Aluminum in drinking water: Guideline technical document for consultation

Download the alternative format
(PDF format, 937 KB, 65 pages)
Organization: Health Canada
Date published: 2019-06-28
Purpose of consultation
This guideline technical document outlines the evaluation of the available information on aluminum with the intent of updating the guideline value(s) for aluminum in drinking water. The purpose of this consultation is to solicit comments on the proposed guideline and operational guidance (OG) value, on the approach used for their development, and on the potential economic costs of implementing them.
The existing guideline technical document on aluminum, developed in 1998, recommended OG values for treatment plants using aluminum-based coagulants as follows: less than 0.1 mg/L (100 µg/L) for conventional treatment plants and less than 0.2 mg/L (200 µg/L) for other types of treatment systems (e.g., direct or in-line filtration plants, lime softening plants). A health-based guideline was not established at that time, as there was no consistent, convincing evidence that aluminum in drinking water could cause adverse health effects in humans.
This document proposes a maximum acceptable concentration (MAC) of 2.9 mg/L (2,900 µg/L) for total aluminum in drinking water, based on neurological effects observed in rats. An OG value of 0.050 mg/L (50 μg/L) is proposed for total aluminum to optimize water treatment and distribution systems.
This document is available for a 60-day public consultation period.
Please send comments (with rationale, where required) to Health Canada via email:
If this is not feasible, comments may be sent by postal mail to:
Water and Air Quality Bureau, Health Canada
269 Laurier Avenue West, A.L. 4903D
Ottawa, ON K1A 0K9
All comments must be received before August 30, 2019. Comments received as part of this consultation will be shared with members of the Federal-Provincial-Territorial Committee on Drinking Water (CDW), along with the name and affiliation of their author. Authors who do not want their name and affiliation shared with CDW members should provide a statement to this effect along with their comments.
It should be noted that this guideline technical document will be revised following the evaluation of comments received, and a drinking water guideline will be established, if required. This document should be considered as a draft for comment only.
Proposed guideline
A maximum acceptable concentration (MAC) of 2.9 mg/L (2900 μg/L) is proposed for total aluminum in drinking water, based on a locational running annual average of a minimum of quarterly samples taken in the distribution system.
An OG value of 0.050 mg/L (50 μg/L) is proposed for total aluminum to optimize water treatment and distribution systems.
Executive summary
This guideline technical document was prepared in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water and assesses all available information on aluminum.
Exposure
Aluminum is a metal widely distributed in nature. It may be present in water from natural sources or as a result of human activities. The metal is used for many purposes: in the production of construction materials, vehicles, aircraft, electronics, pharmaceuticals and personal care products; as food additives; and as components of food packaging materials. Aluminum salts are commonly added as coagulants during water treatment to remove turbidity, organic matter and microorganisms. Aluminum is also an impurity found in other water treatment chemicals and can leach into drinking water from cement mortar pipes or linings.
The Canadian population is exposed to aluminum from its presence in the environment and in a variety of products and processes. The main source for Canadians' exposure is through food, followed sequentially by exposure through soil, drinking water and air. Aluminum concentrations in water vary across Canada, with surface water generally presenting higher concentrations than groundwater. Intake of aluminum from drinking water is not expected to occur through either skin contact or inhalation.
Health effects
Aluminum is not an essential element. Studies in humans have found possible associations between aluminum ingestion and diseases of the nervous system. However, these studies have a number of design limitations and do not provide strong evidence that aluminum can cause these diseases. Studies in animals have consistently observed adverse effects on the nervous system following ingestion of high levels of aluminum, which supports effects seen in human studies. The proposed MAC of 2.9 mg/L is based on neurological effects observed in rats.
Operational and aesthetic considerations
Aluminum can act as an accumulation sink for such other contaminants as arsenic, chromium, manganese and nickel and can influence the concentrations of lead and copper. An OG of 0.050 mg/L is proposed for both the entry point and distribution system to minimize the potential accumulation and release of aluminum and co-occurring contaminants.
Aluminum can coat watermains, service lines and water meters, resulting in pressure losses, meter malfunctions or turbid/discoloured water. An OG of 0.050 mg/L is proposed for both the entry point and the distribution system to avoid these issues as well.
Analytical and treatment
Several methods are available for analyzing total aluminum in drinking water at concentrations well below the proposed MAC and OG. Online or portable colorimetric analyzers are important tools for obtaining a rapid indication of changes to aluminum concentrations. These measurements can be used to make quick treatment adjustments, which are critical for effective plant operation. Water utilities should confirm with the responsible drinking water authority in the affected jurisdiction whether results from these units can be used for compliance reporting.
Water treatment strategies should minimize the aluminum concentration that enters the distribution system from the treatment plant. For water treatment plants using aluminum-based coagulants, the aluminum residual is an important process parameter (like pH, temperature, turbidity and other measurements) to practice optimum coagulation. Strict pH control and adequate coagulant dosing are necessary to optimize coagulation and minimize aluminum residual concentrations. It is important to note that coagulant under-dosing can result in substantial deterioration of pathogen removal capability. Strategies to minimize residual aluminum concentrations should not compromise the removal effectiveness of pathogen or natural organic matter (NOM) (i.e., disinfection by-product precursors).
Measures should also be in place to minimize the contribution of aluminum from other water treatment chemicals.
For naturally occurring aluminum in source water, the only known effective treatment technology is coagulation, which is not typically undertaken in small systems or private water supplies. In cases where aluminum removal is required and coagulation is not feasible, the responsible drinking water authority in the affected jurisdiction should be contacted to discuss possible options.
Distribution system
It is recommended that water utilities develop a distribution system management plan to minimize the accumulation and release of aluminum and co-occurring contaminants in the system. This typically involves minimizing the aluminum concentration entering the distribution system and implementing best practices to maintain stable chemical and biological water quality conditions throughout the system, as well as to minimize physical and hydraulic disturbances.
Application of the guideline
Note: Specific guidance related to the implementation of drinking water guidelines should be obtained from the appropriate drinking water authority in the appropriate jurisdiction.
Due to the effect of pH, temperature and NOM on aluminum concentrations, seasonal trends can be highly relevant, even for systems that do not add coagulants. Treatment modifications or other operational practices can also impact aluminum concentrations. Thus, water utilities should carefully monitor total aluminum concentrations, from the source through to the distribution system, as concentrations can change. Site-specific monitoring plans should be developed to capture all seasonal water quality conditions for comparison with the proposed OG of 0.050 mg/L.
Total aluminum in drinking water, based on a locational running annual average of a minimum of quarterly samples taken in the distribution system, should be calculated for comparison with the proposed MAC of 2.9 mg/L.
International considerations
Other national and international organizations have drinking water guidelines, standards and/or guidance values. Variations in these values can be attributed to the age of the assessments or to differing policies and approaches, including the choice of key study and the use of different consumption rates, body weights and source allocation factors.
The United States Environmental Protection Agency (U.S. EPA), the European Union and Australia's National Health and Medical Research Council have not established health-based regulatory limits for aluminum in drinking water. Rather, these agencies and other international agencies have set OG values ranging from 0.050 mg/L to 0.20 mg/L, based on aesthetic or operational considerations.
In its 2010 assessment of aluminum in drinking water, the World Health Organization (WHO) has calculated a non-regulatory health-based value of 0.9 mg/L but has highlighted the importance of not exceeding the practicable levels of 0.1-0.2 mg/L. The proposed Canadian guideline differs from the WHO's health-based value because Canada takes into consideration advancements in science since 2010. The WHO assessment is based on the Joint Food and Agriculture Organization of the United Nations/WHO Expert Committee on Food Additives's (JECFA) previous Provisional Tolerable Weekly Intake (PTWI) for aluminum of 1 mg/kg body weight per day (JECFA, 2007). JECFA has since revised their PTWI to 2 mg/kg body weight per day (JECFA, 2012) based on the key study, Poirier et al. 2011, that is used in the Canadian guideline.
Table of Contents
- Proposed guideline
- Executive summary
- Exposure
- Health effects
- Operational and aesthetic considerations
- Analytical and treatment
- Distribution system
- Application of the guideline
- International considerations
- 1.0 Exposure Considerations
- 2.0 Health Considerations
- 3.0 Derivation of the health-based value
- 4.0 Analytical and Treatment Considerations
- 5.0 Control strategies 5.2 Monitoring
- 6.0 International Considerations
- 7.0 Rationale
- 8.0 References
- Appendix A: List of acronyms
- Appendix B: Provincial and territorial anticipated impacts
- Appendix C: Canadian water quality data
1.0 Exposure Considerations
1.1 Sources and uses
Aluminum is the third most abundant metal in the earth's crust. Mining and weathering of minerals results in the release of aluminum; consequently, it is found naturally in soils, groundwater, surface water and agricultural products such as vegetables, grains and meat. Canada is the world's third largest producer of aluminum. The metal is used widely in construction materials (e.g., for buildings and infrastructure), vehicles, aircrafts, electronics and packaging materials (NRCan, 2018). Aluminum compounds are also used by the pharmaceutical industry in personal care products, in food packaging and as a food additive. In addition, aluminum is used widely in treatment plants for drinking water, wastewater and industrial water. In drinking water treatment, aluminum salts are applied to remove turbidity, organic material and microorganisms. Statistics Canada (2013) reports that aluminum-based coagulants are used in the treatment process for 69.2% of surface waters and 6.7% of groundwater/GUDI (groundwater under the direct influence of surface water). Aluminum has been found to leach from cement mortar pipes or linings into drinking water (Leroy et al., 1996) and is also an impurity found in other chemicals used in water treatment (e.g., for pH adjustment).
1.2 Substance identity
Aluminum (Chemical Abstracts Service Registry No. 7429-90-5) is a ductile metal with a molecular weight of 26.98 and a vapour pressure of 1 mm Hg at 1,284 °C (ATSDR, 2008). The chemistry of aluminum in the aquatic environment is complex. The speciation, mobility and partitioning of aluminum are affected by numerous environmental characteristics, including the temperature, the presence/type of various ligands, and the pH (ATSDR, 2008). Due to its reactive nature, dissolved aluminum does not exist in its elemental state but rather binds with either inorganic ligands (e.g., hydroxide, fluoride, sulphate) or organic ligands (e.g., natural organic matter (NOM)) to form numerous types of complexes. At low pH, the complex hydrated aluminum cation [Al(H2O)6]3+, also commonly known as "free aluminum" and abbreviated as Al3+, is the most soluble form of aluminum (Environment Canada and Health Canada, 2010). At high pH, the complex anion (Al(OH)4-) is most prevalent and is highly soluble. (See Section 4.2 for further information on the effects of pH.)
1.3 Exposure
As indicated in a Priority Substances List Assessment Report (Environment Canada and Health Canada, 2010), the main source for Canadians' exposure to aluminum is through food, followed sequentially by exposure through soil, drinking water and air (Table 1). Exposure through drinking water constitutes less than 10% of the average daily intake of aluminum across all age groups (Table 1).
| Estimated mean daily intake of total aluminum (μg/kg bw per day) | |||||||
|---|---|---|---|---|---|---|---|
| Source of exposure | Infant (0-6 months) | Toddler (0.5-4 years) | Child (5-11 years) | Teen (12-19 years) | Adult (20-59 years) | Senior (>60 years) |
|
| Breastfed exclusively | Non-breastfed | ||||||
| Drinking waterFootnote a | 0 | 16.75 | 7.09 | 5.5 | 3.17 | 3.32 | 3.49 |
| Food and beverages | 12.2 | 85.0 | 268 | 341 | 270 | 143 | 113 |
| Ambient air | 0.05 | 0.10 | 0.08 | 0.05 | 0.04 | 0.03 | |
| Indoor air | 0.37 | 0.78 | 0.61 | 0.35 | 0.30 | 0.26 | |
| Soils | 166 | 268 | 87 | 21 | 18 | 17 | |
| TOTAL | 179 | 268 | 544 | 434 | 295 | 165 | 134 |
Note: Adapted from Environment Canada and Health Canada, 2010
|
|||||||
Water monitoring data from the provinces and territories (municipal and non-municipal supplies; Table 2), the National Drinking Water Survey (Health Canada, 2017) (Appendix C) and Environment and Climate Change Canada (Environment and Climate Change Canada, 2017) show that total aluminum
- is detected in all water types but is variable across Canada;
- concentrations tend to be higher in surface water than in groundwater;
- concentrations are higher in rivers, likely due to high total particulate matter content;
- concentrations are generally low for raw, treated and distributed water, but the median, mean and 90th percentile levels of total aluminum in municipal surface water (treated and/or distributed) can exceed 0.05 mg/L;
- the 90th percentile for non-municipal supplies (usually untreated groundwater) tends to be greater than the municipal raw groundwater concentration in the same jurisdiction; and
- maximum concentrations for non-municipal supplies and municipal surface water (treated and/or distributed) can exceed the proposed MAC.
| Jurisdiction (MDL mg/L) | Water type (Non-municipal: ground/not specifiedFootnote a and municipal: ground/surface-raw, treated, distributedFootnote b) | No. detects/ samples | Values above MDL (mg/L) | |||
|---|---|---|---|---|---|---|
| Median | Mean | 90th percentile | Max | |||
| NewfoundlandFootnote 1 (N/A) |
Municipal: | |||||
| Ground-raw | 42/102 | 0.020 | 0.044 | 0.109 | 0.280 | |
| Ground-distribution | 629/1,686 | 0.016 | 0.044 | 0.082 | 1.000 | |
| Surface-raw | 600/646 | 0.080 | 0.109 | 0.240 | 0.800 | |
| Surface-distribution | 2,820/3,178 | 0.080 | 0.145 | 0.300 | 6.600 | |
| Nova ScotiaFootnote 2 (0.005-0.010) |
Non-municipal: ground | 574/574 | 0.005 | 0.039 | 0.057 | 3.400 |
| Municipal: | ||||||
| Ground-raw | 77/133 | 0.009 | 0.009 | 0.013 | 0.032 | |
| Ground-treated | 29/50 | 0.009 | 0.016 | 0.020 | 0.089 | |
| Ground-distribution | 35/52 | 0.013 | 0.015 | 0.022 | 0.060 | |
| Surface-raw | 88/88 | 0.082 | 0.101 | 0.212 | 0.501 | |
| Surface-treated | 180/187 | 0.056 | 0.088 | 0.180 | 0.724 | |
| Surface-distribution | 197/204 | 0.025 | 0.081 | 0.112 | 5.700 | |
| New BrunswickFootnote 3 (0.001-0.025) | Non-municipal: ground | 90/443 | 0.003 | 0.027 | 0.061 | 0.580 |
| Municipal: | ||||||
| Ground-raw | 289/924 | 0.003 | 0.007 | 0.014 | 0.120 | |
| Ground-distribution | 225/550 | 0.011 | 0.015 | 0.022 | 0.270 | |
| Surface-raw | 104/139 | 0.037 | 0.046 | 0.090 | 0.228 | |
| Surface-distribution | 338/391 | 0.020 | 0.029 | 0.061 | 0.300 | |
| QuebecFootnote 4 (0.005-0.025) |
Municipal: | |||||
| Ground-raw | 77/147 | 0.011 | 0.022 | 0.059 | 0.160 | |
| Ground-treated | 1/2 | 0.037 | 0.037 | 0.037 | 0.037 | |
| Ground-distribution | 32/67 | 0.009 | 0.018 | 0.036 | 0.110 | |
| Surface-raw | 6/6 | 0.275 | 0.244 | 0.330 | 0.330 | |
| Surface-treated | 6/6 | 0.029 | 0.084 | 0.200 | 0.360 | |
| OntarioFootnote 5 (0.001) | Municipal: | |||||
| Ground and surface-treated | 1,316/1,438 | 0.026 | 0.051 | 0.101 | 1.500 | |
| Ground and surface-distributed | 1,212/1,387 | 0.029 | 0.049 | 0.114 | 1.340 | |
| ManitobaFootnote 6 (0.0002-0.409) |
Non-municipal: ground | 51/144 | 0.004 | 0.021 | 0.017 | 0.266 |
| Municipal: | ||||||
| Ground-raw | 309/877 | 0.002 | 0.022 | 0.021 | 2.490 | |
| Ground-treated | 194/606 | 0.005 | 0.016 | 0.006 | 0.381 | |
| Ground-distribution | 72/96 | 0.002 | 0.010 | 0.007 | 0.392 | |
| Surface-raw | 392/413 | 0.149 | 0.429 | 0.673 | 32.400 | |
| Surface-treated | 396/443 | 0.042 | 0.189 | 0.337 | 7.970 | |
| Surface-distribution | 71/72 | 0.022 | 0.152 | 0.284 | 3.900 | |
| SaskatchewanFootnote 7(0.0005-0.025/0.005-0.101 for non-municipal) | Non-municipal: ground | 1,938/4,128 | 0.003 | 0.046 | 0.031 | 14.000 |
| Municipal: | ||||||
| Ground-raw | 216/216 | 0.001 | 0.010 | 0.008 | 0.740 | |
| Ground and surface-treated | 293/293 | 0.011 | 0.106 | 0.272 | 2.030 | |
| Ground and surface-distribution | 2,102/2,102 | 0.003 | 0.056 | 0.052 | 1.420 | |
| Surface-raw | 148/148 | 0.040 | 1.746 | 0.210 | 3.173 | |
| AlbertaFootnote 8 (0.003-0.020) |
Non-municipal: ground | 1,355/1,686 | 0.005 | 0.032 | 0.025 | 5.100 |
| Municipal: | ||||||
| Surface-raw | 147/148 | 0.184 | 0.631 | 1.746 | 6.200 | |
| Surface-treated | 278/286 | 0.062 | 0.074 | 0.130 | 0.301 | |
| Surface-distribution | 462/474 | 0.062 | 0.068 | 0.120 | 0.304 | |
| British ColumbiaFootnote 9 (0.005-0.050) | Non-municipal: not specified | 313/352 | 0.025 | 0.065 | 0.060 | 3.000 |
| YukonFootnote 10 (0.001-0.050) |
Municipal: | |||||
| Ground-raw | 48/219 | 0.005 | 0.009 | 0.012 | 0.061 | |
| Ground-treated | 11/68 | 0.003 | 0.005 | 0.010 | 0.019 | |
| Surface-treated | 0/10 | N/A | N/A | N/A | N/A | |
| CanadaFootnote c | Municipal: | |||||
| Ground-treated | 0.016 | |||||
| Ground-distribution | 0.033 | |||||
| Surface-treated | 0.157 | |||||
| Surface-distribution | 0.123 | |||||
MDL = method detection limit
|
||||||
Aluminum is naturally present in many foods, and certain aluminum-containing substances also have permitted uses as food additives. The highest concentrations of aluminum (>10 µg/g) among the composite food samples analyzed in the Canadian Total Diet Study between 2008 and 2012 were found in herbs and spices, baking powder, various baked goods, processed chicken products and chewing gum (Health Canada, 2007). The study's analysis of aluminum in infant formula found 0.040-0.171 µg/g in milk-based formula and 0.258-0.476 µg/g in soy-based formula (Health Canada, 2016). Comparable results were reported in the Canadian Food Inspection Agency's Children's Food Project and other targeted surveys (CFIA, 2018).
Canadians are also exposed to aluminum through consumer products (e.g., deodorants, creams, makeup and hair or nail products) and medications (e.g., antiulcer, antidiarrheal, antiperspirants for hyperhidrosis). Notably, aluminum is present in antacids (~300-600 mg aluminum hydroxide per tablet) and represents an important source of exposure to individuals who consume antacids on a regular basis (ATSDR, 2008).
Aluminum concentrations in Canadian soil vary according to the sampling location, with average values ranging from 12,000 mg/kg in Nova Scotia to 87,633 mg/kg in British Columbia. The mean total aluminum concentration in Canadian soils, calculated from over 40 studies covering 10 provinces, was approximately 41,000 mg/kg (Environment Canada and Health Canada, 2010).
The levels of aluminum in ambient Canadian air also varyConcentrations range from the detection limit (not provided) up to 24.94 μg/m3 with a mean total aluminum concentration in PM10 of 0.17 µg/m3 (Environment Canada and Health Canada, 2010). Indoor air concentrations of aluminum are expected to be higher than outdoor air; however, they still do not constitute a significant exposure source (Environment Canada and Health Canada, 2010).
2.0 Health Considerations
2.1 Kinetics
Absorption: Aluminum ingested via the oral route is poorly absorbed through the gastrointestinal tract. While the acidic environment in the stomach favours the formation of the most soluble aluminum ion [Al(H2O)6]3+, the more neutral pH of the intestine results in the formation of insoluble aluminum hydroxide complexes, which are then generally excreted in the feces. However, small amounts of aluminum that complexed with organic molecules in the stomach will still remain soluble at the higher pH of the small intestine (Health Canada, 1998). Absorption is generally greater with more soluble aluminum compounds; however, the absorption of aluminum through the stomach or intestines varies and depends heavily on the presence of chemical constituents from the diet and the types of complexes that aluminum forms with the dietary ligands (Zhou and Yokel, 2005). Intestinal absorption of aluminum may increase in the presence of anions, carboxylates (including citrate and lactate), fluoride, and vitamin D supplements. Citrate (the conjugate base of citric acid) is one of the most important complexing agents relevant to aluminum uptake in humans. Blood and tissue levels of aluminum can be substantially increased through the intake of citric acid without further increasing the intake of aluminum itself. Conversely, the absorption of aluminum may decrease due to the presence of phosphates, silicones, polyphenols, and folic acid supplements (ATSDR, 2008). The bioavailability of aluminum in drinking water has been measured in both human and animal studies. In humans, the absorption of aluminum complexed with citrate, chloride, hydroxide or lactate has been found to range between 0.01% and 0.65%. In experimental animals, the reported values range between 0.01% and 5.1% (Environment Canada and Health Canada, 2010). A likely estimate for aluminum bioavailability in both humans and animals is 0.3%, based on human studies by Stauber et al. (1999) and a critical review of animal data by Krewski et al. (2007).
Distribution: Aluminum primarily binds to transferrin; it is slowly taken up by tissues and organs and accumulates primarily in bone. To a lesser extent, aluminum can accumulate in the brain either by crossing the blood-brain barrier or through the choroid plexus in the cerebrospinal fluid of the cerebral ventricles. Aluminum is also detected in the lungs, skin, lower gastrointestinal tract, lymph nodes, adrenals, parathyroid glands, and most soft tissue organs (EFSA, 2008; Environment Canada and Health Canada, 2010). Aluminum may also distribute to the placenta, fetus and breast milk (ATSDR, 2008). The distribution of aluminum may be influenced by other metals, including iron (negatively correlated with aluminum tissue accumulation), calcium, and magnesium (deficiency may contribute to aluminum accumulation in the brain and bone) (EFSA, 2008).
Metabolism: The free form of aluminum (Al3+) binds easily to many substances; as a consequence, it is the affinity to the ligand and the metabolic fate of the complex that determines the metabolism of aluminum. Aluminum can form low-molecular-weight complexes with organic acids, amino acids, nucleotides, phosphates, and carbohydrates that are quite stable. Aluminum can also form stable macromolecular complexes with proteins, polynucleotides, and glycosaminoglycans. Some complexes are so stable that the aluminum cation cannot be exchanged for another cation. Because aluminum has a high affinity for these organic ligands, much of the aluminum in the body exists in the form of macromolecular complexes (ATSDR, 2008).
Elimination: In humans, upwards of 95% of the mobile aluminum is eliminated by the kidneys (Krewski et al., 2007). Individuals with compromised kidney function thus have an increased risk for aluminum toxicity (Willhite et al., 2014). The majority of the remaining portion is eliminated via biliary excretion in the feces. To a much lesser extent, sweat, saliva and seminal fluid can also contribute to the elimination of aluminum (Krewski et al., 2007). The elimination half-life of aluminum from the whole body is highly variable, ranging from hours to years (Priest et al., 1995; Talbot et al., 1995; Priest, 2004). The rate of elimination is influenced by a number of factors, including the presence of chemical complexes in the blood (e.g., aluminum citrate complexes are more readily eliminated than transferrin bound aluminum) (ATSDR, 2008). In addition, slow elimination and increased exposure with age contribute to the accumulation of aluminum in the body (NSCFS, 2013). In animal studies, elimination in rats was observed to occur more rapidly in well-perfused tissues (such as kidneys and lungs) than in poorly perfused tissues (such as bone and spleen), with half-lives of 2.3-113 days (Environment Canada and Health Canada, 2010). However, aluminum had a slower elimination rate from the brain, despite its being a well-perfused organ, with half-lives of 13-1,635 days (Krewski et al., 2007). The retention times of aluminum appear to be shorter in rodents than in humans, but information for allometric scaling is not available (EFSA, 2008).
Physiologically based pharmacokinetic modelling: No models applicable to the current risk assessment were identified.
2.2 Health effects
The database for the oral toxicity of aluminum is extensive, covering numerous endpoints (e.g., effects in bone, kidney, the nervous system and the immune system) and various types of exposure in both animals and humans (see Krewski et al. (2007), ATSDR (2008) and Willhite (2014) for a more thorough review). The preponderance of the literature, however, focuses on neurotoxicity and reproductive/developmental toxicity; the emphasis on these endpoints is likely driven by findings in human case studies (i.e., encephalopathy in renal patients exposed to aluminum in dialysate and/or aluminum phosphate binders; cognitive impairment of preterm infants exposed to aluminum in parenteral nutritional solutions). An evaluation of the overall database clearly identifies the nervous system as the most sensitive target for aluminum toxicity (ATSDR, 2008). Other reviews also support this conclusion (EFSA, 2008; Environment Canada and Health Canada, 2010; JECFA, 2012). Consequently, studies examining neurological endpoints are the focus of the subsequent sections in this document. In addition, emphasis is placed on oral studies, as these are the most relevant for drinking water risk assessment. The previous review on aluminum by Environment Canada and Health Canada (2010) covers the literature up to 2008. All of the previous data is considered in the current assessment of aluminum in drinking water; however, the data presented herein focus on material published from 2009 to 2017.
2.3 Effects in humans
Despite aluminum's abundance in the environment, it is generally accepted that aluminum is not required by biological systems and does not participate in any essential biological processes (Exley, 2013). In terms of acute exposures, reports of short-lived nausea, vomiting, diarrhea, mouth ulcers, skin ulcers, skin rashes and arthritic pain were noted when up to 20,000 people were exposed to aluminum concentrations 500-3,000 times the WHO OG value (0.200 mg/L) in an accidental contamination of water supplies in Camelford, UK (Lowermoor Incident Health Advisory Group, 1989). A number of follow-up studies to this acute exposure were conducted but did not demonstrate conclusive evidence of long-term effects (McMillan et al., 1993a, 1993b; Altmann et al., 1999; Exley, 2006; UK Committee on Toxicology, 2013).
Regarding longer-term exposures, a limited number of studies have investigated the effects of aluminum in healthy populations (see reviews in Krewski et al. (2007), ATSDR (2008) and Environment Canada and Health Canada (2010)). Several cross-sectional and ecological studies published after 2009 have investigated associations between aluminum and effects in bone (Dahl et al., 2014; Callan et al., 2015), kidney (Callan et al., 2015; Panhwar et al., 2016), reproduction and development (Huang et al., 2011; Giaccio et al., 2012; Karakis et al., 2014), body composition (Skalnaya et al., 2014; Cetin et al., 2017) and other endpoints in humans (Lv et al., 2011; Lindquist et al., 2011; Lind et al., 2012; Guo et al., 2013; Vandenplas et al., 2014).
The neurotoxicity of aluminum is well documented in human studies; however, many of these studies have been cases of medical treatment for specific disease conditions (e.g., patients with impaired kidney function). The association between exposure to aluminum and neurotoxicity endpoints in otherwise healthy individuals is less conclusive; such an association, specifically with Alzheimer's disease (AD), is the subject of much research and debate (Lidsky, 2014; Walton, 2014). Studies examining the link between aluminum and AD have focused on a number of areas, including the evaluation of aluminum concentrations in the body as it relates to AD. In the past, the results of these studies tended to be mixed-positive associations were noted in some studies but not in others-and this continues to be the finding of more recent investigations (Baum et al., 2010; Akatsu et al., 2011; Rusina et al., 2011; Bhattacharjee et al., 2013; Virk and Eslick, 2015; Mirza et al., 2017; Xu et al., 2018). Other studies that examined the concentration of aluminum in the brain in relation to the occurrence of beta-amyloid plaques and neurofibrillary tangles (primary features of AD pathogenesis) also noted mixed results (Strozyk et al., 2009; Walton, 2010; Exley et al., 2012).
Other recent studies examined the link between aluminum and the occurrence of various neurological diseases or disorders, other than AD, including amyotrophic lateral sclerosis (Garzillo et al., 2014), multiple sclerosis (Arain et al., 2015; Tamburo et al., 2015), attention deficit hyperactivity disorder (Nicolescu et al., 2010), autistic spectrum disorders (Albizzati et al., 2012), learning disabilities (do Nascimento et al., 2014) and cognitive dysfunction (Bakar et al., 2010). Most of these studies did not find a significant positive association between aluminum concentrations in the body and the respective neurological endpoint. Unfortunately, many of the studies were small in size, did not adjust for confounders and/or did not have adequate control populations.
A review outlined several epidemiological studies and investigated the association between exposure to aluminum in drinking water and the development of AD and other neurodegenerative disorders (JECFA, 2012). A large prospective study by Rondeau et al. (2009) found a significant association between high exposures to aluminum in drinking water (>0.1 mg/d) and the risks of cognitive decline, dementia and AD. However, the power of this study was low, with only 13 subjects (6 cases) having exposure ≥ 0.1 mg/day. In addition, there was a lack of information on exposure to aluminum through the diet, which was considered to account for 95% of the total oral exposure. A recent meta-analysis of cohort and case-control studies (including the study by Rondeau et al., 2009) found that chronic exposure to aluminum was associated with a 71% increased risk of AD (Wang et al., 2016). Of note are the facts that only eight studies were considered and that half these studies evaluated occupational exposures rather than drinking water exposures.
Overall, the epidemiological database provides only uncertain indications of an association between aluminum exposure and neurological diseases, including AD. Although recent reviews and international assessments consistently conclude that there is insufficient evidence for a causal link between exposure to aluminum and AD, there is also consensus that the hypothesis should not be dismissed (ATSDR, 2008; EFSA, 2008; Environment Canada and Health Canada, 2010; JECFA, 2012; Willhite et al., 2014). In addition to the absence of a clear point of departure needed for dose-response analysis, limitations in the epidemiological studies include a lack of individual exposure data, small sample sizes, poor disease ascertainment, and failure to control for confounders. These limitations prevent the ability to use their results in a quantitative risk assessment. However, the results of these studies can be used qualitatively to support the choice of the key endpoint used for quantitative assessment in animals.
2.4 Effects in animals
Exposure to aluminum is well known to result in a number of health effects in animal models. Reviews of these studies are found in Krewski et al. (2007), ATSDR (2008) and Willhite (2014). Acute oral exposures of rats and mice to various aluminum compounds have resulted in LD50 levels of 222-980 mg Al/kg (Ondreicka et al., 1966; Yokel and McNamara, 1985; Llobet et al., 1987; Vucetic-Arsic et al., 2013; Yu et al., 2016). Other studies, published after 2009, have noted effects in the bone at doses of AlCl3 >100 mg/L (Li et al., 2011a, 2011b, 2015; Sun et al., 2015, 2016, 2017), in the liver at doses of AlCl3 >34 mg/kg bw per day (Turkez et al., 2010; Bhasin et al., 2012; Zhu et al., 2013; Abdel Moneim et al., 2013; Belaid-Nouira et al., 2013b; She et al., 2015; Ghorbel et al., 2016a) and in the kidneys at doses of AlCl3 >34 mg/kg bw per day (Abdel Moneim et al., 2013; Belaid-Nouira et al., 2013a; Wasana et al., 2015; Liu et al., 2016; Ghorbel et al., 2016b).
The vast majority of animal studies have investigated the potential for aluminum to cause neurotoxic, neurobehavioural and reproductive/developmental effects (including neurodevelopmental toxicity). Many of these experiments were designed to investigate the role of aluminum in the development of neurodegenerative diseases (including mechanistic studies), peripheral markers of aluminum neurotoxicity, or the protective properties of various agents against aluminum-induced toxicity. A summary of the recent studies (published after 2009) most relevant to the present assessment of aluminum is presented in Table 3. Included in this table are studies with exposure durations greater than 30 days in which aluminum was administered via drinking water. Studies conducted with gavage or dietary exposures were included only if they investigated multiple doses. In Table 3, as well as throughout this document, where sufficient data was provided in the study the doses of the aluminum compound were also expressed as mg aluminum to facilitate comparisons between studies. It should be noted that in many studies it was not clear whether the reported dose was reflective of the aluminum ion or of the aluminum compound (e.g., AlCl3·6H2O). Consequently, the dose as worded by the authors of the study is reported in the table unless otherwise indicated.
Neurotoxicity: The endpoints considered in the neurotoxicity studies listed in Table 3 include both histopathological effects (e.g., neuronal degeneration, vacuolization around the neuron, congestion in the blood vessels) and biochemical effects (e.g., oxidative stress responses, metal ion imbalances, altered neurotransmitter function). In these investigations, rats, mice and rabbits were exposed to aluminum for periods of 30 days to 18 months. The aluminum compounds investigated included aluminum chloride, aluminum sulphate, and aluminum maltolate. Most of the studies investigated doses of aluminum that were significantly higher than human exposures would be under normal conditions. The lowest dose at which adverse neurotoxicity effects were observed was 10 mg/kg bw per day AlCl3 (≈2 mg Al/kg bw per day) (Rui and Yongjian, 2010). No data were found that addressed the reversibility of neurotoxic effects upon cessation of the exposure.
Neurobehavioural: The endpoints considered in the neurobehavioural studies include changes to reflexes, motor activity, learning, memory, and sensory parameters. In these investigations rats and mice were exposed to aluminum chloride for periods of 42 days to 14 months. Treatment with 100 mg/kg bw per day of aluminum chloride for 6 weeks (42 days) is a well-known model for inducing dementia (impaired spatial memory) in animals. The lowest dose at which adverse neurobehavioural effects were observed (i.e., lowest LOAEL (lowest-observed-adverse-effect level)) is 1.5 mg Al/kg bw per day, which was considered equivalent to human dietary aluminum exposure levels (Martinez et al., 2017a).
Reproductive/developmental toxicity (including neurodevelopmental toxicity): In the reproductive and developmental studies in Table 3, rats, mice and guinea pigs were exposed to various concentrations of aluminum chloride, aluminum citrate, aluminum sulphate and aluminum ammonium sulphate. These studies show that aluminum may affect reproductive parameters, including reproductive hormone levels, sperm counts, sperm motility, sperm morphology, and testis histology. In addition, gestational and/or lactational exposure to aluminum can result in developmental effects that include decreased pup weight (often in the presence of maternal effects), delayed maturation, impaired neurobehaviour and changes to brain biochemistry. The lowest doses at which no adverse effects were observed are 8-14 mg Al/kg bw per day of aluminum sulphate (Hirata-Koizumi, 2011a) and 5-9 mg Al/kg bw per day of aluminum ammonium sulphate (Hirata-Koizumi, 2011b). These no-observed-adverse-effect levels (NOAELs) were based on decreased body weight gain and a slight but significant delay of the vaginal opening at the highest dose level in both studies. Unfortunately, interpretation of the study outcomes was confounded by treatment-related reductions in food and fluid consumption (likely due to the astringent taste and decreased palatability of the aluminum treated water). The authors stated that they could not separate the effects of the decreased water intake from the effects associated with aluminum treatment. Further, since other hormone-dependent events, such as those governing estrous cyclicity and post-natal anogenital distance, were not impacted in the aluminum-treated groups, the authors indicated that it was unlikely that aluminum had a clear impact on hormonal messaging during development. Therefore, if these results were disregarded, the next NOAEL dosage is 30 mg Al/kg bw per day of aluminum citrate (Poirier et al., 2011). In addition, the LOAEL for reproductive effects is 1.5 mg Al/kg bw per day (Martinez et al., 2017b).
| NOAEL/LOAEL (mg Al/ kg-day)Footnote a | Species, sex, number | Exposure duration | Compound and dose(s)Footnote b | Critical effect(s) | Key strength and/or weaknessFootnote c |
Ref |
|---|---|---|---|---|---|---|
| N/2 | Mice, ICR, (15/group) | 100 days | AlCl3; 0, 10, 50, 300 mg/kg bw per day via the diet | Neurotoxicity: Increased lipid peroxidation (MDA); decreased SOD; increased DNA damage (comet assay); increased mitochondrial DNA oxidative damage (8-OHdG) | Limited endpoints | (Rui and Yongjian, 2010) |
| N/10 | Mice, Balb-c, M (10/group) | 5 weeks | AlCl3; 0, 50 mg/kg bw per day |
Neurotoxicity: Increased lipid peroxidation (MDA); decreased antioxidant (GSH); decreased AChE and butyrylcholinesterase activity; activation of brain monoamine oxidase (MAO-A and MAO-B) but inhibition of cerebellar MAO-B | Single dose | (Linardaki et al., 2013) |
| N/20 | Rats, Wistar, M (10/group) | 6 weeks | AlCl3; 0, 100 mg/kg bw per day |
Neurotoxicity: Increased brain AchE; decreased acetylcholine, dopamine, noradrenaline, adrenaline and SOD; increased nitric oxide and H2O2, cortisol and adrenocorticotropic hormone; formation of amyloid plaques and necrosis of neurons | Single high dose | (ElBaz. et al., 2017) |
| N/20 | Rats, Sprague-Dawley, M (6-8/group) |
8 weeks | AlCl3; 0, 100 mg/kg bw per day |
Neurotoxicity: Decreased neurotransmitters, AchE; increased L-citrulline, nitric oxide and monoamine oxidase; increased tau, amyloid precursor protein, glial fibrillary acidic protein, ubiquitin, α-synuclein and Hsp 70; alterations in neurohistoarchitecture (loss of pyramidal and Purkinje cells) | Single high dose | (Singla and Dhawan, 2017) |
| 100/N | Mice, Tg2576 and Tg2576 /tau, F (6/group) |
4 or 10 months | AlCl3; 0, 100 mg/kg bw per day (as Al) |
Neurotoxicity: Long-term Al intake did not accelerate the accumulation of Aβ in Tg2576 mice or accumulation of Aβ and tau in Tg2576/tau mice. | Single high dose | (Akiyama et al., 2011) |
| N/101 | Rats, Wistar, M | 30 days | AlCl3; 0, 500 mg/kg bw per day |
Neurotoxicity: Reduced catalase and GSH levels; mild degenerative changes in the prefrontal cortex; no evidence of amyloid deposits | Single high dose | (Akinola et al., 2015) |
| N/? | Rats, Wistar, F (10/group) | 5 months | AlCl3; 0, 500 mg/kg bw per day i.g. for 1 month, then 1,600 ppm in drinking water for 4 months | Neurotoxicity: Increased lipid peroxidation in posterior brain; altered lipid metabolism | Single high dose | (Belaid-Nouira et al., 2012) |
| N/? | Rats, Wistar, F (10/group) | 5 months | AlCl3; 0, 500 mg/kg bw per day i.g. for 1 month, then 1,600 ppm in drinking water for 4 months | Neurotoxicity: Reduced production of interleukin-6 (marker of inflammation) in the posterior brain; reduced immunoreactivity to GFAP (marker of astroglia activation) in the hippocampus and cerebral cortex; reduced number of GFAP-positive cells | Single high dose | (Belaid-Nouira et al., 2013c) |
| N/? | Rats, Wistar, M (5/group) | 6, 12 or 18 months | AlC3; 0.18, 0.72, 3.6 g/L | Neurotoxicity: Accumulation of aluminum in the brain varied by doses and exposure duration. Histopathological alterations in the dentate gyrus: destructive effect on subgranular layer and granular layer | No data on water consumption to calculate doses | (Hichem et al., 2014) |
| N/35 | Rats, Sprague-Dawley, M (10/group) | 12 weeks | AlCl3; 0, 2,000 mg/L | Neurotoxicity: Increased Aβ in the hippocampus and cerebral cortex; histological evidence of shrunken and swollen neurons; reduced density of normal neurons | Single dose | (Zhang et al., 2013a) |
| N/71 | Rats, Wistar (10/group) | 3 months | AlCl3; 0, 0.2%, 0.4%, 0.6% | Neurotoxicity: Decreased activities of protein kinase C and mitogen-activated protein kinase; reduced expression of extracellular signal-regulated kinases (ERK1/2) and Ca2+-calmodulin dependent protein kinase II (CaMKII) in hippocampus; attenuation of population spike amplitude of long-term potentiation (indicator of synaptic plasticity) from the hippocampal CA1 region | Actual daily Al doses not reported | (Wang et al., 2010) |
| N/72 | Rats, Wistar (20/group) | 3 months | AlCl3; 0, 0.2%, 0.4% 0.6% | Neurotoxicity: Impact on Ras/ERK signal pathway: increased protein and mRNA expression of Ras; decreased expression of Raf1 and ERK2 in the hippocampi | Actual daily Al doses not reported | (Cui et al., 2012) |
| N/? | Rats, Wistar, M (5/group) | 30 days | AlCl3; 0, 10, 100 ppm | Neurotoxicity: Oxidative stress induction (increased MDA, decreased SOD levels); activation of astroglia, microglia and infiltration of B-cells in the prefrontal cortex Some evidence of dose-response | Short-term study | (Akinrinade et al., 2015) |
| N/N | Rabbits, New Zealand, M (5/group) | 10 weeks | Al sulphate; 0, 0.36 ppm (as Al) | Neurotoxicity: No increase in the number of beta-amyloid reactive neurons, but increased number of ABCA1-immunopositive neurons, in Al-treated rabbits fed a 2% cholesterol diet | Single dose | (Schreurs and Sparks, 2016) |
| N/? | Mice, T 44 tau Tg and wild-type (5-13/ group) | 3, 6, 9, 12 months | Al maltolate; 0, 2 mM |
Neurotoxicity: Accelerated tau aggregation, apoptosis and neurological dysfunction in mouse model with slow progressive tau accumulation | Single dose | (Oshima et al., 2013) |
| N/1.5 | Rats, Wistar, M (6/group) | 60 days, 42 days | AlCl3·6H2O 1.5, 8.3, 100 mg/kg bw per day (as Al) | Neurobehaviour: Impaired recognition memory (object recognition memory test) | Well-conducted study | (Martinez et al., 2017a) |
| N/10 | Mice, Balb-c, M (10/group) | 5 weeks | AlCl3; 0, 50 mg/kg bw per day |
Neurobehaviour: Impaired long-term memory (passive avoidance task) | Single dose | (Linardaki et al., 2013) |
| N/50 | Mice, Balb-c, M (5-9/group) | 42 days | AlCl3; 0, 250 mg/kg bw per day |
Neurobehaviour: Deficits in learning and higher anxiety (fear extinction and open field tests) | Single high dose | (Farhat et al., 2017a) |
| N/50 | Mice, Balb-c, M (5-9/group) | 42 days | AlCl3; 0, 250 mg/kg bw per day |
Neurobehaviour: Impaired recognition memory (novel object recognition test), reduced sociability (social novelty preference test) | Single high dose | (Farhat et al., 2017b) |
| N/71 | Rats, Wistar (10/group) | 3 months | AlCl3; 0, 0.2%, 0.4%, 0.6% | Neurobehaviour: Impaired memory (step-down test) | Actual daily Al doses not reported | (Wang et al., 2010) |
| N/35 | Rats, Sprague-Dawley, M (10/group) | 12 weeks | AlCl3; 0, 2,000 mg/L | Neurobehaviour: Impaired spatial learning and memory (Morris water maze) | Single dose | (Zhang et al., 2013a) |
| N/13 | Rats, Wistar, M (10/group) | 120 days | AlCl3; 0, 64.18, 128.36, 256.72 mg/kg bw per day |
Reproductive: Suppression of testosterone and luteinizing hormone; decreased androgen receptor protein and mRNA expression | Did not account for Al in diet or for water consumed | (Sun et al., 2011) |
| N/13 | Rats, Wistar, F (10/group) | 120 days | AlCl3; 0, 64.18, 128.36, and 256.72 mg/kg bw per day | Reproductive: Decreased levels of estrogen, progestogen, follicle-stimulating hormone, and luteinizing hormone in serum | Did not account for Al in diet or for water consumed | (Wang et al., 2012) |
| N/13 | Rats, Wistar, F (20/group) | 120 days | AlCl3; 0, 64, 128, 256 mg/kg bw per day |
Reproductive: Damaged ovarian structure; altered iron, zinc and copper levels; decreased activities of Na(+)-K(+)-ATPase, Mg(2+)-ATPase and Ca(2+)-ATPase in the ovary; decreased follicle-stimulating hormone, and luteinizing hormone protein expression | Did not account for Al in diet | (Fu et al., 2014) |
| N/13 | Rats, Wistar, M (10) | 120 days | AlCl3; 0, 64.18, 128.36, 256.72 mg/kg bw per day |
Reproductive: Decreased sperm count; increased sperm malformations; decreased testicular enzymes; altered iron, zinc and copper levels | Did not account for Al in diet or for water consumed | (Zhu et al., 2014) |
| N/? | Rats, diabetic and non-diabetic, Wistar, M (10/group) |
30 days | AlCl3; 0, 250 ppm |
Reproductive: Reduced sperm count and motility; decreased follicle-stimulating hormone; elevated estradiol levels | Single dose | (Akinola et al., 2016) |
| N/? | Guinea pigs, M (13/group) | 13 weeks | AlCl3; 0, 300 mg/L |
Reproductive: Decreased sperm count; increased sperm malformations; decreased testosterone; reduced gene and protein expression of StAR and P450scc | Single dose | (Dong et al., 2016) |
| N/1.5 | Rats, Wistar, M (6/group) | 60 days, 42 days |
AlCl3·6H2O; 1.5, 8.3, 100 mg/kg bw per day | Reproductive: Decreased sperm count, daily sperm production, sperm motility and normal morphological sperm; impaired testis histology; increased oxidative stress in reproductive organs; inflammation in testis | Well-conducted study | (Martinez et al., 2017b) |
| N/? | Rats, Wistar, M (7-10/ group) | 6 months (3 generations) | Al sulphate; 0, 200, 400, 1,000 ppb | Reproductive: Lower testosterone levels; decreased sperm counts; higher percentages of immobile and abnormal sperm; decrease in testis weight; alterations in the histoarchitecture of the testes | Minimal study details reported | (Muselin et al., 2016) |
| 8-14/31-56 | Rats, Sprague- Dawley, M and F (24/sex/group) | 2 generations | Al sulphate; 0, 120, 600, 3,000 ppm | Reproductive/Developmental: No adverse effects on reproductive and fertility parameters; delay of the vaginal opening. | Confounding effects of decreased water intake | (Hirata-Koizumi et al., 2011a) |
| 5-9/36-61 | Rats, Sprague-Dawley, M and F (24/sex/group) | 2 generations | Al ammonium sulphate; 0, 50, 500, 5,000 ppm | Reproductive/Developmental: No adverse effects on reproductive and fertility parameters; delay of the vaginal opening | Confounding effects of decreased water intake | (Hirata-Koizumi et al., 2011b) |
| N/N | Rats, Wistar, M and F (10/sex/group) | M: 28 days; F: 37-53 days | AlCl3; 0, 3.6, 18, 90 mg/kg bw per day (as Al); via gavage |
Reproductive/Developmental: No reproductive, breeding or early post-natal developmental effects | GLP study | (Beekhuijzen, 2007, as reported in JECFA, 2012) |
| 30/100 | Rats, Sprague-Dawley, M and F (80/sex/group) | Gestation, lactation, until one year of age | Al citrate; 0, 30, 100, 300 mg/kg bw per day (as Al) | Developmental: Deficits in fore- and hind-limb grip strength and foot splay | GLP study | (Poirier et al., 2011) |
| N/? | Rats, Wistar, M and F (5-10/ group) | Gestation, lactation, then direct exposure for 4 months | AlCl3; 0, 0.3% | Developmental: Reduced locomotor activity; increased anxiety; changes in the glial system; increased glial fibrillary acidic protein labelling and increased numbers of astrocytes in the brain; reduced locomotor activity; effects on dopaminergic neurons | Single dose | (Erazi et al., 2010; Erazi et al., 2011) |
| N/60 | Mice, Swiss Webster, M and F | Gestation and lactation (PND 15) | AlCl3; 0, 300, 600 mg/kg bw per day | Developmental: Pre-weaning: dose-dependent decline in body weight gain and delays in eye opening and appearance of body hair fuzz; dose-dependent suppression of righting, rotating and cliff avoidance reflexes Post-weaning: dose-dependent deficits in locomotor activity and learning Various time points: dose-dependent decline in neurotransmitters in the forebrain |
No data on fluid consumption despite clear reduction of consumption | (Abu-Taweel et al., 2012) |
| N/231 | Rats, Wistar, M and F (6-10/ group) |
Lactation, then direct exposure for 3 months | AlCl3; 0, 0.2%, 0.4%, 0.6% | Developmental: Impaired spatial memory; changes to neuronal and synaptic ultrastructures in the hippocampus; electrophysiological impairment of late-phase long-term potentiation | Well-designed study, but comparatively high LOAEL | (Zhang et al., 2013b) |
| N/231 | Rats, Wistar, M and F (6-10/ group) |
Lactation, then direct exposure for 3 months | AlCl3; 0, 0.2%, 0.4%, 0.6% | Developmental: Long-term memory damage; changes to the neuronal and synaptic ultrastructure and repression of the cAMP-PKA-CREB signaling pathway | Well-designed study, but comparatively high LOAEL | (Zhang et al., 2014) |
| N/10 | Rats, Wistar, F (6/group) | Gestation and lactation (PND 14) | AlCl3; 0, 50 mg/kg bw per day |
Developmental: Decreased body weight, decreased enzymatic and non-enzymatic antioxidant levels, decreased AChE activities and increased levels of malondialdehyde and advanced oxidation protein product | Single dose | (Ghorbel I. et al., 2016c) |
| N/? | Rats, Wistar, M and F (5/group) | Gestation lactation, then direct exposure for 4 months | AlCl3: 0, 3% | Developmental: Effects on serotonin neurotransmission in the brain; decreased RF glycoprotein (involved in the detoxification of cerebrospinal fluid) | Single dose | (Laabbar et al., 2014) |
Aβ: beta-amyloid; AChE: acetylcholinesterase; GLP: good laboratory practice; GSH: glutathione; i.g.: intragastric; MDA: malondialdehyde; PND:post-natal day; SOD: superoxide dismutase
|
||||||
2.5 Genotoxicity and carcinogenicity
Aluminum compounds are not generally considered to be gene mutagens but appear to act as clastogens and likely act through indirect mechanisms of action. As summarized in EFSA (2008), the potential mechanisms of action include the cross-linking of DNA with chromosomal proteins, interactions with the microtubule assembly and mitotic spindle functioning, the induction of oxidative damage and the damaging of lysosomal membranes with the liberation of DNase. Despite producing DNA damage, the EFSA panel considered the genotoxicity of aluminum likely to be irrelevant for humans, given the presumed threshold and the low oral exposures. No conclusive evidence exists for the carcinogenicity of aluminum in animal models (Hackenberg, 1972; Schroeder and Mitchener, 1975a, 1975b; Oneda et al., 1994). The International Agency for Research on Cancer (IARC) has not reviewed the carcinogenicity of aluminum itself but has determined that there is sufficient evidence for carcinogenicity in humans following occupational exposures to various chemicals during "aluminum production" (i.e., Group 1 classification). Of note is that the hazard is based on exposure to known carcinogens (primarily polycyclic aromatic hydrocarbons) and not aluminum (IARC, 2012).
2.6 Mode of action
Although numerous studies have attempted to elucidate the mode of action of aluminum toxicity, no one mechanism has been identified, and it is likely that several processes are involved. The mechanisms implicated in aluminum neurotoxicity are summarized in several reviews (ATSDR, 2008; Environment Canada and Health Canada, 2010; Willhite et al., 2014) and include, among others, oxidative damage, inflammatory responses, changes in neuronal cytoskeletal proteins (neurofilament aggregates), altered cholinergic activity, effects on signal transduction pathways, membrane effects and metal ion imbalances. The literature to date provides no indication of a difference between the mode of action in animals and that in humans. A full analysis of the mode of action of aluminum toxicity was not conducted as it is not critical to the derivation of a health-based value (HBV) for aluminum.
2.7 Selected key study
Two key studies were considered in the risk assessment for aluminum: a neurodevelopmental study in rats by Poirier et al. (2011) and a reproductive (sperm quality) study in rats by Martinez et al. (2017b).
In the Poirier et al. (2011) study, the effects of aluminum citrate in rats were investigated in accordance with good laboratory practice (GLP) specifications and with a design based on OECD Test Guideline 426: Developmental Neurotoxicity. Aluminum citrate was specifically selected as the most soluble and the most bioavailable aluminum compound able to cross the blood-brain barrier. In this double-blind study, male and female Sprague-Dawley rats were exposed to 30, 100 and 300 mg Al/kg bw per day in utero, through lactation, and then in drinking water post-weaning until one year of age. Low concentrations (<10µg/g) were present in the diet. There was significant morbidity and mortality in the male pups of the highest dose group, and renal pathology associated with aluminum treatment was also observed in these male pups. In terms of reproductive outcomes, no effects on gestational length or on the number of offspring and litters were observed in the aluminum-treated groups as compared with controls. Delayed sexual development of both male and female pups was observed in the high-dose aluminum citrate group. This effect was considered treatment-related, but as body weights were also depressed, the authors were uncertain as to whether the developmental effects were secondary to decreases in body weight. In terms of neuromuscular development, the study noted a deficit in fore- and hind-limb grip strength and, to a lesser extent, in foot splay in the mid- and high-dose groups. For the present assessment, individual neuromuscular data were obtained and reviewed. A re-analysis of the data confirmed a significant deficit of the three neuromuscular endpoints in female rats, and a deficit in hind-limb grip strength for male rats. Since the aluminum citrate was delivered through the drinking water, the dose of aluminum varied with the extent of water consumption. In general, doses were at (or slightly under) target level at gestation, higher than target level during lactation, and lower than target level during the remainder of the study. Although reduced water consumption affected aluminum uptake in the later stages of the study and may have confounded results, the effect on grip strength was still prominent in younger animals when exposure to aluminum was primarily due to in utero or lactational exposures when dams received appropriate or higher-than-target-level doses. Based on clinical observations, clinical biochemical changes and effects on renal pathology and neuromuscular function, the authors assigned a LOAEL of 100 mg Al/kg per day (target concentration) and a NOAEL of 30 mg Al/kg per day (target concentration).
In the Martinez et al. (2017b) study, the reproductive effects of low doses of aluminum chloride were investigated in male rats. In this non-GLP and non-OECD guideline study, rats were divided into two groups. The first group of rats was exposed for 60 days to 1.5 and 8.3 mg Al/kg bw per day as AlCl3‧6H20 via drinking water (equivalent to human dietary levels), whereas the second group was exposed for 42 days to 100 mg Al/kg bw per day as AlCl3‧6H20 via gavage (a known neurotoxicity model). In terms of reproductive effects, exposure to aluminum resulted in a dose-related decrease in sperm count, daily sperm production and normal morphological sperm. Decreased sperm motility and impaired testis histology were also observed. Using the same study design, the authors also investigated the neurobehavioural effects associated with the same doses (Martinez et al., 2017a). No effects were observed on exploratory/locomotor activity (open field test), anxiety (elevated plus maze test) or pain threshold (hot plate test). However, exposure to both low aluminum doses, as well as the known neurotoxic dose, resulted in recognition memory impairment in the object recognition memory test. Of note is that in the reproductive toxicity study, the effects seen at the 8.3 mg Al/kg bw per day dose were occasionally greater and more significant than the effects observed at the neurotoxic dose (100 mg/kg bw per day). Although the authors state that further studies are necessary to explain these results, they suggest that the dose may not be the most important determinant of aluminum toxicity; rather, exposure conditions, intrinsic and individual characteristics, distribution and bioavailability through the body may also be important. In both the neurobehavioural and reproductive studies, increased markers of oxidative stress were noted in association with the effects and were highlighted as a potential mechanism of action.
Ultimately, the Poirier et al. (2011) study was chosen as the most appropriate study for the risk assessment. While the Martinez et al. (2017b) study was well conducted and had a lower point of departure, concerns were raised over the magnitude of the changes in sperm quality. The changes were often smaller at the 100 mg Al/kg bw per day dose than at the 8.3 mg Al/kg bw per day dose, despite the higher peak dose and cumulative exposure at the 100 mg Al/kg bw per day dose. No scientifically founded explanation could be offered for this finding. Confidence is placed in the study by Poirier et al. (2011) as it was a large blinded study conducted according to GLP standards and OECD guidelines. The study was specifically designed and undertaken to address previously identified research needs (JECFA, 2007), and it evaluated multiple doses as well as multiple endpoints related to both developmental toxicity and neurotoxicity. The selection of the Poirier et al. (2011) study is supported by the JECFA (2012) study, which used the same key study and point of departure for its calculation of the provisional tolerable weekly intake of aluminum in the diet. The critical effect of decreased neuromuscular function, and specifically a decline in fore- and hind-limb grip strength, has been used in another international assessment (ATSDR, 2008) and is supported by previous research studies. Maternal exposure to aluminum during gestation and lactation has consistently resulted in decreases in grip strength in rodent pups (Donald et al., 1989; Golub et al., 1992a, 1995; Golub and Germann, 2001; Colomina et al., 2005). Studies examining adult exposures to aluminum have reported mixed results for grip strength (Donald et al., 1989; Golub et al., 1992a, 1992b, 1995, 2000; Oteiza et al., 1993; Drobyshev et al., 2018).
3.0 Derivation of the health-based value
Consistent with the Environment Canada and Health Canada (2010) report, the present assessment considers neurotoxicity and reproductive/developmental toxicity to be the critical endpoints of concern. These effects were observed across a variety of species (including humans), doses and exposure times, as well as through a variety of experimental assays. Since the release of the 2010 report, several robust key studies have been published, filling data gaps previously identified (JECFA, 2007; Environment Canada and Health Canada, 2010). As noted above, the study by Poirier et al. (2011) was selected as the basis for the current risk assessment. The NOAEL of 30 mg Al/kg bw per day identified by the authors is based on a dose-related decline in neuromuscular parameters (i.e., foot splay, hind- and fore-limb grip strength) as well as clinical observations, clinical biochemical changes and effects on renal pathology. Neuromuscular effects have been previously observed in both young and adult animals with similar points of departure, and it is unlikely that the young are more sensitive than adult populations (ATSDR, 2008).
Aluminum compounds in general have very low bioavailability (~0.3%) through drinking water. In the study by Poirier et al. (2011) aluminum citrate was used, as it was considered to be the most soluble and most bioavailable aluminum compound crossing the blood-brain barrier. A health-based value based on the point of departure with aluminum citrate is considered to be conservative and protective of exposure to all aluminum species. Potential modifying factors that could affect the bioavailability and consequent toxicity of aluminum in humans include the chemical form of aluminum, the presence of other chemical constituents from the diet and the types of complexes that aluminum forms with these dietary ligands. For example, aluminum absorption may increase in the presence of carboxylic acids such as citrate (naturally present in many foods and fruit juices) but may decrease in the presence of silicone-containing compounds (likely due to the formation of insoluble hydroxyaluminosilicate). Depending on what's present in the gastrointestinal tract, the oral absorption of aluminum can vary at least 10-fold based on the type of complex alone (ATSDR, 2008; EFSA, 2008).
Modelling of the dose-response data from the Poirier et al. (2011) study is considered inappropriate, given the low number of doses (for males: two doses plus the control due to significant mortality in the high dose group) and variability in the doses across the different study stages (the dosages received varied according to the animal's water consumption). Consequently, the data are not amenable to approaches such as benchmark dose modelling to calculate the point of departure. Therefore, the NOAEL of 30 mg/kg bw per day was retained as the point of departure.
Using the NOAEL of 30 mg/kg bw per day, the tolerable daily intake (TDI) for aluminum is calculated as follows:
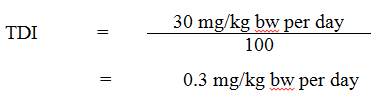
Equation 1
The TDI is 0.3 mg/kg bw per day. This is calculated by dividing 30 mg/kg bw per day by the uncertainty factor of 100.
where:
- 30 mg/kg bw per day is the NOAEL from Poirier et al. (2011), based on neuromuscular effects (i.e., a deficit in foot splay and fore- and hind-limb grip strength occurring at 100 mg/kg bw per day); and
- 100 is the uncertainty factor, selected to account for interspecies variation (×10), intraspecies variation (×10).
Using this TDI, the HBV for aluminum in drinking water is calculated as follows:

Equation 2
The HBV is 2.9 mg/L. This is calculated by multiplying 0.3 mg/kg bw per day by 74 kg, then by 0.20. This product is then divided by 1.53 L/day.
where
- 0.3 mg/kg bw per day is the TDI derived above;
- 74 kg is the average body weight for an adult (Health Canada, in preparation);
- 0.2 is the allocation factor for drinking water. Given that food represents the main source of exposure, and drinking water was a minor contributor to the total aluminum exposure, a floor value of 0.2 was applied as a health-protective approach (Krishnan and Carrier, 2013);
- 1.53 L per day is the drinking water intake rate for a Canadian adult (Health Canada, in preparation). Due to its low volatility and low dermal absorption (Flarend et al., 2001; Pineau et al., 2012), exposure to aluminum from showering or bathing is unlikely to be significant; Consequently, a multi-route exposure assessment, as outlined by Krishnan and Carrier (2008), was not performed.
4.0 Analytical and Treatment Considerations
4.1 Analytical methods to detect aluminum
4.1.1 Standardized methods
Standardized methods available for the analysis of total aluminum in drinking water and their respective method detection limits (MDL) are summarized in Table 4. MDLs are dependent on the sample matrix, instrumentation, and selected operating conditions and will vary between individual laboratories. Analyses for aluminum should be carried out as directed by the responsible drinking water authority in the affected jurisdiction. Water utilities should confirm that the method reporting limits are low enough to ensure accurate quantitation at concentrations below the proposed MAC and the OG.
4.1.2 Online and portable colorimetric analyzers
Commercial online and portable analyzers are available for quantifying dissolved aluminum in source and drinking water, and analysis is generally based on SM 3500-Al C (APHA et al., 1995). Acidification of the sample prior to analysis is needed for the measurement of total aluminum. These analyzers can be used to obtain a rapid or continuous (online units only) indication of changes to aluminum concentrations, which are critical for process monitoring within a water treatment plant (Haught and Fabris, 2002). In general, commercial online methods are capable of measuring aluminum concentrations in the range of 5-1,500 µg/L, with higher concentrations requiring dilution. The detection limits range from 1 µg/L to 10 µg/L. To accurately measure aluminum using these units, water utilities should develop a quality assurance and quality control (QA/QC) program such as those outlined in SM 3020 (APHA et al., 2017). In addition, periodic verification of results using an accredited laboratory is recommended. Water utilities should check with the responsible drinking water authority in the affected jurisdiction to determine whether results from these units can be used for compliance reporting.
| Method (Reference) |
Methodology | MDL (µg/L) | Interferences/Comments |
|---|---|---|---|
| U.S EPA Methods | |||
| EPA 200.5 Rev. 4.2 (U.S. EPA, 2003) |
Axially viewed inductively coupled atomic emission spectrometry (AVICP-AES) | 2.2 | Matrix interferences: calcium, magnesium and sodium >125 mg/L and silica >250 mg/L |
| EPA 200.7 Rev. 4.4 (U.S. EPA, 1994a) |
Inductively coupled plasma-atomic emission spectrometry (ICP-AES) | 20 | Matrix interferences: total dissolved solids >0.2% weight per volume (w/v) |
| EPA 200.8 Rev. 5.4 (U.S. EPA, 1994b) |
Inductively coupled plasma-mass spectrometry (ICP-MS) | 1Footnote a-1.7Footnote b | Matrix interference: total dissolved solids >0.2% w/v |
| EPA 200.9 Rev 2.2 (U.S. EPA, 1994c) |
Graphite furnace atomic absorption (GFAA) | 7.8 | Use of hydrochloric acid may cause chloride ion vapour state interferences. Elevated aluminum in palladium matrix will cause elevated blank absorbances. |
| APHA Standard Methods (APHA et al., 2017, except where noted) | |||
| SM 3111D and SM 3111E |
Direct (SM 3111D) or extraction (3111E) nitrous oxide-acetylene flame atomic absorption spectrometry | 100 | SM 3111E: Applicable for determination of aluminum concentrations <900 µg/L; matrix interference: iron >10 mg/L |
| SM 3113B | Electrothermal atomic absorption spectrometry | 3 | |
| SM 3120B | Inductively coupled plasma-mass atomic emission spectrometry (ICP-AES) | 40 | Matrix interference: total dissolved solids >1,500 mg/L |
| SM 3125 | Inductively coupled plasma mass spectrometry (ICP-MS) | 0.03 | Matrix interference: total dissolved solids >0.5% w/v |
| SM 3500-Al B | Colorimetric method using eriochrome cyanine R dye and spectrophotometer (535 nm) | 6 | Fluoride, phosphates and ferric iron may cause interferences. Procedures and correction factors may be needed to obtain accurate measurements. |
| SM 3500-Al C (APHA et al., 1995) |
Colorimetric method using pyrocathechol violet and spectrophotometer (580 nm) | 7-10 | |
|
|||
4.1.3 Sample preservation and preparation
Total aluminum includes both the dissolved and particulate (suspended) fractions of aluminum in a water sample and is analyzed using methods for total recoverable aluminum. Analysis of total aluminum is needed for comparison to the MAC and OG. Determining the concentration of both the dissolved and particulate fractions may be necessary for process monitoring (see Section 4.2.1.1).
Sample processing considerations for analysis of aluminum in drinking water can be found in the references listed in Table 4. Accurate quantification of dissolved, particulate and total aluminum in samples is dependent on the proper sample preservation and processing steps. SM 3030B provides guidance on filtration and preservation (acidification) procedures for the determination of dissolved or particulate metals (APHA et al., 2017). It is important to note that in order to determine dissolved aluminum concentrations, samples should be filtered and the filtrate acidified to pH <2 at the time of collection (not at the laboratory). Delineation between dissolved and particulate fractions in a sample is dependent on the filter type and pore size; therefore, water utilities that may have smaller particles or colloids present in the water should consider whether the standard filter size (0.4-0.45 µm pore-diameter membrane) will be suitable.
Currently, EPA methods 200.7 and 200.8 and SM 3111D, SM 3113B, SM 3120B do not require hot acid digestion for total recoverable metals unless the turbidity of the sample is greater than 1 nephelometric turbidity unit (NTU). APHA et al. (2017) recommends verifying whether adequate recovery of metals has occurred in different sample matrices by comparing digested and undigested samples. Microwave-assisted digestion (SM 3030K) is recommended for analysis of total recoverable metals using SM methods that are based on ICP-MS.
4.2 Treatment considerations
The form of aluminum (e.g., particulate or dissolved) that will be present depends on a wide variety of environmental parameters, including pH, temperature, NOM and the presence of inorganic ligands such as fluoride, sulphate, silicate and phosphorous (Environment Canada and Health Canada, 2010). Aluminum is highly insoluble in the near neutral pH range (Appelo and Postma, 1996). Depending on water quality conditions various chemical precipitates may form, involving oxide, hydroxide, silicate or phosphate (Snoeyink et al., 2003; Friedman et al., 2010). In low pH or high pH conditions, most forms of aluminum become highly soluble. Aluminum solubility is also influenced by temperature. For aluminum sulphate (alum), the pH of minimum solubility occurs at 6.2 at 20 °C and shifts to 6.7 at 5 °C (see Figure 1). At the pH of minimum solubility, soluble aluminum concentrations of 0.005-0.014 mg/L are expected (-6.7 M and -6.3 M, respectively, in Figure 1). This increases dramatically to 27 mg/L at pH 9.7 and 20 °C (-3 M) (Van Benschoten et al., 1992). For pre-hydrolyzed forms of aluminum (e.g., polyaluminum chloride; PACl), the pH of minimum solubility for a coagulant with high basicity occurs at 6.4 at 20 °C and shifts to 6.9 at 5 °C (see Figure 2). As a result, PACl coagulants can generally be used at higher pH values (Pernitsky, 2003) and over a wider temperature range at lower coagulant doses (Matilainen et al., 2010). However, at pH values less than the pH of minimum solubility, dissolved aluminum concentrations increase much more steeply than for alum (e.g., 27 mg/L around pH 6 and 20 °C (-3 M in Figure 2)) (Pernitsky and Edzwald, 2006). Thus, pH and temperature will have an important influence on the aluminum concentration in treated water and potentially on aluminum deposition and accumulation within distribution systems.
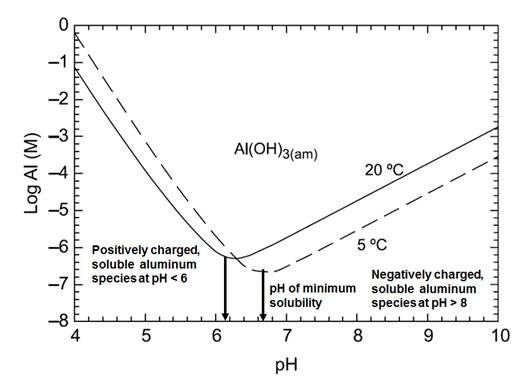
Figure 1
A graph that shows the solubility for alum. The vertical axis shows the aluminum concentration on a log scale in moles ranging from -8 to 0 and the horizontal axis shows pH ranging from 4 to 10. The solubility curve is shaped like a parabola with the point of minimum solubility at the base. The left side of the parabola raises at a slope of 2.5 while the right side raises at a slope of 2. Two solubility curves are provided - one for 20 °C with the base at pH 6.2 and one for 5 °C shifted to the right with the base at pH 6.7.
Figure 1. Alum solubility curves based on theory and experimental data presented in Pernitsky and Edzwald (2003, 2006)
(Adapted from JWSRT - AQUA Volume 55, Issue 2, pp. 121-141, with permission from the copyright holders, IWA Publishing)
pH of minimum solubility (high basicity sulphated coagulant)
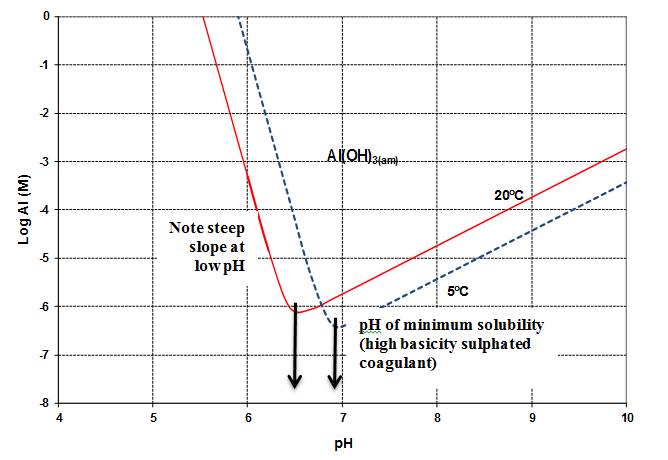
Figure 2
A graph that shows the solubility for alum. The vertical axis shows the aluminum concentration on a log scale in moles ranging from -8 to 0 and the horizontal axis shows pH ranging from 4 to 10. The solubility curve is shaped like a parabola with the point of minimum solubility at the base. The left side of the parabola raises at a slope of 2.5 while the right side raises at a slope of 2. Two solubility curves are provided - one for 20 °C with the base at pH 6.2 and one for 5 °C shifted to the right with the base at pH 6.7.
Figure 2. PACl solubility curves based on theory and experimental data presented in Pernitsky and Edzwald (2003, 2006)
(Adapted from JWSRT - AQUA Volume 55, Issue 2, pp. 121-141, with permission from the copyright holders, IWA Publishing)
- aluminum concentrations can exceed the proposed MAC (Kim et al., 2011; Cantwell et al., 2012; Locco et al., 2018; Table 2);
- aluminum precipitates can trap and protect microorganisms, potentially impairing the efficacy of disinfection processes at the treatment plant and within the distribution system (Letterman and Driscoll, 1988);
- aluminum precipitates in the distribution system can influence the concentration of lead and copper (Kvech and Edwards, 2001), adsorb and release arsenic and chromium (Kim et al., 2011) and act as an accumulation sink for other contaminants (Snoeyink et al., 2003; Friedman et al., 2010);
- precipitates of aluminum can adsorb or co-precipitate lead and copper, contributing to co-accumulation and the risk of particulate lead/copper releases (Knowles et al., 2015; Cantor, 2017);
- aluminum hydroxide has a strong affinity for manganese at pH >7.5 (Wang et al., 2012a);
- aluminum can interfere with lead and copper corrosion control strategies involving orthophosphate passivation by preventing the formation of protective scales (AWWA, 2011a; Wasserstrom et al., 2017).
The precipitation of aluminum in the distribution system can also result in operational issues:
- decreased carrying capacity of watermains and associated pressure loss or increased pumping costs (Baylis, 1953; Hudson, 1966; Cooper and Knowles, 1975; Foley, 1980; Costello, 1984; Kriewall et al., 1996; Grigg, 2010);
- aluminum deposition on water meters, causing them to malfunction and in-service lines causing low household water pressure (Halton, 2001);
- the appearance of turbid or discoloured water (e.g., "milk-coloured" or "cloudy" water) (Costello, 1984; Dietrich, 2015; NHMRC and NRMCC, 2011; Locco et al., 2018).
At high concentrations (5-6 mg/L) aluminum may cause an unpleasant taste, and at very high concentrations (100-500 mg/L) the water may feel "sticky" (Hrudey and Hrudey, 2014). High concentrations have occurred as a result of accidents at full-scale water treatment plants using aluminum-based coagulants. This highlights the need for standard operating procedures, alarms and interlocks, and contingency plans when dealing with water treatment chemicals.
4.2.1 Municipal-scale treatment
For naturally occurring aluminum in source water, the only known effective treatment technology is coagulation. This is a complex treatment technology that is typically not used for small systems or groundwater supplies. In cases where aluminum removal is required and coagulation is not feasible, the responsible drinking water authority in the affected jurisdiction should be contacted to discuss possible options.
4.2.1.1 Use of aluminum-based coagulants
Coagulation has multiple objectives, and optimum coagulation conditions necessitate a coagulant dose and a pH that
- maximize the removal of turbidity (particles) by downstream processes;
- maximize the removal of NOMs (disinfection by-product precursors); and
- minimize the coagulant residual in treated water.
When aluminum-based coagulants are added to the water, chemical reactions occur with particles, as well as with the organic matter naturally present in the source water. The NOM acts as a ligand that complexes the positively charged aluminum ions, exerting a coagulant demand that must be overcome before flocculation can occur (Edzwald and Haarhoff, 2012). If the coagulant dose is insufficient to overcome this demand, aluminum remains in dissolved form, resulting in elevated aluminum residuals and suboptimal particle removal (Jekel and Heinzmann, 1989; Edzwald and Van Benschoten, 1990; Van Benschoten and Edzwald, 1990a; Srinivasan et al., 1999; Edzwald and Kaminski, 2009). Under acidic conditions, overdosing can also increase the aluminum residual (Van Benschoten and Edzwald, 1990a). Post-treatment precipitation of particles causing turbidity, as well as deposition and accumulation within distribution systems, can occur with changes in pH and temperature (Snoeyink et al., 2003; Pernitsky and Edzwald, 2006).
A review of paired raw and treated water samples for surface water treatment plants in three provinces (Nova Scotia, Manitoba, Alberta) found a decrease in aluminum concentrations for 70-82% of the samples. Increased concentrations in treated water tended to occur when raw water aluminum concentrations were low (see Table 5). The concentration increases clearly highlight the impacts of improper coagulation on aluminum residuals and the potential for treated water concentrations to exceed the proposed MAC, although the use of other water treatment chemicals may have contributed to the increase (see Section 4.2.1.3). The decreased concentrations provide an indication of the low concentrations (0.010-0.032 mg/L) that can be achieved.
| Jurisdiction | Number of paired samples | Increased concentrations | Decreased concentrations |
|---|---|---|---|
| Nova ScotiaFootnote 1 | 54 samples from 24 facilities | n 15/54 (28%) Raw 0.071 mg/L Treated 0.724 mg/L % increase 920% |
n 39/54 (72%) Raw 0.180 mg/L Treated 0.010 mg/L % decrease 94.4% |
| ManitobaFootnote 2 | 154 samples from 34 facilities | n 46/154 (30%) Raw 0.047 mg/L Treated 7.97 mg/L % increase 16,714% |
n 108/154 (70%) Raw 32.4 mg/L Treated 0.032 mg/L % decrease 99.9% |
| AlbertaFootnote 3 | 136 samples from 3 facilities | n 24/136 (18%) Raw 0.052 mg/L Treated 0.256 mg/L % increase 392% |
n 112/136 (82%) Raw 5.68 mg/L Treated 0.025 mg/L % decrease 99.6% |
|
|||
A review of 10 full-scale case studies assessed the achievable aluminum residual concentration for the range of temperatures experienced in Canada (Health Canada, 2018a). Findings are summarized in Table 6 and show that water treatment plants adding aluminum-based coagulants can exceed 0.05 mg/L at some time, during either cold or warm water conditions. Plants coagulating at acidic pH tend to experience higher aluminum concentrations in cold water conditions, because this is when they are operating furthest from the point of minimum solubility (see Figures 1 and 2). Conversely, plants coagulating at alkaline pH generally experience higher aluminum concentrations in warm water conditions, because this is when they are operating furthest from the point of minimum solubility. Other published literature documented similar findings (Van Benschoten and Edzwald, 1990b; Anderson et al., 1998; Halton, 2001; Kundert et al., 2004). In addition, the review found that water treatment plants with changes in NOM content experienced elevated aluminum residual concentrations due to inadequate coagulant dose. Increasing the coagulant dose decreased aluminum residual concentrations from 0.16-0.50 mg/L to 0.06-0.07 mg/L (Srinivasan et al., 1999; Anderson et al., 2017; Health Canada, 2018a).
| pH conditions | Water temperature at which high aluminum residuals occur | Aluminum concentrations |
|---|---|---|
| Constant acidic pH (i.e., no seasonal adjustment) | ≤5°C | Increase from <0.05 mg/L to ~0.300 mg/L |
| pH seasonally adjusted | N/A | Below 0.06 mg/L for all seasonsFootnote a except for two measurementsFootnote b |
| Constant alkaline pH (i.e., no seasonal adjustment) | ≥15°C | Increase from <0.05 mg/L to ~0.400 mg/L |
|
||
Strict pH control and adequate coagulant dosing are necessary to minimize aluminum residual concentrations in treated water (Driscoll and Letterman, 1995). When optimizing coagulation, it is recommended that a filter effluent turbidity goal of 0.1 NTU be established to minimize aluminum residuals (Jekel and Heinzmann, 1989; Van Benschoten et al., 1992). Jar testing can be a helpful tool to optimize the coagulation process and test alternate coagulants and/or flocculant aids (AWWA, 2011b). Process monitoring of residual aluminum should include total and dissolved aluminum concentrations. Dissolved aluminum provides an indication of the suitability of the coagulation pH, while particulate aluminum indicates the performance of filter operations. In this case, it is acceptable to consider particulate aluminum to be the difference between total and dissolved aluminum.
The use of alternative iron-based coagulants to minimize residual aluminum concentrations should be considered with caution, as iron is reported to add to adverse health effects (Rao and Adlard, 2018). It is also important to note that coagulant under-dosing can result in substantial deterioration of pathogen removal capability (Huck et al., 2001). Thus, it is critical that efforts to minimize residual aluminum concentrations not compromise the effectiveness of pathogen log removal capability or interfere with the removal of NOM (i.e., disinfection by-product precursors).
4.2.1.2 Orthophosphate
Orthophosphate added during the rapid mix after coagulant addition has been identified as a possible strategy to decrease aluminum residuals, because it can form aluminum-phosphate precipitates that can be removed by filtration (Frommel et al., 2004; Wang et al., 2012b; Health Canada, 2018a). This approach is not recommended, because the addition of phosphorous generates competing chemical reactions during water treatment. Depending on water quality conditions, aluminum-phosphate precipitates are formed, resulting in the loss of orthophosphate for corrosion control, and/or the phosphorous introduces a negative charge to aluminum hydroxide flocs, resulting in poor filtration. Thus, if orthophosphate is added for corrosion control, it should be at a location downstream of the clearwell to avoid introducing phosphorous to the filters during backwashing (Edzwald, 2018).
Caution is also recommended when using orthophosphate for corrosion control, as aluminum can interfere with the passivation of lead (Cantor, 2017). Theoretical solubility models for the lead carbonate-orthophosphate system typically assume the formation of hydroxypyromorphite (Pb5(PO4)3OH), but orthophosphate may precipitate with residual aluminum (range of 29-110 μg/L for this study), forming porous aluminum- and phosphorus-rich deposits that adhere poorly to pipe surfaces and do not effectively inhibit lead release (Wasserstrom et al., 2017). Cantor (2017) reviewed the phosphate-based corrosion control strategies for 12 municipal and non-municipal water systems using ground and surface water and found a strong association between particulate lead and copper release and particulate aluminum. Aluminum-phosphate precipitates can also contribute to distribution system deposits (see section 4.3), turbidity and milky-white colour at the point of use. A target maximum aluminum concentration of 0.05 mg/L is recommended for both the entry point and the distribution system to avoid these issues (AWWA, 2011a).
4.2.1.3 Use of certified chemicals with minimal aluminum content
Health Canada commissioned a report to determine the potential aluminum contribution to drinking water from five commercially available types of chemicals certified to NSF International (NSF)/American National Standards Institute (ANSI) Standard 60 (NSF/ANSI, 2017) that may have contained aluminum as an impurity: ammonium sulphate (chloramination), calcium hypochlorite (disinfectant), calcium hydroxide (pH adjustment), calcium oxide (pH adjustment), and sodium silicate (corrosion control). Data were compiled from product certification and ongoing surveillance evaluations conducted between 2016 and 2017 (NSF, 2018). For compliance to NSF/ANSI Standard 60, chemical products must not exceed an at-the-tap concentration of 2 mg/L aluminum.
The aluminum concentrations measured in the chemical product types are summarized in Table 7. From these results, estimates of the amount of aluminum added to drinking water at the maximum use level of the product can be calculated (i.e., normalized). These normalized results are summarized in Table 8. It is important to note that these are estimated concentrations, not actual concentrations measured in treated drinking water.
A review of the data in Table 8 indicates that a facility adding both calcium hydroxide and sodium silicate could add up to 51 μg/L of aluminum to the drinking water. Although these values are significantly lower than allowed under NSF/ANSI Standard 60, they may result in the accumulation of aluminum in the distribution system (see Section 4.3). To minimize the amount of aluminum added to treated water, the maximum anticipated dose that will be applied at the treatment facility should be considered when specifying chemical products.
| Chemical product type | No. detects/ samples | Minimum (mg/kg) |
Median (mg/kg) |
95th percentile (mg/kg) |
Maximum (mg/kg) |
|---|---|---|---|---|---|
| Ammonium sulphate | 0/25 | 0 | 0 | 0 | 0 |
| Calcium hypochlorite | 21/23 | 121 | 223 | 437 | 485 |
| Calcium hydroxide | 30/31 | 0.6 | 11 | 33 | 93 |
| Calcium oxide | 25/27 | 0.1 | 1.0 | 22 | 30 |
| Sodium silicate | 31/36 | 46 | 99 | 392 | 550 |
| Chemical product type | No. detects/ samples | Minimum (μg/L) |
Median (μg/L) |
95th percentile (μg/L) |
Maximum (μg/L) |
|---|---|---|---|---|---|
| Ammonium sulphate | 0/25 | 0 | 0 | 0 | 0 |
| Calcium hypochlorite | 21/23 | 0.7 | 2.5 | 5.8 | 6.1 |
| Calcium hydroxide | 30/31 | 0.7 | 8 | 21 | 28 |
| Calcium oxide | 25/27 | 0.3 | 2.6 | 11 | 15 |
| Sodium silicate | 31/36 | 1.9 | 3.9 | 15 | 23 |
Note: Concentrations were calculated assuming the product is used at its maximum use level. |
|||||
4.2.1.4 Other treatment options for naturally occurring aluminum
There is a paucity of literature regarding technologies other than coagulation for the removal of naturally occurring aluminum. Aluminum is known to foul reverse osmosis membranes (Allenby, 2004); cation exchange resins must be modified and used at an extremely low pH (Vanloot et al., 2007); lime softening may increase aluminum concentrations (Reijnen et al., 1991; Alabdula'aly, 1998; Kettunen and Keskitalo, 2000; AWWA, 2011a); and chemical oxidants, such as chlorine, are ineffective because the oxidation state of aluminum does not change (Edzwald, 2018). Based on the occurrence data presented in Table 2, 90th percentile concentrations are below the proposed MAC, while some maximum values are above it. For sources with aluminum concentrations above the proposed MAC, a site-specific assessment would be necessary to determine the most appropriate treatment option if coagulation is not feasible. Pilot testing is recommended to ensure the source water can be successfully treated. Alternatively, a safe alternate drinking water supply could be used.
4.2.2 Residential-scale treatment
In cases where aluminum removal is desired at the household level-for example, when a household obtains its drinking water from a private well-treatment is expected to be challenging, based on the information presented in Section 4.2.1. The responsible drinking water authority in the affected jurisdiction should be contacted to discuss possible options.
4.3 Distribution system considerations
4.3.1 Aluminum deposition and accumulation
Observations of aluminum deposits on distribution system piping have been reported in the literature since 1953 (Baylis, 1953; Hudson, 1966; Cooper and Knowles, 1975; Foley, 1980; Costello, 1984; Kriewall et al., 1996; Halton, 2001; Muylwyk and MacDonald, 2001; Schock and Holm, 2003; Lytle et al., 2004; Schock, 2005; Friedman et al., 2010; Grigg, 2010; Li et al., 2018). Aluminum can accumulate on all pipe materials (Hudson, 1966) and be released, along with other health-based contaminants, when water quality conditions change (e.g., pH or temperature) (Fuge et al., 1992; Kriewall et al., 1996; Halton, 2001; Snoeyink et al, 2003; Kim et al., 2011). Physical/hydraulic disturbances may also cause poorly adhered deposits to detach (e.g., road work, hydrant flushing, watermain breaks, meter installation, leak repair, firefighting activity) (Friedman et al., 2010; Hill et al., 2010; Del Toral et al., 2013; Wasserstrom et al., 2017).
Additionally, changes in pH and temperature in the distribution system can cause aluminum to go in and out of solution and be transported and deposited throughout the system (Driscoll et al., 1987; Halton, 2001; Snoeyink et al., 2003; Munk and Faure, 2004).
Table 9 quantifies the accumulation of aluminum in various system types. The majority of results presented in Table 9 are for groundwater systems, which tend to have lower aluminum concentrations. Nonetheless, Lytle et al. (2004) and Friedman et al. (2010) reported 90th percentile aluminum concentrations in pipe section solids that were comparable in groundwater and surface water systems, whereas maximum concentrations were 5.5 and 1.8 times higher in groundwater systems than in surface water. Although surface water data are limited, these data demonstrate that aluminum accumulates in all water systems.
With respect to hydrant flush solids (see Table 9), Lytle et al. (2004) reported the highest aluminum concentration (144,265 μg/g) in a groundwater system with alum addition. This system also had the highest copper, lead and nickel concentrations. In groundwater systems with no alum addition, the maximum aluminum concentration was 19 times lower. Li et al. (2018) measured an aluminum concentration of 55,000 μg/g for unidirectional flush solids from a cast-iron pipe for a surface water system adding PACl (average aluminum residual = 0.050 mg/L). The authors reported that aluminum (and manganese) contribute to the formation of loose deposits that are more easily released by hydraulic disturbances.
| Deposit type | Water type | No. of samples | Min (μg/g) |
Median (μg/g) |
90th (μg/g) |
Max (μg/g) |
|---|---|---|---|---|---|---|
| Pipe section solids | Lytle et al., 2004Footnote a | |||||
| Groundwater | 35 | 28 | 718 | 2,789 | 7,286 | |
| Surface water with alum addition | 1 | 1,324 | 1,324 | 1,324 | 1,324 | |
| Friedman et al., 2010Footnote b | ||||||
| Groundwater | 22 | 105 | 536 | 3,294 | 8,880 | |
| Mixed-groundwater and surface waterFootnote c | 8 | 374 | 1,422 | 8,322 | 20,256 | |
| Mixed-surface waterFootnote d and groundwater | 3 | 561 | 759 | 944 | 990 | |
| Surface water with alum addition | 2 | 4,373 | 4,669 | 4,906 | 4,965 | |
| Hydrant flush solids | Lytle et al., 2004Footnote a | |||||
| Groundwater | 22 | 96 | 375 | 2,905 | 7,512 | |
| Groundwater with alum addition | 4 | 11,708 | 103,602 | 139,252 | 144,265 | |
| Friedman et al., 2010Footnote b | ||||||
| Groundwater | 21 | 33 | 446 | 1,066 | 1,659 | |
| Mixed-groundwater and surface waterFootnote e | 2 | 1,545 | 5,911 | 9,403 | 10,276 | |
|
||||||
Li et al. (2018) discussed the cumulative process of deposit mixtures and suggested that aluminum and manganese served as the main scavengers to adsorb other metals. The authors stated that measures to minimize aluminum and manganese deposits in the distribution system were essential to reduce heavy-metal-related risks. On average, aluminum ranked eighth out of 13 elements (manganese was seventh) in terms of deposit concentrations in Lytle et al. (2004), whereas aluminum ranked third out of 12 elements (manganese was fifth) in Friedman et al. (2010). Schock (2005) published metal accumulation in lead service lines and iron pipe scales for a variety of water types. On average, aluminum ranked fourth out of 13 elements (manganese was sixth) in terms of deposit concentrations. These data highlight that aluminum and manganese solids can represent a significant portion of legacy deposits in the distribution system.
Health-based contaminants that have accumulated may be released to distributed water as dissolved or particulate species when changes to water chemistry occur (Schock, 2005; Hill et al., 2010; Kim et al., 2011; Trueman and Gagnon, 2016; Cantwell et al., 2012). Kim et al. (2011) measured aluminum concentrations in the order of 2-7 mg/L between pHs 9 and 10 in batch dissolution tests of corrosion products from a lead pipe. Cantwell et al. (2012) reported aluminum concentrations of 1,060-4,610 μg/L between pHs 7.1 and 7.6 for a pipe loop study. Co-releases of arsenic, chromium and lead were also reported (Kim et al., 2011; Cantwell et al., 2012).
These observations highlight a complication in pH-dependent strategies for controlling contaminant releases: while some contaminants are released in response to a pH decrease (e.g., lead, manganese), others are released in response to a pH increase (e.g., arsenic, copper, chromium) (Kim et al., 2011). For aluminum, solubility characteristics can vary seasonally due to changes in temperature, pH and NOM concentrations. Higher temperatures in the summer, for example, may allow aluminum to stay in dissolved form and not precipitate. If the temperature increase is high enough to cause the system to experience subsaturation conditions, previously accumulated aluminum deposits (i.e., legacy deposits) can dissolve and release co-precipitated contaminants. Seasonal variations in other parameters (e.g., phosphate, silicate) can also impact chemical equilibrium processes. Thus, a comprehensive control strategy (see Section 5) is required to meet concomitant water quality goals related to aluminum, corrosion products and other health-based contaminants that may accumulate in the distribution system (Cantor, 2017; Li et al., 2018).
4.3.2 Leaching of aluminum from cement-based materials
Aluminum may enter the distribution system through leaching from cement-based materials and linings (Leroy et al., 1996) even when using certified materials and linings applied according to industry standards (U.S. EPA, 2002). Mlynska and Zielina (2017) conducted a bench-scale study to compare the aluminum leaching from two pipe specimens coated with different cement linings: a prefabricated pipe cement coating and a coating prepared onsite during a pipe renovation. Both pipe specimens were filled with water collected from a water treatment plant (aluminum concentration not reported). Water samples were collected from each pipe specimen following specific periods of time for up to 56 days. At the end of the experiment, the aluminum concentrations were approximately 0.03 mg/L and 8 mg/L in the pipe specimen with the prefabricated pipe coating and in the onsite applied coating specimen, respectively. However, it is important to note that this study represents stagnation conditions that generally do not occur in distribution systems. At full-scale testing, Zielina et al. (2015) reported the leaching of aluminum after the application of a cement mortar lining inside a 500 mm steel pipe (length 614.5 m). Aluminum concentrations increased from 0.043 mg/L to 0.293 mg/L after 3 hours and decreased to 0.052 mg/L after 11 hours. Berend and Trouwborst (1999) reported aluminum concentrations of 650 μg/L 6 weeks after 2,200 metres of ductile iron pipe was coated with a cement mortar lining. Given that aluminum concentrations can increase when lining watermains with cement mortar material, water quality monitoring should be considered.
Additional guidance regarding the leaching of aluminum from cement-based materials and linings is available in U.S. EPA (2002).
4.4 Residuals management
Treatment technologies may produce a variety of residuals that contain aluminum (e.g., backwash water, reject water/concentrate). If residuals are discharged directly to a water body or if the residuals treatment process involves a discharge to a water body, the responsible drinking water authority in the affected jurisdiction should be contacted to confirm the requirements that will apply. Guidance can be found elsewhere (CCME, 2003; CCME, 2007). In some cases, aluminum-rich residual streams (e.g., filter backwash, thickener supernatant) are recycled to the head of the treatment plant to improve water recovery rates. Where feasible, these streams should be treated prior to the recycling to remove solids (including aluminum particles and co-precipitated contaminants), improving and stabilizing the treated water quality (Confluence Engineering, 2018). Recycled residual streams should, in all cases, be treated prior to recycling to reduce risks from enteric protozoa and viruses (Health Canada, 2019a, 2019b).
5.0 Control strategies
All water utilities should implement a risk management approach, such as the source-to-tap or water safety plan approach, to ensure water safety (CCME, 2004; WHO, 2011, 2012). These approaches require a system assessment to characterize the source water, describe the treatment barriers that prevent or reduce contamination, identify the conditions that can result in contamination, and implement control measures. Operational monitoring is then established, and operational/management protocols are instituted (e.g., standard operating procedures, corrective actions and incident responses). Compliance monitoring is determined and other protocols to validate the water safety plan are implemented (e.g., record keeping, consumer satisfaction). Operator training is also required to ensure the effectiveness of the water safety plan at all times (Smeets et al., 2009).
5.1 Control strategies
As it is difficult to control the accumulation and release of aluminum and other health-based contaminants in the distribution system, the control strategy should minimize the aluminum concentration that enters the distribution system from the treatment plant. Secondly, the distribution system should be managed such that drinking water is transported from the treatment plant to the consumer with minimum loss of quality. As source waters, treatment plants and distribution systems can differ significantly, a system-specific control strategy would be necessary.
5.1.1 Treatment
There is extensive guidance available to assist water utilities in understanding the mechanisms associated with coagulation (Edzwald, 1993; Pernitsky, 2003; Dempsey, 2006; O'Melia, 2006; Pernitsky and Edzwald, 2006; Shin et al., 2008; Edzwald and Kaminski, 2009; AWWA, 2011b; Davis and Edwards, 2014). Jar testing is preferred for optimization studies, as it is relatively easy to perform experiments using various coagulant types, dose, pH, and mixing speeds. The choice of coagulant will depend on the characteristics of the water to be treated. For many water supplies, coagulant dosing will be controlled by the amount of NOM present rather than the turbidity (Edzwald and Van Benschoten, 1990; Pernitsky and Edzwald, 2006; Edzwald and Kaminski, 2009; Health Canada, 2018b).
Strict pH control is necessary to minimize the residual aluminum concentrations leaving a treatment plant. Table 10 highlights the optimum pH ranges that are most applicable for alum and PACl coagulants for cold and warm water conditions (Edzwald, 2018). The achievable residual aluminum concentration is also noted (e.g., <0.03 mg/L at temperatures <10 °C and <0.05 mg/L at temperatures >10 °C). Water utilities should thus aim to decrease total aluminum to <0.05 mg/L in filtered water (prior to fluoride or phosphorous addition) and further strive to achieve a target of <0.03 mg/L when temperatures are <10 °C (see Table 10).
When implementing pH control, water utilities should be aware of the impact of post-chlorination on pH (e.g., decrease with chlorine gas or increase with sodium hypochlorite), particularly if the dose is adjusted on a seasonal basis (Larson and Sollo, 1967; Costello, 1984; Reijnen et al., 1991). When selecting a coagulant dose, water utilities should be aware that under-dosing to reduce the aluminum residual can result in substantial deterioration of the pathogen removal capability (Huck et al., 2001). Adequate coagulant dosing and strict pH control are necessary to practice optimum coagulation and minimize residual aluminum.
| Coagulant | Cold water (<10°C) | Warm water (>10°C) | ||
|---|---|---|---|---|
| Optimum pH range | Achievable aluminum concentration |
Optimum pH range | Achievable aluminum concentration | |
| Alum | 6.5 to 7 | 0.01-0.03 mg/L | 6.0 to 6.5 | 0.02-0.05 mg/L |
| PACl | 6.8 to 7.3 | 0.02-0.03 mg/L | 6.3 to 6.8 | 0.02-0.05 mg/L |
5.1.2 Distribution system
There is increasing recognition that distribution systems represent a complex and dynamic environment, where numerous interactions and reactions capable of impacting aluminum concentrations at consumer taps can occur. Seasonal source water quality fluctuations, process control modifications or other causes can ultimately affect the fate and transport of aluminum in the distribution system, resulting in an increase in aluminum concentrations at the tap. Other events or water utility practices may also result in water chemistry changes (e.g., blending of different sources, nitrification) (Hill et al., 2010).
To minimize the degradation of water quality, water utilities should maintain stable water chemistry conditions that promote consistent equilibrium-based solubility of aluminum, preferably subsaturation to reduce the risk of precipitation and the accumulation of aluminum throughout the distribution system. Stable water chemistry conditions also minimize the risk of desorption (release) of aluminum and co-occurring health-based contaminants that can be complexed or co-precipitated on or within the legacy aluminum deposits (as well as manganese deposits or other solids). Key water quality parameters relevant to these mechanisms include pH, temperature, oxidation-reduction potential, NOM, sulphate, dissolved inorganic carbon, fluoride, and residual concentrations of orthophosphate or silicate (when applied for corrosion control). In addition, water quality that is non-aggressive towards concrete and cement pipe types and cement mortar linings should be maintained to minimize leaching of aluminum (and calcium, etc.) from these matrices (Leroy et al., 1996). Water utilities should determine the baseline water quality entering and within their distribution systems and subsequently establish boundary conditions outside of which an excursion could be expected to trigger a release event (Friedman et al., 2016).
Depending on the situation, there are a variety of methods to improve the stability of these parameters, such as installation of treatment, modification of existing treatment processes, enhanced process monitoring and control (at the treatment plant and/or in the distribution system), slow and controlled introduction of new or seasonal sources, and controlled blending of dissimilar sources (Confluence Engineering, 2018). Prior to the introduction of a new source and/or the application of a new or modified treatment process for an existing source, pilot testing should be conducted using harvested pipe specimens from the system to consider the following points and avoid unintended consequences (Hill and Giani, 2017):
- assess the occurrence and inventory of aluminum and other health-based contaminants in the pipe scales;
- identify a pipe and deposit/scale response to the new source or water chemistry; and
- evaluate approaches to mitigate any observed adverse responses.
Distribution system pH variability should be minimized to ±0.2 units (Muylwyk and MacDonald, 2001; Friedman et al., 2010; Health Canada, 2015).
Biostability in the distribution system is another important requirement to minimize contaminant accumulation and release. Biostability can be achieved by minimizing nutrients in the water (e.g., organic carbon, ammonia, nitrate/nitrite, total phosphorus), managing water age and maintaining a sufficient disinfectant residual (Cantor, 2017; Health Canada, 2018b).
Other measures that contribute to maintaining stable chemical and biological conditions in the distribution system include pipe cleaning (e.g., unidirectional flushing, pipe pigging), pipe replacement, and appropriate treatment to minimize the loading of other contaminant sinks (e.g., iron, manganese) and decrease the concentrations of contaminants entering the distribution system (e.g., arsenic, barium, chromium, manganese) (Friedman et al., 2010; Cantor, 2017).
For systems that use orthophosphate for corrosion control, the orthophosphate should be applied at all system entry points and a consistent residual concentration should be maintained throughout the distribution system to promote the stability of phosphate-based scales (Friedman et al. 2010). It should be noted that polyphosphates (i.e., blended ortho/poly products) can soften cementitious matrices and leach aluminum (calcium, etc.) into the distribution system (Leroy et al., 1996).
5.2 Monitoring
Aluminum concentrations can vary in source water and within treatment plants and distribution systems; therefore, monitoring programs should be established that enable water utilities to obtain a good understanding of aluminum concentrations from source to tap. Monitoring programs should be designed to verify that control strategies are operating as intended and to consider risk factors that contribute to the likelihood of aluminum being elevated within the drinking water system.
5.2.1 Source water characterization
Source water characterization should be part of routine system assessments and should include an understanding of aluminum concentrations in the source water (both groundwater and surface water) and conditions that can lead to changes in these concentrations. Source water monitoring should be conducted quarterly in conjunction with treated and distribution system monitoring, as discussed below.
5.2.2 Operational monitoring
As aluminum is an important process parameter to practice optimum coagulation, water utilities that use aluminum-based coagulants should conduct daily or more frequent monitoring of total aluminum (Edzwald, 2018). These measurements should be conducted onsite using an online or portable colorimetric analyzer (Edzwald, 2018). An appropriate QA/QC and verification program should also be in place. To minimize interferences, samples should be collected after filtration before any fluoride or phosphate addition. Monitoring of dissolved aluminum concentrations is also recommended for process control. Water utilities that use aluminum-based coagulants should aim to achieve an OG of 0.050 mg/L and further strive to achieve a target of 0.030 mg/L for total aluminum.
Measures should also be in place to assess the contribution of aluminum from other water treatment chemicals. This can be determined by comparing aluminum concentrations in the filter effluent and treated water when aluminum-based coagulants are used or by comparing raw and treated aluminum concentrations for other systems.
5.2.3 Distribution system monitoring
Given that aluminum concentrations can change throughout the distribution system (Halton, 2001), appropriate distribution system monitoring should be conducted (Friedman et al., 2010) in conjunction with paired source- and treated-water sampling. Given the important links between NOM removal, coagulation optimization and aluminum residuals, it is recommended that aluminum concentrations be measured on a quarterly basis in free-flowing samples, in conjunction with disinfection by-product monitoring (Health Canada, 2018c). Monitoring should include dissolved and total aluminum concentrations, pH, temperature, and orthophosphate residual (if relevant) (Cantor, 2017). A locational running annual average of a minimum of quarterly samples should be calculated for comparison with the MAC and OG. To minimize the potential for the accumulation and release of aluminum and co-occurring contaminants, for interference with orthophosphate (where applicable) and for aesthetic issues (e.g., colour, turbidity), water utilities should strive to maintain aluminum concentrations below 0.050 mg/L throughout the distribution system.
In addition, event-based monitoring should be conducted during conditions where the risk of release is increased, such as following hydraulic disturbances (e.g., watermain flushing) or changes in water chemistry (e.g., changes to pH, temperature, source water type, chlorine residual) as well as when discolouration of water has been reported (Friedman et al., 2016). Some samples should be collected from sites within the distribution system (such as hydrants or valves) as well as from drinking water taps in public or private buildings to help determine the cause of the event and the aluminum concentrations at the point of use (i.e., tap). Event-based samples should also be analyzed for other metals that can co-occur in the distribution system and be released with aluminum (e.g., arsenic, chromium, copper, iron, lead, manganese, nickel).
When lining watermains onsite with cement mortar materials, water quality monitoring should be conducted to assess whether aluminum is leaching into the drinking water.
5.2.4 Compliance monitoring
Total aluminum in drinking water based on a locational running annual average of a minimum of quarterly samples taken in the distribution system should be calculated for comparison with the proposed MAC. The responsible drinking water authority in the affected jurisdiction should be contacted to confirm how the proposed OG of 0.050 mg/L will be applied to facilities using aluminum-based coagulants and distribution system management plans.
Water utilities that undertake preventive measures with stable hydraulic, physical, chemical and biological water quality conditions and that have baseline data indicating that aluminum does not occur in the system may conduct less frequent monitoring.
5.2.5 Deposit characterization and inventory
There are limited data suggesting that health-based contaminants measured at the tap (e.g., lead) originate from aluminum deposits. More work is required to determine whether these interactions are similar to those between lead and iron in drinking water systems. Characterization of pipe deposits may help in gaining a better understanding of aluminum interactions with other elements. Speciation of aluminum (i.e., particulate and dissolved) and other elements at the point of use may identify pathways by which trace inorganic contaminants are mobilized (e.g., aluminum-rich particulate matter with adsorbed lead). This work involves specialized methods that may require a partnership between water utilities and universities or advanced commercial laboratories.
Establishing the mass inventory (i.e., mass per pipe wall area) of aluminum and other contaminants contained within distribution system deposits is also encouraged to obtain site-specific concentration increases that could occur under a release scenario (Brandhuber et al., 2015). The Friedman et al. (2010) study provides guidance on sampling pipe specimens to establish an inventory of distribution system solids mass and composition.
6.0 International Considerations
This section presents drinking water guidelines, standards and/or guidance from other national and international organizations. Variations in these values can be attributed to the age of the assessments or to differing policies and approaches, including the choice of key study and the use of different consumption rates, body weights and source allocation factors.
With the exception of the California EPA, no other national or international agencies have established limits for aluminum in drinking water based on health considerations. Rather, non-regulatory guidance values have been set based on aesthetic or operational considerations. The WHO has set practicable values of 0.1-0.2 mg/L based on optimization of the coagulation process in drinking water plants (WHO, 2010). The U.S. EPA has set a secondary maximum contaminant level of 0.05-0.2 mg/L (U.S. EPA, 2018), while Australia has chosen an aesthetic objective of 0.2 mg/L (NHMRC and NRMMC, 2011) and New Zealand has a guideline value of 0.1 mg/L for aesthetic considerations (New Zealand Ministry of Health, 2008).The European Union lists aluminum as an indicator parameter in its drinking water directive with a value of 0.2 mg/L (EU, 1998).
In its assessment of aluminum in drinking water, the WHO (2010) did calculate a health-based value of 0.9 mg/L (rounded) but has highlighted the importance of not exceeding the practicable levels of 0.1-0.2 mg/L to ensure optimization of the coagulation process, in order to prevent microbial contamination and minimize deposition of aluminum floc in the distribution system. The proposed guideline differs from the WHO's health-based value because Canada takes into consideration advancements in science since 2010. The WHO assessment is based on the JECFA's previous PTWI for aluminum of 1 mg/kg body weight per day (JECFA, 2007). JECFA has since revised their PTWI to 2 mg/kg body weight per day (JECFA, 2012) based on the key study, Poirier et al. 2011, that is used in the Canadian guideline.
The California EPA (2008) has established a non-regulatory public health goal for aluminum of 0.6 mg/L, based on elevated serum levels of aluminum in a human balance study (Greger and Baier, 1993), as well as on impaired neurological development in premature infants given aluminum parenterally (Bishop et al. 1997).
7.0 Rationale
The proposed MAC of 2.9 mg/L (2,900 μg/L) is protective of potential health effects and can be reliably measured by available analytical methods and achieved by coagulation. However, the presence of aluminum at low concentrations can cause operational and aesthetic issues in the distribution system. Therefore, an OG of 0.050 mg/L (50 μg/L) is also proposed for total aluminum to avoid these issues.
Aluminum is present in drinking water sources both naturally and as a result of human activities. Aluminum concentrations in water vary across Canada, with surface water generally presenting higher concentrations than groundwater. Aluminum salts are commonly added as coagulants during water treatment to remove turbidity, organic matter and microorganisms. Aluminum is also an impurity found in other chemicals used in water treatment and has been found to leach from cement mortar pipes or linings into drinking water. Based on aluminum's chemical properties, the intake of aluminum from drinking water is by ingestion and is not expected to occur through either skin contact or inhalation while showering and bathing. The nervous system is generally considered to be the major target for aluminum toxicity. Studies in animals have consistently observed adverse neurological effects following ingestion of high levels of aluminum, which supports effects seen in human studies. Studies in humans have found possible associations between aluminum ingestion and neurological diseases such as dementia and Alzheimer's disease; however, design limitations prevent the use of these studies as a basis to develop the HBV. The HBV of 2.9 mg/L (2,900 μg/L) for total aluminum is established based on neurological effects observed in rats. The HBV is based on the latest science, and in particular on rigorous studies that were not available for the calculation of previous HBVs (e.g., the WHO's HBV of 0.9 mg/L and the California EPA's HBV of 0.6 mg/L). For the purposes of this risk assessment, the HBV is designated as the MAC because the HBV is achievable by treatment and reliably measured. The proposed OG of 0.050 mg/L (50 μg/L) is related to minimizing the potential for the accumulation and release of aluminum and co-occurring contaminants in the distribution system as well as its interference with orthophosphate (where applicable).
As part of its ongoing guideline review process, Health Canada will continue to monitor new national and international research in this area and will recommend any change to the guideline or OG value that is deemed necessary.
8.0 References
Abdel Moneim, A., Othman, M.S., Mohmoud, S.M. and El-Deib, K.M. (2013). Pomegranate peel attenuates aluminum-induced hepatorenal toxicity. Toxicol. Mech. Method., 23(8): 624-633.
Abu-Taweel, G.M., Ajarem, J.S. and Ahmad, M. (2012). Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol. Biochem. Be., 101(1): 49-56.
Akatsu, H., Hori, A., Yamamoto, T., Yoshida, M., Mimuro, M., Hashizume, Y., Tooyama, I. and Yezdimer, E.M. (2011). Transition metal abnormalities in progressive dementias. Biometals, 25(2): 337-350.
Akinola, O.B., Biliaminu, S.A., Adediran, R.A., Adeniye, K.A. and Abdulquadir, F.C. (2015). Characterization of prefrontal cortex microstructure and antioxidant status in a rat model of neurodegeneration induced by aluminium chloride and multiple low-dose streptozotocin. Metab. Brain Dis., 30(6): 1531-1536.
Akinola, O.B., Biliaminu, S.A., Adedeji, O.G., Oluwaseun, B.S., Olawoyin, O.M. and Adelabu, T.A. (2016). Combined effects of chronic hyperglycaemia and oral aluminium intoxication on testicular tissue and some male reproductive parameters in Wistar rats. Andrologia, 48(7): 779-786.
Akinrinade, I.D., Memudu, A.E., Ogundele, O.M. and Ajetunmobi, O.I. (2015). Interplay of glia activation and oxidative stress formation in fluoride and aluminium exposure. Pathophysiology, 22(1): 39-48.
Akiyama, H., Hosokawa, M., Kametani, F., Kondo, H., Chiba, M., Fukushima, M. and Tabira, T. (2011). Long-term oral intake of aluminium or zinc does not accelerate Alzheimer pathology in ABPP and ABPP/tau transgenic mice. Neuropathology, 32(4): 390-397.
Alabdula'aly, A. (1998). Trace metals in groundwater and treatment plant product water of the central region of Saudi Arabia. Desalination, 120(1-2): 163-168.
Alberta Environment and Parks (2017). Personal communication with Donald Reid, Operations Division.
Albizzati, A., More, L., Di Candia, D., Saccani, M. and Lenti, C. (2012). Normal concentrations of heavy metals in autistic spectrum disorders. Minerva Pediatr., 64(1): 27-31.
Allenby, M. (2004). Aluminum compounds cause operational problems in a reverse osmosis plant. Mater. Perform., 43(5): 44-46.
Altmann, P., Cunningham, J., Dhanesha, U., Ballard, M., Thompson, J. and Marsh, F. (1999). Disturbance of cerebral function in people exposed to drinking water contaminated with aluminium sulphate: Retrospective study of the Camelford water incident. BMJ, 319(7213): 807-811.
Anderson, W.B., Jasim, S.Y., Urfer, D. and Huck, P.M. (1998). Coagulant and pH control alternatives for soluble aluminum control. In: Proceedings of the American Water Works Association Water Quality Technology Conference, San Diego, California. American Water Works Association, Denver, Colorado.
Anderson, L.E., Krkošek, W.H., Stoddart, A.K., Trueman, B.F. and Gagnon, G.A. (2017). Lake recovery through reduced sulfate deposition: A new paradigm for drinking water treatment. Environ. Sci. Technol., 51(3): 1414-1422.
APHA, AWWA and WEF (1995). Standard methods for the examination of water and wastewater. 19th edition. American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC.
APHA, AWWA and WEF (2017). Standard methods for the examination of water and wastewater. 23rd edition. American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC.
Appelo, C.A.J. and Postma, D. (1996). Geochemistry, groundwater and pollution. Third edition. A.A. Balkema, Rotterdam, Netherlands.
Arain, M.S., Afridi, H.I., Kazi, T.G., Talpur, F.N., Arain, M.B., Kazi, A., Arain, S.A. and Ali, J. (2015). Correlation of aluminum and manganese concentration in scalp hair samples of patients having neurological disorders. Environ. Monit. Assess., 187(2): 10.
ATSDR (2008). Toxicological profile for aluminum. Agency for Toxic Substances and Disease Registry, Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA.
AWWA (2011a). Internal corrosion control in water distribution systems: Manual of water supply practices M58. First edition. American Water Works Association, Denver, Colorado.
AWWA (2011b). Operational control of coagulation and filtration processes: Manual of water supply practices M37. Third edition. American Water Works Association, Denver, Colorado.
Bakar, C., Karaman, H., Baba, A. and Sengunalp, F. (2010). Effect of high aluminum concentration in water resources on human health, case study: Biga Peninsula, northwest part of Turkey. Arch. Environ. Contam. Toxicol., 58(4): 935-944.
Baum, L., Chan, I.H., Cheung, S.K., Goggins, W.B., Mok, V., Lam, L., Leung, V., Hui, E., Ng, C., Woo, J., Chiu, H.F., Zee, B.C., Cheng, W., Chan, M.H., Szeto, S., Lui, V., Tsoh, J., Bush, A.I., Lam, C.W. and Kwok, T. (2010). Serum zinc is decreased in Alzheimer's disease and serum arsenic correlates positively with cognitive ability. Biometals, 23(1): 173-179.
Baylis, J.R. (1953). Cast-iron pipe coatings and corrosion. J. Am. Water Works Assoc., 45(8): 807-831.
Belaid-Nouira, Y., Bakhta, H., Bouaziz, M., Flehi-Slim, I., Haouas, Z. and Cheikh, H.B. (2012). Study of lipid profile and parieto-temporal lipid peroxidation in AlCl3 mediated neurotoxicity. Modulatory effect of fenugreek seeds. Lipids Health Dis., 11(1): 16.
Belaid-Nouira, Y., Bakhta, H., Haouas, Z., Flehi-Slim, I. and Cheikh, H.B. (2013a). Fenugreek seeds reduce aluminum toxicity associated with renal failure in rats. Nutr. Res. Pract., 7(6): 466.
Belaid-Nouira, Y., Bakhta, H., Haouas, Z., Flehi-Slim, I., Neffati, F., Najjar, M.F. and Cheikh, H.B. (2013b). Fenugreek seeds, a hepatoprotector forage crop against chronic AlCl3 toxicity. BMC Vet. Res., 9(1): 22.
Belaid-Nouira, Y., Bakhta, H., Samoud, S., Trimech, M., Haouas, Z. and Cheikh, H.B. (2013c). A novel insight on chronic AlCl3 neurotoxicity through IL-6 and GFAP expressions: Modulating effect of functional food fenugreek seeds. Nutr. Neurosci., 16(5): 218-224.
Berend, K. and Trouwborst, T. (1999). Cement-mortar pipes as a source of aluminum. J. Am. Water Works Assoc., 91(7): 91-100.Bhasin, P., Singla, N. and Dhawan, D.K. (2012). Protective role of zinc during aluminum-induced hepatotoxicity. Environ. Toxicol., 29(3): 320-327.
Bhattacharjee, S., Zhao, Y., Hill, J.M., Culicchia, F., Kruck, T.P.A., Percy, M.E., Pogue, A.I., Walton, J.R. and Lukiw, W.J. (2013). Selective accumulation of aluminum in cerebral arteries in Alzheimer's disease (AD). J. Inorg. Biochem., 126: 35-37.
Bishop, N.J., Morley, R., Day, J.P. and Lucas, A. (1997). Aluminum neurotoxicity in preterm infants receiving intravenous-feeding solutions. N. Engl. J. Med., 336: 1557-1561.
Brandhuber, P., Craig, S., Friedman, M., Hill, A., Booth, S. and Hanson, A. (2015). Legacy of manganese accumulation in water systems. Water Research Foundation, Denver, Colorado.
British Columbia Ministry of Health (2017). Personal communication with Drinking Water Manager David Fishwick.
Cal EPA (2001). Public health goal for aluminum in drinking water. Office of Environmental Health Hazard Assessment, California Environmental Protection Agency. April 2001.
Callan, A.C., Devine, A., Qi, L., Ng, J.C. and Hinwood, A.L. (2015). Investigation of the relationship between low environmental exposure to metals and bone mineral density, bone resorption and renal function. Int. J. Hyg. Environ. Health, 218(5): 444-451.
CFIA (2018). Chemical residues in food: Children's food project report 2013-2014 and Arsenic, cadmium, lead, mercury, and aluminum in infant formulas meal replacements, and nutritional supplements 2012-2013. Food Safety Science Directorate,
Canadian Food Inspection Agency, Ottawa. Available at: http://www.inspection.gc.ca/food/chemical-residues-microbiology/chemical-residues/eng/1324258929171/1324264923941
Cantor, A. (2017). Optimization of phosphorus-based corrosion control chemicals using a comprehensive perspective of water quality. Water Research Foundation, Denver, Colorado.
Cantwell, R.E., Muylwyk, Q., Waller, M., Stuart, J., Rossi, G. and Snoeyink, V.L. (2012). Role of pH for phosphate-based lead control in Windsor, Ontario, Canada. In: Proceedings of the American Water Works Association Water Quality Technology Conference, Toronto, Ontario. American Water Works Association, Denver, Colorado.
CCME (2003). Guidance on the site-specific application of water quality guidelines in Canada: Procedures for deriving numerical water quality objectives. Canadian Council of Ministers of the Environment, Winnipeg, Manitoba.
CCME (2004). From source to tap: Guidance on the multi-barrier approach to safe drinking water. Canadian Council of Ministers of the Environment, Winnipeg, Manitoba.
CCME (2007). A protocol for the derivation of water quality guidelines for the protection of aquatic life. Canadian Council of Ministers of the Environment, Winnipeg, Manitoba.
Cetin, I., Nalbantcilar, M.T., Tosun, K. and Nazik, A. (2017). How trace element levels of public drinking water affect body composition in Turkey. Biol. Trace Elem. Res., 175(2): 263-270.
Colomina, M.T., Roig, J.L., Torrente, M., Vicens, P. and Domingo, J.L. (2005). Concurrent exposure to aluminum and stress during pregnancy in rats: Effects on postnatal development and behavior of the offspring. Neurotoxicol. Teratol. 27(4): 565-574.
Confluence Engineering (2018). Personal communication. Seattle, Washington.
Cooper, R.E. and Knowles, W.L. (1975). Loss in capacity of a large diameter water pipeline. In: Proceedings of the American Water Works Association Water Quality Technology Conference, Minneapolis, Minnesota. American Water Works Association, Denver, Colorado.
Costello, J.J. (1984). Postprecipitation in distribution systems. J. Am. Water Works Assoc., 76(11): 46-49.
Cui, X., Wang, B., Zong, Z., Liu, S. and Xing, W. (2012). The effects of chronic aluminum exposure on learning and memory of rats by observing the changes of ras/raf/ERK signal transduction pathway. Food Chem. Toxicol., 50(2): 315-319.
Dahl, C., Sogaard, A.J., Tell, G.S., Flaten, T.P., Hongve, D., Omsland, T.K., Holvik, K., Meyer, H.E. and Aamodt, G. (2014). Do cadmium, lead, and aluminum in drinking water increase the risk of hip fractures? A NOREPOS study. Biol. Trace Elem. Res., 157(1): 14-23.
Davis, C.C. and Edwards, M. (2014). Coagulation with hydrolyzing metal salts: Mechanisms and water quality impacts. Crit. Rev. Environ. Sci. Technol., 44(4): 303-347.
Del Toral, M.A., Porter, A. and Schock, M.R. (2013). Detection and evaluation of elevated lead release from service lines: A field study. Environ. Sci. Technol., 47(16): 9300-9307.
Dempsey, B.A. (2006). Coagulant characteristics and reactions. Chapter 2 in: Interface science in drinking water treatment: Theory and applications. G. Newcombe and D. Dixon (eds). Academic Press, London, United Kingdom. pp. 5-24.
Dietrich, A.M. (2015). EPA secondary maximum contaminants levels: A strategy for drinking water quality and consumer acceptability. Water Research Foundation, Denver, Colorado.
Donald, J.M., Golub, M.S., Gershwin, M.E. and Keen, C.L. (1989). Neurobehavioral effects in offspring of mice given excess aluminum in diet during gestation and lactation. Neurotoxicol. Teratol. 11(4): 345-351.
do Nascimento, S.N., Charao, M.F., Moro, A.M., Roehrs, M., Paniz, C., Baierle, M., Brucker, N., Gioda, A., Barbosa, F., Bohrer, D., Avila, D.S. and Garcia, S.C. (2014). Evaluation of toxic metals and essential elements in children with learning disabilities from a rural area of southern Brazil. Int. J. Environ. Res. Public. Health., 11(10): 10806-10823.
Dong, C., Cao, J., Cao, C., Han, Y., Wu, S., Wang, S. and Wang, J. (2016). Effects of fluoride and aluminum on expressions of StAR and P450scc of related steroidogenesis in guinea pigs' testis. Chemosphere, 147: 345-351.
Driscoll, C.T. and Letterman, R.D. (1995). Factors regulating residual aluminium concentrations in treated waters. Environmetrics, 6(3): 287-305.
Driscoll, C.T., Letterman, R.D. and Fitch, D.E. (1987). Residual aluminum in filtered water. American Water Works Association, Denver, Colorado.
Drobyshev, E.J., Solovyev, N.D., Gorokhovskiy, B.M. and Kashuro, V.A. (2018). Accumulation patterns of sub-chronic aluminum toxicity model after gastrointestinal administration in rats. Biol. Trace Elem. Res., 185(2): 384-394.
Edzwald, J.K. (1993). Coagulation in drinking water treatment: Particles, organics and coagulants. Water Sci. Technol., 27(11): 21-35.
Edzwald, J.K. (2018). Personal communication. University of Massachusetts, Potsdam, New York.
Edzwald, J.K. and Van Benschoten, J.E. (1990). Aluminum coagulation of natural organic matter. In: Chemical water and wastewater treatment: Proceedings of the 4th Gothenburg Symposium, Madrid, Spain. pp. 341-359.
Edzwald, J.K. and Kaminski, G.S. (2009). A practical method for water plants to select coagulant dosing. J. N. Engl. Water Works Assoc., 123(1): 15-31.
Edzwald, J.K. and Haarhoff, J. (2012). Dissolved air flotation for water clarification. McGraw Hill, New York.
EFSA (2008). Safety of aluminium from dietary intake - scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC). European Food Safety Authority. EFSA Journal, (7): 1-34.
ElBaz, F.K., Aly, H.F. and Ali, G.H. (2017). Haematococcus pluvialis modulating effect on neurotransmitters, hormones and oxidative damage-associated with Alzheimer's disease in experimental rat's model. Int. J. Pharm. Pharm. Sci., 9(2): 198-206.
Environment and Climate Change Canada (2017). National long-term water quality monitoring data. available at: http://donnees.ec.gc.ca/data/substances/monitor/national-long-term-water-quality-monitoring-data/
Environment Canada and Health Canada (2010). Priority substances list assessment report. Follow-up to the state of science report 2000. Aluminum chloride, aluminum nitrate, aluminum sulphate. Ottawa. Available at: https://www.ec.gc.ca/lcpe-cepa/default.asp?lang=En&n=AA0EAAFE-1
Erazi, H., Sansar, W., Ahboucha, S. and Gamrani, H. (2010). Aluminum affects glial system and behavior of rats. C. R. Biol., 333(1): 23-27.
Erazi, H., Ahboucha, S. and Gamrani, H. (2011). Chronic exposure to aluminum reduces tyrosine hydroxylase expression in the substantia nigra and locomotor performance in rats. Neurosci. Lett., 487(1): 8-11.
EU (1998). Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Official journal of the European communities, L 330, 5/12/1998. pp.32-54.
Exley, C. (2006). Severe cerebral congophilic angiopathy coincident with increased brain aluminium in a resident of Camelford, Cornwall, UK. J. Neurol. Neurosur. Ps., 77(7): 877-79.
Exley, C. (2013). Aluminum in biological systems. In: Encyclopedia of metalloproteins. Aluminum in biological systems. Springer, New York. pp. 33-34.
Exley, C., House, E., Polwart, A. and Esiri, M.M. (2012). Brain burdens of aluminum, iron, and copper and their relationships with amyloid-beta pathology in 60 human brains. J. Alzheimers Dis., 31(4): 725-730.
Farhat, S.M., Mahboob, A. and Ahmed, T. (2017a). Cortex- and amygdala-dependent learning and nicotinic acetylcholine receptor gene expression is severely impaired in mice orally treated with AlCl3. Biol. Trace Elem. Res., 179(1): 91-101.
Farhat, S.M., Mahboob, A., Iqbal, G. and Ahmed, T. (2017b). Aluminum-induced cholinergic deficits in different brain parts and its implications on sociability and cognitive functions in mouse. Biol. Trace Elem. Res., 177(1): 115-121.
Flarend, R., Bin, T., Elmore, D. and Hem, S.L. (2001). A preliminary study of the dermal absorption of aluminium from antiperspirants using aluminium-26. Food Chem. Toxicol., 39(2): 163-168.
Foley, P.D. (1980). Experience with direct filtration at Ontario's Lake Huron Treatment Plant. J. Am. Water Works Assoc., 72(3): 162-164.
Friedman, M.J., Hill, A.S., Reiber, S.H., Valentine, R.L. and Korshin, G.V. (2010). Assessment of inorganics accumulation in drinking water system scales and sediments. Water Research Foundation, Denver, Colorado.
Friedman, M., Hill, A., Booth, S., Hallett, M., McNeill, L., McLean, J., Sorensen, D., Hammer, T., De Haan, M., MacArthur, K. and Mitchell, K. (2016). Metals accumulation and release within the distribution system: Evaluation and mitigation. Water Research Foundation, Denver, Colorado.
Frommell, D.M., Feld, C.M., Snoeyink, V.L., Melcher, B. and Feizoulof, C. (2004). Aluminum residual control using orthophosphate. J. Am. Water Works Assoc., 96(9): 99-109.
Fu, Y., Jia, F.B., Wang, J., Song, M., Liu, S.M., Li, Y.F., Liu, S.Z. and Bu, Q.W. (2014). Effects of sub-chronic aluminum chloride exposure on rat ovaries. Life Sci., 100(1): 61-66.
Fuge, R., Pearce, N.J.G. and Perkins, W.T. (1992). Unusual sources of aluminium and heavy metals in potable waters. Environ. Geochem. Health, 14(1): 15-18.
Garzillo, E.M., Lamberti, M., Genovese, G., Pedata, P., Feola, D., Sannolo, N., Daniele, L., Trojsi, F., Monsurro, M.R. and Miraglia, N. (2014). Blood lead, manganese, and aluminum levels in a regional Italian cohort of ALS patients. J. Occup. Environ. Med., 56(10): 1062-1066.
Ghorbel, I., Chaabane, M., Elwej, A., Boudawara, O., Abdelhedi, S., Jamoussi, K., Boudawara, T. and Zeghal, N. (2016a). Expression of metallothioneins I and II related to oxidative stress in the liver of aluminium-treated rats. Arch. Physiol. Biochem., 122(4): 214-222.
Ghorbel, I., Maktouf, S., Fendri, N., Jamoussi, K., Ellouze, C.S., Boudawara, T. and Zeghal, N. (2016b). Co-exposure to aluminum and acrylamide disturbs expression of metallothionein, proinflammatory cytokines and induces genotoxicity: Biochemical and histopathological changes in the kidney of adult rats. Environ. Toxicol., 31(9): 1044-1058.
Ghorbel I., Amara I.B., Ktari N., Elwej A., Boudawara O., Boudawara T. and Zeghal, N. (2016c). Aluminium and acrylamide disrupt cerebellum redox states, cholinergic function and membrane-bound ATPase in adult rats and their offspring. Biol. Trace Elem. Res., 174(2): 335-346.
Giaccio, L., Cicchella, D., Vivo, B.D., Lombardi, G. and Rosa, M.D. (2012). Does heavy metals pollution affect semen quality in men? A case of study in the metropolitan area of Naples (Italy). J. Geochem. Explor., 112: 218-225.
Golub, M.S. and Germann, S.L. (2001). Long-term consequences of developmental exposure to aluminum in a suboptimal diet for growth and behavior of Swiss Webster mice. Neurotoxicol. Teratol., 23(4): 365-372.
Golub, M.S., Keen, C.L. and Gershwin, M.E. (1992a). Neurodevelopmental effect of aluminum in mice: Fostering studies. Neurotoxicol. Teratol. 14(3): 177-182.
Golub, M.S., Han, B., Keen, C.L. and Gershwin, M.E. (1992b). Effects of dietary aluminum excess and manganese deficiency on neurobehavioral endpoints in adult mice. Toxicol. Appl. Pharmacol., 112(1): 154-160.
Golub, M.S., Han, B., Keen, C.L., Gershwin, M.E. and Tarara, R.P. (1995). Behavioral performance of Swiss Webster mice exposed to excess dietary aluminum during development or during development and as adults. Toxicol. Appl. Pharmacol., 133(1): 64-72.
Golub, M.S., Germann, S.L., Han, B. and Keen, C.L. (2000). Lifelong feeding of a high aluminum diet to mice. Toxicology, 150(1-3): 107-117.
Greger, J.L. and Baier, M.H. (1983). Excretion and retention of low or moderate levels of Al by human subjects. Food Chem. Toxicol., 21: 473-477.
Grigg, N.S. (2010). Secondary impacts of corrosion control on distribution system and treatment plant equipment. Water Research Foundation, Denver, Colorado.
Guo, C., Chen, P., Hsia, S., Hsu, G.W. and Liu, P. (2013). The relationship of plasma aluminum to oxidant-antioxidant and inflammation status in asthma patients. Environ. Toxicol. Pharmacol., 35(1): 30-38.
Hackenberg, U. (1972). Chronic ingestion by rats of standard diet treated with aluminum phosphide. Toxicol. Appl. Pharmacol., 23(1): 147-158.
Halton (2001). Investigation of material deposits in the Burlington distribution system. Regional Municipality of Halton, Oakville, Ontario.
Haught, R. and Fabris, M. (2002). Inorganic monitors. Chapter 6 in: Online monitoring for drinking water utilities. American Water Works Association, Denver, Colorado. pp. 133-162.
Health Canada (1998). Guidelines for Canadian Drinking water quality: Guideline technical document: Aluminum. Health Canada, Ottawa.
Health Canada (2007). Concentration of contaminants and other chemicals in food composites. Retrieved from: https://www.canada.ca/en/health-canada/services/food-nutrition/food-nutrition-surveillance/canadian-total-diet-study/concentration-contaminants-other-chemicals-food-composites.html
Health Canada (2015). Guidelines for Canadian drinking water quality: Guideline technical document: pH. Water Quality and Health Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/content/dam/hc-sc/documents/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-ph-eng.pdf
Health Canada (2016). Concentration of contaminants and other chemicals in food composites: Trace elements. Canadian Total Diet Study. Health Products and Food Branch, Health Canada, Ottawa. Available at: https://www.canada.ca/en/health-canada/services/food-nutrition/food-nutrition-surveillance/canadian-total-diet-study/concentration-contaminants-other-chemicals-food-composites.html
Health Canada (2017). Personal communication with Anca-Maria Tugulea, Environmental and Health Sciences Research Bureau.
Health Canada (2018a). Assessment of the impacts of temperature on the efficacy of the coagulation/flocculation process in drinking water treatment. Prepared by Y.S. Vadasarukkai for Health Canada, Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Ottawa, Ontario. Available upon request.
Health Canada (2018b). Guidance on natural organic matter in drinking water. Water Quality and Health Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario.
Health Canada (2018c). Assessment of the impacts of aluminum in drinking water distribution systems. Prepared by Armview Engineering Limited for Health Canada, Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Ottawa, Ontario. Available upon request.
Health Canada (2019a). Enteric protozoa: Giardia and Cryptosporidium. Water Quality and Health Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/water-quality/enteric-protozoa-giardia-cryptosporidium.html
Health Canada (2019b). Enteric viruses. Water Quality and Health Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-enteric-viruses.html
Health Canada (in preparation). Canadian exposure factors used in human health risk assessments. Health Canada, Ottawa, Ontario.
Hichem, N., May, M.E., Ladhari, N., Mrabet, A. and Gharbi, R. (2014). Aluminum chloride impacts dentate gyrus structure in male adult albino Wistar rats. Tissue Cell, 46(6): 409-414.
Hill, A. and Giani, R. (2017). Magnified manganese release after switching sources to the Sacramento River. In: Proceedings of the American Water Works Association Water Quality Technology Conference, Portland, Oregon. American Water Works Association, Denver, Colorado.
Hill, A.S., Friedman, M.J., Reiber, S.H., Korshin, G.V. and Valentine, R.L. (2010). Behaviour of trace inorganic contaminants in drinking water distribution systems. J. Am. Water Works Assoc., 102(7): 107-118.
Hirata-Koizumi, M., Fujii, S., Ono, A., Hirose, A., Imai, T., Ogawa, K., Ema, M. and Nishikawa, A. (2011a). Two-generation reproductive toxicity study of aluminium sulfate in rats. Food Chem. Toxicol., 31(2): 219-230.
Hirata-Koizumi, M., Fujii, S., Ono, A., Hirose, A., Imai, T., Ogawa, K., Ema, M. and Nishikawa, A. (2011b). Evaluation of the reproductive and developmental toxicity of aluminium ammonium sulfate in a two-generation study in rats. Food Chem. Toxicol., 49(9): 1948-1959.
Hrudey, S.E. and Hrudey, E.J. (2014). Ensuring safe drinking water: Learning from frontline experience with contamination. American Water Works Association, Denver, Colorado.
Huang, J., Wu, J., Li, T., Song, X., Zhang, B., Zhang, P. and Zheng, X. (2011). Effect of exposure to trace elements in the soil on the prevalence of neural tube defects in a high-risk area of China. Biomed. Environ. Sci., 24(2):
94-101.
Hudson, W.D. (1966). Studies of distribution system capacity in seven cities. J. Am. Water Works Assoc., 58(2): 157-164.
Huck, P.M., Emelko, M.B., Coffey, B.M., Maurizio, D. and O'Melia, C. (2001). Filter operation effects on pathogen passage. American Water Works Association Research Foundation, Denver, Colorado (Report No. 90874).
IARC (2012). Chemical agents and related occupations. A review of human carcinogens. Occupational exposures during aluminum production. International Agency for Research on Cancer Monograph Vol 100F. IARC, Lyon.
JECFA (2007). Evaluation of certain food additives and contaminants. Sixty-seventh report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Technical Report Series, 940, Geneva.
JECFA (2012). Safety evaluation of certain food additives. Prepared by the Seventy-Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA). WHO Food Additives Series, 65, Geneva.
Jekel, M.R. and Heinzmann, B. (1989). Residual aluminum in drinking-water treatment. J. Water Supply Res. Technol. AQUA, 38: 281-288.
Karakis, I., Sarov, B., Landau, D., Manor, E., Yitshak-Sade, M., Rotenberg, M., Hershkovitz, R., Grotto, I., Gurevich, E. and Novack, L. (2014). Association between prenatal exposure to metals and neonatal morbidity. J. Toxicol. Env. Health. A., 77(21): 1281-1284.
Kettunen, R. and Keskitalo, P. (2000). Combination of membrane technology and limestone filtration to control drinking water quality. Desalination, 131(1-3): 271-283.
Kim, E.J., Herrera, J.E., Huggins, D., Braam, J. and Koshowski, S. (2011). Effect of pH on the concentrations of lead and trace contaminants in drinking water: A combined batch, pipe loop and sentinel home study. Water Res., 45(9): 2763-2774.
Knowles, A.D., Nguyen, C.K., Edwards, M.A., Stoddart, A., McIlwain, B. and Gagnon, G.A. (2015). Role of iron and aluminum coagulant metal residuals and lead release from drinking water pipe materials. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng., 50(4): 414-423.
Krewski, D., Yokel, R.A., Nieboer, E., Borchelt, D., Cohen, J., Harry, J., Kacew, S., Lindsay, J., Mahfouz, A.M. and Rondeau, V. (2007). Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health, Pt. B, 10(supp1): 1-269.
Kriewall, D., Harding, R., Maisch, E. and Schanz, L. (1996). The impact of aluminum residual on transmission main capacity. Public Works, 127(12): 28-31.
Krishnan, K. and Carrier, R. (2008). Approaches for evaluating the relevance of multiroute exposures in establishing guideline values for drinking water contaminants. J. Environ. Sci. Health. C. Environ. Carcinog. Ecotoxicol. Rev., 26(3): 300-316.
Krishnan, K. and Carrier, R. (2013). The use of exposure source allocation factor in the risk assessment of drinking-water contaminants. J. Toxicol. Environ. Health B Crit. Rev., 16(1): 39-51.
Kundert, K., Meilke, L., Elford, T., Maksymetz, B. and Pernitsky, D.J. (2004). Evaluating pH adjustment to investigate seasonal changes in aluminum residuals at a large conventional water treatment plant. In: Proceedings of the American Water Works Association Water Quality Technology Conference, San Antonio, Texas. American Water Works Association, Denver, Colorado.
Kvech, S. and Edwards, M. (2001). Role of aluminosilicate deposits in lead and copper corrosion. J. Am. Water Works Assoc., 93(11): 104-112.
Laabbar, W., Elgot, A., Kissani, N. and Gamrani, H. (2014). Chronic aluminum intoxication in rat induced both serotonin changes in the dorsal raphe nucleus and alteration of glycoprotein secretion in the subcommissural organ: Immunohistochemical study. Neurosci. Lett., 577: 72-76.
Larson, T.E. and Sollo Jr., F.W. (1967). Loss in water main carrying capacity. J. Am. Water Works Assoc., 60(12): 1565-1572.
Leroy, P., Schock, M.R., Wagner, I. and Holtshulte, H. (1996). Cement-based materials. In: Internal corrosion of water distribution systems. Second edition. American Water Works Association Research Foundation and DVGW-Technologiezentrum Wasser. pp. 313-388.
Letterman, R.D. and Driscoll, C.T. (1988). Survey of residual aluminum in filtered water. J. Am. Water Works Assoc., 80(4): 154-158.
Li, G., Ding, Y., Xu, H., Jin, J. and Shi, B. (2018). Characterization and release profile of (Mn, Al)-bearing deposits in drinking water distribution systems. Chemosphere, 197: 73-80.
Li, P., Luo, W., Zhang, H., Zheng, X., Liu, C. and Ouyang, H. (2015). Effects of aluminum exposure on the bone stimulatory growth factors in rats. Biol. Trace Elem. Res., 172(1): 166-171.
Li, X., Hu, C., Zhu, Y., Sun, H., Li, Y. and Zhang, Z. (2011a). Effects of aluminum exposure on bone mineral density, mineral, and trace elements in rats. Biol. Trace Elem. Res., 143(1): 378-385.
Li, X., Zhang, L., Zhu, Y. and Li, Y. (2011b). Dynamic analysis of exposure to aluminum and an acidic condition on bone formation in young growing rats. Environ. Toxicol. Pharmacol., 31(2): 295-301.
Lidsky, T.I. (2014). Is the aluminum hypothesis dead? J. Occup. Environ. Med., 56(5 Suppl): S73-9.
Linardaki, Z.I., Orkoula, M.G., Kokkosis, A.G., Lamari, F.N. and Margarity, M. (2013). Investigation of the neuroprotective action of saffron (crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem. Toxicol., 52: 163-170.
Lind, P.M., Olsen, L. and Lind, L. (2012). Circulating levels of metals are related to carotid atherosclerosis in elderly. Sci. Total Environ., 416: 80-88.
Lindquist, B., Lingstrom, P., Fandriks, L. and Birkhed, D. (2011). Influence of five neutralizing products on intra-oral pH after rinsing with simulated gastric acid. Eur. J. Oral Sci., 119(4): 301-304.
Liu, J., Wang, Q., Sun, X., Yang, X., Zhuang, C., Xu, F., Cao, Z. and Li, Y. (2016). The toxicity of aluminum chloride on kidney of rats. Biol. Trace Elem. Res., 173(2): 339-344.
Llobet, J.M., Domingo, J.L., Gomez, M., Tomas, J.M. and Corbella, J. (1987). Acute toxicity studies of aluminium compounds: Antidotal efficacy of several chelating agents. Pharmacol. Toxicol., 60(4): 280-283.
Locco, D., Waller, M., Giani, R., Nikolica, P., Hill, A. and Friedman, M. (2018). Pilot testing for optimized unidirectional flushing in the Hamilton distribution system. In: Proceedings of the American Water Works Association Annual Conference and Exposition, Las Vegas, Nevada. American Water Works Association, Denver, Colorado.
Lowermoor Incident Health Advisory Group. (1989). Water pollution at Lowermoor, North Cornwall. Report of the Lowermoor Incident Health Advisory Group. Chairman: Professor Dame Barbara Clayton. Truro: Cornwall and Isles of Scilly Health Authority.
Lv, J., Wang, W., Krafft, T., Li, Y., Zhang, F. and Yuan, F. (2011). Effects of several environmental factors on longevity and health of the human population of Zhongxiang, Hubei, China. Biol. Trace Elem. Res., 143(2): 702-716.
Lytle, D.A., Sorg, T.J. and Frietch, C. (2004). Accumulation of arsenic in drinking water distribution systems. Environ. Sci. Technol., 38(20): 5365-5372.
Manitoba Sustainable Development (2017). Personal communication with Kim Philip, Office of Drinking Water.
Martinez, C.S., Alterman, C.D.C., Pecanha, F.M., Vassallo, D.V., MelloCarpes, P.B., Miguel, M. and Wiggers, G.A. (2017a). Aluminum exposure at human dietary levels for 60 days reaches a threshold sufficient to promote memory impairment in rats. Neurotox. Res., 31(1): 20-30.
Martinez, C.S., Escobar, A.G., Uranga-Ocio, J.A., Pecanha, F.M., Vassallo, D.V., Exley, C., Miguel, M. and Wiggers, G.A. (2017b). Aluminum exposure for 60 days at human dietary levels impairs spermatogenesis and sperm quality in rats. Reprod. Toxicol., 73: 128-141.
Matilainen, A., Vepsäläinen, M. and Sillanpää, M. (2010). Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci., 159(2): 189-197.
McMillan, T.M., Dunn, G. and Colwill, S.J. (1993a). Psychological testing on schoolchildren before and after pollution of drinking water in North Cornwall. J. Child Psychol. Psychiatry, 34(8): 1449-1459.
McMillan, T.M., Freemont, A.J., Herxheimer, A., Denton, J., Taylor, A.P., Pazianas, M., Cummin, A.R. and Eastwood, J.B. (1993b). Camelford water poisoning accident: Serial neuropsychological assessments and further observations on bone aluminium. Hum. Exp. Toxicol., 12(1): 37-42.
Ministère du Développement durable, de l'Environnement et de la Lutte contre les changements climatiques (2017). Personal communication with Caroline Robert, Direction de l'eau potable et des eaux souterraines.
Mirza, A., King, A., Troakes, C. and Exley, C. (2017). Aluminium in brain tissue in familial Alzheimer's disease. J. Trace Elem. Med. Biol., 40: 30-36.
Mlynska, A. and Zielina, M. (2017). The influence of prefabricated pipe cement coatings and those made during pipe renovation on drinking water quality. In: E3S Web of Conferences. 17. 00061. 10.1051/e3sconf/20171700061.
Munk, L. and Faure, G. (2004). Effects of pH fluctuations on potentially toxic metals in the water and sediment of the Dillon Reservoir, Summit County, Colorado. Appl. Geochem., 19(7): 1065-1074.
Muselin, F., Cristina, R.T., Igna, V., Dumitrescu, E., Brezovan, D. and Trif, A. (2016). The consequences of aluminium intake on reproductive function in male rats: A three-generation study. Turk. J. Med. Sci., 46(4): 1240-1248.
Muylwyk, Q. and MacDonald, J. (2001). Aluminum deposits in the distribution system: What can you do? In: Proceedings of the American Water Works Association Water Quality Technology Conference, Nashville, Tennessee. American Water Works Association, Denver, Colorado.
New Brunswick Department of Environment and Local Government (2018). Personal communication with Kevin Gould, Healthy Environment Branch.
Newfoundland and Labrador Department of Municipal Affairs and Environment (2017). Personal communication with Haseen Khan, Water Resources Management Division.
New Zealand Ministry of Health (2008). Drinking-water standards for New Zealand 2005 (Revised 2008). Ministry of Health, Wellington.
NHMRC and NRMMC (2011). Australian drinking water guidelines. Paper 6. National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra, Version 3.4. Updated October 2017. Available at: https://www.nhmrc.gov.au/_files_nhmrc/file/publications/nhmrc_adwg_6_version_3.4_final.pdf. Nicolescu, R., Petcu, C., Cordeanu, A., Fabritius, K., Schlumpf, M., Krebs, R., Kramer, U. and Winneke, G. (2010). Environmental exposure to lead, but not other neurotoxic metals, relates to core elements of ADHD in Romanian children: Performance and questionnaire data. Environ. Res., 110(5): 476-483.
Nova Scotia Environment (2018). Personal communication with Angelina Polegato, Drinking Water Management Unit.
NRCan (2018). Aluminum facts, Natural Resources Canada. Last Updated: April 20, 2018. Government of Canada, Ottawa. Available at: http://www.nrcan.gc.ca/mining-materials/facts/aluminum/20510
NSCFS (2013). Risk Assessment of the exposure to aluminum through food and the use of cosmetic products in the Norwegian population. Opinion of the Panel on Food Additives, Flavourings, Processing Aids, Materials in Contact with Food and Cosmetics and of the Panel on Contaminants of the Norwegian Scientific Committee for Food Safety. Doc. No. 11-504_final. Available at: https://vkm.no/download/18.175083d415c86c573b59c179/1501678206406/a729a67e65.pdf
NSF (2018). Aluminum contaminant concentrations in water treatment chemicals. Prepared for Health Canada, Ottawa, Ontario.
NSF/ANSI (2017). NSF International/American National Standards Institute Standard 60: Drinking water treatment chemicals: Health effects. NSF International, Ann Arbor, Michigan.
O'Melia, C.R. (2006). Fundamentals of particle stability. Chapter 18 in: Interface science in drinking water treatment: theory and applications. G. Newcombe and D. Dixon (eds). Academic Press, London, United Kingdom. pp. 317-362.
Ondreicka, R., Ginter, E. and Kortus, J. (1966). Chronic toxicity of aluminium in rats and mice and its effects on phosphorus metabolism. Br. J. Ind. Med., 23(4): 305-312.
Oneda, S., Takasaki, T., Kuriwaki, K., Ohi, Y., Umekita, Y., Hatanaka, S., Fujiyoshi, T., Yoshida, A. and Yoshida, H. (1994). Chronic toxicity and tumorigenicity study of aluminum potassium sulfate in B6C3F1 mice. In Vivo, 8(3): 271-278.
Ontario Ministry of the Environment and Climate Change (2017). Selected water quality results for treated and distribution water from the drinking water surveillance program in Ontario treatment plants (2013-2017). Available at: https://www.ontario.ca/data/drinking-water-surveillance-program.
Oshima, E., Ishihara, T., Yokota, O., Nakashima-Yasuda, H., Nagao, S., Ikeda, C., Naohara, J., Terada, S. and Uchitomi, Y. (2013). Accelerated tau aggregation, apoptosis and neurological dysfunction caused by chronic oral administration of aluminum in a mouse model of tauopathies. Brain Pathol., 23(6): 633-644.
Oteiza, P.I., Keen, C.L., Han, B. and Golub, M.S. (1993). Aluminum accumulation and neurotoxicity in Swiss-Webster mice after long-term dietary exposure to aluminum and citrate. Metabolism, 42(10): 1296-1300.
Panhwar, A.H., Kazi, T.G., Afridi, H.I., Arain, S.A., Arain, M.S., Brahaman, K.D., Naeemullah and Arain, S.S. (2016). Correlation of cadmium and aluminum in blood samples of kidney disorder patients with drinking water and tobacco smoking: Related health risk. Environ. Geochem. Health, 38(1): 265-274.
Pernitsky, D.J. (2003). Coagulation 101. In: Proceedings of the Technology Transfer Conference, University of Calgary, Alberta, Canada.
Pernitsky, D.J. and Edzwald, J.K. (2003). Solubility of polyaluminium coagulants. J. Water Supply Res. Technol. Aqua, 52(6): 395-406.
Pernitsky, D.J. and Edzwald, J.K. (2006). Selection of alum and polyaluminum coagulants: Principles and applications. J. Water Supply Res. Technol. AQUA, 55(2): 121-141.
Pineau, A., Guillard, O., Favreau, F., Marrauld, A. and Fauconneau, B. (2012). In vitro study of percutaneous absorption of aluminum from antiperspirants through human skin in the Franz diffusion cell. J. Inorg. Biochem., 110: 21-26.
Poirier, J., Semple, H., Davies, J., Lapointe, R., Dziwenka, M., Hiltz, M. and Mujibi, D. (2011). Double-blind, vehicle-controlled randomized twelve-month neurodevelopmental toxicity study of common aluminum salts in the rat. Neuroscience, 193: 338-362.
Priest, N.D. (2004). The biological behaviour and bioavailability of aluminium in man, with special reference to studies employing aluminium-26 as a tracer: Review and study update. J. Environ. Monit., 6(5): 375-403.
Priest, N., Newton, D., Day, J., Talbot, R. and Warner, A. (1995). Human metabolism of aluminium-26 and gallium-67 injected as citrates. Hum. Exp. Toxicol., 14(3): 287-293.
Rao, S.S. and Adlard, P.A. (2018). Untangling tau and iron: Exploring the interaction between iron and tau in neurodegeneration. Front. Mol. Neurosci., 11: 276.
Reijnen, G.K., Kostense, A., Verdouw, J. and Hiemstra, P. (1991). Aluminum in groundwater, origin, sampling, analysis and removal. In: Proceedings of the International Water Supply Association, Copenhagen, Denmark. International Water Supply Association, The Netherlands.
Rondeau, V., Jacqmin-Gadda, H., Commenges, D., Helmer, C. and Dartigues, J.F. (2009). Aluminum and silica in drinking water and the risk of Alzheimer's disease or cognitive decline: Findings from 15-year follow-up of the PAQUID cohort. Am. J. Epidemiol., 169(4): 489-496.
Rui, D. and Yongjian, Y. (2010). Aluminum chloride induced oxidative damage on cells derived from hippocampus and cortex of ICR mice. Brain Res., 1324: 96-102.
Rusina, R., Matej, R., Kasparova, L., Kukal, J. and Urban, P. (2011). Higher aluminum concentration in Alzheimer's disease after box-cox data transformation. Neurotox. Res., 20(4): 329-333.
Saskatchewan Water Security Agency (2017). Personal communication with Sam Ferris, Environmental and Municipal Management Services Division.
Schock, M.R. (2005). Distribution systems as reservoirs and reactors for inorganic contaminants. Chapter 6 in: Distribution system water quality challenges in the 21st century: A strategic guide. American Water Works Association, Denver, Colorado. pp. 105-140.
Schock, M.R. and Holm, T.R. (2003). Are we monitoring in the right places for inorganics and radionuclides? J. N. Engl. Water Works Assoc., 117(2): 102-116.
Schreurs, B.G. and Sparks, D.L. (2016). Dietary high cholesterol and trace metals in the drinking water increase levels of ABCA1 in the rabbit hippocampus and temporal cortex. J. Alzheimers Dis., 49(1): 201-209.
Schroeder, H.A. and Mitchener, M. (1975a). Life-term effects of mercury, methyl, mercury, and nine other trace metals on mice. J. Nutr., 105(4): 452-458.
Schroeder, H.A. and Mitchener, M. (1975b). Life-term studies in rats: Effects of aluminum, barium, beryllium and tungsten. J. Nutr., 105(4): 421-427.
She, Y., Zhao, H., Zhu, Y., Han, Y., Xia, S., Bai, C., Zhang, J. and Li, Y. (2015). Aluminum trichloride disorders bile acid secretion and induces hepatocyte apoptosis in rats. Cell Biochem. Biophys., 71(3): 1569-1577.
Shin, J.Y., Spinette, R.F. and O'Melia, C.R. (2008). Stoichiometry of coagulation revisited. Environ. Sci. Technol., 42(7): 2582-2589.
Singla, N. and Dhawan, D.K. (2017). Zinc improves cognitive and neuronal dysfunction during aluminium-induced neurodegeneration. Mol. Neurobiol., 54(1): 406-422.
Skalnaya, M.G., Tinkov, A.A., Demidov, V.A., Serebryansky, E.P., Nikonorov, A.A. and Skalny, A.V. (2014). Hair toxic element content in adult men and women in relation to body mass index. Biol. Trace Elem. Res., 161(1): 13-19.
Smeets, P.W.M.H., Medema, G.J. and Van Dijk, J.C. (2009). The Dutch secret: How to provide safe drinking water without chlorine in The Netherlands. Drink. Water Eng. Sci., 2(1): 1-14.
Snoeyink, V.L., Schock, M.R., Sarin, P., Wang, L., Chen, A.S-C. and Harmon, S.M. (2003). Aluminium-containing scales in water distribution systems: Prevalence and composition. J. Water Supply Res. Technol. AQUA, 52(7): 455-474.
Srinivasan, P.T., Viraraghavan, T. and Bergman, J. (1999). Factors influencing residual aluminium levels at the Buffalo Pound Water Treatment Plant, Saskatchewan, Canada. J. Water Supply Res. Technol. AQUA, 48(4): 167-175.
Statistics Canada (2013). Survey of drinking water plants 2011. Minister of Industry, 16-403-X, Ottawa.
Stauber, J., Florence, T., Davies, C., Adams, M. and Buchanan, S. (1999). Bioavailability of Al in alum-treated drinking water. J. Am. Water Works Assoc., 91(11): 84-93.
Strozyk, D., Launer, L.J., Adlard, P.A., Cherny, R.A., Tsatsanis, A., Volitakis, I., Blennow, K., Petrovitch, H., White, L.R. and Bush, A.I. (2009). Zinc and copper modulate Alzheimer Abeta levels in human cerebrospinal fluid. Neurobiol. Aging, 30(7): 1069-1077.
Sun, H., Hu, C., Jia, L., Zhu, Y., Zhao, H., Shao, B., Wang, N., Zhang, Z. and Li, Y. (2011). Effects of aluminum exposure on serum sex hormones and androgen receptor expression in male rats. Biol. Trace Elem. Res., 144(1): 1050-1058.
Sun, X., Cao, Z., Zhang, Q., Liu, S., Xu, F., Che, J., Zhu, Y., Li, Y., Pan, C. and Liang, W. (2015). Aluminum trichloride impairs bone and downregulates Wnt/β-catenin signaling pathway in young growing rats. Food Chem. Toxicol., 86: 154-162.
Sun, X., Liu, J., Zhuang, C., Yang, X., Han, Y., Shao, B., Song, M., Li, Y. and Zhu, Y. (2016). Aluminum trichloride induces bone impairment through TGF-β1/Smad signaling pathway. Toxicology, 371: 49-57.
Sun, X., Wang, H., Huang, W., Yu, H., Shen, T., Song, M., Han, Y., Li, Y. and Zhu, Y. (2017). Inhibition of bone formation in rats by aluminum exposure via Wnt/β-catenin pathway. Chemosphere, 176: 1-7.
Talbot, R.J., Newton, D., Priest, N.D., Austin, J.G. and Day, J.P. (1995). Inter-subject variability in the metabolism of aluminium following intravenous injection as citrate. Hum. Exp. Toxicol., 14(7): 595-599.
Tamburo, E., Varrica, D., Dongarra, G. and Grimaldi, L.M. (2015). Trace elements in scalp hair samples from patients with relapsing-remitting multiple sclerosis. PLoS One, 10(4): e0122142.
Trueman, B.F. and Gagnon, G.A. (2016). A new analytical approach to understanding nanoscale lead-iron interactions in drinking water distribution systems. J. Hazard. Mater., 311: 151-157.
Turkez, H., Yousef, M.I. and Geyikoglu, F. (2010). Propolis prevents aluminium-induced genetic and hepatic damages in rat liver. Food Chem. Toxicol., 48(10): 2741-2746.
UK Committee on Toxicology. (2013). Subgroup report on the Lowermoor water pollution incident. Available at: https://cot.food.gov.uk/sites/default/files/cot/lwpiapp811.pdf
U.S. EPA (1994a). Method 200.7 revision 4.4. Determination of metals and trace elements in water and wastes by inductively coupled plasma-atomic emission spectrometry. Environmental Monitoring Systems Laboratories. Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
U.S. EPA (1994b). Method 200.8 revision 5.4. Determination of trace elements in waters and wastes by inductively coupled plasma - mass spectrometry. Environmental Monitoring Systems Laboratories, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
U.S. EPA (1994c). Method 200.9. revision 4.2. Determination of trace elements by stabilized temperature graphite furnace atomic adsorption. Environmental Monitoring Systems Laboratories, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
U.S. EPA (2002). Distribution system issue paper - Permeation and leaching. Prepared by AWWA with assistance from Economic and Engineering Services, Inc. for U.S. EPA, Office of Water, Office of Ground Water and Drinking Water.
U.S. EPA (2003). Method 200.5 revision 4.2. Determination of trace elements in drinking water by axially viewed inductively coupled plasma - atomic emission spectrometry. EPA 600-R-06-115. National Exposure Research Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
U.S. EPA (2018). Secondary drinking water standards: Guidance for nuisance chemicals. Available at: https://www.epa.gov/dwstandardsregulations/secondary-drinking-water-standards-guidance-nuisance-chemicals
Van Benschoten, J.E. and Edzwald, J.K. (1990a). Chemical aspects of coagulation using aluminum salts-II. Coagulation of fulvic acid using alum and polyaluminum chloride. Water Res., 24(12): 1527-1535.
Van Benschoten, J.E. and Edzwald, J.K. (1990b). Measuring aluminum during water treatment. Methodology and application. J. Am. Water Works Assoc., 82(5): 71-78.
Van Benschoten, J.E., Edzwald, J.K. and Rahman, M.A. (1992). Effects of temperature and pH on residual aluminium for alum and polyaluminium coagulants. Water Supply, 10(4): 49-54.
Vandenplas, Y., Castrellon, P.G., Rivas, R., Gutierrez, C.J., Garcia, L.D., Jimenez, J.E., Anzo, A., Hegar, B. and Alarcon, P. (2014). Safety of soya-based infant formulas in children. Br. J. Nutr., 111(8): 1340-1360.
Vanloot, P., Boudenne, J-L., Brach-Papa, C., Sergent, M. and Coulomb, B. (2007). An experimental design to optimize the flow extraction parameters for the selective removal of Fe(III) and Al(III) in aqueous samples using salicylic acid grafted on Amberlite XAD-4 and final determination by GF-AAS. J. Hazard. Mater., 147(1-2): 463-470.
Virk, S.A. and Eslick, G.D. (2015). Aluminum levels in brain, serum, and cerebrospinal fluid are higher in Alzheimer's disease cases than in controls: A series of meta-analyses. J. Alzheimers Dis., 47(3): 629-638.
Vucetic-Arsic, S., Radonjic, N.V., Jovanovic, M., Selakovic, V., Nikolic, T., Velimirovic, M., Stojkovic, T., Milovanovic, A., Milovanovic, J. and Petronijevic, N. (2013). Oxidative stress precedes mitochondrial dysfunction in gerbil brain after aluminum ingestion. Environ. Toxicol. Pharmacol., 36(3): 1242-1252.
Walton, J.R. (2010). Evidence for participation of aluminum in neurofibrillary tangle formation and growth in Alzheimer's disease. J. Alzheimers Dis., 22(1): 65-72.
Walton, J.R. (2014). Chronic aluminum intake causes Alzheimer's disease: Applying Sir Austin Bradford Hill's causality criteria. J. Alzheimers Dis., 40(4): 765-838.
Wang, B., Xing, W., Zhao, Y. and Deng, X. (2010). Effects of chronic aluminum exposure on memory through multiple signal transduction pathways. Environ. Toxicol. Pharmacol., 29(3): 308-313.
Wang, N., She, Y., Zhu, Y., Zhao, H., Shao, B., Sun, H., Hu, C. and Li, Y. (2012). Effects of subchronic aluminum exposure on the reproductive function in female rats. Biol. Trace Elem. Res., 145(3): 382-387.
Wang, W., Zhang, X., Wang, H., Wang, X., Zhou, L., Liu, R. and Liang, Y. (2012a). Laboratory study on the adsorption of Mn2+ on suspended and deposited amorphous Al(OH)3 in drinking water distribution systems. Water Res., 46(13): 4063-4070.
Wang, W., Li, H., Ding, Z., Wang, X. and Liu, R. (2012b). Effects of humic acid on residual Al control in drinking water treatment plants with orthophosphate addition. Front. Environ. Sci. Eng. China, 6(4): 470-476.
Wang, Z., Wei, X., Yang, J., Suo, J., Chen, J., Liu, X. and Zhao, X. (2016). Chronic exposure to aluminum and risk of Alzheimer's disease: A meta-analysis. Neurosci. Lett., 610: 200-206.
Wasana, H.M., Perera, G.D., De Gunawardena, P.S. and Bandara, J. (2015). The impact of aluminum, fluoride, and aluminum-fluoride complexes in drinking water on chronic kidney disease. Environ. Sci. Pollut. Res. Int., 22(14): 11001-11009.
Wasserstrom, L.W., Miller, S.A., Triantafyllidou, S., Desantis, M.K. and Schock, M.R. (2017). Scale formation under blended phosphate treatment for a utility with lead pipes. J. Am. Water Works Assoc., 109(11): E464-E478.
WHO (2010). Aluminium in drinking-water. Background document for development of WHO guidelines for drinking-water quality. World Health Organization, WHO/HSE/WSH/10.01/13, Geneva.
WHO (2011). Guidelines for drinking-water quality. Fourth edition. World Health Organization, Geneva, Switzerland. Available at: http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/
WHO (2012). Water safety planning for small community water supplies. World Health Organization, Geneva, Switzerland. Available at: http://www.who.int/water_sanitation_health/publications/small-comm-water_supplies/en/
Willhite, C.C., Karyakina, N.A., Yokel, R.A., Yenugadhati, N., Wisniewski, T.M., Arnold, I.M., Momoli, F. and Krewski, D. (2014). Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit. Rev. Toxicol., 44 (Suppl. 4): 1-80.
Xu, L., Zhang, W., Liu, X., Zhang, C., Wang, P. and Zhao, X. (2018). Circulatory levels of toxic metals (aluminum, cadmium, mercury, lead) in patients with Alzheimer's disease: A quantitative meta-analysis and systematic review. J. Alzheimers Dis., 62(1): 361-372.
Yokel, R.A. and McNamara, P.J. (1985). Aluminum bioavailability and disposition in adult and immature rabbits. Toxicol. Appl. Pharmacol., 77(2): 344-352.
Yu, L., Zhai, Q., Liu, X., Wang, G., Zhang, Q., Zhao, J., Narbad, A., Zhang, H., Tian, F. and Chen, W. (2016). Lactobacillus plantarum CCFM639 alleviates aluminium toxicity. Appl. Microbiol. Biotechnol., 100(4): 1891-1900.
Yukon Health and Social Services. (2017). Personal communication with Pat Brooks, Drinking Water Program Coordinator.
Zhang, C., Li, Y., Wang, C., Lv, R. and Song, T. (2013a). Extremely low-frequency magnetic exposure appears to have no effect on pathogenesis of Alzheimer's disease in aluminum-overloaded rat. PLoS One, 8(8): e71087.
Zhang, L., Jin, C., Liu, Q., Lu, X., Wu, S., Yang, J., Du, Y., Zheng, L. and Cai, Y. (2013b). Effects of subchronic aluminum exposure on spatial memory, ultrastructure and L-LTP of hippocampus in rats. J. Toxicol. Sci., 38(2): 255-268.
Zhang, L., Jin, C., Lu, X., Yang, J., Wu, S., Liu, Q., Chen, R., Bai, C., Zhang, D., Zheng, L., Du, Y. and Cai, Y. (2014). Aluminium chloride impairs long-term memory and downregulates cAMP-PKA-CREB signalling in rats. Toxicology, 323(Suppl. C): 95-108.
Zhou, Y, and Yokel, R.A. (2005). The chemical species of aluminum influences its paracellular flux across and uptake into Caco-2 cells, a model of gastrointestinal absorption. Tox. Sci. 87(1), 15-26.
Zhu, Y., Han, Y., Zhao, H., Li, J., Hu, C., Li, Y. and Zhang, Z. (2013). Suppressive effect of accumulated aluminum trichloride on the hepatic microsomal cytochrome P450 enzyme system in rats. Food Chem. Toxicol., 51(Suppl. C): 210-214.
Zhu, Y.Z., Sun, H., Fu, Y., Wang, J., Song, M., Li, M., Li, Y.F. and Miao, L.G. (2014). Effects of sub-chronic aluminum chloride on spermatogenesis and testicular enzymatic activity in male rats. Life Sci., 102(1): 36-40.
Zielina, M., Mlynska, A. and Zaba, T. (2015). Experimental research on deterioration of drinking water quality after cement mortar pipe lining. Technical Transactions: Civil Engineering, 4-B: 145-152.
Appendix A: List of acronyms
- Aβ
- Beta-amyloid
- AChE
- Acetylcholinesterase
- AD
- Alzheimer's disease
- Al
- Aluminum
- ANSI
- American National Standards Institute
- APHA
- American Public Health Association
- ATSDR
- Agency for Toxic Substances and Disease Registry
- AVICP-AES
- Axially viewed inductively coupled atomic emission spectrometry
- AWWA
- American Water Works Association
- AWWARF
- American Water Works Association Research Foundation
- CCME
- Canadian Council of Ministers of the Environment
- EFSA
- European Food Safety Authority
- GLP
- Good Laboratory Practice
- GSH
- Glutathione
- HBV
- Health-based value
- HC
- Health Canada
- IARC
- International Agency for Research on Cancer
- ICP-AES
- Inductively coupled plasma-atomic emission spectroscopy
- ICP-MS
- Inductively coupled plasma-mass spectrometry
- JECFA
- Joint Food and Agriculture Organization of the United Nations/World Health Organization Expert Committee on Food Additives
- LOAEL
- Lowest-observed-adverse-effect level
- M Moles
- MAC
- Maximum acceptable concentration
- MDA
- Malondialdehyde
- MDL
- Method detection limit
- NHMRC
- National Health and Medical Research Council (Australia)
- NOAEL
- No-observed-adverse-effect level
- NRMCC
- Natural Resources Management Ministerial Council (Australia)
- NSF
- NSF International
- NTU
- Nephelometric turbidity unit
- OECD
- Organization for Economic Co-operation and Development
- OG
- Operational guidance value
- PACl
- Polyaluminum chloride
- PM10
- Particulate matter, 10 micrometers in diameter or less
- QA/QC
- Quality assurance and quality control
- SM
- Standard Method
- SOD
- Superoxide dismutase
- TDI
- Tolerable daily intake
- U.S. EPA
- United States Environmental Protection Agency
- WHO
- World Health Organization
- w/v
- Weight per volume
Appendix B: Provincial and territorial anticipated impacts
Please note that this information is not available in both official languages because the source of the information is not subject to the Official Languages Act.
Prince Edward Island
As no drinking water supply systems in the province of PEI employ conventional water treatment processes with the addition of aluminum bearing additives, and naturally occurring aluminum levels are low in the groundwater from which all potable water is derived, no impact of this guideline is expected.
Newfoundland and Labrador
Monitoring-The Province of Newfoundland and Labrador is responsible for extensive monitoring for inorganic parameters including aluminum in the province. Aluminum monitoring is conducted semi-annually for all surface water public water supplies in the province, annually for all groundwater public water supplies, and quarterly for populations larger than 5,000.
Cost of Implementing Guideline-A total of 6 public surface water drinking water supplies have had an aluminum exceedance of the proposed MAC of 2.9 mg/L. This analysis is based on individual values and not locational running annual averages. Of the 6 supplies, 5 were from supplies that utilize alum-based coagulants. Of the 6 supplies, only 2 have semi-regular exceedances that could cause potential exceedances of the proposed MAC based on the locational running annual averages. Optimization of these two systems would require only minor infrastructure upgrades and operator training at a minimal cost.
Quarterly aluminum sampling for all public water supplies would have high cost implementation due to additional analysis cost and field time for existing staff. Due to workload constraints, this additional sampling requirement would not be possible using existing staff only.
Other Comments-The 6 drinking water supplies that have had aluminum levels above the proposed MAC are currently sampled 2 times per year for aluminum and other metals.
Action Items-Continue to monitor for aluminum at the current frequency with the exception of the two supplies that have semi-regular exceedances. These two systems to be monitored quarterly to assess the locational running annual averages.
Nova Scotia
Health Canada is proposing to establish a maximum acceptable concentration (MAC) of 2.9 mg/L for total aluminum in drinking water and to lower the operational guideline (OG) to 0.050 mg/L.
Nova Scotia's drinking water program consists of both public and private supplies. Public systems include municipal and registered facilities. Nova Scotia Environment (NSE) requires public drinking water supplies to comply with the health-based criteria for parameters listed in the Guidelines for Canadian Drinking Water Quality. Municipal supplies are also required by their operating approval to comply with the OG for aluminum.
The proposed MAC will have a negligible impact; however, the reduction to the OG will have a significant impact for our municipal drinking water systems.
Based on 2015 data obtained from annual reports, 15 of our 31 municipal water treatment facilities using aluminum-based coagulants will exceed an OG of 0.050 mg/L. Aluminum concentrations in the source water ranged from 0.006 - 0.501 mg/L with an average of 0.101 mg/L. Treated water concentrations ranged from <0.005 - 0.724 mg/L with an average of 0.053 mg/L. Facilities exceeding the proposed OG provide water to approximately 40% of the population served by a municipal drinking water facility. No treated water samples for aluminum from municipal facilities exceed the proposed MAC.
Of the 1668 registered water supplies, aluminum data is available for 741 facilities. Based on available data, <1% of facilities will exceed the proposed MAC and approximately 10% will exceed the OG.
While NSE does not regulate water quality at private water supplies, <1% are expected to exceed the proposed MAC.
NSE supports the establishment of a health-based guideline for total aluminum in drinking water; however, the proposed OG will have a significant impact for our municipal systems. While it is likely that facilities can improve the aluminum concentration in their filtered water through optimization of their coagulation process (e.g. strict pH control and adequate coagulant dosing), others (e.g. direct filtration plants) will not be able to achieve the proposed OG without impacting their filtration process.
New Brunswick
Based on the analytical data that we have available, it is not anticipated that establishing a MAC for Aluminum would result in impacts that would require additional treatment for most (if not all) New Brunswick drinking water systems. However, we do not monitor specifically for Aluminum and the introduction of an Aluminum MAC would likely result in additional sampling and monitoring requirements. Achieving the Operational Guideline for water quality in all distribution systems (e.g., ground and surface water systems) based on a locational running annual average of a minimum of quarterly samples taken in the distribution system could have implications. Based on the analytical data that we have, potential exists for some systems to exceed the OG, but this would need to be confirmed with additional sampling and monitoring. Corrosion Control is not a mandated operational practice in New Brunswick, and most ground water systems do not use any form of pH adjustment.
Quebec
Au Québec, étant donné que l'aluminium ne fait pas l'objet d'une norme au Règlement sur la qualité de l'eau potable, les résultats d'aluminium dont dispose le ministère de l'Environnement et de la Lutte contre les changements climatiques découlent des campagnes d'échantillonnage réalisées par le Ministère dans le cadre du Programme de surveillance de la qualité de l'eau potable. De 2012 à 2016, près de 230 analyses de l'aluminium ont été réalisées dans 60 installations de production d'eau potable majoritairement alimentées en eau souterraine. Peu de donnée sont disponibles actuellement en ce qui concerne les installations de production d'eau potable alimentées en eau de surface.
Aucun des échantillons prélevés par le Ministère n'a présenté un résultat supérieur à la CMA proposée de 2.9 mg/L (2900 μg/L). Par ailleurs, 6 (5,5 %) des échantillons prélevés à l'eau produite dépassaient la valeur de référence opérationnelle proposée de 0.05 mg/L (50 μg/L), ce qui concerne 4 (6,7 %) des installations visitées.
Considérant les résultats d'analyse disponibles, les impacts attendus de l'ajout d'une norme pour l'aluminium au Règlement sur la qualité de l'eau potable, en fonction de la révision de la recommandation publiée par Santé Canada, seraient faibles.
Ontario
Ontario supports the derivation of the health-protective guideline value for aluminum. The more stringent operational guideline will require more monitoring and optimization to ensure that aluminum removal is effective. The impact of the proposed health guideline value is minimal.
Manitoba
No impact paragraph has been provided by the province.
Saskatchewan
The Water Security Agency (WSA) has reviewed the proposed guideline technical document for Aluminum in drinking water and supports the proposed Maximum Acceptable Concentration (MAC) of 2.9 mg/L. An operational guideline value (OG) of 0.050 mg/L for Water Treatment Plants (WTPs) also proposed and is based on 1) a running annual average of monthly values determined from daily measurements of filtered water entering the distribution system and 2) on a locational running annual average of quarterly samples taken in the distribution system; the WSA noted that the new proposed OG is nearly 50% reduction of the existing operational guidance value of 0.100 mg/L aluminum in drinking water. Although most of the WTPs regulated by the WSA meet the new proposed OG, some of the WTPs may face challenges in meeting the proposed OG for aluminum in drinking water.
A review of the provincial water quality database showed that there are 2363 samples submitted for aluminum levels in treated drinking water and water from the distribution system over for the last 5 years (2013 to 2018) and data analysis revealed that aluminum levels in drinking water at the WSA regulated waterworks across the province are well below the proposed MAC of 2.9 mg/L.
In terms of drinking water treatment, the WSA will ensure that all the WTPs regulated by the WSA that use aluminum-based coagulants adopt appropriate water treatment strategies, such as strict pH control, adequate coagulant dosing etc to minimize the aluminum levels in treated water if there are any exceedances of regulated level. Considering aluminum levels in treated water, use of innovative Best Available Treatment (BAT) systems and adoption of appropriate operational controls by the WTPs in the province, the Water Security Agency believes that the new MAC for aluminum, if eventually adopted as a drinking water standard in the province, may not pose a significant compliance challenge. A comprehensive cost estimation for treatment plant upgrades at regulated waterworks cannot be developed or may not be needed at this time. Before formal adoption of any drinking water standard for aluminum in Saskatchewan, WSA will conduct further studies/data analysis to determine aluminum levels in treated water of selective water treatment systems in the province and compliance.
Alberta
In Alberta, municipal drinking water systems serve about 85% of the population and are regulated by the ministry of Environment and Parks under the Potable Water Regulation, a regulation within the Environmental Protection and Enhancement Act (EPEA). For Alberta's larger municipalities, the source of drinking water is typically a river, many of which have considerable variability in flow rates, water quality, and water chemistry depending on seasonal conditions. For example, during spring months when ice cover is breaking up on the North Saskatchewan River (Edmonton's source water), the concentration of suspended solids or particulates can increase by 3 orders of magnitude (or x 1,000) to over 2,000 mg/L compared to values as low as 2 mg/L during winter ice cover. For the City of Calgary, the Bow River also has varying water quality depending on the seasonal condition but with lesser extremes that pose water treatment challenges. The treatment process for these highly variable surface water surfaces has of course been optimized for each seasonal condition. Alum is used by both major cities in Alberta as a coagulant in the water clarification process. For 2017/2018, both waterworks systems had typical average concentrations of about 70 ppb for total aluminum in the treated water. This meets the current Operational Guidance value of 100 ppb.
In reviewing the aluminum consultation document, the following comments serve as the impact statement for the Alberta regulatory jurisdiction for drinking water on behalf of Environment and Parks. Treatment processes to produce safe, reliable drinking water quality are fundamentally about risk management and weighing costs, benefits, and different risks - namely infectious risks versus trace chemical hazard risks. This is well understood for chlorine as a chemical disinfectant, for example, and managing risks of waterborne pathogens and production of disinfection by-products. Ideally there should be a minimum of total aluminum in the finished treated water. The proposed MAC for total aluminum is 2,900 ppb and the new Operational Guideline value is 50 ppb. For Alberta drinking water facilities, the proposed MAC of 2,900 ppb should not pose any issue. This value is quite high (based on the neurological animal study findings), and Alberta drinking water facilities would be expected to not exceed this MAC of 2,900 ppb by using optimized alum dosing strategies. On the other hand, the new proposed O.G. value of 50 ppb will be a challenge to meet on a monthly basis, especially given challenges with seasonally variable source water quality.
Material impacts from trying to meet the lower 50 ppb O.G. value for total aluminum could include treatment process challenges, an increased risk for carry through of particulates and related increased risk for waterborne pathogens, more complexity in treatment if pH acidification is required and additional OH&S risks for operators. For Edmonton and Calgary's waterworks systems, for example, the annual average for total aluminum was about 70 ppb in 2017/2018. It should be advised that multi-year piloting studies would be needed to try to further optimize the coagulation treatment process, minimize trace levels aluminum in the finished treated water, without compromising treatment removal efficiencies. This would entail additional cost for water utilities and municipalities which would likely pose an additional fiscal challenge to the smaller municipal drinking water systems in Alberta.
British Columbia
A review of available data on aluminum in BC drinking water show less than one percent of water systems have aluminum values above the proposed health-based MAC of 2.9 mg/L. About 9% may have challenges with meeting the proposed operational guidelines of 0.05 0mg/L, most of which would likely be related to carryover from alum applications. It is not known how difficult it may be for these systems to adjust their operations to reduce values to below this operational guideline.
Yukon
It is not possible to quantify the impact of the proposed technical document and MAC for aluminum at this point in time for Yukon.
Yukon drinking water regulatory framework is primarily focused on water treatment and provision of safe drinking water as it leaves the water treatment plant to the distribution system. Provisions of the Drinking Water Regulation specify specific monitoring requirements for raw and treated water (at the point of water leaving the plant). While there is a requirement for monitoring of free chlorine, total coliforms and E. coli within the distribution system, there are currently no specific requirements for routine monitoring of other parameters.
Environmental Health Services (EHS) regulatory mandate extends to curb stop for large public drinking water systems. Routine compliance monitoring and regulatory requirements for large public drinking water system owners beyond this point is not within the current mandate. Further policy development would be needed to incorporate testing at the tap.
Currently all Yukon large public drinking water systems (LPDWS) meet the proposed MAC for aluminum entering the distribution system.
EHS is doing policy development in terms of monitoring of the distribution system for aluminum and other metals. LPDWS owners will be required to do an annual sample for specific parameters including aluminum in the upcoming calendar year.
Northwest Territories
No impact paragraph has been provided by the territory.
Nunavut
No impact paragraph has been provided by the territory.
Indigenous Services Canada
Based on the review of available data for First Nations south of 60° (excluding transferred communities in SK) a small number public or semi-public water treatment systems could be affected by the proposed maximum acceptable concentration (MAC) of 2.9 mg/L. It is noted that no system was identified as currently exceeding this value.
Workload and costs for drinking water monitoring conducted by Environmental Public Health Officers (EPHOs) is expected to increase as a result of the recommendation to assess compliance with a locational running annual average of a minimum of quarterly samples taken in the distribution system. EPHO sampling of drinking water is meant to validate operational monitoring conducted by the Water System Operator (WSO) and aluminum is not currently in the list of parameters that EPHOs are to sample quarterly. Any costs associated with addressing MAC exceedances will depend on the treatment system and the cause of the exceedance.
The proposed operational guidance (OG) of 0.05 mg/L is recommended to be applied to locational running annual averages of monthly values for filtered water in treatment plants using aluminum-based coagulants and to all distribution systems based on a locational running annual average of quarterly values. This OG may be difficult to achieve in small plants that have elevated levels of naturally-occurring aluminum in the source water and/or in small plants where an aluminum-based coagulant is used and coagulation pH is not strictly controlled.
ISC is not responsible for and does not regularly monitor private wells or systems with fewer than five connections where the public does not have access. As such, the impacts on these systems or private wells are difficult to quantify. It is noted that, for the timeframe studied (2012-2017), none of the available private system results were above the health based guideline of 2.9 mg/L.
Appendix C: Canadian water quality data
| Water Type | Summer (μg/L)a | Winter (μg/L)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detects/ samples |
Median | Mean | 90th | Max | Detects/ samples |
Median | Mean | 90th | Max | |
| Well-raw | 7/17 | 8 | 10 | 17 | 17 | 6/9 | 8 | 28 | 70 | 130 |
| Well-treated | 9/16 | 9 | 12 | 24 | 32 | 7/9 | 6 | 12 | 26 | 36 |
| Well-distribution | 6/17 | 19 | 17 | 27 | 31 | 6/9 | 15 | 16 | 24 | 31 |
| Lake-raw | 16/16 | 27 | 59 | 146 | 310 | 10/11 | 16 | 39 | 72 | 230 |
| Lake-treated | 16/16 | 21 | 34 | 71 | 120 | 10/11 | 14 | 52 | 114 | 280 |
| Lake-distribution | 21/21 | 16 | 56 | 130 | 330 | 8/8 | 23 | 43 | 99 | 140 |
| River-raw | 22/22 | 175 | 462 | 1,172 | 2,600 | 11/11 | 91 | 357 | 370 | 2,800 |
| River-treated | 22/22 | 35 | 89 | 220 | 390 | 9/11 | 53 | 74 | 122 | 270 |
| River-distribution | 26/26 | 34 | 68 | 155 | 330 | 9/10 | 43 | 55 | 95 | 210 |
Source: Health Canada, 2017, |
||||||||||
| Region | River basin | No. of samples | No. of detectsa | Median (μg/L) |
Mean (μg/L) |
90th percentile (μg/L) |
Maximum (μg/L) |
|---|---|---|---|---|---|---|---|
| East | Maritime Coast | 583 | 583 | 168 | 337 | 335 | 84,800 |
| Newfoundland-Labrador | 1,127 | 1,126 | 82 | 128 | 216 | 4,120 | |
| North Shore-Gaspé | 42 | 42 | 113 | 140 | 166 | 887 | |
| Saint John-St. Croix | 89 | 88 | 35 | 72 | 153 | 634 | |
| Central | Winnipeg | 53 | 53 | 166 | 173 | 248 | 347 |
| Prairie | Assiniboine-Red | 829 | 827 | 320 | 875 | 2,348 | 16,100 |
| Churchill | 292 | 280 | 38 | 97 | 235 | 1,880 | |
| Lower Saskatchewan-Nelson | 394 | 394 | 161 | 362 | 960 | 3,120 | |
| Missouri | 94 | 94 | 280 | 1,052 | 1,744 | 22,800 | |
| North Saskatchewan | 491 | 491 | 105 | 525 | 1,060 | 19,300 | |
| South Saskatchewan | 750 | 748 | 66 | 925 | 1,440 | 58,500 | |
| Pacific | Columbia | 4,418 | 4,395 | 25 | 138 | 348 | 9,850 |
| Fraser | 3,689 | 3,689 | 167 | 617 | 1,580 | 24,800 | |
| Okanagan-Similkameen | 1,153 | 1,152 | 41 | 287 | 542 | 21,200 | |
| Pacific Coastal | 2,789 | 2,789 | 123 | 693 | 1,762 | 25,900 | |
| Peace-Athabasca | 393 | 393 | 121 | 776 | 1,896 | 21,000 | |
| Arctic | Arctic Coast | 136 | 136 | 392 | 2,357 | 6,275 | 26,600 |
| Keewatin-Southern Baffin Island | 39 | 39 | 11 | 13 | 24 | 39 | |
| Lower Mackenzie | 919 | 916 | 73 | 577 | 1,510 | 12,800 | |
| Yukon | 642 | 632 | 42 | 165 | 454 | 3,080 | |
Source: Environment Canada, 2017, |
|||||||