Biocides Fee Proposal
Table of contents
- 1. Introduction
- 2. Background
- 3. Context
- 4. Fee Proposal
- 5. International Analysis
- 6. Consultation Process
- 7. Conclusion
- Annex A: Costing
- Annex B: Proposed Fees and Performance Standards
1. Introduction
Health Canada is the federal department responsible for helping the people of Canada maintain and improve their health. One of the roles the Department plays in carrying out this mandate is that of a regulator of drugs, including disinfectants, and medical devices. This role is achieved through the scientific evaluation of products before they are authorized for sale, the monitoring of these products once made available to people in Canada, and acting on non-compliance. Fees are charged to industry in relation to these regulatory activities, as per authorities granted to the Minister in the Food and Drugs Act.
On May 7, 2022, Health Canada published in Canada Gazette, Part I proposed regulations under the Food and Drugs Act (FDA) to regulate disinfectants and sanitizers for use on hard or soft non-living or non-liquid surfaces (biocides) under a single regime. The new framework would transfer disinfectants that are currently regulated under the Food and Drug Regulations (FDR) and certain surface sanitizers that are currently regulated under the Pest Control Products Act (PCPA), to the proposed Biocides Regulations.
The proposed Regulations would create a new, modern authorization and regulatory framework with safety, efficacy and quality requirements tailored to biocides, including the use of a foreign decision pathway. While the scientific review of surface disinfectants and surface sanitizers are currently subject to fees under separate regimes, this proposal lays out the new fees for biocides that would support activities under the proposed Regulations.
2. Background
Biocides are currently regulated under separate regulatory frameworks in Canada with surface sanitizers and disinfectants having different associated requirements, fees and timelines, despite having similar risks, benefits, uses, and ingredients. As the federal authority responsible for regulating the safety, efficacy and quality of all products affecting the health and safety of Canadians, Health Canada currently regulates these products. Surface disinfectants and surface sanitizers are currently regulated under the FDR and PCPA, respectively.
The COVID-19 pandemic has created an unprecedented demand on Canada's health care system. The need for health products, including biocides, has exponentially increased as a result of the pandemic. Certain biocides are able to kill SARS-CoV-2, the virus that causes COVID-19. These products are an essential part of a coordinated approach to help prevent the spread of COVID-19. Since biocides are regulated under separate regulatory frameworks, having different requirements for these similar products has not provided a consistent approach to their regulation, resulting in challenges for industry. The proposed Biocides Regulations aim to reduce burden on industry and the government by more closely aligning international and domestic requirements. The proposal also aligns with the intent of the Health and Biosciences Regulatory Review Roadmap to identify and address regulatory barriers to economic growth and innovation.
Scope
The scope of products that would be subject to the proposed Biocides Regulations would include disinfectants currently regulated under the FDR and surface sanitizers that meet the FDA definition of a drug. This includes disinfectants and sanitizers for use on hard or soft non-living and non-liquid surfaces. The following products would be excluded from the proposed Biocides Regulations:
- Sanitizers that do not meet the definition of a drug under the FDA. This includes algaecides, slimicides, material preservatives and products for odor control. These products would continue to be regulated under the PCPA;
- Sanitizers and disinfectants that meet the definition of a drug, but that are for use in air or water. This includes pool and spa disinfectants, water sanitizers, and air sanitizers. These higher-risk products would continue to be regulated under the PCPA;
- High-level disinfectant and sterilant solutions. This includes contact lens disinfectants and disinfectants intended for use on medical devices that are classified by Health Canada as invasive medical devices. These products would continue to be regulated under the Medical Devices Regulations;
- Disinfectants and surface sanitizers exclusively used directly on the surface of a food, such as fruits, vegetables and meat products. These products would continue to be regulated under the FDR as food processing aids;
- Drugs with antimicrobial activity for use on humans or animals, such as human use antiseptic drugs (e.g., hand sanitizers). These products would continue to be regulated under the FDR or the Natural Health Products Regulations; and
- Cleaners. These would continue to be regulated under the Canadian Consumer Product Safety Act and the Hazardous Products Act.
Sanitizers and disinfectants for use in commercial or household settings to disinfect and sanitize surfaces that may come into contact with food would be included within the scope of the proposed Biocides Regulations.
The proposed Biocides Regulations would have many benefits:
- For industry, the proposal would help streamline the pre-market review process by transferring similar biocides under a single regime; reduce costs; encourage companies to bring their products to Canada; and harmonize regulatory requirements, which would increase the predictability of the regulatory process for applicants.
- For consumers and users, the proposal would facilitate and maintain timely access to biocides that have been authorized for sale in other countries, beginning with the United States, to address needs for consumer and commercial product choice. The proposal would also include the maintenance of a lifecycle approach to the regulation of biocides while supporting requirements and authorities for the continuous post-market monitoring of a biocide's risks and benefits.
- For Health Canada, the proposed Biocides Regulations would introduce the ability for one market authorization to include multiple brand names or multiple versions of the biocide. It would allow for a more efficient application submission and review process, and enable Health Canada to leverage the authorization from foreign jurisdictions through the Use of Foreign Decisions pathway to bring biocides to the Canadian market sooner.
Further, the FDAand PCPA provide Health Canada the authority to charge fees for activities related to the scientific review, compliance activities and continued surveillance of those products requiring authorization for sale in Canada. Health Canada has charged fees for the scientific review of disinfectants and sanitizers since 1995. Disinfectant fees (along with fees for drugs and medical devices) were updated in 2011 under the User Fees Act, and again in 2020. A new fee category was created at that time to accommodate labelling updates to disinfectants, as well as a separate, lower Right to Sell fee specifically for disinfectant products compared to Over-The-Counter (OTC) and prescription products, based on the lower cost for associated regulatory services. Fees for sanitizers were last updated in 2017 as part of a larger update for products regulated under the PCPA.
As the proposed Biocides Regulations fall under the FDA, Health Canada exercises the Ministerial authorities to set fees under this legislation for biocides. While exempt from the Service Fees Act, the same accountability provisions will be applied. This document presents the proposed fees under the proposed Biocides Regulations based on the current approach to fees related to drugs and medical devices, including its fee setting ratios, small business mitigation and penalty for missed performance standards.
For further details on the regulatory framework itself, please consult the proposed Biocides Regulations.
3. Context
Historical Fee Context
Fees for sanitizers were most recently updated in 2017 under the PCPA. Details on this update can be found here. For sanitizers that would be transferred to the FDA under the proposed Biocides Regulations and for which data on fees were available, the average fee for a new application review was $2,150. However, these applications go back over 20 years, and most of these applications were made prior to the 2017 update.
As part of the 2020 update for Fees for Drugs and Medical Devices the following changes were made to disinfectant fees:
- The fee for a full disinfectant review increased by 28%, commensurate with the increase in costs for such reviews since the previous update in 2011.
- The fee for a labelling standard review for a Drug Identification Number - Disinfectant (DIN D) submission decreased by 7% due to a similar change in costs.
- The Right to Sell fee increased by 7% based on costs. The creation of separate Right to Sell fees for the three main sectors in human drug regulation (i.e. prescription, OTC and disinfectant) resulted in a lower increase for the disinfectant Right to Sell fee, in comparison to the other two sectors. This was due to the lower level of effort associated with disinfectants.
Current Proposal
This fee proposal applies to regulatory activities related to biocides, for the following proposed fee lines:
- Pre-Market Submission Evaluation fees: Before a health product is authorized for sale in Canada, Health Canada reviews it to assess safety, efficacy and quality. Companies are required to pay this fee, regardless of whether or not the product is authorized by Health Canada for sale to the Canadian market.
- Right to Sell fee: Health Canada monitors products on the Canadian market through post-market surveillance, monitoring and compliance and enforcement activities. These activities are partially funded through a Right to Sell fee. This is an annual charge paid by companies for each product they are actively authorized to sell in Canada. Companies with disinfectants licensed under the FDR already pay this fee for each product they are marketing in Canada. For sanitizers currently regulated under the PCPA, the Right to Sell fee would replace the current annual charge under those regulations.
Disinfectants and surface sanitizers are currently excluded from Establishment Licensing requirements under the FDR,so no establishment license fees are charged. This would continue for all products under the proposed Biocides Regulations.
4. Fee Proposal
This section presents the proposed changes that affect all fee lines, as well as the individual changes to specific fee lines.
| Current Approach to Disinfectant Fees Under FDA | Current Approach to Sanitizer Fees Under PCPA | Proposed Approach to Biocide Fees | |
|---|---|---|---|
| Fee Setting Ratios | Evaluation fees based on 75% fee setting ratio Right to Sell fee based on 67% fee setting ratio |
Application fees based on approx. 30% fee setting ratio | Same as current approach to disinfectant fees |
| Annual Adjustments | Annual fee adjustment tied to the Consumer Price Index (CPI) of previous year, rounded to nearest dollar | Annual pre-market fee adjustment of 2% annually, rounded upwards Annual post-market fee adjustment tied to the Consumer Price Index (CPI) of previous year, no rounding |
Same as current approach to disinfectant fees |
| Fee Mitigation | 1) Companies meeting Health Canada's small business definition will be eligible to receive their first pre‐market submission free regardless of fee amount; 50% mitigation for all subsequent pre-market submission Evaluation fees and 25% for Right to Sell fee 2) Waiver for submission evaluation fee for products for urgent public health need (on the List of Drugs for an Urgent Public Health Need, as per the Access to Drugs in Exceptional Circumstances Regulations) 3) All fees waived for publicly funded health care institutions |
Based on annual gross revenue from previous year Application fees: If company's gross revenue is less than 10 times the application fee, company will pay the higher of 10% of gross revenue or 10% of the application fee Annual charge: If 4% of company's gross revenue is less than the annual charge stated in regulations, company will pay the higher of 4% of gross revenue or the minimum annual charge stated in regulations |
Same as current approach to disinfectant fees |
| Performance Standard | Each existing fee category has a performance standard | Submissions are assigned a category during processing. Performance standards are based on category and category subdivision |
Performance standards for disinfectants would be maintained for biocides All new fee categories have an appropriate service standard |
| Penalty Provision | An individual submission that exceeds the performance standard will receive a remission of 25% of the fee paid; and a 'Pause the Clock' provision to limit the standard to the time spent by Health Canada on that submission | Penalty remissions are based on a sliding scale from 10%-25%, depending on how much the performance standard was missed | Same as current approach to disinfectant fees |
| Pre-Market Submission Evaluation fees | Individual fees for different types of applications (i.e. fee categories) | Individual fees for different application components. Fees may be charged for one or more of these components in a given application | Labelling Only - Disinfectant, Labelling Standard and Administrative would be carried over from Fees Order for Drugs and Devices and renamed Disinfectant - Full Review category from Fees Order would be split into five different categories:
|
| Right to Sell fee | Annual fee for each individual DIN assigned to a company | Annual charge for each individual product marketed in Canada by a company | Same as current approach to disinfectant fees |
Fee-Setting Ratio
Fees are set based on costs incurred by the Department, in accordance with Treasury Board Secretariat's costing guidelines. The 2017 fee proposal for drugs (including disinfectants) and medical devices set fees at 90% of costs for pre-market review and Right to Sell. Following consultations, the fee setting ratios for pre-market product submissions were revised to 75% of costs, while the Right to Sell fees were set at 67% of costs due to the creation of tiered fees to reflect the lower level of effort related to disinfectants and OTC products compared to prescription drugs. The updated fees were implemented on April 1, 2020. The final fee-setting ratios for drugs and medical devices will be used for all fees contained in this proposal.
Small Business Mitigation
Mitigation for small businesses was implemented in the April 1, 2020, fee update for drugs (including disinfectants) and medical devices. Qualifying small businesses (fewer than 100 employees or between $30,000 and $5 million in gross annual revenue), were entitled to the following:
- No charge for a business's first qualifying pre-market submission review
- All subsequent pre-market submissions receive 50% remission
- 25% remission on annual Right to Sell fee
This model differs from existing mitigation under the Pest Control Product Fees and Charges Regulations (PCPFCR), which offers mitigation as a function of a company's gross revenue. This mitigation strategy allows companies to pay fees as a percentage of their gross revenue if it falls under a certain threshold.
The small business mitigation approach for drugs (including disinfectants) and medical devices is being maintained in this fee proposal to align cost recovery practices across health products.
Performance Standards and Penalties
Currently, each existing fee has a related performance standard. As part of this proposal, it is proposed that existing disinfectant performance standards be maintained, and that each new fee has an appropriate performance standard (details are presented in Annex B). Existing and proposed performance standards are generally internationally comparable and will be reported publically on annual basis through the annual Health Canada Fees Report.
Under the current fees for drugs (including disinfectants) and medical devices, an individual submission that exceeds the performance standard receives a rebate of 25% of the fee paid. This method is used in conjunction with a Pause the Clock provision, which pauses the performance standard count in defined circumstances. Under the PCPR, a penalty remission is made to a company for any submission which is completed at least 10% outside of the performance standard. The remission is equal to the percentage by which the standard was missed, to a maximum of 25%.
The approach to performance standards and penalties for drugs (including disinfectants) and medical devices is being maintained in this fee proposal, to align cost recovery practices across health products.
Fees
This is a summary of all proposed fees under the proposed Biocides Regulations. For further details, please refer to Annex B.
Evaluation Fees - Existing
The following existing fee categories for disinfectant submission evaluation would be carried over to the proposed Biocides Regulations:
- Labelling Only (Disinfectants): Would become Biocide Labelling Only
- Labelling Standard: DIN D applications under this fee category would become Biocide Monograph
- Administrative: DIN D applications under this fee category would become Biocide Administrative
Evaluation Fees - New
In addition to the existing fees, Health Canada is proposing new review pathways and associated fees, which would allow for greater access to and confidence in biocides products for Canadians:
-
1. Use of Foreign Decisions pathway
One of the new pathways under the proposed Biocides Regulations would be the Use of Foreign Decisions pathway for new products, and for amendments to products already approved through this pathway. This new pathway would facilitate market entry of foreign products into the Canadian market, beginning with products sold in the United States. By doing so, this pathway would reduce review costs and the length of time to make a regulatory decision. As such, the fee for this pathway would be lower than that for a full biocide review, but higher than a Biocides Labelling Only application due to additional review steps (e.g. confirmation of active foreign registration, screening of data provided to the foreign authority).
- 2. Novel Biocides
Currently, submissions for novel disinfectants are filed under Division 8 of the FDR, and are charged the same Disinfectant - Full Review fee as Division 1 disinfectants. However, Division 8 submissions require significantly more time to review and assess. Canada is currently the only major jurisdiction that does not charge a separate fee for the review of novel disinfectants.
Health Canada is proposing to create a separate review pathway specifically for novel biocides. Applications under this pathway would include novel active ingredients, a novel combination of active ingredients, a novel use or purpose, or a novel physical form. Common examples of requirements to review novel biocides would be additional safety (e.g. exposure data, acute toxicity) and quality (chemistry & manufacturing) information. This would harmonize the review done by other jurisdictions where New Active Ingredient fees are charged, including those reviewed under the PCPA.
Fees for novel biocides would be based on costs of reviewing past disinfectant submissions filed under Division 8. As such, given the longer review time for these applications, they would have a higher fee than other biocide full reviews, but still lower than other human drug submissions filed under Division 8 as a New Active Substance, given the differing requirements for a biocide application compared to a human drug submission.
- 3. Tiered Full Review
Under the existing disinfectant fee structure, all full reviews of disinfectants are charged the same fee, regardless of the number of pieces of efficacy data included in a given application. The United States Environmental Protection Agency's fee structure includes a tiered approach to such submissions, with fees increasing at 25 and 50 claims. An analysis of full review disinfectant submissions filed with Health Canada over the past three years revealed a linear relationship between efficacy data and Health Canada's level of effort for review. This was due to the increased data requirements and resulting review for each piece of data.
As a result, Health Canada is proposing to create separate fees for products containing 25 or fewer, 26-50, and 51 or more pieces of efficacy data. These fees would also apply for products seeking a post-authorization change, if those products include additional efficacy data to what had been authorized by Health Canada in the original submission.
Full review biocides fees would be based on costs of reviewing past disinfectant submissions filed under Division 1 (including both new and revised DIN submissions). The fee to be charged would be based on the number of new pieces of efficacy or confirmatory data included in the application. In the case of a post-authorization change, only the additional number of pieces of efficacy data would apply when calculating the fee. For example, a product which originally included 25 pieces of efficacy data, but is being revised to include a total of 28, would be charged the lowest tier fee for the revised application, due to the 3 additional pieces of efficacy data requiring review; it would not be charged the second-tier fee for the total of 28 pieces of efficacy data to support the market authorization.
- 4. Major Changes
Under the current regime, all changes to authorized disinfectants are classified as full reviews, regardless of the number of additional pieces of efficacy data included in the application for revision, and charged the applicable fee. However, Health Canada recognizes that revisions with no additional efficacy data have a lower level of effort to review. As a result, a new pathway for Major Changes is being created to replace the current filing structure for revising DINs, with a lower associated fee. This is similar to how changes to sanitizers are handled under the PCPA.
As stated above, any post-authorization changes which include additional efficacy data would be required to be filed as a full review, and are not eligible for this pathway.
Fees for this new pathway would be based on Health Canada's costs of reviewing past DIN revisions containing no additional pieces of efficacy or confirmatory data.
- 5. Minor Changes
Currently, Health Canada does not charge for post-DIN changes to disinfectant submissions under the FDR. Given the significant increase in these submissions being received by Health Canada, and the level of effort involved in their review, these submissions are being reclassified as Minor Changes under the proposed Biocides Regulations, and would have a fee associated with them, as is done with minor changes to sanitizers under the PCPA.
Fees for Minor Changes would be based on costs of reviewing past disinfectant post-DIN change submissions completed by Health Canada.
Transfer of Products
Health Canada would also facilitate the transfer of existing products to the proposed Biocides Regulations:
- 1. Transfer of Sanitizers and Disinfectants
Existing authorization holders of disinfectants and registrants of surface sanitizers who wish to continue to market their product as a biocide would be required to file an abbreviated application, containing no safety or efficacy data, under the proposed Biocides Regulations. No fees would apply to these abbreviated applications. Market authorization would need to be received within four years of the coming into force date of the aforementioned regulations.
- 2. Products Making Claims Under Multiple Regulations
Not all sanitization claims would be regulated under the Biocides Framework (e.g., air/water sanitization claims). It is possible that some products would make efficacy claims under both sets of regulations, and so would require authorization and registration under both the Biocides Regulations and the PCPA.
Should authorization and registration under both sets of regulations be required, appropriate fee(s) under both the proposed Biocides Regulations and the Pest Control Products Fees and Charges Regulations would apply.
Biocide Right to Sell
Health Canada currently monitors disinfectants and sanitizers on the Canadian market through post‐market surveillance, compliance and enforcement oversight, and quality management oversight. Industry pays an annual fee for the right to maintain and sell disinfectants and sanitizers in Canada.
There would be a Biocides Right to Sell (BRTS) fee applicable to all products under the proposed Biocides Regulations. Products that transition from the PCPA to the proposed Biocides Regulations would be charged the new BRTS fee instead of their current fee under the PCPR. Similar to pre-market review, any products making claims under both the proposed Biocides Regulations and the PCPA would have those claims considered separately by each framework, requiring an authorization, registration and annual fees under both.
| Category | Existing Fee (Disinfectants) - April 1, 2024 | Proposed Fee (to take effect April 1, 2024) |
|---|---|---|
| Novel Biocide | $11,743 | $39,928 |
| Biocide full review - 1-25 pieces of efficacy or confirmatory data, including post-authorization changes | $8,233 | |
| Biocide full review - 26-50 pieces of efficacy or confirmatory data, including post-authorization changes | $10,986 | |
| Biocide full review - 51+ pieces of efficacy or confirmatory data, including post-authorization changes | $12,953 | |
| Biocide Use of Foreign Decisions pathway | N/A | $2,969 |
| Biocide Labelling Only | $2,687 | $2,632 |
| Biocide Monograph | $1,732 | $2,557 |
| Biocide Administrative | $889 | $1,820 |
| Biocide Major Change - no efficacy or confirmatory data | N/A | $4,746 |
| Biocide Minor Change | N/A | $1,306 |
| Biocides Right to Sell | $1,538 (under FDA) $4,029 (under PCPA) |
$1,470 |
|
Note: Analysis of sanitizers expected to be transferred to the proposed Biocides Regulations indicates an average fee for the review of a new registration is $2,150. However, most of these applications were made prior to the introduction of new fees under the PCPA in 2017. | ||
5. International Analysis
Health Canada's fees are determined based on the cost of providing service to industry (see Annex A for costing information). In developing this proposal Health Canada reviewed several international regulatory regimes, which charge fees for their biocide regulatory services. Health Canada focused its analysis on four comparable international regulators: Australia's Pesticides and Veterinary Medicines Authority (APVMA), the European Chemicals Agency (ECHA), the United Kingdom Health and Safety Executive (HSE), and the United States Environmental Protection Agency (US EPA).
Since the definition of a biocide varies and the fee structure for reviews is not harmonized across all foreign jurisdictions, a direct comparison cannot always be made. That said, the fees proposed by Health Canada in most cases are lower than their international counterparts, and the department is committed to maintaining competitive services and fees. See summary tables below for estimated fees and small business policy across jurisdictions (all dollar figures in CDN$).
| Health Canada (proposed) | US EPA | ECHA | HSE | APVMA | |
|---|---|---|---|---|---|
| Novel Biocide | $39,928 | $80,000 - $240,000 | $176,000 | $220,000 | $80,000 -$110,000 |
| Regular review submissions | 1-25 claims: $8,233 26-50 claims: $10,986 51+ claims: $12,953 |
1-25 claims: $6,500 26-50 claims: $12,000 51+ claims: $19,000 |
$59,000 - $117,000 | $25,000 - $60,000 | $18,000 |
| Post-market changes | $1,306 - $12,953 | $5,000 - $17,000 | $3,000 -60,000 | $850 -$11,000 | $1,800 - $34,000 |
| Annual fee | $1,470 | ~$4,300 | Renewal of authorization: $7,000 (single product), $11,000 (product family) | Varies | $800 - $1,600 |
| Small Business Mitigation - Pre-market Evaluation | First submission free; 50% mitigation for qualifying companies | None | Tiered based on company size (20-60% reduction) | Tiered based on company size (30-90% reduction) | Varies |
| Small Business Mitigation - Right to Sell | 25% mitigation for qualifying companies | Annual company cap depending on company size and number of products | 25% reduction | None found | Varied, not based on predetermined percentage |
6. Consultation Process
As part of its ongoing commitment to meaningful consultations, Health Canada is providing stakeholders an opportunity to provide feedback and identify concerns with the Biocides Fee Proposal.
Health Canada will be gathering and considering this feedback for the finalization of the fees for biocides. Health Canada will be accepting feedback on all aspects of the proposed fees, except for the costing methodology.
Stakeholders are invited to provide feedback on this document via the generic email account CRI_IRC_Consultations@hc‐sc.gc.ca by July 16, 2022 (70 day consultation period). A report summarizing the feedback, along with the response from Health Canada, will be published on Health Canada's website at a later date.
In addition to the written feedback process, Health Canada will be organizing a virtual stakeholder meeting in spring 2022 to discuss all aspects of the proposed Biocides Regulations, including this Fee Proposal.
7. Conclusion
The fees outlined throughout this document reflect Health Canada's costs for providing services to industry, aligns with the government's position that industry should pay a fair share for services that benefit them while relieving taxpayers, and considers the cost recovery practices of international counterparts. The introduction of the proposed Biocides Regulations would provide Canadians with access to a greater variety of products, increased predictability of the regulatory process for the biocides industry, and improved efficiencies in Health Canada's regulatory oversight. This is an important step forward to ensure that Health Canada can continue to provide reliable service to industry while continuing to serve the interests of Canadians.
Annex A: Costing
Costing Methodology
Health Canada's approach to costing has not changed from the 2020 drug and medical device fee update. It uses an integrated approach to determine costs for program activities. It has implemented a program‐wide time tracking system (the Cross‐Application Timesheet - Project System; CATS‐PS) to collect level of effort by activity (including tracking time spent reviewing individual submissions and applications). Costs are derived by applying employee salaries to this level of effort, based on the highest pay level for the employee's classification using the most recent rates of pay. This system allows both direct program costs and indirect program costs to be assigned to activities based on their use of resources.
The Department has also developed and implemented a detailed activity structure that provides consistent definitions of key activities to allow for costs to be compared across programs and product lines. The information contained in both the activity structure and time tracking systems are aligned with the data in the departmental Financial System (SAP), which allows for more accurate mapping. The information from these structures and systems was used to establish and validate the proposed new fees.
In conducting this activity based costing exercise, Health Canada respected the Treasury Board Secretariat's Guide to Cost Estimating and determined total costs per fee line.
The total costs were determined by totalling the costs described above (program direct and indirect costs, corporate costs, and capital costs). A costing model was developed that maps costs to specific activities within a branch, and allows Health Canada to calculate fully loaded costs by fee line. The last four years of data from the time tracking system were used. These total costs include both the direct costs in support of actual submissions and applications as well as indirect costs proportionally applied to those activities. Direct service support costs were allocated proportionally to all activities within the associated fee. Program indirect costs are allocated proportionally to all activities within the given program.
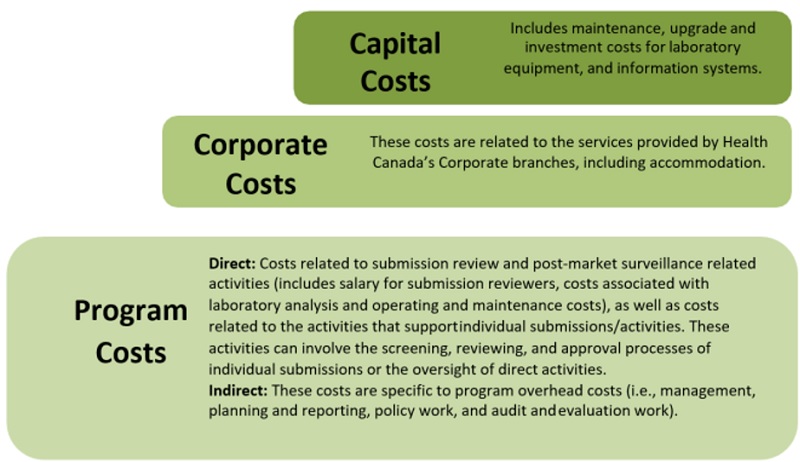
Figure - Text description
Direct program costs are costs related to submission review and post-market surveillance activities, including salary for submission reviewers, costs associated with laboratory analysis and operating and maintenance costs, as well as costs related to the activities that support individual submissions or activities. These activities can involve the screening, reviewing, and approval processes of individual submissions or the oversight of direct activities.
Indirect program costs are specific to program overhead costs. These include management, planning and reporting, policy work, and audit and evaluation work. Corporate costs are related to the services provided by Health Canada's corporate branches , including accommodations.
Capital costs include maintenancy, upgrade and investment costs for laboratory equipment and information systems.
Evaluation Fees
Activities such as individual submission review, submission screening and processing, submission coordination and management, and scientific/technical management are included in these fee lines.
For proposed fees based on pre‐market authorization fees in the current Fees Order (Biocide Labelling Only, Biocide Monograph, and Biocide Administrative), an hourly rate was determined using the costing method described above as well as the volume and average time spent on submissions within each existing fee activity (Labelling Only - Disinfectant, Labelling Standard, and Administrative, respectively). This data was based on all submissions received and completed between April 2017 and March 2021 (the reference period). The hourly rate for each fee line was determined by dividing the total costs by the calculated total direct hours spent on each fee line. To arrive at the unit costs for each fee line the hourly rate was multiplied by the mean sample hours required to complete a submission. All costs attributed to COVID-19 submissions or related work (e.g. COVID-19 policy) were excluded from the dataset since flexibilities were offered that impacted performance targets, which would not reflect the typical level of effort for disinfectant submissions.
New Fee Categories Based on Existing Fees
These fees do not currently exist in the Fees in Respect of Drugs and Medical Devices Order, but are based on existing pathways:
- Novel Biocide: Based on Division 8 submissions in the Disinfectant – Full Review fee category in the reference period
- Biocide Full Review - Tiers I-III: Based on Division 1 submissions in the Disinfectant – Full Review category in the reference period, and subdivided according to number of pieces of efficacy data included in the application for the product under review (1-25 for Tier I, 26-50 for Tier II, and 51 or more for Tier III)
- Biocide Minor Change: Based on Post-DIN change submissions, which do not currently have a fee associated with them, during the reference period
For Novel Biocide and Biocide Minor Change, the unit costs were calculated using the methodology described above.
For the tiered Biocide full review fees, the hourly rate for the existing Disinfectant - Full Review category was calculated, then unit costs for each tier were calculated using level of effort for submissions in the reference period which fit each tier, based on number of pieces of efficacy data.
New Review Pathways
The following fees do not currently exist, and are entirely new pathways. However, they were based on existing data from comparable applications with similar level of effort:
- Biocide Use of Foreign Decisions pathway
- Biocide Major Change
Both of these fees used Labelling Only - Disinfectant as a proxy for hourly rate, as this was believed to be the most similar fee category to both.
For the Use of Foreign Decisions pathway, projected level of effort was estimated to be 1.5 hours more than the level of effort for a Labelling Only submission. The unit cost was calculated based on this level of effort.
For Major Change submissions, projected level of effort was estimated using existing Disinfectant - Full Review submissions with zero additional pieces of efficacy data. These submissions match what a Biocide Major Change application without additional efficacy data is expected to contain.
Right To Sell Fee
Activities such as compliance verification and post‐market surveillance are included in this fee line.
The Right to Sell fee for biocides was calculated using a combination of 2020-21 costs in SAP PS for compliance verification, and costs based on an estimate of FTEs required for future post-market surveillance of biocides. Health Canada determined that the single year of historical costs for compliance verification best represented the scope of this work on biocides going forward.
The combined costs were divided by the estimated number of marketed biocides to arrive at the unit cost.
Costing Tables
| Activity | Total Costs | Avg. Hourly Rate | Unit Cost |
|---|---|---|---|
| Biocide Full Review - Novel Biocide | $216,000 | $338.56 | $49,487 |
| Biocide Full Review - 25 or fewer pieces of efficacy or confirmatory data | $2,510,000 | $344.99 | $10,312 |
| Biocide Full Review - 26-50 pieces of efficacy or confirmatory data | $13,617 | ||
| Biocide Full Review - 51 or more pieces of efficacy or confirmatory data | $16,222 | ||
| Biocide Review - Use of Foreign Decisions pathway | N/A | $279.44 (est.) | $3,680 |
| Biocide Labelling Only | $937,000 | $279.44 | $3,261 |
| Biocide Monograph | $1,129,000 | $243.42 | $3,169 |
| Biocide Administrative | $462,000 | $344.41 | $2,256 |
| Biocide Major Change - No additional pieces of efficacy or confirmatory data | N/A | $279.44 (est.) | $5,882 |
| Biocide Minor Change | N/A | $209.37 | $1,618 |
| Activity | Estimated Total Costs | Estimated Volume | Unit Cost |
|---|---|---|---|
| Biocide Right to Sell | $2,447,000 | 1,200 | $2,039 |
| Fee Category | Description | Current Disinfectant Fee (2022) | Scheduled Fee - April 1, 2024Footnote * | Proposed FeeFootnote * | Current Performance Standard | Proposed Performance Standard | |||
|---|---|---|---|---|---|---|---|---|---|
| Evaluation | |||||||||
| Biocide Full Review - Novel Biocide | Applications for biocides that include a novel active ingredient, a novel combination of active ingredients, a novel physical form, or have a novel use or purpose | $9,211 | $11,743 | $39,928 | 300 calendar days to complete Review 1 | 300 calendar days to complete Review 1 | |||
| Biocide Full Review - 25 or fewer pieces of efficacy or confirmatory data | Applications that include 25 or fewer pieces of efficacy or confirmatory data in support of a biocide, including post-authorization applications | $9,211 | $11,743 | $8,233 | 210 calendar days to complete Review 1 | 180 calendar days to complete Review 1 | |||
| Biocide Full Review - 26-50 pieces of efficacy or confirmatory data | Applications that include between 26 and 50 pieces of efficacy or confirmatory data in support of a biocide, including post-authorization applications | $9,211 | $11,743 | $10,986 | 210 calendar days to complete Review 1 | 195 calendar days to complete Review 1 | |||
| Biocide Full Review - 51 or more pieces of efficacy or confirmatory data | Applications that include 51 or more pieces of efficacy or confirmatory data in support of a biocide, including post-authorization applications | $9,211 | $11,743 | $12,953 | 210 calendar days to complete Review 1 | 210 calendar days to complete Review 1 | |||
| Biocide Use of Foreign Decisions pathway and post-authorization pathway | Applications in support of a biocide based on a comparison to a foreign biocide | N/A | N/A | $2,969 | N/A | 90 calendar days to complete Review 1 | |||
| Biocide Labelling Only | Applications in support of a market authorization that requires a review of labelling material due to deviations from the previously authorized labelling and/or product (including post-authorization applications) | $2,588 | $2,687 | $2,632 | 90 calendar days to complete Review 1 | 90 calendar days to complete Review 1 | |||
| Biocide Monograph | Applications that attest to compliance with a Monograph for a biocide and that do not include efficacy data (including post-authorization applications) | $1,668 | $1,732 | $2,557 | 60 calendar days to complete Review 1 | 60 calendar days to complete Review 1 | |||
| Biocide Administrative | Applications in support of a market authorization that does not require a review of labelling material (including post-authorization applications) | $698 | $889 | $1,820 | 45 calendar days to complete Review 1 | 45 calendar days to complete Review 1 | |||
| Biocide Major Change - No additional pieces of efficacy or confirmatory data | Applications in support of a change to the product that significantly affects the benefit-harm profile of the product given any uncertainties, with no additional pieces of efficacy or confirmatory data included in the revised application | $9,211 | $11,743 | $4,746 | N/A | 60 calendar days to complete Review 1 | |||
| Biocide Minor Change | Applications in support of a change to the product that does not significantly affect the benefit-harm profile of the product given any uncertainties | N/A | N/A | $1,306 | N/A | 30 calendar days to complete Review 1 | |||
| Right to sell | |||||||||
| Biocide Right to Sell | Annual fee for the right to maintain a biocide on the Canadian market | $1,449(products under FDR) $3,872.61(products under PCPR) |
$1,538 | $1,470 | 20 calendar days to update database following receipt of a complete Annual Notification Package | 20 calendar days to update database following receipt of a complete Annual Notification Package | |||
|
|||||||||