Risk management scope for phenol, methylstyrenated
Official title: Risk management scope for phenol, methylstyrenated
Chemical Abstracts Service Registry Number (CAS RN):
68512-30-1
Environment and Climate Change Canada
Health Canada
November 2021
Summary of proposed risk management
This document outlines the risk management options under consideration for phenol, methylstyrenated (MSP) (CAS RN 68512-30-1), which has been proposed to be harmful to the environment.
In particular, the Government of Canada is considering:
- Regulations to prohibit the manufacture, use, sale, offer for sale, and import of MSP and products containing the substance
- Regulatory and non-regulatory initiatives to prevent or limit releases of MSP from notified activities during an interim phase-out period, if warranted
- Rescission of the Significant New Activity (SNAc) Order and Ministerial Conditions for MSP once regulations have come into effect
Moreover, because certain data gaps to inform risk management decision-making remain, the following information should be provided (ideally on or before January 5, 2022), to the contact details identified in section 8 of this document, to inform risk management decision-making (more details on these topics can be found in section 3.5):
- Socio-economic and technical impacts of the proposed risk management
- Alternative substances to MSP in paints and coatings products
- Alternative paints and coatings products
- Analytical methods to detect levels of MSP and/or its representative component in the aquatic environment and in paints and coatings products
The risk management options outlined in this Risk Management Scope document may evolve through consideration of assessments and risk management options or actions published for other Chemicals Management Plan (CMP) substances as required to ensure effective, coordinated, and consistent risk management decision-making.
Note: The above summary is an abridged list of options under consideration to manage this substance and to seek information on identified gaps. Refer to section 3 of this document for more complete details in this regard. It should be noted that the proposed risk management options may evolve through consideration of additional information obtained from the public comment period, literature, and other sources.
1. Context
The Canadian Environmental Protection Act, 1999 (CEPA) provides the authority for the Minister of the Environment and the Minister of Health (the Ministers) to conduct assessments to determine if substances are toxic to the environment and/or human health as set out in section 64 of CEPAFootnote 1 Footnote 2 , and if so, to manage the associated risks.
As part of the first phase of the Chemicals Management Plan, the Ministers published the Final decision on the screening assessment of 145 substances on the Domestic Substances List (subsection 77(6) of the Canadian Environmental Protection Act, 1999) (Canada 2008b; Canada 2008c), a screening assessment of 145 substances with similar hazardous properties. The substance phenol, methylstyrenated, Chemical Abstracts Service Registry Number (CAS RN)Footnote 3 68512-30-1, referred to throughout this document as MSP Footnote 4 , was one of the 145 substances included in the screening assessment.
At that time, it was concluded that MSP did not meet the criteria of section 64 of CEPA because it was not entering the environment at levels that could have posed a risk to human health or to the environment (Canada 2008b). The conclusion was based on the fact that, according to a survey issued pursuant to section 71 of CEPA, no industrial activities (import or manufacture) in relation to the substance were identified in Canada above the reporting threshold of 100 kg for the specified reporting year and, therefore, there was no known exposure to humans or to the environment (Canada 2006). However, given the persistent, bioaccumulative, and inherently toxic (PBiT) properties of this substance, there was concern that new activities, which had not been identified or assessed, could lead to the substance meeting the criteria of section 64 of CEPA.
On the basis of the report’s conclusion, the Significant New Activity (SNAc) provisions specified under subsection 81(3) of CEPA were applied to MSP (Canada 2008a). The SNAc Order for this substance requires notification, for the purpose of assessment, of the manufacture, import, or use of MSP in Canada in quantities at or above 100 kg. In response to the SNAc Order, multiple Significant New Activity Notifications (SNANs) have been received since 2015, leading to the current assessment of MSP.
2. Issue
Health Canada and Environment and Climate Change Canada conducted a new joint screening assessment of MSP in Canada. A notice summarizing the scientific considerations of the draft screening assessment for this substance was published in the Canada Gazette, Part I, on November 5, 2021 (Canada 2021). For further information on the draft screening assessment for MSP, refer to the draft screening assessment of phenol, methylstyrenated.
2.1 Draft screening assessment conclusion
On the basis of the information available, the draft screening assessment proposes that MSP is toxic under paragraph 64(a) of CEPA because it is entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity (Canada 2021).
The draft screening assessment also proposes that MSP meets the criteria for persistence and meets the criteria for bioaccumulation, as defined in the Persistence and Bioaccumulation Regulations made under CEPA (Canada 2000).
The exposures of concern identified in the draft screening assessment are based on the releases of MSP from activities notified through the received SNANs. The notified activities include the use of paints and coatings containing MSP in industrial and commercial applications. These activities were estimated to result in releases of the substance into the aquatic environment that would be of ecological concern. This document focuses on the notified activities of concern associated with paints and coatings containing this substance (refer to sections 4 and 5).
2.2 Proposed recommendation under CEPA
On the basis of the findings of the draft screening assessment conducted pursuant to section 68 of CEPA, the Ministers propose to recommend that MSP be added to the List of Toxic Substances in Schedule 1 of the Act Footnote 5 .
Given that MSP meets one or more criteria in section 64, and is both persistent and bioaccumulative in accordance with the Persistence and Bioaccumulation Regulations, it is proposed that this substance be virtually eliminated Footnote 6 taking into account relevant environmental or health risks and social, economic and technical matters, such as may be outlined in section 6 of this document.
The Ministers will take into consideration comments submitted by stakeholders during the 60-day public comment period on the draft screening assessment for MSP (Canada 2021) and its associated Risk Management Scope document.
If the Ministers finalize the recommendation to add MSP to Schedule 1, risk management instruments will be proposed within 24 months from the date on which the Ministers recommended that MSP be added to Schedule 1 of CEPA, and finalized within 18 months from the date on which the risk management instruments are proposed (refer to section 8 for publication timelines applicable to this group of substances)
3. Proposed risk management
3.1 Proposed environmental objective
Proposed environmental objectives are quantitative or qualitative statements of what should be achieved to address environmental concerns related to a substance.
MSP is proposed to meet the criteria for virtual elimination set out in the Toxic Substances Management Policy, 1995 Footnote 7 . As such, the proposed environmental objective for MSP is virtual elimination in order to prevent or minimize negative effects on the aquatic environment. A Level of Quantification (LoQ) or a predicted no-effect concentration (PNEC) may be used as a quantitative objective.
The LoQ is the lowest concentration that can be accurately measured using sensitive but routine sampling and analytical methods, and is a tool to determine whether virtual elimination has been achieved. Alternatively, the PNEC of 0.23 µg/L in surface water for dimers of C9 monomer, the most toxic and most significant component of MSP, may be used if it has a lower value than the LoQ.
3.2 Proposed risk management objective
Proposed risk management objectives set quantitative or qualitative targets to be achieved by the implementation of risk management regulations, instruments and/or tools for a given substance or substances. In this case, in order to achieve the proposed environmental objective of virtual elimination, the proposed risk management objective for MSP is to prevent releases of the substance to the aquatic environment.
Such objectives will be refined on the basis of consultation with stakeholders, the proposed risk management, consideration of further information received, the outcome of the screening assessment, and socio-economic and technical considerations (such as may be outlined in section 6 of this document). Revised environmental and risk management objectives will be presented in the Risk Management Approach document that will be published concurrently with the final screening assessment for this substance, or in subsequent risk management documents (e.g., consultation document on proposed instrument), as the case may be.
3.3 Proposed risk management options under consideration
To achieve the proposed risk management objective and to work towards achieving the proposed environmental objective, the risk management options under consideration for MSP are:
- Regulations to prohibit the manufacture, use, sale, offer for sale, and import of MSP and products containing the substance
- Regulatory and non-regulatory initiatives to prevent or limit releases of MSP from notified activities during an interim phase-out period, if warranted
- Rescission of the Significant New Activity (SNAc) Order and Ministerial Conditions once the regulations have come into effect
Note that the proposed risk management options described in this document are preliminary and subject to change. Following the publication of this document, additional information obtained during the public comment period and from other sources will be considered, along with the information presented in this document, in the instrument selection and development process Footnote 8 . The risk management options outlined in this document may also evolve through consideration of assessments and risk management options or actions published for other CMP substances to ensure effective, coordinated, and consistent risk management decision-making.
3.4 Performance measurement and evaluation
Performance measurement evaluates the ongoing effectiveness and relevance of the actions taken to manage risks from toxic substances Footnote 9 . The aim is to determine whether the environmental objective has been met and whether there is a need to revisit the risk management approach for the substance to ensure that risks are managed effectively over time. To achieve this, the Government of Canada will consider reviewing the effectiveness of the risk management actions for MSP.
The Government of Canada plans to measure the effectiveness of the risk management actions by collecting and analyzing data to measure progress towards meeting the environmental and risk management objectives. Consideration may be given to conducting baseline verification. As it may be difficult to detect the presence of the substance in the environment, alternate approaches and indicators to measure performance of risk management and achievement of the environmental objective will also be explored.
The results of performance measurement and evaluation will be used to inform whether further risk management action is warranted and will be made available to Canadians along with recommendations for further action, if applicable.
3.5 Risk Management Information Gaps
Interested stakeholders are invited to provide further information, such as may be outlined below, to inform risk management decision-making regarding MSP:
- Socio-economic and technical impact:
- Anticipated economic and technical impacts should the manufacturing and importation of MSP and products containing the substance be prohibited or restricted in Canada
- Alternatives to MSP and products containing MSP:
- The trade names and safety data sheets (SDS) of anticorrosive coatings that do not contain MSP for use in harsh outdoor conditions, and/or when surface preparation is less than optimal, and/or for use on seafaring ships and industrial equipment and machinery
- Performance requirements or specifications (i.e., durability) of high-performance protective/anticorrosive coatings used for seafaring ships or industrial equipment and machinery
- The trade names, SDS, and performance specifications of products that contain MSP
- The name, CAS RN, and SDS of other substances that can act as alternatives to the function of MSP in paints and coatings
- Time, costs, and other anticipated constraints associated with replacing paints and coatings containing MSP with alternatives
- Analytical methods to detect levels of MSP and/or its representative component (dimers of C9 monomer) in the aquatic environment and in paints and coating products
Should stakeholders have further information to help address these gaps, they should provide it ideally on or before January 5, 2022 to inform the risk management decision-making process, within the timelines (and to the contact) identified in section 8 of this document.
4. Background
4.1 General information on phenol, methylstyrenated (MSP)
MSP is an organic substance of Unknown or Variable composition, Complex reaction products and Biological materials (UVCB) and is composed of multiple components Footnote 10 . Three major components (monomethylstyrenated phenol, dimethylstyrenated phenol, and dimers of C9 monomer) are expected to represent the largest fraction of the composition of MSP in imported products.
Among these three major components of MSP, monomethylstyrenated phenol is expected to persist in the environment and not bioaccumulate in organisms, while dimethylstyrenated phenol and dimers of C9 monomer are expected to persist in the environment and to bioaccumulate in organisms. All of these three major components are highly inherently toxic to aquatic organisms.
4.2 Previous assessment of MSP
MSP was identified during the categorization of the Domestic Substances List (DSL) in 2006 as a high priority for assessment on the basis of concerns associated with its persistence, bioaccumulation, and inherent toxicity.
In 2008, during the first phase of the CMP, the Ministers published the Final decision on the screening assessment of 145 substances on the Domestic Substances List (subsection 77(6) of the Canadian Environmental Protection Act, 1999) (Canada 2008b). The screening assessment examined the potential ecological and health risks associated with 145 substances, including MSP, that were identified as meeting the persistence and bioaccumulation criteria set out in the Persistence and Bioaccumulation Regulations (Canada 2000) and the inherent toxicity criteria applied during categorization.
At the time, a survey issued pursuant to section 71 of CEPA found that no import or manufacture of the substance was reported in Canada above the reporting threshold of 100 kg for the specified reporting year (Canada 2006). Therefore, it was concluded that MSP did not meet the criteria of section 64 of CEPA because it was not entering the environment at levels that could pose a risk to human health or to the environment (Canada 2008b).
However, given the persistent, bioaccumulative, and inherently toxic properties of this substance, there was concern that new activities, which had not been identified or assessed, could lead to the substance meeting the criteria of section 64 of CEPA. Therefore, this substance was subject to Significant New Activity provisions specified under subsection 81(3) of CEPA (Canada 2008a). The SNAc Order for this substance, which has been in place since 2008, requires notification for the purpose of assessment of all manufacture, import, or use of the substance in Canada in quantities at or above 100 kg.
4.3 Uses and identified sectors
Currently, MSP is manufactured outside of Canada and is imported in paints and coatings products.
In response to the SNAc Order applied to the substance in 2008, multiple Significant New Activity Notifications (SNANs) have been received since 2015, marking the beginning of notified domestic activity for MSP in Canada. In total, the SNANs indicate the intention to import the substance as an ingredient in industrial coatings in the range of 10 000 to 100 000 kg per year. These coatings would be applied to large industrial equipment and machinery, and transportation vessels such as ships. Ministerial Conditions applicable to certain notifiers permit the manufacture or import of the substance in accordance with specified conditions (see Section 7). The SNANs have not indicated any intention to manufacture the substance in Canada.
A consumer adhesives product containing MSP for home repair projects was previously available to the public from Canadian retailers; however, available information indicates that this product has been reformulated to no longer contain the substance.
Based on information available on international uses of MSP and uses of structurally similar substances, MSP has the potential to be used in tire manufacturing, the formulation of polymeric surfactants, and the formulation of paints and coatings, although these uses are not known to be occurring in Canada at this time. Such uses could result in increases in domestic demand of this substance, which could lead to its manufacture taking place in Canada. These activities could lead to future exposures if these uses were to occur in Canada.
5. Exposure sources and identified risks
Releases of MSP to the Canadian aquatic environment are expected from notified activities.
Direct release into receiving surface water may occur when paints and coatings are applied for small surface repairs and anticorrosion maintenance to seafaring ships while in transit or docked. The application of paints and coatings to ships in transit is expected to result in a highly diluted concentration of MSP in receiving water. Alternatively, application to ships while they are docked can be expected to result in less diluted concentrations due to the fact that water in docks is relatively more stagnant.
Application of paints and coatings to industrial equipment in specialised facilities may lead to indirect releases to receiving surface water through wastewater effluent.
MSP is toxic to aquatic organisms at very low exposure concentrations. Some components are associated with estrogenic activity and endocrine effects and are highly bioaccumulative in aquatic organisms.
Once released to water, each component of MSP will distribute separately in the environment. Components of MSP are expected to remain in the water column or adsorb in sediment and are not expected to undergo significant biodegradation. If released to soil, all major components of MSP are expected to remain in that compartment.
Once a coating containing MSP has cured, it is expected that the substance will be contained and its release from the cured coating is unlikely, even during disposal or recycling of the substrate to which the coating was applied. MSP is also not expected to be released to air.
6. Risk management considerations
6.1 Alternatives and alternate technologies
MSP has been reported to be in commerce in Canada since 2015 as an anticorrosive coating for industrial equipment, machinery, and seafaring ships subject to harsh outdoor conditions and in situations where surface preparation is less than optimal. Paints and coatings formulated without MSP have been used for these activities prior to 2015, and continue to be widely used in Canada. It is expected that the coatings in commerce in Canada containing MSP represent a small fraction of the total available paints and coatings used for these activities.
A potential substitute for MSP is phenol, styrenated (CAS RN 61788-44-1) a UVCB structurally similar to MSP that possesses a similar variety of industrial applications. Phenol, styrenated is currently undergoing a screening assessment as part of the Substituted Phenols Group in the third phase of CMP.
6.2 Socio-economic and technical considerations
Socio-economic and technical barriers to no longer using MSP and products that contain the substance are expected to be minimal given the limited commercial activity of MSP in Canada at present. There is little information available on whether products containing the substance have a significant advantage over similar products that do not contain this substance.
Anticorrosive coatings, which may or may not contain MSP, are applied to equipment and machinery used by end users in various sectors such as energy, transportation, and industrial/outdoor equipment manufacturing. In North America, the demand for high-performance anticorrosive coatings is expected to continue to grow moderately overall while higher growth is expected in energy markets, chemical plants, and infrastructure (IHS 2015; IHS Markit 2019). Higher demand is expected for applications such as wind turbine blades and housing, wastewater treatment pipes, industrial flooring, and pipelines and rail cargo linings for shipments of certain corrosive oil products such as those from Canada’s oil sands (IHS 2015; IHS Markit 2019). As environmental standards for paints and coatings become more stringent in Canada, the US, and Europe, industry research has been focused on developing compliant paints and coatings (IHS 2015).
7. Overview of existing risk management
7.1 Related Canadian risk management context
A SNAc Order was published in 2008 indicating that the SNAc provisions of CEPA apply to this substance (Canada 2008a). This action can be taken by the Government of Canada when there is reasonable suspicion that new activities with respect to a substance may result in new or increased risks to the environment or human health. The SNAc Order triggers an obligation for a person to notify the Government of Canada and provide specific information when proposing an activity that meets the definition of a significant new activity as defined in the SNAc Order. The Government of Canada will assess the information provided to determine whether the substance, could pose a risk to the environment or to human health and, if so, whether risk management is required.
In response to the SNAc Order, multiple SNANs have been received since 2015. In 2019, Ministerial Conditions, which place restrictions on activities and quantities or concentrations of the substance, were applied to two of the SNAN notifiers and any person to whom they may transfer the substance. The Ministerial Conditions require that the substance only be used as a component in epoxy-based coatings applied in a spray booth or enclosed area designed to capture overspray, or be applied for minor maintenance and repair purposes in quantities not exceeding 10 kg per day, per site (Canada 2019). Persons subject to the Ministerial Conditions must also follow certain disposal, release, and record-keeping practices. Notifiers must only transfer the substance to persons who will comply with the above conditions. For further information on the Ministerial Conditions, refer to Ministerial Condition No. 19668 and Ministerial Condition No. 19768.
7.2 Pertinent international risk management context
7.2.1 United States
At the federal level in the United States (US), MSP is labeled as an active commercial substance in the Toxic Substance Control Act (TSCA) Inventory (US EPA 2019). Manufacturers and importers, should they meet a certain quantity threshold, may be required to report information on this substance to the United States Environmental Protection Agency (US EPA) under the TSCA’s Chemical Data Reporting (CDR) Rule.
At the state level, MSP is designated a “chemical of concern” in Maine and a “chemical of high concern” in Minnesota (Maine Department of Environmental Protection 2018; Minnesota Department of Health 2016). MSP is on the candidate list but not the “chemicals of concern” list maintained by California’s Department of Toxic Substances Control under the Safer Consumer Products Program (California Department of Toxic Substances Control 2019). The candidate list is used to help identify priority products for further screening. The substance’s inclusion on the candidate list is based on its PBiT properties as described in the Government of Canada’s Final decision on the screening assessment of 145 substances on the Domestic Substances List.
7.2.2 European Union
MSP is registered under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulations of the European Union (EU) Footnote 11 . The substance has not been identified as a substance of very high concern (SVHC) and it is not on the restriction list (ECHA 2019).
The substance is classified as hazardous under the Classification, Labeling and Packaging (CLP) regulations which are legally binding classification, labeling, and packaging requirements for hazardous substances and mixtures applicable to manufacturers, importers, or downstream users in EU Member States (ECHA [date unknown]). As such, regulatees must also comply with the information and communication requirements for hazardous substances under REACH.
The substance is included on the 2012-2014 Community Rolling Action Plan (CoRAP) list for evaluation by Denmark on the initial grounds that the substance may potentially be persistent, bioaccumulative and toxic, be an endocrine disruptor, have a high (aggregated) tonnage, and/or have wide dispersive use (ECHA 2018). A final conclusion is pending and may lead to a recommendation to take further risk management measures.
7.2.3 Australia
In Australia, MSP is listed on the Australian Inventory of Chemical Substances (AICS) and can only be used commercially by registered manufacturers and importers, though registered manufacturers and importers are not required to notify the Australian Government of their activities with the substance under the National Industrial Chemicals Notification and Assessment Scheme (NICNAS) (Australian Government 2018).
7.3 Risk management alignment
There is very limited risk management alignment between actions proposed to be undertaken in Canada and those currently undertaken in the United States, the European Union, and Australia. While the substance is suspected of being toxic and identified as a potential chemical of concern in other jurisdictions (United States, the European Union, and Australia), few restrictions or controls on manufacture, importation and use of the substance currently apply to MSP, aside from certain reporting requirements. However, its inclusion on the EU’s CoRAP list indicates that the substance has or will undergo evaluation by a Member State which may lead to EU-wide risk management measures such as restrictions or other actions (ECHA 2018).
The Government of Canada would be the first to aim for a significant restriction or prohibition of activities with MSP and products that contain the substance.
8. Next steps
8.1 Public comment period
Industry and other interested stakeholders are invited to submit comments on the content of this Risk Management Scope or other information that would help to inform decision-making (such as outlined in section 3.5). Please submit additional information and comments prior to January 5, 2022.
The Risk Management Approach document, which will outline and seek input on the proposed risk management instruments, will be published at the same time as the screening assessment. At that time, there will be further opportunity for consultation.
Comments and information submissions on the Risk Management Scope should be submitted to the address provided below:
Program Development and Engagement Division
Environment and Climate Change Canada
Gatineau, Quebec K1A 0H3
Telephone: 1-800-567-1999 (in Canada) or 819-938-3232
Fax: 819-938-5212
Email: substances@ec.gc.ca
Companies who have a business interest in MSP and/or products that contain the substance, including companies that import, manufacture, sell, and/or use paints and coatings products such as anticorrosive coatings designed for use in harsh outdoor conditions or when surface preparation is less than optimal, are encouraged to identify themselves as stakeholders. The stakeholders will be informed of future decisions regarding MSP and may be contacted for further information.
8.2 Timing of actions
Electronic consultation on the draft screening assessment and Risk Management Scope: November 6, 2021 to January 5, 2022. This should include the submission of public comments, additional studies and/or information on MSP.
Publication of responses to public comments on the draft screening assessment and Risk Management Scope: Concurrent to the publication of the screening assessment and, if required, the Risk Management Approach document.
Publication of responses to public comments on the Risk Management Approach, if applicable and if required, the proposed instruments: At the latest, 24-months from the date on which the Ministers recommended that MSP be added to Schedule 1 of CEPA.
Consultation on the proposed instruments, if required: 60-day public comment period starting upon publication of each proposed instrument.
Publication of the final instruments, if required: at the latest, 18-months from the publication of each proposed instrument.
These are planned timelines, and are subject to change. Please consult the schedule of risk management activities and consultations for updated information on timelines.
9. References
Australian Government, Dept. of Health. 2018. Chemical Inventory; search results for CAS RN 68512-30-1 Phenol, Methylstyrenated. [accessed 2019 Apr 5].
California Department of Toxic Substances. 2019. CalSAFER: Candidate Chemical List entry for phenol, methylstyrenated. [accessed 2019 Sept 27].
Canada, Dept. of the Environment. 1995. Toxic Substances Management Policy. [accessed 2019 Jun 17].
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations [PDF, 1975 KB], P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette. Part II, vol. 134, no. 7, p. 607-612.
Canada, Dept. of the Environment, Dept. of Health. 2006. Canadian Environmental Protection Act, 1999: Notice with respect to selected substances identified as priority for action. Canada Gazette, Part I, vol. 140, no. 9, p. 435–459.
Canada. 2008a. 2008-87-01-01 Amending the Domestic Substances List, Canadian Environmental Protection Act, 1999 [PDF 5.85 MB]. June 25 2008, SOR/2008-192, Canada Gazette Part II, vol. 142, vo. 13. [accessed 2019 Apr 5].
Canada. 2008b. Final decision on the screening assessment of 145 substance on the Domestic Substances List (subsection 77(6) of the Canadian Environmental Protection Act, 1999) [PDF]. Canada Gazette Part I, vol. 142, no. 23.
Canada. 2008c. Final Screening Assessment of Potentially Toxic Substances. [accessed 2019 Apr 5].
Canada. 2015. Treasury Board of Canada Secretariat. Red Tape Reduction Act. S.C. 2015, c.12.
Canada, Dept. of the Environment. 2019. Ministerial Condition No. 19668 and Ministerial Condition No. 19768.Canada Gazette, Part I, vol. 153, no. 5. [accessed 2019 Apr 5].
Canada, Department of the Environment, Department of Health. [2021]. Draft Screening Assessment for Phenol, Methylstyrenated.
[ECHA] European Chemicals Agency. 2018. Substance Evaluation – CoRAP; Oligomerisation and alkylation reaction products of 2-phenylpropene and phenol [Previously registered as Phenol, methylstyrenated - EC N. 270-966-8 and CAS N. 68512-30-1]. [accessed 2019 Apr 5].
[ECHA] European Chemicals Agency. 2019. REACH Substance Information Infocard; CAS RN 68512-30-1 Phenol, methylstyrenated. [updated 2019 Feb 6; accessed 2019 Apr 5].
[ECHA] European Chemicals Agency. Date unknown. Understanding CLP. [accessed 2019 Apr 5].
[HC and ECCC] Health Canada & Environment and Climate Change Canada. 2016. Significant New Activity Notification: Phenol, Methylstyrenated. [accessed 2019 Apr 5].
[IHS] Information Handling Services. 2015. Specialty Chemicals Update Program: High-Performance Anticorrosion Coatings [PDF]. IHS Chemical. [accessed 7 Mar 2019; restricted access].
[IHS Markit] Information Handling Services. 2019. Specialty Chemicals Update Program: Coatings, High-Performance Anticorrosion [PDF]. IHS Chemical. [accessed 21 May 2019; restricted access].
Maine Department of Environmental Protection. c2018. Chemicals of Concern. [accessed 2019 Apr 5].
Minnesota Department of Health. 2016. Chemicals of High Concern List, 2016 [PDF 1.25 MB]. [accessed 2019 Apr 5].
[TBS] Treasury Board of Canada Secretariat. 2012. Red Tape Reduction Action Plan. Ottawa (ON): Government of Canada. [accessed 2018 Aug 29].
[TBS] Treasury Board of Canada Secretariat. 2018. Cabinet Directive on Regulation. Ottawa (ON): Government of Canada. [accessed 2018 Aug 29].
[US EPA] U. S. Environmental Protection Agency. 2019. ChemView entry search results; CAS RN 68512-30-1 Phenol, methylstyrenated. [updated 2019 Apr 5; accessed 2019 Apr 5].
Annex A. Synonyms
| CAS RN | DSL name (English) | Other names and acronyms | Other identifiers |
|---|---|---|---|
| 68512-30-1 | Phenol, methylstyrenated | MSP; Methylstyrenated Phenol; Isopropenylbenzene; Phenol, methylstyrolisiert; OAPP; Oligomerisation and alkylation reaction products of 2-phenylpropene and phenol | EINECS/EC (European Community) number: 270-966-8; EPA SRS (Substance Registry Services) tracking number: 444125 |
Annex B. Major components of phenol, methylstyrenated (MSP)
| CAS RN | DSL name (English) | Other names | Chemical structure |
|---|---|---|---|
| 599-64-4 | Phenol, 4-(1-methyl-1-phenylethyl)- | Monomethylstyrenated phenol | 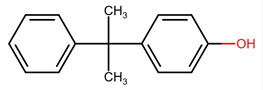 |
| 2772-45-4 | Phenol, 2,4-bis(1-methyl-1-phenylethyl)- | Dimethylstyrenated phenol | 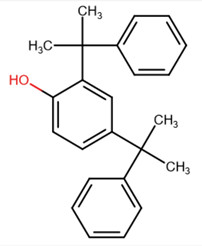 |
| CAS RN | Chemical name | Representative chemical structure |
|---|---|---|
| 3910-35-8 | 2,3-Dihydro-1,1,3-trimethyl-3-phenyl-1H-indene | 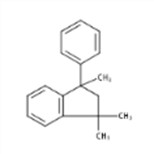 |
| 6258-73-7 | Benzene, 1,1'-(1,3,3-trimethyl-1-propene-1,3-diyl)bis- | 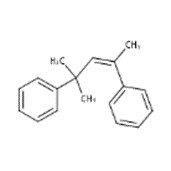 |
| 6362-80-7 | Benzene, 1,1'-(1,1-dimethyl-3-methylene-1,3-propanediyl)bis- | 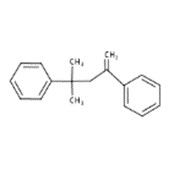 |
