The COSEWIC Summaries of Terrestrial Species Eligible for Addition or Reclassification on Schedule 1 - January 2018
The following section presents a brief summary of the reasons for the COSEWIC status designation of individual species, and their biology, threats, distribution and other information. For a more comprehensive explanation of the conservation status of an individual species, please refer to the COSEWIC status report for that species, also available on the Species at Risk Public Registry
or contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment Canada
Ottawa ON K1A 0H3
- Anticosti Aster
- Blanding's Turtle (Great Lakes / St. Lawrence population)
- Bullsnake
- Caribou (Barren-ground population)
- Caribou (Eastern Migratory population)
- Caribou (Torngat Mountains population)
- Eastern Banded Tigersnail
- Evening Grosbeak
- Golden-eye Lichen (Great Lakes population)
- Golden-eye Lichen (Prairie / Boreal population)
- Harris's Sparrow
- Lark Bunting
- Long's Bulrush
- Magdalen Islands Grasshopper
- Monarch
- Pink-footed Shearwater
- Rusty Cord-moss
- Sonora Skipper
- Spotted Wintergreen
- Transverse Lady Beetle
- Western Painted Turtle (Pacific Coast population)

Photo: © Atlantic Canada Conservation Data Centre
Long description
Symphyotrichum anticostense
Vascular Plants
Special Concern
Quebec, New Brunswick
This clonal plant is restricted to calcareous shores of larger rivers (and occasionally lakes) in Eastern Québec and New Brunswick. At least 95% of its small global range occurs in Canada. Invasive species threaten habitat quality and there is some evidence that localized hybridization and deer browsing may minimally affect population persistence at local scales. Since the species’ last assessment of Threatened in 2000, extensive searching resulted in the documentation of several new subpopulations. The subpopulations appear to be stable.
Anticosti Aster is a 10 to 75 cm tall, herbaceous species that spreads by long rhizomes to form loose clonal colonies. The stiff, narrowly linear leaves are somewhat leathery in texture, often arched, and have smooth or minutely toothed margins. Flowers are in long-stalked flower heads composed of purple ray (petal-like) florets and yellow disk florets. Anticosti Aster is a Holocene (<11,700 year old) species that originated by hybridization of New York Aster and Rush Aster. Identification based solely on morphology is not entirely reliable, particularly in New Brunswick due primarily to similarity with narrow leaved forms of New York Aster. It is of interest as a rare regional endemic species of postglacial origin that grows in association with many other plant species of conservation concern within regionally significant calcareous river shore communities.
Anticosti Aster is a rare northeastern North American endemic species occurring in three distinct regions: 1) Anticosti Island, QC, 2) Lac Saint-Jean, QC and 3) the southern and eastern portions of the Gaspé Peninsula, QC, northwestern New Brunswick (Restigouche and Saint John river systems), and northeastern Maine (Aroostook River, a Saint John River tributary). Each of these distinct regions could represent an independent hybrid origin for the species, but there is currently insufficient evidence of genetic distinctiveness to warrant considering them as separate designatable units.

Global distribution of Anticosti Aster (aerial imagery source: Esri World Imagery Basemap).
Anticosti Aster is found on the open shores of larger rivers within the zone of annual flooding, and sometimes on similar lakeshores. It is strongly associated with underlying calcareous sedimentary bedrock and surface materials (mainly limestone). Plants are most often found on wide, low gradient rock, cobble, gravel and sand shores in unvegetated or sparsely vegetated areas between the highest and lowest water marks. At one site, Anticosti Aster has extensively colonized the gravelly roadside and railroad bed adjacent to a river, indicating potential to take advantage of disturbed habitats.
Anticosti Aster is a colonial perennial species, spreading vegetatively via rhizomes, with genetic individuals likely capable of persisting for many years. Lifespan of individual shoots or rhizome segments is unknown. It is likely dependent on insect pollination. It flowers from late July to late September and disperses seed from mid-August to late fall. Transport by water flow is likely the most significant mode of dispersal. Plants can probably produce flowers within the first year, but in the field, time to sexual maturity is likely greater.
The total population of Anticosti Aster in Canada is roughly estimated at 410,000 to 1,063,000 stems, distributed at 18 subpopulations for which identification is considered reliable (1 at Lac Saint-Jean, 7 on Anticosti Island and 10 in the Gaspé Peninsula / western New Brunswick region). Collectively, subpopulations in the Gaspé / western New Brunswick region of occurrence contain at least 95% of the total known global population. The Restigouche River (NB and QC) supports the largest known occurrence (hundreds of thousands of stems), extending over roughly 80 km of river. Gaspé Peninsula’s Grande Rivière, Bonaventure and Petit Pabos rivers are the next largest subpopulations (>68,000, >20,000 and >5000, respectively). All other known subpopulations are estimated at a few thousand stems or fewer. Subpopulations are not believed to have changed significantly since the last status assessment in 2000.
Historically, a substantial amount of potential habitat has been lost through construction of large dams in the Saint John River system, NB, and at Lac Saint-Jean, QC. Competition from exotic invasive plant species, particularly Reed Canary Grass (Phalaris arundinacea), represents a significant threat to subpopulations on New Brunswick’s Saint John River. Invasive exotic plants may be impacting other subpopulations to a lesser degree, and this threat is likely to increase in severity and extent in the future. On Anticosti Island, browsing by over-abundant introduced White-tailed Deer appears to be having a considerable negative impact. Continued hybridization with New York Aster may be causing localized loss of genetic integrity in New Brunswick and Gaspé Peninsula subpopulations. Beach activity from nearby housing and cottage development is a moderate threat at the isolated Lac Saint-Jean population. Other postulated threats appear very minor. Habitat specificity is an important limiting factor for Anticosti Aster, as suitable habitat represents a very small portion of the landscape within the species’ range.
Anticosti Aster is a Schedule 1 species listed as Threatened under the federal Species at Risk Act. It is provincially Endangered and legally protected in New Brunswick under the New Brunswick Species at Risk Act and provincially Threatened and protected in Quebec under the Loi sur les Espèces Menacées ou Vulnérables. The species is ranked as globally Vulnerable (G3) with national status ranks of Vulnerable (N3) in Canada and Critically Imperiled (N1) in the United States, and subnational status ranks of Vulnerable (S3) in Quebec, Imperilled to Vulnerable (S2S3) in New Brunswick and Critically Imperiled (S1) in Maine.
Source: COSEWIC. 2017. COSEWIC assessment and status report on the Anticosti Aster Symphyotrichum
anticostense in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 58 pp.

Photo: © Ryan M. Bolton
Long description
Emydoidea blandingii
Reptiles
Endangered
Ontario, Quebec
This population, although widespread, is declining because of several observed, inferred, and projected threats. The most serious threats include: road and rail mortality; illegal collection for the pet, food and traditional medicine trades; habitat loss due to invasive European Common Reed; development and wetland alterations; and, increasing numbers of predators. Quantitative analyses estimate that the total number of mature individuals in this population has declined > 60% over the last three generations (due to large-scale wetland drainage after European arrival) and will decline 50% over the next three generations because of road mortality alone.
The Blanding’s Turtle, Emydoidea blandingii, is the only representative of the genus Emydoidea. It is a medium-sized freshwater turtle with a characteristic bright yellow throat and a highly domed black shell with yellowish spots and flecks. It has one of the smallest global ranges compared to most other North American turtles and only ~20% of its global range occurs in Canada.
In its Canadian range, the Great Lakes/St. Lawrence population of the Blanding’s Turtle occurs primarily in southern Ontario (with isolated reports as far north as Timmins) and southern Québec (with isolated reports occurring as far north as the Abitibi-Témiscamingue region and as far east as the Capitale-Nationale region in Québec). The much smaller Nova Scotia population occurs in the southern portion of the province and represents the most isolated population within the species’ range.
In the United States, the Blanding’s Turtle occurs in the northeastern states, and is mainly concentrated around the Great Lakes; however, it occurs as far west as Nebraska and South Dakota and there are small isolated populations along the Atlantic seaboard in New York, Massachusetts, New Hampshire and Maine.

North American distribution of Blanding’s Turtle.
Source: Map provided by NatureServe, 2008 (NatureServe).
In Nova Scotia, Blanding’s Turtles tend to prefer darkly-coloured water, indicative of relatively higher secondary productivity. In the Great Lakes/St. Lawrence population, however, Blanding’s Turtles are often observed using clear water eutrophic wetlands. Blanding’s Turtles have strong site fidelity but may use several connected water bodies throughout the active season. Turtles of all ages occur primarily in shallow water habitats. Females nest in a variety of substrates including sand, organic soil, gravel, cobblestone, and soil-filled crevices of rock outcrops. Adults and juveniles overwinter in a variety of water bodies that maintain pools averaging about 1 m in depth; however, hatchling turtles have been observed hibernating terrestrially during their first winter. Reported mean home ranges generally fall between 10-60 ha (maximum 382 ha) or 1000-2500 m (maximum 7000 m); however, most studies likely underestimate Blanding’s Turtle home range size because few have utilized GPS loggers to track daily movements throughout one or more entire active seasons.
The Blanding’s Turtle is an exceptionally long-lived and late-maturing species, even for a turtle. Blanding’s Turtles mature between 14-25 years of age and can continue to reproduce successfully until at least 75 years old. Mature females produce one clutch of eggs every 1-3 years and female fecundity and reproductive frequency are positively correlated with age. Females carry out long-distance nesting migrations and can make overland movements of >10 km. The Blanding’s Turtle’s ability to make long-distance movements facilitates gene flow among wetlands and may substantially increase reproductive success. The mean generation time for Canadian Blanding’s Turtles is ~40 years.
Across the North American range, Blanding’s Turtles mainly occur in small, isolated subpopulations that maintain a few dozen to approximately 100 turtles. In Canada, most monitored subpopulations appear to maintain fewer than 150 adults, with none exceeding 1000.
The size of the Blanding’s Turtle Great Lakes/St. Lawrence population is impossible to estimate accurately, given that very few mark-recapture studies have been conducted throughout the region, but is believed to harbour < 50,000 adults. It is estimated that over the last three generations > 60% of the population was lost due to large-scale wetland drainage after European arrival, and a further decline of > 50% is projected over the next three generations based on observed trends for monitored subpopulations and road mortality models. The long-term mark-recapture program in Québec has found fewer than 200 adults to date; although no trends have been confirmed for this subpopulation, it has likely also declined due to historical wetland loss and ongoing anthropogenic threats.
The total number of mature individuals in the Blanding’s Turtle, Nova Scotia population is believed to be < 500. The longest studied subpopulations show very late maturity (20-25 years) and great longevity (> 70 years). Without management intervention, models predict that the Nova Scotia population faces a high extinction risk despite occurring in a protected area.
This species faces numerous threats, the most serious of which include:
i.road/rail mortality and associated road effects;
ii.habitat loss due to the invasive European Reed, various types of development and wetland modifications;
iii.illegal collection for the pet, food and traditional medicine trades; and
iv.increased mortality of individuals and nests from subsidized predators.
Additional potential threats include: mortality from aggregate, forestry, energy production and recreational activities; wetland pollution; climate change and the introduction of other invasive species. The most serious threats to Blanding’s Turtle subpopulations are those that result in the mortality or loss of adults.
The main limiting factors for this species are its slow life-history (extreme longevity, very late age of maturity, low annual reproductive output, low juvenile recruitment, and a dependency on high annual adult survival) and short, cool summers at the northern periphery of the range, which reduce turtle reproductive frequency and nest success. These limiting factors make the Blanding’s Turtle highly vulnerable to even small increases (< 5%) in annual adult mortality. Because the Blanding’s Turtle matures much later than other Canadian turtles, its vulnerability to decline is exacerbated compared to other turtle species. Therefore, population stability and persistence are critically dependent on high adult survivorship.
In 2016, COSEWIC designated the Nova Scotia population and the Great Lakes/St. Lawrence population as Endangered. The Blanding’s Turtle is legally protected under the federal Species at Risk Act, 2002 (S.C. 2002, c. 29), the Ontario Endangered Species Act, 2007 (S.O. 2007, c. 6), the Ontario Fish and Wildlife Conservation Act, 1997 (S.O. 1997, c. 41), the Québec Loi sur la conservation et la mise en valeur de la faune, 2002 (RLRQ, c. C-61.1), and the Nova Scotia Endangered Species Act, 2000 (1998, c. 11, s. 1). In 2013, it was listed as a ‘CITES Appendix II’ species and its international trade is now regulated.
Its General Status Rank in Canada, Ontario, Québec and Nova Scotia is ‘At Risk’. Across all 18 jurisdictions within the North American range, the Blanding’s Turtle is only considered apparently secure (S4) in one state, Nebraska, where the species has benefited from nearly a century of large-scale habitat protection. It is officially designated as Endangered or Threatened in 13 of the 18 provinces and states in which it occurs and in 2010, the IUCN up-listed the Blanding’s Turtle to Endangered based on global population size reductions of ≥ 80% over the last three generations.
Source: COSEWIC. 2016. COSEWIC assessment and status report on the Blanding’s Turtle Emydoidea blandingii, Nova Scotia population and Great Lakes/St. Lawrence population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xix + 110 pp.

Photo: © Leagh Vermeylen
Long description
Pituophis catenifer sayi
Reptiles
Special Concern
Alberta, Saskatchewan
Like other large snakes, this species is affected by habitat loss and roadkill and may become Threatened if threats are not mitigated. The species relies on communal wintering dens, which may be scarce on the landscape. Although the severity of threats across the species’ range is not fully understood, the impact of those threats is potentially significant. The species is especially vulnerable to increased mortality because of its low abundance, late maturity, and low rate of productivity.
Bullsnake is one of three subspecies of gophersnakes in Canada. It is one of the largest species of snake in Canada, occasionally exceeding 2 m in length, and it has inspired countless reptile enthusiasts. Adults are yellowish with black, brown, or reddish brown blotches on their dorsal and lateral scales. Distinguishing features include a narrow scale at the tip of the snout that is raised above the nearby scales, a dark line that crosses the head in front of the eyes, a dark band from the eye to the angle of the jaw, and a dark vertical spot below the eye. Bullsnake is non-venomous.
Bullsnake’s range in North America extends from Alberta and Saskatchewan in the north, through central United States to northeastern Mexico in the south. In Alberta, Bullsnake occurs from north and west of Drumheller along the Red Deer River coulee system, distributed mainly in the mixed grassland region in the Lower Red Deer, South Saskatchewan, and Milk river valleys. In Saskatchewan, the Bullsnake’s range extends east to the Big Muddy Valley and north to the South Saskatchewan River.
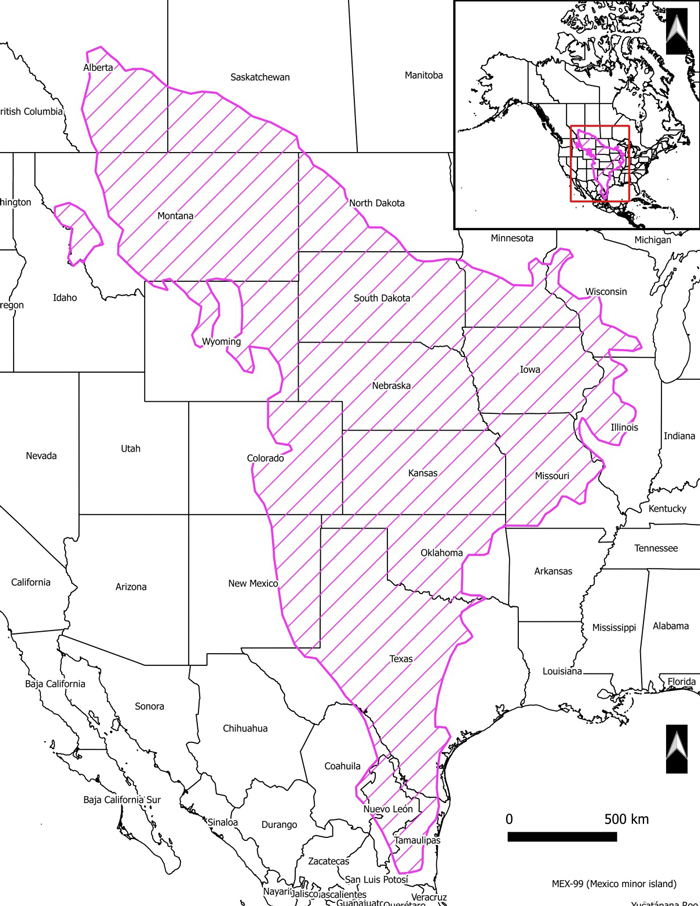
North American distribution of Bullsnake. Adapted from Conant and Collins (1998) and Kissner and Nicholson (2003).
In Canada, Bullsnake occurs in short- and mixed-grass prairie, commonly in association with brushy and sandy areas and around badlands along major river valleys. The snakes often use mammal burrows for foraging, protection from predators, moulting of the skin, temperature regulation, and as hibernation sites. Bullsnakes hibernate communally, often with other snake species, in mammal burrows, slump blocks, meander scarps and fissures, sinkholes, and rocky outcrops on slopes with warm exposure. For nesting, female Bullsnakes excavate burrows or modify existing mammal burrows in sandy or friable soils on south-facing bluffs within the coulees and gorges of river valleys.
Mating generally occurs in May, soon after the snakes emerge from hibernation. Females may reproduce annually or every other year, laying an average of 16 eggs in June or July, with hatchlings emerging from mid-August to mid-September. The age at sexual maturity is unknown; however, the closely related Great Basin Gophersnake probably does not reproduce until four years of age. Generation time for Bullsnakes in Canada is probably approximately eight years. Bullsnakes are active during the day, foraging mainly on small mammals. They are adept climbers and will also eat birds and bird eggs.
Insufficient data exist to document abundance or population trends. Declines from historical levels are inferred from road mortality and habitat loss. Habitat in the grassland regions has been lost and degraded throughout the range of the Bullsnake in Alberta and Saskatchewan. However, the snakes appear to persist across their wide Canadian range.
Threats to the Bullsnake include road mortality, certain types of agricultural practices and overgrazing, and alteration of prairie habitat from oil and gas drilling. The impact of these threats on Bullsnakes overall is considered to be low. Additional threats determined to have overall negligible, but potentially important local impacts on Bullsnakes include the following: persecution; human disturbance in the form of recreational and military activities; natural system modifications such as wildfires; residential and commercial development; and pollution, specifically from rodent control measures. Potential threats with unknown impacts on Bullsnake include landslides (slumping) and habitat alteration by invasive plants.
Globally, NatureServe lists the Bullsnake as secure (G5T5), with subnational rankings of S3 (vulnerable) for Alberta and S4 (apparently secure) for Saskatchewan. In Alberta, Bullsnake is designated as a sensitive species by Alberta Environment and Parks, with the population described as stable or possibly declining. Bullsnakes are afforded general protection as native wildlife under the Alberta and Saskatchewan wildlife acts; hibernation sites are seasonally protected under the provincial Wildlife Act in Alberta. In national and provincial parks, the species and its habitats are protected under the Canada National Parks Act, The Provincial Parks Act (Alberta), and The Parks Act (Saskatchewan).
Source: COSEWIC. 2017. COSEWIC assessment and status report on the Bullsnake Pituophis catenifer sayi in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 34 pp

Photo: © Anne Gunn
Long description
Rangifer tarandus
Mammals
Threatened
Yukon, Northwest Territories, Nunavut, Alberta, Saskatchewan, Manitoba
Members of this population give birth on the open arctic tundra, and most subpopulations (herds) winter in vast subarctic forests. Well-known for its large aggregations, lengthy migrations, and significant cultural and social value to northern Aboriginal Peoples and other Canadians, its 14-15 subpopulations range from northeastern Alaska to western Hudson Bay and Baffin Island. Numbering more than 2 million individuals in the early 1990s, the current population is estimated at about 800,000. Most subpopulations have declined dramatically, but two are increasing, including the Porcupine Caribou Herd. For 70% of the population with sufficient data to quantify trends, the decline is estimated at 56% over the past three generations (since 1989), with several of the largest herds having declined by >80% from peak numbers. Available survey data for an additional 25% of the total population also indicate declines. Evidence from both local Aboriginal people and scientific studies suggests that most herds have undergone natural fluctuations in numbers in the past; however, available demographic data indicate no sign of rapid recovery at this time and cumulative threats are without historical precedent. Status meets criteria for Endangered because of a reduction in numbers of ≥50%, but Threatened is recommended because, overall, this population does not appear to be facing imminent extinction at this time. Despite worrisome declines across most of the range, the current numerical abundance of the Porcupine Caribou Herd and the initiation of numerous management actions by governments, wildlife management boards, and communities support Threatened as a more appropriate conservation status. The status of these subpopulations will have to be carefully monitored and may warrant re-assessment within five years.
All the world’s caribou and reindeer belong to a single cervid species, Rangifer tarandus, and are found in arctic and subarctic regions as well as in northern forests. Barren-ground Caribou are characterized by long migrations and highly gregarious behaviour, often travelling in groups of hundreds or thousands. As a relatively large herbivore with an extensive distribution and high numbers, Barren-ground Caribou is a keystone species, playing a key ecological and cultural role in northern ecosystems.
The significance of Barren-ground Caribou to the peopling of northern Canada is evident from archaeological findings tracking the distribution of people and Barren-ground Caribou relative to the retreating glaciers some 8,000 years ago in the central barrens and as long as 12-15,000 years ago in the central range of the Porcupine subpopulation. Barren-ground Caribou have been and continue to be a key resource for people in northern Canada; in some cases these animals have such importance that families would follow their migration. They have significant direct economic value from harvest, primarily for subsistence use. They also contribute to the northern economy through wildlife tourism and recreational hunting; beyond this, they have incalculable cultural value for people throughout the subpopulation ranges.
The global range of Barren-ground Caribou extends from Alaska to western Greenland, and is continuous across northern continental mainland Canada, from northwestern Yukon to Baffin Island. The northern extent is the Arctic mainland coast; the southern extent is northern Saskatchewan, Alberta and Manitoba. Sampling efforts and methods have varied among subpopulations, leading to differences in interpreting subpopulation structure; 14-15 are recognized in this report. Some are combined for the purposes of generating population abundance and trend estimates, for a total of 13 units. Ten subpopulations have been consistently identified for the past several decades, mainly through fidelity to calving areas.
Fluctuating abundance of individual subpopulations affects distribution; as Barren-ground Caribou decline in abundance their distribution (especially during winter) changes, reducing the length of fall and pre-calving migration. Mainland subpopulations of Barren-ground Caribou generally migrate toward the Arctic coast to calve, and occur during summer and fall on the tundra of the Southern Arctic ecozone. Western and central mainland subpopulations usually winter in the boreal forests of the Taiga Cordillera, Taiga Plains or Taiga Shield ecozones.
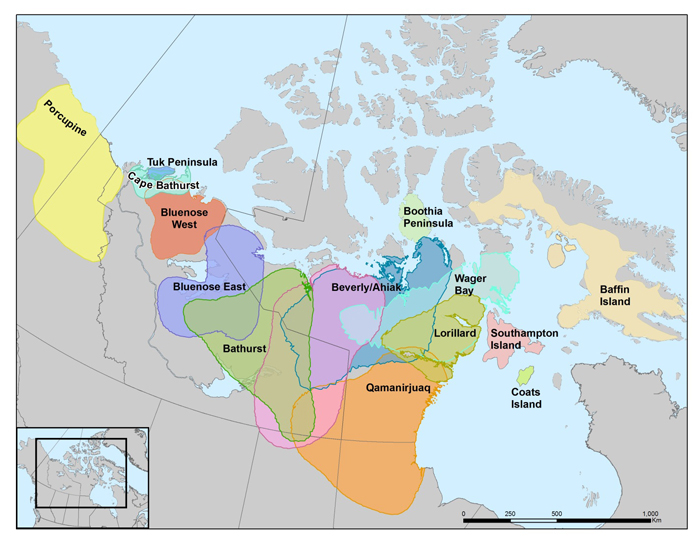
Distribution of Caribou subpopulations in the Barren-Ground Caribou designatable unit. Map by Bonnie Fournier, GNWT.
Habitat requirements are partly driven by the need for forage, which depends on the timing of the caribou’s annual breeding cycle and its nutritional costs relative to the brief plant growing season and long winters of the sub-arctic and arctic regions. Caribou are generalist foragers, especially in summer, and select among grasses, sedges, shrubs and forbs for nutrient content according to the stage of plant growth rather than plant species. Barren-ground Caribou require large annual ranges (several hundred thousand square kilometres in size) to enable selection of alternative habitats in response to annual variations in the environment, such as snow cover, plant growth, and/or predation or parasite risk. Habitat attributes that are important for calving include those that reduce predation risk and maximize nutrition intake; these vary among calving grounds. Forage requirements depend on the timing of the annual breeding cycle relative to the brief plant growing season and long winter that is characteristic of the sub-arctic and arctic regions. On summer ranges, caribou seek habitats that reduce exposure to insect harassment, while obtaining high-quality forage. While most subpopulations winter in the boreal forest, several remain in tundra habitats at that time.
Within the previous three generations, there has been some reduction in habitat as a consequence of the natural fragmentation of the winter ranges caused by forest fires and increasing human presence (i.e., infrastructure) on the caribou ranges. However, habitat outside the forested winter range is still largely intact at the landscape scale. The generally increasing trends in human population will increase economic development (industrial development, roads and traffic) within Barren-ground Caribou ranges in the future.
Caribou usually first calve at three years of age, although they can calve at two years when conditions are favourable. Females give birth to a single calf and may breed every year, although if nutritionally stressed they do not conceive every year. Calving is highly synchronized, generally occurring over a 2-week period in June. The breeding system is polygynous. Annual migrations and gregarious behaviour are the most conspicuous characteristics of most Barren-ground Caribou subpopulations. They are adapted to a long winter season when cold temperatures, wind chill and snow impose high energetic costs. Those costs are met through reducing their maintenance energy requirements and mobilizing fat and protein reserves.
Predation is an important factor affecting many facets of caribou ecology, as caribou movements and habitat choices are often made to minimize exposure to predators. An array of predators and scavengers depend on Barren-ground Caribou: Grizzly Bears (Ursus arctos) are effective predators on newborn calves, while Gray Wolves (Canis lupus, hereafter referred as Wolves) are predators of all sex and age classes throughout the year. Pathogens (including viruses, bacteria, helminths and protozoa) together with insects, play an important role in caribou ecology with effects ranging from subtle effects on reproduction through to clinical disease and death.
The current population of Barren-ground Caribou is estimated at about 800,000 individuals. Between 1986 and mid-1990s, the overall trend was an increase to > two million, followed by a decline, which has persisted through today. Of 13 subpopulation units used to derive abundance estimates, eight are declining, two are increasing, and three are unknown. The median three-generation percentage decline in the total number of Barren-ground Caribou was 56.8% (range = -50.8 – -59.0%), based on the summed population change for seven subpopulations with sufficient survey data, which comprise almost 70% of the total current population. Four of these seven subpopulations declined by >80% during this period, one had a median decline of -39%, characterized by marked variability, whereas the remaining two increased. Available survey data for three additional subpopulations, representing about 25% of the total population, also suggest declines; the current trajectories of another three subpopulations are unknown, due to lack of recent surveys.
Evidence from ATK and scientific study suggests that Barren-ground Caribou subpopulations undergo periods of high and low numbers (fluctuations) that might resemble population cycles. The evidence is, however, insufficient to consistently infer a naturally occurring cyclic increase across the full range of subpopulations. Available demographic data, cumulative changes to the environment, habitats, and harvest regimes for many of these subpopulations are without historical precedent, such that it would be risky to assume there will be a naturally occurring recovery, at least to numbers recorded in the 1990s, for many of the subpopulations.
Climate and weather influence other limiting factors important for Barren-ground Caribou, including forage availability, predation, parasites and diseases – in complex non-linear and cascading ways. So many aspects of caribou ecology are affected by weather that a warmer climate could have a significant but complicated suite of positive and negative effects.
Industrial exploration and development in Barren-ground Caribou ranges has increased over the past several decades, such that there are several new mines and hundreds of prospecting permits, mineral claims and mineral leases on several subpopulation ranges. Subsistence and sport harvest can be significant causes of mortality that can increase the rate of decline and lead to a lower population size after populations have been reduced for other reasons. Chemical contaminant levels in tissues are generally low at present. The changing conditions on the caribou ranges also include the administrative and political complexity of a mix of settled and unsettled land claims, with changes in jurisdictional boundaries and mandates. The implementation of management actions is challenged by the inter-jurisdictional complexity between political, land management and wildlife management agencies, combined with the migratory nature of caribou and their use of extensive seasonal ranges.
Protection of Barren-ground Caribou subpopulations by territorial and provincial jurisdictions is through harvest regulation and habitat protection. The co-management regime is a shared management responsibility among governments and bodies established through land claim legislation and through renewable multi-jurisdictional agreements among public governments (for the Porcupine, Beverly and Qamanirjuaq subpopulations). The Porcupine Caribou subpopulation is the only subpopulation of Barren-ground Caribou covered by an international agreement signed between Canada and the United States in 1987. The Barren-ground Caribou designatable unit (DU) was assessed for the first time by COSEWIC as Threatened in November 2016. It is currently not scheduled under the federal Species at Risk Act (SARA). The 2015 national general status for Caribou in Canada will not be available until the 2015 General Status Report is published August 2017. This Canada-wide rank will apply to all DUs of Caribou combined, with nothing specific to Barren-ground Caribou. The 2015 territorial rank for Yukon for Barren-ground Caribou is Vulnerable to Apparently Secure, and for Northwest Territories is Sensitive. At present, there is no specific rank for Barren-ground Caribou for Nunavut; however, for all DUs combined, the territory-specific general status rank for Caribou in Nunavut is Apparently Secure. Federal protected areas that exclude industrial land uses but allow continued subsistence hunting cover about 6% of Barren-ground Caribou ranges, including eight national parks.
Source: COSEWIC. 2016. COSEWIC assessment and status report on the Caribou Rangifer tarandus, Barren-ground population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 123 pp.

Photo: © Steeve Côté
Long description
Rangifer tarandus
Mammals
Endangered
Manitoba, Ontario, Quebec, Newfoundland and Labrador
This migratory caribou population exists as four subpopulations from coastal western Hudson Bay to Labrador. The present population estimate of 170,636 mature animals indicates there has been an 80% overall decline in number over three generations (18-21 years). The decline is predicted to continue because of overharvest, and a decrease in habitat quality associated with climate change and development. Two declining subpopulations contain about 99% of the Eastern Migratory population; the George River has declined by 99% over 3 generations, and the Leaf River by 68% over two generations. Although migratory caribou populations fluctuate in abundance, there is concern that recent and predicted threats will limit population growth in a population that presently is at its lowest recorded level. Threats appear to be less prevalent in the two western subpopulations which represent only about 4% of the existing total population. Most of the remaining caribou reside in the Leaf River subpopulation, which continues to decline.
Caribou (Rangifer tarandus) are a medium-sized member of the deer family. Their relatively long legs and large hooves facilitate living in deep snow associated with northern environments. Caribou are central to the culture, spirituality, and subsistence of many northern Aboriginal communities, and are also important to non-Aboriginal people across Canada. Caribou exhibit high variability in morphology, ecology, and behaviour across their circumpolar range. In 2011, COSEWIC recognized 12 designatable units (DUs); this report assesses the Eastern Migratory population (EM; DU4), and the Torngat Mountains population (TM; DU 10).
The EM contains four subpopulations: Cape Churchill, which is found along the Hudson Bay coast at the Manitoba-Ontario border; Southern Hudson Bay, found in a similar area, but mainly further south and east into northern Ontario; Leaf River (in French; Rivièreaux- Feuilles), in northern Quebec; and George River (Rivière-George), in Quebec and Labrador. The combined range is over 1.5 million km2. The TM Caribou exist as one population and occupy a range of approximately 28,000 km2 in the Torngat Mountains in upper Labrador, Quebec, and Nunavut (Killiniq and adjacent islands).
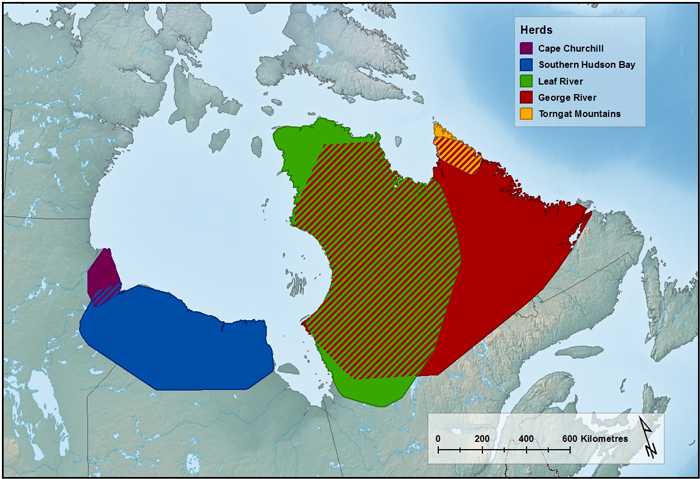
Approximate distribution of the Torngat Mountain Caribou population and the four subpopulations of the Eastern Migratory Caribou. Hash lines indicate overlap of subpopulations. The George River subpopulation illustrates the past range (< 1999), before range retraction. (Source: Pond et al. 2016, unpub. data from Caribou Ungava, Governments of Ontario, Québec, Newfoundland and Labrador). (Map created by A. Filion, COSEWIC Secretariat).
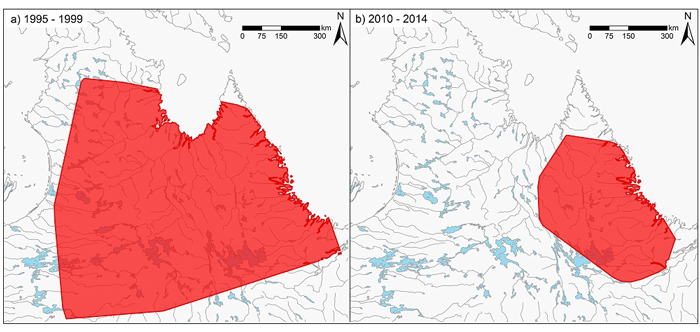
Range of the George River subpopulation in the late 1990s based on 100% MCP polygons of satellite-tagged animals, compared to range between 2010 – 2014, indicating a range decrease of approximately 85% (Source: Government of Québec, Government of Newfoundland and Labrador, Caribou Ungava).
Eastern Migratory Caribou mainly use tundra during calving and summer periods, and use taiga and mainly boreal forest during winter. The TM use alpine areas on mountain plateaus and adjacent valleys in the Torngat Mountains, and seashore areas. Caribou use hillsides, islands, and alpine plateaus for calving.
Typical longevity in Caribou is < 10 years for males and < 15 years for females. Most females ≥ 3 years old give birth to a single calf annually, resulting in a lower reproductive rate than other North American Cervid species. Primiparity can occur at 2 years of age in good habitat conditions. Generation length is estimated as a range of 6 - 7 years.
The minimum population size for the EM is 227,513 Caribou of all ages, based on the most recent total estimates for the Leaf River (2016) and George River (2016) subpopulations, and most recent minimum estimates for the Cape Churchill (2007) and Southern Hudson Bay (2011) subpopulations. The estimated number of mature animals is 170,636. The population estimate for mature Caribou of the EM three generations (18 – 21 years) ago is 833,774 Caribou, suggesting a decline of 80% over three generations. ATK supports that a decline has occurred in the George River subpopulation.
The subpopulations in eastern EM range are known to fluctuate (based on ATK, and historical data) but it is unclear if the populations will increase again because of novel threats. Caribou in these DUs associate with lichen and grass-dominated tundra but the tundra landscape is changing due to climate warming. The number of George River subpopulation Caribou (until recently, the largest-sized subpopulation in the EM) is lower than previously recorded and threats are considered to be significant for the George River and Leaf River subpopulations.
The population of the TM was estimated as approximately 5,000 Caribou in the 1980s, and at 930 Caribou (698 mature animals) in spring 2014, an estimated reduction of >80% in approximately 35 years (approximately 4 – 5 generations). ATK supports that a decline has occurred. Data do not exist on population changes over a three-generation time period.
Caribou are sensitive to disturbance. Industrial development, particularly mining and associated road networks, present threats to EM Caribou. Human overharvest of EM and TM Caribou is contributing to population declines. Populations generally are limited by food availability, but subsistence and sport hunting can be limiting at low population size, or in a declining population. A parasite, Besnoitia tarandi, became evident in the eastern subpopulations of the EM in the mid-2000s and may impact Caribou productivity. Climate change, through impacts on habitat quality and resource availability, also appears to be a threat for Caribou populations as the amount of shrubs increase on tundra landscapes. The threats calculator exercise concluded that the threat level was ‘Very High to High’ for the EM and ‘High’ for the TM Caribou.
COSEWIC assessed the conservation status of the EM Caribou (Endangered) in April 2017, and TM Caribou (Endangered) in November 2016. In 2016, the IUCN changed its assessment for the global population of Caribou from Least Concern to Vulnerable. The global NatureServe rank for Caribou is G5 (Secure; last updated in 2012) but ranks have not been determined for separate DUs recognized by COSEWIC. The draft 2015 rank for Caribou in Labrador (mainly the George River subpopulation) is S1S2 (critically imperiled to imperiled).
Source: COSEWIC. 2017. COSEWIC assessment and status report on the Caribou Rangifer tarandus, Eastern Migratory population and Torngat Mountains population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xvii + 68 pp.

Photo: © Charles Jutras
Long description
Rangifer tarandus
Mammals
Endangered
Nunavut, Quebec, Newfoundland and Labrador
This population is restricted to the Ungava Peninsula of eastern Québec, northern Labrador, and Nunavut (Killiniq and adjacent islands). A quantitative trend is not available because survey data are limited, but the total population was estimated to be 5,000 individuals in 1980 and 930 individuals in 2014, suggesting a significant decline. Aboriginal Traditional Knowledge also indicates a decline. The population meets Endangered status because the estimated 698 mature animals exist in a single population, a population decline is evident, and a decline is predicted to continue because of harvest and a decrease in habitat quality associated with climate change. The population may be facing imminent extinction because of the low numbers remaining.
Caribou (Rangifer tarandus) are a medium-sized member of the deer family. Their relatively long legs and large hooves facilitate living in deep snow associated with northern environments. Caribou are central to the culture, spirituality, and subsistence of many northern Aboriginal communities, and are also important to non-Aboriginal people across Canada. Caribou exhibit high variability in morphology, ecology, and behaviour across their circumpolar range. In 2011, COSEWIC recognized 12 designatable units (DUs); this report assesses the Eastern Migratory population (EM; DU4), and the Torngat Mountains population (TM; DU 10).
The EM contains four subpopulations: Cape Churchill, which is found along the Hudson Bay coast at the Manitoba-Ontario border; Southern Hudson Bay, found in a similar area, but mainly further south and east into northern Ontario; Leaf River (in French; Rivièreaux- Feuilles), in northern Quebec; and George River (Rivière-George), in Quebec and Labrador. The combined range is over 1.5 million km2. The TM Caribou exist as one population and occupy a range of approximately 28,000 km2 in the Torngat Mountains in upper Labrador, Quebec, and Nunavut (Killiniq and adjacent islands).
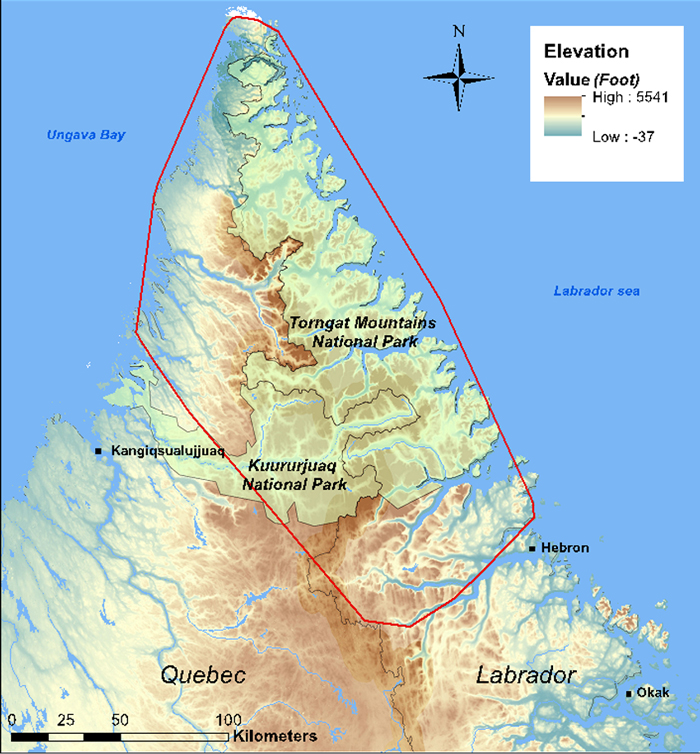
Estimated range of the Torngat Mountains Caribou subpopulation (red polygon), based on 100% minimum convex polygon of locations of 35 satellite-tagged adult Caribou monitored between 2011 and 2015. (Source: Courturier and Mitchell Foley 2014; Caribou Ungava).
Eastern Migratory Caribou mainly use tundra during calving and summer periods, and use taiga and mainly boreal forest during winter. The TM use alpine areas on mountain plateaus and adjacent valleys in the Torngat Mountains, and seashore areas. Caribou use hillsides, islands, and alpine plateaus for calving.
Typical longevity in Caribou is < 10 years for males and < 15 years for females. Most females ≥ 3 years old give birth to a single calf annually, resulting in a lower reproductive rate than other North American Cervid species. Primiparity can occur at 2 years of age in good habitat conditions. Generation length is estimated as a range of 6 - 7 years.
The minimum population size for the EM is 227,513 Caribou of all ages, based on the most recent total estimates for the Leaf River (2016) and George River (2016) subpopulations, and most recent minimum estimates for the Cape Churchill (2007) and Southern Hudson Bay (2011) subpopulations. The estimated number of mature animals is 170,636. The population estimate for mature Caribou of the EM three generations (18 – 21 years) ago is 833,774 Caribou, suggesting a decline of 80% over three generations. ATK supports that a decline has occurred in the George River subpopulation.
The subpopulations in eastern EM range are known to fluctuate (based on ATK, and historical data) but it is unclear if the populations will increase again because of novel threats. Caribou in these DUs associate with lichen and grass-dominated tundra but the tundra landscape is changing due to climate warming. The number of George River subpopulation Caribou (until recently, the largest-sized subpopulation in the EM) is lower than previously recorded and threats are considered to be significant for the George River and Leaf River subpopulations.
The population of the TM was estimated as approximately 5,000 Caribou in the 1980s, and at 930 Caribou (698 mature animals) in spring 2014, an estimated reduction of >80% in approximately 35 years (approximately 4 – 5 generations). ATK supports that a decline has occurred. Data do not exist on population changes over a three-generation time period.
Caribou are sensitive to disturbance. Industrial development, particularly mining and associated road networks, present threats to EM Caribou. Human overharvest of EM and TM Caribou is contributing to population declines. Populations generally are limited by food availability, but subsistence and sport hunting can be limiting at low population size, or in a declining population. A parasite, Besnoitia tarandi, became evident in the eastern subpopulations of the EM in the mid-2000s and may impact Caribou productivity. Climate change, through impacts on habitat quality and resource availability, also appears to be a threat for Caribou populations as the amount of shrubs increase on tundra landscapes. The threats calculator exercise concluded that the threat level was ‘Very High to High’ for the EM and ‘High’ for the TM Caribou.
COSEWIC assessed the conservation status of the EM Caribou (Endangered) in April 2017, and TM Caribou (Endangered) in November 2016. In 2016, the IUCN changed its assessment for the global population of Caribou from Least Concern to Vulnerable. The global NatureServe rank for Caribou is G5 (Secure; last updated in 2012) but ranks have not been determined for separate DUs recognized by COSEWIC. The draft 2015 rank for Caribou in Labrador (mainly the George River subpopulation) is S1S2 (critically imperiled to imperiled).
Source: COSEWIC. 2017. COSEWIC assessment and status report on the Caribou Rangifer tarandus, Eastern Migratory population and Torngat Mountains population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xvii + 68 pp.

Photo: © Annegret Nicolai
Long description
Anguispira kochi kochi
Molluscs
Endangered
Ontario
This large terrestrial snail remains in small isolated habitat patches on Middle and Pelee islands, in Lake Erie. The loss of subpopulations on some smaller islands was probably due to habitat destruction from overabundant Double-crested Cormorants, which colonized the islands in the early 1980s, as well as human activities. Habitat loss and alteration on Pelee Island likely led to subpopulation declines and fragmentation. Climate change is the most serious threat.
Banded Tigersnail is a large land snail (adult shell width 2.0 – 2.5 cm) with a globular, yellow to brown shell that has an opening in the centre when viewed from below and a light-coloured spiral band bordered by a darker band on either side. Variations include size, shell thickness, and colour of the shell, as well as the visibility of bands. Two subspecies are currently recognized: Anguispira kochi kochi on the Lake Erie islands in Ontario, and A. k. occidentalis in British Columbia. The Eastern and Western subspecies are part of the unique faunas of the Carolinian and northern Columbia Basin ecosystems, respectively, and have significance for biodiversity, research, and conservation. As part of the gastropod community in forest ecosystems, Banded Tigersnail plays a role in litter decomposition and nutrient cycling.

Photo: © Annegret Nicolai
Long description
The distribution of Banded Tigersnail is disjunct, consisting of an eastern and western North American component, and extends from southern Canada southward to Tennessee in the east and to Oregon in the west. In Canada, the Ontario and British Columbia populations are separated by over 2000 km with no connections through the US. In Ontario, Eastern Banded Tigersnail is currently known to occur on two islands in Lake Erie (Pelee and Middle islands). In British Columbia, Western Banded Tigersnail occurs in the southeastern part of the province with most records from the West Kootenay region.

Distribution of Eastern Banded Tigersnail in Ontario. Map prepared by Alain Filion (COSEWIC Secretariat) based on records compiled for this report.
In Ontario, Chinquapin Oak-Nodding Onion treed alvar, dry-fresh Hackberry deciduous forest, dry-fresh Sugar Maple-White Ash deciduous forest, and dry Black Oak woodland are preferred habitats of the Banded Tigersnail. These habitats, encompassing approximately 98 ha in total, are characterized by the proximity of limestone bedrock to topsoil or a sandy soil with a substantial leaf litter layer. Pelee Island is largely developed for agriculture, and habitat loss is historical. Habitats continue to be affected by flooding and management measures such as invasive species control and prescribed burning, as well as erosion of the tip of Fish Point on Pelee Island. Middle Island has been uninhabited by humans since the 1980s, but habitats continue to be modified by storms and overabundant Double-Crested Cormorants.
In British Columbia, the snails inhabit moist, well-vegetated mixed-wood forests and are often found in riparian areas along lakes, rivers, and creeks, especially where Cottonwoods are present. A well-developed litter layer and coarse woody debris on the forest floor provide hiding places and refuges from inclement weather. Historically, land conversions for residential and industrial developments and for agriculture have resulted in loss of habitat at lower elevations, especially along river valleys, lake shores, and highways. Habitats across the snails’ range continue to be modified and fragmented by forestry, road networks, expanding urban development, and increasing frequency and duration of droughts projected under climate change.
Banded Tigersnail is an air-breathing (pulmonate), simultaneous hermaphrodite (possesses both male and female reproductive organs), egg-laying snail. Few details of the life history of the species in Canada are known. Mating probably occurs in mid-spring and mid-summer, and egg-laying in late spring and late summer. Hibernation extends from early October until April in temperate regions. Snails are prone to freezing in winter and dehydration in summer. They rely on sheltered refuges and snow cover to buffer them from freezing during winter. Dormancy in summer may occur during prolonged drought. Sexual maturity is probably reached at 2 – 3 years of age. The generation time is probably 5 – 6 years. Active dispersal for colonization of new areas is in the order of tens of metres over several years. Passive dispersal by flooding of rivers or transportation by birds is possible but has not been documented. There is no evidence that the species is transported by humans.
Eastern Banded Tigersnail could be confirmed only on Middle Island and Pelee Island during fieldwork in 2013 – 2015; historical habitat disturbance suggests a reduction in abundance in some sites on these islands. The species has apparently disappeared from Middle Sister Island, East Sister Island, and a property near Alvinston in Lambton County on the mainland. The persistence of the species on Hen and North Harbour islands is uncertain. The population is currently estimated at about 800,000 mature individuals. Recruitment was observed in most sites where the species was found alive. Rescue from outside Canada is not possible due to Lake Erie acting as a barrier.
Nothing is known of densities and population trends of Western Banded Tigersnail, but it is probable that the species was historically more widespread and abundant than currently, particularly in larger river valleys. Most distribution records are recent (since the 1990s), and there are insufficient historical records to allow for comparisons. Threats to habitats continue from various sources and may result in declines in the future. Several records of the species exist from the vicinity of the Canada – US border, and where habitat is continuous, there is potential for rescue. However, due to poor dispersal ability of the snails and habitat fragmentation, rescue of British Columbia subpopulations from the US is of limited importance.
In Canada, Banded Tigersnail exists at the northern limit of its range. Low dispersal ability and low physiological resistance to fluctuating environmental factors such as temperature and humidity are considered limiting factors.
In Ontario, climate change represents an important but poorly understood threat to the snails through storms on Middle Island and erosion and flooding of forest on Pelee Island. Moreover, risk of droughts and extreme temperatures, resulting in spring frost, are a threat at all sites. Other threats include competition with introduced snails and slugs and increased predation pressure from introduced omnivorous Wild Turkeys and Ring-necked Pheasants on Pelee Island. On Middle Island, nesting native Double-crested Cormorants have severely altered habitats, resulting in alteration of soil chemistry, tree dieback, reduced plant species’ richness, and an increase in exotic species. Exotic plants and earthworms on Pelee Island also contribute to modification of the litter layer and habitat structure. Prescribed fire affects potential habitat.
In British Columbia, threats include habitat loss, alteration, and fragmentation by logging, roads, urban development, and wildfires, as well as increased frequency and intensity of droughts, storms and flooding, as predicted under climate change. Prolonged summer droughts associated with climate change are likely to exacerbate the effects of logging and wildfires. Climate change and forest disturbance may facilitate the spread of introduced invertebrates such as slugs, snails, and ground beetles, which may compete with or prey on tigersnails.
Banded Tigersnail has no legal designations. It is ranked as globally secure and nationally secure in the US but vulnerable in Canada. It is ranked as imperilled in Ontario and vulnerable in British Columbia. In Ontario, most of the species’ range is on protected lands managed by Parks Canada, Nature Conservancy Canada, or Ontario Ministry of Natural Resources and Forestry. In British Columbia, land ownership varies across the species’ range, but most records are from unprotected provincial forestry lands. In British Columbia, the species has been recorded from five provincial parks; several other provincial parks and other protected areas exist within its range.
Source: COSEWIC. 2017. COSEWIC assessment and status report on the Eastern Banded Tigersnail Anguispira kochi kochi and the Western Banded Tigersnail Anguispira kochi occidentalis, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xv + 82 pp.

Photo: © Carl Savignac
Long description
Coccothraustes vespertinus
Birds
Special Concern
Yukon, Northwest Territories, British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, New Brunswick, Prince Edward Island, Nova Scotia, Newfoundland and Labrador
This large finch is widely distributed across Canada’s forests, but has exhibited significant long-term declines (77-90%) over most of its range, since 1970. Over the past decades, some data suggest a further decline of nearly 40%, while other data indicate stabilization at a lower level. Threats to the species include reduced availability of mature and old-growth mixed wood and conifer forests, collisions with windows, and mortality associated with feeding on grit and salt along roads in winter.
Evening Grosbeak is a stocky, boldly coloured songbird, with a massive greenish-yellow bill. Adult males have a dark brown head with a brilliant yellow supercilium; the brown of the head transitions to yellow upperparts and belly, contrasting with a black tail and black wings, with a distinct patch of all-white secondaries. Adult females and juveniles are generally greyish-brown with some yellow on the nape and flanks and black and white wings and tail. In summer, this species can be a major predator of the Spruce Budworm and helps in the natural control of this insect pest. In winter it is a familiar visitor to bird feeders.
Evening Grosbeak breeds in Canada, the United States, and Mexico. In Canada, its distribution includes all Canadian provinces and territories except Nunavut. In the United States, the species breeds primarily in northern New England and some western states. In winter, it is nomadic and can range widely, depending on the quantity of seeds produced in the boreal forest. Historically, this species was restricted to western North America, but expanded eastward in the late 19th and early 20th centuries.
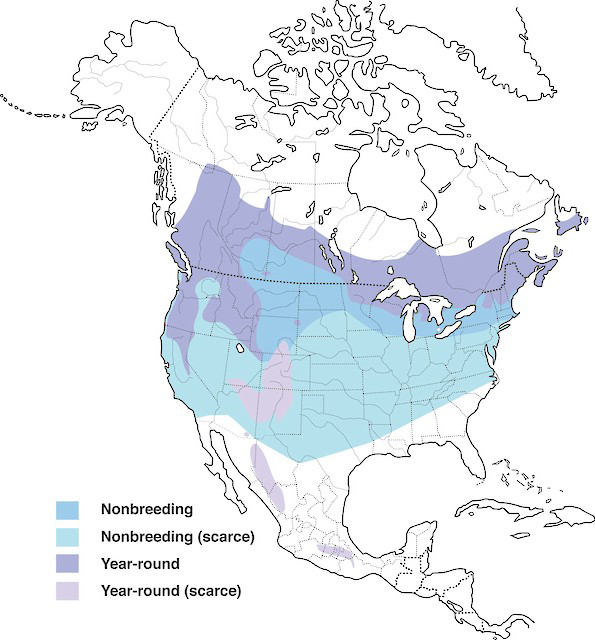
Distribution of Evening Grosbeak showing nonbreeding, and year-round ranges in North America.
Source: Map provided by Birds of America online (Birds of North America), Cornell Lab of Ornithology.
Optimal Evening Grosbeak breeding habitat generally includes open, mature mixedwood forests, where fir species and/or White Spruce are dominant, and Spruce Budworm is abundant. Outside the breeding season, the species seems to depend largely on seed crops from various trees such as firs and spruces in the boreal forest, but is also attracted to ornamental trees that produce seeds or fruit, and bird feeders stocked with sunflower seeds.
Evening Grosbeak is socially monogamous and is not territorial during the breeding period. Pairs typically arrive on their breeding grounds from mid- to late May, and the nesting season can extend until early September. The nest is an open cup made of twigs or rootlets located in the canopy of trees, with conifers preferred over deciduous trees. There is one clutch per year with an average size of 3 to 4 eggs; re-nesting may occur if the initial clutch fails. Incubation typically lasts 12 to 14 days, and fledglings leave the nest at 13 to 14 days old. The age at first breeding is one year.
The Canadian Evening Grosbeak population is estimated to be approximately 2,200,000 mature individuals. Trends are difficult to evaluate for nomadic species, but data from the Christmas Bird Count (CBC) and Breeding Bird Survey (BBS) show similar long-term declines. The CBC, which samples sites throughout the entire wintering range, indicates a significant overall decline of 3.4% per year from 1970 to 2012, corresponding to a cumulative decline of 76.6%, although from 2002 to 2012 there was a non-significant increase of 3.1% per year. The BBS primarily monitors the southern portion of the Evening Grosbeak’s breeding range, and indicates a significant annual decline of 5.2% between 1970 and 2014, for a population decline of 90% over 44 years. BBS data for the most recently available ten-year period (2004 to 2014) show an ongoing significant decrease of 5.0% per year in Canada, for a cumulative decline of 42%. Short-term (2004-2014) BBS trends are also negative in all provinces, but the trend is significant only in Manitoba, Ontario, and Quebec. Provincial breeding bird atlases, the Étude des populations d’oiseaux du Québec (ÉPOQ), and Project FeederWatch also generally show declining trends. Observatoire d’oiseaux de Tadoussac (QC) data suggest that Evening Grosbeak numbers were low from 1998 to 2011, but have increased considerably from 2012 to 2015. The Fort Liard Songbird Monitoring Project in the southern Northwest Territories showed a stable trend for 1998 to 2011. Overall, long-term trends are strongly negative across many sources of data; there is more variability among short-term trends, with some indicating ongoing declines, and others reflecting stability or increasing numbers in certain regions.
Fluctuations of Spruce Budworm populations, which naturally occur every 25-40 years in eastern Canada and every 26 years in western Canada, are likely a key factor in fluctuations of the Evening Grosbeak population since 1970. Known threats to Evening Grosbeak include mortality caused by window strikes while birds are visiting feeders in winter, reduction of mature and old-growth mixedwood forests due to commercial forest management, and mortality due to road collisions when individuals feed on grit and road salt. Mortality related to ingestion of sodium chloride along roadsides may also be a threat. Over the long term, there may be a contraction of breeding habitat due to climate change.
In Canada, Evening Grosbeak and its nests and eggs are protected under the Migratory Birds Convention Act, 1994. NatureServe considers Evening Grosbeak secure in Canada, imperilled in Prince Edward Island, and vulnerable in Yukon, Manitoba, and New Brunswick; in other provinces and territories, the species is considered either secure or probably secure.
Source: COSEWIC. 2016. COSEWIC assessment and status report on the Evening Grosbeak Coccothraustes vespertinus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 64 pp.

Photo: © Sam Brinker
Long description
Teloschistes chrysophthalmus
Lichens
Endangered
Ontario
This population now consists of a single individual on a single Red Oak tree found in Sandbanks Provincial Park on Lake Ontario. Trend data are limited, but suggest that this population, which is associated with deciduous host trees, was likely always rare in this province. The number of mature individuals of this lichen has declined due to a combination of threats, which include air pollution, human disturbance, invasive species and severe weather. A single natural or human-induced event could lead to the loss of the entire population.
The Golden-eye Lichen, Teloschistes chrysophthalmus, is a distinctive bright orange to greenish-grey, tree-inhabiting macrolichen. The thallus has a tufted, shrubby habit often with flattened branches held to surfaces by a central holdfast. The abundant orange fruiting bodies (apothecia) with ciliate margins and the lack of vegetative propagules such as isidia or soredia, distinguish this species within the genus.
In Canada, the Golden-eye Lichen occurs in localized areas of south-central Manitoba, northwestern Ontario, and the southern portion of the Great Lakes region of Ontario. In the USA, the Golden-eye Lichen is known from the interior Midwest, the Great Plains south to Texas, and from coastal California and Mexico. On the east coast of the USA, there are historical records from Maine south to New Jersey with recent sightings only in North Carolina.
The Golden-eye Lichen occurs in the Northern and Southern Hemisphere on five continents (except for Asia and Antarctica). Records include southern portions of Australia and New Zealand, North Africa, the Canary and Cape Verde Islands as well as western, central and southern Europe. There are also scattered occurrences in South America, especially Argentina and Chile.
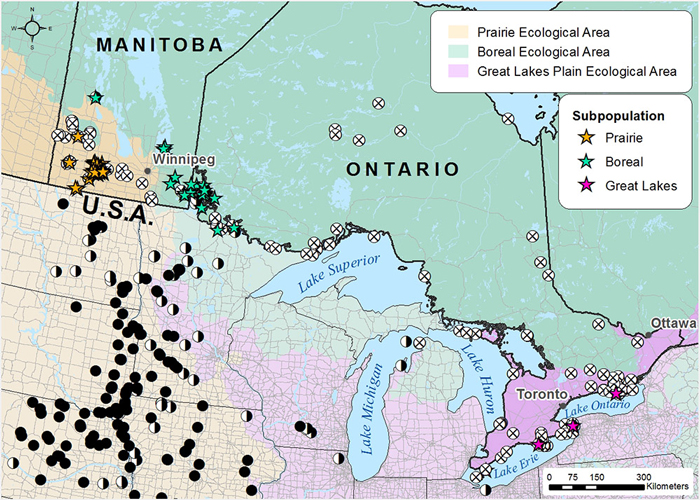
Search effort and occurrence of the Golden-eye Lichen in each COSEWIC National Ecological Area. Stars (☆) indicate Canadian occurrences of the Golden-eye lichen: yellow and green stars indicate the Prairie/boreal population and red stars the Great Lakes population. White circles with an x (⊗) represent unsuccessful searches for the Golden-eye Lichen in Canada over the period 2013-2015. Black circles (●) represent recent Golden-eye Lichen records in the USA from literature sources. Half black and half white circles (◑) represent historical USA records (>20 yrs).
The Golden-eye Lichen requires well-lit, humid environments in temperate to Mediterranean climates, and is often found near shorelines and coastal areas. In Canada, it is most common on the branches and twigs of several host tree species. In south-central Manitoba, numerous thalli are found on mature White Spruce that grow loosely clustered in “islands” within mixed-grass prairie in the Assiniboine Delta region over calcareous sands. In southeastern Manitoba and northwestern Ontario, the Golden-eye Lichen grows at very low density in relatively open, conifer-dominated woods and rocky barrens on White Spruce, Trembling Aspen, Jack Pine, Balsam Fir and Bur Oak. In the southern Great Lakes region of Ontario, the only extant site for the Golden-eye Lichen is in a remnant old-growth coastal deciduous forest of Sugar Maple, Eastern Hop-hornbeam and Red Oak along Lake Ontario growing over limestone bedrock. Here, it grows on well-lit bark of Red Oak.
Sexual reproduction in the Golden-eye Lichen occurs via the dispersal of fungal ascospores that must germinate and encounter a compatible green alga of the genus Trebouxia. Short distance dispersal by asexual reproduction as a result of thallus fragmentation is common in lichens and is assumed to occur in the Golden-eye Lichen. This speciesis a mesotrophic lichen that tolerates moderate amounts of nitrogen but not the high levels tolerated by nitrophytic lichens such as the related Maritime Sunburst Lichen. Growth rates of the Golden-eye Lichen are quite rapid, likely because of its preference for well-lit, nutrient-enriched substrata resulting in a shorter generation time than many other species of lichen. However, the Golden-eye Lichen is sensitive to acid rain and sulphur dioxide, partially because of its shrubby nature that gives it a high surface area to volume ratio.
Twenty-five Golden-eye Lichen occurrences have been documented in Canada representing three subpopulations: Prairie, Boreal, and Great Lakes. Six occurrences comprise the Prairie subpopulation; 14 occurrences form the Boreal subpopulation (one of which is historical); and five occurrences comprise the Great Lakes subpopulation (four of which are historical and likely extirpated). The Great Lakes subpopulation is considered to be a separate designatable unit because it is geographically isolated and ecologically distinct, growing on deciduous trees.
The total abundance in 2013 of the Golden-eye Lichen in Canada was estimated to be greater than 15 million individuals. The number of lichen colonies on White Spuce trees was estimated by counting colonies on individual branches. Then the number of branches occupied by the lichen on each tree was counted. Using these data, it was estimated that individual trees were each host to between 10,000-20,000 lichen colonies. Thus, while the number of individuals in the total population of the Golden-eye Lichen is very high, they could be accommodated by as few as 7,000 to 15,000 White Spruce trees.
Approximately 99% of the known Golden-eye Lichen population occurs in the Prairie subpopulation, more specifically within 15 km of Spruce Woods Provincial Forest in south-central Manitoba. Outside this core area, the occurrences are few, small and fragmented, and likely represent a former more continuous range. The Boreal subpopulation contains approximately 0.03-0.05% of the total population (estimated at 5,000-7,000 individuals) and occurs from southern Lake Winnipeg through Lake of the Woods to Rainy Lake in northwestern Ontario. The Great Lakes subpopulation, is a separate DU and now consists of a single individual found in Sandbanks Provincial Park along Lake Ontario. Trend data from this region, while scant, suggests that the species was likely always rare in this area, but has declined due to human-induced factors.
The results of the threats calculator assessment indicate that the impacts of the threats to the Golden-eye Lichen in Canada are considered to be “medium to high.” The main threats to the very large Prairie subpopulation are fire and fire suppression, climate change, recreational activities and livestock grazing. The Boreal subpopulation may be affected by cottage development while the very small Great Lakes subpopulation, now reduced to a single host tree, could be affected by several threats including severe weather, human disturbance, air pollution, and invasive species.
Currently, the Golden-eye Lichen has no formal legal protection or status in Canada or the United States. It has a global rank of G4G5 (Apparently Secure to secure) and a Canadian national rank of N3N4 (Vulnerable to Apparently Secure). However, its provincial conservation status in Ontario is S2S3 (Imperiled to Vulnerable), and S3S4 (Apparently Secure to Vulnerable) in Manitoba.
The largest Canadian subpopulation occurs in the Prairie Ecological Area of south-central Manitoba where much of the suitable habitat is found in the Spruce Woods Provincial Park and adjacent Provincial Forest where it is afforded some protection. A portion of this subpopulation is also found in the adjacent federally managed Canadian Forces Base Shilo. The Boreal subpopulation mainly occurs on Crown land along lake shores and has no formal protection. The only extant occurrence in the Great Lakes subpopulation, a separate DU located in Sandbanks Provincial Park, is afforded some protection through the Provincial Parks and Conservation Reserves Act, although no formal monitoring program is in place to assess the impact of threats or the persistence of the Golden-eye Lichen here.
Source: COSEWIC. 2016. COSEWIC assessment and status report on the Golden-eye Lichen Teloschistes chrysophthalmus, Prairie / Boreal population and Great Lakes population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xv + 50 pp.

Photo: © Sam Brinker
Long description
Teloschistes chrysophthalmus
Lichens
Special Concern
Manitoba, Ontario
Approximately 99% of the known population for this lichen occurs within 15 km of Spruce Woods Provincial Forest in south-central Manitoba, but scattered occurrences extend from southern Lake Winnipeg in Manitoba to Rainy Lake in northwestern Ontario. Threats to this population include changes in the frequency and severity of fires, climate change, recreational activities and livestock grazing. These threats are expected to contribute to a further decline in the lichen, its habitat and its preferred White Spruce host.
The Golden-eye Lichen, Teloschistes chrysophthalmus, is a distinctive bright orange to greenish-grey, tree-inhabiting macrolichen. The thallus has a tufted, shrubby habit often with flattened branches held to surfaces by a central holdfast. The abundant orange fruiting bodies (apothecia) with ciliate margins and the lack of vegetative propagules such as isidia or soredia, distinguish this species within the genus.
In Canada, the Golden-eye Lichen occurs in localized areas of south-central Manitoba, northwestern Ontario, and the southern portion of the Great Lakes region of Ontario. In the USA, the Golden-eye Lichen is known from the interior Midwest, the Great Plains south to Texas, and from coastal California and Mexico. On the east coast of the USA, there are historical records from Maine south to New Jersey with recent sightings only in North Carolina.
The Golden-eye Lichen occurs in the Northern and Southern Hemisphere on five continents (except for Asia and Antarctica). Records include southern portions of Australia and New Zealand, North Africa, the Canary and Cape Verde Islands as well as western, central and southern Europe. There are also scattered occurrences in South America, especially Argentina and Chile.
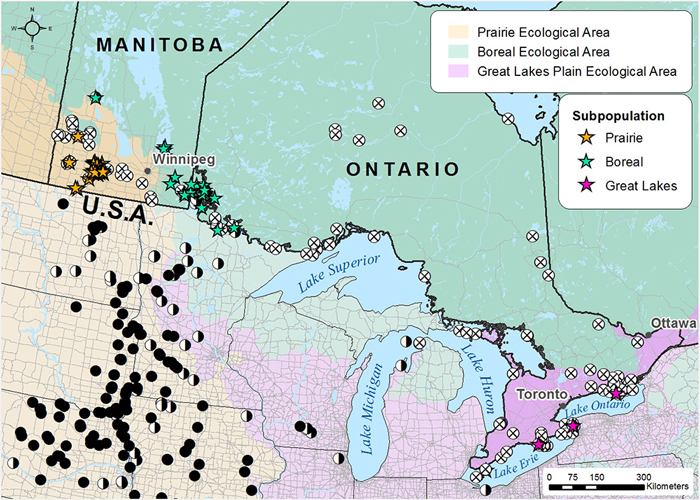
Search effort and occurrence of the Golden-eye Lichen in each COSEWIC National Ecological Area. Stars (☆) indicate Canadian occurrences of the Golden-eye lichen: yellow and green stars indicate the Prairie/boreal population and red stars the Great Lakes population. White circles with an x (⊗) represent unsuccessful searches for the Golden-eye Lichen in Canada over the period 2013-2015. Black circles (●) represent recent Golden-eye Lichen records in the USA from literature sources. Half black and half white circles (◑) represent historical USA records (>20 yrs).
The Golden-eye Lichen requires well-lit, humid environments in temperate to Mediterranean climates, and is often found near shorelines and coastal areas. In Canada, it is most common on the branches and twigs of several host tree species. In south-central Manitoba, numerous thalli are found on mature White Spruce that grow loosely clustered in “islands” within mixed-grass prairie in the Assiniboine Delta region over calcareous sands. In southeastern Manitoba and northwestern Ontario, the Golden-eye Lichen grows at very low density in relatively open, conifer-dominated woods and rocky barrens on White Spruce, Trembling Aspen, Jack Pine, Balsam Fir and Bur Oak. In the southern Great Lakes region of Ontario, the only extant site for the Golden-eye Lichen is in a remnant old-growth coastal deciduous forest of Sugar Maple, Eastern Hop-hornbeam and Red Oak along Lake Ontario growing over limestone bedrock. Here, it grows on well-lit bark of Red Oak.
Sexual reproduction in the Golden-eye Lichen occurs via the dispersal of fungal ascospores that must germinate and encounter a compatible green alga of the genus Trebouxia. Short distance dispersal by asexual reproduction as a result of thallus fragmentation is common in lichens and is assumed to occur in the Golden-eye Lichen. This species is a mesotrophic lichen that tolerates moderate amounts of nitrogen but not the high levels tolerated by nitrophytic lichens such as the related Maritime Sunburst Lichen. Growth rates of the Golden-eye Lichen are quite rapid, likely because of its preference for well-lit, nutrient-enriched substrata resulting in a shorter generation time than many other species of lichen. However, the Golden-eye Lichen is sensitive to acid rain and sulphur dioxide, partially because of its shrubby nature that gives it a high surface area to volume ratio.
Twenty-five Golden-eye Lichen occurrences have been documented in Canada representing three subpopulations: Prairie, Boreal, and Great Lakes. Six occurrences comprise the Prairie subpopulation; 14 occurrences form the Boreal subpopulation (one of which is historical); and five occurrences comprise the Great Lakes subpopulation (four of which are historical and likely extirpated). The Great Lakes subpopulation is considered to be a separate designatable unit because it is geographically isolated and ecologically distinct, growing on deciduous trees.
The total abundance in 2013 of the Golden-eye Lichen in Canada was estimated to be greater than 15 million individuals. The number of lichen colonies on White Spruce trees was estimated by counting colonies on individual branches. Then the number of branches occupied by the lichen on each tree was counted. Using these data, it was estimated that individual trees were each host to between 10,000-20,000 lichen colonies. Thus, while the number of individuals in the total population of the Golden-eye Lichen is very high, they could be accommodated by as few as 7,000 to 15,000 White Spruce trees.
Approximately 99% of the known Golden-eye Lichen population occurs in the Prairie subpopulation, more specifically within 15 km of Spruce Woods Provincial Forest in south-central Manitoba. Outside this core area, the occurrences are few, small and fragmented, and likely represent a former more continuous range. The Boreal subpopulation contains approximately 0.03-0.05% of the total population (estimated at 5,000-7,000 individuals) and occurs from southern Lake Winnipeg through Lake of the Woods to Rainy Lake in northwestern Ontario. The Great Lakes subpopulation, is a separate DU and now consists of a single individual found in Sandbanks Provincial Park along Lake Ontario. Trend data from this region, while scant, suggests that the species was likely always rare in this area, but has declined due to human-induced factors.
The results of the threats calculator assessment indicate that the impacts of the threats to the Golden-eye Lichen in Canada are considered to be “medium to high.” The main threats to the very large Prairie subpopulation are fire and fire suppression, climate change, recreational activities and livestock grazing. The Boreal subpopulation may be affected by cottage development while the very small Great Lakes subpopulation, now reduced to a single host tree, could be affected by several threats including severe weather, human disturbance, air pollution, and invasive species.
Currently, the Golden-eye Lichen has no formal legal protection or status in Canada or the United States. It has a global rank of G4G5 (Apparently Secure to secure) and a Canadian national rank of N3N4 (Vulnerable to Apparently Secure). However, its provincial conservation status in Ontario is S2S3 (Imperiled to Vulnerable), and S3S4 (Apparently Secure to Vulnerable) in Manitoba.
The largest Canadian subpopulation occurs in the Prairie Ecological Area of south-central Manitoba where much of the suitable habitat is found in the Spruce Woods Provincial Park and adjacent Provincial Forest where it is afforded some protection. A portion of this subpopulation is also found in the adjacent federally managed Canadian Forces Base Shilo. The Boreal subpopulation mainly occurs on Crown land along lake shores and has no formal protection. The only extant occurrence in the Great Lakes subpopulation, a separate DU located in Sandbanks Provincial Park, is afforded some protection through the Provincial Parks and Conservation Reserves Act, although no formal monitoring program is in place to assess the impact of threats or the persistence of the Golden-eye Lichen here.
Source: COSEWIC. 2016. COSEWIC assessment and status report on the Golden-eye Lichen Teloschistes chrysophthalmus, Prairie / Boreal population and Great Lakes population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xv + 50 pp.

Photo: © Mark Gilmore
Long description
Zonotrichia querula
Birds
Special Concern
Northwest Territories, Nunavut, Alberta, Saskatchewan, Manitoba, Ontario
This northern ground-nesting bird is the only songbird that breeds exclusively in Canada. Data from Christmas Bird Counts in the US Midwest wintering grounds show a significant long-term decline of 59% over the past 35 years, including 16% over the past decade. The species may be affected by climate change on the breeding grounds, while threats on the wintering grounds include habitat loss, pesticide use, road mortality, and predation by feral cats.
Harris’s Sparrow is a large sparrow with a distinctive black hood and bib. Both sexes have similar plumage. Non-breeding and first-year birds are similar to each other in plumage, lacking much of the black bib and facial patterning found in breeding individuals. Harris’s Sparrow is the only passerine that breeds exclusively in Canada.
Harris’s Sparrow is a long-distance temperate migrant that is found exclusively in North America. The species breeds along the tree-line in northern Canada (Northwest Territories, Nunavut, Saskatchewan, Manitoba, and irregularly Ontario), and winters in the central Midwest region of the United States (regularly in Nebraska, Kansas, Oklahoma, Texas, and irregularly in Arkansas, Missouri, Iowa, and South Dakota). Because of limited accessibility of the breeding range, relatively little is known about the species in Canada.
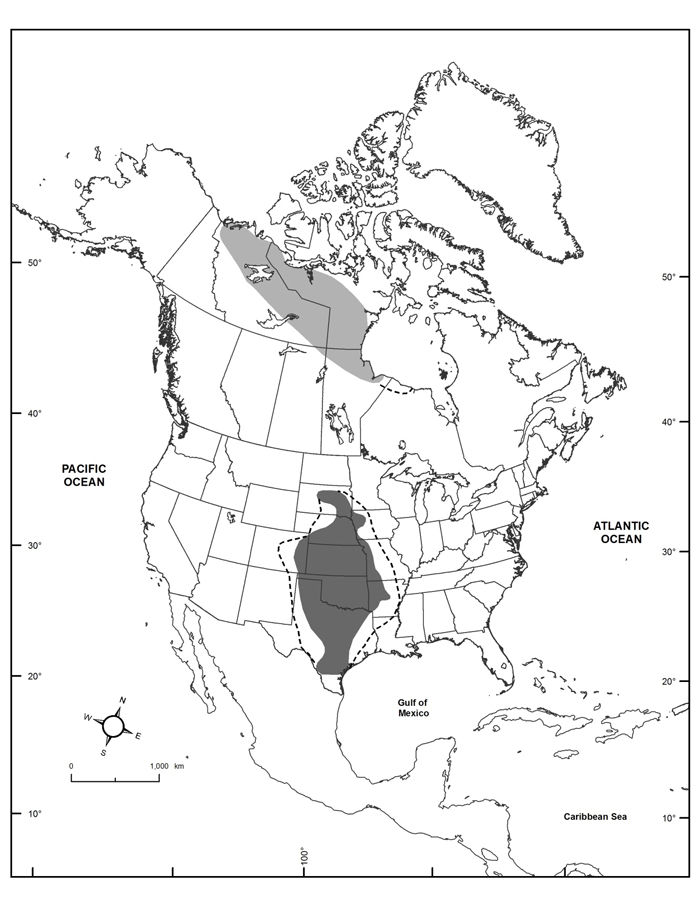
Global distribution of Harris’s Sparrow during breeding (light gray; adapted from James et al. 1976; Cadman 2007) and wintering (dark gray; adapted from National Audubon Society 2015; eBird 2016; Norment et al. 2016) seasons, including irregular occurrences within dashed lines.
Harris’s Sparrow favours a mosaic of upland and tundra, with scattered lakes. Breeding territories typically include coniferous trees; densities are highest where forest stands are dominated by spruce or tamarack, interspersed with shrubs typically <1 m tall. In winter and during migration, the species frequents a variety of habitats, with riparian thickets, grasslands, woodland edges, hedgerows, and willow thickets commonly used.
Harris’s Sparrow is a socially monogamous breeder that consumes fruits, seeds, and insects. Throughout the breeding season, the species is initially heavily dependent on fruits, before switching its diet as the breeding season progresses to include more insects and seeds as snow cover disappears. Nests are constructed and incubated by the female and are placed on the ground in densely concealed ground vegetation. Average clutch size is 4.07 eggs, with a range of 3 – 5 eggs. Incubation lasts 12 – 13.5 days and young fledge after 8.5 – 10 days. Research on the Thelon River in Northwest Territories documented a hatching rate of 76%, fledging rate of 62.5%, and overall nest success rate of 47.5%, with 2.07 fledged young per pair.
The global population, which breeds exclusively in Canada, is estimated at 500,000 – 5,000,000 individuals, with the most recent estimates indicating ~2,000,000 individuals.
Christmas Bird Count (CBC) data indicate a significant long-term rate of annual decline of -2.58% between 1980 and 2014, amounting to a total population loss of 59% over the last 35 years. Over the most recent 10-year period (2004 to 2014), CBC data show a decline of -1.77% per year amounting to a cumulative loss of 16%.
Throughout the wintering grounds in the Midwestern United States, the conversion of grassland and fringe lands for agricultural purposes is thought to be a factor in the decline of Harris’s Sparrow. Pesticide use throughout the wintering grounds has been linked to declines in Harris’s Sparrow; while the relative influence of this factor is unknown, it is anticipated to be negative and potentially considerable in severity.
Within the breeding range, concerns include habitat loss linked to deforestation near the northern edge of the species’ range associated with forest fires, quarries and mine development, and climate change, which may reduce suitable breeding habitat while allowing ectoparasites and mammalian predators, such as Red Fox, to spread north.
More studies are needed to assess the species throughout its annual life cycle and assess the relative importance of different threats on its breeding and wintering grounds.
Harris’s Sparrow and its nest and eggs are protected in Canada under the Migratory Birds Convention Act. The Act prohibits the sale or possession of migratory birds and their nests, and any activities that are harmful to migratory birds, their eggs, or their nests, except as permitted under the Migratory Birds Regulations. It is protected in the United States under the Migratory Birds Treaty Act.
Harris’s Sparrow is ranked as globally secure by NatureServe. Within Canada the species is ranked as secure nationally, secure in Alberta and Saskatchewan and vulnerable in Manitoba and the Northwest Territories. COSEWIC assessed this species as Special Concern in April 2017.
Source: COSEWIC. 2017. COSEWIC assessment and status report on the Harris’s Sparrow Zonotrichia querula in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 36 pp.

Photo: © Marcel Gahbauer
Long description
Calamospiza melanocorys
Birds
Threatened
Alberta, Saskatchewan, Manitoba
This grassland songbird is at the northern edge of its range in the Canadian Prairies. It is nomadic, with breeding populations shifting considerably from year to year to track favourable conditions across the regional landscape, seeking peak abundance of grasshoppers. Population estimates therefore fluctuate substantially and complicate the estimation of short-term trends, but long-term data show a decline of 98% since 1970. Over most of the past decade, the trend has remained strongly negative. Conversion of grassland habitat and insecticide use are believed to be the primary threats to this species.
Lark Bunting is a large chunky sparrow with a short tail and relatively large bill. Males have a distinctive black and white breeding plumage, but resemble females in the nonbreeding season. Females are greyish-brown with black streaking on their upperparts and dark brown wings with a whitish patch. Juveniles are similar to females in pattern, but are buff-coloured with more streaking.
No subspecies have been described for the species and it is the only member of its genus. Lark Bunting is the state bird of Colorado.

Photo: © Marcel Gahbauer
Long description
Lark Bunting is restricted to breeding in the grasslands of west-central North America, from the southern Canadian prairies through the Great Plains of the central US into northern Mexico. In Canada, Lark Buntings are found in southeastern Alberta, southern Saskatchewan, and southwestern Manitoba. Lark Buntings spend the non-breeding season in the southwest US and north-central Mexico.
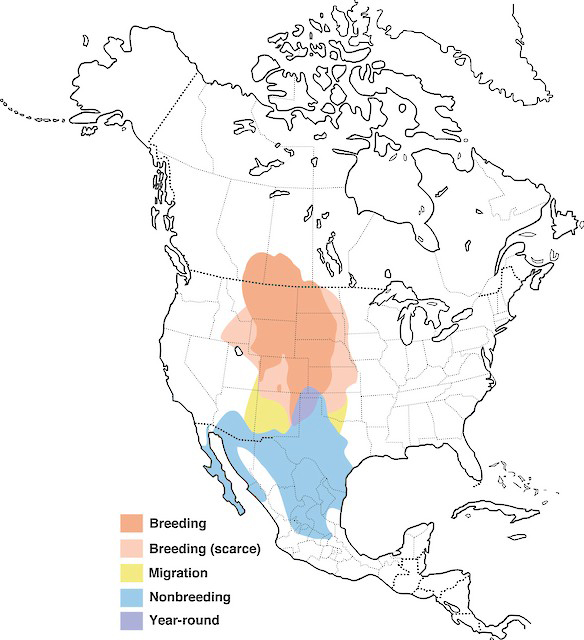
Distribution of Lark Bunting showing breeding, migration, nonbreeding, and year-round ranges in North America.
Source: Map provided by Birds of America online (Birds of North America), Cornell Lab of Ornithology.
Lark Buntings occur in a variety of grassland habitats, including shortgrass and mixed-grass prairie, weedy fallow fields, pastures, and croplands. They prefer habitat with a combination of grass, shrubby vegetation and bare ground for nesting. Shrubs or tall grasses near the nest provide shading and concealment from predators. In Canada, the species appears to use managed agricultural areas such as hayfields, cultivated grasslands and roadside ditches, in addition to native grasslands. During the non-breeding season, Lark Buntings are found in flat open areas including plains, cropland, fields and desert flats.
Lark Buntings are believed to nest once per year, laying 3-5 eggs per clutch. The mean number of young fledged per nest ranges from 1.2 to 3.1 depending on habitat type.
Lark Buntings have evolved several adaptations to deal with the environmental instability that characterizes their grassland habitat. They are highly nomadic from year to year, a behaviour which appears to have evolved to track favourable habitat conditions across a changing landscape. Lark Buntings also time nesting to coincide with peak abundance of grasshoppers, a major component of their diet. Nest-site selection is linked to minimizing heat stress for eggs and nestlings, as well as for the dark-plumaged incubating male. Once chicks leave the nest, male and female parents divide the brood and continue parental care separately, a strategy that reduces predation and increases foraging efficiency, especially during droughts.
Lark Buntings are frequent hosts of Brown-headed Cowbirds but do not appear to have evolved any avoidance strategies against this brood parasite. Numerous predators feed on Lark Buntings, including owls, raptors, cats, Coyotes, ground squirrels, weasels, and snakes.
Lark Buntings evolved with American Bison and other large native herbivores on their breeding grounds, and depend to some degree on grazing to maintain their habitat, particularly in taller grasslands.
The total global breeding population of Lark Buntings is estimated to be 10 million individuals, with approximately 160,000 individuals breeding in Canada. Between 1970 and 2014, Lark Buntings declined by approximately 3.2% per year across North America and 8.6% per year in Canada, amounting to cumulative losses of 77% and 98%, respectively. Rates of decline have accelerated more recently, with a 6% per year decline across North America and a 14% per year decline in Canada between 2005 and 2015; the species is projected to lose half of its overall remaining population over the next 16 years. However, the inter-annual variability in Lark Bunting distribution and abundance caused by the highly nomadic nature of the species may result in misleading snapshots of short-term regional population trends. An examination of rolling 10-year trends in Canada (in which one point per year represents the average annual percent change over the previous10-year period) shows a tendency toward strongly negative trends over both the short- (2005-2015) and long-term (1980-2015), although there is considerable variability over time. Interpolating from the long-term decline, the decline of the Canadian population over the past decade is estimated to be 59%.
Little is known about threats specific to the Canadian Lark Bunting population. Over much of the Great Plains, habitat loss, degradation, and fragmentation due to agriculture, urbanization and resource extraction are considered the primary threats to the species, along with effects of pesticides.
Grassland habitat is one of the most endangered ecosystems in North America. In Canada, over 70% of the prairie landscape has been degraded or lost since European settlement due primarily to agriculture and urbanization, and much of the remainder is highly fragmented.
Although some Lark Buntings breed in agricultural landscapes, their success may be lower in these habitats due to plowing, mowing and pesticide application. Pesticides may be of serious concern to Lark Buntings not only through direct lethal effects, but also through depleting populations of prey such as grasshoppers.
Oil and gas development on the prairies has also contributed to habitat loss and fragmentation for Lark Buntings, and associated sensory disturbance is also a concern. An increase in wind and solar farms poses a growing threat. Associated power lines can facilitate the presence of avian predators and cowbirds that pose threats to Lark Bunting survival and productivity.
Climate change is predicted to be an increasing threat for Lark Buntings. Continued warming, coupled with more frequent and intense droughts and large storm events, is likely to negatively affect the species. Lark Buntings are expected to lay fewer eggs, and have lower egg and chick survival under these conditions, while flooding from extreme rainfall may also lead to greater adult mortality.
A number of limiting factors make Lark Buntings susceptible to decline. They rely heavily on the availability of vegetative cover to minimize thermal stress while nesting. They are sensitive to drought conditions, when their main food (grasshoppers) is less abundant, and they experience increased competition with other grassland bird species and a resultant lower rate of recruitment. Conversely, they are also vulnerable to heavy rainfall events on the breeding grounds, and to fluctuations in seed availability on their wintering grounds.
Lark Bunting is protected under the federal Migratory Birds Convention Act (1994) in Canada, under the federal Migratory Bird Treaty Act in the US and under the Convention for the Protection of Migratory Birds and Game Mammals in Mexico. Lark Bunting also receives provincial protection, under the Alberta Wildlife Act, the Saskatchewan Wildlife Act, and the Manitoba Wildlife Act. None of the preceding legislation has specific provisions for habitat protection.
Lark Bunting is classified as a species of least concern on the IUCN Red List. NatureServe designates it as globally secure and secure on the US breeding and nonbreeding grounds, because it is common and widespread. In Canada, it is ranked by NatureServe as apparently secure to secure overall, recognizing that there is cause for long-term concern because of declines or other factors. It is considered secure in Alberta, apparently secure to secure in Saskatchewan, and critically imperilled in Manitoba.
Lark Bunting is designated a species of continental importance under the Partners in Flight North American Landbird Conservation Plan because it is a common bird in steep decline.
Source: COSEWIC. 2017. COSEWIC assessment and status report on the Lark Bunting Calamospiza melanocorys in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 39 pp.

Photo: © Atlantic Canada Conservation Data Centre
Long description
Scirpus longii
Vascular Plants
Special Concern
Nova Scotia
This globally vulnerable, long-lived wetland plant is restricted in Canada to a small region of Nova Scotia that supports nearly half of the world’s population. The species is increasingly threatened by competition and shading from the invasive Glossy Buckthorn and native shrubs. Peat mining could be a future threat. Limited sexual reproduction and hybridization may also reduce survival of this sedge.
Long’s Bulrush is a robust, perennial sedge of peatlands. It forms circular clones of vegetative shoots from stout underground rhizomes. Flowering stems, infrequent in most occurrences, are 100-180 cm long and terminate in a much-branched cluster of up to 1,000 spikelets, each containing up to 60 tiny flowers that develop a woolly appearance at maturity. The flower cluster is subtended by three leaflike bracts, which are dark and sticky at the base. In addition to these bracts, thick rhizomes, large stature and red-brown fruits (seed-like achenes), distinguish the species from co-occurring relatives.
Long’s Bulrush is a globally Vulnerable (G2G3) species with a restricted world distribution, for which Canada bears a high conservation responsibility. Canadian occurrences (46+% of the global total) are in a much less disturbed landscape than most in the United States, and may be especially significant because they are at the northern limit of the species’ global distribution. Long’s Bulrush is one of many disjunct, Atlantic Coastal Plain plants that are rare in Canada, and of public interest in southern Nova Scotia. It is a locally dominant species in peatlands and its impressive 400+ year clone longevity is often mentioned in Coastal Plain flora nature interpretation.
Long’s Bulrush has a restricted global range extending from southern New Jersey, U.S.A. to southern Nova Scotia, Canada. No records are more than 70 km from the coast. Historical occurrences of this plant in Connecticut and New York have been lost to human development resulting in an almost 300 km gap in the range between New Jersey and eastern New England in Rhode Island, southern New Hampshire and southern Maine. In Canada, Long’s Bulrush is known from 37 subpopulations in a 94 km by 90 km area of southwestern Nova Scotia, where there is strong evidence that many undiscovered occurrences exist.
Canadian distribution of Long’s Bulrush in southern Nova Scotia. Protected areas are shaded yellow.
Long’s Bulrush is a species of wet, acidic, nutrient-poor, open peatlands with limited cover of shrubs or trees taller than the herbaceous shoots. Occurrences are especially frequent and subpopulations are generally larger in peatlands subject to annual flooding from adjacent streams, rivers and lakes, but the species is also found in peatlands away from watercourses, mostly within seasonally wet areas with low standing biomass.
Long’s Bulrush is a clonal perennial. Vegetative reproduction via rhizomes is the primary mode of growth and clones can be extremely long-lived, with some large clones estimated to be several hundred years old. Flowering is infrequent in most subpopulations and is often induced by disturbance such as fire and Muskrat herbivory. Flowering occurs in late May and June. Pollen is dispersed by wind and possibly also by insects. The mating system and self-compatibility have not been investigated. Seed-like achenes mature in mid- to late summer and may germinate immediately. Germination and establishment are limited unless atypical ecological conditions, such as fire, reduce plant and litter cover. Seed dispersal via wind and water occurs primarily in late summer and autumn, continuing into winter if stalks remain standing. Internal or external dispersal by waterfowl may be important for longer distance movement. Time to maturity is likely at least several years, although flowering in the first year has been observed in New Jersey. Long-term seed banking could be significant given infrequency of flowering and increased seedling establishment associated with potentially infrequent disturbances. Rhizome fragmentation by ice or Muskrats appears to be important for dispersal along watercourses but is likely infrequent in peatlands away from water bodies.
Population size is difficult to quantify because it is difficult to determine “mature individuals”. The documented Canadian population is estimated at 2,700 clones containing 718,000 shoots, with the population of mature individuals probably best represented for status assessment by a number closer to 2,700. It is likely that undiscovered occurrences in southern Nova Scotia support additional clones and shoots at least equivalent in abundance to those currently documented.
The Canadian population appears to be relatively stable. All subpopulations documented in the last status report are extant, and with one possible exception there are no indications of significant declines. Glossy Buckthorn, natural succession, and potentially also localized all-terrain vehicle or development impacts, will likely cause low magnitude declines over the coming decades.
Threats to Long’s Bulrush are mostly slow-moving or spatially limited. Shading by the invasive exotic shrub Glossy Buckthorn is not yet significant but is the largest and most widespread short-term threat, with 20 of 37 subpopulations occurring within 15 km of known invaded sites. At least four of these 20 subpopulations have Glossy Buckthorn on their immediate margins and Glossy Buckthorn can be expected throughout the Canadian range of Long’s Bulrush (though not necessarily in all occupied habitat) within one to three times the presumed generation time of the bulrush.
Introgressive hybridization with the native and much more abundant Woolgrass Bulrush was detected at two of five subpopulations surveyed in a genetic analysis, and is believed to be an ongoing threat to genetic integrity of Long’s Bulrush. This threat is heightened by the increased occurrence of Woolgrass Bulrush in disturbed sites such as logging road ditches, but the longevity of clones and infrequency of flowering in Long’s Bulrush substantially limits the threat’s immediacy. Flooding by hydroelectric development undoubtedly eliminated subpopulations between 1900 and 1950 but is not expected to increase in the short-term. All-terrain vehicle use and natural succession are threats at some subpopulations. Peat mining is a potential future threat.
Infrequent flowering and resulting limited seed production, dispersal and establishment are significant limiting factors. The extent to which these are reduced in Canada from levels occurring prior to European settlement, because of human fire suppression or other factors, is not well understood.
Long’s Bulrush is listed as Special Concern under Schedule 3 of Canada’s Species at Risk Act, and Vulnerable under the Nova Scotia Endangered Species Act, with each status conferring limited protection. Long’s Bulrush is provided some legal protection under state endangered species acts in New Jersey, Rhode Island, New Hampshire, and Massachusetts. It is a Species of Special Concern in Connecticut, where it is presumed extirpated. Nature Serve status ranks are G2G3 (Imperiled to Vulnerable) globally, Imperiled in United States (N2) and Imperilled to Vunerable in Canada (N2N3), SX (Presumed Extirpated) in New York, SH (Potentially Extirpated) in Connecticut, S1 (Critically Imperiled)in Rhode Island and New Hampshire, S2 (Imperiled) in New Jersey, Massachusetts and Maine, and S2S3 (Imperilled to Vulnerable) in Nova Scotia.
Source: COSEWIC. 2017. COSEWIC assessment and status report on the Long’s Bulrush Scirpus longii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiv + 61 pp.

Long description
Photo: © Cory Sheffield
Melanoplus madeleineae
Arthropods
Special Concern
Quebec
This Canadian endemic is restricted to the Magdalen Islands in Quebec, where it is known to occur on seven of the eight main islands. Threats to this species are low, but recreational activities, road mortality and habitat loss through predicted coastal erosion may impact this species or its habitat.
Magdalen Islands Grasshopper (Melanoplus madeleineae) is a large (21 – 29 mm) nondescript species endemic to the Magdalen Islands, Québec, Canada. The main diagnostic features are on the hind femur, which is dark crimson on the lower surface, and uniformly dark coloured (i.e., non-banded) on the outer surface.
The Magdalen Islands Grasshopper is a relic of the Wisconsinan ice age. The species may have derived from the smaller, yet morphologically similar and closely related Northern Spur-throat Grasshopper, which does not occur on the Magdalen Islands yet is widespread on the mainland.

Photo: © Cory Sheffield
Long description
The global and Canadian range of the Magdalen Islands Grasshopper is restricted to the Magdalen Islands within the Gulf of Saint Lawrence, Québec. The Magdalen Islands archipelago is composed of eight main islands and several other small uninhabited islands. This grasshopper has been recorded from seven of the main islands. Six of these (Île du Havre Aubert, Île d’Entrée, Île du Cap aux Meules, Grosse Île, Île du Havre aux Maisons and Pointe aux Loups) are connected by sand bars and smaller islands, whereas Île d’Entrée is separated by about 4 km from the main group. This species is not known from Brion Island, which is approximately 13 km from Grosse Île.

Global distribution of Magdalen Islands Grasshopper. The species is confirmed most recently from Île du Havre aux Maisons and Île d’Entrée. Map GoogleEarth.
The Magdalen Islands Grasshopper occurs within open maritime meadow and grass/sedge hillside habitats containing plant communities.
The Magdalen Islands Grasshopper overwinters as an egg, hatching as a nymph in the early spring. The species may have a two-year diapause, as has been reported with its sister taxon, the Northern Spur-throat Grasshopper. Growth is by gradual metamorphosis, with each of the five nymphal instars getting progressively larger, and with more pronounced morphological adult characteristics, as they moult. Both nymphs and adults share feeding habits. Adults are active from mid-July through to late September and mating and oviposition occur during this time. Females oviposit egg pods within soft soil substrates, on potentially bare ground such as trails and dirt roads. Like most spur-throated grasshoppers, this species probably feeds on a wide range of plant hosts, though specific feeding preferences (if any) are unknown.
There is no information on subpopulation size(s) and trends for the Magdalen Islands Grasshopper. Approximately 80 specimens have been collected and deposited as museum vouchers. Surveys to date have focused on recording new subpopulations, collecting natural history and habitat information, and genetic studies. Strategic surveys for this species have been primarily by sweep-netting for grasshoppers and collecting in suitable habitats. It is difficult to time collection events to correspond with peak adult emergence, suggesting that detection success and perceptions of rarity could vary considerably.
There are several potential threats to the Magdalen Islands Grasshopper. Road mortality is potentially high for grasshoppers where roadways bisect suitable habitat or where adults may oviposit within the soft substrate of dirt roads. Recreational activities such as all-terrain vehicle operations on pathways through meadow habitats can cause direct mortality as well as compaction of soil and grasshopper forage plants. The species may also be subject to a range of natural predators, parasites and pathogens that regulate orthopteran populations. Overall threat impact is considered Low.
There are no federal or provincial laws that specifically protect the Magdalen Islands Grasshopper, mitigate specific threats to grasshoppers, or protect this species’ habitat. The global conservation status rank is G2 (Imperilled), the Canadian national status rank is N2 (Imperilled) and the Québec provincial status is S2 (Imperilled). Approximately 30% of the main island group of the Magdalen Islands is public land, although the portion that is occupied by the Magdalen Islands Grasshopper is unknown.
Source: COSEWIC. 2016. COSEWIC assessment and status report on the Magdalen Islands Grasshopper Melanoplus madeleineae in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 44 pp.

Photo: © Figment Films; photo: John Mitchell
Long description
Danaus plexippus
Arthropods
Endangered
British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, New Brunswick, Prince Edward Island, Nova Scotia, Newfoundland and Labrador, Northwest Territories
This large showy species is one of the most well-known butterflies in the world. The Canadian population is migratory with two distinct pathways and cumulative threats at both overwintering sites and along the long migratory routes. The migratory group west of the Rocky Mountains moves between coastal California and southern British Columbia. The group east of the Rocky Mountains represents the vast majority of the Canadian population and moves between the Oyamel Forest of central Mexico and southern Canada east of Alberta. The overwintering sites in central Mexico are extremely small, and threats to these areas include illegal logging and agricultural development, and increased frequency and severity of storms during key congregation times. Declines of greater than 50% have occurred over the past decade.
The Monarch is a conservation icon and one of the most well-known and well-studied butterflies in the world. The species has four life stages. The adult Monarch is a large (wingspan 93 – 105 mm), showy butterfly with predominantly orange wings outlined by a broad black border and two rows of circular white spots. The caterpillar is distinctively white, yellow, and black-banded, with a pair of black filaments at its head and tail. The chrysalis is green and gold. The eggs are approximately 1 mm long, oval with a flat base and bluntly pointed apex.
The Monarch is one of a few butterflies that migrate and their migration from southern Canada to Mexico has been described as an endangered biological phenomenon. The Monarch is used in classrooms all over North America to teach children about biology, metamorphosis, conservation, and an appreciation for nature.
The Monarch is a migratory butterfly. The overall native range of the Monarch occurs from Central America northward through the continental United States to southern Canada, and from the Atlantic Coast westward to the Pacific Coast. The Canadian range of occurrence includes portions of all ten provinces and the Northwest Territories. Monarchs are loosely divided into eastern and western subgroups based on their migratory routes and overwintering sites. Eastern Monarchs breed from Alberta east to Nova Scotia and migrate south to overwinter in the mountains of Central Mexico. Western Monarchs breed in southern British Columbia and migrate south to overwinter in coastal California. The breeding range in Canada is south of the 50° latitude in Ontario, Quebec and the Maritimes and extends north to the 54° latitude in Manitoba, Saskatchewan and Alberta.
The Monarch is being assessed as one designatable unit in Canada. There is some exchange of individuals between the eastern and western migratory routes and no genetic or morphological evidence to suggest two subspecies.
Monarchs have also colonized continental Europe, North Africa, Australia and many Pacific islands within the last 200 years but these colonized populations do not migrate.
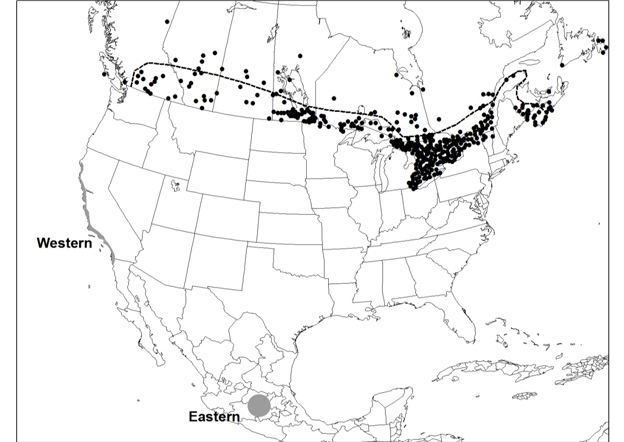
The Canadian range extent of the Monarch. The dashed line is the approximate northern distribution limit for caterpillar food plants Swamp Milkweed, Common Milkweed, and Showy Milkweed. Dots north of the line and in coastal B.C. represent non-breeding vagrants and occurrences at isolated patches of milkweeds planted outside their native range. Overwintering areas of eastern and western Monarchs are shown (grey areas).
Milkweeds (numerous species) are the sole food plant for Monarch caterpillars. These plants grow predominantly in open and periodically disturbed habitats such as roadsides, fields, wetlands, prairies, and open forests. Milkweeds are often planted outside their native range, and sometimes wayward Monarchs are observed at these patches.
Overwintering Monarchs require a cool, humid microclimate that is protected from frost, excessive sunlight, wind, and heavy precipitation. These conditions are found along the Pacific coast of California and the high elevation forests of central Mexico. Eastern Monarchs overwinter at elevations of 2900 - 3300 m in the Oyamel Fir forests in Mexico. Western Monarchs overwinter within a few kilometres of the Pacific coast of California, mainly in stands of non-native eucalyptus trees that replaced native pines starting in the 1850s, which were planted to replace native tree species.
Monarchs require staging areas which are used to rest, feed, and avoid inclement weather during migration. In Canada, they are found along the north shores of the Great Lakes where Monarchs roost in trees before crossing large areas of open water.
Adults mate during the winter or early spring at the overwintering sites in Mexico or California and begin flying north in late February or early March. About 10% of eastern Monarchs arriving in Canada fly the entire journey but most females that leave the overwintering sites breed in the southern United States. Female Monarchs lay 300 - 400 eggs singly on the undersides of milkweed leaves. The eggs hatch in three to eight days and the caterpillars feed almost continuously as they increase their body weight 2000-fold. After 9 - 14 days of feeding, the caterpillar transforms into a chrysalis and the adult emerges 9 - 15 days later. Adults of the following generations continue the migration north, many of which breed, reproduce and complete another generation in the central United States. Most Monarchs that reach Canada are the great-grandchildren of those that left Mexico. Monarchs in southern Ontario and Quebec produce two to three generations between June and October each year. Monarchs in southern British Columbia produce at least one generation each summer. Summer adults live for two to five weeks, but overwintering adults live up to nine months. The late summer adults migrate south to Mexico or California, where they overwinter and the yearly migration begins again.
Monarch caterpillars sequester the chemicals present in milkweed plants, which make them, as well as adult butterflies, unpalatable to most birds and other vertebrates.
Population size estimates are not available for Monarchs in Canada. Each fall hundreds of thousands of Monarchs migrate through Long Point in southern Ontario but it’s unknown what proportion of the Canadian population these individuals represent. Population estimates are available for the overwintering sites, which include Monarchs from both Canada and the United States. The total overwintering population size in Mexico (eastern Monarch) was estimated at 66 million individuals in 2014-2015 and 200 million in 2015-2016. A storm in March 2016 killed a large but unknown number of Monarchs at the eastern overwintering sites. Fewer than 500,000 Monarchs currently overwinter in California (western Monarch), and only a tiny percentage of these breed in Canada. In some years, the western Canadian breeding population (in British Columbia) is so small as to be undetectable.
The overwintering population in Mexico, as measured by area of occupied habitat (hectares), declined significantly over the period 1994-2015. A log-linear regression of the time series indicates an 83% decline. The 2012-2014 estimates were the lowest in the time series. The area of occupied habitat in 2015 (4 ha) was higher than the previous three years but below the time series average of 6 ha. The decline rate of the occupied habitat over a 10-year period was calculated using the slope of a log-linear regression of the entire time series applied to a period of 10 years. The estimated 10-year change was estimated to be -59%.
A long-term migration monitoring study at Long Point, Ontario showed modest declines when numbers were adjusted for weather effects but similar studies at Cape May (New Jersey) and the Peninsula Point (Michigan) showed no evidence of decline.
Most North American Monarchs are concentrated in a few hectares in the winter and are vulnerable to extreme weather events, fire, diseases, predation, and anthropogenic threats. Overwintering habitat in Oyamel Fir forest in Mexico has been fragmented and degraded by conversion to agriculture, fire, logging, and forest thinning. These practices increase the exposure of overwintering Monarchs to winter storms, cold temperatures and wet conditions, resulting in increased mortality. Climate change models predict that the area of suitable forest at the overwintering sites in Mexico will decline and the frequency of winter storms will increase resulting in catastrophic mortality of Monarchs. Degradation to the western Monarch overwintering habitat is caused mainly by real estate development along the California coast and by elimination of introduced eucalyptus upon which the butterflies overwinter.
The increased use of herbicides and subsequent decline in milkweeds is a significant threat facing Monarchs throughout their North American range. Increased herbicide use may also cause declines in nectar supplies needed by migrating Monarchs and reduce overwinter survival. Neonicotinoid pesticides are an emerging threat, the magnitude of which is poorly understood.
The Monarch was assessed as Special Concern by COSEWIC in 1997. The status was re-examined and confirmed in 2001 and 2010 and designated Endangered in 2016. Monarchs are classified as Special Concern under the Ontario Endangered Species Act and the New Brunswick Species at Risk Act. The Monarch is listed as “under special protection” in Mexico and in the United States, Monarchs are under review for listing under the United States Endangered Species Act. Breeding populations of Monarchs are ranked as Apparently Secure (G4) globally and Secure for Canada and the United States. Migratory concentration areas are ranked as Vulnerable to Imperiled in the United States.
Source: COSEWIC. 2016. COSEWIC assessment and status report on the Monarch Danaus plexippus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 59 pp.

Photo: © David Fraser
Long description
Ardenna creatopus
Birds
Endangered
British Columbia, Pacific Ocean
This long-lived seabird nests on only three islands off the coast of Chile, where it has suffered significant declines due to nest predation by introduced predators, exploitation by humans and habitat degradation. It also experiences mortality due to incidental take by fisheries across its range, including important foraging areas off the coast of British Columbia. Bycatch risk from fisheries has increased over the last three generations. This species is also sensitive to offshore oil spills.
The Pink-footed Shearwater is a stocky seabird about the size of a medium gull. In flight individuals appear heavy, with laboured wingbeats alternating with glides. It is distinguished from other North Pacific shearwaters by a combination of greyish-brown plumage above, variably mottled pale grey underparts with white wing linings, and a dusky head. The plumage of adult and juvenile birds is alike and there are no seasonal differences, although males are larger than females on average. Its pinkish-yellow, dusky-tipped bill and pink legs and feet are distinctive. The Pink-footed Shearwater is a globally threatened species that is known to breed at only three sites worldwide.

Photo: © Ben Lascelles
Long description
The Pink-footed Shearwater is known to breed on three islands off the coast of Chile: Mocha, Robinson Crusoe, and Santa Clara islands. At sea, the Pink-footed Shearwater primarily occupies waters of the continental slope, shelf-break, and shelf of the eastern Pacific. Its range extends from its breeding islands north along the coast of South and North America to the Gulf of Alaska and the southern Bering Sea, but only a few individuals occur north of Haida Gwaii. In Canada, the Pink-footed Shearwater occurs exclusively off the coast of British Columbia, with observations concentrated off the west coast of Vancouver Island, the entrance of the Strait of Juan de Fuca, and in Queen Charlotte Sound. Numbers in Canada peak from June to October.
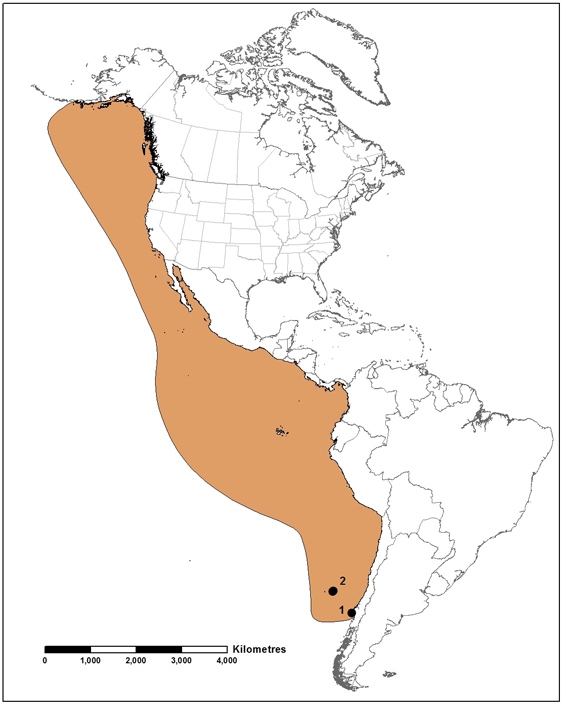
Global distribution of the Pink-footed Shearwater (to the east of line), based on maps from BirdLife International (2003). 1 – Mocha Island colony, 2 – Juan Fernández Archipelago colonies (Robinson Crusoe and Santa Clara islands). This species is also regularly reported from New Zealand (not shown; intervening range not known). Data source: BirdLife International and NatureServe (2015). Map prepared by Alain Filion, COSEWIC.
Pink-footed Shearwaters nest in burrows that they excavate in the soil of their breeding colonies. On Mocha Island, burrows are located in dense native forest along the seaward sides of upper slopes and ridgetops, while on Robinson Crusoe and Santa Clara islands, nests are located in remnant native forests or open terrain with grassy vegetation or bare soils. In the marine environment, Pink-footed Shearwaters display a preference for biologically productive waters associated with the continental slope, shelf and shelf-break.
Pink-footed Shearwaters breed during the austral spring and summer, with birds returning to their colonies from early to mid-October. They lay a single egg per year, with egg-laying occurring from late November to mid-December. Eggs hatch from late January to mid-February after a prolonged incubation period, and fledging primarily occurs in May. Both parents share in incubation. After chicks fledge, post-breeders migrate north to their wintering grounds off Peru and the Pacific coast of the US and Canada.
The global population size of the Pink-footed Shearwater is estimated at 28,000 breeding pairs. At Mocha Island, the population is believed to have declined considerably over the 20th century due to illegal chick harvesting and introduced predators. A study at Mocha Island in the late 1990s estimated a substantial decline in the number of breeding pairs (~40%) from an estimate in the late 1980s, although methods differed between surveys. There is plausible evidence of decline on Robinson Crusoe Island within the past 55 years (3 generations) due to predation of adults and chicks by Coatimundis and feral cats. However, the Robinson Crusoe population is thought to have been stable over the past 15 years, and monitoring at Mocha Island since 2010 suggests a stable population over that time. Trends within the Canadian range of the species are unknown.
Threats facing this species at its colonies include human exploitation and disturbance, predation, disturbance and competition from introduced mammals; and habitat loss and destruction, particularly via erosion compounded by vegetation loss. At sea, the species is threatened by interactions with fisheries, oil and other pollution, plastic ingestion, and likely by competition with humans for prey fish.
The Pink-footed Shearwater is listed as Threatened in Canada, as Endangered in Chile, and as Vulnerable by the IUCN. The British Columbia Conservation Data Centre ranks it as Vulnerable. In 2015, it was added to Annex 1 of the Agreement on the Conservation of Albatrosses and Petrels, an agreement under the Convention on the Conservation of Migratory Species (Bonn Convention), under which it is also listed. The Juan Fernández Archipelago is a Chilean national park, and a national reserve protects the portion of Mocha Island occupied by nesting Pink-footed Shearwaters. In Canada, the species occurs within the Gwaii Haanas National Marine Conservation Area.
Source: COSEWIC. 2016. COSEWIC assessment and status report on the Pink-footed Shearwater Ardenna creatopus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 43 pp.

Photo: © Marc Jones
Long description
Entosthodon rubiginosus
Mosses
Special Concern
British Columbia, Saskatchewan
The known distribution and abundance of this moss has increased significantly due to field and collection research since the species was first assessed by COSEWIC in 2004, resulting in decreased extinction risk. It is now known from both British Columbia and Saskatchewan, and considerable unexplored potential habitat exists. Small declines have been observed, and potential threats, including, livestock use, climate change, conversion of natural habitat for agricultural use, and alien invasive species, have been identified. The species remains at risk and could become Threatened unless threats are mitigated with demonstrable effectiveness.
Rusty Cord-moss is a small, pale green to green moss that grows as individual stems or in tiny patches. It grows to 2–3 mm high and is inconspicuous and often hidden among other mosses. Rusty Cord-moss is endemic to North America where it is rare across its total range. The Canadian occurrences represent the northernmost extent of its range in North America. In addition to its Canadian occurrences, there are seven known occurrences in the United States (one of which is historical). Rusty Cord-moss can be distinguished from the similar Entosthodon fascicularis by microscopic characters in the capsule wall.
Rusty Cord-moss is endemic to western North America and is found in arid and semiarid regions of British Columbia, Saskatchewan, Montana, Arizona, New Mexico, Texas, and Washington. In Canada, Rusty Cord-moss has been found at 17 sites, including 12 in the southern interior of British Columbia and five in southwestern Saskatchewan.
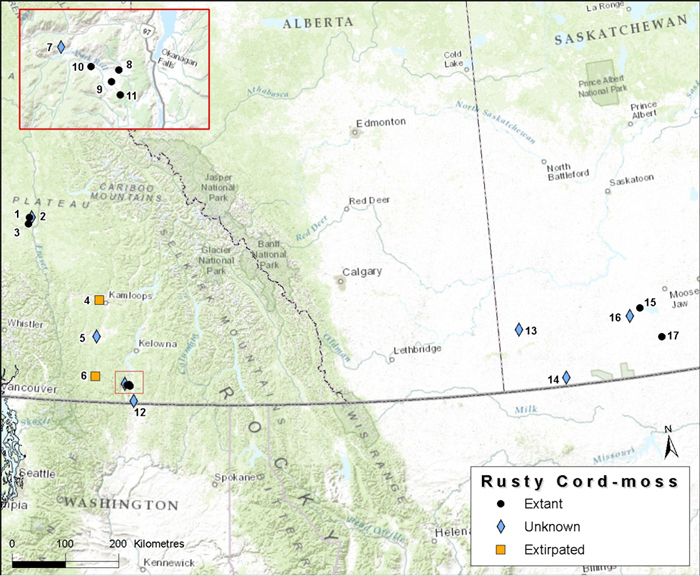
Canadian distribution of Rusty Cord-moss. Sites are: 1. Roundup Lake; 2. Lost Lake; 3. Riske Creek; 4. Cooney Bay; 5. Quilchena; 6. Princeton; 7. Twin Lakes; 8. Observatory; 9. White Lake; 10. Park Rill; 11. Grasslands; 12. Strawberry Creek; 13. Maple Creek; 14. Climax; 15. Courval; 16. Gravelbourg; 17. Lake of the Rivers.
In Canada, Rusty Cord-moss is restricted to seasonally damp, saline, usually silt- or clay-rich soil at the edges of open ponds, lakes, sloughs, and seepage slopes in relatively dry environments. It grows on bare soil and tolerates some accumulation of litter and vascular plants. Rusty Cord-moss is most often found within a narrow band around the edges of wetlands where the topography is flat to very slightly sloping. It has not been found in saline sites where tall rushes and sedges dominate. The saline nature of these areas arises from evaporation of water during warmer months over many years, leaving minerals behind.
Rusty Cord-moss may be an annual or short-lived perennial (~2 years) that regularly produces sporophytes. Short-range dispersal of spores, spore persistence in the soil, and the spread of vegetative fragments all likely contribute to the persistence of Rusty Cord-moss subpopulations. Rusty Cord-moss has physiological traits that allow it to survive in arid and semi-arid environments, such as prolonged dormancy, curled leaf margins, and leaf hair points.
Rusty Cord-moss is known from 17 sites, 12 of which are in the southern interior of British Columbia and five are in southwestern Saskatchewan. It may be extirpated from two sites in British Columbia. Continuing bryophyte surveys have increased the number of known sites: only four sites were documented at the time of the initial status report in 2004. Most known sites of Rusty Cord-moss are small patches containing fewer than 10 individuals, but two recently discovered sites in British Columbia, Park Rill in the White Lake Basin and Roundup Lake on the Chilcotin Training Area, support larger subpopulations of more than 1000 individuals. Based on resurveys, most subpopulations in the White Lake Basin and Chilcotin appear to be stable. However, many of the sites with small subpopulations were not relocated in 2015, making it difficult to estimate trends in abundance.
Rusty Cord-moss is limited to seasonally damp, bare soil usually associated with saline lakes, ponds, sloughs, and seeps. Threats include livestock use, changing hydrological regimes associated with climate change, conversion of wetlands to agricultural uses, alien invasive species, off-road vehicular use, and disturbance due to Canada Geese. Heavy livestock use, which can directly and indirectly affect Rusty Cord-moss through trampling and soil disturbance, is a medium-low threat to the species. Most known Rusty Cord-moss sites are accessible to livestock, and livestock use at some sites is quite high. The effects of changes in wetland hydrology caused by altered temperature and precipitation on Rusty Cord-moss are unknown. Rusty Cord-moss habitats may become drier, more ephemeral, and subject to greater hydrological extremes due to climate change. Conversion of wetlands to agriculture is a negligible threat to Rusty Cord-moss sites in the prairies, although none of the sites documented in Saskatchewan appears to be threatened by this currently. Other threats are likely to have negligible or unknown impacts.
Rusty Cord-moss was assessed as Endangered by COSEWIC and is listed as Endangered on Schedule 1 of the Species at Risk Act. NatureServe ranks Rusty Cord-moss’s global conservation status as G1G3, ranging from Critically Imperilled to Vulnerable (high to moderate risk of extirpation or extinction). In British Columbia, it is Blue-listed (defined as a taxon of special concern that is particularly sensitive or vulnerable to human activities or natural events) and is ranked by the BC Conservation Data Centre as S2S3, ranging from Imperilled to Vulnerable (high to moderate risk of extirpation or extinction). It is considered historical (SH, known only from records over 50 years ago, and it may be rediscovered) by the Montana Natural Heritage Program and is unranked in Arizona, New Mexico and Washington. Occurrences of Rusty Cord-moss on the Chilcotin Training Area and in the White Lake Basin have some protection under current management guidelines in these areas.
Source: COSEWIC. 2017. COSEWIC assessment and status report on the Rusty Cord-moss Entosthodon rubiginosus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 41 pp.

Photo: © Tom Murray
Long description
Polites sonora
Arthropods
Not at risk
British Columbia
This butterfly has a small range in the southern interior of British Columbia. Since it was last assessed, new information has been gathered on its distribution, habitat, host plants, natural history and threats. It is now known to occur in a greater number of natural and disturbed sites, including meadows, roadsides and clearcuts that typically have wet seepages or some form of standing water. It also has much broader host plant preferences than previously known and some ability to use non-native species to complete life stages. Threats remain low and some potential impacts such as clear-cut logging can result in habitat creation and corridors for dispersal for this butterfly.
Sonora Skipper (Polites sonora) is in the family Hesperiidae, the skippers. Adults have a wingspan of 25 to 30 mm. The wing uppersides are a combination of rusty orange and brown with blackish wing borders. The forewing undersides have a basal black patch, tawny and pale areas in the median area, and a dark brown border. The ventral surface of the hindwings is ochre brown with a distinct semicircular band of pale spots. There are at least eight possible Sonora Skipper subspecies across its range. The Canadian population may belong to a ninth and undescribed subspecies although the taxonomic work has not been completed. Regardless of the subspecies-level taxonomy, only one subspecies exists in Canada, and the entire species is the subject of this status report.
Sonora Skipper, as a species, is widely distributed in western North America, from southern British Columbia (BC) south to Baja California and east in the United States to Wyoming and Colorado. The Canadian population has a small restricted range in the north Cascade Mountains and adjacent Thompson Plateau within southern BC and adjacent to the international border. There are twelve extant subpopulations of Sonora Skipper in Canada; some subpopulations are composed of multiple sites.
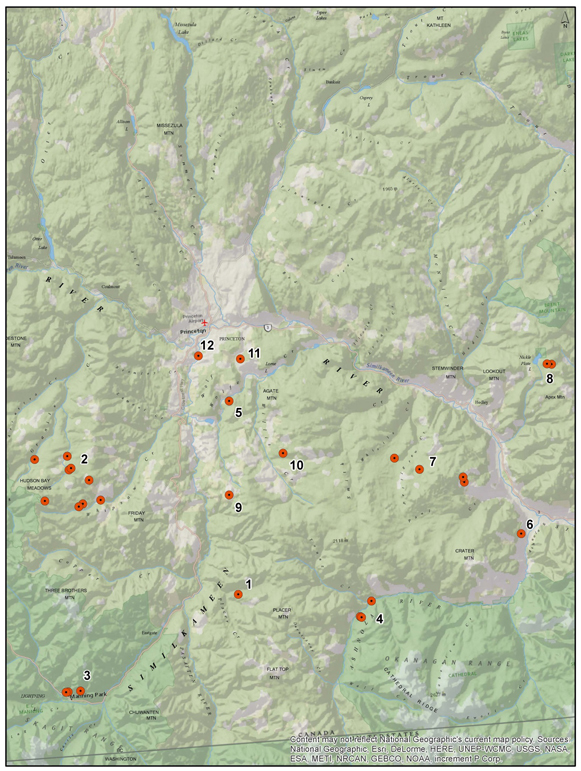
Distribution of Sonora Skipper in British Columbia. Subpopulations are: 1. Placer Creek; 2. Corral Creek (Whipsaw Creek); 3. Twenty Minute Lake, E.C. Manning Provincial Park; 4. 2.6 km north of McBride Creek; 5. Verde Creek (Wolfe Creek); 6. Red Bridge Lake (Crater Mountain); 7. North of Paul Creek; 8. Apex Mountain; 9. 1.8 km east of Sunday Creek; 10. Southwest of Wilbert Hills; 11. South of August Lake; 12. Northwest of Allenby.
Sonora Skipper habitats include open moist and mesic grassy forest openings and flowery meadows, gentle slopes, open roadside areas, open streamside banks, fallow agricultural meadows, grassy forest openings of southern exposure, some bordered by forest, clear-cuts or denser vegetation. Sonora Skipper has been recorded from anthropogenic semi-natural areas such as hay fields and old logged areas that have turned to meadows. Sites are typically on a level bench or gentle slope and have wet seepages with some form of standing water (i.e., puddles, pools, dripping seepages).
Sonora Skipper has four life stages, one generation per year and an adult life-span of seven to ten days. In BC, adults have been recorded from late June to mid-August. Females lay eggs while flying low, within close proximity to host plants. The larval host plants are unconfirmed in BC. However, females were observed at one BC. site dropping multiple eggs onto non-native Redtop Bentgrass and non-native Common Timothy grass. Elsewhere within the skipper’s global range, larvae have been successfully reared on non-native Yellow Bristlegrass, native Idaho Fescue, non-native Common Timothy and native Kentucky Bluegrass. Eggs hatch into larvae within 7-8 days of oviposition. The larvae build and take refuge in silken shelters, emerging to feed on their host plants. Each larval instar builds a new shelter. Larvae overwinter at the fourth instar, break diapause in the spring (April/May) and continue to feed for approximately a month before pupation (fifth instar).
To date, surveys have focused on recording new populations, natural history and habitat information and there are few data from which to estimate Sonora Skipper abundance, population size or trends at extant sites. Most records are one or two individuals. The largest number of Sonora Skipper was recorded in 2014 from a site along Granite Forest Service Road (#2 Corrall Creek) where 25 butterflies were observed on July 24, 2014. The natural population fluctuations in butterflies are a result of factors such as parasites, predators, and the previous years’ weather. Sonora Skipper does not likely experience extreme fluctuations although there is insufficient information to estimate population fluctuations or trends for Sonora Skipper in Canada or elsewhere in the species’ range. Many sites have been visited over multiple years to confirm the species’ presence and record number of individuals. However, these sites were only visited on one or two dates of a field season.
Threats to Sonora Skipper subpopulations are considered low and site-specific without any immediate range-wide threat. Specific lows threats include habitat loss from recreational ski hill development; timing of annual or biannual haying that could kill both eggs and larvae; and inappropriate cattle grazing regimes.
Most of the range of Sonora Skipper (excluding provincial parks) is within active timber supply areas. Clear-cut logging may provide temporary habitat (< 10 years), provided the appropriate host plants and suitable moisture conditions are present. These same habitats may provide corridors with other suitable habitats or isolated subpopulations. At some extant sites, past logging appears to have opened habitat: the forest has not regenerated and remained a wet meadow in which Sonora Skipper subpopulations have remained.
Sonora Skipper subpopulations are protected at E.C. Manning Provincial Park under the BC Park Act. The BC Forest and Range Practices Act lists the Sonora Skipper as Identified Wildlife enabling the species to be protected from forestry and grazing threats through the establishment of Wildlife Habitat Areas. The species is listed as Special Concern under the federal Species at Risk Act.
Source: COSEWIC. 2016. COSEWIC assessment and status report on the Sonora Skipper Polites sonora in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 35 pp.

Photo: © Thomas G. Barnes @ USDA-NRCS PLANTS Database
Long description
Chimaphila maculata
Vascular plants
Threatened
Ontario, Quebec
This low-growing perennial plant is restricted to sandy soils in southern Ontario. Since the last assessment, this species has been found at two new sites and lost at two others. The overall population has remained fairly stable but the five subpopulations are under threat from recreational activities and the possibility of wildfire.
Spotted Wintergreen (Chimaphila maculata) is a small, low-growing, evergreen perennial that is woody at the base and spreads by rhizomes to form colonies. Each stem consists of a whorl of thick, blue-green, toothed leaves with a white stripe along the mid-rib and white areas extending from the mid-rib. Topping the whorl of leaves is a stalk supporting one to five nodding white or pinkish flowers. In a given year, only some of the stems in a subpopulation produce flowers. The rounded seed capsules become erect after flowering, and contain numerous tiny seeds.
Spotted Wintergreen occurs in eastern North America, Mexico, and Central America. Its range in eastern North America extends from southern Michigan and Ontario, east to southern New Hampshire and Maine, and south to Mississippi and northern Florida. Historically, Spotted Wintergreen was more widely distributed in southern Ontario and into southwestern Quebec. It is now restricted to a few subpopulations in southern Ontario and is considered extirpated in Quebec.
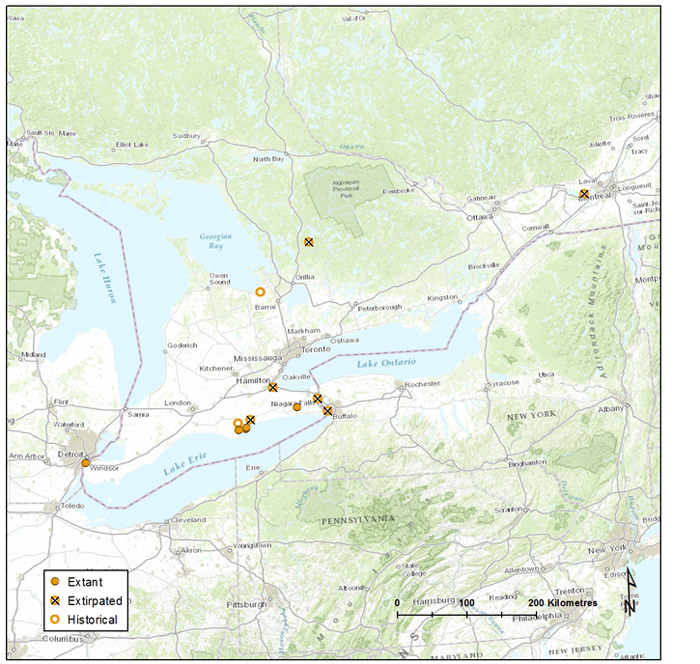
Canadian distribution of Spotted Wintergreen. This figure shows all documented subpopulations in Canada to date. Sources: NHIC 2015, CDPNG 2015.
Spotted Wintergreen is a woodland understorey species typically associated with dry– fresh oak and oak–pine mixed forests and woodlands. The plant tends to occur on well-drained sandy soils free of coarse fragments, with low organic content and poor nutrient status.
Spotted Wintergreen flowers in late July to early August. It can reproduce either clonally or by seed. As stems arise from creeping rhizomes, clumps or contiguous groupings of stems likely represent ramets rather than unique genetic individuals. The tiny, dust-like seeds in this family are dispersed mainly by wind.
In Canada, there are currently five extant subpopulations. Surveys between 2011 and 2014 show a total Canadian population of at least 3587 (~3600) stems. The number of genetic individuals is not known, although it is presumably smaller. Previously reported population sizes are in the vicinity of a few hundred stems; however, two of the extant subpopulations (and several smaller sites) have been discovered since the most recent status report, and both are significantly disjunct from other extant sites. These probably do not represent newly established subpopulations, but may reflect increased survey effort and reporting of observations. Most sites known since around 2000 have remained at least stable, while some have increased in abundance and extent, evidenced by regular monitoring.
There are additionally two historical and six extirpated subpopulations. There is a possibility that plants persist at either historical site. Most of the extirpated records are only known through vague locality or population information and have never been relocated. One small subpopulation discovered near Montréal in 1992 may have been planted and is now believed to be extirpated.
Recreational activities are probably the predominant threat to extant subpopulations of Spotted Wintergreen; however, fire has the potential to have the greatest impact as this species appears to not persist after fire. Most extant sites are in public ownership and are protected from loss due to development but many sites are publicly accessible, and a few may be vulnerable to ATV damage and soil compaction from adjacent walking trails. Many sites do not appear to have any imminent threats, although their small size and spatial extent make them vulnerable to even localized disturbances. Invasive species are present at or near a few sites, but do not appear to negatively affect ramet (or shoot) numbers within these subpopulations. Habitat degradation (e.g., by garbage dumping) may also have limited impacts on some Spotted Wintergreen subpopulations. This species may be limited to some degree by its dependence on soil mycorrhizae and its reproductive biology.
Spotted Wintergreen was first assessed as Endangered by COSEWIC in 1987. This status was re-examined and confirmed in 1998 and 2000. The species is currently listed as Endangered under the Species at Risk Act and under Ontario’s Endangered Species Act (ESA). The species and its habitat in Ontario are protected under the ESA. The global conservation status rank for Spotted Wintergreen is G5, secure. In Canada, Spotted Wintergreen is ranked N2 (imperilled). In Ontario, it is ranked S2 and in Quebec is it ranked SX. Spotted Wintergreen is considered secure (N5) in the United States, but within the U.S., it is considered critically imperilled (S1) in Illinois, and imperilled (S2) in Vermont, Maine, and Mississippi. The species is also legally protected in Illinois, where it has been designated as Endangered.
Source: COSEWIC. 2017. COSEWIC assessment and status report on the Spotted Wintergreen Chimaphila maculata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 39 pp.

Photo: © Tamra Feenstra
Long description
Coccinella transversoguttata
Arthropods
Special Concern
Yukon, Northwest Territories, Nunavut, British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, New Brunswick, Prince Edward Island, Nova Scotia, Newfoundland and Labrador
This species was once common and broadly distributed throughout most of Canada. Declines started in the 1970s and the species is now absent in southern Ontario and the Maritimes. In some parts of its western and northern range, the species is still commonly recorded. The spread of non-native lady beetles is considered one of the possible threats to this species through competition, intraguild predation, or introduction of pathogens. Non-native lady beetles are less commonly found in places where this species remains.
Transverse Lady Beetles are small, round beetles (5.0 to 7.8 mm) that are native to North America. Adults have orange to red wing covers with black markings, consisting of a black band and four elongate spots, which distinguish them from other species. This charismatic species was once one of the more common and widespread lady beetles in North America, playing an important role as a biological control agent of aphids and other insect pests.
The Transverse Lady Beetle is a wide-ranging species occurring from coast to coast across Canada and the United States. The Canadian range of the Transverse Lady Beetle stretches from St. John’s, Newfoundland and Labrador, west to Vancouver Island. The northernmost extent of its range includes Yukon, the Northwest Territories and likely Nunavut.
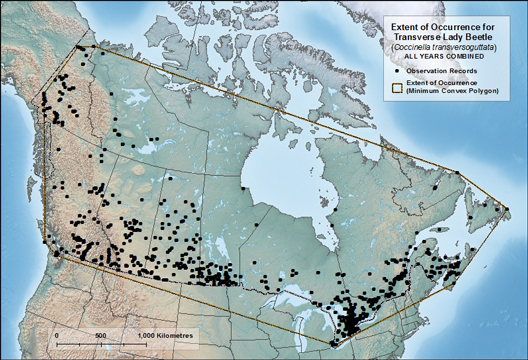
Canadian distribution of Transverse Lady Beetle based on museum collections and recent surveys (1889-2015). The map shows the Extent of Occurrence, the area included in a polygon without concave angles that encompasses the geographic distribution of all known populations of a wildlife species.
Transverse Lady Beetles are habitat generalists, primarily feeding on aphids and occurring across a wide range of habitats. This lady beetle inhabits agricultural areas, suburban gardens, parks, coniferous forests, deciduous forests, prairie grasslands, meadows, riparian areas and other natural areas. This broad habitat range reflects their ability to exploit seasonal changes in prey availability across different vegetation types.
Transverse Lady Beetles have four life stages: egg, larva, pupa and adult, and can have two generations per year. Adults of the spring generation can undergo aestivation to avoid high summer temperatures, and lay eggs in early autumn. Adults of the autumn generation congregate to overwinter and undergo diapause; becoming active and reproducing when temperatures warm in the early spring. This species occupies a wide ecological niche across a wide variety of habitats and temperature regimes in Canada. In general lady beetles are very mobile, display low site fidelity, and readily engage in short (few hundred metres) and long (18 – 120 km) distance dispersal. This species does not migrate. Both adult and larval stages are predatory and primarily prey on aphids. In turn, this species is also subject to predation by other invertebrates, vertebrates, and is susceptible to parasitoids and pathogens.
The historically broad geographic range and abundance of the Transverse Lady Beetle stands in stark contrast to its current distribution. Prior to 1986, this species was widely distributed and abundant across North America and was one of the most common lady beetles collected. Currently, in many parts of its range this species is either absent or below detection thresholds where it was formerly common. In other regions it persists in low numbers. In Yukon, the Northwest Territories and British Columbia, however, this species seems to be abundant and common. These regions also have a smaller proportion of non-native lady beetle species, which are considered one of the potential threats to this species and other native lady beetles.
The specific range-wide causes of decline in the Transverse Lady Beetle are currently unknown. Possible threats to this species may include negative interactions with recently arrived non-native species, such as the Seven-spotted Lady Beetle and Multicolored Asian Lady Beetle through competition, intraguild predation or indirect effects through introduction of pathogens. Other possible localized and cumulative threats include land use changes, such as direct and indirect effects of agricultural pesticide/chemical use to control their prey species, habitat loss through urban expansion, conversion of farmland to forest, and other human disturbances.
There are no laws in Canada that protect the Transverse Lady Beetle. This species has not yet been ranked globally or nationally. The Conservation Data Centres across Canada have assigned conservation status ranks as follows: ON: S1, YT: S4; NT: S4S5; BC: S5; AB, SK, MB: S4S5; ON: S1; QC: S4; NB, NS, PE: SH; NF: SU; NF (Labrador only): S5.
Source: COSEWIC. 2016. COSEWIC assessment and status report on the Transverse Lady Beetle Coccinella transversoguttata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 57 pp.

Photo: © Kym Welstead
Long description
Chrysemys picta bellii
Reptiles
Threatened
British Columbia
The distribution of this population overlaps with an area of dense human population in southwestern British Columbia, including the Lower Fraser Valley, where wetland loss has been extensive. Across its range, this population continues to face multiple threats from habitat loss and alteration, road mortality, and introduced species, such as Bullfrog and introduced turtles. Survey efforts within the past 10 years have revealed many new localities, bringing the total number of occupied waterbodies to over 80, grouped within 39 clusters. However, the Canadian population and local subpopulations are small and many, especially in the Lower Fraser Valley, are declining or considered not viable. The long-term persistence of the Canadian population remains precarious.
Western Painted Turtles (Chrysemys picta bellii, Gray 1831) are relatively large-bodied painted turtles with adults reaching up to 25 cm in carapace (dorsal shell) length. The subspecies has a distinct bright orange plastron (ventral shell) with a complex pattern of reticulated black lines.
In Canada, Western Painted Turtles occur in three discrete broad areas, corresponding to the following designatable units: Pacific Coast and Intermountain – Rocky Mountain populations in British Columbia, and Prairie/Western Boreal – Canadian Shield population east of the Rocky Mountains. Western Painted turtles in British Columbia show extensive genetic differentiation, sometimes over short distances. This report recognizes these differences but maintains the three designatable units from the previous COSEWIC assessment (2006), based on considerations of discreteness and significance of the variation.
The distribution of the Prairie/Western Boreal – Canadian Shield population extends from the Algoma region of northern Ontario, west across the southern prairies to southeastern Alberta. Its natural range in Alberta is limited to the Milk River watershed, but numerous introduced subpopulations exist, some of which have been well established for close to a century or longer. The Intermountain – Rocky Mountain population occurs primarily in major valley bottoms between mountain ranges across the Southern Interior of British Columbia. Major population centres include the Thompson and Okanagan valleys, the southern East Kootenay Trench, and the southern Cariboo Region. The Pacific Coast population occurs in the Lower Fraser Valley from about Chilliwack to Greater Vancouver, Sunshine Coast north to Powell River, Texada Island, and parts of Vancouver Island including the Capital Regional District, Nanaimo area, and Alberni Valley.
Distribution of Western Painted Turtle in Pacific Coast Population. Small yellow circle: <10 individuals; mid-sized blue circle: 10-100 individuals; large green circle: >100 individuals; red square: extirpated. Note that there is much uncertainty about population sizes, particularly for Vancouver Island north of the Capital Regional District.
Western Painted Turtles are highly aquatic and are found in shallow waters of ponds, lakes, oxbows, and marshes, in slow-moving stream reaches, and in quiet backwater sloughs of rivers. Usually, their habitat contains muddy substrates with emergent aquatic vegetation, exposed vegetation root mats, floating logs, and open banks. Painted Turtles prefer floating logs, branches, or other emergent objects for basking. Nesting habitats are on land adjacent to aquatic foraging habitat, usually within 200 m of the water body, typically on gentle south-facing slopes. Eggs are laid in well-drained sites with soil, sand or gravel substrates that have minimal or no plant cover.
Adult Western Painted Turtles are omnivorous and forage on aquatic vegetation, carrion, and small live prey in shallow waters during the active season from spring to autumn. Younger turtles tend to be more carnivorous and feed on a variety of invertebrates and tadpoles. Eggs hatch in autumn, but hatchlings usually remain in the nest for their first winter. Hatchlings are freeze-tolerant to at least -10°C. Painted Turtles are considered to be one of the most tolerant vertebrates of hypoxic (oxygen-poor) conditions. Predation on eggs and hatchlings can be very high. Age at maturity is thought to range from 4 to 10 years for males and from 6 to 15 years for females. Generation time is estimated to be approximately 25 to 30 years for the Pacific Coast population and 30 to 40 years for the Intermountain – Rocky Mountain and Prairie/Western Boreal – Canadian Shield populations.
There are no accurate estimates of population size for any of the three populations. The Pacific Coast population probably consists of approximately 3000 or fewer adults. The Intermountain – Rocky Mountain population may have 5,000 – 10,000 adults, while the Prairie/Western Boreal – Canadian Shield population may have 10,000s of adults. None of these estimates are based on robust methods. Inferred from habitat loss, the Pacific Coast population has most likely declined from historical levels by an unknown percentage. The number of known localities has increased greatly over the past ten years due to search efforts. The Intermountain – Rocky Mountain population has also likely suffered declines from historical levels, especially in the Okanagan Valley, based on habitat trends. Population trends for the Prairie/Western Boreal – Canadian Shield population are unknown, but localized declines are likely.
The main threats to Western Painted Turtles are from habitat loss and alteration, and road mortality. Habitat loss and alteration result from a variety of threats including residential and industrial development, agricultural activities that drain or infill water bodies, and free-ranging cattle that degrade water bodies. Turtles face threats from road mortality during seasonal migrations, when females move from water bodies to terrestrial nesting areas and when both males and females disperse. Other threats include invasive species, such as American Bullfrogs on the coast that prey on hatchlings and plants that reduce the quality of their nesting grounds. Recreational use can disturb basking and nesting turtles. Off-road vehicle use may degrade ponds and adjacent riparian habitats or damage nesting sites. Pollution runoff may affect water quality; sources include agricultural and septic tank runoff and industrial pollutants. Combined, threats for the Pacific Coast population were scored as “Very High”, for the Intermountain – Rocky Mountain population as “High”, and for the Prairie/Western Boreal – Canadian Shield population as “Medium”.
Western Painted Turtle was assessed by COSEWIC as three populations in 2006: Pacific Coast - Endangered; Intermountain-Rocky Mountain - Special Concern; and Prairie/Western Boreal – Canadian Shield - Not at Risk. The Pacific Coast and Intermountain – Rocky Mountain populations were placed on Schedule 1 of the Species at Risk Act (SARA) in their respective designations. A provincial recovery plan for the Pacific Coast population was finalized in June 2016. Critical Habitat has been drafted but not yet identified under SARA. There are no specific habitat protection measures in place for Western Painted Turtles. The species does benefit from some municipal planning measures designed to maintain environmentally sensitive areas and certain water bodies. These measures have limited scope and application.
Source: COSEWIC. 2016. COSEWIC assessment and status report on the Western Painted Turtle Chrysemys picta bellii, Pacific Coast population, Intermountain – Rocky Mountain population and Prairie/Western Boreal – Canadian Shield population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xxi + 95 pp.