Final Assessment
4. Environment
4.1 Releases and Presence of Triclosan in the Environment
There are no known natural sources of triclosan; its presence in the environment is due solely to human activity. The possible pathways for releases of triclosan to the environment are presented in Figure 4-1; they are based on a conceptual diagram proposed by Bound and Voulvoulis (2005) for pharmaceuticals in the environment.

Long description for figure 4-1
Possible pathways for releases of triclosan to the environment. This figure shows that manufacture or use of products that contains triclosan, whether this happen in industries or in domestic households, can result in down-the-drain releases (wastewater) that will end up in wastewater treatment plants. From wastewater treatment plants, triclosan can reach surface water if it is released as part of their effluent. It can also reach land if sewage sludge from these plants is spread on soil. Triclosan in landfills or that reached land through sewage sludge application could potentially leach to groundwater. Triclosan in biosolids spread on land could also reach surface water through runoff.
Triclosan can be released to the environment as a result of its use in many products used by consumers, or as a result of the industrial manufacture or formulation of products containing triclosan. Use in products is considered to be the major contributor to releases of triclosan down the drain. Triclosan released into wastewater reaches WWTPs, where it is partly removed from wastewater, depending on the type of treatment. Triclosan is released to surface water as part of WWTP effluents. Some triclosan partitions to sludge during the wastewater treatment process. As a result, triclosan also reaches soils by way of biosolids amendment to agricultural land. Other possible pathways included in Figure 4-1 are expected to be less important in terms of environmental releases of triclosan (see sections below).
Additional details on potential sources of triclosan for the aquatic and soil compartments are provided in the following sections. Releases of triclosan to water and soil are described in sections 4.1.1 and 4.1.2. Presence of triclosan in surface waters, sediments and soil in Canada and in other countries is described in section 4.1.3. Metabolites of triclosan, methyl-triclosan and lower-chlorinated dioxins, are also described and considered in the following sections as appropriate.
Triclosan is not expected to be released to air based on the documented uses of triclosan in Canada and on its physical/chemical properties (e.g., low volatility). Air monitoring data for triclosan and its metabolite methyl-triclosan were not identified. Therefore, exposure to triclosan in air is not considered further in this assessment, and additional information is limited to the prediction of the environmental fate that included the air compartment, and estimation the half-life in air (see section 4.2.2).
4.1.1 Releases to water
4.1.1.1 Releases from Industry/Household to Wastewater Treatment Plants
Triclosan is used in a variety of products used by consumers, mainly soaps and skin cleansers. These products are for the most part released down the drain, discharged into sewers and carried to WWTPs. Triclosan is not manufactured in Canada; however, it is imported by a number of companies to manufacture products that contain triclosan. Industrial activities associated with manufacturing of these products may also release some triclosan into sewers. Based on an analysis of the results obtained through the survey conducted under section 71 of CEPA (Environment Canada 2013), the overall relative contribution from manufacturing facilities compared to households in terms of releases of triclosan to WWTPs is expected to be minor.
Also, triclosan that is present in products such as drugs, cleansers and toothpaste can be absorbed orally by humans and then excreted (up to 83% of the oral dose; Sandborgh-Englund et al. 2006) or directly released into the sink. The excreted triclosan is then carried to WWTPs through sewers. Triclosan is also applied on textiles such as T-shirts to prevent emissions of undesirable odours. Based on published studies, it is estimated that the washing of these T-shirts during their use life can release 1.5% of the mass of triclosan that they contain (22 mg per shirt) to sewers (Walser et al. 2011). Junker and Hay (2004) showed that only trace amounts of triclosan are desorbed from plastic when exposed to water in a laboratory setting. Considering that as of December 31, 2014, triclosan is no longer registered in Canada as a pest control product (i.e., can no longer be used to treat textiles, leather, paper, plastics or rubber materials manufactured in Canada), any potential environmental contribution from triclosan-treated articles is expected to be reduced.
Measured concentrations of triclosan in the influent (i.e., in wastewater at point of entry into WWTP) or effluent of several WWTPs located across Canada are shown in Table 4-1. Most of the wastewater systems listed in Table 4-1 use a secondary level of treatment to treat wastewater while two of these systems use a primary level of treatment, and five of the systems are lagoons. The Capital Regional District of Victoria has no wastewater treatment. It can be noted that the WWTP that has a concentration of 20 750 ng/L of triclosan in its influent receives wastewater from a soap manufacturer that reported using triclosan (Environment Canada 2013). The concentration of triclosan in the effluent of this WWTP is however very low due to a high removal efficiency of the wastewater treatment.
Table 4-1. Concentration of triclosan in the influent and effluent of certain WWTPs in Canada
| Location of WWTPs | Sampling year | Conc. in influent (min.-max. or average, ng/L) |
Conc. in effluent (min.-max. or average, ng/L) |
Reference |
|---|---|---|---|---|
| Montreal (population served 1 620 693) |
2005-2006 | 102-811 | 55-662 | Lajeunesse and Gagnon 2007 |
| 1 WWTPa in Quebec | 2010-2012 | 500 | 360 | Pers. comm.b,c |
| 1 WWTPa in Quebec | 2011-2013 | 2050 | 525 | Pers. comm.c |
Table Notes
Note: For table abbreviations and footnotes, see Table 4-1c.
| Location of WWTPs | Sampling year | Conc. in influent (min.-max. or average, ng/L) |
Conc. in effluent (min.-max., or average, ng/L) |
Reference |
|---|---|---|---|---|
| Hamilton (population served 352 000) |
2002 | 1150 | 520-740 | Lee et al. 2003 |
| Toronto (4 WWTPs; (population served 75 000-1 750 000) |
2002 | 380-1320 | 140-210 | Lee et al. 2003 |
| Burlington (population served 144 130) |
2002 | 790 | 130 | Lee et al. 2003 |
| Guelph (population served 100 000) |
2002 | 740 | 110-130 | Lee et al. 2003 |
| Dundas (population served 27 800) |
2002 | 2910 | 30-50 | Lee et al. 2003 |
| Waterdown (population served is NA) | 2002 | 2260 | 120-150 | Lee et al. 2003 |
| Windsor (population served 78 500) |
2003-2004 | 4530 | Mean prior to UV disinfection: 80-330 Mean after UV disinfection: 63 |
Hua et al. 2005; McPhedran et al. 2013 |
| 12 WWTPsa along the Thames River (receiving a mix of residential and industrial wastewater) (population served 2475-182 000) |
2002 | 410-3640 | Mean: 108 Max.: 324 |
Lishman et al. 2006 |
| 8 WWTPsa in southern Ontario (population served 77 225 - 1 750 000) |
2004 | 870-1830 | 50-360 | Lee et al. 2005 |
| 1 WWTPa in Ontario | 2010-2013 | 1073 | 109 | Pers. comm.b,c |
| 1 WWTPa in Ontario | 2010-2011 | 1908 | 90 | Pers. comm.b |
| 1 WWTPa in Ontario | 2011-2012 | 2440 | 40 | Pers. comm.c |
| 1 WWTPa in Ontario | 2011-2012 | 2600 | 20 | Pers. comm.c |
| 1 WWTPa in Ontario | 2011-2013 | 20 750 | 12 | Pers. comm.c |
| 1 WWTPa in Ontario | 2011-2013 | 865 | 40 | Pers. comm.c |
Table Notes
Abbreviations: NA, not applicable.
Note: For table abbreviations and footnotes, see Table 4-1c.
| Location of WWTPs | Sampling year | Conc. in influent (min.-max. or average, ng/L) |
Conc. in effluent (min.-max. or average, ng/L) |
Reference |
|---|---|---|---|---|
| 1 WWTPa in British Columbia | 2010-2013 | 1673 | 167 | Pers. comm.b,c |
| 1 WWTPa in British Columbia | 2011-2013 | 1350 | 865 | Pers. comm.c |
| Capital Regional District Victoria outfall | 2006 | NA | 2200-4160 | Pers. comm.d |
Table Notes
Abbreviations: conc., concentration; max., maximum; min., minimum; NA, not available; pers. comm., personal communication; UV, ultraviolet; WWTP, wastewater treatment plant.
a Identity cannot be divulged. Certain WWTPs are the same across studies.
b 2011 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
c 2013 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
d 2008 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
Some of the concentrations measured in influent and effluent cited in Table 4-1 as personal communication have been summarized by Guerra et al. (2014).
4.1.1.2 Removal by WWTPs
The fate of triclosan within WWTPs is somewhat complex and has been the subject of several investigations (Bester 2003, 2005; Sabaliunas et al. 2003; Thomas and Foster 2005; Waltman et al. 2006). Studies show that WWTPs are quite efficient in removing triclosan from wastewater, if they have secondary wastewater treatment system. Thomas and Foster (2005) reported that the majority of triclosan removal occurs during secondary treatment (55-88%) and that a smaller proportion (10-44%) is removed during the primary treatment.
In Canada, Lishman et al. (2006) reported 74-98% removal of triclosan in WWTPs located along the Thames River in Ontario. Most of these plants have at least secondary treatment with activated sludge as part of their process. Lee et al. (2003) also reported a median removal efficiency of 81% (range: 49-94%) in WWTPs located in southern Ontario, where most of the plants surveyed employed at least a secondary treatment. It is noted that, based on data from 2004, 26% of the 22 million Canadians serviced by sewer systems were provided with primary wastewater treatment or less (Environment Canada 2007). In 2012, federal Wastewater Systems Effluent Regulations were put in place. These Regulations set national baseline effluent quality standards achievable through secondary wastewater treatment and require wastewater systems with no or little wastewater treatment to be upgraded (Canada 2012).
Canadian removal efficiencies compare with those measured in other countries. In the United States, triclosan removal of 95-96% (McAvoy et al. 2002), and up to 99% (Thomas and Foster 2005), was reported at WWTPs that use secondary treatment. In Europe, WWTP removal efficiencies for triclosan in the range of 87-96% were reported for Germany (Bester 2003, 2005), 94 % for Switzerland (Singer et al. 2002), and 95% for the United Kingdom (Sabaliunas et al. 2003), also for plants that have secondary treatment. These numbers show that efficient removal of triclosan is attributed to secondary treatment.
The removal mechanisms of triclosan from wastewater were investigated in a few studies. Thomas and Foster (2005) showed that adsorption to particulate matter is a likely removal mechanism for triclosan. Bester (2003) reported that 96% of triclosan was removed from wastewater, of which 22-43% was adsorbed to the sludge. This is in line with the moderately sorptive nature of this compound (log Koc up to 4.67; see Table 2-2). Federle et al. (2002) conducted a continuous activated sludge test aimed at examining the degradation of triclosan. In this test, 14C-labelled triclosan was used to establish a material balance. The authors reported that, at steady state, between 1.5% and 4.5% of triclosan was sorbed to solids, whereas 81-92% was mineralized to carbon dioxide or incorporated into microbial biomass. The 14C present in the effluent consisted of extractable (in ethyl acetate) and non-extractable polar intermediates (0.4-7.2% and 2.3-10.5%, respectively). Overall, removal of the parent compound exceeded 98.5%. A second set of experiments was conducted by Federle et al. (2002) and showed that shock loading with triclosan, representative of a situation in which a WWTP receives a consistent low level of triclosan (e.g., from down-the-drain disposal of products used by consumers) with periodical pulses of higher levels (e.g., from a manufacturing facility), did not significantly change the removal pattern. Finally, in a batch activated sludge mineralization test, Federle et al. (2002) observed that 31-52% of triclosan had degraded to 14CO2 in 71 days after its addition to the sludge. Following a lag period of 3-10 days, triclosan was spiked again in the test system, resulting in 79-81% of this second dose being recovered as 14CO2 after 52 days.
Even though triclosan is removed efficiently by WWTPs, it may also be methylated to methyl-triclosan during the treatment process, likely during secondary treatment. The contribution of this reaction to the overall removal of triclosan from wastewater has not been quantified, but a decrease in triclosan levels has been associated with an increase in methyl-triclosan levels during secondary treatment (Lozano et al. 2013). Generally, the levels of methyl-triclosan in effluent from WWTPs are very low (Lindström et al. 2002; McAvoy et al. 2002), partly because this substance partitions to wastewater sludge (Lozano et al. 2013).
In addition, triclosan can react with chloramines which are used either as an alternative disinfectant to free chlorine in drinking water treatment or formed during the chlorination of non-nitrified wastewater effluent. Greyshock and Vikesland (2006) examined triclosan reactivity in chloraminated waters over a pH range of 6.5-10.5. The reactivity of triclosan in the presence of chloramines is low. The products of these reactions included three chlorinated forms of triclosan as well as 2,4-dichlorophenol and 2,4,6-trichlorophenol.
Impacts of triclosan exposure on bacterial communities in municipal digesters have not been extensively studied; however the few laboratory studies to date indicate that triclosan can alter bacterial community structure and proliferate antimicrobial resistance. It is noted that conditions and concentrations used in the laboratory studies differ from the actual environmental conditions and exposure concentrations used are generally higher than those measured in the environment. Therefore, the extent of antimicrobial resistance and impacts on bacterial community structures in WWTP from present levels of triclosan are not clear. It has been shown that triclosan can decrease oxygen uptake and inhibit nitrification in activated sludge biomass (Stasinakis et al. 2008a). In a study using lab-scale anaerobic digesters, exposure to triclosan at 5, 50 and 500 mg/kg affected bacterial community structures and digester function, and resulted in proliferation of antimicrobial resistance genes (McNamara et al. 2014). Both the Bacteria and Archaea communities used in the McNamara (2014) study were observed to diverge from the control communities, overall digester function, assessed by means of methane production, diminished, with 50 mg/kg exposure concentration observed to be the point at which function began to fail in some communities, and the proliferation of triclosan resistance gene (mexB) increased at the exposure concentration of 500 mg/kg in previously unexposed communities (McNamara et al. 2014). In aerobic bacteria, alteration of community structure and selection for resistant bacteria in aerobic sediments and in aerobic activated sludge were also observed (Drury et al. 2013; Son et al. 2010).
4.1.1.3 Releases from WWTPs to surface water
4.1.1.3.1 In Canada
Results of several surveys have indicated that triclosan is released from Canadian WWTPs in the effluent (12-4160 ng/L; see Table 4-1). The wide range of concentrations measured in effluent reflects mainly the differences in the population served by the WWTPs as well as the various treatment levels used by the plants (from no treatment to secondary wastewater treatment). Given the multiple products containing triclosan and their ubiquity, a fairly consistent use pattern is expected across Canada.
4.1.1.3.2 In other countries
Concentrations of triclosan in WWTP influent and effluent were measured internationally, in the United States, Switzerland, Scandinavian countries, Spain, and Germany, and generally reflect the levels found in Canada. Monitoring of methyl-triclosan was also undertaken in the United States and Switzerland.
In the United States, samples of influent, primary effluent and final effluent were collected from five WWTPs and analyzed for triclosan and methyl-triclosan in a monitoring study (McAvoy et al.2002). The plants sampled served populations of 2 445-398 000. The concentrations of triclosan in the final effluent sample ranged between 240 and 410 ng/L, and 1610 and 2700 ng/L for plants using activated sludge or trickling filter treatments, respectively. Methyl-triclosan, a transformation product, was qualitatively detectable in all samples and was estimated to be present in the range of 2-50 ng/L.
The trickling filter treatment involves the use of a bed of crushed rock or synthetic media to support a film of aerobic microorganisms. This method is recognized as being less effective than the activated sludge treatment. Less than 2% of WWTPs in Canada use this process.
In Switzerland, samples of primary and final effluent from WWTPs were collected in 1997 and 2001 from WWTPs that employed a biological treatment process (secondary treatment, but exact method not specified). The sampled WWTPs served populations of 4500-36 000 persons. Triclosan in the primary effluent was found at concentrations of 600-1300 ng/L, whereas methyl-triclosan was detected in much lower concentrations, from less than 1 to 4 ng/L. The corresponding final effluent concentrations were between 70 and 650 ng/L for triclosan and between less than 2 and 11 ng/L for methyl-triclosan. The higher concentrations of methyl-triclosan in the final effluent compared with the primary effluent indicate that this transformation product is formed during biological treatment.
A monitoring program in Denmark examined triclosan concentrations in the final effluent of a WWTP serving both a population of 750 000 and with industrial input. This WWTP included a biological treatment as part of its wastewater treatment process. The average triclosan concentration measured in the effluent was below the detection limit of 1000 ng/L (Pedersen and Nielsen 2003). In Sweden, the final effluent from the three largest WWTPs in the country were sampled and analyzed for several organic pollutants, including triclosan (Paxéus 1996). In two of the plants, triclosan was measured at a concentration of 500 ng/L; it was not detected in the effluent of the third plant (method detection limit [MDL] not specified).
International and domestic monitoring data for WWTP effluent was also summarized by the US EPA (US EPA 2008e). According to US EPA (2008e), triclosan concentrations in WWTP effluent ranged from 10 to 2700 ng/L in the United States, from 80 to 269 000 ng/L in Spain, and from 10 to 600 ng/L in Germany.
4.1.2 Releases to soil
Some of the reported uses for triclosan in Canada may lead to this substance reaching landfills as part of solid wastes (e.g., products made of textile or rubber). Landfills that do not collect and treat their leachate may potentially release substances to soil, eventually reaching ground or surface water via leaching. However, no data on the quantity of triclosan following this disposal pathway are available.
The application of biosolids from wastewater treatment plants to agricultural lands can result in the presence of triclosan in soil. Considering this route of exposure, the presence of triclosan in sludge and biosolids was investigated.
4.1.2.1 Concentrations in wastewater treatment sludge and biosolids in Canada
Triclosan was readily found in sludge and biosolids collected from WWTPs across Canada as described in numerous studies and monitoring initiatives (Table 4-2).
Between 2011 and 2013, sludge from six Canadian WWTPs was sampled by Environment Canada; average triclosan concentrations ranged between 3.5 and 26.0 μg/g dw (median: 8.9 μg/g dw) (2013 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced). In a study conducted for the Canadian Council of Ministers of the Environment to document the occurrence of emerging substances of concern in biosolids, samples were collected in 2009 at 11 WWTPs located across Canada (CCME 2010a; Table 4-2). Overall, triclosan was found in 97% of the samples collected; the median concentration for all samples was 6.1 µg/g dw (range: less than 0.1-46.4 µg/g dw), the highest median value among all of the 82 substances analyzed in this study. According to the study, aerobic treatment processes appeared successful in reducing the input mass of triclosan in the feed sludges (residual wastewater solids delivered to the treatment processes studied). This substance was not well reduced by anaerobic digestion. Chu and Metcalfe (2007) measured similar levels of triclosan, in the range of 0.68-11.55 μg/g dw, in treated biosolids collected in 2006 from four WWTPs located in southern Ontario. Concentrations of triclosan in wastewater sludge sampled in 25 WWTPs across Canada, from Vancouver to Moncton, were reported by Lee and Peart (2002). Most of the samples collected were from digested sludge (i.e., following secondary wastewater treatment). Triclosan was detected in all sludge samples in the range of 0.90-28.2 μg/g dry weight (dw) (median: 12.5 μg/g dw). According to Lee and Peart (2002), triclosan is likely to be the most abundant polychlorinated phenol found in wastewater sludge, since only 3 out of 35 samples taken contained less than 5 μg/g dw of triclosan.
No monitoring data could be found for concentrations of methyl-triclosan in wastewater sludge from WWTPs in Canada.
| WWTP location | Sampling period | Concentration (min.-max. or average, µg/g dw) |
Reference |
|---|---|---|---|
| Vancouver (BC) | 1994 and 1999 | 8.41-24.7 | Lee and Peart 2002 |
| Calgary (Bonny Brook) (AB) | 1999 | 12.8 | Lee and Peart 2002 |
| Calgary (Fish Creek) (AB) | 1999 | 19.5 | Lee and Peart 2002 |
| Edmonton (AB) | 2000 | 22.0 | Lee and Peart 2002 |
| Regina (SK) | 2000 | 18.9 | Lee and Peart 2002 |
| Saskatoon (SK) | 2000 | 9.9 | Lee and Peart 2002 |
| Adelaidea (ON) | 1998 | 8.9 | Lee and Peart 2002 |
| Burlington (ON) | 2001 | 19.4 | Lee and Peart 2002 |
| Galt (ON) | 1996 | 7.48 | Lee and Peart 2002 |
| Guelph (ON) | 1999 | 28.2 | Lee and Peart 2002 |
| Hamilton (ON) | 1997 | 16.2 | Lee and Peart 2002 |
| Ingersoll (ON) | 1998 | 11.5 | Lee and Peart 2002 |
| Kitchener (ON) | 1997 | 16.1 | Lee and Peart 2002 |
| Ottawa (ON) | 2000 | 18.6 | Lee and Peart 2002 |
| Waterloo (ON) | 1996 | 11.7 | Lee and Peart 2002 |
| Windsor (ON) | 1997 | 8.84 | Lee and Peart 2002 |
| Toronto (Ashbridges Bay) (ON) | 2000 | 20.3 | Lee and Peart 2002 |
| Toronto (Highland Creek)a(ON) | 2000 | 16.5 | Lee and Peart 2002 |
| Toronto (Humber) (ON) | 2000 | 16.6 | Lee and Peart 2002 |
| Toronto (North) (ON) | 2000 | 5.4 | Lee and Peart 2002 |
| Montreala (QC) | 1999 | 6.1 | Lee and Peart 2002 |
| Granby (QC) | 1996 | 0.90 | Lee and Peart 2002 |
| Quebeca (QC) | 2000 | 5.5-9.8 | Lee and Peart 2002 |
| Moncton (NB) | 1997 | 1.92 | Lee and Peart 2002 |
| Truro (NS) | 1996 | 7.53 | Lee and Peart 2002 |
| Windsor (ON) | 2004 | 5.29 | McPhedran et al. 2013 |
| 4 WWTPs in southern Ontario (ON) | 2006 | 0.68-11.55 | Chu and Metcalfe 2007 |
| Salmon Arm (BC) | 2009 | Min.-max.: 21.3-24.0 Median: 21.5 |
CCME 2010a |
| Red Deer (AB) | 2009 | Min.-max.: 11.7-13.9 Median: 12.7 |
CCME 2010a |
| Saskatoon (SK) | 2009 | Min.-max.: 5.6-6.3 Median: 6.1 |
CCME 2010a |
| Prince Albert (SK) | 2009 | Min.-max.: 2.3-5.6 Median: 4.0 |
CCME 2010a |
| Eganville (ON)b | 2009 | Min.-max.: 0.6-30.6 Median: 3.1 |
CCME 2010a |
| Smiths Falls (ON)b | 2009 | Min.-max.: 11.8-11.9 Median: 11.8 |
CCME 2010a |
| Gatineau Valley (QC)b | 2009 | Min.-max.: 27.6-46.4 Median: 38.6 |
CCME 2010a |
| Gatineau Valley (QC)c | 2009 | Min.-max.: less than 0.1-0.92 Median: 0.78 |
CCME 2010a |
| Saguenay (QC)b | 2009 | Min.-max.: 0.9-2.8 Median: 1.3 |
CCME 2010a |
| Moncton (NB)d | 2009 | Min.-max.: 5.9-7.3 Median: 7.0 |
CCME 2010a |
| Moncton (NB)c | 2009 | Min.-max.: 0.60-0.96 Median: 0.63 |
CCME 2010a |
| Halifax (NS)e | 2009 | Min.-max.: 4.8-6.5 Median: 6.1 |
CCME 2010a |
| Gander (NL) | 2009 | Min.-max.: 9.2-20.3 Median: 9.6 |
CCME 2010a |
| 3 WWTPsf in Ontario | 2011-2013 | 3.5-14.5 | Pers. comm.g |
| 2 WWTPsf in British Columbia | 2011-2013 | 6.5b-26.0 | Pers. comm.g |
| 1 WWTPf in Quebecb | 2011-2012 | 7.7 | Pers. comm.g |
Table Notes
Abbreviations: dw, dry weight; pers. comm., personal communication; max., maximum; min., minimum; WWTP, wastewater treatment plant.
a In raw sludge.
b In dewatered biosolids cake.
c Composted biosolids.
d Lime-stabilized biosolids.
e This plant also treats sludge from Herring Cove, Bedford, Dartmouth and Aerotech.
f Identity cannot be divulged. Certain WWTPs are the same across studies.
g 2013 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
Some of the concentrations measured in biosolids cited in Table 4-2 as personal communication have been summarized by Guerra et al. (2014).
4.1.2.2 Concentrations in wastewater treatment sludge in other countries
Data on triclosan occurrence in sludge were available for the United States, Sweden, and Australia. Methyl-triclosan, and chlorinated derivates of triclosan were also measured in samples from the United States. Triclosan sludge concentrations found in samples from both the United States and Sweden were within the range found in Canadian samples (presented in Table 4-2).
Triclosan and methyl-triclosan were measured in sludge samples taken from WWTPs in the United States (McAvoy et al. 2002). It was found that triclosan was rapidly removed during the aerobic sludge digestion process, whereas samples from a trickling filter treatment plant showed little or no removal of triclosan during anaerobic sludge digestion. Triclosan concentrations ranged from 0.5 to 15.6 μg/g dw, whereas those for methyl-triclosan ranged from below the limit of quantification (LOQ) to 1.03 μg/g dw, and concentrations of chlorinated derivatives were up to 0.42 μg/g dw (McAvoy et al. 2002). McClellan and Halden (2010) measured an average triclosan concentration of 12.6 μg/g dw and a maximum concentration of 19.7 μg/g dw in archived biosolids collected in 2001 from 94 WWTPs in the United States as part of a national survey. Among the 38 compounds that were detected in the sludge samples, triclosan was found at the second highest mean concentration after triclocarban, which is another antimicrobial agent.
In Sweden, Svensson (2002) sampled sludge from 19 WWTPs in 2001-2002. Concentrations of triclosan in the sludge samples ranged from 0.028 to 6.4 μg/g dw. Another investigation of sludge samples from four Swedish WWTPs in 2001 revealed similar triclosan levels in the range of 2.8-4.4 μg/g dw in anaerobically digested sludge (Remberger et al. 2002). For one of the plants surveyed, both a primary sludge and an anaerobically digested sludge sample were analyzed. The results of these analyses supported the findings of McAvoy et al. (2002) that little or no removal of triclosan occurs during anaerobic digestion.
In Australia, Langdon et al. (2011) sampled biosolids from 13 WWTPs and found triclosan concentrations ranging from 0.22 to 9.89 μg/g dw, with an average of 3.77 μg/g dw.
4.1.3 Environmental concentrations
Continuous releases of triclosan from products that contain it, most notably through wastewater, result in the ubiquitous presence of this chemical in the environment. Concentrations of triclosan have been found in surface waters, sediments, and soil in the range of ppt to ppb. Monitoring of triclosan in the Canadian surface waters between early 2000 and until the latest available data for 2014 indicate that triclosan continues to be present at constant levels.
Available monitoring and surveillance data for water, sediments, and soil for Canada and other countries are summarized below.
4.1.3.1 Measured concentrations in surface waters
4.1.3.1.1 In Canada
Table 4-3 presents the range of triclosan concentrations measured in surface waters in Canada. The large portion of this data was generated by the Water Science and Technology Directorate, Environment Canada (personal communication, Table 4-3b-h, unreferenced). Data were available for all provinces and territories, except Prince Edward Island, from 2002 to 2013. Certain locations across Canada continued to be sampled in 2014. Levels reported spanned almost four orders of magnitude, from below the method detection limit (MDL) to 874 ng/L (reported method detection limits ranged from 4 to 42 ng/L); the highest median concentration was calculated as 139 ng/L. Since surface water in both heavily and lightly populated areas was sampled, this range is expected to be representative of the Canadian inland waters. The data for locations sampled over 8-10 years generally indicate that triclosan continues to be present at constant levels.
Table 4-3. Concentrations of triclosan in surface water in Canada
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)a | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| Detroit River, 600 m downstream of Little River WWTP (City of Windsor) | 2003 | 3 | NA | 8 (mean) | NA | Hua et al. 2005 |
| Mouth of Niagara River (Niagara-on-the-Lake) | 2004-2005 | 10 | 0.34 | 0.69 | 3.20 | Pers. comm.b |
| Head of Niagara River (Fort Erie) | 2004-2005 | 11 | less than MDL | less than MDL | 0.43 | Pers. comm.b |
| St. Lawrence River (south channel) at outlet of Lake Ontario (Wolfe Island) | 2004-2005 | 11 | less than MDL | 0.11 | 0.25 | Pers. comm.b |
| Thames River | 2002 | 86 | less than MDL | less than MDL | 691 | Pers. comm.c |
| Hamilton Harbour | 2003-2004 | 59 | less than MDL | 12 | 626 | Pers. comm.c |
| Grand River | 2003-2004 | 72 | less than MDL | 11 | 260 | Pers. comm.c |
| Andrews Creek | 2005 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.c |
| Blyth Brook | 2005 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.c |
| Egbert Creek | 2005 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.c |
| Indian Creek | 2005 | 4 | less than MDL | less than MDL | 599 | Pers. comm.c |
| Kerrys Creek | 2005 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.c |
| Laurel Creek | 2005 | 5 | less than MDL | less than MDL | 65 | Pers. comm.c |
| Little Ausable River | 2005 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.c |
| Middle Maitland River | 2005 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.c |
| Mill Creek | 2005 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.c |
| Nineteen Creek | 2005 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.c |
| Nissouri Creek | 2005 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.c |
| North Maitland River | 2005 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.c |
| Nottawasaga River | 2005 | 6 | less than MDL | less than MDL | 22 | Pers. comm.c |
| Spring Creek | 2005 | 6 | less than MDL | less than MDL | 93 | Pers. comm.c |
| Stokes River | 2005 | 6 | less than MDL | less than MDL | 43 | Pers. comm.c |
| Twenty Mile Creek | 2005 | 15 | less than MDL | less than MDL | 433 | Pers. comm.c |
| Vineland Creek | 2005 | 5 | less than MDL | 34 | 66 | Pers. comm.c |
| West Don River | 2005 | 6 | less than MDL | 23 | 64 | Pers. comm.c |
| 6 rivers and 3 lakes in Ontario | 2009-2010 | 22 | less than MDL | less than MDL | 74 | Pers. comm.d |
| Niagara River (at Niagara-on-the-Lake) | 2012-2013 | 5 | less than MDL | less than MDL | 7.53 | Pers. comm.e |
| Wolfe Island | 2012-2013 | 5 | less than MDL | less than MDL | less than MDL | Pers. comm.e |
| Mimico Creek | 2012-2014 | 19 | less than MDL | less than MDL | 80.4 | Pers. comm.e |
| Highland Creek | 2012-2014 | 18 | less than MDL | 5.38 | 22.6 | Pers. comm.e |
| Grand River (upstream of Kitchener WWTP) | 2012-2014 | 16 | less than MDL | less than MDL | 6.7 | Pers. comm.e |
| Grand River (downstream of Kitchener WWTP) | 2012-2014 | 19 | less than MDL | 12.5 | 44.2 | Pers. comm.e |
| Thames River (upstream of London Greenway WWTP) | 2012-2014 | 17 | less than MDL | less than MDL | 19.1 | Pers. comm.e |
| Thames River (downstream of London Greenway WWTP) | 2012-2014 | 17 | less than MDL | 8.18 | 16.9 | Pers. comm.e |
| 4 sites in Hamilton Harbour | 2012-2014 | 60 | less than MDL | 5.92 | 268 | Pers. comm.e |
| Taylor Creek | 2012-2014 | 19 | less than MDL | 20.8 | 58.8 | Pers. comm.e |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a Ontario: MQL = 4 ng/L for the Detroit River; MDL = 0.10 ng/L for the mouth and head of the Niagara River and St. Lawrence River; MDL = 5 ng/L for the Grand River and Hamilton Harbour; MDL = 20 ng/L for other water bodies referenced as personal communication;c MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng /L (average of 6.02 ng/L) for water bodies referenced as personal communication.e,f
b 2006 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
c 2007 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
d 2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
e 2015 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)a | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| Ottawa River (Carillon) | 2006-2008 | 10 | less than MDL | less than MDL | 9 | Pers. comm.f |
| St. Maurice River (at Trois-Rivières) | 2007-2008 | 4 | less than MDL | less than MDL | less than MDL | Pers. comm.f |
| St. Lawrence River (at Lavaltrie) | 2006-2009 | 11 | less than MDL | 16 | 29 | Pers. comm.f |
| St. Lawrence River (at Bécancour) | 2006-2009 | 10 | less than MDL | 5 | 25 | Pers. comm.f |
| Richelieu River (at Sorel) | 2006-2009 | 11 | less than MDL | less than MDL | 11 | Pers. comm.f |
| St. Lawrence River (at Lévis) | 2007-2009 | 11 | less than MDL | 6.9 | 34 | Pers. comm.f |
| 3 rivers and 1 lake in Québec | 2009-2010 | 11 | less than MDL | 41 | 146 | Pers. comm.d |
| St. Lawrence River (at Lévis) | 2012-2014 | 10 | less than MDL | less than MDL | 7.65 | Pers. comm.a |
| St. Lawrence River (Lavaltrie) | 2012-2014 | 17 | less than MDL | 7.52 | 15.8 | Pers. comm.e |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a Quebec: MDL = 6 ng/L for water bodies referenced as personal communication;g MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng/L (average of 6.02 ng/L) for water bodies referenced as personal communication.e.
b 2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
e 2015 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
f 2010 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)a | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| 2 rivers and 2 lakes in Manitoba | 2009-2010 | 8 | less than MDL | less than MDL | less than MDL | Pers. comm.d |
| Red River (at Highway 4) | 2013 | 2 | less than MDL | NA | 5.73 | Pers. comm.e |
| Red River (Selkirk) | 2013-2014 | 5 | less than MDL | less than MDL | 14 | Pers. comm.e |
| Red River (Winnipeg) | 2013 | 2 | less than MDL | NA | 37.1 | Pers. comm.e |
| Red River (Emmerson) | 2013 | 2 | less than MDL | less than MDL | less than MDL | Pers. comm.e |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a Manitoba: MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng/L (average of 6.02 ng/L) for water bodies referenced as personal communication.e,f.
d 2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
e 2015 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)d | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| Columbia River (at Waneta) | 2009 | 1 | NA | NA | less than 147 | Pers. comm.g |
| Fishtrap Creek | 2009-2010 | 2 | less than 66 | NA | less than 69 | Pers. comm.g |
| Fraser River | 2008 | 2 | less than 236 | NA | less than 240 | Pers. comm.g |
| Mill Creek (Kelowna) | 2008–2010 | 18 | less than 63 | NA | less than 249 | Pers. comm.g |
| Okanagan River | 2008–2010 | 16 | less than 62 | NA | less than 248 | Pers. comm.g |
| Still Creek (Burnaby) | 2008, 2010 | 3 | less than 64 | NA | less than 241 | Pers. comm.g |
| Sumas River | 2008–2010 | 4 | less than 64 | NA | less than 245 | Pers. comm.g |
| BX Creek (Vernon) | 2009–2010 | 3 | less than 70 | NA | less than 120 | Pers. comm.g |
| Ellis Creek (Penticton) | 2009–2010 | 4 | less than 64 | NA | less than 131 | Pers. comm.g |
| Hastings Creek (North Vancouver) | 2010 | 1 | NA | NA | less than 63 | Pers. comm.g |
| Osoyoos Lake | 2009–2010 | 2 | less than 67 | NA | less than 111 | Pers. comm.g |
| 3 rivers and 3 lakes in British Columbia | 2009–2010 | 12 | less than MDL | less than MDL | less than MDL | Pers. comm.d |
| Mill Creek (upstream) | 2012–2014 | 15 | less than MDL | less than MDL | 7.7 | Pers. comm.e |
| Mill Creek (middle) | 2012–2014 | 15 | less than MDL | less than MDL | 20.7 | Pers. comm.e |
| Mill Creek (reference) | 2012–2014 | 11 | less than MDL | less than MDL | 35.3 | Pers. comm.e |
| Okanagan River (North) | 2012–2014 | 6 | less than MDL | NA | 17 | Pers. comm.e |
| Okanagan River | 2012–2014 | 6 | less than MDL | less than MDL | 8.9 | Pers. comm.e |
| Osoyoos Lake | 2012–2013 | 4 | less than MDL | less than MDL | less than MDL | Pers. comm.e |
| Serpentine River | 2012–2014 | 15 | less than MDL | less than MDL | 11.3 | Pers. comm.e |
| Still Creek | 2012–2014 | 18 | less than MDL | less than MDL | 20.2 | Pers. comm.e |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a British Columbia: Values are presented as less than SDL for water bodies referenced as personal communication.h The SDL varies by sample and can be lower or higher than the MDL depending on the sample's cleanness (i.e., presence or absence of interfering constituents). MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng/L (average of 6.02 ng/L) for water bodies referenced as personal communication.e,f
d2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
e2014 personal communication from Water Science and Technology Directorate, Environment Canada to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
g2011 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)e | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| Wascana Creek (downstream of Regina) | 2002-2003 | 23 | 12 | 87 | 602 | Pers. comm.g |
| Wascana Creek (upstream to downstream of Regina) | 2006 | 5 | less than MDL | 139 | 178 | Pers. comm.g |
| Wascana Creek (upstream to downstream of Regina) | 2006-2007 | 10 | less than MDL | 43 | 112 | Waiser et al. 2011 |
| Qu'Appelle River (upstream to downstream of confluence with Wascana Creek) | 2006 | 5 | less than MDL | 22 | 26 | Pers. comm.g |
| Pasqua Lake | 2006 | 1 | NA | NA | 15 | Pers. comm.g |
| 2 rivers in Saskatchewan | 2009-2010 | 4 | less than MDL | less than MDL | less than MDL | Pers. comm.d |
| Wascana Creek (downstream) | 2012-2014 | 12 | less than MDL | 63.3 | 874 | Pers. comm.e |
| Wascana Creek (upstream) | 2012-2013 | 9 | less than MDL | less than MDL | less than MDL | Pers. comm.e |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a Saskatchewan: MDL = 25 ng/L (Waiser et al. 2011), MDL = 5 ng/L for water bodies referenced as personal communication;h MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng/L (average of 6.02 ng/L) for water bodies referenced as personal communication.e,f
e 2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
d 2014 personal communication from Water Science and Technology Directorate, Environment Canada to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
g 2011 personal communication from Water Science and Technology Directorate, Environment Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)a | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| 1 lake and 3 rivers in Alberta | 2009-2010 | 8 | less than MDL | less than MDL | less than MDL | Pers. comm.d |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a Alberta: MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng/L (average of 6.02 ng/L) for water bodies referenced as personal communication.
d 2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)a | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| 1 river and 2 lakes in Newfoundland | 2009-2010 | 6 | less than MDL | less than MDL | 34 | Pers. comm.d |
| Waterford River | 2012-2014 | 12 | less than MDL | NA | 17 | Pers. comm.e |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a Newfoundland: MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng/L (average of 6.02 ng/L) for water bodies referenced as personal communication.
d 2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
e 2015 personal communication from Water Science and Technology Directorate, Environment Canada to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)a | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| 1 river and 1 lake in New Brunswick | 2009-2010 | 4 | less than MDL | less than MDL | less than MDL | Pers. comm.d |
| Napan River | 2012-2013 | 7 | less than MDL | less than MDL | less than MDL | Pers. comm.e |
| St. John River (upstream) | 2012-2014 | 16 | less than MDL | less than MDL | 8.0 | Pers. comm.e |
| St. John River (downstream) | 2012-2014 | 16 | less than MDL | less than MDL | 6 | Pers. comm.e |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a New Brunswick: MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng/L (average of 6.02 ng/L) for water bodies referenced as personal communication.
d 2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
e 2015 personal communication from Water Science and Technology Directorate, Environment Canada to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)a | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| 2 lakes in Nova Scotia | 2009-2010 | 4 | less than MDL | less than MDL | less than MDL | Pers. comm.d |
| Little Sackville River | 2012-2013 | 5 | less than MDL | 12 | 25.4 | Pers. comm.e |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a Nova Scotia: MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng/L (average of 6.02 ng/L) for water bodies referenced as personal communication.
d 2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
e 2015 personal communication from Water Science and Technology Directorate, Environment Canada to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)a | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| 1 lake in Yukon | 2009-2010 | 2 | less than MDL | less than MDL | less than MDL | Pers. comm.d |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a Yukon: MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng/L (average of 6.02 ng/L) for water bodies referenced as personal communication.
d 2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)a | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| 1 river and 2 lakes in Northwest Territories | 2009-2010 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.d |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a Northwest Territories: MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng/L (average of 6.02 ng/L) for water bodies referenced as personal communication.
d 2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
| Water body | Sampling period | No. of samples | Min. conc. (ng/L)a | Median conc. (ng/L)a | Max. conc. (ng/L)a | Reference |
|---|---|---|---|---|---|---|
| 3 lakes in Nunavut | 2009-2010 | 6 | less than MDL | less than MDL | less than MDL | Pers. comm.d |
Table Notes
Abbreviations: conc., concentration; max., maximum; MDL, method detection limit; min., minimum; MQL, method quantification limit; NA, not available; No., number; SDL, sample detection limit; WWTP, wastewater treatment plant.
a Nunavut: MDL = 10 ng/L for water bodies referenced as personal communication;d MDL varied between sample batches and ranged from 4.06 to 41.9 ng/L (average of 6.02 ng/L) for water bodies referenced as personal communication.
d2014 personal communication from Environmental and Radiation Health Sciences Directorate, Health Canada, to Science and Risk Assessment Directorate, Environment Canada; unreferenced.
In addition, methyl-triclosan monitoring data were identified for Ontario and Saskatchewan. Methyl-triclosan was measured at concentrations of approximately 1 ng/L and 0.1 ng/L in water samples from Hamilton Harbour and Lake Ontario, respectively (Andersen et al. 2007). In Saskatchewan, Waiser et al. (2011) measured concentrations ranging from 3 to 17 ng/L in Wascana Creek downstream of Regina’s WWTP. The Wascana Creek downstream sampling location is associated with the highest measured levels of triclosan (see Table 4-3).
4.1.3.1.2 In other countries
Levels of triclosan have been monitored in the United Sates. In a national reconnaissance survey of 139 streams across 30 states during 1999 and 2000, the maximum and median measured concentrations of triclosan were 2300 ng/L and 140 ng/L, respectively (Kolpin et al. 2002). In Texas, Coogan et al. (2007) measured triclosan and methyl-triclosan concentrations of 60-120 ng/L and 50-80 ng/L, respectively, in a creek receiving an effluent from a WWTP.
Okumura and Nishikawa (1996) measured triclosan at concentrations of 50-150 ng/L in a river in Japan. In Switzerland, concentrations of triclosan in rivers and lakes ranged from 1.4 to 74 ng/L, as reported by Lindström et al. (2002). Still in Switzerland, Singer et al. (2002) measured a methyl-triclosan concentration of about 0.5 ng/L (between the method quantification limit [MQL] and MDL) in water sampled in both the epilimnion and hypolimnion of a lake.
Brausch and Rand (2011) reviewed all studies conducted on triclosan that were published before April 2010 and calculated that this compound has been detected in 56.8% of the surface water samples analyzed (n = 710), with a median concentration of 48 ng/L (range: less than 0.1-2300 ng/L). Their review included data for surface water in the United States, Romania, the United Kingdom, the Republic of Korea and Switzerland, to name a few.
4.1.3.2 Measured concentrations in sediments
4.1.3.2.1 In Canada
Monitoring data for triclosan and methyl-triclosan are available for the years 2012 and 2013 (personal communication, 2015 email from Water Quality Monitoring and Surveillance Division, Environment Canada to Ecological Assessment Division, Environment Canada; unreferenced). Surface sediment samples were collected from the Pacific and Atlantic regions, Lake Erie, and St. Lawrence River. Overall, Canadian surface sediment concentrations of triclosan were in the range of less than 1-47 ng/g, and in the range of less than 2-22 ng/g for methyl-triclosan. Samples of core sediment at different depths from Lake Ontario were analyzed; the maximum concentrations of triclosan and methyl-triclosan were measured at 9 cm core depth, and were 9 and 15 ng/g, respectively. Suspended sediment was measured at varying distances from a WWTP located along the St. Lawrence River; the maximum triclosan concentration in the range of nearly 1000-2000 ng/g was found at a distance 4 km. Canadian monitoring data of triclosan and methyl-triclosan are presented in Table 4-4 below.
| Location (sample size) | Sample type | Triclosan range (ng/g) | Triclosan geometric mean (ng/g) | Methyl-triclosan range (ng/g) | Methyl-triclosan geometric mean (ng/g) |
|---|---|---|---|---|---|
| Pacific region (3) | Surface sediment | less than 1-9 | 2.1 | less than 2 | NA |
| Great Lakes (2) | Surface sediment | 7 | 7.0 | less than 2-14 | NA |
| St. Lawrence River (7) | Surface sediment | 14-47 | 27.4 | less than 2-22 | 4.5 |
| Atlantic region (9) | Surface sediment | less than 1-18 | 1.9 | less than 2-3 | NA |
| Lake Ontario (1) | Core sediment (1 cm depth) |
7 | NA | 14 | NA |
| Lake Ontario (1) | Core sediment (3 cm depth) |
8 | NA | less than 2 | NA |
| Lake Ontario (2) | Core sediment (5-7 cm depth) |
less than 1 | NA | less than 2 | NA |
| Lake Ontario (1) | Core sediment (9 cm depth) |
9 | NA | 15 | NA |
| Lake Ontario (11) | Core sediment (11-32 cm depth) |
less than 1 | NA | less than 2 | NA |
| St. Lawrence River (2) | Suspended sediment (1 km from WWTP) |
15-21 | 17.8 | less than 2-9 | NA |
| St. Lawrence River (2) | Suspended sediment (4 km from WWTP) |
990-2000 | 1427 | 17-24 | 20.2 |
| St. Lawrence River (4) | Suspended sediment (7 km from WWTP) |
29-150 | 70.4 | 12-19 | 17 |
| St. Lawrence River (6) | Suspended sediment (15 km from WWTP) |
26-150 | 72 | 9-22 | 15.7 |
Table Notes
Abbreviations: NA, not available; WWTP, wastewater treatment plant.
a Source: Unpublished data, Quality Monitoring and Surveillance Division, Environment Canada.
4.1.3.2.2 In other countries
Sediment monitoring data for triclosan were available for Switzerland, Sweden, the United States and China. Singer et al. (2002) analyzed a sediment core taken from a lake in Switzerland that receives effluent from WWTPs. The profile in the core showed triclosan concentrations ranging from less than 5 ng/g dw in 1960-1961 to 53 ng/g dw in 1992-1993. In Sweden, Remberger et al. (2002) reported triclosan concentrations of 8-17 ng/g dw in sea sediments sampled in an industrial area. Triclosan was also detected by Miller et al. (2008) in cored estuarine sediments from Jamaica Bay, New York. The peak concentrations were 600-800 ng/g dw in sediments deposited between the mid-1960s and late 1970s; they then declined to less than 50 ng/g dw in the following years. Zhao et al. (2010) measured triclosan concentrations ranging from 56.5 to 739 ng/g dw in sediments sampled from three rivers flowing in a heavily populated area of China.
4.1.3.3 Measured concentrations in soils
No monitoring data for concentrations of triclosan or methyl-triclosan in soil were found for Canada. In Sweden, Remberger et al. (2002) measured triclosan concentrations in two contaminated (industrial) areas and in one pristine forest area. Triclosan concentrations in the contaminated sites ranged from less than 3 to 15 μg/kg dw, while they were less than 3 μg/kg dw (detection limit) in the forest soil. In the United States, Wu et al. (2010b) measured triclosan in soils that had been amended with biosolids. The concentrations of triclosan in amended soils ranged from 1.6 to 11 μg/kg dw.
4.2 Environmental Fate
This section contains information on the environmental distribution and fate of triclosan in the environmental media. Environmental distribution to water, soil, sediment and air is evaluated using the Multispecies Model (version 1.0; Cahill 2008), and considers the ionizing properties of triclosan at pH 7 and 8. Environmental persistence of triclosan is evaluated in water, sediment and soil using empirical data. Degradation of triclosan in air is evaluated using modelled data generated from AOPWIN (2008). Information on abiotic and biotic degradation pathways and transformation products is organized based on the environmental compartment.
4.2.1 Environmental Distribution
When a substance is able to ionize in water at environmentally relevant pH, its neutral and ionic forms will co-exist in the environment (water, sediment and soil). With a pKa of 8.1 (see Table 2-2), triclosan will ionize to some extent in most of the natural water bodies in Canada. The ionization of triclosan proceeds as the proton attached to the phenolic group dissociates from the structure forming an anionic molecule. At pH values of 6, 7, 8 or 9, the fraction of ionized triclosan in pure water will be 1%, 7%, 44% or 89%, respectively, using the equation Fi = 1 - (1/(1+10pH-pKa)) × 100%, where Fi is the fraction ionized.
Table 4-5 summarizes the distribution of the neutral and anionic forms of triclosan among environmental compartments based on the Multispecies Model (version 1.0; Cahill 2008). More specifically, the results provide the proportion (fraction of the total mass emitted to the environment - 1000 g per hour to each of air, water and soil compartments, as default model input) of each form present in each compartment upon a continuous release to water or soil, at an environmental pH of 7. The model was also run at an environmental pH of 8, since this value is also relevant for many aquatic and terrestrial ecosystems in Canada. The proportion modelled is determined with respect to the total quantity released, so the sum of all proportions adds up to 100%. The physical/chemical properties and half-life values presented in Tables 2-2 and 4-6, respectively, were used as input for the model. The input values for the physical/chemical properties of the ionized form of triclosan were based on the corresponding values for the neutral form, after applying correction factors, while the input values for half-lives were the same as for the neutral form. The results in Table 4-5 represent the net effect of chemical partitioning, intermedia transport and loss by both advection (out of the modelled region, but not out of the wider ecosystem) and degradation or transformation processes. In spite of loss processes, the sum of all proportions still adds up to 100% given that the predictions are based on the assumption that steady state is reached among the four compartments after triclosan is being released on a continuous basis.
| Triclosan released to: | Form | Percentage of triclosan partitioning into each compartment | |||
|---|---|---|---|---|---|
| Water (100%) at pH 7 | Neutral | Air: 0.0 | Water: 72.9 | Soil: 0.0 | Sediment: 19.8 |
| Water (100%) at pH 7 | Ionized | Air: 0.0 | Water: 5.8 | Soil: 0.0 | Sediment: 1.5 |
| Soil (100%) at pH 7 | Neutral | Air: 0.0 | Water: 0.1 | Soil: 92.6 | Sediment: 0.0 |
| Soil (100%) at pH 7 | Ionized | Air: 0.0 | Water: 0.0 | Soil: 7.3 | Sediment: 0.0 |
| Water (100%) at pH 8 | Neutral | Air: 0.0 | Water: 50.6 | Soil: 0.0 | Sediment: 5.2 |
| Water (100%) at pH 8 | Ionized | Air: 0.0 | Water: 40.1 | Soil: 0.0 | Sediment: 4.1 |
| Soil (100%) at pH 8 | Neutral | Air: 0.0 | Water: 0.2 | Soil: 55.6 | Sediment: 0.0 |
| Soil (100%) at pH 8 | Ionized | Air: 0.0 | Water: 0.1 | Soil: 44.1 | Sediment: 0.0 |
In a scenario where triclosan is exclusively released to water, it is expected to reside in both water (79-91%) and sediment (9-21%) at pH 7 and 8. If released only to soil, triclosan remains almost exclusively in this compartment (greater than 99%). At an environmental pH of 7, triclosan will mainly be present in its neutral form in water, sediment and soil. At a pH of 8 in these same compartments, about 55% of triclosan will be in its neutral form and about 45% in its ionized (anionic) form. In the prairie provinces, for instance, where soil is alkaline (pH 9), triclosan would be present primarily in its anionic form.
4.2.2 Fate in Air
Modelled environmental distribution profile using the Multispecies Model (version 1.0; Cahill 2008) summarized in Table 4-5 indicated that triclosan in unlikely to partition to air if released into the environment. Model results using the model AOPWIN (2008) indicated that triclosan degrades fast via reactions with hydroxyl radicals, with a half-life of 0.66 day. Triclosan is not likely to be subject to long-range transport given its unlikely distribution into air, and the predicted short air residence time.
4.2.3 Fate in Water
4.2.3.1 Abiotic Processes
Triclosan is a phenolic compound that ionizes at environmentally relevant pH (pKa of 8.1; see Table 2-2). The speciation, or ionization state, of a weak organic acid, such as triclosan, will influence its fate in the environment and its bioavailability. For instance, the ionized form of triclosan has a different light absorption spectrum than the neutral form. Also, organisms may more readily take up the neutral form; this was highlighted by Orvos et al. (2002), who showed that, for the same species, the toxicity of triclosan decreased with increasing pH. More generally, the results obtained by Erickson et al. (2006a, b) suggest that the ionized form of weak organic acids is also available for uptake through a variety of mechanisms. Hence, ionized triclosan could also accumulate in organisms.
In natural waters, triclosan may form complexes with dissolved organic matter, which could influence the concentrations of freely dissolved triclosan. Assuming that the dissolved organic matter-triclosan complexes cannot cross a cell membrane, only the fraction of total triclosan present in the freely dissolved form in the water column could be bioavailable. No studies quantifying the effect of dissolved organic matter on the bioaccumulation of triclosan in aquatic organisms could be found in the literature. According to a mass balance fish model, the predicted bioavailable fraction of triclosan in the water column is approximately 99%, based on its log Koc of 4.7 (see Section 4.3.1).
Laboratory studies have shown that triclosan is hydrolytically stable at pH 4, 7 and 9 (US EPA 2008e). It is also stable against strong acids and bases (Singer et al. 2002). Its low Henry's law constant of 5.05 × 10-4 Pa·m3/mol (see Table 2-2) indicates that it should not volatilize from a water surface.
Triclosan is susceptible to phototransformation in surface waters, as shown in many studies (Lindström et al. 2002; Singer et al. 2002; Tixier et al. 2002; Mezcua et al. 2004; Latch et al. 2005; Lores et al. 2005). Tixier et al. (2002) quantified the phototransformation of triclosan under laboratory and field conditions for a small lake in Switzerland. They highlighted the fact that pH, by affecting the speciation of triclosan (pKa = 8.1), has an impact on its absorption of sunlight. Indeed, the direct phototransformation rate of triclosan increases with pH, i.e., with the proportion of the ionized form of triclosan present in solution. Indirect phototransformation (e.g., photosensitization by organic matter) was a negligible process. The study authors estimated that, during the summer season, direct phototransformation accounted for 80% of the observed total elimination of triclosan from the study lake. The remaining major sink for triclosan was the loss in the outflow. The authors also predicted triclosan phototransformation rates for a variety of environmental conditions, including time of year and latitude. The resulting primary degradation half-life values spanned from 2 to 2000 days. For latitudes modelled by the authors that are equivalent to southern Canada (~45-50°N) and for a pH of 8.0, the effective annualized phototransformation half-lives obtained for triclosan in water were less than 100 days throughout the year. For water bodies with a lower pH, somewhat longer half-lives would be expected (but still less than 100 days), and the relative importance of other removal processes, such as biodegradation and sedimentation, would increase.
Latch et al. (2005) performed experiments in both natural and deionized water under natural sunlight and showed that triclosan was rapidly degraded by direct photolysis (half-life of 5 hours at pH 8, midsummer sunlight, 45°N latitude).
Lindström et al. (2002) conducted a photolysis experiment in which triclosan was exposed to natural sunlight in lake water at different pH values. While triclosan was stable at pH 5.6, it degraded rapidly at pH 8.0 (half-life of about 20 minutes). Methyl-triclosan, which reaches surface water as part of WWTP effluent, was also tested in this study; it did not photodegrade at either pH.
Different degradation products can be formed by the photolysis of triclosan. For instance, in addition to showing a short half-life for triclosan (41 minutes), a study conducted under laboratory conditions indicated that 2,4-DCP was formed as a major transformation product (up to 97%; US EPA 2008f). This substance has been the subject of a SIDS Initial Assessment Report under the OECD HPV Chemicals Programme. This report indicates that 2,4-DCP is likely not persistent, not bioaccumulative and moderately toxic to aquatic organisms (OECD 2007).
Mezcua et al. (2004) measured 2,7/2,8-DCDD as major phototransformation products of triclosan under natural sunlight. Two phototransformation experiments were conducted at two different pH values (pH 5 and 7). It was shown that triclosan transformed to dioxin at pH 7 only, confirming the results obtained by Tixier et al. (2002) regarding the high transformation rate of the ionized form compared with the neutral form. Mezcua et al. (2004) also measured 2,7/2,8-DCDD in the effluent of a WWTP (4-400 ng/L), thereby revealing its input to receiving surface waters. The phototransformation of triclosan to DCDD was confirmed by Lores et al. (2005) and by Sanchez-Prado et al. (2006) using photo-solid-phase microextraction. Latch et al. (2005) also measured 2,8-DCDD as well as 2,4-DCP as transformation products of triclosan in a photolysis experiment. Yields of these products ranged from 3% to 12%. Finally, the phototransformation of triclosan to 2,8-DCDD was also reported in seawater (Aranami and Readman 2007).
Data available on the degradation of 2,7/2,8-DCDD and the aquatic toxicity of 2,8-DCDD indicate that these compounds should be less harmful to the environment than other dioxins, such as their tetrachlorinated congeners (e.g., 2,3,7,8-TCDD). 2,7/2,8-DCDD are not on the list of 17 dioxins and furans that are of the greatest concern based on international toxicity equivalency factors (NATO 1988). The photolability of 2,7/2,8-DCDD is reported in several studies (Mezcua et al. 2004 [half-life less than 20 hours]; Latch et al. 2005; Sanchez-Prado et al. 2006; Aranami and Readman 2007), as is the aerobic microbial degradation of both 2,7- and 2,8-DCDD (e.g., 16-33% within 7 days; Field and Sierra-Alvarez 2008) (Parsons and Storms 1989; Parsons 1992). The toxicity of 2,8-DCDD to fish appears to be low, as suggested by the results of a study in which embryos of the Japanese medaka (Oryzias latipes) hatched and survived for 3 days post-hatch (full exposure duration) when exposed to 50 000 ng/L (Wisk and Cooper 1990). The toxicity of 2,7-DCDD is unknown. Given their probable transient state in the environment and low toxicity, these DCDDs are not likely to be of environmental concern.
Buth et al. (2009) showed that chlorinated triclosan derivatives formed during the disinfection of wastewater can further phototransform to PCDDs, as well as to 2,4-DCP, in natural water. These dioxin congeners (1,2,8-TriCDD, 2,3,7-TriCDD and 1,2,3,8-TCDD) were detected in sediments from the Mississippi River at levels that trended with the historical use of triclosan (Buth et al. 2010).
These compounds may be more toxic than 2,7/2,8-DCDD due to their increased chlorine substitution. Buth et al. (2010) estimated that the mass contribution of triclosan-derived dioxins could represent up to 30% of the total dioxin pool in the sediment cores that they analyzed.
4.2.3.2 Biotic processes
4.2.3.2.1 WWTP-Related Conditions
Based on its chemical structure, triclosan is not expected to biodegrade rapidly. Results obtained for the standardized OECD test guideline 301C (modified MITI test (I)) test indicate that triclosan is not readily or inherently biodegradable (0% degradation after 4 weeks at a test concentration of 100 mg/L) (NITE 2002). In this kind of test, which measures ultimate degradation (measured by the formation of carbon dioxide), an aqueous solution of the test substance is inoculated and incubated under aerobic conditions in the dark or in diffuse light. These results are consistent with previous work by Voets et al. (1976), who observed no loss of triclosan in test systems that were inoculated with a soil extract. However, Federle et al. (2002) suggested that the negative results obtained in these tests are unreliable as a consequence of the likely bacterial toxicity of triclosan at the high concentrations used (1-100 mg/L). This statement is supported by the results of a ready biodegradability study in which triclosan was applied at a rate of 0.2 mg/L to a microbial inoculum in sandy loam soil and activated sludge. Triclosan degraded with an average half-life of 5.2 days (US EPA 2008e). Results of aerobic biodegradation tests conducted at various concentrations (10-500 000 µg/L) for various durations (21-91 days) indicated 18-70% degradation for triclosan (NICNAS 2009). More specifically, Stasinakis et al. (2008b) conducted a biodegradability test with triclosan (at 10 µg/L) using the OECD test guideline 301F method (manometric respirometry test). In this 28-day test, 52% ultimate degradation was achieved, and the calculated half-life was 1.8 days. Federle et al. (2002) conducted biodegradation tests with activated sludge at triclosan concentrations of 20–200 µg/L. By the end of the tests (71 days), 31-52% of triclosan had mineralized to carbon dioxide. For comparison purposes, concentrations of triclosan in the influent of WWTPs in Canada are in the range of 0.102-20.7 µg/L (Table 4-1)--that is, much lower than those tested in the biodegradation tests mentioned above.
Voets et al. (1976) conducted tests with triclosan under anaerobic conditions for sludge digestion in WWTPs. Results of two anaerobic biodegradation tests conducted at 200 and 1000-5000 µg/L for 147 and 21 days, respectively, indicated 10% and 50% degradation, respectively.
4.2.3.2.2 Environmental conditions
In an aerobic aquatic metabolism study conducted at 20°C, triclosan disappeared rapidly from the water layer in river water-sandy loam sediment and pond water-silty clay loam sediment systems (US EPA 2008e). In the water layer (pH 7.2-7.3), [14C]triclosan declined from an average 88-93% of the applied radioactivity at time 0 to 49-53% at 1 day to less than or equal to 0.3% at 56-104 days post-treatment. Volatilized carbon dioxide for the whole system was 21-29% of the applied radioactivity by study termination (day 104). [14C]Triclosan dissipation half-lives for the water layer (resulting from degradation and partitioning) were 1.3-1.4 days based on extractable residues only. Half-lives for sediments and total systems were 54-60 days and 40-56 days, respectively. More details are provided in Section 4.2.4.2 below.
Considering the results above for ultimate biodegradation (i.e., mineralization to carbon dioxide) of triclosan under aerobic conditions, there is evidence that this substance is not persistent in water. Results from the aerobic aquatic metabolism study also indicate that triclosan is not persistent in this environmental compartment.
| Medium: fate process (test conditions) | Degradation value | Degradation endpoint (units) | Reference |
|---|---|---|---|
| Air: atmospheric oxidation | 0.66a | Half-life (d) | AOPWIN 2008 |
| Water: hydrolysis | Stable | NA | Singer et al. 2002 |
| Water: hydrolysis (pH 4, 7 and 9, 50°C, for 5 d) | Stable | NA | US EPA 2008e |
| Water: photodegradation (field conditions, pH 8.0, year-round, 50°N) | less than 100 | Primary half-life (d) | Tixier et al. 2002 |
| Water: photodegradation (laboratory conditions, pH 8.0, summer sunlight, 45°N) | 5 | Primary half-life (h) | Latch et al. 2005 |
| Water: photodegradation (laboratory conditions, pH 8.0, summer sunlight, 47°N) | 0.37 | Primary half-life (h) | Lindström et al. 2002 |
| Water: photodegradation (laboratory conditions, pH 7.0, artificial light) | 41 | Half-life (min) | US EPA 2008e |
| Water: biodegradation, WWTP-related conditions (aerobic conditions, various test concentrations and durations) | 18-70 | Degradation (%) | NICNAS 2009 |
Water: biodegradation and partitioning (aerobic conditions, 20°C, in darkness, for 104 d):
|
Range for both systems (water layer): 1.3-1.4a | Dissipation half-life (d) | US EPA 2008e |
Sediment: biodegradation and partitioning (aerobic conditions, 20°C, in darkness, for 104 d):
|
Ranges for both systems: sediment: 54-60a whole system: 40-56 |
Dissipation half-life (d) Degradation half-life (d) |
US EPA 2008e |
Soil: biodegradation (aerobic conditions, 20°C, in darkness, for 124 d):
|
2.9 3.8 3.7 |
Half-life (d) | US EPA 2008e |
| Soil: biodegradation (aerobic conditions, loam, pH 7.4, 22°C) | 18a | Primary half-life (d) | Ying et al. 2007 |
Soil: biodegradation (aerobic conditions, room temperature):
|
58 32 |
Primary half-life (d) | Wu et al. 2009 |
Soil: biodegradation (aerobic conditions, 20°C in darkness, for 45 d):
|
14 16 14 13 |
Primary half-life (d) | Xu et al. 2009 |
| Soil: biodegradation (anaerobic conditions, loam, pH 7.4, 22°C) | much greater than 70 | Primary half-life (d) | Ying et al. 2007 |
Table Notes
Abbreviations: d, days; h, hours; NA, not available; WWTP, wastewater treatment plant.
a Value used for fugacity modelling with Multispecies Model.
4.2.4 Fate in Sediment
4.2.4.1 Abiotic Processes
Triclosan is susceptible to rapid oxidation by manganese oxides, which are present in aerobic sediments and soils (Zhang and Huang 2003). Under environmentally relevant pH and manganese dioxide concentrations, the primary degradation half-life of triclosan was calculated to be less than 21 hours. Degradation products were reported to include 2,4-DCP (less than 1% of triclosan loss). However, dissolved metal ions and natural organic matter in water and soil would likely increase this value by competitively adsorbing and reacting with manganese dioxide.
Given its moderate log Koc values of 3.34-4.67 (see Table 2-2), it can be expected that triclosan (especially the neutral form) will adsorb to organic matter present in effluent or in receiving surface waters. As the substance is released to aquatic ecosystems through WWTP effluent, a portion could be removed from the water column through sedimentation. Once in aerobic sediments, triclosan could react with manganese oxides to a certain extent. The balance of these two processes-i.e., input to sediment through sedimentation and output through oxidation-would be difficult to quantify.
4.2.4.2 Biotic Processes
As noted previously, triclosan degraded rapidly in river water-sandy loam sediment and pond water-silty clay loam sediment systems under aerobic conditions (US EPA 2008e). In the water layer, [14C]triclosan declined from an average 88-93% of the applied radioactivity at time 0 to less than or equal to 0.3% at 56-104 days post-treatment. In the sediment, [14C]triclosan increased from an average 39-40% of the applied radioactivity at time 0 to 69-75% at 7-14 days and was 21-22% at 104 days post-treatment. In the total system, [14C]triclosan decreased steadily from 88-93% of the applied radioactivity at time 0 to 52-68% at 28 days and to 21.5-21.8% at 104 days post-treatment. [14C]Triclosan dissipation half-lives (degradation and partitioning) were 1.3-1.4 days (water layer) and 54-60 days (sediment) for both water-sediment systems; degradation half-lives were 40-56 days in total systems. Non-extractable residues (not included in half-life calculations) were 32-33% at study termination, and volatilized carbon dioxide was 21-29%. Methyl-triclosan was identified as a minor transformation product, with a maximum mean of 0.1% of the applied radioactivity at 28 days post-treatment in the water and a maximum mean of 3.4-4.8% at 104 days in the sediment and total system.
No experimentally measured half-lives for triclosan in sediments under anaerobic conditions could be found. However, evidence of the persistence of triclosan in buried anaerobic sediments is shown by monitoring data. Singer et al. (2002) analyzed a sediment core taken from a lake in Switzerland that receives effluent from WWTPs. The concentration profile in the core showed that triclosan has been accumulating in sediments, from less than 5 ng/g dw in 1960-1961 to 42 ng/g dw in 1970-1971 to 53 ng/g dw in 1992-1993. This increase was likely due to its continual input into the lake, showing that it accumulates in anaerobic sediments more rapidly than it degrades. The fact that a relatively high amount of triclosan was contained in the approximately 30-year-old sediment layer (1970-1971) points to a slow degradation rate for triclosan. Triclosan was also detected in cored estuarine sediments from Jamaica Bay, New York. Indeed, Miller et al. (2008) measured peak triclosan concentrations of 600-800 ng/g dw in sediments deposited in that bay between the mid-1960s and late 1970s. For the following years, the concentrations declined to less than 50 ng/g dw, probably due to the introduction of an activated sludge treatment process to the Jamaica Bay WWTP. In China, Zhao et al. (2010) measured triclosan concentrations ranging from 56.5 to 739 ng/g dw in sediments sampled from three rivers flowing in a heavily populated area. As a whole, these sediment core data point to the persistence of triclosan in buried anaerobic sediments.
Given that organisms live mostly under aerobic conditions (even endobenthic fauna), a greater weight is attributed to half-lives measured under these conditions. Triclosan that is present in buried anaerobic sediments is considered of less significance in terms of biological exposure. In addition, if triclosan in these sediments were to be resuspended, it would likely come in contact with oxygen as a result of mixing and could then be subject to biodegradation processes. Half-life values for ultimate degradation under aerobic conditions are not available for sediments. The study conducted with two water-sediment systems indicated half-lives of 40-56 days in those systems. These half-lives represent a mix of primary and ultimate degradation processes, since carbon dioxide was 21-29% of the applied radioactivity by study termination. In this study, a portion of triclosan is not available for biodegradation given its partitioning to sediments (i.e., bound to residues). Based on empirical evidence for rapid primary biodegradation in water and soil (half-lives of days to a few weeks; Table 4-6) and half-lives of approximately 30-70 days for ultimate degradation in water, it is expected that triclosan will not be persistent in sediment. Methyl-triclosan is a transformation product of triclosan in sediment.
4.2.5 Fate in soil
4.2.5.1 Abiotic processes
As mentioned previously, hydrolysis is not an important transformation process for triclosan. Also, its Henry's Law constant value (see Table 2-2) indicates that it should not volatilize from moist soil surfaces. Its log Koc values (3.34-4.67) suggest that it should generally not be mobile in soil, especially if the organic carbon content in soil is high. Other abiotic processes, such as phototransformation, have not been documented for triclosan in the soil compartment. Since its main entry route in soil would likely be through spreading of biosolids on agricultural fields followed by ploughing (see Section 4.5.3), a portion of triclosan will likely be incorporated in the deeper soil layers and hence would not be exposed to light. If spread on wood lots or in the forest, triclosan in biosolids could be exposed to light in the absence of ploughing. Prior to biosolid application, some WWTPs may have placed biosolids on a sludge pad or open field for further drying, leaving triclosan susceptible to phototransformation and possible production of degradation products that could be released in the environment.
The leaching potential of triclosan from soil was examined using both the criteria of Cohen et al. (1984) and the groundwater ubiquity score (Gustafson 1989). These two approaches allow for a semiquantitative determination of the leaching potential of a chemical. Table 4-7 shows how physical/chemical properties and certain fate data for triclosan compare with the values for the criteria of Cohen et al. (1984). This comparison does not allow for a clear indication regarding the leaching potential of triclosan. In the Prairies, where soils tend to be alkaline, the anionic form of triclosan is expected to predominate, thus increasing its potential for leaching.
| Property | Criteria of Cohen et al. (1984) indicating a potential for leaching | Triclosan value | Meets criterion for leaching |
|---|---|---|---|
| Solubility in water | greater than 30 mg/L | 10 mg/L | No |
| Kd | less than 5 and usually less than 1 or 2 | 10-282 | No |
| Koc | less than 300 | 2188-46 774 | No |
| Henry's law constant | less than 10-2atm·m3/mol (less than 1013 Pa·m3/mol) |
4.99 × 10-9atm·m3/mol (5.05 × 10-4 Pa·m3/mol) |
Yes |
| pKa | Negatively charged (either fully or partially) at ambient pH | 8.1 | Yes (varies with ambient pH) |
| Hydrolysis half-life | greater than 20 wk (greater than 140 d) | Stable to hydrolysis | Yes |
| Soil phototransformation half-life | greater than 1 wk (greater than 7 d) | NA | NA |
| Half-life in soil | greater than 2-3 wk (greater than 14-21 d) | Aerobic: 2.9-58 d Anaerobic: much greater than 70 d |
Yes |
Table Notes
Abbreviations: d, days; Kd, soil/water partition coefficient; Koc, soil organic carbon/water partition coefficient; NA, not available; pKa, dissociation constant; wk, week.
The method of Gustafson (1989) may also be used to estimate the leaching potential of chemicals. Gustafson's assessment method uses a groundwater ubiquity score (GUS), which is based on the persistence and mobility of the compound and is expressed as:
GUS = log10(t½ soil) × (4 - log10(Koc))
The GUS value indicates the leachability of the compound. The persistence term in the GUS equation, t½ soil, is the field dissipation time (DT50), as determined in field dissipation studies, and is meant to include dissipation by volatilization, phototransformation and biological transformation. Instead of the field dissipation DT50, however, the laboratory aerobic soil DT50 or t½value was used in the GUS equation; this is because the field dissipation DT50 may also include dissipation from leaching and runoff and therefore may underestimate leaching potential when used in the equation. The GUS classification scheme is as shown in Table 4-8.
| GUS | Probable attributes |
|---|---|
| greater than 2.8 | Leacher |
| greater than 1.8 and less than 2.8 | Borderline leacher |
| less than 1.8 | Non-leacher |
For triclosan, a half-life value of 58 days in aerobic soil and a Koc value of 2188 were used to calculate a conservative value of the GUS index. According to the leachability classification presented in Table 4-8, triclosan is non-leacher (GUS = 1.16).
GUS for triclosan = log10(58) × (4 - 3.34) = 1.16
When present in soil, triclosan is expected to have a low potential to leach based on the mobility classification (Koc: 2188–46 774: immobile to slight mobility, as per McCall et al. 1981) and the GUS score indicating that it is a non-leacher. It should be noted, however, that triclosan has been detected in groundwater at low levels in various monitoring studies, suggesting that other mechanisms, such as facilitated transport (particle facilitated or macropore/fractures), may contribute to its detection in groundwater (Gottschall et al. 2012; Edwards et al. 2009). In a national reconnaissance of contaminants present in groundwater in the United States in 2000, triclosan was detected in 15% of the 47 sites sampled by Barnes et al. (2008). The concentrations were all below the reporting level of 1 µg/L. The sampling sites consisted mainly of wells and of a few springs and sumps. They were located in areas suspected to be susceptible to contamination from either animal or human wastes (i.e., down-gradient of a landfill, unsewered residential development or animal feedlot). In China, Chen et al. (2011) measured triclosan in groundwater that served to irrigate agricultural fields; concentrations of 1.2-10.8 ng/L were measured at three different sites. Triclosan was below the LOQ (1.6 µg/kg) in the corresponding irrigated soils.
Triclosan may also enter into the terrestrial environment through the disposal of products in landfills. Leaching is expected to be limited for products in which triclosan is embedded into solid material, such as plastics. However, for materials like textiles, triclosan is more likely to leach out given its application on the surface of the material. Personal care products are also disposed of in landfills and are expected to contribute to triclosan residues in landfill leachate. Leachate from 94% of the larger landfills in Canada is collected and treated (on-site and/or off-site) before being released to the environment. Monitoring data were collected under the Government of Canada's Chemicals Management Plan monitoring program in 2010, 2011, 2012 and 2013 at 4 to 12 of the larger landfills in Canada. These data indicate that triclosan concentrations in leachate before any treatment ranged from below MDL to 1.4 µg/L. Three of the 12 overall landfills sampled are treating their leachate on-site before either sending it to a WWTP or releasing it to the environment. For these landfills, triclosan concentrations in leachate after treatment ranged from below MDL to 0.16 µg/L. For landfills that send their leachate (treated or not) to a WWTP, the removal of triclosan during wastewater treatment (primary or secondary) followed by the dilution of the WWTP effluent in the receiving watercourse will likely result in low releases of triclosan in aquatic ecosystems (Conestoga-Rovers and Associates 2015). Based on this information, landfills are not a likely source of triclosan to the environment.
There is also evidence that triclosan can reach surface water and groundwater through runoff and drainage. Following broadcast application of either liquid or dewatered wastewater biosolids to soil and simulating a rainfall, Topp et al. (2008) and Sabourin et al. (2009) measured triclosan concentrations in runoff of 258 ng/L and 110 ng/L, respectively, one day after biosolids application. In the study by Topp et al. (2008), the concentration of triclosan in runoff was still above the LOQ on day 266 following application. To explain this persistence, the authors suggested that sorptive and diffusive processes in the soil had sequestered a portion of the chemical, reducing its availability for biodegradation. Lapen et al. (2008) and Edwards et al. (2009) measured maximum triclosan concentrations of 3680 ng/L and 240 ng/L in tile drainage following application of liquid and dewatered wastewater biosolids, respectively, which points to the potential of triclosan to reach groundwater. Gottschall et al. (2012) detected triclosan in the tile water at 73 ng/L, and at 19 ng/L in ground water at the depth of 2 meters, but not at depths of 4 to 6 meters, following application of dewatered waste water biosolids. These studies on runoff and tile drainage were conducted in Ontario, Canada.
4.2.5.2 Biotic processes
In an aerobic soil metabolism study conducted at 20°C, triclosan degraded rapidly, with half-lives of 2.9 days (sandy loam), 3.8 days (clay loam) and 3.7 days (loam) (US EPA 2008e). [14C]Triclosan declined from an average 92-95% of the applied radioactivity at time 0 to 42-58% at 2-3 days and to 1.1-4.3% at 61-124 days post-treatment. Non-extractable residues (not included in half-life calculations) were 61-76% of the applied radioactivity at study termination, and volatilized carbon dioxide was 11-16%. The major transformation product was methyl-triclosan, at maximum averages of 13-24% of the applied dose at 14-28 days post-treatment. Methyl-triclosan then decreased by study termination. Dissipation time (DT50) values for methyl-triclosan in these soils, as provided in NICNAS (2009), ranged from 39 to 153 days. A supplementary experiment was conducted at 10°C with the sandy loam described above. The DT50 value obtained for triclosan was 10.7 days versus 2.5 days for the same soil at 20°C, as provided in NICNAS (2009). The former value is still low in terms of persistence of triclosan in soil.
Ying et al. (2007) studied the biological degradation of triclosan in soil under both aerobic and anaerobic conditions in the laboratory. For the aerobic experiments, triclosan was added to a loam soil (at 1 mg/kg), which was then incubated in darkness for 70 days. The anaerobic experiments were conducted the same way but were carried out in an anaerobic incubation chamber filled with nitrogen. At each sampling time during the experiment, soil samples were extracted with acetone, and triclosan present in the extracted fraction was measured by high-performance liquid chromatography. Sterile soil samples were also incubated to assess abiotic transformation processes; no degradation occurred in these samples. The results obtained showed that triclosan degraded in aerobic soil, with a half-life of 18 days. However, it had not degraded under anaerobic conditions by the end of the study period (i.e., half-life much greater than 70 days). Additional measurements indicated that triclosan did not have negative effects on soil microbial activity in the aerobic soil samples; similar measurements were not made in the anaerobic soil. This study indicates that triclosan is not persistent in aerobic soil; however, the extent to which it degrades was not characterized by the study authors (e.g., primary vs. ultimate degradation). Indeed, no attempts were made to identify or quantify degradation products in soil, and no traps were used to collect volatile degradation compounds, such as carbon dioxide. In addition, the fraction of triclosan bound to soil residues (i.e., not extracted with acetone) was not quantified; however, the figures provided in the paper indicate that concentrations of extractable triclosan in sterile soil were rather stable over the study duration. The fact that these concentrations remained stable indicates that the bound residues formed in the non-sterile soil were likely transformation products of triclosan and not parent triclosan, since the latter did not bind to the soil under sterile conditions. In a similar study, Wu et al. (2009) incubated under aerobic conditions two types of soil to which triclosan had been added. The incubation period was 60 days. The half-lives obtained were 58 days and 32 days, respectively, for a silty clay and a sandy loam. The authors also measured the biodegradation rate of triclosan in the same soils that had been amended with biosolids; the corresponding half-lives were found to be 41 days and 20 days. Finally, Xu et al. (2009) incubated four types of soil with triclosan under aerobic conditions for 45 days and observed half-lives of 13-16 days.
In a study comparing the transformation of triclosan in soils that had never received biosolids application and in the same soils to which biosolids were applied in the laboratory, Kwon et al. (2010) observed that the presence of biosolids significantly slowed the transformation of triclosan, likely due to physical and chemical interactions such as adsorption. Half-lives in two different soils were 2 days and 13 days without biosolids; half-lives in the same two soils were 50 days and 108 days, respectively, following biosolids application. Because biosolids are likely the main source of triclosan to the terrestrial environment, these longer half-lives can be expected under field conditions. Lozano et al. (2010) reported a dissipation half-life of 107 days for triclosan for a field that had received one application of biosolids. An additional study by Lozano et al. (2012) studied triclosan and its transformation product methyl-triclosan over a period of three years following application of biosolids to a sandy loam soil under field conditions. Triclosan disappearance corresponded with methyl-triclosan appearance, suggesting in situ formation. Dissipation half-lives were estimated to be 104 days for triclosan and 443 days for methyl-triclosan, respectively.
Similarly to sediments, given that organisms live mostly under aerobic conditions in soil, a greater weight is attributed to half-lives measured under these conditions. Half-life values for ultimate degradation in soil are not available. The only aerobic soil metabolism study in which carbon dioxide was trapped and measured indicates triclosan half-lives of 2.9-3.8 days and a production of 11-16% carbon dioxide after 124 days. These half-lives represent a mix of primary and ultimate degradation processes. Generally, carbon dioxide is not expected to reach high levels, because a large proportion of triclosan partitions to soil residues and hence is not available for degradation. Based on the evidence for rapid primary biodegradation in the various aerobic soil studies described above (half-lives of 2.9-58 days), triclosan is not considered persistent in soils.
4.2.6 Relevance of environmental fate of triclosan
Residence time and fate of a chemical in the environment are factors that directly affect levels of exposure to that chemical and associated risk, i.e., the likelihood of adverse effects from contact or uptake of a chemical. In general, long persistence can contribute to prolonged exposure and thus greater risk (Mackay et al. 2014).
Triclosan is not likely to persist in the environment as indicated by its half-lives in the various environmental compartments and its environmental distribution in each compartment (Tables 4-5 and 4-6). For the aquatic compartment, however, its continual input to surface waters through WWTP effluent results in its continuous presence in the receiving aquatic ecosystems. As noted by Mackay et al. (2014), when there is a constant and widespread input of a chemical into the environment, it leads to its continuous presence in the environment near field (i.e., in proximity to emission sources), and exposure to a chemical can occur well before its degradation processes are able to take place. Therefore, in this case, the half-life of a chemical as an indicator of the overall persistence is largely irrelevant, because of the short time to exposure (Mackay et al. 2014). Indeed, for triclosan, time to exposure in aquatic ecosystems may be shorter than the time needed for its degradation. Therefore, long-term exposures to triclosan in water and sediments are expected, especially near field, closer to effluent sources. In terrestrial ecosystems, exposure to triclosan can occur from the periodic land applications of biosolids. Since the field dissipation half-lives measured for triclosan are relatively long (greater than 100 days), exposure levels in soil are also expected to be somewhat constant.
Exposure to triclosan transformation products is also expected in the environment, and the associated risk is dependent on their properties. In water, photolysis of triclosan leads to formation of dichlorophenol (DCP), and other phototransformation products of triclosan include lower (di-) chlorinated dioxins. DCP and di-chlorinated dioxins are not likely to be of environmental concern due to their moderate toxicity and a transient state in the environment. Disinfection of wastewater can also lead to formation of DCP as well as tri-chlorinated dioxin congeners. While the tri-chlorinated dioxins may be more toxic than DCP and di-chlorinated dioxins due to higher chlorine substitution, they are not considered as harmful as the higher chlorinated dioxins such as the tetra-chlorinated congeners.
Methylation of triclosan during biological process at WWTPs and in soil and in sediments leads to formation of methyl-triclosan. This transformation product is known to have longer half-lives in the environment, and, similarly to triclosan, it is also highly toxic to aquatic organism. Methyl-triclosan is expected to be ubiquitous in the environment, and co-exposure to both triclosan and methyl-triclosan is likely in the environment.
Long-range transport of triclosan in the environment is not expected because of its relatively short half-life in aquatic ecosystems, the short modelled half-life in air, and its predicted distribution in the environment (results presented in Table 4-5).
4.3 Bioaccumulation
Bioaccumulation is the process that causes an increased chemical concentration in an organism through all routes of exposure, i.e., diet and ambient environmental sources, compared to that in its environment (Arnot and Gobas 2006; Burkhard et al. 2012). It is the net result of competing processes of the chemical uptake into the organism, from the diet and bioconcentration from the respiratory and dermal surfaces, and of the chemical elimination from the organism, through metabolic biotransformation of the parent compound, respiratory exchange, fecal egestion, and growth dilution (Arnot and Gobas 2006). On the ecosystem level, bioaccumulation of a chemical in organisms can lead to its biomagnification across trophic levels.
Numerous metrics can be used to assess the bioaccumulation potential of a chemical including the bioconcentration factor (BCF), bioaccumulation factor (BAF), the log Kow (the partition coefficient between n-octanol (a surrogate for lipid tissue) and water), and biomagnification factor (BMF). Bioavailability and biotransformation of the parent compound through metabolism are also important considerations in determining the extent and potential of a chemical to bioaccumulate. Characterization of the bioaccumulation potential of a chemical is also important in evaluating its toxicity. Bioaccumulation to levels that surpass the internal narcotic toxicity thresholds can lead to adverse effects and mortality in organisms.
Bioaccumulation potential of triclosan was characterized using its physical chemical properties, BCF and BAF studies, metabolism, fugacity ratio and fugacity capacity calculations, and modelling using the model BASL4 (2011). Data were available for numerous aquatic organisms, and some terrestrial species. Bioaccumulation of methyl-triclosan in aquatic species is also described. The available bioaccumulation studies in fish and the potential for metabolism of triclosan presented in this section were also reviewed in an unpublished report (Arnot 2015) submitted to Environment and Climate Change Canada. In addition, an unpublished report (Arnot 2016), also submitted to Environment and Climate Change Canada, describes the use of in vitro to in vivo extrapolations (IVIVE) to estimate the whole body biotransformation rate constants (kB) from published in vitro bioassay experiments for use in BCF calculations. According to Arnot (2016), the range of the calculated BCFs using the IVIVE data were comparable to the reliable quality measured BCFs, as well as the field BCFs from various species and to the BCF predictions from various models.
Triclosan is available for uptake by organisms as demonstrated by its presence in tissues of exposed aquatic organisms. Triclosan can also be readily metabolized by organisms. BCF values ranging from low to high were available for two fish species and a moderate BCF value was determined in mussels (Böttcher 1991; Schettgen et al. 1999; Schettgen 2000; NITE 2006; Gatidou et al. 2010; Gonzalo-Lumbreras et al. 2012). There are numerous uncertainties associated with some of the BCF studies; the highest reported BCF values are thought to be overestimated. Triclosan BAF values reported for algae and snails were low to moderate (Coogan et al. 2007; Coogan and La Point 2008). BAF values reported for methyl-triclosan were moderate to high (Coogan et al. 2007; Coogan and La Point 2008; Balmer et al. 2004). Moderate bioaccumulation of triclosan in fish can lead to concentrations that surpass the internal toxicity thresholds, as demonstrated by the fugacity capacity calculations. Triclosan is unlikely to biomagnify in aquatic and terrestrial food webs, primarily because it can be metabolised by organisms.
4.3.1 Aquatic Organisms
4.3.1.1 Concentrations measured in wild aquatic organisms
Although limited information could be found on the levels of triclosan in wild aquatic organisms in Canada, experimental data on the presence or bioaccumulation of triclosan in organisms were available in the literature for other countries. Adolfsson-Erici et al. (2002) reported accumulation of triclosan in the bile of fish exposed in different ways to effluent from WWTPs in Sweden (Table 4-9). Some fish were exposed to effluent in the laboratory for 3-4 weeks, whereas others were caged for three weeks downstream from a WWTP. Wild fish were also caught, for which the exposure period is uncertain. When taken together, the concentrations measured in the bile of fish for all exposure types ranged from 0.24 to 120 mg/kg wet weight (ww). The highest concentrations were measured for fish exposed to wastewater in the laboratory, followed by fish that were caged downstream a WWTP. The lowest concentrations were measured in wild fish collected downstream a WWTP. These measurements are for the bile, which likely overestimate the concentration that would be expected for the whole body. Although no bioaccumulation factor (BAF) can be calculated from this study, the results show that triclosan is bioavailable when released in water. The data also highlight the potential for excretion of unmetabolized triclosan by fish. Results reported by Valters et al. (2005) show that triclosan is present to a much lesser extent in the plasma of fish (0.750-10 ng/g ww; Table 4-9). Boehmer et al. (2004) measured triclosan concentrations up to 3.4 ng/g ww in the muscle of fish sampled in numerous rivers in Germany. Corresponding concentrations of methyl-triclosan in the same samples were up to about 90 times higher than the triclosan concentrations (Table 4-9).
Fair et al. (2009) collected blood plasma from wild bottlenose dolphins in South Carolina and Florida. Triclosan concentrations in plasma ranged from 0.025 to 0.27 ng/g ww, with up to 31% of the sampled individuals having detectable levels of triclosan.
For the marine environment, triclosan and methyl-triclosan tissue residue data are available for the mussel species Mytilus galloprovincialis (Kookana et al. 2013). Triclosan and methyl-triclosan were measured in mussels at the mean concentrations of 9.87 and 6.99 ng/g ww, respectively, following exposure for 70 days at four marine locations in Adelaide, South Australia, that receive effluents from WWTPs (Kookana et al. 2013).
Given its pKa of 8.1, triclosan can be partly ionized at environmentally relevant pH values, which can influence its bioaccumulation potential. pH values were not available for the studies described above.
| Test organism | Endpoint | Value (based on wet weight) | Reference |
|---|---|---|---|
| Rainbow trout (Oncorhynchus mykiss) | Concentration in bile | 34-120 mg/kga | Adolfsson-Erici et al. 2002 |
| Rainbow trout (Oncorhynchus mykiss) | Concentration in bile | 17-47 mg/kgb | Adolfsson-Erici et al. 2002 |
| Roach (Rutilus rutilus) | Concentration in bile | 4.4 mg/kgc | Adolfsson-Erici et al. 2002 |
| Eelpout (Zoarces viviparus) | Concentration in bile | 0.24-0.90 mg/kgc | Adolfsson-Erici et al. 2002 |
| Perch (Perca fluviatilis) | Concentration in bile | 0.44 mg/kgc | Adolfsson-Erici et al. 2002 |
| 13 fish species collected in the Detroit River (near Windsor, Ontario) | Concentration in blood plasma | 0.750-10 ng/g | Valters et al. 2005 |
| Bottlenose dolphins (Tursiops truncatus) | Concentration in blood plasma | 0.025-0.27 ng/g | Fair et al. 2009 |
| Bream (Abramis brama) | Concentration in muscle | less than 0.25-3.4 ng/g | Boehmer et al. 2004 |
| Marine mussel (Mytilus galloprovincialis) | Whole body concentration | 9.87 ng/g | Kookana et al. 2013 |
Table Notes
Abbreviations: WWTP, wastewater treatment plant.
a Fish exposed to effluent from WWTPs in tanks in laboratory.
b Test organisms caged downstream from a WWTP.
c Organisms in the wild collected downstream from WWTPs.
4.3.1.2 Molecular size and bioconcentration
Molecular size and cross-sectional diameters are useful parameters to consider as weight of evidence for bioaccumulation potential and are commonly used by international jurisdictions such as the EU (ECHA 2008). Recent investigations relating fish BCF data and molecular size parameters (Dimitrov et al., 2002, 2005) suggest that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum diameter. The probability of passive diffusion via the gills decreases appreciably when the maximum diameter of a chemical is greater than about 1.5 nm, and much more so for molecules having a maximum diameter greater than 1.7 nm. Sakuratani et al. (2008) also investigated the effect of cross-sectional diameter on passive diffusion in a BCF test set of about 1200 new and existing chemicals. They observed that substances that do not have a very high bioconcentration potential (BCF less than 5000 L/kg ww) often have a maximum diameter of greater than 2.0 nm and an effective diameter of greater than 1.1 nm. For triclosan, the maximum diameter of 1.3 nm and effective diameter of 0.81 nm were determined, and suggest that triclosan will be passively diffused without restriction through the lipid bilayer.
4.3.1.3 Metabolism and toxicokinetics in fish
Studies on the metabolism and distribution of triclosan in fish suggest that triclosan can be biotransformed, predominantly through Phase II glucuronide conjugate transformation, and subsequently cleared from the exposed organisms (James et al. 2012; Newsome et al. 1975). Mammalian metabolism and toxicokinetics studies are discussed in section 3.1.1; the glucuronide conjugate of triclosan was also noted to be the major metabolite of triclosan in numerous studies (US EPA 2008b; SCCP 2009; Sandborgh-Englund et al. 2006). In addition, it was estimated using ACD Labs Percepta software (ACD/Percepta c1997-2012), that triclosan has a low to moderate volume of distribution (Vd) at 2.4 L/kg compared with more hydrophobic compounds, and a high predicted potential for protein plasma binding (log Ka human serum albumin = 4.1). This suggests that triclosan may be distributed in blood as well as in lipophilic tissues. Using the same software, it was predicted that triclosan is highly permeable in human jejunum (intestine) tissues with a high rate of passive diffusion (ka = 0.06 min-1 via a 100% transcellular route). This is consistent with the size of maximum and effective diameter estimates for triclosan discussed in subsection 4.3.1.2.
Newsome et al. (1975) investigated the absorption, distribution, metabolism and excretion of triclosan in goldfish (Carassius auratus). In the study, a group of six goldfish (weight of 4 g to 107 g) was exposed to radiolabelled triclosan at 2 mg/L for two hours, or at 0.5 mg/L for eight hours of uptake (pH 7.8 to 8.2). After the two-hour uptake of triclosan, the greatest concentration of radioactivity was found in the gall bladder, with a concentration factor of 2500 over the bathing solution, and approximately 60% of the activity was found in the bile. Triclosan was eliminated rapidly during the excretion period. After 24-hour excretion, 60% of activity in water was identified as metabolite(s), with 40% remaining as the parent compound. At least one metabolite was identified, which was speculated to be a glucuronide conjugate. Newsome et al. (1975) stated that both kidney and body reached equilibrium with the bathing solution about two hours after initial exposure, whereas the liver and gall bladder concentrations continued to rise steeply. It is thus likely that steady-state had not been reached in the liver and gall bladder. Although a whole body steady-state was not reached in this study, the results generally suggest a short half-life of triclosan in goldfish, estimated at approximately 1-2 days.
In an in vitro study, triclosan was found to be rapidly glucuronidated and sulfonated in the liver and intestine of channel catfish (Ictalurus punctatus) (James et al. 2012). Triclosan glucuronidation and sulfonation were assayed in the microsomal fraction from liver and proximal intestine. The Km values for methyl-triclosan ranged from 80 to 250 µM, with Vmax values for O-demethylation ranging from 30 to 150 pmol/min/mg protein (at 21 oC). Triclosan at 1 µM could be glucuronidated at a rate of 23 pmol/min/mg protein in liver and 3.2 pmol/min/mg protein in intestine, and sulfonated at rates of 277 and 938 pmol/min/mg protein in liver and intestine, respectively. James et al. (2012) concluded that triclosan could be rapidly cleared in catfish tissues following dietary uptake based on these rates.
Metabolism of triclosan was also modelled using the BCFMax Model with Mitigating Factors (Dimitrov et al. 2005); the potential metabolites predicted by the model are shown in Figure 4-2. The Phase II glucuronide conjugate transformation was the dominant pathway, characterized by a nearly 100% probability of occurrence of the glucuronide conjugate metabolite of triclosan or a complete transformation of the parent molecule (i.e., 1:1 molar ratio). Phase I arene oxidation was predicted to have a lower probability of occurrence, but it may also be a likely elimination pathway. Using SMARTCyp, a HTML-based Cytochrome P450 Quantitative Structure-Activity Relationship (QSAR) from the University of Copenhagen's Department of Drug Design and Pharmacology (Rydberg et al. 2010a, 2010b; Rydberg and Olsen 2012a, 2012b; Rydberg et al. 2013a, 2013b), 3-4 sites of Phase I arene oxidation were predicted with high likelihood on the structure of triclosan.
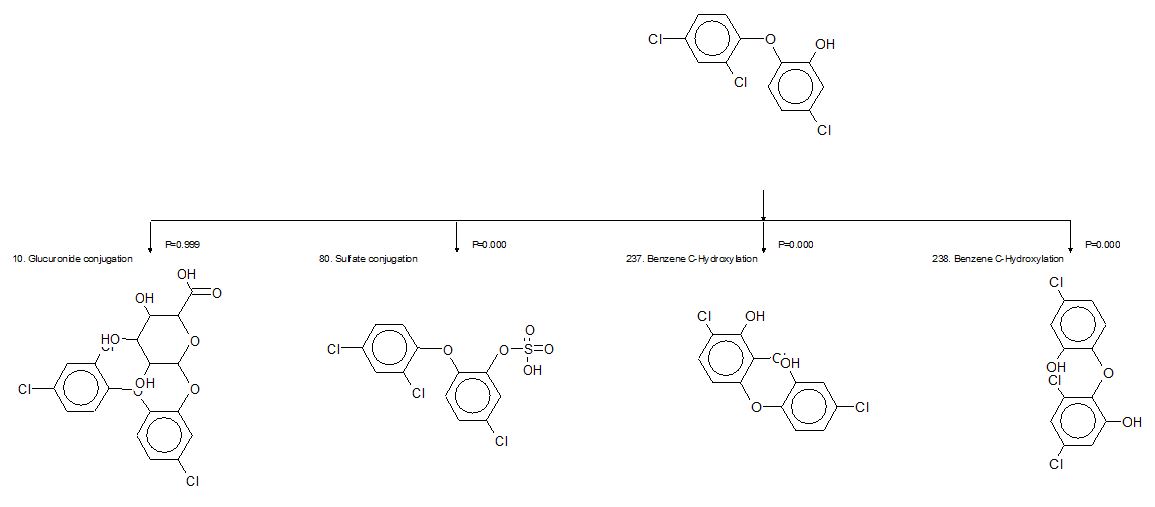
Long description for figure 4-2
Prediction of potential metabolites from triclosan using the BCFMax Model with Mitigating Factors. This figure shows the structure of triclosan and the four structures of its potential metabolites predicted by the model. Glucuronide conjugation was found to be the dominant pathway with the occurrence of the glucuronide conjugate metabolite predicted to have a 99.9% probability of occurrence, while sulfate conjugation and two types of benzene C-hydroxylation were predicted to have a 0% probability of producing metabolites.
4.3.1.4 Bioconcentration factors (BCF) and bioaccumulation factors (BAF) in aquatic species
Studies measuring the bioconcentration factors (BCF) in fish and mussels (Gonzalo-Lumbreras et al. 2012; Gatidou et al. 2010; Schettgen et al. 1999; Schettgen 2000; NITE 2006; Böttcher 1991), and the bioaccumulation factor (BAF) in algae and snails (Coogan et al. 2007; Coogan and La Point 2008) are described below and summarized in Table 4-10.
| Test organism | Endpoint | Value (L/kg, based on wet weight) | Reference |
|---|---|---|---|
| Zebrafish (Danio rerio) (larvae) | BCF | 2018-2630 (based on 15% lipid content) |
Gonzalo-Lumbreras et al. 2012 |
| Zebrafish (Danio rerio) | BCF | 2532-4157 (at pH 7.7-8.0) |
Böttcher 1991 |
| Zebrafish (Danio rerio) | BCF | 3700-8700 (at pH 6, 7, 8 and 9) |
Schettgen et al. 1999; Schettgen 2000 |
| Common carp (Cyprinus carpio) | BCF | 16-90 | NITE 2006 |
| Algae (field samples, various species) | BAF | 900-2100a | Coogan et al. 2007 |
| Daphnia resting eggs (ephippia) | BCF | 74; 4970b | Chiaia-Hernandez et al. 2013 |
| Mussel (Mytilus galloprovincialis) | BCF | 1700c | Gatidou et al. 2010 |
| Mussel (Mytilus galloprovincialis) | BCF | 646;c 13 490b | Kookana et al. 2013 |
| Snail (Helisoma trivolvis) | BAF | 500d | Coogan and La Point 2008 |
Table Notes
Abbreviations: WWTP, wastewater treatment plant.
a Organisms in the wild collected downstream from WWTPs.
b Lipid normalized.
c Dry weight.
d Test organisms caged downstream from a WWTP.
Böttcher (1991) conducted a bioconcentration test with zebrafish (Danio rerio) in a flow-through test system based on methods modified from OECD test guideline 305C. Zebrafish were exposed to either 3 or 30 μg/L of triclosan in the test water. The test compound concentrations were well maintained (at 2.95-3.18 μg/L and 26.4-27.66 μg/L for 3 and 30 μg/L nominal concentrations, respectively). Fish had an average weight of 0.33 g at study initiation. Uptake and depuration periods were 5 and 2 weeks, respectively. [14C]Triclosan was used for the experiment, and results were based on total radioactivity measured in water and fish tissues. The experiment was conducted at pH 7.7-8.0; given the pKa of 8.1 for triclosan, almost half of the test substance was dissociated resulting in exposure to both the neutral and ionogenic forms of triclosan. Steady state did not appear to be reached during the 5-week uptake period, as bioconcentration factor (BCF) values fluctuated during this period at both exposure concentrations. The maximum BCF values were reached at week 3, but then decreased until week 5. The causes for the decrease in BCF values are unknown, but are related to fluctuations in tissue residues rather than in exposure concentrations as the latter were very stable throughout the uptake phase. The average BCFs over the 5-week uptake period were calculated to be 4157 L/kg ww at 3 µg/L and 2532 L/kg ww at 30 μg/L (Table 4-10); maximum BCF values were 5337 L/kg ww and 3408 L/kg ww, respectively. Because such measurements based on total radioactivity cannot distinguish between the parent compound and possible metabolites, this might have led to overestimation of BCF values. Depuration rate constants (k2) at 3 µg/L and 30 µg/L were 0.142/day and 0.141/day, respectively. It should be noted that the higher exposure concentration used in this test is 5.6% of the 96-hour median lethal concentration (LC50) for zebrafish (540 µg/L; unreviewed study cited in NICNAS 2009). OECD test guideline 305 recommends that the highest concentration be set at 1% of the acute asymptotic LC50 to avoid toxic effects that could affect fish bioaccumulation kinetics. Given the deficiencies of this study, mainly the lack of equilibrium during the uptake phase, its results are uncertain and thus have questionable reliability. As such, these uncertainties were considered in the weight of evidence approach used to characterize the bioaccumulation potential of triclosan.
Schettgen et al. (1999) conducted a bioconcentration study with triclosan at different pH values (6-9) based on OECD test guideline 305E. Zebrafish (Danio rerio) were exposed to either 35 or 50 μg/L of triclosan for about 150 hours before being transferred to clean water for an additional 100 hours for the depuration phase. The average lipid content was 5.32%, 6.18%, 3.86% and 7.55% for fish tested at pH 6, 7, 8 and 9, respectively. Triclosan was dissolved in methanol, and the concentration of methanol was 0.05% in the accumulation tank (Schettgen 2000). This concentration exceeded the maximum solvent concentration of 0.01% (equivalent of 0.1 mL/L) specified in the OECD test guideline 305E. The high concentration of methanol might have increased the bioavailability of triclosan resulting in a higher degree of uptake than might be expected under natural conditions. Concentrations of triclosan in fish and water were analyzed by gas chromatography-electron capture detection, and rate constants for uptake (k1) and clearance (k2) were calculated. Based on the uptake and elimination curve obtained, equilibrium seems to have been reached during the experiment. The exposure period for the uptake phase (150 hours) exceeded the time to reach 80% of steady state (80% time to steady state = 1.6/k2 = 1.6/(0.0347/hour) = 46 hours), which is an additional indication that steady state was reached. The BCF values (± standard deviation) were determined as the ratio of the rate constants and were as follows: 8700 (±2632) L/kg ww, 8150 (±1417) L/kg ww, 6350 (±963) L/kg ww and 3700 (±1232) L/kg ww at pH 6, 7, 8 and 9, respectively (Table 4-10). These values show the expected decrease in uptake rate with increasing ionization of triclosan from pH 6 to 9; the clearance rate constant had similar values for all pH values tested, ranging from 0.0347/hour to 0.0413/hour. The metabolic rate constant (kM) will be slightly lower than these depuration values, but the half-life estimated based on the average depuration rate is approximately 18 days, somewhat longer than other studies. The uptake rate constants decreased from 356/hour to 129/hour with increasing pH values. As for one exposure concentration used in the study by Böttcher (1991), the exposure concentrations used in this test are higher than 1% of the acute asymptotic 96-hour LC50 for zebrafish (540 µg/L; unreviewed study cited in NICNAS 2009); the limit of 1% is recommended in the OECD test guideline 305 to avoid toxic effects that could affect fish bioaccumulation kinetics. In this experiment, the uptake and depuration processes, and hence the BCF values, may have been slightly affected by the concentrations of triclosan used. The recovery rate of triclosan was 168% in fish and was 93% in water; the greater than 100% recovery rate in fish suggests experimental error and possible tissue contamination, which might have led to overestimation of BCF. Because of the deficiencies in the experimental study, the reliability of this study is questionable, and this is considered in the weight of evidence approach used to characterize the bioaccumulation potential of triclosan.
A study using zebrafish larvae was conducted by Gonzalo-Lumbreras et al. (2012) as an alternative to the OECD technical guideline 305 based on ethical and economic considerations. Zebrafish larvae were exposed to 3 and 30 μg/L, corresponding to 0.1 and 1% of their LC50, and similar to the concentrations used in studies by Böttcher (1991) and Schettgen et al. (1999), described above. The BCF values were determined as 2018 and 2630 at the lower and higher exposure concentrations, respectively, over 72 hours. Exposure solutions were changed every 24 hours as per the OECD 305 guideline requirement, to avoid fluctuations of the nominal exposure concentrations. Steady state was not reached during the uptake phase, and it was explained that a longer exposure time would be required. It is noted that at 15%, zebrafish larvae have a higher lipid content than adult fish which may impact the degree of bioaccumulation of hydrophobic chemicals, compared to results in adult fish which typically have 5% lipid content. A direct comparison with BCF values for adult fish would require application of a conversion factor to account for the differences in the lipid content. Given that in the natural environment fish would be exposed to triclosan at all life stages, this study is considered a valid representation for bioaccumulation potential at an early life stage.
The Japanese National Institute of Technology and Evaluation (NITE) conducted a bioconcentration study with carp (Cyprinus carpio) in which fish were exposed to either 3 or 30 μg/L of triclosan for 8 weeks under flow-through conditions (NITE 2006). The protocol followed the NITE test guideline for bioaccumulation in carp, which corresponds to OECD test guideline 305C. Measured concentrations of triclosan in test water over the study duration slightly fluctuated from 22.4 to 26.0 μg/L and from 2.00 to 2.46 μg/L for the 30 μg/L and 3 μg/L exposure concentrations, respectively. The study report did not mention whether a depuration phase occurred during the experiment, but this is likely to have been performed. Also, the pH of the test water was not reported. Average BCF values at the 3 μg/L exposure concentration were 55, 69, 56, 39 and 80 L/kg ww at 1, 2, 4, 6 and 8 weeks, respectively. At the 30 μg/L exposure concentration, average BCF values were 36, 36, 30, 36 and 18 L/kg ww at 1, 2, 4, 6 and 8 weeks, respectively. The minimum and maximum BCF values were 16 and 90, respectively, when considering both exposure concentrations and all data (Table 4-10). As in the study by Böttcher (1991), BCF values fluctuated somewhat during the NITE study, but only nominally from the grand average BCF of 45. Since the measured concentrations in water were relatively stable, fluctuations in BCF values could be due to fluctuations in fish tissue concentrations; however, these data were not available. No values were reported for the uptake or depuration rate constants (k1), but using the grand average BCF value from the test and extrapolating kM using procedures outlined in Arnot et al. (2008), a metabolic rate constant would be approximately 8.8 d-1 or a half-life less than 1 day.
The large differences observed between the fish BCF values reported by Böttcher (1991), Schettgen et al. (1999), Gonzalo-Lumbreras et al. (2012) and NITE (2006) could be due to differing uptake rates, metabolic rates, different weights of fish used, different lipid contents, etc., in addition to the study deficiencies mentioned.
There are no BAF values available for fish; however, the Koc and Kow of triclosan suggest that the BAF should closely approximate BCF. Indeed, at a log Koc of 4.7, the predicted bioavailable fraction of triclosan in the water column according to mass balance fish models is approximately 99%, which means that practically all of the total water concentration of triclosan will be in the dissolved phase. This suggests that uptake from water via the gills is a very relevant exposure for this substance. This also suggests that the contribution of the diet to the total body burden of triclosan in aquatic organisms is likely quite low. In fact, the calculated BAF using the Arnot-Gobas mass balance (version 1.11) (Arnot and Gobas 2003) is only 3% greater than the BCF.
Two bioconcentration studies are available for a marine species, the mussel Mytilus galloprovincialis. Gatidou et al. (2010) measured a BCF of 1700 L/kg dry weight (reported as 1.7 L/g) for mussel M. galloprovincialis. Mussels were exposed to 300 ng/L of triclosan for 28 days (pH not reported), during which the tissue concentrations constantly increased, and steady state was not observed. The BCF was determined as the ratio of the uptake and depuration rate constants. The experiment revealed that the depuration rate of triclosan was lower than its uptake rate and the biological half-live of triclosan was reported as 12 days. Given this half-life value, a higher BCF would have been expected. Kookana et al. (2013) investigated bioconcentration of triclosan and methyl-triclosan in the same species of mussels. Mussels were exposed to triclosan at a concentration of 100 ng/L for 30 days is seawater aquaria. Steady state was reached at about 24-30 days. The lipid content of mussels remained constant, at about 5.4%. BCF for triclosan was determined as 646, and 13 490 when lipid normalized. BCF (lipid normalized) for methyl-triclosan was determined as 15 488.
Bioconcentration in daphnia resting eggs (ephippia) were investigated by Chiaia-Hernandez et al. (2013). Dapnia magna ephippia were exposed to triclosan at concentrations between 150 and 250 µg/L for up to 120 hours. The lipid content of test organisms was measured as 1.5% of the wet weight. The BCF in ephippia was determined as 74 (wet weight), and 4970 when lipid normalized.
Coogan et al. (2007) calculated a BAF ranging from 900 to 2100 L/kg ww for algae collected in a creek receiving the effluent from a WWTP, while a BAF of 500 was calculated for snails that had been caged in the same creek for 2 weeks (Coogan and La Point 2008). It is unknown but likely that a steady state in triclosan concentration in snails was reached by the end of the exposure period. The exposure pH was not reported in either study.
4.3.1.5 Biota sediment accumulation factors (BSAF) in sediment species
The route and degree of uptake of triclosan by the sediment-dwelling worm, Lumbriculus variegatus, were investigated using 14C-labelled triclosan (Karlsson et al. 2015). Feeding and non-feeding worms (where the head anterior segments were removed) were used in the study to assess the kinetics of uptake. Nominal triclosan exposure concentrations over 48-hours ranged between approximately 625 to 650 nmol/kg in sediment, and up to nearly 5 nmol/L in water. The 14C activity of triclosan in the 48 hour uptake phase in the water column was observed to decrease, and was attributed to the uptake of the parent triclosan and any transformation products into the study organisms since the measurement of 14C activity may represent not only the parent compound but also transformation products in the test systems. Significantly greater uptake of triclosan was observed in the feeding worms compared to the non-feeding worms; the increased observed uptake was attributed to the hydrophobicity of triclosan and resulting adsorption to sediments. The biota sediment accumulation factors (BSAF) were calculated based on the 48-hour uptake and depuration measurement using the first order one compartment model. The 48-hour BSAF for feeding worms was 9.0, and 6.6 for the non-feeding worms, for which uptake is thought to be dominated via the epidermis (Karlsson et al. 2015). These BSAF values represent a combination of parent and transformation products.
4.3.1.6 Water to biota fugacity ratio
Fugacity of a chemical is a thermodynamic equilibrium criterion that can be used to assess the relative chemical activity in a system comprised of multiple compartments or phases (such as water, sediment or diet). At equilibrium, the chemical fugacities in the different phases are equal, and fugacity ratios between an organism and a reference phase are equal to one. A fugacity ratio between an organism and a reference phase that is greater than one indicates an increase (i.e., magnification) in the activity of the chemical in the organism compared to the reference phase. The fugacity ratio approach can be used to show a biomagnification potential of a chemical; fugacity ratios that exceed one indicate increases of chemical residues in organisms through trophic levels.
The fugacity ratio of triclosan for biota and water was calculated using the equation modified from Burkhard et al. (2012):
Fbiota-water = BCF (L/kg) × Dbiota (kg/L) × Zwater ÷ Zbiota
where
BCF = bioconcentration factor
Zwater, fugacity capacity in water = 1/HLC (Henry's law constant)
Zbiota, fugacity capacity in biota = %lipid × (Dbiota ÷ Dlipid) × Kow × Zwater
Dbiota = 1 kg/L and Dlipid= 0.9 kg/L (2012 personal communication from Frank A.P.C. Gobas, Simon Fraser University, to Science and Risk Assessment Directorate, Environment Canada; unreferenced).
A geometric mean of BCF values (from the whole body BCF fish studies by Böttcher (1991), Schettgen et al. (1999), Schettgen 2000, NITE (2006), summarized in Table 4-10) of 887 L/kg ww and a log Kow (log Dow) of 5.2 (at pH 7.4) were used in this calculation, resulting in a fugacity ratio of 0.13 at pH 7.4 (~blood). The result is less than one, and indicates that triclosan has a low potential for biomagnification (Burkhard et al. 2012). If maximum reported BCF values are used in this calculation, the fugacity ratio slightly exceeds one. Given that triclosan is readily metabolized by fish, and that the high BCF values (reported in Schettgen et al. 1999 and Schettgen 2000) are uncertain and may be overestimated due to experimental error, biomagnification across trophic levels is unlikely.
4.3.1.7 Bioaccumulation and the internal narcotic toxicity threshold
Bioaccumulation of a chemical to levels that surpass the internal toxicity thresholds can lead to mortality in the exposed organisms. The value of 5 mmol/kg is considered to be the internal neutral narcotic toxicity threshold for toxicity in fish from acute exposure. This value is based on findings from numerous studies which show that internal concentrations of neutral narcotic chemicals in fish causing death are fairly constant at about 2-8 mmol/kg for acute exposures, with a median of 5 mmol/kg, and 0.2-0.8 mmol/kg for chronic exposures (McCarty 1986, 1987a, 1987b, 1990; McCarty and Mackay 1993; McCarty et al. 1985, 1991, 2013; Van Hoogen and Opperhuizen 1988).
The equation for fugacity (F = C ÷ Z; Burkhard et al. 2012) and the fugacity ratio of biota to water (Fbiota-water) (see section 4.3.1.4) can be used to calculate the maximum internal concentration of a chemical according to bioconcentration factor (BCF) data as follows:
C = F × Zbiota
where
F = fugacity (Pa)
Zbiota = BCF (L/kg) × Dbiota (kg/L) × Zwater ÷ Fbiota-water (see section 4.3.1.4)
This calculation assumes that octanol is a reasonable surrogate for lipids in fish and that the chemical's diffusion partitioning in fish is driven by hydrophobicity (i.e., no other mechanisms such as covalent binding, which is unlikely for triclosan).
For triclosan, if fugacity capacity using the vapour pressure of 0.00053 Pa is considered and 5% lipid in fish, the maximum concentration that can be achieved at pH 7.4 is approximately 30 mmol/kg (a fugacity of 0.000067 Pa), which is 6 times the median acute internal narcotic threshold of 5 mmol/kg. This indicates that based on its intrinsic properties, triclosan can bioaccumulate to levels exceeding internal narcotic thresholds.
4.3.1.8 Bioconcentration and bioaccumulation of methyl-triclosan in aquatic organisms
Given that methyl-triclosan has often been detected in aquatic organisms in waters contaminated with triclosan, BCF and BAF values for methyl-triclosan were also considered in the overall weight-of-evidence analysis. Miyazaki et al. (1984) were the first to report accumulation of methyl-triclosan in aquatic biota. They detected various levels of this compound in species of fish and shellfish sampled in the Tama River and Tokyo Bay in Japan. The concentrations ranged from 1 to 38 μg/kg and from 3 to 20 μg/kg in fish and shellfish, respectively (Table 4-11). The authors attributed the presence of this compound to biological methylation of triclosan in the environment.
Balmer et al. (2004) measured methyl-triclosan in white fish, roach and lake trout from lakes in Switzerland that receive effluents from WWTPs, as well as in reference lakes not influenced by WWTPs. They also sampled water using semipermeable membrane devices in order to derive a concentration for dissolved methyl-triclosan. The concentrations of methyl-triclosan in fish were up to 35 μg/kg on a wet weight basis and up to 365 μg/kg on a lipid basis. No methyl-triclosan was detected in fish from the reference lakes (less than 1 and less than 2 μg/kg). The concentrations of methyl-triclosan in fish correlated well (r2 = 0.85) with the ratio of the human population in the watershed to the water flow of the lakes, which is considered to be a measure of the domestic burden from WWTPs to a lake. A BAF was estimated for methyl-triclosan using the concentrations in fish as well as the water concentrations derived from the semipermeable membrane devices; the resulting BAF was in the order of 100 000-260 000 L/kg (lipid basis). Assuming an average fat content in fish of 2%, the study authors estimated the BAF for methyl-triclosan to be 2000-5200 L/kg (log BAF of 3.3-3.7) on a wet weight basis.
BAF values of 700-1500 L/kg were reported for methyl-triclosan for algae collected in a creek receiving the effluent from a WWTP (Coogan et al. 2007), while a BAF of 1200 L/kg was calculated for snails that had been caged in the same creek for 2 weeks (Coogan and La Point 2008). It is unknown but likely that steady state was reached in this experiment, given the length of the exposure period.
| Test organism | Endpoint | Value (based on wet weight) | Reference |
|---|---|---|---|
| Topmouth gudgeon (Pseudorasbora parva) | Concentration in whole body | 1-38 μg/kg | Miyazaki et al. 1984 |
| Goby (Acanthogobius flavimanus) | Concentration in whole body | less than 1-2 μg/kg | Miyazaki et al. 1984 |
| Short-necked clam (Tapes philippinarum) | Concentration in whole body | 3 μg/kg | Miyazaki et al. 1984 |
| Thin-shelled surf clam (Mactra veneriformis) | Concentration in whole body | 5 μg/kg | Miyazaki et al. 1984 |
| Oyster (Crassostrea gigas) | Concentration in whole body | 13 μg/kg | Miyazaki et al. 1984 |
| Blue mussel (Mytilus edulis) | Concentration in whole body | 20 μg/kg | Miyazaki et al. 1984 |
| White fish (Coregonussp.) | Concentration in whole body | 4-211 μg/kga,b | Balmer et al. 2004 |
| Roach (Rutilus rutilus) | Concentration in whole body | less than 2-365 μg/kga,b | Balmer et al. 2004 |
| Lake trout (Salmo trutta) | Concentration in whole body | less than 1 μg/kga-c | Balmer et al. 2004 |
| 13 fish species collected in the Detroit River (near Windsor, Ontario) | Concentration in plasma | less than 0.000 010 μg/kg | Valters et al. 2005 |
| Bream (Abramis brama) | Concentration in muscle | 3.8-26.1 ng/g | Boehmer et al. 2004 |
| White fish (Coregonus sp.) and roach (Rutilus rutilus) | BAF | 2000-5200 L/kga,b | Balmer et al. 2004 |
| Algae (field samples, various species) | BAF | 700-1500 L/kgb | Coogan et al. 2007 |
| Snail (Helisoma trivolvis) | BAF | 1200 L/kgd | Coogan and La Point 2008 |
Table Notes
Abbreviations: WWTP, wastewater treatment plant.
a Values on a lipid basis.
b Wild fish caught, or algae collected, downstream from WWTPs. Levels in fish from reference lakes were less than 1 and less than 2 μg/kg.
c This species was only found in the reference lake.
d Snails caged downstream from a WWTP.
4.3.2 Bioaccumulation in terrestrial organisms
Kinney et al. (2008) sampled earthworms from agricultural soils that had been amended with biosolids from WWTPs. Based on the ratio of triclosan concentrations measured in earthworm tissues and in soil, BAF values of 10 and 27 (unitless) were calculated at 31 and 156 days following soil amendment, respectively. It is not known but likely that steady state in triclosan body concentrations was reached by 156 days, even though data are available for only two sampling times. Under field conditions similar to those in this case, the exposure is dynamic rather than static, given the pulses created by biosolids application followed by dissipation of triclosan through various processes. Pannu et al. (2012b) found similar BAF values (4.3 to 12, unitless) for earthworms exposed to biosolids-amended soils in the laboratory (28 days) and on the field.
Wu et al. (2010a) grew soybean plants in a sandy soil that had been either amended with biosolids or irrigated with wastewater containing triclosan. The BCF values (root/soil) measured after 60 and 110 days of growth in the soil amended with biosolids were about 2.5 and 5.9 (unitless), respectively. No BCF values could be calculated for plants grown in the soil irrigated with wastewater, as triclosan was not detected in the soil; however, triclosan did accumulate in plant tissues (root, stem, leaf and bean; 24.2-80.1 ng/g after 110 days). Again, it is unknown whether steady state was reached in this experiment.
The bioconcentration of triclosan in two species of wetland macrophytes was measured by Stevens et al. (2009). They exposed the organisms for 28 days to concentrations of triclosan ranging from 0.4 to 1000 µg/L in water-only flow-through systems. They measured BCF values ranging from 0.4 to 2.8 L/kg ww and from 1.4 to 101 L/kg ww in plant shoots and roots, respectively. These values would likely be different in a natural environment where plants would be rooted in soil.
Potential for secondary poisoning by triclosan in terrestrial food chains was assessed using the model BASL4 (BASL4 2011; see Section 4.5.3 for more details). In this model, the exposure of earthworms to triclosan present in soil following the application of biosolids to fields, and the subsequent accumulation of triclosan in earthworms, is estimated based on factors such as soil ingestion, lipid content and growth dilution, among others. As a conservative estimate, it is assumed that no metabolism of triclosan will occur in organisms. The bioaccumulation of triclosan in shrews consuming these earthworms is then estimated based on similar factors. Two biosolids application scenarios were run (a lower-end and an upper-end; see Section 4.5.3). In both scenarios, peak concentrations in soils (118 µg/kg dw and 222 µg/kg dw for lower-end and upper-end scenarios, respectively) occur right after biosolid application. Concentrations of triclosan in soil between biosolid applications averages 11 μg/kg dw and 110 μg/kg dw, respectively, for these lower-end and an upper-end scenarios. Based on the highest modelled concentrations in soil, earthworms (~11 100 µg/kg dw and ~21 000 µg/kg dw for lower-end and upper-end scenarios, respectively) and shrews (~531 000 µg/kg dw and ~994 000 µg/kg dw for lower-end and upper-end scenarios, respectively), the modelled BAF values for earthworms (i.e., concentration in earthworms divided by concentration in soil) are approximately 95, while the modelled BAF values for shrews (i.e., concentration in shrews divided by concentration in soil) are approximately 4500. The modelled biomagnification factor values (i.e., concentration in shrews divided by concentration in earthworms) are ~48. Field data cited above indicate BAFs of 4.3-27 for earthworms sampled in biosolid-amended fields (Kinney et al. 2008, Pannu et al. 2012b). The model results indicate that triclosan concentrations will increase from soil to earthworms, and will further increase from earthworms to shrews. The modelled BAF values for shrew are unexpectedly high given that triclosan is extensively metabolized in mammals. Indeed, there is no evidence that triclosan bioaccumulates in mammals, although there may be retention of triclosan and/or its metabolites in the liver (NICNAS 2009, SCCP 2009). Triclosan is extensively metabolized via glucuronide and sulfate conjugation. The high modelled BAF values are probably due to the fact that BASL4 assumes that there is no metabolism occurring in organisms.
4.3.3 Relevance of triclosan bioaccumulation
The available information is reflective of the complex behaviour of triclosan in the environment and in organisms, and precludes precise description of the magnitude of bioaccumulation of triclosan. BCF studies conducted some time ago are of speculative reliability resulting in equivocal lines of evidence for determining an absolute factor for bioconcentration. What is more clear and consistent from the available evidence is that triclosan can be rapidly taken up in aquatic and terrestrial organisms and likely reaches steady-state within a few days given its low log Kow, as is seen with many pharmaceuticals. Thus, triclosan is a highly bioavailable chemical in vivo with pH likely having a dramatic effect on the fugacity potential of triclosan and its distribution among tissues. In the environment, at pH of 7.0, triclosan will mostly occur in the neutral form and will tend to partition more readily into organisms than the ionized species. At pH 7.4 of blood, triclosan will occur largely in the neutral form as well, but will also occur at a lower fraction (20%) in the ionized state. Thus, triclosan is likely distributed between lipophilic and non-lipophilic tissues within biota and may undergo protein plasma binding in albumin due to its hydrogen donor/acceptor properties, particularly in the ionized form. The range of BCF values (noting their equivocal status) and field BAF values suggests that the rate of metabolism within and among higher and lower trophic level organisms naturally differs, and, given the high bioavailability of triclosan, is the predominant reason explaining the variation in bioaccumulation potential of triclosan as well as the pH of exposure waters. Considering the in vivo, in silico and in vitro information, there is a degree of consistency in the evidence to suggest that most organisms can eliminate triclosan relatively quickly via Phase II and possibly Phase I metabolic transformations. The uptake of methyl-triclosan and subsequent demethylation can also add to the body burdens of triclosan, but this may be hard to distinguish from the uptake of triclosan itself in real world exposures.
There is evidence to suggest that the bioconcentration potential of triclosan from water can vary depending on the exposure conditions and organisms exposed. Compared with other chemicals of similar or more hydrophobic nature, the potential for triclosan to bioaccumulate is generally low to moderate, and it is predominantly mitigated by biotransformation (see for comparison Figure 7 in Arnot and Gobas (2006)). In this assessment, however, the absolute factor of bioconcentration or bioaccumulation is of less importance than triclosan’s intrinsic ability to partition from water and into tissues. Importantly, triclosan has a sufficient bioconcentration potential and fugacity to result in internal body burdens that exceed narcotic or polar narcotic thresholds of toxicity, given a sufficient concentration in water. This becomes highly relevant because considering that the chemical activityFootnote3 of triclosan in water in some cases can exceed the chemical activity of narcotic chemicals by more than a factor of ten, it suggests that the toxicity of triclosan can approach that of the more reactive chemicals (like some drugs) under chronic exposure. Thus, bioconcentration, even at low to moderate levels, becomes a critical factor for understanding the potential for adverse effects in the Canadian environment.
4.4 Ecological Effects
4.4.1 Mode of action
Triclosan cellular modes of action (MOA) through binding molecular targets have been demonstrated in bacteria, plants, and rodents (Jang et al. 2008; McMurry et al. 1998; Heath et al. 1999; Hoang and Schweizer 1999; Levy et al. 1999; Zhang et al. 2006; Serrano et al. 2007). Triclosan has numerous intracellular and cytoplasmic target sites and may influence the transcription of genes involved in amino acid, carbohydrate and lipid metabolism as well as signalling pathways, as shown in the bacteria Staphylococcus aureus (Jang et al. 2008). Triclosan blocks lipid biosynthesis in bacteria by specifically inhibiting the enzyme enoyl-acyl carrier protein reductase, which is involved in type II bacterial fatty acid synthesis (McMurry et al. 1998; Heath et al. 1999; Hoang and Schweizer 1999; Levy et al. 1999). Plants share similar fatty acid synthesis pathways with bacteria (Zhang et al. 2006). Experiments conducted with the plant Arabidopsis (in the family Brassicaceae) have shown that enoyl-acyl carrier protein reductase is a possible target of triclosan (Serrano et al. 2007). In the mouse, activation of PPARα is the primary MOA for triclosan-induced hepatocarcinogenesis (see Section 3.1.8). Triclosan may also disrupt thyroid-mediated processes; triclosan has been shown to alter thyroid hormone-associated gene expression in amphibians in vitro(Veldhoen et al. 2006) (see Section 3.1.10 and Section 4.4.2.1). It is suspected that triclosan can uncouple oxidative phosphorylation (Newton et al. 2005, 2014 personal communication from Beate Escher, The University of Queensland, to the Science and Risk Assessment Directorate, Environment Canada, unreferenced).
The molecular structure of triclosan with its two phenol functional groups resembles those of several non-steroidal estrogens, such as diethylstilbestrol and bisphenol A. This suggests the potential to act as an endocrine-disrupting agent through estrogen receptor binding (Ishibashi et al. 2004; see Section 4.4.2). The Profiler function of the OECD QSAR Toolbox (QSAR 2008) identified structural alerts for high-toxicity classification for triclosan that suggest that triclosan exerts toxicity beyond a baseline narcotic MOA. These included estrogen receptor binding (strong binder), acute aquatic toxicity by OASIS (phenols and anilines) and high hazard class according to Cramer rules.
The chemical activity of triclosan (i.e., fraction of solubility in water eliciting adverse effects) is about 0.00004 (based on the predicted no-effect concentration (PNEC) for aquatic organisms; see Section 4.4.2.1 below), which is far less than the chemical activity expected for baseline narcotic chemicals (usually 0.1 to 0.01) (Mackay et al. 2014). The toxicity ratios are far greater than 10 indicating a specific mode of toxic action (Escher et al. 2011).
Triclosan ionizes at environmentally relevant pH levels. Some studies conducted with daphnids (Ceriodaphnia dubia) and algae (Scenedesmus subspicatus) have shown that the pH of the test solution may influence the toxicity of triclosan (Orvos et al. 2002, Roberts et al. 2014). Tests conducted at lower pH values, corresponding to a higher proportion of the neutral form of triclosan in solution, generally showed higher toxicity although this effect was not demonstrated consistently at all pH levels. This may be due to the fact that the bioavailability of the neutral form, in terms of its capacity to cross cellular membranes, is higher than that of the ionogenic form, due to an electronic barrier at the membrane surface for ionogenic species. Thus, the toxicokinetics of triclosan would be influenced by pH, but not its intra-cellular toxicity. At the site of toxic action, the pH of the cytoplasm matters due to an ion trapping phenomenon (Neuwoehner and Escher 2011) and thus this pH would govern the species of triclosan inducing the effect. This may explain the lack of a consistent relationship between exposure medium pH and effects in one of the two studies mentioned above (Roberts et al. 2014). Also, comparison of different studies conducted with the same species or other species does not clearly point to an influence of pH on toxicity. Given a strongly suspected MOA for triclosan as an uncoupler, an adjustment of ecotoxicity data for a pH-dependent effect (of the medium) was not done in this assessment. Weak acid uncouplers are known to have the highest potency when internal pH equals the pKa (i.e., 50:50 ratio of neutral and ionized species) and thus both forms are believed to contribute to the toxicodynamics of triclosan. It is indeed believed that internal toxic effects are independent of pH of the test medium. It is acknowledged that the latter can affect the toxicokinetics of triclosan; however, the ultimate relationship between the pH of the test medium and the toxicity observed cannot be quantified.
4.4.2 Ecotoxicity
An extensive toxicity data set for triclosan was compiled by Environment and Climate Change Canada, and includes data for aquatic, benthic and terrestrial organisms. Studies with both acute and longer term or chronic exposure durations were available. The determination of whether an endpoint is acute or chronic was based on the lifespan of each species considered. Effects data for aquatic species are presented in subsection 4.4.2.1, for benthic organisms in subsection 4.4.2.2, and for terrestrial organisms in sub-section 4.4.2.3. Sub-section 4.4.2.1 also includes a description of the species sensitivity distribution (SSD) for aquatic species based on the chronic effects studies using the 2007 guidance protocol by the Canadian Council of Ministers of the Environment (CCME) (CCME 2007), a derivation of the predicted no-effect concentration (PNEC), and a summary of effects data for methyl-triclosan in aquatic species. Sub-section 4.4.2.3 also includes a derivation of the PNEC for terrestrial organisms. Antimicrobial resistance is addressed in section 3.6. There is indication that resistance to triclosan and multidrug resistance can increase in the environmental microbial communities exposed to triclosan (Carey and McNamara 2015); however few studies are available and they are generally limited to laboratory settings and high exposure concentrations. Environmental hazard due to impacts from microbial resistance to triclosan based on the measured concentrations of triclosan has not been identified.
4.4.2.1 Aquatic organisms
4.4.2.1.1 Algae, macrophytes and bacterial communities
Single-species toxicity tests as well as community-level studies have been conducted with bacteria, algae and macrophytes exposed to triclosan. Orvos et al. (2002) tested five algal species. The blue-green alga Anabaena flos-aquae was the most sensitive species, with an EC10 value of 0.97 μg/L (Table 4-12). It is worth noting that the only marine species tested (the diatom Skeletonema costatum; 96-hour EC25 greater than 66 μg/L) was the least sensitive among the five algal species tested, which could suggest that the salinity of the test water may have had an impact on triclosan speciation and bioavailability (i.e., higher proportion of the ionized form). However, DeLorenzo and Fleming (2008) measured a 96-hour EC50 of 3.55 μg/L for a marine phytoplankton species (Dunaliella tertiolecta), which is comparable with the toxicity measured by Orvos et al. (2002) for certain freshwater algae. Yang et al. (2008) measured a 72-hour EC50 of 0.53 μg/L for Pseudokirchneriella subcapitata, which is much lower than the one reported by Orvos et al. (2002) for the same species (96-hour EC50 of 4.46 μg/L). This variation in toxicity values from different tests conducted with the same algal species is likely due to pH and illumination. Indeed, the test pH influences the fraction of neutral and ionized forms of triclosan present in solution, which may exert different levels of toxicity (Roberts et al. 2014). Also, illumination of the test medium induces quick photolysis of triclosan by UV rays, especially of the ionized form, causing exposure concentrations to decline during the test period. This may lead to underestimation of triclosan toxicity if concentrations are not measured throughout the test period. Fulton et al. (2009) obtained a similar 7-d MATC for growth inhibition for Lemna gibba as the one obtained in Study Submission (2013) (17 and 28 μg/L, respectively, Table 4-12).
Wilson et al. (2003) reported an algal community structure shift at triclosan levels as low as 0.015 μg/L. This study used natural algal assemblages as well as natural water, making the outcome of the bioassays more environmentally realistic. However, because insufficient data were reported, such as measurements of exposure concentrations, there is uncertainty about the actual threshold of effects and about the reliability of the study in general. Hence, the results of this study were not used for the derivation of a chronic toxicity threshold for triclosan. Lawrence et al. (2009) investigated the effects of triclosan on the structure and function of river biofilm communities, which are a key component of whole ecosystem function. Using South Saskatchewan River water as a source of inoculum and nutrients, they employed a variety of techniques, including microscale analyses, molecular probes and physiological determinations, to determine the effects of a continuous 8-week exposure to triclosan at 10 μg/L. Analyses of the biofilm communities indicated shifts in the algal and bacterial composition, as well as a significant reduction in algal biomass, in test systems containing triclosan as compared with controls. The general shift observed was towards a more heterotrophic community, which may have significant ecological implications for carbon and energy flow. The actual exposure level in this study is however uncertain as triclosan concentration was not stable. Using pure cultures of protozoa, the same authors found effects of triclosan on certain species of algae, cyanobacteria and protozoa exposed to 0.5 and 10 μg/L for 14 days. However, the effects observed were not quantified and exposure concentrations were likely not maintained given the use of a static system. These results were not further considered. Miyoshi et al. (2003) reported deleterious effects of triclosan on two Paramecium species at concentrations of 1564 and 400 μg/L after 5 days. However, lack of experimental information, notably exposure concentrations, makes the reliability of this study questionable; hence, the results were not further considered.
Table 4-12. Chronic toxicity of triclosan to freshwater aquatic organismsa
| Organism | Endpoint (duration) | Effect | Conc. (µg/L) | Used in SSD | Reference |
|---|---|---|---|---|---|
| Scenedesmus subspicatus | MATC (72 h) | Growth | 0.77 | No | Orvos et al. 2002 |
| Scenedesmus subspicatus | NOEC (96 h) | Growth | 0.69 | No | Orvos et al. 2002 |
| Scenedesmus subspicatus | EC10 (72 h) | Growth | 0.5 | Yes | Roberts et al. 2014 |
| Scenedesmus vacuolatus | EC10 (24 h) | Growth | 1.09 | Yes | Franz et al. 2008 |
| Anabaena flos-aquae | EC10 (96 h) | Growth | 0.97 | Yes | Orvos et al. 2002 |
| Pseudokirchneriella subcapitata | EC25 (96 h) | Growth | 2.44 | Yes | Orvos et al. 2002 |
| Pseudokirchneriella subcapitata | MATC (72 h) | Growth | 0.28 | No | Yang et al. 2008 |
| Pseudokirchneriella subcapitata | NOEC (72 h) | Growth | 0.53 | No | Tamura et al. 2012 |
| Navicula pelliculosa | EC25 (96 h) | Growth | 10.7 | Yes | Orvos et al. 2002 |
| Nitzschia palea | EC10 (72 h) | Photosynthetic activity | 194 | Yes | Franz et al. 2008 |
| Closterium ehrenbergii | MATC (96 h) | Growth | 354 | Yes | Ciniglia et al. 2005 |
| Lemna gibba | MATC (7 d) | Growth | 28 | Yesb | Study Submission 2013 |
| Lemna gibba | MATC (7 d) | Growth | 17 | Yesb | Fulton et al. 2009 |
Table Notes
Abbreviations: conc.. concentration; ECx, the concentration of a substance that is estimated to cause some effect on x% of the test organisms; LCx, the concentration of a substance that is estimated to be lethal to x% of the test organisms; LOEC, lowest-observed-effect concentration; MATC, maximum allowable toxicant concentration, generally presented as the range between NOEC and LOEC or as the geometric mean of the two measures; NOEC, no-observed-effect concentration; SSD, species sensitivity distribution.
a All data in this table are from reliable studies as evaluated with Robust Study Summaries, which are available upon request from Environment and Climate Change Canada.
b Since these endpoints are equivalent and are for the same species, these values were used to calculate a geometric mean for these species (22 µg/L for L. gibba and 39 µg/L for C. dubia), which were used in the SSD.
| Organism | Endpoint (duration) | Effect | Conc. (µg/L) | Used in SSD | Reference |
|---|---|---|---|---|---|
| Hyalella azteca | LC10 (10 d) | Survival | 5 | Yes | Dussault et al. 2008 |
| Hyalella azteca | EC10 (10 d) | Growth | 50 | No | Dussault et al. 2008 |
| Ceriodaphnia dubia | MATC (7 d) | Reproduction | 8.5 | Yesb | Orvos et al. 2002 |
| Ceriodaphnia dubia | MATC (7 d) | Survival and reproduction | 177 | Yesb | Tatarazako et al. 2004 |
| Ceriodaphnia dubia | NOEC (8 d) | Survival and reproduction | 30 | No | Tamura et al. 2012 |
| Daphnia magna | NOEC (21 d) | Survival of parental generation | 200 | No | Orvos et al. 2002 |
| Daphnia magna | MATC (21 d) | Reproduction | 89 | Yes | Orvos et al. 2002 |
Table Notes
Abbreviations: conc.. concentration; ECx, the concentration of a substance that is estimated to cause some effect on x% of the test organisms; LCx, the concentration of a substance that is estimated to be lethal to x% of the test organisms; LOEC, lowest-observed-effect concentration; MATC, maximum allowable toxicant concentration, generally presented as the range between NOEC and LOEC or as the geometric mean of the two measures; NOEC, no-observed-effect concentration; SSD, species sensitivity distribution.
a All data in this table are from reliable studies as evaluated with Robust Study Summaries, which are available upon request from Environment and Climate Change Canada.
b Since these endpoints are equivalent and are for the same species, these values were used to calculate a geometric mean for these species (22 µg/L for L. gibba and 39 µg/L for C. dubia), which were used in the SSD.
| Organism | Endpoint (duration) | Effect | Conc. (µg/L) | Used in SSD | Reference |
|---|---|---|---|---|---|
| Chironomus dilutus | LC10 (10 d) | Survival | 20 | Yes | Dussault et al. 2008 |
| Chironomus dilutus | EC10 (10 d) | Growth | 80 | No | Dussault et al. 2008 |
Table Notes
Abbreviations: conc.. concentration; ECx, the concentration of a substance that is estimated to cause some effect on x% of the test organisms; LCx, the concentration of a substance that is estimated to be lethal to x% of the test organisms; LOEC, lowest-observed-effect concentration; MATC, maximum allowable toxicant concentration, generally presented as the range between NOEC and LOEC or as the geometric mean of the two measures; NOEC, no-observed-effect concentration; SSD, species sensitivity distribution.
a All data in this table are from reliable studies as evaluated with Robust Study Summaries, which are available upon request from Environment and Climate Change Canada.
| Organism | Endpoint (duration) | Effect | Conc. (µg/L) | Used in SSD | Reference |
|---|---|---|---|---|---|
| Physa acuta | MATC (42 d) | Growth | 3.2 | Yes | Brown et al. 2012 |
Table Notes
Abbreviations: conc.. concentration; ECx, the concentration of a substance that is estimated to cause some effect on x% of the test organisms; LCx, the concentration of a substance that is estimated to be lethal to x% of the test organisms; LOEC, lowest-observed-effect concentration; MATC, maximum allowable toxicant concentration, generally presented as the range between NOEC and LOEC or as the geometric mean of the two measures; NOEC, no-observed-effect concentration; SSD, species sensitivity distribution.
a All data in this table are from reliable studies as evaluated with Robust Study Summaries, which are available upon request from Environment and Climate Change Canada.
| Organism | Endpoint (duration) | Effect | Conc. (µg/L) | Used in SSD | Reference |
|---|---|---|---|---|---|
| African clawed frog (Xenopus laevis) | NOEC (32 d) | Growth and postembryonic development | greater than 29.6 | Yes | Fort et al. 2011 |
| African clawed frog (Xenopus laevis) | NOEC (14 d) | Growth and endocrine biomarkers | greater than 200 | No | Matsumura et al. 2005 |
| Bullfrog (Rana catesbeiana) | NOEC (18 d) | Growth and postembryonic development | greater than 11.2 | Yes | Veldhoen et al. 2006 |
| Bullfrog (Rana catesbeiana) | LOEC (6 d) | Gene expression | 0.12 | No | Veldhoen et al. 2006 |
| Pacific tree frog (Pseudacris regilla) | MATC (21 d) | Postembryonic development | 0.95 | Yes | Marlatt et al. 2013 |
Table Notes
Abbreviations: conc.. concentration; ECx, the concentration of a substance that is estimated to cause some effect on x% of the test organisms; LCx, the concentration of a substance that is estimated to be lethal to x% of the test organisms; LOEC, lowest-observed-effect concentration; MATC, maximum allowable toxicant concentration, generally presented as the range between NOEC and LOEC or as the geometric mean of the two measures; NOEC, no-observed-effect concentration; SSD, species sensitivity distribution.
a All data in this table are from reliable studies as evaluated with Robust Study Summaries, which are available upon request from Environment and Climate Change Canada.
| Organism | Endpoint (duration) | Effect | Conc. (µg/L) | Used in SSD | Reference |
|---|---|---|---|---|---|
| Rainbow trout (Oncorhynchus mykiss) | MATC (61 d) | Fry survival | 49.3 | Yes | Orvos et al. 2002 |
| Mosquitofish (Gambusia affinis) | MATC (35 d) | Sperm count | 76.6 | Yes | Raut and Angus 2010 |
| Fathead minnow (Pimephales promelas) | NOEC (21 d) | Growth | greater than 0.450 | No | Schultz et al. 2012 |
| Japanese medaka (Oryzias latipes) | NOEC (21 d) | Fecundity, fertility | greater than 137 | Yes | Ishibashi et al. 2004 |
Table Notes
Abbreviations: conc.. concentration; ECx, the concentration of a substance that is estimated to cause some effect on x% of the test organisms; LCx, the concentration of a substance that is estimated to be lethal to x% of the test organisms; LOEC, lowest-observed-effect concentration; MATC, maximum allowable toxicant concentration, generally presented as the range between NOEC and LOEC or as the geometric mean of the two measures; NOEC, no-observed-effect concentration; SSD, species sensitivity distribution.
a All data in this table are from reliable studies as evaluated with Robust Study Summaries, which are available upon request from Environment and Climate Change Canada.
4.4.2.1.2 Invertebrates
Regarding freshwater crustaceans, Orvos et al. (2002) measured acute and chronic toxic effects for daphnids at 390 μg/L and 89 μg/L, respectively. Their results for the reproduction of Ceriodaphnia dubia indicate a MATC of 8.5 μg/L (Table 4-12), while Tatarazako et al. (2004) obtained a MATC for the reproduction of the same species of 177 µg/L of triclosan. Flaherty and Dodson (2005) observed that Daphniamagna exposed to 10 μg/L of triclosan on a chronic basis produced more than twice as many male individuals as their control counterparts. However, when Daphnia was exposed to triclosan in a mixture of pharmaceuticals, there was a decrease in sex ratio, with 20% fewer male offspring. No endpoints could be calculated for the triclosan-only experiment because only one concentration was tested.
Borgmann et al. (2007) tested the effects of a mixture of pharmaceuticals, including triclosan, on the freshwater amphipod Hyalella azteca. Survival, mating, body size and reproduction were monitored over three generations. No effects were observed on any of the endpoints measured. The mean measured concentration of triclosan over the experiment was 127 ng/L. Dussault et al. (2008) conducted a chronic toxicity test with this amphipod and obtained LC10 and EC10 values of 5 µg/L and 50 μg/L for survival and growth, respectively. The same authors also tested larvae of the aquatic dipteran Chironomus dilutus and obtained similar LC10 and EC10 values (20 and 80 μg/L). Even though Hyalella and Chironomus are benthic organisms, the tests mentioned above were conducted using spiked water only (and not spiked sediments).
Triclosan was found to have genotoxic and cytotoxic effects in vivo in hemocytes of the freshwater zebra mussel (Dreissena polymorpha). Several biomarkers were assessed over a 96-hour exposure period. Significant increases in all genetic biomarkers (e.g., micronucleus test, apoptotic frequency) as well as a clear destabilization of lysosomal membranes were observed following exposure to triclosan at 290-870 ng/L (Binelli et al. 2009). Brown et al. (2012) exposed the freshwater snail Physa acuta to various concentrations of triclosan for 42 days. Snails exposed to 5 μg/L of triclosan and higher had decreased growth rate compared to control.
4.4.2.1.3 Amphibians
Fraker and Smith (2004) observed a decreased activity in Rana pipiens tadpoles exposed to triclosan for 24 days at concentrations of 0.230 µg/L and higher though not in a dose-response manner. Survival of tadpoles was significantly lower when exposed to 230 µg/L but it was similar to controls at 23 µg/L and below. In contrast, Smith and Burgett (2005) observed an increased activity in Bufo americanus tadpoles exposed to triclosan at 230 µg/L for 14 days. They did not observe any effect on growth or survival at the highest concentration tested (230 µg/L). However, exposure concentrations in both studies are uncertain since they were not verified analytically and the test medium was only renewed on a weekly basis. For these reasons, it is not possible to determine meaningful endpoints for growth and survival from these studies.
Based on acute LC50 values, Palenske et al. (2010) concluded that amphibian larvae were most sensitive to triclosan during early developmental stages. The study was conducted on one larval stage of three North American species, Acris crepitans blanchardii, Bufo woodhousii woodhousii and Rana sphenocephala, and on four larval stages of the African clawed frog, Xenopus laevis. The 96-hour LC50 values for these species were 367, 152, 562 and 259-664 µg/L (for four stages of X. laevis), respectively. There was a significant difference between the LC50 values for the North American species, and there was a significant difference between the LC50 values for the earlier versus later larval stages of X. laevis. Metabolic rate and heart rate in amphibian larvae were also monitored and seemed to be affected at various triclosan concentrations, but not in a clear dose-dependent manner.
Matsumura et al. (2005) showed no significant effect on growth and no significant difference in the levels of endocrine biomarkers such as plasma vitellogenin and testosterone in male adult clawed frogs exposed to 20-200 μg/L of triclosan in a 14-day waterborne exposure test.
Studies have also been conducted to assess the influence of triclosan on thyroid hormone-mediated metamorphosis in frogs. Veldhoen et al. (2006) studied the effects of triclosan on precocious metamorphosis in bullfrog (Rana catesbeiana) tadpoles. Premetamorphic tadpoles were either not injected or injected with T3 to induce metamorphosis and were exposed to measured triclosan concentrations of 0.12-11.2 µg/L for 18 days. A reduction in body weight was observed after 18 days for the frogs exposed to 0.12 µg/L triclosan with T3, but not in the frogs exposed to higher concentrations or to concentrations of triclosan alone. Snout-vent length (SVL) and tail length were not significantly affected in any of the triclosan treatment exposures. The development of tadpoles, based on differences in developmental stages as defined by Nieuwkoop and Faber (1994), was advanced in all T3/triclosan exposures but not in triclosan-only exposures. Although R. catesbeiana is not the species used in standardized protocols for testing amphibian metamorphosis, this species is native to eastern Canada. Using a X. laevis cell line, the same authors reported that exposure to low levels of triclosan (30-300 ng/L) resulted in altered (i.e., increased) TRα and TRβ mRNA expression. An increase in TRβ transcript levels may be indicative of advanced metamorphosis.
In contrast, Fort et al. (2010, 2011) concluded that triclosan does not alter the normal course of metamorphosis of X. laevis. In a 21-day test where prometamorphic tadpoles (NF stage 51) were exposed to triclosan concentrations of 0.6, 1.5, 7.2 and 32.2 µg/L, Fort et al. (2010) observed that larval growth (i.e., whole body length and weight, snout-vent and hind limb length) was reduced at 1.5 µg/L, but not at the other treatment levels. Based on developmental stages, the postembryonic development of X. laevis was advanced, although not in a dose-related manner. Indeed, a significant induction in TRβ mRNA expression occurred in the 1.5 and 7.2 µg/L treatments only. Such a lack of a dose-response relationship is not unusual. For instance, in recent studies conducted with chemicals that are known to alter endocrine function (reviewed in Welshons et al. 2003), the effects observed were not necessarily manifested following a linear dose-response relationship and, in several instances, were found to follow a non-monotonic response curve. In a similar 32-day test, NF stage 47 X. laevis (premetamorphic) tadpoles were exposed to triclosan concentrations of 0.3, 1.3, 5.9 and 29.6 µg/L (Fort et al. 2011). Effects on growth endpoints such as a significant increase in mean whole body length and weight as well as SVL were observed at concentrations of 0.3 µg/L and 1.3 µg/L, respectively. Such effects are not necessarily detrimental from an ecological perspective. Contrary to the 21-day study, the postembryonic development of X. laevis was delayed in the treatment groups when compared with the control, but no statistical significance was detected. Although minimal, occurrences of thyroid gland hypertrophy and congestion were noted in all treatment levels, with the number of cases increasing with exposure concentration. Thyroid histology (e.g., follicle count, follicle size, colloid content/follicle) was not significantly different from that of control; however, the variability among individuals was high in the highest treatment levels for some parameters. Finally, TRβ mRNA expression was not significantly affected at any of the concentrations tested in this 32-day test. The authors of these two studies concluded that triclosan seems capable of increasing tadpole growth during their development, but not advancing thyroid-mediated metamorphosis (Fort et al. 2011). The authors suggested that increased growth was due to non-thyroidal mechanisms, such as reduced bacterial stressors in culture. It can be noted that in this study, the tadpoles were not injected with thyroid hormones (like T3 or T4) to induce metamorphosis as was done in the study by Veldhoen et al. (2006).
Marlatt et al. (2013) observed disrupted coordination of postembryonic tadpole development in the Pacific tree frog (Pseudacris regilla) in a 21-day adapted Amphibian Metamorphosis Assay (AMA). AMA is a standard test guideline (OECD TG 231) that was developed for X. laevis and that is designed to identify chemicals that disrupt thyroid hormone-mediated biological processes. In this study, the test protocol was modified and applied to a frog species that is relevant to North America, Pseudacris regilla. Prematamorphic tadpoles were injected or not with T4 and were exposed for 21 days to nominal concentrations of triclosan of 0.3, 3 and 30 µg/L. Significant effects of triclosan on progression of developmental stage and on morphological endpoints such as body length, hindlimb length, snout-vent ratio and wet weight were observed at various days during the experiment, for both the triclosan-only test concentrations and the combined T4/triclosan test concentrations. In addition, the expression of thyroid-hormone-responsive genes was altered at all combination of exposure concentrations, especially at the beginning of the exposure period (day 2). Some of these effects were transient, yet necessary to metamorphosis, according to the authors. They suggested that triclosan was responsible for uncoupling in the timing and progression of tadpole tissues (acceleration). It can be noted that mean mortality was up to 17% in the tadpoles exposed to T4 only or to a combination of T4 and 0.3 µg/L of triclosan; this rate is higher than the 10% limit recommended in the test guideline. Triclosan did not seem to be the cause of the mortality. Based on the endpoints identified in the OECD TG 231 as indicators of thyroid activity, a MATC of 0.95 µg/L was determined for this study based on significant effects of triclosan (in absence of T4; in line with OECD TG 231) on hindlimb length/SVL at day 7. Developmental stage was not affected by triclosan-only exposures at days 7 or 21.
Overall, these studies do not demonstrate a consistent effect of triclosan on thyroid-mediated amphibian metamorphosis. However, they demonstrated effects on developmental stage, certain morphological endpoints and gene expression. It seems like triclosan alone does not significantly alter thyroid-mediated processes, however it seems to alter these processes when metamorphosis is induced by T4 and T3 hormones. These effects suggest that triclosan may interfere with the action of natural thyroid hormone in amphibians. As mentioned by Marlatt et al. (2013), altered development may translate into decrease fitness for amphibians; however, long-term exposure to triclosan would need to be tested to evaluate development through complete metamorphosis.
4.4.2.1.4 Fish
Orvos et al. (2002) determined acute toxicity (96-hour LC50) values of 260 µg/L and 370 μg/L of triclosan for the fathead minnow and bluegill sunfish, respectively. For chronic toxicity, they measured a no-observed-effect concentration (NOEC) and lowest-observed-effect concentration (LOEC) of 34.1 µg/L and 71.3 μg/L, respectively, for the rainbow trout in an early life cycle test. An acute study (Oliveira et al. 2009) concluded that triclosan had deleterious effects on adult and early life stages of zebrafish (Danio rerio). Effects included embryotoxicity and hatching delay. The authors attributed the high embryo mortality to the incorporation of triclosan into the eggs. The 96-hour LC50 value for embryo survival was 420 µg/L. Embryotoxicological effects, such as spine malformation and reduced size, were observed after 4 days of exposure to 500 µg/L of triclosan.
In a study conducted on male western mosquitofish (Gambusia affinis), Raut and Angus (2010) observed a significant increase in normally female-limited vitellogenin mRNA expression at a triclosan treatment of 101 µg/L. In this study, which suggested that triclosan has the potential to act as an endocrine disruptor in male mosquitofish, it was also found that triclosan both decreased sperm counts and increased the mean hepatosomatic index at 101 µg/L. Other concentrations tested were 29 and 58 µg/L. Decreased sperm counts could have an impact at the population level; hence, it is considered an ecologically relevant endpoint.
A few published studies (Tamura et al. 2012, Tatarazako et al. 2004, Ishibashi et al. 2004) were conducted following the OECD 212 test guideline "Fish, Short-term Toxicity Test on Embryo and Sac-fry Stages". These studies were not considered to assess the effects of triclosan on fish growth and reproduction since an OECD report recommended that this test is no longer scientifically valid because the time to start feeding is too late and the test is considered relatively insensitive (OECD 2012). It is also considered as a sub-chronic test rather than a true, life-cycle, chronic test for fish. That being said, some findings are still relevant to note. In particular, Ishibashi et al. (2004) observed that gonadosomatic and hepatosomatic indices were significantly higher in adult Japanese medaka (Oryzias latipes) exposed to concentrations of 20 μg/L and higher. Also, concentrations of hepatic vitellogenin were increased significantly in males exposed to 20 and 100 μg/L.
Investigations by Foran et al. (2000) of possible estrogenic properties of triclosan on Japanese medaka (Oryzias latipes) indicated that this substance does not display estrogenic activity at levels ranging from 1 to 100 µg/L. However, based on the evaluation of changes in secondary sexual characteristics (slight increase in dorsal and anal fins in the high treatment group), these authors suggested that triclosan is potentially weakly androgenic. The observed effects could also have been induced by an anti-estrogenic MOA.
4.4.2.1.5 Species sensitivity distribution
A species sensitivity distribution (SSD) was developed to identify the critical toxicity value (CTV) for triclosan according to the 2007 guidance protocol provided by the Canadian Council of Ministers of the Environment (CCME) (CCME 2007). The CCME guidance (2007) indicates that toxicity endpoints obtained through regression-based statistical data evaluation (i.e., ECx values identifying no- or low-effects thresholds) are preferred over endpoints obtained through hypothesis-based statistical data evaluation (i.e., NOEC and LOEC values). In addition, endpoints representing no-effects thresholds for a given species are preferred over endpoints representing low-effects thresholds, when available. Given these two aspects (i.e., data evaluation technique and effects threshold), acceptable endpoints were considered using the following tiered approach: most appropriate ECx/ICx representing a no-effects threshold greater than EC10/IC10 greater than EC11-25/IC11-25 greater than MATC greater than NOEC greater than LOEC greater than EC26-49/IC26-49 greater than non-lethal EC50/IC50 (CCME 2007). Robust study summariesFootnote4 were completed for all the endpoints included in the SSD to ensure that they came from reliable studies. Only chronic toxicity data were chosen to derive the SSD, given that chronic exposure to triclosan is expected in the receiving ecosystems. The SSD comprises endpoints for three fish, three amphibian, five invertebrate, one macrophyte and seven algal species; the resulting distribution is shown in Figure 4-3. When more than one endpoint was available for a single species, the most preferred endpoint according to the CCME guidance (2007) was chosen. If multiple similar endpoints were available, the lowest value was chosen, or the geometric mean of these endpoints was calculated if these endpoints were deemed the same (e.g., effect, duration).
Several of the data mentioned above were not used in the derivation of the SSD for reasons other than not being the preferred endpoint. The toxicity values for the algae Skeletonema costatum and Dunaliella tertiolecta were not considered for the SSD because they are marine species and the exposure data available are for fresh water. The toxicity value for Hyalella azteca from Borgmann et al. (2007) was not used, as this test was conducted with a mixture of substances. The EC10 values for growth inhibition of Hyalella azteca and of Chironomus dilutus from Dussault et al. (2008) were not used in the SSD since lethality (LC10) occurred at lower concentrations for these organisms in this study. This means that amphipods and chironomids that survived exposure to triclosan were able to grow well up to their respective EC10. The fathead minnow study (Schultz et al. 2012) was also not included in the SSD since it provided a very low unbounded NOEC (i.e., two orders of magnitude lower than NOEC for other fish species). This NOEC is not considered toxicologically meaningful, as concentrations of triclosan tested were most likely too low. For amphibians, the endpoints relevant to population dynamics (e.g., growth and development) were used in the SSD. Two of those endpoints were unbounded NOECs (i.e., "greater than" values); nonetheless, they were included in the SSD as they did not over-estimate toxicity, and were based on relatively high test concentrations.
Endpoints based on biochemical responses (e.g., gene expression) available for amphibians and molluscs were not used in the SSD because they are difficult to relate to and to evaluate impacts on population dynamics. Although they were excluded from the SSD, they were still used as a valuable line of evidence to characterize the ecological effects of triclosan.
The values chosen for the SSD were not adjusted for the pH to reflect the ionizing potential of triclosan in the environment (see section 4.4.2). This is because, such an adjustment could only be done if the relationship between the pH and the degree of toxicity of triclosan was known, or if it were assumed to be linear. Assumption of linearity is a gross simplification of the physical-chemical processes that can take place, and in effect, would lead to a great uncertainty with the calculated results.
The software SSD Master Version 3.0 (CCME 2013) was used to plot the SSD. Several cumulative distribution functions (normal, logistic, extreme value, Gumbell, and Weibull) were fit to the data using regression methods. Model fit was assessed using statistical and graphical techniques. The best model was selected based on consideration of goodness of fit and model feasibility. Model assumptions were verified graphically and with statistical tests. The normal model was selected (Anderson-Darling Statistic [A2] for goodness of fit = 0.242), and the 5th percentile (HC5, i.e., hazardous concentration to 5% of species) of the SSD plot is 376 ng/L, with lower and upper confidence limits of 263 and 538 ng/L, respectively. Figure 4-3 shows the plot for the triclosan SSD.
Long description for figure 4-3
Species sensitivity distribution (SSD) for triclosan based on selected chronic toxicity data for freshwater aquatic organisms. The normal model fit to the data is shown on the graph along with the 95% confidence intervals. The data used in the SSD are identified in Table 4-12. This figure shows that the sensitivity of organisms to triclosan is distributed following an S-shaped curve. The most sensitive organisms to triclosan are some plants, amphibians and invertebrates. Fish and other types of invertebrates and plants are less sensitive. Of the 19 data points, 3 points fall outside the 95% confidence intervals of the curve fit. They are located at both extremities of the fit.
It is noted that numerous SSDs for triclosan using endpoints for aquatic species were developed by other authors (Capdevielle et al. 2008, Lyndall et al. 2010, Belanger et al. 2013) using approaches other than the CCME guideline (2007). The HC5 values obtained in these studies range from 534 to 1550 ng/L. Differences in the HC5 values can be attributed to the selection of endpoints and species as well as different data-fitting approaches.
4.4.2.1.6 Derivation of the predicted no-effect concentration (PNEC) for aquatic species
The 5th percentile value (HC5) of 376 ng/L calculated from the SSD for aquatic freshwater species was selected as the critical toxicity value (CTV) for triclosan. This value is below the lowest endpoint value used in the SSD (500 ng/L for Scenedesmus subspicatus).
Since the CTV was based on no- or low-effect chronic SSD that included toxicity endpoints for numerous species, an assessment factor (AF) of 1 was used to derive a predicted no-effect concentration (PNEC). Consequently, the value of 376 ng/L was chosen as the PNEC in the risk analysis of triclosan (see section 4.5.1). It is noted that the SSD includes endpoints that may be susceptible to endocrine influence, including effects on growth and reproduction in fish and amphibians. Therefore, the PNEC is expected to be encompassing of endocrine disrupting effects.
The aim of the approach taken for the derivation of the PNEC for triclosan was to capture inter-species variation in sensitivity. It is considered that the PNEC of triclosan reflects accurately the observed no- to low-effect levels, and is not overly conservative.
4.4.2.1.7 Methyl-triclosan
A study conducted to assess the toxicity of methyl-triclosan to Daphnia magna indicates that the 48-hour NOEC for immobilization is greater than or equal to 180 µg/L. In another study, the 72-hour EC50 values for biomass and growth rate for the alga Scenedesmus subspicatus were 120 µg/L and 170 µg/L, respectively. The corresponding EC10 values were 55 µg/L and 76 µg/L, respectively (Study Submissions 2009). These results suggest that methyl-triclosan is less toxic to aquatic organisms than triclosan, but is nonetheless of high inherent toxicity.
4.4.2.2 Benthic organisms
The toxicity of triclosan to benthic organisms was assessed using a test with chironomids (Chironomus riparius) in accordance with OECD test guideline 218. After 28 days, no adverse effects were observed on the emergence ratio or development rate at any of the concentrations tested (Study Submissions 2009). Based on these results, the NOEC for triclosan is greater than or equal to 100 mg/kg dw, the highest concentration tested. The concentrations of triclosan in sediments were measured in the control, middle and highest treatment levels and were constant throughout the test. The concentrations of triclosan residues in the overlying water column were very low throughout the test period (less than 1% of applied radiolabelled triclosan). Similarly, very low amounts of radioactivity were measured in the pore water samples (0.1% of applied radioactivity). This indicates that triclosan was mainly bound to the sediment, but most of this fraction was extractable.
There are differences in the observed triclosan binding with sediments. Results from the aerobic aquatic metabolism study described in Section 4.2.4.2, indicate that about one third of the triclosan that was bound to sediment by study termination (104 days) was not extractable. The differences between the metabolism study and the chironomid toxicity study may result from differences in protocols used in each study, study durations or different types of sediments. The sediments used in the toxicity study were mainly composed of sand silica, a substrate that has a low adsorption capacity.
4.4.2.3 Terrestrial organisms
Effects data for triclosan in several terrestrial organisms including microorganisms, plants, invertebrates, birds and small mammals were available. A summary of toxicity data for low level, chronic effects of triclosan on terrestrial organisms is presented in Table 4-13. Soil invertebrates were most sensitive to triclosan exposure followed by dicotyledonous plants (e.g., tomato, lettuce, soybean, and cucumber) and monocotyledonous plants (e.g., garlic chive, corn, wheat, rice) (Wang et al. 2015). The PNEC for the soil compartment was calculated based on a critical toxicity value (the most sensitive, reliable endpoint) divided by an assessment factor (see subsection 4.4.2.3.5).
Table 4-13. Toxicity of triclosan to terrestrial organisms
| Organism | Endpoint (duration) | Effect | Conc. (mg/kg dw) | Reference |
|---|---|---|---|---|
| Soil microorganisms | NOEC (1 h to 28 d) | Respiration, nitrification, phosphatase, glucosidase, chitinase | 1 | Waller and Kookana 2009 |
Table Notes
Abbreviations: conc., concentration; ECx, the concentration of a substance that is estimated to cause some effect on x% of the test organisms; LCx, the concentration of a substance that is estimated to be lethal to x% of the test organisms; LDx, the dose of a substance that is estimated to be lethal to x% of the test organisms; LOEC, lowest-observed-effect concentration; NOAEL, no-observed-adverse-effect level; NOEC, no-observed-effect concentration; LOAEL, lowest-observed-adverse-effect level.
| Organism | Endpoint (duration) | Effect | Conc. (mg/kg dw) | Reference |
|---|---|---|---|---|
| Ryegrass (Lolium perenne)a | NOEC (21 d) | Root weight | 0.162 | Study Submissions 2009b |
| Corn (Zea mays)a | NOEC (21 d) | Root length; Shoot length; Fresh weight |
30; 60; 60 |
Wang et al. 2015c |
| Corn (Zea mays)a | EC10 (21 d) | Root length; Shoot length; Fresh weight |
26; 52; 41 |
Wang et al. 2015c |
| Garlic chives (Allium tuberosum)a | NOEC (21 d) | Root length; Shoot length; Fresh weight |
40; 40; 40 |
Wang et al. 2015c |
| Garlic chives (Allium tuberosum)a | EC10 (21 d) | Root length; Shoot length; Fresh weight |
40; 33; 25 |
Wang et al. 2015c |
| Wheat (Triticum aestivum)a | NOEC (21 d) | Shoot weight | 0.162 | Study Submissions 2009b |
| Wheat (Triticum aestivum)a | EC10 (21 d) | Survival | 142 | Amorim et al. 2010c |
| Rice (Oryza sativa)a | NOEC (20 d) | Root length; Shoot height |
1; 70 |
Liu et al. 2009c |
| Rice (Oryza sativa)a | EC10 (20 d) | Root length; Shoot height |
27; 37 |
Liu et al. 2009c |
| Soybean (Glycine max)d | NOEC (21 d) | Root length; Shoot length; Fresh weight |
20; 20; 10 |
Wang et al. 2015c |
| Soybean (Glycine max)d | EC10 (21 d) | Root length; Shoot length; Fresh weight |
20; 29; 22 |
Wang et al. 2015c |
| Field mustard (Brassica rapa)d | EC10 (21 d) | Survival | 3 | Amorim et al. 2010c |
| Lettuce (Lactuca sativa)d | NOEC (21 d) | Root length; Shoot length; Fresh weight |
8 | Wang et al. 2015c |
| Lettuce (Lactuca sativa)d | EC10 (21 d) | Root length; Shoot length; Fresh weight |
4; 14; 6 |
Wang et al. 2015c |
| Cucumber (Cucumis sativus)d | NOEC (21 d) | Shoot length | 0.065 | Study Submissions 2009b |
| Cucumber (Cucumis sativus)d | NOEC (20 d) | Root length; Shoot height |
10; 10 |
Liu et al. 2009c |
| Cucumber (Cucumis sativus)d | EC10 (20 d) | Root length; Shoot height |
17; 6 |
Liu et al. 2009c |
| Tomato (Solanum lycopersicum)d | NOEC (21 d) | Root and shoot weight | 0.162 | Study Submissions 2009b |
| Tomato (Solanum lycopersicum)d | NOEC (21 d) | Root length; Shoot length; Fresh weight |
8; 8; 8 |
Wang et al. 2015c |
| Tomato (Solanum lycopersicum)d | EC10 (21 d) | Root length; Shoot length; Fresh weight |
11; 14; 9 |
Wang et al. 2015c |
Table Notes
Abbreviations: conc., concentration; ECx, the concentration of a substance that is estimated to cause some effect on x% of the test organisms; LCx, the concentration of a substance that is estimated to be lethal to x% of the test organisms; LDx, the dose of a substance that is estimated to be lethal to x% of the test organisms; LOEC, lowest-observed-effect concentration; NOAEL, no-observed-adverse-effect level; NOEC, no-observed-effect concentration; LOAEL, lowest-observed-adverse-effect level.
a Monocotyledonous plants.
b Based on time-weighted mean measured concentrations.
c Based on nominal concentrations.
d Dicotyledonous plants.
| Organism | Endpoint (duration) | Effect | Conc. (mg/kg dw) | Reference |
|---|---|---|---|---|
| Earthworm (Eisenia fetida) | NOEC (56 d) | Reproduction; Survival | 2; greater than 64 |
Wang et al. 2015c |
| Earthworm (Eisenia fetida) | EC10 (56 d) | Reproduction | 1.05 | Wang et al. 2015c |
| Earthworm (Eisenia fetida) | NOEC (14 d) | Survival | greater than 1026 | Reiss et al. 2009 |
| Earthworm (Eisenia fetida) | NOEC (56 d) | Reproduction | 10 | Lin et al. 2014c |
| Earthworm (Eiseniafetida) | LOEC (56 d) | Reproduction | 50 | Lin et al. 2014c |
| Tiger worm (Eisenia andrei) | NOEC (14 d) | Survival | 32 | Amorim et al. 2010c |
| Tiger worm (Eisenia andrei) | EC10 (56 d) | Reproduction | 0.6e | Amorim et al. 2010c |
| White worm (Enchytraeus albidus) | NOEC (42 d) | Reproduction; Survival |
3.2 | Amorim et al. 2010c |
| Collembolan (Folsomia candida) | NOEC (28 d) | Reproduction; Survival |
3.2; greater than or equal to 320 |
Amorim et al. 2010c |
| Terrestrial snail(Achatina fulica) | NOEC (28 d) | Inhibition of food intake; growth of biomass; growth of shell diameter | 24 | Wang et al. 2014 |
| Terrestrial snail(Achatina fulica) | NOEC (28 d) | Survival | 200 | Wang et al. 2014 |
Table Notes
Abbreviations: conc., concentration; ECx, the concentration of a substance that is estimated to cause some effect on x% of the test organisms; LCx, the concentration of a substance that is estimated to be lethal to x% of the test organisms; LDx, the dose of a substance that is estimated to be lethal to x% of the test organisms; LOEC, lowest-observed-effect concentration; NOAEL, no-observed-adverse-effect level; NOEC, no-observed-effect concentration; LOAEL, lowest-observed-adverse-effect level.
c Based on nominal concentrations.
e EC10 = 0.6 mg/kg was chosen as the critical toxicity value (CTV) in derivation of the predicted no effect concentration (PNEC) for the soil compartment.
| Organism | Endpoint (duration) | Effect | Conc. (mg/kg dw) | Reference |
|---|---|---|---|---|
| Mallard duck (Anas platyrhynchos) | LD50 (14 d) (acute oral) | Survival | greater than or equal to 2150 | US EPA 2008f |
| Bobwhite quail (Colinus virginianus) | LD50 (14 d) (acute oral) | Survival | 825 | US EPA 2008f |
| Bobwhite quail (Colinus virginianus) | LD50 (8 d) (dietary) | Survival | greater than 5000 | US EPA 2008f |
Table Notes
Abbreviations: conc., concentration; ECx, the concentration of a substance that is estimated to cause some effect on x% of the test organisms; LCx, the concentration of a substance that is estimated to be lethal to x% of the test organisms; LDx, the dose of a substance that is estimated to be lethal to x% of the test organisms; LOEC, lowest-observed-effect concentration; NOAEL, no-observed-adverse-effect level; NOEC, no-observed-effect concentration; LOAEL, lowest-observed-adverse-effect level.
| Organism | Endpoint (duration) | Effect | Conc. (mg/kg dw) | Reference |
|---|---|---|---|---|
| Rat (Rattus norvegicus) | LD50 (acute oral) | Survival | greater than 5000 | NICNAS 2009 |
| Rat (Rattus norvegicus) | NOAEL (90 d) (dietary exposure) |
Survival, reproduction or growth | greater than 433 (males) greater than 555 (females) |
NICNAS 2009 |
| Mouse (Mus musculus) | NOAEL (90 d) (dietary exposure) |
Decrease in body weight gain | 750 | NICNAS 2009 |
| Mouse (Mus musculus) | LOAEL (90 d) (dietary exposure) |
Decrease in body weight gain | 900 | NICNAS 2009 |
Table Notes
Abbreviations: conc., concentration; ECx, the concentration of a substance that is estimated to cause some effect on x% of the test organisms; LCx, the concentration of a substance that is estimated to be lethal to x% of the test organisms; LDx, the dose of a substance that is estimated to be lethal to x% of the test organisms; LOEC, lowest-observed-effect concentration; NOAEL, no-observed-adverse-effect level; NOEC, no-observed-effect concentration; LOAEL, lowest-observed-adverse-effect level.
4.4.2.3.1 Microorganisms
The effect of triclosan on microbial activity was studied by Waller and Kookana (2009) in two types of soils (sandy loam and clay). Substrate-induced respiration and nitrification were decreased at a concentration of 50 mg/kg and 5 mg/kg, respectively. The activities of four enzymes - namely, the acid and alkali phosphatase, β-glucosidase and chitinase - were also measured, but did not seem affected by triclosan, except for the β-glucosidase in the sandy soil. No adverse effects were noted on any of the microbial processes at the lowest concentration tested of 1 mg/kg. In a study by Liu et al. (2009), soil respiration in a paddy soil was inhibited after 22 days of incubation at triclosan concentrations of 10 mg/kg and above. The phosphatase activity seemed to decrease with increasing triclosan concentrations in soil; however, the differences were not significant.
4.4.2.3.2 Plants
Triclosan effects studies were available for several terrestrial plant species. Wang et al. (2015) observed that dicotyledonous plants tend to be more sensitive to triclosan exposure than monocotyledonous plants, based on tests with corn, garlic chives, soybean, tomato and lettuce. Similar observations were made by Liu et al. (2009) and Amorim et al. (2010) in tests with cucumber, rice, field mustard, and wheat. Low level (i.e., NOEC, EC10), chronic effects presented in Wang et al. (2015), Amorim et al. (2010) and Liu et al. (2009) were summarized in Table 4-13b. Observed effects on the root and shoot growth, and plant survival in these studies point to moderate toxicity of triclosan; effects ranged between 3 mg/kg (survival for the dicotyledonous field mustard) to 142 mg/kg (in the monocotyledonous common wheat).
In addition, three unpublished studies, submitted to Environment and Climate Change Canada (Study Submissions 2009), assessed the effects of triclosan on terrestrial plants, and show low toxicity of triclosan to the most sensitive species. In the first study, six plant species (corn, ryegrass, wheat, cucumber, soybean and tomato) were exposed to triclosan in quartz sand at nominal concentrations of 0.01-1 mg/kg dw for 21 days. Cucumber was the most sensitive species, with a measured time-weighted average NOEC of 0.065 mg/kg dw soil for shoot length (Study Submissions 2009). In the second study, the seed germination and seedling growth of cucumber exposed to triclosan in a sandy loam at nominal concentrations of 0.01-1 mg/kg dw were studied over 28 days. No adverse effects were observed at the highest concentration tested, resulting in a time-weighted average NOEC of 0.446 mg/kg dw based on measured concentrations (Study Submissions 2009). In the third study, 10 plant species (corn, ryegrass, wheat, cucumber, soybean, tomato, lettuce, radish, vetch and pea) were exposed for 14 days (post–median control emergence) to triclosan in a sandy loam at nominal concentrations ranging from 0.2 to 1000 mg/kg, following OECD test guideline 208 (Büche et al. 2009). The most sensitive species was lettuce, with NOEC and LOEC values for shoot weights of 50 and 75 mg/kg, respectively, based on nominal concentrations. The NOEC for shoot weight for all other tested species was 1000 mg/kg.
Lastly, the effects of triclosan on seed germination and seedling development of three wetland plants, Sesbania herbacea, Euphorbia prostrata, and Bidens frondosa, were studied by Stevens et al. (2009). These plants are also commonly found in terrestrial habitats. Plants were exposed to triclosan for 28 days at concentrations ranging from 0.0004 to 1 mg/kg in water-only flow-through systems. While germination and shoot weight were not affected at the highest concentration tested, root length was affected at 0.0006 mg/L for two of the species tested. Liu et al. (2009) found that in the plant growth test, the shoot growth was also a less sensitive endpoint than root elongation. Given that plants were tested in water flow-through systems, it is possible that the observed effects thresholds could be different if plants were rooted in soil.
4.4.2.3.3 Invertebrates
Soil invertebrates are sensitive to triclosan exposure. However, length of exposure and soil characteristics such as pH impact the extent of effects. Short and long-term effects studies were conducted for numerous species, including three soil-dwelling worms Eisenia fetida, Eisenia andrei, and Enchytraeus albidus, the collembolan Folsomia candida, and a terrestrial snail Achatina fulica(Reiss et al. 2009; Wang et al. 2014, 2015; Amorim et al. 2010; Lin et al. 2014).
Short-term 14-day exposure to triclosan does not cause mortality in earthworms, E. fetida, at the nominal exposure concentrations of up to 1026 mg/kg (dw soil) (Reiss et al. 2009), rather adverse effects of triclosan have been observed in long-term exposure studies. Wang et al. (2015) measured a 56-day EC10 of 1.05 mg/kg for reproduction in the earthworm, E. fetida. Similarly, a 56-day EC10 of 0.6 mg/kg for reproduction was determined for the tiger worm, Eisenia andrei (Amorim et al. 2010). The white worm, E. albidus, and the collembolan, F. candida, were also tested for effects on reproduction and survival in the Amorim et al. (2010) study. No clear dose–response curves were obtained for these two species; however, it is clear that juveniles were significantly affected at the highest concentration tested (320 mg/kg dw soil) based on a visual inspection of the curves representing results of chronic bioassays.
Lin et al. (2014) tested the effect of triclosan on the reproduction of the earthworm E. fetida exposed to a coastal alkali-saline soil (fine clay soil based on soil composition). The NOEC and LOEC values were 10 and 50 mg/kg, respectively, based on number of juveniles and cocoons produced after 56 days. This is much higher than the EC10 of 0.6 mg/kg obtained by Amorim et al. (2010) for E. Andrei. This difference in toxicity could be due to different sensitivity of the test species but also to the differences in test pH. The pH of the soil used by Amorim et al. (2010) and by Lin et al. (2014) were 5.8 and 8.1, respectively. This means that earthworms in the Amorim et al. (2010) study were likely exposed exclusively to the neutral form of triclosan. This form has been suggested to cause most of the toxicity in two aquatic species (Orvos et al. 2002; Roberts et al. 2014). Another factor to explain the difference in toxicity could be the difference in bioavailability due to binding of triclosan to organic matter. However, the percentage of organic matter in test soils would suggest a lower bioavailability in the soil used by Amorim et al. (2010) (4.4% versus 2.2% in the soil used by Lin et al. (2014)).
Effects of triclosan on the growth of biomass and shell-dimeter, inhibition of food intake and survival were studied in the terrestrial snail A. fulica (Wang et al. 2014). Moderate toxicity of triclosan was observed: the 28-day NOEC for growth, and inhibition of food intake was determined as 24 mg/kg, and the 28-day NOEC for survival was 200 mg/kg (Wang et al. 2014).
In addition, biochemical responses associated with oxidative stress and harmful stress conditions were studied in A. fulica (Wang et al. 2014) and in E. fetida (Lin et al. 2010, 2014), at triclosan exposure concentrations ranging from 1 to 300 mg/kg. Although indicative of specific cellular responses or pathways, these sublethal effects were observed at levels of triclosan unlikely to be reached in soils (i.e., greater than or equal to 12.5 mg/kg).
4.4.2.3.4 Birds and mammals
Based on a limited data set, triclosan seems not toxic to slightly toxic to birds (median lethal dose [LD50] greater than or equal to 2150 mg/kg bw and 825 mg/kg bw for mallard duck and bobwhite quail, respectively) and not toxic to mammals (rat, LD50 greater than 5000 mg/kg bw) on an acute oral basis. Subchronic oral toxicity data indicate a NOAEL of 750 mg/kg bw per day based on decrease in body weight gain observed in mice. Oral toxicity studies were also conducted with dogs and baboons, but the results of these studies were not considered in this assessment due to a number of factors (see Section 3.2.3). There were no indications of adverse effects on thyroid function in mammals (see Section 3.1.10).
4.4.2.3.5 Derivation of the predicted no-effect concentration (PNEC) for terrestrial species
The calculation of a PNEC for the soil compartment is based on the most sensitive acceptable endpoint identified for terrestrial organisms (reproduction in the earthworm E. andrei, EC10 = 0.6 mg/kg; see Table 4-13). Another endpoint (growth in cucumber, 0.065 mg/kg) is lower than the one for earthworms; however, the cucumber study was conducted using quartz sand which, while it has low adsorption capacity and therefore maximizes the bioavailability of triclosan, is not representative of agricultural soils to which biosolids-borne triclosan would be applied. Agyin-Birikorang et al. (2010) measured similar partition coefficients (Kd and Koc) for triclosan in soils and biosolids-amended soils, while these coefficients were higher in biosolids alone. This suggests that toxicity studies conducted with soils spiked with triclosan may adequately simulate the bioavailability of triclosan in biosolids-amended soils. No toxic effects of triclosan in soils amended with biosolids for six crop species were observed, suggesting a low bioavailability of triclosan in these soils (Prosser et al. 2014). The concentrations achieved in Prosser et al. (2014) were much lower than the concentrations tested in laboratory assays, but they were based on realistic agronomic rates. Overall, the EC10 of 0.6 mg/kg for earthworm reproduction selected as the critical toxicity value (CTV) is considered as being a conservative yet realistic endpoint. An assessment factor of 1 was applied since a no-effect, chronic endpoint is used. In addition, the test pH used in this study likely maximized the presence of the neutral form of triclosan in soil, which has proven to be the most toxic form to certain aquatic organisms (most likely due to greater bioaccumulation). The triclosan PNEC for soil is calculated as 0.6 mg/kg.
4.4.3 Relevance of the effects data for triclosan
Effects studies determine the ability a chemical to cause adverse effects in the tested species. Some effects studies can also help elucidate the mechanisms underlying the observed effects, i.e, the modes of action. Ecological effects of triclosan were characterized based on several toxicity data available for numerous species belonging to a variety of taxa. Toxicity of triclosan to the aquatic species was most extensively characterized through numerous studies on algae, invertebrates and vertebrates. Effects studies, particularly in aquatic species, served as one of the key lines of evidence in the risk assessment of triclosan. Predicted no-effect concentration (PNEC) values were determined for the aquatic and terrestrial species based on the available effects data.
There is consistency between the chemical activity, fugacity, and the measured toxicity values, all of which suggest that triclosan is a potent chemical acting through specific modes of action such as receptor mediated interactions. There is some uncertainty regarding the occurrence and the threshold for endocrine disruption in amphibians. Nonetheless, these subtle effects levels have likely been captured in the PNEC for the aquatic compartment, and it is difficult to determine if the current potency thresholds would change with additional new endocrine disrupting data. Effects data are available for only one benthic species and therefore, it is not fully representative of the potential effects in the sediment compartment.
Methylation of triclosan in aerobic soil and in WWTPs, leads to formation of methyl-triclosan. Limited effects data are available for methyl-triclosan; it seems to have a high inherent toxicity to aquatic organisms, and no toxicity data are available for terrestrial organisms. Co-exposure to both triclosan and methyl-triclosan is expected in the environmental media, but such combined effects data are also not available.
4.5 Ecological Exposure and Risk Assessment
Sources of triclosan in the Canadian environment and measured environmental concentrations of triclosan and methyl-triclosan in Canada and other countries were described in section 4.1. The main receiving environmental medium for triclosan is water; triclosan is released to the aquatic ecosystems via effluents from WWTPs. Once in the water column, results from the Multispecies Model (version 1.0; Cahill 2008) indicate that triclosan will to a large extent remain in water (79-91%) and will also partition to sediments (9-21%). Triclosan has been detected in surface water samples collected across Canada, and other countries. Triclosan can also reach soils through spreading of biosolids to agricultural lands. Monitoring data for soil were not available; therefore predicted environmental concentrations were modelled.
The environmental risk assessment includes risk quotients analysis. The risk quotient is an important line of evidence in characterizing the potential of a substance to cause harm to ecosystems. A risk quotient is the ratio of a predicted environmental concentration (PEC) to a toxicity endpoint (predicted no-effect concentration [PNEC]) determined for each medium of concern.
For triclosan, a risk quotient analysis was done for key compartments of concern, namely water and soil. A qualitative risk assessment was done for sediment. Risk from exposure to methyl-triclosan was also evaluated for the aquatic compartment (see sections below).
Risk assessment of triclosan and methyl-triclosan is presented below, based on the environmental compartment.
4.5.1 Water
4.5.1.1 Risk analysis based on measured concentrations of triclosan in surface water
Because measured concentrations of triclosan in surface water are available for numerous water bodies in both densely and lightly populated areas of Canada, and because these concentrations integrate simultaneous fate processes occurring in surface water, these data are considered to provide a realistic representation of levels of triclosan in Canada (Table 4-3). It is recognized however that such measurements often provide only a snapshot of concentrations in time and space. For instance, Price et al. (2010) showed that triclosan concentrations measured over a single month at one site in a river in England varied from 21 to 195 ng/L. This was mainly due to variations in river discharges. The data available for Canada are based on sampling episodes that occurred at different sites on the same water body (except for small water bodies) and at different times over each sampling year. As such, it is believed that these data are representative of levels of triclosan in Canada. Several of the sampling sites are closely associated with WWTPs.
The data available date from 2002 to 2014, and the concentrations reported span almost four orders of magnitude, from below the lowest MDL (0.10 ng/L) to 874 ng/L; median concentrations for each site range from below the MDL to 139 ng/L. Most of the water bodies have relatively low maximum concentrations of triclosan. Five water bodies had maximum concentrations above the PNEC of 376 ng/L (Table 4-3). Recent data (2010 to 2013) collected for three of these five water bodies indicate that the maximum concentration of triclosan in two of them is now below the PNEC. The other water body has a maximum concentration (874 ng/L) much above the PNEC. There are no recent data available for the fourth and fifth water bodies. Data available for these two water bodies are from 2005 and show maximum concentrations of 433 ng/L and 599 ng/L. The water bodies where high concentrations of triclosan were measured are receiving effluent from WWTPs that have secondary treatment or that are lagoons.
The data presented in Table 4-3 are considered representative of conditions occurring across Canada, with no outliers identified. At those sites with high measured concentrations of triclosan, it is likely that there is a low dilution of the WWTP effluent by the receiving watercourse at certain times of the year and/or high inputs of triclosan. As noted in Anger et al. (2013), exposure to triclosan is dynamically linked to the size of the receiving water body and degree of wastewater impact, where a small-scale wastewater-impacted water body tends to be burdened with long-term exposure to the substance at elevated levels. Such variability with respect to the size and flow of the water bodies has been observed for the sampled WWTPs listed in Table 4-3.
Assuming that the data available for concentrations of triclosan in surface water are representative of those for the entire country, for some locations where triclosan is prevalent, i.e., near populated areas across Canada, the PEC values are expected to exceed the PNEC for triclosan.
4.5.1.2 Risk analysis based on quantities in industrial use
A survey conducted under section 71 of CEPA requested information on the manufacture, import, use and release of triclosan for the year 2011 (see Section 2.3.5). Results from this survey indicate that triclosan was not manufactured in Canada in 2011. Twenty-nine companies reported importing between 10 000 and 100 000 kg of triclosan as either the pure substance or in products. Some companies reported using triclosan to manufacture products such as antibacterial hand soap, dentifrice, cleaners, etc. This use of triclosan to manufacture products could result in releases of this substance to WWTPs through industrial wastewater as a result of washing residues from tanks, and spills. Depending on removal efficiency in WWTPs and on dilution in the receiving water bodies, the manufacturing facilities could represent sources of triclosan to the environment.
To assess whether these potential sources may be of ecological concern, locations of WWTPs for which data on measured concentrations of triclosan in influents are available (Table 4-1) were matched with locations of those facilities that reported the top quantities (greater than 400 kg) for use of triclosan to manufacture products in 2011. Of these facilities, three could be matched with a WWTP having measured data. These three manufacturing facilities together used about 40% of the total quantity of triclosan reported as being used to manufacture products. The analysis indicated that the WWTP that has the highest triclosan concentration in its influent (20 750 ng/L, in 2011-2013; Table 4-1) receives wastewater from a soap manufacturer. In this case, due to high removal efficiency, the concentration of triclosan in the effluent was quite low (12 ng/L; Table 4-1). Two other WWTPs that receive industrial wastewater from facilities that used triclosan in 2011 had concentrations in their influent of 1260 and 1430 ng/L and concentrations in their effluent of 190 and 240 ng/L, respectively. These effluents would be further diluted once released to surface water. These effluent concentrations date from 2004 so it is uncertain whether the facilities were actually using triclosan at that time and whether the quantities used were similar to the ones reported for 2011, however, these values are not particularly different from other WWTP effluent concentrations (Table 4-1). Based on these data, triclosan releases from the manufacturing facilities are not likely to be of ecological concern.
4.5.1.3 Risk analysis for methyl-triclosan
The potential risk posed by methyl-triclosan to aquatic ecosystems was also assessed, since this substance is released in WWTP effluent and there is evidence of its presence in certain water bodies in Canada. For this substance, the worst-case scenario would be equivalent to assuming 100% transformation of triclosan to methyl-triclosan. Although it was not quantified, the portion of triclosan actually biotransformed to methyl-triclosan is expected to be much lower than this, as suggested by the results of the studies conducted on the fate of triclosan in WWTPs. A more realistic scenario would be to take into account all the fate pathways-that is, to base the PEC on monitoring data for water. Monitoring data for methyl-triclosan in Canada were available only for Hamilton Harbour, Lake Ontario and Wascana Creek (Saskatchewan); the highest value was 17 ng/L for Wascana Creek. Therefore, the PEC for methyl-triclosan in water was chosen to be 17 ng/L. This is considered to be a realistic worst-case since Wascana Creek is known for its low capacity to dilute the WWTP effluent that it receives.
The results of only two aquatic toxicity studies are available for methyl-triclosan (acute tests with daphnids and algae). The lowest endpoint from these studies was selected as the CTV-that is, a 72-hour EC10 value of 55 µg/L for biomass of the alga Scenedesmus subspicatus (Study Submissions 2009).
An assessment factor of 100 was chosen to derive a PNEC from this value, given the very limited data set from which it was taken. Dividing the PEC of 17 ng/L by the PNEC of 550 ng/L results in a risk quotient (RQ) of 0.03, indicating that methyl-triclosan would be unlikely to represent a risk to aquatic organisms. However, this does not take into account the possible chronic toxicity of methyl-triclosan due to its bioaccumulation. Indeed, BAFs up to 5200 were measured for aquatic organisms (Table 4-11). It is also acknowledged that combined exposure to methyl-triclosan and triclosan is likely in certain aquatic ecosystems. The overall impact is uncertain, given the limited monitoring and effects data for methyl-triclosan. The risk associated with this co-exposure is likely somewhat higher than that from triclosan alone. Given that the PEC for triclosan is much higher, the realistic worse-case scenario presented in section 4.5.1.2 for triclosan likely encompasses risk from the potential combined exposure of methyl-triclosan and triclosan.
4.5.2 Sediment
The available toxicity data for triclosan in benthic organisms are limited to only one study using chironomids (Chironomus riparius). The NOEC in this study was established as greater than or equal to 100 mg/kg dw (Study Submissions 2009). The available data are not fully representative of the sediment compartment as other benthic organisms could be more sensitive to triclosan.
Triclosan and methyl-triclosan were measured in surface, suspended and core sediment samples collected at different locations across Canada in 2012-2013 (see Table 4-4). The highest concentrations of triclosan were found in suspended sediment samples collected in the St. Lawrence River at different distances away from a WWTP. At 4 km away, triclosan was measured in two samples, at 990 and 2000 ng/g (0.99-2 mg/kg). At distances of up to 15 km away from the WWTP, the measured concentrations did not exceed 150 ng/g (0.15 mg/kg). It is noted that suspended sediment is not considered to be the primary route of exposure to sediment-dwelling organisms. Concentrations found in surface sediment were up to 47 ng/g (0.047 mg/kg), and in core sediment up to 9 ng/g (0.009 mg/kg). These measured concentrations are well below the NOEC value determined for a sediment-dwelling species (Chironomus riparius).
Methyl-triclosan was found in sediment samples at levels much lower than triclosan; the highest concentrations reported for surface, suspended and core sediment were 22, 24 and 15 ng/g, respectively (see Table 4-4). Due to the lack of suitable toxicity data on benthic organisms for methyl-triclosan, no further analysis was conducted.
4.5.3 Soil
4.5.3.1 Risk analysis for triclosan
The main release of triclosan to soil is via the spreading of biosolids from WWTPs. In Canada, about 40% of this type of biosolids is applied to various types of land (i.e., agricultural, forest or dedicated land) (Apedaile 2001). A PEC based on monitoring data (i.e., triclosan concentrations in soil) cannot be determined, as such data were not found for Canada. However, numerous monitoring data were available for triclosan in wastewater sludge and biosolids; these data can be used to derive a PEC for soil. As presented in Section 4.1.2.1, the concentration of triclosan in wastewater sludge and biosolids from various WWTPs across the country ranges from less than 1 to 46.4 μg/g dw. In Canada, the worst-case conditions for biosolids application to an agricultural soil are a maximum application rate of 8300 kg dw/ha per year (based on the highest existing provincial regulatory limit; such limits are only available for four provinces) with a mixing depth of 0.2 m (plough depth) and a soil density of 0.0017 kg/cm3 (Environment Canada 2006). The following equation was used for deriving a soil PEC:
PEC = ([triclosan]sludge × application rate) ÷ (depth × density)
Taking the 95th percentile of triclosan concentrations found in biosolids (28 μg/g dw; Table 4-2) as a realistic worst-case concentration and the maximum application rate described above for biosolids spreading, a PEC of 68 μg/kg dw is obtained. Assuming a yearly application of biosolids over 10 years, the cumulative triclosan concentration in soil would be 684 μg/kg dw. This PEC value is based on the highly conservative assumption that triclosan will not degrade further once mixed into soil and that it will not leach or run off. In order to estimate more realistic PEC values, the Biosolids-Amended Soil Level 4 (BASL4) model was used (BASL4 2011). This model is a fugacity-based model and uses equilibrium partitioning principles to deduce the overall fate of a chemical in the soil. In this model, a chemical can be removed from the soil by volatilization, degradation, leaching, runoff and erosion processes.
Two scenarios were modelled in BASL4 to simulate the lower and upper ends of a range of possible PECs in soil based on two triclosan half-lives, two biosolids application rates and the 95th percentile of triclosan concentrations found in biosolids (28 μg/g dw). In the first scenario (lower-end), a half-life of 18 days was used based on results from laboratory biodegradation experiments (Table 4-6), and an application rate of 5000 kg dw/ha per year was used based on average existing provincial regulatory limits (such limits are only available for four provinces). BASL4 requires the use of a degradation half-life in soil that may represent biodegradation, photolysis, hydrolysis and oxidation. For triclosan, biodegradation is expected to be the major degradation process in soil (see Section 4.2.5). In the second scenario (upper-end), a half-life of 200 days was arbitrarily chosen as an estimate of a field degradation half-life. Lozano et al. (2010) reported a dissipation half-life of 107 days for a field that had received one application of biosolids. Chen et al. (2014) reported first-order dissipation half-lives of 258 and 106 days for a field that had received either one or repeated application of biosolids, respectively. Since these reported half-lives likely include contributions from processes such as leaching and volatilization, in addition to degradation processes, the value of 200 days was conservatively chosen to account for degradation only, as required for BASL4. Still in that second scenario, an application rate of 8300 kg dw/ha per year was used based on the highest existing provincial regulatory limit. A 10-year period was simulated with a yearly application during the first three years of this period. Yearly applications over ten years were also modelled; they generated similar results given that triclosan does not build up in soil.
The results obtained for the lower-end scenario show that the highest triclosan concentrations in soil would be reached at the time of ploughing, right after biosolid applications-i.e., on days 2, 367 and 732 (average of 118 μg/kg). This average is higher than the value of 68 μg/kg obtained above assuming no dissipation, probably because the equation used to calculate the latter assumes instantaneous mixing in the soil layer. The average triclosan concentration in soil between biosolid applications is estimated to be 11 μg/kg. There is no buildup in soil concentrations due to cumulative applications because of the relatively rapid rate of loss of triclosan from soil. The results for the upper-end scenario show that the highest concentrations in soil would again be reached at the time of ploughing, right after biosolid applications (average of 222 μg/kg). The average triclosan concentration in soil between biosolid applications is estimated to be 110 μg/kg.
For comparison purposes, Fuchsman et al. (2010) conducted a terrestrial risk assessment for triclosan and modelled concentrations in soil using two half-lives (2 weeks, based on laboratory studies, and 16 weeks, based on soil dissipation studies) and two application frequencies (1 and 3 times a year; average application rate of 19 ;000 kg/ha per year). Their modelling exercise showed that there is no buildup of triclosan in soil, except for one of the four scenarios tested (one application and half-life of 16 weeks), in which the concentration of triclosan stabilizes over the years at approximately 110% of the initial soil concentration.
Measurements of triclosan in soils that were amended with biosolids are available from the literature. Wu et al. (2010b) measured triclosan in soils that had been amended with biosolids in Ohio. The soil for which the highest concentration of triclosan was measured (11 µg/kg dw in November 2008) is a clay that had historically received two biosolids applications (0.76 µg/g dw in biosolids), one in December 2006 and the other in November 2008. For comparison with the numbers provided above for Canada, the application rates for these two dates were 11 600 and 9900 kg dw/ha, respectively. In another study conducted in Virginia, Lozano et al. (2010) measured triclosan concentrations in soils that had been amended once with biosolids (average of 15.6 µg/g dw in biosolids) to vary between 4.1 and 4.5 µg/kg dw and between 24 and 67 µg/kg dw, 16 months and less than a year after application, respectively. In fields where there had been multiple applications of biosolids containing triclosan, there was a slight buildup in concentrations observed over the years, but these were much lower than the predictions made by the authors using an equation similar to the one above. In an additional study conducted by Lozano et al. (2012) in Maryland, triclosan concentration in a soil that was amended once with biosolids (average triclosan concentration of 19.1 µg/g dw in biosolids, and application rate of 72 000 kg ww/ha) peaked at 64 µg/kg dw two months after application. In the Midwestern United States, Kinney et al. (2008) found triclosan concentrations of 160 and 96 µg/kg dw in soil samples that were collected 31 and 156 days following biosolids application, respectively. The biosolids were applied once at a rate of 18 000 kg dw/ha, and its triclosan concentration was 10.5 µg/g dw. Finally, Sánchez-Brunete et al. (2010) measured triclosan concentrations of 4.7 and 1.7 µg/kg dw in agricultural soil sampled 1 day and 6 months following biosolids application (12 000 kg dw/ha; triclosan concentration in biosolids not mentioned), respectively. The same authors measured methyl-triclosan concentrations of 1.7 and 3.8 µg/kg dw in the same soil samples. Overall, when compared with results from soil biodegradation studies, these data suggest that the persistence of triclosan in soil is greater when it is applied in biosolids, likely because it is present as bound residues. As such, its bioavailability to soil organisms is probably lower as compared with laboratory conditions.
Use of treated wastewater to irrigate agricultural fields, as well as other types of field (e.g., golf courses), can also contribute to the introduction of triclosan in the terrestrial environment. This practice is used worldwide, including in Canada (Hogg et al. 2007). However, no data are available to quantify the relative importance of this source as compared with biosolids application.
The PNEC for the soil compartment of 0.6 mg/kg was based on the critical toxicity value, as the most sensitive acceptable endpoint identified for terrestrial organisms (reproduction in earthworm E. andrei, EC10 = 0.6 mg/kg (Amorim et al. 2010); see Table 4-13), divided by an assessment factor of 1 (see sub-section 4.4.2.3).
The risk quotients based on the average peak soil concentrations obtained for the lower-end and upper-end scenarios modelled in BASL4 are 118 μg/kg / 600 μg/kg = 0.20 and 222 μg/kg / 600 μg/kg = 0.37, respectively. Based on these results, there is low potential of risk to soil organisms from the application of biosolids that contain triclosan.
The potential risk of exposure of terrestrial wildlife to triclosan was not quantitatively assessed, since results from repeated oral dose toxicity studies in mammals showed low effects (e.g., NOAEL and LOAEL of 750 and 900 mg/kg bw per day, respectively, in mice; Table 4-13). Acute exposure also showed low toxicity to mammals (LD50 greater than 5000 mg/kg bw per day in rat; Table 4-13).
In addition, the BAF values in terrestrial organisms, such as earthworms and shrews (modelled BAFs of ~95 and ~4500 based on BASL4; see Section 4.3.2), coupled with some metabolism of triclosan that would occur following prey ingestion, would both mitigate exposure levels in top predators.
4.5.3.1 Risk analysis for methyl-triclosan
Methyl-triclosan is a major transformation product of triclosan in soil under aerobic conditions. A risk quotient analysis for terrestrial organisms was not performed due to lack of methyl-triclosan effects data for the soil compartment.
4.5.4 Characterization of ecological risk
Properties of triclosan relevant to ecological risk assessment have been described in this assessment report. Lines of evidence that characterize ecological risk of triclosan in Canada are summarized below.
In Canada, triclosan is used in many products used by consumers that end up in wastewater. A portion of triclosan is removed from wastewater before being released to surface water as part of an effluent. During the wastewater treatment process, a portion of triclosan partitions to sludge in WWTPs. Biosolids from WWTPs may eventually be spread on land, hence potentially releasing triclosan to the terrestrial environment. A portion of triclosan may also be methylated during wastewater treatment to form methyl-triclosan. Methyl-triclosan is also formed in soils that receive application of biosolids.
When in surface water, triclosan is found either under a neutral or ionized form, depending on ambient pH. The ionized form is rapidly photodegraded (within hours) if exposed to sunlight. Potential transformation products resulting from this reaction include dichlorophenol (2,4-DCP) and lower chlorinated dioxins (2,7/2,8-DCDD).
Triclosan is not likely to persist in water and sediments in the long term, should releases of this chemical be halted. However, its continual input to surface water through WWTP effluents results in its continuous presence in receiving aquatic ecosystems. The relatively short half-life of triclosan in aquatic ecosystems means that triclosan will not be subject to long-range transport. Therefore, long-term exposures to triclosan in water and sediments are expected to be in the near field, closer to emission sources.
The evidence for bioaccumulation of triclosan in water is variable depending on exposure conditions and organisms exposed. Bioaccumulation of triclosan in organisms is partly dictated by its ionization state. The neutral form of triclosan has a greater potential for bioaccumulation compared to the ionized form. In fish, triclosan is rapidly taken up from the water phase. The physical and chemical properties of triclosan suggest that the contribution of the diet to the total body burden of triclosan in fish is likely quite low. While it bioconcentrates rapidly, triclosan also metabolizes rapidly. Triclosan also accumulates in algae and invertebrates with BCF/BAF ranging from 500 to 2100. BCF values ranging from 16 to 8700 have been reported for fish and have been shown to be influenced by pH of exposure and internal tissues. Fugacity ratio calculations in fish suggest that triclosan bioconcentrates sufficiently in fish to cause chronic adverse effects.
Triclosan is known to act through specific modes of action. Triclosan likely functions as an uncoupler of oxidative phosphorylation. The molecular structure of triclosan resembles that of several non-steroidal estrogens which suggests the potential to act as an endocrine-disrupting agent. Studies show that triclosan may disrupt the thyroid hormone in amphibians. Triclosan blocks fatty acids synthesis in bacteria. Plants share similar fatty acid synthesis pathways with bacteria, which may explain their high sensitivity to triclosan.
Triclosan is highly inherently toxic to aquatic organisms; observed adverse effects at very low exposure concentrations include reduction in growth, reproduction and survival. Triclosan may also interfere with the action of certain hormones in amphibians, fish and mammals. Algae are the most sensitive group of organisms, followed by invertebrates and vertebrates.
A species sensitivity distribution (SSD) based on no- to low-effects chronic endpoints for 19 aquatic species was used to determine a predicted no-effect concentration (PNEC) of 376 ng/L for triclosan. This SSD also includes endpoints which may be susceptible to endocrine influence such as growth, postembryonic development and reproduction in fish and amphibians. Because this PNEC is based on sensitive endpoints that were measured under chronic exposure, which likely allowed for bioaccumulation of triclosan and subsequent occurrence of adverse effects, it is not considered to be overly conservative.
The widespread use of triclosan in products used by consumers is reflected through its ubiquitous presence in water bodies located in populated areas across Canada. Of the available surface water monitoring data, a large number of the measured triclosan concentrations are below the toxic effects threshold (i.e., the PNEC of 376 ng/L), but there are a few instances where this level is exceeded. This indicates that the measured concentrations of triclosan in surface water in Canada can reach levels where there is a potential for triclosan to cause harmful effects to aquatic ecosystems.
A portion of triclosan partitions to sediments when released to the water compartment. Triclosan is expected to degrade relatively rapidly under aerobic conditions, but it degrades slowly in buried anaerobic sediment. Given its continuous presence in the water column, due to its continual release to surface water, and given its rapid partitioning to sediments, benthic organisms are likely exposed to triclosan on a steady-state long-term basis. Triclosan bioavailability in sediments may partly be mitigated by its partitioning to the solids phase. Based on the very limited data available for benthic toxicity and for levels of triclosan in sediments, there seems to be a low concern for triclosan to cause harmful effects to benthic organisms.
Generally, there is a similar level of toxicity of triclosan to marine algae and invertebrates compared to chronic toxicity for freshwater organisms. However, triclosan is not expected to cause harm to marine organisms, due to low exposure in marine ecosystems. Exposure concentrations of triclosan are expected to be lower than those for freshwater ecosystems because a high dilution is expected at many of these sites. Therefore, further risk assessment specific to marine ecosystems was not conducted.
The main route of entry of triclosan into soil is through the spreading of WWTP biosolids to agricultural lands. Experimental evidence shows that triclosan is not persistent in aerobic soil (half-life ranging from 3 to 58 days) under laboratory conditions. However, when applied as part of biosolids, field dissipation half-lives are 50 to 258 days. Releases of triclosan to terrestrial ecosystems are not continuous like those in aquatic ecosystems, but rather episodic. Even though triclosan is not expected to build-up in soil, it is likely to be present in this environmental compartment long enough to result in chronic exposure for soil organisms. Triclosan reaching small water bodies through runoff following broadcast application of biosolids to soil could be of concern. Indeed, as mentioned previously in this report, triclosan concentrations up to 258 ng/L have been measured in runoff one day after biosolids application, which is close to the aquatic PNEC of 376 ng/L.
Triclosan does not bioaccumulate in soil organisms to a great extent based on BCF/BAF values of 2.5-27 measured for earthworms and soybean plants. BAF values modelled for earthworms and shrews were approximately 95 and 4500 (assuming no metabolism), respectively. Toxicity of triclosan to soil organisms varies depending on the species; observed effects include reduction in growth and reproduction, at both low and high levels of exposure. Triclosan is slightly toxic to birds and of low toxicity to mammals on an acute and subchronic oral basis. Risk quotients of less than or equal to 0.37 were calculated for terrestrial organisms based on measured concentrations of triclosan in biosolids in Canada and measured half-lives in soil, as well as on effects data for the most sensitive organism (earthworm). Based on the toxicity levels (NOAEL of 750 mg/kg bw per day in mice), effects in wildlife are not likely to occur. Overall, there is a low concern for triclosan to cause harmful effects in terrestrial organisms.
Transformation products of triclosan have been characterized. Triclosan is a precursor to lower chlorinated dioxins, namely 2,7/2,8-DCDD. Given their probable transient state in aerobic environments and their low inherent toxicity, 2,7/2,8-DCDD are not likely to be of environmental concern. Other persistent polychlorinated dioxins, e.g., 1,2,8-TriCDD, 2,3,7-TriCDD and 1,2,3,8-TCDD, present in sediments as a result of the phototransformation of chlorinated triclosan derivatives formed during wastewater disinfection, could be of concern, depending on their inherent toxicity (Buth et al. 2010).
Another transformation product of triclosan in water-sediment systems and in soil is methyl-triclosan. Methyl-triclosan seems to be persistent in wastewater sludge, likely as bound residues due to the high organic carbon content in sludge, and it also seems persistent in anaerobic sediments. Limited data on effects to aquatic organisms indicated that methyl-triclosan is highly toxic. It is also highly bioaccumulative, with a reported BAF greater than 5000 in fish (Balmer et al. 2004). In a field study in which both triclosan and methyl-triclosan were measured in fish muscles, the latter was found at concentrations 90 times higher than triclosan (Boehmer et al. 2004). Methyl-triclosan is likely present in surface waters over wide areas associated with triclosan, since it is formed in WWTPs. Methyl-triclosan can reach soil through the application of biosolids to land. Triclosan and methyl-triclosan were measured in two field studies; triclosan and methyl-triclosan ranged from less than the MDL to 112 ng/L and from 3 to 17 ng/L, respectively, in Wascana Creek in Saskatchewan (Waiser et al. 2011). The risk quotient analysis for aquatic ecosystems suggests that methyl-triclosan does not reach levels that would be harmful to aquatic organisms, but would contribute somewhat to the total toxicity of triclosan and its transformation products. A risk quotient analysis could not be done for terrestrial ecosystems due to a lack of effects data on methyl-triclosan for terrestrial organisms.
4.6 Consideration of the Lines of Evidence and Uncertainties
Technical information for various lines of evidence for ecological risk of triclosan was examined to develop a conclusion as required under CEPA. A weight of evidence approach, where several lines of evidence are used in the decision-making in all portions of the risk assessment, as well as precaution (as appropriate) were applied. Uncertainties underlying the lines of evidence were identified and their impacts on the assessment were considered. Uncertainties often result from data gaps characteristic of limited or incomplete data sets, or lack of data; assumptions, grounded in sound science, have to be made to address the data gaps. This in turn can lead to an over or underestimation of risk, or impacts can remain unknown.
The fate of triclosan in the environment, its bioaccumulation potential, ecological effects, environmental levels and risk quotient analyses for key environmental compartments were described in the assessment report to characterize the potential of triclosan to cause adverse effects in the Canadian environment. Consideration of the lines of evidence in an integrated manner led to the risk assessment conclusion under CEPA (see sections 4.7 and 5.1).
To effectively assess the impacts of the identified uncertainties on the risk assessment of triclosan, qualitative criteria were used. This qualitative analysis served to determine the overall confidence in the decision-making process that led to the assessment conclusion. The level of uncertainty was judged based on the abundance and quality of data, and its suitability. The analysis also included consideration of the relevance of each line of evidence and the qualitative assessment of the weight for each line of evidence to determine their impact on the overall conclusion. Qualifiers used in the analysis ranged from low to high.
Lines of evidence in the assessment of triclosan, associated uncertainties, and their analysis using the qualitative criteria are presented in Table 4-14.
| Theme | Line of evidence | Level of uncertainty | Relevance in assessmenta | Weight assignedb |
|---|---|---|---|---|
| Environmental Fate | Primary half-life in water | Moderate | Moderate | Moderate |
| Environmental Fate | Primary half-life in sediments | Moderate | Moderate | Moderate |
| Environmental Fate | Primary half-life in soil | Low | Moderate | Moderate to High |
| Environmental Fate | Impact of transformation product - methyl-triclosan | Moderate to high | Moderate | Low to moderate |
| Environmental Fate | Impact of transformation products - PCDDs | Moderate to high | Moderate | Low to moderate |
| Bioaccumulation | Bioconcentration in aquatic organisms | Moderate | High | Moderate to high |
| Bioaccumulation | Critical body residue analysis in aquatic organisms | Moderate | High | Moderate to high |
| Bioaccumulation | Bioaccumulation in terrestrial organisms | Moderate | Moderate | Moderate |
| Toxicity | Mode of toxic action/receptor binding/chemical activity | Low | High | High |
| Toxicity | PNEC aquatic | Low | High | High |
| Toxicity | PNEC soil | Moderate | High | Moderate to high |
| Toxicity | Mammalian and avian toxicity | Low | Low to moderate | Moderate |
| Environmental exposure | Exposure in water | Low | High | High |
| Environmental exposure | Exposure in soil | Moderate to high | Moderate to high | Moderate |
| Risk quotient analysis | RQ aquatic | Low | High | High |
| Risk quotient analysis | RQ soil | Moderate to high | High | Moderate |
Table Notes
Abbreviations: PCDD, polychlorinated dibenzodioxin; PNEC, predicted no-effect concentration; RQ, risk quotient.
a Relevance refers to the impact of the evidence in the assessment, from a scientific and a regulatory point of view.
b Weight is assigned to each line of evidence and it is directly related to its relevance in the assessment and to its uncertainty.
The themes described in Table 4-14 are interconnected in how they contribute to the overall risk, where characteristics stemming from one contribute to or influence others. Some of these relationships are the nature of release, the residence time and fate of the substance which can affect levels of exposure; the transformation and degradation products or metabolites with toxic profiles that can add to exposure thorough co-exposure or similar mode of action; the bioaccumulation which can contribute to overall toxicity when internal toxicity thresholds levels are surpassed; and the specific modes of action that can trigger toxicity responses in numerous species at low exposure concentrations. Considerations of the relevance of these the themes for triclosan are presented in sections 4.2.6 (Fate), 4.3.3 (Bioaccumulation) and 4.4 (Ecological Effects), and summarized in section 4.5.4 (Characterization of Ecological Risk).
Although triclosan is unlikely to persist in the environment, based on a relatively robust set of degradation data, chronic exposure to triclosan is expected in water, as triclosan is continuously released from products used by consumers that get released down-the-drain. Measured concentrations of triclosan in water and in sediment samples across Canada indicate the ubiquitous and dispersive nature of this chemical. In soil, triclosan half-lives are known to be longer and, even though exposure in this medium results from intermittent rather than continuous releases, chronic exposure to triclosan is also expected for terrestrial organisms.
Triclosan transforms to methyl-triclosan and lower chlorinated dioxins in the environment. Limited data characterizing these compounds, including their potential for exposure in the Canadian environment, are available. Methyl-triclosan seems to be highly toxic to aquatic species and has a longer residence time in the environment than triclosan. Similarly to triclosan, chronic exposure is expected in the aquatic compartment; ultimately, organisms are expected to be co-exposed to both triclosan and methyl-triclosan. The overall impact is uncertain, but the risk associated with this co-exposure is likely somewhat higher than that from triclosan alone. Lower chlorinated dioxins formed from triclosan are generally characterized by low toxicity and tend to be transient in the environment.
Triclosan is a bioavailable chemical, readily taken up by organisms. It is highly toxic to aquatic organisms, as demonstrated by a wealth of reliable studies for numerous species. There is also convincing evidence, based on toxicity studies, fish kinetics studies, and QSAR modelling, that triclosan is a very reactive chemical with specific modes of action. These factors are highly relevant in the risk assessment of triclosan and demonstrate that triclosan causes adverse effects at low exposure concentrations. Despite its ability to rapidly biotransform in fish, triclosan likely accumulates sufficiently to result in body burdens that exceed thresholds of toxicity, based on robust calculations of fugacity capacity. This is also highly relevant to the overall impact of triclosan in aquatic ecosystems because under chronic exposure, even at low to moderate levels, bioconcentration of a reactive chemical like triclosan will lead to adverse effects in aquatic organisms.
Triclosan shows high toxicity in chronic studies with soil organisms, although data are available for only a few species and results are variable. It is uncertain whether triclosan accumulates to exceed internal toxicity thresholds in terrestrial organisms; fugacity capacity and critical body residue calculations are currently not possible to verify. Measured and modelled bioaccumulation (BAF) and bioconcentration (BCF) factors are low to moderate, except for one modelled BAF in mammals where metabolism was not considered, likely resulting in an overestimation.
Predicted no-effect concentrations (PNECs) were determined for the aquatic and soil compartments and serve as very important lines of evidence in the risk assessment of triclosan. For the aquatic compartment, the fifth percentile value of a species sensitivity distribution, representative of the level that affects 5% of species, was used as the critical toxicity value, to obtain the PNEC value. This approach considers the inter-species variation in sensitivity, and is based on a broad range of data. Considering this and recognizing that the PNEC is based on no- and low-effects endpoints, it is not overly conservative. Due to a more limited data set for soil organisms, the most sensitive endpoint was chosen as the critical toxicity value to determine the PNEC in soil. Finally, there is evidence that shows that triclosan is moderately toxic to birds and mammals. Effects data for these organisms are limited but results are robust. Since low environmental exposure to triclosan is expected for birds and mammals, this line of evidence is overall less relevant in the risk assessment of triclosan.
There are numerous, reliable, and consistent measurements of triclosan in water bodies and in wastewater at WWTPs across Canada. As triclosan is present in biosolids from WWTPs, soil amendment of biosolids can result in exposure to soil organisms. Concentrations of triclosan in soils in Canada were modelled due to the lack of monitoring data. There are uncertainties associated with the modelling as well as the soil amendment practices across Canada.
Risk quotient analyses for aquatic and terrestrial ecosystems are of high importance in the risk assessment of triclosan. For the aquatic compartment, there is high confidence associated with the risk quotient analysis as both the exposure levels of triclosan in water and the PNEC for aquatic organisms have low uncertainty. This analysis indicated that the higher exposure levels of triclosan determined for aquatic ecosystems in Canada slightly exceed the PNEC; however, given the high potency of triclosan, precaution is needed when interpreting the aquatic risk quotient analysis in terms of the potential for triclosan to cause harm in the environment. For terrestrial ecosystems, more assumptions were used in the risk quotient analysis due to limitations in exposure data and in information on soil amendment practices. The impact of these conservative but realistic assumptions is uncertain, but may ultimately contribute to an overestimation of risk for the terrestrial ecosystems.
Overall, exposure in the aquatic compartment is of high importance in the risk assessment of triclosan. Therefore, a higher weight was assigned to those lines of evidence that describe continuous release of triclosan to the aquatic environment in Canada (Table 4-14). Given that triclosan is a very potent chemical acting through specific modes of action, low levels of exposure and bioconcentration can cause harm in organisms. The level of uncertainty associated with the key lines of evidence is viewed as low and therefore has little impact on the characterization of risk for triclosan.
4.7 Conclusion of Risk to the Environment
Triclosan is a man-made chemical that has been measured in numerous water bodies across Canada at concentrations in the range of ng/L. Though it tends to degrade relatively quickly in the environment, it is always present in aquatic ecosystems near sources of release across the country because it is continuously released when products containing triclosan are disposed of or washed down-the-drain.Triclosan is a very potent chemical that can cause effects in organisms even at low exposure levels in the environment in the ng/L range. These effects include reduction in growth, reproduction and survival as have been observed in studies with aquatic invertebrates and vertebrates, and terrestrial organisms including plants. Triclosan is known to have anti-microbial properties. There is evidence that triclosan can elicit effects associated with endocrine disruption at environmentally relevant concentrations. Triclosan is also known to accumulate in aquatic organisms to levels that can cause adverse effects.
While it is recognized that there are uncertainties in the exposure assessment of triclosan, a precautionary approach is required considering the potency of this biocide and its ubiquitous presence in the Canadian environment. In addition, the combined exposure from its transformation product, methyl-triclosan, and from chemicals such as triclocarban that have similar mode of action and use patterns, could also contribute to the potential for harm.
Considering the potency of triclosan and current exposure levels observed in the Canadian environment, it is concluded that a potential for harm exists from exposure to triclosan in aquatic ecosystems.
It is considered that sufficient robust data are available for the key lines of evidence that support the conclusion. Considering both the sources of uncertainty and overall confidence in the available data, it is anticipated that the above conclusion would not be highly sensitive to refinement if additional data were provided for the key lines of evidence. Additional data to elucidate other modes of toxic action or receptor mediated effects for triclosan and its transformation product methyl-triclosan would be beneficial for better understanding of the endocrine disruption potential of this substance.