COSEWIC Assessment and Status Report on the Pygmy Pocket Moss Fissidens exilis in Canada - 2016

- Table of contents
- Assessment summary
- Executive summary
- Technical summary
- Preface
- Wildlife species description and significance
- Distribution
- Habitat
- Biology
- Population sizes and trends
- Threats and limiting factors
- Protection, status and ranks
- Acknowledgements and authorities contacted
- Information sources
- Biographical summary of report writer(s)
- Collections examined
- Figure 1. Photograph of Pygmy Pocket Moss on a clay bank in Franquelin, QC. Plants (leaves plus immature spore capsules) are less than 1 cm tall.
- Figure 2. Approximate known North American distribution of Pygmy Pocket Moss, based on all available sources for Canada, and on herbarium records for the United States. It is likely that more US herbarium records exist than were found for this report. However, specimens were found for all states for which literature reports exist, except for Illinois. The Illinois report is credible (Pursell 2007), but no dot has been added for Illinois in the above figure.
- Figure 3. Known Canadian distribution of Pygmy Pocket Moss (Fissidens exilis) in A. eastern Canada and B. western Canada, based on 20 known Canadian specimens and/or literature reports.
- Table 1. Summary of occurrences of Pygmy Pocket Moss in Canada, including collections examined. Records that are newly reported since the original status assessment are in bold. Records were sought via contact with collectors listed in the "Authorities Consulted" section of this report, as well as with herbaria (National Herbarium of Canada, Canadian Museum of Nature (CANM), Herbier Marie-Victorin, Montréal Botanical Garden (MT), New Brunswick Museum (NBM), University of Guelph (OAC), Herbier Louis-Marie, Université du Québec (QFA), Royal Ontario Museum (TRT), Beaty Biodiversity Museum, University of British Columbia (UBC), University of Western Ontario (UWO) and Devonian Botanic Garden, University of Alberta (ALTA-DBG), and conservation data repositories (Ontario Natural Heritage Information Centre, Atlantic Canada Conservation Data Centre), and via searches of online databases of Canadensys, Prairie and Northern Plant Diversity Centre, Acadia University herbarium (ACAD), and the herbarium of the New York Botanical Garden (NY). Specimens flagged with ⁺ were examined by the writer. All specimens in the table (including the missing voucher from North Dumfries ON) have been reliably verified by Jennifer Doubt, Steve Joya, Ron Pursell, Linda Ley, Tom Neily, Wilf Schofield, and/or Jean Faubert. No specimen has been found for the Montréal occurrence.
- Appendix 1. Threats calculator for Fissidens exilis
COSEWIC
Committee on the Status
of Endangered Wildlife
in Canada

COSEPAC
Comité sur la situation
des espèces en péril
au Canada
COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2016. COSEWIC assessment and status report on the Pygmy Pocket Moss Fissidens exilis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 28 pp. (Species at Risk Public Registry website).
COSEWIC 2005. COSEWIC assessment and status report on the pygmy pocket moss Fissidens exilis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 18 pp.
COSEWIC would like to acknowledge Jennifer Doubt for writing the status report on the Pygmy Pocket Moss (Fissidens exilis) in Canada, prepared under contract with Environment Canada. This report was overseen and edited by René Belland, Co-chair of the COSEWIC Mosses and Lichens Subcommittee.
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment and Climate Change Canada
Ottawa, ON
K1A 0H3
Tel.: 819-938-4125
Fax: 819-938-3984
E-mail: COSEWIC E-mail
Website: COSEWIC
Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Fissident pygmée (Fissidens exilis) au Canada.
Photograph of Pygmy Pocket Moss on a clay bank in Franquelin, QC, by bryologist Stéphane Leclerc. Plants (leaves plus immature spore capsules) are less than 1 cm tall.
Pygmy Pocket Moss (Fissidens exilis) is an ephemeral moss, periodically producing minute (up to 2 mm), 4- to 8-leaved plants from a mat of undifferentiated green filaments, or “protonemata”, persisting between periods of reproductive activity on and in the surface soil layer. It can be identified using microscopic features of the leafy plants (gametophores), but the protonemata, which persist between periods of reproductive activity, cannot be visually identified by any means. Spore-filled capsules, supported on 2 – 9 mm stalks, are attached to the apex of each successfully fertilized, mature plant. Pygmy Pocket Moss is most likely to be detected when capsules are present, especially in large colonies.
Pygmy Pocket Moss is known from Europe, Asia, Africa, the West Indies, New Zealand and North America. Some authors speculate that it may have been introduced to the last three of these, but conclusive evidence is lacking. Pygmy Pocket Moss was first discovered in North America in 1947, in Cleveland, Ohio, and it is known from at least fifteen eastern US states, as well as from the Canadian provinces of Nova Scotia, Quebec, Ontario, and British Columbia. Some experts believe the species may be introduced in British Columbia.
Search effort for Pygmy Pocket Moss requires specific, intensive approaches that address challenges associated with ephemeral mosses, which can be visually recognized under only certain, sporadic conditions. These measures have not been undertaken, and most known subpopulations were opportunistically discovered.
In North America, most Pygmy Pocket Moss has been found largely on bare, moist, at least partly shaded, clay-based soil or loam. It has been collected on the forested banks of streams and ravines, floodplains, bluffs, beaches, roadsides, trails and other environments where bare soil is exposed. Habitat patches are transient and may be unpredictable, resulting from a variety of natural and human-related phenomena. No broad trends in the preferred habitat of Pygmy Pocket Moss are known.
Pygmy Pocket Moss is ephemeral and exhibits a “fugitive” life history strategy: the life and reproductive cycles of its leafy plants are short (less than a year), not seasonally dependent, and driven largely by abiotic factors. Reduced size allows such species to reach maturity sooner than larger mosses with more protracted developmental processes. It expends relatively high reproductive effort, with virtually every tiny plant producing a spore-filled capsule, and its small spores (less than 20 μm) are characteristic of species with longevity in the spore bank. These traits equip plants to complete their life cycles in transient, early-successional environments, and avoid stress during periods of habitat unsuitability by persisting in forms (spores and underground filaments) that are less vulnerable to unfavourable conditions.
Spores are dispersed from less than 1 cm above the substrate, and most collections of this moss have been made from at least partly sheltered environments, so long-distance spore dispersal may be very infrequent. Dispersal of moss- or spore-laden soil via a range of possible biotic and abiotic vectors may be important.
Population sizes and trends are unknown for Pygmy Pocket Moss, and efforts to establish both must take into account challenges presented by the species' ephemeral nature and tiny size.
Some threats can be inferred with reference to the general biology of mosses and the habitats in which Pygmy Pocket Moss has been collected, but no research has demonstrated any specific threats to this species. Some human activities that routinely threaten other plant species may have a neutral or beneficial effect on this species, which relies on localized soil disturbance.
Pygmy Pocket Moss is currently listed as a species of Special Concern under the Canadian Species at Risk Act. It is also protected under the Ontario Endangered Species Act, and at least half of the sites where it has been found are managed by the federal or Ontario government, or by conservation-oriented organizations. Some North American jurisdictions, including British Columbia, have ranked Pygmy Pocket Moss SE (exotic).
| Is there an [observed, inferred, or projected] continuing decline in number of mature individuals? | No |
| Estimated percent of continuing decline in total number of mature individuals within [5 years or 2 generations] | Not applicable |
| [Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over the last [10 years, or 3 generations]. | Unknown |
| [Projected or suspected] percent [reduction or increase] in total number of mature individuals over the next [10 years, or 3 generations]. | Unknown |
| [Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over any [10 years, or 3 generations] period, over a time period including both the past and the future. | Unknown |
Are the causes of the decline
|
Not applicable |
| Are there extreme fluctuations in number of mature individuals? Extreme fluctuations are characteristic of ephemeral mosses, which opportunistically respond to local microclimate | Suspected, but only at a local scale |
| Summary items | Information |
|---|---|
| Estimated extent of occurrence | 2 030 000 km² |
| Index of area of occupancy (IAO) (Always report 2x2 grid value). |
84 km² (likely a gross underestimate of actual IAO) |
| Is the population “severely fragmented” i.e. is >50% of its total area of occupancy in habitat patches that are (a) smaller than would be required to support a viable population, and (b) separated from other habitat patches by a distance larger than the species can be expected to disperse? | Probably not |
| Number of "locations"? (Note: See Definitions and Abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) (use plausible range to reflect uncertainty if appropriate) (use plausible range to reflect uncertainty if appropriate) In absence of known imminent threats, it is not expected that any threat would affect more than one known occurrence of Pygmy Pocket Moss at a time. The number of locations is therefore equivalent to the number of known occurrences. More occurrences are expected with additional / customized search effort (see Distribution and Population Sizes and Trends sections) |
21 but more expected |
| Is there an [observed, inferred, or projected] decline in extent of occurrence? | No |
| Is there an [observed, inferred, or projected] decline in index of area of occupancy? | No |
| Is there an [observed, inferred, or projected] decline in number of subpopulations? | No |
| Is there an [observed, inferred, or projected] decline in number of “locations”? (Note: See Definitions and Abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
No |
| Is there an [observed, inferred, or projected] decline in [area, extent and/or quality] of habitat? | Unknown |
| Are there extreme fluctuations in number of subpopulations? | No |
| Are there extreme fluctuations in number of "locations"? (Note: See Definitions and Abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
Unknown |
| Are there extreme fluctuations in extent of occurrence? | No |
| Are there extreme fluctuations in index of area of occupancy? | Unknown |
| Subpopulations (give plausible ranges) | N Mature Individuals |
|---|---|
| Total | Unknown |
| Summary items | Information |
|---|---|
| Probability of extinction in the wild is at least [20% within 20 years or 5 generations, or 10% within 100 years]. | Not calculated |
| Summary items | Information |
|---|---|
| The individual and combined impacts of all plausible threats considered for this species are unknown, either because no specific instance of a threatening event is known, or because the response of Pygmy Pocket Moss to the potential threat is not fully understood. Was a threats calculator completed for this species and if so, by whom? |
Yes D. Fraser (chair of threats discussion), R. Belland (Co-chair of Mosses and Lichens Specialist Subcommittee), J. Doubt (report writer), R. Boles (Canadian Wildlife Service), J. McKnight (Canadian Wildlife Service), E. Snyder (Ontario), S. Bureau (Canadian Wildlife Service), K. Golinski (Mosses and Lichens Specialist Subcommittee), N. Fenton (Mosses and Lichens Specialist Subcommittee) |
| Summary items | Information |
|---|---|
| Status of outside population(s) most likely to provide immigrants to Canada. Allen et al. (2004) suspect that the North American population is expanding, but low detectability makes it difficult to know which newly documented populations represent newer populations | Stable or expanding |
| Is immigration known or possible? | Not demonstrated, but likely |
| Would immigrants be adapted to survive in Canada? | Yes |
| Is there sufficient habitat for immigrants in Canada? | Yes |
| Are conditions deteriorating in Canada? See Table 3 ( Guidelines for modifying status assessment based on rescue effect) |
Unlikely |
| Are conditions for the source population deteriorating? See Table 3 ( Guidelines for modifying status assessment based on rescue effect) |
Unknown |
| Is the Canadian population considered to be a sink? See Table 3 ( Guidelines for modifying status assessment based on rescue effect) |
No |
| Is rescue from outside populations likely? | Yes |
| Summary items | Information |
|---|---|
| Is this a data sensitive species? | No |
| Summary items | Information |
|---|---|
| COSEWIC: Designated Special Concern in May 2005. | Status re-examined and designated Not at Risk in April 2016. |
| Summary items | Information |
|---|---|
| Status | Not at Risk |
| Alpha-numeric codes | Not applicable |
| Reasons for designation | This species has a very large extent of Canadian occurrence, occurring on both Pacific and Atlantic coasts, and in central Canada. Despite low detectability that confounds attempts to quantify population sizes and trends, the number of known occurrences has increased from seven to 21 since 2005, and it is expected that more occurrences will be documented with ongoing search effort. Although it is found in some densely populated regions of Canada, including southern Ontario, no declines or direct imminent threats are known for this species. Localized soil disturbance is required for suitable habitat, such that some kinds of human disturbance may actually benefit the species. Although data are lacking in many aspects of its biology, ecology, distribution, and abundance, no evidence suggests that this species is at risk in Canada. |
| Summary items | Information |
|---|---|
| Criterion A (Decline in Total Number of Mature Individuals) | Not applicable. No evidence of decline in numbers of mature individuals. |
| Criterion B (Small Distribution Range and Decline or Fluctuation) | Not applicable. EO is above thresholds for all criteria. IAO is below the threshold for Endangered but the number of locations exceeds thresholds for at-risk status. This species does not undergo extreme fluctuations, its population is not severely fragmented and there is no evidence of population decline. |
| Criterion C (Small and Declining Number of Mature Individuals) | Not applicable. The number of mature individuals is not known and for known occurrences, cannot be enumerated without intensive long-term study. |
| Criterion D (Very Small or Restricted Population) | Not applicable. The number of mature individuals is not known and neither IAO nor number of known locations meet thresholds for at-risk status. |
| Criterion E (Quantitative Analysis) | Not applicable. No quantitative analysis has been done. |
Pygmy Pocket Moss was first assessed in 2005 (COSEWIC 2005). Since that time, pre-existing Nova Scotia and Quebec records have become evident, and occurrences have been newly reported in Nova Scotia (Anderson and Neily 2010), Quebec, Ontario, and British Columbia (SQB 2014, herbarium records), bringing the national total from seven to 21, so far.
The known North American distribution also has expanded (though less dramatically) since the original assessment, and the species has received new designations in several national and subnational jurisdictions.
After considering a draft Status Appraisal Summary in 2013, the COSEWIC Mosses and Lichens Subcommittee determined that our new understanding of the species' range and potential abundance warranted an update Status Report that could support consideration of various status options.
The subcommittee recognized that, given the natural limitations to detectability of this species, fieldwork on the scale feasible for Status Report preparation would not resolve the species' EO, IAO, or number of locations with confidence. There is, however, enough information to recommend a status.
A management plan for Pygmy Pocket Moss was published for comment in June 2015 (Environment Canada 2015).
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
The Canadian Wildlife Service, Environment and Climate Change Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Pygmy Pocket Moss, Fissidens exilis Hedw., a member of the moss family Fissidentaceae, belongs to subgenus Aloma Müll. Hal. (Pursell 2007, Beever 1999).
The genus’ Latin name means “split-tooth,” referring to the teeth surrounding the open end of the spore-bearing capsule, while its common name – “Pocket Moss” – refers to the more immediately obvious, unique doubling of the upper half of each leaf, forming a pocket that cups the lower half of the leaf above it. The specific epithet “exilis” means small, or slender.
Synonyms of F. exilis include F. bloxamii Wilson, Bryum viridulum Dicks., Dicranum exile (Hedw.) Muhl., Schistophyllum exile (Hedw.) Lindb., Skitophyllum exile (Hedw.) Bach. Pyl., and Hypnum minutum Wilson (Steere 1950, Allen 2005, Missouri Botanical Garden 2014). In the past, the species has also been classified as a variety of F. bryoides, F. viridulus, and Dicranum palmatum.
Detailed descriptions by Steere (1950) and Crum and Anderson (1981) are summarized in COSEWIC (2005), and more recent descriptions are found in Allen (2005), Pursell (2007), and Faubert (2013). A photo of the species is in Figure 1.
Pygmy Pocket Moss produces minute (up to 2 mm), 4- to 8-leaved plants from a mat of undifferentiated green filaments, or “protonemata” on and in the surface soil layer. Sexual structures are generated on the leafy plants.
Leafy plants are identifiable and distinguishable from other pocket mosses using microscopic cellular characteristics, but their dark green or brown colour contrasts poorly with the species’ preferred bare soil substrate and its protonemal mat. Between periods of reproductive activity, the protonemal mat, which cannot be identified to species visually by any means, persists on the soil.
Spore-filled capsules, supported on 2 – 9 mm stalks, are attached to the apex of each successfully fertilized, mature plant. Pygmy Pocket Moss is most likely to be detected when capsules are present, especially in large colonies. Spores are classified as small (11–14 μm) among bryophyte spores, which range from 5 μm to 310 μm (Crum 2001).

Long description for Figure 1
Photo of Pygmy Pocket Moss on a clay bank. This moss produces tiny (up to two millimetres), four- to eight-leaved plants from a mat of undifferentiated green filaments on and in the surface soil layer. Spore-filled capsules are supported on stalks (two to nine millimetres) attached to the apex of each successfully fertilized, mature plant.
The Canadian population has not been studied sufficiently to provide reliable information on population structure and variability.
There is not sufficient information on the species' genetic structure, distribution, dispersal, or ecology to adequately assess the discreteness or significance of any portion of the Canadian population. It is assessed as a single designatable unit.
Ephemeral mosses like Pygmy Pocket Moss, particularly those of wooded habitats, are uncommon in Canada. Canadian subpopulations mark the species' northern range limit in North America.
Pygmy Pocket Moss is known from Europe (British Isles, central and northern Europe, Scandinavia (Steere 1950, Pursell 2007)), Asia (Japan (Iwatsuki and Noguchi 1973), Kashmir (NatureServe 2014)), and Africa (Algeria (Pursell 2007)). Although NatureServe (2014), citing Smith (1978), also lists it for South America, Smith (2004) does not mention South America. It occurs also in New Zealand (Beever 1999) and the West Indies (Pursell 2007), where it is thought to have been introduced. In many parts of its range, it is considered to be rare.
Pygmy Pocket Moss was first discovered in North America in 1947, in Cleveland, Ohio (Steere 1950). Some recent authors (Allen et al. 2004, Pursell 2007, Faubert 2013) have conjectured that the species was introduced from Europe, based on its relatively recent discovery in regions of the continent that are densely populated, subject to human disturbance, and relatively well-botanized. To date, conservation ranks for North Carolina (NatureServe 2014) and British Columbia (B.C. Conservation Data Centre 2016) reflect the opinion that Pygmy Pocket Moss was introduced. There is no evidence to suggest that this is the case. While the sites where the species is found are within urban areas, the species is not associated with anthropogenic habitats as one would expect for an introduced species. Further the species is opportunistic, growing on disturbed soils, and will grow on these substrates whether the habitat is anthropogenic or not (see further discussion, below).
However, there are recently discovered native North American bryophyte species with greater detectability (e.g., larger plant size and perennial habit) than that exhibited by Pygmy Pocket Moss, suggesting that recent discovery is not clear evidence of introduction. Schleicher's Silk Moss (Entodon schleicheri), for example, was first reported for North America in the 1970s (Buck and Crum 1978, based on material collected in 1938), but was previously known from Europe. McIntosh (1989) more recently reported four North American species for the first time, as well as two genera and seven species previously unknown in Canada, in a single British Columbia study.
Furthermore, occurrences of Pygmy Pocket Moss are not clustered around ports, in urban settings, or on anthropogenic linear disturbances as many introduced (Schofield 1988) or rapidly expanding (Hassel and Söderström 1998) bryophyte species tend to be. Instead, it occurs both in natural areas and urban settings with various disturbance histories. Its requirement for exposed mineral soil makes it a good candidate to colonize areas of human activity, whether or not human activity brought it to this continent.
In the United States (Figure 2), Pygmy Pocket Moss has been reported from at least fifteen eastern states (Alabama, Illinois, Indiana, Kentucky, Maine, Maryland, Michigan, Missouri, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Tennessee, Vermont and West Virginia (Allen et al. 2004, Pursell 2007)). No Pacific coast populations have been reported for the US, unlike Canada.

Long description for Figure 2
Map of the North American distribution of the Pygmy Pocket Moss. In the United States, Pygmy Pocket Moss has been reported from at least 15 eastern states (Alabama, Illinois, Indiana, Kentucky, Maine, Maryland, Michigan, Missouri, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Tennessee, Vermont, and West Virginia). In Canada, it has been reported from Nova Scotia, Quebec, Ontario, and British Columbia.
Canadian specimens of Pygmy Pocket Moss have been collected in Nova Scotia, Quebec, Ontario, and British Columbia (Table 1, Figure 3). It has been found in Mixedwood Plains, southern Boreal Shield, Atlantic Maritime and Pacific Maritime ecozones (Ecological Stratification Working Group 1996). British Columbia is the only province with no populations yet reported in natural settings; the two known sites are in the Vancouver area.
| # | Location | First Observed | Later Search | Specimen or Record | Substrate, habitat | Land tenure |
|---|---|---|---|---|---|---|
| 1 | Richmond, BC. Sea Island | March 17, 2012 | May 18, 2015: absent | UBC B212546 | Clay bank of slough, under shrubs | Municipality of Richmond? |
| 2 | Vancouver, BC. Point Grey, Wreck Beach | March 24, 2010, | March 9, 2012: present; May 18 2015: absent | UBC B211597, B218058 | On lump of clay by edge of path behind beach | Pacific Spirit Regional Park, Greater Regional Vancouver District |
| 3 | Dunnville, ON. Ruigrok Tract Conservation Area | November 18, 2012 | - | CANM 335576⁺ | On clay in transition from upland deciduous woods to track of swampy humic thicket | Niagara Peninsula Conservation Authority |
| 4 | Port Dover, ON. | November 23, 2011 | - | CANM 331159⁺ | On clay encrusted upturned root mass of fallen white pine, near a ploughed field | Agricultural enterprise |
| 5 | Chatham-Kent, ON. Sinclair’s Bush Conservation Area | August 16, 2002 | - | ALTA-DBG B-14643⁺ | On bare mud in maple/beech forest | Lower Thames Valley Conservation Authority |
| 6 | North Dumfries Twp., ON. Sudden Tract | September 23, 1995 | - | Specimen missing, likely at NB or ALTA-DBG (Bradley, pers. comm. 2015) | Fresh – Moist Sugar Maple – Hardwood Deciduous Forest | Ontario Ministry of Natural Resources (Regional Forest) |
| 7 | Walsingham Township, ON. Deer Creek Conservation Area | June 22, 1995 | - | ALTA-DBG B-6969 | Dry – Fresh Hardwood – Hemlock Mixed Forest | Long Point Region Conservation Authority |
| 8 | Anderdon Twp., ON. “Canard River Kentucky Coffee Tree Woods” | March 24, 1984 | August 2002: not found | CANM 290756⁺ | On lumps of clay in floodplain woods | Essex Region Conservation Authority |
| 9 | Colchester South Twp., ON. | March 26, 1981 | August 2002: not found | CANM 275055⁺, UBC B24863, NY 113173 | Mature deciduous woods, oak- dominated | Ontario Ministry of Natural Resources |
| 10 | Sainte-Foy, QC. | June 3, 1987 | - | Jean Faubert private herbarium 7965 | Bare soil, deciduous forest | Laval University Campus |
| 11 | Gatineau, QC. | June 1982 | - | CANM 291533⁺ | Not recorded | Gatineau Park (National Capital Commission) |
| 12 | Montréal, QC. | Autumn 1973 | July 2002 : not found | Molnar 1975 (literature report only) | On clay soil in a planted spruce-tamarack association | Morgan Arboretum, McGill University |
| 13 | Franquelin, QC | October 12, 2014 | July 17 2015: present but less abundant | SQB (2014); specimen STL-0053 verified by Jean Faubert | Clay soil along edges of stream through boreal forest with dry granite rocks | Province of Quebec; managed by municipality of Franquelin |
| 14 | Teare Brooke, NS. | April 26, 2012 | - | CANM 331676⁺ | Exposed soil on slope to brook | Private |
| 15 | White Head, NS. | May 7 & 8 2010 | - | CANM 331674⁺, NB BB-21566, ACAD ECS03857 | On soil behind gypsum bluff | Private |
| 16 | Herbert River, NS. | April 3, 2012 | - | CANM 331675⁺ | On soil, on stream bank | Private |
| 17 | Glen Brook, NS. | April 23, 2012 | - | ACAD ECS039057 | On soil, ravine slope | Unknown |
| 18 | Glendyer, NS. | October 10, 2013 | - | ACAD ESC039909 | Shaded damp soil, sinkhole | Private |
| 19a | Big Harbour, NS. | October 10, 2013 | - | ACAD ECS039908 | Clay soil, under hardwood trees | Crown |
| 19b | Big Harbour, NS. | October 10, 2013 | - | ACAD ECS039907 | Clay soil, under hardwood trees | Crown |
| 20 | Bishop Brook, NS. | January 15, 2014 | - | ACAD ECS039911, CANM 335560⁺ | On rock, under alders on slope | Private |
| 21 | Belle Isle, NS. | July 16, 1987 | - | UBC B114963 | Second-growth spruce-fir forest; clayey moist soil in forest | Unknown |
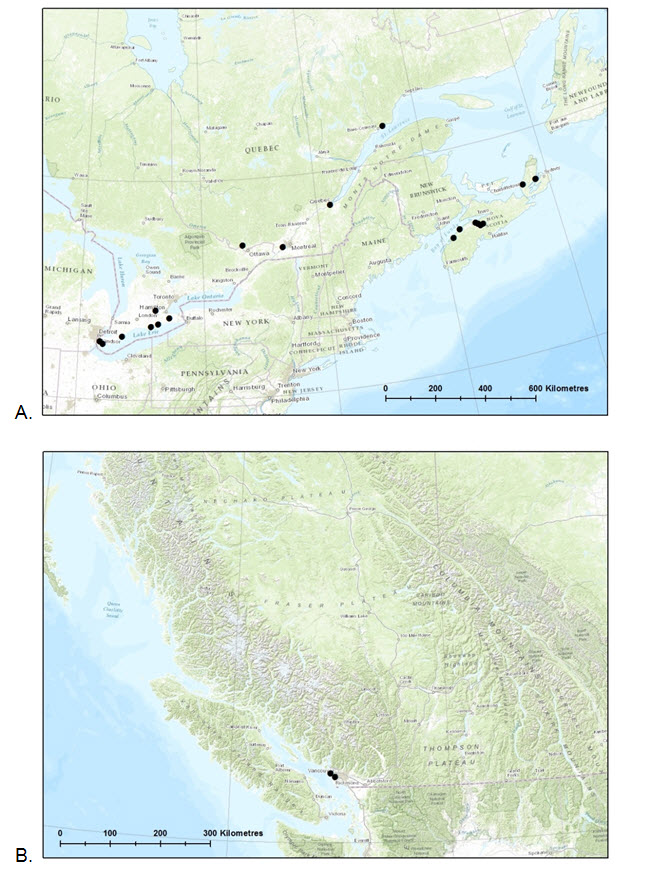
Long description for Figure 3
Two maps illustrating the known Canadian distribution of Pygmy Pocket Moss eastern Canada (map A) and western Canada (map B).
With the documentation of occurrences that were not known at the time of the previous Status Report (COSEWIC 2005), the known extent of occurrence for Pygmy Pocket Moss has increased from 30 000 km2 to 2.03 million km2. The IAO, calculated on a 2 km x 2 km grid is 84 km2, likely underestimates the area occupied by this species because it is less likely to be detected than most other mosses, and many areas of potentially suitable habitat have yet to be searched.
A dedicated search associated with the preparation of the original Status Report for Pygmy Pocket Moss was conducted in 2002. This search targeted one Ontario site and two Quebec sites that were described on herbarium labels and in literature reports, and also included the searched sites for two species (Bryoandersonia illecebra, southern Ontario and Helodium paludosum, southern Ontario and Quebec) for which Status Reports were concurrently being completed. Out of 36 sites visited, minute pocket mosses were collected from eleven, yielding 46 samples (COSEWIC 2005) for microscopic examination. Pygmy Pocket Moss was found once, at a previously undocumented site (Table 1). In contrast, the remaining 19 known Canadian collections of Pygmy Pocket Moss were documented by botanists conducting general biodiversity surveys or projects targeting species other than Pygmy Pocket Moss.
No dedicated search effort is associated with the current Update Status Report. Pygmy Pocket Moss presents several challenges to directed search effort:
- The small stature of Pygmy Pocket Moss makes it less conspicuous than many other bryophytes. It is most likely to be noticed when its short-lived spore capsules are fully formed, and liable to be missed at other stages of development (e.g., Allen et al. 2004, Allen 2005, Faubert 2013).
- Pygmy Pocket Moss persists as protonemata (and likely also as spores) when leafy plants and capsules are not visible, such that it may frequently be undetectable for unknown and potentially long periods of time, even where it’s present. If apparently suitable habitat exists, it is not possible to base presence, absence, or an apparent trend in Pygmy Pocket Moss abundance on a single site visit.
- Unlike some vascular plants, for which seasons of detectability are reasonably well-documented, the optimum time for detecting Pygmy Pocket Moss is not well understood and (as in other ephemeral species) likely varies geographically and temporally according to local climate, microclimate, and disturbance, without reliable links to the annual seasonal cycle. Spore capsules are reported to mature in the winter (e.g., Steere 1950, Smith 2004, Atherton et al. 2010), when moisture is high, making spring and fall detection most likely. Canadian collections have been made in every month except for February and December, with one quarter collected between May and August (Table 1).
- In wooded areas where Pygmy Pocket Moss is found, protracted damp conditions may be more common in the spring and fall, but could occur at any time of year, and may occur more frequently in the northern parts of its range than further south. Similarly, localized disturbances (e.g., tree fall, stream flooding) that expose soil suitable for Pygmy Pocket Moss growth occur unpredictably within and among years.
Allen et al. (2004) suggest that Pygmy Pocket Moss is more common than occurrence records suggest, due to its small size and short window of collecting opportunity. This is often said of other ephemeral mosses (Allen 1979, Risk 2002, Glime 2007). Vanderpoorten and Engels (2002) found that ephemeral bryophytes defied predictive distribution modelling based on environmental variables. Nonetheless, attentive observation of the species’ preferred habitat and carefully targeted / timed site visits permitted Risk (2002) to document fifty new US localities of the related species, Hyaline Pocket Moss (Fissidens hyalinus), bringing the number of known sites to 65 from 15. Similar focused effort may prove valuable for Pygmy Pocket Moss.
As Beever (1999) points out, pocket mosses are typical of most mosses in their association with specific micro-environmental conditions. In North America, most Pygmy Pocket Moss has been found on at least partly shaded, bare, moist clay-based soil or loam (e.g., Allen 2005, Faubert 2013, herbarium records). It has been collected on the forested banks of streams and ravines, floodplains, bluffs, roadsides, trails and other environments where bare soil is exposed (Crum and Anderson 1981, Pursell 2007, Anderson and Neily 2010, herbarium records, Table 1). It may be associated with other ephemeral mosses such as Ephemerum and Micromitrium (Crum and Anderson 1981), which are known to prefer temporary habitat patches. Tom Neily (pers. comm. 2015) notes that Nova Scotia sites for Pygmy Pocket Moss occur in karst and basalt areas.
As a species linked to soil exposure, broad habitat trends may not be as important as local-scale trends. In general, however, natural disturbances resulting from tree fall and flooding are less common in highly populated and managed regions, including the Canadian cities where Pygmy Pocket Moss has been documented. At the same time, human disturbance has potential to expose fresh soil through the construction and use of terrestrial transportation and service corridors, as well as through activities related to farming and forestry. Sites where Pygmy Pocket Moss has been collected are subject to natural and/or human disturbance (Table 1).
Very little has been published on the biology of Pygmy Pocket Moss. COSEWIC (2005) outlines some of the generalized characteristics of all acrocarpous, autoicous, soil-dwelling mosses.
Pygmy Pocket Moss is described by most authors (e.g., Allen et al. 2004, Allen 2005, Pursell 2007) as ephemeral, meaning that the life and reproductive cycles of leafy plants are short (less than a year), not seasonally dependent, and driven largely by abiotic factors (During 1979). It may therefore share some biological characteristics with certain members of other moss families – Ephemeraceae, Micromitraceae, Funariaceae, Buxbaumiaceae, and Polytrichaceae – that also have ephemeral plants (with or without perennial protonemata). Reduced size allows these species to mature more quickly than larger mosses with more protracted developmental processes (e.g., Goffinet et al. 2011).
In During’s (1979) classification of bryophyte life history strategies, Pygmy Pocket Moss is a “fugitive”: it is short-lived (less than one year) and reproduces sexually very soon after leafy plants are initiated. It expends relatively high reproductive effort, with virtually every plant producing a spore-filled capsule, and its spores are small (anything less than 20 μm qualifies as small in During’s classification). These traits equip the plants to complete their life cycles in unpredictable, early-successional environments, and avoid stress during periods of habitat unsuitability by persisting in forms – such as spores and persistent protonemata – that are less vulnerable.
With each leafy plant producing male and female gametangia, Pygmy Pocket Moss likely self-fertilizes. Stalks topped by spore-filled capsules result from successful fertilization. Virtually every plant produces a capsule.
In short-lived species such as ephemerals, with life cycles triggered by transient conditions in their immediate microclimate, it is possible for more than one generation to be produced per year (e.g., Gray 1935 in Glime 2007, Furness and Hall 1981), and under optimal conditions, each developmental stage may be very brief. Allen et al. (2004), for example, noted marked reduction in abundance along a trail in a city park between March 20 and 29, 2003.
During periods that are not conducive to reproductive activity, Pygmy Pocket Moss persists in soil as undifferentiated protonemata (juvenile gametophytic tissue appearing similar to algal filaments) that are not identifiable to species, and as spores, which (owing to the small stature of the moss) fall close to the parent plants. Although experiments have not been conducted with Pygmy Pocket Moss, ephemeral species with small spores (<20 μm) are thought to have much greater longevity in soil than those with large spores (>25 μm). The latter are more characteristic of predictably disturbed environments (During 1979).
Furness and Hall (1981) documented the abundance of the large-spored ephemeral moss Physcomitrium sphaericum (in which protonemata are not persistent) at two sites in Britain over the course of more than forty years. They found that spores in pond sediments persisted for more than a decade, and could initiate thriving populations as soon as lake levels lowered sufficiently to expose the species’ preferred uncolonized mud substrate. However, no studies have yet been completed to document the longevity of spores in Pygmy Pocket Moss.
The protonemal mat is probably very important for local vegetative propagation of Pygmy Pocket Moss colonies. The needs and longevity of the protonemal mat are not known. Embedded in their soil substrate, protonemata probably avoid water loss and minor surface disturbance better than leafy plants. In moist soil, some species’ protonemata may survive for fifty years (Bristol 1916 in Schofield 1981).
The generation time for Pygmy Pocket Moss is unknown. However, ephemeral species such as this one, typically have short-lived (<1 yr) sporophytes, but have persistent protonemata of unknown longevity
Pygmy Pocket Moss relies on exposed mineral soil, making it vulnerable to successional changes in its habitat. The fugitive life history strategy of the gametophores suits it better to avoid rather than tolerate this stress. In places where moisture and disturbance return periodically (e.g., stream and river banks, floodplains), soil may be kept bare, or new patches may open as old ones are covered with vegetation, giving opportunities for the persistent protonemata within the soil to tolerate periods of habitat unsuitability. Where the disturbance is not repeated, colonizers of bare soil may be eliminated over time.
Spore production is very important to fugitive species, which rely upon patchy, temporary, unpredictably recurring substrates (e.g., During 1979). However, spores released from less than 1 cm above the soil, in sheltered habitats, are unlikely to result in significant long-distance dispersal. Fragmentation of plants (virtually any moss cell can regenerate a clone) or of embedded protonemata is also possible with repeated soil disturbance. Dispersal of spores or fragments in the soil by water or animal / machine vectors is likely. Although no specific animal vectors are documented, many species could plausibly pick up spores or fragments from the soil of creek banks, in particular. Pygmy Pocket Moss is also a good candidate for dispersal among reforestation or other land management projects where machinery used to prepare soil travels from one site to the next.
Ephemeral mosses avoid competition by relying on fresh substrates and completing their life cycles very quickly (Slack 1990). The establishment of other mosses and vascular plants renders their habitat patches unsuitable until fresh disturbance “resets” the successional clock.
Pygmy Pocket Moss may be associated with any localized soil disturbance, which could result, for example, from direct (e.g., digging, trampling by turkeys or deer) or indirect (e.g., flooding by beaver) soil disturbance from animal activity. Similarly, human disturbance of natural substrates (e.g., trail use) could create or maintain bare mineral soil patches suitable for Pygmy Pocket Moss. The proliferation of non-native earthworms in many parts of Canada likely also increases the availability of mineral soil substrate (e.g., Sackett et al. 2013). However, soil is naturally disturbed by other means (e.g., flooding, erosion, windfall) as well, and Pygmy Pocket moss is probably not reliant on any other species.
Hallingbäck and Hodgetts (2000) recommend that localities for ephemeral bryophytes be searched repeatedly, at appropriate times of the year, over several years to take account of population fluctuations and the invisible persistence of species in the diaspore bank (in the case of Pygmy Pocket Moss, persistent protonemata also form part of the species’ propagule bank). This kind of survey data has not been reported for Pygmy Pocket Moss. Some of the factors that may challenge attempts to quantify population sizes and trends have already been outlined (see Search Effort). Most documented occurrences of Pygmy Pocket Moss were discovered incidentally by botanists working on other projects. Furthermore, the necessity of microscopic examination to identify this species introduces a delay between collection and positive identification. For these reasons, abundance has not been assessed even for the point in time at which each subpopulation was first observed. Return visits – even quite soon after the initial observation – are likely to capture different results (e.g., Allen et al. 2004), creating a dynamic understanding of the species’ abundance and conditions optimal for reproductive activity.
Most authors describe Pygmy Pocket Moss as rare or undercollected (e.g., Crum and Anderson 1981, Allen et al. 2004, Allen 2005, NatureServe 2014, Faubert 2013). The recent discovery of previously undocumented populations suggests that considerable, well-strategized additional search effort in intervening areas (Environment Canada 2015) is required to adequately estimate EO, IAO, or the number of mature individuals (see also Sampling Effort and Methods).
Local population fluctuations are characteristic of ephemeral species (e.g., Furness and Hall 1981, Allen et al. 2004). Allen et al. (2004) suspect that the eastern North American Pygmy Pocket Moss population is expanding, based on increasing range and number of collections, but no studies have addressed this question.
The northern US population, which is the nearest potential source of Pygmy Pocket Moss outside Canada, would likely provide rescue for the Canadian population, should it disappear. Although its plants and spores are very small, the spread of Pygmy Pocket Moss (see Dispersal and Migration section) within the US and across the border into adjacent Canada seems equally likely, particularly because the Canadian and US distributions overlap in latitude (Figure 2). Natural migration may be slowed by the Great Lakes in central Canada, although without in-depth understanding of dispersal mechanisms, the significance of this potential barrier remains unknown. Individuals in the United States would likely be adapted to life in Canada.
Movement of Pygmy Pocket Moss between US and Canadian habitats is likely, via spores or vegetative fragments carried by water or animal vectors. Restrictions on the movement of soil across Canada’s international borders probably limit artificial transport of this species more today than in the past.
Like all plants, this species has a characteristic range of tolerance for moisture, nutrients, and light. Beyond the habitats and substrates with which it has been associated (see Habitat), however, the specific conditions required by Pygmy Pocket Moss have not been investigated. Thus, it is expected that Pygmy Pocket Moss would be threatened by activities that alter the existing moisture, nutrient, light, or disturbance regime of its habitat in the long term. Large-scale removal of soil substrate or destruction of habitat by development, for example, is expected to negatively impact local populations. Similarly, the cessation of disturbance that generates fresh, uncolonized mineral soil would reduce the suitability of its habitat.
The IUCN-CMP Threats Classification Scheme (IUCN-CMP 2006) provides a systematic basis for considering the impacts of a wide variety of threats to a species. The headings below correspond to IUCN threats categories that may be relevant to Pygmy Pocket Moss (Appendix 1). None is known with certainty to present an imminent threat, however. This species occurs in both natural areas and developed urban or recreational settings. There is no evidence for a decline in the population, and Allen et al. (2004) suspect an ongoing population increase in North America. Some human activities, such as trail and road building, and certain kinds of forestry, mining, and agricultural development that disturb soil while leaving it available for colonization by plant life may favour the dispersal and proliferation of Pygmy Pocket Moss. An overall threat impact for this species could not be calculated with the available information, and appears to be minimal (Table 1).
At least seven Canadian occurrences of Pygmy Pocket Moss are in publicly accessible parks and/or managed natural areas. The construction / use of trails on native soil favours Pygmy Pocket Moss by maintaining exposed soil substrate, although it has been found both on and away from trails. On the other hand, the application of chemical pesticides, elimination of suitable substrate by buildings and other infrastructure, and excessive trampling would negatively impact its success.
At least three Canadian specimens of Pygmy Pocket Moss were collected from stream banks or flood plains. Water regulation in these kinds of habitats has potential to reduce the availability of suitable disturbed-soil substrate at appropriate (more moist) times of the year. No proposed new water management developments are known for the sites where Pygmy Pocket Moss has been observed.
One Canadian occurrence of Pygmy Pocket Moss is near the edge of a ploughed field (Table 1). None have yet been reported within agricultural fields, possibly due to higher light and/or lower moisture than is typical for its known habitats, but these habitats are also often under-surveyed. Some ephemeral bryophytes are closely associated with agricultural activity (e.g., Porley 2008). Based on European studies, fall cultivation of fields may disrupt the development of ephemeral bryophytes, whereas fields that overwinter as stubble may allow greater opportunity for plants to begin their reproductive cycle after damp conditions increase in the autumn, and complete it before the field is reactivated in the spring (Porley 2008, Hallingbäck and Hodgetts 2000). As bryophytes, in general, are highly sensitive to chemical changes in their environments, the use of fertilizers or herbicides is not expected to favour Pygmy Pocket Moss.
One Canadian occurrence of Pygmy Pocket Moss is in a regional forest (Table 1). It is possible that machinery used to conduct forestry activities (harvest, reforestation) may help to disperse Pygmy Pocket Moss from one nearby site to the next. The degree to which forestry activities help or hinder Pygmy Pocket Moss by creating fresh disturbed-soil habitat probably depends on the degree to which the overall moisture, nutrient and light conditions of the site are changed at the same time.
In general, the construction and maintenance of land-based transportation corridors (railroads, roads) that result in peripheral soil disturbance are likely to favour Pygmy Pocket moss where they pass through suitable habitat, assuming that chemical inputs such as road salt and herbicides are minimal.
Removing or covering substrate, or introducing intensive management (mowing, application of fertilizers or pesticides/herbicides) could threaten Pygmy Pocket Moss, but no such developments are planned for Canadian sites where this species has been documented. Furthermore, some sites in British Columbia and Quebec where this species has been collected, as well as many US sites, are in urban environments, indicating some degree of tolerance of or affinity for human activity.
Activities that involve large-scale removal of soil substrate and/or unnatural chemical concentrations are expected to negatively impact Pygmy Pocket Moss. No mining or energy development is known to be planned at sites where this species has been observed.
COSEWIC (2005) cited climate as a potential limiting factor, but the climate tolerance of Pygmy Pocket Moss seems to be broad, based on its global distribution. The impact of climate warming on this species through changes in habitat parameters such as moisture, temperature, natural disturbance, and the activity of possible dispersal vectors, is unknown.
None of sites where Pygmy Pocket Moss has been observed are likely to be impacted by the same plausible threatening event as the nearest neighbouring site. Twenty-one occurrences of Pygmy Pocket Moss have been documented since 1973 (Table 1), therefore this is considered to be the number of documented locations. Five of these occurrences have been revisited since the time they were first discovered; only two were found when revisited (Table 1). However, given the ongoing presence of apparently suitable substrate, the intermittent visibility of the species, and the absence of very specific locality information, the species is assumed to be resident at all sites.
Pygmy Pocket Moss is currently listed as a species of Special Concern on Schedule 1 of the Canadian Species at Risk Act, and on Ontario’s official list of Species at Risk in Ontario. A Management Plan aimed at more confidently characterizing its distribution and abundance, by creating and implementing specific inventory and monitoring protocols, has been drafted (Environment Canada 2015).
Pygmy Pocket Moss is ranked G3/G4 (Vulnerable) globally and N1 in Canada (NatureServe 2014). Subnationally, it is ranked S1 (Critically Imperilled) in Tennessee and Vermont (NatureServe 2014), S1? in Nova Scotia (ACCDC 2014), S1S2 in Quebec (CDPNQ 2015), and S2 (Imperilled) in Ontario (NHIC 2014) and New York (NatureServe 2014). In British Columbia (Penny, pers. comm. 2015), and North Carolina (NatureServe 2014), it is considered to have been introduced. Elsewhere it is considered unrankable (Missouri) or is simply not ranked (NatureServe 2014).
The General Status list for mosses, to be updated in 2015, lists Pygmy Pocket Moss as S2 in Ontario and S1? in Quebec (Belland, pers. comm. 2015). General status ranks for British Columbia and Nova Scotia were not available at the time of writing.
Available ownership information for Canadian occurrences of Pygmy Pocket Moss is summarized in Table 1. Approximately three quarters of the sites are publicly accessible, and half are either managed by conservation-oriented organizations or by governments that administer legal protections under federal and Ontario species at risk acts.
All authorities contacted in assembling data for this report, along with the hard-working and good-humoured members of the COSEWIC Mosses and Lichens Subcommittee, are gratefully acknowledged for sharing data, wisdom, and expert opinions concerning Pygmy Pocket Moss in Canada. Alain Filion, Julie Perrault, and Shirley Sheppard of the COSEWIC Secretariat provided stellar administrative support, encouragement, and mapping.
Allan Aubin, Bryologist, Simcoe, ON.
Annie St-Louis, Technicienne en travaux d’enseignement et de recherche, Herbier Louis-Marie, Université Laval, Québec, QC.
Bruce Bagnell, Bryologist, NB.
Carole Ann Lacroix, Assistant Curator, Herbarium, University of Guelph (OAC), Guelph, ON.
Deb Metsger, Assistant Curator, Herbarium, Royal Ontario Museum (TRT), Toronto, ON.
David Bradley, Botanist / Bryologist, Ontario Natural Heritage Information Centre, Ontario Ministry of Natural Resources and Forestry, Peterborough, ON.
Emmanuelle Fay, Coordinator – Species at Risk Recovery, Canadian Wildlife Service, Environmental stewardship branch, Environment Canada, Québec, QC.
Geoffrey Hall, Collection Manager, Herbier Marie-Victorin (MT), Montréal, QC.
Jean Faubert, Bryologist et Président, Société Québecoise de Bryologie, Rimouski, QC.
Jenifer Penny, Program Botanist, British Columbia Conservation Data Centre, Victoria BC.
Linda Ley, Bryologist, Ottawa, ON.
Michael Oldham, Botanist / Herpetologist, Ontario Natural Heritage Information Centre, Ontario Ministry of Natural Resources and Forestry, Peterborough, ON
Olivia Lee, Bryophyte, Fungi and Lichen Collections Manager, University of British Columbia Herbarium, Beaty Biodiversity Museum (UBC), Vancouver, BC.
René Belland, Bryologist and Curator, Plant Herbarium, Devonian Botanic Garden (ALTA-DBG); Faculty Service Officer, Department of Renewable Resources, University of Alberta, Edmonton, AB.
Ruth Newell, Curator, Acadia University Herbarium, Wolfville, NS.
Sandra Mackin, Herbarium Technician, University of Western Ontario Herbarium (UWO), London, ON.
Scott Schuette, Treasurer and Copyright Licences, American Bryological and Lichenological Association
Sean Blaney, Botanist and Assistant Director, Atlantic Canada Conservation Centre, Sackville, NB.
Stephane Leclerc, Bryologist, Sainte-Anne-de-Beaupré, QC.
Steve Joya, Bryologist and Herbarium Technician, Beaty Biodiversity Museum, University of British Columbia (UBC), Vancouver, BC.
Stephen Clayden, Curator, Herbarium of the New Brunswick Museum, Saint John, NB.
Terry McIntosh, Bryologist and Consultant, Vancouver, BC.
Tom Neily, Bryologist/lichenologist and Consultant, Middleton, NS.
ACCDC (Atlantic Canada Conservation Data Centre). 2014. Rarity ranks for non-vascular plants. (accessed December 29, 2014).
Allen, B.H. 1979. Additional distributional records of mosses in Pennsylvania. The Bryologist 82:289–291.
Allen, B.H., R.A. Pursell, and C. Darigo. 2004. Fissidens exilis and a key to the species of Fissidens in Missouri. Evansia 21:111–115
Allen, B.H. 2005. Maine Mosses: Sphagnaceae – Timmiaceae. Memoirs of the New York Botanical Garden 93. The New York Botanical Garden Press, New York. 419 pp.
Atherton, D.M., S.D.S. Bosanquet, and M. Llawley. 2010. Mosses and Liverworts of Britain and Ireland: A Field Guide. British Bryological Society, England. 856 pp.
ACCDC (Atlantic Canada Conservation Data Centre). 2014. List of species ranks. (accessed December 31 2014)
Anderson, F. and T. Neily. 2010. A reconnaissance level survey of calciphilous lichens in selected karst topography in Nova Scotia with nots on incidental bryophytes. Report to Nova Scotia Department of Natural Resources. Mersey Tobeatic Research Institute, August 2010.16 pp.
B.C. Conservation Data Centre. 2016. BC Species and Ecosystems Explorer. B.C. Ministry of Environment, Victoria B.C. (last assessed April 2016)
Beever, J.E. 1999. Studies of Fissidens (Bryophyta: Musci) in New Zealand: a synopsis and key to taxa. New Zealand Journal of Botany 37: 659–670.
Bryoquel (PDF; 136 KB) (Disponible en Francais seulement). 2015. Liste des taxons rare au Qc-Labr. (up-to-date listing of species ranks resulting from ongoing, consultative information gathering and refinement; Centre de données sur le patrimoine naturel du Québec rankings, which are official, are co-ordinated with those on Bryoquel). (accessed August 2015)
Buck, W.R. and H.A. Crum. 1978. Entodon schleicheri new to North America. The Bryologist 81:429–432.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada) 2005. COSEWIC assessment and status report on the pygmy pocket moss Fissidens exilis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 18 pp.
Crum, H.A. 2001. Structural Diversity of Bryophytes. University of Michigan Herbarium, Ann Arbor, 379 pp.
Crum, H.A. and L.E. Anderson. 1981. Mosses of Eastern North America. Columbia University Press, New York. 1328 pp.
During, H.J. 1979. Life strategies in bryophytes : a preliminary review. Lindbergia 5 :2–18.
Ecological Stratification Working Group. 1996. A National Ecological Framework for Canada. Agriculture and Agri-Food Canada, Research Branch, Centre for Land and Biological Resources Research, and Environment Canada, State of the Environment Directorate, Ecozone Analysis Branch, Ottawa/Hull. Report and national map at 1:7,500,000 scale.
Environment Canada. 2015. Management Plan for the Pygmy Pocket Moss (Fissidens exilis) in Canada [Draft]. Species at Risk Act Management Plan Series. Environment Canada, Ottawa. iv + 16 pp. (accessed August 2015)
Faubert J. 2007. Catalogue des bryophytes du Québec et du Labrador. Provancheria N°30, Mémoire de l’Herbier Louis-Marie, Université Laval, 138 pp.
Faubert, J. 2013. Flore des bryophytes du Québec-Labrador. Volume 2 : Mousses, première partie. Société québécoise de bryologie, Saint-Valérien, Québec, xiv + 402 pp.
Furness, S.B. and R.H. Hall. 1981. An explanation of the intermittent occurrence of Phycomitrium sphaericum (Hedw.) Brid. Journal of Bryology 11:733–742.
Glime, Janice M. 2007 Bryophyte Ecology. Volume 1. Physiological Ecology. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. (accessed January 14 2015)
Goffinet, B., J.M. Budke, and L.C. Newman. 2011. Micromitraceae: A new family of highly reduced mosses. Taxon 60:1245–1254.
Hallingbäck, T. and N. Hodgetts (compilers). 2000. Mosses, Liverworts and Hornworts. Status Survey and Conservation Action Plan for Bryophytes. International Union for Conservation of Natuer and Natural Resources (IUCN) Bryophyte Specialist Group, Gland, Switzerland and Cambridge, UK. x + 106 pp.
Hassel, K. and L. Söderström. 1998. The presence of Pogonatum dentatum (Brid.) Brid. In roadside diaspore banks in Sweden. Lindbergia 23:113–118.
IUCN-CMP (International Union for Conservation of Nature and Conservation Measures Partnership). 2006. IUCN – CMP unified classification of direct threats, ver. 1.0 – June 2006. Gland, Switzerland. 17 pp. (Accessed January 14 2015)
Iwatsuki, Z. and A. Noguchi. 1973. Index muscorum Japonicarum. Journal of the Hattori Botanical Laboratory 37:299–418.
McIntosh, T.T. 1989. Bryophyte records from the semiarid steppe of northwestern North America, including four species new to North America. The Bryologist 92:356–362.
Missouri Botanical Garden. 2014. Tropicos’ details and synonyms for Fissidens exilis. (accessed December 31, 2014).
Molnar, L. 1975. New distribution data on two mosses, Fissidens exilis and Thuidium pygmaeum, in Québec. The Canadian Field Naturalist 89: 324–325.
NatureServe. 2014. Arlington, VA. U.S.A. (Accessed December 29 2014).
NHIC (Natural Heritage Information Centre). 2014. List of species ranks. (Accessed December 29 2014).
Porley, R. 2008. Arable Bryophytes: A Field Guide to the Mosses, Liverworts and Hornworts of Cultivated Land in Britain and Ireland. WILDGuides Ltd., Hampshire. 140 pp.
Pursell, R.A. 2007. Fissidentaceae, pg. in Flora of North America Editorial Committee, Eds. Flora of North America North of Mexico. Vol 27. Oxford University Press, New York and Oxford. 713 pp.
Risk, A.C. 2002. The distribution, commonness and habitat characteristics of Fissidens hyalinus in the United States. The Bryologist 105:43–47.
Sackett, T.E., S.M. Smith, and N. Basiliko. 2013. Indirect and direct effects of exotic earthworms on soil nutrient and carbon pools in North American temperate forests. Soil Biology & Biochemistry 57:459–467.
Schofield, W.B. 1981. Ecological significance of morphological characters in the moss gametophyte. The Bryologist 84:149–165.
Schofield, W.B. 1988. Bryophyte disjunctions in the Northern Hemisphere: Europe and North America. Botanical Journal of the Linnean Society (1988). 98:211–224.
Slack, N.G. 1990. Bryophytes and ecological niche theory. Botanical Journal of the Linnean Society 104:187–213.
Smith, A.J.E. 1978. The Moss Flora of Britain and Ireland. Cambridge University Press, Cambridge. 706 pp.
Smith, A.J.E. 2004. The Moss Flora of Britain and Ireland (2nd edition). Cambridge University Press, Cambridge.1012 pp.
SQB (Societe québécoise de bryologie) 2014. Online « fil de nouvelles » (accessed January 19 2015).
Steere, W.C. 1950. Notes on Fissidens. II. The discovery of Fissidens exilis in North America. The Bryologist 53: 131–136.
Vanderpoorten, A. and P. Engels. 2002. The effects of environmental variation on bryophtyes at a regional scale. Ecography 25:513–522.
Jennifer Doubt is Bryologist and Curator of Botany at the National Herbarium of Canada, Canadian Museum of Nature.
See Table 1.
| Threat Impact | Threat Impact (descriptions) | Level 1 Threat Impact Counts: high range |
Level 1 Threat Impact Counts: low range |
|---|---|---|---|
| A | Very High | 0 | 0 |
| B | High | 0 | 0 |
| C | Medium | 0 | 0 |
| D | Low | 0 | 0 |
| - | Calculated Overall Threat Impact: | - | - |
| # | Threat | Impact (calculated |
Impact (description) |
Scope (next 10 Yrs) |
Severity (10 Yrs or 3 Gen.) |
Timing | Comments |
|---|---|---|---|---|---|---|---|
| 1 | Residential and commercial development | - | - | - | - | - | - |
| 1.1 | Housing and urban areas | - | - | - | - | - | Not a threat. |
| 1.2 | Commercial and industrial areas | - | - | - | - | - | Not a threat. (Line 542): residential and commercial development could be a threat but there is no known development in the plans right now. |
| 1.3 | Tourism and recreation areas | - | - | - | - | - | Not a threat. Same as 1.2 (above): could be a threat if planned, but we have no evidence of any development right now. - construction of trails could be a benefit for this species. |
| 2 | Agriculture and aquaculture | - | Unknown | Unknown | Unknown | Unknown | - |
| 2.1 | Annual and perennial non-timber crops | - | Unknown | Unknown | Unknown | Unknown | Not a threat. (Line 512): Studies in Europe find management of agriculture could be both beneficial and a threat. In Canada, the species is not found in cultivated fields (only in Europe). |
| 2.2 | Wood and pulp plantations | - | - | - | - | - | Not a threat - could be a benefit. |
| 2.3 | Livestock farming and ranching | - | - | - | - | - | Not a threat. |
| 2.4 | Marine and freshwater aquaculture | - | - | - | - | - | Not a threat. |
| 3 | Energy production and mining | - | - | - | - | - | Not a threat |
| 3.1 | Oil and gas drilling | - | - | - | - | - | - |
| 3.2 | Mining and quarrying | - | - | - | - | - | Most NS sites are associated with karst and basalt areas, which are, in general, targeted for resource extraction. However, no specific mining plans for sites where Pygmy Pocket Moss occurs are known. |
| 3.3 | Renewable energy | - | - | - | - | - | - |
| 4 | Transportation and service corridors | - | Unknown | Unknown | Unknown | Unknown | - |
| 4.1 | Roads and railroads | - | Unknown | Unknown | Unknown | Unknown | (Line 537): roads could be detrimental (i.e. road salts) but we just don't have evidence of it. |
| 4.2 | Utility and service lines | - | Unknown | Unknown | Unknown | Unknown | Same as 4.2 (above): utility lines could be a threat but unknown. |
| 4.3 | Shipping lanes | - | - | - | - | - | Not a threat. |
| 4.4 | Flight paths | - | - | - | - | - | Not a threat. |
| 5 | Biological resource use | - | Unknown | Unknown | Unknown | Unknown | - |
| 5.1 | Hunting and collecting terrestrial animals | - | - | - | - | - | Not a threat. |
| 5.2 | Gathering terrestrial plants | - | - | - | - | - | Not a threat. |
| 5.3 | Logging and wood harvesting | - | Unknown | Unknown | Unknown | Unknown | (Line 528): both positive and negative effects. Forestry would disturb soil but have potential to disperse mosses as well. There are so many unknowns; habitat could be created but the site might not be suitable for a few years afterwards. |
| 5.4 | Fishing and harvesting aquatic resources | - | - | - | - | - | - |
| 6 | Human intrusions and disturbance | - | Unknown | Unknown | Unknown | High (Continuing) | - |
| 6.1 | Recreational activities | - | Unknown | Unknown | Unknown | High (Continuing) | Species like some disturbances but not much. - there is no evidence that trampling wiped out any subpopulation - species can be found along trails (BC = 1 site; ON = 1 site; QC = possible 2 sites) |
| 6.2 | War, civil unrest and military exercises | - | - | - | - | - | - |
| 6.3 | Work and other activities | - | Unknown | Unknown | Unknown | High (Continuing) | - |
| 7 | Natural system modifications | - | Unknown | Unknown | Unknown | High (Continuing) | - |
| 7.1 | Fire and fire suppression | - | - | - | - | - | Not a threat. Many of the sites where the species occur have fire suppression. |
| 7.2 | Dams and water management/use | - | Unknown | Unknown | Unknown | High (Continuing) | Disturbances from dikes create habitat, but how they are managed determines if it's a threat or not. |
| 7.3 | Other ecosystem modifications | - | - | - | - | - | Gravelling of trails could be a problem, but unknown if there are any major trail gravelling programs planned. |
| 8 | Invasive and other problematic species and genes | - | Unknown | Unknown | Unknown | Unknown | - |
| 8.1 | Invasive non-native/alien species | - | Unknown | Unknown | Unknown | High (Continuing) | Nothing has been reported. |
| 8.2 | Problematic native species | - | Unknown | Unknown | Unknown | High (Continuing) | - |
| 8.3 | Introduced genetic material | - | - | - | - | - | - |
| 9 | Pollution | - | Unknown | Unknown | Unknown | Moderate (Possibly in the short term, < 10 yrs) | General pollution concerns near agricultural fields and roads that would change the soil content. |
| 9.1 | Household sewage and urban waste water | - | - | - | - | - | - |
| 9.2 | Industrial and military effluents | - | - | - | - | - | - |
| 9.3 | Agricultural and forestry effluents | - | Unknown | Unknown | Unknown | Moderate (Possibly in the short term, < 10 yrs) | - |
| 9.4 | Garbage and solid waste | - | - | - | - | - | - |
| 9.5 | Air-borne pollutants | - | - | - | - | - | - |
| 9.6 | Excess energy | - | - | - | - | - | - |
| 10 | Geological events | - | - | - | - | - | - |
| 10.1 | Volcanoes | - | - | - | - | - | - |
| 10.2 | Earthquakes/ tsunamis | - | - | - | - | - | - |
| 10.3 | Avalanches/landslides | - | - | - | - | - | Could be a potential benefit. |
| 11 | Climate change and severe weather | - | Unknown | Unknown | Unknown | Unknown | - |
| 11.1 | Habitat shifting and alteration | - | - | - | - | - | - |
| 11.2 | Droughts | - | Unknown | Unknown | Unknown | Unknown | Might be a threat. - droughts in the longer term could be an issue, but not in the next decade. |
| 11.3 | Temperature extremes | - | - | - | - | - | - |
| 11.4 | Storms and flooding | - | Unknown | Unknown | Unknown | Unknown | - |