Deputy Minister Briefing Material – September 2019
- Overview of Department
- Health Portfolio Organizational Chart
Health Canada Organizational Chart - Branches
- Strategic Policy Branch
- Health Products and Food Branch
- Controlled Substances and Cannabis Branch
- Healthy Environments and Consumer Safety Branch
- Regulatory Operations and Enforcement Branch
- Pest Management Regulatory Agency
- Communications and Public Affairs Branch
- Corporate Services Branch
- Chief Financial Officer Branch
- Departmental Legal Services Unit
- Office of Audit and Evaluation (reports through Public Health Agency of Canada [PHAC])
- Office of International Affairs (reports through PHAC)
- Departmental budget and financial overview
- Health Portfolio Organizational Chart
- Governance and Operations
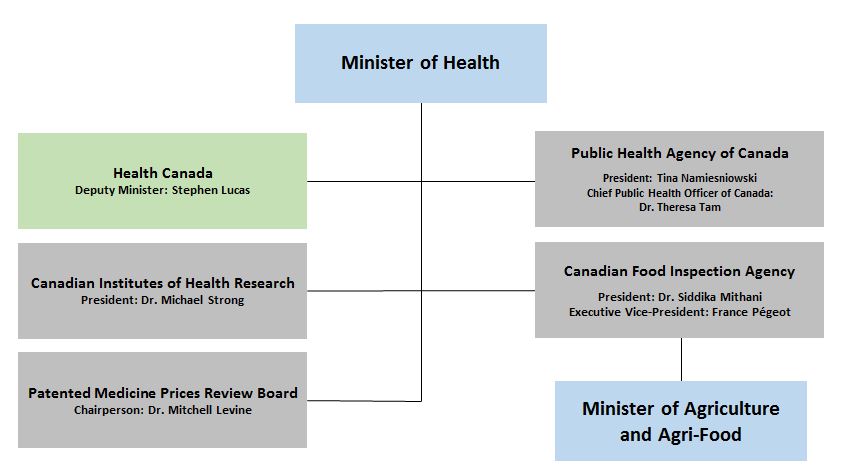
Health Portfolio Organizational Chart - Text Equivalent
The top box of the Health Portfolio Organizational Chart contains the Health Minister and then shows the five organizations that make up the Health Portfolio as well as the most senior officials of each organization: Health Canada with Deputy Minister: Stephen Lucas; the Public Health Agency of Canada with president: Tina Namiesniowski and the Chief Public Health Officer of Canada: Dr. Theresa Tam; the Canadian Institutes of Health Research with president: Dr. Michael Strong; the Patented Medicine Prices Review Board with Chairperson: Dr. Mitchell Levine; and the Canadian Food Inspection Agency with President: Dr. Siddika Mithani and Executive Vice-President: France Pégeot. The organizational chart also shows that the Canadian Food Inspection Agency is also under the purvue of the Minister of Agriculture and Agri-Food.
Health Canada Organizational Chart
Deputy Minister

Stephen Lucas
Deputy Minister of Health
(effective September 3, 2019)
Vacant
Associate Deputy Minister
Assistant Deputy Minister


Strategic Policy Branch (SPB)
ADM and Associate ADM
Abby Hoffman and Marcel Saulnier
Chief Financial Officer Branch (CFOB)
ADM & Chief Financial Officer
Randy Larkin


Health Products and Food Branch (HPFB)
ADM and Associate ADM
Pierre Sabourin and Kendal Weber

Corporate Services Branch (CSB)
ADM
Debbie Beresford-Green

Healthy Environments and Consumer Safety Branch (HECS)
ADM
Robert Ianiro

Communications and Public Affairs Branch (CPAB)
ADM
Jennifer Hollington


Controlled Substances and Cannabis Branch (CSCB)
ADM and Associate ADM
Jacqueline Bogden and Eric Costen
Legal Services
Executive Director and Senior General Counsel
Samantha Maislin Dickson
(effective September 3, 2019)

Pest Management Regulatory Agency (PMRA)
Executive Director
Richard Aucoin

Regulatory Operations and Enforcement Branch (ROEB)
ADM
Stefania Trombetti

Office of International Affairs (OIA)
Director General Footnote 1
Michael Pearson

Office of Evaluation & Audit (OAE)
Director General Footnote 1
Shelley Borys
Strategic Policy Branch (SPB) Background Deck
September 2019
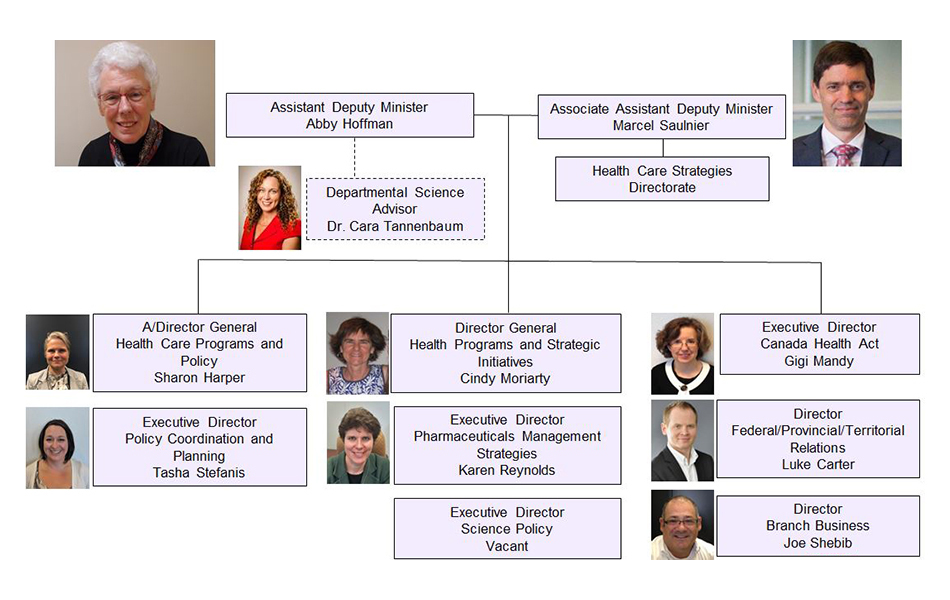
Strategic Policy Branch (SPB) Organizational Structure - Text Equivalent
The Strategic Policy Branch (SPB) is led by the Assistant Deputy Minister, Abby Hoffman, and the Associate Assistant Deputy Minister, Marcel Saulnier. There are nine directorates and divisions in the branch. Marcel Saulnier is also the head of the Health Care Strategies Directorate. Sharon Harper is the Acting Director General of the Health Care Programs and Policy Directorate. Tasha Stefanis is the Executive Director of the Policy Coordination and Planning Directorate. Cindy Moriarty is the Director General of Health Programs and Strategic Initiatives. Karen Reynolds is the Executive Director of the Office of Pharmaceuticals Management Strategies. Gigi Mandy is the Executive Director of the Canada Health Act Division. Luke Carter is the Director of the Federal/Provincial/Territorial Relations Division. Joe Shebib is the Director of the Branch Business Division. The position of Executive Director of the Science Policy Directorate is vacant. SPB also provides support for the Departmental Science Advisor, Dr. Cara Tannenbaum. Photographs for each individual are also included.
SPB - Key Functions
SPB has three main functions:
- Federal focal point for health care policy, with leadership and oversight responsibilities for health care on a pan-Canadian scale.
- Delivery of broad array of grants and contributions programs, including transfers to P/Ts, in support of federal health care policy objectives.
- Coordination and delivery of cross-cutting corporate functions for HC (and, in some cases, the Health Portfolio) and leadership on targeted horizontal policy files.
While SPB provides services similar to those of OGD strategic policy shops, it is more like a program branch than a typical corporate policy shop:
- Similar in that SPB houses Cabinet and Parliamentary Affairs, handles portfolio affairs, oversees annual submissions to the federal Budget process, supports departmental governance, reports on results and delivery, oversees integration of gender-based analysis and Indigenous perspectives and does early work ups on 'orphan' files.
- However, because the Portfolio and Health Canada branches tend to operate on a decentralized basis, SPB does not do policy development for all of HC or Health Portfolio (i.e., SPB does some quality control and central agency/MO liaison, but there is no centralized Memoranda to Cabinet function, nor challenge function on non-SPB policy files).
- Level of staff effort and resources devoted to policy and programs closer to what is done in a "line" branch in OGDs, with primary responsibility in SPB for a wide range of health care policy issues and programs.
- SPB has the largest G and C budget in the Health Portfolio, used to advance pan-Canadian health care policies and partnerships needed for a strong publicly-funded health care system.
- Legislative and regulatory role, once minimal, has been growing, in support of key policy files.
1 - Federal Focal Point for Health Care Policy
- SPB provides policy leadership, analysis and advice on federal strategy for health care system issues and the federal stewardship and oversight role in health care.
- Interprets and administers the Canada Health Act (CHA) and supports the Minister as steward of Canada's universal publicly-funded health care system.
- Negotiates and oversees federal/provincial/territorial (F/P/T) accords/agreements (e.g., 2017 F/P/T Common Statement of Principles - or CSOP - on Shared Health Priorities, accompanied by bilateral funding agreements with P/Ts).
- Responsible for the full scope of activities (including health research and analysis functions) associated with development and implementation of health care policy initiatives in priority areas. E.g.:
- Pharmaceutical and medical technology policy (including pharmacare, price regulation, intellectual property, trade)
- Health care innovation
- Health information and digital health
- Mental health and addictions
- Home, palliative care and end of life care (including Medical Assistance in Dying)
- Health workforce issues
- Organ Donation and Transplantation
- Provides secretariat support for federal panels and task forces, including advising prominent panel members, on significant health sector issues (e.g., 2018-19 Advisory Council on the Implementation of National Pharmacare, 2018 expert panel on blood plasma, 2015 Advisory Panel on Healthcare Innovation; 2015 Panel on Physician Assisted Dying).
Health Care Policy - 2019-20 Priorities
- Improve access to pharmaceuticals by lowering drug prices: modernize PMPRB regulations; leverage buying power of existing public drug plans; develop federal game plan for national pharmacare in consultation with Finance Canada; work with P/Ts and other key partners to implement Budget 2019 commitments to a Canadian Drug Agency, a national formulary, and a national strategy for high-cost drugs for rare diseases.
- Advance work on F/P/T shared health priorities set out in the 2017 CSOP (home and community care, mental health and addiction services, health care innovation, and pharmaceuticals), including work with Canadian Institute for Health Information on common indicator reporting and with P/Ts to strengthen linkages between their bilateral agreement work plans and HC funding programs.
- Address CHA compliance issues through oversight in existing and new areas, and proceeding with new federal policy initiatives on reimbursement, reporting and diagnostic services; oversee P/T Action Plans under Reimbursement Policy where required; support litigation process on Cambie Charter challenge.
- Lead reforms of the PCHO suite aimed at improving the coherence of their work and their contributions to federal and F/P/T health care system priorities.
- Undertake a comprehensive suite of policy reviews in areas of SPB responsibility in support of HC medium-term planning process; develop health diagnostique for SPB and Health Portfolio.
- Work with P/Ts to support the delivery of medical assistance in dying (MAID) services; support the mandatory review of the 2016 MAID legislation (starting June 2020).
- Strengthen outreach and policy capacity on mental health and addictions issues; work toward integrated Health Portfolio approach.
- Oversee implementation of the federal framework and action plan for palliative care.
- Advance Canadian Health Information Forum (CHIF); support efforts to accelerate adoption of digital health and virtual approaches to health care delivery.
2 - Grants and Contributions Programs
- SPB manages a wide array of grants and contributions programs in support of health care objectives and acts as HC centre of expertise (~$1.5B in 2019-20; see Appendix A for full breakdown).
- Largest part of SPB's budget is now targeted transfers to P/Ts, reflecting recent shift away from the Canada Health Transfer as the sole vehicle to support shared health priorities; federal funding is complemented by SPB work in related policy areas.
- Bilateral agreements with P/Ts on home and community care and mental health and addiction services ($11B/10 years to 2026-27)
- Territorial Health Investment Fund ($108M/4 years ending in 2020-21 for Territorial innovation and medical travel)
- Considerable resources dedicated to managing funding to (and relationships with) suite of federally created pan-Canadian health organizations known as the PCHOs (~$285M in 2019-20).
- Canadian Institute for Health Information
- Canada Health Infoway
- Canadian Partnership Against Cancer
- Canadian Agency for Drugs and Technologies in Health
- Canadian Foundation for Healthcare Improvement
- Mental Health Commission of Canada
- Canadian Centre on Substance Use and Addiction
- Canadian Patient Safety Institute
- Also responsible for programs designed to support specific federal interests (~$140M in 2019-20):
- Official Languages Health Program (for Minority OL communities)
- Health Care Policy Contribution Program - covers broad range of health care policy priorities
- Research funding to Canadian Blood Services for blood research and development of an organ and tissue donation and transplantation system for Canada
- Canadian Thalidomide Survivors Support Program
Grants and contributions - 2019-20 priorities
- Advance priorities for health care modernization through the eight pan-Canadian health organizations (PCHOs) funded by Health Canada, and complete the review of the organizations begun in 2017 (process improvements from the review are underway but structural decisions related to their future function and composition paused pending further consideration of options post-election).
- Implement Budget 2019 funding commitments for: expanded federal role in data/performance initiative for organ donation and transplantation; Brain Canada; Terry Fox Foundation; support for ovarian cancer. Includes related policy development, [REDACTED], and new and/or revised funding agreements with recipients.
- Consider approach to flowing next 5 years of 10-year targeted funding to P/Ts for home and community care and mental health and addictions in advance of expiration of current agreements in 2021-22, and examine possibility of renewal of Territorial Health Investment Fund in advance of 2020-21 sunset (currently on fourth continuous iteration of initiative).
- Oversee implementation ofthenew Canadian Thalidomide Survivors Support Program and manage on-going litigation related to former program.
- Continue efforts to reduce blood donor deferral times for men who have sex with men (MSM) through work with Canadian Blood Services.
- Reset Health Care Policy Contribution Program priorities to align with current federal and F/P/T agenda and make process improvements to streamline and speed up solicitation, decision-making, and oversight/monitoring processes (including modifications for delivery to Indigenous organizations).
3 - Coordination of Cross-cutting Corporate Functions & Leadership on Horizontal Policy Files
- SPB provides strategic oversight and coordination for cross-cutting corporate functions:
- Provides advice to Minister and Deputy Minister on Cabinet and parliamentary business.
- Prepares HC annual submission to federal Budget process, supports Minister for Mains and Supplementary Estimates processes (with CFOB), reports on Results to PCO.
- Coordinates HC governance structures (e.g., Executive Committees, DG Policy committee, Medium-Term Planning and Transition exercises).
- Manages departmental briefing functions (e.g., Ministerial Briefing Unit).
- Coordinates Governor in Council appointments.
- SPB also provides leadership and plays integration role on key horizontal functions and files:
- Focal point for HC's federal/provincial/territorial (F/P/T) relations (with some Health Portfolio-wide coordination)
- HC focal point for engagement on Indigenous health matters and inter-departmental work.
- Develops and administers a framework of policies for the conduct of science in HC (research ethics, scientific integrity, Open Science, scientific publications), coordinates science policy issues within HC and with other government departments, supports new Departmental Science Advisor.
- SPB plays these roles for the broader Health Portfolio when an integrated approach is needed for the Minister (e.g., Cabinet and parliamentary business, federal Budget submissions, preparation for F/P/T Ministerial meetings).
Cross-cutting Corporate Functions and Horizontal Policy Files - 2019-20 Priorities
- Design and lead the HC medium-term planning and transition processes and co-ordinate roll-out of Health Portfolio transition activities; support DM's HC Transition Advice and Planning (TAP) Committee of regulatory and program/policy ADMs; liaise with PCO and support HC contributions to Deputy-level and whole-of-Government process (e.g., MTP-oriented DMCs); be ready for immediate post-election roll-out.
- Co-ordinate Health Portfolio Budget 2020 proposals, reflecting platform commitments, priorities of next Government and Mandate Letter commitments.
- Support F/P/T dimensions of SPB's files (with an emphasis on supporting engagement on national pharmacare and implementation of the PCHO review); lead Health Portfolio preparation for F/P/T Health Ministers Meeting (HMM) early in mandate of next Government.
- Support recently appointed inaugural Departmental Science Advisor as part of priority to strengthen HC role in science policy.
- Following recent shift of First Nations and Inuit Health Branch from HC to ISC, develop HC/Health Portfolio game plan to support Indigenous health policy; advance F/P/T and ISC-HC-Indigenous providers/stakeholder process on cultural safety.
Emerging function: regulatory, legislative and litigation activities
- SPB is, fundamentally, not a regulatory or legislative branch but approach to addressing recent priorities has significantly increased SPB's role in (and resources dedicated to) regulatory, legislative and legal activities.
- Lead role in developing regulations in support of broader health objectives under SPB's remit (e.g., developing, and implementing the MAID Reporting Regulations, modernization of the PMPRB's drug price regulatory regime);
- Directly engaged in Criminal Code Amendments for MAID and amendments to the Federal-Provincial Fiscal Arrangements Act (related to the Canada Health Act reimbursement policy); and
- Growing engagement in litigation related to health care issues and SPB files (e.g., Hepatitis C compensation, legal challenges to the Thalidomide Support Program, federal role in Cambie (CHA) Charter Challenge).
- Role anticipated to continue, with legal decisions on MAID and the Cambie Charter challenge expected in coming year, and new litigation on the horizon (e.g., pharmaceutical companies challenge to the PMPRB regulatory changes).
SPB Financial Overview - 2019-20
- Total SPB 2019-20 budget is $1.586 B.
- SPB's $30M salary budget supports an average head count of approximately 270 employees.
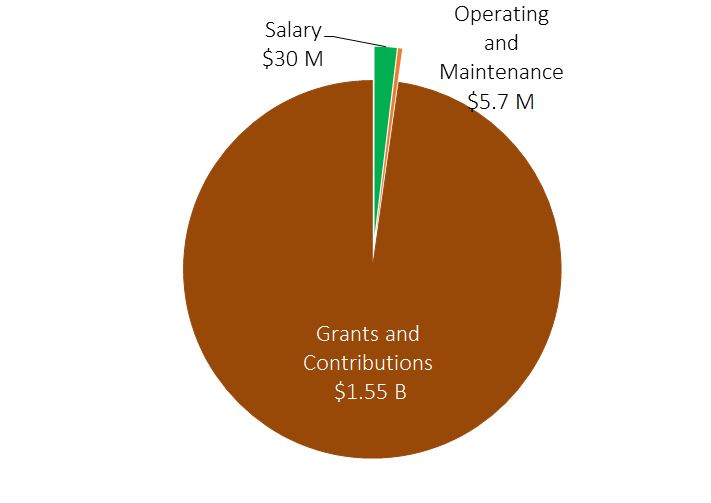
Strategic Policy Branch Expenditures - Text Equivalent
The pie chart shows a break-down of Strategic Policy Branch's expenditures for 2019-2020. Expenditures for 2019-20 include $1.55 billion for grants and contributions, $30 million for salary, and $5.7 million for operating and maintenance.
| Grants / Contributions | 2019-20 Amount ($000) |
|---|---|
| Funding to Provinces and Territories | 1,127,000 |
| Funding to the Pan-Canadian Health Organizations | 285,568 |
| Multi-project targeted funding | 106,853 |
| Funding for health research | 21,755 |
| Canadian Thalidomide Survivors Support Program | 13,419 |
| Total | 1,554,595 |
- Total SPB 2019-20 budget is $1.586 B.
- SPB's $30M salary budget supports an average head count of approximately 270 employees.
| Grant/Contribution | Funding Authority | 2019-20 Amount ($000) |
|---|---|---|
| Provinces and Territories | ||
| Bilateral agreements with P/Ts (home and community care and mental health and addictions services) | 10-year commitment (2017-18 to 2026-27) |
1,100,000 |
| Territorial Health Investment Fund | Four-year commitment (2017-18 to 2020-21) |
27,000 |
| Pan-Canadian health organizations | ||
| Canadian Institute for Health Information | Ongoing | 87,659 |
| Canada Health Infoway | Five-year commitment (2017-18 to 2021-22) |
75,000 |
| Canadian Partnership Against Cancer | Five-year commitment (2017-18 to 2021-22) |
51,000 |
| Canadian Agency for Drugs and Technologies in Health | Ongoing | 23,059 |
| Canadian Foundation for Healthcare Improvement | Ongoing | 17,000 |
| Mental Health Commission of Canada (MHCC) | Four-year commitment (2017-2021) |
14,250 |
| Canadian Centre on Substance Use and Addiction (CCSA) | Ongoing | 10,000 |
| Canadian Patient Safety Institute | Ongoing | 7,600 |
| Multi-project targeted funding | ||
| Health Care Policy Contribution Program (e.g., Heart & Stroke Foundation, Choosing Wisely, Pallium Canada, Canadian Virtual Hospice, Institute for Safe Medication Practices, Centre for Addiction and Mental Health) | Ongoing | 26,874 |
| Official Languages Health Program | Five-year commitment (2018-19 to 2022-23) |
37,380 |
Substance Use and Addictions Program
|
Ongoing | 38,828 |
|
Five-year commitment (2018-19 to 2022-23) |
2,405 |
|
Five-year commitment (2018-19 to 2022-23) |
1,366 |
| Health Research | ||
Canadian Blood Services
|
Ongoing | 5,000 |
|
Ongoing | 3,580 |
| Canada Brain Research Fund Program (Brain Canada) | Ending in 2019-20; being renewed following Budget 2019 announcement | 13,175 |
| Canadian Thalidomide Survivors Support Program | Ongoing | 13,419 |
| Total (may not add up due to rounding) | - | 1,554,595 |
- In addition, a 3-year funding commitment (2019-20 to 2021-22) for Innovative Solutions Canada ($1.4 million in 2019-20) is in approvals.
- Note: Substance Use and Addictions Program funds transferred to CSCB may also be included in the financial overview slide of the CSCB presentation.
Health Products and Food Branch (HPFB)
Background Deck
September 2019
Mission
- Minimizing health risk factors to Canadians while maximizing the safety provided by the regulatory system for health products and food.
- Providing information to Canadians so they can make healthy, informed decisions about their health.
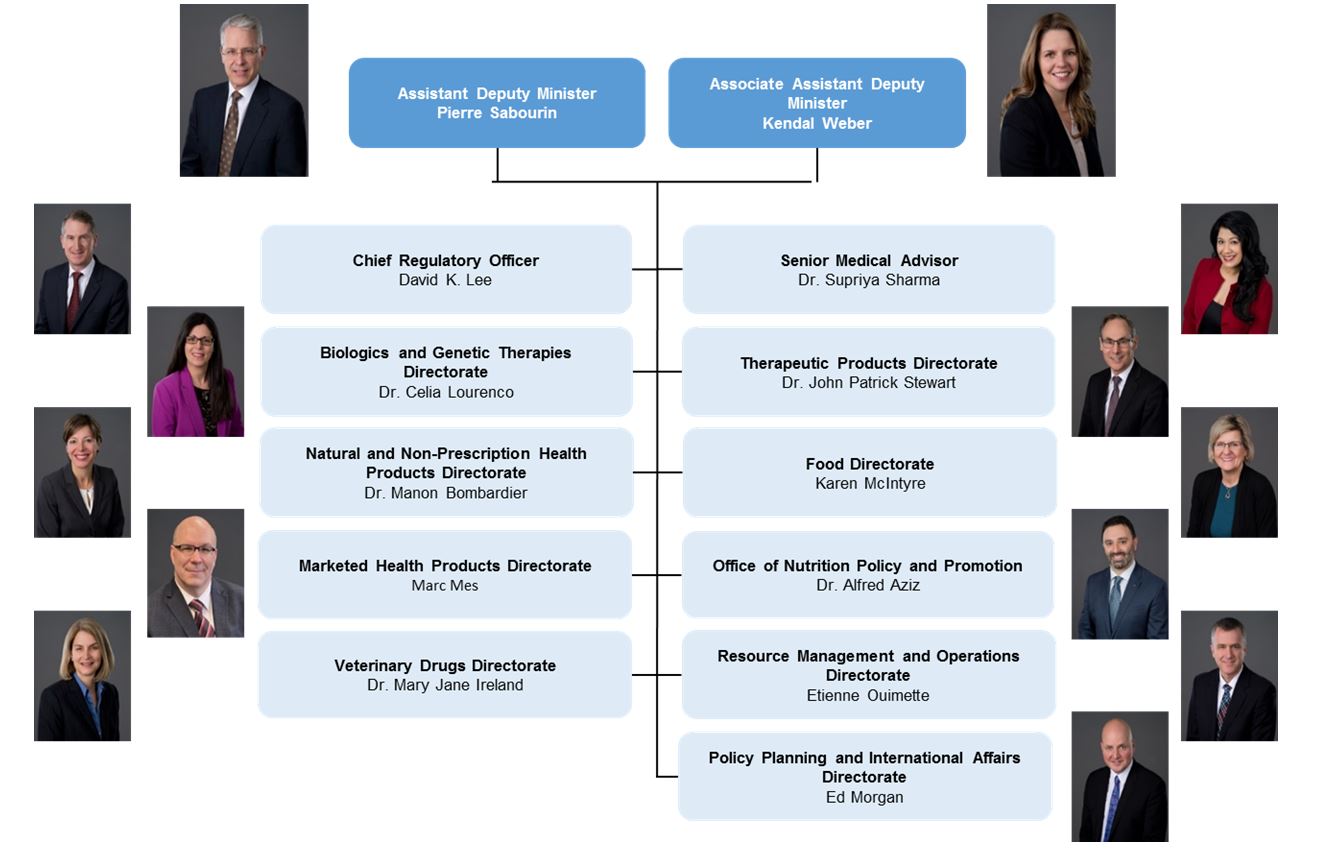
Current Branch Organizational Structure - Text Equivalent
Health Products and Foods Branch senior management organizational structure:
- Assistant Deputy Minister: Pierre Sabourin; Associate Assistant Deputy Minister: Kendal Weber
- Chief Regulatory Officer: David K. Lee; Senior Medical Advisor: Doctor Supriya Sharma
- Director General of Biologics and Genetic Therapies Directorate: Doctor Celia Lourenco; Director General of Therapeutic Products Directorate: Doctor John Patrick Stewart
- Director General of Natural and Non-Prescription Health Products Directorate: Doctor Manon Bombardier; Director General of Food Directorate: Karen McIntyre
- Director General of Marketed Health Products Directorate: Marc Mes; Director General of Office of Nutrition Policy and Promotion: Doctor Alfred Aziz
- Director General of Veterinary Drugs Directorate: Doctor Mary Jane Ireland; Director General of Resource Management and Operations Directorate: Etienne Ouimette
- Director General of Policy, Planning and International Affairs Directorate: Ed Morgan
Domestic and International Roles
Areas of responsibilities
- Food (including the food guide)
- Prescription drugs
- Non-prescription drugs
- Natural health products
- Medical devices
- Veterinary drugs
- Blood
- Assisted human reproduction
International collaboration
- Build and maintain working relationships with key partners: US, EU, UK, Japan and Australia
- Increase multilateral engagement in targeted areas on advanced technologies and medical devices
Legislative framework
Food and Drugs Act and its Regulations
Assisted Human Reproduction Act and its Regulations
Key Stakeholders
- Food Industry
- Health Products Industry
- Canadian Blood Services (CBS)/ Héma-Québec
- Provincial/Territorial health partners
- Canadian Agency For Drugs And Technologies In Health (CADTH)/ Institut national d'excellence en santé et en services sociaux (INESSS)
- Healthcare professional and Patient organizations
- Academia
Core Business Lines
Food
- Develop evidence-based dietary guidance (e.g. Canada's Food Guide).
- Establish regulations for all foods sold in Canada and provide pre-market assessment and authorization of food additives, novel foods, and infant formula.
- Provide health risk assessments to support CFIA's food safety investigations.
Health Products
- Clinical Trials
- Regulate, review application
- Product Submission Review and Market Authorization
- Assess health product submissions for scientific evidence and potential risks.
- Authorize products for sale if the benefits outweigh the risks.
- Post-Market Surveillance
- Monitor health product safety, and communicate emergent risks to Canadians.
- Collect adverse event reports, address advertising complaints and conduct post-market safety reviews.
Blood
- Regulate the safety of blood and blood components collected for transfusion and for manufacture into therapeutic products.
- Review and authorize submissions filed under the Blood Regulations to ensure the safety of the blood establishments' operational procedures.
Assisted Human Reproduction
- Establish regulations to protect Canadians making use of third party assisted human reproduction technology.
Significant accomplishments over the last four years
Over the last four years, HPFB has delivered on an unprecedented number of high profile initiatives. This volume of activity was unique, as was the amount of funding the Branch received. We published 23 regulatory package and some major accomplishments included:
New Canada Food Guide
- Canada's new Food Guide was launched in 2019.
Opioids
- New pathway to permit the Minister to add/amend terms and conditions to an authorization, require patient information handout and warning stickers, and restrict marketing of opioids.
Implementing Vanessa's Law - Protecting Canadians from Unsafe Drugs Act
- Implementing changes to the Food and Drug Act to improve the ability to collect post-market safety information, take appropriate actions when a serious risk to health is identified (e.g. recall), and promote greater confidence in the oversight by increasing transparency.
Improving the regulations of drugs and devices
- Parallel review with CADTH and INESSS, scientific capacity investments (i.e., digital health).
Cost Recovery Renewal
- Increased fees for regulatory activities related to human drugs, veterinary drugs, and medical devices to help sustain programs and provide the Branch with a source of funds to address existing and emerging gaps. The revised fees will be implemented April 1, 2020.
Strengthening the Assisted Human Reproduction Act
- Regulations published to reduce the risks to human health and safety and make clear what expenses may be reimbursed to donors and surrogates.
Will come in force in 2020.
Blood
- Reduce MSM deferral to 3 months.
Priorities
New healthy eating initiatives to reduce the burden of chronic disease
- Canada is facing a growing burden of chronic disease. Two in five Canadian adults live with at least one of the ten most common chronic diseases, including heart disease, stroke, diabetes and cancer.
- The economic burden of chronic diseases is estimated at $26.7 billion annually. By 2025, it will cost Canada over $34 billion annually to treat chronic disease.
- Poor diets are a primary cause of chronic disease in Canada. Initiatives to improve diet and enhance information to help Canadians make better choices could help Canada create a more sustainable healthcare system: [REDACTED].
Regulatory Reviews (led by TBS)
- Health and Biosciences: advanced therapeutics, use of foreign reviews, modernized clinical trial regulations, and risk based approval pathways.
- Agri-food led by CFIA: supplemental foods, use of foreign reviews, human milk fortifiers, food labelling coordination, and biotechnology products approval pathways.
Determining whether to establish a pathway for cannabis health products that do not require physician oversight
- Exploring whether to establish a pathway for cannabis-based products which do not require the oversight of a health practitioner (natural health products, over the counter drugs).
- Analysis of the results of the Summer 2019 consultation are underway and we will provide advice on next steps, including the launch of an external scientific advisory panel.
Priorities
Enhancing post-market authorities for natural health products (NHPs)
- Health Canada does not currently have the same post-market tools for NHPs as it does for other products like drugs, devices or consumer products. [REDACTED].
- [REDACTED].
- Office of the Auditor General audit is taking place in 2019-20 to review Health Canada's oversight and enforcement of responsibilities as they relate to NHP programs and NHPs.
Medical Devices Action Plan
- Recent safety issues with medical implants have resulted in significant domestic and international media attention regarding the evidence required to approve a medical device, post-market oversight of devices, and the transparency of medical device regulations. Industry, clinicians and patients began calling for more openness and increased transparency concerning the approval, oversight and performance of medical devices.
- In response, Health Canada introduced the Action Plan on Medical Devices, which includes concrete actions to continuously improve the safety, effectiveness and quality of medical devices in Canada. Work is underway and needs to continue in order to meet our commitments to Canadians.
Prevent antimicrobial resistance (led by PHAC)
- Antimicrobial resistant (AMR) infections are a growing public health threat in Canada and around the world. The misuse and over use of antimicrobials in humans and animals is a major contributor to the emergence and spread of AMR, threatening our ability to treat infections.
- [REDACTED].
Overview of Substance Use in Canada and the Controlled Substances and Cannabis Branch
Background Deck
September, 2019
Purpose
- Provide an overview of substance use in Canada, and the approach being taken to address it
- Discuss short and longer term substance use issues, and key forthcoming decisions
- Provide a snapshot of Health Canada's Controlled Substances and Cannabis Branch
Substance Use in Canada
Rates of substance use (2017)
- Alcohol - 78% (23.3 million Footnote 2)
- Tobacco - 18% (5.3 million Footnote 3)
- Cannabis - 15% (4.4 million Footnote 4)
- Illegal DrugsFootnote 5 - 3% (987,000 Footnote 6)
- Vaping Products - 3% (863,000 Footnote 7)
- Problematic Prescription Drug Use - 1% (336,000 Footnote 8)
Key Substance Use Trends
- Generally speaking, overall rates of substance use tend to be higher in one's youth than in later years.
- Riskier/more harmful forms of use (e.g. binge use) also tend to be undertaken more by youth than adults
- Men tend to use substances more than women
- Many people will use multiple substances at once (poly-substance use)
- Overall, only a small percentage of people who use substances will develop a substance use disorder
- Estimates range from 4% to over 10% in any given year, depending on the substance and age of the person
- Over one's lifetime, it's estimated that more than 1 in 5 (21.6%) Canadians will meet the criteria for a substance use disorder
- Underlying factors often include experience with trauma, poverty, mental health issues, physical and emotional pain, and other socio-economic factors
- Problematic substance use causes significant health harms and associated costs
- Opioids crisis - over 4,400 deaths in 2019 (1 life lost every two hours)
- Tobacco and alcohol claim tens of thousands of deaths each year (over 47,500 and 14,800 respectively)
- Each day, over 400 Canadians are hospitalized because of alcohol or drug-related harms
- In 2017, 4% (820,000) of Canadians aged 15 years and older reported experiencing at least one harm in the past year due to their illegal drug use
Rates of Cannabis Use Footnote 9 (2019, Q2)
- Men
- 21%
- Women
- 12 %
- Aged 15-24
- 27.4 %
- Aged 25-44
- 24.2 %
- Aged 45-64
- 12.2 %
Patterns of Substance Use
- Generally speaking, substance use occurs across a spectrum
- Understanding patterns of use and associated risks/harms helps to better design policies and programs
- Federal focus is on minimizing use (youth) or reducing the harms of use (adults), preventing problematic use patterns before they begin, and supporting treatment and recovery
- Problematic substance use: the use of any drug or substance in a manner, situation, amount, or frequency that is harmful to the individual or to society
- For those with substance use disorder, the focus shifts to harm reduction, treatment and recovery
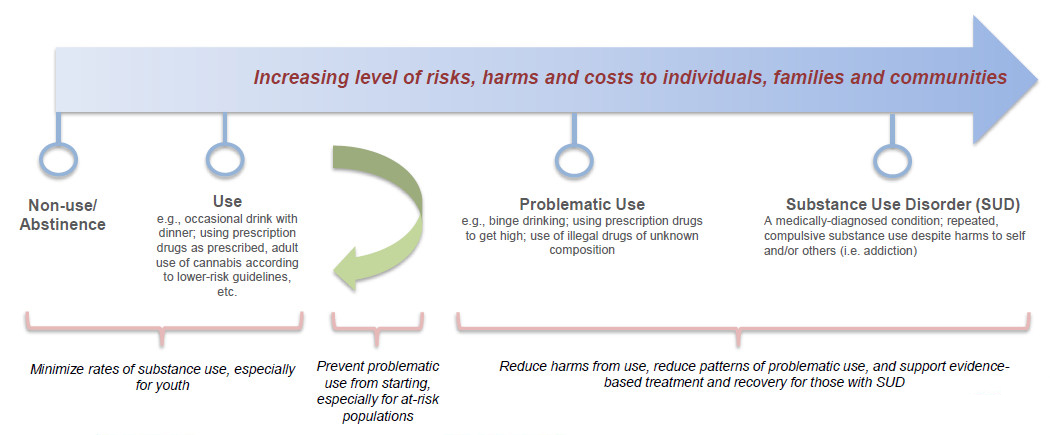
Continuum of substance use - Text Equivalent
The continuum depicts the increasing level of risks, harms and costs to individuals, families and communities as users move from non-use/abstinence to Substance Use Disorder
- Non-Use/Abstinence and
- Use indicate an opportunity to minimize rates of substance use, especially for youth.
- A Green Arrow showing a change in direction indicates the optimal point on the continuum at which to prevent problematic use, especially for at-risk populations.
- Problematic Use and 5. Substance Use Disorder (SUD) are areas where increased costs occur because reduction in harms from drug use or effort to reduce patterns of problematic use, require the use of evidence-based treatment and recovery
Harms Related to Substance Use
- A number of factors contribute to the overall level of harm related to substance use:
- the nature of the substance itself (lower risk substances such as cannabis, vs. higher risk substances such as tobacco, cocaine, opioids, etc.)
- the method by which the substance is consumed (e.g. ingestion tends to be less harmful than injection; vaping less harmful than smoking, etc.)
- the manner in which the substance is used (infrequently vs frequently, in small amounts vs "binge" use, alone vs. with others who can provide help in case of emergency, etc.)
- the total number of people in society using a substance
- See Appendix A for further details on the costs and harms of specific substances in Canada
| Associated Harms (2014 Data) |
Alcohol | Tobacco Footnote 1 | Cannabis | Opioids |
|---|---|---|---|---|
| Deaths | 14,826 | 47,562 | 850 Footnote 2 | 2,395 Note: 4460 deaths in 2018. |
| Emergency Dept. Visits | 649,412 | 239,512 | 27,761 | 31,686 |
| Hospitalizations | 87,911 | 145,801 | 3,835 | 6,981 |
| Unintentional Injuries | 42,774 | 113 | 98 | 2,204 |
| Lost Productivity Footnote 3 | 110,048 | 74,622 | 6,892 | 49,533 |
| Counts of Policing | 252,227 | - | 126,492 | 99,052 |
|
||||
Sources: Canadian Substance Use Costs and Harms, 2018 (data from 2014); Canadian Tobacco, Alcohol and Drugs Survey 2017.
Costs of Substance Use in Canada
Apart from deaths, other costs in Canada are increasing. The estimated cost of substance use in Canada (for 2014) is approximately $38.4 billion per year Footnote 10.
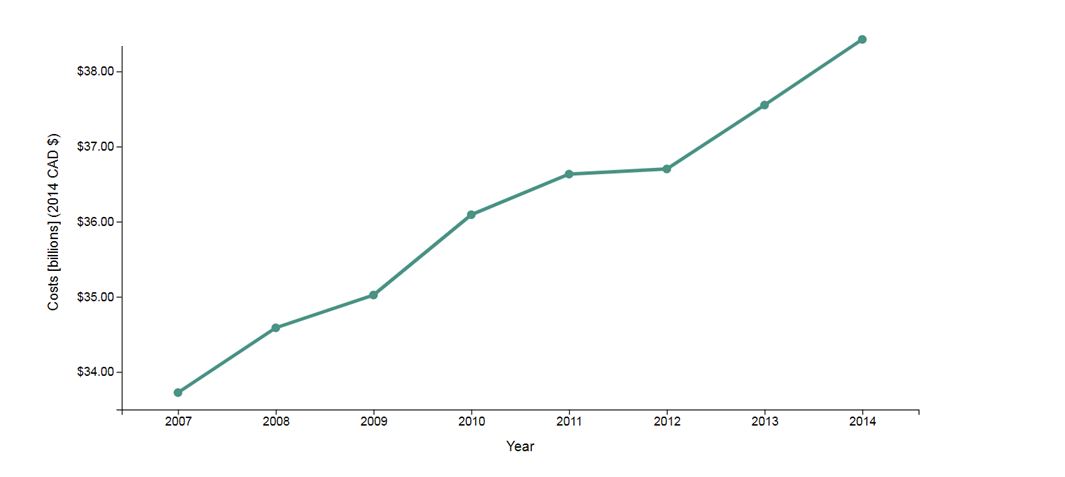
Substance use-attributable overall costs, Canada 2007-2014 - Text Equivalent
Substance use-attributable overall costs in Canada 2007 – 2014. The line graph demonstrates the increasing costs from 2007 to 2014. In 2007, costs were reported at less than $34 billion and increase sharply over the following seven years to exceed $38 billion by 2014.
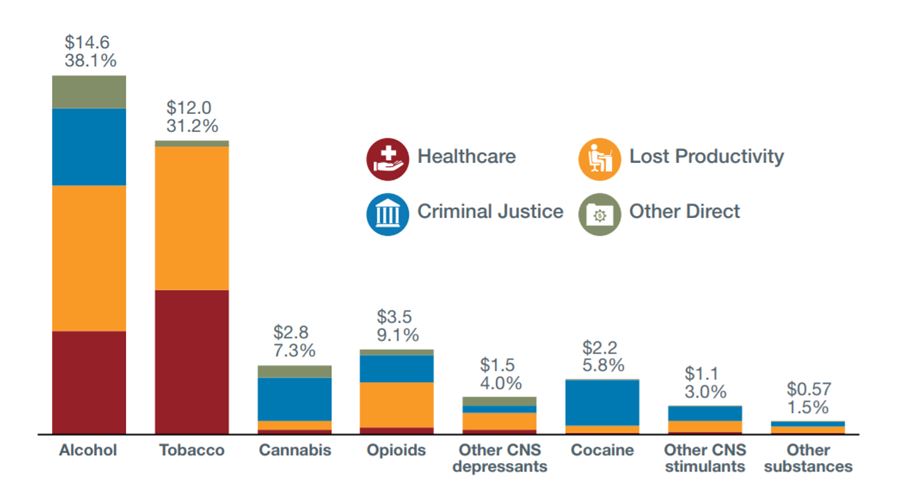
Costs of substance use in Canada (2014) - Text Equivalent
Costs of substance use in Canada in 2014. The bar graph depicts 2014 substance use costs, broken out by substance and further broken out in four specific implicated sectors: Healthcare, Criminal Justice, Lost Productivity and Other Direct.
- Alcohol comprises 38.1% of total costs (approximately $14.6 billion)
- Tobacco comprises 31.2% of total costs (approximately $12.0 billion)
- Cannabis comprises 7.3% of total costs (approximately $2.8 billion)
- Opioids comprises 9.1% of total costs (approximately $3.5 billion)
- Other CNS Depressants comprises 4.0% of total costs (approximately $1.5 billion)
- Cocaine comprises 5.8% of total costs (approximately $2.2 billion)
- Other CNS Stimulants comprises 3.0% of total costs (approximately $1.1 billion)
- Other substances comprises 1.5% of total costs (approximately $0.57 billion)
- Most costs are associated with alcohol and tobacco
- Considerable regional variations (highest total costs in ON and QC; highest per capita costs in the Territories)
- These numbers are from 2014 and so do not reflect the recent increases in costs related to the ongoing opioids crisis
Addressing Substance Use
Substance use is a multifaceted, and often complex, problem:
- A significant number of Canadians use substances
- People use substances in different ways (experimental, occasional, repeated/habitual) and for different reasons (for enjoyment, curiosity, to "fit in", to reduce stress, to deal with physical/emotional pain, life events or trauma)
- In the case of people with a substance use disorder, they may have a uncontrollable compulsion to use substances, or a need to use them to ward off the painful effects of withdrawal
- Patterns of substance use often vary by region, requiring close collaboration with P/Ts and local organizations/stakeholders
- No one level of government, nor one specific action or intervention, can solve the problem
- Actions need to be taken at all levels: population level, targeted interventions (e.g. youth, at-risk populations) and at the individual level
- Reducing rates and patterns of harmful substance use can be done, but it takes time and effort (e.g. reducing rates of tobacco use has taken decades of action at all levels)
Key Federal Directions in Addressing Substance Use
- Prevention: Reducing rates of use and preventing problematic use, especially for youth and at-risk populations
- Treatment: Supporting evidence-based treatment and recovery services
- Reducing harms and improving health outcomes for people who currently use substances
- Enforcement and regulatory measures that address organized drug crime and support authorized uses of controlled substances (e.g. the manufacture and distribution of some prescription medications)
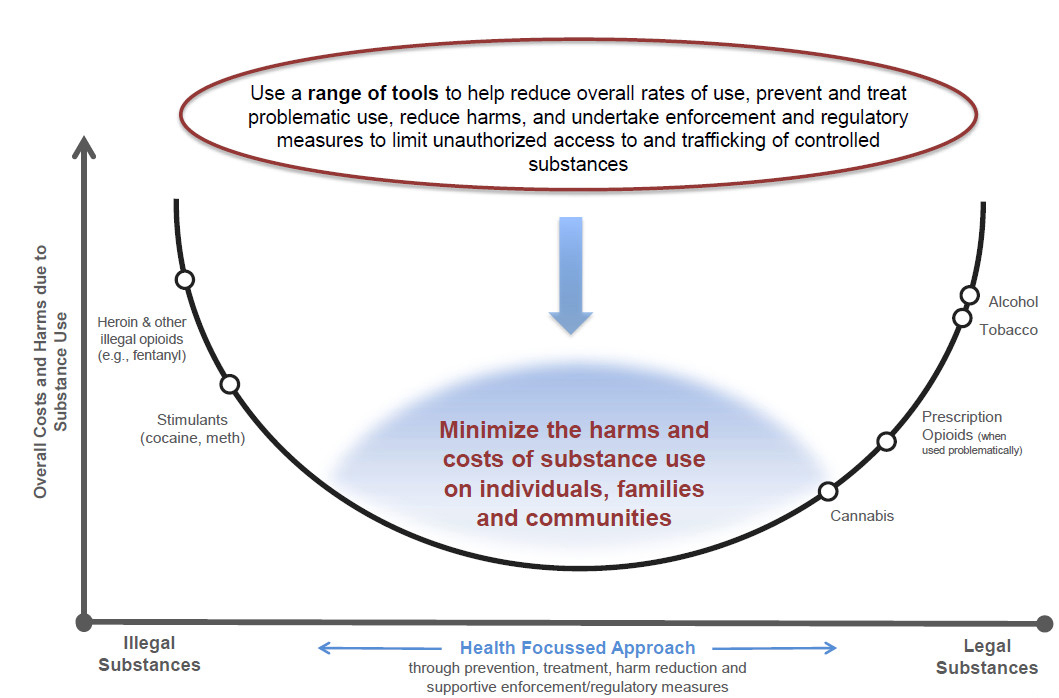
Our Goal - Text Equivalent
A u-shape graph outlines a theoretical model for a health-focused policy approach for drug control. The overall goal of the model is to show that a comprehensive health-focused policy approach to substance use (both legal and illegal drugs) involves minimizing the level of costs and harms to both individuals and society as a whole. For illustrative purposes only, on the vertical axis a range of illegal and legal drugs are placed to demonstrate the harms and costs of different drugs (e.g. both harms and costs felt by the individual and those felt more broadly, at community and societal levels). Substances with greater costs and harms appear at the upper ends of the curve. The ranking of each of these substances is illustrative and not an objective assessment of the relative harms of each substance to the individual and/or society.
The horizontal axis of the graph shows that a comprehensive, health-focused approach to dealing with substance use can result in decreased overall costs and harms. The graph notes that a range of tools are required to help reduce overall rates of use, prevent and treat problematic substance use, reduce harms and undertake enforcement and regulatory measures to limit unauthorized access to and trafficking of controlled substances.
Roles and Responsibilities
Substance use cannot be addressed through federal action alone. Collaboration and coordination with provincial and territorial governments is essential, as is engagement with a range of key non-governmental players.
Federal Government
- Drug control legislation (Controlled Drugs and Substances Act, Tobacco and Vaping Products Act, Cannabis Act, Food and Drugs Act), and enforcement of regulations (through Health Canada's Regulatory Operations and Enforcement Branch)
- National leadership and collaboration through the Canadian Drugs and Substances Strategy (CDSS) and Canada's Tobacco Strategy (CTS) - see Appendix B
- Grants and contributions funding programs (e.g. Substance Use and Addictions Program)
- Data collection, surveillance and research
- Drug risk education/health promotion activities (e.g. public education campaigns)
- Services provided to federal populations (Indigenous peoples, members of armed forces and veterans, federal inmates)
- International engagement on tobacco and drug control/policy issues
Provincial/Territorial Governments
- Responsible for the delivery of health services to Canadians, including treatment and harm reduction services
- Undertake targeted education/ prevention campaigns
- Responsible for local law enforcement
- Set regulations for sale and access to certain substances (e.g. age restrictions on alcohol, tobacco and cannabis)
- Collection of provincial data (e.g. drug use trends, hospitalization data, overdose data).
Key Stakeholders Footnote 11
People with lived/living experience
- Patient organizations, organizations representing people who use/have used drugs and their family members,
Medical community (doctors, nurses, medical regulatory colleges)
- Responsible for the practice of medicine, including delivery of treatment programs and related medical/operational protocols.
Other key stakeholders
- Drug policy advocacy organizations, public health associations, Indigenous leadership and communities, professional associations, researchers/academics, etc.
Industry
- Compliance with laws and regulations on regulated substances (alcohol, tobacco, vaping products, cannabis, prescription drugs)
- Engagement with some national industry associations (cannabis, alcohol)
Moving Forward: Priority Areas and Challenges
While a wide range of substance use issues are currently being addressed by Health Canada and federal partners, there are a number of key priority issues causing significant harms and costs to individuals and communities:
- The opioid overdose crisis is one of the most serious public health crises and continues to claim thousands of Canadian lives each year
- While illegal drugs have always posed a risk of overdose, the illegal market is now increasingly toxic as a result of the introduction of more potent or lethal and difficult to detect synthetic substances, such as fentanyl. This may be a permanent change to the composition of the illegal drug market, leading to a higher risk overdose death for thousands of Canadians each year.
- Methamphetamine use is rising at alarming rates in some provinces, particularly in the Prairies
- Presents unique health risks to people who use it, public safety challenges to first responders and communities, and is challenging to treat
- Alcohol is the most costly substance to Canadian society, and causes nearly 15,000 deaths a year
- Alcohol use is normalized in Canada, including some forms of problematic use, such as binge drinking
- Our approach to alcohol lags behind actions taken on tobacco and opioids, and also lags behind those of other like-minded countries
- Tobacco use is the leading cause of premature death and disease in Canada
- Vaping holds promise as a less harmful alternative to smoking cigarettes, and may help further reduce the number of Canadians who smoke. However, youth vaping has risen rapidly and poses risks, including nicotine addiction that could lead to future tobacco use
- Cannabis legalization and regulation is still in its early days - need to continue to monitor use rates for cannabis, especially for youth
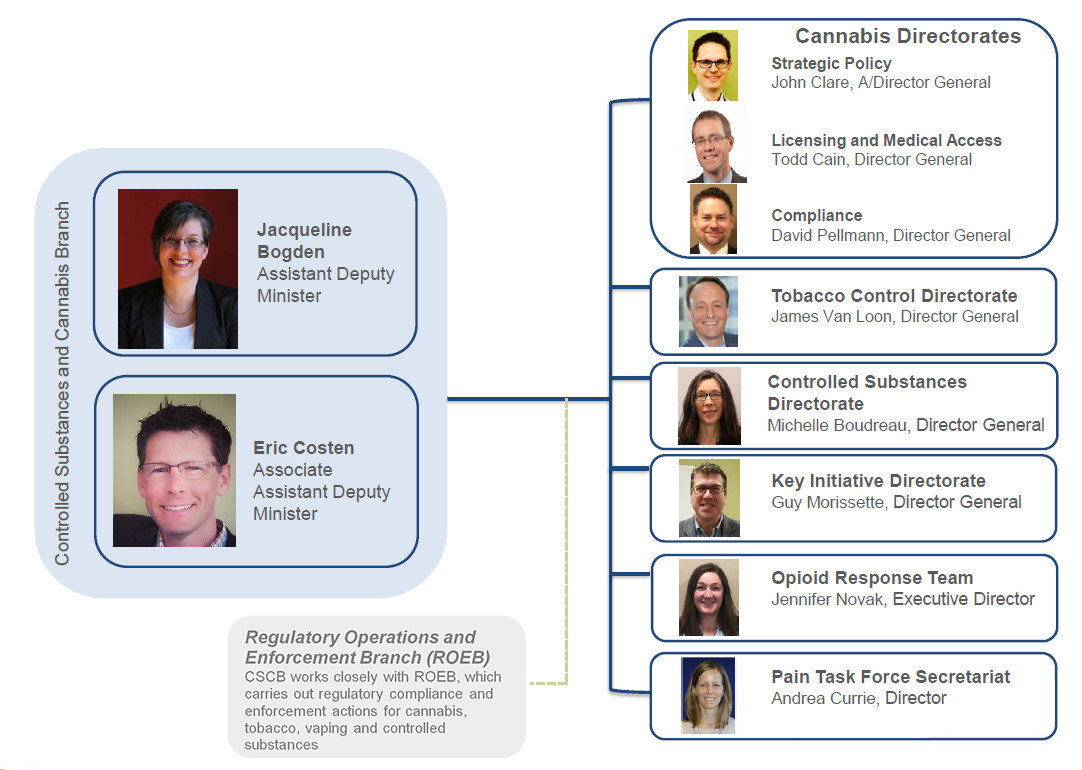
Controlled Substances and Cannabis Branch: Organizational Structure - Text Equivalent
The chart shows the Assistance Deputy Minister, Jacqueline Bogden, and Associated Assistant Deputy Minister, Eric Costen, leading the Branch and the eight direct reports:
- John Clare, A/Director General, Strategic Policy, Cannabis Directorate
- Todd Cain, Director General, Licensing and Medical Access, Cannabis Directorate
- David Pellmann, Director General, Compliance, Cannabis Directorate
- James Van Loon, Director General, Tobacco Control Directorate
- Michelle Boudreau, Director General, Controlled Substances Directorate
- Guy Morissette, Director General, Key Initiatives Directorate
- Jen Novak, Executive Director, Opioid Response Team
- Andrea Currie, Director, Pain Task Force Secretariat
Cannabis Directorates
Mandate: To support the government's commitment to legalize and strictly regulate cannabis
- Core business lines include:
- Setting cannabis policy direction and regulations, supported by a coordinated approach to research and data collection, in collaboration with stakeholders
- Granting licences and other authorizations (cultivation, processing, research, import/export permits and personal production registration certificates)
- Promoting, monitoring and verifying compliance with the regulations
- Cannabis directorates provide support to:
- the Minister of Border Security and Organized Crime Reduction as the Minister responsible for the administration of the Cannabis Act, and
- the Minister of Health for public education relating to the health effects of cannabis and research and surveillance (the review and approval of prescription drugs containing cannabis is supported by the Health Products and Food Branch)
- The Program works with other Branches and federal departments in the design of the legal framework, in implementing the program, and monitoring and enforcing compliance with the regulations
- e.g., ROEB which leads cannabis inspection activities in support of compliance and enforcement
- e.g., Justice and Public Safety in the design and implementation of the cannabis framework
Tobacco Control Directorate
Mandate: To lead and implement Canada's Tobacco Strategy in collaboration with federal partners, provinces and territories, the health community, and other stakeholders
- Core business lines include:
- Setting tobacco and vaping policy direction and regulations, supported by a coordinated approach for research and data collection, in collaboration with stakeholders
- Administering and monitoring compliance with the Tobacco and Vaping Products Act and its regulations (with ROEB)
- Providing public education resources, information and programming on smoking prevention/cessation, health effects, and youth vaping
- The directorate liaises with several other federal departments who play a critical role in the area of tobacco control, including:
- The Public Safety portfolio and the Canada Revenue Agency, which administer programs related to the excise duty and combatting contraband tobacco
- Indigenous Services Canada, which engages with and funds Indigenous organizations in tobacco programming
- The Public Health Agency of Canada, which funds targeted projects for populations with high tobacco use prevalence
Controlled Substances Directorate
Mandate: To regulate controlled substances and promote initiatives that reduce or prevent harm associated with substance use, including alcohol
- Core business lines include:
- Setting drug and substance policy direction and regulations, supported by a coordinated approach to research and data collection, in collaboration with partners and stakeholders through the Canadian Drugs and Substances Strategy
- Providing guidance and leadership on harm reduction activities such as supervised consumption sites (SCS) and urgent public health need sites (UPHNS) also known as overdose prevention sites (OPS) as entry points to treatment and saving lives by preventing and reversing overdose deaths related to substance use
- With ROEB, administering and monitoring compliance of the Controlled Drugs and Substances Act and its regulations (authorization of licences, exemptions, and a compliance program)
- Acting as the competent authority with respect to the United Nations Drug Control Conventions
- The Canadian Drugs and Substances Strategy sets out the overall federal approach to drug and substance use policy, and takes a comprehensive, collaborative, compassionate and evidence-based approach under the pillars of prevention, treatment, harm reduction and enforcement
Key Initiatives Directorate
Mandate: To deliver opioid-related programs and support federal/provincial/territorial (FPT) relations on matters related to drug and substance use issues
- Core business lines include:
- [REDACTED]
- Fostering and maintaining intergovernmental collaboration on opioid issues and initiatives, including the Emergency Treatment Fund, through bilateral and multilateral engagements and via the FPT Committee on Problematic Substance Use and Harms
- Administering and overseeing the Substance Use and Addictions Program (SUAP), which provides contribution funding to other levels of government, community-based and not-for-profit organizations to respond to drug and substance use issues
- The SUAP has an annual budget of approximately $50 million to fund projects that support federal priorities related to cannabis, tobacco, and controlled drugs and substances
Opioid Response Team
Mandate: To act as the central coordinating hub for the federal response to the opioid crisis
- Core business lines include:
- Developing strategic advice and recommendations pertaining to the various factors guiding the federal opioid response
- Synthesizing and integrating research and analysis on the opioid crisis and problematic substance use to promote an accurate understanding of the opioid crisis
- Providing a focal point for engagement with partners and stakeholders related to the opioid crisis in Canada, including other federal departments and levels of government, health professionals and organizations, substance use experts, and people with lived and living experience with drug use
- Addressing attitudes and behaviors that can interfere with recovery and delivery of substance use services
Pain Task Force
Task Force Mandate: Work with members of the chronic pain community, FPT governments, professional associations, and other stakeholders to:
- assess how chronic pain is currently addressed in Canada (March to July 2019);
- identify best practices and elements of an improved approach to prevent and manage chronic pain in Canada (July 2019 to June 2020); and
- disseminate information about best practices to facilitate their uptake across the country (July 2020 to December 2021)
The opioid crisis has drawn attention to longstanding challenges faced by the 1 in 5 Canadians living with chronic pain and stakeholder calls for a national pain strategy to better address pain in Canada.
The Task Force will provide advice and information to increase Canadians' access to the range of pharmacological, psychological and physical therapies needed to effectively manage pain.
| Costs and Impacts by Substance | ||||
|---|---|---|---|---|
| Substance | % Past-year use by Canadians aged 15 and up (CTADS 2017) |
Impact | ||
| Costs (CCSA, 2018) |
Annual Deaths Footnote 1 | Emergency Department Visits | ||
| Alcohol | 78% | $14.6B annually | 14,827 | 649,412 |
| Tobacco (all tobacco products) |
18% | $12.0B annually | 47,562 | 239,512 |
| Cannabis | 15% | $2.8M annually | No known deaths (850 deaths where cannabis was a known factor Footnote 2) | 3,835 |
| Cocaine | 2% | $2.2M annually | No national figures | No national figures |
| Hallucinogens (e.g., LSD, magic mushrooms) | 1% | Unknown | No national figures | No national figures |
| Opioids | >1% Footnote 3 | $3.5B annually | 4,460 (2018) | 31,686 opioid-related visits |
| Amphetamine | >1% | $1.1M annually (includes other stimulants like ecstasy) | No national figures (346 meth-related deaths in BC in 2017) | No national figures |
|
||||
Appendix B: Federal Strategies
Canadian Drugs and Substances Strategy (CDSS)
- A comprehensive, collaborative, compassionate and evidence-based approach to drug policy
- Four pillar approach (prevention, treatment, harm reduction, enforcement)
- Encompasses a wide range of legal and illegal substances and guides the federal approach to opioids, cannabis, and other drugs and substances
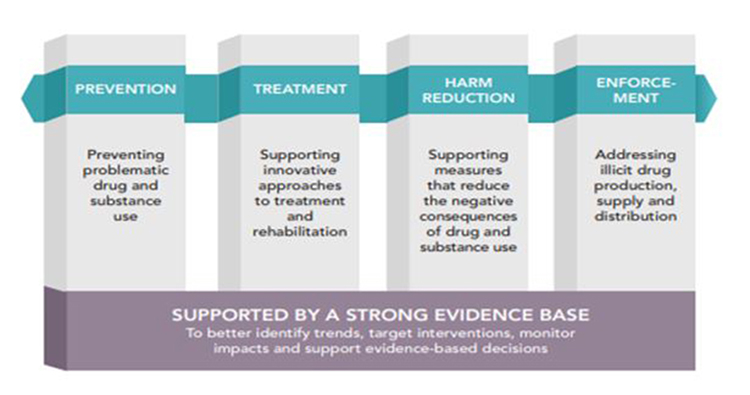
Canadian drugs and substances strategy - Text Equivalent
There are four pillars of the strategy:
Prevention: Preventing problematic drug and substance use
Treatment: Supporting innovative approaches to treatment and rehabilitation
Harm Reduction: Supporting measures that reduce the negative consequences of drug and substance use
Enforcement: Addressing illicit drug production, supply and distribution
Supported by a strong evidence base: To better identify trends, target interventions, monitor impacts and support evidence-based decisions.
Canadian Tobacco Strategy (CTS)
- Outlines the federal approach to reaching the goal of less than 5% tobacco use in Canada by 2035
- Aims to:
- help Canadians quit tobacco
- protect youth and non-tobacco users from nicotine addiction
- work with Indigenous groups to create specific plans for Indigenous people
- strengthen our science, surveillance and partnerships
Appendix C: Key Stakeholder Groups
Drug Policy/Research Organizations
- Canadian Centre on Substance Use and Addiction (CCSA): funded by Health Canada, research based organization with a focus on substance use and addiction, health and public safety, people and communities, and data trends.
- Canadian Institute for Health Information (CIHI): focus on health data and information to accelerate improvements in health care, health system performance and population health across the continuum of care. Includes the Canadian Research Institute on Substance Misuse (CRISM), a 5-year initiative with a focus on clinical and community-based interventions for substance use disorders, evidence regarding prevention and treatment services, and supporting improvement in the quality of care and quality of life for Canadians living with substance use.
- British Columbia Centre on Substance Use (BCCSU): provides provincial leadership in substance use and addiction research, education and clinical care guidance.
- Canadian Institute for Substance Use Research (CISUR): network of individuals and groups dedicated to the study of substance use and addiction in support of community-wide efforts to promote health and reduce harm. Located in Victoria, BC.
- Centre for Addiction and Mental Health (CAMH): Canada's largest mental health teaching hospital and one of the world's leading research centres. Offers both clinical care to patients and engages in research, training, and development of promotion and prevention strategies related to mental health and addictions.
- Canadian Drug Policy Coalition (CDPC): research and advocacy organization that works to advance and realize drug policies grounded in compassion and guided by science, and shift the public narrative on substance use and people who use drugs.
People with Lived/Living Experience with Substance Use
- Canadian Association of People Who Use Drugs (CAPUD): national advocacy organization formed by and representing the interests of people who use drugs.
- Moms Stop the Harm (MSTH): network of mothers and families whose loved ones use drugs or who have died due to substance use. They call for new approaches based on reducing harm, where people who use drugs are treated with respect, compassion and support.
- Community Addictions Peer Support Association (CAPSA): Provides opportunities for people affected by addiction to connect with their local community through collective volunteerism and community participation.
Health Professional and Regulatory Bodies
- College of Family Physicians of Canada (CFPC): leads family medicine to improve the health of all people in Canada by setting standards for education, certification and support of family physicians, championing advocacy and research, and honouring the patient-physician relationship.
- National Association of Pharmacy Regulatory Authorities (NAPRA): Provides leadership to enhance patient care by regulating the practice of pharmacy and operation of pharmacies in their respective jurisdictions in Canada.
- Federation of Medical Regulatory Authorities of Canada (FMRAC): FMRAC's mission is to advance medical regulation on behalf of the public through collaboration, common standards and best practices.
- A wide range of professional medical associations (e.g. Canadian Medical Association, Canadian Nurses Association, Canadian Pharmacists Association, etc.)
Other Key Stakeholder Groups:
Indigenous organizations - Assembly of First Nations, Inuit Tapiriit Kanatami, Metis National Council, Native Women's Association of Canada. Thunderbird Partnership Foundation
Pain groups - organizations representing the interests of pain patients (e.g. Canadian Pain Society, Chronic Pain Association of Canada)
Cannabis - Cannabis Council of Canada, Canadian Hemp Trade Alliance, Canadians for Fair Access to Medical Marijuana
Alcohol - Mother's Against Drunk Driving, Post-Secondary Education Partnership on Alcohol Harms
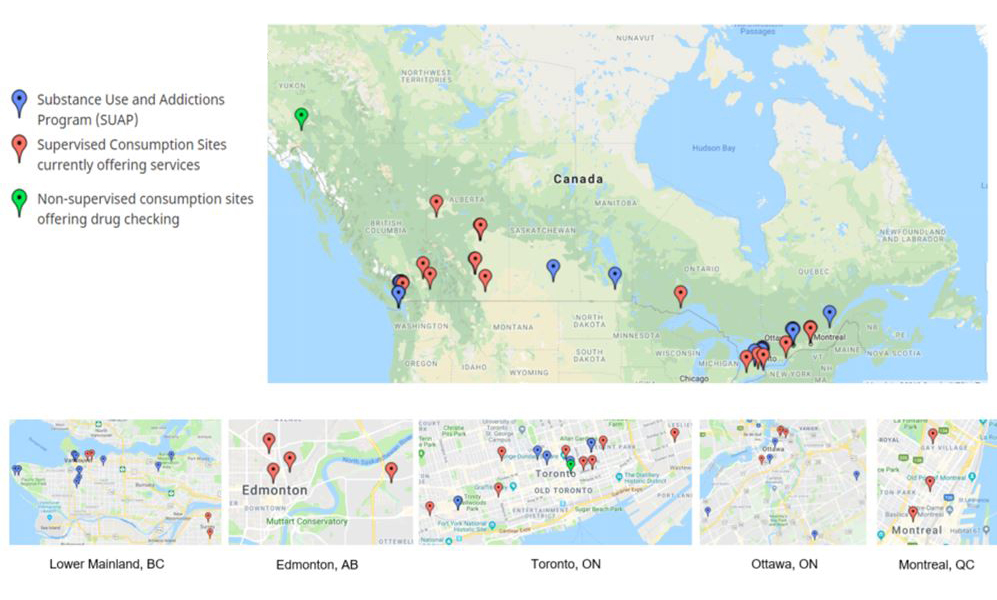
Use and Addictions Program Funding Recipients - Text Equivalent
- Map of Canada shows 13 supervised consumption sites, 8 Substance Use and Addictions Programs and 1 non-supervised consumption site/drug checking locations across Canada
- Map of Lower Mainland, British Columbia shows 4 supervised consumption sites and 10 Substance Use and Addictions Programs locations across the region
- Map of Edmonton, Alberta shows 4 supervised consumption site locations across the city
- Map of Toronto, Ontario shows 8 supervised consumption sites, 5 Substance Use and Addictions Programs and 1 non-supervised consumption site/drug checking locations across the city
- Map of Ottawa, Ontario shows 3 supervised consumption sites and 5 Substance Use and Addictions Programs locations across the city
- Map of Montreal, Quebec shows 3 supervised consumption sites locations across the city
Appendix E: Location of Cannabis Licence Holders
As of August 2019, there are 211 cannabis license holders (cultivators, processors and sellers) in Canada
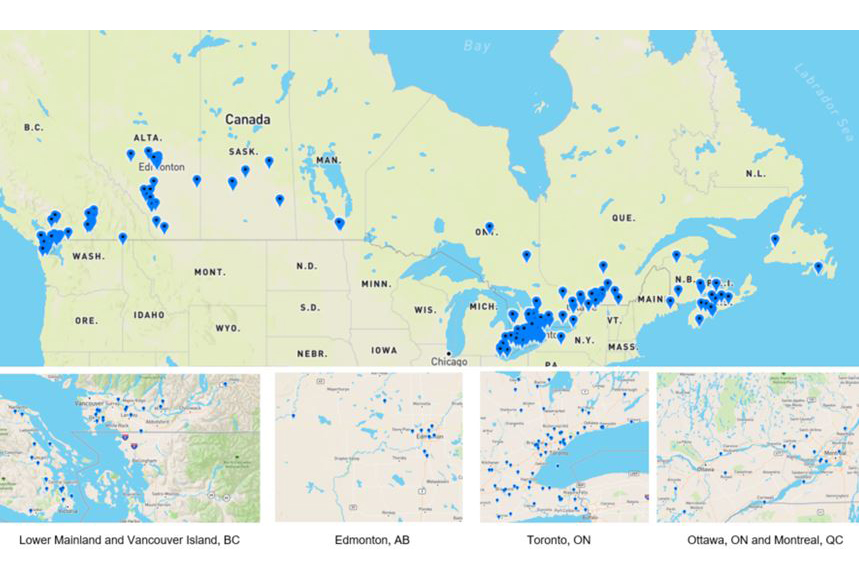
As of August 2019, there are 211 cannabis license holders (cultivators, processors and sellers) in Canada - Text Equivalent
- Map of Canada shows all 211 cannabis license holders (cultivators, processors and sellers) locations across Canada
- Map of Lower Mainland and Vancouver shows approximately 30 cannabis license holders locations across these areas
- Map of Edmonton, Alberta shows approximately 10 cannabis license holders locations across the city
- Map of Toronto, Alberta shows approximately 50 cannabis license holders locations across the city
- Map of Ottawa, Ontario and Montreal, Quebec shows approximately 15 cannabis license holders locations across these areas
Healthy Environments and Consumer Safety Branch
Background Deck
September 2019
Mission of the Healthy Environments and Consumer Safety Branch (HECSB)
- HECSB helps to maintain and improve the health of all Canadians by:
- promoting healthy living, working and recreational environments
- minimizing the harm caused by environmental factors, workplace hazardous materials, and unsafe consumer products and cosmetics
- HECSB works with Canadians, and other partners, to help prevent problems before they occur, target the highest risk areas to maximize our impact, and respond quickly when necessary to protect Canadians
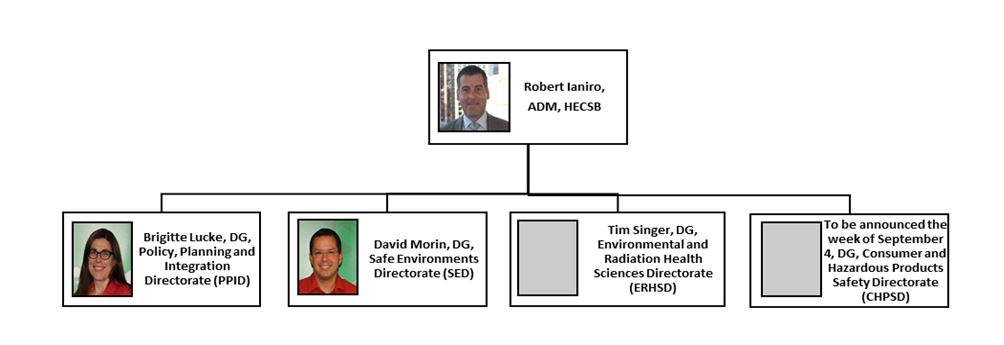
HECSB Organizational Structure - Text Equivalent
The organizational structure for HECSB begins with the Assistant Deputy Minister, Robert Ianiro, and under his leadership are 4 Directors General that oversee different directorates. These directorates and their associated Directors General include: Brigitte Lucke, the Director General for the Policy, Planning and Integration Directorate; David Morin, the Director General for the Safe Environments Directorate; Tim Singer, the Director General for the Environmental and Radiation Health Sciences Directorate; and an additional Director General for the Consumer and Hazardous Products Safety directorate who was to be announced the week of September 4, 2019.
HECSB Core Business Lines
Safe Environments
- Assesses and manages potential risks to human health posed by new and existing chemicals under the Chemicals Management Plan (CMP), with Environment and Climate Change Canada (ECCC)
- Works with P/Ts to develop guidelines for Canadian drinking and recreational water quality, and national standards and guidelines for indoor and outdoor air quality
- Provides expert advice for Impact Assessments of major resource projects (e.g., pipelines, mines) and federal contaminated sites on the health effects related to air quality, drinking water, noise and radiation, and traditional foods
- Undertakes activities under the Canadian Environmental Protection Act, 1999, the Canadian Environmental Assessment Act and the Impact Assessment Act
- Helps Canadians and health authorities understand the impacts of climate change on health, and inform adaptation strategies to minimize the health effects
Environmental and Radiation Health Sciences
- Conducts research, monitoring and surveillance on the health impacts of environmental factors, chemicals, and radiation
- Leads the Federal Nuclear Emergency Plan, Canada's plan for coordinating the federal government response to a radiological or nuclear emergency, and contributes to fulfilling the requirement of the Comprehensive Nuclear Test-Ban Treaty Implementation Act through the operation of a laboratory and radiation monitoring stations
- Maintains 24/7 readiness and capacity to respond to nuclear and chemical emergencies
- Manages the National Dosimetry Service to provide radiation exposure measurement services for workers
- Administers the Radiation Emitting Devices Act and Regulations, which support regulation of devices that emit electromagnetic or acoustical energy including industrial/commercial/security products (e.g. airport security screening equipment, smart meters); consumer products (e.g. cellphones, laser pointers); and medical devices (e.g. medical x-ray and ultrasounds)
- Manages horizontal science and science policy issues for the Branch including open science, research governance, and science promotion
Consumer and Hazardous Products Safety
- Protect Canadians from hazards associated with consumer products, including toys, appliances, mobile phones, and cosmetics, using a post-market, risk-based system
- Relies on authorities in the Canada Consumer Product Safety Act (CCPSA), the Food and Drugs Act (FDA), and various specific regulations (e.g. Cosmetics, Toys, Consumer Chemicals, Lead)
- Ensures workers are warned about the hazards, appropriate precautions, and first aid treatment associated with dangerous workplace chemicals using authorities under the Hazardous Products Act and the Hazardous Materials Information Review Act (HMIRA)
Policy, Planning and Integration
- Coordinates planning, accountability, horizontal policy and business functions for HECSB
- Provides leadership to fulfill Health Canada's responsibilities under the Federal Sustainable Development Act (e.g. input to the Federal Sustainable Development Strategy, development of a departmental strategy, contributions to 'green' departmental operations), and supports the Department's compliance with Strategic Environmental Assessment requirements
HECSB Science
Science is foundational to HECSB's policy, programmatic, legislative and regulatory work
- Monitoring and Surveillance:
- Biomonitoring through longitudinal Maternal-Infant Research and Environmental Chemicals Study and Canadian Health Measures Survey, as examples, are used to risk assessments and risk management actions
- Gathering and distributing poison centre data through the Pan-Canadian Poison Surveillance System to provide near real-time safety signals, high quality surveillance data and ready access to medical toxicology expertise
- Research:
- Investigating emerging issues of concern related to chemicals, plastics, vaping, air, water and radiation (with partners - domestic and/or international)
- Informing Risk Communication:
- Using our science to communicate risk, such as helping people reduce their exposure to air pollutants through the Air Quality Health Index, raising awareness of risks from chemicals and other pollutants around the home through the Healthy Home Campaign, and providing information on radon risks through National Radon Action Month
- Laboratories:
- HECSB laboratories support compliance and enforcement work, test and methodology development and research on products, cosmetics and radiation
- HECSB laboratories are part of the TerraCanada cluster of the Laboratories Canada initiative
HECSB Priorities
Advance current CMP work to reduce the risks posed by chemicals, including:
- Assessing and managing the risks of chemical substances, toward the goal of addressing 4,300 priority substances by 2020, and addressing new substances entering Canada
- Engaging internationally on global chemical issues and providing leadership for the development of a global framework on chemicals and waste beyond 2020, the future of the Strategic Approach to International Chemicals Management. [REDACTED].
Protecting Canadians from the health impacts of climate change through:
- [REDACTED].
- Coordinating federal efforts on health under the Pan-Canadian Framework on Clean Growth and Climate Change to ensure health adaptation is a core component of federal action on climate change.
- Building the capacity of 10 pilot health authorities (PT and local) across Canada to understand climate change risks to health and the health system and develop adaptations through the HealthADAPT Gs & Cs program ($3M)
[REDACTED]
[REDACTED]
Continuing Regulatory Review initiatives including modernizing the Hazardous Materials Information Review Act (HMIRA) and optimizing choice of instruments for consumer products.
Key Files
- [REDACTED]
- Memorandum of Understanding between Health Canada and the Impact Assessment Agency of Canada to support the Impact Assessment Act
- [REDACTED]
- [REDACTED]
- [REDACTED]
Regulatory Operations and Enforcement Branch (ROEB)
Background Deck
September 2019
Branch Mandate
- The Regulatory Operations and Enforcement Branch (ROEB) is Health Canada's dedicated compliance and enforcement (C&E) branch.
- ROEB was established to strengthen oversight of the Department's acts and regulations, modernize C&E activities to be more risk-based, strengthen inspection capacity and enhance the ability to respond to emerging issues.
Our vision
To be a world class compliance and enforcement organization
Our mission
To be a compliance and enforcement leader that informs and protects Canadians from health risks associated with products, substances, and their environment
Who We Are
- ROEB was created in April 2016 as a dedicated compliance and enforcement (C&E) branch, bringing together all of Health Canada's C&E functions under one accountable ADM.
- ROEB delivers its core business through a national program delivery model.
- ROEB is one of the largest federal C&E organizations, with more than 1,300 employees in 26 locations across Canada.
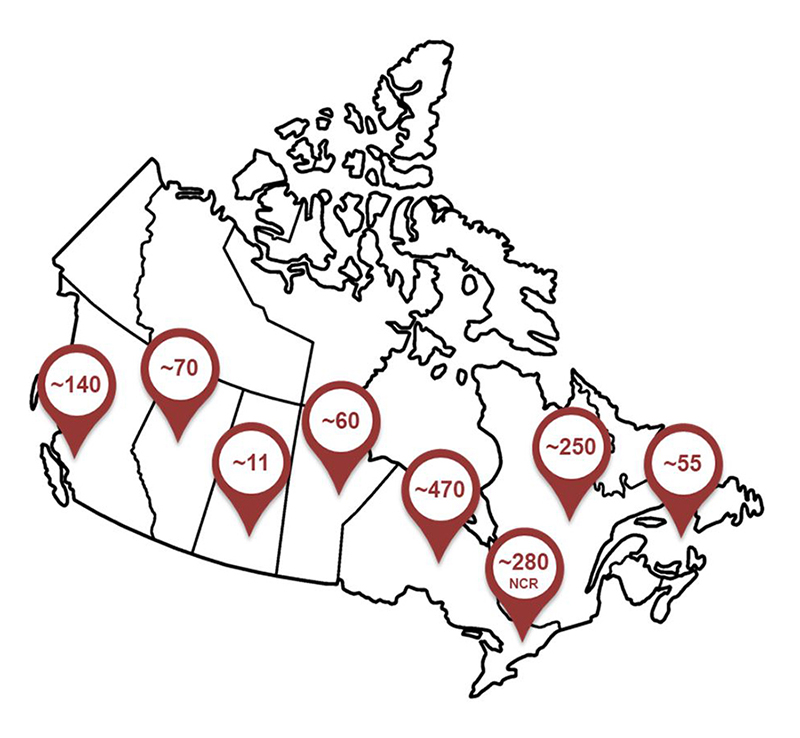
Roeb employees across canada - Text Equivalent
Map showing ROEB employees across Canada. As one of the largest federal compliance and enforcement organizations, ROEB has employees in 26 locations across Canada. The approximate number of employees in the various locations are: 140 in British Columbia, 70 in Alberta, 11 in Saskatchewan, 60 in Manitoba, 470 in Ontario and an additional 280 in the National Capital Region, 250 in Quebec, and 55 in the Atlantic Region.
What We Do
- ROEB carries out the following C&E actions:
- Compliance promotion - educating and promoting compliance with the law;
- Compliance monitoring - monitoring and verifying that products, processes and activities comply with the law; and
- Enforcement - bringing regulated parties into compliance with the law and preventing future non-compliance.
- ROEB provides strategic policy advice and support for the department's regulatory agenda, as well as legislative and regulatory changes.
- ROEB delivers complementary scientific programs, such as laboratory services and environmental health expert advice, and delivers the Internationally Protected Persons Program for international dignitaries and international summits.
- ROEB works closely with partner branches across Health Canada to deliver its mandate (see Annex A for roles and responsibilities)
Core Business
- Compliance and Enforcement
- Cannabis and controlled substances
- Consumer product safety and cosmetics, pest control products, and tobacco/ vaping
- Health products (prescription drugs, non-prescription drugs, natural health products, veterinary drugs)
- Medical devices and biological products (blood, donor sperm and ova, cells/tissues/organs), clinical trials and border operations
- Laboratories and Scientific Analysis
- Illegal drugs and substances, cannabis, health products, food, pesticides and microbiology analysis
- Air quality, chemical assessments and human health risk assessments for contaminated sites
- Health Promotion, Protection and Outreach
- Environmental health
- Internationally protected persons
- Domestic and international collaboration
- Policy and Operations
- Border integrity and surveillance
- Establishment licensing
- Regulatory policy development as well as internal operations and services
Operating Context
- ROEB operates in a complex and rapidly evolving environment. The following drivers inform the way ROEB conducts its business.
- Globalization:
- The evolving global supply chain requires increased harmonization and collaboration
- Innovation:
- The rapid pace of innovation requires the development of new regulatory frameworks and state-of-the-art laboratory equipment
- Technological Advancement:
- Innovative products require establishing new compliance and enforcement measures
- Credibility as a Regulator:
- The protection of Canadians requires providing risk-based oversight and information on health and safety risks
Strategic Direction
- Comprehensive Review (2017)
- The department conducted a comprehensive review of its programs in 2017. Compliance and enforcement was identified as an area for improvement.
As a result, ROEB is implementing a plan to stabilize, modernize and transform its compliance and enforcement function. This includes increasing inspections, strengthening oversight and modernizing tools and training.
- ROEB 2019-2022 Strategic Plan
- The branch released its Strategic Plan in April 2019. The plan outlines three strategic priorities for the branch:
- Modern Program Delivery - strengthen partnerships, invest in technology and ensure national consistency.
- Compliance and Enforcement Transformation - leverage data, target resources and increase foreign and domestic C&E capacity, and modernize statutory frameworks.
- People and Workplace Excellence - create an innovative workplace, develop talent, foster employee engagement, and support a healthy and productive workplace.
- The plan will be monitored through the branch operational planning exercise and the development of a performance framework.
- The branch released its Strategic Plan in April 2019. The plan outlines three strategic priorities for the branch:
Key Priorities
Core Business Activities
Inspections: Conduct over 14,000 inspections annually across product lines.
Management of Non-Compliance: Proactive monitoring activities and collecting risk intelligence.
Provide Information: Recalls, product seizures, stop sales, adverse events, shortages, etc.
Scientific and Technical Services: Analysis of Illicit drugs, pesticides, food products and health products.
Environmental Health: Identify and assess health risks posed by environmental factors.
Compliance and Enforcement Modernization
Transformation of Compliance and Enforcement Activities:
- Increase domestic and foreign surveillance.
- Establish a national training program for inspectors.
- Develop an investigation capacity.
- Leverage data to be more proactive and risk-based.
- Implement the revised C&E Policy 001.
- Implement the C&E transformation framework.
Regulatory Frameworks and Modernization
The Regulatory Agenda: Facilitate the management of the department's regulatory agenda.
Regulatory Roadmaps: Coordinate the implementation of the Regulatory Roadmaps developed through TBS-led Regulatory Review.
Compliance and Enforcement Approaches: Modernize legislative, regulatory and policy frameworks to keep pace with the changing operating context.
Key Issues - First 30 Days
Bulk Importation
- Stakeholder and media attention has increased because of recent developments in the United States regarding the bulk importation of drugs and the potential impact on Canadian drug prices and supply.
New Cannabis Classes
- New compliance and enforcement measures are required for the legislative and regulatory changes to legalize cannabis edibles, extracts, beverages and topicals (e.g., skin creams).
Drug Shortages
- There is significant advocacy from stakeholders as drug shortages frequently occur and can be disruptive to Canadians.
Cabinet Directive on Regulation Implementation
- Cabinet Directive on Regulation regulatory transparency administrative burden baseline reporting requirement needs to be posted by September 30, 2019. The Health Canada Regulatory Stock Review will also be posted in September.
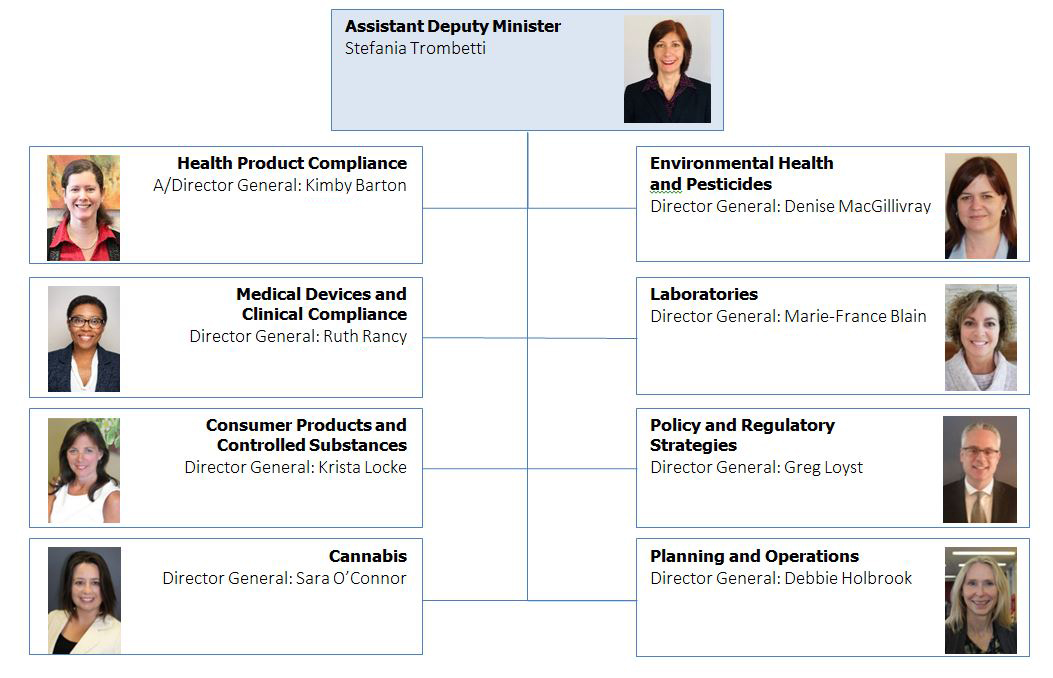
Organizational Structure - Text Equivalent
The organizational structure for ROEB begins with the Assistant Deputy Minister, Stefania Trombetti, and under her leadership are 8 Directors General that oversee different directorates. These directorates and their associated Directors General include: Kimby Barton the Acting Director General for Health Product Compliance; Denise MacGillivray the Director General for Environmental Health and Pesticides; Ruth Rancy the Director General for Medical Devices and Clinical Compliance; Marie-France Blain the Director General for Laboratories; Krista Lock the Director General for Consumer Products and Controlled Substances; Greg Loyst the Director General for Policy and Regulatory Strategies; Sara O'Connor the Director General for Cannabis; and Debbie Holbrook the Director General for Planning and Operations.
ROEB - Financial Overview
Annual Reference Level Update (in millions)
- Salaries and Wages represent 85% of ROEB's overall budget
- Operating represents 13% of ROEB's budget
- 23% of the budget is related to the collection of revenues, which adds to the Salary and Operating budget
- ROEB has a small capital budget of $3M
Financial Snapshot ($ millions)
Salary - $ 113.4
O&M - $ 16.7
Capital - $ 3.0
TOTAL - $ 133.1
# of FTEs - 1,285
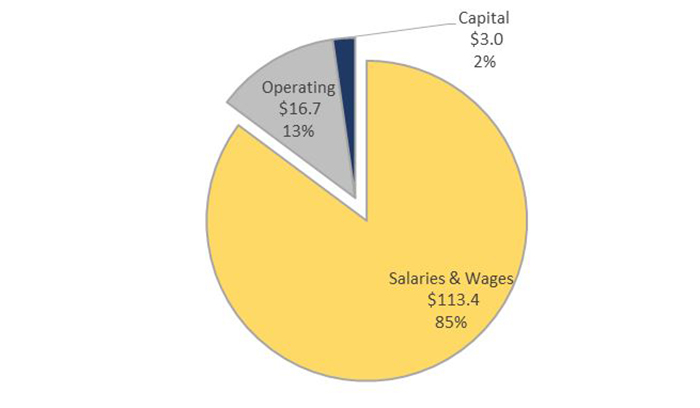
ROEB Budget - Text Equivalent
ROEB Budget pie chart. ROEB's budget is comprised of Salaries and Wages which represents 85% of the Branch's budget, Operating which represents 13%, and Capital which represents 2% of the overall budget.
Pest Management Regulatory Agency (PMRA) Background Deck
September 2019
PMRA Mandate
As the federal regulator of pesticides:
- PMRA's primary objective is to prevent unacceptable risks to Canadians and the environment from the use of pesticides. This is accomplished through pre- and post-market regulatory activities and the establishment of food safety standards (i.e., pesticide maximum residue limits).
- PMRA's legislative mandate stems from its administration of the Pest Control Products Act (PCPA) and its regulations.
- PMRA's mission is to protect the health and environment of Canadians by using modern evidence-based scientific approaches to pesticide regulation, in an open and transparent manner.
About Pesticides:
- Pesticides are, for the most part, inherently hazardous chemicals designed to eliminate a specific pest and released purposefully into the environment.
- Regulated pesticides include: agricultural chemicals such as herbicides, insecticides and fungicides; industrial chemicals such as wood preservatives and anti-fouling paints for boats; and, consumer products such as personal insect repellents, sanitizers and swimming pool disinfectants.
- Agriculture accounts for 60% of pesticide use by volume.
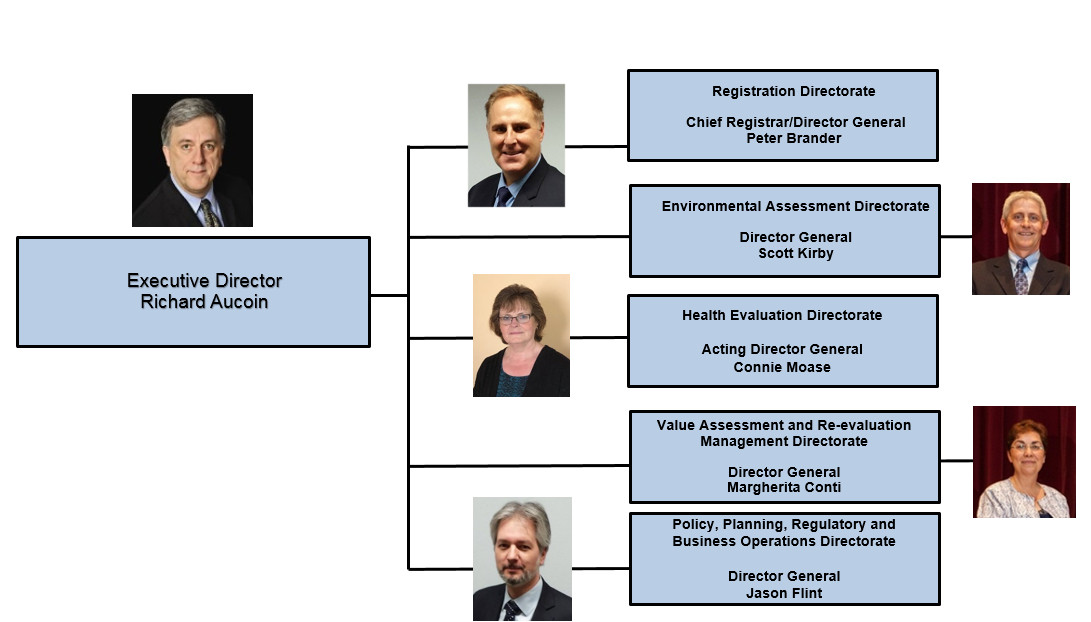
PMRA Organizational Structure - Text Equivalent
The organizational chart lists the senior management of the Pest Management Regulatory Agency
- The Executive Director is Richard Aucoin.
- There are 5 senior managers reporting to the Executive Director.
- Peter Brander, Chief Registrar/Director General for the Registration Directorate.
- Scott Kirby, Director General of the Environmental Assessment Directorate.
- Connie Moase, Acting Director General of the Health Evaluation Directorate.
- Margherita Conti, Director General of the Value Assessment and Re-evaluation Management Directorate.
- Jason Flint, Director General of the Policy, Planning, Regulatory and Business Operations Directorate.
Core Business Lines
New Product Evaluation (Pre-Market)
Prior to registering a product for use, PMRA conducts a scientific assessment to ensure that any risks to the health of Canadians and the environment are acceptable and that the product has value (e.g., will meet a need to control a pest problem).
PMRA regulates pesticide products under the PCPA. Pesticides must be registered or otherwise authorized before they can be sold or used in Canada.
Registered Product Evaluation (Post-market)
To ensure that pesticides meet modern standards for protection of health and the environment, all pesticides must be re-evaluated on a 15 year cycle.
Special reviews must be initiated if there are reasonable grounds to believe that the health and environmental risks posed by the product may no longer be acceptable. Monitoring activities such as incident reporting and collection of sales data are included under this business line.
Science and Business Innovation
- PMRA develops innovative science policy and IM/IT solutions to: address emerging technologies; keep pace with scientific developments; improve information sharing with federal and international regulators; improve the timeliness, predictability and transparency of regulatory processes; and, support the global alignment of regulatory science policies and approaches.
PMRA Key files/priorities/issues
1. Review of the Pesticide Post-Market Review Program:
- PMRA's workload for the re-evaluation of pesticides is compromising the integrity of the program
- The PCPA requires re-evaluation of every pesticide on a 15-year cycle.
- The Commissioner of the Environment and Sustainable Development, and Environmental Non-Governmental Organizations (ENGOs), have been critical of PMRA's ability to make and implement timely regulatory decisions to protect Canadians health and the environment.
- While workload has doubled since the new PCPA came into force in 2006, funding for the program has not kept pace resulting in a growing backlog.
- Stakeholders want increased engagement and time to provide data and information
- Pesticide regulatory decisions can have serious economic implications for agriculture and other use sectors.
- Industry stakeholders expect: a high level of engagement prior to publication of proposed and final decisions; the opportunity to generate new data to avoid negative decisions; and, when required, long phase-out periods for economically important chemicals.
- All stakeholders expect science-based decisions that reflect key information/data such as real-world use and environmental monitoring, although such information is no longer collected by Federal departments with relevant mandates.
- Through extensive consultations PMRA is exploring ways to transform the pesticide post-market review program including:
- Examining new approaches to re-evaluation (including international comparisons);
- Legislative and regulatory modernization through Parliamentary review of the PCPA, as early as Fall 2020;
- Changes to appropriations and the cost recovery regime for pesticides; and
- Enhanced linkages with Other Government Departments (OGDs).
- PMRA will propose a path forward early in the new mandate.
2. Neonicotinoids (Neonics):
- Neonics are insecticides used extensively in agriculture.
- Neonics have received worldwide attention due to their potential risks to bees.
- There are three neonic pesticides approved in Canada currently being re-evaluated.
- Imidacloprid, thiamethoxam and clothianidin.
- In April of 2019, Health Canada published the final pollinator re-evaluations for the three neonic pesticides. To protect pollinators some uses were cancelled with restrictions applied to other uses.
- Neonics were also being measured at levels in some aquatic environments that would pose risks to certain insect populations.
- In 2016, PMRA proposed a 3 to 5 year phase-out of all agricultural uses of imidacloprid to protect aquatic insects. The Special Review of clothianidin and thiamethoxam in August of 2018 also proposed to cancel all outdoor uses due to this same risk.
- Final decisions related to aquatic organisms are expected in January 2020.
3. Glyphosate:
- Public interest in glyphosate remains high due to political level (European) decisions, high profile court cases in the United States and recent initiation of class action cases in Canada linking glyphosate exposure and presence of Non-Hodgkins lymphoma.
- In April 2017, PMRA published the final re-evaluation decision for glyphosate, concluding that it does not pose unacceptable risks to human health or the environment.
- Health Canada's finding with respect to carcinogenicity is consistent with other countries, as no pesticide regulatory authority in the world considers glyphosate to be a cancer risk to humans.
- Health Canada continues to monitor for new information related to glyphosate, including regulatory actions from other governments, and will take appropriate action if risks of concern to human health or the environment are identified
4. International Regulatory Cooperation (IRC)
- PMRA works with international counterparts (e.g., US EPA) to align science and policy approaches, which has allowed us to participate in international joint reviews of pesticides.
- Other key IRC activities include:
- Chairing or participating in a number OECD steering & expert groups
- Leadership at CODEX, a multilateral body that develops international food standards (e.g. Maximum Residue Limits - MRLs).
- Participate in the negotiation and implementation of multi-lateral environmental and trade agreements (e.g., Stockholm, Rotterdam Conventions, CUSMA, CPTPP, CETA).
- Continuing working relationship within the CUSMA framework in relation to Pesticides (continuing from the NAFTA Working Group on Pesticides).
- Regulatory Cooperation Council plan for crop protection products.
- Support for federal partners by providing scientific regulatory support on trade-related files
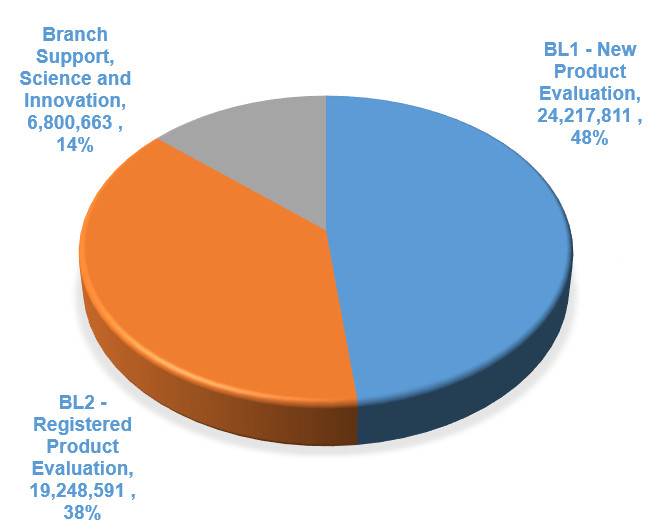
PMRA Financial Overview - Text Equivalent
This pie chart describes the financial overview of the Pest Management Regulatory Agency in three segments
- Branch Support - Science and Innovation, 6,800,663, 14%
- Business Line 1 - New Product Evaluation, 24,217,811, 48%
- Business Line 2 - Registered Product Evaluation, 19,248,591, 38%]
Total budget and total full time equivalents
- Total Budget (Main Estimates 2019-2020)
- $37,390,000
- Total Full Time Equivalents
- 396
| Financement | Business Line 1 - New Product Evaluation | Business Line 2 - Registered Product Evaluation | Branch Support - Science and Innovation, | Total |
|---|---|---|---|---|
| A- Base | $15.7 | $1.7 | $6.8 | $24.3 |
| B-Base - Chemicals Management Plan - expires March 2021 | N/A | $4.9 | N/A | $4.9 |
| B-Base - Canadian Agricultural Partnership - expires March 2023 | $3.3 | N/A | N/A | $3.3 |
| Departmental Pressure Funding S&W(incl. EBP): 89%, O&M: 11%- expires March 2020 | N/A | $4.8 | N/A | $4.8 |
| Respendable Revenue | $5.1 | $7.7 | N/A | $12.9 |
| Total | $24.2 | $19.2 | $6.8 | N/A |
| Gross Budget | - | - | - | $50.3 |
Communications and Public Affairs Background Deck
September 2019
CPAB Mission
- The Communications and Public Affairs Branch (CPAB) is a full-service communications branch that directly supports Health Canada and the Public Health Agency of Canada (PHAC) in their missions, through a Shared Services Partnership
- CPAB provides a broad range of communications services, including strategic communications and issues management, risk communications, marketing and advertising, digital communications, public opinion research and consultations, internal communications and executive correspondence
- CPAB is the primary liaison with Privy Council Office (PCO) Communications and with Communications in the Minister's Office
- Our work contributes to ensuring that Canadians have access to the information they need to take action on their health
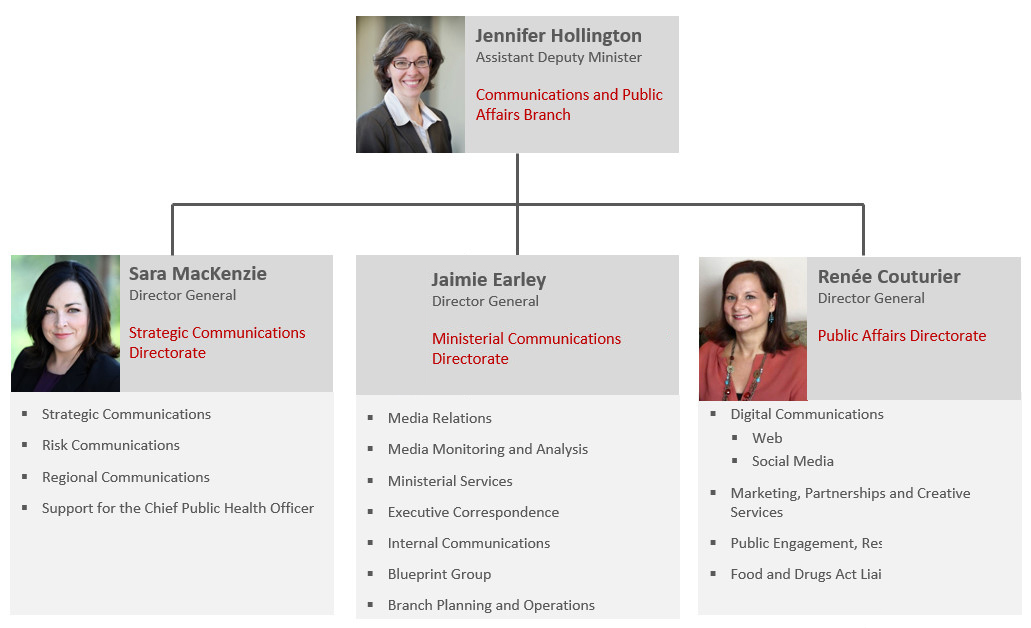
CPAB Organizational Structure - Text Equivalent
The Communications and Public Affairs Branch Organizational chart description
- Assistant Deputy Minister: Jennifer Hollington. Directorates under the Assistant Deputy Minister:
- Strategic Communications Directorate. Sara Mackenzie, Director General. Areas of responsibility: Strategic Communications; Risk Communications; Regional Communications; and Support for the Chief Public Health Officer.
- Ministerial Communications Directorate. Jaimie Earley, Director General. Areas of responsibility: Media Relations; Media Monitoring and Analysis; Ministerial Services; Executive Correspondence; Internal Communications; the Blueprint Group; and Branch Planning Operations.
- Public Affairs Directorate. Renée Couturier, Director General. Areas of responsibility: Digital Communications (web and social media); Marketing, Partnerships and Creative Services; Public Engagement, Research and Analysis; and the Food and Drugs Act Liaison Office.
CPAB Services
Strategic and Ministerial Communications
- Provide strategic communications advice, planning and issues management to support the Minister of Health Footnote 12 and Health Canada, including:
- Ministerial announcements, including speechwriting
- Communications plans and products
- Media relations
- Media monitoring and analysis
- Regional communications
Risk Communications
- Communicate important health and safety information to Canadians, including advisories about health and consumer products
Marketing, Advertising, Partnerships and Creative Services
- Develop and deliver marketing and advertising campaigns and partnerships to advance program objectives
- Provide design, publishing, video, copyright, printing and distribution services to all branches, and manage the Service Canada contract for the Health Canada general enquiries line
Digital Communications
- Manage Health Canada's digital presence on Canada.ca
- Manage Health Canada social media accounts as well as the official Minister of Health Twitter account
Public Opinion Research and Consultations
- Provide full-service public opinion research contracted through external firms or using survey software managed internally
- Support external consultations and the establishment of external advisory bodies
- Manage Health Canada's Consultation and Stakeholder Information Management System (CSIMS), and provide stakeholder intelligence and mapping services
Internal Communications
- Lead internal communications to all Health Canada employees, including providing advice to branches
- Support the Deputy Minister in employee engagement
Blueprint and Beyond 2020
- Support Health Canada's efforts to modernize and transform the workplace through a range of initiatives
- Manage the Health Canada Solutions Fund, which supports employee-led experimentation
- Develop and coordinate implementation of Health Canada's Beyond 2020 plan
Executive Correspondence
- Manage correspondence for the Minister and the Deputy Minister
Food and Drugs Act Liaison Office (FDALO)
- Provide dispute-resolution services through an impartial, confidential office to improve relations between external stakeholders and representatives of Health Canada, as well as to increase openness and transparency in the regulatory process
Financial Profile
- For FY 2019-20, CPAB has an opening reference level of $44.5M (including EBP) Footnote 13
- CPAB's total salary budget covers 306 FTEs, 72 of which are funded through PHAC's contribution to the
Shared Service Partnership (SSP) - Funding from PHAC represents 24% of CPAB's reference-level budget
- CPAB also receives in-year funding from TB submissions, advertising campaigns and Branch Transfer Agreements. The following is anticipated for the 2019-20 fiscal year:
- HC: $2.0M salary / $7.0M O&M
- PHAC: $0.3M salary / $1.2M O&M
| Funding source | Full-time equivalents (FTEs) | Funds |
|---|---|---|
| HC Salary & Operating (Vote 1) | - | $30.6M |
| EBP | - | $2.8M |
| FTEs | 234 | - |
| PHAC Shared Services Partnership | - | $9.4M |
| EBP | - | $1.7M |
| FTEs | 72 | - |
| Total Salary & Operating | - | $44.5M |
(Does not include EBP)
2019-20 Opening Reference Level - Text Equivalent
The graph is a pie chart illustrating the opening reference level for funding sources within Communications and Public Affairs Branch. The chart does not include the Employee Benefit Plan.
- Health Canada salary: $18.4 million
- Public Health Agency of Canada salary: $6.2 million
- Health Canada operations and maintenance budget: $12.2 million
- Public Health Agency of Canada operations and maintenance budget: $3.2 million
Corporate Services Branch (CSB) Overview
Background Deck
September 2019
Mandate
- The Corporate Services Branch (CSB) provides support/services to Health Canada (HC) in
- Human Resources
- Information Management/Information Technology
- Real Property and Security
- Access to Information and Privacy
- Enterprise Architecture and Data
- These services are also provided to the Public Health Agency of Canada pursuant to a Shared Services Partnership Agreement established in 2012.
- Some services also continue to be provided to HC's former First Nations and Inuit Health Branch that transferred to Indigenous Services Canada through a MOU established in 2017.
- CSB also provides Specialized Health services (Occupational Health, Employee Assistance) to various Government of Canada institutions through cost recovery and other arrangements.

CSB Organizational Structure - Text Equivalent
CSB Organizational Structure
Senior management:
- Assistant Deputy Minister, Debbie Beresford-Green, Chief Security Officer among other roles
Directorates:
- Human Resources Services Directorate, Director General, Daryl Gauthier
- Information Management Services Directorate, Acting Chief Information Officer and Director General, Tracey Sampson
- Specialized Health Services Directorate, Director General, Nancy Porteous
- Real Property and Security Directorate, Acting Director General, Greg MacMillan
- Planning, Integration and Management Services Directorate, Director General, Jean-François Luc
- Business Renewal and Enterprise Architecture Directorate, Director General, Scott McKenna
Core Business/Key Files
Human Resources Services Directorate
Scope: Provides human resource services, strategic advice and policy guidance; supports a healthy, respectful, inclusive, safe and productive workplace; engages Public Services and Procurement Canada (PSPC) to provide compensation advice and support; and manages executive recruitment, staffing, organizational design and classification as well as performance/talent management.
Key Files:
Pay
- Implement PSPC's Pod Model to better support employees and adjust tools and processes to meet new HR-to-Pay Timeliness requirements from Treasury Board Secretariat (TBS).
- In-house pay team of 65 employees working on pay issues under MOU with PSPC.
Workplace Wellness Service Centre
- Continue efforts to implement 'one-stop access' for Disability Management, Duty to Accommodate and Occupational Health and Safety Services (best practice as per GoC/Clerk's Annual Report).
- Risk-based Conflict of Interest regime implemented for the cannabis program.
Information Management Services Directorate
Scope: Manages, develops and delivers information management and information technology (IM/IT) strategies, policies, software/solutions and services; engages Shared Services Canada (SSC) to advance departmental IM/IT priorities; supports the implementation of TBS/SSC-driven enterprise initiatives; and protects information and systems from cyber attacks.
IT Key Files:
Enterprise initiatives
- Complete implementation of Wi-Fi, Windows 10 and migration of applications/servers running Windows 2008 and in data centres' scheduled closures; these initiatives have varying timelines, with the earliest coming up in December 2019 (Windows 2008).
IT Planning
- Lead annual planning process (Fall start) and engage SSC on priority IT projects. Includes HC's evolving 'science IT' needs, as well as Aging IT and Mission Critical Applications.
IM Key Files:
- Implementing GCDocs across HC and PHAC (replace current shared drive and RDIMS repositories).
Real Property and Security Directorate
Scope: Manages custodial and office space nationally; partners with PSPC to deliver real property and accommodations services; leads GCworkplace office modernization projects; develops and maintains policies, systems and procedures for managing buildings; keeps personnel, assets and information safe and secure; manages plans to ensure preparedness and business continuity in the event of emergencies; and facilitates security clearances, awareness, contracting and travel briefings.
Key Files:
Security
- Developing a new three-year strategic Integrated Departmental Security Plan (including security and cybersecurity) and update policies and practices, consistent with new TBS policy.
Laboratory Renewal
- Developing a national Laboratory Infrastructure Renewal Strategy for HC custodial laboratories.
Laboratories Canada Initiative
- Lead for HC which is part of two clusters (Terra Canada + Regulatory Science and Security).
Planning, Integration and Management Services Directorate
Access to Information and Privacy
Scope: Manages compliance under the Access to Information Act, Privacy Act and related TBS policies. The volume/scientific nature of ATI requests present unique challenges relative to most other federal institutions (in 2018-2019, HC received 1,942 ATI requests, representing an increase of 7.5% compared to 2017-18). While steps taken in recent years to improve performance (demand management, program accountability and increased investments) enabled HC to close 2,255 ATI requests last FY (20%+ than previous year), a significant backlog of requests remain.
Key Files:
A permanent investment was made at the beginning of 2016-17 to significantly increase time performance on the management of new files.
Backlog reduction: using in-year investments to eliminate the backlog (consultants) and continue to work with programs to explore ways to reduce incoming levels through proactive disclosure (e.g. adverse drug reactions).
Business Renewal and Enterprise Architecture Directorate
Scope: Collaborates with branches to bring a broad, enterprise-wide view to investment decisions; leads the development of a collaborative data strategy for HC; and fosters innovation through a network of virtual and physical workspaces designed for collaboration.
Key Files:
Enterprise Architecture
- Representing HC & PHAC on GC Enterprise Architecture Review Board
Data Strategy
- Leading the development of the HC Data Strategy (a key part of the Government of Canada's broader digital agenda).
- Developing an integrated data strategy/roadmap for HC & collaborate with PHAC in developing its data strategy.
Specialized Health Services Directorate
Scope: Public Service Occupational Health Program (PSOHP) - delegated to HC by Treasury Board in 1984, this program supports Deputy Heads in meeting their obligations under the Canada Labour Code by ensuring that public servants, especially those in high risk and safety-sensitive positions, are physically and psychologically fit to perform their duties, and that they will not pose any danger to their colleagues or the citizens they serve. PSOHP also provides domestic and overseas occupational health advice and leadership in response to emerging issues and hazards e.g. lead, asbestos, legionella, air quality, noise levels, and exposure to toxins such as fentanyl and beryllium.
Employee Assistance Program, which provides 24/7/365 confidential bilingual services to support employees and their families in identifying and resolving personal and work-related problems. Services are provided on a cost-recovery basis to 85+ federal institutions, including RCMP, Canadian Air Force and Veterans. Also includes Specialized Organizational Services, which provides counselling for grief and loss, mental health training, workplace wellness and mental health supports, and Psycho-social emergency response.
Key Files:
PSOHP Sustainability
Continue TBS engagement to ensure implementation of a sustainable funding model that keeps pace with Public Service growth/renewal, rising costs and evolving/complex client needs.
HC-PHAC Shared Services Partnership (SSP)
In June 2012, a Shared Services Partnership (SSP) agreement was signed by Deputy Heads of HC and PHAC to consolidate resources in selected areas; under this agreement, each organization retains responsibility for a different set of internal services and corporate functions, however, each service/function is accountable to both Deputy Heads.
HC responsibility
- Communications and Public Affairs
- Corporate Services (Human Resources, IM/IT, Real Property and Security, ATIP and Enterprise Architecture)
- Financial Operations/Procurement
- Ombudsman, Values and Ethics, Informal Conflict Management
PHAC responsibility
- Audit and Evaluation
- Emergency Preparedness and Response
- International Affairs
The SSP is overseen by the Partnership Executive Committee (PEC), which consists of: you and the President, PHAC as co-chairs, the Associate DM (HC) and the Chief Public Health Officer of Canada (PHAC) as members, and the ADM, CSB as Secretary.
- There have been no formal meetings of the PEC in recent years owing to the evolution/maturing of SSP and reliance on existing HC/PHAC governance mechanisms
The SSP Agreement (Appended) Footnote 14, which was last revised in August 2016, is being updated and also the subject of an internal audit.
| 2019-20 Initial Budget ($M)Footnote 1 | |
|---|---|
| Salary & Operating (Vote 1) | 127.3 |
| Revenue | 30.9 |
| Capital (Vote 5) | 4.3 |
| Sub-Total | 162.5 |
| Shared Services Partnership Funding | 33.0 |
| Total | 195.5 |
| 2019-20 FTEsFootnote 1 | 1,156 |
|
|
Revenue sources:
- Employee Assistance Program (cost recovered)
- MOU with Indigenous Services Canada
- Internal Services revenue received from the Health Products and Food Branch, Regulatory Operations and Enforcement Branch, Specialized Health Services Directorate and the Pest Management Regulatory Agency.
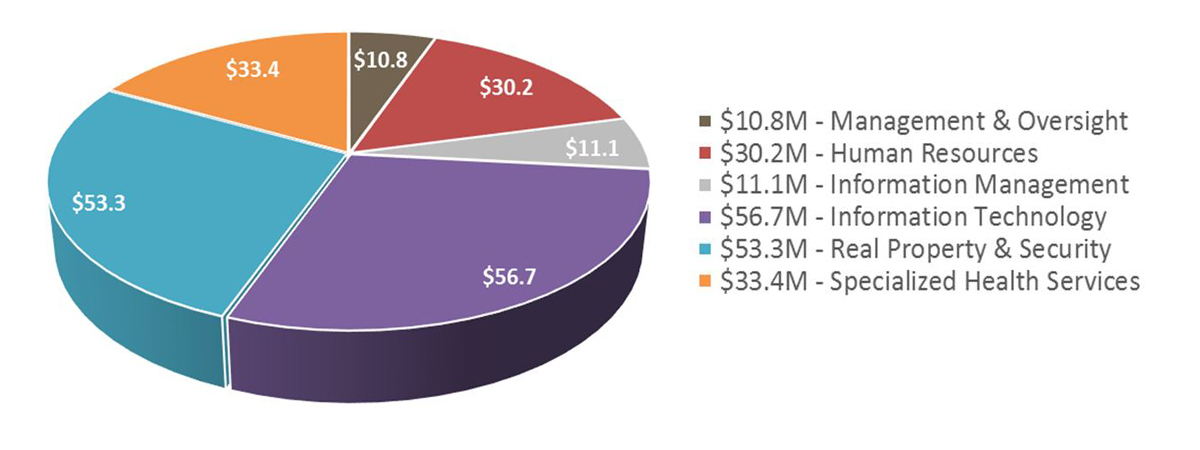
CSB Initial Budget by Function ($195.5M) - Text Equivalent
Pie Chart is a multi-coloured visual representation of the financial distribution of the initial budget ($195.5M) for the Corporate Services Branch Directorates.
The financial distribution among the directorates is broken down as follows:
- $10.8M - Management and Oversight (brown)
- $30.2M - Human Resources (red)
- $11.1M - Information Management (grey)
- $56.7M - Information Technology (purple)
- $53.3M - Real Property and Security (blue)
- $33.4M - Specialized Health Services (orange)
Chief Financial Officer Branch (CFOB)
Background Deck
September 2019
Purpose
- To provide an initial overview of the Chief Financial Officer Branch, including its mandate and key activities
- To flag a number of files that will require your attention in the near-term
Mandate
The Chief Financial Officer Branch (CFOB) is the Departmental focal point for accountability to ensure rigorous stewardship of resources and managing for results.
Key Activities:
- Resource Management: Strategic advice, monitoring and oversight of the departmental financial situation and coordination of TB subs
- Financial Operations: Core financial services including accounting services, financial systems, travel, and financial policies
- Procurement and Asset Management: Providing procurement and asset management services in accordance with government contracting policies
- Planning and Performance Reporting: Departmental results reporting including the DRF and publication of the DP/DRR
Organizational Structure
The Chief Financial Officer (CFO) provides the Minister, Deputy Minister and Departmental executives with strategic advice on all financial matters.
- The CFO is the lead executive with Central Agencies for overall financial management, and has a functional reporting relationship to the Comptroller General of Canada.
- CFOB has a budget of $27.2M and 353 FTEs
Organizational structure - Text Equivalent
Organizational chart showing the Chief Financial Officer and Directors General. The Chief Financial Officer and Assistant Deputy Minister is Randy Larkin and there are three reporting Directors General. Edward de Sousa the Director General of Planning and Resource Management. Todd Mitton the Director General of Financial Operations. Serena Francis the Director General of Cost Recovery and Investment Planning. The Chief Financial Officer is functionally accountable to the Comptroller General to Canada.
CFOB's Shared Services
- HC and the Public Health Agency of Canada (PHAC) share a number of internal services and functions
- CFOB provides financial operations, systems, procurement, asset and materiel management services to PHAC
- However PHAC retains its own CFO
- CFOB also hosts a number of other Departments on its financial system
- Indigenous Services Canada (ISC)
- Crown-Indigenous Relations and Northern Affairs Canada (CIRNA)
- Patented Medicine Price Review Board (PMPRB)
- Canadian Northern Economic Development Agency (CanNor)
These Departments are hosted through a series of MOUs and buy-back agreements
Cfob shared functions and services - Text Equivalent
Visual diagram showing the various functions and services provided by the Chief Financial Officer Branch to its client Departments. The Chief Financial Officer Branch provides accounting services, procurement and asset management and materiel management to the Public Health Agency of Canada. The Chief Financial Officer Branch also provides financial systems (SAP) to Indigenous Services Canada, Crown-Indigenous Relations and Northern Affairs Canada, Patented Medicine Price Review Board, Canadian Northern Economic Development Agency and the Public Health Agency of Canada.
Files requiring attention in the near-term
Financial signing authorities
- Minister and DM sign-off of the financial delegation matrix following the election
- Separate briefing to be scheduled for your DM-level authority
Upcoming approvals / tablings
- A number of public documents will require sign-off and tabling:
- Financial Statements, Departmental Results Report, Fees Report, Supplementary Estimates
Treasury board submissions
- Several submissions targeting early fall/winter approval:
- [REDACTED]
Proactive disclosure
- Requirement to disclose various expenditures on a regular basis:
- Contracts over $10K
- Gs&Cs over $25K
- Travel and Hospitality
A more detailed presentation on the Department's financial situation will be provided upon your arrival
Next Steps
- Following your arrival at HC we will provide you with the necessary internal briefings to authorize your financial delegation
- However Ministerial sign-off is required to activate your DM-level signing authorities
- Presentation on the departmental financial situation
[REDACTED]
Office of Audit and Evaluation
Background Deck
September 2019
Office of Audit and Evaluation (OAE) Core Business Lines
The Office of Audit and Evaluation (OAE) provides independent and objective advice and assurance to Health Canada (HC) and the Public Health Agency of Canada (PHAC) senior management on the effectiveness of risk management, controls, and governance, as well as the relevance and performance of programs.
Internal Audit and Special Examinations (IASE)
- Audits currently scheduled focus on a combination of program activities, primarily regulatory, and corporate services. Audits of internal services which are on the HC-PHAC shared services platform are taken to the Departmental Audit Committees of both HC and PHAC.
- IASE also conducts special examinations related to losses of money, fraudulent activities, or other illegal actions against the Crown. These examinations are conducted on an ad-hoc basis when allegations are made.
Evaluation
- Almost half of the planned evaluations over the next five years touch on Health Canada's regulatory responsibilities, while the rest cover the Department's grants and contributions agreements, and other functional work. We are also heavily engaged in horizontal evaluations, specifically with Environment Canada on its climate change agenda.
Performance Measurement Planning and Integration
- The Performance Measurement team is continuing to advance the culture of performance measurement to ensure useful information is collected for program and senior management decision-making and reporting, to support robust evaluations by providing advice and guidance on performance measurement, and to carry out evaluation readiness assessments.
Practice Management
- The Practice Management team is responsible for quality assurance, annual audit and evaluation planning, governance of audit and evaluation committees, liaising and coordinating with external assurance providers, and following up on action plans to respond to recommendations.
More about OAE
- OAE is part of the Shared Services Platform which has enabled the audit and evaluation functions to integrate and transfer knowledge and lessons learned from both organizations.
- As of today, we have 71 indeterminate employees within OAE not including casuals (3), students (8) and contractors (3). Our target end state is 76 FTEs.
- OAE places importance on professional development and supports team members in the pursuit of credentials, as well as the specialized training needed to maintain them. Official languages capacity has also been enhanced through ensuring a culture that recognizes and supports development in this area.
- A concerted focus on workplace wellness has had a positive impact on the environment within OAE. This was validated in the 2018 PSES results.
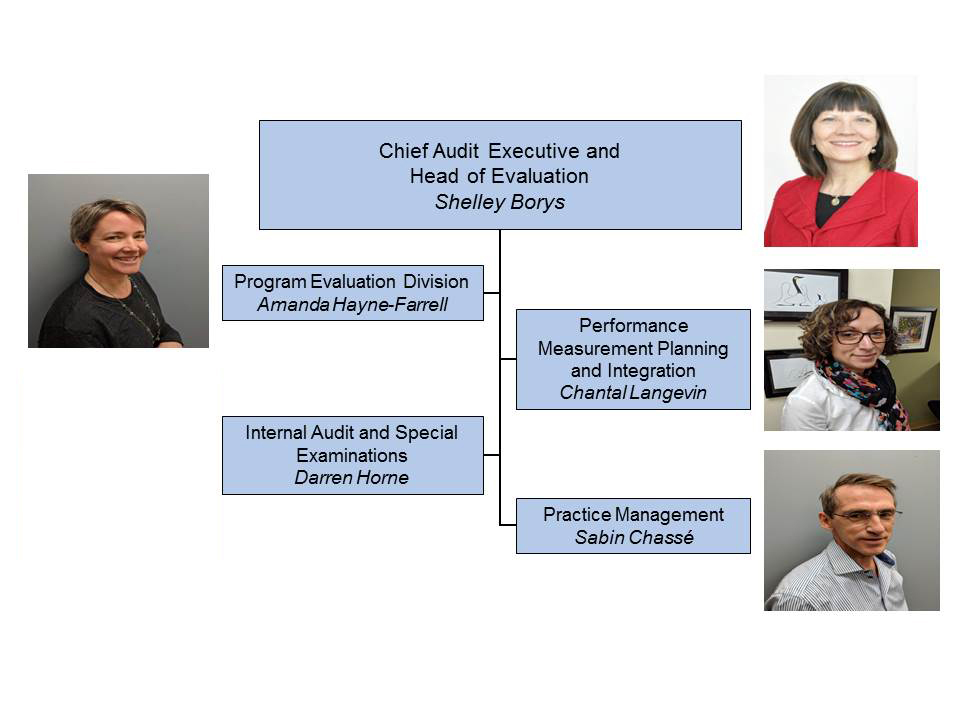
Organizational Structure - Text Equivalent
OAE Organizational Structure. Organization chart showing the Office of Audit and Evaluation by Director under the DG.
- Chief Audit Executive and Head of Evaluation is Shelley Borys
- Program Evaluation Division: Amanda Hayne-Farrell
- Internal Audit and Special Examinations: Darren Horne
- Performance Measurement Planning and Integration: Chantal Langevin and
- Practice Management: Sabin Chassé
| Health Canada Engagements | ||
|---|---|---|
| 2019-20 | Type of Engagement | Branches and Other Government Departments |
| Natural Health Products (September 2019 - November 2020) |
External Audit by the Office of the Auditor General |
|
| Management of Grants and Contributions (Phase II) (April 2018 - October 2019) |
Audit |
|
| Controlled Substances (October 2018 - December 2019) |
Audit |
|
| Inspection Activities (April 2018 - March 2020) |
Audit |
|
| Program Implementation of the Cannabis Act (April 2019 - March 2020) |
Audit Consulting Engagement |
|
| Medical Devices (March 2019 - March 2020) |
Joint Audit and Evaluation |
|
| Chemicals Management Plan (August 2018 - October 2019) |
Evaluation |
|
| Thalidomide Survivors Contribution Program (September 2019 - December 2019) |
Evaluation (FAA Deadline: March 2020) |
|
| Drug Safety and Effectiveness Network (DSEN) (December 2018 - December 2019) |
Evaluation (FAA Deadline: March 2020) |
|
| Workplace Hazardous Products (April 2019 - March 2020) |
Evaluation |
|
| Tobacco Control (January 2019 - October 2019) |
Evaluation Readiness Assessment |
|
| Consumer Product Safety (February 2019 - December 2019) |
Evaluation Readiness Assessment |
|
| Health Canada Engagements | ||
|---|---|---|
| 2019-20 | Type of Engagement | Branches and Other Government Departments |
| IT Systems Development (August 2018 - November 2019) |
Audit |
|
| Procurement and Contracting (November 2018 - December 2019) |
Audit |
|
| Shared Services Partnership Agreement (May 2019 - December 2019) |
Audit |
|
| IT Asset Management (December 2018 - January 2020) |
Audit |
|
| Management of Privacy Practices (January 2019 - February 2020) |
Audit |
|
OAE is also responsible for conducting ten projects that focus strictly on PHAC activities over the current fiscal year (two audits, one joint audit and evaluation, four evaluations and three evaluation readiness assessments).
Departmental Audit Committee
- The Departmental Audit Committee (DAC) is comprised of four external members: Jim Mitchell (Chair); Vicki Harnish; John McWhinnie; and Lorraine Maheu (appointed in May 2019).
- Our DAC members have an excellent rapport with Health Canada's senior management. This positive relationship allows for authentic discussions resulting in sound advice for the department.
- The Deputy Minister is an internal member and the Chief Financial Officer and the Chief Audit Executive are ex-officio members.
- The DAC meets three times a year. A meeting was held on July 9, 2019, and there are meetings scheduled for November 7, 2019 and March 19, 2020.
- An orientation/refresher session is planned for this Fall, which will include a visit to a laboratory. Branches will present an overview of their activities and priorities.
Performance Measurement, Evaluation and Results Committee (focused on Evaluation)
- The Performance Measurement, Evaluation and Results Committee (PMERC) is comprised of Executive Committee (EC) members and meets four times a year on evaluation topics (the Head of Performance Measurement schedules other PMERC meetings that focus on performance measurement).
- Each evaluation is discussed at three stages: scoping; preliminary findings; and final report and management response and action plan (MRAP). The forward agenda for 2019-20 is as follows:
- October 21, 2019:
- Final Report and MRAP for the Evaluation of Health Canada's Chemicals Management Plan
- Preliminary Findings for the Drug Safety Effectiveness Network (DSEN)
- Evaluation Readiness Assessment for Tobacco Control
- December 16, 2019:
- Final Report and MRAP for the Evaluation of DSEN
- Preliminary Findings for the Audit and Evaluation of Medical Devices
- Preliminary Findings for the Evaluation of Workplace Hazardous Products
- Scoping Discussion on the Evaluation of the Pesticides Program
- Consultation on the draft schedule for the Five Year Departmental Evaluation Plan (DEP)
- Evaluation Readiness Assessment for Consumer Product Safety
- March 9, 2020:
- Final Report and MRAP for the Audit and Evaluation of the Medical Devices Program
- Final Report and MRAP for the Evaluation of Workplace Hazardous Products
- Scoping Discussion on the HC component for the Evaluation on Climate Change
- Final consultation on the DEP
- October 21, 2019:
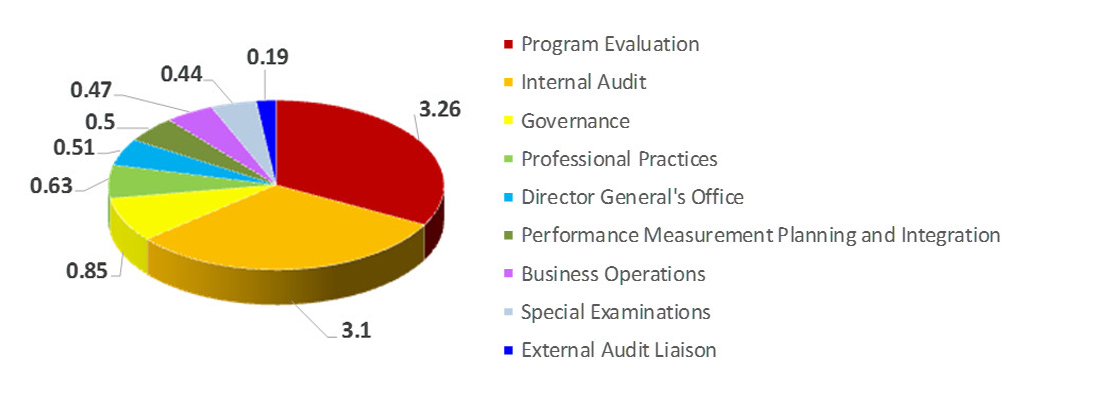
OAE Financial Overview - Text Equivalent
OAE Financial Overview - Budget 2019-20 by Category. Pie chart showing the total budget by Division for 2019-2020.
Program Evaluation = 3.26; Internal Audit = 3.1; Governance = 0.85; Professional Practices = 0.63; Director General's Office = 0.51; Performance Measurement Planning and Integration = 0.5; Business Operations = 0.47; Special Examinations = 0.44; External Audit Liaison = 0.19
Budget and Full Time Equivalents
- Total Budget (Main Estimates 2019-2020)
- $9.94M
- Total Full Time Equivalents
- 76
Key financial considerations
- OAE is jointly funded by Health Canada and PHAC. In 2019-2020, Health Canada's contribution was 55% of the total budget.
Upcoming Priorities
- The OAG audit of Natural Health Products will begin the first week of September, 2019.
- A DAC teleconference will be held on September 12, 2019, to seek recommendation for your approval of the MRAP for the Audit of Procurement and Contracting.
- Consistent with Treasury Board guidance, data collection for two projects is ongoing until mid-October. The previous Deputy Minister was comfortable with this approach and your confirmation will be sought. This includes:
- A survey with 80 Provincial and Territorial inspectors (accredited by Health Canada to conduct inspections under the Hazardous Products Act) for the Evaluation of the Workplace Hazardous Products Program; and
- Interviews (n=25) with academics, community organizations, members of the public and industry for the Audit and Evaluation of the Medical Devices Program.
- A PMERC meeting will occur on October 21, 2019.
- A DAC Orientation session to welcome the new member followed by a regular DAC meeting are scheduled to take place in early November, 2019.
- A review exercise to support future OAG audits was initiated by the former DM, to be completed by late Fall, 2019, concentrating on assessing the realistic or aspirational nature of performance measurement targets. The review will also ensure that the rationale for aspirational targets is clearly articulated.
Key International Priorities and Engagement Opportunities
Office of International Affairs
September 2019
Purpose
- To provide the global health landscape overview, as well as why and how we engage internationally on health.
- To highlight key global health issues and international engagement opportunities over the next 12 months.
- To consider how these opportunities could be best leveraged to advance Canadian interests.
Context: The Global Health Landscape
- Health issues are increasingly complex (e.g. multiple sectors and actors; proliferation of new fora)
- Geopolitical tensions and instability threaten recent gains; impact on the global community's ability to respond to key issues
- Obligation to meeting global commitments
- Binding: International Health Regulations, Tobacco
- Political Endorsement: Sustainable Development Goals; G7/G20 Statements (e.g. UHC)
- Opportunity to make a difference/influence international health agenda
- Areas where global action is required - WHO 10 Threats to Global Health
- Fill gaps in global leadership
Context: Why Should Canada Engage in Global Health?
Strategic engagement provides opportunities to:
- Protect the health and safety of Canadians
- Establish and share expertise and success stories
- Advance Canada's interests through global leadership on issues where we can make a difference
- Use health as a "soft diplomacy tool" to advance Canada's broader foreign policy objectives
- Feminist International Assistance Policy (FIAP)
- UN Security Council Campaign (UNSC)
- Trade negotiations and economic interests
- Canada's international reputation
OIA's Role Footnote 15 in the Health Portfolio/Government of Canada
- Lead Canada's engagement in global health governance (with GAC)
- Focal point to coordinate global health issues across the HP and GoC (shared service)
- Single window for PM, Ministerial and senior official engagement
- Coordinate Canada's policy positions
- Provide protocol, international event and official visit logistics support
- Collaborate with domestic partners to:
- leverage Canadian expertise/experiences; and
- position Canada's engagement priorities on the global stage
- Manage the International Health Grants Program (base $1M per year)
Overview: HP/GoC Roles in Global Health - Text Equivalent
Overview of the Health Portfolio and Government of Canada's roles in global health.
The Health Portfolio and several Government departments collectively contribute to the Federal Government's role in global health.
The Health Portfolio is comprised of the Public Health Agency of Canada, Health Canada, the Canada Food Inspection Agency, the Canadian Institute for Health Research, and the Patented Medicine Price Review Board.
Within the Public Health Agency of Canada, there are three branches that contribute to global health-the Health Promotion and Chronic Disease Prevention Branch; the Health Security Infrastructure Branch; and the Infectious Diseases Prevention and Control Branch. An example of a global health issue the Health Promotion and Chronic Disease Prevention Branch addresses is healthy aging and dementia, which includes the implementation of the WHO Global Strategy and Action Plan on Ageing and Health. The Health Security Infrastructure Branch addresses the issue of health security, including developing an international health security strategy and the Infectious Diseases Prevention and Control Branch deals with issues such as HIV including target reporting for HIV 90/90/90.
Some examples of branches within Health Canada that contribute to global health include the Strategic Policy Branch, the Health Products and Food Branch, and the Controlled Substances and Cannabis Branch. Examples of other government departments contributing to global health include Public Safety and Environment, and Climate Change Canada. Additionally, Global Affairs Canada has a significant role in global health, which includes foreign policy, development, and trade negotiations.
All programs within the above listed agencies and departments lead on technical international activities drawing on their internal international expertise. Furthermore, the Office of International Affairs for the Health Portfolio consults with the Health Portfolio and Government of Canada counterparts on issues that fall within their domestic mandate in order to develop cohesive policy positions and highlight accomplishments. Moreover, the Office of International Affairs for the Health Portfolio is the focal point between Health Portfolio member agencies and Global Affairs Canada by representing the Health Portfolio's positions and providing input on health issues to Global Affairs Canada. The Office of International Affairs for the Health Portfolio also supports Global Affairs Canada by bringing their foreign policy objectives forward during the Health Portfolio's global health engagements.
| Issue | Int'l Pressures | Domestic Priorities | |
|---|---|---|---|
| WHO 10 Threats to GH in 2019 | MoH Mandate Letter | [REDACTED] | |
| Immunization (e.g. Access; Vaccine Confidence) | yes | yes | [REDACTED] |
Non-communicable diseases (NCDs)
|
yes | yes | [REDACTED] |
| (Global) Health Security (e.g. pandemic influenza, and other emergencies) | yes | yes | [REDACTED] |
| Antimicrobial Resistance (AMR) | yes | no | [REDACTED] |
| Problematic Substance Use (e.g. Opioids) | no | yes | [REDACTED] |
| Sexually Transmitted Blood Borne Infections (STBBIs) (e.g. HIV/AIDs) | yes | no | [REDACTED] |
Universal Health Coverage (UHC)
|
yesFootnote 2 | yes | [REDACTED] |
| Health Impacts of Climate Change | Yes | no | [REDACTED] |
|
|||
Key Players - International Fora
Key Fora
Multilateral Fora: Focus on UN System
- UN General Assembly (UNGA)
- UN Special Procedures (UN Special Rapporteur visits)
- World Health Organization (WHO)
- International Agency for Research on Cancer (IARC)
- Framework Convention on Tobacco Control (FCTC)
- Pan American Health Organization (PAHO)
Political / Economic Fora:
- G7 / G20
- Organisation for Economic Co-operation and Development (OECD)
- World Bank
Engagement:
- Represent Canada at meetings and events
- Provide oversight on governance and administration (e.g. budget review)
- Negotiate Canadian positions, political declarations
- Fund priority projects and Canada's membership to FCTC
- Support work of health working groups/ committees
Key Players - Bilateral / Regional Partners
- Strategic bilateral engagement occurs directly with key countries or on the margins of international events, participation in regional bodies (e.g. APEC) and in international fora (e.g. G7, OECD)
- Bilateral engagement supported by formal mechanisms (e.g. Memoranda of Understanding and policy dialogues) and informal technical cooperation
[REDACTED]
[REDACTED]
[REDACTED]
Key Players - Stakeholders
Stakeholder / Partners
International
- Gates Foundation, GAVI
- Wellcome Trust
- Alliances/coalitions of willing partners
- International Civil Society
- World Economic Forum
Domestic
- NGOs (Canadian Society for International Health, MDSC, MHCC)
- Professional Associations (CMA, CPHA)
- Academia (Global Health Schools - McGill, Dalla Lana)
- Youth and People with Lived/Living Experience
- Provinces/Territories (P/Ts)
- Industry (arm's length)
Engagement
Strategic and meaningful engagement to formulate policy positions is key.
- Stakeholders make-up an important part of Canadian Delegations (e.g. youth, P/Ts for annual WHA)
- Funding to NGOs and international partners for priority projects under the IHGP
- Gathering intel and sharing of info with NGOs/GH schools
Canada in Global Health: Preparing for the next 12 months
- Continue fulfilling core mandate; ongoing engagement throughout transition (keeping in mind potential impact of caretaker role)
- Continue progress in fulfilling obligations/commitments, including implementation of Sustainable Development Goals
- Prepare strategic recommendations on areas Canada can continue to make a difference, focusing on:
- Advancing legacy issues and initiatives with demonstrated impact
- Strengthening and leveraging key relationships
- Providing strategic advice and bringing policy coherence to key global health issues
ANNEX A: Office of International Affairs - Key Functions
Global Health Policy Analysis, Advice & Support
- Global Health policy development, analysis and negotiations
- Global Health issues and trends analysis
- Connections with foreign policy and trade agendas
International Relations & Engagement
- Government-to-government bilateral relations
- Lead on governing body multilateral relations (e.g. UN, WHO, PAHO)
- Protocol, International Events and Official Visits
- Interface with other government departments on Global Health
- Stakeholder engagement of Global health issues
Priority-Setting & Strategic Planning
- Coordinating and contributing to International priority-setting
- Planning, monitoring and reporting on deliverables and commitments
- Managing the International Health Grants Program
- Portfolio secretariat for international working groups
Health Canada Departmental Budget and Financial Situation Departmental Budget
- Per the 2019-20 Main Estimates, Health Canada has a budget of approximately $2.5B. The budget is broken down by branch as follows:
| - | FY 2019-20 Budget by Vote | ||||
|---|---|---|---|---|---|
| - | FTEs | Vote 1 | Vote 5 | Vote 10 | Total |
| Strategic Policy Branch | 307 | 52.6 | - | 1,515.8 | 1,568.4 |
| Health Products and Food Branch | 2,038 | 234.0 | 0.5 | - | 234.5 |
| Healthy Environments and Consumer Safety Branch | 1,020 | 114.1 | 1.5 | 1.7 | 117.3 |
| Controlled Substances and Cannabis Branch | 732 | 80.2 | 0.2 | 38.8 | 119.2 |
| Regulatory Operations and Enforcement Branch | 1,285 | 140.5 | 1.5 | - | 142.0 |
| Pest Management Regulatory Agency | 394 | 41.4 | 0.2 | - | 41.6 |
| Chief Financial Officer Branch | 353 | 27.1 | 0.0 | - | 27.2 |
| Corporate Services Branch | 1,156 | 158.2 | 4.3 | - | 162.5 |
| Executive Services | 158 | 43.3 | - | - | 43.3 |
| Communications and Public Affairs Branch | 234 | 33.1 | - | - | 33.1 |
| Departmental Reserve | 0 | 18.4 | 12.2 | 0.1 | 30.7 |
| TOTAL | 7,677 | 942.9 | 20.4 | 1,556.4 | 2,519.8 |
Financial Situation
- At P3, the Department is forecasting a surplus of $23.8M across all Votes
| - | Vote 1 | Vote 5 | Vote 10 | Total |
|---|---|---|---|---|
| P3 Forecasted Surplus | 13.5 | 1.7 | 8.5 | 23.8 |
Key Financial Considerations
- The department has a number of initiatives scheduled to sunset over the next four years. Programs are working with stakeholders and partner departments to determine the path forward in seeking renewed funding
- [REDACTED]
| Year of Sunset | Program Description | Total |
|---|---|---|
| (millions) | ||
| 2019-20 | Brain CanadaFootnote 1 | 13.2 |
| Program IntegrityFootnote 1 | 35.0 | |
| 2019-20 Total | 48.2 | |
| 2020-21 | Chemicals Management Pan | 66.0 |
| Heart & Stroke | 1.2 | |
| Mental Health Commission of Canada | 14.3 | |
| Territorial Health Investment Fund | 27.0 | |
| 2020-21 Total | 108.4 | |
| 2021-22 | Legalization of Cannabis | 109.7 |
| Canada Health Infoway | 80.0 | |
| Regulatory Review for Drugs and DevicesFootnote 1 | 17.3 | |
| 2021-22 Total | 207.0 | |
| 2022-23 | Canadian Agricultural Partnership | 4.0 |
| Impact Assessment | 6.8 | |
| 2022-23 Total | 10.8 | |
| 2023-24 | Bringing Innovation to RegulationsFootnote 1 | 13.9 |
| Bovine Spongiform Encephalopathy | 1.2 | |
| 2023-24 Total | 15.1 | |
|
||
Health Canada Executive Committee Structure - Text Equivalent
The committee structure charts shows the Deputy Minister and Associate Deputy Minister at the top of the chart with several committees reporting up to the Deputy Minister and Associate Deputy Minister.
- Executive committee and Executive Committee (Look Ahead) which both meet weekly. Their chair is the Deputy Minister and membership includes: Associate Deputy Minister, Assistant Deputy Ministers and Associate Assistant Deputy Ministers, Chief Financial Officer, Executive Director, Pest Management and Regulatory Agency, Senior General Counsel (Legal Services), Executive Director, Departmental Secretariat. There are three subcommittees, which report to the Executive Committee: Executive Committee on Regulatory Strategies; the Executive Sub-Committee on Finance, Investment Planning and Transformation and the Executive committee on People Management.
- Performance Measurement, Evaluation and Result Committee. The Chair is the Deputy Minister.
- Deputy Minister's Policy Committee (not active). The Chair is the Deputy Minister
- Transition Advice and Planning (time-limited committee). The Chair is the Deputy Minister.
Another committee is the Departmental Audit Committee. The Chair is Jim Mitchell and the Deputy Minister is a member of this committee.
| Membership | Executive Committee (EC) |
Executive Committee - Look Ahead (EC-LA) |
Performance Measurement, Evaluation and Results Committee (PMERC) |
Executive Committee on Regulatory Strategies (EC-RS) |
Executive Sub-Committee on Finance, Investment Planning and Transformation (EC-FIPT) | Executive Committee on People Management (EC-PM) | Deputy Minister's Policy Committee (DMPC) | Transition Advice and Planning (TAP) (time limited) |
|---|---|---|---|---|---|---|---|---|
| BRANCH RESPONSIBLE | SPB/ECS | SPB/ECS | ECS/OAE | HECSB | CFOB | CPAB | SPB/ECS | SPB/ECS |
| Deputy Minister | Chair | Chair | Chair | Not a member | Not a member | Not a member | Chair | Chair |
| Associate Deputy Minister | Alternate Chair | Alternate Chair | Alternate Chair | Alternate Chair | Alternate Chair | Not a member | Member | Alternate Chair |
| Abby Hoffman | ADM, SPB | Member | Member | Member | Not a member | Not a member | Not a member | Alternate Chair | Member |
| Marcel Saulnier | AsADM, SPB | Member | Member | Member | Member | Not a member | Not a member | Member | Member |
| Pierre Sabourin | ADM, HPFB | Member | Member | Member | Member | Member | Member | Member | Member |
| Kendal Weber | AsADM, HPFB | Member | Member | Member | Not a member | Not a member | Not a member | Member | Member |
| Stefania Trombetti | ADM, ROEB | Member | Member | Member | Chair | Member | Member | Member | Member |
| Debbie Beresford-Green | ADM, CSB | Member | Member | Member | Not a member | Member | Member | Not a member | Not a member |
| Robert Ianiro | ADM, HECSB | Member | Member | Member | Member | Member | Member | Member | Member |
| Jacqueline Bogden | ADM, CSCB | Member | Member | Member | Member | Not a member | Not a member | Member | Member |
| Eric Costen | AsADM, CSCB | Member | Member | Member | Member | Not a member | Not a member | Member | Member |
| Jennifer Hollington | ADM, CPAB | Member | Member | Member | Member | Member | Chair | Member | Member |
| Randy Larkin | ADM and CFO, CFOB | Member | Member | Member | Not a member | Chair + DG, Planning and Resource Mgm't & Chief Information Officer |
Not a member | - | Not a member |
| Richard Aucoin | ED, PMRA | Member | Member | Member | Member | Not a member | Not a member | Not a member | Member |
| Samantha Maislin Dickson | Senior General Counsel | Member | Member | Member | Observer Status | Not a member | Not a member | Not a member | Not a member |
| Shelley Borys | OAE | Member | Not a member | Member | Not a member | Not a member | Not a member | Not a member | Not a member |
| Daryl Gauthier | DG HRSD | Not a member | Not a member | Not a member | Member | Not a member | Not a member | Not a member | Not a member |
| PHAC | Vice-President | Not a member | Not a member | Not a member | Member | Not a member | Not a member | Not a member | Not a member |
| Dr. Supriya Sharma | Chief Medical Advisor | Not a member | Not a member | Not a member | Member | Not a member | Not a member | Member | Not a member |
| Karen Shepherd | Ombudsman | Observer Status | Not a member | Not a member | Not a member | Not a member | Member | - | Not a member |
Health Canada Executive Committee
Terms of Reference
1. Authority:
The Executive Committee (EC) functions under the authorities of the Deputy Minister (DM) and the Associate Deputy Minister (AsDM) to set the strategic direction of the department, make key policy and management decisions, and coordinate cross-departmental activities.
2. Role and mandate:
EC is Health Canada's senior decision-making, direction setting and oversight body.
EC convenes at two weekly meetings: Executive Committee and Executive Committee Look Ahead.
At EC meetings, members generally focus on policies, legislation and regulations, as well as emerging issues and trends that have departmental, portfolio or government implications. Members also ensure appropriate management oversight and accountability on progress, activities and performance of the department in financial management, program and service delivery, and human resources management.
At EC Look Ahead meetings, members generally focus on short-term departmental business planning such as upcoming Cabinet business, parliamentary activities, Treasury Board submissions, communications, as well as ongoing business and issues requiring senior management attention.
The Executive Committee also acts as the departmental evaluation and performance measurement committee. In this role, it serves as an advisory body to the deputy head related to the departmental evaluation plan, resourcing, and final evaluation reports and may also serve as the decision-making body on other evaluation and evaluation-related activities of the department. (See Appendix D - Roles and Responsibilities of Departmental Evaluation Committee).
3. Guiding principles
Health Canada's governance structure is based on the following principles:
- Leadership - achieving an department-wide commitment to good governance through leadership
- Accountability - being answerable for decisions and having meaningful mechanisms in place to ensure the department adheres to all applicable standards
- Integrity - acting impartially, ethically and in the interests of the department
- Stewardship - using every opportunity to enhance the value of the public assets and institutions that have been entrusted to care
- Efficiency - ensuring the best use of resources to further the aims of the department, with a commitment to evidence-based strategies for improvement
- Transparency/openness/predictability - having clear roles and responsibilities and clear procedures for making decisions
- Engagement - using all opportunities to create a learning organization and to foster staff engagement
- Agility - ensuring a flexible, responsive, adaptive organization
EC and its sub-committees commit to operate with due regard to:
- Public service values, as described in the Values and Ethics Code for the Public Sector Footnote 16 (i.e. democratic, professional, ethical and people values) and the Health Canada Code of Conduct;
- The Treasury Board Secretariat Framework Footnote 17 for the Management of Risk and the Health Canada Decision-Making Framework Footnote 18;
- Senior management responsibility to foster a work environment that is conducive to the effective use of both official languages, as stated in the Treasury Board Secretariat Policy on Official Languages and Health Canada's Policy on Language of Work;
- Horizontal collaboration within the department and portfolio;
- Compliance with laws and regulations; and
- Departmental and government priorities.
4. Position with governance structure Footnote 19 :
EC is supported by the following sub-committees:
- EC - Finance, Investment Planning and Transformation (EC-FIPT)
- EC - People Management (EC-PM)
- Executive Committee on Regulatory Strategies (EC-RS)
- Performance Measurement, Evaluation and Results Committee (PMERC)
An additional sub-committee made up of Assistant Deputy Ministers, called ADM-DAC, meets quarterly to review audits, progress reports against MRAPs and audit plans being tabled at Departmental Audit Committee (DAC) meetings.
It should be noted that the structure of EC and its sub-committees should not preclude departmental management from creating and dissolving committees based on operational need. However, EC shall remain the most senior horizontal decision-making body in Health Canada.
5. Agenda setting:
The business of EC is captured in the Forward Agenda of EC meetings as well as the fixed agenda of EC Look Ahead meetings.
The Forward Agenda of EC meetings are populated by items:
- proposed by the Chairs of the sub-committees and approved by the DM and AsDM;
- proposed by Branches and approved by the DM and AsDM; and
- requested by the DM and AsDM.
Guidance for Scheduling Items at EC Meetings
- The scope of the issue suggests that it would benefit or requires input from multiple Branches; or would require implementation by multiple Branches (corporate policies, changes in internal/external service delivery, etc.).
- The issue directly implicates the authority or accountability of multiple ADMs, the DM, the Minister, Cabinet or Treasury Board (e.g. Memoranda to Cabinet, regulatory amendments, compliance with Central Agency policies, etc.).
- The issue involves or could involve significant financial materiality (e.g. financial transactions, resource allocations, capital planning, etc.).
- The issue has policy implications with potentially significant impact to the department or the health of Canadians.
Branches are encouraged to use the same criteria in proposing items for EC consideration and are encouraged to interact with the EC Services for clarification as needed.
6. Membership
- Chair:
- Deputy Minister/ Associate Deputy Minister
- Alternate Chair:
- ADM if designated by DM or AsDM
- Members:
- All Assistant Deputy Ministers (ADMs) and Associate ADMs
Executive Director, Pest Management and Regulatory Agency Senior General Counsel (Legal Services) - Observers:
- Director General of Evaluation and Chief Audit Executive Footnote 20
Chief of Staff to the Deputy Minister
Advisors to the Associate Deputy Minister
Director General, Policy Coordination and Planning Directorate (PCPD)
Director, Strategic Policy, Priorities and Portfolio Affairs
Members are expected to attend all meetings. Where this is not possible, EC Members may designate a DG-level substitute for EC meetings, subject to the Chair's approval.
Guest presenters or observers may accompany a member for the presentation of an item, subject to the Chair's approval.
EC Members are responsible for the preparation and timely submission of materials to Executive Committee Services (ECS).
7. Frequency of meetings
EC meetings will be held weekly, or at the call of the Chair.
EC - Look Ahead meetings will be held weekly, or at the call of the Chair.
Additional special EC meetings may be organized at the call of the Chair.
8. Secretariat and administration
The Director General of Policy Coordination and Planning Directorate, with support from the Strategic Policy Priorities and Portfolio Affairs Division, and Executive Committee Services are responsible for overall secretariat support for EC meetings, which includes:
- maintaining a Forward Agenda;
- providing guidance on the development of EC documents to be presented by EC Members as needed;
- providing advice to the Deputy and Associate Deputy Minister on EC meeting agenda items;
- following up with Branches, post-meeting, as requested by the Chair, to support the further development of the work presented;
- taking notes and preparing the Records of Decisions for EC meetings;
- coordinating overall logistics and distribution of documents; and records management.
Footnotes
- Footnote 1
-
Reports through PHAC.
- Footnote 2
-
Past 12-month use (CTADS 2017).
- Footnote 3
-
Past 30-day use of any tobacco product (CTADS 2017).
- Footnote 4
-
Past 12-month use (CTADS 2017).
- Footnote 5
-
Includes: cocaine or crack, ecstasy, speed or methamphetamines, hallucinogens and heroin.
- Footnote 6
-
Past 12-month use (CTADS 2017).
- Footnote 7
-
Past 30 day use.
- Footnote 8
-
Past 12-month use (CTADS 2017).
- Footnote 9
-
Past 3-month use (NCS 2019).
- Footnote 10
-
Source: Canadian Institute for Substance use Research/Canadian Centre on Substance Use and Addictions, 2018. Note: "CNS" = central nervous system.
- Footnote 11
-
See Appendix B for details on key stakeholder groups.
- Footnote 12
-
And the Minister of Border Security and Organized Crime Reduction in relation to the Cannabis Act.
- Footnote 13
-
CPAB reference levels are from the 2019-20 ARLU.
- Footnote 14
-
The SSP Agreement was not included in the material provided to the Deputy Minister.
- Footnote 15
-
See Annex A for key functions.
- Footnote 16
-
http://www.tbs-sct.gc.ca/pubs_pol/hrpubs/tb_851/vec-cve-eng.asp
- Footnote 17
-
http://www.tbs-sct.gc.ca/pol/doc-eng.aspx?id=12254
- Footnote 18
-
http://www.hc-sc.gc.ca/ahc-asc/pubs/hpfb-dgpsa/risk-risques_tc-tm-eng.php
- Footnote 19
-
See Governance Structure at Appendix A.
- Footnote 20
-
The, Director General, Evaluation and Chief Audit Executive attends Executive Committee but is not required to attend Executive Committee Look Ahead.