Evaluation of the Thalidomide Survivors Contribution Program 2015 to 2019
Download the alternative format
(PDF format, 248 KB, 14 pages)
Organization: Public Health Agency of Canada
Published: 2020-08-19
Prepared by
the Office of Audit and Evaluation
Health Canada and the Public Health Agency of Canada
May 2020
Table of contents
- Executive summary
- Introduction
- History of Government Supports to Thalidomide Survivors
- Creation of the Thalidomide Survivors Contribution Program (TSCP)
- Needs of Thalidomide Survivors as they Age
- How TSCP Helped Thalidomide Survivors Age with Dignity
- Access to the TSCP by Bona Fide Thalidomide Survivors
- Delivery of the TSCP
- Conclusions
- Appendix A – Logic Model
- Endnotes
List of figure and tables
- Figure 1. EAP Eligibility Criteria
- Table 1. Proportion of Survey Respondents who Perceived that their Ability to Access Care, Treatment and Support Improved since Receiving Ongoing TSCP Payments
- Table 2. Forecasted and Actual Expenses of TSCP Contribution Agreement Funding*
List of Acronyms
- CTSSP
- Canadian Thalidomide Survivors Support Program
- EMAF
- Extraordinary Medical Assistance Fund
- EAP
- Extraordinary Assistance Plan
- TSCP
- Thalidomide Survivor Contributions Program
Executive Summary
This report examined how the Thalidomide Survivors Contribution Program (TSCP) helped thalidomide survivors and the extent to which program activities were delivered efficiently. This evaluation was required under the Financial Administration Act and the Treasury Board of Canada's Policy on Results (2016)
The TSCP was replaced in June 2019 by the Canadian Thalidomide Survivors Support Program. As such, the evaluation did not include recommendations, and relied on a review of the literature, program files, and results from annual surveys of thalidomide survivors.
Program Context
The TSCP was established in 2015, after the House of Common adopted a unanimous motion in December 2014 calling for the Government of Canada to support thalidomide survivors. The TSCP provided about $10M a year to eligible thalidomide survivors in the form of a one-time ex gratia payment, ongoing yearly support payments, and access to an Extraordinary Medical Assistance Fund (EMAF) to pay for extraordinary health supports, such as home and vehicle adaptations, or specialized surgery not covered by provincial or territorial health plans. Program intake and financial support were administered by a third-party organization under a contribution agreement with Health Canada.
Key Conclusions
The objective of the TSCP was to help thalidomide survivors age with dignity, as well as access care, treatment, and supports. Studies showed that thalidomide survivors have faced significant hardships all their lives and these challenges have increased as they age. Challenges include physical deterioration (e.g., from performing daily tasks using teeth or feet) leading to secondary problems, such as chronic pain. They are also experiencing a loss of independence or loss of support networks as their families are becoming older.
A majority of thalidomide survivors surveyed reported that the TSCP has improved their ability to age with dignity. A slight majority of respondents also indicated that the program has improved their ability to purchase aids (e.g., for better mobility) to improve their quality of life, and their ability to access private care or treatment services (e.g., massage, personal helper, chiropractic). More than half of the respondents reported having peace of mind, as the TSCP benefits have provided them with improved financial stability. Respondents whose needs were not met by the program reported that the financial assistance provided could not fund the full costs of massage or physiotherapy treatments, nor could it fund the full costs of the health care supports they require.
The TSCP was implemented in a relatively short timeframe. Policy and program authorities were approved six months following the unanimous motion from the House of Commons, while the first ongoing payments were made to thalidomide survivors in the following 16 months. Administration costs represented about eight percent of the total contribution agreement spending in 2016-17, and two percent in the following two fiscal years.
Only 35% of funding allocated to the EMAF was used in 2018-19, based on eligible claims submitted and approved. This is a notable decrease compared to fiscal years 2016-17 and 2017-18, where 67% and 80% of the funds were used, respectively. It would be appropriate for Health Canada to continue monitoring the variance between budgeted and actual expenses for the new program. This could help determine if reallocation of funding or other relevant changes would be advisable.
Of note, the annual survey of thalidomide survivors conducted by the TSCP third-party administrator gave the program a strong foundation for measuring its performance and collecting information to monitor service delivery. It would be worth maintaining this survey moving forward, as well as identifying key questions to repeat over time, in order to monitor trends on the challenges faced by thalidomide survivors.
Introduction
Operating from June 2015 to June 2019, the Thalidomide Survivors Contribution Program (TSCP) provided approximately $10 million annually to thalidomide survivorsEndnote 1. This evaluation examined the extent to which the TSCP helped thalidomide survivors to age with dignity, as well as to access care, treatment, and supports (see logic model in Appendix A). It also examined the extent to which program activities were delivered efficiently.
This evaluation was required under the Financial Administration Act and the Treasury Board of Canada's Policy on Results (2016). No recommendations were made, as the TSCP was replaced in June 2019 by the Canadian Thalidomide Survivors Support Program (CTSSP). The evaluation was based on a review of literature and program files, and annual surveys of thalidomide survivorsFootnote a.
History of Government Supports to Thalidomide Survivors
Thalidomide was released in 1957 by a German pharmaceutical company, Chemie Grünenthal, as a non-addictive sedative. The drug was authorized for use in Canada in sample format in June 1959, and subsequently authorized as a prescription medication in April 1961. It was also found to be effective at treating morning sickness in pregnant women and was used off-label for this purpose. Thalidomide was withdrawn from markets around the world after it was associated with a wide range of profoundly damaging in-utero effects (e.g., missing or stunted limbs, missing or damaged organs, death of the foetus) when taken during the first trimester of pregnancy. West Germany, the United Kingdom, and Australia withdrew the drug in December of 1961, but it remained officially available in Canada until March 2, 1962. Over ten thousand children were born worldwide with severe congenital harm as a result of thalidomide, in addition to an unknown number of deaths in utero, miscarriages, and stillbirthsEndnote 2.
In the months following the withdrawal of thalidomide, the Department of National Health and Welfare (now Health Canada), in collaboration with provinces, hospitals, professional associations, and physicians, conducted an epidemiological study to determine the number of children affected by thalidomide. This led to the creation of the 1963 Registry, which identified 115 thalidomide survivors.
Figure 1. EAP Eligibility Criteria
Thalidomide survivors had to fulfill one of the following criteria in order to be eligible for the EAP:
- verifiable information of the receipt of a settlement from the drug company;
- documentary proof (e.g., medical or pharmacy records) of the maternal use of thalidomide (brand names Kevadon or Talimol) in Canada during the first trimester of pregnancy; or
- listing on an existing government registry of thalidomide survivors (e.g., 1963 Registry).
In 1991, the Government of Canada introduced the Extraordinary Assistance Plan (EAP) to provide a one-time payment ranging between $56,000 and $83,000 to 109 thalidomide survivors who fulfilled the eligibility criteria (see Figure 1).
Creation of the Thalidomide Survivors Contribution Program (TSCP)
The TSCP was established in 2015, after concerns were raised at a national levelEndnote 3 on the increasing deterioration of health experienced by thalidomide survivors, and their need for support to age with dignity. A campaign to raise awareness culminated in a unanimous motion adopted by the House of Commons in December 2014, calling for government support for thalidomide survivorsEndnote 4. The TSCP provided the following supports to eligible thalidomide survivors:
- a one-time, tax-free ex gratia payment of $125,000 to support immediate health needs;
- ongoing yearly tax-free support payments based on level of disability, set at three levels: $25,000, $75,000, and $100,000 (indexed at two percent annually); and
- access to an Extraordinary Medical Assistance Fund (EMAF), set at $500,000 per year (indexed at two percent annually), to pay for extraordinary health support such as home and vehicle adaptations or specialized surgery.
Health Canada established a contribution agreement with a third-party administrator responsible for reviewing the eligibility of applicants, determining the disability level of thalidomide survivorsEndnote b, and administering financial assistance.
The TSCP eligibility criteria mirrored those of the EAP (see Figure 1). Thalidomide survivors who received support through the 1991 EAP were automatically admitted to the TSCP (97 individuals), while other individuals who believed they were thalidomide survivors had six months to apply. In total, 193 new individuals applied to the TSCP, of whom 25 were deemed eligible and 168 were denied. Five of the denied applicants requested judicial reviews. Two were ultimately deemed eligible under the TSCP further to court decisions rendered on March 9, 2018. As of September 2019, the TSCP recognized 122 thalidomide survivors. More than half were female (69), as opposed to male (53).
While thalidomide survivors from other countries have received financial compensation, the approaches taken have varied. For example, in the United Kingdom, thalidomide survivors have received compensation payments from childhoodEndnote 5. In Australia, compensation to thalidomide survivors has principally been in the form of settlements with the drug company. However, an Australian Senate committee concluded in 2019 that more comprehensive supports were needed to meet the needs of thalidomide survivors in that countryEndnote 6.
Needs of Thalidomide Survivors as they Age
Studies have demonstrated that thalidomide survivors have faced significant hardships, which have increased as they age. Thalidomide survivors have typically experienced significant physical deterioration (e.g., from performing daily tasks using teeth or feet) leading to secondary problems such as chronic pain. A 2011 Canadian study on thalidomide survivors found that 76% of respondents felt that their general health had become worse or much worse over the previous five yearsEndnote 7. These results were echoed by recent surveys of thalidomide survivors receiving TSCP funding. These results show that an increased proportion of respondents reported that their level of health has worsened both physically (an increase from 26% in 2017 to 57% in 2019) and functionally (an increase from 21% in 2017 to 45% in 2019).
When asked about the factors that have contributed to the change in their health status, 62% of the 2019 survey respondents indicated experiencing a physical decline due to the increased stress that performing daily tasks had put on their body. Forty-seven percent also indicated experiencing a physical decline due to pre-existing medical conditions, while 35% attributed their physical decline to a new medical condition.
In other studies, thalidomide survivors have reported a progressive loss of independence (i.e., ability to prepare meals, maintain personal hygiene, maintain upkeep of a house). In the 2011 Canadian study, 46% of respondents felt that they had lost abilities in the past five yearsEndnote 8. A 2019 inquiry in Australia highlighted that thalidomide survivors in different countries foresaw that they would need increased access to specialized therapies or to move into a long-term care facility at a younger age, when compared to their age-group peers in the general populationEndnote 9. The abilities of family members to support thalidomide survivors have also diminished with time as their parents age and their children leave home, increasing their need for servicesEndnote 10.
While many thalidomide survivors have achieved high levels of education and pursued careers, they have also reported difficulties in finding and maintaining employment due to health issues, as well as discrimination from employersEndnote 11. Specialized care, housing adaptations, and assistive technologies are also expensive, leading to financial anxietyEndnote 12. A 2017 study from Germany found that mental disorders occurred about twice as often in thalidomide survivors when compared to the general populationEndnote 13.
How TSCP Helped Thalidomide Survivors Age with Dignity
Data collected from the annual survey of thalidomide survivors shows that the program helped most of them to age with dignity. As shown in Table 1, a majority of survey respondents agreed that accessing ongoing payments under the TSCP has improved their ability to age with dignity, their ability to purchase aids (e.g., for better mobility) to improve their quality of life, and their access to private care or treatment services (e.g., massage, personal helper, chiropractic).
| Year | Slightly better or much betterTable 1 - Footnote a |
|---|---|
| Ability to age with dignity | |
| 2017 | 65% |
| 2018 | 81% |
| 2019 | 76% |
| Improved access to private care/treatments (e.g. massage, chiropractic, personal helper etc.) | |
| 2017 | 56% |
| 2018 | 63% |
| 2019 | 55% |
| Ability to purchase aids to improve my quality of life (e.g. mobility aids, hearing, visual assistive devices etc.)Table 1 - Footnote b |
|
| 2017 | 68% |
| 2019 | 59% |
Source: TSCP beneficiary survey.
|
|
In 2019, over half (54%) of the respondents reported experiencing a positive impact due to peace of mind, as the TSCP benefits provided them with financial stability. Thirty-eight percent also reported experiencing a positive impact due having an improved access to health care and support as a result of the TSCP. According to 2019 survey respondents, the ongoing TSCP support was mainly used to fund ongoing treatments or prescriptions (57%), to purchase personal care items (47%), or to hire help for daily personal activities (e.g., bathing, dressing, home maintenance) (40%). Respondents were asked to explain why the program did or did not make a difference. They were also asked about needs left unaddressed by the program. Although the number of responses for these questions was low, answers provided show that thalidomide survivors who had needs unmet by the program mainly thought that the TSCP financial assistance did not fully fund massage or physiotherapy, nor did it fund the full costs of health care supports required by thalidomide survivors.
In addition to the ongoing support, eligible thalidomide survivors were able to access the EMAF to pay for home and vehicle adaptations or specialized surgery not covered by provincial and territorial health plans. Between 20 and 38 applications were submitted annually to the EMAF from 2016-17 to 2018-19. These applications were predominantly from women, representing 55% to 75% of the EMAF annual applications, although they represented 57% of thalidomide survivors enrolled in the TSCP. The amount of funding provided under the EMAF varied greatly over the years (from $188K to $417K). According to survey responses, it was mainly used for home adaptations, followed by medical expenses.
Access to the TSCP by Bona Fide Thalidomide Survivors
Since its creation, the TSCP has been subject to criticism in the House of Commons, by individuals, and in the media. In addition, judicial reviews were initiated in Federal Court for those whose applications to the TSCP were rejected, asking for reconsideration on the basis that the eligibility criteria were unreasonably restrictive.
Concerns were raised that it may be difficult to fulfill the program eligibility criteria, as mothers of thalidomide survivors may not have been aware that they had taken thalidomideEndnote 14, or records from hospitals, doctor offices, or pharmacies may have been lost or disposed of over timeEndnote 15. Concerns were also raised about the possibility that not all thalidomide survivors were recorded in the 1963 RegistryEndnote 16.
In 2017, the House of Commons Standing Committee on Health recommended that the Minister of Health review and reconsider TSCP eligibility criteria, with a focus on the United Kingdom Thalidomide Trust's model. The United Kingdom model screens applicants on the principle of a balance of probabilities that they had been exposed to thalidomide during pregnancy.
The TSCP was replaced by the CTSSP in the spring of 2019. The new program has expanded eligibility criteria to align with international practices by incorporating a probabilities-based approach. Thalidomide survivors who had previously received support under the TSCP were automatically enrolled in the new program. Those who have previously been denied under the TSCP or who have never applied for available supports can apply to this new program.
Delivery of the TSCP
Program Implementation
The TSCP was implemented in a relatively short timeframe. Policy and program authorities were approved six months following the unanimous motion from the House of Commons calling for government support to thalidomide survivors in December 2014. The first ongoing payments were made to thalidomide survivors starting on March 30, 2016, 16 months after the motion was adopted.
During that period, about 10 full-time-equivalent staff from Health Canada undertook the tasks of locating approximately 100 known thalidomide survivors and administering ex gratia payments to them, developing terms and conditions for the program, and launching a request for proposals to select the third-party administrator. The contribution agreement with the third-party administrator was signed in October 2015.
Program implementation occurred with a certain degree of complexity, as Health Canada had to coordinate with provinces to ensure that payments to thalidomide survivors were not clawed back, and to ensure the privacy of sensitive personal information. In a press release from May 2015, the Thalidomide Victims Association of Canada, on behalf of the Thalidomide Survivor Task ForceFootnote c, noted that they were very appreciative of all the hard work that the Minister and Health Canada had put into this program over the previous six monthsEndnote 17.
Program Administration
The program was administered at a relatively low cost. In the last year of the TSCP, its operations were overseen by approximately one full-time-equivalent staff member at Health Canada. The administrative costs of delivering the program by the third-party administrator represented eight percent of the total program expenditures in the first year of program implementation (2016-17), and then decreased to two percent in the following two years (2017-18 and 2018-19). As shown in Table 2, in those two years, the third-party administrator spent only about 40% to 42% of the funding budgeted for program administration.
Funding for the EMAF was underspent in all years. As mentioned previously, between 20 and 38 applications were submitted in a given year to the EMAF. Reasons explaining the uptake of the EMAF were explored in the beneficiary survey, but the number of respondents who provided an explanation was not large enough to draw meaningful conclusions. Responses provided suggest that a few respondents felt that the application rules were not clear, or that the process was too cumbersome. The most frequently provided response was that the respondent did not need the funding at that point.
| Budget Items | Forecasted Expenses (in Millions $) | Actual Expenses (in Millions $) | Variance Forecast & Actual Expenses | Ratio of Administrative Cost over Total Expenses |
|---|---|---|---|---|
| 2016-17 | ||||
| Ongoing payments to thalidomide survivors | 9.2 | 9.1 | 99% | - |
| EMAF | 0.51 | 0.34 | 67% | - |
| Cost to administer the program | 0.72 | 0.84 | 117% | 8% |
| Total | 10.4 | 10.3 | ||
| 2017-18 | ||||
| Ongoing payments to thalidomide survivors | 9.1 | 7.8 | 86% | - |
| EMAF | 0.52 | 0.42 | 80% | - |
| Cost to administer the program | 0.35 | 0.14 | 40% | 2% |
| Total | 10 | 8.4 | ||
| 2018-19 | ||||
| Ongoing payments to thalidomide survivors | 8.6 | 7.9 | 92% | - |
| EMAF | 0.53 | 0.19 | 35% | - |
| Cost to administer the program | 0.34 | 0.14 | 42% | 2% |
| Total | 9.5 | 8.1 | - | |
|
Source: Program financial reports and progress reports.
|
||||
Ease of Program Navigation
Overall, the service and information provided by the third-party administrator was viewed as helpful by thalidomide survivors. The majority of respondents to the 2017 and 2019 surveys (73% and 72%, respectively) found that the level of service received from calling the administrator was either helpful or very helpful. Up to two-thirds of respondents also reported that the level of service received from emailing the administrator was either helpful or very helpful (56% in 2017 and 66% in 2019). Sixty-nine percent of 2019 respondents also felt that the third-party administrator's website was easy to navigate.
Performance Measurement
The annual survey of thalidomide survivors has been a useful source of information for measuring the program's performance in achieving its expected outcomes. It also allowed for monitoring of the satisfaction of thalidomide survivors with the services and information provided to them. However, the wording of some survey questions has been modified between survey versions, limiting the ability to compare certain results over time.
Conclusions
Thalidomide survivors have experienced the disastrous effects of this drug all of their lives and the hardships they have always faced have increased as they age. Supporting Canadian thalidomide survivors is a need that has been recognized by the House of Commons. As shown by available data, the TSCP made a notable contribution to help thalidomide survivors age with dignity and access to care, treatment, and support. The program was also delivered efficiently, with low administrative costs.
While the TSCP has been replaced by the CTSSP, a few observations identified in this evaluation are worth considering moving forward. In the context where funding for program administration and EMAF was underspent, especially in 2018-19, it would be appropriate for Health Canada to continue monitoring the variance between budgeted and actual expenses for the new program, as well as the possible reasons to explain the variance. This could help determine if reallocation of funding or other relevant course corrections would be advisable. The annual survey conducted by the third-party administrator provided the TSCP with a strong foundation for performance measurement and for collecting information to monitor service delivery. It would be worth maintaining this survey moving forward, as well as identifying a few key questions to repeat over time, in order to collect trend data on the situation of thalidomide survivors.
Appendix A – Logic Model
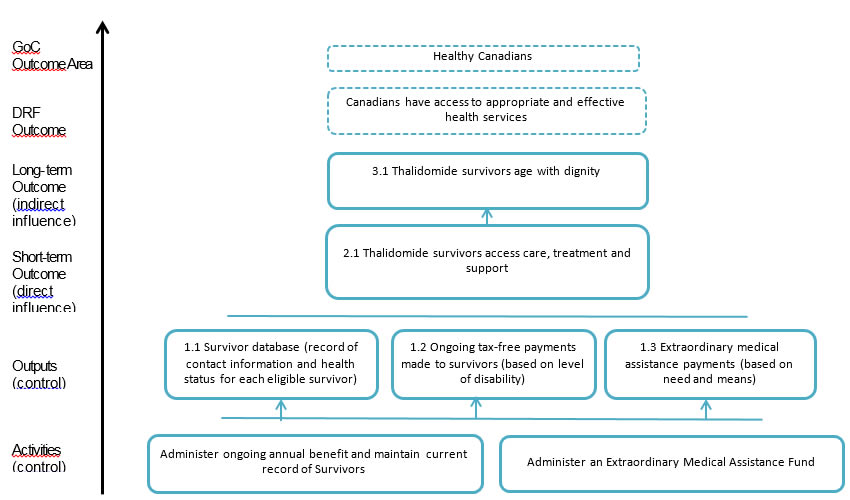
Appendix A – Logic model for Thalidomide
Activities (control)
- Administer ongoing annual benefit and maintain current record of Survivors
- Administer an Extraordinary Medical Assistance Fund
Outputs (control)
1.1Survivor database (record of contact information and health status for each eligible survivor)
1.2Ongoing tax-free payments made to survivors (based on level of disability)
1.3Extraordinary medical assistance payments (based on need and means)
Short-term Outcome (direct influence)
2.1Thalidomide survivors access care, treatment and support
Long- term Outcome (indirect influence)
3.1Thalidomide survivors age with dignity
DRF Outcome
- Canadians have access to appropriate and effective health services
GoC Outcome Area
- Healthy Canadians
Footnotes
- Footnote a
-
The third-party administrator responsible for administrating the TSCP sent annual surveys to thalidomide survivors from 2016 to 2019 to gauge their satisfaction with the program, changes in health status, and services received. Data from the 2016 survey were not included in this report, as they were not fully consistent with other cohorts. Results discussed in the report should be interpreted with caution, as the number of respondents varied significantly across cohorts (i.e., 2017 (n=34); 2018 (n=52) and 2019(n=74)).
- Footnote b
-
The TSCP provided thalidomide survivors with the ability to request a reassessment of their disability level in recognition of increased health issues due to aging.
- Footnote c
-
The Thalidomide Survivor Task Force was created in 2013 to build the case for long-term financial support for thalidomide survivors in Canada. The Task Force consisted of representatives from the Thalidomide Victims Association of Canada, as well as lawyers and a government relations firm. (Information from: https://thalidomide.ca/en/right-the-wrong-campaign-victory/)
Endnotes
- Endnote 1
-
2017-18 Departmental Plan: Health Canada. Retrieved from: https://www.canada.ca/en/health-canada/corporate/transparency/corporate-management-reporting/departmental-performance-reports/2017-18-supplementary-information-tables/page-8-2017-18-supplementary-information-tables.html#a10
- Endnote 2
-
Vargesson, N. 2015. Thalidomide-Induced Teratogenesis: History and Mechanisms. Volume 105: Birth Defects Research Part C: Embryo Today: Reviews, p. 142. Doi: 10.1002/bdrc.21096. Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/26043938
- Endnote 3
-
Thalidomide Victims Association of Canada. 2015. Victory of the “Right the Wrong” Campaign. Retrieved from: https://thalidomide.ca/en/right-the-wrong-campaign-victory/
- Endnote 4
-
Parliament of Canada. 41st Parliament, 2nd Session Edited Hansard. Number 150, Thursday, November 27, 2014. Retrieved from: https://www.ourcommons.ca/DocumentViewer/en/41-2/house/sitting-150/hansard
- Endnote 5
-
Newbronner E, Glendinning C, Atkin K, Wadman R. 2019. The health and quality of life of Thalidomide survivors as they age – Evidence from a UK survey. PLoS ONE 14(1): e0210222. Retrieved from: https://doi.org/10.1371/journal.pone.0210222
- Endnote 6
-
The Senate, Community Affairs References Committee, Commonwealth of Australia. 2019. Support for Australia's thalidomide survivors: Interim report, Chapter 4, Compensation and international government responses. Retrieved from: https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/ThalidomideSurvivors/Interim_Report
- Endnote 7
-
Thalidomide Victims Association of Canada. 2013. Study on the Current Living Conditions of Canadian Thalidomide survivors and their Projections for their Future, p. 38. Retrieved from: https://thalidomide.ca/wp-content/uploads/2017/12/2013-study-report.pdf
- Endnote 8
-
Thalidomide Victims Association of Canada. 2013. Study on the Current Living Conditions of Canadian Thalidomide survivors and their Projections for their Future, p. 32. Retrieved from: https://thalidomide.ca/wp-content/uploads/2017/12/2013-study-report.pdf
- Endnote 9
-
The Senate, Community Affairs References Committee, Commonwealth of Australia. 2019. Support for Australia's thalidomide survivors: Interim report, Chapter 2, The impact of thalidomide injuries. Retrieved from: https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/ThalidomideSurvivors/Interim_Report
- Endnote 10
-
Thalidomide Victims Association of Canada. 2013. Study on the Current Living Conditions of Canadian Thalidomide survivors and their Projections for their Future, p. 9. Retrieved from: https://thalidomide.ca/wp-content/uploads/2017/12/2013-study-report.pdf
The Senate, Community Affairs References Committee, Commonwealth of Australia. 2019. Support for Australia's thalidomide survivors: Interim report, Chapter 2, The impact of thalidomide injuries, Carer Responsibilities, p. 39. Retrieved from: https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/ThalidomideSurvivors/Interim_Report - Endnote 11
-
Newbronner E, Glendinning C, Atkin K, Wadman R. 2019. The health and quality of life of Thalidomide survivors as they age – Evidence from a UK survey. PLoS ONE 14(1): e0210222. Retrieved from: https://doi.org/10.1371/journal.pone.0210222
Standing Committee on Health, House of Commons Canada. HESA, NUMBER 054, 1st SESSION, 42nd PARLIAMENT EVIDENCE, Thursday, May 11, 2017, p. 2. Retrieved from: https://www.ourcommons.ca/DocumentViewer/en/42-1/HESA/meeting-54/evidence - Endnote 12
-
Thalidomide Victims Association of Canada. 2013. Study on the Current Living Conditions of Canadian Thalidomide survivors and their Projections for their Future, p. 43. Retrieved from: https://thalidomide.ca/wp-content/uploads/2017/12/2013-study-report.pdf
- Endnote 13
-
Niecke, A; Peters, K; Samel, C; Forster, K; Lüngen, M; Pfaff, H; Albus, C. 2017. Mental Disorders in People Affected by Thalidomide. Deutsches Ärzteblatt International; 114: 168–74. Retrieved from: https://www.aerzteblatt.de/int/archive/article/186661/Mental-disorders-in-people-affected-by-thalidomide-a-cross-sectional-study-of-prevalence-and-psychosocial-needs
- Endnote 14
-
Standing Committee on Health, House of Commons Canada. HESA, NUMBER 054, 1st SESSION, 42nd PARLIAMENT EVIDENCE, Thursday, May 11, 2017, p. 1. Retrieved from: https://www.ourcommons.ca/DocumentViewer/en/42-1/HESA/meeting-54/evidence
- Endnote 15
-
Standing Committee on Health, House of Commons Canada. HESA, NUMBER 054, 1st SESSION, 42nd PARLIAMENT EVIDENCE, Thursday, May 11, 2017, p. 1. Retrieved from: https://www.ourcommons.ca/DocumentViewer/en/42-1/HESA/meeting-54/evidence
- Endnote 16
-
The War Amputations of Canada. 1989. Report of the Thalidomide Task Force, Synopsis, p. 8. Retrieved from: https://thalidomide.ca/wp-content/uploads/2018/01/synopsis-war-amps-report.pdf
- Endnote 17
-
Thalidomide Victims Association of Canada. Press release – May 22, 2015. Official Statement from the Thalidomide survivors Taskforce concerning Health Canada's Program to Assist the Canada's 94 Living Victims of Thalidomide. Retrieved from: https://thalidomide.ca/en/may-22-2015-press-release-about-health-canadas-program-for-thalidomide-survivors/
Page details
- Date modified:
