Draft screening assessment - Alkyl halides Group
Official title: Draft screening assessment - Alkyl halides Group
Chemical Abstracts Service Registry Numbers
74-96-4
75-00-3
106-94-5
156-60-5
Environment and Climate Change Canada
Health Canada
March 2022
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment on four substances referred to collectively under the Chemicals Management Plan as the Alkyl Halides Group. The Chemical Abstracts Service Registry Numbers (CAS RN Footnote 1 ), their Domestic Substances List (DSL) names and their common names are listed in the table below.
| CAS RN | DSL name | Common name |
|---|---|---|
| 74-96-4 | Ethane, bromo- | Bromoethane |
| 75-00-3 | Ethane, chloro- | Chloroethane |
| 106-94-5a | Propane, 1-bromo- | 1-bromopropane |
| 156-60-5b | Ethene, 1,2-Dichloro-, (E)- | Trans-1,2-Dichloroethene |
a This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
b This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was determined to be a priority as a result of Identification of Risk Assessment Priorities (IRAP).
All of the substances in the Alkyl Halides Group are synthesized commercially. In addition, bromoethane and 1-bromopropane are naturally occurring. According to the information submitted in response to a survey under section 71 of CEPA, 1 000 kg to 10 000 kg of 1-bromopropane was reported to be manufactured in Canada in 2008. No manufacturing activity was reported for the other substances. Bromoethane, chloroethane and 1-bromopropane were reported to be imported in Canada in total quantities up to 1 000 000 kg in 2008, and trans-1,2-dichloroethene in a quantity of 382 744 kg in 2011. Three of the four substances in the Alkyl Halides Group, chloroethane, 1-bromopropane, and trans-1,2-dichloroethene, may be found in a number of products available to consumers, including liquid or aerosol cleaners or degreasers, aerosol starting fluids (engine starting aid), air conditioning refrigerant flush, silicone mold release spray, and spray foam insulation.
The ecological risks of substances in the Alkyl Halides Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate, or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, substances in the Alkyl Halides Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from four substances in the Alkyl Halides Group. It is proposed to conclude that bromoethane, chloroethane, 1-bromopropane and trans-1,2-dichloroethene do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The risk to human health for substances of the Alkyl Halides Group was characterized on the basis of available health effects and exposure information. Assessments from the World Health Organization, the Organization for Economic Cooperation and Development, the International Agency for Research on Cancer, as well as the United States Environmental Protection Agency were used to inform the health effects characterization in this screening assessment.
The general population of Canada may be exposed to bromoethane primarily from indoor and ambient air. Bromoethane is not anticipated to be found in products available to consumers. For bromoethane, critical effects were considered to be olfactory epithelium respiratory metaplasia of the nasal cavity and cancer. Based on the anticipated incremental lifetime cancer risk from exposure through air, cancer is not anticipated to present a health risk of concern. Margins between estimates of exposure and the critical effects observed in animal studies are considered to be adequate to address uncertainties in the health effects and exposure databases for the non-cancer endpoint.
The general population of Canada may be exposed to chloroethane primarily from indoor and ambient air, and from the use of starting fluid spray. For chloroethane, critical effects were determined to be developmental toxicity and cancer. Based on the anticipated incremental lifetime cancer risk from exposure through air, cancer is not anticipated to present a health risk of concern. Margins between estimates of exposure and the critical effects observed in animal studies are considered to be adequate to address uncertainties in the health effects and exposure databases for the non-cancer endpoint.
The general population of Canada may be exposed to 1-bromopropane primarily from indoor air, and from the use of silicone mold release spray, electronic cleaner spray, and automotive air conditioning (A/C) flush, with the primary route of exposure being inhalation. For 1-bromopropane, critical effects were determined to be cancer, developmental toxicity, and neurotoxicity. Based on the anticipated incremental lifetime cancer risk from exposure through air, cancer is not anticipated to present a health risk of concern. Margins between estimates of exposure and the critical effects observed in animal studies are considered to be potentially inadequate to address uncertainties in the health effects and exposure databases for the non-cancer endpoint. Specifically, the margins between estimate of exposure and the developmental endpoints from the use of silicone mold release spray, electronic cleaner spray, and automotive A/C flush are anticipated to present a health risk.
The general population of Canada may be exposed to trans-1,2-dichloroethene primarily from indoor and ambient air, and from the use of textile spot cleaners. For trans-1,2-dichlorothene, immunotoxicity was considered to be the critical effect for chronic exposure, and developmental effects were considered to be the critical effect for acute exposures. Margins between estimates of exposure and critical effects observed in animal studies are considered to be adequate to address uncertainties in the health effects and exposure databases for non-cancer endpoints.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that bromoethane, chloroethane and trans-1,2-dichloroethene do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that 1-bromopropane meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that 1-bromopropane meets one or more of the criteria set out in section 64 of CEPA, and that bromoethane, chloroethane and trans-1,2-dichloroethene do not meet any of the criteria set out in section 64 of CEPA.
It is also proposed to conclude that 1-bromopropane meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment on four of eight substances, referred to collectively under the Chemicals Management Plan as the Alkyl or Aryl Halides Group, to determine whether these four substances present or may present a risk to the environment or to human health. Three of these four substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. One substance in this group, trans-1,2-dichloroethene (CAS RN 156-60-5), did not meet categorization criteria; however, it was included in this assessment because it was determined to be a priority as a result of the approach described for Identification of Risk Assessment Priorities (IRAP), based on a large volume in commerce and a low reference dose (RfD) (ECCC 2009). IRAP is an approach developed by ECCC and HC and consists of a cyclical process by which both departments compile new information on specific substances, evaluate this information, and determine if further action on the substances may be warranted (ECCC, HC 2015).
The other four substances (Table 1‑1) are Aryl Halides and were considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016), and in either the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Science Approach Document (Health Canada 2016), or via the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018a), and were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this report. Conclusions for these four substances are provided in the assessments listed below (Table 1-1). The four substances addressed in this screening assessment will hereinafter be referred to as the Alkyl Halides Group.
| CAS RN | Domestic Substances List name | Approach under which the substance was addressed | References |
|---|---|---|---|
| 74-88-4 | Methane, iodo- | ERC/Rapid Screening | ECCC, HC 2018a |
| 77-47-4 | 1,3-Cyclopentadiene, 1,2,3,4,5,5-hexachloro- | ERC/TTC | ECCC, HC 2018b |
| 126-99-8 | 1,3-Butadiene, 2-chloro- | ERC/Rapid Screening | ECCC, HC 2018a |
| 630-20-6 | Ethane, 1,1,1,2-tetrachloro- | ERC/Rapid Screening | ECCC, HC 2018a |
The ecological risks of substances in the Alkyl Halides Group were characterized using the ERC approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
For the human health risk assessment, the four substances in the Alkyl Halides Group were considered separately. Substances in the Alkyl Halides Group currently being evaluated have been reviewed internationally through the Organization for Economic Cooperation and Development (OECD) High Production Volume (HPV) Chemicals Programme Screening Information Data Set (SIDS) Initial Assessment Reports (SIARs), the World Health Organization (WHO) International Programme of Chemical Safety Program, the United States Environmental Protection Agency (US EPA), and by the International Agency for Research on Cancer (IARC). These assessments undergo rigorous review (including peer-review) and endorsement. The WHO Concise International Chemical Assessment Document (CICAD), OECD SIDS SIARs, IARC monographs, as well as the US EPA assessments were used to inform the health effects characterization in this screening assessment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses, and exposures, including additional information submitted by stakeholders. Relevant data were identified up to March 2019. However, more recent studies or information provided via internal and external peer consultation may also be cited. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external peer review and as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this draft screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The CAS RN, Domestic Substances List (DSL) names, common names, and acronyms for the individual substances in the Alkyl Halides Group are presented in Table 2‑1.
| CAS RN | DSL name (common name) | Chemical structure and molecular formulaa | Molecular weight (g/mol)a |
|---|---|---|---|
| 74-96-4 | Ethane, bromo- (bromoethane) | 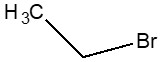 C2H5Br
C2H5Br
|
108.97 |
| 75-00-3 | Ethane, chloro- (chloroethane) | 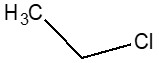 C2H5Cl
C2H5Cl
|
64.51 |
| 106-94-5 | Propane, 1-bromo- (1-Bromopropane) | 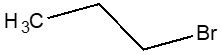 C3H7Br
C3H7Br
|
122.99 |
| 156-60-5 | Ethene, 1,2-dichloro-, (E)- (trans-1,2-dichloroethene) | 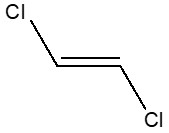 C2H2Cl2
C2H2Cl2
|
96.94 |
a Information is from ChemIDplus (2018).
3. Physical and chemical properties
A summary of the key physical and chemical properties of the substances in the Alkyl Halides Group are presented in Table 3‑1. Additional physical and chemical properties are reported in ECCC (2016b).
| Common Name | Vapour pressure (Pa)a,b,c | Henry’s law constant (Pa·m3/mol)a,b,c | Water solubility (mg/L)a,b,c | Log Kow (dimensionless)a,b |
|---|---|---|---|---|
| Bromoethane | 62 262 | 750d | 9000 | 1.61 |
| Chloroethane | 134 389e | 1125f | 6710 | 1.43 |
| 1-bromopropane | 14 772e | 742d,e | 2450e | 2.1 |
| Trans-1,2-dichloroethene | 44 130d | 950f | 4520 | 2.09 |
Abbreviation: Kow, octanol-water partition coefficient
a Data from PhysProp (2013)
b Based on experimental data, unless otherwise noted
c At 25°C, unless otherwise noted
d Value was extrapolated based on experimental measurement outside of the temperature range of the reported value
e Data from US EPA (2020); at 20°C
f At 24°C
4. Sources and uses
Bromoethane and 1-bromopropane are naturally occurring. Bromoethane and 1-bromopropane are produced by algae (HSDB 2002, 2013). Bromoethane can also be released to the environment from volcanic gases (HSDB 2002). All of the substances in the Alkyl Halides Group are synthesized commercially and have been included in surveys issued pursuant to section 71 of CEPA (Canada 2009, 2012). Table 4‑1 presents a summary of information reported on the total manufacture and total import quantities for the Alkyl Halides Group.
| Common name | Total manufacturea (kg) | Total importsa (kg) | Reporting year | Survey reference |
|---|---|---|---|---|
| Bromoethane | NR | 10 000 - 100 000 | 2008 | Canada 2009 |
| Chloroethane | NR | 100 000 - 1 000 000 | 2008 | Canada 2009 |
| 1-bromopropane | 1 000 - 10 000 | 105 600 - 256 200 | 2008 | Canada 2009 |
| Trans-1,2-dichloroethene | NR | 382 744 | 2011 | Canada 2012 |
Abbreviations: NR, Not reported above the reporting threshold of 100 kg.
a Values reflect quantities reported in response to CEPA section 71 surveys (Canada 2009, 2012). See surveys for specific inclusions and exclusions (schedules 2 and 3).
In the US, the ChemView website reported the national production volume (2015 reporting year) for 1-bromopropane as between 10 000 000 and 50 000 000 pounds (approximately 4 500 000 to 22 500 000 kg), and between 1 000 000 and 10 000 000 pounds (approximately 450 000 to 4 500 000 kg) for trans-1,2-dichloroethene (US EPA 2018a).
Table 4‑2 presents a summary of the major uses (including commercial and consumer uses) of the Alkyl Halides Group according to information reported pursuant to CEPA section 71 surveys (Canada 2009, 2012).
| Major usesa | Bromoethane | Chloroethane | 1-bromopropane | Trans-1,2-dichloroethene |
|---|---|---|---|---|
| Cleaning and furnishing care | N | N | Y | Y |
| Munitions | N | N | Y | N |
| Lubricant and greases | N | N | Y | N |
| Industrial intermediate | Y | N | N | N |
| Petrochemical process moderator | N | Y | N | N |
| Foam insulation | N | N | N | Y |
| Medical devices | N | N | N | Y |
| Degreaser | N | N | Y | N |
| Cleaner | N | N | Y | N |
| Automotive care | N | N | Y | N |
Abbreviations: Y, yes this use was reported for this substance; N, no this use was not reported for this substance
a Non-confidential uses reported in response to CEPA section 71 surveys (Canada 2009, 2012). See surveys for specific inclusions and exclusions (schedules 2 and 3).
On the basis of publically available product material safety data sheets (MSDSs) and other publically available information, no product available to consumers in Canada was identified for bromoethane. Chloroethane may be found in starting fluid (MSDS 2017a); 1-bromopropane may be found in silicone mold release spray (MSDS 2016a), electronic cleaner spray (MSDS 2017b) and automotive air conditioning (A/C) flush (MSDS 2017c) as a solvent; and trans-1,2-dichloroethene may be found in powdered spot cleaner for textiles (MSDS 2017d), cleaner or degreaser for electrical equipment or electronic parts (MSDS 2010, 2014), A/C refrigerant flush (MSDS 2015a) and spray foam insulation (MSDS 2016b, 2016c). 1-Bromopropane may also be used in commercial settings for dry cleaning (US EPA 2020), and living in close proximity to a dry cleaner may also present a potential route of exposure.
5. Environmental fate and behaviour
According to models used in ERC (ECCC 2016b), bromoethane and 1-bromopropane are expected to persist in air but not in water, sediment, or soil.
According to models used in ERC (ECCC 2016b), chloroethane and trans-1,2-dichloroethene are expected to persist in air, water, sediment and soil; however, given the high vapour pressure and Henry’s law constant of chloroethane and trans-1,2-dichloroethene, these substances are expected to volatilize to air from soil and water.
Given their low Kow and low bioconcentration factors (ECCC 2016b), bromoethane, chloroethane, 1-bromopropane and trans-1,2-dichloroethene are not expected to significantly bioaccumulate in organisms.
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risks of the substances in the Alkyl Halides Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization.
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), and from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate, or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity, and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Alkyl Halides Group, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
The hazard and exposure classifications for the four substances in the Alkyl Halides Group are summarized in Table 6‑1.
| Common Name | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Bromoethane | moderate | low | low |
| Chloroethane | moderate | high | moderate |
| 1-Bromopropane | moderate | low | low |
| Trans-1,2-dichloroethene | low | high | low |
According to information considered under ERC, bromoethane and 1-bromopropane were classified as having low exposure potentials. Bromoethane and 1-bromopropane were classified as having moderate hazard on the basis of structural alerts from OECD (Q)SAR Toolbox (2014) which identified these substances as being potential DNA and protein binders. Bromoethane and 1-bromopropane were classified as having a low potential for ecological risk. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of these substances. On the basis of current use patterns, bromoethane and 1-bromopropane are unlikely to be resulting in concerns for the environment in Canada.
According to information considered under ERC, chloroethane was classified as having a high exposure potential on the basis of a critically long half life in air and a large annual import quantity according to information reported in response to a CEPA section 71 survey (Environment Canada 2013). Chloroethane was classified as having a moderate hazard on the basis of structural alerts from OECD (Q)SAR Toolbox (2014 which identified this substance as being a potential DNA and protein binder. Chloroethane was classified as having a moderate potential for ecological risk. Given its overall classification as having a moderate potential for ecological risk, it is unlikely that this substance is resulting in concerns for the environment in Canada. As chloroethane is currently being used in high quantities in Canada, fluctuations in use patterns are unlikely to result in a significant increase in risk to the environment.
According to information considered under ERC, trans-1,2-dichloroethene was classified as having a high exposure potential on the basis of a critically long half life in air and a large annual import quantity according to information reported in response to a CEPA section 71 survey (Environment Canada 2013). Trans-1,2-dichloroethene was classified as having a low hazard potential and consequently was classified as having a low potential for ecological risk. Considering current use patterns, trans-1,2-dichloroethene is unlikely to be resulting in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Bromoethane
7.1.1 Exposure assessment
7.1.1.1 Environmental media
No reliable environmental monitoring data were identified for bromoethane in water, soil, dust, or food in Canada or elsewhere. Given its very high vapour pressure and Henry’s law constant, bromoethane is expected to volatilize to air from soil and water. On this basis, exposure to bromoethane from environmental media is expected to occur primarily from air.
Concentrations of bromoethane measured in ambient and indoor air in Canada are presented in Appendix A, and are summarized below.
Bromoethane was monitored by the National Air Pollution Surveillance (NAPS) program between 1991 and 2013, where mean ambient air concentrations measured from various sites across Canada ranged from not detected (laboratory detection limit [DL] = 0.043 to 0.058 µg/m3) to 0.068 µg/m3 and 95th percentile concentrations ranged from not detected to 0.050 µg/m3 (ECCC 2019). Ambient air concentrations for bromoethane were also measured in seven Canadian air quality studies conducted in Windsor, Regina, Halifax, Edmonton, Montreal, Sault-Ste-Marie and Ottawa (Health Canada 2010a, 2010b, 2010c, 2012, 2013, [Personal communication from Tim Shin and Ron Garson, WAQB, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Dales et al. 2013, Mallach et al. 2017)]). The geometric mean and 95th percentile bromoethane concentrations in ambient air in these Canadian studies were below the laboratory detection limit (DL = 0.022 to 0.074 µg/m3) in all of the studies.
Indoor air concentrations for bromoethane were also measured across the same seven Canadian studies referred to above, as well as in Swan Lake, Manitoba, as part of the First Nations Indoor Air Quality Study (FNIAS) (Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere [i.e., Weichenthal et al. 2012]). The geometric mean and 95th percentile bromoethane concentrations in indoor air were at the DL in all of the studies (0.074 µg/m3).
Personal exposure to bromoethane was also measured in a Windsor, Ontario air study (Health Canada 2010c). Personal air samples take into account exposures to bromoethane from both indoor and ambient air from various locations including the home, office and during transit. Bromoethane was not detected in all personal exposure samples collected in both winter and summer (DL = 0.070 µg/m3).
In regards to the treatment of results below the laboratory detection limit (DL) for Canadian air quality studies conducted in Halifax, Edmonton, Montreal, Sault-Ste-Marie, Ottawa (Health Canada 2010a, 2012, 2013, [Personal communication from Tim Shin and Ron Garson, WAQB, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Dales et al. 2013, Mallach et al. 2017)]), the values were used as reported in the statistical analysis. The results were considered valid as the retention time and target ions/qualifier ions in the gas chromatography–mass spectrometry (GC-MS) chromatogram met the analytical criteria of the laboratory. With respect to the Canadian air quality studies conducted in Regina and Windsor (Health Canada 2010b, 2010c), one-half of the DL was reported in the statistical analysis for values below the DL. In addition, when the laboratory did not observe any peak or target ions/qualifier ions in the GC-MS chromatogram, concentration of bromoethane was reported as zero as the quantitation criteria were not met. The results were considered valid and replaced by a near zero value (0.0001 µg/m3) or one-half of the corresponding MDL to calculate the descriptive statistics using log transformation.
The highest laboratory detection limit from the above studies was used to estimate general population exposure to bromoethane in air (i.e., 0.074 µg/m3) since the highest 95th percentile bromoethane concentration was below the DL.
7.1.1.2 Products available to consumers
No product available to consumers was identified for bromoethane; thus, exposure to bromoethane through products available to consumers is not expected to occur for the general population of Canada.
7.1.2 Health effects assessment
The WHO (2002) summarized the health effects literature and characterized the hazard for bromoethane. WHO (2002) was used to inform the health effects characterization in this screening assessment. IARC has classified bromoethane as Group 3 (not classifiable as to its carcinogenicity to humans) (IARC 1999). The European Chemicals Agency (ECHA) has classified bromoethane as Global Harmonisation System (GHS) Carc. 2 (Warning; H351: suspected of causing cancer) (ECHA 2018). A literature search was conducted from the year prior to WHO 2002 to March 2019. No health effects studies which could impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated in WHO 2002, NTP 1989, Great Lakes Chemical Corporation 2002 and IARC 1999) were identified.
Limited information is available on the toxicokinetics of bromoethane (WHO 2002). In animals, absorption can occur following oral, inhalation and dermal exposure; however, the extent to which bromoethane is absorbed is not known (Schwander 1936; Miller and Haggard 1943 as cited in WHO 2002). Bromoethane is distributed in animals at least to the brain and liver following inhalation (Leuze 1922; Abreu and Emerson 1940 as cited in WHO 2002). Most absorbed bromoethane may be eliminated unchanged via exhaled air, urine, or faeces. Early studies indicated that debromination and glutathione conjugation may occur (Heppel and Porterfield 1948; Thomson et al. 1958; Barnsley et al. 1964; Johnson 1965; Jones 1973 as cited in WHO 2002).
No formal reproductive toxicity studies have been carried out in animals; however, the occurrence of severe testicular atrophy in all male rats (F344/N) exposed for 14 weeks to 7 200 mg/m3 in a 14-week inhalation study indicates a potential for effects on male fertility (WHO 2002). Therefore, a no-observed-adverse-effect-concentration (NOAEC) of 3600 mg/m3 and lowest-observed-adverse-effect concentration (LOAEC) of 7200 mg/m3 are retained for the reproductive toxicity endpoint (Roycroft 1989 as cited in WHO 2002).
Bromoethane is considered to be genotoxic in the Ames assay, with and without metabolic activation (WHO 2002). Bromoethane is mutagenic in an in vitro Sister chromatid exchange assay using Chinese Hamster Ovary cells in culture, with and without metabolic activation. Overall, WHO and IARC concluded there is concern for genotoxicity, but it is not currently possible to reliably quantify the level of risk to human health (WHO 2002). No in vivo genotoxicity data in either animals in laboratory or humans were available.
An inhalation carcinogenicity study was conducted in both rats (F344/N) and mice (B6C3F1) via the inhalation route of exposure. Test animals were exposed for two years to 0, 450, 900 and 1 800 mg/m3 (6 hours/day, 5 days/week) (50/sex/concentration). In rats, the NOAEC identified in male rats was 450 mg/m3 based on a non-cancer effect, olfactory epithelium respiratory metaplasia of the nasal cavity, at the LOAEC of 900 mg/m3 (Roycroft 1989 as cited in WHO 2002). In mice, a dose-related increase in incidence of uterine tumours (adenomas, adenocarcinomas or squamous cell carcinomas combined) was observed (0/50, 4/50, 5/47, 27/48 compared to the historical incidence range for chamber controls at the study laboratory of mean +/- SD): 4/335 [1% +/- 2%]) (Roycroft 1989 as cited in WHO 2002). A lower limit of the benchmark concentration associated with a 10% incidence of increased uterine tumours (BMCL10) of 448 mg/m3 was derived using benchmark dose software (BMDS version 3.1 USEPA). The mechanism of tumour formation remains unclear. There are no data available on the ability of bromoethane to cause cancer in potentially exposed human population (WHO 2002). Overall, the WHO concluded there is concern for carcinogenicity based on the results from Roycroft (1989) as cited in WHO (2002) which are likely, the basis for the ECHA GHS Carc 2 classification as well.
A human cancer slope factor of 0.0073 (mg/kg bw/day)-1 based on uterine tumours in mice derived by the California Environmental Protection Agency (Cal EPA 2012) was used to derive quantitative cancer risk estimates for bromoethane for the Canadian population (Appendix B). This human cancer slope factor was derived by fitting the multistage model to the dose-response data from the National Toxicity Program (NTP) (1989) study in female mice and by using the applicable interspecies scaling factor (Cal EPA, 2012).The lifetime average daily dose (LADD) for bromoethane in air was modified using age-dependent adjustment factors (ADAFs) (Appendix B, Table B-1).
7.1.3 Characterization of risk to human health
Table 7‑1 provides the relevant exposure and hazard values for bromoethane, as well as resultant margins of exposure (MOEs), for determination of non-cancer risk.
| Exposure scenario | Exposure concentration | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Inhalation exposure from air | 0.000074 mg/m3 | BMCL10 adj = 80 mg/m3 in 2- year inhalation study in ratsa | Olfactory epithelium respiratory metaplasia at 900 mg/m3 | 1 086 000 |
Abbreviations: MOE; Margin of Exposure; BMCL10 adj: benchmark concentration associated with a 10% incidence of increased of the effect.
a A BMCL10 of 448 mg/m3 was determined from this study. When exposure is amortized to 24 hours/day, 7 day/week, this BMCL10 is adjusted to 80 mg/m3.
For bromoethane, with respect to inhalation exposure, comparison of non-cancer critical effects to estimates of exposure concentrations in air resulted in an MOE that is considered adequate to account for uncertainties in the health effects and exposure databases.
The LADD for bromoethane in air was used to calculate the Incremental Lifetime Cancer Risk (ILCR). The LADD was determined to be 1.88 x 10-5 mg/kg bw/day (Appendix B, Table B-2). These factors were then applied to the cancer risk calculation for each age group (see Appendix B, Table B-3). The resulting lifetime cancer risk using the cancer slope factor of 0.0073 (mg/kg bw/day)-1 and applying the appropriate ADAFs to the exposure estimates results in a carcinogenic risk of 4.3 x 10-7 which is considered acceptable given the uncertainties in the exposure and hazard databases.
While exposure of the general population to bromoethane is not of concern at current levels, this substance is considered to have a health effect of concern on the basis of its potential carcinogenicity (ECHA GHS Carc 2 classification). Therefore, there may be a concern for human health if exposure were to increase.
7.1.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| No reliable Canadian environmental monitoring data in water, soil, dust, or food were available. | +/- |
| Lack of developmental toxicity studies | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7.2 Chloroethane
7.2.1 Exposure assessment
7.2.1.1 Environmental media
No reliable environmental monitoring data were identified for chloroethane in water, soil, dust, or food in Canada or elsewhere. Given its very high vapour pressure and Henry’s law constant, chloroethane is expected to volatilize from soil and water. On this basis, exposure to chloroethane from environmental media is expected to occur primarily from air.
Concentrations of chloroethane measured in ambient and indoor air in Canada are presented in Appendix A, and are summarized below.
Chloroethane was monitored by the NAPS program between 1991 and 2016. Mean ambient air concentrations measured from various sites across Canada ranged from not detected (DL = 0.027 to 0.069 µg/m3) to 0.176 µg/m3 and 95th percentile concentrations ranged from not detected (DL = 0.044 to 0.069 µg/m3) to 0.368 µg/m3 (ECCC 2019). Ambient air concentrations for chloroethane were also measured in seven Canadian air studies conducted in Windsor, Regina, Halifax, Edmonton, Montreal, Sault-Ste-Marie and Ottawa (Health Canada 2010a, 2010b, 2010c, 2012, 2013, [Personal communication from Tim Shin and Ron Garson, WAQB, dated May 27, 2021; unreferenced. The details on the study designs have been presented elsewhere (i.e., Dales et al. 2013, Mallach et al. 2017)]). The geometric mean concentrations in ambient air in these Canadian cities were below the DL (0.027 to 0.115 µg/m3) in all of the studies, and the 95th percentile concentrations ranged from not detected to 0.060 µg/m3 (DL: 0.039 µg/m3).
Indoor air concentrations for chloroethane were also measured across the same seven Canadian studies referred to above, as well as in Swan Lake, Manitoba, as part of the First Nations Indoor Air Study (FNIAS) (Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere [i.e., Weichenthal et al. 2012]). The geometric mean and 95th percentile chloroethane concentrations measured in indoor air in these Canadian cities ranged from not detected (DL = 0.027 to 0.115 µg/m3) to 0.092 µg/m3 and from not detected (DL = 0.062 to 0.115 µg/m3) to 0.233 µg/m3, respectively.
Chloroethane was also measured in personal air in the Windsor, Ontario air study (Health Canada 2010c). The geometric mean concentrations in both winter and summer were at the DL, and the 95th percentile concentrations were not detected (DL = 0.115 µg/m3) and 0.160 µg/m3 in the summer and winter, respectively.
In regards to the treatment of results below the laboratory detection limit (DL) for Canadian air quality studies conducted in Halifax, Edmonton, Montreal, Sault-Ste-Marie, Ottawa (Health Canada 2010a, 2012, 2013, [Personal communication from Tim Shin and Ron Garson, WAQB, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Dales et al. 2013, Mallach et al. 2017)]), the values were used as reported in the statistical analysis. With respect to the Canadian air quality studies conducted in Regina and Windsor (Health Canada 2010b, 2010c), one-half of the DL was reported in the statistical analysis for values below the DL. In addition, when the laboratory did not observe any peak or target ions/qualifier ions in the GC-MS chromatogram, concentration of chloroethane was reported as zero as the quantitation criteria were not met. The results were considered valid and replaced by a near zero value (0.0001 µg/m3) or one-half of the corresponding DL to calculate the descriptive statistics using log transformation.
The highest 95th percentile concentration measured in either ambient or indoor air in Canada (i.e., 0.368 µg/m3 from the NAPS program; 2252 samples measured in 1996) (ECCC 2019) was used to estimate general population exposure to chloroethane from air.
7.2.1.2 Products available to consumers
The use of products available to consumers (e.g., engine starting aid or starting fluid) containing chloroethane may result in general population exposure via the dermal and/or inhalation route. Starting fluids are expected to be used intermittently and by adults.
The estimated exposures from starting fluids represents the highest level of potential dermal and inhalation exposure (referred to as sentinel scenarios) for relevant age groups and are presented in Table 7‑3. Potential exposure was estimated based on conservative assumptions. Details are presented in Appendix C.
| Substance | Product scenario | Concentration (%)a | Route of exposure | Per event internal exposure (mg/kg bw) | Mean event concentration (mg/m3) |
|---|---|---|---|---|---|
| Chloroethane | Starting fluid (spray) | 1 | Dermal | 0.0027 | - |
| Chloroethane | Starting fluid (spray) | 1 | Inhalation | 0.00065 | 4.2 |
a MSDS 2017a
7.2.1.3 Biomonitoring
Biomonitoring data were identified in the United States (US) National Health and Nutrition Examination Survey (NHANES) for chloroethane (2013-2014) (CDC 2014). Chloroethane was not detected in whole blood analyzed in the US population. No Canadian biomonitoring data are available.
7.2.2 Health effects assessment
The OECD (2006) summarized the health effects literature and characterized the hazard for chloroethane. OECD (2006) was used to inform the health effects characterization in this screening assessment. IARC has classified chloroethane as Group 3 (not classifiable as to its carcinogenicity to humans) (IARC 1999). ECHA has classified chloroethane as GHS Carc. 2 (Warning; H351: suspected of causing cancer) (ECHA 2018b).
A literature search was conducted from the year prior to OECD (2006) (i.e., April 2005) to March 2019. No health effects studies which could impact the risk characterization (i.e., result in different critical endpoints or lower points of departure) than those stated in OECD (2006), ATSDR (2018), NTP (1989), and IARC (1999) were identified.
Chloroethane is rapidly absorbed by the lungs following inhalation (OECD 2006). In humans exposed briefly by inhalation to chloroethane, 30% of the retained dose was excreted in the breath within 1 hour (Morgan et al. 1970 in ATSDR 2018). The highest concentration found in the animal body from toxicity studies was found in fatty tissue around the kidney and the lowest concentration found in cerebrospinal fluid (Konietzko 1984 in ATSDR 2018). In mice and rats, metabolism involves conjugation with glutathione (GSH) and oxidation by cytochrome P-450 dependent monooxygenases to produce s-ethyl-glutathione and acetaldehyde, respectively (ATSDR 1998). Some metabolites are excreted in the urine, while unmetabolized chloroethane is exhaled.
An 11-day inhalation study was conducted in mice (B6C3F1). Test animals were exposed for 11 days to 0, 660, 3 250 or 13 088 mg/m3 (23 hours/day) (7 /sex/concentration). The NOAEC was 3 250 mg/m3 based on an increase in mean relative liver weights in both males and females and an increase in hepatocellular vacuolation (glycogen or fat) and the LOAEC was 13 088 mg/m3 (IRIS 1991, Landry et al. 1989 as cited in OECD 2006).
A 13-week inhalation study was conducted in rats (F344/N) and mice (B6CF1). Test animals were exposed for 13 weeks to 0, 6 544, 13 088, 26 000 or 50 000 mg/m3 (6 hours/day, 5 days/week) (10 /sex/concentration). The NOAEC was 26 000 mg/m3 based on an increase in liver to body weight ratio in male rats and female mice and a decrease of body weight in rats of both sexes at the LOAEC of 50 000 mg/m3 (NTP 1989 in OECD 2006).
A developmental study was conducted in mice (CF-1), via the inhalation route of exposure. Test animals were exposed on days 6 through 15 of gestation to 0, 1 308, 3 926 or 13 088 mg/m3 (6 hours/day) (30 /sex/concentration). The NOAEC was 3 926 mg/m3 based on delayed fetal ossification and the LOAEC was 13 088 mg/m3 (Scortichini et al. 1986 in OECD 2006).
In vitro studies show that chloroethane is mutagenic in the Ames assay, with and without metabolic activation (OECD 2006). Chloroethane also induced gene mutations in mammalians systems, with and without metabolic activation. In vivo studies (e.g., clastogenicity and DNA damage) did not show any genotoxicity of chloroethane (OECD 2006). Therefore, it is possible to conclude that chloroethane is genotoxic in vitro but not in vivo.
An inhalation carcinogenicity study was conducted in both rats (F344/N) and mice (B6C3F1) via the inhalation route of exposure. Test animals were exposed for two years to 0 and 39 264 mg/m3 (6 hours/day, 5 days/week) (50 /sex/concentration). In mice, there was carcinogenic activity for female at 39 264 mg/m3, as indicated by uterine tumours (carcinomas) (0/49, 43/50 for 0 and 39 264 mg/m3 respectively) and the historical incidence range for chamber controls at the study laboratory (mean +/- SD) is 4/335 (1% +/- 2%) (NTP 1989 as cited in OECD 2006). Since animals were exposed to only one concentration, a dose-response increase based on different concentrations could not be examined. Other types of tumours were observed in test animals, including tumours of the brain, skin, hematopoetic system, kidney, urogenital tract, liver, and lung. It should be noted that the study in male mice was considered to be an inadequate study of carcinogenicity because of reduced survival in the exposed group due to an ascending urinary tract infection (OECD 2006). However, the incidence of nonneoplastic lesions of different systems was also noted in male mice (i.e. alimentary system, cardiovascular system, endocrine system, general body system, genital system, musculoskeletal system, nervous system and, respiratory system). With respect to neoplastic lesions, a NOAEC could not be identified from this study. However, a LOAEC equal to 39 264 mg/m3 was identified in female mice based on an increase in uterine tumours (NTP 1989 in OECD 2006). It should be noted that results from the NTP (1989) as cited in OECD (2006) are likely the basis for the ECHA GHS Carc 2 classification. IARC (1999) reviewed the same carcinogenicity study and concluded that chloroethane is not classifiable as to its carcinogenicity in humans (Group 3).
A human cancer slope factor of 0.0025 (mg/kg bw/day)-1 based on uterine tumours in mice derived by the California Environmental Protection Agency (Cal EPA) (2001) was used to derive quantitative cancer risk estimates for chloroethane for the Canadian population (Appendix B). This human cancer slope factor was derived by using a particular case of the multistage model (one-hit model), in which only one stage is used, and the applicable interspecies scaling factor that results from the updated interspecies scaling equation (qhuman = qanimal • (bwh / bwa)1/4. It should be noted that the original regulations for Proposition 65 (the Safe Drinking Water and Toxic Enforcement Act of 1986) were updated in 2011. Therefore, the ratio of human to animal body weight is raised to the 1/4 power instead of 1/3 power (Personal communication from Office of Environmental Health Hazard Assessment (OEHHA), California Environmental Protection Agency to Water and Air Quality Bureau (WAQB), Health Canada, dated July 3, 2019; unreferenced).
7.2.3 Characterization of risk to human health
Table 7‑4 provides all relevant exposure and hazard values for chloroethane, as well as resultant MOEs, for determination of non-cancer risk.
| Exposure scenario | Exposure concentration | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Inhalation exposure from air | 0.000368 mg/m3 | NOAEC adj = 982 mg/m3 based on a developmental inhalation study of 10 days of gestationa | Delayed fetal ossification in mice at 13 088 mg/m3 | 2 700 000 |
| Per event inhalation exposure to starting fluid (spray) | 4.2 mg/m3 | NOAEC = 3 926 mg/m3 based on a developmental inhalation study of 10 days of gestation | Delayed fetal ossification in mice at 13 088 mg/m3 | 935 |
| Per event dermal exposure to Starting fluid (spray) | 0.0027 mg/kg bw (internal) | NOAEC(dermal) = 478 mg/kg bw based on a developmental inhalation study of 10 days of gestationb | Delayed fetal ossification in mice at 13 088 mg/m3 | 177 000 |
Abbreviations: MOE, Margin of Exposure; NOAECadj, no-observed-adverse-effect-concentration adjusted to account for daily exposures of 24 h.
a NOAEC of 3 926 mg/m3 was determined in this study. When exposure is amortized to 24 hours/day, 7 day/week, this NOAEC is adjusted to 982 mg/m3.
b A dermal equivalent NOAEC of 478 mg/kg bw was determined as follows: NOAEC(dermal) = NOAEC(inhalation) x ventilation rate ¸ body weight, where NOAEC(inhalation) = 3926 mg/m3, ventilation rate = 1.5 m3/hour for 6 hours, and body weight = 74 kg.
For chloroethane, with respect to inhalation exposure, comparison of non-cancer critical effects to estimates of exposure concentrations in air and from use of starting fluid resulted in MOEs that are considered adequate to account for uncertainties in the health effects and exposure databases. It should be noted that the per-event scenarios did not need to be quantified as the 13 088 mg/m3 concentration was excessive. In regards to dermal exposure to starting fluid, comparison of non-cancer critical effects to estimates of exposure intake resulted in a MOE that is considered adequate to account for uncertainties in the health effects and exposure databases. These MOEs are also considered to account for potential exposures for by-standers (i.e., non-users).
The lifetime average daily dose (LADD) for chloroethane in air was used to calculate the Incremental Lifetime Cancer Risk (ILCR). The LADD was determined to be 6.35 x 10-5 mg/kg bw/day (Appendix B, Table B-4). Age-dependent adjustment factors (ADAFs) recommended by the US EPA (2005) were considered and adjusted to the Health Canada age groups (see Appendix B, Table B-1). These factors were then applied to the cancer risk calculation for each age group (see Appendix B, Table B-5). The resulting lifetime cancer risk using the cancer slope factor of 0.0025 (mg/kg bw/day)-1 and applying the appropriate ADAFs to the exposure estimates results in a carcinogenic risk of 5.0 x 10-7 which is considered acceptable given the uncertainties in the exposure and hazard databases.
While exposure of the general population to chloroethane is not of concern at current levels, this substance is considered to have a health effect of concern on the basis of its potential carcinogenicity (ECHA GHS Carc 2 classification). Therefore, there may be a concern for human health if exposures were to increase.
7.2.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| No reliable Canadian environmental monitoring data in water, soil, dust, or food were available. | +/- |
| No dermal absorption data for chloroethane | - |
| Lack of dermal toxicity studies | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7.3 1-Bromopropane
7.3.1 Exposure assessment
7.3.1.1 Environmental media
No reliable environmental monitoring data were identified for 1-bromopropane in water, soil, dust, or food in Canada or elsewhere. Given its very high vapour pressure and Henry’s law constant, 1-bromopropane is expected to volatilize from soil and water. On this basis, exposure to 1-bromopropane from environmental media is expected to occur primarily from air.
Canadian indoor air monitoring data were identified for 1-bromopropane. Concentrations of 1-bromopropane measured in indoor air in Canada are presented in Appendix A, and are summarized below.
1-Bromopropane was measured in the national Canadian indoor air study conducted in 2012-2013 as part of cycle 3 of the Canadian Health Measures Survey (CHMS) (Canada 2013). Li et al. (2019) discussed the results of this study that covers a 24-month period to monitor 88 volatile organic compounds (VOCs) in 3 524 Canadian residential homes. This substance was detected in indoor air at a low frequency (0.3%), with a method detection limit (MDL) of 0.29 µg/m3 and a maximal concentration of 3.1 µg/m3. It was thus not detected in almost all indoor air measurements (99.7%).
Living in close proximity to a dry-cleaner may also be a potential source of exposure, as dry-cleaning has been identified as an occupational exposure scenario (US EPA 2020); however, Canadian data are lacking for this exposure scenario.
The US Department of Health and Human Services used air dispersion modeling to estimate 1-bromopropane concentrations in ambient air in the vicinity of industrial foam manufacturing facilities where this substance is used (Morris and Wolf 2003, cited in NTP 2016). However, in a response to a CEPA section 71 survey (Canada 2009), foam manufacturing is not a use in Canada (see Table 4‑2), and this modelling data was not considered relevant to Canadian exposures.
As a conservative approach, the maximal concentration from the CHMS indoor air study (Canada 2013) (3.1 µg/m3) was used to estimate the general population exposure to 1-bromopropane in Canadian homes.
7.3.1.2 Products available to consumers
Exposure of the general population to 1-bromopropane can result from the use of products available to consumers including silicone mold release spray, electronic cleaner spray, and automotive A/C flush. These products can be found on the current Canadian market. Only intermittent uses by adults are expected for available products. No uses intended for children were reported for 1-bromopropane in response to CEPA section 71 surveys (Canada 2009, 2012).
Estimated exposures from products available to consumers that were determined to be relevant to the general population of Canada and represent the highest level of potential dermal and inhalation exposure (referred to as sentinel scenarios) for relevant age groups are presented in Table 7‑6 and Table 7‑7. Potential exposures were estimated based on conservative assumptions. Details are presented in Appendix C.
While the primary route of exposure is considered to be inhalation, dermal exposure may also occur through skin contact for liquid consumer product formulations (e.g., automotive A/C flush), vapor or mist deposition onto the skin from spray product formulations (e.g., silicone mold release spray, electronic cleaner spray). A limited in vitro study using human epidermis reported dermal absorption of 1-bromopropane to be 0.16% for finite exposure scenarios (e.g., splashing on skin where it is expected to rapidly evaporate and exposure does not continue) (Frasch et al. 2011). The same study shows that dermal absorption for infinite or continuous exposures (e.g., direct contact with the material during product use or after application), where 1-bromopropane cannot readily evaporate, may be substantial. However, the study did not measure skin-bound residues of 1-bromopropane, and reported a large standard deviation, likely indicative of the difficulty in spreading a small, rapidly evaporating dose evenly over the exposed skin surface. In addition, US EPA (2020) notes that the indoor air wind speed used in the study is high and may underestimate dermal absorption. Adjusting for more typical wind speed likely to be found indoors, the US EPA (2020) estimates dermal absorption at 0.29%.
| Substance | Product scenario | Concentration (%) | Route of exposure | Per event internal exposure (mg/kg bw/day) |
|---|---|---|---|---|
| 1-bromopropane | Silicone mold release spray | 30a | Dermal | 0.0031 |
| 1-bromopropane | Electronic cleaner spray | 44b | Dermal | 0.0071 |
| 1-bromopropane | Automotive A/C flush | 100c | Dermal | 0.00047 |
a MSDS 2016a
b MSDS 2017b
c MSDS 2017c
| Substance | Product scenario | Concentration (%) | Route of exposure | Mean event concentration (mg/m3) | 6-hr TWA air concentration (mg/m3) |
|---|---|---|---|---|---|
| 1-bromopropane | Silicone mold release spray | 30a | Inhalation | 2 490 | 208 |
| 1-bromopropane | Electronic cleaner spray | 44b | Inhalation | 1 980 | 165 |
| 1-bromopropane | Automotive A/C flush | 100c | Inhalation | 1 100 | 92 |
Abbreviation: TWA, time-weight average
a MSDS 2016a
b MSDS 2017b
c MSDS 2017c
7.3.1.3. Biomonitoring
N-Acetyl-S-(n-propyl)-L-cysteine (AcPrCys) has been proposed as a biomarker of exposure to 1-bromopropane. AcPrCys has been identified in urine of occupationally exposed workers, and has been shown to be correlated with ambient air occupational concentrations of 1-bomopropane (US EPA 2020). AcPrCys has also been detected in the urine of the general population. For example, it has been detected in urine samples of children and adults in the National Health and Nutrition Examination Survey (NHANES) at approximately 3-4 µg/L (geometric mean). It was also measured in the US National Children’s Vanguard Study (2009-2010) in the urine of pregnant women in their third trimester (detected in 99% of the 488 samples) at a median concentration of 2.6 ng/ml (Boyle et al. 2016) which is significantly lower than the geometric mean reported in the NHANES. While occupational studies indicate that AcPrCys is the most predictive biomarker of 1-bromopropane exposure available, the ubiquitous nature of AcPrCys in the general population also suggests that this metabolite may not be specific to 1-bromopropane as general population exposure is expected to be limited (US EPA 2020).
7.3.2 Health effects assessment
US EPA (2020) summarized the health effects literature and characterized the hazard for 1-bromopropane, which was used to inform the health effects characterization in this screening assessment. IARC has classified 1-bromopropane as Group 2B (possibly carcinogenic to humans due to sufficient data in animals and inadequate in humans) (IARC 2018). ECHA has classified 1-bromopropane as GHS Repr. 1B (H360FD: may damage fertility; may damage the unborn child) (ECHA 2018c).
A literature search was conducted from the year prior to US EPA (2020) to May 2019. No new health effects studies which would impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated in US EPA (2020) were identified.
Limited toxicokinetic information is available for 1-bromopropane. Studies in humans and animals indicate that 1-bromopropane is readily absorbed via inhalation, dermal and oral routes. Inhalation is anticipated to be the predominant route of exposure, and following uptake, based on its fat:blood partition coefficient, it is anticipated to partition to fat. 1-bromopropane can directly conjugate with glutathione or can be oxidized by CYP2E1and then further oxidized and/or conjugated with glutathione and eventually excreted as mercapturic acid derivatives in the urine, including N-Acetyl-S-(n-propyl)-L-cysteine. CYP2E1 metabolism of 1-bromopropane is known to form mutagenic metabolites. Excretion of 1-bromopropane primarily occurs via exhalation, with lesser amounts excreted in urine and feces (US EPA 2020).
A behavioural neurotoxicity study was conducted in male rats (F344), via the inhalation route of exposure. Test animals were exposed for 3 weeks (8hours/day) to 10, 50, 200 and 1 000 ppm (50, 251, 1 006 and 5 028 mg/m3) (Honma et al. 2003 as cited in US EPA 2020). The critical effect level and corresponding hazard endpoint was a BMCL1SD equal to 18.2 ppm (91.5 mg/m3), for decreased traction time (i.e., time hanging from a suspended bar) (where BMCL1SD is BMCL at a benchmark response [BMR] of 1 standard deviation (1SD)). The LOAEC for this endpoint was 200 ppm (1 006 mg/m3). This endpoint is consistent with peripheral neurotoxicity, which has also been reported in human studies and other studies in experimental animals (Honma et al. 2003 as cited in US EPA 2020).
A 2-generation reproductive study was conducted in rats (SD), via the inhalation route of exposure. Test animals (25/sex/concentration) were exposed for ≥ 70 days (6hours/day) prior to mating, throughout gestation through gestation day 20 at concentrations of 100, 250, 500 or 750 ppm (503, 1258, 2 515 or 3 773 mg/m3). Females exposed to 750 ppm were non-gravid, therefore F1 exposures were limited to the three lower doses groups only (i.e., 100, 200 and 500 ppm; 503, 1 258 and 2 515 mg/m3). All Fo and F1 females were allowed to deliver and rear their pups until weaning on lactation day 21. Effects to male and female reproductive parameters and to the developing fetus were observed, beginning at 250 ppm (1 259 mg/m3). Effects in males included decreases in sperm motility, changes in sperm morphology and decreased mating and fertility indices. Effects in females included decreased number of corpora lutea, and antral follicles, and implantation sites. Increased estrous cycle length was also observed as well as increasing numbers of F0 females with evidence of mating without delivery. Developmental effects included decreased live litter size, decreased pup body weight, and decreased brain weight. The critical effect level and corresponding hazard endpoint was a BMCL1 equal to 23 ppm (116 mg/m3), for post-implantation loss in F0 females (where BMCL1 is the BMCL at a BMR of 1%). This study also showed histopathological changes in the liver (increased incidence of vacuolization of centrilobular hepatocytes; BMCL10 = 143.5 ppm; 721.8 mg/m3) and kidney (increased incidence of pelvic mineralization; BMCL10 = 135 ppm; 679.1 mg/m3) (WIL Research 2001 as cited in US EPA 2020).
A 2-year cancer study was conducted in rats (F344) and mice (B3C3F1), via the inhalation route of exposure. Test animals were exposed for 105 weeks (6 hours/day, 5 days/week) to 0, 125, 250 or 500 ppm (0, 269, 1 258 or 2 515 mg/m3; rats) and 62.5, 125 and 250 ppm (0, 314, 629 or 1 258 mg/m3; mice) (50/sex/concentration). In female mice, a significant increase in the incidence of alveolar/bronchiolar adenomas or carcinomas was observed at all doses tested (1/50, 9/50, 8/50, 14/50 for combined adenoma and carcinoma at 0, 314, 629 or 1 258 mg/m3, respectively). The US EPA derived a benchmark concentration (BMC10) of 78.6 ppm (396 mg/m3) and benchmark concentration 95% lower confidence limit (BMCL10) of 54.1ppm (272 mg/m3) for combined alveolar/bronchiolar adenoma or carcinoma in female mice from this study (NTP 2011 as cited in US EPA 2020). A significant increase in incidences of combined keratoacanthoma/squamous cell carcinoma (male rat) and large intestine adenoma (female rat) was also observed, but provided higher points of departure. Based on the observed increase in tumour incidence in both rats and mice, at multiple sites, and the occurrence of rare tumours (e.g., intestinal adenomas), there is sufficient evidence of carcinogenicity in animals.
IARC has classified 1-bromopropane as Group 2B (possibly carcinogenic to humans, based on sufficient data in animals, inadequate in humans) (IARC 2018). IARC (2018) also concluded that there is moderate evidence that 1-bromopropane is genotoxic. US EPA (2020) states that the evidence for a mutagenic mode of action for 1-bromopropane carcinogenicity is suggestive but inconclusive. There is some evidence for mutagenicity and DNA damage in vitro; however, there is uncertainty whether it is genotoxic in vivo. In vitro genotoxicity results are mixed (equivocal in bacteria, positive mouse lymphoma, and Comet assays), however, in vivo tests (dominant lethal, micronuclei, and gene mutation in transgenic mice) are negative. There is also evidence that 1-bromopropane contributes to a multi-stage process of carcinogenesis via oxidative stress, immunosuppression and cell proliferation.
Risk from exposure to 1-bromoproprane from the use of products available to consumers was assessed by the US EPA under the Toxic Substances Control Act (TSCA) program, and a final risk evaluation was published in 2020 (US EPA 2020). Exposure estimates for acute inhalation exposure to 1-bromopropane for users and non-users (by-standers) were derived for the following consumer uses: adhesive accelerant, general spray cleaner, spot cleaner/stain remover, mold cleaning/release, general spray cleaner/degreaser, electronics spray cleaner/degreaser, coin and scissors cleaner, automobile AC flush and insulation. Acute dermal exposures to 1-bromopropane were evaluated for users only and estimates were derived for all consumer uses mentioned above except insulation. The US EPA found that there were inadequate MOEs for acute inhalation exposure to eight consumer uses: adhesive accelerant, general spray cleaner, spot cleaner/stain remover, mold cleaning/release, general spray cleaner/degreaser, electronics spray cleaner/degreaser, coin and scissors cleaner and automobile AC flush. The basis involved concerns for adverse developmental effects that may occur as a result of a single exposure to 1-bromopropane during a critical window of susceptibility (US EPA 2020). Based on the same concerns, the US EPA found that there were inadequate MOEs for four acute dermal consumer use exposures: general spray cleaner, spot cleaner/stain remover, general spray cleaner/degreaser, and automobile AC flush. However, with the exception of the silicone mold release spray, electronic cleaner spray, and automotive A/C flush, no evidence was found to indicate that the products specified by the US EPA are available to consumers in Canada.
7.3.2 Characterization of risk to human health
Table 7‑8 provides all relevant exposure and hazard values for 1-bromopropane, as well as resultant margins of exposure (MOEs), for determination of non-cancer risk.
| Exposure scenario | Exposure concentration | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily inhalation exposure from air | 0.0031 mg/m3 | BMCL0.1(adj) = 30.5 mg/m3 based on a behavioural neurotoxicity study in ratsa | Neurotoxicity (decreased traction time) in male rats at 1 006 mg/m3 | 9800 |
| Per event inhalation exposure to silicone mold release spray | 208 mg/m3 | BMCL1SD = 116 mg/m3 based on a 2-generation reproductive inhalation study in rats | Developmental (post-implantation loss) at 2 515 mg/m3 | 1 |
| Per event inhalation exposure to electronic cleaner spray | 165 mg/m3 | BMCL1SD = 116 mg/m3 based on a 2-generation reproductive inhalation study in rats | Developmental (post-implantation loss) at 2 515 mg/m3 | 1 |
| Per event inhalation exposure to automotive A/C flush (liquid) | 120 mg/m3 | BMCL1SD = 116 mg/m3 based on a 2-generation reproductive inhalation study in rats | Developmental (post-implantation loss) at 2 515 mg/m3 | 1 |
| Per event dermal exposure to electronic cleaner spray | 0.0071 mg/kg bw (internal) | BMCL1SD(dermal) = 14.1 mg/kg bw/day based on a 2-generation reproductive inhalation study in ratsb | Developmental (post-implantation loss) at 2 515 mg/m3 | 2000 |
Abbreviations: BMCL, benchmark concentration lower confidence limit; MOE, Margin of Exposure
a A BMCL0.1 of 91.5 mg/m3 was determined in this study. When exposure is amortized to 24 hours/day, 7 days/week, the BMCL0.1 was adjusted to 30.5 mg/m3
b A dermal equivalent BMCL1SD of 14.1 mg/kg bw/day was determined as follows: BMCL1SD(dermal) = BMCL1SD(inhalation) x ventilation rate ¸ body weight, where BMCL1SD(inhalation) = 116 mg/m3, ventilation rate = 1.5 m3/hour for 6 hours, and body weight = 74 kg.
For 1-bromopropane, with respect to inhalation exposure from air, comparison of non-cancer critical effects to estimates of exposure concentrations in air resulted in an MOE that is considered adequate to account for uncertainties in the health effects and exposure databases. However, due to the reproductive toxicity (ECHA GHS Repr. 1B classification) of this substance, there may be concern for human health if exposure were to increase. For inhalation exposure to products used by consumers, the margins of exposure between critical effects and the estimate of intake of 1-bromopropane, which are also considered to account potential exposures for by-standers (i.e., non-users), are considered potentially inadequate to account for uncertainties in the databases. For dermal exposure to products used by consumers, the margins of exposure between critical effects and the estimate of intake of 1-bromopropane are considered adequate to account for uncertainties in the health effects and exposure databases.
A human inhalation unit risk of 1 x 10-6 (µg/m3)-1 based on alveolar/bronchiolar adenoma or carcinoma in female mice, derived by the US EPA (2020), was used to derive quantitative cancer risk estimates for 1-bromopropane for the Canadian population (Appendix B). This inhalation unit risk was derived by fitting the multistage model to the dose-response data from the NTP (2011) study in female mice (US EPA 2020). The lifetime average daily dose (LADD) for 1-bromopropane in air was modified using age-dependent adjustment factors (ADAFs) (Appendix B, Table B-1). The LADD for 1-brompropane in air was used to calculate the Incremental Lifetime Cancer Risk (ILCR). The LADD was determined to be 0.69 µg/kg bw/day (Appendix B, Table B-6). These factors were then applied to the cancer risk calculation for each age group (see Appendix B, Table B-7). The resulting lifetime cancer risk using the inhalation unit risk of 1 x 10-6 (µg/m3)-1 and applying the appropriate ADAFs to the exposure estimates results in a carcinogenic risk of 4.3 x 10-6, which is considered acceptable given the uncertainties in the exposure and hazard databases. However, due to the potential carcinogenicity (IARC 2B classification) of this substance, there may be a concern for human health if exposure were to increase.
7.3.3 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| No reliable Canadian environmental monitoring data in ambient air, water, soil, dust, or food were available. | +/- |
| No information on exposure that may arise from living in close proximity to a dry-cleaner | - |
| Lack of dermal toxicity studies | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7.4 Trans-1,2-dichloroethene
7.4.1 Exposure assessment
7.4.1.1 Environmental media
No reliable environmental monitoring data were identified for trans-1,2-dichloroethene in water, soil, dust or food in Canada or elsewhere. Given its very high vapour pressure and Henry’s law constant, trans-1,2-dichloroethene is expected to volatilize from soil and water. On this basis, exposure to trans-1,2-dichloroethene from environmental media is expected to occur primarily from air.
Concentrations of trans-1,2-dichloroethene measured in ambient and indoor air in Canada are presented in Appendix A, and are summarized below.
Trans-1,2-dichloroethene was monitored by the NAPS program between 1990 and 2013, where mean ambient air concentrations measured from various sites across Canada ranged from not detected (DL range from 0.013 to 0.083 µg/m3) to 0.065 µg/m3 (DL: 0.013 µg/m3) and 95th percentile concentrations ranged from not detected to 0.060 µg/m3 (DL: 0.013 µg/m3 ) (ECCC 2019). Ambient air concentrations for trans-1,2-dichloroethene were also measured in seven Canadian air studies conducted in Windsor, Regina, Halifax, Edmonton, Montreal, Sault-Ste-Marie and Ottawa (Health Canada, 2010a, 2010b, 2010c, 2012, 2013, [Personal communication from Tim Shin and Ron Garson, WAQB, dated May 27, 2021; unreferenced. The details on the study designs have been presented elsewhere (i.e., Dales et al. 2013, Mallach et al. 2017)]). The geometric mean and 95th percentile concentrations measured in ambient air in these Canadian cities ranged from not detected (DL ranged from 0.018 to 0.042) to 0.042 µg/m3 and from not detected to 0.314 µg/m3, respectively.
Indoor air concentrations for trans-1,2-dichloroethene were also measured across the same seven Canadian studies referred to above, as well as in Swan Lake, Ontario, as part of the FNIAS (Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere [i.e., Weichenthal et al. 2012]). The geometric mean and 95th percentile trans-1,2-dichloroethene concentrations measured in indoor air in these Canadian cities ranged from not detected (DL ranged from 0.018 to 0.055) to 0.535 µg/m3 and from not detected to 0.255 µg/m3, respectively.
Trans-1,2-dichloroethene was also measured in personal air in the Windsor, Ontario air study (Health Canada 2010c). The geometric and 95th percentile concentrations in both winter and summer were at the DL (0.069 µg/m3).
In regards to the treatment of results below the laboratory detection limit (DL) for Canadian air quality studies conducted in Halifax, Edmonton, Montreal, Sault-Ste-Marie, Ottawa (Health Canada 2010a, 2012, 2013, [Personal communication from Tim Shin and Ron Garson, WAQB, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Dales et al. 2013, Mallach et al. 2017)]), the values were used as reported in the statistical analysis. The results were considered valid as the retention time and target ions/qualifier ions in the GC-MS chromatogram met the analytical criteria of the laboratory. With respect to the Canadian air quality studies conducted in Regina and Windsor (Health Canada 2010b, 2010c), one-half of the DL was reported in the statistical analysis for values below the DL. In addition, when the laboratory did not observe any peak or target ions/qualifier ions in the GC-MS chromatogram, concentration of trans-1,2-dichloroethene was reported as zero as the quantitation criteria were not met. The results were considered valid and replaced by a near zero value (0.0001 µg/m3) or one-half of the corresponding DL to calculate the descriptive statistics using log transformation.
The highest 95th percentile concentration measured in either ambient or indoor air in Canada (i.e., 0.314 µg/m3 from the Regina study; 5-day sampling in 101 homes) (Health Canada 2010b) was used to estimate general population exposure to trans-1,2-dichloroethene from air. This air monitoring concentration was converted to an internal dose for comparison with the critical effect level (refer to Appendix D for details).
7.4.1.2 Products available to consumers
The use of products available to consumers containing trans-1,2-dichloroethene may result in general population exposure via the dermal and/or inhalation route. Based on the types of products used by consumers (e.g., cleaner or degreaser for electrical equipment or electronic parts, powdered spot cleaner for textiles, automotive A/C refrigerant flush and spray foam insulation), they are expected to be used intermittently and by adults. Furthermore, no uses intended for children were reported for trans-1,2-dichloroethene in response to CEPA section 71 surveys (Canada 2009, 2012).
Low-pressure two-component spray foam insulation products containing trans-1,2-dichloroethene are available for purchase off the shelf from retailers in systems (kits) providing up to approximately 0.04 cubic meters (15 board feet) of spray foam (i.e., 0.99 kg package). While the label of the products indicates that it is "for professional use", based on the availability and small volume of the product, use by do-it-yourself (DIY) applicators cannot be excluded. As such, a scenario was developed for homeowners using spray foam insulation products for small projects (e.g., to insulate gaps, cracks, expansion joints and other air leakage).
Estimated exposures from powdered textile spot remover were determined to be relevant to the general population of Canada and represent the highest level of potential dermal and inhalation exposure (referred to as sentinel scenarios) for relevant age groups and are presented in Table 7‑10. Potential exposures were estimated based on conservative assumptions. Details are presented in Appendix C. Additional potential use scenarios for trans-1,2-dichloroethene were considered (refrigerant, cleaner or degreaser, foam insulation) but resulted in lower exposure estimates than those presented in Table 7‑10.
| Substance | Product scenario | Concentration (%)a | Route of exposure | Internal dose on day of exposure (mg/kg bw) | Mean concentration on day of exposure (mg/m3) |
|---|---|---|---|---|---|
| Trans-1,2-dichloroethene | Powdered textile spot remover (spray) | 60 | Dermal | 0.259 | - |
| Trans-1,2-dichloroethene | Powdered textile spot remover (spray) | 60 | Inhalation | 4.40 | 22.0 |
a MSDS 2017d
-: not applicable
7.4.1.3. Biomonitoring
Biomonitoring data were identified in the US National Health and Nutrition Examination Survey (NHANES) for trans-1,2-dichloroethene (2003-2012) (CDC 2013). 1,2-Dichloroethene was not detected in whole blood analyzed in the US population. No Canadian biomonitoring data are available.
7.4.2 Health effects assessment
US EPA (2010) summarized the health effects literature and characterized the hazard for trans-1,2-dichloroethene. A literature search was conducted from the year prior to US EPA (2010) to March 2019. No health effects studies, which could impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated in US EPA (2010)), were identified. This section provides critical endpoints and corresponding effect levels to be used for risk characterization, as cited directly from US EPA (2010).
Limited information is available on the toxicokinetics of trans-1,2-dichloroethene (US EPA 2010). Studies in animals indicate that it is readily absorbed by inhalation, and while there are no studies of dermal absorption, its physical chemical properties indicate that it is also likely to be absorbed following dermal exposure. Trans-1,2-dichlorethene is primarily metabolized by CYP2E1 to dichloroacetaldehyde, which is readily converted to dichloroacetic acid and 1,2-dichoroethanol. Further metabolism by GSH does not likely occur. No information is available on the elimination of trans-1,2-dichloroethene.
A 13-week, repeated-exposure study was conducted in mice (CD-1), via the oral route of exposure. Test animals (8-12/sex/dose) were exposed for 90 days to 0, 17, 175, or 287 mg/kg bw/day (males), or 0, 23, 224 or 452 mg/kg bw/day (females) via drinking water. The BMDL1SD was 65 mg/kg bw/day for immunotoxicity, measured as a decrease in the number of spleen antibody forming cells (AFCs) against sheep red blood cells in male mice (Shopp et al. 1985, as cited in US EPA 2010), and the LOAEL was 175 mg/kg bw/day. The US EPA (2010) deemed this effect to be indicative of suppression of the humoral immune system.
Liver and thymus effects were also reported in other subchronic oral studies but provided higher points of departure (i.e., 190 and 138.5 mg/kg bw/day for liver and thymus effects respectively) (Barnes et al. 1985 as cited in US EPA 2010; NTP 2002 as cited in US EPA 2010).
A 16-week repeated-exposure study was conducted in female rats (SPR Wistar) via the inhalation route of exposure. Test animals were exposed for 1, 2, 8 or 16 weeks (8 hours/day, 5 days/week) to air containing 792 mg/m3 (6/concentration). Effects including liver effects were noted at the test concentration of 792 mg/m3 and were observed as fat accumulation in liver lobules and Kupffer cells, an effect which also increased in incidence and severity with increasing exposure duration(Freundt et al. 1977, as cited in US EPA 2010).
A 90-day repeated-exposure study was conducted in rats (Crl:CD®BR) via the inhalation route of exposure. Test animals were exposed for 6 hours/day, 5 days/week to 0, 792, 3 960 or 15 800 mg/m3 (15/sex/concentration). Decreased lymphocyte counts were reported (significant at the high dose males only), but were of uncertain toxicological significance due to a high level of variability in controls animals for this endpoint across studies, and the possibility that this effect was due to stress rather than exposure to trans-1,2-dichloroethene. However, it was also reported that trans-1,2-DCE was irritating in rats at a concentration of 7940 mg/m3 (Hurtt et al. 1993 as cited in US EPA 2010). Therefore, it is possible that the decrease of lymphocytes count reflects a stress-related increase in glucocorticoid levels and redistribution of white blood cells (WBC) between the blood and other immune components in the rat. The critical effect level was therefore a NOAEC of 15 800 mg/m3 (Dupont et al. 1998 as cited in US EPA 2010).
While liver effects are the most commonly observed effect following oral exposure to trans-1,2-dichloroethene, the US EPA (2010) determined that evidence for liver toxicity is inconsistent; the 16-week inhalation study in rats (SPR Wistar) showed liver effects at 792 mg/m3, whereas the 13-week inhalation study in rats (Crl:CD®BR) did not show liver effects at this or higher concentrations. Therefore, data from the two subchronic inhalation studies described above (i.e., Freundt et al. 1977 and Dupont et al. 1998, respectively) were deemed insufficient by the US EPA for deriving an inhalation reference concentration.
A developmental study was conducted in rats (Crl:CD®BR) via the inhalation route of exposure. Pregnant females (N=24) were exposed from GD 7-16 (6 hours/day) to 0, 7 920, 23 760 and 47 520 mg/m3. A developmental NOAEC of 23 760 mg/m3 for decreased fetal body weight was identified from this study (Dupont 1988 as cited in US EPA, 2010), which was observed at 47 520 mg/m3 in the presence of maternal toxicity (i.e., decreased maternal body weight and food consumption) (Dupont 1988 as cited in US EPA, 2010).
Studies assessing the carcinogenic potential of trans-1,2-dichloroethene are not available (e.g., human epidemiological studies, or chronic studies in experimental animals) and in vivo and in vitro genotoxicity studies are generally negative (US EPA 2010). Weak, but positive results were observed in human lymphocytes for the micronucleus and comet assays (Tafazoli and Kisch-Volders, 1996 as cited in US EPA 2010); however, these results are inconsistent with the overall genotoxicity database and need further confirmation.
7.4.3 Characterization of risk to human health
Table 7‑11 provides the relevant exposure and hazard values for trans-1,2-dichloroethene, as well as resultant margins of exposure (MOEs), for determination of non-cancer risk.
| Exposure scenario | Exposure concentration | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Inhalation exposure from air | 0.00023 mg/kg bw/daya | BMDL1SD = 65 mg/kg bw/day based on a 90-day repeated dose drinking water study in male mice | Immunotoxicity, measured as a decrease in the number of AFCs against sheep red blood cells at 175 mg/kg bw/day | 283 000 |
| Inhalation exposure to powdered textile spot cleaner on day of exposure (liquid) | 22.0 mg/m3 | NOAEC = 23 760 mg/m3 based on an inhalation developmental study in rats | Decreased fetal body weight at 47 520 mg/m3 | 1100 |
| Dermal exposure to powdered textile spot cleaner (spray) | 0.259 mg/kg bw (internal) | NOAEC(dermal) = 2 890 mg/m3 based on an inhalation developmental study in ratsb | Decreased fetal body weight at 47 520 mg/m3 | 11 000 |
Abbreviations: AFCs, antibody forming cells; MOE, Margin of Exposure; NOAEC, no observed adverse effect concentration; BMDL1SD, benchmark dose lower confidence limit at a benchmark response of 1 standard deviation change, adjusted
a Estimated potential daily intake from inhalation exposure in air from the highest exposed subpopulation (i.e., 1 year old children) calculated from the highest 95th percentile concentration measured in Canada. The estimated potential daily intakes for all subpopulations are presented in Appendix D.
b A dermal equivalent NOAEC of 2980 mg/kg bw was determined as follows: NOAEC(dermal) = NOAEC(inhalation) x ventilation rate ¸ body weight, where NOAEC(inhalation) = 23 760 mg/m3, ventilation rate = 1.5 m3/hour for 6 hours, and body weight = 74 kg.
For trans-1,2-dichloroethene, with respect to inhalation exposure from air and inhalation and dermal exposure to products used by consumers, comparison of non-cancer critical effects to estimates of exposure concentrations resulted in MOEs that are considered adequate to account for uncertainties in the health effects and exposure databases. The MOEs derived for inhalation exposure to products used by consumers are also considered to account for potential exposures for by-standers (i.e., non-users).
7.4.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| No adequate Canadian environmental monitoring data in water, soil, dust, or food were available. | +/- |
| Lack of reproductive toxicity and cancer studies | +/- |
| Lack of dermal toxicity studies | +/- |
| The database is unclear on species/strain sensitivity with respect to liver effects | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
8. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from the four substances in the Alkyl Halides Group. It is proposed to conclude that bromoethane, chloroethane, 1-bromopropane and trans-1,2-dichloroethene do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that bromoethane, chloroethane and trans-1,2-dichloroethene do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that 1-bromopropane meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that 1-bromopropane meets one or more of the criteria set out in section 64 of CEPA, and that bromoethane, chloroethane and trans-1,2-dichloroethene do not meet any of the criteria set out in section 64 of CEPA.
It is also proposed to conclude that 1-bromopropane meets the persistence criteria but does not meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
[ATSDR] Agency for Toxic Substances and Disease Registry. 2018. Toxicology profile for chloroethane [PDF] [accessed 2019 January 30].
Boyle EB, Viet SM, Wright DJ, Merrill LS, Alwis KU, Blount BC, Mortensen ME, Moye Jr. J, Dellarco M. 2016. Assessment of Exposure to VOCs among Pregnant Women in the National Children’s Study. Int J Environ Res Public Health. 13(4):376.
Boublík T, Fried V, Hála E. 1984. The vapour pressures of pure substances: Selected values of the temperature dependence of the vapour pressures of some pure substances in the normal and low pressure region (2nd Revised ed.). Amsterdam, The Netherlands: Elsevier Science Publishers.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2956.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Canada. 2013. Canadian Health Measure Survey. (CHMS). Cycle 3. 2012-2013.
[Cal EPA] California Environmental Protection Agency. 2012. No significant risk level (NSRL) for the proposition 65 carcinogen bromoethane [PDF] [accessed 2019 March 4].
ChemIDplus [database]. 2018. Bethesda (MD): US National Library of Medicine. [updated NS; accessed 2018 October 30].
[ConsExpo Web] Consumer Exposure Web Model. 2018. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Dales R, Kauri LM, Sabit C, Mamun M, Weichenthal SA, Ryswyk KV, Kumarathasan P, Thomson E, Vincent R, Broad G, Liu L. 2013. Acute changes in lung function associated with proximity to a steel plant: A randomized study. Environ Int. 55:15-19.
[ECCC] Environment and Climate Change Canada. 2009. Substances search. [accessed 2019 October 24].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification and Risk Assessment Priorities: Results of the 2015 Review [PDF]. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018a. Rapid screening of substances with limited general population exposure. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018b. Screening assessment: substances identified as being of low concern using the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2019. NAPS Data Products [accessed 2019 April 16].
[ECHA] European Chemical Agency. 2019. Summary of classification and labelling - bromoethane. [accessed 2019 October 24].
[ECHA] European Chemical Agency. 2019. Summary of classification and labelling – chloroethane. [accessed 2019 October 24]
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Frasch HF, Dotson GS and Barbero AM. 2011. In vitro human epidermal penetration of 1-bromopropane. J Tox Env Health, Part A. 74(19):1249-1260.
Great Lake Chemical Corporation. 2002. Test Plan for Ethyl bromide (CAS# 74-96-4) [PDF]. [accessed 2019 December 19].
[HSDB] Hazardous Substances Data Bank, 2002 [Database]. Ethyl bromide. Bethesda (MD): National Library of Medicine. [accessed 2018 November 28].
[HSDB] Hazardous Substances Data Bank, 2013 [Database]. 1-bromopropane. Bethesda (MD): National Library of Medicine. [accessed 2018 November 28].
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate
Health Canada 2010a. Montreal Congestive Heart Failure Study. 2008-2010.
Health Canada. 2010b. Regina Indoor Air Quality Study (2007): Data Summary for Volatile Organic Compound Sampling [PDF]. Ottawa (ON): Government of Canada. [accessed 2019 April 24].
Health Canada. 2010c. Windsor Exposure Assessment Study (2005-2006): Data Summary for Volatile Organic Compound Sampling [PDF]. Ottawa (ON): Government of Canada. [accessed 2019 April 24].
Health Canada. 2012. Halifax Indoor Air Quality Study (2009): Volatile Organic Compounds (VOC) Data Summary. Ottawa (ON): Government of Canada.
Health Canada. 2013. Edmonton Indoor Air Quality Study (2010). Volatile Organic Compounds (VOC) Data Summary [PDF]. Ottawa (ON): Government of Canada. [accessed 2019 April 24].
Health Canada. 2013. Interim Guidance on Human Health Risk Assessment for Short-term Exposure to Carcinogens at Contaminated Sites [PDF]. Ottawa (ON): Government of Canada. [accessed 2019 May 7].
Health Canada. 2015. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa (ON): Government of Canada.
Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
Health Canada. 2018. Draft backgrounder document on updated default body surface areas for use in ESRAB assessments. Unpublished report. Ottawa (ON): Government of Canada.
[IARC] International Agency for Research on Cancer. 1999. Bromoethane (Groupe 3) [PDF]. [accessed on 2019 Feb 25].
[IARC] International Agency for Research on Cancer. 1999. Chloroethane (Groupe 3) [PDF]. [accessed on 2019 Feb 25].
[IARC] International Agency for Research on Cancer. 2018. Some industrial chemicals. IARC Monogr Eval Carcinog Risk Chem Hum. 115:37-67.
Konietzko H. 1984. Chlorinated ethanes: Sources, distribution, environmental impact and health effects. Hazard Assessment of Chemicals. 3:401-448.
Li Y, Cakmak S, Zhu J. 2019. Profiles and monthly variations of selected volatile organic compounds in indoor air in Canadian homes: results of Canadian national indoor air survey 2012-2013. Environ Int. 126:134–144.
Mallach G, St-Jean M, MacNeill M, Aubin D, Wallace L, Shin T, Van Ryswyk K, Kulka R, You H, Fugler D, et al. 2017. Exhaust ventilation in attached garages improves residential indoor air quality. Indoor Air. 27(2):487-499.
Morgan A, Black A, Belcher DR. 1970. The excretion in breath of aliphatic halogenated hydrocarbons following administration by inhalation. Ann Occup Hyg. 13:210-233.
[MSDS] Material Safety Data Sheet. 2010. HFE Super Cleaner Degreaser [PDF]. Burlington (ON): MG Chemicals. [accessed 2019 April 11].
[MSDS] Material Safety Data Sheet. 2014. Contact Cleaner 2000 Precision Cleaner [PDF]. Warminster, (PA): CRC Industries Inc. [accessed 2019 February 8].
[MSDS] Material Safety Data Sheet. 2015a. Dura II A/C Flush Solvent [PDF]. Lewisvill (TX): MicroCare Corporation. [accessed 2019 April 11].
[MSDS] Safety Data Sheet. 2016a. Silicone Mold Release [PDF]. Warminster (PA): CRC Industries Inc. [accessed September 2019].
[MSDS] Material Safety Data Sheet. 2016b. Touch ‘n Foam Professional System 15 CCMC Part-A [PDF]. Pacific (MO): Convenience Products. [accessed 2019 April 11].
[MSDS] Material Safety Data Sheet. 2016c. Touch ‘n Foam Professional System 15 CCMC Part-B [PDF]. Pacific (MO): Convenience Products. [accessed 2019 April 11].
[MSDS] Material Safety Data Sheet. 2017a. Ace Starting Fluid [PDF]. Brampton (ON): Kleen-Flo Tumber Ind. Ltd. [accessed 2018 November 21].
[MSDS] Safety Data Sheet. 2017b. Tool Crib Electric Contract Cleaner [PDF]. Sycamore (IL): Seymour of Sycamore. [accessed September 2019].
[MSDS] Safety Data Sheet. 2017c. Johnsen’s Premium A/C Flush Non-Flammable 1 Gallon [PDF]. Cleburne (TX): Technical Chemical Company. [accessed September 2019].
[MSDS] Material Safety Data Sheet. 2017d. AlbaChem Spot Lifter II [PDF]. Long Island City (NY): Albatross USA Inc. [accessed 2019 February 8].
[NTP] National Toxicology Program (US). 1989. Toxicology and carcinogenesis studies of bromoethane (ethyl bromide) (CAS No. 74-96-4) in F344/N rats and B6C3F1 mice (inhalation studies) [PDF]. [accessed 2018 December 20].
[NTP] National Toxicology Program (US). 1989. Toxicology and carcinogenesis studies of chloroethane (ethyl chloride) (CAS No. 75-00-3) in F344/N rats and B6C3F1 mice (inhalation studies) [PDF]. [accessed 2019 January 10].
[NTP] National Toxicology Program (US). 2016. Reports on Carcinogens, Fourteenth Edition. 1-Bromopropane [PDF]. [accessed 2019 February 22].
[OECD] Organization for Economic Cooperation and Development. 2006. SIDS Initial Assessment Report for SIAM22 [PDF] [accessed 2019 Feb 1].
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[PhysProp] Interactive PhysProp Database [database]. 2013. Syracuse (NY): SRC, Inc. [accessed 2018 May 25].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2014. General fact sheet: general default parameters for estimating consumer exposure: updated version for ConsExpo 4 [PDF]. Bilthoven (NL): RIVM. Report No.: 090013003/2014. [accessed 2019 September].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2018. Cleaning products fact sheet: default parameters for estimating consumer exposure: updated version for ConsExpo 4 [PDF]. Bilthoven (NL): RIVM. Report No.: 2016-0179. [accessed September 2019].
[US EPA] United States Environmental Protection Agency. 1987. Household solvent products: A national usage survey [PDF]. (EPA-OTS 560/5-87-005). Washington (DC): US EPA, Office of Toxic Substances, Office of Pesticides and Toxic Substances.
[US EPA] United States Environmental Protection Agency. 1989. Bromomethane; CASRN 74-83-8 Carcinogenicity Assessment (II.). Washington (DC): US EPA. [accessed 2019 March 15].
[US EPA] United States Environmental Protection Agency. 2005. Supplemental guidance for assessing susceptibility from early-life exposure to carcinogens [PDF]. Washington (DC): US EPA. [accessed 2019 March].
[US EPA] United States Environmental Protection Agency. 2010. Toxicological Review of cis-1,2-dichloroethylene and trans-1,2-dichloroethylene. Washington (DC): US EPA. EPA/635/R-09/006F.
[US EPA] United States Environmental Protection Agency. 2011. Chapter 6: Inhalation Rates. Exposure Factors Handbook 2011 Edition (Final). Washington (DC): US EPA. EPA/600/R-09/052F.
[US EPA] United States Environmental Protection Agency. 2016. TSCA Work Plan Chemical Risk Assessment PEER REVIEW DRAFT 1-Bromopropane: (n-Propyl Bromide) Spray Adhesives, Dry Cleaning, and Degreasing Uses CAS RN: 106-94-5 [PDF]. Washington (DC): US EPA. [accessed 15 April 2019].
[US EPA] United States Environmental Protection Agency. 2018a. ChemView [database on the Internet]. Washington (DC): US EPA. [updated 2018 October 26; accessed 31 October 2019].
[US EPA] United States Environmental Protection Agency. 2018b. Problem Formulation of the Risk Evaluation for 1-Bromopropane [PDF]. Washington (DC): US EPA. [accessed 15 April 2019].
[US EPA] US Environmental Protection Agency. 2020. Risk Evaluation for 1-Bromopropane [PDF]. Washington (DC): US EPA, Office of Chemical Safety and Pollution Prevention. [accessed 2020 August 12].
Weichenthal S, Mallach G, Kulka R, Black A, Wheeler A, You H, St-Jean M, Kwiatkowski R, Sharp D. 2012. A randomized double blind cross-over study of indoor air filtration and acute changes in cardiorespiratory health in a First Nations community. Indoor Air. 23(3):175-184.
[WHO] World Health Organization. 2002. Concise International Chemical Assessment Document 42 Bromoethane [PDF]. [accessed 2019 January 14].
Appendix A. Concentrations of substances in the Alkyl Halides Group measured in air in Canada
| Sampling year | Lab detection limit (µg/m3) | No. of samples | % samples >DL | Conc. range (µg/m3) | Geo. mean conc. (µg/m3) | 95th perc. (µg/m3) |
|---|---|---|---|---|---|---|
| 1990 | 0.043 | 0 | ‒ | ‒ | ‒ | ‒ |
| 1991 | 0.043 | 443 | 0.0 | <DL | ‒ | <DL |
| 1992 | 0.043 | 3 040 | 0.3 | <DL-0.065 | <DL | <DL |
| 1993 | 0.043 | 2 588 | 0.4 | <DL-0.211 | 0.068 | <DL |
| 1994 | 0.043 | 2 803 | 1.4 | <DL-0.580 | 0.066 | <DL |
| 1995 | 0.043 | 2 179 | 0.6 | <DL-0.152 | <DL | <DL |
| 1996 | 0.043 | 2 252 | 1.4 | <DL-0.094 | <DL | <DL |
| 1997 | 0.043 | 2 000 | 5.2 | <DL-0.563 | <DL | 0.043 |
| 1998 | 0.043 | 2 287 | 0.0 | <DL-0.121 | <DL | <DL |
| 1999 | 0.043 | 2 252 | 19.2 | <DL-0.080 | <DL | 0.050 |
| 2000 | 0.043 | 2 469 | 5.3 | <DL-0.100 | <DL | 0.050 |
| 2001 | 0.043 | 3 027 | 4.6 | <DL-0.150 | <DL | <DL |
| 2002 | 0.043 | 3 437 | 1.9 | <DL-0.125 | <DL | <DL |
| 2003 | 0.043 | 6 095 | 0.1 | <DL-0.098 | <DL | <DL |
| 2004 | 0.043 | 3 253 | 0.0 | <DL | <DL | <DL |
| 2005 | 0.043 | 3 135 | 0.6 | <DL-0.225 | <DL | <DL |
| 2006 | 0.043 | 2 849 | 1.1 | <DL-2.931 | <DL | <DL |
| 2007 | 0.043 | 2 740 | 0.3 | <DL-0.188 | <DL | <DL |
| 2008 | 0.043 | 3 330 | 0.1 | <DL-0.174 | <DL | <DL |
| 2009 | 0.043 | 3 296 | 1.8 | <DL-20.318 | <DL | <DL |
| 2010 | 0.043 | 2 772 | 0.1 | <DL-0.393 | <DL | <DL |
| 2011 | 0.043 | 2 459 | 0.1 | <DL-0.063 | <DL | <DL |
| 2012 | 0.043 | 2 548 | 0.1 | <DL-0.098 | <DL | <DL |
| 2013 | 0.058 | 1 381 | 0.1 | <DL-0.084 | <DL | <DL |
| 2014 | 0.059 | 0 | ‒ | ‒ | ‒ | ‒ |
| 2015 | 0.075 | 0 | ‒ | ‒ | ‒ | ‒ |
| 2016 | 0.093 | 0 | ‒ | ‒ | ‒ | ‒ |
Abbreviation: DL, detection limit.
| Season | Type of sample, sample duration, location | DL (µg/m3) | No. of samples | Conc. range (µg/m3) | % homes >DLa | Geo. mean (µg/m3) | 95th perc. (µg/m3) |
|---|---|---|---|---|---|---|---|
| Summer | Indoor, 24-hr, Edmontonc | 0.065 | 328 | <DL | 0.0 | <DL | <DL |
| Summer | Outdoor, 24-hr, Edmontonc | 0.065 | 324 | <DL | 0.0 | <DL | <DL |
| Winter | Indoor, 24-hr, Edmontonc | 0.035 | 337 | <DL-0.067 | 2.0 | <DL | <DL |
| Winter | Outdoor, 24-hr, Edmontonc | 0.035 | 332 | <DL | 0.0 | <DL | <DL |
| Summer | Indoor, 24-hr, Halifaxd | 0.036 | 331 | <DL | 0.0 | <DL | <DL |
| Summer | Outdoor, 24-hr, Halifax | 0.036 | 324 | <DL-0.154 | 2.0 | <DL | <DL |
| Winter | Indoor, 24-hr, Halifaxd | 0.036 | 312 | <DL | 0.0 | <DL | <DL |
| Winter | Outdoor, 24-hr, Halifaxd | 0.036 | 287 | <DL | 0.0 | <DL | <DL |
| Summer | Indoor, 24-hr, Reginae | 0.034 | 105 | <DL | 0.0b | <DL | <DL |
| Summer | Indoor, 5-d, Reginae | 0.034 | 101 | <DL | 0.0b | <DL | <DL |
| Summer | Outdoor, 24-hr, Reginae | 0.034 | 108 | <DL | 0.0b | <DL | <DL |
| Summer | Outdoor, 5-d, Reginae | 0.034 | 97 | <DL | 0.0b | <DL | <DL |
| Winter | Indoor, 24-hr, Reginae | 0.034 | 105 | <DL | 0.0b | <DL | <DL |
| Winter | Indoor, 5-d, Reginae | 0.034 | 89 | <DL | 1.1b | <DL | <DL |
| Winter | Outdoor, 24-hr, Reginae | 0.034 | 94 | <DL | 0.0b | <DL | <DL |
| Summer | Indoor, 24-hr, Windsorf | 0.070 | 217 | <DL | 0.0 | <DL | <DL |
| Summer | Outdoor, 24-hr, Windsorf | 0.070 | 216 | <DL | 0.0 | <DL | <DL |
| Summer | Personal, 24-hr, Windsorf | 0.070 | 207 | <DL | 0.0 | <DL | <DL |
| Winter | Indoor, 24-hr, Windsorf | 0.070 | 232 | <DL-0.070 | 2.1 | <DL | <DL |
| Winter | Outdoor, 24-hr, Windsorf | 0.070 | 201 | <DL | 0.0 | <DL | <DL |
| Winter | Personal, 24-hr, Windsorf | 0.070 | 225 | <DL | 0.0 | <DL | <DL |
| Summer | Indoor, 24-hr, Windsorf | 0.074 | 211 | <DL | 0.0 | <DL | <DL |
| Summer | Outdoor, 24-hr, Windsorf | 0.074 | 214 | <DL | 0.0 | <DL | <DL |
| Winter | Indoor, 24-hr, Windsorf | 0.074 | 224 | <DL | 0.0 | <DL | <DL |
| Winter | Outdoor, 24-hr, Windsorf | 0.074 | 214 | <DL | 0.0 | <DL | <DL |
| NA | Indoor, 24-hr, Montrealg | 0.065 | 285 | <DL | 0.0 | <DL | <DL |
| NA | Outdoor, 24-hr, Montrealg | 0.036 | 200 | <DL | 0.0 | <DL | <DL |
| Summer | Outdoor, 24-hr, Sault Ste-Marieh | 0.029 | 108 | <DL | 0.0 | <DL | <DL |
| Winter | Indoor, 24-hr, Swan Lakei | 0.025 | 67 | <DL | 0.0 | <DL | <DL |
| Winter | Indoor, 7-d, Swan Lakei | 0.025 | 54 | <DL | 0.0 | <DL | <DL |
| Winter | Indoor, 48-hr, Ottawaj | 0.022 | 166 | <DL | 3.0 | <DL | <DL |
| Winter | Outdoor, 48-hr, Ottawaj | 0.022 | 151 | <DL | 0.0 | <DL | <DL |
| Winter | Garage, 48-hr, Ottawaj | 0.022 | 206 | <DL | 0.0 | <DL | <DL |
Abbreviations: DL, detection limit; NA, not available
a Percentage of homes with at least one sample greater than the detection limit.
b Percentage of samples greater than the detection limit.
c Health Canada 2013
d Health Canada 2012
e Health Canada 2010b
f Health Canada 2010c
g Health Canada 2010a
h Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Dales et al. 2013).
i Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Weichenthal et al. 2012).
j Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Mallach et al. 2017).
| Sampling year | Lab detection limit (µg/m3) | No. of samples | % samples >DL | Conc. range (µg/m3) | Geo. mean (µg/m3) | 95th perc. (µg/m3) |
|---|---|---|---|---|---|---|
| 1990 | 0.027 | 0 | ‒ | ‒ | ‒ | ‒ |
| 1991 | 0.027 | 443 | 24.4 | <DL-2.680 | 0.176 | 0.258 |
| 1992 | 0.027 | 3 040 | 22.2 | <DL-2.857 | 0.085 | 0.235 |
| 1993 | 0.027 | 2 588 | 13.6 | <DL-2.728 | 0.158 | 0.183 |
| 1994 | 0.027 | 2 803 | 33.4 | <DL-49.673 | 0.117 | 0.317 |
| 1995 | 0.027 | 2 179 | 42.3 | <DL-4.613 | 0.062 | 0.230 |
| 1996 | 0.027 | 2 252 | 67.0 | <DL-2.664 | 0.059 | 0.368 |
| 1997 | 0.027 | 2 000 | 90.4 | <DL-5.729 | 0.084 | 0.198 |
| 1998 | 0.027 | 2 287 | 77.8 | <DL-3.100 | 0.061 | 0.180 |
| 1999 | 0.027 | 2 253 | 98.8 | <DL-1.890 | 0.086 | 0.180 |
| 2000 | 0.027 | 2 475 | 99.6 | <DL-2.350 | 0.082 | 0.160 |
| 2001 | 0.027 | 6 537 | 59.1 | <DL-4.355 | 0.040 | 0.113 |
| 2002 | 0.027 | 6 451 | 51.2 | <DL-0.724 | 0.031 | 0.089 |
| 2003 | 0.027 | 9 995 | 14.4 | <DL-0.422 | <DL | 0.047 |
| 2004 | 0.027 | 8 530 | 6.4 | <DL-2.390 | <DL | 0.030 |
| 2005 | 0.027 | 7 716 | 8.6 | <DL-0.937 | <DL | 0.032 |
| 2006 | 0.027 | 7 536 | 10.7 | <DL-0.388 | <DL | 0.037 |
| 2007 | 0.027 | 6 412 | 14.2 | <DL-0.330 | <DL | 0.040 |
| 2008 | 0.027 | 7 066 | 13.6 | <DL-0.183 | <DL | 0.038 |
| 2009 | 0.027 | 6 621 | 9.9 | <DL-2.927 | <DL | 0.034 |
| 2010 | 0.027 | 7 532 | 8.2 | <DL-0.909 | <DL | 0.032 |
| 2011 | 0.027 | 6 933 | 12.8 | <DL-0.144 | <DL | 0.038 |
| 2012 | 0.027 | 6 506 | 8.5 | <DL-0.324 | <DL | 0.032 |
| 2013 | 0.067 | 3 437 | 0.7 | <DL-0.190 | <DL | <DL |
| 2014 | 0.069 | 4 811 | 1.6 | <DL-0.176 | <DL | <DL |
| 2015 | 0.044 | 4 435 | 1.5 | <DL-0.436 | <DL | <DL |
| 2016 | 0.065 | 2 192 | 0.2 | <DL-0.101 | <DL | <DL |
Abbreviation: DL, detection limit
| Season | Type of sample, sample duration, location | DL (µg/m3) | No. of samples | Conc. range (µg/m3) | % homes >DLa | Geo. mean (µg/m3) | 95th perc. (µg/m3) |
|---|---|---|---|---|---|---|---|
| Summer | Indoor, 24-hr, Edmontonc | 0.062 | 328 | <DL-0.105 | 12.0 | <DL | <DL |
| Summer | Outdoor, 24-hr, Edmontonc | 0.062 | 324 | <DL-0.071 | 2.0 | <DL | <DL |
| Winter | Indoor, 24-hr, Edmontonc | 0.028 | 337 | <DL-0.493 | 52.0 | <DL | 0.053 |
| Winter | Outdoor, 24-hr, Edmontonc | 0.028 | 332 | <DL-0.258 | 14.0 | <DL | <DL |
| Summer | Indoor, 24-hr, Halifaxd | 0.028 | 331 | <DL-0.132 | 90.0 | <DL | 0.064 |
| Summer | Outdoor, 24-hr, Halifaxd | 0.028 | 324 | <DL-0.230 | 56.0 | <DL | 0.036 |
| Winter | Indoor, 24-hr, Halifaxd | 0.028 | 312 | <DL-0.110 | 90.0 | <DL | 0.053 |
| Winter | Outdoor, 24-hr, Halifaxd | 0.028 | 287 | <DL-0.102 | 58.0 | <DL | 0.044 |
| Summer | Indoor, 24-hr, Reginae | 0.039 | 105 | <DL-0.383 | 53.3b | <DL | 0.100 |
| Summer | Indoor, 5-d, Reginae | 0.039 | 101 | <DL-0.415 | 64.2b | 0.040 | 0.113 |
| Summer | Outdoor, 24-hr, Reginae | 0.039 | 108 | <DL-0.125 | 12.0b | <DL | 0.052 |
| Summer | Outdoor, 5-d, Reginae | 0.039 | 97 | <DL-0.187 | 124.4b | <DL | 0.060 |
| Winter | Indoor, 24-hr, Reginae | 0.039 | 105 | <DL-0.273 | 43.8b | <DL | 0.113 |
| Winter | Indoor, 5-d, Reginae | 0.039 | 89 | <DL-0.450 | 52.8b | <DL | 0.100 |
| Winter | Outdoor, 24-hr, Reginae | 0.039 | 94 | <DL-0.080 | 3.2b | <DL | 0.020 |
| Summer | Indoor, 24-hr, Windsorf | 0.115 | 217 | <DL-1.120 | 48.9 | <DL | 0.230 |
| Summer | Outdoor, 24-hr, Windsorf | 0.115 | 216 | <DL-0.165 | 2.2 | <DL | <DL |
| Summer | Personal, 24-hr, Windsorf | 0.115 | 207 | <DL-1.020 | 38.6 | <DL | 0.160 |
| Winter | Indoor, 24-hr, Windsorf | 0.115 | 232 | <DL-1.150 | 2.1 | <DL | <DL |
| Winter | Outdoor, 24-hr, Windsorf | 0.115 | 201 | <DL | 0.0 | <DL | <DL |
| Winter | Personal, 24-hr, Windsorf | 0.115 | 225 | <DL-0.360 | 8.5 | <DL | <DL |
| Summer | Indoor, 24-hr, Windsorf | 0.110 | 211 | <DL-0.390 | 23.9 | <DL | 0.123 |
| Summer | Outdoor, 24-hr, Windsorf | 0.110 | 213 | <DL-0.162 | 6.7 | <DL | <DL |
| Winter | Indoor, 24-hr, Windsorf | 0.110 | 224 | <DL-0.115 | 2.1 | <DL | <DL |
| Winter | Outdoor, 24-hr, Windsorf | 0.110 | 213 | <DL | 0.0 | <DL | <DL |
| NA | Indoor, 24-hr, Montrealg | 0.028 | 285 | <DL-0.433 | 77.8 | 0.059 | 0.147 |
| NA | Outdoor, 24-hr, Montrealg | 0.062 | 200 | <DL-0.136 | 55.6 | <DL | <DL |
| Summer | Outdoor, 42-hr, Sault Ste-Marieh | 0.027 | 108 | <DL-0.068 | 100.0 | <DL | 0.040 |
| Winter | Indoor, 24-hr, Swan Lakei | 0.045 | 67 | <DL -0.300 | 81.0 | 0.071 | 0.220 |
| Winter | Indoor, 7-d, Swan Lakei | 0.045 | 54 | <DL-0.267 | 95.2 | 0.092 | 0.233 |
| Winter | Indoor, 48-hr, Ottawaj | 0.027 | 166 | <DL-0.080 | 97.0 | <DL | 0.036 |
| Winter | Outdoor, 48-hr, Ottawaj | 0.027 | 151 | <DL-0.036 | 69.7 | <DL | <DL |
| Winter | Garage, 48-hr, Ottawaj | 0.027 | 206 | <DL-0.108 | 97.0 | <DL | 0.040 |
Abbreviation: DL, detection limit; NA, not available
a Percentage of homes with at least one sample greater than the detection limit.
b Percentage of samples greater than the detection limit.
c Health Canada 2013
d Health Canada 2012
e Health Canada 2010b
f Health Canada 2010c
g Health Canada 2010a
h Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Dales et al. 2013).
i Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Weichenthal et al. 2012).
j Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Mallach et al. 2017).
| Type of sample | % detected | MDL (µg/m3) | Maximal concentration (µg/m3) |
|---|---|---|---|
| Indoor | 0.3 | 0.29 | 3.1 |
Abbreviation: MDL, method detection limit
| Sampling year | Lab detection limit (µg/m3) | No. of samples | % samples >DL | Conc. range (µg/m3) | Geo. mean (µg/m3) | 95th perc. (µg/m3) |
|---|---|---|---|---|---|---|
| 1990 | 0.013 | 1 326 | ‒ | <DL-0.000 | ‒ | <DL |
| 1991 | 0.013 | 1 327 | 2.1 | <DL-0.139 | 0.036 | <DL |
| 1992 | 0.013 | 3 040 | 2.7 | <DL-0.223 | <DL | <DL |
| 1993 | 0.013 | 2 588 | 0.9 | <DL-0.267 | 0.065 | <DL |
| 1994 | 0.013 | 2 803 | 0.9 | <DL-0.075 | 0.014 | <DL |
| 1995 | 0.013 | 2 179 | 17.8 | <DL-0.098 | 0.017 | 0.028 |
| 1996 | 0.013 | 2 252 | 31.3 | <DL-0.135 | 0.023 | 0.039 |
| 1997 | 0.013 | 2 000 | 44.7 | <DL-0.220 | 0.030 | 0.060 |
| 1998 | 0.013 | 2 287 | 8.2 | <DL-0.060 | 0.018 | 0.018 |
| 1999 | 0.013 | 2 219 | 67.9 | <DL-0.070 | 0.035 | 0.050 |
| 2000 | 0.013 | 2 469 | 96.2 | <DL-0.830 | 0.028 | 0.040 |
| 2001 | 0.013 | 3 027 | 93.4 | <DL-0.070 | 0.029 | 0.040 |
| 2002 | 0.013 | 3 437 | 84.8 | <DL-0.102 | 0.023 | 0.041 |
| 2003 | 0.013 | 6 095 | 1.0 | <DL-0.817 | <DL | <DL |
| 2004 | 0.013 | 3 253 | 1.0 | <DL-0.849 | <DL | <DL |
| 2005 | 0.013 | 3 135 | 1.3 | <DL-0.233 | <DL | <DL |
| 2006 | 0.013 | 2 849 | 1.2 | <DL-0.398 | <DL | <DL |
| 2007 | 0.013 | 2 740 | 3.2 | <DL-0.516 | <DL | <DL |
| 2008 | 0.013 | 3 330 | 3.7 | <DL-0.371 | <DL | <DL |
| 2009 | 0.013 | 3 296 | 5.6 | <DL-0.349 | <DL | 0.014 |
| 2010 | 0.013 | 2 772 | 23.2 | <DL-2.013 | <DL | 0.044 |
| 2011 | 0.013 | 2 459 | 39.4 | <DL-0.587 | <DL | 0.058 |
| 2012 | 0.013 | 2 548 | 31.3 | <DL-0.277 | <DL | 0.048 |
| 2013 | 0.041 | 1 381 | 8.3 | <DL-2.230 | <DL | 0.059 |
| 2014 | 0.042 | 0 | ‒ | ‒ | ‒ | ‒ |
| 2015 | 0.052 | 0 | ‒ | ‒ | ‒ | ‒ |
| 2016 | 0.083 | 0 | ‒ | ‒ | ‒ | ‒ |
| Season | Type of sample, sample duration, location | DL (µg/m3) | No. of samples | Conc. range (µg/m3) | % homes >DLa | Geo. mean (µg/m3) | 95th perc. (µg/m3) |
|---|---|---|---|---|---|---|---|
| Summer | Indoor, 24-hr, Edmontonc | 0.042 | 328 | <DL-0.135 | 28.0 | <DL | 0.055 |
| Summer | Outdoor, 24-hr, Edmontonc | 0.042 | 324 | <DL-0.114 | 34.0 | <DL | 0.046 |
| Winter | Indoor, 24-hr, Edmontonc | 0.021 | 337 | <DL-0.484 | 50.0 | <DL | 0.120 |
| Winter | Outdoor, 24-hr, Edmontonc | 0.021 | 332 | <DL-0.292 | 58.0 | <DL | 0.056 |
| Summer | Indoor, 24-hr, Halifaxd | 0.018 | 331 | <DL-5.084 | 28.0 | <DL | 0.028 |
| Summer | Outdoor, 24-hr, Halifaxd | 0.018 | 324 | <DL-0.550 | 28.0 | <DL | 0.024 |
| Winter | Indoor, 24-hr, Halifaxd | 0.018 | 312 | <DL | 0.0 | <DL | <DL |
| Winter | Outdoor, 24-hr, Halifaxd | 0.018 | 287 | <DL | 0.0 | <DL | <DL |
| Summer | Indoor, 24-hr, Reginae | 0.019 | 105 | <DL-2.045 | 10.5b | <DL | 0.205 |
| Summer | Indoor, 5-d, Reginae | 0.019 | 101 | <DL-3.305 | 10.9b | <DL | 0.255 |
| Summer | Outdoor, 24-hr, Reginae | 0.019 | 108 | <DL-0.605 | 7.4b | <DL | 0.098 |
| Summer | Outdoor, 5-d, Reginae | 0.019 | 97 | <DL-0.413 | 10.3b | <DL | 0.314 |
| Winter | Indoor, 24-hr, Reginae | 0.019 | 105 | <DL-0.933 | 1.0b | <DL | <DL |
| Winter | Indoor, 5-d, Reginae | 0.019 | 89 | <DL-1.190 | 1.1b | <DL | <DL |
| Winter | Outdoor, 24-hr, Reginae | 0.019 | 94 | <DL | 0.0b | <DL | <DL |
| Summer | Indoor, 24-hr, Windsorf | 0.069 | 217 | <DL | 0.0 | <DL | <DL |
| Summer | Outdoor, 24-hr, Windsorf | 0.069 | 216 | <DL | 0.0 | <DL | <DL |
| Summer | Personal, 24-hr, Windsorf | 0.069 | 207 | <DL | 0.0 | <DL | <DL |
| Winter | Indoor, 24-hr, Windsorf | 0.069 | 232 | <DL | 0.0 | <DL | <DL |
| Winter | Outdoor, 24-hr, Windsorf | 0.069 | 201 | <DL | 0.0 | <DL | <DL |
| Winter | Personal, 24-hr, Windsorf | 0.069 | 225 | <DL | 0.0 | <DL | <DL |
| Summer | Indoor, 24-hr, Windsorf | 0.055 | 211 | <DL-0.110 | 2.2 | <DL | <DL |
| Summer | Outdoor, 24-hr, Windsorf | 0.055 | 214 | <DL-0.065 | 2.2 | <DL | <DL |
| Winter | Indoor, 24-hr, Windsorf | 0.055 | 224 | <DL | 0.0 | <DL | <DL |
| Winter | Outdoor, 24-hr, Windsorf | 0.055 | 214 | <DL | 0.0 | <DL | <DL |
| NA | Indoor, 24-hr, Montrealg | 0.042 | 285 | <DL-1.263 | 55.6 | <DL | 0.060 |
| NA | Outdoor, 24-hr, Montrealg | 0.018 | 200 | <DL-0.084 | 53.7 | 0.019 | 0.053 |
| Summer | Outdoor, 42-hr, Sault Ste-Marieh | 0.021 | 108 | <DL-2.228 | 100.0 | 0.042 | 0.088 |
| Winter | Indoor, 24-hr, Swan Lakei | 0.020 | 67 | <DL-0.013 | 0.0 | <DL | <DL |
| Winter | Indoor, 7-d, Swan Lakei | 0.020 | 54 | <DL-0.027 | 9.5 | <DL | <DL |
| Winter | Indoor, 48-hr, Ottawaj | 0.017 | 166 | <DL-20.672 | 36.4 | 0.535 | 0.184 |
| Winter | Outdoor, 48-hr, Ottawaj | 0.017 | 151 | <DL-0.132 | 33.3 | <DL | 0.028 |
| Winter | Garage, 48-hr, Ottawaj | 0.017 | 206 | <DL-16.764 | 42.4 | 0.460 | 0.136 |
Abbreviations: DL, detection limit; NA, not available
a Percentage of homes with at least one sample greater than the detection limit.
b Percentage of samples greater than the detection limit.
c Health Canada 2013
d Health Canada 2012
e Health Canada 2010b
f Health Canada 2010c
g Health Canada 2010a
h Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Dales et al. 2013).
i Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Weichenthal et al. 2012).
j Personal communication from Tim Shin and Ron Garson, WAQB, Health Canada, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Mallach et al. 2017).
Appendix B. Calculation of cancer risk for bromoethane, chloroethane and 1-bromopropane
For the purpose of estimating the risk of cancer from exposure to bromoethane, chloroethane and 1-bromopropane, a lifetime average daily dose (LADD) from air was calculated using the following equation (Health Canada 2013):
LADD = exposure rate x exposure duration / lifetime
where,
Exposure rate = daily intake in µg/kg bw/day
Exposure duration = exposure duration during life stage (years)
Lifetime = years in a lifetime = 80 years
Estimates of daily intake and assumptions for various age groups within the general population of Canada:
- 0–5 months: Assumed to weigh 6.3 kg (Health Canada 2015) and to breathe 3.7 m3 of air per day (US EPA 2011).
- 6–11 months: Assumed to weigh 9.1 kg (Health Canada 2015), to breathe 5.4 m3 of air per day (US EPA 2011).
- 1 year: Assumed to weigh 11.0 kg (Health Canada 2015) and to breathe 8.0 m3 of air per day (US EPA 2011).
- 2–3 years: Assumed to weigh 15 kg (Health Canada 2015) and to breathe 9.2 m3 of air per day (US EPA 2011).
- 4-8 years: Assumed to weigh 23 kg (Health Canada 2015) and to breathe 11.1 m3 of air per day (US EPA 2011).
- 9-13 years: Assumed to weigh 42 kg (Health Canada 2015) and to breathe 13.9 m3 of air per day (US EPA 2011).
- 14-18 years: Assumed to weigh 62 kg (Health Canada 2015) and to breathe 15.9 m3 of air per day (US EPA 2011).
- ≥19 years: Assumed to weigh 74 kg (Health Canada 2015) and to breathe 15.1 m3 of air per day (US EPA 2011).
For non-threshold carcinogens acting through a mutagenic mode of action, it is recommended that age-dependent adjustment factors (ADAFs) be applied to the cancer slope factor with exposure averaged over a lifetime to account for the sensitivity of the age-specific exposure period (Health Canada 2013). Accordingly, the ADAFs (US EPA, 2005) were adjusted to the Health Canada age groups (see Table B-1) and applied.
| Life stage | Age (years) | ADAF |
|---|---|---|
| Infant | 0 to 5 months | 10 |
| Infant | 6 to 11 months | 10 |
| Toddler | 1 | 10 |
| Toddler | 2 to 3 | 3 |
| Child | 4 to 8 | 3 |
| Pre-teen | 9 to 13 | 3 |
| Teenager | 14 to 18 | 1.8a |
| Adult | 19 + | 1 |
a ADAF14–18 = (ADAF14–15 x D14-15/D14-18)+(ADAF16-18 x D16-18/D14-18) = 1.8, where Di = exposure duration (years) [ADAF14–18 = (3 x 2/5) + (1x3/5) = 1.8]
Bromoethane:
| Route of exposure | 0-5 months | 6-11 months | 1 year | 2 to 3 years | 4 to 8 years | 9-13 years | 14-18 years | 19 + years |
|---|---|---|---|---|---|---|---|---|
| Ambient aira | 5.43E-03 | 5.49E-03 | 6.73E-03 | 5.67E-03 | 4.46E-03 | 3.06E-03 | 2.37E-03 | 1.89E-03 |
| Indoor aira | 3.80E-02 | 3.84E-02 | 4.71E-02 | 3.97E-02 | 3.12E-02 | 2.14E-02 | 1.66E-02 | 1.32E-02 |
| Total intake | 4.35E-02 | 4.39E-02 | 5.38E-02 | 4.54E-02 | 3.57E-02 | 2.45E-02 | 1.90E-02 | 1.51E-02 |
a Estimated to be 0.074 µg/m3 daily exposure when using the highest DL from Canadian air monitoring data (i.e., NAPS program and seven Canadian air quality studies conducted in Windsor, Regina, Halifax, Edmonton, Montreal, Sault-Ste-Marie and Ottawa (Health Canada 2010a, 2010b, 2010c, 2012, 2013, [Personal communication from Tim Shin and Ron Garson, WAQB, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Dales et al. 2013, Mallach et al. 2017)]). Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
The estimates of daily intake from air as shown in Table B-2 were used to derive a lifetime average daily dose for the general population.
LADD from air:
LADD: (4.35E-02x0.5/80) + (4.39E-02x0.5/80) + (5.38E-02x1/80) + (4.54E-02x2/80) + (3.57E-02x5/80) + (2.45E-02x5/80) + (1.90E-02x5/80) + (1.51E-02x61/80)
=1.88E-02 µg/kg bw/day or 1.88E-05 mg/kg bw/day
The ADAFs from Table B-1 were then applied to the cancer risk calculation for each age group and used to derive lifetime cancer risk (see Table B3).
| Age-group | Age-specific cancer riska |
|---|---|
| 0 to 5 months | 1.98E-08 |
| 6 to 11 months | 2.00E-08 |
| 1 yr | 4.91E-08 |
| 2 to 3 yr | 2.48E-08 |
| 4 to 8 yr | 4.89E-08 |
| 9 to 13 yr | 3.35E-08 |
| 14 to 18 yr | 1.51E-07 |
| 19 + yr | 8.41E-08 |
| Lifetime cancer riskb = | 4.3E-07 |
a Age-specific cancer risk = [cancer slope factor (mg/kg bw/day)-1 x ADAF x exposure estimate (mg/kg bw/day) x averaging timeage group]; Example for infants 0 to 5 months old = 0.0073 (mg/kg bw/day)-1 x 10 x (4.35E-5 mg/kg bw/day x 0.5/80)/1000 = 1.98E-08
b Lifetime cancer risk = sum of all age-specific risk calculations
Chloroethane:
| Route of exposure | 0-5 months | 6-11 months | 1 year | 2 to 3 years | 4 to 8 years | 9-13 years | 14-18 years | 19 + years |
|---|---|---|---|---|---|---|---|---|
| Ambient aira | 2.70E-02 | 2.73E-02 | 3.35E-02 | 2.82E-02 | 2.22E-02 | 1.52E-02 | 1.18E-02 | 9.39E-03 |
| Indoor airb | 1.20E-01 | 1.21E-01 | 1.48E-01 | 1.25E-01 | 9.84E-02 | 6.75E-02 | 5.23E-02 | 4.16E-02 |
| Total intake | 1.47E-01 | 1.48E-01 | 1.82E-01 | 1.53E-01 | 1.21E-01 | 8.27E-02 | 6.41E-02 | 5.10E-02 |
a Estimated to be 0.368 µg/m3 daily exposure when using the highest 95th percentile concentration measured in ambient air in Canada (i.e., NAPS program). Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
b Estimated to be 0.233 µg/m3 daily exposure when using the highest 95th percentile concentration measured in indoor air in Canada (Health Canada 2010a, 2010b, 2010c, 2012, 2013, [Personal communication from Tim Shin and Ron Garson, WAQB, dated May 27, 2021; unreferenced. The details on the study design have been presented elsewhere (i.e., Dales et al. 2013, Mallach et al. 2017, and Weichenthal et al. 2012)]
The estimates of daily intake from air as shown in Table B-4 were used to derive a lifetime average daily dose for the general population.
LADD from air:
LADD: (1.47E-01x0.5/80) + (1.48E-01x0.5/80) + (1.82E-01x1/80) + (1.53E-01x2/80) + (1.21E-01x5/80) + (8.27E-02x5/80) + (6.41E-02x5/80) + (5.10E-02x61/80)
=6.35E-02 µg/kg bw/day or 6.35E-05 mg/kg bw/day
The ADAFs from Table B-1 were then applied to the cancer risk calculation for each age group and used to derive lifetime cancer risk (see Table B-5).
| Age-group | Age-specific cancer riska |
|---|---|
| 0 to 5 months | 2.29E-08 |
| 6 to 11 months | 2.32E-08 |
| 1 yr | 5.68E-08 |
| 2 to 3 yr | 2.87E-08 |
| 4 to 8 yr | 5.65E-08 |
| 9 to 13 yr | 3.88E-08 |
| 14 to 18 yr | 1.75E-07 |
| 19 + yr | 9.72E-08 |
| Lifetime cancer riskb = | 5.0E-07 |
a Age-specific cancer risk = [cancer slope factor (mg/kg bw/day)-1 x ADAF x exposure estimate (mg/kg bw/day) x averaging timeage group]; Example for infants 0 to 5 months old = 0.0025 (mg/kg bw/day)-1 x 10 x (1.47E-04 mg/kg bw/day x 0.5/80)/1000 = 2.29E-08
b Lifetime cancer risk = sum of all age-specific risk calculations
1-Bromopropane:
| Route of exposure | 0-5 months | 6-11 months | 1 year | 2 to 3 years | 4 to 8 years | 9-13 years | 14-18 years | 19 + years |
|---|---|---|---|---|---|---|---|---|
| Indoor aira | 1.59 | 1.61 | 1.97 | 1.66 | 1.31 | 8.98 | 0.696 | 0.553 |
a Estimated to be 3.1 µg/m3 daily exposure when using the maximal concentration measured in Canadian homes (Li et al. 2019).
The estimates of daily intake from air as shown in Table B-6 were used to derive a lifetime average daily dose for the general population.
LADD from air:
LADD: (1.59x0.5/80) + (1.61x0.5/80) + (1.97x1/80) + (1.66x2/80) + (1.31x5/80) + (0.898x5/80) + (0.696x5/80) + (0.553x61/80)
=0.690 µg/kg bw/day
The ADAFs from Table B-1 were then applied to the cancer risk calculation for each age group and used to derive lifetime cancer risk (see Table B3).
| Age-group | Age-specific cancer riska |
|---|---|
| 0 to 5 months | 1.70E-07 |
| 6 to 11 months | 1.70E-07 |
| 1 yr | 3.39E-07 |
| 2 to 3 yr | 2.03E-07 |
| 4 to 8 yr | 5.09E-07 |
| 9 to 13 yr | 5.09E-07 |
| 14 to 18 yr | 3.05E-07 |
| 19 + yr | 2.07E-06 |
| Lifetime cancer riskb = | 4.3E-06 |
a Age-specific cancer risk = age-specific cancer slope factor (µg/kg bw/day)-1 x ADAF x exposure estimate (µg/kg bw/day) x averaging timeage group], where age specific cancer slope factor (µg/kg bw/day)-1 = inhalation unit risk (µg/m3)-1 x body weight (kg) / inhalation rate (m3/day). (Example for infants 0 to 5 months old: Age specific cancer slope factor = 1 x 10-6 (µg/m3)-1 x 6.3 kg / 3.7 m3/day = 1.7 x 10-6 (µg/kg bw/day)-1, and age-specific cancer risk = 1.7 x 10-6 (µg/kg bw/day)-1 x 10 x (1.7x 10-6 µg/kg bw/day x 0.5/80) = 1.7x 10-7
b Lifetime cancer risk = sum of all age-specific risk calculations
Appendix C. Estimated inhalation and dermal exposures to humans from products available to consumers – Sentinel Scenarios
Assumptions used in scenarios to estimate the exposure to substances in the Alkyl Halides Group from products that may be available to consumers in Canada are summarized in Tables C-1. These products are not expected to be used by children. Thus, exposures were estimated based on the assumed weight (74 kg) of an adult (Health Canada 2015), inhalation rate of an adult (15.1 m3/day) (US EPA 2011) and use behaviours of an adult. Exposures were estimated using ConsExpo Web version 1.0.5 (ConsExpo Web 2018). Default parameters from ConsExpo Web version were used unless otherwise specified.
| Scenario | Substance | Assumptions |
|---|---|---|
| Starting fluid (engine starting aid) (spray) | Chloroethane |
Maximum concentration: 1% (MSDS 2017a) Frequency: 8 times per year (professional judgement) Inhalation: exposure to spray, instantaneous release Released mass: 14.4 g (professional judgement) Exposure duration: 1 min (professional judgement) Room volume: 34 m3 (garage) Ventilation rate: 1.5/hour (garage) Adult inhalation rate: 15.1 m3/day (US EPA 2011) Dermal: Direct contact, constant rate Exposed area: 2 190 cm2 (adult, sum of surface area for hands and forearms, Health Canada 2018b) Contact rate: 100 mg/min (for aerosol spray cans, RIVM 2018) Release duration: 12 seconds (based on released mass of 14.4 g and mass generation rate of 1.2 g/sec for aerosol spray cans (RIVM 2018)) |
| Silicone mold release spray | 1-bromopropane |
Maximum concentration: 30% (MSDS 2016a) Frequency: 12 times per year (professional judgement) Inhalation: exposure to vapour, instantaneous release Released mass: 192 g (US EPA 1987 in US EPA 2020) Exposure duration: 30 minutes (US EPA 1987 in US EPA 2020) Room volume: 20 m³ (unspecified room in RIVM 2014; aligns with the selection of utility room (20 m³) in US EPA 2020) Ventilation rate: 0.6 per hour (for unspecified room, RIVM 2014) Adult inhalation rate: 15.1 m³/day (US EPA 2011) Dermal: Direct contact, constant rate Exposed area: 2 190 cm2 (adult, sum of surface area for hands and forearms, Health Canada 2018b) Contact rate: 100 mg/min (for aerosol spray cans, RIVM 2018) Release duration: 160 seconds (based on released mass of 192 g and mass generation rate of 1.2 g/sec for aerosol spray cans (RIVM 2018)) Absorption fraction: 0.29% (US EPA 2020) |
| Automotive A/C flush (liquid) | 1-bromopropane |
Maximum concentration: 100% (MSDS 2017c) Frequency: 1 time per year (professional judgement) Inhalation: exposure to vapour, evaporation – constant release Product amount: 573 g (US EPA 2020) Exposure duration: 30 minutes (US EPA 1987 in US EPA 2020) Room volume: 34 m³ (garage in RIVM 2014) Ventilation rate: 1.5 per hour (for garage, RIVM 2014) Adult inhalation rate: 15.1 m³/day (US EPA 2011) Application temperature: 20 °C (US EPA 2019) Vapour pressure: 110.8 mmHg (Boublik et al. 1984 in US EPA 2020) Mass transfer coefficient: 3.47 m/hr (US EPA 2020) Release area: 730 cm² (US EPA 2020) Emission duration: 30 minutes (duration of activity of loading/flushing the fluid; US EPA 1987 in US EPA 2020) Dermal: Direct contact, instant application Exposed area: 2 190 cm² (adult, sum of surface area for hands and forearms, Health Canada 2018b) Product amount: 0.0121 g (based on the ConsExpo default of 0.01 mL, multiplied by the product density of 1.21 g/cm3; RIVM 2018) Absorption fraction: 0.29% |
| Electronic contact cleaner (spray) | 1-bromopropane |
Maximum concentration: 44.4% (MSDS 2017b) Frequency: 2 times per year (professional judgement) Inhalation: Exposure to vapour – Instantaneous release Released mass: 293 g (US EPA 1987 in US EPA 2020) Exposure duration: 30 minutes (US EPA 1987 in US EPA 2020) Room volume : 58 m³ (living room in RIVM 2014; aligns with the selection of living room in US EPA 2020) Ventilation rate : 0.5 per hour (for living room, RIVM 2014) Adult inhalation rate : 15.1 m³/day (EPA 2011) Application temperature: 20 °C (US EPA 2020) Vapour pressure: 110.8 mmHg (Boublik et al. 1984 in US EPA 2020) Dermal: Direct contact, constant rate Exposed area: 2 190 cm2 (adult, sum of surface area for hands and forearms, Health Canada 2018b) Contact rate: 100 mg/min (for aerosol spray cans, RIVM 2018) Release duration: 244 seconds (based on released mass of 293 g and mass generation rate of 1.2 g/sec for aerosol spray cans (RIVM 2018)) Absorption fraction: 0.29 % (US EPA 2020) |
| Powdered textile spot cleaner (spray) | Trans-1,2-dichloroethene |
Maximum concentration: 60% (MSDS 2017d) Frequency: 12 times per year (professional judgement) Inhalation: exposure to spray, instantaneous release Released mass: 23 g (professional judgement) Exposure duration: 1 hour (professional judgement) Room volume: 20 m3 (unspecified room) Ventilation rate: 0.6/hour (unspecified room) Adult inhalation rate: 15.1 m3/day (US EPA 2011) Dermal: Direct contact, constant rate Exposed area: 2 190 cm2 (adult, sum of surface area for hands and forearms, Health Canada 2018b) Contact rate: 100 mg/min (for aerosol spray cans, RIVM 2018) Release duration: 19.2 seconds (based on released mass of 23 g and mass generation rate of 1.2 g/sec for aerosol spray cans [RIVM 2018]) |
Appendix D. Estimated daily intake from inhalation exposure to trans-1,2-dichloroethene in air
| Age Group | 0 to 5 months | 6 to 11 months | 1 yr | 2 to 3 yr | 4 to 8 yr | 9 to 13 yr | 14 to 18 yr | 19+ yr |
|---|---|---|---|---|---|---|---|---|
| Air Concen-tration (95th perc.) (0.314 µg/m3) | 0.18 | 0.19 | 0.23 | 0.19 | 0.15 | 0.10 | 0.08 | 0.06 |
a The highest 95th percentile concentration measured in air in Canada was converted to estimated daily intakes for each population subgroup using the following equation: Internal dose (µg/kg bw/day) = concentration (µg/m3) × 100% inhalation absorption × inhalation rate (IR) (m3/day) ÷ body weight (BW) (kg), where the exposure factors for age group were: 0 to 5 months: BW = 6.3 kg, IR = 3.7 m3/day; 6 to 11 months: BW = 9.1 kg, IR = 5.4 m3/day; 1 yr: BW = 11 kg; IR = 8.0 m3/day; 2 to 3 yr: BW = 15 kg, IR = 9.2 m3/day; 4 to 8 yr: BW = 23 kg, IR = 11.1 m3/day; 9 to 13 yr: BW = 42 kg, IR = 13.9 m3/day; 14 to 18 yr: BW = 62 kg, IR= 15.9 m3/day; 19+ yr: BW = 74 kg, IR = 15.1 m3/day (Health Canada 2015, US EPA 2011)
