Risk management scope for 2-Pyrrolidinone, 1-methyl- (NMP)
Official title: Risk management scope for 2-Pyrrolidinone, 1-methyl- (NMP)
(CAS RN 872-50-4)
Environment and Climate Change Canada
Health Canada
January 2024
Summary of proposed risk management
This document outlines the risk management options under consideration for 2-pyrrolidinone, 1-methyl-, commonly known as N-methylpyrrolidone (NMP), which has been proposed to be harmful to human health in Canada but not to the environment.
In particular, the Government of Canada is considering:
- Regulatory or non-regulatory actions to help reduce inhalation and dermal exposure of people in Canada, including persons of childbearing age, to NMP in adhesive products that are available to consumers, including deck construction adhesives.
Information on the following items should be provided (on or before March 27, 2024) to the contact details identified in section 8 of this document, to inform risk management decision-making:
- How widely NMP is used in adhesives and sealants available to consumers (for example, number or products containing NMP, volume relative to other products) and at what concentrations is NMP typically present within these adhesives and sealants;
- The ease/difficulty of reformulating NMP-containing adhesives available to consumers with a different solvent or at a lower concentration of NMP, and any associated socio-economic impacts of doing so; and
- Alternatives to NMP in adhesives available to consumers, and the potential for NEP to be used as a replacement for NMP.
The risk management options outlined in this Risk Management Scope may evolve through consideration of assessments and risk management options published for other Chemicals Management Plan (CMP) substances as required to ensure effective, coordinated, and consistent risk management decision-making.
Note: The above summary is an abridged list of options under consideration to manage this substance and to seek information on identified information gaps and uncertainties. Refer to section 3 of this document for more complete details in this regard.
1. Context
The Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999) provides the authority for the Minister of the Environment and the Minister of Health (the ministers) to conduct assessments to determine if substances are toxic to the environment and/or to human health as set out in section 64 of CEPA,Footnote 1, Footnote 2 and, if so, to manage the associated risks.
The substances 2-pyrrolidinone, 1-methyl-, Chemical Abstracts Service Registry Number (CAS RN)Footnote 3 872-50-4, referred to throughout this document as N-methylpyrrolidone (NMP) and 2-pyrrolidinone, 1-ethyl- (CAS RN 2687-91-4), referred to throughout this document as N-ethylpyrrolidone (NEP), were included in the updated draft assessment for NMP/NEP as part of the third phase of the Chemicals Management Plan (CMP) (Canada 2024).
2. Issue
In 2017, Health Canada and Environment and Climate Change Canada conducted and published a draft scientific assessment of NMP and NEP (ECCC, HC 2017). New information was received after the publication that led to changes in the proposed conclusions for NMP. As a result, Health Canada and Environment and Climate Change Canada have updated the draft scientific assessment, and a notice summarizing the scientific considerations of the updated draft assessment for these substances was published in the Canada Gazette, Part I. For further information, refer to the draft assessment report for NMP and NEP.
2.1 Updated draft assessment conclusion
A draft screening assessment for NMP and NEP was originally published in February 2017. Since then, significant new information became available regarding exposure to products available to consumers containing NMP and NEP.
On the basis of the updated information available, the updated draft assessment proposes that NMP meets the criteria under section 64(
Although risk to human health or the environment has not been identified at current levels of exposure, there may be a concern if exposure to NEP were to increase. As a result, this substance may be considered in future initiatives to track its commercial status or identify new uses or exposures.
The updated draft assessment also proposes that NMP does not meet the criteria for persistence or bioaccumulation, as defined in the Persistence and Bioaccumulation Regulations made under CEPA (Canada 2000).
The exposure source of concern, as identified in the updated draft assessment, is potential combined inhalation and dermal exposure to NMP from the use of deck construction adhesive products available to consumers.
2.2 Proposed recommendation under CEPA
On the basis of the findings of the updated draft assessment pursuant to CEPA, the Ministers propose to recommend that NMP be added to Part 2 in Schedule 1 to CEPAFootnote 4 . Addition of a substance to Schedule 1 to CEPA enables the Government to propose certain risk management measures under CEPA to manage potential ecological and human health risks associated with the substance.
Until regulations specifying criteria for the classification of substances that pose the highest risk or that are carcinogenic, mutagenic or toxic to reproduction are available, NMP is proposed to be recommended for addition to Part 2 of Schedule 1. Following the availability of the aforementioned criteria, the substance may be moved to Part 1 of Schedule 1, if applicable.
CEPA sets out a 2-track approach for managing risks.
Under sub-section 77(3), the Ministers are required to propose recommending the addition of a substance that poses the highest risk, as defined in paragraph (a), (b) or (c), to Part 1Footnote 5 of Schedule 1 of the Act and, in developing a proposed regulation or instrument respecting preventive or control actions, to give priority to the total, partial or conditional prohibition of activities in relation to the substance or to the release of the substance into the environment.
For other substances recommended for addition to Part 2 of Schedule 1 of the Act, the Ministers shall give priority to pollution prevention, and this could include regulatory or non-regulatory measures such as prohibition if warranted.
The ministers will take into consideration comments made by stakeholders during the 60-day public comment period on the updated draft assessment for NMP and its associated Risk Management Scope document.
If the Ministers finalize the recommendation to add NMP to Part 2 of Schedule 1, risk management instruments must be proposed within 24 months from the date on which the Ministers recommended that NMP be added to Schedule 1 to CEPA, and finalized within 18 months from the date on which the risk management instruments are proposed, as outlined in sections 91 and 92 of CEPA (refer to section 8 for publication timelines applicable to this group of substances).
3. Proposed risk management
3.1 Proposed human health objective
Proposed human health objectives are quantitative or qualitative statements of what should be achieved in order to address human health concerns.
The proposed human health objective for this substance is to reduce exposure of the general population to NMP to levels that are protective of human health. The risks and exposure sources of concern are outlined in section 5 of this document.
3.2 Proposed risk management objective
Proposed risk management objectives set quantitative or qualitative targets to be achieved by the implementation of risk management regulations, instrument(s) and/or tool(s) for a given substance or substances.
In this case, the proposed risk management objective for NMP is to reduce dermal and inhalation exposures to the general population, including persons of childbearing age, to this substance from the use of adhesive products available to consumers, including deck construction adhesives, to levels that are protective of human health.
This proposed risk management objective may be refined on the basis of consultation with stakeholders, the proposed risk management, consideration of further information received, the outcome of the final assessment, and socio-economic and technical considerations (outlined in section 6 of this document).
The revised human health and risk management objectives would be presented in the risk management approach that would be published concurrently with the final assessment for NMP and NEP, or in subsequent risk management documents (for example, consultation document on the proposed instrument).
3.3 Proposed risk management options under consideration
To achieve the proposed risk management objective and to work towards achieving the proposed human health objective, the risk management options under consideration for NMP are as follows:
- Regulatory and/or non-regulatory measures to reduce dermal and inhalation exposure of persons, including those of childbearing age, to NMP under certain use conditions (such as duration of use and quantity used) in adhesive products available to consumers, including deck construction adhesives.
Note that the proposed risk management option is preliminary and subject to change. Following the publication of this document, additional information obtained from the public comment period and from other sources will also be considered in the instrument selection and development processFootnote 6 . The risk management option may also evolve through consideration of assessments and risk management options or actions published for other CMP substances such as NEP to ensure effective, coordinated, and consistent risk management decision-making.
3.4 Performance measurement and evaluation
Performance measurement evaluates the ongoing effectiveness and relevance of the actions taken to manage risks from toxic substancesFootnote 7 . Environment and Climate Change Canada and Health Canada have developed a Performance Measurement Evaluation Strategy that sets out the approach to evaluate the effectiveness of actions taken on substances found toxic under CEPA.The aim is to determine whether human health and/or environmental objectives have been met, and whether there is a need to revisit the risk management approach for that substance, to ensure that risks are managed effectively over time. To achieve this, the Government of Canada plans to evaluate the effectiveness of the risk management action(s) for NMP.
The Government of Canada plans to evaluate the effectiveness of risk management by collecting and analyzing data, such as data obtained from mandatory or voluntary surveys on the presence of NMP in products available to Canadian consumers.
The results of the performance measurement evaluation will be used to inform whether further risk management action is warranted. These results will be made available to Canadians along with recommendations for further action, if applicable.
3.5 Risk management information gaps
Interested stakeholders are invited to provide further information regarding the following, to inform risk assessment and management decision-making for NMP:
- How widely NMP is used in adhesives and sealants available to consumers (for example, number or products containing NMP, volume relative to other products) and at what concentrations is NMP typically present within these adhesives and sealants.
- The ease/difficulty of reformulating NMP-containing adhesives available to consumers with a different solvent or at a lower concentration of NMP, and any associated socio-economic impacts of doing so.
- Alternatives to NMP in adhesives available to consumers, and the potential for NEP to be used as a replacement for NMP.
Should stakeholders have further information to help address these gaps, they should provide it to the contact identified in Section 8 of this document on or before March 27, 2024 to inform the risk management decision-making process.
4. Background
4.1 General information on NMP
NMP was included in a survey issued pursuant to section 71 of CEPA (Environment Canada 2012). According to information submitted for NMP, there was no manufacturing in Canada above the reporting threshold of 100 kg, and between 100 000 kg to 1 000 000 kg were reported to be imported into Canada in 2011.
4.2 Current uses and identified sectors
In response to a CEPA section 71 survey NMP is used in the manufacture of agricultural products, electrical and electronic products, metal and mining products, paper products, mixtures or manufactured items, and plastic and rubber materials. Canadian businesses in the chemical manufacturing and automotive, aircraft and transportation sectors also reported involvement with NMP. Products in Canada that contain NMP and that may be available to the general population include adhesives and sealants, auto interior cleaners, cleaning and degreasing products, paints and coatings, and paint removers. NMP may be present in certain personal care products, including as a non-medicinal ingredient in pharmaceuticals, and in a limited number of nail care, synthetic nail or eyelash adhesives and adhesive removers, and hair products. NMP is also present in certain registered pest control products.
5. Exposure sources and identified risk
According to the updated draft assessment, NMP does not occur naturally. In Canada, individuals may be exposed to NMP via dermal and inhalation exposure to a wide range of consumer products including: sealants for eavestroughs, automotive seams and residential driveways, engine cleaners and degreasers, automotive interior cleaners, paints and coatings as well as paint and coating removers, and adhesives. Exposure to NMP may also occur through cosmetics, where notifications for NMP have been received under the Cosmetic Regulations as adhesives (and adhesive removers) for synthetic eyelashes and nails.
Reproductive and developmental effects were the critical human health effects for NMP identified in the updated draft assessment based on a high-quality, 2-generation rat study. A comparison of estimated dermal and inhalation exposure to NMP, from the use of deck construction adhesives to the critical health effect level, resulted in margins of exposure which were considered inadequate to address uncertainties in the health effects and exposure databases (Canada 2024) for persons of childbearing age.
No sources of exposure of concern, other than the dermal and inhalation exposure to deck construction adhesive products available to consumers were identified.
6. Risk management considerations
6.1 Alternatives and alternate technologies
There are currently other deck construction adhesives available on the market which do not contain NMP. As NMP is used mainly as a solvent, it is expected that different solvents are available to replace or reduce the concentration of NMP in the deck adhesives that currently contain NMP. Information on alternatives to NMP in adhesives available to consumers is requested in Section 3.5.
6.2 Socio-economic and technical considerations
Socio-economic factors will be considered in the selection of a regulation and/or instrument respecting preventive or control actions, and in the development of the risk management objectives(s). Socio-economic factors will also be considered in the development of regulations, instrument(s) and/or tool(s) as identified in the Cabinet Directive on Regulation (TBS 2018) and the guidance provided in the Treasury Board document Assessing, Selecting, and Implementing Instruments for Government Action (TBS 2007).
7. Overview of existing risk management
7.1 Related Canadian risk management context
Domestically, the following relevant risk management actions for NMP include:
- Pest Control Products Formulant Database – NMP is listed as a formulant under List 3. List 3 substances (Formulants That Do Not Meet the Criteria of Lists 1, 2, 4A and 4B) may be subject to reassessment, pursuant to the Pest Control Products Act and Regulations.
- Ontario Air Contaminants Benchmark List – NMP is listed with a 24-hour average benchmark of 40 mg/m3.
- Volatile Organic Compound Concentration Limits for Certain Products Regulations - the Regulations establish VOC concentration limits for the total amount of VOCs in approximately 130 product categories and subcategories including adhesives.
7.2 Pertinent international risk management context
In the US, the relevant risk management actions for NMP include:
- 40 CFR §59 Subpart E—National Volatile Organic Compound Emission Standards for Aerosol Coatings (Mar. 9, 2012) – NMP has a reactivity factor of 2.56 g O3/g VOC.
- NMP is subject to export notification.
- The US EPA has identified risks of concern with NMP in adhesives, including consumer and occupational uses, and is considering risk management actions (US EPA, 2022).
In Europe, the following relevant risk management actions for NMP include:
- Not permitted in cosmetics since January 2019 (Annex II, Regulation EC 1223/2009);
- Not permitted for use in components that come in contact with food ( 5(2)(c)(i) of Commission Regulation (EC) 450/2009/EC);
- Not permitted as a formulant in plant protection products since March 2021 (Commission Regulation (EU) 2021/383).
8. Next steps
8.1 Public comment period
Industry and other interested stakeholders are invited to submit comments on the content of this Risk Management Scope or other information that would help to inform decision-making (such as outlined in section 3.5).
Should the final assessment confirm that NMP is harmful to human health, the Risk Management Approach document, which would outline and seek input on the proposed risk management instrument(s), would be published at the same time as the final assessment. At that time, there would be further opportunity for consultation.
Comments and information submissions on the Risk Management Scope should be submitted to the address provided below:
Environment and Climate Change Canada
Chemicals Management Division
Gatineau Quebec K1A 0H3
Tel: 1-800-567-1999 | 819- 938-3232
Fax: 819-938-5212
Email: substances@ec.gc.ca
Companies that have a business interest in NMP are encouraged to identify themselves as stakeholders. Stakeholders will be informed of future decisions regarding NMP and may be contacted for further information.
8.2 Timing of actions
Electronic consultation on the updated draft assessment report and Risk Management Scope: January 27, 2024 to March 27, 2024. This should include the submission of public comments, additional studies and/or information on NMP.
Publication of responses to public comments on the updated draft assessment and Risk Management Scope: concurrent to the publication of the final assessment and, if required, the Risk Management Approach document.
Publication of responses to public comments on the risk management approach, if applicable and, if required, on the proposed instrument(s): At the latest, 24 months from the date on which the ministers recommended that 2-pyrrolidinone, 1-methyl- be added to Schedule 1 of CEPA.
Consultation on the proposed instrument(s), if required: 60-day public comment period starting upon publication of each proposed instrument.
Publication of the final instrument(s), if required: At the latest, 18 months from the publication of each proposed instrument.
These are planned timelines, and are subject to change. Please consult the schedule of risk management activities and consultations for updated information on timelines.
References
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March 2000, SOR/2000-107.
Canada, 2015. Red Tape Reduction Act.
Canada. 2023. Dept. of the Environment, Dept. of Health. Updated Draft Screening Assessment for NMP and NEP.
ECCC, HC 2017. Dept. of the Environment, Dept. of Health. Draft Screening Assessment for NMP and NEP.
Environment Canada. 2012 . DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EC] European Commission, 2009. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. [Accessed June 2021].
[EU] European Union, 2009. Commission Regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. [Accessed June, 2021].
[EU] European Union, 2021. Commission Regulation (EU) 2021/383 of 3 March 2021 amending Annex III to Regulation (EC) No 1107/2009 of the European Parliament and of the Council listing co-formulants which are not accepted for inclusion in plant protection products. [Accessed June, 2021].
Ontario, 2016. Ontario’s Ambient Air Quality Criteria. Ontario Ministry of the Environment, Conservation and Parks. [accessed 2021 Jun].
PMRA [modified 2010 Aug 31]. PMRA List of Formulants: list of formulants that are found in pest control products currently registered in Canada under the Pest Control Products Act and Regulations. Ottawa (ON): Pest Management Regulatory Agency. [accessed 2021 Jun].
[TBS] Treasury Board of Canada Secretariat. 2007. Assessing, Selecting, and Implementing Instruments for Government Action.
[TBS] Treasury Board of Canada Secretariat. 2012. Red Tape Reduction Action Plan.
[TBS] Treasury Board of Canada Secretariat. 2018. Cabinet Directive on Regulation.
TSCA [modified August 2022]. Toxic Substances Control Act section 12(b) Export Notification List [accessed 2022 Jun].
[US eCFR, 2012]. United States Code of Federal Regulations. Title 40, Part 59, Subpart E – National Volatile Organic Compound Emission Standards for Aerosol Coatings. [accessed 2021 Jun].
US EPA. 2022. Risk Evaluation for n-Methylpyrrolidone (NMP)
Appendix A. Substance targeted for risk management
| CAS RN | DSL name | Common Name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|---|
| 872-50-4 | 2-Pyrrolidinone, 1-methyl- | N-methylpyrrolidone (NMP) |
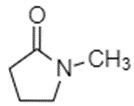 C5H9NO |
99.1 |
