Screening assessment Anthraquinones Group
Official title: Screening assessment
Anthraquinones Group
Chemical Abstracts Service Registry Numbers
81-48-1
81-77-6
6408-72-6
14233-37-5
17418-58-5
72391-24-3
74499-36-8
Environment and Climate Change Canada
Health Canada
July 2021
Cat. No.: En84-228/2021E-PDF
ISBN: 978-0-660-38279-1
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 7 of 15 substances referred to collectively under the Chemicals Management Plan as the Anthraquinones Group. These 7 substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. The other 8 substances were determined to be of low concern through other approaches, and decisions for these substances are provided in separate reports.Footnote 1 ,Footnote 2 Accordingly, this screening assessment addresses the 7 substances listed in the table below. The 7 substances addressed in this screening assessment will hereinafter be referred to as the Anthraquinones Group.
| CAS RNa | Domestic Substances List name | Common name |
|---|---|---|
| 81-48-1 | 9,10-Anthracenedione, 1-hydroxy-4-[(4- methylphenyl)amino]- | Solvent Violet 13 |
| 81-77-6 | 5,9,14,18-Anthrazinetetrone, 6,15-dihydro- | Pigment Blue 60 |
| 6408-72-6 | 9,10-Anthracenedione, 1,4-diamino-2,3- diphenoxy- | Solvent Violet 59 |
| 14233-37-5 | 9,10-Anthracenedione, 1,4-bis[(1-methylethyl)amino]- | Solvent Blue 36 |
| 17418-58-5 | 9,10-Anthracenedione, 1-amino-4- hydroxy-2-phenoxy- | Disperse Red 60 |
| 72391-24-3 | Benzenesulfonic acid, [[(chloroacetyl)amino]methyl][4-[[4- (cyclohexylamino)-9,10-dihydro-9,10- dioxo-1- anthracenyl]amino]phenoxy]methyl-, monosodium salt | Acid Blue 239 |
| 74499-36-8b, c | 9,10-Anthracenedione, 1,4-diamino-,N,N’-mixed 2-ethylhexyl and Me and pentyl derivs. | NA |
Abbreviations: NA, not available.
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society, and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy is not permitted without the prior written permission of the American Chemical Society.
b This substance was not identified under subsection 73(1) of CEPA, but was included in this assessment as it was considered a priority on the basis of other human health concerns.
c This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
The substances in the Anthraquinones Group are used as colourants in products available to consumers, including cosmetics (e.g., body creams, lipsticks/lip balms, make-up, hair products and face paint), food packaging materials, arts and crafts materials (i.e., stampers), toys, do-it-yourself products (e.g., specialty lubricants, epoxy coatings), and textiles. According to information submitted in response to a survey under section 71 of CEPA, the following quantities were imported in the 2011 calendar year: between 1 000 and 10 000 kg of Solvent Violet 13, between 10 000 and 100 000 kg of Pigment Blue 60, between 1000 and 10 000 kg of Solvent Violet 59, less than 100 kg of Solvent Blue 36, between 100 and 1000 kg of each of Disperse Red 60 and Acid Blue 239, and between 1000 and 10 000 kg of CAS RN 74499-36-8. No manufacturing quantities were reported for any of the substances in this group above the reporting threshold of 100 kg in the 2011 calendar year.
The ecological risks of the substances in the Anthraquinones Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk- based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, substances in the Anthraquinones Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from Solvent Violet 13, Pigment Blue 60, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239 and CAS RN 74499-36-8. It is concluded that Solvent Violet 13, Pigment Blue 60, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239 and CAS RN 74499-36-8 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the general population of Canada, the predominant source of exposure to substances in the Anthraquinones Group is the use of products available to consumers that contain these substances. For each substance, estimates of exposure were derived for uses with the greatest potential for exposure. The predominant route of exposure is dermal, with some uses also resulting in oral or inhalation exposure. Estimates of potential exposure to Solvent Violet 13 were derived from use of cosmetics. Estimates of potential exposure to Pigment Blue 60 were derived from use of craft products (i.e., stampers). Estimates of potential exposure to Solvent Violet 59 were derived from mouthing of plastic toys. Estimates of potential exposure to Solvent Blue 36 were derived from use of hair conditioner and specialty lubricants. Estimates of potential exposures to Disperse Red 60 and Acid Blue 239 were derived from contact with textiles. Estimates of potential exposure to CAS RN 74499-36-8 were derived from application of epoxy coating products.
Pigment Blue 60 has been reviewed internationally through the Joint Food and Agriculture Organization of the United Nations / World Health Organization Expert Committee on Food Additives (JECFA). In laboratory studies, Pigment Blue 60 was a reproductive toxicant, but was not genotoxic or carcinogenic. Solvent Blue 36 was considered to be a developmental toxicant. Given some limitations in the health effects information for other substances in the Anthraquinones Group, a read-across approach based on health effects information for Solvent Blue 36 and anthraquinone informed the health effects characterization of these substances. Based on anthraquinone, the critical non-cancer health effects for Solvent Violet 13, Solvent Violet 59, and Disperse Red 60 include kidney, liver, spleen, and bone marrow toxicity, and based on Solvent Blue 36, the critical non-cancer health effects for Acid Blue 239 and CAS RN 74499-36-8 are developmental effects. With respect to cancer effects, all substances in the Anthraquinones Group except Pigment Blue 60 are considered to be possibly carcinogenic given their common structural backbone anthraquinone. Margins between levels of exposure of the general population from daily use of Solvent Violet 13 in certain cosmetics (body cream, spray perfume) and levels associated with non-cancer health effects were considered potentially inadequate to address uncertainties in the health effects and exposure databases. Margins between levels of exposure of the general population from daily use of Solvent Violet 13 in certain cosmetics (lip balm, lipstick, body cream, permanent hair dye, spray perfume and face paint) and cancer effects were also considered potentially inadequate. Margins of exposure were, however, considered adequate for other uses of Solvent Violet 13 and for other substances in the Anthraquinones Group.
On the basis of the information presented in this screening assessment, it is concluded that Solvent Violet 13 meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this screening assessment, it is concluded that Pigment Blue 60, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239, and CAS RN 74499-36-8 do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that Solvent Violet 13 meets one or more of the criteria set out in section 64 of CEPA. It is concluded that Pigment Blue 60, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239 and CAS RN 74499-36-8 do not meet any of the criteria set out in section 64 of CEPA.
It is concluded that Solvent Violet 13 meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 7 of the 15 substances referred to collectively under the Chemicals Management Plan as the Anthraquinones Group to determine whether they present or may present a risk to the environment or to human health. These 7 substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
The other 8 substances (listed in Table 1-1, below) were considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a) and in either the Threshold of Toxicological Concern (TTC)- based Approach for Certain Substances Science Approach Document (Health Canada 2016) or the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018a) and were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this report. Conclusions for these 8 substances are provided in either the Substances Identified as Being of Low Concern using the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Screening Assessment (ECCC, HC 2018b) or in the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018a). The 7 substances addressed in this screening assessment will hereinafter be referred to as the Anthraquinones Group.
| CAS RNa | Domestic Substances List Name | Approach under which the substance was addressed | References |
|---|---|---|---|
| 2379-79-5 | Anthra[2,3-d]oxazole-5,10- dione, 2-(1-amino-9,10- dihydro-9,10-dioxo-2-anthracenyl)- | ERC/TTC | ECCC, HC 2018b |
| 2475-45-8 | 9,10-Anthracenedione, 1,4,5,8-tetraamino- | ERC/Rapid Screening | ECCC, HC 2018a |
| 4051-63-2 | [1,1’-Bianthracene]- 9,9’,10,10’-tetrone, 4,4’-diamino- | ERC/Rapid Screening | ECCC, HC 2018a |
| 13676-91-0 | 9,10-Anthracenedione, 1,8- bis(phenylthio)- | ERC/Rapid Screening | ECCC, HC 2018a |
| 15791-78-3 | 9,10-Anthracenedione, 1,8- dihydroxy-4-[[4-(2- hydroxyethyl)phenyl]amino]- 5-nitro- | ERC/TTC | ECCC, HC 2018b |
| 19286-75-0 | 9,10-Anthracenedione, 1- hydroxy-4-(phenylamino)- | ERC/Rapid Screening | ECCC, HC 2018a |
| 19720-45-7 | 9,10-Anthracenedione, 1,4- bis[(2-methylpropyl)amino]- | ERC/TTC | ECCC, HC 2018b |
| 28173-59-3 | Carbonic acid, 2-[(1-amino- 9,10-dihydro-4-hydroxy- 9,10-dioxo-2-anthracenyl)oxy]ethyl phenyl ester | ERC/TTC | ECCC, HC 2018b |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society, and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior written permission of the American Chemical Society.
The ecological risks of substances in the Anthraquinones Group were characterized using the ERC approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses, and exposures, including additional information submitted by stakeholders. Relevant data were identified up to April 2017, and targeted literature searches were conducted up to October 2020. Additional data were submitted by stakeholders up to July 2019. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. The human health portion of this assessment has undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Ms. Theresa Lopez, Ms. Jennifer Flippin, and Dr. Joan Garey at Tetra Tech. Additionally, the draft of this screening assessment (published November 3, 2018) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 3 This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The CAS RN, Domestic Substances List (DSL) names and common names for the individual substances and representative structures in the Anthraquinones Group are presented in Table 2-1.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 81-48-1 | 9,10-Anthracenedione, 1- hydroxy-4-[(4- methylphenyl)amino]- (Solvent Violet 13; also called Disperse Blue 72) | 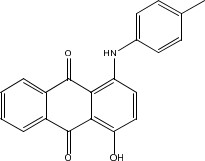 C21H15NO3 | 329.35 |
| 81-77-6 | 5,9,14,18-Anthrazinetetrone, 6,15-dihydro-(Pigment Blue 60) | ![Representative chemical structure Pigment Blue 60, SMILES: c12c3c([nH]c4c5c(c(c6ccccc6c5=O)=O)ccc4[nH]3)ccc1c(c1ccccc1c2=O)=O](/content/dam/eccc/images/pded/anthraquinones/20210406-Table2.1.2.jpg) C28H14N2O4
C28H14N2O4 | 442.43 | 6408-72-6 | 9,10-Anthracenedione, 1,4- diamino-2,3-diphenoxy- (Solvent Violet 59; also called Disperse Violet 31 and Disperse Violet 26) |  C26H18N2O4 | 422.44 |
| 14233-37-5 | p;9,10-Anthracenedione, 1,4- bis[(1-methylethyl)amino]- (Solvent Blue 36) | 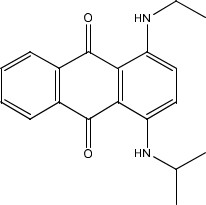 C20H22N2O2 | 322.41 |
| 17418-58-5 | 9,10-Anthracenedione, 1- amino-4-hydroxy-2-phenoxy- (Disperse Red 60) |  C20H13NO4 | 331.33 |
| 72391-24-3 | Benzenesulfonic acid, [[(chloroacetyl)amino]methyl][ 4-[[4-(cyclohexylamino)-9,10- dihydro-9,10-dioxo-1- anthracenyl]amino]phenoxy] methyl-, monosodium salt (Acid Blue 239) | 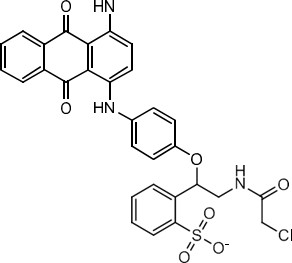 C36H34ClN3O7S.Na C36H34ClN3O7S.Na | 710.18 |
| 74499-36-8a | 9,10-Anthracenedione, 1,4-diamino-, N,N’-mixed 2- ethylhexyl and Me and pentyl derivs.(NA) | 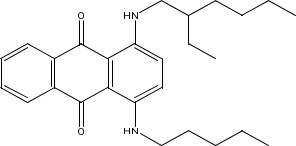 C27H36N2O2 | 420.60 |
Abbreviation: NA, not available.
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials). The representative structure and molecular formula shown here are for the 2-ethylhexyl and pentyl derivative.
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, has been used to inform the human health assessment for substances in the Anthraquinones Group associated with limited data for certain endpoints. Analogues were selected based on similar structures and properties (similar physical-chemical properties, toxicokinetics) and on availability of relevant empirical data. Details of the read-across data chosen to inform the human health assessment of the Anthraquinones Group are further discussed in the relevant sections of this report. One of the analogues selected was anthraquinone, which is the common structural backbone shared between substances in the Anthraquinones Group and their analogues. It is presented in Table 2-2 below. Solvent Blue 36, which is part of the Anthraquinones Group, was also used to inform the human health hazard for Acid Blue 239 and CAS RN 74499-36-8.
| CAS RN | DSL or other name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 84-65-1 | 9,10-Anthracenedione (anthraquinone) | 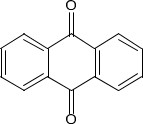 C14H8O2 | 208.22 |
3. Physical and chemical properties
A summary of physical and chemical properties of the substances in the Anthraquinones Group is presented in Table 3-1. Experimental information regarding the physical and chemical properties of these substances is limited. Modelled values are based on data from (Q)SAR models. Additional physical-chemical properties are reported in ECCC (2016b).
| Substance | Water solubility (mg/L) | log Kow | Vapour pressure (Pa) | Key references |
|---|---|---|---|---|
| Solvent Violet 13 | 0.001799 (estimated)a | 6.5 | 1.44E-9 (estimated)b | BASF Corporation 2015; EPI Suite c2000-2012 |
| Pigment Blue 60 | “Insoluble”; 0.005 | 1 | “0”c | ECHA 2017;Haynes 2017 |
| Solvent Violet 59 | “Insoluble”; 0.053 | 5.19 | 1.34E-11 (estimated)b | Sijm et al. 1999; EPI Suite c2000- 2012; Brown 1983 |
| Solvent Blue 36 | 0.004586 (estimated)a | 6.07 (estimated)d | 5.18E-7 (estimated)b | EPI Suite c2000- 2012 |
| Disperse Red 60 | 0.00064 | 1.77 | 9.53 E-10 (estimated)b | Yen et al. 1989; ECHA c2007-2017; EPI Suite c2000-2012 |
| Acid Blue 239 | 3.09 (estimated) | 0.96 (estimated) | 1E-19 (estimated) | ECCC 2016b |
| CAS RN 74499-36-8 | 1.157E-6 (estimated)a | 9.58 (estimated)d | 9.71E-10 (estimated)b | EPI Suite c2000- 2012 |
a Water solubility was modeled using WSKOW (EPI Suite c2000-2012) using the experimental log Kow of 6.5 as input.
b Vapour pressure was modeled using the Modified Grain method (EPI Suite c2000-2012).
c Given the absence of significant figures, a modeled value of 1.25E-16 using the Modified Grain method (EPI Suite c2000-2012) was also considered.
d log Kow was modeled using KOWWIN (EPI Suite c2000-2012).
4. Sources and uses
All of the substances in the Anthraquinones Group were included in a survey issued pursuant to section 71 of CEPA (Canada 2012). Table 4-1 presents a summary of the total import quantities for the substances in the Anthraquinones Group. No manufacturing activities were reported above the reporting threshold.
| Common name | Total importsa (kg) |
|---|---|
| Solvent Violet 13 | 1000 – 10 000 |
| Pigment Blue 60 | 10 000 – 100 000 |
| Solvent Violet 59 | 1000 – 10 000 |
| Solvent Blue 36 | Under 100 |
| Disperse Red 60 | 100 – 1000 |
| Acid Blue 239 | 100 – 1000 |
| CAS RN 74499-36-8 | 1000 – 10 000 |
a Values reflect quantities reported in response to a CEPA section 71 survey (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
In Canada and globally, substances in the Anthraquinones Group are used as colourants in a variety of applications. Table 4-2 presents a summary of the major uses of the Anthraquinones Group based on information submitted in response to a CEPA section 71 survey (Environment Canada 2013). Other uses were also reported but are not indicated in this assessment as they were identified as being confidential business information.
| Substance | Usesa |
|---|---|
| Solvent Violet 13 | Manufacture of candles |
| Pigment Blue 60 | Paints and coatings; automotive, aircraft and transportation |
| Solvent Violet 59 | Toys, playground and sporting equipment |
| Solvent Blue 36 | Automotive care; anti-freeze and de-icing |
| Disperse Red 60 | Fabric, textile and leather articles |
| Acid Blue 239 | Fabric, textile and leather articles |
| CAS RN 74499-36-8 | Lubricants and greases; fuels and related products, mixtures or manufactured items |
a Non-confidential uses reported in response to a CEPA section 71 survey (Environment Canada 2013). See surveys for specific inclusions and exclusions (schedules 2 and 3).
In Canada, Solvent Violet 13, Pigment Blue 60, Solvent Violet 59, and Disperse Red 60 may be used as components in food packaging materials. Solvent Violet 13 and CAS RN 74499-36-8 may also be used as components in incidental additives used in food processing establishments: Solvent Violet 13 may be used as a component in sanitizers for hands with water rinse before handling food and in cleaners with water rinse after treatment, while CAS RN 74499-36-8 may be used in synthetic lubricants with non-food contact (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated 2016; unreferenced).
According to notifications submitted under the Cosmetic Regulations to Health Canada, Solvent Violet 13 and Solvent Blue 36 are present in cosmetics. Solvent Violet 13 is used in a variety of cosmetics, including body creams, bath products, lipsticks/lip balms, make-up, nail products, shampoos and conditioners, permanent hair dyes, hair styling products, perfumes, and face painting products, while Solvent Blue 36 is listed in notifications as being present in hair conditioners only (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated 2016, 2019; unreferenced).
Solvent Violet 13 is listed in the Natural Health Products Ingredients Database with a non-medicinal role for external use only as colour additive in natural health products. It is also listed in the Licensed Natural Health Products Database as being present as a non-medicinal ingredient in a limited number of currently licensed topical natural health products, such as acne therapy products (e.g., gels and liquids), antiseptic skin cleansers (e.g., cream, foam, gel, liquid and spray), and anti-dandruff products (e.g., shampoos) (LNHPD [modified 2016]; NHPID [modified 2017]; personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated 2016 and 2017; unreferenced). Pigment Blue 60 is listed in the internal Drug Product Database as a non-medicinal ingredient in disinfectants in Canada (personal communication, email from the Therapeutic Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated 2016; unreferenced). However, this use is limited to food premises, health care facilities and/or hospitals.
Solvent Violet 13 and Pigment Blue 60 are listed on the Health Canada Pest Management Regulatory Agency (PMRA) List of Formulants (personal communication, email from the PMRA, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated 2016; unreferenced). None of the substances in the Anthraquinones Group are registered active ingredients in pest control products in Canada (personal communication, email from the PMRA, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated 2016; unreferenced).
None of the substances in the Anthraquinones Group are on the List of Prohibited and Restricted Cosmetics Ingredients nor are they permitted food additives (Health Canada [modified 2015a]; Health Canada [modified 2015b]; personal communications, emails from the Consumer and Hazardous Products Safety Directorate and Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated 2016; unreferenced).
Additional consumer uses for some substances in the Anthraquinones Group were identified in Canada from publicly available sources and are listed in Table 4-3.
| Substance | Uses | References |
|---|---|---|
| Solvent Violet 13 | Pet shampoos | MSDS 2007a, 2015a |
| Pigment Blue 60 | Arts and crafts (i.e., stampers); artist paints | MSDS 2009a,b,c, 2015b, 2018 |
| Solvent Blue 36 | Dent repair; speciality lubricants; nozzle gels | MSDS 2002, 2008, 2015c |
| CAS RN 74499-36-8 | Scented candles; furniture cleaners; epoxy coatings | MSDS 2007b,c,d, 2011, 2015d |
Globally, Solvent Violet 13 was also identified as a colourant in non-plastic toys (Danish EPA 2015), and in other products including textiles, paper, and plastics (ECHA c2007-2019a). Pigment Blue 60 was found in inks and toners and in products with material based on fabrics, textiles and apparel (e.g., clothing, mattress, curtains or carpets, textile toys) (ECHA 2017).
5. Environmental fate and behaviour
5.1 Environmental persistence
According to models used in ERC (ECCC 2016b), Solvent Violet 13, Pigment Blue 60, Solvent Violet 59, Solvent Blue 36, Disperse Red 60 and CAS RN 74499-36-8 are expected to persist in water, sediment, and soil. In addition, Pigment Blue 60 is expected to persist in air. Acid Blue 239 is not expected to persist in water, sediment, soil, or air.
5.2 Potential for bioaccumulation
Predicted bioconcentration factors (BCFs) with metabolism correction for this group of substances were all low (ECCC 2016b). In addition, modelled cross-sectional diameters ranged from 1.3 to 2.4 nm for these substances (Catalogic 2014), indicating that steric hindrance at cell membranes may reduce the uptake of these substances from water, ultimately limiting bioavailability and bioconcentration potential (Dimitrov et al. 2005).
Modelled BCFs were considered unreliable due to the structural and/or property domains of these substances falling outside of the models’ domains (Environment Canada 2000). Therefore, read-across empirical bioconcentration data from selected analogues were also considered. Read-across empirical data indicate low bioaccumulation potential of Solvent Violet 13. Both Disperse Blue 77 and Solvent Blue 36 were identified as analogues of Solvent Violet 13 (OECD QSAR Toolbox 2017). The empirical BCF study for Disperse Blue 77 determined BCFs of less than 100. Greater weight was assigned to this study as it is considered reliable based on the evaluation of experimental protocol (Hu and Shen 2008), and Disperse Blue 77 is more structurally similar to Solvent Violet 13 than is Solvent Blue 36. The BCF study summary on Solvent Blue 36, which determined BCFs of 5300 to 5400 (J-CHECK c2010-), was given a lower overall weight due to lack of sufficient study details to evaluate its reliability. Furthermore, an empirical biomagnification study for Solvent Blue 36 determined that it has a low biomagnification potential (Inoue et al. 2012).
6. Potential to cause ecological harm
The ecological risks of the substances in the Anthraquinones Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. Since CAS RN 74499-36-8 is a UVCB substance and could not be suitably represented by a single chemical structure, a manual judgement-based approach to classification was used. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), and from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high.
Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of CAS RN 74499-36-8, hazard and exposure could not be fully profiled because of the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed through examination of the UVCB constituents and information submitted in response to CEPA section 71 surveys, and decisions were based on consideration of similar substances and application of expert judgement.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard, exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is estimated to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Anthraquinones Group, and the hazard, exposure and risk classification results, are presented in ECCC (2016b). The hazard and exposure classifications for the seven substances in the Anthraquinones Group are summarized in Table 6-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Solvent Violet 13 | high | low | low |
| Pigment Blue 60 | high | low | moderate |
| Solvent Violet 59 | high | low | low |
| Solvent Blue 36 | high | low | low |
| Disperse Red 60 | high | low | low |
| Acid Blue 239 | high | low | low |
| CAS RN 74499-36-8 | high | low | low |
According to information considered under ERC, Solvent Violet 13, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239, and CAS RN 74499-36-8 were classified as having a low exposure potential. Solvent Violet 13, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239, and CAS RN 74499-36-8 were classified as having a high hazard potential on the basis of a reactive mode of action and potential to cause adverse effects in aquatic and terrestrial food webs. In addition, structural alerts from the OECD toolbox identified Solvent Violet 13 and Disperse Red 60 as being potential endocrine receptor binders. CAS RN 74499-36-8 also had an elevated ecotoxicity ratio, which suggests that this chemical is likely of high potency. These six substances were initially classified as having a moderate potential for ecological risk; however, the risk classification was decreased to low potential for ecological risk following the adjustment of risk classification on the basis of current use quantities (see section 7.1.1 of the ERC approach document, ECCC 2016a). The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of these substances. On the basis of current use patterns, these substances are unlikely to be resulting in concerns for the environment in Canada.
According to information considered under ERC, Pigment Blue 60 was classified as having a low exposure potential. Pigment Blue 60 was classified as having a high hazard potential on the basis of the agreement between reactive mode of action and elevated ecotoxicity ratio, both of which suggest that this chemical is likely of high potency. In addition, Pigment Blue 60 was profiled to have a high potential to cause adverse effects in aquatic food webs. Pigment Blue 60 was classified as having moderate potential for ecological risk. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. Considering current use patterns, this substance is unlikely to be resulting in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Exposure assessment
Substances in the Anthraquinones Group were not identified or measured in any environmental media in Canada or elsewhere. Overall, given their limited commercial quantities in Canada, their very low volatility, their low water solubility (except for Acid Blue 239), the dispersion of any potential releases, and expected removal by water treatment systems, exposure from environmental media that could impact human health of the general population is considered to be minimal for the substances in this group.
Although substances in the Anthraquinones Group were not reported to be present in food, potential for direct food contact was identified for Solvent Violet 13, Pigment Blue 60, Solvent Violet 59, and Disperse Red 60 because of their use as components in the manufacture of food packaging material in Canada (personal communication, email from the Risk Management Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated February 17, 2016; unreferenced). However, exposure to these substances from food packaging is considered negligible (personal communication, email from the Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated April 24, 2017; unreferenced).
Exposures from use of products available to consumers were evaluated. Exposure estimates for uses that result in the highest levels of potential exposure for each substance by the oral and dermal routes, hereinafter referred to as sentinel scenarios, are presented in Tables 7-1 and 7-2, respectively. Potential exposures were estimated using conservative assumptions and default values. See Appendix A for details on assumptions, default values, and algorithms or models used for generating exposure estimates. Systemic exposure estimates for each scenario are expressed on a per-event and/or daily basis, depending on the exposure frequency and the critical health effects (see section 7.3, Characterization of risk to human health).
For characterizing risks from cancer effects, daily systemic exposures estimated on an age-group-specific basis were used, except where lifetime adjustment was undertaken as a refinement or was most appropriate to the scenario (see section 7.3, Characterization of risk to human health). For those scenarios where such an adjustment was used, lifetime average daily doses (LADDs) were derived.
| Substance | Product scenario | Age group | Per-event systemic exposure (mg/kg bw) | Daily systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Solvent Violet 13 | Lip balm | Toddler | 0.0065 | 0.0038 |
| Solvent Violet 13 | Lipstick | Adult | NA | 0.0034 |
| Solvent Violet 13 | Face paint | Toddler | 0.53 | NA |
| Pigment Blue 60 | Arts and crafts (i.e., stampers) | Toddler | 1.29 | 0.0645 |
| Solvent Violet 59 | Plastic toy, mouthing | Toddler | 8.5E-06a | 3.4E-06a |
| Disperse Red 60 | Textiles, mouthing | Infant | 0.0027b | 0.00027b |
| Acid Blue 239 | Textiles, mouthing | Infant | 0.0027b | 0.00027b |
Abbreviation: NA, not applicable.
a Although toy mouthing is considered to occur daily, the per-event and daily systemic exposure estimates correspond to use of a maximum and a mean concentration in simulant, respectively (see Appendix A).
b Although textile mouthing is considered to occur daily, the per-event and daily systemic exposure estimates correspond to use of an initial “acute” and a longer-term “chronic” migration fraction, respectively (see Appendix A).
To estimate the potential cancer risk of either daily oral exposure (lip balm use for a toddler and older age groups, lipstick use for adults) or intermittent per-event oral exposure (face paint use for toddlers), LADDs of 0.00342 mg/kg bw/day and 0.00102 mg/kg bw/day were derived for Solvent Violet 13 from the use of lip balm or lipstick and face paint, respectively. For all other oral exposure scenarios where potential cancer risks were estimated, the daily systemic exposures on an age-group-specific basis were used (see section 7.3, Characterization of risk to human health).
For estimated potential exposures by the dermal route, the maximum flux (Jmax) approach (Williams et al. 2016) was used for Solvent Blue 36 and Acid Blue 239 to characterize systemic exposures as a refinement. For Disperse Red 60, a dermal uptake fraction of 0.02 in areas of high perspiration was used as a conservative assumption in estimating exposure from clothing, following the recommendation of the Textiles Working Group at the German Federal Institute for Risk Assessment (BfR 2007). This recommendation is based on analyses of studies performed by the Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers (ETAD 1994, 1995) on dermal absorption of several disperse dyes, including Disperse Red 60, using porcine and human skin examined over 55 hours. Although the study designs did not account for skin-bound residues of the dyes at termination of the experiments, the data suggest relatively low dermal absorption of Disperse Red 60 in these studies.
In an in vitro dermal absorption study of Solvent Violet 13 using human skin, cumulative absorption was measured over a 24-hour period of exposure under occluded conditions for two test preparations (Charles River Laboratories 2017). A potentially absorbable dose (which includes measurements of the substance in tape strips 3 to 20 of the stratum corneum, viable epidermis, dermis, receptor fluid, and receptor chamber washes) of 1.63 µg/cm2 was measured for a test preparation consisting of an Oilatum® cream (containing 1% by weight radiolabelled Solvent Violet 13). This dose corresponds to the mean potentially absorbable dose of 0.45 µg/cm2, plus two standard deviations of 0.59 µg/cm2 to account for high variability. In the same study, a potentially absorbable dose of 41.33 µg/cm2 was measured for a test preparation consisting of olive oil (containing 10% by weight radiolabelled Solvent Violet 13). This dose corresponds to the maximum potentially absorbable dose, as the mean dose plus two standard deviations (accounting for high variability) exceeded the maximum dose. To assess exposures to Solvent Violet 13 from body cream, the dose of 1.63 µg/cm2 corresponding to the Oilatum® cream test preparation was used given the similarity between product type and experimental formulation. For all other dermal exposure scenarios, as neither experimental formulation was a direct match to the product types, a dose range of 1.63 to 41.33 µg/cm2 was used. Dermal absorption was conservatively assumed to be equivalent to absorption by the gastrointestinal tract for the other substances in the group.
No information was identified indicating the presence of Solvent Violet 59 in products available to consumers that would lead to dermal exposure of the substance. Therefore, exposure to Solvent Violet 59 via the dermal route is not expected.
| Substance | Product scenario | Age group | Per-event systemic exposure (mg/kg bw)a | Daily systemic exposure (mg/kg bw/ day)a |
|---|---|---|---|---|
| Solvent Violet 13 | Body cream | Adult | NA | 0.389 |
| Solvent Violet 13 | Spray perfume | Adult | NA | 0.00230 – 0.0583 |
| Solvent Violet 13 | Permanent hair dye | Teen | 0.0175 –0.444 | NA |
| Solvent Violet 13 | Face paint | Toddler | 0.0457 – 1.16 | NA |
| Pigment Blue 60 | Arts and crafts (i.e., stampers) | Toddler, teenb | 1.29, 0.337 | 0.0645, 0.0168 |
| Solvent Blue 36 | Specialty lubricants | Adult | 3.82E-05 | NA |
| Solvent Blue 36 | Hair conditioner | Adult | NA | 1.84E-04 |
| Disperse Red 60 | Textiles, personal apparel | Infant | 0.00805c | 8.05E-04c |
| Disperse Red 60 | Textiles, personal apparel | Adult | 0.00513c | 5.13E-04c |
| Acid Blue 239 | Textiles, personal apparel | Infant | 1.33E-05d | 1.33E-05d |
| Acid Blue 239 | Textiles, personal apparel | Adult | 8.49E-06d | 8.49E-06d |
| CAS RN 74499-36-8 | Epoxy coating product, application | Adult | 0.0035 | NA |
Abbreviation: NA, not applicable.
a Dermal absorption of Pigment Blue 60 and CAS RN 74499-36-8 was conservatively assumed to be equivalent to absorption by the gastrointestinal tract. Dermal absorption of Disperse Red 60 was assumed to be 2%. Dermal absorption of Solvent Violet 13 was estimated to be either 1.63 µg/cm2 or 1.63 to 41.33 µg/cm2, depending on the scenario. Dermal exposures of Solvent Blue 36 and Acid Blue 239 were estimated using a maximum flux approach (see Appendix A).
b Although the use of these products by teens may also result in incidental oral ingestion, the estimated dermal exposure is assumed to be significantly higher than the potential oral exposure for this age group.
c Although wearing textiles is considered to occur daily, the per-event and daily systemic exposure estimates correspond to use of an initial “acute” and a longer-term “chronic” migration fraction, respectively (see Appendix A).
d Unlike the scenario for Disperse Red 60 above, distinction between “acute” and “chronic” scenarios for exposure to Acid Blue 239 by wearing textiles is not applicable to the dermal exposure estimate given the use of the maximum flux approach (see Appendix A).
To estimate potential cancer risk, LADDs were also derived for daily systemic dermal exposures to Solvent Violet 13 from a number of products, to Solvent Blue 36 from the use of hair conditioner, and to Disperse Red 60 and Acid Blue 239 from wearing textiles. These exposure estimates are provided in Table 7-3.
| Substance | Product scenario and age groups | LADD (mg/kg bw/day)a |
|---|---|---|
| Solvent Violet 13 | Body cream (child, teen, adult) | 0.368 |
| Solvent Violet 13 | Spray perfume (child, teen, adult) | 0.00226 – 0.0558 |
| Solvent Violet 13 | Permanent hair dye (teen, adult) | 2.4E-04 – 0.00579 |
| Solvent Violet 13 | Face paint (toddler, child, teen, adult)b | 4.53E-04 – 0.0115 |
| Solvent Blue 36 | Hair conditioner (toddler, child, teen, adult) | 1.83E-04 |
| Disperse Red 60 | Textiles, wearing clothes (infant, toddler, child, teen, and adult) | 5.45E-04 |
| Acid Blue 239 | Textiles, wearing clothes (infant, toddler, child, teen, and adult) | 9.01E-06 |
Abbreviation: LADD, lifetime average daily dose
a Dermal absorption of Disperse Red 60 was assumed to be 2%. Dermal absorption of Solvent Violet 13 was estimated to be either 1.63 µg/cm2 or 1.63 to 41.33 µg/cm2, depending on the scenario. Dermal exposures of Solvent Blue 36, and Acid Blue 239 were estimated using a maximum flux approach (see Appendix A).
b Although only toddlers were considered when calculating an LADD from oral exposures to face paint (because of potential for hand-to-mouth contact), children, teens and adults were also considered when calculating an LADD from dermal exposures (see Appendix A).
Aggregate daily exposures for infants wearing and mouthing their sleepers were also derived for Disperse Red 60 and Acid Blue 239 of 0.00108 and 2.83E-04 mg/kg bw/day, respectively. Other potential aggregate exposures (e.g., oral and dermal exposures to Solvent Violet 13 from face paint and co-exposures from use of multiple cosmetics containing Solvent Violet 13) were considered but are not presented because of the potential inadequacy of the margins of exposure (MOEs) of the individual route- and product-specific exposure estimates (see section 7.3, Characterization of risk to human health).
Dermal exposure to Pigment Blue 60 from the use of artist paints (MSDS 2015b, 2018) and to Solvent Blue 36 from the use of dent repair products (MSDS 2002) and nozzle gels (MSDS 2015c) was also considered, but any such contact was determined to be incidental and less than other dermal exposure estimates presented herein.
Exposure via inhalation to Solvent Violet 13 from the use of spray perfumes (aerosol) was considered, and a daily systemic exposure estimate of 1.72E-05 mg/kg bw/day was derived for adults. Inhalation exposure to the remaining substances in the Anthraquinones Group was not considered to be of concern because of their very low volatility and their use pattern (i.e., non-aerosolized products).
7.2 Health effects assessment
There were limited chemical-specific health effects data for substances in the Anthraquinones Group. Potential analogues were identified using the OECD QSAR toolbox (2014) and were considered for similarities in their physical and chemical properties, metabolism, and structure. Using a read-across approach, health effects data from analogues were used to inform certain endpoints where chemical-specific data for substances in the Anthraquinones Group were not available.
The chemical-specific health effects data for each substance in the Anthraquinones Group will be presented first, followed by health effects information for the source chemicals (i.e., analogues) used in read-across and a summary of the points of departure selected for the characterization of risk to human health.
Solvent Violet 13
In a 28-day oral repeated dose toxicity study (OECD test guideline 407), Wistar rats (5/sex/dose) were administered Macrolex Violett B (containing 97% Solvent Violet 13) via gavage at doses of 0, 250, 500 or 1000 mg/kg bw/day. Statistically significant changes in some hematological parameters were observed in males and females of the highest dose group (e.g., increased reticulocytes and decreased mean corpuscular hemoglobin (MCH), erythrocyte, hemoglobin, hematocrit and platelet values), but there were no histopathological changes in either the spleen or bone marrow. There was no effect on body weight or body weight gain for males or females at any dose level. Histopathological examination of tissues did not reveal any changes associated with administration of the chemical. For females in the 1000 mg/kg bw/day dose group, decreased adrenal weight was observed, but no similar decrease was found for males at this dosage. In the absence of any supporting histopathological changes, these findings were considered not to represent an adverse effect of treatment by the study authors. A no observed adverse effect level (NOAEL) of 1000 mg/kg bw/day, the highest dose tested, was identified by the study authors (ECHA c2007-2019a).
In a reproduction/developmental toxicity screening test (OECD test guideline 421), Wistar rats (12/sex/dose) were administered Macrolex Violett B (containing 97% of Solvent Violet 13) via gavage at doses of 0, 250, 500 or 1000 mg/kg bw/day for approximately 6 weeks for males and up to 8 weeks for females, including a 2-week pre-pairing phase, pairing, gestation and early lactation for females. There were no treatment-related effects on body weight or thyroid weight in either sex. No effect on male reproductive organ weights was observed at any dose levels. Histopathological examination of reproductive tissues (testes, epididymis and ovaries) from the control and 1000 mg/kg bw/day animals did not reveal any treatment-related findings. One male at 1000 mg/kg bw/day showed a mass on the right epididymis and microscopic examination revealed moderate sperm granuloma. However, in the absence of any similar occurrence in this dose group, this finding was considered by the study authors to be incidental and unrelated to treatment. Evaluation of thyroxine (T4) in adult males and offspring at day 13 of age did not identify any treatment-related effect at any dose level. There was no treatment-related effect on oestrous cycles, mating performance, fertility and gestation length at doses up to 1000 mg/kg bw/day. There was no effect on the number of implantations, post-implantation loss, litter size and sex ratio at birth and subsequent offspring survival to day 13 of age at doses up to 1000 mg/kg bw/day. There were no treatment-related effects observed in the offspring from birth to termination on day 13 at any dose level. A NOAEL of 1000 mg/kg bw/day, the highest dose tested, for reproductive and developmental effects was identified by the study authors (ECHA c2007-2019a).
Solvent Violet 13 was not mutagenic in vitro in a bacterial reverse mutation assay (i.e., Ames test) (Muzzal and Cook 1979). It also tested negative in vivo in a chromosome aberration assay in mice (ECHA c2007-2019a). Solvent Violet 13 did not show a carcinogenic effect when mouse skin was painted with 0.1 mL of a 1% solution (approximately 4.7 mg/kg bw/day) once per week for 18 months (Carson 1984). However, this study is considered limited because of its methodology (single unoccluded low dose, weekly dosing regime) and uncertainties (purity, effect of 0.1% sodium lauryl sulphate vehicle). Given the limited substance-specific hazard data for chronic toxicity and carcinogenicity endpoints, an analogue, anthraquinone, was used to inform this part of the hazard assessment (as described later).
Pigment Blue 60
Pigment Blue 60 was reviewed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 1974), which reported that it had low acute oral toxicity and was not a dermal sensitizer (Lu and Lavalleé 1964; Bär and Griepentrog 1960). No systemic effects were observed in rats administered 50 mg/kg bw/day in the diet for 6 months (Umeda 1956). In a combined repeated-dose toxicity study with the reproduction/developmental toxicity screening test (OECD test guideline 422), rats (10/sex/dose) were administered doses of 0, 100, 300 or 1000 mg/kg bw/day by gavage for 2 weeks premating for a total of approximately 5 (parental males) to 7 weeks (dams were dosed up to post-natal day [PND] 4) (Heucotech Ltd 2013). At 1000 mg/kg bw/day, there was a complete litter loss in one dam, increased post-implantation loss, decreased maternal food consumption (13% decrease during gestation), slightly decreased maternal body weight (during gestation), and decreased maternal body weight gain (20% decrease between gestation days 0 and 7), with no effects observed at 100 or 300 mg/kg bw/day. Although historical records noted one other control dam with a complete litter loss and two dams with 93% litter loss, complete litter loss was unusual. It was recognized that the biological significance of the reproductive effects were unclear considering that they occurred at the limit dose, that no effects were observed in pups, and that both reproductive effects were influenced entirely or largely by one dam. However, since the single dam represented 10% of the sampled group and the extent of post-implantation loss exceeded both concurrent and historical controls, the reproductive NOAEL was 300 mg/kg bw/day (Heucotech Ltd 2013). The parental NOAEL was determined to be 300 mg/kg bw/day on the basis of decreased maternal food consumption (statistically significant during gestation) and decreased maternal body weight gain (statistically significant gestation days 0 to 7) at the lowest observed adverse effect level (LOAEL) of 1000 mg/kg bw/day (Heucotech Ltd 2013).
In support of the reproductive NOAEL of 300 mg/kg bw/day, rats (20 to 23/sex/dose) were administered 0 or 500 mg/kg bw/day by diet for 6 months prior to mating, and for up to 2 years (except during the lactation period) in a combined reproductive toxicity and carcinogenicity study. Decreased body weight was noted (30 g versus 50 g in control) for the remainder of the 2-year period (Oettel et al. 1965). Unfortunately, limited available study details regarding reproductive parameters reduced confidence in the use of this combined reproductive and carcinogenicity study to establish a reproductive NOAEL (Oettel et al. 1965).
The in vitro genotoxicity results for Pigment Blue 60 were mixed. Regardless of metabolic activation, Pigment Blue 60 was not genotoxic in Ames assays (ETAD 1988) nor in a chromosomal aberration assay, but it was genotoxic in a sister chromatid exchange assay (NTP [modified 2017]). Consistently, no tumours or systemic toxicity were observed after 2 years in rats administered doses of 0, 50 or 500 mg/kg bw/day in the diet in a carcinogenicity study (DFG 1957) or a dose of 500 mg/kg bw/day in the diet in a combined reproductive toxicity and carcinogenicity study (Oettel et al. 1965). Although there were study methodology limitations, including few animals (up to 23/sex/dose in the carcinogenicity studies, lack of detail), the two limited studies suggested that Pigment Blue 60 was neither genotoxic nor carcinogenic.
Solvent Blue 36
In a combined repeated-dose toxicity study with the reproduction/developmental toxicity screening test (OECD test guideline 422), rats (12/sex/dose) were administered Solvent Blue 36 via gavage at doses of 0, 12, 60 or 300 mg/kg bw/day for approximately 6 to 7 weeks up to PND 4 (MHLWJ 2012). In addition, a non-breeding group (5/sex/dose) were given 0 or 300 mg/kg/day for 6 weeks by gavage and were examined after a 2-week recovery period. At the low dose of 12 mg/kg bw/day and above, dams in the post-natal phase had decreased food consumption (16% to 20%) and decreased body weight gain (13% or 60%). At 60 mg/kg bw/day and above, parental males exposed for 6 weeks had liver toxicity (increased absolute and relative liver weights, liver hypertrophy), while dams had increased toxicity, including 5% decreased body weight, decreased absolute and relative pituitary weights (15% and 13%, respectively), decreased white blood cells (leucocyte, neutrophil, basophil, monocyte), and increased thyroid hormone levels (T3, T4, TSH). At 300 mg/kg bw/day, there was increased toxicity in all adults exposed for 6 weeks, including increased alanine transaminase (ALT) levels, atrophy of the thymus gland and cortical cell hypertrophy in the adrenal gland in both non-pregnant and pregnant females, as well as other signs of toxicity (inflammatory cell infiltration of prostate and decreased eosinophils in males; liver hypertrophy, increased adrenal weight in non-pregnant females; decreased hematocrit and blood urea nitrogen levels, tubular regeneration in kidneys, microgranuloma in liver, extramedullary hematopoiesis in spleen in dams). Since decreased food consumption and decreased body weight gain at 12 mg/kg bw/day were not reflected in body weight changes in dams and could be reversible changes, it was not selected as a parental LOAEL. The parental NOAEL was determined to be 12 mg/kg bw/day on the basis of the LOAEL of 60 mg/kg bw/day at which there was increased liver weight and hypertrophy in males exposed for 6 weeks, as well as decreased body weight, body weight gain, and food consumption in dams.
At 12 mg/kg bw/day and above, PND 4 pups had decreased body weight in both sexes relative to controls (12% at 12 mg/kg bw/day, 35% to 36% at 60 mg/kg bw/day). Although this may have been secondary to maternal effects, 12 mg/kg bw/day was selected as a developmental LOAEL for Solvent Blue 36 considering the increased severity of effect at 60 mg/kg bw/day, at which there was decreased pup birth weight for 5%, 8% (statistically significant), and 13% (statistically significant) at low, mid, and high doses, respectively, in both sexes, decreased body weight gain (statistically significant 90% to 93% decrease relative to controls in both sexes PND 0 to 4), and decreased % of PND 4 pup viability (mean value), with 86%, 87%, 30% (statistically significant), and 0.6% (statistically significant) viability at the control, low, mid and high dose, respectively. At 300 mg/kg bw/day, there were entire litter losses except for 1 male pup. Discoloration in PND 4 pups at all doses (blue fat in all, some with blue skin and gastrointestinal content) was not considered adverse but suggested transfer via milk. As 12 mg/kg bw/day was the lowest tested dose, the developmental NOAEL was not determined.
Solvent Blue 36 is positive (with activation) in vitro in an Ames test and in a chromosome aberration test in Chinese hamster V79 lung cells (MHLWJ 2012).
Disperse Red 60
Rats (20/sex/dose) administered 0 or 1000 mg/kg bw/day Disperse Red 60 via gavage for 4 weeks (once per day, 5 days per week) were not affected other than discoloration of urine and tissues (Leist 1982). The latter was considered a reflection of absorption and not adverse, and a NOAEL of 1000 mg/kg bw/day was thus established.
The genotoxicity profile of Disperse Red 60 in vitro was mixed (Ames negative with or without activation, Ames positive with or without activation, positive and negative in a mouse lymphoma TK assay), but it was not a clastogen in chromosome aberration and sister chromatid exchange assays (ECHA c2007-2019b; ETAD 1988; Seifried et al. 2006; Crompton & Knowles Corp. 1988; NTP [modified 2017]).
Solvent Violet 59
In a short-term repeated dose toxicity study (OECD test guideline 407), Wistar rats (5/sex/dose) were administered Macrolex Rotviolett R (containing 94% to 100% Solvent Violet 59) via gavage at doses of 0, 100, 300 or 1000 mg/kg bw/day for 28 consecutive days. There were no treatment-related changes in body or organ weights, nor were any treatment-related hematological changes observed. Histopathological examination of tissues did not reveal any changes associated with administration of the substance. A NOAEL of 1000 mg/kg bw/day, the highest dose tested, was identified by the study authors (ECHA c2007-2019c).
In a reproduction/developmental toxicity screening test (OECD test guideline 421), Wistar rats (12/sex/dose) were administered Macrolex Rotviolett R (containing a nominal concentration of 91% to 103% Solvent Violet 59) via gavage at doses of 0, 100, 300 or 1000 mg/kg bw/day for approximately 6 weeks for males and up to 8 weeks for females (including a 2-week pre-pairing phase, pairing, gestation, and lactation to day 13). There were no treatment-related effects on body weight or thyroid weight in either sex. No effect on male reproductive organ weights was observed at any dose level. At 300 mg/kg bw/day, two males showed small and flaccid testes and small epididymides, and both of these males failed to mate with their female partners. In the absence of any similar finding at the high dose of 1000 mg/kg bw/day, these findings were considered by the study author to be incidental and unrelated to treatment. Evaluation of thyroxine (T4) in adult males and offspring at day 13 of age did not identify any treatment-related effects at any dose level. There was no treatment-related effect on estrous cycles, mating performance, fertility or gestation length at doses up to 1000 mg/kg bw/day. There was no effect on the number of implantations, post-implantation loss, litter size, sex ratio at birth or subsequent offspring survival to day 13 of age at doses up to 1000 mg/kg bw/day. There were no treatment-related effects observed in the offspring from birth to termination on day 13 at any dose level. A NOAEL of 1000 mg/kg bw/day, the highest dose tested, for reproductive and developmental effects was identified by the study authors (ECHA c2007-2019c).
Solvent Violet 59 was mutagenic in vitro in a modified Ames assay using Salmonella typhimurium strains YG1041 and YG1042 (derived from TA 98 and 100, respectively), with and without metabolic activation (Health Canada 2017).
Acid Blue 239 and CAS RN 74499-36-8
Health effects information was minimal for the other two substances in the Anthraquinones Group. The ECHA registration dossier for Acid Blue 239 contains an Ames test which shows no evidence of mutagenicity with and without metabolic activation, although the purity of the test material is only 65.6% (ECHA c2007-2019d). CAS RN 74499-36-8 was mutagenic in a modified Ames assay using Salmonella typhimurium strains YG 1040 and YG 1042 (derived from TA 98 and 100, respectively), with and without metabolic activation (Health Canada 2017).
Selection of analogues and corresponding health effects information for read-across to substances in the Anthraquinones Group
Points of departure for per-event exposures in Table 7-4 for Solvent Violet 13, Pigment Blue 60, Solvent Violet 59, and Solvent Blue 36 were derived from substance-specific studies that are described earlier in this section; read-across was not required. For Solvent Violet 13 and Solvent Violet 59, the points of departure were each based on OECD test guideline 421 studies with reproductive/developmental NOAELs of 1000 mg/kg bw/day (ECHA c2007-2019a, ECHA c2007-2019c). The points of departure for Pigment Blue 60 and Solvent Blue 36 were each based on OECD test guideline 422 studies with a reproductive/parental NOAEL of 300 mg/kg bw/day (Heucotech Ltd 2013) and a developmental LOAEL of 12 mg/kg bw/day (MHLWJ 2012), respectively.
For Disperse Red 60, systemic toxicity was informed by a substance-specific 4-week study, and the NOAEL of 1000 mg/kg bw/day (Leist 1982) is identified in Table 7-4 as the point of departure for per-event exposures. For reproductive and developmental effects, there was an OECD test guideline 422 study identified in the ECHA registration dossier for this substance; it was based on read-across to an analogue (ECHA c2007-2019b), in which no reproductive or developmental effects were observed up to the highest dose tested of 1000 mg/kg bw/day.
For Acid Blue 239 and CAS RN 74499-36-8, systemic and reproductive/developmental effects for Acid Blue 239 and CAS RN 74499-36-8 were based on read-across to a previously described OECD test guideline 422 study in rats administered Solvent Blue 36 by gavage (MHLWJ 2012). The point of departure for systemic effects consisted of a parental NOAEL of 12 mg/kg bw/day based on adverse effects identified with 60 and 300 mg/kg bw/day Solvent Blue 36 (including increased liver toxicity in both sexes, increased adrenal gland weights and hypertrophy, atrophy of the thymus gland, and decreased spleen and ovary weights in females). For reproductive and developmental toxicity, there were no reproductive effects up to the highest tested dose of 300 mg/kg bw/day for Solvent Blue 36, but developmental effects were considered to have occurred at the lowest tested dose of 12 mg/kg bw/day. The points of departure from this study consisted of a parental NOAEL and a developmental LOAEL of 12 mg/kg bw/day. These were determined to be the most sensitive endpoints among the potential analogues considered for systemic and developmental effects, with the developmental LOAEL of 12 mg/kg bw/day being the most sensitive.
The point of departure for daily exposures (systemic) for Pigment Blue 60 presented in Table 7-4 is the same as that selected for per-event exposures, as the reproductive/parental NOAEL of 300 mg/kg bw/day (Heucotech Ltd 2013) is more sensitive than the NOAEL of 500 mg/kg bw/day that could potentially have been used from applicable studies of longer duration (DFG 1957; Oettel et al. 1965).
For daily exposures (systemic) for other substances in the Anthraquinones Group, chronic systemic effects were informed by the 2-year chronic/carcinogenicity study conducted on anthraquinone (NTP 2005), in which observations included decreased body weight and effects in the kidneys, liver, spleen, and bone marrow in rats, resulting in a LOAEL of 20 mg/kg bw/day. Additional details on this study are provided below. This LOAEL of 20 mg/kg bw/day is described in Table 7-4 as the point of departure for daily exposures (systemic) for Solvent Violet 13, Solvent Violet 59, and Disperse Red 60. For Solvent Blue 36, Acid Blue 239 and CAS RN 74499-36-8, however, the OECD test guideline 422 study for Solvent Blue 36 (MHLWJ 2012) was used to derive the point of departure for daily exposures (systemic) presented in Table 7-4. Developmental toxicity effects were considered to have occurred at the lowest tested dose of 12 mg/kg bw/day, as previously described, and the LOAEL of 20 mg/kg bw/day with anthraquinone cannot be achieved without exceeding the per-event LOAEL of 12 mg/kg bw/day.
Predictions for in vitro genotoxicity using (Q)SAR models of Acid Blue 239 provided mixed results: in the statistical Leadscope model, the substance was predicted to be positive (Leadscope Model Applier 2016); in the TIMES structural alert model, it was predicted to be positive (TIMES 2016); in the Derek Nexus expert knowledge-based model, no structural alerts were found (Derek Nexus 2016). In vitro genotoxicity data from other substances in the Anthraquinones Group were mixed: Solvent Violet 13 was not genotoxic in vitro (negative Ames test) (Muzzal and Cook 1979); Pigment Blue 60 was not genotoxic overall (negative Ames assay, mouse lymphoma assay, chromosomal aberration assay; positive sister chromatid exchange) (ETAD 1988; NTP 2005); Solvent Blue 36 was genotoxic (positive Ames test and chromosomal aberration assay) (MHLWJ 2012); equivocal results were reported for genotoxicity of Disperse Red 60 (mixed results in Ames and mouse lymphoma assays, negative chromosomal aberration and sister chromatid exchange assays) (ECHA c2007-2019b; ETAD 1988; Seifried et al. 2006; Crompton & Knowles Corp. 1988; NTP [modified 2017]); and Solvent Violet 59 and CAS RN 74499-36-8 were genotoxic (positive modified Ames assays) (Health Canada 2017). Available information suggests that Acid Blue 239 may be genotoxic in vitro.
Although there were mixed in vitro and in vivo genotoxicity results for anthraquinone (CAS RN 84-65-1)—the common structural backbone of substances in the Anthraquinones Group—positive results have been attributed to potential impurities that were mutagenic (e.g., 9-nitroanthacene) and which may have occurred when anthraquinone was manufactured by oxidation of anthracene distilled from coal tar (ECHA 2015a; Alay 2012). It has also been noted that potential mutagenicity due to mutagenic metabolites cannot be excluded (ECHA 2015b), but on the basis of a weight of evidence approach ECHA determined that the in vitro and in vivo tests generally indicate no effect on mutation frequency (ECHA 2015a).
Considering potential analogues, the substances in the Anthraquinones Group other than Pigment Blue 60 are potentially carcinogenic. Pigment Blue 60 was not identified as a potential analogue for this endpoint using the OECD QSAR Toolbox, likely because of differences in structural alerts between Pigment Blue 60 and the other substances in the Anthraquinones Group. Six potential analogues identified using the OECD QSAR Toolbox had increased liver (CAS RNs 82-28-0, 84-65-1, 117-10-2, 129-15-7, 129- 43-1) and urinary bladder tumours (CAS RN 81-54-9) in rats and/or mice (NCI 1978; NTP 2005; Mori et al. 1985, 1986, 1990, 1991; Yoshimi et al. 1995; Tanaka et al. 1991; Bionetics Research Labs 1968; Krishna Murthy et al. 1977; CPDB [modified 2007]). The chronic points of departure were quite diverse, with LOAELs ranging from 20 mg/kg bw/day (see Table 7-4) for anthraquinone (CAS RN 84-65-1) to 500 mg/kg bw/day (only tested dose for CAS RN 117-10-2, 81-54-9, 129-43-1) in rats, and from 39 mg/kg bw/day (CAS 129-15-7) to 260 mg/kg bw/day (CAS RN 117-10-2) in mice.
Anthraquinone (CAS RN 84-65-1) was tumorigenic in both rats and mice by the dietary route. It was selected as the source chemical for read-across of carcinogenic potential and non-cancer systemic effects for some substances in the Anthraquinones Group, since it had the lowest LOAEL where tumours or systemic effects were observed and since there were limitations in the methodology of some of the other studies (e.g., single dose, irregular dosing, short duration, low purity). Because anthraquinone increased kidney tumours in rats at the lowest tested dose of 20 mg/kg bw/day, no NOAEL was determined for anthraquinone (NTP 2005). This LOAEL was considered protective of tumours observed in other dietary carcinogenicity studies with analogues, including 2-methyl-1-nitroanthraquinone (CAS RN 129-15-7), which induced subcutaneous fibromas in male rats at a slightly higher LOAEL (lowest tested dose) of 30 mg/kg bw/day in a 78-week dietary study (NCI 1978).
The International Agency for Research on Cancer (IARC) has classified anthraquinone as Group 2B (sufficient data in animals, inadequate in humans) (IARC 2013). It was not considered mutagenic in vitro or in vivo and was classified as a Globally Harmonized System (GHS) 1B Carcinogen by the European Chemicals Agency (EU 2017; ECHA 2015a). In a 2-year carcinogenicity study in rats and mice by the National Toxicology Program (NTP), F344/N rats (50/sex/dose) were administered anthraquinone in the diet at doses of 0, 469, 938, 1875 or 3750 ppm (equivalent to 0, 20/25, 45/50, 90/100, 180/200 mg/kg bw/day, males/females respectively) (NTP 2005). At 20/25 mg/kg bw/day (males/females) and above, observations included: decreased body weight (6% males/11% females); effects on the kidneys (increased absolute and relative weight, nephropathy, hyaline droplet accumulation, pigmentation, mineralization in renal medulla, and transitional epithelial hyperplasia in both sexes), liver (increased absolute and relative weight in both sexes), spleen (incidence congestion, pigmentation, and hematopoietic cell proliferation in both sexes), and bone marrow (increased hyperplasia and increased atrophy in females); increased renal tubule adenomas in both sexes; and combined adenomas and carcinomas in females. Incidences of renal tubular adenomas were 1/0, 3/4, 9/9, 5/7, 3/12 for males/females, out of 50/sex/dose (except for 49 high-dose females), which were all above historical control ranges for both sexes (0% to 4% in males, 0% to 2% in females). These increases were statistically significant relative to controls for males at 45 and 180 mg/kg bw/day, and in females at 50 mg/kg bw/day and above. At 45/50 mg/kg bw/day or above, there were increased urinary bladder (transitional epithelial hyperplasia, papillomas, and/or carcinomas) and liver effects (hepatocellular adenomas in females, equivocal combined increased adenomas and carcinomas in males). NTP concluded that there was some evidence of carcinogenicity in the male F344/N rat based on increased incidences of renal tubule adenoma and of transitional epithelial papillomas of the kidney and urinary bladder, and that hepatocellular neoplasms may be related. There was clear evidence of carcinogenicity in female F344/N rats based on increased incidences of renal tubule neoplasms, of urinary bladder transitional epithelial papillomas/carcinomas (combined) and of hepatocellular adenomas (NTP 2005).
The respective incidences of combined renal adenomas and carcinomas in female rats for doses of 0, 25, 50, 100 or 200 mg/kg bw/day were 0, 6, 9, 8 and 14 out of 50 female rats, except for the highest dosed group, which only had 49 female rats (NTP 2005). Using the US EPA’s Benchmark Dose Software (BMDS, ver. 2.6.0) and selecting the LogLogistic model, which had the lowest Akaike’s Information Criterion value and a lack of warning in benchmark dose level (BMDL) computation, the benchmark dose (BMD) for 10% extra risk was 41 mg/kg bw/day, and the lower limit for 10% extra risk (BMDL10) was 30.3 mg/kg bw/day (personal communication, email from the Biostatistics Unit, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated April 12, 2017; unreferenced).
In the 2-year NTP (2005) study with dietary anthraquinone in B6C3F1 mice (50/sex/treated dose, 60/sex/control) fed 0, 833, 2500 or 7500 ppm anthraquinone (equivalent to 0, 90/80, 265/235, 825/745 mg/kg bw/day in males/females), there were effects at the lowest tested dose of 90/80 mg/kg bw/day (male/female) and above in the liver (including hypertrophy, hepatoblastomas, adenomas and carcinomas) and thyroid gland (follicular cell hyperplasia and equivocal increases in neoplasms). NTP (2005) determined that this was clear evidence of carcinogenicity in male and female B6C3F1 mice, based on increased incidence of liver neoplasms, and considered that thyroid gland follicular cell neoplasms might be related. This was in contrast to an earlier carcinogenicity study with no clear tumour increases in B6C3F1 or B6AKF1 mice with 464 mg/kg bw/day for 4 weeks by gavage followed by 157 mg/kg bw/day up to 18 months by diet (Bionetics Research Labs 1968). The rationale for the discrepancy was unclear but could be related to methodology since the test article purity or stability was unclear, there were fewer animals (18/sex/strain, with 4/sex/BC3F1 and 3 female B6AKF1 mice dying from pneumonia), and the study duration was shorter. The tumours observed in the NTP studies could not be attributed entirely to the mutagenic impurities in the anthraquinone used, which included 0.1% 9-nitroanthracene (NTP 2005; ECHA 2015a).
Points of departure from these oral studies were considered applicable to dermal and inhalation routes of exposure in the absence of adequate route-specific hazard data for either route. A 4-month whole body inhalation study in which a total of 96 rats were exposed to 0, 5.5 or 12.2 mg/m3 anthraquinone for 5 to 6 hours/day was considered supportive data since there was limited detail. At 12 mg/m3, rats had decreased body weight, hemoglobin levels, and erythrocyte counts and increased reticulopenia (Volodchenko et al. 1970).
Summary of points of departure selected for characterization of risk to human health
The points of departure selected for the characterization of risk to human health for each substance (see section 7.3, Characterization of risk to human health) are summarized in Table 7-4. Unless chemical-specific empirical health effects data were available, health effects data from an analogue were used.
| Substance | Per-event exposure | Daily exposure (systemic) |
|---|---|---|
| Solvent Violet 13 | OECD test guideline 421 (rats, gavage) Reproductive/developmental NOAEL of 1000 mg/kg bw/day, no effects at the highest dose tested | Based on 2-year chronic/carcinogenicity study conducted on anthraquinone (rats, diet) LOAEL of 20 mg/kg bw/day (decreased BW and effects in kidneys, liver, spleen, bone marrow) |
| Pigment Blue 60 | OECD test guideline 422 (rats, gavage)Reproductive/parental NOAEL of 300 mg/kg bw/day, LOAEL of 1000 mg/kg bw/day (litter loss, increased post-implantation loss, decreased maternal FC and BWG) | Based on the per-event point of departure reproductive/parental NOAEL of 300 mg/kg bw/day |
| Solvent Violet 59 | OECD test guideline 421 (rats, gavage) Reproductive/developmental NOAEL of 1000 mg/kg bw/day, no effects at the highest dose tested | Based on 2-year chronic/carcinogenicity study conducted on anthraquinone (rats, diet) LOAEL of 20 mg/kg bw/day (decreased BW and effects in kidneys, liver, spleen, bone marrow) |
| Solvent Blue 36 | OECD test guideline 422 (rats, gavage) Parental NOAEL of 12 mg/kg bw/day, LOAEL of 60 mg/kg bw/day (increased liver weight, hypertrophy day 42 males; decreased BW, BWG, FC dams)Developmental LOAEL of 12 mg/kg bw/day (LTD): decreased BW PND4 pups | Based on the per-event point of departure developmental LOAEL of 12 mg/kg bw/day (LTD) |
| Disperse Red 60 | 4-week study (rats, gavage) NOAEL of 1000 mg/kg bw/day (HTD) | Based on 2-year chronic/carcinogenicity study conducted on anthraquinone (rats, diet) LOAEL of 20 mg/kg bw/day (decreased BW and effects in kidneys, liver, spleen, bone marrow) |
| Acid Blue 239 | See Solvent Blue 36 | See Solvent Blue 36 |
| CAS RN 74499-36-8 | See Solvent Blue 36 | See Solvent Blue 36 |
Abbreviations: BW(G), body weight (gain); FC, food consumption; HTD, highest tested dose; PND, postnatal day; LOAEL, lowest observed adverse effect level; LTD, lowest tested dose; NOAEL, no observed adverse effect level; OECD test guideline 422, Combined Repeated Dose Toxicity Study with the Reproduction/Developmental Toxicity Screening Test.
As previously described, the BMDL10 of 30.3 mg/kg bw/day based on increased renal adenomas and carcinomas in female rats administered anthraquinone was used to estimate the cancer risk for all dermal and oral daily exposure scenarios of substances in the Anthraquinones Group, except for Pigment Blue 60. An estimate of the cancer risk to Pigment Blue 60 was not derived given its toxicity profile (e.g., negative carcinogenicity up to 500 mg/kg bw/day and negative genotoxicity).
7.3 Characterization of risk to human health
Tables 7-5 and 7-6 provide relevant oral and dermal exposure estimates and hazard points of departure (PODs) for substances in the Anthraquinones Group, as well as resultant MOEs, for determination of risk.
| Exposure scenario | Systemic exposure (mg/kg bw [/d]) | Critical effect level (mg/kg bw [/d]) | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Lip balm, toddler, per event, Solvent Violet 13 | 0.0065 | NOAEL 1000 | Reproductive/developmental toxicity screening test, no adverse effects at the highest tested dose | 150 000 |
| Lip balm, toddler, daily, Solvent Violet 13 | 0.0038 | LOAEL 20 | 2-year chronic/carcinogenicity study, decreased body weight and effects in kidneys, liver, spleen, and bone marrow | 5263 |
| Face paint, toddler, per event, Solvent Violet 13 | 0.53 | NOAEL 1000 | Reproductive/developmental toxicity screening test, no adverse effects at the highest tested dose | 1887 |
| Lipstick, adult, daily, Solvent Violet 13 | 0.0034 | LOAEL 20 | 2-year chronic/carcinogenicity study, decreased body weight and effects in kidneys, liver, spleen, and bone marrow | 5882 |
| Arts and crafts (i.e., stampers), toddler, per event, Pigment Blue 60 | 1.29 | NOAEL 300 | Combined repeated-dose study with reproduction/ developmental screening test, decreased maternal food consumption and decreased body weight gain | 233 |
| Arts and crafts (i.e., stampers), toddler, daily, Pigment Blue 60 | 0.0645 | NOAEL 300 | Combined repeated-dose study with reproduction/ developmental screening test, decreased maternal food consumption and decreased body weight gain | 4651 |
| Plastic toys, mouthing, toddler, daily, Solvent Violet 59 | 3.4E-06 | LOAEL 20 | 2-year chronic/carcinogenicity study, decreased body weight and effects in kidneys, liver, spleen, and bone marrow | 5 900 000 |
| Textiles, mouthing, infant, daily, Disperse Red 60 | 0.00027 | LOAEL 20 | 2-year chronic/carcinogenicity study, decreased body weight and effects in kidneys, liver, spleen, and bone marrow | 74 000 |
| Textiles, mouthing, infant, per event, Acid Blue 239 | 0.0027a | LOAEL 12 | Combined repeated-dose study with reproduction/developmental screening test, decreased body weight in PND 4 pups | 4444 |
Abbreviations: LOAEL, lowest observed adverse effect level; MOE, margin of exposure; NOAEL, no observed adverse effect level.
a Although mouthing of textiles would occur on a daily basis, the “per-event” systemic oral exposure estimate based on use of the initial “acute” migration fraction was used to be protective of the developmental endpoint.
On the basis of the conservative parameters used in modelling exposures to products, the resulting MOEs for non-cancer systemic effects are considered adequate to address uncertainties in the health effects and exposure databases for oral exposure scenarios for Solvent Violet 13, Pigment Blue 60, Solvent Violet 59, Disperse Red 60, and Acid Blue 239.
| Exposure scenario | Systemic exposure (mg/kg bw [/d]) | MOE (based on BMDL10)a |
|---|---|---|
| Lip balm or lipstick, LADD (toddler, child, teen, adult), Solvent Violet 13 | 0.00342b | 8860 |
| Face paint, LADD (toddler), Solvent Violet 13 | 0.00102 | 30 000 |
| Plastic toys, mouthing, toddler, daily, Solvent Violet 59 | 3.4E-06 | 9 000 000 |
| Textiles, mouthing, infant, daily, Disperse Red 60 | 0.00027 | 100 000 |
| Textiles, mouthing, infant, daily, Acid Blue 239 | 0.00027 | 100 000 |
Abbreviations: LADD, lifetime average daily dose; MOE, margin of exposure.
a BMDL10 of 30.3 mg/kg bw/day from increased renal adenomas and carcinomas in female rats (NTP 2005).
b The LADD represents use of lip balm by toddlers, children, teens, and adults and is protective of potential cancer effects from the use of a lipstick.
The MOEs for oral exposure scenarios for cancer effects were considered adequate to address uncertainties in the health effects and exposure databases for Solvent Violet 59, Disperse Red 60 and Acid Blue 239, but are potentially inadequate for Solvent Violet 13 from the use of lip balm or lipstick, even when adjusted for lifetime exposure.
| Exposure scenario | Systemic exposure (mg/kg bw [/d]) | Critical effect level (mg/kg bw [/d]) | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Body cream, adult, daily, Solvent Violet 13 | 0.389 | LOAEL 20 | 2-year chronic/carcinogenicity study, decreased body weight and effects in kidneys, liver, spleen, and bone marrow | 51 |
| Permanent hair dye, teen, per event, Solvent Violet 13 | 0.0175 – 0.444 | NOAEL 1000 | Reproductive/developmental toxicity screening test, no adverse effects at the highest tested dose | 2 252 – 57 143 |
| Spray perfume, adult, daily, Solvent Violet 13 | 0.00230 –0.0583 | LOAEL 20 | 2-year chronic/carcinogenicity study, decreased body weight and effects in kidneys, liver, spleen, and bone marrow | 343 – 8 696 |
| Face paint, toddler, per event, Solvent Violet 13 | 0.0457 – 1.16 | NOAEL 1000 | Reproductive/developmental toxicity screening test, no adverse effects at the highest tested dose | 862 – 21 882 |
| Arts and crafts (i.e., stampers), toddler, per event, Pigment Blue 60 | 1.29 | NOAEL 300 | Combined repeated-dose study with reproduction/developmental screening test, decreased material food consumption and decreased body weight gain | 233 |
| Arts and crafts (i.e., stampers), teen, per event, Pigment Blue 60 | 0.337 | NOAEL 300 | Combined repeated-dose study with reproduction/developmental screening test, increased or complete litter loss | 890 |
| Arts and crafts (i.e., stampers), toddler, daily, Pigment Blue 60 | 0.0645 | NOAEL 300 | Combined repeated-dose study with reproduction/developmental screening test, decreased material food consumption and decreased body weight gain | 4651 |
| Arts and crafts (i.e., stampers), teen, daily, Pigment Blue 60 | 0.0168 | NOAEL 300 | Combined repeated-dose study with reproduction/developmental screening test, increased or complete litter loss | 18 000 |
| Specialty lubricants, adult, per event, Solvent Blue 36 | 3.82E-05 | LOAEL 12 | Combined repeated-dose study with reproduction/developmental screening test decreased body weight in PND 4 pups | 310 000 |
| Hair conditioner, adult, daily, Solvent Blue 36 | 1.84E-04 | LOAEL 12 | Combined repeated-dose study with reproduction/developmental screening test, decreased body weight in PND 4 pups | 65 000 |
| Textiles, wearing clothing, infant, daily, Disperse Red 60 | 8.05E-04 | LOAEL 20 | 2-year chronic/carcinogenicity study, decreased body weight and effects in kidneys, liver, spleen, and bone marrow | 25 000 |
| Textiles, wearing clothing, adult, daily, Disperse Red 60 | 5.13E-04 | LOAEL 20 | 2-year chronic/carcinogenicity study, decreased body weight and effects in kidneys, liver, spleen, and bone marrow | 39 000 |
| Textiles, wearing clothing, infant, per event, Acid Blue 239 | 1.33E-05 | LOAEL 12 | Combined repeated-dose study with reproduction/developmental screening test, decreased body weight in PND 4 pups | 902 000 |
| Textiles, wearing clothing, adult, per event, Acid Blue 239 | 8.49E-06 | LOAEL 12 | Combined repeated-dose study with reproduction/developmental screening test, decreased body weight in PND 4 pups | 1 400 000 |
| Epoxy coating product, application, adult, per event, CAS RN 74499-36-8 | 0.0035 | LOAEL 12 | Combined repeated-dose study with reproduction/developmental screening test, decreased body weight in PND 4 pups | 3429 |
Abbreviations: LOAEL, lowest observed adverse effect level; MOE, margin of exposure; NOAEL, no observed adverse effect level.
On the basis of the conservative parameters used in modelling exposure to products, the resulting MOEs for non-cancer systemic effects are considered adequate to address uncertainties in the health effects and exposure databases for dermal exposure scenarios for Solvent Violet 13 from permanent hair dye and face paint, Pigment Blue 60, Solvent Blue 36, Disperse Red 60, Acid Blue 239, and CAS RN 74499-36-8. However, the resulting MOEs for non-cancer systemic effects are not considered adequate to address uncertainties for dermal exposure scenarios for Solvent Violet 13 from body cream and spray perfume.
Aggregate daily oral and dermal exposures of 0.00108 and 2.83E-04 mg/kg bw/day were derived for Disperse Red 60 and Acid Blue 239, respectively, for infants wearing and mouthing their sleepers. This resulted in MOEs of 18 000 and 42 000, respectively, for non-cancer effects when compared to LOAELs of 20 and 12 mg/kg bw/day, respectively. These MOEs were considered adequate to address uncertainties in the health effects and exposure databases.
| Exposure scenario | LADD(mg/kg bw [/d]) | MOE(based on BMDL10)a |
|---|---|---|
| Body cream (child, teen, adult), Solvent Violet 13 | 0.368 | 82 |
| Permanent hair dye (teen, adult), Solvent Violet 13 | 2.4E-04 – 0.00579 | 5233 – 130 000 |
| Spray perfume (child, teen, adult), Solvent Violet 13 | 0.00226 – 0.0558 | 543 – 13 000 |
| Face paint, (toddler, child, teen, adult)b, Solvent Violet 13 | 4.53E-04 – 0.0115 | 2635 – 67 000 |
| Hair conditioner (toddler, child, teen, adult), Solvent Blue 36 | 1.83E-04 | 166 000 |
| Textiles, wearing clothing (infant, toddler, child, teen, adult), Disperse Red 60 | 5.45E-04 | 56 000 |
| Textiles, wearing clothing (infant, toddler, child, teen, adult), Acid Blue 239 | 9.01E-06 | 3 400 000 |
Abbreviations: LADD, lifetime average daily dose; MOE, margin of exposure.
a BMDL10 of 30.3 mg/kg bw/day from increased renal adenomas and carcinomas in female rats (NTP 2005).
b Although only toddlers were considered when calculating an LADD from oral exposures to face paint (due to potential for hand-to-mouth contact), children, teens and adults were also considered for dermal exposures when calculating an LADD (see Appendix A).
Although the risks to the cancer endpoint for dermal exposure scenarios were considered adequate to address uncertainties in the health effects and exposure databases for Solvent Blue 36, Disperse Red 60 and Acid Blue 239, they were potentially inadequate to address uncertainties in the health effects and exposure databases for Solvent Violet 13 from body cream, permanent hair dye, spray perfume, and face paint.
The inhalation risk for daily exposure to Solvent Violet 13 in spray perfume (1.72E-05 mg/kg bw/day) was considered adequate when compared to the LOAEL of 20 mg/kg bw/day on the basis of decreased body weight and effects in kidneys, liver, spleen, and bone marrow, with an MOE of 1 200 000. The MOE of 1 800 000 using a BMDL10 of 30.3 mg/kg bw/day for cancer effects was also adequate for daily inhalation exposure to Solvent Violet 13 from spray perfume.
Given the conservative parameters used in modelling exposure to products, the resulting MOEs for systemic and cancer effects in inhalation scenarios are considered adequate to address uncertainties in the health effects and exposure databases for Solvent Violet 13. However, as the non-cancer and cancer MOEs for dermal systemic exposure were potentially inadequate for Solvent Violet 13 from spray perfume, MOEs for aggregate inhalation and dermal exposures would be potentially inadequate as well.
While exposures of the general population to Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239, and CAS RN 74499-36-8 are not of concern at current levels, these substances are considered to have a health effect of concern because of the potential carcinogenicity of their analogue anthraquinone. Therefore, there may be a concern for human health if exposures to these substances were to increase.
7.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key sources of uncertainty | Impact |
|---|---|
| Oral exposure to Pigment Blue 60 was estimated from incidental ingestion of craft products (i.e., stampers) by toddlers. The oral bioavailability of this pigment is unknown. To be protective of human health, it was assumed that systemic absorption following ingestion occurs. | + |
| Dermal absorption was conservatively assumed to be equivalent to absorption by the gastrointestinal tract for Pigment Blue 60 and CAS RN 74499-36-8. | + |
| There are no metabolism studies for substances in the Anthraquinones Group except dermal absorption for Disperse Red 60 and for Solvent Violet 13. | +/- |
| There are no in vivo genotoxicity studies for substances in the Anthraquinones Group, except for Solvent Violet 13. | +/- |
| There are no repeated-dose or chronic animal studies for oral exposure except for Pigment Blue 60 (repeated-dose, chronic), Solvent Blue 36 and Disperse Red 60 (repeated-dose). | +/- |
| There are no repeated-dose or chronic animal studies for inhalation exposure for Solvent Violet 13. | +/- |
| There are no repeated-dose or chronic animal studies for dermal exposure for substances in the Anthraquinones Group except for a limited skin painting study with Solvent Violet 13. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
8. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from Solvent Violet 13, Pigment Blue 60, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239 and CAS RN 74499-36-8. It is proposed to conclude that Solvent Violet 13, Pigment Blue 60, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239 and CAS RN 74499-36-8 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that Solvent Violet 13 meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this screening assessment, it is concluded that Pigment Blue 60, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239 and CAS RN 74499-36-8 do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that Solvent Violet 13 meets one or more of the criteria set out in section 64 of CEPA. It is also concluded that Pigment Blue 60, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239 and CAS RN 74499-36-8 do not meet any of the criteria set out in section 64 of CEPA.
It is concluded that Solvent Violet 13 meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
Alay GM. 2012. Anthraquinone, genotoxicity and carcinogenicity potential of different manufacturing origins. Barcelona (ES): REACH Monitor SLNE.
Bär F, Griepentrog F. 1960. Die allergenwirkung von fremden stiffen in den lebensmitteln. Med U Ernähr. 1:9. [cited in JECFA 1974].
BASF Corporation. 2015. Notice in accordance with TSCA Section 8(e): Results of a log Pow measurement of 1-Hydroxy-4-(p-toluidino)anthraquinone (CAS No. 81-48-1). Letter from Boucher A, BASF Corp. to US EPA TSCA.
[BfR] Bundesinstitut für Risikobewertung. 2007. Introduction to the problems surrounding garment textiles. Berlin (DE): German Federal Institute for Risk Assessment (BfR). BfR Information No. 018/2007, 1 June 2007. Available upon request.
Bionetics Research Labs Inc. 1968. Evaluation of carcinogenic, teratogenic, & mutagenic activities of selected pesticides and industrial chemicals. Volume I: Carcinogenic study. Bethesda (MD): National Cancer Institute. Report No.: NCI-DCCP-CG-1973-1-1.
Bremmer HJ, Prudhomme de Lodder LCH, van Engelen JGM. 2006. Cosmetics fact sheet. RIVM report 320104001/2006 [PDF].
Bremmer HJ, van Veen MP. 2002. Children’s toys fact sheet to assess the risks for the consumer. RIVM report 612810012/2002 [PDF].
Brown D (ICI Group Environmental Laboratory, Brixham, UK). 1983. Environmental assessment of dyestuffs. Prepared for the Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers, Basel, Switzerland. ETAD ecological sub-committee project E3020. Submitted to Environment Canada May 9, 2008.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List. Canada Gazette, Part I, vol. 146, no. 48, Supplement [PDF].
Carson ST. 1984. Skin painting studies in mice on 11 FD&C and D&C colors: FD&C Green No. 3, Red No. 2, Red No. 4, Yellow No. 6, and External D&C No. 7, D&C Orange No.4, Violet No. 2, Red No. 17, Red No. 34, and Yellow No. 7. J Toxicol. 3(3):309-331.
CATALOGIC [environmental fate and ecotoxicity model]. 2014. Ver. 5.11.15. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
Charles River Laboratories. 2017. The in vitro percutaneous absorption of radiolabelled solvent violet 13 in two test preparations through human skin. Draft report. Elphinstone (UK): Charles River Laboratories Edinburgh Ltd. 67 p. Report No.: 38617. Sponsored by [ESRAB] Environmental Health Science and Research Bureau, Health Canada, Ottawa (ON). [restricted access].
[ConsExpo] Consumer Exposure Model [Internet]. 2006 Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[CPDB] Carcinogenic Potency Database. [modified 2007 Oct 3].
Crompton & Knowles Corp. 1988. Health and safety data for six primary aminoanthraquinones selected for reporting by the EPA with attachments and cover letter dated 020188. Submission to the US EPA TSCA. Document number 86880000132. Microfiche No.: OTS0514022.
[Danish EPA] Danish Environmental Protection Agency. 2008. Survey and health assessment of chemical substances in hobby products for children. Survey of chemical substances in consumer products, No 93. Copenhagen (Denmark): Danish Environmental Protection Agency.
[Danish EPA] Danish Environmental Protection Agency. 2015. CMR substances in toys – Market surveillance and risk assessment. Copenhagen (Denmark).
Derek Nexus [toxicity prediction module]. 2016. Ver. 5.0.2. Leeds (UK): Lhasa Limited. [restricted access].
[DFG] Deutsche Forschungsgemeinschaft. 1957. Kommission zur Bearbeitung des Lebensmittelfarbstoffproblems. Mitteilung. 6. Toxikologische Daten von Farbstoffen und ... für Lebensmittel in verschiedenen Ländern. Bonn (DE): DFG. [cited in JECFA 1974].
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531-554.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC). ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2017 Apr 25].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018a. Rapid Screening of Substances with Limited General Population Exposure. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018b. Screening Assessment: Substances Identified as Being of Low Concern using the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances. Ottawa (ON): Government of Canada.
[ECHA] European Chemical Agency. 2015a. Committee for Risk Assessment: RAC Opinion proposing harmonised classification and labelling at EU level of Anthraquinone. Helsinki (FI): ECHA [accessed 2017 May 4].
[ECHA] European Chemical Agency. 2015b. CLH proposed report for anthraquinone (CAS No. 84-65-1). Helsinki (FI): ECHA.
[ECHA] European Chemicals Agency. 2017. Brief profile: 6,15-dihydroanthrazine-5,9,14,18-tetrone; CAS RN 81-77-6. Helsinki (FI): ECHA. [updated 2017 Apr 4; accessed 2017 Apr 18].
[ECHA] European Chemicals Agency. c2007-2017. Registered substances database; search results for CAS RN 81-77-6 and 17418-58-5. Helsinki (FI): ECHA. [updated 2017 May 12; accessed 2017 Jun 14].
[ECHA] European Chemicals Agency. c2007-2019a. Registered substances database; search results for CAS RN 81-48-1. Helsinki (FI): ECHA. [accessed 2019 May 30].
[ECHA] European Chemicals Agency. c2007-2019b. Registered substances database; search results for CAS RN 17418-58-5 Helsinki (FI): ECHA. [accessed 2020 Oct 19].
[ECHA] European Chemicals Agency. c2007-2019c. Registered substances database; search results for CAS RN 6408-72-6. Helsinki (FI): ECHA. [accessed 2019 May 30].
[ECHA] European Chemicals Agency. c2007-2019d. Registered substances database; search results for Acid Blue 239. Helsinki (FI): ECHA. [accessed 2019 Aug 14].
[Environ] ENVIRON International Corporation. 2003. Voluntary Children’s Chemical Evaluation Program Pilot (VCCEPP)-Tier 1 assessment of the potential health risks to children associated with exposure to the commercial octabromodiphenyl ether product and appendices. Emerville (CA): ENVIRON International Corporation.
Environment Canada. 2000. Environmental categorization for persistence, bioaccumulation and inherent toxicity of substances on the Domestic Substances List using QSARs. Unpublished final report. Gatineau (QC): Environment Canada, Existing Substances Division. On the cover: Results of an international QSAR workshop hosted by the Chemicals Evaluation Division of Environment Canada, November 11-12, 1999, Philadelphia, Pennsylvania. Available on request.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1983. Final report on extractability of dyestuffs from textiles. Basel (CH): ETAD. Project No. A 4007.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1988. Toxicological testing of major colorants. Basel (CH): ETAD. Project T 2015. OTS0000621.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1994. In vitro absorption of various dyes through human and pig epidermis Basel (CH): ETAD. Project T 2030, Part 1. OTS0001293.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1995. In vitro absorption of two disperse dyes from synthetic perspiration and five formulations. Basel (CH): ETAD. Project T 2030, Part 2.
[EU] European Union. 2017. Commission Regulation (EU) 2017/776 of 4 May 2017 amending, for the purposes of its adaptation to technical and scientific progress Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures. Off J Eur Union L 116:1-19.
Haynes WM, editor. 2017. CRC Handbook of Chemistry and Physics: Physical constants of organic compounds. [Internet]. 97th ed. Boca Raton (FL): CRC Press/Taylor & Francis.
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations. Ottawa (ON): Government of Canada.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. [modified 2015 Oct 7]a. List of permitted colouring agents. [Internet]. Ottawa (ON): Government of Canada. [cited 2016 Aug].
Health Canada. [modified 2015 Dec 14]b. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Government of Canada. [accessed 2016 Aug].
Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
Health Canada. 2017. Assessment of mutagenicity for selected Chemicals Management Plan-directed needs compounds with the plate incorporation Ames mutagenicity assay. Ottawa (ON): Health Canada, Environmental Health Sciences and Research Bureau. 33 p. Prepared for the Existing Substances Risk Assessment Bureau, Health Canada.
Heucotech Ltd. 2013. Notice in accordance with TSCA section 8(e) – toxicological results for 6,15- dihydroanthrazine-5,9,14,18-tetrone (CASRN 81-77-6). Letter to the US Environmental Protection Agency dated 2013 Jun 7.
Hu S, Shen G (Environmental Testing Laboratory, Shanghai Academy of Environmental Sciences, Shanghai, China). 2008. Bioconcentration test of C.I. Disperse Blue 77 in fish. Prepared for Dystar on behalf of Ecological and Toxicological Association of the Dyes and Organic Pigments Manufacturers (ETAD) Basel, Switzerland. Report No. S-071-2007. Submitted to Environment Canada in April 2008.
[IARC] International Agency for Research on Cancer. 2013. Some chemicals present in industrial and consumer products, food and drinking-water: Anthraquinone. IARC Monogr. 101:41-70.
Inoue Y, Hashizume N, Yoshida T, Murakami H, Suzuki Y, Koga Y, Takeshige R, Kikushima E, Yakata N, Otsuka M. 2012. Comparison of bioconcentration and biomagnification factors for poorly water-soluble chemicals using Common Carp (Cyprinus carpio L.). Arch Environ Contam Toxicol. 63:241–248.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1974. Indanthrene Blue RS. Toxicological evaluation of certain veterinary drug residues in food. WHO Food Additives Series, No. 6. Prepared by the eighteenth meeting of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization, Geneva (CH).
[J-CHECK] Japan CHEmicals Collaborative Knowledge Database [database]. c2010- . Tokyo (JP): National Institute of Technology and Evaluation (NITE). [accessed 2020 06 18].
Krishna Murthy AS, Baker JR, Smith ER, Wade GG. 1977. Development of hemangiosarcomas in B6C3F1 mice fed 2-methyl-1-nitroanthraquinone. Int J Cancer. 19(1):117-121.
Leadscope Model Applier [prediction module]. 2016. Ver. 2.1. Columbus (OH): Leadscope, Inc. [restricted access].
Leist KH. 1982. Subacute toxicity studies of selected organic colorants. Ecotoxicol Environ Saf. 6:457- 463.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, et al. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol. 46:1516-1524.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, et al. 2006. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44:2008-2018.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, et al. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43:279-291.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Health Canada. [accessed 2017 Jan 9].
Lu FC, Lavalleé A. 1964. The acute toxicity of some synthetic colours used in drugs and foods. Can Pharm J. 97:30. [cited in JECFA 1974].
[MHLWJ] Ministry of Health, Labour and Welfare of Japan. 2012. 1,4 bisu(isopropylamino)anthraquinone: Reverse mutation test using bacteria. Final Report. Tokyo (JP): MHLWJ Pharmaceutical Foods Division, Administration Division, & Chemical Substance Safety Division. [translated].
Mori H, Sugie S, Niwa K, Takahashi M, Kawai K. 1985. Induction of intestinal tumours in rats by chrysazin. Br J Cancer. 52:781-783.
Mori H, Sugie s, Niwa K, Yoshimi N, Tanaka T, Hirono I. 1986. Carcinogenicity of chrysazin in large intestine and liver of mice. Gann. 77:871-876.
Mori H, Yoshimi N, Iwata H, Mori Y, Hara A, Tanaka T, Kawai K. 1990. Carcinogenicity of naturally occurring 1-hydroxyanthraquinone in rats: induction of large bowel, liver and stomach neoplasms. Carcinogenesis. 11(5):799-802.
Mori Y, Yoshimi N, Iwata H, Tanaka T, Mori H. 1991. The synergistic effect of 1-hydroxyanthraquinone on methylazoxymethanol acetate-induced carcinogenesis in rats. Carcinogenesis. 12(2):335-338.
[MSDS] Material Safety Data Sheet [PDF]. 2002. Pullmelt 7B. Appenzell (CH): Beulentechnik AG. [accessed 2017 Jan 10].
[MSDS] Material Safety Data Sheet 2007a. No rinse natural cat shampoo. Dallas (TX): OUT! International Inc. [accessed 2016 Jan 26].
[MSDS] Material Safety Data Sheet 2007b. 665098876496 (Lotus Blossom). Mayfield (KY): MVP Group International. [accessed 2016 Jan 26].
[MSDS] Material Safety Data Sheet. 2007c. 665098866503 (Fresh Cut Grass). Mayfield (KY): MVP Group International. [accessed 2016 Jan 26].
[MSDS] Material Safety Data Sheet 2007d. 665098866480 (Ocean Spray). Mayfield (KY): MVP Group International. [accessed 2016 Jan 26].
[MSDS] Material Safety Data Sheet [PDF]. 2008. Cam & lifter installation lube. Memphis (TN): Comp Cams. [accessed 2017 Jan 10].
[MSDS] Material Safety Data Sheet. 2009a. Ink (green color) in Christmas 6 pack stamper. Hong Kong (HK): G T Plus Limited. [accessed 2016 Jan 26]. Available upon request.
[MSDS] Material Safety Data Sheet. 2009b. Ink in 6pk Valentine stamper. Hong Kong (HK): G T Plus Limited. [accessed 2016 Jan 26]. Available upon request.
[MSDS] Material Safety Data Sheet. 2009c. Ink in Easter stamper set assortment. Hong Kong (HK): G T Plus Limited. [accessed 2016 Jan 26]. Available upon request.
[MSDS] Material Safety Data Sheet [PDF]. 2011. Furniture cleaner & restorer aerosol. Hickory (NC): RPM Wood Finishes Group, Inc. [accessed 2017 Jan 11].
[MSDS] Material Safety Data Sheet. 2015a. Groomer’s best puppy shampoo. Secaucus (NJ): Hartz Mtn. Corp. [accessed 2016 Jan 26].
[MSDS] Material Safety Data Sheet [PDF]. 2015b. Utrecht studio series oil colors. Brooklyn (NY): Utrecht Manufacturing. [accessed 2016 Jan 04].
[MSDS] Material Safety Data Sheet [PDF]. 2015c. Nozzle gel. Oakville (ON): Techniweld Corporation. [accessed 2017 Jan 10].
[MSDS] Material Safety Data Sheet. 2015d. MCS 7000 Component A. Clifton (NJ): DriTac Flooring Products, LLC. [accessed 2017 Jan 11].
[MSDS] Material Safety Data Sheet [PDF]. 2018. Series 13 – PRIMAcryl. Erkrath (DE): H. Schmincke & Co. GmbH & Co. KG. [accessed 2017 Jan 10, and modified 2018 Oct. 25].
Muzzal JM, Cook WL. 1979. Mutagenicity of dyes used in cosmetics with the Salmonella/mammalian- microsome test. Mutat Res. 67:1-8.
[NCI] National Cancer Institute. 1978. Bioassay of 2-methyl-1-nitroanthraquinone for possible carcinogenicity (CAS No. 129-15-7). Bethesda (MD): NCI, Division of Cancer Cause and Prevention. Report No.: 29.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2017 Jan 10]. Ottawa (ON): Health Canada. [accessed 2017 May 19].
[NTP] National Toxicology Program. 2005. Toxicology and carcinogenesis studies of anthraquinone (CAS No. 84-65-1) in F344/N rats and B6C3F1 mice (feed studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Report No.: TR 494.
[NTP] National Toxicology Program. [modified 2017 May 17]. Testing status of C.I. Disperse Red 60 – M88155 Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. [accessed 2017 May 18].
Noguerol-Cal R, López-Vilariño JM, González-Rodríguez MV, Barral-Losada L. 2011. Effect of several variables in the polymer toys additive migration to saliva. Talanta 85(4):2080-2088.
Norris B, Smith S. 2002. Research into the mouthing behaviour of children up to 5 years old [Internet]. Report commissioned by UK Department of Trade and Industry, London, UK.
OECD QSAR Toolbox [read-across tool]. 2014. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
OECD QSAR Toolbox [read-across tool]. 2017. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Oettel H, Frohberg H, Nothdurft H, Wilhelm G. 1965. Testing some synthetic dyes for their suitability for food coloring. Arch Toxicol. 21:9-29. [cited in JECFA 1974].
Pesticide Label Search [database]. [modified 2016 Jan 25]. Ottawa (ON): Health Canada. [accessed 2015 Sep 30].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2006. Cosmetics fact sheet [PDF]: To assess the risks for the consumer: updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No.: 320104001/2006.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2007. Do-it-yourself products fact sheet [PDF]: To assess the risks for the consumer. Bilthoven (NL): RIVM. Report No.:320104007/2007.
[SCCS] Scientific Committee on Consumer Safety. 2012. The SCCS’s notes of guidance for the testing of cosmetic ingredients and their safety evaluation, 8th Revision [PDF]. [Internet]. Scientific Committee on Consumer Safety. [accessed 23 Feb 2017].
Seifried HE, Seifried RM, Clarke JJ, Junghans TB, San RHC. 2006. A compilation of two decades of mutagenicity test results with the Ames Salmonella typhimurium and L5178Y mouse lymphoma cell mutation assays. Chem Res Toxicol. 19:627-644.
Sijm DTHM, Schüürmann G, de Vries PJ, Opperhuizen A. 1999. Aqueous solubility, octanol solubility, and octanol/water partition coefficient of nine hydrophobic dyes. Environ Toxicol Chem. 18(6):1109-1117. [cited in Epi Suite c2000-2012].
Statistics Canada. 2012. Canadian Health Measures Survey (CHMS) - Cycle 1. Ottawa (ON): Statistics Canada. Available upon request.
Tanaka T, Kojima T, Yoshimi N, Sugie S, Mori H. 1991. Inhibitory effect of the nonsteroidal anti- inflammatory drug, indomethacin on the naturally occurring carcinogen, 1-hydroxyanthraquinone in male ACI/N rats. Carcinogenesis. 12:1949-1952. [cited in IARC 2002].
[TIMES] TIssue MEtabolism Simulator [prediction module]. 2016. Ver. 2.27.19. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry. [restricted access].
Umeda M. 1956. Experimental study of xanthene dyes as carcinogenic agents. Gann. 47:51. [cited in JECFA 1974].
[US EPA] US Environmental Protection Agency. 2011. Age dependent Adjustment Factor (ADAF) application. Final Report. Washington (DC): US EPA, Office of Water Policy.
[US EPA] US Environmental Protection Agency. 2012. Standard operating procedures for residential exposure assessments [PDF]. Washington (DC): US EPA, Office of Chemical Safety and Pollution Prevention, Office of Pesticide Programs, Health Effects Division.
Versar Inc. 1986. Standard scenarios for estimating exposure to chemical substances during use of consumer products. Prepared for US EPA Office of Toxic Substances, Exposure Evaluation Division.
Volodchenko VA, Gudz ZA, Timchenko AN. 1970. Materialy k obosnovaniju predelno dopustimoj koncentraciji antrachinona v vozduche rabocej zony. Gig Tr Prof Zabol. 15(2):58-59. [cited in ECHA 2015b].
Williams FM, Rothe H, Barrett G, Chiodini A, Whyte J, Cronin MTD, Monteiro-Riviere NA, Plautz J, Roper C, Westerhout J, et al. 2016. Assessing the safety of cosmetic chemicals: consideration of a flux decision tree to predict dermally delivered systemic dose for comparison with oral TTC (Threshold of Toxicological Concern). Regul Toxicol Pharmacol. 76:174-186.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48:3109-3119.
Yen CC, Perenich TA, Baughman GL. 1989. Fate of dyes in aquatic systems II. Solubility and octanol/water partition coefficients of disperse dyes. Environ Toxicol Chem. 8(11):981-986. [cited in EPI Suite c2000-2012].
Yoshimi N, Ino N, Suzui M, Tanaka T, Nakashima S, Nakamura M, Nozawa Y, Mori H. 1995. The mRNA overexpression of inflammatory enzymes, phospholipase A2 and cyclooxygenase, in the large bowel mucosa and neoplasms of F344 rats treated with naturally occurring carcinogen, 1- hydroxyanthraquinone. Cancer Lett. 97:75-82.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. 1988. Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environ Mol Mutagen. 11(S12):1-158. [cited in NTP 2005].
Zeilmaker MJ, Kroese ED, van Haperen P, van Veen MP, Bremmer HJ, van Kranen HJ, Wouters MFA, Janus JA. 1999. Cancer risk assessment of azo dyes and aromatic amines from garment and footwear Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. RIVM Report No.: 601503014.
Zeilmaker MJ, van Kranen HJ, van Veen MP, Janus JA. 2000. Cancer risk assessment of azo dyes and aromatic amines from tattoo bands, folders of paper, toys, bed clothes, watch straps and ink [internet]. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (RIVM) [National Institute for Public Health and the Environment]. Report No.: 601503 019.
Appendix A. Estimated potential exposures to substances in the Anthraquinones Group
Sentinel exposure scenarios were used to estimate the potential exposure to substances in the Anthraquinones Group; scenario assumptions are summarized in Table A-3. Exposures were estimated using ConsExpo version 4.1 or algorithms from the model (ConsExpo 2006), unless noted otherwise. An overall retention factor (RF) of 1 was used unless otherwise specified.
Exposures were estimated for different age groups based on body weights (BW) from Health Canada’s exposure factors for the general population of Canada (Health Canada 1998):
Infants (newborn to 6 months): 7.5 kg
Toddlers (0.5–4 years): 15.5 kg
Children (5–11 years): 31.0 kg
Teen (12–19 years): 59.4 kg
Adults (20+ years): 70.9 kg
Dermal exposures to Solvent Violet 13
The potential absorbable dose(s) of Solvent Violet 13 from the Charles River study (2017) was/were used to characterize systemic exposures for each dermal scenario. The following parameters, algorithms and considerations were used.
AV: skin surface area exposed
PAA: potential absorbable dose (over 24 hours of exposure)
F: exposure frequency
Conc: concentration
RF: retention factor
Per-Event Systemic Exposure = (AV x PAA)/BW
For mass balance check:
(Total) Dermal Load = Conc x Product Amount x RF x F
(where “F” is only incorporated if >1)
If the per-event systemic exposure was less than the (total) dermal load, the per-event systemic exposure was used to characterize systemic exposure given the lack of full dose depletion, otherwise the (total) dermal load was used (due to full dose depletion). Where “F” is greater than once per day, the per-event systemic exposure can be used as a daily systemic exposure estimate. In other words, no adjustment for exposure frequency would be needed regardless of the number of product applications within a 24-hour period given that PAA represents the cumulative amount absorbed over 24 hours. For scenarios where F is less than once per day and as needed, exposure frequency was incorporated to (also) generate a daily systemic exposure estimate.
| Sentinel exposure scenario | Age group | Dermal load (mg/cm2) | Potential absorbable dose (mg/cm2)b |
|---|---|---|---|
| Body cream | Child | 0.00260 | 0.00163 |
| Body cream | Teen | 0.00255 | 0.00163 |
| Body cream | Adult | 0.00257 | 0.00163 |
| Spray perfume | Child | 0.0330 | 0.00163 – 0.04133 |
| Spray perfume | Teen | 0.0561 | 0.00163 – 0.04133 |
| Spray perfume | Adult | 0.0561 | 0.00163 – 0.04133 |
| Permanent hair dye | Teen | 0.157 | 0.00163 – 0.04133 |
| Permanent hair dye | Adult | 0.157 | 0.00163 – 0.04133 |
| Face paint | Toddler | 0.0897 | 0.00163 – 0.04133 |
| Face paint | Child | 0.0893 | 0.00163 – 0.04133 |
| Face paint | Teen | 0.0893 | 0.00163 – 0.04133 |
| Face paint | Adult | 0.0893 | 0.00163 – 0.04133 |
a See exposure scenarios in Table A-3 for frequency (F), if relevant.
b Differences in absorbable dose are a result of differences in the formulations and concentrations used in the study test preparations. The potential absorbable dose presented for the body cream scenario corresponds to the Oilatum cream formulation, whereas all other dermal exposure scenarios use a range corresponding to the Oilatum cream and olive oil formulations as neither experimental formulation are a direct match to the product types.
Maximum Flux Approach
As a refinement, the maximum flux (Jmax) approach as conducted in Williams et al. (2016) was used to estimate dermal exposures for Solvent Blue 36 and Acid Blue 239. Jmax represents the theoretical upper limit to steady-state flux of a given substance across the skin, independent of vehicle (barring potential penetration-retarding or -enhancing effects of certain formulations). Its use does not account for the presence of potentially absorbable skin-bound residues following termination of exposure. However, its use is conservative with respect to the assumptions that a given substance is present at its solubility limit in the “in-use” vehicle of a product and that the absorption is entirely steady-state (i.e., ignores slower absorption during the lag phase).
The equations used are provided below, and the water solubility, log Kow and molecular weight (MW) values were obtained from Tables 2-1 and 3-1 of this screening assessment report. A mass balance check was also done; this equation varies slightly according to the exposure scenario and is therefore provided in Table A-3 below.
Kp (Potts and Guy equation, based on aqueous vehicle):
log Kp (in cm/h) = -2.71 + (0.71)(log Kow) - (0.0061)(MW, in g/mol)
Jmax:
Jmax (in mg/cm2/h) = Kp (in cm/h) x Water solubility (in mg/cm3)
Maximum theoretical amount absorbed per day (Qabs):
Qabs (in mg) = Jmax (in mg/cm2/h) x Surface area of skin contact (in cm2) x Exposure duration (in h)
Dermal Systemic Exposure = Qabs/BW
The dermal systemic exposure estimate shown above represents a “per-event” estimate where F was less than once per day and a “daily” estimate where F was equal to or greater than once per day. Where F was less than once per day, exposure frequency was incorporated as needed to (also) generate a daily systemic exposure estimate. As Qabs represents the theoretical maximum amount absorbed over a 24-hour period for the sentinel scenarios considered for Solvent Blue 36 and Acid Blue 239, no adjustment for number of product applications was required to generate the daily systemic exposure estimates when F was greater than once per day.
| Substance and sentinel exposure scenario | Age group(s) | Jmax (mg/cm2/h) | Qabs (mg) |
|---|---|---|---|
| Solvent Blue 36, speciality lubricants | Adult | 5.00E-7 | 0.00273 |
| Solvent Blue 36, hair conditioner (wash-off) | Toddler | 5.00E-7 | 0.00521 |
| Solvent Blue 36, hair conditioner (wash-off) | Child | 5.00E-7 | 0.0101 |
| Solvent Blue 36, hair conditioner (wash-off) | Teen, adult | 5.00E-7 | 0.0131 |
| Acid Blue 239, textiles, wearing clothing | Infant | 1.38E-9 | 9.99E-5 |
| Acid Blue 239, textiles, wearing clothing | Toddler | 1.38E-9 | 1.91E-4 |
| Acid Blue 239, textiles, wearing clothing | Child | 1.38E-9 | 3.20E-4 |
| Acid Blue 239, textiles, wearingclothing | Teen | 1.38E-9 | 5.36E-4 |
| Acid Blue 239,textiles, wearing clothing | Adult | 1.38E-9 | 6.02E-4 |
a See exposure scenarios in Table A-3 for frequency (F), if relevant.
Lifetime average daily dose (LADD)
The LADD was derived for all oral and dermal exposures to Solvent Violet 13 as a refinement and also for certain dermal exposures to Solvent Blue 36, Disperse Red 60 and Acid Blue 239 to account for product use by other age groups. The assumptions and equation are provided below:
DSE: daily systemic exposure
Average lifetime (AL): 70 years (US EPA 2011)
Age group durations (AD): 0.5 years for infants (0 to 6 months), 4.5 years for toddlers (7 months to 4 years), 7 years for children (5 to 11 years), 8 years for teen (12 to 19 years) and 50 years for adults (20+ years) (Health Canada 1998)
LADD = [(DSEinfant x ADinfant) + (DSEtoddler x ADtoddler) + (DSEchild x ADchild) + (DSEteen x ADteen) + (DSEadult x ADadult)] / [AL]
| Substance(s) | Sentinel exposure scenario | Assumptions |
|---|---|---|
| Solvent Violet 13 | Lip balm or lipstick (toddler, child, teen and adult) | Conc: ≤1% (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated 2016; unreferenced) F: 0.59/day and 0.89/day for toddler and child, respectively (Wu et al. 2010); 2.4/day for teen and adult (Loretz et al. 2005) Product amount: 0.01 g/application for all age groups (Loretz et al. 2005), 100% assumed to be ingested |
| Solvent Violet 13 | Body cream (child, teen and adult) | Conc: ≤0.9% (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated 2016; unreferenced) F: 1.1/day (Loretz et al. 2005) Product amount per application (mean): 2.2 g for child, 3.8 g for teen and 4.4 g for adult (Loretz et al. 2005; using surface area adjustment factors for child and teen) AV: 8390 cm2 for child, 14740 cm2 for teen and 16925 cm2 for adult (Health Canada 1995) PAA: 1.63 µg/cm2 (Charles River Laboratories 2017) |
| Solvent Violet 13 | Spray perfume (aerosol) (child, teen and adult) | Conc: ≤1% (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated 2016; unreferenced) F: 0.58/day or 17.4/month for child (Wu et al. 2010); 1.7/day for teen and adult (Loretz et al. 2006)Product amount: 0.33 g/application for all age groups (Loretz et al. 2006) AV: 100 cm2 for all age groups (ConsExpo 2006) PAA: 1.63 to 41.33 µg/cm2 (Charles River Laboratories 2017) Inhalation rate (adult): 16.2 m3/day (Health Canada 1998) |
| Solvent Violet 13 | Permanent hair dye (wash-in) (teen and adult) | Conc: ≤1% (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated 2016; unreferenced) F: 0.01/day for teen and 0.02/day or 7.99/year for adult (Statistics Canada 2012) For both age groups: Product amount: 100 g/application (RIVM 2006) RF: 0.10 (SCCS 2012) AV: 638 cm2 (Health Canada 1995) PAA: 1.63 to 41.33 µg/cm2 (Charles River Laboratories 2017) |
| Solvent Violet 13 | Face paint (toddler, child, teen and adult) | Conc: ≤3% (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated 2019; unreferenced) F: 0.03/day or 12/year for toddler, child and teen; 0.02/day or 6/year for adult (Bremmer et al. 2006) For estimated dermal exposure: Product amount per application: 1.3 g for toddler, 1.8 g for child and 1.9 g for teen and adult (Bremmer et al. 2006) AV: 435 cm2 for toddler, 605 cm2 for child and 638 cm2 for teen and adult (Health Canada 1995) PAA: 1.63 to 41.33 µg/cm2 (Charles River Laboratories 2017) Oral exposure to face paint (indirect hand to mouth) was also estimated for toddlers. Ingestion rate: 0.44 mg/min (Bremmer and van Veen 2002) Duration: 480 min (Bremmer and van Veen 2002) |
| Pigment Blue 60 | Arts and crafts (i.e., stampers) (toddler and teen) | Conc: 40% (MSDS 2009a,b,c) Daily ink line: 25 cm/day (personal communication, emails from the Art and Creative Materials Institute to the Existing Substances Risk Assessment Bureau, Health Canada, dated 2009; unreferenced) Ink laydown rate: 100 µg/cm (90th percentile level ink laydown of writing instruments; personal communication, emails from the Art and Creative Materials Institute to the Existing Substances Risk Assessment Bureau, Health Canada, dated 2016; unreferenced) Estimated amount of ink per exposure: 50 mg (conservatively assumed to be the same as that from a marker; Danish EPA 2008)Fraction absorbed: 1 (as worst-case) Estimated per-event oral/dermal exposure = (Conc x Estimated amount of ink per exposure x Fraction absorbed)/BW Estimated daily oral/dermal exposure= (Daily ink line x Ink laydown rate x Conc)/(BW) |
| Solvent Violet 59 | Plastic toys, mouthing (toddler) | Concentration in simulant (Conc): 0.004 mg/L (mean concentration used for daily systemic exposure)a; 0.01 mg/L (maximum concentration used for “per- event” exposure)a (Noguerol-Cal et al. 2011) Salivary flow rate (Vs): 0.00022 L/min (Environ 2003) Fractional extraction (FR; unitless fraction): 1 Oral absorption factor (AFo; unitless fraction): 1 F: 60 min/day (Norris and Smith 2002) Estimated oral exposure = (Conc x Vs x FR x AFo x F)/BW (adapted from Environ 2003) |
| Solvent Blue 36 | Specialty lubricants (adult) | Conc: 1% (MSDS 2008) Surface area of skin contact: 227.5 cm2 (corresponds to one fourth of the hand surface area in Health Canada 1995) Film thickness on skin (T): 15.88E-03 cm (value for initial film thickness of mineral oil on skin after immersion) (Versar 1986) Product density (DSY): 1 g/cm3 (water density used as rough approximation in absence of product- specific density) Exposure duration: 24 h For mass balance check: Dermal load = Conc x T x DSY |
| Solvent Blue 36 | Hair conditioner (wash-off) (toddler, child, teen, adult) | Conc: ≤3% (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated 2016; unreferenced) Surface area of skin contact: 435 cm2 for toddler, 845 cm2 for child, and 1092.5 cm2 for teen and adult (Health Canada 1995) F: 0.44/day for toddler (Wu et al. 2010), 0.49/day for child (Wu et al. 2010), and 1.10/day for teen and adult (Loretz et al. 2008) Product amount: 8.9 g/application for toddler (based on surface area adjustment factor) and 13.1 g/application for child, teen, and adult (Loretz et al. 2008) Exposure duration: 24 h/day RF: 0.01 (SCCS 2012) For mass balance check: (Total) Dermal Load = Conc x Product Amount x RF x F |
| Disperse Red 60, Acid Blue 239 | Textiles, mouthing (infant) | Conc: 1% (BfR 2007) F: 1/day Surface area (SA) of object mouthed: 20 cm2 (Zeilmaker et al. 2000) Area weight of textile (AW): 20 mg/cm2 (US EPA 2012)c Migration fraction (MF): 0.005 (acute); 0.0005 (chronic) (BfR 2007)d Estimated oral exposure = (Conc x SA x AW x MF x F)/BW |
| Disperse Red 60 | Textiles, wearing clothes (infant, toddler, child, teen, adult) | Conc: 1% (BfR 2007) F: 1/day SA of skin contact: 3020 cm2 for infant (baby sleeper), 5780 cm2 for toddler, 9660 cm2 for child, 16 200 cm2 for teen, and 18 200 cm2 for adult (personal apparel) (Health Canada 1995) AW: 20 mg/cm2 (US EPA 2012)b MF: 0.005 (acute); 0.0005 (chronic) (BfR 2007)c Skin contact factor (SCF): 1 Dermal absorption (DA) for Disperse Red 60: 2% (areas of high perspiration) (Bfr 2007) Estimated dermal exposure = (Conc x SA x AW x MF x F x SCF x DA)/BW |
| Acid Blue 239 | Textiles, wearing clothes (infant, toddler, child, teen, adult) | Conc: 1% (BfR 2007) SA of skin contact: 3020 cm2 for infant (baby sleeper), 5780 cm2 for toddler, 9660 cm2 for child, 16 200 cm2 for teen, and 18 200 cm2 for adult (personal apparel) (Health Canada 1995) Exposure duration: 24 h/day AW: 20 mg/cm2 (US EPA 2012)b MF: 0.005 (acute); 0.0005 (chronic) (BfR 2007)c For mass balance check: Dermal load = Conc x SA x AW x MF |
| CAS RN 74499-36-8 | Epoxy coating product, application (adult) | Conc: 0.1% (MSDS 2015d) Product amount: 0.25 g (RIVM 2007) Exposure estimated using ConsExpo Web (2016) |
Abbreviations: AV, skin surface area exposed; PAA, potential absorbable dose (over 24 hours of exposure); F, exposure frequency; Conc, concentration; RF, retention factor.
a In the absence of chemical-specific empirical migration data for Solvent Violet 59, the measured concentration of Solvent Blue 35 (CAS RN 17354-14-2), an anthraquinone dye, from polymeric commercial toys (n=3) in contact with saliva simulant (pH of 6.8; room temperature; 10-day contact time) was used as surrogate data, due to similarity in product type and given that both are of the same chemical class and dye application class. Solvent Blue 35 is anticipated to be a conservative analogue for migration given that its molecular weight of 350.45 g/mol is less than the molecular weight of Solvent Violet 59 (422.44 g/mol).
b The area weight of textile corresponds to cotton, which is representative of the worst-case scenario textile relative to synthetic fabric area weight (i.e., 1 mg/cm2) and is considered to be protective on this basis.
c The migration of dyes from textiles varies considerably depending on the type of fibre, the type of dye used, the dye load, dyeing technology and colour intensity and aftertreatment process. The exposure from textiles is partly dictated by the amount of dye that migrates from textile material onto human skin (ETAD 1983) or via mouthing.
The Textiles Working Group (BfR 2007) uses a peak initial migration fraction of 0.005 to estimate exposure to dyes from newly bought unwashed garments, and the chronic migration fraction is assumed to be one-tenth of the value measured for the first migration to reflect exposure after initial washes. It is assumed that the sweat migration fraction is similar to the salivary migration fraction; this is consistent with observations of leaching behaviours of dyes from textiles reported by Zeilmaker et al. (1999). Accordingly, the fraction of dye that migrates from a textile material per day was assumed to be 0.0005 for estimating both dermal and oral exposures on a chronic basis.
