State of the science report certain organic flame retardants substance grouping benzene, 1,3,5-tribromo-2-(2-propenyloxy)- (ATE)
Official title: State of the science report certain organic flame retardants substance grouping benzene, 1,3,5-tribromo-2-(2-propenyloxy)- (ATE)
Chemical Abstracts Service Registry Number 3278-89-5
Environment and Climate Change Canada Health Canada
May 2019
Cat. No.: En14-371/2019E-PDF
ISBN 978-0-660-30310-9
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have prepared a state of the science (SOS) report on benzene, 1,3,5-tribromo-2-(2-propenyloxy)- (ATE) (CAS RN 3278-89-5).
The purpose of this report is to review the current science on ATE and provide an updated analysis of potential for harm to the Canadian environment and human health.
This substance is included in the Certain Organic Flame Retardants (OFR) Substance Grouping, which includes ten organic substances having a similar function: application to materials to slow ignition and spread of fire. ATE was identified as a priority for action on the basis of potential ecological concerns identified from an evaluation conducted in response to notification received pursuant to the New Substances provisions of CEPA. While this substance is not on the Domestic Substances List (DSL) and therefore is subject to section 81 of the Act, it has been in commerce in Canada since the transitional period between the establishment of the DSL and the coming into force of the New Substances Notification Regulations (Chemicals and Polymers) (between January 1, 1987 and July 1, 1994).
ATE does not occur naturally in the environment. ATE is not currently manufactured in Canada. A survey conducted under section 71 of CEPA determined that in 2011, fewer than five respondents imported a total of between 100 000 and 1 000 000 kg of ATE into Canada. Uses of ATE in Canada are presumed to be consistent with those internationally including as a flame retardant for expandable polystyrene (EPS), polyolefin, electronic products, polyamide/polyimide wire insulation, adhesives, coatings and industrial textiles.
According to the United States Environmental Protection Agency’s Inventory Update Report, 4.5 to 230 tonnes (10 000 to 500 000 lbs) of ATE was produced nationally in the United States in 2006. The number of manufacturing, processing, and use sites was reported in the range of 1 to 99. ATE is estimated to have a low production volume (LPV) in the European Union (EU), where LPV is defined as being between 10 to 1000 tonnes per year.
ATE has a low predicted vapour pressure and moderate Henry’s Law Constant, high experimental and predicted log Kow, and log Koc and very low modelled and empirical water solubility.
ATE has been measured in the Canadian environment (air, water and biota) and internationally (air, water, sediment, biosolids and biota). On the basis of the results gathered from a modelling data, ATE is expected to reside predominantly in soil and sediment, depending on the compartment of release, with less than 3% residing in water. ATE has a short atmospheric half-life with rapid degradation after release to air when in the gas phase. Physical and chemical properties suggest that in the air, a low percentage of the substance will be adsorbed on particles and the majority will be present in the gas phase (99%). Long-range transport models indicate that ATE is not expected to be subject to long-range transport in the environment.
Experimental and modelled biodegradation data indicate that ATE exhibits moderate persistence in water, soil and sediment. Empirical data suggest that ATE is persistent when adsorbed to soils or sediment. Modelled data suggest that ATE will mineralize in months, likely within less than a year.
Modelled data indicate that ATE may bioaccumulate in biota and that it has the potential for biomagnification.
On the basis of the results gathered from an empirical aquatic toxicity testing, ATE has the potential to cause adverse effects to pelagic organisms (fish and crustaceans). Modelling also suggests potential effects for aquatic organisms at low concentrations. No soil, sediment or wildlife toxicity data were available. No effects (oral LD50) at levels greater than 2000 mg/kg-bw/day in Sprague-Dawley rats suggests that harm to mammalian wildlife is unlikely for current industrial release.
Four potential ATE transformation products were predicted using environmental fate modelling. Three of the four substances can be identified: 3-(2,4,6-tribromophenoxy)propane-1,2-diol (CAS RN 51286-98-7), benzene, 2,4-dibromo-1-(2-propenyloxy)- (CAS RN 69227-61-8), and 2,4,6-tribromophenol (CAS RN 118-79-6). Results of modelling indicated that some of these transformation products may have potential to accumulate to some extent in fish and one is also expected to be moderately to highly toxic to algae, daphnids and fish. Two potential metabolites of ATE were predicted, 2,4,6-tribromophenol (2,4,6-TBP) and acrolein. However, there is low confidence in the metabolic prediction as ATE was outside the model domain. Acrolein is not expected to persist or bioaccumulate in the environment but is acutely toxic to aquatic organisms. 2,4,6-TBP was determined to be persistent in air and biosolids. The potential for bioconcentration of the substance was determined to be moderate and acutely toxic to aquatic organisms.
ATE is found in commercial products and products available to consumers as an additive and reactive flame retardant. As a reactive flame retardant, release from electronic products is not expected; however, release from products where ATE is used additively (e.g., expandable polystyrene or EPS) would be expected but would be minimal and diffuse. Greatest releases of ATE to the environment are expected as a result of industrial use (i.e., product manufacturing). Industrial release scenarios developed to provide estimates of exposure to the aquatic environment, including sediment and biosolids media, indicated that the risk of harm to organisms in these media from ATE exposure is low based on current levels. To evaluate potential ecological effects of ATE, critical body residue (CBR) calculations were conducted for fish on the basis of the estimated concentration in water from the industrial release scenario. The estimated CBR values were found to be below the threshold for lethality under both acute and chronic exposures. However, using the water solubility limit for ATE, these CBR thresholds are exceeded showing that a toxic hazard towing to its lethality is nevertheless possible at higher concentrations in water.
Considering all available lines of evidence presented in this SOS report, there is currently a low potential of harm to the environment from ATE.
For the human health evaluation, exposure of the general population to ATE from environmental media (air, water and food) is estimated to be low. Exposure to the general population from use of products available to consumers (i.e., electronic products and expandable polystyrene) is expected to be minimal based on its properties as a reactive flame retardant in plastic and low potential for exposure with expandable polystyrene containing ATE as an additive flame retardant.
No classifications of the health effects of ATE by national or international regulatory agencies were identified. Limited empirical health effect data for ATE were available. Analyses from several lines of evidence were inconclusive with respect to the potential for genotoxicity or carcinogenicity. Exposure of the general population through environmental media and products available to consumers in Canada is expected to be low, and therefore the potential harm to human health is considered to be low. As an additional line of evidence, it is also noted that the estimated intake of ATE from environmental media and food for the general population is below the lowest threshold of toxicological concern value established.
Overall outcome
Although present estimated levels of exposure of ATE are not indicative of harm to the environment or to human health, there may be concerns if import and use quantities were to increase in Canada.
As ATE is a commercial alternative to other flame retardants, there is a possibility that quantities could increase in Canada. Given that ATE is not on the DSL, the substance will continue to be subject to section 81 of the Act and the New Substances Notifications Regulations (Chemicals and Polymers) of CEPA, which will ensure pre-market notification of any new import or manufacture of this substance and will allow restrictions to be put in place, as needed.
1. Introduction
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted an evaluation of one of ten substances, referred to collectively under the Chemicals Management Plan as the Certain Organic Flame Retardant Substance Group, to determine whether this substance present or may present a risk to the environment or to human health.
The Substance Groupings Initiative is a key element of the Government of Canada’s Chemicals Management Plan. The Certain Organic Flame Retardants Substance Grouping consists of ten substances identified as priorities for assessment as they met the categorization criteria under section 73(1) CEPA or were considered as a priority on the basis of ecological or human health concerns (Environment Canada, Health Canada 2007). All of these substances have a similar function: the application to materials to prevent the ignition and spread of fire. These substances are potential alternatives for other flame retardants which are presently subject to regulatory controls or phase-out in Canada and/or globally.
This state of the science (SOS) report focuses on the substance benzene, 1,3,5-tribromo-2-(2-propenyloxy)- (ATE) (CAS RN 3278-89-5). The substance is specified on the Non-Domestic Substances List (NDSL). The NDSL is an inventory of substances that are not on the Domestic Substances List (DSL) but are accepted as being in use internationally. As ATE is not present on the DSL, it is subject to section 81 of the Act and the New Substances Notification Regulations (Chemicals and Polymers) pursuant to CEPA (Canada 2005). Following New Substances ecological and human health risk assessments, conducted in December 2000, this substance was suspected of meeting the criteria for toxicity under CEPA. ATE has been in commerce in Canada since the transitional period between the establishment of the Domestic Substances List and the coming into force of the New Substance Notification Regulations (between January 1, 1987 and July 1, 1994).
The purpose of this report is to review the science on ATE and provide an updated analysis of potential for harm to the Canadian environment and human health.
This SOS report includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to January 2017 for Environment and Climate Change Canada and Health Canada. Empirical data from key studies, as well as some results from models were used to reach the outcome. When available and relevant, information presented in assessments from other jurisdictions was considered.
This SOS report was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological and human health portions of this report have undergone external written peer review and/or consultation. Comments on the technical portions relevant to the environment were received from Dr. Jon Arnot (Arnot Research and Consulting), Dr. Adrian Covaci (University of Antwerp), Dr. Miriam Diamond (Diamond Environmental Research Group), Michael Francis (Nova Chemicals), and Linda Santry (Nova Chemicals). Comments on the technical portions relevant to human health were received from scientific experts selected and directed by Toxicology Excellence for Risk Assessment (TERA), including Michael Jayjock (LifeLine Group), Bernard Gadagbui (TERA) and Patricia McGinnis (Independent Consultant). Additionally, the draft of this SOS report was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the SOS report remain the responsibility of Health Canada and Environment and Climate Change Canada.
This SOS report focuses on the critical studies and lines of evidence pertinent to the evaluation by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 1 . This SOS report presents the critical information and considerations on which the evaluation is based.
2. Substance identity
For the purposes of this document, benzene, 1,3,5-tribromo-2-(2-propenyloxy)- will be referred to as ATE. ATE is an aromatic compound, and is considered a brominated flame retardant. It is synthesized by condensation of one mole of 2,4,6-tribromophenol (TBP; a flame retardant in itself) with 3-bromo-1-propene (allyl bromide) (Ma et al. 2012). Information on the substance identity of ATE is presented in Table 2-1.
| CAS RN | Molecular weight (g/mol) | Molecular formula | Chemical structure and molecular formula |
|---|---|---|---|
| 3278-89-5 | 370.86 | C9H7Br3O | 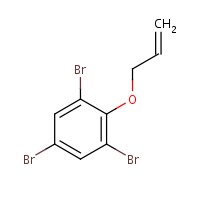 |
2.1 Selection of analogues
Guidance on the use of a read-across approach and Quantitative Structure-Activity Relationships or (Q)SAR models for filling data gaps has been prepared by various organizations such as the Organisation for Economic Co-operation and Development (OECD). These methods have been applied in various regulatory programs including the European Union’s (EU) Existing Substances Programme. An analysis was conducted to identify appropriate analogues for ATE; however, no suitable environmental analogues for ATE were available.
3. Physical and chemical properties
Physical and chemical properties determine the overall characteristics of a substance and are used to determine the suitability of different substances for different types of applications. Such properties also play a critical role in determining the environmental fate of substances (including their potential for long-range transport), as well as their toxicity to humans and non-human organisms.
A summary of experimental and modelled physical and chemical properties of ATE that are relevant to its environmental fate and ecotoxicity are presented in Table 3-1. A detailed table of physical and chemical properties of ATE (empirical and modelled) can be found in Appendix A. Empirical physical and chemical property data for ATE are limited. Empirical data available from Great Lakes Solutions (2010) for the flame retardant product PHE-65 (described as CAS RN 3278-89-5) and Study Submission (1996d-g) are presented in Table 3-1. Percent purity of PHE-65 (ATE) is not known. ATE is produced in the form of a dry powder (US EPA 2006). Empirical data indicates that the melting point for ATE ranges between 74 - 76°C (Study Submission 1996a) and the substance has low water solubility (0.24 mg/L) (Study Submission 1996g). Empirical vapour pressure and octanol-water partition coefficients are available; however, as these values are unbounded, they are not included as inputs for fate and toxicity modeling. Results from an ultraviolet and visible absorption spectrum study indicated that no absorption peaks were observed in the visible wavelength range (Study Submission 1996i). Absorption peaks were observed in the ultraviolet wavelength range suggesting that ATE may be susceptible to photodegradation from ultraviolet radiation.
(Q)SAR models were used to generate data for the physical and chemical properties of ATE (Appendix A). ATE exhibits a low predicted water solubility of 0.078 mg/L at 25°C. ATE has a low predicted vapour pressure of 0.00854 to 0.0135 Pa, as well as a moderate predicted Henry’s Law constant of 2.65 to 2.68 Pa·m3/mol at 25°C. ATE is characterized by a high octanol-water partition coefficient (modelled log Kow of 5.59; empirical greater than 4.86) and a moderate to high organic carbon-water partition coefficient (modelled log Koc of 3.12 to 4.97).
As ATE is a solid, the sub-cooled liquid properties for the vapour pressure and water solubility values are estimated and compared to the empirical and QSAR results. Estimates of the fugacity ratio are used to determine the sub-cooled properties from the solid state properties. The sub-cooled results do not differ substantially from the solid values with the exception of ECOSAR values.
ATE is within the QSAR EPI suite model domain of applicability. The structural and/or property parameter domains are represented in the training set used for the model.
| Property | Modelled value | Reference | Empirical value | Reference |
|---|---|---|---|---|
| Water solubility (mg/L) | 0.078 | WSKOWWIN 2010 v1.42 | 0.24 | Study Submission 1996g |
| Henry Law’s Constant ((Pa.m3/mol) | 2.68 | HENRYWIN 2010 v3.20 (Bond Est) | NA | NA |
| Log Kow (dimensionless) | 5.59 | KOWWIN 2010 v1.68 | >4.86 | Study Submission 1996e |
| Vapour pressure (Pa) | 0.0135 | MPBPVPWIN 2010 v1.43 (Modified Grain Method – selected VP) | <10 | Study Submission 1996d |
| Log Koc | 3.12-4.97 | KOCWIN 2010 | 3.5-4.8 | Study Submission 1996f |
| Log Koa | 8.55-9.05 | KOAWIN 2010 V1.10 | NA | NA |
| pKaa | NA | NA | NA | NA |
Abbreviations: log Kow: octanol-water partition coefficient;
logKoc, organic carbon-water partition coefficient;
log Koa, octanol-air partition coefficient;
pKa, acid dissociation constant;
NA: not available.
a Not available as ATE has no ionisable groups
4. Sources
Basing its evidence on the available literature, ATE is not known to occur naturally. In Canada, ATE was formerly produced by Chemtura Corporation under the trade name PHE-65 (Ma et al. 2012; Covaci et al. 2011). ATE is not currently manufactured in Canada (ECCC 2013-2014)
A few companies are known to have imported ATE into Canada in 2011 (Environment Canada 2013). Between 100 000 and 1 000 000 kg of ATE were imported into Canada in 2011 (Environment Canada 2013, ECCC 2013-2014). This information was acquired through a section 71 survey (Canada 2013).
According to the United States Environmental Protection Agency’s Inventory Update Report (US EPA 2006), less than 230 tonnes (less than 500,000 lbs) of ATE was produced nationally in the United States in 2006. The number of manufacturing, processing, and use sites was reported as being in the range of 1 to 99, however, it is noted this may be underestimated (US EPA 2006). ATE is estimated to have a low production volume (LPV) in the European Union (EU) (Harju et al. 2009). LPV is defined as being between 10 to 1000 tonnes per year) (UK Environment Agency 2010).
5. Uses
Globally, ATE can be used as a flame retardant in various polymers, such as polyester, polypropylene, polystyrene and other polycarbonates (Kolic et al. 2009). Most commonly, these polymers are used in polyolefin and polyamide/polyimide wire insulation and in expandable polystyrene (EPS) that may be used in building or packaging materials (Ash and Ash 2003; Nova Chemicals 2012). ATE is also used as a flame retardant for electronic products, adhesives, coatings and industrial textiles (Ash and Ash 2003; IPC [modified 2011 March 10]).
When added to plastics, ATE may be added as a reactive flame retardant, meaning it is added during the polymerization procedure to become part of the polymer (Fisk et al. 2003; Harju et al. 2009). This results in a modified polymer having flame retardant properties. The process minimizes the release of the flame retardant from leaving the polymer because it is covalently reacted with the polymer; this keeps the flame retarding properties intact for a longer period, apparently with lower emissions to the environment (Harju et al. 2009).
ATE is also an additive flame retardant when used in EPS and polystyrene (PS) foam (rigid and flexible) (WHO 1997; Harju et al. 2009). ATE may also be used as a synergist for aromatic bromine‑containing flame retardants in applications where maximum process temperatures do not exceed 150˚C (Chemtura 2007). It is additionally used as a flame retardant in the production of polyamide/polyimide wire insulation, polyester, polyethylene, polypropylene, polystyrene, and polycarbonates (Clement et al. 2012). Uses of ATE in Canada as a flame retardant are in line with international uses (ECCC 2013-2014).
ATE is listed by the United Nation Environment Programme Persistent Organic Pollutants Review Committee as a chemical alternative to HBCD in the two-step EPS process (UNEP 2012). The USEPA Design for Environment (DfE) report indicates that ATE is recommended in patents as a potential alternative to HBCD (US EPA 2014). However, the DfE report does not recommend ATE as a potential alternative for the use of HBCD in XPS foam owing to its poor thermal stability at operating temperatures. It is not considered a cost-effective alternative in EPS because it is only viable in the less-economic two-step manufacturing process. ATE was also reported to interfere with the styrene polymerization process, resulting in a product with a lower average molecular weight and residual unreacted styrene in the product. The resulting foam would lack the strength to meet building code requirements.
ATE is not listed as an approved food additive in the Lists of Permitted Food Additives, which have been incorporated by reference into their respective Marketing Authorizations issued under the Food and Drugs Act (Health Canada [modified 2017 May 3]), nor has it been identified as being used/present in formulations of food packaging materials or incidental additives (Health Canada 2013, 2013 email from Food Directorate [Health Canada] to Risk Management Bureau [Health Canada]; unreferenced). ATE is not listed in the Drug Products Database, the Therapeutic Products Directorate's internal Non-Medicinal Ingredient Database, the Natural Health Products Ingredients Database or the Licensed Natural Health Products Database as a medicinal or non-medicinal ingredient present in final pharmaceutical products, natural health products or veterinary drugs in Canada (DPD [modified 2017], NHPID [modified 2016], LNHPD [modified 2017]; 2013 email from the Therapeutic Products Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). On the basis of the notifications submitted under the Cosmetic Regulations to Health Canada, ATE is not anticipated for use in cosmetic products in Canada (personal communication, 2014 email from the Consumer Product Safety Directorate [Health Canada] to the Existing Substances Risk Assessment Bureau [Health Canada]; unreferenced).
6. Releases to the environment
ATE has a potential for release to the environment, especially when it is used as an additive flame retardant. Releases to the Canadian environment may occur during industrial use (i.e., product manufacturing), consumer or commercial use of the product, service life and disposal of the substance and product containing ATE. Releases may occur in both indoor and outdoor environments (Shoeib et al. 2012).
ATE can be found in commercial products and products available to consumers. Additive use of ATE in EPS and PS foam (both rigid and flexible) suggests diffuse releases may occur from commercial products or products available to consumers, and although there are uncertainties, the rate is assumed to be low in comparison to industrial point sources during incorporation of the substance into products. Reactive use of ATE indicates that the substance is chemically bound to the polymer matrices and will have limited potential to leach out into the environment (Stapleton 2010).
Environmental release, via leaching, of the substance from polymers is considered to be very low as the substance will be incorporated into the polymer and ultimately contained within the solid matrices of the product. Products containing ATE, e.g. EPS foam (both rigid and flexible PS foams), are not expected to be in contact with water. The potential release of flame retardants used as additives in plastics during service life is estimated at 0.05% over lifetime to water if the substance is for indoor use or 0.16% over lifetime outdoor use (OECD 2009). The majority of ATE-containing products would be enclosed and used indoor; therefore, the release rate of 0.05% is most applicable and may likely be an overestimate since contact with water is not expected. Overall, releases from products are expected to be geographically dispersed and spread out over the duration of the products service life and end-of-life of these products.
In general, wastewater is a potential point of entry of ATE to water and a potential point of entry to soil through the land application of biosolids. Although the vapour pressure of ATE is low, monitoring results indicate that once in the air, emissions may result in atmospheric deposition to soil and water.
ATE has been measured in house dust in Vancouver, Canada. ATE was detected in 81% of the dust samples, in concentrations ranging from less than 0.04 to 52 ng/g (Shoeib et al. 2012). Further consideration is given to ATE levels in dust in Section 9.1.1.3. These measurements reflect disperse releases of ATE to the indoor environment. Releases from products available to consumers could be contained within household dust and this dust could make its way to wastewater treatment systems. The accumulation of flame retardants, including ATE, by clothing from indoor air and their transfer via laundering to the outdoors were investigated by Saini et al. (2016). ATE was not detected in laundry wastewater and was not found sorbed to cotton and polyester fabrics.
7. Environmental concentrations
7.1 Measured environmental concentrations
Data concerning concentrations of ATE in Canadian air, water, vegetation and biota have been identified (ECCC 2016). According to the use of ATE in Canada, measured environmental concentrations likely reflect disperse release from products or from industrial activities.
7.1.1 Air
ATE was monitored in outdoor air (passive and active) around the Great Lakes region and in the urban area of Toronto, Ontario in 2011 (Diamond et al. 2013). The mean concentration for ATE from active high volume sampling was less than 1 pg/m3 from one location operating 24 hours bi-monthly. Daily passive ambient air monitoring during a 3-month period in the summer and winter seasons over a one-year period at 6 locations in Toronto indicated no detection of ATE in the winter months. ATE concentrations between 1 to 15 ng/PUF disc (polyurethane foam disc sampler) were reported during spring and summer months. ATE has been detected in air samples in the Great Lakes (Shoeib and Jantunen 2014, ECCC 2016). ATE was detected in 100% of samples around Lake Ontario in 2008 and 88% of samples around Lake Huron in 2012, and was detected at a field site in urban Toronto (87%). Concentrations were mainly detected in the gas phase and ranged from 0.04 to 11.1 pg/m3 (Shoeib and Jantunen 2014). Median concentrations of ATE at the three locations ranged from 0.11 to 2.5 pg/m3.
ATE was analyzed in air samples collected at a location in the sub-Arctic (Little Fox Lake, Yukon Territory) from August 2011 to December 2014 (Yu et al. 2015). ATE was detected in 26% of all samples ranging from 0.007 to 0.107 pg/m3.
Lee et al. (2016) conducted a retrospective analysis on air samples that were collected in 2005 under the GAPS program. For ATE, the levels in the Canadian atmosphere ranged from 0.008 to 0.98 pg/m3. On the basis of the concentration data for all sampling periods, ATE had the second highest frequency of detection (71%) on a global basis.
ATE has also been measured in air in the United States, Norway, and Sweden (see ECCC 2016) at concentrations ranging from 0.012 to 15 pg/m3.
7.1.2 Water
ATE has been detected in the surface waters of Lake Ontario at 0.22 pg/L and Lake Opeongo at 0.07 pg/L (Muir et al. 2011). ATE was below detection limits for the Lake Erie and Lake Siskiwit samples (Muir et al. 2012). Xie et al. (2011) also collected seawater samples aboard a cruise ship in the Atlantic and Southern Ocean in 2008. All 16 samples collected during the sampling period of two months measured below the detection limit (less than 0.1 to less than 0.5 pg/L) (see ECCC 2016).
7.1.3 Sediment and soil
No sediment or soil concentrations of ATE are available for Canada.
ATE was detected in all soil samples over a 6 month period from 8 sites along a transect in Birmingham, United Kingdom between June 2012 and January 2013 (Drage et al. 2016). Concentrations in the soils ranged from 0.01 to 0.69 ng/g (organic matter).
ATE was sampled between August 2012 and August 2013 in surface river sediments from the UK East coast (n=23) and the river Elbe, Germany, but was below the limit of detection (59 pg/g dw) (Sühring.et al. 2015a).
Sediment samples were collected from urban sites in Denmark, Faroe Islands, Finland, Norway, and Sweden (TemaNord et al. 2011). All samples were below detection limits with respect to ATE except for an urban site sample collected in 2009 from Asefjorden, Norway which measured 0.092 ng/g dry weight (dw). No data are available characterizing levels of ATE in soil (see ECCC 2016).
7.1.4 Wastewater and biosolids
Although it is recognized that wastewater system effluent and biosolids are not considered “environment”, they represent a direct source to the environment and are included in the discussion.
An analysis of brominated flame retardants was conducted of clothing and laundry wastewater by Saini et al. (2016), and determined that ATE was not detected on cotton or polyester clothing or in laundry water (instrument detection limit of 1.1 pg)
ATE was not detected in an analysis of a total of 186 liquid influent, effluent (detection limit of 0.0062 ng/L) and 58 biosolid (detection limit not provided) samples from 8 Canadian wastewater treatment systems (Shanmuganathan et al. 2017 submitted).
ATE has been measured in wastewater biosolids samples from Reykjavik, Iceland (11 ng/g dw), a wastewater treatment plant in Reykjavik, Iceland (27 ng/g dw), and from two wastewater treatment plants in Alesund, Norway (2.6 ng/g dw and 1.2 ng/g dw) in 2009 (TemaNord 2011). The researchers also collected biosolids samples at wastewater treatment plants, urban sites, and recycling sites from Denmark, Faroe Islands, Finland, Sweden, Iceland, and Norway, but levels of ATE were below analytical detection limits.
ATE has been detected in 15 of 18 wastewater biosolids samples in Germany from ten different wastewater treatment plants (Weisser 1992). The levels ranged from <5 to 91 µg/kg dw.
7.1.5 Biota
ATE has been detected in a number of organisms such as caribou, wolves, harp seals, glaucous-wing and herring gulls, European starlings, American eels, European eels, zooplankton, Lake trout, sculpin, and blue mussels from the North Alaska coast, Nunavut, Barents Sea, Greenland Sea, Lake Ontario and Lake Opeongo. (ECCC 2016).
ATE was one of the most abundant non-PBDE halogenated flame retardants in samples in a terrestrial food chain (vegetation-caribou-wolves) study in the Bathurst region, Nunavut (Morris et al. 2014 unpubl). ATE was detected regularly in caribou muscle and liver tissue (2.1; 3.3 ng/g lipid weight (lw), respectively), although levels were highest in wolf muscle and liver tissue (4.0 and 0.99 ng/g lw, respectively).
ATE has been detected in American eel (Anguilla rostrata) samples from Nova Scotia, New Brunswick, Quebec, and Ontario, ranging from 0.25 ± 0.13 ng/g to 1.3 ± 0.4 ng/g (Byer et al. 2010). ATE was quantifiable in 55 of 58 samples collected from seven locations in 2007 and 2008 (Byer et al. 2010).
Muir et al. (2014) analyzed mysid samples collected from Lake Ontario in June 2013. Concentrations of 0.063 ng/g ww ATE was detected in mysids. In 2005 to 2010, Muir et al. (2011) collected samples of zookplankton from Lakes Erie, Ontario, and Opeongo, and lake trout and sculpin from Lake Ontario. ATE was detected at 0.0024 ng/g ww and 0.0032 ng/g ww in zooplankton from Lake Ontario and Lake Opeongo, respectively. Lake trout and sculpin measured 0.327 ng/g ww and 0.032 ng/g ww, respectively. ATE concentrations in zooplankton collected from Lake Erie were below detection limit.
A biomonitoring study of ATE in eggs of three gull species in 19 colonies was conducted across Canada in 2009 (Martin and Hughes 2016a). Maximum concentrations of 0.12 and 0.16 ng/g ww were reported for the glaucous-winged gull (Larus glaucescenus) and herring gull (Larus argentatus) at Mitlenatch Island, BC and Manawagonish Island, NB, respectively. Twenty percent of ATE samples were greater than the detection limit (0.02 ng/g ww). A 2009 biomonitoring study of European starling eggs (Sturnus vulgaris) indicated that ATE was found below the limit of detection in samples from 17 urban sites across Canada (Martin and Hughes 2016b).
ATE concentrations were determined to be below the detection limit (<3 ng/g ww) in tree swallows (Tachycineta bicolor) in a study where they were sampled in the vicinity of two wastewater treatment systems and a reference reservoir in Ontario, Canada (2007 to 2010) (Gilchrist et al. 2014).
The eggs of 3 marine seabird species (common eider (Somateria mollissima), European shag (Phalacrocorax aristotelis), and European herring gull (Larus argentatus) were analyzed from the Norwegian coastal environment for a range of legacy and emerging pollutants to assess chemical mixture exposure profiles. ATE was found at concentrations below the limit of detection (0.01 ng/g) (Huber et al. 2015).
Von Der Recke and Vetter (2007) detected 5.4 µg/kg wet weight (ww) of ATE in blubber and 3.1 to 10 µg/kg ww in brain tissue of harp seals (Phoca groenlandica) from the Barents Sea and Greenland Sea. This study indicated ATE was able to penetrate the blood-brain barrier. 2,3-Dibromopropyl-2,4,6-tribromophenyl ether (DPTE) was the predominant organobromine compound in these samples (blubber 322 to 470 ng/g ww, brain 130 to340 ng/g ww). 2-Bromoallyl-2,4,6-tribromophenylether (BATE) was also present in the samples at about the same concentrations as ATE. The ATE/DPTE and BATE/DPTE ratios were 0.018 and 0.015 respectively in blubber and 0.030 and 0.019 respectively in brain. The general co-occurrence of ATE and BATE supports the hypothesis that the source for ATE in these samples was from the biotransformation of DPTE. Anaerobic transformation studies of DPTE with super-reduced corrinoids resulted in the formation of ATE.
Sühring et al. (2015b) analyzed the maternal transfer of chlorinated flame retardants in European eels in two German drainage systems (Ems River and Schlei Fjord). Studies showed that ATE has a significant uptake from the surrounding water, rather than just food and may be formed by metabolism or biotransformation processes. ATE was detected in various tissue types of eels, including the muscle (0.7 to 6.2 ng/g), eggs (0.16 to 0.80 ng/g) and gonads (0.19 to 2.9 ng/g).
Blue mussel composite samples were collected in 2009 from surface water at two urban stations in Åse, Norway (TemaNord 2011). ATE was detected in a sample from one of the stations at 0.0045 ng/g ww.
8. Environmental fate and behaviour
8.1 Environmental distribution
ATE is expected to be released primarily in the industrial effluent of facilities that use the substance in the manufacture of EPS and electronics. No ATE landfill leachate data have been reported to date, but such data could help interpret end of life releases.
The mass-fraction distribution of ATE using the Level III fugacity modeling (EQC 2012) is given in Table 8-1 using individual steady-state emissions to air, water and soil.
The results of Level III fugacity modelling indicate that ATE is expected to predominantly reside in soil, and sediment, depending on the compartment of release.
| ATE | Air | Water | Soil | Sediment |
|---|---|---|---|---|
| Air (100%) | Negligible | Negligible | 78.6 | 20.7 |
| Water (100%) | Negligible | 2.1 | Negligible | 97.9 |
| Soil (100%) | Negligible | Negligible | 100 | Negligible |
a EQC v1.00 2012.
b Physical chemical properties and half-lives (t1/2) of ATE in environmental media are required for modelling and are listed in Appendix A.
If released to air, a negligible amount of ATE is expected to reside in air in the gas phase because of its rapid degradation because of reactions with hydroxyl radicals (t½ = 5.88 hours) and affinity to partition to the atmospheric particles (high log Koa). Therefore, ATE is not expected to reside in air long enough to undergo long range transport to remote regions in air. However, monitoring results indicate that ATE is more persistent when associated with particulates (Section 7.1.1). The particulate phase is deposited to land and water as wet and dry deposition. The majority of ATE that partitions to air will transfer from air to soil (78.6%) and a small fraction will partition to sediment (20.7%). ATE has a low predicted vapour pressure of 0.0085 to 0.013 Pa and a moderate Henry’s Law constant of 2.68 to 64.08 Pa·m3/mol; model results indicate that negligible amounts of the substance will partition to air.
If released to water, the majority of ATE will partition to sediment (97.9%) and strongly adsorb to suspended solids and eventually sink to sediment. With a low vapour pressure (0.0135 Pa), volatilization from surface water to air is not expected. ATE is expected to adsorb onto particles. Therefore, loss of ATE from aqueous systems is anticipated to be to sediments where it will remain as biodegradation is expected to be very slow (2% degradation in sediment). ATE is not likely to reside in water to any large degree towing to its low empirical water solubility of 0.24 mg/L, with only a small amount potentially remaining dissolved in water (i.e., ~2%).
If released exclusively to soil, it is expected that the ATE will remain in the soil (100%) compartment because of its hydrophobic nature. Evaporation from soil to air is not expected because of a low vapour pressure. ATE is also anticipated to be stable in soil and resistant to mineralization (Table 8-2) and loss processes for soil will be driven by soil burial or surface runoff of soil particles.
8.1.1 Long-range transport potential
The models for gas-aerosol partitioning are based on vapour pressure or Koa. Herzke et al. (2010) conducted a study regarding the potential for ATE to undergo long range transport (LRT) and found that a log Koa of 9.05 and a log Kaw of -2.96 (Table 3-1) indicates that ATE has the potential to undergo LRT. However, its short atmospheric half-life suggests that transport will be limited to the near source environment since the substance is expected to incur degradation soon after release to air when in the gas phase. Ma et al. (2012) reported that ATE was present in both the particle and vapour phases in the Great Lakes region (ECCC 2016). When associated with particulates (aerosol), it is expected that ATE would be more persistent and amenable to long range transport.
The Transport and Persistence Level III Model (TaPL3) (TaPL3 2000) was used to estimate the Characteristic Travel Distance (CTD) defined as the maximum distance traveled in air by 63% of the substance. Beyer et al. (2000) have proposed CTDs of greater than 2000 km as representing high long-range atmospheric transport potential (LRATP), 700 to 2000 km as moderate LRATP, and less than 700 km as low LRATP. On the basis of the CTD estimate of 130 km, the long-range atmospheric transport potential of ATE is considered to be low.
The OECD POPs Screening Model can be used to help identify chemicals with high persistence and long-range transport potential (OECD 2006). The Characteristic Travel Distance (CTD) calculated for ATE using the OECD model is 120.0 to 122.0 km indicating that ATE still has the potential for transport in air, but this is below the boundary (5097 km, CTD of PCB-28) suggested for global pollutants by Klasmeier et al. (2006) and Liagkouridis et al. (2015). The model also calculates an overall persistence (Pov) of 260 days, as well as the transfer efficiency (TE), which is the percentage of emission flux to air that is deposited to the surface (water and soil) in a remote region. The TE for ATE was calculated to be 8.18x10-3%, which is below the boundary of 2.248% (PCB-28) established on the basis of the model’s reference substances empirically known to be deposited from air to soil or water. The TE for ATE of 0.01% was estimated by Liagkouridis et al. (2015) using the OECD Overall Persistence (Pov) and Long-Range Transport Potential (LRTP) Screening Tool.
The low TE suggests that ATE is not deposited to a high degree to remote regions. Detection of ATE in the Great Lakes area, Vancouver and urban centres in the US (Shoeib and Jantunen 2014; Ma et. al. 2012, Shoeib et al. 2012) suggests that there are local (urban) sources of ATE or the substance is transported in the gas phase.
The OECD POPS tool models a particulate (aerosol) matter sub-compartment in air, whereas the TaPL3 model considers the liquid phase sub-compartment in air (i.e., partitioning from the gas phase into water phase and then as rain deposition to soil and water) but does not consider particulate. On the basis of the vapour pressure of 0.013 Pa and the Log Koa of 9.055, the majority of ATE is expected to reside in the gas phase (~99%).
In summary, ATE is expected to predominantly reside in the gas phase in the atmosphere, soil and sediment. On the basis of the physical chemical properties and some models, ATE is not expected to be a high concern for long range transport. With ATE’s low predicted transfer efficiency and its short atmospheric half-life, the transport will be limited to the near source environment since the substance is expected to incur degradation soon after release to air when in the gas phase.
Empirical data indicated that ATE will not undergo photodegradation in the visible range; rather, photodegradation should occur from ultraviolet radiation (Study Submission 1996i.
8.2 Environmental persistence
On the basis of the expected releases of ATE and partitioning characteristics, environmental persistence is relevant for the water, soil and sediment compartments. However, because of frequent detection of ATE in air, this media will also be considered. Modelled predictions for ATE in air indicate a half-life of less than a day (gas phase) and persistence (Pov) of 171 days (4107 hours) (Scheringer et. al 2009). Empirical data indicate that ATE is persistent in soils and sediments; however, the modelled data suggest that ATE will mineralize in months and likely within less than a year in soil and sediments. These conflicting results are likely owing to the fact that if ATE is adsorbed on to particulates, degradation is longer (Table 8-2). Environmental monitoring in Canada reflects levels of ATE in the indoor and outdoor environment. Empirical data, and Level III fugacity modelling results in conjunction with their physical-chemical properties, indicate that soil and sediment are the key environmental reservoirs for ATE. Empirical and modelled data for ATE were considered in order to provide the best possible weight-of-evidence for the persistence of ATE and its metabolites or transformation products. Potential ATE metabolites were reviewed based on metabolism modeling. Table 8-2 presents fugacity modeling and abiotic modelled degradation date for ATE.
8.2.1 Abiotic degradation
Hydrolysis of ATE was determined using the OECD Test Guideline 111 (Study Submission 1996g) (Table 8-2). Results showed less than 10% hydrolytic degradation after 5 days at 50˚C under acidic, neutral and alkaline conditions (pH 4, 7, 9). The corresponding half-life (t½) at 50˚C is greater than 1 year.
Results from an ultraviolet-visible absorption spectrum study of ATE (Study Submission 1996i) provided an indication of the wavelengths at which ATE may be susceptible to direct natural sunlight photo degradation (limited to the region between 290 and 800 nm). No absorption peaks were observed in the visible wavelength range Results from a study on the photochemical behaviour of flame retardants, indicated that ATE can undergo photochemical transformation under simulated sunlight irradiation (Zhang et al. 2016). Calculated direct photolysis half-life values relevant with solar irradiation for ATE ranged from 1.9 days in summer to 21.9 days in winter.
The predicted half-life for atmospheric degradation of ATE owing to its reaction with the hydroxyl radical is 0.33 days (12-hr day, AOPWIN 2010) overall OH rate constant of 32.42 x10-12 cm3/molecule-sec) (Table 8-2). The results of AEROWIN (2010) predicts a small fraction of ATE absorption to airborne particles (Phi = 0.007). This is consistent with the OECD Pops model which finds approximately 99% of ATE in air is present in the gas or aerosol phase, and has an overall persistence of 171 days for the substance.
| Medium | Fate process | Degradation value | Degradation endpoint / units | Methods | Reference |
|---|---|---|---|---|---|
| Air | Atmospheric oxidation (OH rate constant) | Half-life (t 1/2) | 0.33 days 32.42 x10-12 cm3/molecule-sec) |
QSAR Model | AOPWIN 2010 v1.92aa |
| Air | Ozone reaction | Half-life (t 1/2) | 0.955 days | QSAR Model | AOPWIN 2010 v1.92aa |
| Air | Atmospheric oxidation (OH rate constant) | Half-life (t 1/2) | 3.96 hours | QSAR Model | Kuramochi et al. 2014 |
| Water | Hydrolysis | Half-life (t 1/2) | > 5 days at pH 4,7,9 | OECD Guideline 111 | Study Submission 1996 |
| Water | Hydrolysis | n/ab | n/ab | QSAR Model | HYDROWIN 2010a |
| Water | Hydrolysis | Half-life (t 1/2) | 2880 hours | QSAR Model | Kuramochi et al. 2014 |
| Water | Photolysis | Half-life (t 1/2) | 1.9-21.9 days | GC-MSc | Zhang et al 2016 |
a EPIsuite (2010-2012).
b Model does not provide an estimate for this type of structure.
c Gas chromatography/Mass spectrometry
8.2.2 Biodegradation
Qualitative modelled primary and ultimate degradation data for ATE (BIOWIN 2010) are presented in Tables 8-2 and 8-3. Modelled data predict a short half-life of 0.330 days in air using AOPWIN suggesting that ATE is not highly stable in the air compartment.
Empirical studies indicate that ATE is unlikely to biodegrade under aerobic conditions. Removal of ATE was evaluated using the semi-continuous activated sludge test (SCAS) on activated sludge from a wastewater treatment plant (Study Submission 1989a). The percent recovery, on the basis of the total amount of the test substance added to the test system, was determined weekly and after 28 days. A high degree of removal of the test substance was attributed to adsorption to biosolids surfaces (91 to 95%), however, the substance was not found to biodegrade on the basis of dissolved organic carbon (DOC) analysis. Removal of biosolids by biodegradation is one of the main processes of ATE loss from water (Table 8-3).
A sediment ready aerobic biodegradation study was carried out according to Japanese Environmental Agency Method 392 (similar to OECD Test Guideline 301C – Modified MITI Test) (Study Submission 1989a) (Table 8-3). A maximum of 2% biodegradation was observed as measured by biological oxygen demand (BOD) indicating that ATE is not readily biodegradable.
There are limited empirical persistence data for ATE, and therefore, a QSAR-based weight-of-evidence approach was applied using the degradation models shown in Table 8-3. Given the ecological importance of the soil and sediment compartment and the fact that this substance is expected to reside in these compartments, biodegradation in soil and sediment were examined. The results of this approach show that ATE is also very stable in soil and sediment and is likely to present long-term exposures in these media.
The modelled persistence data from BIOWIN Sub-model 4 indicate that for ATE in water, significant primary degradation will not take place. Modelled data from BIOWIN Sub-models 3 and 6 also suggest that ATE will take months or longer to completely mineralize in water. However, results from BIOWIN Submodel 5 indicate that ATE will mineralize within months in water. Probability results from TOPKAT and CATABOL are contradictory with the TOPKAT model suggesting a faster rate of mineralization compared to CATALOGIC. CATALOGIC aerobic and anaerobic values indicate a slow rate of mineralization which is consistent with results from the empirical data.
Using an extrapolation ratio of 1:1:4 for water: soil: sediment biodegradation half-life (Boethling et al. 1995), it is expected that ATE is persistent in soil and sediment.
Hydrolysis could not be estimated for the ATE as there are no chemicals of structural comparability are contained in the training set of HYDROWIN 2010.
In summary, empirical data indicate minimal biodegradation and sorption of the substance on sediments or soils which will lengthen the half-life. The modelled data indicates that ATE has the ability to degrade to a small degree in the aqueous phase but is more recalcitrant in soil and biosolids. Aerobic results, including empirical and modelled data indicate that biodegradation will occur in the range of months rather than years. Overall, ATE is considered moderately persistent in water, air, soil, sediments and air.
| Medium | Fate process | Degradation value | Degradation endpoint / units | Methods | Reference |
|---|---|---|---|---|---|
| Activated sludge | Bio-degradation | 2% | 28-day Biodegradation BOD/% |
Semi-Continuous Activated Sludge (SCAS) Removability Test | Study Submission 1989a |
| Activated sludge | Bio-degradation | 91-95% adsorbed to sludge | 90-day Biodegradation/% |
Semi-Continuous Activated Sludge (SCAS) Removability Test | Study Submission 1989a |
| Water | Primary Bio-degradation (aerobic) | 2.93a “persistent” |
persistent | QSAR Model | BIOWIN 2010c |
| Water | Bio-degradation (aerobic) | 1.91a | Biodegrades in months | QSAR Model | BIOWIN 2010d |
| Water | Bio-degradation (aerobic) | 0.389b | not persistent | QSAR Model | BIOWIN 2010e |
| Water | Bio-degradation (aerobic) | 0.168b | persistent (does not biodegrade fast) | QSAR Model | BIOWIN 2010f |
| Water | Bio-degradation (aerobic) | 0.96b “biodegrades slowly” |
biodegrades | QSAR Model | TOPKAT 2004 |
| Water | Bio-degradation (aerobic) | % BOD = 2.1 | “biodegrades slowly” | QSAR Model | Catalogic 2012 |
a Output is a numerical score from 0 to 5.
b Output is a probability score for rapid biodegradation.
c Sub-model 4: Expert Survey (qualitative results)
d Sub-model 3: Expert Survey (qualitative results)
e Sub-model 5: MITI linear probability
f Sub-model 6: MITI non-linear probability
8.2.3 Transformation products
The model CATALOGIC (2012) predicted four possible transformation products (less than 5% each, and less than 0.05 mole versus 1 mole of parent) of ATE that demonstrate a less hydrophobic nature than their parent, and exhibit Log Kow values ranging from 3.6 to 5.10. The potential transformation products are presented in ECCC 2016, in decreasing order of molar ratios versus parent. Three of the four substances can be identified: 3-(2,4,6-tribromophenoxy)propane-1,2-diol (CAS RN 51286-98-7), benzene, 2,4-dibromo-1-(2-propenyloxy)- (CAS RN 69227-61-8), and 2,4,6-tribromophenol (CAS RN 118-79-6). All four transformation products are not expected to undergo ultimate biodegradation (EPISUITE 2012). The CATABOL model results also suggest that the four products exhibit no or low ultimate biodegradation potential, i.e., biodegrades slowly. The MITI Linear model (BIOWIN 5) results, however, indicate that the transformation products are expected to undergo fast primary biodegradation.
The persistence, of the two potential metabolites of ATE, 2,4,6-tribromophenol (2,4,6-TBP) and acrolein were evaluated. Vapour-phase 2,4,6-TBP was degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals with a half-life reaction in air estimated to be 34 days (WHO 2005). 2,4,6-TBP reached 49% of its theoretical biochemical oxygen demand in 28 days using an activated sludge inoculum at 30 mg/litre in the Japanese MITI test, a result that fails the criterion for ready biodegradability (CITI 1992) Acrolein is highly reactive in air and water and has short half lives in these media. Acrolein is also unlikely to partition from these compartments to soil or sediments.
8.3 Potential for bioaccumulation
The properties of the substance (log Kow, log Koa, molecular size and cross-sectional diameters) as well as bioaccumulation modelling and results from an empirical ATE biomagnification study were considered for evaluation of ATE.
No empirical studies on bioconcentration factors (BCFs) are available in the literature at this time. Results from a terrestrial food chain study (vegetation-caribou-wolves) (Morris et al. 2014 unpubl) indicated that ATE did not biomagnify (Table 8-5). Metabolism corrected kinetic mass-balance modelling was used for modeling bioaccumulation, thereby filling the corresponding empirical data gap. The results of the BAF and BCF modelling for ATE are 59 440 and 8 965 L/kg, respectively.
There is support for the hypothesis that ATE is a biotransformation product of 2,3-Dibromopropyl-2,4,6-tribromophenyl ether (DPTE CAS 35109-60-5) (Von Der Recke and Vetter 2007 and Ma et al. 2012). DPTE has reported use as a flame retardant in extrusion grade polypropylene (ICPS, 1997). Owing to the co-occurrence of ATE in seal samples contaminated with DPTE and relatively constant ratios of ATE/DPTE, Von Der Recke & Vetter (2007) proposed that the residues of ATE measured in seals likely originate from transformation of DPTE, with the main resulting product being ATE making up 68% of the initial pool of DPTE. It is noted that the production of DPTE ceased in the mid-1980s (Ma et al. 2012).
Bioaccumulation potential of the four transformation products of ATE, ranged from BCFs of 189 to 2 571 L/Kg wet-wt to bioaccumulation factors (BAFs) ranging from high 190 to 3 967 L/Kg wet-wt suggesting that there is some potential for these transformation products to accumulate in aquatic organisms l. Measured BCF values of 513 and 83 were measured in zebra fish (Brachydanio rerio) and fathead minnow (Pimephales promelas), respectively, for 2,4,6-TBP, suggest that the potential for bioconcentration of 2,4,6-TBP in aquatic organisms is moderate (WHO 1995). Exposure of bluegills (Lepomis macrochirus) to 2,4,6-TBP for 28 days resulted in a 20-fold bioaccumulation in edible tissue and 140-fold bioconcentration in viscera. Of the potential metabolites of ATE, acrolein is rapidly metabolized by organisms and does not bioaccumulate (Environment Canada, Health Canada 2000). A bioconcentration factor of 344 was reported for acrolein (Environment Canada, Health Canada 2000).
A modelled log Kow value of 5.59 for ATE (Table 3-1) suggests that this chemical has a high potential to bioaccumulate and biomagnify in biota as it is within the highly bioavailable and hydrophopic range of log Kow. In addition, the combination of log Kow of 5.59 and the modelled log Koa of 8.55 suggests that a terrestrial dietary exposure may be an important route for uptake in mammals. Gobas et al. (2003) suggest screening criteria for potential bioaccumulation of organic chemicals in air breathing animals of log Kow greater than or equal to 2 and log Koa greater than or equal to 6.
The results of the ATE BCF and BAF modelling are presented in Table 8-4. Bioconcentration factors ranging from ~2 300 to 8 965 L/kg and a BAF of 59 440 L/kg ww for mid-trophic level fish for ATE suggests that ATE has the potential to be highly bioaccumulated by aquatic biota. Since empirical BCF data are not available at this time to derive an empirically derived kM, the kM for ATE was estimated at 0.02/day for a 10 g fish at 15°C using the BCFBAF model v3.01 of EPIsuite (2012). The derivation of kM can be found in Arnot et. al (2009). The BCF and BAF of ATE were estimated using both structure-based models and a three trophic level kinetic mass-balance model. All estimates of BCF and BAF, except sub-model 1 of the BCFBAF model in EPIWIN v4.0, were corrected for metabolism because it represents a fundamental elimination pathway for many chemicals.
Investigations relating fish BCF data and molecular size parameters (Dimitrov et al. 2005, Sakuratani et al. 2008) suggest that the probability of a molecule crossing gill cell membranes as a result of passive diffusion declines significantly with increasing maximum diameter (Dmax). Results from the studies suggest that the probability decreases appreciably when Dmax is greater than ~1.5 nm and much more so for molecules having a Dmax of greater than 1.7 nm. On the basis of the3D analysis of ATE conformers calculated using the BCFmax Model with Mitigating Factors (Dimitrov et al. 2005), the maximum and effective molecular diameters of ATE range from 1.07 nm to 1.32 nm. This suggests that uptake of ATE is unlikely to be restricted from steric effects at the gill surface owing to its molecular size.
Bioaccumulation factor (BAF)
There are no empirical bioaccumulation data for ATE. High bioaccumulation factors obtained from modelling indicate that there is a potential for ATE to bioaccumulate in biota from exposure through both diet and water. Physical and chemical data for ATE suggest it will also have the potential to be bioavailable in the environment. It is unlikely to be limited by uptake restrictions across the gills owing to its steric hindrance. This indicates the potential for bioaccumulation and food chain transfer of this substance and exposure to wildlife. It may further indicate increased ecotoxicity potential.
Biomagnification factor (BMF)
BMF values describe the process in which the concentration of a chemical in an organism reaches a level that is higher than that in the organism’s diet, owing to dietary absorption (Gobas and Morrison 2000). A BMF exceeding 1 indicates that biomagnification is potentially occurring, and may be considered an indicator of the potential for uptake and accumulation in biota. Table 8-5 presents empirical BMF data for ATE. The BMFs are on the basis of lipid corrected arithmetic mean concentrations of ATE in caribou and wolf-caribou in the Bathurst region (Northwest Territories and Nunavut) (Morris et al. 2014 unpubl). The BMFs ranged from 0.072 to 0.16 for Caribou diet and 0.34 to 5.2 for the wolf; caribou liver and muscle, respectively. However, although the BMF values for wolf-caribou muscle exceeded 1, the values were determined to not be statistically significantly and ATE is considered to not biomagnify according to this study. The authors suggested that biomagnification of ATE was influenced by both environmental and metabolic transformation. A project carried out by Muir et al. (2014), however, illustrates that caribou to wolf biomagnification factors (BMFs) for ATE were greater than one (4.3) (Muir et al. 2014).
Trophic magnification factor (TMF)
The TMF is a measure of the averaged biomagnification potential of a substance within a studied food web under field conditions, and is estimated by correlating the normalized substance concentration in biota at different trophic levels.
There is a lack of trophic magnification of ATE in the terrestrial food web (0.57) compared to the high TMF value in the Lake Ontario pelagic food web (Muir et al. 2014). Muir et al. 2014 reported a trophic magnification factor of 3.1 for ATE in a Lake Ontario pelagic food web.
| kM (days–1) | Model and model basis | Endpoint | Value wet weight (L/kg) | Reference |
|---|---|---|---|---|
| ~0.02 /day (10 gram fish) | BCFBAF Sub-model 1 (linear regression) |
BCFa | 2 270 | BCFBAF 2010 v3.01 |
| ~0.02 /day (10 gram fish) | BCFBAF Sub-model 2 (mass balance) (Arnot-Gobas BCF for mid trophic fish) |
BCFb | 8 965 | BCFBAF 2010 v3.01 |
| ~0.01 | BCFmax with mitigating factors | BCFc | 5 623 | Dimitrov et al. 2005 |
| ~0.02 /day (10 gram fish) | BCFBAF Sub-model 3 (Gobas-mass balance) (Arnot-Gobas BAF for mid trophic fish) |
BAFb | 59 440 | BCFBAF 2010 v3.01 |
Abbreviations: kM, metabolic rate constant, BCF, bioconcentration factor; BAF, bioaccumulation factor.
a Result generated using weight, lipid and temperature from Arnot and Gobas 2003a study.
b Results generated using weight, lipid and temperature for a middle trophic level fish.
c Possible mitigating factors include ionization, molecular size, metabolism and water solubility.
| Test organism | Ratio of arithmetic mean ±Standard error | Reference |
|---|---|---|
| Caribou diet (Fall/winter) | 0.086 | Morris et al. 2014 unpubl. |
| Caribou diet (spring) | 0.072 | Morris et al. 2014 unpubl |
| Caribou diet (summer) | 0.16 | Morris et al. 2014 unpubl. |
| Wolf-Caribou muscle | 5.2 (not statistically significant) | Morris et al. 2014 unpubl. |
| Wolf-Caribou Liver | 0.34 | Morris et al. 2014 unpubl. |
| Wolf-Caribou Total body burden | 4.3 (not statistically significant) | Morris et al. 2014 unpubl. |
8.4 Environmental fate summary
ATE releases to the Canadian environment may occur during industrial use (i.e., product manufacturing), consumer or commercial use of the product, service life and disposal of the substance and product containing ATE. Releases may occur in both indoor and outdoor environments (Shoeib et al. 2012), and some releases are expected to wastewater. While there is some potential for ATE releases from in-service products where ATE is used as an additive flame retardant, there is an absence of data precluding accurate quantitation of environmental exposure owing to the leaching from commercial products or products available to consumers.
On the basis of its high sorption characteristics, it is expected that ATE will reside in biosolids, sediments, and soil. ATE exhibits faster primary degradation with slower ultimate degradation. Sorption will result in longer half-lives in soils and sediments. The high persistence of ATE means that there is a potential for levels to build-up over time in near-field in sediment and soil environments as a result of continuous emissions. ATE is expected to be bioavailable and an elevated bioaccumulation potential indicates that ATE may accumulate in organisms while the potential for biomagnification cannot be ruled out.
9. Potential to cause ecological harm
9.1 Ecological effects
Physical and chemical properties, such as the log Kow and log Koa indicate that ATE has the potential to be bioavailable to aquatic and terrestrial organisms. According to the OECD QSAR Toolbox (2012) profile, the mode of action for ATE is classified as “reactive unspecified”. The extremely reactive vinyl group has potential for harm to aquatic, soil and benthic organisms.
Using an in silico approach, Kharlyngdoh et al. (2015) and Pradhan et al 2015 showed that ATE is a potential potent androgen receptor antagonist (RA). Kharlyngdoh et al (2015) also reported that ATE impacted the expression of L-type amino acid transporter system (LAT) genes that are needed for amino acid uptake across the blood-brain barrier. The disruption of LAT gene function has been impacted in several brain disorders.
Pradhan et al. (2015) analyzed ATE for zebrafish AR modulating properties. Using in silico analysis with two softwares, Molecular Operating Environment (MOE) and Internal Coordinate Mechanics (ICM), the results indicated that ATE has the potential to act as a zebrafish AR antagonist.
The avian embryonic hepatocyte in vitro screening method was used to assess the effects of organic flame retardants, including ATE, on cytotoxicity and mRNA expression of genes associated with xenobiotic metabolism, the thyroid hormone pathway, lipid metabolism, and growth (Porter et al. 2014). Exposure of ATE to chicken and herring gull embryonic hepatocytes was shown to decrease viability of chicken embryonic hepatoctyes (CEH) with an LC50 value of 115 ±36 µM. No adverse effect was reported for herring gull hepatocyte cell viability. ATE was also reported to bind to and activate the aryl hydrocarbon receptor (AhR), and to unregulate the Cytochrome P450 1A4 mRNA (CYP1A4) (a phase I metabolizing enzyme) expression by 11-fold, in chicken hepatocytes.
Crump et al. (2016) used methods developed for chicken embryonic hepatocytes to compare endpoints with a fish-eating bird that would be naturally exposed to environmental pollutants, the double-crested cormorant (Phalacrocorax auritus). ATE was shown to decrease cormorant hepatocyte viability in a similar manner to that observed in studies with CEH.
There is experimental evidence that ATE causes harm to aquatic freshwater organisms following short-term (acute) exposure at low concentrations. Empirical aquatic toxicity tests results for Daphnia magna and fish are available (Study Submission 1989b, Study Submission 1990a, 1990b). Values ranged from greater than 0.019 to 0.40 mg/L (Table 9-1).
Although the majority of ATE is expected to reside in soil, or sediment compartments or the lipid fraction of biota, e.g. bioavailable solute fraction of ATE is 0.32% (Arnot and Gobas 2008), only aquatic toxicity data are available for ATE. No empirical data are available for chronic effects to ATE.
9.1.1 Aquatic empirical studies
The acute toxicity of ATE to Daphnia magna and Bluegill sunfish (Lepomis macrochiris) were assessed. The results of the 48-hour static Daphnia magna toxicity studies were 0.26 mg/L and 0.40 mg/L (Study Submission 1989b, Study Submission 1990b) (Table 9-1) resulting in immobility and abnormal effects (e.g., Daphnids were observed surfacing, clumping together, and at the bottom of the test chambers). The results of the static Bluegill sunfish toxicity study using ATE indicated a 96-hour no-observed effect concentration of 0.21 mg/L which was on the basis of the lack of mortality and abnormal effects at this concentration.
9.1.2 Aquatic modelled studies
Modelled aquatic toxicity data determined by EPI Suite (2012) and TOPKAT (2004) have been summarized in Table 9-2. Sub-cooled values were used for EPIsuite modelling. Modelled values are above the water solubility of 0.76 mg/L and are not considered in this report. Vinyl/allyl ether acute effect for fish and Daphnid were an order of magnitude lower than the water solubility (0.089 to 0.16) mg/L EPI Suite 2012). Chronic modelled values for neutral organics ranged from 0.023 to 0.041 mg/L for fish and Daphnid, respectively.
9.1.3 Critical body residue (CBR) estimation
Critical Body Residue (CBR) analysis were undertaken to address exposures to fish via the food web and uptake from water as ATE has the potential to bioaccumulate. The toxicity potential from dietary uptake was investigated on the basis of the behaviour of ATE to highly partition to sediment and soil coupled with a high degree of environmental stability and bioaccumulation potential via the diet. Exposure via the dietary intake is the scenario of most concern for the ATE as it has been identified as bioavailable. Although there are toxicity studies for water, the CBR was applied to confirm results from dietary sources.
The CBR concept was therefore applied to investigate the potential for lethality in fish from the dietary uptake of bioavailable ATE. This concept considers whether the uptake of a chemical from the environment can accumulate to critical body burden levels associated with mortality. McCarty (1986, 1987a, 1987b, 1990), McCarty and Mackay (1993), McCarty et al. (1985, 1991), Van Hoogen and Opperhuizen (1988), and McCarty et al. (2013) have shown that internal concentrations of neutral narcotic chemicals in fish causing death are fairly constant at about 2 to 8 mmol/kg for acute exposures and 0.2 to 0.8 mmol/kg for chronic exposures. McCarty and MacKay 1993 and Escher et al. (2011) provide the mathematical formula as follows:
CBR = [BAF (5% lipid) x water concentration of chemical] / MW
Where:
CBR = critical body residue in fish (mmol/kg)
BAF 5% lipid = can be BAF or BCF lipid normalized to 5% (50 685 L/kg)
MW = molecular weight of the substance (370 g/mol)
Predicted Chemical concentration in water near an industrial site using ATE (4. x 10-6gm/L) (Section 8.2.2)
The CBR for ATE was estimated to be 5.5x10-4 mmol/kg which is below the internal narcotic thresholds for acute and chronic lethality. However, this does not rule out the potential for non-lethal effects which cannot be quantified following this methodology. Moreover, the fugacity ratios for biota-diet (1.65) indicate that ATE has the potential to biomagnify in fish via the diet. The fugacity capacity values (Zwater = 0.37 vs Zbiota = 8005 vs Zdiet12 007 mol/(m3 · Pa) show that the greatest exposure presented to aquatic biota is via the diet.
Although the CBR results indicate that ATE does not have the potential to bioaccumulate to levels in tissues that exceed lethality thresholds for narcotic chemicals, when the empirical water solubility (0.24 mg/L) is considered as the predicted environmental concentration (PEC), there is a potential for toxic effects.
Consistent with the fact that the majority of ATE is expected to reside in soil or sediment compartments, i.e. bioavailable solute fraction of ATE is 0.32% (Arnot and Gobas 2003a), fugacity results indicate that aquatic toxicity results are not the most environmentally relevant for this substance.
No measured toxicity data are available for other environmental compartments.
9.1.4 Transformation products
ECOSAR (2012) classified the transformation products of ATE as neutral organics, vinyl/allyl ethers and phenols on the basis of its chemical structure. The acute and chronic toxicity of the transformation products of ATE are estimated to range from 0.04 mg/L to 1.47 and 0.06 to 0.26 mg/L, respectively, indicating moderate to high toxicity.
Aquatic toxicity studies for the two potential metabolites of ATE, 2,4,6-TBP and acrolein, resulted in acute values ranging from 1.3 mg/L and 1.1 mg/L for Daphnia magna and fish (Cyprinus carpio), respectively for 2,4,6-TBP and a 96-hour LC50 of 0.007 mg/L for acrolein (Holcome et al. 1987) for the frog tadpole, Xenopus laevis, suggesting that both substances are acutely toxic. Studies indicate that terrestrial organisms are less sensitive to acute exposure to acrolein (Eisler 1994).
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Daphnia magna | Acute immobilization (48 hours) | EC50 | >0.019 | CHRIP-Japan 2008 (cites MOE) |
| Daphnia magna | Acute immobilization (48 hours) | EC50 | 0.40 | Study Submission 1990a |
| Daphnia magna | Acute immobilization (48 hours) | EC50 | 0.26 | Study Submission 1990b |
| Daphnia magna | 48 hour (absence of immobility) | NOEL | 0.23 | Study Submission 1990a |
| Daphnia magna | 48 hour (absence of immobility) | NOEL | 0.16 | Study Submission 1990b |
| Bluegill Sunfish (Lepomis macrochiris) | Acute (96 hour) | Acute (96 hour LC50) | >0.21 | Study Submission 1996 |
| Bluegill Sunfish (Lepomis macrochiris) | Acute (96-hour) | NOEL | 0.21 | Study Submission 1996 |
| Fish | Acute (96-hour) | Acute (96 hour LC50) | >0.025 | CHRIP/NITE 2005 |
Abbreviations: EC50, the concentration of a substance that is estimated to cause an effect on 50% of the test organisms;
LC50, the concentration of a substance that is estimated to be lethal to 50% of the test organisms;
NOEL, no-observed-effect level, exposure level at which there are no statistically or biologically significant increases in the frequency or severity of any effect between the exposed population and its appropriate control.
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Fish | Acute (96 hours) | LC50 | 0.26 | ECOSAR 2012 v1.11a(neutral organic SAR – baseline toxicity) |
| Fish | Chronic | NS | 0.023 | ECOSAR 2012 v1.11 (neutral organic SAR – baseline toxicity) |
| Fathead minnow (Pimephales promelas) | Acute (96 hours) | LC50 | 0.26 | ACD/Labs v14.0 |
| Fathead minnow (Pimephales promelas) | Acute (96 hours) | LC50 | <0.098 | CPOPS 2012 |
| Daphnia | Acute | 48 hr EC50 | 0.09 | ECOSAR 2012 v1.11 (neutral organic SAR – baseline toxicity) |
| Daphnia | Acute | 48 hr EC50 | <0.045 | CPOPs 2012 |
| Daphnia | Chronic | NS | 0.040 | ECOSAR 2012 v1.11 (Vinyl/allyl ethers) |
| Daphnia | Chronic | NS | 0.041 | ECOSAR 2012 v1.11 (neutral organic SAR – baseline toxicity) |
| Green algae | Acute | 96-hr EC50 | 0.26 | ECOSAR 2012 v1.11 (Vinyl/Allyl ethers) |
| Green algae | Chronic | NS | 0.14 | ECOSAR 2012 v1.11 (Vinyl/allyl ethers) |
Abbreviations: EC50, the concentration of a substance that is estimated to cause an effect on 50% of the test organisms; LC50, the concentration of a substance that is estimated to be lethal to 50% of the test organisms; NS: not specified
9.1.5 Derivation of the PNEC and rationalization of the assessment factors
9.1.5.1 Water
A predicted no-effect concentration (PNEC) was derived from the acute toxicity value of 0.26 mg/L (as the most sensitive, valid experimental value) for Daphnia magna. The PNEC was obtained by dividing the acute toxicity value by an assessment factor of 100 to account for interspecies and intraspecies variability in sensitivity (10) and to account for short-term to long-term effects (10) to give a value of 0.0026 mg/L.
9.1.5.2 Soil
As no soil toxicity data were available for ATE and no acceptable analogues with soil toxicity data were located, no quantitative results were determined for the substance.
9.1.5.3 Sediment
As no sediment toxicity data were available for ATE and no acceptable analogues with soil toxicity data were located, no quantitative results were determined for the substance.
9.1.5.4 Wildlife
An oral acute toxicity study in Sprague-Dawley rats reported a lethal dose, (LD50) greater than 2000 mg/kg-bw/day (Study Submission 2013). No mortality or treatment-related changes in body weight were observed over the 14-day observation period after treatment. Clinical signs were observed after treatment, including decreased activity, wobbly gait, faecal/urine staining, soft/mucoid stools and dark material around the facial area, but disappeared by day 4 post-treatment. No treatment-related abnormalities were noted at necropsy. Studies of ATE in rats suggest that ATE may not be bioavailable for uptake.
The low oral rat toxicity and current low aquatic concentrations of ATE in the Canadian environment indicates that a wildlife predicted environmental concentration is not required at this time.
9.2 Ecological exposure
9.2.1 Exposure scenarios and predicted environmental concentrations (PECs) in Canada
ATE can be added as a flame retardant during the preparation of expanded polystyrene in Canada. An exposure scenario was developed on the basis of the use of the pure substance with off-site secondary treatment prior to wastewater effluent discharge to a variety of surface waters including rivers of varying size and a lake.
As ATE is used in the manufacture of commercial products and products available to consumers and can be present in industrial effluent, an aquatic exposure scenario was developed to estimate the concentration in aquatic ecosystems. Aquatic exposure to ATE is expected if the substance is released from industrial manufacture and formulation or to a wastewater system that discharges its effluent to a receiving surface water body. The estimated concentration of the substance in the receiving water near the discharge point of the wastewater system is used as the predicted environmental concentration (PEC) in evaluating the aquatic risk of the substance. Further details on the equation used to calculate the concentration in the aquatic environment are available in ECCC 2016.
Table 9-3 presents the range of the inputs used to estimate aquatic concentrations resulting from site-specific industrial uses close to industrial point discharge. The predicted environmental concentration (PEC) of ATE in the receiving water bodies was estimated to be in the range of 2.0x10-7 to 4.2x10-6 mg/L.
| Input | Value(s) | Justification and reference |
|---|---|---|
| Quantity (kg) | 100 to 10 000 | Section 71 survey information from one importer; quantity is assumed to be used by different expected clients |
| Loss to wastewater (%) | 0.1 | Professional assumption based on use |
| Wastewater system removal efficiency (%) | 91 | Predicted for secondary treatment (Study Submission 1989a) |
| Number of annual release days (days) | 250 | EC standard assumption for continuous activity within industrial facilities |
| Wastewater system effluent flow (m3/d) | 700 000 to 325 627 000 | Site specific wastewater treatment system data |
| Dilution factor (–) | 10.0 | Assuming an instantaneous dilution of the effluent, the dilution factor of a receiving watercourse was calculated by dividing the flow of either the facility effluent (in case of direct discharge to a watercourse) or the wastewater treatment (WWT) effluent (connected to the facility) by the 10th percentile of the annual distribution of the flow of the receiving watercourse. When this dilution factor was greater than 10, a maximum default value of 10 was used. A dilution factor of 10 was also used for those releases that occur in a lake, bay or basin. This maximum dilution factor represents exposures near the discharge point of the effluent |
An equilibrium sediment-water partition approach was used to estimate the concentration of ATE in bottom sediment. This approach is based on a partitioning principle described by the European Chemicals Agency (ECHA 2010) and incorporates two additional calculation methods. The first method is to estimate the substance’s concentration in the aqueous phase (dissolved) of the overlying water from its total concentration, according to studies by Gobas (2003 and 2010). The second method is to estimate a substance’s concentration in bottom sediment from its concentration in the aqueous phase of the overlying water on the basis of an equilibrium partitioning assumption between bottom sediment and overlying water described by the USEPA’s National Center for Environmental Assessment (USEPA 2003). At equilibrium, the predicted environmental concentration (PEC) in bottom sediment can linearly correlate with the concentration in the aqueous phase of the overlying water. Sediment exposure scenarios were developed as an extension of the industrial aquatic release scenarios described above to determine equilibrium sediment PECs, standardized to 4% organic carbon (typical organic carbon content in bottom sediment for rivers and lakes). The resulting PEC in bottom sediment ranged from 3.45x10-4 to 1.0x10-2 mg/kg dw.
An approach described by the European Chemicals Agency (ECHA 2010) was used to estimate predicted environmental concentrations in soil (soil PECs) resulting from the land application of wastewater biosolids. This approach employed the quantity of biosolids accumulated within the top 20 cm layer (ploughing depth) of soil over 10 consecutive years as the basis for soil PECs. One underlying assumption of the approach was that substances were subject to no loss because of degradation, volatilization, and leaching and soil run-off upon their entry into soil via biosolids land application. This assumption, therefore, yielded conservative soil PECs. Soil exposure scenarios were developed as an extension of the aquatic and sediment release scenarios described above, using biosolids concentration and production rates on the basis of site specific wastewater treatment plants. Concentrations in biosolids ranged from were1.0x10-6 to 1.0x10-3 in Canada. Soil PECs were standardized to 2% OC.
| Use/Sector | PEC water (mg/L) | PEC sediment (4%OC) (mg/kg dw) |
PEC soil (2%OC) (mg/kg dw) |
|---|---|---|---|
| Preparation of expanded polystyrene | 2.010-7 to 4.2x10-6 | <3.56x10-4 to 1.0x10-2 | <1.0x10-6 to 3.0x10-3 |
9.3 Characterization of ecological risk
The approach taken in this ecological evaluation is to examine various supporting information and develop conclusions on the basis of a weight-of-evidence approach and using precaution as required under CEPA. Lines of evidence considered include results from a conservative risk quotient calculation, as well as information on persistence, bioaccumulation, inherent or ecological toxicity, and sources, fate of the substance and presence and distribution in the environment.
9.3.1 Risk quotient analysis
A risk quotient analysis, integrating conservative estimates of exposure with toxicity information, was performed for the aquatic medium to determine whether there is potential for ecological harm in Canada. The industrial scenario presented above yielded predicted environmental concentrations (PEC) of 2.0x10-7 to 4.2x10-6 mg/L. A predicted no-effect concentration (PNEC) of 0.0026 mg/L was derived from the acute toxicity value of 0.26 mg/L (see the Ecological Effects section). The resulting risk quotients (PEC/PNEC) are 7.7x10-5 to 1.6x10-3. Therefore, harm to aquatic organisms is not likely near an industrial facility using ATE in Canada.
| Compartment | Scenario | PNEC | PEC | RQ |
|---|---|---|---|---|
| Water | Industrial release to water | 0.0026 mg/L | 2.0x10-7 -4.2x10-6 mg/L | 7x10-5 to 1.6X10-3 |
As there are no soil toxicity data or appropriate analogues available for ATE, a risk quotient analysis cannot be determined for soil organisms. As no sediment toxicity data or acceptable analogue data were available for ATE, a risk quotient was not estimated for benthic organisms.
A Wildlife PEC was not derived as the results of the available mammalian toxicity data (i.e., relevant to the oral acute toxicity study in Sprague-Dawley rats) indicated a low level of oral toxicity and this suggested that harm to wildlife is unlikely for these industrial scenarios.
9.3.2 Consideration of lines of evidence
Current releases of ATE levels to the environment as a result of industrial uses as an additive and reactive flame retardant are expected to be low. Release from the use of commercial products or products available to consumers are expected to be minimal and diffuse. All industrial exposure calculations were on the basis of the current Canadian industrial use quantity data. A table summarizing the major lines of evidence for risk characterization is presented in Appendix B.
ATE is not expected to be persistent in air and is anticipated to mineralize within a year in water. ATE is expected to be found mainly in soil and sediment where it is expected to be highly persistent. There is however a lack of measured exposure data in soil or sediment in Canada.
ATE has the potential for high bioaccumulation and modelling results suggest biomagnification is possible. ATE may be used to replace other flame retardants and as such importation volumes may increase. However, current uses and sampling of wastewaters and biosolids at wastewater treatment systems across Canada, do not suggest the potential for widespread release to the Canadian environment. Models indicate that ATE does not have the potential for long range transport; however, monitoring data show that ATE has been measured in northern Canada. The source of the substance may be as a result of industrial releases, weathering of products available to consumers, biotransformation of other tribromophenoxy compounds or gas phase transport. Empirical and modelled data indicate that ATE has a high potential for toxicity to aquatic organisms.
An analysis was conducted of potential stable transformation products and potential metabolites. The results showed that the ATE transformation products are expected to represent only a minor fraction (up to 4.68%) relative to parent form. Modelling results indicated that the transformation products are predicted to have low to moderate potential to accumulate in aquatic organisms and have moderate to low toxicity.
Both modelled and empirical aquatic toxicity data indicate that ATE has the potential to cause adverse at low concentrations. However, the PEC in water estimated for industrial release is below the PNEC for aquatic organisms. Using the water PEC, a critical body residue (CBR) analysis for fish showed that the resultant ATE tissue residues were below the internal narcotic thresholds for both acute and chronic lethality. However, using the water solubility limit for ATE, thresholds for both acute and chronic lethality were exceeded showing that a toxic hazard because of lethality is nevertheless possible. No soil, sediment or wildlife toxicity data are available. No effect (oral LD50) greater than 2000 mg/kg-bw/day in Sprague-Dawley rats suggests that harm to wildlife is unlikely for current industrial scenarios. This information indicates that ATE has low potential to cause ecological harm in Canada.
9.3.3 Uncertainties in evaluation of ecological risk
For ATE, there is uncertainty in several key lines of evidence. Although there is a lack of experimental data for some key experimental data, the substance is within the QSAR dataset. There is a moderate to high level of confidence with these properties.
The exposure evaluation focuses on industrial point sources as being most relevant for ATE in the environment. The absence of landfill leachate data presents an uncertainty in terms of assessing the validity of this assumption. No ATE landfill leachate concentrations have been reported to date, but such data could help interpret end-of-life releases. Additive flame retardants, such as ATE, would be expected to migrate from both in service and end-of-life manufactured products (e.g., in EPS foam) as shown by concentrations in household dust (see section10.1.1.3). Additional manufactured item exposure scenarios (e.g., landfill leachate to address end of life manufactured items and dust disposed of as solid waste) could not be developed with the information currently available, adding uncertainty to the overall exposure characterization. Finally, while the majority of landfills in Canada treat their leachate through wastewater treatment systems, landfills that do not collect and treat their leachate may potentially release substances to ground or surface water via leachate.
Although data indicates that ATE may cross the blood-brain barrier, data is lacking to develop this reasoning.
Further empirical investigation is warranted to determine levels of ATE in dust, landfill leachate and environmental media since the information currently available is limited and uncertain. Similarly, releases from industrial transport container cleaning were not considered in a quantitative manner because of a high degree of uncertainty. Conservative assumptions were made, as described in ECCC 2016, but overall there is a moderate confidence with the exposure scenarios used to generate PEC values.
Although there is a lack of empirical data for bioaccumulation and bioconcentration, the substance is within the model domain. The major uncertainty surrounds the metabolic rate constant, kM and whether biomagnification is expected to be a significant factor. There is moderate to high confidence with the bioconcentration and bioconcentration data.
The critical body residue (CBR) analysis relies on the modelled BAF as the primary model input for calculation of tissue residues in fish. Given the lack empirical data, there is a low to moderate level of confidence in estimates of CBR.
On the basis of the predicted partitioning behaviour of ATE, there is uncertainty related to the soils and sediment exposure scenarios. Also, with the physical and chemical data, ATE is expected to partition to soils and sediment; however, no soil or sediment toxicity data are available. Thus, the risk to the environment may be underestimated because of this data gap.
There are data gaps on the toxicity of ATE to wildlife. The oral mammalian toxicity data available for ATE was not applicable for use in the wildlife model therefore there is high uncertainty regarding the potential effects of ATE on wildlife.
There is also uncertainty in the standard emission scenarios used to calculate PECs in water, soil and sediment. In addition, estimation was carried out on only one source of use. A number of the model parameters are known to be variable (emission factors, removal rate in WWTPs, biosolids adsorption, effluent release limits) and will contribute to a range of predicted environmental concentrations. There is a low to moderate level of confidence with the emission scenarios used to generate PEC values. Emission scenario values portray a relative comparison of exposure potential to ATE.
Risk quotient analysis was conducted to account for exposure to water contaminated with ATE. Given that the uncertainties in the PEC values are moderate to high, the confidences in the data are low to moderate.
10. Potential to cause harm to human health
10.1 Exposure
10.1.1 Environmental media and food
ATE is typically used as a flame retardant in electronic products, cable and wire coating and expandable polystyrene (EPS) with potentially similar uses in Canada. As a reactive flame retardant, ATE may be considered to be chemically bound to the polymer matrix for some products (e.g., polyolefin and polyamide/polyimide wire insulation) which would limit the potential environmental emission over the service life of the product. With other products where ATE is an additive flame retardant (e.g. EPS foam), there may be a greater potential for environmental emission from the product. ATE has been monitored in various environmental compartments in Canada and these study results are presented above (section 7.1.1).
Based on the environmental monitoring data presented below, the highest total daily intake of ATE from environmental media and food is estimated to be 0.041 ng/kg-bw per day for children 0.5 to 4 years old (Appendix C).
10.1.1.1 Air
Ambient air
In Canada, Diamond et al. (2013) monitored outdoor air (no storage issue at that time, see Indoor Air section below) around the Great Lakes region and in the urban area of Toronto, Ontario. Diamond et al. (2013) collected passive and active outdoor air samples from various locations in Toronto in 2011. The mean concentration for ATE from active high volume sampling was <1 pg/m3 from one location operating 24 hours bi-monthly. Daily passive sampling occurred during a 3-month period in the summer and winter seasons over a one-year period at 6 locations in Toronto. Passive ambient air concentrations of ATE were not detected in the winter months, but ranged between 1-15 ng/PUF disc (polyurethane foam disc sampler) during spring and summer months (Diamond et al. 2013). ATE was detected in 100% of samples around Lake Ontario, in 88% of samples around Lake Huron, and in 87% of samples at a field site in Toronto, ON (Shoeib and Jantunen 2014). Ambient air concentrations ranged from 0.04-11.1 pg/m3, with the highest concentration found in Toronto, ON (Shoeib and Janunten 2014). Monitoring in Chicago, Illinois and Cleveland, Ohio yielded vapour and particle concentrations from 24-hr sampling ranging from 0.012 to 15 pg/m3 (Ma et al. 2012). Concentrations in U.S. rural areas around the Great Lakes were found to be lower, ranging from 0.012 to 2.5 pg/m3 (Ma et al. 2012).
Ambient air concentrations from Norway, Sweden and Denmark were measured in the TermaNord European study between 2009 and 2010. The highest outdoor air mean concentration reported for an urban area (Oslo, Norway) was 0.27 pg/m3. During the same period, the lowest mean concentration was <0.016 pg/m3 in Copenhagen, Denmark (TemaNord 2011). The Global Atmospheric Passive Sampling (GAPS) program, which monitored several ‘new’ brominated flame retardants at various urban, rural and agricultural sites from each continent (n=31), measured ATE with a detection frequency between 60-78% globally. No further quantitative data are available from this study (Lee et al. 2010).
The maximum mean concentration reported in ambient air (11 pg/m3) from a Canadian urban area, i.e., Toronto (Shoeib and Janunten 2014) was used to derive an estimate of daily intake for the Canadian population.
Indoor air
ATE has been detected in indoor air in Canada and other northern European countries. In Canada, indoor air measurements from over 20 homes and offices in Toronto between 2010 and 2012 were collected and analysed for ATE (Diamond et al. 2013). Air samplers were placed in the bedroom, living room and/or kitchens of the homes. Storage methods limited the ability to quantify ATE levels due to the retention of ATE on the glass and container walls; however, the results indicated the presence of ATE in indoor air. ATE mean concentrations inside an office building in Oslo, Norway, ranged from <1.3 to 1.7 pg/m3 (TemaNord 2011). Given the lack of Canadian data, the indoor air level of 1.7 pg/m3 (TemaNord 2011) found in Norway was selected to derive an estimate of total daily intake of ATE (Appendix C).
10.1.1.2 Soil and sediment
There were no identified reports of ATE in soil or sediment in Canada or elsewhere in North America. The low volatility and moderate to high KOW suggest that ATE will partition to biosludges, sediments and soil more readily than to water. Assuming biosolids from wastewater treatment systems is applied to soil in an agricultural field, the BASL4 model was used to predict a soil concentration of <1.0x10-6 to 3.0x10-3 mg/kg dw (section 8.2.2.3). The total daily intake was derived based on the upper bound PEC estimate.
10.1.1.3 Dust
ATE has been monitored in dust in multiple studies in Canada. A potential source of ATE in household dust is from electronic equipment (e.g. wire insulation) (Diamond et al. 2013, Shoeib et al. 2012). Despite the reactive nature of ATE, continued use of electronic equipment may lead to a breakdown of the polymer containing ATE into the dust stream.
ATE was targeted in the Canadian baseline study of halogenated flame retardants in household dust collected in 2007-2010 across13 Canadian cities within the Canadian House Dust Study (CHDS) as per the method described by Fan et al. (2016). ATE was detected in 67.7% of samples (n=631), and concentrations ranged from not detected (method detection limit [MDL] = 0.5 ng/g) to 1060 ng/g, with a median of 0.98 ng/g and 95th percentile of 26.3 ng/g (Kubwabo et al., manuscripts in preparation, Environmental Health Science and Research Bureau (EHSRB), Health Canada; unreferenced, dated June 5, 2017).
In a Vancouver study, a mean ATE dust concentration of 0.4 ng/g was measured, with a 95th percentile of 7.84 ng/g across 116 samples (Shoeib et al. 2012). In Toronto, dust was sampled in several homes over 3 years. In 2010, 4 homes were sampled, with an additional residence sampled in 2011. The maximum dust concentration in 2010/2011 was 110 ng/g (n=20) (Diamond et al. 2013). An additional study of 20 homes in 2012 did not detect concentrations in dust (detection limit of 5.3 pg/g [n: 28]) (Diamond et al. 2013). The difference in ATE dust levels between the 2 study campaigns may be due to differences in home age or age of articles in the monitored homes (Diamond et al. 2013). A recently published Canadian study measured ATE in dust in 35 homes and 10 offices in Toronto in 2012 (Abbasi et al. 2016). Concentrations of ATE in dust ranged from not detected (method detection limit = 0.015) to 30 ng/g with a higher mean concentration and higher detection frequency in offices (6.0 ng/g; 60%) than in homes (1.5 ng/g; 40%). Abbasi et al. (2016) also analyzed the association of ATE in dust from the 2012 study with dust on products in the same locations. ATE was detected at levels close to the method detection limit (0.015 ng) in about half of the products sampled (n=65), including flat screen TVs, PCs and household appliances. The authors showed that there was a positive correlation between the geometric mean concentrations of halogenated flame retardants (including ATE) in home and office dust with those in dust from the surfaces of electronic products.
The upper-bounding estimate of daily intake for the Canadian population from dust was derived using the 95th percentile of ATE concentrations in household dust from the Canadian House Dust Study (26.3 ng/g) (personal communication from EHSRB, Health Canada, dated June 5, 2017).
10.1.1.4 Water
Given its low water solubility (0.24 mg/L), ATE is expected to be found in low concentrations in water (Covaci et al. 2011). One report of a study monitoring ATE in surface water in Canada was identified. Muir et al. (2011) sampled 4 lakes in Ontario between 2005 and 2010 and measured low concentrations (up to 0.22 pg/L). ATE was also reported to be present in surface water in five areas of Norway below the method detection limit of 1.41 ng/L (DNV 2010) (section 8.2.1.2). The highest concentration found in Canadian surface water (0.22 pg/L) was used to derive an estimated intake from water.
10.1.1.5 Food
No reports of ATE in Canadian food were identified. In two European studies, monitoring of ATE in fish and shellfish was conducted (Appendix C). ATE levels in fish muscle tissue (char and perch) and bivalve shellfish (blue mussel) were reported by TemaNord (2011) in Norway. The reported ATE concentrations ranged from not detected (detection limit = 0.00096ng/g) to < 0.0046 ng/g and not detected (detection limit = 0.0019 ng/g) to 0.0045 ng/g in finfish and shellfish, respectively. ATE had previously been detected in a study by Von Der Recke (2007) in the blubber (5.4 – 9.1 ng/g) and brain (3.1 – 10 ng/g) of harp seals in the Greenland and Barents Seas (Covaci et al. 2011; EFSA 2012a). Given the potential for ATE to be present in foods consumed by humans, most notably fish, at concentrations up to 0.0045 ng/g, an upper-bounding estimate of daily intake from food for the Canadian general population was estimated to be 0.016 ng/kg-bw per day (for children aged 0.5-4 yrs). This is a conservative estimate and assumes that all seafood and fish consumed would contain ATE; although certain northern populations or other subpopulations in Canada may consume larger quantities of seafood or fish in their diet, this estimate is considered conservative enough to account for this variability.
10.1.2 Products available to consumers
ATE is primarily used as a flame retardant in expandable polystyrene (EPS) and polyamide insulation for cables and wiring (Ash and Ash 2002). Depending on the product, ATE may be classified as a reactive or additive flame retardant, a factor in the potential for exposure to this substance (Covaci et al. 2001). ATE is considered a reactive flame retardant for use in cable coatings and wire insulation. It is added during the polymerization process and becomes an integral part of the polymer (Harju et al. 2009). The resulting polymer containing ATE has a different molecular structure and properties. The flame retardant properties are retained; however, the modified structure inhibits migration from the product into the environment (Harju et al. 2009). During normal use of wires and cables in the home, direct exposure to ATE is not expected.
Globally, ATE is also commonly used as an additive flame retardant in EPS and polystyrene foam (Covaci et al. 2011). Uses of ATE in Canada are presumed to be in line with international uses. EPS has a number of consumer uses. EPS may be used in construction and building materials in such products as wall insulation and packaging material (Nova Chemicals 2012). Packaging material made from modified EPS is used only as protective foam for shipping. The packaging may be introduced into the home but is typically thrown away shortly after unpacking, limiting potential exposure to ATE.
Installation of wall insulation boards is typically an occupational activity but can occasionally be done by homeowners in renovation projects. Dermal exposure to ATE from such use is expected to be minimal based on the limited duration of contact with the product (only during installation) and based on the limited access to the ATE- containing EPS (insulation boards are typically bonded between oriented strand board or have paper or foil lining). This product is designed to be installed behind a drywall barrier. Modification to the boards (i.e. cutting) may result in a limited release of foam particulates containing ATE into the air, where it would partition to the dust stream. The general population may be exposed to ATE from dust in homes where modified EPS insulation is being handled and installed, which is accounted for in section 9.1.1.3.
Based on the physical and chemical properties of ATE, bioavailability of ATE is likely to be low. Exposure to ATE from products available to consumers is expected to be minimal based on its properties as a reactive flame retardant in polymers used in cable coatings and wire insulation and based on low potential for contact with modified EPS containing ATE.
10.2 Health effects
A limited number of empirical toxicity studies for ATE were identified.
An oral acute toxicity study in rats suggested low concern for acute toxicity, with a 50% lethal dose, (LD50), greater than 2000 mg/kg-bw/day (Study submission 2013). During the 14-day observation period after treatment, no mortality or treatment-related changes in body weight were observed. Transient clinical signs were observed after treatment, but disappeared by day 4 post-treatment. No treatment-related abnormalities were noted at necropsy.
ATE was found to be a weak skin sensitizer in guinea pigs (Study submission 2013).
Genotoxicity studies were limited to in vitro Ames assays conducted in bacteria. Results were negative in Salmonella typhimurium TA98, TA100, TA1535 and TA1557 in the presence and the absence of metabolic activation (Study submission 2013). Results were also negative in E. coli WP2 uvrA in the presence and the absence of metabolic activation (Study submission 2013).
No reproductive or developmental toxicity studies were identified. Ezechias et al. (2012) examined the potential for ATE to influence estrogen and androgen receptors using yeast reporter-gene assays in vitro. ATE was not found to exhibit any estrogenic or androgenic activity. One recent study reported ATE exhibit antiandrogenic activity in in vitro luciferase expression assay (Kharlyngdoh et al. 2015).
No repeated-dose toxicity or toxicokinetic studies were identified.
Several lines of evidence were investigated to help characterize the potential of ATE to be genotoxic or carcinogenic (Appendix D). Three approaches were used: the analogue approach, the quantitative structural activity (QSAR) approach and the structural alert approach.
In the analogue approach, the OECD(Q)SAR Toolbox (OECD 2009b, 2011, 2013) and OASIS TIMES (TIMES 2013) were used to help identify and evaluate potential analogues. No appropriate analogues were found using OECD(Q)SAR Toolbox. The OASIS TIMES predicted qualitatively a number of possible metabolites for ATE, two of which, 2,4,6-tribromophenol (2,4,6-TBP) and acrolein, are associated with available hazard data described in the next sections. There is low confidence in the metabolic prediction as ATE was outside the model domain; however, the predicted metabolic transformation appeared plausible based on commonly observed phase I metabolic transformation. With the lack of empirical toxicokinetic data, it should be noted that there is uncertainty whether ATE can metabolize to these two metabolites and the quantities of these metabolites.
In the QSAR approach, several statistically based QSAR models were used to assess the potential for ATE to induce chromosomal damage (in vitro and in vivo) and carcinogenicity. Limited results were obtained. In some cases, ATE or partial structure of ATE was outside the model domain. In other cases, only a low number of similar chemicals were within the model training set. For instance, the QSAR model, OASIS TIMES (TIMES 2013), identified alerts for ATE to potentially metabolize to epoxide intermediate and acrolein; however, 70% of the structural fragments of ATE were not covered within the structural domain of the model.
The third approach was used to identify any ATE structural alerts associated with genotoxicity or carcinogenicity in mammals. Genotoxicity models did not trigger any alerts. The carcinogenicity alert profiler triggered a non-genotoxic alert based on polyhalogenated aromatic structure (DEREK Nexus 2013). However, the confidence of this prediction was considered low. The mode of action for polyhalogenated aromatics and carcinogenicity is not well understood.
Overall, there was low confidence in the results from these other lines of evidence. As mentioned, although there is uncertainty associated with this prediction, ATE may have the potential to metabolize to 2,4,6-TBP and acrolein, and these substances are examined further in the next sections.
10.2.1 2,4,6-TBP
The International Program on Chemical Safety (IPCS) has published a Concise International Chemical Assessment Document (CICAD) on 2,4,6-TBP and other simple brominated phenols (IPCS 2005). The document described a number of genotoxicity tests (Ames, in vitro chromosomal aberration and in vivo micronucleus tests) of 2,4,6-TBP that were conducted according to OECD guidelines. All of the tests, except for the mammalian cell in vitro (chromosomal aberration) test, were negative. There was no evidence of genotoxicity in an in vivo micronucleus assay, up to the maximum tolerated dose. No long-term or carcinogenicity studies were identified. Described in the IPCS (2005) and Hamers et al. (2006), 2,4,6-TBP was found to be potent in binding to human plasma transport protein transthyretin (TTR) in vitro compared to the natural thyroxine (T4) ligand. TTR is one of the thyroid hormone binding transport proteins in plasma of vertebrates. Some in vitro reporter gene assays suggested that 2,4,6-TBP might exhibit antiestrogenic (Hamers et al. 2006; Ezechias et al. 2012) and antiandrogenic (Ezechias et al. 2012) activity. Hamers et al. (2006) also found 2,4,6-TBP to be a potent E2SULT (sulfation by estradiol sulfotransferase) inhibitor in vitro, suggesting that 2,4,6-TBP might influence estradiol. In an oral combined repeated-dose toxicity study with a reproduction/developmental toxicity screening test in rats, conducted according to OECD guidelines (using doses of 0, 100, 300 or 1000 mg/kg-bw/day of 2,4,6-TBP) (Tanaka et al. 1999 cited in IPCS 2005), no adverse effects were observed on estrous cycle, copulation index, fertility index and duration of gestation period, number of corpora lutea, and delivery findings as well as number of implants, number of total pups and live pups born, implantation index and delivery index in any of the substance-treated groups.
10.2.2 Acrolein
The Government of Canada previously assessed acrolein in a Priority Substances List report (Environment Canada, Health Canada 2000). A CICAD report and an OECD SIDS Initial assessment report were also available (IPCS 2002; OECD 2000). Acrolein is a highly reactive substance that will bind primarily at the site of contact. Following acute or repeated inhalation exposure, acrolein is cytotoxic and can induce histopathological effects in the bronchi and/or trachea. Acrolein is mutagenic in vitro but not in vivo. It was found that acrolein can react directly with DNA and proteins to form stable adducts in vitro; however, there was no increase in DNA-protein cross-links in the nasal mucosa of rats exposed in an acute inhalation study. Environment Canada, Health Canada (2000) and IPCS (2002) considered that there was inadequate data to assess whether acrolein has the ability to induce tumours or interact directly with DNA at the site of contact following inhalation. IPCS (2002) and OECD (2000) concluded that acrolein was not an oral carcinogen. For the oral route, three chronic toxicity or carcinogenicity studies were available, conducted with rats and mice. There was no significant increase in the incidences of tumours of any type in these studies.
10.3 Characterization of risk to human health
Limited empirical health effect data for ATE were identified. Analyses from other lines of evidence were inconclusive with respect to the potential for genotoxicity or carcinogenicity of ATE. Metabolic prediction tools qualitatively predicted potential metabolites of ATE, including 2,4,6-TBP and acrolein, for which hazard data are available.
Evidence suggests that 2,4,6-TBP is unlikely to be genotoxic. In vitro studies suggested that 2,4,6-TBP may have the potential to influence thyroid, estrogen and androgen hormonal systems but no associated adverse effects were observed in in vivo reproductive or developmental toxicity studies. Acrolein is a reactive substance that is associated with adverse effects primarily at the site of contact via the oral and inhalation routes. Acrolein was found to be mutagenic in vitro but not in vivo. IPCS (2002) and OECD (2000) concluded that acrolein is not an oral carcinogen.
Daily intake of ATE by the general population through environmental media (air, water, dust) and food was estimated to be less than a nanogram (i.e.,0.13 ng/kg bw/day). Direct exposure to ATE from products available to consumers is expected to be minimal based on its low bioavailability, its properties as a reactive flame retardant in polymers used in cable coatings and wire insulation, and a low potential for contact with modified EPS containing additive ATE. As intake of ATEby the general population through environmental media is estimated to be low and exposure from products available to consumers in Canada is expected to be negligible, the potential of harm to human health is considered to be low.
As an additional line of evidence, it is also noted that the estimated intake of ATE from environmental media and food for the general population in Canada of 0.13 ng/kg-bw/day is below the threshold of toxicological concern (TTC) value of 2.5 ng/kg bw per day originally proposed by Kroes et al. (2004). The TTC provides a generic reference point against which the range of estimated intakes can be compared. TTC values, which are derived using probabilistic approaches, establish generic chronic oral human exposure threshold values, below which it is expected that the probability of adverse effects of any substance is low. A TTC value of 0.15 µg/day (equivalent to 2.5 ng/kg bw per day) has been established for potentially carcinogenic substances with structural alerts for genotoxicity. Although this TTC value may not be applicable to ATE (ATE may not be genotoxic or carcinogenic), it is used because very limited hazard data is available for ATE and it is the lowest reference point against which exposure to ATE can be compared. Other higher TTC values have been established for different classes of non-genotoxic substances based on no-effect levels from chronic oral exposure (Munro et al. 1996a, b; Kroes et al. 2004; EFSA 2012b; Dewhurst and Renwick 2013).
Based on the above, the potential of harm to human health from exposure to ATE is considered to be low.
10.4 Uncertainties in evaluation of risk to human health
There are some uncertainties associated with the estimated intake of ATE from environmental media. While there are Canadian studies available for household dust, surface water and outdoor air, no Canadian indoor air studies were identified. There is uncertainty in the estimate of intake from food for the general population since it is based on data from Northern Europe for fish only. However, the estimate of total daily intake from exposure to environmental media and food is based on conservative assumptions (e.g., all seafood consumed would contain ATE).
Very limited empirical data on the toxicity of ATE were identified. No repeated-dose toxicity studies, chronic/carcinogenicity study, reproductive and developmental toxicity, toxicokinetics or human studies were identified. Due to the limited number of empirical health effects data and the low confidence in the other lines of evidence analysis including uncertainty in metabolic prediction, confidence in the health effect database for ATE is low. However, the collective evidence indicates that exposure of the general population to this substance is low.
11. Outcome
Considering all available lines of evidence presented in this SOS report, there is currently a low potential for harm to organisms and the broader integrity of the environment from ATE.
Based on the information presented in this SOS report, there is currently a low potential of harm to human health from exposure to ATE.
Although present estimated levels of exposure of ATE are not indicative of harm to the environment or to human health, there may be concerns if import and use quantities were to increase in Canada.
As ATE is a commercial alternative to other flame retardants, there is a possibility that quantities could increase in Canada. Given that ATE is not on the DSL, the substance will continue to be subject to section 81 of the Act and the New Substances Notifications Regulations (Chemicals and Polymers) of CEPA, which will ensure pre-market notification of any new importation or manufacturing of this substance and will allow restrictions to be put in place, as needed.
References
Abbasi G, Saini A, Goosey E, Diamond ML. 2016. Product screening for sources of halogenated flame retardants in Canadian house and office dust. Sci. Total Environ. 545–546:299–307.
ACD/Percepta [Prediction Module]. c1997-2012. Toronto (ON): Advanced Chemistry Development. [accessed 2014 July].
[AEROWIN] AEROWIN Program for Windows [Estimation Model]. 2010. Version 1.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [accessed 2014 January].
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2010. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Arnot JA, Gobas FAPC. 2003a. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337–345.
Arnot JA, Gobas FAPC. 2003b. Categorization of organic substances on the Domestic Substances List for bioaccumulation potential. Report to Environment Canada, Existing Substances Branch. June 2003. Gatineau (QC): Environment Canada.
Arnot JA, Mackay D, Bonnell M. 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 14: 257-297.
Arnot JA, Mackay D, Parkerton TF,Bonnell M. 2008a.Database of fish biotransformation rate for organic chemicals. Environmental Toxicology and Chemistry, Vol. 27, No. 11, pp. 2263–2270,
Arnot JA, Mackay D, Bonnell M. 2008b. Estimating metabolic biotransformation rates in fish from laboratory data. Environmental Toxicology and Chemistry, Vol. 27, No. 2, pp. 341–351
Arnot JA, Meylan W, Tunkel J, Howard PH, Mackay D, Bonnell M, Boethling RS. 2009. A quantitative structure-activity relationship for predicting metabolic biotransformation rates for organic chemicals in fish. Environ Toxicol Chem 28(6): 1168–1177.
Ash M and Ash I. 2003. Handbook of cosmetic and personal care additives. 2nd Edition. Synapse Information Resources Inc. ISBN-10: 1-890595-38-1.
Beyer A, Mackay D, Matthies M, Wania F, Webster E. 2000. Assessing long-range transport potential of persistent organic pollutants. Environ Sci Technol 34(4):699–703
[BCFBAF] Bioaccumulation Program for Windows [Estimation Model]. 2010. Version 3.01. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2010. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation].
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741−752.
Byer, J, Alaee, M, Brown, S, Lebeuf, M, Trottier, S, Backus, S, Blunt, S, Keir, M, Konefal, M, Pacepavicius, G, Hodson, P. 2010. Brominated flame retardants in American eel: a reason for the eel’s decline BFR 2010.
Canada. 1999. Canadian Environmental Protection Act, 1999 [PDF]. S.C., 1999, c. 33, Canada Gazette. Part III, vol. 22, no. 3.
Canada. 2005. Canadian Environmental Protection Act, 1999: New Substances Notification Regulations (Chemicals and Polymers). P.C. 2005-1484. SOR/2005-247. [accessed 2005 August 31]
Canada, Dept. of the Environment. 2013. Canadian Environmental Protection Act, 1999: Notice with respect to certain organic flame retardant substances [PDF]. Canada Gazette, Part I, vol. 147, no. 13, p. 613–633.
CASEUltraT [Prediction module]. 2013. Version 1.5.0.1. Beachwood (OH): Multicase Inc. [cited 2014 April 16]. www.multicase.com/products/prod03.htm [restricted access].
[CATALOGIC] [Computer model]. 2012. Version 5.11.6 Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry.
Chemtura. 2007. Technical information: PHE-65 halogenated flame retardant. Chemtura - Flame Retardants Overview [PDF, archived]. Chemtura Corporation.
[CHRIP] Chemical Risk Information Platform [database]. 2005. Tokyo (JP): National Institute of Technology and Evaluation, Chemical Management Centre (CMC). [accessed 2017 April 20].
[CHRIP] Chemical Risk Information Platform [database]. 2008. Tokyo (JP): National Institute of Technology and Evaluation, Chemical Management Centre (CMC). [accessed 2013 Nov 27].
[CITI].1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL. Japan. Tokyo, Chemical Inspection and Testing Institute.
Clement, RE, Reiner, EJ, Bhavsar, SP. 2012. Organohalogen contaminants of emerging concern in Great Lakes fish: a review. Analytical and Bioanalytical Chemistry, 404; p. 2639-2658.
Covaci A, Harrad S, Abdallah MA-E, Ali N, Law RJ, Herzke D, de Wit CA. 2011. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environment International 37: 532-556.
[CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada, Ecological Assessment Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Environment Canada, Ecological Assessment Division.
Crump D, Farhat A, Chiu S, Williams KL, Jones SP. 2016. Use of a novel double-crested cormorant ToxChip PCR array and EROD assay to determine effects of envornmental contamints in primary hepatoctyes. Environ. Sci. Technol. 50(6): 3265-3274.
DEREK Nexus [Toxicity Prediction Module]. 2013. Version 3.0.1. Leeds (UK): Lhasa Limited. [accessed 2014 April 15]. [restricted access].
Dewhurst I, Renwick AG. 2013. Evaluation of the threshold of toxicological concern (TTC)—Challenges and approaches. Regul Toxicol Pharmacol 65(1):168–177.
Diamond M, Gooset E, Saini A, Chaudhuri S. 2013. Exposure to new flame retardants in Canadian indoor environments. Contract prepared for Health Canada: October 2013.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
[DNV] The Norwegian Veritas AS. 2010. Environmental screening of selected new brominated flame retardants and selected polyfluorinated compounds.
[DPD] Drug Product Database. [modified 2017 Feb 17]. Ottawa (ON): Government of Canada. [accessed 2014 April 24].
Drage DS, Newton S, de Wit CA, Harrod S. 2016. Concentrations of legacy and emerging flame retardants in air and soil on a transect in the UK West Midlands. Chemosphere 148: 195-203.
Dwyer, F.J., D.K. Hardesty, C.G. Ingersoll, J.L. Kunz, and D.W. Whites. 2000. Assessing contaminant sensitivity of American shad, Atlantic sturgeon, and shortnose sturgeon. Action plan project Hudson River Estuary. United States Geological Survey.
[ECCC] Environment and Climate Change Canada. 2016. Supporting Documentation: Information in support of the State of the Science report for benzene, 1,3,5-tribromo-2-(2-propenyloxy)-. Gatineau (QC): ECCC. substances@ec.gc.ca
[ECHA] European Chemicals Agency. 2010. Guidance on information requirements and chemical safety assessment. Chapter R.16: Environmental exposure estimation. May 2010. Guidance for the implementation of REACH. Helsinki (FI): European Chemicals Agency
[ECOSAR] Ecological Structure Activity Relationships Class Program [Estimation Model]. 2012. Version 1.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [accessed 2014 January].
[EFSA] European Food Safety Authority. 2012a. Scientific opinion on brominated flame retardants in food: brominated phenols and their derivatives. EFSA Journal 10(4): 2534.
[EFSA] European Food Safety Authority. 2012b. Scientific opinion on exploring options for providing advice about possible human health risks based on the concept of threshold of toxicological concern (TTC). [PDF] EFSA J 10(7):2750.
Eisler R. 1994. Acrolein hazards to fish, wildlife, and invertebrates: a synoptic review. National Biological Survey, U.S. Department of the Interior, Washington, D.C. 29 pp. (Biological Report 23: Contaminant Hazard Reviews Report 28).
[ECCC] Environment and Climate Change. 2013-2014. Data for certain organic flame retardants substance grouping collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain organic flame retardant substances. Data prepared by: Environment and Climate Change Canada, Health Canada, Existing Substances Program
Environment Canada 2014. Legacy and current use flame retardants in Great Lakes atmosphere. Science and Technology Directorate, Burlington, Ontario, Canada
Environment Canada, Health Canada. 2000. Priority substances list assessment report: Acrolein: Chemical Abstracts Service Registry Number 3278-89-5 [Internet]. [PDF] Ottawa (ON): Environment Canada, Health Canada. [2014 May 6].
Environment Canada, Health Canada. 2007. Chemical substances: Categorization [Internet]. Ottawa (ON): Government of Canada. [updated May 25, 2013; cited July 31, 2013].
[EPISuite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2012. Version 4.1. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. In text reference: (EPISuite 4.1)
[EQC] Equilibrium Criterion Model. 2012. Version 1.0.Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. [accessed 2013 June ].
Escher BI, Ashauer R, Dyer S, Hermens JLM, Lee J-H, Leslie HA, Mayer P, Meador JP, Warne MSJ. 2011. Crucial role of mechanisms and modes of toxic action for understanding tissue residue toxicity and internal effect concentrations of organic chemicals. Integr. Environ Assess Manag. 7 (1): 28-49
[ESIS] European Chemical Substances Information System [database]. c1995–200[9]. European Chemical Bureau (ECB). [accessed 2014 December].
European Chemicals Bureau, Institute for Health and Consumer Protection. Technical Guidance Document on Risk Assessment [PDF] Report No.: EUR 20418 EN/2. 328p. Luxembourg: Office for Official Publications of the European Communities.
Ezechias M, Svobodova K, Cajthaml T. 2012. Hormonal activities of new brominated flame retardants. Chemosphere. 87(7):820-824.
Fan X, Kubwabo C, Rasmussen PE, Wu F. 2016. Non-PBDE halogenated flame retardants in Canadian indoor house dust: sampling, analysis, and occurrence. Environ. Sci. Pollut. Res. 23:7998–8007.
Fisk PR, Girling AE, Wildey RJ. 2003. Prioritisation of flame retardants for environmental risk assessment. UK Environment Science Agency: 2003.
Gilchrist TT, Letcher R, Thomas P, Fernie, KJ. 2014. Polybrominated diphenyl ethers and multiple stressors influence the reproduction of free-ranging tree swallows (Tachycineta bicolor) nesting at wastewater treatment plants. Sci. Total Environ. 472: 63-71.
Gobas FAPC, Kelly BC, Arnot JA. 2003. Quantitative Structure Activity Relationships for Predicting the Bioaccumulation of POPs in Terrestrial Food-Webs. QSAR Comb. Sci. 22: 329-336.
Gobas FAPC. 2010. Comments on approach to sediment exposure. Burnaby (BC): Simon Fraser University, School of Resource & Environmental Management, March 25.
Gobas FA, Morrison HA. 2000. Bioconcentration and biomagnification in the aquatic environment. In: Boethling RS, Mackay D, editors. Handbook of property estimation methods for chemicals, environmental and health sciences. Boca Raton (FL): CRC. p 189C. p
Great Lakes Solutions. 2010. Flame Retardants Product Guide. Philadelphia (PA): Chemtura Corporation. [accessed 2013 Aug 21]. Archived.
Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A. 2006. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci 92(1):157-73.
Harju, M, Heimstad, E, Herzke, D, Sandanger, T, Posner, S, Wania, F. 2009. Current state of knowledge and monitoring requirements; Emerging “new” brominated flame retardants in flame retarded products and the environment. Oslow (Norway): Norwegian Pollution Control Authority.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. [modified 2017 May 3]. Lists of Permitted Food Additives. Ottawa (ON): Health Canada Food Directorate. [accessed 2014 April 24].
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2010. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Herzke D, Harju M, Heimstad E, Dandanger T, Posner S, Wania F. 2010. Emerging “new” brominated flame retardants [PDF]: sources and transport.
Holcome GW, Phipps GL, Sulaiman AH, Hoffman AD. 1987. Simultaneous multiple species testing: Acute toxicity of 13 chemicals to 12 diverse freshwater amphibian, fish and invertebrate families. Arch. Environ. Contam. Toxicol. 16: 697-710.
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2010. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Huber S, Warner NA, Nygard T, Remberger M, Harju M, Uggerud HT, Kaj L, Hanssen L. 2015. A broad cocktail of environmental pollutants found in eggs of three seabird species from remote colonies in Norway. Environ. Toxicol. Chem. 34(6): 1296-1308.
IPC. [modified 2011 March 10]. Joint Industry Guide: Material Composition Declaration for Electrotechnical Products, JIG-101 Ed. 4.0 [PDF]. US: Consumer Electronics Association. [accessed 2018 Feb 21].
IPCS (1997). Flame Retardants: A general introduction. Environmental Health Criteria 192. International Program of Chemical Safety. World Health Organization.
[IPCS] International Programme on Chemical Safety. 2005. Concise International Chemical Assessment Document 66: 2,4,6-tribromophenol and other simple brominated phenols [PDF]. Geneva (CH): United Nations Environment Programme; International Labour Organisation; World Health Organization.On the cover: First draft prepared by Mr P.D. Howe, Dr S. Dobson, and Mr H.M. Malcolm, Centre for Ecology & Hydrology, Monks Wood, United Kingdom.
[IPCS] International Programme on Chemical Safety. 2002. Concise International Chemical Assessment Document 43: Acrolein. [PDF] Geneva (CH): United Nations Environment Programme; International Labour Organisation; World Health Organization. On the cover: First draft prepared by R. Gomes and M.E. Meek, Existing Substances Division, Bureau of Chemical Hazards, Health Canada, Ottawa, Canada, and M. Eggleton, Environment Canada, Hull, Quebec.
Kharlyngdoh JB, Pradhan A, Asnake S, Walstad A, Ivarsson P, Olsson PE. 2015. Identification of a group of brominated flame retardants as novel androgen receptor antagonists and potential neuronal and endocrine disrupters. Environ Int. 74:60-70.
[KOAWIN] Octanol Air Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2010. Version 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[KOCWIN] The Soil Adsorption Coefficient Program [Estimation Model]. 2010. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2010. Version 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Kolic T, Shen L, MacPherson K, Fayez L, Gobran T, Helm P, Marvin C, Arsenault G, Reiner E. 2009. The analysis of halogenated flame retardants by GC-HRMS in environmental samples. J. Chromatographic Science. 47: 83-91
KroesR, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, Schilter B, Schlatter J, van Schothorst F, Vos JG, Würtzen G. 2004. Structure-based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food Chem Toxicol 42(1):65-83.
Kuramochi H, Takigami H, Scheringer M, Sakai S-s. 2014. Estimation of physicochemical properties of 52 non-PBDE brominated flame retardants and evaluation of their overall persistence and long-range transport potential. Science of the Total Environment. 491–492: 108–117.
Leadscope Model Applier [Prediction module]. 2013. Version 1.7.4. Columbus (OH): Leadscope, Inc. [accessed 2014 April 16]. [restricted access].
Lee SC, Sverko E, Harner T, Pozo K, Barresi E, Schachtschneider J, Zaruk D, DeJong M, Narayan J. 2016. Retrospective analysis of “new” flame retardants in the global atmosphere under the GAPS Network. Environ. Pollution. 217: 62-69.
Lee SC, Sverko E, Harner T, Schachtschnieder J, Zaruk D, DeJong M, Barresi E. 2013. “New” flame retardants in the global atmosphere under the GAPS Network. Abstract presented at BFR 2013 conference. San Francisco, CA. [accessed 2013 April].
Liagkouridis I, Cousins AP, Cousins IT. 2015. Physical–chemical properties and evaluative fate modelling of ‘emerging’ and ‘novel’ brominated and organophosphorus flame retardants in the indoor and outdoor environment. Science of the Total Environment. 524–525: 416–426.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Government of Canada. [accessed 2014 April 24].
Ma, Y, Venier, M, Hites, RA. 2012. Tribromophenoxy flame retardants in the Great Lakes atmosphere. Environmental Science & Technology, 46; p. 13112-13117.
Martin P, Hughes K. 2016a. Flame retardants in eggs of four gull species in Canada, 2008-2013. Summary report (August 2014). Ecotoxicology and Wildlife Health Division, Enviornment and Climate Change Canada.
Martin P, Hughes K. 2016b. Flame retardants I eggs of European starlings (Sturnus vulgaris) in Canada, 2008-2013. Summary report (August 2014). Ecotoxicology and Wildlife Health Division, Enviornment and Climate Change Canada.
McCarty LS. 1986. The relationship between aquatic toxicity QSARs and bioconcentration for some organic chemicals. Environ Toxicol Chem. 5:1071-1080.
McCarty LS. 1987a. Relationship between toxicity and bioconcentration for some organic chemicals: I Examination of the relationship. In: QSAR in Environmental Toxicology-II, KLE Kaiser (ed). D Reidel Publishing Co, Dordecht, The Netherlands. 207-220 pp.
McCarty LS. 1987b. Relationship between toxicity and bioconcentration for some organic chemicals: II Application of the relationship. In: QSAR in Environmental Toxicology-II, KLE Kaiser (ed). D Reidel Publishing Co, Dordecht, The Netherlands. 221-229 pp.
McCarty LS. 1990. A kinetics-based analysis of quantitative structure-activity relationships in aquatic toxicity and bioconcentration bioassays with organic chemicals. [Ph.D. thesis]. University of Waterloo. Waterloo, Ontario, Canada.
McCarty LS, Hodson PV, Craig GR, Kaiser KLE. 1985. On the use of quantitative structure-activity relationships to predict the acute and chronic toxicity of chemicals to fish. Environ Toxicol Chem. 4:595-606.
McCarty LS, Mackay D, Smith AD, Ozburn GW, Dixon DG. 1991. Interpreting aquatic toxicity QSARs: the significance of toxicant body residues at the pharmacologic endpoint. Science of the Total Environment, Special Issue: QSAR in Environ Toxicol 109:515-525.
McCarty LS, Mackay D. 1993. Enhancing ecotoxicological modeling and assessment: critical body residues and modes of toxic action. Environ Sci Technol 27:1719-1728.
McCarty LS, Arnot JA, Mackay D. 2013. Evaluation of critical body residue for acute narcosis in aquatic organisms. Environ Sci Technol 32(10).
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: A QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1–2):103−133.
Morris AD, Muir DCG, Solomon KR, Teixeira C, Duric M, Wang X. 2014. Unpublished. Bioaccumulation and trophodynamics of current use halogenated flame retardants in the Bathurst Region vegetation-caribou-wolf food chain in Canadian Arctic.
Morris AD, Muir DCG, Solomon KR, Teixeira C, Duric M, Wang X. 2014. Unpublished. Supporting information for Morris et al. 2014. Bioaccumulation and trophodynamics of current use halogenated flame retardants in the Bathurst Region vegetation-caribou-wolf food chain in Canadian Arctic.
[MPBPVPWIN] Melting Point Boiling Point Vapour Pressure Program for Microsoft Windows [Estimation Model]. 2010. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Muir D, Teixeira C, Epp J, Young T, Wang X, Keir M, Backus, S. 2011. Bioaccumulation of selected halogenated organic flame retardants in remote lakes and in the Great Lakes [Informational poster]. Burlington (ON): Environment Canada.
Muir D, Tomy G, de Jourdan B, Orihel D, Morris A. 2014. Unpublished.Using remote environments to detect new persistent and bioaccumulative chemicals in commerce. Aquatic Contaminants Research Division (ACRD), Environment Canada, Burlington, Ontario.
Munro IC, Ford RA, Kennepohl E, Sprenger JG. 1996a. Correlation of a structural class with no-observed-effect-levels: a proposal for establishing a threshold of concern. Food Chem Toxicol 34:829–867.
Munro IC, Ford RA, Kennepohl E, Sprenger JG. 1996b. Thresholds of toxicological concern based on structure–activity relationships. Drug Metab Rev 28(1–2):209–217.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2017 June 21]. Ottawa (ON): Government of Canada. [accessed 2014 April 24].
Nova Chemicals Corp. 2012. Technical data: Expandable polystyrene resins product chart. Nova Chemicals Corporation. Archived.
[OECD] Organisation for Economic Co-operation and Development. 2000. SIDS Initial Assessment Report for: Acrolein [PDF]; CAS RN 107-02-8. SIDS Initial Assessment Meeting [accessed 2000 March 10]
[OECD] Organisation for Economic Co-operation and Development. 2009a. Emission scenario document on plastics additives [PDF]. Paris (FR): OECD, Environment Directorate. Series on Emission Scenario Documents No. 3. Report No. ENV/JM/MONO(2004)8, JT00166678. [accessed 2014 March].
[OECD] Organisation for Economic Co-operation and Development. 2009b. Guidance Document for Using the OECD (Q)SAR Application Toolbox to Develop Chemical Categories According to the OECD Guidance on Grouping of Chemicals [PDF]. Paris (FR): OECD, Environment Directorate. Series on Series on Testing and Assessment No. 102. Report No. ENV/JM/MONO(2009)5, JT03260091.
[OECD] Organisation for Economic Co-operation and Development. 2011. QSAR Toolbox User Manual: Strategies for grouping chemicals to fill data gaps to assess genetic toxicity and genotoxic carcinogenicity [Internet]. [PDF]
OECD QSAR Toolbox. [Read across tool]. 2012. Version 3.0. Paris (FR): Organisation for Economic Co-oporation and Development, Environment Directorate.
[OECD] Organisation for Economic Co-operation and Development, Environment Directorate. 2013. OECD QSAR Toolbox [Read across tool]. Version 3.1. Paris (FR): Organisation for Economic Co-operation and Development, Environment Directorate. [accessed 2014 April 10].
Porter E, Crump D, Egloff C, Chiu D, Kennedy SW. 2014. Use of an avian hepatocyte assay and the avian toxchip polymerase chain reaction array for testing priorization of 16 organic flame retardants. Environ. Toxicol. Chem. 33(3): 573-582.
Pradhan A, Asnake S, Kharlyngdoh JB, Modig C, Olsson P-E. 2015. In silico and biological analysis of anti-androgen activity of the brominated flame retardants ATE, BATE and DPTE in zebrafish. Chemico-Biological Interactions 233, 35-45.
Saini A, Thaysen C, Jantunen L, McQueen RH, Diamond ML. 2016. From clothing to laundry water: investigating the fate of phthalates, brominated flame regardants, and organophosphate esters. Environ. Sci. Technol. 50(17): 9289-9297.
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol. 29(1):89–92.
Schenker U, MacLeod M, Scheringer M, Hungerbuhler K. 2005. Improving data quality for environmental fate models: A least-squares adjustment procedure for harmonizing physicochemical properties of organic compounds. Environ Sci Technol 39: 8434-8441.
Scheringer M, MacLeod M, Wegmann F. 2009. The OECD POV and LRTP Screening Tool. Version 2.2 [PDF]. Organisation for Economic Cooperation and Development; Zurich (CH): Swiss Federal Institute of Technology. Distributed at OECD/UNEP Workshop on Application of Multimedia Models for Identification of Persistent Organic Pollutants, Ottawa, Canada, May 31 – June 2, 2006. [accessed 2013 November 29].
Shanmuganathan M, Zhang Z, Sverko E, Brymer R, Gill B, Smyth SA, Marvin CH. 2017. Analysis of halogenated flame retardants in Canadian wastewater treatment plants using gas chromatography-tandem mass spectrometry. Analytical and Bioanalytical Chemistry, submitted.
Shoeib M, T. Harner, G.M. Webster, E. Sverko, Y Cheng. 2012. Legacy and current-use flame retardants in house dust from Vancouver, Canada. Environmental Pollution. 169: 175-182.
Shoeib M and L Jantunen. 2014. Legacy and current use flame retardants in Great Lakes atmosphere. Science and Technology, Environment Canada, Ontario, Canada.
Stapleton HM. 2010. Flame retardants: are they a health risk for children? Child Task Force Meeting. Raleigh, NC. January 11, 2010.
Study Submission 1989a. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 1. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission 1989b. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 2. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 1990a. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 1. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 1990b. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 2. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission 1996a. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 1 (WS). Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 1996b. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 2]. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 1996c. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 3]. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 1996d. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 4]. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 1996e. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 5]. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 1996f. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 6]. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 1996g. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 7]. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 1996h. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 8]. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 1996i. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. [Available as Robust Study Summary, Identification No. 9]. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 1997. Toxicological studies on 1,3,5-tribromo-2-(2-propenyloxy)-benzene CAS No. 3278-89-5. Unpublished confidential data submitted to Health Canada under the New Substances Program. Ottawa (ON): Health Canada, New Substances Assessment and Control Bureau.
Study Submission. 2013. Toxicological studies on 1,3,5-tribromo-2-(2-propenyloxy)-benzene CAS No. 3278-89-5. Unpublished confidential data submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Sühring R, Barber JL, Wolschke H, Kötke D. 2015a. Fingerprint analysis of brominated flame retardants and Dechloranes in North Sea sediments. Environmental Research. 140: 569-578.
Sühring R, Frees M, Schneider M, Schubert S, Pohlmann J, Alaee M, Wolschke H, Hanel R, Ebinghaus, Marohn L. 2015b. Maternal transfer of emerging brominated and chlorinated flame retardants in European eels. Science of the Total Environment. 530-531: 209-218.
Tanaka R, Yamada R, Oba K, Iga T, Mikami S. 1999. Combined repeat dose and reproductive/developmental toxicity screening test of 2,4,6-tribromophenol by oral administration in rats. Shizuoka, Japan Ministry of Health & Welfare, Biosafety
Research Centre (Toxicity Testing Reports of Environmental Chemicals No. 7). [cited in IPCS 2005]
[TaPL3] Long Range Transport and Persistence Level III model. 2000. Version 2.10. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre.
[TemaNord] Nordic Council of Ministers. 2011. Schlabach M, Remberger M, Brorström-Lundén E, Norström k, Kaj L, Andersson H, Herzke D, Borgen A and Harju M, 2011. Brominated Flame Retardants (BFR) in the Nordic Environment. TemaNord 2011: 528. Nordic Council of Ministers, Copenhagen, Denmark.
[TIMES] TIssue MEtabolism Simulator [Prediction module]. 2013. Version 2.27.5. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [cited 2014 April 10].
[TOPKAT] TOxicity Prediction by Komputer Assisted Technology [prediction module]. 2004. Version 3.1. San Diego (CA): Accelrys Software Inc.
UK Environment Agency. 2010. Environmental prioritization of low production volume substances under REACH: PBT screening [PDF].
[UNEP] United Nations Environment Programme. 2012. Report of the Persistent Organic Pollutants Review Committee on the work of its eighth meeting [PDF]. Stockholm Convention on Persistent Organic Pollutants. Geneva, 15–19 October. UNEP/POPS/POPRC.8/16/Add.3.
[US EPA] US Environmental Protection Agency. 2006. Inventory update reporting, past IUR data: Non-confidential 2002 IUR company/chemical records: Benzene, 1,3,5-tribromo-2-(-propen-1-yloxy)-, CAS RN 3278-89-5 [Internet]. Washington (DC): US Environmental Protection Agency; [cited 2013 Aug 15]. Archived.
[US EPA] US Environmental Protection Agency. 2014. Flame retardant alternatives for hexabromocyclododecane (HBCD). Final Report. EPA Publication 740R14001.
Van Hoogen G, Opperhuizen A. 1988. Toxicokinetics of chlorobenzenes in fish [PDF]. Environ Toxicol Chem 7:213-219.
VEGA] Virtual models for property Evaluation of chemicals within a Global Architecture. 2013. Version 1.0.8. Milan (IT): Istituto di Ricerche Farmacologiche Mario Negri, Laboratory of Environmental Toxicology and Chemistry. [accessed 2014 April 16].
Von der Recke R & Vetter W. 2007. Synthesis and characterization of 2,3-dibromopropyl-2,4,6-tribromophenyl ether (DPTE) and structurally related compounds evidenced in seal blubber and brain. Environmental Science & Technology, 41; p. 1590-1595.
[WHO] World Health Organisation. 1997. Flame Retardants: a general introduction. Environmental health criteria 192. International Program for Chemical Safety. 995. Geneve.
[WHO] World Health Organisation. 2005. 2,4,6-Tribromophenol and other simple brominated phenols. Concise international chemical assessment document; 66
Weisser M. 1992. Investigations on the contamination of municipal sewage sludges with organic pollutants. Universitat Karlsruhe (Schriftenreihe ISWW, 63)
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Human and Ecological Risk Assessment 19(1): 158-188.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2010. Version 1.42. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Xie, Z, Möller, A, Ahrens, L, Sturm, R, Ebinghaus, R. 2011. Brominated flame retardants in seawater and atmosphere of the Atlantic and the Southern Ocean. Environmental Science & Technology, 45; p. 1820-1826.
Yu Y, Hung H, Alexandrou N, Roach P, Nordin K. 2015. Multiyear measurements of flame retardants and organochlorine pesticides in air in Canada’s Western Sub-Arctic. Environ. Sci. Technol. 49(14) : 8623-8630.
Zhang Y-N, Chen J, Xie Q, Li Y, Zhou C. 2016. Photochemical transformation of five novel brominated flame retardants: kinetics and photoproducts. Chemosphere. 150: 453-460.
Appendix A. Detailed list of physical and chemical properties for ATE
| Property | Type | Value | Temperature (°C) | Reference |
|---|---|---|---|---|
| Melting point (ºC) | Experimental | 74-76°C§ | NA | Great Lakes Solutions 2010 |
| Melting point (ºC) | Experimental | 74-77.6 | NA | Study Submission 1996a |
| Melting point (ºC) | Modelled | 101.74 | NA | MPBPVPWIN 2010 v1.43 (weighted value) |
| Boiling point (ºC) | Modelled | 323.15 | NA | MPBPVPWIN 2010 v1.43 (Adapted Stein and Brown Method) |
| Boiling point (ºC) | Experimental | 210 | NA | Study Submission 1996b |
| Density (kg/m3) | Experimental | 2020 (2.02 g/cm3) | NA | Study Submission 1996c |
| Vapour pressure (Pa) | Experimental | <10 | 20 | Study Submission 1996d |
| Vapour pressure (Pa) | Modelled | 0.00854 | 25 | MPBPVPWIN 2010 v1.43 (Antoine Method) Modelled |
| Vapour pressure (Pa) | Modelled | 0.0135§ | 25 | MPBPVPWIN 2010 v1.43 (Modified Grain Method – “selected VP”) Modelled |
| Vapour pressure (Pa) | Modelled | 0.075 | NA | Sub-Cooled Liquid Property |
| Vapour pressure (Pa) | Modelled | 0.049 | 25 | ACD/Percepta [Prediction Module]. c1997-2012 |
| Henry’s Law constant (Pa·m3/mol) | Modelled | 2.68 | 25 | HENRYWIN 2010 v3.20 (Bond Est) |
| Henry’s Law constant (Pa·m3/mol) | Modelled | 2.65 | 25 | HENRYWIN 2010 v3.20 (Bond Est) Sub-cooled |
| Log Kow (dimensionless) | Experimental | >4.86 | 25 | Study Submission 1996e |
| Log Kow (dimensionless) | Modelled | 5.59§* | 25 | KOWWIN 2010 v1.68 |
| Log Kow (dimensionless) | Modelled | 6.09 | NA | Sub-Cooled Liquid Property |
| Log Koc (dimensionless) | Experimental | 3.5 – 4.8 | NA | Study Submission 1996f |
| Log Koc (dimensionless) | Modelled | 3.12 | 25 | KOCWIN 2010 v2.00 (MCI Est) |
| Log Koc (dimensionless) | Modelled | 3.12 | 25 | KOCWIN 2010 v2.00 (MCI Est) Sub-cooled |
| Log Koc (dimensionless) | Modelled | 4.07 | 25 | KOCWIN 2010 v2.00 (Log Kow Est) |
| Log Koc (dimensionless) | Modelled | 4.35 | 25 | KOCWIN 2010 v2.00 (Log Kow Est) Sub-cooled |
| Log Koc (dimensionless) | Modelled | 4.97 | NA | ACD/pKaDB v9.04 2005 |
| Log Kaw (dimensionless) | Modelled | -2.96§ | NA | KOAWIN 2010 v1.10 |
| Log Koa (dimensionless) | Modelled | 8.55 | 25 | KOAWIN 2010 v1.10 |
| Log Koa (dimensionless) | Modelled | 9.05 | 25 | KOAWIN 2010 v1.10 Sub-cooled |
| Water solubility (mg/L) | Experimental | 0.24§* | 20-22 | Study Submission 1996g |
| Water solubility (mg/L) | Modelled | 0.06 | NA | Sub-Cooled Liquid Property |
| Water solubility (mg/L) | Modelled | 0.078 | 25 | WSKOWWIN 2010 v1.42 |
| Dmin (nm)a | Modelled | 10.73 | NA | CPOPS 2008 |
| Dmax (nm)b | Modelled | 13.18 | NA | CPOPS 2008 |
| pKa (dimensionless) | - | No pKa as ATE has no ionisable groups | - | - |
Abbreviations: log Kow, octanol-water partition coefficient;
log Koc, organic carbon-water partition coefficient;
log Kaw, air-water partition coefficient;
log Koa, octanol-air partition coefficient;
pKa, acid dissociation constant;
NA, not available.
§Indicates selected value for modelling.
a minimum diameter of cell membrane
b maximum diameter of cell membrane
Appendix B. Weight of evidence in the ecological risk assessment
| Line of evidence | Level of uncertaintya | Relevancy in assessmentb | Weight assignedc |
|---|---|---|---|
| Physical chemical properties | Low to Moderate | High | Moderate to high |
| Persistence | Low to Moderate | Moderate | Moderate to High |
| Potential degradation products | High | Moderate | Low to Moderate |
| Bioaccumulation | Moderate | Moderate to High | Moderate to High |
| Biomagnification | Low to moderate | Moderate to High | Moderate to High |
| Mammalian toxicity | High | High | Moderate |
| Critical body residue | Moderate | High | Moderate to High |
| Monitoring data | Low | High | High |
| Risk quotient analysis | Moderate | High | Moderate to High |
a Ability to infer truth from the data given the level of uncertainty and power of the data
b Describes how relevant the data are scientifically and to this regulatory assessment
c Final weight assigned to a line of evidence which is a function of the outcomes assigned to the Strength of Inference and Relevancy
Appendix C. Estimates of daily intake of ATE by various age groups within the general population of Canada
| Route of exposure | 0–6 moa (breast milk fed) b | 0–6 moa (formula fed)c | 0–6 moa (not formula fed)d | 0.5–4 yre | 5–11 yrf | 12–19 yrg | 20–59 yrh | 60+ yri |
|---|---|---|---|---|---|---|---|---|
| Ambient Airj | 3.9E-07 | 3.9E-07 | 3.9E-07 | 8.3E-07 | 6.4E-07 | 3.7E-07 | 3.1E-07 | 2.7E-07 |
| Indoor Airk | 4.2E-07 | 4.2E-07 | 4.2E-07 | 8.9E-07 | 7.0E-07 | 4.0E-07 | 3.4E-07 | 3.0E-07 |
| Drinking Waterl | N/A | 2.3E-08 | 8.8E-09 | 9.9E-09 | 7.8E-09 | 4.4E-09 | 4.7E-09 | 4.9E-09 |
| Foodm | NI | NI | NI | 1.6E-05 | 1.3E-05 | 7.4E-06 | 7.1E-06 | 4.6E-06 |
| Dustn | 1.3E-04 | 1.3E-04 | 1.3E-04 | 7.0E-05 | 2.6E-05 | 9.7E-07 | 9.3E-07 | 9.1E-07 |
| Soilo | N/A | N/A | N/A | 2.7E-06 | 2.0E-06 | 7.1E-08 | 6.8E-08 | 6.3E-08 |
| Total Intake | 1.3E-04 | 1.3E-04 | 1.3E-04 | 9.0E-05 | 4.3E-05 | 9.2E-06 | 8.7E-06 | 6.1E-06 |
Abbreviations: N/A, not applicable; NI, data not identified in the literature; mo, months; yr, years.
a Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day (Health Canada 1998), and to ingest 38 and 0 mg of dust and soil per day, respectively (Wilson et al. 2013).
b Exclusively for breast milk-fed infants, assumed to consume 0.742 L of breast milk per day (Health Canada 1998), and breast milk is assumed to be the only dietary source. No quantitative data were identified for concentrations of ATE in breast milk.
c Exclusively for formula-fed infants, assumed to drink 0.8 L of water per day (Health Canada 1998), where water is used to reconstitute formula. No monitoring data on ATE in formula were identified; therefore, dietary intakes are only those from water. See footnote on drinking water for details.
d Exclusively for not formula-fed infants, assumed to drink 0.7 L of water per day (Health Canada 1998), and approximately 50% of non-formula-fed infants are introduced to solid foods by 4 months of age, and 90% by 6 months of age (Health Canada 1998).
e Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day, to consume 54.7 g of fish per day (Health Canada 1998), and to ingest 41 and 14 mg of dust and soil per day, respectively (Wilson et al. 2013).
f Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day, to consume 89.8 g of fish per day (Health Canada 1998), and to ingest 31 and 21 mg of dust and soil per day, respectively (Wilson et al. 2013).
g Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day, to consume 97.3 g of fish per day (Health Canada 1998), and to ingest 2.2 and 1.4 mg of dust and soil per day, respectively (Wilson et al. 2013).
h Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day, to consume 111.7 g of fish per day (Health Canada 1998), and to ingest 2.5 and 1.6 mg of dust and soil per day, respectively (Wilson et al. 2013).
i Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day, to consume 72.9 g of fish per day (Health Canada 1998), and to ingest 2.5 and 1.5 mg of dust and soil per day, respectively (Wilson et al. 2013).
j The highest Canadian concentration of ATE (11.1 × 10-6 µg/m3), measured in Toronto, Ontario (Shoeib and Jantunen 2014), was selected for deriving upper-bounding estimates of daily intake for ambient air exposure. Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
k No Canadian indoor air monitoring data were identified. The maximum concentration of ATE (1.7 × 10-6 µg/m3) measured in indoor office air in Norway (TemaNord, 2011) was selected for deriving upper-bounding estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
l No drinking water monitoring data were identified. The maximum concentration of ATE (0.22 pg/L) in surface water in Lake Ontario (Muir et al. 2011), was selected for deriving upper-bounding estimates of daily intake for drinking water exposure.
m No monitoring data on marketed foods in Canada were identified; however environmental fish data were available. The maximum concentration of ATE (0.0045 µg/kg fresh weight), measured in blue mussel in Norway (TemaNord 2011), was selected for deriving upper-bounding estimates of daily intake for exposure to all fish-related food items in the fish food group. Amounts of daily food consumption by each age group over 12 food groups were obtained from the 1970–1972 Nutrition Canada Survey (Health Canada 1998).
n The 95th percentile concentrations of ATE (26.3 ng/g) from the Canadian House Dust Study (n=631) was selected for deriving upper-bounding estimates of daily intake for dust exposure.
o No appropriate or relevant soil and sediment studies on ATE monitoring in North America were identified; therefore, the soil maximum PEC of 3.0 µg/kg dw was selected for deriving upper-bounding estimates of daily intake for soil exposure.
Appendix D. Analysis of other lines of evidence for genotoxicity and carcinogenicity potential of ATE
Analogue approach (read-across)
Analogue of ATE
The OECD (Q)SAR Toolbox (v3.1) was used to help identify potential analogues for predicting the outcome for ATE in the in vitro mammalian chromosomal aberration assay, the in vivo micronucleus assay and in carcinogenicity (OECD 2009, 2011, 2013). An initial pool of analogues was created on the basis of similar organic functional groups (aryl halide and alkene). According to the function group analysis, no appropriate analogues were found.
Metabolites of ATE
No toxicokinetic data for ATE was found. The in vivo rat metabolic simulator available in the OASIS TIMES software (TIMES 2013) was used to predict (qualitative) oral metabolism for ATE. Only phase I metabolic transformations were shown. The confidence in the metabolic prediction was considered low as ATE was outside the model domain (on the basis of unknown structural features of ATE that were not covered in the training set). Nevertheless, the predicted metabolic transformations appeared plausible on the basis of commonly observed phase I metabolic transformations. Two of the predicted metabolites: 2,4,6-tribromophenol and acrolein, were found to have genotoxicity or carcinogenicity studies in the OASIS TIMES database (Table D-1).
| CAS RN and chemical name | Structure | In vitro mammalian chromosomal aberration assay | In vivo assay | Carcinogenicity |
|---|---|---|---|---|
118-79-6 2,4,6-tribromophenol (2,4,6-TBP) |
 |
Human peripheral lymphocytes: positive ± S9 (rat) Chinese Hamster Lung (CHL) cells: positive ± S9 |
Mouse Micronucleus (i.p.): negative | no data |
107-02-8 Acrolein |
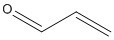 |
Chinese Hamster Ovary (CHO) cells: negative ± S9 CHO cells: |
Rat Chromosome Aberration (i.p.): negative | Rat: Negative Mouse: Negative |
Quantitative structural activity relationship (QSAR) approach
Several statistical QSAR models (Leadscope Model Applier 2013, TIMES 2013, CASEUltra 2013, TOPKAT 2004; VEGA 2013) were utilized to predict genotoxicity (chromosomal aberration and micronucleus) and carcinogenicity. Results are presented in Table D-2 to D-8. Overall, there were no predictions with high confidence
| QSAR model | Prediction result | Reliability | Remarks |
|---|---|---|---|
| In vitro chromosomal aberration, human lymphocyte cells | Negative | Unreliable | The predicted result was mostly on the basis of the high log Kow value of ATE. The model did not predict accurately for the closest analogue in the training set. The training set contained a low number of similar chemicals and did not cover the terminal double bond of ATE. |
| In vitro sister chromatid exchange, Chinese hamster ovary cells (CHO) | Negative | Unreliable | The predicted result was mostly on the basis of the high log Kow value of ATE. The model did not predict accurately for the closest analogue in the training set. The training set contained a low number of similar chemicals and did not cover the terminal double bond of ATE. |
| In vitro chromosomal aberration, Chinese hamster lung cells (CHL) | Out of domain | Not applicable | Not applicable |
| In vitro chromosomal aberration, Chinese hamster ovary cells (CHO) | Out of domain | Not applicable | Not applicable |
| QSAR model | Prediction result |
|---|---|
| In vivo chromosomal aberration, rat | Out of domain |
| In vivo micronucleus, mouse | Out of domain |
| In vivo micronucleus, rodent | Out of domain |
| QSAR model | Prediction result | Reliability | Remarks |
|---|---|---|---|
| Carcinogenicity rat (male) | Negative | Low | The model did not predict accurately for the closest analogue in the training set. The training set contained a low number of similar chemicals and did not cover the terminal double bond of ATE. |
| Carcinogenicity rat (female) | Negative | Low | The model did not predict accurately for the closest analogue in the training set. The training set contained a low number of similar chemicals and did not cover the terminal double bond of ATE. |
| Carcinogenicity mouse (male) | Negative | Low | The model did not predict accurately for the closest analogue in the training set. The training set contained a low number of similar chemicals and did not cover the terminal double bond of ATE. |
| Carcinogenicity mouse (female) | Negative | Low | The model did not predict accurately for the closest analogue in the training set. The training set contained a low number of similar chemicals and did not cover the terminal double bond of ATE. |
| QSAR model | Prediction result | Reliability | Remarks |
|---|---|---|---|
| In vitro chromosomal aberration (S9 metabolic simulator) (v5.05) | Active (out of domain) | Low | ATE was within the physical chemical property domain but outside the structural domain of the model. Only 30% of the structural fragments were recognized by the model (70% were classified as unknown fragment). The “active” prediction was because of the predicted metabolism that can generate an epoxide intermediate metabolite and acrolein. |
| In vivo micronucleus (in vivo rat metabolic simulator) (v2.02) | Active (out of domain) | Low | ATE was within the physical chemical property domain but outside the structural domain of the model. Only 30% of the structural fragments were recognized by the model (70% were classified as unknown fragment). The “active” prediction was because of the predicted metabolism that can generate an epoxide intermediate metabolite and acrolein. |
| QSAR model | Prediction result | Reliability | Remarks |
|---|---|---|---|
| Carcinogenicity rat (male) (AF1) (v1.4.4.6) | Positive | Low | The training set contained a low number of similar chemicals. |
| Carcinogenicity rat (female) (AF2) | Negative | Low | The training set contained a low number of similar chemicals. |
| Carcinogenicity mouse (male) (AF3) | Negative | Low | The training set contained a low number of similar chemicals. |
| Carcinogenicity mouse (female) (AF4) | Positive | Low | The training set contained a low number of similar chemicals. |
| QSAR model | Prediction result | Reliability | Remarks |
|---|---|---|---|
| NTP Carcinogenicity rat (male) (v3.2) | Positive | Low | No analogues in the training set with similarity above 0.8. |
| NTP Carcinogenicity rat (female) (v3.2) | Negative | Low | No analogues in the training set with similarity above 0.8. |
| NTP Carcinogenicity mouse (male) (v3.2) | Positive | Low | No analogues in the training set with similarity above 0.8. |
| NTP Carcinogenicity mouse (female) (v3.2) | Negative | Low | No analogues in the training set with similarity above 0.8. |
| FDA Carcinogenicity rat (male) (v3.2) | Out of domain | Not applicable | Not applicable |
| FDA Carcinogenicity rat (female) (v3.2) | Positive | Low | No analogues in the training set with similarity above 0.8. |
| FDA Carcinogenicity mouse (male) (v3.2) | Negative | Low | No analogues in the training set with similarity above 0.8. |
| FDA Carcinogenicity mouse (female) (v3.2) | Positive | Low | No analogues in the training set with similarity above 0.8. |
| QSAR model | Prediction result | Reliability | Remarks |
|---|---|---|---|
| CAESAR Rat Carcinogenicity (v2.1.8) | Non-carcinogenic (out of domain) | Low | According to the algorithm of the model, ATE was regarded as out of domain. |
Structural alerts (mechanistic alerts)
OECD QSAR Toolbox (v3.1) (OECD 2013)) and DEREK Nexus (v3.0.1) (DEREK Nexus 2013) structural alert models were used to identify any structural alerts of ATE associated with genotoxicity and carcinogenicity. No genotoxicity-related alerts were identified for ATE (Table D-9). ATE triggered a non-genotoxic alert for carcinogenicity (Table D-9). However, there was low confidence for this prediction. The mode of action for polyhalogenated aromatics and carcinogenicity is not well understood.
| Structural alerts package | Structural alert profiler | Structural alert | Reference |
|---|---|---|---|
| OECD QSAR Toolbox (v3.1) | In vivo mutagenicity (micronucleus) alerts by ISS | No alerts | TIMES 2013 |
| OECD QSAR Toolbox (v3.1) | DNA alert by OASIS (v1.1) | No alert | TIMES 2013 |
| DEREK Nexus (v3.0.1) | Chromosomal damage (in vivo / in vitro) | No alert | DEREK Nexus 2013 |
| DEREK Nexus (v3.0.1) | Carcinogenicity Mammal | Plausible. Polyhalogenated aromatic. Non-genotoxic alert with unknown mechanism. Low confidence for the prediction | DEREK Nexus 2013 |
