COSEWIC Assessment and Status Report on the Black-foam Lichen Anzia colpodes in Canada - 2015

Photo © Environment Canada, 2015
Long description for the cover figure
Photo of a patch of the Black-foam lichen (Anzia colpodes), a foliose lichen with thalli that form greenish-grey rosettes. The solid thallus lobes rest on a dense, thick spongy cushion of black fungal filaments (the hypothallus). Stout but sparse rhizines grow from the lower cortex to the substrate, through the spongy hypothallus.
- Document Information
- Assessment Summary
- Executive Summary
- Technical Summary
- Wildlife Species Description and Significance
- Distribution
- Habitat
- Biology
- Population Sizes and Trends
- Threats and Limiting Factors
- Number of Locations
- Protection, Status and Ranks
- Acknowledgements and Authorities Contacted
- Information Sources
- Biographical Summary of Report Writers
- Collections Examined
- Figure 1. Close-up photograph of the thallus of A. colpodes showing an apothecium, with its brown disc (on the right), and the black pycnidia on the lobe ends (on the left). The black foam-like hypothallus underlies the greenish-grey thallus (photo: Troy McMullin)
- Figure 2. The distribution of Anzia colpodes in the United States. The record in Utah is very probably an error
- Figure 3. The distribution of A. colpodes in Canada. The occurrences currently known to be extant, from fieldwork carried out for this report, are shown as yellow dots (these occurrences were found post-1995). The black stars show occurrences that were found before 1995 and were not revisited, and revisited occurrences where the lichen was absent are red squares
- Figure 4. Search and collecting activity (red circles) with respect to the lichen family Parmeliaceae, the family to which Anzia colpodes belongs (see Classification). This family includes genera like Parmelia, Hypogymnia and Melanelia that are found on deciduous trees
- Figure 5. Sites that have been searched for epiphytic lichens, including Anzia colpodes, during fieldwork for this report and in recent surveys (black dots)
- Figure 6. The modelled ideal climate for Anzia colpodes using annual precipitation and mean maximum monthly temperature. The map on the left is for climate data observed from 1961 to 1990 and the map on the right is for climate data predicted to 2099. The yellow, orange and red zones indicate areas of increasingly more ideal climate for this lichen. The blue and dark blue areas show less ideal climates
- Figure 7. Grazing damage on thalli of A. colpodes; note also the pycnidia at the end of the lobes, the contents of which seem to have been eaten (compare with Figure 1). (Photo: Frances Anderson.)
- Table 1. General surveys for lichens which included searches for Anzia colpodes. This table does not include sites that were revisited or new sites from targeted searches (see Table 2) or the historical surveys undertaken by Wolfgang Maass (see Table 3)
- Table 2. Occurrences of Anzia colpodes that were revisited during fieldwork in 2013 and 2014 and those discovered since 1995 that were not revisited*
- Table 3. Pre-1983 records of Anzia colpodes from localities that have not been revisited for this report. The records for Nova Scotia result from collections made by Wolfgang Maass. These data were compiled after the completion of the 2013 surveys described above (see Table 2). The data came to light following transfer of the W. Maass herbarium to the New Brunswick Museum, where the collection is being curated
COSEWIC
Committee on the Status
of Endangered Wildlife
in Canada

COSEPAC
Comité sur la situation
des espèces en péril
au Cananda
COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2015. COSEWIC assessment and status report on the Black-foam Lichen Anzia colpodes in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 47 pp. (Species at Risk Public Registry website).
COSEWIC would like to acknowledge David Richardson, Frances Anderson, Robert Cameron, Stephen Clayden and Troy McMullen for writing the status report on the Black-foam Lichen, Anzia colpodes, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Rene Belland, co-chair of the COSEWIC Mosses and Lichens Specialist Subcommittee
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment Canada
Ottawa, ON
K1A 0H3
Tel.: 819-938-4125
Fax: 819-938-3984
E-mail: COSEWIC E-mail
Website: COSEWIC
Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur L'anzie mousse-noire (Anzia colpodes) au Canada.
Black-foam Lichen -- Provided by author
The Black-foam Lichen, Anzia colpodes, is a leafy lichen that grows as greenish grey rosettes up to 20 cm across on the trunks of deciduous trees. The 1-2 mm wide solid lobes rest on a thick spongy black tissue made of fungal filaments. The reddish-brown fruit bodies on the upper surface contain sacks that are unusual in containing a large number of tiny spores that provide its only means of reproduction.
The Black-foam Lichen is thought to be endemic to North America, although there is one report of its being found in eastern Russia. In the USA, it has been collected in the Appalachian Mountains from Georgia to Maine, but also on the Ozark Plateau and in Illinois, Wisconsin and Michigan. In Canada, this lichen is growing at the northern end of its distribution range and has been found in Ontario, Québec, New Brunswick and Nova Scotia. Recent surveys indicate that the Black-foam Lichen no longer occurs in the first two of these provinces and has not been recorded in New Brusnwick in the last decade. This lichen is widespread but not common in Nova Scotia.
The Black-foam Lichen grows on the trunks of mature deciduous trees growing on level or sloped land where high humidity is supplied by nearby wetlands, lakes or streams. The most common host is Red Maple but it also occurs on White Ash, Sugar Maple, Red Oak and very occasionally on other species.
Fruit bodies are frequent on the Black-foam Lichen and provide the only means of reproduction. The spores ejected from the fruit bodies need to land on a host tree trunk and encounter a compatible green alga. The algae become enveloped by fungal strands and eventually these grow into visible lichen. The generation time for this lichen is probably around 17 years. Unlike many other leafy lichens which grow on tree trunks, the Black-foam Lichen has no specialized vegetative propagules to provide a means of asexual reproduction.
The Black-foam Lichen seems always to have been less common in Ontario and Québec and in the adjacent US states than in New Brunswick and Nova Scotia. In the first two provinces there are only four records for this lichen; all the sites were revisited, but it was not found. In New Brunswick there are 12 records for this lichen, and it was not found again during searches at six of these sites done in 2013.
In Nova Scotia, the Black-foam Lichen is not common, but it is widespread. Thirty-five occurrences have been documented in the province since 1995. The population was enumerated at the nine occurrences where the Black-foam Lichen was found during the fieldwork for this status report. On the basis of the enumeration, it is estimated that the total population of this lichen in Canada could be as high as 3,700 individuals, with almost all being in Nova Scotia. In addition, the lichen was no longer present at three of the seven post-2006 revisited occurrences, indicating a ~40% decline over the last ten years.
In Ontario and Québec, the main threat to the Black-foam Lichen appears to be habitat disturbance. The few sites where it was recorded historically have been subject to the spread of suburbia, building sites, highways and trails that have removed the forest where this lichen was once found. Other likely threats in these provinces are air pollution and changing weather patterns. The cause of the disappearance of the Black-foam Lichen from these provinces is uncertain, but it is significant that declines have also been observed in adjacent states of the USA.
In New Brunswick and Nova Scotia, the main current threat is harvesting of older hardwood forests. The grazing impact of introduced molluscs is another threat with an unknown impact. The Black-foam Lichen with its low content of secondary substances lacks anti-herbivory effectiveness. Changing weather patterns are thought to have enhanced the spread and impact of grazing molluscs and may have affected the ability of this lichen to reproduce. Its tiny spores have little stored energy to provide the fungal germ tube with the means to search extensively for a compatible algal partner, a process required at every generation. Furthermore, its stout but sparse holdfasts which fasten small thalli firmly to the tree bark loosen as the lichen grows, making it vulnerable to removal by wind, rain or animals.
Over the longer term, climate change and alterations of weather pattern are predicted to result in reduced precipitation or enhanced evaporation. These are likely to affect the survival of the Black-foam Lichen as this species requires the right combination of climate and forest stand features. It is largely limited to growing on trees close to water bodies that include swamps, swamp margins, lakes and streams.
The Black-foam Lichen is listed by NatureServe Global Status asG3 (vulnerable)/ G5 (secure). The Rounded Global Status is G4 - Apparently secure. In the USA it has a national status of NNR (unranked). In Michigan, North Carolina and Pennsylvania it is SNR (not yet assessed), but in Wisconsin it is SX (presumed extirpated) and is also thought to be extirpated in Ohio. In Canada, the Black-foam Lichen is ranked by NatureServe as NNR (unranked). In Ontario it is SH (possibly extirpated) and in Québec it is SNR (not yet assessed).
Currently the Black-foam Lichenhas no legal protection or status in Canada, although a number of occurrences in Nova Scotia are protected as they occur in provincially protected wilderness areas or National Parks.
| Question | Information |
|---|---|
| Generation time (usually average age of parents in the population; indicate if another method of estimating generation time indicated in the IUCN guidelines(2008) is being used). Uncertain but between 10-30 years with 17 being the best estimate. | 17 years |
| Is there an [observed, inferred, or projected] continuing decline in number of mature individuals? | Yes, observed and projected |
| Estimated percent of continuing decline in total number of mature individuals within [5 years or 2 generations] (52-60 years) | Unknown |
| [Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over the last [10 years, or 3 generations] (78-90 years). The lichen was no longer present at 2 of five post-2006 occurrences which were revisited. | ~40% |
| [Projected or suspected] percent [reduction or increase] in total number of mature individuals over the next [10 years, or 3 generations] (78-90 years). Unknown as to precise magnitude, but a continuing decline is inferred based on evidence of recent declines and ongoing decline in available mature deciduous forest, a key habitat for the lichen. | ~30% |
| [Observed, estimated, inferred or suspected] percent [reduction or increase] in total number of mature individuals over any [10 years, or 3 generations] period, over a time period including both the past and the future. | Unknown |
| Are the causes of the decline clearly reversible and understood and ceased? | No |
| Are there extreme fluctuations in number of mature individuals? | Probably not |
| Question | Information |
|---|---|
| Estimated extent of occurrence (This was calculated based on occurrences found since 1995 or older occurrences that were revisited. It does not include the pre-1983 occurrences in Table 3 because none of these occurrences are georeferenced and it is unknown if the lichen is currently present.) | 30,555 km² |
| Index of area of occupancy (IAO, 2 x 2 km2 grid values) This was calculated based on occurrences found since 1995 or older occurrences that were revisited, and does not include older occurrences that were not revisited, e.g., W. Maass sites in Table 3, because none of these occurrences are georeferenced and it is unknown if the lichen is currently present. | 108 km² |
| Is the population severely fragmented? The distribution is widely scattered and its dispersal distance probably limited. | Probably not |
| Number of Locations. The total number of occurrences in Canada is about 100, but the lichen is no longer present at some. It is possible more occurrences may be found in Nova Scotia, hence the total likely number is estimated as <100. Each occurrence considered a location because the threats are varied and the key ones leading to the disappearance of this lichen are not well understood. | <100 |
| Is there an [observed, inferred, or projected] continuing decline in extent of occurrence? | Yes |
| Is there an [observed, inferred, or projected] continuing decline in index of area of occupancy? | Yes |
| Is there an [observed, inferred, or projected] continuing decline in number of populations? Observed decline of 77% was observed in the revisited populations in Canada (the lichen was absent at 17 of the 22 revisited occurrences). | Yes |
| Is there an [observed, inferred, or projected] continuing decline in number of locations? | Yes |
| Is there an [observed, inferred, or projected] continuing decline in [area, extent and/or quality] of habitat? Projected decline inferred due to climate change. | Yes |
| Are there extreme fluctuations in number of populations? | No |
| Are there extreme fluctuations in number of locations? | No |
| Are there extreme fluctuations in extent of occurrence? | No |
| Are there extreme fluctuations in index of area of occupancy? | No |
| Population: | N Mature Individuals |
|---|---|
| Known extant colonies Ontario = 0, Québec = 0, New Brunswick = 0, Nova Scotia = 3,700, if recent and historical occurrences in Nova Scotia are included in the estimate. |
Uncertain, likely <4,000 |
| Total | <4,000 |
| Question | Information |
|---|---|
| Probability of extinction in the wild is at least [20% within 20 years or 5 generations, or 10% within 100 years]. | Not done |
| Forest harvesting, Habitat fragmentation and decline in quality of habitat, changing weather patterns and grazing by introduced gastropods. |
| Question | Information |
|---|---|
| Status of outside population(s) Anzia colpodes appears to be extirpated from Wisconsin and Ohio and very rare in all bordering US states except Maine where 5 occurrences are known. | Declining and extirpated from two states. |
| Is immigration known or possible? Possible but unlikely because of distances involved. | Very unlikely |
| Would immigrants be adapted to survive in Canada? | Potentially if there were suitable host trees |
| Is there sufficient habitat for immigrants in Canada? | Yes, at least in the Maritimes |
| Is rescue from outside populations likely? | Possible, but unlikely |
| Question | Information |
|---|---|
| Is this a data-sensitive species? | No |
Designated Threatened in May 2015
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
The Canadian Wildlife Service, Environment Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Lichen colpodes Ach. Lichenographiae Sueciae Prodromus: 124, 1798.
Parmelia colpodes (Ach.) Ach. Methodus qua Omnes Detectos Lichenes Secundum Organa Carpomorpha ad Genera, Species et Varietates Redigereatque Observationibus Illustrare Tentavit Erik Acharius: 251, 1803.
Anzia colpodes (Ach.), Stizenberger, Flora (Regensburg), 45: 243, 1862
Anzia is a member of the Parmeliaceae, a large family of foliose lichens. Recent attempts to understand the phylogenetic relationships in this family have revealed that 88 genera are monophyletic and six well-supported clades can be distinguished. The phylogenetic position of eight genera including Anzia remains unresolved using both ribosomal markers and ITS, tubulin and other sequences (Thell et al. 2004; Crespo et al. 2007).
The genus Anzia is divided into two sections. Anzia sect. Anzia is confined to east Asia and eastern North America. Anzia sect. Nervosa occurs in the tropics with centres in southeastern Asia and tropical America, and is differentiated by having an axis like Usnea (Yoshimura 1987, Yoshimura et al. 1997). Anzia colpodes belongs to the section Anzia and is one of three species found in North America, the other two being A. americana and A. ornata (Esslinger and Eagan 1995; Hinds and Hinds 2007). According to Jayalal et al. (2013), of 11 species of Anzia studied, A. colpodes is phylogenetically most closely related to A. opuntiella, a species found in Japan.
The name “Black-foam Lichen” (Brodo et al. 2001) refers to the spongy tissue that underlies the thallus.
French Common Name: Anzie mousse-noire (Fournier 2006)
Anzia colpodes is a foliose lichen. The thalli grow to form greenish-grey rosettes that are up to about 20cm in diameter (Brodo et al. 2001).The solid thallus lobes are 1-2 mm wide and rest on a dense, thick spongy cushion of black fungal filaments called the hypothallus. Stout but sparse rhizines grow from the lower cortex to the substrate, through the spongy hypothallus. Its lobe shape and the black colour of the undersurface make it somewhat resemble Hypogymnia, a genus that has hollow thalli. The meristematic zone, at the tips of the Anzia lobes, initially produce a small amount of lower cortex and then the spongy tissue, sometimes termed the spongiostratum (Lawrey 1984; Hensen and Dobelman 1987; Jahns 1988).
Apothecia are common on thalli of A. colpodes. They have a brown disc with a thick in-rolled thalline margin that becomes thin and is less evident as the apothecia mature. The contained asci have an apical cushion and outer layer (Budel and Scheidegger 2008). Most lichens produce eight spores per ascus, but in this lichen, the asci contain more than a hundred tiny spores that are only about 1x3µm in size. Black pycnidia are also frequent and occur near the ends of the lobes (Figure 1) and contain simple curved pycnidiospores (see Life Cycle and Reproduction).

Long description for Figure 1
Close-up photograph of the thallus of A. colpodes showing an apothecium, with its brown disc and the black pycnidia on the lobe ends. The black foam-like hypothallus underlies the greenish-grey thallus.
The photobiont is green and reported to be Trebouxia jamesii (Ahmadjian 1993), but molecular methods have shown that all the algae assigned to this species should be included in the Trebouxia simplex aggregate and that is the latest name for it (Beck 2002; Opanowicz and Grube 2004).
Anzia colpodes has no specialized vegetative propagules, unlike the other two Anzia species found in North America.
The upper cortex is K+Yellow with spot tests and the medulla UV+ white, due respectively to the presence of atranorin and divaricatic acid. Divaricatic acid amounts to 1.4% dry weight of the thallus (Hale 1955; Culberson 1961), although a more recent analysis gave a lower figure of 0.42% (Yoshimura and Kurokawa 1977). Some specimens may also contain traces of sekikaic acid that is derived from divaricatic acid (Yoshimura 1987).
Little is known about the population structure and its variability in Anzia colpodes, but dispersal is likely very limited and separate site occurrences are assumed to represent different subpopulations (see Dispersal and Migration)
Historically, Anzia colpodes occurred across at least two and possibly threeNational Ecological Areas (Boreal, Great Lakes Plains, and Maritimes), and across a disjunction of at least several hundred kilometres (from western Québec to central New Brunswick, see Distribution) the Black-foam Lichen subpopulations in Ontario/Québec and the Maritimes likely meet the discreteness criterion for distinct designatable units (DUs). Given, however, that: (i) there is no evidence of phenotypic differences between lichen from these areas, (ii) no molecular work has been completed on the species, and (iii) the Ontario/Québec subpopulations are historical in nature, the Black-foam Lichen is treated as a single DU.
Anzia colpodes, apart from a possible record from eastern Russia, is considered a North American endemic. It is unusual because of its foam-like under-thallus and the fact that the spore-containing sacks contain many tiny spores rather than the usual eight. It is found in the Ozark plateau and Appalachian mountain chain, but rarely elsewhere. In Canada it now seems restricted to Nova Scotia.
Anzia is an ancient genus of lichens with a fossilized specimen dated to approximately 40 million years before present (Rikkinen and Poinar 2002). Thirty-four species of Anzia have been described from the Eastern and Western hemispheres, mostly in subtropical and tropical regions (Jayalal et al. 2013). During the Tertiary, a similar vascular plant vegetation extended from North America through the then adjoining Europe across to Asia to Japan, but this and its lichen flora were subsequently left as relict populations (Culberson 1972) which were further reduced by glaciation (Poelt 1963). Evolution of these populations led to closely related species that varied in their chemistry or morphology, e.g., A. colpodes in North America and A. colpota in Japan and Korea (Culberson 1972, Park 1990). A. colpodes is thought to be endemic to North America, but there is a recent record of this species from the Nogeevskaya Pass on the boundary of the Lazo Reserve, eastern Russia (Leif Tibell, 1991 in GBIF-Sweden 2014). However, Tibell (pers. comm. 2014) was unable to confirm the identification as it lacked apothecia. Anzia ornata is another North America species with a disjunct distribution in Asia. This, and the European fossil evidence, suggests that Anzia sect. Anzia was once circum-Laurasian, but later became extinct in Europe (Yoshimura 1987; Rikkinen and Poinar 2002).
Anzia colpodes is the most common of the three species of the genus in the USA; the other two are A. ornata and A. americana. Its distribution in the USA (Figure 2) includes a record from Utah, an outlier collected on Bull Mountain in the Henry Mountains, that is probably in error and cannot be verified as the specimen and location details are missing from the Arizona State University Herbarium. A. colpodes is reported to have become increasingly rare. In Maine, it is now restricted to five old-growth forests (Hinds and Hinds 2007). It is probably extirpated from Ohio and critically imperilled in Michigan and Wisconsin (Showman and Flenniken 2004, NatureServe 2014, Bennett and Will-Wolf (pers. comm. 2014). Records from the Consortium of North American Herbaria indicate that the distribution of A. colpodes is centred in eastern North America and is more common in warmer regions of the USA such as Tennessee and Arkansas. In the Ozarks, it has shown no evidence of decline in the past two decades (Doug Ladd pers. comm. 2014).
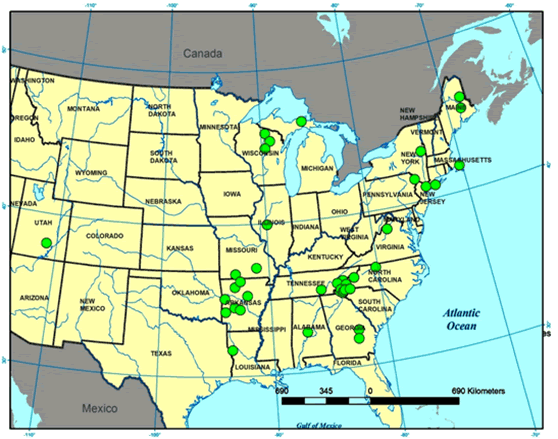
Long description for Figure 2
Map showing the distribution of Anzia colpodes in the United States, where it is centred in the eastern part and more common in warmer regions such as Tennessee and Arkansas. The map includes a record from Utah that is probably in error.
Anzia colpodes is known historically from four provinces in Canada: Ontario, Québec, New Brunswick and Nova Scotia (Figure 3). The historical and current occurrences of A. colpodes in Canadarepresent about half of the known world total.
Anzia colpodes collections from the nineteenth century were made by John Macoun and the Rev. G. Ducharme from four occurrences in Ontario and Québec (listed in Macoun 1902). These were at Rigaud Mountain, Vaudreuil Co. and Kings Mountain, Chelsea in Québec, and Central Ontario Junction, Hastings Co. and Prescott in Grenville County near Ottawa, Ontario. There is also a collection of A. colpodes from Grand Manan Island, New Brunswick, made by Henry Willey in 1879 and lodged in the herbarium of the University of Minnesota (MIN).
Since the 1970s, collections have been made in central and southern mainland New Brunswick and widely in Nova Scotia (Figure 3). Historical collections were made in the late 19th and early 20th century and then there was further significant collecting in the 1980s and very little collecting in the early 1990s. Tom Neily began more extensive searches in the 1990s. The break between the two phases of collection (1980-1995) amounts almost to one generation of A. colpodes. For this reason the year 1995 provides a logical point at which to separate historical occurrences from those at which the lichen was most likely still extant.
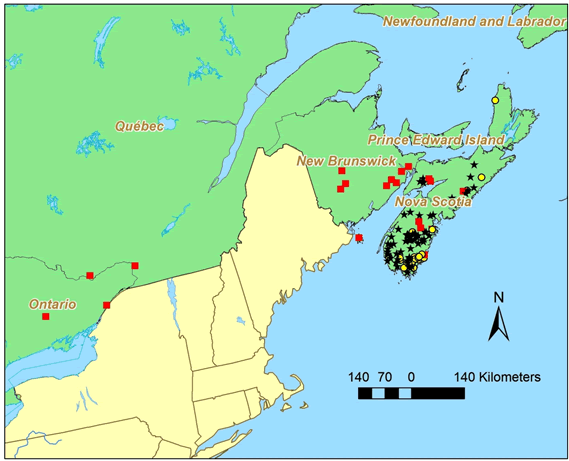
Long description for Figure 3
Map of the distribution of Anzia colpodes in Canada, where the species is known historically from four provinces: Ontario, Quebec, New Brunswick, and Nova Scotia. The map indicates occurrences currently known to be extant from fieldwork carried out for this report, occurrences that were found before 1995 and were not revisited, and revisited occurrences where the lichen was absent.
The extent of occurrence in Canada was found to be about 30,555 km² and was based on records ofA. colpodes found since 1995 and older occurrences that were revisited. It does not include pre-1983 occurrences that were not revisited for this report as they were not georeferenced and it is unknown if the lichen is still present at these sites. The same approach was used for calculating the index of area of occupancy which was estimated to be about 108 km².
There is a long history of lichen collecting in eastern Canada (Goward et al. 1998; p. 6., Clayden 2010, p. 157) and Ontario (Figure 4). Searches prior to the start of fieldwork for this report did not focus on A. colpodes although it was well known to those undertaking the general surveys mentioned in the references cited above (Table 1).
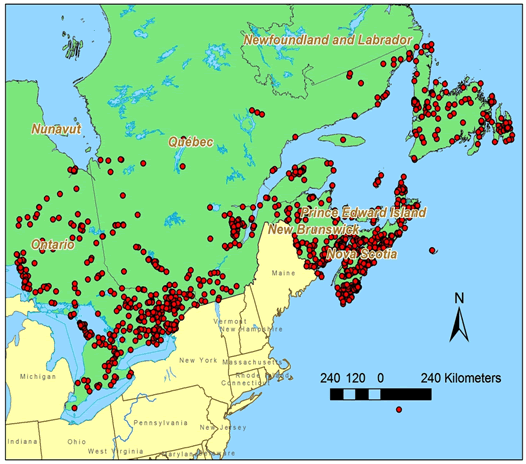
Long description for Figure 4
Map showing localities in Canada that have been searched for lichens in the Parmeliaceae family, the family to which Anzia colpodes belongs.
| Province | Areas searched | Searcher | Date |
|---|---|---|---|
| Ontario | Sites within a square bounded by Ottawa, Peterborough, Haliburton, Watertown | McMullin | 2013 |
| Ontario | Bruce National ParkFootnote star* | Various | 2008 |
| Ontario | Ottawa and Environs | Brodo | 1970 to 2014 |
| Ontario | S. shore Georgian Bay, Awenda Provincial Park | McMullin | 2013 |
| Ontario | Nature Reserve, Guelph | McMullin | 2012 |
| Québec | Mount Orford | S. Clayden | 2004 |
| Ontario | Mount Gosford | Clayden | 2004 |
| Ontario | Eastern Townships | F. LeBlanc | 1960,1963 |
| Ontario | Parc de la GaspésieFootnote star* | various | 2012, 2013 |
| Ontario | Parc du Bic | Anderson | 2013 |
| New Brunswick | Grand Lake Protected Natural Area | Clayden | 2013 |
| New Brunswick | Saint John River floodplain | Clayden | 2013 |
| New Brunswick | Fundy National Park | Clayden, Anderson` | 2013 |
| New Brunswick | Fundy NP | Clayden | 1976 |
| New Brunswick | Grand Falls FlowageFootnote star* | Various | 2011 |
| New Brunswick | Love Lake BrookFootnote star* | Various | 2011 |
| New Brunswick | Fundy NP Mycological Foray | Various | 2013 |
| New Brunswick | Kouchibouquac National Park | Clayden, Koffman | 2001 |
| New Brunswick | CFB Gagetown | Clayden | 2013 |
| New Brunswick | Currie Mountain | Clayden | 2013 |
| New Brunswick | Keswick Ridge | Clayden | 2013 |
| New Brunswick | Near Iroquois River, Madawaska | Clayden | 2013 |
| New Brunswick | Gounamitz River | Clayden | 2013 |
| Nova Scotia | Western end Cobequid mountainsFootnote star* | Various | 2004 |
| Nova Scotia | Cape Chignecto Provincial Park | Anderson | 2004,2005,2006,2007,2008,2009 |
| Nova Scotia | Mount Uniacke | Richardson | 2013 |
| Nova Scotia | Blandford Nature Reserve | Anderson | 2005, 2006 |
| Nova Scotia | Abraham Lake Bioblitz | Anderson | 2012 |
| Nova Scotia | Long Lake Bioblitz | Richardson, Anderson | 2008 |
| Nova Scotia | West Ironbound Island | Anderson | 2009 |
| Nova Scotia | Antigonish and area | Anderson Neily | 2009,2010 |
| Nova Scotia | Tobeatic Game Sanctuary | Anderson | 2010 |
| Nova Scotia | Blandford Nature Reserve | Anderson | 2005,2006 |
| Nova Scotia | Crown Land Shelburne County | Neily | 2006-2014 |
| Nova Scotia | Crown Land Queens County | Neily | 2006-2014 |
| Nova Scotia | Crown Land Guysborough County | Neily | 2006-2014 |
| Nova Scotia | East side Cape Breton Island, Bras d’Or Lake, St. Ann’s Bay | Neily Anderson | 2009-2010,2013 |
| Nova Scotia | Lockhart’s Brook, Cape Breton island | Anderson | 2010 |
| Prince Edward Island | Western end, Prince County | McMullin | 2009 |
| Post-1995 Occurrences not revisited | Occurrence | Date Found | Re-survey date | Re-survey pres-ence | Est. # of col-onies | Host ree |
Grazing damage | Ownership/ Protection |
Possible Threats |
|---|---|---|---|---|---|---|---|---|---|
| Nova Scotia - Annapolis | Bowater Lands McGill | 2005 | - | - | - | Red Maple | - | Crown Land | F,G,H,C |
| Nova Scotia - Digby | Great Pubnico Lake | 2012 | - | - | - | Red Maple | - | Private Land | F,G,H,C |
| Nova Scotia - Guysborough | Melopsoketch/West River Lakes | 2012 | - | - | - | Red Maple | - | Crown Land | F,G,H,C |
| Nova Scotia - Hants | Near Baker Falls | 2013 | - | - | 4 | Ash | N | Private Land | F,G,H,C |
| Nova Scotia - Lunenburg | Rhodes Corner, Beck’s Lake | 2011 | - | - | - | Ash, Red Maple | Y | Private Land | F,G,H,C |
| Nova Scotia - Lunenburg | Sherbrooke Lake, Barss Corner Road | 2014 | - | - | 3 | Red Maple | N | Private Land | F,G,H,C |
| Nova Scotia - Lunenburg | Bowater Timber Lake | 2007 | - | - | - | Red Maple | - | Private Land | F,G,H,C |
| Nova Scotia | Rocky Lake, Clyde River | 2007 | - | - | - | Red Maple | - | Private Land | F,G,H,C |
| Nova Scotia - Queens | Lake Rossignol | 2006 | - | - | 75+ | Red Maple | N/A | Wilderness Area | G,C |
| Nova Scotia - Queens | Pebbeloggitch Lake Kejimkujik | 2011 | - | - | - | Red Maple | - | National Park | G,C |
| Nova Scotia - Shelburne | Bowers Meadow Wilderness Area |
2012 | - | - | - | Red Maple | - | Wilderness Area | G,C |
| Nova Scotia - Shelburne | Northwest Brook | 2007 | - | - | - | Red Maple | - | Crown Land | F,G,H,C |
| Nova Scotia - Shelburne | Birchtown Brook | 2007 | - | - | - | Red Maple | - | Private Land | F,G,H,C |
| Nova Scotia - Shelburne | Misery Lake | 2013 | - | - | 8+ | Red Maple | Y | Crown Land | F,G,H,C |
| Nova Scotia - Shelburne | Misery Brook | 2012 | - | - | - | Red Maple | - | Crown Land | F,G,H,C |
| Nova Scotia - Shelburne | North Lake, N of Canada Hill Lake | 2012 | - | - | - | Red Maple | - | Crown Land | F,G,H,C |
| Nova Scotia - Shelburne | West Jordan River | 2012 | - | - | - | Red Maple | - | Crown Land | F,G,H,C |
| Nova Scotia - Shelburne | North of Jordan Falls | 2008 | - | - | - | Red Maple | - | Crown Land | F,G,H,C |
| Nova Scotia - Shelburne | East Sable River | 2011 | - | - | - | Red Maple | - | Crown Land | F,G,H,C |
| Nova Scotia - Shelburne | Johnstons Pond | 2011 | - | - | - | Red Maple | - | Private Land | F,G,H,C |
| Nova Scotia - Yarmouth | Rushy Lake | 2008 | - | - | - | Red Maple | - | Crown Land | F,G,H,C |
| Nova Scotia - Yarmouth | Great Pubnico Lake | 2012 | - | - | - | Red Maple | - | Private Land | F,G,H,C |
| Nova Scotia - Yarmouth | Louis Lake | 2013 | - | - | - | Red Maple | - | Crown Land | F,G,H,C |
| Province and County | Occurrence | Date Found | Host Tree | Ownership/Protection |
|---|---|---|---|---|
| New Brunswick - Albert | Rat Trail, Fundy National Park | 1981 | - | National Park |
| New Brunswick - Kings | Weldon Brook | 1-Jun-77 | Sugar Maple | Private Land |
| New Brunswick - York | Nashwaaksis stream | 11-May-77 | Eastern Hemlock | Private Land |
| New Brunswick - York | Love's Sugar Bush, Mactaquac | 1-Apr-73 | Sugar Maple | Private Land |
| New Brunswick - ? | Walker Road | 5-Nov-77 | Sugar Maple | Private Land |
| New Brunswick - ? | Maple Vale Organic Farm, Greenhill | 1-Sep-71 | Red Maple | Private Land |
| Nova Scotia - Annapolis | Jakes Landing | 13 June 1981 | Maple | Kejimkujik National Park |
| Nova Scotia - Annapolis | Peskowesk Brook, Kejimkujik | 19 Nov. 1981 | Maple | National Park |
| Nova Scotia - Annapolis | Maitland Bridge, Kejimkujik | 15 April 1982 | Maple | National Park |
| Nova Scotia - Annapolis | New Albany | 22 May 1982 | Red Oak | Private Land |
| Nova Scotia - Annapolis | Nichols Road, Clarence | 20-May-94 | Sugar Maple | Private Land |
| Nova Scotia - Cumberland | New Canaan | 8 June 1981 | Red Maple | Private Land |
| Nova Scotia - Cumberland | Wentworth Brook | 17 April 1982 | Maple | Private Land |
| Nova Scotia - Cumberland | Philip Lake | 26 April 1982 | Ash and Maple | Private Land |
| Nova Scotia - Cumberland | Northside of Cobequid Mountains | 18 September 1982 | Sugar Maple | Private Land |
| Nova Scotia - Digby | Meteghan Station | 19 June 1981 | Maple | Private Land |
| Nova Scotia - Digby | St. Joseph | 17 April 1982 | Red Maple | Private Land |
| Nova Scotia - Digby | Maxwellton Station | 24 April 1982 | Maple | Private Land |
| Nova Scotia - Digby | Weaver Settlement/Havelock | 24 April 1982 | Maple | Private Land |
| Nova Scotia - Digby | Shelburne River/Kedge River | 25 April 1982 | Maple | Private Land |
| Nova Scotia - Guysborough | Main Road, Liscomb Game Sanctury | 1 July 1981 | Maple | Private Land |
| Nova Scotia - Guysborough | Bridge over Birch Hill Creek Branch | 20 June 1981 | Maple | Private Land |
| Nova Scotia - Halifax | Devon | 11 March 1982 | Maple | Private Land |
| Nova Scotia - Halifax | Porters Lake Highway 7/107 | 10 April 1982 | Maple | Private Land |
| Nova Scotia - Halifax | Porters Lake Headwaters | 25 April 1982 | Maple | Crown Land |
| Nova Scotia - Halifax | Sheet Harbour, Highway 224 | 25 April 1982 | Birch | Private Land |
| Nova Scotia - Halifax | Lake Charlotte/Mooseland | 26 April 1982 | Maple | Private Land |
| Nova Scotia - Halifax | Moose River | 22 May 1982 | Sugar Maple | Private Land |
| Nova Scotia - Kings | Fales River | 11-May-77 | Red Maple | Private Land |
| Nova Scotia - Lunenburg | Snake Lake, Kejimkujik | 19 November 1981 | Maple | National Park |
| Nova Scotia - Lunenburg | Stanburne/East Dalhousie | 5 September 1982 | Red Maple | Private Land |
| Nova Scotia - Queens | Burnaby Brook, Medway River | 16 March 1982 | Red Oak | Private Land |
| Nova Scotia - Queens | Molega | 10 April 1982 | Maple | Private Land |
| Nova Scotia - Queens | Tupper Lake | 10 April 1982 | Red Maple | Private Land |
| Nova Scotia - Queens | Harmony Mills/Westfield | 10 April 1982 | Ash and Maple | Private Land |
| Nova Scotia - Queens | Shelburne River | 25 April 1982 | Red Oak | Private Land |
| Nova Scotia - Queens | Porcupine Brook, Lake Rossignol | 1 May 1982 | Maple | Private Land |
| Nova Scotia - Queens | Porcupine Brook, Lake Rossignol | 16 May 1982 | Maple | Private Land |
| Nova Scotia - Queens | Cameron Lake | 22 May 1982 | Ash | Provincial Picnic Park |
| Nova Scotia - Queens | Snake Lake, McGinty Lake, Kejimkujik | 26 July 1982 | Red Oak | National Park |
| Nova Scotia - Queens | Ten Mile Lake, Middlefield | 5 Sept. 1982 | Red Oak | Provincial Picnic Park |
| Nova Scotia - Queens | Medway River/Burnaby Brook | 1-Sept 1971 | Red Oak | Private Land |
| Nova Scotia - Shelburne | Deception Lake/ west branch of Roseway River | 16 May 1981 | Maple | Crown Land |
| Nova Scotia - Shelburne | Clyde River/middle Clyde River | 20 June 1981 | Red Maple | Private Land |
| Nova Scotia - Shelburne | Deception Lake, Clyde River/Shelburne | 20 June 1981 | Maple | Crown Land |
| Nova Scotia - Shelburne | Welshtown/Upper Clyde | 20 June 1981 | Maple | Private Land |
| Nova Scotia - Shelburne | Twenty Click, Clyde River | 11 July 1981 | Balsam Fir | Private Land |
| Nova Scotia - Shelburne | Middle Clyde River | 11 October 1981 | Maple | Private Land |
| Nova Scotia - Shelburne | Pug Lake | 11 March 1982 | Maple | Private Land |
| Nova Scotia - Shelburne | Oak Park | 18 April 1982 | Maple | Private Land |
| Nova Scotia - Shelburne | Harlow Brook, SW Lake Rossignol | 22 May 1982 | Maple | Private Land |
| Nova Scotia - Yarmouth | Glenwood | 11 May 1981 | Maple | Private Land |
| Nova Scotia - Yarmouth | Quinan/Tusket | 22 August 1981 | Maple | Private Land |
| Nova Scotia - Yarmouth | East Kemptville/ Indian Fields | 19 November 1981 | Maple | Private Land |
| Nova Scotia - Yarmouth | Little Tusket Lake/Tusket Lake | 17 April 1982 | Maple | Private Land |
| Nova Scotia - Yarmouth | Kegeshook Lake, East Quinan | 18 April 1982 | Maple | Private Land |
| Nova Scotia - Yarmouth | Lake Jesse | 18 April 1982 | Maple | Private Land |
| Nova Scotia - Yarmouth | Port Maitland Beach | 4 September 1982 | Maple | Private Land |
| Nova Scotia - ? | Lynn | 11 July 1981 | Red Maple | Private Land |
Most of the recent discoveries of A. colpodes in Atlantic Canada occurred during fieldwork for this report and as a result of surveys by Tom Neily. Surveys of three sites in Prince Edward Island, where there are no historical records for A. colpodes, failed to discover the species (Figure 5). Furthermore, A. colpodes has not been found in Newfoundland and Labrador in spite of the fact that modelling indicates that suitable climates exist there (see Habitat Trends).

Long description for Figure 5
Map of Canadian sites that were searched for epiphytic lichens, including Anzia colpodes, during fieldwork for this report and in recent surveys.
Anzia colpodes was not found at either of the historical sites that were revisited (Figure 3). The first area searched was at Prescott, an area that probably had a greater amount of forest cover in 1861 when the lichen was found there. Now it is the end of a suburb and is surrounded by developments on three sides and by the 401 highway on the other. The second site was at the Ontario Central Junction which was an old train station and is now the confluence of a major snowmobile trail and the Trans-Canada trail. One part of this site still has a rich upland old-growth deciduous stand, potentially suitable habitat. Finally, in a field survey for Physconia subpallida, potentially good habitat for A. colpodes was examined in eleven areas within a square bounded by Ottawa, Waterton, Peterborough and Haliburton (McMullin 2013 and pers. comm. 2014). A. colpodes was not found at any of the sites visited (Figure 5). Incidentally, A. colpodes occurs but is very rare in northern Wisconsin and Michigan. There is a possibility that this lichen might be found in adjacent areas in Canada, possibly near Sault Ste. Marie, the north shore of lake Superior, the Almaguin Highlands, Manatoulin Island, Lake Temiskaming, Temagami and Abitibi (S. Brinker pers. comm. 2014).
Two sites where the lichen was previously known were visited in Québec again in 2013 without A. colpodes being found. One was on King Mt. in Gatineau, QC, which was visited twice. The lichen flora was rich and interesting, especially at the top with many large old deciduous trees. The other site visited was at Mt. Rigaud, a rich old mixed-wood forest. It is fairly heavily disturbed by many trails and human activity. The forest also backs onto a large college campus.
There are no known A. colpodes sites in southeastern Québec (east of the St. Lawrence valley) in spite of surveys in the region including searches of Sugar Maple (Acer saccharum)-dominated stands around Mt. Orford and Mt. Gosford (Clayden, pers. comm. 2014). There have also been studies of the epiphytic lichens and bryophytes in the hardwood forests in the Eastern Townships but A. colpodes was not recorded (LeBlanc 1960, Figs. 1-3, LeBlanc 1963).
Twelve A. colpodes sites are known historically in New Brunswick, including Henry Willey’s 1879 record from Grand Manan Island. In 2013, six of these sites were visited. Two of the remaining six historical sites could not be revisited because one had been clear-cut of trees (Walker Road) and the other no longer had its access trail maintained (Rat-tail Trail, Fundy Park). The lower boles of trees (to 2 m above the ground), and many fallen branches were surveyed. A visit was made to Grand Manan Island to re-find Willey’s 1879 site. Searches along the northwest side of the island in a mixed forest with Sugar Maple and other trees were examined for A. colpodes, without success (Table 2).
Eight other potential sites within and outside the documented range of Anzia in the province were also searched. The Weldon site in Albert County is close to the Caledonia Gorge Protected Natural Area, where several weeks of intensive survey work were done in 2011 and 2012. The mature hardwood stands were searched in stands near Fredericton, at Bear Island, in Mactaquac Provincial Park, around Ayers Lake, and in various areas near the Meduxnekeag River. The site on Nashwaaksis Stream was found to have been encroached upon by low-density, high-end suburban residential development, so it was not feasible to get to it. The Grand Lake Protected Natural Area was visited for 10 days of fieldwork as part of a biodiversity survey. The mixed, mesic to wet, forests had a lot of Red Maple (Acer rubrum), but none had A. colpodes. Not much searching has been done in the upper Nashwaak River valley (Greenhill site) but there has been intensive, widespread strip-cutting and clear-cutting of mature hardwoods going on in this area, as in the upland areas of Albert County.
With a very modest lichenological effort in the 1970s (Stephen Clayden, Hal Hinds, and Hinrich Harries) were able to find widely scattered occurrences of A. colpodes in southern NB. In the past several years, with much more intensive searching for this species, it has not been found anywhere.
At the time when fieldwork began for this status report in 2013, it was believed that prior to 2000, A. colpodes had only been collected from Colchester, Cumberland and Queens Counties by Harries in 1977 and 1994 with three specimens also being collected by Maass and Hoisington (1982) and lodged in the NS Museum. There was also a record of A. colpodes being found in Queens County during the 1999 Tuckerman workshop (LaGreca and Lay pers. comm. 2013). However, these records were not published and the first papers on A. colpodes in Nova Scotia were by McMullin et al. (2008) and McMullin (2009).
Twelve of the 35 known occurrences were visited in 2013 and 2014 (Table 2). A drumlin was searched at Reid Hill, Halifax County where the lichen was found to be common and an upland hardwood stand where it was not re-found. One of two sites at Kejimkujik National Park was visited and the lichen was present, but not common. Former sites at Franey’s Corner and Granite Village were visited as was the Hinrich Harries site in Colchester County, the Ash Brook site in Lunenburg County and the Thomas Raddall site in Queens County, but without finding the lichen. Economy Falls area where Stephen Clayden had found A. colpodes in 1992 and nearby areas were searched in 2013, as was a drumlin in the Mount Uniacke area without finding A. colpodes.
The private herbarium of Wolfgang Maass was transferred to the New Brunswick Museum and the collection was curated and searched in 2013 by Stephen Clayden for A. colpodes records. The results became available only after the fieldwork described above had been completed. Maass collected widely in Nova Scotia over his very long career. Among his collections from Nova Scotia are 53 records of A. colpodes, mostly from the 1980s and all pre-1995 (Table 3). Based on these data and the more recent collections, it is clear that A. colpodes is far from being common in the province but it is widespread (Tom Neily pers. comm. 2014). Out of eighteen counties, A. colpodes is recorded in all but five: Antigonish, Cape Breton, Pictou, Richmond, and Victoria.
There are no historical records of A. colpodes from Newfoundland. Although climate modelling suggests Newfoundland may have a favourable climate for A. colpodes (see Habitat Trends below), the comparative absence of suitable substrata (e.g., bark of Acer spp.) makes it unlikely this species occurs there. Furthermore, extensive lichen surveys by W. Maass and T. Ahti in Newfoundland and the survey of the Avalon Peninsula by the Tuckerman Group of lichenologists have failed to record its presence. Finally, studies by Eugene Conway and Ian Goudie (The Newfoundland Lichen Education and Research Group), Mac Pitcher and Claudia Hanel (Government of Newfoundland and Labrador) and John McCarthy have also not found this species, although they mostly focused on cyanolichens.
Anzia colpodes requires mature deciduous tree habitats with high humidity and high light levels. The required humidity is supplied by wetlands, nearby brooks, lakes or by the host’s position on upland slopes above a water body. In southwest Nova Scotia, where the lichen is quite common, the annual precipitation ranges from 1400 to 1500 mm with a mean monthly temperature of 10 to 12 °C while in northern Nova Scotia there is an annual precipitation of 1000 to 1400 mm with a mean monthly temperature of 10 to 11 °C. In the USA, A. colpodes is commonest in the Smokey Mountains and the Ozarks, which respectively have a similar rainfall to the above two areas of Nova Scotia but with higher temperatures of 16-19 and 19-23°C. In Nova Scotia A. colpodes has been found within a kilometre of the coast at Thomas Raddall Provincial Park. However, proximity to a water body or water source appears to be more important than proximity to the coast.
In Nova Scotia, host tree trunks (in forest stands) are usually free of dense undergrowth and the lichen usually occurs at or above the height of the undergrowth (in swamps and fens). Hale (1979) suggested that A. colpodes is “probably overlooked because it grows high up on tree trunks.” A few of the Anzia collections from New Brunswick and Nova Scotia are reported to be from the canopy of Red Maple (Acer rubrum) trees. Recent searches have found that A. colpodes occurs from 20 cm above the ground to 2 m up the tree trunks. Careful attention to possible high level niches failed to detect any high-up thalli.
A. colpodes appears to require moderately rough bark, at a stage of tree maturity where the canopy is still fairly open. If the trees become too large, or the canopy too dense, this lichen seems less able to thrive. Gaps in the forest, or adjacent to it, can allow mature trees with appropriate bark to grow. A. colpodes with its high spore production then has a good chance of colonizing. The most common host for A. colpodes is Red Maple (Acer rubrum), but it has also been found in Canada on Red Oak (Quercus rubra), White Ash (Fraxinus americana), Sugar Maple, and Shadbush (Amelanchier species). Single collections are known from Balsam Fir (Abies balsamea), Yellow Birch (Betula alleghaniensis), Beech (Fagus grandifolia), and Hemlock (Tsuga canadensis).
An Anzia colpodes habitat model was created for Nova Scotia using Classification and Regression Tree (CART) (Cameron, unpubl. data). Dependent variables in the model were the sites known since 1995, used as presence and absence data derived by randomly selecting 250 locations from the lichen location database maintained by Nova Scotia Department of Environment. Predictive variables included elevation, mean annual precipitation, mean July temperature, and forest attributes. CART divides data, based on a statistical test that best explains variation, into a hierarchical sequence of groups. These groups and divisions are presented graphically in the form of a tree diagram. Unfortunately the majority of the recently discovered records by Wolfgang Maass do not have accurate enough data on the exact collection site to incorporate them into the model.
Results indicate that the model was very good at predicting occurrences (87.2%) and absences (96.3%) of A. colpodes and had an area under the Receiver Operating Curve (AUC) of 0.983 (AUC is a common metric of model performance with 0.5 being no better than random and 1.0 being a perfect fit). The model suggests there are about 120,000 ha of predicted habitat in Nova Scotia, which represents about 2.2% of the province. The habitat is scattered throughout the province with the exception of the eastern Valley and the Highlands in Cape Breton. However, there is one site known in the lowlands of Inverness County, Cape Breton.
Despite a relatively high density of A. colpodes sites in the southwest of Nova Scotia there is a low density of predicted habitat. This is because of the low density of hardwood forest stands in this area relative to northern Nova Scotia. The model may indicate that despite suitable climate in southwest Nova Scotia, there are few forest stands with suitable attributes. In the north the forest stands with the right conditions occur more frequently, but the climate is not as ideal.
The model also indicates that the lack of the right combination of climate and forest stands with suitable attributes may be limiting this species. Although the majority of stands in which A. colpodes occurs are quite open (low crown closure), the model suggests under certain conditions high crown closure may be tolerated at higher elevations (> 60 m asl) in mature older forest (e.g., Cobequid Hills) and at lower elevations with lower mean July temperatures (e.g., Eastern Shore). Anzia was found to be absent when the Cobequid Hill sites and the Fundy National Park sites were revisited. This may reflect the negative impact of crown closure both in inhibiting lichen grown and encouraging mollusc grazing damage (see Threats). Overall, the model indicates that the right forest conditions with the right climate occur very rarely for A. colpodes in Nova Scotia.
The lichen database was overlaid on the habitat model to determine if any of the predicted habitat had been previously surveyed and if A. colpodes had been found. The results showed that lichens have been surveyed in 24 of the predicted Anzia habitat polygons in the past. Two Anzia occurrences were found as a result of these searches. This suggests that only about 8% of the predicted habitat actually contains Anzia and that 92% of the habitat is unoccupied. The low predicted habitat occupancy may be because it is an infrequent species or because the habitat model is predicting more area than is actually habitat. It is estimated that no more than 9600 ha or about 0.17% of the province would contain Anzia.
A climate model for Canada was also created for A. colpodes following the methods of Cameron et al. (2011). All the current and known historical occurrences (at the time the model was created), which included data from Ontario, Québec, New Brunswick and Nova Scotia, were used to produce the output (Figure 6). This indicates that suitable conditions for A. colpodes could occur in Newfoundland but it has not been found there (See Search Effort). A Mahalanobis D2 statistic was calculated using annual precipitation and mean monthly maximum temperature for 1961 to 1990 obtained from Price et al. (2004). The D2 statistic is a measure of the distance between one point and another in multivariate space; in this case precipitation and temperature. This approach can be applied using presence-only data and is robust enough for use with non-normally distributed data. This model suggested that the ideal climate has an average annual precipitation of greater than 1400 mm and a mean monthly temperature between 6 and 12°C.
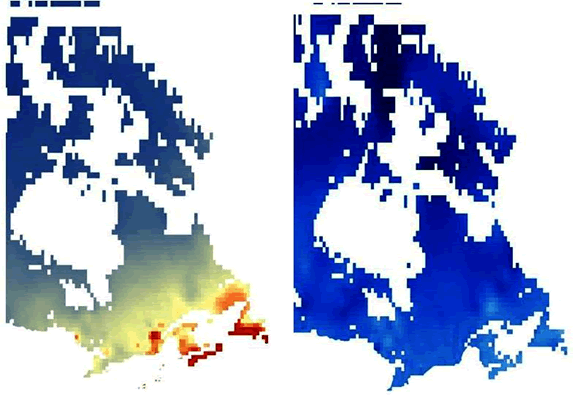
Long description for Figure 6
Two maps showing the results of climate modelling for Canada based on annual precipitation and mean maximum monthly temperature. One map is for climate data observed from 1961 to 1990, while the other is for climate data predicted to 2099. The maps indicate the relative suitability of climatic zones for Anzia colpodes. Comparison of the maps suggests that by the year 2099 there may be no optimal climate for this lichen in Canada.
A second climate model was created using future climate scenarios created by Price et al. (2004). A comparison of the current climate model with the future model (Figure 6) suggests that by the year 2099 all of Canada will have a climate that is outside the historical mean for A. colpodes, so that by 2099 there may be no optimal climate (see above) for this lichen in Canada. One reason for this is that according to Lines et al. (2009), Nova Scotia will have wetter springs and falls and warmer drier summers in the future. Price et al. (2004) suggest a slight increase in annual precipitation for many areas to the north of the existing range for A. colpodes but thiswill not be enough to reach the 1400 mm minimum that the model suggests is the optimal amount. Other areas, e.g., Ontario, where A. colpodes has occurred in the past will have a different pattern of climate change but the chance of immigrants coming from the nearest USA occurrence is very small because of the distance involved. An assumption of the Mahalanobis modelling technique is that the ideal environment can be described as a multivariate mean. This may not always be the case for all variables. Another assumption is that the original samples used to characterize the multivariate mean reflect optimal conditions. With limited climate data available to create these models, this may not be met so caution is needed when interpreting the results. They nevertheless suggest that climate change will negatively affect A. colpodes.
It is uncertain how A. colpodes populations are affected by natural stand dynamics. The upland sites are dominated by tolerant deciduous trees where there is single tree and small gap replacement disturbance dynamics (Forcier 1974). Ice storms and hurricane winds may create larger gaps in the forest (Davis and Browne 1996). If these forests are undisturbed, it is likely that old forest conditions are maintained for centuries with a fairly even microclimate. However, human activity can alter the natural stand dynamics, significantly. For example, a large area adjacent to the Economy River, Nova Scotia, occurrence was recently clear-cut and planted with coniferous trees. In lowland wet forests, the stand dynamics are less well understood. These forests appear to be made up of fairly old Red Maple with younger Balsam Fir and sometimes Black Spruce or Yellow Birch. The Balsam Fir component may mature and die, while the Red Maple is maintained, resulting in a continuous forest cover over time. Cameron (2009) found that these forests often had younger trees in the understory, shrub and herb layer. He speculated that these forests regenerate themselves if left undisturbed. Even if most or all of the Balsam Fir die in a pulse, sunlight levels do not increase significantly because the Red Maple still maintains a canopy. The additional light may provide a window of opportunity for A colpodes growth and reproduction until a new cohort of Balsam Fir grows to maturity.
Anzia colpodes reproduces via fungal ascospores ejected from the fruiting bodies which grow on the upper surface of the thallus. The spores germinate on suitable substrata, the bark of deciduous trees, and will grow towards and envelop compatible algae identified as Trebouxia simplex. A lichen thallus then develops. An unusual feature of A. colpodes is that the ascospores are tiny in comparison to those of most other lichens and therefore contain limited resources to support the growth of the germinating hypha in search of a compatible alga.
Little is known about the growth rate of A. colpodes although the lobes of foliose lichens are known to grow between 1.5 and 5.0 mm per year, with about 3 mm being a common value (Lawrey, 1984; Palmqvist et al. 2008). Once the lichen thallus reaches a few cm in diameter, pycnidia, small flask-shaped structures, develop on the ends of the lobes (see Figure 1.) Pycndiospores from the pycnidia ooze out under moist conditions and become attached to trichogynes projecting from the thallus surface. Once nuclei from the pycnidiospores have migrated into the trychogyne, development of the fruit bodies (apothecia) takes place, asci form during which meiosis occurs and spores form. The spores are ejected and the life cycle is complete (Honegger and Scherrer 2008). Very little is known about how this fertilization process is affected by prevailing environmental conditions.
The generation time for lichens varies from ten years in rapidly colonizing lichens such as Xanthoria parietina that are common in eutrophicated situations to more than 17 years for lichens such as Lobaria pulmonaria that grow in mature forests (Scheidegger and Goward 2002; Larsson and Gauslaa 2010). As A. colpodes occurs on mature tree trunks where Lobaria pulmonaria is also found, its generation time is likely around 17 years.
The dispersal distance for lichen vegetative propagules under forest conditions is only 15 to 30 m (Juriado et al. 2011). A. colpodes doesnot produce such propagules and fragmentation of thalli would likely be dispersed even less readily. Lichen ascospores are ejected from apothecia to a distance of about one centimetre and dispersal then depends on wind and season of discharge. Within mature forests with very low within forest wind speeds, dispersal distances are unlikely to be greater than propagule dispersal.
Lichens contain a spectrum of compounds with antibiotic and antiherbivory activity known to deter grazing insects or molluscs. Some lichens contain high levels of these compounds, up to 10% or more dry weight, and particular lichen compounds are known to deter grazing insects or molluscs (Asplund and Wardl 2013). As mentioned earlier, A. colpodes contains atranorin and divaricatic acid, the latter amounting to between 0.42% and 1.4% dry weight of the thallus, which is a low level compared to many other epiphytic lichens where amounts as high as 5-10% are common and 20% can occur (Muggia et al. 2009). Some specimens of A. colpodes also contain traces of sekikaic acid, derivative of divaricatic acid (Yoshimura 1987). None of these compounds are among those known to be particularly effective in antiherbivory (see Threats section).
Adaptations for water conduction and storage have been studied in other parmelioid lichens (Jahns 1988; Rundel 1988; Rikkinen 1997; Rikkinen and Poinar 2002) but there has been little research on A. colpodes. It has stout rhizoids for attachment to the substratum while the spongy hypothallus helps to retain moisture and extend the period of photosynthesis in a lichen that grows on tree trunks in fairly open forest stands. Other species of Anzia generally also favour relatively high light levels. For example, in the dense forests of Tasmania Anzia thalli are often found on canopy branches, but in more open areas there and in eastern North America they occur on tree trunks (Bratt et al.1976).
Dispersal in A. colpodes depends on the ejected ascospores being carried by the wind to the mature bark of deciduous trees, most commonly Red Maple. If the germinating ascospore encounters a suitable alga, a new thallus is formed. As mentioned earlier, the strategy of A. colpodes is to produce a large number of tiny spores with limited resource to germinate, grow and make contact with a suitable alga. In woodlands, it is likely that the spores are normally dispersed only a few hundred metres.
A. colpodes has no means of specialized vegetative reproduction (soredia or isidia), unlike many other lichens. The lobes of young thalli are firmly attached to the bark, but as thalli grow, the hypothallus lifts the lobe ends away from the bark. Portions of dislodged thallus may break away, but dispersal and attachment are likely inhibited by the sparse rhizinae and the additional weight of the hypothallus, particularly when wet. Hence, it is unlikely that pieces which fall off re-attach elsewhere on tree trunks very often or disperse any distance. No genetic data have been collected that can be used to estimate dispersal distances, but the life history attributes discussed above suggest that dispersal limited and that each site occurrence acts as an independent subpopulation at least across a single or a few generations.
Lichens are used as a source of nutrients by a wide range of invertebrates including Thysanurans, Collembolans, Psocopterans, Lepidopteran larvae, orbatid mites and gastropods (Seaward 2008), and also by lichenicolous fungi. However, no studies appear to have focused on associations between these organisms and A. colpodes. There have been observations of extensive grazing on this lichen (Figure 7) by gastropods which are well known to cause damage (Lawrey 1984). In southwestern Norway, the greater number of gastropods caused more lichen grazing and contributed to the reported extinction of Pseudocyphellaria crocata (Gauslaa 2008). In Norway, mollusc grazing of Lobaria pulmonaria was 17 times greater under a shaded canopy, where conditions were moister than in the dryer more open areas (Asplund and Gauslaa 2008). Asplund and Gauslaa (2008) also observed that young lichen thalli are most vulnerable and that for a given consumption, a small thallus is destroyed whereas larger mature thalli have a greater ability to regenerate (See Threats Section).

Long description for Figure 7
Close-up photo showing grazing damage on thalli of A. colpodes. The contents of the pycnidia at the end of the lobes appear to have been eaten.
Field surveys took place during the summer and autumn of 2013. In order to include a few of the discoveries made by Maass which came to light after completion of the work in 2013, some additional fieldwork was done in 2014. (See Table 1 and 2 and section on Search Effort (above). Twenty-eight sites were searched in Ontario and one hundred and sixty-nine sites in Québec and the Maritimes. Since 2003 ongoing inventories for Erioderma pedicellatum, the Boreal Felt Lichen, have resulted in searches of several hundred lowland Red Maple swamps by those who are able to identify A. colpodes. The species has also been searched for in nearby mixed deciduous forest habitat (Neily pers. comm. 2014). Finally, the Nova Scotia Department of Environment established 50 permanent lichen sampling air quality plots between 2004 and 2008 in upland tolerant hardwood forest but to date A. colpodes has not been found in the plots.
Currently, it appears that all the extant occurrences of A. colpodes are in Nova Scotia as revisits to the 19th century occurrences in Ontario and Québec and the ones in New Brunswick dating back a decade or more have failed to confirm the presence of A. colpodes in these provinces (Tables 1 and 2). The occurrences in Nova Scotia include 12 revisited occurrences, 35 recent discoveries and 53 pre-1983 discoveries (Table 3) which came to light after completion of the fieldwork for this report. Thus the best estimate of the maximum number of extant occurrences is 88. The population of mature individuals was enumerated at the nine occurrences. One hundred and fifty-nine colonies were found with a mean population of size of 18 colonies at each occurrence and a standard deviation of 24. Using these figures and extrapolating them to the 88 above occurrences suggests that the size of the A. colpodes population in Canada is 1,584 but could be as much as 3,696 (18+24x88). It is likely that A. colpodes is no longer present at a significant number of the pre-1983 discoveries, but further fieldwork in Nova Scotia will likely reveal additional occurrences that could compensate for these losses.
Anzia colpodes was clearly never abundant in Ontario and Québec and is no longer found in either province. The same applies to New Brunswick where there are only a few historical records and all date back more than a decade. The absence of new occurrences in Ontario, Québec and New Brunswick and the failure to re-find the lichen at previous sites that have been resurveyed suggests a real decline in eastern Canadian populations. The widespread, but not common occurrence of Anzia in Nova Scotia and its disappearance from some recent (since 1995) sites suggest a slower rate of decline in this province. If only the more recent discoveries in Nova Scotia are considered, the lichen was no longer present at three of seven post-2006 occurrences which were revisited, a decline of about 40% over the last decade. If those found since 1995 are included the decline is 50% (5/10 Table 2). However, these declines are based on examination of a small number of occurrences. Furthermore, 23 historical sites discovered since 1995 and an additional 51 discovered between 1980 and 1994 across Nova Scotia (and one in NB) have not been revisited (Table 2, 3) so the decline rates should be treated with caution. Given the observations of decline of some colonies, current threats and habitat trends, a continuing decline in the lichen population of at least 30% is inferred.
In most of the USA, the distribution is declining and it is becoming less frequent, with occurrences consisting of smaller thalli with fewer or no apothecia. It is presumed to be extirpated from Ohio and its presence in New York State requires confirmation (Harris 2004, Appendix 2). It is critically imperilled in Wisconsin and Michigan where only one specimen has been found in each state since 1997 (Bennett and Will-Wolf, pers. comm. 2014). The reasons for this change in abundance in the US states bordering Canada are uncertain but may be related to habitat disturbance and changing weather patterns (See Threats Section). Hinds and Hinds (2007) listed 22 species, including A. colpodes, that are rare and declining in New England. Of particular concern was the failure to find A. colpodes,in Acadia National Park, Maine, where it was formerly found (Cleavitt et al. 2009), possibly as a result of changing forest practices there (Root et al. 2007).
In the warmer southern regions of the USA it has shown no evidence of declines in the past two decades (Doug Ladd pers. comm. 2014). In the Ozark Plateau, A. colpodes usually occurs on the upper boles and lower branches of canopy oaks but it is never abundant.In the past it seems to have been more common, with over 100 specimens being lodged in the New York Botanical Garden Herbarium (James Lendemer, pers. comm. 2013). Older collections (mid-twentieth century and earlier) are more commonly fertile than contemporary collections, even though there has been no decline in occurrence in this area of the USA (Ladd pers. comm. 2014).
Anzia colpodes could conceivably be carried to suitable sites in Canada from Ohio, Michigan, Wisconsin, New York or Maine. However, the species is thought to be extirpated from Wisconsin and Ohio; in addition, there is only one site from Michigan and New York and five sites in Maine (Figure 2) (Hinds and Hinds 2007). The distances concerned are also large and the lack of vegetative propagules means that a compatible alga would have to be present on the bark upon which the lichen ascospores land. This makes rescue from US populations, which are reported to be declining anyway, very unlikely.
The impact of the various threats to A. colpodes was assessed using the threats calculator and rated as high to very high (Appendix 1). Logging and wood harvesting was considered the greatest threat while that posed by native and alien gastropods and by climate change was considered pervasive although their overall impact was unknown. The nature and severity of the possible threats leading to the disappearance of this lichen at particular occurrences are not well understood (see Table 2). The impact of forestry includes harvest for biomass energy production, pulp and saw-logs. These threats to A. colpodes are discussed below.
In Nova Scotia, forestry activities threaten A. colpodes by removing the host trees or those available for colonization in nearby forest stands as has been documented for epiphytic lichens elsewhere (Edman et al. 2008). Between 1990 and 2000 the annual hardwood harvest in Nova Scotia increased from 400,000 cubic metres to over 800,000 cubic metres. This resulted from 1998 to 2003 in a significant decrease in the amount of forest area with trees greater than 60 years of age (Nova Scotia Department of Natural Resources 2008). At least 50 years is likely required before regenerating trees have the mature bark that seems to be the required substratum for this species. The forest stands in Guysborough and adjacent counties are currently under particular pressure. Recently, one forestry company requested access to an additional 500,000 green metric tonnes of fibre from crown land in southwestern Nova Scotia, where A. colpodes is most frequent, but the government limited them to a previously agreed additional 125,000 green metric tonnes (Gorman and Zaccagna 2014). The shortfall will likely come from private woodlots.
In New Brunswick there has been a decline in the amount of old hardwood forests of more than 20% between the 1980s and the present, based on an analysis of forest inventory data on Crown Land and small private freehold forests which together make up more than 80% of forested lands in NB (NB DNR unpubl. data). Strip-cutting of late-successional tolerant hardwood forests has been occurring in upland areas of New Brunswick within the known range of Anzia. Second-entry selection cuts are starting to take place so negative effects are likely to increase as the amount of old tolerant hardwood declines (Clayden 2014).
Cameron et al. (2013) concluded that forest harvesting adjacent to and within the landscape negatively affected microclimate of a habitat. Microclimate changes for A. colpodes are likely to impede the ability of the tiny spores to germinate or the ability of the required algae to grow on available tree trunks with suitable bark.
The use of biomass to supply energy generation in Nova Scotia has resulted in the removal of trees for which there has previously been little commercial interest. These include Red Maple, White Ash and Amelanchier ssp. growing both in upland hardwood slopes which are more accessible and in mixed deciduous/coniferous red maple swamps. Elimination of these host tree species will restrict the available habitat and thus the long-term establishment and survival of A. colpodes. The increased need for biomass arises from the commissioning of the Port Hawkesbury 60 megawatt electricity co-generating plant. This requires up to 500,000 tonnes of biomass fuel annually (Simpson and Plourde 2011, Bundale 2012a, King 2012, Erskine 2013). Harvesting of both Crown and private land will be required to provide the fuel.
In Nova Scotia, homeowners are also increasingly turning to wood pellets for home heating. Wood pellet production uses primarily hardwoods, again placing demand on previously unharvested tree species, often at an earlier growth stage. As the cost of oil and natural gas increases, the demand for wood pellets and firewood grows. Such harvest is associated with rapid changes to the landscape which is further thought to severely limit lichen presence owing to their requirement for mature bark for colonization (T. Neily, pers. comm. 2014).
The establishment of wind farms requires extensive road development, potentially removing or damaging hardwood stands in upland sites. Hydraulic fracturing (currently under a moratorium in both New Brunswick and Nova Scotia) also requires road infrastructure and can affect groundwater (see below).
In New Brunswick, herbicides are used to limit hardwood regeneration in softwood stands and in Nova Scotia in Christmas tree growing areas. The impact of this on adjacent areas of forest and on A. colpodes is unknown but negative effects on occurrence and biomass have been observed elsewhere (McMullin et al. 2011, 2013). Herbicides limit hardwood regeneration and may be a threat in the long term as they prevent the development of mature deciduous trees, the host for A. colpodes.
Gastropod grazing can play an important part in shaping the epiphytic vegetation of deciduous forests. Juvenile thalli seem to be at particular risk (Asplund and Gauslaa 2008). Extreme slug damage to epiphytic lichens has also been reported in some coastal areas of southern New Brunswick over the past few summers, at Fundy National Park and at in the Caledonia Gorge PNA (Clayden, pers. comm. 2013). Nova Scotia and New Brunswick have experienced unusually wet summers, especially in June and July over the past few years. This may have resulted in higher than average slug numbers that may represent a threat to lichens. Slugs are active in the morning and evening when it is moist (Annegret Nicolai, pers. comm.2014). With respect to A. colpodes in Nova Scotia, the occurrences in the southwest of the province where moist forest conditions pertain are most at risk of slug damage, especially in swampy areas, less so in wetland margins and least in upland areas like drumlins. At Economy Falls, Nova Scotia, extensive searches in 2013, both below and above the falls where A. colpodes was found in 1992 failed to find the lichen. Despite the tree bark being dry near the falls, there was frequent slug-browsing on the epiphytic lichens. A. colpodes seems to be a species that is susceptible to grazing (Figure 7). This may be due to the low levels of anti-herbivory lichen compounds in its thallus which would not deter snails (Asplund and Wardl 2013; Asplund pers. comm. 2014) (see Physiology and Adaptability and Interspecific Interactions).
In addition to increased abundances of native grazing slugs, the Maritimes now has two introduced slug species, Arion subfuscus and Deroceras reticulatum. These are larger and more aggressive than the native species. Arion subfuscus was frequently observed on lichens in 2007 (Asplund pers. comm. 2014). These introduced slugs have been observed feeding on rare lichens including the Boreal Felt Lichen in Nova Scotia (Cameron, 2009; R. Forsyth pers. comm. 2014).
Cameron (2009) found, in Nova Scotia, that 80% of the arboreal gastropods he surveyed were introduced species, the majority from the genus Arion. The dominance and abundance of A. fuscus/subfuscus suggests that it is a relatively new threat to lichens and could be filling a previously unoccupied niche. Thus it may be a new threat to lichens or be outcompeting the native slug species. Either way it is likely a problem for lichens in the Maritimes (Cameron pers. comm. 2014).
The occurrences in Ontario and Québec where A. colpodes was found in the 19th century have undergone great change. Suburban spread, building sites and highways have removed the forest where this lichen was once found. The occurrences in Québec also seemed to have suffered much disturbance as the forest is disturbed by many trails and human activity (see also Search Effort).
The New Brunswick and Nova Scotia occurrences where A. colpodes has been found in the past have not suffered the same because of the smaller, and lower density, of the human population and the slow urban spread has not encroached much on Anzia habitat.
The search for new energy sources including biomass, natural gas through hydrofracturing, and wind farms all require access roads through woodlands. Although these activities often use existing forest roads with short spur roads, they can change the hydrology of watersheds. This is important because A. colpodes grows on Red Maples and other deciduous trees growing in swamps, swamp margins and upland areas kept moist by nearby water bodies. In addition, hydrofracturing activities have the potential to alter rock stability and groundwater patterns (Entrekin et al. 2011; Bundale 2012b). Currently hydrofracturing is on hold in Nova Scotia and New Brunswick. Finally, a pipeline is planned to bring crude oil to New Brunswick from Alberta and this will require a new pipeline corridor from the Québec border to Saint John and forest clearance along the pathway (Anon. 2014a).
Air pollution which can acidify the bark or adversely affect lichen growth may have contributed to the disappearance of A. colpodes from Ontario and Québec or from occurrences near Saint John, New Brunswick. The pollutants include transboundary acid rain and local emissions of sulphur dioxide. Trees such as Red Maple and various ash have bark with a high pH but the above pollutants can acidify bark and overcome its buffering capacity so that it is no longer suitable for colonization (Nieboer et al. 1984). A. colpodes, which has a green algal symbiont, is less sensitive to the direct effects of these pollutants than cyanolichen species like Degelia plumbea and Erioderma pedicellatum. A. colpodes appears to need a delicate balance between substratum age and suitability linked with appropriate microclimate conditions involving a combination of sufficient moisture and appropriate temperature. These factors are required for algal strains to thrive on the tree bark and be colonized by the germinating tiny spores of A. colpodes.
As indicated earlier (see Habitat Trends, above) there are clear predictions for significant climate change in Nova Scotia (Figure 6) in the areas colonized by A. colpodes. An increased number of extreme weather events, storms and flooding, are also anticipated. The impact of these is uncertain as the resultant blowdown of trees could, if not too widespread, enhance light levels and make conditions more suitable for the lichen. On the other hand, increases in precipitation may enhance the spread and impact of grazing molluscs while higher summer temperatures may negatively affect the ability of this lichen to reproduce. A. colpodes is more susceptible than many other lichens to changes in weather patterns because it has no means of vegetative reproduction and its tiny spores have little resources to grow and find a suitable algal partner (see Life Cycle and Reproduction). In New Brunswick, both warmer temperatures and increased precipitation are also expected together with more extreme storm and rainfall events (Anon. 2014b).
It is not possible to predict which occurrences will be affected by the various threats at a given time, and the key ones leading to the disappearance of this lichen are not well understood. Thus, the number of locations is best assessed as the total number of known occurrences. Currently, it appears that all the extant occurrences are in Nova Scotia as revisits to the 19th century occurrences in Ontario and Québec and the ones in New Brunswick dating back a decade or more have failed to confirm the presence of A. colpodes in these provinces (Tables 1 and 2).
The occurrences in Nova Scotia include 12 revisited sites, 35 recent discoveries and 53 pre-1983 discoveries (Table 3) which came to light after completion of the fieldwork for this report. Thus the best estimate of the maximum number of extant occurrences is 88, hence <100, and as It is not possible to predict which occurrences will be affected by the various threats at a given time, each occurrence is considered a separate location.
A. colpodes is uncommon but widely scattered. There is no genetic data to estimate dispersal distances, and the life history attributes (see Biology and Dispersal above), suggest that dispersal is limited. Hence it is concluded that each occurrence acts as an independent subpopulation at least across a single or a few generations
The NatureServe Global Status isG3 (vulnerable) / G5 (secure), the status was last reviewed on05 Dec 2000 and last changedon21 Jan 2001. The Rounded Global Status is G4 (Apparently secure).
In Canada, A. colpodes is ranked by NatureServe as NNR (unranked), while in Ontario it is SH (possibly extirpated). In Québec A. colpodes is SNR (not yet assessed)
(CESCC 2011; NatureServe 2014).
Anzia colpodes has a national status of NNR (unranked), and in Michigan and North Carolina and Pennsylvania it is SNR (not yet assessed), but in Wisconsin it is SX (presumed extirpated) as it is in Ohio (Showman and Flenniken 2002). However, there is a recent record (2002) from Wisconsin so the status may be changed to S1 (critically imperilled) (Jim Bennett pers. comm 2014). There are only a few records of A. colpodes from Georgia and it is likely that the species will be evaluated as S1 (critically imperilled) or S2 (imperilled) in 2015/2016 after consideration by Georgia's Department of Natural Resources (Malcolm Hodges pers. comm.).
Currently A. colpodes has no legal protection or status in Canada.
Some occurrences in Nova Scotia are protected as they occur in provincially protected Wilderness Areas or National or Provincial Parks.
Assistance with documenting the occurrence and distribution of A. colpodes in Canada and the USA has been provided by a number of colleagues. The writers thank the following people for their generous help and time.
We thank Claude Roy, botanist at l’Herbier Louis-Marie, Université Laval.
We thank Wayne Clifford, Steven Selva, Martin Turgeon, and Reginald Webster for joining Stephen Clayden on some field excursions, and Gerry Redmond for providing access to his woodlot.
We thank Rosemary Curley for facilitating lichen surveys, especially in the Townshend Woodlot.
We especially thank Tom Neily and Chris Pepper for making available their records of A. colpodes from extensive surveys of lichens in Nova Scotia and for accompanying Frances Anderson on some of the field excursions.
Assistance with documenting the occurrence and distribution of Anzia colpodes has been provided by members of the Tuckerman Lichen Workshop and a large number of colleagues including:
Canada: Irwin Brodo, Jennifer Doubt
Finland: Teuvo Ahti;
Norway: Johan Asplund and Yngvar Gauslaa
New Zealand: David Galloway
Sweden: Leif Tibell
Switzerland: Christoph Scheidegger
USA: Jim Bennett, Bill Buck, Bob Dirig, Scott LaGreca, Alan Friday, Dick Harris, Alan Fryday, Malcom Hodges, Doug Ladd, Elizabeth Lay, Jim Hinds, Malcolm Hodges, Doug. Ladd, James Lawrey, Les Landrum, James Lendemer, Tom Nash, Michaela Schmull, Larry St. Clair
UK: Mark Seaward, Rebecca Yahr, Heidi Doring
Ahmadjian, V 1993. The Lichen Symbiosis. John Wiley and Sons, New York, p. 33.
Anon. 2008a. The Public Forest: State of the Forest Report [PDF], New Brunswick.
Anon. 2008b. A strategy for crown lands forest management [PDF; 508 KB].
Anon. 2014a. Energy East pipeline Project.
Anon. 2014b. New Brunswick climate change action plan, 2014-2020 [PDF; 382 KB].
Asplund, J. and Wardle, D.A. 2013 The impact of secondary compounds and functional characteristics on lichen palatability and decomposition. Journal of Ecology 101:689-700.
Asplund, J. and Gauslaa, Y. 2008 Mollusc grazing limits growth and early development of the old forest lichen Lobaria pulmonaria in broadleaved deciduous forests. Oecologia, 155, 93–99.
Beck, A. 2002. Selektivat der Symbiotenschwer-metalltoeranterFlechen.Dissertation, Munich.
Bratt, G.C., Blackman, A.J., and Cashin, J.A. 1976.The genus Anzia in Tasmania. Lichenologist 8;69-77.
Brodo, I.M., Sharnoff, S,D., and Sharnoff, S. 2001. Lichens of North America. Yale University Press, New Haven, 795pp.
Budel, B. and Scheidegger, C. 2008. Thallus morphology and Anatomy. In Lichen Biology (T.H.Nash, ed.) Cambridge University Press, Cambridge, p. 62.
Bundale, B. 2012a. Mill gets millions in N.S. cash. August 20.
Bundale, B. 2012b. Energy Company: Exploration didn’t cause N.B. quake. Chronicle Herald, March 13: C1.
Cameron, R.P. 2009. Red maple, Acer rubrum, wetland composition and structure in Nova Scotia. Canadian Field Naturalist 123: 221-229.
Cameron, R.P. 2009. Are non-native gastropods a threat to endangered lichens? Canadian Field Naturalist 123: 169-171.
Cameron, R.P., Neily, T. and Clayden, S.R. 2011. Distribution model for Erioderma mollissimum in Atlantic Canada. The Bryologist 114: 231-238.
Cameron, R.P., Neily, T., and Clapp, H. 2013. Forest harvesting impacts on mortality of an endangered lichen at the landscape and stand scales. Canadian Journal of Forest Research 43: 507-511.
Clayden, S. R. 2010. Lichens and allied fungi of the Atlantic Maritime Ecozone. Pp. 153-178. In D. F. McAlpine and I. M. Smith (eds.), Assessment of Species Diversity in the Atlantic Maritime Ecozone. NRC Research Press, National Research Council Canada, Ottawa.
Clayden, S.R. 2014. Old tolerant hardwood forests in New Brunswick going down fast. New Brunswick Naturalist pp. 60-66.
Cleavitt, N.L., Dibble, A.C. and Werier, D.A. 2009. Influence of tree composition upon epiphytic macrolichens and bryophytes in old forests of Acadia National Park, Maine. Bryologist 112.467-487.
Culberson, W.L. 1961 A second Anzia in North America. Brittonia13:381-384.
Crespo. A., Lumbsch, H.T., Mattsson, J., Blanco, O., Divakar, P.K., Articus, K., Wiklund,
E., Bawingan, P.A. and Wedin, M. 2007. Testing morphology-based hypotheses of phyologenetic relationships in Parmeliaceae (Ascomycota) using three ribosomal markers and the nuclear RPB1 gene. Molecular Phylogenetics and Evolution, 44:812-824.
Culberson, W.L. Disjunctive distributions in the lichen-forming fungi. 1972. Annals of the Missouri Botanical Garden 59:165-173.
Culberson, W.L. 1961. A second Anzia in North America. Brittonia13:381-384.
Davis, D. and S. Browne. 1996. Natural History of Nova Scotia 2 volumes. Nimbus Publishing. Halifax. 502 pp.
Edman, M., Eriksson, A. and Villard, M. 2008. Effects of selection cutting on the abundance and fertility of indicator lichens Lobaria pulmonaria and Lobaria quercizans. Journal of Applied Ecology 45:26-33.
Entrekin, S., Evans-White, M., Johnson, B. and Hagenbuch, E. 2011. Rapid expansion of natural gas development poses a threat to surface waters. Frontiers in Ecology and the Environment 9:503–511.
Erskine, B. 2013. NSP biomass site aims for 4%of power needs of N.S: plant in operation after test. Chronicle Herald, Halifax, B2, July 3rd.
Esslinger, T.L. and Egan, R.S. A sixth checklist of the lichen-forming, lichenicolous and allied fungi of the Continental United States and Canada. Bryologist 98:467-549.
Forcier, L.K. 1975. Re-productive strategies and the co-occurrence of climax tree species. Science 189 : 808-810.
Fournier, R. 2006. Liste des lichens de l'est du Canada [PDF; 152 KB]. Université de Moncton, Campus d'Edmundston. 38 p. (Téléchargé en avril 2010).
Gauslaa, Y. (2008) Mollusc grazing may constrain the ecological niche of the old forest lichen Pseudocyphellaria crocata. Plant Biology 10: 711–717.
GBIF (Global Biogeographic Information Facility) 2014. Anzia Colpodes.
Gorman, M. and Zaccagna, R. 2014. No Bail-out for Northern Pulp. Chronicle Herald January 30.
Goward, T., Brodo, I.M. and Clayden, S.R. 1998. Rare lichens ofCanada: a review and provisional listing. Committee on the Status of Endangered Wildlife in Canada, Ottawa. 74 pp.
Hale, M.E. 1955. Studies on the chemistry and distribution of North American lichens (1-5) The Bryologist 58:242-246.
Hale, M. E. 1979. How to Know the Lichens. Second Edition.
Harris, R.C. 2004 A Preliminary List of the Lichens of New York. Opuscula Philolichenum 1: 55-74, see p. 71.
Hensen, A. and Dobelmann, A. 1987.Development of the spongiostratum in Anzia and Pannoparmelia.In Progress and Problems in Lichenology in the Eighties. Bibliotheca Lichenologica 25:103-108.
Hinds, J.W.and Hinds, P.L. 2007.The Macrolichens of New England. New York Botanical Garden Press, New York, pp 51 and 123.
Honegger, R and Schearrer, S. 2008 Sexual Reproduction in lichen-forming Ascomycetes. In Lichen Bology, T.H. Nash, ed. Cambridge University Press, Pp. 94-103.
Jahns, H.M. 1988.The lichen thallus. In CRC Handbook of Lichenology. ( M. Galun ed.) CRC Press, Boca Raton, Vol 1, p. 127.
Jayalal, U., Wolseley, P., Gueidan, C., Aptroot, A., Wijesundara, S. and Karunaratne, V. 2012. Anzia mahaeliyensis and Anzia flavotenuis, two new lichen species from Sri Lanka. Lichenologist 44:381-389.
Juriado, I., Liira, J., Csencsics, D., Widmer, I., Adolph, C., Kohu, K. and Scheidegger, C, 2011. Dispersal ecology of the endangered woodland lichen bggg in a managed hemiboreal forest landscape. Biodiversity and Conservation 20:1803-1819.
King, N. 2012. Nova Scotia Power wants to talk to potential biomass suppliers. Cape Breton Post Business Section, 20th September.
Larsson, P. and Gauslaa, Y. 2010. Rapid juvenile development in old forest lichens. Botany 89:65-72. Error! Hyperlink reference not valid.
Lawrey, J.D. 1984. Biology of Lichenized Fungi, Praeger Scientific, New York, pp 5-8; 236-244.
LeBlanc, F. 1960. Écologie et phytosociologie des épiphytes corticoles du sud du Québec. PhD thesis. Université de Montréal.
LeBlanc, F. 1963. Quelques sociétés ou unions d’épiphytes du sud du Québec. Canadian Journal of Botany41: 591-638.
Lines, G.S., Pancura, M., lander, C. and Titus, L. 2009. Climate change scenarios for Atlantic Canada ustilizing a statistical downscaling model based on two global climate models. Meterological Service of Canada, Atlantic Region, Science Report Series 2009-01, Dartmouth 17 pp.
Maass, W.S.G. Hoisington, B.L. and Harries, H. 1986. Pannaria lurida in Atlantic Canada. Proceedings of the Nova Scotian Institute of Science 36:131-135.
Macoun, J. 1902. Catalogue of Canadian Plants, Part VII- Lichenes and Hepaticae, Geological Survey of Canada, Government Printing Bureau, Ottawa, p.71.
McMullin, R.T. 2013.An Interim Survey of Pysconia subpallida in Canada A report prepared for the Canadian Wildlife Federation, 36 pp.
McMullin, R.T.; Thompson, I.D.; Newmaster, S.G. 2013. Lichen conservation in heavily managed boreal forests. Conservation Biology 27:1020-1030.
McMullin, R.T., Bell, W.F., and Newmaster, S.G. 2011. The effects of triclopyr and glyphosate on lichens. Forest Ecology and Management 264: 90-97.
McMullin, R.T., Duinker, P.N., Cameron,R.P. Richardson, D.H.S. and Brodo, I.M. 2008. Lichens of coniferous old-growth forests of southwestern Nova Scotia, Canada; diversity and present status. The Bryologist 111: 620-637.
McMullin, R.T. 2009. Lichens of Kejimkujik National Park and National Historic Site, Nova Scotia, Canada (Provisional List).OpusculaP hilolichenum 7: 71-78.
Muggia, L., Schmitt, I. and Grube, M. 2009. Lichens as treasure chests of natural products. SIM News, May/June, pp. 85-97.
Nieboer, E., McFarlane, J.D. and Richardson, D.H.S. 1984. Modification of plant cell buffering capacities by gaseous air pollutants. pp. 313-333, In Koziol, M., and F.R. Whatley (eds.), Gaseous Air Pollutants and Plant Metabolites, Butterworths, London.
Nova Scotia Department of Natural Resources. 2008. State of the Forest Report 1995-2005. Report FOR 2008-3. Nova Scotia Department of Natural Resources, Halifax, p. 40.
Opanowicz, M. andGrube, M. 2004.Photobiont variation in Flavocetraria nivalis from Poland (Parmeliaceae, Lichenized Ascomycota).Lichenologist 36:125-131.
Palmqvist, K., Dahlman, L., Jonsson, A and Nash, T.H. 2008. The carbon economy of lichens. In Lichen Biology, (T.H. Nash Ed.). Cambridge University Press, pp 182-215.
Park, Y.S. 1990. The macrolichen flora of South Korea. Bryologist 93:105-160.
Phillips, H.C. 1970. An annotated list of foliose and fruticosse lichens in land between the lakes. Journal of the Tennessee Academy of Science 45:97-109.
Poelt J. 1963. Flechtenflora und Eiszeit in Europa.PhytonAnnalesReiBotanicae 10:206-215.
Price D.T., McKenney, D.W.,. Papadopol, P., Logan, T., and Hutchinson., M.F. 2004. High Resolution Future Scenario Climate Data for North America.26th Conference on Agricultural and Forest Meteorology. Sunday, 22 August 2004.
Rikkinen, J. 1997. Habitat shifts and morphological variation
Rikkinen, J and Poinar, G.O. 2002.FossilisedAnzia (Lecanorales, lichen-forming Ascomycota) from European Tertiary amber. Mycological Research 106:984-990.
Root, H.T., McGee, G.G. and Nyland R.D. 2007. Effects of two silvicultural regimes with large tree retention on epiphytic macrolichen communities in Adirondack northern hardwoods, New York, USA. Canadian Journal of Forest Research 37:1854-1866.
Rikkinen, J. (1997) Habitat shifts and morphological variation of Pseudevernia furfuracea along a topographic gradient, Symbolae Botanicae Upsalienses 32:223-245
Rikkinen, J and Poinar, G.O. 2002. FossilisedAnzia (Lecanorales, lichen-forming Ascomycota) from European Tertiary amber. Mycological Research 106:984-990.
Rundel, P.W. 1988. Water Relations.In CRC Handbook of Lichenology,( M. galun ed.) CRC Press, Boca Raton,Vol 2:17-36.
Scheidegger, C. and Goward, T. (2002) Monitoring lichens for conservation: red lists and conservation action plans. In Monitoring with lichens – Monitoring lichens (P.L. Nimis, C. Scheidegger and P.A. Walseley eds.) Kluwer Academic, Dordrecht pp. 163-181
Seaward, M.R.D. 2008. Environmental role of lichens. In Lichen Biology, 2nd edition. (T.H. Nash, ed), Cambridge University Press, Cambridge, pp. 274-298.
Showman, R.E. and Flenniken, D.G. 2004. The Macrolichens of Ohio. Ohio Biological Survey, Columbus, p.29.
Simpson, J and Plourde, R. 2011. Nova Scotia achieves the worst possible scenario for biomass energy. Ecology Action Centre, Jan. 26. Halifax.
Thell, A., Feurer, T., Karnfelt, I., Myllys, L. and Stenroos, S. 2004. Monophyletic groups within the Parmeliaceae identified by ITS rDNA, B-tubulin and GAPDH sequences. Mycological Progress 34:297-314.
Yoshimura, I and Kurokawa T. 1977. Sensitivities of thin-layer chromatographic tests for some lichen substances. Bulletin Kochi Gakuen Junior College 8:27-31.
Yoshimura, I. 1987. Taxonomy and speciation of Anzia and Pannoparmelia.In Progress and Problems in Lichenology in the Eighties. Bibliotheca lichenologica 25:185-195.
Yoshimura, I., Singh, K.P. and Elix, J.A. 1997. The genus Anzia (lichenizedAscomycetes) in India. Journal of the Hattoria Botanical Laboratory 82:342-352.
David Richardson is Dean Emeritus at Saint Mary’s University. He has studied lichens since 1963 and as sole author written two books on lichens: The Vanishing Lichens and Pollution Monitoring with Lichens. He has also completed over twenty book chapters and 100 research papers on various aspects of lichenology. He has studied lichens in Australia, Canada, Ireland and the United Kingdom.
Frances Anderson is a Research Associate at the Nova Scotia Museum of Natural History, Halifax. She has been carrying out fieldwork on lichens in Nova Scotia for more than five years and has extensive experience in doing field inventories. She is currently working on a macrolichen checklist for the Province.
Robert Cameron has been studying lichens for over ten years beginning with a Master’s degree in Biology at Acadia University studying the effects of forestry practices on lichens. More recently, Mr. Cameron has been studying the effects of air pollution on lichens, coastal forest cyanolichens and more specifically boreal felt lichen. He is currently the ecologist with Protected Areas Branch of Nova Scotia Environment and Labour, responsible for the protected areas research program.
Stephen Clayden is Curator of Botany at the New Brunswick Museum; He is a life-long Naturalist and became interested in lichens during his undergraduate studies at Mount Allison University. He investigated the dynamics of lichen rich communities in the Abitibi region of Québec for his M.Sc. and earned his Ph.D. at Kings College, London, U.K, for studies of the life histories of Rhizocarpon species. He has collected and researched lichens widely in Atlantic Canada and Québec and co-authored The Rare Lichens of Canada: A Review and Provisional Listing with Trevor Goward and Irwin Brodo.
Troy McMullin is a postdoctoral fellow at the University of Guelph. He began studying lichens in 2004 during his Master’s at Dalhousie University where he examined lichens in old-growth Acadian forests. During his PhD and currently he examined the conservation and management of lichens in Ontario’s boreal forest. As a consultant, Troy regularly works with a variety of organizations doing lichen inventories and ecological monitoring with lichens. His lichen studies have ranged from California and Haida Gwaii to the Maritimes and the Everglades. Troy is also the lichen collections manager at Biodiversity Institute of Ontario Herbarium.
The following herbaria/websites were consulted with respect to records of A. colpodes:
Canadian Museum of Nature, Ottawa
The Consortium of North American Lichen Herbaria CNALH
Herbier Louis-Marie, Université Laval, Québec City
The Nova Scotia Museum, Halifax
The New Brunswick Museum, Saint John
In addition, the American and international lichenologists listed above in the Acknowledgements, and members of the Tuckerman Workshop were contacted for details of A. colpodes collections lodged in their private or university herbaria
Species or Ecosystem Scientific Name: Anzia colpodes, Black-foam Lichen
Element ID:
Elcode:
Date (Ctrl + ";" for today's date): 04/06/2014
Assessor(s): David Richardson, Dwayne Lepitzki, Dave Fraser, Mary Sabine, Vivian Brownell, Sherman Boates, Jean Gagnon, Marie-France Noel, Stephen Clayden, Frances Anderson, Robert Cameron, Julie Perrault
References:
| Threat Impact | Level 1 Threat Impact Counts high range |
Level 1 Threat Impact Counts low range |
|---|---|---|
| A - Very High | 0 | 0 |
| B - High | 2 | 0 |
| C - Medium | 0 | 1 |
| D - Low | 2 | 3 |
Calculated Overall Threat Impact:
- Level 1 Threat Impact Counts - high range: Very High
- Level 1 Threat Impact Counts - low range: High
Assigned Overall Threat Impact: AB = Very High - High
Impact Adjustment Reasons: Of 22 revisited sites, the lichen was no longer extant at 17, a decline of c. 70% in N.S and it's no longer present in Ont., Quebec or N.B.
Overall Threat Comments: This widespread but not common lichen species which appears to be disappearing in much of its range in Canada and the adjacent USA. The combination of threats which led to this are difficult to define.
| [No.] | Threat | Impact (calculated) | Impact (calculated) | Scope (next 10 Yrs) | Severity (10 Yrs or 3 Gen.) | Timing | Comments |
|---|---|---|---|---|---|---|---|
| 1 | Residential and commercial development | - | Negligible | Negligible (<1%) | Extreme (71-100%) | High (Continuing) | - |
| 1.1 | Housing and urban areas | - | Negligible | Negligible (<1%) | Extreme (71-100%) | High (Continuing) | Habitat destruction as a result of housing development seems to have had a serious impact on populations of this lichen in the past in Ontario and Quebec. |
| 1.2 | Commercial and industrial areas | - | - | - | - | - | N/A |
| 1.3 | Tourism and recreation areas | - | Negligible | Negligible (<1%) | Moderate (11-30%) | High (Continuing) | The development of trails in Nova Scotia has and is having an impact which is small in area but still ongoing. |
| 2 | Agriculture and aquaculture | - | - | - | - | - | Not Applicable |
| 2.1 | Annual and perennial non-timber crops | - | - | - | - | - | - |
| 2.2 | Wood and pulp plantations | - | - | - | - | - | - |
| 2.3 | Livestock farming and ranching | - | - | - | - | - | - |
| 2.4 | Marine and freshwater aquaculture | - | - | - | - | - | - |
| 3 | Energy production and mining | - | Negligible | Negligible (<1%) | Extreme (71-100%) | Moderate (Possibly in the short term, < 10 yrs) | - |
| 3.1 | Oil and gas drilling | - | Unknown | Small (1-10%) | Unknown | Moderate (Possibly in the short term, < 10 yrs) | Fracking can affect stability and groundwater patterns leading to drainage of Red maple swamps where the Black-foam Lichen is common, while the associated necessary roads can also lead to changes in hydrology that cause drying of the habitat that harm the lichen. Ground water pumping is also a factor in this context and is part of the assessment here rather than in water management 7.2. |
| 3.2 | Mining and quarrying | - | Negligible | Negligible (<1%) | Extreme (71-100%) | Moderate (Possibly in the short term, < 10 yrs) | This threat considers the footprint of gold mines in Nova Scotia which have recently become operational. and could affect some populations of the Black-foam Lichen |
| 3.3 | Renewable energy | - | - | - | - | - | Wood harvesting biomass energy production. Assessment of the impact is included in Wood Harvesting while the associated roads are considered under Road threats. Also considered under road threats are roads association with the expansion of wind farms. |
| 4 | Transportation and service corridors | D | Low | Small (1-10%) | Moderate (11-30%) | High (Continuing) | - |
| 4.1 | Roads and railroads | D | Low | Small (1-10%) | Moderate (11-30%) | High (Continuing) | This includes the construction of roads for mining, forestry, biomass harvesting, wind farm construction and access etc. Edge effects are important and are included. |
| 4.2 | Utility and service lines | - | Negligible | Negligible (<1%) | Moderate (11-30%) | Moderate (Possibly in the short term, < 10 yrs) | The proposed new pipeline in New Brunswick could have an impact on habitats that might be colonized by the Black-foam Lichen, both via direct impact along the corridor for the pipeline during laying of the pipeline and via edge effects in adjacent forest areas. |
| 4.3 | Shipping lanes | - | - | - | - | - | - |
| 4.4 | Flight paths | - | - | - | - | - | - |
| 5 | Biological resource use | BC | High - Medium | Large - Restricted (11-70%) | Serious (31-70%) | High (Continuing) | - |
| 5.1 | Hunting and collecting terrestrial animals | - | - | - | - | - | - |
| 5.2 | Gathering terrestrial plants | - | - | - | - | - | - |
| 5.3 | Logging and wood harvesting | BC | High - Medium | Large - Restricted (11-70%) | Serious (31-70%) | High (Continuing) | In upland sites, in particular, harvesting of hardwood trees for saw-logs and wood pellet production and firewood can result in loss of host trees and trees colonized by the Black-foam Lichen. Another major threat is wood harvesting biomass energy production, amounting to >500.000 tons a year. The proportion of this activity that involves hardwood trees, not only removes the host for the Black-foam Lichen but the associated Roads can alter drainage patterns in adjacent woodlands and lead to drying of the habitat with harmful effects on this lichen. Assessment of the impact of this latter factor is included under Roads. Although there are regulations in place, riparian areas are not being sufficiently during harvesting especially on private woodlots where they are difficult to enforce. |
| 5.4 | Fishing and harvesting aquatic resources | - | - | - | - | - | - |
| 6 | Human intrusions and disturbance | - | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | - |
| 6.1 | Recreational activities | - | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | - |
| 6.2 | War, civil unrest and military exercises | - | - | - | - | - | - |
| 6.3 | Work and other activities | - | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | Collecting for research on lichens species and communities |
| 7 | Natural system modifications | - | - | - | - | - | - |
| 7.1 | Fire and fire suppression | - | - | - | - | - | - |
| 7.2 | Dams and water management/use | - | - | - | - | - | Groundwater pumping is included under oil development. |
| 7.3 | Other ecosystem modifications | - | - | - | - | - | - |
| 8 | Invasive and other problematic species and genes | - | Unknown | Pervasive (71-100%) | Unknown | High (Continuing) | - |
| 8.1 | Invasive non-native/alien species | - | Unknown | Pervasive (71-100%) | Unknown | High (Continuing) | Two invasive alien slugs have become widespread and abundant in New Brunswick and Nova Scotia and can cause grazing damage to ephiphytic lichens in the red maple swamps which are a common habitat for the Black-foam Lichen. This assessment applies to the percentage of the population affected by non-native slugs. The impact of grazing is available for some lichens but not yet for the Black-foam Lichen and is discussed in the report. |
| 8.2 | Problematic native species | - | - | - | - | - | There is one common native slug species and several other lichen feeding gastropods found on lichens in Nova Scotia that also damage the Black-foam Lichen. However there is no evidence to date which shows that populations of the Black-foam Lichen are negatively affected. |
| 8.3 | Introduced genetic material | - | - | - | - | - | - |
| 9 | Pollution | D | Low | Restricted (11-30%) | Slight (1-10%) | High (Continuing) | - |
| 9.1 | Household sewage and urban waste water | - | - | - | - | - | - |
| 9.2 | Industrial and military effluents | - | Negligible | Negligible (<1%) | Slight (1-10%) | High (Continuing) | Tailings ponds from mining activity |
| 9.3 | Agricultural and forestry effluents | - | - | - | - | - | - |
| 9.4 | Garbage and solid waste | - | - | - | - | - | - |
| 9.5 | Air-borne pollutants | D | Low | Restricted (11-30%) | Slight (1-10%) | High (Continuing) | Air pollution may be one of the factors that contributed to the disappearance of the Black-foam Lichen from Ontario, Quebec and may also have had an effect in New Brunswick and may be in Nova Scotia. The pollutants include acid rain as well as those from forestry and mining activities. The lack of specific impact data on this lichen or quantification of acidification of hardwood tree bark on which the Black-foam lichen grows is lacking for Nova Scotia or New Brunswick. There are very pollution sensitive cyanolichen species to be found in many sites where the Black-foam Lichen is found, so the impact on this species is low. |
| 9.6 | Excess energy | - | - | - | - | - | - |
| 10 | Geological events | - | - | - | - | - | - |
| 10.1 | Volcanoes | - | - | - | - | - | - |
| 10.2 | Earthquakes/tsunamis | - | - | - | - | - | - |
| 10.3 | Avalanches/landslides | - | - | - | - | - | - |
| 11 | Climate change and severe weather | BD | High - Low | Pervasive - Large (31-100%) | Serious - Slight (1-70%) | Moderate (Possibly in the short term, < 10 yrs) | Predicted dryer summers could have an impact on the ability of the Black-foam Lichen to complete its life cycle while increased spring or autumn rainfall may increase grazing by the aggressive species of introduced molluscs. |
| 11.1 | Habitat shifting and alteration | - | Unknown | Pervasive - Large (31-100%) | Unknown | Moderate (Possibly in the short term, < 10 yrs) | Global warming leading to milder winters may increase the survival and activity of introduced molluscs that graze on the Black-foam Lichen. No evidence to date to show whether NO the population of slugs will increase with increasing temperatures. The threat is scored for the next 10 years but models have shown that could be a 100% change of climate in 50 years and a much greater impact in 30 years, although there is uncertainty about the timing and severity of the threat. In addition changes in humidity could have a positive effect on the survival of the Black-foam Lichen. |
| 11.2 | Droughts | - | - | - | - | - | Droughts may not be an impact in Nova Scotia as models predict an increase in precipitation, although fog levels have decreased. |
| 11.3 | Temperature extremes | - | - | - | - | - | - |
| 11.4 | Storms and flooding | BD | High - Low | Pervasive - Large (31-100%) | Serious - Slight (1-70%) | Moderate (Possibly in the short term, < 10 yrs) | There is uncertainty about the timing and severity of this threat. In addition increases in precipitation and humidity could have a positive effect on this lichen. The impact is unknown in the short term but predicted to be more serious in the long term. The impact is scored for the next ten years. However models have shown that there will be 100% increase in 50 years and a great impact is expected in 30 years. |