Tobacco Products Appearance, Packaging and Labelling Regulations
Backgrounder
June 2023
Tobacco use in Canada
Tobacco use continues to be the leading preventable cause of illness and premature death in Canada, killing approximately 48,000 Canadians each year.
Smoking is linked to more than 40 diseases and conditions, including cancer and heart disease. Many of these health effects can be reversed or reduced after a person quits tobacco use.
Tobacco products contain nicotine, a highly addictive substance responsible for tobacco dependence. Young persons are particularly susceptible to the risk of dependence and report symptoms of dependence even at low levels of cigarette use.
The health and economic costs associated with tobacco use in Canada were estimated at $12.3 billion in 2017, with direct health care costs of $6.1 billion. While tobacco prevalence in Canada is at 13% currently, the health care costs associated with tobacco use represent 47% of all health care costs associated with substance use in Canada.
Canada’s Tobacco Strategy aims to reach less than 5% tobacco use by 2035.
The evolution of tobacco product packaging and labelling in Canada
Health-related messages on tobacco product packaging continue to be recognized as one of the best approaches to informing people in Canada of the health hazards of tobacco use.
Canada first adopted pictorial warning requirements for tobacco product packages in 2000 under the Tobacco Product Information Regulations to increase awareness of the health hazards and health effects associated with tobacco use. The warnings combined strong images with messages that were noticeable, informative and credible.
The 2011 Tobacco Products Labelling Regulations (Cigarettes and Little Cigars) introduced strengthened labelling requirements for cigarettes and little cigar packages. The requirements included:
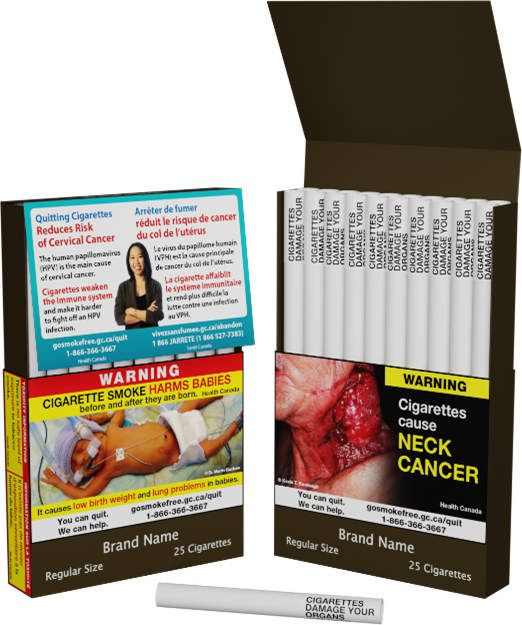
- pictorial health warnings that cover 75% of the front and back of packages and include a pan-Canadian quit line number and web address;
- health information messages enhanced with colour and images; and
- easy to understand toxic emissions and toxic constituents statements.
The Tobacco Products Regulations (Plain and Standardized Appearance) were adopted in 2019. These regulations standardized the appearance of tobacco packages and products through general requirements applicable to all tobacco products, as well as through specific requirements applicable to individual tobacco product types. For instance, all tobacco product packages have to be of the same drab brown colour, bearing only the permitted text displayed in a standard location, font style, colour and size.
Each of these regulations exist under the Tobacco and Vaping Products Act, which regulates the manufacture, sale, labelling and promotion of tobacco products, as well as vaping products.
Strengthened labelling regulations for tobacco products including renewed health warnings
Building on the positive impact of existing tobacco product labelling regulations, updates will help maintain the messages’ effectiveness at raising public awareness about tobacco-related health hazards and reflect the latest research and science available.
Informed by a series of public consultations, as well as by feedback received between June and August 2022 following pre-publication of the proposal in the Canada Gazette, Part I, the new Tobacco Products Appearance, Packaging and Labelling Regulations build on existing regulatory requirements by introducing the following measures:
- Renewed health-related messages (health warnings, health information messages and toxicity information).
- Health warning and toxicity information requirements extended to all tobacco product packaging.
- A minimum size of 75% of the main display area of the packaging for health warnings for most tobacco products.
- A new location for the health information messages on cigarette packages to make these messages more noticeable.
- A health warning printed directly on individual cigarettes, little cigars that have tipping paper, and tubes to inform users, in particular young persons who may not be exposed to the packaging, of the health hazards of tobacco use.
- A rotation scheme that aims to enhance the novelty and relevance of the messages on tobacco products and packages by rotating sets of messages on a pre-determined schedule.
- The ability to update the content of health-related messages (such as images or text) to reflect the most up to date science and research available without updating the regulations.
The regulations bring Canada into full compliance with its labelling obligations under Article 11 of the World Health Organization Framework Convention on Tobacco Control.
Implementation
The new regulations will come into force on August 1, 2023 and will be implemented through a phased approach that will see most measures on the market within the next year, including health warnings printed directly on most cigarettes sold in Canada.
| Proposed Measure | Manufacturers | Retailers |
|---|---|---|
| New Health-related Messages for All Tobacco Product Packages | 6 months (January 31, 2024) |
9 months (April 30, 2024) |
| HW on Individual King Size Cigarettes | 9 months (April 30, 2024) |
12 months (July 31, 2024) |
| HW on Individual Regular Size Cigarettes, Little Cigars with Tipping Paper, and Tubes | 18 months (January 31, 2025) |
21 months April 30, 2025) |
| New Placement for HIM on Extended Upper Slide-flap for Cigarette Packages | 36 months (July 31, 2026) |
39 months (October 31, 2026) |
The full suite of messages are currently available upon request through pregs@hc-sc.gc.ca. The first rotation of messages will also become available on Canada.ca in the coming weeks.
More Information
The new regulations will be published in the June 7, 2023 edition of the Canada Gazette, Part II. In the interim, copies of the new regulations can be obtained upon request through pregs@hc-sc.gc.ca.