Recovery Strategy for the Eastern Musk Turtle (Sternotherus odoratus) in Canada - 2016 [Proposed]

Photo: © Joe Crowley
- Document Information
- Recommended Citation
- Preface
- Acknowledgments
- Executive Summary
- Recovery Feasibility Summary
- 1. COSEWIC Species Assessment Information
- 2. Species Status Information
- 3. Species Information
- 4. Threats
- 4.1 Threat Assessment
- 4.2 Description of Threats
- Land Conversion for Development and Agriculture, & Shoreline Alteration
- Boating Mortality
- Fishing By-catch
- Water Control Structures
- Human-subsidized Predators
- Illegal Collection
- Contamination and Nutrient Loading
- Exotic and Invasive Species
- Road Networks
- Disease Outbreaks
- Climate Change
- Potential Threats
- 5. Population and Distribution Objectives
- 6. Broad Strategies and General Approaches to Meet Objectives
- 7. Critical Habitat
- 8. Measuring Progress
- 9. Statement on Action Plans
- 10. References
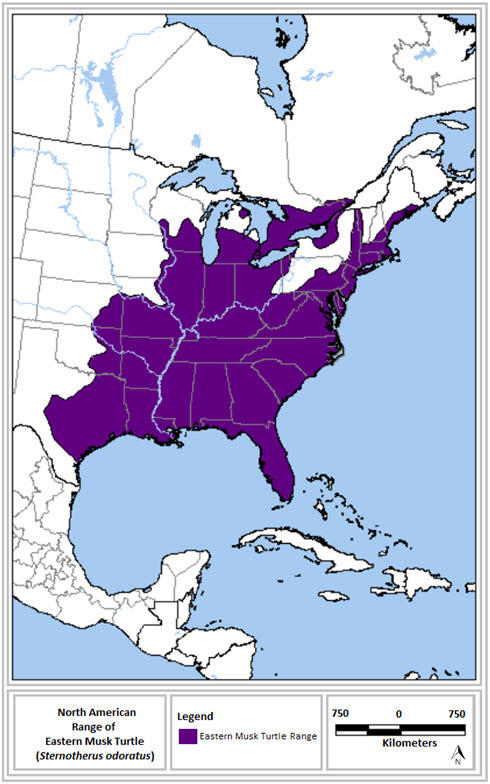
- Figure 1. North American range of the Eastern Musk Turtle (adapted from NatureServe 2008). This map represents the general range of the species, and does not depict detailed information on the presence and absence of observations within the range. Please refer to the text for further details on the distribution of the species in Ontario and Quebec.
- Figure 2. Schematic of Critical Habitat Criteria for the Eastern Musk Turtle. A critical habitat unit is identified where the habitat occupancy criterion applies. Within the critical habitat unit, critical habitat is identified as the areas that contain the detailed biophysical attributes (described in Table 3) that are required for a specific life cycle activity. The maximum extent of biophysical attributes is determined by ecological and behavioural knowledge specific to the Eastern Musk Turtle (i.e., the watercourse or waterbody extending to a maximum of 1.5 km parallel to the shoreline in both directions from an observation and the adjacent suitable habitat[s] within 50 m of the watercourse or waterbody; OR the wetland up to a maximum radial distance of 1.5 km from the valid observation and the adjacent suitable habitat[s] within 50 m of the wetlands; OR a known nesting site comprising an area extending a radial distance of 50 m from a valid nesting observation). The critical habitat unit is extended to include dispersal corridors where two valid observations occur within a continuous hydrological network and are separated by a maximum distance of 4.5 km (Habitat Connectivity Criterion).
- Figure 3. Grid squares that contain critical habitat for the Eastern Musk Turtle in Canada. Critical habitat for the Eastern Musk Turtle occurs within these 50 x 50 km standardized UTM grid squares (red squares) where the description of habitat occupancy (section 7.1.1), habitat suitability (section 7.1.2) and habitat connectivity (section 7.1.3) are met.
- Table 1. Threat Assessment Table
- Table 2. Recovery Planning Table for the Eastern Musk Turtle
- Table 3a: Detailed biophysical attributes of suitable habitat for specific life cycle activities of the Eastern Musk Turtle in Canada.
- Table 3b: Detailed biophysical attributes of suitable habitat for specific life cycle activities of the Eastern Musk Turtle in Canada.
- Table 4.Critical Habitat for the Eastern Musk Turtle in Canada occurs within these 50 x 50 km standardized UTM grid squares where the description of habitat occupancy (section 7.1.1), habitat suitability (section 7.1.2) and habitat connectivity (section 7.1.3) are met.
- Table 5. Schedule of studies
- Table 6: Examples of activities likely to result in the destruction of critical habitat for Eastern Musk Turtle
- Appendix A: Subnational Conservation Ranks of Eastern Musk Turtle (Sternotherus odoratus) in Canada and the United States
- Appendix B. Effects on the Environment and Other Species
- Table B-1. Some of the species at risk that may benefit from conservation and management of turtle habitat in those areas where Eastern Musk Turtle occur.

Environment Canada. 2016. Recovery Strategy for the Eastern Musk Turtle (Sternotherus odoratus) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. viii + 58 pp..
For copies of the recovery strategy, or for additional information on species at risk, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry.
Cover illustration: Eastern Musk Turtle: © Joe Crowley
Également disponible en français sous le titre
« Programme de rétablissement de la tortue ponctuée (Sternotherus odoratus) au Canada [Proposition] »
Content (excluding the illustrations) may be used without permission, with appropriate credit to the source.
The federal, provincial and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of recovery strategies for listed Extirpated, Endangered, and Threatened species and are required to report on progress within five years after the publication of the final document on the SAR Public Registry.
The Minister of the Environment and Minister responsible for the Parks Canada Agency is the competent minister under SARA for the Eastern Musk Turtle and has prepared this strategy, as per section 37 of SARA. To the extent possible, it has been prepared in cooperation with the Province of Ontario (Ministry of Natural Resources and Forestry Footnote1) and the Province of Quebec (Ministère des Forêts, de la Faune, et des Parcs).
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy and will not be achieved by Environment Canada, the Parks Canada Agency, or any other jurisdiction alone. All Canadians are invited to join in supporting and implementing this strategy for the benefit of the Eastern Musk Turtle and Canadian society as a whole.
This recovery strategy will be followed by one or more action plans that will provide information on recovery measures to be taken by Environment Canada, the Parks Canada Agency and other jurisdictions and/or organizations involved in the conservation of the species. Implementation of this strategy is subject to appropriations, priorities, and budgetary constraints of the participating jurisdictions and organizations.
The recovery strategy sets the strategic direction to arrest or reverse the decline of the species, including identification of critical habitat to the extent possible. It provides all Canadians with information to help take action on species conservation. When the recovery strategy identifies critical habitat, there may be future regulatory implications, depending on where the critical habitat is identified. SARA requires that critical habitat identified within a national park named and described in Schedule 1 to the Canada National Parks Act, the Rouge National Urban Park established by the Rouge National Urban Park Act, a marine protected area under the Oceans Act, a migratory bird sanctuary under the Migratory Birds Convention Act, 1994 or a national wildlife area under the Canada Wildlife Act be described in the Canada Gazette, after which prohibitions against its destruction will apply. For critical habitat located on other federal lands, the competent minister must either make a statement on existing legal protection or make an order so that the prohibition against destruction of critical habitat applies. For any part of critical habitat located on non-federal lands, if the competent minister forms the opinion that any portion of critical habitat is not protected by provisions in or measures under SARA or other Acts of Parliament, or the laws of the province or territory, SARA requires that the Minister recommend that the Governor in Council make an order to prohibit destruction of critical habitat. The discretion to protect critical habitat on non-federal lands that is not otherwise protected rests with the Governor in Council.
This document was developed by Rachel deCatanzaro, Krista Holmes, Angela McConnell, Marie-Claude Archambault, Lee Voisin (Environment Canada, Canadian Wildlife Service – Ontario Region), Barbara Slezak, Carollynne Smith, Bruna Peloso, Kari Van Allen and Louis Gagnon (formerly Environment Canada, Canadian Wildlife Service – Ontario Region). The Recovery Strategy benefited from input, review and suggestions from the following individuals: Madeline Austen, Elizabeth Rezek, Lesley Dunn (Environment Canada, Canadian Wildlife Service – Ontario Region), Paul Johanson (Environment Canada, Canadian Wildlife Service - National Capital Region), Sylvain Giguère, Gabrielle Fortin (Environment Canada, Canadian Wildlife Service – Quebec Region), Amelia Argue, Joe Crowley, Gillian Ferguson-Martin, Jay Fitzsimmons, Amanda Fracz, Aileen Wheeldon, Dana Kinsman, Jim Saunders, Rhonda Donley (Ministry of Natural Resources and Forestry), Joanne Tuckwell, Gary Allen, Josh Van Wieren, Tammy Dobbie, Andrew Promaine, Tracy Allison (Parks Canada Agency) and staff from Quebec Ministère des Forêts, de la Faune et des Parcs.
Numerous other individuals contributed to an earlier draft multi-turtle recovery strategy including Patrick Galois (Amphibia-Nature), David Seburn (Seburn Ecological Service), and Scott Gillingwater (Upper Thames River Conservation Authority). Contributions from staff at the Ministry of Natural Resources and Forestry, Quebec Ministère des Forêts, de la Faune et des Parcs, Canadian Wildlife Service, and various universities and other organizations are also gratefully acknowledged. Further, recovery documents developed by the Équipe de rétablissement des tortues du Quebec and the Ontario Multi-Species Turtles at Risk Recovery Team formed the foundation for earlier drafts of this document and are gratefully acknowledged.
Acknowledgment and thanks are given to all other parties that provided advice and input used to help inform the development of this recovery strategy including various Aboriginal organizations and individual citizens, and stakeholders who provided input and/or participated in consultation meetings.
The Eastern Musk Turtle (Sternotherus odoratus), also known as the Stinkpot, is listed as Threatened on Schedule 1 of the Species at Risk Act (SARA). It is a small-sized, highly aquatic turtle with a highly arched, grey-brown to black carapace Footnote2. Eastern Musk Turtles typically inhabit stagnant or slow-moving shallow wetlands that are connected to larger permanent water bodies or shallow bays of lakes and rivers.
The species' range extends from southern Ontario and Quebec, south to Florida and from central Texas east to Maine. In Ontario, the Eastern Musk Turtle has been recorded primarily on and near the shores of Lakes Huron, Erie, and Ontario, and along the southern edge of the Canadian Shield. In Quebec, its range appears to be restricted to the St. Lawrence River as well as one other major river system. It is estimated that roughly 5% of the global distribution of the Eastern Musk Turtle occurs in Canada.
Within the range of the Eastern Musk Turtle in Canada, habitat loss and fragmentation have been most severe in southwestern Ontario and the Golden Horseshoe. In these areas, population declines have occurred and the Eastern Musk Turtle appears to have been extirpated from several locations where it had historically been recorded, based on negative survey results and habitat loss. Elsewhere in the species' range (particularly the southern edge of the Canadian Shield), habitat is abundant and widespread and populations appear to be stable, though there is virtually no information on population trends. The overall abundance of the Eastern Musk Turtle in Canada is unknown. A rough estimate indicates the total population in Canada is greater than 10,000 individuals.
The main threats faced by the species are: land conversion; water control structures; boating mortality; fishing by-catch; human-subsidized predators Footnote3; illegal collection; contamination and nutrient loading; and exotic and invasive species. The Eastern Musk Turtle is highly vulnerable to any increases in rates of mortality of adults or older juveniles since the species has a long-term reproductive success strategy (e.g. delayed sexual maturity, slow reproductive rate).
There are unknowns regarding the feasibility of recovery of Eastern Musk Turtle. In keeping with the precautionary principle, a recovery strategy has been prepared as per section 41(1) and 41(3) of SARA for this species, as would be done when recovery is determined to be feasible.
The population and distribution objective is to maintain, and if feasible, increase the abundance and area of occupancy of the Eastern Musk Turtle to ensure the persistence of self-sustaining local populations in areas where it occurs in Canada. The broad strategies to be taken to address the survival and recovery of the species are presented in the section on Strategic Direction for Recovery (section 6.2).
Critical habitat for Eastern Musk Turtle is identified in this Recovery Strategy using three criteria: 1. Habitat occupancy; 2. Habitat suitability; and 3. Habitat connectivity. Application of the critical habitat criteria to available data identifies 180 units that contain critical habitat for the Eastern Musk Turtle in Canada, totalling up to ~75,000 ha. There are other locations that may still support Eastern Musk Turtle, however, these locations have not been surveyed recently or adequately. For this reason, critical habitat for Eastern Musk Turtle has only been partially identified in this recovery strategy. The Schedule of Studies (section 7.2) outlines the activities required to complete the identification of critical habitat in support the population and distribution objectives. As additional information becomes available, critical habitat may be refined or more units meeting the critical habitat criteria may be added.
One or more action plan(s) will be completed for the Eastern Musk Turtle and posted on the Species at Risk Public Registry by December 2022.
Based on the following four criteria that Environment Canada uses to establish recovery feasibility, there are unknowns regarding the feasibility of recovery of the Eastern Musk Turtle. In keeping with the precautionary principle, this recovery strategy has been prepared as per section 41(1) of SARA, as would be done when recovery is determined to be feasible. This recovery strategy addresses the unknowns surrounding the feasibility of recovery.
- Individuals of the wildlife species that are capable of reproduction are available now or in the foreseeable future to sustain the population or improve its abundance.
Yes. There are individuals capable of reproduction remaining across Ontario and Quebec which may be able to sustain the population or improve its abundance. Populations along the southern edge of the Canadian Shield appear to be stable (Crowley pers. comm. 2012). The total Canadian population of Eastern Musk Turtle is estimated to be greater than 10,000 individuals (COSEWIC 2012). Rescue effect Footnote4 is also a possibility from neighbouring U.S. states. There is potential for Canadian populations of Eastern Musk Turtle in a major river system to be recolonized by individuals from populations in New York, where the species is ranked "Secure" (NatureServe 2013; COSEWIC 2012).
- Sufficient suitable habitat is available to support the species or could be made available through habitat management or restoration.
Unknown. The primary threats to the species include land conversion for agriculture and development, water control structures, boating mortality, fishing by-catch, predation by human-subsidized predators and illegal collection. While the effects of land conversion and water control structures in some areas is likely irreversible or difficult to reverse, it may be possible to restore some former wetland areas, and to mitigate or avoid further habitat destruction through public education and conservation/ protection of current habitat. Public education and enforcement may also help to lessen human disturbance and illegal collection of the species. There are several available techniques to reduce the threat posed by recreational and commercial fisheries by-catch that could be implemented through best management practices (BMPs) or effective regulation, such as turtle exclusion devices (Reference removed Footnote5) and seasonal regulation. To mitigate boating mortality, regulations could be implemented regarding motorized boat use in habitats with high turtle densities (Lester et al. 2013) and educating boaters about impacts of boats to aquatic wildlife. Some techniques are available to control invasive species (such as invasive Common Reed (Phragmites australis)) and to lessen the impacts of nest predation. It is unknown if threats can be mitigated to the extent required to meet the population and distribution objective for Eastern Musk Turtle in Canada.
- The primary threats to the species or its habitat (including threats outside Canada) can be avoided or mitigated.
Unknown. The primary threats to the species include land conversion for agriculture and development, water control structures, boating mortality, fishing by-catch, predation by human-subsidized predators and illegal collection. While the effects of land conversion and water control structures in some areas is likely irreversible or difficult to reverse, it may be possible to restore some former wetland areas, and to mitigate or avoid further habitat destruction through public education and conservation/ protection of current habitat. Public education and enforcement may also help to lessen human disturbance and illegal collection of the species. There are several available techniques to reduce the threat posed by recreational and commercial fisheries by-catch that could be implemented through best management practices (BMPs) or effective regulation, such as turtle exclusion devices (Reference removed) and seasonal regulation. To mitigate boating mortality, regulations could be implemented regarding motorized boat use in habitats with high turtle densities (Lester et al. 2013) and educating boaters about impacts of boats to aquatic wildlife. Some techniques are available to control invasive species (such as invasive Common Reed (Phragmites australis)) and to lessen the impacts of nest predation. It is unknown if threats can be mitigated to the extent required to meet the population and distribution objective for Eastern Musk Turtle in Canada.
- Recovery techniques exist to achieve the population and distribution objectives or can be expected to be developed within a reasonable timeframe.
Unknown. Recovery techniques such as habitat protection through land acquisition, regulations, zoning, and landscape planning, along with stewardship techniques have been successfully used for some local populations (Seburn and Seburn 2000). Some BMPs have been developed and implemented and it is likely that others could be developed and implemented in a reasonable timeframe to help conserve vulnerable populations from habitat loss and degradation, and accidental mortality (see 3. above). However, it is unknown how effective these practices might be at preventing population declines. Public awareness/ educational materials have been developed and will continue to be an integral part of the recovery of this species. Techniques such as the use of nest cages to reduce nest predation, and by-catch reduction devices to reduce mortality from fishing by-catch have been successfully implemented in some locations and could be used more broadly to mitigate the threats to the species. However, it is unknown whether these techniques will be successful in achieving the population and distribution objective in a reasonable timeframe.
Date of Assessment: November 2012
Common Name (population): Eastern Musk Turtle or Stinkpot
Scientific Name: Sternotherus odoratus
COSEWIC Status: Special Concern
Reason for Designation: This species occupies shallow waters of lakes, rivers, and ponds. In southwestern Ontario, the species has declined substantially and is now restricted to a few tiny, scattered populations. Throughout its Canadian range, this species is vulnerable to increased mortality of adults and juveniles from recreational boating, development and loss of shoreline habitat, and fisheries by-catch. The species has delayed maturity and a low reproductive rate with a small clutch size. Since the previous assessment in 2002, increased survey effort has found more populations in eastern Ontario and adjacent areas of Quebec. The species distribution range remains unchanged, but losses in the southern half of its range make it near Threatened.
Canadian Occurrence: Ontario, Quebec
COSEWIC Status History: Designated Threatened in May 2002. Status re-examined and designated Special Concern in November 2012.
In Canada, the Eastern Musk Turtle is currently listed as Threatened Footnote7 on Schedule 1 of the Species at Risk Act (SARA). The latest COSEWIC assessment for this species (COSEWIC 2012) is Special Concern; however, the species' legal status under SARA had not changed at the date of writing. In Ontario, the species was formerly listed as Threatened Footnote8 under the Endangered Species Act, 2007 (S.O. 2007, c. 6) (ESA) since 2008, but it was downlisted to Special Concern in June of 2014. The Eastern Musk Turtle is also listed as a Specially Protected Reptile under the Ontario Fish and Wildlife Conservation Act (S.O. 1997, c.41). In Quebec, it is listed as Threatened Footnote9 under the Act Respecting Threatened or Vulnerable Species (CQRL., c. E-12.01) (ARTVS).
The global rank for Eastern Musk Turtle is Secure (G5) (NatureServe 2013). It is Nationally Vulnerable in Canada (N3) and Nationally Secure (N5) in the United States (NatureServe 2013). The species is ranked as Critically Imperiled (S1) in Quebec and Vulnerable (S3) in Ontario (NatureServe 2013) (Appendix A). The International Union for Conservation of Nature (IUCN) lists the Eastern Musk Turtle as "Least Concern" (van Dijk 2013). Canada has approximately 5% of the global distribution of the Eastern Musk Turtle (NatureServe 2013).
The Eastern Musk Turtle is a small-sized (maximum plastron Footnote10 length of 15 cm; Ernst and Lovich 2009) highly aquatic turtle. The species has a highly arched, grey-brown to black carapace, often obscured by a layer of algae (Behler and King 2002). The skin is grey to black, with two light stripes on either side of the head (eye to neck); stripes may be faded, broken (mottled) or absent in some individuals (Ernst and Lovich 2009). There are tiny fleshy projections on the throat and chin (barbels) and four musk glands at the margins of the plastron, which produce and release a liquid with a foul musky odor, characteristic of the species (Behler and King 2002). The plastron is small, beige with a single inconspicuous hinge, which allows the turtle to raise the front portion of plastron and partially close the shell (Ernst and Lovich 2009). Eastern Musk Turtles exhibit sexual dimorphism Footnote11. Males have relatively longer, thicker tails with a blunt terminal nail; more exposed skin around plastron and display two conspicuous patches of scales on the inner surface of each hind leg; while none of those characteristics are present on the females (Carr 1952; Ernst and Lovich 2009).
Eastern Musk Turtles live between 20 to 30 years. Age at maturity varies according to the location of the population. In the southern U.S., it is 2-7 years, in populations in Michigan the age increases to 9-11 years (Edmonds 1998). In a population along Georgian Bay, males matured at an average carapace length of 63.6 mm (between 5 and 6 years old) and females matured at an average carapace length of 80.7 mm (between 8 and 9 years old) (Edmonds 1998). Northern individuals mature at a later age and attain a larger size than southern individuals (Edmonds 1998).
The Canadian range of the Eastern Musk Turtle (Figure 1) extends from southern Ontario north to North Bay and Sudbury and east into extreme southern Quebec. In the U.S., this species occurs as far south as Florida and from central Texas in the west to Maine in the east (Ernst and Lovich 2009; NatureServe 2013).
In Ontario, the Eastern Musk Turtle has been recorded primarily on and near the shores of Lakes Huron, Erie, and Ontario, and along the southern edge of the Canadian Shield (Ontario Reptile and Amphibian Atlas 2013). In Quebec, its range appears to be restricted to the St. Lawrence River (where two individuals were found in 2014) as well as one other major river system (Chabot and St-Hilaire 1991; Belleau 2008; Desrosiers and Giguère 2008; Saumure 2009; Atlas des amphibiens et des reptiles du Québec 2013; S. Giguère unpulished data).
The Extent of Occurrence of the Eastern Musk Turtle in Canada is 132,205 km2, and the species' Index of Area of Occupancy Footnote12 is 1,408 km2 (COSEWIC 2012). COSEWIC (2012) reported 36 "new" Eastern Musk Turtle local populations (for a total of 113 populations Footnote13 in 32 census divisions) since the previous COSEWIC report (2002); however, this increase did not reflect an increase in abundance or range, but simply better survey efforts (COSEWIC 2012). Despite this effort, the species is not well surveyed across its range. As of 2012, provincial conservation data centres held a total of 521 Eastern Musk Turtle records (480 in Ontario, 41 in Quebec), which identify 129 element occurrences Footnote14 (126 in Ontario, 3 in Quebec) (CDPNQ 2012; NHIC 2012). There is a high number of additional observation records that have not been formally assessed (i.e., using NatureServe methodologies) in Ontario (over 4000 records) and Quebec (578 records) and will likely result in the establishment of new element occurrences and/or modifications to existing element occurrences. The overall abundance of the Eastern Musk Turtle in Canada is unknown, although data are available for some local populations, ranging in population size estimates from 84 (± 77) individuals (Reference removed) to 1440 (± 633) (Laverty 2010). A rough estimate indicates the total population in Canada is greater than 10,000 individuals (COSEWIC 2012).
Within the range of the Eastern Musk Turtle in Canada, habitat loss and fragmentation have been most severe in southwestern Ontario and the Golden Horseshoe (COSEWIC 2012). In these areas, population declines have occurred and the Eastern Musk Turtle appears to have been extirpated from several locations where it had historically been recorded, based on negative survey results and habitat loss (Edmonds 2002; COSEWIC 2012; Ontario Reptile and Amphibian Atlas 2013). Throughout the rest of the species' range (including along the southern edge of the Canadian Shield, and in Quebec), habitat is abundant and widespread and populations appear to be stable (Crowley pers. comm. 2012; Giguère pers. comm. 2015) and the species may be observed in high abundance (DeCatanzaro and Chow-Fraser 2010); however, there is virtually no information on population trends. At some locations, declines in populations may be inferred based on known threats (e.g., fisheries by-catch) (e.g., Laverty 2010; Larocque et al. 2012b). Ongoing and projected loss of habitat suggests that declines of mature individuals will continue into the future (COSEWIC 2012).
Long description for the Figure 1
Figure 1 represents the range of Eastern Musk Turtle in North America. In the North, it includes southern Ontario at North Bay and Sudbury, extreme southern Quebec, central Wisconsin, central Michigan, and western New York. It also extends to eastern Maine in the east. The ranges stretches south to reach central Texas in the west and Florida in the east.
The Eastern Musk Turtle is a highly aquatic species that undertakes only limited overland travel because it moves slowly on land and is prone to rapid dehydration (Ernst 1968). In aquatic habitats, daily movements are generally limited (25 to 131 m/day) (Edmonds 1998; Reference removed) but daily movements of 1 km (maximum) have been observed in individual Eastern Musk Turtles in Quebec (Belleau 2008).
Eastern Musk Turtles commonly inhabit stagnant or slow-moving shallow wetlands that are connected to larger permanent waterbodies or shallow bays of lakes and rivers (Edmonds 2002; Reference removed; Belleau 2008). In Canada, Eastern Musk Turtles have been found in different types of water bodies, such as lakes, ponds, marshes, rivers, and streams (Edmonds 2002). Nevertheless, the species has been described as a habitat specialist (Belleau 2008), since it seems to require water with abundant emergent, floating, and submerged aquatic vegetation that provides surface cover, which may be important for foraging, adult and juvenile refuge, and thermoregulation Footnote15 (Edmonds 2002; Belleau 2008; Rowe et al. 2009; Picard et al. 2011). They are often found in areas with a soft substrate such as sand or organic mud where they can readily bury themselves (Reference removed; Belleau 2008), and also areas with gravel bottoms (Harding 1997).
The Eastern Musk Turtle primarily inhabits the littoral zone Footnote16, up to 2 m in depth (Edmonds 2002; Belleau 2008; Rowe et al. 2009). At one small Ontario lake it has been observed to retreat up to 3 m in depth, although it uses shallower waters to forage and thermoregulate (Gillingwater pers. comm. 2012). The average depth at which the species was caught in one study area of a large river system was 0.43 m (Belleau 2008). Occupied areas also commonly contain underwater shelters such as rocks and submerged logs, as well as Muskrat or Beaver lodges (Belleau 2008; Ernst and Lovich 2009). Although the species prefers shallow water, it may occur in waters up to 9 m deep (Ernst and Lovich 2009).
Overwintering sites are typically located in shallow water up to 3 m deep (Reference removed) where organic bottoms allow the turtles to bury themselves up to 30 cm deep in mud (Edmonds 2002; Ernst and Lovich 2009). They may also use burrows, Beaver and Muskrat lodges, as well as stumps or rocks near water (Ernst and Lovich 2009). In Quebec, this species has been observed overwintering in low-vegetated areas with sand, gravel, and rocky substrates (Belleau 2008; Belleau unpub. data). Eastern Musk Turtles are intolerant of sustained periods of anoxia Footnote17 (Ultsch 2006) and select overwintering sites that remain oxygenated during winter (e.g., shoreline exposed to wind with moderate water flow) (Belleau unpub. data). The species has been known to overwinter communally in large numbers (e.g., 450 individuals; Thomas and Trautman 1937 in COSEWIC 2012). Eastern Musk Turtles have been known to show site fidelity Footnote18 to their overwintering sites (Ernst and Lovich 2009; Belleau unpub. data).
Eastern Musk Turtles begin burrowing when the surrounding water temperature is below 10°C (COSEWIC 2012). The timing of overwintering varies amongst populations according to their geographical location (Ernst and Lovich 2009); in Quebec, Eastern Musk Turtles overwinter between November and April (Belleau 2008); in Pennsylvania, the species overwinters between November and March (Ernst 1986); in Florida, the species may be active all year long (Iverson and Meshaka 2006).
Mating may occur any time during the active season, but usually occurs in the spring (April to May) or fall (September to October) and in water where individuals are congregated at overwintering sites (Risley 1933; McPherson and Marion 1981; Ernst 1986; Mendonça 1987; Ernst and Lovich 2009). In Québec, copulations have been observed in fall only (September) (Saumure 2009).
In Canada, Eastern Musk Turtles have been observed laying eggs from early June to late July (Lindsay 1965; Edmonds 1998). Nests are located in sunny or partially shaded areas (Edmonds 2002). Nesting substrates are variable, but commonly include decaying vegetation (e.g., in leaf mold, or beneath rotting stumps or logs), Beaver or Muskrat lodges, between tufts of grass in beach areas, on shallow gravel and soil-filled rock crevices (Edmonds 2002; Gillingwater pers. comm. 2012). Females often share nesting sites and may return to the same general area to nest (Edmonds 2002; Ernst and Lovich 2009). Little data are available on distances travelled to nesting sites in Canada. However, Eastern Musk Turtles are known to nest close to the shoreline throughout their range. In the U.S., nests have been found to be located up to 50 m (mean 5.5 m) from the water (Steen et al. 2012).
Unlike other turtle species, Eastern Musk Turtles do not always dig a hole in the substrate for their nests. Most nests are shallow, and are formed by scraping away debris such as decaying vegetable matter, leaf mold, and rotting wood; they can also be under leaves, and on top of leaf litter (Ernst and Lovich 2009). Also, while other turtle species lay at least 4 or 5 eggs per nest, Eastern Musk Turtles usually lay only a couple of eggs (Tucker et al. 2008). However, females may produce one to six clutches per year, depending on location (Iverson and Meshaka 2006). Females in the southern portion of the species' North American range often lay two to four clutches per year and females in the northern portion of the species' range lay one per year at most, and may not nest every year (Edmonds 1998), which can likely be explained by climatic difference amongst these regions (i.e., southern populations receive more sun exposure/heat-units than northern populations). Incubation ranges from 65 to 86 days and hatchlings emerge in August and September (Ernst and Lovich 2009). There is a knowledge gap regarding the specific needs of the hatchlings once they leave their egg, which will need to be addressed by research (see Table 2 - Recovery Planning Table).
Turtles regulate their body temperature using the surrounding environment: they are able to modify or maintain their temperature by varying their exposure to sun (known as basking), shade and water (Bulté and Blouin-Demers 2010a). Aquatic thermoregulation sites may include floating or protruding objects (e.g., rocks, logs, floating vegetation, or floating debris), and the species may sometimes thermoregulate while floating at the water surface (Ernst and Lovich 2009). Eastern Musk Turtles often bask just under the surface of the water, usually when floating among or under aquatic vegetation such as lily pads. (Reference removed) conducted a study in an Ontario lake, and reported that Eastern Musk Turtles were most often observed burrowed in the mud in less than 1 m of water (44% of sightings) and only once was an individual observed while basking out of water. Edmonds (2002) also reported that it is uncommon to find an Eastern Musk Turtle basking out of water.
Eastern Musk Turtles are primarily omnivorous Footnote19 feeders. They typically walk along the bottom of the waterbody using their head to probe into soft mud, sand, and rotting vegetation to find their food (Edmonds 2002; Ernst and Lovich 2009). Smaller turtles (carapace length <5 cm) typically feed on aquatic insects, algae, and carrion. Larger individuals consume a variety of food, including leeches, clams, snails, aquatic insects, spiders, crayfish, fish (eggs, larvae, and adults), filamentous algae, parts of higher plants, and carrion (Schneider 1998; Ford and Moll 2004; Iverson and Meshaka 2006; Ernst and Lovich 2009). Eastern Musk Turtles only feed when water temperatures are between 13 and 35°C (Mahmoud 1969). The majority of foraging occurs in the water; however, individuals have been known to occasionally leave the water at dusk to feed on terrestrial slugs (Ernst and Lovich 2009).
Eastern Musk Turtles regularly move between different aquatic habitat types to access required resources (e.g., nesting sites, overwintering sites, food sources) (Belleau 2008). As a result, it is important that the different habitats they use are linked (including aquatic corridors), or in reasonable proximity to one another so that individuals can move between them with ease to carry out all specific life stages (Belleau 2008). To access multiple core areas within a home range, Eastern Musk Turtles move through water (rarely over land) either within a shallow vegetative littoral zone (<2 m deep) (Rowe 2003; Belleau 2008; Rowe et al. 2009) or through relatively deep water with little to no vegetation (Carr 1952) up to 9 m (Edmonds 1998).
The home range size for Eastern Musk Turtles varies considerably throughout the Canadian population, with a mean home range area between 6.2 and 115.4 ha (minimum 0.08 and maximum 430 ha) (Edmonds 1998; Reference removed; Belleau 2008; Picard 2008; Laverty 2010). It appears that home range area estimates are larger for northern populations than those found in the southern portions of the North American range (Mahmoud 1969; Ernst 1986) and likely a product of habitat fragmentation (Edmonds 1998; Belleau 2008), decreased habitat productivity farther north (Harestad and Bunnell 1979) and differential habitat selection for overwintering sites and active season habitats (Ultsch 2006). For example, the exceptionally large home ranges (10.64 ha to 430 ha) reported by Edmonds (1998) in Georgian Bay occurred as an outcome of scattered habitat patches (i.e., shallow areas around islands widely separated by large expanses of deep water). While home range length for Eastern Musk Turtle has not been well documented in Ontario, studies in Quebec have noted an average home range length of 1.5 km for the species (Équipe de rétablissement des tortues du Québec, unpublished data; Belleau 2008).
Studies suggest that daily movements tend to be 25 to 131 m (Belleau 2008; Reference removed; Laverty 2010), although daily movements as far as 1 km have been observed (Belleau 2008). Long distance movements occur through water and at night, when the species is more active (Reference removed). In Quebec, one individual was found 14 km upstream of its former location (Belleau 2008; Reference removed). In a study conducted in Ontario, (Reference removed) found that Eastern Musk Turtles were more likely to move greater distances along the shoreline to find necessary resources (e.g., for foraging) than moving to a neighbouring bay overland, probably due to the species' high vulnerability to desiccation (Ernst 1968). The study also reports that the majority of Eastern Musk Turtles surveyed were located close to shore (mean = 5.0 ± 0.3 m), with one individual moving as far as 25 m from water (Reference removed).
Populations are believed to be "isolated" if they are "separated by more than 10 km of riverine habitat, 5 km of other aquatic habitat (lakes, marshes, etc.) and 1 km of land" (COSEWIC 2012). Locks and dams can limit dispersal of freshwater turtles (Bennett et al. 2010) and would likely limit the movement of Eastern Musk Turtles given their highly aquatic nature.
Turtles have certain common life history traits that can limit their ability to adapt to high levels of disturbance and that help explain their susceptibility to population declines (Congdon et al. 1993; Gibbons et al. 2000; Turtle Conservation Fund 2002). They have a reproductive strategy that depends on high adult survival rates to counterbalance the low recruitment rates because of:
- Late sexual maturity (8 - 9 years old for females from northern populations, and life span over 20 years);
- high rate of natural predation on eggs and juveniles under the age of two; and,
- dependence on environmental conditions for the internal development of eggs and external incubation of eggs without parental care.
As a consequence of these life history traits, turtle populations, including Eastern Musk Turtles, cannot adjust to an increase in adult mortality rates. Long-term studies indicate that high survival rates of adults (particularly adult females) are critical to the maintenance of turtle populations. Even a 2 to 3% increase in the annual adult mortality rate over natural mortality rates could result in population declines (Congdon et al. 1993, 1994; Cunnington and Brooks 1996).
The climatic ranges within which Eastern Musk Turtles can survive limit its range in northern areas (Bleakney 1958; McKenney et al. 1998). Climate plays a vital role in recruitment Footnote21, as Eastern Musk Turtles rely on the external environment for incubation of eggs. Incubation time constitutes a major limitation for northern turtle populations (Brooks 2007), as the short northern summer typically makes it possible to produce only one clutch per year. Recruitment can vary from one year to the next depending on weather conditions, particularly during the summer. Sex determination for Eastern Musk Turtle is temperature-dependent and occurs during incubation (Ernst and Lovich 2009).
In Canada, the Eastern Musk Turtle is at the northern limit of its range (Seburn and Seburn 2000). Because fewer heat-units Footnote22 are available the further north the species occurs, the shorter nesting and development period in Canada constitutes a limiting factor for this species (Brooks 2007). Another important limiting factor could also be the availability of suitable hibernation sites. The species is relatively intolerant of anoxic conditions during winter and ice cover lasts longer in the northern portion of their North American range (Ultsch and Cochran 1994).
Turtles play an important role in Aboriginal spiritual beliefs and ceremonies. To the First Nations peoples, the turtle is a teacher, possessing a great wealth of knowledge. It plays an integral role in the Creation story, by allowing the Earth to be formed on its back. For this reason, most First Nations people traditionally call North America "Turtle Island". Aboriginal peoples also use the turtle shell to represent a lunar calendar, with the 13 scutes Footnote23 representing the 13 full moons of the year. Turtle rattles, made from turtle shells are used in traditional ceremonies and often represent the turtle in the Creation story. Turtles also appear in other traditional stories including the Anishinaabe story "How the turtle got its shell" and the Haudenosaunee story "Turtle races with beaver" (Bell et al. 2010).
Threats to the Eastern Musk Turtle may vary regionally and locally across its distribution within Canada. However, the information presented in Table 1 is an overall assessment of threats to the Eastern Musk Turtle in Canada. Where information is known on the significance of threat at the local scale, additional information is provided in the threat description below Table 1.
The threats presented in Table 1 are in overall decreasing order of concern within each threat category.
| Threat | Threat Description | Threat Information Level of Concern Table Footnotea |
Threat Information Extent |
Threat Information Occurrence |
Threat Information Frequency |
Threat Information Severity Table Footnoteb |
Threat Information Causal Certainty Table Footnotec |
|---|---|---|---|---|---|---|---|
| Habitat Loss, Degradation, or Fragmentation | Land conversion for development and agriculture & shoreline alteration | High | Widespread | Historic/ Current | Recurrent | High | High |
| Habitat Loss, Degradation, or Fragmentation | Water control structures | Medium/ High |
Localized | Historic/ Current | Recurrent | Medium | Medium |
| Accidental Mortality | Boating mortality | High | Localized | Current | Seasonal | High | High |
| Accidental Mortality | Fishing by-catch | High | Widespread | Current | Seasonal | High | High |
| Accidental Mortality | Road networks | Low | Widespread | Current | Seasonal | Low | Low |
| Changes in Ecological Dynamics or Natural Processes | Human-subsidized predators | Medium | Localized | Current | Seasonal | Unknown | Medium |
| Biological Resource Use | Illegal collection | Medium | Widespread | Current | Seasonal | Medium | Medium |
| Pollution | Contamination and nutrient loading | Medium/ Low |
Localized | Current | Continuous/ Seasonal |
Unknown | Low |
| Exotic, Invasive, or Introduced Species | Exotic and invasive species | Medium/ Low |
Localized | Current/ Anticipated |
Continuous | Unknown | Medium/ Low |
| Natural Processes or Activities | Disease outbreaks | Unknown | Localized | Anticipated | Continuous | Unknown | Unknown |
| Climate and Natural Disasters | Climate change | Unknown | Widespread | Current | Continuous | Unknown | Unknown |
This section highlights the threats outlined in Table 1, emphasizes key points, and provides additional information. Although threats are listed individually, an important concern is the long-term cumulative effect of a variety of threats posed on local Eastern Musk Turtle populations. It should be noted that some of these threats apply only during the active season since they lead to direct mortality, mutilation, or illegal collection of individuals. Among mechanisms through which threats can impact Eastern Musk Turtle populations, isolation through habitat loss and fragmentation is of particular concern, as it leads to a breakdown of metapopulation dynamics and limits possibility of rescue effect. Threats are presented in decreasing order of level of concern.
The loss of habitat to agriculture and development is significant to Eastern Musk Turtle (Edmonds 2002). Infilling or draining of wetlands for such purposes effectively eliminates turtle habitat such as basking and foraging sites (Reference removed). Habitat fragmentation, through the construction of associated infrastructure such as roads and bridges, may isolate local turtle populations (Reference removed). Isolation of populations has the potential to compromise rescue effect, which would lead to a higher likelihood of elimination of local populations (Stockwell et al. 2003; Marchand and Litvaitis 2004). In the long-term, a reduced ability for successful dispersal of individuals can result in loss of genetic variation (Rizkalla and Swihart 2006; Gray 1995). Loss of genetic variation in small, isolated populations can in turn cause loss of population fitness and adaptability, and increase the risk of extinction in the wake of a catastrophic or epidemic event (Frankham 1995; Reed and Frankham 2003).
Shoreline habitat degradation reduces the availability of suitable nesting and basking sites (Edmonds 2002; Reference removed; Carrière and Blouin-Demers 2010). Such habitat degradation can also reduce the number of overwintering sites and increase the number of predators (e.g., Ernst and Lovich 2009). In many areas, shorelines are reinforced to prevent erosion, often using metal or concrete walls or rip rap Footnote24 (Reference removed). This hardening of the shoreline may prevent turtles from carrying out critical life functions (such as nesting, foraging, hibernating, and basking) along large stretches of formerly available habitat (Reference removed). For example, natural shorelines possess more emergent and aquatic vegetation than developed shorelines (Radomski and Goeman 2001), and these habitat configurations are crucial to Eastern Musk Turtles throughout the active season (Picard et al. 2011). Construction activities associated with this type of development can also lead to direct turtle deaths.
Dredging may affect turtles directly or indirectly. Individuals may be extracted from overwintering sites and/or killed by heavy equipment during dredging. Overwintering sites might be destroyed by dredging. Alterations in water quality (due to sediment loading in rivers) and changes in river morphology could potentially alter prey composition and availability (Bodie 2001).
Some techniques commonly used for the management of streams and riparian zones, such as reduction of snags/log jams, riparian draining, channelization, or impoundment, may have negative effects on turtles (Bodie 2001).
While in the water, turtles, including Eastern Musk Turtle, are at risk of being injured or killed by collisions with boats and/or propellers (Bancroft et al. 1983; Edmonds 1998; Burger and Garber 1995; Smith et al. 2006; Reference removed; Bulté et al. 2010). Death due to collisions with motorboats, even in water bodies with low to moderate (versus high) boat traffic, may lead to a decline in the local freshwater turtle population (Bulté et al. 2010). Eastern Musk Turtles are at a greater risk of significant injury from boats, since this species basks at the surface and can be severely wounded or killed by propellers and boat hull impacts (Bancroft et al. 1983; Edmonds 1998; Reference removed; Bulté et al 2010, Bennett & Litzgus 2014). One study in the Georgian Bay area found that there are more incidences of boat-injured Eastern Musk Turtles in areas of high recreational use (Laverty 2010). It may be hard to measure how much the turtles are being impacted by boating because the species' small size usually results in a boat impact causing death rather than injury (Laverty 2010).
Lester et al. (2013) suggest the implementation of regulations regarding motorized boat use in habitats with high turtle densities and educating boaters about impacts of boats to aquatic wildlife.
By-catch in commercial and recreational freshwater fishing is an under-appreciated but real threat to turtles (Raby et al. 2011). Turtles can be accidentally hooked on recreational fishing lines or caught in commercial or scientific fish traps or nets and drown. Because nets are often not checked for several days, the rate of drowning among turtles is high. Mortality rates are sufficient to cause extirpation of local turtle populations (Midwood et al. 2014). Those turtles that survive without drowning in nets can show signs of harm that puts them at risk of later mortality (Stoot et al. 2013).
Extensive research has been undertaken in eastern Ontario in recent years on the rates of turtle by-catch in these nets, and has found that Eastern Musk is one of the most common turtle species caught (Larocque et al. 2012b; Midwood et al. 2014; Stoot et al 2013). Studies conducted in eastern Ontario and on the Mississippi River (U.S.) found that passive fishing techniques (e.g., Fyke nets) can result in significant by-catch of turtles, in particular Eastern Musk Turtles (e.g., Barko et al. 2004; Reference removed; Laroque et al. 2012a). In 2005, at least sixteen Eastern Musk Turtles drowned in underwater hoop-nets used for commercial fishing at a site in Eastern Ontario (Reference removed). Even when care is taken to ensure that a portion of the trap remains above water, turtles tend to travel to the last compartment, which is anchored to the bottom and might be completely submerged (Thompson pers. comm. in Seburn 2007).
In addition to the risk of by-catch in commercial fisheries' nets, turtles also risk injury and mortality from ingestion of recreational anglers' hooks. As turtles that get caught in fishing lines are often released by cutting the line, the hook remains in the turtle (Reference removed; Reference removed). Eastern Musk Turtles have been observed to be frequently hooked in the mouth by anglers using baited hooks (David Steen, pers. obs. cited in Steen et al. 2014). The hook and nylon line can lead to serious lacerations in the digestive tract and lead weights can cause poisoning (Borkowski 1997). Eastern Musk Turtles are often caught, and are frequently killed either by the fishermen or as a result of injury from the hooks (Edmonds 2002).
Water control structures can impede the movement of turtles in aquatic environments, thereby increasing habitat fragmentation and preventing access to suitable habitats (Bennett et al. 2010). This is of particular concern for highly aquatic turtle species, such as the Eastern Musk Turtle, which almost always uses aquatic habitat for movement, and for which water control structure construction could potentially contribute to the isolation of populations (Edmonds 2002; Bennett et al. 2010). Isolation of populations has the potential to compromise rescue effect which would lead to a higher likelihood of elimination of local populations (Stockwell et al. 2003; Marchand and Litvaitis 2004). A reduced ability for successful dispersal of individuals can result in loss of genetic variation (Gray 1995). Loss of genetic variation in small, isolated populations can in turn cause loss of population fitness and adaptability, and increase the risk of extinction in the wake of a catastrophic event or epidemic Footnote25 (Frankham 1995; Reed and Frankham 2003).
Some water control operations also impact turtle habitat by altering upstream and downstream water levels, thereby impacting water depth over overwintering sites, availability of nesting, basking, and foraging habitats. For example, the use of dams for flood control may negatively impact the species by reducing the scouring effects of peak flows on the shoreline (removal of vegetation on shorelines), and thus the amount of exposed soil that is suitable for nesting (Seburn 2007). Water control can also affect the downstream flow regime that alters sediment transport, thermal properties, water levels, and oxygen concentrations, all of which can affect the habitat suitability, especially during hibernation.
The fluctuation in water levels caused by water control can cause direct mortality through the following mechanisms: increase of water levels during the spring and summer may drown nests (killing embryos), since nests are usually dug close to water; and a decrease of water levels during the winter may lead to freezing (and death) of overwintering turtles (Ewert 1979).
In many areas, the low density or absence of top predators and increased food availability from human sources (e.g., food handouts, garbage, crops) have led to a greater abundance of turtle predators than natural conditions would have historically supported (Mitchell and Klemens 2000; COSEWIC 2012). The main predators of Eastern Musk Turtle eggs include Raccoons (Procyon lotor), Striped Skunks (Mephitis mephitis), crows (Corvus), and foxes (Urocyon cinereoargenteus, Vulpes vulpes), (Harding 1997; Marchand et al. 2002; Ernst and Lovich 2009). In the Great Lakes region, Eastern Musk Turtle nest mortality often exceeds 80% (Harding 1997).
Methods to counteract elevated predation rates have been developed and used with varying degrees of success (Seburn 2007; Riley and Litzgus 2013). However, in many cases, it is impossible to implement these methods, such as predator exclusion devices over turtle nests, on the scale required to protect the population from this threat.
Worldwide, many turtle species are impacted by casual and large-scale systematic illegal collection for use as pets, food and traditional remedies (Bodie 2001; Reference removed; Moll and Moll 2004). The rate of export of freshwater turtles, for both pet and food trades, is high in the U.S. (Mali et al. 2014). For example, between 1999 to October 2014, around 750,000 Eastern Musk Turtle individuals were legally exported from the United States for commercial purposes, from which around 40% were declared as wild caught (U.S. Fish and Wildlife 2014). It is believed that this number is higher due to the illegal pet trade. The rate of illegal trade can be expected to also be high in Canada given the lucrative trade demand. Reptile species are more likely to be involved in the international pet trade if they are categorized as at risk than if they are not considered at risk (Bush et al. 2014), consistent with a general demand for rare wildlife (Courchamp et al. 2006).
In Canada, the collection, trade, and possession of Eastern Musk Turtle is illegal under federal and provincial legislation. Nevertheless, the illegal sale of Eastern Musk Turtle has been increasing through online websites such as Kijiji (Gillingwater pers. comm. in COSEWIC 2012). Between 2008 to 2012, the Ministry of Natural Resources and Forestry led more than 25 investigations of the online illegal sale of Eastern Musk Turtle (Miller pers comm. 2012 in COSEWIC 2012; Zacher pers. Comm. 2012 in COSEWIC 2012). This type of activity may indicate a high demand for the species in the pet trade.
Illegal collection of Eastern Musk Turtles may not directly cause mortality, but removes individuals from the population which, given the species' reproductive strategy (extreme longevity, low recruitment rates), may greatly reduce recruitment (Congdon et al. 1993, 1994; Burger and Garber 1995). The annual removal of even just a few adults from a local population can have a significant impact to the health and viability of local populations. The extent of illegal organized turtle harvest is poorly documented in Canada for the Eastern Musk Turtle and requires further study.
Aquatic habitat of the Eastern Musk Turtle can be impacted by the degradation of water quality caused by the runoff of contaminated water from agricultural (nutrients and pesticides) and industrial zones (industrial waste), roads (e.g., de-icing salt), and urban areas (e.g., heavy metals) (Mitchell and Klemens 2000; Bishop et al. 2010). Eastern Musk Turtles could be vulnerable to contaminant accumulation, although the long-term impact of this threat is poorly understood. Individuals absorb contaminants in the environment through various physiological processes (e.g., feeding, breathing, and absorption through tissues or membranes such as eggshells). A study has shown that there has been a shift in the diet of Eastern Musk Turtles towards the consumption of zebra mussels and away from their natural prey items (i.e., leeches, clams, snails, aquatic insects, spiders, crayfish, fish) (Patterson and Linderman 2009). This could lead to increased exposure to contaminants because zebra mussels are known to accumulate high levels of toxins due to the nature of their filter feeding (Hogan et al. 2007).
Recent studies indicate that reliance on benthic food items has little effect on mercury accumulation in painted and musk turtles (Reference removed) and that concentration of mercury in blood and scutes does not affect parasitism level in Painted Turtles (Slevan-Tremblay 2013). However, mercury exposure could be detrimental to the immune system of Eastern Musk Turtles by reducing the number of lymphocytes. Two studies, undertaken in the Great Lakes basin, detected several industrial-based contaminants in Snapping Turtle eggs. It was also noted that abnormal embryo development increased with exposure to polychlorinated aromatic hydrocarbons (Bishop et al. 1998; Van Meter et al. 2006). Although these studies focused on other species, the potential for similar effects on Eastern Musk Turtle exists as they share similar habitats and feeding behaviours.
Inputs of sediments and organic matter through erosion and runoff can also alter water quality and habitat structure and threaten local populations of Eastern Musk Turtles. Siltation of deep pools has been linked to the decline of several turtle species (see Bodie 2001), and could degrade Eastern Musk Turtle overwintering habitat by exposing individuals to freezing. Inputs of organic matter and nutrients can increase water turbidity and reduce dissolved oxygen content, which could affect respiration in winter. To what extent such conditions affect the Eastern Musk Turtle is unknown.
The augmentation of nutrient loads associated with human activity can lead to blue-green algal blooms in waters frequented by turtles (Carpenter et al. 1998), and this can threaten turtles through ingestion of toxins from the algae. In addition, nutrient loading can lead to increased oxygen consumption by bacteria, which, in turn, can result in periods of low dissolved oxygen levels (hypoxia) or even a total absence of oxygen (anoxia) during winter. Eastern Musk Turtle are known to be intolerant of hypoxia during overwintering (Ultsch 2006); therefore, if they hibernate in areas where oxygen levels are decreased, they could be at risk of dying during hibernation due to hypoxia or anoxia.
Groundwater contamination related to discharge at and maintenance of overwintering sites is also of concern. Studies to determine if there are effects on turtles are needed to help identify the level of risk to a population.
The introduction of invasive, exotic plants can alter the availability and quality of Eastern Musk Turtle habitat. In some areas, particularly around Lake Erie, Lake Huron, and Lake St. Clair, and along some major rivers, non-native Common Reed has invaded wetlands, lakes, and rivers forming a monoculture Footnote26 that has altered conditions of foraging habitat and nesting habitat, forcing female Eastern Musk Turtles to use other egg laying sites (Reference removed; Gillingwater unpub. data in COSEWIC 2012). The expansion of road networks also facilitates the spread of invasive plant species, especially in southern Ontario (Gelbard and Belnap 2003).
Turtles nest in open, unshaded areas receiving adequate solar heat. In a study conducted at a site on Lake Erie, Ontario, it was found that non-native Common Reed had reduced the amount of suitable nesting habitat for many turtle species, because growth of the plant altered the microenvironment (particularly temperature) of turtle nests during the incubation period (Reference removed). Evidence was found at another site on Lake Erie that non-native Common Reed reduced or eliminated Eastern Musk Turtle access to nesting sites. Immediately after Common Reed invasion, nests became concentrated in breaks in the vegetation stands, making them more vulnerable to predation or accidental trampling by humans who use the area, and previous nesting sites were no longer used (Reference removed). The loss of suitable nesting habitat for turtle species due to invasive plants including non-native Common Reed, as well as Japanese Hops (Humulus japonicas), and Purple Loosestrife (Lythrum salicaria) have also been observed at many other locations throughout southern Ontario (Gillingwater pers. comm. 2012). Reed Mannagrass (Glyceria maxima) might also have an impact on Eastern Musk Turtles.
The introduction of other non-native species may also have a negative effect on the Eastern Musk Turtle. For example, the release of exotic pet turtles (e.g., Red-eared Slider (Trachemys scripta ssp. elegans)) in natural environments following a period of captivity can result in competition and/or the transmission of diseases to native turtle populations (Cadi and Joly 2003, 2004). These non-native turtles are known to occur in high numbers in some locations of the province and may successfully reproduce where habitat conditions are suitable (OMNRF 2014, unpublished data).
Death from collisions with road vehicles is noted as a growing concern in herpetofaunal Footnote27 studies (e.g. Andrews et al. 2006), especially for roads which run through wetlands or along streams and lakes, and are heavily travelled. Although some collisions with turtles are accidental, drivers intentionally driving over turtles are also a threat (Ashley et al. 2007). This study found evidence that reptile decoys were hit at a higher rate than by chance alone, with approximately 2.7% of motorists intentionally hitting them. In Ontario, the road network is developing rapidly, especially in the southern portion of the province, where the length of major roads has increased by 28,000 km within 60 years (Fenech et al. 2005). Road mortality is of major concern in this province and road sections with high mortality rates of freshwater turtles have been identified in many areas, including national and provincial parks (Reference removed; Crowley and Brooks 2005; Ontario Road Ecology Group 2010). Although mortality of Eastern Musk Turtle on roads does occur, it is less common compared to other species of turtles, as Eastern Musk Turtle movement is largely aquatic and it rarely ventures far from water (van Dijk 2013). Eastern Musk Turtle is at some risk of road mortality during the nesting season in Ontario (Haxton 2000).
Females tend to be at greater risk of road mortality because they travel overland during the nesting season (Haxton 2000), may use road shoulders Footnote28 to nest (e.g., Aresco 2005; Reference removed), and, as a result, females are more frequently encountered on roads than males (Steen et al. 2006). One study revealed that during certain times as many as 72% of all Eastern Musk Turtles crossing a road were female (Aresco 2005). This increased female road mortality rate may be the reason that, in wetlands surrounded by a dense road network, some studies have reported a male-biased sex ratio of turtle populations (Marchand and Litvaitis 2004; Steen and Gibbs 2004; Gibbs and Steen 2005). Also, hatchlings emerging from nests located on road shoulders may be killed as they attempt to reach aquatic habitats. This mortality also increases the likelihood of population decline as there are reduced recruitment rates.
Maintenance of roads and trails can pose a threat to individuals and nests when grading and vegetation removal/control is required throughout the summer, autumn and winter. Roads are also identified as barriers to movement and may lead to habitat fragmentation by decreasing turtle dispersal ability (Rizkalla and Swihart 2006; Bennett et al. 2010). Eastern Musk Turtles are vulnerable to desiccation when they are out of water (Ernst 1968) which further limits their dispersal in fragmented landscapes.
Disease outbreaks have the potential to affect a large number of species and to spread rapidly through international transportation modes (Daszak et al. 2000); these outbreaks reduce survival and can severely affect turtle populations (COSEWIC 2012). In Virginia, Eastern Musk Turtles have suffered from a necrotic shell disease which damages skin and shell scutes (Ernst et al. 1999). Although this disease has not been reported in the Eastern Musk Turtle population in Canada, the disease has already been found in Canadian Snapping Turtles and Painted Turtles (Brooks pers. comm. 2012 in COSEWIC 2012). Therefore, this disease could potentially impact Eastern Musk Turtle populations at some point in the near future.
Climate is the main limiting factor of the distribution of turtles in the northern part of their range. Given the effect of climate on recruitment rates, it seems likely that global climate change will have an impact on turtle populations, although the overall nature and extent of the impact is unclear (COSEWIC 2012). An increase in the annual average temperature in Ontario of 2.5 to 3.7ºC by 2050 (compared to 1961-1990) is expected, along with changes in seasonal precipitation patterns (Expert Panel on Climate Change Adaptation 2009).
Sex determination for Eastern Musk Turtle is temperature-dependent and occurs during incubation (Ernst and Lovich 2009). Incubation temperatures at or above 28°C produce nearly all females, between 25°C-28°C produce a mixture of males and females, and below 25°C produce up to 80% males (COSEWIC 2012). One report indicates that a global temperature increase of 4°C or more may lead to the elimination of the production of male turtles and increases of 2°C or less may still lead to dramatic shifts in the sex ratios of turtles (Janzen 1994 in COSEWIC 2012). This could threaten the viability of the species in the future.
Hydrological effects could be marked by lower water levels during summer (Lemmen et al. 2008), and these lower levels could in turn increase the availability of nesting sites. However, in the absence of increased precipitation, higher temperatures and increased evaporation could lead to low water runoff (Expert Panel on Climate Change Adaptation 2009) and dry out wetlands that were once permanent. Decreasing water levels in the Great Lakes may result in significant loss of coastal wetland habitats used by Eastern Musk Turtle.
Hydrological effects could be marked by an increase of extreme rainfall events, which would cause more flooding of eggs on the shoreline; and by longer, more severe droughts that would result in desiccation of eggs. If the frequency and intensity of extreme rainfall events increase in the future as predicted (Expert Panel on Climate Change Adaptation 2009), there is a risk that nesting sites will be flooded even more often, which would reduce hatching success Further studies are needed to determine the expected impacts of climate change on the Eastern Musk Turtle.
There are other threats that could potentially affect the Eastern Musk Turtle. For example, human activity can affect turtles in many ways. Simply approaching basking individuals can cause them to leave their basking sites. The resulting heat loss, should the disturbance become repetitive, can delay the development of eggs in females, and affect other life cycle needs in both sexes and in all age classes (e.g., food metabolism, spring emergence) (Bulté and Blouin-Demers 2010b). The presence of humans and/or boats can delay or interrupt nesting, and females may abandon their nest, making them more subject to predation (Horne et al. 2003; Moore and Seigel 2006; References removed). Recreation on nesting beaches (e.g., use of off-road vehicles) can also lead to trampling of nests or hatchlings (Reference removed). Turtle species have also been subject to deliberate harassment and persecution by humans, including throwing rocks, and shooting with firearms (e.g., Horne et al. 2003). However, there are no known published studies to date (as of October 2014) that have analysed/quantified the effects of these activities on Eastern Musk Turtle.
The population and distribution objective is to:
- Maintain, and if feasible, increase the abundance and area of occupancy of the Eastern Musk Turtle to ensure the persistence of self-sustaining local populations in areas where it occurs in Canada.
There is limited information on the overall size of the Canadian population of Eastern Musk Turtle. A rough estimate indicates the total population in Canada is greater than 10,000 mature individuals, and the Index of Area of Occupancy is estimated at 1,408 km2; however, there are still a number of areas within the species' range which have not been surveyed fully (COSEWIC 2012). Declines in abundance and distribution have occurred in southwestern Ontario and the Golden Horseshoe, where habitat loss and fragmentation have been most severe. The primary goal of this recovery strategy is to halt the population decline and to maintain the overall population and distribution (i.e., abundance and area of occupancy) of the species in Canada. To the extent possible, efforts to increase the abundance and area of occupancy of the species in Canada will be focused in areas of known decline, particularly southwestern Ontario and the Golden Horseshoe, where suitable habitat is being lost/degrade, and/or where threats are documented to be high and negatively affecting populations. It may be feasible to increase abundance of local populations where: recruitment is extremely low, threats are evident and not irreversible, and proven recovery techniques can mitigate the threats (and threat mitigation measures may be put in place). In some areas, the quality of the habitat will need to be improved for recovery to be achieved.
This long-lived species has specific ecological requirements, complex life cycle needs, and a limited ability to compensate for the loss of individuals through reproduction or through recruitment from adjacent populations. As a result, active approaches and strategies undertaken on several fronts and over large regions will be required to achieve this objective. These approaches and strategies include: protection of suitable habitat, protection of individuals (particularly breeding females), improving management practices, threat mitigation, inventory and monitoring of local populations, increasing public awareness, and filling knowledge gaps through research.
Sufficient habitat and habitat linkages (movement corridors) are critical to ensuring local populations have the necessary elements required for survival and recovery. Without movement corridors, individuals may not be able to access different habitats within their home range to complete necessary life cycle activities (e.g., nesting, overwintering) or to migrate to neighbouring populations, which facilitates rescue effect and gene flow. The broad strategies along with the identification of critical habitat will help ensure such habitat is maintained.
At the national scale, the Canadian Herpetology Society (CHS) is the main non-profit organization devoted to the conservation of amphibians and reptiles, including turtles, and conducts the following activities: scientific investigations, public education programs and community projects, compilation and analysis of historical data and the undertaking of projects that support conservation or habitat restoration.
The Government of Canada has been funding projects related to Eastern Musk Turtle conservation throughout Quebec and Ontario through the Habitat Stewardship Program (HSP) and Aboriginal Fund for Species at Risk (AFSAR) since 2001 and the Interdepartmental Recovery Fund (IRF) since 2004. Projects have included activities such as: undertaking targeted surveys for the species; identifying important habitat of local populations; studying the severity of and/or mitigating threats such as fishing by-catch; soliciting observations/ encouraging public reporting of sightings; and educating landowners and/or the public on species identification, threats, and stewardship options.
An Ontario Multi-Species Turtles at Risk Recovery Team was established in the early 2000s by a group of people interested in turtle recovery, and focused on 6 turtle species at risk: Blanding's Turtle (Emydoidea blandingii); Eastern Musk Turtle (Sternotherus odoratus); Northern Map Turtle (Graptemys geographica), Spiny Softshell (Apalone spinifera); Spotted Turtle (Clemmys guttata); and Wood Turtle (Glyptemys insculpta). This group has coordinated and initiated a number of recovery efforts including conducting educational and outreach programs on reptiles and various management initiatives such as nest protection projects and nest site rehabilitation projects (Seburn 2007).
The Ministry of Natural Resources and Forestry (MNRF) has funded numerous turtle conservation and stewardship projects across Ontario through the Ontario Species at Risk Stewardship Fund and other provincial funding programs. In 2010, the MNRF released the Forest Management Guide for Conserving Biodiversity at the Stand and Site Scales (The Stand and Site Guide) (OMNR 2010). This tool, designed for forest managers, provides direction on planning and conducting forest operations at the stand and site level (i.e., 10s of m2 to 100s of km2) so that forest biodiversity will be conserved, and it includes standards, guidelines and best management practices for turtle species found in the Area of the Undertaking Footnote29 including the Eastern Musk Turtle.
Since 2009, Ontario Nature has been coordinating the development of a new Ontario Reptile and Amphibian Atlas and is working with the Natural Heritage Information Centre (NHIC) and other organizations. By soliciting occurrence records from the public, researchers, government and non-government organizations, this project is improving our knowledge of the distribution and status of reptiles and amphibians, including the Eastern Musk Turtle, in Ontario (Ontario Nature 2012; Crowley pers. comm. 2013).
There have been several large-scale inventory, survey, or monitoring programs targeting turtles, including Eastern Musk Turtle, in Ontario (e.g., Ontario Turtle Tally (Toronto Zoo), Kawartha Turtle Watch (Trent University), survey or monitoring initiatives from Nature Conservancy of Canada, Ontario Nature, and Parks Canada Agency) as well as many local survey and monitoring programs. In addition, research has been conducted on Eastern Musk Turtle in Ontario to fill knowledge gaps, including studies on home ranges, population sizes, demographics, habitat use, and ecology have been conducted in various parts of Ontario (e.g. Edmonds 1998; Ultsch 2006; Reference removed; Picard 2008; Laverty 2010).
Various habitat restoration, threat mitigation, and other conservation initiatives have been undertaken in Ontario to benefit Eastern Musk Turtle (e.g., by Parks Canada Agency within National Parks, Nature Conservancy of Canada, and numerous other organizations). This has included, for example, protection of nests and hatchlings (e.g., Parks Canada Agency, Kawartha Turtle Trauma Centre), and Common Reed removal in nesting areas (Parks Canada Agency). The Kawartha Turtle Trauma Centre (KTTC) in Peterborough rehabilitates wild turtles that were injured in the hopes of recovering and releasing them (Kawartha Turtle Trauma Centre). The number of turtles that the centre treats annually is rising.
There are many organizations and agencies that offer outreach/educational programs about turtle species at risk to school groups, First Nations, and the general public (e.g., Scales Nature Park, Reptiles at Risk on the Road Project, The Georgian Bay Biosphere Reserve (and previously the Georgian Bay Reptile Awareness Program), Ontario Nature, MNRF, Ontario Parks, the Parks Canada Agency, Toronto Zoo, Upper Thames River Conservation Authority). The Toronto Zoo Adopt-A-Pond program is one of several projects that have developed turtle conservation curricula for schools, while the Toronto Zoo Turtle Island Conservation program promotes turtle conservation and awareness among First Nation and non-aboriginal groups. Turtle SHELL (Safety, Habitat, Education and Long Life) has prepared booklets and installed turtle crossing signs.
Many projects are being carried out as a requirement under the Ontario Endangered Species Act, 2007 that are directly benefitting Eastern Musk Turtle local populations. For example, turtle fencing and eco-passages are now incorporated into the design of most new highways whenever they bisect at-risk turtle habitat (Ontario Road Ecology Group 2010; OMNRF 2013).
The Quebec Turtles Recovery Team was created in 2005. One of its mandates was to develop and implement a recovery plan for five species of turtles: the Wood Turtle (Glyptemys insculpta), the Northern Map Turtle (Graptemys geographica), the Blanding's Turtle (Emydoidea blandingii), the Eastern Musk Turtle (Sternotherus odoratus) and the Spotted Turtle (Clemmys guttata) (Équipe de rétablissement des tortues du Québec 2005). In 2012, this team merged with the Spiny Softshell (Apalone spinifera) Recovery Team, thus including a sixth species of turtle. To ensure the implementation of the recovery actions, four Implementation Groups were established, each working on a specific turtle species or group of species. One of these groups is the Blanding's Turtle and Eastern Musk Turtle Implementation Group.
The efforts of this Implementation Group, made up of partners from various organizations (including, over the years, MFFP Footnote30, Environment Canada, Hydro-Québec, National Capital Commission, Nature Conservancy Canada, and McGill University) and independent consultants, made it possible to acquire knowledge on various aspects of the Eastern Musk Turtle in Quebec, to identify threats, to implement protection measures for the species and its habitat, and to raise awareness.
Over the past few decades, inventories have been conducted along a major river in Quebec (Chabot and St-Hilaire 1991, Desrosiers and Giguère 2008, Caron 2010, Toussaint and Caron in prep., MFFP unpublished data) and research on habitat selection, movement patterns, and demography of Eastern Musk Turtles (Belleau 2008) has been undertaken in the province. All sightings of the species in the province are collected and archived in the Centre de données sur le patrimoine naturel du Québec (Quebec's Conservation Data Center). Moreover, the Eastern Musk Turtle's element occurrences are in the process of being mapped, as well as the species' habitat mapping under the Act Respecting Threatened or Vulnerable Species (ARTVS). In addition, a protection plan for the Eastern Musk Turtle is also currently being produced by the Wildlife Protection branch of the MFFP. The recovery plan for the Eastern Musk Turtle is also being updated.
Several land acquisition projects along a major river in Quebec have been carried out by the MFFP and partners such as Nature Conservancy Canada to protect habitat for a variety of species, including the Eastern Musk Turtle. Meanwhile, stewardship and communication initiatives have been put forward to protect Eastern Musk Turtles and their habitat (e.g., distribution of brochures and pamphlets to the public, presentations in schools, general public information days, and development of a web page). All these actions have been conducted by government and non-government organizations, conservation organizations, research or zoological institutions or volunteers.
To work towards achieving the population and distribution objectives, seven broad strategies for recovery have been established. The broad strategies are:
- Use legislative and administrative tools to conserve Eastern Musk Turtle individuals and habitat;
- Reduce individual mortality, injury, and illegal collection across the range of the Eastern Musk Turtle in Canada;
- Protect, manage, and restore habitat across the range of the Eastern Musk Turtle in Canada;
- Improve recruitment in locations where local Eastern Musk Turtle populations are in decline or viability is deemed compromised;
- Conduct communication, outreach, and stewardship activities;
- Survey and monitor local Eastern Musk Turtle populations, habitat, and threats; and
- Conduct research on population demographics, habitat characterization and use, and threats/threat mitigation to fill knowledge gaps.
Research and management approaches are recommended for each strategy (Table 2). Threats/limitations in the first column are numbered as follows for concise presentation:
- Land conversion for agriculture and development & shoreline development;
- Boating mortality;
- Fishing by-catch;
- Water control structures;
- Human-subsidized predators;
- Illegal collection;
- Contamination and nutrient loading;
- Exotic and invasive species;
- Road networks;
- Disease outbreaks;
- Climate change.
| Threat or Limitation | Broad Strategy for Recovery | Priority Table Footnoted | General Description of Research and Management Approaches |
|---|---|---|---|
| 1,4,6 | Legislative and administrative tools to conserve Eastern Musk Turtle individuals and habitat | High |
|
| 2,3,5,6,9 | Reduce individual mortality, injury, and illegal collection | High |
|
| 1,4,7,8,9, | Protect, Manage or Restore Habitat | High |
|
| 1-10 | Improve recruitment in locations where Eastern Musk Turtle is declining or viability is deemed compromised | High | This strategy must be implemented concurrently with two aforementioned broad strategies: "Reduce Adult Mortality, Injury, and Collection" and "Protect, Manage or Restore Habitat"
|
| All Threats | Communication, Outreach and Stewardship | Medium |
|
| All Threats | Surveying and Monitoring | Medium |
|
| All Threats | Research | Medium |
|
Considering the Eastern Musk Turtle's reproductive strategy (see section 3.4), maintaining the highest possible adult survival rate, especially for females, remains the primary need of the species to achieve recovery. Unfortunately, some biological traits of the Eastern Musk Turtle (i.e., basking by floating on the surface of the water, nesting in beaches) make it very sensitive to many human activities (e.g., water sports, boating, illegal collection, recreational activities at beaches) so it will be important that a proactive, integrated approach be taken to limit threats on adult Eastern Musk Turtles.
Recovery approaches should focus on those areas and times of year when most of the adult mortality occurs. Habitat protection, management, and restoration are also key to recovery since such approaches contribute to maintaining, improving or creating suitable habitat, and also contributing to reducing adult mortality (i.e., reducing threat severity). Habitat protection and restoration should focus primarily on the aquatic zone and shorelines identified as critical habitat (see section 7) where most of the adults are found. These approaches must be implemented via an integrated approach engaging various groups (e.g., land owners, land users, land planners, First Nations, non-government organizations, and governments). In order to inform these groups, as well as begin to mitigate specific threats (e.g., boating mortality, and fishing by-catch), specific communication and outreach approaches need to be undertaken. Population surveys and monitoring are also necessary to help gather information on the species in order to help inform further conservation efforts. On another front, it is necessary to fill the knowledge gaps which surround this species through a wide range of specific studies to help meet the population and distribution objective.
Under SARA, critical habitat is defined as "the habitat that is necessary for the survival or recovery of a listed wildlife species and that is identified as the species' critical habitat in the recovery strategy or in an action plan for the species". Section 41 (1)(c) of SARA requires that recovery strategies include an identification of the species' critical habitat to the extent possible, as well as examples of activities that are likely to result in its destruction.
This federal recovery strategy identifies critical habitat to the extent possible, based on the best available information for the Eastern Musk Turtle as of December 2013 Footnote31. It is recognized that the critical habitat identified may be insufficient to achieve the population and distribution objectives for the species. A schedule of studies has been included to outline the activities necessary to complete the identification of critical habitat (see section 7.2). Following the publication of this recovery strategy, additional critical habitat may be identified if additional research supports the inclusion of areas beyond those currently identified. In some of the areas identified as critical habitat, the quality of the habitat will need to be improved to support recovery.
Critical habitat for the Eastern Musk Turtle is based on three general criteria: habitat occupancy, habitat suitability, and habitat connectivity (between occupied areas), which are described in detail below.
This criterion refers to areas where there is a reasonable degree of certainty of the presence and current use of a habitat by the species. Habitat is considered occupied when:
- at least one Eastern Musk Turtle individual has been observed in any single year in the last 40 years Footnote32.
A 40-year period has been chosen for the habitat occupancy criterion. It is appropriate given the long generation time Footnote33 of the species (approximately 14 to 20 years) (COSEWIC 2012). This longevity trait makes the entire life span of the species difficult to study, by complicating the acquisition of an adequate amount of accurate life history data. The species is not well surveyed across its range. Application of a 40 year timeframe allows for the inclusion of local populations that likely persist but for which Eastern Musk Turtle individuals may not have been detected in recent years. The habitat occupancy criterion for Eastern Musk Turtle is based on the premise that a single observation may be indicative of a local population. This is appropriate for Eastern Musk Turtle which has a relatively small home range, is typically confined to a single waterbody or watercourse and its adjacent wetlands, and has low survey detection rates. Habitat occupancy is based on professional surveys and telemetry studies, nest site and overwintering site observations, observations of dead individuals, and incidental sightings of Eastern Musk Turtle. These observational data must be spatially precise (≤ 150 m) or provide enough detail to be associated to a specific suitable water feature (e.g., a river, lake or wetland) to be considered adequate to identify critical habitat. Because Eastern Musk Turtle's terrestrial movements are limited and they remain close to water (Ernst and Lovich 2009) it is usually possible to associate the observation with a corresponding suitable aquatic habitat feature. Critical habitat is not identified for locations where sufficient survey efforts, following appropriate timing and methods have been carried out over multiple years but have failed to confirm Eastern Musk Turtle persistence or habitat use and local extinction is presumed.
Habitat suitability refers to areas possessing a specific set of biophysical attributes that allow individuals to carry out essential life cycle activities (i.e., overwintering, mating, thermoregulation, nesting, foraging) as well as their movements. It is important that all required habitat areas are linked aquatically or semi-aquatically, and in reasonable proximity to one another so that turtles can move between them with ease. Suitable habitat for the Eastern Musk Turtle can therefore be described as a mosaic of aquatic and terrestrial habitats, in which specific biophysical attributes can be associated with essential life cycle activities. Within the area of suitable habitat, the biophysical attributes required by Eastern Musk Turtles will vary over space and time with the dynamic nature of ecosystems. In addition, particular biophysical attributes will be of greater importance to turtles at different points in time (e.g., during different life processes or at various times over the year). The biophysical attributes of suitable habitat for the Eastern Musk Turtle are detailed in Table3a and table3b. Given that there is no available information on the amount of habitat that is required for the Eastern Musk Turtle to complete its life cycle activities within a home range, the following approach was used to identify extent of suitable habitat for the Eastern Musk Turtle. This description of suitable habitat reflects the fact that certain biophysical attributes do not need to be immediately adjacent to each other, as long they remain connected so that individuals can move between them to meet all their biological needs and respond to or avoid disturbance. The distances determining the extent of suitable habitat are specific to the Eastern Musk Turtle and based on the species' biological and behavioural requirements (see section 3.3). Suitable habitat for the Eastern Musk Turtle consists of overwintering, mating, thermoregulation, nesting, and foraging habitat, and habitat for movement (commuting and dispersal) between these areas, and is defined as:
- An occupied suitable watercourse or waterbody (up to the high water mark) including in-stream wetlands OR suitable portion of the watercourse or waterbody (i.e., littoral zone, as measured from the high water mark to a maximum depth of 9 m) AND extending a linear distance of 1.5 km parallel to the shoreline in both directions from a valid record Footnote34 of the Eastern Musk Turtle (resulting in a total site length of 3 km); OR
- An occupied suitable wetland (or wetland complex Footnote35) not recognized as a watercourse nor a waterbody AND extending a radial distance of up to 1.5 km from a valid record of the Eastern Musk Turtle; AND
- The adjacent aquatic and terrestrial suitable habitat extending up to 50 m on either side of the occupied watercourse, waterbody, or wetland (measured landward from the boundary of the watercourse, waterbody, or wetlands).
In addition, suitable habitat includes confirmed nesting sites wherever they occur (regardless of the distance to the nearest suitable aquatic feature), as defined by:
- An area extending a radial distance of 50 m from a valid nesting record of Eastern Musk Turtle.
Eastern Musk Turtles are highly aquatic, rarely leave the water, and most home ranges are associated to a permanent waterbody, watercourse, or wetland (Ernst and Lovich 2009), although they regularly move to adjacent or connected streams, ponds and wetlands. The 1.5 km distance is selected based on the average home range length calculated for Eastern Musk Turtle in Quebec (Équipe de rétablissement des tortues du Québec, unpublished data; Belleau 2008). This distance creates a 3 km site length, which captures the movement distances observed for Eastern Musk Turtle in Canada (Edmonds 1998; Belleau 2008). The 50 m distance on either side of a waterbody, watercourse, or wetland is based on the maximum distance from aquatic features reported for Eastern Musk Turtle nesting sites in the United States (Steen et al. 2012), as little information is available on nest locations in Canada. Thus, this criterion will capture the vast majority of potential nesting habitat, which is important considering few precise locations are known. This 50 m distance may also capture some adjacent or connected streams, ponds, or wetlands containing suitable habitat for Eastern Musk Turtle, as well as the habitat suitable for movement to access them. Nest site availability and selection are likely to be especially important for local population persistence given the nature of known factors limiting Eastern Musk Turtle (e.g., long-term reproductive success strategy, climatic conditions – see section 3.4). Due to the rarity of these habitats, confirmed nesting sites are also identified as critical habitat wherever they occur, including the suitable terrestrial and aquatic habitat for Eastern Musk Turtle within a 50 m radial distance around valid nesting observations. This area allows for nesting and staging and may also provide for a protective movement corridor for females and hatchlings to migrate from and to suitable aquatic habitat.
| Habitat Feature(s) | Characteristics | Life Cycle Activities | References |
|---|---|---|---|
| Watercourses (e.g., rivers, streams), or waterbodies (e.g., lakes, bays, ponds, canals), or wetlands (e.g., shallow water, marsh) |
|
Foraging/ Thermoregulation/ Mating | Harding 1997; Belleau 2008; Ernst and Lovich 2009; Picard et al. 2011 |
| Watercourses (e.g., rivers, streams), or waterbodies (e.g., lakes, bays, ponds, canals), or wetlands (e.g., shallow water, marsh) |
|
Overwintering/ Mating | Reference removed; Belleau 2008; Rowe et al. 2009 |
| Watercourses (e.g., rivers, streams), or waterbodies (e.g., lakes, bays, ponds, canals), or wetlands (e.g., shallow water, marsh) |
|
Commuting and dispersal movements | Belleau 2008; Ernst and Lovich 2009; Rowe et al. 2009 |
| Habitat Feature(s) | Characteristics | Life Cycle Activities | References |
|---|---|---|---|
| Open shoreline areas (e.g., river banks, mudflats, sandbars, beaches, rocky outcrops, islands) |
|
Nesting | Lindsay 1965; Edmonds 2002 |
| Shoreline and terrestrial habitat (e.g., river banks, forest, grassland) |
|
Commuting movement | Edmonds 2002 |
Maintaining the natural linkages between habitat types required by the Eastern Musk Turtle is necessary for the persistence of local populations. Connectivity between local populations is required for immigration and emigration (movement into and out of local populations respectively) which increases gene flow (maintaining genetic diversity within and between local populations), allows for rescue effect which will help support local populations, and allows the species to react to environmental stressors (e.g., water level changes, pollution, anoxic environments) by moving to another location. In Canada, habitat loss and fragmentation are threats to local Eastern Musk Turtle populations (see 4.2; Edmonds 2002; COSEWIC 2012). This threat can result in the loss of dispersal corridors, isolating local populations and causing reductions in genetic diversity.
To allow short-distance movements needed to carry out Eastern Musk Turtle life cycle activities (commuting habitat), connectivity is provided within the defined areas of suitable habitat (seasonal movements between habitats as required to complete an annual life cycle) (section 7.1.2, see also Table 3; Figure 2). To allow long-distance movements such as immigration or emigration (dispersal movement – see section 3.3), the habitat connectivity criterion connects local populations by their hydrological corridors based on the documented tendencies of Eastern Musk Turtle to undertake aquatic movements for dispersal (Reference removed).
The habitat connectivity criterion is defined as:
- the hydrological corridor consisting of surface water features (watercourses, waterbodies, or wetlands) (up to the high water mark), OR portions of the feature (extending from the high water mark to a maximum water depth of 9 m) intervening between two valid records of Eastern Musk Turtle that are separated by a maximum linear distance of 4.5 km.
The 4.5 km distance is three times the average linear home-range length (1.5 km) and based on the maximum separation distance between element occurrences recommended by NatureServe (2013) to maintain connectivity and reduce the probability of genetic isolation. The distance is also consistent with documented movements by Eastern Musk Turtle in Canada (>3 km; Edmonds 1998).
Critical habitat for the Eastern Musk Turtle is identified as the extent of suitable habitat (Section 7.1.2) where the habitat occupancy criterion (Section 7.1.1) is met. At the present time, suitable habitat boundaries of permanent watercourses, waterbodies and wetlands are available for most local populations in Ontario and Quebec and can be used to define the area within which critical habitat is found, herein referred to as the critical habitat unit. Where the habitat connectivity criterion is applied (in cases where two valid observation records are within a network of continuous surface water features and are separated by a maximum distance of 4.5 km) the critical habitat unit is extended to identify a larger aquatic habitat complex for the Eastern Musk Turtle (see Figure 2). Thus, the critical habitat unit represents the maximum extent of critical habitat at a given location. Urban areas and/or human-made structures do not possess the biophysical attributes of suitable habitat for the Eastern Musk Turtle (Section 7.1.2) and are therefore not identified as critical habitat.
Application of the critical habitat criteria to available data identifies 180 units that contain critical habitat for the Eastern Musk Turtle in Canada (Table 4) totalling up to ~75,000 ha. This identification includes critical habitat for 105 known element occurrences in Canada (101 in Ontario and 4 in Quebec) as well as additional locations, not yet assessed. This is considered a partial identification of critical habitat as there are 25 locations (25 in Ontario, 0 in Quebec) that have not been surveyed recently or adequately and/or where there is a lack of certainty in the data needed to identify critical habitat or where data sharing agreements are required. A schedule of studies (section 7.2) has been developed to provide the information necessary to complete the identification of critical habitat that will be sufficient to meet the population and distribution objectives.
Due to the sensitivity of the Eastern Musk Turtle to illegal collection, critical habitat has been presented using 50 x 50 km Standardized Universal Transverse Mercator (UTM) grid squares (Figure 3, see also Table 4). The UTM grid squares are part of a standardized grid system that indicates the general geographic areas containing critical habitat, for land use planning and/or environmental assessment purposes. Critical habitat within each grid square occurs where the description of habitat occupancy (section 7.1.1), habitat suitability (section 7.1.2) and habitat connectivity (section 7.1.3) are met. More detailed information on the location of critical habitat, to support protection of the species and its habitat may be requested on a need-to-know basis by contacting Environment Canada – Canadian Wildlife Service at ec.planificationduretablissement-recoveryplanning.ec@canada.ca.
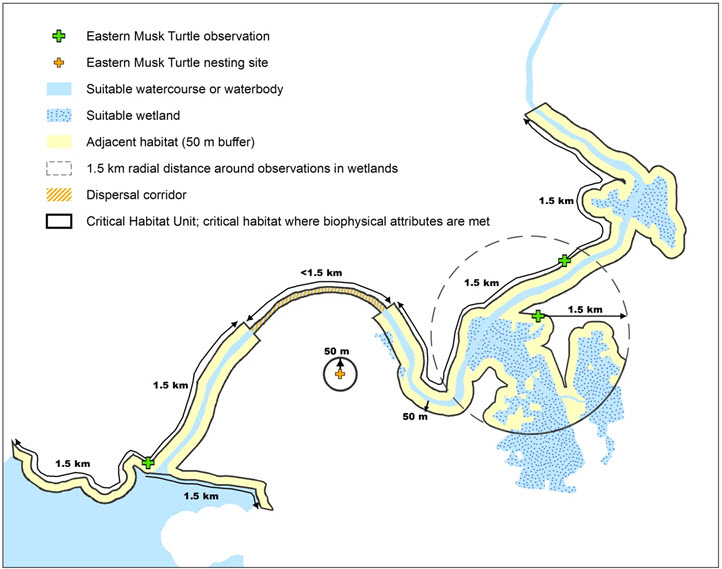
Long description for the Figure 2
Figure 2 is a schematic representation of critical habitat criteria for the Eastern Musk Turtle. It displays 3 turtle observations and 1 nesting site. Critical habitat units include the watercourse or waterbody extending to a maximum of 1.5 km parallel to the shoreline in both directions from an observation and the adjacent suitable habitat[s] within 50 m of the watercourse or waterbody. It may also include the wetland up to a maximum radial distance of 1.5 km from the valid observation and the adjacent suitable habitat[s] within 50 m of the wetlands. Moreover, it may include known nesting site comprising an area extending a radial distance of 50 m from a valid nesting observation. Where two valid observations occur within a continuous hydrological network and are separated by a maximum distance of 4.5 km, then dispersal corridors are included extending critical habitat units.
| 50 x 50 km standardized UTM grid square ID Table Footnoteg | Province/Territory | UTM Grid Square Coordinates Table Footnoteh Easting |
UTM Grid Square Coordinates Table Footnoteh Northing |
|---|---|---|---|
| 17TLGB | Ontario | 300000 | 4650000 |
| 17TLGC | Ontario | 350000 | 4600000 |
| 17TLGD | Ontario | 350000 | 4650000 |
| 17TMHA | Ontario | 400000 | 4700000 |
| 17TMHB | Ontario | 400000 | 4750000 |
| 17TMHD | Ontario | 450000 | 4750000 |
| 17TMMA | Ontario | 400000 | 5100000 |
| 17TNHA | Ontario | 500000 | 4700000 |
| 17TNHB | Ontario | 500000 | 4750000 |
| 17TNJC | Ontario | 550000 | 4800000 |
| 17TNKC | Ontario | 550000 | 4900000 |
| 17TNKD | Ontario | 550000 | 4950000 |
| 17TNLA | Ontario | 500000 | 5000000 |
| 17TNLB | Ontario | 500000 | 5050000 |
| 17TNLC | Ontario | 550000 | 5000000 |
| 17TNLD | Ontario | 550000 | 5050000 |
| 17TNMC | Ontario | 550000 | 5100000 |
| 17TPHB | Ontario | 600000 | 4750000 |
| 17TPJA | Ontario | 600000 | 4800000 |
| 17TPKB | Ontario | 600000 | 4950000 |
| 17TPKC | Ontario | 650000 | 4900000 |
| 17TPLA | Ontario | 600000 | 5000000 |
| 17TQJB | Ontario | 700000 | 4850000 |
| 17TQKA | Ontario | 700000 | 4900000 |
| 17TQKB | Ontario | 700000 | 4950000 |
| 18TTPB | Ontario | 258527 | 4850000 |
| 18TTQA | Ontario | 260346 | 4900000 |
| 18TUPB | Ontario | 300000 | 4850000 |
| 18TUPD | Ontario | 350000 | 4850000 |
| 18TUQA | Ontario | 300000 | 4900000 |
| 18TUQB | Ontario | 300000 | 4950000 |
| 18TUQC | Ontario | 350000 | 4900000 |
| 18TUQD | Ontario | 350000 | 4950000 |
| 18TURA | Ontario | 300000 | 5000000 |
| 18TURB | Ontario | 300000 | 5050000 |
| 18TURC | Ontario & Quebec | 350000 | 5000000 |
| 18TUSA | Ontario | 300000 | 5100000 |
| 18TVQA | Ontario | 400000 | 4900000 |
| 18TVQB | Ontario | 400000 | 4950000 |
| 18TVRA | Ontario & Quebec | 400000 | 5000000 |
| 18TVRC | Ontario & Quebec | 450000 | 5000000 |
| 18TWQB | Ontario & Quebec | 500000 | 4950000 |

Long description for the Figure 3
Figure 3 represents a map of southeastern Ontario with standardized 50 × 50 km grid squares that contain critical habitat for the Eastern Musk Turtle. Critical habitat occurs where the description for critical habitat is met. There is one location with one grid square close to Sudbury and Elliot Lake. There is another location with 9 grid squares at the Georgian Bay stretching from North Bay south to Barrie and from the middle of Georgian Bay east until close to Kearney. Close to Lake Ontario exist 21 grid squares that cover the area between north of Pembroke south to the middle of Lake Ontario, and from Cornwall in the east to Peterborough in the west. On the land between Lake Erie and Lake Huron exist 11 grid squares in the area extending from Detroit and Windsor in the southwest, to Toronto and Niagara Falls in the northeast, and down to the middle of Lake Erie.
The identification of critical habitat for Eastern Musk Turtle in this recovery strategy is considered partial because it is unknown whether it is sufficient to meet the population and distribution objectives (section 5) for the species. There are some locations (e.g., extant or historic element occurrences) that may still support Eastern Musk Turtle but where there is a lack of certainty in the data or where data sharing agreements are required before an identification of critical habitat can be completed. Studies are required to confirm whether these areas contribute to the overall local population viability.
| Description of Activity | Rationale | Timeline |
|---|---|---|
| Confirm habitat occupancy in locations where Eastern Musk Turtle records are spatially imprecise or cannot be associated to specific locations. | This activity is needed to complete critical habitat identification. | 2015 - 2025 |
| Conduct population surveys and habitat assessments at historical sites to confirm species' presence in areas that have received insufficient survey effort. | Information on the recent presence (including nesting) is required to support the identification of critical habitat (i.e., determination of habitat occupancy). | 2015 – 2025 |
Understanding what constitutes destruction of critical habitat is necessary for the protection and management of critical habitat. Destruction is determined on a case by case basis. Destruction would result if part of the critical habitat was degraded, either permanently or temporarily, such that it would not serve its function when needed by the species. Destruction may result from a single activity or multiple activities at one point in time or from the cumulative effects of one or more activities over time.
Destruction of critical habitat for the Eastern Musk Turtle can happen at a variety of scales and in both aquatic and terrestrial habitats. It may occur from an activity taking place either within or outside of the critical habitat boundary, and may occur in any season of the year. Within the critical habitat boundary, activities may affect habitats that provide suitable conditions for mating, nesting, foraging, thermoregulation, or overwintering. Certain activities may also affect dispersal and commuting corridors that connect these habitats. Within these corridors it is most important to maintain habitat permeability (movement through connective habitat to access adjacent suitable habitats) and, as a result, certain activities that are likely to cause destruction in habitats suitable for foraging, mating, nesting, overwintering, and thermoregulation may not cause destruction in corridors so long as sufficient habitat permeability is maintained. Activities taking place outside of the critical habitat boundary are also less likely to cause destruction of critical habitat than those taking place within the critical habitat boundary.
Activities described in Table 6 are examples of those likely to cause destruction of critical habitat for the species; however, destructive activities are not necessarily limited to those listed.
| Description of Activity | Description of Effect | Location of the activity likely to destroy critical habitat Within CH Unit Nesting, Foraging, Mating, Overwintering, OR Thermoregulation habitat |
Location of the activity likely to destroy critical habitat Within CH Unit Nesting, Foraging, Mating, Overwintering, OR Thermoregulation habitat |
Location of the activity likely to destroy critical habitat Outside CH Unit |
|---|---|---|---|---|
| Activities that result in the drainage or filling of wetlands | Complete or partial draining or filling of wetlands at any time of the year is likely to cause permanent or temporary loss or degradation of thermoregulation, overwintering, mating, nesting, foraging, and movement habitat(s). Even activities conducted outside of the critical habitat boundary may indirectly drain wetlands that form part of critical habitat. If these activities were to occur outside the bounds of critical habitat, it could result in destruction or degradation of critical habitat if the wetland characteristics that contribute to critical habitat suitability are not maintained (e.g., hydrology of critical habitat). A single event could cause critical habitat destruction. | X | X | X |
| Activities such as residential and/or industrial development; habitat conversion for agriculture | Complete or partial conversion of aquatic (e.g., wetland) or terrestrial habitats for other uses (e.g., development, agriculture) at any time of year may cause permanent loss or degradation of thermoregulation, overwintering, mating, nesting and/or foraging habitat(s). Such conversion may also remove or degrade movement habitat, thus potentially reducing access to key areas (e.g., nesting sites) as well as isolating populations. If these activities were to occur outside the bounds of critical habitat, it could indirectly result in destruction of critical habitat if the characteristics that contribute to critical habitat suitability are not maintained (e.g., hydrology of critical habitat). Currently, all such activities within critical habitat are likely to result in destruction of critical habitat. A single event could cause critical habitat destruction. | X | X | X |
| Shoreline alteration (e.g., re-profiling, linearization or hardening of stream banks) | Changes to the structure and composition of shorelines/banks (e.g., excessive removal of native vegetation, addition of stabilizing materials such as concrete) at any time of the year may create permanent unsuitable conditions for nesting, thermoregulation, overwintering, and foraging habitat(s). Shoreline hardening may also impede movement. A single event could cause critical habitat destruction. These activities would have to occur within the boundaries of critical habitat to impact the habitat. Currently, all shoreline development within critical habitat is likely to result in destruction of critical habitat. |
X | X | - |
| Activities that alter water flow and/or fragment aquatic habitat, such as the creation and operation of water control structures) | Alteration/ disruption of water flow, such as through the creation and operation of dams or other water control structures, may lead to temporary or permanent degradation or elimination of nesting, overwintering, foraging, and thermoregulation habitat(s). Stabilization of water levels may permanently diminish flood plain habitat availability (e.g., wetlands, open shoreline areas) upon which Eastern Musk Turtles rely for overwintering, nesting, foraging and/or thermoregulation. High water levels can saturate nesting substrates, thereby affecting the possibility of successfully using the site. Recurrent low water levels can promote the growth of vegetation on nesting sites, preventing their use for egg laying. Destruction of overwintering habitat can result if water depth is altered to a point where overwintering requirements are no longer met. Additionally, the construction and operation of water control structures is likely to create a barrier that impedes movements of the Eastern Musk Turtle, thereby fragmenting habitat and preventing the species from accessing suitable habitat areas within a home range, as well as preventing dispersal to adjacent populations. The creation and operation of water control structures within and outside the bounds of critical habitat could result in destruction of critical habitat if the water levels that contribute to critical habitat suitability are not maintained (i.e., hydrology of critical habitat). There is an increased likelihood that such activities could result in the destruction of critical habitat during the nesting and overwintering periods. Further studies are required to set thresholds/conditions to which such activities within and outside of critical habitat are likely to result in habitat destruction. |
X | X | X |
| Construction of roads and bridges | Construction of roads or bridges at any time of the year may degrade or permanently destroy suitable nesting, overwintering, or movement habitat. If construction of water crossings (culverts, bridges, etc.) is conducted in the winter, there is the possibility of negatively impacting overwintering sites through the use of cofferdams to remove water from an area as well as the use of heavy machinery which can impact suitable habitat below the high water mark. Construction of roads may also impede commuting movement (e.g., access to nesting sites). A single event could cause destruction of critical habitat. Such activities would have to occur within boundaries of critical habitat to impact the habitat. Existing roads and bridges are not included in the description of critical habitat and therefore the continuation of maintenance activities on the roads and bridges are not likely to result in destruction of critical habitat. | X | X | - |
| Activities that cause degradation of water quality | Discharges of domestic, commercial, industrial or municipal liquid or solid waste in water are some of the activities that could contaminate water with hazardous chemical and biological materials or heavy metals or lead to eutrophication. Activities leading to siltation or runoff of pesticides and fertilizers (e.g., agricultural activities) can also degrade water quality. The degradation of water quality and/or reduction of oxygen levels (creating anoxic conditions) in aquatic habitats within or outside critical habitat, at any time of the year, could permanently or temporarily alter or destroy foraging, overwintering, and thermoregulation habitats. Continuous, sporadic, or recurrent episodes of such discharges could lead to habitat destruction. Studies are necessary to set thresholds/conditions for these activities. | X | - | X |
| Activities that introduce exotic and/or invasive species (e.g., planting non-native plant species, moving fill) | Introduction of exotic and/or invasive species may lead to degradation or complete loss of habitat through the reduction of nesting, thermoregulation, foraging, overwintering, and movement habitat. For example, dense stands of non-native Common Reed can overgrow nesting sites thereby preventing turtles from nesting, and/or can impede movements to and from nesting, overwintering, or foraging habitats. They can also decrease sun exposure, altering thermoregulation habitat. Such stands can also fill in wetland habitat and alter overwintering sites and/or prevent turtles from being able to forage easily for food. A single event within, or adjacent to, critical habitat could lead to its destruction because once seeds are introduced it can lead to rapid expansion of invasive species. | X | X | X |
The performance indicator presented below provides a way to define and measure progress toward achieving the population and distribution objectives.
- The abundance and area of occupancy of the Canadian population of Eastern Musk Turtle is maintained or increased, helping to ensure the persistence of self-sustaining local populations in areas where it occurs.
Eastern Musk Turtles continue to persist in self-sustaining local populations throughout their Canadian range.
One or more action plans will be posted on the SAR Public Registry for the Eastern Musk Turtle by December 2022.
Due to the vulnerability of some species to illegal collection, specific references providing sensitive information have been removed from this version of the recovery strategy. To support protection of the species and its habitat, the exhaustive list of references may be requested on a need-to-know basis by contacting Environment Canada's Recovery Planning section at ec.planificationduretablissement-recoveryplanning.ec@canada.ca.
Andrews, K. M., J.W. Gibbons, and D.M. Jochimsen. 2006. Literature synthesis of the effects of roads and vehicles on amphibians and reptiles. Federal Highway Administration, U.S. Department of Transportation, Report No. FHWA-HEP-08-005. Washington, D.C. 151 p.
Aresco, M.J. 2005. The effect of sex-specific terrestrial movements and roads on the sex ratio of freshwater turtles. Biological Conservation 123:37-44.
Ashley, P.E., A. Kosloski, and S.A. Petrie. 2007. Incidence of intentional vehicle-reptile collisions. Human Dimensions of Wildlife 12:137-143.
Atlas des amphibiens et des reptiles du Québec. 2013. Data from Atlas des amphibiens et des reptiles du Québec. [accessed January 2013].
Bancroft G.T., Godley. J.S., Gross D.T., Rojas N.N., Sutphen D.A, and McDiarmud R.W. 1983. The herpetofauna of Lake Conway: species accounts. U.S. Army Corps Eng., Misc. Pap., A-83-5: 164207.
Barko, V.A., J.T. Briggler, and D.E. Ostendorf. 2004. Passive fishing techniques: a cause of turtle mortality in the Mississippi River. Journal of Wildlife Management 68:1145-1150.
Behler, J.L. and F. W. King. 2002. The Audubon Society Field Guide to North American Reptiles and Amphibians. Chanticleer Press, Inc. New York.
Bell, N., E. Conroy, K. Wheatley, B.Michaud, C. Maracle, J. Pelletier, B. Filion, B. Johnson. 2010. The ways of knowing guide. Toronto Zoo. Gage Printing.
Belleau, P. 2008. Habitat selection, movement patterns, and demography of common musk turtles (Sternotherus odoratus) in southwestern Quebec. Master's thesis, McGill University, 71 p.
Belleau, P. Unpublished Data.
Bennett, A.M., M. Keevil, and J.D. Litzgus. 2010. Spatial ecology and population genetics of Northern Map Turtles (Graptemys geographica) in fragmented and continuous habitats in Canada. Chelonian Conservation and Biology 9(2):185-195.
Bennett, A. M. and J. D. Litzgus. 2014. Injury Rates of Freshwater Turtles on a Recreational Waterway in Ontario, Canada. Journal of Herpetology In-Press.
Bishop, C.A., P. Ng, K.E. Pettit, S.W. Kennedy, J.J. Stegeman, R.J. Norstrom, and R.J. Brooks. 1998. Environmental contamination and developmental abnormalities in eggs and hatchlings of the common Snapping Turtle (Chelydra serpentina serpentina) from the Great Lakes-St. Lawrence River basin (1989-1991). Environmental Pollution 101:143-156.
Bishop, B.E., B.A. Savitzky, T. Abel-Fattah. 2010. Lead bioaccumulation in emydid turtles of an urban lake and its relationship to shell disease. Ecotoxicology and Environmental Safety 73(4):565-571.
Bleakney, J.S. 1958. A zoogeographical study of the amphibians and reptiles of eastern Canada. National Museum of Canada Bulletin 155:1-119.
Bodie, J.R. 2001. Stream and riparian management for freshwater turtles. Journal of Environmental Management 62, 443-455p.
Borkowski, R. 1997. Lead poisoning and intestinal perforations in a Snapping Turtle (Chelydra serpentina) due to fishing gear ingestion. Journal of Zoo and Wildlife Medicine. 28:109-113.
Brooks, R.J. 2007. The biology, status and conservation of Canadian freshwater turtles. In Seburn C.N.L., Bishop C.A., editors. Ecology, conservation and status of reptiles in Canada. Herpetological Conservation, Vol. 2. Salt Lake City, Utah, Society for the Study of Amphibians and Reptiles. Pp. 57-84.
Brooks, R.J. 2012. pers. comm. In: COSEWIC. 2012. Update COSEWIC Status Report on Eastern Musk Turtle Sternotherus odoratus in Canada. Prepared for the Committee on the Status of Endangered Wildlife in Canada. Ottawa xiii + 76 p.
Bulté, G. and G. Blouin-Demers. 2010a. Estimating the energetic significance of basking behaviour in a temperate-zone turtle. Ecoscience 17(4):387-393.
Bulté, G, and G. Blouin-Demers. 2010b. Implications of extreme sexual size dimorphism for thermoregulation in a freshwater turtle. Oecologia 162(2):313-322.
Bulté, G., M.-A. Carrière, and G. Blouin-Demers. 2010. Impact of recreational power boating on two populations of northern map turtles (Graptemys geographica). Aquatic Conservation: Marine and Freshwater Ecosystems 20:31-38.
Burger, J. and Garber, S.D. 1995. Risk assessment, life history strategies, and turtles: could declines be prevented or predicted. Journal of Toxicology and Environmental Health, 46:483-500.
Bush, E.R.; Baker, S.E. and MacDonald, D.W. 2014. Global Trade in Exotic Pets 2006–2012. Conservation Biology 28(3):663–676.
Cadi, A. and P. Joly. 2003. Competition for basking places between the endangered European pond turtle (Emys orbicularis galloitalica) and the introduced red-eared slider (Trachemys scripta elegans). Canadian Journal of Zoology 81(8):1392-1398.
Cadi, A. and P. Joly. 2004. Impact of the introduction of the red-eared slider (Trachemys scripta elegans) on survival rates of the European pond turtle (Emys orbicularis). Biodiversity & Conservation 13(13):2511-2518.
Caron, J. 2010. Inventaire faunique multispécifique de la rivière des Outaouais de Portage-du-Fort à Norway Bay en juillet 2010. Ministère des Ressources naturelles et de la Faune, Direction de l'expertise Faune-Forêts de l'Outaouais, rapport interne. Gatineau. 3 pp.
Carpenter, S., N.F. Caraco, D.L. Correll, R.W. Howarth, A.N. Sharpley, and V.H. Smith. 1998. Nonpoint Pollution of Surface Waters with Phosphorus and Nitrogen. Issues in Ecology N3, 12pp.
Carr, A. 1952. Handbook of Turtles. Comstock, Ithica, New York. 542pp.
Carrière, M.-A. and G. Blouin-Demers. 2010. Habitat selection at multiple spatial scales in Northern Map Turtles (Graptemys geographica). Canadian Journal of Zoology 88:846-854.
Centre de données sur le patrimoine naturel du Québec (CDPNQ). 2012.
Chabot, J., and D. St-Hilaire, 1991. Première mention de la tortue musquée, Sternotherus odoratus, au Québec. Canadian Field-Naturalist 105:411-412.
Congdon, J.D., A.E. Dunham, and R.C. van Loben Sels. 1993. Delayed sexual maturity and demographics of Blanding's turtles (Emydoidea blandingii): implications for conservation and management of long-lived organisms. Conservation Biology 7:826-833.
Congdon, J.D., A.E. Dunham, and R.C. van Loben Sels. 1994. Demographics of common snapping turtles (Chelydra serpentina): implications for conservation and management of long-lived organisms. American Zoologist 34:397-408.
COSEWIC. 2002. COSEWIC assessment and status report on the stinkpot Sternotherus odoratus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 18p.
COSEWIC. 2009. Guidelines for use of the Index of Area of Occupancy (IAO) in COSEWIC Assessments [accessed June 2014].
COSEWIC. 2012. Update COSEWIC Status Report on Eastern Musk Turtle Sternotherus odoratus in Canada. Prepared for the Committee on the Status of Endangered Wildlife in Canada. Ottawa xiii + 76 p.
Courchamp, F., Angulo, E., Rivalan, P., Hall, R.J., and Signoret, L. 2006. Rarity value and species extinction: The anthropogenic Allee effect. PLoS Biol 4(12): e415. DOI: 10.1371/journal.pbio.0040415
Crowley, J.F., Brooks, R.J. 2005. Protected areas and the conservation of Ontario's reptile species at risk: safe havens or false hopes? Proc. Ontario. Parks Research Forum 8:10-17.
Crowley, J. pers. comm. 2012. Information received by CWS-ON through technical review. Species at Risk Herpetology Specialist. Ministry of Natural Resources and Forestry, Peterborough, Ontario.
Crowley, J. pers. comm. 2013. Information received by CWS-ON through technical review. Species at Risk Herpetology Specialist. Ministry of Natural Resources and Forestry, Peterborough, Ontario.
Cunnington, D.C., and R.J. Brooks. 1996. Bet-hedging theory and eigenelasticity: a comparison of the life histories of loggerhead sea turtles (Caretta caretta) and snapping turtles (Chelydra serpentina). Canadian Journal of Zoology 74:291-296.
Daszak, P., A.A. Cunningham, and A.D. Hyatt. 2000. Emerging infectious diseases of wildlife - Threats to biodiversity and human health. Science 287:443-449.
DeCatanzaro, R., and P. Chow-Fraser. 2010. Relationship of road density and marsh condition to turtle assemblage characteristics in the Laurentian Great Lakes. Journal of Great Lakes Research 36(2):357-365.
Desrosiers, A. and S. Giguère. 2008. Inventaire de la tortue musquée (Sternotherus odoratus) dans le tronçon Waltham – Gatineau de la rivière des Outaouais au printemps 2007. Ministère des Ressources naturelles et de la Faune, Faune Québec et Environnement Canada, Service canadien de la faune, Région du Québec, 42 p.
Edmonds, J.H. 1998. Population ecology of the stinkpot turtle (Sternotherus odoratus) in Georgian Bay, Ontario. Master's thesis, University of Guelph, Guelph, Ontario, Canada. viii + 108pp.
Edmonds, J. 2002. COSEWIC status report on the stinkpot Sternotherus odoratus in Canada, in COSEWIC assessment and status report on the stinkpot Sternotherus odoratus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-18 pp.
Équipe De Rétablissement Des Tortues Du Québec. 2005. Plan de rétablissement de cinq espèces de tortues au Québec pour les années 2005 à 2010 : la tortue des bois (Glyptemys insculpta), la tortue géographique (Graptemys geographica), la tortue mouchetée (Emydoidea blandingii), la tortue musquée (Sternotherus odoratus) et la tortue ponctuée (Clemmys guttata). Ministère des Ressources naturelles et de la Faune, Québec. 57 pp.
Équipe de rétablissement des tortues du Québec. Unpublished data.
Ernst, C.H. 1968. Evaporative water-loss relationships of turtles. Journal of Herpetology 2(3/4):159-161.
Ernst, C.H. 1986. Ecology of the turtle, Sternotherus odoratus, in southeastern Pennsylvania. Journal of Herpetology 20:341-352.
Ernst, C.H., T.S.B. Akre, J.C. Wilgenbusch, T.P. Wilson, and K. Mills. 1999. Shell disease in turtles in the Rappanock River, Virginia. Herpetological Review 30:214-215.
Ernst, C.H. and J.E. Lovich. 2009. Turtles of the United States and Canada. Second edition. Johns Hopkins University Press, Baltimore.
Ewert, M.A. (1979) The embryo and its eggs: Development and Natural History. Les références dans Harless, M. et Morlock, H. (eds) Turtles Perspectives and research, John Wiley & Sons, 695 p.
Expert Panel on Climate Change Adaptation. 2009. Adapting to Climate Change in Ontario: Towards the Design and Implementation of a Strategy and Action Plan. Report to the Minister of the Environment, Queen's Press for Ontario, November 2009. 88pp.
Fenech, A., B. Taylor, R. Hansell, and G. Whitelaw. 2005. Major road changes in southern Ontario 1935-1995: Implications for protected areas. www.utoronto.ca/imap/papers/major_road_changes.pdf.
Ford, D.K. and D. Moll. 2004. Sexual and seasonal variation in foraging patterns in the stinkpot, Sternotherus odoratus, in southwestern Missouri. Journal of Herpetology 38(2):296-301.
Frankham, R. 1995. Conservation genetics. Annual Review of Genetics 29:305-327.
Gelbard, J. L., and J. Belnap. 2003. Roads as conduits for exotic plant invasions in a semiarid landscape. Conservation Biology, 17(2), 420-432.
Gibbons, J.W., D.E. Scott, T.J. Ryan, K.A. Buhlmann, T.D. Tuberville, B.S. Metts, J.L. Greene, T. Mills, Y. Leiden, S. Poppy, and C.T. Winne. 2000. The global decline of reptiles, déjà vu amphibians. BioScience 50:653-666.
Gibbs, J.P., and D.A. Steen. 2005. Trends in sex ratios of turtles in the United States: implications of road mortality. Conservation Biology 19:552-556.
Giguère, S. Unpublished data.
Giguère, S. pers. comm. 2015. Information received by Canadian Wildlife Service-Ontario through technical review. Species at Risk Biologist. Canadian Wildlife Service- Quebec.
Gillingwater, S.D. 2008. Science, education and sympathy, a strategy for successful stewardship of turtles in Ontario. Turtle Stewardship and Management Workshop, Scarborough, Ontario.
Gillingwater, S.D. pers. comm. 2012. Information received by CWS-ON through technical review. Species at Risk Biologist. Upper Thames River Conservation Authority, London, Ontario.
Gillingwater, S.D. unpub. Data. In: COSEWIC. 2012. Update COSEWIC Status Report on Eastern Musk Turtle Sternotherus odoratus in Canada. Prepared for the Committee on the Status of Endangered Wildlife in Canada. Ottawa xiii + 76 p.
Government of Canada. 2009. Species at Risk Act Policies, Overarching Policy Framework [Draft]. Species at Risk Act Policy and Guidelines Series. Environment Canada. Ottawa. 38 pp.
Gray, E.M. 1995. DNA Fingerprinting Reveals a Lack of Genetic Variation in Northern Populations of the Western Pond Turtle (Clemmys marmorata). Conservation Biology 9(5):1244-1255.
Harding, J.H. 1997. Amphibians and Reptiles of the Great Lakes Region. Univ. of Mich. Press, Ann Arbor, MI. 378 pp.
Harestad, A.S., and F.L. Bunnell. 1979. Home range and body weight - a reevaluation. Ecology 60:389-402.
Haxton, T. 2000. Road mortality of snapping turtles, Chelydra serpentina, in central Ontario during their nesting period. Canadian Field-Naturalist 114:106-110.
Hogan, L.S., E. Marschall, C. Folt, and R.A. Stein. 2007. How non-native species in Lake Erie influence trophic transfer of mercury and lead to top predators. Journal of Great Lakes Research 33(1):46-61.
Horne, B.D., R.J. Brauman, M.J. C. Moore, and R.A. Seigel. 2003. Reproductive and nesting ecology of the yellow-blotched map turtle, Graptemys flavimaculata: implications for conservation and management. Copeia 2003:729-738.
IUCN Standards and Petitions Subcommittee. 2014. Guidelines for Using the IUCN Red List Categories and Criteria. Version 11. Prepared by the Standards and Petitions Subcommittee. Downloadable from PDF.
Iverson, J.B. and Meshaka, W.E. 2006. Sternotherus odoratus - Common Musk Turtle or Stinkpot. In: P.A. Meylan (ed.), Biology and Conservation of Florida Turtles, pp. 201-223. Chelonian research Foundation, Lunenburg, MA.
Janzen, F.J. 1994. Climate change and temperature-dependant sex determination in reptiles. Proceedings of the National Academy of Sciences of the United States of America 91(16):7487-7490.
Kawartha Turtle Trauma Centre. 2014. [Accessed July 2014].
Laroque, S.M., P. Watson, G. Blouin-Demers, and S.J. Cooke. 2012a. Accidental Bait: Do deceased fish increase freshwater turtle bycatch in commercial fyke nets? Environmental Management 50:31-38.
Larocque, S.M.; Colotelo, A.H.; Cooke, S.J.; Blouin-Demers, G.; Haxton, T. and Smorowski, K.E. 2012b. Seasonal patterns in bycatch composition and mortality associated with a freshwater hoop net fishery. Animal Conservation 15:53-60.
Laverty, J.F. 2010. Measuring the effects of water-based recreation on turtle populations in an Ontario Park. M.Sc. Thesis, Laurentian University, Sudbury, Ontario, Canada. xv + 131pp.
Lemmen, D.S., F.J. Warren, J. Lacroix, and E. Bush (Eds). 2008. From Impacts to Adaptation: Canada in a Changing Climate. Government of Canada, Ottawa, 448 p.
Lester, L.A.; Avery, H.W.; Harrison A.S.; Standora E.A. 2013. Recreational Boats and Turtles: Behavioral Mismatches Result in High Rates of Injury. PLoS ONE 8(12): e82370. doi: 10.1371/journal.pone.0082370
Lindsay, R.V. 1965. Egg-laying habits of the musk turtle. Ontario Field Biologist 19:9-10.
Litzgus, J.D., J.P. Costanzo, R.J. Brooks, and R.E. Lee, Jr. 1999. Phenology and ecology of hibernation in spotted turtles (Clemmys guttata) near the northern limit of their range. Canadian Journal of Zoology 77:1348-1357.
Mahmoud, I.Y. 1969. Comparative ecology of the Kinosternid turtles of Oklahoma. Southwestern Naturalist 14(1):31-66.
Mali, I., Vandewege, M.W., Davis, S.K., Forstner, M.R.J. 2014. Magnitude of the Freshwater Turtle Exports from the US: Long Term Trends and Early Effects of Newly Implemented Harvest Management Regimes. PLoS ONE 9(1): e86478. doi:10.1371/journal.pone.0086478.
Marchand, M.N., J.A. Litvaitis, T.J. Maier, and R.M. DeGraaf. 2002. Use of artificial nests to investigate predation on freshwater turtle nests. Wildlife Society Bulletin 30(4):1092-1098.
Marchand, M.N., and J.A. Litvaitis. 2004. Effects of habitat features and landscape composition on the population structure of a common aquatic turtle in a region undergoing rapid development. Conservation Biology 18:758-767.
McKenney, D.W., B.G. Mackey, J.P. Bogart, J.E. McKee, M.J. Oldham, and A. Check. 1998. Bioclimatic and spatial analysis of Ontario reptiles and amphibians. Ecoscience 5(1):18-30.
McPherson, R.J., and K.R. Marion. 1981. Seasonal testicular cycle of the stinkpot turtle (Sternotherus odoratus) in Central Alabama. Herpetologica 37(1):33-40.
Mendonça, M.T. 1987. Photothermal effects on the ovarian cycle of the Musk Turtle, Sternotherus odoratus. Herpetologica 43(1):82-90. Midwood, J.D.; Cairns, N.A.; Stoot, L.J.; Cooke, S.J. and Blouin-Demers, G. 2014. Bycatch mortality can cause extirpation in four freshwater turtle species. Aquatic Conserv: Mar. Freshw. Ecosyst. In Press. DOI: 10.1002/aqc.2475
Miller, V., pers. Comm. 2012. IN: COSEWIC. 2012. Update COSEWIC Status Report on Eastern Musk Turtle Sternotherus odoratus in Canada. Prepared for the Committee on the Status of Endangered Wildlife in Canada. Ottawa xiii +76p.
Ministère des Forêts, de la Faune et des Parcs. 2014.
Ministère des Forêts, de la Faune et des Parcs (MFFP). unp. data.
Mitchell, J.C., and M.W. Klemens. 2000. Primary and secondary effects of habitat alteration. In M.W. Klemens (Ed.). Turtle Conservation. Smithsonian Institution Press, Washington, D.C. Pp. 5-32.
Moll, D., and E.O. Moll. 2004. The ecology, exploitation and conservation of river turtles. Oxford University Press, Oxford, UK, 393 p.
Moore, M. J. C. and R. A. Seigel. 2006. No place to nest or bask: effects of human disturbance on yellow-blotched map turtles (Graptemys flavimaculata). Biological Conservation 130:386-393.
Natural Heritage Information Centre (NHIC). 2012. Raw data, up to 2012 observations, for Eastern Musk Turtle provided by NHIC to Canadian Wildlife Service.
NatureServe. 2008. Eastern Musk Turtle Range Map. [accessed September 2014].
NatureServe. 2013. [accessed October 2013].
Ontario Ministry of Natural Resources (OMNR). 2010. Forest Management Guide for Conserving Biodiversity at the Stand and Site Scales. Toronto: Queen's Printer for Ontario. 211 pp.
Ontario Ministry of Natural Resources (OMNR). 2013. Reptile and Amphibian Exclusion Fencing: Best Practices, Version 1.0. Species at Risk Branch Technical Note. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. 11 pp.
Ontario Ministry of Natural Resources (OMNR). 2014. Unpublished data.
Ontario Nature. 2012. [accessed December 2012].
Ontario Reptile and Amphibian Atlas. 2013. Data from Ontario Reptile and Amphibian Atlas Program. [accessed January 2013].
Ontario Road Ecology Group. 2010. A Guide to Road Ecology in Ontario. Prepared for the Environment Canada Habitat Stewardship Program for Species at Risk. PDF [accessed October 10, 2014].
Patterson, J.C., and P.V. Lindeman. 2009. Effects of zebra and quagga mussel (Dreissena spp) invasion on the feeding habits of the stinkpot (Sternotherus odoratus) on Presque Isle, northwestern Pennsylvania. Northeastern Naturalist 16:365-374.
Picard, G. 2008. Does thermal quality of the environment affect habitat selection by musk turtles (Sternotherus odoratus)? B. Sc. Thesis, University of Ottawa, Ottawa, Ontario, Canada. 55pp.
Picard, G., M.A. Carrière, G. Blouin-Demers. 2011. Common Musk Turtles (Sternotherus odoratus) select habitats of high thermal quality at the northern extreme of their range. Amphibia-Reptilia 32:83-92.
Raby, G.D., A.C. Colotelo, G. Blouin-Demers, and S.J. Cooke. 2011. Freshwater commercial bycatch: an understated conservation problem. Bioscience 61:271-280.
Radomski P. and T.J. Goeman. 2001. Consequences of Human Lakeshore Development on Emergent and Floating-Leaf Vegetation Abundance. North American Journal of Fisheries Management. Vol 21:46-61.
Reed, D.H., and R. Frankham. 2003. Correlation between fitness and genetic diversity. Conservation Biology 17:230-237.
Risley, P.J. 1933. Observations on the natural history of the common musk turtle, Sternotherus odoratus. Papers of the Michigan Academy of Science, Arts, and Letters 17:685-711.
Riley, J. L., and Litzgus, J. D. 2013. Evaluation of predator-exclusion cages used in turtle conservation: cost analysis and effects on nest environment and proxies of hatchling fitness. Wildlife Research 40, 499–511.
Rizkalla, C.E., and R.K. Swihart. 2006. Community structure and differential responses of aquatic turtles to agriculturally induced habitat fragmentation. Landscape Ecology 21:1361–1375.
Rowe, J.W. 2003. Activity and movements of midland painted turtles (Chrysemys picta marginata) living in a small marsh system on Beaver Island, Michigan. J. Herpetol 37:342–353.
Rowe, J.W., G.C. Lehr, P.M. McCarthy and P.M. Converse. 2009. Activity, Movements and Activity Area Size in Stinkpot Turtles (Sternotherus odoratus) in a Southwestern Michigan Lake. The American Midland Naturalist. 162(2):266-275.
Saumure, R.A. 2009. Rapport sur la situation de la tortue musquée (Sternotherus odoratus) au Québec. Ministère des Ressources Naturelles et de la Faune du Québec, Direction du développement de la faune. 21pp.
Schneider, J.C. 1998. Fate of dead fish in a small lake. American Midland Naturalist 140(1):192-196.
Seburn, D.C. 2007. Recovery Strategy for Species at Risk Turtles in Ontario. Ontario Multi-Species Turtles at Risk Recovery Team, 73 p.
Seburn, D.C., and C.N.L. Seburn. 2000. Conservation priorities for the amphibians and reptiles of Canada. Prepared for World Wildlife Fund Canada and Canadian Amphibian and Reptile Conservation Network. 92 p.
Slevan-Tremblay, G. 2013. Effects of mercury contamination on the immune system and on parasitism in painted turtles (Chrysemys picta). Honours thesis. University of Ottawa, Ottawa, Ontario, Canada. 20 pp.
Smith, G.R., J.B. Iverson, and J.E. Rettig. 2006. Changes in a turtle community from a northern Indiana lake: a long-term study. Journal of Herpetology 40:180-185.
Steen, D.A., and J.P. Gibbs. 2004. Effects of roads on the structure of freshwater turtle populations. Conservation Biology 18:1143-1148.
Steen, D.A., M.J. Aresco, S.G. Beilke, B.W. Compton, E.P. Condon, C.K. Dodd Jr., H. Forrester, J.W. Gibbons, J.L. Greene, G. Johnson, T.A. Langen, M.J. Oldham, D.N. Oxier, R.A. Saumure, F.W. Shueler, J.M. Sleeman, L.L. Smith, J.K. Tucker, and J.P. Gibbs. 2006. Relative vulnerability of female turtles to road mortality. Animal Conservation 9:269-273.
Steen D. A., J. P. Gibbs, K. A. Buhlmann, J. L. Carr, B. W. Compton, J. D. Congdon, J.S. Doody, J. C. Godwin, K. L. Holcomb, D. R. Jackson, F. J. Janzen, G., Johnson, M. T. Jones, J.T. Lamer, T. A. Langen, M. V. Plummer, J. W. Rowe, R. A. Saumure, J. K. Tucker, and D. S. Wilson. 2012. Terrestrial habitat requirements of nesting freshwater turtles. Biological Conservation. 150:121-128.
Steen D.A., Hopkins B.C., Van Dyke J.U., Hopkins W.A. 2014. Prevalence of Ingested Fish Hooks in Freshwater Turtles from Five Rivers in the Southeastern United States. PLoS ONE 9(3): e91368. doi: 10.1371/journal.pone.0091368
Stockwell, C.A.; Hendry, A.P. and Kinnison, M.T. 2003. Contemporary evolution meets conservation biology. Trends in Ecology and Evolution 18(2):94-101.
Thomas E.S., and M.B. Trautman. 1937. Segregated hibernaculum of Sternotherus odoratus (Latreille). Copeia 1937(4): 231 In COSEWIC. 2012. Update COSEWIC Status Report on Eastern Musk Turtle Sternotherus odoratus in Canada. Prepared for the Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 76 p.
Thompson, S. pers. comm. In Seburn, D.C. 2007. Recovery Strategy for Species at Risk Turtles in Ontario. Ontario Multi-Species Turtles at Risk Recovery Team, 73 p.
Tucker, John K.; Dolan, Chad R.; Lamer, James T. 2008. Sternotherus odoratus (Stinkpot) Minimum Size/ Growth. Herpetological Review 39 (1):83-84.
Turtle Conservation Fund. 2002. A global action plan for conservation of tortoises and freshwater turtles. Strategy and funding prospectus 2002-2007. Conservation International and Chelonian Research Foundation, Washington, D.C., 30 p.
Ultsch, G.R. 2006. The ecology of overwintering among turtles: where turtles overwinter and their consequences. Biological Reviews 81:339-367.
Ultsch, G.R., and D.C. Cochran. 1994. Physiology of northern and southern musk turtles (Sternotherus odoratus) during simulated hibernation. Physiological Zoology 67(1):263-281.
U.S. Fish and Wildlife Service. 2014. Exports on Spiny Softshell, Blanding's Turtle and Eastern Musk Turtle from 1999 to October 2014. U.S. Fish and Wildlife Service, Office of Law Enforcement – LEMIS (Law Enforcement Management Information System), Arlington, TX. (unpublished data).
van Dijk, P.P. 2013. Sternotherus odoratus. The IUCN Red List of Threatened Species. Version 2014.2. Downloaded on 24 July 2014.
Van Meter, R.J., J.R. Spotila, and H.W. Avery. 2006. Polycyclic aromatic hydrocarbons affect survival and development of common snapping turtle (Chelydra serpentina) embryos and hatchlings. Environmental Pollution 142:466-475.
Zacher, G., pers. Comm. 2012. IN: COSEWIC. 2012. Update COSEWIC Status Report on Eastern Musk Turtle Sternotherus odoratus in Canada. Prepared for the Committee on the Status of Endangered Wildlife in Canada. Ottawa xiii +76p.
| Global (G) Rank | National (N) Rank (Canada) | Sub-national (S) Rank (Canada) | National (N) Rank (United States) | Sub-national (S) Rank (United States) |
|---|---|---|---|---|
| G5 | N3 | Ontario (S3) Quebec (S1) |
N5 | Alabama (S5), Arkansas (S5), Connecticut (S4), Delaware (S5), District of Columbia (S4), Florida (S5), Georgia (S5), Illinois (S5), Indiana (S4), Iowa (S2), Kansas (S4), Kentucky (S5), Louisiana (S5), Maine (S3), Maryland (S5), Massachusetts (S4S5), Michigan (S5), Minnesota (SNR), Mississippi (S5), Missouri (S5), New Hampshire (S5), New Jersey (S5), New York (S5), North Carolina (S5), Ohio (SNR), Oklahoma (S4), Pennsylvania (S4), Rhode Island (S4), South Carolina (SNR), Tennessee (S5), Texas (S5), Vermont (S2), Virginia (S5), West Virginia (S5), Wisconsin (S4) |
S1: Critically Imperilled: At very high risk of extirpation in the jurisdiction due to very restricted range, very few populations or occurrences, very steep declines, severe threats, or other factors.
S2: Imperilled: At high risk of extirpation in the jursidction due to restricted range, few populations or occurrences, steep declines, severe threats, or other factors.
N3/S3: Vulnerable: At moderate risk of extirpation in the jurisdiction due to a fairly restricted range, relatively few populations or occurrences, recent and widespread declines, threats, or other factors.
S3?: Vulnerable (inexact): An inexact numeric rank. At moderate risk of extirpation in the jurisdiction due to a fairly restricted range, relatively few populations or occurrences, recent and widespread declines, threats, or other factors.
S4: Apparently Secure: At a fairly low risk of extirpation in the jurisdiction due to an extensive range and/or many populations or occurrences, but with possible cause for some concern as a result of local recent declines, threats, or other factors.
G5/N5/S5: Secure: At very low risk of extinction or elimination due to a very extensive range, abundant populations or occurrences, and little to no concern from declines or threats).
A strategic environmental assessment (SEA) is conducted on all SARA recovery planning documents, in accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals. The purpose of a SEA is to incorporate environmental considerations into the development of public policies, plans, and program proposals to support environmentally sound decision-making and to evaluate whether the outcomes of a recovery planning document would affect any component of the environment or any of the Federal Sustainable Development Strategy's (FSDS) goals and targets.
Recovery planning is intended to benefit species at risk and biodiversity in general. However, it is recognized that strategies may also inadvertently lead to environmental effects beyond the intended benefits. The planning process based on national guidelines directly incorporates consideration of all environmental effects, with a particular focus on possible impacts upon non-target species or habitats. The results of the SEA are incorporated directly into the strategy itself, but are also summarized below in this statement.
Activities undertaken to protect Eastern Musk Turtle and its habitat will also be beneficial to other species that use similar habitat. The protection of wetland habitats will contribute to maintaining the rich biodiversity supported by those habitats. Moreover, threat reduction and mitigation measures targeting the Eastern Musk Turtle can contribute to reduce mortality in other animal species (e.g., use of ecopassages to reduce road mortality, efforts to eliminate pollution from aquatic environments). Some of these measures are likely to be found in other recovery documents, particularly those that deal with aquatic and riparian species. Table B-1 presents examples of species that may benefit from the recovery of the Eastern Musk Turtle population in Canada.
| Common Name | Scientific Name | SARA Status |
|---|---|---|
| Eastern Foxsnake | Pantherophis gloydi | Endangered |
| Fowler's Toad | Anaxyrus fowleri | Endangered |
| King Rail | Rallus elegans | Endangered |
| Lake Erie Watersnake | Nerodia sipedon insularum | Endangered |
| Jefferson Salamander | Ambystoma jeffersonianum | Endangered |
| Blanding's Turtle (Great Lakes/St. Lawrence population) | Emydoidea blandingii | Threatened |
| Least Bittern | Ixobrychus exilis | Threatened |
| Eastern Hog-nosed snake | Heterodon platirhinos | Threatened |
| Spiny Softshell | Apalone spinifera | Threatened |
| Cutlip Minnow | Exoglossum maxillingua | Threatened |
| Eastern Sand Darter | Ammocrypta pellucida | Threatened |
| American Eel | Anguilla rostrata | Threatened |
| Snapping Turtle | Chelydra serpentina | Special Concern |
| Milksnake | Lampropeltis triangulum | Special Concern |
| Eastern Ribbonsnake | Thamnophis sauritus | Special Concern |
| Grass Pickerel | Esox americanus vermiculatus | Special Concern |
These examples do not represent an exhaustive list. Given that specific needs may differ between species, implementation of recovery actions should be evaluated for impacts on all co-occurring species. Wherever possible, natural ecosystem processes should be maintained and allowed to evolve without human interference, because these are the processes to which species are adapted.
The possibility that the present recovery strategy will inadvertently generate negative effects on the environment and on other species was considered. The recommended actions are non-intrusive in nature, focussing on habitat protection, surveys and outreach. It was therefore concluded that the recovery strategy is unlikely to produce significant negative effects.