Food and Drugs Act Liaison Office: Report on Activities April 2015 – March 2016

Download the entire report
(PDF format, 417 KB, 18 pages)
Organization: Health Canada
Type: Report
Date published: April 2015-March 2016
Message from Deputy Minister
As a regulator, Health Canada plays an important role in protecting the health and safety of Canadians. We are committed to greater openness and transparency to serve the public interest, foster better accountability and further strengthen trust in our regulatory decisions.
Under the Food and Drugs Act, our regulatory responsibilities call for fast-paced, complex decision-making anchored in sound science and regulations. The Food and Drugs Act Liaison Office (FDALO) is integral to making the regulatory process more open, transparent and understandable.
The Office’s mandate is to deliver services that foster respectful and constructive communications between Health Canada and external parties with an interest in how we administer the Food and Drugs Act. It is a neutral body with the capacity to resolve disputes and enhance client service experiences.
From its unique neutral and impartial perspective, FDALO provides feedback on concerns or improvements stakeholders have noted with the regulatory process. As outlined in this annual report, the Office has led significant changes to enhance the impartiality, transparency and openness of the prescription drug reconsideration process. The report also captures stakeholder feedback gathered over the April 2015 – March 2016 period, as well as the Office’s commitments for future improvements.
Simon Kennedy
Summary of Accomplishments
- Launched a new reconsideration process for prescription drug reviews
- Managed 135 complaint and inquiry cases
- Developed and launched 2 new competency-building workshops
- Delivered 14 training sessions
Year in Review
Launching a New Reconsideration Process
The reconsideration process is a redress mechanism within Health Canada for companies who disagree with a decision made during the drug review process. FDALO was tasked with enhancing the impartiality and transparency of the reconsideration process. This was in response to some stakeholder complaints that the very directorate that made the decision was also running the reconsideration process.
FDALO is located in the Communications and Public Affairs Branch of Health Canada, and is therefore at arm’s length from the regulatory areas responsible for drug reviews. FDALO led the redesign and began managing the process as outlined in the Guidance Document: Reconsideration of Decisions Issued for Human Drug Submissions. The new process took effect in April 2015. FDALO is now responsible for convening a reconsideration panel and the meeting to resolve the objection. We can convene an internal process by drawing on Health Canada experts who were not previously involved in the submission or enter into a contract with external experts if outside expertise or perspective is required. The process results in a recommendation to the Director General responsible for the drug review.
Over 2015-2016, FDALO received seven requests for reconsideration. One case was deemed not eligible as it was filed after the 30-day time limit. Below is a breakdown of the outcomes. “Innovator” refers to companies who file new drug submissions, while “Generic” refers to companies who manufacture a generic version of a brand name drug.
| Internal Process | External Panel | |||
|---|---|---|---|---|
| Original decision upheld | Original decision modified and sent for further review | Original decision upheld | Original decision modified and sent for further review | |
| Innovator | N/A | N/A | 2 | 1 |
| Generic | 2 | 1 | N/A | N/A |
| Total | 2 | 1 | 2 | 1 |
Evaluation and Feedback on Changes
As part of its commitment to continuous improvement, FDALO solicited feedback from all participants in the new reconsideration process. Evaluation questionnaires were sent out immediately following the reconsideration meeting, before the decision was issued. Fifteen responses were received. The following table outlines some of the feedback received (possible responses were 1=Strongly Disagree, 2=Disagree, 3=Agree, 4=Strongly Agree).
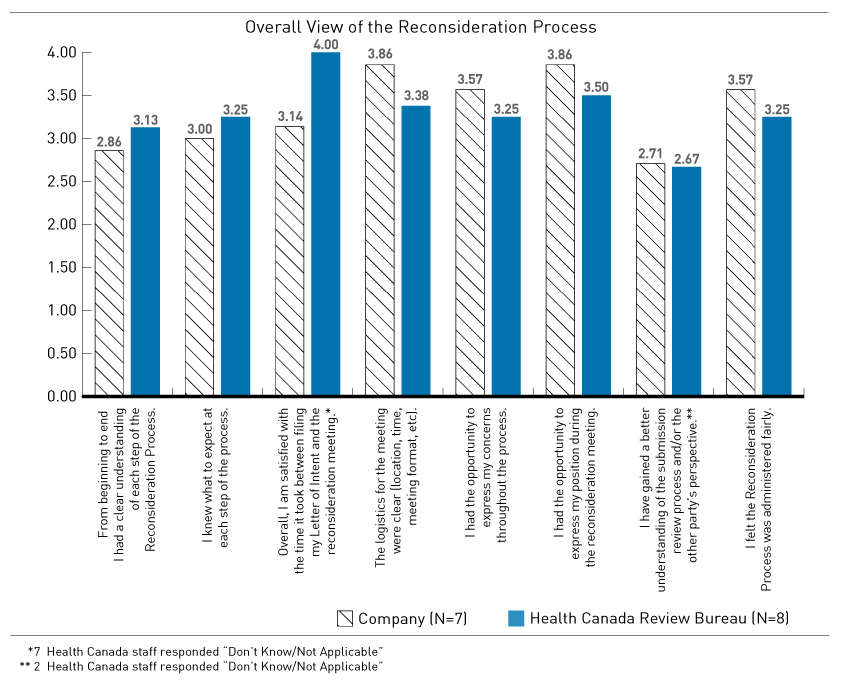
Figure 2 - Text Description
Overall View of the Reconsideration Process
Vertical bar chart detailing the summary of questionnaire responses on the reconsideration process. Responses are broken down into two categories: those from the company and those from the Health Canada Review Bureau. Seven representatives from the companies and eight representatives from Health Canada completed the questionnaire.
The range of satisfaction is zero to four (with four being the highest level of satisfaction). For the question "From beginning to end I had a clear understanding of each step of the Reconsideration Process", the level of satisfaction for the companies was 2.86 and for Health Canada it was 3.13.
For the second question "I knew what to expect at each step of the process", the level of satisfaction for the companies was 3.00 and for Health Canada it was 3.25.
For the third question "Overall, I am satisfied with the time it took between filing my Letter of Intent and the reconsideration meeting", the level of satisfaction for the companies was 3.14 and for Health Canada it was 4. To note, seven Health Canada representatives responded "Don't know/Not applicable" for this question.
For the fourth question "The logistics for the meeting were clear (location, time, meeting format, etc)", the level of satisfaction for the companies was 3.86 and for Health Canada it was 3.38.
For the fifth question "I had the opportunity to express my concerns throughout the process", the level of satisfaction for the companies was 3.57 and for Health Canada it was 3.25.
For the sixth question "I had the opportunity to express my position during the reconsideration meeting", the level of satisfaction for the companies was 3.86 and for Health Canada it was 3.50.
For the seventh question "I have gained a better understanding of the submission review process and/or the other party's perspective", the level of satisfaction for the companies was 2.71 and for Health Canada it was 2.67. To note, two Health Canada representatives responded "Don't know/Not applicable" for this question.
For the eighth question "I felt the Reconsideration Process was administered fairly", the level of satisfaction for the companies was 3.57 and for Health Canada it was 3.25.
*7 Health Canada staff responded “Don’t Know/Not Applicable”
** 2 Health Canada staff responded “Don’t Know/Not Applicable”
Based on feedback and FDALO’s own experience over the past year, additional improvements will be made to the reconsideration process to include:
- a “frequently asked questions” sheet for participants;
- a code of conduct for the meeting;
- guidance for internal and external panel members on how to manage the reconsideration meeting;
- preparatory assistance for companies and staff who require more information;
- standardized reports for clarity and consistency; and
- updated forms.
FDALO is applying the lessons learned from redesigning the drug reconsideration process to other such processes under the Food and Drugs Act. In April 2016, the Office began work to redesign the process for natural health and non-prescription health products. We will consult and engage with interested stakeholders, both external and internal to the department, as we strive to improve the process.
Case Management
Case Management in Brief
- 135 cases managed
- 63 Issues management (complaints), representing 47% of all cases
- 72 Information seeking (inquiries), representing 53% of all cases
In addition to launching a new reconsideration process, FDALO staff managed 135 cases in 2015-2016 (See Figures 3 - 7 in the Case Statistics Section). We classify our cases broadly under “issues management” or “information seeking.” Issues management cases typically involve a complaint. Our dispute resolution expertise is best used to resolve conflict early and at the lowest level possible. However, stakeholders have told us they find our assistance in navigating the complex regulatory environment and departmental structure very helpful. We have included a few case studies at the end of this report to illustrate how we help to resolve complaints and inquiries.
Over this reporting period, we experienced a 35% increase over the previous year. We attribute the rise in cases to our targeted outreach efforts to the public and Health Canada staff who can benefit from our services. We enhanced our Web presence; conducted outreach sessions with industry associations and staff; and sent information electronically to those on the departmental Stakeholder Registry. The consultation and engagement activities we conducted in redesigning the drug reconsideration process also helped to raise the profile of the Office.
A previously identified trend has continued over the past three years: more businesses are contacting the Office. Eighty-six (86) cases are from businesses and industry associations, which is 64% of all cases. Our services are also available to individuals, including patients and consumers, who have questions or complaints about the regulatory process. We heard from 25 individuals.
What We Heard
The following themes capture the main trends we heard from stakeholders this past year.
Positive Feedback
Engagement Across Sectors
A first-of-its-kind engagement session with a cross-section of stakeholders was held in June 2015. It brought together, among others, members from industry associations, health care, patient and consumer groups, and nutrition and healthy living advocates. Invitees had an opportunity to hear from departmental officials and interact with each other on how to achieve better results for Canadians in matters related to food and health care products. Departmental officials shared information about regulatory challenges and priorities, and invited feedback on how to update and renew the strategic plan to deal with the priorities. Participants with seemingly competing interests had an opportunity to engage with each other and the department over these matters. FDALO heard from several participants that they appreciated the session and hoped this type of dialogue would continue in the future.
Stakeholders Requested Improvements
Growing Desire for a New Consumer Health Products Framework
FDALO continues to receive complaints from companies about the current approach to regulating consumer health products. Industry stakeholders continue to call for a new framework that aligns the regulatory approach of non-prescription drugs, natural health products, cosmetics and disinfectants.
Under the current approach, products with similar risk profiles receive different levels of regulatory scrutiny based on how they are classified by the department. For example, a lip balm that has an SPF (sun protection factor) claim may be regulated as a drug or natural health product depending on the active ingredients and labelling claims. However, if the SPF claim is removed, the product may be regulated as a cosmetic. A lip balm regulated as a drug has to undergo a far more rigorous, time-consuming and costly process, despite the fact that it has a similar risk profile to a natural health product or cosmetic. Industry stakeholders welcome further follow-up and action to implement changes to the Consumer Health Products Framework following feedback they provided in the consultation that took place from November 2014 to February 2015. Thirty-one responses were received, mainly from industry. They expressed hope that new regulations, as well as operational changes, would soon be introduced to streamline how consumer health products are regulated. They indicated that improvements would attract more manufacturers to the Canadian marketplace and encourage the launch of more innovative products for consumers.
In 2016, Health Canada carried out additional activities to solicit feedback on the “self-care” regulatory framework from a broader cross-section of stakeholders, including consumers and patients.
Professional Use Category or Special Access Programme for Natural Health Products
Traditional medicine and natural health practitioners note that there is no regulatory framework for a class of natural health products that require oversight by a health practitioner to be made available to a consumer or patient. Under the current Natural Health Product Regulations, once a product meets the safety and efficacy standards for approval, it is available for purchase directly by consumers. Traditional and natural health practitioners are calling for a “professional use” category for natural health products so they can use these naturally sourced medicines as an alternative to prescription drugs to treat conditions that require monitoring and oversight by a qualified practitioner. Given that a “professional use” category does not currently exist, practitioners in this field request access to these natural medicines under Health Canada’s existing Special Access Programme.
Limited Redress Processes for Compliance and Enforcement Actions
Regulated parties know that Health Canada’s compliance and enforcement activities are critical to safeguarding the drugs and health products available to Canadians, as well as the integrity of Canadian brands. However, there are times when the department and its regulated parties disagree as to what type of compliance and enforcement activity is warranted. Disagreements can arise over the inspection results of a manufacturing facility, or about a specific product, either manufactured in or imported into Canada. FDALO opened 14 cases over this reporting period where the stakeholders requested a redress mechanism for various compliance and enforcement actions.
There are limited processes available for dealing with compliance and enforcement disagreements. Where “an opportunity to be heard” or a reconsideration process is available, such as for a refusal or possible suspension of a drug establishment licence, the information is difficult to find and the process not clearly understood.
The approach FDALO uses to manage all types of compliance and enforcement complaints is to broker better communication between the regulated party and the department. We help stakeholders in explaining their perspective to promote understanding. We also help to ensure that the department communicates the rationale for its decisions clearly, and explains the supporting laws and statutes in an accessible way. However, there are times when the regulated party does not agree with the actions of the operational staff, feeling perhaps that an error has been made.
These disputes can be time and resource intensive to manage for all concerned. Stakeholders have requested an administrative redress process that is clearly defined and procedurally fair for dealing with compliance and enforcement disputes.
Implementing Draft Guidance Documents
To keep up with an ever-changing regulatory and scientific environment, Health Canada must continuously review and update various guidance documents. Stakeholders have commented that Health Canada sometimes implements draft guidance processes before the department has communicated externally that the new guidance is finalized. This creates confusion, a lack of predictability and time delays to resolve. Stakeholders want to be consulted and informed before a new guidance document is implemented. Additionally, where appropriate (no major and immediate safety issue at stake), they want a reasonable transitional period to comply with the new requirements.
FDALO in Action: Case Studies
| Issue | Our office received a request from Health Canada staff for assistance in managing complaints from a citizen who kept writing and calling repeatedly despite efforts to address his concerns. The citizen’s complaint was that he suffered from a serious medical condition but was misdiagnosed by his treating practitioners. He indicated he needed access to medication not authorized for sale in Canada. His doctors would not support his request for this medication through Health Canada’s Special Access Programme. |
|---|---|
| Intervention | FDALO staff thoroughly reviewed correspondence between the citizen and the department. It was clear that this was a complex story with allegations by the citizen of negligence by health care practitioners, questioning of hospital practices, and complaints of lack of access to medication. FDALO engaged with the citizen to identify the specific issues that were of concern to him. The Office clarified all of the different issues and the proper complaints processes for each of them, which involved multiple jurisdictions. The role and scope of Health Canada’s regulatory responsibilities were also explained in clear and accessible language. |
| Outcome | The citizen wrote to FDALO to thank staff for their efforts and the clarification provided. He stated that he felt heard by our staff. A few months later, FDALO followed up with Health Canada and found that the letters and phone calls from the stakeholder had stopped. |
| Issue | Over the last several years, regulators around the world have become aware that increasing numbers of people are developing skin sensitivity to the preservative ingredients methylisothiazolinone and methylchloroisothiazolinone - MI/MCI. Health Canada added these substances to the Cosmetic Ingredient Hotlist in December of 2015, prohibiting their use in leave-on products such as body lotion. However, many companies felt they were not given sufficient notice to reformulate their products. |
|---|---|
| Intervention | FDALO addressed several complaints from small and large companies about Health Canada’s approach. Companies wanted a transitional period to allow for finding new preservative ingredients for leave-on products. They felt that existing labelling requirements would provide sufficient information for consumers to make informed decisions about whether to buy these products while they reformulated. Europe had given industry such a transitional period. The US Food and Drugs Administration chose to issue warnings to the public with no requirement to stop using the ingredients. |
| Outcome | FDALO helped industry to communicate with senior officials in the department. Using the “Recalls and Alerts” database for communicating risk to Canadians, the department issued a public advisory to consumers. Health Canada postponed the stop-sale order to give industry a brief transitional period to reformulate leave-on products. |
| Issue | A small business owner had been importing various personal care products commonly used by his cultural group for years. He was surprised one day when border officials, on the instruction of Health Canada, denied entry of his products and seized them. Like many small business operators, this person lacked knowledge and awareness of applicable laws and regulations, which put his business in peril. He was going to experience significant financial losses. He was very upset and contacted FDALO, as Health Canada compliance and enforcement officials denied his request to have a meeting. |
|---|---|
| Intervention | FDALO contacted the compliance and enforcement officials to get their perspective. For them, the issue was clear-cut: the products were not compliant with Regulations and therefore not permissible for import. They felt that they had outlined this information sufficiently in their written communication. FDALO explained that this individual would benefit from a meeting given cultural and language differences, and the complexity of the Regulations. Compliance and enforcement officials agreed to meet with him. |
| Outcome | The outcome did not change and the non-compliant products were still denied entry into the country. However, the business owner found the meeting informative and helpful. He was grateful for the time officials spent demystifying the Regulations. Armed with this knowledge, he could take steps to ensure compliance and continue operating his business without fear of further disruption. |
| Issue | A Health Canada employee contacted FDALO for assistance in resolving a conflict with a company. The company had filed a submission with a specific directorate on the assumption that the directorate was responsible for this product. Review staff classified the product differently and transferred the submission to another directorate. Staff did not clearly inform the company of the transfer and that the new review process had significantly higher fees associated with it. Reviewers in the second directorate rejected the submission in the screening phase of the review. It was at this point that staff notified the company of the transfer and the higher fee. The company objected to both the reclassification and the higher fee. |
|---|---|
| Intervention | FDALO inquired about the decision-making process resulting in the change of directorates. Specifically, we examined what staff had communicated to the company before transferring the file. Staff agreed that the rationale for the transfer had not been clearly explained. The company had not been given a chance to respond to the reclassification or the option to withdraw its submission. FDALO engaged extensively with both directorates to explore various options for resolving the matter quickly and fairly. |
| Outcome | FDALO was able to assist Health Canada in addressing the company’s concerns and prevent the issue from escalating unnecessarily. We facilitated discussions among staff from the two directorates to build consensus and agreement for how to proceed. Staff were able to work things out with the company so that it would pay only the original fee it had expected to pay, and make an informed decision about how it wished to proceed with its submission. |
Case Statistics
Who Contacted Us
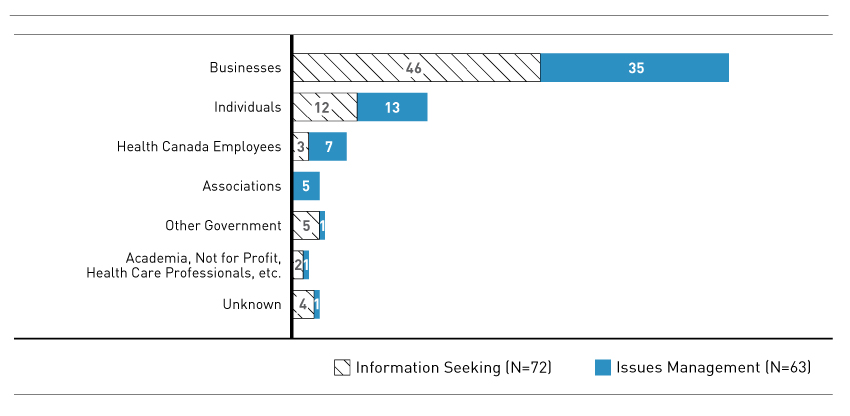
Figure 3 - Text Description
Horizontal Bar chart detailing the type of stakeholder that contacted FDALO. The chart is broken down according to Information Seeking and Issues Management Cases.
The following lists the contacts for the 72 information seeking cases: Individuals: 12; Businesses: 46; Health Canada employees: 3; Academia, not for profit, health care professionals, etc: 2; Other Government: 5; Associations: 0; Unknown: 4.
The following lists the contacts for the 63 Issues Management cases: Individuals: 13; Businesses: 35; Health Canada employees: 7; Academia, not for profit, health care professionals, etc: 1; Other Government: 1; Associations: 5; Unknown: 1.
Breakdown of Themes
We have broken down the analysis of cases further under the following four themes:
- Communication issues (For example, information-seeking inquiries, unreturned calls, unclear correspondence, or correspondence that does not address the stakeholder’s concerns.)
- Policy issues (For example, disagreements with the interpretation or application of the law, policies or regulations, such as product classification, risk assessment, policy coherence.)
- Procedural issues (For example, dissatisfaction with the processes used in regulatory decision-making, such as timeliness, openness, transparency, predictability, advance notice of changes to rule making.)
- Interpersonal issues (For example, stakeholder treatment by staff, or staff requests for assistance in dealing with difficult stakeholder communications.)
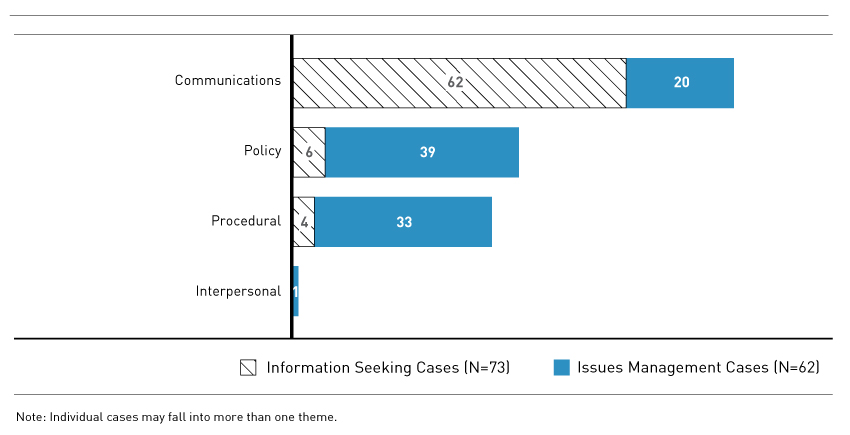
Figure 4 - Text Description
Horizontal Bar chart detailing the General Themes Identified by FDALO. Individual cases may fall into more than one theme.
The following themes were identified in the 73 information seeking cases: Communication Issues: 62 cases; Policy Issues: 6 cases; Procedural Issues: 4 cases; Interpersonal Issues: 0 cases.
The following themes were identified in the 62 Issues Management cases: Communication Issues: 20 cases; Policy Issues: 39 cases; Procedural Issues: 33 cases; Interpersonal Issues: 1 case.
Directorates Involved in our Cases
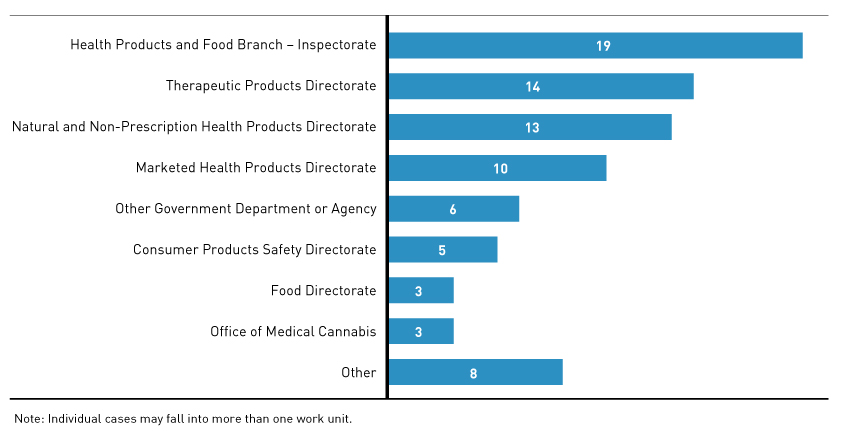
Figure 5 - Text Description
Horizontal bar graph detailing which work units were involved in resolving our 62 complaint cases. Individual cases may fall into more than one work unit.
Health Products and Food Branch Inspectorate: 19; Therapeutic Products Directorate: 14; Natural and Non-Prescription Health Products Directorate: 13; Marketed Health Products Directorate: 10; Other Government Department or Agency: 6; Consumer Products Safety Directorate: 5; Food Directorate: 3; Office of Medical Cannabis: 3; Other: 8.
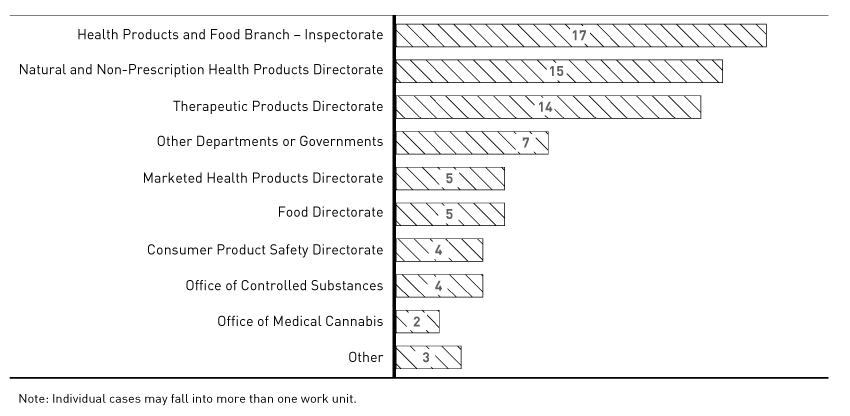
Figure 6 - Text Description
Horizontal bar graph detailing to whom the Food and Drugs Act Liaison Office made referrals in our 73 information seeking cases. Individual cases may fall into more than one unit.
Health Products and Food Branch Inspectorate: 17; Natural and Non-Prescription Health Products Directorate: 15; Therapeutic Products Directorate: 14; Other Departments or Governments: 7; Marketed Health Products Directorate: 5; Food Directorate: 5; Consumer Product Safety Directorate: 4; Office of Controlled Substances: 4; Office of Medical Cannabis: 2; Other: 3.
Geographic Origin of our Cases
Figure 7 - Geographic Origin of Cases
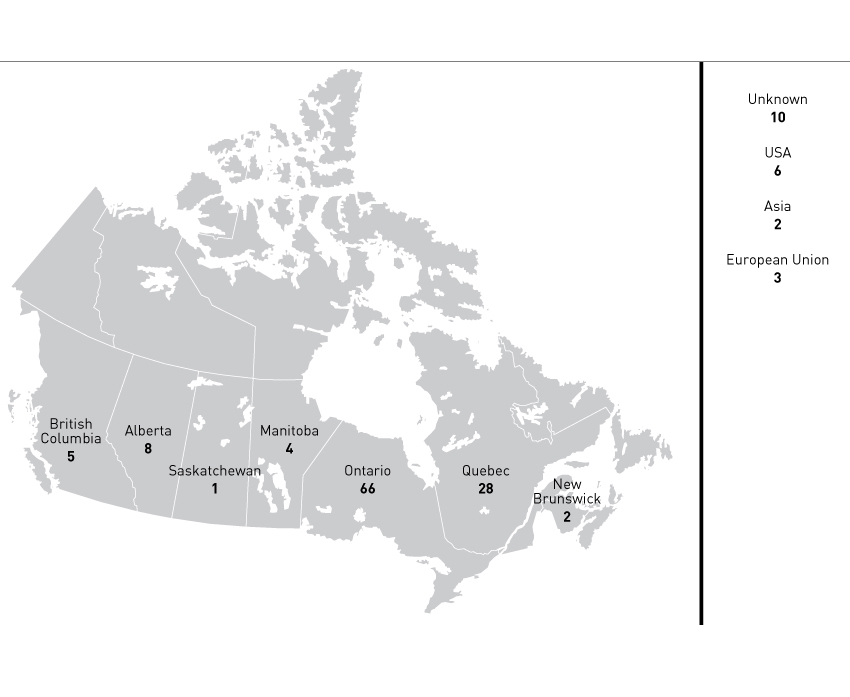
Figure 7 - Text Description
Map of Canada detailing geographic origin of cases opened by the Food and Drugs Act Liaison Office.
Ontario: 66; Quebec: 28; Alberta: 8; British Columbia: 5; Manitoba: 4; New Brunswick: 2; Saskatchewan: 1; other provinces and territories: 0; United States of America: 6; European Union: 3; Asia: 2; Unknown: 10.
Building Competency
Training in Brief
- Hosted 10 sessions of “Making the Most of Difficult Communications with Stakeholders,” with 186 attendees in total.
- Launched 2 new mini workshops, which were presented 4 times to a total of 56 attendees.
FDALO continues to play a significant role at Health Canada in building staff competencies to manage stakeholder relations within a complex and ever-changing regulatory system. Our two-day course “Making the Most of Difficult Communications with Stakeholders” continues to be very popular. We periodically open seats to other federal government staff through the Community of Federal Regulators to give participants an opportunity to share knowledge and best practices across organizations.
In 2015-2016, we launched two interactive half-day workshops. Entitled “Listening for what matters” and “Recognizing our stories”, they are designed to deepen thinking and refine the approach to resolving disputes with stakeholders. They can be delivered during staff retreats, team meetings, or as a refresher to our two-day training.
Moving Ahead
Health Canada capitalized on FDALO’s neutral and impartial position making the Office responsible for managing the drug submission reconsideration process. In the coming year, FDALO will be applying lessons learned as it revamps the reconsideration process for natural and non-prescription health products. We will conduct an external consultation before a new process is launched. The Office will also continue its role of helping to address complaints and inquiries from external stakeholders, and acting as an intermediary with Health Canada staff to resolve these efficiently and fairly.