Consultation: Proposed Residential Indoor Air Quality Guidelines for Acrolein
Current status: Closed
Closed to new input on August 11, 2020.
Download the alternative format
(PDF format, 1.2 MB, 71 pages)
Organization: Health Canada
Published: 2020-06-12
Purpose of consultation
The present document reviews the epidemiological, toxicological, and exposure research on acrolein as well as the conclusions from a number of comprehensive reviews from internationally recognized health and environmental organizations. The intent is to propose new short- and long-term indoor air exposure limits, which would minimize risks to human health, and recommend various risk mitigation measures to reduce exposure to acrolein. The purpose of this consultation is to solicit comments on the proposed Residential Indoor Air Quality Guidelines (RIAQG) for Acrolein.
Health Canada undertook a health risk assessment of acrolein because levels in Canadian homes are generally higher than the recommended Indoor Air Reference Level (IARL) for acrolein established by Health Canada in 2017, and in order to more fully characterize sources of acrolein in the indoor environment.
The document proposes (1) a short-term (one hour) exposure limit of 38 µg/m3 to protect against eye irritation and (2) a long-term exposure limit of 0.44 µg/m3 (based on a 24-hour average) to protect against adverse respiratory effects. Levels of acrolein in a typical Canadian home are likely below the short-term, but above the long-term exposure limits, and accordingly may pose a health risk, specifically related to adverse respiratory effects.
This document is available for a 60-day public consultation period. Please send comments (with rationale, where required) to Health Canada:
hc.air.sc@canada.ca
or
Water and Air Quality Bureau, Health Canada
269 Laurier Avenue West, A.L. 4903B
Ottawa, ON K1A 0K9
All comments must be received before August 11, 2020.
It should be noted that this document may be revised following the evaluation of comments received, and RIAQG for Acrolein will be established, if required. This document should be considered as a draft for comment only.
Preamble
Health Canada assesses the health risks posed by specific indoor pollutants in residential environments and provides recommendations on how to reduce those risks. Residential Indoor Air Quality Guidelines (RIAQG) summarize the known health effects, pollutant sources, and exposure levels in Canadian homes and characterize the risks to health, based on the best scientific data available. Proposed exposure limits (also referred to as guideline values) for short- and/or long-term exposure to the pollutant are developed, representing indoor air concentrations below which health effects are unlikely to occur. The proposed exposure limits take into account the reference concentrations (RfC) for the pollutant and the feasibility of achieving such levels through control of indoor sources, and may be established for short-term exposure, long-term exposure or both. The RIAQG also include recommendations for controlling sources or other actions to reduce exposure to the pollutant.
For some pollutants, a proposed exposure limit may not be developed, although the available scientific evidence justifies reducing Canadians' exposure to the pollutant. In this case, a guidance document that focuses on actions to control sources and reduce exposure is developed.
The RIAQG and guidance documents serve as a scientific basis for activities to evaluate and reduce the risk from indoor air pollutants including, but not limited to:
- assessments by public health officials of health risks from indoor air pollutants in residential or similar environments;
- performance standards that may be applied to pollutant-emitting materials, products, and devices, so that their normal use does not lead to air concentrations of pollutants exceeding the proposed exposure limits; and
- communication products informing Canadians of actions they can take to reduce their exposure to indoor air pollutants and to help protect their health.
The RIAQG and guidance documents replace a series of exposure limit values for indoor air pollutants from a report entitled Exposure Guidelines for Residential Indoor Air Quality (Health Canada 1987). In addition to updates for the substances included in the 1987 report, guidelines or guidance documents will be developed for other substances that are identified as having the potential to affect human health in the indoor environment.
The focus of this document is acrolein, which was identified as a priority for the development of RIAQG, because indoor air concentrations measured in Canadian homes were found to exceed the indoor air reference level (IARL) of 0.35 µg/m3 (Health Canada 2017). The IARL is based on respiratory epithelial lesions in rats from an assessment published by the California Environmental Protection Agency (CalEPA 2008).
In addition to relevant literature, the present document draws from a number of comprehensive reviews of the health effects of acrolein, including:
- Proposition de valeurs guides de qualité d'air intérieur : L'acroléine, published by the Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES; France) in 2013
- Acrolein Reference Exposure Levels, published by the California Environmental Protection Agency in 2008 (cited hereafter as CalEPA 2008)
- Toxicological Profile for Acrolein, published by the Agency for Toxic Substances and Disease Registry in 2007 (cited hereafter as ATSDR 2007)
- Toxicological Review of Acrolein, published by the US Environmental Protection Agency in 2003 (cited hereafter as US EPA 2003)
- Concise International Chemical Assessment Document 43: Acrolein, published by the World Health Organization in 2002 (cited hereafter as WHO 2002)
- Priority Substances List Assessment Report: Acrolein, published by Environment Canada and Health Canada in 2000 (cited hereafter as Environment Canada and Health Canada 2000)
Relevant literature was identified through the aforementioned comprehensive reviews and a web-based search through October 2018, with an emphasis on those published since the most recent comprehensive review (i.e., ANSES 2013). The original articles of direct relevance to evaluating exposure to acrolein in the indoor environment and its associated health effects were reviewed. The scope of this document is limited to the inhalation of acrolein, and does not consider dietary sources or oral routes of exposure. Key studies underlying the derivation of the proposed exposure limits are presented, and where appropriate, supporting information is summarized. In addition, information on acrolein concentrations in Canadian homes as well as factors influencing these concentrations was obtained from Health Canada research studies.
Table of contents
- List of tables
- List of figures
- Executive summary
- 1.0 Physical and chemical characteristics
- 2.0 Sources in the air
- 3.0 Concentrations in indoor and outdoor air
- 4.0 Toxicokinetics
- 5.0 Health effects
- 6.0 Derivation of short- and long-term reference concentrations
- 7.0 Proposed guidelines
- 8.0 References
- Appendix A: List of acronyms and abbreviations
- Appendix B: Human exposure studies
- Appendix C: Toxicological studies
- Appendix D: Other guidelines
- D1. Short-Term Exposure Guidelines
- D2. Exposure Guidelines for Non-Neoplastic Chronic Effects
List of tables
- Table 1. Physical and chemical properties of acrolein
- Table 2. Concentrations (µg/m3) of acrolein in indoor and outdoor air in Canada
- Table 3. Proposed exposure limits for acrolein for indoor environments
- Table B1. Short-term exposure
- Table B2. Epidemiological studies
- Table C1. Acute (single) exposure studies
- Table C2. Repeat exposure studies (3 days to 6 weeks)
- Table C3. Repeat exposure studies (6 weeks to 18 months)
- Table D1. Other short-term exposure guidelines
- Table D2. Other exposure guidelines for non-neoplastic chronic effects
List of figures
- Figure 1. Distribution of indoor acrolein concentrations by season across studies conducted by Health Canada
- Figure 2. Distribution of I/O ratios by season across studies conducted by Health Canada
- Figure 3. Proposed pathway for the metabolism of acrolein (adapted from WHO 2002 and Burcham 2016)
Executive summary
| Exposure Limit | Concentration | Critical effect(s) | |
|---|---|---|---|
| µg/m3 | ppb | ||
| Short-term (1 h) |
38 | 17 | Eye irritation in healthy volunteers |
| Long-term (24 h) |
0.44 | 0.19 | Lesions in the respiratory epithelium of the rat nasal cavity |
The proposed short-term (one hour) exposure limit for acrolein is 38 µg/m3 and the proposed long-term exposure limit is 0.44 µg/m3 (based on 24-hour average).
Levels of acrolein in a typical Canadian home are likely below the short-term, but above the long-term exposure limits, and accordingly may pose a health risk, specifically related to adverse respiratory effects. It is therefore recommended to reduce exposure to acrolein by ensuring adequate ventilation and controlling indoor sources.
Background
Acrolein is a very reactive and volatile α,β-unsaturated aldehyde, which is found in both indoor and outdoor air. In the Priority Substances List Assessment Report: Acrolein published in 2000, Environment Canada and Health Canada derived a tolerable concentration based on changes in cells of the nasal respiratory epithelium of rats following inhalation exposure to acrolein. A number of key studies have been published since the Priority Substances List Assessment Report, along with health risk assessments from several international organizations. Health Canada established an Indoor Air Reference Level (IARL) for acrolein in 2017. IARLs represent concentrations that are associated with acceptable levels of risk after long-term exposure for a specific volatile organic compound (VOC), as determined by the organization or jurisdiction that performed the risk assessment. As levels in Canadian homes are generally higher than the recommended IARL, and in order to more fully characterize sources of acrolein in the indoor environment and review recent health effects literature, this substance was prioritized for a full health risk assessment and development of Residential Indoor Air Quality Guidelines (RIAQG).
The proposed RIAQG review the epidemiological, toxicological, and exposure research on acrolein as well as the conclusions from a number of comprehensive reviews from internationally recognized health and environmental organizations. They are intended to provide proposed short- and long-term indoor air exposure limits for acrolein, which would minimize risks to human health, and to support the development of actions to limit acrolein emissions. The proposed RIAQG also show that levels in Canadian houses may potentially present a health risk when compared to the proposed exposure limits and recommend various risk mitigation measures to reduce exposure to acrolein.
Sources and Exposure
Acrolein is ubiquitous throughout the ambient environment. The primary natural source of acrolein is incomplete combustion of organic matter during forest fires. The principal anthropogenic source of atmospheric acrolein is the combustion of organic matter and fuels, with motor vehicles (including aircraft) generating most of the acrolein emissions. Industrial processes such as incineration, pulp and paper and oriented-strand board production, and coal electricity generation also contribute to acrolein emissions, though much less than mobile sources.
Acrolein levels in residential indoor air are generally greater than outdoor levels. Some of the sources of acrolein in indoor air are smoking, using gas stoves, wood-burning fireplaces, burning incense, cooking with oils, and secondary formation by oxidation of other VOCs from products and building materials. However, no information is available on the relative contributions of these various sources to the total indoor air concentration of acrolein.
Acrolein is one of the most difficult chemicals to measure in air due to its reactivity. Health Canada studies have collected acrolein measurements in air using the following two most common methods: 2,4-dinitrophenylhydrazine cartridges for sampling coupled with high performance liquid chromatography for analysis; and passivated canisters for sampling coupled with gas chromatography mass spectrometry for analysis. While both methods have limitations, the scientific literature and work carried out by Environment and Climate Change Canada and Health Canada suggest that passivated canisters provide the most accurate estimate of indoor acrolein levels available.
Median acrolein levels measured using passivated canisters in Edmonton, Halifax, Regina, and Windsor during winter and summer from 2005 to 2010 ranged from 1.3 to 8.1 µg/m3 indoors and from 0.2 to 2.2 µg/m3 outdoors (Health Canada 2010a, 2010b, 2012, 2013). In Windsor, personal exposure measurements were also collected, with a median range of 1.1 to 4.3 µg/m3. In these studies, the ratio of indoor-to-outdoor acrolein concentrations was in general consistently above 2.5, which is indicative of a predominance of indoor sources of acrolein.
Health Effects
Health effects of exposure to acrolein have been examined in toxicological and controlled human exposure studies, with very little epidemiological evidence related to indoor acrolein exposure. Based on the evidence from these studies, the effects of short- and long-term acrolein inhalation exposures are observed at the site of entry. Key health effects include eye and respiratory irritation, and tissue damage in the respiratory tract.
In this assessment, the short-term exposure limit is derived from the results of a controlled human exposure study, whereas the long-term exposure limit is based on toxicological data from a study in a rodent model. Supporting evidence is provided by the results of other toxicological and controlled human exposure studies.
Human studies
Studies with human participants reported that acute exposure induced eye irritation at acrolein concentrations as low as 0.21 mg/m3 (210 µg/m3), nasal irritation starting at 0.35 mg/m3 (350 µg/m3), and respiratory irritation (measured by decreased respiration rate) starting at 0.69 mg/m3 (690 µg/m3) (Darley et al. 1960; Weber-Tschopp et al. 1977; Dwivedi et al. 2015; Claeson and Lind 2016). Epidemiological data on the long-term effects in humans are limited to two studies in France: one study showed a positive association between acrolein levels in schools and allergic asthma in the previous year, and between acrolein levels and exercise-induced asthma, but a negative association between acrolein levels and non-allergic asthma (Annesi-Maesano et al. 2012); in the other study, no significant relationship was identified between acrolein levels measured in homes and asthma in the previous year (Billionnet et al. 2011). Neither study showed a relationship between acrolein levels and rhinitis.
Toxicological studies
In laboratory animals, acute acrolein exposure induced irritant effects such as decreased respiration, bronchoconstriction/increased flow resistance, and increased mucus secretion in multiple species at concentrations as low as 0.7 mg/m3 (700 µg/m3). Changes in cell composition in the respiratory tracts of guinea pigs, hamsters, and rats were observed at higher concentrations, starting at 2.1 mg/m3 (2100 µg/m3) (Leikauf 1991; Roemer et al. 1993; Cassee et al. 1996; Cassee, Groten and Feron 1996; Arumugan et al. 1999; US EPA 2003; CalEPA 2008).
Repeated inhalation exposures to acrolein produced similar effects as single exposures. Studies in mice and rats have shown that exposure to acrolein for 3 days to 13 weeks results in increased mucus secretion, and inflammation and cell proliferation in the respiratory epithelium accompanied by basal cell hyperplasia and squamous cell metaplasia (Lyon et al. 1970; Feron et al. 1978; Kutzman et al. 1981, 1985; Costa 1986; Roemer et al. 1993; Cassee, Groten and Feron 1996; Dorman et al. 2008). The severity of the effects appears to increase with exposure concentration but not with duration of exposure. In experimental animals, acrolein reacts mainly in the nasal area and upper respiratory tract, but there may be increased penetration and damage to the lower respiratory tract at higher concentrations. In most studies, effects were observed at the lowest test concentration, starting at 0.9 mg/m3 (900 µg/m3); however, one study identified a no observed adverse effect level (NOAEL) of 0.46 mg/m3 (460 µg/m3) for pathology of the rat nasal respiratory epithelium, including inflammation, hyperplasia, and squamous metaplasia (Dorman et al. 2008).
Acrolein has been shown to be mutagenic and genotoxic in vitro, but there were no indications of genotoxicity in limited in vivo studies (Kutzman 1981; Lam et al. 1985; Environment Canada and Health Canada 2000; US EPA 2003; ATSDR 2007; Wang et al. 2012; Lee et al. 2014). Conclusions regarding its carcinogenicity potential cannot be drawn from the limited studies available.
Susceptible populations
Sensitized individuals such as asthmatics as well as individuals with chronic pulmonary disease or bronchitis may be more susceptible to the effects of acrolein on the respiratory tract. Children, especially those with asthma, may be more likely to show adverse respiratory effects following exposure to acrolein due to higher prevalence rates of asthma in children as compared to other age groups, the small size and immature state of their airways, and the exacerbation that toxic air contaminants have been demonstrated to have on asthma in children. In general, pre-existing nasal allergies can also intensify the response to nasal irritants; and individuals with decreased glutathione synthesis or impaired glutathione-S-transferase activity may be more susceptible to the effects of acrolein.
Mode of action of toxicity
Acrolein is a sensory irritant that activates defense mechanisms to reduce penetration further into the respiratory tract, such as a decrease in breathing rate, an increase in mucus secretion, and bronchoconstriction. As it is highly reactive, acrolein becomes rapidly and irreversibly bound to sulfhydryl groups at the site of first contact, causing a decrease in glutathione and other reducing agents, as well as changes in enzyme activities resulting in a decrease in protective activity in the respiratory nasal epithelium. These changes also induce an inflammatory response through the recruitment of immune cells and stimulation of the production and/or release of proinflammatory cytokines.
Derivation of the proposed exposure limits
The determination of the proposed exposure limits is carried out in two stages. First, a reference concentration (RfC) is derived by applying uncertainty factors to the concentrations at which the most sensitive adverse health endpoint was observed. The RfC approach is used for the determination of proposed exposure limits to reduce potential health impacts such as those observed in key toxicological, controlled human exposure, and indoor epidemiological studies.
For the short-term exposure RfC, the exposure period is specified; in the present case, one hour. For the long-term exposure RfC, the exposure is considered to occur over months or years, up to a lifetime.
In the second stage, the short- and long-term exposure RfCs are compared with measured exposures in residential indoor air, and evaluated with respect to their technical feasibility. In general, if the RfC is considered attainable where reasonable control measures are followed, the proposed exposure limit is set equal to the RfC. If the RfC is considered unattainable with currently available risk management technology and practices, the proposed exposure limit may be set at a higher concentration. Setting the proposed exposure limit at a higher concentration than the RfC results in a smaller margin of exposure between the proposed exposure limit and the concentration at which effects have been observed in health studies. Nonetheless, a proposed exposure limit derived in this manner does provide a measure of health protection, while remaining an achievable target for improving indoor air quality when evaluating risk management measures.
Proposed short-term residential indoor air quality exposure limit
For short-term exposure to acrolein, the most sensitive endpoint was eye irritation in studies with healthy volunteers. A NOAEL of 115 µg/m3 was selected as the point of departure (Dwivedi et al. 2015) and an uncertainty factor of 3 was applied to account for sensitive individuals. Thus, the acute RfC is 38 µg/m3.
The Health Canada residential indoor air exposure studies provide a 24-hour integrated sample of acrolein measurements, which does not represent acute or peak exposure (Health Canada 2010a, 2010b, 2012, 2013). These 24-hour measurements show that the short-term reference exposure level is higher than the range of median indoor air concentrations. Therefore, as this exposure limit is achievable in Canadian homes, the proposed short-term exposure limit for acrolein is 38 µg/m3.
It is recommended that the short-term exposure limit be compared to a one-hour air sample.
Proposed long-term residential indoor air quality exposure limit
For long-term exposure to acrolein, the most sensitive endpoint was degenerative lesions in the respiratory epithelium of the rat nasal cavity. A NOAEL of 460 µg/m3 was selected as the point of departure, based on inflammation, hyperplasia, and squamous metaplasia at higher test concentrations (Dorman et al. 2008). This concentration was adjusted for continuous exposure, and toxicokinetic differences between rats and humans were accounted for by applying a regional gas dose ratio, giving a human equivalent NOAEL of 11 µg/m3. Uncertainty factors of 2.5 for toxicodynamic differences between rats and humans and 10 for sensitivity in the human population were applied. Thus, the long-term RfC is 0.44 µg/m3.
Median acrolein concentrations measured inside Canadian homes from the Health Canada residential indoor air exposure studies for a 24-hour averaging period ranged between 1.3 and 8.1 µg/m3, and the 95th percentile ranged between 3.5 and 21.0 µg/m3 (Health Canada 2010a, 2010b, 2012, 2013). This indicates that even considering uncertainties in the measurement of acrolein, there will likely be Canadian homes in which the RfC is exceeded. However, the RfC was derived using the most recent scientific information, and is consistent with both the Health Canada IARL of 0.35 µg/m3 and values from other jurisdictions (Environment Canada and Health Canada 2000, US EPA 2003, CalEPA 2008, ANSES 2013). In addition, reduction of acrolein levels in the home through ventilation and source control is considered possible. Therefore, the proposed long-term exposure limit for acrolein is 0.44 µg/m3.
When comparing a measured acrolein concentration with the long-term exposure limit, the sampling time should be at least 24 hours.
Risk Management Recommendations
Strategies for reducing indoor exposure to acrolein include the following:
- Increasing ventilation by opening windows (when possible) or by employing mechanical ventilation strategies.
- Using a range hood exhaust fan with outside venting, preferably on the high setting, when cooking, especially with oils.
- While cooking, in addition to using a range hood exhaust fan (or if no range hood exhaust fan is available), using back burners instead of front burners, opening windows or running the fan in the furnace or ventilation system.
- Not smoking or burning candles or incense inside the home, and ensuring proper ventilation to the outside during use of combustion appliances (e.g., gas stoves, woodstoves or fireplaces).
- Decreasing volatile organic compound (VOC) levels in the home to reduce secondary formation of acrolein. This can be done by choosing low-emission products whenever possible; opening windows to ensure good ventilation when using products such as glues, paints, varnishes, and cleaning products; and minimizing the use of scented products, such as plug-in or aerosol deodorizers (air fresheners).
1.0 Physical and chemical characteristics
Acrolein is a clear or yellow flammable liquid with a burnt, sweet, pungent odour. It is a volatile α,β-unsaturated aldehyde, with low water solubility and vapour pressure. Its physical and chemical properties are summarized in Table 1 (US EPA 2003; CalEPA 2008).
| Property | Value | Chemical structure |
|---|---|---|
| Molecular formula | C3H4O | 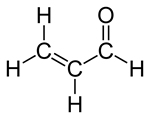 |
| Molecular weight | 56.06 g/mol | |
| CAS registry number | 107-02-8 | |
| Density | 0.843 g/cm3 | |
| Vapour pressure | 29.3 kPa at 20 °C | |
| Solubility | Soluble in ethanol and diethyl ether, and up to ~20% w/v in water | |
| Boiling point | 52.3 °C at 101.3 kPa | |
Odour threshold |
0.37 mg/m3 (370 µg/m3) (0.16 ppm) |
|
| Octanol/water partition coefficient | -0.01 | |
| Common synonyms | Acrylaldehyde, acrylic aldehyde, allyl aldehyde, ethylene aldehyde, 2-propenal, prop-2-en-1-al |
2.0 Sources in the air
This section focuses on sources of acrolein in outdoor and indoor air. Additional sources contribute to exposure to acrolein in media other than air - such as food (Environment Canada and Health Canada 2000) - but these are beyond the scope of this document.
2.1 Outdoor Sources
Acrolein is found throughout the ambient environment, emitted through both natural and anthropogenic sources. The primary natural source of acrolein is incomplete combustion of organic matter during forest fires. Acrolein is also formed as a photooxidation product of various hydrocarbon pollutants found in air (including propylene and 1,3-butadiene) (ATSDR 2007; CalEPA 2008). Fermentation and ripening processes also release small amounts of acrolein (Environment Canada and Health Canada 2000).
The principal anthropogenic source of atmospheric acrolein is the combustion of organic matter and fuels. On-road motor vehicles were estimated to emit up to 3 000 000 kg/year and off-road motor vehicles (including aircraft) emit perhaps even greater amounts (Environment Canada and Health Canada 2000). The use of biodiesel (soy and animal blends) increased the acrolein emissions compared to petroleum-derived diesel (ultralow sulfur diesel) (Karavalakis et al. 2010; Cahill and Okamoto 2012). Industrial processes such as incineration, pulp and paper, oriented-strand board production, and coal electricity generation also contribute to acrolein emissions, though much less than mobile sources (Health Canada and Environment Canada 2000). Between 2013 and 2015, industrial air emissions of acrolein reported to the National Pollutant Release Inventory ranged between 102 and 111 tonnes (NPRI 2017).
No information is available on the relative contributions of various sources to the total ambient air concentration of acrolein.
2.2 Indoor Sources
Acrolein levels in residential indoor air have been found to be between 2- and 20-fold greater than outdoor levels (Environment Canada and Health Canada 2000; WHO 2002; ATSDR 2007; Health Canada 2010a, 2010b, 2012, 2013). Some of the primary sources of acrolein in indoor air are from activities such as smoking and cooking with oils (Environment Canada and Health Canada 2000; WHO 2002). However, no information is available on the relative contributions of the various sources to the total indoor air concentration of acrolein.
Tobacco smoke has been shown experimentally to generate 3 to 220 µg of acrolein per burned cigarette, a large proportion of which can be inhaled in the mainstream smoke or increase the level of acrolein in a typical room by 0.4 to 2 ppb (0.9-4.6 µg/m3) (WHO 2002; ATSDR 2007). In Canada, studies in Prince Edward Island and Regina, Saskatchewan reported an association between increased acrolein levels and the presence of environmental tobacco smoke in the home; however, the differences were not statistically significant due to the small sample sizes, and there is some uncertainty in the measurement methods used in these studies (see section 3) (Gilbert et al. 2005; Héroux et al. 2010). Other studies have shown higher acrolein concentrations in indoor environments where combustion of tobacco products occurs (ATSDR 2007). In addition, significantly higher levels of acrolein metabolites were detected in the urine of tobacco smokers compared to non-smokers in the general population of the United States (Alwis et al. 2015).
Recent studies have demonstrated that electronic cigarettes (e-cigarettes, vaping) emit acrolein at less than 0.02 to 21 µg per puff in the mainstream vapour (Herrington and Myers 2015; McRobbie et al. 2015; Gillman et al. 2016; Farsalinos and Gillman 2018; Farsalinos et al. 2018). A predictive model reported that heavy use of an electronic cigarette in a residential setting contributed more than 0.88 ppb (2 µg/m3) to the indoor air levels (Logue et al. 2017). There are certain cases where electronic cigarette emissions have been shown to exceed that of tobacco smoking, such as the "dry puff," where the electronic-cigarette liquid is overheated (Farsalinos and Gillman 2018).
The overheating of animal and vegetable fats or oils during cooking can result in acrolein emissions (ATSDR 2007). Seaman et al. (2009) showed that cooking or frying several different types of foods in a variety of cooking oils produced significantly greater acrolein emissions compared to frying foods in a "no oil" control. The acrolein indoor air levels 5 minutes after frying food with these various cooking oils in a small (188 m3), well ventilated apartment (sampling 6 metres from emissions source) ranged from 26.4 to 64.5 µg/m3. In a study of commercial kitchens in Hong Kong, cooking with oil was associated with acrolein in the kitchen range hood exhaust (Ho et al. 2006). Similarly, in a study in homes in California, acrolein concentrations were correlated with cooking events (Seaman et al. 2007). The air exchange rate was found to be the most significant chemical removal process for acrolein generated by cooking with oils (Seaman et al. 2009).
The presence of a gas stove in the residence has been identified as a predictor of increased acrolein levels. The mean personal exposure to acrolein was significantly higher (p < 0.05) for participants that lived in homes with gas stoves (2.68 µg/m3) compared to those with electric stoves (2.03 µg/m3) (Stocco et al. 2008). Acrolein is also found in wood smoke and increased concentrations may be associated with the use of wood stoves or wood-burning fireplaces (IARC 1995; Seaman et al. 2009). The acrolein emission rate from burning paraffin candles was experimentally measured to be 0.18 µg/kg of candle consumed (Lau et al. 1997), an emission rate more than 1000 times less than cigarettes. Burning incense also increases the acrolein concentration in indoor air (Ho and Yu 2002).
Contributions to the indoor air concentration of acrolein may also come from building materials by off-gassing or secondary formation (oxidation of other volatile organic compounds emitted). In chamber emissions testing in California, acrolein was found in emissions from some building materials (paints and particle boards), lumber used in home construction, and newly built uninhabited homes (Seaman et al. 2007). Similarly, in a Canadian study, acrolein was detected in some wood, insulation, and paint products (Won et al. 2014). However, the study authors noted that the presence of acrolein needs to be interpreted with caution due to the limitations of the sampling methodology (i.e., the use of pentafluorophenylhydrazine-coated thermal desorption tubes), which resulted in high background levels and low capturing efficiency (see section 3 for more information on measurement methods).
The 14.4 hour half-life of acrolein in the indoor environment is similar to values found in the ambient environment (15-20 hrs) (ASTDR 2007; Seaman et al. 2009).
3.0 Concentrations in indoor and outdoor air
Acrolein is one of the most difficult chemicals to measure in air due to its high volatility and reactivity. Health Canada studies (Health Canada 2010a, 2010b, 2012, 2013) have collected indoor residential acrolein measurements using the following two most common methods: 2,4-dinitrophenylhydrazine (DNPH) cartridges for sampling coupled with high-performance liquid chromatography for analysis; and passivated canisters for sampling coupled with gas chromatography mass spectrometry for analysis. Both methods have their limitations, as described below.
Numerous problems have been reported for the 2,4-DNPH method, including the instability of the DNPH-acrolein hydrazone during collection and storage, reactions with chemicals such as ozone that interfere with accurate acrolein measurements, and poor chromatographic separation of the complex carbonyl mixtures typically found in air (Tejada 1986; Possanzini and Di Palo 1996; Schulte-Ladbeck et al. 2001; Seaman et al. 2006; Knighton et al. 2007; Wang et al. 2009; Uchiyama et al. 2010; Ho et al. 2011; Herrington and Hays 2012). Data collected by Health Canada are consistent with the findings reported in the literature, as approximately 80% of the samples collected were found to be below the limit of detection (personal communication, Health Canada 2018, unreferenced). The unreliability of this method for quantifying acrolein is well established, with the US EPA issuing an addendum in 1999 to Method TO-11A for the removal of acrolein from the list of analytes covered by this method. As a result of these issues, no Health Canada exposure data from 2,4-DNPH cartridges have been reported in this proposed guideline document.
Issues with the passivated canister method have also been reported, with both acrolein growth (Swift et al. 2007; US EPA 2010) and acrolein reductions (ERG 2005) in canisters over time being reported. Furthermore, investigations have shown that background acrolein can be elevated in cleaned canisters, which may lead to overestimates (US EPA 2010). Finally, canister cleaning technique can also influence acrolein background concentrations and growth over time (Dann and Wang 2007; Shelow et al. 2009). Despite these issues, the passivated canister method has been deemed superior to the 2,4-DNPH method by the US EPA, and used in ambient sampling networks such as the US EPA's Urban Air Toxics Monitoring Program and National Air Toxics Trends Stations program in the United States.
In order to ensure that the data collected by Health Canada through the passivated canister method could be used as a reasonable estimate of true indoor air acrolein concentrations, some investigations were conducted. The first one examined the effect of time to analysis (i.e., number of days between canister collection and canister analysis by the analytical laboratory) in historical data. The results of this investigation showed a small but statistically significant effect of time to analysis (0.66% increase per day). Adjustment for air exchange rate, temperature, and indoor humidity at the collection site resulted in a higher although still relatively small increase (1.22% increase per day). For the second investigation, Health Canada and Environment and Climate Change Canada tested the stability of acrolein in passivated canisters. Overall results demonstrated that measured concentrations were generally slightly higher at day 21 compared with the known day 0 concentrations in both the SummaTM and SiloniteTM canisters. The median absolute difference between day 0 and day 21 was 0.34 µg/m3 (0.11 to 5.8 µg/m3) for the SummaTM canisters and 0.44 µg/m3 (0.02 to 6.6 µg/m3) for the SiloniteTM canisters. In both types of canisters, the greatest changes in acrolein concentrations between day 0 and day 21 were observed at the highest concentration (12 μg/m3).
These results suggest that while there may be some measurement error associated with the passivated canister method, they provide the most accurate estimate of indoor acrolein concentrations available at this time. Canadian indoor and outdoor exposure concentrations of acrolein from Health Canada studies are presented in Table 2. These studies, which collected data from over 200 households in four cities across Canada in both summer and winter, are considered to be the most recent and representative data available for quantifying long-term indoor exposure to acrolein in Canadian single-family homes.
Median acrolein levels measured by Health Canada in Edmonton, Halifax, Regina, and Windsor during winter and summer from 2005 to 2010 ranged from 1.3 to 8.1 µg/m3 indoors and from 0.2 to 2.2 µg/m3 outdoors. The 95th percentile values ranged from 3.5 to 21 µg/m3 indoors and from 0.5 to 7.4 µg/m3 outdoors (Health Canada 2010a, 2010b, 2012, 2013). In Windsor, personal exposure measurements were also collected in 2005, with a median range of 1.1 to 4.3 µg/m3 and a 95th percentile range of 3.1 to 8.2 µg/m3 (Health Canada 2010b).
| Location | Sampling period | Sampling methodFootnote a | Season | No. of homes | Smoking status | No. of samplesFootnote b | Concentration (μg/m3) | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Median | 95th %ile | Max | ||||||||
| Indoor | |||||||||||
| Edmonton, Alberta | 2010 | Passivated canisters
(7 days × 24 hours) |
Summer
Winter |
50
50 |
Non-smokers | 328
337 |
< MDL
< MDL |
8.1
6.2 |
21.0
15.6 |
26.4
39.1 |
Health Canada (2013) |
| Halifax, Nova Scotia | 2009 | Passivated canisters
(7 days × 24 hours) |
Summer
Winter |
50
50 |
Non-smokers | 331
312 |
< MDL
< MDL |
4.1
2.8 |
11.4
9.7 |
21.4
334.0 |
Health Canada (2012) |
| Regina, Saskatchewan | 2007 | Passivated canisters
(24 hours) |
Summer
Winter |
111
106 |
Non-smokers
Smokers Non-smokers Smokers |
91
13 83 21 |
0.01
1.8 0.17 0.3 |
4.3
7.0 1.8 2.5 |
11.3
16.0 3.5 10.1 |
13.8
16.0 6.8 12.7 |
Health Canada (2010a) |
| Windsor, Ontario | 2006 | Passivated canisters
(5 days × 24 hours) |
Summer
Winter |
46
47 |
Non-smokers | 211
224 |
1.1
0.4 |
6.2
1.6 |
10.3
3.5 |
12.6
13.3 |
Health Canada (2010b) |
| Windsor, Ontario | 2005 | Passivated canisters
(5 days × 24 hours) |
Summer
Winter |
45
48 |
Non-smokers | 217
232 |
0.01
0.01 |
5.9
1.3 |
10.2
3.5 |
20.2
7.5 |
Health Canada (2010b) |
| Overall range from all studies | < MDL-
1.8 |
1.3-
8.1 |
3.5-
21.0 |
6.8-
334.0 |
|||||||
| Outdoor | |||||||||||
| Edmonton, Alberta | 2010 | Passivated canisters
(7 days × 24 hours) |
Summer
Winter |
50
50 |
- | 324
332 |
0.5
0.2 |
2.2
1.0 |
7.4
2.5 |
30.7
5.0 |
Health Canada (2013) |
| Halifax, Nova Scotia | 2009 | Passivated canisters
(7 days × 24 hours) |
Summer
Winter |
50
50 |
- | 324
286 |
0.2
0.1 |
0.6
0.6 |
1.6
1.4 |
6.9
3.6 |
Health Canada (2012) |
| Regina, Saskatchewan | 2007 | Passivated canisters
(24 hours) |
Summer
Winter |
111
106 |
- | 108
94 |
0.07
0.02 |
1.0
0.2 |
1.9
0.9 |
2.8
2.4 |
Health Canada (2010a) |
| Windsor, Ontario | 2006 | Passivated canisters
(5 days × 24 hours) |
Summer
Winter |
46
47 |
- | 214
215 |
0.1
0.1 |
0.6
0.3 |
1.1
0.5 |
3.2
0.9 |
Health Canada (2010b) |
| Windsor, Ontario | 2005 | Passivated canisters
(5 days × 24 hours) |
Summer
Winter |
45
48 |
- | 216
200 |
0.16
0.01 |
0.6
0.2 |
1.2
0.5 |
2.2
1.0 |
Health Canada (2010b) |
| Overall range from all studies | 0.01-
0.5 |
0.2-
2.2 |
0.5-
7.4 |
0.9-
30.7 |
|||||||
| Personal | |||||||||||
| Windsor, Ontario | 2005 | Passivated canisters
(5 days × 24 hours) |
Summer
Winter |
45
48 |
- | 206
225 |
1.1
0.3 |
4.3
1.1 |
8.2
3.1 |
19.1
10.1 |
Health Canada (2010b) |
Notes: MDL = minimum detection limit Footnotes
|
|||||||||||
The distribution of indoor acrolein concentrations in studies conducted by Health Canada is presented in Figure 1. It should also be noted that for the studies in Edmonton, Halifax, and Windsor, multiple measurements were made at each home and these values have been averaged to present one value per home, while for the Regina study a single measurement was made at each home. Acrolein levels were higher in the summer than in winter in each of the four cities (Health Canada 2010a, 2010b, 2012, 2013). This is likely due to warmer temperatures in summer and is consistent with data collected by the Canadian Health Measures Survey, which found that aldehydes, ketones, and alcohols generally had higher levels in the warm months and lower levels in the cold months (Li et al. 2019).
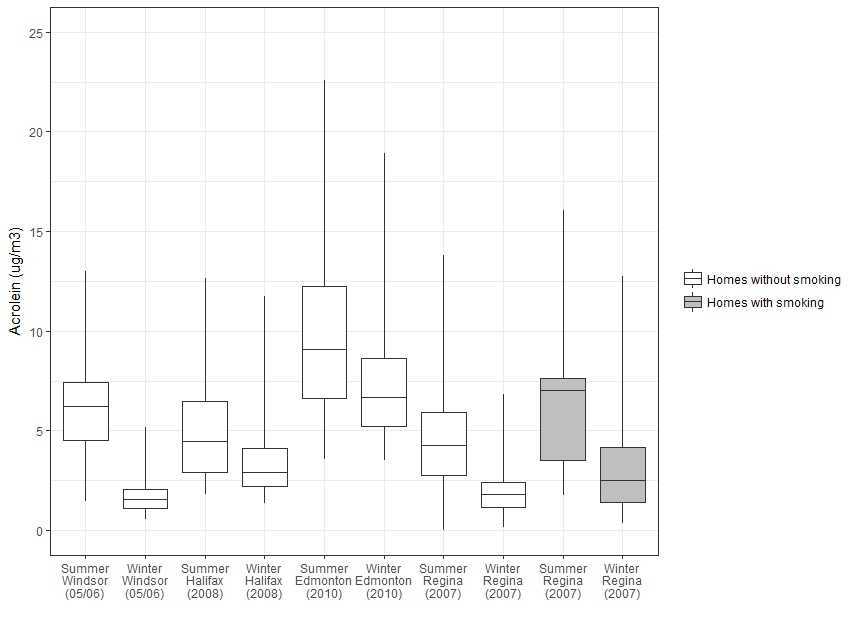
Figure 1 - Text description
Figure 1 is a box and whisker plot that shows the distribution of indoor acrolein concentrations in Health Canada studies. The vertical axis shows the acrolein concentration in µg/m3 ranging from 0 to 25 and the horizontal axis shows the study city, year and season. For each study, a box is shown with the 75th, 50th, and 25th percentiles represented by the top, middle, and bottom of the boxes, and whiskers representing the 90th and 10th percentiles. Homes without smoking are shown for summer and winter for Windsor in 2005-2006, Halifax in 2008, Edmonton in 2010, and Regina in 2007. Homes with smoking are shown for summer and winter for Regina in 2007. The minimum, maximum, median and 95th percentile for each study are shown in Table 2.
Source data: Health Canada (2010a, 2010b, 2012, 2013)
The 75th, 50th, and 25th percentiles are represented by the top, middle, and bottom of the boxes. The whiskers represent the 90th and 10th percentiles. Outlier measurements from Halifax were not used for this plot.
The distribution of indoor/outdoor (I/O) ratios for each home is presented in Figure 2. An I/O ratio compares levels of acrolein measured inside a given home to levels measured directly outside the same home. In these studies, the I/O ratios much greater than 2.5 were generally consistent across cities and seasons and are indicative of a predominance of indoor sources of acrolein.
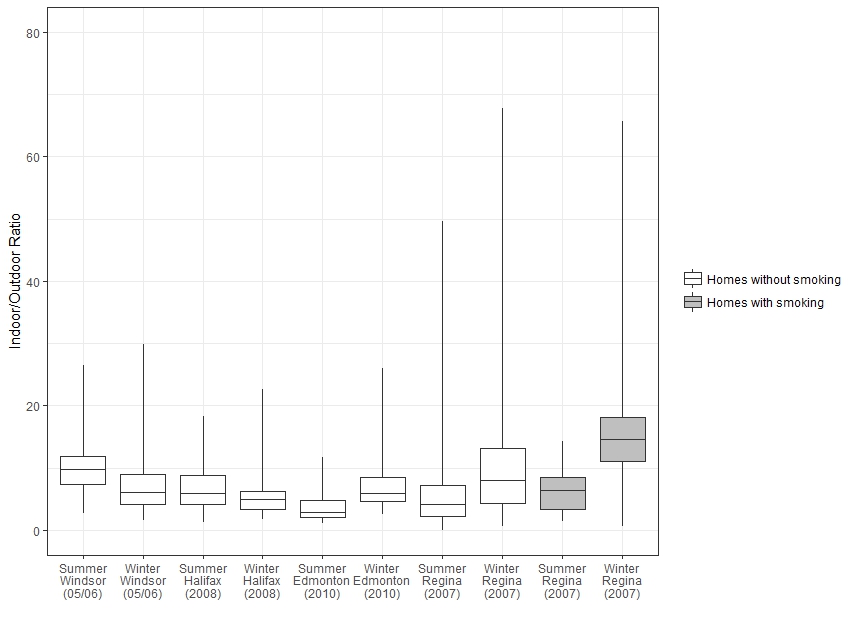
Figure 2 - Text description
Figure 2 is a box and whisker plot that shows the distribution of indoor/outdoor ratios of acrolein concentration in Health Canada studies. The vertical axis shows the indoor/outdoor ratio (unitless) ranging from 0 to 80 and the horizontal axis shows the study city, year and season. For each study, a box is shown with the 75th, 50th, and 25th percentiles represented by the top, middle, and bottom of the boxes, and whiskers representing the 90th and 10th percentiles. Homes without smoking are shown for summer and winter for Windsor in 2005-2006, Halifax in 2008, Edmonton in 2010, and Regina in 2007. Homes with smoking are shown for summer and winter in Regina in 2007. The minimum, maximum, median and 95th percentile for each study are shown in Table 2. Indoor/Outdoor ratios are greater than 2.5 for all cities and seasons.
Source data: Health Canada (2010a, 2010b, 2012, 2013)
The 75th, 50th, and 25th percentiles are represented by the top, middle, and bottom of the boxes. The whiskers represent the 90th and 10th percentiles. Outlier measurements from Halifax were not used for this plot.
4.0 Toxicokinetics
4.1 Absorption, Distribution, Metabolism, and Excretion
It has been demonstrated in various animal species that acrolein is effectively removed from inhaled air by the respiratory tract (ATSDR 2007). For example, Egle (1972) observed that acrolein uptake by the entire respiratory tract of anaesthetized dogs averaged 80 to 85% of the inhaled dose; only about 20% of the inhaled dose reached the lower respiratory tract (Egle 1972 cited in ATSDR 2007; US EPA 2003). Similarly, in mice and rats, inhaled acrolein was absorbed almost entirely into the upper respiratory tract (Morris 1996 cited in ATSDR 2007; Morris et al. 2003; Struve et al. 2008).
In rats, uptake of acrolein in the upper respiratory tract decreased with increasing exposure concentration or flow rate, and the uptake efficiency also decreased over time during 40- or 80-minute exposures (Morris 1996; Struve et al. 2008), suggesting a saturable process. Data in multiple experimental animal species have shown that acrolein mainly reacts in the nasal area, but can penetrate into the lower respiratory tract at higher concentrations (reviewed in US EPA 2003). This may be due to the decreased removal of acrolein in the upper respiratory tract as concentration increases. There are also likely to be species differences in the level of deposition in the upper and lower respiratory tract: deposition is expected to be primarily in the nasal passages for rodents as they are obligate nose breathers and have a large surface area in their nasal passages, whereas some penetration may occur to the lower respiratory tract for humans during mouth breathing (Kimbell et al. 2001; Overton et al. 2001; Corley et al. 2012).
In rats dosed with acrolein intravenously or orally by gavage, almost all of the administered radiolabel was detected in the excreta within 24 hours, 54 to 59% of the radioactivity being found in urine, 22 to 27% in expired carbon dioxide, and 1 to 12% in feces. Tissue concentrations were very low (< 1.2%), indicating a lack of systemic distribution (Parent et al.1996, 1998 cited in US EPA 2003). By inhalation, acrolein did not reduce the concentration of liver glutathione (GSH), again suggesting a lack of systemic distribution (McNulty et al. 1984 cited in CalEPA 2008; Lam et al. 1985). No other data on distribution following inhalation exposure were identified.
Consistent with the highly reactive nature of acrolein, following inhalation, the effects observed tend to be restricted to the initial site of contact (i.e., the respiratory tract). Inhaled acrolein is retained at the site of exposure, and becomes rapidly and irreversibly bound to protein and non-protein sulfhydryl groups and to primary and secondary amines in proteins and nucleic acids (WHO 2002; US EPA 2003). More specifically, it is proposed that acrolein binds with protein cysteine residues and GSH, forming a GSH-acrolein adduct (ATSDR 2007; CalEPA 2008).
The predominant metabolic pathway proposed for acrolein starts with the glutathione-S-transferase (GST)-catalyzed addition of GSH to the activated double bond of acrolein. This is followed by processing of the acrolein-GSH adducts to mercapturic acid derivatives by alcohol and aldehyde dehydrogenases (reviewed in WHO 2002, US EPA 2003). The reduced mercapturic acid derivative, 3-hydroxypropylmercapturic acid (3-HPMA, see Figure 3), is the predominant metabolite and has been detected in the urine of rats administered acrolein by inhalation or by intraperitoneal (IP) or subcutaneous injection (Linhart et al. 1996 cited in US EPA 2003). Similarly, 3-HPMA was detected in urine of mice exposed to acrolein by inhalation (Tully 2014; Conklin et al. 2017). Linhart et al. (1996) also measured a minor metabolite in rat urine, 2-carboxethylmercapturic acid (CEMA, see Figure 3), accounting for approximately 10% of the two mercapturic acids.
Two other proposed minor pathways involve either epoxidation of acrolein and addition of GSH on the epoxide, or addition of water to acrolein to form 3-hydroxypropionaldehyde, which is subsequently oxidized to malonic acid and oxalic acid (Parent et al. 1998).
Acrolein is formed endogenously as a product of lipid peroxidation and the metabolism of α-hydroxyamino acids and polyamines. Lipid peroxidation occurs during inflammation, which is a characteristic of some respiratory diseases such as chronic obstructive pulmonary disease and asthma (reviewed in ATSDR 2007; Bein and Leikauf 2011; Burcham 2016). Acrolein has been detected in expired breath condensate and induced sputum; concentrations were higher in subjects with chronic obstructive pulmonary disease or asthma compared with healthy subjects (Deshmukh et al. 2008 cited in Bein and Leikauf 2011). Similarly, acrolein metabolites 3-HPMA and CEMA were detected in the urine of over 98% of the general population of the United States, with significantly higher levels among tobacco smokers (Alwis et al. 2015).
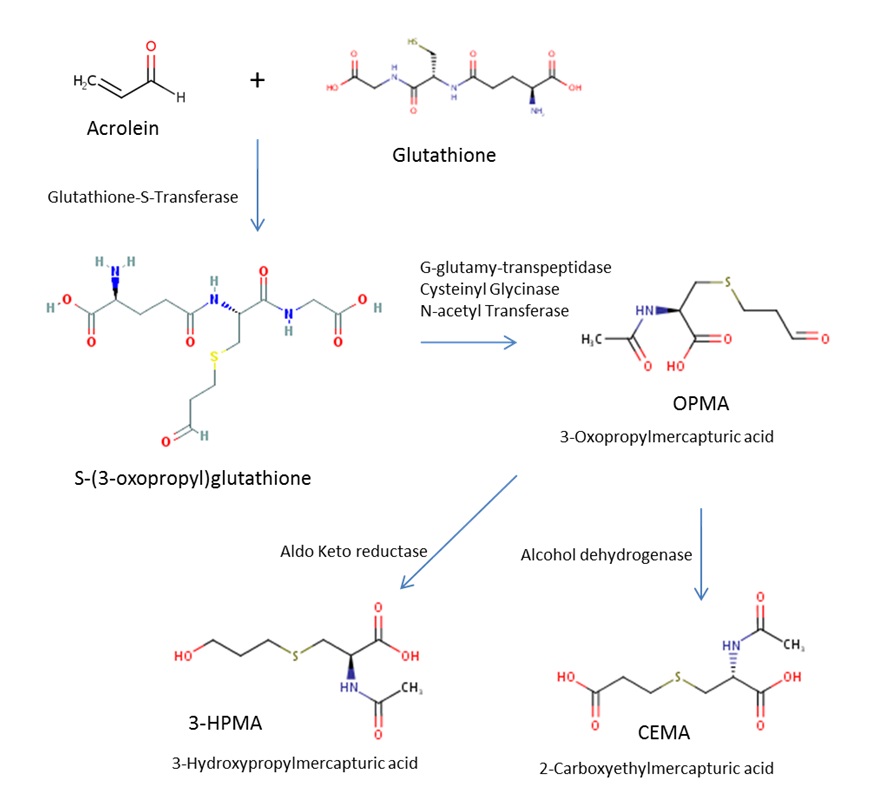
Figure 3 - Text description
Figure 3 shows the main pathway of acrolein metabolism, with acrolein as the starting point at the top left. To the right of acrolein is shown a plus sign with glutathione, and leading down from the plus sign is an arrow pointing down with the words "Glutathione-S-transferase" beside it. The arrow leads to s-(3-oxopropyl)glutathione, and from there is an arrow to the right with the words "G-glutamyl-transpeptidase, Cysteinyl Glycinase and N-acetyl Transferase" above it. This leads to 3-oxopropylmercapturic acid (OMPA), and from there arrows go down to the left and right showing reduction by aldo keto reductase to form 3-hydroxypropylmercapturic acid (3-HPMA) and by alcohol dehydrogenase to form 2-carboxylethylmercapturic acid (CEMA).
4.2 Physiologically Based Pharmacokinetic Modelling
Schroeter et al. (2008) developed a combined computational fluid dynamics (CFD)-physiologically based pharmacokinetic (PBPK) model for acrolein. The CFD model was based on three-dimensional models of rat and human nasal passages; and a two-compartment PBPK model (mucus and epithelial tissues; perfused subepithelial tissues) was applied. The rat model was optimized using pharmacokinetic (PK) data from Struve et al. (2008) and Morris (1996) studies (i.e., PK parameters were adjusted to better match empirical data from these studies), but not fully validated (i.e., model results were not compared to any rat PK studies that were not also used in the calibration process). As the model was not calibrated or validated for humans, the authors used the 99th percentile results to be conservative. Saturation or non-linear kinetics appear to exist at relevant concentrations.
Corley et al. (2012) extended the Schroeter et al. (2008) CFD model to include both the upper and lower respiratory tract. The two-compartment PBPK model was maintained, but a new value for the maximal metabolic rate (Vmax) was derived to account for aldehyde dehydrogenase saturation. Relative acrolein uptake in rats, monkeys, and humans were presented only for an air concentration of 0.6 ppm (1.38 mg/m3), for nasal tissues, and for the entire respiratory tract (considering both nasal and oral human models). Acrolein uptake in nasal tissues was lower in humans than animals (69.5%, 54.7%, and 24% in rats, monkeys, and humans, respectively at 0.6 ppm [1.38 mg/m3]). When the entire airway was considered, humans had similarly low relative acrolein uptake (98.5%, 95.8%, 45.2%, and 34.8% in rat, monkey, human nasal, and human oral models, respectively, at 0.6 ppm [1.38 mg/m3]). The model was calibrated against PK data from rat studies (Morris 1996; Struve et al. 2008), but was neither fully validated for rats, nor calibrated or validated for humans.
As formaldehyde is a related gas, the relative flux of formaldehyde has also been modelled in rats and humans by Kimbell et al. (2001), who used three-dimensional, anatomically realistic, CFD models to estimate flux in regions or "bins" of the nasal passages. The average flux across 20 bins was approximately double in humans compared to rats; the peak flux was about 25% higher in rats than in humans (see section 6.2).
5.0 Health effects
This section provides a review of the effects of acrolein in humans (see section 5.1) as well as relevant toxicological studies in experimental animals, with supporting information from in vitro test systems (see section 5.2). A concise summary of the health effects of inhalation exposure to acrolein, along with a discussion on the mode of action, is also presented (see section 5.3). Details of the human exposure and toxicological studies presented below can also be found in appendices B and C.
Relevant studies on the health effects of acrolein published up to October 2018 were reviewed. Although acrolein is a component of tobacco smoke, studies of tobacco smoke were excluded as tobacco smoke is a complex mixture that contains many known toxins and carcinogens, and its health effects are not addressed in this document. Other routes of exposure (i.e., ingestion and dermal) were not considered physiologically relevant. Health Canada evaluated the original studies identified as key in the derivation of these proposed exposure limits for acrolein (see section 6). Other relevant information was drawn from previous authoritative reviews of the health effects of acrolein: (a) ANSES's (2013) Proposition de valeurs guides de qualité d'air intérieur : L'acroléine; (b) CalEPA's (2008) Acrolein Reference Exposure Levels; (c) ATSDR's (2007) Toxicological Profile for Acrolein; (d) US EPA's (2003) Toxicological Review of Acrolein; (e) WHO's (2002) Concise International Chemical Assessment Document 43: Acrolein; and (f) Environment Canada and Health Canada's (2000) Priority Substances List Assessment Report: Acrolein.
5.1 Effects in Humans
5.1.1 Short-term exposure
Several studies describe the acute effects of acrolein on human volunteers. In these studies, eye irritation was the most sensitive endpoint, occurring at concentrations of 0.06 to 0.1 ppm (0.14-0.23 mg/m3) for exposure durations as short as 5 minutes (Darley et al. 1960; Weber-Tschopp et al. 1977; Dwivedi et al. 2015; Claeson and Lind 2016). Nasal, throat, and respiratory irritation occurred at higher concentrations (Weber-Tschopp et al. 1977).
Darley et al. (1960) exposed the eyes only of 36 volunteers to acrolein for 5 minutes, at concentrations of 0.06, 1.3 to 1.6, or 2.0 to 2.3 ppm (0.14, 2.99-3.68 or 4.60-5.29 mg/m3, respectively). Some subjects reported eye irritation even at the lowest test concentration (0.14 mg/m3), but the overall irritation score at this concentration was still considered in the range of "no irritation."
In a first experiment, Weber-Tschopp et al. (1977; publication in German) exposed 53 volunteers to continuously increasing acrolein concentrations for 40 minutes (up to 0.6 ppm [1.4 mg/m3]); significantly higher incidence of eye irritation (as reported by subjects) was first observed at 0.09 ppm (0.21 mg/m3). Eye irritation as measured by eye blink frequency was observed starting at 0.26 ppm (0.60 mg/m3). The study authors also noted a significant increase in subjective reports of nasal irritation starting at 0.15 to 0.26 ppm (0.35 to 0.60 mg/m3), throat irritation starting at 0.43 ppm (1.0 mg/m3), and a decrease in respiration rate at 0.6 ppm (1.4 mg/m3). In a second experiment, Weber-Tschopp et al. (1977) exposed 42 subjects to acrolein for 1.5 minutes at concentrations of 0.15 to 0.6 ppm (0.35 to 1.4 mg/m3), with a recovery period between exposures. The incidence of volunteer-reported eye irritation was significantly increased at 0.3 ppm (0.69 mg/m3) and nasal irritation was increased at 0.6 ppm (1.4 mg/m3). Finally, Weber-Tschopp et al. (1977) exposed 46 volunteers to acrolein for 60 minutes at 0.3 ppm (0.69 mg/m3). Eye, nose, and throat irritation increased during the first 10 to 20 minutes, and there was a significant decrease in respiration rate (Weber-Tschopp et al. 1977 cited in US EPA 2003).
Other studies reported similar effects. Sim and Pattle (1957) observed that exposures of 0.8 ppm (1.84 mg/m3) for 10 minutes, or 1.2 ppm (2.76 mg/m3) for 5 minutes were "extremely irritating" and caused lacrimation (Sim and Pattle 1957 cited in US EPA 2003). Claeson and Lind (2016) found that volunteers reported eye irritation starting about 7 minutes into a 15-minute eye-only exposure to 0.36 mg/m3 acrolein. Irritation continued for 10 minutes after cessation of exposure. No difference in eye irritation was found between control exposures and a 45-minute exposure to 0.16 mg/m3 or a 60-minute exposure to 0.07 mg/m3. Dwivedi et al. (2015) studied irritation in 18 subjects exposed to 0.05 or 0.1 ppm (0.12 or 0.23 mg/m3) acrolein for 2 hours. Subjective eye irritation and blink frequency were slightly increased at 0.1 ppm (0.23 mg/m3) but not 0.05 ppm (0.12 mg/m3) acrolein. There was no difference between controls and exposed subjects in terms of breathing frequency, pulmonary function, or inflammatory markers in blood or sputum.
All of these studies had small numbers of volunteers and used self-reporting for symptoms of irritation.
Several case studies describe the effects of acute exposure to acrolein; however, exposures are often to multiple substances, and acrolein concentrations are generally unknown. A two-year-old boy was hospitalized for acute respiratory failure following exposure for about an hour to acrid smoke from vegetable oil burning. Lung effects were still visible eighteen months following exposure (Mahut et al. 1993 cited in CalEPA 2008). A chemical worker was exposed to a sudden release of acrolein in the workplace, causing chemical pneumonia and eye irritation, both of which were resolved with treatment (Champeix et al. 1966 cited in US EPA 2003). The Centers for Disease Control and Prevention (CDC) (2013) conducted a review of acute poisonings to acrolein from occupational use of pesticides and identified eight cases in the United States between 1993 and 2009. Symptoms observed included respiratory distress, eye irritation, headache, dyspnea, and skin irritation/burns.
5.1.2 Long-term exposure
Several recent studies were identified which examined the relationship between acrolein air concentrations and health effects in humans. It is important to note that no causality can be determined as all of the available studies are cross-sectional. Two of the studies identified included concentrations of acrolein in schools or homes in France, measured with DNPH-coated passive diffusion samplers; however, subjects were exposed to multiple pollutants, and health endpoints (asthma and rhinitis) were based on questionnaires rather than medical diagnoses (Billionnet et al. 2011; Annesi-Maesano et al. 2012). Another study modelled ambient acrolein levels based on emissions data and compared these levels to the prevalence of asthma in different regions of the United States (deCastro 2014). There were no actual exposures measured, and no individual asthma cases or control subjects. Limitations of the acrolein measurement methods are described in section 3. Additional details of these studies are outlined below.
Annesi-Maesano et al. (2012) measured concentrations of acrolein and other air contaminant in 401 primary school classrooms in 108 schools across six cities in France. The acrolein concentrations were put into 3 tiers: low (< LOD-not specified), medium (> LOD but < 1.55 µg/m3), and high (> 1.55 µg/m3). Health endpoints were asthma and rhinitis, as measured by a health questionnaire completed by parents and a medical visit that included a skin prick test for allergies and a test for exercise-induced asthma. After adjustment for possible confounders (including passive smoking and family history), odds ratios (OR) for asthma in the previous year were 1.23 (95% confidence interval [CI] of 1.02 to 1.45) and 1.22 (95% CI 1.09 to 1.38) for medium and high acrolein concentrations, respectively, compared to low concentration. When the subjects were separated by skin prick reactivity, acrolein was positively related to allergic (atopic) asthma (OR of 1.22 and 1.28 for medium and high exposure groups, respectively), and negatively related to non-allergic (non-atopic) asthma. Acrolein was also found to be significantly correlated with exercise-induced asthma (p < 0.025). There was no association between acrolein concentration and rhinoconjunctivitis in the previous year. This study has multiple limitations, including the narrow range of concentrations measured, no information on distribution of classroom acrolein levels, short-term (5 day) monitoring without accounting for exposure to acrolein in the home, and parental definition of asthma in the previous year rather than medical diagnosis. Given the weak, non-significant association between acrolein and asthma in the whole study group, and the inverse relationship between acrolein and asthma in non-atopic children as well as the lack of concentration-response relationship, the authors' claim that acrolein plays a role in the development of asthma must be viewed with caution.
Billionnet et al. (2011) sampled air in 490 homes in France, and measured acrolein and other indoor air contaminants over one week. Acrolein concentrations were divided into quartiles; the range of concentrations was < limit of detection (LOD, 0.1) to 12.9 µg/m3, with a median of 1.0 µg/m3. Concentrations above the third quartile of distribution were considered "elevated" (1.51 µg/m3). Health endpoints (asthma in the previous year and rhinitis in the past month) were evaluated by questionnaire. After adjustment for possible confounders (including smoking status and home characteristics), no significant relationship was identified between elevated acrolein level and asthma or rhinitis. The main focus of the paper was the effects of combined pollutant concentrations.
A more recent study was identified, which looked at modelled ambient acrolein levels and prevalence of asthma in the population in the United States. deCastro (2014) estimated acrolein exposure concentrations in each census tract based on data from the National Emissions Inventory and US EPA's air monitoring, and models incorporating population density, physical topography, and climate. The modelled concentrations were divided into quintiles, with the lowest being 0.00014 to 0.011 µg/m3 and the highest being 0.055 to 0.457 µg/m3. The authors then compared these with the results of the National Health Interview Survey, which gives national estimates of disease prevalence across the country by year and age group. There was an increase in the 12-month asthma attack prevalence OR in the top quintile relative to the lowest quintile for all subjects (n = 271 348), never smokers, and never + former smokers; the authors defined the increases as "marginally significant," with p-values between 0.05 and 0.15. No trend was observed for the lower four quintiles. A major limitation of this study is that acrolein concentrations were not actually measured.
Two recent biomonitoring studies examined levels of 3-HPMA (main acrolein metabolite) in urine and disease risk. Limitations of these studies include lack of acrolein exposure measurements or source attribution (includes exposure from all sources, including endogenous production); and no actual health endpoints were studied. DeJarnett et al. (2014) examined the concentration of 3-HPMA in urine and the risk of cardiovascular disease in 211 subjects with moderate-to-high cardiovascular disease risk. After adjusting for confounders, urine 3-HPMA concentration was found to be significantly associated with cardiovascular disease risk (higher Framingham Risk Score). The relationship was even more pronounced in non-smokers. Park et al. (2015) measured urinary 3-HPMA in approximately 2200 adult smokers from five ethnic groups. After adjusting for possible confounders, 3-HPMA was highest in Native Hawaiians and lowest in Latinos. The authors note that compared to Whites, Native Hawaiians have a higher risk of lung cancer and Latinos a lower risk, and suggest that differences in acrolein metabolism may account for some of the differences in risk.
5.1.3 Carcinogenicity
The International Agency for Research on Cancer (IARC) considers acrolein "not classifiable as to its carcinogenicity to humans" (Group 3; IARC 1995), due to inadequate evidence in both humans and experimental animals. The US EPA also considers the acrolein database inadequate for the assessment of its carcinogenicity potential (US EPA 2003).
One occupational case-control study was identified (Ott et al.1989 cited in IARC 1995 and US EPA 2003), in which worker exposure to multiple chemicals was classified as "ever" or "never" by job category. Exposure to acrolein was reported for two men who had died with non-Hodgkin's lymphoma, one with multiple myeloma, and three with nonlymphocytic leukaemia. There was no statistically significant increase in cancer cases for workers exposed to acrolein, and concurrent exposure to chemicals other than acrolein is likely. Therefore, the results of this study are insufficient to conclude on the carcinogenic potential of acrolein.
No additional studies on the carcinogenic potential of inhaled acrolein were identified in the literature.
5.2 Toxicological Studies
5.2.1 Respiratory effects
5.2.1.1 Acute (single) exposure
Acrolein has high acute toxicity, inducing acute inflammatory reactions, lung injury, and death in rats, mice, guinea pigs, and rabbits following inhalation exposure (reviewed in US EPA 2003). The LC50 for 4- or 6-hour exposures ranges from 8 to 66 ppm (18.4 to 151.8 mg/m3) for rats, mice, and hamsters (Environment Canada and Health Canada 2000). At lower concentrations, acrolein is a respiratory irritant, inducing effects such as decreased respiration, bronchoconstriction, and increased mucus secretion.
The concentration of acrolein required to lower the respiration rate by 50% (RD50) (an indication of the irritant potential, see Shusterman 2011) has been calculated as 1.0 to 2.9 ppm (2.3 to 6.7 mg/m3) in mice and 4.6 to 9.2 ppm (10.6 to 21.2 mg/m3) in rats (US EPA 2003; CalEPA 2008), indicating that mice are more sensitive than rats to the irritant effects. Co-exposure to acrolein with acetaldehyde and/or formaldehyde led to a more pronounced breathing rate decrease (Cassee et al. 1996).
Decreased respiration rate, and increased flow resistance and tidal volume, were observed in guinea pigs exposed to acrolein by inhalation at 0.35 to 17 ppm (0.8 to 39.1 mg/m3) (Murphy et al. 1963 cited in US EPA 2003; Davis et al. 1967 cited in US EPA 2003). The increased airway resistance was transient at 0.3 ppm (0.7 mg/m3); however, following exposure to 0.9 ppm (2.1 mg/m3) acrolein, bronchial hyperresponsiveness remained for at least 24 hours following cessation of exposure (Leikauf 1991). In mice, a single acrolein exposure at 1.1 to 1.6 ppm (2.5 to 3.7 mg/m3) induced a decrease in breathing frequency and an increase in airway flow resistance, effects which were enhanced in mice previously sensitized by IP injection of ovalbumin (a model of allergic airway disease), suggesting that sensitized animals may be more susceptible to the effects of acrolein on the respiratory tract (Morris et al. 2003).
Changes in cell composition of the trachea were observed in guinea pigs exposed for 2 hours to 0.9 ppm (2.1 mg/m3) acrolein. These changes were transient, with recovery occurring within 24 hours (Leikauf 1991). In hamsters, exfoliation in bronchi, proliferation of basal cells, irregular areas of epithelium, and hyperplasia were observed 4 days after a 4-hour exposure to 6 ppm (13.8 mg/m3) acrolein (Kilburn and McKenzie 1978 cited in US EPA 2003; Environment Canada and Health Canada 2000). In rats, Arumugan et al. (1999) observed desquamized and mononuclear cells in the bronchioles, hyperemia, and emphysema following a 4-hour exposure to 2 ppm (4.6 mg/m3) acrolein. Increased cell proliferation was observed in the nose, trachea, and lung of rats after a 6-hour exposure to 0.2 to 0.6 ppm (0.46 to 1.38 mg/m3) acrolein (Roemer et al.1993). These effects were not replicated by Cassee, Groten and Feron (1996), who did not observe any nasal lesions or cell proliferation in rats exposed for 6 hours to 0.67 or 1.4 ppm (1.54 or 3.22 mg/m3) acrolein.
5.2.1.2 Repeat exposure
Repeated inhalation exposures to acrolein produced similar effects as single exposures. Studies in mice and rats have shown that exposure to acrolein for 3 days to 13 weeks results in increased mucus secretion, and inflammation and cell proliferation in the respiratory epithelium accompanied by basal cell hyperplasia and squamous cell metaplasia. The severity of the effects appears to increase with exposure concentration but not with duration of exposure.
Several short-term (3 days to 4 weeks) studies in mice and rats were identified. Effects observed were consistent with a site-of-contact irritant exposure. In mice, a 4-day exposure (3 hours per day) to 0.5 or 1.7 ppm (1.2 or 3.9 mg/m3) acrolein decreased the respiratory rate further than a single exposure (Kane and Alarie 1977 cited in US EPA 2003). Buckley (1984) observed lesions (exfoliation, erosion, ulceration, necrosis, inflammation, and squamous metaplasia in the respiratory epithelium) in the upper, but not lower, respiratory tract of mice exposed to 1.7 ppm (3.9 mg/m3) acrolein for 6 hours per day for 5 days. In rats, similar effects were observed in a 3-week exposure study (5 days per week at 3 ppm [6.9 mg/m3]) (Leach et al. 1987). In mice and rats exposed to 2 to 3 ppm (4.6 to 6.9 mg/m3) acrolein for 2 to 4 weeks, mucus hypersecretion and goblet cell metaplasia were observed in the lungs (Borchers et al. 1998 cited in US EPA 2003; Borchers, Carty and Leikauf 1999 cited in US EPA 2003; Borchers et al. 1999 cited in US EPA 2003; Borchers et al. 2008; Chen et al. 2010, 2013). Roemer et al. (1993) observed an increase in cell proliferation in rat nasal, tracheal, and lung epithelium after a 3-day exposure (6 hours per day) to 0.2 or 0.6 ppm (0.46 or 1.38 mg/m3) acrolein; effects were less pronounced than after a single exposure. Similarly, Cassee, Groten and Feron (1996) reported an increase in cell proliferation in the rat nose following a nose-only 3-day exposure (6 hours per day) to 0.25 or 0.67 ppm (0.57 or 1.54 mg/m3). The authors also observed lesions in the nasal epithelium, which increased in incidence and severity with increasing concentration (lowest observed adverse effect level (LOAEL) = 0.25 ppm [0.57 mg/m3], but no NOAEL)]. The Government of Canada (Environment Canada and Health Canada 2000) has previously derived benchmark concentrations (BMC05) of 0.14 mg/m3 for "disarrangement, necrosis, thickening and desquamation of the respiratory/transitional epithelium," and 0.68 mg/m3 for basal cell hyperplasia. The BMC05 represents "the concentration associated with a 5% increase in the incidence of lesions in the nasal respiratory epithelium." The BMC05 for the most sensitive endpoint was used to derive the tolerable concentration.
Several subchronic studies (6 to 13 weeks) of acrolein inhalation toxicity were identified, which confirmed the effects observed in short-term and acute studies. In mice, rats, guinea pigs, rabbits, hamsters, monkeys, and dogs, acrolein exposure caused irritant effects in the respiratory system, from mild inflammation at low concentrations to metaplasia and hyperplasia at higher concentrations. Depending on the species and exposure regimen, target tissues included both the upper and lower respiratory tract. The most sensitive region appears to be the nasal cavity as a site of first contact.
Lyon et al. (1970) exposed rats, guinea pigs, monkeys (males only), and dogs (males only) to acrolein vapour at 0.7 or 3.7 ppm (1.6 or 8.5 mg/m3) for 6 weeks (8 hours per day, 5 days per week). In the low concentration exposure group, all species showed eye and nasal irritation and discharge as well as mild, chronic inflammation of the lung tissue. These effects were more pronounced in dogs and monkeys. In the high concentration exposure group, dogs and monkeys had squamous metaplasia and basal cell hyperplasia in the trachea; monkeys also had necrosis and squamous metaplasia in the bronchi. In the same study, the investigators exposed animals continuously to acrolein vapour at concentrations of 0.22, 1.0 or 1.8 ppm (0.5, 2.3 or 4.1 mg/m3) for 90 days. At the low concentration, two out of four dogs showed moderate emphysema, acute lung congestion, focal vacuolization of bronchiolar epithelial cells, and some constriction of bronchioles. In guinea pigs and rats, pulmonary inflammation was observed starting at 1 ppm. Monkeys exposed to 1.8 ppm acrolein had squamous metaplasia and basal cell hyperplasia in the trachea. According to the US EPA (2003), histopathology of the nasal passages was not conducted in this study, and there were no concurrent controls.
In another multi-species study, Feron et al. (1978) exposed hamsters, rats, and rabbits to acrolein vapour at 0, 0.4, 1.4 or 4.9 ppm (0, 0.9, 3.2 or 11.3 mg/m3) for 13 weeks (6 hours per day, 5 days per week). Within the first four weeks of exposure, six rats in the high concentration group had died. Autopsies revealed lung damage (hemorrhage, edema). Surviving rats in this group had severe lung damage, including hemorrhage, edema, bronchopneumonia, bronchitis, hyperplasia, and metaplasia of the bronchial epithelium. Rabbits exposed to the high concentration of acrolein had lung lesions of similar types, but not as severe (authors ranked it as "moderate"). Hamster lungs were not affected. All three species had lesions in the trachea at the high concentration; however, the severity varied from slight hyperplasia in rabbits to moderate hyper- and metaplasia in hamsters, to severe damage in rats. This study also included nasal histopathology (three sections). At the low concentration, only one rat was affected, with metaplastic and inflammatory changes observed. At the middle concentration, the lesions in rats were ranked as moderate (incidence data were not shown), and hamsters also had slight changes. At the high concentration, rats and hamsters had severe alterations, while rabbits had moderate changes. The US EPA (2003) considered 0.4 ppm a minimal LOAEL for rats, the most sensitive species. This study was selected as the critical study by the US EPA for derivation of an inhalation RfC. It was chosen over the 3-day study by Cassee, Groten and Feron (1996) because of the greater number of test animals of multiple species and both sexes, the longer exposure duration, the use of three test concentrations over a wider range, and the characterization of multiple endpoints.
Several studies of acrolein exposure were conducted in F344 rats (Kutzman 1981; Kutzman et al. 1985; Costa et al. 1986). Male and female rats were exposed to 0, 0.4, 1.4 or 4.0 ppm (0, 0.9, 3.2 or 9.2 mg/m3) acrolein for 62 days (6 hours per day, 5 days per week). High mortality (56%) at the high concentration was observed in males, while all females survived. Many of the animals that died had severe acute bronchopneumonia. Surviving animals had lesions in the lung and trachea, of which the severity varied highly between individual animals. Lesions included focal alveolar edema with sloughed cells in bronchi and bronchioles, epithelial necrosis in bronchioles, and tracheal edema with erosion of mucosal epithelium. The authors described the effects as obstructive lung disease, as lesions were observed in both large and small airways. Lung function tests showed evidence of decreased lung function. At the middle concentration, rat lungs showed necrosis and hyperplasia, again with a high degree of variability; however, functionally, this group showed no difference from controls. No lung lesions were observed in the low concentration group; however, these animals showed functional deficits suggestive of restrictive lung lesions. The authors noted that functional tests appeared to be the most sensitive indicator of change, and that lung composition tests supported functional observations. In the previous Health Canada risk assessment of acrolein, a BMC05 of 0.76 mg/m3 for lesions in the rat nasal turbinates was derived from this study (Environment Canada and Health Canada 2000).
Dorman et al. (2008) exposed male F344 rats to 0, 0.02, 0.06, 0.2, 0.6 or 1.8 ppm (0, 0.05, 0.14, 0.46, 1.4 or 4.1 mg/m3) acrolein for 13 weeks (6 hours per day, 5 days per week). Histopathology was conducted on exposure days 4, 14, 30, and 65, and after a 60-day recovery period on six sections of the nasal cavity. Lesions were graded for severity, and the number of animals affected in each group was noted. Pathology of the nasal respiratory epithelium included inflammation, hyperplasia, and squamous metaplasia, with mild effects at 0.6 ppm (1.4 mg/m3) and more severe effects at higher concentrations. At the highest concentration, effects were observed within days of starting exposure. At some sites, inflammation and hyperplasia were transient and were replaced by metaplasia, which persisted even after exposure stopped. The authors determined a NOAEL of 0.2 ppm (0.46 mg/m3) for nasal pathology. Increased cell proliferation was also observed at 0.6 and 1.8 ppm (1.4 and 4.1 mg/m3), but not at 0.2 ppm (0.46 mg/m3). Pathology of the nasal olfactory epithelium was observed at 1.8 ppm (4.1 mg/m3) and included inflammation, degeneration, and atrophy. Squamous metaplasia was also seen in the larynx and trachea; however this was mild and reversible. No treatment-related pathology was observed in the lungs. This was the only acrolein inhalation study identified for which both a NOAEL and a LOAEL could be determined. The NOAEL of 0.2 ppm (0.46 mg/m3) was considered the critical effect level for long-term exposure in the risk assessments of acrolein conducted by CalEPA (2008) and ANSES (2013).
The CFD-PBPK model by Schroeter et al. (2008) was applied for a dosimetric analysis of the 13-week rat inhalation toxicology study by Dorman et al. (2008). In the frontmost regions of the nasal passages (Levels I and II), very high incidence of lesions occurred, so no correlation could be found between predicted dose metrics and lesion incidence; however, strong correlations were observed in Levels III and IV (at 1.8 ppm [4.14 mg/m3]), which led the authors to conclude that 0.6 ppm (1.38 mg/m3) was a NOAEL and 1.8 ppm (4.14 mg/m3) a LOAEL. The threshold flux associated with the NOAEL was 72 pg/cm2-s, which in humans was associated with a concentration of 45 ppb (NOAELhec = 0.1 mg/m3).
Two additional studies were identified that examined acrolein exposure for at least one year. Feron and Kruysse (1977) exposed hamsters to 4 ppm (9.2 mg/m3) acrolein for 52 weeks (7 hours per day, 5 days per week), with an additional 29-week recovery period. No tumours were found in the respiratory tract or other organs; however, inflammation and epithelial metaplasia of the respiratory tract were observed in about 20% of animals (IARC 1995). Le Bouffant et al. (1980) exposed rats to acrolein for 18 months (1 hour a day at 8 ppm [18.4 mg/m3]) and found no tumours. These studies are of limited value due to the single exposure concentration, the small number of animals per group, and the limited endpoints examined.
5.2.2 Immunological effects
The inflammatory response induced by acrolein has been shown in several short-term studies and includes recruitment of immune cells and stimulation of the production and/or release of proinflammatory cytokines. A 3-hour exposure to 3 ppm (6.9 mg/m3) acrolein (but not 0.3 ppm; 0.69 mg/m3) increased lymphocytes in mouse bronchial alveolar lavage fluid (BALF) (Thompson et al. 2017). The total cell number, and the number of neutrophils and eosinophils in the BALF were unchanged. Macrophages accumulated in mouse BALF following a 3-week exposure to 3 ppm (6.9 mg/m3) acrolein (Borchers, Carty and Leikauf 1999 cited in US EPA 2003; Borchers et al. 1999 cited in US EPA 2003).
Acrolein promotes sensitization in vitro and in vivo. Roux et al. (1999 cited in CalEPA 2008) showed that in previously sensitized lung tissue, preincubation with acrolein significantly increased the bronchiole contractile response to several antigens, including dust mites and histamine. In mice exposed to 5 ppm (11.5 mg/m3) acrolein in addition to inhaled ovalbumin (OVA) during a 2-week sensitization period followed by a 3-day challenge to OVA, O'Brien et al. (2016) observed an increase in OVA-specific IgG and neutrophils (compared to exposure to OVA only). Lung inflammation was also increased in animals exposed to acrolein and OVA compared to those exposed to OVA only. The authors suggest that acrolein appears to promote sensitization to inhaled OVA, as observed by the increased IgG, but they also note that because of the exposure protocol (repeated exposures to two antigens), the results could also indicate inhalation tolerance.
Prior exposure to acrolein has also been shown to cause desensitization (tolerance). Preexposure of mice to inhaled acrolein (3 days for 3 hours per day at 0.5 or 1.7 ppm [1.2 or 3.9 mg/m3]), followed by a 10-minute challenge with 0.4 to 11.2 ppm (0.9 to 25.8 mg/m3) acrolein, increased the RD50 from 1.7 to 3 ppm (3.9 to 6.9 mg/m3) (Kane and Alarie 1977 cited in US EPA 2003). Similarly, in rats, prior inhalation exposure to another aldehyde (9-day exposure to 15 ppm formaldehyde) increased the RD50 by 5-fold (Babiuk et al. 1985 cited in US EPA 2003). A 4-hour exposure of mice to 5 ppm (11.5 mg/m3) acrolein resulted in a decreased airway response to subsequent allergen challenge, as measured by a decreased release of specific cytokines (IL-33, IL-25, and IL-1a) in the BALF (Danyal 2016). A decrease in cytokine release in response to allergen challenge was also observed in vitro in human and mouse epithelial cells that were pretreated with acrolein.
Acrolein can also reduce allergic inflammation in sensitized animals. In mice previously sensitized to OVA by IP injection (allergic asthma model), a 4-day exposure to 5 ppm acrolein reduced allergic airway inflammation (suppressed mucus production, leukocyte infiltration and cytokine levels). Decreased goblet cell hyperplasia was also noted (Spiess et al. 2013).
O'Brien et al. (2016) proposed an explanation for the conflicting observations: "Acrolein exposure can affect allergic airway disease in different ways depending on when exposure occurs during the course of disease pathogenesis," suggesting it can promote allergic sensitization (as in asthma development) and neutrophilic inflammation, but it can also suppress allergic inflammation (as in ongoing asthma).
5.2.3 Cardiovascular effects
The blood pressure and heart rate of anesthetized rats exposed to acrolein for one minute increased with exposure concentration from 22 ppm (50 mg/m3); however, at higher concentrations of 1100 or 2200 ppm (2530 or 5060 mg/m3), the heart rate decreased (Egle and Hudgins 1974 cited in US EPA 2003).
In rats exposed to 3 ppm (6.9 mg/m3) acrolein for 3 hours, an increased incidence of cardiac arrhythmias was observed during exposure and one hour after exposure. Acrolein also depressed the baroreflex sensitivity in both normotensive and spontaneously hypertensive rats (Hazari et al. 2014). In another study by the same researchers, a 3-hour exposure to 3 ppm (6.9 mg/m3) acrolein was found to increase blood pressure compared to when the same rats were exposed to air (no concurrent control animals were included in the study) (Perez et al.2013). Hypertensive rats were more sensitive to acrolein exposure in these studies. Another recent study from Kurhanewicz et al. (2017) showed that mice exposed to 3 ppm (6.9 mg/m3) acrolein for 3 to 4 hours had increased heart rate variability and incidence of arrhythmias.
Thompson et al. (2017) reported that mice had decreased myocardial performance following a 3-hour exposure to 0.3 ppm (0.69 mg/m3) acrolein. This effect was not observed when the acrolein concentration was 3 ppm (6.9 mg/m3). However, at the higher concentration, stroke volume and cardiac output increased. In addition, there was an increase in regional circumferential strain delay, indicating intraventricular myocardial dyssynchrony.
5.2.4 Reproductive and developmental effects
The studies by Kutzman (1981) and Kutzman et al. (1985) included a reproductive toxicity component. Six days after the 62-day exposure regime (see section 5.2.1.2 for details), treated rats (male and female) were mated with untreated rats. There was no change in the pregnancy rate, and the number of corpora lutea, viable embryos, fetal death or preimplantation loss. There were also no morphological sperm abnormalities. Bouley et al. (1976) exposed rats (3 male and 21 female) continuously to 0 or 0.55 ppm (1.3 mg/m3) acrolein for 4 days prior to mating and for an additional 22 days after mating. No difference was found in the number of pregnant animals and in the number and weight of fetuses between exposed and control animals. These studies are limited by the small number of animals and few endpoints. In addition, the study by Bouley et al. (1976) only used a single exposure concentration.
5.2.5 Genotoxicity
In vitro, acrolein has been shown to induce DNA mutations in bacteria; and mutations and DNA damage, including chromosome aberrations (CA), sister chromatid exchange (SCE), and DNA breaks and cross-links, in mammalian cells (reviewed in Environment Canada and Health Canada 2000; US EPA 2003; ATSDR 2007; Wang et al. 2011, 2012; Mohammad et al. 2012; Luo et al. 2013; Lee et al. 2014). Often, genotoxicity was only observed under conditions which limit cytotoxicity.
Repair-deficient cells are more susceptible to acrolein-induced genotoxicity, and acrolein also appears to inhibit DNA repair. Acrolein increased mutations in repair-deficient human fibroblast cells, but not in repair-proficient cells (Curren et al. 1988 cited in US EPA 2003). It also induced mutations in repair-deficient V79 cells (Chinese hamster lung), although normal V79 cells were not tested (US EPA 2003). Wang et al. (2012) showed that acrolein decreased DNA repair in cultured human bronchial epithelial cells and lung fibroblasts. Lee et al. (2014) found that acrolein inhibits both nucleotide excision repair and base excision repair, and induces repair protein degradation in urothelial cells.
A limited amount of in vivo genotoxicity data is available for acrolein. In particular, only two studies were identified that examined genotoxicity endpoints following inhalation exposure. No evidence of SCE or CA was observed in rat peripheral blood following a 62-day exposure to 4 ppm (9.2 mg/m3) acrolein. Bone marrow was also negative for SCE (Kutzman 1981). No DNA-protein cross-linking occurred in rat nasal mucosa following a 6-hour inhalation exposure to 2 ppm (4.6 mg/m3) acrolein (Lam et al. 1985). Acrolein was also negative in a dominant lethal assay in mice (exposure by IP injection) and in a sex-linked recessive lethal assay in drosophila (ATSDR 2007).
Acrolein forms adducts with DNA, and deoxyguanosine (dG), deoxyadenosine, and deoxycytidine adducts with acrolein have been observed in mammalian cells in culture following incubation with acrolein (US EPA 2003; Moghe et al. 2015; Randall et al. 2016). Some research suggests that adducts form preferentially at CpG sites and that methylation at these sites enhances acrolein binding (Wang et al. 2013). Acrolein-DNA adducts have been found in lungs of current and former smokers (Zhang 2007 cited in Burcham 2016), and increased acrolein-dG adducts were also observed in BALF, lung, and bladder of mice exposed to cigarette smoke (Lee et al. 2015). Feng et al. (2006) found that the locations of acrolein-DNA adducts in the p53 tumour suppressor gene are similar to the mutational hotspots observed in lung tumours of smokers. In a recent study comparing bladder tumours with normal bladder tissue, Lee et al. (2014) found more acrolein-dG adducts in the tumours compared to normal tissue; most of the tumours were from current or former smokers. However, in another study, Zhang et al. (2011) found that the total acrolein-dG level in blood leukocytes was the same in smokers and non-smokers. DNA-acrolein adducts have also been identified in liver DNA of unexposed humans and rats, indicating some level of background acrolein exposure or endogenous production (Nath et al. 1996 cited in US EPA 2003). DNA adducts were observed in aortas of chickens exposed to 1 ppm (2.3 mg/m3) acrolein for 6 hours; however, 10 days later, the levels of DNA adducts were comparable to controls, suggesting a repair mechanism (Penn et al. 2001 cited in CalEPA 2008).
5.2.6 Carcinogenicity
Very few studies have been conducted on acrolein with exposure durations greater than 13 weeks. Feron and Kruysse (1977) exposed hamsters to 4 ppm (9.2 mg/m3) acrolein for 52 weeks (7 hours per day, 5 days per week). No tumours were found in the respiratory tract or any other organs after a 29-week recovery period. The authors also found that this acrolein inhalation regimen followed by the 29-week recovery period did not increase the incidence of respiratory tract tumours in animals co-exposed to benzo(a)pyrene by intratracheal instillation or N-nitrosodiethylamine by subcutaneous injection (Feron and Kruysse 1977 cited in IARC 1995). Le Bouffant et al. (1980) exposed rats to 8 ppm (18.4 mg/m3) acrolein for 18 months (1 hour a day) and found no tumours. Conclusions regarding the carcinogenicity potential of acrolein cannot be drawn from these studies due to their short durations and single exposure concentrations.
No evidence of carcinogenicity has been observed in several oral studies of acrolein exposure in multiple species (reviewed in ATSDR 2007; IARC 1995). No treatment-related tumour increase was observed in gavage studies in mice (18 months) or rats (24 months) (Parent et al. 1991, 1992 cited in IARC 1995), or in a rat 24 to 28-month drinking water study (Lijinski and Reuber1987; Lijinski 1988, both cited in IARC 1995).
In an initiation/promotion study in rats, IP injection of acrolein alone for 21 weeks did not induce urinary bladder tumours. However, rats given acrolein injections twice weekly for 6 weeks followed by 20 weeks of uracil in the diet had a statistically significant increase in bladder papillomas compared to the group given twice weekly injections of water for 6 weeks followed by 20 weeks of uracil in the diet (incidence of 18/30 and 8/30 respectively, p < 0.05) (Cohen et al. 1992 cited in IARC 1995).
In vitro, Lee et al. (2015) found that acrolein induces anchorage-independent growth of human bronchial epithelial and urothelial cells, indicating neoplastic transformation.
5.3 Summary of Health Effects and Mode of Action
Based on the evidence from human and animal toxicology studies, the effects of single and repeated acrolein inhalation exposures are observed at the site of contact. More specifically, key health effects are eye, nose, and respiratory tract irritation. Sensory irritants, including aldehydes, act by directly stimulating trigeminal nerve endings in the nasal mucosa. This activates defense mechanisms to reduce penetration further into the respiratory tract, such as a decrease in breathing rate, an increase in mucus secretion, and bronchoconstriction. More severe damage to the respiratory tract occurs with increasing exposure concentration.
In experimental animals, acrolein reacts mainly in the nasal area and upper respiratory tract, but at higher concentrations, there may be increased penetration to the lower respiratory tract. In humans, acrolein may reach the lower respiratory tract during mouth breathing. The uptake of acrolein appears to be a saturable process.
Due to its highly reactive (electrophilic) nature, acrolein becomes rapidly and irreversibly bound to protein and non-protein sulfhydryl groups and to primary and secondary amines in proteins and nucleic acids (WHO 2002; US EPA 2003). Acrolein causes a decrease in reduced GSH and other reducing agents as well as changes in enzyme activities, resulting in an overall decrease in protective activity in the respiratory nasal epithelium (US EPA 2003; CalEPA 2008).
In studies with human volunteers, a single exposure to acrolein induced irritation of the eyes, nose, and throat. The most sensitive effect was eye irritation, reported by subjects at concentrations as low as 0.14 to 0.36 mg/m3 following exposures of 5 to 60 minutes (Darley et al. 1960; Weber-Tschopp et al. 1977, both cited in US EPA 2003 and CalEPA 2008; Dwivedi et al. 2015; Claeson and Lind 2016). Subject-reported nasal irritation was observed starting at 0.35 to 0.60 mg/m3, throat irritation at 0.69 mg/m3, and respiratory irritation as measured by decreased respiration rate at 0.69 mg/m3 (10 to 15% decrease) and 1.4 mg/m3 (25% decrease) (Weber-Tschopp et al. 1977 cited in CalEPA 2008 and US EPA 2003).
Epidemiological data on the long-term effects of acrolein on humans are limited to two French cross-sectional studies on indoor air quality and asthma and rhinitis. Annesi-Maesano et al. (2012) measured acrolein in school classrooms and found higher acrolein concentration (> LOD) was associated with increased incidence of asthma, but not rhinoconjunctivitis in the previous year (as reported by parents on a questionnaire), compared to lower acrolein concentration (< LOD). The LOD was not specified, and most students (72%) had exposures < LOD. The authors also reported a correlation between acrolein exposure and exercise-induced asthma. Billionnet et al. (2011) found no significant relationship between acrolein levels in homes and asthma in the previous year or rhinitis in the previous month (as reported on a questionnaire). These studies both have significant limitations, which are described in section 5.1.2.
Signs of irritation as well as inflammation and damage to the airways occurred in laboratory animals exposed to inhaled acrolein. In mice, rats, guinea pigs, rabbits, hamsters, monkeys, and dogs, acrolein exposure caused irritant effects in the respiratory system, from mild inflammation at low concentrations to metaplasia and hyperplasia at higher concentrations. Reduced respiration, as an indication of sensory irritation, was observed in all species tested; the lowest RD50 identified for a single exposure was 2.3 mg/m3 in mice (US EPA 2003; CalEPA 2008). Other respiratory irritant effects observed were increased airway resistance and bronchial hyperresponsiveness in guinea pigs, and mucus hypersecretion in mice and rats (Murphy 1963 cited in US EPA 2003; Leikauf 1991; Borchers et al. 1998; Borchers et al. 1999 cited in US EPA 2003).
With respect to histopathology, the rat nasal respiratory epithelium is the most sensitive target organ, with lesions observed at exposure concentrations as low as 0.46 to 1.38 mg/m3 in multiple studies, from a single exposure to repeated exposure for up to 13 weeks (Feron et al. 1978; Roemer et al. 1993; Cassee, Groten and Feron 1996; Dorman et al. 2008). Inflammatory, degenerative, and metaplastic lesions in the rat nasal epithelium have been characterized as disarrangement, necrosis, thickening, desquamation, basal cell hyperplasia, and squamous metaplasia; the severity of the lesions increases with exposure concentration but not with duration of exposure (Feron et al. 1978; Cassee, Groten and Feron 1996; Dorman et al. 2008). At low concentrations, effects are generally limited to the nasal cavity; but at higher concentrations, damage to the lower respiratory tract occurs, with lesions in the rat trachea and lung observed above 1.6 mg/m3 (Lyon et al. 1970; Feron et al. 1978; Kutzman 1981; Arumugan et al. 1999; Dorman et al. 2008; Chen et al. 2010, 2013). Dorman et al. (2008) also found inflammation, degeneration, and atrophy of rat nasal olfactory epithelia as well as olfactory neuronal loss following exposure to 4.1 mg/m3 acrolein for 13 weeks. The authors noted that the respiratory epithelium was the most sensitive tissue based on the observed NOAEL and LOAEL; however, based on a dosimetry model from Schroeter et al. (2008), olfactory epithelium actually had a lower delivered tissue dose.
Inflammation and histopathology in the lower respiratory tract (trachea and lungs) were also reported in guinea pigs, hamsters, rabbits, dogs, and monkeys at exposure concentrations of 1.61 mg/m3 and above, and, as in rats, the severity of the lesions increased with concentration (Lyon et al, 1970; Feron et al. 1978; Kilburn and McKenzie 1978 cited in US EPA 2003; Leikauf 1991). In mice, similar lesions to rats (exfoliation, erosion, ulceration, necrosis, inflammation, and squamous metaplasia in the respiratory epithelium) were observed in the upper, but not lower, respiratory tract in a 5-day exposure study at 3.9 mg/m3 (Buckley 1984); indications of metaplasia in the lungs were observed in a 4-week exposure study at 4.6 mg/m3 (Borchers et al. 2008). No lower concentration studies were available in mice.
In vitro, acrolein has shown to be mutagenic, inhibit DNA repair, and cause DNA damage, including DNA adducts, breaks and cross-links. However, limited information is available on the formation and mutagenicity of DNA adducts in vivo. Inhalation of acrolein did not cause DNA damage (SCE or CA) in rat blood or bone marrow in a 62-day exposure study (Kutzman 1981), and no DNA-protein cross-linking occurred in rat nasal mucosa following a single inhalation exposure (Lam et al. 1985).
No evidence of acrolein-induced tumours was identified in limited carcinogenicity studies in experimental animals. However, the related aldehydes, formaldehyde and acetaldehyde, induce nasal tumours in rats. Mode of action analyses have determined that the tumorigenic responses to these aldehydes are non-linear and result primarily from cytotoxicity and proliferation rather than mutagenicity (Environment Canada and Health Canada 2000; Health Canada 2017). The same pattern of tissue damage and cell proliferation induced by other aldehydes is also observed with acrolein exposure in animal models. Cytotoxicity (degeneration), proliferation, and metaplasia in the respiratory epithelium were consistently observed in laboratory animals exposed to inhaled acrolein, in some cases after just a single exposure. Therefore, although inhalation of acrolein has not been shown positively to induce tumours in the respiratory system and acrolein is not being considered a carcinogen in this assessment, the carcinogenic potential of acrolein requires further investigation.
5.4 Susceptible populations
Sensitized individuals such as asthmatics as well as individuals with chronic pulmonary disease or bronchitis may be more susceptible to the effects of acrolein on the respiratory tract. Although no human studies conducted in susceptible populations are available to show this for acrolein specifically, it has been demonstrated for other aldehydes including acetaldehyde (Health Canada 2017), and formaldehyde (Health Canada 2006). Also, in general, pre-existing nasal allergies can intensify the response to nasal irritants (Shusterman 2011).
Animal studies showing bronchial hyperresponsiveness and inflammation following acrolein inhalation as well as in vitro studies indicating that acrolein increases mucin and pro-inflammatory mediators provide evidence that acrolein can exacerbate asthma and that sensitized individuals may be more susceptible to the adverse effects of acrolein. The effects of inhalation exposure to acrolein, including a decrease in breathing rate, an increase in mucus secretion, and bronchoconstriction, are enhanced in mice previously sensitized by IP injection of OVA (a model of allergic airway disease) (Morris et al. 2003). Roux et al. (1999 cited in CalEPA 2008) showed that in previously sensitized lung tissue, preincubation with acrolein significantly increased the bronchiole contractile response to several antigens, including dust mites and histamine.
Children, especially those with asthma, may be more likely to show adverse respiratory effects following exposure to acrolein due to higher prevalence rates of asthma in children as compared to other age groups, the small size and immature state of their airways, and the exacerbation that toxic air contaminants have been demonstrated to have on asthma in children (Delfino et al. 2003; CalEPA 2008).
Individuals with hypertension may also be more susceptible to the effects of acrolein. Perez et al. (2013) found that hypertensive rats had an increase in breathing frequency and minute volume at a concentration that did not affect normotensive rats. The hypertensive rats were also more sensitive to cardiovascular effects, including increased heart rate, blood pressure, and heart rate variability.
In a study with Dahl rats that were either resistant (DR) or susceptible (DS) to hypertension, DS rats were found to be more sensitive to acrolein-induced mortality and lung lesions than DR rats (Kutzman et al. 1984).
Contact lens wearers could be more susceptible to ocular exposure and irritation by acrolein, as contact lenses can trap and concentrate volatile compounds, and extend the exposure time by limiting the eye's normal self-cleansing (CalEPA 2008).
Due to the key involvement of GSH in the detoxification of acrolein, individuals with decreased GSH synthesis may be more susceptible to its effects. Similarly, some individuals may be more susceptible to acrolein toxicity due to impaired GST activity. For example, one of the four allelic variants of the GST P1-1 isoenzyme has a significantly lower catalytic efficiency in GSH conjugation of acrolein (Pal et al. 2000 cited in Stevens and Maier 2008).
6.0 Derivation of short- and long-term reference concentrations
6.1 Short-Term Reference Concentration
For short-term exposure to acrolein, several studies investigating eye and respiratory irritation in human volunteers were available for consideration as the point of departure for a short-term reference concentration (see section 5.1). The LOAELs for eye irritation identified in multiple studies were similar, ranging from 0.21 to 0.36 mg/m3 (Weber-Tschopp et al. 1977; Dwivedi et al. 2015; Claeson and Lind 2016). The study by Weber-Tschopp et al. (1977) used multiple exposure protocols, including a 40-minute exposure with increasing acrolein concentrations (up to 1.4 mg/m3) and a 60-minute exposure to a fixed acrolein concentration (0.69 mg/m3). The original paper is in German; therefore, the US EPA report is cited. No NOAEL for eye irritation was derived in this study as subjects reported eye irritation at the lowest tested concentration of 0.21 mg/m3 (LOAEL). In a more recent study, Dwivedi et al. (2015) exposed healthy volunteers to acrolein for 2 hours. There was a slight increase in eye irritation at 0.23 mg/m3, but not 0.12 mg/m3 (LOAEL and NOAEL, respectively). The NOAEL from this study was similar to those identified in two other eye-only exposure studies (Darley et al. 1960 where the NOAEL = 0.14 mg/m3; Claeson and Lind 2016 where the NOAEL = 0.16 mg/m3).
The lowest LOAEL for respiratory irritation is from the study by Weber-Tschopp et al. (1977). During a 60-minute exposure to 0.69 mg/m3 acrolein, subjects reported eye, nose, and throat irritation, and respiration rates were reduced by 10 to 15%, indicating a slight respiratory irritation. No NOAEL for respiratory irritation was identified in this study. Dwivedi et al. (2015) noted there was no difference in breathing frequency, pulmonary function, nasal swelling, or inflammatory markers in blood or sputum after a 2-hour exposure to 0.23 mg/m3 (NOAEL). No LOAEL was identified for these effects.
Therefore, eye irritation is the most sensitive endpoint, and the lowest LOAELs identified for this endpoint were 0.21 mg/m3 from Weber-Tschopp et al. (1977) and 0.23 mg/m3 from Dwivedi et al. (2015). As Weber-Tschopp et al. (1977) did not identify a NOAEL, the NOAEL of 0.12 mg/m3 (115 µg/m3) for eye irritation from Dwivedi et al. (2015) was selected as the point of departure for the acute RfC. This point of departure is also below the LOAEL and NOAEL for respiratory effects observed by Weber-Tschopp et al. (1977) and Dwivedi et al. (2015), respectively. An uncertainty factor (UF) of 3 was applied to account for sensitive individuals and is considered sufficient as eye irritation due to contact is not expected to vary greatly across the population (NRC 2001; US EPA 2008). No UF for database deficiencies was applied as the critical study and the database for acute toxicity were adequate. Thus, the acute RfC is 38 µg/m3.
6.2 Long-Term Reference Concentration
Few epidemiological studies have been conducted on acrolein and its health effects; and due to study limitations, in particular the lack of causality established, none of the available studies were appropriate for use as key studies for deriving a long-term reference value.
Inhalation studies in multiple species of laboratory animals have consistently shown respiratory tract effects following exposures of 3 days to 13 weeks to acrolein concentrations in the range of 0.46 to 1.38 mg/m3. Effects were observed at the lowest test concentration in most studies; only one 13-week study was able to identify both a NOAEL and a LOAEL. Dorman et al. (2008) found a NOAEL of 0.46 mg/m3 and a LOAEL of 1.38 mg/m3 for degenerative lesions in the respiratory epithelium of the rat nasal cavity. A NOAEL of 1.38 mg/m3 and a LOAEL of 4.14 mg/m3 for olfactory neuronal loss were also derived.
Dorman et al. (2008) noted that the modelled tissue dose in rats (from Schroeter et al. 2008) associated with the effect on olfactory epithelium was lower than that of the respiratory epithelial lesions. However, the NOAEL of 0.46 mg/m3 (460 µg/m3) for lesions of the respiratory epithelium was selected as the point of departure because it was the lowest exposure concentration associated with an adverse effect. This concentration was adjusted from the animal study exposure (6 hours per day, 5 days per week) to continuous exposure (24 hours per day, 7 days per week), resulting in an adjusted NOAEL of 82 µg/m3.
Toxicokinetic differences between rats and humans were accounted for by applying a regional gas dose ratio (RGDR) of 0.13 for a Category 1 gas with extrathoracic respiratory effects (US EPA 1994), giving a human equivalent NOAEL of 11 µg/m3. This approach was also used by the US EPA (2003) and ANSES (2013) to derive RfCs for acrolein and is considered appropriate in the absence of chemical-specific information. Although the CFD-PBPK model by Corley et al. (2012) showed that acrolein uptake in the respiratory tract in humans was lower than in rats, this model was not applied for interspecies extrapolation in this assessment as it has not been calibrated or validated for humans. CalEPA (2008) used the dosimetric adjustment factor (DAF) for formaldehyde in their derivation of an RfC for acrolein. This DAF was based on the average of the mean flux and peak flux of formaldehyde in the upper respiratory tract of rats relative to humans (Kimbell et al. 2001). The CalEPA approach introduces additional uncertainty due to the use of a chemical analogue (i.e., formaldehyde) as well as from the averaging of mean and peak flux.
Uncertainty factors (UFs) of 2.5 for toxicodynamic differences between rats and humans, and 10 for sensitivity in the human population were applied to the point of departure (human equivalent NOAEL of 11 µg/m3). Although the Dorman et al. (2008) study was of less than chronic duration, no additional UF was applied, as there is no indication that the severity or incidence of lesions increases with longer exposure durations; exposure concentration appears to be the driving factor. Also, no UF for database deficiencies was applied as the critical study and the health effects database were adequate. A detailed justification for the selection of UFs for the long-term RfC can be found in Ritter et al. (2007). Thus, the long-term RfC is 0.44 µg/m3.
6.3 Exposure in Canadian Homes in Relation to Reference Concentration and Determination of Proposed Exposure Limits
In the past decade, Health Canada has completed several exposure studies in multiple Canadian cities (Health Canada 2010a, 2010b, 2012, 2013). These studies are considered the most recent and most representative data available for quantifying long-term levels of exposure in Canadian homes (see section 3.0).
Short- and long-term RfCs are based on the characterization of the concentration-response relationship and the application of UFs to account for variability and data gaps. The context within which these RfCs are to be applied, technical feasibility, and availability of risk mitigation measures do not enter into their determination. However, these issues are relevant to the determination of short- and long-term exposure limits.
In order to determine the proposed exposure limits, the short- and long-term RfCs are first compared to available exposure data from Canadian homes. The feasibility of achieving the RfC through the control of indoor sources is then evaluated. If the RfC is judged to be feasible, the same value is set as the proposed exposure limit. If not, a higher concentration may be selected, while still targeting an exposure limit that is protective of health in consideration of current evidence.
In the present assessment, the criteria guiding the determination of the value for both the proposed short- and long-term exposure limits for acrolein are:
- a value that is potentially achievable in Canadian homes in the absence of significant sources of indoor acrolein; and
- a value that is not associated with appreciable health effects, considering the derived reference exposure levels and currently available evidence.
6.3.1 Short-term reference concentration and proposed exposure limit
The literature database provided sufficient information on the effects in humans for development of a short-term RfC, which was determined for acrolein to be 38 µg/m3. The range of median indoor air acrolein concentrations measured in Canadian homes from the Health Canada residential indoor air exposure studies for a 24-hour averaging period was 1.3 to 8.1 µg/m3, with the 95th percentile ranging from 3.5 to 21.0 µg/m3 (see Table 2) (Health Canada 2010a, 2010b, 2012, 2013). The 24-hour integrated samples collected in these studies do not represent acute or peak exposures. However, short-term acrolein peaks may occur with behaviours such as smoking or cooking as well as with the use of fireplaces and wood-burning stoves. Based on the 24-hour sampling data and the expected sources present, the short-term RfC should be achievable in Canadian homes. Therefore, the proposed short-term exposure limit for acrolein is 38 µg/m3.
6.3.2 Long-term reference concentration and proposed exposure limit
From the literature database, a chronic RfC of 0.44 µg/m3 was derived based on lesions in the respiratory epithelium. The Health Canada residential indoor air exposure studies (Health Canada 2010a, 2010b, 2012, 2013) provide the best measure of chronic exposure in Canadian homes although uncertainty still remains in terms of accuracy of exposure data due to methodological difficulties in measuring acrolein in indoor air. The range of median indoor air acrolein concentrations measured in Canadian homes from the Health Canada residential indoor air exposure studies for a 24-hour averaging period was 1.3 to 8.1 µg/m3, with the 95th percentile ranging from 3.5 to 21.0 µg/m3 (see Table 2) (Health Canada 2010a, 2010b, 2012, 2013). This indicates that even considering uncertainties in the measurement of acrolein, there will likely be Canadian homes in which the RfC is exceeded. However, the RfC was derived using the most recent scientific information, and is in line with the Health Canada IARL of 0.35 µg/m3 and reference values from other jurisdictions (see Appendix D). Sources of acrolein in Canadian homes include smoking, cooking with oils, and secondary formation from reactions of other VOCs. Based on the limited available source information, reduction of acrolein levels in the home through ventilation and source control is considered possible. Therefore, the proposed long-term exposure limit for acrolein is 0.44 µg/m3.
6.4 Uncertainties and Areas of Future Research
As only limited data were available on repeated inhalation exposure to acrolein in humans, animal data were used as a point of departure when deriving the RfC. The evidence in multiple species clearly shows that acrolein is a reactive substance which exerts effects at the site of first contact (i.e., the nasal cavity in rats). Various approaches for comparing the physiology of the respiratory tracts to account for toxicokinetic differences between rats and humans were considered. Assumptions are made in each approach, and each has uncertainties. Further research on the kinetics of acrolein deposition in humans would be useful. Moreover, data that would allow for the calibration and validation of a human CFD-PBPK model would facilitate a chemical-specific extrapolation between animals as well as intermittent to continuous exposure.
Although the nature of effects (irritation) is likely to be the same across species, quantitative differences in sensitivity were accounted for using default values for the toxicodynamic UF (rats to humans) and an intraspecies uncertainty factor (for sensitive individuals). No studies could be found on the effects of acrolein in sensitive individuals such as asthmatics-such studies would reduce the uncertainty in the RfC.
Studies on the effects of long-term inhalation exposure to acrolein are limited. There were also significant limitations, as described in section 5.1.2, to the few epidemiological studies examining associations between acrolein exposure and asthma or rhinitis. Similarly, most studies in experimental animals did not go beyond a subchronic duration, and those few chronic studies available were inadequate to draw conclusions about the carcinogenicity of acrolein.
Existing exposure studies have evaluated 24-hour sampling times to give an average daily exposure. Exposures to peak concentrations over shorter durations have not been evaluated. As described in section 3, acrolein is difficult to quantify accurately, and available methods have limitations. Therefore, there is uncertainty with respect to the actual concentration of acrolein in indoor air. However, the Health Canada data collected by the passivated canister method are believed to provide a realistic estimate of acrolein concentrations in Canadian homes.
Minimal data are available on quantitative estimates of indoor acrolein sources. Additional information on source attribution would enable a more detailed strategy for reducing indoor concentrations of acrolein and a determination of whether the proposed exposure limits can be achieved in a typical Canadian home.
7.0 Proposed guidelines
7.1 Proposed Exposure Limits
| Exposure Limit | Concentration | Critical effect(s) | |
|---|---|---|---|
| µg/m3 | ppb | ||
| Short-term (1 h) |
38 | 17 | Eye irritation in healthy volunteers |
| Long-term (24 h) |
0.44 | 0.19 | Lesions in the respiratory epithelium of the rat nasal cavity |
It is recommended that the short-term (acute) exposure limit be compared to a 1-hour air sample.
When comparing a measured acrolein concentration with the long-term exposure limit, the sampling time should be at least 24 hours, taken under normal conditions. Moreover, the averaging of results of repeated samples taken at different times of the year will provide a more representative estimate of the long-term exposure.
7.2 Risk Management Recommendations
Some homes in Canada may have levels of acrolein above the long-term reference exposure limit derived for protection against respiratory irritation and degeneration of the respiratory epithelium. Therefore, sources of acrolein in the home should be controlled to limit exposure as much as reasonably possible, given that air quality testing in individual homes is neither practical nor recommended in most instances. Furthermore, many of the measures outlined below will also contribute to reducing the concentrations of other indoor air contaminants, generally improving indoor air quality.
Exposure to acrolein indoors can be reduced by ensuring adequate ventilation and controlling for indoor sources. Strategies for reducing indoor exposure to acrolein include the following:
- Increasing ventilation by opening windows (when possible) or by employing mechanical ventilation strategies. For more information, refer to the Factsheet: Ventilation and the indoor environment (Health Canada 2018a).
- Using a range hood exhaust fan with outside venting (preferably on the high setting) when cooking, especially with oils.
- While cooking, in addition to using a range hood exhaust fan (or if no range hood exhaust fan is available), using back burners instead of front burners, opening windows or running the fan in the furnace or ventilation system. For more information, refer to the Factsheet: Cooking and Indoor Air Quality (Health Canada 2018b).
- Not smoking or burning candles or incense inside the home, and ensuring proper ventilation to the outside during use of combustion appliances (e.g., gas stoves, woodstoves or fireplaces).
- Decreasing VOC levels in the home to reduce secondary formation of acrolein. This can be done by choosing low-emission products when possible; opening windows to ensure good ventilation when using products such as glues, paints, varnishes, and cleaning products; and minimizing the use of scented products, such as plug-in or aerosol deodorizers (air fresheners).
8.0 References
Alwis, K.U., deCastro, B.R., Morrow, J.C. and Blount, B.C. (2015) Acrolein Exposure in U.S. Tobacco Smokers and Non-Tobacco Users: NHANES 2005-2006. Environmental Health Perspectives, 123(12): 1302-1308.
Annesi-Maesano, I., Hulin, M., Lavaud, F., Raherison, C., Kopferschmitt, C., De Blay, F., Charpin, D.A. and Denis, C. (2012) Poor air quality in classrooms related to asthma and rhinitis in primary schoolchildren of the French 6 Cities Study. Thorax, 67(8): 682-688.
ANSES (2013) Proposition de valeurs guides de qualité d'air intérieur. L'acroléine. Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail, http://www.anses.fr/en/documents/AIR2011sa0354Ra.pdf.
Arumugam, N., Sivakumar, V., Thanislass, J., Pillai, K.S., Devaraj, S.N. and Devaraj, H. (1999) Acute pulmonary toxicity of acrolein in rats--underlying mechanism. Toxicology Letters, 104(3): 189-194.
ATSDR (2007) Toxicological Profile for Acrolein. Agency for Toxic Substances and Disease Registry, Public Health Service, US Department of Health and Human Services, Atlanta, GA.
Babiuk, C., Steinhagen, W.H. and Barrow, C.S. (1985) Sensory irritation response to inhaled aldehydes after formaldehyde pretreatment. Toxicology and Applied Pharmacology, 79(1): 143-149.
Bein, K. and Leikauf, G.D. (2011) Acrolein - a pulmonary hazard. Molecular Nutrition and Food Research, 55(9): 1342-1360.
Billionnet, C., Gay, E., Kirchner, S., Leynaert, B. and Annesi-Maesano, I. (2011) Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environmental Research, 111(3): 425-434.
Borchers, M.T., Carty, M.P. and Leikauf, G.D. (1999) Regulation of human airway mucins by acrolein and inflammatory mediators. The American Journal of Physiology, 276(4 Pt 1): L549-55.
Borchers, M.T., Wert, S.E. and Leikauf, G.D. (1998) Acrolein-induced MUC5ac expression in rat airways. The American Journal of Physiology, 274(4 Pt 1): L573-81.
Borchers, M.T., Wesselkamper, S.C., Eppert, B.L., Motz, G.T., Sartor, M.A., Tomlinson, C.R., Medvedovic, M. and Tichelaar, J.W. (2008) Nonredundant functions of alphabeta and gammadelta T cells in acrolein-induced pulmonary pathology. Toxicological Sciences: An Official Journal of the Society of Toxicology, 105(1): 188-199.
Borchers, M.T., Wesselkamper, S., Wert, S.E., Shapiro, S.D. and Leikauf, G.D. (1999) Monocyte inflammation augments acrolein-induced Muc5ac expression in mouse lung. The American Journal of Physiology, 277(3): L489-97.
Bouley, G., Dubreuil, A., Godin, J., Boisset, M. and Boudene, C. (1976) Phenomena of adaptation in rats continuously exposed to low concentrations of acrolein. The Annals of Occupational Hygiene, 19(1): 27-32.
Buckley, L.A., Jiang, X.Z., James, R.A., Morgan, K.T. and Barrow, C.S. (1984) Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicology and Applied Pharmacology, 74(3): 417-429.
Burcham, P.C. (2016) Acrolein and Human Disease: Untangling the Knotty Exposure Scenarios Accompanying Several Diverse Disorders. Chemical Research in Toxicology, 30(1): 145-161.
Cahill, T.M. and Okamoto, R.A. (2012) Emissions of Acrolein and Other Aldehydes from Biodiesel-Fueled Heavy-Duty Vehicles. Environmental Science & Technology, 46(15): 8382-8388.
CalEPA (2008) TSD [Technical Support Document] for Noncancer RELs: Appendix D. Individual Acute, 8-Hour and Chronic Reference Exposure Level Summaries. D.1 Summaries using this version of the Hot Spots Risk Assessment Guidelines. California Environmental Protection Agency, Office of Environmental Health Hazard Assessment. Available from: http://www.oehha.ca.gov/air/hot_spots/2008/AppendixD1_final.pdf#page=42.
Cassee, F.R., Arts, J.H.E., Groten, J.P. and Feron, V.J. (1996) Sensory irritation to mixtures of formaldehyde, acrolein, and acetaldehyde in rats. Archives of Toxicology, 70(6): 329-337.
Cassee, F.R., Groten, J.P. and Feron, V.J. (1996) Changes in the nasal epithelium of rats exposed by inhalation to mixtures of formaldehyde, acetaldehyde, and acrolein. Fundamental and Applied Toxicology, 29(2): 208-218.
CDC (2013) Acute pesticide-related illness resulting from occupational exposure to acrolein - Washington and California, 1993-2009. Centers for Disease Control and Prevention - Morbidity and Mortality weekly report, 26(16).
Champeix, J., Courtial, L., Perche, E. and Catalina, P. (1966) Acute broncho-pneumopathy from acrolein vapors. Archives des maladies professionnelles de médecine du travail et de sécurité sociale, 27(10): 794-796.
Chen, P., Deng, Z., Wang, T., Chen, L., Li, J., Feng, Y., Zhang, S., Nin, Y., Liu, D., Chen, Y., Ou, X. and Wen, F. (2013) The potential interaction of MARCKS-related peptide and diltiazem on acrolin-induced airway mucus hypersecretion in rats. International Immunopharmacology, 17(3): 625-632.
Chen, Y.J., Chen, P., Wang, H.X., Wang, T., Chen, L., Wang, X., Sun, B.B., Liu, D.S., Xu, D., An, J. and Wen, F.Q. (2010) Simvastatin attenuates acrolein-induced mucin production in rats: Involvement of the Ras/extracellular signal-regulated kinase pathway. International Immunopharmacology, 10(6): 685-693.
Claeson, A.S. and Lind, N. (2016) Human exposure to acrolein: Time-dependence and individual variation in eye irritation. Environmental Toxicology and Pharmacology, 45: 20-27.
Cohen, S.M., Garland, E.M., St John, M., Okamura, T. and Smith, R.A. (1992) Acrolein initiates rat urinary bladder carcinogenesis. Cancer Research, 52(13): 3577-3581.
Conklin D.J., Malovichko M.V., Zeller I., Das T.P., Krivokhizhina T.V., Lynch B.H., Lorkiewicz P., Agarwal A., Wickramasinghe N., Haberzettl P., Sithu S.D., Shah J., O'Toole T.E., Rai S.N., Bhatnagar A., Srivastava S. (2017) Biomarkers of Chronic Acrolein Inhalation Exposure in Mice: Implications for Tobacco Product-Induced Toxicity. Toxicological Sciences. 158(2):263-274.
Corley, R.A., Kabilan, S., Kuprat, A.P., Carson, J.P., Minard, K.R., Jacob, R.E., Timchalk, C., Glenny, R., Pipavath, S., Cox, T., Wallis, C.D., Larson, R.F., Fanucchi, M.V., Postlethwait, E.M. and Einstein, D.R. (2012) Comparative computational modeling of airflows and vapor dosimetry in the respiratory tracts of rat, monkey, and human. Toxicological Sciences: An Official Journal of the Society of Toxicology, 128(2): 500-516.
Costa, D.L., Kutzman, R.S., Lehmann, J.R. and Drew, R.T. (1986) Altered lung function and structure in the rat after subchronic exposure to acrolein. The American Review of Respiratory Disease, 133(2): 286-291.
Curren, R.D., Yang, L.L., Conklin, P.M., Grafstrom, R.C. and Harris, C.C. (1988) Mutagenesis of xeroderma pigmentosum fibroblasts by acrolein. Mutation Research, 209(1-2): 17-22.
Dann & Wang (2007) Canada's Experience with Acrolein Measurements Using Canisters - Preliminary Results. NESCAUM Monitoring and Assessment Committee Meeting.
Danyal, K., de Jong, W., O'Brien, E., Bauer, R.A., Heppner, D.E., Little, A.C., Hristova, M., Habibovic, A. and van der Vliet, A. (2016) Acrolein and thiol-reactive electrophiles suppress allergen-induced innate airway epithelial responses by inhibition of DUOX1 and EGFR. American Journal of Physiology. Lung Cellular and Molecular Physiology, 311(5): L913-L923.
Darley, E.F., Middleton, J.T. and Garber, M.J. (1960) Plant damage and eye irritation from ozone-hydrocarbon reactions. Agricultural and Food Chemistry, 8(6): 483-485.
Davis, T.R., Battista, S.P. and Kensler, C.J. (1967) Mechanism of respiratory effects during exposure of guinea pigs to irritants. Archives of Environmental Health, 15(4): 412-419.
deCastro, B.R. (2014) Acrolein and asthma attack prevalence in a representative sample of the United States adult population 2000-2009. PloS One, 9(5): e96926.
DeJarnett, N., Conklin, D.J., Riggs, D.W., Myers, J.A., O'Toole, T.E., Hamzeh, I., Wagner, S., Chugh, A., Ramos, K.S., Srivastava, S., Higdon, D., Tollerud, D.J., DeFilippis, A., Becher, C., Wyatt, B., McCracken, J., Abplanalp, W., Rai, S.N., Ciszewski, T., Xie, Z., Yeager, R., Prabhu, S.D. and Bhatnagar, A. (2014) Acrolein exposure is associated with increased cardiovascular disease risk. Journal of the American Heart Association, 3(4): 10.1161/JAHA.114.000934.
Delfino, R.J., Gong, H., Linn, W.S., Hu, Y. and Pellizzari, E.D. (2003) Respiratory symptoms and peak expiratory flow in children with asthma in relation to volatile organic compounds in exhaled breath and ambient air. Journal of Exposure Analysis and Environmental Epidemiology, 13(5): 348-363.
Deshmukh, H.S., Shaver, C., Case, L.M., Dietsch, M., Wesselkamper, S.C., Hardie, W.D., Korfhagen, T.R., Corradi, M., Nadel, J.A., Borchers, M.T. and Leikauf, G.D. (2008) Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. American Journal of Respiratory Cell and Molecular Biology, 38(4): 446-454.
Dorman, D.C., Struve, M.F., Wong, B.A., Marshall, M.W., Gross, E.A. and Willson, G.A. (2008) Respiratory tract responses in male rats following subchronic acrolein inhalation. Inhalation Toxicology, 20(3): 205-216.
Dwivedi, A.M., Johanson, G., Lorentzen, J.C., Palmberg, L., Sjogren, B. and Ernstgard, L. (2015) Acute effects of acrolein in human volunteers during controlled exposure. Inhalation Toxicology, 27(14): 810-821.
Egle, J.L.,Jr (1972) Retention of inhaled formaldehyde, propionaldehyde, and acrolein in the dog. Archives of Environmental Health, 25(2): 119-124.
Egle, J.L.,Jr and Hudgins, P.M. (1974) Dose-dependent sympathomimetic and cardioinhibitory effects of acrolein and formaldehyde in the anesthetized rat. Toxicology and Applied Pharmacology, 28(3): 358-366.
Environment Canada and Health Canada (2000) Priority Substances List Assessment Report: Acrolein. Government of Canada, Ottawa, ON, Canada.
ERG (2005) Carbonyl recovery and stability in canisters. Eastern Research Group. EPA 68-D-00-264.
Farsalinos, K.E. and Gillman, G. (2018) Carbonyl emissions in e-cigarette aerosol: A systematic review and methodological considerations. Frontiers in Physiology, 8(JAN).
Farsalinos, K.E., Yannovits, N., Sarri, T., Voudris, V., Poulas, K. and Leischow, S.J. (2018) Carbonyl emissions from a novel heated tobacco product (IQOS): comparison with an e-cigarette and a tobacco cigarette. Addiction, 113(11): 2099-2106.
Feng, Z., Hu, W., Hu, Y. and Tang, M.S. (2006) Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proceedings of the National Academy of Sciences of the United States of America, 103(42): 15404-15409.
Feron, V.J. and Kruysse, A. (1977) Effects of exposure to acrolein vapor in hamsters simultaneously treated with benzo[a]pyrene or diethylnitrosamine. Journal of Toxicology and Environmental Health, 3(3): 379-394.
Feron, V.J., Kruysse, A., Til, H.P. and Immel, H.R. (1978) Repeated exposure to acrolein vapour: Subacute studies in hamsters, rats and rabbits. Toxicology, 9(1-2): 47-57.
Gilbert, N.L., Guay, M., Miller, J.D., Judek, S., Chan, C.C. and Dales, R.E. (2005) Levels and determinants of formaldehyde, acetaldehyde, and acrolein in residential indoor air in Prince Edward Island, Canada. Environmental Research, 99(1): 11-17.
Gillman, I.G., Kistler, K.A., Stewart, E.W. and Paolantonio, A.R. (2016) Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regulatory Toxicology and Pharmacology, 75: 58-65.
Hazari, M.S., Griggs, J., Winsett, D.W., Haykal-Coates, N., Ledbetter, A., Costa, D.L. and Farraj, A.K. (2014) A single exposure to acrolein desensitizes baroreflex responsiveness and increases cardiac arrhythmias in normotensive and hypertensive rats. Cardiovascular Toxicology, 14(1): 52-63.
Health Canada (2018a) Factsheet: Ventilation and the Indoor Environment. https://www.canada.ca/en/health-canada/services/publications/healthy-living/factsheet-ventilation-indoor-environment.html.
Health Canada (2018b) Factsheet: Cooking and Indoor Air Quality. https://www.canada.ca/en/health-canada/services/publications/healthy-living/factsheet-cooking-and-indoor-air-quality.html.
Health Canada (2017) Summary Document: 2012-2015 Indoor Air Reference Levels for Chronic Exposure to Volatile Organic Compounds. Cat.: H144-48/2017E-PDF ISBN: 978-0-660-23532-5, Government of Canada, Ottawa, Canada.
Health Canada (2016) Proposed Re-evaluation Decision: Acrolein. PRVD2016-16. Health Canada, Pest Management Regulatory Agency. Ottawa, Ontario.
Health Canada (2013) Edmonton Exposure Assessment Study: VOC Sampling Data Summary (2010). Health Canada, Ottawa, Ontario.
Health Canada (2012) Halifax Indoor Air Quality Study (2009): Volatile Organic Compounds (VOC) Data Summary. Health Canada, Ottawa, Ontario.
Health Canada (2010a) Regina Indoor Air Quality Study 2007: VOC Sampling Data Summary. Health Canada, Ottawa, Ontario.
Health Canada (2010b) Windsor Exposure Assessment Study (2005-2006): Data Summary for Volatile Organic Compound Sampling. Health Canada, Ottawa, Ontario.
Health Canada (2006) Residential Indoor Air Quality Guideline: Formaldehyde. http://www.hc-sc.gc.ca/ewh-semt/pubs/air/formaldehyde-eng.php.
Health Canada (1987) Exposure Guidelines for Residential Indoor Air Quality. Cat. No.: H46-2/90-156E.
Héroux, M.E., Clark, N., van Ryswyk, K., Mallick, R., Gilbert, N.L., Harrison, I., Rispler, K., Wang, D., Anastassopoulos, A., Guay, M., Macneill, M. and Wheeler, A.J. (2010) Predictors of indoor air concentrations in smoking and non-smoking residences. International Journal of Environmental Research and Public Health, 7(8): 3080-3099.
Herrington, J.S. and Hays, M.D. (2012) Concerns regarding 24-h sampling for formaldehyde, acetaldehyde, and acrolein using 2,4-dinitrophenylhydrazine (DNPH)-coated solid sorbents. Atmospheric Environment, 55: 179-184.
Herrington, J.S. and Myers, C. (2015) Electronic cigarette solutions and resultant aerosol profiles. Journal of Chromatography A, 1418: 192-199.
Ho, S.S.H., Ho, K.F., Liu, W.D., Lee, S.C., Dai, W.T., Cao, J.J. and Ip, H.S.S. (2011) Unsuitability of using the DNPH-coated solid sorbent cartridge for determination of airborne unsaturated carbonyls. Atmospheric Environment, 45(1): 261-265.
Ho, S.S.H., Yu, J.Z., Chu, K.W. and Yeung, L.L. (2006) Carbonyl emissions from commercial cooking sources in Hong Kong Journal of the Air & Waste Management Association, 56(8): 1091-1098.
Ho, S.S.H. and Yu, J.Z. (2002) Concentrations of formaldehyde and other carbonyls in environments affected by incense burning. Journal of Environmental Monitoring, 4(5): 728-733.
IARC (1995) Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, 63: 75.
Kane, L.E. and Alarie, Y. (1977) Sensory irritation to formaldehyde and acrolein during single and repeated exposures in mice. American Industrial Hygiene Association Journal, 38(10): 509-522.
Karavalakis, G., Bakeas, E., and Stournas, S. (2010) Influence of Oxidized Biodiesel Blends on Regulated and Unregulated Emissions from a Diesel Passenger Car. Environmental Science & Technology, 44(13): 5306-5312.
Kilburn, K.H. and McKenzie, W.N. (1978) Leukocyte recruitment to airways by aldehyde-carbon combinations that mimic cigarette smoke. Laboratory Investigation; a Journal of Technical Methods and Pathology, 38(2): 134-142.
Kimbell, J.S., Overton, J.H., Subramaniam, R.P., Schlosser, P.M., Morgan, K.T., Conolly, R.B. and Miller, F.J. (2001) Dosimetry modeling of inhaled formaldehyde: binning nasal flux predictions for quantitative risk assessment. Toxicological Sciences: An Official Journal of the Society of Toxicology, 64(1): 111-121.
Kimbell, J.S., Subramaniam, R.P., Gross, E.A., Schlosser, P.M. and Morgan, K.T. (2001) Dosimetry modeling of inhaled formaldehyde: comparisons of local flux predictions in the rat, monkey, and human nasal passages. Toxicological Sciences: An Official Journal of the Society of Toxicology, 64(1): 100-110.
Knighton, W.B., Herndon, S.C., Shorter, J.H., Miake-Lye, R.C., Zahniser, M.S., Akiyama, K., Shimono, A., Kitasaka, K., Shimajiri, H. and Sugihara, K. (2007) Laboratory evaluation of an aldehyde scrubber system specifically for the detection of acrolein. Journal of the Air and Waste Management Association, 57(11): 1370-1378.
Kurhanewicz, N., McIntosh-Kastrinsky, R., Tong, H., Ledbetter, A., Walsh, L., Farraj, A. and Hazari, M. (2017) TRPA1 mediates changes in heart rate variability and cardiac mechanical function in mice exposed to acrolein. Toxicology and Applied Pharmacology, 324: 51-60.
Kutzman, R.S. (1981) A subchronic inhalation study of Fischer 344 rats exposed to 0, 0.4, 1.4 or 4.0 ppm acrolein. BNL 30222, Brookhaven National Laboratory for the National Toxicology Program, Upton, NY, 11973.
Kutzman, R.S., Popenoe, E.A., Schmaeler, M. and Drew, R.T. (1985) Changes in rat lung structure and composition as a result of subchronic exposure to acrolein. Toxicology, 34(2): 139-151.
Kutzman, R.S., Wehner, R.W. and Haber, S.B. (1984) Selected responses of hypertension-sensitive and resistant rats to inhaled acrolein. Toxicology, 31(1): 53-65.
Lam, C.W., Casanova, M. and Heck, H.D. (1985) Depletion of nasal mucosal glutathione by acrolein and enhancement of formaldehyde-induced DNA-protein cross-linking by simultaneous exposure to acrolein. Archives of Toxicology, 58(2): 67-71.
Lau, C., Fiedler, H., Hutzinger, O., Schwind, K.H. and Hosseinpour, J. (1997) Levels of selected organic compounds in materials for candle production and human exposure to candle emissions. Chemosphere, 34(5-7): 1623-1630.
Le Bouffant, L., Martin, J.C., Daniel, H., Henin, J.P. and Normand, C. (1980) Action of intensive cigarette smoke inhalations on the rat lung. Role of particulate and gaseous cofactors. Journal of the National Cancer Institute, 64(2): 273-284.
Leach, C.L., Hatoum, N.S., Ratajczak, H.V. and Gerhart, J.M. (1987) The pathologic and immunologic effects of inhaled acrolein in rats. Toxicology Letters, 39(2-3): 189-198.
Lee, H.W., Wang, H.T., Weng, M.W., Chin, C., Huang, W., Lepor, H., Wu, X.R., Rom, W.N., Chen, L.C. and Tang, M.S. (2015) Cigarette side-stream smoke lung and bladder carcinogenesis: inducing mutagenic acrolein-DNA adducts, inhibiting DNA repair and enhancing anchorage-independent-growth cell transformation. Oncotarget, 6(32): 33226-33236.
Lee, H.-., Wang, H.-., Weng, M.-., Hu, Y., Chen, W.-., Chou, D., Liu, Y., Donin, N., Huang, W.C., Lepor, H., Wu, X.-., Wang, H., Beland, F.A. and Tang, M.S. (2014) Acrolein- and 4-Aminobiphenyl-DNA adducts in human bladder mucosa and tumor tissue and their mutagenicity in human urothelial cells. Oncotarget, 5(11): 3526-3540.
Leikauf, G.D. (1991) Mechanisms of aldehyde-induced bronchial reactivity: role of airway epithelium. Research Report (Health Effects Institute), 49: 1-35.
Li, Y., Cakmak, S., and Zhu, J. (2019) Profiles and monthly variations of selected volatile organic compounds in indoor air in Canadian homes: Results of Canadian national indoor air survey 2012-2013. Environment International, 126: 134-144.
Lijinsky, W. (1988) Chronic studies in rodents of vinyl acetate and compounds related to acrolein. Annals of the New York Academy of Sciences, 534: 246-254.
Lijinsky, W. and Reuber, M.D. (1987) Chronic carcinogenesis studies of acrolein and related compounds. Toxicology and Industrial Health, 3(3): 337-345.
Linhart, I., Frantik, E., Vodickova, L., Vosmanska, M., Smejkal, J. and Mitera, J. (1996) Biotransformation of acrolein in rat: excretion of mercapturic acids after inhalation and intraperitoneal injection. Toxicology and Applied Pharmacology, 136(1): 155-160.
Logue, J.M., Sleiman, M., Montesinos, V.N., Russell, M.L., Litter, M.I., Benowitz, N.L., Gundel, L.A. and Destaillats, H. (2017) Emissions from Electronic Cigarettes: Assessing Vapers' Intake of Toxic Compounds, Secondhand Exposures, and the Associated Health Impacts. Environmental Science & Technology, 51(16): 9271-9279.
Luo, C., Yang, L., Feng, Z., Li, Y., Long, J. and Liu, J. (2013) A cigarette component acrolein induces accelerated senescence in human diploid fibroblast IMR-90 cells. Biogerontology, 14(5): 503-511.
Lyon, J.P., Jenkins, L.J.,Jr, Jones, R.A., Coon, R.A. and Siegel, J. (1970) Repeated and continuous exposure of laboratory animals to acrolein. Toxicology and Applied Pharmacology, 17(3): 726-732.
Mahut, B., Delacourt, C., de Blic, J., Mani, T.M. and Scheinmann, P. (1993) Bronchiectasis in a child after acrolein inhalation. Chest, 104(4): 1286-1287.
McNulty, M., Heck, H.D. and Casanova-Schmitz, M. (1984) Depletion of glutathione in rat respiratory mucosa by inhaled acrolein. Fed Proc, 43: 575.
McRobbie, H., Phillips, A., Goniewicz, M.L., Smith, K.M., Knight-West, O., Przulj, D. and Hajek, P. (2015) Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prevention Research, 8(9): 873-878.
Moghe, A., Ghare, S., Lamoreau, B., Mohammad, M., Barve, S., McClain, C. and Joshi-Barve, S. (2015) Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicological Sciences: An Official Journal of the Society of Toxicology, 143(2): 242-255.
Mohammad, M.K., Avila, D., Zhang, J., Barve, S., Arteel, G., McClain, C. and Joshi-Barve, S. (2012) Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicology and Applied Pharmacology, 265(1): 73-82.
Morris, J.B. (1996) Uptake of Acrolein in the Upper Respiratory Tract of the F344 Rat. Inhalation Toxicology, 8(4): 387-403.
Morris, J.B., Symanowicz, P.T., Olsen, J.E., Thrall, R.S., Cloutier, M.M. and Hubbard, A.K. (2003) Immediate sensory nerve-mediated respiratory responses to irritants in healthy and allergic airway-diseased mice. Journal of Applied Physiology (Bethesda, Md.: 1985), 94(4): 1563-1571.
Murphy, S.D., Klingshirn, D.A. and Ulrich, C.E. (1963) Respiratory response of guinea pigs during acrolein inhalation and its modification by drugs. The Journal of Pharmacology and Experimental Therapeutics, 141: 79-83.
Nath, R.G., Ocando, J.E. and Chung, F.L. (1996) Detection of 1, N2-propanodeoxyguanosine adducts as potential endogenous DNA lesions in rodent and human tissues. Cancer Research, 56(3): 452-456.
[NPRI] National Pollutant Release Inventory (2017) Ottawa (ON): Government of Canada. Search results for acrolein. [accessed Dec 18, 2018]. https://pollution-waste.canada.ca/national-release-inventory/archives/index.cfm?do=query&lang=en.
NRC (National Research Council) (2001) Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
O'Brien, E., Spiess, P.C., Habibovic, A., Hristova, M., Bauer, R.A., Randall, M.J., Poynter, M.E. and van der Vliet, A. (2016) Inhalation of the reactive aldehyde acrolein promotes antigen sensitization to ovalbumin and enhances neutrophilic inflammation. Journal of Immunotoxicology, 13(2): 191-197.
Ott, M.G., Teta, M.J. and Greenberg, H.L. (1989) Lymphatic and hematopoietic tissue cancer in a chemical manufacturing environment. American Journal of Industrial Medicine, 16(6): 631-643.
Overton, J.H., Kimbell, J.S. and Miller, F.J. (2001) Dosimetry modeling of inhaled formaldehyde: the human respiratory tract. Toxicological Sciences: An Official Journal of the Society of Toxicology, 64(1): 122-134.
Pal, A., Hu, X., Zimniak, P. and Singh, S.V. (2000) Catalytic efficiencies of allelic variants of human glutathione S-transferase Pi in the glutathione conjugation of alpha, beta-unsaturated aldehydes. Cancer Letters, 154(1): 39-43.
Parent, R.A., Caravello, H.E. and Long, J.E. (1992) Two-year toxicity and carcinogenicity study of acrolein in rats. Journal of Applied Toxicology: JAT, 12(2): 131-139.
Parent, R.A., Caravello, H.E. and Long, J.E. (1991) Oncogenecity study of acrolein in mice. Journal of the American College of Toxicology, 10(6): 647-659.
Parent, R.A., Caravello, H.E. and Sharp, D.E. (1996) Metabolism and distribution of [2,3-14C]acrolein in Sprague-Dawley rats. Journal of Applied Toxicology: JAT, 16(5): 449-457.
Parent, R.A., Paust, D.E., Schrimpf, M.K., Talaat, R.E., Doane, R.A., Caravello, H.E., Lee, S.J. and Sharp, D.E. (1998) Metabolism and distribution of [2,3-14C]acrolein in Sprague-Dawley rats. II. Identification of urinary and fecal metabolites. Toxicological Sciences: An Official Journal of the Society of Toxicology, 43(2): 110-120.
Park, S.L., Carmella, S.G., Chen, M., Patel, Y., Stram, D.O., Haiman, C.A., Le Marchand, L. and Hecht, S.S. (2015) Mercapturic Acids Derived from the Toxicants Acrolein and Crotonaldehyde in the Urine of Cigarette Smokers from Five Ethnic Groups with Differing Risks for Lung Cancer. PloS One, 10(6): e0124841.
Penn, A., Nath, R., Pan, J., Chen, L., Widmer, K., Henk, W. and Chung, F.L. (2001) 1,N(2)-propanodeoxyguanosine adduct formation in aortic DNA following inhalation of acrolein. Environmental Health Perspectives, 109(3): 219-224.
Perez, C.M., Ledbetter, A.D., Hazari, M.S., Haykal-coates, N., Carll, A.P., Winsett, D.W., Costa, D.L. and Farraj, A.K. (2013) Hypoxia stress test reveals exaggerated cardiovascular effects in hypertensive rats after exposure to the air pollutant acrolein. Toxicological Sciences, 132(2): 467-477.
Possanzini, M. and Di Palo, V. (1996) Short-term measurements of acrolein in ambient air. Chromatographia, 43(7-8): 433-435.
Randall, M.J., Haenen, G.R., Bouwman, F.G., van der Vliet, A. and Bast, A. (2016) The tobacco smoke component acrolein induces glucocorticoid resistant gene expression via inhibition of histone deacetylase. Toxicology Letters, 240(1): 43-49.
Ritter, L., Totman, C., Krishnan, K., Carrier, R., Vézina, A. and Morisset, V. (2007) Deriving uncertainty factors for threshold chemical contaminants in drinking water. Journal of Toxicology and Environmental Health - Part B: Critical Reviews, 10(7): 527-557.
Roemer, E., Anton, H.J. and Kindt, R. (1993) Cell proliferation in the respiratory tract of the rat after acute inhalation of formaldehyde or acrolein. Journal of Applied Toxicology: JAT, 13(2): 103-107.
Roux, E., Hyvelin, J.M., Savineau, J.P. and Marthan, R. (1999) Human isolated airway contraction: interaction between air pollutants and passive sensitization. American Journal of Respiratory and Critical Care Medicine, 160(2): 439-445.
Schroeter, J.D., Kimbell, J.S., Gross, E.A., Willson, G.A., Dorman, D.C., Tan, Y.M. and Clewell III, H.J. (2008) Application of physiological computational fluid dynamics models to predict interspecies nasal dosimetry of inhaled acrolein. Inhalation Toxicology, 20(3): 227-243.
Schulte-Ladbeck, R., Lindahl, R., Levin, J.-. and Karst, U. (2001) Characterization of chemical interferences in the determination of unsaturated aldehydes using aromatic hydrazine reagents and liquid chromatography. Journal of Environmental Monitoring, 3(3): 306-310.
Seaman, V.Y., Bennett, D.H., and Cahill, T.M. (2007) Origin, occurrence, and source emission rate of acrolein in residential indoor air. Environmental Science &Technology, 41(20): 6940-6946.
Seaman, V.Y., Charles, M.J. and Cahill, T.M. (2006) A Sensitive Method for the Quantification of Acrolein and Other Volatile Carbonyls in Ambient Air. Analytical Chemistry, 78(7): 2405-2412.
Seaman, V.Y., Bennett, D.H. and Cahill, T.M. (2009) Indoor acrolein emission and decay rates resulting from domestic cooking events. Atmospheric Environment, 43(39): 6199-6204.
Shelow, D., Rice, J, Jones, M., Camalier, L, and Swift, J. (2009) Acrolein Measurements. Presentation at 2009 National Ambient Air Monitoring Conference, Nashville, TN.
Shusterman, D. (2011) The effects of air pollutants and irritants on the upper airway. Proceedings of the American Thoracic Society, 8(1): 101-105.
Sim, V.M. and Pattle, R.E. (1957) Effect of possible smog irritants on human subjects. Journal of the American Medical Association, 165(15): 1908-1913.
Spiess, P.C., Kasahara, D., Habibovic, A., Hristova, M., Randall, M.J., Poynter, M.E. and van der Vliet, A. (2013) Acrolein exposure suppresses antigen-induced pulmonary inflammation. Respiratory Research, 14: 107-9921-14-107.
Stevens, J.F. and Maier, C.S. (2008) Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Molecular Nutrition and Food Research, 52(1): 7-25.
Stocco, C., MacNeill, M., Wang, D., Xu, X., Guay, M., Brook, J. and Wheeler, A.J. (2008) Predicting personal exposure of Windsor, Ontario residents to volatile organic compounds using indoor measurements and survey data. Atmospheric Environment, 42(23): 5905-5912.
Struve, M.F., Wong, V.A., Marshall, M.W., Kimbell, J.S., Schroeter, J.D. and Dorman, D.C. (2008) Nasal uptake of inhaled acrolein in rats. Inhalation Toxicology, 20(3): 217-225.
Swift, J.L., Howell, M., Tedder, D. & Merrill, R. (2007) Collection and Analysis of Acrolein using Compendium Method TO-15. Presented at the QA Region 6 Conference, Dallas TX, October 2006.
Tejada, S.B. (1986) Evaluation of Silica Gel Cartridges Coated In Situ With Acidified 2, 4-Dinitrophenylhydrazine for Sampling Aldehydes and Ketones in Air. International Journal of Environmental Analytical Chemistry, 26(2): 167-185.
Thompson, L.C., Ledbetter, A.D., Haykal-Coates, N., Cascio, W.E., Hazari, M.S. and Farraj, A.K. (2017) Acrolein Inhalation Alters Myocardial Synchrony and Performance at and Below Exposure Concentrations that Cause Ventilatory Responses. Cardiovascular Toxicology, 17(2): 97-108.
Tully, M., Zheng, L., Acosta, G., Tian, R. and Shi, R. (2014) Acute systemic accumulation of acrolein in mice by inhalation at a concentration similar to that in cigarette smoke. Neuroscience Bulletin, 30(6): 1017-1024.
Uchiyama, S., Inaba, Y. and Kunugita, N. (2010) Determination of acrolein and other carbonyls in cigarette smoke using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine. Journal of Chromatography A, 1217(26): 4383-4388.
US EPA (1994) Methods for derivation of inhalation reference concentrations and application of inhalation dosimetry. EPA/600/8-90/066F.
US EPA (2003) Toxicological Review of Acrolein (107-02-8). In Support of Summary Information on the Integrated Risk Information System (IRIS). EPA/635/R-03/003, United States Environmental Protection Agency, Washington, D.C. http://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0364tr.pdf.
US EPA (2008) Reregistration Eligibility Decision: Acrolein. https://archive.epa.gov/pesticides/reregistration/web/pdf/acrolein_red.pdf.
US EPA (2010) Data Quality Evaluation Guidelines for Ambient Air Acrolein Measurements. https://www3.epa.gov/ttnamti1/files/ambient/airtox/20101217acroleindataqualityeval.pdf.
Wang, H.T., Hu, Y., Tong, D., Huang, J., Gu, L., Wu, X.R., Chung, F.L., Li, G.M. and Tang, M.S. (2012) Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. Journal of Biological Chemistry, 287(15): 12379-12386.
Wang, H.T., Weng, M.W., Chen, W.C., Yobin, M., Pan, J., Chung, F.L., Wu, X.R., Rom, W. and Tang, M.S. (2013) Effect of CpG methylation at different sequence context on acrolein- and BPDE-DNA binding and mutagenesis. Carcinogenesis, 34(1): 220-227.
Wang, H., Zhang, X. and Chen, Z. (2009) Development of DNPH/HPLC method for the measurement of carbonyl compounds in the aqueous phase: Applications to laboratory simulation and field measurement. Environmental Chemistry, 6(5): 389-397.
Wang, L., Sun, Y., Asahi, M. and Otsu, K. (2011) Acrolein, an Environmental Toxin, Induces Cardiomyocyte Apoptosis via Elevated Intracellular Calcium and Free Radicals. Cell Biochemistry and Biophysics, 61(1): 131-136.
Weber-Tschopp, A., Fischer, T., Gierer, R. and Grandjean, E. (1977) Experimentally induced irritating effects of acrolein on men (author's transl). International Archives of Occupational and Environmental Health, 40(2): 117-130.
WHO (2002) Acrolein - Concise International Chemical Assessment Document 43. World Health Organization, Geneva.
Won, D., Nong, G., Yang, W. and Collins, P. (2014) Material Emissions Testing: VOCs from Wood, Paint, and Insulation Materials. A1-000342.2, NRC Institute for Research in Construction, Ottawa, ON.
Zhang, S., Balbo, S., Wang, M. and Hecht, S.S. (2011) Analysis of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human leukocyte DNA from smokers and nonsmokers. Chemical Research in Toxicology, 24(1): 119-124.
Zhang, S., Villalta, P.W., Wang, M. and Hecht, S.S. (2007) Detection and quantitation of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human lung by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chemical Research in Toxicology, 20(4): 565-571.
Appendix A: List of acronyms and abbreviations
- ANSES
- Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (France)
- ATSDR
- Agency for Toxic Substances and Disease Registry
- BALF
- Bronchioalveolar lavage fluid
- BMC05
- Benchmark concentrations associated with a 5% increase of an effect
- CA
- Chromosome aberration
- CalEPA
- California Environmental Protection Agency
- CDC
- Centers for Disease Control and Prevention
- CEMA
- 2-Carboxethylmercapturic acid
- CFD
- Computational fluid dynamics
- CI
- Confidence interval
- DAF
- Dosimetric adjustment factor
- dG
- Deoxyguanosine
- DNA
- Deoxyribonucleic acid
- DNPH
- Dinitrophenylhydrazine
- GSH
- Glutathione (free or reduced)
- GST
- Glutathione-S-transferase
- HEC
- Human equivalent concentration
- 3-HPMA
- 3-Hydroxypropylmercapturic acid
- I/O
- Indoor/outdoor
- IARC
- International Agency for Research on Cancer
- IARL
- Indoor air reference level
- IP
- Intraperitoneal
- LC50
- Lethal concentration that kills 50% of the test population
- LOAEL
- Lowest observed adverse effect level
- LOD
- Limit of detection
- MDL
- Minimum detection limit
- NOAEL
- No observed adverse effects level
- OR
- Odds ratio
- OVA
- Ovalbumin
- PBPK
- Physiologically based pharmacokinetic
- PK
- Pharmacokinetic
- ppb
- Parts per billion
- ppm
- Parts per million
- RD50
- Concentration required to reduce the respiratory rate by 50%
- RfC
- Reference concentration
- RGDR
- Regional gas dose ratio
- RIAQG
- Residential Indoor Air Quality Guidelines
- SCE
- Sister chromatid exchange
- UF
- Uncertainty factor
- US EPA
- United States Environmental Protection Agency
- VOC
- Volatile organic compound
- WHO
- World Health Organization
Appendix B: Human exposure studies
| Study | Participants | Exposure | Results | NOAEL/LOAEL |
|---|---|---|---|---|
| Claeson and Lind 2016 | 26 volunteers (18 women, 8 men) | Eye-only exposure for 15, 45 or 60 min to 0.07, 0.16, 0.36 mg/m3 acrolein | Eye irritation in more than half the subjects at 0.36 mg/m3 for 15 min (starting at 6.8 min)
No difference at 0.16 mg/m3 for 45 min or 0.07 mg/m3 for 60 min |
NOAEL = 0.16 mg/m3
LOAEL = 0.36 mg/m3 (eye irritation) |
| Darley et al. 1960 | 36 volunteers (26 men, 10 women) | Eye-only exposure for 5 min to 0, 0.06, 1.3-1.6 or 2.0-2.3 ppm (0.14, 2.99-3.68 or 4.60-5.29 mg/m3) acrolein | Subjective measure of irritation (none = 0, medium = 1, severe = 2)
Average maximum irritation scores: air = 0.361, 0.06 ppm = 0.471, 1.3-1.6 ppm = 1.182, 2.0-2.3 ppm = 1.476 |
LOAEL = 0.14 mg/m3 (slight eye irritation at the lowest test concentration) Used in the derivation of an acute reference level by CalEPA (2008) |
| Dwivedi et al. 2015 | 18 volunteers (9 women, 9 men) | Whole body exposure for 2 hours to 0.05 or 0.1 ppm (0.12 or 0.23 mg/m3) acrolein | Eye blink frequency and irritation increased slightly at high but not low concentration.
No difference in breathing frequency, pulmonary function, nasal swelling, and inflammatory markers in blood or sputum. |
NOAEL = 0.12 mg/m3
LOAEL = 0.23 mg/m3 (slight eye irritation) |
| Weber-Tschopp et al. 1977 |
|
Whole body exposures:
|
|
LOAEL = 0.21 mg/m3 (slight eye irritation at the lowest test concentration) Used in the derivation of an acute exposure guideline limit by US EPA (2010) LOAEL = 0.69 mg/m3 (reduced respiration rate/irritation) Used in the derivation of an acute minimal risk level by ATSDR (2007) |
| Study | Participants | Exposure | Results | NOAEL/LOAEL |
|---|---|---|---|---|
| Annesi-Maesano et al. 2012 | 6590 children in six cities in France (401 classrooms in 108 primary schools) | Concentrations were measured "during class time" (does not specify for how long although a "5-day mean" concentration is mentioned elsewhere).
All concentrations were put into 3 tiers: low < LOD, medium = LOD-1.55 µg/m3, high > 1.55 µg/m3 (LOD not specified). |
Medical visit included skin prick test for allergies and test for exercise-induced asthma.
Health questionnaire completed by parents (rhinitis and asthma in the previous year). Confounders included passive smoking and family history (adjusted). Previous year rhinoconjunctivitis OR < 1 for medium/high, previous year asthma OR = 1.23 for medium, and OR = 1.22 for high. When population stratified by skin prick reactivity, acrolein significantly related to allergic asthma (atopic) (OR of 1.22 and 1.28 for medium and high, p = 0.1665) and negatively associated with non-allergic asthma (non-atopic) (OR of 0.94 and 0.60, p = 0.0741). The association with asthma was stronger during the spring-summer (OR 1.37). Acrolein was significantly correlated with exercise-induced asthma (p < 0.025). |
None derived Authors suggest that acrolein plays a role in asthma development in atopic children. |
| Billionnet et al. 2011 | 1012 individuals ( > = 15 years old) from 490 homes in France | Sampling in main (parents) bedroom for one week; used mean for analysis LOD 0.1, limit of quantification (LOQ) 0.3 µg/m3 0.8% homes < LOD, 3.5% between LOD and LOQ Range: < LOD to 12.9 µg/m3 Median = 1.0 µg/m3 3rd quartile = 1.51 µg/m3 Threshold value of above 3rd quartile of distribution (considered "elevated" high/low) |
Questionnaire on home characteristics, activity level/time at home
Questionnaire on asthma (previous year) and rhinitis (previous month)-diagnosis not confirmed by physician Controlled for confounders (including smoking, outdoor pollution, and pets) No relationship between acrolein and asthma (OR of 0.83, 95% CI 0.5-1.5) No relationship between acrolein and rhinitis (OR of 1.08, 95% CI 0.8-1.7) |
None derived Results suggest no relationship between acrolein exposure above 1.5 µg/m3 and asthma in the previous year. |
| deCastro 2014 | ~270 000 subjects in the U.S.
National Health Interview Survey (CDC) |
Examined ambient acrolein levels (2005 National Air Toxics Assessment-NATA-concentrations)
NATA: data from National Emissions Inventory and EPA's air monitoring and uses models to estimate outdoor concentrations (accounting for population density and physical topography of census tracts) then exposure concentrations (considers demographics, activity, climate)-from quintile 0.000138-0.010900 µg/m3 to quintile 0.0555101-0.457000 µg/m3. |
Interview of nationally representative cross-section of households, designed to produce national estimates of disease prevalence (i.e., estimation of asthma prevalence across the U.S. over time and age group). Included confounders (urban/rural, smoking). Self-reported asthma attacks in previous year (standard CDC definition for evaluating asthma attack prevalence). Exposures geographically linked at census tract level with residences of participants. Authors state "marginally significant" (p = 0.1) increase in asthma attack in the top quintile with OR 1.08 (95% CI 0.98-1.19) for all subjects, never smokers (OR 1.13), and never + former smokers (OR 1.09). No trend in lower 4 quintiles. |
None derived EPA estimates that outdoor acrolein is responsible for about 75% of non-cancer respiratory health effects attributable to air toxics in the U.S. |
Appendix C: Toxicological studies
| Study | Species, Sex and Number | Exposure | Results | NOAEL/LOAEL |
|---|---|---|---|---|
| Arumugan et al. (1999) | Male Wistar rats, 5 per group | 0, 1 or 2 ppm (2.3 or 4.6 mg/m3) acrolein for 4 hours (head only) | In lungs: reduced GSH, ascorbic acid, α-tocopherol; decreased activity of catalase, glutathione peroxidase; desquamized and mononuclear cells in bronchioles; hyperemia; emphysema | LOAEL = 2.3 mg/m3
(enzyme and cellular changes in respiratory epithelium at the lowest test concentration) |
| Cassee, Groten and Feron (1996) | Male Wistar rats, 5-6 per group | 0, 0.25, 0.67 or 1.4 ppm (0.57, 1.54 or 3.22 mg/m3) acrolein for 6 hours (nose only) | Decreased glutathione reductase activity in nasal respiratory epithelium
No nasal lesions or cell proliferation |
LOAEL = 0.57 mg/m3
(enzyme changes in respiratory epithelium at the lowest test concentration) |
| Lam et al. (1985) | Male Fischer 344 rats, 4 per group | 0, 0.1, 0.5, 1.0, 2.5, 5 ppm (0.23, 1.2, 2.3, 5.8, 11.5 mg/m3) acrolein for 3 hours (nose only) | Dose-dependent depletion of non-protein sulfhydryl groups in respiratory mucosa (significant from 1.2 mg/m3)
No DNA-protein cross linking in nasal mucosa |
LOAEL = 1.2 mg/m3
(depletion of sulfhydryl groups in respiratory mucosa) |
| Leikauf (1991) | Male Hartley guinea pigs, 5-7 per group | 0, 0.31, 0.67, 0.91, 1.26 ppm (0.7, 1.54, 2.1, 2.9 mg/m3) for 2 hours (whole body) | Bronchial hyperresponsiveness/increased airway resistance transient at 0.7 mg/m3, but remaining for at least 24 hours following cessation of exposure to 2.1 mg/m3
Histopathology of the trachea at 2.1 mg/m3, with recovery occurring within 24 hours |
LOAEL = 0.7 mg/m3
(bronchial hyperresponsiveness and increased airway resistance at the lowest test concentration) |
| Morris et al. (2003) | C57Bl/6J mice, 3-8 per group
Naive and sensitized (OVA) (number of males and females per group not specified) |
0.3, 1.6, 3.9 ppm (0.69, 3.7, 9.0 mg/m3) acrolein for 10 min (nose only)
(no separate control group) |
Decrease in breathing frequency; increase in airway flow resistance
Effects enhanced in mice previously sensitized by IP injection of OVA |
None derived; no control group
Effects seen at 0.69 mg/m3 (lowest test concentration) |
| Perez et al. (2013) | Spontaneously hypertensive (SH) and Wistar Kyoto normotensive (NT) male rats, 6 per group | 3 ppm (6.9 mg/m3) acrolein for 3 hours (whole body)
(no separate control group; 5 days between baseline test and exposure for individual animals) |
SH rats had increased heart rate, blood pressure, heart rate variability (only at hour 3), breathing frequency (only at hour 3), and minute volume (only at hour 3). NT rats had only increased blood pressure during acrolein exposure, but this increase was not as large as in SH rats. | None derived; no control group
Effects seen at 6.9 mg/m3 (lowest test concentration) |
| Roemer et al. (1993) | Male Sprague Dawley rats, 3-5 per group | 0, 0.2 or 0.6 ppm (0.46 or 1.4 mg/m3) acrolein for 6 hours (head only) | Increased DNA synthesis and cell proliferation in the nose, trachea and lung | LOAEL = 0.46 mg/m3
(proliferation in respiratory epithelium at the lowest test concentration) |
| Thompson et al. (2017) | Male C57Bl/6J mice, 6 per group | 0.3 or 3 ppm (0.7 or 6.9 mg/m3) acrolein for 3 hours (whole body)
(no separate control group; the 30 min acclimation period was considered baseline for individual animals) |
At high but not low concentration, decreased respiration frequency, increased tidal volume, increased lymphocytes in lungs (no change in total cells, neutrophils, eosinophils); increased stroke volume (20%) and cardiac output (10%) at 24-hour post-exposure; increased delay in cardiac cycle "dyssynchrony" (i.e., change in timing of contractions) at 1-hour and 24-hour post exposure At low but not high concentration, decreased heart rate (5% at 1-hour but not at 24-hour post-exposure), decreased myocardial performance (at 1- and 24-hour post-exposure) |
None derived; no control group
Effects seen at 0.7 mg/m3 (lowest test concentration) |
| Study | Species, Sex and Number | Exposure | Results | NOAEL/LOAEL |
|---|---|---|---|---|
| Borchers et al. (2008) | Female C57Bl/6J mice, 8 per group | 0, 0.5 or 2 ppm (1.2 or 4.6 mg/m3) acrolein, 6 hours per day for 1, 2 or 4 weeks (whole body) | Increased mucus index (high concentration, 2 weeks, 4 weeks)-mucus cell metaplasia in lungs
Increased BAL epithelial cells (airway damage) (high concentration, 1, 2, 4 weeks) Macrophage accumulation (low and high concentrations, 2 weeks and 4 weeks) |
LOAEL = 1.2 mg/m3
(changes in BALF at the lowest test concentration) |
| Bouley et al. (1976) | SPF OFA rats (3 male and 21 female) | Continuous exposure to 0 or 0.55 ppm (1.3 mg/m3) acrolein for 4 days prior to mating and for an additional 22 days after mating | No difference was found in number of pregnant animals, and number and weight of fetuses between exposed and control animals | NOAEL (reproductive/developmental toxicity) = 1.3 mg/m3 (single test concentration; limited endpoints examined) |
| Buckley (1984) | Male Swiss-Webster mice, 16-24 per group (8-10 in control) | 0 or 1.7 ppm (3.9 mg/m3) acrolein, 6 hours per day for 5 days (whole body) | Exfoliation and squamous metaplasia in respiratory epithelium, and moderate ulceration in the olfactory epithelium. No lesions in lower respiratory tract. | LOAEL = 3.9 mg/m3
(lesions in respiratory and olfactory epithelium at the lowest test concentration) |
| Cassee, Groten and Feron (1996) | Male Wistar rats, 5-6 per group | 0, 0.25, 0.67 or 1.4 ppm (0.57, 1.54 or 3.22 mg/m3) acrolein, 6 hours per day for 3 days (nose only) | Concentration-related histopathological changes (including disarrangement, necrosis, thickening, desquamation, and basal cell hyperplasia) in the nasal respiratory/transitional epithelium, but not in the olfactory epithelium. Severity of lesions increased with concentration.
Glutathione reductase decreased at low and mid concentrations. GST and aldehyde dehydrogenase were reduced at mid concentration. Increased cell proliferation at low and mid concentration. |
LOAEL = 0.57 mg/m3
(lesions in nasal respiratory epithelium at the lowest test concentration) Health Canada has previously derived a BMC05 of 0.14 mg/m3, which was used to derive the tolerable concentration. |
| Leach et al. (1987) | Male SD rats, 40 per group | 0, 0.1, 1.0 or 3.0 ppm (0.23, 2.3 or 6.9 mg/m3) acrolein, 6 hours per day, 5 days per week for 3 weeks | In the high concentration group, there was exfoliation, erosion, and necrosis in the respiratory epithelium of the nasal turbinates, squamous cell metaplasia, but no lung histopathology changes. | None derived
Only 12 rats from the control group and 12 from the high concentration group were used for histopathology testing. |
| O'Brien et al. (2016) | Male C57BL/6 mice | 0 or 5 ppm (11.5 mg/m3) acrolein, 4 hours per day, 4 days per week for 2 weeks
(+OVA, also OVA alone-inhaled) Challenged with 3 days inhaled OVA after 1 week recovery |
Exposure to acrolein increased OVA-specific IgG compared to OVA alone (acrolein promotes sensitization). Exposure to acrolein increased lung inflammation compared to OVA alone. No differences in lung leukocyte number, macrophage number or ILs, TNFα in BAL in any group. Also, there was no difference in lung tissue cytokine mRNA Increased lung IL-17F mRNA in acrolein exposed animals (+/- OVA). The authors state that this IL has been associated with asthma. |
None derived
Effects observed at 11.5 mg/m3 (single test concentration) |
| Roemer et al. (1993) | Male Sprague Dawley rats, 3-5 per group | 0, 0.2 or 0.6 ppm (0.46 or 1.38 mg/m3) acrolein, 6 hours per day for 3 days (head only) | Increase in cell proliferation in rat nasal, tracheal, and lung epithelium; effects were less pronounced than after a single exposure. | LOAEL = 0.46 mg/m3
(cell proliferation in respiratory epithelium at the lowest test concentration) |
| Spiess et al. (2013) | Male C57BL/6 mice sensitized to OVA by IP injection, 3-4 per group | 0 or 5 ppm (11.5 mg/m3) acrolein, 6 hours per day for 4 days (whole body)
(during challenge phase, animals were also exposed by inhalation to OVA for 30 min) |
Acrolein exposure reduced allergic airway inflammation (suppressed mucus production, leukocyte infiltration and cytokine levels). Decreased goblet cell hyperplasia was also noted. | None derived
Effects observed at 11.5 mg/m3 (single test concentration) |
| Study | Species, Sex and Number | Exposure | Results | NOAEL/LOAEL |
|---|---|---|---|---|
| Dorman et al. (2008) | Male Fischer 344 rats, 12 per group | 0, 0.02, 0.06, 0.2, 0.6 or 1.8 ppm (0, 0.05, 0.14, 0.46, 1.4 or 4.1 mg/m3) acrolein, 6 hours per day, 5 days per week for 13 weeks (whole body) Histopathology of respiratory tract conducted at days 4, 14, 30, and 65 as well as after a 60-day post-exposure recovery period. Lesions were graded on a scale of 1 to 5 (minimal to severe). Nasal cavity was divided into 6 sections. |
Some body weight reductions-significant (20%) in high concentration group. For all other exposed animals, the reduction was less but significant and remained throughout exposure. Partial recovery after exposure. Pathology of nasal respiratory epithelium: inflammation, hyperplasia, squamous metaplasia. Mild effects at 1.4 mg/m3 at day 4 and later. More severe effects at higher concentrations. At 4.1 mg/m3, effects were observed within days of starting exposure. At some sites, inflammation and hyperplasia were transient and were replaced by metaplasia, which persisted even after exposure stopped. Increased cell proliferation was observed at 1.4 or 4.1 mg/m3, but not 0.46 mg/m3. Inflammatory response in olfactory epithelium, degeneration and atrophy at 4.1 mg/m3, starting at day 4 |
NOAEL = 0.46 mg/m3
LOAEL = 1.4 mg/m3 (lesions in the respiratory epithelium of the nasal cavity) The NOAEL of 0.46 mg/m3 was considered the critical effect level for long-term exposure in the risk assessments of acrolein conducted by CalEPA (2008) and ANSES (2013). NOAEL = 1.4 mg/m3 LOAEL = 4.1 mg/m3 (inflammation in the olfactory epithelium) |
| Feron and Kruysse (1977) | Syrian golden hamsters, 18 per sex per exposure group Additional animals were also given NaCl and benzo(a)pyrene by tracheal instillation weekly, or N-nitrosodiethylamine by subcutaneous injection every 3 weeks. |
0 or 4 ppm (9.2 mg/m3) acrolein, 7 hours per day, 5 days per week for 52 weeks (whole body)
+/- recovery period of 29 weeks |
Decreased body weight, but difference began to disappear post-exposure
Inflammation and epithelial metaplasia in nasal cavity (slight to moderate). Lesions still observed after recovery period in 20% of animals. No respiratory tract tumours No clear enhancement of B(a)P induced tumours; no impact on DENA induced tumours |
None derived
No evidence of carcinogenicity at 9.2 mg/m3 |
| Feron et al. (1978) | Male and female Syrian golden hamsters (20 per group), Wistar rats (12 per group), Dutch rabbits (4 per group) | 0, 0.4, 1.4 or 4.9 ppm (0.9, 3.2, 11.3 mg/m3) acrolein, 6 hours per day, 5 days per week for 13 weeks
(whole body) Histopathology of respiratory system for all animals; lesions graded as slight, moderate or severe |
Significant weight gain reduction at high concentration for hamsters and rabbits, and mid and high concentration for rats
Nasal histopathology: at low concentration, one rat had metaplastic and inflammatory changes; at mid concentration, there were changes in rats (moderate; incidence not shown) and hamsters (slight), but not rabbits; at high concentration, rabbits moderate, rats and hamsters severe Tracheal epithelia histopathology: at high concentration, slight hyperplasia in rabbits; moderate hyperplasia and metaplasia in hamsters; and severe damage in rats Lung histopathology: at high concentration, severe hemorrhage, edema, bronchopneumonia, bronchitis, hyperplasia and metaplasia of bronchi, and wide range of degree of lesions between individuals in rats; similar effects in rabbits but less severe (moderate) than rats |
LOAEL = 0.9 mg/m3 (lowest test concentration)
US EPA considered 0.9 mg/m3 a minimal LOAEL, which was used to derive an RfC |
| Kutzman (1981); Kutzman et al. (1985); Costa et al. (1986) | Fischer 344 rats: 24 males per group for pulmonary function testing and lung pathology; 8 males per group for pathology only; 10 males per group for cytology; 8 males and 8 females per group for reproductive test | 0, 0.4, 1.4 or 4.0 ppm (0.9, 3.2 or 9.2 mg/m3) acrolein, 6 hours per day, 5 days per week for 62 exposure days, + 1 week recovery
(whole body) Histopathology on lung, trachea and nasal turbinates (no sections); lesions scored on 0-5 scale |
During exposure, body weights at high concentration were significantly less than controls; other concentration groups were not different from controls. At high concentration, mortality (bronchopneumonia), bronchiolar epithelial necrosis and sloughing, and bronchiolar and pulmonary edema were observed. Severity of tracheal edema was highly variable. There was decreased lung function, including in animals with no histologic lesions. There were no pulmonary lesions at mid concentration. Some rats had bronchiolar necrosis and hyperplasia, but functionally they were the same as controls. Some functional deficits were observed at low concentration, but no lung lesions. There were no sperm abnormalities, and no change in pregnancy rate, corpora lutea, viable embryos, fetal death or preimplantation loss. |
LOAEL = 0.9 mg/m3 (decreased lung function at the lowest test concentration)
The US EPA considered 0.9 mg/m3 support for a minimal LOAEL, which was used to derive an RfC. NOAEL = 9.2 mg/m3 (reproductive toxicity at the highest test concentration; limited endpoints examined) |
| Kutzman et al. (1984) | Female Dahl rats, hypertension susceptible (DS) and resistant (DR), 10 per group | 0, 0.4, 1.4 or 4.0 ppm (0.9, 3.2 or 9.2 mg/m3) acrolein, 6 hours per day, 5 days per week for 62 exposure days, + 1 week recovery
(whole body) |
At high concentration, all DS rats died within 11 days from severe airway epithelial necrosis with edema and haemorrhage. 4 out of 10 DR rats died by day 62; surviving rats lost weight and developed primarily a proliferative change in lung tissue.
At mid and low concentrations, both strains had mild bronchiolar epithelial hyperplasia and squamous metaplasia as well as acute inflammation in nasal turbinates. |
LOAEL = 0.9 mg/m3
(proliferative changes in respiratory tract and nasal inflammation at the lowest test concentration) DS strain more susceptible than DR at high concentration |
| Le Bouffant et al. (1980) | Female SD rats, 20 per group | 8 ppm (18.4 mg/m3) acrolein, 1 hour per day, 5 days per week for 10 or 18 months (whole body) | No tumours, no body weight change | None derived (study primarily on cigarette smoke inhalation) |
| Lyon et al. (1970) | Male and female SD rats (15 per group), male and female guinea pigs (15 per group), male monkeys (9 per group), male dogs (2 per group) |
0.7 or 3.7 ppm (1.6 or 8.5 mg/m3) acrolein, 8 hours per day, 5 days per week for 6 weeks 0.22, 1.0 or 1.8 ppm (0.5, 2.3 or 4.1 mg/m3) acrolein, continuously for 90 days No concurrent controls No nasal histopathology Descriptive only, no scoring of lesions |
6-week study: At 0.7 ppm, all animals had chronic inflammation (mild, focal to diffuse, no definite alteration of respiratory epithelium) and occasional emphysema (mild, patchy), which were more prominent in dogs and monkeys. In dogs and monkeys, significant morphologic changes in trachea (squamous metaplasia and basal cell hyperplasia). In monkeys, necrosis of bronchi, squamous metaplasia in lungs (bronchi), and repair and regeneration of bronchi epithelium. 2 monkeys died (days 6 and 9), but unclear if treatment related. 90-day study: At 0.22 ppm, dogs had moderate emphysema, acute lung congestion, focal vacuolization of bronchiolar epithelial cells, and some constriction of bronchioles. There were non-specific inflammatory changes in lungs of monkeys, guinea pigs, and dogs, and non-specific histopathology in rats (only lung and trachea, not nasal cavity, of only half the rats were examined). At 1 ppm, guinea pigs had pulmonary inflammation, and one dog bronchiolitis and early bronchopneumonia. At 1.8 ppm, monkeys had squamous metaplasia and basal cell hyperplasia in the trachea, and dogs bronchopneumonia. |
None derived (no concurrent controls) Effects observed at 0.5 mg/m3 (lowest test concentration) |
Appendix D: Other guidelines
D1. Short-Term Exposure Guidelines
In the Environment Canada and Health Canada's Priority Substances List Assessment Report: Acrolein, no guideline for short-term exposure to acrolein was derived (Environment Canada and Health Canada 2000).
For acute exposures, the California EPA (CalEPA) (2008) derived an acute (1 hour) reference exposure level of 2.5 µg/m3. This reference level is based on the geometric mean of effect levels for eye irritation in humans from the following two studies: a LOAEL of 138 µg/m3 in a study of 36 volunteers exposed (eye only) to acrolein for 5 minutes (Darley et al. 1960), and a LOAEL of 210 µg/m3 in a study of 53 volunteers exposed to increasing acrolein concentrations for 40 minutes (Weber-Tshopp et al. 1977). Uncertainty factors of 6 for the use of LOAELs and 10 for intraspecies variation were applied, giving a total UF of 60.
The US EPA (2010) derived an acute exposure guideline limit (AEGL-1) of 70 µg/m3 for non-disabling effects for timeframes of 10 minutes to 8 hours, based on eye irritation at 210 µg/m3 in humans exposed to increasing acrolein concentrations for 40 minutes (Weber-Tschopp et al. 1977). An uncertainty factor of 3 was applied to account for intraspecies variability.
In their pesticide evaluations, the US EPA (2008) and Health Canada's Pest Management Regulatory Agency (2016) derived a concentration of concern for short-term exposure of 7µg/m3, using a LOAEL of 210 µg/m3 for eye irritation with uncertainty factors of 10 for intraspecies sensitivity and 3 for lack of NOAEL, and a LOAEL of 700 µg/m3 for nasal and throat irritation with uncertainty factors of 10 for intraspecies sensitivity and 10 for lack of NOAEL.
ANSES (2013) derived a short-term exposure guideline of 6.9 µg/m3 for a 1-hour time frame, based on a LOAEL of 0.7 mg/m3 for eye, nose, and throat irritation in volunteers exposed to acrolein for 60 minutes (Weber-Tschopp et al. 1977). Uncertainty factors of 10 for the use of a LOAEL and 10 for intraspecies variability were applied, giving a total UF of 100.
ATSDR (2007) derived an acute (1 to 14 day) minimal risk level of 3 ppb (6.9 µg/m3), based on a LOAEL of 0.3 ppm (0.7 mg/m3) for an increase in eye, nose, and throat irritation, and a decrease in respiration rate in a study of 46 volunteers exposed to acrolein for 60 minutes (Weber-Tschopp et al. 1977). Uncertainty factors of 10 for the use of a LOAEL and 10 for intraspecies variation were applied, giving a total UF of 100.
| Organization | Exposure guideline | Health effect |
|---|---|---|
| California EPA (2008) | 2.5 µg/m3 (1 h) | Eye irritation |
| US EPA (2010) | 70 µg/m3 (10 min to 8 h) | Eye irritation |
| US EPA (2008), Health Canada (2016) | 7 µg/m3 | Eye, nose, and throat irritation |
| ANSES (2013) | 6.9 µg/m3 (1 h) | Eye, nose, and throat irritation |
| ATSDR (2007) | 6.9 µg/m3 (3 ppb) (1 to 14 days) | Eye, nose, throat, and respiratory irritation |
D2. Exposure Guidelines for Non-Neoplastic Chronic Effects
Previous assessments have developed proposed exposure limits for chronic or long-term acrolein exposure based on lesions in the nasal respiratory epithelium in rats.
The Government of Canada (Health Canada and Environment Canada 2000) derived a tolerable concentration of 0.4 µg/m3, based on a BMC05 of 0.14 mg/m3 from a 3-day study (Cassee, Groten and Feron 1996), which was adjusted for continuous exposure (6 hours/24 hours). Uncertainty factors of 10 for interspecies extrapolation and 10 for sensitive human populations were applied, giving a total UF of 100.
The US EPA (2003) derived an inhalation RfC of 0.2 µg/m3, based on a LOAEL of 0.9 mg/m3 from a 13-week rat study (Feron et al. 1978). The LOAEL was adjusted for continuous exposure (6 hours/14 hours and 5 days/7 days), and a human equivalent concentration (HEC) was calculated using an RGDR conversion factor of 0.13 (HEC = 0.02 mg/m3). This ratio accounts for pharmacokinetic but not pharmacodynamic differences between animals and humans; an uncertainty factor of 3 was also applied for pharmacokinetic differences between species. Uncertainty factors of 10 for sensitive human populations, 10 to account for the use of a subchronic study, and 3 for the use of a LOAEL were also applied, giving a total UF of 1000.
The CalEPA (2008) derived a chronic reference exposure level of 0.35 µg/m3, based on a NOAEL of 0.46 mg/m3 in a 13-week study (Dorman et al. 2008). The NOAEL was adjusted for continuous exposure (6 hours/14 hours and 5 days/7 days), and an HEC was calculated using a DAF of 0.85 based on a model for the analogue formaldehyde (HEC = 0.07 mg/m3). Uncertainty factors of 2 for pharmacokinetics (use of a DAF from a chemical analogue), 3 for pharmacodynamics, 10 for sensitive human populations, and 3 for use of a subchronic study, giving a total UF of 200.
ANSES (2013) also used the NOAEL of 0.46 mg/m3 from Dorman et al (2008) to derive a long-term exposure guideline of 0.8 µg/m3. No duration adjustment was made, and an HEC was calculated using an RGDR conversion factor of 0.13 (HEC = 60 µg/m3). This ratio accounts for pharmacokinetic but not pharmacodynamic differences between animals and humans; an uncertainty factor of 2.5 was also applied for pharmacokinetics. Uncertainty factors of 10 for sensitive human populations and 3 to account for the use of a subchronic study were also applied, giving a total UF of 75.
| Organization | Exposure guideline | Health effect |
|---|---|---|
| Health Canada and Environment Canada (2000) | 0.4 µg/m3 | Lesions in nasal respiratory epithelium |
| US EPA (2003) | 0.2 µg/m3 | Lesions in nasal respiratory epithelium |
| California EPA (2008) | 0.35 µg/m3 | Lesions in nasal respiratory epithelium |
| ANSES (2013) | 0.8 µg/m3 | Lesions in nasal respiratory epithelium |