Screening assessment - lotus corniculatus, extract
Official title: Screening assessment - Lotus corniculatus, extract
Chemical Abstracts Service Registry Number
84696-24-2
Environment and Climate Change Canada
Health Canada
Cat. No.: En84-281/2021E-PDF
ISBN: 978-0-660-39846-4
August 2021
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of Lotus corniculatus, extract. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1 ) for Lotus corniculatus extract is 84696-24-2. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA.
Lotus corniculatus is a plant that is also known by the common name of bird’s-foot trefoil. According to information submitted in response to a CEPA section 71 survey, Lotus corniculatus extract was not manufactured or imported into Canada above the reporting threshold of 100 kg. Notifications submitted under the Cosmetic Regulations to Health Canada identified Lotus corniculatus seed and flower extracts as being present in cosmetic products in Canada.
The ecological risk of Lotus corniculatus extract was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, Lotus corniculatus extract is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from Lotus corniculatus extract. It is concluded that Lotus corniculatus extract does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The general population of Canada may be exposed to Lotus corniculatus extract through the use of cosmetics, including body lotion and lip balm. Some phenotypes of Lotus corniculatus are known to produce cyanogenic glycosides, and the possibility exists that products containing Lotus corniculatus extract could expose consumers to hydrogen cyanide. Although all plant extracts are multiconstituent mixtures of various phytochemicals, based on the known chemistry of Lotus corniculatus, hydrogen cyanide is considered the most toxicologically relevant substance.
Hydrogen cyanide is a systemic toxicant that interferes with the ability of cells to use oxygen by disrupting the electron transport chain, thereby inhibiting cellular respiration. In rodent studies, hydrogen cyanide has effects on the male reproductive system, whereas low levels of hydrogen cyanide exposure are associated with neuropathies and thyroid disturbances in humans. Margins between estimates of cyanide exposure from Lotus corniculatus extract used in cosmetics and critical effect levels are considered adequate to address uncertainties in the health effects and exposure databases for all endpoints.
Considering all the information presented in this screening assessment, it is concluded that Lotus corniculatus extract does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that Lotus corniculatus extract does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of Lotus corniculatus, extract. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA (ECCC and HC [modified 2007]).
The ecological risk of Lotus corniculatus extract was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of action, chemical reactivity, food web-derived internal toxicity, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to October 2018. Targeted literature searches were also conducted up to October 2018. Empirical data from key studies as well as results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the Product Safety Program at Health Canada and the CEPA Risk Assessment Program at Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. The human health portion of this assessment has undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Theresa Lopez, M.S.; Jennifer Flippin, M.S.; and Joan Garey, Ph.D. Additionally, the draft of this screening assessment (published December 7, 2019) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether the substance meets the criteria set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of Lotus corniculatus extract
Lotus corniculatus is a near globally distributed flowering plant of the pea family that is known by the common name of bird’s-foot trefoil. As a botanical derivative, Lotus corniculatus extract is a complex mixture of various phytochemicals, or what is referred to by the acronym for Unknown or Variable composition, Complex reaction products and Biological material (UVCB). These materials are derived from natural sources or complex reactions and cannot practicably be synthesized by simply combining individual constituents. A UVCB is not an intentional mixture of discrete substances and is considered a single substance.
To inform the ecological and human health assessments of Lotus corniculatus extract as a whole, the components of the extract with potential for toxicological relevance were identified on the basis of available empirical data. Specifically, Lotus corniculatus is notable for being polymorphic in the ability to produce cyanogenic glycosides, which are plant secondary compounds involved in metabolism and predator defense. Cyanogenic phenotypes produce the glycoside linamarin and its methylated form lotaustralin, both of which may liberate hydrogen cyanide (HCN) upon enzymatic hydrolysis. The highest glycoside concentrations are observed in the leaves of immature plants, while the seeds as well as leaves of mature plants generally contain low concentrations (Grant and Sidhu 1967). Flowers have been reported to contain glycoside levels similar to developing leaves (Gebrehiwot and Beuselinck 2001). The chemical structures of these compounds as well as their hydrolysis product appear in Table 2‑1.
| CAS RN | Name | Representative chemical formula | Representative chemical structure | Molecular weight (g/mol) |
|---|---|---|---|---|
| 554-35-8 | Linamarin | C10H17NO6 | ![CC(C)(O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)C#N](/content/dam/eccc/images/pded/lotus-corniculatus/20210714-f21a.jpg) | 247.25 |
| 534-67-8 | Lotaustralin | C11H19NO6 | (O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)C#N](/content/dam/eccc/images/pded/lotus-corniculatus/20210714-f21b.jpg) | 261.27 |
| 74-90-8 | Hydrogen cyanide; Prussic acid | HCN | 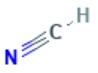 | 27.03 |
If cyanogenic phenotypes of Lotus corniculatus were used to derive the botanical extracts, the possibility exists that these products might contain linamarin and lotaustralin. The potential toxicity of these substances depends principally on their capacity to generate HCN, which can also interconvert with the cyanide anion (CN-) depending on pH and temperature (EFSA 2004). Acyanogenic plants may lack the glycosides, the enzymes to hydrolyze them, or both (Compton and Jones 1985) and are therefore potentially a source of cyanide.
3. Physical and chemical properties
A summary of physical and chemical property data of the key components in Lotus corniculatus extract are presented in Table 3‑1. When experimental information was limited or not available for a property, (quantitative) structure-activity relationship ([Q]SAR) models were used to generate predicted values for the substance. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Linamarin | Lotaustralin | HCN | Key reference(s) |
|---|---|---|---|---|
| CAS RN | 554-35-8 | 534-67-8 | 74-90-8 | N/A |
| Physical state | Solid | Solid | Liquid or gas | Kim et al. 2019; WHO 2004 |
| Melting point (°C) | 145 | 147 | -13.24 to -13.4 | PhysProp 2013; Gail et al. 2012 |
| Boiling point (°C) | 434 | 446 | 25.6 to 25.7 | PhysProp 2013; Gail et al. 2012 |
| Vapour pressure (Pa) (at 25°C) | 8.17 x 10-12 | 1.08 x 10-12 | 98 900 to 100 000 | PhysProp 2013; Chatwin et al. 1987; Daubert and Danner 1985 |
| Henry’s law constant (Pa·m3/mol) | 7.6 x 10-9 | 1.01 x 10-8 | 13.5 to 5167.6 | PhysProp 2013; Gaffney et al. 1987; Yoo et al. 1986 |
| Water solubility (mg/L) | 5.65 x 105 | 1.8 x 105 | 1 x 106; miscible | PhysProp 2013; Lide 1990 |
| Log Kow (dimensionless) | -1.91 | -1.41 | -0.25 to 0.66 | PhysProp 2013; US EPA 1984; Hansch et al. 1995 |
Abbreviations: N/A, Not Applicable; Kow, octanol-water partition coefficient
4. Sources and uses
Lotus corniculatus extract was included in a survey issued pursuant to section 71 of CEPA (Canada 2012). For the 2012 calendar year, there were no reports of manufacture or import into Canada above the reporting threshold of 100 kg (Environment Canada 2013).Footnote 3
Lotus corniculatus is listed in the Natural Health Products Ingredients Database with a homeopathic role, with whole fresh flowering plant as the source material and a minimum homeopathic potency of 12CH (i.e., a 10-24 dilution); however, no homeopathic medicines containing Lotus corniculatus are currently listed as being licensed as natural health products in the Licensed Natural Health Products Database (NHPID 2018; LNHPD 2018).
Notifications submitted under the Cosmetic Regulations to Health Canada identified Lotus corniculatus seed and flower extracts as being present in cosmetic products, including facial cleanser, skin moisturizer, face make-up, facial peel, shampoo and conditioner, and lip balm. Lotus corniculatus seed and flower extracts are listed in the Personal Care Products Council's Cosmetic Ingredient Identification Database, which contains all information published in the current edition of the International Cosmetic Ingredient Dictionary and Handbook, with the reported functions of skin-conditioning agents – miscellaneous (Nikitakis and Lange 2016).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of Lotus corniculatus extract was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. Since Lotus corniculatus extract is a UVCB substance and could not be suitably represented by a single chemical structure, a manual judgement-based approach to classification was used. The following summarizes the approach, which is described in detail in ECCC (2016a).
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate or high. Additional rules were applied (for example, classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of this UVCB, hazard and exposure could not be fully profiled because of the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed by examining the UVCB constituents and information submitted in response to a CEPA section 71 survey (Environment Canada 2013), making decisions on the basis of consideration of similar substances, and application of expert judgement.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics, such as structural profiling of mode of action, reactivity and/or estrogen-binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for Lotus corniculatus extract and the hazard, exposure and risk classification results are presented in ECCC (2016b).
According to information considered under ERC, Lotus corniculatus extract was classified as having a low exposure potential. Lotus corniculatus extract was classified as having a high hazard potential on the basis of structural alerts from OECD (Q)SAR Toolbox (2014), which identified this substance as being a potential endocrine receptor binder. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. It is unlikely that Lotus corniculatus extract is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Between January 2015 and January 2018, Lotus corniculatus extract was notified as being present in cosmetics in Canada. The products notified included rinse-off and leave-on products for the face (cleanser, moisturizer, make-up, and facial peel), hair products (shampoo / conditioner), skin moisturizer, which could be used as a body lotion, as well as lip balm.
Lotus corniculatus extract is potentially a source of the cyanogenic glycosides linamarin and lotaustralin and/or their hydrolysis product HCN (see Appendix A for additional details). In a survey of various Lotus corniculatus cultivars, the highest HCN concentrations observed were between 70 and 139 µg/g fresh weight and between 296 and 784 µg/g dry weight (Borsos et al. 1976). These values are generally in agreement with Gebrehiwot and Beuselinck (2001), who reported that, on a whole-plant basis, the HCN content of cyanogenic cultivars averaged 636 µg/g dry weight when field grown and 800 µg/g dry weight when greenhouse grown. Similarly, the average cyanide content in leaves of cyanogenic cultivars (n=158) surveyed by Briggs and Schultz (1990) was approximately 810 µg/g dry weight.
The highest cyanide concentration in Lotus corniculatus identified in the literature was 1000 µg/g wet weight, which was the maximum value observed among 204 strains harvested from 33 countries around the world (Ross and Jones 1983). Based on the ratios of Borsos et al. (1976) above, this value is estimated to correspond to approximately 5000 µg/g dry weight. Assuming Lotus corniculatus extract is not deliberately enriched for cyanide content and no measures are taken to prevent loss of HCN during the extraction processFootnote 4 , the HCN concentration in extracts would not be expected to exceed the maximum observed concentration on a dry weight basis. Therefore, an HCN content of 5000 µg/g (0.5%) for Lotus corniculatus extract was used for exposure estimation.
Exposure estimates related to the use of cosmetics were derived on the basis of expected use patterns by the general population. The highest concentration in product notifications submitted to Health Canada (or the upper bound in the case of a range) was multiplied by the maximum total cyanide concentration (free and bound) in plant matter on a dry weight basis (5000 µg/g) in order to estimate the maximum cyanide content of products. In the case of products applied to the skin, body moisturizer was selected as a sentinel exposure scenario, with the maximum amount of HCN applied dermally estimated to be 250 µg/day, corresponding to a surface load of 0.014 µg/cm2/day for adults. Additional details of the HCN content of notified products as well as estimated exposure may be found in Appendix B.
Assuming an adult body weight of 70.9 kg and complete absorption, a dermal load of 250 µg/day corresponds to a systemic dose of 3.5 µg/kg bw/day. However, HCN is a highly volatile substance, with a boiling point of ~25oC. Therefore, at a default skin surface temperature of 32oC (Freitas 1999), the substance will rapidly volatilize. The Finite Dose Skin Permeation Calculator (NIOSH 2013), which implements solutions to Kasting and Miller's (2006) model of the kinetics of finite dose absorption through skin for volatile compounds, was used to predict the disposition of an applied surface load and estimate the fractional total mass that is potentially available for percutaneous absorption versus that lost to evaporation. The HCN quantities estimated to be distributed to the stratum corneum, viable epidermis, dermis and systemic compartment were summed to yield a percutaneous dose of 3.4 x 10-5 µg/cm2, which corresponds to a systemic HCN dose of 0.009 µg/kg bw/day (Appendix C).
HCN that evaporates from the skin surface rather than being absorbed may result in inhalation exposure. Therefore, HCN concentrations in air were derived using ConsExpo exposure modelling (ConsExpo 2016). The theoretical maximum HCN concentration in air was estimated to average 21 µg/m3 during application of the body lotion, corresponding to a mean daily HCN exposure concentration of 0.15 µg/m3 and a systemic dose of 0.03 µg/kg bw/day, assuming 100% absorption via the inhalation route. Additional default parameters used in the exposure scenarios and further details of the calculations are provided in Appendix B.
The absorption of volatile substances through the skin directly from the gas phase can also be an important route of exposure (Weschler and Nazaroff 2014). However, Gaskin et al. (2013) have demonstrated that transdermal uptake of HCN gas is relatively poor. Therefore, dermal absorption from the vapour phase due to cosmetic use is considered to be negligible and no quantitative estimates were derived.
Use of Lotus corniculatus extract in lip balm was also notified, where potential exists for the product to be ingested. For the purposes of this screening assessment, it was assumed that lip balm contained Lotus corniculatus extract at up to 1%, which is the upper bound of the highest concentration range notified to Health Canada, and that all product applied is ultimately ingested. The maximum potential systemic HCN exposure via the oral route from use of lip balm containing Lotus corniculatus extract is estimated to be 0.03 µg/kg bw/day for a 70.9 kg adult. Lip balm may also be used by younger children and toddlers, and the maximum systemic dose for a toddler was estimated to be 0.07 µg/kg bw/day. Additional details of the assumptions and default parameters used to estimate exposure via the oral route may be found in Appendix B.
Empirical data on concentrations of Lotus corniculatus extract in environmental media in Canada were not identified, but are expected to be negligible. Lotus corniculatus extract is not expected to be found in food or beverages.
6.2 Health effects assessment
Plant-associated cyanogenic glycoside toxicity depends on enzymatic hydrolysis of the parent molecules to liberate HCN. Cyanide toxicity has been studied extensively both in humans and laboratory animals and the biochemical mechanisms of cyanide action are generally consistent in most mammalian species (NRC 2002). Cyanide disrupts cellular respiration leading to a state of hypoxia by binding to cytochrome c oxidase, the final enzyme complex of the electron transport chain (located in the membrane of the mitochondria of eukaryotic cells). Essentially, cyanide toxicity results from the inability of cells to utilize oxygen as an electron receptor, thereby disrupting synthesis of adenosine triphosphate, the primary source of cellular energy (Nelson 2006). Cyanide is a systemic toxicant and the organ systems most sensitive to low oxygen levels are also the most susceptible to acute cyanide toxicity, in particular the nervous, cardiovascular and respiratory systems. The following description is not intended to be exhaustive, but rather provides an overview of existing health-based guidance values for HCN and the lowest published doses associated with adverse effects. The health effects of HCN were previously reviewed by Health Canada (Canada 2018), and free/simple cyanides are the subject of various international assessments, most recently by the United States Environmental Protection Agency (US EPA 2010) and Food and Agricultural Organization/World Health Organization (FAO/WHO 2012).
The United States National Research Council has established an Acute Exposure Guideline (or AEGL-1) for HCN of 1 ppm (1 mg/m3), based on human monitoring studies in workers occupationally exposed to HCN (NRC 2000). AEGL values are intended to represent exposure thresholds that are applicable to the general public, including susceptible subpopulations such as infants, children, the elderly, persons with asthma, and those with other illnesses (NRC 2002). The AEGL-1 threshold for HCN is based on a weight of evidence evaluation that concluded an 8-hour exposure to HCN at 1 mg/m3 would be without adverse health effects for the general population. Because the value is based on exposure over extended work periods (generally 8 h/day), it is thought to represent a conservative approach to AEGL derivation (NRC 2002). The 8-hour AEGL-1 value was derived from a consideration of the dose response data obtained from several monitoring studies cited and subsequently time-scaled to the shorter AEGL exposure durations (see NRC 2002). Regression analyses have determined that the exposure duration (t) - concentration (C) relationship for HCN toxicity in a nonhuman primate is described by the equation C2 × t = k (NRC 2002), where k denotes a constant. Therefore, an 8-hour AEGL-1 of 1 ppm can be extrapolated to a 10-minute AEGL-1 of 6.9 mg/m3.
In rodent studies, subchronic cyanide exposure via drinking water did not cause clinical signs associated with neurotoxicity or histopathological evidence of brain or thyroid effects in rats and mice at doses up to 12.5 and 26 mg cyanide/kg bw/day, respectively (NTP 1993). However, subtle changes in the male reproductive tract were observed, including decreased cauda and whole epididymis weights, decreased testes weight, and altered sperm parameters, with rats appearing to be the more sensitive species. There were no changes in epididymal sperm concentrations relative to controls and the authors did not consider the effect to be biologically relevant for the rodent species. Humans, however, have lower rates of sperm production compared to rats and the potential impact of sperm quality decrements is greater for humans than for rats (US EPA 2010). Therefore, the lower 95% confidence limit on the dose corresponding to a change in cauda epididymis weight in male rats equal to one standard deviation from the control mean (benchmark dose lower confidence limit or BMDL1SD) was selected by the US EPA as the point of departure for determining a chronic oral reference dose (RfD). Uncertainty factors totalling 3000 were applied to the BMDL1SD of 1.9 mg/kg bw/day to yield a final chronic oral RfD of 0.6 µg/kg bw/day (US EPA 2010). The Joint (FAO/WHO) Expert Committee on Food Additives also derived a provisional maximum tolerable daily intake for HCN based on the same point of departure (JECFA 2011).
Chronic cyanide exposure via the inhalation route has also been documented in occupationally-exposed workers. The US EPA (2010) has derived a chronic inhalation RfC on the basis of thyroid effects and neurological symptoms in workers in three electroplating factories (El Ghawabi et al. 1975). Male workers (n = 36) chronically exposed to HCN for 5-15 years showed significantly altered rates of iodine incorporation by the thyroid, enlargement of the thyroid, as well as central nervous system (CNS) symptoms such as headache, weakness, and sensory changes for taste and smell (El Ghawabi et al. 1975). The results of individual breathing zone measurements indicated the mean HCN concentration across factories ranged from 7.07 to 11.5 mg/m3, and urinary excretion of thiocyanates was strongly correlated with individual exposure measurements. Twenty of the exposed workers (56%) were observed to have mild to moderate thyroid enlargement and altered iodide uptake. Neither exposure duration (nor air concentrations were correlated with the incidence nor magnitude of effect, although given the small sample size and relatively narrow range of exposure concentrations this is not entirely unexpected. The US EPA (2010) selected the lowest mean air concentration of 7.07 mg/m3 as the lowest observed adverse effect level (LOAEL). This number was adjusted to a continuous exposure of 2.5 mg/m3 for use as the point of departure for RfC derivationFootnote 5 . Uncertainty factors totalling 3000 were applied to the adjusted LOAEL to yield a final chronic inhalation RfC of 0.8 µg/m3 (US EPA 2010).
The available data are not sufficient to assess the carcinogenic potential of cyanide in humans. A two-year feeding study in which groups of 10 male and 10 female rats were administered food fumigated with HCN at estimated doses of 4.3 and 10.8 mg cyanide/kg bw/day yielded no evidence of histopathologic lesions attributable to cyanide (Howard and Hanzal 1955), although the study has important methodological limitations. No studies evaluating the carcinogenicity of HCN via the inhalation route were identified.
6.3 Characterization of risk to human health
Consumer exposure to Lotus corniculatus extract is expected to be limited to the use of cosmetic products. Lotus corniculatus extract may contain cyanogenic glycosides and/or the hydrolysis product HCN. Although Lotus corniculatus extract may be present in skin products intended for topical application, given the very high volatility of HCN the principle route of exposure is expected to be inhalation. Ingestion may also occur through the use of lip balm containing Lotus corniculatus extract.
Chronic exposure to relatively low levels of cyanide has been associated with neuropathy and thyroid effects in occupationally-exposed workers as well as depressed growth and male reproductive dysfunction in rodent studies. Acute exposure to minimally toxic cyanide levels is known to cause mild CNS effects, especially headache (NRC 2000; Canada 2018). Critical effect levels associated with acute (inhalation) and chronic (oral, inhalation) cyanide toxicity were selected as points of departure for comparison with upper bounding estimates of cyanide exposure from the use of cosmetic products containing Lotus corniculatus extract.
Table 6‑1 provides relevant exposure and hazard values for potential HCN exposure from the use of sentinel cosmetic products containing Lotus corniculatus extract, as well as the resultant margins of exposure (MOEs).
| Exposure scenario | Estimated exposure | Critical effect level | Critical health effect | MOE |
|---|---|---|---|---|
| Body lotion - adult (daily inhalation) | 0.15 µg/m3 | 2.5 mg/m3 (LOAEL)a | Thyroid enlargement and altered iodide uptake | >16 000 |
| Body lotion - adult (daily dermal) | 0.009 µg/kg bw/day | 1.9 mg/kg bw/day (BMDL)b | Decreased cauda epididymis weight in male rats | >200 000 |
| Body lotion - adult (per event inhalation) | 21 µg/m3 | 6.9 mg/m3 (AEGL-1)c | The AEGL-1 is considered a “safe value” based on occupational studies | 329 |
| Lip Balm - adult (daily oral) | 0.03 µg/kg bw/day | 1.9 mg/kg bw/day (BMDL)b | Decreased epididymis weight in male rats | >60 000 |
| Lip Balm - toddler (daily oral) | 0.07 µg/kg bw/day | 1.9 mg/kg bw/day (BMDL)b | Decreased epididymis weight in male rats | >25 000 |
Abbreviations: MOE, margin of exposure
a El Ghawabi 1975; US EPA 2010
b NTP 1993; US EPA 2010.
c NRC 2002. The 10-minute AEGL-1 was derived using the 8-hour AEGL-1 of 1 ppm and extrapolating for 10 min of exposure based on the equation of C2 x t = k, where C is concentration, t is time and k is a constant.
Based on the use of conservative parameters in estimating exposure from cosmetic products, the margins between estimates of exposure and critical effect levels observed in both human epidemiological as well as animal studies are considered adequate to account for any uncertainties in the toxicological and exposure databases.
6.4 Uncertainties in evaluation of risk to human health
Although Canadian data were available concerning the levels of Lotus corniculatus extract used in cosmetics some significant limitations in the exposure database remain. Similarly, the toxicity of cyanide and its mode of action are well documented, yet some uncertainties in the health effects were identified. The key sources of uncertainty are presented in Table 6‑2 below.
| Key source of uncertainty | Impact |
|---|---|
| The maximum potential HCN level in Lotus corniculatus extract is unknown. | +/- |
| There is no information available concerning whether Lotus corniculatus extracts are prepared from cyanogenic phenotypes. For the purposes of this assessment, all extracts were considered to be cyanogenic. | + |
| Due to the volatility of HCN, it is unknown whether the final product would contain any HCN even if the extract was from a cyanogenic phenotype. For the purposes of this assessment, it was considered that no HCN was lost between processing of the extract and consumer use of the product and there was full conversion of parent glycosides to HCN. | + |
| No information on cyanide carcinogenicity via the inhalation route was identified and the lone oral study has methodological limitations, precluding assessment of the carcinogenic potential of HCN. | +/- |
| There is uncertainty concerning the extent to which cyanide-induced reproductive toxicity in male rats is relevant to humans as the cyanide database lacks human male reproductive studies. | +/- |
| There are no chronic animal studies for oral exposure, nor subchronic or chronic animal studies for inhalation exposure. | +/- |
| The 90-day National Toxicology Program (NTP 1993) study for oral exposure in rat did not assay for thyroid endpoints. | +/- |
| The chronic human study used for inhalation exposure (El Ghawabi et al. 1975) did not assay for male reproductive endpoints (focus was on thyroid gland and iodine uptake). Further, there was no temporal correlation between the time of exposure and the degree of thyroid enlargement in this study. | +/- |
| There are numerous other sources of HCN other than Lotus corniculatus extract as well as the potential for interaction with other chemical exposures such as carbon monoxide. | - |
+ = uncertainty with potential to cause over-estimation of risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
Although no data were identified concerning the levels of cyanogenic glycosides or HCN in either Lotus corniculatus extract or finished products, the assumptions used are believed to be sufficiently conservative.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from Lotus corniculatus extract. It is concluded that Lotus corniculatus extract does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that Lotus corniculatus extract does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that Lotus corniculatus extract does not meet any of the criteria set out in section 64 of CEPA.
References
Berrin JG, McLauchlan WR, Needs P, Williamson G, Puigserver A, Kroon PA, Juge N. 2002. Functional expression of human liver cytosolic β‐glucosidase in Pichia pastoris: Insights into its role in the metabolism of dietary glucosides. Eur J Biochem. 269(1):249-258.
Borsos O, Haraszti E, Vetter J. 1976. Nehany Lotus corniculatus fajta cianglikozid szintjenek valtozasa a vegetacios idoszak folyaman. Botanikai kozlemenyek.
Briggs MA, Schultz JC. 1990. Chemical defense production in Lotus corniculatus L. II. Trade-offs among growth, reproduction and defense. Oecologia. 83(1):32-37.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List. Canada Gazette, Part I, vol. 146, no. 48, Supplement [PDF].
Canada. 2018. Draft screening assessment cyanides. Ottawa (ON): Environment and Climate Change Canada, Health Canada. [accessed 2018 November 21].
Chatwin TD, Trepanowski J, Wadsworth ME. 1987. Attenuation of cyanide in soils. Phase I Report. Resource Recovery and Conservation Company and the University of Utah [cited in AGDH 2010].
Compton SG, Jones DA. 1985. An investigation of the responses of herbivores to cyanogenesis in Lotus corniculatus L. Biol J Linn Soc Lond. 26(1):21-38.
[ConsExpo] Consumer Exposure Model. 2016. Web version. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Daubert TE, Danner RP. 1985. Data compilation tables of properties of pure compounds. New York: Design Institute for Physical Property Data (US) and American Institute of Chemical Engineers.
Dorr RT, Paxinos J. 1978. The current status of laetrile. Ann Intern Med. 89:389-397.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[EFSA] European Food Safety Authority. 2004. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on hydrocyanic acid in flavourings and other food ingredients with flavouring properties. Question number EFSA-Q-2003-145. EFSA Journal. 105:1-28.
[EFSA] European Food Safety Authority. 2007. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to cyanogenic compounds as undesirable substances in animal feed. Question N° EFSA-Q-2003-064. EFSA Journal. 434:1-67.
El Ghawabi SH, Gaafar MA, El-Saharti AA, Ahmed SH, Malash KK, Fares R. 1975. Chronic cyanide exposure: a clinical, radioisotope, and laboratory study. Occup Environ Med. 32(3):215-219.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[FAO/WHO] Food and Agricultural Organization/World Health Organization. 2012. Cyanogenic Glycosides (addendum). Safety Evaluation of Certain Food Additives and Contaminants. Prepared by the Seventy-fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additive Series 65.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: frequency data. Food Chem Toxicol. 78:159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, et al. 2016. Consumption of cosmetic products by the French population second part: amount data. Food Chem Toxicol. 90:130-141.
Frakes RA, Sharma RP, Willhite CC. 1986. Comparative metabolism of linamarin and amygdalin in hamsters. Food Chem Toxicol. 24:417-420.
Freitas RA Jr. 1999. Nanomedicine, Volume I: Basic Capabilities. Austin (TX): Landes Bioscience. 524 pp.
Gaffney JS, Streit GE, Spall WD, Hall JH. 1987. Beyond acid rain. Do soluble oxidants and organic toxins interact with SO2 and NOx to increase ecosystem effects? Environ Sci Tech. 21(6):519.
Gail E, Gos S, Kulzer R, Lorosch J, Rubo A, Sauer M, Kellens R, Reddy J, Steir N, Hasenpusch W. 2012. Cyano Compounds, Inorganic, Ullmann’s Encyclopedia of Industrial Chemistry.
Gaskin S, Pisaniello D, Edwards JW, Bromwich D, Reed S, Logan M, Baxter C. 2013. Chlorine and hydrogen cyanide gas interactions with human skin: In vitro studies to inform skin permeation and decontamination in HAZMAT incidents. J Hazard Mater. 262:759-765.
Gebrehiwot L, Beuselinck PR. 2001. Seasonal variations in hydrogen cyanide concentration of three Lotus species. Agron J. 93(3):603-608.
Grant WF, Sidhu BS. 1967. Basic chromosome number, cyanogenetic glucoside variation, and geographic distribution of Lotus species. Can J Bot. 45(5):639-647.
Hansch C, Leo A, Hoekman D. 1995. Exploring QSAR: hydrophobic, electronic and steric constants. Washington (DC): American Chemical Society. 348 pp.
Hernández T, Lundquist P, Oliveira L, Cristià RP, Rodriguez E, Rosling H. 1995. Fate in humans of dietary intake of cyanogenic glycosides from roots of sweet cassava consumed in Cuba. Nat Toxins. 3(2):114-117.
Howard JW, Hanzal RF. 1955. Pesticide Toxicity, Chronic Toxicity for Rats of Food Treated with Hydrogen Cyanide. J Agric Food Chem. 3:325-329.
[JECFA] Joint Expert Committee on Food Additives. 2011. Evaluation of Certain Food Additives and Contaminants. World Health Organization (WHO) Technical Report Series 966 [PDF]. [accessed 2019 March 12].
Jones DA, Turkington R. 1986. Biological flora of the British Isles. Lotus corniculatus L. J Ecol. 74(4):1185-1212.
Kasting GB, Miller MA. 2006. Kinetics of finite dose absorption through skin 2: Volatile compounds. J Pharm Sci. 95(2):268–280.
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE. 2019. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 47(D1):D1102-1109.
Lide DR, editors. 1990. CRC Handbook of Chemistry and Physics. 72nd ed. Boca Raton (FL): CRC Press.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 06]. Ottawa (ON): Government of Canada. [accessed 2018 November 20].
Meesters JAJ, Nijkamp MN, Schuur AG, Te Biesebeek JD. 2018. Cleaning Products Fact Sheet. Default parameters for estimating consumer exposure - Updated version 2018. RIVM Report 2016-0179.
Nelson L. 2006. Acute cyanide toxicity: mechanisms and manifestations. J Emerg Nurs. 32(4):S8-S11.
Németh K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA. 2003. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr. 42(1):29-42.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2018 Nov 09]. Ottawa (ON): Government of Canada. [accessed 2018 November 20].
[NIOSH] National Institute for Occupational Safety and Health. 2013. Finite Dose Skin Permeation Calculator. Atlanta (GA): Centers for Disease Control and Prevention. [accessed 2018 November 8].
[NRC] National Research Council. 2000. Hydrogen cyanide. In: Spacecraft Maximum Allowable Concentrations for Selected Airborne Contaminants, Volume 4. Washington (DC): National Academy Press. 330-365 pp.
[NRC] National Research Council. 2002. Acute Exposure Guideline Levels for Selected Airborne Chemicals, Volume 2. Subcommittee on Acute Exposure Guideline Levels Committee on Toxicology. [accessed 2018 November 21].
[NTP] National Toxicology Program. 1993. NTP technical report on toxicity studies of sodium cyanide (CAS No. 143-33-9) administered in drinking water to F344/N rats and B6C3F1 mice. [PDF] Washington (DC): US Department of Health and Human Services. NIH Publication 94-3386. [accessed 2018 November 21].
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Nikitakis J, Lange B, editors. 2016. International cosmetic ingredient dictionary and handbook. 16th ed. Washington (DC): Personal Care Products Council.
[PhysProp] Interactive PhysProp Database [database]. c2013. Syracuse (NY): SRC, Inc.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu (Dutch National Institute for Public Health and the Environment). 2006. Cosmetics Fact Sheet: To assess the risks for the consumer. RIVM report no.: 320104001/2006. [PDF] [accessed 2018 November 19].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu (Dutch National Institute for Public Health and the Environment). 2014. General Fact Sheet - General default parameters for estimating consumer exposure - Updated version 2014. [PDF] RIVM report no.: 090013003/2014. [accessed 2018 November 19].
Ross MD, Jones WT. 1983. A genetic polymorphism for tannin production in Lotus corniculatus and its relationship to cyanide polymorphism. Theor App Genet. 64(3):263-268.
[US EPA] US Environmental Protection Agency. 1984. Health effects assessment for cyanide. Washington (DC):: US EPA. EPA540186011.
[US EPA] US Environmental Protection Agency. 2010. Toxicological Review of Hydrogen Cyanide and Cyanide Salts. In Support of Summary Information on the Integrated Risk Information System (IRIS) [PDF]. Washington (DC): US EPA [accessed 2018 November 21].
[US EPA] US Environmental Protection Agency. 2011. Exposure Factors Handbook: 2011 Edition [PDF]. Washington (DC): Office of Research and Development, National Center for Environmental Assessment (NCEA), US EPA. EPA/600/R-090/052F. 1436 pp.
[US EPA] US Environmental Protection Agency. 2012. Air Pollution Training Institute, Course 414: Control of Gaseous Emissions [PDF]. Research Triangle Park (NC): Office of Air and Radiation, Office of Air Quality Planning and Standards, US EPA. 503 pp. [accessed 2018 November 19].
Vetter J. 2000. Plant cyanogenic glycosides. Toxicon. 38:11-36.
Weschler CJ, Nazaroff WW. 2014. Dermal uptake of organic vapors commonly found in indoor air. Environ Sci Tech. 48(2):1230-1237.
[WHO] World Health Organization. 2004. Concise International Chemical Assessment Document 61: Hydrogen Cyanide and Cyanides, Human Health Aspects. Geneva (CH): WHO. 73 pp.
Yoo KP, Lee SY, Lee WH. 1986. Ionization and Henry’s Law Constants for Volatile, Weak Electrolyte Water Pollutants. Korean J Chem Eng. 3:67.
Appendix A. Cyanogenic glycosides and the potential for HCN exposure from cosmetics containing Lotus corniculatus extract
Cyanogenic glycosides are relatively small molecules, which are typically found in low concentrations in plants (Briggs and Schultz 1990), although concentrations vary seasonally with spring and summer having consistently higher HCN levels than fall and winter (Gebrehiwot and Beuselinck 2001). The highest cyanide levels are found in developing leaves, and decrease as leaves mature and toughen, while the seeds contain little to no cyanogenic glycosides (Jones and Turkington 1986; Vetter 2000). There is less information on cyanide content of Lotus flowers, although in one greenhouse-grown cultivar cyanide concentration in flowers was similar to that of leaves (Gebrehiwot and Beuselinck 2001).
In intact plant material, the cyanogenic glycosides are stored in separate cellular compartments from hydrolytic enzymes such as ß-glucosidases. Damage to the plant through chewing by herbivores or processing to produce extracts may disrupt this compartmentalization and initiate the formation of HCN. On hydrolysis, one gram of linamarin can liberate 109.3 mg HCN (equivalent to 105.2 mg CN-) and one gram of lotaustralin can liberate 103.4 mg HCN (equivalent to 99.6 mg CN-). Humans and other mammals were long thought to lack the endogenous hydrolytic enzymes relevant to the metabolism of dietary glucosides (Dorr and Paxinos 1978; Frakes et al. 1986; Hernandez et al. 1995), although partial hydrolysis of glycosides and the release of HCN may be accomplished by the action of microbial enzymes in the gastrointestinal tract (EFSA 2007). Evidence of endogenous human β-glucosidases has since emerged (Berrin et al. 2002; Németh et al. 2003), although these enzymes do not appear to have any significant capacity to hydrolyze linamarin, the dominant glycoside produced by Lotus corniculatus as well as several other plant species that are important human foodstuffs. Therefore, if not metabolized by gut microbiota, the parent glycosides are absorbed and excreted in urine without causing HCN exposure (EFSA 2007).
It is reasonable to assume that HCN may be liberated from cyanogenic glycosides during the production and processing of Lotus corniculatus extract if plant material is homogenized or otherwise subject to technical processes that compromise the integrity of the material. Extracts derived from acyanogenic phenotypes that produce the glycoside but lack the enzyme may also contain intact glycosides. Although no data were identified concerning the capacity of skin microflora to hydrolyze cyanogenic glycosides, the possibility exists that unhydrolyzed glycosides that remain in plant extracts may also liberate HCN via this mechanism. As per the oral route, any cyanogenic glycosides that are percutaneously absorbed without being metabolized by skin biota are not expected to result in HCN exposure due to the lack of endogenous hydrolytic enzymes in mammals.
Appendix B. Default parameters and assumptions in estimating hydrogen cyanide exposure from cosmetics via the inhalation and oral routes
Sentinel exposure scenarios were used to estimate the potential exposure to cyanide from use of cosmetic products containing Lotus corniculatus extract; scenario assumptions are summarized in Tables B-1. Exposures were estimated on the basis of the assumed weight of 70.9 kg for adults and 15.5 kg for toddlers (Health Canada 1998) as well as an adult inhalation rate of 16.2 m3/day. Air concentrations were estimated using ConsExpo Web version or algorithms from the model (ConsExpo Web 2016).
| Exposure scenario | Assumptions |
|---|---|
Body lotion | Max HCN concentration in product: 25 mg/kg (max cyanide concentration in Lotus corniculatus extract [see section 5.1] multiplied by max concentration of extract in product per cosmetic notifications). Product amount: 10 g (Ficheux et al. 2016) Frequency: 1/day (Ficheux et al. 2015) Retention factor: 1 (RIVM 2006) Room volume: 10 m3 (RIVM 2014) Ventilation rate: 2/hr (RIVM 2014) Application duration: 1 minute (expert judgement) Exposure duration: 10 minutes (US EPA 2011) Release area: 17 530 cm2 (Ficheux et al. 2016) Temperature: 32oC (default skin temperature NIOSH 2013). Vapour pressure: 959 torr at 32oC (calculated using Antoine equation – see Appendix C) Mass transfer rate: 0.167 m/min (Meesters et al. 2018) |
Lip Balm | Max HCN concentration in product: 50 mg/kg (max cyanide concentration in Lotus corniculatus extract (see section 5.1) multiplied by max concentration of extract in product per cosmetic notifications). Product amount: 0.022 g (Ficheux et al. 2016) Max HCN conc. (mg/kg): 50 Frequency: 2/day adults; 1/day toddlers (Ficheux et al. 2015) Amount ingested: 100% (theoretical worst case) Retention factor: 1 (RIVM 2006) |
Appendix C. Finite dose skin permeation of volatile substances
The Antoine equation may be used to estimate vapour pressure within a specific range of temperatures. The equation is
where p is the vapour pressure (mmHG), T is temperature (oC) and A, B and C are component-specific constants. The Antoine constants for hydrogen cyanide were obtained from US EPA (2012).
| A | B | C | Tmin oC | Tmax oC |
|---|---|---|---|---|
7.52823 | 1329.49 | 260.418 | -16.4 | 46.2 |
Accordingly, the vapour pressure of hydrogen cyanide at 32oC (default skin surface temperature) may be calculated as follows:
The finite dose skin permeation calculator (NIOSH 2013) solves for the disposition of an applied surface load and can be used to estimate fluxes, skin concentrations and the amount of a chemical that is absorbed. In the case of highly volatile substances such as cyanide, exposure via the dermal route will depend not only on the surface load but also the rate of evaporation relative to the rate of absorption. Indeed, the finite dose skin permeation calculator predicts that at a skin temperature of 32oC the maximum evaporative flux will vastly exceed the maximum absorptive flux (Table C-1).
| Parameter | Value |
|---|---|
| Maximum Absorptive Flux (max[Jabs]) | 1.645E-03 μg/cm²/h¹ |
| Time to Max Abs Flux | 0.471 hours |
| Maximum Evaporative Flux | 2789.242 μg/cm²/h¹ |
| Time to Max Evap Flux | 2.778E-10 hours |
The finite skin permeation calculator was used to estimate permeant distribution following dermal application (Table C-2). The sum of the total absorbed solution in all skin layers plus that systemically absorbed was estimated to be 6.9 x 10-3 µg/cm2. Assuming an HCN concentration of 0.5%, this corresponds to a dose per unit area of 3.4 x 10-5 µg/cm2. This value may be multiplied by a body surface area of 17 530 cm2 and divided by an adult body weight of 70.9 kg to yield a systemic HCN dose of 0.009 µg/kg bw/day.
| HCN Disposition | Mass per unit area (µg/cm2) |
|---|---|
Evaporated | 0.564 |
Skin surface | 0.0 |
Stratum corneum | 1.57 x 10-06 |
Viable epidermis | 4.18 x 10-06 |
Dermis | 5.71 x 10-05 |
Systemically absorbed | 6.84 x 10-03 |
