Screening assessment - Epoxides and Glycidyl Ethers Group
Official title: Screening assessment - Epoxides and Glycidyl Ethers Group
Chemical Abstracts Service Registry Numbers
106-92-3, 1139-30-6, 2210-79-9, 2451-62-9, 120547-52-6
Environment and Climate Change Canada
Health Canada
August 2020
Cat. No.: En14-415/2020E-PDF
ISBN 978-0-660-35321-0
Synopsis
Pursuant to sections 68 and 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment on five of twelve substances referred to collectively under the Chemicals Management Plan as the Epoxides and Glycidyl Ethers Group. These five substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. The other seven substances were determined to be of low concern through other approaches, and decisions for these substances are provided in separate reportsFootnote 1 ,Footnote 2 . Accordingly, this screening assessment addresses the five substances listed in the table below. The five substances addressed in this screening assessment will hereinafter be referred to as the Epoxides and Glycidyl Ethers Group. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 3 ), their Domestic Substances List (DSL) names and their common names and acronyms are listed in the table below.
| CAS RN | Domestic Substances List name | Common name (acronym) |
|---|---|---|
| 106-92-3a | Oxirane, [(2-propenyloxy)methyl]- | Allyl glycidyl ether (AGE) |
| 1139-30-6 | 5-Oxatricyclo[8.2.0.04,6]dodecane, 4,12,12-trimethyl-9-methylene-, [1R-(1R,4R,6R,10S)]- | Beta-caryophyllenoxide(BCPO) |
| 2210-79-9a | Oxirane, [(2-methylphenoxy)methyl]- | o-Cresol glycidyl ether (o-CGE) |
| 2451-62-9a | 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-tris(oxiranylmethyl)- | Triglycidyl isocyanurate (TGIC) |
| 120547-52-6b | Oxirane, mono[(C12-13-alkyloxy)methyl] derivs. | Alkyl (C12-C13) glycidyl ether (C12-C13 AGE) |
a This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
b This substance is a UVCB (which stands for substances of unknown or variable composition, complex reaction products or biological materials).
With the exception of BCPO, which is naturally present in some plant species and essential oils, the substances in the Epoxides and Glycidyl Ethers Group are not known to occur naturally. All of the substances in the Epoxides and Glycidyl Ethers Group were included in surveys issued pursuant to section 71 of CEPA. AGE, BCPO, o-CGE, and TGIC were not reported to be manufactured in Canada above the reporting threshold of 100 kg in 2011. Imported quantities of AGE, BCPO, o-CGE, and TGIC were 100 to 10 000 kg, <100 kg, 79 000 kg, and 407 000 kg, respectively, in the 2008 or 2011 reporting year. C12-C13 AGE was not reported to be manufactured or imported above the reporting threshold of 100 kg in 2011.
The ecological risks of the substances in the Epoxides and Glycidyl Ethers Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, substances in the Epoxides and Glycidyl Ethers Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from AGE, BCPO, o-CGE, TGIC and C12-C13 AGE. It is concluded that AGE, BCPO, o-CGE, TGIC and C12-C13 AGE do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
AGE is used as a reactive diluent in epoxy resin systems; however, its applications are primarily as an industrial intermediate and no products available to consumers were identified. Exposure of the general population to AGE from environmental media is expected to be minimal due to the low quantities reported in commerce and the rapid degradation of the substance in the environment. AGE is associated with health effects of concerns as it has been classified as suspected of causing genetic defects, suspected of causing cancer, and suspected of damaging fertility. However, since exposure to the general population is expected to be minimal, the risk to human health from exposure to AGE is low.
BCPO is reported to be used in cosmetic products as a fragrance ingredient. It is not an approved food additive in Canada; however, the substance may be present in foods as a flavouring agent as it is reported to be used as such in the United States and Europe. Exposure of the general population to BCPO from environmental media is expected to be minimal due to the low quantities reported to be in commerce. Adverse effects on the liver and the mesenteric lymphatic system observed in laboratory studies were identified as the critical effects for risk characterization. Comparison of estimates of exposure from the use of cosmetic products containing BCPO with the critical effect level resulted in margins of exposure that were considered adequate to address uncertainties in the exposure and health effects databases. Estimated intakes derived by both the Joint FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization) Expert Committee on Food Additives and the European Food Safety Authority for the use of BCPO as a food flavouring agent are several orders of magnitude lower than the critical effect level for this substance and the risk to human health from exposure to BCPO from its use as a food flavour is considered low.
o-CGE is used predominantly as a reactive diluent in the formulation of epoxy resins and was identified in a limited number of do-it-yourself products including a flooring adhesive, a floor coating for garages, a two-component epoxy resin, and an arts and crafts/hobby resin. General population exposure to o-CGE from environmental media is expected to be negligible. Carcinogenicity observed in laboratory studies conducted with structurally-related substances as well as non-cancer effects observed in short-term studies with o-CGE (e.g., nasal mucosa inflammation) were identified as the critical effects for risk characterization. Comparison of estimates of exposure from the use of certain do-it-yourself products containing o-CGE with the critical effects levels resulted in margins of exposure that were considered adequate to address uncertainties in the exposure and health effects databases.
The predominant use of TGIC is as a crosslinking agent in the formulation of polyester resins used in the manufacture of polyester powder coatings. Exposure of the general population to TGIC from environmental media is expected to be minimal as the substance is expected to be rapidly hydrolyzed if released. Exposure from contact with painted manufactured items is not expected as the substance would be fully cross-linked and cured. TGIC is associated with health effects of concern as it has been classified as potentially causing genetic defects. However, given the current levels of exposure to the general population, the risk to human health from exposure to TGIC is expected to be low.
C12-C13 AGE was identified in a limited number of do-it-yourself products including a two-component epoxy adhesive, an epoxy filler sold in tube packaging, and in a multi-purpose low-viscosity epoxy resin, used to seal and coat various surfaces. Exposure to C12-C13 AGE from environmental media is not expected. Critical effects associated with short-term dermal exposure are limited to reversible site-of-contact effects and the risk to human health from dermal exposure to C12-C13 from use of these products is considered low. Comparison of estimates of inhalation exposure to C12-C13 AGE with levels associated with adverse effects in laboratory animals resulted in margins of exposure that were considered to be adequate to address uncertainties in the exposure and health effects databases.
On the basis of the information presented in this screening assessment, it is concluded that AGE, BCPO, o-CGE, TGIC, and C12-C13 AGE do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that AGE, BCPO, o-CGE, TGIC, C12-C13 AGE do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 and 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment on five of twelve substances referred to collectively under the Chemicals Management Plan as the Epoxides and Glycidyl Ethers Group to determine whether these five substances present or may present a risk to the environment or to human health. These five substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
The other seven substances (listed in Table 1-1) were considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a), and in either the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Science Approach Document (Health Canada 2016), or via the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018a), and were identified as being of low concern to both human health and the environment. As such they are not further addressed in this report. Conclusions for these seven substances are provided in the Substances Identified as Being of Low Concern based on the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Screening Assessment (ECCC, HC 2018b) and the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018a).
| CAS RNa | Domestic Substances List (DSL) name | Approach under which the substance was addressed | References |
|---|---|---|---|
| 101-90-6 | Oxirane, 2,2’-[1,3-phenylenebis(oxymethylene)]bis- | ERC/Rapid Screening | ECCC, HC 2018b |
| 556-52-5 | Oxiranemethanol | ERC/Rapid Screening | ECCC, HC 2018b |
| 28768-32-3 | Oxiranemethanamine, N,N’-(methylenedi-4,1-phenylene)bis[N-(oxiranylmethyl)- | ERC/Rapid Screening | ECCC, HC 2018b |
| 61788-72-5 | Fatty acids, tall-oil, epoxidized, octyl esters | ERC/TTC | ECCC, HC 2018b |
| 61789-01-3 | Fatty acids, tall-oil, epoxidized, 2-ethylhexyl esters | ERC/TTC | ECCC, HC 2018a |
| 66072-38-6 | Oxirane, 2,2’,2’’-[methylidynetris(phenyleneoxymethylene)]tris- | ERC/Rapid Screening | ECCC, HC 2018a |
| 68082-35-9 | Fatty acids, soya, epoxidized, Me esters | ERC/TTC | ECCC, HC 2018b |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
The other five substances addressed in this screening assessment will hereinafter be referred to as the Epoxides and Glycidyl Ethers Group.
The ecological risks of the substances in the Epoxides and Glycidyl Ethers Group were characterized using the ERC approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to June 2017. Empirical data from key studies as well as some results from models were used to reach the conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Bernard Gadagbui, Department of Environmental Health, College of Medicine, University of Cincinnati and Michael Jayjock, Jayjock & Associates. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this screening assessment (published November 24, 2018) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA, by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 4 . The screening assessment presents the critical information and considerations upon which the conclusions are made.
2. Identity of substances
The CAS RN, DSL names and common names and acronyms for the individual substances in the Epoxides and Glycidyl Ethers Group are presented in Table 2‑1 and Table 2-2.
| CAS RN (acronym) | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 106-92-3 (AGE) | Oxirane, [(2-propenyloxy)methyl]-(Allyl glycidyl ether) | 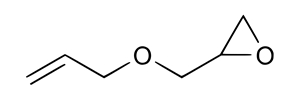 C6H10O2 C6H10O2 | 114.14 |
| 1139-30-6 (BCPO) | 5-Oxatricyclo[8.2.0.04,6]dodecane, 4,12,12-trimethyl-9-methylene-, [1R-(1R,4R,6R,10S)]-(Beta-caryophyllenoxide) | 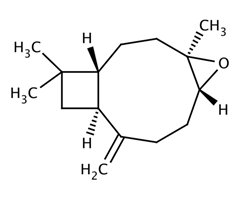 C15H24O C15H24O | 220.25 |
| 2210-79-9 (o-CGE) | Oxirane, [(2-methylphenoxy)methyl]-(o-Cresol glycidyl ether) | 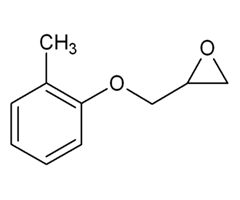 C10H12O2 C10H12O2 | 164.20 |
| 2451-62-9 (TGIC) | 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-tris(oxiranylmethyl)-(Triglycidyl isocyanurate) | 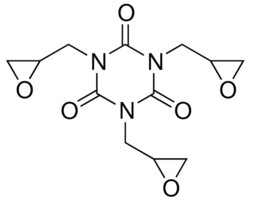 C12H15N3O6 C12H15N3O6 | 297.27 |
| CAS RN (acronym) | DSL name | Representative chemical name (formula) | Representative chemical structurea |
|---|---|---|---|
| 120547-52-6 (C12-C13 AGE) | Oxirane, mono[(C12-13-alkyloxy)methyl] derivs. | (Alkyl (C12-C13) glycidyl ether) | 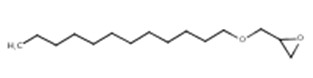 C15H30O2 C15H30O2 |
a This is a substance of unknown or variable composition, complex reaction products and biological materials (UVCB). The representative structure, as described in the US EPA (2010) is depicted.
C12-C13 AGE represents a substance of unknown or variable composition, complex reaction products and biological materials (UVCB). Gas chromatography with flame ionization detection shows the composition to be approximately 49% n-dodecyl glycidyl ether and 39% n-tridecyl glycidyl ether (Steidemann et al. 1996, as cited in Society of the Plastics Industry Inc 1997a). Therefore, the main component (i.e., C12 AGE) was considered to be the representative structure for this substance. This is consistent with the representative structure established by the US EPA (2010).
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues informs the human health assessment for o-CGE. Analogues were selected that were structurally similar and/or functionally similar to these substances (e.g., based on physical-chemical properties, toxicokinetics, reactivity), and that had relevant empirical data for use in hazard characterization. Appendix A provides further details on the factors considered in the identification of analogues. A list of analogues used to inform this assessment is presented in Table 2‑3. For further information on the physical-chemical properties of the analogues of o-CGE, refer to Appendix B. Details of the read-across data to inform the human health assessments are further discussed in the relevant sections of this report.
| CAS RN | Common name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 26447-14-3 | Cresyl glycidyl ether (CGE) | 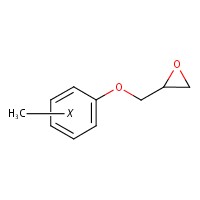 C10H12O2 C10H12O2 | 165.21 |
| 122-60-1 | Phenyl glycidyl ether (PGE) | 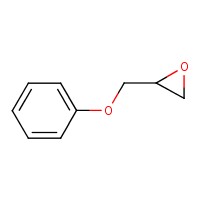 C9H10O2 C9H10O2 | 150.18 |
| 96-09-3 | Styrene oxide (SO) | 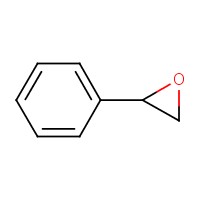 C8H8O C8H8O | 120.15 |
3. Physical and chemical properties
A summary of physical and chemical properties of the substances in the Epoxides and Glycidyl Ethers Group are presented in Table 3‑1 with the range in values indicated for each property. When experimental information was limited or not available for a property, data from analogues were used for read-across and/or (Q)SAR models were used to generate predicted values for the substance. Additional physical chemical properties are presented in ECCC (2016b).
| Property | AGE | BCPO | o-CGE | TGIC | C12-C13 AGE |
|---|---|---|---|---|---|
| Physical state | Colourless liquid | Colourless crystals | Colourless liquid | White solid | Liquid |
| Melting point (°C) | -100 (ECHA 2017a) | 61-63 (SDS 2014a) | -69 (ECHA 2017b) | 92-95 (ECHA 2017c) | NA |
| Boiling point (°C) | 154 (ECHA 2017a) | 263 (EpiSuite c2000-2012) | 260 (ECHA 2017b) | 240 (ECHA 2017c) | NA |
| Vapour pressure (Pa) | 480 (ECHA 2017a) | 1.3 (EpiSuite c2000-2012) | 0.82 (ECHA 2017b) | <0.007 (ECHA 2017c) | 0.28 (EpiSuite c2000-2012) |
| Henry’s law constant log(Pa·m3/mol) | -5.6 (EpiSuite c2000-2012) | -3.1 (EpiSuite c2000-2012) | -6.1 (EpiSuite c2000-2012) | < -7 (EpiSuite c2000-2012) | -1.6 (EpiSuite c2000-2012) |
| Water solubility (mg/L) | 140 000 (ECHA 2017a) | 2.2-4.6 (EpiSuite c2000-2012) | 840 (ECHA 2017b) | 9000 – 10 000 (ECHA 2017c) | 0.027 (EpiSuite c2000-2012) |
| Log Kow | 0.45 (ECHA 2017a) | 4.9 (EpiSuite c2000-2012) | 2.5 (ECHA 2017b) | -0.8 (ECHA 2017c) | 7.25 (EpiSuite c2000-2012) |
Abbreviations: NA, not available
4. Sources and uses
4.1 Sources
With the exception of BCPO, the substances in the Epoxides and Glycidyl Ethers Group are not known to occur naturally. BCPO occurs naturally as the oxidation product of the parent compound, beta-caryophyllene, which can be present in plants such as basil, cinnamon, black pepper, cannabis, lavender, oregano, rosemary, salvia, and Syzgium cordatum. BCPO is the primary component in essential oils such as geranium rose-scented oil, melissa oil and guava leaf oil (Burdock 2009, Fidty et al. 2016).
AGE is manufactured through the condensation of allyl alcohol and epichlorohydrin with subsequent dehydrochlorination to form the epoxy ring (Pottenger et al. 2012). In the European Union, manufacture of AGE occurs in closed batch processes (ECHA 2017a). o-CGE can be made through reactions with allyl chloride via epoxidation or with epichlorohydrin with subsequent dehydrochlorination (Pottenger et al. 2012). TGIC is industrially produced by reacting cyanuric acid with excess epichlorohydrin (WHO 1998).
All of the substances in the Epoxides and Glycidyl Ethers Group were included in surveys issued pursuant to section 71 of CEPA (Environment Canada 2009, Environment Canada 2012). Table 4‑1 presents a summary of the reported total manufacture and total import quantities for the Epoxides and Glycidyl Ethers Group for the years 2008 or 2011. AGE, BCPO, and TGIC were not reported to be manufactured in Canada above the reporting threshold of 100 kg (Environment Canada 2009, Environment Canada 2013). C12-C13 AGE was not reported to be manufactured or imported into Canada at greater than the reporting threshold of 100 kg in the 2011 reporting (Environment Canada 2013).
| Common name(Acronym) | Total manufacturea (kg) | Total importsa (kg) | Reporting year | Survey reference |
|---|---|---|---|---|
| Allyl glycidyl ether (AGE) | - | 100 – 10 000 | 2008 | Environment Canada 2009 |
| Beta-caryophyllenoxide(BCPO) | - | < 100 | 2011 | Environment Canada 2013 |
| o-Cresol glycidyl ether (o-CGE) | 100 – 1 000 | 79 000 | 2008 | Environment Canada 2009 |
| Triglycidyl isocyanurate (TGIC) | - | 407 000 | 2008 | Environment Canada 2009 |
| Alkyl (C12-C13) glycidyl ether (C12-C13 AGE) | - | - | 2011 | Environment Canada 2013 |
a Values reflect quantities reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2009, Environment Canada 2013). See surveys for specific inclusions and exclusions (schedules 2 and 3)
“-“ Not reported above the reporting threshold of 100 kg
In the United States (US), manufacture or import volumes ranged from approximately 1 to 10 million lbs (450 000 to 4 500 000 kg) for AGE, o-CGE, TGIC and C-12-C13 AGE (ChemView 2016a,b,c,d). In the European Union, manufacture or import volumes of AGE, o-CGE, and TGIC ranged from 100 000 to over 1 000 000 kg (ECHA 2017a,b,c).
4.2 Uses
AGE is primarily used in commercial applications as a resin intermediate, and may also be used as a stabilizer of other chemicals, resins, and rubbers (Pottenger et al. 2012). In the United States, AGE is reported to be used as an industrial chemical intermediate in adhesive and sealant manufacturing, synthetic rubber manufacturing, paint and coatings manufacturing, however these uses were reported as industrial or commercial (ChemView 2016a). In the European Union, the substance is used at industrial sites and in manufacturing (ECHA 2017a).
The European Commission has listed this substance under Annex II, List of Substances Prohibited in Cosmetic products, indicating that it must not form part of the composition of a cosmetic product in the European Union (CosIng 2017). In Canada, AGE has not been notified as an ingredient used in cosmetics, and the substance is not listed on Health Canada’s Cosmetic Ingredient Hotlist, which describes substances that are restricted or prohibited from cosmetics in Canada (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated May 2016; unreferenced; Health Canada modified 2015). AGE was not identified to be used in products available to consumers in Canada. AGE is not listed in the Internal Drug Product Database, Natural Health Products Ingredients Database, or the Licensed Natural Health Products Database (DPD modified 2015, LNHPD modified 2014, NHPID modified 2015). AGE is not a permitted food additive in Canada. No definitive information is available concerning the potential use of AGE as a component in food packaging materials and incidental additives in Canada; however, since the substance is known to be used a component in food packaging materials in the United States, it is possible that it may be is used in food packaging materials for foods sold in Canada (personal communication, emails from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated August 2019; unreferenced). AGE is not used in pest control products in Canada (personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 2016; unreferenced).
In Canada, BCPO is not listed in the Internal Drug Product Database, Natural Health Products Ingredients Database, or the Licensed Natural Health Products Database (DPD modified 2015, LNHPD modified 2014, NHPID modified 2015). The substance is used as a formulant in pest control products registered in Canada (personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 2016; unreferenced). Information submitted in response to a CEPA section 71 survey (Environment Canada 2012) reported BCPO to be used as a fragrance ingredient in cosmetic products such as body lotions, shower gels, hand soaps and fragrance products at a final concentration of less than 0.2 ppm (ECCC 2016b).
BCPO is not a permitted food additive in Canada, nor has it been reported to be used as a component in food packaging materials or incidental additives (personal communication, emails from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated March 2016; unreferenced). In the United States, BCPO is permitted under 21 CFR 172.515, as synthetic flavour that is used at minimum quantity required to produce intended effect and in accordance with good manufacturing practices (GMP) (US eCFR 2017). This substance is also permitted in the European Union as a flavouring substance under EU No. 872/2012 (EFSA 2014). Therefore, it is possible that the substance may be present in foods as a flavouring agent in Canada.
o-CGE is not a permitted food additive in Canada, nor are there any reported uses of the substance as a component in food packaging materials or incidental additives, or in pest control products in Canada (personal communication, emails from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated March 2016; unreferenced; personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 2016; unreferenced). In Canada, o-CGE is not listed in the Internal Drug Product Database, the Natural Health Products Ingredients Database, or the Licensed Natural Health Products Database (DPD modified 2015, LNHPD modified 2014, NHPID modified 2015). o-CGE has not been notified as an ingredient used in cosmetics in Canada; however, as a cresol derivative, this substance is “prohibited for use in cosmetic products” on the Cosmetic Ingredient Hotlist for mixed cresols and derivatives (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated May 2016; unreferenced; Health Canada modified 2015).
o-CGE is primarily used as a reactive diluent for liquid epoxy resins (Pottenger et al. 2012). Reactive diluents allow epoxy resins to be less viscous and easier to handle in an uncured state (Dow 2012). Owing to the presence of the epoxide functional group, o-CGE participates in polymerization and crosslinking reactions, and is thus covalently bound to the matrix upon curing (Bosch et al. 1985; Lee 1983; Hamerton 1996, as cited in Environment Canada, Health Canada 2010).
According to data submitted in response to a CEPA section 71 survey, o-CGE may be used in Canada as a chemical intermediate, adhesive and sealant substance, or viscosity adjustor in applications such as the manufacture of adhesives and sealants, paints and coatings, or building or construction materials (Environment Canada 2009). Through publicly available information, o-CGE has been identified in a limited number of do-it-yourself (DIY) products available to consumers in Canada. The substance was identified in a flooring adhesive, a floor covering epoxy compound, and an epoxy finish resin for sealing surfaces (SDS 2015a,b; SDS 2017). o-CGE was also identified in an epoxy resin used for making resin products out of molds for arts and crafts applications (SDS 2014b).
TGIC is not a permitted food additive in Canada. No definitive information is available concerning the potential use of TGIC as a component in food packaging materials and incidental additives in Canada; however, since the substance is known to be used a component in food packaging materials in the United States, it is possible that it may be used in food packaging materials for foods sold in Canada (personal communication, emails from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated August 2019; unreferenced). TGIC is not used as a component in in pest control products in Canada (personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 2016; unreferenced). In Canada, TGIC is not listed in the Internal Drug Product Database, Natural Health Products Ingredients Database, or the Licensed Natural Health Products Database (DPD modified 2015, LNHPD modified 2014, NHPID modified 2015). The substance is not notified to be an ingredient used in cosmetics in Canada (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated May 2016; unreferenced).
According to data reported in response to a CEPA section 71 survey, TGIC may be used as an adhesive and sealant substance, chemical filler, or additive in the manufacture of paints and coatings, adhesive and sealants, or formed metal articles including automotive parts (Environment Canada 2009). The main use of TGIC globally is as a three-dimensional cross-linking agent or curing agent in the manufacture of powder coating paints (WHO 1998). TGIC polyester powder coatings manufactured for industrial uses appear to be available in consumer size quantities. Application of powder coatings require specialized equipment and consumer application of such product is expected to be limited. C12-C13 AGE is not a permitted food additive in Canada, nor has it been reported to be used as a component in food packaging materials or incidental additives, or in pest control products in Canada (personal communication, emails from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated March 2016; unreferenced; personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 2016; unreferenced). In Canada, C12-C13 AGE is not listed in the Internal Drug Product Database, Natural Health Products Ingredients Database, or the Licensed Natural Health Products Database (DPD modified 2015, LNHPD modified 2014, NHPID modified 2015). The substance is not notified to be an ingredient used in cosmetics in Canada (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated May 2016; unreferenced).
Uses of C12-C13 AGE are primarily industrial as a chemical intermediate for applications such as paints and coatings or adhesives and sealants (ChemView 2016d). The substance was identified in a limited number of epoxy adhesive compounds available to consumers in Canada (SDS 2013, SDS 2014c, SDS 2016).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the substances in the Epoxides and Glycidyl Ethers Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, on the basis of weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014) and from information reported in response to CEPA section 71 surveys, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances which had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is known to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the Epoxides and Glycidyl Ethers Group, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
The hazard and exposure classifications for the Epoxides and Glycidyl Ethers Group are summarized in Table 5‑1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| AGE | low | low | low |
| BCPO | moderate | low | low |
| o-CGE | high | low | low |
| TGIC | moderate | high | moderate |
| C12-C13 AGE | high | low | low |
According to information considered under ERC, AGE and BCPO were classified as having low exposure potential. AGE and BCPO were classified as having low and moderate hazard potential, respectively. BCPO was profiled to have a moderate potential to cause adverse effects in aquatic food webs given its moderate bioaccumulation potential. As such, AGE and BCPO were classified as having low potentials for ecological risk. On the basis of current use patterns, it is unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under ERC, o-CGE was classified as having a low exposure, although with greater potential for local-scale exposures. o-CGE was classified as having a high hazard potential on the basis of a reactive mode of action and elevated toxicity ratio, both of which suggest that this chemical is likely of high potency, and on the basis of structural alerts from the OECD (Q)SAR Toolbox (OECD QSAR Toolbox 2014) which identified this substance as being a potential DNA and/or protein binder. o-CGE was classified as having a moderate potential for ecological risk; however, the risk classification was decreased to low potential for ecological risk following the adjustment of risk classification based on current use quantities (see section 7.1.1. of the ERC approach document ECCC 2016a). The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, it is unlikely that this substance is resulting in concerns for the environment in Canada.
According to information considered under ERC, TGIC was classified as having a high exposure potential on the basis of a long overall persistence and large reported use volume according to information reported in response to a CEPA section 71 survey (Environment Canada 2013). TGIC was classified as having a moderate hazard potential on the basis of a reactive mode of action, with structural alerts from OECD (Q)SAR Toolbox which identified this substance as being a potential DNA and protein binder (OECD QSAR Toolbox 2014). TGIC was classified as having a moderate potential for ecological risk. Given its overall classification as having a moderate potential for ecological risk, it is unlikely that this substance is resulting in concerns for the environment in Canada. As this substance is currently being used in high quantities in Canada, fluctuations in use patterns are unlikely to result in a significant increase in risk to the environment. The potential effects and how they may manifest in the environment were not further investigated.
According to information considered under ERC, C12-C13 AGE was classified as having a low exposure potential. C12-C13 AGE was classified as having a high hazard potential on the basis of a reactive mode of action and elevated toxicity ratio, both of which suggest that this chemical is likely of high potency. C12-C13 AGE was profiled to have a moderate potential to cause adverse effects in aquatic food webs given its moderate bioaccumulation potential. C12-C13 AGE was classified as having a moderate potential for ecological risk; however, the risk classification was decreased to low following the adjustment of risk classification based on current use quantities (see section 7.1.1. of the ERC approach document ECCC 2016a). The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, this substance is unlikely to be resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Allyl glycidyl ether (AGE)
6.1.1 Exposure assessment
No measured concentrations of AGE in air, water, or soil were identified. Based upon the uses of AGE as an intermediate in the manufacture of other chemicals and products, environmental releases to water or air may occur as the substance has a high vapour pressure and high water solubility. AGE has a short hydrolysis half-life ranging from approximately 7-13 days at pH ranges of 4-9 (ECHA 2017a) and a short estimated photodegredation half-life in air of less than 5 hours (AOPWIN 2010). In consideration of the limited quantities (100 to 10 000 kg) reported for AGE in Canada according to a CEPA section 71 survey (Environment Canada 2009) and the physical-chemical properties of the substance (rapid hydrolysis and photodegredation), exposure to AGE from environmental media are expected to be minimal.
AGE may be used in the formulation of resins as a chemical intermediate; no studies were identified on potential exposure to residual AGE from cured epoxy resins. The International Agency for Research on Cancer (IARC) noted that in resin manufacturing the epoxy group of glycidyl ethers react during the curing process and are therefore generally no longer present in completely cured products (IARC 1989). The substance may also have applications in manufacturing adhesives and sealant, synthetic rubber, or paint and coatings. However, no products available to consumers were identified.
6.1.2 Health effects assessment
A comprehensive summary of the health effects associated with AGE exposure has been generated by the OECD in a SIDS Initial Assessment Profile (SIAP) document (OECD 2007). AGE has been classified by the EU as suspected of causing genetic defects (Muta 2), suspected of causing cancer (Carc 2), and suspected of damaging fertility (Repr 2) (ECHA 2017a).
In a 2-year inhalation study, Osborne-Mendel rats (n=50/sex/dose) and B6C3F1 mice (n=50/sex/dose) were exposed to 0, 5, or 10 ppm AGE (equivalent to approximately 0, 23, or 47 mg/m3), 5 days/week for 102-103 weeks (NTP 1990). Nasal tumours accompanied with non-cancer effects such as inflammation, squamous metaplasia, respiratory metaplasia, and degeneration of the olfactory epithelium were identified. Although not statistically significant, these tumours were considered to be biologically significant since primary nasal tumours are rare in rodents. The authors concluded that there was equivocal evidence of carcinogenicity in male rats, no evidence in female rats, some evidence in male mice, and equivocal evidence in female mice.
AGE has been found to be genotoxic in the majority of the in vitro assays conducted (AMES, sister chromatid exchange, chromosome aberration tests) and was reported to cause the formation of micronuclei, reciprocal translocations and sex-linked recessive lethality in vivo (Wade et al. 1979; Hemminki et al. 1980; Allied Corporation 1982; Department of Health & Human Services 1984; NIH 1984; Shell Oil Company 1984; Yoon et al. 1985; Canter et al. 1986; NTP 1990; von der Hude et al. 1990; von der hude et al. 1991). Also, AGE was observed to form DNA-adducts in vivo following dermal and intraperitoneal administration (Plna and Segerback 1997; Perez and Osterman-Golkar 2000).
Several acute and repeated-dose inhalation assays have demonstrated respiratory irritation and effects on the nasal passage in experimental animals at levels as low as 4 ppm (equivalent to approximately 18.7 mg/m3) (Shell Chemical Company 1956; DOW Chemical Company 1978; Gagnaire et al. 1987; NTP 1990; Zissu 1995).
6.1.3 Characterization of risk to human health
On the basis of the available information on sources and uses of AGE, exposure to the general population is expected to be minimal. As such, at current levels of exposure, the risk to human health is considered low.
While exposure of the general population to AGE is not of concern at current levels, this substance has been associated with health effects of concern on the basis of its classification by the EU (Muta 2, Carc 2, and Repr 2) (ECHA 2017a). Therefore, there may be a concern for human health if exposures were to increase.
6.2 Beta-caryophyllene oxide (BCPO)
6.2.1 Exposure assessment
Environmental media and food
No measured concentrations of BCPO in air, water, or soil were identified. In consideration of the low quantities (<100 kg) of the substance reported to be used in Canada (ECCC 2016c), and the limited number of plant species for which BCPO may be a major component, chronic exposure to BCPO from environmental media is expected to be minimal.
In Canada, potential exposure to BCPO may arise from its use as a food flavouring agent. The substance is permitted for use as a food flavouring agent in the United States and in Europe.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated a flavouring group of epoxides at its 65th meeting (WHO 2006). As part of the evaluation, the Committee estimated the per capita intake of BCPO from its use as a food flavouring agent to be 0.002 µg/kg bw/day for the U.S. population. This intake estimate which used a maximized survey-derived daily intake approach, was derived by assuming that the reported annual production amount of BCPO in the United States was consumed by 10% of the U.S. population (“eaters only”), and that only 80% of the annual production volume amount of 0.9 kg was reported in the poundage surveys (National Academy of Science 1987; International Organization of the Flavour Industry 1995, Lucas et al. 1999; as cited in WHO 2006). In the assessment, the JECFA also noted that BCPO would be consumed predominantly from foods that contained it as a natural ingredient, citing an annual quantity in naturally occurring foods during 1987 to be 488 kg (WHO 2006, Stofberg and Grundschober 1987). The JECFA concluded there is “no safety concern at estimated levels of intake” for BCPO, when used as a food flavouring agent.
The European Food Safety Authority (EFSA) panel considered five epoxides evaluated by the JECFA (at its 65th meeting) for use as food flavouring substance in Europe (EFSA 2014). In the 2014 report, the EFSA provided information on the use levels of BCPO as a flavour in food (specific food items were not provided). The EFSA panel derived a modified theoretical added maximum daily intake based on the absence of accurate use information for the substances in the evaluation group (EFSA 2014). This approach used to estimate intakes assumes that all foods in an entire food category contain the average use level of the substance and that the entire food category is consumed daily (personal communication, emails from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated March 2016; unreferenced). This is expected to generate an exaggerated estimate of intake (personal communication, emails from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated March 2016; unreferenced). In this evaluation the EFSA panel agreed with the JECFA conclusion of “No safety concern at estimated levels of intake as a flavouring substance based on the maximized survey-derived daily intake approach” (EFSA 2014).
Cosmetics
In Canada, BCPO is used as a fragrance ingredient in cosmetic products such as body lotions, shower gels, hand soaps, and fragrance products at a final concentration of less than 0.2 ppm (ECCC 2016c). Use of fragrance products and body lotions that may contain BCPO were considered to be the scenarios associated with the highest inhalation and dermal exposure potential and estimates of exposure were derived using algorithms from ConsExpo Web (2016).
Inhalation exposure from the use of a fragrance spray containing BCPO was estimated to be in the nanogram range and would result in negligible exposure. Body lotion containing BCPO was estimated to result in dermal exposure ranging from 8.2 x 10-6 mg/kg-bw/day to 3.8 x10-5 mg/kg-bw/day, with infants representing the age group associated with the highest exposure (See Appendix C for details).
6.2.2 Health effects assessment
There are currently no hazard classifications designated by the International Agency for Research on Cancer (IARC), the United States Environmental Protection Agency (U.S. EPA) or the European Chemicals Agency (ECHA) for BCPO.
The short-term effects of BCPO have been investigated in a 14-day, range-finding dietary study in which Hsd:SD rats (n=3/sex/dose) were administered 0, 3000, 9000 or 18000 ppm BCPO in the diet (corresponding to 0, 279.1, 788.8, 1558.1 mg/kg-bw/day in males and 0, 267.6, 815.7, 1586.0 mg/kg-bw/day in females) (Product Safety Labs 2012). Animals were observed for clinical signs, body weight changes, and behavioural changes. Gross necropsies were also performed on all animals at the end of the study. There were no treatment-related, adverse effects on clinical signs, body weight, body weight gain, food consumption, food efficiency, or gross findings. The NOAEL was determined to be 18000 ppm (the highest dose tested, equivalent to approximately 1500 mg/kg-bw/day). No short-term studies of BCPO via the dermal or inhalation routes were identified.
In a 90-day, subchronic dietary study in Sprague-Dawley rats, animals (n=10/sex-dose) were administered 0, 1750, 10500 or 21000 ppm in the diet (corresponding to 0, 109, 672, 1398 mg/kg-bw/day for males and 0, 137, 800, 1660 mg/kg-bw/day in females) (Product Safety Labs 2013). Animals were observed for clinical signs, body weight changes, and behavioural changes. Animals were also subjected to gross examination, histopathology, blood chemistry testing, and urinalyses. Nephropathy and tubular cytoplasmic droplets, accompanied by increased absolute and relative kidney weights were observed in males at all dietary levels. The authors indicated that these findings were consistent with the presence of α2u-globulin nephropathy syndrome. However, since there is no evidence of production of α2u-globulin in humans, the authors did not consider these findings to be relevant to human health. Other treatment-related, dose-dependent, adverse effects included those affecting the liver (e.g., increases in absolute and relative liver weights, hepatocyte hypertrophy) and the mesenteric lymph nodes (e.g., presence of erythrocytes within sinuses) at the mid- and high-dose groups. There were no treatment-related, adverse effects on the reproductive organs. Under the conditions of the study, the authors indicated that the NOAEL was less than 109 mg/kg-bw/day in males on the basis of adverse effects in the kidneys while the NOAEL was 137 mg/kg-bw/day in females on the basis of histologic evidence of hepatocyte hypertrophy. However, given that the kidney effects in males are not relevant to humans, a NOAEL of 109 mg/kg-bw/day was determined to be appropriate on the basis of findings in the liver and mesenteric lymph nodes at the next dose level. This effect level was consistent with that adopted by the EFSA for the use of BCPO as a flavouring substance (EFSA 2014). No subchronic studies of BCPO via the dermal or inhalation routes were identified.
No chronic studies were identified. However, there is some in vitro evidence suggesting that BCPO may be anti-tumorigenic (Fidyt et al. 2016). Specifically, BCPO has been reported to inhibit proliferation of CaCo-2 cells (derived from the human colon) and enhance the activity of doxorubicin, an anti-cancer drug (Ambroz et al. 2015). Furthermore, BCPO has been shown to be cytotoxic to various cancer cell lines such as HepG2 (human leukemia cancer cells), AGS (human lung cancer cells), SNU-1 (human gastric cancer cells) and SNU-16 (human stomach cancer cells). In terms of genotoxicity, BCPO was consistently non-mutagenic in bacterial assays (Richold et al. 1979, as cited in EFSA 2014; Di Sotto et al. 2013) and did not result in increases of micronuclei frequency in human peripheral lymphocytes in vitro (Di Sotto et al. 2013). No in vivo genotoxicity assays were identified.
6.2.3 Characterization of risk to human health
The 90-day sub-chronic, dietary rat study was identified as the most relevant study for the characterization of risk from exposure to BCPO. A NOAEL of 109 mg/kg-bw/day was selected as the critical effect level on the basis of effects observed in the liver (e.g., increases in absolute and relative liver weights, hepatocyte hypertrophy) and the mesenteric lymph nodes (e.g., presence of erythrocytes within sinuses) at the mid- and high-dose groups (Product Safety Labs 2013).
Table 6‑1 provides the relevant estimates of exposure to BCPO, and critical effect levels for BCPO, as well as the resultant margins of exposure (MOEs).
| Exposure scenario | Systemic exposure | Critical effect level | MOE(s) |
|---|---|---|---|
| Daily dermal exposure from use of body lotion (infants) | 3.8 x 10-5 mg/kg-bw/day | NOAEL = 109 mg/kg-bw/dayLiver and mesenteric lymph node effects were observed at the next dose level (672 mg/kg-bw/day) in a 90-day, dietary rat study. | > 2 000 000a |
a assuming that dermal absorption is equivalent to oral absorption
Comparison of the critical effect level to estimated dermal exposure to BCPO from its use in cosmetic products resulted in an MOE > 2 000 000, which is considered adequate to address the uncertainties in the exposure and health effects databases.
With respect to potential dietary exposure to BCPO from its use as a food flavouring agent, the JECFA and EFSA have concluded that there are no safety concerns for BCPO. Furthermore, estimated intakes derived by both the JECFA and ESFA for food flavouring use of BCPO are several orders of magnitude lower than the relevant critical effect level and the risk to human health from exposure to BCPO from foods that naturally contain it and from its use as a food flavouring agent is considered to be low.
6.2.4 Uncertainties in evaluation of risk to human health
Although there are limitations in the health effects database for BCPO, given that the estimated margins of exposure are sufficiently large (several orders of magnitude) there is confidence in the risk characterization approach for this substance.
6.3 o-Cresyl glycidyl ether (o-CGE)
6.3.1 Exposure assessment
No measured concentrations of o-CGE in air, water or soil were identified. o-CGE is not stable in water as the substance has a very short hydrolysis half-life of 10.5 to 8.9 hours over a pH range of 4-9 (ECHA 2017b). The substance has a low Henry’s law constant indicating that upon release to the environment it would generally partition to the water compartment where it would then be hydrolysed. Modelled environmental exposure estimates (ChemCAN 2003) based upon the upper end of reported quantities (79 000 kg) of the substance in Canada (Environment Canada 2009) indicate that concentrations of o-CGE in air, water, and soil would be in the nanogram range and are expected to result in negligible exposure to the general population.
In Canada, o-CGE is used as a chemical intermediate, adhesive and sealant substance, or viscosity adjustor in applications such as the manufacture of adhesives and sealants, paints and coatings, or building or construction materials (Environment Canada 2009). o-CGE, like other glycidyl ethers, has applications primarily as a reactive diluent for the formulation of epoxy resin compounds (IARC 1989). No studies were identified on the potential exposure to residual o-CGE from cured epoxy resins. However, it is generally assumed that the glycidyl ether is no longer present in the cured product (IARC 1989). In addition, the Dow Chemical Product Safety Assessment report on reactive diluents, including o-CGE, indicated that although reactive diluents can be used in a variety of consumer applications, the levels of unreacted material remaining in consumer goods is negligible (Dow 2012).
Products available to consumers
Although o-CGE is primarily used in industrial applications as a reactive diluent, the substance has been identified in a limited number of DIY products available to consumers in Canada (i.e, in a flooring adhesive for use prior to installing flooring material, an epoxy flooring product for use on garage floors, an epoxy finish resin for sealing and finishing surfaces (e.g., model crafts), and an epoxy compound used for making moulds and decorative items) (SDS 2015a, b; SDS 2017).
The flooring adhesive is a two-component product, designed to be used for interior or exterior applications and may be used in residential or commercial buildings (TDS 2010). The product is used by first mixing the two components which are then applied using a trowel within one hour of mixing under a flooring material. Dermal and inhalation exposure may arise from mixing and applying the flooring adhesive. Post-application exposure to o-CGE is expected to be minimal as it would chemically react with other ingredients in the product once cured and the product would be adhered to a floor covering.
o-CGE was identified in another flooring product for use on garage floors. The product is a two-component epoxy floor coating for use on concrete garage floors (TDS 2012) applied with a brush or roller, within 2 hours of mixing. After application, the coating is allowed to cure before the surface may be used. Exposure to o-CGE may occur during use of this product via both the inhalation and dermal route. Potential post-application exposure to o-CGE from the use of this product is expected to be minimal as the substance chemically reacts with other ingredients in the product to form the hardened epoxy once cured.
o-CGE was identified to be an ingredient in a two-component epoxy resin used on various surfaces to provide a clear finish. The product is intended to be used on plastic and hard surfaces for hobby products such as model crafts (planes, cars, boats, etc) and would be applied with a brush or roller, within two hours of mixing. Dermal and inhalation exposure from the use of this product may occur during mixing, loading or application of the two epoxy components (resin and hardener). o-CGE was also identified as an ingredient in a hobby-use epoxy resin for creating epoxy resin castings from moulds. Dermal and inhalation exposure from this use may arise during mixing and loading, and pouring of the resin into a mould, and inhalation exposure may arise from the potential evaporation of o-CGE during the curing process.
Inhalation and dermal exposures to o-CGE were estimated using ConsExpo Web (ConsExpo 2016) and are considered to be conservative owing to the constraints of the model. As these products are applied, it is expected that the amount of unreacted o-CGE would decrease as a result of the epoxy formulation curing. However, the ConsExpo model does not account for the physical transformation of a substance, and it was assumed that all of the o-CGE present in the product before application is volatilized. The amount of free o-CGE released to the air during application would likely be lower than estimated by ConsExpo. ConsExpo estimates of air concentration are based on the physical-chemical properties of o-CGE but not the physical transformation of o-CGE when applying the product.
The mass transfer coefficient is a key parameter in the evaporation calculation of inhalation exposures used in ConsExpo (McCready & Fontaine 2010). A method developed by Sparks et al. (1996) to estimate mass transfer coefficients was used to characterize inhalation exposure to o-CGE as it correlated to indoor evaporation to the air flow in rooms, temperature, and molecular diffusivity.
Inhalation exposure to o-CGE from the use of an epoxy floor adhesive, an epoxy floor coating, a two-component coating/sealant and an arts and craft hobby resin was estimated using ConsExpo Web and the Sparks et al. (1996) derived mass transfer coefficient. Dermal exposure to o-CGE from use of these products was also estimated and is presented in Table 6-2 (See Appendix C for details).
| Product scenario | Inhalation exposure (24-hr mean event concentration) | Dermal exposure (per event)a |
|---|---|---|
| Epoxy flooring adhesive | 0.061 mg/m3 | 0.79 mg/kg-bw |
| Epoxy floor coating | 0.25 mg/m3 | 1.37 mg/kg-bw |
| Two-component epoxy coating/sealant | 0.29 mg/m3 | 2.1 mg/kg-bw |
| Arts and Craft/ Hobby resin | 0.031 mg/m3 | 0.22 mg/kg-bw |
a Dermal exposure value presented represents exposure from both mixing & loading and application of the products, where applicable, based on use instructions.
Use of the epoxy flooring adhesive, epoxy floor coating, and the two-component epoxy coating/sealant is expected to be infrequent (i.e., mainly improve, fix, and repair type tasks and less than or equal to one event per year) (ConsExpo 2007). The arts and crafts/hobby resin is used for hobby rather than DIY purposes and may be used more frequently. A conservative assumption of one use event per week was made for the evaluation of the arts and craft/hobby resin (ConsExpo 2007). Exposure from use of the arts and craft/hobby resin is expected to be of long-term and intermittent duration and estimates of inhalation and dermal exposure amortized over a lifetime (LADD, expressed as an inhalation concentration or dermal dose) were also derived and are presented in Table 6-3.
| Product scenario | LADD inhalationa (mg/m3) | LADD dermala (mg/kg-bw/day) |
|---|---|---|
| Arts and Craft/ Hobby resin | 9.15 x10-3 | 1.12 x 10-2 |
a Details on these calculations are presented in Appendix C
6.3.2 Health effects assessment
o-CGE is currently included in the Community Rolling Action Plan (CoRAP) by ECHA and is being evaluated by a member state in Europe. This substance is classified as suspected of causing genetic defects (Muta 2) according to the harmonised classification and labelling approved by the European Union (ECHA 2017b). There are no hazard classifications by other organizations.
The short-term effects of o-CGE have been investigated in a 21-day inhalation study, whereby RAI f SPF rats (n=10/sex/dose) were exposed to 0, 53, 152 or 305 mg/m3, 6 hours/day, 5 days/week, for 3 weeks (Ciba-Geigy Limited 1978). At the lowest dose (53 mg/m3), there was a reduction of food consumption and body weight in male rats between days 3 and 10. Other observations included dyspnoea, exophthalmos, and ruffled fur, but these effects were also observed in control animals. At the mid-dose level (152 mg/m3), in addition to the effects reported at the low dose level, there was congestion and purulent inflammation with ulceration of the nasal mucosa throughout the entire exposure period. These nasal changes were reversible after a recovery period of 21 days. At the highest dose (305 mg/m3), there was statistically significant lower body weights and mortality in 75% of the animals, beginning on day 6. There was also occasional hemorrhage in the myocardium, lungs, liver, kidneys, adrenals, pituitary, ovaries, and brain. Furthermore, the animals displayed marked congestion, purulent inflammation with ulceration of the nasal mucosa and showed depletion of thymocytes, atrophy of lymphoid tissue, and reduced spermatogenesis. The authors concluded that the NOEC was below 53 mg/m3. However, since the only treatment-related effects observed at 53 mg/m3 were reduction in food consumption and body weight, the NOAEC was determined to be 53 mg/m3.
The results of another short-term inhalation study were reported in a REACH registration dossier. In this study, Fischer 344 rats (n=5/sex/dose) were exposed to 0, 0.6 or 4 ppm (equivalent to approximately 0, 4, and 27 mg/m3) of o-CGE, 5 days/week, for 4 weeks. The report stated “no effects observed” or “no adverse effects observed” and identified the dose of 4 ppm (27 mg/m3, highest dose tested) as a NOEC (Anonymous 1991, as cited in ECHA 2017d).
No short-term studies of o-CGE via the dermal or oral routes of exposure were identified. Information on the potential health effects of the analogues cresyl glycidyl ether (CGE, CAS RN 26447-14-3), phenyl glycidyl ether (PGE, CAS RN 122-60-1) and styrene oxide (SO, CAS RN 90-96-03) was taken into consideration in the health effects characterization of o-CGE, on the basis of their similarity in terms of chemical structure, physical chemical properties, reactivity, and toxicokinetics. The chemical structures of these analogues are provided in Table 2‑3.
In an oral study investigating the short-term effects of SO on cell proliferation in the forestomach, male F344 rats (n=5/dose) were administered doses of 0, 137, 275 or 550 mg/kg-bw/day of SO via gavage, 3 times/week for 4 weeks (Cantoreggi et al. 1993). There were marginal morphological changes (i.e., thickness of the squamous epithelium, slightly enhanced keratinisation of the forestomach) and statistically significant increases in cell proliferation in certain sections of the stomach and upper small intestine. A LOAEL of 137 mg/kg-bw was determined on the basis of significant increases in cell proliferation observed in the forestomach.
Chronic toxicity/carcinogenicity
There are currently no long-term/chronic studies examining the effects of o-CGE through the oral, dermal, or inhalation routes. Information on the analogues PGE and SO was taken into consideration. Both of these analogues have been classified by the International Agency for Research on Cancer (IARC) (IARC 1989; IARC 1994; IARC 1999). PGE has been classified in the Group 2B category (possibly carcinogenic to humans) while SO has been classified in the Group 2A category (probably carcinogenic to humans).
In an oral carcinogenicity study conducted by Conti et al. (1988), Sprague-Dawley rats (n=40/sex/dose) were administered doses of 0, 50 or 250 mg/kg-bw/day of SO (in pure olive oil) by gavage, 4-5 days per week, for 52 weeks. A dose-related increase in total and malignant tumours was observed in the treated groups (statistical analyses not reported). In particular, SO produced squamous cell carcinomas, papillomas, acanthomas, and precursor lesions in the forestomach.
The carcinogenic potential of SO was also investigated in a two-year study, whereby F344 rats (n=52/sex/dose) and B6C3F1 mice (n=52/sex/dose) were given SO (in corn oil) by oral gavage, 3 times per week for 104 weeks (Lijinsky 1986). The rats were administered doses of 0, 275 or 550 mg/kg-bw/day while mice were administered doses of 0, 375 or 750 mg/kg-bw/day. In rats, the major histopathological findings were high incidences of squamous cell carcinomas and papillomas in the forestomach. In mice, there was a statistically significant increase of squamous cell carcinomas and papillomas (combined) in the forestomach of male and female animals at all dose levels. There was also a statistically significant increase in the incidence of liver carcinomas and adenomas (combined) in male mice at the mid-dose level, but this was not observed in female mice. Overall, these findings suggest that the target organ following oral exposure to SO is the forestomach in laboratory animals, suggesting a site-of-contact effect.
For the dermal route, only two studies (Weil et al. 1963; Van Duuren et al. 1963) associated with significant limitations (e.g., single dose, insufficient details on dose, vehicle selection) were identified and were not of utility for the health effects characterization.
For the inhalation route of exposure, investigations on chronic toxicity/carcinogenicity have been performed for PGE. In a two-year inhalation study, Sprague-Dawley rats (n=100/sex/group) were exposed to 0, 1 or 12 ppm PGE for 6 hours/day, 5 days/week, for 24 months (Lee et al. 1983). This corresponded to approximately 0, 6.14 or 73.68 mg/m3 (IARC 1999). Nasal tumours were observed in rats exposed to the high-dose (11% in males and 4.4% in females), while none were observed at the low-dose. Furthermore, when both male and female animals were considered, there were dose-dependent increases in the incidences of rhinitis and squamous metaplasia, which were correlated with nasal tumours. Although no statistical analyses were performed, the authors noted that the nasal tumours developed from direct contact and absorption of PGE through the respiratory epithelium in the nasal cavity.
o-CGE was considered to be a weak genotoxin by Gardiner et al. (1992). In the EU, o-CGE is classified as “suspected of causing genetic defects” (ECHA 2017b). Taking into consideration the health effects profile of o-CGE, any carcinogenic effects arising from chronic exposure to o-CGE would be expected to occur at the site-of-contact (similar to the analogues PGE and SO).
Reproductive/developmental toxicity
With regards to reproductive and developmental toxicity, no studies investigating the effects of o-CGE were identified for the relevant routes of exposure (i.e., oral, dermal, and inhalation). Health effects data from analogues were therefore considered.
For the oral route, the reproductive and developmental effects of the analogue SO have been investigated in a study whereby female BDIV rats (n=14/group) were administered a single dose of 200 mg/kg-bw SO (in olive oil) by gavage on gestation day (GD) 17 (Ponomarkov et al. 1984). The progeny of these animals were then given 100-150 mg/kg-bw of SO, once per week for 96 weeks from weaning until termination of the study. No treatment-related, adverse effects on the maternal animals were reported following a single dose of 200 mg/kg-bw (highest dose tested). In the offspring, there were statistically significant increases in forestomach carcinomas. The results of this study indicate that the target organ during development would be the forestomach, which is consistent with the target organ identified in the chronic/carcinogenicity studies.
In a dermal study investigating the dominant lethal effects of the analogue CGE, male B6D2F1 mice (n=10) were administered a dose of 1500 mg/kg-bw/day CGE, 3 times/week for 8 weeks (Pullin 1977; Gardiner et al. 1992). Following the treatment period, the male mice were mated with untreated females. Approximately 2 weeks after the mating period, the females were sacrificed and examined for pregnancy rates, total number of implantations and fetal death per pregnancy. Although there was a statistically significant decrease in the proportion of pregnant animals, the effects of CGE on the number of implantations/pregnancy and their implications on dominant lethality were unclear.
Inhalation studies investigating the reproductive and developmental endpoints have been conducted using the analogue PGE. In a two-generation reproduction and dominant lethal assay, male rats (n=8/group) were exposed to 0, 2, 6 or 11 ppm (equivalent to approximately 0, 12, 37 or 68 mg/m3) PGE, 6 hours/day for 19 consecutive days, and subsequently mated with untreated females (Terrill et al. 1982). There were no changes in body weight or mortality status in these male rats. Furthermore, the fertility of the male rats was observed to be similar to controls and the resulting pups showed normal growth with no gross structural anomalies. In the developmental portion of this study, pregnant dams (n=25/group) were also exposed to 0, 2, 6 or 11 ppm (equivalent to approximately 0, 12, 37, or 68 mg/m3) PGE, 6 hours/day, between GD 4 and 15. There were no treatment-related, adverse effects in the treated dams or fetuses. The authors concluded that rats exposed up to 11 ppm (equivalent to 68 mg/m3) showed no significant developmental or reproductive outcomes (NOAEC=68 mg/m3, highest dose tested).
Inhalation studies investigating the reproductive and developmental effects of the analogue SO were also taken into consideration. Sikov et al. (1986) exposed female Wistar rats (n=106/group) to SO vapour at 0, 100, or 300 ppm (equivalent to approximately 0, 614, 1842 mg/m3), 7 hours/day, 5 days/week, for 3 weeks prior to gestation. Owing to severe toxicity, the 300 ppm group was terminated from the study. The remaining animals were then mated and exposed to either 0 or 100 ppm SO for 7 hours/day, 5 days/week from GD 0-18. Animals were sacrificed at GD 20 and necropsied. At 100 ppm (614 mg/m3), there were significant increases in mortality, significant reductions in food consumption/body weight gain, and significant increases in lung weights in maternal animals exposed both before and during gestation. The same dose during gestation resulted in increased preimplantation loss. Fetuses from exposed dams had significantly increased incidences of ossification defects of the sternebrae and of the occipital bones. The authors could not establish whether these were direct effects or the result of maternal toxicity. A LOAEC of 100 ppm (614 mg/m3) was determined for both reproductive and developmental effects in rats on the basis of the observations of increased maternal mortality, increased percentage of litters with resorptions, decreased fetal body weights, and increased ossification effects in the foetuses.
In the same study, New Zealand White female rabbits (n=24/group) were also exposed to SO at concentrations of 0, 15 or 50 ppm (equivalent to approximately 0, 92 or 307 mg/m3), 7 hours/day from GD 1 to 24. Animals were killed at GD 30 and necropsied, with the same examinations as described previously for rats. Exposure to SO resulted in statistically significant increases in mortality and significant decreases in mean body weights and food consumption. There was also a dose-dependent, statistically significant increase in the percentage of litters with resorptions. There were no significant differences in fetal body weights when compared to controls and no indications of increased malformations as a result of exposure. A LOAEC of 15 ppm (92 mg/m3) was determined for maternal toxicity and reproductive success in rabbits on the basis of increased maternal mortality and increased percentage of litters with resorptions. A NOAEC of 50 ppm (307 mg/m3) was determined for developmental toxicity in rabbits (highest dose tested).
Other effects
Other effects associated with o-CGE exposure include the potential for skin sensitization. Reports of guinea pig maximization tests indicated that o-CGE could cause skin sensitization in guinea pigs (Anonymous 1976, as cited in ECHA 2017d; Anonymous 1989, as cited in ECHA 2017d; Ullmann et al. 1991, as cited in Gardiner et al. 1992). Available data from human studies and case reports in occupational settings also support the potential for skin sensitization. Volunteers that have been previously diagnosed with allergic contact dermatitis or other skin conditions had positive patch tests when they were exposed to 0.25% (w/w) o-CGE in petrolatum (Jolanki et al. 1990; Jolanki et al. 1991; Tosti et al. 1993; Chieregato et al. 1994; Angelini et al. 1996).
6.3.3 Characterization of risk to human health
On the basis of the health effects data available for the analogues SO, and PGE, the critical effect associated with exposure to o-CGE is expected to be carcinogenicity.
The 2-year study investigating the carcinogenic effects of PGE exposure to rats (Lee et al. 1983) via inhalation was considered appropriate for use in the characterization of risk of cancer effects from exposure to o-CGE. Benchmark concentration (BMC) modelling was performed to derive a point of departure for critical cancer effects. The dose-response curve was used to derive a lower one-sided 95% confidence limit for the benchmark concentration (BMCL) predicted to result in a 10% incidence of tumours (BMCL10). The BMCL10 levels were estimated for the tumour data using the dichotomous models available in the US EPA Benchmark Dose Software (BMDS, version 2.5). A model was selected on the basis of fit (Appendix E). An analysis of the dose-response data yielded a BMCL10 of 11.4 ppm (76.6 mg/m3) on the basis of nasal tumours.
The inhalation exposure estimate of the arts and craft/hobby resin for the general population was adjusted over a lifetime to calculate a lifetime average daily dose (LADD, expressed in mg/m3) and was determined to be 9.15 x10-3. Comparison of the critical effect level (BMCL10=76.6 mg/m3) with the estimate of lifetime average daily exposure through inhalation to o-CGE from the use of an arts and craft/hobby resin resulted in a MOE of 8372. Based upon the conservative nature of the assessment (assumption of use of hobby product once a week for a lifetime) and on the substance profile (site-of-contact carcinogenicity) this margin is considered adequate to address the uncertainties in the health effects and exposure databases.
There were no dermal studies examining the effects of repeated exposure to o-CGE. With respect to the analogues PGE and SO, the data examining the effects of long-term, dermal exposure did not contain sufficient details to derive effect levels for a quantitative characterization of chronic risk. The hazard datasets associated with the analogues PGE and SO indicate that exposure results in mainly site-of-contact effects, with limited to no systemic toxicity. Although tumours have been observed at the site-of-contact in other organs such as the forestomach and nasal cavity, the skin typically represents a less sensitive barrier when compared to the gastrointestinal tract and the respiratory tract (IGHRC 2006). Site-of-contact effects are thus likely to occur at higher doses on the skin than on internal mucosae. On the basis of this information and on the basis of adequate MOE from chronic inhalation exposure to o-CGE, it is not expected that dermal exposure from use of o-CGE in arts and craft product would represent a concern for human health.
A NOAEC of 53 mg/m3 on the basis of treatment-related adverse effects observed at the next dose level (i.e., congestion, purulent inflammation with ulceration of the nasal mucosa) in laboratory animals was identified as the critical effect level for characterization of risk to human health from short-term inhalation exposure to o-CGE from the use of products available to consumers. Table 6-4 provides all relevant inhalation exposure estimates, critical effect level, as well as resultant MOEs for determination of risk.
| Exposure scenario | 24-hr Mean event concentration (mg/m3) | Critical effect level (NOAEC, mg/m3)a | MOE |
|---|---|---|---|
| Epoxy floor adhesivea | 0.061 mg/m3 | 53 | 868 |
| Epoxy floor coating | 0.25 mg/m3 | 53 | 212 |
| Two-component epoxy coating/sealanta | 0.29 mg/m3 | 53 | 183 |
| Arts and craft/hobby resin | 0.031 mg/m3 | 53 | 1709 |
a On the basis of congestion, purulent inflammation with ulceration of the nasal mucosa observed at the next dose (152 mg/m3) in a 21-day inhalation study on rats.
Comparison of the critical effect level with the estimates of inhalation exposure to o-CGE from the use of an epoxy floor adhesive, an epoxy floor coating, a two-component coating/sealant, and an arts and craft hobby resin resulted in MOEs ranging from 183 to 1 709, which are considered adequate to address the uncertainties in the health effects and exposure databases.
Toxicity studies associated with short-term, dermal exposure to o-CGE were not identified. However, available information from the 21-day inhalation study indicates that site-of-contact effects would be expected to be reversible following short-term exposure.
While exposure of the general population to o-CGE is not of concern at current levels, this substance is considered to have a health effect of concern based on its potential mutagenic effects, with a respective classification of Muta 2 in the EU. Therefore, there may be a concern for human health if exposures were to increase.
6.3.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
6.4 Triglycidyl isocyanurate (TGIC)
6.4.1 Exposure assessment
No measured concentrations of TGIC in air, water, or soil were identified. Based upon the uses of TGIC as a chemical crosslinking agent in the production of polyester paint coatings, much of the TGIC used in these powder coating applications would be immobilized through crosslinking and thus not available for release to the environment (AGDH 1994, 2001). Environmental exposure to TGIC from use in commercial or industrial applications is expected to be negligible as electrostatic spray is an efficient application method, and excess powders (overspray) would be removed using dust extractors (AGDH 1994, OECD 2004). TGIC may be released to water at facilities that manufacture, process, or use the substance; however, it is expected to be rapidly hydrolyzed when released (WHO 1998, AGDH 1994). Aqueous discharges of TGIC from commercial or industrial powder coating applications do not occur (OECD 2004).
The primary use of TGIC is as a crosslinking agent in the formulation of polyester resins which are used in the manufacture of polyester powder coatings. In the manufacture of polyester powder coatings, TGIC may be formulated with other resins, pigments, fillers and additives at levels between 4-10% by weight of the final powdered coating (AGDH 1994). Post-application exposures to TGIC on finished metal articles are not expected as during the application process TGIC is cured at high temperatures and is fully cross-linked and bound to a solid matrix (WHO 1998).
6.4.2 Health effects assessment
Given that current exposure to TGIC is expected to be minimal in the general population in Canada, only a brief overview of the main human health effects associated with TGIC is provided.
TGIC been classified by the EU as “may cause genetic defects” (Muta 1B) (ECHA 2017c). The short-term effects of TGIC have been reported for the oral, dermal, and inhalation routes of exposure. In a report of a 7-day oral study, renal tubular damage and hemorrhagic/degenerative changes of the gastric and duodenal mucosa were observed in rats (Shell 1971, as cited in WHO 1998). In a document submitted to the US EPA, a NOEL of 43/56 mg/kg-bw (females/males) was identified (Ciba-Geigy Corporation 1990a). In the same document, a NOEL of 130 mg/kg-bw was established for a 7-day dermal rat study. In a 5-day inhalation study, male CD-1 mice (n=12/dose) were exposed to 0, 10, 40, or 140 mg/m3. Adverse clinical signs, increased body weight loss and higher mortality occurred at the mid- and high-dose levels (Nissan Chemical American Corporation 1992a). The NOAEL was determined to be 10 mg/m3. Several short-term studies examining the effects of inhalation exposure to TGIC on mouse spermatogonial cells were conducted. The lowest NOAEC identified was 1.79 mg/m3 in a spermatogonial chromosome aberration test, whereby male Crl:CD-1 (ICR)BR mice (n=10/dose) were exposed for 5 days (Ciba-Geigy Corporation 1992a).
TGIC has been investigated in a 13-week combined toxicity and fertility study on male Sprague-Dawley rats (n=10/dose). Animals were given TGIC at daily dietary levels of 0, 10, 30 or 100 ppm (corresponding to approximately 0, 0.72, 2.08, 7.32 mg/kg-bw/day). A NOAEL of 30 ppm (2.08 mg/kg-bw/day) was established by the study authors on the basis of the effects observed in mesenteric lymph nodes (Fabreguettes 1995; Ciba-Geigy Corporation 1995). In the fertility protocol of this study, no treatment-related, adverse effects were observed for the parental animals or their offspring. However, this study only administered TGIC to males. Therefore, reproductive/developmental effects arising from maternal exposure was not investigated.
The effects of long-term/chronic exposure from TGIC have been investigated in a cancer bioassay in male Sprague-Dawley rats (n=50/dose) at dietary levels of 0, 10, 30, 100, or 300 ppm (corresponding to approximately 0. 0.430, 1.30, 4.36, and 13.6 mg/kg-bw/day). The authors concluded that TGIC did not exhibit carcinogenic potential (Fabreguettes 1999). TGIC was also not reported to promote skin tumour formation in a dermal initiation-promotion bioassay (Shell 1971, as cited in AGDH 1994).
TGIC has demonstrated both skin sensitization and respiratory sensitization potential. In occupational settings, allergic contact dermatitis has been reported (Mathias 1988; Nishioka et al. 1988; Foulds and Koh 1992; Munro and Lawrence 1992; McFadden and Rycroft 1993; Jolanki et al. 1994; Pirilla et al. 1997; Wigger-Alberti et al. 1997; Craven et al. 1999; Meuleman et al. 1999; Aalto-Korte and Suuronen 2016). Occupational asthma has also been reported in a series of case reports (Pirila et al. 1997; McCoach et al. 1998; Meuleman et al. 1999; Quirce et al. 2004; Anees et al. 2011; Quirce et Sastre 2011; Sastre et al. 2011).
6.4.3 Characterization of risk to human health
Based upon available use exposure information for TGIC, exposure to the general population from environmental media is expected to be minimal. Consumer exposure to finished painted articles containing TGIC is not expected as the substance would be fully cross-linked and cured. On the basis of this information, the risk to human health from exposure to TGIC for the general population is expected to be low.
While exposure of the general population to TGIC is not of concern at current levels, this substance is considered to have a health effect of concern based on its potential mutagenic effects, with a respective classification of Muta 1B in the EU (ECHA 2017c). Therefore, there may be a concern for human health if exposures were to increase.
6.4.4 Uncertainties in evaluation of risk to human health
Although there are some uncertainties in the health effects database (e.g., insufficient details on effect levels from epidemiological data), and some limitations in the exposure databases (e.g., data on the availability of specialty products required to use powder coatings containing TGIC), given that the sources, uses, and properties of TGIC are well characterized, a qualitative approach to risk characterization is considered appropriate for this assessment.
6.5 C12-C13 Alkyl glycidyl ether (C12-C13 AGE)
6.5.1 Exposure assessment
No measured concentrations of C12-C13 AGE in air, water, or soil were identified. Quantities of C12-C13 AGE in Canada were below the reporting threshold of 100 kg based upon information from a CEPA section 71 survey (Environment Canada 203). As the substance does not naturally occur in the environment, indirect exposure to C12-C13 AGE from environmental media is expected to be minimal based on the low reported quantities in commerce (Environment Canada 2013).
Primary uses of C12-C13 AGE are as an ingredient in epoxy resins, where it likely functions as a reactive diluent similar to other glycidyl ethers (IARC 1989). The substance was identified in a limited number of all-purpose two-component epoxy adhesive products available to consumers in Canada. It was identified in a two-component epoxy adhesive (SDS 2016) and an epoxy filler sold in tube packaging (SDS 2014c). C12-C13 AGE was also identified to be an ingredient in a multi-purpose low-viscosity epoxy resin, which may be used to seal and coat various surfaces such as furniture or boats (SDS 2013).
Acute inhalation and dermal exposure to C12-C13 AGE may occur during mixing and loading and application of certain epoxy adhesives reported to contain it as an ingredient. Post-application exposures are not expected to be significant as the substance chemically reacts with other ingredients and would be cross-linked to a solid matrix upon curing. Using ConsExpo Web, dermal and inhalation exposures from using these epoxy adhesives were estimated and are presented in Table 6-6 below. The Sparks method for deriving a mass transfer coefficient for the substance was used to estimate inhalation exposures (Sparks et al. 1996, as cited in McCready & Fontaine 2010). The inhalation estimates of exposure to C12-C13 from these uses are considered to be conservative since, as with the o-CGE exposure estimates, the model does not take into consideration the epoxy formulation curing after it is applied (further detail is provided in in the exposure assessment of o-CGE in section 6.3.1, above). Details on each product scenario can be found in Appendix C.
| Product scenario | Acute inhalation exposure (24-hr mean event concentration) | Acute dermal exposurea,b |
|---|---|---|
| Two-component epoxy adhesive | 0.0085 mg/m3 (0.0044 mg/kg-bw) | 0.07 mg/kg-bw |
| Epoxy filler from a tube | 0.0069 mg/m3 (0.0036 mg/kg-bw) | 0.035 mg/kg-bw |
| Coating with low-viscosity resin | 0.069 mg/m3 (0.036 mg/kg-bw) | 1.84 mg/kg-bw |
a Dermal exposure value presented represents exposure from both mixing and loading and application of the products, where applicable, based on use instructions
b Dermal systematic exposure based on an assumed dermal absorption value of 100%
6.5.2 Health effects assessment
The short-term effects of C12-C13 AGE have been investigated in an oral toxicity study, where Fischer 344 rats (n=10/sex/group) were given 0, 100, 500 or 2500 mg/kg-bw/day C12-C13 AGE by gavage, 7 days/week for 28 days (Shell Oil Company 1991). At the end of the study, all animals were necropsied and tissues were examined histopathologically. Treatment-related, diffuse or focal hyperplasia of the non-glandular stomach was observed in the rats exposed to 500 or 2500 mg/kg-bw/day. The maturation of cells was ordered and there was no cellular atypia or invasion noted below the muscularis mucosa, suggesting that these effects would likely be reversible upon cessation of exposure. No systemic effects were observed. The authors established a NOAEL of 100 mg/kg-bw/day on the basis of gastric (non-glandular) hyperplasia observed at the higher doses.
For the dermal route of exposure, a range-finding study was conducted in Fischer 344 rats (n=2/sex/dose) over a period of 2 weeks (Society of the Plastics Industry Inc 1997a). The animals were dermally exposed to 0, 10, 100 or 1000 mg/kg-bw/day C12-C13 AGE (in acetone), 5 days/week, for 2 weeks. The dermal site was evaluated daily for dermal irritation. Gross pathology and limited histopathology was also performed. At the lowest dose, the only effects observed were slight scaling. At the mid- and high-doses, treatment-related histopathological effects of the skin (e.g., erythema, eschar, edema, scaling, and fissuring) were observed, which were also associated with a loss of skin integrity at the highest dose. The authors established a NOAEL of 10 mg/kg-bw/day based upon the local, dermal effects occurring at the next dose level, however, no systemic effects were identified at any of the doses tested. No short-term, inhalation studies were identified.
Based upon the aforementioned range-finding study, subchronic toxicity from dermal exposure to C12-C13 AGE was investigated in a 13-week study, whereby Fischer 344 rats (n=10/sex/dose) were dermally exposed to 0, 1, 10 or 100 mg/kg-bw/day C12-C13 AGE (in acetone), 5 days/week, for 13 weeks (Society of the Plastics Industry Inc 1997a). The dermal site was evaluated daily for the first week, and weekly thereafter for dermal irritation. Animals were also examined for clinical signs, hematology, and clinical chemistry. At the end of the study, animals were necropsied and underwent histopathological examination. There were no apparent treatment-related, adverse effects other than the local effects observed at the dermal site. The authors established a NOAEL of 1 mg/kg-bw/day on the basis of dose-dependent dermal changes (i.e., scaling, thickening of the skin, epidermal hyperplasia, hyperkeratosis, and hyperplasia of the sebaceous glands) observed at the mid- and high-doses, however, no systemic effects were identified at any of the doses tested.
In another subchronic study investigating neurotoxicity, Fischer 344 rats (n=12/sex/dose) were dermally exposed to 0, 1, 10 or 100 mg/kg-bw/day C12-C13 AGE (in acetone), 5 days/week for 13 weeks (Society of the Plastics Industry Inc 1997b). Dermal evaluations were conducted daily for the first week, then weekly thereafter. A Functional Observation Battery focused on grip performance, landing foot splay and motor activity was also conducted. Electrodiagnostic tests were used to screen for dysfunction of peripheral nerves, spinal cord, brainstem, cerebellum, and cerebrum by evaluating evoked potentials from these anatomical sites. Necropsy and histopathological examinations were conducted on external tissues. Animals at the mid- and high-dose groups exhibited erythema, edema, scaling, moderate scabbing. The skin condition of animals in the low-dose group was comparable with those in the control group. In terms of the neurotoxicity parameters examined, significant effects were only observed for tests of the visual pathway, expressed as flash evoked potentials (FEPs), although these effects were not associated with histopathological findings. There were no gross pathologic observations or histopathologic effects in the central and peripheral nervous systems. On the basis of this data, the authors identified a NOEL of 1 mg/kg-bw/day on the basis of mild skin effects in mid-dose rats and mild FEP alterations in mid-dose male rats. Owing to the uncertainty of the mechanism of the FEP differences, and because early visual processing in albino rats is known to be different than in pigmented animals, the toxicological significance of FEP differences observed in this study are uncertain. Therefore, FEP changes will not be considered relevant in the risk characterization of C12-C13 AGE to human health in this assessment. There were no subchronic studies conducted through the oral or inhalation routes of exposure.
Chronic toxicity/carcinogenicity/genotoxicity
There are currently no long-term studies investigating the effects of C12-C13 AGE for any of the relevant routes of exposure (i.e., oral, dermal, inhalation).
C12-C13 AGE has demonstrated genotoxic potential in bacterial mutagenicity assays. It resulted in positive findings in Salmonella typhimurium TA1535 with and without metabolic activation. However, it was negative for mutagenicity in all other tested strains such as TA98, TA100, TA1537 with and without metabolic activation. It also did not result in mutagenic findings in E. coli WP2 (Society of the Plastics Industry Inc 1997c). An in vitro mammalian cell gene mutation test conducted on Chinese Hamster Ovary (CHO) cells yielded negative findings (Society of the Plastics Industry Inc 1998). In vivo, intraperitoneal exposure to C12-C13 AGE at doses of 0, 1000, 2000 or 4000 mg/kg-bw in ICR mice (n=5/sex/dose) did not induce a significant increase of micronucleated polychromatic erythrocytes in bone marrow and was concluded to be negative for genotoxicity (Society of the Plastics Industry Inc 1997d). Consideration of the available information on genotoxicity indicates that C12-C13 AGE is not likely to be genotoxic.
Reproductive/developmental toxicity
With respect to reproductive and developmental toxicity, C12-C13 AGE is not expected to cause significant adverse effects through the dermal route. In a developmental toxicity screen, female Crl:CD(SD)BR rats (n=8/group) were dermally exposed to 0, 1, 10, 50, 100 or 200 mg/kg-bw/day C12-C13 AGE (in acetone), 6 hours/day from GD 6-15 (Epoxy Resin Systems Task Group 1997). Clinical signs, dermal irritation, body weights, and food consumption were recorded throughout the study. On GD 20, sacrifice with examination of uterine contents was performed, which was followed by examination of the thoracid, abdominal, and pelvic cavity contents. The uterus and ovaries were excised and the number of corpora lutea counted. All fetuses, early and late resorptions, number of implantation sites were also recorded. An external examination of the fetuses was conducted. Treatment-related, adverse effects on maternal animals were limited to those situated at the site of dermal exposure, and there was no indication of systemic toxicity. Dermal irritation, characterized by erythema, edema and desquamation, was observed in the 50, 100, and 200 mg/kg-bw/day dose groups. At 10 mg/kg-bw/day, there were only 2 female animals exhibiting slight erythema. However, these were not definitive indications of treatment-related dermal irritation. No other dermal findings were observed. For reproductive parameters, no treatment-related, adverse effects were observed in female reproductive organs. This finding is consistent with the lack of effects on the reproductive organs in the aforementioned subchronic studies (Society of the Plastics Industry Inc 1997b). Furthermore, post-implantation loss, intrauterine growth, pup survival, viable litter size, fetal sex ratio, the number of corpora lutea and implantation sites were comparable to the controls. In terms of fetal morphological data, no external malformations and external developmental variations were observed. The authors indicated that the NOEL for dermal irritation was considered to be 10 mg/kg-bw/day and the NOEL for maternal and developmental toxicity to be 200 mg/kg-bw/day (the highest dose tested).
6.5.3 Characterization of risk to human health
Exposure of the general population to C12-C13 AGE may occur through the use of DIY products available to consumers, (i.e., a two-component epoxy adhesive, an epoxy filler from a tube, and a coating with low-viscosity resin). Exposure is expected to occur on a short-term basis via the inhalation and dermal route.
With respect to cancer effects, although the possibility of a carcinogenic response of C12-C13 cannot be excluded, no long-term studies investigating this effect were identified. Consideration of the available information on genotoxicity indicates that C12-C13 AGE is not likely to be genotoxic. As exposure scenarios associated with use of this substance are expected to be short-term only, carcinogenicity is not considered to be a critical effect for characterization of the risk to human health for C12-C13 AGE.
With respect to short term exposure via the dermal route, only local effects on the skin were observed at the site-of-contact following exposure to C12-C13 AGE. Systemic effects arising from dermal exposure to C12-C13 AGE have not been observed in a short-term, range-finding study in rats (Society of the Plastics Industry Inc 1997a), in subsequent subchronic studies (Society of the Plastics Industry Inc 1997a,b), or in a study investigating reproductive and developmental toxicity (Epoxy Resin Systems Task Group 1997) at doses up to 1000 mg/kg-bw/day. On the basis of the available information, this substance is considered to be associated with non-adverse, reversible local effects following short-term dermal exposure and the risk to human health associated with exposure to C12-C13 via the dermal route is low.
No studies investigating the effects of C12-C13 AGE via inhalation were identified. Therefore, a NOAEL of 100 mg/kg-bw-day on the basis of site-of-contact effects on the forestomach observed in an oral rat study at the next dose level (i.e., 500 mg/kg-bw/day) was used as the critical effect for the risk characterization of short-term inhalation exposure to C12-C13 AGE (Shell Oil Company 1991),
| Exposure scenario | Exposure concentration (converted to external dose, mg/kg-bw) | Critical effect level (mg/kg-bw/day)a | MOE |
|---|---|---|---|
| Two-component epoxy adhesive | 0.0044 | 100 | 22727 |
| Epoxy filler from a tube | 0.0036 | 100 | 27778 |
| Coating with low-viscosity resin | 0.036 | 100 | 2778 |
a Focal hyperplasia of the non-glandular stomach observed at the next dose level (i.e., 500 mg/kg-bw/day) in a 28-day oral study on rats.
Comparison of the critical effect level to the estimates of inhalation exposure from the use of DIY-products containing C12-C13 AGE resulted in MOEs that ranged between 2 778 to 27 778. These MOEs are considered adequate to address uncertainties in the health effects and exposure databases for these scenarios. Although there is uncertainty in the use of a critical effect from an oral study to characterize risk from inhalation, based upon the overall toxicological profile of C12-C13, it is expected that effects via inhalation would likely be limited to site-of-contact irritation. In addition, this assessment is considered to be conservative as it compares an effect level (site-of-contact irritation of the forestomach mucosa) occurring after 28-days of exposure in animal laboratories, with an exposure from short-term use of products.
6.5.4 Uncertainties in the evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from AGE, BCPO, o-CGE, TGIC and C12-C13 AGE. It is concluded that AGE, BCPO, o-CGE, TGIC and C12-C13 AGE do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based upon the information presented in this screening assessment, it is concluded that AGE, BCPO, o-CGE, TGIC, and C12-C13 AGE do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that AGE, BCPO, o-CGE, TGIC, C12-C13 AGE do not meet any of the criteria set out in section 64 of CEPA.
References
Aalto-Korte K, Suuronen K. 2016. Occupational contact allergy to components of polyester resin systems. Contact Dermatitis. 75(1): 14-19.
[AGDH] Australian Government Department of Health. 1994. Triglycidylisocyanurate (TGIC) [Word]. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme (NICNAS). Priority Existing Chemical (PEC) Assessment Report No. 1.
Allied Corporation. 1982. Evaluation of allyl glycidyl ether for enzyme mediated mutagenicity in salmonella typhimurium with cover letter. Microfiche No. 215156. Doc ID: 878221062. Old Doc ID: 8DS.
Ambroz M, Bousova I, Skarka A, Hanusova V, Kralova V, Matouskova P, Szotakova B, Skalova L. 2015. The influence of sesquiterpenes from Myrica rubra on the antiproliferative and pro-oxidative effects of doxorubicin and its accumulation in cancer cells. Molecules. 20(8): 15343-15358.
Anees W, Moore VC, Croft JS, Robertson AS, Burge PS. 2011. Occupational asthma caused by heated triglycidyl isocyanurate. Occupational Medicine. 61(1): 65-67.
Angelini G, Rigano L, Foti C, Grandolfo M, Vena GA, Bonamonte D, Soleo L, Scorpiniti AA. 1996. Occupational sensitization to epoxy resin and reactive diluents in marble workers. Contact Dermatitis. 35(1): 11-16.
[AOPWIN] Atmospheric Oxidation Program for Microsoft Windows [estimation model]. 2010. Ver. 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Asakawa Y, Taira Z, Takemoto T. 1981. X-ray crystal structure analysis of 14-hydroxycarylophyllene oxide, a new metaboilite of (-)-caryophyllene, in rabbits. Journal of Pharmaceutical Sciences. 70(6): 710-711.
Asakawa Y, Ishida T, Toyota M, Takemoto T. 1986. Terpenoid biotransformation in mammals IV biotransformation of (+)-longifolene, (-)-carylophyllene, (-)-caryophyllene oxide, (-)-cyclocolorenone, (+)-nootkatone, (-)-elemol, (-)-abietic acid and (+)-dehydroabietic acid in rabbits. Xenobiotica. 16(8): 753-767.
Burdock. 2009. Fenaroli’s Handbook of Flavour Ingredients. 6th ed. Boca Raton (FL): CRC Press.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canter DA, Zeiger E, Haworth S, Lawlor T, Mortelmans K, Speck W. 1986. Comparative mutagenicity of aliphatic epoxides in salmonella. Mutation Research. 172(2): 105-138.
Cantoreggi S, Dietrich DR, Lutz WK. 1993. Induction of cell proliferation in the forestomach of F344 rats following subchronic administration of styrene 7,8-oxide and butylated hydroxyanisole. Cancer Research 53(15): 3505-3508.
ChemCAN 2003. Level III fugacity model of 24 regions of Canada. Ver. 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
ChemIDplus [database]. 1993-. Bethesda (MD): US National Library of Medicine.
ChemView. [database]. 2016a. Search results for CAS RN 106-92-3. Washington (DC): EPA Office of Pollution Prevention and Toxics. [updated 2016 Dec 17; accessed 2017 May 29].
ChemView. [database]. 2016b. Search results for CAS RN 2210-79-9. Washington (DC): EPA Office of Pollution Prevention and Toxics. [updated 2016 Dec 17; accessed 2017 May 29].
ChemView. [database]. 2016c. Search results for CAS RN 2451-62-9. Washington (DC): EPA Office of Pollution Prevention and Toxics. [updated 2016 Dec 17; accessed 2017 May 29].
ChemView. [database]. 2016d. Search results for CAS RN 120547-52-6. Washington (DC): EPA Office of Pollution Prevention and Toxics. [updated 2016 Dec 17; accessed 2017 May 29].
Chieregato C, Vincenzi C, Guerra L, Farina P. 1994. Occupational allergic contact dermatitis due to ethylenediamine dihydrochloride and cresyl glycidyl ether in epoxy resin systems. Contact Dermatitis. 30: 120.
Ciba-Geigy Corporation. 1990a. Initial submission: summary of toxicology data of Araldite PT 810 with cover letter dated 101992. Microfiche No: OTS0571432. New Doc ID: 88-920009782. Old Doc ID: 8EHQ-1092-11501.
Ciba-Geigy Corporation. 1992a. Support: 1,3,5-triglycidylisocyanurate: chromosomal aberrations assay in mouse spermatogonial cells with cover letter dated 112592. Microfiche No: OTS0503914-15. New Doc ID: 89-930000035. Old Doc ID: 8EHQ-1292-0490.
Ciba-Geigy Corporation. 1995. Support: 13-week toxicity study and fertility study of Araldite PT-810 by oral route (dietary admixture) in male rats, with cover letter dated 4/26/96. Microfiche No: OTS0503914-17. New Doc ID: 000811668U. Old Doc ID: 8EHQ-0596-0490.
Ciba-Geigy Limited. 1978. TK 10’410, 21-day aerosol inhalation study in rats. Document No: 8EHQ-92-0530. 08930000012.
Conti B, Maltoni C, Perino G, Ciliberti A. 1988. Long-term carcinogenicity bioassays on styrene administered by inhalation, ingestion and injection and styrene oxide administered by ingestion in Sprague-Dawley rats, and para-methylstyrene administered by ingestion in Sprague-Dawley rats and Swiss mice. Annals New York Academy of Sciences. 534(1): 203-234.
[CosIng] Cosmetic Ingredients and Substances. [database]. 2017. v.2. Search results for CAS RN 106-92-3. Brussels (BE). DG Internal Market, Industry, Entrepreneurship and SMEs. [accessed 2017 May 29].
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Craven NM, Bhushan M, Beck MH. 1999. Sensitization to triglycidyl isocyanurate, epoxy resins and acrylates in a developmental chemist. Contact Dermatitis. 40(1): 54.
De Rooij BM, Commandeur JNM, Hommes JW, Aalbers T, Groot EJ, Vermeulen NPE. 1998. Urinary metabolite profile of phenyl and o-cresyl glycidyl ether in rats : identification of a novel pathway leading to n-acetylserine o-conjugates. Chemical Research in Toxicology. 11(2): 111-118.
Department of Health & Human Services. 1984. The salmonella/microsome mutagenicity test system with cover letter dated 060484. Microfiche No. OTS0522514. New Doc ID: 40-8440544. Old Doc ID: 42051 B3-267.
Di Sotto A, Maffei F, Hrelia P, Castelli F, Sarpietro MG, Mazzanti G. 2013. Genotoxicity assessment of β-caryophyllene oxide. Regulatory Toxicology and Pharmacology 66(3): 264-268.
DOW Chemical Company. 1978. Acute oral, acute percutaneous absorption, and acute inhalation toxicity of allyl glycidyl ether. Microfiche No: OTS0206671. Doc ID: 878214853. Old Doc ID: 8DS.
[Dow] Dow Chemical Company. 2012. Product safety assessment: DOW reactive diluents. [revised 2012 May 2; cited 2017 May 30]. Available upon request.
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Health Canada. [updated 2015 Jul 17; accessed 2017 April 2]
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2016c. Data submitted pursuant to the Canadian Environmental Protection Act, 1999, section 70. Data prepared by ECCC, Health Canada; Existing Substances Program.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2007 Apr 20]. Categorization. Ottawa (ON): Government of Canada. [accessed 2017 April 01].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of Risk Assessment Priorities: Results of the 2015 Review. Ottawa (ON): Government of Canada [accessed 2017 April 01]
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018. Rapid Screening of Substances with Limited General Population Exposure. Ottawa (ON): Government of Canada. [accessed 2017 April 01].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018. Screening Assessment: Substances Identified as Being of Low Concern based on the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. 2017a. Brief profile: Allyl 2,3-epoxypropyl ether; CAS RN 106-92-3. Helsinki (FI): ECHA. [updated 2017 May 5 ; accessed 2017 May 24].
[ECHA] European Chemicals Agency. 2017b. Brief profile: 2,3-epoxypropyl o-tolyl ether; CAS RN 2210-79-9. Helsinki (FI): ECHA. [updated 2017 May 5 ; accessed 2017 May 24].
[ECHA] European Chemicals Agency. 2017c. Brief profile: 1,3,5-tris(oxiranylmethyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione; CAS RN 2451-62-9. Helsinki (FI): ECHA. [updated 2017 May 5 ; accessed 2017 May 24].
[ECHA] European Chemicals Agency. 2017d. Guidance on safe use: 2,3-epoxypropyl o-tolyl ether; CAS RN 2210-79-9. Helsinki (FI): ECHA. [updated 2017 May 26 ; accessed 2017 June 19].
[EFSA] European Food Safety Authority. 2014. Scientific opinion: scientific opinion on flavouring group evaluation 82, Revision 1 (FGE.82Rev1): consideration of epoxides evaluated by the JECFA (65th meeting). EFSA Journal. 12(6):3708.
Environment Canada, Health Canada. 2010. Screening assessment for the Challenge: Oxirane, (butoxymethyl)-(n-Butyl glycidyl ether): Chemical Abstracts Service Registry Number 2426-08-6. Ottawa (ON): Government of Canada. [accessed 2017 May 30].
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2012. Domestic Substances List Inventory Update Phase 2 (DSL IU2). Guidance for responding to the Notice with respect to certain substances on the Domestic Substances List (the notice) published in the Canada Gazette, Part I, on December 1, 2012.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Epoxy Resin Systems Task Group. 1997. Final report, a dermal developmental toxicity screening study of alkyl glycidyl ethers in rats, with cover letter dated 10/19/97. Microfiche No: OTS0558897. New Doc ID: 44645. Old Doc ID: 42185 F1-17 44645.
Fabreguettes C. 1995. 13-week toxicity study and fertility study by oral route (dietary mixture) in male rats. CIT/Study No. 11099 TCR/PT 810 (TGIC)/Ciba-Geigy and Nissan. Microfiche No: OTS0503914-17. New Doc ID: 000811668U. Old Doc ID: 8EHQ-0596-0490.
Fabreguettes C. 1999. Carcinogenicity study in male rats. CIT/Study No. 11117 TCR. Microfiche No: OTS0573828-1. New Doc ID: 89990000269. Old Doc ID: 8EHQ-0799-14351.
Fidyt K, Fiedorowicz A, Strzadala L, Szummy A. 2016. βcaryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Medicine. 5(10):3007-3017.
Foulds IS, Koh D. 1992. Allergic contact dermatitis from resin hardeners during the manufacture of thermosetting coating paints. Contact Dermatitis. 26(2): 87-90.
Gagnaire F, Zissu D, Bonnet P, De Ceaurriz J. 1987. Nasal and pulmonary toxicity of allyl glycidyl ether in mice. Toxicology Letters. 39(2): 139-145.
Gardiner TH, Waechter JM, Wiedow MA, Solomon WT. 1992. Glycidyloxy compounds used in epoxy resin systems: a toxicology review. Regulatory Toxicology and Pharmacology. 15(2):S1-S77.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2013. Interim guidance on human health risk assessment for short-term exposure to carcinogens at contaminated sites [PDF]. Ottawa (ON): Government of Canada. [accessed 2017 Jun 19].
Health Canada. [modified 2015 Dec 14]. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Health Canada, Consumer and Hazardous Products Safety Directorate. [accessed 2016 May 29].
Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
Hemminki K, Falck K, Vainio H. 1980. Comparison of alkylation rates and mutagenicity of directly acting industrial and laboratory chemicals: epoxides, glycidyl ethers, methylating and ethylating agents, halogenated hydrocarbons, hydrazine derivatives, aldehydes, thiuram and dithiocarbamate derivatives. Arc. Toxicol. 46(3-4): 277-285.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1989. Some organic solvents, resin monomers and related compounds, pigments and occupational exposures in paint manufacture and painting. IARC Monogr Eval Carcinog Risks Hum 47.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1994. Styrene-7,8-oxide. IARC Monogr Eval Carcinog Risks Hum. 60: 321-346.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1999. Phenyl glycidyl ether. IARC Monogr Eval Carcinog Risks Hum. 71: 1525-1527.
[IGHRC] The Interdepartmental Group on Health Risks from Chemicals. 2006. Guidelines on route-to-route extrapolation of toxicity data when assessing health risks of chemicals. Bedfordshire (UK): Institute of Environment and Health.
Jolanki R. 1991. Occupational skin diseases from epoxy compounds. Acta Dermato-Venereologica. Supplementum 159: 1-80.
Jolanki R, Estlander T, Kanerva L. 1987. Occupational contact dermatitis and contact urticaria caused by epoxy resins. Acta Derm Venereol Stockholm. 134(supplementum): 90-94.
Jolanki R, Kanerva L, Estlander T, Tarvainen K. 1994. Concomitant sensitization to triglycidyl isocyanurate, diaminodiphenylmethane and 2-hydroxyethyl methacrylate from silk-screen printing coatings in the manufacture of circuit boards. Contact Dermatitis. 30(1): 12-15.
Jolanki R, Kanerva L, Estlander T, Tarvainen K, Keskinen H, Henriks-Eckerman ML. 1990. Occupational dermatoses from epoxy resin compounds. Contact Dermatitis. 23(3): 172-183.
Langmuir I. 1913. The vapor pressure of metallic tungsten. Phys Rev 2(329): 42
Lee KP, Schneider PW, Trochimowicz HJ. 1983. Morphologic expression of glandular differentiation in the epidermoid nasal carcinomas induced by phenylglycidyl ether inhalation. American Journal of Pathology. 111(2):140-148.
Lijinsky W. 1986. Rat and mouse forestomach tumors induced by chronic oral administration of styrene oxide. JNCI. 77(2): 471-476.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol 43: 279-291.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, et al. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol 46: 1516-1524.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2014 Feb 27]. Ottawa (ON): Health Canada. [accessed 2017 April 2].
Mathias CGT. 1988. Allergic contact dermatitis from triglycidyl isocyanurate in polyester paint pigments. Contact Dermatitis. 19(1): 67-68.
McCready D, Fontaine D. 2010. Refining ConsExpo evaporation and human exposure calculations for REACH. Human and Ecological Risk Assessment. 16(4): 783-800.
McCoach JS, Burge PS. 1997. Occupational asthma due to triglycidyl isocyanurate (TGIC). Thorax. 52(Suppl 6): P173.
McCoach JS, Robertson AS, Burge PS. 1998. Occupational asthma due to triglycidyl isocyanurate tgic. Annual Meeting of the European Academy of Allergology and Clinical Immunology, Birmingham, England, Uk, June 21-26, 1998. Allergy (Copenhagen) 53(SUPPL. 43): 218.
McFadden JP, Rycroft RJG. 1993. Occupational contact dermatitis from triglycidyl isocyanurate in a powder paint sprayer. Contact Dermatitis. 28(4): 251.
Meuleman L, Goossens A, Linders C, Rochette F, Nemery B. 1999. Case report: sensitization to triglycidylisocyanurate (TGIC) with cutaneous and respiratory manifestations. Allergy. 54(7): 752-756.
Munro CS, Lawrence CM. Short communications: occupational contact dermatitis from triglycidyl isocyanurate in a powder paint factory. Contact Dermatitis. 26(1): 59.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2015 Nov 23]. Ottawa (ON): Health Canada. [accessed 2017 April 2].
[NIH] National Institutes of Health. 1984. Comparative mutagenicity of aliphatic diesters in salmonella. Microfiche No: OTS0522514. New Doc ID: 40-8440414. Old Doc ID: 42051 B3-266.
Nishioka K, Ogasawara M, Asagami C. 1988. Short communications: occupational contact allergy to triglycidyl isocyanurate (TGIC, Tepic). Contact Dermatitis. 19(5): 379-380.
Nissan Chemical American Corporation. 1992a. Supplemental information from Nissan Chemical America Corp to USEPA concerning triglycidyl isocyanurate: 5-day repeat exposure inhalation toxicity study in the male mouse w—attach. Microfich No: OTS0503914-13. New Doc ID: 89-920000049. Old Doc ID: 8EHQ-0292-0490.
Nissan Chemical American Corporation. 1992b. Supplement: Triglycidyl isocyanurate: chromosome analysis in mouse spermatogonial cells, comparative inhalation study with cover letter dated 091892. Microfiche No: OTS0503914-14. New Doc ID: 89-920000133. Old Doc ID: 8EHQ-0992-0490.
[NTP] National Toxicology Program (US). 1990. NTP technical report on the toxicology and carcinogensis studies of allyl glycidyl ether (CAS No. 106-92-3) in Osborne-mendel rats and B6C3F1 mice (inhalation studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Report No.: 376.
[NTP] National Toxicology Program (US). 2000. Report on carcinogens background document for Styrene-7,8-oxide. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program, Public Health Service.
[NTP] National Toxicology Program (US). 2010. o-Cresyl glycidyl ether. Salmonella: Study Summary. Study ID: 141757.
[OECD] Organisation for Economic Co-operation and Development. 2004. Emission scenario document on metal finishing [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 12; Report No.: ENV/JM/MONO(2004)23, JT03263583). [accessed 2017 July 15].
[OECD] Organisation for Economic Co-operation and Development. 2007. SIDS Initial Assessment Profile (SIAP): Allyl 2,3-epoxypropyl ether [ PDF]. SIAM [SIDS Initial Assessment Meeting] 25; 2007 October; Paris, France. [accessed 2017 Jun 19].
OECD QSAR Toolbox.2014 Ver. 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Opdycke DLJ, Letizia C. 1983. Monographs on fragrance raw materials, Caryophyllene oxide. Food Chem Toxicol. 21(5): 661-662.
Pérez HL, Osterman-Golkar S. 2000. Biotransformation of the double bond in allyl glycidyl ether to an epoxide ring. Evidence from hemoglobin adducts in mice. Chemico-Biological Interactions 125(1): 17-28.
Piirila P, Eslander T, Keskinen H, Jolanki R, Laakkonen A, Pfaffli P, Tupasela O, Tuppurainen M, Nordman H. 1997. Occupational asthma caused by triglycidyl isocyanurate (TGIC). Clinical and Experimental Allergy. 27(5): 510-514.
Plna K, Segerback. 1997. 32P-Postlabelling of DNA adducts formed by allyl glycidyl ether in vitro and in vivo. Carcinogenesis. 18(8): 1457-1462.
Ponomarkov V, Cabral JRP, Wahrendorf J, Galendo D. 1984. A carcinogenicity study of styrene-7,8-oxide in rats. Cancer Letters 24(1): 95-101.
Pottenger LH, Boverhof DR, Waechter JM. 2012. Epoxy Compounds—Olefin Oxides, Aliphatic Glycidyl Ethers, and Aromatic Monoglycidyl Ethers. Patty’s Toxicology. 82:425-490.
Product Safety Labs. 2012. Beta-caryophyllene epoxide: palatability/toxicity study: a 14-day dietary study in rats. Study Number: 31086. [restricted access].
Product Safety Labs. 2013. Beta-caryophyllene epoxide: a 90-day dietary study in rats. Study number: 33329. [restricted access].
Pullin TG. 1977. Report to the DOW Chemical Company. Integrated mutagenicity testing program on several epoxy compounds. Microfiche No: OTS0206671. Doc ID: 878214859. Old Doc IC: 8DS.
Quirce S, Fernandez-Nieto M, de Gorgolas M, Renedo G, Carnes J, Sastre J. 2004. Hypersensitivity pneumonitis caused by triglycidyl isocyanurate. Allergy. 59(10): 1128.
Quirce S, Sastre J. 2011. New causes of occupational asthma. Current Opinion in Allergy and Clinical Immunology. 11(2): 80-85.
Sastre J, Carnes J, Garcia del Potro M, Manso L, Aguado E, Fernandez-Nieto M. 2011. Occupational asthma caused by triglycidyl isocyanurate. Int Arch Occup Environ Health. 84(5): 547-549.
[SDS] Safety Data Sheet. 2013. MAS Epoxies Low Viscosity Epoxy Resin [PDF]. South Saint Paul (MN): Endurance Technologies, Inc. [accessed 2017 June 1].
[SDS] Safety Data Sheet. 2014a. (−)-Caryophyllene oxide. Oakville (ON): Sigma-Aldrich Canada Co. [accessed 2017 June 1].
[SDS] Safety Data Sheet. 2014b. EASYCAST EPOXY RESIN PART A [PDF]. Fields Landing (CA): Environmental Technology Inc. [accessed 2017 May 30].
[SDS] Safety Data Sheet. 2015a. EPOXY 1-GLK 2PK GARAGE FLOOR GRAY Kit Part A [PDF]. Concord (ON): Rust-Oleum Consumer brands Canada (RCBC). [accessed 2017 May 30].
[SDS] Safety Data Sheet. 2015b. Z-POXY FINISHING RESIN KIT – RESIN [PDF]. Ontario (CA): Pacer Technology. [accessed 2017 May 30].
[SDS] Safety Data Sheet. 2016. PC-SUPEREPOXY, Part A [PDF]. Allentown (PA): Protective Coating Company. [accessed 2017 May 30].
[SDS] Safety Data Sheet. 2017. ULTRABOND G-21 PART A [PDF]. Laval (QC): Mapei Inc. [accessed 2017 May 30].
Shell Chemical Company. 1956. The toxicology of glycidol and some glycidyl ethers. Microfiche No: OTS0523687. New Doc ID: 40-5640496. Old Doc ID: 42051 B3-206.
Shell Oil Company. 1984. Toxicity of fine chemicals: genotoxicity studies with allyl glycidyl ether with cover letter dated 103086. Microfiche No. OTS0513375. New Doc ID: 86870000029.
Shell Oil Company. 1991. Initial submission: histopathologic evaluation of tissues and organs from rats on a twenty-eight day oral toxicity evaluation of G23-09 (draft report) w-cover letter dated 102291. Microfiche No: OTS0535219. New Doc ID: 88-920000035. Old Doc ID: 8EHQ-1091-1389.
Sikov MR, Cannon WC, Carr DB, Miller RA. 1986. Reproductive toxicology of inhaled styrene oxide in rats and rabbits. Journal of Applied Toxicology. 6(3): 155-164.
Society of the Plastics Industry Inc. 1997a. Alkyl glycidyl ether: 2-week range finding and 13-week repeated dose dermal toxicity study in fischer 344 rats, with cover letter dated 10/29/97. Microfiche No: OTS0558896. New Doc ID: 44645. Old Doc ID: 42185 F1-18 44645.
Society of the Plastics Industry Inc. 1997b. Alkyl glycidyl ether: 13-week neurotoxicity study in fischer 344 rats, with cover letter dated 2/12/1998. Microfiche No: OTS0559329. New Doc ID: 44647. Old Doc ID: 42185 F1-021 44647.
Society of the Plastics Industry Inc. 1997c. Final report, bacterial reverse mutation assay with an independent repeat assay of alkyl glycidyl ether, with cover letter dated 11/18/97. Microfiche: OTS0558898. New Doc ID: 44645. Old Doc ID: 42185 F1-19 44645.
Society of the Plastics Industry Inc. 1997d. Final report, micronucleus cytogenetic assay in mice of alkyl glycidyl ether, with cover letter dated 5/16/97. Microfiche No: OTS0558882. New Doc ID: 44641. Old Doc ID: 42185 F1-16 44641.
Society of the Plastics Industry Inc. 1998. Final report, in vitro mammalian cell gene mutation test with independent repeat assay (alkyl glycidyl ether), with cover letter dated 3/18/1998. Microfiche No: OTS0559330. New Doc ID: 44648. Old Doc ID: 42185 F1-022 44648.
Sparks LE, Tichenor BA, Chang J, Guo Z. 1996. Gas-phase mass transfer model for predicting volatile organic compound (VOC) emission rates from indoor pollutant sources. Indoor Air. 6:31-30
Stofberg J, Grundschober F (1987). Consumption Ratio and Food Predominance of Flavouring Materials. Perfumer and Flavorist 12 (4): 27
[TDS] Technical Data Sheet. 2010. ULTRABOND G21 [PDF]. Laval (QC): Mapei Inc. [accessed 2017 May 30].
[TDS] Technical Data Sheet. 2012. EPOXYSHIELD [PDF]. Vernon Hills (IL): USA. Rust-Oleum Corperation. [accessed 2017 April 2]
Terrill JB, Lee KP, Culik R, Kennedy GL. 1982. The inhalation toxicity of phenylglycidyl ether: reproduction, mutagenic, teratogenic, and cytogenetic studies. Toxicology and Applied Pharmacology 64(2): 204-212.
Tosti A, Guerra L, Vincenzi C, Peluso AM. 1993. Occupational skin hazards from synthetic plastics. Toxicology and Industrial Health. 9(3): 493-502.
[US eCFR] Code of Federal Regulations Title 21. [database]. 2017. Search Results for beta-caryophyllene oxide. [updated 2016 Apr 1; accessed 2017 May 29].
[US EPA] US Environmental Protection Agency. 2005. Guidelines for carcinogen risk assessment. [PDF] Washington (DC): US EPA, Risk Assessment Forum. [accessed 2017 Jun 19].
[US EPA] US Environmental Protection Agency. 2010. Screening-level hazard characterization. Sponsored chemical: Alkyl (C12-C14) glycidyl ether (CAS RN 68609-97-2). Supporting chemical: Alkyl (C12-C13) glycidyl ether (CAS RN 120547-52-6) [PDF]. Washington (DC): US EPA, Office of Pollution Prevention and Toxics [accessed 2017 Jun 19].
Van Duuren. 1967. Carcinogenic epoxides, lactones and halo-ethers and their mode of action. Annals of the New York Academy of Sciences. 163(2): 633-651.
Van Duuren BL, Nelson N, Orris L, Palmes ED, Schmitt FL. 1963. Carcinogenicity of epoxides, lactones and peroxy compounds. Journal of the National Cancer Institute. 31(1): 41-55.
von der Hude W, Seelbach A, Basler A. 1990. Epoxides: comparison of the induction of SOS repair in Escherichia coli PQ37 and the bacterial mutagenicity in the Ames test. Mutation Research. 231(2): 205-218.
von der Hude W, Carstensen S, Obe G. 1991. Structure-activity relationships of epoxides: induction of sister chromatid exchanges in Chinese hamster V79 cells. Mutation Research. 249(1): 55-70.
Wade MJ, Moyer JW, Hine CH. 1979. Mutagenic action of a series of epoxides. Mutation Research. 66(4):367-371.
Weil CS, Condra N, Haun C, Striegel JA. 1963. Experimental carcinogenicity and acute toxicity of representative epoxides. American Industrial Hygiene Association Journal. 24(4): 305-325.
[WHO] World Health Organization. 1998. Concise International Chemical Assessment Document 8: Triglycidyl Isocyanurate [PDF]. Geneva (CH): World Health Organization.
[WHO] World Health Organization. 2006. Joint FAO/WHO Expert Committee on Food Additives [PDF]. 2006. Evaluation of certain food additives. Geneva (CH): World Health Organization. (WHO Technical Report Series 934). Sixty-fifth report of the JECFA.
Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. 2006. What are the sources of exposure to eight frequently used pthalic acid esters in Europeans? Risk Anal 26(3): 803-824.
Wigger-Alberti W, Hofmann M, Elsner P. 1997. Contact dermatitis caused by triglycidyl isocyanurate. American Journal of Contact Dermatitis. 8(2): 106-107.
Yoon JS, Mason JM, Valencia R, Woodruff RC, Zimmering S. 1985. Chemical mutagenesis testing in drosophila. IV. Results of 45 coded compounds tested for the National Toxicology Program. Environmental Mutagenesis 7(3): 349-367.
Zissu D. 1995. Histopathological changes in the respiratory tract of mice exposed to ten families of airborne chemicals. Journal of Applied Toxicology. 15(3): 207-213.
Appendix A. Considerations applied for the identification of relevant analogues
| Consideration | Rationale |
|---|---|
1. Chemical structure. Emphasis was placed on analogues with a benzene ring. The benzene ring may or may not be substituted with a short alkane group, but it must be substituted with a glycidyl ether or an epoxide group. | Analogues that have similar chemical structure are expected to have similar toxicity profiles. |
2. Similar metabolites (predicted or observed). The metabolism of o-CGE mainly occurs through GSH conjugation or through epoxide hydrolysis. In rats, the metabolite from GSH conjugation is o-cresyl glycidyl ether mercapturic acid (o-CGEMA). The metabolite from epoxide hydrolysis is a typical 1,2-diol metabolite, which undergoes subsequent oxidative reactions. | Analogues that are metabolized through similar pathways to similar degradation products are expected to have similar toxicity profiles. |
3. Common structural alerts | Analogues with similar structural alerts are expected to share greater similarity in terms of toxicity. |
4. Similar physical-chemical properties. Emphasis was placed on chemical structures with similar molecular weight, water solubility, vapour pressure, and log Ko/w. | Analogues with similar physical chemical properties may potentially share similar toxicological profiles. |
The analogues cresyl glycidyl ether (CGE, CAS RN 26447-14-3), phenyl glycidyl ether (PGE, CAS RN 122-60-1) and styrene oxide (SO, CAS RN 90-96-03) were taken into consideration on the basis of their similarity to o-CGE in terms of chemical structure, physical chemical properties, reactivity, and toxicokinetics. CGE exhibits the highest similarity to o-CGE and the available data associated with this substance was used first. CGE is a racemic mixture of different isomers of o-CGE, whereby the methyl functional group is situated at different positions of the aromatic ring relative to the glycidyl ether moiety. o-CGE was considered to be the predominant isomer manufactured in these mixtures (Gardiner et al. 1992). In the absence of CGE data however, toxicity data associated with the analogue PGE were taken into consideration. PGE and o-CGE only differ by presence or absence of a single methyl functional group on the aromatic ring. In addition, there is evidence demonstrating that PGE and o-CGE are metabolized through similar pathways (de Rooij et al. 1998). When data for PGE was not available, information from the next closest analogue (i.e., SO) was used to inform the data gap. SO and o-CGE differ by the presence or absence of a methyl group on the aromatic ring and an ether functional group separating the aromatic ring from the epoxide moiety.
Appendix B. Physical-chemical property values for o-CGE and its analogues
| Properties | Stryrene oxide CAS RN 96-09-3 | Phenyl glycidyl ether CAS RN 122-60-1 | o-Cresyl glycidyl ether CAS RN 2210-79-9 | Cresyl glycidyl ether CAS RN 26447-14-3 |
|---|---|---|---|---|
| MW (g/mol) | 120.15 | 150.18 | 164.20 | 165.21 |
| Vapour pressure (mm Hg) | 0.58b | 4.36 x 10-4b | 6.2x10-3b | 0.13a |
| Henry’s Law Constant (atm·m3/mol) | 1.78 x 10-5a | 2.16 x 10-5a | -6.1 | 5.29 x 10-4a |
| Water Solubility (mg/L) | 3000b | 3172a | 840b | 5.64 x 10-3a |
| LogKo/w (unitless) | 1.59-1.61a | 1.61-1.74a | 2.5b | 3.37a |
a Estimated using software (e.g., EpiSuite c2000-2012)
b Experimental
Appendix C. Estimated potential human exposure to substances in the Epoxide and Glycidyl Ether Group
Exposure scenarios were used to estimate the potential exposure to substances in the Epoxides Glycidyl Ethers Group; scenario assumptions are summarized in Table C-1. Exposures were estimated using ConsExpo Web or algorithms from the model (ConsExpo 2006, ConsExpo Web 2016), unless noted otherwise. An overall retention factor (RF) of 1 was used unless otherwise specified.
Exposures were estimated for different age groups based upon body weights (BW) from Health Canada’s exposure factors for the general population of Canada (Health Canada 1998):
Adults (20–59 years): 70.9 kg
| Substance(s) | Exposure scenario | Assumptions |
|---|---|---|
o-CGE | Epoxy flooring adhesive | Model: ConsExpo Web (2016) Inhalation model: Exposure to vapour, evaporation C: 2.5% (SDS 2017) F: 0.25 per year Exposure duration (min): 75 Product amount (kg): 10 (TDS 2010) Room Volume (m3): 58 Ventilation Rate (/hour): 0.5 Inhalation rate: 1.53 m3/hr Mass Transfer Coefficient (m/hr): 2.10a (Sparks et al 1996, as cited in McCready & Fontaine 2010) Molecular weight matrix: 3000 g/mol Release area (m2): 4 Dermal model: Exposed area (cm2): 110 Contact Rate (mg/min): 30 Release duration (min): 75 Dermal uptake: 1 |
o-CGE | Epoxy floor coating | Model: ConsExpo Web (2016) Inhalation model: Exposure to vapour, evaporation C: 2.5% (SDS 2015a) F: 0.33/year Product amount: 4850 g (TDS 2012) Exposure duration: 135 min Product amount (kg): 4.85 Room volume: 34 m3 Ventilation rate: 2 air exchanges per hour Inhalation uptake of 100% Inhalation rate: 1.53 m3/hr Mass Transfer Coefficient (m/hr): 1.55a (Sparks et al 1996, as cited in McCready & Fontaine 2010) Molecular weight matrix: 3000 g/mol Release area (m2): 23 Dermal model: Exposed area: 228 cm2 (half of one hand) Contact Rate: 30 mg/min Release duration: 120 min Dermal uptake: 1 |
o-CGE | Two-component epoxy coating/sealant | Model: ConsExpo Web (2016) Inhalation model: Exposure to vapour, evaporation C: 15% (SDS 2015b) F: 1/year Product amount (g): 400 Exposure duration (min): 132 (B) Room volume (m3): 20 Ventilation rate: 0.6 air exchanges per hour Inhalation uptake of 100% Inhalation rate: 1.53 m3/hr Mass Transfer Coefficient (m/hr): 2.64a (Sparks et al 1996, as cited in McCready & Fontaine 2010) Molecular weight matrix: 3000 g/mol Release area (m2): 0.5 Dermal model: Exposed area (cm2) : 228 Contact Rate mg/min: 30 Release duration (min): 30 Dermal uptake: 1 |
o-CGE | Arts & Craft/ Hobby resin | Model: ConsExpo Web (2016) C: 15% F: 52/year Product amount: 205 g Inhalation model: Exposure to vapour, evaporation Exposure duration(min): 240 Product amount (g): 205 Room volume (m3): 20 Ventilation rate: 0.6 air exchanges per hour Inhalation uptake of 100% Inhalation rate: 1.53 m3/hr Mass Transfer Coefficient (m/hr): 4.93a (Sparks et al 1996, as cited in McCready & Fontaine 2010) Molecular weight matrix: 3000 g/mol Release area (cm2): 178 Dermal model: Exposed area: 2 cm2 (fingertips) Product amount (g): 0.05 Dermal uptake: 1 |
C12-C13 AGE | Two-component epoxy adhesive | Model: ConsExpo Web (2016) C: 2.5% Product amount (g): 20 Inhalation model: Exposure to vapour, evaporation Exposure duration (min): 240 Room volume (m3): 20 Ventilation rate: 0.6 air exchanges per hour Inhalation uptake of 100% Inhalation rate: 1.53 m3/hr Mass Transfer Coefficient (m/hr): 3.22a (Sparks et al 1996, as cited in McCready & Fontaine 2010) Molecular weight matrix: 3000 g/mol Release area (m2): 0.05 Dermal model: Exposed area (cm2): 43 Product amount (g): 0.1 |
C12-C13 AGE | Epoxy filler from a tube | Model: ConsExpo Web (2016) C: 5% Product amount: 40 g Inhalation model: Exposure to vapour, evaporation Exposure duration(min): 240 Room volume (m3): 20 Ventilation rate: 0.6 air exchanges per hour Inhalation uptake of 100% Inhalation rate: 1.53 m3/hr Mass Transfer Coefficient (m/hr): 3.79a (Sparks et al 1996, as cited in McCready & Fontaine 2010) Molecular weight matrix: 3000 g/mol Release area (m2): 0.012 Dermal model: Exposed area: 228 cm2 (half of one hand) Product amount (g): 0.05 |
C12-C13 AGE | Coating with low-viscosity resin | Model: ConsExpo Web (2016) Inhalation model: Exposure to vapour, evaporation C: 30% F (per year): 0.33 Product amount (g): 220 Exposure duration (min): 240 Room volume (m3): 34 Ventilation rate: 0.6 air exchanges per hour Inhalation uptake of 100% Inhalation rate: 1.53 m3/hr Mass Transfer Coefficient (m/hr): 1.78a (Sparks et al 1996, as cited in McCready & Fontaine 2010) Molecular weight matrix: 3000 g/mol Release area (m2): 2 Dermal model: Exposed area: 228 cm2 Contact rate (mg/min): 30 Release duration (min): 30 Dermal uptake: 1 |
BCPO | Dermal exposure, body lotion/cream/moisturizer | C: < 0.2ppm (ECCC 2016b) F : 1.1/day for adults (Loretz et al. 2006), 1.7/day for infants (Wormuth et al. 2006) Product amount per application (mean): 4.4 g for adults (Loretz et al. 2005) and 1.4 g for infants (Wormuth et al. 2006) Dermal absorption (%): 100 |
BCPO | Inhalation exposure spray perfume (aerosol) | Model: ConsExpo Web (2016) C: <0.2ppm (ECCC 2016b) Exposure duration: 5 min Released mass: 0.5 g Room Volume: 10 m3 Inhalation rate: 1.53 m3/hr Ventilation rate: 0 |
a Estimated based on an assumed characteristic length equal to the square root of the release area for each scenario.
Lifetime average daily dose (LADD)
The LADD was calculated to estimate lifetime exposure to o-CGE from its use in arts and craft/hobby resin, cited from Health Canada (2013). The assumption that adults will be exposed to the product for 50 years is considered to be conservative, and the risk characterization is considered to be protective of exposures that may occur in individuals under 20.
The equation is as follows:
LADD = [(DSEinfant x ADinfant) + (DSEtoddler x ADtoddler) + (DSEchild x ADchild) + (DSEteen x ADteen) + (DSEadult x ADadult)] / [AL]
Where:
DSE= daily systemic exposure
Average lifetime (AL) = 70 years
Age group durations (AD)= 50 years for adults (20+ years)
Appendix D. BMC modelling of data from a 2-year study investigating the carcinogenic effects of PGE exposure to rats (Lee et al. 1983)
Benchmark concentration (BMC) modelling was conducted using the US EPA Benchmark Dose Software (BMDS, version 2.5). The results from 10 models available in the software are presented in Table D-1. A best-fit model was selected based upon considerations of the P-value, scaled residuals, Akaike’s Information Criterion (AIC) value, and visual inspection of the model fit. The LogLogistic, LogProbit, and Weibull models yielded optimal fit with scaled residuals of 0, comparable AIC values, and visual inspection. As a conservative measure, the LogProbit model was selected as it provided the lowest BMCL estimate (i.e., 11.4 ppm).
| Model name | AIC | P-value | BMC | BMCL | Scaled residual for dose group near BMC |
|---|---|---|---|---|---|
| Gamma | 112.146 | NA | 13.0155 | 11.5035 | 0.001 |
| Logistic | 110.451 | 0.266 | 13.2218 | 11.7009 | 0.014 |
| LogLogistic | 112.146 | NA | 12.6436 | 11.5315 | 0 |
| LogProbit | 112.146 | NA | 13.3094 | 11.4026 | 0 |
| Multistage-Cancer | 112.186 | 0.1567 | 18.4445 | 11.8606 | 0.294 |
| Multistage-Cancer | 110.323 | 0.3032 | 14.2987 | 11.4782 | 0.025 |
| Multistage-Cancer | 110.161 | 0.3218 | 13.4565 | 11.5492 | 0.002 |
| Probit | 110.52 | 0.2562 | 13.5611 | 11.6255 | 0.022 |
| Weibull | 112.146 | NA | 12.6217 | 11.5511 | 0 |
| Quantal-Linear | 112.186 | 0.1567 | 18.4445 | 11.8606 | 0.294 |
NA: Not applicable. The p-values associated with these models were close to or equal to 1
