COSEWIC Assessment and Status Report on the Western Bumble Bee Bombus occidentalis in Canada - 2014
List of Figures
- Figure 1. Female (worker) Western Bumble Bee occidentalis subspecies (note there is more than one form, and this photo is considered the typical form). Photo by Sheila Colla. Specimen housed at the Packer Bee Collection, York University, Toronto.
- Figure 2. Male Western Bumble Bee occidentalis subspecies (note there is more than one form, and this photo is considered the typical form). Photo by Sheila Colla. Specimen housed at the Packer Bee Collection, York University, Toronto.
- Figure 3. Female (worker) Western Bumble Bee mckayi subspecies (Bombus occidentalis mckayi). Photograph by Cory Sheffield. Specimen housed at the Royal Saskatchewan Museum, Regina, Saskatchewan.
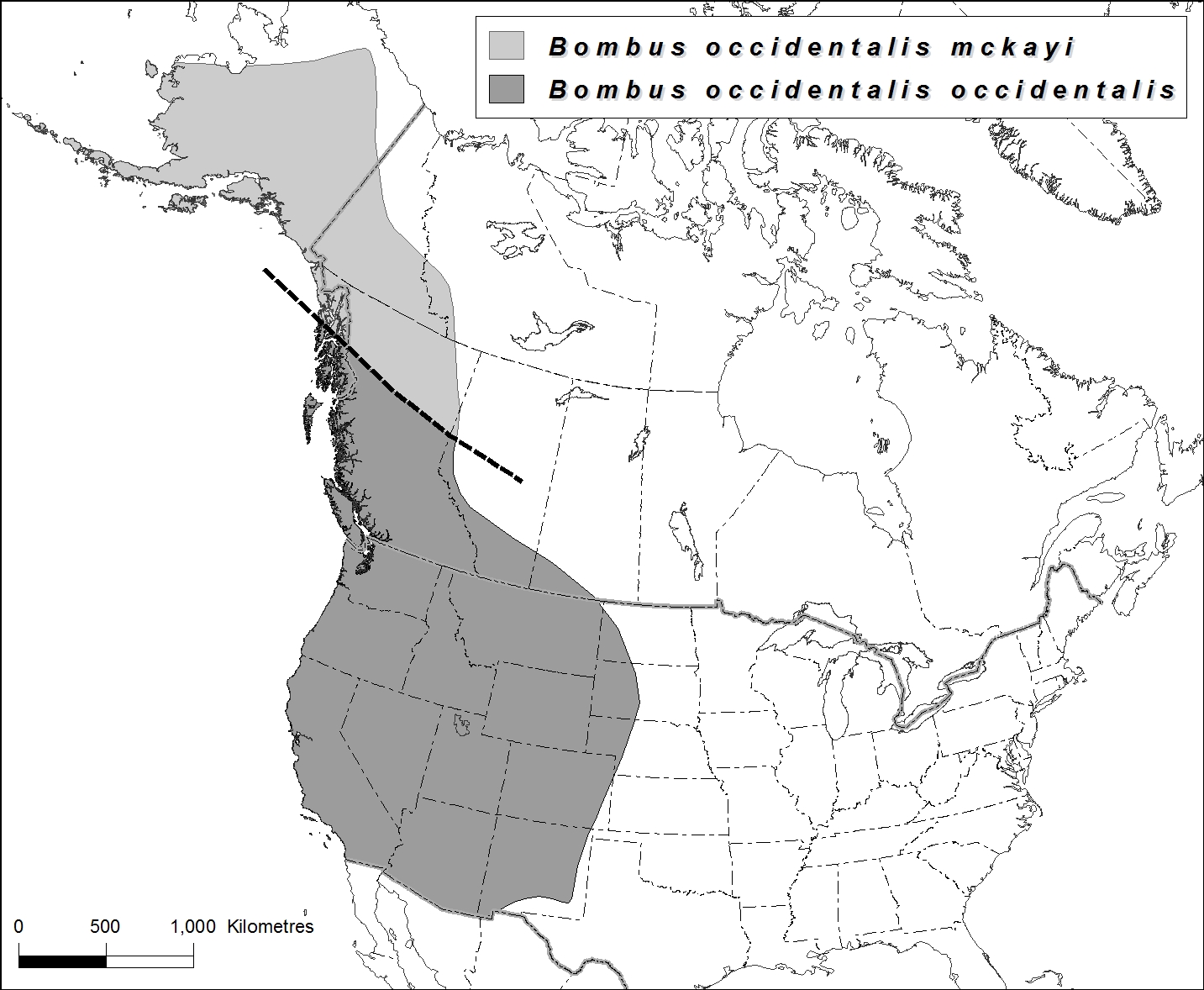
- Figure 4. Global range map of Bombus occidentalis showing the distribution of both subspecies, B. o. occidentalis (below line) and B. o. mckayi (above line). Note that the southern boundary of B. o. mckayi and the northern boundary of B. o. occidentalis are not well-defined. Map created using data from Sheffield et al. 2013, and Sheffield et al. in prep.
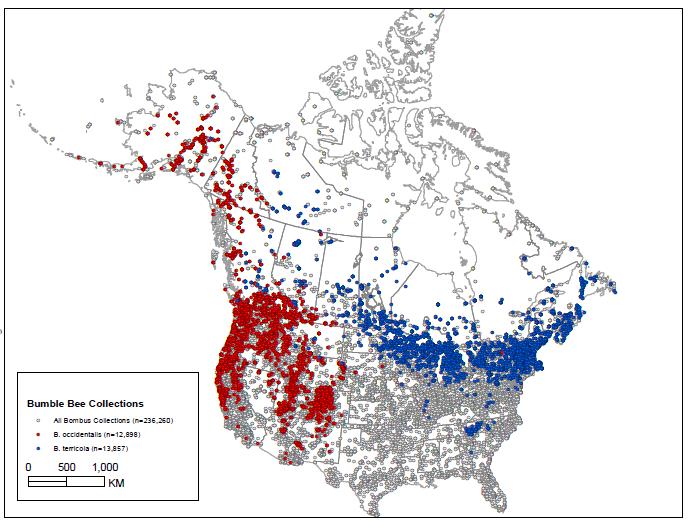
- Figure 5. Bumble bee collection points (all dots total 236,260) for North America from 1892 - 2012. Red dots = Western Bumble Bee (Bombus occidentalis (12,898 records) both subspecies); blue dots = closely related Yellow-banded Bumble Bee (B.terricola) (13,857 records) including regions of overlap (see Wildlife Species Description and Significance). Note there have been taxonomic issues with B. occidentalis and B. terricola, and it is not guaranteed that all specimens used in these maps are correctly identified. The one eastern specimen of B. occidentalis is presumed to be an error. These maps should be used as general range maps and outliers further investigated. No data exists for areas without points. More than 70 individuals and institutions contributed to the dataset, and are listed at: www.leifrichardson.org/bbna.html. Map compiled by Leif Richardson. Specimens compiled in a dataset for Williams et al. 2014.
- Figure 6. Spatial distribution of sampling records of Western Bumble Bee in Canada from 1882 - 2010. Each circle is proportional to the number of observations recorded at that site between 1882 and 2010, inclusive (including non-independent samples and sites where multiple observations were recorded within a year). Data are mapped on a 50 x 50 km grid. Grey squares show records prior to 1996 (128 squares); yellow squares show sites collected pre/post 1996 (27 squares); green squares show collections after 1996 (13 squares) (n= 1706 specimens). Data is compiled from CBIF and contributors listed in the Acknowledgements section. This map is not possible to update.
- Figure 7. Relative abundance (RA) of Western Bumble Bee across southern Alberta during two time periods (combined sample sizes shown in parentheses). All data from southern Alberta are included (i.e., surveys of 2000 and 2010, as well as the additional study sites sampled at other times). Except for two sites where Western Bumble Bee did not occur in either period (Innisfail and Trunk Rd.), RA declined from 2000 to 2010. Habitats in which the species was collected are: Calgary (urban), Clarseholm (rural), Barrier Lk. (natural), Drumheller (edge of species’ range), Fortress Mt. (higher elevation), Coleman (rural), and Innisfail (out of subspecies’ range) and Trunk Rd. (out of subspecies’ range).
- Figure 8. Predicted probability of collecting Western Bumble Bee, based on data from 14 sites in southern Alberta sampled between 1985 and 2010. Points indicate average probabilities (with 95% confidence interval) from sites sampled during a given year. Dashed line is a quadratic regression fit; between 2010 and 2018, the line represents projected values from the regression equation.
- Figure 9. Relative abundance (RA) of Western Bumble Bee (WBB) based on all databased Bombus records in Canada (1882 – 2011). The left Y-axis (shaded portions of bars) indicates WBB specimens and the right Y-axis (triangles) represents the proportion of WBB specimens by ten-year intervals. Linear regression was used to examine trends in RA in WBB across ten-year intervals: the line represents a best fit of the data. See also Table 3. Graphs generated using Minitab ® software.
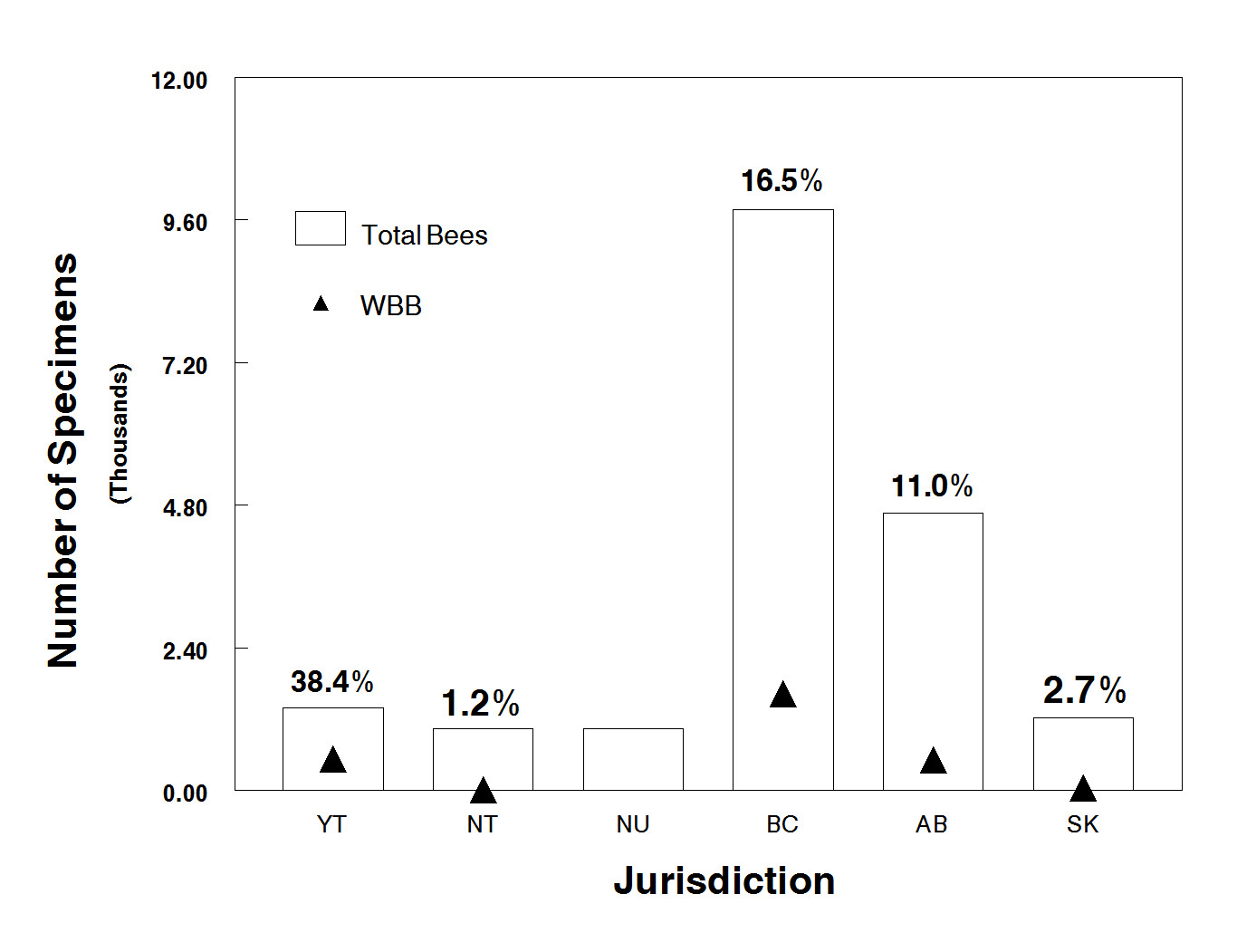
- Figure 10. Total number of databased bumble bee specimens in Canada (1882 – 2011) from each province and territory; triangles represent the number of Western Bumble Bee (WBB) specimens. Values above each bar represent the percentage of specimens within the collection which are WBB. See also Table 3. Graphs generated using Minitab ® software.
List of Tables
- Table 1. Summary statistics related to area and elevation of the distribution of Western Bumble Bee across four sampling intervals spanning 1882 and 2010. See also Figure 6.
- Table 2. Recent (since 2002) surveys targeting bumble bees within the range of Western Bumble Bee.
- Table 3. Relative abundance (RA) of Western Bumble Bee compared with databased Bombus collection data (1882 – 2011) in Canada. More than 70 individuals and institutions contributed to the dataset. Specimens compiled in a dataset for Williams et al. 2014. RA of Western Bumble Bee is given in ten-year intervals (graphical representation in Figure 9 and Figure 10). Manitoba records are not considered natural populations. Most BC records are from the southern third of the province and considered subspecies occidentalis.
- Table 4. Threat classification table for Western Bumble Bee subspecies occidentalis (Bombus occidentalis occidentalis) across its geographic range in Canada and based on the IUCN-CMP (World Conservation Union–Conservation Measures Partnership) unified threats classification system. For a detailed description of the threat classification system, see the Conservation Measures Partnership website (CMP 2006). For information on how the values are assigned, see Master et al. (2009). Threat calculator completed by J. Heron and C. Sheffield with input from D. Fraser, S. Colla and L. Richardson.

Committee on the Status of Endangered Wildlife in Canada (COSEWIC) status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2014. COSEWIC assessment and status report on the Western Bumble Bee Bombus occidentalis, the occidentalis subspecies (Bombus occidentalis occidentalis) and the mckayi subspecies (Bombus occidentalis mckayi) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 52 pp. (Species at Risk Public Registry website).
COSEWIC would like to acknowledge Sheila Colla, Michael Otterstatter, Cory Sheffield and Leif Richardson for writing the status report on the Western Bumble Bee (Bombus occidentalis) in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Jennifer Heron, Co-chair of the COSEWIC Arthropods Specialist Subcommittee.
For additional copies contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment Canada
Ottawa, ON
K1A 0H3
Tel.: 819-953-3215
Fax: 819-994-3684
COSEWIC E-mail
COSEWIC web site
Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Bourdon de l'Ouest (Bombus occidentalis) de la sous-espèce occidentalis (Bombus occidentalis occidentalis) et la sous-espèce mckayi (Bombus occidentalis mckayi) au Canada.
Cover illustration/photo:
Western Bumble Bee - Cover photograph by David Inouye, Western Bumble Bee worker robbing an Ipomopsis flower.
© Her Majesty the Queen in Right of Canada, 2014.
Catalogue No. CW69-14/694-2014E-PDF
ISBN 978-1-100-22392-6

Assessment Summary - May 2014
Assessment Summary - May 2014
Western Bumble Bee, Bombus occidentalis Greene, is one of five North American members of the subgenus Bombus sensu stricto. It is a medium-sized (1 – 2 cm) bumble bee with a short head. The abdomen is colour variable, but all individuals have a transverse band of yellow hair on the thorax in front of the wing bases, and the tip of the abdomen is almost always white.
Bumble bee taxonomy is widely debated, including the taxonomic history of Western Bumble Bee. The species was once considered synonymous with Yellow-banded Bumble Bee; however, recent genetic work confirms these two species as separate. Additional recent taxonomic work further splits Western Bumble Bee into two separate subspecies: Bombus occidentalis occidentalis and Bombus occidentalis, based on genetic, morphological and distributional information.
Western Bumble Bee ranges throughout most of western North America. Subspecies occidentalis, ranges from central California north to northern British Columbia, and east into southern Saskatchewan and South Dakota. Subspecies mckayi ranges from central-northern British Columbia northward into the Yukon, Northwest Territories and Alaska.
Western Bumble Bee lives in a diverse range of habitats, including mixed woodlands, farmlands, urban areas, montane meadows and into the western edge of the prairie grasslands. Subspecies mckayi is seemingly restricted to the Boreal and Cordilleran Ecological Areas. Western Bumble Bee has been recorded gathering pollen and nectar from the flowers of a variety of plant genera. Like many bumble bees, it typically nests underground in abandoned rodent burrows or within hollows in decaying wood.
Western Bumble Bee has an annual life cycle. Mated queens (colony founders) emerge from wintering sites in the spring and search for potential nest sites. Once a nest site is chosen, the queen then forages for pollen and nectar, returning to the nest site to lay eggs which will eventually produce a brood of workers. Workers emerge and take over nest care, pollen and nectar foraging. In late summer, males and new queens are produced. These reproductive individuals leave the colony, mate, and only the mated queens enter hibernation while all other castes, including the old queen, perish at the onset of colder temperatures.
Subspecies occidentalis continues to be recorded throughout most of its historical range in Canada, although at fewer sites and with lesser abundance: relative abundance data within the past ten years suggests a probable decline of more than 30%. In the regions in Canada where subspecies occidentalis has been most studied (i.e., southern BC and AB), significant declines in relative abundance have occurred at all surveyed sites within the last three decades. Subspecies mckayi is more commonly observed, and with a constant abundance, although there is little historical data for this subspecies from which to derive trends.
Possible threats to subspecies occidentalis may include the transfer of pathogens from managed bees used for greenhouse pollination that have escaped. Additional regional threats include agricultural pesticide and chemical use, and habitat loss.
There is currently no legal protection in Canada for either subspecies of Western Bumble Bee. All members of subgenus Bombus appear to be globally declining.
Bombus occidentalis occidentalis
Demographic Information
Extent and Occupancy Information
| Population | N Mature Individuals |
|---|---|
| subspecies occidentalis | Unknown |
| Total | Unknown |
Quantitative Analysis
Threats (actual or imminent, to populations or habitats)
Subspecies occidentalis has among the highest parasite loads (particularly the microsporidian Nosema bombi) of any bumble bee in North America. Ongoing threats to the subspecies, particularly within the southern portions of its range, include pathogen spillover and the transmission of disease from exotic and commercially managed bumble bee colonies introduced for greenhouse pollination, pesticide use (including neonicotinoid compounds), and more intensive agricultural land use practices and overall habitat change in some parts of its range.
Rescue Effect (immigration from outside Canada)
Status History
COSEWIC: Designated Threatened in May 2014.
Status and Reasons for Designation:
Applicability of Criteria
Bombus occidentalis mckayi
Demographic Information
Extent and Occupancy Information
| Population | N Mature Individuals |
|---|---|
| subspecies mckayi | Unknown |
| Total | Unknown |
Quantitative Analysis
Threats (actual or imminent, to populations or habitats)
Recent studies in Alaska suggest that this subspecies has among the highest parasite loads (particularly the microsporidian Nosema bombi) of any bumble bee species in North America. Other potential threats include the unknown transmission of disease from exotic bumble bee species introduced for pollination in greenhouses (ongoing in the Yukon), pesticide use (including neonicotinoid compounds), and habitat change.
Rescue Effect (immigration from outside Canada)
Status History
COSEWIC: Designated Special concern in May 2014.
Status and Reasons for Designation:
Applicability of Criteria

The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
A species, subspecies, variety, or geographically or genetically distinct population of animal, plant or other organism, other than a bacterium or virus, that is wild by nature and is either native to Canada or has extended its range into Canada without human intervention and has been present in Canada for at least 50 years.
The Canadian Wildlife Service, Environment Canada, provides full administrative and financial support to the COSEWIC Secretariat.
The genus Bombus includes approximately 250 species found primarily in temperate regions of North America, Central America, South America, Europe and Asia. Western Bumble Bee (Bombus occidentalis) belongs to the subgenus Bombus sensu stricto, one of 15 subgenera of bumble bees recognized globally. In North America, subgenus Bombus contains four additional species: Bombus affinis Cresson, B. cryptarum (Fabricius), B. franklini Frison, and B. terricola Kirby.
Bumble bees are primarily identified using colour patterns at the adult life stage, although in many species colour patterns are variable. This variation has contributed to historical and recent taxonomic difficulties with many bumble bee species, including Western Bumble Bee.
Bombus occidentalis was first described as a distinct species by Greene (1858), and subsequently recognized as conspecific with B. terricola at the species’ level (e.g., Milliron 1971; Cameron et al. 2007 [though Milliron considered it a distinct subspecies]), and by other authors as a distinct species (e.g., Stephen 1957; Thorp et al. 1983).
Cameron et al. (2007) recently compared DNA sequences from the 16S mitochondrial gene and found the two taxa (i.e., B. occidentalis and B. terricola) to be conspecific. However, Williams et al. (2012) reported that mitochondrial cytochrome c oxidase (COI) gene sequences (i.e., DNA barcodes) were sufficiently different to consider B. occidentalis a distinct species. These results support those of Bertsch et al. (2010), with an overall COI sequence divergence of approximately 5% between the two species. Furthermore, Owen and Whidden (2013) found consistent morphological and molecular characters supporting two distinct taxa. Thus, B. occidentalis is considered distinct and separate from B. terricola.
Williams et al. (2012) also found divergence in COI sequences within samples of Western Bumble Bee correlated with geography and recognized two subspecies: B. o. occidentalis and B. o. mckayi. For further information on the molecular phylogeny by Williams et al. (2012) which verified the division of Western Bumble Bee into two distinct genotypes. Current research based on molecular, morphological, and distributional data supports this conclusion (Sheffield et al. 2013). In recognition of this, B. occidentalis is presented in this status report as two subspecies: subspecies occidentalis and subspecies mckayi.
Bumble bees are holometabolous insects. They have four developmental stages (e.g., egg, larvae, pupae, and adult) and are primitively eusocial with three adult forms or castes: the queen (reproductive female), female workers (non-reproductive) and males. Western Bumble Bee adults are highly colour variable, primarily on the scutellum (the hard plate on the dorsal side of the bumble bee thorax, usually where the wings attach), and on the second and third abdominal segments, which can range from black to yellow (details below).
Females: Queens and workers show a similar range in colour patterns, although they differ in size (queen length 1.6-1.9 cm, worker length 1.1-1.3 cm). The head is entirely black (Figure 1) and the malar space (i.e. area between the lower edge of the compound eye and the base of the mandible) is short (i.e. approximately as long as broad). All individuals have a transverse band of yellow hair anterior to the wing bases. Abdominal colouration is variable, ranging from all black on the first four segments (Figure 1) to individuals having the third (and sometimes part of the second) segment with yellow. Most individuals have white (more rarely yellowish) hairs on the apical terga (i.e. abdominal tip). Subspecies occidentalis is the most colour variable. Subspecies mckayi consistently has yellow hairs on the third abdominal segment (Figure 3).
Figure 1. Female (worker) Western Bumble Bee occidentalis subspecies (note there is more than one form, and this photo is considered the typical form). Photo by Sheila Colla. Specimen housed at the Packer Bee Collection, York University, Toronto.

Photo: © S. Colla
Long description for Figure 1
Photo of a specimen of a worker Western Bumble Bee in the occidentalis subspecies. Western Bumble Bee workers and queens show a similar range of colour patterns. The head is entirely black and the malar space is short (approximately as long as broad). There is a transverse band of yellow hair anterior to the wing bases, and the abdomen is black on the first four segments. Most individuals have white (more rarely yellowish) hairs on the abdominal tip.
Males: Similar in appearance to females, with variability in colour pattern (Figure 2), and intermediate in body size (1-2 cm length). Male-specific colouring includes the pale yellow hairs intermixed with black hairs on the face. The malar space is short.
Figure 2. Male Western Bumble Bee occidentalis subspecies (note there is more than one form, and this photo is considered the typical form). Photo by Sheila Colla. Specimen housed at the Packer Bee Collection, York University, Toronto.

Long description for Figure 2
Photo of a specimen of a male Western Bumble Bee in the occidentalis subspecies. Males are similar in appearance to females (see report text), with variable colour pattern. Body size is 1 to 2 centimetres in length. Male-specific colouring includes pale yellow hairs intermixed with black hairs on the face. The malar space is short.
Figure 3. Female (worker) Western Bumble Bee mckayi subspecies (Bombus occidentalis). Photograph by Cory Sheffield. Specimen housed at the Royal Saskatchewan Museum, Regina, Saskatchewan.

Long description for Figure 3
Photo of a specimen of a worker Western Bumble Bee in the mckayi subspecies. Western Bumble Bee workers and queens show a similar range of colour patterns: The head is entirely black and the malar space is short (approximately as long as broad). There is a transverse band of yellow hair anterior to the wing bases, and the abdomen is black on the first four segments. Most individuals have white (more rarely yellowish) hairs on the abdominal tip. Subspecies mckayi (in photo) consistently has yellow hairs on the third abdominal segment.
A full account of the different adult colour variants of Western Bumble Bee is shown in Koch et al. (2012) and Williams et al. (2014). Bumble bees (and this is true for most bees) are not typically observed or identified by their immature life stages (i.e., egg, larvae, pupae). These stages are largely unobserved for most bees and the nesting biology and immature stages of most bee species have not been studied. General accounts of the life stages of bees are found in Stephen et al. (1969) and Michener (2007). Stephen and Koontz (1973a, 1973b) provided more discussion specific to the larval stages of bumble bees. The immature life stages of Western Bumble Bee are not described in the literature, and species-specific characters are largely lacking within genera.
Genetic diversity and population stability in US populations of Western Bumble Bee (applicable to subspecies occidentalis) were recently studied using 8-11 microsatellite loci (Cameron et al. 2011). Results suggest this subspecies has low among-subpopulation genetic diversity (n=93, loci = 8, total HE=0.584) and may be susceptible to steeper population declines than other bumble bee species studied due to increased inbreeding potential and genetic drift in small effective populations.
Many specimens of Western Bumble Bee from throughout its natural range have had DNA barcodes (i.e., COI) sequenced, which are available on the Barcode of Life Data Systems (BOLD) (see Williams et al. 2012). These sequences were used to support the designation of two subspecies: B. o. mckayi and B. o. occidentalis (Williams et al. 2012). Further morphological and biogeographical data supporting the two subspecies of Western Bumble Bee recognized by Williams et al. (2012) were presented by Sheffield et al. (2013); these data are also currently under preparation for publication (Sheffield et al. in prep.).
Two designatable units are proposed based on the subspecies recognized by Williams et al. (2012). In Canada, subspecies occidentalis occurs within the COSEWIC National Ecological Areas - Pacific, Southern Mountain, Prairie, and the southernmost part of the Northern Mountain (COSEWIC 2010). Subspecies mckayi occurs in the COSEWIC Northern Mountain National Ecological Area, though may extend into the far northwestern area of the Boreal National Ecological Area. The two subspecies appear to have a morphological, molecular and ecological division between 55°N and 60°N (Sheffield pers. data) (see Distribution) supporting two designatable units.
Prior to its decline, Western Bumble Bee was considered one of the most commonly observed bumble bees within western Canada (Hobbs 1968; Richards 1978). Like all bees, Western Bumble Bee is ecologically significant, providing pollination services to various native plant species throughout its range (see Ascher and Pickering 2013 for plant list). Bumble bees are active throughout the growing season, flying during inclement weather conditions often not suitable for most other insects (Heinrich 2004). As pollinators, bees facilitate plant reproduction, which ultimately provides shelter and food for other animals, as well as sustainability of native ecosystems (Goulson 2010; Heinrich 2004). Extensive background on the ecosystem goods and services of bumble bees is written in Goulson (2010) and Heinrich (2004).
Managed bumble bee colonies are shipped throughout the globe and used to pollinate greenhouse crops (e.g., cucumbers, sweet peppers, and tomatoes) (Patten et al. 1993; Macfarlane and Patten 1997). These crops are grown and flower year round and require continuous pollination. Western Bumble Bee was once available as a managed pollinator for greenhouse pollination in North America before problems rearing the species in captivity arose in the early 2000s (e.g., Whittington and Winston 2003). Up to this time, commercial bumble bee producers (i.e. Koppert® and BioBest®) reared Common Eastern Bumble Bee (B. impatiens) for pollination in eastern North America, and Western Bumble Bee for the west. At present, only Common Eastern Bumble Bee is available for greenhouse pollination in North America, and no restrictions prevent the importation of Common Eastern Bumble Bee to greenhouses for pollination services throughout Canada; it is used in BC, where it has established feral populations (Ratti and Colla 2010). Common Eastern Bumble Bee is suspected to be shipped for use in private greenhouses in Northwest Territories (Carriere pers. comm. 2014). Some states, such as Oregon, have banned the importation of extralimital species of Bombus (i.e. Common Eastern Bumble Bee).
Bees provide pollination services for wild fruit production and natural ecosystem sustainability. See Ascher and Pickering (2013) for a partial list of plant genera/species visited by Western Bumble Bee. Bees are also of high cultural significance to Aboriginal groups. Some of the noted plants visited by Western Bumble Bee that are of importance to First Nations people include species in the aster family (Asteraceae; e.g., Helianthus), honeysuckle family (Caprifoliaceae), blueberry family (Ericaceae; Vaccinium spp.), rose family (Rosaceae; Rosa spp., Rubus spp.), and many others (Turner 1975).
The subgenus Bombus sensu stricto in North America is represented by five species (just over 10% of the 46 species; Williams et al. 2014). For reasons that are not clear (though highly overlapping areas of high urbanization/agriculture) the subgenus shows a higher inherent risk of vulnerability than any other subgenus on the continent -- four of the species are currently of concern. Two of the species are considered at risk: the Rusty-patched Bumble Bee (Bombus affinis Cresson) was assessed as Endangered by COSEWIC (2010), and Franklin’s Bumble Bee (B. franklini Frison; found in USA only) could possibly be extinct as it has not been seen since 2008. The Yellow-banded Bumble Bee (B. terricola Kirby) is currently having a COSEWIC report prepared. In addition, Western Bumble Bee is host to two cuckoo bumble bees: Gypsy Cuckoo Bumble Bee (B. bohemicus Cresson) and Suckley’s Cuckoo Bumble Bee (B. suckleyi Greene), the former of which appears to have undergone significant declines.
Western Bumble Bee ranges in western North America (Figure 4 and Figure 5). Subspecies occidentalis ranges from central California (CA) north to central British Columbia (BC), east in Alberta (AB), southern Saskatchewan (SK), and south through North Dakota (ND), South Dakota (SD), Idaho (ID), Montana (MO), Wyoming (WY), Utah (UT), Colorado (CO), New Mexico (NM), northern Arizona (AZ) and Nevada (NE). Subspecies mckayi ranges from northern BC, north into Yukon (YT), western Northwest Territories (NT) and Alaska (AK). The species has been recorded from elevations at sea level to 1350 metres, although the elevations of collection sites vary with latitude: the species ranges at higher latitudes in southern parts of its global range.
Figure 4. Global range map of Bombus occidentalis showing the distribution of both subspecies, B. o. occidentalis (below line) and B. o. mckayi (above line). Note that the southern boundary of B. o. mckayi and the northern boundary of B. o. occidentalis are not well-defined. Map created using data from Sheffield et al. 2013, and Sheffield et al. in prep.
Map: © Environment Canada
Long description for Figure 4
Map of North America showing the range of the Western Bumble Bee subspecies occidentalis and mckayi. Subspecies occidentalis ranges from central California north to central British Columbia, eastern Alberta, southern Saskatchewan, and south through North Dakota, South Dakota, Idaho, Montana, Wyoming, Utah, Colorado, New Mexico, northern Arizona, and Nevada. Subspecies mckayi ranges from northern British Columbia, north into Yukon, western Northwest Territories, and Alaska.
Figure 5. Bumble bee collection points (all dots total 236,260) for North America from 1892 - 2012. Red dots = Western Bumble Bee (Bombus occidentalis (12,898 records) both subspecies); blue dots = closely related Yellow-banded Bumble Bee (B.terricola) (13,857 records) including regions of overlap (see Wildlife Species Description and Significance). Note there have been taxonomic issues with B. occidentalis and B. terricola, and it is not guaranteed that all specimens used in these maps are correctly identified. The one eastern specimen of B. occidentalis is presumed to be an error. These maps should be used as general range maps and outliers further investigated. No data exists for areas without points. More than 70 individuals and institutions contributed to the dataset, and are listed at: www.leifrichardson.org/bbna.html. Map compiled by Leif Richardson. Specimens compiled in a dataset for Williams et al. 2014.
Map: © Environment Canada
Long description for Figure 5
Map of North America showing the range (collection points) of Western Bumble Bee subspecies occidentalis and mckayi and the closely related terricola. Subspecies occidentalis ranges within western Canada throughout southern British Columbia to about 55 degrees north and west through southern Alberta and Saskatchewan. Subspecies mckayi ranges north of about 55 degrees north within British Columbia, Yukon, and likely western Northwest Territories.
Subspecies occidentalis ranges within western Canada throughout southern BC to ca 55°N and west through southern AB and SK. Subspecies mckayi ranges north of ca 55°N within BC, YK, and likely western NT (Sheffield et al. in prep) (Figure 4 and Figure 5).
Extent of occurrence (EO) for the two Western Bumble Bee subspecies are approximate: subspecies occidentalis is 720,170 km2 and subspecies mckayi is 623,837 km2. There are some minor uncertainties in delimiting the range interface between subspecies occidentalis and mckayi due to insufficient sampling in this critical range (Sheffield pers. comm. 2013).
An index of area of occupancy (IAO) is not possible to calculate. The population size and the widespread, scattered records across a large area suggest the IAO values for both subspecies to be > 2000 km2.
Museum and collection records for Western Bumble Bee date from 1882 to 2013. Bumble bee collection events for North America were plotted and represent both indirect and direct search effort for Western Bumble Bee (Figure 5, all dots total 236,260; all Bombus). Surveys have not been systematic or comprehensive over time and across the range of Western Bumble Bee; however, it is assumed if the species were present it would have been collected during bumble bee collection events. There are large areas and time periods with little data.
Search effort for bumble bees (as a group) in some areas of North America has been extensive in the past decade. Search effort in the past ten years within the range of Western Bumble Bee is partially summarized below by province and listed in Table 2.
Within the range of subspecies occidentalis, surveys from 2009 - 2013 recorded specimens from southern Vancouver Island, lower mainland, lower Fraser Valley, Okanagan region, and the Kootenay region (Table 2). Cumulative search effort in 2010 found only 17 observations over extensive surveys (> 575 hours; > 115 sites and > 800km) (Table 2).
In the past decade, there have been minimal surveys or bumble bee collection events from the central and northern parts of British Columbia. In 2013, surveys (minimum of 281 hours cumulative search effort over approximately 104 sites; additional sites and samples are still being processed) were conducted in BC (Sheffield et al. 2013; data being used in a manuscript in prep.). These surveys yielded a minimum of 6447 Bombus specimens (additional samples are still being processed), of which 115 specimens (or 1.7% of total examined) were Western Bumble Bee (Sheffield et al. in prep.), which was recorded at only 36 of at least 104 sites (sites are still being tallied) (Sheffield pers. data 2013).
During the 1960s and 1970s, researchers investigating bumble bee ecology placed thousands of artificial nests in natural areas in southern Alberta, attracting hundreds of Western Bumble Bee queens (Hobbs 1968; Richards 1978). During the spring of 1985 and 1986, researchers engaged in daily collecting of all local Bombus queens in the Calgary and Kananaskis Valley regions of southern Alberta, resulting in well over 200 queens each year, and a total of 126 Western Bumble Bee records (Owen 1988). During the summers of 1997-2000, researchers conducted regular sampling of bumble bee workers and males in the Kananaskis Valley and southern Alberta, collecting over 700 Western Bumble Bee records (Otterstatter and Whidden 2004). The later work in Kananaskis (1985-2000) represents at least 1500 person-hours of collecting.
Search effort in the past decade has been minimal. In 2007 surveys in southeastern AB recorded Western Bumble Bee in the Cypress Hills (ca. 60 queens; Sheffield pers. data). In 2013, surveys were conducted in four areas of the province. Specimens were collected, albeit uncommonly from near Dinosaur Provincial Park, Red Cliff (south of Medicine Hat) and Cypress Hills areas (Sheffield pers. data). No Western Bumble Bee was recorded in 2013 surveys around Edmonton (Sheffield pers. data), though this area is thought to be out of the range of this species.
There are few historic surveys or museum collections from SK. Recently subspecies occidentalis (i.e., 2012-2013) has been recorded in the southern third of SK. Extensive, summer-long insect surveys were conducted by the Royal Saskatchewan Museum in four geographic areas in southwestern SK (i.e., Grasslands National Park, Saskatchewan Landing Provincial Park, Great Sand Hills, Big Muddy Valley, and Cypress Hills Provincial Park). Subspecies occidentalis was detected in several of these sites, and in other parts of the province, though the samples are still being processed (Sheffield pers. comm.). The subspecies appears rather uncommon compared to other bumble bees (Sheffield pers. comm. 2013). Prior to these surveys, there are no historic records databased from these areas although this subspecies may not have been as common on the prairies as in western parts of its range.
Bumble bee surveys along major highways have been ongoing for the past three years, for a minimum for four days each year. Subspecies mckayi was present at many sites surveyed in 2009, 2010 and 2013 (Cannings pers. comm. 2013; Sheffield pers. comm. 2013). Subspecies mckayi is still considered common in adjacent Alaska, where in one study it accounted for over 30% of all bumble bees observed (Koch and Strange 2012). Prior to these surveys there are very few records from Yukon.
Very few records exist for subspecies mckayi in the NT, these from the extreme western part of the jurisdiction. There is only one record from pre-2011 (August 4, 1944 – exact location not given). The remaining eight specimens are from various sites on the South Nahanni River, collected on various dates in August 2011 (Stotyn and Tate 2012).
Western Bumble Bee requires habitat with abundant floral resources and suitable nesting sites. The species is a habitat generalist, inhabiting open coniferous, deciduous and mixed-wood forests, wet and dry meadows, montane meadows and prairie grasslands, meadows bordering riparian zones, and along roadsides in taiga adjacent to wooded areas, urban parks, gardens and agricultural areas, subalpine habitats and more isolated natural areas.
There are few studies on natural nesting preferences of Western Bumble Bee. In Alberta, Hobbs (1968) attracted 37 queens to nest in underground artificial nests. Three additional queens established colonies in aboveground nest boxes, indicating a preference for underground nests (supported by Kearns and Thomson 2001), but also suggesting some behavioural plasticity (Hobbs 1968). Similarly, Richards (1978) placed artificial nests in a variety of habitats in southeastern Alberta and found that 88 Western Bumble Bee queens (12% of 709 queens across 15 Bombus species) established nests. The best location for Western Bumble Bee was in underground nests connected to the surface with downward-sloping tunnels (open west-southwest) (Hobbs 1968). Queens exhibited a clear preference for wooded and transitional (wooded to meadow) nesting areas over open meadows (Richards 1978).
All bumble bees hibernate as solitary mated queens and usually overwinter by burrowing in loose soil or rotting trees (Benton 2006). Hobbs (1968) describes one Western Bumble Bee hibernaculum two inches deep in the steep west slope of a mound of earth.
In general, bumble bees require floral resources, suitable nest sites at which a colony can thrive over a season, and protected overwintering sites. No studies have specifically related habitat trends to Western Bumble Bee populations. However, widespread and cumulative habitat conversion has likely caused a decline in portions of its range. The major urban centres of the lower mainland, greater Victoria area and Calgary, combined with the large-scale agriculture within these areas, have led to cumulative habitat quality decline.
Habitat fragmentation, new agricultural development, including the conversion of insect-pollinated crops to wind-pollinated or greenhouse systems), and/or agricultural intensification, possibly in combination with increased pathogen rates, have likely contributed to the decline of this subspecies in much of its range in southwestern Canada (primarily BC and western AB).
In more recent decades, agricultural development in southern BC and AB has led to declines in wildlife (including pollinator) habitat suitability (see Javorek and Grant 2011). Logging, grazing and drying out of wetlands may have adversely altered suitable habitats. In AB, for example, the foothills habitat of Western Bumble Bee is changing at a rapid pace due to substantial energy and forestry industry development, as well as intensification of recreational use, agriculture, and acreages. These uses create significant land and water disturbance and habitat fragmentation (Gardner 2007), and presumably have negative effects Western Bumble Bee.
This subspecies may not have been as common on the prairies as in western parts of its range. Much of the natural prairie habitat conversion occurred decades ago, and there are few historical Western Bumble Bee records. For this reason it is difficult to assess trends.
Habitat does not appear to have substantially changed historically, although the cumulative effects of resource development and climate change may lead to changes in the timing of the floral resources necessary to sustain populations over the season.
Information is compiled from general bumble bee references (Alford 1975; Goulson 2003; Benton 2006) and where applicable references are provided specifically for Western Bumble Bee.
Western Bumble Bee is a primitively eusocial species with queen and worker castes, where the workers are the offspring of the founding queen and together live in a colony. Colonies are annual, with one generation per year. Mating occurs in the fall, males die and only the queen overwinters and emerges in the spring to found a new colony.
Queens typically emerge from April to May and immediately start looking for suitable nest sites. Nests are established in abandoned rodent burrows, grassy hummocks, rotted logs or openings in dead wood. Queens in southern parts of its range emerge sooner than in northern parts, and depending on the temperature and climate, queens will emerge at different times. Queens from Alberta established nests in mid- to late May (Hobbs 1968).
A few weeks after the queen’s initial egg-laying period, female workers emerge and begin foraging for the colony and feeding the brood. As summer progresses the colony reaches maximum worker production and begins producing males and potential queens. These reproductive individuals leave the nest and mate. After mating, young queens enter diapause and overwinter. The old queen, the males and workers decline as fall approaches, and ultimately die with the first frost. Little is known about mating behaviour and colony dynamics in Western Bumble Bee. In the closely related Common Eastern Bumble Bee, females mate with a single male during a single mating event and, as with all bees, the sperm is stored in a spermatheca until used in fertilization (Greeff and Schmid-Hempel 2008).
Eggs hatch after approximately four days and larvae feed on pollen and nectar brought to the nest by workers. The larval stage of bumble bees has four instars. After almost two weeks larvae spin cocoons and pupate. Pupae develop for two weeks before hatching as adults. In total, development from egg to adult takes approximately five weeks, but varies with temperature and food supply (Alford 1975). Western Bumble Bee is a pollen-storer, meaning the larvae live in cells and are fed individually by adults that open the brood clump regularly as the larvae develop. Pollen-storing adults emerge relatively equal in size compared to pocket-making bumble bee species, in which workers vary greatly in size due to unequal food distribution within the brood clumps. Western Bumble Bee queens may require more pollen than other bumble bee species to initiate worker production (Hobbs 1968).
A wide variety of invertebrates parasitize Western Bumble Bee at all stages of the colony cycle (Schmid-Hempel 1998). Spring queens can be infected by nematodes (Sphaerularia bombi) or protozoa (Apicystis bombi) rendering them incapable of founding colonies. During the summer, workers may acquire parasites (e.g., Crithidia bombi), while foraging on flowers contaminated by infected bees.
Cuckoo bumble bees (subgenus Psithyrus) specialize in usurping queens. Adult females enter the colony, occasionally killing the queen, and lay their own eggs, which are cared for by the remaining host workers. Western Bumble Bee is host to two cuckoo bumble bees: Gypsy Cuckoo Bumble Bee (B. bohemicus Cresson) and Suckley's Cuckoo Bumble Bee (B. suckleyi Greene).
The internal mite Locustacarus buchneri is a common parasite that lives within the respiratory tubes of most (perhaps all) Bombus species. A survey in southern Alberta showed this mite primarily occurs in Western Bumble Bee, with up to 50% of queens and workers infected (Otterstatter and Whidden 2004). The reasons for this specificity are unclear; however, this parasite may pose a threat to Western Bumble Bee populations.
Nosema bombi is a microsporidian gut and tissue parasite of bumble bees. Nosema bombi is considered low among wild bumble bees in Canada (average infection rates = 5-10%), but recent field surveys across the United States (Cameron et al. 2011) found the highest levels of N. bombi infection (i.e., over 35%) among declining bumble bee species, particularly Western Bumble Bee, which supports the hypothesis that this parasite is a serious threat. However, Koch and Strange (2012) found similarly high rates (i.e., 44%) in Western Bumble Bee (subspecies mckayi) in Alaska where it remains the most common bumble bee recorded in this area. As such, high rates of Nosema infection may be part of the normal host-pathogen dynamics of Western Bumble Bee (Koch and Strange 2012) and declines in subspecies occidentalis may involve multiple cumulative threats (see above). However, this pathogen is believed responsible for the mid-1990’s collapse of commercial Western Bumble Bee in North America (Thorp and Shepherd 2005).
Predators of adult Western Bumble Bee include robber flies (Family Asilidae) and crab spiders (Family Thomicidae). Thickheaded flies (Family Conopidae) are parasitoids of adult bumble bees. Raccoons, skunks, bears and other mammals are known to destroy and consume bumble bee colonies (Breed et al. 2004).
Bumble bees overwinter as adult queens, which emerge early in the spring and require early flowering plant species to initiate the colony, and additional floral resources for colony development throughout the spring and summer months. As these bees are obligatorily social, they are dependent on diverse plant communities, requiring ample pollen and nectar resources throughout the active period of the colony. Therefore, only habitats supporting rich plant communities provide the nutrition to support bumble bee colonies.
Bumble bees are found throughout most of Canada, and many (including Western Bumble Bee) appear to be relatively cold-tolerant during the active adult period, having been found at elevations as high as 3800 m in southern parts of its global range (USDA 2010). Bumble bees have the physiological capability to thermoregulate (Heinrich 2004); they are able to “shiver” to generate heat in their thoracic muscles to warm up to reach the required minimum body temperature (approx. 30°C) during low ambient temperatures (Heinrich 2004). Given that bumble bees fly in the spring and fall in temperate and arctic regions, internal temperatures generated by shivering can be well above ambient temperature. Since Western Bumble Bee is an early emerging species and occurs at high latitudes and altitudes, thermoregulation is likely an extremely important adaptation.
There is little information on natural dispersal rates for bumble bees. Dispersal occurs primarily in spring by queens while searching for suitable nest sites (Goulson 2003). There is some evidence that bumble bees are able to disperse relatively long distances. Males of the closely related Buff-tailed Bumble Bee (B. terrestris) are reported to fly between 2.6 and 9.9 km from the colony of origin (Kraus et al. 2008). Buff-tailed Bumble Bee was introduced to Tasmania in the early 1990s and has since spread at a rate of approximately 10 km per year (Stout and Goulson 2000). Dispersal is likely important to survival based on studies that have examined the patchiness of bumble bee habitat (e.g., Hatfield and LeBuhn 2007) and increased problems associated with small effective population sizes in haplodiploid insects (Zayed and Packer 2005) (see Limiting Factors).
Western Bumble Bee is a generalist forager; it naturally co-forages and competes with many other bee species for food pollen and nectar. This species also can nectar-rob (i.e., carry out illegitimate flower visits usually not resulting in pollination; bees bite holes in the base of flowers to reach the nectar without contacting the anthers and/or stigma) allowing workers to forage for nectar from long-tubed flowers. This allows the species to feed for nectar on a large variety of flowering plant species.
Western Bumble Bee likely has important mutualistic relationships with many early spring and montane meadow flowering plant species, which may rely on it and other bumble bee species for pollination. However, the extent of interdependence of individual plant species on Western Bumble Bee for pollination is unknown. Where its range overlaps with the closely related Yellow-banded Bumble Bee, Western Bumble Bee is usually more common in montane habitats and Yellow-banded Bumble Bee more common at lower elevations (Hobbs 1968), though both species are sympatric in parts of the prairies.
Western Bumble Bee is host to Gypsy Cuckoo Bumble Bee and Suckley’s Cuckoo Bumble Bee (see Predation and Parasitism).
Four different datasets are used to show declines in the relative abundance of Western Bumble Bee. Relative abundance (RA) is the number of individuals of one species (e.g., Western Bumble Bee) divided by the total number of individuals (e.g., Bombus) collected, and is often used as a proxy of abundance when data are not amenable to other analysis. The RA is also used as an index of search effort for Western Bumble Bee, and it is assumed that if the species was within an area during a collection event, that it would likely have been collected. It is noted that measuring the RA of a species may not reflect actual population abundance. For ease of reference between the next sections, these studies are numbered.
1) The first study uses a dataset of bumble bees for Canada, with 44,706 museum and observation records databased, ranging in dates from 1882 – 2011 (this dataset does not yet include data from 2012 and 2013 [e.g., from Sheffield et al. in prep.]). The RA of Western Bumble Bee was plotted in ten-year increments and for each jurisdiction where found in Canada (Table 3; Figure 9 and Figure 10).
2) In a second study (and using an older and less complete version of the dataset of bumble bees mentioned above), Canada-based distribution data were mapped on a 50 x 50 km grid, from 1882-1995 and 1996-2010 (including non-independent samples and sites where multiple observations were recorded within a year). Figure 6 is the graphical representation of this data.
Figure 6. Spatial distribution of sampling records of Western Bumble Bee in Canada from 1882 - 2010. Each circle is proportional to the number of observations recorded at that site between 1882 and 2010, inclusive (including non-independent samples and sites where multiple observations were recorded within a year). Data are mapped ona 50 x 50 km grid. Grey squares show records prior to 1996 (128 squares); yellow squares show sites collected pre/post 1996 (27 squares); green squares show collectionsafter 1996 (13 squares) (n= 1706 specimens). Data is compiled from CBIF and contributors listed in the Acknowledgements section. This map is not possible to update.

Map: © Environment Canada
Long description for Figure 6
Map showing the spatial distribution of sampling records of Western Bumble Bee in Canada from 1882 to 2010. It is based on the study designated number 2 in the report text. In the study, Canada-based distribution data from 1882 to 1995 and from 1996 to 2010 were mapped on a 50 by 50 kilometre grid (including non-independent samples and sites where multiple observations were recorded within a year). Symbols indicate 128 records prior to 1996, 27 sites where the species was collected both before and after 1996, and 13 collections after 1996.
Figure 7. Relative abundance (RA) of Western Bumble Bee across southern Alberta during two time periods (combined sample sizes shown in parentheses). All data from southern Alberta are included (i.e., surveys of 2000 and 2010, as well as the additional study sites sampled at other times). Except for two sites where Western Bumble Bee did not occur in either period (Innisfail and Trunk Rd.), RA declined from 2000 to 2010. Habitats in which the species was collected are: Calgary (urban), Clarseholm (rural), Barrier Lk. (natural), Drumheller (edge of species’ range), Fortress Mt. (higher elevation), Coleman (rural), and Innisfail (out of subspecies’ range) and Trunk Rd.(out of subspecies’ range).

Map: © Environment Canada
Long description for Figure 7
Bar chart showing the relative abundance (RA) of Western Bumble Bees across southern Alberta during two time periods: 1985 to 2000 and 2010. The data show that, except for two sites where Western Bumble Bee did not occur in either period (Innisfail and Trunk Road), RA declined from 2000 to 2010. Declines are as follows: Calgary (urban), 59 to 4 percent; Clarseholm (rural), 21 to 3 percent; Barrier Lake (natural), 15 to 0 percent; Drumheller (edge of species’ range), 5 to 3 percent; Fortress Mountain, 5 to 4 percent; Coleman (rural), 4 to 0 percent; and Innisfail (out of subspecies’ range) and Trunk Road, 0 percent in both periods.
Two additional studies, one in southern AB and one in the Fraser Valley of BC, are regional in scope and used to show RA declines within each respective region. Note that specimens collected during these studies are assumed to be subspecies occidentalis based on the geography of the collection sites.
3) The southern Alberta study compares data collected in 1985-2000 and repeats similar data collection methods at these sites in 2010. The Fraser Valley study compares populations in 1981-82 with those in 2003-04. During preparation of this status report, these studies were analyzed to determine changes in the RA of Western Bumble Bee over time. The proportion of the total bee catch composed of Western Bumble Bee was analyzed using logistic regression and included time period (early or late years of the study) and study site (for the southern Alberta surveys only). This approach estimates the change over time in the probability of collecting Western Bumble Bee at a study site.
In the Alberta study, bumble bee workers and males were surveyed at eight sites across southern Alberta during the summer of 2000 (all species n=1672), and repeated using the same methods at the same sites in 2010 (n=775). Details of the 2000 survey are published elsewhere (Otterstatter 2001), whereas the second survey was conducted specifically to provide information for this report (surveys conducted by R. Owen [pers. data]; R. Longair [pers. data]). Collecting locations, seasonal timing and search effort was the same in both 2000 and 2010; and both studies are considered highly comparable.
These data were supplemented with additional surveys conducted in the same geographic region, but at different times or at different sites. These additional data include: a 1985-86 study of bumble bee queens (n=442) in the Kananaskis Valley and Calgary areas (Owen 1988); intensive surveys of bumble bees near Barrier Lake, Kananaskis, during 1997-2000 (n=4376) and in Calgary during 1998 (n=367) (Otterstatter et al. 2002; Otterstatter 2004; Otterstatter and Whidden 2004); a study of pollen foraging by bumble bee queens and workers (n=99) at three sites in the foothills of southwestern Alberta during 1991-92 (Rasheed and Harder 1997); and opportunistic surveys of workers and males (n=109) at five sites in southern Alberta during 2000 (R. Owen pers. data).
4) In the Fraser Valley, wild bumble bees were collected in commercial berry fields in 1981-82, and again in 2003-04 (details in Winston and Graf 1982; MacKenzie and Winston 1984; Ratti 2006; Ratti et al. 2008; Colla and Ratti 2010). In the recent study twelve sites were surveyed using sweep nets and pan traps. Both studies (1981-82 and 2003-04) were carried out in the same area, with similar methods and are considered highly comparable.
1) The RA of Western Bumble Bee appears to decline within each jurisdiction (Table 3, Figure 9) and overall when all records are combined across the species’ range in Canada (Table 3; Figure 10). In BC, the RA declines from approximately 43% (1992 – 2001) to 3% within the last ten-year increment (2002 – 2011) with an overall decline of more than 85%. In AB, RA of Western Bumble Bee declines from 83% (1992 – 2001) to less than 10% (2002 – 2011). In SK, in general there are few historical records of bumble bees; however, there is a decline in RA from 9% to 3% within the last ten-year increment.
2) This study shows the spatial distribution of sampling records of Western Bumble Bee in Canada from 1882 – 2010 (Figure 6). Each circle is proportional to the number of observations recorded at that site between 1882 and 2010, inclusive (including non-independent samples and sites where multiple observations were recorded within a year) (n= 1706 specimens). Grey squares show records prior to 1996 (128 squares); yellow squares show sites collected pre/post 1996 (27 squares); green squares show collections after 1996 (13 squares) (n= 1706 specimens).
Two regionally specific studies show declines in RA for subspecies occidentalis.
3) In Alberta, RA declined from 16.9% (n=1017 [subspecies occidentalis] / 6006 [Bombus collected]) during 1985-2000 to 3.2% (n=25 [subspecies occidentalis] / 775 [Bombus collected]) in 2010 (Figure 8 and Figure 9). The highest number of Western Bumble Bee recorded in this study was at Barrier Lake (n=4924) and one of the most notable changes is the complete (or very near) disappearance of this bee from one site at Barrier Lake (Kananaskis) where it was formerly the 3rd or 4th most common Bombus species collected. Across six sites sampled in 2000 and again in 2010 (eight sites total but two sites excluded because Western Bumble Bee was not recorded in either time period), RA declined 80% from 14% to 0.7% in 10 years.
Figure 8. Predicted probability of collecting Western Bumble Bee, based on data from 14 sites in southern Alberta sampled between 1985 and 2010. Points indicate average probabilities (with 95% confidence interval) from sites sampled during a given year. Dashed line is a quadratic regression fit; between 2010 and 2018, the line represents projected values from the regression equation.

Map: © Environment Canada
Long description for Figure 8
Chart plotting the average probability of collecting Western Bumble Bee (y-axis) against year (x-axis), based on data from 14 sites in southern Alberta sampled between 1985 and 2010. Points indicate average probabilities from sites sampled during a given year. A line connects the points from 1985 to 2010 and is extended to 2018, based on projected values from a regression equation. The chart shows a steep decline in the probability of collecting Western Bumble Bee from 1985 to 2010 in Alberta, with average probability falling from almost 0.4 to just over 0.05.
Figure 9. Relative abundance (RA) of Western Bumble Bee (WBB) based on all databased Bombus records in Canada (1882 – 2011). The left Y-axis (shaded portions of bars) indicates WBB specimens and the right Y-axis (triangles) represents the proportion of WBB specimens by ten-year intervals. Linear regression was used to examine trends in RA in WBB across ten-year intervals: the line represents a best fit of the data. See also Table 3. Graphs generated using Minitab ® software.

Map: © Environment Canada
Long description for Figure 9
Five chart panels illustrating relative abundance (RA) of the Western Bumble Bee based on all databased Bombus records in Canada (from 1882 to 2011, with data presented in 10-year intervals) (see also Table 3). One y-axis indicates the number of Western Bumble Bee specimens, and the other y-axis represents the proportion of Western Bumble Bee specimens. Panels are provided for Yukon, Northwest Territories, British Columbia, Alberta, and Saskatchewan. The relative abundance of the Western Bumble Bee appears to decline within each jurisdiction (see discussion under “Abundance” in report text).
Figure 10. Total number of databased bumble bee specimens in Canada (1882 – 2011) from each province and territory; triangles represent the number of Western Bumble Bee (WBB) specimens. Values above each bar represent the percentage of specimens within the collection which are WBB. See also Table 3. Graphs generated using Minitab ® software.
Map: © Environment Canada
Long description for Figure 10
Chart with bars indicating the total number of databased bumble bee specimens in Canada, from 1882 to 2011, in each province and territory. Symbols represent the number of Western Bumble Bee (WBB) specimens, and the percentage of WBB is given above each bar. (See also Table 3.) WBB represent 38.4 percent of specimens in Yukon, 1.2 percent in the Northwest Territories, 0 percent in Nunavut, 16.5 percent in British Columbia, 11 percent in Alberta, and 2.7 percent in Saskatchewan.
Western Bumble Bee does not appear to have shifted its geographic range northward: the two northernmost sites in the AB data set (i.e. Forestry Trunk Road and Innisfail) did not record the bee in the most recent period. Classifying the sites according to ecoregion (as defined by Strong 1992) suggests that the largest declines have occurred in parkland regions (areas around and south of Calgary, RA decreased from 58.6% to 6.3%). Substantial change has also occurred in montane areas (Kananaskis Valley and areas south between grasslands and Rocky Mountains, 14.8% declined to 0%). The change observed for grasslands was less pronounced (12.7% declined to 3.1%). Subalpine habitats showed no significant change (5.0% compared to 5.2%); however, Western Bumble Bee abundance is typically low in subalpine areas.
The change in the probability of collecting Western Bumble Bee during 1985-2010 in Alberta shows a steep decline over the past 25 years (estimated from the logistic regression of Western Bumble Bee RA across all 14 sites) (Figure 8). Given that these predictions are based on data aggregated across disparate regions, the margin of error is large (as reflected by the large confidence intervals during the earliest years) and considers differences in search effort and methods. For example, the search effort in Alberta was greater during 1985-2000 (n=6006, resulting from numerous person-hours across several studies) than during 2010 (n=775, resulting from 2 collectors in a single study).
4) The study in the Fraser Valley showed RA in berry fields declined from 33.3% (n=608/1828) in 1981-82 to 0.7% (30/4221) in 2003-04 (details in Winston and Graf 1982; MacKenzie and Winston 1984; Ratti 2006; Ratti et al. 2008; Colla and Ratti 2010).
In the United States, population declines have occurred in some of the most historically abundant bumble bees (including Western Bumble Bee), which formerly occupied wide ranges (Cameron et al. 2011). Of total of 16,788 bumble bees collected throughout the United States except Alaska (e.g., within the range of subspecies occidentalis) from 2007-2009, only 129 Western Bumble Bee individuals were collected (Cameron et al. 2011). All detections occurred in the Intermountain West (i.e. region of North America lying between the Rocky Mountains to the east and the Cascades and Sierra Nevada to the west) and Rocky Mountains and the species was largely absent from the western part of its range (historically the Pacific west, CA, OR and WA) (Cameron et al. 2011). The detected range-area reduction in this study was estimated at 28% over more than 100 years.
In YK and NT, RA appears stable (Table 3; Figure 10). There are few historical specimens and thus there is minimal comparable data from which to draw trends in RA. Based on 2013 surveys (at least 20 sampling sites, half hour minimum sample duration; not yet in Canadian bee dataset) this bee was recorded from many sites throughout the range in the YT (Sheffield pers. data 2013). Although known from the western NT, no trend data exists for this species as almost all of the records are from 2011 (and see Stotyn 2012).
In Alaska, Koch and Strange (2012) indicate that Western Bumble Bee is the most common bumble bee. The species commonly occurs on roadsides from taiga to boreal forests and was more abundant in August 2010 than in surveys from the same year throughout the lower western states (i.e. the range of subspecies occidentalis) (Strange pers. comm. 2010).
Little is known about natural bumble bee population fluctuations and trends. Western Bumble Bee was formerly one of the most common bumble bees in western North America and the recent decline of subspecies occidentalis over much of its global range suggests that this trend is not likely a natural fluctuation.
There appears to have been a reduction in the range of subspecies occidentalis (i.e., based on comparisons of pre-1996 and post-1995, see Figure 6). Frequency distributions in combination with changes in spatial distribution of records suggest that declines in Western Bumble Bee occurred after the 1970s (Figure 4). However, measures for estimating sampling effort are not available; thus, spatial trends may also reflect sampling effort biases as well as spatial inaccuracies associated with data georeferencing of specimens and observations. Patterns of declines based on sites with recorded samples over the three time intervals suggest approximately 60% decline in occupied area (Table 1). Sites as a 50 km grid cell were selected to be consistent with previous Bombus work (e.g., Fitzpatrick et al. 2007). Further, the mean elevation of Western Bumble Bee records was lowest for the pre-1996 period and highest for the post-1996 period (Table 1). However the difference in mean elevation between these two periods was only ~100 m, and relative sampling intensity was not consistent across periods (n=128, 27 and 13), making it difficult to conclude a consistent trend towards higher elevations.
| Pre-1996 | Pre-1996 and continuing | Post-1995 | Current distribution (all records since 1995) | |
|---|---|---|---|---|
| Area (km2) | 320,000 | 67,500 | 32,500 | 100,000 |
| No. of sites (each site = 50 km2) | 128 | 27 | 13 | 40 |
| Mean elevation (m) | 1114 | 1210 | 1218 | - |
| Elevation SD (m) | 520 | 600 | 441 | - |
| Elevation range (m) | 0 – 2615 | 20 - 2177 | 396 - 1920 | - |
Large areas within the range of Western Bumble Bee in Canada are undersampled. Within respective geographic ranges for both subspecies, populations of Western Bumble Bee within suitable natural habitats could potentially disperse and recolonize areas where the bee has declined and habitat was suitable. However, the source-sink dynamics of this rescue effect are unknown. Rescue effect between subspecies occidentalis and mckayi is not applicable.
Much of the northern range of subspecies occidentalis in Canada is suitable and largely unmodified, though it remains undersampled. Recent (2013) survey work shows the subspecies is still present in the central interior of BC (see Search Effort) although at small proportions (1.7% of total examined in 2013 study; specimens still being processed) are Western Bumble Bee (Sheffield et al. in prep; Sheffield et al. 2013). Subspecies occidentalis extends into the western United States, where it has also declined (Cameron et al. 2011). Thus although there may be suitable habitat, the US populations may not be abundant enough to support rescue to Canadian habitats.
Subspecies mckayi is more commonly collected throughout its range in northern BC, YK, and western NT. Rescue effect from Alaska is possible for subspecies mckayi.
The International Union for Conservation of Nature–Conservation Measures Partnership (IUCN-CMP) threats calculator (Salafsky et al. 2008; Master et al. 2009) was used to classify and list threats to subspecies occidentalis with an overall low threat impact (Table 3). Despite the apparent low impact, subspecies occidentalis appears to be declining based on abundance during recent collection events (see Search Effort and Population Sizes and Trends). It is thought that the cumulative impacts of numerous threats have contributed to the decline of the subspecies. A threats assessment for subspecies mckayi is not completed.
Bees are more vulnerable to habitat fragmentation than other animal species due to genetic elements inherent to haplodiploidy (Packer and Owen 2001) (see Limiting Factors). Additionally, bumble bees require large inputs of floral resources (i.e., pollen and nectar) over the entire growing season, as new queens for establishing the next generations are only produced towards the end of the colony cycle. Threats that impact floral resources, nesting sites (during the growing season) and overwintering sites can have huge impacts on local bumble bee populations.
Habitat loss from intensive residential and commercial developments within the urban areas may be contributing to local declines of subspecies occidentalis. However, there are a few recent (within the past five years) records of the subspecies in Victoria (2012), Delta (2010), and other urban areas (see Table 2). This threat only applies to the highly urban and human populated areas: BC – the lower mainland and lower Fraser valley and greater Victoria areas; AB – Calgary and surrounding areas. This threat is does not appear applicable to subspecies mckayi.
| Province | General region | Year | Search Effort | Observations | Reference |
|---|---|---|---|---|---|
| BC | Southeastern Vancouver Island | 2010 | 332 km 106 hours |
0 | Page, Lilley and Heron 2010 |
| Lower Mainland | 2010 | 64 sites; 271 hours; 355 km |
1 (subspecies occidentalis) | Parkinson and Heron 2010 | |
| Lower Fraser Valley | 2010 | 46 sites 18 days |
6 (subspecies occidentalis) | Knopp, Larkin and Heron 2010 | |
| Okanagan | 2010 | 40 sites 158 hours 147 km |
4 (subspecies occidentalis) | Marks and Heron 2010 | |
| West Kootenays | 2010 | 11 sites 19 km 40 hours |
6 (subspecies occidentalis) | Westcott and Heron 2010 | |
| Throughout southern parks of range in BC, AB and SK | 2010 | Unknown. | Yes (subspecies occidentalis) | Best pers. data. 2010 | |
| Victoria area, southern Vancouver Island | 2012 | 7 sites; all municipal parks in urban setting | 9 (subspecies occidentalis) | Wray pers. comm. 2013 | |
| Central interior BC | 2013 | 281 hours | In progress, both subspecies |
Sheffield pers. data 2013; Heron pers. data 2013 | |
| Northern portions along the Alaska Highway from Fort St. John to Atlin | 2013 | May 28 – August 2; 55 sites; minimum ½ hour search effort per site. | Yes. Numerous specimens in YK and northern BC. | Cannings pers. data 2013 | |
| AB | Edmonton | 2013 | in progress | Yes, in progress (subspecies occidentalis) | C. Sheffield pers. data 2013 |
| SK | Cypress Hills and well into the prairies (Leader, Eastend, Shaunovan, Swift Current, and as far east as Regina) | 2013 | in progress | Yes, in progress (subspecies occidentalis) | C. Sheffield pers. data 2013 |
| NT | South Nahanni River from Moose Ponds to Blackstone Landing on the Liard River, including Nahanni National Park Reserve | 2011 | Opportunistic bumble bee collections at 19 sites from July 5 – 26. | Yes. Three Bombus occidentalis mckayi (of 78 collected bumble bees). | Stotyn and Tate 2012 |
| YT | Throughout the southern portions | 2013 | May 28 – August 2; 16 sites; minimum ½ hour search effort per site. | Yes. Numerous specimens of Bombus occidentalis mckayi in YK and northern BC. | Cannings pers. data 2013 |
| YT (subspecies mckayi) | NT (subspecies mckayi) | NT (subspecies mckayi) | BC (subspecies occidentalis and subspecies mckayi) | AB (subspecies occidentalis) | SK (subspecies occidentalis) | SK (subspecies occidentalis) | Overall change in RA from previous decade | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of specimens in database | All Bombus | 1882-1891 | 0 | 0 | 1 | 15 | 39 | 2 | 57 | |
| Number of specimens in database | WBB | 1882-1891 | 0 | 0 | 0 | 6 | 7 | 0 | 13 | |
| Number of specimens in database | RA | 1882-1891 | - | - | - | 0.4 | 0.179487 | - | 0.22807 | |
| Number of specimens in database | All Bombus | 1892-1901 | 0 | 0 | 0 | 59 | 34 | 0 | 93 | |
| Number of specimens in database | WBB | 1892-1901 | 0 | 0 | 0 | 10 | 3 | 0 | 13 | |
| Number of specimens in database | RA | 1892-1901 | 0 | - | - | 0.169492 | 0.088235 | - | 0.139785 | 39 |
| Number of specimens in database | All Bombus | 1902-1911 | 2 | 1 | 3 | 166 | 119 | 8 | 299 | |
| Number of specimens in database | WBB | 1902-1911 | 1 | 0 | 0 | 49 | 11 | 0 | 61 | |
| Number of specimens in database | RA | 1902-1911 | 0.5 | - | - | 0.295181 | 0.092437 | - | 0.204013 | 46 |
| Number of specimens in database | All Bombus | 1912-1921 | 21 | 9 | 44 | 668 | 166 | 31 | 939 | |
| Number of specimens in database | WBB | 1912-1921 | 8 | 0 | 0 | 149 | 24 | 0 | 181 | |
| Number of specimens in database | RA | 1912-1921 | 0.380952 | - | - | 0.223054 | 0.144578 | - | 0.192758 | 0.055169 |
| Number of specimens in database | All Bombus | 1922-1931 | 3 | 10 | 13 | 313 | 372 | 0 | 711 | |
| Number of specimens in database | WBB | 1922-1931 | 2 | 0 | 0 | 76 | 74 | 0 | 152 | |
| Number of specimens in database | RA | 1922-1931 | 0.666667 | - | - | 0.242812 | 0.198925 | - | 0.213783 | -11 |
| Number of specimens in database | All Bombus | 1932-1941 | 1 | 70 | 15 | 88 | 39 | 70 | 283 | |
| Number of specimens in database | WBB | 1932-1941 | 0 | 0 | 0 | 27 | 6 | 4 | 37 | |
| Number of specimens in database | RA | 1932-1941 | - | - | - | 0.306818 | 0.153846 | 0.057143 | 0.130742 | -38 |
| Number of specimens in database | All Bombus | 1942-1951 | 157 | 226 | 92 | 513 | 135 | 56 | 1179 | |
| Number of specimens in database | WBB | 1942-1951 | 29 | 1 | 0 | 152 | 41 | 1 | 224 | |
| Number of specimens in database | RA | 1942-1951 | 0.184713 | - | - | 0.296296 | 0.303704 | 0.017857 | 0.189992 | -0.45318 |
| Number of specimens in database | All Bombus | 1952-1961 | 126 | 4 | 33 | 377 | 211 | 8 | 759 | |
| Number of specimens in database | WBB | 1952-1961 | 15 | 0 | 0 | 137 | 38 | 0 | 190 | |
| Number of specimens in database | RA | 1952-1961 | 0.119048 | - | - | 0.363395 | 0.180095 | - | 0.250329 | 32 |
| Number of specimens in database | All Bombus | 1962-1971 | 348 | 219 | 135 | 478 | 201 | 67 | 1448 | |
| Number of specimens in database | WBB | 1962-1971 | 239 | 0 | 0 | 239 | 32 | 1 | 511 | |
| Number of specimens in database | RA | 1962-1971 | 0.686782 | - | - | 0.5 | 0.159204 | 0.014925 | 0.352901 | 41 |
| Number of specimens in database | All Bombus | 1972-1981 | 198 | 33 | 1 | 174 | 120 | 9 | 535 | |
| Number of specimens in database | WBB | 1972-1981 | 71 | 0 | 0 | 76 | 15 | 0 | 162 | |
| Number of specimens in database | RA | 1972-1981 | 0.358586 | - | - | 0.436782 | 0.125 | - | 0.302804 | -14 |
| Number of specimens in database | All Bombus | 1982-1991 | 37 | 31 | 10 | 253 | 59 | 1 | 391 | |
| Number of specimens in database | WBB | 1982-1991 | 13 | 0 | 0 | 115 | 1 | 0 | 129 | |
| Number of specimens in database | RA | 1982-1991 | 0.351351 | - | - | 0.454545 | 0.016949 | - | 0.329923 | 9 |
| Number of specimens in database | All Bombus | 1992-2001 | 2 | 12 | 0 | 58 | 6 | 22 | 100 | |
| Number of specimens in database | WBB | 1992-2001 | 1 | 0 | 0 | 25 | 5 | 2 | 33 | |
| Number of specimens in database | RA | 1992-2001 | 0.5 | - | - | 0.431034 | 0.833333 | 0.090909 | 0.33 | 0 |
| Number of specimens in database | All Bombus | 2002-2011 | 116 | 140 | 59 | 4573 | 2103 | 263 | 7254 | |
| Number of specimens in database | WBB | 2002-2011 | 73 | 12 | 0 | 128 | 142 | 8 | 363 | |
| Number of specimens in database | RA | 2002-2011 | 0.62931 | 0.085714 | - | 0.02799 | 0.067523 | 0.030418 | 0.050041 | -85 |
| Threat Impact | Threat Impact (descriptions) | Level 1 Threat Impact Counts high range |
low range |
|---|---|---|---|
| Threat Impact Reasons Subspecies occidentalis is primarily threatened by the cumulative effects (in order of greatest threat): 8.1) Invasive non-native/alien species: Pathogen spillover (the use of infected commercial bumble bees [e.g., use of Common Eastern Bumble Bee in western Canada] for greenhouse pollination may facilitate pathogen spillover into wild populations of bumble bees foraging nearby. Lab studies show the parasite species Crithidia bombi and Nosema bombi (suspected) have adverse effects on Bombus colony-founding queens, foraging workers and entire nests. 9.3) Agricultural and forestry effluents: Imidacloprid (a neonicotinoid) pesticides are harmful at concentrations in the parts per billion (ppb). These pesticides are systemic, cumulative and travel throughout the plant, reaching pollen and nectar and are commonly used on golf courses and agricultural lands. 2.1) Annual and perennial non-timber crops: Cumulative reductions of floral resources for wild bees in landscapes dominated by monocultures, particularly those that do not require insect-pollination. |
|||
| A | Very High | 0 | 0 |
| B | High | 0 | 0 |
| C | Medium | 0 | 0 |
| D | Low | 2 | 2 |
| Calculated Overall Threat Impact: | Low | Low | |
| # | Threat | Impact (calculated) |
Scope (next 10 Yrs) |
Severity (10 Yrs or 3 Gen.) |
Timing | Comments |
|---|---|---|---|---|---|---|
| 1 | Residential & commercial development | Negligible | Negligible(<1%) | Slight (1-10%) | High (Continuing) | Negligible scope because there are large areas of natural habitat where development is not ongoing. Slight severity because cumulative impacts of housing and industrial development surrounding the urban centres of western Canada, specifically in southern regions approximately 200km from the US border, often result in complete loss of habitat. However, subspecies occidentalis is still recorded (e.g., Victoria, Delta, Edmonton, Regina). High timing because the practice is continuing. |
| 1.1 | Housing & urban areas | Negligible | Negligible(<1%) | Slight (1-10%) | High (Continuing) | Habitat loss as a result of increased urbanization, although this only affects a small proportion of the subspecies’ range. |
| 1.2 | Commercial & industrial areas | Negligible | Negligible(<1%) | Slight (1-10%) | High (Continuing) | Habitat loss as a result of increased urbanization, although this only affects a small proportion of the subspecies’ range. |
| 1.3 | Tourism & recreation areas | N/A; some recreational development may cause bee habitat loss, but overall other tangential impacts may affect bee habitat (e.g., pesticide use on golf courses, water diversion, reduction of floral resources, etc.) and these threats are accounted for elsewhere in this threats calculator. | ||||
| 2 | Agriculture & aquaculture | Negligible | Negligible(<1%) | Slight (1-10%) | High (Continuing) | Negligible scope because there are large areas of natural habitat where agricultural practices do not apply; slight severity because there are agricultural areas where bees are abundant and widespread; high timing because the practice is continuing. |
| 2.1 | Annual & perennial non-timber crops | Negligible | Negligible(<1%) | Slight (1-10%) | High (Continuing) | Agricultural intensification in lower elevation areas, especially within the southern parts of the range (e.g., BC - Okanagan, Kootenays, Vancouver Island, lower mainland; AB - Calgary and southern AB). Although this threat is historical, agricultural intensification has occurred within the past 25 years and resulted in lower habitat quality (e.g., see Javorek and Grant 2011). Semi-bee friendly agricultural habitats (e.g., hayfields and leafy crops) are being replaced by enclosed greenhouse crops, vineyards, and other agricultural systems that may not require insect pollination. |
| 4 | Transportation & service corridors | Negligible | Negligible(<1%) | Negligible (<1%) | High (Continuing) | Negligible scope because there are large areas of natural habitat where road building and utility/service lines are not planned. Negligible severity because in many cases transportation corridors may leave habitat more open for bees (provided the transportation corridor is not paved). High timing because the practice is continuing. |
| 4.1 | Roads & railroads | Negligible | Negligible(<1%) | Negligible (<1%) | High (Continuing) | The threat is considered negligible. May temporarily increase habitat adjacent to roadsides - areas need to be cleared. Although there could be cumulative herbicide impacts (this threat is captured elsewhere though). |
| 4.2 | Utility & service lines | Negligible | Negligible(<1%) | Negligible (<1%) | High (Continuing) | The threat is considered negligible. May temporarily increase habitat adjacent to roadsides - areas need to be cleared. Although there could be cumulative herbicide impacts (this threat is captured elsewhere though). |
| 5 | Biological resource use | Negligible | Negligible(<1%) | Negligible (<1%) | High (Continuing) | N/A |
| 5.3 | Logging & wood harvesting | Negligible | Negligible(<1%) | Negligible (<1%) | High (Continuing) | The threat is considered negligible. Logging may temporarily increase available habitat if there are habitat connections. |
| 6 | Human intrusions & disturbance | Negligible | Negligible(<1%) | Slight (1-10%) | High (Continuing) | Negligible scope because there are large areas of natural habitat where recreational activities are not ongoing; slight severity because recreational activities may trample or decrease nest site suitability; high timing because the practice is continuing. |
| 6.1 | Recreational activities | Negligible | Negligible(<1%) | Slight (1-10%) | High (Continuing) | The threat is considered negligible. Some recreational activities may cause local extirpations of nests; although across the subspecies’ range this threat is likely minor overall. |
| 8 | Invasive & other problematic species & genes | Low | Small (1-10%) | Extreme (71-100%) | High (Continuing) | Small scope because the spread of invasive species is primarily within the urban and agricultural areas of Canada. The natural habitats do not appear to have non-native bees present. Pathogen spillover and impacts remain unstudied in much of the ssp. range. Extreme severity because these practices impact bees. High timing because these practices are continuing. |
| 8.1 | Invasive non-native/alien species | Low | Small (1-10%) | Extreme (71-100%) | High (Continuing) | The introduction and use of Common Eastern Bumble Bee for greenhouse pollination services in western Canada may further impact wild populations of Western Bumble Bee. Escaped and established populations of the Common Eastern Bumble Bee may out-compete native bumble bees for nesting habitat and/or floral resources, and introduced colonies may serve as a pathogen or disease vector. The status of establishment of wild populations of Common Eastern Bumble Bee in western Canada is unknown, but is likely to have a negative impact on native bumble bee species, as has been documented in other parts of the world for other managed species (Williams and Osborne 2009). Lab studies have shown the parasite species Crithidia bombi and Nosema bombi (suspected) have devastating effect on Bombus colony-founding queens, foraging workers and entire nests (Brown et al. 2000, 2003; Otterstatter et al. 2005). |
| 9 | Pollution | Low | Small (1-10%) | Serious (31-70%) | High (Continuing) | |
| 9.3 | Agricultural & forestry effluents | Low | Small(1-10%) | Serious (31-70%) | High (Continuing) | Neonicotinoids pose a particular threat to bees (compared to other pesticides) because they are harmful even at concentrations in the parts per billion (ppb) (Environmental Protection Agency [EPA] 1994; Marletto et al. 2003). These pesticides are systemic and travel throughout the plant, reaching pollen and nectar. |
| 11 | Climate change & severe weather | Not Calculated | Pervasive(71-100%) | Unknown | High (Continuing) | Pervasive scope because climate change is ongoing across the entire species’ range. Unknown severity because impacts are unstudied at a large scale. High timing because the threat is continuing. |
| 11.2 | Droughts | Not Calculated | Pervasive(71-100%) | Unknown | High (Continuing) | Climate change is another possible threat (Williams and Osborne 2009). Bumble bee species shown to have narrow climatic tolerances are more vulnerable to extrinsic threats (Williams et al. 2009). Climatic tolerances for Western Bumble Bee are not currently unknown. |
Classification of Threats adopted from IUCN-CMP (International Union for Conservation of Nature - Conservation Measures Partnership), Salafsky et al. (2008).
Habitat loss as a result of agricultural intensification is ongoing throughout southern portions of subspecies occidentalis range in BC, AB and SK, which have some of the most highly urbanized/farmed regions in Canada. The increased reliance on intensive agriculture over the past few decades has resulted in decreased quality foraging habitat for bumble bees globally (e.g., Williams 1989; Kosior et al. 2007). Agricultural intensification is less and not perceived as a high threat for subspecies mckayi. The use of chemicals (e.g., pesticides) in agricultural areas adds an additional, potentially severe threat to all bees. This is discussed in the pollution section below (Threat 9).
Pathogen spillover has been implicated in the significant declines of many animals (Morton et al. 2004; Power and Mitchell 2004) but is a poorly understood threat for bumble bees. Pathogen spillover occurs when pathogens spread from a heavily infected ‘reservoir’ host population to a sympatric ‘non-reservoir’ host population (Power and Mitchell 2004). The use of infected commercial bumble bees (Common Eastern Bumble Bee in Canada) for greenhouse pollination is known to cause pathogen spillover into populations of wild bumble bees foraging nearby (Colla et al. 2006; Otterstatter and Thomson 2008). In western Canada, greenhouses using managed bees are present mostly across southern BC and to a lesser extent in the Lacombe and Redcliff regions of AB. The parasite species involved (Crithidia bombi), or suspected to be involved (Nosema bombi), in spillover to wild Bombus have detrimental effects on colony-founding queens, foraging workers and entire nests (Brown et al. 2000, 2003; Otterstatter et al. 2005). These parasites are found in a variety of bumble bee species (Macfarlane 1974; Macfarlane et al. 1995; Colla et al. 2006), but their virulence in wild Western Bumble Bee remains unknown. Nonetheless, the increased use of bumble bees in greenhouse operations in recent decades has been implicated in the decline of members of the subgenus Bombus, including B. affinis and Western Bumble Bee (Thorpe and Shepherd 2005; NRC 2007; Evans et al. 2008).
Recent surveys in Alaska (Koch and Strange 2012) found Nosema infection in subspecies mckayi to be on par with to slightly higher than in populations of the subspecies occidentalis in the United States. Commercial bumble bee colonies are used less in the north, and high levels of Nosema may be reflective of natural host-parasite levels (Koch and Strange 2012), and may not be spillover from greenhouses.
The introduction and use of the highly successful Common Eastern Bumble Bee (B. impatiens) in western Canada for pollination services may further impact Western Bumble Bee populations. The importation of Common Eastern Bumble Bee to greenhouses in BC may be a potential competitor and threat to declining populations, since this species has appeared to escape greenhouses the wild (Ratti and Colla 2010). This species may out-compete Western Bumble Bee for nesting habitat or forage resources. It may also serve as a pathogen or disease source. The status of wild populations of this introduced eastern species in western Canada is unknown, but may have adverse impacts on native species, as has been documented elsewhere (Williams and Osborne 2009).
In highly agricultural landscapes it likely competes for nectar with the introduced and managed European Honey Bee (Apis mellifera L.). However, competition is difficult to quantify under natural conditions (Thomson 2006), so the impact in agricultural landscapes is unknown. Honeybees have been in North America for hundreds of years making it difficult to ascribe recent reductions in Western Bumble Bee to impacts of direct competition with honeybees.
Pollution via agrochemicals (i.e.. pesticides) is known to have detrimental effects on bees and other beneficial insects (e.g., Whitehorn et al. 2012; Baron et al. 2014). Many agrochemicals are known to impact bees, though much attention in recent decades has implicated neonicotinoides as a major risk to pollinators (Marandin and Winston 2003; Laycock et al. 2012; Whitehorn et al. 2012). At the approximate time that declines of Western Bumble Bee and other bumble bees in this subgenus were first noted, a new Imidacloprid pesticide (a neonicotinoid) was registered for use in the US (1994) and Canada (1995) (Cox 2001; PMRA 2001). Neonicotinoids are systemic pesticides that travel and accumulate throughout the plant, including pollen and nectar. These pesticides are detrimental to bees (compared to other pesticides) at concentrations in the parts per billion (ppb) (EPA 1994; Marletto et al. 2003). Imidacloprid is non-lethal to bumble bees when used as directed (e.g., Tasei et al. 2001). However, studies of its effects on bumble bees only tested one species, B. impatiens, as the representative for all North American species (Gels et al. 2002; Morandin and Winston 2003). Further study showed neonicotinoids had negative impacts on a European bumble bee species in the same subgenus as Western Bumble Bee (Tasei et al. 2001). Various life history traits of Western Bumble Bee (such as large body size, early emergence, long colony cycle, etc.) may make it especially vulnerable to accumulation of pesticides in the colony.
The widespread use of neonicotinoid pesticides throughout the range of the both subspecies has not been quantified and therefore is speculative only. Neonicotinoids are commonly used on golf courses and agricultural lands (Sur and Stork 2003). Large treated areas used for golf courses may expose bumble bees to large quantities of pesticides in otherwise suitable habitat (Tanner and Gange 2004).
Climate change is another possible threat to bumble bees (Williams and Osborne 2009). Bumble bee species with narrow climatic tolerances are more vulnerable to extrinsic threats (Williams et al. 2009).
Bumble bees require a constant suite of floral resources throughout the growing season to support colony growth, and without these resources, emerging queen, worker and colony growth is limited. Abundant food resources throughout the colony growth period ensure that local populations will persist. Only mated queens overwinter, so lack of abundant early season floral resources will cause colonies to die, or newly emerged queens to disperse.
Bumble bees are haplodiploid organisms with complementary sex determination which makes them extremely susceptible to extinction when effective population sizes are small (Zayed and Packer 2005). This is due to the ‘diploid male extinction vortex’ (Zayed and Packer 2005). Sex in bees, and most other haplodiploids, is determined by genotype at a single “sex locus”: hemizygotes (haploids) are males, heterozygotes are female and homozygotes are diploid males. Diploid males are usually sterile. The number of sex alleles in a population determines the proportion of diploids that are male and is itself determined primarily by the effective size of the population. This means that as bumble bee population size decreases, the frequency of diploid males increases. As diploid males are attempts at female production, their increasing production in smaller populations increases the rate of population decline causing a special case of the extinction vortex: “the diploid male extinction vortex.” This special form of genetic load is the largest known (Hedrick et al. 2006). In practical terms, if a bee population decreases to a few reproducing individuals, it is certain to become extinct even under stable environmental conditions unless its number increases within a few generations.
There are no federal or provincial laws that protect Western Bumble Bee, mitigate threats to this group or protect the species’ nest sites or habitat.
Some Canadian provinces are considering a legislated ban on neonicotinoid pesticides, which would mitigate this threat to bees (see Threats, Pollution). At present, none of the governments within the range of Western Bumble Bee have proposed legislation.
The provincial and territorial conservation status ranks, using NatureServe criteria, will be part of Wild Species 2015 (Hebert pers. comm. 2013). The Canada National Status Ranks (Canadian Endangered Species Conservation Council [CESCC 2011]) are: BC and AB - N3 (sensitive), YT and SK - N5 (undetermined). The global conservation status rank is GU (not ranked based on lack of information) (NatureServe 2013). The Xerces Society for Invertebrate Conservation (2013) Red-list assessment is Imperiled. The IUCN Red list (2013): None; and the species has not been reviewed or listed under the USA - federal Endangered Species Act.
In a review of global bumble bee declines globally, Williams and Osborne (2009) assess Common Eastern Bumble Bee (including its western form Western Bumble Bee) as Endangered using IUCN criteria: “A2: >50% population reduction since 1995 (inferred), causes may not be reversible and may not yet have ceased, based on very few records of individuals in the last four years, at least in the southwestern quarter of its range”.
The decline of Western Bumble Bee, and other members of the subgenus Bombus s. str., appears to have started in the mid-1990s (NRC 2007). Bombus franklini has disappeared from its range in western USA and is listed by the IUCN as critically endangered (Evan et al. 2008). Bombus affinis has recently been assessed as Endangered by COSEWIC in Canada. Bombus terricola also appears to have declined (NRC 2007, Evans et al. 2008). These declines have not yet been attributed to any one cause (NRC 2007, Evans et al. 2008).
There has not been an analysis that determines land ownership status across the species’ range. Much of land within the populated areas adjacent to the international border is privately owned, urban and/or managed intensively for agricultural purposes.
The Canadian range of Western Bumble Bee spans numerous provincial and national parks and protected areas. Recent (since 2003) records from protected areas include:
- British Columbia: Subspecies occidentalis has been recorded from local government parks: Boundary Bay Regional Park (2010, Metro Vancouver), Mount Douglas Park (2012, Saanich), Mount Tolmie Park (2012, Saanich), Christmas Hill Nature Sanctuary (2012, Saanich), Highrock (Cairn) Park (2012, Esquimalt), Brentwood Bay (Gardens) (2012, Central Saanich). Provincial parks include Manning Provincial Park and federal parks include Mount Revelstoke National Park and Yoho National Park. In 2013 surveys included provincial parks within the range of subspecies mckayi although data has not yet been analyzed (Sheffield pers. comm. 2013).
- Alberta: Subspecies occidentalis has been recorded in Cypress Hills Inter-provincial Park (AB-SK), Banff National Park, Dinosaur Provincial Park.
- Saskatchewan: Subspecies occidentalis has been recorded in Cypress Hills Inter-provincial Park (AB-SK), Grasslands National Park.
- Northwest Territories: Subspecies mckayi has been recorded in Nahanni National Park Reserve, Naats'ihch'oh National Park Reserve.
- Yukon: No records from parks or protected areas, although subspecies mckayi likely occurs in these areas.
The following people and their institutions are acknowledged for the data and specimens provided for this assessment: Elizabeth Elle (Simon Fraser University), Xerces Society for Invertebrate Conservation, Jamie Strange (USDA), Jonathan Koch (Utah State University), John Ascher (American Museum of Natural History), Ralph Cartar (University of Calgary), Robin Owen (Mount Royal College), Lincoln Best (York University), Cory Sheffield (York University), Jason Gibbs (York University), Jennifer Heron (BC Ministry of the Environment), Syd Cannings (Environment Canada), Rob Longair (University of Calgary), David Larson, Katrina Stipec (BC Ministry of Environment), Alison Leslie, Claudia Ratti (York University), Dave Fraser (BC Ministry of the Environment), Julie Wray (Simon Fraser University), Leif Richardson (Dartmouth College). We especially thank Ilona Naujokaitis-Lewis (University of Toronto) for analyzing the spatial data.
Cover photograph by David Inouye, Western Bumble Bee worker robbing an Ipomopsis flower.
Much recent (2013) data provided from projects funded by Earth Rangers and other funding to C. Sheffield (Royal Saskatchewan Museum). Resources and staff time from Canadian Wildlife Service and British Columbia Ministry of Environment.
- Alford, D.V. 1975. Bumble bees. London: Davis-Poynter.
- Ascher, J. and J. Pickering. 2013. Discover Life’s bee species guide and world checklist. Species account for Bombus occidentalis. Accessed November 12, 2013.
- Baron, G.L., Raine, N.E., Brown, M.J.F. (2014), Impact of chronic exposure to a pyrethroid pesticide on bumblebees and interactions with a trypanosome parasite. Journal of Applied Ecology. doi: 10.1111/1365-2664.12205
- Benton, T. 2006. Bumble bees. Harper-Collins, UK.
- Bertsch, A., M. Hrabé de Angelis, and G.K.H. Przemeck. 2010. A phylogenetic framework for the North American bumblebee species of the subgenus Bombus sensu stricto (Bombus affinis, B. franklini, B. monderatus, B. occidentalis and B. terricola) based on mitochondrial DNA markers (Hymenoptera: Apidae: Bombus). Beiträge zur Entomologie 60: 229-242.
- Best, L. 2010. York University, Toronto, ON.
- Breed, M.D., E. Guzman-Novoa, and G. J. Hunt. 2004. Defensive Behavior of honey bees: Organization, Genetics, and Comparisons with Other Bees. Annual Review of Entomology 49: 271-298.
- Brown M.J.F., R. Loosli and P. Schmid-Hempel. 2000. Condition-dependent expression of virulence in a trypanosome infecting bumble bees. Oikos 91: 421–427.
- Brown M., R. Schmid-Hempel and P. Schmid-Hempel. 2003. Strong context-dependent virulence in a host-parasite system: reconciling genetic evidence with theory. Journal of Animal Ecology 72: 994–1002.
- Cameron, S.A., J.D. Lozier, J.P. Strange, J.B. Koch, N. Cordes, L.F. Solter and T. Griswold. 2011. Patterns of widespread decline in North America bumble bees. Proceedings of the National Academy of Science 108:662-667.
- Cameron, S.A.; Hines, H. M. and Williams, P. 2007: A comprehensive phylogeny of the bumble bees (Bombus). Biological Journal of the Linnean Society 91: 161-188.
- Carriere, S. 2014. Department of Environment and Natural Resources (ENR) Government of the Northwest Territories, Yellowknife, NT. Personal communication to J. Heron.
- Colla, S.R., M.C. Otterstatter, R.J. Gegear and J.D. Thomson. 2006. Plight of the bumble bee: Pathogen spillover from commercial to wild populations. Biological Conservation 129:461-467.
- Colla, S.R. and Packer, L. 2008: Evidence for decline in eastern North American bumble bees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodiversity and Conservation 17: 1379-1391.
- Colla, S.R. and C.M. Ratti. 2010. Evidence for the decline of Western Bumble Bee (Bombus occidentalis Greene) in British Columbia Pan-Pacific Entomologist 86:32-34.
- COSEWIC 2010. COSEWIC assessment and status report for the Rusty-patched Bumble bee, Bombus affinis, in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa.
- Cox, C. 2001. Insecticide factsheet: Imidacloprid. Journal of Pesticide Reform 21:15-22.
- Environmental Protection Agency (EPA), U.S.A. 1994. Pesticide fact sheet: Imidacloprid, Washington, D.C. Mar. 18
- Evans, E., Thorp, R., Jepson, S., and S.H. Black. 2008. Status review of three formerly common species of bumble bee in the subgenus Bombus, (PDF Format, 830 Kb). Prepared for the Xerces Society for Invertebrate Conservation.
- Fitzpatrick, U., T.E. Murray, R.J. Paxton, J. Breen, D. Cotton, V. Santorum and M.J.F. Brown. 2007. Rarity and decline in bumble bees – A test of causes and correlates in the Irish fauna. Biological Conservation 136: 185-194.
- Gardner, A. 2007. The changing landscape of the southern Alberta foothills. Southern Foothills Study.
- Gels, J.A., D.W. Held, and D.A. Potter. 2002. Hazards of insecticides to the bumble bees Bombus impatiens (Hymenoptera: Apidae) foraging on flowering white clover in turf. Journal of Economic Entomology 95:722-728.
- Gillespie, S. 2010. Factors affecting parasite prevalence among wild bumble bees. Ecological Entomology 35: 737-747.
- Goulson, D. 2003. Bumble bees, Their Behaviour and Ecology. Oxford University Press, Oxford, 235 pp.
- Greef, M. and P. Schmid-Hempel. 2008. Sperm reduces female longevity and increases melanization of the spermatheca in the bumble bee Bombus terrestris. Insectes Sociaux 55: 313-319.
- Greene, J.W. 1858. Descriptions of several new hymenopterous insects from the northwest coast of America. Annals of Lyceum of Natural History of New York 7: 11-12.
- Hatfield, R.G. and G. LeBuhn. 2007. Patch and landscape factors shape community assemblages of bumble bees, Bombus spp. (Hymenoptera:Apidae), in montane meadows. Biological Conservation 139: 150-158.
- Heinrich, B. 2004. Bumble Bee Economics. Harvard University Press, U.S.A. 245 pp.
- Heron, J. 2013. BC Ministry of Environment, Vancouver, BC. Personal communication to Sheila Colla.
- Hobbs, G.A. 1968. Ecology of species of Bombus Latr. (Hymenoptera: Apidae) in southern Alberta. VI. Subgenus Bombus. Canadian Entomologist 100: 156-164.
- Javorek, S.K. and M.C. Grant. 2011. Trends in wildlife habitat capacity on agricultural land in Canada, 1986 – 2006. Canadian Biodiversity: Ecosystem Status and Trends 2010, Technical Thematic Report No. 14. Canadian Councils of Resource Ministers. Ottawa, ON. vi + 46 p.
- Knopp, D., L. Larkin and J. Heron. 2010. Surveys for Dun Skipper (Euphyes vestris) and Western Bumble Bee (Bombus occidentalis) in the Lower Fraser Valley, B.C., B.C. Ministry of Environment, Terrestrial Conservation Sciecne Section, UBC Office, 315 – 2202 Main Mall, Vancouver, BC. 53 pp.
- Koch, J.B., and J. Strange. 2009. Constructing a species database and historic range maps for North American bumble bees (Bombus sensu stricto Latrielle) to inform conservation decisions. Uludag Bee Journal 9:97-108.
- Koch, J.B., and J.P. Strange. 2012. The status of Bombus occidentalis and B. moderatus in Alaska with special focus on Nosema bombi incidence. Northwest Science 86: 212-220.
- Kosoir, A., W. Celary, P. Olejniczak, J. Fijal, W. Krol, W. Solarz, and P. Plonka. 2007. The decline of the bumble bees and cuckoo bees (Hymenoptera: Apidae: Bombini) of Western and Central Europe. Oryx 41:79-88
- Kraus, F.B., S. Wolf and R.F.A. Moritz. 2008. Male flight distance and population substructure in the bumble bee, Bombus terrestris. Journal of Animal Ecology 78:247-252.
- Laycock, I., Lenthall, K.M., Barratt, A.T., and Cresswell, J.E. (2012). Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology, 21(7), 1937-1945.
- Lilley, P., N. Page and J. Heron. 2010. Surveys for butterfly species at risk on private and municipal lands on southeastern Vancouver Island and the Gulf Islands, British Columbia (2010). B.C. Ministry of Environment, Terrestrial Conservation Science Section, UBC Office, 315 – 2202 Main Mall, Vancouver, BC.
- Longair, R. 2010. University of Calgary, Calgary, AB. Personal communication to Sheila Colla.
- Macfarlane, R.P. 1974. Ecology of Bombinae (Hymenoptera: Apidae) of Southern Ontario, with emphasis on their natural enemies and relationships with flowers. PhD, thesis, University of Guelph, Guelph, ON, Canada.
- Macfarlane, R.P., J.J. Lipa, and H.J. Liu. 1995. Bumble bee pathogens and internal enemies. Bee World 76: 130-148.
- Macfarlane, R.P. and Patten, K.D. 1997. Food sources in the management of bumble bee populations around cranberry marshes. In Proceedings of the 7th International Symposium on Pollination. K.W. Richards (ed.) Acta Horticulturae: 239-244.
- MacKenzie K.E. and M.L. Winston. 1984. Diversity and abundance of native bee pollinators on berry crops and natural vegetation in the lower Fraser valley, British Columbia. Canadian Entomologist 116: 965-974.
- Marks, D. and J. Heron. 2010. Surveys for Western Bumble Bee (Bombus occidentalis) and other arthropod species at risk on private and municipal lands in the south Okanagan – Similkameen River valleys, British Columbia 2010. B.C. Ministry of Environment, Terrestrial Conservation Sciecne Section, UBC Office, 315 – 2202 Main Mall, Vancouver, BC.
- Marletto, F., A. Patetta, and A. Manino. 2003. Laboratory assessment of pesticide toxicity to bumble bees. Bulletin of Insectology 56:155-158.
- Milliron, H.E. 1971: A monograph of the western hemisphere bumble bees (Hymenoptera: Apidae; Bombinae). I. The genera Bombus and Megabombus Subgenus Bombias. – Memoirs of the Entomological Society of Canada 82: 1-80.
- Morandin, L.A., and M.L. Winston. 2003. Effects of novel pesticides on bumble bee (Hymenoptera: Apidae) colony health and foraging ability. Community and Ecosystem Ecology 32:555-563.
- Morton, A., Routledge, R.C. Peet, and A. Ladwig. 2004. Sea lice infection rates on juvenile pink and chum salmon in the nearshore environment of British Columbia, Canada. Canadian Journal of Fish and Aquatic Sciences 61:147-158.
- National Research Council (NRC). 2007. Status of Pollinators in North America. The National Academies Press, Washington, DC.
- Otterstatter, M.C. 2001. The incidence of parasitism by conopid and phorid flies and its effects on the behavior of bumble bees (Hymenoptera: Apidae). M.Sc. thesis, University of Calgary.
- Otterstatter, M.C. 2004. Patterns of parasitism among conopid flies parasitizing bumble bees. Entomologia Experimentalis et Applicata 111: 133-139.
- Otterstatter, M.C., R.J. Gegear, S.R. Colla and J.D. Thomson. 2005. Effects of parasitic mites and protozoa on the flower constancy and foraging rate of bumble bees. Behavioral Ecology and Sociobiology, 58: 383-389.
- Otterstatter, M.C. and T.L. Whidden. 2004. Patterns of parasitism by tracheal mites (Locustacarus buchneri) in natural bumble bee populations. Apidologie 35: 351-357.
- Otterstatter, M.C., T.L. Whidden and R.E. Owen. 2002. Contrasting frequencies of parasitism and host mortality among phorid and conopid parasitoids of bumble-bees. Ecological Entomology 27: 229-237.
- Otterstatter, M.C., and J.D. Thomson. 2008 Does Pathogen Spillover from Commercially Reared Bumble Bees Threaten Wild Pollinators? PLoS One 3: e2771.
- Owen, R.E. 1988. Body size variation and optimal body size of bumble bee queens (Hymenoptera: Apidae). Canadian Entomologist 120: 19-27.
- Owen, R.E. 2010. Mount Royal College, Calgary, AB. Personal communication to Sheila Colla.
- Owen, R.E., T.L. Whidden. 2013. Discrimination of the bumble bee species Bombus occidentalis Greene and B. terricola Kirby by morphometric, colour and RAPD variation. Zootaxa 3608: 328–344.
- Packer, L. and R. Owen. 2001. Population genetic aspects of pollinator decline. Ecology and Society 5:4.
- Parkinson, L. and J. Heron. 2010. Surveys for Western Bumble Bee (Bombus occidentalis) and other arthropod species at risk in the lower mainland, British Columbia (2010). B.C. Ministry of Environment, Terrestrial Conservation Science Section, UBC Office, 315 – 2202 Main Mall, Vancouver, BC.
- Patten, K.D., C.H. Shanks, and D.F. Mayer. 1993. Evaluation of herbaceous plants for attractiveness to bumble bees for use near cranberry farms. Journal of Apicultural Research 32: 73-79.
- Pest Management Regulatory Agency (PMRA). 2001. Imidacloprid. Regulatory Note. REG2001-11. Ottawa: Health Canada, Pest Management Regulatory Agency. Available at
- Power, A.G., and C.E. Mitchell. 2004. Pathogen Spillover in Disease Epidemics. American Naturalist 164:S79-S89.
- Rasheed, S.A. and L.D. Harder. 1997. Economic motivation for plant species preferences of pollen-collecting bumble bees. Ecological Entomology 22: 209–219.
- Ratti, C.M. 2006. Bee abundance and diversity in berry agriculture. M.Sc. thesis, Simon Fraser University.
- Ratti, C.M., H.A. Higo, T.L. Griswold, and M.L. Winston. 2008. Bumble bees influence berry size in commercial Vaccinium spp. cultivation in British Columbia. The Canadian Entomologist 140: 348-363.
- Ratti, C.M. and S.R. Colla. 2010. Discussion of the presence of an eastern bumble bee species (Bombus impatiens Cresson) in western Canada. Pan-Pacific Entomologist 86:29.31.
- Richards, K.W. 1978. Nest site selection by bumble bees (Hymenoptera: Apidae) in southern Alberta. The Canadian Entomologist 110: 301-318.
- Schmid-Hempel, P. 1998. Parasites in social insects. Princeton University Press, Princeton, NJ.
- Sheffield, Cory. 2013. Royal Saskatchewan Museum, Regina, SK. Personal communication to J. Heron.
- Stephen, W.P. 1957. Bumble Bees of Western America. Oregon State College, Agricultural Experiment Station, Technical Bulletin 40.
- Stephen, W.P., Bohart, G.E., and Torchio, P.F. 1969. The Biology and External Morphology of Bees, with a Synopsis of the Genera of Northwestern America. Agricultural Experiment Station, Oregon State University, Corvallis, Oregon.
- Stephen, W.P., and Koontz, T. 1973a. larvae of the Bombini. I. Interspecific variation in larval head characteristics of Bombus (Humenoptera: Apoidea). Melanderia 13: 1-12.
- Stephen, W.P., and Koontz, T. 1973b. Larvae of the Bombini. II. Developmental changes in the preadult stages of Bombus griseocollis (Degeer). Melanderia 13: 13-29.
- Stephen, W.P., and Koontz, T. 1973. Larvae of the Bombini. I. Interspecific variation in larval head characteristics of Bombus (Humenoptera: Apoidea). Melanderia 13: 1-12.
- Stephen, W.P., and Koontz, T. 1973. Larvae of the Bombini. II. Developmental changes in the preadult stages of Bombus griseocollis (Degeer). Melanderia 13: 13-29.
- Stotyn, S. and D. Tate. 2012. Wild Bumble Bees of the Nahanni River Region, Northwest Territories. Report submitted to Parks Canada Agency Nahanni Park Reserve of Canada. 9 pp.
- Strange, J. 2010. United States Department of Agriculture, Bee Lab, Utah. Personal communication to Sheila Colla.
- Stout, J.C and D. Goulson. 2000. Bumble bees in Tasmania: their distribution and potential impact on Australian flora and fauna. Bee World 81: 80-86.
- Strong, W. 1992. Ecoregions of Alberta. Alberta Forestry, Lands and Wildlife, Edmonton, AB.
- Sur, R. and A. Stork. 2003. Uptake, translocation and metabolism of imidacloprid in plants. Bulletin of Insectology 1:35-40.
- Tanner, R.A. and A,C, Gange, 2004. Effects of golf courses on local biodiversity. Landscape and Urban Planning 71: 137-146.
- Tasei, J.N., G. Ripault, and E. Rivault. 2001. Hazards of Imidacloprid seed coating to Bombus terrestris (Hymenoptera: Apidae) when applied to Sunflower. Journal of Economic Entomology 94:623-627.
- Thomson, D.M. 2006. Detecting the effects of introduced species: a case study of competition between Apis and Bombus. Oikos 114: 407-418.
- Thorp, R.W., D.S. Horning and L.L. Dunning. 1983. Bumble bees and cuckoo bumble bees of California (Hymenoptera: Apidae). Bulletin of the California Insect Survey 23: viii.
- Thorp, R.W. and M.D. Shepherd. 2005. Subgenus Bombus Latreille 1802 (Apidae: Apinae: Bombini). In Shepherd, M.D., D.M. Vaughan, and S.H. Black (Eds.) Red List of Pollinator Insects of North America, (PDF Format, 23 Kb). CD-ROM Version 1 (May 2005). Portland, OR: The Xerces Society for Invertebrate Conservation. Accessed November 12, 2013.
- Turner, N. 1975. Food plants of British Columbia Indians Part 1 Coastal Peoples. Handbook 34. British Columbia Provincial Museum, Victoria, BC. 264 pp.
- United States Department of Agriculture (USDA) (2010) U.S. National Pollinating Insects Database, Agriculture Research Service, Bee Biology and Systematics Laboratory, Logan, Utah (Accessed 2010-30-11)
- Westcott, L. and J. Heron. 2010. Surveys for Western Bumble Bee (Bombus occidentalis) and other arthropod species at risk on private and municipal lands in the West Kootenay region, British Columbia, August 2010. B.C. Ministry of Environment, Terrestrial Conservation Science Section, UBC Office, 315 – 2202 Main Mall, Vancouver, BC.
- Whidden, T. and Owen, R.E. 2011. Frequencies of diploid males in natural populations of three North American Bumble Bee (Bombus) species (Hymenoptera:Apidae). Annals of the Entomological Society of America 104:83-87.
- Whitehorn, P.R., O’Connor, S., Wackers, F.L., and Goulson, D. (2012). Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science, 336 (6079), 351-352.
- Whittington, R. and M.L. Winston. 2003. Effects of Nosema bombi and its treatment fumagillin on bumble bee Bombus occidentalis colonies. Journal of Invertebrate Pathology 84: 54 – 58.
- Whittington, R. and M.L. Winston. 2003. Are bumble bee colonies in tomato greenhouses obtaining adequate nutrition? The Canadian Entomologist 135: 883 -892.
- Williams, P.H. 2010. Bombus of the World, Accessed November 30, 2010.
- Williams, P.H., and J.L. Osborne. 2009. Bumble bee vulnerability and conservation world-wide. Apidologie 40:367-387.
- Williams, P.H., S.R. Colla and Z. Xie. 2009. Bumble bee vulnerability: common correlates of winners and losers across three continents. Conservation Biology 23: 931-940
- Williams, P.H., R.W. Thorp, L.L. Richardson, and S.R. Colla. (2014). Guide to the bumble bees of North America. Princeton University Press. 208 pp.
- Williams P.H. 1989. Bumble bees - and their decline in Britain. Ilford: Central Association of Bee-Keepers. 15 pp. [Online].
- Williams, P.H., Brown, M.J.F., Carolan, J.C., Goulson, D., An, Jiandong, Aytekin, A.M., Best, L.R., Byvaltsev, A.M., Cederberg, B., Dawson, R., Huang, J., Ito, M., Monfared, A., Raina, R.H., Schmid-Hempel, P., Sheffield, C.S., Šima, P., Xie, Z. 2012. Unveiling cryptic species of the bumblebee subgenus Bombus s. str. worldwide with COI barcodes (Hymenoptera: Apidae). Systematics and Biodiversity 10: 21-56. doi: 10.1080/14772000.2012.664574
- Winston, M.L. and L.H. Graf. 1982. Native bee pollinators of berry crops in the Fraser Valley of British Columbia. Journal of the Entomological Society of British Columbia 79:14-20.
- Wray, J. 2013. Simon Fraser University, Burnaby, BC. Personal communication to Jennifer Heron.
- Zayed, A. and L. Packer. 2005. Complementary sex determination substantially increases extinction proneness of haplodiploid populations. Proceedings of the National Academy of Sciences 102:10742-10746.
Sheila R. Colla has studied various aspects of bumble bee ecology and behaviour throughout North America. Previously she worked as a research assistant to Dr. James Thomson, Dr. Michael Otterstatter, and Dr. Robert Gegear at the University of Toronto, St. George Campus looking at pathogen spillover from managed to wild bumble bee populations. She is currently a doctorate student and recipient of the NSERC Alexander Graham Bell Canadian Graduate Scholarship at York University, Toronto, ON under the supervision of Dr. Laurence Packer. Her dissertation examines changes in bumble bee communities over the past century and looks into some of the causes for observed declines. In addition, she is a member of the North American Pollinator Protection Campaign and her research has been featured in The Washington Post, Canadian Gardening, The Toronto Star, BioScience, CBC’s Quirks and Quarks, and The Daily Planet for Discovery Channel Canada.
Michael C. Otterstatter has worked on bumble bees and their parasites since 1997. This work has been done in conjunction with noteworthy bumble bee researchers, including Robin Owen, Lawrence Harder, James Thomson, Robert Gegear, and Sheila Colla. Michael has done several studies on the bumble bees of Alberta, with particular attention to parasitic flies and mites (MSc work), and on the bumble bees of southern Ontario, with particular attention to parasitic protozoa and the spillover of disease from commercial to wild populations (PhD work). This latter work has been featured in New Scientist, Scientific American, and the New York Times. Additional studies have included an investigation of the natural enemies of bumble bees in southern Chile. Michael currently works as an epidemiologist and pursues projects in bumble bee conservation with a variety of collaborators.
Leif L. Richardson is a PhD candidate at Dartmouth College, Hanover, NH, where he examines the effects of floral nectar chemistry on bees and their parasites. Additionally, he combines bee surveys with museum collections data to study changes in the distribution of North American bumble bees. With Sheila R. Colla and other co-authors he produced a Guide to the Bumble Bees of North America, to be published by Princeton University Press in 2014.
Cory S. Sheffield has been studying bees and pollination since 1993, as part of undergraduate honours studies at Acadia University, Wolfville, NS. He continued graduate studies (MSc) of insect-plant interactions at Acadia, and at Agriculture and Agri-Food Canada (AAFC), Kentville, NS from 1994 - 2006. Cory did graduate studies (PhD) at the University of Guelph, ON while continuing to work at the AAFC. These studies focused on the bee fauna of Nova Scotia, including their diversity and contributions to crop pollination. During this time, Cory and several co-authors published on the re-discovery of Epeoloides pilosulus in Nova Scotia, which was thought extinct. Cory then worked on post-doctoral studies at York University, ON in bee taxonomy and DNA barcoding, followed by a research associate position in bee taxonomy with the Canadian Pollination Initiative (CANPOLIN). He is now research scientist and curator of invertebrate zoology at the Royal Saskatchewan Museum in Regina, SK. His research continues to focus on bees: he has published on the taxonomy of Canadian/North American bees, the utility of DNA barcoding for bees, bee physiology, pollination contributions and diversity of the Canadian bee fauna.
Data from numerous North American collections were used for analysis presented in this report. More than 70 individuals and institutions contributed to the dataset, and are listed at: www.leifrichardson.org/bbna.html.