Draft Guidance on asbestos in drinking water
Document for public comment
Consultation period ends: March 24, 2026
Download in PDF format
(777 KB, 64 pages)
Purpose of consultation
This document has been developed with the intent to provide regulatory authorities and decision-makers with guidance on asbestos in Canadian drinking water supplies.
This document is available for a 60-day consultation period. Please send comments (with rationale, where required) to Health Canada via email to: water-consultations-eau@hc-sc.gc.ca
All comments must be received before March 24, 2026. Comments received as part of this consultation will be shared with members of the Federal-Provincial-Territorial Committee on Drinking Water (CDW), along with the name and affiliation of their author. Authors who do not want their name and affiliation to be shared with CDW should provide a statement to this effect along with their comments.
It should be noted that this guidance document on asbestos will be revised following evaluation of comments received, and a final guidance document will be posted. This document should be considered as a draft for comment only.
Background on guidance document
The main responsibility of the Federal-Provincial-Territorial Committee on Drinking Water (CDW) is to work in collaboration with Health Canada to develop and update the Guidelines for Canadian Drinking Water Quality (GCDWQ). This role has evolved over the years, and Health Canada and the CDW also develop guidance documents. Guidance documents provide advice on issues related to drinking water quality for substances that do not require a formal Guideline for Canadian Drinking Water Quality.
There are two reasons for which Health Canada, in collaboration with the CDW, may choose to develop guidance documents. The first would be to provide operational or management guidance related to specific drinking water-related issues (such as boil water advisories or corrosion control), in which case the document would provide only limited scientific information or a health risk assessment.
The second reason would be to make risk assessment information available when a guideline is not deemed necessary. Guidelines for Canadian Drinking Water Quality are developed specifically for substances that meet all of the following criteria:
- exposure to the substance could lead to adverse health effects
- the substance is frequently detected or could be expected to be found in a large number of drinking water supplies throughout Canada
- the substance is detected, or could be expected to be detected, at a level that is of possible health significance
If a substance of interest does not meet all these criteria, Health Canada, in collaboration with the CDW, may choose not to establish a numerical guideline or develop a guideline technical document. In that case, a guidance document may be developed.
Guidance documents undergo a similar process as guideline technical documents, including public consultations through the Health Canada website. They are offered as information for drinking water authorities, and in some cases to help provide guidance in spill or other emergency situations.
Executive summary
Asbestos can enter drinking water through natural sources (erosion and runoff from soil and rock), emissions from human activities (such as mining), and releases from aging asbestos-cement (A-C) pipes in drinking water distribution systems. Asbestos fibres have no detectable odour or taste, and they do not dissolve in water or evaporate. Canadian data are limited but indicate that there was no asbestos detected in most samples. A maximum acceptable concentration (MAC) for asbestos in drinking water is not recommended since there is no consistent, convincing evidence that oral exposure to asbestos causes adverse effects in humans and animals.
Given public concern with asbestos and the goal of minimizing particle loading in treated drinking water to effectively operate the distribution system, it is recommended to implement best practices to minimize the concentrations of asbestos fibres in drinking water. Monitoring for asbestos can help provide a condition assessment of A-C pipes and inform infrastructure replacement schedules.
Health Canada has completed its review of asbestos in drinking water. This guidance document was prepared in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water (CDW) and assesses the available information on asbestos in the context of exposure from drinking water.
Assessment
The health effects of asbestos related to inhalation exposure are well established and extensively researched. In contrast, oral exposure studies have not clearly demonstrated adverse health outcomes when considering the weight of evidence and the strength of the available studies. This guidance document provides an assessment of the available human and animal studies involving oral exposure to asbestos in drinking water.
The toxicity of asbestos is influenced by many factors including duration and frequency of exposure, tissue-specific dose over time, persistence of the fibres in the tissue (influenced by the absorption, distribution and clearance of fibres), individual susceptibility, and, most importantly, the type and size of the fibres. Another factor influencing toxicity is the physiology of the digestive tract. Stomach acidity aids in the degradation of certain asbestos fibres (chrysotile) to smaller, less toxic fibres, while the intestinal mucosal barrier and cellular tight junctions limit the penetration and uptake of fibres. Studies in animals and humans report that nearly all of the ingested asbestos fibres (greater than 99%) pass through the digestive system and are excreted within 48 hours. Furthermore, the few fibres that do cross the intestinal barrier are generally less than 1 µm in length, a size that is not considered to be carcinogenic.
Standardized methods are available for the analysis of asbestos in source and drinking water. However, there are no accredited laboratories conducting asbestos analysis in drinking water in Canada.
At the municipal scale, conventional coagulation and filtration treatment can effectively remove asbestos fibres from source water. More than 99% of asbestos fibres can be removed by optimizing coagulation and filtration processes. At the residential and small scale, there are certified drinking water treatment devices capable of removing asbestos fibres from drinking water. The technologies certified to the NSF standards include carbon-based filters and reverse osmosis (RO) systems.
Water mains can be composed of A-C. Most A-C mains were installed many decades ago (from the 1940s until the late 1970s, with the use of products containing asbestos prohibited in Canada in 2018) and are at or near the end of their useful life span. The existing A-C mains eventually deteriorate, and the erosion of the pipe material can lead to the release of asbestos fibres, loss of mechanical stability and possibly pipe failure. Corrosion, or dissolution, as well as flow rate (low or high) and water quality (such as low pH, soft water and high sulphate) conditions impact the integrity of A-C pipes and can also lead to the release of asbestos fibres.
A MAC for asbestos in drinking water is not recommended since there is no consistent, convincing evidence that oral exposure to asbestos causes adverse effects in humans and animals. Due to significant limitations in study design and the absence of clear health outcomes, the available data on oral exposure to asbestos are insufficient for deriving a health-based value. Furthermore, asbestos fibres present in drinking water are generally smaller than those considered to be of concern for human health and greater than 99% of fibres in drinking water are excreted following ingestion.
Part A. Guidance on asbestos in drinking water supplies
A.1 Scope and aim
The intent of this document is to provide guidance on the health considerations for exposure to asbestos from drinking water. This document provides information on how people in Canada are exposed to asbestos in drinking water and summarizes the current available health data from human and animal oral ingestion studies. It outlines treatment strategies to remove naturally occurring asbestos. Management strategies to evaluate the release of asbestos fibres from asbestos-cement (A-C) pipes and assess potential exposure, the loss of mechanical stability in these pipes as well as the potential for pipe failure are also addressed in this document.
A.2 Application
A.2.1 Introduction
Asbestos refers to a family of six naturally occurring fibrous silicate minerals falling into either the serpentine or amphibole groups, based on their physical and chemical properties. Chrysotile, the only member of the serpentine group, has fibres that are flexible and curved. Crocidolite, amosite, actinolite, anthophyllite and tremolite are members of the amphibole group that have stiff and straight fibres. Asbestos fibres have no detectable odour or taste, do not dissolve in water and are non-volatile.
People in Canada can be exposed to asbestos mainly from drinking water and air. Asbestos can enter drinking water sources by erosion and runoff from natural deposits in soil and rock in some geological areas or by emissions from human activities. Asbestos fibres can also be present in drinking water as a post-treatment contaminant from degrading A-C water distribution pipes or from disintegrating asbestos roofing materials when rainwater is collected into cisterns. Ambient outdoor air can contain small quantities of asbestos fibres, with urban areas or locations near industrial sources having higher concentrations than rural areas. Indoor air can also contain low levels of asbestos. Historically, the most significant exposures to asbestos have come from chronic inhalation in occupational settings, such as in the mining and milling of asbestos minerals, the manufacture of products containing asbestos, construction and automotive industries and the asbestos-removal industry. Asbestos fibres may be present in foods through contamination with soil particles, dust or other dirt containing asbestos fibres. However, the presence of asbestos fibres in foods has not been well studied.
A.2.2 Health considerations
The toxicity of asbestos fibres is influenced by many factors, including duration and frequency of exposure, tissue-specific dose over time, persistence of the fibres in the tissue (influenced by the absorption, distribution and clearance of fibres), individual susceptibility, and the type and size of the fibres. Fibre size is the most important determinant of carcinogenicity, where fibres longer than 5 µm and thinner than 0.25 µm have been shown to be more toxic. Another factor influencing toxicity is the physiology of the digestive tract. Stomach acidity aids in the degradation of certain asbestos fibres (chrysotile) to smaller, less toxic fibres, while the intestinal mucosal barrier and cellular tight junctions limit penetration and uptake of fibres. Studies in animals and humans report that nearly all of the ingested asbestos fibres (greater than 99%) pass through the digestive system and are excreted within 48 hours. Furthermore, the few fibres that do cross the intestinal barrier are generally less than 1 µm in length, a size that is not considered to be carcinogenic.
The health hazards associated with inhaled asbestos are well known. Asbestos is a known carcinogen through the inhalation route, causing mesothelioma and other cancers, including lung, laryngeal and ovarian. Inhalation exposure in occupational settings has also been associated with colorectal, stomach and pharyngeal cancers. Oral exposure to asbestos, however, has not been clearly shown to cause adverse effects in humans and animals. Overall, human and animal oral exposure data are insufficient to support a dose-response analysis and the determination of a point of departure for non-cancer or cancer health outcomes due to significant limitations in the design of all available studies as well as an absence of clear health outcomes. In addition, given the physiological differences between the lungs and the gastrointestinal (GI) tract, which affect the retention and absorption of fibres, extrapolation from inhalation to oral exposure is not recommended.
A.2.3 Management considerations
A maximum acceptable concentration (MAC) for asbestos in drinking water is not recommended for the following reasons:
- the available data on oral exposure to asbestos in both humans and animals are insufficient for deriving a health-based value (HBV) in drinking water due to significant limitations in study design and an absence of clear health outcomes
- historical data indicate that asbestos fibres present in drinking water are generally smaller (less than 1 µm) than those typically associated with adverse health effects in humans
- after ingestion, small fibers present in drinking water are further degraded in the stomach and are largely excreted, since the GI tract serves as an effective barrier to their absorption
As part of its ongoing drinking water guideline/guidance review process, Health Canada will continue to monitor new research on the health outcomes associated with oral exposure to asbestos and recommend any change(s) to this guidance that it deems necessary.
A.2.3.1 Analytical and treatment
Three standardized methods are available for the quantification of asbestos in source and drinking water, based primarily on transmission electron microscopy. However, there are no accredited laboratories conducting asbestos analysis in drinking water in Canada.
At the municipal scale, conventional coagulation and filtration treatment can effectively remove asbestos fibres from source water. Greater than 99% of asbestos fibres can be removed by optimizing coagulation and filtration processes. At the residential and small scale, there are certified drinking water treatment devices capable of removing asbestos fibres from drinking water. The technologies certified to the NSF standards include carbon-based filters and reverse osmosis (RO) systems.
A.2.3.2 Factors affecting A-C pipes
A-C water mains can deteriorate, and the erosion of the pipe material can lead to the release of asbestos fibres, loss of mechanical stability and possibly pipe failure. Corrosion, or dissolution, of A-C pipes is governed by solubility considerations such as soft distributed water (for example, water with low mineral content) and pH levels below 7.5. Very high sulphate and polyphosphate concentrations are especially corrosive to A-C pipes. Pipes experiencing low flow conditions or long residence times can also lead to their deterioration. However, high water flows from main flushing can also lead to high asbestos fibre concentrations in the distributed water due to the mobilization of fibres in dead ends or shear forces on deteriorated A-C pipes.
A.2.3.3 Management strategies
Although a MAC is not recommended, given public concern with asbestos and the goal of minimizing particle loading in treated drinking water to effectively operate the distribution system, it is recommended to implement best practices to minimize the concentrations of asbestos fibres in drinking water. In water sources with high asbestos fibres concentrations, conventional water treatment can be implemented and optimized for asbestos fibre removal. Non-treatment options such as alternative water supplies can also be considered. Where aging A-C pipes are in use, degradation and release of fibres into drinking water should be minimized by controlling water corrosivity or by coating A-C pipes with suitable structural linings.
As A-C pipes reach the end of their useful lifespan and begin to fail or deteriorate significantly, they should be replaced with new asbestos-free materials. The use of products containing asbestos has been prohibited in Canada since 2018. Water treatment facilities may consider monitoring to investigate the presence and contribution of older A-C pipes to numbers, types, size and shape of fibres in drinking water (WHO, 2021). Information from the monitoring of asbestos fibres in drinking water can inform decisions about infrastructure replacement plans as well as support communication with consumers about water quality.
If the effectiveness of asbestos removal is to be assessed, paired samples of source and treated water should be collected to confirm the efficacy of treatment. Measurements of asbestos fibre concentrations obtained from hydrant mains samples in conjunction with water quality results can provide an indication of the integrity and condition of A-C pipes. Structural integrity of water mains can be monitored using destructive and non-destructive testing as well as predictive models based on historical data. Detailed information on management strategies and the monitoring of asbestos fibres and A-C pipe deterioration are found in Part B.5.
Part B. Supporting information
B.1 Exposure considerations
B.1.1 Identity, use, sources and environmental fate
Asbestos (Chemical Abstracts Service Registry Number [CAS RN] 1332-21-4) is the generic name for a family of six naturally occurring fibrous silicate minerals that have been used commercially. These fibrous minerals are composed of sheets or chains of fibres that have the silicate tetrahedron (SiO4) as the basic chemical unit, which can be associated with other chemical elements such as magnesium, calcium, aluminum, iron, potassium or sodium. Asbestos fibres are classified into two groups based on their physical and chemical properties: serpentine and amphibole. The serpentine group consists of only one member, chrysotile. Chrysotile is a magnesium silicate with thin and flexible fibres arranged in sheets that curl in a spiral manner. Chrysotile (CAS RN 12001-29-5) has a net positive surface charge, forms a stable suspension in water and degrades in weak acids but is largely resistant to alkali. The amphibole group (crocidolite, amosite, actinolite, anthophyllite and tremolite), however, have stiff, straight, needle-like fibres that do not dissolve, are resistant to acid and have a negative surface charge.
Asbestos fibres have no detectable odour or taste, are not soluble in water, nor do they evaporate. Asbestos-containing minerals occur as organized bundles of parallel fibres that can be separated into thinner strands. The length of fibre bundles can vary from several millimetres to more than 10 cm in length (IPCS, 1986; ATSDR, 2001; Virta, 2011; IARC, 2012). The Canada Occupational Health and Safety Regulations (Canada Labour Code, 1986) defines an asbestos fibre as a particle having a length of greater than 5 µm and an aspect ratio (length to width) equal or greater than 3:1.
Asbestos minerals have been historically used for numerous industrial applications because of their strong, long-lasting properties that are resistant to fire, heat and chemicals. They are also resistant to biodegradation and exhibit low electrical conductivity. Asbestos has been used in construction products (cement and plaster, building insulation, floor and ceiling tiles, house siding), friction materials (car and truck brake pads and transmission components), and anti-fire/heat applications (protective wear, heat, sound and electrical insulation, packing materials) (ATDSR, 2001; IARC, 2012). In Canada, the Prohibition of Asbestos and Products Containing Asbestos Regulations (2018) under the authority of the Canadian Environmental Protection Act, 1999 (CEPA) (1999), prohibit the import, sale, and use of asbestos, as well as the manufacture, import, sale and use of products containing asbestos, with some exceptions. Legacy asbestos materials are still present in older buildings and other products and are gradually being replaced with substitute materials or alternative products. Asbestos that is undisturbed or sealed to prevent release into environmental media are not considered a concern to health (ATDSR, 2001; IARC, 2012).
Asbestos fibres in water can come from natural sources, such as soil and rock, or from anthropogenic sources. Mineral fibres may be released into surface water by erosion and runoff of natural deposits and waste piles. They naturally settle out of air and water to deposit in soil or sediment. Fibre migration depends on a variety of factors including site and geographical characteristics in combination with key physico-chemical characteristics such as particle size and morphology, solubility and surface charge (ATDSR, 2001; IARC, 2012). Small fibres (0.1 to 1 µm) can stay suspended in air and water, allowing them to be transported over long distances. Following release into the environment, asbestos degrades very slowly, with leaching of minerals from the fibre surface or breakdown into shorter lengths (U.S. EPA, 2018).
Asbestos fibres can also be present in drinking water from deterioration of A-C water mains or from disintegrating asbestos roofing materials when rainwater is collected into cisterns (ATDSR, 2001; IARC, 2012). In North America, A-C was commonly used for the construction of potable water mains starting in the 1940s. Its use was discontinued in the late 1970s due to health concerns associated with the manufacturing process and it was estimated that A-C pipes made up 16% to 18% of water distribution pipes in the United States (U.S.) and Canada (Hu et al., 2008). Data from the Core Public Infrastructure Survey published by Statistics Canada indicate that in 2022, less than 14,000 km of A-C pipe were in use in Canadian drinking water distribution systems (see Appendix A, Table A1). This accounts for approximately 6% of the total length (in km) of all types of water pipes in use across Canada (Statistics Canada, 2025).
A-C is made out of Portland cement, with or without silica, mixed with asbestos fibres (Hu et al., 2008). Portland cement contains calcium silicates, calcium aluminates, iron calcium aluminates and gypsum (Leroy et al., 1996). Asbestos fibres are the aggregate materials that provide stress and pressure resistance and represent about 20% of the pipe by weight (Hu et al., 2008; Leroy et al., 1996). Chrysotile and crocidolite are the two types of asbestos fibres that were used in A-C pipes (Hu et al., 2008), with chrysotile asbestos being the main one used in North America (Cook et al., 1974).
The shedding of fibres from these distribution pipes occurs due to many factors. These include the physical characteristics of the piping (age, size, quality of manufacturing) and the local environment (season, temperature of the water, pH and other water chemistry parameters). A-C pipe degradation is associated with low pH, low alkalinity, increased age, and the presence of any internal pipe coatings. The primary cause of fibre release into drinking water is pipe softening due to calcium leaching from the A-C material as it degrades (Zavasnik et al., 2022).
Natural erosion is another potential source of asbestos fibres. In natural waters, Webber and Covey (1991) reported that levels are generally less than 1 million fibres per litre (MFL). However, in certain regions, like eastern North America, high concentrations of chrysotile have been measured in surface waters in areas of serpentinized bedrock. A study by Monaro et al. (1983) reported concentrations of 1 MFL as part of an investigation to assess the influence of mining on asbestos pollution of the Bécancour river in Quebec. In the analysis of 1 500 water samples in the U.S., chrysotile asbestos was the most commonly found, though some samples contained amphibole asbestos (Millette et al., 1980). The study also suggested that the size distribution of the fibres depends on their source. For example, fibres released from A-C pipes tended to be longer than those resulting from natural erosion, averaging approximately 4 µm and 1 µm, respectively.
B.1.2 Exposure
People in Canada can be exposed to asbestos mainly from drinking water and air. Exposure from drinking water is expected to occur primarily from the oral route. Asbestos fibres are non-volatile. However, they have been shown to transfer to air by aerosolization at very low concentrations. It is possible that inhalation exposure to aerosolized asbestos could occur during showering and bathing with water containing high concentrations of fibres. Since asbestos fibres in drinking water have been shown to be smaller than those considered to be a health concern (less than 1 µm in length, see section B.2.6), adverse health outcomes from this exposure are not expected. Additionally, asbestos fibres are not able to pass through skin; thus, skin contact with asbestos fibres in drinking water is not an expected route of exposure.
B.1.2.1 Water
Limited water monitoring data was available from the provinces and territories (PTs) for the concentration of asbestos fibres in drinking water. Data was obtained from Newfoundland and Labrador, Saskatchewan and British Columbia. Other PTs as well as the First Nations and Inuit Health Branch (FNIHB) did not have any information on the concentration of asbestos fibres in drinking water or in source water (Indigenous Services Canada, 2023; Manitoba Department of Environment and Climate, 2023; Ministère de l'Environnement, de la Lutte contre les changements climatiques, de la Faune et des Parcs du Québec, 2023; New Brunswick Department of Environment and Local Government, 2023; Northwest Territories Department of Health and Social Services, 2023; Nova Scotia Environment, 2023; Nunavut Department of Health, 2023; Ontario Ministry of the Environment, 2023; Prince Edward Island Department of Environment, 2023). The detection limit used in the analysis of asbestos is defined as the analytical sensitivity (AS).
Overall, the limited data received from the PTs demonstrated a very low detection frequency of asbestos fibres in drinking water. This indicates that either the samples had no detectable asbestos fibres or that concentrations were below the AS. When there is less than 10% detection, the 90th percentile is presented as being below the AS. The range of ASs, number of detects, number of samples, 90th percentile asbestos concentration and maximum asbestos fibres concentration are presented in Table 1 for the provincial data. Overall, for asbestos fibres concentration, the dataset showed that:
- most of the samples were below the AS
- limited information is available concerning asbestos fibre concentration in raw, treated and distributed water.
| Jurisdiction
(AS MFL) |
Water type | No. detects/ samples |
Median (MFL) |
Mean (MFL) |
90th percentile (MFL) |
Max (MFL) |
|---|---|---|---|---|---|---|
| Newfoundland and Labrador Footnote 1 (0.18–1.9) [2012–2021] |
Not specified; distribution Footnote a |
1/21 | < AS | < AS | < AS | 0.58 |
| Saskatchewan Footnote 2 (0.18–2.11) [2024–2025] |
Not specified; distribution | 0/44 | < AS | < AS | < AS | < AS |
AS – analytical sensitivity; MFL – million fibres per litre. |
||||||
In Saskatchewan, water samples were collected to determine asbestos concentrations in municipal drinking water distribution systems in areas known to have A-C pipes. A total of 102 asbestos samples were collected from 47 communities from November 2024 to February 2025. Samples were collected under both normal operating conditions (n = 95) and following A-C pipe break and repair conditions (n = 7). As noted in Table 1, no asbestos fibres were detected in any of the distribution system water samples.
In British Columbia, testing has been conducted by health authorities. Data from one health authority had values mainly below the AS. One water treatment facility that was conducting regular testing (number of samples and testing frequency were not provided) also found all values were below the AS (British Columbia Ministry of Health, 2023).
Additional data were obtained for a small number of water treatment facilities, either through direct communication or in online versions of reports. These are summarized in Table 2. In each case, the facilities reported that the concentrations of asbestos fibres, quantified using the U.S. Environmental Protection Agency's (EPA's) EPA Method 100.2, were below the ASs. The City of Regina, SK sampled water from one location in their distribution system annually from 2016 to 2019. They subsequently increased the sampling to 11 locations from 2020 to 2022. The locations selected were connected to A-C water mains that were installed between 1956 and 1987. The analytical method used in Regina only considers fibres that are greater than 10 µm in length (City of Regina, 2023). The City of Medicine Hat, AB estimated that, in 2022, 32% of underground pipes were A-C pipes (City of Medicine Hat, 2023), and no detectable levels of asbestos fibres were measured in six water samples (ALS Laboratory Group, 2023). Water samples were collected in 2018 and 2023 to evaluate the concentration of asbestos in water in Edmonton, Alberta. Fourteen samples were collected in 2018 (two sources, two treated, and 10 distributed water samples) and 2023 (two treated and 12 distributed water samples). The sampling locations in the distribution system were selected from areas known to have A-C pipes and low water flow/high water age. No asbestos was detected in any sample in both studies (EPCOR, 2018; 2024).
| Jurisdiction (AS MFL) [year sampled] |
Water type | No. detects/ samples |
90th percentile (MFL) |
Max (MFL) |
|---|---|---|---|---|
| Regina, Saskatchewan Footnote 1 (0.16 to 0.17) [2016 to 2022] |
Surface; distribution | 0/37 | < AS | < AS |
| Guelph, Ontario Footnote 2 (0.18) [2023] |
Ground; ground/raw | 0/1 | NC | < AS |
| Medicine Hat, Alberta Footnote 3 (0.18) [2023] |
Surface; distribution | 0/6 | NC | < AS |
| Edmonton, Alberta Footnote 4 (0.17) [2018] |
Surface; distribution | 0/14 | NC | < AS |
| Edmonton, Alberta Footnote 5 (0.145) (rounded) [2023] |
Surface; distribution | 0/14 | NC | < AS |
AS – analytical sensitivity; MFL – million fibres per litre; NC – not calculated. |
||||
Historical concentrations of asbestos fibres in Canadian drinking water are reported elsewhere (Bacon et al., 1986; Chatfield and Dillon, 1979; Cunningham and Pontefract, 1971; Toft et al., 1981; Wigle, 1977; Wigle et al., 1986). These concentrations are unlikely to be representative of the current situation as these publications are decades old and the characteristics of the drinking water distribution systems may have changed. Also, sources of asbestos fibres in drinking water distribution systems (that is, the A-C pipes) may have been removed. Sample preparation methods and analysis also differ from current approved methods since the studies took place prior to the establishment of the EPA methods and American Public Health Association (APHA) Standard Method (SM). In discussing sizing of asbestos fibres in these studies, differences in sensitivities can be observed between studies that also influence the results (Millette et al., 1983).
Samples from raw, treated and distributed water were collected in 71 Canadian municipalities during August and September 1977 (Toft et al., 1981). The study by Toft et al. (1981) is based on a national survey published by Chatfield and Dillon (1979). Analytical methods used were similar to EPA Method 100.1 (Chatfield et al., 1978). Chrysotile asbestos was the major type of asbestos found in drinking water, whereas amphibole asbestos was not significant with detectable levels found in 7% of samples (Chatfield and Dillon, 1979; Toft et al., 1981). This study found that 5% of water supplies contained asbestos at concentrations greater than 10 MFL. Concentrations of asbestos fibres were also significantly higher in samples collected from a distribution system than in treated water samples (Toft et al., 1981). In cases where the asbestos concentration was greater than 5 MFL in the distribution water, the median fibre lengths were between 0.5 and 0.8 µm (Toft et al., 1981).
Concentrations of asbestos fibres were measured in the tap water of eight cities located in Quebec and Ontario and one river sample in Ontario. It was found that fibres in most filtered tap waters were less than 1 µm in length. Where water was treated in a municipal drinking water treatment plant (DWTP), concentrations of asbestos fibres in the tap water ranged between 2.0 MFL and 5.9 MFL. The highest concentration was attributed to the source water being located in a small lake within an asbestos mining area (Cunningham and Pontefract, 1971).
Concentrations of asbestos fibres were quantified in southeastern Quebec (the drainage basins of the Richelieu, Yamaska, Magog, Missisquoi [north branch] and Sutton Rivers) where the source water was impacted by asbestos-bearing railway ballast and naturally occurring asbestos deposits (Bacon et al., 1986). No samples from treated drinking water were collected during this study. Detectable levels of asbestos fibres were found in all the source water samples. For groundwater samples (four sampling locations), concentrations ranged between 2.2 MFL and 23.0 MFL. For surface water samples (14 sampling locations), concentrations ranged between 0.6 MFL and 147.8 MFL (Bacon et al., 1986).
Four communities in Quebec (Asbestos, Drummondville, Plessisville and Thetford Mines) either adjacent to asbestos deposits or using rivers that drain from regions with asbestos deposits as their drinking water source were sampled by Wigle (1977). In the four raw water samples, the concentrations varied considerably, with one sample containing 13 MFL while the other three samples ranged between 680 and 1 300 MFL.
In Christchurch, New Zealand, an average of 0.9 MFL was measured for asbestos fibres > 10 µm in length while shorter fibres (> 0.5 µm in length) had an average concentration of 6.2 MFL in 20 samples. Samples were collected at hydrants and were representative of water mains. Household tap samples were also collected and the authors found that long chrysotile fibres were detected in three of 15 household tap samples, averaging 0.3 MFL. Short asbestos fibres were also detected in these same locations, but concentrations were considerably higher with an average of 3.5 MFL. Additional household samples were obtained to determine if results at the tap reflected distribution system samples. However, only two hydrants were able to be paired as a direct supply to household taps. In one set of hydrant samples, concentrations of 1 MFL for short fibres were detected in the hydrant samples, but no fibres were detected in the samples collected from household taps. In the second paired samples, concentrations from the hydrant and at household taps were 4.1 MFL and 2.2 MFL, respectively. The occurrence of positive, high-fibre counts in hydrant samples was significantly greater than observed in household tap samples (Mager et al., 2022). Although tap sampling may inform exposure, the limited results indicate that it may not inform the state of A-C pipe deterioration as well as hydrant sampling.
In the U.S., the typical concentration of asbestos measured in drinking water is less than 1 000 fibres/L, even in areas with asbestos deposits or A-C water supply pipes—although very high concentrations have also been reported (10 to 300 MFL or more) (IARC, 2012). Measured asbestos levels in U.S. drinking water from 2006 to 2011 were reported to range from 0.10 to 6.8 MFL (5th and 95th percentiles, respectively) (U.S. EPA, 2016). Water samples were collected from 538 A-C water distribution systems, located throughout the ten U.S. Environmental Protection Agency (EPA) regions. Results showed that the average length of chrysotile fibres found in was less that 5 µm (Millette et al., 1980; 1983).
Although asbestos fibres are non-volatile, they have been found in water aerosols generated from contaminated drinking water. Roccaro and Vagliasindi (2018) investigated the release of asbestos fibres from a portable home humidifier and shower. The humidifier was filled with groundwater containing 24 687 asbestos fibres/L. Air samples were found to contain fibres longer than 5 µm with a width less than 3 µm and with a length-to-width ratio greater than 3:1. The authors reported that 0.04% to 0.07% of fibres were transferred from the humidifier to the air. For the shower, the authors reported a transfer of 4.3% to 10.8% of fibres from tap water containing natural levels of 8 229 fibres/L. An earlier study by Hardy et al. (1992) determined a similar release of asbestos-like fibres from a room humidifier where 0.03% to 4.7% of fibres from the water (57 to 280 000 million asbestos structures/L) used to fill the humidifier were transferred to the air. In a controlled experiment designed to simulate the migration of asbestos fibres from water to air during the collapse of bubbles and foams from polluted natural waters, Avataneo et al. (2022) reported that the minimum waterborne asbestos fibre concentration required to release at least one fibre/L (the alarm threshold limit set by the World Health Organization [WHO] for airborne asbestos) into the air is 40 MFL. Avataneo et al. (2022) indicated that the higher migration of fibres in the studies by Hardy et al. (1992) and Roccaro and Vagliasindi (2018) may be due in part to a more effective system to generate fibre migration to air (humidifier/showering versus bubbling), differences in fibre sizes and lower relative humidity in these studies compared to the bubbling study (Avataneo, 2022).
B.1.2.2 Air
Inhalation is the primary route of exposure to asbestos. Ambient outdoor air contains small and highly variable quantities of asbestos fibres, with urban areas or sites in close proximity to industrial sources having higher concentrations (approximately 0.1 fibres/L) than rural locations (approximately 0.001 fibres/L) (ATSDR, 2001; IARC, 2012; U.S. EPA, 2018). Indoor air can also contain low levels of asbestos (IARC, 2012). Historically, most inhalation of asbestos has been shown to occur through chronic occupational exposure, such as in the mining and milling of asbestos minerals, the manufacture of products containing asbestos, construction and automotive industries and the asbestos removal industry (IARC, 2012). With the decline of asbestos manufacture and use, more recent occupational exposures to asbestos mainly occur in the construction industry and associated occupations (for example, carpenters, trades helpers and labourers and electricians). From 2006 to 2016, approximately 235 000 Canadian workers were reported as having been occupationally exposed to asbestos (Fenton et al., 2023).
Some inhaled asbestos fibres can collect in the air passages of the respiratory system and become deposited in the ciliated portion of the airway. These fibres are removed through mucociliary expulsion from the lungs to the throat and are then swallowed. Approximately 28% of inhaled particulate matter, including asbestos, is reportedly transported to the GI tract (Gross et al., 1974).
B.1.2.3 Food
There are no recent data for asbestos fibre levels in food or beverages. Rowe (1983) suggested that foods contaminated with soil particles, dust, or dirt may also contain asbestos fibres, and could be a significant source of exposure to ingested asbestos relative to drinking water. However, due to the lack of a simple and reliable analytical method, asbestos measurement in food has not been well studied.
B.1.3 Climate change considerations
Climate change is projected to have impacts on temperature, precipitation patterns, soil moisture and occurrence of extreme weather events (Olmstead, 2014). Temperature, moisture and precipitation are some of the climatic conditions influencing water main deterioration (Ahmad et al., 2023; Hu et al., 2008).
For example, climate change can have an impact on the moisture content of soils through severe heat waves and drought or by increasing precipitation. This can affect the shrinking and swelling of the soil, which causes stress on pipes and can lead to increases in water main failures (Ahmad et al., 2023). In particular, significant moisture content changes can occur in montmorillonitic clay soils, creating stress on buried infrastructures such as A-C pipes (Hudak et al., 1998; Hu and Hubble, 2007).
The deterioration of the outside surface of the pipe can be influenced by the groundwater and soil surrounding it (Hu et al., 2008). The aggressiveness of the soil towards A-C pipes depends on its pH and the amount of sulphate present (Hu et al., 2008). At least one climate change modelling study suggests that an increase in mean annual air temperature and annual precipitation could increase soil pH in eastern Canada (Houle et al., 2020).
B.2 Health effects
The toxicity of asbestos fibres is dependent on fibre characteristics (such as asbestos type, dimensions, fibre size, surface area and charge) and exposure considerations (such as dose, duration and route of exposure). Asbestos is a known carcinogen through inhalation exposure, causing mesothelioma and cancers of the lung, larynx and ovaries. In occupational settings, colorectal, stomach, liver and pharyngeal cancers have also been reported as being associated with inhalation exposure (IARC, 2012; Brandi and Tavolari, 2020). Non-cancer effects following inhalation exposure include fibrotic lung disease (asbestosis), pleural plaques and thickening of the pleura (ATSDR, 2001). The evidence for health effects from oral exposure in animals and humans, however, is inconsistent. This guidance document presents the available health information associated with oral exposure to asbestos fibres (largely chrysotile fibres) mainly from drinking water (epidemiological studies) and to a lesser extent food (animal studies). A weight of evidence analysis and quality assessment of the available studies has also been conducted.
B.2.1 Effects in humans
A systematic review of the available epidemiological data examined the associations between ingesting asbestos-contaminated drinking water and the risk of adverse health outcomes (Go et al., 2024). From an initial total of 7 044 references identified in the published literature, 25 references (assessing 17 studies) were retained while the rest of the studies were not relevant for risk assessment for drinking water exposure. Fourteen studies were of ecological design, two were case-control studies, and one was a cohort study. The main sources of asbestos fibres in drinking water in these studies were from A-C distribution pipes, A-C roofs from which drinking water was collected, lakes contaminated by industrial waste containing asbestos or from natural water sources. When reported, the concentration of asbestos fibres ranged from below the limit of detection to 7.1 x 103 MFL.
Tables 3a and 3b provide a summary of the relevant information on study design, exposure information and health outcomes reported in each of the studies retained for review. An assessment of confidence in the data from these studies was conducted using the Office of Health Assessment and Translation (OHAT) Risk of Bias Rating (ROB) Tool, developed by the U.S. National Toxicology Program (NTP) and the National Institute of Environmental Health Sciences (NTP, 2015). The outcome of these bias assessments were used to rank the studies into three Tiers (see Figure 1) (Go et al., 2024).
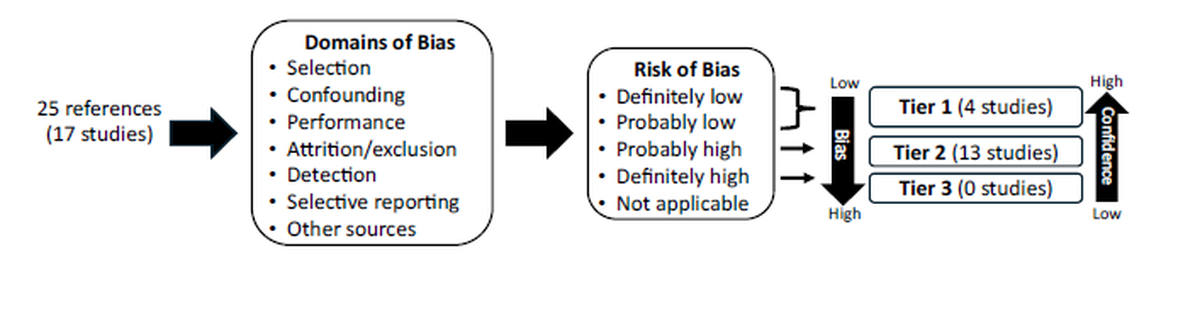
Figure 1 - Text description
This figure illustrates the assessment of confidence of 25 references that assessed 17 studies. Individual studies were assessed using the Office of Health Assessment and Translation Risk of Bias Tool.
The assessment considers the following 7 domains of bias:
- selection
- confounding
- performance
- attrition/exclusion
- detection
- selective reporting
- other sources of bias
The assessment of each study results in a risk of bias rating of:
- definitely low
- probably low
- probably high
- definitely high
- not applicable
Based on their risk of bias rating, studies are categorized into tiers:
- Tier 1 studies have a low risk of bias or probably a low risk of bias indicating the highest confidence
- Tier 2 studies have a probable high risk of bias indicating a low confidence
- Tier 3 studies have a high risk of bias indicating a very low confidence [KH1]
Individual studies were assessed using the Office of Health Assessment and Translation (OHAT) Risk of Bias Rating (ROB) Tool (NTP, 2015). Studies are ranked by tier based on evaluating seven domains of bias. Tier 1 studies have a low risk of bias or probably a low risk of bias (highest confidence), Tier 2 studies have a probable high risk of bias (low confidence) and Tier 3 studies have a high risk of bias (very low confidence).
| Reference | Study design; location (country, region) | Sample size | Source | Asbestos fibre type; asbestos fibre concentration (fibres/L) | Health outcome(s) |
|---|---|---|---|---|---|
| Kanarek et al. (1980); Conforti et al. (1981); Conforti (1983) | Ecological study; United States, San Francisco, Oakland, California |
Approximately 3 000 000 people | Naturally occurring | Chrysotile; 25 x 103 to 36 x 106 |
Significant positive association with incidence of cancer of the digestive tract (M/F), esophagus (M/F), pancreas (M/F), stomach (M/F), large intestine (M), rectum (F), respiratory system (F), lung (M), trachea/bronchus/lung/pleura (F), breast (F), prostate (M), retroperitoneum (F) |
| Polissar et al. (1982) | Ecological study; United States, Western Washington, Puget Sound Region |
Major cities: 3 Water sources: 5 Water samples: 95 |
Sultan River Cedar River Tolt River Green River Lakewood wells |
Chrysotile: Sultan River: 206.5 x 104 Other areas: 7.3 x 104 |
Significant positive association with mortality from cancer of genital (F), multiple myeloma (F) Significant positive association with incidence of cancer of the prostate (M), multiple myeloma (M), eye (M) and soft tissue (M) |
| Millette et al. (1983) | Ecological study; United States, Escambia County, Florida |
Potential high exposure: 46 123 people Low exposure: 86 897 people No exposure: 51 378 people |
A-C pipes | Amphibole, chrysotile; Amphibole (range): 0.1 to 0.5 x 106 Chrysotile (range): 0.1 to 0.5 x 106 |
No significant positive associations with mortality from cancer of the bladder, kidneys, pancreas, liver, lungs, and GI tract (esophagus, stomach, intestines, colon, rectum, liver, gallbladder, pancreas, peritoneum) |
| Polissar et al. (1983a, 1983b, 1984) | Case-control study; United States, Everette, Washington |
Cases: 382 people Controls: 462 people |
Sultan River | Chrysotile; Approximately 200 000 000 |
Significant positive association with incidence of cancer of the stomach (M) and pharynx (M) |
A-C – asbestos-cement; F – females; GI – gastrointestinal; M – males. |
|||||
| Reference | Study design; location (country, region) |
Sample size | Source | Asbestos fibre type; asbestos fibre concentration (fibres/L) |
Health outcome(s) |
|---|---|---|---|---|---|
| Masson et al. (1974) | Ecological study; United States, Duluth, Minnesota, Hennepin County |
Duluth: 105 759 people Minnesota: 3 371 603 people Hennepin County: 825 986 people |
Industrial waste | NR; NR |
Significant positive association with mortality from cancer of the digestive tract (M/F), esophagus (M), stomach (M/F), pancreas (F), liver (F), large intestine (F), rectum (M/F) and lung (M) |
| Wigle (1977) | Ecological study; Canada, Quebec |
(Municipalities; Population) Group 1 (2; 31 714 people) Group 2 (6; 93 620 people) Group 3 (14; 294 396 people) |
Industrial waste | Chrysotile; Asbestos: Raw: 1 200 x 106 Filtered: 200 x 106 Thetford Mines: Raw: 172 x 106 Raw: 1 300 x 106 Drummondville: Raw: 680 x 106 Filtered: 1.1 x 106 Plessisville: Raw: 13 x 106 |
Significant positive association with mortality from cancer of the upper GI tract (F), stomach (M), pancreas (F), colorectal (M), large intestine (F), lung (M), uterus (F), prostate (M), kidney (F), lymphoma (F), brain (M) Significant positive association with mortality from non-cancer diseases (disease types not specified) (M/F) |
| Harrington et al. (1978); Harrington and Craun (1979) | Ecological study; United States, Connecticut |
Approximately 576 800 people | A-C pipes | Chrysotile; Less than LOD to 7 x 105 |
Significant positive association with incidence of cancer of the large intestine (M/F), stomach (M/F) and rectum (M/F) |
| Meigs et al. (1980) | Ecological study; United States, Connecticut |
Group 1: 82 towns Group 2: 11 towns Group 3: 76 towns |
Group 1: A-C pipes Group 2: Naturally occurring Group 3: N/A |
Chrysotile; Group 1: less than 0.1 x 106 Group 2: NR Group 3: approximately 0.005 x 106 |
Significant positive association with incidence of cancer of the pancreas (M) and lung (M) |
| Levy et al. (1976); Sigurdson et al. (1981); Sigurdson (1983) | Ecological study; United States, Duluth, Minnesota |
100 578 people | Industrial waste | Amphibole; 1 to 30 x 106 |
Significant positive association with incidence of cancer of the stomach (M), large intestine (M/F), corpus uteri (F), prostate (M), peritoneum/ retroperitoneum (M) Significant positive association with mortality Footnote 1 from cancer of the GI (F), stomach (M/F), pancreas (F), small intestine (M/F) and rectum (M/F) |
| Toft et al. (1981) | Ecological study; Canada |
71 municipalities | Naturally occurring Industrial waste A-C pipes |
Chrysotile (major type), amphibole (minor type); Amphibole: 13 x 106 (max) Chrysotile: greater than 10 x 106 in approximately 5% of population receiving water; greater than 100 x 106in approximately 0.6% of population receiving water; 1800 x 106(max) |
Significant positive association with mortality from cancer of the digestive system (M), stomach (M) and lung (M) Significant positive association with mortality from respiratory system disease (non-cancer) (M) |
| Sadler et al. (1984) | Ecological study; United States, Utah |
Exposed: 14 communities Non-exposed: 27 communities |
A-C pipes | NR Less than LOD |
Significant positive association with incidence of cancer of the gall bladder (F), kidney (M) and leukemia (M/F) |
| Wigle et al. (1986) | Ecological study; Canada |
71 cities | Sherbrooke: Naturally occurring Other cities: NR |
Chrysotile; Filtered: Raw: 0.7 to 83.0 x 106 Distribution system: 0.03 to 3.0 x 106 Unfiltered: Raw: 0.3 to 280 x 106 Distribution system: 1.9 to 153 x 106 |
No significant positive association with mortality from cancer of the tongue, mouth, and pharynx, esophagus, stomach, large intestine except rectum, large intestine including rectum, rectum, liver, pancreas, total GI tract, breast, ovary, prostate, bladder, kidney, and brain |
| Howe et al. (1989) | Ecological study; United States, Woodstock, New York |
2 679 people | A-C pipes | Chrysotile, crocidolite; 3.2 to 304.5 x 106 Fibres greater than10 µm (1-10%): 0.9 to 15.1 x 106 |
Significant positive association with incidence of cancer of the buccal cavity (M) and prostate (M) |
| Andersen et al. (1993); Kjaerheim et al. (2005) | Cohort study; Rural regions in Norway |
726 people | A-C tiles | Chrysotile (92%), amphibole (8%); 1.8 x 109 to 7.1 x 1010 |
Significant positive association with incidence of cancer of the GI tract (M), stomach (M) and large intestine (M) |
| Browne et al. (2005) | Ecological study; United States, Woodstock, New York |
Exposed: 1 852 people All cohort: 2 936 people | A-C pipes | Greater than 90% chrysotile: Greater than 10 x 106 in 4/5 samples |
Significant positive association with incidence of cancer of the pancreas (M) |
| Fiorenzuolo et al. (2013) | Ecological study; Italy, Senigallia |
NR | A-C pipes | Amosite; 3/20 samples had asbestos concentrations less than 2 680 |
No significant positive association with incidence of and mortality from cancer of the GI tract |
| Mi et al. (2015) | Case-control study; China, Dayao County |
Cases: 54 people Controls: 108 people |
Naturally occurring | Crocidolite; Well water: 8.6 x 106 Surface water: 1.37 x 108 |
Significant positive association with mortality from cancer of the GI tract (sex not indicated) |
A-C – asbestos-cement; F – females; GI – gastrointestinal; LOD – limit of detection; M – males; N/A – not available; NR – not reported; OHAT ROB – Office of Health Assessment and Translation (OHAT) Risk of Bias Rating (ROB). |
|||||
The OHAT framework was also used to evaluate the confidence in the epidemiological data for cancer outcomes for 15 organ systems as well as the data for respiratory and cardiovascular diseases (non-cancer outcomes). The organ systems assessed were the upper aerodigestive tract, digestive tract, digestive organs, mesothelium, abdominal cavity, respiratory system, kidney, urothelium, nervous system and eye, female breast, reproductive system and tract, male reproductive system, endocrine system, lymphoid and hematopoietic system, skin and connective tissues. The results of the organ system confidence analysis are summarized in Go et al., 2024. Twelve of the 17 studies were of ecological design that carry a low level of confidence in the reported health outcomes, whereas 5 of the 17 studies were cohort and case-control design that have a moderate confidence in the reported health outcomes. With further evaluation of the factors that can increase (large magnitude of response, dose response, low residual confounding, consistency across populations) or decrease (risk of bias, unexplained inconsistency, imprecision, publication bias) confidence, the final confidence ratings across all organ systems are largely considered as very low (see Go et al., 2024). Overall, all organ systems examined either displayed very low confidence levels or lacked sufficient evidence indicating a health outcome.
Of note, 15 of the 17 studies (Tables 3a and 3b) assessed stomach cancer with eight studies reporting at least one statistically significant positive association for mortality or incidence. The case-control and cohort studies by Polissar et al. (1984) and Kjaerheim et al. (2005) reported increased stomach cancer incidence among males with an odds ratio of 1.78 (95% confidence interval lower bound of 1.04) and a standardized incidence ratio of 1.6 (95% confidence interval of 1.0 to 2.3), respectively. Additionally, Kjaerheim et al. (2005) reported an increased standardized incidence ratio of 1.7 (95% confidence interval of 1.1 to 2.7) among male lighthouse keepers who were exposed for over 20 years. The remaining 13 ecological studies are considered as providing insufficient evidence for stomach cancer. Given that only two moderately strong studies indicate a potential for stomach cancer, the weight of evidence is considered insufficient to assess whether oral exposure to asbestos fibres in drinking water causes stomach cancer.
Limitations in the current epidemiological evidence for cancer and non-cancer outcomes have also been discussed by agencies and organizations in other countries, including the U.S. (ATSDR, 2001; OEHHA, 2003) and France (ANSES, 2021) as well as in another weight of evidence review by Cheng et al. (2021). These limitations are:
- ecological-design studies are not effective in demonstrating associations between exposure and health impacts since the duration and levels of exposure are not precisely determined. Therefore, without a dose-response relationship, determining causality between asbestos exposure and health outcomes is not feasible
- there is a lack of consistency in the reporting of asbestos exposures (numbers versus mass of fibres), and for most studies, fibre size was not measured or discussed
- potential confounding factors such as occupational exposures to asbestos, co-exposure to other carcinogens, ethnicity, employment, socioeconomic status and lifestyle factors (such as smoking, alcohol consumption and diet) are often not considered
- the use of cancer death certificates to identify cancer outcomes can lead to non-differential bias due to poor coding and different definitions of cancer sites
- often these studies cover a time period that is insufficient for investigating cancer outcomes
- many of the studies use different statistical methods, lack statistical power or do not report p-values or confidence intervals which further limits any interpretations of health outcomes
In summary, the epidemiological data for both cancer and non-cancer health outcomes following oral exposure to asbestos are considered insufficient for establishing a point of departure for risk assessment:
- the majority of the reported associations between health outcomes and oral exposure do not show a large magnitude of effect
- there are no clear dose-response relationships over multiple levels of exposure or exposure durations
- none of the studies accounted for all of the important potential confounders
- higher degrees of bias in most studies lead to an overall low confidence in the reported health outcomes
- there was no clear evidence of associations across different populations and locations for any of the health outcomes
B.2.2 Effects on experimental animals
Health Canada searched current scientific literature for studies of non-human mammals that were orally exposed to asbestos, published up to June 2023. Details of the screening approach are in Go et al., 2024. A total of 34 publications were determined to be relevant for further review. These publications were dated between 1974 and 2008, and covered 60 different types of asbestos oral exposure experiments. The systematic review is discussed in greater detail in Go et al., 2024.
Oral exposure to asbestos did not impact body weights, survival rates or mortality in chronic studies with doses ranging from 10 to 360 mg/week by gavage, 45 to 13 000 MFL in water and 20 to 300 mg/day (and 0.003% to 10%) in food. No observed systemic, reproductive, developmental, or neurological effects from oral exposure to different asbestos fibre types and sizes were reported in several large chronic exposure studies in rats and hamsters conducted by the U.S. NTP (NTP, 1983; 1985; 1988; 1990a; 1990b; 1990c). A lack of adverse reproductive and developmental outcomes from chrysotile ingestion was also reported by Schneider and Maurer (1977), Rita and Reddy (1986) and Haque et al. (2001), further supporting the NTP study findings.
The focus of the animal data presented below is on cancer outcomes, specifically in the GI tract. Guidance from the Organisation for Economic Co-operation and Development (OECD) (2018; Guideline #451) dictates that animal carcinogenicity studies should include large groups of more than 50 rodents per sex and administer a minimum of 18 to 24 months of duration of exposure with three test doses and a concurrent control. Of the 34 chronic carcinogenicity publications identified in the systematic review, 10 studies met the criteria for animal number and exposure length. Table 4 presents the key information from these studies and provides an indication as to whether the findings support carcinogenicity from oral exposure. For the 24 chronic carcinogenicity publications excluded for not meeting the OECD guidance, 19 studies showed no effects, two showed benign tumour and non-precursor outcomes, and the remaining two publications showed possible cancer outcomes. It is difficult to draw meaningful interpretations from the 19 studies that showed no effect due to significant weaknesses in their experimental designs, including small samples sizes, often single-dose exposures as well as inadequate latency periods for determining cancer outcomes.
| Reference | Exposure | Asbestos characteristics | Results | Support for carcinogenicity |
|---|---|---|---|---|
| Donham et al. (1980) | Chrysotile, 10% in food pellets (rat, N = 189) | UICC "B", not washed or treated | No significant differences in the number of neoplastic and non-neoplastic lesions of any one type in the colon compared to controls; authors observed 4 tumours in asbestos-dosed animals and 5 tumours in control animals | Negative |
| NTP (1983) | Amosite, 1% in food (hamster, N = 252 M / 254 F) | S-33 (Transvaal), single milled median length: 4.37 μm; range 0.85 to 995 μm; 24.6% greater than 100 μm long; many greater than 1 000 μm |
No increase in the incidence of GI tumours or tumours in any other site | Negative |
| NTP (1985) | Chrysotile, 1% in food, SR length of fibres | SR: COF-25 median length: 0.66 μm; range: 0.88 to 51.1 μm; 98% less than 10 μm |
No neoplastic or nonneoplastic disease was associated with exposure to SR fibres | Negative |
| NTP (1985) | Chrysotile, 1% in food, IR length of fibres (rat 88 to 250 M / 88 to 250 F) |
IR: Plastobest-20 median length: 0.82 μm; Range: 0.104 to 783.4 μm; 65% greater than 10 μm; 14% greater than 100 μm |
IR chrysotile significantly increased the incidence of benign epithelial neoplasms (adenomatous polyps) in the large intestine of males (9/250) when compared to pooled NTP asbestos study controls, but not when compared to concurrent controls; authors noted that this finding was of biological importance | Positive |
| NTP (1988) | Crocidolite, 1% in food (rat, N = 250 M / 250 F) | ML-6, milled twice; mean length: 10 μm |
Crocidolite did not increase the incidence of neoplastic or nonneoplastic disease | Negative |
| NTP (1990a) | Amosite, 1% in food (rat, N = 200 to 250 M / 250 to 400 F) | S-33 (Transvaal), single milled; median length: 4.37 μm; range 0.85 to 995 μm; 24.6% greater than 100 μm; many greater than 1 000 μm | Amosite was not carcinogenic at this concentration | Negative |
| NTP (1990b) | Chrysotile, 1% in food, SR length of fibres (hamster, N = 253 M / 252 F) Chrysotile, 1% in food, IR length of fibres (hamster, N = 251 M / 252 F) |
SR: COF-25 median length: 0.66 μm; range: 0.88 to 51.1 μm; 98% less than 10 μm IR: Plastobest-20 median length: 0.82 μm; range: 0.104 to 783.4 μm; 65% greater than 10 μm; 14% greater than 100 μm |
Significant increase in adrenal cortical adenomas in males exposed to SR and IR chrysotile, and in females receiving IR chrysotile, compared to pooled NTP study controls, but not significant when compared with concurrent controls Authors noted that the biological importance of this finding was questionable |
Negative |
| NTP (1990c) | Tremolite, 1% in food (rat, N = F0: 70 M / 140F; F1: 250 M / 250 F) | Governeur Talc Company, crushed and milled; 72% tremolite, 25% serpentine, 3% other; 93.6% less than 10 μm; 75% less than 4 μm |
No increase in the incidence of tumours | Negative |
| Smith et al. (1980) | Amosite: 0.5, 5, 50 mg/L in water (130, 1 300, 13 000 MFL), (hamster, N = 30 M / 30 F) | UICC, untreated; mean length 2.4μm; 91.5% less than 5 μm; 97.8% less than 10 μm; 2.6% greater than 10 μm | No Treatment-related increases in the incidence of tumours | Negative |
| Smith et al. (1980) | Taconite ore tailings: 0.5, 5, 50 mg/L in water (45, 450, 4 500 MFL) (hamster, N = 30 M / 30 F) | Mean length 2.1 μm; 95.8% less than 5 μm; 99.7% less than 10 μm; 0.3% greater than 10 μm | No significant increase in tumours | Negative |
| Truhaut and Chouroulinkov (1989) | Chrysotile, 10, 60, 360 mg/day in palm oil (rat, N = 70 M / 70 F) |
UICC | No differences in tumour frequency with respect to localization, type of fibre, dose and sex | Negative |
| Truhaut and Chouroulinkov (1989) | Chrysotile/ Crocidolite (75%/25%), 10, 60, 360 mg/day in palm oil (rat, N = 70 M / 70 F) |
UICC | No evidence of carcinogenic effects | Negative |
F – female; GI – gastrointestinal; IR – intermediate range; M – male; MFL – million fibres per litre; N – number; NTP – National Toxicology Program; SR – short range; UICC – Union for International Cancer Control (asbestos standard). |
||||
Limitations of the available animal oral carcinogenicity studies have also been identified by international risk assessment organizations (OEHHA, 2003; ANSES, 2021). These limitations include:
- the majority of studies available in the literature exposed small groups of animals, which make it difficult to determine the statistical significance of digestive system tumour development in rats, which is a rare event; the NTP studies, however, with sufficient group sizes, are considered the most informative of all available studies
- the majority of studies implemented a low number of doses (often a single dose), which limits any interpretation of dose-response relationships
- the use of different exposure media (water versus food) limits the comparison of results given their influence on the availability and residence time of asbestos in the GI tract
- in some studies, the latency period is inadequate for determining cancer outcomes
- the NTP studies use different control groups (some comparisons were made with experimental controls whereas other comparisons were made with controls combined over several experiments), which can influence the statistical significance and interpretation of the observed results
In summary, there are a limited number of animal studies providing quality data on the health outcomes from oral exposure to asbestos. Several NTP chronic toxicity studies in rats and hamsters did not report any health outcomes of biological significance following oral exposure to high doses of amosite, chrysotile, crocidolite, or tremolite fibres. Although benign adenomatous polyps were observed in the colon of male rats only that ingested intermediate length chrysotile fibres at a concentration of 1% in food (NTP, 1985), a higher dose of chrysotile (10%) did not cause an increase in cancerous lesions in the rat colon (Donham et al., 1980); therefore, the evidence for this health outcome is inconclusive. The authors of the NTP (1985) study noted that there was no indication of progression from benign adenomatous polyps to cancer over the lifetime length of the study and that there was no incidence of malignant epithelial neoplasms in the large intestine. In a similar study (NTP, 1990b), neither intermediate nor short range chrysotile fibres were found to be carcinogenic in hamsters. Overall, the lifetime animal studies do not provide consistent and conclusive evidence that oral exposure to asbestos fibres causes cancer in any specific digestive organs.
B.2.3 Effects in vitro
In vitro studies can aid in understanding how toxicity can occur at the cellular level. However, they do not serve as an appropriate indicator of the potential health effects from drinking water exposure. The design of such studies does not accurately reflect the complex human physiological environment, given that cells are directly and continuously exposed, cells or tissues are studied in isolation and the physiological processes that metabolize or remove the contaminant are absent.
The limited available in vitro evidence shows that fibre size, type and morphology can impact inflammatory, oxidative and immune responses. Hong and Choi (1997) treated Chinese hamster lung fibroblasts (V79 cells) with crocidolite and chrysotile at doses ranging from 0.16 to 20 µg/ml for 72 hours. They observed that the fibres were cytotoxic after phagocytosis induced multinucleate giant cell formation by interfering with mitosis. The authors also reported that, at higher doses, chrysotile was more potent at inducing multinucleate giant cells than crocidolite. Duncan et al. (2010) demonstrated that inflammatory marker expression (IL-8 and COX-2) in human bronchial epithelial cells was equally induced by 24-hour exposure to two different unfractionated amphibole fibre types (Libby-type versus amosite). However, when exposed to smaller-sized fibres (less than 2.5 um), the small-sized amosite was four times more potent than the Libby-type. Khaliullin et al. (2020) investigated asbestos fibre morphology and its influence on cytotoxicity, cytokine secretion, and transcriptional changes in murine alveolar macrophages (MPI cells) following exposure to asbestos and non-asbestos riebeckite or tremolite mineral particles for 24 hours. The dosage was based on mass, surface area, and particle number equivalent concentrations. Asbestos particles were observed to be more cytotoxic; however, both asbestos and non-asbestos particles of equal surface area or particle number induced similar lactate dehydrogenase leakage and impaired cell viability. All treatments increased chemokines, but not pro-inflammatory cytokines. Gene expression dysregulation patterns for several genes were also evaluated and found to differ (upregulation versus downregulation as well as degree of effect) depending on the asbestos mineral type.
B.2.4 Absorption, metabolism, distribution and excretion
B.2.4.1 Absorption
The absorption of ingested asbestos fibres has been demonstrated as being very low. Cook (1983) analyzed exposures from several laboratory animal and human environmental exposure studies and reported that only a small fraction of ingested fibres are likely to penetrate the GI tract; Millette et al. (1981) estimated absorption across the GI tract at approximately one in 1 000 fibres (0.1%). Factors that may influence the passage and uptake of asbestos fibres in the GI tract include total exposure time, the type of foods or fluids present with the asbestos fibres, the permeability of the GI mucosa, the presence of mucosal abnormalities, altered intestinal motility and the intestinal tract microbiota present (Cook, 1983; Pambianchi et al., 2022). In addition, the GI tract serves as a strong barrier against the absorption of asbestos fibres due to its robust structure consisting of mucin-covered, tightly packed columnar epithelial cells, sub-mucosal connective tissue and muscular layers. Following a review of animal and human studies on intestinal transport of macromolecules in food, Weiner (1988) proposed four mechanisms by which macromolecules ranging from 0.2 to 20 µm in size could be transported through the intestinal barrier. The mechanisms are: uptake into specialized epithelial cells (with fewer microvilli) and/or macrophages of the Peyer's patches or gut-associated lymphoid tissue; endocytosis of particles less than 2 µm in length into columnar epithelial cells by membrane-bound vesicles; possible uptake into goblet cells; and paracellular transport through "leaky" tight junctions between the cells (persorption) for larger particles like asbestos.
Dermal absorption of asbestos fibres is expected to be negligible (OEHHA, 2003).
B.2.4.2 Distribution
Ingested fibres, once absorbed, can be transported to various organs. Once past the cells lining the stomach or intestine, absorbed fibres reach the bloodstream or lymphatic system, which can carry them to other tissues where they are deposited or cleared. The fibres found beyond the GI tract are generally shorter in length than those originally ingested. It has been suggested that fibres shorter than 1 µm can cross the intestinal barrier by persorption (para-cellular passage); fibres of this size, according to the available mode of action data, are unlikely to be a health concern.
In mice exposed to asbestos in drinking water, fibres were detected in the stomach, intestines, blood and liver (Zheng et al., 2019). Hasanoglu et al. (2008) provided evidence that ingested asbestos fibres migrated to internal organs and caused histopathological changes. In rats that drank water containing chrysotile fibres (1.5 and 3.0 g/L) for 6, 9 or 12 months, fibres were found in the spleen and lung, likely reaching these organs through the lymphohematological route. There is limited evidence available on placental transfer of asbestos following oral exposure. Haque et al. (2001) investigated placental transfer in pregnant mice exposed (by gavage) to chrysotile asbestos at a concentration of 50 µg/0.2 ml of saline. The authors reported that the lungs of pups were found to contain a mean fibre count of 780 fibres/g, and the mean fibre count in the liver was 214 fibres/g.
B.2.4.3 Metabolism
Asbestos fibres are not metabolized. However, certain fibre types can be altered or degraded by the acidity of digestive fluids and physical processes of the GI tract, which can serve as a mechanism for reducing toxicity. Seshan (1983) showed that exposure of chrysotile fibres to strong acids and simulated gastric juices caused physical and chemical alterations such as changes in crystal structure, magnesium loss and changes in surface charge (from positive to negative); however, crocidolite (an amphibole type) was unchanged with acid exposure. The alteration or degradation of asbestos fibres by GI mechanisms supports the observation of shorter fibres in tissues (compared to the exposure media) analyzed following oral exposures (ANSES, 2021).
B.2.4.4 Excretion
Nearly all fibres pass through the digestive system within a few days and are excreted in the feces after approximately 48 hours following oral ingestion in rats (ATSDR, 2001). An estimated 0.1% of fibres in drinking water containing an unspecified high asbestos content were eliminated through urine in a human ingestion study (Cook and Olson, 1979). The eliminated fibres were less than 1 µm in length.
B.2.5 Genotoxicity and carcinogenicity
Asbestos is a known carcinogen through the inhalation route and has been assessed by the International Agency for Research on Cancer (IARC) as a Group 1 carcinogen (carcinogenic to humans). It causes mesothelioma as well as other cancers including lung, laryngeal and ovarian. Colorectal, stomach and pharynx cancers have also been shown to be positively associated with inhalation exposure in occupationally exposed workers (IARC, 2012). The genotoxicity of inhaled fibres has been reported in occupational and non-occupational populations exposed to different types of asbestos fibres (ATSDR, 2001); however, no population studies have investigated the genotoxicity of asbestos following ingestion.
A limited number of in vivo studies in animal models have reported no increased genotoxicity following oral exposure to asbestos. Lavappa et al. (1975) observed no increased frequency of micronuclei in bone marrow from mice gavaged with 4 to 400 mg/kg of chrysotile as well as no increase in the number of chromosomal aberrations in the bone marrow of monkeys gavaged with 100 or 500 mg/kg of chrysotile. Rats administered 50 mg/kg of anthophyllite or crocidolite showed no increased frequency of sister chromatid exchange in bone marrow cells 24 hours following a single gavage dose (Varga et al., 1996a; 1996b).
Evidence from in vitro studies shows that genotoxic or mutagenic changes can vary depending on the target cell. Chromosomal aberrations have been demonstrated in human mesothelial cells, lymphocytes, and amniotic fluid cells following exposure to chrysotile fibres (Valerio et al., 1980; Olofsson and Mark, 1989; Emerit et al., 1991; Korkina et al., 1992; Pelin et al., 1995; Dopp et al., 1997; Dopp and Schiffmann, 1998; Takeuchi et al., 1999), but not in fibroblasts or promyelocytic leukemia cells (Sincock et al., 1982; Takeuchi et al., 1999). Mutagenicity is dependent on differences in phagocytic activity of the cell (Both et al., 1994; 1995) with human phagocytic cells, such as mesothelioma cells, being more susceptible to asbestos injury than non-phagocytic cells like lymphocytes (Takeuchi et al., 1999). Amount of exposure is also important whereby a threshold concentration must be exceeded before chromosomal aberrations are observed (DiPaolo et al., 1983; Oshimura et al., 1984; Jaurand et al., 1986; Palekar et al., 1988). Although genotoxic and mutagenic changes are indirect effects of asbestos toxicity (see section B.2.6), fibres have also been shown to physically interfere with chromosome segregation during mitosis and cause clastogenic effects (ATSDR, 2001; IARC, 2012). Asbestos exposure has led to chromosomal aberrations such as aneuploidy, fragmentation, breaks, rearrangements, gaps, dicentrics, inversions and rings in Chinese hamster ovary cells and Syrian hamster embryo cells, as well as rat and human mesothelial cells, lymphocytes and amniotic fluid cells. It was suggested that asbestos fibres physically interfere with chromosome segregation during mitosis to cause clastogenic effects (ATSDR, 2001; IARC, 2012).
Finally, dose-response studies have shown that a threshold concentration (ranging from 7 to 40 µg/ml) must be attained before chromosomal aberrations are seen (Dipaolo et al., 1983; Jurand et al., 1986; Oshimura et al., 1984; Palekar et al., 1988).
B.2.6 Mode of action
The toxicity of asbestos exposure is influenced by many factors, including duration and frequency of exposure, tissue-specific dose over time, persistence of the fibres in the tissue (influenced by the absorption, distribution and clearance of fibres), individual susceptibility, and the type and size of the fibres. The mechanisms associated with inhaled asbestos toxicity are influenced by two key fibre features: physical attributes—such as length, width, aspect ratio and surface area—and surface chemical composition and reactivity (Aust et al., 2011).
Pepelko (1991) evaluated animal data comparing the effectiveness of oral versus inhalation exposure and the risk of cancer. Of the 29 known carcinogens evaluated, three insoluble forms of particulate matter (including asbestos) showed an increased risk of cancer only through the inhalation route, while the other 26 chemicals showed similar cancer risks from both the oral and inhalation routes of exposure. Furthermore, in the case of asbestos, inhalation exposure was estimated as 100 times more potent than exposure via the oral route. It is important to note that the oral exposure study used for comparison (Donham et al., 1980) reported tumours in the colons of four dosed animals and five control animals, therefore providing inconclusive evidence of cancer following oral exposure. Nonetheless, Pepelko (1991) showed that, for asbestos, inhalation exposure is more hazardous than oral exposure, which is likely due to deposition and longer retention of fibres in the lung tissue compared to the GI tract where asbestos fibres are rapidly eliminated.
Several studies have investigated the mechanisms of asbestos toxicity in the lung. Stanton et al. (1977) reports that the physical nature of asbestos, rather than the chemical structure, is responsible for its carcinogenicity. In an inhalation study, Stayner et al. (2008) determined that fibres longer than 10 μm were a strong predictor of lung cancer mortality in humans. In a review of 59 datasets, Wylie and Korchevskiy (2023) analyzed the relationship between the dimensions of elongate mineral particles and mesothelioma or lung cancer risk, confirming that carcinogenicity was mainly driven by fibre dimension (particularly width). The authors observed that amphibole fibres longer than 5 µm and thinner than 0.25 µm were the most carcinogenic, whereas fibres having lengths shorter than 5 µm were not associated with an increased cancer risk.
Early research suggests that asbestos fibres do not directly interact with DNA causing gene mutations or cell transformation (Williams, 1979; Shelby, 1988). Instead, these outcomes appear to result from the activation of cellular pathways that are linked to cell death from high dose asbestos-induced reactive oxygen species (ROS). For example, asbestos fibres with high iron content, such as crocidolite and amosite, have been shown to generate ROS, causing oxidative damage, DNA mutations, and inflammatory responses linked to carcinogenesis (Chao and Aust, 1994; Ghio et al., 1997). IARC (2012) has proposed a mechanism for the carcinogenicity of asbestos fibres from the inhalation route of exposure. Asbestos fibres greater than 5 μm in size are difficult for macrophages to engulf, resulting in either impaired clearance or the activation of an inflammatory response. These pathways cause the release of ROS, reactive nitrogen species, cytokines, chemokines and growth factors that promote DNA damage, apoptosis and persistent inflammation. As a consequence, mechanisms of fibrosis (activation of intracellular signaling pathways, resistance to apoptosis, cellular proliferation) and DNA damage (insufficient DNA repair, chromosomal and epigenetic alterations, activation of oncogenes, inactivation of tumour suppressor genes) can ultimately lead to lung cancer or mesothelioma. The mechanism for carcinogenicity proposed by the IARC is supported by an analysis of human and animal in vivo and in vitro data by Krewski et al. (2019), which shows that asbestos has the ability to induce epigenetic alterations, oxidative stress and chronic inflammation, and causes cell immortalization as well as altered cell proliferation, cell death and altered nutrient supply.
Finally, early mechanistic studies in animals investigating the effects of ingested asbestos on DNA, RNA and protein synthesis as well as impacts on enzyme changes in the GI tract organs have produced variable results. Amacher et al. (1974; 1975) conducted oral exposure studies in rats to determine if chrysotile could alter DNA synthesis in the GI tract. In both a two-week dose-response study and on days 1, 7, 14, 28 and 63 of a time-response study, a single gavage dose of chrysotile caused transient, non-dose-related changes in DNA synthesis showing increased synthesis in the small intestine and colon, and decreased synthesis in the liver. In a second experiment, minor changes in DNA synthesis were observed in stomach, duodenum and jejunum (increased) and liver (decreased) three days after chrysotile exposures ranging from 5 to 100 mg/kg. Similarly, these GI changes were observed by Jacobs et al. (1977), who exposed rats to chrysotile in feed and measured nucleic acid metabolism in the mucosal and gut lumen cells of the small intestine. After 10 months, lumen cells exhibited increased cellular DNA and decreased RNA levels. This study was expanded to examine nucleic acid metabolism in a wider selection of GI tract tissues after short (one week) and chronic (five to 15 months) oral exposures (Jacobs et al., 1978). After the short period of exposure, DNA metabolism was increased in the small intestine, esophagus, caecum, colon and rectum, stomach and spleen, but decreased in the liver. Similar increases in DNA metabolism were observed after 15 months of exposure in the small intestine, colon and rectum, stomach and spleen, along with a decrease in the liver. In addition, alterations in RNA metabolism in the liver and lung were noted. In monkeys, pancreatic DNA replication was increased nine days after receiving an acute dose of chrysotile, but, unlike in rats, DNA synthesis was not increased in the stomach, small intestine, colon, liver or kidney. Furthermore, no notable histopathological changes were observed (Epstein and Varnes, 1976). The authors suggest that the observed increase in pancreatic DNA replication may be a response to asbestos-induced cytotoxicity, or the result of direct stimulation by asbestos.
B.3 Risk assessment
Asbestos is a known carcinogen through the inhalation route. However, oral exposure studies have not clearly demonstrated adverse cancer and non-cancer health outcomes.
The toxicity of asbestos fibres is influenced by many factors, including duration and frequency of exposure, tissue-specific dose over time, persistence of the fibres in the tissue (influenced by the absorption, distribution and clearance of fibres), individual susceptibility, and the type and size of the fibres. Fibre size is the most important determinant of carcinogenicity, where fibres longer than 5 µm and thinner than 0.25 µm have been shown to be more toxic (see section B.2.6). Asbestos toxicity is also influenced by the physiology of the digestive tract. Stomach acidity helps degrade certain asbestos fibres (including chrysotile) to smaller sizes that are less toxic, while the intestinal mucosal barrier and cellular tight junctions limit penetration and uptake of fibres. Studies in animals and humans indicate that most ingested asbestos (greater than 99%) passes through the digestive system with excretion occurring within approximately 48 hours of ingestion (Millette et al., 1981; Cook, 1983; ATSDR, 2001). Furthermore, the few fibres that do cross the intestinal barrier are likely less than 1 µm in length, which, according to the available mode of action data, is a size that is unlikely to be a health concern.
A weight of evidence analysis of the available epidemiological data indicates that the data for both cancer and non-cancer health outcomes following oral exposure to asbestos are insufficient for determining a HBV for asbestos in drinking water. While eight studies reported possible associations with stomach cancer in some individuals, there are few high-quality studies available to clearly demonstrate a causal relationship for this health endpoint. Limitations in the available data include: the majority of the reported associations between health outcomes and oral exposure do not show a large magnitude of effect; there are no clear dose-response relationships in the studies assessing multiple levels and/or durations of exposure; none of the studies accounted for all of the important potential confounders; high bias in many of the studies have led to an overall low confidence in the reported health outcomes; and there is no clear evidence of associations across different populations and locations for any of the health outcomes.
A limited number of animal studies provided data on the health outcomes from oral exposure to asbestos fibres. Six NTP lifetime exposure studies in rats and hamsters reported no adverse health outcomes following oral exposure to high doses of various types of asbestos fibres. Benign polyps developed in the colon of male rats only that ingested intermediate length chrysotile fibres at a concentration of 1% in food. However, higher-dosed animals in the same study did not show an increased incidence of cancerous lesions in the colon; therefore, the evidence for this health outcome is inconclusive. Overall, the lifetime animal studies do not provide consistent and conclusive evidence that oral exposure to asbestos fibres causes cancer in any specific digestive organs.
International assessments have drawn similar conclusions from the available scientific evidence on oral exposure to asbestos. For example, the Agency for Toxic Substances and Disease Registry (ATSDR) (2001), Agence Nationale de Sécurité Sanitaire de l'alimentation, de l'environnement et du travail (ANSES) (2021) and WHO (2021) conclude that human and animal exposure studies are insufficient for dose-response analysis and determining points of departure for cancer and non-cancer endpoints. Limitations of the epidemiological evidence identified by these assessments include: little to no information on duration and levels of exposure in most studies rendering dose-response and causality interpretations impossible; inconsistent reporting of exposure levels and fibre sizes; inadequate consideration of important confounding factors; insufficient time periods assessed for investigating cancer outcomes; and inconsistent or low power statistical evaluations limiting the interpretation of health outcomes. Limitations in the animal evidence include: small dose groups (except in the NTP studies) reducing statistical power; low number of doses tested (often a single dose); different media of exposure (water versus food) limiting the ability to compare study results; inadequate latency period for determining cancer outcomes; and the use of different control groups, which can influence the statistical significance and interpretation of the observed results.
A MAC for asbestos in drinking water is not recommended for the following reasons:
- the available data on oral exposure to asbestos in both humans and animals are insufficient for deriving a HBV in drinking water because of significant limitations in study design and an absence of clear health outcomes
- historical data indicate that asbestos fibres present in drinking water are generally smaller (less than 1 µm) than the those typically associated with adverse health effects in humans
- after ingestion, small fibres present in drinking water are further degraded in the stomach and largely excreted, since the GI tract serves as an effective barrier to their absorption
There are limitations and data gaps in the scientific evidence for the health outcomes associated with oral exposure to asbestos. As part of its ongoing drinking water guideline/guidance review process, Health Canada will continue to monitor new research in this area and recommend any change(s) to this guidance that it deems necessary.
B. 4 Analytical methods
B.4.1 Standardized methods
Standardized methods available for the analysis of asbestos in source and drinking water and their respective AS are summarized in Tables 5a and 5b. ASs are dependent on the sample matrix, instrumentation, and selected operating conditions and will vary between individual laboratories. Quantitative determination of asbestos in water is achieved usingtransmission electron microscopy (TEM) and identification is achieved by energy-dispersive X-ray analysis and selected-area electron diffraction (SAED). These methods are subject to a variety of interferences, which are outlined in the respective references.
As there are no accredited laboratories conducting asbestos analysis in drinking water in Canada, no laboratory was contacted to determine their ASs. There are laboratories located in the U.S. providing analysis for asbestos fibres in drinking water.
Analysis of asbestos fibres should be carried out as directed by the responsible drinking water authority. DWTPs should discuss sampling requirements with the laboratory conducting the analysis to ensure that quality control procedures are met. They should also have an understanding of what the laboratory's minimum reporting levels are, to ensure accurate monitoring at concentrations below the benchmark concentration they select for asbestos.
| Method (Reference) |
Methodology | Analytical sensitivityFootnote a (MFL) | Comments |
|---|---|---|---|
| EPA 100.1 (U.S. EPA, 1983) and recommended modifications (U.S. EPA, 1994b) | TEM, SAED and EDXA | NR | Considers fibres of greater than 0.5 µm in length. In samples with a turbidity of 0.1 NTU or lower, 0.01 MFL can be detected. |
| EPA 100.2 (U.S. EPA, 1994a) | TEM, SAED and EDXA | 0.2 | Quantifies structures over 10 µm in length (that is, those relevant to the U.S. EPA Max Contaminant Level). |
EDXA – energy dispersive X-ray analysis; MFL – million fibres per litre; NTU – nephelometric turbidity unit; NR – not reported; SAED – selected area electron diffraction; TEM – transmission electron microscopy; U.S. EPA – United States Environmental Protection Agency. |
|||
| Method (Reference) |
Methodology | Analytical sensitivity Footnote a (MFL) |
Comments |
| SM 2570 (APHA et al., 2018) | TEM, SAED and EDXA | NR | Quantifies structures over 10 µm in length. |
APHA – American Public Health Association; EDXA – energy dispersive X-ray analysis; MFL – million fibres per litre; NR – not reported; SAED – selected area electron diffraction; TEM – transmission electron microscopy. |
|||
The main difference between EPA Method 100.1 and EPA Method 100.2 is the size cutoff used in the definition of an asbestos fibre. EPA Method 100.1 defines fibres as being greater than 0.5 µm in length while EPA Method 100.2 uses 10 µm as the cutoff (U.S. EPA, 1983; 1994a). However, in both cases, fibres must have an aspect ratio equal or greater than 3:1 (length to width) with parallel sides. SM 2570 is similar to EPA Method 100.2 and only fibres greater than 10 µm in length are counted (APHA et al., 2018). Counting rules are specified in the different methods.
B.4.2 Sample preservation and preparation
Sample processing considerations for the analysis of asbestos in drinking water (for example, sample preservation, storage, preparation) can be found in the references listed in Tables 5a and 5b.
EPA Method 100.1 and its recommended modifications, EPA Method 100.2 and SM 2570 specify the steps required to prepare sampling bottles and to avoid contamination of the samples. Key considerations include using new, pre-cleaned glass or polyethylene bottles (not polypropylene); avoiding the use of preservatives when sampling; transporting the samples to ensure that they are filtered in the laboratory within 48 hours after collection; and avoiding freezing the sample. Refrigeration of the sample is recommended to minimize algal and bacterial growth.
The recommended modifications to EPA Method 100.1 and EPA Method 100.2 state that, for compliance analysis, the required AS is 0.2 MFL. For samples having a turbidity of 0.1 nephelometric turbidity unit (NTU) or lower, this method allows for the detection of a concentration as low as 0.01 MFL.
B.5 Treatment considerations
Sources of asbestos fibres in drinking water may include the source water, by leaching from asbestos mineral deposits, and the distribution system, where fibres can be released from A-C pipes. Control strategies such as maintaining water quality are discussed in section B.6 and can be implemented to minimize the release of asbestos fibres from A-C pipes in drinking water distribution systems.
B.5.1 Municipal-scale treatment
Municipal-scale treatment can be effective at removing asbestos fibres from source water. Conventional treatment (coagulation and filtration) is the main treatment technology proven to remove asbestos fibres from source water. More than 99% of asbestos fibres, either naturally occurring or from anthropogenic sources, can be removed by optimized coagulation and filtration (WHO, 2021). Considering the small size of asbestos fibres, their removal by granular media is unlikely (Logsdon et al., 1983). Instead, Logsdon et al. (1983) identified that asbestos fibres are removed by sedimentation or interception in the filter bed followed by their attachment. For the fibres to adhere to the filter media, they must first have been destabilized sufficiently.
The selection of an appropriate treatment process will depend on many factors, including the raw water source and its characteristics, the operational conditions of the selected treatment method and the water utility's treatment goals. Treatment goals may require that pH be adjusted post-treatment to address corrosion issues in the distribution system (Health Canada, 2015). Bench and pilot-scale testing is critical to ensure the source water can be successfully treated and to optimize operating conditions.
B.5.1.1 Conventional coagulation
An overview of studies based on coagulation for asbestos fibres removal is presented in Table 6. Many of the studies are based in the U.S. in the Lake Superior and Cascade Mountain regions in Washington in the 1970s. Concentration of asbestos fibres in the source water (Lawrence and Zimmermann, 1976; McGuire et al., 1983) and the surface charge of the asbestos fibres have been identified as factors influencing their removal by coagulation (Bales and Morgan, 1985; Lawrence et al., 1975; Parks, 1967; Webber and Covey, 1991). Source water alkalinity, pH and temperature also influence the effectiveness of conventional coagulation for removing asbestos fibres (Lawrence and Zimmermann, 1976). Fibre size also affects their removal, with a greater number of smaller fibres found in the treated water than in the raw water following filtration (Bales et al., 1984; Logsdon and Symons, 1977). Small chrysotile fibres (0.1 to 3.0 µm) could pass through DWTPs designed for turbidity removal (Bales et al., 1984). Logsdon et al. (1983) noted that, for a DWTP to effectively remove asbestos fibres, each unit of the process must be operated effectively. Treated water quality is significantly influenced by coagulant and polyelectrolyte concentrations used during treatment (Lawrence and Zimmermann, 1976).
For chrysotile, the zero point of charge was determined to be around pH 10 to 11, and around pH 5 for cummingtonite, an amphibole asbestos (Logsdon and Symons, 1977; Parks, 1967). Bales and Morgan (1985) observed that suspended chrysotile fibres in an inorganic electrolyte have a positive surface charge at a pH below 8.9. Fibres can also adsorb natural organic matter, at concentrations encountered in natural waters, to acquire a negative charge. Webber and Covey (1991) suggested that this enhances removal by coagulation and sedimentation processes. In water with pH ranging between 6 and 8, it was observed that amphibole fibres had a negative surface charge, while chrysotile fibres had a positive surface charge (Logsdon et al., 1983; Logsdon and Symons, 1977). However, Logsdon et al. (1983) questioned whether the same surface charge would be observed in natural waters. Pilot-scale testing showed that anionic polymers might not be the most effective for the removal of chrysotile asbestos. Lawrence and Zimmermann (1976) obtained the same effectiveness for the removal of chrysotile and cummingtonite when combining non-ionic, cationic or anionic polyelectrolytes with alum. The authors suggested that asbestos fibres are physically removed by alum. For treatment to be effective, Logsdon (1983) summarized that the control of the coagulant dose is important to destabilize the particle charge as well as control the pH during the process.
One study found that reaching a turbidity of 0.1 NTU or less in the treated water did not always result in low asbestos fibre concentrations (< 1 MFL) in the treated water (McGuire et al., 1983). However, work by Logsdon (Logsdon, 1979; 1983; Logsdon et al., 1981; 1983) suggests that turbidity can be a useful indicator of the treated water quality or the filter performance, despite the lack of correlation between turbidity and fibre counts in raw water (Logsdon, 1983; Logsdon et al., 1981). When the filtered water turbidity increases, the likelihood of higher asbestos fibre counts also increases (Logsdon, 1983).
Lawrence et al. (1975) tested the impact of different types of polyelectrolytes on the removal of asbestos fibres through water filtration processes, in combination with ferric chloride: anionic, non-ionic and cationic coagulants. The charge of the polyelectrolyte did not influence the floc formation. Larger flocs, which could better capture the asbestos fibres, were formed with raw water instead of with water filtered by sand filtration. The authors explained that the sand filtration decreased nucleation centres. An optimal dose of 6 to 8 ppm of ferric chloride was determined. Lawrence et al. (1975) also tested the use of bentonite clay and a cationic polyelectrolyte (1 ppm), which resulted in a 99% removal of asbestos fibres, with only chrysotile fibres remaining. Using an anionic polyelectrolyte yielded poor removals. The results can be explained by the surface charges of the different types of asbestos fibres, with chrysotile having a positive surface charge (Lawrence et al., 1975; Parks, 1967). In a Seattle, Washington pilot plant that used liquid aluminum sulphate as the main coagulant, a non-ionic polymer was successfully used for the removal of amphibole fibres, while an anionic polymer performed better for removal of chrysotile fibres (Logsdon, 1979).
Different filter media reported in the studies are listed in Table 6, including anthracite, sand, ilmenite and garnet (Logsdon et al., 1983).
| Influent concentration (MFL) | % removal or treated water (MFL) | Coagulant type and dose | Information on treatment | Information on location and study scale | Reference |
|---|---|---|---|---|---|
| Mean: 690 Footnote a Range: 230 to 1 300 Footnote a | Mean: 8.3 MFL Range: 0.37 to 31 MFL |
Alum: 3.8 to 9.9 mg/L Cationic polymer: 1.4 to 3.0 mg/L |
Dosing of Cl2, alum and cationic polymer, rapid mix, flocculation, sedimentation, dosing of Cl2 and polymer, filtration (coal and sand), addition of Cl2, pH adjustment | F. E. Weymouth filtration plant Full scale |
McGuire et al. (1983) |
| Mean: 650 Range: 280 to 1 300 |
Mean: 35 MFL Range: 0.25 to 200 MFL |
Alum: 2.5 to 6.6 mg/L Cationic polymer: 1.6 to 2.4 mg/L |
Dosing of Cl2, alum and cationic polymer, rapid mix, flocculation, sedimentation, dosing of Cl2 and polymer, filtration (coal and sand), addition of Cl2, pH adjustment | Robert B. Diemer filtration plant Full scale |
McGuire et al. (1983) |
| Mean: 5.7 Range: 0.19 to 45 |
Mean: 0.41 MFL Range: less than DL to 5.6 MFL |
Alum: 0 to 5.0 mg/L Cationic polymer: 0.5 to 1.9 mg/L |
Dosing of Cl2, alum and cationic polymer, rapid mix, flocculation, sedimentation, dosing of Cl2 and polymer, filtration (coal and sand), addition of Cl2, pH adjustment | Joseph Jensen filtration plant Full scale |
McGuire et al. (1983) |
| Mean: 1.9 Range: less than DL to 6.4 |
Mean: 0.31 MFL Range: less than DL to 2.3 MFL |
Alum: 2.6 to 6.4 mg/L Cationic polymer: 0.3 to 3.1 mg/L |
Dosing of Cl2, alum and cationic polymer, rapid mix, flocculation, sedimentation, dosing of Cl2 and polymer, filtration (coal and sand), addition of Cl2, pH adjustment | Robert A. Skinner filtration plant Full scale |
McGuire et al. (1983) |
| Mean: 770 Range: 230 to 1900 |
Mean: 6.7 MFL Range: 0.11 to 0.5 MFL |
Alum: 5.4 to 10.5 mg/L Cationic polymer: 1.5 to 3.2 mg/L |
Dosing of Cl2, alum and cationic polymer, rapid mix, flocculation, sedimentation, dosing of Cl2 and polymer, filtration (coal and sand), addition of Cl2, pH adjustment | Henry J. Mills filtration plant Full scale |
McGuire et al. (1983) |
| Amphibole: 19 to 356 | Amphibole: less than 0.07 to 2.3 MFL 97.1% to 99.9% |
Alum: Dose NR | Direct filtration plant two-stage rapid mix, two-stage flocculation, filtration through mixed media 2.6 mgd |
Two Harbors, Minnesota (Lake Superior) Full scale |
(1979) |
| Amphibole: 1.8 to 25.3 | Amphibole: less than 0.13 to 0.33 MFL 92.8% to 99.29% | Alum: Dose NR | Rapid mixing, 2 flocculation basins in series, filtration on dual media filter (anthracite and sand) | Silver Bay, Minnesota (Lake Superior) Full scale | Logsdon (1979) |
| 1.2 to 15.8 | 0.02 to 0.1 MFL 87.2% to 99.4% |
Ferric chloride: 13.3 mg/L | Rapid mixing where coagulation takes place, flocculation basin, sedimentation basin, rapid sand filters | Torresdale, Philadelphia Full scale | Logsdon (1979) |
| 2.9 to 19.5 | Less than 0.01 to 0.84 MFL 93.1% to greater than 99.7% | Ferric chloride: 7.3 mg/L | Rapid mixing, flocculation basin, sedimentation tanks, rapid sand filters | Queen Lane, Philadelphia Full scale |
Logsdon (1979) |
| 2.5 to 15.2 | Less than 0.01 to 0.28 96.7% to greater than 99.8% | Alum: 15.5 mg/L | Rapid mixing, flocculation basins, sedimentation basins, rapid sand filters | Belmont, Philadelphia Full scale |
Logsdon (1979) |
| Chrysotile: 143 Amphibole: 4.68 |
Chrysotile: 0.07 Amphibole: less than 0.01 | NR | Rapid mix, tapered flocculation, sedimentation, rapid sand filtration | Everett, Washington Pilot scale |
Watkins et al. (1978) |
| Amphibole: Mean: 1.6 Range: less than 0.04 to 5.7 Chrysotile: Mean: 7.1 Range: 1.2 to 25.8 |
Amphibole: less than 0.01 to 0.72 MFL Chrysotile: less than 0.01 to 11.2 MFLv 9.5% to 99.8% |
Alum: 2 to 16 mg/L Cationic polymer: 0.2 to 3.2 mg/L |
Hydraulic rapid mixing, flocculation, tube settlers, granular media filter pH 6.1 to 6.7 | Seattle, Washington Pilot scale |
Logsdon (1979) |
| 1 300 Footnote c | 0.05 MFL Footnote d | Alum: 50 ppm Non-ionic polyelectrolyte: 1 ppm |
Coagulation: 3 min at 100 rev/min Flocculation: 30 min at 30 rev/minv Filtration: on sand or sand anthracite filters at 0.9 L/s |
NR Pilot scale | Lawrence and Zimmermann (1976) |
| 12.3 | 99.8% | Ferric chloride: 10 ppm | After mixing, added 1 ppm polyelectrolyte 100 rev/min, 10 min. Mixing 40 rev/min for 30 min. pH 7.5 plus/minus 0.1 | Lake Superior water Bench scale |
Lawrence et al. (1975) |
| 12.3 | 0.9 MFL (9 x 105 fibres/L) | Ferric chloride: 10 ppm | Sand filtration prior to coagulation | Lake Superior water Bench scale |
Lawrence et al. (1975) |
Cl2 – chlorine; DL – detection limit; MFL – million fibres per liter; NR – not reported; Rev – revolution. |
|||||
A 1979 national survey of Canadian municipalities for asbestos in drinking water quantified the concentration of asbestos fibres in raw and treated water (if greater than 5 MFL) in seven DWTPs (Chatfield and Dillon, 1979). From these data points, Toft et al. (1981) reported removals ranging from 85.9% to 100% after treatment, suggesting that coagulation and filtration were effective at removing chrysotile asbestos fibres. Based on the data from Chatfield and Dillon (1979), removal efficacies greater than 99% were calculated for the DWTPs. It was also noted that the removal efficacy may be related to the time since the last backwash of the filter bed (Toft et al., 1981). No information regarding the treatment processes was provided.
At bench scale, the use of diatomaceous earth filtration was found to be effective for removing asbestos fibres from test waters (Logsdon, 1979). Filtration using different media and particle size ranges lowered the asbestos fibres concentration in samples collected from Lake Superior (see Table 7) (Lawrence et al., 1975).
| Influent (MFL) | Treated water (MFL) | Filter media | Information on filtration |
|---|---|---|---|
| 12.3 | 3.5 | Sand, 0.45 to 0.55 mm particles size range | 25 mm diameter glass tubes Flow rate: 9.7 L/s/m2 |
| 12.3 | 3.5 | Sand, 0.67 to 0.80 mm particles size range | 25 mm diameter glass tubes Flow rate: 9.7 L/s/m2 |
| 12.3 | 3.5 | Sand, 0.81 to 1.55 mm particles size range | 25 mm diameter glass tubes Flow rate: 9.7 L/s/m2 |
| 12.3 | 0.4 | "Brick" sand, 0.0003 to 2.3 mm particle size range | 25 mm diameter glass tubes Flow rate: L/s/m2 |
| 12.3 | 0.33 | Diatomaceous earth filters | Not specified |
MFL – million fibres per litre. |
|||
Alum can be used as a coagulant for the removal of asbestos fibres from source water but can carry over aluminum into the distributed water if coagulation is not optimized. Concentrations of aluminum entering the distribution system from the DWTP should be minimized. For more detailed discussion, refer to Health Canada's Guidelines for Canadian Drinking Water Quality, Guideline Technical Document on Aluminum (Health Canada, 2021).
B.5.2 Residential-scale treatment
In cases where asbestos fibres removal is desired at the household level for example, when a household obtains its drinking water from a private well a residential drinking water treatment unit may be an option. Systems classified as residential scale may have a rated capacity to treat volumes greater than that needed for a single residence. Thus, these systems may also be used in small systems.
Before a treatment unit is installed, the water should be tested to determine the general water chemistry and asbestos fibre concentration in the source water. Periodic testing by an accredited laboratory should be conducted on both the water entering the unit and the treated water, to verify that the treatment unit is effective. Units can lose removal capacity through use and time and need to be maintained and/or replaced. Consumers should verify the expected longevity of the components in the treatment unit according to the manufacturer's recommendations and service it when required. Choosing a unit with a warning system (for example, an alarm or light indicator) will indicate when servicing is required.
Health Canada does not recommend specific brands of drinking water treatment units. However, it is strongly recommended that consumers use units that have been certified by an accredited certification body. The certification makes sure the drinking water treatment unit meets the appropriate NSF International/American National Standards Institute (NSF/ANSI) standards. The purpose of these standards is to establish minimum requirements for the materials, design and construction of drinking water treatment units. The certification of treatment units is conducted by a third party. This certification ensures that materials in the unit do not leach contaminants into the drinking water (in other words, material safety). In addition, the standards include performance requirements that specify the removal that must be achieved for specific contaminants (for example, a reduction claim) that may be present in water supplies.
Certification organizations (in other words, third parties) provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada. Accredited organizations in Canada (SCC, 2025) include:
- CSA Group
- NSF International
- Water Quality Association
- UL Solutions
- Bureau de normalization du Québec (available in French only)
- International Association of Plumbing and Mechanical Officials
- ALS Laboratories
An up-to-date list of accredited certification organizations can be obtained from the Standards Council of Canada.
Certified residential treatment units for the reduction of asbestos fibres from drinking water are currently available.
NSF/ANSI Standard 53 (Drinking water treatment units – Health effects) and NSF/ANSI Standard 58 (Reverse osmosis drinking water treatment systems) are applicable to the reduction of asbestos fibres from drinking water. For a drinking water treatment unit to be certified to Standard 53 and Standard 58 for asbestos fibre reduction, it must be capable of reducing an average influent (incoming) concentration of 107 to 108 fibres per liter by at least 99%. The reduction claim is based on fibres exceeding 10 µm in length, using U.S. EPA Method 100.1 or by analyzing asbestos fibres using TEM or X-ray diffraction. The influent water must be constituted of a 50/50 blend of chrysotile and anthophyllite asbestos (NSF International, 2023a; 2023b).
Water that has been treated using RO and distillation is more likely to be corrosive to internal plumbing components. Also, as large quantities of influent water are needed to obtain the required volume of treated water, these devices are generally not practical for point-of-entry installation. Therefore, these devices should be installed only at the point-of-use.
B.5.3 Distribution system considerations
Distribution systems are complex and the related water quality concerns are inherently challenging. Additional information on distribution system issues and maintenance can be found elsewhere (Health Canada, 2022a; 2022b). A-C pipes are subject to deterioration after prolonged exposure to aggressive water (for example, soft water with very low ion content), due either to the dissolution of lime and other soluble compounds or to the chemical attack by ions such as high sulphate concentrations, which can react with free lime and hydrated calcium aluminates to form calcium sulphate (gypsum) and calcium (Leroy et al., 1996; Chowdhury et al., 2012; Hu and Hubble, 2005; 2007). These factors may contribute to the possible structural failure of A-C pipes. Other factors that influence A-C pipe breaks include their age, diameter, the type of soil present around the pipes and the maintenance methods used (Chowdhury et al., 2012; Hu and Hubble, 2005; 2007).
B.5.3.1 Factors affecting deterioration of A-C pipes
A-C water mains can deteriorate and there is some evidence that erosion of the pipe material can lead to the release of asbestos fibres (Toft et al., 1981; Webber et al., 1989). Asbestos fibres from A-C pipes tend to be longer than asbestos fibres that are naturally eroded in the environment and potentially present in source water (Millette et al., 1980). In some drinking water systems, Toft et al. (1981) observed a significant difference in asbestos fibre concentrations between samples collected in treated water and in distribution systems. In the Winnipeg, Manitoba water system, a progressive increase in asbestos fibre was observed as water travelled through the distribution system. Asbestos fibre concentration in the treated water was between 0.5 and 1.3 MFL and increased to 6.5 MFL in the distribution system. The authors suggested that erosion of A-C pipes was the source of asbestos fibres. Webber et al. ( 1989) found that deteriorated A-C pipes contributed to asbestos fibres concentrations of up to 1 850 MFL in the Woodstock, New York distribution system. The authors suggested that the corrosive groundwater could have deteriorated the A-C pipes.
Deterioration of A-C pipes that were installed in Christchurch, New Zealand was observed by Mager et al. (2022). An average corrosion rate of 0.20 mm per year was observed along with leaching of asbestos fibres and was attributed to the soft and highly aggressive distributed water. For fibres greater than 10 µm, concentrations ranging from under the detection limit and 3.7 MFL were measured in hydrants and from under the detection limit to 6 MFL in households or public taps. The authors attributed the difference in concentrations between hydrants and households to the high flow rate used when flushing the hydrants prior to sampling, which can mobilize asbestos fibres. These limited results seem to indicate that tap sampling may not be an appropriate indicator of A-C pipe deterioration.
A-C pipe corrosion
Corrosion, or dissolution, of A-C pipes is governed by solubility considerations. The A-C pipe matrix is predominated by three phases: tricalcium silicate (Ca3SiO5), dicalcium silicate (Ca2SiO4) and tricalcium aluminate (Ca3Al2O6) (Schock and Buelow, 1981; Schock et al., 1981). Free lime or calcium hydroxide [Ca(OH)2] is also present in the matrix. Water with low pH (less than approximately 7.5 or 8.0, unless they contained high calcium and alkalinity concentration) and very high sulphate and polyphosphate concentrations are especially corrosive to A-C pipes (Leroy et al., 1996).
Hard waters are generally less corrosive than soft waters. High levels of calcium help stabilize calcium silicate phases in A-C pipes and promote the formation of calcium carbonate to block the release of free lime (calcium hydroxide) (Leroy et al., 1996). In soft waters with low mineral content, the calcium hydroxide is first released from the pipe by dissolution. Calcium carbonate is dissolved or not formed, depending on the acid content of the water. The hydrated calcium silicates are then converted to calcium carbonate (Leroy et al., 1996). When calcium is leached from the A-C pipes, asbestos fibres can be released in the water (Zavasnik et al., 2022).
The dissolution of A-C pipes can have the following effect on the distributed water (Leroy et al., 1996):
- increase in levels of calcium, aluminum and silicate species in solution
- increase in pH due to several of the dissolution reactions and the dissociations of silicic acid; dissolution of calcium hydroxide also releases OH-
- increase in alkalinity of the water in the distribution system, as the pH increase favours the formation of bicarbonate and carbonate ions
- other ions such as OH-, SiO(OH)3- and Si2O2(OH)22- also contribute directly to the alkalinity
A loss in the mechanical stability of the A-C pipes can occur as the inner wall of the pipe deteriorates (Zavasnik et al., 2022). This can result in fractures and leakages of the A-C pipes (Slaats et al., 2004). Microorganisms can also cause structural damage to A-C pipes by generating organic acids promoting the leaching of free lime and dissolution of calcium (Wang et al., 2011).
Precipitation reactions in distribution systems
The effects of precipitation reactions might be difficult to predict in drinking water distribution systems. Change in pH in the distributed water may also be mitigated by the buffering capacity of the water. Very large and localized pH increases may cause the deposition of CaCO3, which would have a protective effect on the A-C pipe. Nevertheless, pH and calcium increases in the distributed water with increasing distance from the DWTP are good evidence of A-C pipe dissolution. The A-C pipe matrix can also stabilize since calcium carbonate deposits can protect the cement material making changes in pH and calcium concentration less significant (Leroy et al., 1996). Although changes in pH and calcium concentration in the distributed water can indicate A-C pipe dissolution, the changes may not be a direct indicator of asbestos fibre release and should be interpreted with caution (Leroy et al., 1996).
A-C pipe age and flow considerations
Assessment of deterioration of A-C pipes should consider the pipe age as well as flow rate in the distribution system. In particular, even in non-aggressive water, water mains located in dead-ends and experiencing long residence time can deteriorate (Webber and Covey, 1991). Asbestos fibres accumulated in dead-ends of distribution systems can also be released during main flushing (Logsdon, 1983; Webber et al., 1989).
High water flows can also lead to high asbestos fibre counts in distribution systems, up to 100 times higher than normal concentrations. Therefore, drinking water main flushing should be conducted in a way that thoroughly removes debris and does not simply result in stirring up sediments (Logsdon, 1983). Webber and Covey (1991) mention that flushing of A-C pipes that are already deteriorated can increase the presence of asbestos fibres. The authors recommend ruling out deteriorated A-C pipes as the source of asbestos fibres prior to implementing a flushing program in a drinking water distribution system containing A-C pipes. When significant deterioration of A-C pipes is observed, flow reversal or elevated water flow can shear off asbestos fibres and is generally not recommended.
B.5.3.2 Indices
When A-C pipes are exposed to water of varying chemistries, there is no simple index that can predict their behaviour (Leroy et al., 1996). Despite this, the prediction of the threshold for calcium carbonate formation is important. When water was at the calcite saturation levels, it was observed that the integrity of the A-C pipe was conserved (Schock and Buelow, 1981).
The Langelier Saturation Index (LSI) is based on the effect of pH on the solubility of CaCO3 at equilibrium. The pH at which a water is saturated with CaCO3 is known as the pH of saturation (pHs). At pHs (LSI equal to 0), a CaCO3 scale is not expected to be deposited nor dissolved. It has also been determined that the pH of saturation may be inaccurate when calcium concentrations and alkalinity are low (Buelow et al., 1980).
The LSI is defined by the equation:
LSI = pH pHs
At an LSI greater than 0: Water is supersaturated and can precipitate a scale layer of CaCO3.
At an LSI equal to 0: Water is saturated (in equilibrium) with CaCO3 and no scale layer is precipitated or dissolved.
At an LSI less than 0: Water is undersaturated and can dissolve solid CaCO3 (Schock and Lytle, 2011).
The Aggressiveness Index (AI) is used as an indicator of the corrosiveness of water but might not be a predictor for release of asbestos fibres (Millette et al., 1984) or predict the behaviour of A-C pipes (Buelow et al., 1980). The original purpose of the AI was to outline water quality conditions that might lead to structural failures (Hu and Hubble, 2007; Schock et al., 1981). Schock et al. (1981) also found that the AI tended to be overly conservative in its prediction of water corrosiveness. The limitations of the AI include (Schock and Buelow, 1981):
- temperature dependence of the solubility constant for calcite
- assumption that dissolution of Portland cement, which is silicious, can be accurately represented by calcite may be false
- it does not consider complexation reactions (that is, with polyphosphate additives) that can limit free ion activities of calcium and carbonate ions
- it does not consider the formation of protective precipitates
- it does not consider the effect of pipe coatings
B.5.3.3 Passivation of A-C pipes
A-C pipes can be protected from dissolution by the formation of a passivation film coating the inside of the pipe. Calcium carbonate can be deposited at the surface of the pipe wall, which can reduce free lime leaching from the A-C pipe. Deposition of calcium carbonate depends on the pH, hardness and alkalinity of the distributed water, with high buffer capacity and alkalinity favouring deposition (Leroy et al., 1996). Leroy et al. (1996) also summarized multiple studies on the internal corrosion of A-C pipes and highlighted that different types of deposits containing iron, manganese, magnesium, calcium carbonate, zinc and colloidal humic matter can act as a barrier for calcium removal. For example, adding chloride, sulphate or orthophosphate salts of zinc can provide a protective zinc coating inside A-C pipes when they are added to the water at the proper concentration and pH ranges and when those chemical conditions are maintained throughout the distribution system. The zinc-hydroxy-carbonate precipitate formed at the pipe surface can protect it and prevent the release of asbestos fibres (Schock and Buelow, 1981). Albertin et al. (1992) observed that A-C pipes covered by inorganic deposits, consisting of carbonates, sulphates and silicates, released significant amounts of asbestos fibres when in contact with aggressive waters.
B.5.3.4 Distribution system repairs
Physical work on A-C pipes can release asbestos fibres in the distributed water, for example, during improper tapping or cutting operations. Tapping operations of degraded A-C pipes have been linked to an increase in asbestos fibres in distribution systems (Webber et al., 1989). Mechanical vibration of the A-C pipe, caused by traffic, construction or other sources, can also affect the release of asbestos fibres in the distributed water by degrading the calcium deposits and structural component of the pipe (Zavasnik et al., 2022). Water main flushing can generally remove fibres that were released (Logsdon, 1983; Webber and Covey, 1991).
B.5.4 Residuals management
Treatment technologies may produce a variety of residuals that contain asbestos (for example, backwash water, reject water/concentrate and media waste). The appropriate authorities should be consulted to ensure that the disposal of liquid and solid waste residuals from the treatment of drinking water meet applicable regulations. Guidance on residuals management can be found elsewhere (CCME, 2003; 2007).
B.6 Management strategies
Although a MAC is not recommended, given public concern with asbestos and the goal of minimizing particle loading in treated drinking water to effectively operate the distribution system, it is recommended to use best practices to minimize the concentrations of asbestos fibres in drinking water. In general, all DWTPs should implement a comprehensive, up-to-date risk water safety plan or other risk-based source-to-tap water management framework (CCME, 2004; WHO, 2012; 2017). These approaches require a system assessment to characterize the source water; describe the treatment barriers that prevent or reduce contamination; identify the conditions that can result in contamination; and implement control measures. Operational monitoring is then established, and operational/management protocols are instituted (for example, standard operating procedures, corrective actions and incident responses). Compliance monitoring is determined and other protocols to validate the water safety plan are implemented (for example, record keeping, consumer satisfaction). Operator training is also required to ensure the effectiveness of the water safety plan (Smeets et al., 2009).
B.6.1 Control strategies
In water sources with high asbestos fibre concentrations, conventional water treatment can be implemented and optimized for asbestos fibre removal. Non-treatment options such as alternative water supplies can also be considered. If a treatment technology is chosen, pilot-scale testing is recommended to ensure that the source water can be successfully treated and the process design established. Attention must be given to the overall water quality of a new source prior to making any changes (that is, switching, blending, and interconnecting) to an existing water supply. For example, if the new water source is more aggressive, it may cause leaching of lead or copper in the distribution system.
Generally, the distribution system should be managed such that drinking water is transported from the DWTP to the consumer with minimum loss of quality. As source waters, DWTPs and distribution systems can differ significantly, control strategies are system-specific.
If A-C pipes are present in a drinking water distribution system, distributed water quality should be protective against their dissolution and subsequent leaching of asbestos fibres into the distributed water (Logsdon, 1983). Low pH and low alkalinity water should be modified to ensure that it is not aggressive towards A-C pipes. Also, pipe walls can be passivated by chemical precipitation, or a cement mortar lining can be applied to the pipe wall to seal the asbestos fibres against erosion (Leroy et al., 1996; Logsdon, 1983). In the Woodstock, New York distribution system, the removal of deteriorated A-C pipes lowered asbestos fibre concentrations where elevated levels had been measured (Webber et al., 1989).
Water main flushing can generally remove fibres that were released into the distributed water -- for example, from cutting an A-C pipe or improper tapping (Logsdon, 1983; Webber and Covey, 1991). Prior to initiating a flushing program in a drinking water distribution system with A-C pipes, DWTPs should determine if they are a source of asbestos fibres. Flushing should remove the debris without stirring up sediments. Flow reversal or elevated water flow are generally not recommended when there is significant deterioration of A-C pipe.
Removal of A-C pipes should follow all relevant health and safety regulations to ensure worker safety. Best practices include minimizing the release of asbestos fibres when the pipes are cut to protect workers from the risk of inhalation.
B.6.2 Monitoring
B.6.2.1 Source water
Water sources should be characterized to determine if asbestos fibres are present, particularly if they are near areas where asbestos is known or suspected to be present geologically.
Monitoring of source water should be conducted yearly. Authorities may consider reduced monitoring when it has been demonstrated that asbestos is not present and/or appropriate treatment is in place.
B.6.2.2 Operational and treatment
Where treatment is required to remove asbestos fibres, operational monitoring should be implemented to confirm whether the treatment process is functioning as required (that is, paired samples of source and treated water to confirm the efficacy of treatment). The frequency of operational monitoring will depend on the treatment process.
B.6.2.3 Distribution system
Information from monitoring of asbestos fibres in drinking water can inform decisions about infrastructure replacement plans, as well as support communication with customers about water quality.
Most A-C pipes were installed many decades ago (from the 1940s until the late 1970s) and are at or near the end of their useful life span (Chu et al., 2008). As such, water systems should work to identify areas where A-C pipes are in use and assess their condition, to inform an infrastructure replacement schedule. Since asbestos fibres can be released from A-C pipes present in drinking water distribution systems, monitoring should be conducted in the distribution system in areas where A-C pipes are known to be present. This is particularly important for systems with aggressive water quality (such as low pH), since aggressive water could affect the integrity of the pipes. Monitoring should also be conducted when asbestos fibres are present in the source and at the entry point to the distribution system or in the distributed water. DWTPs that have baseline data indicating that asbestos fibres are not present within the distribution system may conduct less frequent monitoring.
Monitoring programs should be designed on a system-specific basis to verify that control strategies are operating as intended and consider risk factors that contribute to the likelihood that asbestos fibres may be elevated within the drinking water system. Factors that influence asbestos fibre release, such as changes to water chemistry, physical/hydraulic disturbances or pipe breaks in the distribution system, could be used as indicators of when and where to monitor for release of asbestos fibres.
Monitoring of water at hydrant mains for the presence of asbestos fibres can provide a good indication of pipe decay (Mager et al., 2022). When the concentration of calcium and the pH increase with increasing distance travelled within distribution systems, it can be an indicator of A-C pipe deterioration (Leroy et al., 1996). However, the pH of the water can be influenced by other reactions in the water and is not necessarily an indication of the release of asbestos fibres (Leroy et al., 1996). Results for asbestos fibres obtained from hydrant mains, in conjunction with water quality results (such as pH and calcium) could be used to prioritize and plan A-C pipe replacement (Leroy et al., 1996; Mager et al., 2022).
Structural integrity of water mains can also be monitored. A sample section of A-C pipes can be removed to determine its level of deterioration and different tests can be carried out (Hu et al., 2008), although this type of testing is destructive. General conditions and defects can be visually observed. A scratch test can be used to evaluate the softening of the interior of an A-C pipe (Buelow et al., 1980). The Barcol hardness test includes a hardness measurement and durometer readings that provide information about the depth to which calcium has been lost from the pipe surface (Hu et al., 2008; Millette et al., 1984). The condition of A-C pipes can also be tested using the phenolphthalein test, which provides information on lime leaching (Leroy et al., 1996). Elemental analysis of the interior of the pipe walls can also provide information on leaching of lime and presence of calcium (Hu et al., 2008). A pipe strength test can provide information about its residual resistance, and it can be compared against standards (Hu et al., 2008).
Non-destructive testing can also be conducted to assess the level of deterioration of A-C pipes, such as the Georadar technique (Slaats et al., 2004). The water mains can remain in operation during this testing, but they must be uncovered (Hu et al., 2008).
Models have been developed to predict water main breaks based on historical information. Such models can be an alternative in order to identify water main sections of the distribution system that could have deteriorated (Hu et al., 2008; Kleiner and Rajani, 2001).
B.6.2.4 Residential
Households with private wells may wish to have their water tested to establish if asbestos is found in their water supply. In cases where removal of asbestos fibres is desired, there are certified drinking water treatment devices capable of removing asbestos fibres from drinking water. The technologies certified to the NSF standards include carbon-based filters and RO systems.
Although there is no MAC for asbestos, if it is detected, well owners may prefer, for personal reasons, to take mitigation measures such as installing a treatment device.
B.7 International considerations
The U.S. EPA has established a maximum contaminant level (MCL) of 7 MFL of water for fibres greater that 10 µm in size. The MCL was established based on the consideration that this level of protection would not cause an increased risk of developing benign intestinal polyps. The MCL was also established at 7 MFL given that this was the lowest level to which water systems could reasonably be required to remove asbestos from drinking water using the current technology and resources at that time (U.S. EPA, 1995).
The WHO has not established a guideline value for asbestos fibres in drinking water since the current available epidemiological studies have limitations that make them unsuitable for deriving a guideline value. However, given the uncertainties in the data, the WHO recommends that the concentrations of asbestos fibres in drinking water be minimized as far as is practical. Where A-C materials are used, such as in pipes and storage containers, degradation and release of fibres into drinking water should be minimized by controlling water corrosivity or by coating A-C pipes with suitable structural linings. As these materials fail or deteriorate significantly, they should be replaced with new asbestos-free materials. The WHO also recommends that, in view of the limited data available on occurrence of asbestos in drinking water, investigative monitoring should be considered, to provide additional information on the contribution of older A-C pipes to numbers, types, size and shape of fibres in drinking water (WHO, 2021).
In Australia, a drinking water guideline for asbestos has not been established, since the data are insufficient to determine a guideline value and it is unlikely that the number of asbestos fibres present in most drinking water supplies would be a health concern. Additionally, the weight of evidence indicates that ingested asbestos is not hazardous to health. The Australian Asbestos Safety and Eradication Agency discusses the management of ageing A-C pipes as an ongoing issue for many Australian water agencies. The factors to be considered when deciding how to eliminate or minimize asbestos exposure risks include the location and condition of A-C pipes, the practicality of different management methods, and the availability of asbestos removalists and asbestos disposal facilities (NHMRC and NRMMC, 2011).
The European Union (EU), following WHO recommendations, determined that it is not necessary to include asbestos fibres in Annex I to the Drinking Water Directive. The EU recommends that A-C pipes should no longer be used or approved for drinking water and that a comprehensive renovation and asbestos removal plan for drinking water distribution networks in European nations be implemented. Member States are encouraged to carry out regular monitoring of source water quality (EU, 2021).
Part C. References, abbreviations and appendices
C.1 References
Ahmad, T., Shaban, I. A., Zayed, T. (2023). A review of climatic impacts on water main deterioration. Urban Clim. 49:101552.
Albertin, P., Bixio, V., Navazio, G., Simioni F. (1992). Water aggressiveness and hydrodynamic effects on the fibres release in asbestos-cement pipes for public water supply. Int J Environ Pollut. 2(1-2):122-133.
ALS Laboratory Group. (2023). TEM Report CG2304329. City of Medicine Hat, Calgary, Alberta.
Amacher, D. E., Alarif, A., Epstein, S. S. (1974). Effects of ingested chrysotile on DNA synthesis in the gastrointestinal tract and liver of the rat. Environ Health Perspect. 9:319-324.
Amacher, D. E., Alarif, A., Epstein, S. S. (1975). The dose-dependent effects of ingested chrysotile on DNA synthesis in the gastrointestinal tract, liver, and pancreas of the rat. Environ Res. 10(2):208-216. doi:10.1016/0013-9351(75)90084-5
Andersen, A., Glattre, E., Johansen, B. V. (1993). Incidence of cancer among lighthouse keepers exposed to asbestos in drinking water. Am J of Epidemiol. 138(9):682-687. Available at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med3&NEWS=N&AN=8237983
ANSES. (2021). Revue systématique de la littérature visant à dresser un état des lieux des connaissances actuelles sur la caractérisation du danger lié à l'ingestion d'amiante (2018-SA0001). Agence Nationale de Sécurité Sanitaire de l'alimentation, de l'environnement et du travail Maisons-Alfort. Anses, 340 p.
APHA, AWWA, WEF. (2018). 2570 Asbestos. Standard Methods For the Examination of Water and Wastewater 23rd edition (Online version). The American Public Health Association, the American Water Works Association and the Water Environment Federation. Amer Pub Health Ass.
ATSDR. (2001). Toxicological Profile for Asbestos (TP-61). Agency for Toxic Substances and Disease Registry. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service. Available at: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=30&tid=4
Aust, A. E., Cook, P. M., Dodson, R. F. (2011). Morphological and chemical mechanisms of elongated mineral particle toxicities. J Toxicol and Environ Health. Part B, 14(1-4):40-75. doi:10.1080/10937404.2011.556046
Avataneo, C., Petriglieri, J. R., Capella, S., Tomatis, M., Luiso, M., Marangoni, G., Lazzari, E., Tinazzi, S., Lasagna, M., De Luca, D. E., Bergamini, M., Belluso, E., Turci, F. (2022). Chrysotile asbestos migration in air from contaminated water: An experimental simulation. J Hazardous Mat. 424(424). doi:10.1016/j.jhazmat.2021.127528
Bacon, D. W. Coomes, O. T., Marsan, A. A., Rowlands, N. (1986). Assessing potential sources of asbestos fibers in water supplies of S.E. Quebec. Water Resources Bulletin, American Water Resources Association. 22(1):29-38.
Bales, R. C., Morgan, J. J. (1985). Surface charge and adsorption properties of chrysotile asbestos in natural waters. Environ Sci Technol. 19(12):1213-1219.
Bales, R. C., Newkirk, D. D., Hayward, S. B. (1984). Chrysotile asbestos in California surface waters: From upstream rivers through water treatment. J Am Water Works Assoc. 76(5):66-74.
Both, K., Henderson, D. W., Turner, D. R. (1994). Asbestos and erionite fibres can induce mutations in human lymphocytes that result in loss of heterozygosity. Inter J Cancer. 59:538-542. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1002/ijc.2910590417
Both, K., Turner, D. R., Henderson, D. W. (1995). Loss of heterozygosity in asbestos‐induced mutations in a human mesothelioma cell line. Environ and Mol Mut. 26(1):1-93. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1002/em.2850260110
Brandi, G., Tavolari, S. (2020). Asbestos and intrahepatic cholangiosarcoma. Cells. 9(2), 421. https://doi.org/10.3390/cells9020421
British Columbia Ministry of Health. (2023). Personal communication with D. Fishwick.
Browne, M. L., Varadarajulu, D., Lewis-Michl, E., Fitzgerald, E. F. (2005). Cancer incidence and asbestos in drinking water, town of Woodstock, New York, 1980-1998. Environ Res. 98(2):224-232. Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med6&NEWS=N&AN=15820729
Buelow, R. W., Millette, J. R., McFarren, E. F., Symons, J. M. (1980). The behaviour of asbestos-cement pipe under various water quality conditions: a processes report. J Am Water Works Assoc. 72(2):91-102.
Canada Labour Code. (1986). Canada Occupational Health and Safety Regulations: SOR/86-304. Canada Gazette Part II, 120(6). Retrieved from the Canada Gazette archives: https://recherche-collection-search.bac-lac.gc.ca/eng/Home/Record?app=cangaz&IdNumber=14146&ecopy=cgc_p2-0_v120_n006_t000_000_19860319_p00264
CCME. (2003). Guidance on the site-specific application of water quality guidelines in Canada: Procedures for deriving numerical water quality objectives. Winnipeg, Manitoba: Canadian Council of Ministers of the Environment.
CCME. (2004). From source to tap: Guidance on the multi-barrier approach to safe drinking water. Winnipeg, Manitoba: Canadian Council of Ministers of the Environment.
CCME. (2007). A protocol for the derivation of water quality guidelines for the protection of aquatic life. Winnipeg, Manitoba: Canadian Council of Ministers of the Environment.
Chao, C. C, Aust, A. E. (1994). Effect of long-term removal of iron from asbestos by desferrioxamine B on subsequent mobilization by other chelators and induction of DNA single-strand breaks. Arch. Biochem. Biophys. 308(1):64-69.
Chatfield, E. J., Dillon, M. J. (1979). A national survey for asbestos fibres in Canadian drinking water supplies. Ottawa, Ontario: Environmental Health Directorate, Health Protection Branch, Minister of National Health and Welfare. No. 79-EHD-34.
Chatfield, E. J., Glass, R. W., Dillon, M. J. (1978). Preparation of water samples for asbestos fiber counting by electron microscopy. Athens, Georgia: Environmental Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency. No. EPA-600/4-78-011.
Cheng, T. J., More, S. L., Maddaloni, M. A., Fung, E. S. (2021). Evaluation of potential gastrointestinal carcinogenicity associated with the ingestion of asbestos. Rev Environ Health. 36(1):15-26.
Chowdhury, R., Hu, Y., Wang, D. (2012). Condition evaluation of asbestos cement water mains. No. 0784-412480.
City of Medicine Hat. (2023). Water test results show no asbestos fibres in Medicine Hat water system.April 24, 2023. Media relations, Medicine Hat, Alberta. Available at: https://www.medicinehat.ca/en/news/water-test-results-show-no-asbestos-fibres-in-medicine-hat-water-system.aspx
City of Regina. (2023). Personal communication with S. Wellman.
Cook, P. M. (1983). Review of published studies on gut penetration by ingested asbestos fibers. Environ Health Persp. 53:121-130.
Cook, P. M., Glass, G. E., Tucker, J. H. (1974). Asbestiform amphibole minerals: Detection and measurement of high concentrations in municipal water supplies. Science. 185(4154):853-855.
Cook, P. M., Olson, G. F. (1979). Ingested mineral fibers: elimination in human urine. Science. 204(4389):195-198.
Cunningham, H. M., Pontefract, R. (1971). Asbestos Fibres in Beverages and Drinking Water. Nature. 232(5309):332-333.
Department of Environment and Conservation of Newfoundland and Labrador. (2012). Asbestos in public drinking water supplies in Newfoundland and Labrador.
Department of Environment and Conservation of Newfoundland and Labrador. (2023). Personal communication with R. Harvey.
DiPaolo, J. A., DeMarinis, A. J., Doniger, J. (1983). Asbestos and benzo(a)pyrene synergism in the transformation of syrian hamster embryo cells. Pharmacol. 27(2):65-73.
Donham, K. J., Berg, J. W., Will, L. A., Leininger, J. R. (1980). The effects of long-term ingestion of asbestos on the colon of F344 rats. Cancer, 45(5 Suppl):1073-1084. doi:10.1002/1097-0142(19800315)45:5
Dopp, E., Schiffmann, D. (1998). Analysis of chromosomal alterations induced by asbestos and ceramic fibers. Toxicol Letters. 96-97:155-162. Available at: https://www.scopus.com/record/display.uri?eid=2-s2.0-0031690962&origin=resultslist&sort=plf-f&src=s&sid=8f7beb8b65750492ffcc4256d754ad0e&sot=b&sdt=cl&s=FIRSTAUTH%28Dopp%29&sl=15&sessionSearchId=8f7beb8b65750492ffcc4256d754ad0e&relpos=9
Dopp, E., Schuler, M., Schiffmann, D., Eastmond, D. A. (1997). Induction of micronuclei, hyperdiploidy and chromosomal breakage affecting the centric/pericentric regions of chromosomes 1 and 9 in human amniotic fluid cells after treatment with asbestos and ceramic fibers. Mut Res. 377(1):77-87. doi:10.1016/s0027-5107(97)00062-6
Duncan, K. E., Ghio, A. J., Dailey, L. A., Bern, A. M., Gibbs-Flournoy, E. A., Padilla-Carlin, D. J., Roggli, V. L., Devlin, R. B. (2010). Effect of size fractionation on the toxicity of amosite and libby amphibole asbestos. Toxicol Sci. 118(2):420-434. doi:10.1093/toxsci/kfq281
Emerit, I., Jaurand, M. C., Saint-Etienne, L., Levy, A. (1991). Formation of a clastogenic factor by asbestos-treated rat pleural mesothelial cells. Agents and Actions. 34(3-4):410-415. doi:10.1007/BF01988737
Epstein, S. S., Varnes, M. (1976). The short-term effects of ingested chrysotile asbestos on DNA synthesis in the pancreas and other organs of a primate. Experientia 32(5):602-604. doi:10.1007/BF01990187
EU. (2021). European Parliament. Protecting workers from asbestos. European Parliament resolution of 20 October 2021 with recommendations to the Commission on protecting workers from asbestos (2019/2182(INL)). European Union. Available at: https://www.europarl.europa.eu/doceo/document/TA-9-2021-0427_EN.pdf
Fenton, S., Rydz, E., Demers, P. A., Peters, C. E. (2023). Prevalence and level of occupational exposure to asbestos in Canada in 2016. Annals of Work Exp and Health. 67:536-545. doi:https://doi.org/10.1093/annweh/wxac077
Ghio, A. J., LeFurgey, A., Roggli, V. L. (1997). In vivo accumulation of iron on crocidolite is associated with decrements in oxidant generation by the fiber. J Toxicol Environ Health. 50(2):125–142.
Go, J., Farhat, N., Leingartner, K., Insel, E. I., Momoli, F., Carrier, R., Krewski, D. (2024). Review of epidemiological and toxicological studies on health effects from ingestion of asbestos in drinking water. Crit Rev in Toxicol. 1–39. https://doi.org/10.1080/10408444.2024.2399840
Gross, P., Harley, R. A., Swinburne, L. M., Davis, J. M., Greene, W. B. (1974). Ingested mineral fibers. do they penetrate tissue or cause cancer? Arch of Environ Health. 29(6):341-347. doi:10.1080/00039896.1974.10666612
Haque, A. K., Ali, I., Vrazel, D. M., Uchida, T. (2001). Chrysotile asbestos fibers detected in the newborn pups following gavage feeding of pregnant mice. J Toxicol and Environ Health. Part A, 62(1):23-31. Available at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=11205533
Hardy, R. J., Highsmith, V. R., Costa, D. L., Krewer, J. A. (1992). Indoor asbestos concentrations associated with the use of asbestos- contaminated tap water in portable home humidifiers. Environ Sci and Technol. 26(4):680-689. doi:10.1021/es00028a004
Hasanoglu, H. C., Bayram, E., Hasanoglu, A., Demirag, F. (2008). Orally ingested chrysotile asbestos affects rat lungs and pleura. Arch of Environ & Occup Health. 63(2):71-75. doi:10.3200/AEOH.63.2.71-75
Health Canada. (1989). Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Asbestos. Health Canada. Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-asbestos.html
Health Canada. (2015). Guidelines for Canadian drinking water quality: Guideline technical document - pH. Ottawa, Ontario: Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada. No. H144-28/2016E-PDF.
Health Canada. (2021). Guidelines for Canadian Drinking Water Quality: Guideline Technical Document - Aluminum. Ottawa, Ontario: Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada. No. H144-13/18-2021E-PDF.
Health Canada. (2022a). Guidance on monitoring the biological stability of drinking water in distribution systems. Ottawa, Ontario: Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada. No. H144-96/2022E-PDF.
Health Canada. (2022b). Guidance on sampling and mitigation measures for controlling lead corrosion. Ottawa, Ontario: Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada.
Hong, Y. C., Choi, S. S. (1997). Cytotoxicity and multinucleate giant cell formation in chinese hamster lung fibroblast caused by crocidolite and chrysotile. J of Korean Medi Sci. 12(2):99-104. doi:10.3346/jkms.1997.12.2.99
Houle, D., Marty, C., Agustin, F., Dermont, G., Gagnon, C. (2020). Impact of climate change on soil hydro-climatic conditions and base cations weathering rates in forested watersheds in Eastern Canada. Front For Global Change. 3:535397.
Hu, Y., Hubble, D. W. (2005). Failure conditions of asbestos cement water mains in Regina. In : Proceedings of the 33rd Annual Conference of the Canadian Society of Civil Engineering (CSCE), Toronto, Ontario, June 2, 2005. Publication date: 2005-2001, p. 2001–2010.
Hu, Y., Hubble, D. W. (2007). Factors contributing to the failure of asbestos cement water mains. Can J Civ Eng. 34(5):608-621.
Hu,Y., Wang, D. L., Cossitt, K. (2008). Asbestos cement water mains: history, current state, and future planning. In: Proceedings of the 60th Annual Western Canada Water and Wastewater Association Conference, Regina, Saskatchewan, 23 September, 2008. Publication date: 2008-2023, p. 2001–2013.
Hudak, P. F., Sadler, B., Hunter, B. A. (1998). Analyzing underground water-pipe breaks in residual soils. Water Eng & Man. 145(12):15-19.
IARC. (2012). Arsenic, metals, fibres, and dusts - a review of human carcinogens. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100C. International Agency for Research on Cancer. World Health Organization, Lyon, France, p. 219-309.
Indigenous Services Canada. (2023). Personal communication with D. Poulin.
IPCS. (1986). Asbestos and other natural mineral fibres. Environmental Health Criteria 53. International Programme on Chemical Safety. Available at: https://www.inchem.org/documents/ehc/ehc/ehc53.htm
Jacobs, R., Dodgson, K. S., Richards, R. J. (1977). A preliminary study of biochemical changes in the rat small intestine following long-term ingestion of chrysotile asbestos. Br J Exp Pathol. 58(5):541-548.
Jacobs, R., Humphrys, J., Dodgson, K. S., Richards, R. J. (1978). Light and electron microscope studies of the rat digestive tract following prolonged and short-term ingestion of chrysotile asbestos. British Journal of Experimental Pathology, 59(5):443-453.
Jacobs, R., Weinzweig, M., Dodgson, K. S., Richards, R. J. (1978). Nucleic acid metabolism in the rat following short-term and prolonged ingestion of chrysotile asbestos or cigarette-smoke condensate. Br J Exp Pathol. 59(6):594-600.
Jaurand, M. C., Kheuang, L., Magne, L.,, Bignon, J. (1986). Chromosomal changes induced by chrysotile fibres or benzo-3,4-pyrene in rat pleural mesothelial cells. Mut Res/Gen Toxicol. 169(3):141-148.
Khaliullin, T. O., Kisin, E. R., Guppi, S., Yanamala, N., Zhernovkov, V., Shvedova, A. A. (2020). Differential responses of murine alveolar macrophages to elongate mineral particles of asbestos and non-asbestos varieties: Cytotoxicity, cytokine secretion and transcriptional changes. Toxicol and Applied Pharmacol. 409, 115302. doi:10.1016/j.taap.2020.115302
Kjaerheim, K., Ulvestad, B., Martinsen, J. I., Andersen, A. (2005). Cancer of the gastrointestinal tract and exposure to asbestos in drinking water among lighthouse keepers (Norway). Can Causes & Control: CCC. 16(5):593-598. Available at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med6&NEWS=N&AN=15986115
Kleiner, Y., Rajani, B. (2001). Comprehensive review of structural deterioration of water mains: statistical models. Urban water. 3(3):131-150.
Korkina, L. G., Durnev, A. D., Suslova, T. B., Cheremisina, Z. P., Daugel-Dauge, N. O., Afanas'ev, I. B. (1992). Oxygen radical-mediated mutagenic effect of asbestos on human lymphocytes: Suppression by oxygen radical scavengers. Mut Res. 265(2):245-253. doi:10.1016/0027-5107(92)90053-5
Krewski, D., Al-Zoughool, M., Bird, M., Birkett, N., Billard, M., Milton, B., Rice, J., Cogliano, V., Hill, M., Little, J., Zielinski, J. M. (2019). Chapter 22, Analysis of key characteristics of human carcinogens. In: Baan R, Stewart B, Straif K, editors. Tumour Site Concordance and Mechanisms of Carcinogenesis - IARC Scientific Publications, No 165. Lyon, France: International Agency for Research on Cancer.
Lavappa, K. S., Fu, M. M., Epstein, S. S. (1975). Cytogenetic studies on chrysotile asbestos. Environmental Research, 10(2):165-173. doi:10.1016/0013-9351(75)90080-8
Lawrence, J., Tosine, H. M., Zimmermann, H. W., Pang, T. W. S. (1975). Removal of asbestos fibres from potable water by coagulation and filtration. Water Res. 9(4):397-400.
Lawrence, J., Zimmermann, H. W. (1976). Potable water treatment for some asbestiform minerals: optimization and turbidity data. Water Res. 10(3):195-198.
Leroy, P., Schock, M.R., Wagner, I., Holtschulte, H. (1996). Chapter 7: Cement-based materials. Internal corrosion of water distribution systems 2nd edition. Denver, Colorado: Amer Water Works Ass Res Found and DVGW Technologiezentrum Wasser.
LEX Scientific. (2023). Technical memorandum, Asbestos in drinking water. Guelph, Ontario.
Logsdon, G. S. (1979). Water filtration for asbestos removal. Cincinnati, Ohio: United States Environmental Protection Agency, Municipal Environmental Research Laboratory. No. EPA-600/2-79-206.
Logsdon, G. S. (1983). Engineering and operating approaches for controlling asbestos fibers in drinking water. Environ Health Persp. 53:169-176.
Logsdon, G. S., Evavold, G. L., Patton, J. L., Watkins, J. (1983). Filter plant design for asbestos fibre removal. J Environ Eng. 109(4):900-914.
Logsdon, G. S., Symons, J. M. (1977). Removal of asbestiform fibres by water filtration. J Am Water Works Assoc. 69(9):499-506.
Logsdon, G. S., Symons, J. M., Sorg, T. J. (1981). Monitoring water filter for asbestos removal. Journal of the Environmental Engineering Division. 107(6):1297-1315.
Mager, S., Knopick, M., Oddy, G. (2022). The concentration and prevalence of asbestos fibres in Christchurch, New Zealand's drinking water supply. Water Supply. 22(4):4445-4456.
Manitoba Department of Evironment and Climate. (2023). Personal communication with S. Janzen.
McGuire, M. J., Bowers, A. E., Bowers, D. A. (1983). Optimizing large-scale water treatment plants for asbestos-fiber removal. J Am Water Works Assoc. 75(7):364-370.
Mi, J., Peng, W., Jia, X., Wei, B., Yang, L., Hu, L., Lu, R. (2015). A case-control study on the relationship of crocidolite pollution in drinking water with the risk of gastrointestinal cancer in Dayao County. Wei Sheng Yan Jiu. 44(1):28-32.
Millette, J. R., Boone, R. L., Rosenthal, M. T., McCabe, L. J. (1981). The need to control asbestos fibers in potable water supply systems. The Science of the Total Environment. 18:91-102. doi:10.1016/s0048-9697(81)80052-6
Millette, J. R., Clark, P. J., Pansing, M. F., Twyman, J. D. (1980). Concentration and size of asbestos in water supplies. Environ Health Persp. 34:13-25. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1568536/
Millette, J. R., Clark, P. J., Stober, J., Rosenthal, M. (1983). Asbestos in Water Supplies of the United States. Environ Health Perspect. 53:45-48.
Millette, J. R., Craun, G. F., Stober, J. A., Kraemer, D. F., Tousignant, H. G., Hildago, E., Duboise, R. L., Benedict, J. (1983). Epidemiology study of the use of asbestos-cement pipe for the distribution of drinking water in Escambia county, florida. Environ Health Persp. 53:91-98. doi:10.1289/ehp.835391
Millette, J. R., Logsdon, G. S., Clark, P. J., Kinman, R. N. (1984). Evaluating the condition of asbestos-cement pipe. Mater Perform. 23(12):20.
Ministère de l'Environnement, de la Lutte contre les changements climatiques, de la Faune et des Parcs du Québec. (2023). Personal communication with P. Cantin.
Monaro, S., Landsberger, S., Lecomte, R., Paradis, P., Desaulnier, G., P'an, A. (1983). Asbestos pollution levels in river water measured by proton-induced X-ray emission (PIXE) techniques. Environ Pollut (Series B). 5:83-90.
New Brunswick Department of Environment and Local Government. (2023). Personal communication with J. Bishop.
NHMRC and NRMMC. (2011). Australian drinking water guidelines - paper 6, version 3.5 updated August 2018. National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council. Commonwealth of Australia, Canberra.
Northwest Territories Department of Health and Social Services. (2023). Personal communication with C. Rohit.
Nova Scotia Environment. (2023) Personal communication with A. Polegato.
NSF International. (2023a). NSF/ANSI Standard 53: Drinking water treatment units - Health effects. Ann Arbor, Michigan: NSF International/American National Standards Institute.
NSF International. (2023b). NSF/ANSI Standard 58: Reverse osmosis - Drinking water treatment systems. Ann Arbor, Michigan: NSF International/American National Standards Institute.
NTP. (1983). NTP lifetime carcinogenesis studies of amosite asbestos (CAS no. 12172-73-5) in syrian golden hamsters (feed studies). National Toxicology Program Technical Report Series, 249:1-81.
NTP. (1985). NTP toxicology and carcinogenesis studies of chrysotile asbestos (CAS no. 12001-29-5) in F344/N rats (feed studies). National Toxicology Program Technical Report Series, 295:1-390.
NTP. (1988). NTP toxicology and carcinogenesis studies of crocidolite asbestos (CAS no. 12001-28-4) in F344/N rats (feed studies). National Toxicology Program Technical Report Series, 280:1-178.
NTP. (1990a). NTP lifetime carcinogenesis studies of chrysotile asbestos (CAS no. 12001-29-5) in syrian golden hamsters (feed studies). National Toxicology Program Technical Report Series, 246:1-192.
NTP. (1990b). NTP toxicology and carcinogenesis studies of amosite asbestos (CAS no. 12172-73-5) in F344/N rats (feed studies). National Toxicology Program Technical Report Series, 279:1-341.
NTP. (1990c). NTP toxicology and carcinogenesis studies of tremolite (CAS no. 14567-73-8) in F344/N rats (feed studies). National Toxicology Program Technical Report Series, 277:1-183.
Nunavut Department of Health. (2023). Personal communication with W. Joy.
OECD. (2018). Test no. 451: Carcinogenicity studies, OECD guidelines for the testing of chemicals, section 4. Paris: Organisation for Economic Co-operation and Development Publishing. Available at: https://doi.org/10.1787/9789264071186-en
OEHHA. (2003). Public Health Goal for Asbestos in Drinking Water. California: Office of Environmental Health Hazard Assessment, Environmental Protection Agency. Available at: https://oehha.ca.gov/media/downloads/water/chemicals/phg/ph4asbestos92603.pdf
Olmstead, S. M. (2014). Climate change adaptation and water resource management: A review of the literature. Energy Econ. 46:500-509.
Olofsson, K., Mark, J. (1989). Specificity of asbestos-induced chromosomal aberrations in short-term cultured human mesothelial cells - ScienceDirect. Can Gen and Cytogen. 41(1):33-39. Available at: https://doi.org/10.1016/0165-4608(89)90105-2
Ontario Ministry of the Environment, Conservation and Parks. (2023). Personal communication with S. Deshpande.
Oshimura, M., Hesterberg, T. W., Tsutsui, T., Barrett, J. C. (1984). Correlation of asbestos-induced cytogenetic effects with cell transformation of syrian hamster embryo cells in culture. Can Res. 44(11):5017-5022.
Palekar, L. D., Most, B. M., Coffin, D. L. (1988). Significance of mass and number of fibers in the correlation of V79 cytotoxicity with tumorigenic potential of mineral fibers. Environ Res. 46(2):142-152. doi:10.1016/s0013-9351(88)80028-8
Pambianchi, E., Pecorelli, A., Valacchi, G. (2022). Gastrointestinal tissue as a "new" target of pollution exposure. IUBMB Life, 74(1):62-73. doi:10.1002/iub.2530
Parks, G. A. (1967). Aqueous surface chemistry of oxides and complex oxide minerals: Isoelectric point and zero point of charge. Equilibrium Concepts in Natural Water Systems. Volume 67. p. 121-160.
Pelin, K., Hirvonen, A., Taavitsainen, M., Linnainmaa, K. (1995). Cytogenetic response to asbestos fibers in cultured human primary mesothelial cells from 10 different donors. Mut Res. 334(2):225-233. doi:10.1016/0165-1161(95)90015-2
Pepelko, W. E. (1991). Effect of exposure route on potency of carcinogens. Reg Toxicol and Pharmacol: RTP. 13(1):3-17. Available at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med3&NEWS=N&AN=2024044
Prince Edward Island Department of Environment, Energy and Climate Action. (2023). Personal communication with G. Somers.
Prince Edward Island Department of Environment, Energy and Climate Action. (2025). Personal communication with B. Lanigan.
Rita, P.,, Reddy, P. P. (1986). Effect of chrysotile asbestos fibers on germ cells of mice. Environ Res. 41(1):139-143. Available at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med2&NEWS=N&AN=3019656
Roccaro, P., Vagliasindi, F. G. A. (2018). Indoor release of asbestos fibers from naturally contaminated water and related health risk. Chemosphere. 202:76-84. doi:10.1016/j.chemosphere.2018.03.040
Rowe, J. N. (1983). Relative source contributions of diet and air to ingested asbestos exposure. Environ Health Perspec. 53:115–120.
Saskatchewan Water Security Agency. (2025). Exposure levels of asbestos in Saskatchewan municipal drinking water. Saskatoon, Saskatchewan: Saskatchewan Water Security Agency, Government of Saskatchewan.
Schneider, U., Maurer, R. R. (1977). Asbestos and embryonic development. Teratology, 15(3):273-279. doi:10.1002/tera.1420150309
Schock, M. R., Buelow, R. W. (1981). The behavior of asbestos-cement pipe under various water quality conditions: Part 2, theoritical considerations. J Am Water Works Assoc. 73(12):636-651.
Schock, M. R., Logsden, G. S., Clark, P. J. (1981). Evaluation and control of asbestos-cement pipe corrosion. In: Proceedings of the Internation Corrosion Forum of the National Association of Corrosion Engineers, Toronto, Ontario, April 6-10. Nat Assoc of Corrosion Eng.
Schock, M. R., Lytle, D. A. (2011). Chapter 20: Internal corrosion and deposition control. In: Edzwald JK, editor. Water quality and treatment:a handbook on drinking water. 6th ed. Denver, Colorado: McGraw Hill and Am Water Works Ass.
Shelby, M. D. (1988). The genetic toxicity of human carcinogens and its implications. Mut Res. 204(1):3-15. doi:10.1016/0165-1218(88)90113-9
Sincock, A. M., Delhanty, J. D., Casey, G. (1982). A comparison of the cytogenetic response to asbestos and glass fibre in chinese hamster and human cell lines. Demonstration of growth inhibition in primary human fibroblasts. Mut Res. 101(3):257-268. doi:10.1016/0165-1218(82)90157-4
Slaats, P. G. G., Mesman, G. A. M., Rosenthal, L. P. M., Brink, H. (2004). Tools of monitor corrosion of cement-containing water mains. Water Sci Technol. 49(2):33-39.
Smeets, P., Medema, G., Van Dijk, J. (2009). The Dutch secret: how to provide safe drinking water without chlorine in the Netherlands. Drinking Water Eng Sci. 2(1):1-14.
Smith, W. E., Hubert, D. D., Sobel, H. J., Peters, E. T., Doerfler, T. E. (1980). Health of experimental animals drinking water with and without amosite asbestos and other mineral particles. J of Environ Pathol and Toxicol. 3(5-6):277-300.
Stanton, M. F., Laynard, M., Tegeris, A., Miller, E., May, M., Kent, E. (1977). Carcinogenicity of fibrous glass: Pleural response in the rat in relation to fiber dimension. J of the Nat Can Inst. 58(3):587-603. doi:10.1093/jnci/58.3.587
Statistics Canada (2025). Source of data: Custom tabulation: Length of asbestos-cement water pipes, 2022. Canada's Core Public Infrastructure Survey (available upon request) and Table 34-10-0287-01, Inventory of core public infrastructure assets. Available at: Inventory of core public infrastructure assets
Stayner, L., Kuempel, E., Gilbert, S., Hein, M., Dement, J. (2008). An epidemiological study of the role of chrysotile asbestos fibre dimensions in determining respiratory disease risk in exposed workers. Occ and Environ Med. 65(9):613-619. doi:10.1136/oem.2007.035584
Takeuchi, T., Nakajima, M., Morimoto, K. (1999). A human cell system for detecting asbestos cytogenotoxicity in vitro. Mut Res. 438(1):63-70. doi:10.1016/s1383-5718(98)00163-6
Toft, P., Meek, M. E., Wigle, D. T., Méranger, J. C., Miller, A. B. (1984). Asbestos in drinking water. Crit Rev in Environ Control. 14(2):151-197. doi:10.1080/10643388409381716
Toft, P., Wigle, D., Meranger, J.C., Mao, Y. (1981). Asbestos and drinking water in Canada. Sci Total Environ. 18:77-89.
Truhaut, R., Chouroulinkov, I. (1989). Effect of long-term ingestion of asbestos fibres in rats. IARC Scientific Publications, (90):127-133. Available at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med3&NEWS=N&AN=2744821
U.S. EPA (1983). Method 100.1 Analytical method for determination of asbestos fibers in water Athens, Georgia: Environmental Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency.
U.S. EPA. (1994a). Method 100.2 Analytical method for determination of asbestos structures over 10 µm in length in drinking water Cincinnati, Ohio: Office of Research and Development, United States Environmental Protection Agency.
U.S. EPA. (1994b). Technical notes on drinking water methods. Cincinnati, Ohio: United States Environmental Protection Agency.
U.S. EPA. (1995). National Primary Drinking Water Regulations: Asbestos. United States Environmental Protection Agency. Available at: https://nepis.epa.gov/Exe/ZyPDF.cgi/9100PO1W.PDF?Dockey=9100PO1W.PDF
U.S. EPA. (2016). The analysis of regulated contaminant occurrence data from public water systems in support of the third six-year review of national primary drinking water regulations: Chemical phase rules and radionuclides rules. Washington, D.C.: United States Environmental Protection Agency. EPA-810-R-16-014.
U.S. EPA. (2018). 2018 edition of the drinking water standards and health advisories. Washington, D.C.: United States Environmental Protection Agency. EPA 822-F-18-001.
Valerio, F., De Ferrari, M., Ottaggio, L., Repetto, E., Santi, L. (1980). Cytogenetic effects of rhodesian chrysotile on human lymphocytes in vitro. IARC Scientific Publications, (30):485-489.
Varga, C., Horvath, G., Timbrell, V. (1996a). In vivo studies on genotoxicity and cogenotoxicity of ingested UICC anthophyllite asbestos. Cancer Lett. 105(2):181-185.
Varga, C., Pocsai, Z., Horvath, G., Timbrell, V. (1996a). Studies on genotoxicity of orally administered crocidolite asbestos in rats: Implications for ingested asbestos induced carcinogenesis. Anticancer Res. 16(2):811-814. Available at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=8687133
Virta, R. (2011). Asbestos. Kirk-othmer encyclopedia of chemical technology. p. 1-40. doi:10.1002/0471238961.0119020510151209.a01.pub3
Wang, D., Cullimore, R., Hu, Y., Chowdhury, R. (2011). Biodeterioration of asbestos cement (AC) pipe in drinking water distribution systems. Int Biodeterior Biodegrad. 65(6):810-817.
Watkins, J. J., Ryder, R. A., Persich, W. A. (1978). Investigation of turbidity, asbestos fibers and particle counting techniques as indices of treatability of a Cascade Mountain water source. In: Proceedings of the American Water Works Association Annual Conference, Atlantic City, New Jersey. American Water Works Association, Denver, Colorado.
Webber, J. S., Covey, J. R. (1991). Asbestos in water. Criti Rev in Environ Control. 21(3-4):331-371.
Webber, J. S., Covey, J. R., King, M. V. (1989). Asbestos in drinking water supplied through grossly deteriorated A-C pipe. J Am Water Works Assoc. 81(2):80-85.
Weiner, M. L. (1988). Intestinal transport of some macromolecules in food. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 26(10), 867-880. doi:10.1016/0278-6915(88)90028-2
WHO. (2012). Water safety planning for small community water supplies. Geneva, Switzerland: World Health Organization.
WHO. (2017). Guidelines for drinking-water quality. Fourth Edition. Incorporating the First Addendum. Geneva, Switzerland: World Health Organization.
WHO. (2021). Asbestos in drinking water, Background document for development of WHO Guidelines for drinking-water quality. Geneva, Switzerland: World Health Organization. No. WHO/HEP/ECH/WSH/2021.4.
Wigle, D. T. (1977). Cancer mortality in relation to asbestos in municipal water supplies. Arch Environ Health. 32(4):185-190.
Wigle, D. T., Mao, Y., Semenciw, R., Smith, M. H., Toft, P. (1986). Contaminants in drinking water and cancer risks in Canadian cities. Can J Public Health. 77(5):335-42.
Wylie, A. G., Korchevskiy, A. A. (2023). Dimensions of elongate mineral particles and cancer: A review. Environ Res. 230:114688. doi:10.1016/j.envres.2022.114688
Zavasnik, J., Sestan, A., Skapin, S. (2022). Degradation of asbestos – Reinforced water supply cement pipes after a long-term operation. Chemosphere. 287:131977.
Zheng, B., Zang, L., Li, W., Li, H., Wang, H., Zhang, M., Song, X. (2019). Quantitative analysis of asbestos in drinking water and its migration in mice using fourier-transform infrared spectroscopy and inductively coupled plasma optical emission spectrometry. Analytica Chimica Acta. 1058:29–38. https://doi.org/10.1016/j.aca.2018.12.022
C.2 Abbreviations
- A-C
- asbestos-cement
- AI
- Aggressiveness Index
- ANSI
- American National Standards Institute
- AS
- analytical sensitivity
- CAS RN
- Chemical Abstracts Service Registry Number
- DL
- detection limit
- DWTP
- Drinking water treatment plant
- GI
- gastrointestinal
- HBV
- health-based value
- IARC
- International Association for Research on Cancer
- IR
- intermediate range
- LSI
- Langelier Saturation Index
- MAC
- maximum acceptable concentration
- MCL
- maximum contaminant level
- MDL
- method detection limit
- MFL
- million fibres per litre
- NSF
- NSF International
- NTP
- National Toxicology Program
- NTU
- nephelometric turbidity unit
- OECD
- Organisation for Economic Co-operation and Development
- OHAT
- Office of Health Assessment and Translation
- pHs
- pH of saturation
- PTs
- provinces and territories
- RNA
- ribonucleic acid
- RO
- reverse osmosis
- ROB
- risk of bias
- ROS
- reactive oxygen species
- SCC
- Standards Council of Canada
- U.S. EPA
- United States Environmental Protection Agency
- WHO
- World Health Organization
C.3 Appendix A
| Geography | Local and regional government organizations Footnote a | All municipalities | All urban municipalities | All rural municipalities |
|---|---|---|---|---|
| Name | Kilometres (data quality) |
Kilometres (data quality) |
Kilometres (data quality) |
Kilometres (data quality) |
| Canada | 13 701.4 Footnote b (B) | 10 684 Footnote b (A) | 8 094.8 (A) | 2 583.6 (B) |
| Newfoundland and Labrador | 69.9 (E) | 69.9 (E) | 10.1 (B) | (F) |
| Prince Edward Island | (F) | 5.6 Footnote c | (F) | (F) |
| Nova Scotia | 82.7 (D) | 65.3 (D) | 30.4 (E) | 34.8 (D) |
| New Brunswick | 12.8 (B) | 12.8 (B) | 11.6 (A) | (F) |
| Quebec | 863.7 (B) | 840.7 (B) | 567 (B) | 273.7 (B) |
| Ontario | 1 258.5 (B) | 937.6 (A) | 659.2 (B) | 278.5 (A) |
| Manitoba | 1 005.7 (A) | 989.8 (A) | 924.2 (A) | 65.6 (B) |
| Saskatchewan | 2 757.3 (B) | 2 757.3 (B) | 1 662.3 (A) | 1 095.1 (C) |
| Alberta | 3 286.3 (E) | 1 827.1 (A) | 1 431.2 (A) | 395.9 (B) |
| British Columbia | 4 261.2 (B) | 3 080.3 (B) | 2 730.5 (B) | 349.8 (A) |
| Yukon | (F) | (F) | (F) | (F) |
| Northwest Territories | (F) | (F) | 0 (A) | (F) |
| Nunavut | 0 (A) | 0 (A) | 0 (A) | 0 (A) |
