Draft screening assessment - Hexamethylenetetramines Group
Official title: Draft screening assessment - Hexamethylenetetramines Group
Chemical Abstracts Service Registry Numbers
4080-31-3
51229-78-8
58713-21-6
Environment and Climate Change Canada
Health Canada
March 2021
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of three substances referred to collectively under the Chemicals Management Plan as the Hexamethylenetetramines Group. Substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1), their Domestic Substances List (DSL) names and their common names and/or acronyms are listed in the table below.
| CAS RN | DSL name | Common name(s) |
|---|---|---|
| 4080-31-3a | 3,5,7-Triaza-1-azoniatricyclo[3.3.1.1³,7]decane, 1-(3-chloro-2-propenyl)-, chloride |
|
| 51229-78-8c | 3,5,7-Triaza-1-azoniatricyclo[3.3.1.1³,7]decane, 1-(3-chloro-2-propenyl)-, chloride, (Z)- |
|
| 58713-21-6c | 1,3,5,7-Tetraazatricyclo[3.3.1.1³,7]decane, hydrochloride |
|
a This CAS RN is a mixture of cis- and trans-1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride (CTAC).
b According to the European Commission database for information on cosmetic substances and ingredients (CosIng), “Quaternium-15” is represented by CAS RN 4080-31-3 or by a mixture containing CAS RN 4080-31-3 and CAS RN 51229-78-8.
c This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
d The Scientific Committee on Consumer Safety in Europe (SCCS) uses the term “Quaternium-15” to refer to cis‑CTAC (CAS RN 51229-78-8).
The substances in the Hexamethylenetetramines Group have been included in a survey issued pursuant to section 71 of CEPA. In the 2011 calendar year, cis/trans-CTAC and cis-CTAC were imported into Canada at volumes of 10 000 to 100 000 kg, and 1 000 to 10 000 kg, respectively. There were no reports of cis/trans-CTAC and cis-CTAC in Canada being manufactured above the reporting threshold of 100 kg. There were no reports of methenamine hydrochloride being manufactured in or imported to Canada above the reporting threshold of 100 kg. In Canada, cis/trans-CTAC and cis-CTAC are used in the automotive, aircraft, and transportation sectors; in automotive care products; and in paints and coatings. Consumer uses for these two substances include cleaning products, paints, adhesives, and self-care products (i.e., cosmetics, non-medicinal ingredients in non‑prescription drugs, and natural health products). Cis/trans-CTAC is also a medicinal ingredient in drug products. In addition, it may be used as a component in food packaging materials, as well as in food contact surface cleaners and hand cleaners used in food processing establishments. Cis-CTAC may be used as a component in hand cleaners and lubricants used in food processing establishments. Cis/trans-CTAC, cis-CTAC, and methenamine hydrochloride have been identified as formulants in registered pest control products in Canada, with cis/trans-CTAC also identified as an active ingredient in pest control products.
The ecological risks of the substances in the Hexamethylenetetramines Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate, or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, cis/trans-CTAC, cis-CTAC, and methenamine hydrochloride are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from cis/trans-CTAC, cis-CTAC, and methenamine hydrochloride. It is proposed to conclude that cis/trans-CTAC, cis-CTAC, and methenamine hydrochloride do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
With respect to the human health assessment, cis/trans-CTAC and cis-CTAC were assessed together as they are both associated with the common International Nomenclature of Cosmetics Ingredients (INCI) name “Quaternium-15”, and contain one or more isomers of the compound 3,5,7-Triaza-1-azoniatricyclo[3.3.1.1³,7]decane, 1-(3-chloro-2-propenyl)-, chloride (CTAC). Effects on the testes and liver were identified to be the critical effects associated with cis/trans-CTAC and cis-CTAC, via the dermal route of exposure, on the basis of available data in laboratory studies. A comparison of the critical effect levels with estimates of exposure from the use of a non-prescription sunscreen lotion or a body moisturizer (as a natural health product) in younger subpopulations (e.g., infants, toddlers, and children) resulted in margins of exposure (MOEs) that were considered potentially inadequate to address uncertainties in the health effects and exposure databases. The resultant MOEs for these products in other subpopulations (e.g., teens and adults) were considered adequate. The resultant MOEs for shampoo, body moisturizer (cosmetic), hair perm/straightener, wall paint, furniture cleaning wipe, and stain remover were considered adequate.
General toxicity (i.e., reduced body weight, food consumption) and developmental effects were identified to be the critical effects associated with cis/trans-CTAC and cis‑CTAC, via the oral route of exposure, on the basis of available data in laboratory animal studies. A comparison of the critical effect levels with estimates of oral exposure (i.e., dietary exposure from use in food packaging applications, drinking water, and hand-to-mouth contact with certain consumer products) resulted in MOEs that were considered adequate.
The health effects dataset for methenamine hydrochloride was considered to be limited, and toxicological data from an analogue (i.e., methenamine) was taken into consideration. Methenamine was assessed as part of the Heterocycles Group screening assessment published in June 2019, in which developmental toxicity and potential skin sensitization were identified as critical effects. With respect to exposure, methenamine hydrochloride has only been identified as an impurity in products available to consumers containing cis/trans-CTAC. Comparison of the critical effect levels with an exposure estimate from the most highly exposed subpopulation to cis/trans-CTAC containing methenamine hydrochloride as an impurity resulted in MOEs that are considered adequate to account for uncertainties in the exposure and health effects databases.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that cis/trans-CTAC and cis-CTAC meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that methenamine hydrochloride does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that cis/trans-CTAC and cis-CTAC meet one or more of the criteria set out in section 64 of CEPA.
It is also proposed to conclude that methenamine hydrochloride does not meet any of the criteria set out in section 64 of CEPA.
It is also proposed that cis/trans-CTAC and cis-CTAC do not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 2012), the Minister of the Environment and the Minister of Health have conducted a screening assessment of three substances referred to collectively under the Chemicals Management Plan as the Hexamethylenetetramines Group to determine whether these substances present or may present a risk to the environment or to human health. The substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
The ecological risks of the substances in the Hexamethylenetetramines Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016b). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
With respect to the human health risk assessment, cis/trans-CTAC and cis-CTAC were assessed together as they are both associated with the common International Nomenclature of Cosmetics Ingredients (INCI) name “Quaternium-15” and contain one or more isomers of the compound 3,5,7-Triaza-1-azoniatricyclo[3.3.1.1³,7]decane, 1-(3-chloro-2-propenyl)-, chloride (CTAC). This approach is similar to that taken by the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) (2014) in a toxicological report and the United States Environmental Protection Agency in a Reregistration Eligibility Decision for Dowicil®CTAC (US EPA 1995), in which the pesticide uses of cis/trans-CTAC and cis-CTAC were re-evaluated. The US EPA (1995) was the basis for the re-evaluation of pesticidal uses of CTAC in Canada (Health Canada 2005).
For methenamine hydrochloride, a read-across approach was applied, whereby toxicological data from the analogue methenamine were used to inform the health effects assessment. Methenamine was previously assessed by Health Canada and Environment and Climate Change Canada as part of the Heterocycles Group screening assessment (ECCC, HC 2019).
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, and uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to September 2019. Targeted literature searches for toxicological data were conducted up to November 2019. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review. Comments on the technical portions relevant to human health were received from Jennifer Flippin, Theresa Lopez, and Dr. Joan Garey, all affiliates of Tetra Tech. The ecological portion of this assessment is based on the ERC document (published on July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this draft screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 3), Domestic Substances List (DSL) names, and common names and/or acronyms for the individual substances in the Hexamethylenetetramines Group are presented in Table 2-1.
| CAS RN | DSL name (common names) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 4080-31-3a | 3,5,7-Triaza-1-azoniatricyclo[3.3.1.1³,7]decane, 1-(3-chloro-2-propenyl)-, chloride (cis/trans-CTAC,Quaternium-15b) | ![[N+]12(CN3CN(C1)CN(C2)C3)C\C=C\Cl.[ClH-]](/content/dam/eccc/images/pded/hexamethylenetetramines/20210203-t21a.jpg) C9H16ClN4·Cl C9H16ClN4·Cl | 251 |
| 51229-78-8 | 3,5,7-Triaza-1-azoniatricyclo[3.3.1.1³,7]decane, 1-(3-chloro-2-propenyl)-, chloride, (Z)- (cis-CTAC, Quaternium-15c) | (C3)C\C=C/Cl.[ClH-]](/content/dam/eccc/images/pded/hexamethylenetetramines/20210203-t21b.jpg) C9H16ClN4·Cl C9H16ClN4·Cl | 251 |
| 58713-21-6 | 1,3,5,7-Tetraazatricyclo[3.3.1.1³,7]decane, hydrochloride (methenamine hydrochloride) | 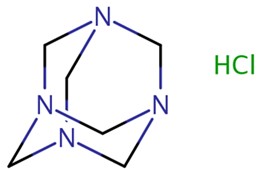 C6H12N4·HCl C6H12N4·HCl | 177 |
aThis CAS RN is a mixture of cis- and trans-1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride (CTAC) (SCCS 2011).
b According to the European Commission database for information on cosmetic substances and ingredients (CosIng), “Quaternium-15” is represented by CAS RN 4080-31-3 or by a mixture containing CAS RN 4080-31-3 and CAS RN 51229-78-8 (EC 2019).
c The Scientific Committee on Consumer Safety in Europe (SCCS 2011) uses the term “Quaternium-15” to refer to cis-CTAC (CAS RN 51229-78-8).
Cis/trans-CTAC and cis-CTAC are both associated with the common INCI name “Quaternium-15” (US EPA 1995; Becker et al. 2010; SCCS 2011). The former substance represents a mixture of isomers that contains approximately 31% to 60% cis-CTAC and 20% to 53% trans-CTAC (US EPA 1995; SCCS 2011). Since toxicological studies conducted on the mixture do not always report the relative concentration of each isomer, it is not possible to definitively distinguish whether health effects are mediated by one isomer or the other. Accordingly, cis/trans-CTAC and cis-CTAC were assessed together in the human health assessment and the toxicological data from both were taken into consideration.
Methenamine hydrochloride represents an impurity in cis/trans-CTAC. It has been reported in some toxicological studies to represent 3.1% of cis/trans-CTAC (Carney et al. 2006; Carney et al. 2008; Hansen et al. 2008, all cited in SCCS 2011) and can reach concentrations of up to 7% (SCCS 2011).
2.1 Selection of analogues
A read-across approach using data from analogues, where appropriate, was used to inform the human health assessments. Analogues were identified on the basis of similarity to substances within this group (e.g., structural similarity, similar physical-chemical properties, toxicokinetics, reactivity) that had relevant empirical data that could be used to read across to endpoints with limited empirical data. Methenamine was the only analogue identified containing relevant toxicological data for cis/trans-CTAC, cis-CTAC, and methenamine hydrochloride with respect to toxicological endpoints with limited data. Information on the identity and chemical structure of methenamine is presented in Table 2-2. For further information on the physical-chemical properties and a summary of toxicological data, refer to Appendix A.
| CAS RN | Common name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 100-97-0 | Methenamine | 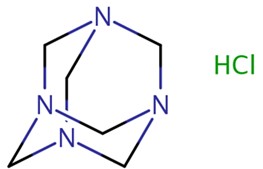 C6H12N4 C6H12N4 | 140 |
All of the substances in the Hexamethylenetetramines Group share a similar backbone in their chemical structure, with 4 nitrogen atoms connected in a ring (considered to be a “quaternary amine”). This structural backbone also exists for the analogue methenamine. Although the substances in the Hexamethylenetetramines Group and methenamine differ with respect to vapour pressure and Henry Law’s constants, they are comparable with respect to water solubility and octanol-water partition coefficients (refer to Appendix A).
The chemical structures of cis/trans-CTAC and cis-CTAC also contain a monohaloalkene functional group, which is not present in the chemical structure of the analogue methenamine. Certain monohaloalkenes have been associated with the induction of DNA adduct formation following cytochrome P450-mediated biotransformations into epoxide metabolites (Guengerich et al. 2003). With respect to cis/trans-CTAC and cis-CTAC, metabolism simulations using modelling software revealed that epoxide metabolites were observed under in vitro conditions, but not in vivo (OECD QSAR Toolbox 2017; TIMES 2016). To examine the impact of the haloalkene functional group, chemicals containing this substructure were taken into consideration.
1-chloropropene was identified as a representative monohaloalkene, despite being dissimilar to cis/trans-CTAC and cis-CTAC with respect to structural similarity and physical-chemical properties. Haloalkenes containing longer carbon chain lengths within a similar range as cis/trans-CTAC and cis-CTAC were not associated with relevant toxicological data. Information on the identity and chemical structure of 1‑chloropropene is presented in Table 2‑3. For further information on the physical-chemical properties and a summary of toxicological data, refer to Appendix A.
| CAS RN | Common name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 590-21-6 | 1-chloropropene | 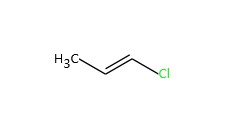 C3H5Cl C3H5Cl | 76 |
3. Physical and chemical properties
A summary of estimated physical and chemical property data of the substances in the Hexamethylenetetramines Group is presented in Table 3-1. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | cis/trans-CTACa | cis-CTACb | Methenamine hydrochlorideb |
|---|---|---|---|
| Physical state | Solid | Solid | NA |
| Vapour pressure (Pa) | 5.59 × 10−7 | 1.74 × 10−6 | 2.46 × 10−5 |
| Henry’s law constant (Pa·m3/mol) | 1.76 × 10−8 | 1.76 × 10−8 | 1.03 × 10−8 |
| Water solubility (mg/L) | 1.0 × 106 | 1.0 × 106 | 1.0 × 106 |
| Log Kow (dimensionless) | -5.29 | -5.29 | -5.67 |
Abbreviations: NA, Not Available; Kow, octanol-water partition coefficient
a ChemID Plus 1993-
b Epi Suite c2000-2012
4. Sources and uses
All of the substances in the Hexamethylenetetramines Group have been included in a survey issued pursuant to section 71 of CEPA (Canada 2012). Table 4-1 presents a summary of information reported on the total manufacture and total import quantities for the Hexamethylenetetramines Group from the reporting year 2011 (Environment Canada 2013).
| Common name | Total manufacturea (kg) | Total importsa (kg) |
|---|---|---|
| cis/trans-CTAC | NR | 10 000 – 100 000 |
| cis-CTAC | NR | 1 000 – 10 000 |
| Methenamine hydrochloride | NR | NR |
Abbreviations: NR, not reported above reporting threshold of 100 kg
a Values reflect quantities reported in response to the surveys issued pursuant to section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3). Confidential quantities are presented as a range of values.
Table 4-2 presents a summary of the major uses of substances in the Hexamethylenetetramines Group according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). Table 4-3 presents additional use information in Canada derived from other sources.
| Major usesa | Cis/trans-CTAC | Cis-CTAC | Methenamine hydrochloride |
|---|---|---|---|
| Automotive, aircraft, and transportation sectors | Y | N | N |
| Automotive care products | Y | N | N |
| Paints and coatings | Y | N | N |
| Personal care products | N | Y | N |
Abbreviations: Y = yes, this use was reported for this substance; N = no, this use was not reported for this substance
a Non-confidential uses reported in response to the survey issued pursuant to section 71 of CEPA (Environment Canada 2013). See surveys for specific inclusions and exclusions (schedules 2 and 3).
| Use | Cis/trans-CTAC | Cis-CTAC | Methenamine hydrochloride |
|---|---|---|---|
| Incidental additivesa | Y | Y | N |
| Food packaging materialsa | Y | N | N |
| Medicinal or non-medicinal ingredients in disinfectant, human, or veterinary drug productsb | Y | N | N |
| Medicinal or non-medicinal ingredients in licensed natural health productsc | Y | N | N |
| Present in cosmetics, based on notifications submitted under the Cosmetic Regulationsd | Y | Ye | N |
| Active ingredient or Formulant in registered pest control productsf | Y (Active and Formulant) | Y (Formulant) | Y (Formulant) |
a Personal communication, email from the Food Directorate (FD), Health Canada (HC), to the Existing Substances Risk Assessment Bureau (ESRAB), HC, dated Feb. 4, 2019; unreferenced.
b Personal communication, email from the Therapeutic Product Directorate (TPD), HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced.
c LNHPD [modified 2019].
d Personal communication, email from the Consumer and Hazardous Products Safety Directorate (CHPSD), HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced.
e Any cosmetic notifications under the INCI name “Quaternium-15” may also refer to cis-CTAC as it is not possible to know if cosmetics contain cis/trans-CTAC or cis-CTAC.
f Personal communication, email from the Pest Management Regulatory Agency (PMRA), HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced.
Cis/trans-CTAC and cis-CTAC are both identified by the INCI name Quaternium-15 (SCCS 2011; EC 2019). Quaternium-15 has a reported function as a preservative in products available to consumers, such as self-care productsFootnote 4 (cosmetics, non-prescription drugs, natural health products), adhesives, and paints (PubChem 2004- ). Quaternium-15 is reported as an ingredient in various cosmetics, in Canada, including rinse-off bath products, hair bleaches, facial cleansers, leave-in conditioners, face powder makeup, mascara, facial and body moisturizers, shampoos, face and body shaving products, and hair styling products (hair perm/straightener) (personal communication, email from the CHPSD, HC, to the ESRAB, HC, dated Feb. 4, 2019, unreferenced). Quaternium-15 is also found in non-prescription drug products in Canada, more specifically sunscreen lotions (personal communication, email from the TPD, HC, to the ESRAB, HC, dated Feb. 4, 2019, unreferenced). In addition, cis/trans-CTAC, listed as Quternium-15, is found in natural health products (NHPs), such as body moisturizers (personal communication, email from the NNHPD, HC, to the ESRAB, HC, dated Feb. 4, 2019, unreferenced).
Additional consumer uses of cis/trans-CTAC include wall paints, wallpaper adhesive, and various cleaning products, including bleach cleaners, toilet bowl cleaners, automotive cleaning wipes, and furniture wipes (ECCC 2016a; personal communication, email from the TPD, HC, to the ESRAB, HC, dated Feb. 4, 2019, unreferenced; SDS 2013; SDS 2015a; SDS 2016). Cis-CTAC has also been identified in a stain remover product (SDS 2018). Internationally, cis/trans-CTAC has been identified in a wood floor polish product in the United States (SDS 2015b); however, this product was confirmed to not be available in Canada (personal communication, email from Church & Dwight Canada Corp. to the ESRAB, HC, dated Feb. 6, 2020, unreferenced).
5. Environmental fate and behaviour
5.1 Environmental persistence
According to models used in ERC (ECCC 2016c), cis/trans-CTAC, cis-CTAC and methenamine hydrochloride are not expected to persist in water, air, sediment, or soil.
5.2 Potential for bioaccumulation
Given their low Kow and low bioconcentration factors (ECCC 2016c), cis/trans-CTAC, cis-CTAC, and methenamine hydrochloride are not expected to significantly bioaccumulate in organisms.
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risks of the substances in the Hexamethylenetetramines Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016b). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), and from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate, or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity, and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Hexamethylenetetramines Group, and the hazard, exposure, and risk classification results, are presented in ECCC (2016b).
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Cis/trans-CTAC | low | moderate | low |
| Cis-CTAC | low | low | low |
| Methenamine hydrochloride | low | low | low |
On the basis of low hazard and low exposure classifications according to information considered under ERC, cis-CTAC and methenamine hydrochloride were classified as having a low potential for ecological risk. It is unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under ERC, cis/trans-CTAC was classified as having a moderate exposure potential on the basis of a long overall persistence and a moderate annual import quantity according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). Cis/trans-CTAC was classified as having a low hazard potential and a low ecological risk potential. Although the use patterns result in a moderate exposure potential, considering the low hazard potential, cis/trans-CTAC is unlikely to be resulting in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Cis/trans-CTAC and cis-CTAC
7.1.1 Exposure assessment
Environmental media
Cis/trans-CTAC and cis-CTAC have low vapour pressures and high water solubility. No Canadian monitoring data in ambient air or drinking water were identified for either substance.
Due to the high water solubility of both cis/trans-CTAC and cis-CTAC, exposures through drinking water are possible. Estimated surface water concentrations were derived using the Environmental Assessment Unit (EAU) drinking water spreadsheet (Health Canada 2015). The resulting modelled maximum 50th percentile surface water concentrations for cis/trans-CTAC and cis-CTAC are 5.1 x 10-4 mg/L, and 7.2 x 10‑5 mg/L, respectively. These concentrations were derived using total import quantity information submitted in response to a CEPA section 71 survey (Environment Canada 2013). The surface water concentrations were used to estimate the daily intake of the two substances through drinking water, where the highest intake (per unit body weight) was 6.7 x 10-5 mg/kg bw/day and 9.4 x 10-6 mg/kg bw/day in formula-fed 0 to 5-month olds, for cis/trans-CTAC and cis-CTAC, respectively. The use of modelled surface water concentrations using the total import volumes into Canada to estimate drinking water intake is considered to be conservative. In the characterization of risk to human health (Table 7-3), an estimate of drinking water intake was derived for each cis/trans-CTAC and cis-CTAC separately. In addition, a combined estimate of daily intake from drinking water and estimates of dietary exposure from use in food packaging applications (see Food section, below) was also derived for cis/trans-CTAC.
Food
Cis/trans-CTAC has potential use in food packaging applications in Canada as an antimicrobial preservative in adhesives, which are separated from food by a functional barrier or for which the quantity of the adhesives that contacts food shall not exceed the trace amount at seams and edges of the packaging articles. The substance may also be used in the manufacture of polyurethane resins in contact with dry foods where migration of the substance into the food is not expected at ambient temperatures. Therefore, exposure from these uses is not expected (personal communication, email from the FD, HC to the ESRAB, HC, dated Feb. 4, 2019; unreferenced).
Cis/trans-CTAC may also be used as a preservative in latex pigment binders used in the manufacture of paper and paperboard that contact aqueous and fatty foods, and in the manufacture of acrylic coatings for paperboard cartons with direct food contact. The probable daily oral intake from these uses is estimated to be 1.3 x 10-4 mg/kg bw/day, which represents dietary exposure for the general population 12 months of age and older (personal communication, email from the FD, HC to the ESRAB, HC, dated Feb. 4, 2019 and Sept. 13, 2019; unreferenced).
Both cis/trans-CTAC and cis-CTAC may also be used as a component in incidental additives, such as food contact surface cleaners, hand cleaners and/or lubricants used in food processing establishments. The use of these cleaners is followed by potable water rinse after treatment, and there is no food contact with the lubricant use. Therefore, exposure through food resulting from these uses is not expected (personal communication, email from the FD, HC to the ESRAB, HC, dated Feb. 4, 2019; unreferenced).
Products available to consumers
Since the ingredient Quaternium-15 can refer to either cis/trans-CTAC or cis-CTAC, products for consumers containing Quaternium-15 were considered to contain either of the two substances. The main route of exposure from self-care products (cosmetics, natural health products (NHPs), and non-prescription drugs) is expected to be dermal due to the low vapour pressure of these substances (range from 5.59 x 10-7 to 1.74 x 10-6 Pa). Dermal exposure estimates from these products are presented in Table 7-1 below. Additional dermal scenarios for the other product types listed in Section 4 were considered, but exposure estimates were lower than those presented in Table 7‑1. Details on the method and parameters used to derive estimates of dermal exposure are provided in Appendix B. Because the same products and concentrations are considered for both substances, the exposures to cis/trans-CTAC and cis-CTAC are considered to be equivalent.
Estimated inhalation exposures to other products for consumers, such as wall paints, cleaning wipes, and stain removers, are negligible (less than 2.5 ng/kg bw/day). Dermal exposures for these products are expected through direct product contact, and in toddlers from contacting treated surfaces following the application of furniture wipes and stain remover. In addition, incidental oral exposure via hand-to-mouth contact for toddlers can occur following the application of furniture wipes and stain remover. Exposure estimates for these products are presented in Table 7‑2; additional scenarios for the other product types listed were considered, but exposure estimates were lower than those presented in Table 7‑2. Details on the method and parameters used to derive exposure estimates are provided in Appendix B.
| Exposure scenario | Maximum concentration | Dermal exposure estimate |
|---|---|---|
| Body moisturizer (cosmetic); dermal; 14-18 year olds – 19+ year oldsa; daily | 0.1%b | 0.13-0.16 mg/kw bw/day |
| Shampoo; dermal; 19+ year oldsa; daily | 10%b | 0.17 mg/kg bw/day |
| Sunscreen lotion; dermal; 6-11 month olds; 19+ year old; daily | 0.07%c | 0.15-0.66 mg/kg bw/day |
| Body moisturizer (NHP)d; dermal; 0-5 month olds; 19+ year olds; daily | 0.1%e | 0.13-0.32 mg/kg bw/day |
| Hair perm/straightener product; dermal; 19+ year oldsa; per event | 0.1%b | 0.11 mg/kg bw per event |
a Product is intended for an older subpopulation only (personal communication, email from the CHPSD, HC, to the ESRAB, HC, dated Sept. 10, 2019; unreferenced).
b Personal communication, email from the CHPSD, HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced.
c Personal communication, email from the TPD, HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced.
d Product is specific to cis/trans-CTAC.
e Personal communication, email from the NNHPD, HC, to the ESRAB, HC, dated Sep. 4, 2019; unreferenced.
| Exposure scenario | Maximum concentration | Dermal exposure estimate (mg/kg bw per event) | Oral exposure estimate (mg/kg bw per event) |
|---|---|---|---|
| Wall paint; 19+ year old; per eventa | 0.1%b | 4.9 x 10-2 | N/A |
| Furniture cleaning wipe; 19+ year old; per eventa | 1%c | 6.8 x 10-3 | N/A |
| Furniture cleaning wipe – post application; 1 year old; per eventa | 1%c | 1.5 x 10-2 | 2.3 x 10-3 |
| Stain remover; 19+ year old; per eventd | 0.05%e | 4.1 x 10-3 | N/A |
| Stain cleaner – post application; 1 year old; per eventd | 0.05%e | 4.0 x 10-2 | 6.1 x 10-3 |
Abbreviations: N/A, Not Applicable
a Product contains cis/trans-CTAC
b ECCC 2016a
c SDS 2015a
d Product contains cis-CTAC
e SDS 2018
7.1.2 Health effects assessment
Since cis/trans-CTAC and cis-CTAC share the common name “Quaternium-15” and are sometimes identified under their common name only, the toxicological data for cis/trans‑CTAC and cis-CTAC were pooled together in the health effects assessment. These substances were previously assessed by the United States Environmental Protection Agency (US EPA) as part of a Reregistration Eligibility Decision document that assessed the potential human health and environmental risks associated with pesticide uses (US EPA 1995). A qualitative approach was taken for the characterization of risk since a toxicological endpoint of concern was not identified by the US EPA at that time. Since the assessment by the US EPA addressed the main formulation types registered in Canada and was relevant to registered Canadian pesticidal uses, the Pest Management Regulatory Agency (PMRA) utilized the US EPA (1995) assessment as a basis for its re-evaluation of cis/trans-CTAC and cis-CTAC (Health Canada 2004; Health Canada 2005). Since then, other international agencies have assessed these substances, namely the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) (2014). Other independent groups such as the Scientific Committee on Consumer Safety in Europe (SCCS) (2011), and the Cosmetic Ingredient Review (CIR) (1986) have also conducted assessments. These assessments reviewed additional health effects studies not captured by the US EPA (1995).
According to the harmonised classification and labelling approved by the European Union, cis-CTAC is harmful if swallowed (Acute Tox 4), suspected of damaging the unborn child (Repr 2), causes skin irritation (Skin Irrit 2), and may cause an allergic reaction (Skin Sens 1) (ECHA 2019). No classifications were found for cis/trans-CTAC. In the following health effects assessment, toxicological data for both cis/trans-CTAC and cis-CTAC were taken into consideration.
Repeated-dose toxicity
The effects of cis/trans-CTAC following subchronic oral exposure were described in a report whereby Sprague-Dawley rats (n = 10/sex/dose) were administered 0, 7.5, 15, 30, or 60 mg/kg bw/day cis/trans-CTAC in the diet for 90 days (Humiston et al. 1972, as cited in SCCS 2011). All dose levels were associated with significantly decreased food consumption and body weight (up to 20%), increased relative brain weight, and increased relative testes weights. At doses equal to or greater than 15 mg/kg bw/day, significantly lower alanine aminotransferase concentrations were also observed. At the highest dose level (60 mg/kg bw/day), there were findings of increased relative liver weights (accompanied by hepatocellular swelling), significantly elevated serum urea nitrogen levels, and significantly decreased alkaline phosphatase concentrations. A no observed adverse effect level (NOAEL) of 15 mg/kg bw/day was identified in the report, but a lowest observed adverse effect level (LOAEL) of 7.5 mg/kg bw/day was considered on the basis of general toxicity (decreased food consumption, body weight), and effects on the brain and testes. Furthermore, effects on the testes were also observed in other toxicological studies within the health effects dataset.
A report of a subchronic oral study in beagle dogs was also identified (Schwetz et al. 1976, as cited in SCCS 2011). In this study, beagle dogs (n = 4/sex/dose) were orally administered 0, 7.5, 15, and 30 mg/kg bw/day cis/trans-CTAC in gelatin capsules for 91 to 92 days. At doses equal to and greater than 15 mg/kg bw/day, lower absolute/relative heart weights were observed, along with significant decreases in aspartate/alanine aminotransferase levels. At the highest dose, one female animal was sacrificed on day 84 due to overt toxicity. In addition, absolute/relative liver weights were significantly greater than controls, which were accompanied by obliterative vasculitis and perivasculitis of hepatic blood vessels, infiltration of mononuclear cells, and hyperplasia of reticuloendothelial cells in the liver. Alterations in the heart tissue (i.e., multifocal myocardial degeneration, necrosis, and inflammation) were also observed in one male dog at the high dose level. Other male dogs exhibited significant reductions in packed cell volume, red/white blood cell counts, and percent haemoglobin. The report indicated that the liver and possibly heart were the target organs and a no observed effect level (NOEL) of 7.5 mg/kg bw/day was reported.
The effects of cis/trans-CTAC have also been examined in studies conducted using the dermal route of administration. Cis/trans-CTAC (in water) was topically applied under occluded conditions to New Zealand White rabbits (n=10/sex/dose) at concentrations of 0, 2.9, 11.8, or 60% (w/v), 6 hours per day for 91 days (Corley et al. 1988, as cited in SCCS 2011). This was equivalent to approximately 0, 50, 200, or 1000 mg/kg bw/day. Skin irritation was observed in a dose-dependent manner. At the highest concentration, increases in white blood cell count and platelets were observed (no further details). Gross pathological findings were limited to the skin (e.g., scabbing), which was accompanied by an inflammatory reaction in the epidermis and dermis (i.e., ulcerative dermatitis). The report suggested that no systemic toxicity was observed up to 1000 mg/kg bw/day.
Cis/trans-CTAC (in water) was also topically applied to mice (under occluded conditions, unknown strain, n=10/sex/dose) at dose levels of 0, 100, 400, and 1200 mg/kg bw/day, 6 hours per day for 90 days (Quast et al. 1996, as cited in SCCS 2011). The report indicated that there were no findings of systemic toxicity up to the highest dose tested (1200 mg/kg bw/day).
In general, the aforementioned dermal studies did not reveal any indications of systemic toxicity up to the highest dose tested for cis/trans-CTAC. However, this was not the case for studies conducted using cis-CTAC with shorter exposure durations. In a report of a short-term dermal study, 0, 10, 25, 50, and 100 mg/kg bw/day of cis-CTAC (in aqueous solution) was topically applied to the intact or abraded skin of sexually immature rabbits (n=5/sex/dose) for 7 hours per day, 5 days per week, for 3 weeks (Dow Chemical Company 1983, as cited in Becker et al. 2010 and CIR 1986). At 50 mg/kg bw/day, rabbits had decreased spermatogenesis and skin irritation. At 100 mg/kg bw/day, there were significant decreases in absolute testes weight, relative testes-to-brain weight, and skin irritation. Decreased spermatogenesis was observed in 4/5 males with intact skin and 3/5 males with abraded skin. No other treatment-related effects with respect to mortality, behaviour, body weight, haematological values, clinical chemistry, urinalyses, gross pathology, histopathology, or organ weights were observed. On the basis of this data, a NOAEL of 25 mg/kg bw/day was considered on the basis of testicular effects at the next dose level of 50 mg/kg bw/day.
In a follow-up study, the abraded backs of sexually mature New Zealand White rabbits (n = 7/dose) were treated topically with cis-CTAC (in water) at doses of 0, 25, 50, or 100 mg/kg bw/day (Lockwood et al. 1978, as cited in SCCS 2011; Dow Chemical Company 1983, as cited in CIR 1986). The test material was left in contact with the skin for 7 hours per day, 5 days per week for 30 days. Local effects such as chronic inflammation, degeneration, and necrosis of the skin were observed at the site of application. With respect to systemic effects, significantly decreased liver weights were observed at the mid- and high-dose levels. At the highest dose, body weight and food consumption were significantly decreased. A NOAEL of 25 mg/kg bw/day was identified by the authors of the study (no further details provided), presumably on the basis of liver effects observed at the next dose level of 50 mg/kg bw/day. Although this follow-up study did not reveal testicular effects, it should be noted that the life stages of the animals were different (sexually immature rabbits in the previous study vs. sexually mature animals in the current study).
Studies examining prototype cosmetic formulations containing cis/trans-CTAC or cis‑CTAC have also been reported (McCollister et al. 1969, as cited in SCCS 2011; Anonymous 1980, as cited in NTP 1991). In general, these studies did not reveal signs of systemic toxicity up to the highest tested dose of 3000 mg/kg bw/day. However, due to the presence of other ingredients in the products, limited details on concurrent control groups, and limited information on analytical chemistry, these studies were considered to be of limited utility for risk characterization.
Reproductive and developmental toxicity
Effects on the testes and spermatogenesis were observed in some of the studies discussed in the Repeated-dose toxicity section above (Humiston et al. 1972, as cited in SCCS 2011; Dow Chemical Company 1983, as cited in Becker et al. 2010). The lowest effect level identified for the oral and dermal routes were 7.5 mg/kg bw/day (LOAEL) and 25 mg/kg bw/day (NOAEL), respectively, on the basis of effects on testicular weights and spermatogenesis at higher dose levels (Dow Chemical Company 1983, as cited in Becker et al. 2010, and CIR 1986).
Studies examining developmental toxicity through the oral route of administration have been identified for both cis/trans-CTAC and cis-CTAC. In a report of a prenatal developmental toxicity study, New Zealand White rabbits (n=26 females/group) were administered by gavage 0, 2.5, 8, and 25 mg/kg bw/day of cis/trans-CTAC (31.3% cis‑CTAC, 32.5% trans-CTAC, in water), from gestation day (GD) 7 to 27 (Carney et al. 2008, as cited in SCCS 2011). At the highest dose level of 25 mg/kg bw/day, maternal toxicity such as decreased body weight gain and food consumption was observed throughout the entire dosing period. There were also observations of lower mean gravid uterine weights and lower mean fetal body weights at the same dose level. The report identified a NOEL of 8 mg/kg bw/day for both maternal and developmental toxicity. No developmental toxicity was observed when there was no maternal toxicity.
With respect to cis-CTAC, the potential for developmental toxicity was evaluated in a prenatal developmental study, whereby Fischer 344 rats (n=33 to 34 females/group) were gavaged 0, 5, 25, or 75 mg/kg bw/day cis-CTAC (97.9% purity, in water) from GD 6 to 15 (John et al. 1982, as cited in SCCS 2011). At the mid-dose level of 25 mg/kg bw/day, dams exhibited transient decreases in body weight and food consumption during the treatment period while the fetuses exhibited a significant increase in the incidence of total malformations (mainly eye anomalies such as microphthalmia [17% of litters] and anophthalmia). At the highest dose level of 75 mg/kg bw/day, dams exhibited a significant decrease in body weight, body weight gain, and food/water consumption. The dams also had an increase in absolute/relative liver weights and a significant increase in the incidence of resorptions. Fetuses in this dose group exhibited a higher incidence of total malformations (e.g., 19% of litters had microphthalmia) and weighed significantly less than controls. Although not significant, it was noted that two fetuses of the low-dose group (5 mg/kg bw/day) showed malformations. One fetus had micrognathia (undersized jaw) and anophthalmia while the other fetus exhibited polydactyly. A third fetus that died in utero had exencephaly, but this case was not included in the statistical evaluation. One case of anophthalmia was also found in the control group. A NOEL of 5 mg/kg bw/day was reported by the authors for maternal and fetal toxicity. However, since malformations were observed in a dose-dependent manner at higher doses, reaching statistical significance, the malformations observed at 5 mg/kg bw/day were considered to be toxicologically relevant. A LOAEL of 5 mg/kg bw/day was considered on the basis of malformations. For maternal toxicity, a NOAEL of 5 mg/kg bw/day was considered on the basis of decreased body weight and food consumption at the next dose level of 25 mg/kg bw/day.
It was reported that Fischer 344 rats had a high propensity for fetal eye defects and that the malformations observed in the previous study were likely related to a spontaneous genetic cluster effect (Carney et al. 2005, as cited in SCCS 2011). To test this hypothesis, another prenatal developmental toxicity study was performed whereby Fischer 344 rats (n=33 females) were administered by gavage 0, 25, or 75 mg/kg bw/day cis-CTAC (98.9% purity, in water) from GD 6 to 15 (Carney et al. 2005, as cited in SCCS 2011). Compared to the previous study, similar decreases in maternal body weight, body weight gains, and feed consumption were observed. In the fetuses, there was a litter incidence of 6.3% and 6.4% for microphthalmia/anophthalmia in the 25 and 75 mg/kg bw/day dose groups, respectively. These incidences were reported to be similar to historical control values and did not exhibit dose-dependence, suggesting that the previous study findings represented a spontaneous genetic cluster effect. However, the interpretation of this study was compromised by the fact that the 5 mg/kg bw/day dose level was not included or examined. Overall, a LOAEL of 5 mg/kg bw/day (on the basis of malformations) was considered for risk characterization purposes as it represented the most sensitive effect level in the dataset with respect to the oral route. This was consistent with the critical effect level identified by ANSES (2014).
With respect to the dermal route of exposure, there was one report of a combined repeated-dose and reproductive/developmental toxicity screening test for cis/trans-CTAC, and one report of a developmental toxicity test for cis-CTAC. In the first report, cis/trans-CTAC (31% cis-CTAC, 32% trans-CTAC, in water) was topically applied to Crl:CD(SD) rats (n=10/sex/dose) at dose levels equivalent to 0, 75, 225, and 750 mg/kg bw/day (Carney et al. 2006, as cited in SCCS 2011). Dermal effects (e.g. scaling, erythema, edema) were reported at all dose levels in the parental animals, and the highest dose group was sacrificed due to the severity of skin lesions. At the mid-dose level, there were significantly decreased body weights (8.1%) in females, significantly reduced food consumption in both sexes, and reduced triglyceride levels (unknown statistical significance). In the pups, there was reduced body weights (7.5-15%), which was statistically significant on post-natal day (PND) 21 in female pups at the mid-dose level. There was also significantly reduced food consumption in the pups. No treatment-related effects on reproductive performance, pup survival, and sex ratio were observed. A NOEL of 75 mg/kg bw/day was reported on the basis of general systemic toxicity (e.g., body weight) and reproductive toxicity (no further details) observed at the next dose level of 225 mg/kg bw/day.
With respect to cis-CTAC, there was a report of a prenatal developmental toxicity study whereby Fischer 344 rats (n=25 females/group) were topically applied (occlusively) 0, 250 or 500 mg/kg bw/day cis-CTAC (in water) from GD 6 to 15 (Dow Chemical Company 1984, as cited in Becker et al. 2010). The authors concluded that there were no maternal toxicity or effects on the offspring up to the highest dose tested of 500 mg/kg bw/day.
Genotoxicity and carcinogenicity
With respect to genotoxicity, cis-CTAC was found to be weakly mutagenic in bacterial mutagenicity assays (Zieger et al. 1988). It was mutagenic in Salmonella typhimurium strains TA98 and TA100, and in E. coli with and without metabolic activation (Kuramochi 1994, as cited in SCCS 2011). Cis/trans-CTAC was found to be mutagenic with metabolic activation in an in vitro mammalian gene cell mutation test, but was not mutagenic in the absence of metabolic activation (Linscombe et al. 1988, as cited in SCCS 2011). Cis-CTAC was demonstrated to be clastogenic in an in vitro mammalian chromosome aberration test conducted on Chinese hamster lung cells, but was not clastogenic in rat whole blood lymphocytes (Murli 1994, as cited in SCCS 2011). Cis-CTAC did not induce unscheduled DNA synthesis in rat hepatocytes (Domoradzki 1981, as cited in SCCS 2011).
Although there were some positive findings in the in vitro assays, cis-CTAC was not observed to be genotoxic in in vivo assays. It did not induce micronuclei in mouse erythrocytes (Day and Shabrang 2000, as cited in SCCS 2011) and did not induce unscheduled DNA synthesis (Cifone 2002, as cited in SCCS 2011) up to doses of 1000 mg/kg bw/day that were associated with signs of toxicity.
Studies examining the effects of cis/trans-CTAC or cis-CTAC following chronic exposure or examining carcinogenicity have not been identified. Toxicological data from the analogue methenamine (CAS RN 100-97-0) were taken into consideration. In order to investigate the impact of the monohaloalkene functional group within the chemical structure of cis/trans-CTAC and cis-CTAC, toxicological data from 1-chloropropene (CAS RN 590-21-6) were also considered. Neither of these substances were associated with any hazard classifications by the International Agency for Research on Cancer (IARC), ECHA, or the US EPA. For further information on the physical-chemical properties and summary health effects data, refer to Appendix A.
With respect to methenamine, no systemic toxicity or indications of carcinogenicity were observed in studies examining chronic dose levels up to 2500 mg/kg bw/day (Brendel 1964; Kewitz 1966, as cited in ECHA 2008; Della Porta et al. 1968; Natvig et al. 1971; Lijinsky and Taylor 1977).
For 1-chloropropene, only one limited carcinogenicity study was identified, whereby male and female Ha:ICR Swiss mice (n=30/group) were subjected to four different treatment regimens over a period of 342 to 649 days: repeated skin application (35 mg/kg bw/day), skin application in a tumour initiation-promotion assay (35 to 83 mg/kg bw/day), subcutaneous injection (5 mg/kg bw/day), and oral gavage (5 mg/kg bw/day) (Van Duuren et al. 1979). Clinical signs and body weights were documented. At the end of the study, complete gross macroscopic examinations were conducted and any abnormal tissues and organs were excised for histopathological examination. No treatment-related increases in tumour incidence were observed in the dermal and subcutaneous experiments. However, intragastric administration was associated with a significant increase in the number of female mice with forestomach tumours (43% vs 17% in the control group). A mode-of-action framework for evaluating the relevance of rodent forestomach tumours in cancer risk assessment has been published, which outlines decision criteria for use in considering tumour data for classification of potential human carcinogenicity (Proctor et al. 2007). According to the framework, tumours that are limited to the forestomach (an organ that is not present in humans) and only observed following oral gavage administration may not be relevant to human exposures. These tumours may result from chronic irritation and hyperplasia of the forestomach, ultimately leading to tumour formation. Chronic irritation of epithelial tissue is generally not consistent with human exposure conditions, which are likely to be below a threshold for irritation (Proctor et al. 2007).
Overall, the available data on 1-chloropropene and the analogue methenamine suggest that cis/trans-CTAC and cis-CTAC are not likely to result in carcinogenicity that would be relevant to human health.
Sensitization
Cis-CTAC resulted in sensitizing responses in a local lymph node assay (LLNA), with a EC3 value (the effective concentration inducing a threefold increase in lymph node cell proliferation compared to controls) of 20.8% (De Jong et al. 2007). BALB/c mice were treated with 25 μL of cis-CTAC on the dorsal skin of both ears for either 3 days or for 3 days, once weekly, followed by an additional 3 days. The animals were then sacrificed and auricular lymph nodes were excised and subjected to lymphocyte stimulation testing. Furthermore, cis/trans-CTAC and cis-CTAC have also been associated with contact dermatitis and are considered to be human allergens, with incidences of 0.6% to 1.9% and 7.1% to 9.6% in epidemiological studies conducted in Europe and the United States, respectively (SCCS 2011).
Formaldehyde release
Cis/trans-CTAC and cis-CTAC can be metabolized to formaldehyde, a substance which has been classified as a Group 1 carcinogen (IARC 2012). In a toxicokinetic study, rats administered up to 47 mg/kg bw/day of cis/trans-CTAC by gavage eliminated a large portion of the dose as carbon dioxide. Less than 0.12% of the administered dose was identified as formaldehyde (SCCS 2011). Some international jurisdictions have also considered Quaternium-15 to be a “formaldehyde releaser” in products available to consumers (SCCS 2011; Becker 2017). Creams containing 0.1 or 2% Quaternium-15 were found to contain 1000 or 2000 ppm formaldehyde, respectively, according to polarographic analysis (Jordan et al. 1979, as cited in CIR 1986). However, no releases were found for other products such as shampoos.
In a study examining formaldehyde release from preservatives, different types of cosmetic products (e.g., shampoo, bath gel, perfume, hand cleaner, toothpaste, and nail polish) were mixed with Quaternium-15 at a concentration of 1 mg per gram of product (Lv et al. 2015). The quantity of formaldehyde released from the products was found to be dependent on pH, time, temperature, and the type of product. More formaldehyde was released from products with higher usage times, higher temperatures, and those associated with more water content (i.e., shampoo, bath gel, hand cleaner). Although no point estimates were provided by the authors, visual inspection of the data revealed that the highest amount of formaldehyde that could be released from these products was approximately 2.5 mg/L.
There is inconsistency with respect to how releases of formaldehyde from preservatives are managed in different international jurisdictions. The US EPA (1995) identified potential exposures to formaldehyde for consumers. However at the time, the agency determined that the risk would be low since exposure in residential settings was minimal.
The Government of Australia did not consider formaldehyde to be a concern and indicated that it was not likely to be volatile from the low-concentration solutions present in products with cis/trans-CTAC or cis-CTAC (AGDH 2014). In the European Union (EU), Quaternium-15 was previously permitted in cosmetics up to a maximum concentration of 0.2%. As of June 2019, however, Quaternium-15 was added to the list of prohibited substances in cosmetics in the EU.
Quaternium-15 is not currently restricted in Canada. However, formaldehyde is restricted in cosmetics in Canada to 0.01% in non-aerosol products that release formaldehyde vapours, 0.1% in oral products, 0.2% in non-oral products, and 5% in nail hardeners (Health Canada 2018). A residential indoor air quality guideline for formaldehyde has also been published (Health Canada 2006). Given the restrictions on formaldehyde in cosmetics and the existence of indoor air quality guidelines, the focus of this assessment was on cis/trans-CTAC and cis-CTAC only, and the risk from exposure to formaldehyde was not characterized.
7.1.3 Characterization of risk to human health
On the basis of the data available, cis/trans-CTAC and cis-CTAC are not expected to be genotoxic in vivo. In addition, they are not expected to result in any carcinogenicity that would be relevant to human health on the basis of the toxicological data available on 1‑chloropropene and the analogue methenamine.
For the characterization of risk, the short-term dermal study conducted using cis-CTAC in developing rabbits was considered for dermal exposure scenarios relevant to infants, toddlers, and children. These scenarios included body moisturizer (NHP), sunscreen lotion, furniture cleaning wipes, and stain remover. A NOAEL of 25 mg/kg bw/day was identified on the basis of testicular effects observed at the next dose level of 50 mg/kg bw/day (Dow Chemical Company 1983, as cited in Becker et al. 2010; CIR 1986).
For dermal exposure scenarios that included teens and adults (i.e., body moisturizer [NHP and cosmetic], shampoo, sunscreen lotion, hair perm/straightener, wall paint, furniture cleaning wipe, and stain cleaner), the short-term dermal study conducted in rabbits of reproductive age was selected for the characterization of risk. A NOAEL of 25 mg/kg bw/day was identified on the basis of liver effects at the next dose level of 50 mg/kg bw/day (Lockwood et al. 1978, as cited in SCCS 2011; Dow Chemical Company 1983, as cited in CIR 1986). This was consistent with the effect level identified by the French Agency for Food, Environmental and Occupational Health & Safety for adult dermal exposure scenarios (ANSES 2014).
For oral exposure scenarios that included teens and adults, the prenatal developmental toxicity study conducted in rats (John et al. 1982, as cited in SCCS 2011) was used for the characterization of risk. A LOAEL of 5 mg/kg bw/day was identified on the basis of fetal malformations occurring in the absence of maternal toxicity. The same study was also selected for oral exposure scenarios relevant to infants, toddlers, and children. However, a NOAEL of 5 mg/kg bw/day was utilized for the characterization of riskbased on maternal toxicity (reduced body weight and food consumption) observed at the next dose level of 25 mg/kg bw/day. These effect levels are within the same order of magnitude as those identified from other toxicity studies. For example, a LOAEL of 7.5 mg/kg bw/day was identified on the basis of general toxicity, and effects on the brain and testes in a subchronic, oral study in rats (Humiston et al. 1972, as cited in SCCS 2011). In another oral study (prenatal developmental toxicity screening test in rabbits), a NOEL of 8 mg/kg bw/day was identified on the basis of maternal and developmental toxicity observed at the next dose level of 25 mg/kg bw/day (Carney et al. 2008, as cited in SCCS 2011).
Table 7‑3 provides all relevant exposure and hazard values for cis/trans-CTAC and cis‑CTAC, as well as resultant margins of exposure (MOEs), for the determination of risk.
| Exposure scenario | Exposure estimate | Critical effect level (mg/kg bw/day) | MOE |
|---|---|---|---|
| Body moisturizer (cosmetic) (0.1% concentration); dermal; 14-18 year olds – 19+ year olds; daily | 0.16 (14-18 year olds) - 0.13 (19+ year olds) mg/kg bw/day | 25a | 156 (14-18 year old) – 192 (19+ year olds) |
| Shampoo (10% concentration); dermal; 19+ year olds; daily | 0.17 mg/kg bw/day | 25a | 147 |
| Body moisturizer (NHP) (0.1% concentration)b; dermal; all sub-populations; daily | 0.32 (0-5 month old) - 0.13 (19+ year old)mg/kg bw/day | 25a,c | 79 (0-5 month old) – 185 (19+ year olds) |
| Sunscreen lotion (0.07% concentration); dermal; 6-11 month olds – 19+ year old; daily | 0.66 (6-11 month old) - 0.15 (19+ year olds) mg/kg bw/day | 25a,c | 38 (6-11 month old) – 167 (19+ year olds) |
| Hair perm/straightener product (0.1% concentration); dermal; 19+ year olds; per event | 0.11 mg/kg bw per event | 25a | 227 |
| Wall paint (0.1% concentration)b; dermal; 19+ year olds; per event | 4.9 x 10-2 mg/kg bw per event | 25a | 510 |
| Furniture cleaning wipe (1% concentration)b; dermal; 19+ year olds; per event | 6.8 x 10-3 mg/kg bw per event | 25a | 3 676 |
| Furniture cleaning wipe – post application (1% concentration)b; dermal; 1 year olds; per event | 1.5 x 10-2 mg/kg bw per event | 25c | 1 729 |
| Furniture cleaning wipe – post application (1% concentration)b; oral; 1 year olds; per event | 2.3 x 10-3 mg/kg bw per event | 5d | 2 304 |
| Stain remover (0.05% concentration)e; dermal; 19+ year olds; per event | 4.1 x 10-3 mg/kg bw per event | 25a | 6 098 |
| Stain remover – post application (0.05% concentration)e; dermal; 1 year olds; per event | 4.0 x 10-2 mg/kg bw per event | 25c | 618 |
| Stain remover – post application (0.05% concentration)e; oral; 1 year olds; per event | 6.1 x 10-3 mg/kg bw per event | 5d | 823 |
| Food packaging and drinking water intake (combined, cis/trans-CTAC)f ; oral; 1 year old – 19+ year olds; daily | 1.4 x 10-4 (19+ year olds) – 1.5 x 10-4 (1 year olds) mg/kg bw/day | 5d,g | 33 333 (1 year old) – 35 714 (19+ year olds) |
| Food packaging (cis/trans-CTAC)f; oral; 1+ years; daily | 1.3 x 10-4 mg/kg bw/day | 5d | 38 462 |
| Drinking water (cis/trans-CTAC); oral; 0-5 month olds; daily | 6.7 x 10-5 mg/kg bw/day | 5d | 74 627 |
| Drinking water (cis-CTAC); oral; 0-5 month olds; daily | 9.4 x 10-6 mg/kg bw/day | 5d | 531 915 |
a NOAEL=25 mg/kg bw/day, on the basis of liver effects observed at the next dose level of 50 mg/kg bw/day in a 30‑day, dermal study in sexually mature rabbits.
b Exposure is specific to cis/trans-CTAC.
c NOAEL of 25 mg/kg bw/day was identified on the basis of testicular effects observed at the next dose level of 50 mg/kg bw/day in a 3-week, dermal study in sexually immature rabbits.
d NOAEL=5 mg/kg bw/day, on the basis of maternal toxicity (reduced body weight and food consumption) in an oral prenatal developmental toxicity study in rats.
e Exposure is specific to cis-CTAC.
f Probable daily intakes from food packaging represent dietary exposure for the general population 12 months of age and older.
g LOAEL=5 mg/kg bw/day, on the basis developmental toxicity (i.e. malformations) in an oral prenatal developmental toxicity study in rats.
With respect to dermal exposure from the use of sunscreen lotion and body moisturizers (NHPs), comparison of the critical effect levels with the estimated exposures resulted in MOEs ranging from 38 to 79 for younger subpopulations (e.g. infants and children), which are considered potentially inadequate to address uncertainties in the exposure and health effects databases. The resultant MOEs for these products for the teen and adult subpopulations were considered adequate. For other products available to consumers (body moisturizer [cosmetic], shampoo, hair perm/straightener product, wall paint, furniture cleaning wipes, and stain removers), the MOEs were considered to be adequate.
With respect to the oral exposure from food packaging uses, drinking water, and hand-to-mouth exposure), comparison of the critical effect levels to the estimated exposures resulted in MOEs that are considered adequate to address uncertainties in the exposure and health effects databases.
7.1.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| The INCI name Quaternium-15 is assigned to both cis/trans-CTAC and cis-CTAC, and it was assumed that cosmetics containing Quaternium-15 could have either substance present as an ingredient | +/- |
| Lack of measured concentrations of cis/trans-CTAC and cis-CTAC in environmental media | +/- |
| Health effects data on trans-CTAC were not identified, precluding a comparison of toxicity between the two isomers (i.e., cis-CTAC vs. trans-CTAC). | +/- |
| No studies on chronic toxicity or carcinogenicity were identified for either cis/trans-CTAC or cis-CTAC. Toxicological data from the analogue methenamine and 1-chloropropene were taken into consideration. | +/- |
| There was insufficient evidence to treat ocular malformations as a genetic cluster effect, as proposed by Carney et al, 2005. | + |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7.2 Methenamine hydrochloride
7.2.1 Exposure assessment
No measured concentrations of methenamine hydrochloride in environmental media were identified, and there were no reports of methenamine hydrochloride being manufacted in or imported to Canada above the reporting threshold of 100 kg based on information submitted in response to a CEPA section 71 survey (Environment Canada 2013). Methenamine hydrochloride has been identified as an impurity at a concentration of 7% in a mixture of cis/trans-CTAC (SCCS 2011); the reported quantities of methenamine hydrochloride in Canada do not account for its presence as an impurity in cis/trans-CTAC. An estimated surface water concentration was derived using the EAU drinking water spreadsheet (Health Canada 2015), using total import information submitted for cis/trans-CTAC in response to a CEPA section 71 survey (Environment Canada 2013), and assuming a 7% impurity of methenamine hydrochloride. The resulting modelled maximum 50th percentile surface water concentration for methenamine hydrochloride is 2.88 x 10-5 mg/L, which results in an estimated daily intake of 3.4 x 10-6 mg/kg bw/day in formula fed 0 to 5 month olds, who were the highest exposed age group (per unit body weight). The use of modelled surface water concentrations, and the assumption that the total volume of cis/trans-CTAC imported into Canada contains 7% methenamine hydrochloride, to estimate exposure via drinking water intake is considered to be conservative.
There are no consumer uses identified for methenamine hydrochloride in Canada. However, the use of products available to consumers containing cis/trans-CTAC (maximum concentrations between 0.1% and 10% from Table 7-1) may result in dermal exposures to methenamine hydrochloride since methenamine hydrochloride can exist as an impurity of up to 7% in the cis/trans-CTAC mixture found in the product. To assess potential exposure to methenamine hydrochloride from its presence as an impurity in cis/trans-CTAC, exposure was calculated for the most highly exposed subpopulation to cis/trans-CTAC, exposure from a sunscreen product at a concentration of 0.07%. Exposure to cis/trans-CTAC was adjusted by 7% to account for the maximum amount of methenamine hydrochloride present, which results in exposure estimates of 1.1 x 10-2 to 4.6 x 10-2 mg/kg bw/day (6 to 11 month olds to 19+ year olds; 0.15 to 0.66 mg/kg bw/day exposure to cis/trans-CTAC * 7%).
7.2.2 Health effects assessment
The health effects dataset for methenamine hydrochloride is limited. To address these limitations, toxicological data from the analogue methenamine (CAS RN 100-97-0) were taken into consideration. This analogue represents the non-ionized form of methenamine hydrochloride. Following dissociation in aqueous solutions, methenamine hydrochloride is expected to share similar toxicity, metabolic pathways, and reactivity as the analogue methenamine.
The Government of Canada assessed methenamine as part of the Heterocycles Group screening assessment (ECCC, HC 2019). The health effects characterization for methenamine was based on international reviews conducted by ECHA (2008) and OECD (2007). A literature search was conducted from one year prior to the Heterocycles Group assessment (i.e., June 2018) to August 2019. No health effects studies, which could impact the health effects assessment (i.e., result in different critical endpoints or lower points of departure than those stated in the assessment) were identified.
The critical effects identified for methenamine were dermal sensitization at high doses and developmental effects (ECCC, HC 2019). For these critical effects, a positive effective concentration (EC3) of 30.6% and a NOAEL of 27 mg/kg bw/day were selected as the points of departures for the characterization of risk, respectively.
7.2.3 Characterization of risk to human health
Exposure of the general population to methenamine hydrochloride may occur through drinking water or through the use of products available to consumers containing cis/trans-CTAC, where methenamine hydrochloride may be present as an impurity (up to 7%). A margin of exposure was derived by comparing the estimates of exposure to methenamine hydrochloride from drinking water and from the use of sunscreen containing 0.07% cis/trans-CTAC to the critical effect level of 27 mg/kg bw/day (NOAEL), as identified in the Heterocycles Group screening assessment (ECCC, HC 2019). This results in margins of exposure ranging from 587 to 2 454 for the sunscreen for infants to adults, and over 1 000 000 for drinking water, which are considered to be adequate to account for uncertainties in the exposure and health effects databases. With respect to dermal sensitization, it was noted that the maximum concentration in products of 10% is approximately 44-fold lower than the EC3 of 30.6%. The products associated with this concentration (i.e., shampoo) represent rinse-off products and are likely to result in lower exposure potential. All other products are associated with concentrations that are at least 400-fold lower than the EC3.
7.2.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Lack of measured concentrations of methenamine hydrochloride in environmental media or products for consumers in Canada | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
8. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from cis/trans-CTAC, cis-CTAC, and methenamine hydrochloride. It is proposed to conclude that cis/trans-CTAC, cis-CTAC, and methenamine hydrochloride do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that cis/trans-CTAC and cis-CTAC meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that methenamine hydrochloride does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that cis/trans-CTAC and cis-CTAC meet one or more of the criteria set out in section 64 of CEPA.
It is therefore proposed to conclude that methenamine hydrochloride does not meet one or more of the criteria set out in section 64 of CEPA.
It is also proposed that cis/trans-CTAC and cis-CTAC do not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
[AGDH] Australian Government Department of Health. 2014. Formaldehyde donors: human health tier II assessment. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme (NICNAS).
[ANSES] Agence nationale de sécurité sanitaire alimentation, environnement, travail. 2014. Profil toxicologique du cis-CTAC (n° CAS 51229-78-8). Rapport d’expertise collective.
Becker LC. 2017. Quaternium-15. Int J Toxicol. 36(5 Supple 2):52S.
Becker LC, Bergfeld WF, Belsito DV, Klaassen CD, Hill R, Leibler D, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Alan Andersen F. 2010. Final report of the amended safety assessment of Quaternium-15 as used in cosmetics. Int J Toxicol. 29(3 Suppl):98S-114S.
Bremmer HF, Prud’homme de Lodder LCH, van Engelen JGM. 2006. Cosmetics Fact Sheet. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. RIVM Report 320104001/2006.
Brendel R. 1964. Untersuchungen an ratten zur verträglichkeit von hexamethylentetramin. Arzneimittel-Forsch. 14:51-53.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [accessed 2019 Mar 04].
[ConsExpo Web] Consumer Exposure Web Model. 2019. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[CIR] Cosmetic Ingredient Review. 1986. Final report on the safety assessment of quaternium-15. J Am Coll Toxicol. 5:61-101.
De Jong WH, De Klerk A, Beek MT, Veenman C, Van Loveren H. 2007. Effect of prolonged repeated exposure to formaldehyde donors with doses below the EC3 value on draining lymph node responses. J Immunotoxicol. 4(3):239-246.
Della Porta G, Colnaghi MI, Parmiani G. 1968. Non-carcinogenicity of methenamine in mice and rats. Food Cosmet Toxicol. 6(6): 707-715.
[EC] European Commission. 2019. Cosmetic Ingredients and Substances (CosIng) Simple Search Database [Internet]. Brussels (BE): EC.
[ECCC] Environment and Climate Change Canada. 2016a. Data collected from a targeted information gathering initiative for assessments under the Chemicals Management Plan (Fall 2016). Data prepared by ECCC, Health Canada; Existing Substances Program.
[ECCC] Environment and Climate Change Canada. 2016b. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016c. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2019. Screening assessment: Heterocycles Group. Chemical Abstracts Service Registry Numbers 100-97-0, 110-91-8, 4174-09-8. Ottawa (ON): Environment Canada, Health Canada. [accessed 2019 September 21].
[ECHA] European Chemicals Agency. 2008. Risk Assessment: Methenamine. Final Approved Version, 27.05.2008. Helsinki (FI): ECHA. p. 1-134.
[ECHA] European Chemicals Agency. 2019. Table of harmonised entries in Annex VI to CLP. Helsinki (FI): ECHA. [accessed 2019 September 21]. [xlsx, 583K]
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC, 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food Chem Toxicol. 78:159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC. 2016. Consumption of cosmetic products by the French population second part: Amount data. Food Chem Toxicol. 90:130-141.
Guengerich FP. 2003. Cytochrome P450 oxidations in the generation of reactive electrophiles: epoxidation and related reactions. Arch Biochem Biophys. 409(1):59-71.
Health Canada. 2004. Proposed Acceptability for Continuing Registration. Reevaluation of 1-(3-chloroallyl)-3,5,7-triaza-1-azonia adamantine chloride. Ottawa (ON): Government of Canada. PACR2004-17.
Health Canada. 2005. Re-evaluation Decision Document. 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride. Ottawa (ON): Government of Canada. RRD2005-03.
Health Canada. 2006. Residential Indoor Air Quality Guidelines: Formaldehyde. Ottawa (ON): Government of Canada. [accessed 2020 March 2].
Health Canada. 2015. Environmental Assessment Unit Drinking Water Spreadsheets [Excel format]. Ottawa (ON): Health Canada. [cited 2019 Sep 18].
Health Canada. 2018. Cosmetic Ingredient Hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Government of Canada. [accessed 2019 October 7].
Health Canada. 2019. Canadian exposure factors used in human health risk assessment. Unpublished report. Ottawa (ON): Government of Canada.
[IARC] International Agency for Research on Cancer. 2012. Chemical Agents and Related Occupations: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100F: Formaldehyde. Lyon (FR): International Agency for Research on Cancer, World Health Organization.
Lijinsky W, Taylor HW. 1977. Feeding tests in rats on mixtures of nitrite with secondary and tertiary amines of environmental importance. Food Cosmet Toxicol. 15(4):269-274.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 06]. Ottawa (ON): Government of Canada. [accessed 2019 Sep 18].
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol. 46:1516-1524.
Lv C, Hou J, Xie W, Cheng H. 2015. Investigation on formaldehyde release from preservatives in cosmetics. Int J Cosmet Sci. 37(5):474-478.
Natvig H, Andersen J, Rasmussen EW. 1971. A contribution to the toxicological evaluation of hexamethylenetetramine. Food Cosmet Toxicol. 9(4):491-500.
[NTP] National Toxicology Program. 1991. Executive summary of safety and toxicity information: N-(3-chloroallyl)hexaminium chloride. CAS Number 4080-31-3. Chemical Evaluation Committee Draft Report. [PDF]. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[OECD] Organisation for Economic Cooperation and Development. 2007. SIDS Initial Assessment Profile Methenamine. SIAM 24. Paris (FR): Organisation for Economic Cooperation and Development.
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
OECD QSAR Toolbox [Read-across tool]. 2017. Ver. 4.2. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Proctor DM, Gatto NM, Hong SJ, Allamneni KP. 2007. Mode-of-action framework for evaluating the relevance of rodent forestomach tumors in cancer risk assessment. Toxicol Sci. 98(2):313-326.
PubChem [database]. 2004- . Bethesda (MD): US National Library of Medicine, National Center for Biotechnology Information. [accessed 2019 Dec 13].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007. Paint products fact sheet: to assess the risks for the consumer. Updated version for ConsExpo4. [PDF, 264KB] Bilthoven (NL): RIVM. Report No.: 320104008/2007. [accessed 2019 Sep 19].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2018. Cleaning products fact sheet: default parameters for estimating consumer exposure – updated version 2018. [PDF, 3.46MB] Bilthoven (NL): RIVM. Report No.: 2016-0179. [accessed 2019 Sep 19].
[SCCS] Scientific Committee on Consumer Safety. 2011. Scientific Committee on Consumer Safety Opinion on Quaternium-15 (cis-isomer). [PDF, 566KB] Adopted by the SCCS during the 13th plenary meeting of 13‑14 December 2011. Brussels (BE): European Commission. Report No. SCCS/1344/10.
[SDS] Safety Data Sheet. 2013. Shur Stick Cellulose Adhesive [PDF]. Mississauga (ON): GH International Sealants ULC [accessed 2019 Jul 07] [restricted access].
[SDS] Safety Data Sheet. 2015a. Furniture Wipes [PDF]. Gurnee (IL): Weiman Products [accessed 2019 Jul 07] [restricted access].
[SDS] Safety Data Sheet. 2015b. Orange Glo Hardwood Refinisher [PDF]. Ewing (NJ): Church & Dwight Co., Inc [accessed 2019 Jul 07] [restricted access].
[SDS] Safety Data Sheet. 2016. Trim Shine Wipes [PDF]. East Rutherford (NJ): CCA Industries [accessed 2019 Jul 07] [restricted access].
[SDS] Safety Data Sheet. 2018. Skout’s Honor Urine Destroyer [PDF]. Irvine (CA): Skout’s Honor L.L.C. [accessed 2019 Jul 07] [restricted access].
[TIMES] TIssue MEtabolism Simulator [prediction module]. 2016. Ver. 2.27.19. Bourgas (BG): University “Prof. Dr. Assen Zlatarov,” Laboratory of Mathematical Chemistry.
[US EPA] US Environmental Protection Agency. 1995. Reregistration Eligibility Decision (RED): Dowicil®CTAC. EPA 738-R-95-017 [PDF, 620KB]. Washington (DC): US EPA, Office of Prevention, Pesticides and Toxic Substances.
[US EPA] United States Environmental Protection Agency. 2012. Standard Operating Procedures for Residential Pesticide Exposure Assessment. [PDF, 4.95MB] Washington (DC): Health Effects Division, Office of Pesticide Programs, Office of Chemical Safety and Pollution Prevention.
Van Duuren BL, Goldschmidt BM, Loewengart G, Smith AC, Melchionne S, Seldman I, Roth D. 1979. Carcinogenicity of halogenated olefinic and aliphatic hydrocarbons in mice. JNCI. 63(6):1433-1439.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48(11):3109-3119.
Zieger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. 1988. Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environ Mol Mutagen. 11(Suppl 12):1-157.
Appendix A. Read-across approach for human health effects assessment
| 1-chloropropene (CAS RN 590-21-6) | Methenamine (CAS RN 100-97-0) | Methenamine hydrochloride (CAS RN 58713-21-6) | Cis/trans-CTAC (CAS RN 4080-31-3) | Cis-CTAC (CAS RN 51229-78-8) | |
|---|---|---|---|---|---|
MW (g/mol) | 76 | 140 | 177 | 251 | 251 |
VP (Pa) | 6.8E4 | 12.1 | 2.46E-5 | 5.59E-7 | 1.74E-6 |
HLC (Pa·m3/mol) | 5.6E3 | 1.7E4 | 1.03E-8 | 1.76E-8 | 1.76E-8 |
WS (mg/L) | 2.4E3 | 4.5E5 | 1E6 | 1E6 | 1E6 |
LogKo/w(unitless) | 2.04 | -4.15 | -5.67 | -5.29 | -5.29 |
Oral LD50(g/kg) | 1.95 | >20 | - | 0.08-2.8 | >2 |
Dermal LD50(g/kg) | 20 | >2 | - | 0.57-0.6 | 0.6-0.9 |
RD toxicity (mg/kg bw/day) | - | Oral: NOAEL (50wks, rats) = 80 (HTD)3 NOAEL (90d, rats) = 1280 (HTD)4 LOAEL=5000 based on death and fur discolouration5 | - | Oral: LOAEL (90d, rats) = 7.5 based on general toxicity (↓BW, FC)9 NOEL (91-92d, dogs) = 7.5 based on liver/heart effects at the next dose10 Dermal: NOAEL (91d, rabbits) =1000 (HTD)11 NOAEL (90d, mice) = 1200 (HTD)12 | Dermal: NOAEL (3wks, immature rabbits) = 25 based on testicular effects at the next dose22 NOAEL (30d, mature rabbits) = 25 based on liver effects at the next dose23 |
Chronic toxicity (mg/kg bw/day) | Oral: LOAEL (623d) = 5mg/kg bw/day based on forestomach tumours1 Dermal: NOAEL (342-468d) = 35mg/kg bw/day (HTD)1 NOAEL (581d) = 35-83 mg/kg bw/day (HTD)1 Subcutaneous: NOAEL (622 d) = 5mg/kg bw/day (HTD)1 | Oral: NOAEL (60 wks, mice) = 2500 (HTD)5 NOAEL (104 wks, rats) = 2000 (HTD)5 NOAEL (333 d, rats) = 1130 (HTD)4 NOAEL (life, rats) = 100 (HTD)6 NOAEL (life, cats) = 61 (HTD)7 | - | - | - |
Cancer | Positive (forestomach tumours)1 | Negative4,5,6,7 | - | - | - |
Repro/devo toxicity (mg/kg bw/day) | - | Oral: NOAEL (humans) = 27 (HTD)8 | - | Oral: NOEL (rabbits) = 8 based on maternal/developmental toxicity at the next dose13 Dermal: NOEL (10 wks,rat) = 75 based on↓BW in dams and fetuses14 | Oral: LOAEL (rats) = 5 based on fetal malformations24 LOAEL = 25 based on maternal toxicity25 Dermal: NOAEL (rats) = 500 (HTD)26 |
Genotoxicity | In vitro: positive2 | Negative8 | - | In vitro: positive15,16,17, negative18,19 In vivo: Negative20,21 | In vitro: positive15,16,17, negative18,19 In vivo: Negative20,21 |
Abbreviations: BW, body weight; d, days; FC, food consumption; HLC, Henry’s Law Constant; HTD, highest tested dose; LD, lethal dose; MW, molecular weight; NOAEL, no observed adverse effect level; RD, repeated-dose; Repro/devo, reproductive and developmental; VP, vapour pressure; WS, water solubility
References:
1Van Duuren et al. 1979; 2OECD QSAR Toolbox 2017; 3Lijinsky and Taylor 1977; 4Brendel 1964; 5Della Porta et al. 1968; 6Natvig et al. 1971; 7Kewitz 1966, as cited in ECHA 2008; 8ECHA 2008; 9Humiston et al. 1972, as cited in SCCS 2011; 10Schwetz et al. 1976, as cited in SCCS 2011; 11Corley et al. 1988, as cited in SCCS 2011; 12Quast et al. 1996, as cited in SCCS 2011; 13Carney et al. 2008, as cited in SCCS 2011; 14Carney et al. 2006, as cited in SCCS 2011; 15Zieger et al. 1988; 16Kuramochi 1994, as cited in SCCS 2011; 17Linscombe et al. 1988, as cited in SCCS 2011; 18Murli 1994, as cited in SCCS 2011; 19Domoradzki 1981, as cited in SCCS 2011; 20Day and Shabrang 2000, as cited in SCCS 2011; 21Cifone 2002, as cited in SCCS 2011; 22Dow Chemical Company 1983, as cited in Becker et al. 2010 and CIR 1986; 23Lockwood et al. 1978, as cited in SCCS 2011, Dow Chemical Company, as cited in CIR 1986; 24John et al. 1982, as cited in SCCS 2011; 25Carney et al. 2005, as cited in SCCS 2011; 26Dow Chemical Company 1984, as cited in Becker et al .2010.
Appendix B. Parameters to estimate human exposures from use of products available to consumers in Canada
Exposure estimates were calculated based on default body weights of 74 kg for 19+ year olds, 62 kg for 14 to 18 year olds, 42 kg for 9 to 13 year olds, 23 kg for 4 to 8 year olds, 15 kg for 2 to 3 year olds, 11 kg for 1 year olds, 9.1 kg for 6 to 11 month olds, and 6.3 kg for 0 to 5 month olds (Health Canada 2019). The estimated dermal exposure parameters for self-care products and other products available to consumers are described in Tables B-1 and B-2, respectively. Exposures for products available to consumers were estimating using ConsExpo Web (ConsExpo Web 2019), unless stated otherwise. Default parameters from ConsExpo Web were used unless otherwise specified in Tables B-1 and B-2. In addition, dermal absorption was assumed to be 100% for all scenarios.
| Product | Assumptions |
|---|---|
Body moisturizer (cosmetic) | Concentration of Quaternium-15: 0.1%a Frequency of useb: 1 time per day (19+ year olds) (Ficheux et al. 2015; Wu et al. 2010), 0.8 per day (14-18 year olds) (Wu et al. 2010) Dermal – Direct contact, instant application Product amount: 10 g (19+ year olds and 14-18 year olds) (Ficheux et al. 2016) Retention factor: 1 |
Shampoo | Concentration of Quaternium-15: 10%a Frequency of usec: 1.1 times per day (19+ year old) (Loretz et al. 2008) Dermal – Direct contact, instant application Product amount: 11.8 g (19+ year olds) (Loretz et al. 2008) Retention factor: 0.01 |
Sunscreen lotion | Concentration of Quaternium-15: 0.07%d Frequency of use: 1.4 times per day (19+ year olds, 14-18 year olds, 9-13 year olds, and 4-8 year olds), 1.6 per day (2-3 year olds, 1 year olds, 6-11 month olds) (Ficheux et al. 2015) Dermal – Direct contact, instant application Product amount: 18.2 g (19+ year olds and 14-18 year olds), 6.3 g (9-13 year olds and 4-8 year olds), and 5.4 g (2-3 year olds, 1 year olds, and 6-11 month olds) (Ficheux et al. 2016) Retention factor: 1 |
Body moisturizer (NHP) | Concentration of Quaternium-15: 0.1%e Frequency of use: 1 time per day (19+ year olds) (Ficheux et al. 2015; Wu et al. 2010), 0.8 per day for all other age groups (Wu et al. 2010) Dermal – Direct contact, instant application Product amount: 10 g (19+ year old ands 14-18 year olds) (Ficheux et al. 2016), 7.7 g (9-13 year olds), 5 g (4-8 year olds), 4.1 g (2-3 year olds), 3.1 g (1 year olds), 2.5 g (6-11 month olds), 2 g (0-5 month olds) (surface area adjustments) Retention factor: 1 |
Hair perm/straightener | Concentration of Quaternium-15: 0.1%a Frequency of usec: 0.016 time per day (19+ year olds) (Wu et al. 2010; Ficheux et al. 2015) Dermal – Direct contact, instant application Product amount: 80 g (19+ year olds) (Bremmer 2006) Retention factor: 0.1 |
a Personal communication, email from the CHPSD, HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced.
b Product intended for use for ages 14 and older (personal communication, email from the CHPSD, HC, to the ESRAB, HC, dated Sept. 10, 2019; unreferenced).
c Product intended for use by adults only (19+ years old) (personal communication, email from the CHPSD, HC, to the ESRAB, HC, dated Sept. 10, 2019; unreferenced).
d Personal communication, email from the TPD, HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced.
e Personal communication, email from the NNHPD, HC, to the ESRAB, HC, dated Sep. 4, 2019; unreferenced.
| Exposure scenario (substance) | Assumptions |
|---|---|
Wall paint (cis/trans-CTAC) | Concentration of cis/trans-CTAC: 0.1%a Subpopulation: Adults (19+ year olds) Scenario: brush/roller painting (application), waterborne wall paint (RIVM 2007) Dermal – direct product contact Contact rate: 30 mg/min Release duration: 120 min |
Furniture cleaning wipe (cis/trans-CTAC) | Concentration of cis/trans-CTAC: 1%b Subpopulation: Adults (19+ year olds) Scenario: All-purpose cleaner wet tissue – cleaning (application) (RIVM 2018) Dermal – direct product contact Exposed area: 225 cm2 Product amount: 0.05 g |
Furniture cleaning wipe – post application (exposure to treated surface) (cis/trans-CTAC) | Concentration of cis/trans-CTAC: 1%b Subpopulation: 1 year olds Dermal Scenario: Post-Application Dermal Exposure Algorithm (mattresses) US EPA Residential SOPs (2012), Section 7 (US EPA 2012) Calculated using the following algorithm (US EPA 2012): Dose (mg/kg bw/day) = [DR * SA/BW * F * Fai * PF * DA] DR: deposited residue (mg/cm2) calculated assuming: DR = (PA/CA) * WCF * ACF * C Where: PA: product amount (g) = 11.5 (for wet wipe to treat leather couch) (RIVM 2018) CA: couch surface area (m2) = 5.5 (area of leather couch to be treated) (RIVM 2018) WCF: weight conversion factor = 1 000 mg/g ACF: area conversion factor = 1 m2/10 000 cm2 C: concentration (fraction): 0.01 SA/BW: surface area / body weight ratio (cm2/kg) = 482 (based on body surface area of 5300 cm2 and body weight of 11 kg for 1 year olds (Health Canada 2019)) F: fraction of body contacting residue = 0.5 (default from US EPA 2012) Fai: fraction of substance available for transfer from treated mattress = 0.06 (default from US EPA 2012) PF: protection factor = 0.5 (default from US EPA 2012) DA: dermal absorption = 100% Incidental oral Calculated using the following algorithm for post-application hand-to-mouth exposure (US EPA 2012): Exposure = [HR * (FM * SAH) * (ET * N_Replen) * (1 – (1 – SE)Freq_HtM/N_Replen)] / BW HR: hand residue loading (mg/cm2); calculated using the following algorithm: HR = [Faihands * Dermal exposure (mg) (calculated above; 1.66 x 10-1 mg)] / (SAH * 2) Faihands: fraction of active ingredient on hands compared to total surface residue from jazzercise study (unitless) = 0.15 (US EPA 2012) SAH: surface area of one hand (cm2) = 150 (US EPA 2012) FM: fraction of hand mouthed per event (unitless) = 0.13 (US EPA 2012) SAH: surface area of one hand (cm2) = 150 (US EPA 2012) ET: exposure time (hours/day) = 4 (in children aged 1-2 years old for carpets) (US EPA 2012) N_Replen: number of replenishment intervals per hour = 4 (US EPA 2012) SE: saliva extraction factor = 0.48 (US EPA 2012) Freq_HtM: number of hand-to-mouth events per hour = 20 (US EPA 2012) BW: body weight (kg) = 11 (for children aged 1 year) (Health Canada 2019) |
Carpet/furniture stain remover (cis-CTAC) | Concentration of cis-CTAC: 0.05% c Subpopulation: Adults (19+ years old) Scenario: Carpet spot remover – removing spots (application) (RIVM 2018) Dermal – direct product contact, instant application Exposed area: 75 cm2 Product amount: 0.6 g |
Carpet/furniture stain remover – post application (crawling on treated surface) (cis-CTAC) | Concentration of cis-CTAC: 0.05%c Subpopulation: 1 year olds Scenario: post-application (rubbing-off) of stain remover from carpet Calculations based on the US EPA Residential SOPs (2012), Section 7 (US EPA 2012) Dermal Calculated using the following algorithm (US EPA 2012): Exposure = [DR * Fai * TC * ET * DA * AF] / BW DR: deposited residue (mg/cm2) calculated assuming: DR = SR * WCF * ACF * C Where: SR: surface residue (g/m2) = 77 (carpet surface residue resulting from the use of removing spots following use of stain remover) (RIVM 2018) WCF: weight conversion factor = 1 000 mg/g ACF: area conversion factor = 1 m2/10 000 cm2 C: concentration (fraction): 0.0005 TC: transfer coefficient (cm2/hr) = 1 927 (adult transfer coefficient (6800 cm2/hr) adjusted for the body surface area of a 1-2 year old (0.28 (5 300 cm2/18 700 cm2) (Health Canada 2019) Fai: fraction available for transfer = 0.06 (default for carpet; US EPA 2012) ET: exposure time = 4 hours (for carpets in children 1-2 years old) DA: dermal absorption = 100% AF: adjustment factor (fraction) = 0.25 (professional judgement, assuming 25% of carpet is spot treated) BW: body weight (kg) = 11 (for children aged 1 year) (Health Canada 2019) Incidental oral (hand to mouth contact) Calculated using the following algorithm for post-application hand-to-mouth exposure (US EPA 2012): Exposure = [HR * (FM * SAH) * (ET * N_Replen) * (1 – (1 – SE)Freq_HtM/N_Replen)] / BW HR: hand residue loading (mg/cm2); calculated using the following algorithm: HR = [Faihands * Dermal exposure (mg) (calculated above; 4.4 x 10-1 mg)] / (SAH * 2) Faihands: fraction of active ingredient on hands compared to total surface residue from jazzercise study (unitless) = 0.15 (US EPA 2012) SAH: surface area of one hand (cm2) = 150 (US EPA 2012) FM: fraction of hand mouthed per event (unitless) = 0.13 (US EPA 2012) SAH: surface area of one hand (cm2) = 150 (US EPA 2012) ET: exposure time (hours/day) = 4 (in children aged 1-2 years old for carpets) (US EPA 2012) N_Replen: number of replenishment intervals per hour = 4 (US EPA 2012) SE: saliva extraction factor = 0.48 (US EPA 2012) Freq_HtM: number of hand-to-mouth events per hour = 20 (US EPA 2012) BW: body weight (kg) = 11 (for children aged 1 year) (Health Canada 2019) |
a ECCC 2016a
b SDS 2015a
c SDS 2018
