Consultation on Amending the List of Species under the Species at Risk Act: Terrestrial Species – January 2016
- Introduction
- Addition of Species to the Species at Risk Act
- The Species at Risk Act Listing Process and Consultation
- Significance of the Addition of a Species to Schedule 1
- The List of Species Eligible for an Amendment to Schedule 1
- The COSEWIC Summaries of Terrestrial Species Eligible for Addition or Reclassification on Schedule 1
- Document Information
- Addition of Species to the Species at Risk Act
- The Species at Risk Act and the List of Wildlife Species at Risk
- COSEWIC and the assessment process for identifying species at risk
- Terms used to define the degree of risk to a species
- Terrestrial and aquatic species eligible for Schedule 1 amendments
- Comments solicited on the proposed amendment of Schedule 1
- Questions to guide your comments
- The Species at Risk Act Listing Process and Consultation
- The purpose of consultations on amendments to the List
- Legislative context of the consultations: the Minister’s recommendation to the Governor in Council
- Figure 1: The species listing process under SARA
- The Minister of the Environment’s response to the COSEWIC assessment: the response statement
- Normal and extended consultation periods
- Who is consulted, and how
- Role and impact of public consultations in the listing process
- Significance of the Addition of a Species to Schedule 1
- The List of Species Eligible for an Amendment to Schedule 1
- Status of the recently assessed species and consultation paths
- Providing comments
- Table 1: Terrestrial species recently assessed by COSEWIC eligible for addition to Schedule 1 or reclassification
- Table 2: Terrestrial species recently reassessed by COSEWIC (no consultations – species status confirmation)
- The COSEWIC Summaries of Terrestrial Species Eligible for Addition or Reclassification on Schedule 1
- Indexes
- Glossary
Please submit your comments by
May 4, 2016, for terrestrial species undergoing normal consultations
and by
October 4, 2016, for terrestrial species undergoing extended consultations.
For a description of the consultation paths these species will undergo, please visit the Species at Risk Public Registry (SAR) website.
Please email your comments to the Species at Risk Public Registry.
Comments may also be mailed to:
Director General
Canadian Wildlife Service
Environment and Climate Change Canada
Ottawa ON
K1A 0H3
For more information on the Species at Risk Act, please visit the Species at Risk Public Registry website.
The Government of Canada is committed to preventing the disappearance of wildlife species at risk from our lands. As part of its strategy for realizing that commitment, on June 5, 2003, the Government of Canada proclaimed the Species at Risk Act (SARA). Attached to the Act is Schedule 1, the list of the species provided for under SARA, also called the List of Wildlife Species at Risk. Extirpated, Endangered and Threatened species on Schedule 1 benefit from the protection of prohibitions and recovery planning requirements under SARA. Special Concern species benefit from its management planning requirements. Schedule 1 has grown from the original 233 to 521 wildlife species at risk.
The complete list of species currently on Schedule 1.
Species become eligible for addition to Schedule 1 once they have been assessed as being at risk by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC). The decision to add a species to Schedule 1 is made by the Governor in Council further to a recommendation from the Minister of the Environment and Climate Change (ECC). The Governor in Council is the formal executive body that gives legal effect to decisions that then have the force of law.
COSEWIC is recognized under SARA as the authority for assessing the status of wildlife species at risk. COSEWIC comprises experts on wildlife species at risk. Its members have backgrounds in the fields of biology, ecology, genetics, Aboriginal traditional knowledge and other relevant fields. They come from various communities, including academia, Aboriginal organizations, governments and non-governmental organizations.
COSEWIC gives priority to those species more likely to become extinct, and then commissions a status report for the evaluation of the species’ status. To be accepted, status reports must be peer-reviewed and approved by a subcommittee of species specialists. In special circumstances, assessments can be done on an emergency basis. When the status report is complete, COSEWIC meets to examine it and discuss the species. COSEWIC then determines whether the species is at risk, and, if so, it then assesses the level of risk and assigns a conservation status.
The conservation status defines the degree of risk to a species. The terms used under SARA are Extirpated, Endangered, Threatened and Special Concern. Extirpated species are wildlife species that no longer occur in the wild in Canada but still exist elsewhere. Endangered species are wildlife species that are likely to soon become extirpated or extinct. Threatened species are likely to become endangered if nothing is done to reverse the factors leading to their extirpation or extinction. The term Special Concern is used for wildlife species that may become threatened or endangered due to a combination of biological characteristics and threats. Once COSEWIC has assessed a species as Extirpated, Endangered, Threatened or Special Concern, it is eligible for inclusion on Schedule 1.
For more information on COSEWIC, visit the COSEWIC website.
On October 6, 2015, COSEWIC sent to the Minister of the ECC its newest assessments of species at risk. Environment and Climate Change Canada (ECCC) is now consulting on changes to Schedule 1 to reflect these new designations for these terrestrial species. To see the list of the terrestrial species and their status, please refer to tables 1 and 2.
The Minister of Fisheries and Oceans conducts separate consultations for the aquatic species. For more information on the consultations for aquatic species, visit the Fisheries and Oceans Canada website.
The Minister of the ECC is conducting the consultations for all other species at risk.
Approximately 57% of the recently assessed terrestrial species at risk also occur in national parks or other lands administered by Parks Canada; Parks Canada shares responsibility for these species with ECCC.
The conservation of wildlife is a joint legal responsibility: one that is shared among the governments of Canada. But biodiversity will not be conserved by governments that act alone. The best way to secure the survival of species at risk and their habitats is through the active participation of all those concerned. SARA recognizes this, and that all Aboriginal peoples and Canadians have a role to play in preventing the disappearance of wildlife species from our lands. The Government of Canada is inviting and encouraging you to become involved. One way that you can do so is by sharing your comments concerning the addition or reclassification of these terrestrial species.
Your comments are considered in relation to the potential consequences of whether or not a species is included on Schedule 1, and they are then used to inform the drafting of the Minister’s proposed listing recommendations for each of these species.
The following questions are intended to assist you in providing comments on the proposed amendments to the List of Wildlife Species at Risk (see Table 1 for the list of species under consultation). They are not limiting, and any other comments you may have are welcome. We also encourage you to share descriptions and estimates of costs or benefits to you or your organization where possible, as well as to propose actions that could be taken for the conservation of these species.
Respondent information
Are you responding as an individual or representing a community, business or organization (please specify)?
Species benefit to people or the ecosystem
Do any or all of the species provide benefits to you or Canada’s ecosystems? If yes, explain how. What is the estimated value of these benefits? Values do not need to be monetary.
For example:
- Do any or all of the species provide benefits by supporting your livelihood, for example, through harvesting, subsistence or medicine?
- Do any or all of the species provide cultural or spiritual benefits, for example, recreation, sense of place or tradition? If yes, how?
- Do any or all of the species provide environmental benefits, for example, pollination, pest control or flood control? If yes, how?
Impact of your activities and mitigation
- Do any or all of the species provide benefits by supporting your livelihood, for example, through harvesting, subsistence or medicine?
- Do any or all of the species provide cultural or spiritual benefits, for example, recreation, sense of place or tradition? If yes, how?
- Do any or all of the species provide environmental benefits, for example, pollination, pest control or flood control? If yes, how?
Impacts of amending the List of Wildlife Species at Risk
Based on what you know about SARA and the information presented in this document, do you think that amending the List of Wildlife Species at Risk with the proposed listing (Table 1) would have no impact, a positive impact or a negative impact on your activities or the species? Please provide as much detail as possible.
For example:
- If any of your activities impact a species or its residence, would you have to avoid or adjust these activities to mitigate their impact? What would be the implications of such avoidance or mitigation?
- Do you think that listing the species would have cultural or social cost or benefits to you, your community or your organization?
- Do you think that listing the species would have economic costs or benefits to you, your community or your organization?
- Do you think that listing the species would have costs or benefits to the environment or Canada's ecosystems?
Additional information for small businesses
If you are responding for a small business, please provide the following details to help ECCC gather information to contribute to the required Small Business Lens analysis that forms part of the Regulatory Impact Analysis Statement that will accompany any future listing recommendation.
- Are you an enterprise that operates in Canada?
- Do you engage in commercial activities related to the supply of services or property (which includes goods)?
- Are you an organization that engages in activities for a public purpose (e.g., social welfare or civic improvement), such as a provincial or municipal government, school, college/university, hospital or charity?
- Is your enterprise owned by a First Nations community?
- How many employees do you have?
- 0–99
- 100 or more
- What was your annual gross revenue in the last year?
- Less than $30,000
- Between $30,000 and $5 million
- More than $5 million
To ensure that your comments are considered in time, they should be submitted before the following deadlines.
For terrestrial species undergoing normal consultations, comments should be submitted by May 4, 2016.
For terrestrial species undergoing extended consultations, comments should be submitted by October 4, 2016.
To find out which consultation paths these species will undergo (extended or normal), please visit the Species at Risk Public Registry.
Comments received by these deadlines will be considered in the development of the listing proposal.
Please email your comments to the Species at Risk Public Registry.
By regular mail, please address your comments to:
Director General
Canadian Wildlife Service
Environment and Climate Change Canada
Ottawa ON
K1A 0H3
The addition of a wildlife species at risk to Schedule 1 of Species at Risk Act (SARA) facilitates providing for its protection and conservation. To be effective, the listing process must be transparent and open. The species listing process under SARA is summarized in Figure 1.
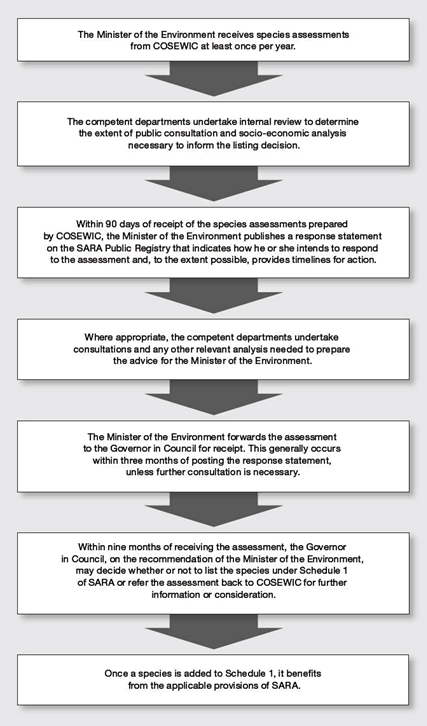
Long description for Figure 1
This figure explains the various steps of the listing process under the SARA. It is a flowchart. Its content is the following:
- The Minister of the Environment and Climate Change (ECC) receives species assessments from COSEWIC at least once a year.
- The competent departments undertake internal review to determine the extent of public consultation and socio-economic analysis necessary to inform the listing decision.
- Within 90 days of receipt of the species assessments prepared by COSEWIC, the Minister of the ECC publishes a response statement on the SARA Public Registry that indicates how he or she intends to respond to the assessment and, to the extent possible, provides timelines for action.
- Where appropriate, the competent departments undertake consultations and any other relevant analysis needed to prepare the advice for the Minister of the ECC.
- The Minister of the ECC forwards the assessment to the Governor in Council for receipt. This generally occurs within three months of posting the response statement, unless further consultation is necessary
- Within nine months of receiving the assessment, the Governor in Council, on the recommendation of the Minister of the ECC, may decide whether or not to list the species under Schedule 1 of SARA or refer the assessment back to COSEWIC for further information or consideration.
- Once a species is added to Schedule 1, it benefits from the applicable provisions of SARA.
When COSEWIC assesses a wildlife species, it does so solely on the basis of the best available information relevant to the biological status of the species. COSEWIC then submits the assessment to the Minister of the ECC, who considers it when making the listing recommendation to the Governor in Council. The purpose of these consultations is to provide the Minister with a better understanding of the potential social and economic impacts of the proposed change to the List of Wildlife Species at Risk, and of the potential consequences of not adding a species to the List.
The comments collected during the consultations inform the Governor in Council’s consideration of the Minister’s recommendations for listing species at risk. The Minister must recommend one of three courses of action. These are for the Governor in Council to accept the species assessment and modify Schedule 1 accordingly, not to add the species to Schedule 1, or to refer the species assessment back to COSEWIC for its further consideration (Figure 1).
After COSEWIC has completed its assessment of a species, it provides it to the Minister of the ECC. The Minister of the ECC then has 90 days to post a response on the Species at Risk Public Registry, known as the response statement. The response statement provides information on the scope of any consultations and the timelines for action, to the extent possible. It identifies how long the consultations will be (whether they are “normal” or “extended”) by stating when the Minister will forward the assessment to the Governor in Council. Consultations for a group of species are launched with the posting of their response statements.
Normal consultations meet the consultation needs for the listing of most species at risk. They usually take two to three months to complete, while extended consultations may take one year or more.
The extent of consultations needs to be proportional to the expected impact of a listing decision and the time that may be needed to consult. Under some circumstances, whether or not a species will be included on Schedule 1 could have significant and widespread impacts on the activities of some groups of people. It is essential that such stakeholders have the opportunity to inform the pending decision and, to the extent possible, to provide input on its potential consequences and to share ideas on how best to approach threats to the species. A longer period may also be required to consult appropriately with some groups. For example, consultations can take longer for groups that meet infrequently but that must be engaged on several occasions. For such reasons, extended consultations may be undertaken.
For both normal and extended consultations, once they are complete, the Minister of the ECC forwards the species assessments to the Governor in Council for the government’s formal receipt of the assessment. The Governor in Council then has nine months to come to a listing decision.
The consultation paths (normal or extended) for the terrestrial species listed in Table 1 will be announced when the Minister publishes the response statements. These will be posted by January 4, 2016, on the Species at Risk Public Registry.
No consultations will be undertaken for those species already on Schedule 1 and for which no change in status is being proposed (Table 2).
It is most important to consult with those who would be most affected by the proposed changes. There is protection that is immediately in place when a species that is Extirpated, Endangered or Threatened is added to Schedule 1 (for more details, see below, “Protection for listed Extirpated, Endangered and Threatened species”). This immediate protection does not apply to species of Special Concern. The nature of protection depends on the type of species, its conservation status, and where the species is found. Environment and Climate Change Canada (ECCC) takes this into account during the consultations; those who may be affected by the impacts of the automatic protections are contacted directly, others are encouraged to contribute through a variety of approaches.
Aboriginal peoples known to have species at risk on their lands, for which changes to Schedule 1 are being considered, will be contacted. Their engagement is of particular significance, acknowledging their role in the management of the extensive traditional territories and the reserve and settlement lands.
A Wildlife Management Board is a group that has been established under a land claims agreement and is authorized by the agreement to perform functions in respect of wildlife species. Some eligible species at risk are found on lands where existing land claims agreements apply that give specific authority to a Wildlife Management Board. In such cases, the Minister of the ECC will consult with the relevant board.
To encourage others to contribute and make the necessary information readily available, this document is distributed to known stakeholders and posted on the Species at Risk Public Registry. More extensive consultations may also be done through regional or community meetings or through a more targeted approach.
ECCC also sends notice of this consultation to identified concerned groups and individuals who have made their interests known. These include, but are not limited to, industries, resource users, landowners and environmental non-governmental organizations.
In most cases, it is difficult for ECCC to fully examine the potential impacts of recovery actions when species are being considered for listing. Recovery actions for terrestrial species usually have not yet been comprehensively defined at the time of listing, so their impact cannot be fully understood. Once they are better understood, efforts are made to minimize adverse social and economic impacts of listing and to maximize the benefits. SARA requires that recovery measures be prepared in consultation with those considered to be directly affected by them.
In addition to the public, ECCC consults on listing with the governments of the provinces and territories with lead responsibility for the conservation and management of these wildlife species. ECCC also consults with other federal departments and agencies.
The results of the public consultations are of great significance to informing the process of listing species at risk. ECCC carefully reviews the comments it receives to gain a better understanding of the benefits and costs of changing the List.
The comments are then used to inform the Regulatory Impact Analysis Statement (RIAS). The RIAS is a report that summarizes the impact of a proposed regulatory change. It includes a description of the proposed change and an analysis of its expected impact, which takes into account the results of the public consultations. In developing the RIAS, the Government of Canada recognizes that Canada’s natural heritage is an integral part of our national identity and history and that wildlife in all its forms has value in and of itself. The Government of Canada also recognizes that the absence of full scientific certainty is not a reason to postpone decisions to protect the environment.
A draft Order (see Glossary) is then prepared, providing notice that a decision is being taken by the Governor in Council. The draft Order proposing to list all or some of the species under consideration is then published, along with the RIAS, in the Canada Gazette, Part I, for a comment period of 30 days.
The Minister of the ECC will take into consideration comments and any additional information received following publication of the draft Order and the RIAS in the Canada Gazette, Part I. The Minister then makes a final listing recommendation for each species to the Governor in Council. The Governor in Council next decides either to accept the species assessment and amend Schedule 1 accordingly; or not to add the species to Schedule 1; or to refer the species assessment back to COSEWIC for further information or consideration. The final decision is published in the Canada Gazette, Part II, and on the Species at Risk Public Registry. If the Governor in Council decides to list a species, it is at this point that it becomes legally included on Schedule 1.
The protection that comes into effect following the addition of a species to Schedule 1 depends upon a number of factors. These include the species’ status under Species at Risk Act (SARA), the type of species and where it occurs.
Responsibility for the conservation of wildlife is shared among the governments of Canada. SARA establishes legal protection for individuals as soon as a species is listed as Threatened, Endangered or Extirpated, and, in the case of Threatened and Endangered species, for their residences. This applies to species considered federal species or if they are found on federal land.
Federal species include migratory birds, as defined by the Migratory Birds Convention Act, 1994, and aquatic species covered by the Fisheries Act. Federal land means land that belongs to the federal government, and the internal waters and territorial sea of Canada. It also means land set apart for the use and benefit of a band under the Indian Act (such as reserves). In the territories, the protection for species at risk on federal lands applies only where they are on lands under the authority of the Minister of the Environment and Climate Change (ECC) or the Parks Canada Agency.
Migratory birds are protected by the Migratory Birds Regulations, under the Migratory Birds Convention Act, 1994, which strictly prohibits the harming of migratory birds and the disturbance or destruction of their nests and eggs.
SARA’s protection for individuals makes it an offence to kill, harm, harass, capture or take an individual of a species listed as Extirpated, Endangered or Threatened. It is also an offence to damage or destroy the residence of one or more individuals of an Endangered or Threatened species or an Extirpated species whose reintroduction has been recommended by a recovery strategy. The Act also makes it an offence to possess, collect, buy, sell or trade an individual of a species that is Extirpated, Endangered or Threatened.
Species at risk that are neither aquatic nor protected under the Migratory Birds Convention Act, 1994, nor on federal lands, do not receive immediate protection upon listing under SARA. Instead, in most cases, the protection of terrestrial species on non-federal lands is the responsibility of the provinces and territories where they are found. The application of protections under SARA to a species at risk on non-federal lands requires that the Governor in Council make an order defining those lands. This can only occur when the Minister is of the opinion that the laws of the province or territory do not effectively protect the species. To put such an order in place, the Minister would then need to recommend the order be made to the Governor in Council. If the Governor in Council agrees to make the order, the prohibitions of SARA would then apply to the provincial or territorial lands specified by the order. The federal government would consult before making such an order.
Recovery planning results in the development of recovery strategies and action plans for Extirpated, Endangered or Threatened species. It involves the different levels of government responsible for the management of the species, depending on what type of species it is and where it occurs. These include federal, provincial and territorial governments as well as Wildlife Management Boards. Recovery strategies and action plans are also prepared in cooperation with directly affected Aboriginal organizations. Landowners and other stakeholders directly affected by the recovery strategy are consulted to the extent possible.
Recovery strategies must be prepared for all Extirpated, Endangered and Threatened species. They include measures to mitigate the known threats to the species and its habitat and set the population and distribution objectives. Other objectives can be included, such as stewardship, to conserve the species, or education, to increase public awareness. Recovery strategies must include a statement of the time frame for the development of one or more action plans that will state the measures necessary to implement the recovery strategy. To the extent possible, recovery strategies must also identify the critical habitat of the species, which is the habitat necessary for the survival or recovery of the species. If there is not enough information available to identify critical habitat, the recovery strategy includes a schedule of studies required for its identification. This schedule outlines what must be done to obtain the necessary information and by when it needs to be done. In such cases, critical habitat can be identified in a subsequent action plan.
Proposed recovery strategies for newly listed species are posted on the Species at Risk Public Registry to provide for public review and comment. For Endangered species, proposed recovery strategies are posted within one year of their addition to Schedule 1, and for Threatened or Extirpated species, within two years.
Once a recovery strategy has been posted as final, one or more action plans based on the recovery strategy must then be prepared. These include measures to address threats and achieve the population and distribution objectives. Action plans also complete the identification of the critical habitat where necessary and, to the extent possible, state measures that are proposed to protect it.
For terrestrial species listed on SARA Schedule 1 as Extirpated, Endangered or Threatened, the Minister of the ECC may authorize exceptions to the Act’s prohibitions, when and where they apply. The Minister can enter into agreements or issue permits only for one of three purposes: for research, for conservation activities, or if the effects to the species are incidental to the activity. Research must relate to the conservation of a species and be conducted by qualified scientists. Conservation activities must benefit a listed species or be required to enhance its chances of survival. All activities, including those that incidentally affect a listed species, its individuals, residences or critical habitat must also meet certain conditions. First, it must be established that all reasonable alternatives to the activity have been considered and the best solution has been adopted. Second, it must also be established that all feasible measures will be taken to minimize the impact of the activity on the listed species. And finally, it must be established that the activity will not jeopardize the survival or recovery of the species. Having issued a permit or agreement, the Minister must then include an explanation on the Species at Risk Public Registry of why the permit or agreement was issued.
While immediate protection under SARA for species listed as Extirpated, Endangered and Threatened does not apply to species listed as Special Concern, any existing protections and prohibitions, such as those provided by the Migratory Birds Convention Act, 1994 or the Canada National Parks Act, continue to be in force.
For species of Special Concern, management plans are to be prepared and made available on the Species at Risk Public Registry within three years of a species’ addition to Schedule 1, allowing for public review and comment. Management plans include appropriate conservation measures for the species and for its habitat. They are prepared in cooperation with the jurisdictions responsible for the management of the species, including directly affected Wildlife Management Boards and Aboriginal organizations. Landowners, lessees and others directly affected by a management plan will also be consulted to the extent possible.
On October 6, 2015, COSEWIC submitted 22 assessments of species at risk to the Minister of the Environment and Climate Change (ECC) for species that are eligible to be added to Schedule 1 of Species at Risk Act (SARA). Nineteen of these are terrestrial species, and 3 are aquatic species. COSEWIC also reviewed the classification of species already on Schedule 1, in some cases changing their status. Two terrestrial species are now being considered for down-listing on SARA (to a lower risk status) and 4 terrestrial species are now being considered for up-listing on SARA (to a higher risk status). In all, 25 terrestrial species that are eligible to be added to Schedule 1 or to have their current status on Schedule 1 changed are included in this consultation (Table 1).
One of these terrestrial species, the Spiked Saxifrage, was originally assessed by COSEWIC as Threatened in May 2013. However, COSEWIC advised the Minister that it must reassess this species, due to new information that was not available at the time of the assessment. This was communicated to Environment and Climate Change Canada (ECCC) when the December 2013 consultation document was already in production and, as a consequence, the Spiked Saxifrage was included in the document, but no consultations were held. COSEWIC reassessed the Spiked Saxifrage in May 2015 as Special Concern, and the species is included in the current consultation document as a terrestrial species eligible for an addition to Schedule 1 of SARA.
COSEWIC also submitted the reviews of species already on Schedule 1, confirming their classification. Twenty of these reviews were for terrestrial species. These species are not included in the consultations because there is no regulatory change being proposed (Table 2).
For more information on the consultations for aquatic species, visit the Fisheries and Oceans Canada website at www.dfo-mpo.gc.ca.
The involvement of Canadians is integral to the listing process, as it is to the ultimate protection of Canadian wildlife. Your comments matter and are given serious consideration. ECCC will review all the comments that it receives by the deadlines provided below.
Comments for terrestrial species undergoing normal consultations must be received by May 4, 2016.
Comments for terrestrial species undergoing extended consultations must be received by October 4, 2016.
Most species will be undergoing normal consultations. For the final consultation paths, please visit the Species at Risk Public Registry.
after January 4, 2016.
For more details on submitting comments, see the section “Comments solicited on the proposed amendment of Schedule 1” of this document.
| Taxon | Species | Scientific Name | Range |
|---|---|---|---|
| Reptiles | Eastern Box Turtle | Terrapene carolina | ON |
| Taxon | Species | Scientific Name | Range |
|---|---|---|---|
| Vascular Plants | Limber Pine | Pinus flexilis | BC AB |
| Vascular Plants | Tall Beakrush | Rhynchospora macrostachya | NS |
| Vascular Plants | Fascicled Ironweed | Vernonia fasciculata | MB |
| Molluscs | Broad-banded Forestsnail | Allogona profunda | ON |
| Molluscs | Proud Globelet | Patera pennsylvanica | ON |
| Birds | Black Swift | Cypseloides niger | BC AB |
| Taxon | Species | Scientific Name | Range |
|---|---|---|---|
| Lichens | Black-foam Lichen | Anzia colpodes | ON QC NB NS |
| Vascular Plants | Griscom’s Arnica | Arnica griscomii ssp. griscomii | QC NL |
| Arthropods | Sable Island Sweat Bee | Lasioglossum sablense | NS |
| Taxon | Species | Scientific Name | Range |
|---|---|---|---|
| Mosses | Tiny Tassel | Crossidium seriatum | BC |
| Vascular Plants | Spiked SaxifrageFootnotea | Micranthes spicata | YT |
| Vascular Plants | Yukon Podistera | Podistera yukonensis | YT |
| Arthropods | Vivid Dancer | Argia vivida | BC AB |
| Arthropods | Yellow-banded Bumble Bee | Bombus terricola | YT NT BC AB SK MB ON QC NB PE NS NL |
| Birds | Cassin's Auklet | Ptychoramphus aleuticus | BC Pacific Ocean |
| Birds | Red-necked Phalarope | Phalaropus lobatus | YT NT NU BC AB SK MB ON QC NB PE NS NL Pacific Ocean Arctic Ocean Atlantic Ocean |
| Reptiles | Prairie Rattlesnake | Crotalus viridis | AB SK |
| Mammals | Caribou (Newfoundland population) | Rangifer tarandus | NL |
| Taxon | Species | Scientific Name | Range |
|---|---|---|---|
| Vascular Plants | Phantom Orchid | Cephalanthera austiniae | BC |
| Arthropods | Poweshiek Skipperling | Oarisma poweshiek | MB |
| Taxon | Species | Scientific Name | Range |
|---|---|---|---|
| Vascular Plants | Blue Ash | Fraxinus quadrangulata | ON |
| Mammal | Eastern Wolf | Canis sp. cf. lycaon | ON QC |
| Taxon | Species | Scientific Name | Range |
|---|---|---|---|
| Vascular Plants | Small White Lady's-slipper | Cypripedium candidum | MB ON |
| Vascular Plants | Toothcup (Great Lakes Plains population)Footnoteb | Rotala ramosior | ON |
| Taxon | Species | Scientific Name | Range |
|---|---|---|---|
| Lichens | Boreal Felt Lichen (Atlantic population) | Erioderma pedicellatum | NB NS |
| Vascular Plants | Red Mulberry | Morus rubra | ON |
| Vascular Plants | Toothcup (Southern Mountain population)Footnotec | Rotala ramosior | BC |
| Arthropods | White Flower Moth | Schinia bimatris | MB |
| Arthropods | Ottoe Skipper | Hesperia ottoe | MB |
| Reptiles | Spotted Turtle | Clemmys guttata | ON QC |
| Mammals | Townsend's Mole | Scapanus townsendii | BC |
| Mammals | Caribou (Atlantic-Gaspésie population) | Rangifer tarandus | QC |
| Taxon | Species | Scientific Name | Range |
|---|---|---|---|
| Reptiles | Western Rattlesnake | Crotalus oreganus | BC |
| Mammals | Caribou (Boreal population) | Rangifer tarandus | YT NT BC AB SK MB ON QC NL |
| Mammals | Ermine haidarum subspecies | Mustela erminea haidarum | BC |
| Taxon | Species | Scientific Name | Range |
|---|---|---|---|
| Lichens | Boreal Felt Lichen (Boreal population) | Erioderma pedicellatum | NL |
| Lichens | Frosted Glass-whiskers (Atlantic population) | Sclerophora peronella | NS |
| Mosses | Banded Cord-moss | Entosthodon fascicularis | BC |
| Mosses | Columbian Carpet Moss | Bryoerythrophyllum columbianum | BC |
| Mosses | Twisted Oak Moss | Syntrichia laevipila | BC |
| Amphibians | Northern Red-legged Frog | Rana aurora | BC |
| Reptiles | Western Skink | Plestiodon skiltonianus | BC |
| Birds | Ancient Murrelet | Synthliboramphus antiquus | BC Pacific Ocean |
| Mammals | Spotted Bat | Euderma maculatum | BC |
The following section presents a brief summary of the reasons for the COSEWIC status designation of individual species, and their biology, threats, distribution and other information. For a more comprehensive explanation of the conservation status of an individual species, please refer to the COSEWIC status report for that species, also available on the Species at Risk Public Registry.
or contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment and Climate Change Canada
Ottawa ON
K1A 0H3

Photo: © Terry Gray
Long description for Figure 1
Long Description: Photo of a Black Swift (Cypseloides niger) on its nest. The plumage is almost entirely black.
Reason for designation
Canada is home to about 80% of the North American population of this bird species. It nests in cliff-side habitats (often associated with waterfalls) in British Columbia and western Alberta. Like many other birds that specialize on a diet of flying insects, this species has experienced a large population decline over recent decades. The causes of the decline are not well understood, but are believed to be related to changes in food supply that may be occurring at one or more points in its life cycle. The magnitude and geographic extent of the decline are causes for conservation concern.
Wildlife Species Description and Significance
The Black Swift is the largest swift in North America. Canada is home to over 80% of the population. It has an almost entirely blackish plumage, has long, pointed wings and is the only North American swift with a notched tail. As well as having many unusual life history traits compared to other landbird species (single egg clutch, extended maturation, remote waterfall and cave-nesting sites), the Black Swift may be a sensitive indicator for climate change. This is because its waterfall nesting sites are likely to be impacted by decreased snow pack and glacial melt. The Black Swift feeds exclusively on flying insects.
Distribution
The global breeding range of the subspecies that occurs in Canada shows a disjunct distribution: a northern range (from southeastern Alaska, northwestern British Columbia and southwestern Alberta, south through northwestern Montana, northern Idaho, and northern Washington), and scattered populations south of this (in Oregon, California, Utah, Colorado, northern New Mexico, southeastern Arizona). This subspecies also breeds in Mexico as far south as Oaxaca and Veracruz and possibly other areas in Mexico. Other subspecies occur elsewhere in Mexico, the Caribbean and Central America.
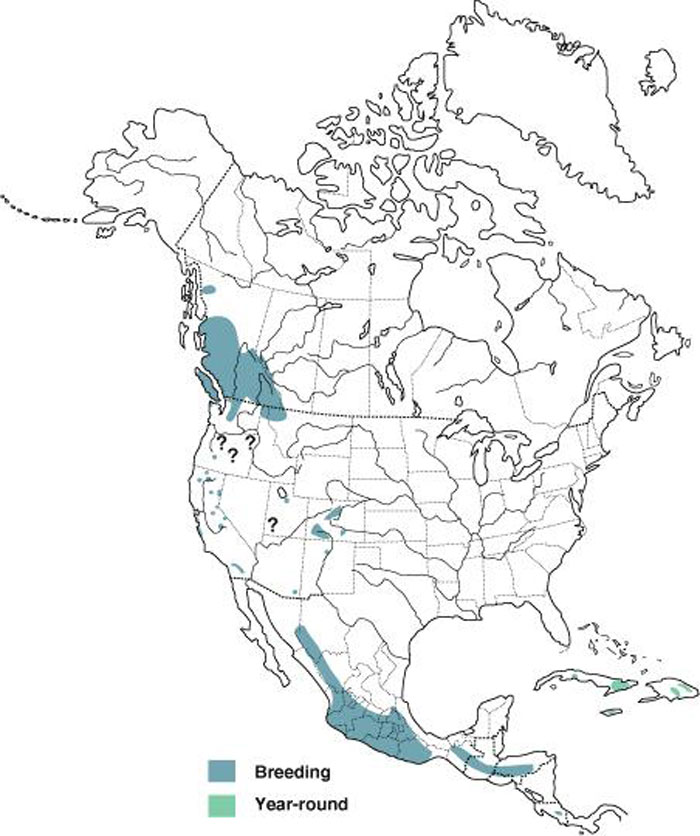
Source: Map courtesy of “Birds of North America Online", maintained by the Cornell Lab of Ornithology, Ithaca, NY.
Long description for Map 1
Map illustrating the breeding and non-breeding range of the Black Swift in North America, Central America, and the Caribbean. The map shows a disjunct distribution, with a large breeding range in British Columbia and Alberta (extending into adjacent U.S. states), scattered subpopulations south of this, and then a continuous large range in Mexico, and through Central America, with scattered permanent localized resident populations in the West Indies.
Habitat
Often foraging at high altitude, Black Swifts fly over open country and forests in mountainous areas and lowlands, pursuing aerial insects. They nest near or behind waterfalls and in caves, located in canyons and sometimes on sea cliffs. Their nest sites are characterized by presence of flowing water, high relief, inaccessibility, darkness, and an unobstructed flight path.
Biology
Little is known about the biology of the Black Swift. The species is believed to be monogamous and long-lived. The oldest known individual was 16 years old. Age at first breeding is unknown but, given other life history characteristics, may be from 3-5 years. It is one of only two landbirds in Canada to lay a single egg clutch, and has an extremely long fledgling period (7 weeks). Canadian birds migrate south, likely to spend the winter in South America. However, the precise winter range of Canadian birds is unknown.
Population Sizes and Trends
The population size of the borealis subspecies in Canada is hard to determine, but is estimated at 15,000 to 60,000 mature individuals. Canada is believed to harbour about 81% of the North American population, the vast majority of which occurs in British Columbia. Less than 0.1% of the North American population occurs in Alberta.
Across their range in Canada and the United States, Black Swifts are showing negative population trends. The Canadian population appears to have declined by more than 50% over the 40-year period between 1973 and 2012. A generation time ranging between 6.25 and 16.5 years yields a cumulative population loss of -72% to -96% over three generations, with expert opinion suggesting that the value is most likely around -89% (average annual trend of -6.5% over 33 years). The rate of decline has lessened in recent years; the 10-year short-term trend (2002-2012) estimate was -4.6% per year, which is equivalent to an overall decline of about 38% over the most recent decade. During this period, there was a 25% probability that the population declined by >50%, and a 45% probability that it declined by 25-50%.
Threats and Limiting Factors
The most important threats to the Black Swift are largely unknown but are believed to
be: 1) airborne pollutants that reduce aerial insect food availability and/or potentially cause reproductive failure in swifts; and 2) climate change that could reduce stream flow at nest sites or lead to temporal mismatches between aerial arthropod phenology and the swift’s breeding cycle. Other threats such as problematic native species, logging, annual and perennial non-timber crops, livestock farming and ranching, hydroelectric dams and water management, and recreational activities were considered as being negligible.
Protection, Status, and Ranks
The Black Swift is considered a continental Watch List species by Partners in Flight and is listed as Special Concern by many bird conservation region and state bird conservation plans. IUCN lists the species as Least Concern and it is a bird of conservation concern in the United States. According to NatureServe, it is considered apparently secure globally and apparently nationally secure in Canada and the United States, but these assessments are dated. It is listed as critically imperilled, imperilled or vulnerable in some states, but apparently secure in British Columbia and unranked in Alberta.

Photo: © David Richardson
Long description for Figure 2
Photo of a patch of the Black-foam lichen (Anzia colpodes), a foliose lichen with thalli that form greenish-grey rosettes. The solid thallus lobes rest on a dense, thick spongy cushion of black fungal filaments (the hypothallus). Stout but sparse rhizines grow from the lower cortex to the substrate, through the spongy hypothallus.
Reason for designation
In Canada, this lichen is at the northern edge of its range, and is known from Ontario, Québec, New Brunswick and Nova Scotia. It appears to be extirpated from Ontario and Quebec and has not been seen in New Brunswick for about a decade. It occurs on sites dominated by mature deciduous trees with high humidity and moderate light. In Nova Scotia, this lichen is widespread but not common. The reasons for its decline are not clear. The main current threat is deforestation. Additional threats may include grazing by molluscs and climate change.
Wildlife Species Description and Significance
The Black-foam Lichen, Anzia colpodes, is a leafy lichen that grows as greenish grey rosettes up to 20 cm across on the trunks of deciduous trees. The 1-2 mm wide solid lobes rest on a thick spongy black tissue made of fungal filaments. The reddish-brown fruit bodies on the upper surface contain sacks that are unusual in containing a large number of tiny spores that provide its only means of reproduction.
Distribution
The Black-foam Lichen is thought to be endemic to North America, although there is one report of its being found in eastern Russia. In the USA, it has been collected in the Appalachian Mountains from Georgia to Maine, but also on the Ozark Plateau and in Illinois, Wisconsin and Michigan. In Canada, this lichen is growing at the northern end of its distribution range and has been found in Ontario, Québec, New Brunswick and Nova Scotia. Recent surveys indicate that the Black-foam Lichen no longer occurs in the first two of these provinces and has not been recorded in New Brunswick in the last decade. This lichen is widespread but not common in Nova Scotia.
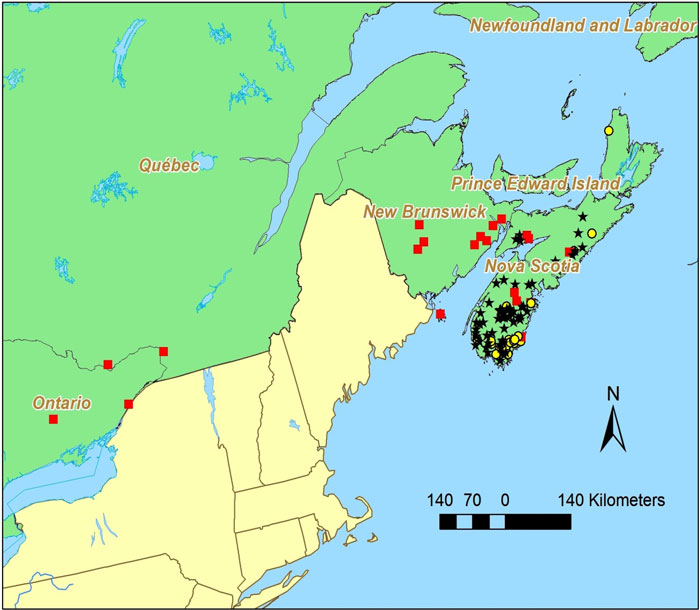
Source: COSEWIC 2015. COSEWIC Assessment and Status Report on the Black-foam Lichen in Canada.
Long description for Map 2
Map of the distribution of Anzia colpodes in Canada, where the species is known historically from four provinces: Ontario, Quebec, New Brunswick, and Nova Scotia. The map indicates occurrences currently known to be extant from fieldwork carried out for this report, occurrences that were found before 1995 and were not revisited, and revisited occurrences where the lichen was absent.
Habitat
The Black-foam Lichen grows on the trunks of mature deciduous trees growing on level or sloped land where high humidity is supplied by nearby wetlands, lakes or streams. The most common host is Red Maple but it also occurs on White Ash, Sugar Maple, Red Oak and very occasionally on other species.
Biology
Fruit bodies are frequent on the Black-foam Lichen and provide the only means of reproduction. The spores ejected from the fruit bodies need to land on a host tree trunk and encounter a compatible green alga. The algae become enveloped by fungal strands and eventually these grow into visible lichen. The generation time for this lichen is probably around 17 years. Unlike many other leafy lichens which grow on tree trunks, the Black-foam Lichen has no specialized vegetative propagules to provide a means of asexual reproduction.
Population Sizes and Trends
The Black-foam Lichen seems always to have been less common in Ontario and Québec and in the adjacent US states than in New Brunswick and Nova Scotia. In the first two provinces there are only four records for this lichen; all the sites were revisited, but it was not found. In New Brunswick there are 12 records for this lichen, and it was not found again during searches at six of these sites done in 2013.
In Nova Scotia, the Black-foam Lichen is not common, but it is widespread. Thirty-five occurrences have been documented in the province since 1995. The population was enumerated at the nine occurrences where the Black-foam Lichen was found during the fieldwork for this status report. On the basis of the enumeration, it is estimated that the total population of this lichen in Canada could be as high as 3,700 individuals, with almost all being in Nova Scotia. In addition, the lichen was no longer present at three of the seven post-2006 revisited occurrences, indicating a ~40% decline over the last ten years.
Threats and Limiting Factors
In Ontario and Québec, the main threat to the Black-foam Lichen appears to be habitat disturbance. The few sites where it was recorded historically have been subject to the spread of suburbia, building sites, highways and trails that have removed the forest where this lichen was once found. Other likely threats in these provinces are air pollution and changing weather patterns. The cause of the disappearance of the Blackfoam Lichen from these provinces is uncertain, but it is significant that declines have also been observed in adjacent states of the USA.
In New Brunswick and Nova Scotia, the main current threat is harvesting of older hardwood forests. The grazing impact of introduced molluscs is another threat with an unknown impact. The Black-foam Lichen with its low content of secondary substances lacks anti-herbivory effectiveness. Changing weather patterns are thought to have enhanced the spread and impact of grazing molluscs and may have affected the ability of this lichen to reproduce. Its tiny spores have little stored energy to provide the fungal germ tube with the means to search extensively for a compatible algal partner, a process required at every generation. Furthermore, its stout but sparse holdfasts which fasten small thalli firmly to the tree bark loosen as the lichen grows, making it vulnerable to removal by wind, rain or animals.
Over the longer term, climate change and alterations of weather pattern are predicted to result in reduced precipitation or enhanced evaporation. These are likely to affect the survival of the Black-foam Lichen as this species requires the right combination of climate and forest stand features. It is largely limited to growing on trees close to water bodies that include swamps, swamp margins, lakes and streams.
Protection, Status, and Ranks
The Black-foam Lichen is listed by NatureServe Global Status as G3 (vulnerable)/ G5 (secure). The Rounded Global Status is G4 - Apparently secure. In the USA it has a national status of NNR (unranked). In Michigan, North Carolina and Pennsylvania it is SNR (not yet assessed), but in Wisconsin it is SX (presumed extirpated) and is also thought to be extirpated in Ohio. In Canada, the Black-foam Lichen is ranked by NatureServe as NNR (unranked). In Ontario it is SH (possibly extirpated) and in Québec it is SNR (not yet assessed).
Currently the Black-foam Lichen has no legal protection or status in Canada, although a number of occurrences in Nova Scotia are protected as they occur in provincially protected wilderness areas or National Parks.

Photo: © John D. Ambrose
Long description for Figure 3
Photograph of Blue Ash (Fraxinus quadrangulata) trees on a rocky cliffside. The trunk of the Blue Ash can be straight or irregular, and the crown is narrow, small and rounded. The bark is light-coloured, reddish-grey or tan-grey, and scaly. The leaves are compound and opposite with seven leaflets, and the twigs have square sides with four distinctive corky ridges or wings.
Reason for designation
This tree has a restricted distribution in the Carolinian forests of southwestern Ontario. Small total population size in a fragmented landscape, combined with increasing potential impact from browsing by White-tailed Deer and infestation by the invasive Emerald Ash Borer, place the species at risk of further declines at most sites. In addition, mature trees on Middle Island are threatened by impacts of nesting Double-crested Cormorants. These factors resulted in a change in status from Special Concern to Threatened.
Wildlife Species Description and Significance
Blue Ash is a medium-sized tree, roughly 20 m in height and up to 80 cm in diameter, and is one of six ash species native to Canada. The trunk can be straight or irregular and the crown is narrow, small and rounded. Trees have light-coloured, reddish-grey or tan-grey, scaly bark. The leaves are compound and opposite with seven (5-11) leaflets and the twigs have square sides with four distinctive corky ridges or wings (hence the scientific epithet quadrangulata). Clusters of small flowers that lack petals are produced in spring, as new leaves are expanding. The fruits are single-seeded samaras that are usually twisted, with a notch in the broad wing. A distinctive feature is the retention of dead lower branches, giving the tree an untidy appearance. The inner bark contains a sticky substance that turns blue upon exposure to air (hence the species’ common name).
Distribution
Blue Ash has a restricted distribution in Canada and occurs only in southwestern Ontario in the counties and municipalities of Elgin, Middlesex, Lambton, Chatham-Kent and Essex. It is found at Point Pelee, Peche Island at the mouth of the Detroit River, and the Erie Islands, as well as in river valleys along the Thames River, Sydenham River, and Catfish Creek. Blue Ash is more widely distributed in the United States, and ranges from Ohio south into Alabama, Georgia and Arkansas and west to Wisconsin, Oklahoma and Kansas.

Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Blue Ash in Canada.
Long description for Map 3
Map of the distribution of extant Blue Ash subpopulations in Ontario. The Blue Ash occurs at Point Pelee, Peche Island, the islands in Lake Erie, and valleys along the Thames River, Sydenham River, and Catfish Creek.
Habitat
Blue Ash grows in a variety of habitats and soil types. In Ontario, it is found in three distinctive habitat types. They include floodplains and river valleys where Blue Ash grows in rich soils in association with a variety of other tree species; shallow soils on alvar and limestone on the Lake Erie Islands; and stabilized beaches at Point Pelee National Park, and Fish Point on Pelee Island. All of these habitats have declined in area and quality over the last 100 years. While the effects of habitat fragmentation on Blue Ash have not been assessed, it is expected that fragmentation will result in ecological degradation and perhaps genetic degradation over a longer timeframe, which may contribute to decreasing the likelihood of persistence of subpopulations.
Biology
Unlike other ash species, flowers of Blue Ash include both male and female reproductive structures. The species reproduces by seed and there is no evidence of clonal spread. Blue Ash trees can live up to 300 years (typically 150 to 200 years) and age of maturity (fruiting age) is approximately 25 years. Seed crops are produced every 3-4 years and seeds are dispersed by wind. Most seeds likely disperse within 10 m of the parental tree, but a small number of seeds may travel up to 200 m. Seeds may be dispersed over larger distances by water or animal transport.
Population Sizes and Trends
In 1983, 14 sites with Blue Ash trees were reported within four regions of southwestern Ontario. By 2000, additional searches resulted in recognition of a total of 37 extant subpopulations. In 2001, an additional 19 sites were documented; combining with the 37 subpopulations above this gives a total of 56 sites. The total Canadian population was estimated at fewer than 1000 mature trees in 2001. Fieldwork conducted during 2012/2013 suggests that Blue Ash is more abundant than previously documented. Information on about half of the known sites was collated (n=26) and 1806 trees were counted. Of these trees, 708 (39%) were considered mature (capable of bearing seed). Large numbers of seedlings and saplings were observed at some sites, especially at Point Pelee National Park, and the McAlpine Tract on the Sydenham River.
Threats and Limiting Factors
Since the last status assessment, the potential for deer browsing to impact recruitment and establishment of Blue Ash has emerged as a greater concern than previously noted. Although a few surveyed sites had very large numbers of seedlings and young trees, at many surveyed sites there was little evidence of regeneration suggesting that deer browsing could be preventing establishment of young trees. In addition, the invasive alien beetle Emerald Ash Borer (EAB) has emerged as a new threat to native ash species, including Blue Ash. First detected in North America in 2002, EAB has since spread rapidly. During surveys in 2012/2013, signs of EAB were found at 45.8% (11 out of 26) of the sites and in 70 (3.7%) Blue Ash trees. Although few Blue Ash trees appear to have been killed so far by EAB (0.26% of surveyed trees) and they appear to show resistance, it is unknown whether the impact of EAB will increase in the future. Additional threats to Blue Ash include forest management practices that may include direct cutting of Blue Ash trees because of misidentification by landowners, or authorities – either deliberately or because of EAB related management; alteration to natural disturbance regimes through fire suppression and water management; impacts of livestock farming and ranching including grazing and trampling in riparian habitats; recreational activities (e.g., all-terrain vehicles in local areas), which could impact regeneration through trampling; and, at Middle Island, nitrification of soils and damage to trees from Double-crested Cormorant guano and nesting activities.
Protection, Status, and Ranks
COSEWIC first assessed Blue Ash as Special Concern in April 1983, confirmed same status in November 2000, and the wildlife species was last assessed Threatened in November 2014. Blue Ash is listed as ‘Special Concern’ under Canada’s Species at Risk Act, 2003 and under Ontario’s Endangered Species Act, 2007. Although Blue Ash is considered globally secure (G5) and nationally secure in the United States (N5), it is considered vulnerable (N3) in Canada and is not ranked in Ontario (S3?). Blue Ash is listed as critically imperiled (S1) in Pennsylvania, West Virginia, Wisconsin and Iowa, as imperiled (S2) in Kansas and Mississippi, and vulnerable (S3) in Virginia. It is listed as critically imperiled to imperiled (S1S2) in Georgia and as imperiled to vulnerable (S2S3) in Oklahoma. It is not ranked (SNR) in all other states where it occurs.

Photo: © Allan Harris
Long description for Figure 4
Photo of a Broad-banded Forestsnail, Allogona profunda, shell showing the low spire. The shell is pale yellow with pale brown bands, and the surface is sculptured with fine grooves oriented across the whorls.
Reason for designation
In Canada, this large terrestrial snail is known to exist only in Point Pelee National Park and on Pelee Island. An overabundance of nesting Double-crested Cormorants has most likely led to the loss of subpopulations on some small Lake Erie islands since the early 1980s; historical losses of woodlands and forests also occurred on the mainland and Pelee Island. Major continuing threats are from recreational activities and shoreline erosion. A possible threat is predation by introduced Wild Turkeys, which are rapidly increasing in numbers.
Wildlife Species Description and Significance
Broad-banded Forestsnail is a large (about 30 mm in diameter) terrestrial snail. Shells usually have a distinctive low tooth inside the lower lip of the aperture (shell opening) and a large open umbilicus (hole at the central part of the underside of the shell). The lip of the aperture is white and flares outward. The shell is pale yellow, often with pale brown bands, and the surface is sculptured with fine grooves. Canadian populations of Broad-banded Forestsnail may be genetically isolated from other populations and have significance for conservation.
Distribution
Broad-banded Forestsnail is distributed from southern Ontario and the Upper Peninsula of Michigan south to northern Alabama and east to Pennsylvania and North Carolina. Fossil shells along the Mississippi River as far south as Louisiana represented its southern range limit during the Pleistocene. In Canada, Broad-banded Forestsnail is restricted to the Carolinian Forest region of Ontario on the north shore and islands of Lake Erie. Known subpopulations are presently restricted to Point Pelee and Pelee Island, but there are historical records from the smaller Lake Erie islands and several mainland sites.
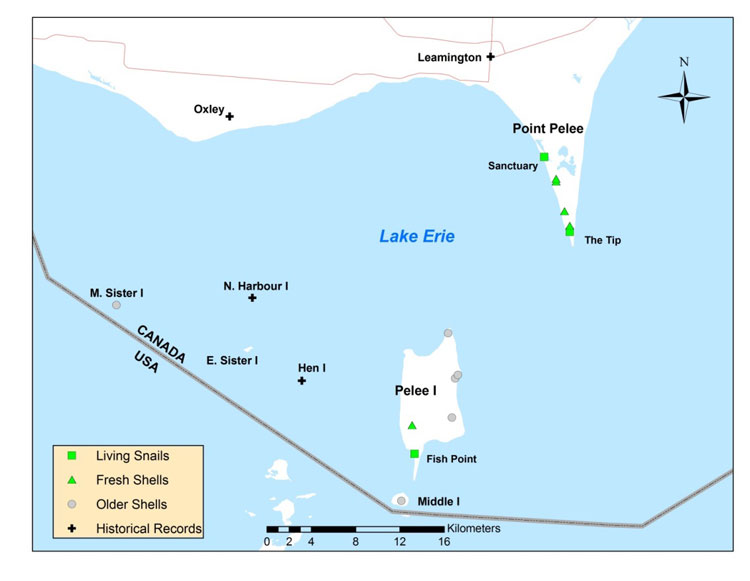
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Broad-banded Forestsnail in Canada.
Long description for Map 4
Map showing the distribution of Broad-banded Forestsnail records at Point Pelee and the Lake Erie islands in 2013. Living and fresh shells are shown at Point Pelee and Pelee Island. There are historical records of the species from Middle Island, Middle Sister Island, East Sister Island, North Harbour Island, Hen Island, Leamington, and Oxley.
Habitat
Broad-banded Forestsnail habitat consists of deciduous forest. In Ontario, extant subpopulations are found primarily in forest and woodland on sandy soil. Empty snail shells were found at some sites extending into wooded alvars (shallow soils over limestone) and shrubby vegetation on sandy soil adjacent to deciduous forest.
Biology
Little information is available about Broad-banded Forestsnail biology. It is an air-breathing, terrestrial snail. Individuals have both male and female reproductive parts (hermaphroditic) and both members of a mating pair exchange sperm and produce eggs. Broad-banded Forestsnail may reach maturity as early as one year, and can live for at least four years. Hibernation occurs buried 5 – 10 cm under the soil or in shallow depressions in the forest floor where leaf litter provides insulation. Broad-banded Forestsnails are active both day and night, but often retire to shelter under leaf litter from mid-morning until late afternoon. Foraging usually takes place on the ground. Green plants and fungus growing on decaying logs are apparently important food sources. Terrestrial snails require damp habitat to feed, move, and reproduce and most species are restricted to forested or wooded habitats that provide shade and retain moisture in the soil and leaf litter. Individuals probably move only a few metres over the course of their lives. Eggs and immature stages are not known to be dispersed by the wind or water.
Population Sizes and Trends
The Canadian population probably declined in the early 1800s, when most of the historical Canadian range was cleared for agriculture. More recently, the number of extant sites has decreased with the apparent loss of subpopulations on Middle Sister, East Sister and Middle islands. The population size is unknown.
Threats and Limiting Factors
Historical and recent threats included forest clearing and Double-crested Cormorants. Most of the forest cover within the mainland range of Broad-banded Forestsnail was cleared decades ago and extant populations are within protected areas where further forest clearing is a negligible threat. Double-crested Cormorant nesting colonies have increased dramatically on the smaller Lake Erie islands since the early 1980s. Associated habitat changes from vegetation dieback and altered soil chemistry probably contributed to the extirpation of snails on these islands. Cormorants prefer to nest on uninhabited islands and are unlikely to colonize Point Pelee and Pelee Island.
Present threats are less well understood. Trampling from recreational use of trails probably kills snails at Point Pelee and Pelee Island. Snails also may be killed in prescribed burns. Altered shoreline processes caused by climate change and shoreline development are causing substantial erosion at Point Pelee and Fish Point. Invasive plants and earthworms occur throughout southern Ontario and may have altered forest ecosystems and snail habitat. Introduced Wild Turkeys and Ring-necked Pheasants may be additional sources of predation.
Protection, Status, and Ranks
Broad-banded Forestsnail is not protected by any Canadian legislation, regulations, customs or conditions except as indicated below. It is not listed under the US Endangered Species Act or under any state or provincial acts. It is not listed under the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). The species and its habitat are protected in Point Pelee National Park and Fish Point Provincial Nature Reserve by federal and provincial park regulations, but threats from invasive species, accidental trampling from recreational use, and similar activities can still occur.
The Global Rank is G5 (Secure) and Subnational Rank in Ontario is S1 (Critically Imperilled). It is not ranked in most states where it occurs.

Photo: © Mike McGrath
Long description for Figure 5a
Photograph of the Caribou, Rangifer tarandus. The photo shows one animal from the Newfoundland population (NP).
Reason for designation
This population was last assessed as Not at Risk in 2002 when the population was 85,000. This population has fluctuated in abundance over the last 100 years and presently has declined by approximately 60% over the last 3 caribou generations. The decline was due to limited forage when the population was at high density, harvest, and predation. Various indices suggest that the population is improving but there is concern that Eastern Coyote, which has recently arrived to Newfoundland, may become a significant predator and influence recruitment such that the population continues to decline.
Wildlife Species Description and Significance
Caribou (Rangifer tarandus) are a medium-sized member of the deer family with relatively long legs and large hooves, which facilitate survival in northern environments. Caribou are central to the culture, spirituality, and subsistence lifestyles of many Aboriginal and non-Aboriginal communities across Canada. Caribou exhibit tremendous variability in morphology, ecology, and behaviour across their circumpolar range. In 2011, COSEWIC recognized 12 designatable units (DUs); this report assesses three DUs: Newfoundland population (NP; DU5); Atlantic-Gaspésie population (GP; DU11); and Boreal population (BP; DU6).
Distribution
Caribou originally inhabited the entire island of Newfoundland, although three areas of higher abundance were identified in the early 20th century: the Humber River Valley; the central portion of the island south of the railway; and the Avalon Peninsula (Prichard 1910, cited in Banfield 1961). Twelve Caribou sub-populations were present before additional sub-populations were established through a series of relocations made in the 1960s-70s (Mercer et al. 1985). Up to 36 sub-populations have existed (Figure 1) but there appear to be approximately 14 sub-populations presently (Pardy Moores pers. comm.). Shifts in Caribou occupancy have been observed in some sub-populations; anecdotal evidence suggests that a small number of Caribou have begun to reoccupy areas (NLDEC, unpubl. data 2013).
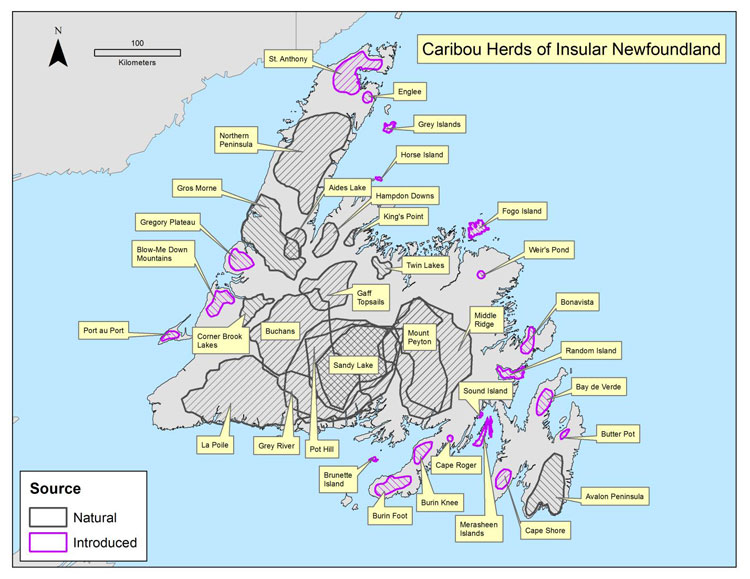
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Caribou, Newfoundland population, Atlantic-Gaspésie population and Boreal population, in Canada.
Long description for Map 5
Map showing the distribution of 36 Caribou subpopulations throughout the island of Newfoundland during the 1990s. Areas containing naturally occurring subpopulations are distinguished from areas with introduced populations.
Habitat
NP Caribou use coniferous forests, barren lands, shrub lands, and wetland complexes. Selection of closed-canopy conifer forests by Caribou generally becomes stronger with increasing disturbance levels. Anthropogenic disturbance tends to lead to the functional loss of residual habitat.
Biology
Typical longevity in Caribou is < 10 years in males and < 15 years in females. Females ≥ 3 years old give birth to a single calf annually, resulting in an overall lower reproductive rate when compared to other North American deer species. Generation time is estimated at 6 years. Reproductive success is closely linked to forage availability.

Photo: © John Blake, Newfoundland Wildlife Division
Population Sizes and Trends
The NP has experienced dramatic fluctuations, at least since the early 1900s; after a peak estimate of 100,000 individuals in the 1900s, the population declined approximately 85% to 10,000-15,000 individuals between 1925 and 1935, then increased approximately 84% over four decades, and reached 94,000 individuals by the mid-1990s. By 2002, the NP declined to 68,000 individuals, and continued to decline, to approximately 32,000 in 2013. The three generation (18 year; 1996-2013) trend is – 62%. The decline is believed to be due to limited forage that reduced juvenile productivity and survival, excessive hunting during the decline phase and, possibly, additive predation. The present decline appears to be part of natural population fluctuations and recently several indices on health and calf survival suggest that the population will increase.
Threats and Limiting Factors
The primary threat to Caribou persistence is habitat loss and excessive mortality rates, factors which often interact because predation increases in disturbed areas. Cumulative anthropogenic (e.g. natural resource extraction and development, roads), and natural disturbances (e.g. forest fire, blowdown) are associated with avoidance behaviour, and decreased recruitment because of increased predation rates. Forest-clearing activities (e.g. forestry, oil and gas development) increases the abundance of alternate prey (e.g. Moose, deer), which can cause increased mortality rates on Caribou. Predation is considered a major proximate threat to Caribou in developed regions of the BP, and in all of GP, and of unknown, but likely lower, significance in the NP. In NP, disturbance appears less significant because fires are rare and much of the range has relatively minimal forestry or mining activity. […] Natural factors, such as climate change and environmental disturbance, can impact Caribou habitat. The NP, BP, and GP are all associated to varying extents with mature - old coniferous stands, which are subject to fire events that are likely to increase in the future, particularly in the BP range. Disease impacts are less well known but there are concerns over spread of brainworm in parts of BP range and several pathogens in BP and GP range. A threats assessment concluded that the overall threat is High-Medium for the NP, Very High-Very High for the GP, and Very High-High for the BP.
Protection, Status, and Ranks
COSEWIC assessed the conservation status of NP in 1984, 2000, and 2002, and recommended that this population was Not at Risk. The NP was ranked as S4 in 2012 at the provincial level. In NP, large areas exist which are of marginal timber value and are not in imminent danger of being disturbed by industrial activity. […] Forest management plans have been modified to assist Caribou in parts of all three DUs, but implementation is variable and efficacy unknown to date. Predator control has been applied annually since 2001 in the GP, and in parts of the BP. In the NP, hunting of Black Bear and Coyote occurs but direct predator control is not applied.

Photo: © Carita Bergman
Long description for Figure 6a
Photo of a Cassin's Auklet, Ptychoramphus aleuticus, from above. The image shows a compact seabird with short wings. The plumage is dark grey and there is a small white crescent above the eye. The irides are white.
Reason for designation
About 75% of the world population of this ground-nesting seabird occurs in British Columbia. Overall, the Canadian population is thought to be declining, but population monitoring has been insufficient to determine size and trends. The species faces threats from mammalian predators that have been introduced to its breeding islands. While predators have been removed from some breeding colonies, it is likely that ongoing predator management is going to be needed to maintain the species. The species also faces other threats when it forages at sea, including large-scale climate change effects on its oceanic prey, and risks from oiling.
Wildlife Species Description and Significance
Cassin’s Auklet is a small grey seabird in the Family Alcidae. About 75-80% of the global population breeds in British Columbia. This species comprises almost half of all seabirds nesting in British Columbia.
Two subspecies are recognized, Ptychoramphus aleuticus aleuticus and P. a. australis. Only the former subspecies is found in Canada.
Distribution
Cassin’s Auklets are found along the Pacific coast of North America. They spend most of their lives at sea and come to land only to breed. Most nest in colonies on coastal islands from the western Aleutian Islands in Alaska to central Baja California; they occasionally nest in Siberia and on the Kuril(e) Islands in Japan/Russia. During the non-breeding season, the birds are found mainly from southeast Alaska through Baja California, with concentrations off California.
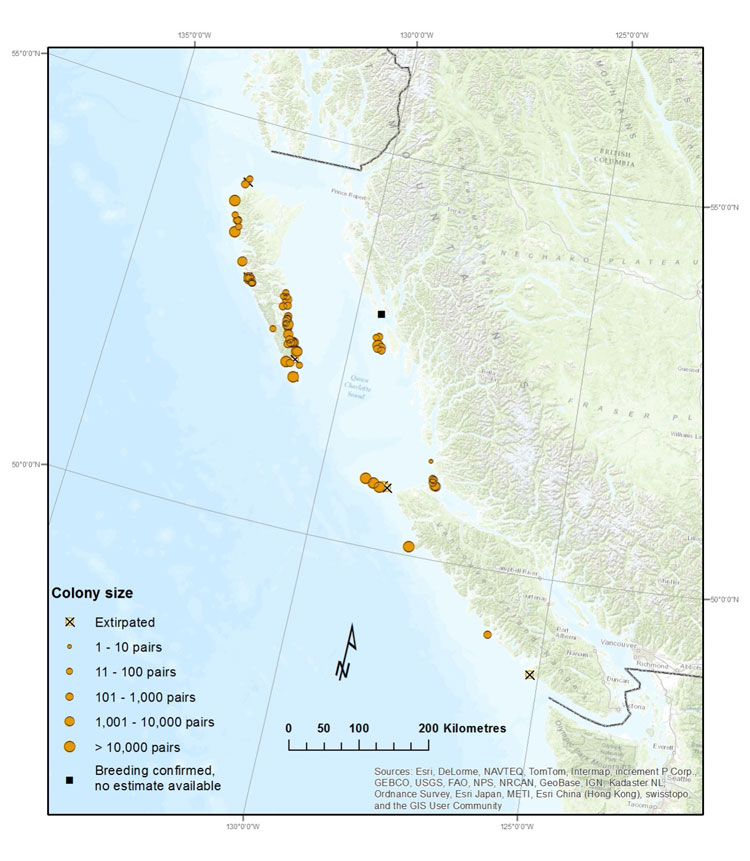
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Cassin’s Auklet in Canada.
Long description for Map 6
Map showing locations and relative sizes (by number of pairs) of colonies of Cassin's Auklets in British Columbia. Cassin's Auklets nest on 62 islands or island groups along coastal Haida Gwaii, the north and west coasts of Vancouver Island and the northern mainland coast.
Habitat
Cassin’s Auklets nest on islands that are free of native mammalian predators, such as raccoons and mink. In British Columbia, the vast majority nest in burrows in forested or treeless habitats. Most burrows are within 100 m of the shoreline. The amount of suitable nesting habitat has declined over the past 75 years due to introductions of mammalian predators to colony islands. Changes to vegetation have also decreased the amount of high-quality nesting habitat on some islands since the 1980s.
At sea, the Cassin’s Auklet inhabits two oceanographic domains: the California Current System, which extends from the northern tip of Vancouver Island through Mexico, and the Alaska Current System farther north. The birds’ marine habitat is highly variable over multiple temporal scales. Atmospheric/oceanographic processes that elevate ocean temperatures (e.g., warm water phases of the Pacific Decadal Oscillation) are associated with reduced Cassin’s Auklet reproductive performance while those that cause extreme climate events (e.g., El Niño events) can lower adult survival rates.

Photo: © Carita Bergman
Long description for Figure 6b
Photo of a Cassin's Auklet, Ptychoramphus aleuticus, in water
Biology
Cassin’s Auklets lay a single-egg clutch, which is incubated by both parents on alternating days for about 38 days. After the egg hatches, parents return to the burrow at night to feed the nestling for about 45 days. The young are independent at fledging.
In the California Current System, Cassin’s Auklet reproductive success and fecundity are reduced during warm water years due to declines in food availability. Reduced reproductive success is attributed to a temporal mismatch between the nestling provisioning period and the birds’ critical zooplankton prey, which peaks in abundance earlier and for a shorter duration during warm water years. In addition, adult survival is reduced during extreme climate events. In contrast, Cassin’s Auklets in the Alaska Current System show reduced survival during El Niño events, but no effects on reproductive performance.
Population Sizes and Trends
The global population of Cassin’s Auklet is estimated at 3.57 million breeding individuals, of which about 2.69 million (75%) nest in Canada. Triangle Island is the world’s largest Cassin’s Auklet colony and alone supports about 55% of the global population. Over the last 75 years, colonies have been extirpated by introduced predators: rats, raccoons and mink. The magnitude of decline is largely unknown because population data are available for fewer than 30 years.
Threats and Limiting Factors
The main threats are climate change, introduced predators and oil spills. Climate change is expected to result in warmer ocean temperatures and more frequent El Niños, both of which have negative consequences for Cassin’s Auklet reproduction and survival. The impacts are expected to be most severe and immediate in the California Current System. Rats, raccoons and mink cause notable destruction to, and possibly extirpations of, colonies. The threat of oil contamination from chronic or catastrophic spills is ongoing and expected to increase if offshore vessel traffic increases.
Protection, Status, and Ranks
Cassin’s Auklet is categorized as a species of “Least Concern” according to the IUCN Red List and its global status is “Apparently Secure”. Nationally and provincially, the breeding population is considered vulnerable to imperilled, whereas the non-breeding population is considered apparently secure. Cassin’s Auklet has been placed on the British Columbia Blue List as a species of Special Concern. It is an Identified Species under the province’s Identified Wildlife Management Strategy in the Forest Range and Practices Act. Only one breeding colony (supporting less than 1% of the population) does not have formal protection in British Columbia.

Photo: © Scott Gillingwater
Long description for Figure 7
Photo of an Eastern Box Turtle, Terrapene carolina, on sparsely vegetated ground, showing side and front of carapace and side of head. The carapace is slightly keeled, high-domed, and brown to black with yellow patterning.
Reason for designation
This turtle occurred historically in Ontario based on archeological evidence and Aboriginal Traditional Knowledge. Habitat modification has been extensive and the species is no longer extant. Considerable search effort has documented fewer than 10 individuals in Ontario, but these individuals all represent released captive individuals from unknown sources and are not considered part of the former Canadian population.
Wildlife Species Description and Significance
The Eastern Box Turtle (Terrapene carolina) is a small terrestrial turtle rarely exceeding 16 cm in straight carapace length. It has a slightly keeled, high-domed carapace, which is usually brown to black with variable yellow to orange patterning. The plastron has a hinge, allowing the two lobes to completely close against the underside of the carapace. The Eastern Box Turtle has special cultural significance to the Iroquois. It is also the largest known freeze-tolerant animal in the world.
Distribution
The Eastern Box Turtle is found across much of eastern North America. It occurs from central Michigan to southern Maine in the north and from eastern Texas to Florida in the south. Disjunct populations occur in two areas of Mexico. No current native populations of the Eastern Box Turtle are known to exist in Canada. The remains of Eastern Box Turtles have been found at 12 archeological sites from Ontario. COSEWIC has previously assessed the Eastern Box Turtle as native to Canada (Ontario).
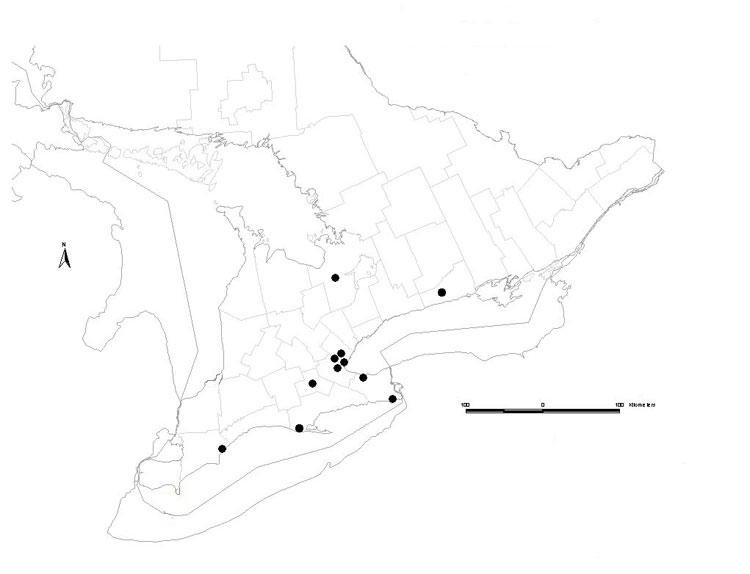
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Eastern Box Turtle in Canada.
Long description for Map 7
Map showing the approximate locations of 12 sites in southern Ontario that contain archeological remains of Eastern Box Turtle.
Habitat
The Eastern Box Turtle is associated with open deciduous or mixed woodlands. It also makes use of adjacent habitats such as old fields, pastures, riparian zones and suburban landscapes. Small wetlands, ponds, seepages or streams are also required. The Eastern Box Turtle typically lays its eggs in open areas with sandy or loamy soil, possibly outside areas used the rest of the year. Many nesting sites are in disturbed areas such as grazed fields, or along roadways. Hibernation usually occurs on land, with turtles burrowing into loose soil or under leaf litter, though some Eastern Box Turtles will overwinter aquatically at the bottom of ponds or streams.
Biology
Eastern Box Turtles typically mature in 5-6 years for males and 7-8 years for females in the southern part of their range. Individuals in the northern part of the range likely take longer to mature.
Most adult females will lay only a single clutch of eggs in a given year, although up to 4 clutches are possible in southern populations. In the northern portion of the range, the eggs are usually laid in June. Clutch size ranges from 1-11 eggs, although 4-7 eggs are more common. Incubation can last 61-90 days in the northern part of the range. The embryos have temperature dependent sex determination. In constant temperatures, males are produced at temperatures of 22.5-27.0oC, whereas above 28.5 females are produced. Hatchlings average 30.3 mm in carapace length and 8.2 g in weight. Individuals can live more than 100 years in the wild.
The Eastern Box Turtle is an omnivore, although juveniles are primarily carnivorous. Confirmed food includes fungi, mosses, roots, stems, seeds, and fruits of various plants, invertebrates (snails and slugs particularly) and vertebrates (usually consumed as carrion). Nests are often depredated by raccoons, foxes and skunks. Juveniles are consumed by a wide range of predators including mammals, snakes and birds. Adults are more protected from predation but can possibly be killed by mammals.
The Eastern Box Turtle usually occupies a small home range of approximately 2 ha, although home ranges >30 ha have been observed. Seasonal movements of 10.0 km have been documented, but are unusual.
Population Sizes and Trends
There are no known extant populations in Canada, but in Ontario, individual box turtles have been found at archeological sites and observed sporadically over at least the past 55 years. During the 20th century, the Eastern Box Turtle was first reported in 1960 from Point Pelee National Park and in 1963 from Rondeau Provincial Park. There are reports of individual Eastern Box Turtles from various locations in southern Ontario (Brant, Essex, Haldimand-Norfolk, Hamilton-Wentworth, Kent, Lambton, Middlesex, Niagara, and Waterloo) ranging up to 2013. There is also a report of an Eastern Box Turtle near Montreal, Québec in 1988. Some individuals are from subspecies from the southern portion of the Eastern Box Turtle’s range and most, if not all, of these reports are widely considered to be released pets.
There are many observations of Eastern Box Turtles from Point Pelee National Park spanning many years, and successful overwintering has been documented in the park. Intensive surveys for freshwater turtles at Point Pelee in 2000-2001 failed to locate any Eastern Box Turtles, though there have been a few records since then. It seems unlikely that these turtles are remnants of a native population given that intensive biological surveys conducted in the park in the early part of the 20th century failed to locate any Eastern Box Turtles.
Populations in the USA vary greatly in size from < 25 to > 1700 individuals, although small or low-density populations have poor viability. The Eastern Box Turtle remains widespread but is declining across much of its range and has disappeared from many areas especially in the northern part of its range. There have been few long-term studies but population declines of 50-75% have been documented despite the fact that annual adult survival rates as high as 96% have been documented. Egg and juvenile mortality are likely quite high. One study found 100% mortality of tracked hatchlings and juveniles.
Threats and Limiting Factors
The Eastern Box Turtle faces a number of threats across its range. Traffic mortality is a major threat for this terrestrial species, which can wander significant distances, and readily nest on roads. Legal international export of Eastern Box Turtles was once a major issue, but this has been halted by the Convention on International Trade in Endangered Species (CITES) with quotas listed at zero. Nevertheless, legal and illegal collection of individuals for sale or for personal use is still a major threat across the species’ range. Habitat loss and fragmentation are also significant threats as large intact woodland areas become lost to development or divided by road construction. Mortality arising from individuals being trapped between train tracks may be significant in some areas. Cutting or mowing hay can also result in mortality. Diseases such as Iridovirus (a Ranavirus), and upper respiratory tract infections have also caused mortality in some populations. Fires, including prescribed burns, can also result in significant mortality of Eastern Box Turtles.
Protection, Status, and Ranks
The Eastern Box Turtle is listed on CITES Appendix II. Globally, it has been listed as Vulnerable, but in the USA, the species is considered Secure nationally (N5). It is listed as Critically Imperilled (S1) in two states: Maine and New Hampshire.

Photo: © Michael Runtz
Long description for Figure 8a
Photograph of an Eastern Wolf (Canis sp. cf. lycaon), side view and walking, from Algonquin Provincial Park, Ontario. Compared with Grey Wolves and Great Lakes-Boreal Wolves, Eastern Wolves typically have coats with a more reddish-brown to tawny colour and reddish forelegs.
Reason for designation
This species is an intermediate-sized canid with a generally reddish-brown/tawny coat. It has a small population size (likely < 1000 individuals) and a restricted range, limited to south-central Ontario and south-central Quebec. Most records come from scattered protected areas, where mortality and rates of hybridization with Eastern Coyotes occurs less frequently than elsewhere in its range. Population expansion is unlikely, owing to competition with Eastern Coyote and increased mortality outside protected areas.
Status history
In 1999, the Eastern Grey Wolf (Canis lupus lycaon) was considered a subspecies of the Grey Wolf and was placed in the Data Deficient category. Status was re-examined (as Eastern Wolf, Canis lupus lycaon) and designated Special Concern in May 2001. New genetic analyses indicate that the Eastern Wolf is not a subspecies of Grey Wolf. In May 2015, a new wildlife species, Eastern Wolf (Canis sp. cf. lycaon) was designated Threatened.
Wildlife Species Description and Significance
The Eastern Wolf (putatively Canis lycaon, formerly Canis lupus lycaon) is an intermediate-sized canid weighing an average 24 kg for females and 29 kg for males. Pelage often is described as reddish-brown/tawny, but is highly variable. The Eastern Wolf is best defined by a combination of genetic distinctiveness, morphological characters, and an ecological role associated with a feeding preference for smaller prey than fed on by Gray Wolf (C. lupus). The Eastern Wolf population has a degree of hybridization with Coyote (C. latrans), and individuals are defined based on having a high level of genetic ‘purity,’ that is, distinctiveness from both Gray Wolf and Coyote as determined by molecular genetic analysis. It is important to note that the Eastern Wolf discussed in this report is not the same Eastern Wolf discussed in the Great Lakes region because those Canis are considered in this report as Great Lakes-Boreal Wolves, a hybrid between the Eastern Wolf and Gray Wolf. Although evidence is strong that the Eastern Wolf is a valid species, the taxonomy of Eastern Wolf is under debate; in this report the Eastern Wolf is considered to be Canis sp. c.f. lycaon, a wildlife species as defined under SARA that is worthy of conservation because of its distinctiveness, persistence, and significance as a large carnivore, and likely part of the last remnant population of the large Canis from eastern North America. Aboriginal traditional knowledge also supports the existence of a medium sized Canis in the region.

Photo: © Michael Runtz
Long description for Figure 8b
Photograph of two Eastern Wolves (Canis sp. cf. lycaon), facing the camera, from Algonquin Provincial Park, Ontario.
Distribution
The current distribution of Eastern Wolves is thought to be restricted to the mixed coniferous-deciduous forests of central Ontario and southwestern Québec, namely the Great Lakes-St. Lawrence Forest Region. Eastern Wolves were extirpated from most of their original range in North America due to eradication of large Canis over much of the past 400 years. Genetic analyses suggest that the current distribution of Eastern Wolves mainly is in central Ontario and southern Québec (north of the St. Lawrence River), with concentrations in core areas, all of which are protected areas.
Source: COSEWIC 2015. COSEWIC Assessment and Status Report on the Eastern Wolf in Canada.
Long description for Map 8
Map delineating the present extent of occurrence (126,573 sq. km) of the Eastern Wolf based on genetic analysis of Canis specimens sampled across much of the Great Lakes region and from Manitoba to the Maritime Provinces.
Habitat
Eastern Wolves typically occur in deciduous and mixed forest landscapes with low human density, south of the Boreal Forest Region. Sandy soils are often preferred for den sites. Both den and rendezvous sites tend to be located in conifer/hardwood-dominated landscapes near a permanent water source. Territory size is often near 200 km².

Photo: © Michael Runtz.
Long description for Figure 8c
Photograph of an Eastern Wolf (Canis sp. cf. lycaon), side view with head turned to face camera, from Algonquin Provincial Park, Ontario.
Biology
Eastern Wolves live in family-based packs composed of a breeding pair and offspring from the current and previous years. Females give birth to an average of five pups in late April - early May and they remain at the den site for6 - 8 weeks. Dispersing juveniles leave the pack after 37 weeks. Eastern Wolves are primarily predators of White-tailed Deer (Odocoileus virginianus). Predator-prey and diet analyses indicate that Eastern Wolves can be effective predators of Moose (Alces americanus), although efficiency varies by pack, season, and year. Beaver (Castor canadensis) also constitutes a substantial portion of Eastern Wolf diet.
Population Sizes and Trends
There have been 170 - 195 Eastern Wolf (all ages) identified in the last 10-15 years. The population size is unknown but likely less than 1000 mature individuals. The estimated minimum population size is 236 mature individuals, mainly located within protected areas. A best-possible-scenario maximum estimate of 1203 mature individuals within the extent of occurrence is based on there being an equally high density of Eastern Wolf outside protected areas. Most records though occur in protected areas and the population size of mature Eastern Wolf likely is closer to 236 individuals. There is no population trend information except for Algonquin Park, the site with the most Eastern Wolf records to date, which appears to be stable.
Threats and Limiting Factors
The main threat and limiting factor for Eastern Wolves outside the protected areas likely is human-caused mortality from hunting and trapping, which is facilitated by road networks. Based on research in Algonquin Park, excessive mortality likely limits dispersal, and alters pack breeding dynamics, leading to another main threat, gene introgression (hybridization) with Eastern Coyotes due to the lack of conspecific mates. Habitat loss and fragmentation associated with road networks and urbanization is expected to continue outside protected areas and likely will deter population expansion. Negative public attitudes towards wolves, and established packs of Eastern Coyote, may limit population expansion.
Protection, Status, and Ranks
The Eastern Wolf is listed as Special Concern under the federal Species at Risk Act (SARA) and Ontario’s Endangered Species Act, 2007. Both listings are as a subspecies of Gray Wolf (Canis lupus lycaon). No wolf species is listed under the Lois sur les espèces menacées ou vulnérables [Act respecting Threatened or Vulnerable Species in Québec]. Hunting and trapping of wolves is permitted in wildlife reserves, but not in national (federal or provincial) parks. In Ontario, wolves are protected from regulated hunting and trapping in Algonquin Park, in the townships surrounding Algonquin Park, and in all provincial Crown Game Preserves. Eastern Wolves are protected from hunting, but not from trapping, in French River Park. Wolves are protected from harvest in national parks. Aboriginal communities retain constitutional rights to harvest Wolves for sustenance and ceremonial purposes, including in protected areas. A small game licence is required to hunt Wolves in Ontario (limit of 2 per year) and Québec (no bag limit). NatureServe ranks Canis lupus lycaon as N4 (apparently secure). At the provincial scale, Eastern Wolf is ranked as S4 (‘apparently secure’) in Ontario, and is not ranked in Québec.

Photo: © Cary Hamel
Long description for Figure 9
Photo of the flower-bearing stems of the Fascicled Ironweed, Vernonia fasciculata. The inflorescence is a flat, dense cluster of flower heads composed of purple disc florets.
Reason for designation
This showy perennial plant has a restricted geographical range in Canada, and occupies small prairie remnants mainly along roadside ditches and riversides in southern Manitoba. The few small subpopulations are at risk from such threats as flood duration/frequency alteration, cultivation, ranching, herbicide use, and road and right-of-way maintenance activities.
Wildlife Species Description and Significance
Fascicled Ironweed is an erect perennial herb with smooth to slightly hairy stems that grow up to two metres tall and support sharply toothed stalkless leaves with conspicuous pits on the underside. The inflorescence is a flat, dense cluster of flower heads composed of purple disc florets. The seed-like fruits called “cypselae” have a crown of elongate bristles and are adapted for wind dispersal.
The species has been used for ornamental plantings, and some cultural and medicinal uses have been reported.
Distribution
The extant Canadian range of Fascicled Ironweed is confined to a small area in south eastern Manitoba. Its North American range extends south through much of the central United States.
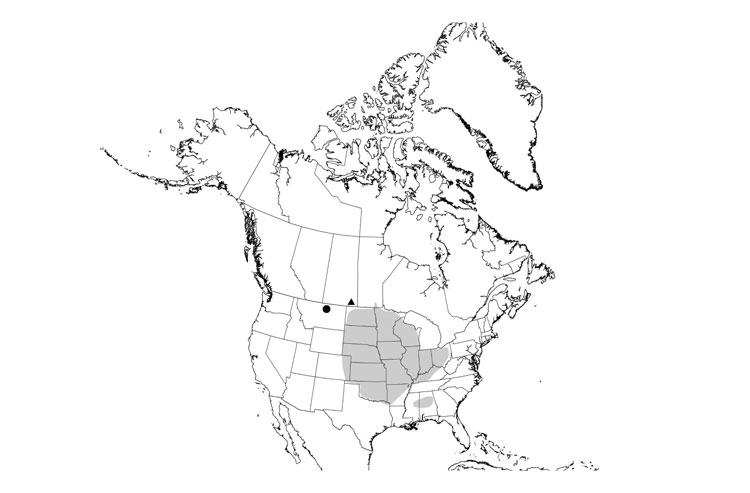
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Fascicled Ironweed in Canada.
Long description for Map 9
Map illustrating the global range of the Fascicled Ironweed in the tallgrass prairies of the midwestern and eastern United States and southern part of Manitoba in Canada.
Habitat
Fascicled Ironweed is typically found in moist to wet prairies and riparian areas. It does not tolerate deep shade. In Manitoba it is found in roadside ditches and open to semi-open riparian areas.
Biology
Fascicled Ironweed is a perennial species which flowers one to two years after germination. Flowers are visited by bees, flies, and butterflies. Seeds are adapted for wind dispersal but may also be dispersed by flowing water. This species can survive seasonal flooding and is generally avoided by mammalian grazers.
Subpopulation Sizes and Trends
There are three known subpopulations in Canada, two of which have fewer than 100 plants each (one has only five plants). There are thousands of plants in the largest subpopulation; the number of plants is coarsely estimated to be 21,000. Overall trends are difficult to assess given the lack of consistent monitoring, though the abundance of at least one subpopulation has decreased in the past decade. Two historical subpopulations in Morris, MB and Weyburn, SK are believed to be extirpated.
Threats and Limiting Factors
Roadside subpopulations are threatened by road and ditch maintenance activities. Riparian plants are threatened by alteration of flood duration and frequency, and cultivation.
Protection, Status, and Ranks
Fascicled Ironweed was assessed by COSEWIC as Endangered in November 2014. In Manitoba, it has been listed as Endangered under the Endangered Species and Ecosystems Act.
The NatureServe global rank of Fascicled Ironweed is G5 (Secure); the national rank in Canada is N1 (Critically Imperilled). Subnational ranks are S1 (Critically Imperilled) in Manitoba and SH (Possibly Extirpated) in Saskatchewan.

Photo: © Michael Burzynski
Long description for Figure 10
Photo of a clump of the Griscom's Arnica, Arnica griscomii ssp. griscomii. This low, showy plant produces a rosette of basal leaves from which flowering stems emerge. The flowers are yellow and consist of a composite flowerhead composed of perfect disk florets surrounded by pistillate ray florets.
Reason for designation
This mat-forming plant is a Canadian Gulf of St. Lawrence endemic found only on small, isolated calcareous cliffs and limestone barrens of Quebec and the Island of Newfoundland, is increasingly under threat due to habitat shift in response to a changing climate. The instability of some sites increases the threat of a stochastic event that could result in the loss of some small subpopulations. ATV use in limestone barrens is of some concern.
Wildlife Species Description and Significance
Griscom’s Arnica (Arnica griscomii ssp. griscomii) is a small perennial herb with bright-yellow daisy-like flowers. It is a Canadian Gulf of St. Lawrence endemic, and is found only in Québec and on the island of Newfoundland. The flowers, which grow on stems about 20 cm tall, arise from a cluster of leaves that lie almost flat on the ground. These plants spread by rhizomes (underground stems), often forming dense clumps. Dense patches of showy flowers may make this a charismatic species for inspiring public interest in preserving calcareous cliffs, limestone barrens, and their plant life.
Distribution
Griscom’s Arnica is endemic to Canada and is known only from five subpopulations on the Gaspé Peninsula of Quebec and from three subpopulations on the island of Newfoundland.
<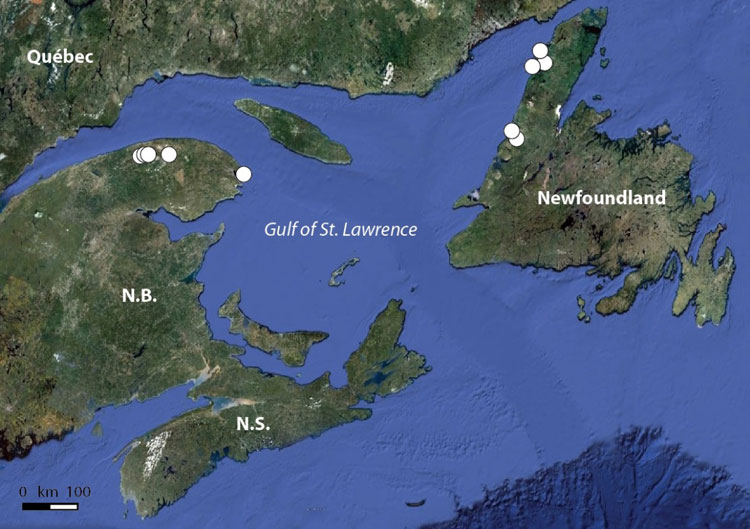
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Griscom’s Arnica in Canada.
Long description for Map 10
Entire range of Arnica griscomii ssp. griscomii. All reported sites are shown. Base map from GoogleEarth Nov. 2012. See Table 2 in COSEWIC Assessment and Status Report on the Griscom’s Arnica in Canada for more information.
Habitat
Griscom’s Arnica grows only on calcium-rich soils. It prefers full sun or partial shade, and is usually found on cliff faces, talus slopes, around rock outcrops, and at the edge of vegetation patches on natural limestone gravel barrens.
Biology
Griscom’s Arnica is adapted to sites that are subjected to extreme weather, and the stems die down to the soil surface in winter. The plant is able to produce seeds without fertilization, and its seeds are wind-borne, like a dandelion’s. Although there are some signs of herbivory, this species does not seem to be palatable to many animals. Because of its strict habitat requirements and inability to compete with faster-growing plants, Griscom’s Arnica does not colonize new sites easily.
Population Sizes and Trends
There are 125 flowering plants in Quebec, and about 10,500 in Newfoundland. The Newfoundland subpopulations seem to be stable, but the Quebec subpopulations may be in decline.
Threats and Limiting Factors
Griscom’s Arnica is limited primarily by competition from faster-growing plants. It can only thrive where other species are handicapped by extreme soil and climatic conditions. Climate change is probably the greatest threat to this plant due to the high potential for other species to take advantage of milder conditions and displace Griscom’s Arnica. Other minor threats include trampling by Moose and Woodland Caribou, trampling and habitat damage by humans and their vehicles, and collecting of plants for horticulture.
Protection, Status, and Ranks
All but one of the subpopulations of Griscom’s Arnica are located in federal parks or provincial protected areas, and are afforded some protection by their regulations. The only subpopulation that does not have legal protection is on St. John Island, off the coast of western Newfoundland. In Quebec the species is designated as Threatened under provincial legislation.
Griscom’s Arnica has a NatureServe global conservation rank of G5T2 (the species overall is Secure, but the Gulf of St. Lawrence subspecies is Imperilled), a national rank of N2 (Imperilled), and a subnational rank of S1 (Critically Imperilled) in Quebec, and S1S2 (Critically Imperilled to Imperilled) in Newfoundland & Labrador. It is ranked as At Risk in Quebec and as May Be At Risk in Newfoundland & Labrador by General Status of Canada.

Photo: © Cindy Smith
Long description for Figure 11a
Photograph of a mature Limber Pine growing on a rocky, exposed site.
Reason for designation
This tree species is imminently and severely threatened throughout its Canadian range by White Pine Blister Rust (an introduced pathogen), Mountain Pine Beetle, and climate change. Surveys at a number of sites in 2009 document an average of 43% and 35% of infected or dead trees, respectively. Repeated survey information leads to an estimated decline in the Canadian population of about 1% per year. At that rate, close to 2/3 of mature individuals are expected to be lost over the next 100 years, and local subpopulations could become extirpated.
Wildlife Species Description and Significance
Limber Pine is a five-needled pine, typically 3-15 m tall, with a much-branched, rounded crown. The seed cones are egg-shaped (7-15 cm long by 4-6 cm wide) and light-brown to greenish-brown. The cones open to release the seeds and then fall to the ground. Its large seeds are brown, 10-15 mm long and usually wingless.
Limber Pine growth rings can provide information on climate and river flows back 500-1000 years, much further than historical records, which are generally 100 years at most. This information is important for understanding and projecting scenarios of climate change, including drought and river flows. Limber Pine is also a “keystone” species, the seeds providing important food for bears, small mammals and birds, and the trees sheltering other species.
Distribution
Limber Pine naturally occurs only in western North America, extending from southeastern British Columbia and southwestern Alberta south to northern Arizona and New Mexico, and southern California. In Canada, it extends in southeastern British Columbia, from near Field, south along the eastern side of the Rocky Mountain Trench nearly to the Canada-United States of America (U.S.) border and, in southwestern Alberta, from near Kootenay Plains south in the Rocky Mountains and Foothills to the Canada-U.S. border.

Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Limber Pine in Canada.
Long description for Map 11
Map illustrating currently occupied range of the Limber Pine in Canada, where it extends from southeastern British Columbia (near Field) south along the eastern side of the Rocky Mountain Trench nearly to the Canada-United States border, and from southwestern Alberta (near Kootenay Plains) to the Canada-United States border in the Rocky Mountains and Foothills.
Habitat
In Canada, Limber Pine occurs typically on warm, dry sites in the lower portions of the mountains and foothills at elevations of ca. 850 m to 1900 m. Some occurrences are as high as around 2000 m and may form mixed stands with Whitebark Pine. Limber Pine can occur at both lower and upper treeline sites. Aspects are usually southerly or westerly and slopes vary from gentle to steep. In British Columbia, most stands are on steep, exposed cliffs and ridges, while in Alberta, some stands are in more gently rolling terrain as well as rocky ridges and outcrops. Limber Pine sites are often exposed to strong winds, which in conjunction with shallow, well to rapidly drained soils and warm aspects, create droughty conditions.
Biology
Limber Pine is a long-lived species, frequently reaching several hundred years and trees over 1000 years old are known. Cones are typically produced at about 50 years of age, although this may be delayed, and the largest cone crops are produced decades later. Cone production is irregular with some years of very low seed production. Seeds are primarily dispersed by birds but also by small mammals. However, most seedlings germinate from seeds dispersed by birds, so dispersal by small mammals likely contributes little to recruitment. Both seedlings and trees are physiologically adapted to tolerate harsh environmental conditions, especially drought.

Photo: © Cindy Smith
Limber Pine is dependent on mycorrhizal fungi, which enable the roots to take up nutrients and also aid in protecting the roots from pathogens. Other fungi can damage seeds, needles, stems and roots. Limber Pine needles are the sole food of a small ermine moth, which is rare in Canada.
Population Sizes and Trends
The number of mature Limber Pine trees in Canada is estimated to be 44.4 million. The Canadian population is declining at an average annual rate of about 1%, which over 100 years is a 66% decline. Rescue from populations in the U.S. is not a realistic possibility because the same threats are affecting those populations, many of which are declining as well.
Threats and Limiting Factors
Limber Pine is imminently and severely threatened throughout its Canadian range by White Pine Blister Rust (an introduced species), Mountain Pine Beetle, and climate change. While each taken singly poses a significant threat, they interact to further increase the severity of the impacts. With climate change, the frequency, intensity and duration of drought is projected to increase, and fire is projected to be more frequent and severe. Stressed trees are likely to be more susceptible to pathogens and insects.
Protection, Status, and Ranks
Limber Pine is listed as Endangered in Alberta under the Wildlife Act, although no provisions exist under that act to provide broad legal protection for either individuals or habitat. A provincial recovery plan is being prepared. In British Columbia, Limber Pine has no legal protection, although it is a Blue-Listed (special concern) species. Some protection is provided in both provinces for small subpopulations in provincial protected areas. Limber Pine also occurs in national parks in Alberta and British Columbia, where both individuals and habitat are protected.
Limber Pine has a NatureServe conservation rank of Imperilled (S2) in Alberta and Vulnerable (S3) in British Columbia.

Photo: © Denis Knopp
Long description for Figure 12
Photo of several flowering stems of the Phantom Orchid, Cephalanthera austiniae. The flowering stem is almost totally white. White sheaths clasp a smooth leafless stock topped by a loose raceme composed of up to 20 white flowers.
Reason for designation
This parasitic orchid occurs in very low numbers at scattered locations in southwestern British Columbia. Losses of some subpopulations, along with continuing habitat fragmentation and declines in habitat quality through new housing development and recreational activities, make future losses of subpopulations likely. The species’ dependency on specific habitat conditions and its inter-dependency on a fungal partner and associated tree species make it more susceptible to extirpation.
Wildlife Species Description and Significance
The Phantom Orchid (Cephalanthera austiniae) is a myco-heterotrophic epiparasite that lacks chlorophyll and derives its food from a three-way partnership with an underground fungus and a tree species. The white flowering stem stands up to 55 cm tall. White sheaths up to 10 cm long clasp a smooth leafless stock topped by up to 20 white flowers. The noticeably vanilla-scented, aromatic flowers have a yellow throat. Fibrous roots branch from a slender rhizome.
Distribution
The Phantom Orchid is the only North American representative of the genus Cephalanthera. It is found only in the Pacific Northwest, in California, Oregon, Washington, Idaho, and British Columbia (BC). In BC, it occurs only in the extreme southwest, with subpopulations reported from southeast Vancouver Island, Saltspring Island, and the lower Fraser Valley.

Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Phantom Orchid in Canada.
Long description for Map 12
Map showing occurrences (extant, presumed extant, extirpated, historical) of the Phantom Orchid in British Columbia, where the species is found in both the Coastal Douglas-fir and the Coastal Western Hemlock biogeoclimatic zones. Occurrences are restricted to the lower Fraser Valley between Mission and Chilliwack, Saltspring Island, and Greater Victoria, including the Saanich Peninsula, on Vancouver Island.
Habitat
In BC, the Phantom Orchid is found in relatively undisturbed old growth, mature and occasionally older second growth forests. It is typically found in coniferous or mixed forests and it requires an intact below-ground (ectomycorrhizal) fungal network. In BC, the Phantom Orchid usually grows in sites with sparse ground cover and thick leaf litter although it is also occasionally found in areas with a high cover of forbs and shrubs. In BC, the Phantom Orchid is found at elevations ranging from 0-550 m, on a range of slopes (0- 92%) and the majority of sites are south to southwest-facing. Some sites in BC occur on soils with elevated pH including bedrock with carbonate materials, shell middens, and limestone quarry tailings. Litter from Bigleaf Maple or other trees may play a role in making the soil pH more alkaline than in other sites.
Biology
Phantom Orchid does not flower every year and although the flowers indicate the presence of the orchid, they do not reflect the full extent of the below-ground plants. Plants may have periods of dormancy and it is unclear what factors trigger the production of flowering stems. Flowering is staggered over the growing season from early May to mid- July with unconfirmed reports of flowering stems emerging as late as September. The pollinators of the Phantom Orchid in BC are not known. The Phantom Orchid can selfpollinate and other Cephalanthera species are known to have substantial levels of inbreeding, suggesting that they also self-pollinate. Like other orchids, Cephalanthera species produce large numbers of very tiny seeds that are dispersed by wind, generally with short dispersal distances (i.e. less than 6 m). In BC, very few of the flowering stems produce capsules or mature seed. The Phantom Orchid receives its food via a parasitic connection to mycorrhizal fungi, which are in turn associated with the roots of a tree species. The health of both the tree species and the mycorrhizal fungus is critical to the survival of the orchid. Molecular studies of populations in the United States found the Phantom Orchid was exclusively associated with a fungus of the family Thelephoraceae.
Population Sizes and Trends
The previous status report (COSEWIC 2000) documented nine subpopulations. Since that time, three sites within two different subpopulations have been extirpated and one subpopulation is presumed extirpated. At two other subpopulations, plants have not been seen since 2000 and 2006. Because these subpopulations have not been consistently surveyed and Phantom Orchids may be dormant at these sites, the subpopulations are presumed extant, but they may also be extirpated. Since the previous status report, nine new Phantom Orchid subpopulations have been found and new sites have also been found within previously known subpopulations. There are currently 20 known Phantom Orchid subpopulations in Canada, with 76 extant sites. In 2013, the number of flowering stems in each subpopulation ranged from 0 (dormant plants) to 76.
Trends in the total number of flowering stems are difficult to determine due to irregular monitoring, periods of dormancy, and annual weather variation, which may influence flowering. Based on 2013 subpopulation estimates during which all but 4 sites were remeasured, the total population included approximately 344 flowering stems. The number of flowering stems represents a slight overestimation of the number of mature individuals because flowering stems that are close to each other may be part of the same individual (this is impossible to determine without excavation, which would kill the plants). However, the total count may also be an underestimation because dormant individuals were not included.
The 2013 population estimate is greater than that reported previously (i.e. 49 flowering stems in the 2000 COSEWIC status report) owing to increased search effort compared to the previous report rather than increasing numbers at previously known sites. The population is severely fragmented because the majority of individuals are found in small and relatively isolated subpopulations, most with low estimated viability.
Threats and Limiting Factors
The primary threat to Phantom Orchid is habitat destruction from the rapid increase of new housing development. The majority of Phantom Orchid sites occur on private property (12 of the 20 subpopulations have some or all sites on private land). Phantom Orchid occurs on private property owned by 22 different landowners and several of the landowners intend to subdivide. Homeowner activities including maintenance and construction of both buildings and gardens, inadvertent mowing and trampling can threaten the Phantom Orchid. The Phantom Orchid is also threatened by forest harvest activities, which can destroy habitat directly and/or by altering hydrology/light conditions, removing host trees, destroying the fungal partner, creating edge effects, and increasing fragmentation. Recreational activities including hiking and dirt-biking can also damage plants and habitat. Other threats include competition from invasive plants, plant collection, overgrazing by deer, impacts associated with small isolated populations, and threats to partner species.
Protection, Status, and Ranks
The Phantom Orchid is protected under Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora and is listed as Threatened under Canada’s SARA on Schedule 1. A draft provincial recovery strategy for the Phantom Orchid has been prepared.
In BC, the Phantom Orchid has a provincial status of Imperilled (S2) and is on the BC Conservation Data Centre Red List. In Canada, the Phantom Orchid has a National NatureServe Status of Imperilled (N2). Globally it is ranked Apparently Secure (G4).
Although 12 of the 20 of Phantom Orchid subpopulations occur either solely or partially on private land, ten of the subpopulations are afforded some protection from development by their locality either entirely or partially within provincial parks, regional parks, provincial Crown land, municipal Crown land, BC Parks Ecological Reserve and federally owned Department of National Defence land. One subpopulation on provincial Crown land is currently protected from logging within a Wildlife Habitat Area.

Photo: © Robert P. Dana
Long description for Figure 13
Photo of the Poweshiek Skipperling, Oarisma Poweshiek, lateral view. The ventral hindwing surfaces have a striking pattern of white scales on the wing veins that contrast with the pale brown background.
Reason for designation
The Canadian population is isolated and disjunct from the populations in United States which are 1000 km to the south. Widespread declines within the past decade on both sides of the border mean Canada holds a significant portion of the species global range. Within Canada this species is restricted to native tall-grass prairie, a habitat that has also undergone similar declines. Although most of the occupied habitat is protected, even with appropriate management, its range is so small that the butterfly is increasingly vulnerable to stochastic events.
Wildlife Species Description and Significance
Poweshiek Skipperling is a small butterfly with a wingspan of 24 to 30 mm. The dorsal wing surfaces are dark brown with orange lines along the wing margins. The ventral hindwing has a striking pattern of white scales on the wing veins that contrast with the pale brown background. The species is easily recognized by its fluttery flight pattern. Poweshiek Skipperling is one of a very small group of specialist butterflies that occurs only in native tall grass prairie habitats in Canada. It now persists in one population in Canada and a series of isolated populations in the United States. The loss of this species from Canada would represent the loss of a significant element of the endangered prairie ecosystem.
Distribution
The historical range of Poweshiek Skipperling extended from southeastern Manitoba through the eastern Dakotas and western Minnesota to Iowa, with isolated populations in southeastern Wisconsin, northwestern Illinois and southern Michigan. Its entire historical range remains uncertain because much of the tall grass prairie went under the plough in the mid-1800s and before most butterfly collections in the region began. The global range of Poweshiek Skipperling has substantially contracted since the early 2000s, and it is currently extant in Manitoba, Michigan and Wisconsin. The Canadian range of Poweshiek Skipperling is disjunct from populations in the United States and restricted to about 40 km² of prairie habitat in southeastern Manitoba.
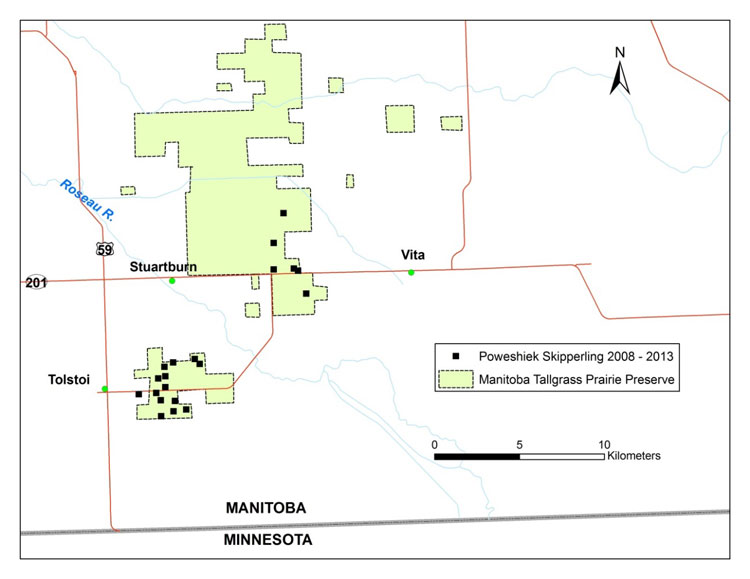
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Powesheik Skipperling in Canada.
Long description for Map 13
Map illustrating the Canadian range of the Poweshiek Skipperling in southeastern Manitoba (based on surveys from 2008 to 2013) and the boundary of the Manitoba Tall Grass Prairie Preserve. There are two main Poweshiek Skipperling population centres, 7 to 10 kilometres apart. One straddles Provincial Road 201 between Stuartburn and Vita, and the other is east of Tolstoi.
Habitat
Poweshiek Skipperling inhabits wet to mesic tall grass prairies in Canada, which range in size from less than 1 ha to several hundred hectares. Prairie habitats often consist of elongated openings among groves of Bur Oak and Trembling Aspen, which provide windbreaks. Habitat patches are a combination of wetter and drier sections of prairie. The wetter areas are dominated by various willows, Tufted Hair Grass, Redtop, Mat Muhly, various sedges, and Slender Spike Rush. The drier areas are dominated by Big Bluestem, Prairie Dropseed, and various forbs. The larval host plants used by Poweshiek Skipperling in Manitoba include Big Bluestem, Indian Grass, and Mat Muhly. Slender Spikerush is also a suspected host plant. The presence of Black-eyed Susan is important because it is the preferred adult nectar plant.
Biology
Poweshiek Skipperling has one generation per year. Flight dates in Manitoba range from late June to late July with peak numbers typically in early to mid-July; adults emerge earlier in warmer years. Adults live for a few days to a week. Males patrol for unmated females by flying low over prairie host plants and grasses. Following mating, oviposition occurs on the upper surface of host plant leaves, and eggs hatch within nine to ten days. Larvae undergo five moults and overwinter as fifth instar larvae on the underside of a blade of grass or on the stem near the base of the host plant. The following spring larvae wake up on warm days, feed and eventually undergo two to four additional moults before pupation begins sometime in early June. Adults emerge after about two weeks. The males disperse 1.0 km to 1.6 km but they are unlikely to disperse across dense woodlands, row crops or habitats not dominated by grasses. Roads may act as barriers between suitable prairie habitat or nectar sources.
Population Sizes and Trends
Population size estimates are unavailable. Changes in population size are difficult to detect due to responses to fire and other disturbance and variation in survey effort. No more than 240 adults have been counted in any given year since 2002. Previous estimates of 5,000 to 10,000 individuals in Canada are likely an overestimate. There is little change in the extent of occurrence or area of occupancy since 2002.
Threats and Limiting Factors
Vegetation succession of open prairie habitats to woody shrubs and trees threatens Poweshiek Skipperling habitat. In the absence of natural disturbance processes such as wildfire or grazing by native Plains Bison, woody species replace prairie vegetation. Prescribed fire and domestic livestock grazing have been used to reduce woody vegetation growth in Poweshiek Skipperling habitat, but excessive, poorly timed, or cumulative disturbance can kill larvae and reduce nectar plant abundance. Wildfires occur at irregular intervals and compound the threat of mortality. Fires with frequencies of less than five years are probably the most serious threats facing Poweshiek Skipperling. Historically, habitat loss and fragmentation were also threats, but now most Canadian sites are protected from habitat conversion. The small extent of occurrence makes the Canadian populations vulnerable to severe weather events.
Protection, Status, and Ranks
Poweshiek Skipperling was assessed Threatened by COSEWIC in 2003 and listed as Threatened under the federal SARA in 2005. Critical habitat has been identified and includes about 99% of the Canadian population. The species is listed as Endangered under the Manitoba Endangered Species Act. The global status is G1 (critically imperiled), national status N2 (imperiled) in Canada and N1 (critically imperiled) in the United States. The General Status rank for Canada is “May Be at Risk”. Most Poweshiek Skipperling habitat is within the Manitoba Tall Grass Prairie Preserve, which is managed for prairie conservation and is unlikely to be developed or converted to other uses.

Photo: © Adam Martinson
Long description for Figure 14
Photo of a Prairie Rattlesnake (Crotalus viridis), showing a heavy-bodied pit viper. The snake is tan in colour with darker bands or blotches along its back and dark tail rings. Like all rattlesnakes, it has a segmented rattle at the end of its tail and heat sensing pits below its eyes.
Reason for designation
The species has undergone declines since the 1930s, primarily resulting from large-scale habitat loss from cultivation and increased road mortality. Some local populations have experienced substantial recent declines and the species still faces serious threats across its Canadian range. The species may become Threatened if factors suspected of negatively influencing its persistence are neither reversed nor managed with demonstrable effectiveness.
Wildlife Species Description and Significance
The Prairie Rattlesnake is a heavy-bodied pit viper. It is tan in colour with darker bands or blotches along its back and dark tail rings which are usually olive to brown. Adults attain an average snout-vent length of 120 cm, and an average mass of 1000 g. Like all rattlesnakes, this species has a segmented rattle at the end of its tail, two heat sensing pits below its eyes and two retractable fangs in its upper jaw. The Prairie Rattlesnake is one of three extant rattlesnake species in Canada and has been the subject of numerous scientific investigations in Alberta and Saskatchewan. The Prairie Rattlesnake is a symbol of the Canadian Prairies, and the protection of its grassland habitat will contribute to the conservation of a globally imperilled ecosystem.
Distribution
The global range of the Prairie Rattlesnake extends from northern Mexico, through the central U.S. and into southern Canada, which supports at least 3% of its global range. The Canadian distribution of this species is limited to southeastern Alberta and southwestern Saskatchewan and is strongly associated with major river valleys. A historical range decline in Canada is presumed; however, over the last 40 years the known range of the species has remained relatively stable. There are ~ 230 unique locations (i.e., hibernacula) of this species in Canada. From increasing search effort, there has been an increase in the number of previously undocumented locations over the last 15 years and this trend is presumed to continue. Despite the discovery of previously undocumented dens, there is a recent and projected continuing decline of ~ 30% in the number of Prairie Rattlesnake locations in Canada.
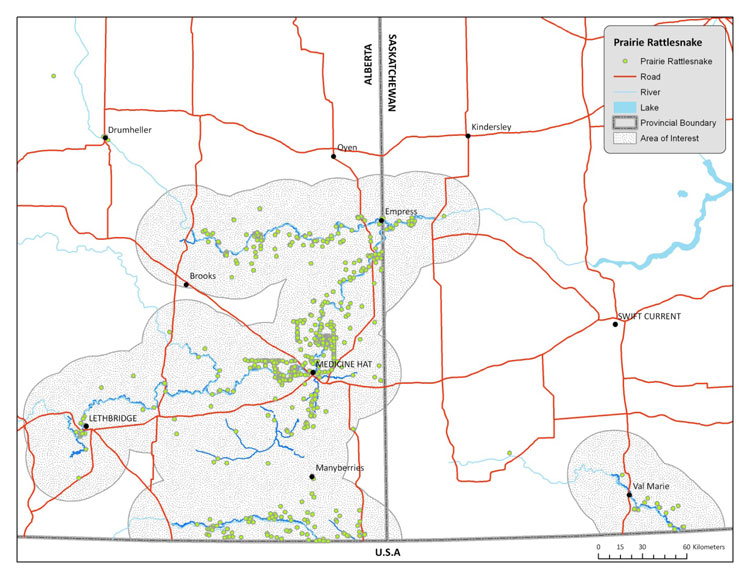
Source: COSEWIC 2015. COSEWIC Assessment and Status Report on the Prairie Rattlesnake in Canada.
Long description for Map 14
Map showing the estimated maximum biological area of occupancy of the Prairie Rattlesnake in Canada based on a 30-km buffer for all watercourses with confirmed hibernacula. This map was produced in 2009 with occurrence data from an unknown timespan. Observation records are indicated, revealing a clear disjunction between the cluster of observations along the Frenchman River, near Val Marie, Saskatchewan, and the rest of the Canadian distribution.
Habitat
Prairie Rattlesnakes require hibernacula, foraging habitat, gestation sites, and movement corridors between these habitats. This species is often associated with river and coulee bottoms, and upland grasslands or badlands. Suitable retreat sites such as animal burrows and shrubs are necessary microhabitat components. Hibernacula are mostly associated with south- or east-facing slopes of major river drainages and consist of features which allow access to a suitable subterranean environment. Gestation sites provide optimum conditions for development of young and protection from predators. Average home range size of the Prairie Rattlesnake in Canada ranges from 4 to 109 ha. The majority of habitat (i.e., grassland) loss in Canada occurred prior to the 1930s as a result of cultivation. Regardless, there is an ongoing and projected continuing decline of 3 - 18% in the amount of available Prairie Rattlesnake habitat in Canada, mostly due to the expansion of intensive agriculture, but also due to combined effects from oil and gas drilling, urbanization, and road networks.
Biology
Several behaviours render the Prairie Rattlesnake vulnerable to human-induced threats. These include: 1) seasonal congregations at overwintering sites and gestation sites, 2) high site fidelity to hibernacula and gestation sites, 3) long-distance migrations between overwintering and foraging grounds, 4) high fidelity to seasonal migration routes, and 5) conspicuous defensive behaviours. Certain biological attributes limit the ability of the species to recover from human-induced declines. These include: delayed age of maturity, long generation time, slow growth, biennial or triennial reproduction, small litter size, and high juvenile mortality rate.
Population Sizes and Trends
The total population size of the Prairie Rattlesnake in Canada is estimated to be at least 22,300 (20,400 – 28,300) individuals, which is estimated to consist of at least 14,900 (13,600 – 18,900) adults. Yearly variation in adult population size at any given location is probably minimal under natural conditions, therefore, substantial variation in abundance over a short time period is likely caused by human activity. Over the past 40 years declines in abundance of Prairie Rattlesnakes at a few Canadian den sites have been inferred based on anecdotal evidence, or documented through empirical studies. Future population declines are also projected. The Prairie Rattlesnake is experiencing a continuing decline in abundance across its Canadian range.
Threats and Limiting Factors
The viability of Prairie Rattlesnake populations in Canada is threatened by many human activities. These activities are associated with the following threat categories: roads and railroads, hunting and collecting, annual and perennial non-timber crops, oil and gas drilling, and housing and urban areas. Combined, threats contribute to the loss, degradation, or fragmentation of habitat and can cause direct and indirect mortality, either individually or en masse (e.g., intentional persecution at hibernacula). Of all threats, those posed by roads are projected to have the greatest impact on the persistence of Prairie Rattlesnakes in Canada over the next 10 years.
Protection, Status, and Ranks
The Prairie Rattlesnake is considered “Secure” globally and in the U.S. In Canada, it is considered “Vulnerable” nationally and a “Species of Special Concern” in Alberta. The species has not previously been assessed by COSEWIC and is not protected under the federal Species at Risk Act. The Saskatchewan Wildlife Act and the Alberta Wildlife Act prohibit any harm or possession of Prairie Rattlesnakes without a permit and also offer some protection of their hibernacula from destruction. At least 4,550 km² of land within the range of the Prairie Rattlesnake is owned by federal and provincial governments, combined.

Photo: © Robert Forsyth
Long description for Figure 15
Three photos providing different views of the shells of Proud Globelets (Patera pennsylvanica). A general characteristic of this genus is the imperforate umbilicus. Unlike other species of Patera, Proud Globelet lacks a parietal tooth-like protuberance on the aperture wall, has a higher spire and has a last whorl that is more markedly descending at the aperture. Shells are thin, regularly striate, yellowish olive with 5.75 to 6 whorls in adults. The lip of the aperture is white and narrowly reflected with a low prominence on the inner rim of the baso-columellar margin.
Reason for designation
This large terrestrial snail is found in the upper mid-west of North America, with Canada’s single recorded occurrence in and near a wooded park in Windsor, Ontario. General snail surveys conducted throughout southern Ontario over the last century have not detected this species anywhere else. Freshly dead shells were found in 1992 and 1996 but only dead, weathered shells were found in extensive surveys in 2013. Human intrusions and disturbances from recreational activities and ecosystem modifications from invasive plants and animals, the surrounding urbanization, pollution from local and regional sources, and climate change may have contributed to the species’ demise; it appears another native snail disappeared from the same area at the same time.
Wildlife Species Description and Significance
Proud Globelet, Patera pennsylvanica is a terrestrial snail in the family Polygyridae. The yellowish, round shell (15-20 mm diameter) lacks a tooth-like protuberance at the shell opening compared to other species of the genus. The sole known Canadian population occurred in and near the Black Oak Heritage Forest owned by the City of Windsor. Although the ecological significance of Proud Globelet is unknown, gastropods, in general, play important roles in forest ecosystem functioning via nutrient cycling and soil building processes.
Distribution
Proud Globelet is found from southwestern Ontario south to Iowa and Missouri and east to Pennsylvania. The species’ entire range, nearly all of which is in the U.S., is about 534,453 km². Canada has less than 0.001% of the global range. Empty, fresh shells were found in 1992 and 1996 in Windsor. Empty, weathered shells were found in 2013 in the same place and nearby. No live individual was ever recorded in Canada. This species was not found elsewhere in southwestern Ontario in gastropod surveys from 1916 to 2013.

Source: COSEWIC 2015. COSEWIC Assessment and Status Report on the Proud Globelet in Canada.
Long description for Map 15
Map outlining the global range of the Proud Globelet, where it occurs or has occurred from southwestern Ontario south to Iowa and Missouri and east to Pennsylvania. The global range covers 534,453 square kilometres based on a minimum convex polygon.
Habitat
Proud Globelet generally occurs on wooded hillsides or in ravines. In Canada, the species has been reported in a sandy oak forest and a disturbed light industrial site. Food requirements for Proud Globelet might be fungi, leaf litter and fresh plant material, but some Polygyridae are carnivorous. In Ontario, trends observed in the habitat of Proud Globelet include a general reduction in oak forests and reduction in biodiversity, the latter potentially affecting the snail communities.
Biology
Very little is known about the biology of Proud Globelet. From other species in the polygyrid family, it has been inferred that mating occurs in fall or early spring and oviposition in spring to late summer. Clutch size ranges between 20 and 80 eggs that hatch about 20 to 60 days after oviposition. Growth rate and, consequently, adult size (reached after 1-2 years) are highly variable. Growth periods correspond to activity periods from spring to fall. Sexual maturity is reached after 2 to 3 years, and lifespan has been estimated to range between 3 and 5 years. Snails are ectotherms and prone to freezing in winter or dehydration in summer. Different strategies have evolved to enable the species to survive extreme temperatures and drought besides going into dormancy. Hibernation in the Polygyridae extends from early October until mid-April in temperate regions. Aestivation occurs occasionally during prolonged heat and drought periods. Snails rely on humiditybuffered refugia and snow cover for dormancy survival. Most Polygyridae are active at dusk or during the night with dispersal for colonization of new habitat being slow, around 35 m in 3 years. Predation and parasites can be a source of mortality for land snails.
Population Sizes and Trends
Seventeen person-hours of search effort in different light and weather conditions were spent trying to find live individuals in a 200 m x 100 m square plot in the Black Oak Heritage Forest and in a nearby former light industrial area in 2013. The plot encompasses the area where the snail had been previously found. Fourteen empty shells (estimated to be 5-15 years old) were found under leaf litter in the upper 5 cm of the soil in addition to other snail and slug species in the forest plot; Proud Globelet was not found elsewhere in the forest but one shell also was found south of the forest in the nearby former light industrial area. The complete absence of live individuals and the age of the shells found in 2013 suggest that the population has substantially declined since 1996; there is a strong likelihood that Proud Globelet has disappeared from this area and from Canada as it has only ever been found in this area in southwestern Ontario. Rescue from the U.S. population is unlikely because the Detroit and St. Clair rivers and Lake Erie are dispersal barriers.
Threats and Limiting Factors
Human impacts such as pollution, garbage accumulation, intensive recreational use and changes to soil composition and hydrology can affect the snail population. Introduced species, such as plants, earthworms and other gastropods can affect native snail populations through alteration of the soil nutrient cycle, reduction of leaf litter and interspecific competition. The introduced slug Dusky Arion, Arion subfuscus, was abundant at the site where Proud Globelet shells were found and was observed feeding on fungi. Climate change can have a large impact on snail survival. In temperate regions, climate change will involve increases in both average temperature and the frequency of extreme weather events such as heat waves, drought, and high precipitation, as well as an absence of insulating snow cover. Snails are limited by their low dispersal or escape capacity, relatively long generation time, low physiological resistance to fluctuating environmental factors, susceptibility to bioaccumulation of toxic agents, and limited genetic flow.
Protection, Status, and Ranks
Global Rank: G4 (Apparently Secure), National Rank (Canada): N1 (Critically Imperilled), National Rank (US): N4 (Apparently Secure). Sub-national ranks are “critically imperilled” (S1) in Ontario and West Virginia, “critically imperilled” to “imperilled” (S1S2) in Pennsylvania. Michigan listed Proud Globelet as a species of special concern.

Photo: © Bree Walpole
Long description for Figure 16
Photo of a female Red-necked Phalarope, Phalaropus lobatus (lateral view), standing in shallow water. The image shows the bird in breeding plumage, with red-orange colouring on the sides and base of its neck. The remainder of its plumage is primarily blue-grey and white. Non-breeding plumage is white along the head, throat, breast and underparts, with dark upperparts, eye stripe, and crown.
Reason for designation
This bird has declined over the last 40 years in an important staging area; however, overall population trends during the last three generations are unknown. The species faces potential threats on its breeding grounds including habitat degradation associated with climate change. It is also susceptible to pollutants and oil exposure on migration and during the winter. This is because birds gather in large numbers on the ocean, especially where currents concentrate pollutants.
Wildlife Species Description and Significance
The Red-necked Phalarope is a small shorebird, easily recognized in breeding plumage by the red-orange colour on the sides and base of its neck. The remainder of its plumage is primarily blue-grey and white. Females are more brightly coloured than males. Non-breeding plumage is white along the head, throat, breast and underparts, with dark upperparts, eye stripe and crown. Unlike most other shorebirds, the Red-necked Phalarope spends much of the non-breeding season at sea.
Distribution
The Red-necked Phalarope breeds across the entire circumpolar sub- and low-Arctic. However, the species’ distribution, in particular while at sea, is not completely understood. The primary over-wintering sites for North American breeding Red-necked Phalaropes are believed to be off the western coast of Peru, with migration along the Pacific and Atlantic coasts of North America, and through the continent’s interior towards the California shoreline. In Canada, the species breeds or migrates through every province and territory.
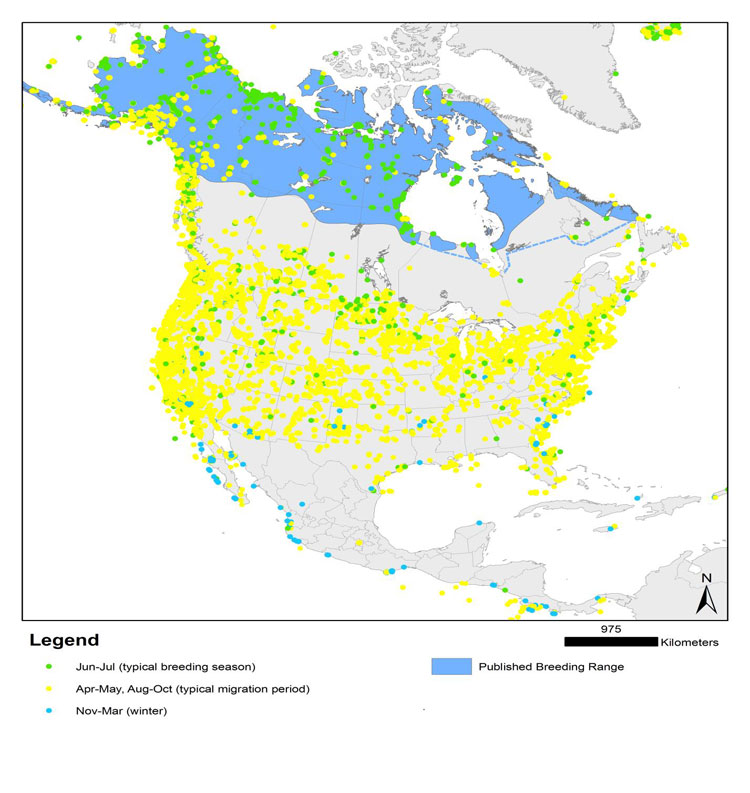
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Red-necked Phalarope in Canada.
Long description for Map 16
Map of North and Central America illustrating localities where Red-necked Phalaropes have been sighted in (a) June to July (breeding season), (b) April to May and August to October (migration period), and (c) November to March (winter). The map is based on data from the Canadian Wildlife Service NWT-NU Checklist Database, eBird, and the most current published range information. A continuous breeding distribution is shown across the northern reaches of the continent, with breeding observations reported as far west as the Alaska Peninsula and as far east as the Labrador coast. Red-necked Phalaropes breed throughout the Yukon Territory, with a concentration of observations along the coast and on Herschel Island. They are also common throughout the Northwest Territories and eastward through Nunavut, as far north as Victoria Island and southern Baffin Island. Overwintering takes place in marine habitats at low latitudes, primarily along the Pacific coast. Some wintering birds are sighted irregularly along the Atlantic coasts of Georgia and Florida and the Gulf of Mexico.
Habitat
While migrating and during the winter months, Red-necked Phalaropes concentrate at sea in areas where prey is forced to the surface (e.g., convergences and upwellings). To a lesser extent, migrants may also stop at lakes and ponds in interior North America, especially saline lakes with abundant aquatic invertebrates. Red-necked Phalaropes breed in low- and sub-Arctic wetlands, near freshwater ponds, lakes, or streams. The drying of freshwater ponds and the expansion of shrubs and trees into low- and sub-Arctic wetland habitats, with a changing climate, is expected to have a significant impact on habitat quality and availability for the species.
Biology
All phalarope species exhibit sex-role reversal, with males undertaking the majority of parental care. Females initiate the selection of a nesting site and may mate with multiple males. Nests are a simple scrape containing 4 eggs. Neither sex defends a territory. Shortly after laying, females desert incubating males in search of other mates. Females then congregate near the coast or leave the breeding grounds entirely, with males remaining until later in the season to tend young.
While at sea, Red-necked Phalaropes form large flocks and prey almost exclusively on zooplankton.
Population Sizes and Trends
Estimates of population size are based largely on expert opinion. The current estimate of abundance within North America is a minimum of 2 500 000 individuals, with about 74% or 1 850 000 individuals occurring in Canada. This is likely an underestimate, as it was derived by approximately summing the estimated number of individuals at known key stopover sites. Migration routes are incompletely known, so some unknown fraction of the population would not be included in this sum.
Trend estimates from various studies are imprecise and capture only a small fraction of the population, offering little insight into population status. Targeted surveys in the outer Bay of Fundy offer the most reliable information, albeit for a restricted area. Millions once passed through the area, with estimates of up to 3 000 000 in the outer Bay of Fundy in the 1970s. By 1990, they had declined drastically. In the most recent surveys (2009-2010), an estimated 550 000 Red-necked Phalaropes occurred between Grand Manan and Brier Island in the Bay of Fundy. Despite the significant uncertainty, experts generally agree that the species is less abundant in the Bay of Fundy than it once was. Declines have also been noted on the breeding grounds (e.g., Churchill and La Perouse Bay, Manitoba; Herschel Island, Shingle Point, and Old Crow Flats, Yukon), although observations are limited.
Threats and Limiting Factors
The many knowledge gaps relating to the species, particularly regarding adaptability, migration and over-wintering biology, make threat identification challenging. A change in climate, and associated habitat and food-web effects, is likely the single greatest threat to Red-necked Phalaropes on their breeding grounds. The build-up of contaminants in the Arctic environment, increase in industrial activities, and denuding of vegetation caused by increasing Snow Goose populations are also likely to have negative impacts on breeding birds and their habitat.
Changes in ocean temperature, salinity, and currents due to climate change are also likely to affect the species during the non-breeding season. A decline in the availability of prey at traditional staging areas and over-wintering sites could also have an impact on the species. Other possible threats during the non-breeding season include increased disturbance (e.g., shipping traffic) and a change in water quality. While at sea, Red-necked Phalaropes are also susceptible to the impacts caused by chronic oiling and point-source oil spills, as well as the ingestion of microplastics.
Protection, Status, and Ranks
The Red-necked Phalarope receives protection under the Migratory Birds Convention Act, 1994. It also receives protection through the Convention on Migratory Species, in which it is included under Appendix II. The species is ranked as ‘moderate concern’ in both the Canadian and United States Shorebird Conservation Plans. The global and national (Canada and United States) conservation status ranks for Red-necked Phalarope indicate that the species is apparently secure. The International Union for Conservation of Nature (IUCN) Red List ranks the species as “least concern” globally.

Photo: © Jason Gibbs
Long description for Figure 17
Photo of a male Sable Island Sweat Bee, Lasioglossum sablense, specimen (lateral view). The bee is a dull metallic colour, with pollen-collecting hairs on the hind legs and abdomen. It has dense punctures on the dorsal surface of the thorax, adjacent to the wing bases.
Reason for designation
This species is globally endemic to Sable Island, Nova Scotia, and occurs as one isolated population with a very small range and no possibility of rescue. The island has only about 13 km² of vegetated area that provides forage/nesting sites for this bee. Nesting likely occurs near or within this vegetated area and sweat bees are not known to travel large distances (i.e. > 200 m) for forage. Increased frequency and severity of storms, in addition to climate change and related sea level rise, are expected to drive change which will further decrease the quality and quantity of bee habitat on the island. Eco-tourism is also a potential future threat, which may also increase the introduction and spread of invasive species. Habitat on the island is also susceptible to invasive plant species, introduced horses, and seawater flooding.
Wildlife Species Description and Significance
The Sable Island Sweat Bee, Lasioglossum sablense Gibbs, is a small (5–6 mm), dullmetallic sweat bee in the family Halictidae. The species is endemic to Canada, occurring solely on Sable Island, Nova Scotia. Both sexes can be distinguished from the three other bee species (two of these sweat bees) on Sable Island by the combination of their small size and the dense lateral punctures on the dorsal part of the thorax.
Distribution
The global and Canadian distribution of the species is confined to Sable Island, Nova Scotia, which is approximately 34 km² in area, excluding the intertidal zone. The island is isolated from mainland Nova Scotia by a distance of approximately 150 km.

Map created by Mark L. Richardson (Environment Canada).
Long description for Map 17
Map showing the location of Sable Island, approximately 150 km off the mainland of Nova Scotia.
Habitat
Sable Island is primarily composed of sand, with low levels of organic material in the sandy soil. Approximately 13 – 15 km² of the island (39%) has vegetation and is considered potential bee habitat. Vegetated areas are composed of a few distinct plant communities, the largest of which are Marram-Forb grasslands, sparse grass lands and heath. Climatic conditions are cool and foggy with high winds during the summer and relatively warm conditions during the winter. Females dig underground nests. Flowering plants are visited for pollen and nectar resources.
Biology
The Sable Island Sweat Bee is a ground-nesting species and a generalist floral visitor. Inseminated females overwinter as adults and emerge in spring to form nests. Reproductive males and females are produced in the late summer. Adults fly from at minimum June 1st to September 11th. Related species are known to have social organization in nests, but the social behaviour of the Sable Island Sweat Bee remains unstudied.
Population Sizes and Trends
The population size of Sable Island Sweat Bee is not possible to estimate given the collection data available for the species. Of the four bee species occurring on Sable Island, the Sable Island Sweat Bee is the least commonly collected. Historical records are too sparse to effectively estimate historical trends. Relative proportions of the two sweat bees on the island, L. novascotiae (Mitchell) to the Sable Island Sweat Bee, collected with nets in 1966 –1967, 2008, and 2013 are comparable (3:1, 1:1, 2:1, respectively).
Population sizes have likely decreased over historical time due to decreases in the spatial vegetated area on Sable Island.
Threats and Limiting Factors
Loss of habitat due to the inundation of vegetation by sand or submersion of low-lying areas with rising sea levels would have negative impacts on population sizes of the Sable Island Sweat Bee. Harsh weather conditions could compound this effect while also reducing adult foraging activity.
Past human influence may have also reduced the extent and diversity of flowering vegetation. Current human activity is minimal due to the isolation of the island and the control of visitors. There is potential with increased future human visitation to the island to introduce non-native bee species.
Protection, Status, and Ranks
The Sable Island Sweat Bee is not protected under federal or provincial legislation. The species has not been assigned a conservation status rank. The species’ habitat is within Sable Island National Park Reserve, which is protected under the Parks Canada Act.

Photo: © Gary Allen
Long description for Figure 18
Close-up photo of the flower of the Small White Lady's-slipper (Cypripedium candidum), a perennial, clonal orchid. The flower is a white, pouch-shaped "slipper," 1.7 to 2.7 cm long, with purplish veins or spots. The surrounding twisted, green to brownish-yellow petals and sepals are also typically streaked or spotted with reddish purple.
Reason for designation
This orchid is known in Canada from Manitoba and Ontario where it grows mainly in tallgrass and mixed grass prairies. These sites require management to prevent encroachment of woody vegetation and to remain suitable for the orchid. Increased search effort has uncovered previously unknown populations in Manitoba, but many populations are small, and some have been lost in recent years. The discovery of additional populations, increased habitat protection, and active management for this species resulted in a change in status from Endangered to Threatened. Because individuals are slow to mature and require a fungal partner, the species is especially vulnerable to local extirpations. In addition to encroachment, the species is threatened by invasive plant species, alteration of hydrology, residential and commercial development, roadside maintenance and illegal collecting.
Wildlife Species Description and Significance
Small White Lady’s-slipper is a perennial, clonal orchid. Each plant produces one to many stems that reach approximately 15 cm when in flower. Three or four simple clasping leaves alternate along each stem. Each flowering stem typically bears one white, pouchshaped “slipper”. In Canada, flowers typically appear between mid-May and mid-June. Fruits are produced by late summer and contain many small seeds.
Distribution
The current range of Small White Lady’s-slipper extends across 18 states and two provinces. Less than 10% of its range is in Canada, with extant subpopulations occurring in southern Ontario and Manitoba. The Manitoba subpopulations are separated from those in Ontario by approximately 1,300 km. Subpopulations in Ontario also show a disjunction, with a single subpopulation in Hastings County separated by approximately 400 km from subpopulations on Walpole Island. Of the 39 known Canadian subpopulations, 22 are considered extant, and roughly half of these have few mature individuals.
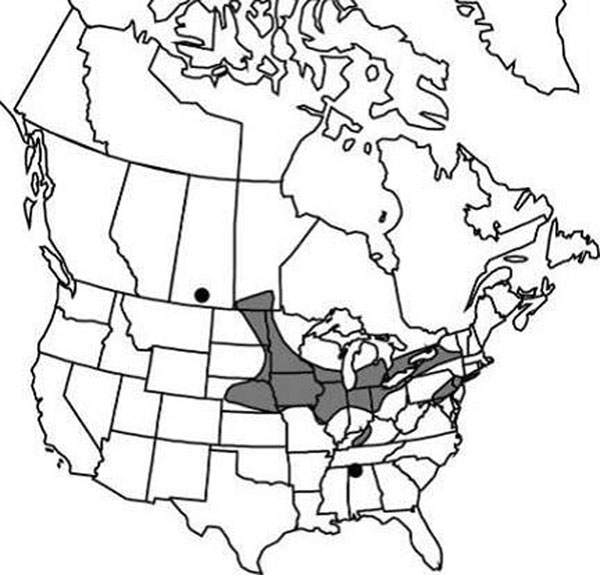
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Small White Lady’s-slipper in Canada.
Long description for Map 18
Map of the global range of the Small White Lady's-slipper in eastern North America. The current range extends across 18 states and two provinces. In the United States, it is considered extant in Alabama, Connecticut, Illinois, Indiana, Iowa, Kentucky, Maryland, Michigan, Minnesota, Missouri, Nebraska, New Jersey, New York, North Dakota, Ohio, South Dakota, Virginia, Wisconsin, and extirpated from Pennsylvania. It is no longer considered extirpated from New Jersey or Missouri. In Canada, extant subpopulations of the Small White Lady's-slipper occur in Manitoba and Ontario.
Habitat
In Canada, Small White Lady’s-slipper typically grows in remnant fragments of moist, calcareous native prairie openings. This includes patches of prairie remnants in roadside ditches surrounded by agricultural fields. Most sites appear to have some sub-surface water seeping through them. When on ridges or adjacent to trees or tall shrubs, its preferred aspect is south or west, as it is shade-intolerant. The subpopulation in Hastings County occurs in a fen.
Biology
Small White Lady’s-slipper requires approximately three years to produce its first leaf and 12 or more years to produce its first flower. The species is capable of extended dormancy, surviving underground for as long as six years, until suitable conditions occur for above ground growth. In Manitoba, late spring frosts are known to reduce fruit production to 1 or 2% relative to usual yields. Although the microscopic wind-dispersed seeds can disperse thousands of kilometres, they require specific soil fungi to provide nutrients for successful germination.
Population Sizes and Trends
In Manitoba, there are approximately 22,000 mature individuals (flowering stems). In Ontario, there are approximately 536 mature individuals in the Hastings County subpopulation. Data are currently unavailable for the Walpole Island subpopulation. Because of the potential for extended below-ground dormancy, and because the number of flowering stems varies among individuals, there is a high degree of uncertainty associated with estimates of Small White Lady’s-slipper abundance and therefore population trends are difficult to assess.
Threats and Limiting Factors
The most imminent, widely documented threats to Small White Lady’s-slipper are related to loss, degradation and fragmentation of its prairie habitat. Natural and anthropogenic factors that contribute to ongoing habitat decline include encroachment by woody vegetation, invasive species, and urban development. Nine of Manitoba’s 19 extant subpopulations are restricted to remnant prairie along roadsides. Plants in these habitats are subject to direct harm from activities such as mowing during flowering and fruiting seasons, maintenance of fence lines and utility cables, spraying of herbicides, and trampling. Illegal collecting is also more likely in these more accessible sites.
Natural limiting factors include light and moisture availability, low seedling survival, long time to maturity, low sexual reproductive rates, low genetic diversity, requirements for specific soil fungi and pollinators, competition with woody and weedy vegetation, browsing, late season frost, and hybridization. Hybridization with Yellow Lady’s-slipper is known to occur throughout the North American Range. However, genetic assimilation of Small White Lady’s-slipper by Yellow Lady’s-slipper does not seem imminent where Small White Lady’s-slipper is locally more abundant (most Canadian subpopulations).
Protection, Status, and Ranks
Small White Lady’s-slipper was first assessed by COSEWIC and designated Endangered in 1981. The status was re-examined and confirmed by COSEWIC in April 1999 and in May 2000. Status was re-examined by COSEWIC in November 2014 and designated Threatened. Small White Lady’s-slipper is currently listed as Endangered on Schedule 1 of Canada’s Species at Risk Act. A draft national Recovery Strategy was submitted to Environment Canada in 2011 that includes designation of proposed critical habitat. It is listed as Endangered under Manitoba’s Endangered Species Act and Ontario’s Endangered Species Act, 2007.
Small White Lady’s-slipper subpopulations occur on private, provincial, and First Nations lands. Most subpopulations are not adequately protected, either due to lack of awareness (often due to changes in land ownership/management) or lack of information and resources to manage habitat for the benefit of Small White Lady’s-slipper.

Photo: © Syd Cannings
Long description for Figure 19a
Photo of the Spiked Saxifrage, Micranthes spicata, in fruit, growing beside a stream. The leaves are mainly basal, with long-petioles, orbicular to reniform, and with sharply toothed and ciliated margins.
Reason for designation
This perennial wildflower grows only in Yukon and Alaska. In Canada it is restricted to small sites in a restricted geographical area where it shows genetic differences from the Alaskan population. It lives along cool, shady creeks and in moist, rocky alpine areas that may be affected by mining activities and the potential effects of climate change.
Wildlife Species Description and Significance
Spiked Saxifrage is a large, showy perennial herb, growing singly or in tufts from short, thick rhizomes. The inflorescence is borne on a stalk 15-70 cm tall.
Spiked Saxifrage is an eastern Beringian endemic, one of a small group of species known globally only from unglaciated areas in Alaska and western Yukon. The Canadian population is at the eastern edge of the species’ range and has been shown to be genetically distinct from the Alaskan population. In Yukon, Spiked Saxifrage appears to occupy a narrow ecological niche, with very specific habitat conditions and a short growing season.
Distribution
Spiked Saxifrage is endemic to Yukon and Alaska. In Alaska, it occurs throughout much of the central part of the state; in Canada it is known from 12 subpopulations in western Yukon. Approximately 10% of its global range is in Canada.

Source: COSEWIC 2015. COSEWIC Assessment and Status Report on the Spiked Saxifrage in Canada.
Long description for Map 19
Map of the global range of the Spiked Saxifrage, which has been found only in Alaska and western Yukon Territory. In Alaska, the species occurs scattered throughout much of the central part of the state from the Yukon border to the west coast.
Habitat
In Canada, Spiked Saxifrage grows in two distinct habitats, both characterized by cool, moist conditions during the growing season: the shores of cool, shady creeks, and moist, rocky alpine meadows. Along creeks, it grows on moist rock shelves of adjacent outcrops and on narrow bordering floodplains. In those places it grows in small piles of siltand moss-covered substrate, and on exposed soil. Plants may grow singly but often form dense clusters of up to several dozen plants. In moist alpine and upper subalpine, it grows among boulders and rock rubble, in turf at the edge of stabilized scree.
Creeks supporting subpopulations of Spiked Saxifrage in Yukon share a number of characteristics: year-round flow of clear, cold water in narrow, rocky beds that are subject to “glaciering” (i.e., aufeis - ice that forms in winter as spring-fed water constantly flows over the frozen creek that may persist into July) and/or permafrost, which helps to maintain a humid, cold microclimate; with rock outcrops bordering the creeks, and abundant shade from forests of Alaska Paper Birch and/or White Spruce, alders and willows.

Photo: © Syd Cannings
Long description for Figure 19b
Photo of Spiked Saxifrage habitat at Spicata Creek, showing waterfalls, rock outcrops bordering the creek and abundant shade from forests of Alaska Paper Birch and/or White Spruce, alders and willows. Other vegetation consists of a diverse assortment of low shrubs, mosses, grasses, forbs, and abundant leaf litter.
Biology
Little is known of the biology of Spiked Saxifrage. Reproduction is by seeds and by rhizomes; conditions for germination are unknown. Self-fertilization is common in the Saxifrage family, and may occur with Spiked Saxifrage. Longevity of the plants and possible seed banks are unknown.
The plant’s ability to withstand and repopulate after disturbance is unknown. It apparently can survive flooding, but severe flood events (e.g., a flash flood) may scour the floodplain and eliminate existing subpopulations and possibly seed banks. However, plants growing on the outcrops above flood level may provide a seed source for repopulation, if essential habitat characteristics have not been altered.
Population Sizes and Trends
Twelve subpopulations totalled 4680+ plants in 2014, of which 3244 are estimated to be mature. Though more plants are expected, it is unlikely the total will exceed 10,000.
Despite over a century of botanical collecting in the region, Spiked Saxifrage was only reported once in Canada (in 1899) until it was rediscovered in 2009, so it seems the species was uncommon or rare even during the gold rush era of the late 1800s and early 1900s. Although no population trends can be derived from data at hand, much of the species lowland habitat was likely altered or destroyed by placer mining, road-building, and wood cutting since the late 1800s. These activities are continuing. Alpine occurrences appear to be pristine.
Threats and Limiting Factors
Placer mining is the most extensive cause of habitat loss for Spiked Saxifrage in Yukon. Placer mining activity fluctuates in rate and scope with changes in gold prices. Subpopulations can be destroyed or diminished as a direct result of mining, or by upstream activities that affect habitat, such as siltation (sediment build-up), damming, stream realignment, etc. As well, natural processes such as flash flooding, forest fires, and landslides may be increasing in frequency and severity due to human-induced climate change. There are no imminent threats to the four alpine subpopulations; however habitat is limited to a small region in southwest Yukon. The effect of climate change and advanced mineral development could threaten these subpopulations in the future.
Protection, Status, and Ranks
Spiked Saxifrage has a NatureServe Global rank of G3G4 (Vulnerable to Probably Secure). Its National Rank in the U.S. is N3N4 (Vulnerable to Probably Secure), and in Canada is N2 (Imperilled). Its Subnational Rank in Alaska is S3S4 (Vulnerable to Probably Secure), and in Yukon is S2 (Imperilled). The National General Status ranks for Canada and Yukon are ‘May be at Risk’.
Spiked Saxifrage currently has no legal protection in Canada, and is not listed under the U.S. Endangered Species Act or the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES).
Active placer and/or quartz mining claims occur on or upstream of the plant’s habitat on seven of the twelve subpopulations representing about 70% of the Canadian population. While there are restrictions on how operations are conducted on those claims, these restrictions target protection of fish habitat, and there is no legal obligation to protect the habitat or existing subpopulations of Spiked Saxifrage.

Photo: © Sean Blaney
Long description for Figure 20a
Photo showing Tall Beakrush, Rhynchospora macrostachya, inflorescences. The inflorescences consist of long-peduncled, dense clusters of elongated brown spikelets that are predominantly in terminal clusters.
Reason for designation
In Canada, this perennial sedge only occurs along two acidic, peaty lakeshores in southwestern Nova Scotia, where it is disjunct from its main U.S. Atlantic Coastal Plain distribution. Its small population size (ca 700 individuals total in two subpopulations) and very specific habitat needs make it vulnerable to lakeshore development, water regulation (for hydroelectric power), and shading and competition from introduced invasive plants such as Glossy Buckthorn, which benefit from increased concentrations of nutrients in these two lakes.
Wildlife Species Description and Significance
Tall Beakrush is a perennial, herbaceous sedge. Flowering stems, arising from a dense clump of basal leaves, reach 150 – 170 cm in the United States and about 100 cm in Canada. Flowers are enclosed within brown scales, with each having male and female parts and six elongate, barbed bristles. Fertilized flowers develop into a hard, flattened achene 5 to 6 mm long, topped by a greatly elongated tubercle.
Tall Beakrush is one of many species of the Atlantic Coastal Plain that are disjunct and nationally rare in southern Nova Scotia, and that have received fairly widespread attention and appreciation in the region through ongoing outreach programs. The Canadian population is isolated from others by 468 km and is the northernmost worldwide, suggesting potential significance to the species’ range-wide genetic diversity. The seed-like achenes of Tall Beakrush can also be an important food for wild ducks in the southern United States.
Distribution
Tall Beakrush is predominantly a species of the Atlantic and Gulf Coastal Plains between southern Maine, northeastern Florida, and Louisiana, but it also occurs in southeast Michigan and adjacent Indiana, eastern Oklahoma and adjacent areas of Kansas, Missouri and Arkansas, and along the Tennessee-Alabama border. Isolated records are reported for Kentucky, and northern New York. Reports from Illinois, Mississippi and Vermont are erroneous. Canadian occurrence is restricted to two lakes 23 km apart in southern Nova Scotia. Canada supports less than 1% of the global population.
Source: Modified from COSEWIC 2014. COSEWIC Assessment and Status Report on the Tall Beakrush in Canada
Long description for Map 20
Map of the Canadian distribution of the Tall Beakrush at Carrigan and Molega Lakes, in the COSEWIC Atlantic National Ecological Area in southwestern Nova Scotia.
Habitat
Tall Beakrush is an obligate wetland plant occurring in Canada on shallow acidic open lakeshores that are fully exposed (or nearly so) during summer low water levels. Substrates are mostly gravelly, often with a thin layer of peaty organic soil on top, but some plants are on deeper peat or on shallow organic soil within cracks in exposed bedrock. In the southern United States, Tall Beakrush also occupies freshwater and slightly saline tidal marshes, swamp forests, and marshes and sloughs within tallgrass prairies, and it can occur in disturbed habitats such as ditches, all-terrain vehicle tracks, pipeline rights-of-way, rice fields and impoundments.

Photo: © Sean Blaney
Biology
In Nova Scotia, Tall Beakrush flowers from July to September. Pollination is presumed to be largely or entirely by wind, as is the case with most sedges. It is believed to be selfcompatible. Seed-like achenes are dispersed from the parent plant in the fall and their long bristles may facilitate dispersal via floatation or on animals. Internal and external dispersal by waterfowl over longer distances is also likely. In a closely related species, germination occurs best in drier periods than are ideal for growth. Reproduction before age one occurs in the United States but probably requires at least two or three years in Nova Scotia, based on observation of mid-sized, non-flowering rosettes. The species is non-rhizomatous but vegetative reproduction occurs over very short distances via production of new rosettes to the side of existing ones. Demographics of vegetative reproduction are unknown, as are longevity of genetic individuals and ramets, and generation time.

Photo: © Sean Blaney
Population Sizes and Trends
A 2013 comprehensive count of the Canadian population found 688 individuals, 648 (95%) of which were in a 1.3 km x 0.7 km area on Carrigan Lake and 36 (5%) of which were in a 30 m stretch of shoreline on Keddy Cove on Molega Lake. Survey effort is sufficient to suggest that it is unlikely that large numbers of additional individuals would be found on these lakes, or that many additional undiscovered subpopulations are present in Canada. Trends are unknown but habitat near current subpopulations suggests stability or small population declines in the past three generations and potentially significant historical subpopulation losses from damming.
Threats and Limiting Factors
Lakeshore development has not yet affected Carrigan Lake plants but 38% of the Canadian population there is adjacent to private land potentially subject to shoreline development, and an additional 39% is on land owned by Nova Scotia Power that might one day be sold. All of the Molega Lake subpopulation (5% of the Canadian population) is in a small, undeveloped zone within shoreline otherwise occupied by cottages, and is under significant threat of further development. All plants at Carrigan Lake (95% of the Canadian population) occur within shoreline for which Nova Scotia Power has flooding rights associated with hydroelectric power generation. Nova Scotia Power believes that anthropogenic flooding has never occurred on Carrigan Lake, and suggests it is unlikely for the foreseeable future. The invasive exotic shrub Glossy Buckthorn is already present immediately around some occurrences at Carrigan Lake and occurs within 950 m of the Molega Lake subpopulation, but is believed unlikely to impact most occupied lakeshore habitat. Competitive exclusion by more aggressive plants responding to eutrophication from mink farm waste or from the cumulative effects of hundreds of additional cottages on Molega Lake is a potential future threat.
Protection, Status, and Ranks
Tall Beakrush has no legal protected status in Canada and no occurrences are within protected areas, but it has legal protection in Maine, Connecticut and Tennessee. Tall Beakrush is Critically Imperilled (N1) in Canada and in Nova Scotia (S1) and is ranked as May Be At Risk in Nova Scotia and Canada under the General Status process. It is globally secure (G4) and nationally secure in the United States (N4), but Critically Imperilled (S1) in Kentucky, Maine, Missouri and Rhode Island, borderline Critically Imperilled (S1S2) in Connecticut and Tennessee, Imperilled (S2) in Arkansas, Indiana and Kansas, Vulnerable (S3 or S3?) in New York, Virginia and North Carolina and marginally Vulnerable (S3S4) in Michigan. Tall Beakrush is Apparently Secure (S4) in Delaware, and is unranked (SNR) in Alabama, Florida, Louisiana, Maryland, Massachusetts, New Jersey, Oklahoma, South Carolina, Texas, and the District of Columbia, and Unrankable (SU) in Georgia. It may be Imperilled in Florida and marginally vulnerable in Alabama, Georgia and Massachusetts.

Photo: © Lyn Baldwin
Long description for Figure 21a
Photo of the Tiny Tassel, Crossidium seriatum, showing a small dark green to golden brown moss.
Reason for designation
This very small moss has a very narrow range in Western Canada. It occurs only in the semiarid shrub steppe of four valleys in the Okanagan region of southernmost central British Columbia. Surveys have confirmed this species from only 20 sites on steep slopes associated with calcareous glacial lake deposits. Threats include erosion due to recreational use of the habitat, and maintenance of road cuts. Climate change may also be a threat, although the potential impacts are unknown. One site has been extirpated due to habitat conversion.
Wildlife Species Description and Significance
Tiny Tassel (Crossidium seriatum) is a small dark green to golden brown moss. It grows to 1-1.5 mm high, sometimes in clumps but more often as scattered individuals among other species of small dryland mosses. The population of Tiny Tassel in British Columbia represents the northernmost extent of the species’ range in North America. It occurs in Canada only within dry grasslands in the southern interior of British Columbia. These grasslands are a rare habitat type that occupies less than 1% of the British Columbia land base.
Distribution
Tiny Tassel occurs throughout western North America. It has been documented in Baja California and Chihuahua in Mexico; Arizona, California, Nevada, New Mexico, Idaho, and Washington in the United States; and British Columbia in Canada. Tiny Tassel is considered by some experts to be present in Europe; however, European taxonomic authorities now consider examples of Tiny Tassel documented from Europe to be a different, closely related species. Tiny Tassel may also be present in China.
In Canada, Tiny Tassel is known only from the southern interior of British Columbia, where it occurs in the valleys of the Fraser, Thompson, Nicola, and Okanagan rivers. Most known occurrences of Tiny Tassel are clustered around the towns of Kamloops and Penticton in the Thompson and Okanagan River Valleys, respectively.
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Tiny Tassel in Canada
Long description for Map 21
Map showning the distribution of the Tiny Tassel in North America, where there are at least 62 recorded occurrences. These include occurrences in Baja California and Chihuahua in Mexico; Arizona, California, Nevada, New Mexico, Idaho, and Washington in the United States; and British Columbia in Canada.
Habitat
Tiny Tassel occurs in semiarid and arid regions of western North America. It has been found in sagebrush, grassland, and desert regions. It occurs primarily on soil, often from calcium-rich parent material. In Canada, Tiny Tassel occurs on fine-textured soils associated with silts in the semiarid shrub steppe of south-central British Columbia. These silts, which tend to occur along the major valleys of the Thompson and Okanagan Rivers, are often calcareous and were derived from lake deposits formed during the most recent glacial period.

Photo: © Lyn Baldwin
Biology
Tiny Tassel reproduces through spores, although it is likely that it can also regenerate from stem and rhizoid tissue. The production of sporophytes in the British Columbia population of Tiny Tassel may be uncommon. Only one occurrence, now extirpated, has been observed with sporophytes. Bryophyte spores are often wind-dispersed and can result in very long-range dispersal. Given Tiny Tassel’s widespread distribution, including islands and post-glaciated environments, it is likely that at least episodic sporophyte production coupled with long- and short-range dispersal plays a role in its reproduction and spread. Tiny Tassel has physiological traits which allow it to survive in arid and semiarid environments, such as prolonged dormancy, curled leaf margins, leaf papillae and filaments, and leaf hair points.
Population Sizes and Trends
Tiny Tassel is currently known from 20 sites in British Columbia. There were previously 15 known sites with Tiny Tassel, 9 of which were recently confirmed as extant and 5 of which were presumed to be extant. One of these sites (Cache Creek) is extirpated. Tiny Tassel has recently been found at 6 additional locations, for a total of 20 currently known sites in British Columbia. Colonies of Tiny Tassel are small, scattered, and interspersed with other species of dryland mosses. It is therefore very difficult to estimate either the current population size of Tiny Tassel or changes in its abundance.
Threats and Limiting Factors
Tiny Tassel is restricted to specific habitats within the southern interior of British Columbia. Much of this habitat is under increasing pressure from human uses and development, including livestock grazing and agricultural and urban conversion. The location of the microsites where Tiny Tassel is most likely to occur, namely steep silt bluffs, may mitigate the direct effect of these uses. Tiny Tassel is likely to be affected by changing temperatures and precipitation patterns associated with climate change. It is difficult to predict, however, whether these changes will benefit or adversely affect Tiny Tassel.
Protection, Status, and Ranks
Tiny Tassel has no legal protection in any jurisdiction at the present time. Its global conservation status, assessed by NatureServe, is imperiled to apparently secure (G2G4). The Nevada Natural Heritage Program ranks it as imperiled (S2) and the British Columbia Conservation Data Centre ranks it as imperiled to vulnerable (S2S3). The species is unranked (SNR) in Arizona, California, Idaho, New Mexico, and Washington. In British Columbia, Tiny Tassel occurs on First Nations, private, and provincially managed Crown lands. No occurrences are on formally protected lands, although some sites are afforded some protection from development due to their geological instability.

Photo: © Jennifer Anderson (USDA-NRCS PLANTS Database)
Long description for Figure 22
Photograph of Toothcup (Rotala ramosior) stems. The leaves are borne in opposite pairs that alternate at right angles along the stem.
Reason for designation
This annual plant is known from the shores of only two lakes at the southern edge of the Canadian Shield in southeastern Ontario. Year-to-year fluctuations in water levels along the lakeshore impact the abundance of plants. Impacts from development, recreational boating activities, and manipulation of water levels have the potential to reduce the number of individuals.
Status history
The species was considered a single unit and designated Endangered in April 1999. Status re-examined and confirmed in May 2000. Split into two populations in November 2014. The Great Lakes Plains population was designated Threatened in November 2014.
Wildlife Species Description and Significance
Toothcup is a low growing annual plant in the loosestrife family (Lythraceae). Its small flowers are sessile, and usually solitary in the leaf axils. Flowers usually have 4 white or pink petals up to 1 mm long. In Canada, Toothcup is at the northern limit of its North American range. Populations at the edge of a species’ range may be genetically distinct.
Distribution
Toothcup is native to North America, Central America, and South America. In North America, it ranges in the east from Massachusetts south to Florida, and west from southern Minnesota, south to Texas and into Mexico. It is found only sparingly in the Midwestern US and Intermountain region, appearing more frequently along the west coast from California, north to south-central British Columbia. It has a disjunct distribution in Canada, known from Ontario and British Columbia.
In Ontario, Toothcup is restricted to shoreline habitat on Puzzle Lake and Sheffield – Long Lake (an enlargement of the Salmon River) and adjoining Clare River. These water bodies are situated along the southern edge of the Canadian Shield in the county of Lennox and Addington.
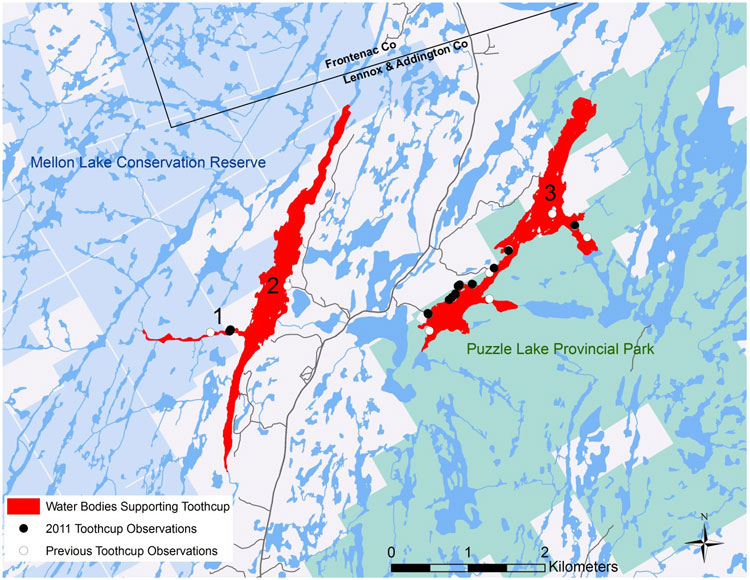
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Toothcup, Great Lakes Plains population, in Canada
Long description for Map 22
Map showing Ontario localities where the Toothcup was observed in 2011 and localities where it was previously observed but not found in 2011. The species is restricted to shoreline habitat on Puzzle Lake and Sheffield - Long Lake (an enlargement of the Salmon River) and adjoining Claire River. These water bodies are situated along the southern edge of the Canadian Shield in the county of Lennox and Addington.
Habitat
Toothcup is a species of open, seasonally wet areas with natural or artificial water level fluctuation. Its habitat includes riverbanks, ditches, pond margins, sandy to muddy shores, interdunal swales, and occasionally, moist edges of cultivated fields. In southcentral Ontario, it grows in moist, shallow bedrock crevices filled with small accumulations of sand, gravel and peat along lake and river shorelines. In southwestern Ontario, it formerly grew in remnant sand prairie within moist old field habitat. In the South Okanagan Valley of British Columbia, Toothcup inhabits moist to wet, sometimes saline, muddy to sandy shorelines of lagoons or ponds, inshore swales, and shallow depressions. In the Kamloops area, it inhabits sandy or silty, shallow depressions and interdunal swales, or muddy silty-sands of exposed channel banks.
Biology
Toothcup is an annual plant associated with periodically flooded areas, and populations may undergo large fluctuations from year to year. It reproduces sexually, producing copious amounts of seed. The large majority of Toothcup seeds are dormant when they mature in autumn, but tend to break dormancy while flooded in late fall or winter.
Population Sizes and Trends
The total Canadian population of Toothcup was estimated to include at least 6,859 individuals in 2011, when it was known from four subpopulations, including two in Ontario (Great Lakes Plains DU) and two in British Columbia (Southern Mountain DU).
In Ontario, counts from 2011 were low relative to counts from previous years. A total of 1,444 mature individuals was recorded (305 mature individuals from Sheffield - Long Lake / Clare River and 1,139 from Puzzle Lake). The highest count was made in 2004, when 4,325-6,325 mature individuals were counted (2,615-4,615 from Sheffield - Long Lake / Clare River and 1610-1710 from Puzzle Lake). In British Columbia, between 5,410 and 5,570 individuals were observed in 2011 at two sites in the Kamloops subpopulation. No individuals were observed from the other previously reported Kamloops site at McArthur Island. No individuals were observed at the South Okanagan Valley subpopulation in 2011, but not all sites were visited, including one which held an estimated 12,000 individuals in 2004. The highest single year estimate here was 12,180 individuals in 2004.
Since the previous assessment, no losses of Toothcup subpopulations have been documented in Ontario. Infrequent counts at both subpopulations suggest fluctuations among years, though census data are insufficient for assessment of trends. In British Columbia, although the Kamloops subpopulation is extant, the South Okanagan Valley subpopulation is believed to be declining and several sites are known to have been extirpated historically. The likelihood of natural immigration of Toothcup from outside Canada is extremely low.
Threats and Limiting Factors
The Canadian range of Toothcup is limited by its restricted occurrence to seasonally flooded habitats. In Ontario, shoreline development and recreational activities are the main threats. In British Columbia, invasive plant species pose the greatest threat to extant populations of Toothcup. Habitat loss through development, habitat degradation and livestock, as well as the modification of natural Osoyoos Lake levels, are also threats in British Columbia.
Protection, Status, and Ranks
Toothcup was originally designated by COSEWIC as Endangered in Canada in 1999 and is listed on Schedule 1 of the federal Species at Risk Act. A federal Recovery Strategy has not yet been finalized for Toothcup. COSEWIC assessed the Great Lakes Plains population of Toothcup as Threatened and the Southern Mountain population as Endangered in November 2014. Toothcup is listed as an Endangered Species under the Ontario Endangered Species Act, 2007, receiving species and habitat-level protection. It also receives protection in Puzzle Lake Provincial Park and Mellon Lake Conservation Reserve. There is no specific legal protection for Toothcup in British Columbia. The General Status rank for Toothcup is “At Risk” for Ontario, British Columbia, and Canada.

Photo: © Alan Harris
Long description for Figure 23a
Photo of an adult male Vivid Dancer, Argia vivida (lateral view), showing the typical bright blue colouring with black markings.
Reason for designation
This damselfly is found in southern British Columbia and Banff, Alberta. Through much of its Canadian range it is restricted to thermal springs, but in the hot valleys of the Okanagan and the Fraser it is also found in cooler, spring-fed creeks. Habitat loss and degradation at most sites suggest subpopulations have declined. The species is threatened by intensive recreational use of thermal springs, livestock trampling at cool springs, and introduced fish. Sites are also vulnerable to potential tourism development and changes in springs caused by events such as droughts, earthquakes and landslides.
Wildlife Species Description and Significance
Vivid Dancer is a robust damselfly (Order Odonata) 29.5 – 35mm long. Adult males are typically bright blue or occasionally violet blue, both forms with black markings. Females resemble males or may have more subdued colours, typically orange or redbrown and black. Vivid Dancer is distinguished from similar damselflies in other genera by wing venation patterns, the shape of reproductive structures, and comparatively longer leg spines. Vivid Dancer larvae are short, stocky and flattened, with broad, heavily pigmented, leaf-like gills.
For much of its Canadian range, Vivid Dancer is a specialist of thermal spring habitats. The species is the only documented odonate adapted to breed in geothermal springs in North America. The floral and faunal communities within thermal springs vary from site to site.
Distribution
The range of Vivid Dancer is within the southern half of British Columbia. The westernmost site is in Pemberton, extending east through the Okanagan Valley, the Kootenays to Banff in western Alberta. The species ranges south through the western United States to the southern tip of the Baja Peninsula in Mexico, east to Nebraska.
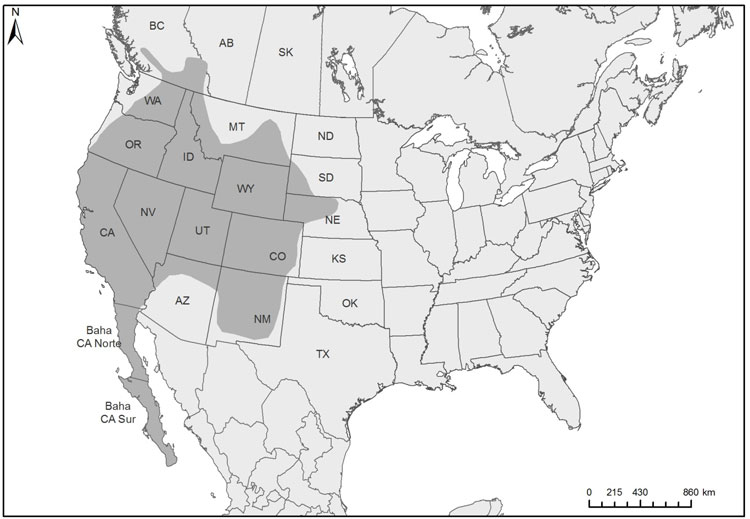
Source: COSEWIC 2015. COSEWIC Assessment and Status Report on the Vivid Dancer in Canada
Long description for Map 23
Map of the global and Canadian distribution of the Vivid Dancer, where it ranges in western North America from south-central British Columbia south through 14 states to the southern tip of the Baja Peninsula in Mexico and east to Nebraska.
Habitat
Vivid Dancer larvae inhabit both thermal and cold springs and associated small streams. In Canada, the species primarily inhabits thermal springs at least 10°C warmer than the mean annual air temperature. The species is also recorded from low elevation cool springs in the Okanagan Valley and near Lytton, which are also two of the warmest regions of Canada during summer months. Adults forage and roost adjacent to water habitats and nearby forests.
Biology
Vivid Dancer overwinters as larvae and emerges as adults from late-April through mid- October, depending on the temperature of the spring. The minimum temperature for egg development is 11°C. Within thermal springs the generation time is one year, but in cooler springs the generation time is up to three years. Adults disperse 700 - 800 m within the surrounding forest and feed on small flying insects, such as mosquitoes, mayflies, and small moths. On cool days adults bask in sunlit forest patches to increase body temperature. In late summer, adults return to the spring to breed. Females lay eggs on emergent vegetation below the water’s surface.
Larvae are aquatic, feeding on small invertebrates and using aquatic vegetation for cover. Adults fall prey to robber flies, dragonflies, spiders, amphibians, and birds. Fish, amphibians, and possibly waterfowl likely consume Vivid Dancer larvae.

Photo : © Jennifer Heron
Long description for Figure 23b
Photograph of a newly emerged Vivid Dancer (teneral stage) on a rock.
Population Sizes and Trends
Vivid Dancer population information is available for one site at Banff, with an estimate of 2,000 to 20,000 adults in 2005. Other springs lack population estimates; however, adult numbers are probably smaller based on available quality or undeveloped habitat. Population trend data are also lacking but most springs have undergone habitat damage from commercial and recreation use, suggesting populations have likely declined.
Threats and Limiting Factors
Habitat loss and degradation are the primary threats to Vivid Dancer. Threats include water diversion and degradation (e.g., cooling, pollution) by commercial thermal spring operations, alteration of springs and drainage channels by recreational users at noncommercial thermal springs, and livestock use at the cool springs sites in the Okanagan. Other potential threats include introduced fish at Banff and altered spring water discharge caused by road building. Sites are also vulnerable to changes in spring water discharge caused by stochastic events such as droughts, seismic activity, and landslides.
Protection, Status, and Ranks
Vivid Dancer is not protected under provincial legislation in BC or AB. Like all wildlife in national parks, Vivid Dancers in Banff National Park are protected under the National Parks Act. Some sites in Banff National Park are offered some habitat protection under the designation of sections of certain springs as critical habitat for the Banff Springs Snail. In BC, Vivid Dancer habitat occurs within heavily developed private lands and provincial parks, both with no specific habitat protection. The global status rank is Secure (G5) and national rank in Canada is Imperilled (N2). Provincially the species is ranked Imperilled (S2) in BC and Critically Imperilled (S1) in Alberta.

Photo: © John Klymko
Long description for Figure 24a
Photo of a Yellow-banded Bumble Bee (Bombus terricola) nectaring. This bee has a medium-sized body with a short head (malar space just shorter than broad). The abdomen displays a distinctive yellow and black pattern, and the body hair is short and even.
Reason for designation
This bee has an extensive distribution in Canada, ranging from the Island of Newfoundland and the Maritime provinces, west to eastern British Columbia, and north into the Northwest Territories and extreme southwestern Yukon. Perhaps 50- 60% of the global range of this species occurs in Canada. This species was historically one of the most common bumble bee species in Canada within its range. However, while this species remains relatively abundant in the northern part of its range, it has recently declined by at least 34% in areas of southern Canada. Causes for declines remain unclear, yet pesticide use, habitat conversion, and pathogen spill over from managed bumble bee colonies are suspected contributing factors.
Wildlife Species Description and Significance
Yellow-banded Bumble Bee is a medium-sized bumble bee with a short head and tongue length relative to other species. The distinctive yellow and black abdominal band pattern is consistent throughout its range. This species is an important pollinator of a variety of agricultural crops and native plant species.
Distribution
Yellow-banded Bumble Bee occurs in eastern North America from New Jersey to Newfoundland and Labrador, and west through the northern United States and most of Canada to southern Northwest Territories, southeastern Yukon, and eastern British Columbia. In the southern part of its range, there are scattered records from upper elevations of the Appalachian Mountains as far south as Georgia.
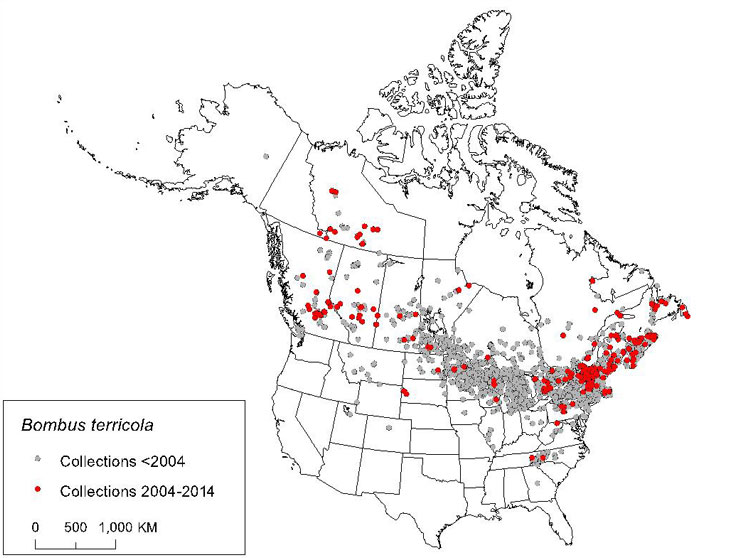
Source: COSEWIC 2015. COSEWIC Assessment and Status Report on the Yellow-banded Bumble Bee in Canada
Long description for Map 24
Map of the global range of the Yellow-banded Bumble Bee, which ranges from Georgia north through the eastern United States to Labrador, and west through the northern United States and Canada to Montana, British Columbia, and the Northwest Territories. The map indicates collections made before 2004 and between 2004 and 2013.
Habitat
Yellow-banded Bumble Bee occurs in a diverse range of habitats, including mixed woodlands, farmlands, urban areas, montane meadows, prairie grasslands and boreal habitats. It has been recorded foraging on flowers for pollen and nectar from a variety of plant genera. Like many bumble bees, it usually nests underground in pre-existing cavities such as abandoned rodent burrows and rotten logs. Yellow-banded Bumble Bee queens overwinter underground and in decomposing organic material such as rotting logs.
Biology
Yellow-banded Bumble Bee has an annual life cycle. Mated queens (colony founders) emerge from wintering sites in the spring and search for potential nest sites. Once a nest site is chosen, the queen then forages for pollen and nectar, returns to the nest site and lays eggs to eventually produce a brood of workers. Workers emerge and take over nest care and foraging for pollen and nectar. In late summer, males and new queens are produced. These reproductive individuals leave the colony, mate, and mated queens enter hibernation while all other castes, including the old queen, perish by fall.

Photo: © Corey Sheffield
Long description for Figure 24b
Photograph of a female Yellow-banded Bumble Bee nectaring and with full pollen basket.
Population Sizes and Trends
Yellow-banded Bumble Bee was once one of the most common species in collections of bumble bees made in Canada. However, in the early 1990s populations began to decline in the southeastern part of their range in Ontario. At many sites, Yellow-banded Bumble Bees once accounted for > 20% of all bumble bees collected, yet in recent studies (in the past ten years), they typically make up < 4%. The Yellow-banded Bumble Bee has declined significantly at nine of 10 sites analyzed across southern and central Canada, with an average of 66.5% reduction in proportional abundance between pre- and post-10-year sampling periods. The species is now thought absent from many historical collection sites in these areas. However, there are few historical and modern collection data across the northern part of the species’ range in the boreal forest.
Threats and Limiting Factors
The specific causes of decline for Yellow-banded Bumble Bee are unknown, although it is likely due to a combination of factors. Possible threats include introduced pathogens from managed bumble bees used in greenhouses and the transfer of these pathogens to native bumble bees when introduced bees escape, pesticide use associated with agriculture (including neonicotinoids), climate change and habitat loss within urban areas and areas of intensive agriculture.
Protection, Status, and Ranks
There are no laws in Canada that specifically protect the Yellow-Banded Bumble Bee, its nest sites or habitat. In Québec, Yellow-banded Bumble Bee is integrated on the Liste des espèces susceptibles d’être désignées menacées ou vulnérables (list of wildlife species likely to be designated threatened or vulnerable). The NatureServe global conservation status rank is G2G4 (Imperiled to Apparently Secure).

Photo: © Syd Cannings
Long description for Figure 25
Photo of a clump of Yukon Podistera, Podistera yukonensis, showing flowering stems bearing compound umbels of small yellow flowers. The erect or ascending basal leaves are blue-green and pinnate with three to six pairs of opposite leaflets. The lowermost leaflets are usually ternate (compound with three equal divisions), while the others are simple.
Reason for designation
This long-lived plant, almost entirely restricted to Canada, is at risk due to projected loss of its alpine habitat as a result of rapidly changing climate. In addition, mining and mineral exploration is occurring at or near several locations.
Wildlife Species Description and Significance
Yukon Podistera is a tufted perennial 10-40 cm tall that often forms clumps from a stout elongate taproot. Blue-green pinnate basal leaves subtend the leafless flowering stems bearing compound umbels of small flowers that are bright yellow when newly opened, but fade to white.
Yukon Podistera is one of just a few species restricted globally to unglaciated areas of Alaska and west-central Yukon (eastern Beringia); approximately 90% of its global range lies within Canada. Yukon Podistera occupies a narrow ecological niche in Yukon.
Distribution
Yukon Podistera is restricted globally to the west-central Yukon and a small area of adjacent eastern Alaska. The 22 known Canadian subpopulations are found in two disjunct regions, the northern one centred in the southern Ogilvie Mountains and the southern in the Dawson and Ruby ranges.
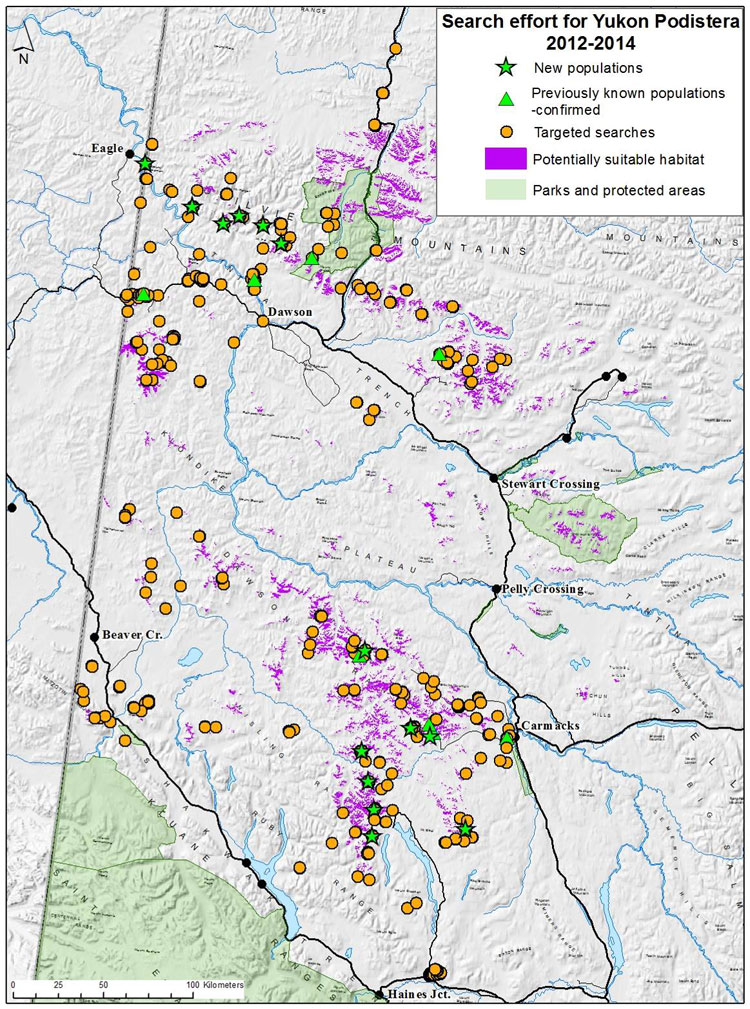
Source: COSEWIC 2014. COSEWIC Assessment and Status Report on the Yukon Podistera in Canada
Long description for Map 25
Map illustrating search effort for the Yukon Podistera in Yukon between 2012 and 2014. Symbols indicate localities where new populations were found, where previously known populations were confirmed, and where targeted searches were conducted.
Habitat
Yukon Podistera is restricted to dry, well-drained, rock-dominated habitat with sparse vegetation and limited soil development. It is shade-intolerant, and prefers substrates where surface materials periodically move downslope, or where there is slow movement through frost action, generally with some degree of slope. Yukon Podistera grows on rocky tors, talus slopes and on river bluffs with exposed bedrock. Most sites occur on southfacing slopes, but in a few sheltered microsites, some individuals were found on east- and west-facing slopes, with low snow accumulation. It grows from 702 to 1,757 m in elevation, with 15 of 22 Yukon subpopulations found between 1450 and 1700 m.
Biology
Little is known about the biology of Yukon Podistera, but it is likely a long-lived species. It is an early-flowering plant that appears to exhibit staggered fruit development. In most subpopulations the majority of plants did not flower, the two lower elevation subpopulations being the exception. Subpopulations visited later in the season all exhibited some degree of failure to set fruit (50-70% of plants in one subpopulation). Reproduction is by seed that likely disperses by wind over short distances. Whether the plant is able to withstand or adapt to disturbance is unknown.
Population Sizes and Trends
The total number of Yukon Podistera plants found in Canadian subpopulations is estimated to be at least 17,143 to greater than 24,093. Seven subpopulations had counts of 1,000 or more, ten subpopulations had 200-1000 individuals, and the remaining five subpopulations had fewer than 200 plants. Nearly 20,000 of the total are considered to be mature. No information is available on trends.
Threats and Limiting Factors
A loss of habitat as a result of climate change is expected to be the greatest threat to Yukon Podistera. Mining activity, which can directly affect subpopulations and habitat, has also been identified as a low threat. Seven of the 22 known Canadian subpopulations of Yukon Podistera occur on active quartz mining claims (18% of the Canadian population), and one of these also falls partially within an active placer mining claim. Seven others (40% of the Canadian population) are located in heavily staked areas and are within 2 km of an active claim; however, direct long-term effects to these populations are not considered imminent. Only one subpopulation occurs in a protected area.
Protection, Status, and Ranks
Yukon Podistera currently has no legal protection or status in Canada or the US. It is not listed under the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES).
NatureServe (2014) considers Yukon Podistera to be vulnerable globally (G3) in Canada (N3) and in Yukon (S3). In the United States, it is considered critically imperilled to imperilled nationally (N1N2) and in Alaska (S1S2). The Canadian and Yukon General Status rank is 2, “May Be At Risk.” While quartz mining is regulated under the Quartz Mining Act (2003), exploration is often conducted under the threshold for a land use permit and environmental assessment. Exploration at this level may pose a serious threat to some small subpopulations.
Lichens (1)
Mosses (1)
Vascular Plants (10)
- Blue Ash
- Fascicled Ironweed
- Griscom’s Arnica
- Limber Pine
- Phantom Orchid
- Small White Lady's-slipper
- Spiked Saxifrage
- Tall Beakrush
- Toothcup (Great Lakes Plains population)
- Yukon Podistera
Arthropods (4)
Molluscs (2)
Reptiles (2)
Birds (3)
Terrestrial Mammals (2)