Medical device meetings draft guidance document: Appendices
On this page
- Device Advice: Meeting process map
- Innovation information meeting record of decision template
- Pre-clinical meeting record of decision template
- Pre-submission meeting record of decision template
Device Advice: Meeting process map
This map outlines the steps for the Device Advice: Medical Device Meeting Program.
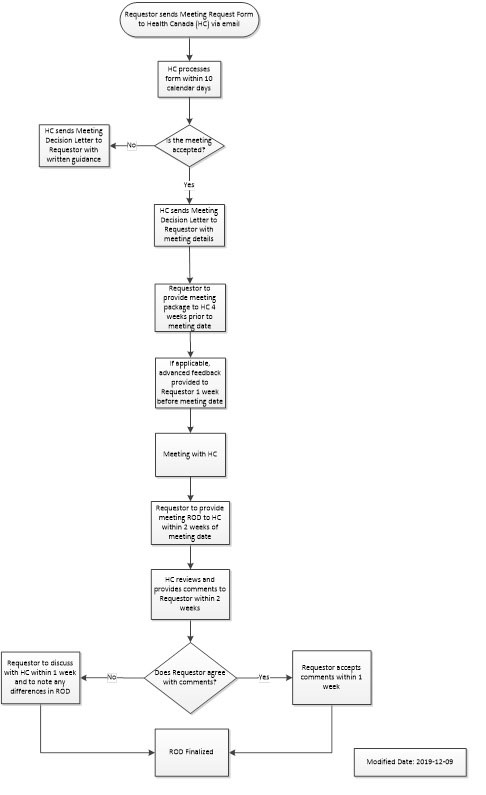
Figure 1 - Text equivalent
Step 1: The Requestor sends a Meeting Request Form to Health Canada via email.
Step 2: Health Canada processes the form within 10 calendar days.
Step 3: Is the meeting accepted by Health Canada?
- No: If the meeting is not accepted, Health Canada will send a Meeting Decision Letter to Requestor with written guidance; process ends here
- Yes: If the meeting is accepted, process continues to step 4
Step 4: Health Canada sends a Meeting Decision Letter to requestor with meeting details.
Step 5: Requestor to provide meeting package, based on meeting type, to Health Canada 4 weeks prior to meeting date.
Step 6: If applicable, Health Canada will provide advanced feedback to requestor 1 week before meeting date.
Step 7: The meeting between Health Canada and requestor takes place.
Step 8: Requestor provides the meeting record of decisions (ROD) to Health Canada within 2 weeks of meeting date.
Step 9: Health Canada reviews and provides comments on the record of decisions to requestor within 2 weeks.
Step 10: Does requestor agree with comments from Health Canada on ROD?
- Yes: Requestor to accept Health Canada comments on ROD within 1 week.
- No: Requestor to communicate concerns regarding comments on ROD to Health Canada via email within 1 week. Any unresolved differences will be noted on the ROD by Health Canada.
Step 11: The record of decision is finalized.
Innovation information meeting record of decision template
Innovation Information Meeting – [DD-MM-YYYY]
Record of Decisions
Name of Manufacturer:
Name of Device:
Location:
Time:
Participants
Health Canada Participants: (Name, Title, Bureau)
Manufacturer Participants: (Name, Title, Organization)
Other Participants: (Name, Title, Organization)
Record of Decisions
Presentation: Questions raised by Health Canada during the presentation and corresponding key points discussed (if applicable):
Action Items (if applicable):
Question 1 (from Innovation Information Meeting Package – if applicable):
Main points of Discussion/Summary of advice:
Action Items (if applicable):
Question 2 (from Innovation Information Meeting Package – if applicable):
Discussion/Summary of advice:
Action Items (if applicable):
Question 3 (from Innovation Information Meeting Package – if applicable):
Discussion/Summary of advice:
Action Items (if applicable):
Note: Duplicate the table above as necessary for additional questions.
Pre-clinical meeting record of decision template
Pre-Clinical Meeting – [DD-MM-YYYY]
Record of Decisions
Name of Manufacturer:
Name of Device:
Location:
Time:
Participants
Health Canada Participants: (Name, Title, Bureau)
Manufacturer Participants: (Name, Title, Organization)
Other Participants: (Name, Title, Organization)
Record of Decisions
Presentation: Questions raised by Health Canada during the presentation and corresponding key points discussed (if applicable):
Action Items (if applicable):
Question 1 (from Pre-Clinical information package):
Main points of Discussion/Summary of advice:
Action Items (if applicable):
Question 2 (from Pre-Clinical information package):
Discussion/Summary of advice:
Action Items (if applicable):
Question 3 (from Pre-Clinical information package):
Discussion/Summary of advice:
Action Items (if applicable):
Note: Duplicate the table above as necessary for additional questions.
Pre-submission meeting record of decision template
Pre-Submission Meeting – [DD-MM-YYYY]
Record of Decisions
Name of Manufacturer:
Name of Device:
Location:
Time:
Participants
Health Canada Participants: (Name, Title, Bureau)
Manufacturer Participants: (Name, Title, Organization)
Other Participants: (Name, Title, Organization)
Record of Decisions
Presentation: Questions raised by Health Canada during the presentation and corresponding key points discussed (if applicable):
Action Items (if applicable):
Question 1 (from Pre-Submission information package):
Main points of Discussion/Summary of advice:
Action Items (if applicable):
Question 2 (from Pre-Submission information package):
Discussion/Summary of advice:
Action Items (if applicable):
Question 3 (from Pre-Submission information package):
Discussion/Summary of advice:
Action Items (if applicable):
Note: Duplicate the table above as necessary for additional questions.