What We Heard
Responses to the Public Consultation on the White Paper "Public Release of Clinical Information in Drug Submissions and Medical Device Applications"
Who provided feedback?
From March 10 to May 26, 2017, Health Canada received 45 individual submissions in response to its white paper entitled "Public Release of Clinical Information in Drug Submissions and Medical Device Applications".
The breakdown of responses by stakeholder groups is shown in Pie chart 1 below.
*Note: the numbers below reflect unique submissions from each stakeholder group; some submissions were
submitted on behalf of multiple individuals or organizations.
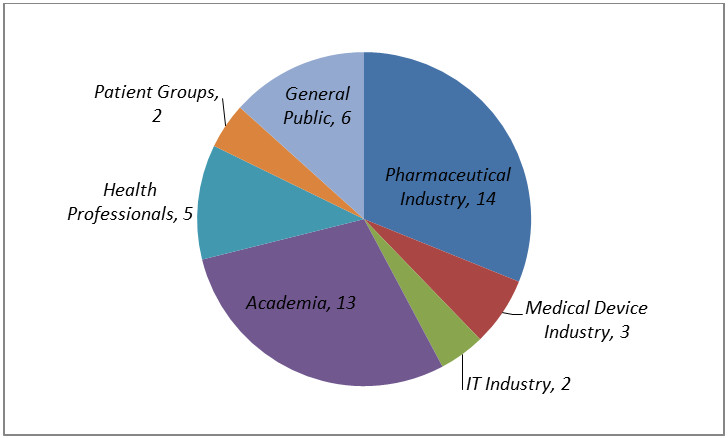
Pie chart 1 - Text Equivalent
This graph illustrates the distribution by stakeholder group of responses to the public consultation on the white paper, "Public Release of Clinical Information in Drug Submissions and Medical Device Applications". Shown is a total of 45 responses, with 14 from the pharmaceutical industry, 3 from the medical devices industry, 2 from the IT industry, 13 from academia, 5 from health professionals, 2 from patient groups, and 6 from the general public.
What did the stakeholders say?
Academia and researchers:
Respondents in this group were supportive of this proposal and advocated for even greater transparency. In summary:
- Majority of the respondents supported public release of clinical information and stated that access to released clinical information needs to be unrestricted.
- Several suggested that proposed provisions for protection of information constituting exploratory endpoints, secondary indications, ongoing development programs, interim results, and methodologies may prevent full release of clinical information.
- Consultation on the scope of the redactions and time allowed for industry should be well defined in order not to prolong the process.
- All non-prescription drugs and natural health products, as well as reviewer's reports should be included in the scope of the proposal.
Health professionals:
Similar to academia and researchers, respondents in this group were also supportive of this proposal. In summary:
- Respondents thought that greater availability of clinical information will benefit doctor-patient interactions and inform decision-making on available treatments.
- Similarly, alignment with international clinical trial transparency initiatives would benefit health professionals and research in Canada.
- Respondents raised concerns with respect to risks of delay in implementation and proper resourcing of this initiative.
Pharmaceutical, medical device and IT industries:
While many industry members were supportive of this proposal and transparency initiatives in general, several comments were raised on the proposal. In summary:
- The pharmaceutical industry encouraged close international alignment of this proposal internationally, and specifically with European Medicines Agency's (EMA) proactive clinical data publication initiative. Many respondents stated that the scope of the documents to be released should be exactly the same as in EMA's Policy 0070.
- Several respondents asked that the proposal apply prospectively from the coming into force date only, some citing technical difficulties with submissions not in common technical document (CTD) format submitted prior to 2001.
- The pharmaceutical industry suggested that the release of individual patient data be aligned with phase 2 of EMA's Policy 070, and adequate time and thought be afforded for the complexities associated with disclosing individual patient data.
- Safeguards against commercial use of disclosed clinical information should be similar to those used by the EMA's portal.
- Several respondents also raised concerns about burden on industry and Health Canada resources.
- Several respondents asked that non-prescription new drug submissions as well as withdrawn and/or negative regulatory decisions be excluded from the scope of the proposal.
- Several respondents believe that the existing frameworks for accessing clinical information (i.e. manufacturers' publications, ATI requests, and CBI requests under s.21.1(3)(c) of the FDA) are sufficient for achieving the transparency objectives.
- Member companies and associations from the medical device industry noted that while drug companies can leverage their EMA policy 070 transparency submissions, there is no equivalent opportunity for medical devices. Respondents noted that the global approach in major jurisdictions (i.e., United States and European Union) to clinical data transparency for medical devices varies and is at different stages of development. Members suggested that the implementation of medical device clinical data disclosure be delayed and aligned with recently published European Union medical device regulations (Regulation 2017/745 and IVD Regulation 2017/746) that are not expected to be implemented prior to 2020.
Patient groups and general public:
Respondents from patient groups and the general public were favourable of this proposal and supported the positions of academics and researchers and health professionals. In summary:
- Most reiterated the foreseen benefits to the healthcare system of greater availability of clinical information.
- Patient groups were optimistic of further benefits to their advocacy and educational efforts, and the respondents from the general public supported the notion that doctor-patient interactions would benefit as well.
- Some respondents were concerned with the limitations on the scope of clinical information being proposed for release. Concerns also focused on evidence that Health Canada is committed to early implementation and a process that avoids the delays experienced through Access to Information.
Next steps - continued engagement & regulatory development
We would like to thank all respondents for providing comments on this proposal. Feedback received will inform the development of draft regulations, internal operational processes, and relevant guidance documents.
Health Canada is also moving ahead with plans to continue external engagement with stakeholders and subject matter experts throughout the summer and fall of 2017.
Health Canada anticipates to propose draft regulations to support public release of clinical information in drug submissions and medical device applications in the fall of 2017. There will be further public consultations after the draft regulations are published.
Contact us
Mailing address:
Office of Information Management
Resource Management and Operations Directorate
Health Products and Food Branch
Health Canada
Graham Spry Building
250 Lanark Avenue
Ottawa, Ontario
K1A 0K9
Telephone: 613-793-4180
Email: hc.rmod_stakeholders-intervenants_dgro@hc-sc.gc.ca
Visit our page to receive updates on our work.