Draft screening assessment - Naphthalene Sulfonic Acids and Salts (NSAs) Group
Official title: Draft screening assessment - Naphthalene Sulfonic Acids and Salts (NSAs) Group
Chemical Abstracts Service Registry Numbers
1321-69-3, 25322-17-2, 25619-56-1, 57855-77-3, 60223-95-2, 68425-61-6
Environment and Climate Change Canada
Health Canada
July 2020
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment on six of seven substances referred to collectively under the Chemicals Management Plan as the Naphthalene Sulfonic Acids and Salts Group. These six substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health or ecological concerns. Although three substances in this group did not meet categorization criteria, they were included in this assessment because they were determined to be priorities as a result of the approach described for Identification of Risk Assessment Priorities. A seventh substance was initially included in the group; however, it was determined to be of low concern through other approaches, and the conclusion for this substance is provided in a separate report.Footnote 1 Accordingly, this screening assessment addresses the six substances listed in the table below. The six substances addressed in this screening assessment are hereinafter referred to as the Naphthalene Sulfonic Acids and Salts (NSAs) Group. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 2 ), their Domestic Substances List (DSL) names and their acronyms are listed in the table below.
| CAS RN | DSL name | Acronym |
|---|---|---|
| 1321-69-3 | Naphthalenesulfonic acid, sodium salt | NaNSA |
| 25322-17-2b | Naphthalenesulfonic acid, dinonyl- | DNNSA |
| 25619-56-1b | Naphthalenesulfonic acid, dinonyl-, barium salt | BaDNNSA |
| 57855-77-3c | Naphthalenesulfonic acid, dinonyl-, calcium salt | CaDNNSA |
| 60223-95-2b | Naphthalenedisulfonic acid, dinonyl- | DNNDSA |
| 68425-61-6 | Naphthalenesulfonic acid, bis(1-methylethyl)-, compd. with cyclohexanamine (1:1) | CDINSA |
a All substances are UVCBs (unknown or variable composition, complex reaction products, or biological materials).
b This substance was determined to be a priority as a result of the approach described for the Identification of Risk Assessment Priorities.
c This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
All six substances in the NSAs Group are commercially produced and do not occur naturally in the environment. The six substances were included in surveys issued pursuant to section 71 of CEPA. According to information submitted, NaNSA was manufactured in a total quantity between 100 000 kg and 1 000 000 kg, and that less than 1000 kg of CaDNNSA was manufactured in Canada. The remaining substances in the group were not manufactured in Canada but were imported into Canada in quantities of 1000 kg to 100 000 kg for each substance. These substances have a variety of uses in fuels, lubricants, oil and natural gas extraction, paints and coatings, rubber materials, and water treatment.
The ecological risk of NaNSA was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Based on the outcome of the ERC analysis, NaNSA is considered unlikely to be causing ecological harm.
The other five substances in the NSAs Group were assessed for ecological risk based on a mixture of empirical and analogue hazard data, which informed the fate and effects of these substances. Due to similarities in their chemical structures and effects, the hazard of these substances was considered as a group. Similarly, their ecological exposure was considered as a group due to the assumed potential for interchangeable industrial uses of the substances. Some of these substances may be persistent and bioaccumulative. The exposure scenarios examined in the ecological assessment included aquatic releases from lubricant oil blending, use of metal working fluids, formulation of paints and coatings, formulation of oil and gas products, and industrial use of paints. Exposure to soil via the application of biosolids to land, and exposure in sediment, were also considered. Low risk was identified from these five NSAs at current levels of exposure.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from the six substances in the NSAs Group. It is proposed to conclude that the six substances in the NSAs Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
With respect to human health, BaDNNSA and CDINSA were evaluated using the approach applied in the Rapid Screening of Substances with Limited General Population Exposure to determine if a substance requires further assessment on the basis of the potential for direct and indirect exposure of the general population. On the basis of this approach, the potential for exposure of the general population to BaDNNSA and CDINSA was considered to be negligible, indicating a low probability of risk to human health. Therefore, BaDNNSA and CDINSA are considered to be a low concern for human health at current levels of exposure.
For the four other substances, Canadians may be exposed to DNNSA, CaDNNSA and DNNDSA mainly through drinking water, while NaNSA is not released to the environment. In addition, DNNSA may be used as an antistatic agent in certain food packaging materials with potential for direct food contact. However, exposure from this food packaging use is expected to be negligible. The general population is not expected to be exposed to NaNSA, DNNSA or DNNDSA from the use of products available to consumers. The use of a general purpose aerosol lubricant containing CaDNNSA may result in intermittent inhalation and dermal exposures to this substance.
NaNSA was not identified as posing a high hazard to human health on the basis of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity, or reproductive toxicity. Further investigation into the potential health effects of NaNSA was not pursued as exposure of the Canadian general population to this substance is not expected. The health effects data for DNNSA, CaDNNSA and DNNDSA were limited; as such, a read-across approach was used to inform the health effects characterization of these substances. On the basis of laboratory studies conducted on structurally-related substances, the critical health effects of DNNSA, CaDNNSA, and DNNDSA are considered to be crystal formation in the kidneys and effects on the thyroid. Comparisons of levels of exposure to DNNSA or DNNDSA from environmental media to levels at which health effects occur result in margins that are considered adequate to address uncertainties in the health effects and exposure databases. Similarly, comparisons of levels of exposure to CaDNNSA from environmental media and from the use of a lubricant containing CaDNNSA to levels at which health effects occur result in margins that were considered adequate to address uncertainties in the health effects and exposure databases.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that the six substances in the NSAs Group do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that the six substances in the NSAs Group do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment on six of seven substances, referred to collectively under the Chemicals Management Plan as the Naphthalene Sulfonic Acids and Salts (NSAs) Group, to determine whether these six substances present or may present a risk to the environment or to human health. Three substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health or ecological concerns (ECCC, HC [modified 2017]). The remaining three substances were included because they were identified as priorities within the Identification of Risk Assessment Priorities approach (ECCC, HC 2015; Environment Canada, Health Canada 2014).
The seventh substance, naphthalenesulfonic acid, butyl-, sodium salt (Chemical Abstracts Service Registry Numbers (CAS RNFootnote 3 ) 25638-17-9) was originally included in the NSAs Group. However, it was considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a) and via the approach applied in the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018) and was identified as being of low concern to both the environment and human health. As such, it is not further addressed in this report. The conclusion for this substance is provided in the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018). The six substances addressed in this screening assessment will hereinafter be referred to as the (NSAs) Group.
The ecological risk of one of the substances in the NSAs Group, NaNSA (CAS RN 1321-69-3), was characterized using ERC (ECCC 2016a; Appendix C), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The ERC identified NaNSA as having low potential to cause ecological harm (ECCC 2016b), thus its ecological risk is not further discussed in this report, though its risk to human health is described.
Given the potential for DNNSA, BaDNNSA, CaDNNSA, DNNDSA and CDINSA to be used in similar ways and applications, the potential for ecological risk was assessed using similar exposure assumptions across the group. The risk to human health was assessed individually for each substance.
With respect to human health, BaDNNSA and CDINSA were considered under the approach applied in the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018). In the approach, the potential for direct exposure was evaluated on the basis of considerations such as evidence of the substance being present in a product used by the general population, and the potential for indirect exposure was adopted from the general approach reported in the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances science approach document (Health Canada 2016). On the basis of the evaluation of both direct and indirect exposure conducted as part of this approach, exposure of the general population to BaDNNSA and CDINSA was considered to be negligible. Therefore, BaDNNSA and CDINSA are considered to be a low concern for human health at current levels of exposure.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures. Relevant data were identified up to April 2019. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external review or consultation. Comments on the technical portions relevant to the environment were received from Geoff Granville (GCGranville Consulting Corp.) and James Armitage (AES Environmental Services, Inc.). Comments on the technical portions relevant to human health were received from Ms. Theresa Lopez, Ms. Jennifer Flippin, and Dr. Joan Garey at Tetra Tech. In addition, the ERC science approach document (ECCC 2016) was peer-reviewed and subject to a 60-day public comment period. The Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 4 This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The CAS RN, Domestic Substances List (DSL) names, common names and acronyms for the six substances in the NSAs Group are presented in Table 2-1.
Each substance in this group is considered to be an Unknown or Variable composition Complex reaction products and Biological material (UVCBFootnote 5 ) as the positions of both the sulfonate and the alkyl groups on the naphthalene are not specified. Furthermore, for DNNSA, BaDNNSA, CaDNNSA and DNNDSA, the dinonyl alkyl groups may exist in both linear and branched forms. For simplicity, the exact geometry (linear or branched) is not shown in the representative structures.
| CAS RN (acronym) | DSL name (common name) | Representative chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 1321-69-3 (NaNSA) | Naphthalenesulfonic acid, sodium salt (sodium naphthalenesulfonate) | ![[Na+].CC(C)c1cc(C(C)C)c(c2ccc(C)cc12)S([O-])(=O)=O](/content/dam/eccc/images/pded/nsas/20200604-t21a.jpg) C10H8O3SNa C10H8O3SNa | 230.22 |
| 25322-17-2 (DNNSA) | Naphthalenesulfonic acid, dinonyl- (dinonylnaphthalenesulfonic acid) | 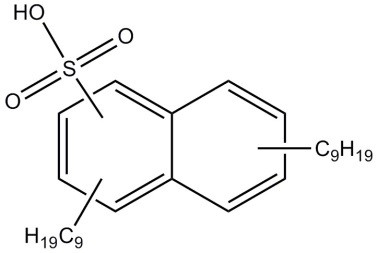 C28H44O3S C28H44O3S | 460.72 |
| 25619-56-1 (BaDNNSA) | Naphthalenesulfonic acid, dinonyl-, barium salt (barium dinonylnaphthalenesulfonate) | 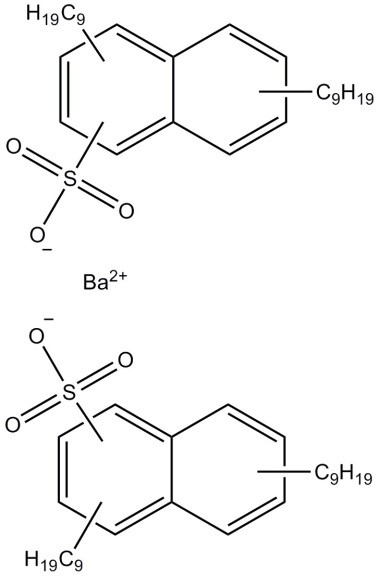 C56H88O6S2Ba C56H88O6S2Ba | 1056.75 |
| 57855-77-3 (CaDNNSA) | Naphthalenesulfonic acid, dinonyl-, calcium salt (calcium dinonylnaphthalenesulfonate) | 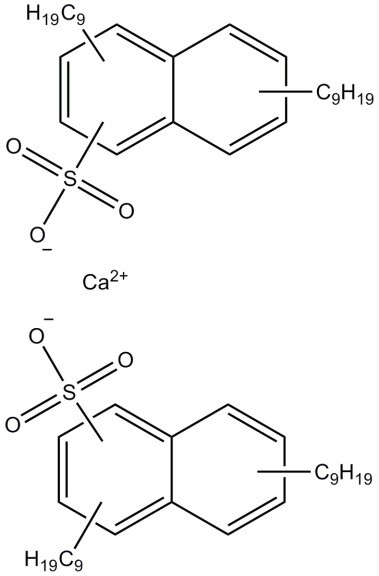 C56H88O6S2Ca C56H88O6S2Ca | 959.50 |
| 60223-95-2 (DNNDSA) | Naphthalenedisulfonic acid, dinonyl-(dinonylnaphthalenedisulfonic acid) | 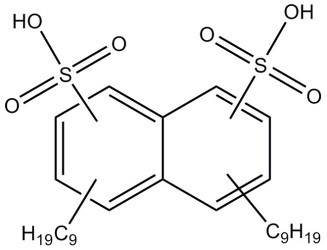 C28H44O6S2 C28H44O6S2 | 540.78 |
| 68425-61-6 (CDINSA) | Naphthalenesulfonic acid, bis(1-methylethyl)-, compd. with cyclohexanamine (1:1) (cyclohexylammonium diisopropylnaphthalenesulfonate) | 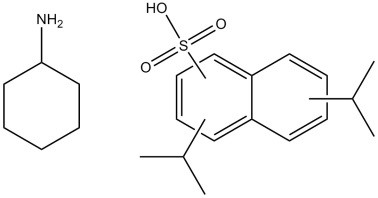 C6H13N.C16H20O3S C6H13N.C16H20O3S | 391.57 |
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, has been used to inform the ecological and human health assessments. Analogues were selected on the basis of structural similarity and/or functional similarity to substances within this group (e.g., similar physical-chemical properties, toxicokinetics) and that had relevant empirical data that could be used to read across to substances with limited empirical data. The applicability of (Q)SAR models was determined on a case-by-case basis. Details of the read-across data and (Q)SAR models chosen to inform the ecological and human health assessments of the NSAs Group are further discussed in the relevant sections of this report, and in Appendix F. Information on the identities and chemical structures of the analogues used to inform this assessment is presented in Table 2-2. Table 2-3 provides an indication of the read-across data available for different parameters.
| CAS RN (acronym) | DSL or other name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 120-18-3 (2-NSA) | 2-Naphthalenesulfonic acid | 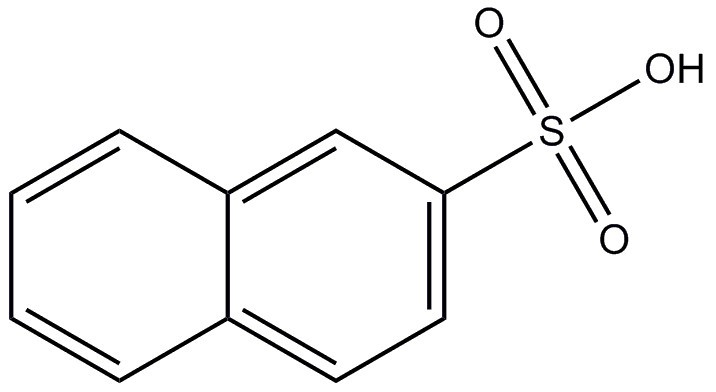 C10H8O3S C10H8O3S | 208.23 |
| 68153-01-5 | Naphthalenesulfonic acids | 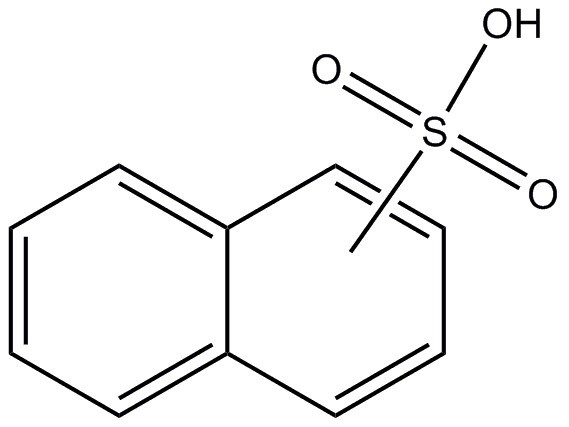 C10H8O3S C10H8O3S | 208.23 |
| 91078-64-7 | Naphthalenesulfonic acids, branched and linear Bu derivs., sodium salts | ![[(C4H9)n]C1=CC2=C(C=C1)C=C([(SO3Na)m])C=C2](/content/dam/eccc/images/pded/nsas/20200604-t22b.jpg) m = 1-2, n = 1-3 m = 1-2, n = 1-3 | 288.29- 551.46 |
| European Community Numbera, b 939-714-0 (C9-rich DANSA) | di C8-C10, branched, C9 rich, alkylnaphthalene sulfonic acid (C9-rich dialkylnaphthalenesulfonic acid) | ![[R]C1=CC=C(C=C(S(=O)(O)=O)C=C2)C2=C1](/content/dam/eccc/images/pded/nsas/20200604-t22d1.jpg) ![[R]C1=C([R])C=C(C=C(S(=O)(O)=O)C=C2)C2=C1](/content/dam/eccc/images/pded/nsas/20200604-t22d2.jpg) ![[R]C1=C([R])C=C(C=C(S(=O)(O)=O)C=C2[R])C2=C1](/content/dam/eccc/images/pded/nsas/20200604-t22d3.jpg) R = C8-C10 R = C8-C10 | N/A |
| European Community Numbera, b 939-718-2 (Ba- C9-rich DANSA) | barium bis(di C8-C10, branched, C9 rich, alkylnaphthalenesulfonate) (barium C9-rich dialkylnaphthalenesulfonate) | ![[R]C1=CC=C2C(C=CC(S(=O)([O-])=O)=C2)=C1.[Ba+2]](/content/dam/eccc/images/pded/nsas/20200604-t22e1.jpg) ![[R]C1=C([R])C=C2C(C=CC(S(=O)([O-])=O)=C2)=C1.[Ba+2]](/content/dam/eccc/images/pded/nsas/20200604-t22e2.jpg) ![[R]C1=C([R])C=C2C(C=CC(S(=O)([O-])=O)=C2[R])=C1.[Ba+2]](/content/dam/eccc/images/pded/nsas/20200604-t22e3.jpg) R = C8-C10 R = C8-C10 | 776.18-1393.39 |
| European Community Numbera, b 939-717-7 (Ca- C9-rich DANSA) | calcium bis (di C8-C10, branched, C9 rich, alkylnaphthalenesulfonate) (calcium C9-rich dialkylnaphthalenesulfonate) | ![[R]C1=CC=C2C(C=CC(S(=O)([O-])=O)=C2)=C1.[Ca+2]](/content/dam/eccc/images/pded/nsas/20200604-t22f1.jpg) ![[R]C1=C([R])C=C2C(C=CC(S(=O)([O-])=O)=C2)=C1.[Ca+2]](/content/dam/eccc/images/pded/nsas/20200604-t22f2.jpg) ![[R]C1=C([R])C=C2C(C=CC(S(=O)([O-])=O)=C2[R])=C1.[Ca+2]](/content/dam/eccc/images/pded/nsas/20200604-t22f3.jpg) R = C8-C10 R = C8-C10 | 678.24-1296.15 |
| European Community Numbera 947-977-8 | Naphthalenesulfonic acid, reaction products with isobutanol, sodium salts | ![CC(C)CC1=CC=C(S(=O)([O-])=O)C2=CC=C(CC(C)C)C=C21.[Na+]](/content/dam/eccc/images/pded/nsas/20200604-t22g.jpg) C18H23O3SNa C18H23O3SNa | 342.43 |
| N/Aa | Alkyl sulfates, alkane sulfonates, and alpha-olefin sulfonates | N/A | N/A |
| N/Aa (C10-C11 LAS) | Linear C10-C11 alkybenzenesulfonates | ![O=S(C1=CC=CC([R])=C1)([O-])=O.[Na+]](/content/dam/eccc/images/pded/nsas/20200604-t22h.jpg) R = C10-C11 R = C10-C11 | 320.42-334.45 |
| N/Aa | C14-C17 alkane sulfonates | N/A | N/A |
| N/Aa (C10-C13 LAS)b | Linear C10-C13 alkylbenzenesulfonates | ![O=S(C1=CC=CC([R])=C1)([O-])=O.[Na+]](/content/dam/eccc/images/pded/nsas/20200604-t22i.jpg) R = C10-C13 R = C10-C13 | 320.42-362.50 |
| 68909-82-0b | Naphthalenesulfonic acid, bis(1-methylethyl)-, Me derivs., sodium salts | ![[Na+].CC(C)c1cc(C(C)C)c(c2ccc(C)cc12)S([O-])(=O)=O](/content/dam/eccc/images/pded/nsas/20200604-t22j.jpg) | 328.40 |
Abbreviation: N/A, Not Applicable
a This substance does not have a CAS RN or the CAS RN is unknown
b Molecular formula has not been included due to structural complexity
| CAS RN for analogue(acronym) | Common name | Physical/chemical and ecological data | Health effects data |
|---|---|---|---|
| 120-18-3 (2-NSA) | 2-Naphthalenesulfonic acid | Persistence | N/Aa |
| 68153-01-5 | Naphthalenesulfonic acids | Ecotoxicity | N/Aa |
| 91078-64-7 | Naphthalenesulfonic acids, branched and linear Bu derivs., sodium salts | Bioaccumulation, ecotoxicity | N/Aa |
| 68909-82-0 | Naphthalenesulfonic acid, bis(1-methylethyl)-, Me derivs., sodium salts | Ecotoxicity | N/Aa |
| European Community Numberb 939-714-0 (C9-rich DANSA) | C9-rich dialkylnaphthalenesulfonic acid | Persistence, ecotoxicity | Reproductive and developmental toxicity, genotoxicity |
| European Community Number 939-718-2 (Ba- C9-rich DANSA) | barium bis(di C8-C10, branched, C9 rich, alkylnaphthalenesulfonate) | Water solubility | Reproductive and developmental toxicity, genotoxicity |
| European Community Number 939-717-7 (Ca- C9-rich DANSA) | calcium bis(di C8-C10, branched, C9 rich, alkylnaphthalenesulfonate) | Water solubility | Subacute toxicity, subchronic toxicity |
| European Community Numberb 947-977-8 | Naphthalenesulfonic acid, reaction products with isobutanol, sodium salts | Persistence | N/Aa |
| N/Ab | Alkyl sulfates, alkane sulfonates, and alpha-olefin sulfonates | Bioaccumulation | N/Aa |
| N/Ab(C10-C11 LAS) | Linear C10-C11 alkybenzenesulfonates | Bioaccumulation | N/Aa |
| N/Ab | C14-C17 alkane sulfonates | Persistence | N/Aa |
| N/Ab(C10-C13 LAS) | Linear C10-C13 alkylbenzenesulfonates | Persistence | N/Aa |
Abbreviation: N/A, Not Applicable
a Health effects data are not needed for these substances as they are not being used as analogues in the human health assessment
b This substance does not have a CAS RN or the CAS RN is unknown
3. Physical and chemical properties
Summaries of physical chemical property data of the substances in the NSAs Group are presented in Table 3‑1 and Table 3‑2, with the selected values indicated for each property. Table 3‑1 displays the selected physical and chemical property values for DNNDSA and DNNSA, which includes the dissociated organic DNNSA components of CaDNNSA and BaDNNSA. Table 3‑2 displays the values for CDINSA and NaNSA. In these tables, values are the result of modelling programs, except where indicated. Modelled results were generated for both the linear and branched structural variations of DNNSA and DNNDSA, where applicable, and when the results differed, an average of the two values was calculated and used in the assessment.
All of these substances have very low acid dissociation constants (pKa) and thus are expected to be completely ionized (i.e., anionic) when in aqueous solutions at ambient pH of 6 to 9. Ionization occurs via loss of a hydrogen ion from each of the sulfonic acid moieties, resulting in a sulfonate anion (ACD/Percepta c1997-2017). However, since many of the QSAR-type models are based on fragment addition methods (e.g., EPI Suite c2000-2012), they typically accept only the neutral form of a chemical as input. Therefore, only the un-ionized form of these substances was modelled, where applicable. For this reason, the physical and chemical properties of BaDNNSA and CaDNNSA were not modelled; rather they were read-across, as needed, from DNNSA, which represents their organic component. Similarly, the data displayed in Table 3-2 for CDINSA and NaNSA are for the neutral forms of their anions. The ionized forms of these substances are expected to be less volatile and to have smaller Henry’s law constants than the neutral forms that were modelled using EPI Suite.
Water solubilities of BaDNNSA, CaDNNSA and DNNDSA (Table 3-1) were measured using the OECD Shake Flask method, but rather than 24 hours of shaking, the samples were shaken for three days, and sonicated for 4 hours/day during those three days. Even with the additional shaking and sonication, the solubilities of these substances were found to be very low to low (personal communication from the Aquatic Contaminants Research Division, Environment and Climate Change Canada (ECCC), to the Ecological Assessment Division, ECCC, June 2019, unreferenced). In the REACH dossiers for DNNDSA and the analogue substances Ba- and Ca- C9-rich DANSA, the reported measured water solubilities were several orders of magnitude higher (Table 3-1). For DNNDSA, few details about the water solubility study were available in its REACH dossier, though it states the measurement was made at a pH of 1.1-2.1, at which an even lower water solubility would be expected (ACD/Percepta c1997-2017). The water solubility measurements for Ba- and Ca- C9-rich DANSA were obtained in the pH range 6.1 to 7.5 (ECHA 2018b, 2018d).
In view of their chemical structures, the substances in the NSAs Group are generally expected to have characteristics typical of anionic surfactants. In water, surfactants have the tendency to aggregate at the interface between two phases (e.g., octanol and water) and, when concentrations are sufficiently high, form micelles. For these reasons, typical test methods used for studying the partitioning of surfactants (i.e., log Kow) as well as their water solubility, such as OECD 117 (HPLC method) and OECD 107 (shake flask method), do not typically give accurate or reliable results and thus are not appropriate for this substance group (McWilliams and Payne 2011). Most of the NSAs have surfactant properties, as they have hydrophobic alkyl chains with chain length between 8 and 18 (Farn 2006), as well as anionic sulfonate groups. However, given the absence of an alkyl group for NaNSA and the short alkyl groups for CDINSA, these two substances are expected to exhibit surfactant properties only to a minimal extent.
The organic carbon-water partition coefficients (log Koc) of DNNSA, DNNDSA and CDINSA were selected based on the equation described in Abraham et al. (1994) and the model output from ACD/Percepta (c1997-2017). This approach uses polyparameter linear free energy relationships (ppLFER) to evaluate the equilibrium partitioning of organic compounds into water versus into organic matter. The ppLFER approach is considered to be more accurate for estimation of Koc for polar compounds and compounds with specific interactions towards organic matter than other traditional methods. This is due to the consideration of multiple types of molecular interactions (with both water and/or organic matter) as contributions towards free energy changes (Nguyen et al. 2005). However, the ppLFER model for estimation of Koc is not ideal, as it does not account for electrostatic interactions that would be present with ionized substances such as NSAs.
| Property | DNNSA(CaDNNSA, BaDNNSA)a | DNNDSA | Reference(s) |
|---|---|---|---|
| Physical state | NA | solid | ECHA 2018a |
| Melting point (°C) | 153 | 121b | Median of models (MPBPWIN 2008, TEST 2016); ECHA 2018a |
| Vapour pressure (Pa) | 1.03x10-10 | 2.33x10-16 | Median of models (MPBPWIN 2008) |
| Henry’s law constant (Pa·m3/mol) | 2.82x10-3 | 1.32x10-9 | HENRYWIN 2008 (bond method) |
| Water solubility (mg/L) | NA (DNNSA)0.0039 (CaDNNSA)0.011 (BaDNNSA) | 2.00 | unpublished ECCC internal report, Aquatic Contaminants Research Division, dated Apr. 26, 2019, unreferenced |
| Water solubility (mg/L) | 0.23 (DNNSA)b0.27 (CaDNNSA)b0.21 (BaDNNSA)b | 2.00x103 b | Read-across from C9-rich DANSA, Ca-C9-rich DANSA, and Ba-C9-rich DANSA (ECHA 2018b, 2018c, 2018d); ECHA 2018a |
| Water solubility of anion (mg/L), pH 5-9 | 0.003 (DNNSA)NA (CaDNNSA)NA (BaDNNSA) | 0.18 | ACD/Percepta c1997-2017 |
| Log Koc (dimensionless) | 5.09 | 4.31 | Abraham et al. 1994 and ACD/Percepta c1997-2017 |
| Dmax (nm) | NA | 19.7 | Simulation from ECHA 2018a |
| pKa1 (dimensionless) | 0.4-0.7 | -2.2-1.1 | ACD/Percepta c1997-2017 |
Abbreviations: NA, Not Available
a Values for BaDNNSA and CaDNNSA are read-across from DNNSA, with the exception of water solubility
b Values are empirical data
| Property | CDINSA | NaNSAa | Reference(s) for CDINSA; NaNSA |
|---|---|---|---|
| Physical state | NA | Solid | ECHA 2019a |
| Melting point (°C) | 164 | 115.5b | Median of models (MPBPWIN 2008, TEST 2016) |
| Vapour pressure (Pa) | 5.07x10-7 | 2.51x10-5 | Median of models (MPBPWIN 2008) |
| Henry’s law constant (Pa·m3/mol) | 9.42x10-5 | NR | HENRYWIN 2008 (bond method) |
| Water solubility (mg/L) | 1.98x102 | 6.01x104 b | Median of models (ACD/Percepta c1997-2017, WATERNT 2010, WSKOWWIN 2010, VCCLab 2005); experimental value (EPI Suite c2000-2012) |
| Log Kow (dimensionless) | 2.92 | 0.85b | Median of models (ACD/Percepta c1997-2017, ppLFER, VCCLab 2005, KOWWIN 2010); Median of experimental values (ACD/Percepta database) |
| Log Koc (dimensionless) | 3.28 | NR | Abraham et al. 1994 and ACD/Percepta c1997-2017 |
| pKa1 (dimensionless) | 0.7 | NR | ACD/Percepta c1997-2017 |
Abbreviations: NA, Not Available; NR, not required for this assessment
a Physical and chemical properties for NaNSA are read-across from empirical and/or modelled data for 2-NSA.
b Values are empirical data
4. Sources and uses
All six substances in the NSAs Group are commercially produced and do not occur naturally.
The six substances were included in surveys issued pursuant section 71 of CEPA (Canada 2012; Canada 2017). Table 4-1 presents a summary of information reported on the total manufacture and total import quantities for the NSAs Group.
| Common name | Total manufacturea (kg) | Total importsa (kg) | Reporting year | Survey reference |
|---|---|---|---|---|
| NaNSA | 100 000 – 1 000 000 | NR | 2015 | ECCC 2018 |
| DNNSA | NR | 10 000 – 100 000 | 2015 | ECCC 2018 |
| BaDNNSA | NR | 37 975 | 2015 | ECCC 2018 |
| CaDNNSA | 110 | 10 000 – 100 000 | 2011 | Environment Canada 2013 |
| DNNDSA | NR | 1000 – 10 000 | 2015 | ECCC 2018 |
| CDINSA | NR | 10 000 – 100 000 | 2011 | Environment Canada 2013 |
NR – not reported at a reporting threshold of 100 kg
a Values reflect quantities reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2013; ECCC 2018). See surveys for specific inclusions and exclusions (schedules 2 and 3).
Table 4-2 presents a summary of the non-confidential major uses of the NSAs Group according to information reported pursuant to CEPA section 71 surveys (Environment Canada 2013; ECCC 2018). The major uses reported for NaNSA are not included in Table 4-2 due to business confidentiality claims.
| Major usesa | DNNSA | BaDNNSA | CaDNNSA | DNNDSA | CDINSA |
|---|---|---|---|---|---|
| Fuels and related products, mixtures or manufactured items | Y | Y | N | N | N |
| Lubricants and greases | N | Y | Y | N | N |
| Oil and natural gas extraction | Y | N | N | N | Y |
| Paints and coatings | Y | Y | N | Y | N |
| Rubber materials | Y | N | N | N | N |
| Water treatment | Y | N | N | N | N |
Abbreviations: Y = yes, this use was reported for this substance; N = no, this use was not reported for this substance
a Non-confidential uses reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2013; ECCC 2018). See surveys for specific inclusions and exclusions (schedules 2 and 3).
In Canada, NaNSA is present as a formulant in registered pest control products (personal communication, email from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated January 2018; unreferenced). DNNSA may be used as an antistatic agent in the production of retention aids for use in the manufacture of paper and paperboard with potential for direct food contact (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated January 2018; unreferenced). CaDNNSA may be used as a lubricant on equipment or machine parts where there is no contact of the lubricant with food (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated August 2016; unreferenced). CaDNNSA is also used as a corrosion inhibitor in certain general purpose lubricants (SDS 2018).
In the United States, major uses of the substances in the NSAs Group include the manufacture of basic organic chemicals, petrochemicals, paints and coatings, petroleum lubricating oils and greases as well as activities in petroleum refineries, oil and gas drilling, extraction and support (Chemview c2013- ). In the European Union, DNNDSA is reported to be used in paints and coatings (ECHA 2018a).
5. Releases to the environment
Potential releases of substances in this group to the environment may occur from industrial facilities that use these substances in lubricant oil blending, as metal working fluids, in the formulation of oil and gas products, or in the formulation of paints and coatings. Most of these uses would result in indirect releases to the environment via wastewater treatment systemsFootnote 6 (WWTSs). Additionally, indirect releases to soil may occur from the application of biosolids to land from WWTSs.
6. Environmental fate and behaviour
6.1 Environmental distribution
Due to the intended uses of the substances in the NSAs Group and their physical-chemical properties, releases of these substances are expected to be predominantly from industrial facilities via wastewater treatment systems.
NSAs are expected to be completely ionized (negatively charged) in the ambient environment, as discussed in Section 3. As such, these substances are expected to have low vapour pressures and to partition to a greater extent to water than to air.
When released to water, it is expected that some of these substances such as CDINSA and DNNDSA will partition to both the water column and to sediments given their physical-chemical properties such as their low to high water solubilities. CDINSA is very soluble and will likely remain mostly in the water column. As these substances are negatively charged under ambient conditions they will likely have lower adsorption to soils and sediments than suggested by their log Koc values, which were derived for the neutral form of these substances.
CDINSA has moderate solubility in water, and as such, is expected to dissolve in water. DNNDSA, DNNSA, and the DNNSA metal salts have low solubility in water and thus are not expected to dissolve significantly in the aquatic compartment. For this reason, DNNDSA, DNNSA and the DNNSA metal salts would be expected to highly partition to sediment when released to water, and stay bound to soil particles when released to soil (i.e., would stay in soil). The very high sorption of CaDNNSA and BaDNNSA to sediment has been confirmed in a sorption/desorption study with a composite sediment and sand (unpublished ECCC internal report, Aquatic Contaminants Research Division, dated Apr. 26, 2019; unreferenced). During the desorption phase of the experiment, aqueous concentrations of CaDNNSA and BaDNNSA were below method detection limits. Due to their low solubilities it was assumed that, at environmentally relevant concentrations, these NSAs will bind to sand or sediment irrespective of the organic carbon or clay content of the adsorbent. In contrast, DNNDSA was detected in both sediment and water during the desorption phase of the experiment. DNNDSA also did not appear to sorb to sand (unpublished ECCC internal report, Aquatic Contaminants Research Division, dated Apr. 26, 2019; unreferenced).
Releases to air are not expected from the intended uses. As these substances all have negligible vapour pressures and low Henry’s Law constants, the likelihood of volatilization occurring from soil or surface waters would be low, indicating that air is not a compartment of interest in this assessment. The physical-chemical properties of these substances, such as negligible vapour pressure, low Henry’s Law constant, and low to moderate mobility in environmental media, indicate that these substances will likely not be subject to long-range transport.
Based on the above information, it is expected that water, sediment, and soil will be compartments of interest for hazard characterization for the NSAs Group.
6.2 Environmental persistence
No empirical biodegradation information on the substances assessed in the NSAs Group was found, however some information was found on the biodegradation of other alkylnaphthalenesulfonates. The biodegradation of alkylnaphthalenesulfonates with branched alkyl groups ranging from isopropyl to isopentyl is described as “marginal at best” (Swisher 1987). However, alkylnaphthalenesulfonates with straight-chain alkyl groups had faster biodegradation, with the longer-chain substances degrading faster. Using a culture of E. coli, Kölbel (1964) found the n-butyl derivative did not degrade in 30 days, whereas the n-hexyl disappeared during days 24-30 and the n-octyl during days 5-15.
Analogue substances showed highly variable degradation potentials: 2-naphthalenesulfonic acid, a close analogue to NaNSA, was shown to biodegrade by >90% in a 28-day test following OECD guideline 301 A (DOC die-away test) and was thus determined to be readily biodegradable (ECHA 2019a). For longer-chain NSAs, C9-rich DANSA was used as read-across. It was found to biodegrade 14-17% in a 29-day CO2 evolution test following OECD guideline 301 B, and was thus determined to not be readily biodegradable (ECHA 2018b). Furthermore, the reaction products of NSA with isobutanol, sodium salts, showed 0% biodegradation in a 28-day closed bottle test following OECD guideline 301 D (ECHA 2018e). However, the study authors noted that the lack of biodegradation does not necessarily indicate that the substance is recalcitrant in nature; rather, the stringency of the closed bottle test procedures may possibly explain the recalcitrance (ECHA 2018e).
The biodegradation of additional anionic surfactants was investigated to further support the potential for biodegradability. In an excerpt on surfactants from Ullmann’s Encyclopedia of Industrial Chemistry (Kosswig 2012), it is said that surfactants with hydrocarbon-derived hydrophobic groups can be oxidized enzymatically and biodegraded under aerobic conditions. The enzymatic attack leading to biodegradation occurs most commonly at the hydrophobic group. Linear C10-C13 alkylbenzene sulfonates and C14-C17 alkane sulfonates (comparable anionic surfactants) have been shown to biodegrade in a variety of tests: in a modified OECD screening test, they showed 94% and 88-96% biodegradation (%DOC) respectively, and in closed bottle tests they showed 55-65% and 63-95% biodegradability (%BOD) respectively. Given that some substances in the NSAs Group have branched aliphatic groups, which can strongly decelerate degradation (Kosswig 2012), data for these anionic surfactants is used as supporting information only. Anaerobic degradation testing is not feasible for this substance group as there are no validated methods for its testing with surfactants (Kosswig 2012).
Modelling approaches, including the CATALOGIC (2014) and BIOWIN (2010) programs were used as an additional line of evidence for biodegradation. Specifically, biodegradation half-life predictions from CATALOGIC (2014) are less than 182 days for DNNSA, DNNDSA, and CDINSA. However, predictions from CATALOGIC (2014) were identified as out of domain and were therefore only used in a weight of evidence approach in conjunction with the aforementioned analogue data. BIOWIN 3 (2010) also supports these predictions, indicating that these three substances will have degradation in the order of weeks to months. To compensate for the conflicting analogue data discussed above, and the fact that the structures of the NSAs can vary (i.e., may contain branched and/or linear alkyl groups), a range of biodegradation half-lives of 92 to 200 days were used in the exposure modelling for soil (Section 7.2.8).
Based on the empirical data on NSA analogues and on branched alkylnaphthalenesulfonates presented above, NSAs are likely to persist in the environment in water, soils and sediments (e.g., have half-lives greater than 182 days in water and soil or greater than 365 days in sediments).
6.3 Potential for bioaccumulation
The octanol-water partition coefficient (log Kow) may be used to inform the bioaccumulation of substances as it gives an indication of a substance’s ability to partition to fatty tissue. However, as the substances in this group are anionic surfactants, they accumulate at the interface between the hydrophilic and hydrophobic regions of a log Kow test. As a result, log Kow does not provide an accurate measurement of their partitioning or bioaccumulation.
Experimental bioconcentration factor (BCF) data for DNNDSA, modelled data, as well as BCF data for other anionic surfactants including LAS were used to characterize the bioaccumulation potential of NSAs. BCF values for DNNDSA following 8-week exposures at 0.1 mg/L and 1 mg/L were <2.0 L/kg and <0.19 L/kg respectively (Table 6-1), which indicate a low potential for bioaccumulation.
| Substance | Test organism | Experimental concentration(duration) | BCF(L/kg) | Reference |
|---|---|---|---|---|
| DNNDSA | Fish (Cyprinus carpio) | 0.1 mg/L (8 weeks) | <2.0 | ECHA 2018a |
| DNNDSA | Fish (C. carpio) | 1 mg/L (8 weeks) | <0.19 | ECHA 2018a |
BCF values for NSAs were modelled using the BIONIC model (2016), a model that is designed for monoprotic ionizing organic substances (Table 6-2). As DNNDSA is diprotic, results for DNNDSA were not included. These values indicate that DNNSA, BaDNNSA and CaDNNSA will bioaccumulate in fish to a high extent, while CDINSA does not appear to be bioaccumulative.
| Substance | BCF (L/kg) |
|---|---|
| DNNSA | 9954 |
| BaDNNSA | 6035 |
| CaDNNSA | 6035 |
| CDINSA | 4 |
Bioaccumulation of other anionic surfactants was examined. General descriptions of the accumulation mechanisms for surfactants are mentioned in EOSCA (2000). For example, uptake from the water column has been shown to be the most significant accumulation mechanism for hydrophobic organic compounds (Bartell et al. 1998). An initial assessment report on alkyl sulfates, alkane sulfonates, and alpha-olefin sulfonates (OECD 2007) states that experimental data (not provided) gave BCFs of ≤73 L/kg, for carbon chains with lengths up to C16. They determined that “any significant bioaccumulation is not expected.” Experimental BCFs for C10 and C11 linear alkyl benzene sulfonates (LAS), commonly used as a representative anionic surfactant, in Pimephales promelas ranged from 1.7-6.1 L/kg, which also indicates low potential for bioaccumulation for these substances (Tolls et al. 1997).
In summary, based on modelled data, DNNSA, BaDNNSA, CaDNNSA and CDINSA appear to be bioaccumulative, while based on experimental data, DNNDSA does not appear to be bioaccumulative.
7. Potential to cause ecological harm
7.1 Ecological effects assessment
Limited experimental data are available for the toxicity of the substances under assessment, in all compartments. For this reason, analogue data comprises a large part of the effects assessment.
7.1.1 Mode/mechanism of action
No information was found in the literature on the mode of action of NSAs. Three profilers were used to profile the mode of action (MoA) of these substances, as seen in the Ecological Risk Classification of Organic Substances (ERC) (ECCC 2016a). It was determined that the ASTER profiler was the most reliable: it indicated that DNNSA, DNNDSA, and CDINSA all have baseline narcosis as a mode of action. As a result it was determined that the NSA group does not have a specific mode of action. This informed the selection of appropriate assessment factors.
7.1.2 Effects on aquatic organisms
Empirical data on DNNDSA is available for the aquatic compartment in a REACH dossier (ECHA 2018a). Relevant analogue data are also available in the REACH dossier for C9-rich DANSA (ECHA 2018b). Fish, invertebrate, and algae studies were reviewed for reliability. Based on the available empirical and analogue data, the most sensitive aquatic organism for DNNDSA and the analogue substance is algae. Table 7-3 summarizes the key aquatic toxicity studies for the substances under assessment in the NSAs Group, and a close analogue.
Invertebrate data for DNNDSA was obtained from its REACH dossier (ECHA 2018a). In a study which followed OECD guideline 202 (Daphnia sp. acute immobilisation test) and EU Method C.2 (acute toxicity for Daphnia), juvenile Daphnia magna offspring were exposed to 5 concentrations of DNNDSA. Concentrations were only reported as nominal values; however, measured concentrations were said to be 97% to 112% of the nominal values. The endpoint used from this study was a nominal 48h EC50 of 87 mg/L.
Algal toxicity data for DNNDSA in its REACH dossier (ECHA 2018a) was informed by C9-rich DANSA (ECHA 2018b), with a noted caveat that the analogue substance is much less soluble than DNNDSA. In two studies which followed OECD guideline 201 (alga, growth inhibition test), Pseudokirchneriella subcapitata was exposed to seven concentrations of C9-rich DANSA, purchased as a 100% UVCB substance. Due to the low solubility of C9-rich DANSA, water-accommodated fractions (WAFs) were used in testing and nominal loading rates were reported along with measured concentrations. The measured concentrations dropped significantly (17 to 76%) after 72 hours, due to adhesion to glassware. Although multiple endpoints were reported (EC50, NOEC, EC10), only the EC10 values were considered in the selection of a critical toxicity value since the study authors consider them to be the most accurate at showing the dose-response pattern of the test.
Fish, algae and invertebrate data were reported for naphthalene sulfonic acids (CAS RN 68153-01-5) as well as additional fish data for branched and linear butyl derivatives of naphthalene sulfonic acids, sodium salt (CAS RN 91078-64-7) (Greim et al. 1994). No background information was provided on test methods as the values were submitted by an external lab; however, the paper was peer-reviewed and as such these data were deemed acceptable for use in a weight of evidence approach in conjunction with other experimental and analogue data. Table A-1 in Appendix A provides this additional analogue aquatic toxicity data.
Two species, the amphipod Hyalella azteca and the snail Planorbella pilsbryi, were exposed to BaDNNSA, CaDNNSA, and DNNSA for 96 hours. Only nominal concentrations were reported, as percentages of saturated NSA solution. CaDNNSA was the most hazardous to both species, followed by DNNSA, then BaDNNSA. All three NSA solutions were more hazardous to H. azteca than to P. pilsbryi (unpublished ECCC internal report, Aquatic Contaminants Research Division, dated Apr. 26, 2019; unreferenced). As no conventional endpoints were reported, these species were not included in the assessment factor approach for derivation of a predicted no-effect concentration (PNEC).
Included in the above report was a 21-day fathead minnow (P. promelas) embryo-larval exposure study, where the organisms were suspended in a cup in water above sediments containing NSAs. Newly fertilized fathead minnow embryos were exposed to nominal concentrations of DNNDSA and CaDNNSA at up to 2000 mg/kg sediment. Exposure to CaDNNSA caused a slight decrease in hatch success, with 98% observed in the control group compared to 81% in the highest exposure group, while no effects were observed as a result of exposure to DNNDSA (unpublished ECCC internal report, Aquatic Contaminants Research Division, dated Apr. 26, 2019; unreferenced). In another study, embryonic frog (Silurana tropicalis) were exposed to water overlaying sand spiked with 75 mg/kg CaDNNSA, from 8 hours after fertilization until the peak of their metamorphosis. Significant developmental delays were observed in the exposed group as opposed to the control group, starting around week 12. In addition, decreased body size was reported, with the average total body weight in the frogs that reached peak metamorphosis dropping from 0.75 g in the control group to 0.60 g in the group exposed to CaDNNSA (unpublished ECCC internal report, Aquatic Contaminants Research Division, dated Apr. 26, 2019, unreferenced); Matten et al. 2018).
The critical toxicity value (CTV) selected for aquatic species was a 72h EC10 of 0.16 mg/L with C9-rich DANSA for inhibition of yield of the algal species P. subcapitata, as reported in its REACH dossier (ECHA 2018b).
To derive the PNEC, the CTV was divided by an assessment factor (AF). AFs account for various extrapolations and sources of uncertainty. An endpoint standardization factor (FES) is considered for extrapolation from a short-term (acute) to a long-term (chronic) time-frame, from lethal effects (i.e., mortality) to sublethal effects (e.g., growth, reproduction), and from median effect levels (e.g., EC50) to low effect levels (e.g., EC10). The AF also accounts for the number of species and organism categories that are represented in the toxicity data set (species variation factor; FSV), and whether the substance has a mode of action that is more toxic than baseline narcosis (mode of action factor, FMOA). The final assessment factor (AF) is derived by multiplying the FES, FSV and the FMOA.
Since the CTV is a chronic study with a low-effects sublethal endpoint, the FES is equal to one. The mode of action for NSAs is non-polar (baseline) narcosis (Section 7.1.1); therefore, the FMOA is also equal to one. The combined aquatic toxicity dataset for DNNDSA and analogue substance C9-rich DANSA includes three species, covering the three species categories (plants, invertebrates and vertebrates); therefore, a FSV of 5 was used. The overall AF of 5 (FES × FSV × FMoA = 1 × 5 × 1) was applied to the CTV, resulting in an aquatic PNEC of 32 µg/L.
| Common name | Test organism | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| DNNDSA | Invertebrate (D. magna) | 48h EC50 | 87 | ECHA 2018a |
| C9-rich DANSA | Fish (C. carpio) | 96h LC50 | >0.28 | ECHA 2018b |
| C9-rich DANSA | Invertebrate (D. magna) | 48h EC50 | >0.27 | ECHA 2018b |
| C9-rich DANSA | Algae (P. subcapitata) | 72h EC50 (growth rate) | >9.60 | ECHA 2018b |
| C9-rich DANSA | Algae (P. subcapitata) | 72h EC50 (yield) | 2.4 | ECHA 2018b |
| C9-rich DANSA | Algae(P. subcapitata) | 72h EC10 (growth rate) | 0.8 | ECHA 2018b |
| C9-rich DANSA | Algae (P. subcapitata) | 72h EC10 (growth rate) | 0.7 | ECHA 2018b |
| C9-rich DANSA | Algae (P. subcapitata) | 72h EC10 (yield) | 0.2 | ECHA 2018b |
| C9-rich DANSA | Algae (P. subcapitata) | 72h EC10 (yield) | 0.16 | ECHA 2018b |
Abbreviations: LCx: Lethal concentration for x% of the population; ECx: Effect concentration for x% of the population
7.1.3 Effects on sediment-dwelling organisms
The effects of DNNDSA, CaDNNSA, and BaDNNSA on two species of invertebrates (H. azteca and Tubifex tubifex) were studied in chronic substrate exposure tests, which followed the ASTM (2010) standard E1706-05. Tests with T. tubifex used a nominal concentration range of 200 to 10 000 mg NSA per kg of dry weight sediment, whereas tests with H. azteca used 100 to 2000 mg NSA per kg dry wt. of sediment. Test organisms were exposed for 28 days (unpublished ECCC internal report, Aquatic Contaminants Research Division, dated Apr. 26, 2019, unreferenced; Matten et al. 2018). Nominal effect concentrations for both species are summarized in Table 7-2.
The CTV selected for sediment is a 28d EC50 of 205 mg/kg for production of young in T. tubifex. To convert the CTV to a PNEC, an overall assessment factor of 100 was applied, which comprised an FES of 5 to account for extrapolation from median effect levels (i.e., EC50) to low effect levels, a mode of action factor of 1 (for nonpolar narcosis) and a FSV of 20, as only one organism category, invertebrates, is represented. This results in a sediment PNEC of 2.05 mg/kg.
| Common name | Test organism | Endpoint | Value (mg/kg dry wt.) |
|---|---|---|---|
| BaDNNSA | Amphipod (H. azteca) | 28d LC50 | 832 |
| BaDNNSA | Amphipod (H. azteca) | 28d EC50 (growth) | 709 |
| BaDNNSA | Amphipod (H. azteca) | 28d EC50 (biomass) | 699 |
| BaDNNSA | Sludge worm (T. tubifex) | 28d LC50 | 1598 |
| BaDNNSA | Sludge worm (T. tubifex) | 28d EC50 (cocoon production) | 803 |
| BaDNNSA | Sludge worm (T. tubifex) | 28d EC50 (young production) | 398 |
| CaDNNSA | Amphipod (H. azteca) | 28d LC50 | 648 |
| CaDNNSA | Amphipod (H. azteca) | 28d EC50 (growth) | 424 |
| CaDNNSA | Amphipod (H. azteca) | 28d EC50 (biomass) | 373 |
| CaDNNSA | Sludge worm (T. tubifex) | 28d LC50 | 1279 |
| CaDNNSA | Sludge worm (T. tubifex) | 28d EC50 (cocoon production) | 419 |
| CaDNNSA | Sludge worm (T. tubifex) | 28d EC50 (young production) | 205 |
| DNNDSA | Sludge worm (T. tubifex) | 28d EC50 (cocoon production) | 3412 |
| DNNDSA | Sludge worm (T. tubifex) | 28d EC50 (young production) | 2336 |
| DNNDSA | Sludge worm (T. tubifex) | 28d LC50 | >10,000 |
Abbreviations: LCx: Lethal concentration for x% of the population; ECx: Effect concentration for x% of the population
a References: Unpublished ECCC internal report, Aquatic Contaminants Research Division, dated Apr. 26, 2019; unreferenced; Matten et al. 2019
7.1.4 Effects on soil-dwelling organisms
Data on the soil toxicity of NSAs were very limited. Data were available for an earthworm study with the analogue substance naphthalenesulfonic acid, bis(1-methylethyl)-, Me derivs., sodium salts (CAS RN 68909-82-0) (ECHA 2019b). Following the OECD test guideline for earthworm reproduction, adult earthworms (Eisenia fetida) were exposed to the test substance at nominal concentrations of 15.63 to 500 mg/kg dry wt. artificial soil, for 8 weeks. Table 7‑3 summarizes the key (nominal) results from this study. There were no statistically significant differences in reproduction or body weight gain for treatment concentrations of up to 250 mg/kg dry wt. However, at 500 mg/kg dry wt., reproduction (measured at 8 weeks) and body weight gain (measured at 28 days) were significantly reduced. No pathological symptoms or behavioural changes were observed over the test period.
The CTV selected for soil was the 8-week NOEC of 250 mg/kg dry wt. for earthworm reproduction (Table 7-3). To convert the CTV to a PNEC, an overall assessment factor of 50 was applied, which comprised an FES of 1, as no extrapolations were required to standardize this endpoint as it is a chronic NOEC, a mode of action factor of 1 (for nonpolar narcosis) and a FSV of 50, as data for only one organism category and species were available. Therefore, the PNEC is 5 mg/kg.
| Endpoint | Value (mg/kg dry wt.) |
|---|---|
| 8 week EC50 (reproduction) | 398 |
| 8 week NOEC (reproduction) | 250 |
| 8 week LOEC (reproduction) | 500a |
| 8 week NOEC (mortality) | 500 |
Abbreviations: NOEC: No observed effect concentration; LOEC: Lowest observed effect concentration; LCx: Lethal concentration for x% of the population; ECx: Effect concentration for x% of the population
a unbounded value
7.2 Ecological exposure assessment
The substances within the NSAs Group could potentially be used interchangeably for industrial applications. Therefore, for the purposes of this assessment, the ecological exposure scenarios consider DNNSA, BaDNNSA, CaDNNSA, DNNDSA and CDINSA collectively with the assumption that any one of the substances could be substituted for another for a given application and therefore the sum of DNNSA, BaDNNSA, CaDNNSA, DNNDSA and CDINSA reported by individual companies is considered. The scenarios are based on information reported for the substances in response to surveys issued pursuant to CEPA section 71 (Environment Canada 2013; ECCC 2018). The exposure scenarios covered in this assessment include aquatic releases from lubricant oil blending, use of metal working fluids, formulation of paints and coatings, formulation of oil and gas products, and industrial use of paints. Exposure to soil via the application of biosolids to land, and exposure in sediment via equilibrium in the water column, are estimated as an extension of the aquatic scenarios. Each of these scenarios is described in more detail below.
An exposure scenario was not prepared for the use of NSAs in lubricants and greases. It was determined that their use as lubricants would result in little to no environmental exposure, as these products typically get recycled or disposed of at waste facilities according to provincial/territorial programs and are therefore not expected to be discharged to the environment.
An exposure scenario was also not prepared for the use of NSAs in oil and natural gas extraction products because during oil field applications the process waters and wastes are not generally discarded to a sewer or the aquatic environment. Injection for well stimulation and deep well injection of the process water are the most common methods of disposal in North America (OECD 2012).
7.2.1 Measured concentrations in environmental media and wastewater
The only data found on measured environmental concentrations of NSAs in Canada were for wastewater effluent from four Canadian domestic wastewater treatment systems (WWTSs). DNNDSA, BaDNNSA and CaDNNSA were not detected in the effluent from these four facilities, which had either primary treatment or lagoon treatment, at method detection limits of 0.46 to 3.6 µg/L (Personal communication, e-mail from CMP Research and Monitoring Section to Ecological Assessment Division, ECCC, dated July 15, 2019, unreferenced). Some metalworking facilities and oil and gas product formulation facilities discharge their effluents to these four WWTSs, however it is not known if these facilities use NSAs, or, assuming they do, if they may have discharged NSAs during periods when the WWTS sampling occurred.
7.2.2 Calculation of PECs and general assumptions
The environmental exposures are estimated and presented in the form of predicted environmental concentrations (PECs). Aquatic PECs were calculated using the following equation:
Where,
PEC = Predicted Environmental Concentration (μg/L)
Q = Quantity used per site per year (kg/year)
L = Losses to wastewater (fraction)
R = WWTS removal efficiency (fraction)
D = Daily dilution volume (L/day)
N = number of days of release (days/year)
109 = conversion factor from kg to μg (μg/kg)
There are differences in the physical/chemical properties of the NSAs that will affect how they partition in the environment. For example, solubility varies by orders of magnitude and sorption potential also varies significantly among the substances in the NSAs Group. These properties were taken into consideration when calculating PECs. Due to the lack of data for NSAs, a WWTS removal efficiency was estimated based on read-across from a group of analogue substances. Linear alkyl benzene sulfonates (LAS), have an average removal rate across different systems of about 90% (OECD 2005). Since there are differences in degradation potential between LAS and NSAs (Section 5.2), the removal rate of more soluble NSAs that bind less strongly to solids (e.g., CDINSA and DNNDSA) is assumed to be half of that of LAS. Therefore, a removal efficiency of 45% was assumed for these substances. To cover the NSAs that have strong affinity to solids, a WWTS removal efficiency of 99% was assumed. Therefore, the exposure estimates were done using both the lower end (45%) and upper end (99%) removal rates to provide a range of possible PECs. Daily dilution volumes are calculated by multiplying the effluent flow of wastewater treatment systems (WWTS) or facilities discharging to a receiving water body by the dilution factor of the receiving water body. In all cases, aquatic PECs were derived using a dilution factor based on the 10th percentile low flow of the receiving water body and capped at a maximum dilution factor of 10.
The aquatic PECs represent potential concentrations of the substances in the receiving water body near the discharge point of a WWTS. The PEC values are presented in each exposure scenario and a summary of key assumptions are provided in Appendix B. Potential releases via container cleaning and transport including loading and unloading are not considered in this assessment.
7.2.3 Exposure scenario 1: Lubricant oil-blending
Based on information reported for the substances in response to surveys issued pursuant to CEPA section 71 (Environment Canada 2013; ECCC 2018), one of the main uses of NSAs is as an additive in lubricants. Therefore, a scenario was developed to reflect the possible releases of NSA to wastewater treatment systems and water bodies from lubricant oil blending facilities in Canada. There are over 10 companies in Canada that manufacture and/or blend lubricant products, located in various regions across Canada.
The aquatic PEC for a generic representative blending facility was calculated based on compiled data from different sources. The scenario is based on import quantities from a number of companies, where an average value was used as a representative number. It is assumed that a representative facility would discharge its effluent via an off-site secondary, tertiary or lagoon WWTS. The daily dilution volume selected is a representative daily dilution volume for the lubricant oil-blending sector. Refer to Table B-1 in Appendix B for a summary of assumptions.
The calculated generic aquatic PECs range between 0.05 to 2.87 µg/L.
7.2.4 Exposure scenario 2: Use of metal working fluids
Based on information reported for the substances in response to surveys issued pursuant to CEPA section 71 (Environment Canada 2013; ECCC 2018), NSAs are used as a corrosion inhibitor/anti scaling agent in metalworking fluids used to coat metal parts. Therefore, a scenario was developed to reflect the possible releases of NSA to wastewater treatment systems from facilities that use metalworking fluids to coat metal parts.
Usage in metalworking fluids may occur in multiple facilities located across Canada, ranging in operation size and location. Specific information on the users of metalworking fluids containing NSAs is unknown. This scenario considers a generic situation where an industrial facility uses metalworking fluids (containing NSAs) throughout the year.
Parameters such as production capacity, emission factor, and days of release were based on data from the OECD emission scenario document on the use of metalworking fluids. The daily dilution volume selected is the 10th percentile value of a distribution of daily dilution volume covering a variety of plants involved in activities requiring use of metalworking fluids. The facilities involved in these activities are assumed to have some on-site treatment of their wastewater in the form of oil/water separator prior to releasing to the sewer system for further treatment at a WWTS. Refer to Table B-2 of Appendix B for a summary of assumptions used to calculate the PECs.
The resulting aquatic PECs from this scenario range between 0.06 to 3.38 µg/L.
7.2.5 Exposure scenario 3: Formulation of paints and coatings
According to information reported for the substances in response to surveys issued pursuant to CEPA section 71 (Environment Canada 2013; ECCC 2018), these substances are used as process regulators as well as oxidizing and reducing agents in the industrial formulation of paints and coatings. This scenario considers the use of NSAs in the formulation of paints and coatings. Releases from these facilities are expected to enter wastewater treatment systems before being released to the environment.
The scenario is based on the largest reported import quantity of NSA by a formulation facility in this sector. The daily dilution volume selected is the 10th percentile value of a distribution of daily dilution volume developed for the paints and coatings sector. A summary of key assumptions for this scenario is provided in Table B-3 of Appendix B.
The calculated aquatic PECs for this scenario range between 0.05 to 2.64 µg/L.
7.2.6 Exposure scenario 4: Formulation of oil and gas products
Based on information reported for the substances in response to surveys issued pursuant to CEPA section 71 (Environment Canada 2013; ECCC 2018), NSAs are used as a processing aid in products used for oil and natural gas extraction. Therefore, this scenario looked at the release of NSAs to wastewater treatment systems from the formulation of products that are used in oil and gas extraction.
The estimated PECs considered a generic scenario where a facility is formulating products for oil and gas extraction and discharging to a secondary or tertiary wastewater treatment facility. The daily dilution volume selected is the 10th percentile value of a distribution of daily dilution volumes for a variety of industrial facilities. Refer to Table B-4 of Appendix B for a summary of assumptions used to calculate the PEC.
The resulting PECs from this scenario range between 0.07 to 3.83 µg/L.
7.2.7 Exposure scenario 5: Industrial use of paints
According to submissions received under section 71 of CEPA and communication with the Canadian Vehicle Manufacturers’ Association (CVMA) (personal communication, email from CVMA to Products Division, ECCC, dated August 2, 2019; unreferenced), NSAs are used industrially in paints, including in the automotive sector. Therefore, a scenario was developed to reflect the possible releases of NSA to wastewater treatment systems from facilities that use paints in the manufacture of original automotive equipment (OEM).
OEM painting is automated and overspray is collected in waterwash booths of downdraft or crossdraft design where water is used almost exclusively to collect overspray in OEM (US EPA 1996). The US EPA generic scenario for automobile spray coating (US EPA 1996) was adapted to calculate the PEC for a site where painting occurs, using the following equation:
Where,
Q = quantity used (kg/year)
TE = Average transfer efficiency for the spraying processes (fraction)
R = wastewater treatment system (WWTS) removal efficiency (fraction)
D = daily dilution volume (L/day)
N = number of release days (days/year)
The aquatic PEC was calculated based on compiled data from different sources. Parameters such as days of release were based on data from the US EPA generic scenario for automobile spray coating (US EPA 1996) while the transfer efficiency was based on the OECD emission scenario document on the coating industry (OECD 2009). Parameters such as discharge methods, on-site and off-site treatment systems, and wastewater flow were based on information representing relevant automotive manufacturing facilities in Canada. The use quantity is the high end of the range of import values reported (ECCC 2018). Refer to Appendix Table B-5 for a summary of assumptions used to calculate the PECs.
The resulting aquatic PECs range between 0.19 – 10.54 µg/L.
7.2.8 Exposure in sediment
A sediment-water equilibrium partitioning approach was used to estimate the PEC of NSAs in bottom sediment. This approach is based on the European Chemicals Agency’s guidance on environmental exposure estimation for suspended sediment (ECHA 2012) and on an equilibrium partitioning approach for bottom sediment described by the US EPA’s National Center for Environmental Assessment (US EPA 2003). At equilibrium, the PEC in bottom sediment linearly correlates with the concentration in the aqueous phase of the overlying water. Typical characteristics of suspended and bottom sediments as suggested by Gobas (2007 and 2010) were used in the estimation. The PEC in bottom sediment (in mg/kg) is typically calculated using the following equation:
Where,
Ctotal = total concentration in the water column (mg/L)
Koc = organic carbon-water partition coefficient for suspended or bottom sediment (L/kgOC)
Ranges of PECs in bottom sediment, standardized to 3% organic carbon (a typical organic carbon content in bottom sediment for rivers and lakes in Canada), were estimated for scenarios 1 to 5 above. A log KOC value of 3.28 was used as a representative value for higher solubility NSAs such as CDINSA, while a log KOC of 5.09 was used to represent the lower solubility NSAs such as DNNSA. In the calculations, the KOC values were paired up with the suitable removal rate (e.g., the high end removal was associated with the high Koc value, and the low end removal was associated with the low Koc value). These KOC values are very conservative, as they represent the neutral forms of these substances. The actual KOC values are expected to be much lower. Sediment PECs are provided in Table 7-4. A summary of additional assumptions used are provided in Tables B-6 to B-10 of Appendix B. Note that the total concentration in the water column was calculated using the 50th percentile flows rather than 10th percentile flows. This was done in order to reflect a more average exposure period in receiving water bodies needed to reach equilibrium in sediment.
| Scenario | PEC (mg/kg) |
|---|---|
| 1- Lubricant oil blending | 0.06 – 0.09 |
| 2- Use of metalworking fluids | 0.08 – 0.13 |
| 3- Formulation of paints and coatings | 0.09 – 0.15 |
| 4- Formulation of oil and gas products | 0.11 – 0.17 |
| 5- Industrial use of paints | 0.38 – 0.59 |
7.2.9 Biosolids application to land
This scenario considered the application of NSAs to soil in the form of biosolids from wastewater treatment systems. A range of soil PECs were calculated for scenarios 1 to 5 above and were calculated as an extension of these aquatic scenarios.
The soil PEC after 10 years of biosolids application and considering biodegradation as a loss mechanism, is calculated by iterating the equations below. Concentrations were determined on a yearly basis immediately after application and at the end of the year (after degradation has occurred, but prior to the subsequent application) over a 10-year period.
At the beginning of the year (directly after application):
(note that )
At the end of the year (after degradation):
Where,
PECbeginning = Predicted Environmental Concentration in soil at the beginning of the year after application of biosolids (before degradation) (mg/kg)
PECend = Predicted Environmental Concentration in soil at the end of the year (after degradation), prior to subsequent application of biosolids (mg/kg)
t = Years of biosolids land application (y), varying from 1 to 10 years
Cs = Concentration of the substance in biosolids (mg/kg dry weight)
A = Annual biosolids land application rate (kg/m2-y)
d = Soil mixing depth (m)
ρ = Dry soil density (kg/m3)
Half-lives of 92 to 200 days were estimated for NSAs. The concentration of NSAs in soil does not greatly increase over the 10-year period and soil concentrations are maximal after application (decreasing significantly afterwards over the year). The calculated PECs at the start of the 10th year for each scenario are provided in Table 7-5. A summary of assumptions used are provided in Tables B-11 to B-16 of Appendix B.
| Scenario | PEC (mg/kg) |
|---|---|
| 1- Lubricant oil blending | 0.11 – 0.33 |
| 2- Use of metalworking fluids | 0.66 – 1.91 |
| 3- Formulation of paints and coatings | 0.63 – 1.81 |
| 4- Formulation of oil and gas products | 0.18 – 0.53 |
| 5- Industrial use of paints | 0.18 – 0.51 |
7.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine assessment information and develop proposed conclusions using a weight-of-evidence approach and precaution. Evidence was gathered to determine the potential for substances in the NSAs Group to cause harm in the Canadian environment. Lines of evidence considered include those evaluated in this assessment that support the characterization of ecological risk in the Canadian environment. Secondary or indirect lines of evidence are considered when available, including regulatory decisions and classification of hazard or fate characteristics made by other regulatory agencies.
7.3.1 Ecological risk classification of organic substances (ERC)
NaNSA was identified as having a low potential to cause ecological harm via the ecological risk classification of organic substances (ERC) (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. The approach is summarized in Appendix C. Critical data and considerations used to develop the substance-specific profile for NaNSA are available in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to ERC, NaNSA was classified as having a low potential for ecological risk. It is therefore unlikely that this substance is resulting in concerns for the environment in Canada.
7.3.2 Risk quotient analysis
Risk quotient analyses were performed by comparing the various estimates of exposure (PECs; see the Ecological Exposure Assessment section) with ecotoxicity information (PNECs; see the Ecological Effects Assessment section) to determine whether there is potential for ecological harm in Canada. Risk quotients (RQs) were calculated by dividing the PEC by the PNEC for relevant environmental compartments and associated exposure scenarios. Table 7‑6 Risk quotient , 7-7 and 7-8 present RQs for aquatic, soil, and sediment compartments for the NSAs group, respectively.
| Exposure scenario | Aquatic PEC (μg/L) | Aquatic PNEC (μg/L) | Aquatic RQ |
|---|---|---|---|
| Lubricant oil blending | 0.05 – 2.87 | 32 | 0 – 0.09 |
| Use of metalworking fluids | 0.06 – 3.38 | 32 | 0 – 0.11 |
| Formulation of paints and coatings | 0.05 – 2.64 | 32 | 0 – 0.08 |
| Industrial formulator of oil and gas products | 0.07 – 3.83 | 32 | 0 – 0.12 |
| Industrial use of paints | 0.19 – 10.54 | 32 | 0.09 – 0.33 |
| Exposure scenario | Sediment PEC (mg/kg dry wt.) | Sediment PNEC (mg/kg dry wt.) | Sediment RQ |
|---|---|---|---|
| Lubricant oil blending | 0.06 – 0.09 | 2.05 | 0.03 – 0.05 |
| Use of metalworking fluids | 0.08 – 0.13 | 2.05 | 0.04 – 0.06 |
| Formulation of paints and coatings | 0.09 – 0.15 | 2.05 | 0.05 – 0.07 |
| Industrial formulator of oil and gas products | 0.11 – 0.17 | 2.05 | 0.05 – 0.08 |
| Industrial use of paints | 0.38 – 0.59 | 2.05 | 0.18 – 0.29 |
| Exposure scenario | Soil PEC (mg/kg) | Soil PNEC (mg/kg) | Soil RQ |
|---|---|---|---|
| Lubricant oil blending | 0.11 – 0.33 | 5 | 0.02 – 0.07 |
| Use of metalworking fluids | 0.66 – 1.91 | 5 | 0.13 – 0.38 |
| Formulation of paints and coatings | 0.63 – 1.81 | 5 | 0.13 – 0.36 |
| Industrial formulator of oil and gas products | 0.18 – 0.53 | 5 | 0.04 – 0.11 |
| Industrial use of paints | 0.18 – 0.51 | 5 | 0.04 – 0.10 |
The above RQs (Tables 7-6, 7-7, 7-8) are all below one, which indicates that NSAs have low potential to cause harm to aquatic, sediment or soil organisms as a result of their potential releases from industry.
7.3.3 Consideration of the lines of evidence
To characterize the ecological risk of the NSAs Group, technical information for various lines of evidence was considered (as discussed in the relevant sections of this report) and qualitatively weighted. The key lines of evidence supporting the assessment conclusion are presented in Table 7-9, with an overall discussion of the weight of evidence provided in section 7.3.4. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence. The relevance refers to the impact the line of evidence has when determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis ranged from low to high, with the assigned weight having five possible outcomes.
| Line of evidence | Level of confidencea | Relevance in assessmentb | Weight assignedc |
|---|---|---|---|
| Similarity in chemical structure for read-across purposes – fate and effects | Moderate | High | Moderate-High |
| Similarity in chemical structure for read-across purposes – DNNSA to BaDNNSA and CaDNNSA | High | High | High |
| Physical-chemical properties | Low | Moderate | Low-Moderate |
| Environmental distribution | Moderate | Moderate | Moderate |
| Persistence in the environment | Low | High | Moderate |
| Long-range transport | Moderate | Low | Low-Moderate |
| Bioaccumulation in aquatic organisms | Moderate | Moderate | Moderate |
| Mode of action and/or other non-apicald data | Low | Moderate | Low-Moderate |
| PNEC for aquatic organisms | Moderate | High | Moderate-High |
| PNEC for soil-dwelling organisms | Low | High | Moderate |
| PNEC for sediment-dwelling organisms | Moderate | High | Moderate-High |
| PEC in water | Moderate | High | Moderate-High |
| PEC in soil | Moderate | High | Moderate-High |
| PEC in sediment | Moderate | High | Moderate-High |
| RQs for water | Moderate | High | Moderate-High |
| RQs for soil | Moderate | High | Moderate-High |
| RQs for sediment | Moderate | High | Moderate-High |
a Level of confidence is determined according to data quality, data variability, data gaps (i.e., are the data fit for purpose).
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the overall combined weights for level of confidence and relevance in the assessment.
d Non-apical endpoints refer to endpoints other than mortality, growth, reproduction (i.e., those endpoints identified with population-level effects).
7.3.4 Weight of evidence for determining potential to cause harm to the Canadian environment
The physical-chemical properties and other parameter values selected for NSAs were informed by a combination of experimental, modelled, and read-across data, depending on availability of information. The weight of evidence supporting the selected parameters varies depending on the source (i.e., experimentally obtained versus modelled) and reflect the limited dataset.
Given the uncertainty associated with the modelling of these substances, which are ionizing and have surfactant properties, the risk assessment was based on read-across and empirical evidence where possible, and ranges of values were used in the exposure assessment, to mitigate the impact of these uncertainties on the overall assessment.
No empirical information was available on whether NSAs undergo long-range transport in the environment. Given their physical-chemical properties (i.e., negligible vapour pressure, low to moderate water solubility, low predicted mobility in soil), it is not expected that NSAs will undergo long-range transport.
Environmental persistence was informed using empirical data for other naphthalene sulfonic acids, analogue substances, as well as modelled results. Some of the empirical biodegradation data, as well as the modelled data indicate that NSAs will persist in the environment long enough to cause chronic toxicity.
Little empirical data were available for bioaccumulation of NSAs. Available data for DNNDSA were used in conjunction with modelled data for the other NSAs. The lack of empirical data for substances other than DNNDSA means that there is only moderate confidence in the determination that some NSAs are bioaccumulative.
The mode of action characterization was informed by chemical profilers (notably the ASTER profiler from the ERC approach), as no information was identified in the literature. The PNECs for aquatic, sediment and soil organisms were determined using fairly small datasets, which included the use of read-across data for aquatic and soil organisms, resulting in low to moderate confidence in the PNECs.
The reliability of the PECs considers a number of factors, including the WWTS removal rate, physical/chemical property data, the usage quantity, the industrial emission factor, and the daily dilution water volume of the receiving environment. The WWTS removal rates, which were based on the removal of the analogue substance class LAS, are of limited reliability as read-across data for NSAs, however, this was mitigated by using a range of values (45-99% of LAS). Ranges of values were also used for other parameters in the exposure scenarios, including the log KOC values in the sediment scenarios and the biodegradation half-lives in the soil scenarios, to compensate for the lack of certainty in these values, as well as to account for possible differences in values between different NSAs.
Usage quantities used in the exposure scenarios were based on information obtained through CEPA section 71 surveys. As there was limited information on the use quantities, import quantities were used for the calculations in the exposure scenarios. Additionally, information was lacking on the clients of the importers of these substances. In the absence of complete data, a number of assumptions were made in order to derive PEC values. For example, it was assumed that reported quantities from two survey years (2011 and 2015) are reflective of quantities used in the current year. In addition, for all exposure scenarios, due to the lack of facility-relevant use quantities, it was assumed that the total import quantity reported by a company could be used at each of its facilities. There was limited information on percent composition of products containing NSAs, so this parameter was derived from relevant material safety data sheets (MSDS) as well as OECD Emission Scenario Documents.
The daily dilution water volume data used in the exposure scenarios are considered to be reliable, as they are based on industrial data taken from Canadian government databases and on measured or calculated data on receiving water body flows. The industrial emission factors are estimates from generic sources (i.e., OECD publications), rather than site-specific data. Due to the limitations in the available data, as described above, the confidence in the PECs is moderate.
The RQs and other information discussed above indicate that the NSAs Group has low potential to cause ecological harm in Canada.
7.3.5 Sensitivity of conclusion to key uncertainties
There was a paucity of experimental physical chemical data available for this group and this assessment was therefore reliant on modelled data and a read-across approach for environmental behaviour, fate, and ecological effects characterization. Each environmental compartment was characterized with suitable data (with either empirical data for a substance in the group or from a close analogue) and as a result any changes to the physical-chemical properties would be unlikely to affect the proposed conclusion.
Substances with ionizing and surfactant properties pose a challenge for risk assessment due to their physical and chemical properties and toxicities being difficult to measure in empirical studies. They are also challenging from a modelling perspective, which adds uncertainty to the assessment conclusions. Reliance on physical chemical properties which are of questionable validity for these substances (such as log Kow) was minimized as much as possible in the evaluation of bioaccumulation potential and persistence, and a range of values were used for relevant parameters in the exposure scenarios, such as for log KOC and biodegradation half-life values, and as such additional information about these properties would likely have a low impact on the proposed conclusion.
As described in Section 7.3.4, there were limited industrial usage data and data on composition in products on which to base the exposure scenarios. Better industrial usage and composition data would have increased the certainty in the PECs. However, this information would not likely have changed the risk conclusion for these exposure scenarios given the very low PECs and RQs.
8. Potential to cause harm to human health
BaDNNSA and CDINSA were considered under the approach applied in the Rapid screening of substances with limited general population exposure screening assessment (ECCC, HC 2018). In the approach, Health Canada determined if the substances required further evaluation of potential to cause harm to human health on the basis of the potential for direct and indirect exposure to the general population. The potential for direct exposure was evaluated on the basis of considerations such as evidence of the substance being present in a product used by the general population, and the potential for indirect exposure was adopted from the general approach reported in the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances science approach document (Health Canada 2016). On the basis of the evaluation of both direct and indirect exposure conducted as part of this approach, exposure of the general population to BaDNNSA and CDINSA was considered to be negligible. Therefore, BaDNNSA and CDINSA are considered to be a low concern for human health at current levels of exposure. Additional details with regards to data and considerations used in the TTC-based approach are presented in the science approach document (Health Canada 2016).
8.1 Exposure assessment
8.1.1 Environmental media and food
NaNSA
No reports of measured concentrations of NaNSA in environmental media or dust in Canada or elsewhere were identified. The only uses reported in response to surveys issued pursuant to CEPA section 71 (Environment Canada 2013; ECCC 2018) were industrial and would not result in environmental releases or exposure for the general population (personal communication, email from a stakeholder to the Existing Substances Risk Assessment Bureau, Health Canada, dated August 2018; unreferenced) (Section 7.3.1).
DNNSA, CaDNNSA and DNNDSA
DNNSA, CaDNNSA and DNNDSA were not identified to be present in environmental media or dust in Canada or elsewhere. As indicated in section 6.1, these substances are expected to partition mainly to water, soil and sediment when released to the environment, on the basis of their physical-chemical properties, and current uses in Canada indicate that water, sediment and soil are compartments of interest in the environment. According to information reported for the substances in response to surveys issued pursuant to CEPA section 71 (Environment Canada 2013; ECCC 2018), and communication with the Canadian Vehicle Manufacturers’ Association (CVMA) (personal communication, email from CVMA to Products Division, ECCC, dated August 2, 2019; unreferenced), these substances are used in industrial settings in Canada and may be released to the environment through treated wastewater and biosolids. The highest predicted environmental concentration (PEC) was 10.54 mg/L for water, which was associated with possible releases of NSAs to wastewater treatment systems from facilities that use paints in the manufacture of original automotive equipment (OEM) (Section 7.2). As a conservative approach, intakes of DNNSA, CaDNNSA and DNNDSA by the general population via drinking water were estimated based on this highest PEC for an industrial-release scenario. Maximal estimates of daily intake from drinking water ranged from 0.19 mg/kg bw/day (9 to 13 year olds and 14 to 18 year olds) to 1.38 mg/kg bw/day (0 to 5 months, formula fed). Exposure from soil is considered to be negligible, and exposure from air is not expected (Appendix D).
Exposure through food to DNNSA from its use as an antistatic agent in the production of a retention aid in the manufacture of paper and paperboard with direct food contact is expected to be negligible (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated October 2018 and April 2019; unreferenced). The highest estimated intake from drinking water (1.38 mg/kg bw/day; 0 to 5 months, formula fed) is carried forward for risk characterization (Appendix D).
Exposure to CaDNNSA through food from its potential use as a lubricant on equipment or machine parts is not expected since there is no contact of the lubricant with food (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated August 2016; unreferenced).
8.1.2 Products available to consumers
NaNSA, DNNSA and DNNDSA
NaNSA, DNNSA and DNNDSA were not identified in products available to consumers in Canada and therefore exposure of the general population to these substances from the use of products available to consumers is not expected.
CaDNNSA
CaDNNSA is present as a corrosion inhibitor (1-5%) in a general purpose aerosol lubricant (SDS 2018). This product is expected to be used by the general population on an intermittent basis, leading to potential exposure via the inhalation and dermal routes. Table 8‑1 summarizes the estimated exposures to CaDNNSA from the use of the aerosol lubricant on a per event basis. Details of the parameters used in the exposure estimation are presented in Appendix E.
| Product scenario (age group) | Product concentration | Inhalation exposurea (mg/kg bw) | Dermal exposurea (mg/kg bw) | Combined inhalation and dermal exposurea (mg/kg bw) |
|---|---|---|---|---|
| General purpose aerosol lubricant, intermittent exposure (adult, aged 19 years or older) | 5%b | 2.2 x 10-3 | 1.1 x 10-2 | 1.4 x 10-2 |
a Dermal and inhalation absorption was assumed to be 100% (that is, equivalent to oral absorption)
b The maximum concentration shown on the SDS was used to estimate exposures
8.2 Health effects assessment
NaNSA
NaNSA was not identified as posing a high hazard to human health on the basis of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity, or reproductive toxicity. It is also not on the European Chemicals Agency’s List, or Candidate List of Substances of Very High Concern for Authorisation (ECHA 2018f). Further investigation of health effects is not warranted given that the general Canadian population is not expected to be exposed to this substance.
DNNSA, CaDNNSA, and DNNDSA
A US EPA screening level hazard characterization document of several dinonylnaphthalene substances is available as part of the US HPV Challenge program, which includes DNNSA and CaDNNSA (US EPA 2012). Both DNNSA and CaDNNSA were evaluated as a sub-category, with data being available for oral, inhalation and dermal acute toxicity, as well as eye and skin irritancy and sensitivity. However, no data were identified for repeat-dose, reproductive, or developmental toxicity, or for genotoxicity or carcinogenicity.
A REACH dossier for DNNDSA with empirical data on acute toxicity is available (ECHA 2018a). The other health effects data available for DNNDSA in the REACH dossier are based on read-across from C9-rich DANSA, Ca- C9-rich DANSA and Ba- C9-rich DANSA (ECHA 2018b, 2018c, 2018d).
DNNSA, CaDNNSA and DNNDSA share similar chemical structures; each has a naphthalene ring with one or two sulfonic acid substituents and two C9 alkyl chains, which may exist in branched or linear configurations. CaDNNSA is an alkaline earth metal salt of DNNSA; it is assumed to dissociate into the DNNSA anion and metal cation upon ingestion and absorption, and is expected to manifest similar toxicological effects to DNNSA. As the overall empirical toxicological database for these substances is limited (no repeat-dose or genotoxicity data available), and given their overall structural similarities, the health effects assessment of DNNSA, CaDNNSA and DNNDSA will be presented together and a read-across approach will be used. (DNNDSA is considered sufficiently similar to DNNSA and CaDNNSA for the purpose of read-across, despite differences in water solubility).
For characterization of human health effects for DNNSA, CaDNNSA and DNNDSA, C9-rich DANSA, Ca- C9-rich DANSA and Ba- C9-rich DANSA were used as analogues. These three C9-rich DANSA substances are considered appropriate analogues as they are mixtures of the corresponding C8 to C10 naphthalenesulfonic acids that contain either DNNSA or CaDNNSA as a major component; the mono- and tri-alkylnaphthalenesulfonic acid components (i.e., with only one or three alkyl substituents) within these DANSA substances are also considered appropriate analogues due to their structural similarity with DNNSA, CaDNNSA and DNNDSA.
Genotoxicity
C9-rich DANSA (ECHA 2018b) and Ba- C9-rich DANSA (ECHA 2018d) were both negative for mutagenicity in the Ames test for all S. typhimurium and E. colicoli strains up to the highest tested concentration (5000 µg/plate), with and without metabolic activation. Cytotoxicity was observed at 1000 µg/plate and above for C9-rich DANSA, and at 333 µg/plate and above for Ba- C9-rich DANSA.
A mouse lymphoma thymidine kinase assay showed that Ba- C9-rich DANSA is negative for mutagenicity in mammalian cells up to the highest tested concentration (90 µg/ml) with and without metabolic activation, with cytotoxicity observed from 50 µg/mL (with activation) and 70 µg/mL (without activation) and above (ECHA 2018d). Ba- C9-rich DANSA was negative for clastogenicity in a chromosome aberration study up to a maximum concentration of 250 µg/mL with and without metabolic activation (ECHA 2018d).
No genotoxicity studies were found for Ca- C9-rich DANSA.
In addition, QSAR predictive modelling did not produce any structural alerts for genotoxicity for representative structures of these substances (Derek Nexus 2018; Leadscope Model Applier 2018; TIMES 2016).
On the basis of these findings, DNNSA, CaDNNSA and DNNDSA are deemed not likely to be genotoxic.
No carcinogenicity studies for C9-rich DANSA or its salts are available.
Repeat dose toxicity
In a 14-day repeat dose study, male and female adult Wistar rats (n=3 for each sex and dose) were given 0, 80, 250 or 750 mg/kg bw/day Ca- C9-rich DANSA in dimethylsulfoxide by oral gavage (ECHA 2018c). Findings included non-significant higher inorganic phosphate levels, alkaline phosphatase (ALP) and alanine aminotransferase (ALT) activities, and lower total protein level in females at the highest tested dose, with no overt adverse effects observed at any dose. The NOAEL established by the study authors is 750 mg/kg bw/day (the highest dose tested). Compared to an OECD guideline 28-day repeat dose study, this study used a lower number of animals per sex and dose, had a shorter duration and fewer examined parameters.
In a 90-day repeat dose study, male and female adult Wistar rats (n=10 for each sex and dose) were given 0, 100, 300 or 1000 mg/kg bw/day Ca- C9-rich DANSA in corn oil by oral gavage (ECHA 2018c). At 1000 mg/kg bw/day, 6 females died and necropsy revealed effects on the gastrointestinal tract (GIT) (e.g., ulceration, squamous epithelial hyperplasia, hyperkeratosis and thickening of the forestomach lining, mucosal atrophy and erosion, and distended intestines), bone marrow atrophy and a small thymus. At 1000 mg/kg bw/day, significant changes in biochemical parameters were observed: ALT activity (decreased in males, increased in females), cholesterol (decreased in males and females), phosphate (increased in males), bile acid (decreased in males), albumin (decreased in females), potassium (decreased in females) and calcium (increased in females). At 300 and 1000 mg/kg bw/day, surviving animals showed irreversible significant reduced mean body weight gains with increased food consumption, ulcerative and inflammatory effects of the GIT, significant changes in relative or absolute weights of the thymus (decreased in males and females), liver (decreased in males, increased in females), kidney (increased in males and females), and adrenal gland (increased in males and females). In addition, significant changes in haematology parameters were observed: clotting time (decreased in males and females), neutrophils (increased in females), lymphocytes, platelets and reticulocytes (decreased in females). Histopathological findings at 300 and 1000 mg/kg bw/day revealed increased lymphocytolysis and lymphoid depletion in the thymus of males and females, an increase in thyroid follicular cell hypertrophy in males, and an increase in the presence of alveolar macrophages in the lungs of males. Furthermore, vaginal atrophy and inactive uteri were observed in females at 300 mg/kg bw/day. Thus, the NOAEL for Ca- C9-rich DANSA is established at 100 mg/kg bw/day based on effects on body and organ weight changes, and alterations in the GIT and hematopoietic system observed at 300 mg/kg bw/day.
Repeat dose studies were conducted for C9-rich DANSA and Ba- C9-rich DANSA in the form of combined repeat dose/reproduction-developmental toxicity screening tests, and results are presented in the next section.
No repeat dose studies for other routes of exposure (i.e., dermal or inhalation) were identified.
No long-term repeat dose studies were identified.
Developmental and reproductive toxicity
In a combined repeat dose toxicity study with a reproduction / developmental toxicity screening test, male and female adult Wistar rats (n=10 for each sex and dose) were given 0, 95, 298 or 893 mg/kg bw/day C9-rich DANSA (analytical doses) in propylene glycol by oral gavage (ECHA 2018b). Males were exposed for 31 days while females were exposed for 41-52 days. Surviving pups were sacrificed on day 5-7 of lactation. At 893 mg/kg bw/day, five animals were sacrificed in extremis. Surviving adult males exhibited lower mean body weight or body weight gains throughout the mating period compared to controls. Males in the highest dose group also showed a statistically significant higher mean white blood cell count. Additionally, both adult males and females exhibited higher ALT and ALP activities and lower cholesterol levels than controls. Histopathological findings were noted in the GIT, thymus, lungs and liver of the surviving adult animals. Microscopic findings observed in early sacrifices were generally similar in nature and severity as those recorded for surviving animals. At 298 mg/kg bw/day, higher ALP activity in adult females and lower cholesterol level in adult males were observed, with one female in extremis sacrificed on day 27 post-coitum. At 893 mg/kg bw/day, female pups at lactation day 4 exhibited significant lower mean body weights compared to controls, which could not be attributed to maternal neglect or as secondary effects due to changes in maternal body weight and food consumption. However, no other developmental parameters examined in this study were adversely affected (i.e., gestation index and duration, parturition, maternal care and early postnatal pup development consisting of mortality, clinical signs and macroscopy). No reproductive toxicity was observed in any of the examined parameters in adult males and female rats (i.e., mating, fertility and conception indices, pre-coital time, spermatogenesis and numbers of corpora lutea and implantation sites). Thus, the NOAEL for parental toxicity is 95 mg/kg bw/day based on changes in clinical biochemistry at 298 mg/kg bw/day and systemic toxicity at 893 mg/kg bw/day, while the NOAEL for developmental effects is 298 mg/kg bw/day based on changes in pup mean body weight at 893 mg/kg bw/day. The NOAEL for reproductive effects is 893 mg/kg bw/day due to the absence of effects at the highest tested dose.
In a combined repeat dose toxicity study with the reproduction / developmental toxicity screening test, male and female adult Wistar rats (n=10 for each sex and dose) were given 0, 17, 55 or 165 mg/kg bw/day (corrected for UVCB final purity) of Ba – C9 rich DANSA by oral gavage (ECHA 2018d). Males were exposed for 29 days, while females were exposed for 42-55 days. Surviving pups were sacrificed on days 5-7 of lactation. In the adults exposed to 165 mg/kg bw/day of Ba- C9-rich DANSA, a statistically non-significant increase in the incidence of tubular crystals in the kidneys was observed in one male and one female, along with minimal or slight degrees of tubular dilatation, epithelial hypertrophy and granular casts in the female. In addition, females experienced reversible lower motor activity, and had a slight increase in hypertrophy and hyperplasia of the thyroid gland epithelium. There were no treatment-related effects in any of the reproductive (i.e., mating, fertility and conception indices, pre-coital time, spermatogenesis and numbers of corpora lutea and implantation sites) or developmental (i.e., gestation index and duration, parturition, maternal care and early postnatal pup development consisting of mortality, clinical signs, body weight and macroscopy) parameters examined in the adults or offspring. Thus, the NOAEL for parental toxicity is 55 mg/kg bw/day based on effects in the kidney and the thyroid at 165 mg/kg bw/day, while the NOAEL for reproductive and developmental toxicity is 165 mg/kg bw/day due to the absence of effects at the highest tested dose.
No reproductive or developmental toxicity studies for Ca- C9-rich DANSA are available.
8.3 Characterization of risk to human health
BaDNNSA and CDINSA
BaDNNSA and CDINSA were considered under the approach applied in the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018). On the basis of the evaluation of both direct and indirect exposure conducted as part of this approach, exposure of the general population to BaDNNSA and CDINSA was considered to be negligible. Therefore, BaDNNSA and CDINSA were considered to be a low concern for human health at current levels of exposure.
NaNSA
The general population is not expected to be exposed to NaNSA through environmental media, food, or from the use of products available to consumers. On the basis of these considerations, the risk to human health is considered to be low.
DNNSA, CaDNNSA and DNNDSA
As the health effects data of DNNSA, CaDNNSA and DNNDSA were limited, a read-across approach using health effects data from the analogues C9-rich DANSA, Ca- C9-rich DANSA and Ba- C9-rich DANSA was used. On the basis of available information on analogues, DNNSA, CaDNNSA and DNNDSA are deemed not likely to be genotoxic. Long term repeat dose studies were not identified for DNNSA, CaDNNSA, DNNDSA or their analogues; however, a NOAEL of 100 mg/kg bw/day was established based on effects on body and organ weight changes, and alterations in the GIT observed in experimental animals at 300 mg/kg bw/day in a 90-day oral study conducted with Ca- C9-rich DANSA. A NOAEL of 55 mg/kg bw/day was identified based on kidney and thyroid effects (tubular crystals in the kidneys and hyperplasia/ hypertrophy of the thyroid epithelium) observed at the next dose of 165 mg/kg bw/day in parental animals in a reproductive / developmental toxicity screening test conducted with the analogue Ba- C9-rich DANSA.
The NOAEL of 55 mg/kg bw/day from the reproductive / developmental toxicity screening test is considered protective of effects observed in studies with longer exposure durations, and was used to characterize risk from daily oral exposures to CaDNNSA, DNNSA and DNNDSA in environmental media and food, and from intermittent exposures to CaDNNSA via inhalation and dermal routes from the use of a general purpose aerosol lubricant. Table 8‑2 provides all relevant exposure and hazard values for the NSAs Group, as well as resultant margins of exposure for determination of risk.
| Exposure scenario (age group) | Substance(s) | Systemic exposure (mg/kg bw/day) | Critical effect level (mg/kg bw/day) | Critical health effect endpoint | MOE |
|---|---|---|---|---|---|
| Environmental media (formula-fed infants, aged 0-5 months) | CaDNNSA, DNNSA and DNNDSA | 1.38 x 10-3(daily) | 55 (NOAEL for analogue: Ba- C9-rich DANSA) | Tubular crystals in the kidneys and hyperplasia/ hypertrophy of the thyroid epithelium | 39 900 |
| General purpose aerosol lubricant, combined inhalation and dermal exposurea (adult, aged 19 years or older) | CaDNNSA | 1.4 x 10-2(per event) | 55 (NOAEL for analogue: Ba- C9-rich DANSA) | Tubular crystals in the kidneys and hyperplasia/ hypertrophy of the thyroid epithelium | 3900 |
Abbreviation: MOE, margin of exposure
a Dermal and inhalation absorption was assumed to be 100% (that is, equivalent to oral absorption)
Comparison of the daily (CaDNNSA, DNNSA, and DNNDSA) and per event (CaDNNSA) exposure estimates to the critical effect level resulted in margins of exposure (MOEs) of approximately 39 900 and 3900, respectively. The calculated margins are considered adequate to address uncertainties in the health effects and exposure databases.
8.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Data on the presence of DNNSA, CaDNNSA and DNNDSA in environmental media are unavailable. | +/- |
| The use of an aerosol lubricant containing CaDNNSA is associated with potential inhalation and dermal exposure; however, there are no route-specific inhalation or dermal toxicity studies on CaDNNSA or its analogues. Characterization of risk from inhalation and dermal exposures to CaDNNSA is based on route-to-route extrapolation. | +/- |
| Substance-specific empirical health effects data, including chronic hazard studies, for DNNSA, CaDNNSA and DNNDSA, and their analogues, were limited or unavailable. | +/- |
| The available health effects data for the analogues are limited and were accessible only as robust summaries submitted in REACH dossiers. | +/- |
| The UVCB nature of the analogues creates uncertainty in identifying which component is driving the observed health effects. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure/risk; +/- = unknown potential to cause over or under estimation of risk.
9. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from the six substances in the NSAs Group. It is proposed to conclude that the six substances in the NSAs Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that the six substances in the NSAs Group do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that the six substances in the NSAs Group do not meet any of the criteria set out in section 64 of CEPA.
References
Abraham MH, Chadha HS, Whiting GS, Mitchell RC. 1994. Hydrogen bonding. 32. An analysis of water-octanol and water-alkane partitioning and the Δlog P parameter of seiler. J Pharm Sci. 83(8):1085-1100.
ACD/Percepta [prediction module]. c1997-2017. Toronto (ON): Advanced Chemistry Development, Inc.
Alberta Environment. 2009. Guidelines for the application of municipal wastewater sludges to agricultural lands [PDF]. Edmonton (Alberta): Alberta Environment. [accessed 2018 December 11].
Bartell SM, LaKind JS, Moore JA, Anderson P. 1998. Bioaccumulation of hydrophobic organic chemicals by aquatic organisms: a workshop summary. Int J Environ Pollut. 9:3-25. [cited in EOSCA 2000].
BIONIC Model. 2016. Ver. 2.0. A mechanistic mass balance model for predicting bioconcentration factors (BCFs) of ionizable organic chemicals in fish. Model developed by: James Armitage and Frank Wania (University of Toronto, Canada), Trevor Brown (Dalhousie University, Canada), Don Mackay (Trent University, Canada), John Arnot (ARC Arnot Research and Consulting, Canada).
[BIOWIN] Biodegradation Probability Program for Microsoft Windows [estimation model]. 2010. Ver. 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4): 741–752.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Canada, Dept. of the Environment. 2017. Canadian Environmental Protection Act, 1999: Notice with respect to substances included as part of the 2017 Inventory Update [PDF]. Canada Gazette, Part 1, vol. 151, no. 2.
CATALOGIC [environmental fate and ecotoxicity model]. 2014. Ver. 5.11.15. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
ChemView [database]. 2013- . Search results for CAS RNs 25322-17-2, 25619-56-1, 57855-77-3, 60223-95-2 and 68425-61-6. Washington (DC): US Environmental Protection Agency. [updated 2018 Oct 26; accessed 2018 Sep 19].
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Derek Nexus [toxicity prediction module]. 2018. Ver. 6.0.1 Leeds (UK): Lhasa Limited. [restricted access].
[ECCC] Environment and Climate Change Canada. 2015. Guidance on how to evaluate surfactants: version 2015. Gatineau (QC): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b.Suporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk. Gatineau (QC). ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@e.gc.ca.
[ECCC] Environment and Climate Change Canada. 2018. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to substances included as part of the 2017 Inventory Update. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of Risk Assessment Priorities: Results of the 2015 Review. [accessed 2020 Mar 5]
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2018 Nov 14].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018. Rapid screening of substances with limited general population exposure. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. 2012. Guidance on information requirements and chemical safety assessment. Ver. 2.1. Helsinki (FI): European Chemicals Agency. (Environmental exposure estimation; Chapter R.16).
[ECHA] European Chemicals Agency. 2016. Guidance on information requirements and chemical safety assessment. Chapter R.16: Environmental exposure estimation, Version 3.0. Helsinki (FI): ECHA.
[ECHA] European Chemicals Agency. 2018a. Registration dossier: Dinonylnaphthalenedisulphonic acid; CAS RN 60223-95-2. Helsinki (FI): ECHA. [accessed 2018 Dec 4].
[ECHA] European Chemicals Agency. 2018b. Registration dossier: di C8-C10, branched, C9 rich, alkylnaphthalene sulphonic acid; EC number 939-714-0. Helsinki (FI): ECHA. [accessed 2018 Dec 3].
[ECHA] European Chemicals Agency. 2018c. Registration dossier: calcium bis(di C8-C10, branched, C9 rich, alkylnaphthalene sulphonic acid); EC number 939-717-7. Helsinki (FI): ECHA. [accessed 2018 Sep 4].
[ECHA] European Chemicals Agency. 2018d. Registration dossier: barium bis (di C8-C10, branched, C9 rich, alkylnaphthalene sulphonic acid); EC number 939-718-2. Helsinki (FI): ECHA. [accessed 2018 Aug 22].
[ECHA] European Chemicals Agency. 2018e. Registration dossier: Naphthalene sulfonic acid, reaction products with isobutanol,sodium salts;EC number 947-977-8. Helsinki (FI): ECHA. [updated 13 June 2018].
[ECHA] European Chemicals Agency. 2018f. Candidate List of Substances of Very High Concern for Authorisation [Internet]. Helsinki (FI): European Chemicals Agency. [accessed 2018 Dec 3].
[ECHA] European Chemicals Agency. 2019a. Registration dossier: 2-Naphthalenesulfonic acid; CAS RN 120-18-3. Helsinki (FI): ECHA. [updated 2019 March 7; accessed 2019 May 2].
[ECHA] European Chemicals Agency. 2019b. Registration dossier: Naphthalene sulfonic acid, bis(1-methylethyl)-, Me derivs., sodium salts; CAS RN 68909-82-0. Helsinki (FI): ECHA. [updated 14 July 2019].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2014. Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA 1999). Ottawa (ON): Government of Canada. [accessed 5 March 2020]
[EOSCA] European Oilfield Speciality Chemicals Association. 2000. Bioaccumulation potential of surfactants: A review. [accessed 2018 September].
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Farn RJ. (Ed.). Chemistry and Technology of Surfactants [PDF]. Blackwell Publishing Ltd. U.K. [cited in ECCC 2015].
Gobas F. 2007. Development and review of a generic water–sediment modelling framework for organic chemicals. Burnaby (BC): Simon Fraser University, Faculty of Environment. Report prepared for Environment Canada.
Gobas F. 2010. Comments on approach to sediment exposure approach. Burnaby (BC): Simon Fraser University, Faculty of Environment. Report prepared for Environment Canada.
Greim H, Ahlers J, Bias R, Broecker B, Hollander H, Gelbke H-P, Klimisch H-J, Mangelsdorf I, Paetz A, Schong N, et al. 1994. Toxicity and ecotoxicity of sulfonic acids: Structure-activity relationships. Chemosphere 28(12): 2203-2236.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2015. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa (ON).
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [estimation model]. 2011. Ver. 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Kim M, Guerra P, Theocharides M, Barclay K, Smyth SA and Alaee M. 2013. Polybrominated diphenyl ethers in sewage sludge and treated biosolids: effect factors and mass balance. Water Res. 47: 6496-6505.
[KOAWIN] Octanol-Air Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Kölbel H, Kurzendörfer P, Zahiruddin M. 1964. Constitution and properties of surfactants. IV. Influence of structure on the aerobic biodegradation of anionic surfactants. Tenside 1: 7-18. [Cited in: Swisher 1987].
Kosswig K. 2012. Surfactants. In Ullmann’s Encyclopedia of Industrial Chemistry. Vol. 35. Weinheim: Wiley-VCH Verlag GmbH & Co. p. 431-505.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Leadscope Model Applier [prediction module]. 2016. Ver. 2.1. Columbus (OH): Leadscope, Inc. [restricted access].
Lee Y, Chen L, Fang H, Hu J, Zhang P, inventors; Dow Global Technologies LLC, assignee. 2011 July 21. Alkyd coating formulations. World Intellectual Property Organization WO 085520.
McWilliams P, Payne G. 2011. Bioaccumulation potential of surfactants: a review. Royal Society of Chemistry and the European Oilfield Speciality Chemicals Association. [accessed September 2018].
Matten KJ, Gillis PL, Toito J, Milani D, Bartlett AJ, Parrott JL, Balakrishnan V, Prosser RS. 2018. Effect characterization of three naphthalene sulfonates (NSAs) on freshwater biota and their environmental concentrations in Ontario river sediments (poster). Society of Environmental Toxicology and Chemistry North America, Sacramento, CA.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [estimation model]. 2010. Ver. 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[MSDS] Material Safety Data Sheet. 2014. NAXAN DI-AN. Harrison, OH: Nease Performance Chemicals LLC. [accessed 2018 spring].
[New EQC] New Equilibrium Criterion Model. 2011. Ver. 1.00 (Beta). Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
Nguyen TH, Goss K-U, Ball WP. 2005. Critical Review: Polyparameter linear free energy relationships for estimating the equilibrium partition of organic compounds between water and the natural organic matter in soils and sediments. Environ Sci Technol. 39(4): 913-924.
[OECD] Organisation for Economic Co-operation and Development. 2004. Emission scenario document on lubricants and lubricant additives [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 10; Report No.: ENV/JM/MONO(2004)21, JT00174617). [accessed 2018 Dec 19].
[OECD] Organisation for Economic Co-operation and Development. 2005. SIDS initial assessment report: Linear Alkylbenzene Sulfonates (LAS) [PDF]. SIAM [SIDS Initial Assessment Meeting]: 20: 2005 April: Paris, France. [accessed 2018 November].
[OECD] Organisation for Economic Co-operation and Development. 2007. SIDS Initial Assessment Profile (SIAP): Alkyl Sulfates, Alkane Sulfonates, and alpha-Olefin Sulfonates category. CoCAM-2 [Cooperative Chemicals Assessment Meeting], 2007 October 17-18. [accessed 2018 September].
[OECD] Organisation for Economic Co-operation and Development. 2009. Emissions Scenario Document on Coating Industry (Paints, Laquers, and Varnishes) [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 22; Report No. ENV/JM/MONO(2009)24). [accessed 2019 January].
[OECD] Organization for Economic Co-operation and Development. 2011. Emission Scenario Document on the use of Metalworking Fluids [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 28, Report No. ENV/JM/MONO(2011)18). [accessed 2019 January].
[OECD] Organization for Economic Co-operation and Development. 2012. Emission Scenario Document on Chemicals used in oil well production [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 31, Report No. ENV/JM/MONO(2012)7). [accessed 2019 March].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2009. The ConsExpo spray model – Modelling and experimental validation of the inhalation exposure of consumers to aerosols from spray cans and trigger sprays [PDF]. Bilthoven (NL): RIVM. Report No.: 320104005/2009. [accessed 2019 Feb 11].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2014. General fact sheet: General default parameters for estimating consumer exposure – Updated version 2014 [PDF]. Bilthoven (NL): RIVM. Report No.: 090013003. [accessed 2019 Feb 11].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2018. Cleaning products fact sheet: Default parameters for estimating consumer exposure – Updated version 2018 [PDF]. Bilthoven (NL): RIVM. Report No.: 2016-0179. [accessed 2019 Feb 11].
[SDS] Safety Data Sheet. 2018. Termin-8R [PDF]. Toronto (ON): Spectra Products [accessed 2019 Feb 11].
Statistics Canada. 2004. Canadian Community Health Survey, Cycle 2.2: General Health Component. Share File
Swisher, RD. 1987. Surfactant Biodegradation, 2nd ed. Marcel Dekker Inc., New York.
[TEST] Toxicity Estimation Software Tool. 2016. Ver. 4.2. Washington (DC): US Environmental Protection Agency.
[TIMES] Tissue MEtabolism Simulator [prediction module]. 2018. Ver. 2.27.19. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
Tolls J, Haller M, De Graaf I, Thijssen MATC, Sijm DTHM. 1997. Bioconcentration of LAS: experimental determination and extrapolation to environmental mixtures. Environ Sci Technol. 31:3426-3431. [cited in EOSCA 2000 [PDF].
Tolls J, Haller M, Labee E, Verweij M, Sijm DTHM. 2000. Experimental determination of bioconcentration of the non-ionic surfactant alcohol ethoxylate. Environ Toxicol Chem 19:646-653. [cited in EOSCA 2000 [PDF].
[US EPA] US Environmental Protection Agency. 1996. Generic scenario for automobile spray coating. Draft report. Washington (DC): US EPA, Office of Pollution Prevention and Toxics.
[US EPA] US Environmental Protection Agency. 2003. Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. Washington (DC): US EPA, National Center for Environmental Assessment. Report No.: EPA/600/P-00/001Cb. Part I: Estimating exposure to dioxin-like compounds; Volume 3: Site-specific assessment procedures; Chapter 4: Estimating exposure media concentrations. 148 pages.
[US EPA] US Environmental Protection Agency. 2011. Exposure factors handbook. Washington (DC): US EPA, National Center for Environmental Assessment, Office of Research and Development. [accessed 2018 Feb 20].
[US EPA] US Environmental Protection Agency. 2012. Screening level hazard characterization of high production volume chemicals: Dinonylnaphthalene category. Washington (DC): US EPA, Office of Pollution Prevention and Toxics. [accessed 2018 Feb 20].
[VCCLab] Virtual Computational Chemistry Laboratory. ALOGPS [non-Java interface]. 2005. Ver. 2.1. Munich (DE): VCCLab. [Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, Palyulin VA, Radchenko EV, Zefirov NS, Makarenko AS, et al. 2005. Virtual computational chemistry laboratory - design and description. J Comput Aid Mol Des. 19:453-463.].
[WATERNT] Water Solubility Program [estimation model]. 2010. Ver. 1.01. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Williams JH. 1999. Regulations on additions of sludge-borne metals to soil and their adaptation to local conditions. In L’Hermite P (editor): Treatment and use of sewage sludge and liquid agricultural wastes, 243-250. London (GB): Commission of the European Communities.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [estimation model]. 2010. Ver. 1.42. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Appendix A. Additional ecological effects data
| Common name (CAS RN) | Test organism | Endpointa | Value (mg/L) |
|---|---|---|---|
| Naphthalene sulfonic acids (68153-01-5) | Fish (Unspecified) | 96h LC50 | 100-500 |
| Naphthalene sulfonic acids (68153-01-5) | Invertebrate (D. magna) | 24h EC50 | 85 |
| Naphthalene sulfonic acids (68153-01-5) | Invertebrate (D. magna) | 48h EC50 | 34 |
| Naphthalene sulfonic acids (68153-01-5) | Algae (Unspecified) | 96h EC10 | 73.3 |
| Naphthalene sulfonic acids (68153-01-5) | Algae (Unspecified) | 96h EC50 | 54.3 |
| Branched and linear butyl derivatives of naphthalene sulfonic acids, sodium salts (91078-64-7) | Fish (Unspecified) | 48h LC0 | 20 |
| Branched and linear butyl derivatives of naphthalene sulfonic acids, sodium salts (91078-64-7) | Fish (Unspecified) | 48h LC100 | 100 |
Abbreviations: NOEC, No effect concentration; LOEC, Low effect concentrationconcentration; LCx, Lethal concentration for x% of the population; ECx, Effect concentration for x% of the population
a Endpoints not specified for invertebrate and algae studies.
Appendix B. Ecological exposure assessment: Summary of assumptions
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Quantity | 2400 | kg/year | ECCC (2013, 2018),), average quantity of NSA used by lubricant blending facilities based on reported import quantities in response to surveys conducted under section 71 of CEPA. It is assumed that the entire quantity imported by a company could be used at a single facility. |
| Emission factor | 0.25 | Percent | OECD (2004), this is the worst-case emission factor for a lubricant blending plant. |
| Days of release | 50 | Days/year | OECD (2004), number of days is determined by converting the total quantity NSA used at a facility to the quantity of product formulated at the facility, and then converting the product tonnage to number of days using relevant reference; this conversion was based on the maximum concentration (1%) of NSA within a lubricant based on MSDS information for one product. |
| Removal rate (on-site) | 0 | Percent | None |
| Removal rate (off-site) | 45 - 99 | Percent | Removal rate was varied between 45 and 99 percent to account for differences in physical and chemical properties between NSAs. |
| Daily dilution volume | 22,982,400 | L/day | Daily dilution volumes are calculated by multiplying the effluent flow of WWTS by the dilution factor of the receiving water body. Maximum DF of 10 is used when actual DF is greater than 10. This value corresponds to a representative value for the lubricant oil-blending sector in Canada. |
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Quantity | 161.24 | kg/year | OECD (2011), estimate of the mass of NSA handled at a facility, determined using the geometric mean volume of oil based metalworking fluid handled at a facility (16,124 L/year), density of the metalworking fluid (1 kg/L), and concentration of NSA in metalworking fluids (1%). |
| Emission factor | 11 | Percent | OECD (2011), emission factor associated with metalworking fluids varies between 11 and 100%, which includes releases from residual oil cleaning on metal surfaces, raw materials handling, finishing and other processes. The lowest emission factor of 11% was used. |
| Days of release | 247 | Days/year | OECD (2011), it is assumed that the default number of release days for facilities using metalworking fluids is equal to the default days of operation. |
| Removal rate (on-site) | 45 | Percent | OECD (2011) indicates that the majority of sites using metalworking fluids use on-site wastewater treatment prior to discharging effluents to WWTS. Therefore, the on-site removal rate was assumed equivalent to the lowest secondary treatment removal rate. |
| Removal rate (off-site) | 45 - 99 | Percent | Removal rate was varied between 45 and 99 percent to account for differences in physical and chemical properties between NSAs. |
| Daily dilution volume | 6,430,000 | L/day | Daily dilution volumes are calculated by multiplying the effluent flow of WWTS by the dilution factor of the receiving water body. Maximum DF of 10 is used when actual DF is greater than 10. This value corresponds to the 10th percentile of the distribution of daily dilution volumes for lagoons, secondary, and tertiary WWTS associated with facilities using metalworking fluid in Canada. |
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Quantity | 3162 | kg/year | ECCC (2013 and 2018), the highest import quantity reported to surveys conducted under section 71 of CEPA was converted to a range of 1000 to 10,000 kg/year with 3162 corresponding to the midpoint value on a logarithmic scale. It is assumed that the entire quantity imported by a company could be used at a single facility. |
| Emission factor | 0.505 | Percent | OECD (2009), emission factor associated with standard batch manufacture of aqueous coatings when raw materials are used in powder form. |
| Days of release | 300 | Days/year | EC 2003, TGD Table B2.10, p.260 |
| Removal rate (on-site) | 0 | Percent | None |
| Removal rate (off-site) | 45 - 99 | Percent | Removal rate was varied between 45 and 99 percent to account for differences in physical and chemical properties between NSAs. |
| Daily dilution volume | 11,105,000 | L/day | Daily dilution volumes are calculated by multiplying the effluent flow of WWTS by the dilution factor of the receiving water body. The value corresponded to the 10th percentile value of the distribution of daily dilution volumes for paints and coatings formulation facilities in Canada, which considers lagoons, secondary and tertiary WWTS. |
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Quantity | 3162 | kg/year | ECCC (2013 and 2018), the highest import quantity reported to surveys conducted under section 71 of CEPA was converted to a range of 1000 to 10,000 kg/year with 3162 corresponding to the midpoint value on a logarithmic scale. It is assumed that the entire quantity imported by a company could be used at any of its facilities. |
| Emission factor | 0.3 | Percent | EC (2003), TGD Table A2.1, p.221. While 2% is the determined emission factor from the TGD tables for the given use quantity, based on the cleaning processes of vessels used for formulation, a value of 0.3% is judged as being more appropriate. It is expected that solvents may be used in the cleaning of vessels, and therefore 2% would overestimate the releases to wastewater. |
| Days of release | 60 | Days/year | EC (2003), TGD Table B2.8, p.256; number of days is determined by converting the total quantity NSA used at a facility to the quantity of product formulated at the facility, and then converting the product tonnage to number of days using relevant reference; this conversion was based on the concentration of 10% of NSA within an oil and gas extraction product based on MSDS information for one product. |
| Removal rate (on-site) | 0 | Percent | None |
| Removal rate (off-site) | 45 - 99 | Percent | Removal rate was varied between 45 and 99 percent to account for differences in physical and chemical properties between NSAs. |
| Daily dilution volume | 22,697,000 | L/day | Daily dilution volumes are calculated by multiplying the effluent flow of WWTS by the dilution factor of the receiving water body. Maximum DF of 10 is used when actual DF is greater than 10. This value corresponds to the 10th percentile of the distribution of daily dilution volumes for secondary and tertiary WWTS associated with a variety of industrial facilities in Canada. |
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Quantity | 1000 | kg/year | ECCC (2018), the highest import quantity reported to the Domestic Substances List Inventory Update was converted to a range of 100 to 1000 kg/year with the top end of the range used. It is assumed that the entire quantity will be used at the facility. |
| Transfer efficiency | 65 | Percent | OECD (2009), this is the average transfer efficiency for spraying processes used in the manufacture of original automotive equipment. |
| Days of release | 21 | Days/year | OECD (2009), US EPA (1996),), number of days is determined by converting the total quantity of NSAs used at a facility to the quantity of product used at the facility, and then converting the product tonnage to number of days using relevant references; this conversion was based on the maximum concentration (1.5%) (Lee et al. 2011) of surfactant within a coating formulation based on patent information. |
| Removal rate (on-site) | 90 | Percent | US EPA (1996). Solids removal efficiency based on a pilot plant operation of paint solids removal in a water booth. |
| Removal rate (off-site) | 45 - 99 | Percent | Removal rate was varied between 45 and 99 percent to account for differences in physical and chemical properties between NSAs. |
| Daily dilution volume (for aquatic calculation) | 86,969,000 | L/day | Daily dilution volumes are calculated by multiplying the effluent flow of WWTS by the dilution factor of the receiving water body. Maximum DF of 10 is used when actual DF is greater than 10. This value corresponds to the 10th percentile of the distribution of daily dilution volume associated to a selected Canadian automotive manufacturing facility and the dilution factor of the site. |
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Daily dilution volume | 40,176,000 | L/d | Daily dilution volumes are calculated by multiplying the effluent flow of WWTS by the dilution factor of the receiving water body. Maximum DF of 10 is used when actual DF is greater than 10. This value corresponds to a representative value for the lubricant oil-blending sector in Canada, based on the 50th percentile flow. |
| Total concentration in the water column (Ctotal) | 0.03 – 1.64 | mg/L | Aquatic concentrations calculated using the daily dilution volume above. Other inputs are the same as for the aquatic scenario. |
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Daily dilution volume | 9,731,600 | L/d | Daily dilution volumes are calculated by multiplying the effluent flow of WWTS by the dilution factor of the receiving water body. Maximum DF of 10 is used when actual DF is greater than 10. This value corresponds to the 10th percentile of the distribution of daily dilution volumes based on 50th percentile flows for lagoons, secondary, and tertiary WWTS associated with facilities using metalworking fluid in Canada. |
| Total concentration in the water column (Ctotal) | 0.04 – 2.23 | mg/L | Aquatic concentrations calculated using the daily dilution volume above. Other inputs are the same as for the aquatic scenario. |
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Daily dilution volume | 11,105,000 | L/d | Daily dilution volumes are calculated by multiplying the effluent flow of WWTS by the dilution factor of the receiving water body. Maximum DF of 10 is used when actual DF is greater than 10. This value corresponds to the 10th percentile of the distribution of daily dilution volumes based on 50th percentile flows for lagoons, secondary, and tertiary WWTS associated with paints and coatings facilities in Canada. |
| Total concentration in the water column (Ctotal) | 0.05 – 2.64 | mg/L | Aquatic concentrations calculated using the daily dilution volumes above. Other inputs are the same as for the aquatic scenario. |
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Daily dilution volume | 29,384,000 | L/d | Daily dilution volumes are calculated by multiplying the effluent flow of WWTS by the dilution factor of the receiving water body. Maximum DF of 10 is used when actual DF is greater than 10. This value corresponds to the 10th percentile of the distribution of daily dilution volumes based on 50th percentile flows for secondary and tertiary WWTS associated with all industrial facilities in Canada. |
| Total concentration in the water column (Ctotal) | 0.05 – 2.96 | mg/L | Aquatic concentrations calculated using the daily dilution volumes above. Other inputs are the same as for the aquatic scenario. |
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Daily dilution volume (for calculation of Ctotal in sediment calculation) | 86,969,000 | L/d | Daily dilution volumes are calculated by multiplying the effluent flow of WWTS by the dilution factor of the receiving water body. Maximum DF of 10 is used when actual DF is greater than 10. This value corresponds to the 10th percentile of the distribution of daily dilution volume associated to a selected Canadian automotive manufacturing facility and the dilution factor of the site. |
| Total concentration in the water column (Ctotal) | 0 – 0.01 | mg/L | Recalculated aquatic concentrations using the 10th percentile of the daily dilution volumes based on the 50th percentile flows. Other inputs are the same as for the aquatic scenario. |
| Variable name | Value | Units | Comments |
|---|---|---|---|
| Fraction of removal via sorption in WWTS (Rsorption) | 45 - 99 | Percent | Removal rate was varied between 45 and 99 percent to account for differences in physical and chemical properties between NSAs. |
| Biosolids generation rate (BP) | 104 | mg/L | Default value based on field data of several secondary treatment systems, Kim et al. (2013); used to calculate concentration of substance in biosolids (Cs) |
| Annual biosolids land application rate (A) | 0.83 | kg/m2-yr | In Canada, the maximum land application rate of biosolids is regulated by provinces/territories and varies. The highest rate occurs in Alberta and is used as a default value (Alberta Environment 2009) |
| Number of years for biosolids land application (N) | 10 | Yr | A period of 10 consecutive years is suggested by the European Chemicals Agency (ECHA 2016) for calculating exposure in biosolids-applied land. |
| Soil mixing depth (d) | 0.2 | M | Default value. A soil mixing depth of 20 cm is suggested by the European Chemicals Agency (ECHA 2016) for calculating exposure in biosolids-applied land. |
| Dry soil density (ρ) | 1200 | kg/m3 | Default value reported for soil density (dry) by Williams (1999) |
| Biodegradation half-life in soil | 92 - 200 | days | CATALOGIC (2014) |
| Variable name | Value | Units | Comments |
|---|---|---|---|
| Concentration of substance in biosolids (Cs) | 30.95 – 68.09 | mg/kg dry weight | Cs is determined by the following equation: Where Qd (kg/day) is the daily mass of substance released to WWTS, Rsorption is the fraction of substance removed via sorption, F is the flow of selected WWTS in L/day, and BP is the biosolids generation rate per litre of wastewater in mg/L. See values below and in Table B-11. |
| Daily mass of substance released to WWTS (Qd) | 0.02 | kg/d | Qd is calculated from the annual quantity of the substance at the facility multiplied by the emission factor (from aquatic scenario) and averaged over the year by dividing by 365 days; used to calculate concentration of substance in biosolids (Cs) |
| Flow of WWTS (F) | 2,298,240 | L/d | This value is based on the same daily dilution volume as in the aquatic scenario and assumes a dilution factor of 10; used to calculate concentration of substance in biosolids (Cs). |
| Variable name | Value | Units | Comments |
|---|---|---|---|
| Concentration of substance in biosolids (Cs) | 179.85 – 395.66 | mg/kg dry weight | Cs is determined by the following equation: Where Qd (kg/day) is the daily mass of substance released to WWTS, Rsorption is the fraction of substance removed via sorption, F is the flow of selected WWTS in L/day, and BP is the biosolids generation rate per litre of wastewater in mg/L. See values below and in Table B-11. |
| Daily mass of substance released to WWTS (Qd) | 0.027 | kg/d | Qd is calculated from the annual quantity of the substance at the facility multiplied by the emission factor (from aquatic scenario) and averaged over the year by dividing by 365 days; used to calculate concentration of substance in biosolids (Cs) |
| Flow of WWTS (F) | 643,000 | L/d | This value is based on the same daily dilution volume as in the aquatic scenario and assumes a dilution factor of 10; used to calculate concentration of substance in biosolids (Cs). |
| Variable name | Value | Units | Comments |
|---|---|---|---|
| Concentration of substance in biosolids (Cs) | 170.46 – 375.01 | mg/kg dry weight | Cs is determined by the following equation: Where Qd (kg/day) is the daily mass of substance released to WWTS, Rsorption is the fraction of substance removed via sorption, F is the flow of selected WWTS in L/day, and BP is the biosolids generation rate per litre of wastewater in mg/L. See values below and in Table B-11. |
| Daily mass of substance released to WWTS (Qd) | 0.04 | kg/d | Qd is calculated from the annual quantity of the substance at the facility multiplied by the emission factor (from aquatic scenario) and averaged over the year by dividing by 365 days; used to calculate concentration of substance in biosolids (Cs) |
| Flow of WWTS (F) | 1,110,500 | L/d | This value is based on the same daily dilution volume as in the aquatic scenario and assumes a dilution factor of 10; used to calculate concentration of substance in biosolids (Cs). |
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Concentration of substance in biosolids (Cs) | 49.54 – 109.0 | mg/kg dry weight | Cs is determined by the following equation: Where Qd (kg/day) is the daily mass of substance released to WWTS, Rsorption is the fraction of substance removed via sorption, F is the flow of selected WWTS in L/day, and BP is the biosolids generation rate per litre of wastewater in mg/L. See values below and in Table B-11. |
| Daily mass of substance released to WWTS (Qd) | 0.03 | kg/d | Qd is calculated from the annual quantity of the substance at the facility multiplied by the emission factor (from aquatic scenario) and averaged over the year by dividing by 365 days; used to calculate concentration of substance in biosolids (Cs) |
| Flow of WWTS (F) | 2,269,700 | L/d | This value is based on the same daily dilution volume as in the aquatic scenario and assumes a dilution factor of 10; used to calculate concentration of substance in biosolids (Cs). |
| Variable name | Value | Units | Comments |
|---|---|---|---|
| Concentration of substance in biosolids (Cs) | 47.71 – 104.96 | mg/kg dry weight | Cs is determined by the following equation: Where Qd (kg/day) is the daily mass of substance released to WWTS, Rsorption is the fraction of substance removed via sorption, F is the flow of selected WWTS in L/day, and BP is the biosolids generation rate per litre of wastewater in mg/L. See values below and in Table B-11. |
| Daily mass of substance released to WWTS (Qd) | 0.10 | kg/d | Qd is calculated from the annual quantity of the substance at the facility and averaged over the year by dividing by 365 days; used to calculate concentration of substance in biosolids (Cs) |
| Flow of WWTS (F) | 8,696,900 | L/d | This value is used to represent the 10th percentile low flow of receiving water body associated with the WWTS where the facility is discharging; this value is based on daily dilution volume used in the aquatic scenario and assuming a dilution factor of 10; used to calculate concentration of substance in biosolids (Cs). |
Appendix C. The Ecological Risk Classification of organic substances (ERC) approach
The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency (hazard) and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. The following paragraphs in this section summarize the approach, which is described in detail in ECCC (2016).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, available empirical databases (e.g., OECD QSAR Toolbox 2016), and from responses to surveys conducted under section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also composed of multiple metrics, including potential emission rate, overall persistence and long-range transport potential. Hazard and exposure profiles were compared to decision criteria to classify the hazard and exposure potentials for each organic substance as low, moderate or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance based on its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high, to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016). The following describes two of the more substantial areas of uncertainty. Error in empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2016). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error of underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is considered to be the current use quantity and may not reflect future trends.
Appendix D. Potential human exposures to DNNSA in environmental media and food
| Route of Exposure | 0 to 5 months, formula feda | 6 to 11 monthsb | 1 yearc | 2 to 3 yearsd | 4 to 8 yearse | 9 to 13 yearsf | 14 to 18 yearsg | 19 years or olderh |
|---|---|---|---|---|---|---|---|---|
| Drinking wateri | 1.38 | 0.88 | 0.34 | 0.30 | 0.24 | 0.19 | 0.19 | 0.22 |
| Food | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Total intake | 1.38j | 0.88 | 0.34 | 0.30 | 0.24 | 0.19 | 0.19 | 0.22 |
Abbreviations: N/A, not applicable
a Assumed to weigh 6.3 kg (Health Canada 2015) and drink 0.826 L of water per day (Health Canada 1998). It is assumed that infants younger than 1 year old consume drinking water through formula exclusively, and that infants younger than 1 year old who are breastfed do not consume any drinking water.
b Assumed to weigh 9.1 kg (Health Canada 2015) and drink 0.764 L of water per day (Health Canada 1998). It is assumed that infants younger than 1 year old consume drinking water through formula exclusively, and that infants younger than 1 year old who are breastfed do not consume any drinking water.
c Assumed to weigh 11 kg (Health Canada 2015) and drink 0.36 L of water per day (Health Canada 1998).
d Assumed to weigh 15 kg (Health Canada 2015) and drink 0.43 L of water per day (Health Canada 1998).
e Assumed to weigh 23 kg (Health Canada 2015) and drink 0.53 L of water per day (Health Canada 1998).
f Assumed to weigh 42 kg (Health Canada 2015) and drink 0.74 L of water per day (Health Canada 1998).
g Assumed to weigh 62 kg (Health Canada 2015) and drink 1.09 L of water per day (Health Canada 1998).
h Assumed to weigh 74 kg (Health Canada 2015) and drink 1.53 L of water per day (Health Canada 1998).
i A maximum concentration of 10.54 mg/L of DNNSA, CaDNNSA and DNNDSA in wastewater was used in estimating drinking water intake of these substances.
j Maximum total intake from all routes of exposure
Appendix E. Parameters used to estimate human exposure to CaDNNSA from the use of a general purpose aerosol lubricant
Exposure estimates for a general purpose aerosol lubricant containing up to 5% of CaDNNSA were estimated using ConsExpo Web (ConsExpo Web 2016). The user was assumed to be an adult aged 19 years or older, with a body weight of 74 kg and an inhalation rate of 15.1 m3/day (Health Canada 2015). Unless otherwise specified, default parameters for the ConsExpo Web model for a penetrating spray lubricant were selected from the General Fact Sheet (RIVM 2014), Cleaning Product Fact Sheet (RIVM 2018) and ConsExpo spray model documentation (RIVM 2009). Absorption from inhalation and dermal routes was conservatively assumed to be 100%.
| Exposure scenario and route of exposure | Parameters used in ConsExpo Web |
|---|---|
| General purpose aerosol lubricant, inhalation | Model: Exposure to spray – spraying Spray duration: 10 seconds (based on product instructions from manufacturer) Exposure duration: 60 minutes (RIVM 2018) Weight fraction: 0.05 (SDS 2018) Room volume: 34 m3 (default for garage,RIVM 2014) Room height: 2.5 m (RIVM 2014) Ventilation rate: 1.5/h (default for garage, RIVM 2014) Mass generation rate: 1.5 g/s (for penetrating spray in a spray can, RIVM 2009) Airborne fraction: 0.2 (RIVM 2018) Density non-volatile: 1.8 g/cm3 (RIVM 2018) Inhalation cut off diameter: 15 mm (RIVM 2018) Aerosol diameter distribution type: log-normal (median diameter: 23.3 mm, arithmetic coefficient of variation: 1.3, maximum diameter: 50 mm; RIVM 2009) |
| General purpose aerosol lubricant, dermal | Model: Direct product contact – constant rate Exposed area: 2185 cm2 (hands and forearms; Statistics Canada 2004 and US EPA 2011) Weight fraction: 0.05 (SDS 2018) Contact rate: 100 mg/min (RIVM 2018) Release duration: 10 seconds (same as spray duration) |
Appendix F. Summary table of read-across for health effects endpoints
| Consideration | Rationale |
|---|---|
| 1. Chemical structure. Emphasis was placed on analogues that contained a naphthalene ring core, one or more alkyl chains, and one or two sulfonate groups. | Analogues that have similar chemical structure and/or are metabolized through similar pathways to similar degradation products are expected to have similar toxicity profiles. Analogues found that have known toxic metabolites which are not expected to result from the metabolism of the target were not considered. |
| 2. Similar metabolites (predicted or observed). Using OASIS TIMES models for autooxidation and rat in vivo and in vitro metabolism all analogues and substances of interest produced similar metabolic profiles. | Analogues that have similar chemical structure and/or are metabolized through similar pathways to similar degradation products are expected to have similar toxicity profiles. Analogues found that have known toxic metabolites which are not expected to result from the metabolism of the target were not considered. |
| 3. Similar physical-chemical properties. Emphasis was placed on chemical structures with similar molecular weight, water solubility, and vapour pressure. | Analogues with similar physical chemical properties may potentially share similar toxicological profiles and bioavailability. |
| 4. Availability of health effects data | Only analogues with hazard data of sufficient quality and coverage of routes and durations of exposure relevant to exposure scenarios were considered applicable for read-across purposes. |
| Chemical name | DNNSA | CaDNNSA | DNNDSA |
|---|---|---|---|
| Acute toxicitya, b | Oral LD50 > 5000 mg/kg bw Inhalation LC50 > 200 mg/l Dermal LD50 > 2000 mg/kg bw | Oral LD50 > 5000 mg/kg bw Inhalation LC50 > 18 mg/l Dermal LD50 > 20000 mg/kg bw | Oral LD50 = 2035 mg/kg bw Dermal LD50 > 1100 mg/kg bw |
| Genotoxicity | Ames: negative [read-across from C9-rich DANSA] TK: negative Chr. Ab: negative [read-across from Ba- C9-rich DANSA] | Ames: negative TK: negative Chr. Ab: negative [read-across from Ba- C9-rich DANSA] | Ames: negative [read-across from C9-rich DANSA] TK: negative Chr. Ab: negative [read-across from Ba- C9-rich DANSA] |
| Short term oral studies | NOAEL= 55 mg/kg bw/day [read-across from Ba- C9-rich DANSA repro/devo study] | NOAEL= 55 mg/kg bw/day [read-across from Ba- C9-rich DANSA repro/devo study] | NOAEL= 55 mg/kg bw/day [read-across from Ba- C9-rich DANSA repro/devo study] |
| Sub-chronic oral studies | NOAEL= 100 mg/kg bw/day [read-across from Ca- C9-rich DANSA] | NOAEL= 100 mg/kg bw/day [read-across from Ca- C9-rich DANSA] | NOAEL= 100 mg/kg bw/day [read-across from Ca- C9-rich DANSA] |
| Reproductive and developmental toxicity oral studies | NOAEL= 165 mg/kg bw/day [read-across from Ba- C9-rich DANSA] | NOAEL= 165 mg/kg bw/day [read-across from Ba- C9-rich DANSA] | NOAEL= 165 mg/kg bw/day [read-across from Ba- C9-rich DANSA] |
| Carcinogenicity | Not available | Not available | Not available |
Abbreviations: LD50, the lethal dose required to kill 50% of the population; LC50, the lethal concentration required to kill 50% of the population; TK, tyrosine kinase; Chr. Ab, chromosome aberration
a US EPA 2012
b ECHA 2018a
