Screening assessment substituted alkyl imidazolines group
Official title: Screening assessment - Substituted Alkyl Imidazolines Group
Chemical Abstracts Service Registry Numbers:
- 95-38-5
- 27136-73-8
- 68442-97-7
- 68966-38-1
Environment and Climate Change Canada
Health Canada
December 2020
Cat. No.: En84-156/2020E-PDF
ISBN 978-0-660-36482-7
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment on four of eight substances referred to collectively under the Chemicals Management Plan as the Alkyl Imidazolines and Salts Group. These four substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. The other four substances were either subsequently determined to be of low concern through other approaches, and decisions for these substances are provided in separate reportsFootnote 1, Footnote 2, or are part of a a separate screening assessment.Footnote 3 The four substances addressed in this screening assessment will hereinafter be referred to as the Substituted Alkyl Imidazolines Group.
| CAS RNa | Domestic Substances List name |
|---|---|
| 95-38-5 | 1H-Imidazole-1-ethanol, 2-(8-heptadecenyl)-4,5-dihydro- |
| 27136-73-8 | 1H-Imidazole-1-ethanol, 2-(heptadecenyl)-4,5-dihydro- |
| 68442-97-7b,c | 1H-Imidazole-1-ethanamine, 4,5-dihydro-, 2-nortall-oil alkyl derivs. |
| 68966-38-1 | 1H-Imidazole-1-ethanol, 4,5-dihydro-2-isoheptadecyl- |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society, and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
b This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
c This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
None of the substances in the Substituted Alkyl Imidazolines Group occurs naturally. According to information submitted in response to a CEPA section 71 survey, no manufacturing activities were reported above the reporting threshold of 100 kg for the substances in the Substituted Alkyl Imidazolines Group, and three of these substances were imported into Canada in total quantities in the range from 10 000 to 1 000 000 kg, in the 2011 reporting year. The substance bearing CAS RN 68966-38-1 had no importing activities reported above the reporting threshold of 100 kg in that same year. These substances may be used in various applications for consumer use in Canada, including do-it-yourself products and hair products. Industrial uses, such as components of certain lubricants and greases, in oil and natural gas extraction, and as raw material to blend into industrial products, were also identified in Canada.
The ecological risks of the substances in the Substituted Alkyl Imidazolines Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, substances in the Substituted Alkyl Imidazolines Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from substances in the Substituted Alkyl Imidazolines Group. It is concluded that the substance bearing CAS RN 95-38-5; CAS RN 27136-73-8; CAS RN 68442-97-7; and CAS RN 68966-38-1 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The predominant sources of exposure to the Canadian general population are paint remover, lubricant and rust blocker, and hair conditioner for the substance bearing CAS RN 95-38-5, CAS RN 27136-73-8, and CAS RN 68966-38-1, respectively. There is also the potential for exposure through environmental media for the substance bearing CAS RN 95-38-5, CAS RN 27136-73-8, and CAS RN 68442-97-7.
With respect to human health, the adverse effects observed in a laboratory study of the substance bearing CAS RN 95-38-5 were point of contact effects, or secondary to such effects, via the oral route. Comparison of the No Observed Adverse Effect Level (NOAEL) to the estimated exposures for this substance for the Canadian general population resulted in margins of exposure that were considered adequate to address uncertainties in the exposure and health effects databases.
In the absence of substance-specific hazard information for the substance bearing CAS RN 27136-73-8 and CAS RN 68966-38-1, CAS RN 95-38-5 was selected as the analogue for these two substances based on the similarity of their chemical structures and physical and chemical properties. Comparison of the NOAEL to the estimated exposures for the Canadian general population resulted in margins of exposure that were considered adequate to address uncertainties in the exposure and health effects databases.
On the basis of available information, reduced body weight was observed in a laboratory study with the substance bearing CAS RN 68442-97-7 via the oral route. Comparison of the NOAEL observed in this study to the estimated exposure for the Canadian general population resulted in a margin of exposure that was considered adequate to address uncertainties in the exposure and health effects databases.
On the basis of the information presented in this screening assessment, it is concluded that the substance bearing CAS RN 95-38-5; CAS RN 27136-73-8; CAS RN 68442-97-7; and CAS RN 68966-38-1 do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that the substance bearing CAS RN 95-38-5; CAS RN 27136-73-8; CAS RN 68442-97-7; and CAS RN 68966-38-1 do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment on four of eight substances, referred to collectively under the Chemicals Management Plan as the Alkyl Imidazolines and Salts Group, to determine whether they present or may present a risk to the environment or to human health. These four substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
Two substances (listed in Table 1‑1 below) were considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a) and in either the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Science Approach Document (Health Canada 2016) or via the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018a) and were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this report. Conclusions for these two substances are provided in the Substances Identified as Being of Low Concern using the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Screening Assessment Report (ECCC, HC 2018b) and the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018a). Two other substances originally part of this group, CAS RNs 67633-57-2 and 68122-86-1, are part of a separate initiative.
The four substances addressed in this screening assessment report will hereinafter be referred to as the Substituted Alkyl Imidazolines Group.
| CAS RNa | Domestic Substances List name | Approach under which the substance was addressed | References |
|---|---|---|---|
| 21652-27-7 | 1H-Imidazole-1-ethanol, 2-(8-heptadecenyl)-4,5-dihydro-, (Z)- | ERC/TTC | ECCC, HC 2018b |
| 31135-57-6 | 1H-Benzimidazolesulfonic acid, 2-heptadecyl-1-[(sulfophenyl)methyl]-, disodium salt | ERC/Rapid Screening | ECCC, HC 2018a |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society, and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
The ecological risks of the four substances in the Substituted Alkyl Imidazolines Group were characterized using the ERC approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to April 2018. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. Comments on the technical portions relevant to human health were received from Dr. Andrew Ingram (independent consultant), Dr. Mercedes Fernández-Serrano (University of Granada), and Dr. Theodore Hogan (Illinois University). The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this screening assessment (published June 21, 2019) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA, by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 4 This screening assessment presents the critical information and considerations upon which the conclusions are made.
2. Identity of substances
The CAS RN and Domestic Substances List (DSL) names for the individual substances and representative structures in the Substituted Alkyl Imidazolines Group are presented in Table 2‑1.
CAS RN 27136-73-8CAS RN 27136-73-8CAS RN 68442-97-7 is of unknown or variable composition, complex reaction products, or biological materials (UVCB). These materials are derived from natural sources or complex reactions and cannot be characterized in terms of constituent chemical compounds because their composition is too complex or variable. A UVCB is not an intentional mixture of discrete substances and is considered a single substance. The chemical structure shown in Table 2-1 is a representative structure of this UVCB. The molecular formula and molecular weight correspond to the structure(s) shown.
| CAS RN | DSL name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 95-38-5 | 1H-Imidazole-1-ethanol, 2-(8-heptadecenyl)-4,5-dihydro- | 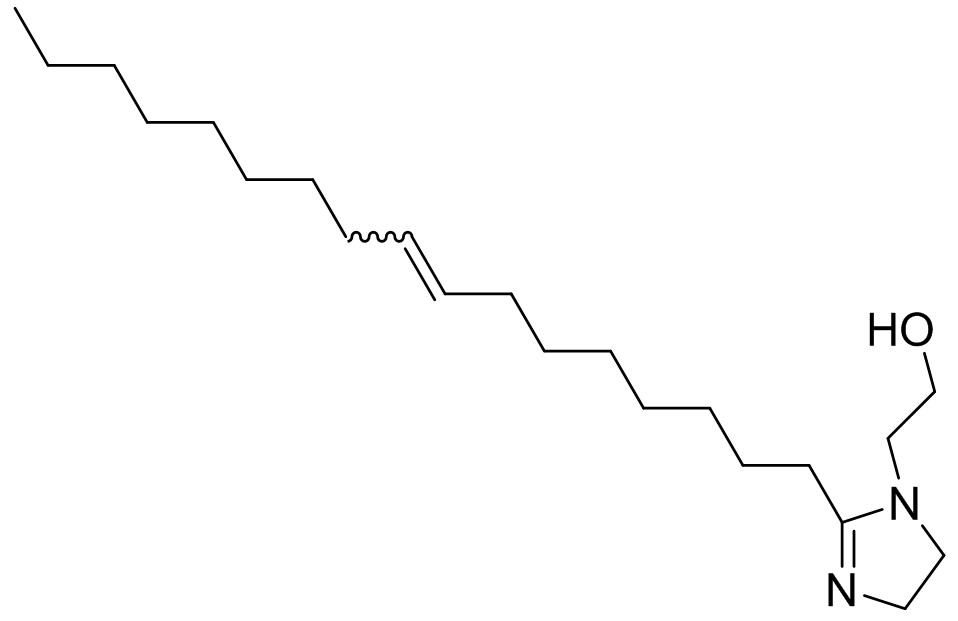 C22H42N2O C22H42N2O |
350.59 |
| 27136-73-8 | 1H-Imidazole-1-ethanol, 2-(heptadecenyl)-4,5-dihydro- | 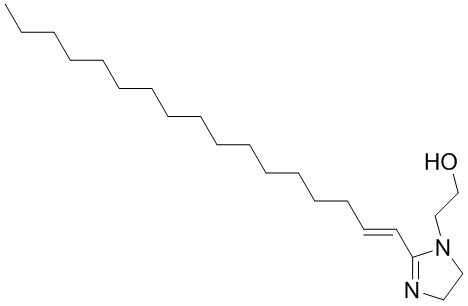 C22H42N2O C22H42N2O |
350.59 |
| 68442-97-7 | 1H-Imidazole-1-ethanamine, 4,5-dihydro-, 2-nortall-oil alkyl derivs. | 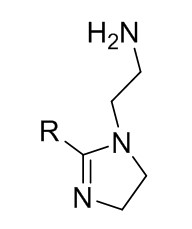 Where R = 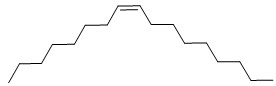 (oleic derivative) or  (linoleic derivative) C22H43N2 or C22H45N3 |
349.61 or 351.62 |
| 68966-38-1 | 1H-Imidazole-1-ethanol, 4,5-dihydro-2-isoheptadecyl- |  C22H44N2O C22H44N2O | 352.61 |
2.1 Selection of analogues
A read-across approach using data from one of the substances in the group as an analogue for two other substances in the group has been used to inform the human health assessment. The analogue, CAS RN 95-38-5, was selected based on the similarity of its chemical structure and physical and chemical properties to CAS RN 27136-73-8 and CAS RN 68966-38-1 (see Appendix B for further information). It had relevant empirical hazard data that was used for read-across to fill in data gaps for the other two substances. Details of the read-across data chosen to inform the human health assessment of the Substituted Alkyl Imidazolines Group are further discussed in section 6.2.
3. Physical and chemical properties
A summary of available physical and chemical property data of the substances in the Substituted Alkyl Imidazolines Group are presented in Table 3‑1. Experimental information regarding the physical and chemical properties of these substances is limited. When experimental information was limited or not available for a property, data from analogues were used for read-across and/or (Q)SAR models were used to generate predicted values for the substance. Additional physical and chemical properties are presented in ECCC (2016b).
| Substance | CAS RN 95-38-5 | CAS RN 27136-73-8 | CAS RN 68442-97-7 | CAS RN 68966-38-1 | Reference(s) |
|---|---|---|---|---|---|
| Vapour pressure (Pa)a | 4.08 ×10-8 | 3.09 ×10-8 | 2.50 ×10-6,b | 1.01 ×10-7 | EPI Suite c2000-2012 |
| Henry’s law constant (atm·m3/mol)c | 1.77 ×10-6 | 5.60 ×10-9 | 1.60 ×10-8,b | 2.01 ×10-6 | EPI Suite c2000-2012 |
|
Water solubility (mg/L)d |
3.10 ×10-3 | 2.35 ×10-3 | 0.594b | 2.29 ×10-3 | EPI Suite c2000-2012 |
| log Kow (dimensionless)e | 7.37 | 7.51 | 5.9b | 7.51 | EPI Suite c2000-2012 |
| log Koc (dimensionless)f | 5.1 | 5.1 | 5.8b | 5.0 | EPI Suite c2000-2012 |
Abbreviation: N/A, not applicable.
a Vapour pressure was modeled using the Modified Grain Method in MPBPVP v1.43 (EPI Suite c2000-2012)
b Mean of results for oleic acid deriv. and linoleic acid deriv.
c Henry’s Law Constant was modeled using HENRYWIN v3.20 (EPI Suite c2000-2012)
d Water solubility was modeled using WSKOW v1.42 (EPI Suite c2000-2012) using the octanol-water partition coefficient (log Kow) that was estimated using KOWWIN v1.68 (EPI Suite c2000-2012)
e The log Kow was modeled using KOWWIN v1.68 (EPI Suite c2000-2012)
f Soil adsorption coefficient (log Koc) was modeled using KOCWIN v2.00 (EPI Suite c2000-2012)
4. Sources and uses
None of the substances in the Substituted Alkyl Imidazolines Group occur naturally. These substances have been included in a survey under section 71 of CEPA (Environment Canada 2013). No importing was reported for CAS RN 68966-38-1 above the reporting threshold of 100 kg in the 2011 calendar year. Table 4‑1 presents a summary of the total reported import quantities for the remaining substances in the Substituted Alkyl Imidazolines Group. No manufacturing activities were reported above the reporting threshold for any of the substances.
| Substance | Total imports (kg) | Reporting year |
|---|---|---|
| CAS RN 95-38-5 | 100000 – 1000000 | 2011 |
| CAS RN 27136-73-8 | 22 072 | 2011 |
| CAS RN 68442-97-7 | 10000 – 100000 | 2011 |
a Values reflect quantities reported in response to a survey conducted under section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
Confirmed uses in Canada based on use codes reported by submitters in response to a CEPA section 71 survey are presented in Table 4‑2. Other uses were also reported but were identified as being confidential business information. These other uses, although not presented in this screening assessment report, were taken into consideration in the risk assessment.
| Major usesa | CAS RN 95-38-5 | CAS RN 27136-73-8 | CAS RN 68442-97-7 | CAS RN 68966-38-1 |
|---|---|---|---|---|
| Lubricants and greases | Y | Yb | N | N |
| Oil and natural gas extraction | Yc | N | N | N |
| Raw material to blend into industrial products | N | N | Yd | N |
Abbreviations: Y = yes this use was reported for this substance; N = no this use was not reported for this substance.
a Non-confidential uses reported in response to the survey conducted under section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
b Expected to be industrial or specialized use only that would not be expected to result in exposures to the Canadian general population.
c No consumer use reported.
d Corrosion inhibitor and anti-scaling agent
The substances in the Substituted Alkyl Imidazolines Group are not listed in the Lists of Permitted Food Additives in Canada (Health Canada [modified 2017]). They were not identified as being used as components in the manufacture of food packaging materials. CAS RN 95-38-5 and CAS RN 27136-73-8 may be used as components in incidental additives in food processing establishments, where CAS RN 95-38-5 may be used as a component in lubricants and degreasers used on non-food contact surfaces only, and CAS RN 27136-73-8 may be used as a component in lubricants with non-food contact (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated January 2017; unreferenced).
On the basis of notifications submitted under the Cosmetic Regulations to Health Canada, CAS RN 68966-38-1 has been notified to be present in a limited number of cosmetics (e.g., hair conditioners) (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, November 2017; unreferenced).
No uses were identified for substances in the Substituted Alkyl Imidazolines Group as either medicinal or non-medicinal ingredients in drugs or natural health products in Canada (LNHPD [modified 2016]; NHPID [modified 2016]; personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated January 2017; unreferenced; personal communication, email from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated January 2017; unreferenced).
The substances in this Group are not on the Pest Management Regulatory Agency (PMRA) Pesticide Formulants List or on PMRA’s List of Active Pesticide Ingredients (Health Canada 2010; personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated January 2017; unreferenced).
Other use information from publically available data has been identified for the substances in the Substituted Alkyl Imidazolines Group. Specific products identified on the market containing CAS RN 95-38-5 are paint removers (e.g. MSDS 2017) and greases (e.g. SDS 2016c, 2017a, b). In addition, specific products identified on the market containing CAS RN 27136-73-8 are lubricants and greases, corrosion inhibitors, and disinfectants for industrial use (e.g. MSDS 2000; SDS 2011, 2013, 2014, 2015, 2016a,b,d, 2017c, 2018).
6. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the substances in the Substituted Alkyl Imidazolines Group were characterized using the ecological risk classification of organic substances (ERC) (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal dose [LC50]) for characterization. Since CAS RN 68442-97-7 is a UVCB substance and could not be suitably represented by a single chemical structure, a manual judgement-based approach to classification was used. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, available empirical databases (e.g., OECD QSAR Toolbox 2014), and from responses to surveys conducted under section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of the CAS RN 68442-97-7, hazard and exposure could not be fully profiled because of the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed through examination of the UVCB constituents and information obtained in response to a CEPA section 71 survey, and decisions were based on consideration of similar substances and application of expert judgement.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Substituted Alkyl Imidazolines Group, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
The hazard and exposure classifications for the four substances in the Substituted Alkyl Imidazolines Group are summarized in Table 5-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| CAS RN 95-38-5 | high | Low | moderate |
| CAS RN 27136-73-8 | high | Low | moderate |
| CAS RN 68442-97-7 | moderate | Low | moderate |
| CAS RN 68966-38-1 | high | Low | low |
According to information considered under ERC, CAS RN 95-38-5 and CAS RN 27136-73-8 were classified as having low exposure potentials. They were classified as having high hazard potentials on the basis of the agreement between reactive mode of action and elevated toxicity ratio, both of which suggest that these chemicals are likely of high potency. These two substances were profiled to have moderate potential to cause adverse effects in aquatic food webs given their bioaccumulation potential, and were classified as having moderate potential for ecological risk. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of these substances. On the basis of current use patterns, these substances are unlikely to be resulting in concerns for the environment in Canada.
According to information considered under ERC, CAS RN 68442-97-7 was classified as having a low exposure potential, although with greater potential for local-scale exposures. It was classified as having a moderate hazard potential with a high potential to cause adverse effects in aquatic food webs given its bioaccumulation potential. This substance was initially classified as having a low potential for ecological risk; however, the risk classification was increased to moderate following the adjustment of risk classification to account for local-scale exposures (see section 7.1.1. of the ERC approach document [ECCC 2016a]). The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, this substance is unlikely to be resulting in concerns the environment in Canada.
According to information considered under ERC, CAS RN 68966-38-1 was classified as having a low exposure potential. CAS RN 68966-38-1 was classified as having a high hazard potential on the basis of the agreement between reactive mode of action and elevated toxicity ratio, both of which suggest that this chemical is likely of high potency. CAS RN 68966-38-1 was also profiled to have a moderate potential to cause adverse effects in aquatic food webs given its bioaccumulation potentialCAS RN 68966-38-1 and was classified as having a moderate potential for ecological risk. However, the risk classification was decreased to low potential for ecological risk following the adjustment of risk classification based on current use quantities (see section 7.1.1. of the ERC approach document [ECCC 2016a]). The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of these substances. On the basis of current use patterns, this substance is unlikely to be resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Potential exposures to substances in the Substituted Alkyl Imidazolines Group from environmental media, food, and products available to consumers are presented in this section. For each substance, exposure scenarios resulting in the highest exposures were selected to characterize risk. Additional details regarding the exposure scenarios are summarized in Appendix A.
Environmental media
Substances in the Substituted Alkyl Imidazolines Group were not identified or measured in any environmental media in Canada.
The uses of CAS RN 95-38-5, CAS RN 27136-73-8, and CAS RN 68442-97-7 identified through information submitted pursuant to a survey under section 71 of CEPA (Environment Canada 2013) indicate that releases of these substances to the Canadian environment may result from various industrial processes. Such releases are expected to occur primarily through wastewater treatment systems (WWTSs), wherein treatment technologies would only partially remove these substances. As such, environmental releases of these substances could contribute to general population exposure through drinking water. There is limited use of these substances in products that would be expected to be poured or washed down-the-drain by consumers.
Given the absence of surface water and drinking water monitoring data for substances in the Substituted Alkyl Imidazolines Group in Canada or elsewhere, industrial release scenarios using the Environmental Assessment Unit (EAU) Drinking Water Spreadsheet were used to derive the theoretical concentration of each of CAS RN 95-38-5, CAS RN 27136-73-8, and CAS RN 68442-97-7 in surface water as a surrogate for drinking water (Health Canada 2015). Total annual usage corresponding to the maximum import quantities identified through section 71 survey data (i.e., 1 000 000 kg for CAS RN 95-38-5, 22 072 kg for CAS RN 27136-73-8, and 100 000 kg for CAS RN 68442-97-7), removal percentages of these substances by wastewater treatment plants of 88% for CAS RN 95-38-5, 82% for CAS RN 27136-73-8, and 81% for CAS RN 68442-97-7 (ECCC 2016b), a flow rate of 21.33 m3/s (50th percentile) as a generic default river, and a conservative worst-case percent release to wastewater of 100% (for industrial release scenarios) were used as inputs. Default dilution factors (i.e., corresponding to waste water flows and receiving water flows) that were most appropriate to the scenarios were used. The resulting surface water concentrations and upper-bounding theoretical intake estimates for drinking water for formula-fed infants (0 – 0.5 years) are provided in Table 6‑1.
| Substance | Surface water concentration from industrial releases (mg/L)a | Daily exposure from drinking water resulting from industrial release scenarios, formula-fed infants (mg/kg bw per day)b |
|---|---|---|
| CAS RN 95-38-5c | 2.7 × 10-1 | 2.9 × 10-2 |
| CAS RN 27136-73-8c | 8.8 × 10-3 | 9.4 × 10-4 |
| CAS RN 68442-97-7 | 4.2 × 10-2 | 4.5 × 10-3 |
a It is acknowledged that these surface water concentrations are highly conservative given that a 100% emission factor was used and that it was assumed that all releases are from a single facility. Therefore, the estimated concentrations would likely overestimate actual concentrations.
b A drinking water intake rate of 0.8 L/day and a body weight of 7.5 kg were used (Health Canada 1998).
c It is noted that the predicted surface water concentration for industrial releases is greater than the water solubility reported in Table 3-1. As such, the actual dissolved concentration in surface water may not be able to achieve the theoretical estimate reported in this table.
For CAS RN 68966-38-1, due to the lack of reported commercial activity in Canada (i.e., above reporting thresholds; see Sources and Uses section), exposure from environmental media that could impact human health of the general population is not expected for this substance.
Food
Substances in the Substituted Alkyl Imidazolines Group were not reported to be present in food or in formulations of food packaging materials; CAS RN 95-38-5 and CAS RN 27136-73-8 may be used as components in incidental additives, i.e., as components in lubricants and degreasers used on non-food contact surfaces (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated January 2017; unreferenced). As there is no potential for food contact, exposure of the general population to these substances from their use in incidental additives is not expected.
Products available to consumers
Exposures to the Canadian general population from the use of products available to consumers containing substances in the Substituted Alkyl Imidazolines Group were estimated and scenarios that resulted in the highest potential exposures for each substance are presented in Table 6-2 and Appendix A.
Oral exposures to the Substituted Alkyl Imidazolines Group are not expected from products available to consumers based on the uses and products identified in Canada.
Estimated dermal exposures from substances in the Substituted Alkyl Imidazolines Group to the Canadian general population are presented in Table 6-2. The dermal maximum fluxes (Jmax) for CAS RN 95-38-5, CAS RN 27136-73-8, and CAS RN 68966-38-1, were predicted using their water solubility values and dermal permeability coefficients estimated using the Potts and Guy (1992) algorithm (adjusted as per Cleek and Bunge 1993), and range from 3.03 x 10-4 to 4.08 x 10-4 μg/cm2·h. Kroes et al. (2007) binned Jmax values into three bins, and the values for these three substances fall into the lowest bin (i.e., <0.1 μg/cm2·h), for which Kroes et al. suggested a 10% default dermal absorption fraction. As such, the dermal absorption of 10% was applied for these three substances.
Estimated inhalation exposures from use of aerosol products are presented in Table 6-2. Due to the very low vapour pressures (10-6 Pa or lower at 25°C) of all four members of the Substituted Alkyl Imidazolines Group, non-aerosol exposures via the inhalation route are not expected.
| Substance | Product scenario | Age group | Concentration | Dermal exposurea | Inhalation exposurea | Total exposure |
|---|---|---|---|---|---|---|
| CAS RN 95-38-5 | Paint removerb | Adult | 3% | 0.083 mg/kg bw per event | 0.098 mg/kg bw per event | 0.18 mg/kg bw per event |
| CAS RN 27136-73-8 | Lubricant and rust blocker (aerosol)c | Adult | 2.5% | 0.026 mg/kg bw per event | 0.047 mg/kg bw per event | 0.073 mg/kg bw per event |
| CAS RN 68966-38-1 | Hair conditioner | Toddler, child, teen, adult | 0.3% | 0.0056 to 0.017 mg/kg bw per event; 0.0061 to 0.0078 mg/kg bw per day | N/A | 0.0056 to 0.017 mg/kg bw per event; 0.0061 to 0.0078 mg/kg bw per day |
Abbreviation: N/A, not applicable.
a A dermal absorption factor of 10% was applied for CAS RN 95-38-5, CAS RN 27136-73-8, and CAS RN 68966-38-1. Inhalation absorption was assumed to be 100% (relative to oral absorption).
b MSDS 2017
c SDS 2011
Potential uses of products containing CAS RN 68442-97-7 are expected to be limited to industrial or specialized applications that would not be expected to result in exposures to the Canadian general population.
6.2 Health effects assessment
There were limited chemical-specific hazard data for the substances in the Substituted Alkyl Imidazolines Group. A read-across approach was used where required. A summary of chemical-specific data and analogue data are presented below.
CAS RN 95-38-5
Limited toxicological studies have been identified for CAS RN 95-38-5. An OECD 422 combined repeated dose toxicity study with reproductive/developmental toxicity screening test in Wistar rats was submitted by BASF to the US EPA and is identified in this assessment as a key study for the hazard identification of the substance (US EPA 2010a). Male and female rats were administered CAS RN 95-38-5, via gavage, at doses of 0, 5, 20 or 100 mg/kg bw/day. Since severe clinical signs (i.e., respiration sounds), reduced body weights and death of one female animal occurred in the high dose group during the first week of the study, the high dose level (100 mg/kg bw/day) was reduced to 60 mg/kg bw/day for the females on study day 8 and for the males on study day 9, and onward to the end of the administration period (at least 28 days).
No exposure related effects on fertility or adverse effects on pups were observed at any dose tested. No parental effects were observed in the low dose and mid dose groups. In the high dose group, significantly decreased food consumption in males during the first two study weeks and in females in the first study week were observed. Significantly decreased body weight was observed in both sexes. In addition, one male and one female adult rat in the high dose group were sacrificed in a moribund state in study week 2 and showed severe dilation of the gastro-intestinal (GI) tract and severe reduction in the size of the thymus (US EPA 2010a). No relevant findings upon histopathological examination of the pups were identified.
Although not discussed in the study, on the basis of the above information it appears that the severe dilation of the GI tract observed in the high dose group may have been GI tract irritation/corrosion-mediated toxicity due to the corrosive nature of this substance (Classification for skin corrosion/irritation: 1C, SDS 2016e). The reduction in size of the thymus appears to be an effect due to stress in dying animals. The decreased body weight is considered likely to be related to the irritation effect and/or secondary to decreased food consumption. Therefore, a NOAEL of 20 mg/kg bw/day based on reduced body weight is considered a conservative point of departure.
CAS RN 95-38-5 tested negative in bacterial mutation assays. It also tested negative in in vitro chromosomal aberration and mammalian cell gene mutation assays (ECHA c2007-2017).
No chronic repeated dose studies and carcinogenicity studies were available for this substance.
CAS RN 27136-73-8 and CAS RN 68966-38-1
In the absence of substance-specific hazard data for CAS RN 27136-73-8 and CAS RN 68966-38-1, CAS RN 95-38-5 is selected as the analogue for these two substances based on the similarity of their chemical structures and physical and chemical properties. The hazard data for this analogue are summarized above.
CAS RN 68442-97-7
CAS RN 68442-97-7 has been evaluated by the U.S. Environmental Protection Agency (US EPA 2010b) as one of the substances in the fatty nitrogen derived imidazoline derivatives category for a screening level hazard characterization. However, no repeated dose hazard data were available for CAS RN 68442-97-7 in that assessment. In 2014, two repeated dose studies were submitted to the US EPA under the Toxic Substances Control Act (US EPA 2014). One study was an OECD 422 combined repeated dose toxicity study with reproductive/developmental toxicity screening test in Wistar rats. Male and female rats were administered CAS RN 68442-97-7 via gavage, at doses of 0, 10, 30 or 100 mg/kg bw/day. Males were exposed to the substance for 28 days, i.e., 2 weeks prior to mating, during mating, and up to termination or start of recovery phase. Females were exposed for at least 42 days, i.e., 2 weeks prior to mating, during mating, during post-coitum, and for at least 4 days of lactation. No reproductive or developmental effects were observed up to the highest dose tested (100 mg/kg bw/day) in this study. Formation of foamy macrophages was observed in various tissues in the experimental animals at all doses (US EPA 2014). However, this observation is considered as an indication of exposure rather than of adverse effects since no toxicologically significant changes were observed in the exposed animals.
CAS RN 68442-97-7 was also tested in a 90-day repeated dose study with the same dose levels as the above OECD 422 study (0, 10, 30 or 100 mg/kg bw/day), via gavage. Significantly reduced body weight was observed in the rats exposed to the substance at 100 mg/kg bw/day and in a dose dependent manner. Foamy macrophages were observed in different tissues, but that is also considered as an indication of exposure rather than of adverse effects (US EPA 2014). Therefore, a NOAEL of 30 mg/kg bw/day is considered as a point of departure based on the reduced body weight.
No chronic repeated dose, carcinogenicity, or genotoxicity studies were identified for CAS RN 68442-97-7. However, (Q)SAR models provided an overall negative prediction for genotoxicity for this substance (Derek Nexus 2016; Leadscope Model Applier 2016; Times 2016).
6.3 Characterization of risk to human health
CAS RN 95-38-5
On the basis of available information, a NOAEL of 20 mg/kg bw/day was identified based on the OECD 422 combined repeated dose and reproductive/developmental toxicity study. A comparison of the NOAEL of 20 mg/kg bw/day to the estimated per event exposure from using a paint remover (0.18 mg/kg bw per event as total exposure) and to the estimated daily oral exposure from drinking water for formula-fed infants (2.9 × 10-2 mg/kg bw per day) results in respective margins of exposure of approximately 110 and 690. These margins are considered adequate to address uncertainties in the exposure and health effects databases.
CAS RN 27136-73-8
No chemical-specific hazard data were available for CAS RN 27136-73-8. CAS RN 95-38-5 was selected as an analogue for this substance based on the similarity of their chemical structures and physical and chemical properties. Therefore, the point of departure for CAS RN 95-38-5, namely the NOAEL of 20 mg/kg bw/day, was used. A comparison of the NOAEL to the estimated per event exposure from using a lubricant and rust blocker aerosol product (0.073 mg/kg bw per event as total exposure) and to the estimated daily oral exposure from drinking water for formula-fed infants (9.4 × 10-4 mg/kg bw per day) results in respective margins of exposure of approximately 270 and 21 000. These margins are considered adequate to address uncertainties in the exposure and health effects databases.
CAS RN 68442-97-7
On the basis of available information, a NOAEL of 30 mg/kg bw/day was identified based on reduced body weight observed in rats exposed to CAS RN 68442-97-7 in a 90-day repeated dose oral study. A comparison of the NOAEL to the estimated theoretical daily oral exposure from drinking water for formula-fed infants (4.5 × 10-3 mg/kg bw per day) results in a margin of exposure of approximately 6700. This margin is considered adequate to address uncertainties in the exposure and health effects databases.
CAS RN 68966-38-1
No chemical-specific hazard data were available for CAS RN 68966-38-1. CAS RN 95-38-5 was selected as an analogue for this substance based on the similarity of their chemical structures and physical and chemical properties. Therefore, the point of departure for CAS RN 95-38-5, namely the NOAEL of 20 mg/kg bw/day, was used. A comparison of the NOAEL to the estimated per event and daily dermal exposures from use in hair conditioners (0.0056 to 0.017 mg/kg bw per event; 0.0061 to 0.0078 mg/kg bw per day) results in margins of exposure ranging from approximately 1200 to 3600. These margins are considered adequate to address uncertainties in the exposure and health effects databases.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 6‑3.
| Key sources of uncertainty | Impact |
|---|---|
| Modelled aquatic concentrations for CAS RN 95-38-5, CAS RN 27136-73-8, and CAS RN 68442-97-7 were used in the absence of monitoring data. | + |
| A default dermal absorption factor of 10% was applied to CAS RN 95-38-5, CAS RN 27136-73-8, and CAS RN 68966-38-1 in the absence of chemical-specific empirical dermal absorption data. | +/- |
| There were no animal studies via dermal and inhalation routes available for the Substituted Alkyl Imidazolines Group. | +/- |
| There were no chronic or carcinogenicity studies available for the Substituted Alkyl Imidazolines Group. | +/- |
| The hazard information used for characterization of risk from exposure to CAS RN 27136-73-8 and CAS RN 68966-38-1 was from their analogue CAS RN 95-38-5. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause
under-estimation of exposure risk+/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from substances in the Substituted Alkyl Imidazolines Group. It is concluded that CAS RN 95-38-5; CAS RN 27136-73-8; CAS RN 68442-97-7; and CAS RN 68966-38-1 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that CAS RN 95-38-5; CAS RN 27136-73-8; CAS RN 68442-97-7; and CAS RN 68966-38-1 do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that CAS RN 95-38-5; CAS RN 27136-73-8; CAS RN 68442-97-7; and CAS RN 68966-38-1 do not meet any of the criteria set out in section 64 of CEPA.
References
Ash, M. 2004. Handbook of Preservatives. Synapse Information Resources, Inc.
Ash, M. 2008. Handbook of Green Chemicals (2nd Edition). Synapse Information Resources, Inc.
Ash, M. 2013a. Handbook of Paper and Pulp Chemicals. Synapse Information Resources, Inc.
Ash, M. 2013b. Handbook of Textile Processing Chemicals. Synapse Information Resources, Inc.
Bajpai D, Tyagi VK. 2006. Fatty imidazolines: chemistry, synthesis, properties and their industrial applications. Journal of oleo science. 55(7):319-329.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
ChemExper. 2018. 95-38-5. [accessed 2018 April 09].
ChemIDplus. 2018. Oleyl hydroxyethyl imidazoline. [accessed 2018 April 09].
ChemSpider. 2015a. 2-Imidazoline-1-ethanol, 2- (8-heptadecenyl)-. [accessed 2018 April 09].
ChemSpider. 2015b. 2-(2-heptadec-1-enyl-2-imidazolin-1-yl)ethanol. [accessed 2018 April 09].
ChemSpider. 2015c. (Z)-2-(8-Heptadecenyl)-2-imidazoline-1-ethanol. [accessed 2018 April 09].
Ciba-Geigy Corporation. 1970. Initial submission: acute oral LD50 study with male and female rats (final report) with cover letter dated 041092. Toxic Substances Control Act Test
Ciba-Geigy Corporation. 1992. Initial submission: evaluation of dermal effects of alrosperse 100 in humans with cover letter dated 100992. Toxic Substances Control Act Test Submission Database (TSCATS) [unpublished health and safety studies submitted to the US Environmental Protection Agency]. Microfich No. OTS0571839. Document No. 88-920010519.
Cleek RL, Bunge AL. 1993. A new method for estimating dermal absorption from chemical exposure. 1. General approach. Pharmaceutical Research 4:497-506.
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Derek Nexus [toxicity prediction module]. 2016. Ver. 5.0.2. Leeds (UK): Lhasa Limited.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization of chemical substances. Ottawa (ON): Government of Canada. [accessed 2018 April 09].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018a. Rapid screening of substances with limited general population exposure. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018b. Screening assessment: substances identified as being of low concern using the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. c2007 -2017. Registered substances database; search results for CAS RN 95-38-5. Helsinki (FI): ECHA. [accessed 2017 June 16].
[ECHA] European Chemicals Agency. c2007-2017. Registered substances database; search results for CAS RN 95-38-5. Helsinki (FI): ECHA. [updated 2017 06 02; accessed 2017 06 16]..
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Envirowise. 2003. Cost-effective paint and powder coating: application technology [PDF]. [accessed 2018 July 25].
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation..
Haz-Map 2017. 27136-73-8 [accessed 2017 June 27]
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
Health Canada. Lists of Permitted Food Additives. [modified 2017 May 3]. Ottawa (ON): Government of Canada. [accessed 2018 April 09].
Home Depot. 2018. How to Choose the Best Paint Sprayer. [accessed 2018 July 25].
Kroes R, Renwick AG, Feron V, Galli CL, Gibney M, Greim H, Guy RH, Lhuguenot JC, van de Sandt JJM. 2007. Application of the threshold of toxicological concern (TTC) to the safety evaluation of cosmetic ingredients. Food and Chemical Toxicology 45:2533-2562.
Leadscope Model Applier [prediction module]. 2016. Ver. 2.1. Columbus (OH): Leadscope, Inc.. [restricted access].
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol 46: 1516-1524.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Government of Canada. [accessed 2018 April 09].
[MSDS] Material Safety Data Sheet. 2000. Liquid Vanish Disinfectant Bowl Cleaner [PDF]. S.C. Johnson Commercial Markets, Inc. [accessed 2018 April 09].
[MSDS] Material Safety Data Sheet. 2010. Jhirmack Leave-In Conditioner. Alleghany Pharmacal. [accessed 2018 April 09].
[MSDS] Material Safety Data Sheet. 2017. CarboLift Sport: Composite Paint Removal [PDF]. Paint Lifting. [accessed 2018 April 09].
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2016 Nov 1]. Ottawa (ON): Government of Canada. [accessed 2018 April 09].
[NPCA] National Paint & Coatings Association. 2004. Exposure to crystalline silica and estimation of the associated human health risks from painting and sanding interior flat latex paint. Final report. Prepared for National Paint & Coatings Association, Inc. by Tim Reinhardt and Edward Fendick, Radian International, Seattle WA. 11 September 11 2000. [errata corrected 26 July 2004]. 48 p.
OECD QSAR Toolbox. 2014 Ver. 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Parchem. 2018. Product Description: Oleyl hydroxyethyl imidazoline. [accessed 2018 April 09].
Potts RO, Guy RH. 1992. Predicting skin permeability. Pharmacol. Res. 9(5):663-669.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007. Do-It-Yourself Products Fact Sheet: to assess the risks for the consumer [PDF]. Bilthoven (NL): RIVM. Report No.: 320104007/2007. [accessed 2018 April 09].
SCCS [Scientific Committee on Consumer Safety]. 2012. The SCCS’s Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation, 8th Revision.
[SDS] Safety Data Sheet. 2011. BullFrog 93692 Lubricant & Rust Blocker Aerosol [PDF]. Cortec Corporation. [accessed 2018 April 09].
[SDS] Safety Data Sheet. 2013. Super Lube Corrosion and Connector Gel [PDF]. Synco Chemical Corporation. [accessed 2018 April 09].
[SDS] Safety Data Sheet. 2014. Red Line Full Synthetic High Temp ATF. Red Line Synthetic Oil. [accessed 2018 April 09].
[SDS] Safety Data Sheet. 2015. RHEOLUBE 380 [PDF]. Nye Lubricants, Inc. [accessed 2018 April 09].
[SDS] Safety Data Sheet. 2016a. BullFrog 93296 Oil Additive [PDF]. Cortec Corporation. [accessed 2018 April 09].
[SDS] Safety Data Sheet. 2016b. Mobil ATF D/M [PDF]. Exxon Mobil Corporation. [accessed 2018 April 09].
[SDS] Safety Data Sheet. 2016c. Multiplex Red Grease [PDF]. Phillips 66 Lubricants. [accessed 2018 April 09].
[SDS] Safety Data Sheet. 2016d. SWEPCO 212 Multigrade Gear Oil 75W140 [PDF]. Southwestern Petroleum Canada Ltd. [accessed 2018 April 09].
[SDS] Safety Data Sheet. 2016e. AMINE O [PDF]. BASF. [accessed 2018 March 15].
[SDS] Safety Data Sheet. 2017a. Dynalife HT Grease [PDF]. Phillips 66 Lubricants. [accessed 2018 April 09].
[SDS] Safety Data Sheet. 2017b. Dynalife L-EP Grease [PDF]. Phillips 66 Lubricants. [accessed 2018 April 09].
[SDS] Safety Data Sheet. 2017c. Red Line D4 ATF Automatic/Manual Transmission Fluid/Transaxle Fluid. Red Line Synthetic Oil. [accessed 2018 April 09].
[SDS] Safety Data Sheet. 2018. Spirax S4 AX 80W-90 [PDF]. Shell Canada Products. [accessed 2018 April 09].
[TIMES] TIssue MEtabolism Simulator [prediction module]. 2016. Ver. 2.27.19. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
Tyagi R, Tyaji VK, Pandey SK. 2007. Imidazoline and its derivatives: an overview. Journal of oleo science 56(5): 211-222.
Union Carbide Corporation. 1961. Progress report on dermal irritation of 2-ethylhexanol in humans. Toxic Substances Control Act Test Submission Database (TSCATS) [unpublished health and safety studies submitted to the US Environmental Protection Agency]. Microfiche No. OTS0515547. Document No. 86-870001385.
[US EPA] US Environmental Protection Agency. 2010a. Letter from the BASF Corporation to the United States Environmental Protection Agency – East Attn: TSCA Section 8(e): Results of a OECD 422 Combined Repeated Dose Toxicity Study with the Reproduction/Developmental Toxicity Screening Test in Wistar Rats with 2-[(2-Heptadec-8-enyl)-4,5-dihydro-imidazol-1 -yl]-ethanol (CAS No. 95-38-5). Document No.: 8EHQ-10-18140.
[US EPA] US Environmental Protection Agency. 2010b. Screening-Level Hazard Characterization - Fatty Nitrogen Derived Imidazoline Derivatives Category.
[US EPA] US Environmental Protection Agency. 2014. Letter from the Akzo Nobel Services Inc. to the US EPA Office of Pollution Prevention and Toxics: TSCA Section 8(e) Notice.
Wilson A, inventor. 1941. Hydroxyalkyl glyoxalidines. United States Patent US 2,267,965.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol 48: 3109-3119.
Appendices
Appendix A. Estimated potential human exposures to Alkyl Imidazolines from products used by consumers
Exposures were estimated on the basis of the default body weights (BW), i.e., 7.5 kg for an infant, 15.5 kg for a toddler, 31.0 kg for a child, 59.4 kg for a teenager, and 70.9 kg for an adult (Health Canada 1998), and anticipated use patterns. When concentrations were used to determine exposure estimates, the upper-bounding values were used as a conservative approach. Table A-1 details the parameters used in the sentinel exposure scenarios. For dermal absorption estimates, a dermal absorption factor of 10% was applied for CAS RN 95-38-5, CAS RN 27136-73-8, and CAS RN 68966-38-1, and an overall retention factor of 1 was used unless otherwise specified. Inhalation absorption was assumed to be 100% (relative to oral absorption).
| Sentinel human exposure scenario | Assumptions |
|---|---|
|
Paint removera (CAS RN 95-38-5) |
Concentration: 3% (MSDS 2017) Age group: Adult For estimated per event dermal exposure, default parameters for direct product contact – constant rate, spray paint product, pneumatic spraying (ConsExpo Web 2016) were used to conservatively estimate exposure during spraying activity and an additional product amount of 500 mg was assumed (based on professional judgement and in alignment with the liquid paint remover scenario presented in RIVM (2007)) to account for potential exposures during the removal of pulps and remnants. The brush-on application of the paint remover is expected to result in lower exposures than the spray application. Product amount in contact with skin as a result of spraying activity: 1463 mg (based on a dermal contact rate of 110 mg/min and a release duration of 13.3 min) Additional product amount in contact with skin during subsequent removal of pulps and remnants: 500 mg Total product amount: 1963 mg For estimated per event inhalation exposure from spray application, it was conservatively assumed that no respirator is worn by the user. The use of an airless sprayer is anticipated to result in higher inhalation exposures compared to use of a high volume low pressure spray gun to apply paint remover based on the lower transfer efficiencies for airless sprayers (ConsExpo Web 2016; Envirowise 2003; Home Depot 2018). The parameters for characterizing exposure from this use are presented here. Breathing zone concentration of respirable paint aerosols: 5.14 mg/m3 (maximum concentration presented in study (NPCA 2004), used as a surrogate for concentration of respirable paint remover aerosols, and without adjustment for the weight fraction as a conservative approach) Inhalation rate: 0.675 m3/hour (Health Canada 1998)Exposure duration: 2 hours (professional judgement) |
|
Lubricant and rust blocker (aerosol)b (CAS RN 27136-73-8) |
Concentration: 2.5% (SDS 2011) Age group: Adult For estimated per event dermal exposure, default parameters for direct product contact – constant rate, spray painting product, spray can (ConsExpo Web 2016) were used unless noted otherwise: Contact rate: 100 mg/minRelease duration: 7.5 min (based on ConsExpo Web 2016 and product specifications (SDS 2011)) For estimated per event inhalation exposure, default parameters for spray model, spray painting product, spray can (ConsExpo Web 2016) were used unless noted otherwise: Spray duration: 7.5 minutes (based on ConsExpo Web 2016 and product specifications (SDS 2011))Exposure duration: 12.5 minutes (based on ConsExpo Web 2016 and product specifications (SDS 2011)) Room volume: 34 m3 Room height: 2.25 m Ventilation rate: 1.5 per hour Inhalation rate: 16.2 m3/day (Health Canada 1998) Density: 0.905 g/cm3 (SDS 2011) Mass generation rate: 0.40 g/s (based on ConsExpo Web 2016 and product specifications (SDS 2011)) Airborne fraction: 0.7 Inhalation cut off diameter: 15 µm |
|
Hair conditioner (CAS RN 68966-38-1) |
Concentration: 0.3% (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated November 2017; unreferenced) Age groups: Toddler, child, teenager, and adult For estimated per event and daily dermal exposures: Frequency: 13.5/month for toddlers, 14.9/month for children, and 1.1/day for teenagers and adults (Loretz et al. 2008; Wu et al. 2010)Product amount: 8.9 g/application for toddlers, and 13.1 g/application for children, teenagers and adults (Loretz et al. 2008) Retention factor: 0.1 (based on the conservative assumption that the particular product notified may be a leave-in conditioner, i.e., transfer factor from hair to scalp of 0.1 and rinse-off factor of 1, as another leave-in conditioner product containing CAS RN 68966-38-1 at a lower concentration was also identified from the public domain (MSDS 2010)) (SCCS 2012) |
a Based on the product description, there are various potential methods of applying the product, including the use of a spray or brush. These various application methods were considered, and the pneumatic spray scenario in ConsExpo Web (2016) was selected to represent the sentinel paint remover scenario as it resulted in the highest levels of exposures (i.e., in comparison to the exposures resulting from the brush-on paint remover scenario from ConsExpo Web 2016).
b Using certain spray can default values in ConsExpo Web (2016) that are based on a paint scenario for a lubricant and rust blocker aerosol product is a source of uncertainty. Product specific adjustments were made where possible including the assumption that one can of the product (200 mL) was used per application event.
Appendix B. Read-across approach
| Criteria | Rationale |
|---|---|
| 1) Similarity of chemical structure. Suitable analogues must contain an imidazoline moiety unless they are found to be significant transformation products/metabolites. They should also otherwise be similar in overall chemical structure (e.g., common functional groups, carbon chain-length). | Chemicals that have similar chemical structures are more likely to exhibit similarity in terms of biological activity (e.g., health effects, bioavailability, toxicokinetics). |
| 2) Similarity of structural alerts identified by toxicological profilers in the OECD QSAR Toolbox that are relevant to the endpoint(s) of interest. Suitable analogues should have similar structural alerts (or lack thereof) in comparison to the target chemical. | Chemicals with similar structural alerts are more likely to exhibit similarity in terms of health effects. |
| 3) Similarity of physical chemical properties. Suitable analogues should have similar molecular weight, water solubility, vapour pressure, and log Kow. | Chemicals with similar physical chemical properties are more likely to exhibit similarity in terms of bioavailability and toxicokinetics. |
| 4) Availability of health effects data. Suitable analogues must have health effects data available for the endpoint(s) of interest. | Only analogues with available hazard data for the endpoint(s) of interest and are of sufficient quality were considered applicable for read-across purposes. |
| CAS RN | 95-38-5 | 27136-73-8 | 68966-38-1 |
|---|---|---|---|
| Structure |  |
 |
 |
| MW (g/mol) | 350.59 | 350.59 | 352.61 |
| Vapour pressure (Pa) | 4.08 ×10-8 | 3.09 ×10-8 | 1.01 ×10-7 |
| Water solubility (mg/L) | 3.10 ×10-3 | 2.35 ×10-3 | 2.29 ×10-3 |
| log Kow (dimensionless) | 7.37 | 7.51 | 7.51 |
| Oral LD50 (mg/kg) | 822-1085a | - | - |
| Dermal LD50 (mg/kg) | 300-1000b | - | - |
| Skin irritation /sensitization | Severe skin irritation/corrosion | Severe skin irritationc | Severe skin sensitizationd |
| Repeated dose toxicity (mg/kg bw/day) | NOAEL=20 (point of contact effect) | - | - |
| Genotoxicity | Negative | - | - |
| Carcinogenicity | - | - | - |
| Reproductive/ Developmental Toxicity (mg/kg bw/day) | No effects at the highest dose tested (i.e., 100) | - | - |
a Ciba-Geigy Corporation 1970; ECHA c2007-2017
b Union Carbide Corporation 1961
c Haz-Map 2017
d Ciba-Geigy Corporation 1992
