Module 3: Strategies to promote and support mandatory reporting

Download the alternative format
(PowerPoint format, 1.1 MB, 29 pages)
Organization: Health Canada
Published: 2019-12-13
Module 3 - Learning Outcomes
Completion of Module 3 will enable you to:
- Identify potential barriers to serious ADR and MDI reporting
- Recognize opportunities to facilitate serious ADR and MDI documentation and reporting
- Describe strategies and systems to support implementation of serious ADR and MDI documentation and reporting
- Describe the shared commitment to health product safety and recognize the key partners involved
Module 3 - Outline
- Barriers and Opportunities to Facilitate Reporting
- Strategies and Systems to Support Implementation
- Shared Commitment to Health Product Safety
- Key Points to Remember
- Abbreviations
- Resources
Conceptual Model of Serious ADR and MDI Reporting by Hospitals
Module 3 describes strategies to promote and support mandatory reporting.
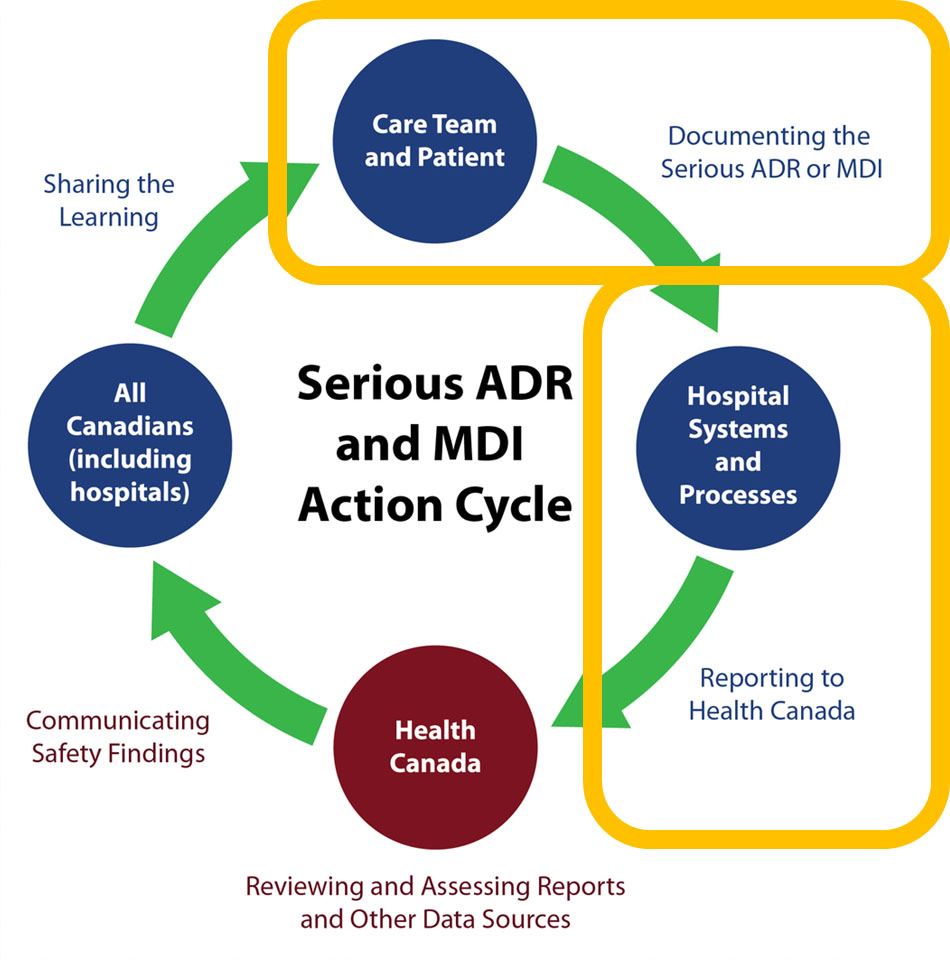
Source: Serious ADR and MDI Action Cycle. ISMP Canada, HSO, CPSI; 2019.
Text description
A circle diagram depicts the action cycle for serious ADR and MDI reporting by hospitals. In summary, the figure illustrates that the care team and patients document the serious ADR or MDI. Reporting a serious ADR or MDI to Health Canada is supported by hospital systems and processes. Health Canada reviews and assesses reports and other data sources. Health Canada communicates safety findings to all Canadians, including hospitals, who are shown to share the learning. This concludes the action cycle.
The diagram superimposes two yellow rectangle shape around specific parts of the action cycle.
The first yellow rectangle is placed around:
- The care team and patient that document the serious ADR or MDI
The second yellow rectangle is placed around:
- Hospital systems and processes that support reporting to Health Canada
Barriers and Opportunities to Facilitate Reporting
Potential Barriers to Reporting
- Resource and time limitations, such as work schedule and staff complement in healthcare settings
- Lack of familiarity with the reporting process and the what, when, and how to report
- Lack of awareness of the value of reporting
- Lack of technology supports to simplify the process of reporting
- Complacency and the assumption that serious ADRs and MDIs have previously been reported
- Uncertainty about the association of a drug or medical device with patient harm
- Fear of consequences and the perception that reporting might have negative repercussions
- Limited feedback since follow-up information may not be available or accessible to those who report
Self-Assessment Questions for Hospitals
The following self-assessment questions may be helpful to identify opportunities to prepare for the implementation of mandatory reporting within your hospital:
- In what way is serious ADR and MDI documentation and reporting promoted?
- Are our hospital policies and procedures aligned with the mandatory reporting requirements?
- Can our hospital systems be leveraged to facilitate documentation and reporting?
- How can awareness and knowledge of serious ADR and MDI documentation and reporting be improved?
- Has our hospital leadership created an environment that supports serious ADR and MDI documentation and reporting?
- Are patients and families empowered to ask questions and be engaged in monitoring their treatments?
- Is serious ADR and MDI documentation and reporting included in orientation or education programs?
- Is the learning or feedback derived from serious ADR and MDI reports shared with health care providers?
Strategies and Systems to Support Implementation
Note: Health Canada does not endorse any particular strategy or system.
The following examples, in alphabetical order, are provided for information sharing only.
Strategic and Operational Considerations for Teams
- Create a multidisciplinary team (e.g., ‘Safety Team’) to support serious ADR and MDI documentation and reporting, and assist with:
- identifying serious ADRs and MDIs through proactive monitoring;
- completing and submitting serious ADR and MDI reports;
- disseminating learning from serious ADR and MDI reporting;
- providing coaching and education;
- enabling continuous quality improvement for serious ADR and MDI reporting processes; and
- providing regular updates to senior leadership.
- Identify individual ‘Champions’ to help lead serious ADR and MDI reporting efforts and/or provide support for identifying and submitting serious ADR or MDI reports to Health Canada.
- Identify networking opportunities to support collaboration and shared commitment to serious ADR and MDI documentation and reporting.
EXAMPLE: Province-Wide Collaboration- Alberta Health Services
- A centralized provincial approach has been developed for serious ADR reporting and for improved MDI reporting.
- Provincial Task Force is in place to coordinate the initiative
- Includes centralized process to receive, review and further report
- Alberta is implementing a province-wide clinical information system
- All sites will use a single electronic health record
- There is a phased roll out across the province over five years
- Serious ADR reporting will be integrated into the electronic health record
- MDI reporting will be linked from the electronic health record, as well as from the Alberta Health Services intranet
Source: Alberta Health Services; 2019.
EXAMPLE: Province-Wide Collaboration - British Columbia
- The BC Patient Safety & Learning System (BCPSLS) has partnered with the BC Ministry of Health, health authorities, and Health Canada to create a provincial strategy for implementing the mandatory reporting requirements.
- The provincial strategy includes:
- the use of an established, province-wide incident reporting system (BCPSLS) to report ADRs and MDIs;
- a steering committee and working groups to guide and implement the approach; and
- a communication and education campaign to promote reporting.
- Members of the steering committee and working groups include representatives from BCPSLS, BC Ministry of Health, Health Canada, pharmacy, biomedical engineering, supply chain, and quality, safety and risk.
Source: British Columbia Patient Safety and Learning System Central Office; 2019.
EXAMPLE: Province-Wide Collaboration - Newfoundland and Labrador Health Authorities
- In Newfoundland and Labrador, the Department of Health and Community Services has partnered with the four regional health authorities in a steering committee to create a provincial strategy for implementing the mandatory reporting requirements.
- The Provincial Steering Committee’s mandate includes:
- establishing the most effective and efficient method for mandatory reporting,
- promoting interdisciplinary collaboration in mandatory reporting, and
- implementing an educational approach.
- Team members of the steering committee include representatives from quality, patient safety and risk management, pharmacy services, nursing, physicians, biomedical services, and information technology.
Source: Department of Health and Community Services, Newfoundland and Labrador; 2019.
EXAMPLE: An Individual Hospital’s Approach - Quebec (CHU Sainte-Justine)
Goal: Collaboration between the pharmacist and medical archivist to lead implementation of mandatory serious ADR reporting.
- A pharmacovigilance coordinator (pharmacist)
- manages the reporting of serious ADRs to Health Canada, and
- reviews and disseminates risk communications from Health Canada.
- A medical archivist (i.e., medical records staff) codes serious ADRs from notes in the patient’s medical record and sends the information to the Pharmacovigilance Coordinator.
Notes:
- A serious ADR may be missed if it is not well documented in the medical record. Providing training to health care providers about documenting ADRs is useful.
- A process to document a serious ADR identified by the medical archivist after discharge is also helpful.
Source: CHU Sainte-Justine, Quebec; 2019.
Strategic and Operational Considerations for Technology
- Integrate the reporting process into workflow and technology systems to make reporting as effective and efficient as possible.
- Explore opportunities to incorporate serious ADR and MDI reporting in electronic health record (or electronic medical record) systems
- Explore opportunities to incorporate serious ADR and/or MDI reporting in electronic incident reporting systems
- Explore opportunities to use the pharmacy information system to record serious ADRs and facilitate reporting to Health Canada
- To facilitate serious ADR and MDI traceability to a specific product, technology systems and documentation practices may need to accommodate product-specific identifiers:
- Drug identification number (DIN) for drugs, disinfectants and biologics / biosimilars
- Device identifier, catalogue number or model name for medical devices
- Brand name for all health products
- Manufacturer’s name for all health products
- Explore the feasibility of support from a designated team (e.g., health record team, patient safety team, or risk management team) to track serious ADRs and MDIs from the hospital’s documentation/coding system(s).
EXAMPLE: Hospital Reporting System for Serious ADRs and MDIs - Alberta Health Services
ADR Reporting
- ADR reporting functionality will be fully integrated into the electronic health record
- Applies to sites that have implemented the new electronic medical record software
- The existing online provincial reporting and learning system will be used as an interim approach
- Applies to sites that have not yet implemented the new electronic medical record software
MDI Reporting
- There are centralized Medical Device Safety support teams to:
- receive incident report forms;
- assess, investigate, and act on reports; and
- track and trend for early detection of larger-scale issues.
- There will be a link to the Medical Device Incident or Problem report form in the electronic health record and the Alberta Health Services intranet.
Source: Alberta Health Services; 2019
EXAMPLE: Hospital Reporting System for ADRs and MDIs - British Columbia Patient Safety & Learning System (BCPSLS)
The BCPSLS is a provincial online reporting system that includes ADR and MDI reporting
Source: British Columbia Patient Safety and Learning System; 2019.
EXAMPLE: Hospital Reporting System for ADRs - British Columbia Research Project
- ActionAde is a software application designed to facilitate ADR reporting and integrate with electronic medical records.
- ActionAde project goals are:
- Improve data to meet the needs of researchers, drug regulators, and decision-makers, and assist health institutions in complying with new federal serious ADR reporting requirements
- Integrate documentation and reporting with existing healthcare practices, electronic medical records, and a provincial database (planned for 2020 with PharmaNet)
- Improve information sharing across healthcare providers (e.g., pharmacists, emergency physicians and general practitioners), and locations (e.g., hospitals and community based pharmacies)
- Provide timely alerts to warn and inform clinicians about a previous ADR (e.g., when clinicians try to prescribe or dispense a medication that previously caused harm)
Source: ActionADE
EXAMPLE: Using ICD-10-CA Diagnostic Coding to Support Serious ADR and MDI Tracking
- Facilities across Canada capture administrative, clinical, and demographic information on all hospital stays for reporting to the Canadian Institute for Health Information’s (CIHI) Discharge Abstract Database (DAD).
- Upon a patient’s discharge, the health record is reviewed and coded, according to national standards, using ICD-10-CA.
- ICD-10-CA is the enhanced Canadian version of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems.
- ICD-10-CA contains codes for diseases, signs and symptoms, abnormal findings, complaints, social circumstances, and external causes of injury or diseases.
- ICD-10-CA coding can be leveraged to support serious ADR and MDI tracking.
- Adverse effect categories in ICD-10-CA:
Adverse effect category codes Definitions for adverse effect categories Additional details about the adverse effect categories Y40-Y59 Adverse effects during therapeutic use of a drug, medicament or biological substance These external causes codes are assigned when the drug/substance is used as prescribed or intended in therapeutic use. Y70-Y82 Adverse events with medical devices These external cause codes are assigned exclusively for unexpected malfunctioning or breakage of a device. - There are some gaps in what is captured to meet DAD reporting requirements and what is needed to comply with mandatory reporting requirements:
- CIHI’s DAD ICD-10-CA coding does not capture specific drug/device names, therefore this information would need to be obtained from the patient’s health record for mandatory reporting.
- CIHI’s DAD ICD-10-CA coding is based on physician*-documented adverse effects that cause harm.
- CIHI’s DAD ICD-10-CA coding is based on actual events and would not identify potential for serious harm in the case of medical device incidents.
*Mandatory reporting regulations do not require physician confirmation of a serious ADR or MDI.
Source: Canadian Institute for Health Information; 2019.
Strategic and Operational Considerations for Education
- Refer to Health Canada’s resources:
- Include education about serious ADR and MDI reporting in:
- orientation programs,
- student teaching programs and curricula, and
- continuing education programs.
- Empower patients and families to ask questions and be engaged in monitoring their treatments
- Share tips for recognizing a serious ADR or MDI with health care providers
- Consider if symptoms experienced by the patient might be due to a serious ADR or MDI
- Develop a process to regularly share within the hospital (e.g., newsletters, intranet) Health Canada’s safety findings related to serious ADRs and MDIs
Shared Commitment to Health Product Safety
Shared Commitment to Health Product Safety
Hospital Leadership
- Ensure processes are implemented to support the requirement for mandatory reporting of serious ADRs and MDIs
- Encourage a culture of safety
Health Care Providers
- Discuss potential harms with patients; identify and document serious ADRs and MDIs; report according to hospital policies and procedures
- Contribute to the planning and implementing of safety initiatives, including education
Patients and Consumers
- Report a serious ADR or MDI to a health care provider, manufacturer and/or Health Canada with as much detail as possible
Health Canada
- Operate Canada's product vigilance system and ensure that methods are in place to collect and evaluate serious ADR and MDI data
- Manage the risks and communicate new health product information to stakeholders to enable informed decisions
Culture of Safety
A culture of safety is defined as the underlying beliefs and values of an organization as they relate to safety as a priority.Footnote 1 A culture of safety facilitates reporting:
- A positive, supportive work environment that recognizes the value of, and acknowledges, reporting efforts can increase the likelihood of reporting.
- Trust, transparency, and open communication, when harm from drugs or medical devices is identified, can shape a health care provider’s approach to reporting.
- Partnering with patients and families is an important component of a culture of safety.
Key Partners in Health Product Safety
Key Partners include:
- International Monitoring Organizations
- Federal and Provincial Governments
- Health Care Product Manufacturers
- Health Care Provider Educators
- Health Care Organizations
- Patients and Consumers
- Health Care Providers
- Media
Key Points to Remember
- Potential barriers to serious ADR and MDI reporting include resource and time limitations, unfamiliarity, uncertainty, unawareness, lack of technology supports, complacency, fear of consequences and limited feedback.
- Strategic and operational considerations (for teams, technology, and education) include strategies and systems to support implementation of serious ADR and MDI documentation and reporting.
- A shared commitment to health product safety includes many key partners with important and complementary roles.
Abbreviations
- ADR:
- Adverse Drug Reaction
- BC:
- British Columbia
- BCPSLS:
- British Columbia Patient Safety & Learning System
- CIHI:
- Canadian Institute for Health Information
- CHU:
- Centre hospitalier universitaire
- DAD:
- Discharge Abstract Database
- DIN:
- Drug Identification Number
- ICD-10-CA:
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Canada
- MDI:
- Medical Device Incident
Resources
- Mandatory reporting of serious adverse drug reactions and medical device incidents by hospitals - Guidance document
- Protecting Canadians from Unsafe Drugs Act (Vanessa’s Law) Amendments to the Food and Drugs Act (Bill C-17)
- Regulations Amending the Food and Drug Regulations (Serious Adverse Drug Reaction Reporting - Hospitals): SOR/2019-190
- Regulations Amending the Medical Devices Regulations (Medical Device Incident Reporting - Hospitals): SOR/2019-191
For additional information, please contact the Canada Vigilance Program at:
Email: hc.canada.vigilance.sc@canada.ca
Telephone: 1-866-234-2345
Acknowledgments
- All materials were developed by the collaborating parties: Health Canada, Institute for Safe Medication Practices Canada (ISMP Canada), Health Standards Organization (HSO), and the Canadian Patient Safety Institute (CPSI).
- Any stakeholder interested in using the materials should acknowledge Health Canada as the owner and source:
Educational Support for Mandatory Reporting. Health Canada; 2019.
- Footnote 1
-
Culture of Safety Defined. ISMP Canada, HSO, CPSI; 2019.
Page details
- Date modified: