Wild species 2010: chapter 8
Vascular plants
Tracheophyta - Plants characterized by the possession of true roots, shoots and leaves containing specialized vascular tissue through which liquids are conducted.

Quick facts
- There are over 352 000 species of vascular plants in the world. More than 95% of vascular plants are flowering plants, also called angiosperms (e.g. grasses, orchids, maple trees). The other types of vascular plants are gymnosperms (cone-bearing trees, e.g. pine trees, spruce trees) and seedless plants (e.g. ferns, horsetails). 5111 species of vascular plants have been found in Canada.
- When excluding species ranked as Extinct, Extirpated, Undetermined, Not Assessed, Exotic or Accidental, the majority (71%) of vascular plants in Canada have Canada General Status Ranks (Canada ranks) of Secure, while 13% have Canada ranks of Sensitive, 12% have Canada ranks of My Be At Risk and 4% have Canada ranks of At Risk.
- A total of 25 species of vascular plants that used to be present in Canada are now Extirpated from the country.
- A large number of species (1252) of vascular plants are Exotic. They represent 24% of all species of vascular plants found in the Canada. This is the highest proportion of any taxonomic group covered in this report.
- All vascular plants were also assessed in the report Wild Species 2005. In the 2000 report, only ferns and orchids were assessed.
Background
Plants play a critical role in maintaining life on earth, because they are one of the few groups of organisms that can make their own food. Through the chemical process of photosynthesis involving green chlorophyll pigments, plants use energy from the sun to convert water and carbon dioxide into oxygen and sugar, which is used as a food source by plants, and by plant-eating animals. Therefore, plants produce two of the resources that all animals need to survive; food and oxygen. In addition, plants play important roles in the environment by helping to regulate climate, providing habitat for wildlife, contributing to nutrient cycles and soil creation, improving air and water quality, and reducing soil erosion.
Most familiar plants, such as ferns, orchids, herbs, grasses, shrubs and trees are vascular plants. All vascular plants have roots, leaves and a vascular system, which transports water, sugars and nutrients throughout the plant. Vascular plants are the largest group of plants in the world, and form the dominant vegetation over much of the earth’s landmass.
The oldest vascular plants are the seedless plants, including the ferns, club mosses, and horsetails. Seedless plants dominated the world in the Carboniferous period, approximately 300 million years ago. Dead plants from this period formed coal beds from which coal is still mined today. All seedless plants reproduce using spores. For more information about seedless plants, please consult the ferns section in the Wild Species 2000 report.
From the seedless plants evolved two groups of seed plants; the cone-bearing plants (the gymnosperms, e.g. pine trees, spruce trees) and the flowering plants (the angiosperms, e.g. grasses, orchids, maple trees, oak trees). As their name suggests, seed plants use seeds rather than spores for reproduction. Seeds are simply embryos surrounded by a seed coat, which protects the embryo from drought, extreme temperatures and other harsh conditions. Most seeds also contain a food source for the developing plant. Angiosperms surround their seeds with an additional layer of protection, the fruit. The fruit protects the seeds, and often provides a mechanism for spreading them over long distances. Fruits can be fleshy (e.g. blueberries, cranberries) or dry (e.g. the keys of the Sugar Maple, Acer saccharum, are actually a type of fruit!).
Vascular plants are usually rooted to one spot, so finding a mate for reproduction can be challenging. Gymnosperms produce pollen (male sex cells) and eggs in separate male and female cones. The pollen is released into the air and carried by the wind to a female cone, where it will fertilize the eggs. The process of transporting pollen from the male cone to the female cone is called “pollination”. The chance of successful pollination is fairly small, so gymnosperms produce large amounts of pollen to increase the chance that some of it will meet with a female cone of the same species.
In angiosperms, all the sex organs are located within flowers (although male and female organs are not necessarily found within the same flower, or even on the same plant). While some angiosperms rely on wind pollination (e.g. grasses), most rely on animal pollinators such as insects, birds and even bats, to carry pollen between their flowers. Angiosperms attract potential pollinators with brightly coloured petals, sweet fragrances, or by producing nectar. Some species have evolved to attract very specific pollinators. For example, the main pollinators of the Cardinal Flower (Lobelia cardinalis) are hummingbirds, which are attracted to the plant by the bright red flowers. The long, narrow tube of cardinal flowers are the perfect shape for a hummingbird to insert its bill and retrieve the nectar. As the hummingbird feeds, pollen is deposited on its head; then the pollen will be brushed off at the next flower, where it will fertilize the eggs. By attracting specific types or species of pollinators, plants can increase the chances that their pollen reaches another flower of the same species.
Plants are amazing chemical factories that make a variety of different products, from defensive chemicals that protect the plant from predation, disease and parasites, to hormones that control the plant’s growth. Humans have long known that many of the chemicals that plants produce have useful medicinal properties. For example, Common Yarrow (Achillea millefolium) is a well known traditional cure for staunching wounds and treating fevers, colds and other ailments. Today, Common Yarrow can be found in more than 20 pharmaceutical products sold in Canada.
Status of knowledge
The study of plants has a long history in Canada, from Aboriginal Peoples who relied on plants for food, shelter, clothing, raw materials and medicines, to the early European settlers, some of whom became famous botanists (e.g. Catherine Parr Trail, John Macoun). Much of today’s research is centered on plants that are important for agriculture, forestry or medicine, using new genetic and molecular tools to study a huge variety of topics including plant physiology, genetics, biotechnology and interactions between plants and pests.
Relative to other species groups covered in this report, the distribution and status of many vascular plant species in Canada is fairly well known, particularly in southern Canada. Nevertheless, systematic surveys are still uncovering new information, such as the recent discovery of a new tree species for Canada, the Swamp Cottonwood (Populus heterophylla, see species spotlight for more details). As well as discovering new species, systematic surveys improve information on the distribution and abundance of vascular plants. For example, in New Brunswick, the first systematic rare plant survey of the upper St. John River in 2001 and 2002, showed that two species of grasses, Mat Muhly (Muhlenbergia richardsonis) and Little Bluestem (Schizachyrium scoparium), thought to be rare in the province, were actually more common than previously believed.
The distribution of vascular plants in remote areas, and in northern Canada is less well known. This is partly because fewer people (both amateurs and professionals) are studying plants in these regions, despite the presence of unique plant communities and endemic species found nowhere else on earth. In addition, many plant specimens from northern Canada have been housed in national collections in southern Canada. While some of these collections have been well documented and catalogued (e.g. the National Herbarium at the Canadian Museum of Nature), others have only recently been fully catalogued to reveal new information about northern vascular plants.
Plant ecology is the study of how plants interact with, and are affected by, the world around them; both the physical world (e.g. temperature, soil type, light levels) and the “biological” world (interactions with other plants, animals, fungi, etc). This is important for understanding a variety of topics, including plant distribution, how plants survive in different environments, and plant productivity. Plant ecology also helps researchers understand how changes in the environment (e.g. climate change, invasion of exotic species) might affect plant communities. For example, researchers in Quebec, working on grasses in pastureland, have shown that exposure to increased levels of carbon dioxide can influence plant succession (changes in community composition through time) and species richness. Knowledge of plant ecology can also help conserve and restore native plant communities. For example, Canadian researchers are working on restoring surface vegetation to bogs that have been mined for peat. This is the first step in restoring mined bogs back to a functioning ecosystem.
Richness and diversity in Canada
Relative to other groups covered in this report, the species richness of vascular plants is high across the country (figure 8), peaking in British Columbia (2127 native species). The flora of British Columbia is particularly diverse within a Canadian context, because many hundreds of native species of vascular plants found there are found nowhere else in Canada. Other regions of Canada known as centers of vascular plant species diversity, and for concentrations of endemic plants include the Central Yukon Plateau, Ellesmere and Baffin Islands, the sand-dune region of Lake Athabasca, Saskatchewan, and the Gulf of St. Lawrence.
The proportion of Exotic plant species is high across the country, but tends to be highest in the provinces of eastern Canada (22% to 36%) and lowest in the territories (2% to 10%).
Species spotlight - Stairstep Moss
The Showy Lady’s-slipper (Cypripedium reginae) is known as the “queen” of the orchids. This species has beautiful pink and white flowers, and grows up to 80 cm tall. Each flower has three petals, the lowest of which is folded into a pouch. This pouch is said to resemble a slipper, giving the lady’s-slipper orchids (genus Cypripedium) their name. Showy Lady’s-slippers require very nutrient-rich soil and are found in fens and wet, open forests throughout eastern and central Canada.
Like all orchids, Showy Lady’s-slippers have an intriguing and complicated life cycle. Orchid seeds are very small, almost microscopic, and do not contain a food source to nourish the germinating seed. In order to survive and grow, the seed must come in to contact with a specific soil fungus, which provides enough nutrients for it to grow into a small plant. Once the plant produces leaves, it can make its own food through photosynthesis. However, it can take up to 12 years for this slow-growing plant to produce flowers! To protect the plant from hungry predators during its long life cycle, the shoots and leaves of Showy Lady’s-slippers are covered in stinging hair-like structures. The stinging “hairs” strongly discourage invertebrates, and larger predators, such as White-tailed Deer (Odocoileus virginianus), from eating the plant.
Showy Lady’s-slippers are pollinated by insects, typically small bees or flies. However, unlike many other angiosperms, lady’s-slipper orchids do not produce nectar to attract visiting insects. Instead, insects are thought to be attracted to the flower by the colours and patterns of its petals, and by its scent. Once an insect enters the flower, it becomes trapped within the folded lower petal, or slipper. To escape, the insect must push past the pistil (the female part of the flower), where pollen is brushed off the insect’s body, to fertilize the eggs. Finally, the insect pushes past the stamen (the male part of the flower), where it picks up more pollen, before leaving the flower.
Due in part to its long, complicated life cycle, this species is particularly vulnerable to increased rates of adult and juvenile mortality. For example, harvesting by gardeners and other collectors has led to the loss of entire populations, despite the fact that this species does not grow at all well in artificial settings. Other concerns include habitat loss, changes in the abundance or distribution of insect pollinators or soil fungi, and trampling of the inconspicuous young shoots and soil compaction by humans attracted by the beauty of the adult plants. Showy Lady’s-slipper is widespread and locally common in much of eastern Canada, and has a Canada rank of Secure.
Species spotlight - carnivorous plants
Carnivorous plants have the fascinating ability to capture and kill insects and other small animals. Carnivorous plants live primarily in nutrient-poor bogs and other habitats with acidic or wet soils. In these habitats, essential nutrients such as nitrogen, are difficult to obtain, so carnivorous plants supplement their nutrient supply by digesting the insects that they capture. Interestingly, this ability has evolved separately in several different plant families, so modern carnivorous plants are quite varied in structure and the methods they use for capturing insects.
There are 20 different species of carnivorous vascular plants in Canada, representing four different groups; pitcher plants (genus Sarracenia, one species), sundews (genus Drosera, five species), butterworts (genus Pinguicula, three species) and bladderworts (genus Utricularia, 11 species). Each group has its own unique method of capturing and digesting prey. For example, sundews have modified leaves covered in red, hair-like structures, each topped with a glistening drop of sticky mucus. Insects are attracted by the sundew’s colourful appearance and sweet-smelling secretions, but once they step onto a leaf, they quickly become stuck. As the insect struggles, more hairs are drawn inwards to help secure the insect. Glands on the hairs secrete enzymes, which digest the prey, allowing the leaf to absorb the nutrients.
The most complicated active traps developed by carnivorous plants are found in the bladderworts, which capture tiny aquatic prey. Aquatic bladderworts float freely in shallow water, without the benefit of roots to draw nutrients from the soil. Their leaves are very finely divided and contain numerous tiny chambers or bladders. Each bladder operates as a vacuum trap, whose door is triggered by hair-like structures. When a prey item brushes against the “hairs”, the door of the bladder flips open and the prey is sucked into the trap along with the surrounding water. Once inside, the prey is digested and the water is pumped back outside, re-creating the vacuum and leaving the trap ready for the next victim. Amazingly, the door of the bladder trap opens in less than 0.002 seconds, one of the fastest response-times in the plant world!
The majority of Canada’s carnivorous plants are ranked Secure, but two species (California Butterwort, Pinguicula macroceras, and Yellowish-white Bladderwort, Utricularia ochroleuca) have a Canada rank of Sensitive and one species (Thread-leaved Sundew, Drosera filiformis) has a Canada rank of At Risk.
Carnivorous plants are an important component of nutrient-poor wetlands across the country. The most important threat is habitat destruction through peat mining, wetland drainage and succession, although collecting for the commercial plant trade is also a concern for all species of carnivorous plants.
Species spotlight - Tamarack
Tamarack (Larix laricina), also known as Hackmatack or Eastern Larch, is found in every province and territory of Canada, and is the official tree of the Northwest Territories. Tamaracks are unusual in the plant world because they are deciduous conifers! Like other conifers, Tamaracks have cones and needle-like leaves, but each autumn, their soft, flexible needles turn a beautiful golden colour and fall off, to be replaced again in spring.
Tamaracks grow in a range of soil conditions, but are typically found in cold, poorly drained soils, in bogs and other peatlands. A small to medium sized tree, mature plants are typically 15 to 23 m tall, up to 40 cm in diameter and can live for about 150 to 180 years. Tamaracks are common in the boreal forest and are considered a very cold-hardy tree. In order to survive the cold winter, Tamaracks take advantage of a process called “extracellular freezing”. As water freezes, ice-crystals are formed, which can damage cells irreparably. However, during extracellular freezing, water is squeezed out of the tree’s cells and stored in the air spaces between the cells. This prevents the cells from being damaged when ice crystals form, allowing Tamaracks to survive as far north as the tree line.
Although Tamaracks are not an important commercial species, they are harvested and sold to make pulp products. The hard, rot-resistant wood is also used to make poles, fence posts and railway ties, while in the past its roots were prized for shipbuilding. Aboriginal Peoples have used Tamarack for many purposes including food, medicine, and construction of canoes and snowshoes. The roots can be used for weaving bags and for sewing bark canoes together.
A variety of animals feed on the leaves, cones, seeds or bark of Tamarack trees, such as Sharp-tailed Grouse (Tympanuchus phasianellus), American Black Bear (Ursus americanus), Snowshoe Hare (Lepus americanus), North American Porcupine (Erethizon dorsatum), and Red Squirrel (Tamiasciurus hudsonicus). Major pests of the Tamarack include Larch Sawfly (Pristiphora erichsonii) and Eastern Larch Beetle (Dendroctonus simplex).
Tamarack has a Canada rank of Secure and is also ranked Secure in each of the provinces and territories. Its native cousins, Subalpine Larch (Larix lyallii) and Western Larch (Larix occidentalis), found only in Alberta and British Columbia, also have Canada ranks of Secure.
Species spotlight - Swamp Cottonwood
In 2002, while carrying out a biological survey of Bickford Woods in southern Ontario, researchers were amazed to discover a new species for Canada. This new species is not small or easily overlooked, but is in fact a stand of over 60 mature trees, growing up to 27 m tall! The new species is Swamp Cottonwood (Populus heterophylla), a deciduous tree belonging to the willow family (family Salicaceae) and closely related to the poplars, aspens and other cottonwoods (genus Populus). Swamp Cottonwood occurs fairly commonly in the southeast United States, but is rarer in the northeast United States.
This medium-sized deciduous tree grows up to 40 m in height, in wet soils of swamps and floodplains. Its leaves are large and rounded, and the bark is thick and rough with a reddish colour. As with other poplars, Swamp Cottonwood flowers grow very early in the spring, even before the leaves appear. The flowers grow in the form of dangling catkins and each tree has either male or female flowers, never both. Pollen is carried by the wind from male to female flowers, where the eggs are fertilized and seeds begin to develop. The seeds are light with hair-like tufts, so they can be carried by wind or float on water. The Swamp Cottonwood’s habitat is often flooded early in the spring, when the seeds are produced. The seeds fall into the water and float until water levels decrease, at which time the seeds are deposited on the wet soil where they can germinate and grow. Swamp Cottonwoods grow best in open areas with little shade, and they are often found along the edges of swamps and rivers. Mature trees occur in low numbers throughout the species’ range and are not a major component of any forest-type.
The story of the discovery of the Swamp Cottonwood in Canada reminds us that there are many discoveries still to be made about Canadian wildlife, even in densely populated regions like southern Ontario. Due to its highly restricted Canadian distribution and small population size, Swamp Cottonwood has a Canada rank of May Be At Risk.
Results of general status assessment
Of the 5111 species of vascular plants found in Canada, 53% have Canada ranks of Secure (2635 species, figure 8 and table 8), while 9% have Canada ranks of Sensitive (484 species), 9% have Canada ranks of May Be At Risk (444 species) and 3% have Canada ranks of At Risk (136 species). Less than 1% of vascular plant species have Canada ranks of Extirpated (25 species), and none have Canada ranks of Extinct. In total, 24% of vascular plant species have a Canada rank of Exotic (1252 species), the highest proportion of Exotic species of any species group covered in this report. Finally, 2% of Canada’s vascular plant species have Canada ranks of Undetermined (112 species), and less than 1% have Canada ranks of Not Assessed (23 species).
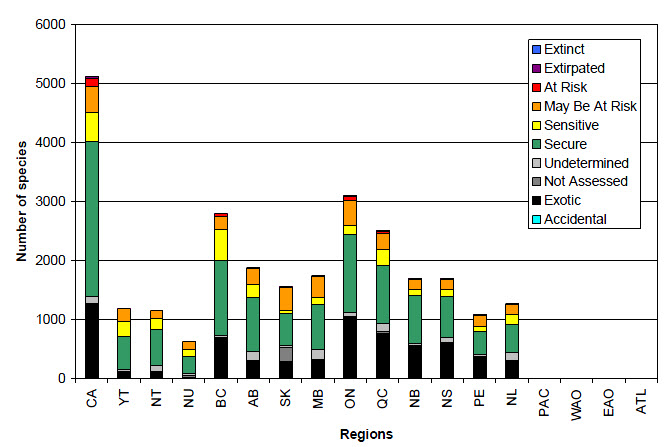
Long description for Figure 8
Figure 8 shows the results of the general status assessments for vascular plant species in Canada in the Wild Species 2010 report. The bar graph shows the number of vascular plant species ranked as Extinct, Extirpated, At Risk, May Be at Risk, Sensitive, Secure, Undetermined, Not assessed, Exotic, and Accidental in Canada, each province and territory and the 4 oceanic regions. Of the 5111 species occurring in Canada, 25 were ranked as Extirpated, 136 as At Risk, 444 as May Be at Risk, 484 as Sensitive, 2635 as Secure, 112 as Undetermined, 23 as Not Assessed, and 1252 as Exotic. Of the 1183 species occurring in the Yukon, 1 was ranked as Extirpated, 1 as At Risk, 218 as May Be At Risk, 256 as Sensitive, 549 as Secure, 38 as Undetermined, 20 as Not Assessed, and 100 as Exotic. Of the 1158 species occurring in the Northwest Territories, 146 were ranked as May Be at Risk, 187 as Sensitive, 602 as Secure, 103 as Undetermined, 3 as Not Assessed and 117 as Exotic. Of the 626 species occurring in Nunavut, 137 were ranked as May Be at Risk, 111 as Sensitive, 296 as Secure, 39 as Undetermined, 29 as Not Assessed and 14 as Exotic. Of the 2801 species occurring in British Columbia, 2 were ranked as Extirpated, 46 as At Risk, 229 as May Be At Risk, 523 as Sensitive, 1282 as Secure, 28 as Undetermined, 17 as Not Assessed and 674 as Exotic. Of the 1874 species occurring in Alberta, 1 was ranked as Extirpated, 6 as At Risk, 270 as May Be At Risk, 221 as Sensitive, 924 as Secure, 148 as Undetermined, 4 as Not Assessed and 300 as Exotic. Of the 1558 species occurring in Saskatchewan, 3 were ranked as Extirpated, 11 as At Risk, 388 as May Be At Risk, 49 as Sensitive, 549 as Secure, 30 as Undetermined, 236 as Not Assessed and 292 as Exotic. Of the 1740 species occurring in Manitoba, 13 were ranked as At Risk, 360 as May Be at Risk, 117 as Sensitive, 768 as Secure, 168 as Undetermined, 1 as Not Assessed and 313 as Exotic. Of the 3099 species occurring in Ontario, 25 were ranked as Extirpated, 62 as At Risk, 427 as May Be at Risk, 149 as Sensitive, 1312 as Secure, 73 as Undetermined and 1051 as Exotic. Of the 2510 species occurring in Quebec, 11 were ranked as Extirpated, 47 as At Risk, 265 as May Be at Risk, 272 as Sensitive, 984 as Secure, 142 as Undetermined, 30 as Not Assessed and 759 as Exotic. Of the 1690 species occurring in New Brunswick, 6 were ranked as Extirpated, 10 as At Risk, 161 as May Be at Risk, 114 as Sensitive, 810 as Secure, 31 as Undetermined and 558 as Exotic. Of the 1695 species assessed in Nova Scotia, 11 were ranked as Extirpated, 9 as At Risk, 160 as May Be at Risk, 126 as Sensitive, 699 as Secure, 74 as Undetermined and 616 as Exotic. Of the 1076 species occurring in Prince Edward Island, 8 were ranked as Extirpated, 1 as At Risk, 188 as May Be at Risk, 86 as Sensitive, 381 as Secure, 44 as Undetermined and 368 as Exotic. Of the 1272 species occurring in Newfoundland and Labrador, 18 were ranked as At Risk, 167 as May Be at Risk, 167 as Sensitive, 485 as Secure, 136 as Undetermined, 15 as Not Assessed and 284 as Exotic. There were no species listed as occurring in the oceanic regions.
Comparison with previous Wild Species reports
The Wild Species 2010 report marks the second national assessment of all vascular plants in Canada (all species of vascular plants were first assessed in the report Wild Species 2005). However, ferns and orchids were also assessed in the Wild Species 2000 report. We then present first a comparison of all species of vascular plants, and then present trends specifically for ferns and orchids.
All species of vascular plants
The rank category of May Be At Risk had the greatest decrease in the number of species of vascular plants, while the category of Secure had the greatest increase in the number of species (table 8). The category Exotic had also a great increase in the number of species. Since the last assessment in 2005, a total of 37 species were added to the national list of vascular plants.
A total of 495 changes were made in the Canada ranks for all species of vascular plants since the last assessment in 2005. Among these changes, 54 species had an increased level of risk, 132 species had a reduced level of risk, 84 species were changed from or to the ranks Undetermined, Not Assessed, Exotic or Accidental, 131 species were added, and 94 species were deleted. Main reasons for changes were improved knowledge of the species and taxonomic changes (table 9).
| Canada rank | Wild Species reports 2000 | Wild Species reports 2005 | Wild Species reports 2010 | Average change between reports | Total change since first report |
|---|---|---|---|---|---|
| 0 Extinct/Extirpated | - | 22 (0%) | 25 (0%) | - | +3 species |
| 1 At Risk | - | 110 (2%) | 136 (3%) | - | +26 species |
| 2 May Be At Risk | - | 552 (11%) | 444 (9%) | - | -108 species |
| 3 Sensitive | - | 460 (9%) | 484 (9%) | - | +24 species |
| 4 Secure | - | 2572 (51%) | 2635 (53%) | - | +63 species |
| 5 Undetermined | - | 112 (2%) | 112 (2%) | - | Stable |
| 6 Not Assessed | - | 30 (1%) | 23 (0%) | - | -7 species |
| 7 Exotic | - | 1216 (24%) | 1252 (24%) | - | +36 species |
| 8 Accidental | - | 0 (0%) | 0 (0%) | - | Stable |
| Total | - | 5074 (100%) | 5111 (100%) | - | +37 species |
| Code | Description | Number of species | Proportion of all changes |
|---|---|---|---|
| B | Change due to biological change in species’ population size, distribution or threats. | 42 | 8% |
| C | Change due to new COSEWIC assessment. | 37 | 7% |
| E | Change due to error in previous ranks. | 8 | 2% |
| I | Change due to improved knowledge of the species. | 301 | 61% |
| P | Change due to procedural changes. | 5 | 1% |
| T | Taxonomic change. | 102 | 21% |
| Total | - | 495 | 100% |
Note: For vascular plants, the ranks of a large number of species changed since the last assessment. We then present only a summary of the reasons for changes.
Fern species only
Ferns were first assessed in the report Wild Species 2000. A total of three species were added to the national list since this first assessment (table 10). Over time, the highest increase was observed in the number of species ranked as May Be At Risk (+3 species) and the highest decrease was observed in the number of species ranked as Secure (-2 species).
| Canada rank | Wild Species reports 2000 | Wild Species reports 2005 | Wild Species reports 2010 | Average change between reports | Total change since first report |
|---|---|---|---|---|---|
| 0 Extinct/Extirpated | 0 (0%) | 0 (0%) | 0 (0%) | Stable | Stable |
| 1 At Risk | 3 (2%) | 5 (4%) | 5 (4%) | +1 species | +2 species |
| 2 May Be At Risk | 18 (15%) | 24 (19%) | 21 (17%) | +2 species | +3 species |
| 3 Sensitive | 20 (16%) | 15 (12%) | 19 (15%) | -1 species | -1 species |
| 4 Secure | 79 (65%) | 78 (63%) | 77 (61%) | -1 species | -2 species |
| 5 Undetermined | 0 (0%) | 0 (0%) | 1 (1%) | +1 species | +1 species |
| 6 Not Assessed | 0 (0%) | 0 (0%) | 0 (0%) | Stable | Stable |
| 7 Exotic | 2 (2%) | 2 (2%) | 2 (2%) | Stable | Stable |
| 8 Accidental | 0 (0%) | 0 (0%) | 0 (0%) | Stable | Stable |
| Total | 122 (100%) | 124 (100%) | 125 (100%) | +2 species | +3 species |
Orchid species only
Orchids were first assessed in the report Wild Species 2000. In total, one species was removed from the national list since this first assessment (table 11). Over time, the highest increase was observed in the number of species ranked as Sensitive (+4 species) and the highest decrease was observed in the number of species ranked as May Be At Risk (-4 species).
| Canada rank | Wild Species reports 2000 | Wild Species reports 2005 | Wild Species reports 2010 | Average change between reports | Total change since first report |
|---|---|---|---|---|---|
| 0 Extinct/Extirpated | 0 (0%) | 1 (1%) | 1 (1%) | +1 species | +1 species |
| 1 At Risk | 7 (9%) | 8 (11%) | 8 (10%) | +1 species | +1 species |
| 2 May Be At Risk | 10 (13%) | 5 (7%) | 6 (8%) | -2 species | -4 species |
| 3 Sensitive | 6 (8%) | 10 (13%) | 10 (13%) | +2 species | +4 species |
| 4 Secure | 50 (64%) | 49 (64%) | 49 (64%) | -1 species | -1 species |
| 5 Undetermined | 0 (0%) | 0 (0%) | 0 (0%) | Stable | Stable |
| 6 Not Assessed | 0 (0%) | 0 (0%) | 0 (0%) | Stable | Stable |
| 7 Exotic | 4 (5%) | 3 (4%) | 3 (4%) | -1 species | -1 species |
| 8 Accidental | 1 (1%) | 0 (0%) | 0 (0%) | -1 species | -1 species |
| Total | 78 (100%) | 76 (100%) | 77 (100%) | -1 species | -1 species |
Threats to Canadian vascular plants
With such a wide diversity of vascular plant species in Canada, it is not surprising that the threats to vascular plants are similarly varied. As with other species groups, habitat loss and degradation are major factors affecting plants. Habitat loss occurs when natural habitats are replaced with land-uses such as agriculture or housing, or as a result of natural processes such as succession, fire or flooding. Habitat degradation can occur in many forms, including pollution, changes in drainage patterns, or trampling by humans or animals. Over-harvesting is another threat for some species, particularly for plants that are valued for their beauty (e.g. Showy Lady’s-slipper), or for medicinal properties.
In recent years, the impact of exotic species has become recognized as a serious threat to native wildlife. Exotic plants can compete with native plants for space to grow and for resources. A well known example of this is Purple Loosestrife (Lythrum salicaria), which was introduced from Europe in the 1800s and has altered many wetlands from systems of high plant diversity to systems dominated almost entirely by a small number of exotic species. This change affects many species including the mammals, reptiles, amphibians and invertebrates that rely on wetlands for survival. For example, Muskrats (Ondatra zibethicus) will not eat Purple Loosestrife, and many birds will not nest in it. Other exotic species, such as the Flowering Rush (Butomus umbellatus), represent important threats along highways in eastern Canada, and some have no natural enemies. Exotic species can also introduce new diseases, which can reduce the health of native plants. Another problem is hybridization, in which an exotic plant interbreeds with a native plant, weakening its gene pool. The native Red Mulberry (Morus rubra), ranked At Risk, has declined partly due to hybridization with the exotic White Mulberry (Morus alba). Every year, millions of dollars are spent on trying to control exotic species like Nodding Thistle (Carduus nutans), Purple Loosestrife, Spotted Knapweed (Centaurea stoebe) and European Buckthorn (Rhamnus cathartica) in natural habitats.
Conclusion
This general status reassessment of all Canada’s 5111 species of vascular plants is an important achievement, involving input from botanists across the country, using the most current information to assess the distribution and general status of Canada’s vascular plants. The results of this reassessment indicate that the majority of vascular plant species in Canada are considered Secure, although many species vascular plants have Canada ranks of May Be At Risk and At Risk. The results also highlight the large proportion of exotic species; 24% of Canada’s vascular plants are ranked Exotic, a much higher proportion than for any other group covered in this report.
Further information
Adrian, S. 2000. Carnivorous plants. Marston House, England: 240 pp.
Agriculture and Agri-food Canada. The vascular plant herbarium. (Accessed February 25, 2010).
Ames, D., Bainard-Acheson, P., Heshka, L., Joyce, B., Neufeld, J., Reeves, R., Reimer, E. and Ward, I. 2005. Orchids of Manitoba. Native Orchid Conservation Inc., Canada: 158 pp.
Bruce-Grey Plant Committee. 1997. A guide to the orchids of Bruce and Grey counties, Ontario, second edition. Stan Brown Printers, Owen Sound: 105 pp.
Burchill, C. 2005. Vascular flora of Manitoba. (Accessed February 25, 2010).
Canadian Botanical Association. (Accessed February 25, 2010).
Cody, W. J. 2000. Flora of the Yukon Territory. National Research Press, Ottawa: 669 pp.
Davis, S. D., Heywood, V. H., Herrera-MacBryde, O., Villa-Lobos, J. and Hamilton, A. (editors). 1997. Centres of plant diversity: A guide and strategy for their conservation. Volume 3: The Americas. IUCN Publications Unit, Cambridge, England. (Accessed February 25, 2010).
E-Flora BC. (Accessed February 25, 2010). Eastman, J. 1992. The book of forest and thicket. Trees shrubs and wildflowers of eastern North America. Stackpole Books, Harrisburg, Pennsylvania: 212 pp.
Farrar, J. L. 1995. Trees in Canada. Fitzhenry & Whiteside, Ontario and Natural Resources Canada, Ottawa: 502 pp.
Flora of North America. (Accessed February 1, 2006).
Henry, J. D. 2002. Canada’s boreal forest. Smithsonian natural history series. Smithsonian Institute Press: 176 pp.
Hinds, H. R. 2000. Flora of New Brunswick: A manual for the identification of the vascular plants of New Brunswick. University of New Brunswick, Fredericton: 699 pp.
Johnston, W. F. 1990. Tamarack. In Silvics of North America: 1. Conifers (R. M. Burns and B. H. Honkala, technical coordinators). Agriculture Handbook 654. U.S. Department of Agriculture, Forest Service, Washington: 877 pp. (Accessed February 25, 2010).
Library and Archives Canada. 2001. Susanna Moodie and Catherine Parr Traill. (Accessed February 25, 2010).
Maunder, J. E. 2001. A digital flora of Newfoundland and Labrador vascular plants. (Accessed February 25, 2010).
McMaster, R. T. 2003. Populus heterophylla L. Swamp cottonwood. Conservation and research plan for New England. New England [PDF, 235.5 KB] Wild Flower Society, Massachusetts. (Accessed January 10, 2006).
Morris, A. 2003. New tree for Carolinian Canada. Carolinian Canada Newsletter [PDF, 742 KB], winter 2003-4. (Accessed January 10, 2006).
National herbarium of Canada. Botany Collections: The National Herbarium of Canada (Accessed February 25, 2010).
PlantWatch. (Accessed February 25, 2010).
Prindle, T. 2000. NativeTech: Native American technology and art. An introduction to tamarack trees & traditions. (Accessed February 25, 2010).
Rice, B. A. 2004. Carnivorous Plant FAQ v10.0. (Accessed February 25, 2010).
Roland, A. E. 1998. The flora of Nova Scotia. Nimbus, Halifax: 1350 pp.
Scoggan, H. J. 1978. The flora of Canada (4 volumes). Canadian Museum of Nature, Ottawa: 1711 pp.
Victorin, M. 1995. Flore Laurentienne, troisième édition. Les presses de l’Université de Montréal, Montréal: 925 pp. (Accessed February 25, 2010). [Avaiable in French only]
Waldron, G., Ambrose, J. and Rodger, L. 2003. Swamp cottonwood (Populus heterophylla), another new tree for Canada. Ontario Natural Heritage Information Centre Newsletter 8: 6. (Accessed February 25, 2010).
White, D. J., Haber, E. and Keddy, C. 1993. Invasive plants of natural habitats in Canada: an integrated review of wetland and upland species and legislation governing their control. Canadian Wildlife Service, Ottawa: 121 pp. (Accessed February 25, 2010).
References
Allaby, M. 1989. Dictionary of the environment. New York University Press, New York: 423 pp.
Chapman, A. D. 2009. Numbers of living species in Australia and the World, second edition. Report for the Australia Biological Resources Study, Canberra, Australia: 80 pp.
Jodoin, Y. 2006. Le roseau commun (Phragmites australis) en bordure des autoroutes du Québec: une étude génétique et biogéographique. M.Sc. thesis, École supérieure d’aménagement du territoire et de développement régional, Faculté d’Aménagement, d’Architecture et des Arts visuels, Université Laval, Québec: 39 pp.
Nature Trust of New Brunswick and Atlantic Canada Conservation Data Centre. 2003. Rare plant survey of the Upper St. John River with focus on Furbish’s Lousewort. The Nature Trust of New Brunswick, Inc. Fredericton: 61 pp.
Smith, D. 2001. Documenting plant domestication: The consilience of biological and archaeological approaches. PNAS 98: 1324-1326.
Vasseur, L. and Potvin, C. 1997. Natural pasture community response to enriched carbon dioxide atmosphere. Plant Ecology 135: 31-41.