Risk management approach for Solvent Violet 13
Official title: Risk management approach for Solvent Violet 13
Chemical Abstracts Service Registry Numbers (CAS RN):
81-48-1
Environment and Climate Change Canada
Health Canada
July 2021
Summary of Proposed Risk Management
This document outlines the proposed risk management actions under consideration for Solvent Violet 13, which has been found harmful to human health.
In particular, the Government of Canada is proposing:
- Measures to reduce exposures to Solvent Violet 13 from certain cosmetics by describing it as a prohibited or restricted ingredient on the Health Canada Cosmetic Ingredient Hotlist. The Hotlist is used to communicate that certain substances may not be compliant with requirements of the Food and Drugs Act or provisions of the Cosmetic Regulations.
The risk management actions outlined in this Risk Management Approach document may evolve through consideration of assessments and risk management options published for other Chemicals Management Plan (CMP) substances as required to ensure effective, coordinated, and consistent risk management decision-making.
Note: The proposed risk management actions may evolve through consideration of additional information obtained from the public comment period, literature and other sources.
1. Context
The Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999) provides the authority for the Minister of Environment and the Minister of Health (the ministers) to conduct assessments to determine if substances are toxic to the environment and/or harmful to human health as set out in section 64 of CEPAFootnote 1 ,Footnote 2, and if so, to manage the associated risks.
The seven substances, listed in Annex A and referred to throughout this document as Solvent Violet 13, Pigment Blue 60, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239 and CAS RN 74499-36-8, are included in the screening assessment of the Anthraquinones Group (ECCC, HC, 2021).
2. Issue
Health Canada and Environment and Climate Change Canada conducted a joint scientific assessment of Solvent Violet 13 as part of the Anthraquinones Group. A notice summarizing the scientific considerations of the screening assessment for the substances in this group was published in the Canada Gazette, Part 1, on July 17, 2021 (Canada, 2021). For further information, please refer to the Screening Assessment Report for the Anthraquinones Group.
2.1 Screening Assessment Conclusion
On the basis of the information available, the screening assessment concludes that Solvent Violet 13 in the Anthraquinones Group, meets the criteria under paragraph 64 (c) of CEPA because it is entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health (Canada 2021). The exposures and sources of concern identified in the screening assessment are oral and dermal exposure to Solvent Violet 13 in certain cosmetics (body creams, lipsticks/lip balms, permanent hair dyes, spray perfumes and face paints). As such, this document will focus on these exposure sources (refer to section 5).
It is concluded that Solvent Violet 13 is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends under paragraphs 64(a) and (b) of CEPA, respectively.
The screening assessment also concludes that the other substances in the Anthraquinones Group, namely Pigment Blue 60, Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239, and CAS RN 74499-36-8, do not meet the criteria under section 64 of CEPA (Canada 2021).
Although a risk to human health or the environment has not been identified at current levels of exposure, there may be a concern if exposure to Solvent Violet 59, Solvent Blue 36, Disperse Red 60, Acid Blue 239, and CAS RN 74499-36 were to increase. As a result, these substances may be considered in future initiatives to track their commercial status or identify new uses.
The screening assessment also concludes that Solvent Violet 13 meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations made under CEPA (Canada 2000).
2.2 Recommendation under CEPA
On the basis of the findings of the screening assessment conducted under CEPA, the Ministers recommend that Solvent Violet 13 be added to the List of Toxic Substances in Schedule 1 of the Act.Footnote 3
The Ministers have taken into consideration comments made by stakeholders during the 60-day public comment period on the draft screening assessment for the Anthraquinones Group and its associated Risk Management Scope for Solvent Violet 13.
As the Ministers finalize the recommendation to add Solvent Violet 13 to Schedule 1, risk management instruments will be proposed and finalized within 24 months from the date on which the Ministers recommend that Solvent Violet 13 be added to Schedule 1 of CEPA, and finalized within 18 months from the date on which the risk management instruments are proposed, as outlined in sections 91 and 92 of CEPA (refer to section 8 for publication timelines applicable to this group of substances).
2.3 Public Comment Period on the draft Screening Assessment Report and the Risk Management Scope
The draft screening assessment for the Anthraquinones Group (ECCC, HC, 2018a) and the associated Risk Management Scope document for Solvent Violet 13 (ECCC, HC, 2018b) summarizing the proposed risk management options under consideration at the time, were published on November 3, 2018. Industry and other interested stakeholders were invited to submit comments on both documents during a 60-day public comment period.
Comments received on the draft screening assessment report and the Risk Management Scope were taken into consideration in the development of this document. A summary of responses to the public comments received is available.
3. Proposed Risk Management
3.1 Proposed Human Health Objectives
Proposed human health objectives are quantitative or qualitative statements of what should be achieved to address human health concerns.
The proposed human health objective for Solvent Violet 13 is focused on addressing the exposure sources of concern outlined in section 5 of this document. As such, the proposed human health objective is to reduce exposure of the general population to Solvent Violet 13 to levels that are protective of human health.
3.2 Proposed Risk Management Objectives
Proposed risk management objectives set quantitative or qualitative targets to be achieved by the implementation of risk management regulations, instrument(s) and/or tool(s) for a given substance or substances.
The proposed risk management objective for Solvent Violet 13 is:
- to reduce dermal and oral exposure to Solvent Violet 13 from certain cosmetics.
3.3 Proposed Risk Management Actions under Consideration
To achieve the proposed risk management objective and to work towards achieving the proposed human health objective, the proposed risk management action for Solvent Violet 13 under consideration is:
- Measures to reduce exposures to Solvent Violet 13 from certain cosmetics by describing Solvent Violet 13 as a prohibited or restricted ingredient on the Health Canada Cosmetic Ingredient HotlistFootnote 4 . The Hotlist is used to communicate that certain substances may not be compliant with requirements of the Food and Drugs Act or provisions of the Cosmetic Regulations.
Following the publication of this Risk Management Approach document, additional information obtained from the public comment period and from other sources will be considered, along with the information presented in this document, in the instrument selection and development processFootnote 5 . The risk management actions outlined in this document may evolve through consideration of assessments and risk management options published for other CMP substances to ensure effective, coordinated, and consistent risk management decision-making.
3.4 Performance Measurement and Evaluation
Performance measurement evaluates the ongoing effectiveness and relevance of the actions taken to manage risks from toxic substancesFootnote 6 . The aim is to determine whether the human health objectives have been met and whether there is a need to revisit the risk management approach for that substance to ensure that risks are managed effectively over time.
The Government of Canada plans to measure the effectiveness of the risk management actions by collecting and analyzing data including data on Solvent Violet 13 in certain cosmetics in order to measure progress towards meeting the risk management objectives.
The results of performance measurement and evaluation will be used to inform whether further risk management action is warranted and will be made available to Canadians along with recommendations for further action, if applicable.
4. Background
4.1 General Information on Solvent Violet 13
Solvent Violet 13 is an organic substance which is part of the Anthraquinones Group. The analogue anthraquinone (CAS RN 84-65-1) is the common structural backbone shared among all the substances in the Anthraquinones Group.
4.2 Current Uses and Identified Sectors
Solvent Violet 13 was included in a survey issued pursuant to section 71 of CEPA (Canada 2012). Total reported imports of Solvent Violet 13 for 2011 ranged from 1000 to 10 000 kg and no manufacturing activities were reported above the reporting threshold of 100 kg. The major use reported in Canada for Solvent Violet 13 according to the above mentioned survey is for the manufacture of candles (Environment Canada 2013).
Solvent Violet 13 is also identified as being used in cosmetics, based on notifications submitted under the Cosmetic Regulations to Health Canada, specifically for a variety of cosmetics, including body creams, bath products, lipsticks/lip balms, make-up, nail products, shampoos and conditioners, hair styling products, perfumes and face painting products (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated February 1, 2016, 2019; unreferenced). Solvent Violet 13 may also be used as a component in food packaging materials; however, exposure is expected to be negligible. The substance may also be used as a component in incidental additives such as hand sanitizers and cleaners, and used in food processing establishments, with no expected food contact (personal communication, e-mail from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated April 24, 2017; unreferenced).
Solvent Violet 13 is also listed in the Natural Health Products Ingredients Database with a non-medicinal role for external use only as colour additive. It is also listed in the Licensed Natural Health Products Database as being present a non-medicinal ingredient in a limited number of currently licensed topical natural health products, such as acne therapy products, anti-dandruff products, and antiseptic skin cleansers (LNHPD [modified 2016], NHPID [modified 2017]). Given its presence in a limited number of natural health products combined with limited information regarding product concentrations, exposure from natural health products has not been identified as a concern at this time.
Solvent Violet 13 is listed on the Health Canada Pest Management Regulatory Agency (PMRA) List of Formulants (personal communication, email from the PMRA, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada (ESRAB), dated 2016; unreferenced).
Additional consumer uses for Solvent Violet 13 identified in Canada from publicly available sources include pet shampoos (MSDS 2007a,b; 2015a,b).
Globally, Solvent Violet 13 was also identified as a colourant in non-plastic toys (Danish EPA 2015) and in other products including textiles, paper, and plastics (ECHA c2007-2019a).
5. Exposure Sources and Identified Risks
Solvent Violet 13 was not identified or measured in any environmental media in Canada or elsewhere. Exposure from environmental media that could impact human health of the general population is considered to be minimal.
Direct exposures from use of products available to consumers were evaluated in the screening assessment. Estimates of potential exposure to Solvent Violet 13 were derived from the use of cosmetics.
Given limited substance-specific hazard data for chronic toxicity and carcinogenicity endpoints, a read-across approach based on health effects information for the substance anthraquinone, was used to inform this part of the hazard assessment.
The critical health effects associated with Solvent Violet 13 identified in the screening assessment (Canada 2019) are carcinogenicity based on the analogue anthraquinone and non-cancer systemic effects (kidney, liver, spleen, and bone marrow toxicity) based on the analogue anthraquinone. In the assessment, exposure of Canadians to Solvent Violet 13 in the following scenarios were identified as a potential concern:
Non-cancer systemic effects:
- Dermal exposure of adults through use of body creams and spray perfumes.
Cancer effects:
- Oral exposure of all age groups through use of lipsticks or lip balms; and
- Dermal exposure of various age groups though the use of body creams, permanent hair dyes, spray perfumes and face paints.
Margins of exposure (MOEs) between levels of exposure of the general population from daily use of Solvent Violet 13 in certain cosmetics (body cream, spray perfume) and levels associated with non-cancer health effects were considered potentially inadequate to address uncertainties in the health effects and exposure databases. MOEs between levels of exposure of the general population from daily use of Solvent Violet 13 in certain cosmetics (lip balm, lipstick, body cream, permanent hair dye, spray perfume and face paint) and cancer effects were also considered potentially inadequate. MOEs were, however, considered adequate for other uses of Solvent Violet 13 (Canada 2019).
No sources of exposure other than cosmetics were identified as a concern in the screening assessment (ECCC, HC, 2021).
6. Risk Management Considerations
6.1 Alternatives and Alternate Technologies
There are alternative substances available that may serve the same function as Solvent Violet 13 (e.g., as colorants or dyes); however, their feasibility as replacements for specific cosmetic products is unknown.
6.2 Socio-economic and Technical Considerations
Socio-economic factors will be considered in the selection process for a regulation and/or instrument respecting preventive or control actions, and in the development of the risk management objectives(s) as per the guidance provided in the Treasury Board document Assessing, Selecting, and Implementing Instruments for Government Action (Treasury Board of Canada Secretariat TBS, 2007). In addition, socio-economic factors will also be considered in the development of regulations, instrument(s) and/or tool(s) to address risk management objective(s) as identified in the Cabinet Directive on Regulatory Management (TBS 2018), Red Tape Reduction Action Plan (TBS 2012) and the Red Tape Reduction Act (Canada 2015).
7. Overview of Existing Risk Management
7.1 Related Canadian Risk Management Context
Domestically, the pertinent risk management actions are as follows:
Food and Drugs Act (F&DA)
Food: The safety of chemicals used in food packaging materials and incidental additives is subject to the provisions of section 4(1)(a) of the F&DA, and food packaging materials are also subject to the provisions in Division 23 of the Food and Drug Regulations. Solvent Violet 13 is not currently included on Health Canada’s Lists of Permitted Food Additives; therefore, it is not an approved food additive in foods sold in Canada.
Cosmetics: Solvent Violet 13 is present in cosmetics based on notifications submitted under the Cosmetic Regulations; it is not currently included on Health Canada’s Cosmetic Ingredient Hotlist.
Drugs: In Canada, Solvent Violet 13 is listed under the Food and Drugs Regulations as a colouring agent permitted in drugs for external use (Canada, 2017).
Natural Health Products (NHPs): NHPs are regulated under the Natural Health Products Regulations. Solvent Violet 13 is listed with a non-medicinal role for external use only as a colour additive in natural health products (NHPID, 2017).
Pest Control Products Act (PCPA)
Solvent Violet 13 is listed on the Pest Management Regulatory Agency (PMRA) List of Formulants (personal communication, 2016 email from the PMRA, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
7.2 Pertinent International Risk Management Context
Internationally, the pertinent risk management actions are as follows:
United States:
- Food and Drug Act, Title 21 of the Code of Federal Regulation (CFR):
- Part 74 – Listing of Color Additives Subject to Certification. Solvent Violet 13 is listed as a color additive that is subject to certification and permitted for use in cosmetics for external use only, and not allowed for eye area or generally (including lipsticks). It is also allowed for use in externally applied drugs and in medical devices not to exceed (NTE) 0.1-0.3% by weight in various absorbable sutures; in amounts NTE the minimum reasonably required to accomplish the intended coloring effect in contact lenses; NTE 0.2% of intraocular lens haptics, NTE 0.15% by weight of meniscal tracks (US eCFR, 2017a).
- Part 82, Listings of Certified Provisionally Listed Colors and Specifications (drugs and cosmetics) (US eCFR, 2017b); and Part 81, General Specification and General Restrictions for Provisional Color Additives for Use in Foods, Drugs and Cosmetics where it is restricted from use in the manufacture of ingested drugs or cosmetics subject to ingestion (US eCFR, 2017c).Threshold for Regulation (TOR) Exemption (TOR No. 1998-015), with use limitations, at levels not to exceed 0.2 ppm in polystyrene food-contact articles. Threshold of Regulation Exemptions are generally applicable and are effective for the food contact substance (FCS).
- Inventory of Effective FCS Notifications - The database lists effective premarket notification for food contact substances that have been demonstrated to be safe for their intended use. Solvent Violet 13 is listed as a colourant in food-contact polystyrene, at levels not to exceed 0.70 ppm, for all food types except for use in contact with infant formula and breast milk. It is also listed as a component in epoxy resin coatings for repeat use in contact with beer, at a maximum level of 1 percent by weight of the cured epoxy coating (US FDA, 2017).
- Additionally, FCS Notifications are effective for the manufacturer or supplier identified in the notification.
- Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), Environmental Protection Agency (EPA) Regulations. Solvent Violet 13 is classified as List 4B -- an inert ingredient in pesticides based upon the ‘reasonable certainty of no harm’ safety finding. It is cleared for use in food and non-food as a dye. This inert ingredient is used pre-harvest with exemptions from the requirement of a tolerance when used in accordance with good agricultural practice. To be exempt from the requirement of a tolerance, it must be limited to not more than 0.005% of the pesticide formulation. It is also an inert ingredient applied to animals (with exemption from the requirement of a tolerance) (US EPA, 2005; US eCFR 2017d).
European Union:
While Solvent Violet 13 is included in Annex IV, List of Colorants Allowed in Cosmetic Products, of European Commission Regulation No 1223/2009 (EC, 2009), it is also listed in Annex II of the List of Substances Prohibited in Cosmetic Products, as per Commission Implementing Regulation No 344/2013 (EC, 2013), specifically when used as a substance in hair dye products.
Other:
- New Zealand - Cosmetic Products Group Standard Solvent Violet - listed in schedule 4, components cosmetic product must not contain when used as a substance in hair dye products (New Zealand, 2006).
- Australia - Australian Government Regulation of Cosmetics (website) - listed as a colouring for use as excipients in medicines for topical use only and does not require evaluation of toxicology data (Australia, 2016).
8. Next Steps
8.1 Public Comment Period
Industry and other interested stakeholders are invited to submit comments on the content of this Risk Management Approach or other information that would help to inform decision-making (such as outlined in section 3.2). Please submit additional information and comments prior to September 15, 2021.
Comments and information submissions on the Risk Management Approach should be submitted to the address provided below:
Environment and Climate Change Canada
Chemicals Management Division
Gatineau Quebec K1A 0H3
Tel: 1-800-567-1999 | 819- 938-3232
Fax: 819-938-5212
Email: substances@ec.gc.ca
Companies who have a business interest in Solvent Violet 13 are encouraged to identify themselves as stakeholders. Stakeholders will be informed of future decisions regarding Solvent Violet 13 and may be contacted for further information.
Following the public comment period on the Risk Management Approach document, the Government of Canada will initiate the development of the specific risk management instrument(s), where necessary. Comments received on the Risk Management Approach document will be taken into consideration in the selection or development of these instrument(s). Consultation will also take place as instrument(s) are developed.
8.2 Timing of Actions
Electronic consultation on the Risk Management Approach: July 17, 2021 to September 15, 2021
Publication of responses to public comments on the Risk Management Approach document: Concurrent to the publication of the proposed instrument(s).
Publication of the proposed instrument(s): At the latest, 24 months from the date on which the Ministers recommended that Solvent Violet 13 be added to Schedule 1 of CEPA.
Consultation on the proposed instrument(s): 60-day public comment period, starting upon publication of the proposed instrument.
Publication of the final instrument(s): At the latest, 18 months from the publication of the proposed instrument.
These are planned timelines and are subject to change. Please consult the schedule of risk management activities and consultations for updated information on timelines.
9. References
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List. Canada Gazette [pdf], Part I, vol. 146, no. 48, Supplement.
Canada. 2015. Red Tape Reduction Act S.C. 2015, c. 12.
Canada. 2017. Consolidated Food and Drug Regulations [pdf]. C.R.C., c. 870. Last amended on June 17, 2017 [Current as of September 14, 2017].
[Danish EPA] Danish Environmental Protection Agency. 2015. CMR substances in toys – Market surveillance and risk assessment [pdf]. Copenhagen (Denmark): Danish Environmental Protection Agency. [accessed 2015 Dec 23].
[EC]. European Commission. 2009. Commission Regulation (EC) No. 1223/2009 of the European Parliament and of the Council on 30 November 2009 concerning cosmetic products.
[EC]. European Commission. 2013. Commission Implementing Regulation (EU) No 344/2013 of 4 April 2013 amending Annexes II, III, V and VI to Commission Implementing Regulation (EC) No 1223/2009.
[ECCC, HC] Environment Canada and Climate Change, Health Canada. [2021]. Screening Assessment for the Anthraquinones Group. [accessed 2021, July, 17]
[ECCC, HC] Environment Canada and Climate Change, Health Canada. 2018a. Draft Screening Assessment for the Anthraquinones Group. [accessed 2018, November, 3]
[ECCC, HC] Environment Canada and Climate Change, Health Canada. 2018b. Risk Management Scope for Solvent Violet 13. [2018, November, 3]
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017. Rapid Screening of Substances with Limited General Population Exposure. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. c2007-2019a. Registered substances databases; search results for CAS RN 81-48-1. Helsinki (FI): ECHA. [accessed 2019 May 30]
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Health Canada. [modified 2015 Dec 14]. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Health Canada, Consumer Hazardous Products Safety Directorate. [accessed 2016 Aug].
Health Canada. 2015. List of permitted colouring agents (lists of permitted food additives) [Internet]. Ottawa (ON): Health Canada. [cited 2016 Aug].
Health Canada. 2016. Science Approach Document: Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances. September 2016. 54 pp.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Health Canada. [accessed 2017 Jan 09].
[MSDS] Material Safety Data Sheet. 2007a. No rinse natural cat shampoo. [Internet]. Dallas (TX): OUT! International Inc. [accessed 2016 Jan 26].
[MSDS] Material Safety Data Sheet. 2007b. No rinse natural cat shampoo. [Internet]. Dallas (TX): OUT! International Inc. [accessed 2016 Jan 26].
[MSDS] Material Safety Data Sheet. 2009. Wax Based Make Up/Face Paint Crayon Products. Orlando (FL): Wolfe Brothers Face Art & FX, LLC. [accessed 2016 Oct 4]. Available upon request.
[MSDS] Material Safety Data Sheet. 2015a. Groomer’s best puppy shampoo. [Internet]. Secaucus (NJ): Hartz Mtn. Corp. [accessed 2016 Jan 26].
[MSDS] Material Safety Data Sheet. 2015b. Groomer’s best puppy shampoo. [Internet]. Secaucus (NJ): Hartz Mtn. Corp. [accessed 2016 Jan 26].
New Zealand. 2006. Cosmetic Products Group Standard 2006 [pdf] (Hazardous Substances and New Organisms Act 1996).
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2017 Jan 10]. Ottawa (ON): Health Canada. [accessed 2017 May 19].
[Pesticide Label Search] [database]. [modified 2016 Jan 25]. Ottawa (ON): Health Canada. [accessed 2015 Sept 30].
[TBS] Treasury Board of Canada Secretariat.2007. Assessing, Selecting, and Implementing Instruments for Government Action.
[TBS] Treasury Board of Canada Secretariat. 2018. Cabinet Directive on Regulation. Ottawa (ON): Government of Canada.
[TBS] Treasury Board of Canada Secretariat. 2012. Red Tape Reduction Action Plan. Available from: http://www.tbs-sct.gc.ca/rtrap-parfa/rtrapr-rparfa-eng.asp
[US eCFR]. United States Code of Federal Regulations. 2017a. Title 21, Part 74 (Listing of Color Additives Subject to Certification).
[US eCFR] United States Code of Federal Regulations. 2017b. Title 21, Part 82 (Listing of Certified Provisionally Listed Colors and Specifications) Subpart 82.1602.
[US eCFR] United States Code of Federal Regulations. 2017c. Title 21, Part 81 (General Specifications and General Restrictions for Provisional Color Additives for Use In Foods, Drugs, and Cosmetics), Subpart 81.30.
[US eCFR]. United States Code of Federal Regulations. 2017d. Title 40, Part 180: Tolerances and Exemptions for Pesticide Chemical Residues in Food.
[US EPA]. United States Environmental Protection Agency. 2005. Memorandum: May 20, 2005 [pdf].
[US FDA]. United States Food and Drug Administration. 2017. Inventory of Effective Food Contact Substance (FCS) Notifications.
Annex A
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 81-48-1 | 9,10-Anthracenedione, 1- hydroxy-4-[(4- methylphenyl)amino]- (Solvent Violet 13; also called Disperse Blue 72) | 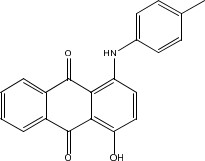 C21H15NO3 | 329.35 |
| 81-77-6 | 5,9,14,18-Anthrazinetetrone, 6,15-dihydro-(Pigment Blue 60) | ![Representative chemical structure Pigment Blue 60, SMILES: c12c3c([nH]c4c5c(c(c6ccccc6c5=O)=O)ccc4[nH]3)ccc1c(c1ccccc1c2=O)=O](/content/dam/eccc/images/pded/anthraquinones/20210406-Table2.1.2.jpg) C28H14N2O4
C28H14N2O4 | 442.43 | 6408-72-6 | 9,10-Anthracenedione, 1,4- diamino-2,3-diphenoxy- (Solvent Violet 59; also called Disperse Violet 31 and Disperse Violet 26) |  C26H18N2O4 | 422.44 |
| 14233-37-5 | p;9,10-Anthracenedione, 1,4- bis[(1-methylethyl)amino]- (Solvent Blue 36) | 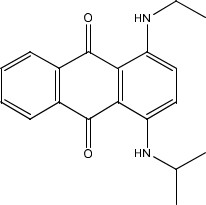 C20H22N2O2 | 322.41 |
| 17418-58-5 | 9,10-Anthracenedione, 1- amino-4-hydroxy-2-phenoxy- (Disperse Red 60) |  C20H13NO4 | 331.33 |
| 72391-24-3 | Benzenesulfonic acid, [[(chloroacetyl)amino]methyl][ 4-[[4-(cyclohexylamino)-9,10- dihydro-9,10-dioxo-1- anthracenyl]amino]phenoxy] methyl-, monosodium salt (Acid Blue 239) | 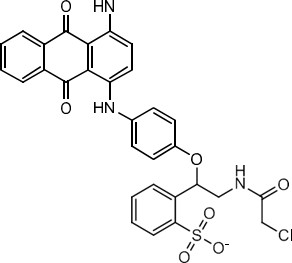 C36H34ClN3O7S.Na C36H34ClN3O7S.Na | 710.18 |
| 74499-36-8a | 9,10-Anthracenedione, 1,4-diamino-, N,N’-mixed 2- ethylhexyl and Me and pentyl derivs.(NA) | 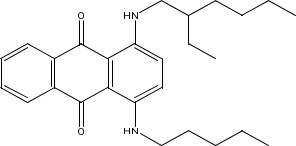 C27H36N2O2 | 420.60 |
