Updated draft screening assessment - Certain organic flame retardants substance grouping - Melamine
Official title: Updated Draft Screening Assessment of Certain Organic Flame Retardants Substance Grouping 1,3,5-Triazine-2,4,6-triamine (Melamine)
Chemical Abstracts Service Registry Number 108-78-1
Environment and Climate Change Canada
Health Canada
October 2020
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 1,3,5-triazine-2,4,6-triamine (CAS RN 108-78-1), commonly known as melamine, a substance included in the Certain Organic Flame Retardants (OFR) Substance Grouping under Canada’s Chemicals Management Plan, which includes ten organic substances having a similar function: application to materials to slow the ignition and spread of fire. Melamine was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA. A draft screening assessment for melamine was published in October 2016. Significant new information subsequently became available regarding exposure to products available to consumers, specifically foam products containing flame retardants such as melamine. As a result, the draft assessment was updated and is presented here.
Melamine does not occur naturally in the environment. It is not manufactured in Canada; however, imports of melamine, as a pure substance or blended into products, in the range of 10 to 100 million kg were reported for the year 2011. In Canada, melamine has numerous industrial applications; its predominant use is in the manufacture of polyurethane foams and melamine-based resins for application in laminates, plastics, paints and coatings. Globally, melamine is used primarily in the synthesis of melamine–formaldehyde resins for similar applications, and in adhesives and moulding compounds. Due to its high nitrogen content, melamine has also been used globally as a fertilizer. In Canada, sources of environmental exposure to melamine are primarily from waste streams or effluents of manufacturing of melamine-based resins, and to a lesser degree from processing plants using melamine to manufacture products with flame retardant properties. Discharges to the environment can be direct or via municipal wastewater treatment systems.
Melamine is a compact and stable molecule characterized by high water solubility, negligible vapour pressure, and low to negligible organic carbon-water and octanol-water partition coefficients. Monitoring of melamine in environmental media has not been conducted in Canada. When released to the environment, melamine is expected to predominantly reside in water and, to a lesser degree, in soil, depending on the compartment of release.
Melamine does not degrade rapidly in the environment; it has a long half-life in air, and has relatively slow biodegradation rates in water and soil. Melamine has a limited potential to bioaccumulate in tissues of organisms. It has very low bioconcentration factors in fish, and residue clearance rates from numerous organisms including mammals, fish and birds are known to be fast.
Based on empirical evidence from short- and long-term studies, melamine has a low toxicity to aquatic and soil-dwelling organisms. As the toxic effects of melamine exposure were more pronounced in long-term studies and those encompassing sensitive life stages, results from these types of studies were generally more informative. In contrast, in the multiple short-term studies, the toxicity limit for melamine could not be defined since the highest concentration tested showed no effects.
It is expected that melamine may be released to the Canadian environment as a result of industrial processing activities. Although melamine can be found in products available for consumer or commercial use, it is expected that release to the environment via this route is minimal. Industrial scenarios, where melamine is released to water, were developed to provide estimates of exposure. Risk quotient analyses, integrating conservative estimates of exposure with toxicity information, were performed for the aquatic compartment. These analyses showed that melamine risk to the environment in Canada is unlikely.
Considering all available lines of evidence presented in this updated draft screening assessment, there is low risk to the environment from melamine. It is proposed to conclude that melamine does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The main sources of exposure to melamine for the general population in Canada are expected to be from the use of products available to consumers, food and environmental media (water, soil). Biomonitoring data were also available from relevant populations (U.S.).
Based principally on the weight of evidence assessments of international agencies and available information, critical effects associated with exposure to melamine are carcinogenicity and effects on the urinary system. Available information indicates that melamine is not genotoxic. For infants, toddlers and young individuals (up to 18 years old), comparisons between levels associated with critical effects in animal studies and estimates of exposure from lying on foam-containing mattresses or furniture containing melamine are considered to be potentially inadequate to address uncertainties in the health effects and exposure databases. For all other types of exposures (from environmental media and food or from use of products available to consumers of all age groups), comparisons between levels associated with critical effects in animal studies and estimates of exposure were considered adequate to address uncertainties in the health effects and exposure databases.
On the basis of the potential inadequacy of the margins between estimates of exposure and critical effect levels in experimental animals in this updated draft screening assessment, it is proposed to conclude that melamine meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Proposed Conclusion
It is therefore proposed to conclude that melamine meets one or more of the criteria set out in section 64 of CEPA. It is also proposed that melamine meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
List of abbreviations and acronyms
- BMD
- Benchmark Dose
- CEPA
- Canadian Environmental Protection Act
- CFIA
- Canadian Food Inspection Agency
- CHO
- Chinese Hamster Ovary
- CMP
- Chemicals Management Plan
- DIY
- Do-it-yourself
- DSL
- Domestic Substances List
- ECHA
- European Chemicals Agency
- EFSA
- European Food Safety Authority
- HD-XRF
- High Definition X-ray Fluorescence
- HLC
- Henry’s Law Constant
- HPLC
- High Performance Liquid Chromatography
- HPVIS
- High Production Volume Information System
- IARC
- International Agency for Research on Cancer
- Kow
- Octanol-water partition coefficient
- LOAEL
- Lowest Observed Adverse Effect Level
- LOD
- Limit of Detection
- Log D
- Distribution coefficient (usually for octanol-water)
- log Kaw
- Air-water partition coefficient
- log Koa
- Octanol-air partition coefficient
- log Koc
- Organic carbon-water partition coefficient
- LOQ
- Limit of Quantitation
- MCI
- Molecular Connectivity Index
- MOEs
- Margins of Exposure
- MSDSs
- Material Safety Data Sheets
- NA
- Not Available
- NGAL
- Neutrophil Gelatinase-Associated Lipocalin
- NHANES
- National Health and Nutrition Examination Survey
- NOAEL
- No Observed Adverse Effect Level
- NOEL
- No Observed Effect Level
- OFR
- Organic Flame Retardants
- PEC
- Predicted Environmental Concentration
- pKa
- Acid Dissociation Constant
- PMRA
- Pest Management Regulatory Agency
- (Q)SARs
- Quantitative Structure-Activity Relationships
- RMB
- Risk Management Bureau
- SAS
- Systems Analysis Software
- SD
- Sprague-Dawley rats
- SWISSI
- Swiss Institute of Safety and Security
- TDI
- Tolerable Daily Intake
- WHO
- World Health Organization
1. Introduction
Pursuant to sections 68 and 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health conduct screening assessments of substances to determine whether they present or may present a risk to the environment or to human health.
The Substance Groupings Initiative is a key element of the Government of Canada’s Chemicals Management Plan (CMP). The Certain Organic Flame Retardants (OFR) Substance Grouping consists of ten substances identified as priorities for assessment as they meet the categorization criteria under subsection 73(1) of CEPA or were considered as a priority based on ecological or human health concerns (Environment Canada and Health Canada 2013). All of these substances have a similar function: the application to materials to slow the ignition and spread of fire. These substances are also potential alternatives for other flame retardants which are presently subject to regulatory controls or phase-out in Canada or globally.
This updated draft screening assessment focuses on 1,3,5-triazine-2,4,6-triamine, commonly known as melamine. Melamine was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA. In addition to its use as a flame retardant, melamine has numerous other (non-flame retardant) applications.
A draft screening assessment for melamine was published in October 2016 (ECCC, HC 2016). It proposed that melamine was not harmful to human health or the environment. Significant new information on the dermal exposure to foam products subsequently became available as a result of consultations with the European Chemicals Agency (ECHA) on their “Screening report – An assessment of whether the use of TCEP, TCPP and TDCP in articles should be restricted” published in 2018. Following further consultation with other jurisdictions, the dermal exposure to foam products containing flame retardants such as melamine, was re-examined and an updated scenario was adopted. On the basis of this information, an updated draft of this assessment is presented here.
This updated draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to March 2017 for both ecological and human health components, and targeted literature searches were conducted up to May 2018 for the human health component of this assessment. However, more recent studies or information provided via internal and external peer consultation for both ecological and human health components may also be cited. Empirical data from key studies, as well as some results from models were used to reach the proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This updated draft screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review or consultation. Comments on the technical portions relevant to the environment were received from Dr. Jon Arnot of Arnot Research and Consulting, Dr. Laurence Deydier of the European Chemicals Agency (ECHA), and Dr. Miriam Diamond of the University of Toronto. Comments on the technical portions relevant to human health were received from Dr. Lynne Haber of Toxicology Excellence for Risk Assessment, Dr. Paul Rumsby of the U.S. National Centre for Environmental Toxicology and Dr. Pam Williams of E Risk Sciences. Additionally, the initial draft of this screening assessment was subject to a 60-day public comment period. Some human health portions of this assessment have undergone additional external written peer consultation. These consultation comments were received from Dr. Richard Manderville of the University of Guelph, Dr. Mohamed Abou-Elwafa Abdallah of the University of Birmingham, United Kingdom, and Dr. Kebede K. Kefeni of Tshwane University of Technology, South Africa. On the basis of these comments as well as new information received, a second draft of this assessment is presented here. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This updated draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 1. This updated draft screening assessment presents the critical information and considerations on which the proposed conclusion is based.
2. Substance identity
The substance 1,3,5-triazine-2,4,6-triamine (CAS RN 108-78-1), hereinafter referred to by its common name, melamine, is a discrete organic chemical characterized by a high nitrogen content. It belongs to the chemical subgroup of substances known as triazines. It is noted that the name melamine for the chemical is also commonly used for the plastic made from it (WHO c2014).
Information regarding substance identity of melamine is summarized in Table 2-1.
| CAS RN | Chemical structure | Molecular mass (g/mol) | Chemical formula |
|---|---|---|---|
| 108-78-1 | 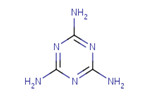 | 126.12 | C3H6N6 |
3. Physical and chemical properties
A summary of experimental and modelled physical and chemical properties of melamine that are relevant to its toxicity and environmental fate is presented in Table 3-1.
Empirical physical chemical property data were gathered from published literature (Hirt et al. 1960), chemistry handbooks (Crews et al. c2012; Rumble 2018) and other sources including database summaries of unpublished studies compiled by other jurisdictions (ECHA c2007-2013). Models based on quantitative structure-activity relationships (QSARs) were also used to generate data for some of the physical and chemical properties of the substance. Most of these models rely on the neutral form of a chemical as input (in SMILES form: c1(nc(nc(n1)N)N)N). Consequently, except where noted, the modelled values shown in Table 3-1 are for the neutral form of substance. Generally, a very good correlation was found between the available empirical physical chemical property values and the modelled values.
Melamine is an odourless, white, fine crystalline powder at room temperature (BASF 2012; ECHA c2007-2013). It is highly soluble in water (Yalkowsky and He 2003; Crews et al. c2012; ECHA c2007-2013) and in ethanol (Rumble 2018). Melamine has very low vapour pressure (~10-9 to 10-7 Pa at room temperature) (Hirt et al 1960; ECHA c2007-2013), and calculated Henry’s Law Constant (HLC) of ~10-9 Pa·m3/mol (EPI Suite 2012). The empirical and modelled log Kow values for melamine are low at -1.37 to -0.38, respectively (Hansch et al. 1995; ECHA c2007-2013; KOWWIN 2010; ECHA c2007-2013). The modelled log Koc values were low at 1.5 (based on the MCI estimation method) and 0.0 (based on the Kow estimation method), respectively (EPI Suite 2012). Melamine is an organic base. Two studies characterizing ionization potential of melamine were identified (Weber 1970; SWISSI 2009). A pKa of 7.3 presented in the SWISSI (2009) report indicates that melamine could ionize to some degree at environmentally relevant pH levels greater than 7, whereas according to Weber (1970), with a pKa of 5, melamine is not expected to ionize appreciably at typical environmentally relevant pH levels (pH 6 to 9). Given limited experimental details, an evaluation of reliability was not feasible for these pKa studies. However, model results, indicating a pKa of 5 for melamine, support the finding that the substance exists predominantly as a neutral chemical at pH 6 to 9 (ACD/Percepta 2005). The modelled log D values did not vary with pH, and were approximately -1.2 at pH levels ranging from 6.5 to 8 (ACD/Percepta 2005). In addition, mammalian toxicity data suggest that melamine is in a neutral form at physiological pH levels (see Human Health section). Although the empirical and modelling data indicate that melamine exists in both the neutral and ionized forms at environmentally relevant pH, the available weight of evidence suggests that melamine will predominantly exist (>~90%) in the neutral form under typical environmental pH.
Property |
Type |
Valuea |
Temperature (°C) |
Reference |
|---|---|---|---|---|
Physical form |
Experimental |
solid, white powder, odourless |
room temperature |
BASF 2012; ECHA c2007-2013 |
Melting point (ºC) |
Experimental |
345*-361 |
NA |
ChemID plus 1993– ; Rumble 2018; BASF 2012 |
Melting point (ºC) |
Modelled |
133 |
NA |
MPBPVP 2010 |
Boiling point (ºC) |
Experimental |
Substance decomposes before boiling |
NA |
BASF 2012 |
Density (kg/m3) |
Experimental |
1.57 |
20 |
ECHA c2007-2013 |
Vapour pressure (Pa) |
Experimental |
7.5x10-9; 4.75x10–8* (3.56 x 10–10 mmHg) |
20 |
Hirt et al. 1960; ECHA c2007-2013 |
Vapour pressure (Pa) |
Experimental |
9.4x10-8; 1.1x 10-7 |
25 |
Hirt et al. 1960; Crew et al. c2012 |
HLC (Pa·m3/mol) |
Modelled |
1.86x10-9 (vapour pressure and water solubility estimate) |
25 |
HENRYWIN 2010 |
Log Kow (dimensionless) |
Experimental |
-1.14* |
25 |
ECHA c2007-2013 |
Log Kow (dimensionless) |
Experimental |
-1.22 |
22 |
SWISSI 2009; ECHA c2007-2013 |
Log Kow (dimensionless) |
Experimental |
-1.37 |
NA |
Hansch et al. 1995 |
Log Kow (dimensionless) |
Modelled |
-0.38 |
NA |
KOWWIN 2010 |
Log Koc (dimensionless) |
Modelled |
1.5* (MCI estimation method) 0 (Kow estimation method) |
NA |
KOCWIN 2010 |
Log D |
Modelled |
-1.22 to -1.18 (at pH 6.5–8.0)
|
NA |
ACD/ Percepta 2005 |
Log Koa (dimensionless) |
Modelled |
10.8 |
NA |
KOAWIN 2010 |
Water solubility (mg/L) |
Experimental |
3190, 3230*,3480 |
20 |
Crews et al. c2012; Yalkowsky and He 2003; ECHA c2007-2013; SWISSI 2009 |
Water solubility (mg/L) |
Experimental |
4850 |
25 |
ECHA c2007-2013 |
pKa (dimensionless) |
Experimental |
5 |
25 |
Weber 1970 |
pKa (dimensionless) |
Experimental |
pKa(base)1=7.3 pKa(base)2=11.4 |
NA |
SWISSI 2009 |
pKa (dimensionless) |
Modelled |
pKa(base)=5.3 |
NA |
ACD/ Percepta 2005 |
Abbreviations: HLC, Henry’s Law constant; log Kow, octanol-water partition coefficient; log Koc, organic carbon-water partition coefficient; log Kaw, air-water partition coefficient; log Koa, octanol-air partition coefficient; Log D, distribution coefficient (usually for octanol-water); pKa, acid dissociation constant; NA, not available; MCI, Molecular Connectivity Index
a Values in parentheses represent the original ones as reported by the authors or as estimated by the models.
* Indicates selected value for modelling.
4. Sources and uses
Melamine does not occur naturally in the environment. Melamine can be produced from urea, dicyandiamide or hydrogen cyanide. Commercially produced melamine is manufactured using urea as a starting material (WHO 2009).
A survey conducted under section 71 of CEPA (Canada 2013) and information obtained from voluntary stakeholder engagement indicated that between 10 and 100 million kg of melamine were imported into Canada in 2011 (ECCC 2013-2014). Melamine was not manufactured in Canada in quantities above the reporting threshold of 100 kg (ECCC 2013-2014).
Canadian import quantities of melamine, of approximately 13 million kg for the year 2011, were reported by the Canadian International Merchandise Trade Database (Statistics Canada 2019).
In 2007, world production of melamine was approximately 1.2 billion kg, and the dominant producers were located in China and Western Europe (WHO 2009). In the U.S., 80 million kg/year are produced, and in 2011-2012, about 1.5-1.6 million kg/year were imported (ICIS c2014). However, the substance is not found in the US EPA High Production Volume Information System (HPVIS) database (US EPA 2007). In the Nordic countries, 12.5 million kg of melamine were used in 2011, whereas use quantities reported for 1999 to 2010 were lower, and ranged between 4.7 million and 10.6 million kg (SPIN c2014).
Uses of melamine are diverse and span numerous industrial sectors, globally and domestically in Canada. The known melamine uses and applications, including instances of adulteration of food products and feed, are summarized below.
In Canada, according to the results of the section 71 survey for the year 2011 and information obtained from voluntary stakeholder engagement (ECCC 2013-2014), melamine was used in consumer and commercial paints and coatings; in foam seating and bedding (which may comprise products such as pillows and mattresses); and in melamine-formaldehyde resin that is used for decorative laminates (ECCC 2013-2014). The substance was also used as a flame retardant in Canada (ECCC 2013-2014). In addition, melamine has applications as a plasticizer in concrete and in automobile brake tubes and hoses (ECCC 2013-2014). An internet search of Canadian products also showed use in thermally-fused melamine paper and shelves, whiteboards and flakeboards, paints, sealants for mechanical, electrical and plumbing applications, and in inkjet ink (The Home Depot Canada 2014; Home Hardware 2014: Formica Corporation 2013 Grand and Toy 2014; Flakeboard Company Ltd. C2012 CSL Silicones Inc. 2014; MSDS 2014). Globally, melamine is used primarily in the synthesis of melamine–formaldehyde resins for the manufacture of laminates (e.g., for kitchen countertops, tabletops), plastics, coatings, commercial filters, products available to consumers such as glues or adhesives, and moulding compounds for melaware (dishware and kitchenware) (WHO 2009; Scorecard c2011).
According to the results of the section 71 survey of CEPA, approximately 4% of all melamine imported into Canada in 2011 was used as a flame retardant (ECCC 2013-2014) which is consistent with what is observed worldwide. Melamine is used as a flame retardant mainly in polyurethane foams (EFRA 2007). Melamine is often used in combination with numerous other flame retardants such as bicyclic phosphate, decabromodiphenyl ether (decaBDE), antimony oxide, Dechlorane Plus (DP), and others, and in polyolefin formulations for use in plastics and elastomers, to improve the overall flame retardant capability of the final product (Weil and Choudhary 1995). Melamine is also used in the production of other flame-retardants, such as melamine cyanurate (CAS RN 37640-57-6), melamine phosphate (CAS RN 20208-95-1), melamine polyphosphate (CAS RN 218768-84-4), and melamine pyrophosphate (CAS RN 15541-60-3) (EFRA 2007).
Other global uses of melamine include its application as an impregnating or adhesive resin in wood-based panels for furniture and flooring, and in paper money, glossy magazines, and textiles (DSM 2010).
Melamine has applications in agriculture. Due to its high nitrogen content, melamine has been tested and used as a slow-release fertilizer (Wehner and Martin 1989; WHO 2009). In addition, melamine is a metabolic by-product of the insecticide, cyromazine, which is an insect growth inhibitor that can be applied as spray or in feed (Roberts and Hudson 1999; Zhu et al. 2009). In Canada, cyromazine is registered for use in products to control the Colorado beetle in potato crops, and insects in greenhouse crops (Health Canada 2012).
Melamine is not listed as an approved food additive in the Lists of Permitted Food Additives issued under the Food and Drugs Act (Health Canada [modified 2017]). Respondents to the section 71 survey under CEPA did not report any uses of melamine in materials that come in contact with food (ECCC 2013-2014), but melamine may be found in various food packaging products in Canada (e.g. the interior coating of cans, excluding infant formula; coating of metallic closures of glass jars for baby foods; glass and plastic bottles for liquid infant formulas; paper used to package bread or margarine; films for milk packaging) (personal communication from Food Directorate to Risk Management Bureau (RMB), Health Canada; August 2013; unreferenced)). Based on notifications submitted to Health Canada under the Cosmetic Regulations, melamine is not used in cosmetics in Canada (June 2013 email from the Consumer Product Safety Directorate, Health Canada to the Risk Management Bureau, Health Canada; unreferenced).
In Europe, melamine is approved for use as a monomer used in the manufacture of resins for food packaging applications, intended to come into contact with food, with a migration limit set at 2.5 mg/kg food (EU 2011). In the U.S., melamine and melamine-formaldehyde copolymer may be used in the formulation of adhesives used as components of articles for use in packaging, transporting, or holding food (indirect food additive) provided that the adhesive is separated from the food by a functional barrier (US eCFR 2014a). Moreover, melamine-formaldehyde resin or polymer may be used as the food contact surface coating (indirect food additive) of articles intended for use in producing, manufacturing, packing, processing, preparing, treating, packaging, transporting, or holding food (US eCFR 2014b).
Melamine is not listed in the Drug Products Database nor the Therapeutic Products Directorate's internal Non-Medicinal Ingredient Database as a medicinal or non-medicinal ingredient present in final pharmaceutical products or veterinary drugs in Canada (DPD [modified 2017]; July 2013 email from the Therapeutic Products Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). Melamine is listed in the Natural Health Products Ingredients Database as a Non-Natural Health Product because it is not a naturally occurring substance included in Schedule 1 to the Natural Health Products Regulations; as such, it is not listed in the Licensed Natural Health Products Database as being present in currently licensed natural health products in Canada (NHPID [modified 2017]; LNHPD [modified 2016]).
Following identification of a melamine-adulterated pet food in the U.S. in 2007, and melamine-adulterated baby formula in China in 2008, standards for melamine in foods were developed internationally to ensure the safety of consumers and to differentiate between the presence of background levels of melamine in food and intentional adulteration (Health Canada 2008, 2016a; Codex Alimentarius 2018). In Canada, interim maximum levels for melamine have been set at 0.5 mg/kg for infant formula and sole source nutrition products, including meal replacement products, and at 2.5 mg/kg in food products containing milk and milk-derived ingredients (Health Canada 2016a; 2018a). These interim maximum levels apply to the presence of both melamine and cyanuric acid, often found together, in order to ensure that foods available for sale in Canada have not been deliberately adulterated with either compound (Health Canada 2016a, 2018a).
5. Releases to the environment
Melamine has many industrial uses and is used in large quantities, both in Canada and worldwide (Du et al. 2010).
Releases to the environment depend upon various losses occurring during the manufacture, industrial use, consumer/commercial use, service life and disposal of a substance. Potential releases are expected to mainly occur during handling of melamine when it is added, as a pure substance, and during industrial processes to make desired products such as melamine resins. These releases are expected to be primarily caused by the diffuse (fugitive) emissions within the industrial facilities during loading/unloading operations, handling or cleaning of processing equipment, and cleaning of transport and storage containers. It is assumed that 0.6% of the total quantity of melamine used could be released into wastewater (OECD 2002).
Melamine release to the environment is most likely to occur during the manufacturing, formulation and/or industrial use stages, rather than when the substance is contained in products. Releases of melamine to the Canadian environment from use and disposal of (commercial or consumer) products or manufactured items containing melamine are expected to be diffuse.
Releases to the environment are expected to occur primarily to wastewater. Although release to the soil could also occur through the application of sewage sludge as biosolids to agricultural and pasture lands, it is expected that these would be minimal given melamine’s limited propensity for partitioning to solids (see Section 6).
Melamine may also be released into the environment from agricultural applications of the pesticide cyromazine, approved for use in Canada. Cyromazine transforms to melamine in soil (major metabolite), plants (minor metabolite) and animals (minor metabolite) (FAO 2007a, b). An environmental and human health re-evaluation of cyromazine is scheduled by the Pest Management Regulatory Agency (PMRA) of Health Canada (Health Canada 2016b, 2018b).
This information is used to further develop exposure characterization scenarios to estimate resulting environmental concentrations.
6. Environmental fate and behaviour
6.1 Environmental distribution
Based on the known industrial applications in Canada (ECCC 2013-2014), melamine is expected to be released primarily to wastewaters. Due to its low Koc and high water solubility melamine is not likely to be efficiently removed by adsorption to sludge in wastewater treatment. Level III fugacity modelling using the New EQC model (2011) was applied to describe the fate of melamine.
The overall results of Level III fugacity modelling suggest that when melamine is released into the environment it is expected to predominantly reside in water and soil, depending on the compartment of release (Table 6‑1).
Substances released to: |
Air(%) |
Water(%) |
Soil(%) |
Sediment(%) |
|---|---|---|---|---|
Air (100%) |
negligible |
27 |
73 |
negligible |
Water (100%) |
negligible |
99.6 |
negligible |
0.4 |
Soil (100%) |
negligible |
18 |
82 |
negligible |
When melamine is released to air, it is expected to quickly partition to the particulate phase in air due to its very high estimated Koa, and as a result, melamine is not expected to reside air. The particulate phase is deposited to land and water as wet and dry deposition. For the amount transferred from air, the majority (more than 70%) will remain in soil, and nearly 30% will be found in water.
When released to surface water, most of the melamine is expected to remain in water due to its high water solubility. Melamine could also potentially be found in sediment pore water due to its high water solubility.
Volatilization from surface water to air is expected to be a very slow process as melamine has a negligible vapour pressure and a very low Henry’s Law Constant. Overall, there are limited rates of advective loss of melamine from aqueous systems.
Melamine has a very low Koc indicating a low soil and sediment sorption potential. However, since melamine is denser than water, it could, to some degree, settle to sediments. When released to soil (e.g., as a function of biosolid application to agricultural lands), melamine is expected to be adsorbed, and also to run off to aqueous systems. Evaporation from soil is not expected as melamine is relatively non-volatile.
In summary, based on the results of fugacity modeling and melamine’s physical and chemical properties, melamine will predominantly reside in water and soil compartments and in pore water associated with sediments. Therefore, aquatic, benthic and soil organisms could potentially be exposed to this substance. High exposure to terrestrial organisms through inhalation is not expected, given the expected low melamine concentrations in air.
6.1.1 Long-range transport potential
Monitoring data for melamine in remote areas (e.g., polar regions) are not available.
It was determined that melamine has long half-lives in air and in water. However, melamine is expected to have very low concentrations in air and releases of it into air are not expected based on the known uses of the substance (see Sources and uses section). With its high water solubility, negligible vapour pressure and Henry’s Law constant, rates of volatilization to air from surface waters are expected to be very low. Modelled results from the OECD POV and LRTP Screening Tool (OECD 2009) confirmed that melamine will not be found in air as a result of emissions to surface waters. Long-range transport in water systems is plausible based on the substance’s characteristics and emission patterns. Results of theTaPL3 model (2003) and the OECD POV and LRTP Screening Tool (OECD 2009) indicated that the characteristic travel distance (CTD) in water for melamine was 120 000 km, and 2 300 km, respectively. It is noted that these CTD results should not be interpreted as the absolute distance that the substance can travel, but rather as an indication that the substance has the potential to move over relatively long distances in water.
6.2 Environmental persistence
Empirical and modelled data were considered to determine the degradation potential of melamine in the environment.
Melamine is produced using a condensation process of urea. Melamine can be reacted with a considerable number of organic and inorganic derivatives, such as formaldehyde, forming highly stable products with wide consumer and industrial applications (Ramusino and Vailati 1982). However, based on melamine industrial uses identified from the section 71 survey of CEPA (ECCC 2013-2014), water is thought to be the main receiving compartment of melamine from effluents.
A catabolic biodegradation pathway of melamine has been elucidated, using bacterial strains of Pseudomonas species, Klebissiela pneumonia, and Rhodococcus corallines, isolated from soil. Metabolism of melamine provides a source of nitrogen for bacterial growth; however, melamine metabolism is generally slow (Shelton et al. 1997). A novel strain of bacteria CY1, closely related to β-proteobacteria Alicycliphilus denitrificans, isolated from melamine-manufacturing factory sludge, was observed to be capable of relatively fast and complete biodegradation of melamine in an in vitro study (Wang et al. 2014).
Melamine is known to be metabolized through three consecutive hydrolytic deamination reactions; first to form ammeline, then ammelide, and then yielding cyanuric acid (Wackett et al. 2002; Shelton et al. 1997). This has been attributed to the presence of specific plasmid-encoded genes encoding melamine-degrading enzymes (Karns and Eaton 1997; Wackett et al. 2002; Takagi et al. 2012; Hatakeyama et al. 2015). In cell free extracts, cyanuric acid was shown to undergo further metabolism to biuret, urea, and ammonia (Cook et al. 1985). A complete mineralization of melamine, through intermediates ammeline, ammelide, cyanuric acid, biulet and allophanate, by a mixed bacterial culture containing a novel Nocardioides species was described in Takagi et al (2012). Formation of melamine-cyanurate complex precipitate, in addition to melamine degradation intermediates ammeline, amelide, cyanuric acid, biuret, allophanate and urea, was detected in biodegradation assays with the CYI bacterial strain (Wang et al. 2014).
Available information regarding degradation and persistence potential of melamine is organized and presented based on the environmental compartment (i.e., air, water, soil and sediment). Empirical biodegradation data are summarized in Table 6-2, and modelled degradation data are presented in Appendix A.
6.2.1 Air
Empirical data for the degradation potential of melamine in air were not available. Modelled results, based on the available QSAR model (AOPWIN 2010), indicated a long half-life of 16.2 days in air. Therefore, it is expected that the substance will not be rapidly degraded by reaction with hydroxyl radicals in the atmosphere. The ozone reaction half-life could not be modelled since the model AOPWIN (2010) does not provide estimates for this class of chemicals. Overall, melamine is considered not readily degradable in air. It is noted that melamine is unlikely to reside in air in high concentrations. Modelled data in air are summarized in Appendix A.
6.2.2 Water
For the water compartment, degradation by hydrolysis, biodegradation by microorganisms found in the sludge, and modelled biodegradation are discussed below.
6.2.2.1 Hydrolysis
Melamine does not undergo hydrolysis under environmentally relevant conditions (ECHA c2007-2013). However, melamine hydrolyzes under conditions catalyzed by strong alkaline and acidic solutions such as mineral acid and inorganic alkali (Crews et al. 2005). This process proceeds stepwise, with the loss of first, second, and then all three amino groups, to produce ammeline, ammelide and cyanuric acid, respectively. The proportion of reaction products can vary with temperature, concentration, and pH (Crews et al. 2005). Melamine hydrolysis rate constants were measured at 100°C, and reported as 3.80 x 10-5 (OH-) and 1.25 x10-4 (H+) (ECHA c2007-2013).
6.2.2.2 Biodegradation by sludge microorganisms
Several inherent and ready biodegradation studies have been conducted using activated sludge and in some studies, pre-adapted sludge, to determine the biodegradation potential of melamine in water.
Biodegradation of melamine using activated sludge was investigated by Xu et al. (2013) in two common treatment processes, the modified Ludzack-Ettinger (MLE) process that is characterized by both anoxic and aerobic conditions, and the continuous stirred tank reactor (CSTR) process, under aerobic conditions. Biodegradation of melamine was monitored for 225 days; dosing with melamine at an influent concentration of 3 mg/L was started on day 125 of the study. Melamine showed limited biodegradation potential by sludge microorganisms, even after the prolonged 100-day exposure to 3 mg/L of melamine that could lead to adaptation. The average removal efficiencies in the MLE and CSTR systems were similar at 20±15%, and 14±10%, respectively, indicating that mixed anoxic/aerobic and solely aerobic conditions did not impact melamine biodegradation. Therefore, it is likely that the enzymes responsible for the hydrolytic deamination of melamine may not be readily induced in activated sludge processes. At a higher melamine concentration of 75 mg/L, decreases in the nitrifying bacterial activities, by 92%±5% in the MLE system, and 82±8% in the CSTR system, and also a decrease in bacterial populations were also observed. An et al (2017) showed a reduction in removal efficiencies in a wastewater treatment system study, where nitrogen removal efficiencies dropped from 94% to 79% and 68% in the presence of 1.0 and 5.0 mg/L melamine, respectively. An et al. (2017) observed that the removal was mainly achieved by activated sludge adsorption instead of biodegradation. These results suggest that melamine may inhibit activated sludge bacterial growth when present at high concentrations.
In earlier studies, biodegradation of melamine based on the biological oxygen demand (BOD) was addressed in publications by Heukelekian and Rand (1955) and Niemi et al. (1987). In these papers, existing published and unpublished data on different classes of chemicals were compiled. Study results were tabulated and descriptions of methodology were limited to general information regarding study protocols or experimental conditions. Heukelekian and Rand (1955) presented two BOD results for melamine, originally published by Swope et al. (1950), which indicated 0 g/g and 0.006 g/g BOD, using sewage and following 5 days of incubation. In Niemi et al. (1987), an existing 1984 study result showing 1% BOD in 5 days of melamine using activated sludge (acclimation was said to not be reported) (Vaishnav 1984) was retested using acclimated activated sludge, also over 5 days. The new results indicated 0% BOD, and verified the previous findings. Test results described in Heukelekian and Rand (1955) and Niemi et al. (1987) point to a slow biodegradation potential of melamine.
Two unpublished industry studies were summarized for the European Union Regulation concerning the Registration Evaluation Authorisation and Restriction of Chemical Substances (REACH), and study summaries were available from ECHA (c2007-2013). Summaries of two inherent biodegradation studies, performed in 1991 and 1993 according to the OECD protocol 302B, were available. In the 1991 study, activated sludge and pre-adapted sludge from an industrial sewage treatment plant were used to test melamine at a concentration of 1000 mg/L (1 g/L). The results from the test using activated sludge indicated 16% dissolved organic carbon (DOC) elimination within 20 days, whereas 10% DOC elimination was observed using adapted inoculum after 14 days. In the 1993 study, melamine was tested twice at a concentration of 100 mg/L using activated sludge. Following 28 days, 0% and less than 10% of DOC were observed in the two trials. In addition, results of a ready biodegradation study conducted according to the OECD protocol 301C (ECHA c2007-2013) were summarized in MITI (1992). Results of this study indicated 0% biodegradation, measured as BOD, when 100 mg/L of melamine was tested over two weeks (MITI 1992).
In addition, biodegradation of melamine in water under various conditions according to a Zahn-Wellens like protocol was the subject of a Master thesis from the University of Salzburg, Austria, completed in 1997 (Fimberger 1997). Key findings from this research were summarized in ECHA (c2007-2013) and OECD (2002). Melamine was tested at 20 mg/L over 28 days. Results indicated no inherent biodegradation using activated sludge from a municipal wastewater treatment system (WWTS). Modifying study conditions by the addition of glucose supported some biodegradation, and the addition of ammonia inhibited biodegradation facilitated by glucose. Melamine was observed to break down rapidly by sludge from an industrial WWTS where melamine was produced, and was no longer detected after 8 hours (Fimberger 1997). Biodegradation was noted to occur by hydrolytic deamination leading to carbon dioxide, which is the pathway presented in other studies that examine microbial degradation of melamine (Karns and Eaton 1997; Shelton et al. 1997; Wackett et al. 2002).
A novel species of bacteria CY1, isolated from the sludge of a melamine-manufacturing factory in China, was observed to completely degrade melamine in an in vitro study. Melamine was tested at a concentration of about 500 mg/L. In the initial 24 hours, approximately 64% of melamine was degraded by CY1, and approximately 94% of melamine was observed to be degraded in 10 days (Wang et al. 2014).
Results from the available empirical studies are summarized in Table 6-2.
Fate process |
Degradation endpoint / units |
Degradation value |
Reference |
|---|---|---|---|
Ready Biodegradation (aerobic) |
% BOD (5 days) |
0; 1 |
Niemi et al. 1987 |
Ready Biodegradation (aerobic) |
BOD (g/g) (5 days) |
0; 0.006 |
Heukelekian and Rand 1955 |
Inherent Biodegradation (aerobic) |
% DOC (28 days) |
0; <10 |
ECHA 2007-2013 |
Inherent Biodegradation (aerobic) |
% DOC (20 days) % DOC (14 days) |
16 10 (pre-adapted inoculum) |
ECHA 2007-2013 |
Ready Biodegradation (aerobic) |
% BOD (14 days) |
0 |
MITI 1992 |
Inherent Biodegradation (aerobic) |
% BOD (28 days) |
0 (sludge from a municipal WWTS) |
Fimberger 1997 |
Abbreviations: BOD, biological oxygen demand; DOC, dissolved organic carbon; WWTS, wastewater treatment system.
In summary, the biodegradation mechanism of melamine proceeds stepwise through hydrolytic deamination, to produce cyanuric acid, and eventually through ring cleavage to yield two final products, ammonia and carbon dioxide. Ready and inherent biodegradation studies indicate that melamine biodegradation in water is slow. Under continuous exposure in industrial wastewater treatment conditions, acclimation of microorganisms may occur, and can lead to a more efficient breakdown of melamine to release ammonia that can be used as an energy source for the resident microorganism population. However, this does not represent a rapid biodegradation potential, but rather a process of adaption by select microorganisms. There is also contrasting evidence suggesting that melamine may be inhibitory to bacterial growth in activated sludge. Given that melamine is a stable molecule, and that its potential for biodegradation in water is limited, as evidenced by numerous studies (see Table 6-2 above), it is considered that the substance is persistent in this environmental compartment.
6.2.2.3 Modelled biodegradation in water
In addition to the available empirical data for the degradation of melamine in water, a QSAR-based weight-of-evidence approach was applied using the degradation models shown in Table 4-3.
The rate of hydrolysis could not be determined for melamine using the model HYDROWIN (2010) since the model cannot provide estimates for triazine structures. BIOWIN sub-models of EPI Suite (2012) were used to evaluate the biodegradation potential of melamine. BIOWIN Sub-model 4 results suggest some potential for primary biodegradation. The results from ultimate biodegradation models, BIOWIN sub-models 3, 5 and 6 (EPI Suite 2012), and CATALOGIC (2012) suggest that melamine biodegrades slowly or not at all. When considered together, the model results indicate a limited potential for ultimate biodegradation, and given the model consensus pointing to slow biodegradation rates, there is insufficient evidence to suggest that melamine undergoes significant primary biodegradation. Model results support findings from empirical biodegradation studies (summarized in Table 6-2), and point to a slow biodegradation of melamine in water. Modelled results for degradation of melamine in water are summarized in Appendix A.
6.2.3 Soil
Biodegradation of melamine in soil proceeds at a very slow rate (Hauck and Stephenson 1964). It may be due in part to the symmetrical resonating structure of the substance; molecular symmetry tends to confer stability (Hauck and Stephenson 1964). A melamine biodegradation mechanism via consecutive hydrolytic deamination reactions was elucidated in vitro in bacteria isolated from soil. This has been attributed to the presence of specific plasmid-encoded genes, known as tri A and trz B, C, D, E genes, that encode enzymes called amidohydrolases which are capable of stepwise conversion of melamine to cyanuric acid to biuret and urea, ultimately leading to degradation to carbon dioxide and ammonia by means of urease (Eaton and Karns 1991 a,b; Karns and Eaton 1997; Wackett et al. 2002). Hatakeyama and Takagi (2016) showed that that while melamine degradation is low, it can increase when bacteria are pre-cultured in a medium containing melamine.
Nitrification of melamine was studied in two types of soil, silty clay loam at pH 8.2, and fine sandy loam at pH 5.2 (Hauck and Stephenson 1964). Melamine was applied to soil samples at a concentration of 0.2 mg/g soil for up to 24 weeks. Results indicated that melamine was nitrified more readily in the silty clay loam, with nearly 8% nitrate formed after 6 days of incubation, and up to 18% after 24 days. In contrast, in the sandy loam soil, 0% nitrification of melamine occurred in the first 12 days of the study, and after 24 weeks, about 9% nitrification was observed. These results suggested that in silty clay loam soil at basic pH, nitrification of melamine proceeds at approximately double the rate of that in the slightly acidic sandy loam. In another test in silty clay loam, melamine was applied at a concentration of 2 mg/g soil. Following 10 weeks incubation, about 1% of the nitrogen from melamine was found as nitrate when incubation proceeded for up to 28 weeks, nitrate was no longer found, possibly due to increased nitrate assimilation by the soil microorganism population (Hauck and Stephenson 1964).
In an earlier study (Konishi and Imanishi 1941), melamine was noted to nitrify very slowly in a paddy soil.
Overall, study results demonstrate a slow biodegradation rate of melamine in soil. Therefore, it is considered that melamine is persistent in soil.
6.2.4 Sediment
No experimental studies were found for the biodegradation of melamine in sediments, and limited modelling is available for this compartment. Therefore, an extrapolation ratio of 1:1:4 for water: soil: sediment biodegradation half-life based on Boethling et al. (1995) was applied. Given that the half-life of melamine in water is long and likely greater than 182 days (based on the BOD and DOC results in melamine biodegradation studies), it follows that the half-life in sediments is expected to be greater than 365 days. This indicates that melamine is likely to persist in sediments.
6.3 Potential for bioaccumulation
Physical and chemical properties, as well as relevant empirical and modelled data, were examined to determine the bioaccumulation potential of melamine.
Melamine is highly soluble in water, indicating that the substance can be readily bioavailable in water. Experimental and modelled log Kow values (in the range of -1.37 to -0.38) for melamine suggest that this chemical is likely to have low potential to bioaccumulate in biota as a function of hydrophobic partitioning. In addition, the combination of two partition coefficient log values, log Kow of -1.37 and log Koa of 10.8, indicates that given a terrestrial dietary exposure, melamine is unlikely to biomagnify in terrestrial food webs, as suggested by Gobas et al. (2003) and Kelly et al. (2007).
Bioconcentration factors (BCFs) of melamine were determined empirically in several fish species (MITI 1992; ECHA c2007-2013). These studies exposed fish to melamine under static conditions. Melamine exposure duration and concentrations varied among the studies but it was noted that a steady state concentration of melamine was reached in fish tissues (MITI 1992; ECHA 2007-2013). Overall, these studies were consistent in showing very low BCFs for melamine. In carp (Cyprinus carpio), BCFs were calculated to be less than 3.8, and less than 0.38 L/kg, following exposure to melamine at concentrations of 0.2 mg/L and 2 mg/L, respectively in a 6-week study (MITI 1992). A 96-hour exposure of fathead minnows (Pimephales promelas) to 0.09 mg/L of melamine resulted in BCF values of 0.48, and 0.26 L/kg in viscera and carcass, respectively (ECHA c2007-2013). When a 72-hour depuration period was considered in the calculations, the BCF values were determined to be marginally lower, at 0.32 in viscera, and 0.2 L/kg in the carcass. Similarly, in rainbow trout (Oncorhynchus mykiss), BCFs were determined to be well below 1 L/kg in muscle and viscera following 64-hour exposure at 0.09 mg/L melamine (BCF were 0.11 L/kg in viscera, and 0.05 L/kg in muscle) and melamine was noted to be rapidly eliminated within the 72-hour depuration period (ECHA c2007-2013). Results from the fish bioconcentration studies are summarized in Table 6-3.
Test organism |
Kinetic and/or steady-state value (L/kg)a |
Reference |
|---|---|---|
Carp (Cyprinus carpio) |
< 3.8 (0.2) <0.38 (2.0) |
MITI 1992 |
Fathead minnow (Pimephales promelas) |
0.26–0.48 (0.09) |
ECHA c2007-2013 |
Rainbow trout (Oncorhynchus mykiss) |
0.05–0.11 (0.09) |
ECHA c2007-2013 |
a Values in parentheses represent the test concentrations in mg/L at which the BCFs were derived.
In addition, the modelled fish BCF values, determined using the BCFBAF model of EpiSuite (2012), were in agreement with the empirical BCFs, and ranged from 0.93 to 3.16 L/kg wet weigh for the middle trophic level fish, depending on application of the metabolic rate constant.
In light of the melamine food adulteration episodes that occurred in 2007 and 2008, numerous feeding studies in livestock, fish, ducks and shrimp, as well as monkeys were conducted to determine the potential for accumulation of melamine in tissues. These studies typically involved adding both low and high levels of melamine to animal feed, and examining effects of melamine-contaminated feed or effects following a single dose of melamine through diet (Qin et al 2010; Andersen et al. 2008, 2011; Lightner et al. 2009; Reimschuessel et al. 2010a; Liu et al. 2010; Phromkunthong et al. 2013, 2015; Suknikom et al. 2016). Generally, the resultant measured residue levels of melamine in tissues were low, and suggested that melamine does not have the potential for significant accumulation in animal tissues following exposure through food. Qin et al. (2010) also noted that melamine residues in animal tissues and milk and eggs were virtually depleted in days and up to two weeks after the melamine-spiked diet was stopped. Similar observations of melamine residue clearance in up to 14 days following a single oral dose were noted in fish (Reimschuessel et al. 2010a); however, adverse effects, including renal failure due to formation of renal crystals were noted when high doses of 20 mg melamine/kg were administered to fish (Reimschuessel et al. 2010b; Phromkunthong et al. 2015).
In summary, there is strong and consistent evidence indicating that melamine does not bioaccumulate to an appreciable degree in aquatic and terrestrial organisms. Melamine is characterised by a combination of physical and chemical properties that suggest low bioaccumulation potential and very low empirical and modelled BCFs in fish. Empirical studies indicated low potential for melamine to accumulate in animal tissues when administered in feed, and a relatively fast residue clearance. Therefore, based on the available evidence, it is considered that melamine has a low potential for bioaccumulation.
6.4 Summary of environmental fate
Melamine is a compact and stable molecule (see Table 2 for structure). Melamine degradation proceeds stepwise through hydrolytic deamination, and eventually through ring cleavage to yield two final products, ammonia and carbon dioxide. In the environment, melamine does not have the potential to degrade quickly. It has a long half-life in air, and relatively slow biodegradation rates in water and soil. Application of the extrapolation factors recommended by Boethling (1995) indicates that melamine is also expected to have a long half-live in sediments. Hydrolysis of melamine does not occur under environmentally relevant pH (6-9). However, under stringent laboratory conditions, melamine can hydrolyze to form cyanuric acid and a complete degradation of melamine to ammonium proceeds rapidly when catalyzed by strong acids.
Melamine is expected to be released primarily to wastewater from industrial sources. Melamine is essentially non-volatile, and has a limited potential to sorb to solid particles. It is not likely to be efficiently removed by wastewater treatment systems, therefore high concentrations of melamine are not expected in biosolids slated for application onto soil from biosolids amendment practices. Long-range transport in air is not expected; however, it may undergo long-range transport in water. Given its releases to water and soil, and its tendency to reside in those two media, exposure to aquatic and soil-dwelling organisms is expected. Melamine does not bioaccumulate appreciably in organisms, and it has relatively fast clearance rates in organisms, including fish and mammals. Therefore, biomagnification in foodwebs is not considered to be a significant process and is not expected to contribute to melamine effects stemming from exposure.
7. Potential to cause ecological harm
7.1 Ecological effects assessment
Ecological effects of melamine were determined through empirical data sourced from both published and unpublished studies. The unpublished industry studies were summarized for the European Union Regulation concerning the Registration Evaluation Authorisation and Restriction of Chemical Substances (REACH), and study summaries were available from the European Chemicals Agency website (ECHA c2007-2013). Limited study details were provided in some of the study summaries, therefore multiple studies were used to compare the results. Given that numerous melamine effects studies for aquatic and soil species were available, modelled effects data were not considered.
Based on the known industrial uses of melamine (ECCC 2013-2014) in Canada, it is expected that the majority of melamine releases would be to surface waters.
Information is presented based on the compartment of exposure. Results from the published and unpublished studies are summarized below and tabulated in Table 7-1 for aquatic microorganisms and invertebrates, and Table 7-2 for fish and for soil-dwelling organisms.
7.1.1 Water
Effects of melamine on sludge microorganisms were determined in several studies based on short-term exposure of up to 2 hours (ECHA c2007-2013; Hockenbury and Grady 1977), and longer-term 72-hour exposure (Xu et al. 2013). Short-term exposure studies suggest that melamine is not appreciably toxic to sludge microorganisms, whereas potential inhibition of activated sludge by melamine was observed in the 72-hour tests (Xu et al. 2013), suggesting that a longer exposure time may be an important factor that is not well characterized in the available dataset. Overall, study results suggest that melamine has a low toxicity to microorganisms.
The effects of melamine on unicellular organisms were studied in the ciliated protozoa, Tetrahymena pyriformis and T. thermophila, and algae species, Scenedesmus pannonicus and Selenastrum capricornutum. Effects of melamine on T. pyriformis were determined in two studies by Wang et al. (2009; 2011), who observed that melamine had a concentration-dependent inhibitory effect. The inhibiting concentrations for 50% effect following 52-hour exposure, i.e., the IC50 values, were determined to be 780 mg/L (Wang et al. 2009) and 820 mg/L (Wang et al. 2011). Similar results were observed by Li et al. (2015) during 20-hour exposure of T. thermophila to melamine. Effects on two algae species S. pannonicus and S. capricornutum were determined in unpublished studies dated 1982 (ECHA c2007-2013) and 1988 (US Testing Company 1988), respectively. Study summaries were available from ECHA (c2007-2013). In the 1982 study, effects of melamine on growth rate were noted, and a no-observed-effect-concentration (NOEC) of 320 mg/L and a 50% effect concentration (EC50) of 940 mg/L were determined following 90.5 hours of exposure. Similarly, in the 1988 study, the NOEC and EC50 at 48-hours were calculated as 97 mg/L and 325 mg/L, respectively, at 72-hours, the NOEC and EC50 were 31 mg/L and 196 mg/L, and at 96-hours, the NOEC and EC50 were 98 mg/L and 325 mg/L, respectively.
The aquatic invertebrate studies testing effects of melamine through water exposure were limited to one species, Daphnia magna. Effects were determined in two unpublished studies dated 1978 (ECHA c2007-2013) and 1988 (ABC Laboratories1988), and included both the short term 24- to 48-hour exposures, and longer-term exposure of up to 21 days. Study summaries were available from ECHA (c2007-2013). In the 1978 study, according to the study summary, D. magna were exposed to melamine for 48-hours, and 7 to 21 days at concentrations of up to 2000 mg/L. The concentration that is estimated to be lethal to 50% of test organisms following 48-hour exposure, i.e., the LC50, was estimated to be greater than 2000 mg/L; however, it was noted that the condition of daphnids was poor at a much lower exposure concentration of 180 mg/L. Therefore, the 48-hour EC50 for behaviour was established to be less than 180 mg/L. The 7-day and 21-day LC50s were estimated to be greater than 32 and less than 56 mg/L, based on survival rates observed at exposure concentrations of 32 and 56 mg/L, where at the exposure concentration of 32 mg/L the survival percentage was over 90%, and at the exposure concentration of 56 mg/L no daphnids survived past day 7 of exposure. The 21-day NOEC for reproduction and mortality was determined to be 18 mg/L. In the 1988 study (ABC Laboratories 1988), melamine was tested at concentrations up to 1000 mg/L for 24 and 48 hours in static conditions. The 48-hour NOEC was estimated to be less than the lowest exposure concentration of 56 mg/L since effects of mortality, and behavioural effects including quiescence, surfacing and/or tending to the bottom of test vessels, were observed at all test concentrations. The 24- and 48-hour EC50 values, based on the total adverse effects, were calculated to be 400 mg/L and 200 mg/L, respectively (ABC Laboratories 1988).
Effects of melamine exposure through diet were observed in black tiger shrimp (Penaeus monodon) and Pacific white shrimp (Penaeus vannamei), resulting from administration of melamine-adulterated feed in shrimp farms (Lightner et al. 2009). Although not representative of realistic exposure concentrations in the environment, this study highlights effects that arise from an alternate route of exposure, through diet. Analysis of feed samples indicated that melamine was present at concentrations of over 100 mg/kg (reported as ppm). Exposure to these levels of melamine through diet resulted in the presence of insoluble crystals of salts of melamine-cyanuric acid in the antennal gland, manifesting as lesions of moderate severity. Increased mortality and prevalence of disease in farmed P. vannamei shrimps where melamine-laced feed had been used were also reported (Lightner et al. 2009).
Results from toxicity studies for sludge microorganisms, unicellular organisms, protozoa, and invertebrates are summarized in Table 7-1.
Test organism |
Test duration |
Endpoint |
Value (mg/L) |
Reference |
|---|---|---|---|---|
Sludge microorganisms |
30 min |
NOEC (respiration) |
2000 |
ECHA c2007-2013 |
Sludge microorganisms |
30 min |
EC10 (respiration) |
>10 000 |
ECHA c2007-2013 |
Sludge microorganisms |
30 min |
EC20 (respiration) |
>1992 |
ECHA c2007-2013 |
Nitrosomonas species |
2 hours |
NOEC Nitrosomonas species |
100 |
Hockenbury and Grady 1977 |
Sludge microorganisms |
100 days |
LOEC (population growth) |
75 |
Xu et al. 2013 |
Ciliated protozoa (Tetrahymena thermophila) |
20 hours |
IC50 (proliferation) |
1000 |
Li et al. 2015 |
Ciliated protozoa (Tetrahymena pyriformis) |
52 hours |
IC50 (generation growth time) |
780 |
Wang et. al 2009 |
Ciliated protozoa (Tetrahymena pyriformis) |
52 hours |
IC05 (generation growth time) |
820 |
Wang et. al 2011 |
Algae (Scenedesmus pannonicus) |
90.5 hours |
NOEC |
320 |
ECHA c2007-2013 |
Algae (Scenedesmus pannonicus) |
90.5 hours |
EC50 |
940 |
ECHA c2007-2013 |
Algae (Selenastrum capricornutum) |
48 hours |
NOEC; EC50 |
97; 325 |
US Testing Company 1988; ECHA c2007-2013 |
Algae (Selenastrum capricornutum) |
72 hours |
NOEC; EC50 |
31; 196 |
US Testing Company 1988; ECHA c2007-2013 |
Algae (Selenastrum capricornutum) |
96 hours |
NOEC; EC50 |
98; 325 |
US Testing Company 1988; ECHA c2007-2013 |
Water flea (Daphnia magna) |
48 hours |
EC50 (mobility) |
200* |
ABC Laboratories 1988; ECHA c2007-2013 |
Water flea (Daphnia magna) |
24 hours |
EC50 (behaviour) |
400 |
ABC Laboratories 1988; ECHA c2007-2013 |
Water flea (Daphnia magna) |
48 hours |
NOEC (mobility and behaviour) |
<56 |
ABC Laboratories 1988; ECHA c2007-2013 |
Water flea (Daphnia magna) |
48 hours |
LC50 |
>2000 |
ECHA c2007-2013 |
Water flea (Daphnia magna) |
48 hours |
EC50 (behaviour) |
<180 |
ECHA c2007-2013 |
Water flea (Daphnia magna) |
7 and 21 days |
LC50 |
>32 and <56 |
ECHA c2007-2013 |
Water flea (Daphnia magna) |
21 days |
NOEC (reproduction and mortality) |
18 |
ECHA c2007-2013 |
Water flea (Daphnia magna) |
21 day |
NOEC, LOEC (reproduction) |
>11 |
ECHA c2007-2017 |
Abbreviations: EC50, the concentration of a substance that is estimated to cause some effect on 50% of the test organisms; LC50, the concentration of a substance that is estimated to be lethal to 50% of the test organisms; IC50, the median inhibition concentration, a point estimate of the concentration of a test substance that causes a 50% reduction in a quantitative biological measurement such as growth rate; NOEC, the no observed effect concentration is the highest concentration in a toxicity test not causing a statistically significant effect in comparison to the controls; LOEC, the low observed effect concentration is the lowest concentration in a toxicity test that caused a statistically significant effect in comparison to the controls
Effects of melamine were determined in numerous fish species based on short- and long-term exposures. Short term studies, with a goal to characterize effects on mortality, included four fish species, the guppy (Poecilia reticulata), rainbow trout (Oncorhynchus mykiss), ide (Leuciscus idus), and the Japanese killifish (Oryzias latipes). Summaries of these unpublished studies (dated 1978, 1982, and 1984) were available from ECHA (c2007-2013) and the Japanese database MITI (1992). Studies were typically performed according to protocols similar to the OECD or US EPA guidelines and included appropriate controls. Based on these studies, it is considered that melamine has low toxicity to fish in short term exposures. Observed endpoints included a 48-hour LC50 of 1000 mg/L and a 96-hour NOEC for mortality of 3000 mg/L. Although some mortality was observed in the tested species, a determination of an acute/short term LC50 for melamine was not feasible in most studies, given that the highest test concentrations used in studies approached the melamine solubility limit of 4850 mg/L (determined at 25ºC). Observations of other physiological effects were not mentioned in the available study summaries. Endpoints determined in these studies are summarized in Table 7-2.
Long-term studies testing effects of melamine were carried out using three fish species, rainbow trout (Salmo gairdneri) and the American flagfish (Jordanella floridae), and fathead minnow (Pimephales promelas). In a 1984 study, summarized in ECHA (c2007-2013), a semi-static test was conducted over 28 days to characterize growth and mortality rates in juvenile rainbow trout (S. gairdneri) exposed to melamine at concentrations ranging from 750 to 3000 mg/L. Approximately 30% mortality was observed at exposure concentrations of 3000 mg/L, therefore, an LC50 for mortality of greater than 3000 mg/L was assigned, and the NOEC for mortality was determined to be 1500 mg/L. Weight loss in fish was also observed at 1500 mg/L and 3000 mg/L exposure concentrations. Therefore, based on these results, a NOEC for growth can be assigned as 750 mg/L. Two other long-term studies, conducted in 1982, examined effects of melamine exposure during early development stages. Ramusino and Vailati (1982) determined effects of melamine on hatching rates and malformations in rainbow trout (S. gairdneri) embryos. Embryos were exposed to melamine at concentrations of 125, 250, 500 and 1000 mg/L until hatched. No mortality was observed in any of the treatments; however, a drop of up to 45% in hatching rates was observed at the highest treatment concentrations. An increase in malformations was also observed in all treatments, with a marked increase to as high as 90% in the 1000 mg/L treatment. It is noted that statistical analyses, that would take factors such as sample size into account, were not performed, and although the percentage drop in the hatching rates and observations of malformation occurrences suggest clear cut effects, it is not certain whether these observations confer statistical differences against controls. Based on the study results, a NOEC for mortality of 1000 mg/L and a LOEC of 125 mg/L for embryonic malformations can be assigned. Effects of melamine on the egg-larval development were also studied in the American flagfish (J. floridae) and study results were summarized in ECHA (c2007-2013). In this study melamine was tested at five concentrations ranging from 100 to 1000 mg/L for 35 days. No effects on hatching ability, appearance and mortality of larvae were noted. Minimal effect on the growth of larvae was noted at the highest concentration tested, but overall the differences in body weights were insignificant (ECHA c2007-2013). The NOEC and EC50 values for survival, growth and condition were determined as 1000 mg/L and greater than 1000 mg/L, respectively. An unpublished 2015 chronic fathead minnow study, summarized in ECHA (ECHA c2007-2015), indicated a LOEC of 10.1 mg/L based on the observations of slight decreases in survival and growth as well as the presence of two deformed individuals. Five concentrations from 0.618-10 mg/L were tested, exposure concentrations were measured throughout the study duration of 36 days (ECHA c2007- 2017).
Addition of melamine to feed also prompted numerous studies in fish used in aquaculture (Liu et al. 2009; Janlek et al. 2009; Xue et al. 2011 a, b; Phromkunthong et al. 2013). As noted in discussion of the shrimp study (Lightner et al. 2009), high dietary exposure to melamine is not expected under typical environmental conditions, rather it is limited to instances where melamine is deliberately added to feed given to farmed animals. Adverse effects were noted and included altered feed efficiency, histopathological changes, as well as statistically lower growth rates in all melamine-fed groups (Phromkunthong et al. 2013). Adverse effects on the fish renal system were noted at higher doses of melamine administered in food, and especially in combination with cyanuric acid. Renal crystal formation and renal failure in trout and catfish was confirmed by Reimschuessel et al. (2010b) following a sequential administration of melamine and cyanuric acid at 20 mg/kg. Similar observations were made in a 2013 unpublished study on red tilapia summarized by ECHA (ECHA c2007-2017).
Results from key studies in fish are summarized in Table 7-2.
Test Organism |
Test duration |
Endpoint |
Value (mg/L) |
Reference |
|---|---|---|---|---|
Japanese Killifish (Oryzias latipes) |
48 hours |
LC50 |
1000 |
MITI 1992 |
Ide (Leuciscus idus melanotus) |
48 hours |
LC50 |
>500 |
ECHA c2007-2013 |
Rainbow trout (Oncorhynchus mykissa) |
96 hours |
LC50 |
>3000 |
ECHA c2007-2013 |
Rainbow trout (Oncorhynchus mykissa) |
96 hours |
NOEC (mortality) |
3000 |
ECHA c2007-2013 |
Guppy (Poecilia reticulata) |
96 hours |
LC50 |
>4400 |
ECHA c2007-2013 |
Guppy (Poecilia reticulata) |
96 hours |
LC50 |
>4590 |
ECHA c2007-2013 |
Guppy (Poecilia reticulata) |
96 hours |
LC50 |
>3000 |
ECHA c2007-2013 |
Rainbow trout (Salmo gairdneri) |
28 days |
NOEC (mortality) |
1500 |
ECHA c2007-2013 |
Rainbow trout (Salmo gairdneri) |
28 days |
LC50 |
>3000 |
ECHA c2007-2013 |
Rainbow trout (Salmo gairdneria) |
28 days |
LOEC (growth) |
750 |
ECHA c2007-2013 |
Rainbow trout (Salmo gairdneria) |
18 to 26 days |
NOEC (mortality) |
1000 |
Ramusino and Vailati 1982 |
Rainbow trout (Salmo gairdneria) |
18 to 26 days |
LOEC (embryonic malformations) |
125 |
Ramusino and Vailati 1982 |
American flagfish (Jordanella floridae) |
35 days |
NOEC (growth, survival, condition) |
1000 |
ECHA c2007-2013 |
American Flagfish (Jordanella floridae) |
35 days |
EC50 (growth, survival, condition) |
>1000 |
ECHA c2007-2013 |
Fathead minnow (Pimephales promelas) |
36 days |
LOEC (survival, growth) |
10.1 |
ECHA c2007-2017 |
Abbreviations: EC50, the concentration of a substance that is estimated to cause some effect on 50% of the test organisms; LC50, the concentration of a substance that is estimated to be lethal to 50% of the test organisms; NOEC, the no observed effect concentration is the highest concentration in a toxicity test not causing a statistically significant effect in comparison to the controls; LOEC, the low observed effect concentration is the lowest concentration in a toxicity test that caused a statistically significant effect in comparison to the controls.
a Salmo gairdneri, commonly known as the rainbow trout, had been reclassified in the genus Oncorhynchus, and therefore, Salmo gairdneri is presently called Oncorhynchus mykiss (Smith and Stearley 1989). Since Latin names that are featured in the original publications and sources are cited, both names mentioned in the table, i.e., Oncorhynchus mykiss and Salmo gairdneri, describe the same fish species.
For the aquatic compartment, a predicted no-effect concentration (PNEC) was derived from the chronic toxicity value of 10.1 mg/L (as the most sensitive, valid experimental value) for the fathead minnow, P. promelas, and by dividing this value by an assessment factor of 5 (to extrapolate from a lethal to sublethal endpoint) to give a value of 2.0 mg/L.
7.1.2 Soil
Limited studies characterizing effects of melamine on soil-dwelling organisms were available. Plant studies including barley (Hordeum vulgare), radish (Raphanus sativus), garden cress (Lepidum sativum) and common wheat (Triticum aestivum) were limited to protocols that involved the use of melamine percolates, and the product Melfasik that contains 2.8% melamine was tested on pea (Pisum sativum) and string bean (Phaseolus vulgaris) (ECHA c2007-2013; OECD 2002). Another study investigated the rates of nitrification by soil microorganism exposed to melamine in soil perfusion experiments (Hauck and Stephenson 1964). Results from these studies generally point to a low toxicity of melamine in the tested species. The toxicity endpoints ranged from a 14-day NOEC of 170 mg/kg, determined for germination and growth of pea and string beans, to a 4-day EC50 of 1100 mg/L for root growth of garden cress.
Given the lack of standard protocols in the existing dataset for the soil medium, and no reliable (Q)SAR models, the PNEC could not be determined for this medium. While some melamine exposure in soil due to biosolids amendment may be expected, it is unlikely that there would be significant melamine concentrations in biosolids, given its low log Koc and log Kow, and high water solubility.
7.1.3 Sediment
Studies addressing the effect of melamine on benthic organisms were not identified.
7.2 Ecological exposure assessment
7.2.1 Measured environmental concentrations
Data concerning concentrations of melamine in the Canadian environment have not been identified. In addition, melamine is not on the list of substances reported to the National Pollutant Release Inventory (Environment and Climate Change Canada 2013).
Globally, baseline concentrations of melamine may be found in the environment as a result of widespread use of materials that contain the substance (WHO 2009). Due to adulteration in animal feed, and the worldwide food trade, animal excrement and urine are suggested to be potential important sources of melamine exposure for environment (Qui et al. 2010). Melamine was measured in water, sediment and in biota in Japan and China (OECD 2002; Qin et al. 2010) as well as in wastewater and soil in China (Qin et al. 2010). Melamine residue deposition and clearance was also studied in livestock (Qui et al. 2010), and provided an insight into melamine contamination of food sources. A summary of melamine concentrations that have been detected worldwide is provided below. In Japan, levels of melamine were surveyed in the environment and biota between years 1986 and 1994 as part of the Environmental Survey and Monitoring of Chemicals led by the Japanese Ministry of Environment (MOE) (CHRIP c2008– ; MOE c2005). Melamine was sampled in surface waters in 1986, 1987, and 1994, and was detected in concentrations ranging between 0.1 to 7.6 µg/L in those years (detection limit was reported as 0.1 µg/L). In those same years, melamine was also sampled and detected in sediment, in concentrations ranging from 0.01 to 0.4 µg/g dry weight (lowest detection limit was reported as 0.01 µg/g dry weight). In wild fish, melamine was detected in concentrations ranging from 0.02 to 0.55 µg/g wet weight (lowest detection limit was reported as 0.02 µg/g wet weight) in years 1987, 1988, and 1986. In 1994, melamine was also measured in air, and was detected in concentrations ranging from 2 to 55 ng/m3 (detection limit was reported as 2 ng/m3) (CHRIP c2008– ; MOE c2005).
In a study supported by the Chinese Ministry of Agriculture, the presence of melamine was assessed in crops, soil, and water, including surface and ground water, and wastewater to determine the extent of melamine contamination in the environment and food products (Qin et al. 2010). In this study, samples were collected in 21 Chinese provinces and included crops (maize, soybean, and wheat), farmland soil (collected at least 150 km from a melamine factory) and soil near industrial operations (100 m from melamine operations), irrigation water (collected either from rivers or underground), and melamine factory wastewater (from sewage disposal at melamine factories).
The maximum melamine concentrations in the Chinese soil and wastewater samples collected near melamine-manufacturing factories were 41.1 and 226.8 mg/kg, respectively (Qin et al. 2010). The highest melamine concentration measured in the irrigation samples was 0.198 mg/L; other concentrations ranged between 21 and 100 µg/L, and many were not detected (detection limit of 20 µg/L). In farmland soil, melamine was detected in only one sample out of 124 tested, at a concentration of 0.176 mg/kg. Analysis of crop samples revealed that less than 20% of samples had melamine at concentrations above 0.1 mg/kg, three samples had melamine more than 1 mg/kg, and the maximum melamine concentration found was 2.05 mg/kg in a wheat sample. The source of the observed melamine levels was attributed to the general presence of melamine in the environment, and residues from the legitimate use of triazine pesticides (such as cyromazine), and melamine-based fertilizers (Qin et al. 2010).
7.2.2 Exposure scenarios and predicted environmental concentrations
No data on measured environmental concentrations (in water, soils or sediments) of melamine in Canada have been identified. Therefore, environmental concentrations have been estimated from available information, including substance quantities used, estimated release rates and characteristics of the receiving environment.
Melamine is a stable chemical, characterized by a slow biodegradation potential in the environment, a low bioaccumulation potential and a low potential for toxicity to aquatic and soil organisms. Melamine is used in high quantities in Canada and worldwide. Its potential for release into the Canadian environment as a result of industrial operations is expected to be mainly to water.
Exposure characterization is focused on scenarios which represent the highest potential for environmental releases and exposure. In general, the magnitude of releases is a direct function of the quantity of a substance manufactured or used and its applicable emission factors. To analyze environmental exposure to melamine in Canada, information for 2011 obtained from the section 71 survey and voluntary stakeholder submissions (ECCC 2013-2014), as well as information obtained from the Canadian International Merchandise Trade Database (Statistics Canada 2019) were considered.
Several companies reported imports of melamine as a pure substance for use in industrial processes to create products such as melamine-formaldehyde resins, plasticizers and products with flame retardant characteristics, as well as imports of melamine already blended into products and as part of manufactured items (ECCC 2013-2014). In these industrial applications, melamine is considered to be chemically bound within the finished product. Although some leaching of melamine out of products (such as plastics) has been identified, this has been limited to releases from melamine dishware and melamine kitchenware, and only when in contact with hot or warm matrices such as food or water (Wu et al. 2013; European Commission 2011). With respect to ecological exposure, the use of melamine in consumer and commercial products is not expected to lead to significant emissions to the environment due to the very low magnitude and dispersed nature of any resultant emissions.
In addition, biosolid amendment to agricultural lands is unlikely to be a significant route of exposure to soil organisms to melamine.Given its high water solubility and the low Log Koc value, melamine will reside in water and is not expected to be associated with biosolids.
Quantitative estimates of melamine concentrations in surface water resulting from the manufacturing of resins and blending to make products with flame retardant qualities are considered in this assessment. These concentrations are based on available information on quantities of melamine, sector-specific emission factors, the characteristics of wastewater treatment systems and the receiving environment.
7.2.3 Exposure scenarios from industrial releases to aquatic medium
To estimate the potential of releases to water resulting from industrial use of melamine in its pure chemical form, conservative scenarios at several industrial facilities were developed. These scenarios were developed by using the substance quantities reported for use in 2011 (ECCC 2013-2014), as well as considering the characteristics of the receiving water bodies, wastewater treatment, and industrial operations at each site. To best characterize the exposure from multiple industrial sites that use melamine to make melamine-formaldehyde resin and melamine compounds, a range of potential situations in Canada is presented as Scenario 1. A second scenario, Scenario 2, for blending of melamine into a product with flame retardant properties is also presented.
Aquatic exposure to melamine could occur if this substance is released from industrial activities to a wastewater system that discharges its effluent to a receiving surface water body. The concentration of the substance in the receiving water near the discharge point of the wastewater system is used as the predicted environmental concentration (PEC) in evaluating the aquatic risk of the substance. It can be calculated using the equation:
C water –ind = [1000 x Q x L x (1 – R)] / N x F x D
where:
Cwater-ind: aquatic concentration resulting from industrial releases, mg/L
Q: total substance quantity used annually at an industrial site, kg/yr
L: loss to wastewater, fraction
R: wastewater system removal rate, fraction
N: number of annual release days, d/yr
F: wastewater system effluent flow, m3/d
D: receiving water dilution factor, dimensionless
Table 7-3 presents the inputs used to estimate resulting aquatic concentrations close to the industrial points of discharge. It is noted that the assumption of loss to water, although based on empirical data, is considered conservative for these specific industrial practices.
Input |
Scenario 1 |
Scenario 2 |
Justification and reference |
|---|---|---|---|
Quantity (kg/site) |
10000-10000000 | 10000-100000 |
Quantity of melamine reported at each site in Canada for 2011 (ECCC 2013-2014) |
Loss to wastewater (%) |
0.6 |
1 |
OECD 2002 (Scenario 1); Environment and Climate Change Canada standard assumption for blending process (Scenario 2) |
Wastewater system removal efficiency (%) |
0.3-2.1 |
2.1 |
STP-EX 2008 (predicted for secondary treatment) |
Number of annual release days (days) |
250-350 |
250 |
National Pollutant Release Inventory database for the years 1992-2013 (October 11, 2013 version) (ECCC 2013) (Scenario 1); Environment and Climate Change Canada standard assumption (Scenario 2) |
Wastewater system effluent flow (m3/d) |
2 652-25 4260
|
45 942 |
Site specific WWTS data |
Dilution factor (–) |
10 |
10 |
Site specific WWTS flow rate/ receiving environment flow rate. When a dilution factor was greater than 10, a maximum default value of 10 was used. |
Based on the above-mentioned assumptions, these scenarios yielded predicted environmental concentrations (PECs) of 3x10-3 mg/L to 1.8 mg/L for scenario 1, and 8x10-3 mg/L for scenario 2. It is noted that the percentage loss of 0.6% to wastewater included in the calculation is based on empirical data from facilities that manufacture and blend melamine (OECD 2002). Since melamine is not manufactured in Canada, this is a very conservative evaluation of melamine releases and may contribute to overestimation of risk.
7.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine assessment information and develop proposed conclusions using a weight-of- evidence approach and precaution. Evidence was gathered to determine the potential for melamine to cause harm in the Canadian environment. Lines of evidence considered include those evaluated in this assessment that support the characterization of ecological risk in the Canadian environment. Secondary or indirect lines of evidence are considered when available, including regulatory decisions and classification of hazard or fate characteristics made by other regulatory agencies. This information suggests that there is low risk of harm to organisms and the broader integrity of the environment in Canada from melamine.
7.3.1 Risk quotient analysis
A risk quotient analysis that integrated conservative estimates of exposure with toxicity information was performed for the aquatic medium. The scenarios for the aquatic medium yielded predicted environmental concentrations (PEC) of 3x10-3 mg/L to 1.8 mg/L for scenario 1 and 8x10-3 mg/L for scenario 2. For both aquatic scenarios, a predicted no-effect concentration (PNEC) of 2.0 mg/L was derived from the chronic toxicity value of 10.1 mg/L for the fathead minnow and by applying an assessment factor of 5. The resulting risk quotients (PEC/PNEC) ranged from 0.0015 to 0.9 for scenario 1. It is noted that the risk quotients obtained from scenario 1, i.e., 0.015 and 0.9, are the lowest and the highest risk quotients, calculated for industrial facilities using melamine, and therefore represent a range of encountered risk quotient values for this most common melamine application in Canada. The risk quotient for scenario 2 was 0.004. Table 7-4 provides a summary of this information. Results indicate that harm to aquatic organisms from releases of melamine in these conservative scenarios is unlikely.
Media |
Scenario |
PNEC |
PEC |
RQ |
|---|---|---|---|---|
Water |
Scenario 1: Industrial releases to water from the use of melamine in its pure chemical form |
2.0 mg/L |
3x10-3-1.8 mg/L |
0.0015-0.9 |
Water |
Scenario 2: Industrial release from blending of melamine into a fire retardant product |
2.0 mg/L |
8x10-3 mg/L |
0.004 |
7.3.2 Consideration of lines of evidence and conclusion
To characterize the ecological risk of melamine, technical information for various lines of evidence was considered (as discussed in the relevant sections of this report) and qualitatively weighted. The key lines of evidence supporting the assessment conclusion are presented in Table 7-5, with an overall discussion of the weight of evidence provided in section 7.3.3. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence. The relevance refers to the impact the line of evidence has when determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis ranged from low to high, with the assigned weight having five possible outcomes.
Line of evidence |
Level of confidencea |
Relevance in assessmentb |
Weight assignedc |
|---|---|---|---|
Environmental fate and behaviour |
High |
High |
High |
Persistence in the environment (water) |
High |
High |
High |
Long-range transport |
Moderate |
Low |
Low to moderate |
Bioaccumulation in aquatic organisms |
High |
Low |
Moderate |
PNEC for aquatic organisms |
High |
High |
High |
PEC in water |
Moderate |
High |
Moderate to high |
RQs for water |
Moderate |
High |
High |
a Level of confidence is determined according to data quality, data variability, data gaps (i.e., are the data fit for purpose).
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the overall combined weights for level of confidence and relevance in the assessment.
7.3.3 Weight of evidence for determining potential to cause harm to the Canadian environment
Melamine is expected to be persistent in water, soil and sediment and to have a low bioaccumulation potential. It has also been demonstrated that melamine has low to moderate potential for toxicity to aquatic organisms. High importation volumes of melamine into Canada, along with information on its uses, indicate potential for widespread release into the Canadian environment. Once released into the environment, melamine is expected to predominantly distribute to water. Predicted environmental concentrations (PECs) resulting from industrial uses of melamine were calculated based on industrial scenarios. Consideration of the PEC values, together with the toxicity of melamine to aquatic organisms, its potential to bioaccumulate and its long residence time in the aquatic media, formed the basis for evaluation of the potential to cause ecological harm. This information indicates that melamine has low potential to cause ecological harm in Canada
7.3.4 Sensitivity of conclusion to key uncertainties
There is uncertainty regarding assumptions used that may have resulted in over-estimation of the risk quotients calculated for the key industrial applications of melamine. In particular, the conservative assumption of substance loss to environmental media of 0.6%, based on empirical data from facilities that manufacture and blend melamine, may have led to an overestimation of risk. Melamine is not manufactured in Canada. Lack of monitoring data in Canada does not allow for direct comparison with PECs to verify whether they have been over- or under-estimated. PECs in water for melamine were determined to be in the range of 8x10-3 to 1.6 mg/L. The measured surface water concentrations of melamine in Japanese rivers were in the range of 1x10-4 – 7.6x10-3 mg/L and up to 1.98x10-1 mg/L in rivers in China that receive irrigation water. Therefore, in comparison with the sparse measured data, the derived PECs are likely conservative.
Although melamine is not expected to be associated with biosludge, some melamine may be removed through biosolids. This process is likely inefficient as dictated by melamine physical chemical properties including the high water solubility and a low soil organic carbon-water partitioning coefficient.
The limited existing empirical dataset for melamine effects in soil-dwelling organisms, and lack of reliable (Q)SAR models for the soil medium, prevented derivation of a reliable PNEC value. The soil scenario was therefore not pursued since too many uncertainties were associated with key information needed for its development. However, given the low observed toxicity of melamine to soil-dwelling organisms, and the likely low concentrations of melamine associated with biosolids, the potential risk to soil-dwelling organisms from this route of exposure is not perceived to be considerable.
There is also uncertainty regarding ecological effects from potential releases of melamine present in melamine-based flame retardants such as melamine cyanurate (CAS RN 37640-57-6), melamine phosphate (CAS RN 20208-95-1), and melamine polyphosphate (CAS RN 218768-84-4). These substances are not on the Domestic Substance List, and were therefore not subject to categorization under subsection 73(1) of CEPA. Potential ecological effects and risk characterization due to melamine contained in these melamine-based flame-retardant substances were not addressed in this screening assessment.
8. Potential to cause harm to human health
8.1 Exposure assessment
The potential for exposure to melamine via environmental media (drinking water, air, soil/dust), food, melamine-containing tableware and dishware, and products available to consumers is discussed in this section, as well as estimates of exposure based on biomonitoring data.
8.1.1 Environmental media and food
8.1.1.1 Air
No data on concentrations of melamine in ambient or residential indoor air were identified.
8.1.1.2 Dust
No data on concentrations of melamine in dust were identified.
8.1.1.3 Soil
No data on melamine concentrations in North American soils were identified and a soil predicted environmental concentration (PEC) was not determined [see section 7.2]. In China, soils were tested at 100 m and approximately 150 km away from melamine manufacturing factories. At 100 m, melamine concentrations in soil ranged from not detected to 41.1 mg/kg. At approximately 150 km, concentrations in farmland soil ranged from not detected to 0.176 mg/kg (Qin et al. 2010) [also discussed in section 7.2.1]. Concentrations measured further away from a melamine manufacturing facility would be more representative of soil concentrations to which the general population could be exposed in Canada. The maximum concentration in farmland soil measured at 150 km away from the manufacturing facility in China was used in a deterministic estimate of daily intake.
The deterministic estimate of daily intake based on exposure to melamine via soil is negligible for all age groups.
8.1.1.4 Water
Canadian occurrence data for melamine in drinking or surface water were not available. The only results for melamine concentrations in drinking water were found in a European Food Safety Authority (EFSA 2010) Panel report. The EFSA report indicates that the data were provided by industry members sourced from various areas around the world (n = 20 tap water samples) and reported melamine concentrations ranging from 10 to 200 µg/kg (mean: 50 µg/kg; values reported as mg/kg for all food groups including tap water in EFSA 2010). Individual results for each sample were not reported and there was no indication as to how many water samples had melamine concentrations below the limit of detection (LOD).
Due to the lack of Canadian data pertaining to this potential source, the EFSA data were used for exposure characterization of melamine from drinking water. Estimated intakes were 5.3 and 21 µg/kg bw/day for 0 to 6 month old infants, formula-fed (highest exposed group), for central tendency and upper-bound concentrations, respectively (see Appendix B).
8.1.1.5 Food
As shown under “Sources and uses” (Section 4), melamine is not listed as an approved food additive, but it may be found in food packaging in Canada. In the USA, melamine is permitted for use as an indirect food additive in the synthesis of melamine-formaldehyde resins intended for use in food processing and packaging.
Various international studies have investigated the migration of melamine from can coatings and jar closures into food and beverages. In the U.K., Bradley et al. (2011) tested migration from resins based on melamine-formaldehyde and related analogues (methylolated melamine) used to cross-link coatings inside food cans and metal closures on glass jars. For 13 coatings tested, concentrations of melamine that migrated into food ranged from < 1.5 to 332 µg/kg depending on the conditions used (i.e., fluid matrix, temperature variation). Using the same experimental conditions, six different laboratories in Europe observed similar migration rates ranging from 1.5 to 327 µg/kg (EFSA 2010). Bradley et al. (2011) also tested migration of melamine into three different food types (food types varied based on acidity, fat content, and presence of meat or fish) in various conditions and observed migration concentrations ranging from < 23 µg/kg to 220 µg/kg. Magami et al. (2015) also tested four different cross-linking substances that contained residual amounts of melamine (< 0.1 to 0.2%) used in epoxy based coatings for food cans. The coatings were heated at a high temperature of 131°C in a food simulant (10% aqueous ethanol) for different periods (30 to 180 min) followed by different storing periods (1 to 30 days), and then reheated at 131°C for 1 h. Migration of melamine from the coatings equated to 7 to 60% on a molar basis of the total melamine content of the cross linker used. The authors were able to show that the migration process is not by diffusion of melamine from the coating. Rather, the melamine formed by hydrolysis of the coating and was released as melamine itself, and it does not have a significant tendency to undergo hydrolysis to analogues such as ammeline, ammelide or cyanuric acid. The aforementioned data show that melamine may migrate from food packaging into food, and that the resulting concentrations in foods will increase if foods are subjected to heating, such as during the processing of food cans and bottles (EFSA 2010).
Melamine has been measured in many foods in many countries, primarily as a follow-up to the identification of melamine-adulterated formula and animal feed incidents in China and North America, respectively (WHO 2009; Hilts and Pelletier 2009; Dorne et al. 2013). Concurrently, there has been an increased demand for more rapid and/or more sensitive and accurate analytical techniques to determine melamine levels in such foods. Recent reviews of advances in analytical techniques to determine melamine in food and milk products reported lower ranges of limits of detection than those reported in the review of Rovina and Siddique (2015) published two years earlier (from 3.7 x 10-12 to 0.6 ng/g for milk products; as low as 1 x 10-5 mg/L for food samples as reported by Lu et al. 2017 and Nascimento et al. 2017).
In Canada, surveys to measure levels of melamine in food were conducted by the Canadian Food Inspection Agency (CFIA) from 2008 to 2012, primarily for the purpose of identifying high concentrations that may have occurred from adulteration and to ensure continued compliance with Health Canada’s Interim Maximum Levels for melamine in foods. As such, sample selection was biased and the methodology resulted in a relatively high limit of detection (LOD). Data from these surveys were not used in this assessment.
In addition, Health Canada has conducted research to measure background levels of melamine in food sold in Canada and has analysed 94 samples of various infant formulas sold (analysed in the “as purchased” form), 246 samples of dairy and soy-based dairy replacement products, and 378 samples of egg-containing products, soy-based meat substitutes, fish and shrimp products, and vegetable products (Tittlemier et al. 2009, 2010a, 2010b). The Health Canada surveys focused on foods with the greatest probability of containing residual levels of melamine. Such foods include dairy foods and other products containing milk, vegetable productsFootnote 2, and marine foodsFootnote 3.
Dietary exposure to melamine from all food sources shown in Table 8-1 was estimated using a semi-probabilistic approach using mean melamine concentration data for each food commodity type sampled by Health CanadaFootnote 4. Mean and 95th percentile exposure estimates for various age groups are provided in Table 8-1 (see Appendix C for melamine concentration levels and dietary exposure assessment methodology)Footnote 5.
Age group (years)a |
Mean |
95th percentile |
|---|---|---|
< 1 |
0.715 |
1.686 |
1 to 4 |
0.464 |
1.200 |
5 to 11 |
0.316 |
1.155 |
12 to 19 |
0.215 |
0.731 |
20 + |
0.209 |
0.803 |
a Males and females are both included in each age category.
Chocolate candy and milk coffeeFootnote 6 combined, account for almost 60 percent of the estimated dietary melamine exposure for the overall population. The high contribution of chocolate candy is the result of one sample that was found to have an elevated concentration of melamine, thereby significantly increasing the mean melamine level used to represent all chocolate candy (i.e., 667.2 µg/kg)Footnote 7. Since the one sample with an elevated concentration of melamine may be an outlier, and the mean concentration of melamine in chocolate candy, excluding this value, is 4.9 µg/kg, the actual contribution of melamine from chocolate candy is likely lower.
Of note, melamine food packaging migration concentrations (< 1.5 to 332 µg/kg) reported in the European studies mentioned above are within the mean range of values reported in the Canadian food monitoring studies (see Appendix C, range: < 4 to 667 µg/kg).
8.1.1.6 Breast milk
Yurdakok et al. (2014, 2015) measured melamine in breast milk of 77 healthy lactating mothers in Ankara, Turkey from June-September 2010 (babies were 3-10 days old). Breast milk samples (10 mL) from each mother were analyzed by HPLC with the LOD and LOQ determined at 10.6 and 41.6 µg/L, respectively. Melamine was detected in 16 of 77 samples (20.8%), with concentrations ranging from 10.1 to 76.4 µg/L (mean: 27.1 µg/L; or between LOD and LOQ), as reported by the authors. There was no influence of body mass index on the distribution of melamine concentrations (mothers grouped into normal, overweight, and obese weight groups).
Since no Canadian data were identified regarding melamine presence in breast milk, the Turkish dataset was used for exposure characterization. Daily intake estimates in infants aged 0 to 6 months, from melamine presence in breast milk are 2.7 and 7.6 µg/kg bw/day for mean and maximum concentrations, respectively.
8.1.2 Migration from melaware
Melamine reacts with formaldehyde to produce a thermoset plastic called “melamine” or “melaware” which can be used as dishware or kitchenware.
Studies on migration of melamine from melaware dishware into foods, water and other beverages were identified. In a Danish study, concentrations of melamine migrating into water from purchased melaware plates (n = 6) and previously used melaware cups (n = 11) ranged from <1500 to 2940 µg/L over a temperature range of 20 to 95°C (Lund and Petersen 2006). Bradley et al. (2010) also analyzed migration in melaware articles (bowls, cups, etc.) purchased in 2010 from retail outlets in Germany, the Netherlands and the UK. Concentrations of melamine migrating into various types of food and liquid matrices (tomato sauces, water, beverages, etc.) ranged from not detected to 4194 µg/kg. Experimental conditions were also varied, i.e., samples were heated to temperature ranges of 40 to 100°C, or put under microwave conditions or unheated samples were exposed to hot foods or liquids. Lynch et al. (2015) tested migration of melamine in 50 children's bowls ordered online and manufactured in China. When the bowls were heated at a temperature of 95°C for 30 min over a series of 10 runs, mean melamine migration into solutions of different pHs ranged from not detected (LOD = 4.1 µg/L) to 76.7 µg/L, and the highest migration rates were noted in solutions of pH 3. Migration rates at all pHs dropped by 85% between runs one and 10.
In a Taiwanese study (Chien et al. 2011), melamine concentrations in a food simulant (3% acetic acid or distilled water), measured in 25 melaware cups, ranged from 30 µg/L (20°C) to 19,030 µg/L (90°C); migration was significantly higher in 3% acetic acid than in distilled water.
In a Malaysian study, melamine concentrations in 3% acetic acid or water, measured in 246 melaware dishware items, ranged from 1.31 to 140 µg/L at 25°C to 4.05-509 µg/L at 100°C (Chik et al. 2011). Additionally, reported mean or median concentrations of melamine migrating into water or other beverages from melaware dishware at room temperature ranged from 22 to 30 µg/L (Chik et al. 2011; Chien et al. 2011). These concentrations fall within the range used for beverages in the dietary intake assessment and are also below the mean drinking water concentration of 50 µg/kg reported by EFSA (2010).
Based on the studies conducted on food and beverages mentioned above, concentrations measured in food items heated (100oC) in melaware dishware appear to be significantly higher than concentrations measured in items left at room temperature. EFSA (2010) examined the migration of melamine from melaware dishware into food and water, citing some of the same data above, as well as additional data provided by other European countries (i.e., Finland, Cyprus, Netherlands). EFSA concluded that migration of melamine from melaware dishware is characterized by high variability, depending on various factors such as manufacturing process, alterations to the surface due to service life, time and temperature conditions of use, as well as characteristics of food (e.g., acidic, aqueous, fatty or dry). Consequently, this may result in higher levels of melamine residue.
EFSA estimated exposure to melamine from migration using “typical and high migration values” for different food classes. Both were considered conservativeFootnote 8. Concentrations in each type of food after migration from a melamine-containing article estimated by EFSA are shown in Table 8-2.
Food Type |
Typical |
High |
|---|---|---|
Acidic foods |
1.0 |
5.0 |
Aqueous foodsa |
0.6 |
3.0 |
Fatty foods |
0.2 |
1.0 |
Dry foods |
0.05 |
0.05 |
a Assumed to have same density as water but not specifically stated in EFSA (2010).
Exposure to melamine from use of melaware dishware was estimated using conservative assumptions, e.g., assuming that all foods and beverages come into contact with melaware dishware. The highest EFSA-derived typical migration levels (acidic and aqueous foods) were used to estimate exposure from this source (see Table 8-2). Although migration studies were not identified for melamine-containing dishware in Canada, it is assumed that conditions and results for migration of melamine from such dishware would be similar to data generated elsewhere. Finally, exposure estimates for different age groups were derived using overall daily food consumption quantities estimated by EFSA (2006) (see Table 8-3).
Age group |
Consumption based on food type (kg)a |
Body weight (kg)b |
Exposure (µg/kg bw/day)c,d |
|---|---|---|---|
Infant aged 6 months (not liquid formula fed) |
Commercial baby foods and drinks and powdered infant formula = 0.5 |
7.5 |
40 |
Child aged 1.5 years |
Beverages = 1.33 Solid foods = 0.67 |
15.5 |
112 |
Adult |
Beverages = 2 Solid foods = 1 |
70 |
37 |
a The consumption scenario considered was taken from EFSA (2006). Values considered for the 6 month infant are the 95th percentile of consumption of commercial baby foods and drinks (other than breast milk or liquid infant formula) and of powdered infant formula observed in 6 month infants in the Dortmund Nutritional and Anthropometrical Longitudinally Designed [DONALD] study (0.5 kg) (Kersting et al. 1998). For the child aged 1.5 years, the daily consumption of 1.33 kg of beverages and 0.67 kg of solid foods were considered (CEC 1993). For adults, daily consumption of 1 kg of solid foods and 2 kg of beverages were considered. Drinks and beverages are assumed to have the same density as water but not specifically stated in EFSA (2006) nor EFSA (2010).
b Infants assumed to weigh 7.5 kg; toddlers assumed to weigh 15.5 kg, and adults assumed to weigh 70 kg (Health Canada 1998).
c Exposure from melaware at highest typical migration level observed for acidic (1 mg/kg) and aqueous foods (0.6 mg/kg) derived by EFSA (2010). For infants 0 to 6 months acidic foods migration rate (1 mg/kg) used for baby food/drink and formula intake calculations. For children 1.5 years old and adults, acidic foods migration rate (1 mg/kg) used for beverage intake calculations and aqueous foods migration rate (0.6 mg/kg) used for solid food intake calculation.
d Exposure = (Migration from melaware articles into food matrix (mg/kg) x Consumption of food type per day) / body weight.
8.1.3 Other products available to consumers
As noted in section 4, melamine has uses in various types of products in Canada. Examples of these products include paints and coatings, sealants, foam seats/backing/mattresses, thermally-fused melamine paper and shelves, whiteboards and flakeboards, inkjet ink, melamine-containing dishware and tableware, etc.
Due to considerations such as limited dermal contact, commercial and industrial use, and low melamine concentrations; exposure to paper and shelves, whiteboards and flakeboards, and inkjet inks were not assessed as exposure is expected to be low especially compared to other scenarios presented below. Additionally, potential exposure to melamine via dishware and tableware is addressed in the previous section.
Do-it-yourself products (paints, sealants, coatings):
Material Safety Data Sheets (MSDSs) from paint products sold in Canada containing melamine show that the maximum concentration in waterborne paints is 13% (MSDS 2013; TDS 2011). MSDSs were also available for sealants sold in Canada for mechanical, electrical and plumbing applications that contain melamine up to a maximum concentration of 60%Footnote 9. Although the MSDSs indicated that the product was for industrial and professional use only, these products are available to general consumers at retail outlets (CSL Silicones Inc. 2014; MSDS 2016).
Both inhalation and/or dermal exposures from “per event use” were considered for users of airless paint spray equipment, brush and roller paint applications, as well as applications of sealing and caulking within the home. However, the following available evidence for melamine was considered:
- Due to the negligible vapour pressure associated with melamine, inhalation exposure from brush and roller paint applications are considered negligible. Further, inhalation from airless paint spray applications would also be negligible as this application would not likely produce respiratory-sized droplets (majority of droplets would be > 15 µm in diameter).
- Acute dermal administration of melamine in experimental animals does not result in skin reactivity (Fassett and Roudabush 1963; Rijcken 1995; Vernon et al. 1990) and structurally similar compounds show only maximum dermal absorptions of 10-16% in human skin (Ademola et al. 1993; Baynes et al. 2005).
- Acute oral, dermal and inhalation studies indicated a lack of acute hazard endpoints of concern in experimental animals (BASF 1969a; US NTP 1983; Vernon et al. 1990; Ubaidullajev et al. 1993; Muijser 1998; OECD 2002).
Based on the above considerations, one-time per event dermal and inhalation exposures to melamine from consumer use of airless paint spray equipment, brush and roller paints, and caulking and sealants in the home were determined to represent a low to negligible risk for the Canadian general population.
Foam products (seating, bedding etc.):
As stated in the “Sources and uses” section, melamine is also found in foam seating and bedding (which may comprise products such as pillows and mattresses) in Canada. Concentrations of melamine in the foam used in seats and backs of metal frame furniture were reported at concentrations of 28 to 29% (ECCC 2013-2014). The US CPSC (2005) analyzed 6 types of upholstery foam for melamine content and found concentrations ranging from not detected (LOD = 0.005% w/w) to 34% w/w. This is consistent with reported melamine concentrations for Canadian foam furniture items and mattresses (bedding is mentioned in the “Sources and uses” section which may comprise products such as pillows and mattresses). Fourteen child restraint seats representing different types and brands manufactured in 2014 were tested for several flame retardants, including melamine, by two different analytical methods. Melamine was detected in 3 of the 14 seats, specifically in polyurethane foam in two seats and in expandable polypropylene foam as well as in the textile of another seat. Concentrations in the seats were not measured (Ecology Center 2015). Melamine was tentatively identified but not quantified using LC/Time-of-Flight MS operated with electrospray ionization analysis, in polyurethane foam and other components from American furniture manufactured between 2000 and 2015 (Petreas et al. 2016). An analysis using LC-MS/MS to analyze six polyurethane foam samples from Canadian sources showed melamine concentrations ranging from not detected (LOD = 0.031% w/w) to 0.43% w/w (Health Canada 2015).
Since melamine is an additive flame retardant and can migrate out of a matrix, dermal exposure may occur from prolonged skin contact with melamine-treated foam furniture and mattresses. This migration may be mediated by sweat since melamine is associated with high water solubility, but migration may also be mediated by non-aqueous skin products such as hair and keratin. Log Kow, commonly used as a measure of hydrophobicity, was also considered.Footnote 10 Data on migration of melamine were not identified. Instead, migration rates for melamine were extrapolated from migration rates in relation to the water solubility or the combination of water solubility and log Kow of other flame retardants, i.e., reported in ECHA (2018) for TDCPP (CAS RN 13674-87-8) and TCEP (CAS RN 115-96-8), as shown in Table 8-4. The ECHA (2018) used TDCPP and TCEP migration rates to estimate potential dermal exposure adapted from exposure studies using treated furniture foam.
Parameter |
TDCPP |
TCEP |
Melamine |
|---|---|---|---|
Water solubility (mg/L) |
18.1 |
7820 |
3230 |
Log Kow (unitless) |
3.69 |
1.78 |
-1.14 |
Rate of migration from covered foam (mg/cm2/hr)a |
0.00297 |
0.0207 |
0.00936b 0.0217c |
a The migration rates for TDCPP and TCEP were determined in migration studies performed on treated furniture foam by the Danish EPA (2015) as reported by ECHA (2018). The migration rates of TCEP and TDCPP were determined using children’s products (i.e., child restraint seats, baby slings, baby mattresses) by submerging pieces of foam from these products (usually with some of the fabric covering included in the samples) in sweat simulant and incubating them at 37°C for 3 hours (Danish EPA 2015). The migration rate for TDCPP used here is the average of the rates found across all samples for this flame retardant while the migration rate for TCEP was from a single item (ECHA 2018).
b Calculated based on plotting a straight line between water solubilities and migration rates for TDCPP and TCEP with equation y = (2E-06 x water solubility) + 2.9E-03.
c Calculated using quadratic equations based on TDCPP and TCEP migration rates, water solubilities, and log Kow: For TDCPP, 0.00297 mg/cm2/hr = x (log of water solubility) + y (log Kow) and for TCEP, 0.0207 mg/cm2/hr = x (log of water solubility) + y (log Kow). Thus, 0.00297 = x (1.26) + y (3.69) and 0.0207 = x (3.89) + y (1.78). Solving for x and y results in: x = 0.005863 and y = -0.0012. For melamine, migration rate = 0.005863 x (log of water solubility) + (-0.0012) (log Kow) = 0.005863 x (3.51) + (-0.0012) (-1.14) = 0.0217 mg/cm2/hr.
Based on this, a migration rate range of 0.00936 to 0.0217 mg/cm2/hr was derived for melamine. Using this range, dermal exposure uptakes were estimated for children and adults in direct contact with fabric-covered foam-containing mattresses and related manufactured items (such as foam-containing furniture). This scenario is considered to also be representative of potential exposure from textile backings in furniture.Footnote 11 As shown in Appendix D, the estimates of dermal exposure to melamine via prolonged dermal contact with foam mattresses or furniture were 0.02 to 0.36 mg/kg bw/day and 0.08 to 1.02 mg/kg bw/day in adults, and infants, respectively. Finally, due to melamine’s negligible vapour pressure, inhalation exposure to melamine contained in foam furniture and mattresses is expected to be negligible.
Based on melamine’s properties (additive flame retardant, high water solubility), it is expected that children may be exposed from mouthing a foam object. Although melamine concentration data in foam products were available, a melamine-specific migration rate from foam was not identified in the literature. In the absence of migration rates for mouthing, the same migration rate used for the dermal scenario was applied. As shown in appendix D, the estimates of oral exposure to melamine via mouthing of a foam object are 0.00031 to 0.0035 mg/kg bw/day for infants and 0.00030 to 0.0034 mg/kg bw/day for toddlers.
8.1.4 Biomonitoring
Panuwet et al. (2012) published the results for melamine measurements in 492 spot samples of human urine collected from the general US population. These samples were collected in 2003-2004 as part of the U.S. National Health and Nutrition Examination Survey (NHANES) and included samples from both males and females aged 6 years and older (US CDC 2010a). Melamine was detected in 76% of the samples (population: 6 + years old, GM: 2.37 ng/mL, 95th percentile: 12 ng/mL, maximum: 161 ng/mL, LOD = 0.66 ng/mL)Footnote 12. Since the relative distribution of age groups (i.e., 6 to 11, 12 to 19 years, etc.) was not available from Panuwet et al. (2012), this information was derived from demographic data collected by NHANES (US CDC 2010b). Geometric mean and 95th percentile concentrations, based on the different age groups, are presented in Table 8-5.
Regarding pharmacokinetic data, no studies were identified showing the fraction of melamine excreted in human urine; however, Mast et al. (1983) showed that 90% of administered melamine was excreted in urine of male rats as melamine. In dogs, Lipschitz and Stokey (1945) showed that 60 to 86.5% of administered melamine was excreted as melamine 24 hr after a single oral dose of melamine. Finally, although Liu et al. (2010) suggested that the amount excreted in monkey urine was much less than the oral dose, they could not document a mass balance for the excretion profile or cite the percentage of melamine dose excreted in the urine (see “Health effects assessment, Oral toxicokinetics” section and Health Canada 2018c: Appendix B, Table B-1).
Due to the variability in urinary excretion between species, the lowest percentage excretion of melamine in urine (60%; Lipschitz and Stokey 1945) was used to calculate biomonitoring intakes. Additionally, since information on urine volume excreted for each participant was not collected; ranges of typical 24 hour mean urine volumes identified from various sources and shown in the table below were also used to calculate intakes (see Appendix E for urine volume ranges and references). Biomonitoring intakes are presented in Table 8-5.
Age Group |
6–11 years |
12–19 years |
20-59 years |
60+ years |
|---|---|---|---|---|
Geometric mean of urinary melamine concentration (ng/mL) |
5.91 |
2.06 |
2.33 |
2.93 |
Upper 95th percentile urinary melamine concentration (ng/mL)e |
6.7 |
10.47 |
12.44 |
11.09 |
No. of participantsa |
6 |
162 |
217 |
107 |
Range of mean urine volumes/day (L/day)b |
0.27-1.14 |
0.44-1.40 |
0.6-2.70 |
0.25-2.4 |
Average body weight (kg)c |
31.8 |
59.4 |
70.9 |
72.0 |
Mean intake (µg/kg bw/day)d |
0.08-0.35 |
0.03-0.08 |
0.03-0.15 |
0.02-0.16 |
95th percentile intakes (µg/kg bw/day)d,e,f |
0.09-0.40 |
0.13-0.41 |
0.18-0.79 |
0.06-0.62 |
a Based on age of participants at time of urine sampling (492 total number of participants).
b Range of average urinary 24 hr volumes reported in the literature (see Appendix E).
c Average body weights for 12-19, 20-59, and 60+ year groups from Health Canada (1998). Average body weight derived for 6-11 year olds, based on US EPA (2011) because Health Canada (1998) does not report body weights based on this age range.
d Based on the lowest reported percentage excretion of melamine in urine in experimental animals 24 hr after dosing = 60% (Lipschitz and Stokey 1945; Mast et al. 1983; Liu et al. 2010).
e For 6 – 11 year olds, 5 individuals showed urinary melamine concentrations between 0.5 and 6.7 ng/mL and the other presented a urinary concentration of 161 ng/mL. Due to small sample size in this age group and the possibility that one of the concentrations was an outlier, a 95th percentile intake calculation would be statistically unstable. Therefore, the second highest concentration was used to determine the upper-bound intake.
f Mean and 95th percentile intake estimates were calculated using the following equation (Aylward et al. 2012): Daily intake (µg/kg bw/day) = [urine concentration (µg/L) x 24 hr urine volume (L/day)] ÷ [fraction excreted in urine x BW (kg)].
For the 12+ years old age groups, mean intakes ranged from 0.02 to 0.16 µg/kg bw/day and 95th percentile intakes ranged from 0.06 to 0.79 µg/kg bw/day. For the group aged 6-11 years old, mean intakes ranged from 0.08 to 0.35 µg/kg bw/day. Due to the small sample size (n = 6) of this age group, 95th percentile intakes were not derived because they would be statistically unstable. Also, one high recorded concentration of 161 ng/mL melamine was recorded for this age group, whereas other concentrations among the 6 individuals ranged from 0.5 to 6.7 ng/mL. Panuwet et al. (2012) did not discuss whether this high value was an artifact.
Other biomonitoring studies have been conducted in Taiwan and Hong Kong (see Appendix F) but the results based on the US population measured by Panuwet et al. (2012) were used as a surrogate for the Canadian population, and were considered most relevant.
8.2 Health effects assessment
Health effects information for melamine is summarized in this section. Further details may be found in the supporting documentation, Health Canada (2018c). In terms of classification, the International Agency for Research on Cancer (IARC) has classified melamine as Group 2B (possibly carcinogenic to humans) (IARC 2019).Footnote 13
8.2.1 Oral toxicokinetics
Melamine was excreted in rat and monkey urine after single oral doses of 1.3 and 1.4 mg/kg bw, respectively, but the following two metabolites were identified in addition to unchanged melamine, after large single oral doses in the rat (250 mg/kg bw) and dog (125 mg/kg bw): dimelamine monophosphate and monomelamine-monooxalate. Also, based on extensive toxicokinetic studies in rats, melamine was not metabolized at lower doses compared to higher doses (1.3 vs. 250 mg/kg bw), which suggests that oral exposure at environmental levels of melamine, it may not be metabolized in the body. In the rats dosed at 1.3 mg/kg bw/day, mass balance calculations showed that 98% of the dose was recovered as melamine 96 hrs after dosing, confirming a lack of metabolism (Mast et al. 1983). In the three monkeys orally gavaged at 1.4 mg/kg bw, the amount excreted in monkey urine was much less than the oral dose, but the authors could not document a mass balance for the excretion profile or cite the percentage of melamine dose excreted in the urine, because melamine was not radiolabelled in this study (Liu et al. 2010).
At 100 mg/kg bw/day oral dosing, there appears to be saturation of excretion. When Wu et al. (2010b) dosed Sprague-Dawley (SD) rats at 100 mg/kg bw, they wrote "About 63.2% of the administered dose was recovered from the urine within 96 h. The previous study indicated a value of 90% elimination [at a dose of 1.3 mg/kg bw] within the first 24 h [Mast et al. 1983], and this discrepancy might result from a much higher dose (100 mg/kg, po) in our study, possibly leading to the saturation of urinary elimination and delayed elimination time. This result suggests that most of the administered melamine was absorbed and eliminated via the urine in unchanged form."
Oral bioavailability of melamine ranged from 73±13% to 98% in rats, confirming high and rapid oral absorption in the gastrointestinal tract (Yang et al. 2009; Wu et al. 2010b). Studies conducted by Yang et al. (2009), Wu et al. (2010b), and Pang et al. (2013) indicate that melamine does not extensively distribute to most tissues in rats after single or repeated oral dosing for 14 days at 100 mg/kg bw, being mostly restricted to the blood. Several authors reported plasma elimination half-lives ranging from 1.3 to 5 hrs from various studies in rats and monkeys upon single oral doses of 1 to 100 mg/kg bw (Wu et al. 2010b; Jacob et al. 2012; Dorne et al. 2013; Pang et al. 2013). Dorne et al. (2013) considered the rates comparable at these oral doses, and in all cases, support the observation of rapid excretion of melamine in urine.
Wang et al. (2013) conducted a repeated dose toxicokinetic study in pigs. Pigs were fed melamine in the diet at doses of 0, 18, or 35 mg/kg bw/day for 42 days with a 5-day recovery period. There was a dose-related increase in residual melamine concentrations in all tissues measured (plasma, brain, duodenum, liver, heart, muscle and kidney), and melamine concentration in kidney was significantly higher than in other tissues (p < 0.01), with tissue residue concentrations decreasing after melamine withdrawal. After 42 days at 35 mg/kg bw/day, the half-life was 9.90 h, with a steady state volume of distribution of 1.07 L/kg. Although slightly longer than the half-lives reported for other species, this study also confirmed that melamine was primarily eliminated via renal filtration. The authors noted that the clearance of melamine was consistent with the clearance observed in pigs subjected to single intravenous doses (0.076 L/h/kg in this study versus 0.061 L/h/kg) conducted by Baynes et al. (2008).
Chu et al. (2013) conducted a toxicokinetic study in pregnant and developing rats. Pregnant females were given a single dose by gavage of 24 mg/kg bw melamine on gestation days (GD) 10, 15, or 20 and by gavage in pups on postnatal weeks 2, 4, 6 or 8 (P2W, P4W, P6W, or P8W, respectively). As reported by the authors, distribution of melamine in maternal serum was about 30% higher in late pregnancy than that in early pregnancy and it was two folds higher in postnatal serum in early pups than in young adulthood (P2W vs. P4W-P8W). Melamine distribution in all postnatal organs was higher than that in prenatal organs. In early pups, postnatal kidneys had the highest maximum concentration and the lowest clearance rate of melamine than the other postnatal organs (Cmax = 10.85 mg/kg vs. 1.06 to 2.36 mg/kg and apparent clearance = 0.62 L/hr vs. 1.85 to 2.75 L/hr in liver, lung, heart, brain and spleen, respectively at P2W). The increased distribution of melamine in serum and kidneys of 2-week old rats compared to other life stages suggests an increased risk of melamine toxicity to the kidney after birth, according to the authors. The developmental toxicity studies of Kim et al. (2011) and Stine et al. (2014; see "Developmental toxicity and fertility studies") showed kidney toxicity in pregnant dams at oral doses of 800 and 1000 mg/kg bw/day melamine; however, no kidney toxicity was observed in fetuses at these doses (although other effects were observed) and the dams were not allowed to litter.
EFSA (2010) also reported that there is indirect evidence that melamine is not metabolized in humans. In a study in which [14C]-ring-labelled hexamethylmelamine was administered to humans orally, 5% of the dose of hexamethylmelamine (as 14C) was excreted as melamine in urine, but no metabolites of melamine were reported The authors conducted the same study in rats dosed intraperitoneally with [14C]-ring-labelled hexamethylmelamine. In this case, 2% of the dose of hexamethylmelamine (as 14C) was excreted as melamine in urine, but no metabolites of melamine were reported. In both the rat and human studies, only 5% of the urinary radioactivity was unidentified.
EFSA (2010) summarized the toxicokinetic data for experimental and farm animals and humans and the overall description of toxicokinetics of melamine is consistent with findings from recent studies mentioned above: 1) Studies in animals indicate that melamine is rapidly absorbed from the gastro-intestinal tract and rapidly eliminated from the body with a plasma-half-life of a few to several hours; 2) The major route of elimination is via the urine, and the limited information available suggests that the substance is hardly metabolised at environmentally relevant doses.
8.2.2 Carcinogenicity/chronic toxicity
In a 2-year study, F344 rats were administered melamine in the diet for 2 years, at doses of 0, 126/262, or 263/542 mg/kg bw/day in males/females, respectively. Transitional-cell carcinomas in the urinary bladder of male rats occurred at a significantly (p ≤ 0.016) higher incidence in the high dose group (8/49) than in the controls (0/45). There was also a statistically significant association (p < 0.001) between bladder stones (observed in 10/49 males) and bladder tumours in male rats fed melamine at the high dose. Urinary bladder tumours were not observed in the low-dose (126 mg/kg bw/day) male rat group, while bladder stones were observed in only one of 50 rats in this low dose group (US NTP 1983; Melnick et al. 1984). Due to the statistically significant association between bladder stones and bladder tumours at the high dose (263 mg/kg bw/day), a re-evaluation of the histopathology from this study was conducted. The results showed a significant increase in the incidence of reflux nephropathy in male rats [7/50 vs. 1/49 in controls] but not of bladder stones [1/50 vs. 0/45 in controls] at 126 mg/kg bw/day (Hard et al. 2009), confirming the lack of tumours at this dose.
In another 2-year study conducted in an unnamed strain, rats were administered melamine in the diet at doses of 0, 67 or 667 mg/kg bw/day. An increase in the incidence urinary bladder stones associated with an increased incidence of benign papillomas at the top dose, was reported (American Cyanamid 1955).
F344 rats were administered melamine in the diet for 24-30 months at doses of 0, 5/5, 25/50, or 50/100 mg/kg bw/day in males/females, respectively. An increased incidence of bladder tumours was not observed. Although a dose-related trend for dilated glands in glandular gastric mucosa and inflammation in non-glandular gastric mucosa was observed at 5 mg/kg bw/day and higher, it was not specified whether this trend applied to one or both sexes. However, the secondary source stated that the NOEL was thought to be the high dose (Hazleton Laboratories 1983 cited in OECD 2002).
Two 36-week studies were conducted on male F344 rats only; in one, animals were administered melamine in the diet at doses of 0, 110, 367 or 1100 mg/kg bw/day (Okumura et al. 1992), and in the other, animals were administered melamine in the diet at doses of 0, 430 or 1200 mg/kg bw/day (Ogasawara et al. 1995). In both studies, dose-related increases in the incidence of transitional cell/urinary carcinomas of the bladder and bladder papillomas were observed, which were associated with dose-related increases in the incidence of papillary/nodulary hyperplasia of the bladder epithelium. These increased incidences, along with a non-dose related increase in bladder stones, resulted in the determination of a LOAEL of 110 mg/kg bw/day in the Okumura et al. (1992) study. Okumura et al. (1992) observed a significant statistical correlation between the incidence of bladder stones and tumours, and concluded that melamine-induced calculi can induce carcinomas in the urinary bladder. Ogasawara et al. (1995) determined that the bladder stones were composed of equal molar ratios of melamine and uric acid, and concluded that proliferative lesions of the urinary tract of F344 male rats were directly due to the irritative stimulation of the calculi, and not to molecular interactions between melamine itself or its metabolites with the bladder epithelium.
B6C3F1 mice were administered melamine in the diet for 103 weeks at doses of 0, 327/523 or 688/1065 mg/kg bw/day in males/females, respectively, which was followed by two weeks of no treatment with melamine. Acute and chronic inflammation and hyperplasia of the bladder, as well as bladder calculi, were observed in male mice at all doses and in females at the high dose. However, no carcinogenic effects were observed in this study (US NTP 1983; Melnick et al. 1984).
In summary, five carcinogenicity studies have been conducted in rats and one in mice; in all cases, melamine was administered through the feed of the animals. In 4 of the rat studies, bladder tumours or papillomas were observed at doses ranging from 263 to 1200 mg/kg bw/day, whereas the other one used lower doses (5 to 100 mg/kg bw/day). No carcinogenic effects were observed in a 2-year mouse feeding study at melamine doses of 327 to 1065 mg/kg bw/day.
8.2.3 Genotoxicity
Many in vitro genotoxicity studies have been conducted on melamine. All gene mutation studies in bacterial (Salmonella typhimurium, Photobacterium phosphoreum, Saccharomyces cerevisiae) and mammalian (mouse lymphoma and Chinese hamster ovary [CHO]) cells, chromosome aberration and sister chromatid exchange assays in CHO cells, a micronucleus study using CHO cells, and unscheduled DNA synthesis studies in rat hepatocytes and bacterial cells (E. coli and S. typhimurium) were negative (Seiler 1973; Litton Bionetics 1977a, 1977b, 1977c; American Cyanamid Co. 1981; American Cyanamid Co. 1982a, 1982b; Mast et al. 1982b; Haworth et al. 1983; Mirsalis et al. 1983; Galloway et al. 1987; Zeiger 1987; McGregor et al. 1988; Elmore and Fitzgerald 1990; Ishiwata et al. 1991; Selden et al. 1994; Yasunaga et al. 2004; Tu et al. 2015). A non-standard test, which measured lambda prophage induction in E. coli as an indicator of DNA damage, was positive both with and without metabolic activation (Rossman et al. 1991). A comet assay was conducted in the unicellular eukaryote, Tetrahymena thermophila. There was a dose-related increase in %DNA damage, percentage of tailed cells, and arbitrary units of DNA damage at all doses, but the increase was statistically significant at 2000 and 4000 mg/L. As shown in Table 7-1, an IC50 was also reported for this organism (Li et al. 2015).
In vivo studies in two micronucleus assays (mice administered melamine via oral gavage and intraperitoneal injection), plus one combining a Pig-a mutation assay (rats administered melamine via oral gavage) were negative for chromosomal aberration in the bone marrow and peripheral blood of mice and no significant differences in micronucleus frequencies or in Pig-a mutation frequencies in red blood cells or in reticulocytes of rats (Pharmakon Research 1981; Mast et al. 1982a; Shelby et al. 1993; Tu et al. 2015). Overall, the genotoxicity database indicates that melamine is not genotoxic.
8.2.4 Carcinogenic mode of action
The mode of action for induction of the observed tumours has not been fully elucidated. It has been postulated, however, that the malignancies in the urinary bladder are based on a threshold mechanism due to reactive hyperplasia that develops in response to a localized tissue irritation effect, which then progresses to bladder neoplasia, and is supported by lack of any mutagenic or genotoxic activity in standard assays as reported by WHO (2009). WHO (2009) also noted that renal papillary mineralization was reported in some studies, but it is unknown if such mineralization may slough and provide a focal point for stones to form in the bladder.
8.2.5 Repeat-dose oral toxicity
In addition to the chronic toxicity/carcinogenicity studies, many repeat-dose oral studies have been conducted in rats, mice, rabbits, cats and dogs for periods ranging from 5 days to 36 weeks. In a 7-day oral feeding study in rats, scattered crystals were observed in renal tubules at the one dose tested, 200 mg/kg bw/day (Jacob et al. 2011). In 14-day oral studies in rats (one via gavage, the other via the diet), one study did not find effects at the highest dose tested, 240 mg/kg bw/day (Kobayashi et al. 2010), and the other showed hard crystalline solids in the urinary bladder of males and females at doses of 835 and 1668 mg/kg bw/day and higher, respectively (both sexes dosed at 0, 417, 835, 1251, 1668 or 2500 mg/kg bw/day). The NOAEL in this study was 417 mg/kg bw/day (US NTP 1983). In male mice administered melamine by gavage, altered sperm morphology and damage to testicular DNA was observed at 412 mg/kg bw/day after an exposure period of 5 days (note that male germ cells were not used in studies discussed under “Genotoxicity”), and increased apoptotic index of spermatogenic cells was observed at 50 mg/kg bw/day after an exposure period of 14 days (Yin et al. 2013).Footnote 14 Bischoff (2017) mentions this study and other studies in mice that show damage to the blood-testis barrier after oral ingestion of melamine, including effects on seminiferous tubules, and sperm development, production and morphology. Recent studies of male mice orally exposed to melamine for periods of 28 and 65 days, respectively, indicated that a dose of 50 mg/kg bw/day resulted in clinical signs of toxicity, changes in testes cell structure and more severe effects on sperm parameters, testosterone synthesis, testicular enzymes and protein levels than at lower doses (Sun et al. 2016a; Khalil et al. 2017).
Studies of 28 days in rats resulted in observations of dose-dependent increases in urinary bladder calculi (containing melamine) and hyperplasia (tissue not specified in source), crystalluria and excretion of acid urine at doses of 266 to 12,678 mg/kg bw/day of melamine (RTI 1982; American Cyanamid Co. 1984), as well as learning and memory deficits in a study in which rats were gavaged with melamine at 300 mg/kg bw/day (An et al. 2011; Yang et al. 2011). In another 28-day gavage study in rats, kidney microstructure was damaged and clinical chemistry parameters were significantly changed in serum (blood urea nitrogen, creatinine), kidney (glutamate, lactate, choline, glucose, amino acid, 3-hydroxybutyrate, pyruvate), liver (N-acetylglycoprotein, choline, creatine, lactate, trimethylamine-N-oxide, glutamate, glucose), and urine (succinate, citrate) at doses of 250 to 1000 mg/kg bw/day. This metabonomic study showed that melamine caused renal dysfunction and disturbed the liver’s glucose, protein and nitrogen metabolism (Sun et al. 2012). In the oral feeding studies, a NOAEL of 133 mg/kg bw/day was determined by RTI (1982), whereas no adverse effects were observed at doses of 40 to 357 mg/kg bw/day in the other study (American Cyanamid Co. 1984). Melamine was administered by gavage to female rats for 28 days and there were no toxic lesions in kidneys at 40 mg/kg bw/day but increased number of atretic follicles, morphological changes and necrosis in the oocyte and granulosa cells as well as increased number of apoptotic granolusa cells were observed in the ovaries at this dose. General health of the animals was not affected (Sun et al. 2016b).
For other species, the doses tested resulted in i) no adverse effects observed in cats treated with doses up to 181 mg/kg bw/day in an 11-day feeding study (Puschner et al. 2007), ii) no adverse effects observed following administration of 126 mg/kg bw/day (only dose tested) in 1 to 4-week feeding studies in both rabbits and dogs (Lipschitz and Stokey 1945a,b), and iii) a NOAEL of 3000 mg/kg bw/day, based on hard crystalline solids in the bladders of mice dosed at 6000 mg/kg bw/day in a 14-day oral gavage study (US NTP 1983).
In mice, short-term studies were conducted to analyze the relationship between bladder hyperplasia and calculi. Three to 4 week old or 5 to 6 week old male and female mice were fed melamine in the diet at doses of 0 or 9373 ppm, equivalent to 0 or 1218.5 mg/kg bw/day, respectively (dose conversion as per Health Canada 1994) for 14 days or 14 days with recovery periods ranging from 4 or 8 days (5-6 wk mice) to 42 days (3-4 wk mice). Another group of 3-4 wk mice were fed melamine in the diet at the same doses for 56 days or 56 days with a 42-day recovery period. Bladder epithelial hyperplasia (BEH) and bladder calculi incidence was 100% in all treated groups with no recovery period. BEH regression (which occurred through rapid ageing/apoptosis of cells in the superficial regions of BEH regression tissue) was observed toward more regression/significant regression in mice dosed 14 days with 8 days recovery compared to 4 days recovery, and there was significant (60 to 63%) and/or complete (38 to 40%) regression in all mice dosed 14 or 56 days with a 42-day recovery. With the rapid regression, bladder calculi completely disappeared. Regression of BEH initiated soon after melamine withdrawal (Sun et al. 2014).
Three different 13-week feeding studies in rats all showed dose-related increases in urinary bladder stones at doses ranging from 63 to 1500 mg/kg bw/day in both sexes, and also increased calcareous deposits in the kidney proximal tubules. The lowest LOAEL was 63 mg/kg bw/day (lowest dose tested) based on the above effects. Relative incidences of bladder stones in both sexes were not changed when rats were administered diet at 1500 mg/kg bw/day, depending on whether 1% ammonium chloride was added to the drinking water or not (US NTP 1983; Melnick et al. 1984). Two 36-week feeding studies in male rats at doses ranging from 110 to 1200 mg/kg bw/day resulted in a dose-related increase in hyperplasia of the bladder epithelium and non-dose related increase in bladder stones, as well as decreased body-weight gain at 430 mg/kg bw/day and higher (Okumura et al. 1992; Ogasawara et al. 1995). Subchronic studies were conducted in other species and effects were noted at the levels tested, i) 1600 mg/kg bw/day (lowest dose tested) based on decreased body weights in males in a 13-week oral feeding study in mice (US NTP 1983; Melnick et al. 1984), and ii) 1200 mg/kg bw/day (only dose tested) based on crystalluria observed after 60-90 days in a 1-year feeding study in dogs (American Cyanamid Co. 1955).
8.2.6 Developmental toxicity and fertility studies
Three oral developmental toxicity studies in rats were available, plus a summary of rabbit maternal toxicity study. In pregnant rats orally gavaged with melamine during gestation days 6 to 20, developmental and maternal toxicity were observed at the top dose of 800 mg/kg bw/day (decreased fetal weights, increased incidence of skeletal variations and delay in fetal ossification; in dams, increased incidences of clinical signs and death, decreased body weight gain and kidney toxicity). No maternal or developmental toxicity were observed at lower doses (200 and 400 mg/kg bw/day) (Kim et al. 2011). Pregnant rats were orally gavaged with melamine during gestation days 10-20 and non-pregnant females were gavaged for 10 consecutive days at 1000 mg/kg bw/day. In both cases, effects included clinical signs of toxicity (listlessness, reluctance to groom, anorexia), decreased body-weight gain, increased kidney weights and renal lesions (tubular dilation with cellular necrosis) which correlated well with the incidence of renal crystals, and melamine was found in the amniotic fluid of pregnant rats. Developmental effects included increased numbers of early and late fetal deaths, and decreases in litter size, average fetal body weight, and average crown rump length (Stine et al. 2014). In pregnant rats fed melamine in the diet during gestation days 6-16, no developmental toxicity was observed but maternal toxicity was observed at the top dose of 1060 mg/kg bw/day (decreased body weight, blood in urine, indrawn flanks) (Hellwig et al. 1996). A summary of a rabbit maternal toxicity study showed effects at 250 and 400 mg/kg bw/day (1/6 females at each dose showing clinical signs of toxicity and decreased body-weight gain), with no effects at 120 mg/kg bw/day; developmental effects were not reported (BASF 2018b). Bischoff (2017) reports a reproductive study in which the mean number of offspring decreased in pregnant mice exposed to 50 mg/kg bw/day in drinking water but the original article by Duan et al. (2015) provides insufficient details on the protocol and only 4 dams/treated and control groups, respectively, were used.
As noted in the "Repeat dose oral toxicity" section, some repeated dose studies identified effects in the testes and ovaries. Several repeat dose oral studies in male mice showed effects on seminiferous tubules; sperm development, production and morphology; spermatogenic cells; testosterone synthesis; and testicular enzymes and protein levels at a dose of 50 mg/kg bw/day for periods ranging from 14 to 65 days. A 28-day oral study in female rats showed effects in the ovaries (oocyte and granulosa cells and increased number of atretic follicles) at dose of 40 mg/kg bw/day. A 17-week inhalation study in rats showed impaired liver function and effects on blood biochemistry parameters at concentrations of 0.058 and 0.5 mg/m3 melamine. Rats were mated after the 17-week exposure period and effects were noted in animals that had been exposed to 0.5 mg/m3 melamine (effects on spermatogenesis and fertility, and fetal deaths observed) (Ubaidellajev et al. 1993). Due to limited information on study protocol in the English translation of this Russian study, it is considered of limited use for hazard characterization.
In summary, the assessment of developmental toxicity showed a critical effect level of 800 mg/kg bw/day melamine in rats for developmental and maternal toxicity, but no validated reproductive toxicity studies were identified. Although potential effects on the ovaries and testes were observed at 40 and 50 mg/kg bw/day, respectively, it is expected that the BMDL of 35 mg/kg bw/day from chronic oral exposure to melamine, will be protective of any potential effects on reproductive organs.
8.2.7 Sensitization
Melamine was not a skin sensitizer in guinea pigs (Fassett and Roudabush 1963); this was the only skin sensitization study identified in experimental animals.
There are a number of human case reports on sensitization observed in occupational settings, as well as one volunteer study in workers. In both the volunteer study and the case reports, patients who did show contact allergy to products containing melamine-formaldehyde, showed no reaction when patch-tested with melamine alone (Soubrier and Burlet 1972; Fregert 1981; Isaksson et al. 1999; Aalto-Korte et al. 2003; Garcia-Gavin et al. 2008).
8.2.8 Epidemiology studies
In humans, a large retrospective case-control study was conducted comparing 683 children with nephrolithiasis (presence of calculi in the kidney) with 6498 children without nephrolithiasis aged < 3 years in Beijing hospitals and their exposure to melamine via adulterated formula (Li et al. 2010). The children in this study were exposed to 4 of 22 brands of melamine-adulterated formula sold in Beijing, and were part of a larger city sample size population of 41 000 children subjected to a Children’s Health and Feeding Status survey. In general, the adjusted odds ratios between melamine dose and nephrolithiasis increased with greater daily level of melamine intake (from 1.7 to 11.3 for daily melamine intakes ranging from >0 to 0.2 mg/kg bw/day to >102.4 mg/kg bw/day), and also increased with the increasing duration of exposure. In children exposed to melamine levels of <0.2 mg/kg bw/day, the adjusted odds ratio expressing the risk for nephrolithiasis was still 1.7 times higher than in those without melamine exposure (Li et al. 2010; EFSA 2010). There were several limitations associated with this study (see “Uncertainties in evaluation of risk to human health”).
A case-control study of children and pregnant women was conducted in Hong Kong. In project one, 152 pregnant women were recruited in 2008, 74 with a dietary history of exposure to melamine-contaminated food products and 78 age-matched controls who did not have a history of consuming melamine contaminated food products. Median daily exposure of the melamine group was 0.0015 (range, 0.0001 to 0.08) mg/kg bw/day. When samples from 20 patients with the highest melamine exposure were compared to 20 control patients, there were no significant differences in melamine concentrations in all the biological samples (mother urine [median = 1.3 vs. 1.2 µg/mmole creatinine in controls], blood [median <5 ppb in both groups], placenta, breast milk [median <50 ppb in both groups], amniotic fluid and cord blood; neonate urine [median <5 ppb in both groups]). Project two tried to determine the usefulness neutrophil gelatinase-associated lipocalin (NGAL) in urine as a surrogate marker for early detection of kidney injury but no significant difference in NGAL concentrations was found between 302 children under 12 years with prolonged melamine-tainted milk product exposure (>0.2 mg/kg bw/day) who had persistent urinary abnormalities and/or clinical features suggestive of renal diseases, and 203 age-matched controls with no history of melamine-tainted milk product consumption (Wong et al. 2013).
8.2.9 Non-cancer mechanisms of toxicity
In terms of non-carcinogenic endpoints, potential mechanisms of toxicity on the urinary system and other physiological systems have been discussed in some reviews. The proposed mechanism of melamine-induced nephrotoxicity involves induction of oxidative stress and an inflammatory response triggered by renal crystals (EFSA 2010; Chu and Wang 2013; Rai et al. 2014). The “Oral toxicokinetics” section showed that there is a potential for increased distribution of melamine in serum and kidneys in the young of experimental animals compared to other life stages of exposure to melamine, including pregnant animals. Dorne et al. (2013) noted that neurotoxic effects occur at doses higher than or subsequent to melamine-induced nephrotoxicity.
8.3 Characterization of risk to human health
Based principally on weight of evidence assessments of international agencies (WHO (2009) and EFSA (2010)), and available information, critical effects associated with exposure to melamine are carcinogenicity and effects on the urinary system. There is evidence to show that these effects are not due to melamine per se but due to its propensity to form calculi or crystals in the kidney and/or bladder and it is the irritative effects of these calculi that lead to other effects, including reactive hyperplasia and bladder tumours in rats.
On the basis of the available information, melamine is not genotoxic, and a threshold mechanism for kidney specific carcinogenicity is supported.
The lowest overall LOAEL was identified in a 13-week feeding study in Fischer 344 rats, in which a dose-dependent increase in the incidence of bladder calculi and increased calcareous deposits in the kidney (not dose-related) were observed in animals fed melamine at all doses tested, the lowest one being 63 mg/kg bw/day (US NTP 1983; Melnick et al. 1984). Also, as shown in the discussion of carcinogenicity in the Health Effects Assessment section, a re-evaluation of the histopathology from the 2-year rat study suggested that increased duration of exposure to melamine at doses below the level at which bladder tumours were observed (263 mg/kg bw/day) does not result in carcinogenicity. Therefore, a quantitative assessment of carcinogenicity is not required.
WHO (2009) calculated a benchmark dose (BMD) and its lower confidence limit (BMDL10), based on this 13-week oral study, of 44.6 and 35 mg/kg bw/day, respectively, for a 10% increased incidence of the observed effects (urolithiasis occurrence and incidence of hyperplasia of the bladder epithelium). The WHO (2009) then derived a tolerable daily intake (TDI) of 0.2 mg/kg bw/day, using this BMDL10 of 35 mg/kg bw/day in combination with a total uncertainty factor of 200, which comprised a factor of 100 for intra- and inter-species variability and an extra uncertainty factor of 2 to “fully account for the potential increased sensitivity of infants and for data uncertainties”. The resultant TDI for melamine was considered applicable to the whole population, including infants. At the time of the melamine infant formula incident of 2008, the World Health Organization (WHO) Collaborating Centre for Food Contamination Monitoring within Health Canada's Food Directorate participated in the WHO's expert toxicology meeting to review melamine and related analogues. This consultation recommended a tolerable daily intake for melamine of 0.2 mg/kg bw/day, which Health Canada supported.
EFSA (2010) also calculated a BMD10 and its lower confidence limit (BMDL10) based on the same study, but applied different assumptions in application of the benchmark dose models than the WHO (2009), which resulted in slightly different values (41 and 19 mg/kg bw/day, respectively, for a 10% increased incidence of the observed effects). EFSA (2010) derived the same TDI of 0.2 mg/kg bw/day from these data for melamine using a default uncertainty factor of 100 (EFSA 2010).
The EFSA Panel also derived a BMD based on the epidemiological study by Li et al. (2010). They calculated a BMD10 of 1.1 mg/kg bw/day and a BMDL10 of 0.74 mg/kg bw/day for a 10% increased incidence of nephrolithiasis. Due to uncertainties in the study design and exposure estimates, the data were not considered robust enough by EFSA for use as the basis for deriving a TDI. However, EFSA considered the human BMDL10 of 0.74 mg/kg bw/day to provide supporting evidence for the adequacy of the TDI of 0.2 mg/kg bw/day derived from animal data (EFSA 2010). The epidemiological study information was not available when the WHO TDI was derived (WHO 2009).
The oral BMDL10 of 35 mg/kg bw/day derived by the WHO (2009) is considered appropriate for use in the characterization of risk from chronic oral exposure to melamine as it is based on the study with the lowest LOAEL of 63 mg/kg bw/day, and it is protective of calculi or crystals formation, since long-term rat feeding studies showed evidence for bladder tumours at 263 mg/kg bw/day of melamine. As the bladder tumours appear to be the result of a progression of events (bladder hyperplasia to irritation to neoplasia) from oral exposure to melamine, this BMDL is also protective of precursor events.
Recent short term studies in rats and mice showed effects in the ovaries and testes at oral LOELs of 40 and 50 mg/kg bw/day, respectively. However, the assessment of developmental toxicity showed a critical effect level of 800 mg/kg bw/day melamine in rats for developmental and maternal toxicity (no validated reproductive toxicity studies were identified). It is expected that the BMDL of 35 mg/kg bw/day from chronic oral exposure to melamine, will be protective of any potential effects on reproductive organs.
The main sources of exposure to melamine for the general population in Canada are expected to be from the use of products available to consumers, food and environmental media. Given the rationale outlined in section 9.1 (negligible vapour pressure, low dermal absorption, low acute toxicity, etc.), dermal and inhalation exposures to melamine from the use of do-it-yourself products is determined to be a negligible risk. Therefore, margins of exposure were not generated for these products.
Comparison of central tendency and upper-bound estimates of intake from environmental media and food, for formula fed infants (highest exposed group), with the BMDL10 of 35 mg/kg bw/day results in MOEs ranging from 1522 - 5833. These MOEs are considered adequate to account for the uncertainties in the exposure and health effects database. See Table 8-6.
Comparison of the upper bound estimate of toddler exposure from use of melaware with the BMDL10 results in an MOE of 313. Given the conservative nature of the assumptions (e.g., all food, water and beverages consumed would be warm and in contact with melaware dishware), exposure from use of melaware is expected to be lower and this MOE is considered adequate to account for uncertainties in the exposure and health effects database. See Table 8-6.
Scenario |
Exposure estimate (mg/kg bw/d) |
Margin of exposure (MOE) based on the BMDL10 of 35 mg/kg bw/ |
|---|---|---|
Environmental media and dietary exposure for an infant less than 1-yr-old. |
0.0060a (0.023)b |
1522 (5833)b
|
Dietary exposure assuming melaware as source (child aged 1-4 years old) |
0.112c |
313 |
Overall exposure based on biomonitoring datad (youth aged 12-19 years) |
0.00041 |
85,400 |
Overall exposure based on biomonitoring datad (adults aged 20-59 years) |
0.00079 |
44,300 |
Overall exposure based on biomonitoring datad (adults aged 60+ years) |
0.00062 |
56,500 |
a Mean estimates from environmental and food summed together (mean values for drinking water summed with mean food intake value for infants from Appendix B).
b This is the upper-end value based on upper-end values for food and drinking water intakes shown in Table B-1, Appendix B.
c Estimate from Table 8-3; includes exposure to food and beverages.
d See Table 8-5 for more information.
Biomonitoring intakes provide an approximation of the exposure estimates from all potential routes and sources of exposure. The highest melamine intakes, based on urinary biomonitoring from the US population, were 0.41, 0.79, and 0.62 µg/kg bw/day for the 12 to 19, 20 to 59, and 60 plus year old age groups, respectively. MOEs based on these intakes range from 44 300 to 85 400 (see Table 8-6). Although biomonitoring intakes were also calculated for the 6-11 year old age group, given the small sample size (n=6) and uncertainty associated with all statistics derived from them, MOEs were not generated for these intakes.
Dermal exposure to foam products, and oral exposure to infants and toddlers from mouthing foam products containing melamine were also considered, as concentrations of melamine in foam seating and bedding have been reported (see “Exposure assessment” section). No repeated dose dermal toxicity studies were identified and the oral 13-week feeding study in rats, which is the basis for BMDL10 of 35 mg/kg bw/day, was used for characterization of risk from both oral and dermal exposure to melamine. MOEs for exposure from foam products are outlined in Table 8-7.
Exposure scenario |
Exposure route and duration |
Estimate of exposure |
Critical effect level |
Type and duration of study |
Margins of exposure |
|---|---|---|---|---|---|
Mouthing of foam product (Infant) |
Oral – long term |
0.0051 to 0.0118 mg/kg bw/day |
BMDL10 = 35 mg/kg bw/day |
13-week oral rat study |
2970 to 6860 |
Mouthing of foam product (Toddler) |
Oral – long term |
0.0049 to 0.0114 mg/kg bw/day |
BMDL10 = 35 mg/kg bw/day |
13-week oral rat study |
3070 to 7140 |
Lying on foam furniture or mattress (Infant) |
Dermal – long term |
0.08 to 1.02 mg/kg bw/day |
BMDL10 = 35 mg/kg bw/day |
13-week oral rat study |
34 to 438 |
Lying on foam furniture or mattress (Toddler) |
Dermal – long term |
0.06 to 0.78 mg/kg bw/day |
BMDL10 = 35 mg/kg bw/day |
13-week oral rat study |
45 to 583 |
Lying on foam furniture or mattress (Child) |
Dermal – long term |
0.04 – 0.54 mg/kg bw/day |
BMDL10 = 35 mg/kg bw/day |
13-week oral rat study |
65 to 875 |
Lying on foam furniture or mattress (Teen) |
Dermal – long term |
0.03 – 0.47 mg/kg bw/day |
BMDL10 = 35 mg/kg bw/day |
13-week oral rat study |
75 to 1170 |
Lying on foam furniture or mattress (Adult) |
Dermal – long term |
0.02 to 0.36 mg/kg bw/day |
BMDL10 = 35 mg/kg bw/day |
13-week oral rat study |
97 to 1750 |
On the basis of the estimated exposures to melamine derived from human biomonitoring data and the conservative assumptions used in modelling dermal exposures to melamine from lying on foam-containing mattresses or furniture for adults, the MOEs are considered adequate to address uncertainties in the in the exposure and health effects database.
Given that these MOEs are based on the BMDL10 (which accounts for a 10% increased incidence of the observed effects at higher doses in the 13-week oral rat study), the margins of exposure for children (including infants, toddlers, and children up to age 18 years) from use of products available to consumers, specifically dermal exposure to melamine from lying on foam-containing mattresses or furniture , are considered potentially inadequate to account for uncertainties in the exposure and health effects databases. Although the MOEs derived from human biomonitoring data are higher than the dermal exposures to melamine from lying on foam-containing matresses or furniture , the biomonitoring data were considered insufficient to derive MOEs for age groups younger than 12 years. This is important as habits of children and infants are different from those of adults and evidence from experimental animals suggests there may be greater exposure to melamine in early life stages. In addition, the “Oral toxicokinetics” section showed an increased distribution of melamine in serum and kidneys of 2-week old rats compared to other life stages (after pregnant rats and 2 to 8 week old rats were dosed with melamine). Furthermore, there are several uncertainties with the biomonitoring data, such as the melamine intakes were not measured directly but retrospectively calculated from urinary biomonitoring data, and the intake calculations were based on data from laboratory animals since human toxicokinetic data were not available (note that a quantitative estimate of 60% was used in determining the lowest urinary excretion rate of melamine based on data in rats and dogs, but Liu et al. (2010) provided qualitative information to suggest a much lower urinary excretion rate in monkeys).
8.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
Key source of uncertainty |
Impact |
|---|---|
No Canadian data on concentrations in drinking water, breast milk or dust/soil were available. |
+ |
Data on concentrations in tap water, breast milk and dust/soil were based on small sample sizes (mean and/or maximum concentrations were used). |
+/- |
Assumptions on frequency and type of melaware used in assessment of exposure to melaware. |
+ |
Epidemiology studies reported melamine intakes that were not measured but retrospectively calculated from consumption data and some had other limitations (e.g. EFSA [2010] noted that for Li et al. [2010], the recruitment of children was done in two phases instead of one, the reference population could be defined in more than one way and there was no information about the distribution of the individual exposure levels when aggregating the exposure data into exposure intervals). |
+/- |
Determination of melamine intake for adults and youth based on urine samples from a non-Canadian population (USA), including assumption on routes of exposure, timing of exposure in relation to timing of sampling, and variability in daily urine volumes both between and within individuals. |
+/- |
Assumption that all melamine measured in urine is associated with direct exposure to melamine. |
+ |
Assumption that melamine levels in human urine represent an excretion of 60% of daily intake (due to lack of human toxicokinetic data). |
+/- |
Very limited hazard studies via the dermal and inhalation routes of exposures. |
+/- |
Lack of a human dermal absorption value for melamine. |
+/- |
Lack of empirical data on the relationship between the migration rate of melamine from foam and its concentration in foam. |
+/- |
The extent of the effect of textile covering on melamine migration from polyurethane foam (PUF) are unknown. |
+/- |
Assumption that PUF would be covered in the estimate for dermal exposure from contact with foam. |
- |
No melamine-specific skin contact factor (SCF) has been identified; a factor of 1 was assumed. |
+ |
Limited occurrence and toxicological data on co-exposure to melamine and cyanuric acid. |
+/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk, - = uncertainty with potential to cause under-estimation of exposure/risk, +/- = unknown potential to cause over or under estimation of risk.
In regards to the last row of the above table, the risk from chronic co-exposure to melamine and cyanuric acid is unknown due to limited occurrence and toxicological information (for additional information on cyanuric acid, see Health Canada 2018c).
9. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is a low risk of harm to the environment from melamine. It is proposed to conclude that melamine does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends.
For infants, toddlers and young individuals (up to 18 years old), comparisons of levels associated between critical effects in animal studies and estimates of exposure from lying on foam-containing mattresses or furniture containing melamine are considered to be potentially inadequate to address uncertainties in the health effects and exposure databases. For all other types of exposures (from environmental media and food or from use of products available to consumers of all age groups), comparisons of levels associated between critical effects in animal studies and estimates of exposure were considered adequate to address uncertainties in the health effects and exposure databases. On the basis of the potential inadequacy of the margins between estimates of exposure and critical effect levels in experimental animals in this updated draft screening assessment, it is proposed to conclude that melamine meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that melamine meets one or more of the criteria set out in section 64 of CEPA. It is also proposed that melamine meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
Aalto-Korte K, Jolanki R, Estlander T. 2003. Formaldehyde-negative allergic contact dermatitis from melamine-formaldehyde resin. Contact Dermatitis. 49(4):194-196.
[ABC Laboratories] Analytical Bio-Chemistry Laboratories Inc. (Columbia, Missouri). 1988. Acute toxicity of CT-338-87 to Daphnia magna. Final report no.: 36645. Unpublished report sponsored by American Cyanamid Company (Wayne, New Jersey). 60 p. [restricted access]
ACD/Percepta. 2005. Prediction Module. Toronto (ON): Advanced Chemistry Development. [cited 2013 Nov 4].
Ademola JI, Sedik LE, Wester RC, Maibach HI. 1993, In vitro percutaneous absorption and metabolism in man of 2-chloro-4-ethylamino-6-isopropylamine-s-triazine (Atrazine). Arch Toxicol. 67:85-91.
American Cyanamid Co. 1955. Melamine: acute and chronic toxicity, Report 55-21, unpublished data. [cited in OECD 2002].
American Cyanamid Co. 1981. Unpublished data. Study number: Raltech Study No. 81560. [cited in OECD 2002].
American Cyanamid Co. 1982a. Unpublished data Report no.: PH 319-AC-002-82. Report date: 1982-05-20. [cited in OECD 2002].
American Cyanamid Co. 1982b. Unpublished data. Report no.: Study No. PH 311-AC-002-82. [cited in OECD 2002].
American Cyanamid Co. 1984. Study. [cited in US EPA 1984].
An L, Li Z, Yang Z, Zhang T. 2011. Cognitive deficits induced by melamine in rats. Toxicol Lett. 206:276-280.
An H, Li X, Yang Q, Wang D, Xie T, Zhao J, Xu Q, Chen F, Zhong Y, Yuan Y, Zeng G. 2017. The behavior of melamine in biological wastewater treatment system. J Hazard Mater. 322:445-453.
Andersen WC, Turnipseed SB, Karbiwnyk CM, Clark SB, Madson MR, Gieseker CM, Miller RA, Rummel NG, Reimschuessel R. 2008. Determination and confirmation of melamine residues in catfish, trout, tilapia, salmon, and shrimp by liquid chromatography with tandem mass spectrometry. J Agric Food Chem. 2008(56):4340-4347.
Andersen WC, Sherri B. Turnipseed SB, Christine M. Karbiwnyk CM, Evans E, Hasbrouck N, Mayer TD, Gieseker CM, Nochetto C, Stine CB, Reimschuessel R. 2011. Bioaccumulation of melamine in catfish muscle following continuous, low-dose, oral administration. J Agric Food Chem. 59:3111-3117.
[AOPWIN] Atmospheric Oxidation Program for Microsoft Windows [estimation model]. 2010. Ver. 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Aylward LL, Kirman CR, Adgate JL, McKenzie LM, Hays SM. 2012. Interpreting variability in population biomonitoring data: Role of elimination kinetics. J Expo Sci Environ Epidemiol. 22:398-408.
BASF. 1969a. BASF AG, Department of Toxicology; unpublished data (XIX/5), 23.04.1969. [cited in OECD 2002 and European Commission 2000]. Note: BASF 1969b is cited in the Supporting documentation (Health Canada 2018c).
BASF. 1969b. BASF AG, Department of Toxicology; unpublished data (XIX/5), 23.04.1969. [cited in OECD 2002 and European Commission 2000]. Note: BASF 1969b is cited in the Supporting documentation (Health Canada 2018c).
BASF. 2012. GPS safety summary: melamine. BASF. [cited 2013 Nov 1]. Note: Search for “melamine” at website.
BASF. 2018b. Notice in Accordance with CEPA Section 70: Results of a maternal toxicity test with CASRN 108-78-1. Section 70 submission to Environment and Climate Change Canada, 5 Nov. 2018. 1 page. Note: BASF 2018a is cited in the Supporting documentation (Health Canada 2018c).
Baynes RE, Yeatts JL, Brooks JD, Riviere JE. 2005. Pre-treatment effects of trichloroethylene on the dermal absorption of the biocide, triazine. Toxicol Lett. 159:252-260.
Baynes, RE, Smith G, Mason SE, Barrett E, Barlow BM, Riviere JE. 2008. Pharmacokinetics of melamine in pigs following intravenous administration. Food Chem Toxicol. 46:1196–1200.
Bischoff K. 2017. Melamine and cyanuric acid. In: Gupta RC, editor. Reproductive and developmental toxicology, 2nd ed. Academic Press, Copyright Elsevier. p. 493-501.
Boethling, RS, Howard, PH, Beauman, JA, Larosche, ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere. 30(4):741-752.
Bradley EL, Castle L, Day JS, Ebner I, Ehlert K, Helling R, Koster S, Leak J, Pfaff K. 2010. Comparison of the migration of melamine from melamine-formaldehyde plastics ("melaware") into various food simulants and foods themselves. Food Addit Contam Part A Chem Anal Control Expos Risk Assess. 27(12):1755-1764.
Bradley EL., Castle L., Day JS, Leak J. 2011. Migration of melamine from can coatings cross-linked with melamine-based resins, into food simulants and foods. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 28(2):243-250.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette. Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2013. Canadian Environmental Protection Act, 1999: Notice with respect to certain organic flame retardant substances [PDF] Canada Gazette, Part I, vol. 147, no. 13, p. 613–633.
CATALOGIC [environmental fate and ecotoxicity model]. 2012. Ver. 5.11.6. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
[CEC] Commission of the European Communities, food-science and techniques. 1993. Nutrient and energy intakes for the European Community. Reports of the Scientific Committee for Food (thirtyfirst series) [cited in EFSA 2006 and EFSA 2010; incorrect date of 2003 given in the references of EFSA 2010].
[CFIA] Canadian Food Inspection Agency. 2010. 2009-2010 melamine residues in fluid milk, milk-based products and soy-based products. Summary. Complete report available upon request to the CFIA.
[CFIA] Canadian Food Inspection Agency. 2011. 2010-2011 melamine in milk-based and soy-based products. Summary. Complete report available upon request to the CFIA.
[CFIA] Canadian Food Inspection Agency. 2012. 2011-2012 melamine in selected foods. Summary. Complete report available upon request to the CFIA.
ChemIDplus [database]. 1993–. Bethesda (MD): National Library of Medicine (US). [cited 2013 Nov 19]. Note: Search for melamine or CAS RN 108-78-1
Cheminfo Services Inc. 2012. Use profile characterization for certain organic flame retardants under the Chemicals Management Plan. Prepared for Environment and Climate Change Canada. 123 pp. (Not for public distribution).
Chien CY, Wu CF, Liu CC, Chen BH, Huang SP, Chou YH, Chang AW, Lee HH, Pan CH, Wu WJ. et al. 2011. High melamine migration in daily-use melamine-made tableware. J Hazard Mater. 188:350-356.
Chik Z, Mohamad Haron DE, Ahmad ED, Taha H, Mustafa AM. 2011. Analysis of melamine migration from melamine food contact articles. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 28(7):967-973.
[CHRIP] Chemical Risk Information Platform (Japan). c2008- . Data Analysis Division, Chemical Management Center, National Institute of Technology and Evaluation, Japan.
Chu CY, Wang CC. 2013. Toxicity of melamine: The public health concern. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 31(4):342-386.
Chu CY, Chu KO, Ho CS, Kwok SS, Chan HM, Fung KP, Wang CC. 2013. Melamine in prenatal and postnatal organs in rats. Reprod Toxicol. 35:40-47.
Codex Alimentarius. 2018. General Standard for Contaminants and Toxins in Food and Feed. CXS 193-1995.
Cook AM, Beilstein P, Grossenbacher H, Hütter R. Ring cleavage and degradative pathway of cyanuric acid in bacteria. 1985. Biochem J. 231:25-30.
Crews GM, Ripperger W, Kersebohm DB, Seeholzer J, Güthner T, Mertschenk B. 2005. Melamine and guanamines. In: Ullmann’s encyclopedia of industrial chemistry. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. 17 pp.
Crews GM, Ripperger W, Kersebohm DB, Guthner T, Mertschenk B. circa (c) 2012. Melamine and Guanamines. In: Ullmann’s Encyclopedia of Industrial Chemistry. Vol. 22. Weinheim (DE): Wiley-VCH Verlag GmbH & Co. KGaA. pp. 377-392.
CSL Silicones Inc. [internet]. 2014. MSDSs and Technical Data Sheets for CSL 557/558/559 Flame Retardant Silicone Sealant [cited 2014 April]. Weblink for these products no longer available. See CSL Silicone Inc. website.
Danish EPA [Environmental Protection Agency]. 2015. Chemical substances in car safety seats and other textile products for children. Survey of chemical substances in consumer products No. 135. Danish Ministry of the Environment.
Davison JM, Noble MCB. 1981. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. British Journal of Obstetrics and Gynaecology 88:10-17.
Dorne JL, Doerge DR, Vandenbroeck M, Fink-Gremmels J, Mennes Knutsen W, Vernazza F, Castle L, Edler L, Benford D. 2013. Recent advances in the risk assessment of melamine and cyanuric acid in animal feed. Toxicol Appl Pharmacol. 370:218-229.
[DPD] Drug Product Database [database]. [modified 2017 Feb 17]. Ottawa (ON): Government of Canada. [accessed 2017 March].
[DSM] Royal DSM N.V. 2010. Life sciences and materials sciences: Staying the course. Annual Report 2009 [PDF]. Heerlen (NL): Royal DSM N.V. 196 pp.
Du X, Chu H, Huang Y, Zhao Y. 2010 Qualitative and quantitative determination of melamine by surface-enhanced raman spectroscopy using silver nanorod array substrates. Appl Spectrosc. 64(7):781-785.
Duan X, Dai X-X, Wang T, Liu H-L, Sun S-C. 2015. Melamine negatively affects oocyte architecture, oocyte development and fertility in mice. Hum Reprod. 30:1643-1652.
Eaton RW, Karns JS. 1991a. Cloning and analysis of s-triazine catabolic genes from Pseudomonas sp. strain NRRLB-12227. J Bacteriol. 173(3): 1215–1222.
Eaton RW, Karns JS. 1991b. Cloning and comparison of the DNA encoding ammelide aminohydrolase and cyanuric acid amidhydrolase from three s-triazine-degrading bacterial strains. J Bacteriol. 173(3):1363–1366.
[ECHA] European Chemicals Agency. c2007-2013. Registered Substances database. Search results for CAS RN 108-78-1. Helsinki (FI): ECHA. [updated 2014 March 19; cited 2017 March 24].
[ECHA] European Chemicals Agency. c2007-2017. Registered Substances database. Search results for CAS RN 108-78-1. Helsinki (FI): ECHA. [cited 2017 March 06].
[ECHA] European Chemicals Agency. 2018. Screening report: An assessment of whether the use of TCEP, TCPP and TDCP in articles should be restricted. Version 3 [PDF]. Helsinki (FI): European Chemicals Agency.
Ecology Center. 2015. Hidden passengers: Chemical hazards in children's car seats. A technical report by Healthystuff.org. The Ecology Center, June 2015. Ann Arbor, Michigan, U.S.A. 24 pp.
[EFRA] European Flame Retardants Association. 2007. Flame retardants; Frequently asked questions. Brussels (BE): EFRA. 37 pp. [cited 2014 June 19]. Weblink no longer available.
[EFSA] European Food Safety Authority. 2006. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to 2,2-bis(4-hydroxyphenyl)propane(Bisphenol A). The EFSA Journal 428:1-75. [cited in EFSA 2010].
[EFSA] European Food Safety Authority. 2010. Scientific Opinion on Melamine in Food and Feed. EFSA Journal 8(4):1573. 145 pp. doi:10.2903/j. efsa.2010.1573. Owner company: EFSA Panel on Contaminants in the Food Chain (CONTAM) and EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF).
Elmore E, Fitzgerald MP. 1990. Evaluation of the bioluminescence assays as screens for genotoxic chemicals. Mutation and the Environment, Part D: 379-387. Testing laboratory: Microbics Corporation, Carlsbad, California.
[ECCC]¸Environment and Climate Change Canada. 2013. National Pollutant Release Inventory and Air Pollutant Emission Summaries and Trends Downloadable Datasets. Facility data (downloadable NPRI data for the year 1993-2012 in Microsoft Access format; Version Oct 11, 2013). [updated 2014 March 11; cited 2014 April].
Environment Canada, Health Canada. 2013. Chemical substances: Categorization of chemical substances [Internet]. Ottawa (ON): Government of Canada. [updated May 25, 2013; cited July 31, 2013].
[ECCC] Environment and Climate Change Canada. 2013-2014. Data for Certain Organic Flame Retardants Substance Grouping collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain organic flame retardant substances. Data prepared by: Environment and Climate Change Canada, Health Canada; Existing Substances Program.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2016. Draft screening assessment of certain organic flame retardants substance grouping: 1,3,5-triazine-2,4,6-triamine (melamine); Chemical abstracts service registry number 108-78-1. Ottawa (ON): Government of Canada. 102 pp.
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2012. Version 4.1. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
European Commission. 2000. IUCLID Dataset, Melamine, CAS No. 108-78-1 [Internet]. Year 2000 CD-ROM edition. [place unknown]: European Chemicals Agency, European Commission. [created 2000 Feb 18]. Weblink no longer available. See IUCLID6 website to search for “.i6z” file on melamine.
European Commission. 2011. The rapid alert system for food and feed (RASFF). 2011 Annual Report [PDF]. Luxembourg: Office for Official Publications of the European Communities. 48 pp.
[EU] European Union. 2011. COMMISSION REGULATION (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food [PDF]. 9 pp.
[FAO] Food and Agriculture Organisation of the United Nations. 2007a. Cyromazine (169) [Evaluation] [PDF].
[FAO] Food and Agriculture Organisation of the United Nations. 2007b. Cyromazine (169): Residue and analytical aspects [Report].
Fassett DW, Roudabush RL. 1963. Laboratory of Industrial Medicine, Eastman Kodak Co. Unpublished observations. [cited in OECD 2002].
Fimberger EA. 1997. Bestimmung von Neben- und Abbauprodukten des Melamins mittels Kapillarelektrophorese und Abbauverhalten des Melamins in Kläranlagen. Diplomarbeit zur Erlangung des Magistergrades an der Naturwissenschaftlichen Fakultät der Universität Salzburg, Austria. Report date: 1997-07-10. [Master thesis in German; cited in ECHA c2007-2013; OECD 2002].
Flakeboard Company Ltd. c2012. Thermally-fused laminates (Melamines) [Internet]. [cited 2014 April]. Original weblink no longer available. See Arauco website.
Formica Corporation. 2013. Our laminate surfaces [Internet]. [cited 2014 April].
Francis WJA. 1960. Disturbances of bladder function in relation to pregnancy. The Journal of Obstetrics and Gynaecology of the British Empire. LXVII(3):353-366.
Fregert S. 1981. Formaldehyde dermatitis from a gypsum-melamine resin mixture. Contact Dermatitis 7:56. [cited in OECD 2002].
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C, Bloom AD, Nakamura F, Ahmed M, Duk S, et al. 1987. Chromosome aberrations and sister chromatid exchange in Chinese hamster ovary cells: evaluation of 108 chemicals. Environmental and Molecular Mutagenesis 10(Suppl.10):1-175.
Garber E, Brewer V. 2010. Enzyme-linked Immunosorbent Assay Detection of Melamine in Infant Formula and Wheat Food Products. J Food Prot. 73(4):701–707.
Garcia-Gavin J, Loureiro Martinez M, Fernandez-Redondo V, Seoane M-J, Toribia J. 2008. Contact allergic dermatitis from melamine formaldehyde resins in a patient with a negative patch-test reaction to formaldehyde. Dermatitis. 19:E5-E6.
Gobas FAPC, Kelly BC, Arnot JA. 2003. Quantitative structure activity relationships for predicting the bioaccumulation of POPs in terrestrial food-webs. QSAR Comb Sci. 22:346-351.
Grand and Toy. 2014. Melamine whiteboard [Internet]. [cited 2014 April].
Grosse Y, Loomis D, Guyton KZ, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Mattock H, Straif K. (on behalf of the IARC Monograph Working Group). 2017. Some chemicals that cause tumours of the urinary tract in rodents. Lancet Oncol. 18(8):1003-1004.
Hansch C, Leo A, Hoekman D. 1995. Exploring QSAR: Hydrophobic, Electronic, and Steric Constants, Washington (DC): American Chemical Society.
Hard GC, Flake GP, Sills RC. 2009. Re-evaluation of kidney histopathology from 13-week toxicity and two-year carcinogenicity studies of melamine in the F344 rat: morphologic evidence of retrograde nephropathy. Vet Pathol. 46:1248-1257.
Hatakeyama T, Takagi K, Yamazaki K, Sakakibara F, Ito K, Takasu E, Naokawa T, Fujii K. 2015. Mineralization of melamine and cyanuric acid as sole nitrogen source by newly isolated Arthrobacter spp. using a soil-charcoal. World J Microbiol Biotechnol. 31:785–793.
Hatakeyama T, Takagi K. 2016. Bacterial biodegradation of melamine-contaminated aged soil: influence of different pre-culture media or addition of activation material. Environ Sci Pollut Res. 23:14997-15002
Hauck RD, Stephenson HF. 1964. Nitrification of triazine nitrogen. J Agric Food Chem. 12(2):147-151.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E. 1983. Salmonella mutagenicity test results for 250 chemicals. Env. Mutagen.(Suppl. 1): 3-142. Testing laboratory: Case Western Reserve University.
Hazleton Laboratories. 1983. Raltech Report for American Cyanamid Company, 2-Year chronic feeding study of melamine in Fisher 344 rats. Unpublished data. [cited in OECD 2002].
Health Canada. 1994. Human health risk assessment for priority substances [PDF]. Ottawa (ON): Health Canada.
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations. Ottawa (ON): Great Lakes Health Effects Program, Health Protection Branch, Health Canada. Available upon request.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate. Available upon request.
Health Canada, 2008. Health Canada’s human health risk assessment supporting standard development for melamine in foods. Prepared by the Bureau of Chemical Safety, Food Directorate, Health Products and Food Branch.
Health Canada. 2012. Pest Management Regulatory Agency Label transcript service [Internet]. E-label search for cyromazine. [modified 2016 Jan 8; cited 2017 Mar 31]. Ottawa (ON): Health Canada, Consumer Product Safety, Pest Management Regulatory Agency.
Health Canada. 2015. Determination of melamine in polyurethane foam and similar consumer products by LC-MS/MS. Project Report number 2105-2122; 9 October 2015. Product Safety Laboratory, Ottawa. 6 pp plus Appendix-1: Method C53.
Health Canada. 2016a. Questions and answers – melamine [Internet]. [updated 2016 May 4].
Health Canada. 2016b. Pest Management Regulatory Agency re-evaluation and special review workplan 2015-2020. Re-evaluation Note REV2016-07. 26 February 2016. [accessed 2017 Mar 31]. Ottawa (ON): Health Canada, PMRA. 6 p.
Health Canada. 2016c. Determination of melamine in electronic boards by LC-MS/MS. Project Report number 2105-2160; 26 January 2016. Product Safety Laboratory, Ottawa. 5 pp plus Appendix-1: Method C53.1.
Health Canada. [modified 2017 May 3]. Lists of Permitted Food Additives. Ottawa (ON): Health Canada Food Directorate. [accessed 2014 April 24].
Health Canada. 2018a. Health Canada's maximum levels for chemical contaminants in foods [Internet]. [updated 2018 Jan 25].
Health Canada. 2018b. Pest Management Regulatory Agency re-evaluation and special review workplan 2018-2023. Re-evaluation Note REV2018-06 [PDF]. 13 April 2018. [accessed 2018 Dec 28]. Ottawa (ON): Health Canada, PMRA. 10 p.
Health Canada. 2018c. Supporting documentation: Information in support of the screening assessment, Certain organic flame retardants substance grouping; 1,3,5-Triazine-2,4,6-triamine (Melamine): Human health supplementary data. Ottawa (ON): Environment and Climate Change Canada. Available on request from: substances@ec.gc.ca
Hellwig J, Gembrandt C, Hildebrandt B. 1996. Melamine - prenatal toxicity in Wistar rats after oral administration (diet), Project No. 32R0242/94007. [cited in OECD 2002].
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2011. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2013 Nov 5].
Heukelekian H, Rand MC. 1955. Biochemical oxygen demand of pure organic compounds: a report of the research committee, FSIWA. Sewage and Industrial Wastes. 27(9):1040-1053.
Higby K, Suiter CR, Phelps JY, Siler-Khodr T, Langer O. 1994. Normal values of urinary albumin and total protein excretion during pregnancy. Am J Obstet Gynecol. 171:984-989.
Hilts C, Pelletier L. 2008. Background paper on the occurrence of melamine in foods and feed. Bureau of Chemical Safety, Food Directorate, Health Products and Food Branch, Health Canada, Ottawa, Ontario, Canada. Prepared for the WHO Expert Meeting on Toxicological and Health Aspects of Melamine and Cyanuric Acid. Geneva Switzerland 2009.
Hirt RC, Steger JE, Simard GL. 1960. Vapour Pressure of 2,4,6-Triamino-s-Triazine (Melamine). J Polym Sci. 43:319-323.
Hockenbury MR, Grady CPL. 1977. Inhibition of Nitrification Effects of Selected Organic Compounds. JWPCF 49(5):768-777.
Home Hardware [internet]. 2014. Products containing (or mentioning) melamine [cited 2014 April].
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [estimation model]. 2010. Ver. 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[IARC] International Agency for Research on Cancer. 1999. Melamine [PDF]. In: Some chemicals that cause tumours of the kidney or urinary bladder in rodents and some other substances. IARC Monogr Eval Carcinog Risks Hum. 73: 329–338.
[IARC] International Agency for Research on Cancer. 2019. Melamine. In: Some chemicals that cause tumours of the urinary tract in rodents. IARC Monogr Eval Carcinog Risks Hum. 119: 115–172.
[ICRP] International Commission on Radiological Protection. 2003. Basic anatomical and physiological data for use in radiological protection: Reference values. ICRP Publication 89. Ann. ICRP 32(3-4).
[ICIS] Independent Chemical Information System. c2014. US Chemical Profile: melamine. Reed Business Information. [cited 2014 June 17].
Isaksson M, Zimerson E, Bruze M. 1999. Occupational dermatoses in composite production. J Occup Environ Med. 41:261-266.
Ishiwata H, Sugita T, Kozaki M, Maekawa A. 1991. Inhibitory effects of melamine on the growth and physiological activities of some microorganisms. J Food Hyg Soc. Japan 32:408-413.
Jacob CC, Reimschuessel R, Von Tungeln LS, Olson GR, Warbritton AR, Hattan DG, Beland FA, Gamboa da Costa G 2011. Dose–response assessment of nephrotoxicity from a 7-day combined exposure to melamine and cyanuric acid in F344 rats. Toxicol Sci. 119:391–397.
Jacob CC, Von Tungeln LS, Vanlandingham M, Beland FA, Gamboa da Costa G. 2012. Pharmacokinetics of Melamine and Cyanuric Acid and Their Combinations in F344 Rats. Toxicol Sci. 126(2):317-324.
Janlek P, Boonyaratpalin M, Phromkunthong W. 2009. Histological changes in hybrid catfish, Clarias macrocepalus (Günther) x Clarias gariepinus (Burchell). Thai Fisheries Gazette. 62:331–340. [In Thai].
Karns JS, Eaton RW. 1997. Genes encoding s-triazine degradation are plasmid born in Klebisiella pneumonia strain 99. J Agric Food Chem. 45:1017-1022.
Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC. 2007. Food Web-Specific Biomagnification of Persistent Organic Pollutants. Science. 317:236-239.
Kersting M, Alexy U, Sichert-Hellert W, Manz F, Schoch G. 1998. Measured consumption of commercial infant food products in German infants: results from the DONALD study. Dortmund Nutritional and Anthropometrical Longitudinally Designed. J Pediatr Gastroenterol Nutr. 27:547-552. [cited in EFSA 2010].
Khalil SR, Awad A, Ali SA. 2017. Melamine and/or formaldehyde exposures affect steroidogenesis via alteration of StAR protein and testosterone synthetic enzyme expression in male mice. Environ Toxicol and Pharmacol. 50:136–144.
Kim SH, Lee IC, Lim JH, Shin IS, Moon C, Kim SH, Park SC, Kim HC, Kim JCl. 2011. Effects of melamine on pregnant dams and embryo-fetal development in rats. J Appl Toxicol. 31:506-514.
[KOAWIN] Octanol Air Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2010. Version 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2013 Nov 5].
Kobayashi, T, Okada a, Fujii Y, Niimi K, Hamamoto S, Yasui t, Tozawa K, Kohri K. 2010. The mechanism of renal stone formation and renal failure induced by administration of melamine and cyanuric acid. Urol Res. 38:117-125.
[KOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2010. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2013 Nov 5].
Kong APS, Choi KC, Ho CS, Chan MHM, Wong CK, Liu EKH, Chu WCW, Chow VCY, Lau JTF, Chan JCN. 2011. Hong Kong Chinese school children with elevated urine melamine levels: A prospective follow up study. BMC Public Health 11:354-358.
Konishi K, Imanishi A. 1941. Fertilizing efficacy of melamines and guanidines. J Sci Soil Manure Jpn. 15:564. [cited in: Hauck and Stephenson 1964].
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2010. Version 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2013 Nov 5].
Lakind JS, Naiman DQ. 2008. Bisphenol A (BPA) daily intakes in the United States: Estimates from the 2003-2004 NHANES urinary BPA data. J Exposure Sci Environ Epidemiol. 18:608-615.
Lentner C. (Ed.). 1981. Geigy scientific tables. Vol. 1: Units of measurement, body fluids, composition of the body, nutrition. Eighth edition. Ciba-Geigy Ltd., Basle, Switzerland.
Li, G, Jiao S, Yin X, Deng Y, Pang X, Wang Y. 2010. The risk of melamine-induced nephrolithiasis in young children starts at a lower intake level than recommended by the WHO. Pediatr Nephrol. 25:135-141.
Li W, Li H, Zhang J, Tian X. 2015. Effect of melamine toxicity on Tetrahymena thermophile proliferation and metallothionein expression. Food Chem Toxicol. 80:1-6.
Lightner DV, Pantoja CR, Redman RM, Hasson KW, Menon JP. 2009. Case of melamine-induced pathology in penaeid shrimp fed adulterated feeds. Dis Aquat Org. 86:107-112.
Lipschitz WL, Stokey E. 1945. The mode of action of three new diuretics: melamine, adenine and formoguanamine. J Pharmacol Exp Ther. 83:235-249.
Litton Bionetics. 1977a. Mutagenicity evaluation of melamine. Unpublished data. Report no.: LBI Project No. 2838. [cited in OECD 2002].
Litton Bionetics. 1977b. Unpublished data. [cited in OECD 2002].
Litton Bionetics. 1977c. Mutagenicity evaluation of melamine. Unpublished data. Report no.: LBI Project No. 2838. [cited in OECD 2002].
Liu, C-C, Wu C-F, Chen BH, Huang SP, Goggins W, Lee HH, Chou YH, Wu WJ, Huang CH, Shiea J, et al. 2011. Low exposure to melamine increases the risk of urolithiasis in adults. Kidney Int. 80:746-752.
Liu G, Li S, Jia J, Yu C, He J, Yu C, Zhu J. 2010. Pharmacokinetic study of melamine in rhesus monkey after a single oral administration of a tolerable daily intake dose. Regul Toxicol. Pharmacil. 56:193-196.
Liu HY, Zhang W, Xue M, Wu XF, Zheng YH, Guo LY, Sheng HJ. 2009. Acute toxicity study for melamine on Japanese sea bass (Lateolabrax japonicus). Acta Hydrobiologica Sinica 33:157–163. [In Chinese].
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Government of Canada. [accessed 2014 April 24].
Lu Y, Xia Y, Liu G, Pan M, Li M, Lee NA, Wang S. 2017. A review of methods for detecting melamine in food samples. Crit Rev Anal Chem. 47(1):51-66.
Lund KH, Petersen JH. 2006. Migration of formaldehyde and melamine monomers from kitchen- and tableware made of melamine plastic. Food Addit Contam. 23(9):948-955.
Lynch RA, Hollen H, Johnson DL, Bartels J. 2015. The effects of pH on the migration of melamine from children’s bowls [PDF]. Int J Food Contam. 2:9. DOI 10.1186/s40550-015-0017-z
Magami SM, Oldring PKT, Castle L, Guthrie JT. 2015. Migration of melamine from thermally cured, amino cross-linked can coatings into an aqueous ethanol food simulant: aspects of hydrolysis, relative reactivity and migration. Food Addit Contam Part A. 32(3):403-409.
Mast RW. Naismith RW, Friedman MA. 1982a. Mouse micronucleus assay of melamine. Environ Mutagen. 4:340-341 (Abstract No. Bi-8).
Mast RW, Friedman MA, Finch RA. 1982b. Mutagenicity testing of melamine. Toxicologist 2: 172 (Abstract No. 602).
Mast RW, Jeffcoat AR, Sadler BM, Kraska RC, Friedman MA. 1983. Metabolism, disposition and excretion of [14C]melamine in male Fischer 344 rats. Food Chem Toxicol. 21(6):807-810.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, Caspary WJ. 1988. Responses of the L5178Y tk+/tk mouse lymphoma cell forward mutation assay: III. 72 coded chemicals. Environ Mol Mutagen. 12:85-154.
Melnick R.L, Boorman GA, Haseman JK, Montali RJ, Huff J. 1984. Urolithiasis and bladder carcinogenicity of melamine in rodents. Toxicol Appl Pharmacol. 72:292-303.
Mirsalis J, Tyson K, Beck J, Loh F, Steinmetz K, Contreras C, Austere L, Martin S, Spalding J. 1983. Induction of unscheduled DNA synthesis (UDS) in hepatocytes following in vivo and in vivo treatment. Environmental Mutagenesis 5: 482; Abstract no. Ef-5 in Abstracts of the Environmental Mutagen Society held at San Antonio, Texas, March 3-6. 1983.
[MITI] Ministry of International Trade & Industry (JP). 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (JP): Japan Chemical Industry Ecology–Toxicology and Information Center.
[MOE] Ministry of the Environment (JP). c2005. Chemicals in the environment [Internet]. Tokyo (JP): MOE, Environmental Health Dept. [cited 2014 Feb 4].
[MSDS] Material Safety Data Sheet. 2013. SUPER SPEC HP® LATEX FLAT FIRE RETARDANT. Montvalue, New Jersey. Benjamin Moore & Co. Revision date: 05 February 2013.
[MSDS] Material Safety Data Sheet. 2014. Cromophtal® Yellow D 1085 (old Cromophtal® Yellow LA2). [cited 2014 April]. BASF [internet].
[MSDS] Material Safety Data Sheet. 2016. 3M Fire Barrier Watertight Sealant 3000 WT. [cited 2017 March]. 3M Company [internet].
Muijser H. 1998. Acute (4-hour) inhalation toxicity study with Melamine in rats. Testing laboratory: Testing laboratory: TNO Nutrition and Food Research Institute, Toxicology Division. Report no.: V98.420. Owner company: DSM N. V. Heerlen, The Netherlands. Study number: 480001/002. Report date: 1998-04-15. Unpublished information submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment and Climate Change Canada, Program Development and Engagement Division. [restricted access].
Nascimento CF, Santos PM, Pereira-Filho ER, Rocha FRP. 2017. Recent advances on determination of milk adulterants. Food Chemistry 221:1232-1244.
Neithardt AB, Dooley SL, Borensztajn J. 2002. Prediction of 24-hour protein excretion in pregnancy with a single voided urine protein-to-creatinine ratio. Am J Obstet Gynecol. 186:883-886.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2017 June 21]. Ottawa (ON): Government of Canada. [accessed 2017 July].
Niemi GJ, Gilman DV, Regal RR, Vaishnav DD. 1987. Structural features associated with degradable and persistent chemicals. Environ Toxicol Chem. 6:515-527.
Norris B, Smith S. 2002. Research into the mouthing behaviour of children up to 5 years old. London, England: Consumer and Competition Policy Directorate, Department of Trade and Industry, London, UK. [cited in US EPA 2011].
[OECD] Organisation for Economic Co-operation and Development. 2002. SIDS Initial Assessment Report for: Melamine; CAS RN 108-78-1. SIDS Initial Assessment Meeting 8; October 1998.
[OECD] Organisation for Economic Co-operation and Development. 2009. OECD Pov and LRTP Screening Tool [Internet]. Version 2.2. A software model for estimating overall persistence (Pov) and long-range transport potential (LRTP) of organic chemicals. [cited 2014 June 3].
Ogasawara H, Imaida K, Ishiwata H, Toyoda K, Kawanishi T, Uneyama C, Hayashi S, Takahashi M, Hayashi Y. 1995. Urinary bladder carcinogenesis induced by melamine in F344 male rats: correlation between carcinogenicity and urolith formation. Carcinogenesis. 16:2773-2777.
Okumura M, Hasegawa R, Shirai T, Ito M, Yamada S, Fukushima S. 1992. Relationship between calculus formation and carcinogenesis in the urinary bladder of rats administered the non-genotoxic agents, thymine or melamine. Carcinogenesis. 13:1043-1045.
Pang J, Li G-Q, Li C-R, Yang X-Y, Lu X, Hu X-X, Zhai Q-Q, Zhang W-X, Jiang J-D, You X-F. 2013. Toxicokinetic study of melamine in the presence and absence of cyanuric acid in rats. J Appl Toxicol. 33:444-450.
Panuwet P, Nguyen JV, Wade EL, D’Souza PE, Ryan PB, Barr DB. 2012. Quantification of melamine in human urine using cation-exchange based high performance liquid chromatography tandem mass spectrometry. J Chromatogr. B 887-888:48-54.
Parboosingh J, Doig A. 1973. Studies of nocturia in normal pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 80:888-895.
Petreas M, Gill R, Takaku-Pugh S, Lytle E, Parry E, Wang M, Quinn J, Park J-S. 2016. Rapid methodology to screen flame retardants in upholstered furniture for compliance with new California labeling law (SB 1019). Chemosphere. 152:353-359.
Pharmakon Research. 1981. Genetic toxicology - Micronucleus test. Report for American Cyanamid Company, Study No. PH 309A-AC-001-81. Unpublished data. [cited in OECD 2002].
Perucca J, Bouby N, Valeix P, Bankir L. 2007. Sex difference in urine concentration across differing ages, sodium intake, and level of kidney disease. Am J Physiol Regul Integr Comp Physiol. 292:R700–R705,
Phromkunthong W, Nuntapong N, Boonyaratpalin M, Kiron V. 2013. Toxicity of melamine, an adulterant in fish feeds; experimental assessment of its effect on tilapia. J Fish Disease. 36:555-568.
Phromkunthong W, Choochuay P, Kiron V, Nuntapong N, Boonyaratpalin M. 2015. Pathophysiological changes associated with dietary melamine and cyanuric acid toxicity in red tilapia. J Fish Disease. 38(2):161-173.
Puschner, B, Poppenga RH, Lowenstine LJ, Filigenzi MS, Pesavento PA . 2007. Assessment of melamine and cyanuric acid toxicity in cats. J Vet Diagnc Invest. 19(6): 616-24.
Qin Y, Lv X, Li J, Qi J, Diao Q, Liu G, Xue M, Wang J, Tong J, Zhang L, Zhang K. 2010. Assessment of melamine contamination in crop, soil and water in China and risks of melamine accumulation in animal tissues and products. Environ Int. 36:446-452.
Rai N, Banerjee D, Bhattacharyya R. 2014. Urinary melamine: Proposed parameter of melamine adulteration of food. Nutrition. 30:380–385.
Ramusino MC, Vailati G. 1982. Modification in Salmo gairdneri due to 2,4,6 triamino 1,3,5 triazine (melamine), Acta Embryol Morphol Exp. N.s. 3(1):41-48. [cited in ECHA c2007-2013].
Reimschuessel R, Evans E, Andersen WC, Turnipseed SB, Karbiwnyk CM, Mayer TD, Nochetto C, Rummel NG, Gieseker CM. 2010a. Residue depletion of melamine and cyanuric acid in catfish and rainbow trout following oral administration. J Vet Pharmacol Ther. 33(2):172-82.
Reimschuessel R, Evans ER, Stine CB, Hasbrouck N, Mayer TD, Nochetto C, Gieseker CM. 2010b. Renal crystal formation after combined or sequential oral administration of melamine and cyanuric acid. Food Chem Toxicol. 48:2898-2906.
Remer T, Fonteyn N, Alexy U, Berkemeyer S. 2006. Longitudinal examination of 24-h urinary iodine excretion in schoolchildren as a sensitive, hydration status-independent research tool for studying iodine status. Am J Clin Nutr. 83:639-646.
Revúsová V, Zvara V, Gratzlová J. 1971. Some laboratory findings in patients with urolithiasis. Int Urol Nephrol. 3(3):251-258.
Rijcken WRP. 1995. Primary skin irritation/corrosion study with melamine in the rabbit, confidential Notox project 146205 for DSM Melamine, 1995. [cited in OECD 2002].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2008. Chemicals in Toys. A general methodology for assessment of chemical safety of toys with a focus on elements. Bilthoven (NL): RIVM. Report No.:320003001/2008. [accessed 2018 July 9].
Roberts TR, Hutson DH. Eds. 1999. Metabolic Pathways of Agrochemicals, Part2: Insecticides and Fungicides. Royal Society of Chemistry, MPG Books, Ltd.: Bodmin, Cornwall, UK. pp. 741–743.
Rossman TG, Molina M, Meyer L, Boone P, Klein CB, Wang Z, Li F, Lin WC, Kinney PL. 1991. Performance of 133 compounds in the lambda prophage induction endpoint of the Microscreen assay and a comparison with S. typhimurium mutagenicity and rodent carcinogenicity assays. Mutat Res. 260:349-367.
Rovina K, Siddiquee S. 2015. A review of recent advances in melamine detection techniques. J Food Compos Anal. 43:25-38.
[RTECS] Registry of Toxic Effects of Chemical Substances. 2013. Datasheet on melamine (108-78-1). Last updated September 2013 [Accessed 13 February 2013].
RTI. 1982. Evaluation of urolithiasis induction by melamine in male weanling Fischer 344 rats, Project No. 31T-2407 for American Cyanamid Company, unpublished data. [cited in OECD 2002].
Rumble JR, editor. 2018. CRC Handbook of Chemistry and Physics. Internet Version 2018. Boca Raton (FL): CRC Press. 1532 pp.
Scorecard (the pollution information site) [Internet]. c2011. Scorecard: Chemical profile for melamine. [cited 2014 Jan 28]. Sponsored by GoodGuide (US).
Seiler JP. 1973. A survey on the mutagenicity of various pesticides. Experienta. 29:622-623.
Selden JR, Dolbeare F, Clair JH, Miller JE, McGettigan K, DiJohn JA, Dysart GR, DeLuca JG. 1994. Validation of a flow cytometric in vitro DNA repair (UDS) assay in rat hepatocytes. Mutat Res. 315(2):147-167.
Shelby MD, Erexson GL, Hook GJ, Tice RR. 1993. Evaluation of a three-exposure mouse bone marrow micronucleus protocol: Results with 49 chemicals. Environ Mol Mutagen. 21:160-179.
Shelton DR, Karns JS, McCarty GW, Durham DR. 1997. Metabolism of Melamine by Klebsiella terragena. Appl Environ Microbiol. 63(7):2832.
Smith GR, Stearley RF. 1989. The classification and scientific names of rainbow trout and cutthroat trouts. Fisheries. 14(1):4-10.
Soubrier R, Burlet P. 1972 Dermatoses provoqué par la mélamine. Arch Mal Prof. 33:202-204. [cited in OECD 2002].
[SPIN] Substances in Preparations in Nordic Countries database [Internet]. c2014. Copenhagen (DE): Nordic Council of Ministers, Chemical Group. [cited 2014 Jan 31].
Statistics Canada, 2004. Canadian Community Health Survey – nutrition (CCHS). Detailed information for 2004 (cycle 2.2). Ottawa (ON): Government of Canada.
Statistics Canada. 2019. Canadian International Merchandise Trade Database [Internet]. Table 990-0029; 29. Import – Organic chemicals (Data selection; 293361 melamine) [updated 2019 Feb. 14; cited 2019 Feb.]. Ottawa (ON): Government of Canada.
Stine CB, Reimschuessel R, Keltner Z, Nochetto CB, Black T, Olejnik N, Scott M, Bandele O, Nemser SM, Tkachenko A et al. 2014. Reproductive toxicity in rats with crystal nephropathy following high doses of oral melamine or cyanuric acid. Food Chem Toxicol. 68:142–153.
[STP-EX] Sewage Treatment Plant Expanded Model. 2008. Windsor (ON): University of Windsor, Dept. of Civil and Environmental Engineering. [Model described in Seth et al. 2008].
Suknikom P, Jermnak U, Poapolathep S, Isariyodom S Giorgi M, Kumagai S, Poapothep A. 2016. Dispositions and tissue depletion of melamine in ducks. J Vet Pharmacol Ther. 39(1)90-94.
Sun Y, Jiang YN, Xu C-F, Du YX, Zhang JJ, Yan Y, Gao XL. 2014. The recovery of bladder epithelial hyperplasia caused by a melamine diet-induced bladder calculus in mice. Food Chem Toxicol. 64:378–382.
Sun YJ, Wang HP, Liang YJ, Yang L, Li W, Wu YJ. 2012. An NMR-based metabonomic investigation of the subacute effects of melamine in rats. J Proteome Res. 11:2544-2550.
Sun J, Cao Y, Zhang X, Zhao Q, Bao E, Lv Y. 2016a. Melamine negatively affects testosterone synthesis in mice. Res Vet Sci. 109:135-141.
Sun J, Zhang X, Cao Y, Zhao Q, Bao E, Lv Y. 2016b. Ovarian toxicity in female rats after oral administration of melamine or melamine and cyanuric acid. PLOS One. DOI:10.1371/journal.pone.0149063
[SWISSI] The Swiss Institute of Safety and Security. 2009. Determination of some physical-chemical properties of Melamine. Report. Assignment no.: 204611.08.0640.02. Basel (CH): SWISSI. 7p. [restricted access].
Swope HG, and Kenna M. 1950. Effect of organic compounds on biochemical oxygen demand. Sew Ind Wastes Eng. 21:467. [cited in Niemi et al. 1987].
[TaPL3] Long Range Transport and Persistence Level III model [Internet]. 2003. Version 3.0. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. [cited 2014 June 1].
Takagi K, Fujii K, Yamazaki K, Harada N, Iwasaki A. 2012. Biodegradation of melamine and its hydroxy derivatives by a bacterial consortium containing a novel Nocardioides species. Appl Microbiol Biotechnol. 94:1647–1656.
Ten Berge W. 2010. A simple dermal absorption model: Derivation and application. Chemosphere. 75:1440-1445.
[TDS] Technical Data Sheet. 2011. SUPER SPEC HP® LATEX FLAT FIRE RETARDANT. Mississauga, Ontario. Benjamin Moore & Co.
The Home Depot Canada [Internet]. 2014. Products containing (or mentioning) melamine [cited 2014 April].
Thorp JM, Norton PA, Lewis Wall L, Kuller JA, Eucker B, Wells E. 1999. Urinary incontinence in pregnancy and the puerperium: A prospective study. Am J Obstet Gynecol. 181:266-273.
Tittlemier S, Lau BP-Y, Ménard C, Corrigan C, Sparling M, Gaertner D, Pepper, K, Feeley M. 2009. Melamine in infant formula sold in Canada: Occurrence and risk assessment. J Agric Food Chem. 57:5340-5344.
Tittlemier S, Lau BP-Y, Ménard C, Corrigan C, Sparling M, Gaertner D, Cao X-L, Dabeka R. 2010a. Baseline levels of melamine in food items sold in Canada. I. Dairy products and soy-based dairy replacement products. Food Addit Contam Part B Surveill. 3(3):135-139.
Tittlemier S, Lau BP-Y, Ménard C, Corrigan C, Sparling M, Gaertner D, Cao X-L, Dabeka R, Hilts C. 2010b. Baseline levels of melamine in food items sold in Canada. II. Egg, soy, vegetable, fish and shrimp products. Food Addit Contam Part B Surveill. 3(3):140-147.
Tu H, Zhang M, Zhou C, Wang Z, Huang P, Ou H, Chang Y. 2015. Genotoxicity assessment of melamine in the in vivo Pig-a mutation assay and in a standard battery of assays. Mutat Res. 777:62-67.
Ubaidellajev RU, et al. 1993. Gigiena i Sanitariya 58:14-16. [cited in RTECS 2013]. [accessed 13 Feb. 2013].
[US CDC] U.S. Centers for Disease Control and Prevention. 2010a. National Health and Examination Survey 2003-2004. Data Documentation, Codebook, and Frequencies. Melamine (Surplus Urine) (SSMEL_C) files. Last revised April 2010. [accessed: March 2014].
[US CDC] U.S. Centers for Disease Control and Prevention. 2010b. National Health and Examination Survey 2003-2004. Demographic Data. [accessed March 2014]. [US CPSC] U.S. Consumer Product Safety Commission. 2005. Analysis of FR Chemicals Added to Foams, Fabric, Batting, Loose Fill, and Barriers. Memorandum dated May 10, 2005. 20 pp.
[US CPSC] U.S. Consumer Product Safety Commission. 2006. CPSC staff preliminary risk assessment of flame retardant (FR) chemicals in upholstered furniture foam. 212 pp.
[US eCFR] U.S. Electronic Code of Federal Regulations [Internet]. 2014a. 21 CFR 175.105 [current as of January 30, 2014]. Washington (DC): National Archives and Record Administration, Office of the Federal Register (OFR); U.S. Government Printing Office. [cited 2014 Jan].
[US eCFR] U.S. Electronic Code of Federal Regulations [Internet]. 2014b. 21 CFR 175.300 and 175.320 [current as of January 30, 2014]. Washington (DC): National Archives and Record Administration, Office of the Federal Register (OFR); U.S. Government Printing Office. [cited 2014 Jan].
[US EPA] U.S. Environmental Protection Agency. 1984. Cyromazine, proposed tolerance, U.S. Federal Register. 49(83):18121-18125.
[US EPA] U.S. Environmental Protection Agency. 2007. High Production Volume Information System (HPVIS) [database on the Internet]. Search results for CAS RN 108-78-1. Washington (DC): U.S. EPA. [cited 2014 Jan 29].
[US EPA] U.S. Environmental Protection Agency. 2011. Exposure factors handbook: 2011 edition. National Center for Environmental Assessment, Washington, DC; EPA/600/R-09/052F.
[US EPA] U.S. Environmental Protection Agency. 2012. Standard Operating Procedures for Residential Pesticide Exposure Assessment [PDF]. Office of Chemical Safety and Pollution Prevention, Washington (DC).
[US EPA] U.S. Environmental Protection Agency. 2015. Flame retardants used in flexible polyurethane foam: An alternatives assessment update. Report No. EPA 744-R-15-002; August 2015. U.S. EPA Design for the Environment. 832 pp.
[US NTP] United States National Toxicology Program. 1983. Carcinogenesis bioassay of melamine in F344/N rats and B6C3F1 mice (feed study); Technical Report Series No. 245 [PDF]. US Department of Health and Human Services.
[US Testing Company] United States Testing Company Inc. (Hoboken, New Jersey). 1988. Algal growth inhibition test (OECD method) using CT-338-87. Test report no.: 07383. Unpublished report conducted for American Cyanamid Company (Wayne, New Jersey). 8 p. [restricted access]
Vaishnav DD. 1984. Biochemical oxygen demand data base. Element 16, Vol. 1. In: Call DJ, Brooke LT, Vaishnav DD, editors. 1984. Aquatic Pollutant Hazard Assessment and Development of Hazard Prediction Technology by Quantitative Structure-Activity Relationships. EPA-CR809234. Superior (WI): University of Wisconsin. [cited in Niemi et al. 1987].
Van Haarst EP, Heldeweg EA, Newling DW, Schlatmann TJ. 2004. The 24-h frequency-volume chart in adults reporting no voiding complaints: defining reference vales and analysing variables. BJU International. 93:1257-1261.
Vernon PA, Deskin R, Dulak LH. 1990. Acute toxicologic evaluation of melamine. Cited in Parent RA, editor. Acute toxicity data. J Am Coll Toxicol Part B. 1:110. Owner company: American Cyanamid Co.
Wackett LP, Sadowsky MJ, Martinez B, Shapir N. 2002. Biodegradation of atrazine and related s-triazine compounds: from enzymes to field studies. Appl Microbiol Biotechnol. 58(1):39–45.
Wang H, Gen C, Li J, Hu A. 2014. Characterization of a novel melamine-degrading bacterium isolated from a melamine-manufacturing factory in China. App Microbiol Biotechnol. 98:3287-3293
Wang W, Chen H, Yu B, Mao X, Chen D. 2013. Tissue deposition and residue depletion of melamine in fattening pigs following oral administration. Food Addit Contam Part A. 31(1):7-14.
Wang Z, Qi X, Zou M, Zhang Z, Xu F. 2009. Cytotoxicity assessment of melamine using the ciliated protozoan Tetrahymena pyriformis. Asian J Ecotoxicol. 4(1):35-39.
Wang Z, Chen L, Al-Kasir R, Han B. 2011. In vitro toxicity of melamine against Tetrahymena pyriformis cells. J Vet Sci. 12(1):27-34.
Weber JB. 1970. Adsorption of s-triazines by montmorillonite as a function of pH and molecular structure. Soil Sci Soc Amer Proc. 34:401-404.
Wehner DJ, Martin DL. 1989. Melamine/urea and oxamide fertilization of Kentucky bluegrass. Urbana (IL): University of Illinois, Dept. of Horticulture. 15 pp.
Weil ED, Choudhary V. 1995. Flame-retarding plastics and elastomers with melamine. J Fire Sci. 13(March/April):104-126.
Williamson MA, Snyder LM, editors. 2011. Wallach’s Interpretation of Diagnostic Tests. 9th Edition, updated 12 Feb. 2014. Philadelphia, Pa: Lippincott Williams and Wilkins.
[WHO] World Health Organization. 2009. Toxicological and Health Aspects of Melamine and Cyanuric Acid. Report of a WHO Expert Meeting in Collaboration with FAO (Food and Agriculture Organization of the United Nations). Supported by Health Canada. 66 pp.
[WHO] World Health Organization. c2014. Health topics: melamine [Internet]. Geneva (CH): WHO. [cited 2014 Feb 3].
Wong CK, Chan MHM, Kwok JSS, Ho CS, Ng PC, Suen SH, Fung KP, Lau CM, Fok TF. 2013. Diagnostic tools for detection of intoxication by melamine and its analogue. Hong Kong Med J. 19(6), Supplement 8:12-15.
Wu AHB. 2006. Tietz clinical guide to laboratory tests. 4th ed. St. Louis (MO): Saunders Elsevier. p. 1102–1104.
Wu CF, Hsieh TJ, Chen BH, Liu CC, Wu MT. 2013. A cross over study of noodle soup consumption in melamine bowls and total melamine excretion in urine. JAMA Inter Med. 173(4):317-319.
Wu CF, Liu CC, Chen BH, Huang SP, Lee HH, Chou YC, Wu WJ, Wu MT. 2010a. Urinary melamine and adult urolithiasis in Taiwan. Clin Chim Acta. 411:184-189.
Wu YT, Huang CM, Lin CC, Ho WA, Lin LC, Chiu TF, Tarng DC, Lin CH, Tsai TH. 2010b. Oral bioavailability, urinary excretion and organ distribution of melamine in Sprague-Dawley rats by high-performance liquid chromatography with tandem mass spectrometry. J Agric Food Chem. 58:108-111.
Xu S, Zhang Y, Sims A, Bernards M, Hu Z. 2013. Fate and toxicity of melamine in activated sludge treatment systems after a long-term sludge adaptation. Water Res. 47:2307-2314.
Xue J, Ai Q, Mai K, Xu W, Yang Y, Liufu Z. 2011a. Effects of melamine on growth performance and skin color of darkbarbel catfish (Pelteobagrus vachelli). Aquaculture. 320:142-146.
Xue M, Qin Y, Wang J, Qui J, Wu X, Zheng Y, Wang Q. 2011b. Plasma pharmacokinetics of melamine and a blend of melamine and cyanuric acid in rainbow trout (Oncorhynchus mykiss). Reg Toxicol Pharmacol. 61:93-97.
Yalkowsky SH, He Y. 2003 Handbook of aqueous solubility data. Boca Raton (FL): CRC Press LLC.
Yang F, Mao Y, Zhang X, Ma Z, Zhang X. 2009. LC-MS/MS method for the determination of melamine in rat plasma: Toxicokinetic study in Sprague–Dawley rats. J Sep Sci. 32:2974-2978.
Yang J, An L, Yao Y, Yang Z, Zhang T. 2011. Melamine impairs spatial cognition and hippocampal synaptic plasticity by presynaptic inhibition of glutamatergic transmission in infant rats. Toxicology. 289:167-174.
Yasunaga K, Kiyonari A, Oikawa T, Abe N, Yoshikawa K. 2004. Evaluation of the Salmonella umu test with 83 NTP chemicals. Environ Mol Mutagen. 44:329-345.
Yin RH, Wang XZ, Bai WL, Wu CD, Yin RL, Li C, Liu J, Liu BS, He JB. 2013. The reproductive toxicity of melamine in the absence and presence of cyanuric acid in male mice. Res Vet Sci. 94(3):618-627.
Yurdakok B, Filazi A, Ekici H, Celik TH, Sireli UT. 2014. Melamine in breast milk. Toxicol Res. 3:242-246.
Yurdakok B, Filazi A, Ekici H, Celik TH, Sireli UT. 2015. Correction: Melamine in breast milk. Toxicol Res. 4:527.
Zeiger E. 1987. Carcinogenicity of mutagens: Predictive capability of the Salmonella Mutagenesis assay for rodent carcinogenicity. Cancer Research 47: 1287-1296. Testing laboratory: Haworth et al. (1983) summarized in table 1.
Zhang Q, Gamboa da Costa G, Von Tungeln LS, Jacob CC, Brown RP, Goering PL. 2012. Urinary biomarker detection of melamine- and cyanuric acid-induced kidney injury in rats. Toxicol Sci. 129:1-8.
Zhu X, Wang, S, Liu Q, Xu Q, Xu S, Chen H. 2009. Determination of residues of cyromazine and its metabolite, melamine, in animal-derived food by gas chromatography-mass spectrometry with derivatization. J Agric Food Chem. 57:11075-11080.
Appendix A. Modelled degradation results for melamine
Fate process |
Model and model basis |
Model result and prediction |
Extrapolated half-life (days) |
|---|---|---|---|
Atmospheric oxidation |
AOPWIN 2010 |
t1/2 = 16.2 daysa |
≥ 2 days |
Ozone reaction |
AOPWIN 2010 |
N/A |
N/A |
Abbreviations: N/A, not available
a Estimation is based on a 12-hour day, and a mean tropospheric OH concentration of 1.5x106 OH/cm3
Fate process |
Model and model basis | Model result and prediction |
Extrapolated half-life (days) |
|---|---|---|---|
Hydrolysis |
HYDROWIN 2010a,b |
N/A |
N/A |
Primary Biodegradation (aerobic) |
BIOWIN 2010b Sub-model 4: Expert Survey (qualitative results) |
3.3c
|
~182 |
Ultimate Biodegradation (aerobic) |
BIOWIN 2010b Sub-model 3: Expert Survey (qualitative results) |
2.3c
|
~182 |
Ultimate Biodegradation (aerobic)
|
BIOWIN 2010b Sub-model 5: MITI linear probability |
0.02d
|
≥182 |
Ultimate Biodegradation (aerobic)
|
BIOWIN 2010b Sub-model 6: MITI non-linear probability |
0d
|
≥182 |
Ultimate Biodegradation (aerobic) |
CATALOGIC 2012 % BOD (biological oxygen demand) |
% BOD = 0 “biodegrades slowly” |
≥182 |
Abbreviations: N/A, not available
a Model does not provide an estimate for this type of structure.
b EPI Suite (2010).
c Output is a numerical score from 0 to 5.
d Output is a probability score.
Appendix B. Estimates of daily intake of melamine (µg/kg-bw per day) by the general population of Canada
Route of expo-sure |
0-6 mo. Breast milk fedd |
0-6 mo. formula fede |
0-6 mo. not formula fede |
0.5–4 yearsf |
5–11 yearsg |
12–19 yearsh |
20–59 yearsi |
60+ yearsj |
|
|---|---|---|---|---|---|---|---|---|---|
Fooda |
- |
0.72b (1.7) |
0.72b (1.7) |
0.46c (1.2) |
0.32 (1.2) |
0.22 (0.73) |
0.21 (0.80) |
0.21 (0.80) |
|
Drinking waterk |
- |
5.3 (21) |
2.0 (8.0) |
2.3 (9.0) |
1.8 (7.1) |
1.0 (4.0) |
1.1 (4.2) |
1.1 (4.4) |
|
Breast milk |
2.7 (7.6) |
- |
- |
- |
- |
- |
- |
- |
|
Dust/soill |
< 0.0001 |
< 0.0001 |
< 0.0001 |
< 0.0001 |
< 0.0001 |
< 0.0001 |
< 0.0001 |
< 0.0001 |
|
Total intake |
2.7 (7.6) |
6.0 (22.7) |
2.7 (9.7) |
2.8 (10.2) |
2.1 (8.3) |
1.2 (4.7) |
1.3 (5.0) |
1.3 (5.2) |
|
a The daily intake from food is based on a semi-probabilistic assessment; mean intake is included here to aid in discussion of Table 8-6. The values in brackets are 95th percentile values. The semi-probabilistic assessment results are discussed in the text in section 8.1.1, and presented in Table 8-1.
b Based on < 1 year old age group instead of 0-6 months; includes both formula-fed and not formula-fed infants.
c Based on 1-4 year old age group instead of 0.5-4 years.
d No data were identified on concentrations of melamine in Canadian breast milk. In Turkey, melamine was measured in the breast milk of 77 healthy lactating mothers. Melamine was detected in 21% of the 77 samples, with concentrations ranging from 10.1 to 76.4 µg/L (mean: 27.1 µg/L) melamine. The mean and maximum concentrations for melamine in breast milk was used in the estimate of daily intake.
e Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, to drink 0.8 L of water per day (powdered formula fed) or 0.2 L/day (not formula fed) and to ingest 30 mg of soil per day (Health Canada 1998).
f Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.2 L of water per day and to ingest 100 mg of soil per day (Health Canada 1998).
g Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 0.4 L of water per day and to ingest 65 mg of soil per day (Health Canada 1998).
h Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 0.4 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
i Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 0.4 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
j Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 0.4 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
k No data were identified on concentrations of melamine in Canadian drinking water. However, concentrations of melamine in drinking water sourced from various areas around the world ranged from 10 to 200 µg/kg (mean and median = 50 µg/kg) based on 20 samples of tap water sampled by industry and reported to EFSA (2010). The mean and maximum concentrations for melamine in drinking water were used for estimating exposure. The values in brackets are maximum values.
l No data on concentrations of melamine in dust and no data on melamine concentrations in North American soils were identified. However, in China, soils were tested at 100 m and approximately 150 km away from melamine manufacturing factories. At 100 m away, melamine concentrations in soil ranged from non-detect to 41.1 mg/kg. At approximately 150 km away from the factories, concentrations in farmland soil ranged from non-detect to 0.176 mg/kg (Qin et al. 2010). Since concentrations measured further away than close to a melamine manufacturing facility would be more representative of melamine soil concentrations in Canada, the maximum concentration in farmland soil measured at 150 km away from the manufacturing facility was used in a deterministic estimate of daily intake.
Appendix C. Summary of melamine occurrence data used to estimate dietary exposure and dietary assessment methodology‡
Food |
# Samples |
Mean melamine concentration in µg/kga (Range in parentheses) |
|---|---|---|
Infant formula – powder (milk, soy) |
64 |
42.9 (<LOD-346) |
Infant formula – concentrate (milk, soy) |
24 |
17.5 (5.5-34.5) |
Infant formula – ready-to-consume (milk, soy) |
6 |
27.5 (<LOD-68.9) |
Milk - whole, 2%,1%, skim |
68 |
4.1 (<LOD-7.4) |
Milk – evaporated, condensed |
17 |
8.3 (<LOD-30.7) |
Milk shake |
9 |
< LOD (<LOD) |
Milk powder |
11 |
6.3 (<LOD-12.3) |
Chocolate milk |
3 |
<LOD (<LOD) |
Other milk beverages |
6 |
23.3 (<LOD-71.9) |
Cream |
4 |
<LOD (<LOD) |
Ice cream/frozen soy dessert |
11 |
<LOD (<LOD) |
Yogurt/yogurt drink |
37 |
4.2 (<LOD-7.3) |
Chocolate/milk candy |
11 |
667.2 (<LOD-7290) |
Milk coffee/coffee drink |
15 |
87.9 (<LOD-282) |
Milk tea |
7 |
16.1 (<LOD-89) |
Meal replacement |
7 |
17.3 (<LOD-53.0) |
Cheese |
7 |
<LOD (<LOD) |
Soy beverage |
46 |
4.1 (<LOD-6.6) |
Soy cereal |
6 |
<LOD (<LOD) |
Soy spread |
4 |
<LOD (<LOD) |
Egg (preserved, liquid, frozen) |
6 |
10.4 (<LOD-42.1) |
Meat substitute |
38 |
5.9 (<LOD-36.4) |
Tofu |
12 |
<LOD (<LOD) |
Sauce (tartar, teriyaki, soy, hoisin) |
13 |
<LOD (<LOD) |
Mayonnaise |
16 |
4.1 (<LOD-5.1) |
Pasta/noodle |
15 |
4.2 (<LOD-5.9) |
Breakfast cereal |
3 |
<LOD (<LOD) |
Energy bar |
14 |
5.4 (<LOD-22.1) |
Pancake, muffin, waffle (mixes) |
17 |
<LOD (<LOD) |
Cookie dough |
7 |
<LOD (<LOD) |
Cake (ready-to-consume, mixes) |
18 |
4.6 (<LOD-8.6) |
Pie |
2 |
<LOD (<LOD) |
Corndog, frankfurter, sausage roll |
3 |
<LOD (<LOD) |
Chicken nugget, strip |
4 |
15.8 (<LOD-27.6) |
Shrimp |
75 |
71.8 (<LOD-1156) |
Fish (tilapia, sole) |
8 |
4.1 (<LOD-4.8) |
Eel |
4 |
52.2 (11.6-92.6) |
Spinach (frozen) |
4 |
8.8 (<LOD-23.1) |
Potatoes (canned, dried) |
26 |
4.9 (<LOD-23.6) |
Mushrooms (canned, dried, pickled) |
38 |
144.6 (<LOD-757) |
Onions (fresh, frozen, dried, pickled, powdered) |
12 |
4.3 (<LOD-8.1) |
Tomatoes (canned, dried, jarred, juice, soup) |
38 |
36.9 (<LOD-153) |
Dried sauce mixes |
9 |
18.4 (<LOD-116) |
‡Data sourced from Tittlemier et al. 2009; Tittlemier et al. 2010a; Tittlemier et al. 2010b.
a Samples where melamine was not detected were assigned a value equivalent to the limit of detection (LOD) of 4 µg/kg.
Dietary exposure assessment methodology and assumptions
Food consumption data were obtained from the 2004 Canadian Community Health Survey (CCHS) cycle 2.2 (Statistics Canada 2004). Measured and self-reported body weights collected through the CCHS were also used. The dietary intake assessment of melamine was conducted by Health Canada's Food Directorate. If consumption figures for certain foods were not available in the CCHS (milk coffee or tea, energy bar), closely related foods were used (instant coffee or tea, granola bars) and certain food types which were analyzed for melamine due to possible presence of milk protein, were used to represent concentrations in all these food types (i.e., all chicken, sausage, and cookie products). In both these cases, exposure may be overestimated due to the higher frequency of consumption of the surrogate foods or due to the presence of melamine in milk protein only. Similarly, the melamine concentrations detected in a limited number of processed spinach, potato, mushroom, onion, and tomato products were conservatively used to represent the melamine concentrations in all food products containing those vegetables, including the fresh vegetables. This is also expected to have overestimated the dietary exposure to melamine.
Appendix D. Exposure estimates of melamine from products
Based on the available information, dermal exposure uptakes were estimated for direct contact with foam-containing mattresses and related manufactured items for infants, toddlers, and adults. Oral exposure estimates were also derived for infants and toddlers from mouthing (sucking) on foam manufactured items intended for children. The exposure parameters and values used to estimate exposures are presented in Tables D-1 and D-2, and are based on conservative assumptions.
Dermal exposure uptake estimates
Uptake = [SA × SCF × TPF x M × ED × DA] / BW
Symbol |
Description |
Value |
|---|---|---|
SAa |
Surface area of skin contact |
545-1840 cm2 (Infant) 792-2890 cm2 (Toddler) 1258-4830 cm2 (Child) 1972-8100 cm2 (Teen) 2033-9100 cm2 (Adult) |
SCFb |
Skin contact factor |
1 |
TPFc |
Textile penetration factor |
0.1 |
| Md | Migration rate |
0.00936 to 0.0217 mg/cm2/hr |
EDe |
Exposure duration |
12 hr/d (Infant) 12 hr/d (Toddler) 10 hr/d (Child) 10 hr/d (Teen) 8 hr/d (Adult) |
DA |
Dermal absorption |
0.10 to 0.16f |
BWg |
Body weight |
7.5 kg (Infant) 15.5 kg (Toddler) 31.0 kg (Child) 59.4 kg (Teen) 70.9 kg (Adult) |
Uptake |
Melamine uptake (mg/kg bw/d)
|
0.082 – 1.02 (Infant) 0.057 – 0.78 (Toddler) 0.038 – 0.54 (Child) 0.031 – 0.47 (Teen) 0.022 – 0.36 (Adult) |
a For this scenario, a range in surface areas (SA) were used to represent dermal contact with a mattress. For the lower SA used, it is assumed that an individual is wearing shorts and a t-shirt that cover half of the limbs. The surface area of exposure is based on exposure to a fraction of the lower half of the limbs (arms and legs) and the back of the head. The surface areas of the limbs (Health Canada 1995) were multiplied by one half to account for clothing coverage and then were multiplied by one third to account for the triangular shape of limbs, where only one side is directly in contact with the mattress (US CPSC 2006). The surface area of the head (Health Canada 1995) was multiplied by a factor of 0.5 to represent exposure to the back of the head only. For the higher SA used, it was assumed that half of the body was in dermal contact with the mattress (US EPA 2012b).
b No melamine-specific skin contact factor (SCF), i.e., the fraction of substance on a surface adhering to skin, was identified in the literature. As such, a value of 1 was selected to assume that all of the chemical in contact with the skin is available for absorption.
c A textile penetration factor (TPF) was applied for melamine to account for the migration rates used for extrapolation (i.e., TDCPP and TCEP) being determined using uncovered foam (ECHA 2018). No melamine-specific textile penetration data were identified in the literature. As such, a value of 0.1 (Driver et al. 2007 as cited in ECHA 2018) was used for the TPF.
d Migration rate from foam to surface of upholstery (extrapolated from TCEP and TDCPP migration rates as shown in Table 8-4).
e Exposure duration for sleeping was adjusted from durations reported in the US CPSC (2006) for leisure sitting to account for longer sleeping durations relative to sitting, and adapted for average sleeping durations as reported in US EPA (2011).
f No dermal absorption data were identified for melamine. Structurally similar compounds show only maximum dermal absorptions of 10-16% in human skin (Ademola et al. 1993; Baynes et al. 2005).
g Health Canada (1998).
Oral exposure intake estimates
Intake = (SA × M × ED) / BW
Symbol |
Description |
Value |
|---|---|---|
SAa |
Surface area of direct mouthing |
10 cm2 (Infant) 20 cm2 (Toddler) |
Mb |
Migration rate |
0.00936 to 0.0217 mg/cm2/hr |
EDc |
Exposure duration |
24.5 min/d (0.408 h/d) |
BW |
Body weight |
7.5 kg (Infant) 15.5 kg (Toddler) |
Intake |
Intake calculated in mg/kg bw/d |
5.09 × 10-3 to 1.18 × 10-2 (Infant) 4.93 × 10-3 to 1.14 ×10-2 (Toddler) |
a Surface area for infants is based on multiple references (RIVM 2008). Surface area for toddlers is based on professional judgment reflecting twice the surface area of the opening of a toddler’s mouth.
b The migration rate range of 0.00936 to 0.0217 mg/cm2/hr as presented in the dermal scenario was also used to estimate oral exposure. It is assumed that melamine is completely absorbed through the oral route and that a textile covering on a foam object would not affect migration (i.e., no textile penetration factor, TPF, applied).
c The mouthing duration for children’s foam products such as nap mats, child restraint seats, small furniture was based on the duration for “other objects” in Norris and Smith (2002) cited in US EPA (2011).
d Health Canada (1998).
Appendix E. Range of typical daily urine volumes
Gender |
Age (years) |
Daily mean urine volumes (L/day) | Reference |
|---|---|---|---|
Males and females |
6 – 11 |
0.274 – 1.14 |
ICRP 2003; Lakind and Naiman 2008; Lentner 1981; Remer et al. 2006; Wu 2006 |
Males and females |
12 – 19 |
0.441 – 1.4 |
ICRP 2003; Lentner 1981; Wu 2006 |
Males and females |
20 – 59 |
0.6 – 2.03 |
Davison and Nobel 1981; Francis 1960; ICRP 2003; Lakind and Naiman 2008; Lentner 1981; Parboosingh and Doig 1973; Perucca et al. 2007; Revúsová 1971; Van Haarst et al. 2004; Wu 2006; |
Pregnant females |
- |
0.8 – 2.7 |
Davison and Nobel 1981; Francis 1960; Higby et al. 1994; Neithardt et al. 2002; Parboosingh and Doig 1973; Thorp et al. 1999; |
Males and females |
60 – 79 |
0.25 – 2.4 |
ICRP 2003; Lentner 1981; Wu 2006 |
Appendix F. International melamine studies
The studies by Kong et al. (2011) and Wu et al. (2010a) are not comparable to the Panuwet et al. (2012) study because different units were used (ng/mL vs µg/mmol of creatinine). The study by Liu et al. (2011) shows concentrations ranging from less than the method detection limit (MDL) to 192 ng/mL in a total of 422 adult subjects from Taiwan. This is comparable to the range of concentrations found in 492 subjects from the US population (< limit of detection– 161 ng/mL).
Wu et al. (2013) conducted a unique biomonitoring study, in which the urinary melamine concentration was measured in 12 Taiwanese adults, who had consumed hot noodle soup from both melamine and ceramic bowls at different periods. The total melamine excretion in urine was 8.35±1.91 µg 12 hr after consuming noodle soup from melamine bowls, and 1.31± 0.44 µg 12 hr after consuming noodle soup from ceramic bowls (urine volumes per person were not reported). Peak urinary excretion occurred at the > 4-6 hr period, and was approximately 6.8 µg/mmol creatinine (interpreted from figure A of article). With a reported half-life of 6 hrs urinary elimination of melamine, this translates to about 12.8 µg/mmol creatinine after 24 hrs. These values fall within the range of 0.02 to 20 µg/mmol creatinine of melamine in urine reported by Wu et al. 2010a for 22 adults in Taiwan shown in Table F-1. Wu et al. (2013) stated that one brand of melamine bowl was chosen for this study, and it was one of 5 brands tested in the Chien et al. (2011) study (see section 8.1.2 “Migration from Melaware”).
Region |
Date of study |
Number of subjects |
Age in years |
Melamine concentrations | Reference |
|---|---|---|---|---|---|
Hong Kong |
2009 |
502: 167 males, 335 females |
6-20 |
Median = 0.8 (ND to 1467) ND in 213 (42%) samples. 47 (9%) samples > 7.1 µg/mmol creatinine (14 males, 33 females). |
Kong et al. 2011 |
Taiwan |
2003-2007 |
22 controls: 20 males, 2 females |
47-61 |
Median = 0.06 (0.02 to 20) µg/mmol creatinine |
Wu et al. 2010a |
Taiwan |
2003-2007 |
11 with uric acid urolithiasis: 10 males, 1 female |
46-62 |
Median = 0.5 (0.07 to 1.18) µg/mmol creatinine |
Wu et al. 2010a |
Taiwan |
2003-2007 |
21 with calcium urolithiasis: 19 males, 2 females |
48-58 |
Median = 0.14 (0.07 to 0.93) µg/mmol creatinine |
Wu et al. 2010a |
Taiwan |
2003-2007 |
211 controls: 132 males, 79 females |
52.4 ± 11.9 (median = 52.0) |
< MDL [0.8] to 56 ng/mL MDL in 168 (80%) samples 23 samples at MDL to 3.11 ng/mL 20 samples ≥ 3.12 ng/mL |
Liu et al. 2011 |
Taiwan |
2003-2007 |
211 with calcium urolithiasis: 132 males, 79 females |
52.3 ± 12.0 (median = 53.0) |
< MDL [0.8] to 192 ng/mL MDL in 80 samples 64 samples at MDL to 3.11 ng/mL 67 samples ≥ 3.12 ng/mL |
Liu et al. 2011 |
Abbreviations: ND, Not detected; MDL, Method detection limit.
