Ministerial Briefing Volume I - Overview of the Health Portfolio – November 2019
Table of contents
- Health Portfolio at a Glance
- Role of Health Portfolio
- The Health Portfolio – a Partner in Health for All Canadians
- Health Canada Overview Deck
- Public Health Agency of Canada (PHAC) Overview Deck
- Canadian Institutes of Health Research (CIHR) Overview Deck
- Canadian Food Inspection Agency (CFIA) Overview Deck
- Patented Medicine Prices Review Board (PMPRB) Overview Deck
- Overview of Legislative and Regulatory Responsibilities in the Health Portfolio
- Overview of Federal-provincial/ Territorial (FPT) Roles and Relations in Health
- List of Provincial/Territorial Ministers Responsible for Health
- An Overview of Pan-canadian Health Organizations
- Key Players in Health
- Relationships With the International Community
Health Portfolio at a Glance
Responsible for Helping Canadians Maintain and Improve Their Health
Under Your Direct Purview
Health Canada (HC)
Promotes and helps protect the health and safety of Canadians by regulating products such as drugs, medical devices, consumer products and food. HC supports universally accessible, publicly funded health care for Canadians through stewardship of the Canada Health Act, leadership on emerging issues and collaboration with provinces and territories on health system improvements.
Public Health Agency of Canada (PHAC)
Protects the health of Canadians by providing national leadership in preparing for and responding to public health events and emergencies. Promotes the well-being of Canadians by preventing the spread of infectious disease, addressing the factors that are important to preventing chronic disease and injury, and providing timely information to the public.
Canadian Food Inspection Agency (CFIA)
Protects Canada and Canadians from food, plant, and animal health risks inherent in the modern environment, while supporting Canadian food businesses as they compete, innovate and grow in domestic and global markets.
Arm's-length Organizations
Canadian Institutes of Health Research (CIHR)
Canada's federal funding agency for health research. Composed of 13 institutes, CIHR collaborates with national and international partners to support discoveries and innovations that improve Canadians' health and strengthen Canada's health care system. CIHR is a source of scientific evidence to inform the Government's decisions.
Patented Medicine Prices Review Board (PMPRB)
Quasi-judicial body that protects consumers and contributes to health care by ensuring that the prices of patented medicines sold in Canada are not excessive. The PMPRB also informs Canadians by reporting on pharmaceutical trends.
Role of Health Portfolio
Strengthening Canada's universal health care system
- Stewardship of universally accessible, publicly-funded health care for Canadians through administration of the Canada Health Act (HC)
- Providing leadership on emerging issues and working multilaterally and bilaterally with provinces and territories on system improvements (HC)
- Investing in Pan-Canadian Health Organizations to drive progress on health system priorities (HC)
- Leading pan-Canadian initiatives on system innovation and quality improvements in new and emerging areas of health care (HC)
- Managing federal grants and contributions programs to support health system innovation and priorities, as well as minority official language communities (HC)
- Enhancing the affordability, accessibility and appropriate use of prescription drugs (HC, PMPRB)
- Improving cultural safety, humility and responsiveness in the health system to improve Indigenous health and address systemic barriers and discrimination (HC)
Supporting health research and science, data collection and surveillance capacity
- Funding research that generates new knowledge, improves health or health services, informs government priorities, and supports health innovation (CIHR)
- Investing in and disseminating research and data to improve the health care system (CIHR)
- Building research capacity in under-developed areas, and training the next generation of health researchers (CIHR)
- Promoting the effective management of health research data and health-related data in Canada (CIHR)
- Supporting Indigenous health research, knowledge translation and capacity-building (CIHR)
- Strengthening surveillance, research and public education on chronic and emerging infectious diseases (PHAC)
- Improving diagnostic and scientific capacity through national laboratories, to detect serious and emerging diseases (PHAC, CFIA)
- Funding six national collaborating centres that synthesize and share knowledge to promote the use of evidence by public health practitioners and policy makers (PHAC)
- Reporting on pharmaceutical trends of all medicines, and research and development spending by patentees (PMPRB)
- Conducting research, monitoring and surveillance on the health impacts of the environment and climate change (HC)
Enabling access to safe and effective health products
- Assessing and regulating health products to ensure their safety, effectiveness and quality (HC)
- Modernizing regulatory regimes to increase access to safe and effective treatment options (HC)
- Overseeing clinical trials to ensure that health products are available to Canadians (HC)
- Monitoring health product safety and adverse events arising from use, and communicating risks to Canadians (HC)
Supporting Canadians in making safe and healthy choices
- Promoting the health and well-being of all Canadians by addressing the root causes of health inequalities and the common risks and protective factors that are important in preventing and avoiding disease (PHAC)
- Creating environments that support healthy choices, and promoting healthy behaviours and practices to improve health (PHAC)
- Examining environmental health by studying the burden of disease and through disease surveillance (PHAC)
- Conducting public awareness campaigns on safe food practices (HC)
- Supporting Canadians in making informed food choices (HC)
- Informing and engaging Canadians by being a trusted source of information on health and safety (HC, PHAC, CFIA)
- Supporting early childhood development programs for Indigenous children and their families living off-reserve (PHAC)
- Supporting research to better define factors leading to healthy aging and to address a wide range of conditions associated with aging (CIHR)
Managing risks to health
- Assessing and managing the health risks of tobacco and controlled substances, consumer products, chemicals and pesticides (HC, PHAC)
- Managing food-related health risks through strong food safety regulations, surveillance and enforcement (HC, PHAC, CFIA)
- Implementing comprehensive approaches to minimize health risks associated with substances (HC, PHAC)
- Overseeing the legal cannabis regime, including implementing cannabis policy direction, granting licences for production, and monitoring compliance with regulations (HC)
- Taking appropriate compliance and enforcement action in relation to acts and regulations administered by HC (HC)
- Working with Indigenous peoples on cannabis, opioids and tobacco strategies (HC)
- Maintaining readiness to respond to public health threats and emergencies:
- Providing domestic and international health security leadership, including pandemic preparedness and public health and nuclear emergency response (PHAC, HC)
- Regulating listed pathogens and toxins such as rabies or anthrax (PHAC)
- Preventing the introduction and spread of communicable diseases, including through border and travel health (PHAC)
Collaboration with Other Federal Government Departments
- Work collaboratively with other government departments on areas of shared priority/responsibility
Provincial/Territorial (PT) Partners
- PTs administer public health insurance plans for medically necessary services; plan and finance hospital care, physician and allied health services; deliver other services (drug plans, home care etc.) on a discretionary basis; administer aspects of public health; and negotiate fee schedules for health professionals
- There is a high level of collaboration between the Health Portfolio and PTs, both bilaterally and multilaterally
Indigenous Partners
- Engage with First Nations, Inuit and Métis to improve health outcomes for Indigenous peoples
Health Partners/Industry Stakeholders
- Work with health stakeholders, including professional associations, regulatory bodies, patient groups and industry to ensure responsive approaches to Canadians' health needs
International Partners
- Engage internationally to protect and advance Canadian health interests
- Participate in multilateral fora, particularly the World Health Organization
- Foster bilateral relationships with key partners and regions
- Partner on health research that affects Canadians and the global community, and position Canadian researchers as leaders
The Health Portfolio – a Partner in Health for All Canadians
I. A Brief Overview of Health in Canada
A. Overall health of Canadians and current health trends
Canadians are among the healthiest people in the world. In general, we live long lives in good health. Canada's health care system is a source of pride for many Canadians. Nevertheless, there are some concerning public health trends and health inequalities.
Certain regions and populations in Canada (Northern, rural and remote communities, Indigenous Peoples, LGBTQ2S+, low-income families, children living in conditions of risk, un- or under-employed adults, older adults, and racialized communities) continue to experience poorer health than the average Canadian.
Chronic diseases continue to be the greatest cause of disease burden in Canada. Close to one-half of Canadian adults over the age of 20 years report that they are living with at least one of ten common chronic diseases or conditions such as cancer, diabetes, heart disease, or hypertension. Childhood obesity rates remain high, and chronic diseases, like type-2 diabetes, are beginning to appear in Canadian children. While progress is being made to reduce rates of some chronic diseases, this remains a major health challenge in Canada.
Did You Know?
- An individual's health is determined by a broad range of personal, social, economic and environmental factors, such as income, housing, education, physical environment, and gender and culture.
- Four major modifiable risk factors for chronic disease are physical inactivity, tobacco use, alcohol use, and unhealthy diet.
- While Canadians are generally healthy, more than TWO OUT OF FIVE Canadians aged 20 years or older have a chronic disease, and over FOUR OUT OF FIVE Canadians aged 20 years or older have at least one preventable risk factor.
While many Canadians are in good mental health, one in three will be diagnosed with a mental health or substance use disorder at some point in their lifetime, and on average, 11 Canadians die by suicide every day. Certain populations (for example, LGBTQ2S+) experience much higher rates of poor mental health.
In addition, Canada is dealing with a number of important trends that are having an impact on the country's progress with respect to health and well-being, including an ongoing overdose death crisis. This crisis is the deadliest in a generation, claiming the lives of at least 12,813 Canadians since January 2016. Its toll is so severe that it has arrested gains in Canadian's overall life expectancy.
There is also an alarming increase in methamphetamine use in some regions of the country, which presents its own devastating challenges.
Efforts across the Canadian health system have reduced the risks of infectious diseases in Canada considerably. Significant progress has been made to increase vaccination rates to protect against vaccine-preventable diseases.
However, there are trends in certain areas that are a growing cause for concern in Canada. Rates of sexually transmitted and blood-borne infections have increased considerably over the past decade. Furthermore, Canada is not meeting its population vaccination targets, and has seen a re-emergence of some vaccine- preventable diseases such as measles. Canadians are also facing an increasing risk of being infected by bacteria that are resistant to antibiotic treatment, and antimicrobial resistance has been identified by the World Health Organization as one of the top 10 risks to global health.
Did You Know?
- Antibiotics are rapidly becoming ineffective because the bacteria they are designed to eliminate are becoming resistant to these drugs, leading to antimicrobial resistance (AMR) – a complex problem that results, in part, from overuse of antibiotics in industrial agriculture.
- In 2018, the estimated number of AMR-related deaths in Canada was 5400. By 2050, the cumulative number of AMR-related deaths is predicted to rise to 390,000.
While smoking rates continue to decline, rates of youth vaping have increased rapidly, which may lead to nicotine addiction and other related harms. The recent emergence of severe pulmonary illness related to vaping in the United States and Canada is also cause for concern.
Canadians are already experiencing health impacts from changes to Canada's climate resulting from more frequent and severe extreme weather events, natural disasters, reduced air quality, and food safety and water quality issues. Specific health impacts include increased injury and fatalities, mental health issues, heat- and food-related illness, and the spread of infectious and vector-borne diseases, such as Lyme disease, all of which place a strain on health services.
B. Health System
Health is a shared responsibility in Canada, with the federal government and the provinces and territories having distinct roles. While primary responsibility for delivery of health care services to Canadians falls within provincial and territorial jurisdiction, the federal government sets and administers national standards for publicly insured health services through the Canada Health Act, and provides funding support for provincial and territorial health care services through the Canada Health Transfer. The federal government also provides health care to certain populations (for example, First Nations on reserve and Inuit communities). Additional federal activities include programs to protect and promote health (for example, through the regulation of drugs and medical devices, consumer safety and public health), the regulation of patented drug prices, managing public health events and emergencies, contributing to the prevention and control of infectious disease, and promoting health and well-being.
For their part, provincial and territorial governments manage, organize and deliver health care services for their residents. Each province or territory has its own public health care insurance plan (based on Canada Health Act principles), which ensures its residents have reasonable access to medically necessary hospital and physician services without paying out of pocket.
Both levels of government must work collaboratively to address health issues where there is shared responsibility. These areas include health care system support and funding, oversight of public health insurance regimes, public health promotion, and chronic and infectious disease prevention.
Canada's health care system faces a number of challenges. The system was designed decades ago, when the population was younger and growing. Despite significant demographic, technological and economic changes, the primary focus continues to be on hospitals and physicians. An aging population and an increase in chronic conditions mean that patients' needs are shifting, with a need for services and care provided in the home and in the community rather than in hospitals. More patients require ongoing, long-term care that does not need to be provided in institutional settings, and can be delivered more cost effectively and efficiently in patients' homes and in the community. Spending on drugs as a proportion of overall national expenditures on health continues to grow.
Mental illness also represents a significant and growing burden on Canadians and the economy. Up to 70 percent of young adults aged 15-24 years with a mood or anxiety disorder reported that their symptoms had started before the age of 15. And yet, Canadians face long wait times for access to mental health services, or are forced to visit emergency rooms when their illness reaches a crisis level.
| Focus | 1975 | 2019 |
|---|---|---|
| Hospitals | 45% | 27% |
| Physicians | 15% | 15% |
| Drugs | 9% | 15% |
| Other | 31% | 43% |
Source: Canadian Institute for Health Information, National Health Expenditure Trends, 1975 to 2019 |
||
C. Key trends and drivers in Canada's health landscape
The Health Portfolio operates in a dynamic and complex environment, with external trends and factors influencing the health landscape in Canada. These key drivers of the health landscape shape the work of the Health Portfolio in helping Canadians maintain and improve their health, as well as areas of current federal, provincial and territorial collaboration.
Evolving demographics and health needs
The demographics of Canada continue to evolve, leading to shifts in the health needs of Canadians. As Canada's population lives longer, chronic diseases such as cancer, cardiovascular disease, and diabetes have become more common. The health needs of Canadians also reflect the increased diversity of Canada's population, including growing populations of immigrants and Indigenous people. Inequalities continue to prevent certain populations, such as First Nations, Inuit, Métis, and Canadians living with low income, from achieving their full health potential. In areas with a high concentration of people who identify as Indigenous, life expectancy at birth is lower, infant mortality is higher, self-rated mental health is lower, and suicide rates are higher. There are also clear disparities in life expectancy, infant mortality, unintentional injury, mortality, mental illness hospitalizations, and chronic disease prevalence for Canadians with lower socioeconomic status (education and income).
Innovation in science and technology
Scientific and technological innovation continues to accelerate the introduction of new or improved products (e.g., drugs, technologies, tools, and software) that are better able to predict, define, treat, and even cure human diseases. This expanding landscape of products and services introduces a range of new challenges and opportunities. Emerging technologies offer the potential to improve patient care and outcomes. For example, artificial intelligence (e.g., machine learning, neural networks, and robotics) has the potential to improve diagnosis, disease onset prediction, prognosis, and service provision, while reducing incidents associated with human errors due to lapses in memory, emotional response, and/or fatigue. Robotics can be applied in surgery, to improve access to health services in rural and remote scenarios, or as care companions to enable a patient's independent living.
Increased precision and personalization in health care can improve patient care through customized therapies that are targeted to individual patients, such as tailoring a therapy based on a patient's DNA. 3D printing is a promising technology to help move health care from its one-size-fits-all approach to small batch or even patient-specific medical devices. These technologies also challenge the Health Portfolio to adapt its approach to regulating health products and devices, which presents the opportunity for Health Canada to modernize its regulatory framework to enable innovation through a flexible, risk-based approach, with appropriate oversight to ensure safety, quality, and efficacy of products.
Emerging technologies have also brought new challenges with respect to public health issues and threats. Technological advancements have made chemical, biological, radiological or nuclear agents (e.g., smallpox, anthrax) easier and less costly to create or obtain, making events involving the accidental or intentional release of these agents a significant public health security threat.
Canadians' evolving expectations of the health system
Alongside these advancements in technology, Canadians' expectations of the health system, and how they seek information regarding their health, are changing. Advancements in health technologies can raise expectations for patients, such as those who seek life-changing treatments made possible by technology, and those who desire greater participation in their health through "anywhere, anytime" monitoring, diagnosis and treatment. Consumers are
seeking a more active role in managing their health through a range of tools such as apps that monitor behaviour like exercise and eating habits, or devices that measure personal health status (e.g., glucose levels in diabetics or sleep interruptions for people with apnea).
Canadians also use social media and websites to seek and share information about health. This has an impact on the flow and quality of information, and on expectations of openness and transparency regarding health information and regulatory decisions made by governments.
The spread of non-evidence-based health information through social media can potentially undermine public health efforts and trust in science. Increases in vaccine hesitancy and recent measles outbreaks are key examples of how the spread of misinformation online can have a public health impact.
New ways data is collected and used
Health systems around the world are generating more data, and the increased accuracy, variety and volume of this health data available in real time can help forecast trends and behaviours to support decision-making, and improve health outcomes and health system efficiency. There is also a demand for real-world evidence, which has great potential to inform decision-making and achieve value for patients. With the rise of "big data" and use of artificial intelligence technologies, there are also challenges in addressing the regulatory, ethical, and privacy concerns regarding the collection and use of this data. However, the capacity of Canada's health systems to collect, analyze, and disseminate policy and clinical practice insights from even the currently available data is limited. As a result, Canada risks falling behind in this area.
Shifts in health care service delivery
Advances in science and technology also lead to shifts in how, where and when health care is delivered, as well as who delivers it. As emerging technologies accelerate the pace of product innovation, many of these technologies may disrupt care delivery. Health system structures and the health care workforce will need to adapt to integrate these new technologies into care delivery. The costs of new technologies will also create significant financial pressure on health budgets.
Growing threat of antimicrobial resistance (AMR)
Global health experts have identified the spread of antimicrobial resistance as the single greatest challenge related to infectious disease prevention and control today. In Canada, more than 18,000 hospital patients acquire an AMR infection every year. The number of bacteria that are resistant to antibiotics is increasing and few new antibiotics are being developed, meaning that treatable illnesses like pneumonia, tuberculosis, gonorrhea and syphilis could become incurable. Given the complexity, scope and wide-ranging effects of AMR, the global community and Canada must continue to mobilize efforts to understand the scope of the issue and take corrective action where possible.
Health effects of climate change
Leading health, environmental and public policy experts identify climate change as one of the greatest challenges of the 21st century. Climate change has major health implications for Canadians, with rising temperatures in Canada already altering the spread and intensity of certain diseases. West Nile virus and Lyme disease are prevalent in some areas of the country and threaten to spread farther north. Other climate-related health threats include heat stress and injury or loss of life related to severe weather events (e.g., floods, snowstorms, wildfires and heat waves). Vulnerable populations - including the poor, elderly, very young and those who are chronically ill, as well as people living in vulnerable geographical areas such as the North – will disproportionately bear the burden of the health effects linked to climate change.
Globalization and the complexity of the global supply chain
Globalization has had a profound effect on public health in Canada. With increased trade and movement of humans and goods across borders, infectious diseases can spread more quickly than ever.
Globalization has also increased the complexity of the pharmaceutical supply chain, resulting in many of today's drug products following a complex, multi-entity path before reaching the Canadian market. Canadian pharmaceutical companies increasingly rely on foreign drug manufacturing activities in countries. The variable integrity of the global supply chain and the international nature of production and distribution processes pose complexities for the Health Portfolio's regulatory oversight to ensure the safety and quality of drugs entering Canada.
Globalization also means that issues with health product safety have the potential to affect a greater number of people, due to the expanded supply chain. Canadians have also been adversely affected by the global supply chain (e.g., through drug shortages and recalls), in which fewer drug manufacturers are serving more markets. To remain effective in this challenging operating context, the Health Portfolio continues to partner with international regulatory authorities to ensure safety in the global supply chain.
II. The Health Portfolio
A Portfolio Approach
As Minister of Health, you are responsible for five dynamic, science-based organizations, each playing a unique and important role in the health and safety of Canadians. Each organization supports you in the delivery of your mandate to help maintain and improve the health of Canadians.Footnote 1
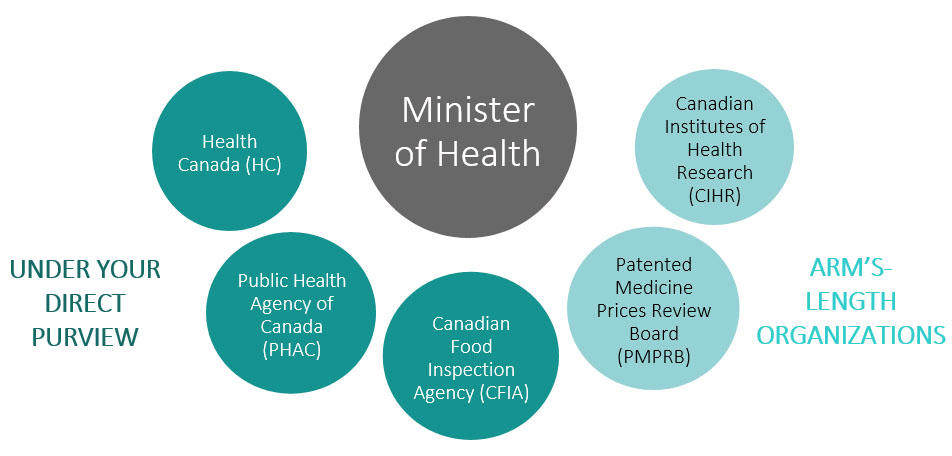
Long Description
The largest of all the circles contains the Minister of Health. There are five smaller circles that contain 5 different organizations within the Health Portfolio. Three are grouped together by colour indicating that they are under the direct purview of the Minister of Health: Health Canada (HC), Public Health Agency of Canada (PHAC) & Canadian Food Inspection Agency (CFIA). Two others are grouped together by colour indicating that they are arm's-length organizations: Canadian Institutes of Health Research (CIHR) & Patented Medicine Prices Review Board (PMPRB).
The Health Portfolio's main activities include:
- Strengthening Canada's universal health care system;
- Enabling access to quality, safe and effective health products;
- Supporting Canadians in making safe and healthy choices;
- Managing risks to health; and
- Supporting health research and science, data collection and surveillance capacity.
Although each organization within the Health Portfolio has its own organization and role, they often have complementary functions in relation to key areas of health. For example, Canada's world-class food safety regime is supported by Health Canada, CFIA, and PHAC. Health Canada sets standards for food safety and nutrition, and carries out research, surveillance and pre-market evaluation for novel foods, human milk fortifiers and infant formula; CFIA is responsible for federal food inspection regimes, including prevention, monitoring, investigation and recalls; and PHAC undertakes food-borne illness outbreak surveillance and leads epidemiological investigations when cases occur in multiple provinces. The sum of these parts creates a comprehensive system that supports Canadians in having access to safe and healthy food choices.
You have a number of other levers and tools at your disposal to help you deliver on your mandate:
How the federal government can act in health

Long Description
Schematic showing how the federal government can act in health
- Legislation and Regulation
- Spending
- Research, Surveillance and Monitoring
- Policy Development, Engagement and Advice
- Communication
- Program and Service Delivery
- Leadership/convening power
Did You Know?
- The Chief Public Health Officer of Canada (CPHO) advises you, as the Minister of Health, on public health related matters.
- The CPHO also provides leadership by communicating with other levels of government, voluntary organizations, the private sector and Canadians on public health issues.
III. Your Responsibilities in the Health Portfolio
A. Strengthening Canada's universal health care system
Stewardship of universally accessible, publicly funded health care for Canadians through administration of the Canada Health Act
The Health Portfolio plays a leadership role as a steward of medicare, including administering the Canada Health Act (CHA), Canada's federal legislation for publicly funded health care insurance. The CHA aims to ensure that all eligible residents of Canada have reasonable access to medically necessary hospital and physician services on a prepaid basis, without direct charges at the point of service. Provinces and territories must meet requirements set out in the CHA (including the principles of public administration, comprehensiveness, universality, portability and accessibility applicable to insured health services) to receive the full amount of their cash entitlement under the Canada Health Transfer.
The Canada Health Transfer (CHT) is the key federal funding vehicle for supporting health care. It is allocated to provinces and territories on an equal-per-capita basis and represents approximately 23% of provincial/territorial government sector health expenditures. The annual growth of the Canada Health Transfer envelope is set in legislation. In 2017-18, the legislated CHT escalator changed from the fixed level of 6%, which had been in place for more than a decade to a three-year moving average of nominal GDP growth with a floor of 3%. In 2019-20, provinces and territories will receive approximately $40 billion in health transfers. While the Canada Health Transfer is administered by the Minister of Finance, the Minister of Health plays a significant role in the strategic management of the file.
As a complement to the Canada Health Transfer, and in recognition of the higher cost of delivering health services in the North, Health Canada also provides funding to the territorial governments through the Territorial Health Investment Fund.
Did You Know?
- Provincial and territorial health care insurance plans must meet the requirements of the CHA to receive full funding under the Canada Health Transfer.
- The CHA contains specific provisions to discourage provinces and territories from allowing extra billing and user charges for insured hospital and physician services.
- Every year, the Minister of Health tables a report in Parliament on the administration of the CHA, to assess compliance and ensure transparency for Canadians on the administration of the Act.
Providing leadership on emerging issues and working multilaterally and bilaterally with provinces and territories on system improvements
As Minister of Health, your collaboration with provinces and territories is maintained through multilateral and bilateral machinery and partnership, including annual Federal-Provincial/ Territorial Health Ministers' Meetings and the pan-Canadian Public Health Network.
One of the more significant recent examples of federal-provincial/ territorial collaboration was the 2017 agreement to the Common Statement of Principles on Shared Health Priorities, which sets out a common menu of action in priority areas, supported by a federal commitment of $11 billion over ten years. The new federal funding is directed to provincial/territorial initiatives in home and community care, and mental health and addictions. Over the next ten years, federal and provincial/territorial Ministers will work together to improve access to home and community care by scaling and spreading evidence-based models that are more integrated with primary care; and enhancing access to palliative and end-of-life care at home or in hospices; increasing support for caregivers; and enhancing digital connectivity and remote monitoring technology.
Did You Know?
- 49% of national health care spending comes from provincial and territorial governments (not counting federal funding that flows through provinces and territories).
- 20% of national health care spending comes from the federal government.
Funding Breakdown by Province and Territory (2017-2027)
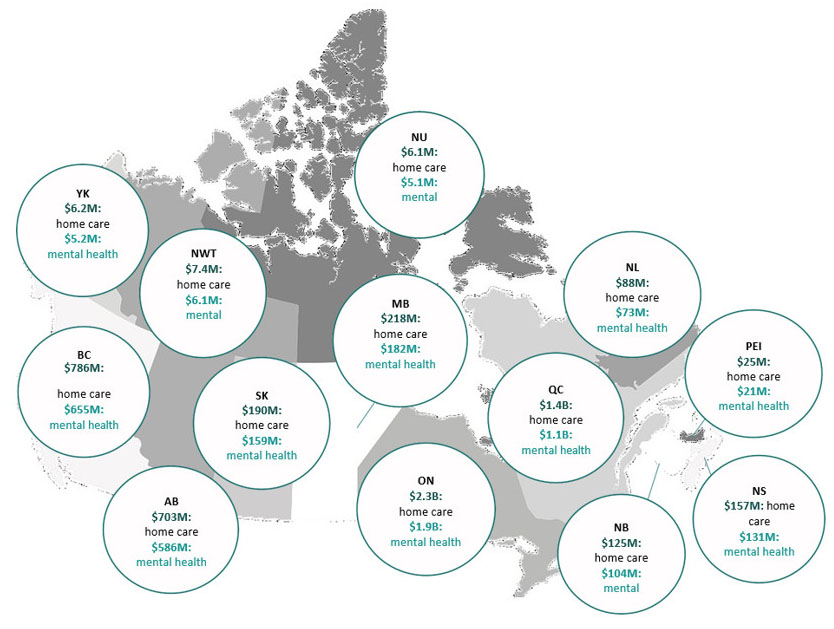
Long Description
The funding breakdown for provinces and territories is as follows: Yukon ($6.2M home care, $5.2M mental health), Northwest Territories ($7.4M home care, $6.1M mental health), Nunavut ($6.1M home care, $5.1M mental health), British Columbia ($786M home care, $655M mental health), Alberta ($703M home care, $586M mental health), Saskatchewan ($190M health care, $159M mental health), Manitoba ($218M home care, $182M mental health), Ontario ($2.3B home care, $1.9B mental health), Quebec ($1.4B home care, $1.1B mental health), New Brunswick ($125M home care, $104M mental health), Nova Scotia ($157M home care, $131M mental health), Prince Edward Island ($25M home care, $21M mental health) & Newfoundland ($88M home care, $73M mental health)
To improve access to mental health and addiction services, individual provinces and territories have developed plans to expand access to community-based mental health and addiction services for children and youth (age 10-25), to spread evidence-based models of care and culturally-appropriate services, and to expand the availability of integrated community-based services for people with complex needs.
In 2017, federal and provincial/territorial Ministers of Health also agreed to work with the Canadian Institute for Health Information (CIHI) to develop a set of common indicators to measure progress on shared priorities. On May 30, 2019, CIHI released the first annual report, which included the first three of 12 common indicators measuring pan-Canadian progress on improving access to mental health and addiction services, and to home and community care.
Did You Know?
Investments through the bilateral agreements for home and community care and mental health and addiction services include the following examples:
- Ontario is increasing access to structured psychotherapy and counselling support programs for people with anxiety and depression
- Saskatchewan is establishing community health centres and teams to shift the delivery of care from hospitals into community settings
- Prince Edward Island is developing culturally appropriate information regarding programs, services and training specific to First Nations continuing care needs
- Through the Yukon's Home First and Complex Client Supports Initiative, Yukon clients will be provided enhanced services at home, with the goal of keeping them out of long term care
- Through its asymmetrical arrangement, Quebec has as one priority increased hours of home care services when patients require more intensive care, to avoid unnecessary emergency room visits or hospitalizations
- New Brunswick is implementing community-based, at-home mental health senior care services across the province to enable seniors to live at home longer
- Governments are improving access to culturally appropriate and integrated mental health and addictions interventions through increasing access to home care services in 125 Indigenous communities across Ontario, the North Zone Indigenous travel team in Alberta, targeted funding to support First Nations Health Authority in British Columbia, and Northwest Territories' territorial suicide prevention and crisis support network
Enhancing the affordability, accessibility, and appropriate use of prescription drugs
Helping Canadians have access to affordable medications is an important priority for the Health Portfolio.
As a first measure to improve affordability, in 2016, the federal government joined provinces and territories as a member of the pan-Canadian Pharmaceutical Alliance, which combines governments' collective buying power to negotiate lower prices on brand name drugs for all public drug plans, and sets the price point for many generic drugs. Federal drug plans now benefit from the lower drug prices that are achieved through these negotiations.
As an arm's-length organization, the PMPRB reviews the prices patentees charge for patented drug products on the Canadian market.Footnote 2 The PMPRB can work with patentees to achieve voluntary price reductions, or hold public hearings to determine whether a price is excessive, and (if so) order price reductions or the offset of excess revenues. As Minister of Health, you have the authority under the Patent Act to direct the PMPRB to inquire into any matter regarding patented medicine prices and report its findings back to you. Additionally, you are responsible for making recommendations to Cabinet on changes to the patented medicines regulations, which inform how the PMPRB fulfills its mandate.
Significant changes were announced in 2019 to modernize the Patented Medicines Regulations in order to bring Canadian drug prices in line with those of other countries that have similar economies and health care systems. These regulatory changes will come into effect on July 1, 2020, and will ensure that the PMPRB has the tools and information needed to fulfill its mandate in the modern context.
To provide more timely access to the new drugs Canadians need, Health Canada is taking steps to streamline regulatory processes to make them more efficient. It is also working to align its review process with health partners, such as the Canadian Agency for Drugs and Technologies in Health (CADTH) to reduce the time between Health Canada approvals and reimbursement recommendations to Canada's public drug plans.
Did You Know?
- High Drug Prices Canada has the third highest patented drug prices in the world.
- Growing Drug Costs Average drug costs per person in Canada have grown from $47 in 1975 to $1078 in 2019, outpacing all other health care costs.
- Specialty Drugs are prescribed to less than 1 per cent of Canadians yet account for 30 percent of all drug spending.
- One in Five Canadians struggle to pay for their prescription medicines.
- Three Million Canadians don't fill their prescriptions because they can't afford to do so.
- One Million Canadians cut spending on food and heat to be able to afford their medicine.
Investing in Pan-Canadian Health Organizations to drive progress on health system priorities
Health Canada funds eight arm's-length pan- Canadian Health Organizations (PCHOs), which were created by the federal government (and in two instances, in partnership with provinces and territories) over the last thirty years to address specific needs and issues. The PCHOs provide national leadership through a diverse range of projects and initiatives on a number of pan- Canadian priorities, including assessing the cost- effectiveness of drugs; electronic prescribing; health system information; national strategies on pressing health issues like cancer control and mental health; patient safety; and health care improvement projects.
In 2019-20, approximately $285 million is being invested in these organizations.
Did You Know?
- The Canadian Agency for Drugs and Technologies in Health provides reimbursement recommendations and advice for all of Canada's federal, provincial, and territorial public drug plans (with the exception of Quebec).
- The Canadian Institute for Health Information was pivotal in leading the development of the 12 pan-Canadian indicators to track progress under the Common Statement of Shared Health Priorities and Bilateral Agreements.
- In 2019, the Canadian Partnership Against Cancer modernized the Canadian Strategy for Cancer Control – a 10-year roadmap to improve equity in the cancer system and deliver world-class cancer care to all Canadians.
Pan-Canadian Health Organizations (PCHOs)
- Canadian Foundation for Healthcare Improvement
- Canada Health Infoway
- Canadian Agency for Drugs and Technologies in Health
- Canadian Institute for Health Information
- Canadian Patient Safety Institute
- Canadian Partnership Against Cancer
- Mental Health Commission of Canada
- Canadian Centre on Substance Use and Addiction
Managing federal grants and contributions programs to support health system innovation and priorities
The Health Portfolio supports many other health care initiatives through targeted project funding to a variety of partners, including health research organizations, professional and regulatory bodies, and partners at various levels of government (i.e., federal, provincial/ territorial or municipal). These targeted federal initiatives address key system challenges, such as health system innovation; home, community and end-of-life care; mental health and addiction services; health promotion and chronic disease prevention; family/gender-based violence prevention; infectious disease prevention and control; and access to health services for official language minority communities. For example, Health Canada has provided funding to Pallium Canada to expand their successful Learning Essential Approaches to Palliative Care program to train more health care providers and others in palliative care so that more Canadians can access palliative care when and where they need it.
Other funding initiatives include PHAC's Multi-sectoral Partnerships to Promote Healthy Living and Prevent Chronic Disease, which test and scales up promising initiatives in areas such as physical activity, healthy eating, smoking cessation and the creation of supportive social and physical environments. PHAC also funds programs in communities across Canada to reach the most at-risk populations, in order to improve health outcomes related to areas such as early childhood development, sexually-transmitted and blood-borne infections, and dementia.
B. Enabling access to safe and effective health products
Assessing and regulating health products to ensure their safety, effectiveness and quality
Health products - including prescription and non-prescription drugs (both pharmaceutical and biologics), veterinary drugs, and medium and high-risk medical devices - are subject to thorough pre-market oversight, which includes drug clinical trials and investigational testing for medical devices.
The purpose of this pre-market oversight is to ensure that the authorization of new products are made in the public interest, and are based on factors such as existing scientific evidence to support their safety, quality and efficacy. Once products are on the market, Health Canada continues its regulatory role by monitoring their safety and effectiveness, as well as conducting compliance and enforcement activities. The graphic below sets out key points on this oversight continuum.

Long description
Schematic showing pre-market on the left and post-market oversight on the right. The following list of key points is from pre-market (left) through to post-market (right): pre-clinical trials, clinical trials, regulatory product submission, submission review, market authorization decision, public access & safety monitoring surveillance inspection compliance verification enforcement.
Regulatory Oversight in the Health Portfolio
Hundreds of new products are introduced by industry every year in Canada. Any health or consumer product, chemical, pesticide or food sold on the Canadian market must meet strict regulatory standards set by Health Canada. The Health Portfolio works to help Canadians lead healthier lives by providing access to products such as pharmaceuticals to improve their health, and by helping to protect them from products that are unsafe or high risk to their health (e.g. illegal opioids, tobacco). The Portfolio regulates tens of thousands of products and monitors them through the administration and enforcement of over 160 regulations in more than 40 Acts.
A number of regulatory tools are used to review, assess and monitor products (see Annex I). The extent of the review of a given product generally depends on its risk level. For example, while health products (including prescription and non- prescription drugs) and pesticides are subject to pre-market oversight (e.g., clinical trials for drugs, exposure modelling for pesticides) as well as post-market measures (e.g. safety monitoring, recalls), consumer products (such as toys and appliances) are managed through a robust post-market regime that includes the development of guidelines and outreach activities for industry, the development of national and international voluntary safety standards, and proactive testing and inspections to support regulatory compliance.
The Health Portfolio also regulates to protect Canadians from threats posed by infectious diseases. PHAC works with the Canadian Border Services Agency to prevent the introduction and spread of communicable diseases that are of significant harm to public health. Risks posed by human pathogens and toxins to human health and safety are also mitigated through the regulation and licensing of facilities working with human pathogens and toxins.
Modernizing regulatory regimes to increase access to safe and effective treatment options for Canadians
The Portfolio operates in a complex and fast-paced environment, which requires continuous adaptation and modernization of its operations. Product innovation, the increased pace of change, the shift from a domestic to a global marketplace and supply chains, and the proliferation of readily accessible health information are all reasons why Health Canada is transforming its regulatory approach through the modernization of its legislative and regulatory frameworks and strengthening its oversight of products for which it is responsible.
For example, Health Canada is currently working on more adaptive and timely regulatory approaches for innovative therapeutic technologies and practices (e.g., advanced cellular therapies, 3D-printed implants, and artificial intelligence-powered diagnostic software). Amendments to key regulatory frameworks will create new oversight to ensure the health and safety of Canadians while reducing delays and barriers in authorizing these products for the Canadian market.
Did You Know?
- 3D Bioprinting is the creation of an object by building many layers of living cells. The process is highly dependent on software, with a variety of printing techniques being used and continuously updated.
- The potential for future uses is staggering, from bioprinting of tissues and organs for transplantation, to pre- clinical testing and personalized drug development.
- Health Canada has developed a new pathway to enable the use of customized regulatory requirements. This will allow for the agility and flexibility necessary for the appropriate oversight of complex technology, such as the application of 3D bioprinting at the point of care.
Did You Know?
Health Canada regulates:
- More than 14,000 prescription and non-prescription drugs
- More than 700 biologics and biotechnology products
- More than 1,500 veterinary drugs and over 1,700 low-risk veterinary health products
- More than 100,000 natural health products (e.g., vitamins, minerals, traditional and homeopathic products)
- More than 35,000 medical devices (e.g., pacemakers)
- More than 7,700 registered pesticides
Health Canada also conducts over 14,000 inspections annually across the above products lines
PHAC regulates:
- More than 1000 facilities that handle human pathogens and toxins
CFIA conducts:
- Approximately 3000 food safety investigations and an average of 240 primary recalls and 428 total recalls each year
Monitoring health product safety and adverse events arising from use and communicating risks to Canadians
By continuing to monitor health products that have been authorized for sale on the Canadian market, Health Canada works to prevent adverse health risks and events for Canadians.
Examples of post-market surveillance include collecting adverse event reports, addressing advertising complaints and conducting post-market safety reviews.
C. Managing Risks to Health
Managing food-related health risks through strong food safety regulations, surveillance and enforcement
Canada is recognized as having one of the strongest food safety systems in the world. The safety of food is vital to all consumers and food businesses. Consumers want to be confident that the food they buy and eat is what they expect, and that it will cause them no harm. Consumer confidence is very important for food businesses. Food safety affects all Canadians. It is the responsibility of all food businesses, no matter how large or small, to ensure anyone who is importing, exporting, or sending food across provincial boundaries has not compromised food safety.
Did You Know?
- The Safe Food for Canadians Regulations
make our food system even safer by focusing on the food industry's prevention of food safety risks during production and allowing for faster removal of unsafe food from the marketplace. - Under these regulations, as of January
15, 2019 new licensing, preventive control and traceability requirements apply to food businesses that import or prepare food for export or to be sent across provincial or territorial boundaries.
Assessing and managing the health risks of consumer products
Similar to other regulators around the world, Health Canada does not pre-approve or pre-test the millions of consumer products and cosmetics available to Canadians before they reach the market. Instead, consumer products (including toys) and cosmetics are regulated under a post- market regime. Under this regime, Health Canada works to promote understanding by regulated parties about their responsibility to protect consumers and their responsibility to ensure that products available in Canada comply with the law and regulations. As well, Health Canada assess product hazards to better understand their existing or potential risks to human health and safety. This work is supported by an active compliance and enforcement program that works to verify that consumer products meet the requirements set out under the Canada Consumer Product Safety Act and its regulations.
Implementing comprehensive approaches to minimize the health risks associated with legal and illegal substances
The Health Portfolio works with partners to address the harms associated with substance use, including both legal substances (e.g., alcohol, cannabis and tobacco/vaping products) and illegal substances (e.g., illegal opioids such as heroin and fentanyl, methamphetamines, and other substances).
Canada continues to experience an unprecedented overdose death crisis that is one of the most significant public health threats in a generation, and has caused the deaths of at least 12,813 Canadians between January 2016 and March 2019. In 2018 alone, there were 4,588 apparent opioid-related deaths in Canada, which corresponds to one life lost every two hours. The issue is so severe that male and female life expectancy in Canada has stopped increasing for the first time in forty years. Health Canada is working with federal partners, provinces and territories to advance a range of actions to help address the crisis, anchored in public education and awareness, prevention, and treatment.
Another key area of focus is tobacco and vaping. Through tight controls on the manufacture, sale, labelling and promotion of tobacco products, Health Canada has succeeded in reducing tobacco use. However, although smoking prevalence has fallen from 35% in 1985 to 15% in 2017, tobacco use is still the leading preventable cause of premature death and disease in Canada.
The recent Tobacco and Vaping Products Act (May 2018) provided Health Canada with new tools and regulatory levers for regulating tobacco and vaping products and protecting youth from nicotine addiction. This includes restrictions on both advertising of tobacco and vaping products as well as prohibiting giving these products to persons under 18 years of age.
While rates of youth smoking have declined in recent years, there has been a rapid rise in youth vaping. Concern about this issue has been amplified by the recent emergence of severe lung illness related to vaping in the United States and Canada.
Lastly, alcohol is the most prevalent substance used in Canada and results in significant public health harms. Its normalization and problematic use leads to nearly 15,000 deaths a year. Public health and other major stakeholders are calling for increased federal leadership to improve regulatory and other controls to minimize harms.
Overseeing the new legal cannabis framework
In October 2018, the Cannabis Act came into force, putting in place a new national control framework for the production, distribution, sale and possession of cannabis in Canada. The Act seeks to protect the health and safety of Canadians by, among other things, restricting youth access to cannabis, protecting young persons and others from inducements to using cannabis, and deterring or reducing illegal activities by providing for the legal production of cannabis and through appropriate sanctions and enforcement.
When the Act came into force last October, only fresh cannabis, dried cannabis, cannabis oil, cannabis seeds and cannabis plants were permitted for legal sale. Parliament established a deadline of October 17, 2019 for additional new products, namely edible cannabis, cannabis extracts and cannabis topicals, to be permitted for legal sale. On that day, amendments to the Cannabis Regulations also came into force, putting in place regulatory controls to help lower the known risks of these products and safeguard the health of adult consumers. Since Health Canada must be notified 60 days before new products are made available for sale, these additional products are expected to only gradually appear in stores, no earlier than December 16, 2019.
Health Canada licences the production of cannabis (i.e., cultivators and processors) and promotes and enforces compliance with the Act and its regulations. As of October 16, 2019, there were 243 licensed sites that cultivate or process cannabis across Canada. Provinces and territories oversee distribution and sales within their jurisdictions.
Maintaining readiness to respond to public health threats and emergencies
i. Developing health security measures / Preparing for and responding to health emergencies
Public health emergency management is a shared responsibility among all levels of government that requires a high degree of engagement and coordination. As Minister of Health, you are responsible, under the Emergency Management Act, for emergency preparedness and response to national and international public health events, such as H1N1 or SARS. As well, you are responsible for coordinating the federal response to a nuclear emergency, such as an emergency at a nuclear power plant in Canada, the United States or abroad. Both PHAC and Health Canada also prepare for large domestic events such as G7, G20 or Olympic Games by providing public health and nuclear emergency response resources and expertise.
The Health Portfolio supports you in your role via a number of health security resources, including the Health Portfolio Operations Centre, National Microbiology Laboratory, and the National Emergency Strategic Stockpile. PHAC also acts as Canada's focal point for the International Health Regulations, which facilitates rapid communication with domestic and international stakeholders in response to global public health risks.
ii. Supporting border and travel health
PHAC works with the Canada Border Services Agency to administer the Quarantine Act to prevent the introduction and spread of communicable diseases in Canada. For example, an ill passenger getting off an international flight in Canada, who displays symptoms compatible with a communicable disease, would be referred to PHAC for assessment.
iii. Providing domestic and international health security leadership
Domestically, the pan-Canadian Public Health Network plays an important role. It provides a governance structure to support the federal government, provinces and territories to collaborate on public health issues and share information across jurisdictions. Federal, provincial and territorial governments work in partnership on planning, training and exercises for public health threats. In addition, Canada has federal-provincial/territorial agreements, frameworks, and plans in place to clarify roles and responsibilities and to facilitate cooperation during emergencies.
Canada also engages with international partners (e.g., the World Health Organization, the United States, the United Kingdom, Mexico and G7 partners), to address health security threats that have the potential to impact Canadians at home and abroad. Canada's strategic and targeted engagement in international efforts, such as responding to the Ebola outbreak in the Democratic Republic of Congo, results in reduced risk that threats will reach the Canadian border; strong relationships and access to global networks and information; and opportunities for PHAC staff to develop and maintain emergency response skills.
Canada's National Microbiology Laboratory is known around the world for its scientific excellence. The NML enables informed public health action through delivery of innovative approaches to advance laboratory science, testing services, lab-based surveillance, outbreak response and national public health laboratory leadership. It provides Canadians with the scientific readiness to respond to infectious disease threats. The National Microbiology Laboratory works closely with key federal and provincial/territorial public health partners and health care stakeholders both internationally and domestically.
Did You Know?
- PHAC has world-class research programs that are essential to Canada's response to national and international infectious disease threats.
- PHAC's National Microbiology Laboratory in Winnipeg works with public health partners in Canada and internationally to prevent the spread of infectious disease. It is the only "Level 4 lab" in Canada, meaning it is able to work with the world's most dangerous pathogens.
iv. Protecting against vaccine-preventable diseases
Vaccinations are one of the most effective public health strategies for protecting populations against infectious disease threats. PHAC collaborates with provincial/territorial governments, academia, and professional associations to maximize the impact of vaccination programs.
Improving vaccination access and uptake further protects Canadians from infectious diseases. PHAC supports bulk procurement of vaccines for provinces and territories and funds capacity- building projects through the Immunization Partnership Fund. PHAC also collaborates to develop recommendations with the National Advisory Committee on Immunization.
v. Responding to the threat posed by antimicrobial resistance
In 2018, the estimated number of antimicrobial resistance (AMR)-related deaths in Canada was 5,400. The Health Portfolio is responding to the rise of AMR, which threatens to make routine treatments of infections ineffective, through a number of activities. Federal departments are working with provincial/territorial partners and other stakeholders to develop the pan- Canadian Action Plan on AMR, conducting surveillance to monitor the spread of AMR, as well as public and professional education awareness campaigns. The Chief Public Health Officer's 2019 Spotlight Report, Preserving antibiotics now and into the future, highlights the importance of responsible antibiotic use in human medicine.
Health Canada has leveraged its regulatory powers to increase oversight of antimicrobials available for use in animals in order to preserve the effectiveness of antimicrobials and prevent the development of resistant organisms. Additionally, CIHR has invested $115M in AMR research over the last five years, and CFIA supports the prudent use of antimicrobials as they relate to animal health and welfare, livestock feeds, and food safety. This multi-sectoral approach recognizes that responding to the threat posed by AMR requires close coordination across human health, animal health and agri-food sectors in order to protect Canadians.
Recognizing the international nature of the threat posed by AMR, Canada also actively engages multilaterally through a number of fora including the Trans-Atlantic Task Force on AMR, and the Global Health Security Agenda. At the 2017 World Health Assembly, Canada joined the Alliance of Champions, a group of Health Ministers and health leaders committed to combatting AMR and to increasing political awareness, engagement and leadership on AMR.
Reducing environmental health risks
The environment has an important impact on human health. Health Canada shares responsibility with Environment and Climate Change Canada (ECCC) for the Canadian Environmental Protection Act, 1999 and the Impact Assessment Act. Health Canada assesses and manages potential risks to human health posed by new and existing chemicals under the Chemicals Management Plan, a joint program with ECCC. As well, Health Canada participates in impact assessments as a federal authority, by providing expert advice on the health effects related to air quality, drinking water, noise and radiation, and traditional foods.
The Health Portfolio works to protect the health of Canadians from environmental risks by conducting research and surveillance, creating guidelines, managing programs and advising other government departments (federal or provincial/territorial) on the potential health effects of environmental risks (for example, water quality guidelines, air quality standards, health impacts from radiation, and noise). Health Canada helps Canadians and health authorities understand the impacts of climate change on health, and informs adaptation strategies to minimize adverse health effects. PHAC actively monitors and responds to the increase in climate-driven zoonotic, food-borne, and water-borne infectious diseases such as Zika and Lyme, through surveillance, funding programs, and education and awareness activities.
Health Canada works closely with Environment and Climate Change Canada to conduct risk assessments and takes risk management actions when necessary (e.g., chemicals used in Canada, such as those in flame retardants). These actions can include the development of new or amended regulations, conditions for industry related to acceptable uses of chemicals, guidelines, or pollution prevention plans.
D. Supporting health research and science, data collection and surveillance capacity
Funding research that generates new knowledge, improves health or health services, informs priorities and supports health innovation
Federal funding for health research is a direct way in which the federal government works to advance innovation and improve the health of Canadians. The Health Portfolio contributes to health research by supporting the work of academics, researchers and research institutions (e.g., universities, hospitals, research centres), and by building capacity through support for the next generation of researchers.
The primary research arm of the Health Portfolio is CIHR, which invests over $1B annually to support more than 13,000 world-class researchers and trainees in a broad range of research, from basic science through knowledge translation and policy. CIHR's work supports:
- Investigator-initiated research (ideas initiated by researchers and trainees);
- Priority-driven research (addressing pressing health issues, knowledge gaps and the health priorities of Canadians); and
- Ensuring capacity and training of the next generation of health researchers.
Did You Know?
- CIHR: Provides support to a network of over 13,000 health researchers and trainees.
- PHAC: Supports research on infectious disease prevention and control, health promotion, and chronic disease prevention, and publishes information for Canadians.
- Health Canada: Supports brain research through the Canada Brain Research Fund, whereby private and charitable funds raised by the Brain Canada Foundation will be matched by Health Canada, up to $120M by 2020.
The Health Portfolio is also involved in international research activities to address emerging global health threats. For example, CIHR is currently engaged with the European-based Joint Programming Initiative on Antimicrobial Resistance and is working with more than 20 partner countries to address knowledge gaps related to AMR. This collaboration allows for alignment of national and international investments towards coordinated global research activities, and will inform future interventions to prevent, limit and control the emergence and spread of antimicrobial resistance in humans, animals and food.
Funding national collaborating centres that promote the use of evidence by public health practitioners and policy makers
PHAC funds National Collaborating Centres for Public Health that synthesize and share public health knowledge, making it useful and accessible to policy makers, program managers and public health practitioners. The centres have an ability to bring together stakeholders and foster relationships that are conducive to further advancing health- related policy development. For example, tools for analyzing public policies, developed through the National Collaborating Centres, have helped inform public health staff and policy makers on the effects of policy implementation in relation to cost, feasibility and equity.
Strengthening surveillance, evidence and public education on chronic and infectious disease
National public health surveillance requires the collection and analysis of data from many sources in order to assess health trends and risk severity, and to report findings. Surveillance data include information relating to diseases, illness and health behaviours, substance-related harms, maternal and child health, and related determinants of health. PHAC surveillance experts work in close collaboration with provincial/ territorial governments and public health agencies to assemble the national picture of health in Canada. PHAC also collaborates with provinces, territories and stakeholders to enhance national laboratory capacity, to strengthen surveillance systems and the capacity to monitor and report on health inequalities, and to leverage new technologies (e.g., artificial intelligence, genomics) to better track, report and address public health concerns.
Did You Know?
PHAC has approximately 50 surveillance systems and data holdings used to generate reports, bulletins and health advisories, and to inform the development of programs and policies. Here are a few:
- Canadian Chronic Disease Surveillance System
- Canadian Integrated Program for Antimicrobial Resistance Surveillance
- Canadian Measles/Rubella Surveillance System
- Canadian Paediatric Surveillance Program
- Canadian Perinatal Surveillance System
- FluWatch - Influenza Surveillance
- FoodNet Canada
- HIV and AIDS Surveillance
- Lyme Disease Enhanced Surveillance System
- Pan-Canadian Health Inequalities Reporting Initiative
- Tuberculosis Prevention and Control Surveillance Reports
E. Supporting Canadians in making safe and healthy choices
Promoting the health and well-being of Canadians by addressing the root causes of health inequalities and the risk factors to prevent disease
The Health Portfolio engages in a range of activities to improve health outcomes for Canadians, including programming focused on the prevention and control of infectious diseases, mental health promotion, injury prevention, and chronic disease prevention. The Portfolio supports the promotion of healthy behaviours to alleviate future costs to our health care system and to foster the development of healthier and more resilient communities.
The Health Portfolio funds programs that support at-risk populations at various life stages. For example, PHAC funds a number of programs that support early child development for vulnerable populations, community-based initiatives, and mental health promotion approaches for children and youth. PHAC's Chief Dental Officer is also a Portfolio resource, advancing population-level oral health, with an emphasis on vulnerable populations. PHAC also produces a range of health promotion materials on topics such as folic acid, pregnancy, postpartum care, prevention of fetal alcohol spectrum disorder, breastfeeding, safe sleep for infants, and parenting, which are used by thousands of community-based stakeholders across Canada each year.
In support of seniors and healthy aging, PHAC launched Canada's first National Dementia Strategy in June 2019, and works with partners to advance understanding and improve the quality of life of those living with dementia and their caregivers. PHAC also supports the Pan-Canadian Fall Prevention Network to reduce the incidence of falls and hospitalization among seniors.
Chronic diseases, such as diabetes, cancer, cardiovascular diseases and chronic respiratory diseases, are among the most common, costly and preventable health problems in Canada and globally. The common causes - unhealthy diets, physical inactivity, tobacco use and harmful use of alcohol - are complex societal issues. Some groups of Canadians are more affected by chronic diseases than others, including Indigenous Peoples and those with lower income and education levels.
CIHR supports research to better understand chronic diseases and the complex societal issues that lead to them.
PHAC helps to protect Canadians from infectious diseases, including sexually-transmitted blood-borne infections (e.g., the Human Immunodeficiency Virus), foodborne illness (e.g., E. coli) and vaccine-preventable diseases (e.g., measles). PHAC works to predict, detect and respond to outbreaks and new threats, contributing to the prevention, control and reduction of these diseases among Canadians.
Did You Know?
CIHR's Canadian Consortium on Neurodegeneration in Aging serves as a research hub for research involving neurodegenerative diseases that affect cognition in aging, including forms of dementia.
Promoting healthy behaviours and practices to improve health
PHAC uses a range of programs and partnerships to promote good physical and mental health and reduce risks for major chronic and infectious diseases, informed by surveillance, science and evidence. Among these are the Healthy Living and Chronic Disease Prevention Multi-sectoral Partnerships program, which tests effective and innovative community- based projects. PHAC also supports the Canadian Task Force on Preventive Health Care to develop clinical practice guidelines that support primary care providers in delivering preventive health services.
Did You Know?
PHAC serves over 279,000 vulnerable prenatal women, children aged 0-6 years, and their families through the Canada Prenatal Nutrition Program, Community Action Program for Children and Aboriginal Head Start in Urban and Northern Communities program.
Supporting Canadians in making better food choices
Healthy eating is fundamental to good health and is equally important in reducing the risk of many chronic diseases. Poor diet is the number one risk factor for obesity and chronic disease burden in Canada. In addition to promoting healthy eating, ensuring Canadians have access to information to make healthier choices and improving food quality are key to achieving healthier outcomes.
Working collaboratively with federal partners, provinces and territories and a range of other stakeholders, Health Canada develops and implements evidence-based policies, guidelines and regulations that apply to foods sold in Canada and promote environments that support Canadians in making healthier food choices. The Department is recognized as an authoritative source of nutrition and food safety information that Canadians trust.
Health Canada sets food safety and nutrition policies, guidelines and regulations to help Canadians make healthier food choices. For example, clear and legible labels are required, which declare the ingredients found in foods as well as instructions for the safe use and handling of certain foods. A Nutrition Facts Table is required on most pre-packaged foods to allow consumers to make informed choices. Food labels must also clearly identify the presence of priority food allergens to help consumers with allergies avoid foods that could trigger adverse reactions.
As part of its role, Health Canada promotes safe and healthy eating using various tools including Canada's Food Guide, food labelling, and safety advisory information, such as how to safely prepare foods at home.
Did You Know?
- A new version of Canada's Food Guide was launched in January 2019, with updated evidence review and web-based tools.
- A Food Guide Snapshot was released in 29 additional languages in July 2019, including nine Indigenous and Inuit languages.
Health Canada also sets standards and promotes the prudent use of veterinary drugs in food- producing animals, and in doing so, works to protect Canada's food supply.
PHAC supports community-based promotion of safe and healthy eating under its multi-sectoral partnership program and integrates safe and healthy eating promotion into other healthy living initiatives. In addition, the CFIA is responsible for overseeing industry compliance with regulations aimed at ensuring that the food supply is safe.
Informing and engaging Canadians by being a trusted source of information on health and safety
The Health Portfolio is responsible for providing timely information about food recalls and safety alerts, drug and health products, public health concerns, pesticides, and nutritional information, as well as consumer products. Canadians look to you and the Portfolio for trustworthy and timely information they need to make informed choices for themselves and their families.
For example, CFIA is responsible for making public food recall warnings via its website. PHAC will similarly ensure the public is alerted when there are any food-related outbreaks that could be harmful to their health. Health Canada implements ongoing, proactive marketing strategies that promote the adoption of safe food handling and preparation practices.
Additionally, Health Canada and PHAC support public awareness regarding the risks associated with cannabis, opioid and other substance use, and continue to work collaboratively to ensure that Canadians, particularly youth, are educated on the negative impacts associated with substance use. For example, in 2018 Health Canada launched the Your Cannabis Questions, Answered: Get the Honest Facts campaign that has garnered more than 69 million online impressions.
Did You Know?
- Health Canada: Canadians can access the Canada.ca/Health website at any time to search for specific recalls and safety alerts.
- CFIA: Food recalls and warnings are updated regularly and are easily accessible through CFIA's inspection.gc.ca website.
- PHAC: Information regarding food-related outbreaks is always available to Canadians on the Canada.ca/Health website.
IV. Engagement With Key Partners
Other Federal Government Departments
At the federal level, the Health Portfolio works collaboratively with a number of other government departments and agencies. Some examples include:
- Public Safety Canada (public health and safety emergencies);
- Justice Canada (medical assistance in dying; controlled substances enforcement);
- Employment and Social Development Canada (seniors, caregivers and social determinants of health);
- Indigenous Services Canada (Indigenous health);
- Environment and Climate Change Canada (chemicals management; environmental health initiatives, including climate change);
- Impact Assessment Agency (environmental assessments);
- Innovation, Science and Economic Development Canada (innovation and emerging technologies);
- Natural Resources Canada (security of the medical isotope supply);
- Women and Gender Equality Canada (gender-based violence); and
- Canadian Heritage (Sport Canada) (concussions).
Health partners and industry stakeholders
The Health Portfolio often works with other partners and stakeholders, including health professionals, institutions, patient groups, labour unions, industry associations, universities, research hospitals, international research organizations, other federal departments and agencies, and the Canadian public. These interactions inform policy development, advance common objectives, and contribute to effective exercise of the Portfolio's regulatory functions.
The Portfolio can also act as a partnership mobilizer, bringing together individuals, communities, the private sector, the charitable sector, other levels of government, and organizations outside the traditional health sector. These partnerships often contribute to increasing the impact and sustainability of new or emerging initiatives.
Indigenous partners
Portfolio engagement with Indigenous organizations and governments at the national and regional level informs the development of programs and policies. The Portfolio also contributes to whole-of- government efforts to implement the Truth and Reconciliation Commission's Calls to Action.
Did You Know?
-
CIHR's Network Environments for Indigenous Health Research program is a national network of centres focused on capacity development, research, and knowledge translation centred on Indigenous Peoples. It aims to provide supportive research environments for Indigenous health research driven by, and grounded in, Indigenous communities in Canada.
International partners
The health of Canadians is linked to complex global issues. As Minister of Health, you play a lead role on a range of international activities that support your mandate to promote, preserve and protect the health of Canadians. You will typically engage multilaterally by leading Canada's delegation at international and political fora, and bilaterally with key counterparts from other countries or regional partners.
Engaging in multilateral fora, such as the World Health Organization, the Organisation for Economic Co-Operation and Development, and meetings of G7 and G20 Health Ministers, allows Canada to maintain awareness of emerging issues and advance health issues that matter to Canadians, and to work with likeminded partners on global health challenges. As Minister of Health, you will also engage with counterparts from other countries or regions to advance bilateral collaboration on health issues of common concern. For example, internationally, Canada has been advancing mental health and well-being as a health priority, by working with key international partners such as the World Health Organization and stakeholders, and launched the Alliance of Champions for Mental Health and Wellbeing with Australia and the United Kingdom.
Annex I: Regulatory Oversight of Products Pre- and Post-market
| "Pre-market": Before a product is on the market |
"Post-market": Once a product is already on the market |
|
|---|---|---|
Prescription drugs (human and veterinary use) |
Health Canada conducts scientific reviews of drugs for:
|
Health Canada:
|
Non- prescription products (human and veterinary use) |
Health Canada conducts scientific reviews of drugs for:
|
Health Canada:
|
Natural health products |
Health Canada reviews products on a risk basis for:
|
Health Canada:
|
Medical Devices |
Health Canada reviews devices on a risk basis for:
|
Health Canada:
|
Biologics and Radiopharma-ceuticals |
Health Canada reviews biologics and radiopharmaceuticals for:
|
Health Canada:
PHAC:
|
Food |
Health Canada:
|
CFIA:
Health Canada:
PHAC:
|
Consumer Products and cosmetics |
Health Canada:
|
Health Canada:
|
Chemical Substances |
Health Canada and Environment and Climate Change Canada:
|
Health Canada and Environment and Climate Change Canada:
|
Pesticides |
Health Canada:
|
Health Canada:
|
Controlled Substances |
Health Canada:
|
Health Canada:
|
Tobacco and vaping products |
Health Canada:
|
Health Canada:
|
Cannabis |
Health Canada:
|
Health Canada:
|
Workplace Hazardous Products |
Health Canada:
|
Health Canada:
|
Health Canada Overview Deck
Health Portfolio
November 2019
Health Canada's Mandate
Health Canada is the federal department responsible for helping the people of Canada maintain and improve their health
Health Canada's Core Business Lines
| Strengthening Canada's health care system | Enabling access to safe and effective health products | Managing risks to health | Supporting Canadians in making safe and healthy choices |
|---|---|---|---|
| Supporting universally accessible, publicly funded health care for Canadians through administration of the Canada Health Act, leadership on emerging issues, and cooperation with provinces and territories on system improvements | Enabling access to safe and effective health products by assessing and regulating health products, such as drugs and medical devices, to ensure their quality, safety, and effectiveness | Managing the health risks of harmful substances, cannabis, consumer products, chemicals and pesticides, reducing environmental health risks, and supporting Canadians in making healthy choices | Supporting Canadians in making safe and healthy choices through public education and awareness campaigns to communicate health and safety information |
Core Business Lines
Strengthening Canada's health care system:
Health Canada acts as the steward of medicare for Canadians and provides leadership and support for Canada's public health care system
- Administers and ensures compliance with the Canada Health Act, in accordance with the key principles of public health insurance: public administration, comprehensiveness, universality, portability, accessibility, and no patient charges
- Works multilaterally and bilaterally with provinces and territories on common health priorities, and oversees bilateral funding agreements to improve access to key services (currently mental health and addictions; and home, community and palliative care)
- Partners with and funds eight Pan-Canadian Health Organizations to catalyze system innovation and improvements in priority areas including cancer, mental health, problematic substance use, patient safety, health care delivery, assessment and appropriate use of drugs and health technologies, health information, and digitization
- Provides advice and leads pan-Canadian initiatives on system innovation and quality improvements in new and emerging areas of health care (e.g., organ donation and transplantation, medical assistance in dying, and virtual care)
- Manages federal health grants and contributions programs supporting health system innovation/priorities and minority official language communities
- Advances the affordability, accessibility, and appropriate use of prescription drugs
- Improves cultural safety, humility and responsiveness in the health care system to address systemic barriers and improve Indigenous health outcomes
Enabling access to safe and effective health products:
Health Canada is the science-based regulator that ensures the safety, effectiveness and quality of health products
- Provides regulatory oversight through pre- and post-market risk assessments, as appropriate, of a wide range of health products including drugs, biologics (such as vaccines and tissues), medical devices, and veterinary drugs
- Ensures that appropriate regulatory pathways are available to bring treatment options to Canadians (e.g., Special Access Program for critical drugs that are not sold in Canada)
- Leads scientific and technical analysis of health product submissions to ensure the safety, efficacy and quality of approved drugs
- Oversees clinical trials conducted by industry to ensure that clinical trials are available to Canadians for cutting edge products
- Monitors health product safety and adverse events arising from real- world use and communicates risks to Canadians
- Optimizes the use of real world evidence for regulatory decision- making to improve the extent and rate of access to medical products in Canada
Managing risks to health:
Health Canada helps Canadians manage the health risks of consumer products, chemicals, pesticides, cannabis and harmful substances
- Regulates and sets safety standards for consumer products, natural health products, food, chemicals and pesticides through pre- and/or post-market risk assessments
- Takes appropriate compliance and enforcement action in relation to acts and regulations administered by Health Canada
- Provides information to Canadians so they can make informed decisions about their health (e.g., recalls, product seizures, drug shortages)
- Conducts research, monitoring and surveillance on environmental health and the health impacts of climate change
- Oversees the legal cannabis regime: implements cannabis policy direction, grants licences for production, and monitors compliance with regulations
- Develops and implements comprehensive approaches to manage the risks associated with harmful substances such as opioids, tobacco and alcohol
Supporting Canadians in making safe and healthy choices:
Health Canada assesses, manages and communicates health and safety risks and benefits to support Canadians in making safe and healthy choices
- Works with domestic and international partners to assess, manage and communicate the health and safety risks and benefits associated with health and consumer products, food, chemicals, pesticides, environmental factors, tobacco and controlled substances
- Conducts public awareness campaigns to educate Canadians on safe food practices
- Supports Canadians in making healthier, safer food choices through the Canada Food Guide and other healthy eating initiatives
- Informs and engages Canadians as a trusted source of information on health and safety
- Undertakes public education and awareness activities, targeting youth and young adults in particular, to provide Canadians with the information they need to make informed decisions and minimize health and safety harms associated with cannabis use
- Provides public education resources, information and programming on smoking prevention, cessation, health effects and youth vaping, to reduce tobacco-related diseases and deaths
Health Canada's Key Partners
- Provincial and Territorial Governments
-
- Multilateral and bilateral machinery (including annual FPT Health Ministers' Meetings and the Pan-Canadian Public Health Network)
- Health System Partners
-
- Eight Pan- Canadian Health Organizations
- National non- governmental organizations (including health professional associations, regulatory and accreditation authorities, disease-specific organizations)
- Patient organizations
- Advocacy groups
- Industry / Consumer Groups
-
- Private sector enterprises (including manufacturers, distributors, and retailers)
- Industry associations
- Consumer groups
- Indigenous Partners
-
- National and regional First Nations, Inuit and Métis partners
- Indigenous Health Professional Organizations
- Federal Departments and Agencies
-
- Agriculture and Agri-Food Canada
- Innovation, Science and Economic Development Canada
- Public Safety Canada
- Canada Border Services Agency
- Environment and Climate Change Canada
- Indigenous Services Canada
- Department of Justice
- Global Affairs Canada
- Shared Services Canada
- Finance Canada
- Natural Resources Canada
- Women and Gender Equality Canada
- International Partners
-
- Multilateral fora (e.g., World Health Organization)
- Bilateral relationships with key partners and regions (e.g., U.S. Federal Drug Administration, European Medicines Agency, and Australian Therapeutic Goods Administration)
Health Canada Financial Overview
Budget by Core Business Line

Budget by Vote
Total Resources: 7,677 FTEs and budget of approximately $2.52B
Notes:
- Budgets based on 2019-20 Main Estimates (Operating expenditures includes Budget 2019 items)
- Internal Services includes Corporate Services, Communications, Finance and Legal Services; Departmental Executive figures are not included
- Totals may not add due to rounding
Long Description
Health Canada's Financial Overview - Budget by Core Business Line and Vote
These two pie charts depict the budget by core business line and budget by vote. The total budget is approximately $2.52 billion and resources include 7,677 full-time employees which is based on the 2019-2020 main estimates with operating expenditures including budget 2019 items. The total may not add due to rounding.
The budget by core business line is comprised of the following:
- 64 percent strengthening the health care system with $1.6 billion and 289 full-time employees;
- 24 percent enabling access to safe and effective health products and reducing health risks with $598 million and 5,690 full-time employees;
- 10 percent internal services including corporate services, communications, finance and legal services and not including departmental executive figures with $262 million and 1,698 full-time employees; and
- 2 percent budget implementation with $51 million.
The budget by vote is comprised of the following:
- 62 percent of vote 10 transfer payments (grants and contributions) with $1,556.4 million;
- 37 percent of vote 1 operating expenditures with $947.0 million; and
- 1 percent of vote 5 capital expenditures with $18.1 million.
Health Canada Organizational Structure
Long description
Health Canada's Organizational Structure begins with the Deputy Minister of Health, Dr. Stephen Lucas, and under his leadership are 8 branches, 1 agency, legal services, and a Chief Medical Advisor.
These branches include:
- Strategic Policy Branch (SPB) with Abby Hoffman as the ADM, and Marcel Saulnier as the Associate ADM;
- Health Products and Food Branch (HPFB) with Pierre Sabourin as the ADM, and Kendal Weber as the Associate ADM;
- Controlled Substances and Cannabis Branch (CSCB) with Jacqueline Bogden as the ADM, and Eric Costen as the Associate ADM;
- Health Environments and Consumer Safety Branch (HECS) with Robert Ianiro as the ADM;
- Regulatory Operations and Enforcements Branch (ROEB) with Stefania Trombetti as the ADM;
- Chief Financial Branch (CFOB) with Randy Larkin as the ADM and Chief Financial Officer;
- Corporate Services Branch (CSB) with Debbie Beresford-Green as the ADM;
- Communications and Public Affairs Branch (CPAB) with Jennifer Hollington as the ADM;
- the Pest Management Regulatory Agency (PMRA) with Peter Brander as the Executive Director;
- Legal Services with Samantha Maislin Dickson as the Executive Director and Senior General Counsel; and
- Chief Medical Advisor being Dr. Supriya Sharma
Public Health Agency of Canada (PHAC) Overview Deck
Health Portfolio
November 2019
PHAC Mandate
- Protect Canadians by preparing for and responding to public health events and emergencies
- Contribute to the prevention, control, and reduction of the spread of infectious diseases
- Promote healthy behaviours and efforts to prevent chronic diseases and injuries
- Collaborate with a wide range of partners to advance its mandate, including provinces, territories, other sectors and international partners
The Agency, created in 2004, is led by a President (Deputy Head). In addition, a Chief Public Health Officer of Canada (CPHO) was created through the Agency's enabling legislation to advise the Minister and President on public health matters
Core Business Lines
| Health Security Infrastructure | Infectious Diseases Prevention and Control | Health Promotion and Chronic Disease Prevention |
|---|---|---|
|
|
|
PHAC advances these responsibilities through: surveillance of diseases, illness and health behaviours; laboratory and scientific expertise; community-based grants and contributions funding; strategic partnerships with provinces and territories (PTs), international and civil society partners; public outreach and communications; and regulation.
Health Security Infrastructure
Protects Canadians from risks to public health by preparing for and responding to public health events and emergencies, addressing health and safety risks associated with the use of pathogens and toxins, and addressing travel-related public health risks
- Maintaining a National Emergency Strategic Stockpile (NESS) to support responses to national emergencies
- Deploying Agency experts domestically and internationally to support other jurisdictions
- Working closely with Federal-Provincial/Territorial (FPT), domestic and international partners to ensure integrated planning, readiness and response to public health emergencies
- Identifying and monitoring emerging global health events that may impact Canada
- Protecting the health and safety of the public against risks posed by pathogens and toxins (Human Pathogens and Toxins Act)
- Preventing entry of travel-related infectious diseases into Canada (Quarantine Act)
- Informing Canadian travellers on how to protect themselves from travel-related public health risks
Infectious Disease Prevention and Control
Protects Canadians from infectious diseases by conducting surveillance, predicting, detecting, assessing, and responding to outbreaks and new threats, and contributes to the prevention, control and reduction of the spread of infectious diseases
- Ensuring preparedness for pandemic influenza (e.g., h1n1), including pandemic vaccine supply management
- Promoting immunization and addressing vaccine hesitancy to reduce the rates of vaccine- preventable diseases (e.g., measles)
- Immunization Partnership Fund (approximately $3.0M/annually, 2016-2021)
- Addressing rising rates of sexually transmitted and blood borne infections (e.g., HIV)
- Community Action Fund ($26.4M/year) and Harm Reduction Fund ($7.0M/year)
- Responding to new and emerging infectious disease risks and threats
- Emerging diseases due to climate change (e.g., Lyme disease)
- Declining efficacy of antimicrobials/antibiotics
- Collaborating with Health Portfolio and PT partners to identify the source of food-borne illness outbreaks (e.g., E-Coli)
- Providing laboratory and scientific leadership
- National Microbiology Laboratory - Level 4 laboratory with the capacity to handle the most dangerous pathogens (e.g., Ebola)
Health Promotion and Chronic Disease Prevention
Promotes the well-being of Canadians by collecting and monitoring data, addressing the causes of health inequalities (e.g., low income, poor housing) and factors that are important to preventing chronic diseases and injury (e.g., physical activity, healthy eating)
- Improving the health of Canada's most vulnerable children and families
- Investing $112M annually in 3,000+ communities across Canada through the Canada Prenatal Nutrition Program, Community Action Program for Children, and Aboriginal Head Start in Urban and Northern Communities
- Reducing the rates of chronic diseases (e.g., cancer, heart disease, diabetes) in Canada through innovative partnerships to test and scale up new approaches to improve health behaviours
- Investing $20M annually through the Multi-sectoral Partnerships Fund
- Improving the mental health of Canadians by advancing initiatives that address key issues, including gender-based violence, post-traumatic stress disorder, and suicide prevention
- Responding to the challenges faced by those living with dementia and their caregivers through the implementation of a National Dementia Strategy
- In collaboration with Portfolio partners, supporting the Government of Canada's efforts to respond to public health concerns such as the opioid crisis and vaping, including conducting surveillance and addressing upstream causes of problematic substance use
PHAC's Partners
| International Partners | Provincial and Territorial Governments | Federal Departments and Agencies | Other Stakeholders | |
|---|---|---|---|---|
Minister's Role |
Plays a leading role in international activities related to public health by advancing priorities at various fora (e.g., G7 and G20 Health Ministerial Meetings, World Health Assembly; and United Nations General Assembly) |
Co-chairs Federal-Provincial/Territorial (FPT) Health Ministers' Meetings and the FPT Ministers of Sport, Physical Activity and Recreation Table |
Works with Cabinet colleagues and bilaterally with counterparts to advance key public health priorities:
|
|
Agency's Role |
|
Collaborates on:
Participates in or supports:
|
Works closely with a number of departments and agencies responsible for ensuring healthy lives and promoting well-being including:
|
|
PHAC Financial Overview
Budget by Core Responsibility
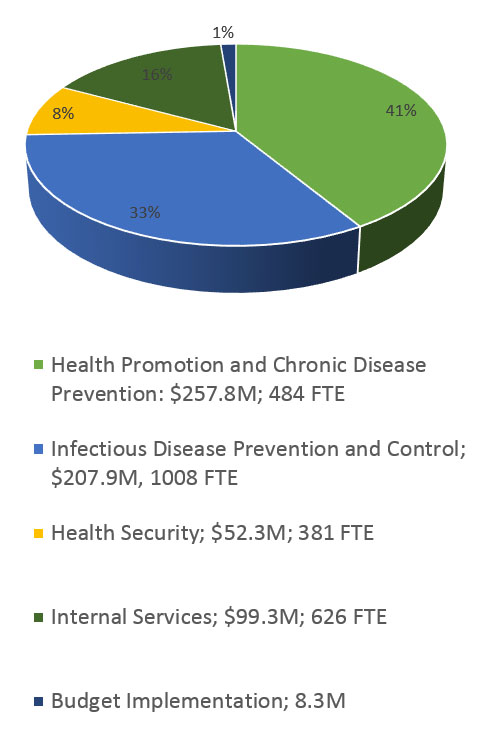
Budget (2019-20 Category) (in millions)

Key financial considerations
- Grants and Contributions (primarily for local program delivery in communities) make up more than one-third of PHAC's total budget
People
- Laboratory presence in Winnipeg (MB), Guelph (ON), and Saint-Hyacinthe (QC)
- Six regional offices with staff in 15 locations across the country support relationships with P/Ts and local stakeholders
Total Resources: 2,499 FTEs and budget of approximately $625.6M
Notes:
- Budgets based on 2019-20 Main Estimates
- Totals may not add due to rounding
Long description
Public Health Agency of Canada's Financial Overview - Budget by Core Responsibility and Category. These two pie charts depict the budget by core responsibility and budget by category. The total budget is approximately $625.6 million and resources include 2,499 full-time employees, based on the 2019-2020 main estimates. The total may not add due to rounding.
The budget by core responsibility is comprised of the following:
- 41 percent health promotion and chronic disease prevention with $257.8 million and 484 full-time employees;
- 33 percent infectious disease prevention and control with $207.9 million and 1,008 full-time employees;
- 16 percent internal services with $99.3 million and 626 full-time employees;
- 8 percent health security with $52.3 million and 381 full-time employees; and
- 1 percent budget implementation with $8.3 million.
The budget by 2019-2020 category is comprised of the following:
- 38 percent grants and contributions with $238.4 million;
- 31 percent salaries with $193.7 million;
- 23 percent operating and management with $142.6 million;
- 7 percent statutory with $43.1 million; and
- 1 percent capital with $7.8 million.
PHAC Organizational Structure

Long description
The organizational structure for PHAC begins with the Minister of Health and continues with Ms. Tina Namiesniowski the President, and Dr. Theresa Tan, the Chief Public Health Officer, who report to the Minister of Health.
Under their leadership are 5 branches.
- Ms. Kim Elmslie, Vice President of Infectious Diseases Prevention and Control;
- Ms. Anna Romano, Vice President of Health Promotion and Chronic Disease Prevention;
- Mr. Carlo Beaudoin, Chief Financial Officer;
- Ms. Sally Thornton, Vice President of Health Security Infrastructure Branch;
- Mr. Stephen Bent, Director General of Office of Strategic Policy and Planning.
There are several areas of shared services with Health Canada:
- Audit and Accountability
- Communications and Public Affairs
- Evaluation Directorate
- Corporate Services Branch
- Office of International Affairs
- Financial Operations Directorate
PHAC also receives Legal Service from the Department of Justice.
Annex A: Chronic Diseases and Conditions
The number of newly diagnosed cases for many chronic diseases are decreasing; however, due to the growing and aging of Canada's population, the number of people living with chronic diseases is increasing
- Cancer: In 2019 alone, a projected 220,400 Canadians will be diagnosed with cancer and 82,100 will die of cancer
- Diabetes prevalence is increasing 3.3% per year; Approximately 10% of children/adolescents and 25% of adults are obese, which increases the risk of chronic diseases such as diabetes
- Mental Illness: 33% of Canadians will be affected by a mental illness or substance use disorder over their lifetimes, and approximately 11 people die by suicide every day in Canada
- Dementia: In 2016-17, over 432,000 Canadians aged 65+ were living with some form of diagnosed dementia; this estimate is expected to increase with Canada's growing and aging population
Common Risk Factors
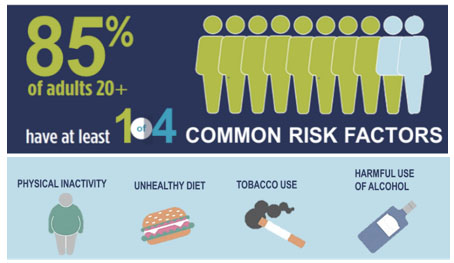
Long description
Common risk factors associated with adults over the age of 20. 85 percent of adults over the age of 20 have at least one of four common risk factors.
The four risk factors are:
- Physical inactivity;
- Unhealthy diet;
- Tobacco use;
- Harmful use of alcohol.
Some groups are more impacted than others, including:
- First Nations, Inuit and Métis
- Those with lower household income and education levels
Annex B: Infectious Diseases
Influenza Pandemics
- A WHO top 10 global threat in 2019; a severe pandemic could reduce Canada's annual GDP growth by up to 1%
Antimicrobial Resistance (AMR)
- Also listed as a WHO top ten global health threat; in Canada, one out of 16 patients admitted to hospital each year is diagnosed with a drug resistant infection
- The annual cost to Canada's healthcare system for just two antibiotic resistant organisms (C. difficile and methicillin-resistant S.aureus) was estimated to be $151 million in 2018; total projected hospital costs estimated at $1.6 billion over the next 10 years
Sexually Transmitted and Blood Borne Infections (STBBIs)
- Canada has endorsed global STBBI targets set by the UN and WHO
- Rates of sexually transmitted infections have risen dramatically over the last decade – chlamydia increased by 49%, gonorrhea by 81%, and syphilis by an alarming 178% between 2007 and 2016
- Even though HIV-related deaths have decreased by 90% since the mid-1990s, HIV transmission continues in Canada
- There have been no further decreases in the rate of reported Hepatitis C cases since 2011
Vaccine-preventable diseases
- Publicly-funded vaccine coverage for Canadian two-year olds ranged from 76-90%, below national vaccination coverage goals of 95% required for population-wide immunity (2017 study)
Public Health Impacts of Climate Change
- Canadian provinces reported 2,025 Lyme disease cases in 2017, compared to 992 cases in 2016; its incidence is increasing rapidly, in part due to rising temperatures
Canadian Institutes of Health Research (CIHR) Overview Deck
Health Portfolio
November 2019
CIHR Mandate
- To excel, according to internationally accepted standards of scientific excellence, in the creation of new knowledge and its translation into improved health for Canadians, more effective health services and products and a strengthened Canadian health care system
- As Canada's federal funding agency for health research, CIHR is using the power of research to improve the health of Canadians, solve health challenges and make our health care system more efficient and effective
A Brief Overview of CIHR
- Invests over $1 billion annually to support over 13,000 world-class researchers and trainees
- Supports the creation of new knowledge, builds research capacity, and promotes the dissemination of research results in order to improve the health of Canadians and make our health care system more efficient
- Offers the Minister of Health tools and resources to:
- develop evidence-based policies and programs
- demonstrate leadership in health innovation
- engage positively with provinces and territories
- Works closely with other federal funders (the Natural Sciences and Engineering Research Council of Canada and the Social Sciences and Humanities Research Council), the Canada Foundation for Innovation and other departments and agencies, to better support Canada's research enterprise
Our Institutes
- A unique model for health research, CIHR Institutes share the responsibility for fulfilling its mandate
- Each institute is a network of researchers brought together to support a broad spectrum of research in its topic areas and, in consultation with its stakeholders, CIHR sets priorities for research in those areas
- The model enables optimal use of existing knowledge to fill research gaps, maximize cooperation and minimize duplication
Long description
This diagram illustrates the institutes of the Canadian Institute for Health Research. They are as follows:
- Aging,
- Cancer Research,
- Circulatory and Respiratory Health,
- Gender and Health,
- Genetics,
- Health Services and Policy Research,
- Human Development, Child and Youth Health,
- Indigenous Peoples' Health,
- Infection and Immunity,
- Musculoskeletal Health and Arthritis,
- Neurosciences, Mental Health and Addiction,
- Nutrition, Metabolism and Diabetes, and
- Population and Public Health.
How CIHR Supports Research
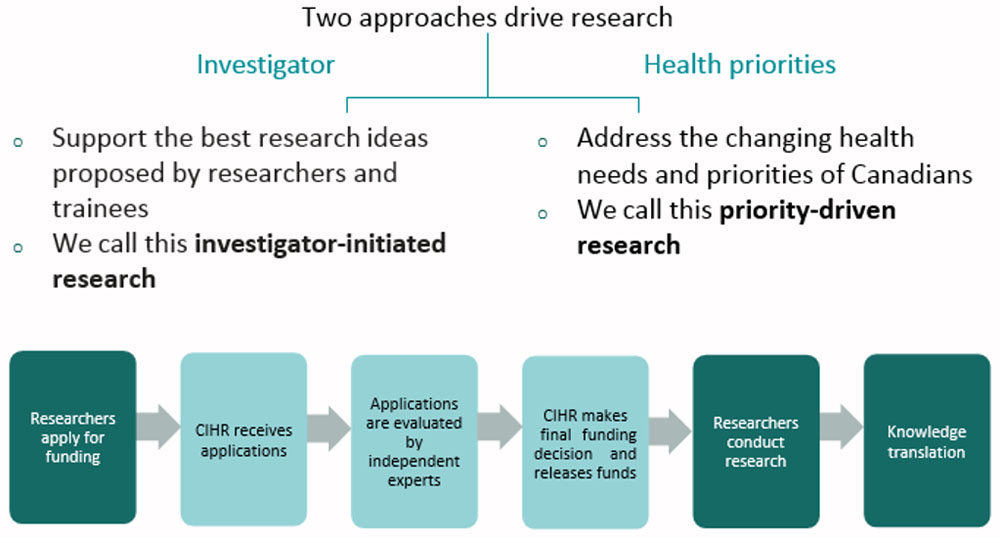
Long description
Diagram showing how Canadian Institute for Health Research Supports Research. Two approaches drive research:
Investigator:
- Support the best research ideas proposed by researchers and trainees
- We call this investigator-initiated research
Health Priorities:
- Address the changing health needs and priorities of Canadians
- We call this priority-driven research
The Canadian Institutes for Health Research supports research using a peer review process whereby:
- Researchers apply for funding;
- The Canadian Institutes of Health Research receives the application;
- Applications are evaluated by independent experts;
- The Canadian Institutes of Health Research makes final funding decision and releases funds;
- Researchers conduct research;
- Knowledge translation.
CIHR Financial Overview
- CIHR's annual budget has remained relatively stable at $1 billion for the last 10 years, but will reach $1.2 billion in 2021-22 as a result of Budget 2018 investments to enhance support for health research
- CIHR's operating budget is approximately 6.0% of its total budget, and CIHR faces increasing pressure to deliver its programs while modernizing its technology
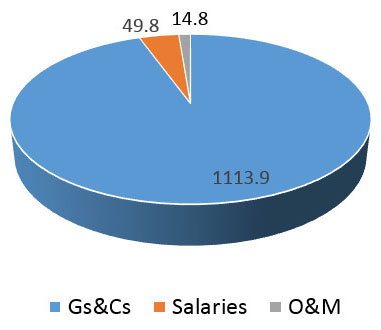
| Description | Amount |
|---|---|
| Total Budget (Main Estimates 2019-2020) | $1,178M |
| Total Full Time Equivalents | 478 |
Notes: CIHR's Terms and Conditions do not allow it to provide contributions
Long description
The Canadian Institutes for Health Research's Financial Overview - Budget by Category. This pie chart depict the budget 2019-2020 by category. The total budget is approximately $1,178 million and resources include 478 full-time employees, which is based on the 2019-2020 main estimates. The Canadian Institutes for Health Research's terms and conditions do not allow it to provide contributions.
The budget by category is comprised of the following:
- grants and contributions with $1,113.9 million;
- salaries with $49.8 million; and
- operating and management with $14.8 million.
Further Financial Information
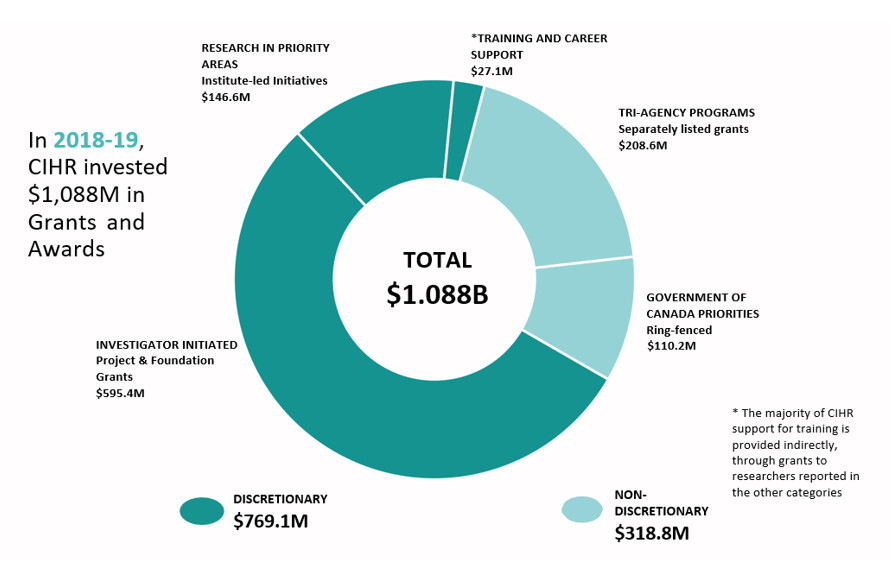
Long description
The graph is a pie chart illustrating additional CIHR financial information. In 2018-19, CIHR invested a total of $1.088B in Grants and Awards broken down into discretionary and non-discretionary investments.
Discretionary investments totaled $769.1M and included:
- Training and Career Support ($27.1M). The majority of CIHR support for training is provided indirectly, through grants to researchers reported in the other categories;
- Research in Priority Areas - Institute-led Initiatives ($146.6M),
- Investigator Initiated - Project & Foundation Grants ($595.4M).
Non-discretionary investments totaled of $318.8M and included:
- Government of Canada Priorities - Ring-fenced ($110.2M);
- Tri-agency Programs - Separately listed grants ($208.6M)
CIHR's Key Priorities
Health Research Priorities
- CIHR is currently developing its 2020-2025 Strategic Plan entitled "Vision 2050: creating a healthier future" with some of the Minister's key stakeholders, including researchers, health research funders, charities, and representatives from the Indigenous community, the general public, and knowledge users and policy-makers, including the provinces and territories
- The new plan will be published in June 2020, to mark CIHR's 20th anniversary
Federal Research Priorities
- Continue to integrate research evidence into Health Portfolio and other government policies (e.g., opioid crisis, AMR Action Plan, HIV/AIDS/ Sexually-Transmitted and Blood-Borne Illnesses, National Dementia Strategy, Post-Traumatic Stress Injuries Action Plan, Post-Traumatic Stress Disorder Framework)
- Work with other members of the Canada Research Coordinating Committee to improve the coordination efforts of Canada's research granting agencies (CIHR, Social Science and Humanities Research Council and Natural Science and Engineering Research Council):
- Strengthen Canada's ability to grow in a rapidly evolving global research landscape
- Advance efforts in key emerging research areas
- Strengthen equity and diversity in research
- Increase the capacity of Indigenous communities to conduct research and partner with the broader research community
- Improve support for the next generation of scientists and scholars
CIHR Organizational Chart
CIHR consists of a single head office in Ottawa, under the lead of the following senior executives:
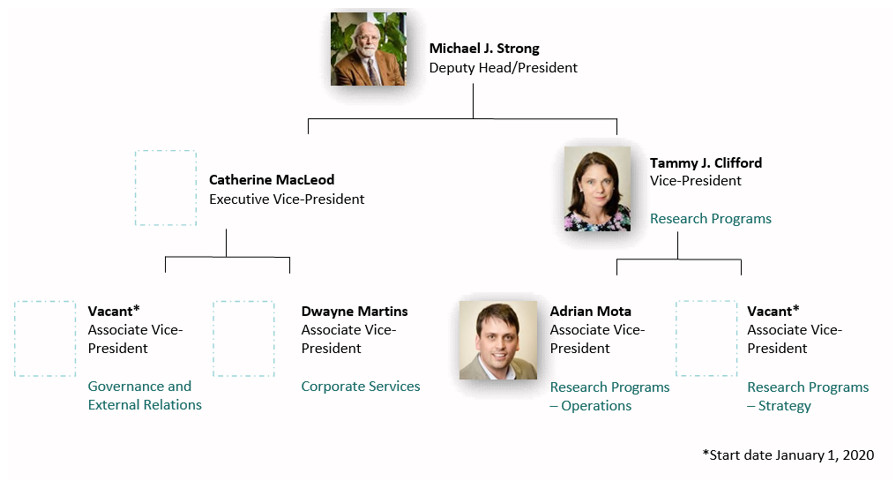
Long description
Chart of CIHR's Organizational Structure. CIHR consists of a single head office in Ottawa, under the lead of the following senior executives:
- Michael J. Strong is the Deputy Head/President. Directly below is Catherine MacLeod, Executive Vice-President and Tammy J. Clifford, Vice-President of Research Programs.
- Two Associate Vice-Presidents report to Catherine MacLeod: Dwayne Martins, Associate Vice-President of Corporate Services and a vacant Associate Vice-President for Governance and External Relations.
- Two Associate Vice-President's report to Tammy J. Clifford: Adrian Mota, the Associate Vice-President of Research Programs for Operations, and a vacant Associate Vice-President of Research Programs for Strategy.
CIHR Governance Structure

Long description
CIHR's governance structure describes the roles and responsibilities that each governance area oversees. At the top of CIHR's governance structure is Parliament and the Minister of Health. Directly under the Minister of Health is CIHR Governing Council (GC), then the CIHR President, and a Science Council (SC) and a Senior Leadership Committee (SLC) that report to the CIHR President.
As a Government of Canada agency within the Health Portfolio, CIHR reports to Parliament (e.g., Departmental Results Report), advises the Minister of Health in respect of any matter relating to health research or health policy, and supports federal government policy directions (e.g., participates in parliamentary committee hearings).
The Governing Council is responsible for:
- Developing strategic directions, goals and policies;
- Evaluating performance/appointments of scientific directors;
- Approving budget/by-laws;
- Establishing, maintaining, terminating and providing mandates for CIHR's institutes.
The President is responsible for:
- Day-to-day management of CIHR;
- Approving funding for research;
- Providing advice to the Minister of Health.
Science Council is responsible for:
- Leadership on research and knowledge;
- Translation strategy;
- Leadership on funding for CIHR.
Senior Leadership Committee is responsible for:
- Leadership on corporate policy and management.
Annex: A Key Player in Federal Science and Technology Investments (2019-20)

Long description
Numerous key players invest in Federal Science and Technology. Below is a list of committees, organizations, departments & agencies along with their investments for 2019-2020.
Canada Research Coordinating Committee members:
- Canadian Institutes of Health Research ($1178M), Natural Sciences and Engineering Research Council ($1346M), Social Sciences and Humanities Research Council ($819M), Canada Foundation for Innovation ($373M).
Research and Technology Organization:
- National Research Council ($1249M)
Federal Agency:
- Canadian Spacy Agency ($283M), Natural Resources Canada ($543M)
Federal Department:
- Statistics Canada ($539M), Global Affairs Canada ($349M), Environment and Climate Change Canada ($708M), Fisheries and Oceans ($332M), National Defence ($341M), Innovation, Science and Economic Development ($538M), Agriculture and Agri-Food Canada ($488M), Health Canada ($532M).
Canadian Food Inspection Agency (CFIA) Overview Deck
Health Portfolio
November 2019
Legislative Mandate
Develop and deliver inspection and other services to:
- Protect plant resources from pests, diseases and invasive species
- Prevent and manage animal diseases, including diseases that threaten human health (e.g., avian influenza)
- Prevent and manage food safety risks (e.g., inspection, food recalls)
- Contribute to consumer protection (e.g., labelling claims)
- Facilitate market access for Canada's food, plants and animals
CFIA Organizational Structure

Long description
CFIA's organizational structure is led by two senior executives. The President is Siddika Mithani, and the Executive Vice-President, France Pégeot. Under the two senior executives are 12 executives that oversee different sections of CFIA.
Delivery of CFIA Mandate
- Colleen Barnes, Vice-President of Policy and Programs, provides strategic policy advice and sets out program policies and procedures.
- Jaspinder Komal, Vice-President of the Science Branch, provides scientific advice, diagnostic and testing services.
- Theresa Luliano, Vice-President of the Operations Branch, delivers inspection programs and takes compliance and enforcement action.
- Fred Gorrell, Assistant Deputy Minister of the International Affairs Branch, leads on market access and international regulatory trade issues.
- Nicole Bouchard-Steeves, Associate Vice-President of Operations, delivers inspection programs and takes compliance and enforcement action.
- Amanda Jane (AJ) Preece, Vice-President of Innovation, Business and Service Development and Chief Information Office, delivers on major projects and priority change initiatives and enables information and information technology.
Corporate Services:
- Dominique Osterrath, Vice-President of Corporate Management and Chief Financial Officer, provides oversight of financial management and assets and security management.
- Jane Hazel, Vice-President of Communications and Public Affairs, delivers internal and external communication services.
- Joanne Butler, Chief Audit Executive and Head of Evaluation, Audit & Evaluation, leads internal audit and evaluation.
- The position of Vice-President of Human Resources is vacant. This section enables talent identification, acquisition and mobilization.
Integrity and Redress Secretariat
- Merril Bawden, Chief Redress Officer, Integrity and Redress Secretariat, serves as the focal point for integrity and redress, including the Complaints and Appeals Office.
Shared Services with Agriculture and Agri-Food Canada:
- Kristine Allen, Executive Director and Senior General Counsel, Legal Services, provides legal services to the CFIA and Agriculture and Agri-Food Canada.
Division of Responsibilities Between Ministers
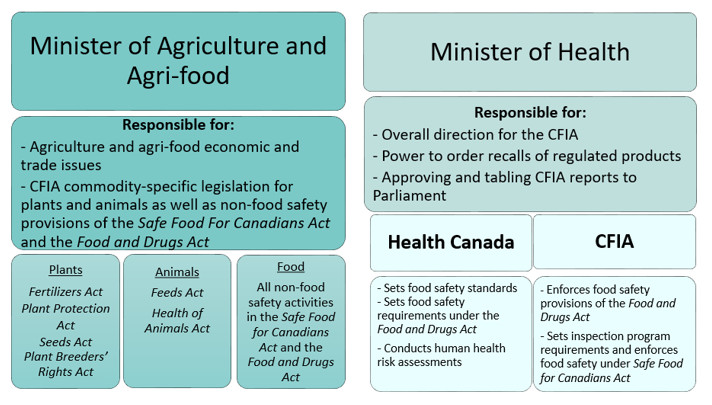
Long Description
Two ministers have responsibilities with respect to the Canadian Food Inspection Agency (CFIA). They are the Minister of Agriculture and Agri-Food and the Minister of Health.
The Minister of Agriculture and Agri-Food is responsible for:
- Agriculture and agri-food economic and trade issues
- CFIA commodity-specific legislation for plants and animals as well as non-food safety provisions of the Safe Food For Canadians Act and the Food and Drugs Act.
Agriculture and Agri-Food administer a number of acts that fall under three main areas:
- Plants: Fertilizers Act, Plant Protection Act, Seeds Act, Plant Breeders' Rights Act
- Animals: Feeds Act, Health of Animals Act
- Food:All non-food safety activities in the Safe Food for Canadians Act, and the Food and Drugs Act
Responsibilities for Minister of Health:
- Overall direction for the CFIA
- Power to order recalls of regulated products
- Approving and tabling CFIA reports to Parliament
Health Canada and CFIA administer a number of standards and acts:
Health Canada:
- Set food safety standards
- Sets food safety requirements under the Food and Drugs Act
- Conducts human health risk assessments
CFIA:
- Enforces food safety provisions of the Food and Drugs Act
- Sets inspection program requirements and enforces food safety under Safe Food for Canadians Act.
Core Responsibilities
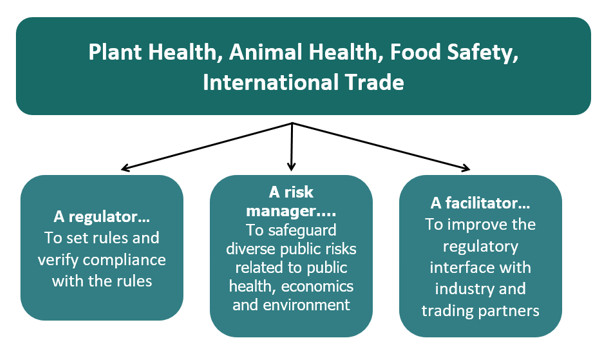
Long description:
This visual flow chart shows the top core responsibilities for CFIA, which are: Plant Health, Animal Health, Food Safety and International Trade.
CFIA Acts as a:
- Regulator… To set rules and verify compliance with the rules.
- A risk manager…To safeguard diverse public health, economics and environment.
- A facilitator… To improve the regulatory interface with industry and trading partners.
Plant Health
Protect Canada's plant resource base
Includes crops, horticulture, nurseries, forest resources and products, greenhouses, seeds, fertilizers, plants with novel traits, invasive alien species
Protect Canada's plant resource base, environment and plant-related industries by:
- Preventing the introduction and spread of pests that could damage Canadian production and the income of Canadian producers
- Verifying farmers have access to safe and effective agricultural inputs (e.g., seed, fertilizer) that support environmental sustainability
- Fostering innovation through protection of intellectual property (i.e., plant breeders' rights)
- Maintaining the reputation of Canadian agricultural products in the global marketplace as being high-quality, pest free and safe
Animal Health
Protect Canada's animal resource base and Canadians from disease
Includes livestock, poultry, animal feeds, and fish and seafood
Minimize risks to Canada's terrestrial and aquatic animal resource base, and ensure the safety of animal feeds, products and vaccines by:
- Protecting Canada's animals, including aquatic animals, from diseases
- Managing animal disease incidents and emergencies (e.g., avian influenza)
- Promoting and regulating animal welfare, in transportation and in slaughter
- Verifying that animal feeds and vaccines are safe and effective
Food Safety
Contribute to safeguarding Canada's food supply
Includes health and safety and labelling
Minimize risks to Canadians by:
- Protecting Canadians from preventable food safety hazards
- Managing food safety investigations and recalls effectively
- Protecting consumers and the marketplace from unfair practices
Contributes to consumer protection by:
- Verifying that information provided to Canadian consumers through labels and advertising is truthful and not misleading
International Trade
Facilitate market access for Canada's plants, animals and food
Contributing to market access for Canadian agriculture by:
- Influencing the development of international rules and standards for plant protection, animal health and food safety through international standard-setting bodies:
- International Plant Protection Convention (IPPC)
- World Organization for Animal Health (OIE)
- Codex Alimentarius Commission (CODEX) (Food)
- Negotiating import / export conditions and technical agreements and standards
- Engaging trading partners
- Working in collaboration with Agriculture and Agri-Food Canada and Global Affairs Canada
CFIA Strategic Plan
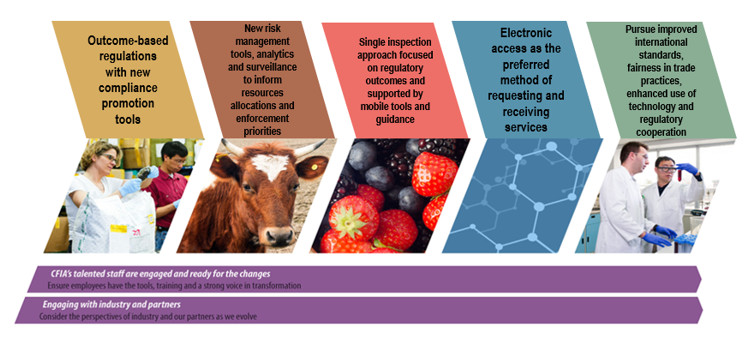
Long description
This flowchart for CFIA's Strategic plan outlines 5 steps and 2 engagements.
The steps are as follows…
- Outcome-based regulations with new compliance promotion tools.
- New risk management tools, analytics and surveillance to inform resource allocations and enforcement priorities.
- Single inspection approach focused on regulatory outcomes and supported by mobile tools and guidance.
- Electronic access as the preferred method of requesting and receiving services.
- Pursue improved international standards, fairness in trade practices, enhanced use of technology and regulatory cooperation
The engagements are …
- CFIA's talented staff are engaged and ready for the changes. This will ensure that employees have the tools, training and strong voice in transformation
- Engaging with industry and partners. This considers the perspectives of industry and out partners as we evolve.
CFIA'S National Presence
13 laboratories : Atlantic (2), Quebec, (2), Ontario (3), Western (6)
Long description
Map showing CFIA's national presence across Canada.
- Atlantic Region: New Brunswick (Fredericton), Nova Scotia (Dartmouth), Prince Edward Island (Charlottetown), Newfoundland and Labrador (St. John's)
- Quebec Region: Montreal East, Montreal West, St. Hyacinthe, Ste. Foy
- Ontario Region: Southwest (London), Central (Guelph), Toronto (Downsview), North East (Nepean)
- Western Region: Manitoba (Winnipeg), Saskatchewan (Regina), Alberta South (Calgary), Alberta North (Edmonton), British Columbia Coast (Burnaby), British Columbia Mainland (Burnaby)
There are 13 laboratories in different areas of Canada: 2 in the Atlantic, 2 in Quebec, 3 in Ontario and 6 in the Western Region.
CFIA Financial Overview
Budget 2019-20 by Core Business
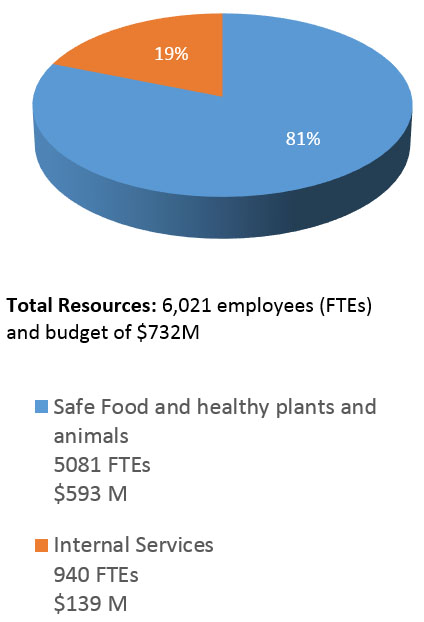
Budget 2019-20 by Vote

Long Description
The graph is a pie chart illustrating CFIA Financial Overview for the 2019-2020 Budget. The financial overview is divided into two pie charts. The first chart is Budget 2019-2020 by Core Business. The second chart is budget 2019-2020 by Vote. The total resources is 6,021 employees Full-Time Equivalents (FTEs) and a budget of $732 million.
Budget 2019-2020 by Core Business describes two segments:
- 81% of the pie chart is devoted to Safe Food and healthy plants and animals, which has 5,081 FTEs and a budget of $593 million.
- 19% of the pie chart is devoted to Internal Services, which has 940 FTEs and a budget of $139 million.
Budget 2019-2020 by Vote divides the pie chart into four segments:
- Voted – Operating expenditures: totals $573 million and 78 percent of the total.
- Voted – Capital expenditures: totals $20 million and 3 percent of the total.
- Statutory revenue – totals $53 million and 7 percent of the total.
- Other statutory – totals $86 million and 12 percent of the total.
CFIA'S Partners

Long description
Canadian Food Inspection Agency's Partners. This diagram shows the multiple partners that work with Canadian Food Inspection Agency which are as follows:
International Partners:
- set import requirements and verify export requirements,
- Comparability and acceptance of relevant systems, and
- Development international science-based rules, standards, etc.
Provincial, Territorial and Municipal Governments:
- enforce jurisdictional food safety, plant and animal health requirements,
- collaborate in responding to food safety incidents, and
- prevent and manage plant and animal health emergencies
Federal Departments and Agencies:
- Health Portfolio,
- Agriculture and Agri-Food Canada Portfolio,
- Global Affairs Canada,
- Canada Border Services Agency,
- Fisheries and Oceans Canada,
- Environment and Climate Change Canada,
- Natural Resources Canada,
- Shared Services Canada, and
- Innovation, Science and Economic Development.
Industry:
- Production of safe food
- Comply with regulatory requirements
- Develop and implement best management practices
Consumers:
- Safe food handling and preparation
- Awareness of plant and animal risks (e.g. transporting infested firewood)
Patented Medicine Prices Review Board (PMPRB) Overview Deck
Health Portfolio
November 2019
PMPRB Vision, Mandate & Jurisdiction
Vision
- A sustainable pharmaceutical system where payers have the information they need to make smart reimbursement choices and Canadians have access to patented drugs at affordable prices
Mandate
The PMPRB has a dual role:
- Regulatory: to ensure that prices of patented medicines sold in Canada are not excessive
- Reporting: to report on pharmaceutical trends
Jurisdiction
- The Patent Act establishes the PMPRB as an independent, quasi-judicial body
- While the PMPRB is part of the Health Portfolio, it carries out its regulatory mandate at arm's length (i.e., independently) from the Minister of Health, due to its quasi-judicial nature
Ministerial Role
The Patent Act authorizes the Minister of Health to:
- Table the Annual Report prepared by the PMPRB before Parliament
- Recommend new or amended regulations to the Governor in Council
- Refer matters to the PMPRB for inquiry
The Minister of Health may (but is not obligated to):
- Participate as a party in a hearing before the PMPRB
- Convene meetings with the PMPRB
- Participate in PMPRB guideline consultations and designate representatives of consumer groups and of the pharmaceutical industry as participants in such consultations
- Enter into agreements with provinces respecting the distribution of excess revenues collected by the PMPRB from pharmaceutical patentees
Business Lines
Regulatory mandate:
- Where PMPRB staff believes that the price of a drug is excessive, a patentee may agree to lower its price and/or pay back excess revenues
- If staff and a patentee disagree, a hearing may be held before PMPRB Board members
- If the Board makes a legal finding that a medicine is excessively priced, it can order the patentee to reduce its price and/or pay back excess revenues
Reporting mandate:
- Under the Patent Act, the PMPRB is required to submit an Annual Report to Parliament that is tabled by the Minister of Health. The report outlines the activities of the PMPRB, and highlights key pharmaceutical trends and reports on research and development investment in Canada
- Pursuant to section 90 of the Patent Act, previous Ministers of Health have asked the PMPRB to report on various other aspects of the pharmaceutical market in Canada, including generic drug prices and key cost drivers in public drug plans
- Under this authority, since 2001, the PMPRB has been producing analytical studies of price utilization and cost trends to FPT health ministries through the National Prescription Drug Utilization Information System (NPDUIS)
Key Files
Modernizing the Regulatory Framework:
- The PMPRB was created in 1987 as part of a major overhaul of Canada's drug patent regime
- Until recently, the PMPRB's regulatory framework has not modernized despite significant changes in the pharmaceutical environment, making it increasingly challenging for the PMPRB to fulfill its consumer protection mandate
- Since June 2016, Health Canada and the PMPRB have been consulting on modernizing the regulatory framework, through changes to Regulations and Guidelines, respectively
- On August 21, 2019, Health Canada published amendments to the Patented Medicines Regulations in Canada Gazette, Part II, which will come into force on July 1, 2020
- The PMPRB is currently consulting its stakeholders on Guidelines that will give effect to these amendments when they come into force
Recent Amendments to Regulations:
Provide modern tools and information to protect Canadians from excessive medicine prices in an era marked by high cost drugs and confidential pricing:
- Benchmarking prices against countries that are more like Canada economically and from a consumer price protection standpoint (Australia, Belgium, the Netherlands, Norway and Japan added to the existing list of France, Germany, Italy, Sweden and the U.K.)
- Regulating at the level of the actual prices being paid in Canada rather than manufacturer list prices (requiring patentees to report confidential rebates and discounts provided to their largest customers – mainly public payers)
- Considering the value (cost effectiveness) and the overall affordability (market size) of a medicine when setting the maximum price (rather than benchmarking ceiling prices to list prices in other countries)
This will bring Canada in line with the policies/practices of most other developed countries
PMPRB Budget by Operational Priorities 2019-20

Total Resources: 90 FTEs and budget of apporximately $16.7 M
Long description
This pie chart describes the 2019-2020 operational priorities budget (in millions) for each branch within PMPRB. PMPRB has six branches. The branches are, Corporate Services, Executive Director, Board Secretariat, Communication and Strategic Planning, Policy and Economic analysis, Regulatory Affairs and Outreach, and Legal Services. The total resources are 90 Full-Time Equivalent (FTEs) and a budget of approximately $16.7 million.
The budget for each branch are as follow:
- Corporate Services has a budget of $3.9 million.
- Executive Director has a budget of $0.3 million.
- Board Secretariat, Communication and Strategic Planning has a budget of $2 million.
- Policy and Economic Analysis has a budget of $3.2 million.
- Regulatory Affairs and Outreach has a budget of $2.9 million.
- Legal Services has a budget of $3.8 million.
In addition, the department's Core Responsibility is to regulate patented medicine prices, and the Department's Result is affordable patented medicine prices. The Department's Budget structure is directed towards two priorities:
- Strategic Outcomes, which encompasses $13.3 million of the budget.
- Internal Services, which encompasses $3.4 million of the budget.
PMPRB Organizational Structure
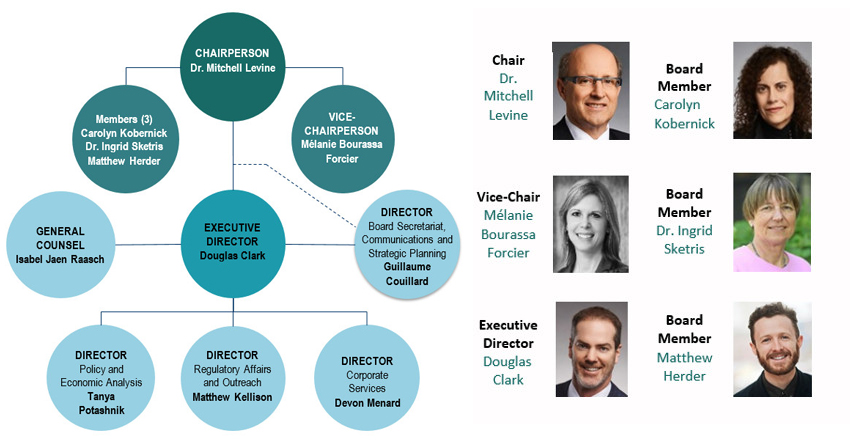
- The Board consists of up to five part-time Governor in Council-appointed members, including a Chairperson and a Vice-Chairperson
- The Chairperson is designated under the Patent Act as the Chief Executive Officer of the PMPRB, with the authority and responsibility to supervise and direct its work
- The Executive Director is responsible for the day-to-day administration of the PMPRB and oversight of its approximately 90 public servants staff
Long Description
The organizational structure lists the senior management of PMPRB. At the top of PMPRB is the chairperson, Dr. Mitchell Levine. Below the chair are three board members: Carolyn Kobernick; Dr. Ingrid Sketris; and Matthew Herder. The Vice Chair Person is Mélanie Bourassa Forcier.
At the next level of the organizational chart is the Executive Director, Douglas Clark as well as the Director of the Board Secretariat, Communication and Strategic Planning, Guillaume Couillard and General Counsel, Isabel Jaen Raasch.
There are 3 directors who report to the Executive Director. They are:
- Director of Policy and Economic Analysis, Tanya Potashnik;
- Director of Regulatory Affairs and Outreach, Matthew Kellison; and
- Director of Corporate Services, Devon Menard.
Health Portfolio Legislative Mandates at a Glance
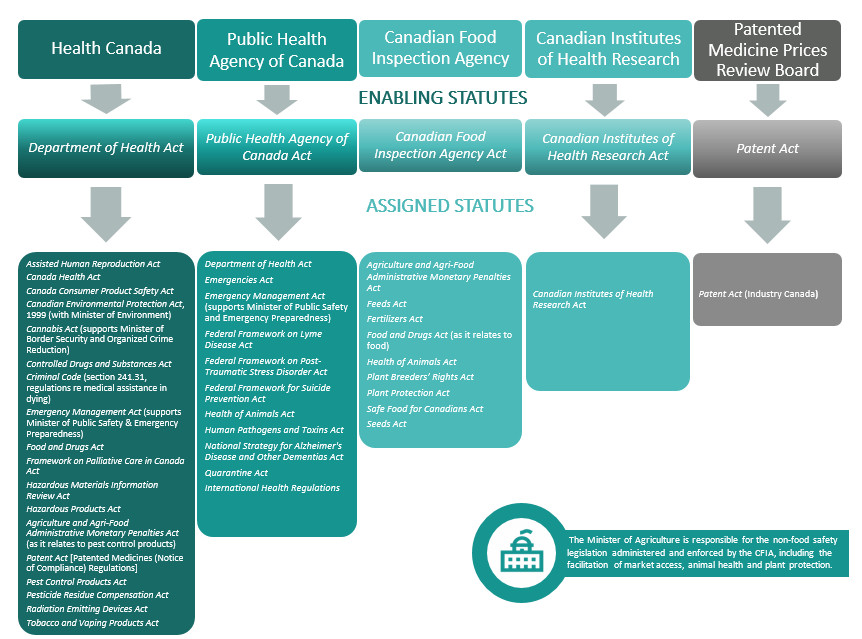
Long description
Placemat depicting the various enabling and assigned statutes for each Health Portfolio organization.
Health Canada's enabling statute is the Department of Health Act and its assigned statutes are:
- Assisted Human Reproduction Act
- Canada Health Act
- Canada Consumer Product Safety Act
- Canadian Environmental Protection Act, 1999 (with Minister of Environment)
- Cannabis Act (supports Minister of Border Security and Organized Crime Reduction)
- Controlled Drugs and Substances Act
- Criminal Code (section 241.31, regulations re medical assistance in dying)
- Emergency Management Act (supports Minister of Public Safety & Emergency Preparedness)
- Food and Drugs Act
- Framework on Palliative Care in Canada Act
- Hazardous Materials Information Review Act
- Hazardous Products Act
- Agriculture and Agri-Food Administrative Monetary Penalties Act (as it relates to pest control products)
- Patent Act [Patented Medicines (Notice of Compliance) Regulations]
- Pest Control Products Act
- Pesticide Residue Compensation Act
- Radiation Emitting Devices Act
- Tobacco and Vaping Products Act
The Public Health Agency of Canada's enabling statute is the Public Health Agency of Canada Act and its assigned statutes are:
- Department of Health Act
- Emergencies Act
- Emergency Management Act (supports Minister of Public Safety and Emergency Preparedness)
- Federal Framework on Lyme Disease Act
- Federal Framework on Post-Traumatic Stress Disorder Act
- Federal Framework for Suicide Prevention Act
- Health of Animals Act
- Human Pathogens and Toxins Act
- National Strategy for Alzheimer's Disease and Other Dementias Act
- Quarantine Act
- International Health Regulations
The Canadian Food Inspection Agency's enabling statute is the Canadian Food Inspection Agency Act and its assigned statutes are:
- Agriculture and Agri-Food Administrative Monetary Penalties Act
- Feeds Act
- Fertilizers Act
- Food and Drugs Act (as it relates to food)
- Health of Animals Act
- Plant Breeders' Rights Act
- Plant Protection Act
- Safe Food for Canadians Act
- Seeds Act
The Canadian Institutes of Health Research's enabling statute is the Canadian Institutes of Health Research Act and its assigned statute is:
- Canadian Institutes of Health Research Act
The Patented Medicine Prices Review Board's enabling statute is the Patent Act and its assigned statute is:
- Patent Act (Industry Canada)
The Minister of Agriculture is responsible for the non-food safety legislation administered and enforced by the CFIA, including the facilitation of market access, animal health and plant protection.
Overview of Legislative and Regulatory Responsibilities in the Health Portfolio
Introduction
In Canada, health is an area of shared jurisdiction. Under the Constitution Act, 1867, provincial responsibilities include the establishment, maintenance and management of hospitals, local matters, and property and civil rights. Over time, courts have interpreted these constitutional provisions to mean that provinces and territories (PTs) are primarily responsible for health care delivery, the administration of provincial health insurance plans, and the regulation of health professions.
Federal authorities in health are grounded in the federal government's constitutional responsibilities for criminal law and taxation, and the federal spending power. These responsibilities provide the basis for helping to protect the health and safety of Canadians through the regulation of drugs, food, medical devices, controlled substances, consumer products, pest control products, and medical assistance in dying.
Parliament also has the authority to spend money raised through taxation, and to attach terms and conditions to the authorized spending. Accordingly, the Canada Health Act establishes the criteria and conditions PT health insurance plans must meet to receive their full cash entitlement under the Canada Health Transfer.
Rooted in the "peace, order and good government" provisions of the Constitution, the federal government also has key functions in relation to national health emergencies, and where public health matters are issues of national concern. Since the 1970s, federal power in public health has been interpreted to also include efforts in health research and promotion, disease prevention and health information.
A number of other federal responsibilities include health elements (not all of which fall within the purview of the Health Portfolio), including economic powers related to commerce and patents, which apply to drugs, medical devices and technologies; responsibilities in foreign affairs and immigration that relate to migration health (e.g., admission of foreign nationals with international credentials, and relations with international bodies and foreign governments); and supplementary benefits and health services for certain populations (First Nations and Inuit, refugees, the military).
Health Portfolio Legislation and Regulation
There is a range of legislative mechanisms that the government can use to meet its desired objectives. Legislative tools include Acts, Regulations, and Orders in Council, all of which are relevant in the Health Portfolio context. While Acts are laws enacted by Parliament, regulations also have legally binding effect. Normally, the power to make regulations is conferred by Parliament to the Governor in Council (Cabinet), a Minister, or, occasionally, an agency.
The Minister of Health is responsible for the administration and enforcement of aspects of approximately 40 Acts (and their associated regulations) that have a direct impact on the health and safety of Canadians.
Four of the Acts are enabling legislation, providing the basis of the activities of the four largest Portfolio organizations -- Health Canada, the Public Health Agency of Canada, Canadian Food Inspection Agency and the Canadian Institutes of Health Research. These Acts include specific responsibilities carried out by these organizations, ranging from the promotion of the physical, social, and mental well-being of Canadians (Department of Health Act), to taking public health measures, identifying and reducing public health risk factors, and supporting national readiness for public health threats (Public Health Agency of Canada Act), and setting non-safety standards for food sold in Canada and enforcing the food provisions of the Food and Drugs Act (Canadian Food Inspection Agency Act and the Public Service Rearrangement and Transfer of Duties Act). The Patent Act provides the legislative basis for the establishment and functioning of the Patented Medicine Prices Review Board.
The Minister also has important responsibilities in relation to the administration of the Canada Health Act (CHA), Canada's federal legislation on insured health services. The CHA defines the national principles that govern the Canadian health care system and aims to "… protect, promote and restore the physical and mental well-being of residents of Canada and to facilitate reasonable access to health services without financial or other barriers." It establishes the criteria and conditions provincial and territorial health insurance plans must meet to receive their full cash contributions under the Canada Health Transfer.
The Public Health Agency of Canada Act mandates the Public Health Agency of Canada and the Chief Public Health Officer to assist the Minister of Health "in exercising or performing the Minister's powers, duties and functions of public health", which includes public health emergency preparedness and response. Under the Emergency Management Act, the Minister of Health has specific responsibilities to identify risks pertinent to his or her mandate, and develop plans to address these risks. Emergency plans have been developed by the Health Portfolio which address a variety of public health risks (for example, pandemic influenza and foodborne illness).
In addition to enabling statutes, there are a number of assigned Acts that establish federal frameworks (e.g., palliative care, suicide prevention, Post-Traumatic Stress Disorder) or national strategies (e.g., dementia), which confer specific responsibilities to the Minister of Health.
The balance of legislation relevant to the Health Portfolio sets out responsibilities to be carried out by the Minister of Health in the context of regulating food, pharmaceutical drugs, controlled substances, pesticides, medical devices, biologics, human toxins and pathogens, and consumer products.
There are significant differences in the nature of these various regulatory regimes. However, some principles of decision-making are common to many of the Acts for which the Minister of Health is identified as exercising a role. The following section sets out some key principles.
"Powers, Duties and Functions" in Legislation
Most Acts of Parliament and associated regulations are administered by individual Ministers, and this responsibility can include a variety of powers, duties, and functions. Depending on the legislation (or regulations), the responsible Minister can be named in the Act itself or designated by the Governor in Council (i.e., Cabinet).
Typically, the various powers, duties and functions set out in an Act or regulations are assigned to the responsible Minister. However, in some circumstances, specific authorities are assigned to other identified individuals or groups of individuals. For example, powers to make regulations and amend Schedules to an Act are often assigned to the Governor in Council. In all cases, the Minister of Health would still be involved in setting overall policy direction for regulatory programs, developing regulations, and approving regulations recommended to the Governor in Council.
Who Makes Regulatory Decisions?
Depending on the legislation, the authority to make decisions may be specifically assigned to the Minister, to other individuals (such as designated inspectors), or, occasionally, to the Governor in Council. The following section explains how these different types of decision- making authorities work.
A. The Minister of Health
1. Decisions made by the Minister or on the Minister's Behalf
Decision-making authority in legislation often resides with the Minister. In the Health Portfolio context, this authority encompasses a large number of possible kinds of regulatory decisions, and on any given day, many of these decisions are made. Accordingly, based on long-standing legal precedent, the vast majority of decisions are made by departmental officials. This has four important advantages:
- Given the volume of regulatory decisions required, it is not practical for a Minister to personally exercise all of his or her authorities;
- The risk of perceived political interference in evidence-based decision-making is minimized;
- Many regulatory decisions are highly technical in nature and require a specialized (often scientific) expertise; and
- In the event that a decision is challenged in a court of law (subject to judicial review), the person who makes the decision may need to give evidence.
At all times, where the decision-making authority in legislation resides with the Minister, the responsible Minister retains the authority to personally make those decisions. However, the practice of allowing officials to exercise regulatory decision-making powers that are appropriate to their functions is common to all regulatory departments.
Regulatory decisions can be scrutinized by industry, the media, the public and the judiciary. Therefore, it is essential that the Minister – or appropriately- placed officials in the Health Portfolio who make those decisions on behalf of the Minister – are able to demonstrate integrity in their decision-making processes. It is important that each decision can be demonstrated to be the result of an objective – and, as applicable, science-based – assessment of all the information available to the regulator.
While routine and uncontroversial regulatory decisions are made every day by officials, if a decision is particularly sensitive in nature, additional background information may be provided so that the Minister is aware of the context and basis for a decision.
2. Ministerial Decision-Making Authority where a Delegation Order is Required
In some specific instances, legislation may include specific provisions that allow the Minister, as head of the institution, to make an order delegating particular powers, duties and functions to officers or employees of the institution (or to another institution within the Portfolio). Relevant examples in the context of the Health Portfolio include the Access to Information Act and the Privacy Act.
The Minister of Border Security and Organized Crime Reduction has been designated as the Minister responsible for the Cannabis Act and its regulations. Health Canada has been designated to support him in this role. As such, the powers, duties and functions given to this Minister have been delegated to Health Canada employees pursuant to the Salaries Act. These powers include the issuance, renewal, suspension, or revocation of licences or permits.
Some examples of the decision-making authorities assigned to the Minister of Health include the power to:
- issue a "Notice of Compliance" permitting the sale of a new drug in Canada (Food and Drug Regulations);
- order the recall of a drug or medical device if the Minister believes it presents a serious or imminent risk of injury to health (Food and Drugs Act);
- issue a registration permitting the sale and use of a pest control product in Canada (Pest Control Products Act);
- issue authorizations for access to controlled substances (Controlled Drugs and Substances Act);
- order a stop sale or stop the import of a non- compliant hazardous product (Hazardous Products Act);
- order a recall of a consumer product that is a danger to human health or safety (Canada Consumer Product Safety Act);
- order the recall of a food, animal or plant product that poses a health risk (Canadian Food Inspection Agency Act); and
- establish a quarantine station at any place in Canada (Quarantine Act).
B. Other Officials
Many Acts confer decision-making powers explicitly on individuals other than the Minister. For example, inspection powers (such as entry, examination of records, detention of substances, etc.) can only be exercised by a designated "inspector" in the Food and Drugs Act, Controlled Drugs and Substances Act, and the Human Pathogens and Toxins Act, to name but a few. Under the Quarantine Act, a "quarantine officer" decides whether to require health assessments of individuals suspected of carrying a communicable disease.
In these instances, the Minister may request a briefing in relation to the decision-making process and discuss the decision with officials, but may not make, nor is directly involved in, the decision itself.
C. Independent Tribunals
Some statutes create tribunals that operate independently of a minister. One such example in the Health Portfolio is the Patented Medicine Prices Review Board (PMPRB). The PMPRB is an independent, quasi-judicial body established under the Patent Act. The Board determines whether the patented drug price set by the manufacturer is excessive and, if so, the Board can order price reductions and/or the offset of excess revenues. Further, the Board has the authority to issue non-binding guidelines regarding the administration of the Board. However, before issuing any such Guidelines, the Board is required to consult with the Minister of Health, as well as other stakeholders.
Although the PMPRB carries out its mandate at arms-length from the Minister of Health and is independent of Health Canada, the Patent Act sets out a number of roles for the Minister of Health in relation to the PMPRB, including recommending new/amending regulations to the Governor in Council in relation to the PMPRB regime, and entering into agreements with any province to disburse funds collected by the PMPRB.
D. Governor in Council (Cabinet)
While not nearly as common, sometimes legislation expressly provides that the Governor in Council will exercise the decision-making power. An example of this in the Health Portfolio is under the Pest Control Products Act, where the Governor in Council may make an order cancelling or amending the registration of a pesticide if considered necessary to implement an international agreement. Another example is under the Quarantine Act, where the Governor in Council can by order prevent any persons from entering Canada if they are from a country which has an outbreak of a communicable disease that could pose a threat to Canadians. Neither the Minister nor departmental officials may make these types of decisions on Cabinet's behalf.
Overview of Federal-provincial/ Territorial (FPT) Roles and Relations in Health
FPT Roles and Responsibilities in Health
Health is an area of shared jurisdiction among the federal government and provincial/territorial governments. Health services delivery, the administration of provincial/territorial health insurance plans, and the regulation of health professions fall within provincial/territorial jurisdiction.
The federal government supports universally accessible, publicly funded health care for Canadians through transfer payment to provinces and territories (PTs) via the Canada Health Transfer (CHT) and administration of the Canada Health Act (CHA). The CHA establishes the requirements that provincial/territorial health insurance plans must meet to receive their full cash contributions under the CHT. As the largest major transfer to PTs, the CHT is intended to provide long-term, predictable funding and currently represents approximately 23 percent public health sector expenditures by PTs.
Federal responsibilities include protecting health and safety through regulation, health security and emergency preparedness and response, health promotion and chronic disease prevention, infectious disease prevention and control as well as support for health research and innovation.
While PTs must provide all residents with universally insured health services, the federal government is also responsible for the financing and administration of a range of health benefits and services for federal populations, specifically for First Nations and Inuit, members of the Canadian Armed Forces and the Royal Canadian Mounted Police, veterans, inmates in federal penitentiaries and refugee claimants.
Overlapping areas of responsibility where both federal and provincial/territorial levers can support health objectives include infectious disease control, health promotion, surveillance, and emergency preparedness. Federal environmental health guidelines and regulations also provide guidance for provincial/territorial implementation and stewardship efforts. Both levels of government and their respective health organizations share responsibility for the collection and analysis of health information, and for funding research and innovation initiatives.
The diagram below summarizes the roles and responsibilities of FPT governments, including areas of overlap:
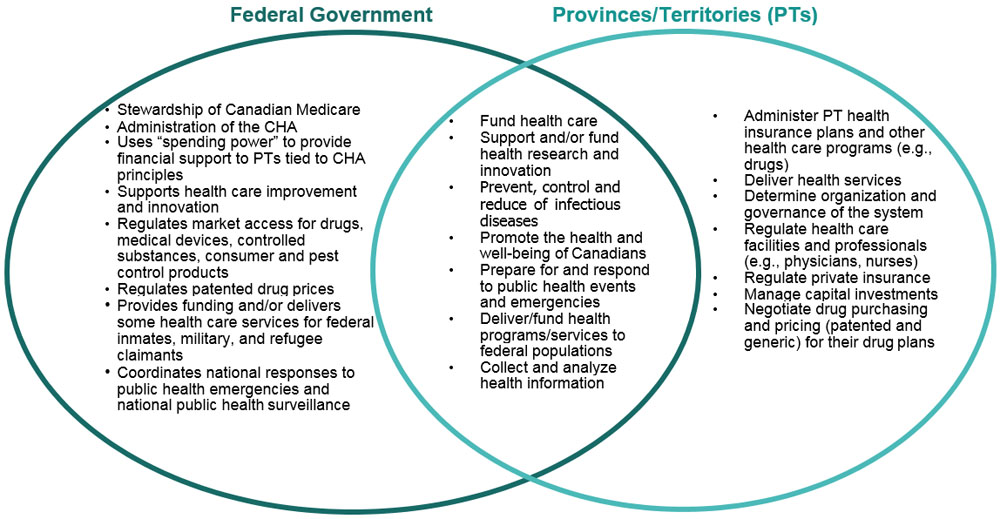
Long Description
The diagram summarizes roles and responsibilities of Federal, Provincial and Territorial governments, including the areas of overlap. The Federal government's roles and responsibilities are:
- Stewardship of Canadian Medicare;
- Administration of the Canada Health Act (CHA);
- Uses "spending power" to provide financial support to Provinces/Territories (PT) tied to CHA principles;
- Supports health care improvement and innovation;
- Regulates market access for drugs, medical devices, controlled substances, consumer and pest control products;
- Regulates patented drug prices;
- Provides funding and/or delivers some health care services for federal inmates, military and refugee claimants;
- Coordinates national responses to public health emergencies and national public health surveillance.
The Provincial and Territories' roles and responsibilities are:
- Administer PT health insurance plans and other health care programs, (for example, drugs);
- Deliver health services;
- Determine organization and governance of the system;
- Regulate health care facilities and professionals (for example, physicians, nurses);
- Regulate private insurance;
- Manage capital investments;
- Negotiate drug purchasing and pricing (patented and generic) for their drug plans.
Shared FPT roles and responsibilities are:
- Fund health care;
- Support and/or fund health research and innovation;
- Prevent, control and reduce of infectious diseases;
- Promote the health and well-being of Canadians;
- Prepare for and respond to public health events and emergencies;
- Deliver/fund health programs/services to federal populations;
- Collect and analyze health information.
FPT Collaboration
Canada's health system has been shaped by key FPT legislative activities and policies spanning over 60 years, and it has evolved to respond to changing population health needs and fiscal capacity. Ongoing FPT collaboration is crucial, as both levels of government must collaborate to address many health issues. This is especially true in areas where responsibilities intersect, such as responding to public health emergencies, preventing the spread of infectious and communicable diseases, and promoting public health.
FPT governments are advancing work in shared priority areas through the Common Statement of Principles (CSOP) on Shared Health Priorities, signed in August 2017 and supported by federal targeted investments in home and community care, and, mental health and addictions services. Following adoption of the CSOP, the federal government negotiated and signed bilateral agreements with each PT that set out details on how each jurisdiction will use federal funding to improve access to home and community care, and, mental health and addiction services.
Governments are currently collaborating on a number of high profile priorities, including opioid response efforts, vaping, cannabis legalization, medical assistance in dying, drug shortages, and the affordability and accessibility of prescription drugs, to name a few. Each of these areas has required ongoing and robust FPT engagement with other sectors (e.g., justice, public safety, trade, public health). Notably, to respond to the ongoing overdose epidemic, a Special Advisory Committee on the Epidemic of Opioid Overdoses was activated through the Public Health Network, a key mechanism for collaboration among senior public health officials.
Although the Health Portfolio generally acts as the primary focal point to engage with PTs on health-related issues, other federal departments, including Indigenous Services Canada; Veterans Affairs; Immigration, Refugees, and Citizenship Canada; Correctional Service of Canada; and the Department of National Defence also engage PTs on health-related matters, given their responsibilities for providing health services or supplementary health benefits.
FPT Machinery
Ongoing collaboration is maintained through well-developed formal structures including: an annual FPT Health Ministers' Meeting (HMM), meetings of Deputy Ministers (known as the Conference of Deputy Ministers, or CDM), and the pan-Canadian Public Health Network (PHN).
The HMM is the key intergovernmental forum through which FPT Ministers of Health discuss and provide collective direction on priority health issues and advance collaborative FPT work. A network of committees (standing and ad hoc) supports the HMM on various files. The federal Health Minister is the co-chair of the HMM, and the Deputy Minister of Health Canada acts as co-chair of the CDM. Provincial/territorial co-chairs are nominated at the provincial/territorial level, and usually rotate annually following the HMM. Ontario assumes the co-chair responsibility the week of November 18, 2019.
The federal Minister of Health, supported by the Public Health Agency of Canada (PHAC), also co-chairs the FPT Ministers of Sport, Physical Activity and Recreation (SPAR) table, alongside the Minister of Heritage and a PT co-chair (currently Yukon). The SPAR table is comprised of three distinct, but interrelated sectors: sport, physical activity and recreation. SPAR Ministers' meetings are an opportunity to highlight current federal leadership on healthy weights and chronic disease prevention. PHAC's President is one of three co-chairs of the FPT Conference of Deputy Ministers of SPAR, with responsibility for physical activity items.
The President of the Canadian Food Inspection Agency (CFIA) participates at the FPT Ministers and Deputy Ministers of Agriculture annual July meeting on matters pertaining to food safety, plant and animal health as well as trade and market access for the agriculture sector.
Through the PHN, jurisdictions work collaboratively on a broad range of issues, including health promotion, chronic disease prevention, public health infrastructure, emergency preparedness and response, and communicable and infectious diseases. The 17-member PHN Council (comprised of FPT government officials responsible for public health) is accountable to the CDM, which provides direction and approves public health policy priorities for Canada. The PHN Council also receives guidance and scientific recommendations from the Council of Chief Medical Officers of Health on technical public health issues relating to its work.
The Canadian Food Safety Information Network is a federal initiative led by CFIA and developed in partnership with Health Canada, PHAC and provincial/territorial food safety authorities. The purpose of the Network is to strengthen the ability of food safety authorities to anticipate, detect, and mitigate food safety hazards and respond quickly and effectively to food safety events. An FPT Food Safety Committee also provides federal and provincial/territorial government leadership and partnership in food safety.
Additional FPT Committees have been established to address the opioid overdose crisis, cannabis legalization and regulation, problematic substance use and harms, antimicrobial resistance, dementia, health workforce issues, medical assistance in dying, drug shortages, and interprovincial health insurance agreements, among others.
Indigenous Health
The provision of health services to Indigenous Peoples is an area of shared responsibility between FPT governments and Indigenous partners. Provincial/territorial governments provide universally accessible and publicly insured health services to all residents, including Indigenous Peoples. Indigenous Services Canada funds or directly provides health programs and services for First Nations (primarily on-reserve) and Inuit that supplement those provided by PTs. In addition, Indigenous Services Canada administers the Non-Insured Health Benefits program, which provides eligible First Nations and Inuit clients with a range of health benefits such as prescription drugs, vision and dental care and medical supplies and equipment and medical transportation to access health services.
Indigenous governments and communities are involved in directing, managing and delivering a range of health programs and services, which vary by PT.
The Health Portfolio is also involved in a range of activities, in collaboration with Indigenous, federal and provincial/territorial partners, to improve Indigenous health outcomes.
The CSOP on Shared Health Priorities commits FPT governments to work together to ensure that health care systems continue to respond to the needs of Indigenous Canadians.
Recognizing the significant disparities in Indigenous health outcomes compared to the Canadian population, FPT governments are committed to working with First Nations, Inuit and Métis to improve access to health services and health outcomes of Indigenous peoples and discuss progress in these areas.
Health Research and Innovation
The Canadian Institutes for Health Research (CIHR) works closely with the National Alliance of Provincial Health Research Organizations as key partners in the Canadian health research enterprise. Canada's Strategy for Patient-Oriented Research (SPOR) is a national coalition of FPT partners dedicated to the integration of research into patient care, whose federal funding is managed through CIHR. SPOR-funded health research provides a collaborative, co-funded FPT platform to improve jurisdiction-specific health care system issues.
List of Provincial/Territorial Ministers Responsible for Health
- British Columbia
Adrian Dix
Minister of Health
(appointed July 18, 2017) - British Columbia
Judy Darcy
Minister of Mental Health and Addiction
(appointed July 18, 2017) - Alberta
Tyler Shandro
Minister of Health
(appointed April 30, 2019) - Alberta
Jason Luan
Associate Minister of Mental Health and Addiction
(appointed April 30, 2019) - Saskatchewan
Jim Reiter
Minister of Health
(appointed August 23, 2016) - Saskatchewan
Warren Kaeding
Minister of Rural & Remote Health/Minister of Seniors
(appointed August 13, 2019) - Manitoba
Cameron Friesen
Minister of Health, Seniors and Active Living
(re-appointed October 23, 2019) - Ontario
Christine Elliott
Minister of Health
(appointed June 29, 2018) - Ontario
Michael A. Tibollo
Associate Minister of Mental Health and Addictions
(appointed June 20, 2019) - Ontario
Merrilee Fullerton
Minister of Long-Term Care
(appointed June 20, 2019) - Québec
Danielle McCann
Minister of Health and Social Services
(appointed October 18, 2018) - Québec
Lionel Carmant
Minister Delegate for Health and Social Services
(appointed October 18, 2018) - New Brunswick
Hugh J.A. (Ted) Flemming
Minister of Health
(appointed November 9, 2018) - Nova Scotia
Randy Delorey
Minister of Health and Wellness
(appointed June 15, 2017) - Prince Edward Island
James S. Aylward
Minister of Health and Wellness
(appointed May 9, 2019) - Newfoundland and Labrador
Dr. John Haggie
Minister of Health and Community Services
(appointed December 14, 2015) - Yukon
Pauline Frost
Minister of Health and Social Services
(appointed December 3, 2016) - Northwest Territories
Diane Thom
Minister of Health and Social Services
(appointed November 5, 2019) - Nunavut
George Hickes
Minister of Health
(appointed August 20, 2018)
An Overview of Pan-Canadian Health Organizations
Overview
At different points over the past thirty years, the Government of Canada created pan-Canadian health organizations (PCHOs) to address specific health care system needs and issues. There are now eight such organizations (See Table A).
PCHOs were created to address priorities in the Canadian health care system, with diverse mandates and activities. For example, the Canadian Agency for Drugs and Technologies in Health (CADTH)'s Common Drug Review assesses the cost-effectiveness of drugs; Canada Health Infoway's PrescribeIT supports electronic prescribing; the Canadian Institute for Health Information (CIHI) reports on health system performance; and the Canadian Partnership Against Cancer and the Mental Health Commission of Canada have developed national strategies on pressing health issues (cancer control and mental health respectively). While most PCHOs were established exclusively through federal investment, two (CADTH and CIHI) were created in partnership with provincial/territorial governments.
The federal government remains the majority funder of all PCHOs, with an annual investment of $285 million (2019-20) accounting for approximately 60-100% of total individual PCHO budgets (see Table A). Based on longstanding agreements, provinces and territories (PTs) provide financial support to CIHI (approximately 20% of its budget) and CADTH (approximately 25% of its budget), while Infoway cost-shares with PTs on the projects it funds.
As not-for-profit corporations, each PCHO is governed by a board of directors on which the federal government generally holds one seat (and in a few cases also designates the board chair). A senior public servant from Health Canada typically serves as the federal representative and PTs generally have public servant representation on PCHO boards. Although PCHOs are operationally independent, they are accountable to their majority funder—the Government of Canada—and have a vested interest in developing products and services that respond to the needs of the federal government and their primary partners, the PTs.
Table A: Pan-Canadian Health Organizations and Federal Funding Commitment
| Organization | Year Established | Health Canada Contribution 2019-20 | % of Budget |
|---|---|---|---|
| Canadian Centre on Substance Use and Addiction (CCSA) | 1988 | $9.5M | 94 |
| Canadian Agency for Drugs and Technologies in Health (CADTH) | 1989 | $23.1M | 60 |
| Canadian Institute for Health Information (CIHI) | 1993 | $87.7M | 80 |
| Canadian Foundation for Health care Improvement (CFHI) | 1996 | $17M | 98 |
| Canada Health Infoway (Infoway) | 2001 | $75M | 100 |
| Canadian Patient Safety Institute (CPSI) | 2003 | $7.6M | 96 |
| Canadian Partnership Against Cancer (CPAC) | 2006 | $51M | 100 |
| Mental Health Commission of Canada (MHCC) | 2007 | $14.3M | 72 |
Mandate and Core Activities
The Canadian Institute for Health Information (CIHI) is the main national body charged with collecting, analyzing and reporting health data (e.g., wait times, quality of care and outcomes, health expenditures, allocation of health professionals). CIHI data and information supports health system improvements, and is used by Canadian governments, policy-makers and health system managers in making health policy decisions and in supporting effective health system management. CIHI relies heavily on PTs for collection of health data.
The Canadian Agency for Drugs and Technologies in Health (CADTH) provides decision-makers with evidence and advice to help provincial/territorial health ministries and federal- provincial/territorial (FPT) drug plans make informed decisions about the effectiveness and efficiency of drugs, medical devices and other health technologies. The Common Drug Review and the Pan-Canadian Oncology Drug Review make recommendations to governments on drugs that are included on public drug plan formularies.
Canada Health Infoway (Infoway) works with PTs, health care providers and other partners to accelerate the development and adoption of electronic health information systems with compatible standards on a pan-Canadian basis. Infoway is currently focused on pan-Canadian initiatives including patient access to digital records, virtual care and an electronic prescribing system.
The Canadian Foundation for Healthcare Improvement (CFHI) accelerates health care improvement by working with governments, health care organizations and providers to identify, implement and measure new approaches to more effective and efficient patient care (for example, by supporting health care teams to adopt best practices in the organization and provision of care for the elderly).
The Canadian Patient Safety Institute (CPSI) provides national leadership on patient safety education, research, tools and interventions that build a culture of patient safety in order to reduce harm to patients caused by preventable patient safety incidents (such as those related to provider care, surgeries, medications, hospital-based infections and medical devices).
The Canadian Partnership Against Cancer (CPAC) provides national leadership to the development and implementation of the Canadian Strategy for Cancer Control (which addresses primary cancer prevention, screening and early detection, standards and cancer guidelines, the cancer journey, health human resources, research, and surveillance), coordinates efforts of PTs, cancer experts and stakeholder groups. CPAC recently led a refresh of the Strategy, released in June 2019.
The Mental Health Commission of Canada (MHCC) acts as a catalyst for improving the mental health system and changing the attitudes and behaviours of Canadians around mental health issues (for example, by reducing the stigma associated with mental health illness and treatment).
The Canadian Centre on Substance Use and Addiction (CCSA) conducts research on emerging issues, promotes increased awareness among Canadians about substance use and addiction, works to increase participation in efforts to reduce the harms of substance use and promotes the use of programs that have been shown to be effective in combating problematic substance use. CCSA is the only PCHO created by federal legislation.
Ministerial Role and Engagement
As PCHOs are operationally independent, the Minister of Health has no direct involvement in their day-to-day activities. The administration of contribution funding to each organization is delegated to Health Canada officials. The Minister of Health has ultimate oversight of federal investments in these organizations, including the use of federal funding to advance priorities and requests through Cabinet for new funding to support emerging federal or FPT priorities.
Most federal board appointments are the prerogative of the Deputy Minister. However, the Minister is charged with nominating the Chair and one additional federal representative to the MHCC Board of Directors, and also recommends Governor in Council appointments for the Chair and up to four other representatives to the CCSA Board of Directors.
Given the close relationship between the department and the organizations, the Minister can also expect PCHOs to seek direct engagement form time to time on matters of relevance to their respective organizations.
Role in Health System
PCHOs play an important role in the health system. [REDACTED]
In 2018, an external review of the role of the PCHOs was conducted. While structural changes were recommended, a decision was taken to proceed initially with a series of process improvements aimed at ensuring the PCHOs, individually and collectively, contribute in a more impactful manner to federal and provincial/territorial priorities for health care system improvement. Work on this agenda is underway with the PCHOs and representatives of PTs.
Key Players in Health
The Health Portfolio works with a variety of key players in health, including provinces and territories, federally funded arm's-length health organizations, non-governmental organizations, professional associations, charities, Indigenous partners, industry, the research community, other federal departments and agencies and Canadians. This work includes partnering on research, consultation and engagement, collaborative policy development, sharing information to support health system improvement, and best practice/knowledge sharing.
- Health Care Stakeholders
- Health Professionals
- Health Institutions
- Patient Groups
- Health Sector Labour Unions
- Public Health Stakeholders
- Health Charities
- National Public Health Associations
- Disease-based Advocacy Groups
- Health Promotion NGOs
- Industry Stakeholders
- Pharmaceutical
- Medical Devices
- Natural Health Products
- Food Producers
- Consumer Products
- Pesticides
- Research Stakeholders
- Universities
- Academic Organizations
- Researchers
- Think Tanks Provincial/Territorial Health Ministries
- Provincial/Territorial Health Ministries
- Pan-Canadian Health Organizations (PCHOs)
- Indigenous Organizations and Governments
- International Organizations
Relationships With the International Community
Increasingly, the health of Canadians is linked to complex global issues that cannot be addressed without collective action. Active international engagement protects and advances Canadian health interests and demonstrates Canada's global leadership on issues where we can make a difference. The Minister of Health plays a leading role internationally through bilateral and multilateral engagement, which includes sharing Canadian experiences and good practices from across sectors and all levels of government with international partners. Recent areas of focus have included health security; emergency preparedness and response; health promotion and chronic disease prevention; mental health and well-being; environmental health including the health impacts of climate change; food and consumer product safety; health research; and regulatory cooperation on pharmaceuticals and medical devices.
Both binding international agreements and non-binding international policy frameworks govern Canada's international engagement on health. Binding agreements include the World Health Organization Framework Convention on Tobacco Control and the International Health Regulations, 2005 (IHR). The IHR require member states to develop and maintain the capacity to detect and respond to outbreaks and other public health events that can have a broader international impact on human health, thereby protecting global health security. A key non- binding international policy framework that guides Canada's foreign policy engagement, including health, is the United Nations Sustainable Development Goals, a broad set of 17 non- binding international commitments adopted as part of the 2030 Agenda for Sustainable Development.
The Minister of Health works closely with the Ministers of Foreign Affairs and International Development to engage on health issues that have a predominant development focus (e.g., maternal and child health). Similarly, the Minister of Health works with the Ministers of Foreign Affairs and International Trade to support trade negotiations, in particular by advocating for the protection of Canadian health interests.
The Minister of Health engages bilaterally with counterparts from other countries, as well as heads of international organizations and multilaterally, including opportunities to lead Canada's delegation at international health fora. Both the United States (U.S.) and Mexico are key bilateral partners, given the role each country needs to play to prepare for and respond to health emergencies that may impact North America.
Multilateral Engagement
Engaging in multilateral fora allows Canada to promote its values, including good governance, accountability and transparency, and advance issues that matter to Canadians. Canada works with likeminded countries and partners in multilateral fora to enhance its ability to respond to a broad range of global health challenges, and to maintain situational awareness of emerging issues. Canada's strength comes from its reputation of being a convener and broker that facilitates agreements on key global health issues, and engagement also supports the advancement of broader foreign policy priorities beyond global health.
The Health Portfolio leads Canada's health-related engagement in a number of fora, including:
The United Nations (UN), which considers specific health issues in high-level meetings and resolutions of the General Assembly, as well as the G7Footnote 3 and G20.Footnote 4 All have active and robust health agendas. The Health Portfolio engages in these fora to build and maintain political momentum and commitment to action on priority health issues (e.g., G20 Leaders' commitments on antimicrobial resistance). These fora also provide a unique opportunity to promote work across sectors to address complex health issues in support of domestic policies and programs (e.g., the 2030 Agenda for Sustainable Development). The Minister of Health attends these meetings to demonstrate Canada's commitment to addressing key global health threats and to endorse political commitments from these meetings.
The World Health Organization (WHO) is the specialized health agency of the UN system. The WHO is responsible for providing leadership on global health matters, setting norms and standards, articulating evidence-based policy options, shaping the health research agenda, and monitoring and assessing health trends. The WHO also plays an important role in declaring and responding to global public health emergencies. Canada is an active member and engages with the WHO to advance domestic health priorities, share Canadian expertise on health issues, and protect the health of Canadians and people around the world. Canada also works closely with the WHO to protect Canadians against international health threats, including the current response to Ebola. By the end of 2019, PHAC will have deployed 16 subject matter experts internationally in support of the WHO response to health emergencies.
The Pan American Health Organization (PAHO) is the Regional Office of the WHO for the Americas and the specialized organization for health of the Organization of the American States (OAS). Engaging with PAHO offers the opportunity for Canada to be a regional leader and partner in advancing health objectives; to assist in finding joint solutions to regional challenges; and to advance health security within the region in order to protect the health of Canadians. Canada is currently serving a three-year term on PAHO's Executive Committee (2018 to 2020) and was the President of the Committee in 2019. The Health Portfolio also supports Canada's engagement with the Asia-Pacific Economic Cooperation (APEC), the Arctic Council and the Organisation for Economic Cooperation and Development (OECD), which discuss a range of health- and safety-related issues.
Bilateral and Regional Engagement
The U.S. is Canada's closest and most important relationship in health. From collaboration on food and drug regulation and health security to tackling youth vaping and associated severe lung illness, the opioid crisis, and pandemic flu risks and vaccine acceptance, and addressing food safety and chemicals management, among many other issues, Canadian and U.S. health authorities work together to protect and improve population health. Early engagement with the U.S. Secretary of Health will provide an opportunity to discuss continued bilateral collaboration and areas for collaboration in multilateral fora.
Other key partners in the Americas region include Mexico (primarily through trilateral engagement with the U.S.); Brazil, one of the largest and most influential players in Latin America; and the Caribbean, a priority region for bilateral engagement, particularly on global health security. Other key partners on health issues include the European Union (E.U.), the United Kingdom (U.K.), the Netherlands, Australia and New Zealand. China also remains a country of interest on health given its increasingly important role on the global health landscape as a main player and key contributor to multilateral fora.
Key Areas of Health Portfolio Engagement
Cooperation with International Regulators
The Health Portfolio works with key partners such as the U.S., the E.U. and various multilateral organizations such as the OECD, WHO, and PAHO to align regulatory approaches for health products including human and veterinary drugs, medical devices, consumer and hazardous products, food safety, pesticides and chemicals management. In an era of complex international trade and global supply chains, this cooperation with trusted regulatory partners helps ensure the safety of the products Canadians use and consume, and helps develop evidence-based risk mitigation approaches. Through various multilateral fora, Health Canada works with the U.S. Food and Drug Administration, the European Medicines Agency, Japan's Pharmaceutical and Medical Devices Agency, Australia's Therapeutic Goods Administration and others, to share information to inform regulatory decisions, harmonize standards, and address current and emerging health regulatory challenges.
Health Security, Emergency Preparedness and Response
The Health Portfolio works closely with WHO and PAHO, and with regional partners such as the U.S. and Mexico, to address threats to Canadian and global health security. Canada is also an active member of the Global Health Security Initiative (composed of G7 countries plus Mexico and the European Commission) and the Global Health Security Agenda (U.S.-led initiative with 44 countries). These two bodies, created in 2001 and 2014 respectively, work to enhance global capacity to prepare and respond to a wide range of chemical, biological, radiological, and nuclear threats through early detection, risk assessment and joint exercises.
Antimicrobial Resistance (AMR)
Recognizing the international nature of health threats, the Health Portfolio works closely with various partners to tackle the spread of AMR in humans, animals and the environment using a collaborative approach, as signaled by the recent declarations of the UN General Assembly, and the 2019 G20 Leaders, Health Ministers and Agriculture Ministers. Canada actively engages on multi-national initiatives including the Trans-Atlantic Task Force on AMR, and the Global Health Security Agenda. At the 2017 World Health Assembly, Canada joined the Alliance of Champions, a group of Health Ministers and health leaders committed to combatting AMR and to increasing political awareness, engagement and leadership on AMR. Currently Canada has the opportunity to demonstrate more global leadership to be more on par with other key partners such as the U.S. and U.K. The Portfolio is working with international regulators to harmonize regulatory requirements for future antimicrobial products, with the objective of streamlining approval and market access of new antimicrobials that could be used to treat antimicrobial-resistant bacteria.
Health Promotion and Chronic Disease Prevention
Chronic, non-communicable diseases such as cancer and diabetes, and their common underlying risk factors (e.g., obesity, tobacco, and physical inactivity), are an ongoing health concern for Canada and around the world. Issues such as dementia and healthy aging are of growing concern. The public health impact of these diseases and conditions have spurred greater international efforts to coordinate research and collaboration. Canada, through PHAC, hosts the WHO/PAHO Collaborating Centre on Chronic Noncommunicable Disease Policy, and works with global partners to share knowledge and tools to prevent chronic disease.
Mental Health and Well-being
Over the past two years, Canada has been advancing mental health and well-being as an international health priority, by working with key international partners such as the WHO and stakeholders. In particular, Canada, with Australia and the U.K., launched the Alliance of Champions for Mental Health and Wellbeing with the objective to increase the visibility of mental health in global health discussions and to accelerate action through increased political engagement. There will continue to be expectations for Canada to continue to play a leadership role to catalyze and sustain momentum. Leadership in this area is an opportunity for Canada to showcase Canadian expertise and best practices globally, as well as to acquire lessons learned from other countries to better address our domestic challenges.
Environment, Climate Change and Health
The Health Portfolio collaborates with key partners such as the WHO, PAHO, the OECD and the UN Environment Program (UNEP) to strengthen approaches needed to address health impacts of climate change. These include non-communicable diseases, such as cancers and respiratory and cardiovascular diseases, attributable to modifiable environmental factors like chemical exposures, air and water pollution, and soil contamination from domestic and international sources. The Portfolio works with international partners to address the current and projected health impacts of climate change on individuals, communities and health systems.
Food Safety
The Health Portfolio engages with international counterparts to establish international common standards, guidelines and recommendations to strengthen food safety and security. The Portfolio participates actively in international standard setting bodies such as the Codex Alimentarius Commission, the APEC Food Safety Cooperation Forum, and the UN Food and Agriculture Organization. There is significant collaboration between Portfolio organizations and bilateral partners, notably the U.S., the E.U., and the Food Safety QUADS (Canada, U.S., Australia, and New Zealand) to more closely align food safety regulations and food inspection practices, the surveillance of foodborne disease outbreaks and to enhance and share technical and scientific information.
Health Research
International collaboration on health research allows the Portfolio to leverage the resources and expertise of other partners to identify solutions to mutual health challenges such as obesity, mental health, dementia, exposure to radiation and environmental contaminants, antimicrobial resistance and emerging infectious diseases. This work has contributed to cutting- edge science and improvements to the health of Canadians and citizens throughout the world.
- Footnote 1
-
CFIA reports to the Minister of Health for many of its functions, and to the Minister of Agriculture and Agri-Food on matters relating to plant and animal health, food labelling and claims, and market access.
- Footnote 2
-
PMPRB regulates the prices of drugs sold directly by manufacturers, and does not have jurisdiction over prices charged by wholesalers or pharmacies, or over pharmacists' professional fees.
- Footnote 3
-
The G7 is a group of major industrial democracies (Canada, France, Germany, Italy, Japan, the U.K., and the U.S.) whose leaders meet annually to address major economic, political, and development issues.
- Footnote 4
-
The G20 brings together the world's leading industrialized and emerging economies to address economic issues requiring global cooperation.