Draft for consultation: Guidelines for Tanning Equipment Owners, Operators and Users
Table of Contents

Download the alternative format (PDF format, 1.35 MB, 37 pages)
Appendices
Acknowledgements
These guidelines have been reviewed by and published in collaboration with the Federal Provincial Territorial Radiation Protection Committee (FPTRPC). The FPTRPC comprises a forum of delegates from the following government organizations: Canadian Nuclear Safety Commission, Department of National Defence, Employment and Social Development Canada (Canada Labour Program), Health Canada and Provincial and Territorial radiation protection programs. The committee was established to support federal, provincial and territorial government radiation protection agencies with their respective mandates in Canada. The mission of the committee is to advance the development and harmonization of practices and standards for radiation protection within federal, provincial and territorial jurisdictions. We wish to thank those organizations for the many hours of research, review and consultation put into preparing the following Guidelines for Tanning Equipment Owners, Operators and Users.
FPTRPC Position Statement on Ultraviolet Radiation
Whereas,
- There is ample scientific evidence demonstrating that excessive exposure to ultraviolet radiation (UVR), from sunlight or from artificial sources, causes acute and chronic adverse health effects;
- The main organs affected by UVR are the skin and the eyes with increasing evidence indicating that UVR also acts as a systemic immunosuppressor;
- Exposure to solar and artificial UVR is widely recognized as an important, and preventable, cause of skin cancer;
- There is significant scientific evidence indicating that long-term exposure to UVR without adequate eye protection also plays a role in the development of some types of cataract and other eye and skin conditions;
- Artificial sources of UVR can be found in work and recreation environments and tanning equipment accounts for significant additional UVR exposure to users;
- The UVR dose to the population can be significantly decreased by applying simple strategies and measures to reduce sun exposure; and,
- The World Health Organization in 2009 classified UVR from tanning equipment as a Class 1 carcinogen and does not recommend the use of UV tanning devices for cosmetic purposes,
The FPTRPC does not endorse artificial tanning and recommends protective measures against excessive exposure to solar and artificial UVR, such as those contained in its overview document, be implemented by health, education, labour and recreation authorities in all provinces and territories and adopted by the general public. The FPTRPC further recommends that tanning and the use of tanning equipment, particularly by minors, be restricted.
Preface
There is no such thing as a safe or healthy tan as tanning results from the skin's natural defense response to damage caused by exposure to ultraviolet radiation (UVR). Exposure to ultraviolet A and B radiation can cause sunburn, premature skin ageing, skin cancers, cataracts and other eye and skin diseases. It has also been shown that UV can weaken the body's immune system.
Individuals choosing to use tanning equipment (sunlamp or tanning bed) should be aware of the hazards and health risks involved so they can make informed decisions about the amount of exposure they receive.
This guideline is designed to give tanning equipment owners, operators and users a fundamental understanding of UVR and its effects on health. It outlines general guidelines in the context of the risks associated with tanning and provides information on certain cosmetic and medicinal products that increase ultraviolet effects.
Only tanning equipment that complies with federal Radiation Emitting Devices Regulations (see Appendix D) should be used for tanning purposes. Owners and operators of tanning equipment should have knowledge of the regulations and refer to them as needed. This document provides further guidance on tanning equipment usage, however it should not be considered sufficient on its own and should not be considered as an endorsement for the use of tanning equipment. Operators are required to take any additional measures necessary to minimize health hazards in their establishment. Additional information is available through regional public health authorities.
This document supersedes the previous version published in 2014.
Tanning Safety Guidelines and Recommendations
Owners and operators of tanning equipment must be aware of and adhere to the requirements for tanning equipment as outlined under the Radiation Emitting Devices Regulations (Tanning Equipment) (Appendix D). In addition, owners, operators and users of tanning equipment should follow the guidelines listed below which have been developed specifically for their adherence.
General
- Children under 18 years of age should not use tanning equipment. Depending on provincial or territorial regulations, a minor may have restricted access to tanning equipment.
- All tanning equipment sold, resold, leased or imported into Canada must comply with the requirements specified for tanning equipment under the federal government's Radiation Emitting Devices Regulations (see Appendix D). Owners should check with their equipment supplier to ensure that tanning equipment and any associated apparatus being purchased and used by either themselves or by others, are in compliance with the Regulations.
- Knowledgeable tanning equipment operators or salon staff members should always be on the premises during business hours to inform and assist the public in the safer use of tanning equipment. Operators and their employees should be familiar with these guidelines and recommendations, as well as municipal and/or provincial/territorial public health and public safety regulations. Tanning salon operators should ensure that their employees receive adequate training including certification programs, where available, on the operation of tanning equipment. Training should include skin typing and exposure procedures.
- Tanning equipment operators should ascertain a client's ability to tan (i.e. skin type), history of sunburns and skin cancer, history of skin infections, rashes or other skin conditions (e.g. open wounds or lesions) as well as any photosensitizing agents (see Appendix C) they may be using prior to or during the time of exposure.
- Tanning salon operators should maintain client records. This information is to be used for exposure planning and to assist clients in understanding how the factors that impact one's ability to tan relate to UVR. Exposure schedules should comply with the Radiation Emitting Devices Regulations (see Appendix D).
- Users should avoid the use of infrared saunas or warming beds for at least 24 h following substantial sun exposures or tanning with artificial sources as it may increase the risk of developing skin cancer. Infrared radiation alone has been shown to induce erythema, skin pigmentation (in certain skin types) and skin photoaging, which can augment the harmful effects of ultraviolet radiation.
- Individuals with photosensitive skin, i.e. light-skinned Type I, who burn easily, severely, and/or never tan, should not use tanning equipment. Tanning equipment operators should inform prospective clients in this regard. Individuals with skin infections, rashes or other skin conditions should not use tanning equipment without first consulting a health care provider.
-
Prior to use of tanning equipment, the owner/operator of the tanning equipment should make users aware of the risks by users of tanning equipment including that a delayed, adverse reaction to UV exposure, such as red, irritated and watering eyes, an itching skin rash or sunburn may result. Delayed reactions can develop anywhere from minutes to a day and a half following exposure. In the event of a serious adverse reaction reported to the tanning establishment, the user should be advised to consult their health care provider. Upon receipt of notification of an adverse reaction, the owner/operator should investigate the incident and implement modifications as necessary.
All such incidents should be documented and made available to an appropriate regulatory official on request. Where an injury to an individual is reported to the owner/operator by a duly qualified health care provider as a result of an exposure to the tanning equipment under the owner's control, the owner shall inform the relevant health authority. See Appendix E for an example injury reporting template.
- Tanning beds are to be controlled by an onsite and trained operator. Self-serve unmanned machines are not to be used in a commercial environment.
- It is recommended that tanning equipment users be informed of these guidelines and advised to consider discussing the risks of artificial tanning with their health care providers.
UV Lamps (also referred to as UV Bulbs)
First and maximum exposure times suggested for different skin types depend on the strength and type of ultraviolet emissions from the lamps used in each individual piece of tanning equipment. There are many different models and brands of ultraviolet lamps available, producing various intensities and emitting different amounts of UVA and UVB radiation. Cases of overexposure and burns from UV radiation have occurred as a result of clients being exposed to tanning equipment which has had its original lamps replaced with newer, more powerful lamps that do not comply with federal regulations.
- All original or replacement ultraviolet lamps must function so that the UVC irradiance (i.e. 200 ≤ λ < 260 nm) does not exceed 0.003 of the UVB irradiance (i.e. 260 ≤ λ ≤ 320 nm)Footnote 1.
- Lamps in tanning equipment should never be replaced by lamps of different, often higher, levels of UVA and UVB than the original lamps. Operators should ensure that replacement lamps are identical or equivalent to the original lamps supplied with this piece of tanning equipment at the time of sale. In order to avoid injury, replacement ultraviolet lamps must function so that maximum exposure time remains within plus or minus 10% of the maximum exposure time originally recommended by the manufacturer. When replacement lamps are identical to the original ones, the client can rely on the manufacturer's information provided with tanning equipment.
- The operator should ensure that:
- The recommended maximum exposure time is not increased to compensate for decreasing UV intensity as lamps age.
- First and maximum exposure times comply with the manufacturer's recommendation. Users should be made aware that UVA tanning equipment exposure times are different than those recommended for higher intensity UVB equipment.
Protective Measures
- UVR warning labels, compliant with the Radiation Emitting Devices Regulations (Tanning Equipment), must be clearly visible on each piece of tanning equipment. Adherence to current labeling requirements, as specified in the Radiation Emitting Devices Regulations, is required for the sale of used tanning equipment with labels designed to warn users about UVR and its harmful effects on health. Labels should remain on the equipment as placed by the manufacturer. Provincial or territorial regulations for commercial tanning establishments may require the use of the latest federal warning label in some manner. Depending on provincial or territorial regulations, additional signage on equipment, doorways, or at point-of-sale may be required. A summary of these guidelines should be available within facilities or in the client reception area.
- Each tanning device must have a control to allow the person being exposed to easily turn off the tanning equipment at any time without the need to disconnect the electrical plug or remove the ultraviolet lamp (as per the Radiation Emitting Devices Regulations (Tanning Equipment)).
- The operator must provide, or make available for purchase, each client/customer with UVR safety eyewear that meets or exceeds the requirements in the Radiation Emitting Devices Regulations (Tanning Equipment) and covers the eyes securely. Instructions should be given on proper use.
- Protective eyewear used with sunlamps or tanning equipment must meet three criteria. The eyewear must have a spectral transmittance that is:
- not more than 0.001 over the wavelength range from 200 to 320 nm;
- not more than 0.01 over the wavelength range from 320 to 400 nm; and
- In addition, eyewear transmittance should be sufficient over wavelengths greater than 400 nm to enable the user to read the labels and use the controls mentioned in the requirements. According to the International Electrotechnical Commission Standard (IEC 60335-2-27, Edition 5.1, 2012-11; Table 101) the maximum transmission between 400 to 500 nm should not exceed 5 percent to in order to minimize retina damage due to extensive blue-light exposure.
- A physical barrier, such as a clear UV-transmitting plexiglass cover, should always be in place between the lamps and the individual being exposed to UV radiation, covering the top and bottom sections of a two-part, hinged tanning bed or covering the side sections of a vertically mounted booth. A physical barrier constructed of acrylic is also acceptable if it prevents direct physical contact between the user and the ultraviolet lamps in a horizontal device. This barrier will prevent injury to the user of the equipment in case of accidental lamp breakage. It will also guard against thermal burns from close contact with the lamps.
- Employees should always turn off tanning equipment during maintenance (e.g., changing UV lamps, cleaning equipment, etc.). In the event that the lamps must remain on, the employees should use protective eyewear and clothing to minimize exposure.
- Adequate ventilation should be provided such that the temperature of the tanning booth does not exceed 30 °C.
- Common contact surfaces, including protective eyewear, should be disinfected between each use, to prevent infection. Disinfectants should not leave films behind after use and the product used must state that it is an effective disinfectant against bacteria and viruses. The product must not damage the plastic in the protective eyewear or the plexiglass barrier. Tanning pillows should be replaced if the outer covering is compromised (i.e. cracked, ripped, split open exposing the inner foam which may encourage infection). Individuals may contact their Provincial/Territorial public health officials for further guidance.
What is Ultraviolet Radiation?
Electromagnetic radiation is the transmission of energy of various frequencies and wavelengths in the form of electromagnetic waves which can range from short wavelength, high energy, ionizing gamma rays to long wavelength, low energy, radio waves. Optical radiation is a form of non-ionizing radiation that is part of the electromagnetic spectrum that can exhibit properties of both waves and particles (photons) which may (e.g. visible light) or may not be (e.g. ultraviolet, infrared) detected by the human eye depending on its particular wavelength.
Ultraviolet (UV) radiation (rays) is defined as the part of the invisible electromagnetic spectrum with wavelengths ranging from 400 to 100 nm (nm stands for nanometer which is one billionth of a meter). While UVR has a longer wavelength and less energy than X-rays, it has a shorter wavelength and is more energetic than visible light. The ultraviolet part of the spectrum is divided into three components; UVA, (400 to 320 nm), UVB, (320 to 280 nm) and UVC (280 to 100 nm). Depending on its wavelength, UV radiation may trigger different biological effects and will penetrate skin tissue to different degrees (more deeply in the case of UVA than UVB or UVC). (See Figure 1.)
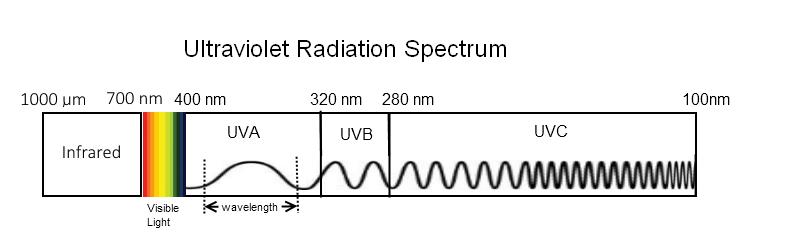
Text equivalent - Figure 1
This diagram depicts the region of the electromagnetic radiation spectrum representing ultraviolet radiation. Visible light and infrared radiation regions are also shown. Ultraviolet radiation is further divided into three portions: high energy ultraviolet C (with shorter wavelengths between 100 and 280 nanometers), ultraviolet B (with wavelengths between 280 and 320 nanometers), and finally lower energy ultraviolet A (with longer wavelengths between 320 and 400 nanometers). Visible light is the only region in the electromagnetic radiation that can be detected by the human eye and is seen as a variety of colours. These colours include violet, blue, cyan, green, yellow, orange and red, with each colour corresponding to a different wavelength. Violet has the shortest wavelength, starting around 400 nanometers, while red has the longest wavelength, ending around 700 nanometers. Wavelengths between 700 nanometers and 1000 nanometers (1 millimeter) make up the infrared region of the electromagnetic spectrum.
- UVA - ultraviolet A radiation (sometimes called "long wave" radiation) has a wavelength range of 320 to 400 nanometres which is closest to the "blue-end" of the light spectrum. UVA has to be 1000 times more intense than UVB to trigger the same erythema level, but UVA penetrates deeper into the dermis potentially causing damage to the underlying vascular system and dermal support structures. It is the most common radiation used in commercial tanning equipment and is responsible for the immediate darkening of the melanin already present in the skin. An intense exposure to UVA can result in burns in sensitive people. UVA rays can cause premature skin ageing due to their penetration in the dermis. Most tanning beds emit between 7-20 mW/cm² UVA, which is 3-8 times more than the sun at noon in the summer. Some facials could emit even more UVA in some cases emitting almost 30 times more than the noon-time summer sun.
- UVB - ultraviolet B radiation (sometimes called "short wave" radiation) has a wavelength range of 280 to 320 nanometres. UVB rays can penetrate the epidermis and are mostly responsible for sunburns (being 1000 times more erythemally effective than UVA) as well as for delayed tanning that appears within 2 or 3 days and lasts for a longer period of time. UVB is found at varying levels in all commercial types of tanning equipment. Always remember that the letter "B" in UVB reminds one of "burn". Some pieces of tanning equipment can emit as much as 10 times more UVB radiation than others so they can cause serious burns in a very short period of time.
- UVC - ultraviolet C radiation (100 - 280 nanometres) is very dangerous to all forms of life, even with only very short exposures. UVC radiation from the sun is completely absorbed by the ozone layer in the higher atmosphere and never reaches the earth's level. Modern tanning equipment should not emit UVC radiation or at least comply to the UVC/UVB ratio outlined in the Radiation Emitting Devices Regulations under Functioning Standards.
The sun is a major source of UVR, emitting mostly UVA and a small amount of UVB. Fortunately, the sun's emissions of the most energetic and harmful form of UVR, UVC, does not reach the Earth's surface. Other than natural sources, UVR can also come from a variety of different man-made products. Artificial sources may include certain lamps (e.g. incandescent, halogen, fluorescent, UV emitting LEDs etc), UV curing devices, welding equipment, UV lasers, and tanning equipment.
Tanning equipment contains tanning lamps that emit mostly UVA radiation with a small amount of UVB. UVR from a tanning lamp is often generated within the fluorescent bulb by exciting the atoms of an inert gas with electricity. Portions of unwanted ultraviolet can be converted into visible light by having it transfer its energy into the phosphors deposited on the inside of the bulb. The percentage of UVA and UVB can vary from bulb to bulb depending on manufacture, operating temperature, wattage, and lamp age.
Tanning Lamps
Low Pressure Lamps
Low pressure fluorescent lamps are the most common in the tanning industry and can vary in size, length and UV output, emitting UVA and UVB, and well as visible light and some heat (infrared). The amount of UVR from low pressure lamps can often exceed the sun's UV emissions several-fold.
High Pressure Tanning Lamps
High pressure lamps, also referred to as High Intensity Discharge (HID) lamps, are less common than low pressure fluorescent lamps and are relatively small in size in comparison. HID lamps produce UVR by means of an electric arc between tungsten electrodes within a quartz or alumina enclosure containing a pressurized gas with some metal salts. HID lamps emit considerably higher amounts of visible, UVA and UVB, than fluorescent lamps and also emit dangerous amounts of UVC radiation. As such, HID lamps require a filter during operation to prevent severe burning and skin damage. It is important to replace any cracked or damaged filters in order to prevent harm to the user. Typically, HID lamps are used for facial tanning and often have shorter exposure times due to their higher UV emissions. The manufacturer's exposure schedule should be adhered to when using HID lamps to prevent injury.
Skin and the UV-induced Tanning Process
Skin is the largest organ of the body; its primary purpose is to protect the underlying tissues against harm from the environment (e.g. infection, toxins, physical injury, UVR, etc.) while maintaining the internal environment (e.g. temperature, humidity, etc.). Skin is made up of basically two layers, the epidermis (outer layer) and the dermis (inner layer). The innermost section, or dermis, is formed of tissues containing nerves, blood vessels, lymphatics and fatty tissue. The outer layer, or epidermis, is made up of a series of layers. Skin cells, mostly consisting of keratinocytes, are created in the bottom or innermost layer of the epidermis. As cells age, they travel from the innermost layer of the epidermis to the outer surface of the skin where they die. The surface layer (or stratum corneum) forms a tough outer protective covering. As the cells move outward, they lose moisture, flatten and eventually flake off the surface of the skin. This process takes about 28 days. It is the epidermis that is primarily involved in the tanning process.
Skin Types
Knowledge of skin type is important when discussing tanning as tanning ability depends on skin type. Skin types are often described using the Fitzpatrick skin type classification system. This system helps to identify an individual's skin sensitivity to UVR based on that individual's natural skin colour. However photosensitivity is not only determined by constitutive skin pigmentation but is subject to other factors including genetic disposition for photosensitivity independent of skin colour, history of skin cancer, frequency of UV exposure, and use of agents (e.g. drugs, cosmetics etc) that may increase one's photosensitivity (refer to Appendix C).
Fitzpatrick classification is grouped into 6 skin types, ranging from very fair (skin type I) to very dark (skin type VI) and is described as follows:
- Skin type I: Always burns, never tans, extremely sensitive skin. Examples of this skin type are often people with red hair, fair to pale skin, freckles and blue eyes. People with this skin type should not use tanning equipment.
- Skin type II: Usually burns, tans minimally. Examples of this skin type are often people with fair-haired, fair skin, and blue or hazel eyes.
- Skin type III: May burn, tans well. This skin type is represented by the average Caucasian with fair skin.
- Skin type IV: Rarely burns, tans well. Exhibits immediate pigment darkening. Examples of this skin type are often people with dark brown hair, naturally tinted or light brown pigmented skin, and dark coloured eyes.
- Skin type V: Very rarely burns, tans easily and well, exhibits immediate pigment darkening. Examples of this skin type are often people with dark brown hair, brown to dark pigmented skin, and dark coloured eyes.
- Skin type VI: Very rarely burns, tans easily and well, exhibits immediate pigment darkening. Examples of this skin type are often people with very dark hair, very dark skin, and dark coloured eyes.
Although subjective, Fitzpatrick skin type classification is used in artificial tanning to determine an individual's exposure schedule and maximum exposure time based on their skin's natural colour to avoid injury from burning or erythema (skin reddening) but still deliver a sufficient UVR dose necessary to develop and maintain a tan.
For this reason, exposure limits to tanning lamps vary for different skin types but also depends on the strength and type of ultraviolet emissions from the light lamps used in each individual piece of tanning equipment. Tanning equipment manufacturers must determine the appropriate exposure schedules and recommended exposure times for each of their products. In addition, manufactures are required to carry specific information for all pieces of tanning equipment. This includes information about first and maximum exposure times based on the user's skin type, the recommendation that the total maximum annual dose of 15 kilojoules per square metre (kJ/m²) must not be exceeded, and the recommendation that the highest dose per session should never exceed 625 Joules per square metre (J/m²) to allow for the maintenance of a desired tan.
It is the responsibility of the operator of the tanning equipment to determine the appropriate exposure time based on the user's skin type by referring to the guide/label provided by the manufacturer for that particular tanning device.
Tanning from Exposure to Ultraviolet Radiation
There are two effects that occur in the skin following exposure to UV radiation. The first effect is referred to as immediate tanning or immediate pigment darkening (IPD) which is a change in skin pigmentation immediately or shortly after exposure to UVR (primarily by UVA). This results from photo-oxidation and darkening of the melanin pigment that is already present in the epidermis. This tan is only temporary, and fades within 3 to 36 hours after exposure.
A second process known as "delayed tanning" occurs in most individuals when the skin is exposed to UVB radiation, but not in people who lack the ability to produce sufficient amounts of melanin in the skin (i.e. Skin type I). There are two processes involved in delayed tanning.
- First, melanocytes (skin cells capable of producing melanin pigment) are produced at the base of the epidermis with each melanocyte producing melanosomes containing the melanin pigment. These melanin containing units begin to spread throughout the layers of the skin as they work their way toward the keratinocytes at the surface of the skin. Melanin-containing cells cause the skin to appear darker in colour.
- Second, the tough outer surface layer of dying skin cells thickens and absorbs more of the hazardous shortwave UVB radiation.
The above processes can take place over the course of a day and produce a noticeable tan within a few days that can last for weeks or even months. Recognizing that a tan is a response to skin damage, the damage has already been done and therefore the protection a tan may offer is insufficient to adequately protect you from further UV damage.
Exposure Schedules
The exposure schedule is designed to allow a client to gradually build a tan, while minimising the risk of erythema. As per the Regulations, the initial dose must not to exceed 100 J/m², weighted according to the ISO 17166:1999 (CIE S 007/E:1998) erythema action spectrum, for the first exposure session for untanned skin, gradually increasing over the following sessions to a maximum of 625 J/m² per session. The total amount of time (minutes) of exposure must correspond to the annual recommended dose of 15 kJ/m², weighted according to the CIE erythema action spectrum. The schedule is based on the skin type of the user and the output of lamps in the tanning unit. The exposure schedule displayed on the equipment provided by the equipment manufacturer should be followed for each particular product and skin type.
The recommended schedule within the international standard IEC 60335-2-27 Ed. 5.1 2012-11 follows that the initial dose should not exceed 100 J/m² for untanned skin and that a waiting period of 48h should be respected between the first and second exposure to allow time to check for unexpected side effects. If any skin reddening (erythema) is visible 16-24h after any exposure, then further exposures should cease and may be continued the following week with the advice of a health care provider. The recommended dose for the second exposure should not exceed 250 J/m². Gradual increments of approximately 100 J/m² per week can be used until a maximum of 625 J/m² per session is reached. It is also recommended that a single tanning course, consisting of a consecutive series of exposures, should not exceed a total dose of 3 kJ/m², weighted according to the erythema action spectrum as suggested by the international standard IEC 60335-2-27 Ed. 5.1 2012-11 and that the annual dose does not exceed 15 kJ/m². Note: avoid sunbathing and the use of the tanning appliance on the same day.
For more information regarding exposure scheduling, refer to the IEC 60335-2-27 Ed. 5.1 2012-11.
Risks of UV-Induced Tanning
Ultraviolet radiation may provide some limited health benefits. For example, it can be used to treat certain skin conditions and specific autoimmune diseases, and it helps form vitamin D in our bodies. However, there is no such thing as a safe or healthy tan. Whether from the sun or from tanning equipment UVR emitted from tanning equipment can pose a risk to one's health.
Overexposure to UV has been linked to the following negative health outcomes:
- sunburns
- premature skin aging
- skin/eye cancer
- eye damage
- weakening of the immune system
The risks associated with UV exposure outweigh the benefits as there is no clear threshold between positive and negative health effects and an individual's response depends on a number of biological or physical variables which are different from person to person and subject to environmental conditions. For example, darkening of the skin (tanning) from exposure to UV is a biological response to UVR damage. The degree of tanning is dependent on a number of biological factors, one of which is the person's skin type. Studies indicate that individuals who frequently tan are at greater risk of developing skin cancer later in life since the damage from UV exposure is cumulative and there is an escalating risk with total hours, sessions or years of tanning equipment use. Age of first use is a factor with both an increased risk of developing melanoma and early onset of the disease being linked to age of first use of tanning equipment.
Adverse health effects from overexposure to UVR include the following:
Sunburn
Sunburn (or erythema) is an inflammatory redness of the skin caused by too much exposure to UV radiation, particularly UVB radiation. The small blood vessels in the skin dilate and increase blood flow to the skin's surface, making it red and painful. This reaction can be almost immediate in severe cases, or may develop up to 24 hours later in less severe cases of overexposure. Delayed reactions, depending on the severity of the burn, include the skin shedding the upper layer of damaged skin and the formation of fluid-filled blisters that may break. These responses to UV may render the skin more susceptible to infection. Keep in mind, individuals with open wounds or lesions are particularly sensitive to UV radiation as the epidermal layer that helps protect against UV damage has been compromised. Studies indicate that people who have suffered severe and frequent sunburns during childhood are at greater risk of developing skin cancer later in life.
Phototoxic and Photoallergic Reactions
A phototoxicity and photoallergy reaction is often an abnormal skin reaction to exposure from light or to UVR. Photoallergic reactions can manifest after 24 to 72 hours following exposure to both a drug and light/UV often resulting in a rash resembling dermatitis. However, phototoxic reactions are more common and can occur rapidly following exposure, often resembling a severe sunburn, and its severity is dependent on the amount of the applied substance or photo-radiation. Examples of photoxic and photoallergic reactions include, but are not limited to, hydroa vacciniforme, actinitic prurigo, solar urticarial (solar 'hives' erythema), and polymorphic light eruption (allergic reaction to UV). Also, pre-existing conditions such as chronic actinic dermatosis, atopic eczema and psoriasis can be exacerbated by either solar or artificial UVR exposure. Individuals with lupus erythematosus, porphyria, or xeroderma pigmentosum are particularly sensitive and are encouraged not to use tanning equipment. In addition, individuals who have the inability to tan or are deemed photosensitive should avoid tanning equipment. A number of drugs and/or cosmetics have also been shown to have photoxic or photoallergic effects. The list of products that can cause photosensitivity is extensive and is thereby described in Appendix C.
Premature Skin Aging
UVR causes actinic elastosis or premature ageing effects such as skin wrinkling, thinning of the skin, pigmentary change and loss of elasticity giving it a more leathery appearance. UVR can also cause the development of actinic keratoses which are scaly or crusty skin growths that resemble warts, often appearing on UV-exposed areas such as the face, scalp, lips, and the back of the hands. If left untreated, these growths may become cancerous.
Skin Cancer
Skin cancer is the most common form of cancer in Canada. In 2013, there were an estimated 81,700 new cases of non-melanoma skin cancers and 6,000 new cases of melanoma--the most dangerous form of skin cancer. More than ever, skin cancer seems to occur in younger individuals. Non-melanoma skin cancers, such as squamous and basal cell carcinomas, are the most common, but are rarely fatal. In most cases, they are caused by UV exposure. Melanoma is a less common, but potentially deadly, type of skin cancer. The main factors that predispose an individual to the development of melanoma seem to be recreational exposure to either solar or artificial UV radiation, history of sunburn or a history of skin cancer.
Non-melanoma skin cancer
Squamous Cell Carcinoma (SCC) is an uncontrolled growth of abnormal squamous cells, which compose most of the skin's upper layers of the epidermis. SCC is more dangerous but not as common as Basal Cell Carcinoma. However, SCCs are less harmful than melanoma. SCCs may occur on any part of the body but may spread to other parts of the body if not treated promptly. SCCs can be identified as elevated growths with a central depression, scaly red patches, open sores, or have a wart-like appearance that may easily bleed, crust or ulcerate. It most often found on areas on skin that is frequently exposed to the sun.
Basal Cell Carcinoma (BCC) is the most common form of non-melanoma skin cancer but is the least fatal of all the skin cancers. Its appearance may be that of a red, pale or pearly colored round or flattened lump or scaly area that may form an ulcer with time. It tends to grow slowly, usually on areas on skin that is frequently exposed to the sun.
Melanoma skin cancer
Melanoma is the third most common type of skin cancer and the most deadly; comprising of cancerous melanocytes (cells containing the melanin pigment). There are two types of melanoma: superficial spreading melanoma and nodular melanoma. Superficial spreading melanoma is the most common form which tends to stay within its area of origin before growing radially across the skin surface. Nodular melanoma is a more invasive form of melanoma that may develop from superficial melanoma, growing downwards through the skin towards the basal layers. Exposure to UVR and heredity plays a role in the development of melanoma.
In 2009, international experts from the World Health Organization's (WHO) International Agency for Research on Cancer (IARC), found significant evidence of an association between artificial tanning and the development of non-melanoma and melanoma skin cancer types. They also determined that the use of tanning equipment can increase the risk of developing cutaneous melanoma by 75% if the age of first exposure was prior to 35 years of age. In light of the scientific evidence, the WHO placed UVR-emitting tanning equipment into its highest cancer risk category to a Group 1 ("carcinogenic to humans"). The risk of developing skin cancer also increases as total exposure to UVR increases, meaning there is an escalating risk with total hours, sessions or years of tanning equipment use. People with fair skin who burn easily are also more at risk of developing skin cancer. The WHO, IARC, the International Commission on Non-ionizing Radiation Protection (ICNIRP), and the Scientific Committee on Consumer Products (SCCP) to the European Commission have recommended that the use of tanning equipment should be avoided for children, adolescents and in individuals who have a diminished ability to tan.
Eye Problems
UVR may cause painful temporary injuries to the cornea and conjunctiva called photokeratitis and photoconjunctivitis. These conditions may develop from 2 to 24 hours after exposure, but usually occur within 6 to 12 hours. UVA radiation may cause eye ageing effects, such as browning of the lens and its loss of elasticity. Overexposure to UVB seems to be partly responsible for the appearance of cataracts in the lens. Repeated exposures can lead to the development of skin cancer around the eyes, pre-cancerous growths (e.g. pterygium) of the outer white part of the eye or may lead to conjunctival cancer or intraocular melanoma. Vision loss due to macular degeneration is also a risk factor but is mostly caused by prolonged exposure to blue light in the visible spectrum which is often a component of non-exclusive UV emitting sources.
Weakened Immune System
There is a scientific consensus that exposure to UV radiation weakens the immune system. UV-induced immunosuppression can take place both locally (i.e. skin) and systemically (i.e. the whole body). This could potentially decrease the body's ability to defend itself against infection and serious illnesses, including skin cancer (i.e. non-melanoma and malignant melanoma) and other non-skin related types of cancer. The immunosuppressive nature of UV can be used to treat different autoimmune diseases. For example, mycosis fungoides (also known as Alibert-Bazin syndrome or granuloma fungoides) is a form of cutaneous T-cell lymphoma that can be treated with UVR exposure under controlled conditions. Similarly, phototherapy can be a treatment option for some types of eczema; an inflammatory skin condition.
Appendix A
| Wavelength | UVC | UVB | UVA |
|---|---|---|---|
| 100-280 nm | 280-320 nm | 320-400 nm | |
| Photon | more energetic | less energetic | |
| Sources |
|
|
|
| Depth of Penetration |
|
|
|
| Effects |
|
|
|
Appendix B
Glossary of Terms
- Actinic elastosis
- Also known as solar elastosis is a disorder associated with photoaging and refers to the accumulation of thickened malformed elastin fibers in the dermis in response to long-term exposure to UVR. The disorder is characterized by thickened, dry, rough, yellowish-colored, skin with deep creases and fine wrinkles.
- Actinic keratosis
- Also known as solar keratosis which is often identified as rough, scaly elevated lesions (resembling a wart) caused by repeated exposure to the UVR from the sun and as a result can be often found on frequently sun exposed areas of the skin. If left unchecked these lesions may develop into skin cancer.
- Delayed tanning
- A type of tanning produced by UVB appearing a few days after exposure and lasting up to a few weeks. This process increases the number of melanocytes in the skin. At the same time, these melanocytes increase their production of melanin which then is oxidized giving the tanned appearance.
- Dermis
- Lowest (innermost) layer of cells in the skin under the epidermis.
- Epidermis
- Outer layers of skin in which melanin is found, and where tanning occurs.
- Erythema
- The medical term for inflammatory redness of the skin. It is the result of an exposure to UVR, particularly UVB. It is commonly called "sunburn".
- Fitzpatrick skin type
- A skin type classification system based on one's natural skin colour (I-VI) that is used to determine tanning ability or photosensitivity.
- Immediate tanning
- Also known as immediate pigment darkening (IPD) is a photo-oxidation process (mainly triggered by UVA) that causes rapid darkening of the melanin pigment already present in the skin with the change in pigmentation often lasting only for a short time (hours to days).
- MED
- Minimum Eythemal Dose is a measure of the accumulated erythemally weighted UV energy that would cause the first perceptible sign of reddening (i.e., erythema). The energy-equivalent depends on the skin type. For the most sensitive skin types, 1 MED is approximately 2 SED. For skin type II 1 MED is approximately 2.5 SED.
- Melanin
- Pigment in the skin that becomes darker when oxidised under the effect of UVR.
- Melanoma
- Also known as Cutaneous Malignant Melanoma (CMM) is the most serious (potentially fatal) but rarest form of skin cancer that occurs in melanocytes (pigment containing cells in the skin).
- Non-Melanoma Skin Cancer (NMSC)
- The most common type of skin cancer that occurs in either basal or squamous cells. Of the two types of non-melanoma skin cancers, Basal Cell Carcinoma (BCC) is the most common often occurring on frequently sun exposed areas of the body and develops in the cells lining the bottom of the epidermis near the dermis. Squamous Cell Carcinoma (SCC) develops in the cells lining the top of the epidermis and is also commonly found on frequently exposed areas of the skin but can spread to other parts of the body.
- Photokeratitis and photoconjunctivitis
- Painful injuries to the cornea (the clear front window of the eye that covers the iris and pupil) and conjunctiva (a thin transparent membrane that covers white part of the eye) caused by overexposure to UVR that can be avoided by wearing protective eyewear.
- Photosensitivity
- A person's biological sensitivity or reactivity to visible light or UVR.
- Pterygium
- A benign growth of the conjunctiva that can invade the cornea which can affect vision.
- SED
- Standard Erythemal Dose is a measure of the accumulated erythemally-weighted UV energy, where 1 SED = 100 J m −2
- Stratum corneum
- Tough outer layer of dead skin cells.
- Ultraviolet (UV) radiation
- Defined as the part of the nonionizing invisible electromagnetic spectrum which ranges from 400 nm to 100 nm. Tanning occurs as a result of exposure to UVR.
Appendix C
Products that Increase Sensitivity to Ultraviolet Radiation
Many products, including prescribed medications, over-the- counter medicines, and a wide range of personal care products can increase the skin's sensitivity to UV radiation, also known as photosensitivity. This is an intense reaction of the skin to UVR which can cause burning (or erythema) in a much shorter period than would normally be expected.
Photosensitivity can be caused by products applied directly to the skin or from medications or other substances that have been ingested. Examples include antidepressants, antibiotics, antihistamines, psoralens, antifungals, antidiabetic, oral contraceptives, tranquilizers, high blood pressure medications, and certain soaps or cosmetics.
List of Photosensitizing Medications
The following list identifies medications and other agents that have been reported to cause photosensitivity reactionsFootnote 2. However, it is provided for information purposes only, may not include all the photosensitive medications and may be subject to change. As there are hundreds of known photosensitizing agents under the following general categories, individuals taking any medications or using any products (some listed below), should be advised to consult a health care provider or pharmacist before using tanning equipment. Pharmaceutical manufacturer's package inserts should also be consulted regarding potential photosensitivity of medications.
* An asterisk is shown next to a drug where photosensitive reactions are more likely to occur.
Anti-cancer
- Capecitabine
- * Dacarbazine
- Dasatinib tyrosine kinase inhibitor
- Farmorubicin
- Fluorouracil (5-FU)
- Flutamide
- Imatinib
- Paclitaxel
- Porphyrins and Metalloporphyrins:
- Photofrin
- Levulan
- Metvix
- Visudyne and others
- Mesna
- Methotrexate
- Tegafur-Uracil (UFT)
- Vinblastine
Anti-depressants
- Amitriptyline
- Amoxapine
- Citalopram
- Clomipramine
- Desipramine
- Doxepin
- Escitalopram
- Fluvoxamine
- Fluoxetine
- Imipramine
- Maprotiline
- Nortriptyline
- Paroxetine
- Phenelzine
- Protriptyline
- Sertraline
- Trazodone
- Trimipramine
- Venlafaxine
Anti-histamines
- Cetirizine
- Cyproheptadine
- Dioxopromethazine
- Diphenhydramine
- Isothipendyl
- Mequitazine
- Trimeprazine
Anti-hypertensives
- Captopril
- Diltiazem
- Enalapril
- Methyldopa
- Minoxidil
- Nifedipine
- Ramipril
- Valsartan
- Verapamil
Anti-inflammatory/anti-arthritic medications
- Celecoxib
- Diclofenac
- Diflunisal
- Hydrocortisone
- Ibuprofen
- Indomethacin
- Ketoprofen
- Mesalazine
- Nabumetone
- Naproxen
- Phenylbutazone
- * Piroxicam
- Sulindac
- Tiaprofenic acid
Anti-microbials
- Fluoroquinolones:
- Ciprofloxacin
- Fleroxacin
- Levofloxacin
- * Lomefloxacin
- Moxifloxacin
- * Nalidixic Acid
- Norfloxacin
- Ofloxacin
- Perfloxacin
- Sparfloxacin
- * Tetracyclines:
- * Demethylchlortetracycline
- * Doxycycline
- Lymecycline
- Oxytetracycline
- Minocycline
- Tetracycline
- Others:
- Chloramphenicol
- Cotrimoxazole
- Dapsone
- Erythromycin
- Griseofulvin
- Pyrazinamide
- Sulfonamides
- Trimethoprim
- Hexachlorophene
Anti-parasitic medications
- Chloroquine
- Halogenated salicylanilides
- Ketoconazole
- Quinine
- * Voriconazole
Anti-psychotic medications
- * Phenothiazines:
- * Chlorpromazine
- Fluphenazine
- Perphenazine
- * Prochlorperazine
- * Promethazine
- * Thioridazine
- Trifluoperazine
- Flupentixol
- Haloperidol
- Olanzapine
- Thioxanthene
Contraceptives, oral and Estrogens (birth control medication, female sex hormones)
- Chlorotrianisene
- Diethylstilbestrol
- Estradiol
- Estrogens, conjugated and esterified
- Ethinyl estradiol
- Medroxyprogesterone
- Megestrol
- Norethindrone acetate
Diuretics
- Acetazolamide
- Amiloride
- * Chlorothiazide
- * Furosemides
- * Hydrochlorothiazide
- Metolazone
- Triamterene
Hypoglycemics and Anti-diabetics
- Chlorpropamide
- Glibenclamide
- Sitagliptin
- Sulfonylureas
- * Tolbutamide
Sunscreens
- Avobenzone
- Benzophenones
- Cinnamates
- Homosalate
- Oxybenzone
- * PABA esters
- * Para-aminobenzoic acid
Others
- Alprazolam
- Amantadine
- * Amiodarone
- Amlodipine
- Atorvastatin
- Azathioprine
- Benzocaine
- Benzoyl peroxide
- * Bergamot oil, oils of citron, lavender, lemon, lime, sandalwood, cedar (used in many perfumes and cosmetics; also topical exposure to citrus rind oils)
- Bumetanide
- Carbamazepine
- Chlordiazepoxide
- Clofibrate
- Clopidogrel
- Desoximetasone
- Disopyramide
- Eculizumab
- Efavirenz
- Epidermal Growth Factor receptor inhibitors:
- Afatinib
- Cetuximab
- Erlotinib
- Gefitinib
- Lapatinib
- Panitumumab
- Vandetanib
- Ethinyl estradiol
- * Etretinate
- Fenofibrate
- Fluorescein
- Gold Salts
- Hydroxychloroquine
- Hydroxyurea
- Indapamide
- Isoniazid
- * Isotretinoin
- Itraconazole
- Leflunomide
- * 6-methylcoumarin (used in perfumes, shaving lotions and sunscreens)
- * Musk ambrette (used in perfumes)
- Paracetamol
- Pravastatin
- Phenytoin
- Pilocarpine
- Pirfenidone
- Pyridoxine
- Quinapril
- Quinidine sulphate and gluconate
- Ranitidine
- Simvastatin
- * Tretinoin
- Vemurafenib
- * Psoralens,
- Trioxsalen
- Methoxsalen
- Coal tar
Appendix D
Radiation Emitting Devices Act - Radiation Emitting Devices Regulations (Tanning Equipment)
A summary of the federal Radiation Emitting Devices Act and Radiation Emitting Devices Regulations for tanning equipment is presented here for general information only. Complete and current copies of the regulations are available at http://laws-lois.justice.gc.ca/eng/regulations/C.R.C.,_c._1370/.
Note to reader: the remainder of the section is unchanged from the previous version and is not part of the consultation.
Appendix E
Tanning Injury Report (Sample)
Tanning Facility Information
- Name
- Telephone
- Address
- City
- Province
- Postal Code
- Operator on duty at time of injury
Owner/Proprietor Information
- Name
- Telephone
- Address
- City
- Province
- Postal Code
Tanning Equipment Information
- Name of Manufacturer
- Model Number
- Serial Number
- Date of Manufacture
- Date of Purchase
- Name of Seller/Distributer
- Type/Model of ultraviolet lamps/bulbs used in device
Injury Information
- Name of injured party (or parent of minor)
- Telephone
- Address
- City
- Province
- Postal Code
- Date of injury
- Duration of UV exposure
- Name of attending physician, if applicable
- Diagnosis/Treatment
- Describe event/injury (attach supplemental sheet if necessary)
Reporting Information
- Date injury reported
- Name of person taking Complaint
- Corrective Action/Repairs Made (including dates and persons responsible)