutson DH. 1985. Genetic toxicology testing of 41 industrial chemicals. Mutat Res. 153(1-2):57-77.
[EAU] Environmental Assessment Unit. 2005. Cosmetics Exposure Workbook. (unpublished, internal Health Canada tool).
[EC] European Commission. 2003. Technical Guidance Document on risk assessment, Part I. 1998. Institute for Health and Consumer Protection, European Chemicals Bureau.
[EC] European Commission. 2016. European Union risk assessment report: 2-aminoethanol: CAS No. 141-43-5. [accessed 2018 Jan 3].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances.Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of Risk Assessment Priorities: Results of the 2015 Review.
[ECCC] Environment and Climate Change Canada. 2017. Data submission under the Canadian Environmental Protection Act, 1999, Section 70. Non-CBI use information for CAS RN 141-43-5 and 85204-21-3.
[ECHA] European Chemicals Agency. c2007-2017a. Registered substances database; search results for CAS RN 141-43-5. Helsinki (FI): ECHA. [updated 2017 Mar 2; accessed 2017 Nov 28].
[ECHA] European Chemicals Agency. c2007-2017b. Registered substances database; search results for CAS RN 111-42-2. Helsinki (FI): ECHA. [updated 2017 Mar 2; accessed 2017 Nov 28].
[ECHA] European Chemicals Agency. c2011-2017. Registered substances database; search results for CAS RN 100-37-8. Helsinki (FI): ECHA. [updated 2017 Mar 2; accessed 2017 Nov 28].
[ECHA] European Chemicals Agency. c2013-2017. Registered substances database; search results for CAS RN 111-05-7. Helsinki (FI): ECHA. [updated 2017 Apr 18; accessed 2018 Jan 15].
[ECHA] European Chemicals Agency. [modified 2017]. Candidate List of substances of very high concern for Authorisation. Helsinki (FI): ECHA. [accessed 2018 Dec 3].
[ECHA] European Chemicals Agency. c2013-2018a. Registered substances database; search results for CAS RN 122-20-3. Helsinki (FI): ECHA. [updated 2017 Mar 2; accessed 2017 Nov 28].
[ECHA] European Chemicals Agency. c2013-2018b. Registered substances database; search results for CAS RN 111-57-9. Helsinki (FI): ECHA. [updated 2018 Mar 16; accessed 2018 Jan 15].
[ECHA] European Chemicals Agency. c2013-2018c. Registered substances database; search results for CAS RN 61791-31-9. Helsinki (FI): ECHA. [updated 2018 Jun 13; accessed 2018 Jan 15].
[ECHA] European Chemicals Agency. c2013-2018d. Registered substances database; search results for CAS RN 93-83-4. Helsinki (FI): ECHA. [updated 2018 Jun 13; accessed 2018 Jan 15].
Edgerton SA, Kenny DV, Joseph DW. 1989. Determination of amines in indoor air from steam humidification. Environ Sci Technol. 23:484-488.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [estimation model]. c2000-2017. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Esher, HJ. 1982. In vivo Cytogenetics Study in Rats. Unpublished Report No. MRI-182-PG-82-58; for The Procter and Gamble Company, Cincinnati, OH, USA; from EG&G/Mason Research Institute, Worcester, MA, USA.
Exxon Biomedical Sciences (1990), Project Number 208618, Sponsored research by SyntheticOrganic Chemicals Manufacturing Association, May 3, 1990.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food Chem Toxicol. 78:159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC. 2016. Consumption of cosmetic products by the French population. Second part: Amount data. Food Chem Toxicol. 90:130-141.
Frauenkron M, Melder J, Ruider G, Rossbacher R, Höke H. 2012. Ethanolamines and propanolamines. In: Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA. Vol. 13, p. 405-431.
Galgano F, Caruso M, Perretti G, Favati F. 2011. Authentication of Italian red wines on the basis of the polyphenols and biogenic amines. Eur Food Res Technol. 232(5):889-897.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C, Bloom AD, Nakamura F, Ahmed M, Duk S, et al. 1987. Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluations of 108 chemicals. Environ Mol Mutagen.10 (Suppl 10):1-175.
Gamer AO, Rossbacher R, Kaufmann W, van Ravenzwaay B. 2008. The inhalation toxicity of di- and triethanolamine upon repeated exposure. Food Chem Toxicol. 46:2173-2183.Giachetti C. 1998. Determination of triethanolamine in air samples by gas chromatography-mass spectrometry. Chromatographia. 48:443-449.
Goater T, Griffiths OD, McElligott TF. 1965. Ninety-day oral toxicity of ethomeen T/12-albino rats. Report No. IHR/173. Industrial Hygiene Research Laboratories, Macclesfield, Cheshire.
González S, Petrovic M, Barceló D. 2004. Simultaneous extraction and fate of linear alkylbenzene sulfonates, coconut diethanol amides, nonylphenol ethoxylates and their degradation products in wastewater treatment plants, receiving coastal waters and sediments in the Catalonian area (NE Spain). J Chromatogr A. 1052:111-120.
Gorzelska K, Galloway JN. 1990. Amine nitrogen in the atmospheric environment over the North Atlantic Ocean. Global Biogeochem Cycles. 4:309-333.
Greaves P. 2011. Histopathology of preclinical toxicity studies: Interpretation and relevance in drug safety evaluation. 4th edition. Amsterdam, Netherlands: Academic Press. p. 115.
Guant IF, Farmer M, Grasso P, Gangolli SD. 1967. Short-term feeding study of lauric diethanolamide in rats. Food Cosmet Toxicol. 5(4):497-503.
Haworth SR. 1981. Salmonella/Mammalian-Microsome Mutagenesis Assay (Ames Test). Report No. 003-468-677-1; prepared for The Procter and Gamble Company, Cincinnati, OH, USA; from EG&G Mason Research Institute, Rockville, MD, USA.
Health Canada. [modified 2018]. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Health Canada, Consumer and Hazardous Products Safety Directorate. [accessed 2018 Jul 10].
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. 2015a. Environmental Assessment Unit Drinking Water Spreadsheets [Excel format]. Ottawa (ON): Health Canada. [cited 2016 Apr 8].
Hellwig J, Liberacki AB. 1997. Evaluation of the pre-, peri-, and postnatal toxicity of monoethanolamine in rats following repeated oral administration during organogenesis. Fundam Appl Toxicol. 40(1):158-62.
Helman RG, Hall JW, Kao JY. 1986. Acute dermal toxicity: in vivo and in vitro comparisons in mice. Fundam Appl Toxicol. 7(1):94-100.
Hinz JP, Thomas JA, Ben-Dyke R. 1992. Evaluation of the inhalation toxicity of diethylethanolamine (DEEA) in rats. Fundam Appl Toxicol. 18:418-424.
Huang X, Deng C, Zhuang G, Lin J, Xiao M. 2016. Quantitative analysis of aliphatic amines in urban aerosols based on online derivatization and high performance liquid chromatography. Environ Sci Process Impacts. 18:796-801.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risk to Human. 2000a. N-Nitrosodiethanolamine. IARC Monogr Eval Carcinog Risks Hum. 77:403-438.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risk to Human. 2000b. Triethnolamine. IARC Monogr Eval Carcinog Risks Hum. 77:381-401.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risk to Human. 2013a. Diethanolamine. IARC Monogr Eval Carcinog Risks Hum. 101:117-140.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risk to Human. 2013b. Coconut oil diethanolamine condensate. IARC Monogr Eval Carcinog Risks Hum. 101:141-148.
Inoue K, Sunakawa T, Okamoto K, Tanaka Y. 1982. Mutagenicity tests and in vitro transformation assays on triethanolamine. Mutat Res. 101(4):305-313.
JETOC. 1996. Mutagenicity Test Data of Existing Chemical Substances, January 1996. Japan Chemical Industry Ecology-Toxicology & Information Center, Japan.
Johansson I. Amides, fatty acid. 2001. In: Kirk-Othmer encyclopedia of chemical technology. Online version. John Wiley & Sons Inc. Vol. 2, p. 442-463.
Kamendulis LM, Klaunig JE. 2005. Species differences in the induction of hepatocellular DNA synthesis by diethanolamine. Toxicol Sci. 87(2):328-36.
Kelly MT, Blaise A, Larroque M. 2010. Rapid automated high performance liquid chromatography method for simultaneous determination of amino acids and biogenic amines in wine, fruit and honey. J Chromatogr A. 1217(47):7385-7392.
Kirby PE. 1980. Test for Chemical Induction of Mutation in Mammalian Cells in Culture – the L5178Y TK+/- Mouse Lymphoma Assay. Report No. 003-692-420-7; for The Procter and Gamble Company, Cincinnati, OH, USA; from EG&G Mason Research Institute, Rockville, MD, USA.
Kirman CR, Hughes B, Becker RA, Hays SM. 2016. Derivation of a No-significant-risk-level (NSRL) for dermal exposures to diethanolamine. Regul Toxicol Pharmacol. 76:137-51.
Kohri N, Matsuda T, Umeniwa K, Miyazaki K, Arita T. 1982. Development of assay method in biological fluids and biological fate of triethanolamine. Yakuzaigaku. 42(4):342-348.
Kraeling MEK, Bronaugh RL. 2003. In vitro absorption of triethanolamine through human skin. J Toxicol Cutan Ocul Toxicol. 22(3):137-145.
Kraeling MEK, Yourick JJ, Bronaugh RL. 2004. In vitro human skin penetration of diethanolamine. 2004. Food Chem Toxicol. 42(10):1553-1561.
Lakritz L, Spinelli AM, Wasserman AE. 1975. Determination of amines in fresh and processed pork. J Agric Food Chem. 23:344-346.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 6]. Ottawa (ON): Health Canada. [accessed 2018 May].
Lehman-McKeeman LD, Gamsky EA, Hicks SM, Vassallo JD, Mar MH, Zeisel SH.2002. Diethanolamine induces hepatic choline deficiency in mice. Toxicol Sci. 67(1):38-45.
Leung HW, Murphy SR. 1998. Developmental toxicity study in Sprague-Dawley rats by whole-body exposure to N,N-diethylethanolamine vapor. J Appl Toxciol. 18:191-196.
Leung HW, Kamendulis LM, Stott WT. 2005. Review of the carcinogenic activity of diethanolamine and evidence of choline deficiency as a plausible mode of action. Regul Toxicol Pharmacol. 43(3):260-271.
Liberacki AB, Neeper-Bradley TL, Breslin WJ, Zielke GJ. 1996. Evaluation of the developmental toxicity of dermally applied monoethanolamine in rats and rabbits. Fundam Appl Toxicol. 31(1):117-23.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43:279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, Re T, Renskers K, Scrafford C, Vater S. 2006. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44:2008-2018.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol 46:1516-1524.
Mader BT, Yu JZ, Xu JH, Li QF, Wu WS, Flagan RC, Seinfeld JH. 2004. Molecular composition of the water-soluble fraction of atmospheric carbonaceous aerosols collected during ACE-Asia. J Geophys Res- Atmos. 109:D06206-n/a.
Maekawa A, Onodera H, Tanigawa H, Furuta K, Kanno J, Matsuoka C, Ogiu T, Hayashi Y. 1986. Lack of carcinogenicity of triethanolamine in F344 rats. J Toxicol Environ Health. 19(3):345-357.
Manetta AC, Di Giuseppe L, Tofalo R, Martuscelli M, Schirone M, Giammarco M, Suzzi G. 2016. Evaluation of biogenic amines in wine: Determination by an improved HPLC-PDA method. Food Control. 62:351-356.
Mathews JM, Garner CE, Black SL, Mathews HB. 1997. Diethanolamine absorption, metabolism and disposition in rat and mouse following oral, intravenous and dermal administration. Xenobiotica. 27:733-746.
Mathews JM, deCosta K, Thomas BF. 1996. Lauramide diethanolamine absorption, metabolism, and disposition in rats and mice after oral, intravenous, and dermal administration. Drug Metab Dispos. 24(7):702-10.
Mayer HK, Fiechter G, Fischer E. 2010. A new ultra-pressure liquid chromatography method for the determination of biogenic amines in cheese. J Chromatogr A 1217(19):3251-3257.
Mayr CM, Schieberle P. 2012. Development of stable isotope dilution assays for the simultaneous quantitation of biogenic amines and polyamines in foods by LC-MS/MS. J Agric Food Chem. 60(12):3026-3032.
Miyazaki Y, Kondo Y, Shiraiwa M, Takegawa N, Miyakawa T, Han S, Kita K, Hu M, Deng ZQ, Zhao Y, Sugimoto N, Blake DR, Weber RJ. 2009a. Chemical characterization of water-soluble organic carbon aerosols at a rural site in the Pearl River Delta, China, in the summer of 2006. J Geophys Res Atmos. 114:14208.
Miyazaki Y, Aggarwal SG, Singh K, Gupta PK, Kawamura K. 2009b. Dicarboxylic acids and water-soluble organic carbon in aerosols in New Delhi, India, in winter: Characteristics and formation processes. J Geophys Res Atmos. 114:19206.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E. 1986. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen. 8 (Suppl 7):1-119.
Mrklas O, Bentley LR, Lunn SRD, Chu A. 2006. Principal component analyses of groundwater chemistry data during enhanced bioremediation. Water Air Soil Pollut. 169:395-411.
[MSDS] Material Safety Data Sheet. 2006. Lysol Brand II Disinfectant Spray, Crisp Linen (Trial Size Aerosol). Parsippany (NJ): Reckitt Benckiser North America, Inc. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2007f. Maja Gel De Bano Perfumado. San Juan (Puerto Rico): Puerto Rico Supplies Group Inc. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2007g. Mothers Leather Conditioner. Huntington Beach (CA): Mothers Polishes Waxes Cleaners. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2007h. Zero by Woolite For All Colours, Fabric Wash (Liquid) – Canadian. Parsippany (NJ): Reckitt Benckiser North America, Inc.”. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2008a. Mr. Clean Multi Surfaces Antibacterial Spray (Orange and Lemon Scents). Cincinnati (OH): The Procter & Gamble Company. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2008f. Magenta color ink for Brother MFC 3100 and MFC 210C, MFC240. Mississauga (ON): Ko-Rec-Type. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2008g. Shampoo Dr. Cabello-Cabellos Teñidos. San Juan (PR): Puerto Rico Merchandising. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2009. Boat Clean Plus. Mississauga (ON): Aurora Marine Insustries Inc. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2010a. Deep Crystal Economy Car Wash. Irving (CA): Meguiar’s USA. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2010b. Coppertone Lotions (Low/No Oil). Pointe Claire (QC): Schering-Plough HealthCare Products Canada. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2010c. Hero Liquid Laundry Detergent. Oakville (ON): Jempak GK Inc. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2011a. Hertel Multi – Ultra Concentrated Cleaner. Montreal (QC): Lavo Inc. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2011b. Lysol Antibacterial Hand Soap No Touch Kitchen System. Parsippany (NJ): Reckitt Benckiser LLC. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2012a. Sunlight Liquid Laundry Detergent 3X - Green Clean Citrus Fresh 946mL. Wilton (CT): Sun Products Corporate Office. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2012b. Coppertone QuickCover Lotion Sprays. Pointe Claire (QC): Merck. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2012c. LePage No More Nails Ultra Heavy Duty Adhesive. Mississauga (ON): Henkel Canada Corporation. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2012d. Liquid Dish Detergent and Antibacterial Hand Soap - premium all variants. Salt Lake City (UT): Sun Products Corporation. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2012e. Grime Eater Extra Strength Laundry Soil & Stain Remover. Mississauga (ON): Grime Eater Products Inc. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2012f. Stoneeffects Countertop Epoxy Coat 4X1.2L. Toronto (ON): Tremco Canada Division. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2013h. Hertel Multi All Purpose Cleaner. Montreal (QC): Lavo Inc. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2013j. Water Pump Lube. Mississauga (ON): Radiator Specialty Co., of Canada. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2013k. BBQ Grill Cleaner. Gurnee (IL): Weiman Products. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2014b. Trewax Gold Label High Gloss Sealer. Kennesaw (GA): Beaumont Products Inc. [accessed 2018 Jun 14].
[MSDS] Material Safety Data Sheet. 2014c. Lemon Suds. Acheson (AB): Sci-Tech Engineered Chemicals. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2014d. Tub-O-Towels. Cleveland (OH): Federal Process Corporation. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2015e. Micro-Gloss. Wilton (IA): Micro-Surface Finishing Products, Inc. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2015h. AN COL THALO BLU (TBL) 1793. Pittsburgh (PA): PPG Industries, Inc. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2015i. Mobil multi-vehicle ATF. Spring (TX): Exxon Mobil Corporation. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2015j. Micro-Gloss. Wilton (IL): Micro-Surface Finishing Products, Inc. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2015k. SA-8 Battery Cleaners 5 Oz. AE. Halton Hills (ON): ITW Permatex Canada. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2015l. Heavy-Duty Oven & Grill Cleaner. Atlanta (GA): Zep Commercial Sales & Service. [accessed 2017 Sep 22].
[MSDS] Material Safety Data Sheet. 2015m. Simple Green® Pro Grade Heavy-Duty Cleaner. Huntington Beach (CA): Sunshine Makers, Inc. [accessed 2018 Mar 1].
[MSDS] Material Safety Data Sheet. 2017c. Goo Gone Oven & Grill Cleaner. Gumee (IL): Goo Gone. [accessed 2018 Jun 13].
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2019 March 21]. Ottawa (ON): Health Canada. [accessed 2018 May].
NICNAS. 2009. Priority Existing Chemical Assessment Report No. 30 Triclosan [Internet]. Sydney (Australia): National Industrial Chemicals Notification and Assessment Scheme.
[NPRI] National Pollutant Release Inventory. 2015. NPRI Datasets. Ottawa (ON): Government of Canada. Search results for diethanolamine. [accessed 2017 Sep 15].
[NTP] National Toxicology Program. 1992. Toxicity studies of diethanolamine (CAS No. 111-42-2) administered topically and in drinking water to F344/N rats and B6C3F1 mice (Tech. Rep. Ser. No. 20; NIH Publication No. 92-3343), Department of Health and Human Services, Research Triangle Park, NC.
[NTP] National Toxicology Program (US). 1999a. NTP toxicology and carcinogenesis studies of diethanolamine (CAS No. 111-42-2) in f344/n rats and b6c3f1 mice (dermal studies). Natl Toxicol Program Tech Rep Ser. 478:1-212.
[NTP] National Toxicology Program (US). 1999b. NTP toxicology and carcinogenesis studies of lauric acid diethanolamine condensate (CAS No. 120-40-1) in F344/N rats and B6C3F1 mice (dermal studies). Natl Toxicol Program Tech Rep Ser. 480:1-200.
[NTP] National Toxicology Program (US). 1999c. NTP toxicology and carcinogenesis studies of oleic acid diethanolamine condensate (CAS No. 93-83-4) in F344/N rats and B6C3F1 mice (dermal studies). Natl Toxicol Program Tech Rep Ser. 481:1-198.
[NTP].National Toxicology Program (US). 1999d. NTP toxicology and carcinogenesis studies of triethanolamine (CAS No. 102-71-6) in F344/N rats and B6C3F1 mice (dermal studies). Natl Toxicol Program Tech Rep Ser. 449:1-298.
[NTP] National Toxicology Program (US). 2001. NTP Toxicology and carcinogenesis studies of coconut oil acid diethanolamine condensate (CAS No. 68603-42-9) in F344/N rats and B6C3F1 mice (dermal studies). Natl. Toxicol. Program Tech. Rep. Ser. 479:5-226.
[NTP] National Toxicology Program (US). 2004. NTP toxicology and carcinogenesis studies of triethanolamine (CAS No. 102-71-6) in B6C3F1 mice (dermal studies). Natl Toxicol Program Tech Rep Ser. 518:5-163.
Ockerman HW. 1978. Source Book for Food Scientists (1978). AVI Publishing Co., Inc., Westport, CT. p. 471.
[OECD] Organisation for Economic Co-operation and Development. 1995. Guidelines for the Testing of Chemicals. TG 105: Water Solubility. OECD, Paris [OECD] Organisation for Economic Co-operation and Development. 2002. SIDS initial assessment report: 2-Diethylaminoethanol: CAS No. 100-37-8. SIAM [SIDS Initial Assessment Meeting] 15; 2002 October; Boston, USA. [accessed 2017 12 15].
[OECD] Organisation for Economic Co-operation and Development. 2007a. SIDS initial assessment report: 2,2′-Iminodiethanol: CAS No. 111-42-2. SIAM [SIDS Initial Assessment Meeting] 24; 2007 April; Paris, France. [accessed 2017 Dec 15].
OECD] Organisation for Economic Co-operation and Development. 2007b. Revised Guidelines for the Testing of Chemicals. TG 105: Water Solubility. OECD, Paris.
[OECD] Organisation for Economic Co-operation and Development. 2008. SIDS Data Set 2,2′-Iminodiethanol: CAS No. 111-42-2.
[OECD] Organisation for Economic Co-operation and Development. 2009. SIDS initial assessment report: 1,1′,1″-nitrilotripropan-2-ol (TIPA). OECD HPV Chemical Programme, SIDS Dossier approved at SIAM 29, 20-23 October 2009. [accessed 2017 Dec 15].
OECD QSAR Toolbox. [read across tool]. 2016. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Pedersen EK, Bjørseth O, Syversen T, Mathiesen M. 2002. Emissions from heated indoor dust. Environ Int. 27:579-587.
Pittermann W. 1994. Embryotoxicity Study (Including Teratogenicity) in the Rat (Segment II). Report number RT920403. Henkel KGaA, Duesseldorf, Germany.
Preti R, Antonelli ML, Bernacchia R, Vinci G. 2015. Fast determination of biogenic amines in beverages by a core-shell particle column. Food Chem. 187:555−562.
Price CJ, Marr MC, Myers CM, Jahnke GD.2005. Postnatal development of rat pups after maternal exposure to diethanolamine. Birth Defects Res. B Dev Reprod Toxicol. 74:243-254.
Rasmussen T, Anthoni U, Christophersen C, Nielsen PH. 1993. Volatile compounds from the marine indicator organism Mytilus edulis. Chemosphere. 27:2123-2125.
Redruello B, Ladero V, del Rio B, Fernández M, Martin MC, Alvarez MA. 2017. A UHPLC method for the simultaneous analysis of biogenic amines, amino acids and ammonium ions in beer. Food Chem. 217:117-124.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006a. Cosmetics fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4 [PDF]. Bilthoven (NL): RIVM. Report No.: 320104001/2006.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006b. General Fact Sheet: Limiting conditions and reliability, ventilation, room size, body surface area. Updated version for ConsExpo 4 [PDF]. Bilthoven (NL): RIVM Report No.: 320104002/2006.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007. Paint products fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4 [PDF]. Bilthoven (NL): RIVM. Report No.: 320104008/2007.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2018. Cleaning products fact sheet: default parameters for estimating consumer exposure (updated version 2018) [PDF]. Bilthoven (NL): RIVM. Report No.: 2016-0179.
[RSI] Risk Sciences International. 2017. Addressing human health hazard data gaps through the use of read-across for a group of alkanol amides. Final report. Ottawa (ON): Health Canada. [restricted access].
Schulte KE, Dreymann H, Möllmann H. 1972. Resorption, Verteilung in den Organen und Metabolisierung von Diäthylaminoäthanol nach oraler Applikation an Ratten. Arzneim-Forsch. 22:1381-1390.
Sci-Tech Inc. 2013. Lemon Suds Technical Data Sheet (Product # 0206). Acheson (AB): Sci-Tech Engineered Chemicals. [accessed 2018 Jun 6].
[SCCS] Scientific Committee on Consumer Safety. 2010. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation, 7th Revision.
[SCCS] Scientific Committee on Consumer Safety. 2012. The SCCS Notes of Guidance for the Testing of Cosmetic Substances and their Safety Evaluation, 8th Revision. European Commission. Report No. SCCS/1501/12. [2018 May].
[SCCS] Scientific Committee on Consumer Safety. 2015. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation, 9th Revision. European Commission. Report No. SCCS/1564/15, Revised version of 25 April 2016. [accessed 2018 May 31].
Sidransky H, Farber E. 1960. Liver choline oxidase activity in man and in several species of animals. Arch Biochem Biophys. 87:129-33.
Simoneit BRT, Oros DR, Elias VO. 2000. Molecular tracers for smoke from charring/burning of chitin biopolymer. Chemosphere Glob Change Sci. 2:101-105.
SimpleTreat [estimation model]. 2015. Ver. 4.0.8. Bilthoven (Netherlands): National Institute of Public Health and the Environment (RIVM).
Statistics Canada. 2012. Custom tabulation of grooming products data from the Canadian Health Measures Survey Cycle 1 (2007-2009). Prepared for Existing Substances Risk Assessment Bureau, Health Canada by Statistics Canada. Unpublished report.
Statistics Canada. 2015. Canadian Community Health Survey (CCHS) 2015 Share File. Ottawa, ON. Canada. Estimates generated using SAS 9.3 [GRID] through SAS EG 5.1.
Statistics Canada. [modified 2017]. Canadian International Merchandise Trade Database (CIMT). Ottawa (ON): Government of Canada. [accessed 2017 Nov 29].
Stott WT, Bartels MJ, Brzak KA, Mar M, Markham DA, Thornton CM, Zeisel SH. 2000a. Potential mechanisms of tumorigenic action of diethanolamine in mice. Toxicol Lett. 114(1-3):67-75.
Stott WT, Waechter JM, Rick DL, Mendrala AL. 2000b. Absorption, distribution, metabolism and excretion of intravenously and dermally administered triethanolamine in mice. Food Chem Toxicol. 38(11):1043-51.
Stott WT, Radtke BJ, Linscombe VA, Mar MH, Zeisel SH. 2004. Evaluation of the potential of triethanolamine to alter hepatic choline levels in female B6C3F1 mice. Toxicol Sci. 79(2):242-7.
Strittholt CA, McMillan DA, He T, Baker RA, Barker ML 2016. A randomized clinical study to assess ingestion of dentrifrice by children. Regul. Toxicol Pharmacol. 75:66-71.
Sun JD, Beskitt JL, Tallant MJ, Frantz SW. 1996. In vitro skin penetration of monoethanolamine and diethanolamine using excised skin from rats, mice, rabbits, and humans. J Toxicol: Cutan Ocul Toxicol. 15(2):131-146.
Thabuis C, Tissot-Favre D, Bezelgues JB, Martin JC, Cruz-Hernandez C, Dionisi F, Destaillats F. 2008. Biological functions and metabolism of oleoylethanolamide. Lipids. 43:887-894.
Thiagar A. 1982. Cytogenicity Study – Chinese Hamster Ovary (CHO) Cells In vitro. Study No. T1807.338; for The Procter & Gamble Company, Cincinnati, OH, USA; from Microbiological Associates Inc., Bethesda, MD, USA.
TSCATS (1990), OTS 0530455, New Doc. I. D. 88-900000214, containing a report dated December 30, 1966 (1 year dog study) and July 31, 1967 (2 year rat study) for Pennsalt Chem. Corp., Date Produced 8/02/90, Atochem N. America, Inc.
[US EPA] U.S. Environmental Protection Agency. 2010a. Screening-level hazard characterization, fatty nitrogen derived (FND) amides category.
[US EPA] U.S. Environmental Protection Agency. 2010b. Screening-level hazard characterization, fatty nitrogen derived (FND) amines category.
[US EPA] United States Environmental Protection Agency. 2011. Exposure Factors Handbook. 2011 Edition. Washington (DC): U.S. Environmental Protection Agency, National Centre for Environmental Assessment.
[US EPA] US Environmental Protection Agency. 2015. EPA’s 2011 national-scale air toxics assessment. Washington (DC): US Environmental Protection Agency. [accessed 2018 Jun 27].
van Gorsel H, Li C, Kerbel EL, Smits M, Kader AA. 1992. Compositional characterization of prune juice. J Agric Food Chem. 40(5):784-789.
Wang Y, Ye D, Zhu B, Wu G, Duan C. 2014. Rapid HPLC analysis of amino acids and biogenic amines in wines during fermentation and evaluation of matrix effect. Food Chem. 163:6-15.
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Hum Ecol Risk Assess. 19(1):158-188.
Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. 2006. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 26(3):803-824.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48:3109-3119.
Yang H, Xu J, Wu W, Wan CH, Yu JZ. 2004. Chemical characterization of water-soluble organic aerosols at Jeju Island collected during ACE-Asia. Environ Chem. 1:13-17.
Yang H, Yu JZ, Ho SSH, Xu J, Wu W, Wan CH, Wang X, Wang X, Wang L. 2005. The chemical composition of inorganic and carbonaceous materials in PM2.5 in Nanjing, China. Atmos Environ. 39:3735-3749.
Zeiger E, Anderson B, Haworth S, Lawlor T. 1987. Mortelmans K, Speck W. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ Mutagen. 9 Suppl 9:1-109.
Zeisel, SH, Blusztajn, JK. 1994. Choline and human nutrition. Annu Rev Nutr. 14:269-296.
Zeisel SH, da Costa KA. 2009. Choline: An essential nutrient for public health. Nutr Rev. 67(11):615-623.
Zha X1, Jay FT, Choy PC. 1992. Effects of amino acids and ethanolamine on choline uptake and phosphatidylcholine biosynthesis in baby hamster kidney-21 cells. Biochem Cell Biol. 70(12):1319-24.
Zhang Q, Anastasio C. 2003. Free and combined amino compounds in atmospheric fine particles (PM 2.5) and fog waters from Northern California. Atmos Environ. 37:2247-2258.
Appendices
Appendix A. Read-across for LME, CDE and LDE
| - | CME | SME | OMIPA | LME |
|---|---|---|---|---|
| Role | Analogue | Analogue | Analogue | Target |
| CAS # | 68140-00-1 | 111-57-9 | 111-05-7 | 142-78-9 |
| Chemical structure |  |
 |
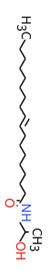 |
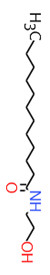 |
| Water solubility (mg/L) | 43.9a | 1b | 0.02-0.75c | 43.9d,pred |
| log Kow | 3.24a,pred | 5.55b,pred | 6.39c,pred, | 3.24d,pred |
| Vapour pressure (mm Hg) | 1.37E-07a | 1.4E-09b | 1.82E-08c | 6.57E-09d,pred |
| Repeat dose toxicity (oral) | NOAEL = 750 mg/kg bw/day (4-wk rat, by gavage; no treatment-related at high tested dose) (CIR 2015b) | NA | NA | NA |
| Repeat dose toxicity (dermal) | NA | NOAEL=2000 mg/kg bw/day (rabbits, no systemic effects) (CIR 2015b) | NA | NA |
| Repeat dose toxicity (inhalation) | NA | NA | NA | NA |
| Reproductive and/or develop-mental toxicity (oral) | NA | NA | NOAEL= 1000 mg/kg bw/day (combined repeated dose toxicity study with reproductive/ developmental toxicity screening test, rats, by gavage, no systemic, reproductive or developmental toxicity at the highest tested dose) (ECHA c2013-2017) | NA |
| Genetic toxicity | NA | NA | In vitro genotoxicity negative (ECHA c2013-2017) | NA |
Abbreviation: Kow, octanol–water partition coefficient; NA, No Available; pred, predicted.
a The Good Scents Company Information System
b ECHA c2013-2018b
c ECHA c2013-2017
d ChemIDplus1993-.
| Chemical name | ODE | LDE | CDE |
|---|---|---|---|
| Role | Analogue | Target | Target |
| CAS# | 93-83-4 | 120-40-1 | 68603-42-9 |
| DEA as an impurity | 0.19% (NTP1999c) | 0.83% (NTP1999b) | 18.2% (NTP 2001) |
| Chemical structure |  |
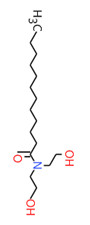 |
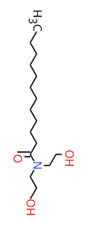 |
| Water solubility (mg/L) | 1a | 226b,pred | 226c,pred |
| Log Kow | >6a | 2.89b,pred | 2.89c,pred |
| Vapour pressure (mm Hg) | 4.5E-07a | 6.70E-09b,pred | 6.70E-09c,pred |
| Metabolism | NA | In rats administered purified LDE, no unchanged LDE, DEA or DEA-derived metabolites were detected, but recovered intermediate products formed after ω- aNA/or ω-1 to 4 hydroxylation (Mathews et al. 1996) |
NA |
| Repeat dose toxicity (dermal) | LOAEL = 50 mg/kg bw/day (13-week dermal study, lowest dose tested, mice; kidney and liver effects, NTP 1999c) | NOAEL = 50 mg/kg bw/day (14-week dermal study, rats, kidney toxicity, NTP 1999b) | NOAEL = 50 mg/kg bw/day (14-week dermal study, rats, kidney toxicity, NTP 2001) |
| Reproductive and/or develop-mental toxicity (oral) | NA | NA | NOAEL= 1000 mg/kg bw/day (no developmental toxicity at the highest tested dose in rats) (Pitterman 1994); % residual DEA not stated |
| Genetic toxicity | Negative (NTP 1999c) | Negative (NTP 1999b) |
Negative (NTP 2001) |
| Carcinogenicity | Negative (NTP 1999c) | Positive (NTP 1999b) | Positive (NTP 2001) |
Abbreviations: Kow, octanol–water partition coefficient; NA, Not Available; pred, predicted.
aECHA c2013-2018d
bChemIDplus 1993-;
cUVCB, properties based on LDE (C12) as typical homologue in mixture (ChemIDplus 1993-).
Appendix B. Environmental media air and soil concentrations and their corresponding human daily intakes and parameters used to estimate concentrations in drinking water
Concentrations reported in the literature were used to derive intake estimates from ambient air and soil where available. Otherwise, concentrations in ambient air and soil were estimated for the neutral/uncharged forms using ChemCAN (2003). For ChemCAN modelling, the Mixed Wood Plains region in Ontario was chosen as it is the most industrialized and populated area of Canada. As input to ChemCAN compartment specific half-lives were derived from EPI Suite (EPI Suite c2000-2017) and loading rates were determined by multiplying the EPI Suite Fugacity Level III partitioning mass % for air, water and soil by the kg quantity in commerce.
| - | MEA | DEEA | LME |
|---|---|---|---|
| Concentration in air (µg/m3) | 3.74 x 10-4(a) | 3.60 x 10-8 (b) | 1.21 x 10-7 (b) |
| Concentration in soil (µg/kg)c | 22.2 | 0.496 | 0.326 |
| Intake from ambient air, toddlers 0.5–4 yrs (mg/kg bw/d)d | 2.78 x 10-8 | 2.70 x 10-12 | 9.08 x 10-12 |
| Intake from indoor air, toddlers 0.5–4 yars (mg/kg bw/d)d | 1.94 x 10-7 | 1.9 x 10-11 | 6.35 x 10-11 |
| Intake from soil, toddlers 0.5–4 yrs (mg/kg bw/d)d | 2.01 x 10-8 | 4.48 x 10-10 | 2.94 x 10-10 |
a Zhang and Anastasio 2003. Dissolved/soluble fraction in PM2.5. Converted from pmol/m3.
b Represents Ontario Mixed Wood Plain - bulk air with degradation – ChemCan 2003, unless otherwise indicated.
c Represents Ontario Mixed Wood Plain - bulk soil with degradation – ChemCan 2003.
d Toddler, assumed to weigh 15.5 kg and to breathe 9.3 m3 of air per day (Health Canada 1998) and to ingest 14 mg of soil per day (Wilson et al. 2013).
| - | DEA | LDE | CDE | CADEA | TADEA |
|---|---|---|---|---|---|
| Concentration in air (µg/m3)a | 4.38 x 10-11 | 2.39 x 10-8 | 6.81 x 10-6 | 5.45 x 10-8 | 3.48 x 10-6 |
| Concentration in soil (µg/kg)b | 4.45 | 0.213 | 61.3 | 0.316 | 32.8 |
| Intake from ambient air, toddlers, 0.5–4 yrs (mg/kg bw/d)c | 3.3 x 10-15 | 1.79 x 10-12 | 5.11 x 10-10 | 4.09 x 10-12 | 2.61 x 10-10 |
| Intake from indoor air, toddlers, 0.5–4 yrs (mg/kg bw/d)c | 2.3 x 10-14 | 1.25 x 10-11 | 3.58 x 10-9 | 2.86 x 10-11 | 1.83 x 10-9 |
| Intake from soil, toddlers, 0.5–4 yrs (mg/kg bw/d)c | 4.02 x 10-9 | 1.92 x 10-10 | 5.54 x 10-8 | 2.85 x 10-10 | 2.96 x 10-8 |
a Represents Ontario Mixed Wood Plain - bulk air with degradation – ChemCan 2003, unless otherwise indicated.
b Represents Ontario Mixed Wood Plain - bulk soil with degradation – ChemCan 2003, unless otherwise indicated.
c Toddler, assumed to weigh 15.5 kg and to breathe 9.3 m3 of air per day (Health Canada 1998), and to ingest 14 mg of soil per day (Wilson et al. 2013).
| - | TEA | TIPA |
|---|---|---|
| Concentration in air (µg/m3)a | 2.15 x 10-12 | 1.78 x 10-12 |
| Concentration in soil (µg/kg)b | 7.00 | 1.51 |
| Intake from ambient air, toddlers, 0.5–4 yrs (mg/kg bw/d)c | 1.61 x 10-16 | 1.34 x 10-16 |
| Intake from indoor air, toddlers, 0.5–4 yrs (mg/kg bw/d)c | 0.00188 | 9.35 x 10-16 |
| Intake from soil, toddlers, 0.5–4 yrs (mg/kg bw/d)c | 6.32 x 10-9 | 1.36 x 10-9 |
a Represents Ontario Mixed Wood Plain - bulk air with degradation – ChemCan 2003, unless otherwise indicated.
b Represents Ontario Mixed Wood Plain - bulk soil with degradation – ChemCan 2003, unless otherwise indicated.
c Toddler, assumed to weigh 15.5 kg and to breathe 9.3 m3 of air per day (Health Canada 1998), and to ingest 14 mg of soil per day (Wilson et al. 2013).
Given the absence of surface water or drinking water monitoring data for DEEA, LME, DEA, LDE, CDE, CADEA, TADEA, TEA and TIPA, and the consumer use profile of these substances, a down-the-drain scenario was used to estimate concentrations in drinking water. The EAU Drinking Water Spreadsheet was used to derive the concentration of the substances in surface water for potential ingestion through drinking water (Health Canada 2015a) using the parameters in Table B-4.
| Parameter | DEEA | LME | DEA | LDE | CDE | CADEA | TADEA | TEA | TIPA |
|---|---|---|---|---|---|---|---|---|---|
Total annual usage (kg) | 347147 | 100000 | 4331373 | 69543 | 20000000 | 100000 | 11000000 | 4695027 | 1000000 |
Estimated removal (%) | 88a | 3a | 94b | 88a | 88a | 88a | 92a | 91c | 68a |
a Estimated using SimpleTreat 4.0.8 (2015).
b OECD 2007b (OECD 303A coupled-units simulation test).
c OECD 1995 (OECD 303A coupled-units simulation test).
Appendix C. MEA food exposure estimates
No Canadian occurrence data for MEA in food were available. Occurrence data from the scientific literature, where appropriate and relevant to the Canadian population, were therefore used for the current assessment. The highest arithmetic mean MEA concentration reported in the scientific literature or the mean concentration calculated using individual data points for a given food type was conservatively applied to represent the concentration of MEA in an entire food or beverage category (Table C-1). The mean MEA concentrations used in the present assessment ranged from 1.2 ppm in honey to 40.3 ppm in orange juice (Table C-1).
| Food category used in dietary exposure assessment | MEA concentration applied (ppm) | Food(s) on which the applied MEA concentration was based | Reference |
|---|---|---|---|
| Cheese | 13.0 | Hard cheeses | Mayer et al. 2010 |
| Yogurt | 4.86 | Yogurt, plain, 3.5% milk fat | Mayr and Schieberle 2012 |
| Chocolate | 7.22 | Chocolate, 75% cocoa | Mayr and Schieberle 2012 |
| Fruit - All types (all) | 1.56 | Unfermented grape must | Wang et al. 2014 |
| Honey | 1.2 | Rhododendron honey | Kelly et al. 2010 |
| Juice – citrus | 40.3* | Navel orange juice | Cerrillo et al. 2015 |
| Juice – prune | 3.4 | Prune juice | van Gorsel et al. 1992 |
| Juice – other | 2.45 | Apricot nectar | Preti et al. 2015 |
| Meat and poultry – cured | 13.4 | Italian salami | Mayr and Schieberle 2012 |
| Meat and poultry – regular | 8 | Pork (ham), cooked | Lakritz et al. 1975 |
| Fish - all types | 6.54 | Tuna, canned | Mayr and Schieberle 2012 |
| Vegetables – fermented | 13.01 | Sauerkraut | Mayr and Schieberle 2012 |
| Wine - red, including rosé and wine coolers | 38.07 | Italian red wine | Galgano et al. 2011 |
| Wine – white | 9.9 | Italian white wine | Manetta et al. 2016 |
| Beer | 8.4 | Dark Belgian ale | Redruello et al. 2017 |
*units converted from mg/L to µg/g (ppm) using the density of orange juice (1.048 g/mL)
Food consumption data from the most recently available Canadian Community Health Survey (CCHS) (Statistics Canada 2015) were employed. HC estimated dietary exposure to MEA from its natural presence in food by multiplying the arithmetic mean MEA concentration assumed for each food category by the quantity of that food reportedly consumed by each respondent in the survey. This yielded a full distribution of MEA exposure estimates for various age groups. Since many of the foods containing MEA are considered to be a regular part of the Canadian diet, the mean and 90th percentile estimates were calculated (Table C-2). These estimates are considered to be conservative.
| Age Group (years)a | Mean | 90th percentile |
|---|---|---|
| 1-3 | 0.20 | 0.47 |
| 4-8 | 0.18 | 0.47 |
| 9-13 | 0.10 | 0.25 |
| 14-18 | 0.08 | 0.22 |
| 19-30 | 0.08 | 0.20 |
| 31-50 | 0.07 | 0.18 |
| 51-70 | 0.07 | 0.19 |
| 71+ | 0.07 | 0.17 |
aMales and females combined
Appendix D. Parameters used to estimate human exposures from products available to consumer
Sentinel exposure scenario assumptions
Sentinel exposure scenarios were used to estimate the potential exposure to substances in the Alkanolamines and Fatty Alkanolamides Group. Scenario assumptions are summarized in Table D-1. Exposures were estimated using ConsExpo Web version or algorithms from the model (ConsExpo Web 2016). Exposures were estimated on the basis of default body weights of 70.9, 59.4, 31, 15.5 and 7.5 kg for adults (20 and older), teenagers (12 to 19 years), children (5 to 11 years), toddlers (6 months to 4 years) and infants (0 to 6 months), respectively (Health Canada 1998). For estimated potential exposures via the dermal route, dermal absorption was 1% for DEA, CDE, LDE and TEA. Otherwise, the determination of risk was based on dermal exposures. Unless specified, the defaults come from the relevant ConsExpo fact sheet for the scenario presented.
| Exposure scenario | Assumptions |
|---|---|
Permanent hair dye (adults 20+ yrs) | Concentration of MEA (30%), CDE (10%) (personal communication, e-mails from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced) Dermal, direct product contact – instant application |
All-purpose spray clceaner – spraying (adults 20+ yrs) | Concentration of MEA (1%), CDE (2%); TEA (10%) (MSDS 2008a, 2013h, 2015m) Frequency of use: 365/year Inhalation, exposure to spray Spray duration: 0.23 minutes Dermal, direct product contact – instant application |
Oven cleaner – trigger spray (adults 20+ yrs) | Concentration of MEA: 2.4% (MSDS 2017c) Frequency of use: 5/year Inhalation, exposure to spray – instantaneous release Room volume: 15 m3 Dermal, direct product contact – instant application |
Floor wax/polish (adults 20+ yrs) | Concentration of DEEA: 10% (MSDS 2014b) Frequency of use: 52/year Inhalation, exposure to vapour – evaporation Room volume: 58 m3 Dermal, direct product contact – instant application |
Body soap liquid (infants 0–6 months, toddlers 0.5–4 yrs, children 5–11 yrs, teenagers 12–19 yrs, adults 20+ yrs) | Concentration of LME (3%; infants and adults), LDE (10%; adults only), CDE (10%), CADEA (5%; children and adults), TEA (10%), (MSDS 2007f; personal communication, e-mails from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced) Dermal, direct product contact – instant application Product amount: 4.6 g/application (infants; Ficheux et al. 2016), 4.6 g/application (toddlers, Ficheux et al. 2016), 5.9 g/application (children, Ficheux et al. 2016), 11 g/application (teenagers and adults, Loretz et al. 2006) Frequency: 0.85 applications/day (infants and toddlers, Ficheux et al. 2015), 0.93 applications/day (children, Ficheux et al. 2015), 1.0 applications/day (teenagers, Ficheux et al. 2015), 1.4 applications/day (adults, Loretz et al. 2006) Exposed area (total body surface area minus head, except for infants include half the head; Health Canada 1995): 3 350 cm2 (infants), 4 910 cm2 (toddlers), 8 450 cm2 (children), 14 740 cm2 (teenagers), 16 925 cm2 (adults) Retention factor (ACI 2010): 0.01 |
Manual dishwashing liquid | Concentration of DEA: 5% (MSDS 2014c) Dermal, direct product contact during handwashing – instant application |
Waterborne wall paint – from colourant diluted in base | Concentration of DEA (in colourant concentrate): 1% (MSDS 2015h) Frequency of use: 2/year Dilution of colourant in paint base: 12.5x (i.e. 8%; RIVM 2007) Dermal, direct product contact (of mixed paint) |
Eye shadow (children 5-11 yrs, teenagers 12-19 yrs, adults 20+ yrs) | Concentration of TEA: 10% (personal communication, e-mails from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced) Dermal, direct product contact – instant application |
Face mask/pack (adults 20+ yrs) | CDE (10%) personal communication, e-mails from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced) Dermal, direct product contact – instant application |
Facial cleansers (children 5-11 yrs, teenagers 12-19 yrs, adults 20+ yrs) | CDE (30%) Personal communication, e-mails from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced) Dermal, direct product contact – instant application |
Hair spray – aerosol | Concentration of LDE (1%), TEA (3%) (personal communication, e-mails from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced) Frequency (Loretz et al. 2006): 1.49 applications/day Oral non-respirable spray model and inhalation exposure to spray – spraying towards person Room volume: 10 m3 |
Hair shampoo (toddlers 0.5–4 yrs, children 5–11 yrs, teenagers 12–19 yrs, adults 20+ yrs) | Concentration of CDE (30%), CADEA (5%), TEA (10%) (Personal communication, e-mails from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018, unreferenced; MSDS 2008g) Dermal, direct product contact – instant application |
Hair conditioner – leave-in (toddlers 0.5-4 yrs, children 5-11 yrs, teenagers 12-19 yrs, adults 20+ yrs) | Concentration of TEA: 3% (personal communication, e-mails from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced) Dermal, direct product contact – instant application |
Do-it-yourself automatic transmission fluid change | Concentration of TADEA: 25% (= weight fraction 0.25) (MSDS 2015i). Based on motor oil change Dermal exposure while changing your own transmission fluid in a personal vehicle Volume of product retained on one hand: Amount of product in contact with skin Short term (acute) dermal intake |
Pre-moistened wet tissues – all-purpose cleaning, application | Concentration of DEA: 1% (MSDS 2014d). Frequency of use: 88/year Inhalation, exposure to vapour – evaporation |
Toothpaste – non-fluoridated (toddlers 0.5-4 yrs and adults 20+ yrs) and whitening (adults 20+ yrs only) | Concentration of TEA: 3% non-fluoridated toothpaste, 10% whitening toothpaste (personal communication, e-mails from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced) Oral – direct exposure (ingestion) |
Body moisturizer/lotion (infants 0-6 months, | Concentration of TEA: 10% (personal communication, e-mails from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced) Dermal, direct product contact – instant application |
Face moisturizer/lotion (teenagers 12-19 yrs, adults 20+ yrs) | Concentration of TEA: 10% (personal communication, e-mails from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced) Dermal, direct product contact – instant application |
Sunscreen (toddlers 0.5-4 yrs, adults 20+ yrs) | Concentration of TEA: 10% (MSDS 2010b) Dermal, direct product contact – instant application |
Hair gel/wax/putty (children 5-11 yrs, teenagers 12-19 yrs, adults 20+ yrs) | Concentration of TEA (10%), TIPA (3%) (personal communication, e-mail from CHPSD, HC, to ESRAB, HC, dates ranging from July 2015 to June 2018; unreferenced) Dermal, direct product contact – instant application |
BBQ grill cleaner spray, application (spraying) | Concentration of TIPA: 5% (MSDS 2013k) Using ConsExpo 4.1 oven cleaner spray scenario (RIVM 2018) Frequency of use: 5/year Dermal, direct product contact – instant application |
All-purpose cleaning spray, application (spraying) | Concentration of TEA: 10% (MSDS 2015m) Frequency of use: 365/year Inhalation - exposure to spray Room volume: 15 m3 |
Lifetime average daily dose (LADD) and lifetime average daily air concentration (LADAC) assumptions and calculations
For the purpose of estimating the risk of cancer from exposure to DEA and TEA, lifetime average daily doses (LADD) and lifetime average daily air concentrations (LADAC) from use of certain products were calculated as follows.
LADD = [Sum of (daily exposure rate (mg/kg bw-d)age group × exposure duration (years)age group)] / Lifetime duration (= 70 years)
LADAC = [Sum of (year-averaged daily air concentration (mg/m3-d)age group × exposure duration (years)age group)] / Lifetime duration (= 70 years)
| Age group | Infant (0-0.5 yrs) |
Toddler (0.5-4 yrs) | Child (5-11 yrs) |
Teenager (12-19 yrs) | Adult 20+ (20-70 yrs) |
|---|---|---|---|---|---|
| Exposure duration (years) | 0.5 | 4.5 | 7 | 8 | 51 |
Appendix E. Estimated DEA dermal absorption values (excluding skin bound residuals) for use in the derivation of systemic critical effect levels
| Critical effect levela | Dermal absorption value (48 hours, excluding skin bound residuals)b | Estimated dermal absorption value (24 hours) | External critical effect levels (mg/kg bw /day, continuous) | Internal critical effect levels (mg/kg bw /day) |
|---|---|---|---|---|
| LOAEL = 32 mg/kg bw/day, in a 13-week repeated dose study in rats administered DEA in 95% ethanol by dermal application | 12% in rats administered 23 mg/kg bw DEA in 95% ethanol by dermal application | 6% | 22.9 | 1.4 |
| LOAEL = 125 mg/kg bw/day, in a 2-week repeated dose study in rats administered DEA in 95% ethanol by dermal application | Approximately 57% [y=0.460x+4.14, established by Kirman et al. 2016, where the dose (x) = 125 mg/kg bw, the total dermal absorption (y) = 62%. To account for the maximum skin bound residuals of 5% (Mathews et al. 1977), the dermal absorption excluding skin bound residuals at 57% (62% - 5%) was estimated] | 29% | 125 | 36.3 |
| BMDL10 = 1.46 mg/kg bw/day, derived from two-year cancer studies in mice exposed to DEA in 95% ethanol via dermal application | 23% in mice administered 8 mg/kg bw DEA in 95% ethanol by dermal application | 12% | 1.04 | 0.12 |
a NTP 1992, 1999a;
b Mathews et al. 1997.
| Product (% DEA) | Dermal Absorption (%) at 24 hours |
|---|---|
| 0.092 or 0.28% DEA shampoos | 0.08 |
| Hair dyes (0.61% DEA) | 0.09 |
| Body lotions (0.02% DEA) | 0.90 |
a Shampoo, hair dye, and body lotion remained on the skin for 5 minutes, 30 minutes and 24 hours, respectively (Kraeling et al. 2004).
| Rats - Applied dermal dose (mg/kg bw) | Rats - Dermal absorption (%, excluding skin bound residuals) at 48 hours | Mice - Applied dermal dose (mg/kg bw) | Mice - Dermal absorption (%, excluding skin residuals) at 48 hours |
|---|---|---|---|
| 2.1 | 2 | 8 | 23 |
| 7.6 | 6 | 23 | 31 |
| 27.6 | 12 | 81 | 56 |
a Mathews et al. 1997
The estimation of systemic dermal critical effect levels: Internal doses for critical effect levels were determined (Table E-1) to consider differences in dermal absorption between humans (Tables E-2) and rodents (Table E-3). It was observed that dermal absorption values were increased with doses administered in mice, as follows: 2% (2.1 mg/kg bw), 6% (7.6 mg/kg bw) and 12% (27.5 mg/kg bw) in 48 hours. As such, each critical effect level was compared to the closest applied dose in the Mathews et al. 1997 study to determine the most appropriate dermal absorption value.
The daily dermal LOAEL of 32 mg/kg bw/day was identified in a 3-month dermal study in rats exhibiting hematological effects (anemia) and kidney toxicity (increased kidney weights). As the critical effect level of 32 mg/kg was closest to the dose of 27.5 mg/kg bw, the dermal absorption of 12% was selected for this specific dose in 48 hours. The amount of DEA absorbed in 24 hours is more than half that absorbed in 48 hours (Kraeling et al. 2004). The dermal absorption in 24 hours was conservatively estimated to be 6%, as half of the dermal absorption (12%) at 48 hours. Therefore, the internal critical effect level is equivalent to 1.4 mg/kg bw/day when converted to a continuous exposure scenario and applied to dermal absorption in 24 hours.
For the per event dermal exposure scenario, the critical effect level LOAEL at 125 mg/kg bw/day was determined on the basis of increased absolute and relative kidney weights in a 2-week study in rats. Since the dermal absorption at the dose of 125 mg/kg bw was out of the range of test doses in the study of Mathews et al. 1997, the dermal absorption of 62% as total dermal absorption in 48 hours was estimated on the basis of the linear regression of absorption values as a function of dose (Kirman et al. 2016). The dermal absorption excluding skin bound residuals at 57% was estimated in accounting for the maximum skin bound residuals of 5% (Mathews et al. 1977). The dermal absorption of 29% in 24 hours in rats was conservatively considered as half the value (57%) in 48 hours. Thus, the internal point of departure of 36.3 mg/kg bw/day ( = 125 mg/kg bw/day x 29%) was estimated.
The dermal BMDL10 of applied residual DEA for the induction of liver tumours was determined to be 1.46 mg/kg bw/day (5 days a week), which is equivalent to an adjusted daily dose of 1.04 mg/kg bw/day (1.46 mg/kg bw/day x 5/7 days). It has been reported that the dermal absorption is 23% in mice administered DEA at 8 mg/kg bw/day (the closest dose to the BMDL) following 48-hour exposure (Mathews et al. 1997) with the same species (mice) and vehicle (95% ethanol) used in NTP dermal cancer studies (NTP 1999abc, 2001). DEA absorption in mice following 24 hours-hour dermal exposure was considered to be half that absorbed in 48 hours (23% x 0.5 = 12%). This dermal absorption of 12% was used in extrapolating the internal BMDL10 from the external BMDL10 of 1.04 mg/kg bw/day, resulting in the internal BMDL10 of 0.12 mg/kg bw/day (1.04 x 12%). The BMDL10 for the external oral dose was considered to be equivalent to the internal BMDL10 of 0.12 mg/kg bw/day by assuming 100% oral absorption in animals from route-to-route extrapolation.
Appendix F. Benchmark dose (BMD) modelling
| Substance | Residual DEA content | Test agent administered [Daily dose (mg/kg bw/day)] | DEA administered [Daily dose (mg/kg bw/day)] | Liver tumoursb | Reference |
|---|---|---|---|---|---|
| DEA | 100% | 0 40 80 160 |
0 40 80c 160 c |
72/100 97/100 100/100 99/100 |
NTP 1999a |
| CDE | 18.20% | 0 100 200 |
0 18.2 36.4 |
62/100 85/100 7/100 |
NTP 2001 |
| LDE | 0.83% | 0 100 200 |
0 0.83 1.7 |
58/100 74/99 73/100 |
NTP 1999b |
| ODE | 0.19% | 0 15 39 |
0 0.029 0.074 |
62/99 71/100 70/100 |
NTP 1999c |
a Adapted from Kirman et al. 2016 (columns relating to liver tumours)
b Number of animals with liver tumours (including hepatocellular adenomas, carcinomas, and hepatoblastomas) in total number of observed animals (data in male and female mice were combined as no significant difference was observed between sexes).
c These groups were not used in the derivation of the BMD as all animals were affected and the incident rate of liver tumours reached 100%.

Long description
The BMD10 and BMDL10 (the 95% lower confidence limit of benchmark dose) for increased liver tumours in mice were determined to be 1.84 and 1.46 mg/kg bw/day, respectively, using the multistage cancer model in BMDS 2.5 (US EPA). The BMD10 of 1.84 mg/kg bw/day and BMDL10 of 1.46 mg/kg bw/day were derived using studies in which animals were exposed 5 days per week. The BMDL10 for continuous exposure (7 days per week) would be 1.04 mg/kg bw/day [1.46 mg/kg bw x (5/7)].

Long description
The doses of TEA applied to the skin of mice was plotted against the incidence of hepatocellular tumours (adenoma and carcinoma) using a multistage cancer model in BMDS 2.5 (US EPA). The BMD10 and BMDL10 were determined to be 60 and 42 mg/kg bw/day, respectively, and were derived using a study in which animals were exposed 5 days per week. The BMDL10 at 42 mg/kg bw/day for continuous exposure (7 days per week) would be 30 mg/kg bw/day [42 mg/kg bw x (5/7)].
