Screening assessment - Fatty amides group
Official Title: Screening Assessment Fatty Amides Group
Chemical Abstracts Service Registry Numbers: 112-84-5, 301-02-0, 68784-17-8
Environment and Climate Change Canada
Health Canada
April 2019
Cat. No.: En14-373/2019E-PDF
ISBN 978-0-660-30371-0
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment on three of twelve substances referred to collectively under the Chemicals Management Plan as the Fatty Amides Group . These three substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA. Five of the twelve substances were subsequently determined to be of low concern through other approaches, and decisions for these substances are provided in a separate reportFootnote 1. Additionally, four substances were placed into other substance groups, to which they were more appropriately suited, on the basis of structural features and/or functionalities of toxicological significanceFootnote 2. The three substances addressed in this screening assessment will hereinafter be referred to as the Fatty Amides Group.
| CAS RNa | Domestic Substances List (DSL) name | Common name |
|---|---|---|
| 112-84-5 | 13-Docosenamide, (Z)- | Erucamide |
| 301-02-0 | 9-Octadecenamide, (Z)- | Oleamide |
| 68784-17-8b | Isooctadecanoic acid, reaction products with tetraethylenepentamine | IODA reaction products with TEPA |
Erucamide and oleamide are naturally occurring substances that may be produced in the environment by abiotic processes (e.g., forest fires) or by biota. In 2011, they were not reported to be manufactured in Canada, but were imported for use primarily for the manufacture of plastic products and rubbers. In the same year, between 1 000 000 and 10 000 000 kg of erucamide and between 100 000 and 1 000 000 kg of oleamide were imported into Canada. The presence of erucamide and oleamide in environmental media, food or products may result from natural or anthropogenic sources.
IODA reaction products with TEPA is not naturally occurring. In 2011, IODA reaction products with TEPA were not reported to be manufactured in Canada and between 100 and 1 000 kg of IODA reaction products with TEPA was imported into Canada. Uses of IODA reaction products with TEPA in Canada are limited to lubricants and greases, primarily as components in 2-cycle marine outboard engine oils. Releases to the environment of this substance are expected to be minimal from industrial and consumer.
The ecological risks of erucamide, oleamide, and IODA reaction products with TEPA were characterized using the ecological risk classification (ERC) of organic substances, which is a risk-based approach that employs multiple metrics for both hazard and exposure based upon weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. . Based on the outcome of ERC analysis,erucamide, oleamide, and IODA reaction products with TEPA are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from erucamide, oleamide, and IODA reaction products with TEPA. It is concluded that erucamide, oleamide, and IODA reaction products with TEPA do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Erucamide and oleamide exhibit low acute toxicity and are not genotoxic. No adverse effects were noted for erucamide in repeated-dose and developmental toxicity studies in laboratory animals and erucamide is therefore considered to be of low hazard potential. Limited health effects information was available for oleamide, however erucamide and oleamide are similar with respect to their chemical structure, physical and chemical properties and toxicokinetics and oleamide is similarly expected to be of low hazard potential.
IODA reaction products with TEPA exhibits low acute toxicity and is not genotoxic. No adverse health effects were noted in a short-term repeated dose toxicity study or in a combined developmental and reproductive toxicity study. IODA reaction products with TEPA is expected to be of low hazard potential.
Considering the low toxicity of erucamide, oleamide, and IODA reaction products with TEPA, the potential risk to human health is considered to be low.
On the basis of information presented in this screening assessment, it is concluded that erucamide, oleamide, and IODA reaction products with TEPA do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that erucamide, oleamide, and IODA reaction products with TEPA do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment on three of twelve substances, referred to collectively under the Chemicals Management Plan as the Fatty Amides Group, to determine whether these three substances present or may present a risk to the environment or to human health. The three substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 20017]).
Five of the other nine substances (listed in Table 1‑1, below) were considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a) and via the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018), and were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this report. Conclusions for these five substances are provided in the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018).
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
The remaining four substances were placed into other substance groups, to which they are more appropriately suited, on the basis of structural features and/or functionalities of toxicological significanceFootnote 3.
The three substances were assessed in this report (hereinafter referred to as the Fatty Amides Group) on the basis of differences in structural features, physical and chemical properties, sources and uses. Erucamide and oleamide were assessed together and IODA reaction products with TEPA was assessed independently.
The ecological risks of erucamide, oleamide, and IODA reaction products with TEPA were characterized using the ERC (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure. Relevant data were identified up to June 2017. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA, by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 4. This screening assessment presents the critical information and considerations on which the conclusion is based.
2. Characterization of ecological risk
The ecological risks of the substances in this screening assessment were characterized using the ecological risk classification of organic substance (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium [e.g., median lethality (LC50)] for characterization. Since IODA reaction products with TEPA is a UVCB substance and could not be suitably represented by a single chemical structure, a manual judgement-based approach to classification was used. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical and chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox), and from responses to surveys conducted under section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were established based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of the UVCBs, hazard and exposure could not be fully profiled due to the lack of a representative structure to estimate needed properties, and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed by examining the UVCB constituents and information obtained from section 71 surveys under CEPA and making decisions on the basis of consideration of similar substances and application of expert judgement.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERCs thus reflect exposure and risk in Canada on the basis of what is believed to be the current use quantity, and may not reflect future trends.
3. Erucamide and oleamide
3.1 Identity of substances
The CAS RNs, DSL names and common names for erucamide and oleamide are presented in Table 3‑1.
| CAS RN | DSL name (common names) | Chemical structure and molecular formula | Molecular weight (Da) |
|---|---|---|---|
| 112-84-5 |
13-Docosenamide, (Z)- (Erucamide; erucyl amide; erucic acid amide) | 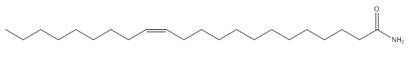 C22H43NO C22H43NO |
337.58 |
| 301-02-0 |
9-Octadecenamide, (Z)- (Oleamide; oleyl amide; oleic acid amide) |
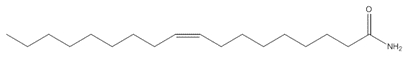 C18H35NO C18H35NO |
281.4 |
3.1.1 Use of read-across and (Q)SAR models
The read across was supported by work completed for Health Canada under contract (RSI, 2017). Read-across using erucamide to address the data gaps of oleamide was considered appropriate as they are structurally similar (both are simple aliphatic unsaturated primary fatty amides) with similar physical and chemical properties (both highly lipophilic) and toxicokinetics (both are known to undergo metabolic hydrolysis to produce corresponding fatty acids).
3.2 Physical and chemical properties
A summary of physical and chemical properties of erucamide and oleamide are presented in Table 3‑2 and Table 3‑3, respectively. When experimental information was limited or not available for a physical-chemical property, models were used to generate predicted values for the substance. Additional physical and chemical properties are presented in ECCC (2016b).
Property |
Value |
Measured or predicted |
Key reference(s) |
|---|---|---|---|
Physical state |
Solid |
N/A |
US EPA 2010a |
Melting point (°C) |
77.5 |
Measured |
SRC 2010 cited in US EPA 2010a |
Boiling point (°C) |
461.05 |
Predicted |
(MPBPWIN 2008) |
Vapour pressure (mm Hg) |
8.2 × 10−8 |
Predicted |
(MPBPWIN 2008) |
Henry’s law constant (atm·m3/mol) |
2.8 × 10−6 |
Predicted |
(HENRYWIN 2008) |
Water solubility (mg/L) |
4.5 × 10−4 |
Predicted |
(WSKOWWIN 2010) |
Log Kow (dimensionless) |
8.44 |
Predicted |
(KOWWIN 2010) |
| Property | Value | Measured or predicted | Key reference(s) |
|---|---|---|---|
| Physical state | Solid | N/A | US EPA 2010a |
| Melting point (°C) | 76 | Measured | US EPA 2010a |
| Boiling point (°C) | 414.63 | Predicted | (MPBPWIN 2008) |
| Vapour pressure (mm Hg) | 1.2 × 10−6 | Predicted | (MPBPWIN 2008) |
| Henry’s law constant (atm·m3/mol) | 9.2 × 10−7 | Predicted | (HENRYWIN 2008) |
| Water solubility (mg/L) | 0.037 | Predicted | NOM05 1987 cited in US EPA 2010a |
| Log Kow (dimensionless) | 6.48 | Predicted | (KOWWIN 2010) |
3.3 Sources and uses
Fatty amides occur naturally and may be produced by abiotic (i.e., combustion) and biotic processes. Fatty amides are synthesized commercially either from reactions of fatty acids with ammonia at elevated temperature and pressure followed by dehydration, or from fatty esters by ammonolysis (Johansson 2001). Erucamide and oleamide are derived from erucic acid and oleic acid, respectively, which are both naturally occurring omega-9 unsaturated fatty acids with high abundance in vegetable/seed oils (e.g., canola/rapeseed) (Johansson 2001). Alkyl amides can be produced from reactions between fatty acids and ammonia during biomass burning (e.g., forest fires) and cooking with oils (Simoneit et al. 2003). Erucamide has been detected in ambient air in Chile (Simoneit et al. 2003) and both erucamide and oleamide have been detected in house dust in Canada (NRC 2011).
Erucamide and oleamide are primary amides with C21 and C17 alkenyl chains, respectively. On the basis of information submitted pursuant to a survey conducted under section 71 of CEPA (Environment Canada 2013) erucamide and oleamide were not reported to be manufactured in Canada above the reporting threshold of 100 kg and are only imported into Canada. Table 3-4 presents a summary of the reported total import quantities for erucamide and oleamide (Environment Canada 2013).
| Common name | Total import range (kg) |
|---|---|
| Erucamide | 1,000,000 – 10,000,000 |
| Oleamide | 100 000 – 1 000 000 |
a Values reflect quantities reported in response to a survey conducted under section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (Schedules 2 and 3).
According to information reported under the section 71 survey, oleamide and erucamide are used in a variety of sectors in Canada, but primarily in the manufacture of plastic and rubber materials (Environment Canada 2013). They function commonly as slip and anti-block agents; these are added to plastics formulations where they gradually bloom to the surface of the material forming a solid lubricating layer at the surface which lowers the friction/adhesion between the polymer and contacting surfaces (Crodapolymeradditives.com c2008; Cooper and Tice 1995).
Table 3‑5 presents a summary of the uses of erucamide and oleamide according to information reported pursuant to the section 71 survey (Environment Canada 2013).
| Use | Erucamide | Oleamide |
|---|---|---|
| Automotive, aircraft and transportation | Y | Y |
| Automotive care | Y | N |
| Compostable bags, compounding | Y | N |
| Food packaging | Y | Y |
| Ink, toner and colourants | Y | N |
| Lubricants and greases | N | Y |
| Plastic and rubber materials | Y | Y |
| Polymer production | Y | N |
| Toys, playground and sporting equipment | Y | N |
Abbreviations: N, no; Y, yes
Table 3‑6 includes information on additional uses of erucamide and oleamide in Canada.
| Use | Erucamide | Oleamide |
|---|---|---|
| Food packaging materialsa | Y | Y |
| Natural Health Products Ingredients Databasea | N | Y |
| Formulant in pest control products registered in Canadab | Y | Y |
Abbreviations: N, no; Y, yes
a Personal communication, emails from the Health Products and Food Branch, Health Canada, to the Existing Substances Risk Assessment Bureau, dated January 21, 2016; unreferenced.
b November 2015 email from the Pest Management Regulatory Agency, Health Canada to the Existing substances Risk Assessment Bureau (ESRAB), Health Canada; unreferenced
In addition to the uses reported above, international use information indicates use of erucamide and/or oleamide in a number of applications including: defoamers in industrial and household synthetic detergent formulations; opacifiers and viscosity control agents in cosmetics; solubilizers for dyestuffs; water repellents in textiles; and wax additives/solvents (Milne 2005; Ash and Ash 2008).
3.4 Potential to cause ecological harm
Critical data and considerations used to develop the substance-specific profiles for erucamide and oleamide and the hazard, exposure and risk classification results are presented in ECCC (2016b).
According to information considered under ERC, erucamide was classified as having a high exposure potential on the basis of long overall persistence and a large annual import quantity according to information reported under a section 71 survey (Environment Canada 2013). According to information considered under ERC, oleamide was classified as having a moderate exposure potential on the basis of a long overall persistence and a moderate annual import quantity according to information reported under a section 71 survey (Environment Canada 2013). The ERC classified both substances as having a low hazard potential and subsequently having a low potential for ecological risk. It is unlikely that these substances are resulting in concerns for the environment in Canada.
3.5 Potential to cause harm to human health
3.5.1 Exposure assessment
The following section provides general information on exposure to erucamide and oleamide. As erucamide and oleamide are considered to be of low hazard potential quantitative estimates of exposure to the general population were not derived.
Environmental media and food
Erucamide and oleamide have not been measured in indoor air, ambient air, soil or drinking water in Canada, but have been detected in household dust in Canada (NRC 2011), and erucamide has been detected in bottled drinking water in the United States (US) (Naidenko et al. 2008). Erucamide and oleamide were quantified in household floor dust in Canada at mean concentrations of 78.6 and 5.3 µg/g, respectively (NRC 2011). Erucic acid and oleic acid are major fatty acids in vegetable/seed oils which can convert in part to their corresponding amides during cooking/deep-frying by reaction with ammonia. Under the Food and Drug Regulations, C22 monoenoic fatty acids (erucic acid) is not permitted at over 5% of the proportion of total fatty acids in cooking oils, salad oils, margarines, and shortening or foods that resemble margarine or shortening (Canada, 1978). Erucamide was detected in the aerosol fraction of ambient air in Santiago, Chile at concentrations ranging from 2 to 1072 ng/m3, reflecting inputs from cooking with seed oils (Simoneit et al 2003). Erucamide and oleamide have very low vapour pressures and so are not expected to be present in the gas phase of air. While erucamide and oleamide have not been reported in Canadian drinking water systems, erucamide was detected in one of nine brands of bottled drinking water tested in the U.S., at a concentration of 1.2 µg/L (Naidenko et al. 2008).
Erucamide and oleamide are not listed as approved food additives in Canada in the Lists of Permitted Food Additives, which have been incorporated by reference into their respective Marketing Authorizations issued under the authority of the Food and Drugs Act (Health Canada [modified 2016 Sept 9]). Erucamide and oleamide are used in the manufacture of certain food packaging materials, but only erucamide has potential for direct food contact by migration. Oleamide may be present as a component in lubricants used on non-food contact surfaces during food processing, but exposure to the general population through this use is not expected (Personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, dated January 21, 2016; unreferenced.).
A European study looked at migration of these two substances to a milk simulant, using the hot fill method for a wide selection of baby bottles on the market, mostly in European Union member states, but also in Canada, the US and Switzerland (Simoneau et. al. 2012). Erucamide migrated from 9 of 149 polypropylene baby bottles (average 303 µg/kg) and oleamide from 2 of 149 polypropylene bottles (average 1357 µg/kg) and 2 of 5 silicone bottles (average 116 µg/kg) (Simoneau et. al. 2012).
Products available to consumers
No publicly available Material Safety Data Sheets (MSDSs) were identified for erucamide or oleamide. However, due to their use as additives in various plastics (e.g., PP, PE, LDPE, PVC, silicone, EVA) and their functional ability to bloom to the product’s surface (Cooper and Tice 1995) exposure of the general population to erucamide and oleamide is expected to occur through the oral and dermal routes from use of products available to consumers. Danish studies have quantified levels of erucamide in the stretch closure part of diapers (max 82 µg/g, DCM extract), the outer material of toddlers' jackets (380 µg/g, artificial saliva simulant) and the plastic fibre (blade portion) of artificial turf (88-177 µg/g, DCM extract); and oleamide in the outer material in children’s mitts (12-83 µg/g, artificial saliva simulant), fetish clothing (max 330 µg/g) and cotton duvet and pillow case bed linens (8 µg/g, prewash, ASE with DCM) (Nilsson et al. 2006; Nilsson et al. 2008; Tønning et al. 2009). Published data from a Japanese manufacturer of disposable diapers indicates inclusion of erucamide in the product at concentrations ranging from 0.2 to 0.4% (i.e. approx. 2000 to 4000 µg/g) (Nonwoven 2003).
Oleamide is listed in the Natural Health Products Ingredients Database with a medicinal role as classified as an NHP under Schedule 1, item 2 (an isolate) of the Natural Health Products Regulations; however, it is not listed in the Licenced Natural Health Products Database as being present in currently licensed natural health products in Canada (Personal communication, emails from the Health Products and Food Branch, Health Canada, to the Existing Substances Risk Assessment Bureau, dated January 21, 2016; unreferenced).
3.5.2 Health effects assessment
Toxicokinetics
Due to their low vapour pressure, inhalation is not expected to be a significant route of exposure.
Erucamide was tested in a four-week digestibility study in Sprague-Dawley (SD) rats. The substance was administered at a level of 10% in a semi-synthetic diet without added fat. Pooled weekly fecal samples were weighed and analyzed for their fat content. The analysis showed absorption of erucamide from the gastrointestinal tract ranged from 52.8 to 72.9% throughout the four week study period (Anonymous 1960a, as cited in ECHA c2007-2016a). However, absorption of the parent amide could be limited as they are expected to undergo hydrolysis to form their respective fatty acid and ammonia in the gastrointestinal tract. In a hydrolysis study, approximately 95% loss of oleamide was measured after a four-hour incubation at 37°C in simulated gastric fluid containing bile salts. Stoichiometric formation of oleic acid was observed (Cooper et al. 1995). The parent amide is also likely to undergo metabolic hydrolysis. In an experiment to examine liver metabolism of erucamide, the compound was incubated with fresh rat liver homogenate to determine the degree of hydrolyzation to the respective fatty acid (erucic acid) and ammonia. Erucamide was found to be efficiently hydrolyzed by rat liver homogenate, with 37.6% hydrolyzed in four hours (Anonymous 1960b, as cited in ECHA c2007-2016a). Both substances are known substrates of fatty acid amide hydrolase (FAAH)-mediated hydrolysis. The relative rates of hydrolysis are similar and have been reported to be 1 to 0.83 for oleamide to erucamide, the reference rate being equal to 0.526 nmol/min/ng FAAH (Boger et al. 2000).
Genotoxicity
Erucamide and oleamide have been tested in Organisation for Economic Co-operation and Development (OECD) guideline studies for genotoxicity and are not mutagenic or clastogenic. Bacterial mutagenicity studies with erucamide or oleamide using Salmonella typhimurium (TA98, TA100, TA1535, TA1537 and TA1538) were negative when tested with and without metabolic activation ((Jones et al. 1990a and Jones et al. 1990b, as cited in ACC 2004); JETOC 2008, as cited in CCRIS 2011). Similarly, oleamide was also negative when tested in Escherichiacoli (JETOC 2008, as cited in CCRIS 2011). In mammalian in vitro systems, erucamide did not induce mutations in mouse lymphoma L5178Y cells (Anonymous 2010a, as cited in ECHA c2007-2016a), and no chromosomal aberrations were observed in Chinese Hamster Lung cells with and without metabolic activation (Anonymous 2010b, as cited in ECHA c2007-2016a).
Acute and repeated-dose toxicity
Erucamide and oleamide both exhibit low acute toxicity in OECD guideline studies. Erucamide was administered via gavage to adult Wistar rats of both sexes in corn oil at a total dose of 5000 mg/kg-bw (dosed twice at 2500 mg/kg-bw within 24 hours). The animals were observed for a period of 14 days. No mortalities occurred during the observation period. At the end of the study all animals were sacrificed and macroscopic examination did not show any treatment-related effects (Reijnders JBJ 1988, as cited in ACC 2004). Erucamide was dermally applied at 2000 mg/kg-bw for a period of 24 hours to male and female rats. The animals were observed over a period of 14 days with no effects on body weight or clinical signs noted. Slight desquamation was noted in a single male. No deaths occurred during the course of the study and no macroscopic findings were apparent at necropsy (Anonymous 2010c, as cited in ECHA c2007-2016a). In a study summary dossier for oleamide submitted to the European Commission, the LD50 cited across multiple studies ranges from >2000 to >10 000 mg/kg-bw in rats, although study details are lacking for all sources cited (European Chemical Bureau 2000).
A study summary of an OECD guideline 90-day repeated-dose study was submitted to the European Chemicals Agency (EChA) under the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Erucamide was administered to male and female Wistar rats via gavage at a dose level of 0, 100, 300 or 1000 mg/kg-bw/day using a corn oil vehicle. No mortalities or specific non-transient treatment related clinical signs of toxicity were noted for any dose group. A slight effect on mean body weight was observed in females of the mid- and high-dose groups when compared with control (101 and 106%, respectively) that correlated with a slight non-statistically significant increase in food consumption in these groups. Decrease in observed platelet counts was noted in mid- and high-dose males, but the change was not dose dependent and the authors noted they are within historical controls. Likewise, the statistically significant lower mean blood cholesterol level of the mid-dose male group alone was considered incidental. No effects were noted in neurobehavioral tests at the end of the study period. In females, there was a statistically significant increase in absolute spleen weight in the high-dose group and relative spleen weights (to brain weight) in the low- and high-dose groups when compared with the controls. The increase was described as minimal. There were no gross pathological or histopathological findings in any examined organs including the spleen, therefore the spleen weight change is not considered to be adverse. Based upon the available information in the summary and the lack of any toxicologically relevant findings at any dose described, the no-observed-adverse-effect level (NOAEL) is considered to be >1000mg/kg-bw/day (Anonymous 2015a, as cited in ECHA c2007-2016a).
Other oral repeated-dose non-guideline rat studies of shorter duration with a dose of 7500 mg/kg-bw/day over 5 days (Molnar 1960, as cited in ACC 2004) and at 10% of diet (~5000 mg/kg-bw/day) over 4 weeks (Anonymous 1960a, as cited in ECHA c2007-2016a) also showed no significant toxicological findings.
Reproductive and developmental toxicity
The previously summarized OECD guideline 90-day repeated-dose study for erucamide included examination of reproductive tissues and organs, as well as fertility parameters. There were no effects on sperm count or morphology. There was a lower percentage of motile and rapidly moving epididymal sperm and a higher static count in two animals of the low-dose group, with no effect seen in the higher dose groups. In the absence of consistency and dose dependency, this finding is considered to be potentially spontaneous and is likely not related to the substance. In female animals, erucamide had no effect on the estrous cycle (Anonymous 2015a, as cited in ECHA c2007-2016a).
Reproductive studies of erucic acid (the metabolite of erucamide) and oleic acid (the metabolite of oleamide) were identified and examined. A study was conducted to examine the influence of erucic acid and oleic acid on fertility in rats (Carroll and Noble 1957). In this study, the male rats (2-6 per group) were on diets with 15% erucic acid or 15% oleic acid (equivalent to 7500 mg/kg-bw/day) for a period of approximately 3 months prior to mating with females (3-12 per group) on the same diet and duration as males or with females on the normal diet without fatty acid supplements. Reduced fertility was observed in males on the diet containing 15% erucic acid when mated with females on the same diet or with females on the normal diet. In contrast, the males on the diet supplemented with 15% oleic acid did not show impairment of fertility. Reyes et al. (1995) observed that the feeding of male and female weanling Wistar rats and Golden Syrian hamsters with a diet containing 25% rapeseed oil (41.5% erucic acid, calculated to be approximately 5200 mg/kg-bw/day) for a period of 90 days prior to mating was not associated with any apparent adverse reproductive or developmental effects. Given the excessive dosing used in the Carrol and Noble (1957) study, the absence of fertility effects observed in the study with rapeseed oil, and the lack of observed effects on the reproductive parameters in the 90-day OECD guideline study up to the limit dose, the substances are unlikely to be reproductive toxicants.
A study summary of an OECD guideline developmental toxicity study was submitted to EChA under REACH. Erucamide was administered to pregnant female Wistar rats at a dose level of 0, 100, 300 or 1000 mg/kg-bw/day during gestational days 5 to19, with sacrifice occurring on gestational day 20. No treatment-related maternal toxicity was noted and no effects were observed for gravid uterine weight, number of corpora lutea, implantations, resorptions, percent pre-implantation loss, and percent post-implantation loss in any dose group. For fetal examinations, no treatment-related effects were observed for sex ratio, number of live or dead fetuses, or mean number of fetuses when compared with controls. Litter weights were generally not affected, with only a statistically significantly lower female litter weight observed (a decrease of 27%) in the low-dose group when compared with controls. As this observation lacked dose dependence and was only shown in females, it is likely incidental in nature. A statistically significant increase in the litter incidence of incomplete ossification of the interparietal bone in the low-dose group was observed, but not in the mid- and high-dose groups. An increased litter incidence of dark discolouration of the right lobe of the liver was observed in the mid-dose groups, but not in the low or high-dose groups. Both of these effects did not exhibit dose dependency and are likely not toxicologically significant. Based upon the information available in the study summary, and the lack of any developmental findings at any dose described, the NOAEL is considered to be >1000mg/kg-bw/day (Anonymous 2015b, as cited in ECHA c2007-2016a).
3.5.3 Characterization of risk to human health
Erucamide and oleamide are not genotoxic and exhibit low acute toxicity. No critical effects were noted for erucamide up to the limit dose of 1000 mg/kg-bw/day in guideline repeated-dose and developmental toxicity studies. Considering that erucamide is not genotoxic and the absence of any significant gross pathological or histopathological effects in the repeated-dose study up to the limit dose, carcinogenicity is not expected to be a concern. Moreover, no effects were observed on the reproductive organs or parameters examined in this study. Limited toxicity data were available for oleamide. However, as erucamide and oleamide are similar when compared to their chemical structure, physical and chemical properties and toxicokinetics, read-across was applied to conclude that oleamide, similar to erucamide, is likely to exhibit low toxicity.
As erucamide and oleamide are considered to be of low hazard potential, derivation of estimates of exposure from sources and uses identified were not considered meaningful and risk to human health is considered to be low.
3.5.4 Uncertainties in evaluation of risk to human health
Studies investigating the health effects of oleamide are limited and read-across from erucamide was applied to address the human health data gaps. This approach increases the uncertainty for the assessment of risk to human health.
4. IODA reaction products with TEPA
4.1 Identity of substances
The CAS RN and DSL name for IODA reaction products with TEPA, an UVCB substance, is presented in Table 4‑1. According to a test plan submitted to the United States Environmental Protection Agency High Production Volume Challenge program, the substance is synthesized by combining liquid IODA with a highly refined lubricant base oil diluent, followed by addition of TEPA (ACC 2003). The chemical structure of the reaction product is described as variable polyamides where the attachments of the fatty acids (n=1-3) occur at various positions (nitrogen at positions 1, 7 and/or 13, respectively) on the polyamine reactant. The substance is not isolated from the refined base oils, but rather blended with other additives where it is used to formulate finished lubricating oils (the concentration of this substance can range from 9 to 34 wt% (ACC 2003). Details on the composition of various components of this substance are not available, including the proportions of unreacted starting material (if any) that may remain in the finished lubricating oil. However, in a publically available dossier submitted to EChA under REACH, the registrant indicated that CAS RN 68784-17-8 is a polyamide dispersant where the average molecular weight is > 1000 Daltons (ECHA c2007-2016b). Representative structures of the components in the mixture are also presented in Table 4‑1.
| CAS RN | DSL name |
Representative structures of starting materials and reaction products | Molecular weight (Da) |
|---|---|---|---|
| 68784-17-8a |
Isooctadecanoic acid, reaction products with tetraethylenepentamine | 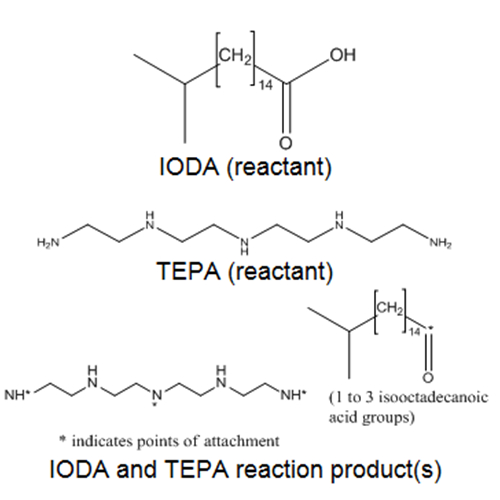 |
284 IODA (reactant) 189 TEPA (reactant) Avg. approx. >1000 IODA and TEPA reaction product(s) |
Abbreviations: IODA, isooctadecanoic acid; TEPA, tetraethylenepentamine
a CAS RN 68784-17-8 is a UVCB substance (unknown or variable composition, complex reaction products, or biological materials).
4.2 Physical and chemical properties
A summary of physical and chemical properties of IODA reaction products with TEPA is presented in Table 4-2 for potentially major reaction products. Reaction of IODA with TEPA will produce a mixture of polyamides with an average molecular weight of approximately 1000 Da. When experimental information was limited or not available for a physical-chemical property, models were used to generate predicted values for the substance. Experimental information is for the mixture and modelled data represents a range of physical and chemical properties for possible products based upon the representative structures in Table 4‑2 (i.e., TEPA products with 1 to 3 IODA groups attached). Additional physical chemical properties are presented in ECCC (2016b).
| Property | Value or rangea | Type of data | Key reference(s) |
|---|---|---|---|
| Physical state | Liquid | N/A | Anonymous 2012a, as cited in ECHA c2007-2016b |
| Melting point (°C) | 1 | Measured | Anonymous 2012b, as cited in ECHA c2007-2016b |
| Boiling point (°C) | 220-350 | Measured | Anonymous 2012c, as cited in ECHA c2007-2016b |
| Vapour pressure (Pa) | 5.26 × 10−26 - 5.99 × 10−11 | Predicted | MPBPWIN 2008b |
| Henry’s Law constant (Pa·m3/mol) | 8.53 × 10-16 - 1.23 × 10−13 | Predicted | HENRYWIN 2008c |
| Water solubility (mg/L) | < 0.05 | Measured | Anonymous 2012d, as cited in ECHA c2007-2016bd |
| Log Kow (dimensionless) | 4.79 - 18.38 | Predicted | ACD/Percepta c1997-2015 |
Abbreviations: Kow, octanol–water partition coefficient; N/A, not applicable.
a the ranges for predicted (i.e. modelled) data represents TEPA with 1 to 3 IODA groups attached
b modified grain method
c bond method
d turbidity measurement technique with UV/Vis spectrophotometry in transmittance mode
4.3 Sources and uses
On the basis of information submitted pursuant to a survey conducted under section 71 of CEPA (Environment Canada 2013), IODA reaction products with TEPA was not reported to be manufactured in Canada above the reporting threshold of 100 kg. It was imported into Canada at a reported total import quantity within the range of 100 to 1 000 kg in 2011 (Environment Canada 2013). According to information reported pursuant to the section 71 survey, IODA reaction products with TEPA is used in lubricants and greases (Environment Canada 2013).
IODA reaction products with TEPA is not naturally occurring. Releases to the environment of this substance is expected to be minimal from industrial and consumer uses in closed systems (ECHA c2007-2016b).
IODA reaction products with TEPA has not been measured in environmental media, is not known to be present in food packaging materials, is not permitted food additives, and is not present in drugs, natural health products, cosmetics or pest control products in Canada (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, the Health Products and Food Branch, Health Canada (HC) and the Pest Management Regulatory Agency to the Existing Substances Risk Assessment Bureau, HC, 2016; unreferenced).
The substance is used to formulate finished lubricating oils used in water-cooled 2-cycle engines as an ashless dispersant to control deposits on the piston and to prevent ring sticking and pre-ignition in the engine (ACC 2004). The concentration of the UVCB in finished oils typically ranges from 9 to 34 wt. % and is then mixed into gasoline at gasoline:oil ratios of 50:1 to 100:1 (ACC 2004). IODA reaction products with TEPA was found in a 2-cycle outboard marine engine oil at concentrations ranging from 9 to 10% (CPID 2016; US HPD 2001-2015) and is available to consumers in Canada.
IODA reaction products with TEPA was also identified in a laundry fabric softener marketed to formulators of fabric conditioning and fabric detergent products, reported in an MSDS at ≤ 100% (MSDS 2011; Crodahomecare.com c2016); however, its availability to consumers in Canada could not be confirmed.
International information indicates use in break fluids, cooling liquids in refrigerators, heat transfer fluids, hydraulic fluids, metal working fluids, motor/engine oils, oil based electric heaters, and processing aids (CPCat 2014; ECHA c2007-2016b).
4.4 Potential to cause ecological harm
Critical data and considerations used to develop the substance-specific profiles for IODA reaction products with TEPA and the hazard, exposure and risk classification results are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, IODA reaction products with TEPA was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
4.5 Potential to cause harm to human health
4.5.1 Exposure assessment
Environmental media
There are no published studies with measured levels of IODA reaction products with TEPA in environmental media in Canada or internationally. Given the low annual amounts imported into Canada in 2011, the intermittent and limited use patterns, and the substance’s relatively low environmental mobility (i.e., predicted low volatility, low water solubility and high logKow), the presence of the substance in environmental media is expected to be minimal, and the risk to human health for the general population from any releases are expected to be low.
Products available to consumers
IODA reaction products with TEPA was found to be used in oils for 2-cycle marine outboard engines. Two-cycle oils are either physically mixed with the fuel or, in the case of direct fuel injection, are combined with the fuel in the combustion chamber from a precisely-metered stream of oil (NMMA.ca c2015). On the basis of the limited use pattern of this substance, oral and inhalation exposure to the general population are not expected. Dermal exposure may occur during refuelling of personal watercraft with 2-cycle engines. Direct injected 2-cycle outboard engines require topping up of the (separate) oil reservoir. Non-direct injected 2-cycle outboards require physical mixing of the fuel and oil in the portable gas tank at each refuelling. Dermal exposure may result from new oil via potential spillage onto the skin in the boat or on the dock prior to or following mixing with the gasoline. Dermal absorption is expected to be minimal based upon the Kow, water solubility and average molecular weight of the mixture. Motor oils are viscous, resulting in a relatively slow diffusion rate from the oil to the skin.
4.5.2 Health effects assessment
Toxicokinetics
Due to its low vapour pressure, inhalation is not expected to be a significant route of exposure. Dermal and oral absorption is expected to be minimal based upon the high average molecular weight (>1000 Da) and high log Kow.
Genotoxicity
IODA reaction products with TEPA have been tested in OECD guideline studies for genotoxicity and are not mutagenic or clastogenic. Bacterial mutagenicity studies using Salmonella typhimurium (TA98, TA100, TA1535, and TA1537) were negative when tested with and without metabolic activation (Anonymous 1986b, as cited in ACC 2003). IODA reaction products with TEPA did not induce chromosomal aberrations in cultured human peripheral blood lymphocytes with and without metabolic activation (Anonymous 2006, as cited in ECHA c2007-2016b).
Acute and repeated-dose toxicity
Acute toxicity was studied in five male and five female SD rats receiving a single oral dose of 5000 mg/kg-bw IODA reaction products with TEPA. During the 14-day observation period, none of the animals died, and no significant signs of toxicity were observed upon necropsy. The LD50 was determined to be greater than 5000 mg/kg-bw (Anonymous 1985, as cited in ACC 2003). In an acute dermal toxicity study, the substance was applied dermally to five male and female New Zealand white rabbits at a dose of 2000 mg/kg-bw for 24 hours. No mortality was noted (LD50 >2000 mg/kg-bw) although skin irritation was observed (microscopic findings included mild hyperkeratosis, epidermal crusting, dermal inflammation and acanthosis) (Anonymous 1986a, as cited in ACC 2003).
A study summary of an OECD guideline short-term repeated-dose toxicity study was submitted to EChA under REACH. IODA reaction products with TEPA was administered to male and female SD rats (14/sex/dose in control and high-dose groups; 7/sex/dose in the low-dose group) via gavage at dose levels of 0, 60, 250, or 1000 mg/kg-bw/day using a corn oil vehicle for 28 days. The study also included a post-treatment 14-day observation period where a subset of animals in the control and high-dose groups received no further administration. No significant findings were reported at any dose level in any of the parameters examined, which included body weight and weight gain, food consumption, hematology and clinical chemistry parameters, neurobehaviour, organ weight, gross pathology and histopathology. The NOAEL was reported to be >1000 mg/kg-bw/day (Anonymous 2007a, as cited in ECHA c2007-2016b).
Reproductive and developmental toxicity
A study summary of an OECD guideline combined developmental and reproductive toxicity study was submitted to EChA under REACH. Groups of 12 male and 12 female SD rats were given IODA reaction products with TEPA from 14 days prior to mating through day 3 of lactation by gavage at doses of 0, 150, 450 or 1000 mg/kg-bw/day in corn oil for a total of 39-43 doses. Parental females that did not show signs of mating were treated to post-cohabitation day 25 (for a total of 52 doses). Parental animals were observed for clinical observations, food consumption and body weights during the study period. At necropsy selected organs were weighed and macroscopic and microscopic observations were recorded (organs not specified in study summary). Reproductive performance of males and females was not significantly affected at any dose level. No adverse effects were noted in any of the parameters examined at any tested dose for parental animals. There were no statistically significant effects on pup viability (number of pups born and live litter size at postnatal day (PND) 0) or sex ratio at any dose level. Likewise, there was no effect on pup body weight or clinical observations on the days examined (PND 0 and 4). The NOAEL was reported to be >1000 mg/kg-bw/day for systemic and reproductive toxicity in the parental generation and for developmental toxicity in the pups (Anonymous 2007b, as cited in ECHA c2007-2016b).
4.5.3 Characterization of risk to human health
IODA reaction products with TEPA are not genotoxic and exhibit low acute oral and dermal toxicity. No adverse health effects were noted in a short-term repeated-dose toxicity study or in the parental animals or pups in a combined developmental and reproductive toxicity study. Both studies were conducted up to the limit dose of 1000 mg/kg-bw/day. Considering that the substance is not genotoxic and in the absence of any significant gross pathological or histopathological effects in the examined toxicity studies (up to the limit dose), carcinogenicity is not expected to be a concern.
The primary source of exposure to IODA reaction products with TEPA is expected to be dermal from 2-cycle marine motor oils available to consumers in Canada. Due to its low annual import quantity, intermittent and limited use pattern and physical and chemical properties demonstrating low environmental mobility (i.e. low volatility and negligible to low water solubility), exposure of the Canadian general population to IODA reaction products with TEPA is expected to be minimal.
Based upon the absence of adverse effects at the highest dose tested and minimal exposure, the potential risk to human health is considered to be low.
4.5.4 Uncertainties in evaluation of risk to human health
On the basis of the molecular weight of IODA reaction products with TEPA exceeding 500 Da and that it is a UVCB, there is greater uncertainty in predicted values for physical chemical properties using models. This uncertainty is considered acceptable acknowledging that this is a UVCB and modelled values are based upon the representative structures of the major reaction products. Concordance of predicted and measured values for key physical and chemical parameters adds confidence to model predictions and the assessment of low potential for dermal absorption and environmental mobility.
This assessment of risk to human health was exclusively on the basis of REACH submitted summaries of limited toxicity studies for IODA reaction products with TEPA. However based upon the limited uses and subsequent minimal exposure expected in the general population, this uncertainty is considered acceptable.
5. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from erucamide, oleamide and IODA reaction products with TEPA. It is concluded that erucamide, oleamide and IODA reaction products with TEPA do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of information presented in this screening assessment, it is concluded that erucamide, oleamide and IODA reaction products with TEPA do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that erucamide, oleamide and IODA reaction products with TEPA do not meet any of the criteria set out in section 64 of CEPA.
References
[ACC] American Chemistry Council. 2004. Fatty Nitrogen Derived Amides Categories [EPA] High Production Volume (HPV) Chemicals Challenge: Assessment of Data Availability and Test Plan . Fatty Nitrogen Derivatives Panel.
[ACC] American Chemistry Council. 2003. Test Plan for Isooctadecanoic acid reaction products with TEPA. Petroleum Additives Panel
ACD/Percepta [prediction module]. c1997-2015 Version 14.0.0 (Build 2726). Toronto (ON): Advanced Chemistry Development, Inc.
Anonymous 2015a. Unnamed [Erucamide Repeated Dose 90-Day Oral Toxicity]. [cited in ECHA c2007-2016a].
Anonymous 2015b. Unnamed [Erucamide Prenatal Developmental Toxicity Study]. [cited in ECHA c2007-2016a].
Anonymous 2012a. Unnamed. [Isooctadecanoic acid reaction products with TEPA appearance/physical state/colour observed behaviour study]. [cited in ECHA c2007-2016b].
Anonymous 2012b. Unnamed. [Isooctadecanoic acid reaction products with TEPA melting point/melting range (OECD Guideline 102) study]. [cited in ECHA c2007-2016b].
Anonymous 2012c. Unnamed. [Isooctadecanoic acid reaction products with TEPA thermal gravimetric analysis boiling point study]. [cited in ECHA c2007-2016b].
Anonymous 2012d. Unnamed. [Isooctadecanoic acid reaction products with TEPA turbidity measurement technique water solubility study]. [cited in ECHA c2007-2016b].
Anonymous 2010a. Unnamed [In vitro mammalian cell gene mutation test in mouse lymphoma L5178Y cells]. [cited in ECHA c2007-2016a].
Anonymous 2010b. Unnamed [Erucamide In vitro mammalian chromosome aberration test in Chinese hamster lung fibroblasts (V79)]. [cited in ECHA c2007-2016a].
Anonymous 2010c. Unnamed [Erucamide Acute Dermal Toxicity Study]. [cited in ECHA c2007-2016a].
Anonymous 2007a. Unnamed [Isooctadecanoic acid reaction products with TEPA Repeated Dose 28-Day Oral Toxicity]. [cited in ECHA c2007-2016b].
Anonymous 2007b. Unnamed [Isooctadecanoic acid reaction products with TEPA Combined developmental and reproductive toxicity study]. [cited in ECHA c2007-2016b].
Anonymous 2006. Unnamed [Isooctadecanoic acid reaction products with TEPA chromosomal aberrations in cultured human peripheral blood lymphocytes]. [cited in ECHA c2007-2015b].
Anonymous 1986a. Unnamed [Isooctadecanoic acid reaction products with TEPA Acute Dermal Toxicity Study]. [cited in ACC 2003].
Anonymous 1986b. Unnamed [Isooctadecanoic acid reaction products with TEPA Bacterial Reverse Mutation Assay]. [cited in ACC 2003].
Anonymous 1985. Unnamed [Isooctadecanoic acid reaction products with TEPA Acute Oral Toxicity Study]. [cited in ACC 2003].
Anonymous 1960a. Unnamed [4-week digestibility diet study on erucaylamide]. [cited in ECHA c2007-2016a].
Anonymous 1960b. Unnamed [Incubation of erucamide with rat liver homogenate]. [cited in ECHA c2007-2016a].
Ash M, Ash I. 2008. Handbook of food packaging chemicals and materials. Second edition. Endicott (NY): Synapse Information Resources, Inc. Pp. 531 and 621.
Boger DL, Fecik RA, Patterson JE, Miyauchi H, Patricelli MP, Cravatt BF. 2000. Fatty acid amide hydrolase substrate specificity. Bioorganic & Mdeicianl Chemistry Ltters(10):2613-2616.
Canada. [1978]. Food and Drug Regulations. C.R.C., c.870.
Carroll KK, Noble RL 1957. Influence of a dietary supplement of erucic acid and other fatty acids on fertility in the rat; sterility caused by erucic acid. Can J Biochem Physiol. 35(11):1093-105
[CCRIS] Chemical Carcinogenesis Research Information System [database]. 2011. Bethesda (MD): US National Library of Medicine. [updated 2011-08-07; accessed 2018-05-10].
Cooper I, Lord T, Tice PA. 1995. Hydrolysis studies on oleamide in simulated gastrointestinal fluids. Food Additives & Contaminants, 12:6, 769-777
Cooper I, Tice PA. 1995. Migration studies on fatty acid amide slip additives from plastics into food simulants. Food Additives and Contaminants. 12(2): 235-244.
[CPCat] Chemical and Product Categories [database]. 2014. Ver. 04. Washington (DC): US Environmental Protection Agency. [updated 2014 May 21; accessed 16 Jul 20]. [Database described in Dionisio KL, Frame AM, Goldsmith MR, Wambaugh JF, Liddell A, Cathey T, Smith D, Vail J, Ernstoff AS, Fantke P, et al. 2015. Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicol Rep. 2:228-237.].
[CPID] Consumer Product Information Database. 2001-2016. McLean (VA): DeLima Associates. [revised 06 Apr 23; accessed 16 Nov 08]. Isooctanoic acid, reaction products with tetraethylenepentamine; CAS RN 68784-17-8.
Crodahomecare.com. c2016. Snaith (UK): Croda International Plc. [accessed 18 May 10].
crodapolymeradditives.com . c2008. Crodamide slip & anti-block: for easier processing & handling of polymers. Snaith (UK): Croda International Plc. [accessed 17 Apr 25]. BP002 05/08.
[ECCC] Environment and Climate Change Canada. 2016a. Ecological science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018. Rapid Screening of Substances with Limited General Population Exposure. Ottawa (ON): ECCC, HC.
[ECHA] European Chemicals Agency. c2007-2016a. Registered substances database. Helsinki (FI): ECHA. [updated 2017 March22; accessed 2018 May 10].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Fedorova I, Hasimoto A, Fecik RA, Hendrick MP, Hanus LO, Boger DL, Rice KC, Basile AS. 2001. Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems. The Journal of Pharmacology and experimental Therapeutics. 299:332-342.
Health Canada. [modified 2016 Sept 9]. Lists of permitted food additives. Ottawa (ON): Health Canada. [accessed 18 May 10].
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [estimation model]. 2008. Ver. 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[JETOC] Japan Chemical Industry Ecology-Toxicology & Information Center. 2008. Mutagenicity test data of existing chemical substances based on the toxicity investigation system of the industrial safety and health law. Supplement 4. [cited in CCRIS 2011].
Johansson I. 2001. Amides, Fatty Acid. Kirk-Othmer encyclopedia of chemical technology. Online version. New York (NY): John Wiley & Sons, Inc. [accessed 18 May 10]. [restricted access].
Jones E, Cook PGS, Grant RA, Kitching J. 1990a. Crodamide ER (Erucamide): Bacterial Mutation Assay. Report number CDA 58A/891761. Huntingdon Research Centra Ltd., Huntingdon, Cambridgeshire, UK. [cited in ACC 2001].
Jones E, Cook PGS, Grant RA, Kitching J. 1990b. Crodamide OR (Oleamide): Bacterial Mutation Assay. Report number CDA 58C/891778. Huntingdon Research Centra Ltd., Huntingdon, Cambridgeshire, UK. [cited in ACC 2001].
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Milne GWA, editor. 2005. Gardener’s commercially important chemicals – Synonyms, trade names, and properties. Hoboken (NJ): John Wiley & Sons, Inc. Pp. 262 and 459.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [estimation model]. 2008. Ver. 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[MSDS] Material Safety Data Sheet. 2011. Cirrasol st ultra-lq-(rb). Snaith (UK): Croda International Plc. [accessed 16 Dec 19]. www.msds.crodadirect.com.
Naidenko O, Leiba N, Sharp R, Houlihan J. 2008. Bottle water quality investigation: 10 major brands, 38 pollutants. Washington (DC): Environmental Working Group.
Nilsson NH, Malmgren-Hansen, Bernth N, Pedersen E, Pommer K. 2006. Survey and health assessment of chemical substances in sex toys. Survey of chemical substances in consumer products No. 77. Copenhagen (DK): Danish Ministry of Environment (Danish EPA).
Nilsson NH, Malmgren-Hansen B, Thomsen US. 2008. Mapping, emissions and environmental health assessment of chemical substances in artificial turf. Survey of chemical substances in consumer products No. 100. Copenhagen (DK): Danish Ministry of Environment (Danish EPA).
NMMA.ca. c2015. Bolton (ON): National Marine Manufacturers Associate Canada. [accessed 2018 May 10].
NOM05. 1987. PC-Nomograph- Programs to Enhance PC-Gems Estimates of Physical Properties for Organic Chemicals. Version 2.0.
Nonwoven for sanitary articles. 2003. Medical Textiles. July: 2-3.
[NRC] National Research Council of Canada. 2011. Chemicals management plan Health Canada moderate priorities: Data gathering on chemicals released to indoor air of residences from building materials and furnishings. Contract Report to Health Canada. Ottawa (ON): Health Canada.
Reijnders JBJ. 1988. Acute Oral Toxicity of UNISLIP 1753 in Rats. Report number 0812/1044. RCC NOTOX. [cited in ACC 2004].
Reyes H, Ribalta J, Hernández I, Arrese M, Pak N, Wells M, Kirsch RE. 1995. Is dietary erucic acid hepatotoxic in pregnancy? An experimental study in rats and hamsters. Hepatology. 21(5):1373-9.
[RSI] Risk Sciences International. 2017. Addressing human health hazard data gaps through the use of read-across for a group of primary amides. Final report. Ottawa (ON): Health Canada. [restricted access].
Simoneau C, van den Eede L, Valzacchi S. 2012. Identification and quantification of the migration of chemicals from plastic baby bottles used as substitutes for polycarbonate. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment. 29 (3): 469-480.
Simoneit BRT, Rushdi AI, Abas MRB, Didyk BM. 2003. Alkyl amides and nitriles as novel tracers for biomass burning. Environmental Science and Technology. 37 (1): 16-21.
SRC. 2010. The Physical Properties Database (PHYSPROP). Syracuse (NY): SRC. [accessed 2018 May 10]
Tønning K, Jacobsen E, Pedersen E, Strange M, Poulsen PB, Møller L, Boyd HB. 2009. Survey and health assessment of 2 year-olds to chemical substances in consumer products. Survey of chemical substances in consumer products No. 102. Copenhagen (DK): Danish Ministry of Environment (Danish EPA).
[US EPA] U.S. Environmental Protection Agency. 2010a. Screening-level hazard characterization document: Fatty Nitrogen Derived (FND) Amides Category. Washington (DC): US EPA, Office of Pollution Prevention and Toxics.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [estimation model]. 2010. Ver. 1.42. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
