Dimethoate and omethoate in drinking water - Guideline technical document for public consultation

Download the alternative format
(PDF format, 1.3 MB, 49 pages)
Organization: Health Canada
Published: 2021-06-18
Guideline Technical Document for Public Consultation
Consultation period ends August 18, 2021
Purpose of consultation
This guideline technical document outlines the evaluation of the available information on dimethoate, and its metabolite omethoate, in order to update the previous guidelines for dimethoate in drinking water. The purpose of this consultation is to solicit comments on the proposed guidelines, the approach used for its development, and the potential impacts of implementation.
The existing guidelines on dimethoate, developed in 1986, based its maximum acceptable concentration (MAC) of 0.02 mg/L (20 µg/L) on decreased cholinesterase levels in whole blood in humans. This document proposes to retain a MAC of 0.02 mg/L (20 µg/L) for dimethoate in drinking water, but it is based on brain cholinesterase inhibition in several repeat-dose animal studies. The document also proposes an additive approach for addressing omethoate in drinking water.
This document is available for a 60-day public consultation period. Please send comments (with rationale, where required) to Health Canada via email to HC.water-eau.SC@canada.ca.
All comments must be received before Month day, year. Comments received as part of this consultation will be shared with members of the Federal-Provincial-Territorial Committee on Drinking Water (CDW), along with the name and affiliation of their author. Authors who do not want their name and affiliation shared with CDW members should provide a statement to this effect along with their comments.
It should be noted that this guideline technical document will be revised following the evaluation of comments received, and final Guidelines for Canadian Drinking Water Quality will be established, if required. This document should be considered as a draft for comment only.
Proposed guideline value
A maximum acceptable concentration (MAC) of 0.02 mg/L (20 μg/L) is proposed for dimethoate in drinking water.
The toxicological effects of dimethoate are the result of omethoate, its oxygen analogue metabolite (oxon). Since omethoate can be formed through the environmental degradation of dimethoate or during treatment of water containing dimethoate, an additive approach should be taken in which the sum of the detected concentrations of dimethoate and omethoate (expressed as a dimethoate equivalent value) does not exceed the MAC for dimethoate.
Executive summary
This guideline technical document was prepared in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water and is based on assessments of dimethoate (which included an assessment of omethoate) completed by Health Canada's Pest Management Regulatory Agency and supporting documents.
Exposure
Canadians can be exposed to dimethoate through the diet, through occupational exposure and, to a lesser extent, through drinking water. Dimethoate is a broad spectrum organophosphate pesticide used to control a wide range of insects and mites on both agricultural and non-agricultural sites. In 2018 (the most recent year for which data are available), more than 25 000 kg of dimethoate as an active ingredient was sold in Canada. Dimethoate can be released into the environment as spray drift during application. Although water-soluble, it rapidly breaks down, is non-persistent in the environment and therefore is unlikely to contaminate groundwater.
Dimethoate is not usually found in drinking water sources in Canada, although low levels of dimethoate have been found in a few Canadian provinces. The maximum reported concentration was well below the proposed MAC.
Omethoate is a breakdown product of dimethoate in the environment. It is also produced during treatment of source water containing dimethoate. However, limited Canadian water monitoring data did not report any omethoate samples above the detection limit.
Health effects
Dimethoate primarily targets the nervous system through its metabolite omethoate, which is more toxic than dimethoate. Dimethoate has also been found to cause increased offspring deaths in animals.
Analytical and treatment considerations
The development of drinking water guidelines takes into consideration the ability to both measure the contaminant and remove it from drinking water supplies. Several analytical methods are available for measuring dimethoate and omethoate in drinking water at concentrations well below the proposed MAC.
At the municipal level, treatment technologies that are available to effectively decrease dimethoate from drinking water include activated carbon adsorption, oxidation, membrane filtration and biological processes. These treatment technologies are capable of achieving treated water concentrations well below the proposed MAC. Although dimethoate may be removed using common oxidants used for disinfection (e.g., chlorine), utilities should ensure that they minimize the formation of by-products, such as omethoate, without compromising the effectiveness of disinfection.
In cases where dimethoate removal is desired at a small or household level, for example, when the drinking water supply is from a private well, a residential drinking water treatment unit may be an option. Although there are no treatment units currently certified for the removal of dimethoate from drinking water, activated carbon adsorption and reverse osmosis technologies are expected to be effective. Since these technologies do not result in the formation of omethoate, only removal of dimethoate is needed at the residential-scale. When using a residential drinking water treatment unit, it is important to take samples of water entering and leaving the treatment unit and to send them to an accredited laboratory for analysis to ensure that adequate dimethoate removal is occurring.
Application of the guidelines
Note: Specific guidance related to the implementation of drinking water guidelines should be obtained from the appropriate drinking water authority.
The proposed guideline value for dimethoate and the additive approach for omethoate are protective against health effects from exposure to dimethoate and omethoate in drinking water over a lifetime. Any exceedance of the proposed MAC should be investigated and followed by the appropriate corrective actions, if required. For exceedances in source water where there is no treatment in place, additional monitoring to confirm the exceedance should be conducted. If it is confirmed that source water dimethoate concentrations are above the proposed MAC, an investigation to determine the most appropriate way to reduce exposure to dimethoate should be conducted. This may include use of an alternate water supply or installation of treatment. Where treatment is already in place and an exceedance occurs, an investigation should be conducted to verify treatment and to determine whether adjustments are needed to lower the treated water concentration below the proposed MAC. When oxidation processes are used to degrade dimethoate, omethoate monitoring should also be conducted to ensure that the sum of their concentrations, calculated using the additive approach, is below the MAC.
Table of contents
- 1.0 Exposure considerations
- 2.0 Health considerations
- 3.0 Derivation of the health-based value
- 4.0 Analytical and treatment considerations
- 5.0 Management strategies
- 6.0 International considerations
- 7.0 Rationale
- 8.0 References
- Appendix A: List of abbreviations
- Appendix B: Canadian water quality data
1.0 Exposure considerations
1.1 Sources and uses
Dimethoate is a broad spectrum organophosphate insecticide and acaricide used to control a wide range of insects and mites on both agricultural and non-agricultural sites (Health Canada, 2011, 2015). It acts by interrupting the transmission of nerve impulses, thereby inhibiting cholinesterase (ChE) (Health Canada, 2011). There are no domestic products or residential uses of dimethoate in Canada (Health Canada, 2011). More than 25 000 kg of dimethoate as active ingredient were sold in Canada in 2018 (the most recent year for which data are available) (Health Canada, 2020a).
Dimethoate can be released into the environment as spray drift and runoff during field application (Health Canada, 2015). It is very soluble in water and is not likely to volatilize from moist soils or surface waters (Health Canada, 2011). It does not adsorb onto soil and is therefore highly mobile in soil (WHO, 2004; Health Canada, 2011, 2015). Despite this, dimethoate is unlikely to contaminate groundwater as it quickly breaks down into omethoate in soil via microbiological and hydrolytic degradation (the main inactivating pathway of dimethoate in the environment) (WHO, 2004; Health Canada, 2011, 2015). Phototransformation is unimportant as a transformation route for dimethoate in soil and water (Health Canada, 2011). Dimethoate has a half-life of 2 to 122 days in soil and 18 hours to 8 weeks in water, although it is relatively stable at pH 2–7 (HSDB, 2010; WHO, 2017). Overall, dimethoate is non-persistent in the environment (WHO, 2004; Health Canada, 2011, 2015).
Omethoate is the major toxic degradation product of dimethoate in the environment (US EPA, 2006a). It is also an organophosphate pesticide that acts by inhibiting ChE. However, it is not registered for use as a pesticide in Canada or the United States (US EPA, 2004; APVMA, 2011). Omethoate has high to very high mobility in soil (EFSA, 2006). With laboratory half-life values of 0.9 to 2.8 days being reported in soil, it is unlikely to be persistent (EFSA, 2006).
In addition to being an environmental degradate, omethoate is produced during chlorination of drinking water, with 11% to 23% of dimethoate being converted to omethoate (Marin, 2010). Omethoate is also a toxicologically important metabolite of dimethoate in mammals and is more toxic than its parent chemical (Health Canada, 2011). For that reason, this assessment of dimethoate also includes information on omethoate.
1.2 Substance identity
Dimethoate (C5H12NO3PS2), or O,O-dimethyl S-methylcarbamoylmethyl phosphorodithioate, is a white crystalline solid belonging to the dithiophosphates chemical group (Health Canada, 2011). Dimethoate can contain low levels of impurities, such as omethoate and isodimethoate, which have a higher ChE inhibition potential than dimethoate (EFSA, 2018).
Omethoate (C5H12NO4PS), or O,O-dimethyl S-(N-methylcarbomoylmethyl) phosphorothioate, is a synthetic, colourless liquid (Lewis et al., 2016). It is an oxygen analogue metabolite (oxon) of dimethoate and plays an important role in the toxic effects of dimethoate in insects and mammals (APVMA, 2010). Some of the physical and chemical properties of dimethoate and omethoate are summarized in Table 1.
| Property | DimethoateFootnote a | Interpretation | OmethoateFootnote b | Interpretation |
|---|---|---|---|---|
| CAS RN | 60-50-1 | Not applicable | 1113-02-6 | Not applicable |
| Molecular weight (g/mol) |
229.4 | Not applicable | 213.2 | Not applicable |
| Water solubility (g/L) |
23.3 at pH 5 | High solubility in water | 500 at 20 °C | High solubility in water |
| Vapour pressure (mPa) |
0.25 at 25 °C | Very low volatility; not likely to volatilize from moist soils or water surfaces | 19.0 at 20 °C | moderately volatile |
| Henry's law constant (Pa m3mol-1) |
1.42 x 10-6 | Not likely to volatilize from moist soils or water surfaces | 4.62 x 10-9 at 25 °C | Not likely to volatilize from moist soils or water surfaces |
| n-octanol:water partition coefficient (log Kow) |
0.704 | Unlikely to bioaccumulate | -0.9 | Unlikely to bioaccumulate |
| Dissociation constant (pKa) |
2.0 at 20 °C | Hydrolyzes moderately under acidic and neutral conditions and hydrolyzes easily under basic conditions | Not available | Not applicable |
1.3 Exposure
Potential exposure to dimethoate is primarily through the diet and from occupational exposure during handling and application of products containing dimethoate (Health Canada, 2011, 2015). Drinking water is a minor source of dimethoate exposure (Health Canada, 2011, 2015). Omethoate exposure is possible through the diet from residues in foods and through drinking water as a result of the oxidation of dimethoate during water treatment and environmental degradation of dimethoate (Health Canada, 2011).
Based on aggregate dietary and drinking water risks assessed by Health Canada (2015), exposure to dimethoate from foods and drinking water is not of concern (Table 2). The World Health Organization (WHO) has estimated a total daily intake of 0.001 μg/kg body weight (bw) (0.000001 mg/kg bw) of dimethoate from food, considerably lower than the 0.00011 mg/kg bw/d estimated by Health Canada's Pest Management Regulatory Agency (PMRA) for the Canadian general population (Health Canada, 2015; WHO, 2017).
| Population Group (in years) | Exposure from FoodFootnote a | Exposure from Food and WaterFootnote a | ||
|---|---|---|---|---|
| mg/kg bw/d | % ADIFootnote b | mg/kg bw/d | % ADIFootnote b | |
| General Population | 0.00011 | 6 | 0.000147 | 7 |
| All Infants (< 1) | 0.000107 | 5 | 0.000230 | 12 |
| Children 1-2 | 0.000262 | 13 | 0.000318 | 16 |
| Children 3-5 | 0.000244 | 12 | 0.000296 | 15 |
| Children 6-12 | 0.000166 | 8 | 0.000202 | 10 |
| Male 13-19 | 0.000105 | 5 | 0.000132 | 7 |
| Male 20-49 | 0.000091 | 5 | 0.000126 | 6 |
| Adults 50+ | 0.000078 | 4 | 0.000115 | 6 |
| Female 13-49 | 0.000088 | 4 | 0.000122 | 6 |
Water monitoring data from the provinces and territories (municipal and non-municipal supplies), the PMRA and Environment Canada and Climate Change (Environment Canada, 2011) were available for dimethoate.
Data provided by the provinces and territories, as well as by the First Nations and Inuit Health Branch (FNIHB) of Indigenous Services Canada, indicate that dimethoate levels are below the method reporting limit (MRL) or method detection limit (MDL) in most samples collected from a variety of water supplies in Canada, including surface water and groundwater as well as treated and distributed water where monitoring occurred (British Columbia Ministry of Health, 2019; Indigenous Services Canada, 2019; Manitoba Sustainable Development, 2019; Ministère de l'Environnement et de la Lutte contre les changements climatiques du Québec, 2019; Nova Scotia Environment, 2019; Prince Edward Island Department of Communities, Land and Environment, 2019; Saskatchewan Water Security Agency, 2019; Ontario Ministry of the Environment, Conservation and Parks, 2020). Table 3 summarizes the monitoring data for jurisdictions in which all samples were reported below the MDL. Table 4 summarizes the data for jurisdictions in which dimethoate detections were reported. The maximum dimethoate concentration reported was 2.5 μg/L in samples from both Saskatchewan and Ontario. There were no monitoring data available in New Brunswick, Newfoundland and Labrador, or Yukon (New Brunswick Department of Environment and Local Government, 2019; Newfoundland and Labrador Department of Municipal Affairs and Environment, 2019; Government of Yukon Environmental Health Services, 2019).
| Jurisdiction (MDL µg/L) |
Monitoring Period | Municipal/Non-municipal | Water Type (Municipal: ground/surface – raw, treated, distributed) |
# Detects/Samples |
|---|---|---|---|---|
| British Columbia (2) |
2013–2018 | Municipal | Surface – raw | 0/18 |
| FNIHB Ontario Region (0.1–2.5) |
2014–2018 | Public water systems | Ground – raw | 0/13 |
| Ground – treated | 0/190 | |||
| Ground – distribution | 0/16 | |||
| Surface – raw | 0/33 | |||
| Surface – treated | 0/308 | |||
| Surface – distribution | 0/23 | |||
| Semi-public water systems | Ground – raw | 0/3 | ||
| Ground – treated | 0/16 | |||
| Ground – distribution | 0/68 | |||
| Surface – raw | 0/1 | |||
| Surface – treated | 0/9 | |||
| Surface – distribution | 0/2 | |||
| Private water systems | Ground – treated | 0/3 | ||
| Ground – distribution | 0/50 | |||
| Surface – treated | 0/5 | |||
| FNIHB Atlantic Region (2.5–10) |
2014–2018 | Public water systems | Ground – treated | 0/4 |
| Ground – distribution | 0/4 | |||
| Surface – treated | 0/1 | |||
| FNIHB Québec (0.01 – 0.03) |
2014–2018 | - | Drinking water system | 0/4 |
| ManitobaFootnote a (0.1) |
2015–2020 | Ambient | Lake | 0/14 |
| River/stream | 0/187 | |||
| Nova Scotia (1.5–5) |
2007–2018 | Municipal | Ground – raw | 0/71 |
| Ground – treated | 0/35 | |||
| Surface – raw | 0/35 | |||
| Surface – treated | 0/40 | |||
| Distributed | 0/1 | |||
| Jurisdiction (MDL µg/L) |
Monitoring Period | Water Type (Municipal: ground/surface - raw, treated, distributed and Non-Municipal: ground) |
# Detects/samples |
Maximum Conc. (µg/L) |
|---|---|---|---|---|
Ontario |
2010–2020 |
Ground – raw (municipal) | 0/194 | - |
| Ground – treated (municipal) | 2/4259 | 0.1 | ||
| Surface – raw (municipal) | 0/154 | - | ||
| Surface – treated (municipal) | 2/4192 | 2.5 | ||
| Distribution (municipal) | 1/60 | 2.5 | ||
| Prince Edward Island (0.04) |
2004–2017 | Ground – raw (municipal) | 0/665 | - |
| Ground – raw (non-municipal) | 1/614 | 0.1 | ||
| Québec (0.01–0.9) |
2012–2018 | Ground – distribution (municipal) | 0/291 | - |
| Surface – distribution (municipal) | 4/1040 | 0.4 | ||
| Ground – rawFootnote a (municipal) | 0/46 | - | ||
| Ground – treatedFootnote a (municipal) | 0/17 | - | ||
| Ground – distributionFootnote a (municipal) | 0/5 | - | ||
| Ground – rawFootnote b (municipal) | 0/82 | - | ||
| Ground – rawFootnote b (non-municipal) | 0/132 | - | ||
| Saskatchewan (0.0001–10) |
2014–2019 |
Ground and surface – distribution (municipal) | 2/32 | 2.5 |
| Ground and surface – treated (municipal) | 0/4 | - | ||
| Ground – raw (municipal) | 0/16 | - | ||
On the basis of an extensive review of available water monitoring data, the PMRA estimated environmental exposure concentrations of 0.03 µg/L and 0.08 µg/L (95th percentile of the mean concentration for each study site, including half level of detection for non-detects) of dimethoate in groundwater and surface water, respectively (Health Canada, 2011). Dimethoate was detected in only 1 of 163 samples taken from a total of 15 reservoirs in Manitoba, Saskatchewan and Alberta (May 2003 to April 2004). The detection limit was 25.10 ng/L. Reservoirs received primarily snowmelt and rainfall runoff from agricultural crop lands. Dimethoate was not detected in treated drinking water samples (n = 28; collection July 2004 and 2005) collected in the same study (Donald et al., 2007).
Canadian omethoate water monitoring data were limited to Nova Scotia Groundwater Observation Network reports (2007–2012, 2015) and consistently showed omethoate below the detection limit of 1 µg/L in up to 40 observation wells monitored annually or biennially (Government of Nova Scotia, 2007–2012, 2015).
In foods, residues of dimethoate and omethoate can potentially occur in plant and animal commodities (Health Canada, 2015). In plants, dimethoate-related residues were found at higher concentrations in the foliage or outer portions of plant samples as compared to the grain and root samples (Health Canada, 2015). In the Canadian Food Inspection Agency's 2015/16 Annual Report (2019), residues of dimethoate and dimethoate plus omethoate were detected in 60 of 1801 samples of fruits and vegetables (fresh, frozen and processed). Of these 60 detects, the highest values were seen in leafy vegetables (e.g., leaf lettuce, kale, fine herbs). The single sample exceeding its maximum residue limit (0.1 ppm) was reported for fine herbs (5.77600 ppm) (CFIA, 2019). In animal commodities (goats and hens), the highest dimethoate-related residue concentrations (primarily omethoate and dimethoate carboxylic acid) were in the liver and kidneys, followed by eggs and milk. The lowest concentrations were in muscle and fat (Health Canada, 2015).
2.0 Health considerations
All pesticides, including dimethoate, are regulated by the PMRA. The PMRA conducts extensive evaluations and cyclical reviews of pesticides, taking into account unpublished and proprietary information as well as foreign reviews by other regulatory agencies, such as the United States Environmental Protection Agency (US EPA). This health assessment of dimethoate, including discussion of omethoate, is based primarily on the PMRA evaluations (Health Canada, 2011, 2015) and supporting documents. Additionally, any reviews and relevant literature available since the PMRA evaluations were completed were also considered.
2.1 Kinetics
Absorption, distribution and elimination of dimethoate were unaffected by sex, dose or route of exposure (Health Canada, 2011). The major metabolites of dimethoate were thiophosphate and phosphate esters (NHMRC, NRMMC, 2011). Although omethoate is a minor metabolite (1%–6%), it is responsible for the ChE inhibition seen following dimethoate exposure (Health Canada, 2011; NHMRC, NRMMC, 2011).
Absorption: Both dimethoate and omethoate were rapidly and almost completely absorbed from the gastrointestinal tract of rats following oral administration (APVMA, 2010; Health Canada, 2011). Dermal absorption of dimethoate ranged from 7% to 11% in rats (APVMA, 2010). In an in vitro study, rat skin absorbed more dimethoate than human skin (Davies, 1999).
Distribution: Dimethoate is rapidly distributed to tissues, especially the liver, bile, kidneys and erythrocytes, but it is unlikely to accumulate (APVMA, 2010; Health Canada, 2011). In rats, tissue retention from single radiolabelled oral doses of either 10 or 100 mg/kg bw of dimethoate was 0.3 and 7 ppm, respectively (Health Canada, 2011).
Omethoate is also widely distributed in tissues (highest levels in thyroid) and showed minimal tissue retention with less than 0.05% of administered doses remaining in tissues 48 hours after oral dosing in rats (Health Canada, 2011; NHMRC, NRMMC, 2011).
Metabolism: Dimethoate is extensively metabolized based on oral exposure studies in rats (Health Canada, 2011). The hydrolytic pathway is the major metabolic pathway and yields dimethoate carboxylic acid (major metabolite; 29%–46%), which is subsequently metabolized to dimethyldithiophosphate, dimethylthiophosphoric acid and dimethyl phosphoric acid (Kirkpatrick, 1995; Health Canada, 2011). The oxidative pathway, a minor pathway, involves the oxidation of dimethoate to its oxon, omethoate (minor metabolite, 1%–6%), which then undergoes limited metabolism (Kirkpatrick, 1995; Health Canada, 2011; NHMRC, NRMMC, 2011). Loss of carbon dioxide by dimethoate is also a minor metabolic pathway (Health Canada, 2011). An in vitro interspecies comparative metabolism study, assessed in conjunction with human in vivo data, did not identify any dimethoate metabolites unique to humans (EFSA, 2018).
Although omethoate is not extensively metabolized (88% is eliminated unchanged within 8 hours), its identified metabolites include N-methyl-methyl-sylphinyl-acetamide (major metabolite, 13%–22%), O-desmethylated omethoate (9%), O,O-dimethyl phosphoric acid and O,O-dimethyl phosphorothioic acid (Health Canada, 2011; NHMRC, NRMMC, 2011).
Elimination: Elimination of dimethoate and its metabolites is rapid in rats, with 91%–97% of a given dose excreted within 5 days mostly in the urine (85%–91%), with lesser amounts in feces (1%–2%), expired air (2%–3%) and the carcass (1%–2%) (Kirkpatrick, 1995; APVMA, 2010; Health Canada, 2011). The major urinary metabolites (53%–87%) are dimethoate carboxylic acid, thiophosphate and phosphate esters; 1%–2% of dimethoate is excreted unchanged in the urine, while omethoate accounts for 1%–6% of the urinary metabolites (Kirkpatrick, 1995; NHMRC, NRMMC, 2011; Health Canada, 2011).
Omethoate is quickly eliminated unchanged (95%–98% as omethoate), primarily in the urine, with another 2%–5% excreted in the feces (Health Canada, 2011). Excretion profiles for omethoate were independent of dose or sex in animal studies, although one study showed male rats excreted more radiolabeled omethoate in the feces at the high dose (10 mg/kg bw) compared to the low (0.5 mg/kg bw) dose (Health Canada, 2011).
2.2 Health effects
The toxicity databases for both dimethoate and omethoate are well characterized in animals, covering several endpoints and various types of exposure, although information regarding toxicity in humans is limited (see US EPA, 2007; APVMA, 2010, 2011; Health Canada, 2011, 2015 for more thorough reviews). Dimethoate primarily targets the nervous system by inhibiting acetylcholinesterase (AChE), an enzyme that breaks down neurotransmitters and that is necessary for normal functioning of the nervous system (Health Canada, 2011). AChE is the primary cholinesterase (ChE) in the body and can be measured in brain tissue, erythrocytes and plasma (WHO, 2004; Health Canada, 2011; EFSA, 2018).
2.3 Effects in humans
The PMRA's assessments and supporting documents (US EPA, 2007; Health Canada, 2011, 2015) did not discuss the effects of dimethoate or omethoate in humans. Data available from the literature were limited to volunteer studies, a few case studies and a prospective study and indicated that acute exposure to dimethoate and omethoate produce typical signs of organophosphate poisoning.
The no-observed-adverse-effect-level (NOAEL) for ChE inhibition was 0.2 mg/kg bw per day in 9 male and female volunteers given dimethoate for 39 days. This NOAEL was supported in seven other studies, each involving 6 to 20 volunteers who received doses ranging from 0.04 to 1.0 mg/kg bw per day for up to 57 days (WHO, 2004).
Case studies and reports of accidental and deliberate ingestion of dimethoate were available from the literature. In a prospective study reviewing 264 cases of intentional ingestion of dimethoate (plasma concentrations of 160.0 to 674.0 µmol/L), acute dimethoate poisoning resulted in hypotensive shock, impaired respiratory function, AChE inhibition (including coma) and, in 61 cases, death (Eddleston et al., 2005). Similar symptoms were seen in individual case studies of dimethoate poisoning and included bradycardia (slowed heart rate), respiratory depression/insufficiency (breathing difficulties), marked gait ataxia (uncoordinated movement), seizures and coma (LeBlanc et al., 1986; De Bleeker et al., 1992; Fonseka et al., 2003; Hoffmann and Papendorf, 2006).
Effects were similar in case studies involving omethoate ingestion (Lotti et al., 1981; Tsatsakis et al., 1998; Pavlic et al., 2002). Omethoate is unlikely to cause delayed neuropathy in humans based on enzyme studies using autopsy material (Lotti et al., 1981).
2.4 Effects in animals
ChE inhibition and pup mortality were the main effects of dimethoate in animal studies and occurred concurrently in key reproductive and developmental studies (Table 5). Dimethoate was not genotoxic, carcinogenic, or teratogenic. In acute studies, dimethoate was moderately toxic via the oral route of exposure and slightly toxic via the dermal and inhalation routes (Table 6) (Health Canada, 2011).
In laboratory animals, oral range-finding, chronic/carcinogenicity, 2-generation reproductive, 1-generation reproductive toxicity, developmental neurotoxicity (DNT), and comparative ChE studies using dimethoate showed effects on the nervous system, particularly the inhibition of ChE in the brain, plasma and erythrocytes of treated animals, both adults and offspring (Hellwig et al., 1986a; Burford et al., 1990a, 1990b; Brooker and Stubbs, 1991; Brooker et al., 1992; Myers, 2001a, 2001b; Mellert et al., 2003; US EPA, 2004, 2006a; APVMA, 2010; Health Canada, 2011). Brain ChE was generally the most sensitive indicator of toxicity, occurring at doses similar to or lower than those causing ChE inhibition in erythrocytes (FIFRA, 2005; US EPA, 2006b; Health Canada, 2011). Erythrocyte ChE inhibition was indicative of adverse changes in peripheral nervous tissue in acute and short-term studies only. In studies of longer duration, erythrocyte ChE inhibition alone was not considered to be a toxicologically adverse effect due to the limitations related to the low rate of re-synthesis of erythrocyte ChE over extended periods of time (US EPA, 2006b; Health Canada, 2011). Plasma ChE was the least affected and was considered a marker of exposure rather than a toxicologically adverse effect (APVMA, 2011; Health Canada, 2011).
In chronic studies using dimethoate, rats were slightly more sensitive than other species to ChE inhibition based on NOAEL values of 0.05 to 1.3 mg/kg bw per day and lowest-observed-adverse-effect-level (LOAEL) values of 0.25 to 3 mg/kg bw per day compared to LOAELs of 3.75 mg/kg bw per day in mice and 0.73 mg/kg bw per day in dogs (Hellwig et al., 1986a; Burford et al., 1990a, 1990b; US EPA, 2006a; APVMA, 2010; Health Canada, 2011). Both young (including fetuses) and adult animals had similar sensitivity to the ChE-inhibiting effects of dimethoate as seen in the range-finding study for the DNT study and the comparative ChE study. In the comparative ChE study adults and offspring had the same NOAEL values (0.5 mg/kg bw per day for acute exposure and 0.1 mg/kg repeat exposure) (Myers, 2001c; US EPA, 2004; Health Canada, 2011). Overall, no pronounced sex differences were apparent in the available database for dimethoate. A comparison of data from rat studies of different durations showed toxicity increased with repeat-dose exposure compared to a single exposure. However, the differences in the methods of exposure (gavage versus dietary) are a confounding factor (Health Canada, 2011).
In addition to ChE inhibition, increased pup mortality was seen in two 2-generation reproductive studies and in a 1-generation range-finding reproductive toxicity study, as well as the range-finding study for the DNT study. However, it was not seen in the DNT study itself as brain ChE was not measured. The DNT study did assess behavioural and neuropathological effects in dams and offspring, with offspring (but not dams) showing effects at ≥ 0.5 mg/kg bw per day. A dose-related increase in pup deaths was observed in the DNT study in the absence of overt signs of maternal toxicity during early lactation in mid- and high-dose pups (including total litter loss in one female in the 0.5 mg/kg bw per day dose group and in three females in the 3.0 mg/kg bw per day dose group). Affected pups were small in size, cold to the touch and had little food in their stomachs. The offspring NOAEL was 0.1 mg/kg bw per day based on increased pup mortality and on changes in motor activity (Myers, 2001b; US EPA, 2004). Increased pup deaths and total litter loss were also seen in the range-finding study for the main DNT study but occurred at 6.0 mg/kg bw per day (Myers, 2001a; APVMA, 2010; Health Canada, 2011).
The comparative ChE study found no significant treatment-related effects on any reproductive or developmental parameters, including number of live births, corpora lutea, implantations, or total resorptions, or on pre- and post-implantation loss, fetal body weights or sex ratio. No clinical signs or increased mortality were observed in adult male or female rats, fetuses or offspring at any dose (Myers, 2001c; US EPA, 2004; Health Canada, 2011).
Given the increased incidence of pup mortality in the DNT study and the potential disruption in dam behaviour related to ChE inhibition, a limited cross-fostering study was conducted to determine the influence of pre- and post-natal maternal exposure on pup mortality. Pregnant dams were dosed with 0, 3 or 6 mg/kg bw per day of dimethoate from gestation day (GD) 6 to postnatal day (PND) 11. On PND 1, some litters were reallocated from control dams to treated dams and vice versa, as described in Table 5. Pups were not directly treated with dimethoate. Treated dams (both doses) had forelimb hair loss and were more restless, scattering their litters; however, neurobehavioural testing did not show any treatment-related effects. The number of pups with no milk in their stomachs was increased in litters cared for by treated dams (both doses). Pup mortality was increased and was positively correlated with the dam's dose level and duration of treatment, but was also somewhat related to maternal restlessness and litter scattering. The cross-fostering study therefore did not resolve whether the pup mortality was due to maternal neglect or dimethoate exposure. The study identified a LOAEL of 3 mg/kg bw per day for both maternal and pup toxicity (Myers, 2004; Health Canada, 2011).
| Study (References) | Method | NOAEL (mg/kg bw per day) |
BMDL10 for brain ChEc inhibition (mg/kg bw per day) |
Effects (mg/kg bw per day) |
|---|---|---|---|---|
DNT main study |
23-24 pregnant CD rats/dose gavaged with 0, 0.1, 0.5 or 3.0 mg/kg bw per day in water from GD 6 to PND 10; Pups exposed in utero and via lactation until PND 10 then gavaged at maternal doses from PND 11 to 21 |
0.1 (offspring) 3.0 (maternal, developmental) |
Not calculated | Dams: no adverse effects Developmental/reproductive: Pups: ≥ 0.5: increased deaths including total litter loss with pups small, cold to the touch and little/no food in stomachs (dams of affected litters were underactive/inattentive), increased horizontal activity (PND 17 in males) 3.0: decreased motor activity, righting reflexes, and rearing Note: ChE not measured in this study; see ChE data in the comparative ChE companion study |
Comparative ChE companion study to DNT study |
Same gavage dosing as main DNT; Acute exposure: 8 PND 11 pups or 8 adult CD rats/sex/dose Repeat exposure: pregnant CD rats 9/dose/day from GD 6–20 and terminated or 10/dose/day GD 6 to PND 10 followed by 1/sex/litter offspring treated from PNDs 11–21, or 8 adults/dose for 11days |
0.5 (acute: pups and adults) 0.1 (repeat: fetuses, pups, maternal, and adults) |
1.3–2 (acute: pups and adults) 0.2–0.7 (repeat: fetuses, pups, maternal, and adults) |
Acute exposure 3.0: decreased brain and plasma ChE (PND 11pups and adults), decreased erythrocyte ChE (adult females), Repeat exposure Maternal/adult ≥ 0.5: decreased brain ChE (GD 20) 3.0: decreased erythrocyte and plasma ChE (GD 20) Fetal/Offspring ≥ 0.5: decreased brain ChE (fetuses, PND 21 pups), decreased erythrocyte ChE (PND 21 female pups) 3.0: decreased brain ChE (PND 4 male pups), decreased erythrocyte ChE (fetuses, PNDs 4 and 21 male pups) |
Range-finding for DNT study |
10 pregnant CD rats/dose gavaged with 0, 0.2, 3.0 or 6.0 mg/kg bw/day from GD 6 to PND 10; plus 5 dams/dose gavaged from GD 6–20 for ChE determination
Pups exposed in utero and via lactation until PND 10 then gavaged from PND 11–21 at maternal doses (2 pups/sex/litter). |
0.2 (maternal, offspring) 3.0 (developmental) |
0.2–0.4 (dams, fetuses, pups) |
Maternal: ≥ 3.0: decreased maternal weight gain during gestation, decreased brain, erythrocyte and plasma ChE (GD 20) Developmental/reproductive: Offspring: ≥ 3: decreased brain, erythrocyte and plasma ChE at GD 20 and PND 21 6.0: increased deaths and total litter loss in PND 1-4, decreased viability index at PND 4 (precull), decreased pup weight gain (PND 1–11) Note: Due to small sample size, parameters were not analysed statistically except body weight |
Cross-fostering |
Pregnant CD rats gavaged with
0 (100 dams), 3 (25 dams) or 6 (50 dams) mg/kg bw/day from GD 6 to PND 10, litters cross-fostered on PND 1 as follows: control litters (¼ reared by their own control dam, ¼ to each of 3 and 6 mg/kg groups and ¼ discarded), 3 mg/kg litters (all to control), 6 mg/kg litters (½ each to control and 6 mg/kg groups) |
LOAEL of 3 |
Not calculated | Maternal 3: forelimb hair loss, marginal decreased weight gain, increased restlessness and scattering of pups Offspring 3: decreased milk consumption, increased blood urea, slight increased mortality 6: increased mortality, hematological and blood chemistry changes, decreased surface righting reflex on PND 10, increased weight gain PND1–11 (post-natal treatment only |
Chronic/Carcinogenicity |
65 Wistar rats/sex/dose fed diets containing 0, 5, 25 or 100 ppm (equal to 0, 0.25, 1.25, or 5 mg/kg bw per day) for 2 years; additional 20 animals/sex given 1 ppm equal to 0.05 mg/kg bw per day to assess ChE inhibition) | 0.05 mg/kg bw/day based on ChE inhibition |
0.22–0.31 | ≥ 0.25: decreased brain ChE and erythrocyte ChE, increased incidence of vascular tumors (combined hemangioma and hemangiosarcoma in the spleen, lymph node and skin) ≥ 1.25: decreased plasma ChE 5: transiently decreased body weight gain in males; increased mortality (females only near end of the study), increased anemia in males, increased leukocytes |
2-generation reproduction – Sprague Dawley |
28 Sprague Dawley rats/sex/dose fed 0, 1, 15 or 65 ppm (equal to 0.08, 1.2 or 5.46 mg/kg bw per day in males and 0, 0.09, 1.3 or 6.04 mg/kg bw per day for females) in diet for 2 generations; 2 litters per generation | 0.08/0.09 males/females 1.2/1.3 males/females |
0.3–0.7 | Parental
Reproductive Offspring |
2-generation reproduction - Wistar |
25 Wistar rats/sex/dose fed 0, 0.2, 1.0 or 6.5 mg/kg bw/day in diet for 2 generations
Brain ChE measured in pups of the controls and 6.5 mg/kg/day groups only |
0.2 6.5 1.0 |
0.2–0.5 | Parental
Reproductive Offspring |
In addition to pup mortality and changes in motor activity, dimethoate also caused reproductive effects (decreased pregnancy rates, decreased litter size, decreased pup weight gain, prostate effects) in two 2-generation reproduction studies (Brooker et al., 1992; Mellert et al., 2003; US EPA, 2004; APVMA, 2010; Health Canada, 2011).
The available toxicology profile of omethoate is similar to that of dimethoate, but omethoate was a more potent ChE inhibitor in subchronic and chronic studies, with NOAEL values of 0.04 mg/kg bw per day for Wistar rats and 0.1 mg/kg bw per day for mice and a LOAEL of ≥ 0.125 mg/kg bw per day for Beagle dogs being reported (Hoffmann and Schilde, 1984; Schladt, 1995, 2001; Health Canada, 2011). Omethoate is also much more acutely toxic than dimethoate based on the median lethal dose 50% (LD50) and median lethal concentration 50% (LC50) values obtained in oral, dermal and inhalation studies (Table 6) (Health Canada, 2011). For both dimethoate and omethoate, clinical signs of acute toxicity were consistent with those of acute organophosphate poisoning (e.g., muscular fibrillation, salivation, lacrimation, urinary incontinence, diarrhea, respiratory distress, prostration, gasping, coma and death) (Health Canada, 2011).
| Acute Value (unit) | Species | Dimethoate | Omethoate | Reference |
|---|---|---|---|---|
| LD50 oral (mg/kg bw) |
Rat | 310–600 | 22–65 | WHO, 2004; EFSA. 2006; Health Canada, 2011 |
| LD50 oral (mg/kg bw) |
Mouse | 150–160 | 27–36 | EFSA. 2006; APVMA, 2010; Health Canada, 2011 |
| LD50 oral (mg/kg bw) |
Rabbit | 300 | 50 | Health Canada, 2011 |
| LD50 dermal (mg/kg bw) |
Rabbit | >2000 | No data | Health Canada, 2011 |
| LD50 dermal (mg/kg bw) |
Rat | >2000 | 145–232 | APVMA, 2010; Health Canada, 2011 |
| LC50 (mg/L) |
Rat | >2 | 0.282 | Health Canada, 2011 |
Omethoate was not teratogenic in two separate studies using pregnant Long Evans FB rats (20 to 24 per dose) and pregnant Wistar rats (25 per dose) gavaged with 0, 0.3, 1.0 or 3.0 mg/kg bw per day of omethoate on GD 6 through 15. However, in Long Evans rats, decreased fetal weight and increased resorptions were observed in the high-dose group in the presence of maternal toxicity. The NOAEL for maternal and developmental effects was 1.0 mg/kg bw per day (Bayer, 1975; Holzum, 1990a; Health Canada, 2011).
Developmental effects (decreased pup weight and increased postnatal loss) and reproductive effects (increased implantation loss, increased pre-coital interval, decreased litter size and increased epithelial vacuolation in epididymides of males) were seen in Wistar rats in the presence of parental toxicity in a 2-generation reproduction study using omethoate. The NOAEL was 0.5 ppm for developmental effects and 3 ppm for reproductive effects, while the LOAEL for parental effects was 0.5 ppm (Dotti et al., 1992; Health Canada, 2011). Developmental studies using rabbits showed increased incidence of contracted joints in pups of Himalayan rabbits but not of New Zealand White rabbits. In the absence of historical control data on Himalayan rabbits and a lack of a dose-response relationship, it is not certain whether the increase was due to omethoate. Other developmental effects included decreased fetal weight (Long Evans rats) and increased resorptions (Long Evans rats and Himalayan rabbits). However, developmental effects were reported in the presence of maternal toxicity (decreased maternal weight gain or decreased cholinesterase activity) (Tesh et al., 1982; Holzum, 1990b; Health Canada, 2011).
2.5 Genotoxicity and carcinogenicity
Dimethoate was not genotoxic in several in vitro (Ames tests, Chinese hamster ovary/ hypoxanthine-guanine phosphoribosyltransferase (HGPRT) gene mutation assay, unscheduled DNA synthesis (UDS) assay) and in vivo (mouse dominant lethal assay, bone marrow cytogenic assay in rats, UDS assay) assays (Health Canada, 2011). Positive results were reported in one in vitro UDS assay but at cytotoxic levels; both a second in vitro UDS assay and an in vivo UDS assay using male Wistar rats were negative (Health Canada, 2011).
Although a 2-year study in which Wistar rats were given dimethoate at 0.5, 4 or 32 ppm (equivalent to 0.04/0.05, 0.30/0.44, 2.92/3.93 mg/kg bw per day in males/females) in drinking water showed an increased incidence of vascular tumours in males only, the results are considered equivocal based on the lack of a dose-response relationship, on the presence of tumours in one sex only, on marginal statistical significances, and on lower than expected incidences in control animals (4%) when compared to historical control data (16% and 22%) (Health Canada, 2011, 2015).
The PMRA has concluded that dimethoate is unlikely to pose a carcinogenic risk to humans (Health Canada, 2015). The Australian Pesticides and Veterinary Medicines Authority (APVMA), analogous to the PMRA, did not consider dimethoate to be genotoxic or carcinogenic (APVMA, 2010).
The US EPA has classified dimethoate as "Group C - possible human carcinogen". This is based on equivocal hemolymphoreticular tumours in male B6C3F1 mice, the weak (no dose response) effect of combined spleen, skin and lymph tumours in male Wistar rats, and positive mutagenic activity (positive gene mutation and structural chromosome aberrations, bacterial mutation and clastogenic effects in vitro and in vivo) associated with dimethoate (US EPA, 1995, 2007). The International Agency for Research on Cancer (IARC) has not reviewed the carcinogenicity of dimethoate.
For omethoate, genotoxicity assays were mostly negative, except for positive results observed in an in vivo mouse spot test, a gene mutation assay at very high dose levels and in in vitro assays for DNA repair and sister chromatid exchange. No effects were observed in in vivo assays of DNA repair and sister chromatid exchange (Health Canada, 2011; NHMRC, NRMMC, 2011).
No carcinogenicity was evident in rats and mice following chronic dosing with omethoate (Health Canada, 2011). The APVMA did not consider omethoate to be genotoxic or carcinogenic (APVMA, 2011). Neither the US EPA nor the IARC have reviewed the carcinogenicity of omethoate.
2.6 Mode of action
Dimethoate causes neurotoxicity when it is activated in vivo to its oxon metabolite, omethoate (US EPA, 2004). Like other organophosphate pesticides, the oxon inhibits ChE through phosphorylation of the enzyme active site. This inhibition leads to an accumulation of acetylcholine and to the continuous stimulation of cholinergic receptors throughout the central and peripheral nervous systems resulting in cholinergic toxicity (US EPA, 2004).
The mode of action of dimethoate in causing pup mortality is not well understood (US EPA, 2006a).
2.7 Selected key endpoint
The toxicological database for dimethoate is extensive and includes prenatal developmental toxicity studies in rats and rabbits, two multi-generation reproduction studies, a DNT study, a comparative ChE study (examining fetuses, pups, pregnant animals and adult animals) and a special cross-fostering study (Health Canada, 2011). From these studies, two potential points of departure (POD) for dimethoate were identified: inhibition of brain ChE and pup mortality (Table 5).
ChE inhibition has been consistently seen in a wide variety of studies (subchronic and chronic dietary, developmental and reproductive, comparative ChE). Rats appeared to be slightly more sensitive than other species (i.e., mouse and dog) to ChE inhibition based on NOAEL/LOAEL values. Overall, no pronounced sex differences were apparent in the available database, despite a few studies in rats that showed females were slightly more sensitive than males to the inhibitory effects of dimethoate. Although no adverse maternal toxicity was seen in the DNT study, a dose-related increase in pup deaths was observed at ≥ 0.5 mg/kg bw per day (LOAEL) and was lower than the pup mortality observed in the DNT range-finding study (LOAEL = 6 mg/kg bw per day) and other reproductive studies. The NOAEL for offspring in the main DNT study was determined by the US EPA and PMRA to be 0.1 mg/kg/day, based on increased pup death and increases in motor activity. In the companion ChE study, the NOAEL for ChE inhibition was 0.1 mg/kg/day following repeated administration; similar inhibition levels were seen in adults and young animals and there were no differences in the NOAELs among age groups. At the lowest dose showing an effect on pup mortality, a comparable level of brain ChE inhibition (10% to 13%) was observed between subpopulations (pregnant dams, fetuses, 4-day old pups, 21-day old pups and adult rats exposed for 11 days). A cross-fostering gavage study of dimethoate in rats showed that pup mortality ensued from either prenatal exposure, post-natal exposure or combined pre- and post-natal exposure. The cross-fostering study therefore did not resolve whether pup mortality was due to maternal neglect (Myers, 2004; US EPA, 2004; Health Canada, 2011).
Benchmark dose (BMD) modelling was undertaken to better reflect the POD for the brain ChE inhibition responses as well as that of pup mortality. The BMD approach is a scientifically more advanced method compared to the NOAEL approach for deriving a POD, since it makes use of all of the available dose-response data from a given study (or studies if using data from multiple studies) and it provides a quantification of the uncertainties in the dose-response data. Detailed BMD calculations are available in Dimethoate: Issues Related to the Hazard and Dose Response Assessment jointly prepared by the US EPA and PMRA (US EPA, 2004). The dataset for BMD analyses was considered robust in that the similarity of protocols of several studies yielded replicate information. Further, robust dose-response curves were attainable from studies of varying modes of administration and durations. BMD analysis was undertaken using an exponential dose-response model and a lower confidence limit of 95% (BMDL). An increase of 5% above background (BMD5) was considered the smallest detectable change for pup mortality. For brain cholinesterase inhibition, a BMD10 was the limit of sensitivity for detecting a statistically significant decrease in ChE activity (Health Canada, 2011).
BMD10 (0.20 to 1.0 mg/kg bw per day) and BMDL10 (0.2 to 0.7 mg/kg bw per day) values for brain ChE inhibition following repeated dosing did not show any age-related differences. BMDL10 (1.3 to 2.0 mg/kg bw) estimates for brain ChE inhibition following single doses of dimethoate were also comparable for pups and adults (Table 5) (US EPA, 2004). PMRA selected a BMDL10 for brain ChE inhibition of 0.2 mg/kg bw per day based on similar values obtained from several repeat-dose oral studies (i.e., 8-day exposures to adult male rats in the comparative cholinesterase gavage study, ~15-day exposures to pregnant dams in the range-finding gavage study, 2-year exposures in the chronic dietary study and > 3-month exposures in the multi-generation reproduction dietary study). The BMD analyses for ChE inhibition showed similar dose-response curves and BMD10 values for all ages following similar exposure durations (Health Canada, 2011).
With respect to potential pre- and post-natal toxicity, prenatal developmental toxicity studies in rats and rabbits provided no indication of increased susceptibility of rat or rabbit fetuses to in utero exposure to dimethoate or omethoate. Similarly, there was no indication of increased susceptibility in the offspring compared to parental animals in the reproduction studies. An increase in pup mortality in repeat-dose studies was observed, with the most sensitive study being the dimethoate DNT study, although no clear association could be made with a specific level of brain ChE inhibition. A meta-analysis of pup mortality in the dimethoate database yielded a BMDL5 of 0.64 mg/kg bw per day. The BMD analyses also indicated that use of the lower 95% confidence limit (BMDL10) for brain cholinesterase inhibition would be protective to the BMDL5 for pup mortality (Health Canada, 2011).
The PMRA and the US EPA jointly consulted the US Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) Scientific Advisory Panel (SAP) regarding its dimethoate hazard and dose-response assessment and its BMD modelling (US EPA and Health Canada, 2004b; Health Canada, 2011). SAP agreed that while the underlying cause of pup mortality could not be determined from the data, the selection of brain ChE inhibition as a critical effect would be protective against pup mortality (FIFRA, 2005; US EPA, 2004; US EPA, 2006b; Health Canada, 2011).
The PMRA has therefore identified the inhibition of brain ChE as the most sensitive indicator of dimethoate toxicity and the BMDL10 of 0.2 mg/kg bw per day as the POD. This value is consistent with NOAELs of 0.2 mg/kg bw per day of dimethoate observed in human volunteer studies previously discussed in Section 2.3.
3.0 Derivation of the health-based value
A BMD approach was used for the determination of the acceptable daily intake (ADI) rather than the NOAEL/LOAEL approach since it offers better dose-response characterization by including all experimental data to determine POD independently of pre-established dose level. The BMDL10 of 0.2 mg/kg bw per day for ChE inhibition was selected as the basis for the current risk assessment as it was protective of both ChE inhibition and pup mortality. An uncertainty factor of 100 was applied to account for interspecies extrapolation (10-fold) and intraspecies variability (10-fold) (Health Canada, 2011).
Using this BMDL10, the ADI for dimethoate (Health Canada, 2011) was calculated as follows:

Equation 1 - Text description
The ADI for dimethoate is 0.002 mg/kg bw per day. This is calculated by dividing the BMDL10 of 0.2 mg/kg bw per day by the uncertainty factor of 100.
where:
- 0.2 mg/kg bw per day is the BMDL10, based on cholinesterase inhibition; and
- 100 is the uncertainty factor, selected to account for interspecies variation (×10), intraspecies variation (×10).
Based on the ADI of 0.002 mg/kg bw per day, a health-based value (HBV) for dimethoate in drinking water was derived as follows:
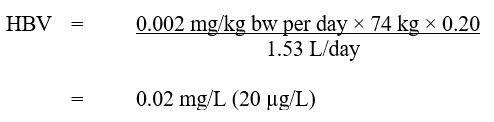
Equation 2 - Text description
The HBV for dimethoate in drinking water is 0.02 mg/L. This is calculated by multiplying the ADI for dimethoate (0.002 mg/kg bw per day) by the allocation factor for water (0.20), then by the average body weight for an adult (74 kg). This product is then divided by the daily volume of water consumed by an adult (1.53 L/day).
where:
- 0.002 mg/kg bw per day is the ADI calculated using a BMDL10 of 0.2 mg/kg bw per day (Health Canada, 2011);
- 74 kg is the adult body weight (Health Canada, in preparation);
- 1.53 L per day is the daily volume of tap water consumed by an adult (Health Canada, in preparation);
- 0.20 is the default allocation factor for drinking water (Krishnan and Carrier, 2013).
This document proposes an additive approach for addressing omethoate in drinking water. Generally, when chemical residues (omethoate) are combined and expressed in parent equivalents (dimethoate), the residues are converted to stoichiometric equivalents by multiplying the chemical residue level by the molecular weight (MW) ratio (parent/residue). The MWs of dimethoate and omethoate are 229 g/mol and 213 g/mol, respectively. This yields a MW ratio of 219/213=1.075. Additionally, the available data showed that omethoate is a more potent ChE inhibitor than dimethoate. To account for this increased toxicity, omethoate (expressed as a stoichiometric equivalent) detected in source or drinking water should be multiplied by a toxicity adjustment factor (TAF). PMRA has calculated a TAF of 3 for omethoate based on ratios of dimethoate and omethoate benchmark doses derived from female rat brain ChE BMD10 responses (Health Canada, 2011). The resulting value for the concentration of omethoate (expressed as a dimethoate equivalent value) is added to the measured concentration of dimethoate. The sum of the detected concentrations of dimethoate and omethoate (with omethoate expressed as a dimethoate equivalent) should not exceed the MAC for dimethoate.
Below is a sample calculation showing the use of the TAF to convert omethoate into a dimethoate equivalent value and the summation of the omethoate (as dimethoate equivalent value) and dimethoate in order to compare the value to the MAC. The calculation assumes a measured concentration of 0.5 µg/L for omethoate and of 0.3 µg/L for dimethoate in drinking water.

Equation 3 - Text description
The dimethoate equivalent value of omethoate is 1.6125 µg/L. This is calculated by multiplying the omethoate measured value 0.5 µg/L by the molecular weight ratio of 1.075 by the toxicity adjustment factor of 3.

Equation 4 - Text description
The sum of dimethoate in example is 1.9125 µg/L rounded to 1.9 µg/L. This is calculated by adding the dimethoate value of 0.3 µg/L and dimethoate equivalent value of 1.6125 µg/L. This sum of 1.9 µg/L is then compared to the MAC of 20 µg/L.
This sum (1.9 µg/L) is then compared to the MAC (20 µg/L).
4.0 Analytical and treatment considerations
Information on analytical and treatment considerations is readily available for dimethoate, but is limited for omethoate. Standardized analytical methods are available for the analysis of dimethoate. However, none are available for omethoate and, therefore, a research method for analysis is presented. In terms of treatment, there are several studies on various technologies for dimethoate removal and only a few studies for the removal of omethoate by adsorption. As omethoate is formed through oxidation of dimethoate, the overall treatment approach should be to remove dimethoate while minimizing the formation of omethoate.
4.1 Analytical methods to detect dimethoate and omethoate
Standardized methods available for the analysis of dimethoate in source and drinking water and their respective MDLs are summarized in Table 7. MDLs are dependent on the sample matrix, instrumentation, and selected operating conditions and will vary between individual laboratories. These methods are subject to a variety of interferences which are outlined in the respective references.
A number of accredited laboratories in Canada were contacted to determine the MDLs and MRLs for dimethoate analysis. The MDLs were in the lower range of those reported in Table 7. The MRLs were between 0.03 and 0.2 μg/L using modified EPA 8270; and 0.5 μg/L using modified EPA 8141 (ALS Environmental, 2019; CARO Analytical Services - Richmond Laboratory, 2019; Element Materials Technology Canada Inc., 2019; SGS Environmental Services, 2019).
The MDLs or MRLs from provincial and territorial data are in the range of 0.0001 to 10 μg/L (see Section 1.3).
Additional analytical methods that are not currently standardized are available for the measurement of dimethoate in water. These methods are based on high performance liquid chromatography (HPLC) with tandem mass spectrometry (Charalampous et al., 2015). MDLs similar to those of the standard methods listed below have been reported and these methods are suitable for use in commercial laboratories (Haiste-Gulde and Sacher, 2019).
There is no standardized method available for the detection of omethoate. A method developed by Cheminova A/S for the determination of dimethoate and omethoate in tap and surface water (US EPA, 2000a) was evaluated by the US EPA. The method is based on gas chromatography and mass spectroscopy and has a level of quantification of 0.05 μg/L. Although the US EPA recognizes that the method may be useful for analysis of omethoate (and dimethoate), they do not consider it an independently validated method (US EPA, 2000b). A few studies present methods for omethoate detection in water using HPLC that use various detection systems (Hayama et al., 2008; Ling et al., 2011; Zheng et al., 2016). A method using HPLC with tandem mass spectrometry was successfully demonstrated to detect a concentration of 0.05 μg/L with appropriate quality control (Hayama et al., 2008).
Drinking water utilities should discuss sampling requirements with the accredited laboratory conducting the analysis to ensure that quality control procedures are met and that MRLs are low enough to ensure accurate monitoring at concentrations below the MAC. Sample processing considerations for the analysis of dimethoate in drinking water (e.g., sample preservation, storage) can be found in the references listed in Table 7. It is important to note that quenching is critical if an oxidant is present in samples in order to prevent additional degradation of dimethoate or omethoate prior to analysis.
| MethodFootnote a (Reference) |
Methodology | MDL (µg/L) |
|---|---|---|
| US EPA Methods | ||
| Method 527 (US EPA, 2005) |
Capillary Column Gas Chromatography/Mass Spectrometry (GC/MS) | 0.025 |
| Method 8141B (US EPA, 2000c) |
Gas Chromatography | 0.26 |
| Method 8270D (US EPA, 1998) |
Gas Chromatography/ Mass Spectrometry (GC/MS) | 20 (estimated quantitation limit) |
4.2 Treatment considerations
Treatment technologies that are available to effectively decrease dimethoate concentrations in drinking water include activated carbon adsorption, membrane filtration (nanofiltration [NF] and reverse osmosis [RO]) and biological filtration. Although chlorination can achieve 100% removal (Ormad et al., 2008; WHO, 2017), omethoate and other by-products are formed in the degradation process (Caregnato et al., 2013; Tian et al., 2014). At the residential scale, certified treatment devices relying on RO or activated carbon adsorption are expected to be effective for removal of dimethoate.
4.2.1 Municipal-scale treatment
The selection of an appropriate treatment process for a specific water supply will depend on many factors, including the raw water source and its characteristics, the operational conditions of the selected treatment method and the water utility's treatment goals. Bench or pilot testing is recommended to ensure the source water can be successfully treated and optimal process design is established.
When using oxidation or advanced oxidation processes or biological processes for pesticide removal in drinking water, it is important to be aware of the potential for formation of by-products due to degradation of the target compound (Ikehata and Gamal El-Din, 2006; Beduk et al., 2012; Li et al., 2019a). Omethoate is an oxidation degradation by-product of dimethoate that is formed through treatment (oxidation), but there is minimal research on the removal of omethoate itself from water. Accordingly, the objective should be both to remove dimethoate and to select a treatment technology that will minimize the formation of omethoate and other by-products. With such an approach, subsequent removal of omethoate and other by-products should not be required. In addition, water utilities should consider the potential for the formation of disinfection by-products depending on the oxidant selected and the source water quality.
4.2.1.1 Conventional treatment
Conventional filtration (chemical coagulation, clarification, and rapid sand filtration) and chlorine disinfection will reduce dimethoate concentrations through oxidation during the disinfection step (Ormad et al., 2008). However, formation of by-products through oxidation may occur.
Coagulation and flocculation alone have limited effectiveness for removing dimethoate. A bench-scale study by Ormad et al. (2008) using river water with an initial dimethoate concentration of 1.108 μg/L and pH of 8.0 was conducted, comparing the removal efficiency with three different coagulant doses of aluminum sulphate (10 mg Al/L, 20 mg Al/L and 40 mg Al/L). Even with a high coagulant dose, a low removal efficiency of only 35% was achieved for all doses.
4.2.1.2 Activated carbon adsorption
Activated carbon adsorption is a widely used technology to reduce the concentration of micropollutants, including pesticides, in drinking water (Haist-Gulde and Happel, 2012; van der Aa et al., 2012). Activated carbon can be applied in two ways: in slurry applications using powdered activated carbon (PAC) or in fixed bed reactors with granular activated carbon (GAC) (Chowdhury et al., 2013).
Data generated through bench-scale testing to determine adsorption coefficients for pesticides are useful in predicting whether activated carbon adsorbs a particular pesticide (US EPA, 2011). In general, pesticides with an adsorption capacity constant (e.g., Freundlich coefficient) greater than 200 µg/g(L/µg)1/n are considered to be amenable to removal by carbon adsorption (Speth and Adams, 1993; Speth and Miltner, 1998; US EPA, 2011). However, it is important to note that the presence of natural organic matter (NOM) adds complexity to activated carbon treatment because NOM competes directly for adsorption sites or fouls the carbon by blocking pores (Chowdhury et al., 2013). Since the capacity of activated carbon can be affected by many factors, including the compound's ionic character and the solution pH, appropriate testing (e.g., jar tests, rapid small scale column tests) should be conducted to confirm removal.
A study by Brauch and Kühn (1988) states that the Freundlich isotherms for atrazine and dimethoate are similar and that the high value of Freundlich isotherm indicates that dimethoate is expected to be removed by activated carbon.
Powdered activated carbon
Many pesticides have been found to strongly adsorb to PAC (Chowdhury et al., 2013), and its use offers the advantage of providing virgin carbon when required (e.g., during the pesticide application season) (Miltner et al., 1989). The capacity of PAC to remove pesticides by adsorption depends on its dose, its characteristics (type, particle size), the contact time, the adsorbability of the contaminant and the competition for adsorption sites from NOM (Haist-Gulde and Happel, 2012; Chowdhury et al., 2013).
Two bench-scale studies were conducted to evaluate the application of PAC in the removal of dimethoate; they are presented in Table 8. The study by Ormad et al. (2008) investigated several treatment options and found that removal by PAC was better than that observed for coagulation/flocculation. The study by Miguel et al. (2008) evaluated two different PACs with two types of water and found that one PAC performed better with distilled water, while the other performed better with natural water. This finding highlights the need to conduct jar tests to assess performance.
The removal of dimethoate from water depends on the water matrix, the PAC type and the contact time; the observed reduction rates ranged from 30% to 60% (Miguel et al., 2008; Ormad et al., 2008).
| Influent (µg/L) |
PAC Dose (mg/L) |
Removal | Process Description | Reference |
|---|---|---|---|---|
| 1.108 | 10 | 60% | Bench-scale: Untreated river water: pH 8.0 Reaction time not provided |
Ormad et al. (2008) |
| 0.5 | 10 (wood-based) |
40% | Bench-scale: Distilled water: pH 5.5; DOC = 0 Residence time = 10 min |
Miguel et al. (2008) |
| 0.5 | 10 (bituminous-based) |
30% | ||
| 0.5 | 10 (wood-based) |
35% | Bench-scale: Natural water: pH 8.0; DOC = 3 mg C/L Residence time = 10 min |
|
| 0.5 | 10 (bituminous-based) |
45% | ||
Granular activated carbon
The use of GAC is an effective approach for treating organic contaminants that are regularly found in source water at concentrations of concern (Chowdhury et al., 2013). The capacity of GAC to remove pesticides by adsorption depends on its characteristics (type, particle size, reactivation method), the filter velocity, empty bed contact time (EBCT), the adsorbability of the contaminant, and the filter run time (Haist-Gulde and Happel, 2012). In addition, because GAC fixed bed adsorbers are typically operated on a continuous basis, the GAC can become fouled (or preloaded) with NOM and may be completely or partially ineffective for pesticide removal (Knappe et al., 1999; Summers et al., 2010; Haist-Gulde and Happel, 2012; Chowdhury et al., 2013).
A pilot-scale study was conducted evaluating removal of 33 organic pollutants (including dimethoate) using GAC (Summers et al., 2014). Four different waters with different pH and dissolved organic carbon (DOC) levels were evaluated and the results are presented in Table 9. The authors stated that dimethoate was moderately adsorbed by GAC.
| Influent (ng/L) | Bed Volumes to 10% Breakthrough | Water | GAC | EBCT (min) | Bed Length (cm) | Operating Conditions |
|---|---|---|---|---|---|---|
| 115 ± 27 | 70 000 | Water type 1: DOC = 3.9 mg/L pH 7.0 |
Bituminous Mean particle diameter = 0.92 mm |
7 | 58 | Bed diameter = 25.4 mm Hydraulic loading = 5 m/h |
| > 46 000 | 15 | 125 | ||||
| 52 000 | Water type 2: DOC = 2.8 mg/L pH 7.7 |
Bituminous Mean particle diameter = 1.29 mm |
7.5 | 63 | ||
| 120 000 | Water type 3: DOC = 2.1 mg/L pH 6.0 |
Bituminous Mean particle diameter = 0.92 mm |
7 | 58 | ||
| 75 000 | Water type 4: DOC = 1.7 mg/L pH 6.2 |
|||||
4.2.1.3 Membrane filtration
In general, NF and RO are effective pressure-driven membrane processes for the removal of pesticides from drinking water. Their effectiveness in removing pesticide is dependent on the membrane characteristics, pesticide properties, feed water composition, operating conditions and membrane fouling (Van der Bruggen and Vandecasteele, 2003; Plakas and Karabelas, 2012).
Since the main mechanism for pesticide removal using NF and RO membranes is size exclusion, the molecular weight cut-off (MWCO) of the membrane is an important characteristic. The molecular weight of dimethoate is 229 Da; membrane technology could be effective if the MWCO of the chosen membrane is appropriate. Retention of small pesticide molecules by larger pore-size membranes can also be influenced by the physicochemical interactions between the pesticide and the membrane surface (Plakas and Karabelas, 2012).
Three bench-scale studies investigated four different polyamide NF membranes (NF90, NF200, NF270 and DK) to assess removal of high concentrations of dimethoate from aqueous solutions (Ahmad et al., 2008a, 2008b; Tan et al., 2019). The membrane separation process can be affected by the surface morphology of the membrane (related to roughness) and the pore-size distribution (related to the pore size and porosity) (Hilal et al., 2015). The membrane characteristics, along with the MWCO, are presented in Table 10. The first bench-scale study illustrated higher rejection with higher operating pressure and lower initial concentration of dimethoate (Ahmad et al., 2008a) (Table 11). The second study showed that for three of the membranes (NF200, NF270 and DK), an increase in pH resulted in increased rejection and decreased permeate flux (Ahmad et al., 2008a), while the fourth membrane (NF90) was relatively unaffected by changes in pH.
The bench-scale study by Tan et al. (2019) investigated removal of atrazine and dimethoate through four different feed waters (deionized, distilled, tap and river; Table 12). The dimethoate rejection results from the deionized and distilled waters were similar when compared to those of the previous studies conducted using deionized water. Increased dimethoate rejection was observed for tap and river waters, and the authors noted that this may be due to binding of other ions and NOM with dimethoate to form larger particles. Atrazine was found to have better rejection in all cases when compared to dimethoate, even though dimethoate has a larger molecular weight. This may be a result of dimethoate being more polar and less hydrophobic than atrazine, thereby decreasing the potential for rejection.
Bellona et al. (2004) present a flow-chart using the characteristics of the pesticide in water (e.g., molecular weight, log Kow, molecular diameter) and those of the membrane properties (e.g., MWCO, pore size) to determine the potential for removal of dimethoate by membrane filtration. It is important to perform appropriate testing prior to full-scale implementation with membrane and source water under the proposed operating conditions to ensure that adequate dimethoate removal is occurring.
| Membranea | MWCO (DFootnote a) | Average Roughness (nm) | Average Porosity (%) | Average Pore Size (nm) |
|---|---|---|---|---|
| NF90 | 90Footnote b Footnote c | 22.7632Footnote d | 17.1Footnote d | 0.55Footnote d |
| NF200 | - | 2.7098Footnote d | 15.5Footnote d | 0.31Footnote d |
| NF270 | 150Footnote c | 3.36Footnote d | 11.7Footnote d | 0.71Footnote d |
| DK | 150-300Footnote b | - | - | 0.55Footnote e |
| Influent (mg/L) |
Rejection (%)Footnote a,Footnote b |
Conditions | Process DescriptionFootnote c | Reference | |||
|---|---|---|---|---|---|---|---|
| NF90 | NF200 | NF270 | DK | ||||
| 2 | 85 | 55 | 25 | 75 | P = 6 x 105 Pa | Bench-scale: Deionized water; 25 ± 2 ˚C Membranes surface charge at pH 7 is negative |
Ahmad et al. (2008Footnote a) |
| 84 | 40 | 22 | 65 | P =12 x 105 Pa | |||
| 20 | 85 | 60 | 35 | 80 | P = 6 x 105 Pa | ||
| 84 | 55 | 34 | 70 | P =12 x 105 Pa | |||
| 10 | 80 | 42 | 22 | 41 | P = 6 x 105 Pa pH 4 |
Ahmad et al. (2008Footnote b) | |
| 82 | 55 | 38 | 40 | pH 7 | |||
| 80 | 68 | 45 | 50 | pH 9 | |||
10 (10 mg/dm3) |
80 | 54 | 40 | 52 | Deionized | Bench-scale: Investigation of dimethoate removal with four different waters (Table 12) P = 6 x 105 Pa |
Tan et al. (2019) |
| 80 | 55 | 41 | 51 | Distilled | |||
| 85 | 70 | 48 | 58 | Tap | |||
| 87 | 70 | 50 | 58 | River | |||
| Parameter | Distilled Water (mg/L) | Tap Water (mg/L) | River Water (mg/L) |
|---|---|---|---|
| COD | Not detected | Not detected | 20.3 |
| Al | 0.013 | 0.075 | 0.011 |
| Ba | 12 | 15 | 21 |
| Ca | 0.11 | 2.94 | 1.22 |
| Cl- | 0.4 | 7.6 | 4.5 |
| Cr | 0.006 | 0.015 | 0.020 |
| Cu | 0 | 4 | 3 |
| Mg | 0.01 | 2.24 | 3.20 |
| NO3- | 0.1 | 0.2 | 0.3 |
| SO42- | 1 | 18 | 12 |
| Zn | 0.04 | 0.05 | 0.10 |
| Pb | 0.001 | 0.005 | 0.009 |
4.2.1.4 Oxidation
Chemical oxidation using chlorine and ozone (O3) can be effective in removing dimethoate from water depending on a variety of factors, including oxidant dose, contact time, disinfectant demand, temperature and pH.
By-products that may be formed from the degradation of dimethoate through the use of chlorine are phosphorothioic acid, N-methyl-2-(methylthio) acetamide and omethoate (Tian et al., 2014). Omethoate was found to comprise 11% to 23% of the degraded dimethoate (see Section 1.1). Omethoate is of concern due to its health impacts.
Several bench-scale studies evaluating various oxidants are presented in Table 13. Chlorine was found to have high removal (100%) in the study by Ormad et al. (2008). Ozone was evaluated through two bench-scale studies by the same authors, in which different removal efficiencies were observed (75% and 25%). The varying performance illustrates the need for pilot-scale testing.
A bench-scale study examining O3 degradation of 23 pesticides found that dimethoate was easily degraded (Meijers et al., 1995). Under all conditions, the degradation of dimethoate achieved a minimum of 83% and a maximum of 97%.
Another bench-scale study was conducted to evaluate dimethoate degradation by chlorine and by-product formation under typical water treatment conditions (Tian et al., 2014). The authors found that bromide and humic acid accelerated the degradation of dimethoate by chlorine. Conversely, the presence of ammonia inhibited degradation due to the formation of monochloramine, which has lower reactivity with dimethoate.
| Oxidant | Influent (µg/L) |
Initial Oxidant Dose (mg/L) or (g O3/g DOC) |
Removal | Process Description | Reference | |
|---|---|---|---|---|---|---|
| Chlorine | 1.108 | 18 mg/L (using NaClO) | 100% | Bench-scale: Untreated river water; pH 8.0; Investigated removal of 44 pesticides including dimethoate Reaction time not provided |
Ormad et al. (2008) | |
| Ozone | 1.108 | 4.3 mg/L | 75% | |||
| 0.5 | 3.0 mg/L | 25% | Bench-scale: River water; pH 8.0; Conductivity 750 μS/cm; DOC = 3 mg/L; Alkalinity = 200 mg CaCO3/L Investigated removal of 44 pesticides including dimethoate Reaction time not provided |
Ormad et al. (2010) | ||
| 0.9–6.4Footnote a | 0.53 g/g | 83% | pH = 7.2; 5 ˚C; C*t10 = 2.0 | Bench-scale: River water; DOC = 2.2 mg C/L; Br- = 100 μg/L, HCO3- = 1.6 mM; 23 pesticides |
Meijers et al. (1995) | |
| 0.55 g/g | 85% | pH = 7.2; 20 ˚C; C*t10 = 1.0 | ||||
| 0.95 g/g | 96% | pH = 8.3; 20 ˚C; C*t10 = 1.0 | ||||
| 1.0 g/g | 97% | pH = 7.2; 5 ˚C; C*t10 = 7.3 | ||||
| 1.0 g/g | 97% | pH = 7.2; 20 ˚C; C*t10 = 3.3 | ||||
| 1.0 g/g | 97% | pH = 8.3; 20 ˚C; C*t10 = 1.1 | ||||
Removal of omethoate
Ling et al. (2011) performed batch experiments on a 1.0 mg/L omethoate solution to determine the degradation by O3 alone as well as with a catalyst. The catalysts that were used in the study were activated carbon (AC) and Fe(III) deposited on activated carbon (Fe-AC). The removal percentages of omethoate under various conditions are presented in Table 14. It was found that the best removal efficiency was for ozone with 5% Fe-AC combined and that removal was much higher than that for ozone alone. The authors examined each catalyst individually without O3 to determine whether omethoate was being removed by adsorption, and both exhibited poor removal efficiencies. Therefore, the authors concluded that O3 in the presence of Fe-AC resulted in the formation of hydroxyl radicals, and it was these radicals that degraded the omethoate.
A later study investigated reaction rates for omethoate degradation using O3 and hydroxyl radicals (Qiang et al., 2013). Omethoate degraded more slowly with the use of ozone (0.04 M-1s-1) than with the use of hydroxyl radicals (5.3x108 M-1s-1) at a pH of 7.5 and temperature of 20 ˚C. The authors stated that the presence of the catalyst, Fe-AC, generated hydroxyl radicals from O3, resulting in the improved degradation of omethoate than achieved by ozone alone.
| Oxidant | Influent Omethoate (mg/L) |
Initial Oxidant Dose (mg/L) |
Catalyst (or Oxidant) Added | Removal | Process Description | Reference |
|---|---|---|---|---|---|---|
| Ozone | 1.0 | 1.0 | None | 37.6% | Bench-scale study; Reaction time = 30 min; pH 7.5; 20 ± 2 ˚C |
Ling et al., 2011 |
| 20 mg/L AC | 58.0% | |||||
| 20 mg/L 5% Fe-AC | 82.4% | |||||
| None | - | 20 mg/L AC | 6.5% | |||
| 20 mg/L 5% Fe-AC | 5.7% | |||||
4.2.1.5 Biological treatment
Biological treatment involves targeting the removal of the biodegradable organic material fraction. The effectiveness of biological treatment depends, therefore, on the initial concentration, source water properties, the microbial community, the contact time, the soil properties and the temperature (Drewes et al., 2009; Diem et al., 2013). The main biological treatment processes for drinking water include riverbank filtration (RBF), rapid granular media filtration without the maintenance of a disinfectant residual across the bed, and slow sand filtration.
Riverbank filtration
Riverbank filtration (RBF) involves locating vertical or horizontal water supply wells near a river in order to use the riverbank and adjacent aquifer as a natural filter to remove contaminants. As water proceeds to the groundwater table, contaminant concentrations are lowered through adsorption, biodegradation and dilution with groundwater (Piet and Zoeteman, 1980; Bize et al., 1981; Kuehn and Mueller, 2000; Ray et al., 2002). Natural attenuation through RBF is one of the most basic and inexpensive methods of water treatment (Verstraeten and Heberer, 2002; Sørensen et al., 2006).
Several studies were conducted to evaluate organic micropollutant (OMP) removal through RBF (Bertelkamp et al., 2015; Bertelkamp et al., 2016a, 2006b). Two different oxic soils and one suboxic/anoxic soil were used in these studies and the properties are presented in Table 15.
| Parameter | Oasen (oxic) | Vitens (oxic) | Vitens (suboxic/deep anoxic) |
|---|---|---|---|
| Porosity | 0.35 | 0.33 | - |
| Cation exchange capacity (meq/kg dry wt) |
9.19 | 42.13 | 14.07 |
| Clay (v/v%) |
0.50 | 3.72 | 1.71 |
| Silt (v/v%) |
0.52 | 3.58 | 2.50 |
| Sand (v/v%) |
98.98 | 92.69 | 95.79 |
| dmedium (μm) |
330.62 | 380.38 | 394.84 |
The first pilot-scale study was conducted to determine the effects of soil type (Bertelkamp et al., 2015) (Table 16). River water spiked with a mixture of 20 OMPs (including dimethoate), each at a concentration of 0.5 μg/L, was used in two pilot tests with different soils, namely Oasen and Vitens. The authors concluded that the microbial community composition seems to be mainly determined by aqueous phase rather than soil type, which is evidenced by the fairly similar biodegradation rates for dimethoate between the two soils (0.42 and 0.37 day-1). From the biodegradation rates, the authors also estimated a 99% dimethoate removal. The required residence time in the oxic zone would be approximately 11.6 days and 13.1 days for the Oasen and Vitens soils, respectively. Prior to moving into the anoxic zones, the water only remains within the oxic zone of an RBF system for the first couple of hours to days (site-dependent) so that less removal of dimethoate would be expected.
A column study was conducted to determine impact on degradation rate under different conditions (Bertelkamp et al., 2016a). Four river feed waters with different organic fractions (hydrophilic, hydrophobic, transphilic and river water organic matter [RWOM]) were used in the evaluation. Three different phases were studied to simulate stable, OMP shock-load and DOC shock-load operations. The first phase represented the RBF under stable operation and had a similar biodegradation rate to that of the previous study. The second phase, with higher initial concentrations of OMPs, resulted in a higher degradation rate for dimethoate. The third phase had differing results depending on the water used.
A pilot-scale study using river water was carried out using three different pilot set-ups to investigate degradation in different redox zones (Bertelkamp et al., 2016b). The first pilot was to evaluate degradation in the oxic zone, the second in the suboxic zone and the third in the anoxic zone. For dimethoate, the highest degradation rate occurred in the oxic zone.
| Influent (µg/L) |
Biodegradation rate (dayFootnote a) | Columna Set-up / Soil | Process Description | Reference | |
|---|---|---|---|---|---|
| 0.5 | 0.42 | Pilot A: 2 columns in series / Oasen | Pilot-scale study; River water: mixture of 20 OMPs – 0.5μg/L each; Hydraulic loading rate = 0.5 L/d |
Bertelkamp et al. (2015) | |
| 0.37 | Pilot B: 2 columns in series / Vitens | ||||
| 0.5 | 0.55 Hydrophilic 0.22 RWOM 0.39 Transphilic |
Phase 1 (Stable operation): 1 column / Oasen |
0.5 μg/L each OMP DOC = 4 mg/L |
|
Bertelkamp et al. (2016a) |
| 2 | 0.60 Hydrophilic 0.42 RWOM 0.43 Transphilic 0.36 Hydrophobic |
Phase 2 (OMP shock-load): 1 column / Oasen |
2 μg/L each OMP DOC = 4 mg/L |
||
| 2 | 0.45 Hydrophilic 1.01 Transphilic |
Phase 3 (DOC shock-load): 1 column / Oasen |
2 μg/L each OMP DOC = 8 mg/L |
||
| 0.5 | 0.39 | Pilot A (Oxic): 2 columns in series / Vitens |
|
Bertelkamp et al. (2016b) | |
| 0.06 | Pilot B (Suboxic): 10 columns in series (Vitens: first 4 oxic/last 6 suboxic/anoxic) | ||||
| 0.11 | Pilot C (Anoxic): 22 columns in series (Vitens: first 4 oxic/last 18 suboxic/anoxic) | ||||
Engineered biological filtration
Engineered biological filtration involves the use of granular media filters (i.e., anthracite/sand or GAC) without the maintenance of a disinfectant residual across the bed. Biological activity within the filters can be influenced by a number of factors, including water quality, temperature, oxidant dose and type, and backwashing procedures (Huck et al., 2001).
A full-scale study investigating two different treatment trains was conducted to determine the impact on dimethoate removal (Yang et al., 2019). The treatment trains varied in the location of the sand filter in relation to the biologically active carbon (BAC) (Table 17). The influent concentrations were very low, but in both treatment trains dimethoate was removed by the BAC. A bench-scale study was conducted to evaluate the overall performance of biological filters in removing 34 OMPs (Zearley and Summers, 2012) including dimethoate. The influent concentration used in this study was quite low; however, good removal of dimethoate occurred, with increasing removal at the higher EBCT.
| Influent (µg/L) | Removal or Effluent Concentration | EBCT (min) | Overall Process Description | Reference |
|---|---|---|---|---|
| 8.65 ng/L | 6.43 ng/L | 16 |
|
Yang et al. (2019) |
| 12.59 ng/L | 5.13 ng/L |
|
||
| 0.038 | 75% | 7.9 |
|
Zearley and Summers (2012) |
| 81% | 15.8 | |||
4.2.1.6 Combined technologies
A full-scale study examining two different treatment trains showed removal of dimethoate for the different processes (Yang et al., 2019). One treatment train had the sand filter prior to ozonation and BAC, whereas the other had the sand filter after the BAC (Table 18).
| Influent (ng/L) | Effluent (ng/L) | Treatment Stage | Other Parameter Information | Overall Process Description |
|---|---|---|---|---|
| 35.17 | 30.19 | Sedimentation | TurbidityFootnote a:
COD:
|
PreO3 dose 0.5 mg/L; aluminum sulphate dose = 40 mg/L; sedimentation time = 105 min; sand filtration velocity = 7.9 m/h; post O3 dose = 1 mg/L; BAC EBCT = 16 min and flow rate = 8.3 m/hr |
| 9.40 | Sand filtration | |||
| 8.65 | Post-ozonation | |||
| 6.43 | BACc | |||
| 17.08 | 18.17 | Sedimentation | TurbidityFootnote a:
COD:
|
PreO3 dose 0.5 mg/L; aluminum sulphate dose = 40 mg/L; sedimentation time = 105 min; sand filtration velocity = 7.9 m/h; post O3 dose = 1 mg/L; BAC EBCT = 16 min and flow rate = 8.3 m/hr |
| 12.59 | Post-ozonation | |||
| 5.13 | BAC | |||
| 3.47 | Sand filtration | |||
The bench-scale study by Ormad et al. (2008) evaluated preoxidation by O3 and chlorine, coagulation and adsorption using PAC as discussed in the previous sections. As part of this study, they also investigated combinations of these technologies (Table 19). Wherever chlorine was one of the applied processes, 100% removal of dimethoate always occurred; however, formation of by-products should be considered. The technology providing the worst removal of dimethoate was that of coagulation.
| Influent (µg/L) | Removal (%) | Treatment Type | Overall Process Description |
|---|---|---|---|
| 1.108 | 100 | Chlorine | Bench-scale study River water: Chlorine demand of 18 mg Cl2/L; pH of 8.0 Description of treatment processes:
Reaction times not provided |
| 100 | Chlorine + coagulation (10 mg Al/L) | ||
| 100 | Chlorine + coagulation (20 mg Al/L) | ||
| 75 | Ozone | ||
| 75 | Ozone + coagulation (20 mg Al/L) | ||
| 85 | Ozone + coagulation (40 mg Al/L) | ||
| 60 | PAC | ||
| 75 | Ozone + PAC | ||
| 35 | Coagulation (10 mg Al/L) | ||
| 35 | Coagulation (20 mg Al/L) | ||
| 35 | Coagulation (40 mg Al/L) | ||
| 100 | Chlorine + PAC + coagulation (20mg Al/L) | ||
| 95 | Ozone + PAC + coagulation (20 mg Al/L) | ||
4.2.2 Residential-scale treatment
In cases where dimethoate removal is desired at the household level (e.g., when a household obtains its drinking water from a private well), a residential drinking water treatment unit may be an option for decreasing dimethoate concentrations in drinking water. Before a treatment unit is installed, the water should be tested to determine the general water chemistry and the dimethoate concentration in the source water.
To verify that a treatment unit is effective, water entering and leaving the treatment unit should be sampled periodically and submitted to an accredited laboratory for analysis. Units can lose removal capacity through use and time and need to be maintained and/or replaced. Consumers should verify the expected longevity of the components in the treatment unit according to the manufacturer's recommendations and service it when required. Systems classified as residential scale may have a rated capacity to treat volumes greater than that needed for a single residence, and thus may also be used in small systems.
Health Canada does not recommend specific brands of drinking water treatment units, but it strongly recommends that consumers use units that have been certified by an accredited certification body as meeting the appropriate NSF International Standard/American National Standard (NSF/ANSI) for drinking water treatment units. The purpose of these standards is to establish minimum requirements for the materials, design and construction of drinking water treatment units that can be tested by a third party. This ensures that materials in the unit do not leach contaminants into the drinking water (i.e., material safety). In addition, the standards include performance requirements that specify the removal that must be achieved for specific contaminants (e.g., reduction claim) that may be present in water supplies. Certification organizations (i.e., third party) provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada (SCC). Accredited organizations in Canada include the following:
- CSA Group
- NSF International
- Water Quality Association
- UL LLC
- Bureau de Normalisation du Québec (available in French only)
- International Association of Plumbing and Mechanical Officials
- Truesdail Laboratories Inc.
An up-to-date list of accredited certification organizations can be obtained from the SCC.
The drinking water treatment technologies that are expected to be effective for dimethoate removal at the residential-scale include adsorption and RO. Currently, dimethoate is not included in the performance requirements (e.g., reduction claims) of NSF/ANSI standards. However, consumers can use a treatment unit that is certified to the standards for adsorption or reverse osmosis to ensure that the material safety has been tested.
Water that has been treated using RO may be corrosive to internal plumbing components. Therefore, these units should be installed only at the point-of-use. Also, as large quantities of influent water are needed to obtain the required volume of treated water, these units are generally not practical for point-of-entry installation.
5.0 Management strategies
All water utilities should implement a risk management approach, such as the source-to-tap or water safety plan approach, to ensure water safety (CCME, 2004; WHO, 2012, 2017). These approaches require a system assessment to characterize the source water, describe the treatment barriers that prevent or reduce contamination, identify the conditions that can result in contamination, and implement control measures. Operational monitoring is then established, and operational/management protocols are instituted (e.g., standard operating procedures, corrective actions and incident responses). Compliance monitoring is determined and other protocols to validate the water safety plan are implemented (e.g., record keeping, consumer satisfaction). Operator training is also required to ensure the effectiveness of the water safety plan at all times (Smeets et al., 2009).
5.1 Monitoring
Dimethoate can be present in groundwater and surface water in areas where it is being used depending on the type and extent of its application, environmental factors (e.g., amount of precipitation, soil type, hydrogeological setting) and environmental fate (e.g., mobility, leaching potential, degradation) in the surrounding area. Water utilities should consider the potential for dimethoate to enter source water (e.g., raw water supply to the drinking water system) based on site-specific considerations.
When it is determined that dimethoate may be present and monitoring is necessary, surface and groundwater sources should be characterized to determine the concentration of dimethoate. This should include monitoring of surface water sources during periods of peak use and rainfall events and/or annual monitoring of groundwater. Where baseline data indicate that dimethoate is not present in source water, monitoring may be reduced.
Where treatment is required to remove dimethoate, operational monitoring should be implemented to confirm whether the treatment process is functioning as required. The frequency of operational monitoring will depend on the water quality, fluctuations of the raw water concentrations and the treatment process. Responsible authorities should be aware of the impact of NOM on activated carbon systems, as it may impact water quality objectives for dimethoate removal.
Where treatment is in place for dimethoate removal, compliance monitoring (i.e., paired samples of source and treated water to confirm the efficacy of treatment) should be conducted, at a minimum, on an annual basis. When routine operational monitoring indicates the potential for contaminant breakthrough, such as with GAC, monitoring should be conducted at least quarterly to plan for change-out of the media. When a degradation process is utilized, like oxidation, monitoring of by-products should also be considered. In particular, omethoate should also be monitored as it is a by-product of dimethoate that is a health concern. An equivalent dimethoate concentration should be calculated and added to the dimethoate concentration measured (see Section 3.0). The sum of these should not exceed the MAC for dimethoate.
6.0 International considerations
Other national and international organizations have drinking water guidelines, standards and/or guidance values for dimethoate and omethoate in drinking water. Variations in these values can be attributed to the age of the assessments or to differing policies and approaches (e.g., BMDL vs NOEL/NOAEL), including the choice of key study and the use of different consumption rates, body weights and source allocation factors (Table 20).
Australia's National Health and Medical Research Council has set a guideline value of 0.007 mg/L for dimethoate in drinking water based on cholinesterase inhibition observed in humans and a value of 0.001 mg/L for omethoate based on inhibition of AChE in a 2-year rat study. For dimethoate, the WHO has a guideline value of 0.006 mg/L (6 µg/L) based on potential effects on reproductive performance in rats (WHO, 2017). The US EPA states that it has made a determination not to regulate dimethoate in drinking water because it thought it did not occur at levels and frequencies of public health concern (US EPA, 2016). Neither the US EPA nor the WHO has a guideline value for omethoate (US EPA, 2006b).
The European Union (EU) does not have a specific chemical parametric value for individual pesticides. Instead, the EU has a value of 0.1 µg/L for any individual (single) pesticide, and a value of 0.5 µg/L for total pesticides found in drinking water. In establishing these values, the EU notes that it did not consider the science related to each pesticide, such as health effects. Rather, its values are based on a policy decision to keep pesticides out of drinking water (European Union, 2020).
| Agency (Year) |
Value (mg/L) |
Key Endpoint | BMDL10/NO(A)EL (mg/kg bw/d) |
UF | ADI (mg/kg bw/d) |
BW (kg) |
DW Intake (L/d) |
AF (%) |
Comments |
|---|---|---|---|---|---|---|---|---|---|
| Health Canada - proposed MAC (2020) |
0.02 | ChE inhibition |
0.2 (BMDL10)Footnote a | 100 | 0.002 | 74 | 1.53 | 20 | Omethoate levels in drinking water are addressed using an additive approach and not a separate MAC |
| WHO (2003) |
0.006 | Reproductive performance in rats | 1.2 (NOAEL) | 500 | 0.002 | 60 | 2 | 10 | UF based on 10 for intra species, 10 for interspecies and 5 for concern regarding whether NOAEL could be a LOAEL. (WHO, 2017) |
| Australia (2010) |
0.007 | ChE inhibition in 57-day human volunteer study | 0.2 (NOEL) | 100 | 0.02 | 70 | 2 | 10 | ADI was established in 1988. No reference given for key study. |
| Australia (2011) Omethoate |
0.001 | ChE inhibition in 2-year rat study | 0.04 (NOEL) | 100 | 0.0004 | 70 | 2 | 10 | ADI was established in 2005. No reference given for key study. |
| EU (1998) |
0.1 µg/L | The EU has a value of 0.1 µg/L for any individual (single) pesticide, and a value of 0.5 µg/L for total pesticides found in drinking water. In establishing these values, the EU did not consider the science related to each pesticide, including health effects. Instead, the values are based on a policy decision to keep pesticides out of drinking water. |
|||||||
7.0 Rationale
Dimethoate is a broad spectrum organophosphate pesticide registered in Canada to control a wide range of insects and mites on both agricultural and non-agricultural sites. Despite its widespread use in Canada, data provided by provinces and territories that monitor for dimethoate in source and drinking water indicate that when detected, levels of dimethoate are well below the proposed MAC. The main target of dimethoate is the inhibition of ChE, although increased pup mortality is also a concern. The anticholinesterase activity of dimethoate is due to its metabolite omethoate. Omethoate is also a major environmental degradate of dimethoate and can be produced during drinking water treatment.
Health Canada, in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water, is proposing to reaffirm a MAC of 0.02 mg/L (20 µg/L) for dimethoate based on the following considerations:
- An HBV of 0.02 mg/L (20 µg/L) based on ChE inhibition in several repeat-dose animal studies;
- The proposed MAC would be protective of both ChE inhibition and pup mortality observed in several repeat-dose animal studies;
- The proposed additive approach for omethoate is protective of health in cases where omethoate is detected in source or treated water;
- Dimethoate and omethoate can be accurately measured at concentrations well below the proposed MAC;
- Drinking water treatment technologies are available to remove dimethoate to below the proposed MAC; and
- Omethoate can be reduced by controlling or minimizing its formation.
The proposed MAC for dimethoate and cumulative approach for omethoate are protective of potential health effects from dimethoate and omethoate exposure. As part of its ongoing guideline review process, Health Canada will continue to monitor new research in this area, including the outcomes of the PMRA's evaluations, and recommend any change to this guideline technical document that it deems necessary.
8.0 References
Ahmad, A.L., Tan, L.S. and Abd Shukor, S.R. (2008a). The role of pH in nanofiltration of atrazine and dimethoate from aqueous solution. J. Hazard. Mater., 154(1-3): 633-638.
Ahmad, A.L., Tan, L.S. and Abd Shukor, S.R. (2008b). Dimethoate and atrazine retention from aqueous solution by nanofiltration membranes. J. Hazard. Mater., 151(1): 71-77.
ALS Environmental. (2019). Personal communication with A. Ganouri-Lumsden. Waterloo, ON.
APVMA (2010). Human Health Risk Assessment of Dimethoate. Australian Pesticides and Veterinary Medicines Authority, Office of Chemical Safety and Environmental Health, Department of Health and Ageing, Canberra, Australia.
APVMA (2011). Review of the Mammalian Toxicology and Metabolism/Toxicokinetics of Omethoate. Australian Pesticides and Veterinary Medicines Authority, Office of Chemical Safety, Office of Health Protection, Department of Health and Ageing, Canberra, Australia.
Bayer (1975). Evaluation of Omethoate for Embryonic and Teratogenic Effects in rats Following Oral Administration. Bayer AG, Report no. 5235, Wuppertal, Germany (as cited in APMA, 2011).
Beduk, F., Aydin, M.E. and Ozcan, S. (2012). Degradation of malathion and parathion by ozonation, photolytic ozonation, and heterogeneous catalytic ozonation processes. Clean - Soil, Air, Water, 40 (2): 179-87.
Bellona, C., Drewes, J.E., Xu, P. and Amy, G. (2004). Factors affecting the rejection of organic solutes during NF/RO treatment - A literature review. Wat. Res., 38 (12): 2795-2809.
Bertelkamp, C., Schoutteten, K., Vanhaecke, L., Vanden Bussche, J., Callewaert, C., Boon, N., Singhal, N., van der Hoek, J.P. and Verliefde, A.R.D. (2015). A laboratory-scale column study comparing organic micropollutant removal and microbial diversity for two soil types. Sci. Total Environ., 536: 632-638.
Bertelkamp, C., van der Hoek, J.P., Schoutteten, K., Hulpiau, L., Vanhaecke, L., Vanden Bussche, J., Cabo, A.J., Callewaert, C., Boon, N., Löwenberg, J., Singhal, N. and Verliefde, A.R.D. (2016a). The effect of feed water dissolved organic carbon concentration and composition on organic micropollutant removal and microbial diversity in soil columns simulating river bank filtration. Chemosphere, 144: 932-939.
Bertelkamp, C., Verliefde, A.R.D., Schoutteten, K., Vanhaecke, L., Vanden Bussche, J., Singhal, N. and van der Hoek, J.P. (2016b). The effect of redox conditions and adaptation time on organic micropollutant removal during river bank filtration: A laboratory-scale column study. Sci. Total Environ., 544: 309-318.
Bize, J., Grenet, B. and Maneglier, H. (1981). Purifying capacity of the alluvial complex bordering a river. Tech. Sci. Munic., 76(7): 393-401.
Brauch, H. and Kühn, W. (1988). Organic micropollutants in the river Rhine and in drinking water treatment. Gwf Wasser Abwasser, 129(3): 189-196.
British Columbia Ministry of Health (2019). Personal communication with Drinking Water Manager David Fishwick.
Brooker, A.J. and Stubbs, A. (1991). Dimethoate: Dietary Range Finding Study in Mature Male and Female Rats and their Juvenile Offspring. Huntingdon Research Center, DTF Doc No: '453-004' Ref: 3-21/Vol. 3-, England (as cited in APVMA, 2010).
Brooker, A.J., Homan, A.B., Parker, C.A., Offer, J.M., Anderson, A. and Dawe, I.S. (1992). The Effect of Dimethoate on Reproductive Function of Two Generations in the Rat. Volume I, II, and III. Huntingdon Research Center, DTF Doc No: '453-003 Ref: 3-22/Vol. 3-7 & 3-8 (as cited in APVMA, 2010).
Burford, P., McLean, T.A., Buist, D.P., Gregson, R.L. and Gopinath, C. (1990a). Dimethoate: 12-Month Dietary Study in Beagle Dogs (Final Report - Repeated Daily Dosage for 52 Weeks). Huntingdon Research Center, DTF Doc No.: '437-011' Ref: 3-7/Vol. 3-4 (as cited in APVMA, 2010).
Burford, P., McLean, T.A., Buist, D.P., Gregson, R.L. and Gopinath, C. (1990b). Individual clinical observations. Supplement to MRID Number 41939801. Dimethoate 12-month dietary study in Beagle dogs (Repeated aily dosage for 52 weeks). Huntingdon Research Center, DTF Doc No.: '437-014' Ref: 3-8/Vol. 3-4 (as cited in APVMA, 2010).
Caregnato, P., Rosso, J.A., Soler, J.M., Arques, A., Mártire, D.O. Gonzalez, M.C. (2013). Chloride anion effect on the advanced oxidation processes of methidathion and dimethoate: Role of Cl 2- radical. Water Res., 47(1): 351-362.
CARO Analytical Services - Richmond Laboratory (2019). Personal communication with K. Fyffe. Richmond, BC.
CCME (2004). From source to tap: Guidance on the multi-barrier approach to safe drinking water. Canadian Council of Ministers of the Environment, Winnipeg, Manitoba. Available at www.ccme.ca/assets/pdf/mba_guidance_doc_e.pdf
CFIA (2019). 2015/16 Annual Report: National Chemical Residue Monitoring Program and Chemistry Oversight Program. Canadian Food Inspection Agency, Ottawa, Ontario.
Charalampous, A.C., Miliadis, G.E. and Koupparis, M.A. (2015). A new multiresidue method for the determination of multiclass pesticides, degradation products and PCBs in water using LC-MS/MS and GC-MS(n) systems. Int. J. Environ. Anal. Chem., 95(13): 1283-1298.
Chowdhury, Z.K., Summers, R.S., Westerhoff, G.P., Leto, B.J., Nowack, K.O. and Corwin, C.J. (2013). Activated carbon: Solutions for improving water quality. Passantino, L. B.(ed.). American Water Works Association. Denver, Colorado.
Davies, D.J. (1999). Dimethoate: In vitro absorption form a 400 g/L EC Formulation through human and rat epidermis. Central Toxicology Laboratory. DTF Doc. No: '469-001' (as cited in APVMA, 2011).
De Bleeker, J., Van Den Neucker, K. and Willems, J. (1992). The intermediate syndrome in organophosphate poisoning: Presentation of a case and review of the literature. J. Toxicol. Clin. Toxicol., 30(3): 321-329.
Diem, S., Rudolf Von Rohr, M., Hering, J.G., Kohler, H-E., Schirmer, M. and Von Gunten, U. (2013). NOM degradation during river infiltration: Effects of the climate variables temperature and discharge. Water Res., 47(17): 6585-6595.
Donald, D.B., Cessna, A.J., Sverko, E. and Glozier, N.E. (2007). Pesticides in surface drinking-water supplies of the northern Great Plains. Environ. Health Perspect., 115(8): 1183-1191.
Dotti, A., Biedermann, K., Luetkemeier, H. and Wright, J. (1992). E 6876 (c.n. Omethoate) Two-Generation Reproduction Study in the Rat. Bayer AG, Project no. 207326.Study no. T 8029476, Itingen, Switzerland (as cited in APVMA, 2011).
Drewes, J.E., Hoppe, C., Oldham, G., McCray, J. and Thompson, K. (2009). Removal of bulk organic matter, organic micropollutants, and nutrients during riverbank filtration. Report number 3180. Water Research Foundation, Denver, Colorado.
Eddleston, M., Eyer, P., Worek, F., Senarathna, L., von Myers, L., Juszczak, E., Hittarage, A., Azher, S., Dissanayake, W., Revzi Sheriff, M.H., Dawson, A.H. and Buckley, N.A. (2005). Differences between organophosphorus insecticides in human self poisoning: A prospective cohort study. Lancet, 366(9495): 1452-1459.
EFSA (2006). Conclusion regarding the peer review of the pesticide risk assessment of the active substance dimethoate. Finalised 23 June 2006. EFSA Scientific Report, 84: 1-102.
EFSA (2018). Peer review of the pesticide risk assessment of the active substance dimethoate. EFSA Journal, 16(10): 5454.
Element Materials Technology Canada Inc. (2019). Personal communication with H. Du. Calgary, AB.
Environment Canada (2011). Presence and Levels of Priority Pesticides in Selected Canadian Aquatic Ecosystems. Water Science and Technology Directorate, Environment Canada, En14-40/2011E-PDF, Ottawa, Ontario.
European Union (2020). Council Directive 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast).
FIFRA (2005). A Set of Scientific Issues Being Considered by the Environmental Protection Agency regarding: Dimethoate: Issues Related to Hazard and Dose-Response Assessment. FIFRA Scientific Advisory Panel, SAP Minutes No. 2005-01. Meeting November 30 and December 1, 2004, Arlington, VA.
Fonseka, M.M.D., Medagoda, K., Tillakaratna, Y., Gunatilake, S.B., de Silva, H.J. (2003). Self-limiting cerebellar ataxia following organophosphate poisoning. Hum. Exp. Toxicol., 22: 107-109.
Government of Nova Scotia (2007). Nova Scotia. Groundwater Observation Network. 2007 Report. Nova Scotia. Environment. Available at https://novaScotia..ca/nse/groundwater/groundwaternetwork.asp.
Government of Nova Scotia (2008). Nova Scotia. Groundwater Observation Network. 2008 Report. Nova Scotia. Environment. Available at https://novaScotia..ca/nse/groundwater/groundwaternetwork.asp.
Government of Nova Scotia (2009). Nova Scotia. Groundwater Observation Network. 2009 Report. Nova Scotia. Environment. Available at https://novaScotia..ca/nse/groundwater/groundwaternetwork.asp.
Government of Nova Scotia (2010). Nova Scotia. Groundwater Observation Network. 2010 Report. Nova Scotia. Environment. Available at https://novaScotia..ca/nse/groundwater/groundwaternetwork.asp.
Government of Nova Scotia (2011). Nova Scotia. Groundwater Observation Network. 2011 Report. Nova Scotia. Environment. Available at https://novaScotia..ca/nse/groundwater/groundwaternetwork.asp.
Government of Nova Scotia (2012). Nova Scotia. Groundwater Observation Network. 2012 Report. Nova Scotia. Environment. Available at https://novaScotia..ca/nse/groundwater/groundwaternetwork.asp.
Government of Nova Scotia (2015). Nova Scotia. Groundwater Observation Network. 2015 Report. Nova Scotia. Environment. Available at https://novaScotia..ca/nse/groundwater/groundwaternetwork.asp.
Government of Yukon Health and Social Services (2019). Personal communication with P. Brooks, Drinking Waters Program.
Haiste-Gulde, B. and Sacher, F. (2019). Personal communication, TZW German Water Centre, Karlsruhe, Germany.
Haist-Gulde, B. and Happel, O. (2012). Removal of pesticides and their ionic degradates by adsorptive processes. Report no. 4022. Water Research Foundation, Denver, Colorado.
Hayama, T., Yoshida, H., Todoroki, K., Nohta, H. and Yamaguchi, M. (2008). Determination of polar organophosphorus pesticides in water samples by hydrophilic interaction liquid chromatography with tandem mass spectrometry. Rapid communications in mass spectrometry. Rapid Commun. Mass Spectrom., 22(14): 2203-2210.
Health Canada (2011). Proposed Re-Evaluation Decision. Dimethoate. Pest Management Regulatory Agency (PMRA); Health Canada, PRVD2011-12, Ottawa, Ontario.
Health Canada (2015). Re-Evaluation Decision Document. Dimethoate. Pest Management Regulatory Agency (PMRA); Health Canada, RVD2015-04, Ottawa, Ontario.
Health Canada (2020a). Pest Control Products Sales Report for 2018. PMRA, Health Canada, Ottawa, Ontario.
Health Canada (2020b). Personal communication from the Health Evaluation Directorate, Pest Management Regulatory Agency (PMRA).
Health Canada (in preparation). Canadian Exposure Factors used in Human Health Risk Assessments. Health Canada, Ottawa, Ontario.
Hellwig, J., Deckhardt, K. and Mirea, D. (1986a). Report on the Study of the Toxicity of Dimethoate in Mice After 78 Week Administration in the Diet. BASF Department of Toxicology, OCS Submission Nos. 150 and 415, Germany (as cited in APVMA, 2010).
Hellwig, J. et al. (1986b). Report on the Study of the Toxicity of Dimethoate in Rats After 24 Months administration in the Diet. BASF Department of Toxicology, Project No. 70. CO 326/8241, OCS Submission Nos. 150 and 415, Germany (as cited in APVMA, 2010).
Hilal, N., Al-Zoubi, H., Darwish, N.A. and Mohammad, A.W. (2005). Characterisation of Nanofiltration Membranes using Atomic Force Microscopy. Desalination, 177 (1-3): 187-199.
Hoffmann, K. and Schilde, B. (1984). S 6876 (Omethoate) Chronic Toxicity to Dogs on Oral Administration (Twelve-Month Stomach Tube Study). Bayer AG Institut für Toxikologie, Report no: 12561 [BA; sub: 239, Vol. 6 of 9. CHA; sub: 12564, Vol. 3-26 of 39], Wiuppertal, Germany (as cited in APVMA, 2011).
Hoffmann, U. and Papendorf, T. (2006). Organophosphate poisonings with parathion and dimethoate. Intensive Care Med., 32: 464-468.
Holzum, B. (1990a). E 6876 (Common Name Omethoate): Study for Embryotoxic Effects on Rats Following Oral Administration. Bayer AG, Study no. T 8030636. Report no. 1922, Wuppertal, Germany (as cited in APVMA, 2011).
Holzum, B. (1990b). E 6876 (Common Nam: Omethoate). Study for Embryotoxic Effects on Rabbits Following Oral Administration. Bayer AG, Study no. T0032834. Report no. 19221, Wuppertal, Germany (as cited in APVMA, 2011).
HSDB (2010). PubChem database. DIMETHOATE, HSDB #1586. National Center for Biotechnology Information, https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1586.
Huck, P.M., Emelko, M.B., Coffey, B.M., Maurizio, D. and O'Melia, C. (2001). Filter operation effects on pathogen passage. Report number 90874. AWWA Research Foundation, Denver, Colorado.
Ikehata, K. and Gamal El-Din, M.G. (2006). Aqueous pesticide degradation by hydrogen peroxide/ultraviolet irradiation and fenton-type advanced oxidation processes: A review. J. Environ. Eng. Sci., 5(2): 81-135.
Indigenous Services Canada. (2019). Personal communication with X. Redhead, First Nations and Inuit Health Branch, Drinking Water Safety Program.
Kirkpatrick, D. (1995). 14C-Dimethoate: The Biokinetics and Metabolism in the Rat. Huntingdon Life Sciences, Ltd. Study Period: January 1993 - December 1995, DTF Doc No: 651-001, Ref: 3-1/Vol. 3-2 (as cited in APVMA, 2010).
Knappe, D.R.U., Snoeyink, V.L., Roche, P., Prados, M.J. and Bourbigot, M-M. (1999). Atrazine removal by preloaded GAC. J. Am. Water Works Assoc., 91(10): 97-109.
Kovács, Z. and Samhaber, W. (2008). Characterization of nanofiltration membranes with uncharged solutes. Membrántechnika, 12(2): 22-36 (as cited in Tan et al., 2019).
Krishnan, K. and Carrier, R. (2013). The use of exposure source allocation factor in the risk assessment of drinking-water contaminants. J. Toxicol. Environ. Health B, 16(1): 39-51.
Kuehn, W. and Mueller, U. (2000). Riverbank filtration: An overview. J. Am. Water Works Assoc., 92(12): 60-69.
LeBlanc, F.N., Benson, B.E. and Glig, A.D. (1986). A severe organophosphate poisoning requiring the use of an atropine drip. J. Toxicol. Clin. Toxicol., 24(1): 69-76.
Lewis, K.A., Tzillivakis, J., Warner, D. and Green, A. (2016). An international database for pesticide risk assessments and management. Hum. Ecol. Risk. Assess., 22(4): 1050-1064.
Li, W., Zhao, Y., Yan, X., Duan, J., Saint, C.P. and Beecham, S. (2019a). Transformation pathway and toxicity assessment of malathion in aqueous solution during UV photolysis and photocatalysis. Chemosphere, 234: 204-214.
Li, N., Wang, X., Zhang, H., Zhang, Z., Ding, J. and Lu, J. (2019b). Comparing the performance of various nanofiltration membranes in advanced oxidation-nanofiltration treatment of reverse osmosis concentrates. Environ. Sci. Pollut. Res., 26(17): 17472-17481.
Ling, W., Qiang, Z., Shi, Y., Zhang, T. and Dong, B. (2011). Fe(III)-loaded activated carbon as catalyst to improve omethoate degradation by ozone in water. J. Mol. Catal. A Chem., 342-343: 23-29.
Lotti, M., Ferrara, S.D., Caroldi, S. and Sinigaglia, F. (1981). Enzyme studies with human and hen autopsy tissue suggest omethoate does not cause delayed neuropathy in man. Arch. Toxicol., 48: 265-270.
Manitoba Sustainable Development (2020). Personal communication with K. Philip, Environmental Stewardship Division.
Marin, J. (2010). Determination of the Effect of Chlorination of the Degradation of Dimethoate in Water. Unpublished study prepared by PTRL West, Inc., Project Number: 1970W (as cited in Health Canada, 2015).
Meijers, R.T., Oderwald-Muller, E.J., Nuhn, P.A.N.M. and Kruithof, J.C. (1995). Degradation of pesticides by ozonation and advanced oxidation. Ozone Sci. Eng., 17: 673-686.
Mellert, W., Hellwig, J., Gembardt, C., Deckardt, K. and van Ravenzwaay, B. (2003). Dimethoate - Two-Generation Reproduction Toxicity Study in Wistar Rats Administration in the Diet. Study Duration: November 2000 - May 2002. BASF Aktiengesellshaft, DTF Doc No; '453-007' Ref: 3-43/Vol. 3-22 to 3-24 (as cited in APVMA, 2010).
Miguel, N., Ormad, M.P., Lanao, M., Mosteo, R. Ovelleiro, J.L. (2008). Influence of the activated carbon nature and the aqueous matrix in the pesticides adsorption. Tecnol. Agua, 28(303): 42-47.
Miltner, R.J., Baker, D.B., Speth, T.F., Fronk, C.A., 1989. Treatment of seasonal pesticides in surface waters. J. Am. Water Works Assoc., 81: 43-52.
Ministère de l'Environnement et de la Lutte contre les Changements climatiques du Québec (2019). Personal communication with P. Cantin, Direction de l'eau potable et des eaux souterraines.
Myers, D.P. (2001a). Dimethoate Dose Finding Study in CD Rats by Oral Gavage Administration Preliminary to Developmental Neurotoxicity Study. Huntingdon Life Sciences, Ltd., Laboratory Project No. CHV/068 (Report 000129); MRID 45529701, Cambridgeshire, England. Available at https://archive.epa.gov/scipoly/sap/meetings/web/html/072904_mtg.html.
Myers, D.P. (2001b). Dimethoate. Developmental Neurotoxicity Study in the CD Rat by Oral Gavage Administration. Huntington Life Sciences, Ltd., Laboratory report number CHV/069; 003881, MRID 44529703, Woodley Road, Alconbury, Huntingdon, Cambridgeshire, England. Available at https://archive.epa.gov/scipoly/sap/meetings/web/html/072904_mtg.html.
Myers, D.P. (2001c). Dimethoate: Effects on Cholinesterase in the CD Rat (Adult and Juvenile) by Oral Gavage Administration. Huntingdon Life Sciences, Ltd., Doc. No. CHV 070/012226. MRID 45529702. Cambridgeshire, England. Available at https://archive.epa.gov/scipoly/sap/meetings/web/html/072904_mtg.html.
Myers, D.P. (2004). Dimethoate Cross Fostering Study in CD Rats. Huntingdon Life Sciences, Ltd., Laboratory Report No. CHV/ 089/033185; MRID 46214501, Cambridgeshire, England. Available at https://archive.epa.gov/scipoly/sap/meetings/web/html/072904_mtg.html.
New Brunswick Department of Environment and Local Government (2019). Personal communication with K. Gould.
Newfoundland and Labrador Department of Municipal Affairs and Environment (2019). Personal communication with H. Khan, Water Resources Management Division.
NHMRC, NRMMC (2011). Australian Drinking Water Guidelines Paper 6 National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra.
Nova Scotia Environment (2019). Personal communication with A. Polegato, Sustainability and Applied Science Division.
Ontario Ministry of the Environment, Conservation and Parks (2020). Personal communication with S. Deshpande, Standards Development Branch.
Ormad, M.P., Miguel, N., Claver, A., Matesanz, J.M. Ovelleiro, J.L. (2008). Pesticides removal in the process of drinking water production. Chemosphere, 71(1): 97-106.
Ormad, M.P., Miguel, N., Lanao, M., Mosteo, R. Ovelleiro, J.L. (2010). Effect of application of ozone and ozone combined with hydrogen peroxide and titanium dioxide in the removal of pesticides from water. Ozone Sci.Eng., 32(1): 25-32.
Pavlic, M., Haidekker, A., Grubwieser, P. and Rabl, W. (2002). Fatal intoxication with omethoate. Int. J. Legal Med., 116: 238-241.
Piet, G.J. and Zoeteman, B.C.J. (1980). Organic water quality changes during sand bank and dune filtration of surface waters in the Netherlands. J. Am. Water Works Assoc., 72(7): 400-404.
Plakas, K.V. and Karabelas, A.J. (2012). Removal of pesticides from water by NF and RO membranes - A review. Desalination, 287: 255-265.
Prince Edward Island Department of Communities, Land and Environment (2019). Personal communication with G. Somers, Environment Division.
Qiang, Z., Ling, W. and Tian, F. (2013). Kinetics and mechanism for omethoate degradation by catalytic ozonation with Fe(III)-loaded activated carbon in water. Chemosphere, 90(6): 1966-1972.
Ray, C., Grischek, T., Schubert, J., Wang, J.Z. and Speth, T.F. (2002). A perspective of riverbank filtration. J. Am. Water Works Assoc., 94(4): 149-160.
Saskatchewan Water Security Agency (2019). Personal communication with S. Ferris, Regulatory Division.
Schladt, L. (1995). E6876 (Folimat®) Study for Chronic Toxicity and Carcinogenicity in Wistar Rats Following Two-Year Administration in Drinking Water. Bayer AG Fachbereich Toxikologie, Bayer study no. T2030748, Friedrich-Ebert-Strasse, Germany (as cited in APVMA, 2011).
Schladt, L. (2001). E 6876 Oncogenicity Study in B6C3F1 Mice (Administration in the Drinking Water Over 24 Months; T1032655). Institute of Toxicology, Bayer AG, Bayer study no. T 1032655. Lab. report no. PH 30972, Friedrich-Ebert-Strasse, Germany (as cited in APVMA, 2011).
SGS Environmental Services (2019). Personal communication with D. Griffin. Lakefield, ON.
Smeets, P.W.M.H., Medema, G.J. and van Dijk, J.C. (2009). The Dutch secret: how to provide safe drinking water without chlorine in the Netherlands. Drinking Water Eng. Sci., 2: 1-14.
Sørensen, S.R., Schultz, A., Jacobsen, O.S. and Aamand, J. (2006). Sorption, desorption and mineralization of the herbicides glyphosate and MCPA in samples from two Danish soil and subsurface profiles. Environ. Pollut., 141(1): 184-194.
Speth, T.F. and Adams, J.Q. (1993). GAC and Air Stripping Design Support for the Safe Drinking Water Act, Strategies and Technologies for Meeting SDWA Requirements. Clark, R. and S. Summers, Eds., Lewis Publishers, Ann Arbor, MI, pp. 47-89.
Speth, T.F. and Miltner, R.J. (1998). Technical note: adsorption capacity of GAC for synthetic organics. AWWA Journal, 90(4): 171.
Summers, R.S., Knappe, D.R.U. and Snoeyink, V.L. (2010). Adsorption of organic compounds by activated carbon, in: Edzwald, J.K. (eds.), Water Quality and Treatment: A Handbook on Drinking Water, 6th edition. McGraw-Hill.
Summers, R.S., Kennedy, A.M., Knappe, D.R.U., Reinert, A.M., Fotta, M.E., Mastropole, A.J., Corwin, C.J. and Roccaro, J. (2014). Evaluation of Available Scale-Up Approaches for the Design of GAC Contactors. Water Research Foundation.
Tan, L. S., A. L. Ahmad, S. R. A. Shukor, and S. P. Yeap (2019). Impact of solute properties and water matrix on nanofiltration of pesticides. Chem. Eng. Tech., 42 (9): 1780-1787.
Tesh, J.M., Ross, F.W., Wgihtman, T.J. and Wilby, O.K. (1982). S 6876: Effects of Oral Administration upon Pregnancy in the Rabbit. 2. Main Study. Bayer AG, LSR Report No. 82/BAG023/111, Friedrich-Ebert-Strasse, Germany (cited in APVMA, 2011).
Tian, F., Liu, W., Guo, G., Qiang, Z. Zhang, C. (2014). Kinetics and mechanism of dimethoate chlorination during drinking water treatment. Chemosphere, 103: 181-187.
Tsatsakis, A.M., Manousakis, A., Tzatzarakis, M., Delaki, C. and Agouridakis, P. (1998). Clinical and toxicological data in fenthion and omethoate acute poisoning. J. Environ. Sci. Health B, 33(6): 657-670.
US EPA (1995). Dimethoate: Pesticide tolerance. Fed. Regist., 60:128 (Wednesday, July 5, 1995): 34945-34947.
US EPA (1998). Method 8270D - Semivolatile organic compounds by gas chromatography/mass spectrometry (GC/MS), Revision 4.0.
US EPA (2000a). ECM - Dimethoate & Omethoate in Water. MRID 46276301. Office of Pesticide Programs. Washington, DC. Available at https://www.EPA..gov/pesticide-analytical-methods/ecm-dimethoate-omethoate-water-mrid-46276301.
US EPA (2000b). DER - Dimethoate & Omethoate in Water. MRID 46276301. Office of Pesticide Programs. Washington, DC. Available at https://www.EPA..gov/pesticide-analytical-methods/ecm-dimethoate-omethoate-water-mrid-46276301.
US EPA (2000c). Method 8141B - Organophosphorus compounds by gas chromatography, Revision 2.0.
US EPA (2002). Dimethoate: 2nd Report of the Hazard Identification Assessment Review Committee. United States Environmental Protection Agency (US EPA.), Hazard Identification Assessment Review Committee (HIARC), Health Effects Division, PC Code 035001; TXR NO. 0050600.
US EPA (2004). Dimethoate: Issues Related to the Hazard and Dose Response Assessment. US Environmental Protection Agency, Office of Pesticide Programs for the FIFRA Scientific Advisory Panel, November 2, 2004. Docket No. OPP-2004-0320, Washington, D.C.
US EPA (2005). Method 527 - Determination of selected pesticides and flame retardants in drinking water by solid phase extraction and capillary column gas chromatography/mass spectrometry (GC/MS), Revision 1.0.
US EPA (2006a). Reregistration Eligibility Decision for Dimethoate. Office of Pesticide Programs, United States Environmental Protection Agency, Docket EPA.-HQ-OPP-2006-0618, Washington, D.C.
US EPA (2006b). Dimethoate: The Revised Post-SAP HED Chapter of the Reregistration Eligibility Decision Document (RED). United States Environmental Protection Agency (US EPA.), Health Effects Division (HED), PC Code: 035001, Case #0088, Washington, D.C.
US EPA (2007). Revised Dimethoate IRED. United States Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Dimethoate Docket EPA.-HGQ-OPP-2005-0084, Washington, D.C.
US EPA (2011). Finalization of Guidance on Incorporation of Water Treatment Effects on Pesticide Removal and Transformation in Drinking Water Exposure Assessments.
US EPA (2016). Announcement of final regulatory determinations for contaminants on the third drinking water contaminant candidate list; EPA-HQ-OW-2012-0155; FRL-9940-64-OW. Fed. Regist., 81:1(Monday, January 4, 2016): 2015-32760.
van der Aa, L.T.J., Kolpa, R.J., Rietveld, L.C. and van Dijk, J.C. (2012). Improved removal of pesticides in biological granular activated carbon filters by pre-oxidation of natural organic matter. J. Water Supply Res. Technol. AQUA, 61(3): 153-163.
Van Der Bruggen, B. and Vandecasteele, C. (2003). Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut., 122(3): 435-445.
Verstraeten, I.M. and Heberer, T. (2002). Organic chemical removal issues. Chapter 17 in: Riverbank Filtration: Improving Source-water Quality. Ray, C., Melin, G. and Linsky, R.B. (eds.). Springer, Kluwer Academic Publishers. Netherlands. pp. 321-330.
WHO (2004). Dimethoate in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality. World Health Organization, WHO/SDE/WSH/03.04/90, Geneva.
WHO (2012). Water Safety Planning for Small Community Water Supplies. World Health Organization, Geneva, Switzerland. Available at http://www.who.int/water_sanitation_health/publications/small-commwater_supplies/en/
WHO (2017). Guidelines for Drinking-Water Quality. Fourth Edition. Incorporating the First Addendum. World Health Organization, Geneva, Switzerland. Available at https://www.who.int/publications/i/item/9789241549950.
Yang, K., Yu, J., Guo, Q., Wang, C., Xia, P., Yang, Y.J. and Yang, M. (2019). Treatment performance comparison between regular O3- BAC and O3- BAC with rear sand filtration: Verification in a full-scale study. Env. Sci. Eur., 31(1).
Zearley, T.L. and Summers, R.S. (2012). Removal of trace organic micropollutants by drinking water biological filters. Environ. Sci. Technol., 46(17): 9412-9419.
Zheng, L., Pi, F., Wang, Y., Xu, H., Zhang, Y. and Sun, X. (2016). Photocatalytic degradation of acephate, omethoate, and methyl parathion by Fe3O4@SiO2@mTiO2 nanomicrospheres. J. Hazard. Mater., 315: 11-22.
Zhu, A., Long, F., Wang, X., Zhu, W. and Ma, J. (2007). The negative rejection of H+ in NF of carbonate solution and its influences on membrane performance. Chemosphere, 67(8): 1558-1565.
Appendix A: List of abbreviations
- AC
- Activated carbon
- AChE
- Acetylcholinesterase
- ADI
- Acceptable daily intake
- ANSI
- American National Standards Institute
- APVMA
- Australian Pesticides and Veterinary Medicines Authority
- BAC
- Biologically active carbon
- BMD
- Benchmark dose
- BMDL
- Benchmark dose lower limit
- BW
- Body weight
- CDW
- Federal-Provincial-Territorial Committee on Canadian Drinking Water
- ChE
- Cholinesterase
- COD
- Chemical oxygen demand
- DNT
- Developmental neurotoxicity
- DOC
- Dissolved organic carbon
- DW
- Drinking water
- EBCT
- Empty bed contact time
- EU
- European Union
- Fe-AC
- Fe(III) deposited on activated carbon
- FIFRA
- Federal Insecticide, Fungicide, and Rodenticide Act
- FNIHB
- First Nations and Inuit Health Branch
- GAC
- Granular activated carbon
- GD
- Gestation day
- HBV
- Health-based value
- HPLC
- High performance liquid chromatography
- IARC
- International Agency for Research on Cancer
- LC50
- Median lethal concentration
- LD50
- Median lethal dose
- LOAEL
- Lowest-observed-adverse-effect-level
- MAC
- Maximum acceptable concentration
- MDL
- Method detection limit
- MRL
- Method reporting limit
- MWCO
- Molecular weight cut-off
- NF
- Nanofiltration
- NOAEL
- No-observed-adverse-effect-level
- NOEL
- No-observed-effect-level
- NOM
- Natural organic matter
- NSF
- NSF International
- OMP
- Organic micropollutant
- PAC
- Powdered activated carbon
- PMRA
- Pest Management Regulatory Agency
- PND
- Postnatal day
- POD
- Point of departure
- RBF
- Riverbank filtration
- RO
- Reverse osmosis
- RWOM
- River water organic material
- SAP
- Scientific Advisory Panel
- SCC
- Standards Council of Canada
- UDS
- unscheduled DNA synthesis
- UF
- Uncertainty factor
- US EPA
- United States Environmental Agency
- WHO
- World Health Organization
Appendix B: Canadian water quality data
| Jurisdiction (year sampled) |
No. Detects/ Samples | MDL (ng/L) |
Range (ng/L) | |
|---|---|---|---|---|
| Min | Max | |||
| Surface Water | ||||
| BC – Lower Fraser Valley and Okanagan Basin (2003–2005) | 15/85 | 0.075 | <0.075 | 604 |
| BC – Lower Fraser Valley (2003–2005) | _ | _ | 1.4 | 604 |
| BC – Lower Fraser Valley (2003–2005)Footnote 1 | _ | _ | 0.02 | 233 |
| ON (2004) | 10/228 | 25.1 | 28.9 | 175 |
| ON (2005) | 2/160 | 25.1 | 24.7 | 33 |
| ON - 10 isolated lakes (2003–2005) | 31/163 | 0.023 | <0.023 | 5.87 |
| QC (2003) | 1/50 | 40 | <40 | 280 |
| QC (2004) | 6/69 | 5-40 | <5 | 90 |
| QC (2005) | 0/62 | 40 | _ | _ |
| NB (2003–2005) | 4/57 | 40-450 | _ | _ |
| PEI (2003–2005) | 0/82 | 50 | _ | _ |
| NS (2003–2005) | 0/19 | 40 | _ | _ |
| Rivers | ||||
| AB, SK, MB – 8 sites (2003) | 0/13 | 25.1 | _ | _ |
| Reservoir Water | ||||
| AB, SK, MB – 15 sites (2003–2004) | 1/30 | 25.10 | 5.98 | 25.1 |
| Runoff | ||||
| BC - Lower Fraser Valley (2003–2005) | _ | _ | 200 | 3000 |
| BC - Okanagan Basin (2003–2005) | _ | _ | 1.2 | 17.5 |
| Air | ||||
| ON – 4 sites (2004–2005) | 0/12 | 0.007 | <0.007 | _ |