Information for Health Care Professionals: Cannabis (marihuana, marijuana) and the cannabinoids
Dried or fresh plant and oil for administration by ingestion or other means
Psychoactive agent
This document has been prepared by the Cannabis Legalization and Regulation Branch at Health Canada to provide information on the use of cannabis (marihuana) and cannabinoids for medical purposes. This document is a summary of peer-reviewed literature and international reviews concerning potential therapeutic uses and harmful effects of cannabis and cannabinoids. It is not meant to be comprehensive and should be used as a complement to other reliable sources of information. This document is not a systematic review or meta-analysis of the literature and has not rigorously evaluated the quality and weight of the available evidence nor has it graded the level of evidence. Despite the similarity of format, it is not a Drug Product Monograph, which is a document which would be required if the product were to receive a Notice of Compliance authorizing its sale in Canada.
This document should not be construed as expressing conclusions or opinions from Health Canada about the appropriate use of cannabis (marihuana) or cannabinoids for medical purposes.
Cannabis is not an approved therapeutic product, unless a specific cannabis product has been issued a drug identification number (DIN) and a notice of compliance (NOC). The provision of this information should not be interpreted as an endorsement of the use of this product, or cannabis and cannabinoids generally, by Health Canada.
Prepared by Health Canada
Date of latest version: Spring 2018
Reporting Adverse Reactions to Cannabis (marihuana, marijuana) Products
Reporting adverse reactions associated with the use of cannabis and cannabis products is important in gathering much needed information about the potential harms of cannabis and cannabis products for medical purposes. When reporting adverse reactions, please provide as much complete information as possible including the name of the licensed producer, the product brand name, the strain name, and the lot number of the product used in addition to all other information available for input in the adverse reaction reporting form. Providing Health Canada with as much complete information as possible about the adverse reaction will help Health Canada with any follow-ups or actions that may be required.
Any suspected adverse reactions associated with the use of cannabis and cannabis products (dried, oils, fresh) for medical purposes should be reported to the Canada Vigilance Program by one of the following three ways:
- Report online
- Call toll-free at 1-866-234-2345
- Complete a Canada Vigilance Reporting Form and:
- Fax toll-free to 1-866-678-6789, or
- Mail to:
Canada Vigilance Program
Health Canada
Postal Locator 0701D
Ottawa, Ontario K1A 0K9
Postage paid labels, Canada Vigilance Reporting Form and the adverse reaction reporting guidelines are available on the MedEffect™ Canada Web site.
Table of contents
- List of figures and tables
- List of abbreviations
- Authorship and acknowledgements
- Overview of summary statements
- 1.0 The Endocannabinoid System
- 2.0 Clinical Pharmacology
- 3.0 Dosing
- 4.0 Potential Therapeutic Uses
- 4.1 Palliative care
- 4.2 Quality of life
- 4.3 Chemotherapy-induced nausea and vomiting
- 4.4 Wasting syndrome (cachexia, e.g., from tissue injury by infection or tumour) and loss of appetite (anorexia) in AIDS and cancer patients, and anorexia nervosa
- 4.5 Multiple sclerosis, amyotrophic lateral sclerosis, spinal cord injury and disease
- 4.6 Epilepsy
- 4.7 Pain
- 4.8 Arthritides and musculoskeletal disorders
- 4.9 Other diseases and symptoms
- 4.9.1 Movement disorders
- 4.9.2 Glaucoma
- 4.9.3 Asthma
- 4.9.4 Hypertension
- 4.9.5 Stress and psychiatric disorders
- 4.9.6 Alzheimer's disease and dementia
- 4.9.7 Inflammation
- 4.9.8 Gastrointestinal system disorders (irritable bowel syndrome, inflammatory bowel disease, hepatitis, pancreatitis, metabolic syndrome/obesity)
- 4.9.8.1 Irritable bowel syndrome
- 4.9.8.2 Inflammatory bowel diseases (Crohn's disease, ulcerative colitis)
- 4.9.8.3 Diseases of the liver (hepatitis, fibrosis, steatosis, ischemia-reperfusion injury, hepatic encephalopathy)
- 4.9.8.4 Metabolic syndrome, obesity, diabetes
- 4.9.8.5 Diseases of the pancreas (diabetes, pancreatitis)
- 4.9.9 Anti-neoplastic properties
- 4.9.10 Emerging potential therapeutic uses
- 5.0 Precautions
- 6.0 Warnings
- 7.0 Adverse effects
- 8.0 Overdose/Toxicity
- References
List of figures and tables
Figures
- Figure 1.
The Endocannabinoid System in the Nervous System - Figure 2.
Pharmacokinetics of THC - Figure 3.
A Proposed Clinical Algorithm for Physicians Considering Supporting Therapeutic Use of Cannabis for a Patient with Chronic, Intractable Neuropathic Pain
Tables
- Table 1.
Selected Pharmacologic Actions of Cannabis/Psychoactive Cannabinoids - Table 2.
Recommendations for the Evaluation and Management of Patients - Table 3.
Relationship between THC Percent in Plant Material and the Available Dose (in mg THC) in an Average Joint - Table 4.
Comparison between Cannabis and Prescription Cannabinoid Medications - Table 5.
Published Positive, Randomized, Double-Blind, Placebo-Controlled, Clinical Trials on Smoked/Vapourized Cannabis and Associated Therapeutic Benefits
List of abbreviations
- 2-AG:
- 2-arachidonoylglycerol
- 5-ASA:
- 5-aminosalicylic acid
- 5-HT:
- 5-hydroxytryptamine
- 2-OG:
- 2-oleoylglycerol
- AA:
- arachidonic acid
- AB:
- Alberta
- ACCESS:
- AIDS Care Cohort to evaluate Exposure to Survival Services
- ACE:
- angiotensin-converting enzyme
- ACMPR:
- Access to Cannabis for Medical Purposes Regulations
- ACTH:
- adrenocorticotropic hormone
- AD:
- Alzheimer's disease
- AED:
- anandamide
- AIDS:
- acquired immune deficiency syndrome
- AKT1:
- AKT Serine/Threonine Kinase 1
- ALS:
- amyotrophic lateral sclerosis
- ALSPAC:
- Avon Longitudinal Study of Parents and Children
- ALT:
- alanine transaminase
- AMP:
- adenosine monophosphate
- AOR:
- adjusted odds ratio
- ApoE:
- apolipoprotein E
- APP:
- amyloid precursor protein
- APRI:
- AST-to-platelet ratio index
- ART:
- anti-retroviral therapy
- AST:
- aspartate transaminase
- AUC:
- area-under-the-curve
- AUC12:
- 12-hour AUC
- Aβ:
- amyloid-beta
- b.i.d.:
- bis in die (i.e. twice per day)
- BAC:
- blood alcohol concentration
- BC:
- British Columbia
- BCOS:
- Bipolar Comprehensive Outcomes Study
- BDNF:
- brain-derived neurotrophic factor
- BDS:
- botanical drug substance
- BHO:
- butane hash oil
- BMI:
- body mass index
- BPI:
- Brief Pain Inventory
- Ca2+:
- calcium
- CADUMS:
- Canadian Alcohol and Drug Use Monitoring Survey
- CAMPS:
- Cannabis Access for Medical Purposes Survey
- CAMS:
- Cannabis in Multiple Sclerosis
- CAPS:
- Clinician-Administered PTSD Scale
- CARDIA:
- Coronary Artery Risk Development In young Adults
- CB:
- cannabinoid
- CBC:
- cannabichromene
- CBD:
- cannabidiol
- CBDA:
- cannabidiolic acid
- CBDV:
- cannabidivarin
- CBG:
- cannabigerol
- CBN:
- cannabinol
- CCL:
- chemokine (C-C motif) ligand
- CDAI:
- Crohn's disease activity index
- CDKL5:
- cyclin-dependent kinase-like 5 gene
- CHS:
- cannabis hyperemesis syndrome
- CI:
- confidence interval
- CINV:
- chemotherapy-induced nausea and vomiting
- CGI-I:
- clinical global impression improvement
- CGI-S:
- clinical global impression scale
- cMAS:
- combined modified Ashworth score
- Cmax:
- Maximal concentration of a drug in the blood
- CNR1:
- cannabinoid receptor 1
- CNR2:
- cannabinoid receptor 2
- CNS:
- central nervous system
- COMT:
- catechol-O-methyltransferase
- COX:
- cyclo-oxygenase
- CRP:
- C-reactive protein
- CRPS:
- complex regional pain syndrome
- CSF:
- cerebrospinal fluid
- CUD:
- cannabis use disorder
- CUPID:
- Cannabinoid Use in Progressive Inflammatory Brain Disease
- CYP:
- cytochrome P450
- D:
- duration of action
- DAG:
- diacylglycerol
- DAGL:
- diacylglycerol lipase
- DAT1:
- dopamine active transporter 1
- DIO:
- diet-induced obesity
- DNA:
- deoxyribonucleic acid
- DNBS:
- dinitrobenzene sulfonic acid
- DSM-5:
- diagnostic and statistical manual of mental disorders (fifth edition)
- DSM-IV:
- diagnostic and statistical manual of mental disorders (fourth edition)
- DUIA:
- driving under the influence of alcohol
- DUIC:
- driving under the influence of cannabis
- ECS:
- endocannabinoid system
- ED50:
- median effective dose
- EDSP:
- Early Developmental Stages of Psychopathology
- EDSS:
- expanded disability status scale
- EEG:
- electroencephalogram
- e.g.:
- for example
- EMBLEM:
- European Mania in Bipolar Longitudinal Evaluation of Medication
- EORTC QLQ-C30:
- European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, Core Module
- EQ-5D:
- EuroQoL five dimensions questionnaire
- ESM:
- experience sampling methodology
- ETA:
- ethanolamine
- FAACT:
- Functional Assessment of Anorexia-Cachexia Therapy
- FAAH:
- fatty acid amide hydrolase
- FEV1:
- forced expiratory volume in one second
- fMRI:
- functional magnetic resonance imaging
- FSH:
- follicle stimulating hormone
- FVC:
- forced vital capacity
- g:
- gram
- GABA:
- gamma-aminobutyric acid
- GAD:
- generalized anxiety disorder
- GI:
- gastrointestinal
- GnRH:
- gonadotropin-releasing hormone
- GPR55:
- G protein-coupled receptor 55
- GRADE:
- Grading of Recommendations, Assessment, Development and Evaluation
- GVHD:
- graft-versus-host disease
- h:
- hour
- H1-MRS:
- proton magnetic resonance spectroscopy
- HD:
- Huntington's disease
- HDL:
- high density lipoprotein
- HIV:
- human immunodeficiency virus
- HMG-CoA:
- 3-hydroxy-3-methyl-glutaryl-coenzyme A
- HMO:
- health maintenance organization
- HOMA-IR:
- homeostatic model assessment of insulin resistance
- HPA:
- hypothalamic-pituitary-adrenal
- HPO:
- hypothalamic-pituitary-ovarian
- HRQoL:
- health-related quality of life
- I.M.:
- intramuscular
- I.P.:
- intraperitoneal
- I.V.:
- intravenous
- IBD:
- inflammatory bowel disease
- IBS:
- irritable bowel syndrome
- IBS-A:
- alternating pattern (alternation constipation/diarrhea) IBS
- IBS-C:
- constipation-predominant IBS
- IBS-D:
- diarrhea-predominant IBS
- IC50:
- median inhibitory concentration
- ICAM-1:
- intercellular adhesion molecule-1
- ICD:
- International Classification of Diseases
- ICM:
- inner cell mass
- IFN:
- interferon
- IL:
- interleukin
- IND:
- investigational new drug
- iNOS:
- inducible nitric oxide synthase
- IOP:
- intraocular pressure
- IQ:
- intelligence quotient
- IQR:
- interquartile range
- IRR:
- incident rate ratio
- K+:
- potassium
- kg:
- kilogram
- L:
- liter
- LCT:
- lipid long-chain triglyceride
- LD50:
- median lethal dose
- LDL:
- low density lipoprotein
- LH:
- luteinizing hormone
- LOX:
- lipo-oxygenase
- MAGL:
- monoacylglycerol lipase
- MB:
- Manitoba
- Met:
- methionine
- mg:
- milligram
- min:
- minute
- miRNA:
- micro ribonucleic acid
- mL:
- milliliter
- MMP:
- matrix metalloproteinase
- MOVE 2:
- Mobility Improvement in MS-Induced Spasticity Study
- mRNA:
- messenger ribonucleic acid
- MS:
- multiple sclerosis
- MSIS-29:
- MS Impact Scale 29
- MUSEC:
- Multiple Sclerosis and Extract of Cannabis trial
- N/A:
- not applicable
- Na+:
- sodium
- NAFLD:
- non-alcoholic fatty liver disease
- NAPE:
- N-arachidonoylphosphatidylethanolamine
- NASEM:
- National Academy of Sciences, Engineering and Medicine
- NB:
- New Brunswick
- NCS:
- National Comorbidity Survey
- NCS-R:
- National Comorbidity Survey-Replication
- NEMESIS:
- Netherlands Mental Health Survey and Incidence Study
- NESARC:
- National Epidemiological Survey on Alcohol and Related Conditions
- ng:
- nanogram
- NHANES:
- National Health and Nutrition Examination Survey
- NK:
- natural killer
- NK-1:
- neurokinin 1
- NL:
- Newfoundland and Labrador
- nM:
- nanomolar
- NMDA:
- N-methyl-D-aspartic acid
- nmol:
- nanomole
- NNT:
- number needed to treat
- NRG1:
- neuregulin 1
- NRS:
- numerical rating scale
- NRS-PI:
- numerical rating scale for pain intensity
- NS:
- Nova Scotia
- NSAIDs:
- nonsteroidal anti-inflammatory drugs
- NSDUH:
- National Survey on Drug Use and Health
- NT:
- Northwest Territories
- NU:
- Nunavut
- O:
- onset of effects
- OA:
- osteoarthritis
- OEA:
- oleoylethanolamide
- ON:
- Ontario
- OR:
- odds ratio
- P:
- peak effects
- PE:
- Prince Edward Island
- P.O.:
- oral administration
- PD:
- Parkinson's disease
- PDQ-39:
- 39-Item Parkinson Disease Questionnaire
- PEA:
- palmitoylethanolamide
- PLD:
- phospholipase-D
- pNRS:
- pain numerical rating score
- PPAR:
- peroxisome proliferator-activated receptor
- PRISMA:
- Preferred Reporting Items for Sytematic Reviews and Meta-Analyses
- PTSD:
- post-traumatic stress disorder
- PWID:
- people who inject drugs
- QC:
- Quebec
- q.i.d.:
- quater in die (i.e. four times per day)
- QoL:
- quality of life
- RA:
- rheumatoid arthritis
- RCT:
- randomized controlled trial
- REM:
- rapid eye movement
- RNA:
- ribonucleic acid
- Rx:
- prescription
- s:
- second
- SAFTEE:
- Systematic Assessment of Treatment Emergent Events
- s.c.:
- subcutaneous
- SCI:
- spinal cord injury
- SD:
- standard deviation
- SDLP:
- standard deviation of lateral position
- SF-36:
- 36-Item Short Form Health Survey
- SIBDQ:
- short IBD questionnaire
- SIV:
- simian immunodeficiency virus
- SK:
- Saskatchewan
- SNP:
- single nucleotide polymorphism
- sNRS:
- subjective numerical rating spasticity scale
- S-TOPS:
- Short-Form Treatment Outcomes in Pain Survey
- SYS:
- Saguenay Youth Study
- t.i.d.:
- ter in die (i.e. three times per day)
- TGCT:
- testicular germ cell tumours
- THC:
- delta-9-tetrahydrocannabinol
- THCA:
- tetrahydrocannabinolic acid
- THCV:
- tetrahydrocannabivarin
- TIA:
- transient ischemic attack
- Tmax:
- Time to maximal blood concentration of a drug
- TNBS:
- trinitrobenzene sulfonic acid
- TNF:
- tumor necrosis factor
- TRH:
- thyrotropin-releasing hormone
- TRP:
- transient receptor potential
- TRPV1:
- transient receptor potential vanilloid channel 1
- TS:
- Tourette's syndrome
- TWSTRS:
- Toronto Western Spasmodic Torticollis Rating Scale
- U.K.:
- United Kingdom
- UPDRS:
- Unified Parkinson's Disease Rating Scale
- Val:
- valine
- VAS:
- visual analogue scale
- VCAM-1:
- vascular cellular adhesion molecule-1
- w/w:
- weight/weight
- WHO:
- World Health Organization
- YT:
- Yukon
- Δ9-THC:
- delta-9-tetrahydrocannabinol
- µg:
- microgram
- μM:
- micromolar
Authorship and acknowledgements
Author: Hanan Abramovici Ph.D.
Co-authors: Sophie-Anne Lamour, Ph.D.: and George Mammen, Ph.D.
Affiliations:
Cannabis Legalization and Regulation Branch, Health Canada, Ottawa, ON, Canada K1A 0K9
Email: hanan.abramovici@canada.ca
Acknowledgements:
Health Canada would like to acknowledge and thank the following individuals for their comments and suggestions with regard to the content in this information document:
Donald I. Abrams, M.D.
Chief, Hematology-Oncology
San Francisco General Hospital
Integrative Oncology
UCSF Osher Center for Integrative Medicine
Professor of Clinical Medicine
University of California San Francisco
San Francisco, CA 94143-0874
USA
Pierre Beaulieu, M.D., Ph.D., F.R.C.A.
Full professor
Department of Pharmacology and Anesthesiology
Faculty of Medicine
University of Montreal
Office R-408, Roger-Gaudry Wing
P.O. Box 6128 - Downtown Branch
Montréal, Québec
H3C 3J7
Canada
Bruna Brands, Ph.D.
Full Professor
Department of Pharmacology and Toxicology
Program Director, Collaborative Program in Addiction Studies
University of Toronto
33 Russell Street
Toronto, ON
M5S 2S1
Canada
Ziva Cooper, Ph.D.
Assistant Professor of Clinical Neurobiology
Division on Substance Abuse
New York State Psychiatric Institute and Department of Psychiatry
College of Physicians and Surgeons Columbia University
1051 Riverside Drive
New York, NY 10032
USA
Paul J. Daeninck, M.D., M.Sc., F.R.C.P.C.
Chair, Symptom Management and Palliative Care Disease Site Group
CancerCare Manitoba
Assistant Professor,
College of Medicine, University of Manitoba
St. Boniface Hospital
409 Taché Ave
Winnipeg, MB
R2H 2A6
Canada
Mahmoud A. ElSohly, Ph.D.
Research Professor and Professor of Pharmaceutics
National Center for Natural Products Research and Department of Pharmaceutics
School of Pharmacy
University of Mississippi
University, MS 38677
USA
Javier Fernandez-Ruiz, Ph.D.
Full Professor of Biochemistry and Molecular Biology
Department of Biochemistry and Molecular Biology
Faculty of Medicine
Complutense University
Madrid, 28040
Spain
Tony P. George, M.D., F.R.C.P.C.
Professor and Co-Director, Division of Brain and Therapeutics
Department of Psychiatry, University of Toronto
Chief, Schizophrenia Division
Centre for Addiction and Mental Health
1001 Queen Street West, Unit 2, Room 118A
Toronto, ON
M6J 1H4
Canada
Manuel Guzman, Ph.D.
Full Professor
Department of Biochemistry and Molecular Biology
Faculty of Chemistry
Complutense University
Madrid, 28040
Spain
Matthew N. Hill, Ph.D.
Assistant Professor
Departments of Cell Biology and Anatomy & Psychiatry
The Hotchkiss Brain Institute
University of Calgary
Calgary, AB
T2N 4N1
Canada
Cecilia J. Hillard, Ph.D.
Professor
Department of Pharmacology and Toxicology
Director of the Neuroscience Research Center
Medical College of Wisconsin
8701 Watertown Plank Road
Milwaukee, Wisconsin 53226
USA
Mary Lynch, M.D., F.R.C.P.C.
Professor of Anaesthesia, Psychiatry and Pharmacology
Dalhousie University
Director, Pain Management Unit-Capital Health
Queen Elizabeth II Health Sciences Centre
4th Floor Dickson Building
5820 University Avenue
Halifax, NS
B3H 1V7
Canada
Jason J. McDougall, Ph.D.
Professor
Departments of Pharmacology and Anaesthesia, Pain Management & Perioperative Medicine
Dalhousie University
5850 College Street
Halifax, NS
B3H 4R2
Canada
Raphael Mechoulam, Ph.D.
Professor
Institute for Drug Research, Medical Faculty
Hebrew University
Jerusalem
91120
Israel
Linda Parker, Ph.D.
Professor and Canada Research Chair
Department of Psychology
University of Guelph
Guelph, Ontario
N1G 2W1
Canada
Roger G. Pertwee, MA, D.Phil. D.Sc.
Professor of Neuropharmacology
Institute of Medical Sciences
University of Aberdeen
Aberdeen
AB25 2ZD
Scotland, United Kingdom
Keith Sharkey, Ph.D.
Professor
Department of Physiology and Biophysics and Medicine
University of Calgary
HSC 1745
3330 Hospital Drive NW
Calgary, AB
T2N 4N1
Canada
Mark Ware, M.D., M.R.C.P., M.Sc.
Associate professor
Departments of Anesthesia and Family Medicine
McGill University
Director of Clinical Research
Alan Edwards Pain Management Unit
A5.140 Montreal General Hospital
1650 Cedar Avenue
Montréal, Québec
H3G 1A4
Canada
Overview of Summary Statements
The following bullet-point statements are meant to summarize the content found within sections 4.0 (Potential Therapeutic Uses) and 7.0 (Adverse Effects) and their respective subsections. The bullet-point statements can also be found in their respective sections and sub-sections in the body of the document itself. Note: most, but not all, clinical studies of cannabis (experimental or therapeutic) have been conducted with dried cannabis containing more THC than CBD and typically, but not always, with lower-potency THC (< 9% THC). Furthermore, the majority of the clinical studies of cannabis (experimental or therapeutic) have administered dried cannabis by smoking. Lastly, the findings from clinical studies of cannabis for therapeutic purposes may not be applicable to other chemotypes of cannabis or other cannabis products with different THC and CBD amounts and ratios.
4.0 Potential Therapeutic Uses
4.1 Palliative care
- The evidence thus far from some observational studies and clinical studies suggests that cannabis (limited evidence) and prescription cannabinoids (e.g. dronabinol, nabilone, or nabiximols) may be useful in alleviating a wide variety of single or co-occurring symptoms often encountered in the palliative care setting.
- These symptoms may include, but are not limited to, intractable nausea and vomiting associated with chemotherapy or radiotherapy, anorexia/cachexia, severe intractable pain, severe depressed mood and anxiety, and insomnia.
- A limited number of observational studies suggest that the use of cannabinoids for palliative care may also potentially be associated with a decrease in the number of some medications used by this patient population.
4.2 Quality of life
- The available clinical studies report mixed effects of cannabis and prescription cannabinoids on measures of quality of life (QoL) for a variety of different disorders.
4.3 Chemotherapy-induced nausea and vomiting
- Pre-clinical studies show that certain cannabinoids (THC, CBD, THCV, CBDV) and cannabinoid acids (THCA and CBDA) suppress acute nausea and vomiting as well as anticipatory nausea.
- Clinical studies suggest that certain cannabinoids and cannabis (limited evidence) use may provide relief from chemotherapy-induced nausea and vomiting (CINV).
4.4 Wasting syndrome (cachexia, e.g., from tissue injury by infection or tumour) and loss of appetite (anorexia) in AIDS and cancer patients, and anorexia nervosa
- The available evidence from human clinical studies suggests that cannabis (limited evidence) and dronabinol may increase appetite and caloric intake, and promote weight gain in patients with HIV/AIDS.
- However the evidence for dronabinol is mixed and effects modest for patients with cancer and weak for patients with anorexia nervosa.
4.5 Multiple sclerosis, amyotrophic lateral sclerosis, spinal cord injury and disease
- Evidence from pre-clinical studies suggests THC, CBD and nabiximols improve multiple sclerosis (MS) associated symptoms of tremor, spasticity and inflammation.
- The available evidence from clinical studies suggest cannabis (limited evidence) and certain cannabinoids (dronabinol, nabiximols, THC/CBD) are associated with some measure of improvement in symptoms encountered in MS and spinal cord injury (SCI) including spasticity, spasms, pain, sleep and symptoms of bladder dysfunction.
- Very limited evidence from pre-clinical studies suggest that certain cannabinoids modestly delay disease progression and prolong survival in animal models of amyotrophic lateral sclerosis (ALS), while the results from a very limited number of clinical studies are mixed.
4.6 Epilepsy
- Anecdotal evidence suggests an anti-epileptic effect of cannabis (THC- and CBD-predominant strains).
- The available evidence from pre-clinical studies suggests certain cannabinoids (CBD) may have anti-epileptiform and anti-convulsive properties, whereas CB1R agonists (THC) may have either pro- or anti-epileptic properties.
- However, the clinical evidence for an anti-epileptic effect of cannabis is weaker, but emerging, and requires further study.
- Evidence from clinical studies with Epidiolex® (oral CBD) suggest efficacy and tolerability of Epidiolex® for drug-resistant seizures in treatment-resistant Dravet syndrome or Lennox-Gastaut syndrome.
- Evidence from observational studies suggest an association between CBD (in herbal and oil preparations) and a reduction in seizure frequency as well as an increase in quality of life among adolescents with rare and serious forms of drug-resistant epilepsy.
- Epidiolex® has received FDA approval (in June 2018) for use in patients 2 years of age and older to treat treatment-resistant seizures associated with Dravet syndrome and Lennox-Gastaut syndrome.
4.7 Pain
4.7.1 Acute pain
- Pre-clinical studies suggest that certain cannabinoids can block the response to experimentally-induced acute pain in animal models.
- The results from clinical studies with smoked cannabis, oral THC, cannabis extract, and nabilone in experimentally-induced acute pain in healthy human volunteers are limited and mixed and suggest a dose-dependent effect in some cases, with lower doses of THC having an analgesic effect and higher doses having a hyperalgesic effect.
- Clinical studies of certain cannabinoids (nabilone, oral THC, levonontradol, AZD1940, GW842166) for post-operative pain suggest a lack of efficacy.
4.7.2 Chronic pain
4.7.2.1 Experimentally-induced inflammatory and chronic neuropathic pain
- Endocannabinoids, THC, CBD, nabilone and certain synthetic cannabinoids have all been identified as having an anti-nociceptive effect in animal models of chronic pain (inflammatory and neuropathic).
4.7.2.2. Neuropathic pain and chronic non-cancer pain in humans
- A few studies that have used experimental methods having predictive validity for pharmacotherapies used to alleviate chronic pain, have reported an analgesic effect of smoked cannabis.
- Furthermore, there is more consistent evidence of the efficacy of cannabinoids (smoked/vapourized cannabis, nabiximols, dronabinol) in treating chronic pain of various etiologies, especially in cases where conventional treatments have been tried and have failed.
4.7.2.3 Cancer pain
- The limited available clinical evidence with certain cannabinoids (dronabinol, nabiximols) suggests a modest analgesic effect of dronabinol and a modest and mixed analgesic effect of nabiximols on cancer pain.
4.7.2.4 "Opioid-sparing" effects and cannabinoid-opioid synergy
- While pre-clinical and case studies suggest an "opioid-sparing" effect of certain cannabinoids, epidemiological and clinical studies with oral THC and nabiximols are mixed.
- Observational studies suggest an association between U.S. states with laws permitting access to cannabis (for medical and non-medical purposes) and lowered rates of prescribed opioids and opioid-associated mortality.
4.7.2.5 Headache and migraine
- The evidence supporting using cannabis/certain cannabinoids to treat headache and migraine is very limited and mixed.
4.8. Arthritides and musculoskeletal disorders
- The evidence from pre-clinical studies suggests stimulation of CB1 and CB2 receptors alleviates symptoms of osteoarthritis (OA), and THC and CBD alleviate symptoms of rheumatoid arthritis (RA).
- The evidence from clinical studies is very limited, with a modest effect of nabiximols for RA.
- There are no clinical studies of cannabis for fibromyalgia, and the limited clinical evidence with dronabinol and nabilone suggest a modest effect on decreasing pain and anxiety, and improving sleep.
- The role of cannabinoids in osteoporosis has only been investigated pre-clinically and is complex and conflicting.
4.9 Other diseases and symptoms
4.9.1 Movement disorders
4.9.1.1 Dystonia
- Evidence from limited pre-clinical studies suggests that a synthetic CB1 and CB2 receptor agonist may alleviate dystonia-like symptoms, and CBD delays dystonia progression.
- Evidence from a limited number of case studies and small placebo-controlled or open-label clinical trials suggests improvement in symptoms of dystonia with inhaled cannabis, mixed effects of oral THC, improvement in symptoms of dystonia with oral CBD, and lack of effect of nabilone on symptoms of dystonia.
4.9.1.2 Huntington's disease
- Evidence from pre-clinical studies reports mixed results with THC on Huntington's disease (HD)-like symptoms.
- Limited evidence from case studies and small clinical trials is mixed and suggests a lack of effect with CBD, nabilone and nabiximols, and a limited improvement in HD symptoms with smoked cannabis.
4.9.1.3 Parkinson's disease
- The evidence from a limited number of pre-clinical, case, clinical and observational studies of certain cannabinoids for symptoms of Parkinson's disease (PD) is mixed.
- One case study of smoked cannabis suggests no effect while an observational study of smoked cannabis suggests improvement in symptoms.
- One small clinical study of nabilone suggests improvement in symptoms, while another clinical study of an oral cannabis extract (THC/CBD) and a clinical study with CBD suggest no improvement in symptoms.
4.9.1.4 Tourette's syndrome
- The limited evidence from small clinical studies suggests that oral THC improves certain symptoms of Tourette's syndrome (TS) (tics).
4.9.2 Glaucoma
- The limited evidence from small clinical studies suggests oral administration of THC reduces intra-ocular pressure (IOP) while oral administration of CBD may, in contrast, cause an increase in IOP.
4.9.3 Asthma
- The limited evidence from pre-clinical and clinical studies on the effect of aerosolized THC on asthmatic symptoms is mixed.
- Inhalation of lung irritants generated from smoking/vapourizing cannabis may worsen asthmatic symptoms.
4.9.5 Stress and psychiatric disorders
4.9.5.1 Anxiety and depression
- Evidence from pre-clinical and clinical studies suggests that THC exhibits biphasic effects on mood, with low doses of THC having anxiolytic and mood-elevating effects and high doses of THC having anxiogenic and mood-lowering effects.
- Limited evidence from a small number of clinical studies of THC-containing cannabis/certain prescription cannabinoids suggests that these drugs could improve symptoms of anxiety and depression in patients suffering from anxiety and/or depression secondary to certain chronic diseases (e.g. patients with HIV/AIDS, MS, and chronic neuropathic pain).
- Evidence from pre-clinical studies suggests that CBD exhibits anxiolytic effects in various animal models of anxiety, while limited evidence from clinical studies suggest CBD may have anxiolytic effects in an experimental model of social anxiety.
- Limited evidence from some observational studies also suggests that cannabis containing equal proportions of CBD and THC is associated with an attenuation of some perturbations in mood (anxiety/dejection) seen with THC-predominant cannabis in patients using cannabis for medical purposes.
4.9.5.2 Sleep disorders
- Human experimental data suggests cannabis and THC have a dose-dependent effect on sleep-low doses appear to decrease sleep onset latency and increase slow-wave sleep and total sleep time, while high doses appear to cause sleep disturbances.
- Limited evidence from clinical studies also suggest that certain cannabinoids (cannabis, nabilone, dronabinol, nabiximols) may improve sleep in patients with disturbances in sleep associated with certain chronic disease states.
4.9.5.3 Post-traumatic stress disorder
- Pre-clinical and human experimental studies suggest a role for certain cannabinoids in alleviating post-traumatic stress disorder (PTSD)-like symptoms.
- However, while limited evidence from short-term clinical studies suggests a potential for oral THC and nabilone to decrease certain symptoms of PTSD, there are no long-term clinical studies for these preparations or any clinical studies of smoked/vapourized cannabis for PTSD.
- Limited evidence from observational studies suggests an association between herbal cannabis use and persistent/high levels of PTSD symptom severity over time.
- There is limited evidence to suggest an association between PTSD and CUD.
4.9.5.4 Alcohol and opioid withdrawal symptoms (drug withdrawal symptoms/drug substitution)
- Pre-clinical studies suggest CB1 receptor agonism (e.g. THC) may help increase the reinforcing properties of alcohol, increase alcohol consumption, and increase risk of relapse of alcohol use, as well as exacerbate alcohol withdrawal symptom severity.
- Pre-clinical studies suggest certain cannabinoids (e.g. THC) may alleviate opioid withdrawal symptoms.
- Evidence from observational studies suggests that cannabis use could help alleviate opioid withdrawal symptoms, but there is insufficient clinical evidence from which to draw any reliable conclusions.
4.9.5.5 Schizophrenia and psychosis
- Significant evidence from pre-clinical, clinical and epidemiological studies supports an association between cannabis (especially THC-predominant cannabis) and THC, and an increased risk of psychosis and schizophrenia.
- Emerging evidence from pre-clinical, clinical and epidemiological studies suggests CBD may attenuate THC-induced psychosis.
4.9.6 Alzheimer's disease and dementia
- Pre-clinical studies suggest that THC and CBD may protect against excitotoxicity, oxidative stress and inflammation in animal models of Alzheimer's disease (AD).
- Limited case, clinical and observational studies suggest that oral THC and nabilone are associated with improvement in a number of symptoms associated with AD (e.g. nocturnal motor activity, disturbed behaviour, sleep, agitation, resistiveness).
4.9.7 Inflammation
4.9.7.1 Inflammatory skin diseases (dermatitis, psoriasis, pruritus)
- The results from pre-clinical, clinical and case studies on the role of certain cannabinoids in the modulation of inflammatory skin diseases are mixed.
- Some clinical and prospective case series studies suggest a protective role for certain cannabinoids (THC, CBD, HU-210), while others suggest a harmful role (cannabis, THC, CBN).
4.9.8 Gastrointestinal system disorders (irritable bowel syndrome, inflammatory bowel disease, hepatitis, pancreatitis, metabolic syndrome/obesity)
4.9.8.1 Irritable bowel syndrome
- Pre-clinical studies in animal models of irritable bowel syndrome (IBS) suggest that certain synthetic cannabinoid receptor agonists inhibit colorectal distension-induced pain responses and slow GI transit.
- Experimental clinical studies with healthy volunteers reported dose- and sex-dependent effects on various measures of GI motility.
- Limited evidence from one small clinical study with dronabinol for symptoms of IBS suggests dronabinol may increase colonic compliance and decrease colonic motility index in female patients with diarrhea-predominant IBS (IBS-D) or with alternating pattern (alternating constipation/diarrhea) IBS (IBS-A), while another small clinical study with dronabinol suggests a lack of effect on gastric, small bowel or colonic transit.
4.9.8.2 Inflammatory bowel diseases (Crohn's disease, ulcerative colitis)
- Pre-clinical studies in animal models of inflammatory bowel disease (IBD) suggest that certain cannabinoids (synthetic CB1 and CB2 receptor agonists, THC, CBD, CBG, CBC, whole plant cannabis extract) may limit intestinal inflammation and disease severity to varying degrees.
- Evidence from observational studies suggests that patients use cannabis to alleviate symptoms of IBD.
- A very limited number of small clinical studies with patients having IBD and having failed conventional treatments reported improvement in a number of IBD-associated symptoms with smoked cannabis.
4.9.8.3 Diseases of the liver (hepatitis, fibrosis, steatosis, ischemia-reperfusion injury, hepatic encephalopathy)
- Pre-clinical studies suggest CB1 receptor activation is detrimental in liver diseases (e.g. promotes steatosis, fibrosis); while CB2 receptor activation appears to have some beneficial effects.
- Furthermore, pre-clinical studies also suggest that CBD, THCV and ultra-low doses of THC may have some protective effects in hepatic ischemia-reperfusion injury and hepatic encephalopathy.
4.9.8.4 Metabolic syndrome, obesity, diabetes
- Pre-clinical studies suggest acute CB1 receptor activation results in increased fat synthesis and storage while chronic CB1 receptor activation (or CB1 receptor antagonism) results in weight loss and improvement in a variety of metabolic indicators.
- Observational studies suggest an association between chronic cannabis use and an improved metabolic profile, while pre-clinical and very limited clinical evidence suggests a potential beneficial effect of THCV on glycemic control (in patients with type II diabetes).
4.9.8.5 Diseases of the pancreas (diabetes, pancreatitis)
- Pre-clinical studies in experimental animal models of certain cannabinoids in the treatment of acute or chronic pancreatitis are limited and conflicting.
- Limited evidence from case studies suggests an association between acute episodes of heavy cannabis use and acute pancreatitis.
- Limited observational studies suggest an association between chronic cannabis use and lower incidence of diabetes mellitus.
- One small clinical study reported that orally administered THC did not alleviate abdominal pain associated with chronic pancreatitis.
4.9.9 Anti-neoplastic properties
- Pre-clinical studies suggest that certain cannabinoids (THC, CBD, CBG, CBC, CBDA) often, but not always block growth of cancer cells in vitro and display a variety of anti-neoplastic effects in vivo, though typically at very high doses that would not be seen clinically.
- While limited evidence from one observational study suggests cancer patients use cannabis to alleviate symptoms associated with cancer (e.g. chemosensory alterations, weight loss, depression, pain), there has only been one limited clinical study in patients with glioblastoma multiforme, which reported that intra-tumoural injection of high doses of THC did not improve patient survival beyond that seen with conventional chemotherapeutic agents.
7.0 Adverse effects
7.1 Carcinogenesis and mutagenesis
- Evidence from pre-clinical studies suggests cannabis smoke contains many of the same carcinogens and mutagens as tobacco smoke and that cannabis smoke is as mutagenic and cytotoxic, if not more so, than tobacco smoke.
- However, limited and conflicting evidence from epidemiological studies has thus far been unable to find a robust and consistent association between cannabis use and various types of cancer, with the possible exception of a link between cannabis use and testicular cancer (i.e. testicular germ cell tumours).
7.2 Respiratory tract
- Evidence from pre-clinical studies suggests that cannabis smoke contains many of the same respiratory irritants and toxins as tobacco smoke, and even greater quantities of some such substances.
- Case studies suggest that cannabis smoking is associated with a variety of histopathological changes in respiratory tissues, a variety of respiratory symptoms similar to those seen in tobacco smokers, and changes in certain lung functions with frequent, long-term use.
- The association between chronic heavy cannabis smoking (without tobacco) and chronic obstructive pulmonary disease, is unclear, but if there is one, is possibly small.
7.3 Immune system
- Pre-clinical studies suggest certain cannabinoids have a variety of complex effects on immune system function (pro-/anti-inflammatory, stimulatory/inhibitory).
- The limited clinical and observational studies of the effects of cannabis on immune cell counts and effect on HIV viral load are mixed, as is the evidence around frequent cannabis use (i.e. daily/CUD) and adherence to ART.
- Limited but increasing evidence from case studies also suggests cannabis use is associated with allergic/hypersensitivity-type reactions.
7.4 Reproductive and endocrine systems
- Pre-clinical evidence suggests certain cannabinoids can have negative effects on a variety of measures of reproductive health. Furthermore, limited evidence from human observational studies with cannabis appears to support evidence from some pre-clinical studies.
- Evidence from human observational studies also suggests a dose- and age-dependent association between cannabis use and testicular germ cell tumours.
- Pre-clinical evidence clearly suggests in utero exposure to certain cannabinoids is associated with a number of short and long-term harms to the developing offspring.
- However, evidence from human observational studies is complex and suggests that while confounding factors may account for associations between heavy cannabis use during pregnancy and adverse neonatal or perinatal effects, heavy cannabis use during pregnancy is associated with reduced neonatal birth weight.
7.5 Cardiovascular system
- Pre-clinical studies suggest that ultra-low doses of THC may be cardioprotective on experimentally-induced myocardial infarction.
- Evidence from case and observational studies suggests that acute and chronic smoking of cannabis is associated with harmful effects on vascular, cardiovascular and cerebrovascular health (e.g. myocardial infarction, strokes, arteritis) especially in middle-aged (and older) users.
- However, a recent systematic review suggests that evidence examining the effects of cannabis on cardiovascular health is inconsistent and insufficient.
7.6 Gastrointestinal system and liver
- Evidence from case reports suggests chronic, heavy (THC-predominant) cannabis use is associated with an increased risk of cannabis hyperemesis syndrome (CHS).
- Limited evidence from observational studies suggests mixed findings between (THC-predominant) cannabis use and risk of liver fibrosis progression associated with hepatitis C infection.
7.7 Central nervous system
7.7.1 Cognition
- Evidence from clinical studies suggests acute (THC-predominant) cannabis use is associated with a number of acute cognitive effects.
- Evidence from observational studies suggests chronic cannabis use is associated with some cognitive and behavioural effects that may persist for varying lengths of time beyond the period of acute intoxication depending on a number of factors.
- Limited evidence from human clinical imaging studies suggests THC and CBD may exert opposing effects on neuropsychological/neurophysiological functioning.
- Evidence from mainly cross-sectional human clinical imaging studies suggests heavy, chronic cannabis use is associated with a number of structural changes in grey and white matter in different brain regions.
- Furthermore, early-onset use and use of high-potency, THC-predominant cannabis, has been associated with an increased risk of some brain structural changes and cognitive impairment.
7.7.2 Psychomotor performance and driving
- Evidence from experimental clinical studies suggests acute use of (THC-predominant) cannabis impairs a number of psychomotor and other cognitive skills needed to drive a motor vehicle.
- While chronic/frequent cannabis use may be associated with a degree of tolerance to some of the effects of cannabis in some individuals, chronic cannabis use can still pose risks to safe driving due, in part, to significant body burden of THC leading to a chronic level of psychomotor impairment.
- Evidence from clinical and epidemiological studies suggests a dose-response effect, with increasing doses of THC increasing the risk of motor vehicle crashes that can lead to injuries and death.
- Combining alcohol with cannabis (THC) is associated with an increased degree of impairment and increased risk of harm.
7.7.3 Psychiatric effects
7.7.3.1 Anxiety, PTSD, depression and bipolar disorder
- Evidence from clinical studies suggests a dose-dependent, bi-phasic effect of THC on anxiety and mood, where low doses of THC appear to have an anti-anxiety and mood-elevating effect whereas high doses of THC can produce anxiety and lower mood.
- Epidemiological studies suggest an association between (THC-predominant) cannabis use, especially chronic, heavy use and the onset of anxiety, depressive and bipolar disorders, and the persistence of symptoms related to PTSD, panic disorder, depressive disorder, and bipolar disorder.
- Preliminary evidence from surveys suggests an association between use of ultra-high-potency cannabis concentrate products (e.g. butane hash oil, BHO) and higher rates of self-reported anxiety and depression and other illicit drug use as well as higher levels of physical dependence than with high-potency herbal cannabis.
7.7.3.2 Schizophrenia and psychosis
- Evidence from clinical studies suggests that acute exposure to (THC-predominant) cannabis or THC is associated with dose-dependent, acute and transient behavioural and cognitive effects mimicking acute psychosis.
- Epidemiological studies suggest an association between (THC-predominant) cannabis use, especially early, chronic, and heavy use and psychosis and schizophrenia.
- The risk of schizophrenia associated with cannabis use is especially high in individuals who have a personal or family history of schizophrenia.
- Cannabis use is also associated with earlier onset of schizophrenia in vulnerable individuals and exacerbation of existing schizophrenic symptoms and worse clinical outcomes.
7.7.3.3 Suicidal ideation, attempts and mortality
- Evidence from epidemiological studies also suggests a dose-dependent association between cannabis use and suicidality, especially in men.
7.7.3.4 Amotivational syndrome
- The available limited evidence for an association between cannabis use and an "amotivational syndrome" is mixed.
Important Note: For the sake of completeness and for contextual purposes, the content in the following document includes information on dried cannabis and other cannabis-based products as well as selected cannabinoids. However, cannabis products and cannabinoids should not be considered equivalent even though the information on such products is presented together within the text. Cannabis and cannabis products are highly complex materials with hundreds of chemical constituents whereas cannabinoids are typically single molecules. Drawing direct comparisons between cannabis products and cannabinoids must necessarily take into account differences in the route of administration, dosage, individual pharmacological components and their potential interactions, and the different pharmacokinetic and pharmacodynamic properties of these different substances.
1.0 The Endocannabinoid System
The endocannabinoid system (ECS) (Figure 1) is an ancient, evolutionarily conserved, and ubiquitous lipid signaling system found in all vertebrates, and which appears to have important regulatory functions throughout the human bodyReference 1. The ECS has been implicated in a very broad number of physiological as well as pathophysiological processes including nervous system development, immune function, inflammation, appetite, metabolism and energy, homeostasis, cardiovascular function, digestion, bone development and bone density, synaptic plasticity and learning, pain, reproduction, psychiatric disease, psychomotor behaviour, memory, wake/sleep cycles, and the regulation of stress and emotional state/moodReference 2-Reference 4. Furthermore, there is strong evidence that dysregulation of the ECS contributes to many human diseases including pain, inflammation, psychiatric disorders and neurodegenerative diseasesReference 5.
Components of the endocannabinoid system
The ECS consists mainly of: the cannabinoid 1 and 2 (CB1 and CB2) receptors; the cannabinoid receptor ligands N-arachidonoylethanolamine ("anandamide") and 2-arachidonoylglycerol (2-AG); the endocannabinoid-synthesizing enzymes N-acyltransferase, phospholipase D, phospholipase C-β and diacylglycerol-lipase (DAGL); and the endocannabinoid-degrading enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) (Figure 1)Reference 2. Anandamide and 2-AG are considered the primary endogenous activators of cannabinoid signaling, but other endogenous molecules, which exert "cannabinoid-like" effects, have also been described. These other molecules include 2-arachidonoylglycerol ether (noladin ether), N -arachidonoyl-dopamine, virodhamine, N -homo-γ-linolenoylethanolamine and N-docosatetraenoylethanolamineReference 2Reference 6-Reference 9. Other molecules such as palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) do not appear to bind to cannabinoid receptors but rather to a specific isozyme belonging to a class of nuclear receptors/transcription factors known as peroxisome proliferator-activated receptors (PPARs)Reference 9. These fatty acyl ethanolamides may, however, potentiate the effect of anandamide by competitive inhibition of FAAH, and/or through direct allosteric effects on other receptors such as the transient receptor potential vanilloid (TRPV1) channelReference 10. This type of effect has been generally referred to as the so-called "entourage effect"Reference 10Reference 11. The term "entourage effect" is also used in the context of the interactions between phytocannabinoids and terpenes in a physiological system (see Section 1.1.2).
Endocannabinoid synthesis
Endocannabinoids are arachidonic acid derivatives which are synthesized "on demand" (e.g. in response to an action potential in neurons or in response to another type of biological stimulus) from membrane phospholipid precursors in response to cellular requirementsReference 2Reference 12-Reference 14. Synthesis of endocannabinoids "on demand" ensures that endocannabinoid signaling is tightly controlled both spatially and temporally. Anandamide is principally, but not exclusively, produced by the transfer of arachidonic acid from phosphatidylcholine to phosphatidylethanolamine by N-acyltransferase to yield N-arachidonoylphosphatidylethanolamine (NAPE). NAPE is then hydrolyzed to form anandamide by a NAPE-specific phospholipase DReference 2Reference 15. Other synthetic routes include acyl-chain removal from NAPE by α/β-hydrolase 4 to yield glycerophospho-N-arachidonoylethanolamine followed by phosphodiester bond hydrolysis of glycerophospho-N-arachidonoylethanolamine by phosphodiesterase 1 to yield anandamideReference 16. In contrast, 2-AG is principally synthesized through phospholipase C-β-mediated hydrolysis of phosphatidylinositol-4,5-bisphosphate, with arachidonic acid on the sn-2 position, to yield diacylglycerol (DAG). DAG is then hydrolyzed to 2-AG by a DAGLReference 2Reference 15. While anandamide and 2-AG are both derivatives of arachidonic acid, they are synthesized by pathways distinct from those used to synthesize eicosanoidsReference 17. Nevertheless, it appears that there may be a certain amount of cross talk between the eicosanoid and endocannabinoid pathwaysReference 17.
Genetics and signaling through the cannabinoid receptors
Endocannabinoids such as anandamide and 2-AG, as well as the phytocannabinoids Δ9-tetrahydrocannabinol (Δ9-THC), Δ8-THC, cannabinol (CBN) and others, bind to and activate (with differing affinities and efficacies) the CB1 and CB2 receptors which are G-protein coupled receptors that activate Gi/Go-dependent signaling cascadesReference 18Reference 19. The receptors are encoded by separate genes located on separate chromosomes; in humans, the CB1 receptor gene (CNR1) locus is found on chromosome 5q15 whereas the CB2 receptor gene (CNR2) locus is located on chromosome 1p36Reference 20. The CNR1 coding sequence consists of one exon encoding a protein of 472 amino acidsReference 21. The CB1 receptor protein shares 97 - 99% amino acid sequence identity across species (human, rat, mouse)Reference 21. As with the CNR1 coding sequence, the CNR2 coding sequence consists of only one exon, but it encodes a shorter protein 360 amino acids in lengthReference 21. The human CB2 receptor shares 48% amino acid identity with the human CB1 receptor; the mouse CB2 receptor shares 82% amino acid sequence identity with the human CB2 receptorReference 21.
Activation of the CB1 or CB2 Gi/o-protein coupled receptors results in inhibition of adenylyl cyclase activity, decreased formation of cyclic AMP with a corresponding decrease in protein kinase A activity, and inhibition of Ca2+ influx through various Ca2+ channels; it also results in stimulation of inwardly rectifying potassium (K+) channels and the mitogen-activated protein kinase signaling cascadesReference 3Reference 13. Anandamide is a partial agonist at cannabinoid receptors, and binds with slightly higher affinity at CB1 compared to CB2 receptorsReference 2Reference 22. 2-AG appears to bind equally well to both cannabinoid receptors (with slightly higher affinity to CB1), but has greater potency and efficacy than anandamide at cannabinoid receptorsReference 2Reference 22.
In the central nervous system (CNS), the overall effect of CB1 receptor activation is suppression of neurotransmitter release (5-hydroxytryptamine (5-HT), glutamate, acetylcholine, GABA, noradrenaline, dopamine, D-aspartate, cholecystokinin) at both excitatory and inhibitory synapses with both short and long-term effectsReference 2Reference 18Reference 23. Inhibition of neurotransmitter release occurs through a retrograde signaling mechanism whereby endocannabinoids synthesized and released from the cell membrane of post-synaptic neurons diffuse backwards across the synaptic cleft and bind to CB1 receptors located on the pre-synaptic terminals (Figure 1)Reference 3. This retrograde signaling mechanism permits the regulation of neurotransmission in a precise spatio-temporal mannerReference 3. In immune cells, activation of CB2 receptors inhibits cytokine/chemokine release and neutrophil and macrophage migration, giving rise to complex modulatory effects on immune system functionReference 19.
Cannabinoid receptor expression and receptor distribution
Most tissues contain a functional ECS with the CB1 and CB2 receptors having distinct patterns of tissue expression. The CB1 receptor is one of the most abundant G-protein coupled receptors in the central and peripheral nervous systemsReference 19. It has been detected in the cerebral cortex, hippocampus, amygdala, basal ganglia, substantia nigra pars reticulata, internal and external segments of the globus pallidus and cerebellum (molecular layer), and at central and peripheral levels of the pain pathways including the periaqueductal gray matter, the rostral ventrolateral medulla, the dorsal primary afferent spinal cord regions including peripheral nociceptors, and spinal interneuronsReference 4Reference 23Reference 24. CB1 receptor density is highest in the cingulate gyrus, the frontal cortex, the hippocampus, the cerebellum, and the basal gangliaReference 5. Moderate levels of CB1 receptor expression are found in the basal forebrain, amygdala, nucleus accumbens, periaqueductal grey, and hypothalamus and much lower expression levels of the receptor are found in the midbrain, the pons, and the medulla/brainstemReference 5. Relatively little CB1 receptor expression is found in the thalamus and the primary motor cortexReference 5. The CB1 receptor is also expressed in many other organs and tissues including adipocytes, leukocytes, spleen, heart, lung, the gastrointestinal (GI) tract (liver, pancreas, stomach, and the small and large intestine), kidney, bladder, reproductive organs, skeletal muscle, bone, joints, and skinReference 25-Reference 43. CB2 receptors are most highly concentrated in the tissues and cells of the immune system such as the leukocytes and the spleen, but can also be found in bone and to a lesser degree in liver and in nerve cells including astrocytes, oligodendrocytes and microglia, and even some neuronal sub-populationsReference 44Reference 45.
Other molecular targets for cannabinoids
Besides the well-known CB1 and CB2 receptors, a number of different cannabinoids are believed to bind to a number of other molecular targets. Such targets include the third putative cannabinoid receptor GPR55 (G protein-coupled receptor 55), the transient receptor potential (TRP) cation channel family, and a class of nuclear receptors/transcription factors known as the PPARs, as well as 5-HT1A receptors, the α2 adrenoceptors, adenosine and glycine receptors. For additional details on this subject please see Section 2.1 and consult the following resourcesReference 8Reference 9Reference 22Reference 46-Reference 49. Modulation of these other cannabinoid targets adds additional layers of complexity to the known myriad effects of cannabinoids.
Signal termination
Endocannabinoid signaling is rapidly terminated by the action of two hydrolytic enzymes: FAAH and MAGLReference 3. FAAH is primarily localized post-synapticallyReference 50Reference 51 and preferentially degrades anandamideReference 14; MAGL is primarily localized pre-synapticallyReference 50Reference 51 and favors the catabolism of 2-AG (Figure 1)Reference 14. Signal termination is important in ensuring that biological activities are properly regulated and prolonged signaling activity, such as by the use of cannabis, can have potentially deleterious effectsReference 52Reference 53.
Dysregulation of the endocannabinoid system and general therapeutic challenges of using cannabinoids
Dysregulation of the ECS appears to be connected to a number of pathological conditions, with the changes in the functioning of the system being either protective or harmfulReference 54. Modulation of the ECS either through the targeted inhibition of specific metabolic pathways, and/or directed agonism or antagonism of its receptors may hold therapeutic promiseReference 13. However, a major and consistent therapeutic challenge confronting the routine use of (THC-predominant) cannabis and psychoactive cannabinoids (e.g. THC) in the clinic has remained that of achieving selective targeting of the site of disease or symptoms and the sparing of other bodily regions such as the mood and cognitive centres of the brainReference 23Reference 54-Reference 57. Despite this significant challenge, emerging evidence from clinical studies of smoked or vapourized (THC-predominant) cannabis for chronic non-cancer pain (mainly neuropathic pain) suggests that use of very low doses of THC (< 3 mg/dose) may confer therapeutic benefits with minimal psychoactive side effectsReference 58Reference 59 (and also see Section 3.0 and 4.7.2.2 for additional details).
Role of the endocannabinoid system in nervous system development
The CB1 receptor is highly expressed in the developing brainReference 60. For example, CB1 receptors are highly expressed from early fetal stages, beginning as early as E12.5 (in mice) and into late fetal stages (E21) with high expression in white matter within a number of different structures including the hippocampus, cerebellum, caudate-putamen and cerebral cortex that continues to increase after birth and into adulthood; in contrast, after birth there is tapering of CB1 receptor expression in other structures such as the corpus callosum, fornix, stria terminalis and the fasciculus retroflexusReference 60. Furthermore, in the adult brain, the CB1 receptor appears to be localized on the axonal plasma membrane and in somatodendritic endosomes, whereas in fetal brain the CB1 receptor is mostly localized to endosomes both in axons and in the somatodendritic regionReference 60. The available evidence suggests a neurodevelopmental role for the ECS including in functions such as survival, proliferation, migration and differentiation of neuronal progenitorsReference 60. CB1 receptor activation, in response to stimulation by endocannabinoids, such as 2-AG and anandamide, promotes these functions but delays the transition from multipotent, proliferating, and migration-competent progenitor phenotype towards a more settled, well-differentiated, post-mitotic neuronal phenotypeReference 60Reference 61. In vitro studies examining the effects of CB1 receptor activation in primary neuronal cultures suggest that the CB1 receptor is mainly a negative regulator of neurite growth since activation of the receptor results in growth cone arrest, repulsion or collapse and thereby influences the ability of axons to reach their targetsReference 60. However, these CB1 receptor-mediated responses may be surmountable by the effects of local growth-promoting effectors at the growth cone and the balance between the effects of endocannabinoids and growth factors would determine the overall outcome of neuronal development. The CB1 receptor appears also to act as a negative regulator of synaptogenesis and in doing so can also affect the fate of neuronal communicationReference 60. Exposure to cannabinoids that activate the CB1 receptor (such as THC) during developmental periods of nervous system development such as during embryonic development in pregnancy could alter the course of normal neuronal development in offspring and negatively affect normal brain function potentially causing long-lasting impairment of a number of cognitive functions and behavioursReference 61 (and also see Sections 2.5 and 7.4 for additional information). For example, a study conducted in pregnant mice using a low dose of THC has been shown to alter the expression level of 35 proteins in the fetal cerebrumReference 62. Furthermore this study concretely identified a specific molecular target for THC in the developing CNS whose modifications can directly and permanently impair the wiring of neuronal networks during corticogenesis by enabling formation of ectopic neuronal filopodia and altering axonal morphologyReference 62. Another in vitro study with retinal ganglion cell explants showed that CBD decreased neuronal growth cone size and filopodia number as well as total projection length and induced growth cone collapse and neurite retraction (i.e. chemo-repulsion) through the GPR55 receptorReference 63.
Figure 1. The Endocannabinoid System in the Nervous System
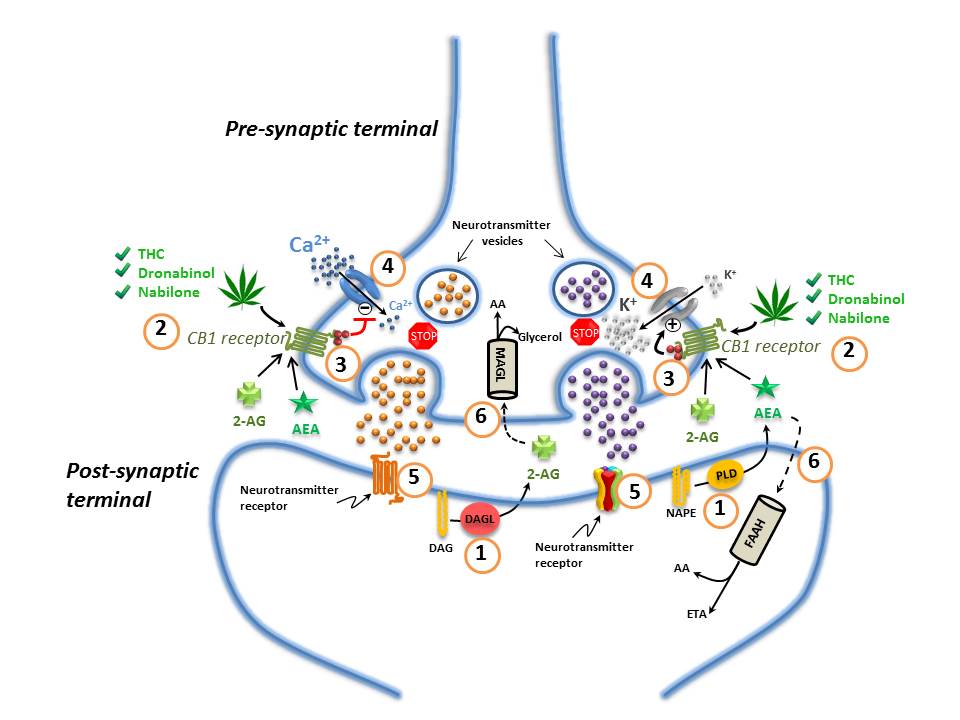
(1) Endocannabinoids are manufactured "on-demand" (e.g. in response to an action potential in neurons) in the post-synaptic terminals: anandamide (AEA) is generated from phospholipase-D (PLD)-mediated hydrolysis of the membrane lipid N-arachidonoylphosphatidylethanolamine (NAPE); 2-AG from the diacylglycerol lipase (DAGL)-mediated hydrolysis of the membrane lipid diacylglycerol (DAG); (2) These endocannabinoids (anandamide (AEA) and 2-AG) diffuse retrogradely towards the pre-synaptic terminals and like exogenous cannabinoids such as THC (from cannabis), dronabinol, and nabilone, they bind to and activate the pre-synaptic G-protein-coupled CB1 receptors; (3) Binding of phytocannabinoid and endocannabinoid agonists to the CB1 receptors triggers Gi/Go protein signalling that, for example, inhibits adenylyl cyclase, thus decreasing the formation of cyclic AMP and the activity of protein kinase A; (4) Activation of the CB1 receptor also results in Gi/Go protein-dependent opening of inwardly-rectifying K+ channels (depicted with a "+") causing a hyperpolarization of the pre-synaptic terminals, and the closing of Ca2+ channels (depicted with a "-"), arresting the release of stored excitatory and inhibitory neurotransmitters (e.g. glutamate, GABA, 5-HT, acetylcholine, noradrenaline, dopamine, D-aspartate and cholecystokinin) which (5) once released, diffuse and bind to post-synaptic receptors; (6) Anandamide and 2-AG re-enter the post- or pre-synaptic nerve terminals (possibly through the actions of a specialized transporter depicted by a "dashed" line) where they are respectively catabolized by fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL) to yield either arachidonic acid (AA) and ethanolamine (ETA), or arachidonic acid (AA) and glycerol. See text for additional details. Figure adapted fromReference 64-Reference 66.
1.1 Cannabis
1.1.1 Chemistry and composition
Cannabis sativa (i.e. cannabis, marihuana, marijuana) is a hemp plant that grows throughout temperate and tropical climatesReference 67. The leaves and flowering tops of Cannabis contain over 500 distinct compounds distributed among 18 different chemical classes, and harbor over 100 different phytocannabinoidsReference 68-Reference 71 The principal phytocannabinoids appear to be delta-9-tetrahydrocannabinol (i.e. Δ9-THC, THC), CBN, and cannabidiol (CBD)Reference 72-Reference 74, although the relative abundance of these and other phytocannabinoids can vary depending on a number of factors such as the Cannabis strain, the soil and climate conditions, and the cultivation techniquesReference 75Reference 76. Other phytocannabinoids found in cannabis include cannabigerol (CBG), cannabichromene (CBC), tetrahydrocannabivarin (THCV) and many othersReference 70. In the living plant, these phytocannabinoids exist as both inactive monocarboxylic acids (e.g. tetrahydrocannabinolic acid, THCA) and as active decarboxylated forms (e.g. THC); however, heating (at temperatures above 120 °C) promotes decarboxylation (e.g. THCA to THC)Reference 77-Reference 79. Furthermore, pyrolysis (such as by smoking) transforms each of the hundreds of compounds in cannabis into a number of other compounds, many of which remain to be characterized both chemically and pharmacologically. Therefore, cannabis can be considered a very crude drug containing a very large number of chemical and pharmacological constituents, the properties of which are only slowly being understood.
Among all the chemical constituents of cannabis, and particularly among the cannabinoids, Δ9-THC is by far the best studied and is responsible for many, if not most, of the physical and psychotropic effects of
cannabisReference 80. Other phytocannabinoids (e.g. CBD, CBC, CBG) are present in lesser amounts in the plant and have little, if any, psychotropic propertiesReference 80. However, Canadian licensed producers of cannabis for medical purposes have now made available a large variety of cannabis strains containing varying levels of THC and CBD, including THC-predominant, CBD-predominant or balanced strains for patients who have received authorization from their healthcare practitioner to access cannabis for medical purposes. For more information, please consult the Health Canada authorized licensed producers of cannabis for medical purposes website.
1.1.2 Other constituents
The large number of compounds found in cannabis spans many chemical classes including phytocannabinoids, nitrogenous compounds, amino acids, proteins, enzymes, glycoproteins, hydrocarbons, simple alcohols, aldehydes, ketones and acids, fatty acids, simple esters and lactones, steroids, terpenes, non-cannabinoid phenols, flavonoids, vitamins, and pigmentsReference 70. Furthermore, differences in the presence and the relative abundance of some of these various components have been investigated, and differences in various components have been noted between cannabis extract, vapour, and smoke, and also between cannabis varietiesReference 81. Of note, cannabis smoke contains many compounds not observed in either extracts or vapour, including a number which are known or suspected carcinogens or mutagensReference 81-Reference 83. Moreover, comparisons between cannabis smoke and tobacco smoke have shown that the former contains many of the same carcinogenic chemicals found in the latterReference 82Reference 84 (see Section 7.1 for more information).
Relatively little is known about the pharmacological actions of the various other compounds found within cannabis (e.g. terpenes, flavonoids). However, it is believed that some of these compounds (e.g. terpenes) may have a broad spectrum of action (e.g. anti-oxidant, anti-anxiety, anti-inflammatory, anti-bacterial, anti-neoplastic, anti-malarial), but this information comes from a few in vitro and in vivo studies and no clinical trials exist to support these claims. Terpenes vary widely among cannabis varieties and are thought to be primarily responsible for differences in fragrance among the different Cannabis strainsReference 75. Furthermore, it is thought that terpenes may contribute to the distinctive smoking qualities and possibly to the character of the "high" associated with smoking cannabisReference 75, but again, this has not been studied in any detail. The concept that terpenes may somehow modify or enhance the physiological effects of the cannabinoidsReference 85Reference 86,i.e. the "entourage effect", is, for the moment, hypothetical as there is little, if any, pre-clinical evidence to support this hypothesis and no clinical trials on this subject have been carried out to date.
1.1.3 Stability and storage
Most of the information on the stability of cannabis does not distinguish between Δ9-THC and its carboxylic acid (Δ9-THCA). The latter is transformed to Δ9-THC by heating during vapourization or cooking, or by pyrolysis during smoking or in the inlet of gas chromatographs used in forensic analysisReference 87. Complete decarboxylation of Δ9-THCA to Δ9-THC has been shown to occur starting at 98 °C and up to a temperature of 200 °C. As the temperature increases, the rate of decarboxylation increases: it takes 4 hours for complete decarboxylation at 98 °C, but only seconds at 200 °CReference 88-Reference 90. Heat, light, humidity, acidity and oxidation all affect the stability of cannabis and phytocannabinoidsReference 91Reference 92. The National Institute on Drug Abuse reports that retention samples of their carefully prepared and standardized cigarettes are stable for months, particularly when stored below 0 oC (-18 °C) in the dark, in tightly-closed containersReference 93. Even when stored at +18 °C, only a third of the Δ9-THC content is lost over a five-year period with some increase in the concentration of CBN. Cannabis cigarettes with lower Δ9-THC content (1.15% THC) appear to lose more Δ9-THC compared to cigarettes with higher Δ9-THC content (2.87% THC)Reference 93. Turner et al. found that the THC content of cannabis decayed at a rate of 3.83, 5.38, and 6.92% per year for cannabis stored at -18 °C, 4 °C and 22 °C respectivelyReference 94. Sevigny has provided the following formula for calculating decay of THC: THC0 = THCa / e-(k)(t) where THC0 is the unknown initial concentration of THC, THCa is the assayed concentration of THC, k is the decay rate constant which can vary according to two conditions: k = 0.0263 (the lower-bound average decay rate for samples stored in darkness at 3 ºC) and k = 0.0342 (the upper-bound average decay rate for samples stored in natural light of a laboratory at 22 °C), and t is the seizure-to-assay analysis lag (in months)Reference 95. For specific stability and storage conditions for cannabis provided by licensed commercial producers in Canada, please consult information provided by the licensed commercial producers.
2.0 Clinical Pharmacology
2.1 Pharmacodynamics
Much of the pharmacodynamic information on cannabis refers to the effects of the major constituent, Δ9-THC, which acts as a partial agonist at both CB receptorsReference 46Reference 48Reference 96, has activity at non-CB receptors and other targetsReference 46Reference 48Reference 97, and is responsible for the psychoactive and potential therapeutic effects of cannabis through its actions at the CB1 receptorReference 46Reference 48Reference 98. Δ8 -THC (an isomer of Δ9-THC) is found in smaller amounts in the plant, but like Δ9-THC, it is a partial agonist at both CB receptors and shares relatively similar efficacy and potency with Δ9-THC in in vitro assaysReference 96. An in vivo animal study and one clinical study suggest Δ8 -THC to be a more potent anti-emetic than Δ9-THCReference 99Reference 100.
CBN is a product of Δ9-THC oxidation and has 10% of the activity of Δ9-THC at the CB1 receptorReference 101. Its effects are not well studied but it appears to have some possible immunosuppressive properties in a small number of in vitro studiesReference 102.
CBG is a partial CB1/2 receptor agonist and a small number of in vitro studies suggest it may have some anti-inflammatory and analgesic propertiesReference 49Reference 101Reference 103Reference 104. For example, in vitro assays have shown that CBG, at a concentration of 100 µg/ml (approximately equivalent to a concentration of 300 µM and above the typical physiological range, and therefore not truly representative of human in vivo conditions), is associated with a greater than 30% inhibition of cyclooxygenase (COX) 1 and 2 enzymes, but only produced weak inhibition (<10%) of prostaglandin production in vivo at concentrations that did not cause cytotoxicityReference 104. Cannabigerolic acid has a similar profile. CBG has also been shown to block 5-HT1A receptors and act as an α2-adrenoceptor agonistReference 105. There is some emerging evidence that suggests CBG can produce signs of analgesia by activation of α2-adrenoceptorsReference 46.
CBD lacks detectable psychoactivity and does not appear to bind to either CB1 or CB2 receptors at physiologically meaningful concentrations, but there is some emerging evidence suggesting it may act as a non-competitive, negative, allosteric modulator of CB1 receptorsReference 106. There is also a considerable body of evidence suggesting CBD also affects the activity of a significant number of other targets including ion channels, receptors, and enzymesReference 18Reference 101Reference 107. For example, CBD has been shown to block the activity of FAAH resulting in an increase in anandamide levels, act as an agonist of the TRPV1 channel, inhibit adenosine uptake by acting as an indirect agonist at adenosine receptors, act as an agonist of 5-HT1A receptors, act as a positive allosteric modulator of glycine receptors, and act as an anti-oxidant and reactive oxygen species scavenger as well as regulating calcium homeostasis via the mitochondrial sodium/calcium (Na+/Ca2+)-exchangerReference 108. The effects of CBD at these and other molecular targets are associated with anti-inflammatory, analgesic, anti-nausea, anti-emetic, anti-psychotic, anti-ischemic, anxiolytic, and anti-epileptiform effectsReference 101Reference 108Reference 109.
THCV acts as a CB1 receptor antagonist and CB2 receptor partial agonist in vitro and in vivoReference 110Reference 111, as well as a 5-HT1A receptor agonistReference 47 and pre-clinical studies suggest it may have anti-epileptiform/anti-convulsant, anti-nociceptive and potential anti-psychotic propertiesReference 47Reference 108Reference 112.
Much of what is known about the beneficial properties of the non-psychotropic cannabinoids (e.g. CBD, THCV) is derived from in vitro and in vivo studies and few well-conducted, rigorous clinical studies of these substances exist. However, the results from these pre-clinical studies point to potential therapeutic indications such as psychosis, epilepsy, anxiety, sleep disturbances, neurodegeneration, cerebral and myocardial ischemia, inflammation, pain and immune responses, emesis, food intake, type-1 diabetes, liver disease, osteogenesis, and cancerReference 18Reference 101Reference 113. For more in-depth information on the pharmacology of cannabinoids, the reader is invited to consult the following resourcesReference 22Reference 46Reference 48Reference 101Reference 114.
Phytocannabinoid-phytocannabinoid interactions and phytocannabinoid differences among cannabis strains
Despite anecdotal claims, there is limited reliable information regarding actual or potential interactions, of biological or physiological significance, among phytocannabinoids, especially Δ9-THC and CBD. The limited information that exists is complex and requires further clarification through additional investigation. The following paragraphs summarize the available information on this subject.
Factors affecting the nature of the potential phytocannabinoid-phytocannabinoid interactions
Various studies have reported either potentiating, opposing, or neutral interactions between Δ9-THC and CBDReference 46Reference 48Reference 106Reference 115-Reference 136. The discrepancies in the nature of the interactions between Δ9-THC and CBD reported in the literature may be explained by differences in the doses and ratios of THC and CBD used in the different studies, differences in the routes of administration, dose ordering effects (CBD pre-treatment vs. simultaneous co-administration with Δ9-THC), differences in the duration or chronicity of treatment (acute vs. chronic), differences in the animal species used, as well as the particular biological or physiological end-points being measuredReference 123.
Pharmacokinetic vs. pharmacodynamic interactions
In general, there appear to be two types of mechanisms which could govern possible interactions between CBD and Δ9-THC: those of apharmacokinetic originReference 123Reference 129, and those of a pharmacodynamic originReference 133Reference 135. Despite the limited and complex nature of the available information, it generally appears that pre-administration of CBD may potentiate some of the effects of THC (through a pharmacokinetic mechanism). Potentiation of THC effects by CBD may be caused by inhibition of THC metabolism in the liver, resulting in higher plasma levels of THCReference 123Reference 129.Simultaneous co-administration of CBD and THC may result in the attenuation of some of the effects of THC (through a pharmacodynamic mechanism). Furthermore, the ratio between the two phytocannabinoids also appears to play a role in determining whether the overall effect will be of a potentiating or antagonistic nature. CBD-mediated attenuation of THC-induced effects may be observed when the ratio of CBD to THC is at least 8: 1Reference 120Reference 134, whereas CBD appears to potentiate some of the effects associated with THC when the CBD to THC ratio is around 2: 1Reference 120. Some emerging pre-clinical evidence suggests combined anti-emetic sub-threshold doses of THC and CBD or cannabidiolic acid (CBDA) may be effective in animal models of acute nausea and/or anticipatory nausea (see Section 4.3 for additional details).
Psychological and physiological effects associated with varying phytocannabinoid concentrations
A number of studies have examined the neurophysiological, cognitive, subjective, or behavioural effects of varying the concentrations of Δ9-THC, CBD, or other cannabinoids such as CBC in smoked cannabisReference 128Reference 137. In one study, 24 healthy men and women who had reported using cannabis at least 10 times in their lifetime were subjected to a double-blind, placebo-controlled, mixed between- and within-subject clinical trial that showed that deliberate systematic variations in the levels of either CBD or CBC in smoked cannabis were not associated with any significant differences in any of the measured subjective, physiological, or performance testsReference 128. In another study, the subjective effects associated with the smoked or oral administration of cannabis plant material were directly compared to those associated with smoked or oral administration of Δ9-THC (using matched doses of Δ9-THC) to normal, healthy subjectsReference 137. This double-blind, placebo-controlled, within-subject, crossover clinical study reported few reliable differences between the THC-only and whole-plant cannabis conditionsReference 137. The authors further concluded that other cannabinoids present in the cannabis plant material did not alter the subjective effects of cannabis, but they speculated that cannabis samples with higher levels of cannabinoids or different ratios of the individual cannabinoids could conceivably produce different results, although no evidence to support this claim was provided in the study. They also hypothesized that whole-plant cannabis and THC alone could differ on other outcome measures more relevant to clinical entities (e.g. spasticity or neuropathic pain). With the possible exception of one studyReference 138, (see Section 4.7.2.3. Cancer Pain), which suggested differences between a whole-plant cannabis extract (i.e. nabiximols, marketed as Sativex®) and THC alone on cancer pain analgesia, no other clinical studies have examined this possibility. One study compared the subjective and physiological effects of oral THC to those of nabiximols in normal, healthy subjectsReference 122. The authors reported the absence of any modulatory effect of CBD (or other components of cannabis) at low therapeutic cannabinoid doses, with the potential exception of the subjective "high"Reference 122.
An internet-based, cross-sectional study of 1 877 individuals with a consistent history of cannabis use reported that those individuals who had indicated using cannabis with a higher CBD to THC ratio had also reported experiencing fewer psychotic symptoms (an effect typically associated with exposure to higher doses of THC)Reference 139. However, the authors noted that the effects were subtle. The study was also hampered by a number of important methodological issues suggesting that the conclusions should be interpreted with caution.
Brunt et al. (2014) conducted a study examining the self-reported therapeutic satisfaction and subjective effects of different strains of pharmaceutical-grade cannabis sold in the NetherlandsReference 118. The authors reported that among the study population of about 100 patients using medical cannabis for conditions such as multiple sclerosis (MS), chronic pain, nausea, cancer and psychological problems, those who used cannabis with cannabinoid concentrations of 6% THC and 7.5% CBD (i.e. "low THC" cannabis) reported significantly less anxiety and dejection (i.e. feeling down, sad, depressed), but also reported less appetite stimulation. Importantly, those patients using the "low THC" condition reported equivalent levels of therapeutic satisfaction as those patients who reported using "high THC" (19% THC, < 1% CBD) and "medium THC" (12% THC, < 1% CBD) cannabis. There was also surprisingly little difference in terms of daily gram amount used between the different THC/CBD varieties with all categories reporting, on average, use of less than one gram of dried cannabis per day. The study findings are also consistent with the rest of the literature in terms of the average daily gram dose of dried cannabis used by patients (i.e. up to 3 g at most, but generally around one gram or less of variable THC content). Taken together, the study suggests that the use of cannabis containing approximately equivalent "lower" levels of THC and "higher" levels of CBD is associated with self-reported therapeutic efficacy and satisfaction across a number of different medical conditions for which dried cannabis is typically used, and also associated with attenuated levels of mood perturbation. The evidence also suggests that cannabis containing higher levels of THC and little CBD is not necessarily more effective than lower dose strains, except for stimulation of appetite. However, the study findings suggest that the use of higher-THC strains is associated with greater mood perturbation than the lower-THC strains. The study carried a number of caveats being that it only looked at a small number of patients, had a limited number of medical conditions and consisted of a self-reported survey.
Two in vivo studies conducted in non-human primates (i.e. rhesus monkeys) showed that CBD attenuated some of the effects of THC including cognitive-impairing effects and disruption of motor inhibitory behaviourReference 115Reference 119.
An in vivo study conducted in non-human primates (i.e. rhesus monkeys) showed that CBD, administered in a 1: 1 ratio with THC, attenuated some of the cognitive-impairing effects of THC, especially effects on spatial memory, but not on THC-induced performance deficits (i.e. non-specific motor and motivational effects)Reference 119. Another in vivo study conducted in non-human primates (i.e. rhesus monkeys) examining the acute and chronic effects of CBD on THC-induced disruption of motor inhibitory behaviour showed that CBD, at ratios of 3: 1 but not 1: 1 relative to THC, attenuated some of the acute and chronic behavioural effects of higher-dose THC on disruption of motor inhibitory behaviourReference 115.
In summary, although it appears that CBD may modulate some of the behavioural effects of THC, further careful study is required to elucidate the influence of CBD, and other phytocannabinoids or terpenoids, on the physiological or psychological effects associated with the use of Δ9-THC, as well as on any medical disorders.
Overview of pharmacological actions of cannabis
Most of the available information regarding the acute and long-term effects of cannabis use comes from studies conducted on non-medical users, with much less information available from clinical studies of patients using cannabis for medical purposes.
The acute effects of smoking or eating cannabis include euphoria (the marijuana "high") as well as cardiovascular, bronchopulmonary, ocular, psychological and psychomotor effects. Euphoria typically occurs shortly after smoking and generally takes longer with oral administrationReference 80. However, some people can experience dysphoria and anxietyReference 140. Tachycardia is the most consistent of the acute physiological effects associated with the consumption of cannabisReference 141-Reference 144.
The short-term psychoactive effects associated with cannabis smoking in non-medical users include the above-mentioned euphoria but also relaxation, time-distortion, intensification of ordinary sensory experiences (such as eating, watching films, and listening to music), and loss of inhibitions that may result in laughterReference 145. This is followed by a depressant periodReference 146. Most reviews note that cannabis use is associated with impaired function in a variety of cognitive and short-term memory tasksReference 102Reference 146-Reference 151 and the levels of Δ9-THC in the plasma after smoking appear to have a dose, time, and concentration-dependent effect on cognitive functionReference 152-Reference 154. Driving and operation of intricate machinery, including aircraft, may be significantly impairedReference 155-Reference 158.
Table 1 (below), adapted from a reviewReference 159, notes some of the pharmacological effects of cannabis in the therapeutic dosage range. Many of the effects are biphasic, with increased activity with acute or smaller doses, and decreased activity with larger doses or chronic useReference 141Reference 160Reference 161. Effects differ greatly among individuals and may be greater in those who are young, severely ill, elderly, or in those taking other drugs.
| Body System/Effect | Detail of Effects |
|---|---|
| Central Nervous System (CNS) | |
| Psychological
(Sections 4.9.5 and 7.7) |
Euphoria ("high"), dysphoria, anxiety, depersonalization, precipitation or aggravation of psychosis, schizophrenia or bipolar disorder (esp. in vulnerable individuals) and suicidal ideation/attempts (esp. among men), limited and mixed evidence in PTSD, mixed evidence for amotivational syndromeReference 80Reference 162-Reference 203. |
| Perception | Heightened sensory perception, distortion of space and time sense, hallucinations, misperceptionsReference 175Reference 179Reference 190Reference 204-Reference 211. |
| Sedative
(Sections 6.2 and 7.7) |
Generalized CNS depression, drowsiness, somnolence (dose-dependent effect on sleep); additive with other CNS depressants (opioids/alcohol)Reference 59Reference 141Reference 162Reference 172Reference 176Reference 179Reference 184Reference 185Reference 195Reference 212-Reference 227. |
| Cognition, psychomotor performance
(Sections 7.7.1 and 7.7.2) |
Fragmentation of thoughts, mental clouding (attention and concentration), memory impairment/amnesia, global impairment of performance especially in complex and demanding tasks and additive effect with other CNS depressants (e.g. alcohol)Reference 128Reference 149-Reference 151Reference 155-Reference 158Reference 185Reference 205Reference 206Reference 227-Reference 235. |
| Motor function
(Sections 4.9.1 and 7.7.2) |
Incoordination, ataxia, falls, dysarthria, weaknessReference 141Reference 172Reference 174Reference 176Reference 180Reference 206Reference 207Reference 222Reference 227Reference 236-Reference 240. Limited and mixed evidence in dystonia, Huntington's disease, Tourette's syndrome and Parkinson's diseaseReference 179Reference 241-Reference 261. |
| Epilepsy | Anti-epileptiform and anti-convulsive properties with CBD (and possibly also with CBDV and THCV)Reference 215Reference 217Reference 262-Reference 264. Mixed pro- and anti-epileptiform and pro- and anti-convulsive effects with THCReference 263Reference 265Reference 266. |
| Analgesic | Limited evidence of mixed effects for acute painReference 267-Reference 274. Modest effect for chronic non-cancer pain (mainly neuropathic)Reference 58Reference 59Reference 108Reference 176Reference 179Reference 184Reference 185Reference 195Reference 218Reference 222Reference 225Reference 226Reference 268Reference 273Reference 275-Reference 281 Modest/mixed effect for cancer painReference 138Reference 282-Reference 285. Mixed "opioid-sparing" effectReference 138Reference 280Reference 284Reference 286-Reference 288. Very limited evidence for mixed effects for headache and migraineReference 289-Reference 293. |
| Anti-nausea/anti-emetic; hyper-emetic (Sections 4.3 and 7.6.1) |
Observed with acute dosesReference 109Reference 286Reference 294-Reference 297. Tolerance may occur with chronic useReference 298. Conversely, nausea and/or vomiting may also be observed with use for medical purposesReference 227. Hyperemesis has also been observed with larger doses or chronic use in non-medical contextsReference 299-Reference 309. |
| Appetite
(Sections 4.4 and 4.9.8.4) |
Increased in normal, healthy subjects, but also in patients suffering from HIV/AIDS-associated anorexia/cachexiaReference 118Reference 179Reference 223Reference 224Reference 227Reference 310-Reference 313. Evidence mixed and modest for loss of appetite in cancerReference 314-Reference 321. Evidence weak for anorexia nervosaReference 322Reference 323. |
| Tolerance | To most behavioural and somatic effects, including the "high" (with chronic use)Reference 181Reference 229Reference 324-Reference 333. |
| Dependence, withdrawal syndrome | Dependence has been produced experimentally, and observed clinically, following prolonged intoxicationReference 145Reference 162Reference 190Reference 329Reference 334-Reference 337. Abstinence leads to withdrawal symptoms which can include anger, anxiety, restlessness, irritability, depressed mood, disturbed sleep, strange dreams, decreased appetite, and weight lossReference 190Reference 329Reference 338-Reference 342. |
| Cardiovascular and Cerebrovascular System | |
| Heart rate/rhythm | Tachycardia with acute dosing; tolerance developing with chronic exposureReference 141-Reference 144Reference 184Reference 185Reference 343-Reference 346. Premature ventricular contractions, palpitations, atrial fibrillation, ventricular arrhythmia also seen with acute dosesReference 144Reference 227Reference 347-Reference 351. |
| Peripheral circulation | Vasodilatation, conjunctival redness, supine hypertension, postural hypotensionReference 219Reference 227Reference 345Reference 347Reference 352-Reference 354. |
| Cardiac output | Increased cardiac outputReference 347 and myocardial oxygen demandReference 352. |
| Cerebral blood flow | Increased with acute dose, decreased with chronic use, region-dependent variationsReference 345Reference 355. |
| Myocardial infarction | Increased risk of acute myocardial infarction within one hour after smoking cannabis especially in individuals with existing cardiovascular diseaseReference 144Reference 352. |
| Stroke | Increased risk of experiencing stroke after an acute episode of smoking cannabisReference 347Reference 356Reference 357. |
| Carcinogenesis/mutagenesis | |
| (Section 7.1) | Cannabis smoke contains many of the same chemicals as tobacco smoke, and cannabis smoke condensates are more cytotoxic and mutagenic than condensates from tobacco smokeReference 82Reference 84. Conflicting evidence linking cannabis smoking and cancerReference 358-Reference 361. Possible link between cannabis smoking and testicular cancerReference 362. |
| Respiratory System | |
| Histopathological changes/ inflammation | Chronic cannabis smoking associated with histopathological changes in the lung (basal cell hyperplasia, stratification, goblet cell hyperplasia, cell disorganization, inflammation, basement membrane thickening, and squamous cell metaplasia)Reference 363. Long-term smoking associated with cough, increased production of phlegm, and wheezeReference 364. |
| Bronchodilatation
(Sections 4.9.3 and 7.2) |
Acute THC exposure causes dilatation; possibly reversed with chronic exposure (by smoking)Reference 364. Smoked/vapourized cannabis may worsen asthmatic symptomsReference 365Reference 366. |
| Pulmonary function (FEV1; FVC) | Acute, low-level exposure possibly stimulatory; long-term, heavy smoking possibly associated with decreased lung functionReference 364Reference 367-Reference 371. |
| Gastrointestinal System | |
| (Sections 4.9.8 and 7.6) | Decreased gastrointestinal motility, decreased secretion, decreased gastric/colonic emptying, anti-inflammatory actions, limited and mixed evidence of benefit in irritable bowel syndrome and inflammatory bowel diseaseReference 33Reference 185Reference 279Reference 372. Abdominal pain, nausea, vomiting, diarrheaReference 227. |
| Liver
(Sections 4.9.8.3 and 7.6.2) |
Increased risk of hepatic steatosis/fibrosis, especially in patients with Hepatitis CReference 35Reference 373-Reference 375. Increased Hepatitis C treatment adherence resulting in a potential sustained absence of detectable levels of Hepatitis C virusReference 376. |
| Pancreas | Risk of acute pancreatitis with chronic, daily, heavy useReference 377-Reference 381. |
| Musculoskeletal system | |
| (Sections 4.5.1, 4.5.3 and 4.8) | Possible positive effect in chronic pain associated with rheumatoid arthritisReference 382-Reference 384 and fibromyalgiaReference 184Reference 385Reference 386. May attenuate spasticity from MS and spinal cord injuryReference 225Reference 226Reference 278Reference 387. May negatively affect bone healingReference 388. |
| Eye | |
| (Section 4.9.2) | Limited evidence for decreased intraocular pressureReference 389-Reference 391. |
| Immune System | |
| (Section 7.3) | Complex immunomodulatory effects with suppressive and/or stimulatory effects (acute and chronic dosing)Reference 26Reference 392. Hypersensitivity/allergic reactionsReference 365Reference 366Reference 393Reference 394. |
| Reproductive System | |
| Males
(Sections 2.5 and 7.4) |
Follicle stimulating hormone (FSH), luteinizing hormone (LH) and testosterone levels either unaffected or decreased with chronic cannabis smokingReference 395 (but seeReference 396 which reports increased testosterone levels). Decreased sperm concentration and sperm count and altered morphology with chronic cannabis smoking in menReference 395Reference 396. Decreased sperm motility, capacitation and acrosome reaction with in vitro THC exposureReference 395. Dose-dependent stimulatory (low-dose) or inhibitory (high-dose) effects on sexual behaviour in menReference 395Reference 397 (but seeReference 398 which suggests increased coital frequency with increased frequency of use in men and women). |
| Females
(Sections 2.5 and 7.4) |
Acute administration of THC suppresses release of gonadotropin-releasing hormone (GnRH) and thyrotropin-releasing hormone (TRH) with decreased release of prolactin and gonadotropins (FSH and LH) in animal and human studiesReference 399. Association between cannabis use and menstrual cycle disruptions in women including: slightly elevated rate of menstrual cycles lacking ovulation (i.e. anovulatory cycles), higher risk of decreased fertility, prolonged follicular phase/delayed ovulation, though evidence is mixedReference 399. Chronic/sub-chronic administration of THC in animals: altered hypothalamic-pituitary-ovarian (HPO) axis function, disruption of follicular development, decreased estrogen and progesterone production, blocking of LH surge, anovulationReference 399. Cannabis can alter HPO axis functionality and ovarian hormones produced by the HPO axisReference 399. Dose-dependent stimulatory (low-dose) or inhibitory (high-dose) effects on sexual behaviour in womenReference 397 (but seeReference 398 which suggests increased coital frequency with increased frequency of use in men and women). |
2.2 Pharmacokinetics
This section covers human pharmacokinetics of smoked and vapourized cannabis, oral preparations including prescription cannabinoid medicines such as dronabinol (Marinol®) and nabiximols (Sativex®), and other routes of administration (e.g. rectal, topical). See Figure 2 (below) for a graphical representation of the pharmacokinetics of THC.
Figure 2. Pharmacokinetics of THC (and other cannabinoids). Figure adapted fromReference 400.
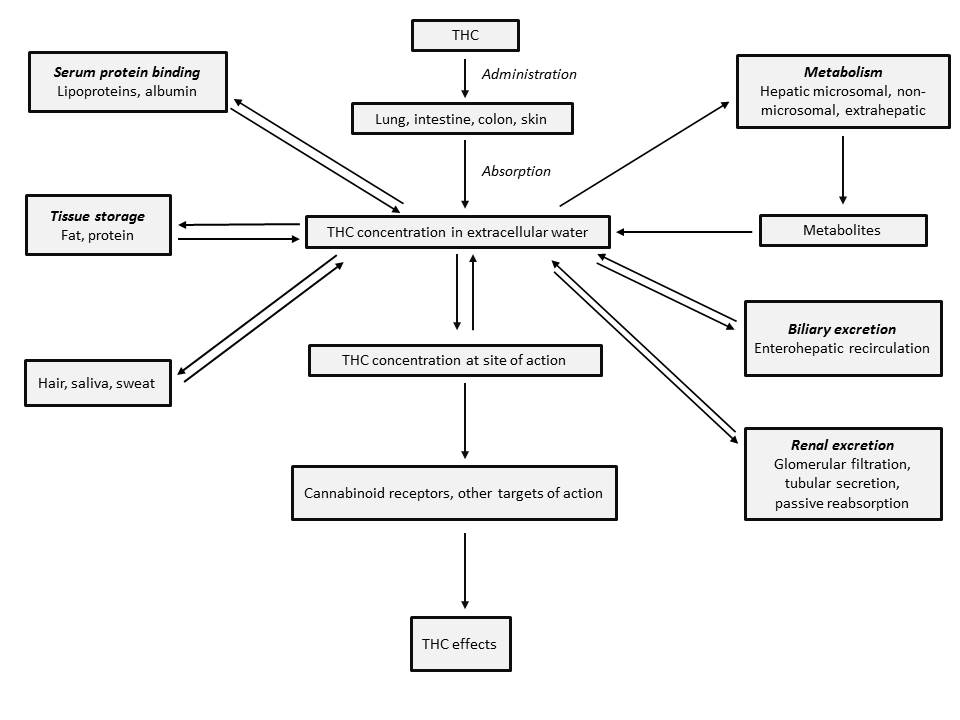
THC (and other cannabinoids) can be administered by inhalation (e.g. smoking/vapourizing), orally (e.g. edibles, capsules, sprays), rectally (e.g. suppositories) or dermally (e.g. topicals) resulting in absorption through the lung, intestine, colon or skin. The concentration of THC (and other cannabinoids) in the extracellular water varies depending on serum protein binding (lipoproteins, albumin), tissue storage (fat, protein), metabolism (hepatic microsomal, non-microsomal, extrahepatic), biliary excretion (enterohepatic recirculation) and renal excretion (glomerular filtration, tubular secretion, passive reabsorption). The metabolism of THC (and other cannabinoids) produces metabolites which can also be found in the extracellular water. The concentration of THC in the extracellular water affects the THC (and other cannabinoids) concentration at the site of action. The effects of THC (and other cannabinoids) are observed when THC (and other cannabinoids) interacts with cannabinoid receptors or other targets of action. THC (and other cannabinoids) can also be detected in hair, saliva and sweat.
2.2.1 Absorption
2.2.1.1 Smoked cannabis
Smoking cannabis results in more rapid onset of action (within minutes), higher blood levels of cannabinoids, and a shorter duration of acute pharmacodynamic effects compared to oral administrationReference 78. The amount of Δ9-THC (and other cannabinoids) delivered from cannabis cigarettes is not uniform and is a major variable in the assessment of absorptionReference 78. Uncontrolled factors include the source of the plant material and the composition of the cigarette/joint, together with the efficiency and method of smoking used by the subjectReference 78Reference 401. While it has been reported that smokers can titrate their Δ9-THC intake, to a certain extent, by adapting their smoking behaviour to obtain desired levels of Δ9-THCReference 402, other reasons may also explain the observed variation in smoking topographyReference 403. As mentioned, Δ9-THC absorption by inhalation is extremely rapid but quite variable, with a bioavailability of 2 to 56% through the smoking route depending on depth of inhalation, puff duration, and breathholdReference 400Reference 404. In practice, a maximum of 25 to 27% of the THC content in a cannabis cigarette is absorbed or delivered to the systemic circulation from the total available amountReference 141Reference 405. It has been estimated that between 2 and 44 µg of THC penetrates the brain following smoking of a cannabis cigarette containing 2 to 22 mg of THC (e.g. 1 g joint containing 0.2 - 2.2% THC, delivering between 0.2 and 5.5 mg of THC based on a smoked bioavailability of 10 to 25%)Reference 406.
The relationships between cannabis Δ9-THC content, dose administered, and resultant plasma levels have been investigated. Mean plasma Δ9-THC concentrations were 7.0 ng/mL and 18.1 ng/mL upon a single inhalation of either a 1.75% "low-dose" Δ9-THC cannabis cigarette (total available dose ~16 mg Δ9-THC), or a 3.55% Δ9-THC "high-dose" cannabis cigarette (total available dose ~34 mg Δ9-THC)Reference 78. Smoking cannabis containing 1.64% Δ9-THC (mean available dose 13.0 mg Δ9-THC) resulted in mean peak THC plasma levels of 77 ng/mLReference 407. Similarly, smoking cannabis joints containing 1.8% Δ9-THC (total available dose ~14 mg Δ9-THC) resulted in mean peak plasma THC levels of approximately 75 ng/mL, whereas with 3.6% Δ9-THC (total available dose ~28.8 mg Δ9-THC), mean peak plasma Δ9-THC levels of 100 ng/mL were attainedReference 408. Smoking a 25 mg dose of cannabis in a pipe containing 2.5, 6, or 9.4% Δ9-THC (total available doses of ~0.6, 1.5, or 2.4 mg Δ9-THC) was associated with mean peak plasma Δ9-THC concentrations of 10, 25, or 45 ng/mL Δ9-THC, respectivelyReference 59. Smoking one cannabis cigarette (800 mg) containing 6.8% THC, (w/w) yielding a total THC content of 54 mg per cigarette was associated with a median whole blood peak THC concentration of approximately 60 ng/mL Δ9-THC (occurring 15 min after starting smoking)Reference 409. Compared to the data available for absorption with smoked THC, there is far less such information available for smoked CBD. In one early clinical study, smoking one cannabis cigarette containing 19 mg CBD (~2.4% CBD) was associated with a mean peak blood plasma level of CBD of 110 ng/mL (range: 42 - 191 ng/mL) at 3 min post-doseReference 410. The estimated systemic bioavailability of CBD by smoking was 31 % (range: 11 - 45%), generally similar to that seen with Δ9-THC.
2.2.1.2 Vapourized cannabis
Vapourization of cannabis has been explored as an alternative to smoking. The potential advantages of vapourization include the formation of a smaller quantity of toxic by-products such as carbon monoxide, polycyclic aromatic hydrocarbons, and tar, as well as a more efficient extraction of Δ9-THC (and CBD) from the cannabis materialReference 402Reference 411-Reference 414. The subjective effects and plasma concentrations of Δ9-THC obtained by vapourization of cannabis are comparable to those obtained by smoking cannabisReference 402. In addition, the study reported that vapourization was well tolerated with no reported adverse effects, and was preferred over smoking by the test subjectsReference 402. While vapourization has been reported to be amenable to self-titration (as has been claimed for smoking)Reference 402Reference 413, the proper use of the vapourizer for optimal administration of cannabis for therapeutic purposes needs to be established in more detailReference 414. The amount and type of cannabis placed in the vapourizer, the vapourizing temperature and duration of vapourization, and, in the case of balloon-type vapourizers, the balloon volume are some of the parameters that can affect the delivery of Δ9-THC and other phytocannabinoidsReference 413. Bioequivalence of vapourization compared to smoking has not been thoroughly established. Inhalation of vapourized cannabis (900 mg of 3.56% Δ9-THC; total available dose of 32 mg of Δ9-THC) in a group of patients taking stable doses of sustained-release morphine or oxycodone resulted in mean plasma Δ9-THC levels of 126.1 ng/mL within 3 min after starting cannabis inhalation, rapidly declining to 33.7 ng/mL Δ9-THC at 10 min, and reaching 6.4 ng/mL Δ9-THC at 60 minReference 280. Peak Δ9-THC concentration (Cmax) was achieved at 3 min in all study participantsReference 280. No statistically significant changes were reported for the AUC12 (12-hour area-under-the-curve) for either morphine or oxycodone, but there appeared to be a statistically significant decrease in the Cmax of morphine sulfate, and a delay in the time needed to reach Cmax for morphine during cannabis exposureReference 280. One clinical study reported that vapourizing 500 mg cannabis containing low-dose (2.9%) THC (~14.5 mg THC), or high-dose (6.7%) THC (~33.5 mg THC) was associated with median whole-blood Cmax values of 32.7 (low-dose) and 42.2 ng/mL (high-dose) THC, and median plasma Cmax values of 46.5 (low-dose) and 62.1 ng/mL (high-dose) THC at 10 min post-inhalation respectivelyReference 206. Median whole-blood Cmax values for 11-hydroxy-THC were 2.8 (low-dose) and 5.0 ng/mL (high-dose) and median plasma Cmax values were 4.1 (low-dose) and 7 ng/mL (high-dose) at 10 - 11 min post-inhalation respectively. Another clinical study reported that vapourizing cannabis with 11 - 12% THC content (administered dose of 300 µg/kg) was associated with mean plasma concentrations of 73.8 ng/mL THC and 6.9 ng/mL 11-hydroxy-THC 5 min post-vapourizationReference 415. A different clinical study showed that inhalation of 8 to 12 puffs of vapourized cannabis containing either 2.9% or 6.7% THC (400 mg each) was associated with a blood plasma Cmax of 68.5 ng/mL and 177.3 ng/mL respectively and median blood plasma concentration of 23 and 47 ng/mL respectivelyReference 416. Plasma Cmax of 11-hydroxy-THC was 5.6 and 12.8 ng/mL for the 2.9 and 6.7% doses, respectively.
2.2.1.3 Oral
Whereas the acute effects on the CNS and physiological effects occur within minutes by the smoking route or by vapourizationReference 149Reference 417, the acute effects proceed on a time scale of hours in the case of oral ingestionReference 417Reference 418. Acute oral administration results in a slower onset of action, lower peak blood levels of cannabinoids, and a longer duration of pharmacodynamic effects compared to smokingReference 78. The psychotropic effect or "high" occurs much more quickly by the smoking than by the oral route, which is the reason why smoking appears to be the preferred route of administration by many, especially among non-medical usersReference 419.
For orally administered prescription cannabinoid medicines such as synthetic Δ9-THC (dronabinol, formerly marketed as Marinol®), only 10 to 20% of the administered dose enters the systemic circulation indicating extensive hepatic first-pass metabolismReference 227. Administration of a single 2.5 mg dose of dronabinol in healthy volunteers was associated with a mean plasma Δ9-THC Cmax of 0.7 ng/mL (range: 0.3 - 1 ng/mL), and a mean time to peak plasma Δ9-THC concentration of 2 h (range: 30 min - 4 h)Reference 227. A single 5 mg dose of dronabinol gave a reported mean plasma Δ9-THC Cmax of 1.8 ng/mL (range: 0.4 - 3.3 ng/mL), whereas a single 10 mg dose yielded a mean plasma Δ9-THC Cmax of 6.2 ng/mL (range: 3.5 - 9 ng/mL)Reference 227. Again, the mean time to peak plasma Δ9-THC concentration ranged from 30 min to 3 h. Twice daily dosing of dronabinol (individual doses of 2.5 mg, 5 mg, 10 mg, b.i.d.) in healthy volunteers yielded plasma Δ9-THC Cmax values of 1.3 ng/mL (range: 0.7 - 1.9 ng/mL), 2.9 ng/mL (range: 1.2 - 4.7 ng/mL), and 7.9 ng/mL (range: 3.3 - 12.4 ng/mL), respectively, with a time to peak plasma Δ9-THC concentration ranging between 30 min and 4 h after oral administrationReference 227. Continuous dosing for seven days with 20 mg doses of dronabinol (total daily doses of 40 - 120 mg dronabinol) gave mean plasma Δ9-THC concentrations of ~20 ng/mLReference 420.
A phase I study evaluating the pharmacokinetics of three oral doses of THC (3 mg, 5 mg and 6.5 mg) in 12 healthy older subjects (mean age 72, range: 65 - 80 years) showed wide inter-individual variation in plasma concentrations of THC and 11-hydroxy-THCReference 180. For those subjects who reached Cmax within 2 hours, the mean THC concentration was 1.42 ng/mL (range: 0.53 - 3.48 ng/mL) for the 3 mg dose, 3.15 ng/mL (range: 1.54 - 6.95 ng/mL) for the 5 mg dose, and 4.57 ng/mL (range: 2.11 - 8.65 ng/mL) for the 6.5 mg dose.
A randomized, double-blind, placebo-controlled, cross-over trial that evaluated the pharmacokinetics of oral THC in 10 older patients with dementia (mean age 77 years) over a 12-week period reported that median time to reach Cmax (Tmax) was between one and two hours with THC pharmacokinetics increasing linearly with increasing dose, but again with wide inter-individual variationReference 421. Patients received 0.75 mg THC orally twice daily over the first six weeks and 1.5 mg THC twice daily over the second six-week period. The mean Cmax after the first 0.75 mg THC dose was 0.41 ng/mL and after the first 1.5 mg THC dose was 1.01 ng/mL. After the second dose of 0.75 mg THC or 1.5 mg THC, the Cmax was 0.50 and 0.98 ng/mL respectively.
Δ9-THC can also be absorbed orally by ingestion of foods containing cannabis (e.g. butters, oils, brownies, cookies), and teas prepared from leaves and flowering tops. Absorption from an oral dose of 20 mg Δ9-THC in a chocolate cookie was described as slow and unreliableReference 401, with a systemic availability of only 4 to 12%Reference 407. While most subjects displayed peak plasma Δ9-THC concentrations (6 ng/mL) between one and two hours after ingestion, some of the 11 subjects in the study only peaked at 6 h, and many had more than one peakReference 78. Consumption of cannabis-laced brownies containing 2.8% Δ9-THC (44.8 mg total Δ9-THC) was associated with changes in behaviour, although the effects were slow to appear and variableReference 418. Peak effects occurred 2.5 to 3.5 h after dosing. Modest changes in pulse and blood pressure were also noted. Plasma concentrations of Δ9-THC were not measured in this study. In another study, ingestion of brownies containing a low dose of Δ9-THC (9 mg THC/brownie) was associated with mean peak plasma Δ9-THC levels of 5 ng/mLReference 137. Ingestion of brownies containing a higher dose of Δ9-THC (~13 mg Δ9-THC/brownie) was associated with mean peak plasma Δ9-THC levels of 6 or 9 ng/mL depending on whether the THC in the brownie came from plant material or was added as pure THCReference 137. Using equivalent amounts of Δ9-THC, inhalation by smoking cannabis yielded peak plasma levels of Δ9-THC several-fold (five to six times or more) higher than when Δ9-THC was absorbed through the oral routeReference 137. Tea made from dried cannabis flowering tops (19.1% Δ9-THCA, 0.6% Δ9-THC) has been documented, but the bioavailability of Δ9-THC from such teas is likely to be smaller than that achieved by smoking because of the poor water solubility of Δ9-THC and the extensive hepatic first-pass effectReference 422.
After oral administration of chocolate cookies containing 40 mg CBD in healthy human subjects, mean plasma CBD levels ranged between 1.1 and 11 ng/mL (mean: 5.5 ng/mL) after one hour and the course of CBD in the plasma over six hours was in the same range as the course after 20 mg THCReference 423. Daily oral doses of 10 mg/kg CBD for six weeks resulted in a mean weekly plasma concentration of 5.9 - 11.2 ng/mLReference 424. Oral intake of 5.4 mg CBD resulted in plasma CBD concentrations ranging between 0.2 and 2.6 ng/mL (mean: 0.95 ng/mL) after one hourReference 425. Bioavailability through the oral route was estimated at 6%Reference 423Reference 426.
While cannabinoids are lipophilic and anecdotal evidence suggests that cannabinoids dissolve better in fats and oils, the influence of various fats on cannabinoid absorption in vivo has been poorly studied. A pre-clinical study examined the effect of dietary fats on THC and CBD absorption in in ratsReference 427. A dose of 12 mg/kg of THC or CBD in either lipid-free formulation or lipid long-chain triglycerides (LCT)-based formulation (sesame oil) was administered to rats by oral gavage. The absolute bioavailability of THC was 2.5 times higher in the lipid-based (Cmax = 172 ng/mL; AUC = 1050 h.ng/mL) versus lipid-free formulation (Cmax = 65 ng/mL; AUC = 414 h.ng/mL). The absolute bioavailability of CBD was three times higher in the lipid-based (Cmax = 308 ng/mL; AUC = 932 h.ng/mL) versus lipid-free formulation (Cmax = 87 ng/mL; AUC = 327 h.ng/mL). Furthermore, an in vitro lipolysis model was used to assess the mechanism by which lipids could enhance the bioavailability of THC and CBD. Results showed that 30% of THC and CBD was solubilized in the micellar layer and therefore was readily available. Incubation studies suggested that cannabinoids have a 70 to 80% association range with natural chylomicrons from rat and human. Chylomicrons act as carriers in the intestine and potentially transfer THC and CBD to the systemic circulation via the intestinal lymphatic system and therefore avoid hepatic first-pass metabolism, which would explain the increased bioavailability with the lipid-based formulation. The authors concluded that administration of cannabinoids with a fatty meal or in the form of a lipid-rich cannabis-containing cookie may increase systemic exposure and therefore change the efficacy of the drug by turning a barely effective dose into a highly effective one, or even, a therapeutic dose into a toxic one.
In vitro and in vivo studies suggest that exposure of CBD to (simulated) gastric fluid results in the conversion of CBD to THC and hexahydrocannabinolsReference 428Reference 429. In mice, it was shown that hexahydrocannabinols could, as is typically observed with THC, produce cataleptogenic effectsReference 429. The clinical implications of this conversion of CBD to THC and hexahydrocannabinols are the subject of heated debate and currently unclear.
Comparing smoked, vapourized and oral administration
A randomized, double-blind, placebo-controlled, double-dummy, cross-over clinical study examined the pharmacokinetics of THC and its phase I and II metabolites between frequent and occasional cannabis smokers after smoked, vapourized and oral cannabis administrationReference 430. Cannabis plant material (800 mg) containing 6.9% THC and 0.20% CBD was used, delivering a maximal THC dose of 51 mg and a maximal CBD dose of 1.5 mg. Vapourization was carried out using the Volcano® vapourizer (210 °C). Cannabis was administered orally by ingestion of cannabis-containing brownies. In frequent cannabis smokers (≥ five times per week over previous three months), the mean baseline-adjusted THC Cmax after smoking was 151 ng/mL, after vapourization it was 85 ng/mL, and after oral consumption it was 15 ng/mL. Mean Tmax was 7 min (smoking), 5 min (vapourization), and 2.5 h (oral). The mean AUC0-72 h (ug ∙ h/L) was 200 (smoking), 174 (vapourization), and 167 (oral). In occasional cannabis smokers (> two times per month but ≤ three times per week), the mean baseline-adjusted THC Cmax after smoking was 52 ng/mL, after vapourization it was 48 ng/mL, and after oral consumption it was 10 ng/mL. Mean Tmax was 7 min (smoking), 7 min (vapourization), and 2.3 h (oral). The mean AUC0-72 h (ug ∙ h/L) was 20 (smoking), 12 (vapourization), and 43 (oral). In frequent cannabis smokers, the mean baseline-adjusted 11-hydroxy-THC Cmax after smoking was 9 ng/mL, after vapourization it was 5 ng/mL, and after oral consumption it was 7 ng/mL. Mean Tmax was 13 min (smoking), 11 min (vapourization), and 2.3 h (oral). The mean AUC0-72 h (ug ∙ h/L) was 31 (smoking), 27 (vapourization), and 52 (oral). In occasional cannabis smokers, mean baseline-adjusted Cmax after smoking was 3 ng/mL, after vapourization it was 2 ng/mL, and after oral consumption, it was 5 ng/mL. Mean Tmax was 13 min (smoking), 6 min (vapourization), and 2.4 h (oral). The mean AUC0-72 h (ug ∙ h/L) was 3 (smoking), 2 (vapourization), and 33 (oral). These findings suggest, among other things, that peak blood THC concentration (THC Cmax) was significantly lower after oral consumption compared to either route of inhalation and time to peak blood THC concentration (Tmax) occurred significantly later for oral consumption compared to inhalation for both frequent and occasional cannabis smokers. In addition, Cmax was significantly higher for the smoking route compared to vapourization, but only among frequent cannabis smokers. In addition, THC Cmax values were significantly greater among frequent smokers compared to occasional smokers after smoking and vapourization only, and 11-hydroxy-THC Cmax values were significantly greater among frequent smokers regardless of route of administration.
2.2.1.4 Oro-mucosal and intranasal
Following a single oro-mucosal administration of nabiximols (Sativex®) (four sprays totalling 10.8 mg Δ9-THC and 10 mg CBD), mean peak plasma concentrations of both THC (~5.5 ng/mL) and CBD (~3 ng/mL) typically occur within 2 to 4 h, although there is wide inter-individual variation in the peak cannabinoid plasma concentrations and in the time to onset and peak of effectsReference 431. When administered oro-mucosally, blood levels of Δ9-THC and other cannabinoids are lower than those achieved by inhalation of the same dose of smoked cannabis, but Δ9-THC blood levels are comparable to those seen with oral administration of dronabinolReference 121Reference 431. Oro-mucosal administration of nabiximols is also amenable to self-titrationReference 122Reference 383Reference 432Reference 433.
A small number of pre-clinical studies have explored intranasal administration of both THC and CBD. In one study in rabbits, intranasal administration of a 1 mg/kg dose of THC in a liquid solution or in a chitosan-based gel formulation produced a Cmax of 20 ng/mL and 31 ng/mL, with Tmax of 20 and 45 min respectively, compared to intravenous administration where the Cmax and Tmax were 1475 ng/mL and 0 min respectivelyReference 434. In rats, intranasal administration of 200 µg/kg CBD in various formulations yielded Cmax values ranging from 20 - 35 ng/mL with Tmax values ranging between 20 and 30 min; by comparison, intravenous administration yielded a Cmax of 3 596 ng/mLReference 435.
2.2.1.5 Rectal
While Δ9-THC itself is not absorbed through the rectal route, the pro-drug Δ9-THC-hemisuccinate is absorbed; this fact, combined with decreased first-pass metabolism through the rectal route, results in a higher bioavailability of Δ9-THC by the rectal route (52 - 61%) than by the oral routeReference 436-Reference 440. Plasma concentrations of Δ9-THC are dose and vehicle-dependent, and also vary according to the chemical structure of the THC esterReference 439. In humans, rectal doses of 2.5 to 5.0 mg of the hemisuccinate ester of Δ9-THC were associated with peak plasma levels of Δ9-THC ranging between 1.1 and 4.1 ng/mL within 2 to 8 h, and peak plasma levels of carboxy-Δ9-THC ranging between 6.1 and 42.0 ng/mL within 1 to 8 h after administrationReference 436.
2.2.1.6 Topical
Cannabinoids are highly hydrophobic, making transport across the aqueous layer of the skin the rate-limiting step in the diffusion processReference 78. No clinical studies have been published regarding the percutaneous absorption of cannabis-containing ointments, creams, or lotions. However, some pre-clinical research has been carried out on transdermal delivery of synthetic and natural cannabinoids using a dermal patchReference 441Reference 442. A patch containing 8 mg of Δ8-THC yielded a mean steady-state plasma concentration of 4.4 ng/mL Δ8 -THC within 1.4 h in a guinea pig model, and this concentration was maintained for at least 48 hReference 441. Permeation of CBD and CBN was found to be 10-fold higher than for Δ8-THCReference 443. Transdermal application of a gel containing CBD with or without permeation enhancers in hairless guinea pigs showed that Cmax without the enhancer was 9 ng/mL, and 36 ng/mL with the enhancer, and that maximal concentrations (Tmax) were reached by 38 and 31 h post-application, respectivelyReference 435. Furthermore, steady-state concentrations were 6 and 24 ng/mL without and with the permeation enhancer, respectively. Another pre-clinical study of a transdermal CBD gel formulation (1% or 10%) applied with increasing daily dose of 0.6, 3.1, 6.2 and 62 mg/day yielded plasma concentrations of 4 ng/mL, 18 ng/mL, 33 ng/mL, and 1 630 ng/mL respectivelyReference 444. Lastly, a pre-clinical study conducted with a 1% CBD cream reported a Cmax of 8 ng/mL, a Tmax of 38 h, and a steady-state plasma concentration of 6 ng/mLReference 445.
2.2.2 Distribution
Distribution of Δ9-THC is time-dependent and begins immediately after absorption. Due to its lipophilicity, it is taken up primarily by fatty tissues and highly perfused organs such as the brain, heart, lung, and liverReference 78. Δ9-THC has a large apparent volume of distribution, approximately 10 L/kg, because of its high lipid solubilityReference 446. The apparent average volume of distribution of CBD is 32.7 L/kg (higher than THC) owing also to its very high lipid solubilityReference 410. CBN has an even higher volume of distribution, 50 L/kgReference 447. The plasma protein binding of Δ9-THC and its metabolites is approximately 97%Reference 448Reference 449. Δ9-THC is mainly bound to low-density lipoproteins (LDL), with up to 10% present in red blood cellsReference 450, while the metabolite, 11-hydroxy-THC is strongly bound to albumin with only 1% found in the free-fractionReference 451.
The highest concentrations of Δ9-THC are found in the heart and in adipose tissue, with levels reaching 10 and 1 000 times that of plasma, respectivelyReference 452. Despite the high perfusion level of the brain, the blood-brain barrier appears to limit the access and accumulation of Δ9-THC in this organReference 78Reference 453Reference 454, and the delay in correlating peak plasma concentration to psychoactive effects may be attributed, in part, to the time required for Δ9-THC to traverse this barrierReference 401. Pre-clinical studies in mice suggest a more rapid penetration of 11-hydroxy-THC into the brain compared to the parent compound, on the order of 6: 1 for 11-hydroxy-THC to THCReference 400Reference 455Reference 456.
As mentioned, Δ9-THC accumulates and is retained in fatty tissue, and its release from this storage site into the blood is slowReference 453. It is also not entirely certain if Δ9-THC persists in the brain (a highly fatty tissue) in the long-term; however, the presence of residual cognitive deficits in abstinent heavy cannabis users suggests this may be the case, at least in the short-termReference 457Reference 458. A study that characterized cannabinoid elimination in blood from 30 male daily cannabis smokers during monitored sustained abstinence for up to 33 days on a closed residential unit found that both THC and its inactive metabolite 11-nor-9-carboxy Δ9-THC were detected in blood up to one month after last smoking, which was reported by the authors as being four times longer than previously describedReference 459. This finding lends further support to the evidence on the distribution, accumulation, and storage of THC (and metabolites) in the adipose tissue and the slow release of THC (and metabolites) from adipose tissue stores back into the bloodstreamReference 229. Residual THC in plasma (likely coming from bodily adipose stores) detected weeks after last smoking episode may be associated with persisting psychomotor impairment in frequent chronic cannabis smokers according to the study authorsReference 229. Lastly, one animal study suggested food deprivation or adrenocorticotropic hormone (ACTH) administration in rats accelerates lipolysis and the release of Δ9-THC from fat stores, however further research is needed to determine if these effects are associated with subsequent intoxication or behavioural/cognitive changesReference 460.
2.2.3 Metabolism
Most cannabinoid metabolism occurs in the liver, and different metabolites predominate depending on the route of administrationReference 78Reference 401. The complex metabolism of Δ9-THC involves allylic oxidation, epoxidation, decarboxylation, and conjugationReference 401. Δ9-THC is oxidized by the xenobiotic-metabolizing cytochrome P450 (CYP) mixed-function oxidases 2C9, 2C19, and 3A4Reference 78. The major initial metabolites of Δ9-THC are the active 11-hydroxy Δ9-THC, and the non-active 11-nor-9-carboxy Δ9-THCReference 78. The psychoactive 11-hydroxy Δ9-THC is rapidly formed by the action of the above-mentioned hepatic microsomal oxidases, and plasma levels of this metabolite parallel the duration of observable drug actionReference 461Reference 462.
CBD undergoes extensive Phase I metabolism, with a reported 30 different metabolites in the urine, and the most abundant metabolites are hydroxylated 7 (or 11)-carboxy derivatives of CBD, with 7 (or 11)-hydroxy CBD as a minor metaboliteReference 78Reference 463Reference 464.
CYP isozyme polymorphisms may also affect the pharmacokinetics of THC (and 11-nor-9-carboxy Δ9-THC). For example, subjects homozygous for the CYP2C9*3 allelic variant displayed significantly higher maximum plasma concentrations of Δ9-THC, significantly higher AUC, and significantly decreased clearance among other measures compared to the CYP2C9*1 homozygote or the *1/*3 heterozygoteReference 465.
Xenobiotics are not only metabolized by CYPs but they also modulate the expression level and activity of these enzymes; CYPs are therefore a focal point in drug-drug interactions and adverse drug reactionsReference 466. Polyaromatic hydrocarbons found in tobacco and cannabis smoke induce the expression of CYP1A2Reference 467, while Δ9-THC, CBD, and CBN inhibit the activity of the CYP1A1, 1A2, 1B1 and 2A6 enzymesReference 74Reference 468. CBD has also been shown to inhibit the formation of Δ9-THC metabolites catalyzed by CYP3A4, with less effect on CYP2C9Reference 446, albeit sufficiently to decrease the formation of 11-hydroxy-THCReference 129Reference 469. Please see Section 6.2 for more detailed information.
Results from in vitro experiments also suggest that Δ9-THC inhibits CYP3A4, CYP3A5, CYP2C9, and CYP2C19, while CBD inhibits CYP2C19, CYP3A4, and CYP3A5; however, higher concentrations than those seen clinically appear to be required for inhibitionReference 74Reference 431. While few clinical studies have specifically sought to evaluate cannabis-drug interactions per se, many, if not most, studies investigating the therapeutic effects of cannabis (e.g. smoked, vapourized, or orally ingested) and cannabinoid-based medicines (e.g. dronabinol, nabilone, nabiximols) have used patients that were concomitantly taking other medications (e.g. nonsteroidal anti-inflammatory agents (NSAIDs), opioids, anti-depressants, anti-convulsants, protease inhibitors) and, in general, did not report significantly increased incidences of severe adverse effects associated with the combination of cannabis or cannabinoids and these other medications. Nevertheless, careful monitoring of patients who are concomitantly consuming cannabis/cannabinoids and other medications that are metabolized by the above-mentioned enzymes may be warranted. Please see Section 6.2 for more detailed information.
The Sativex® product monograph cautions against combining Sativex® with amitriptyline or fentanyl (or related opioids) which are metabolized by CYP3A4 and 2C19Reference 431. One clinical study that investigated the effects of rifampicin, ketoconazole, and omeprazole on the pharmacokinetics of THC and CBD delivered from Sativex® reported that co-administration of rifampicin with Sativex® is associated with slight decreases in the plasma levels of THC, CBD and 11-hydroxy-THC, while co-administration of ketoconazole with Sativex® is associated with slight increases in plasma levels of THC, CBD, and 11-hydroxy-THCReference 470. No significant effects on plasma levels of THC, CBD or 11-hydroxy-THC were noted with omeprazole.
Cannabis smoking, as well as orally administered dronabinol may also affect the pharmacokinetics of anti-retroviral medications, although no clinically significant short-term impacts on anti-retroviral effects were notedReference 471. Concomitant administration of cannabis as a herbal tea (200 mL, 1 g per liter; 18% THC, 0.8% CBD) with 600 mg i.v. irinotecan or 180 mg i.v. docetaxel for 15 consecutive days did not significantly affect the plasma pharmacokinetics of irinotecan or docetaxelReference 472.
In addition, and as seen with tobacco smoke, cannabis smoke has the potential to induce CYP1A2 thereby increasing the metabolism of xenobiotics biotransformed by this isozyme such as theophyllineReference 473 or the anti-psychotic medications clozapine or olanzapineReference 474. Further detailed information on drug-drug interactions can be found in Section 6.2.
2.2.3.1 Inhalation
Plasma values of 11-hydroxy-THC appear rapidly and peak shortly after Δ9-THC, at about 15 min after the start of smokingReference 475. Peak plasma concentrations of 11-hydroxy-THC are approximately 5% to 10% of parent THC, and the AUC profile of this metabolite averages 10 to 20% of the parent THCReference 462. Similar results were obtained with intravenous THC administrationReference 476. Following oxidation, the phase II metabolites of the free drug or hydroxylated-THC appear to be glucuronide conjugatesReference 401.
Peak plasma values of the psycho-inactive metabolite, 11-nor-9-carboxy THC, occur 1.5 to 2.5 h after smoking, and are about one third the concentration of parent THCReference 475.
2.2.3.2 Oral
In contrast to the limited metabolism of Δ9-THC to the 11-hydroxy metabolite through pulmonary administration, oral administration of Δ9-THC results in a significantly greater metabolism of Δ9-THC to the 11-hydroxy metabolite resulting in similar plasma concentrations of Δ9-THC and 11-hydroxy Δ9-THC through the oral routeReference 404Reference 418Reference 477. The plasma levels of active 11-hydroxy metabolite, achieved through oral administration, are about three times higher than those seen with smokingReference 462. Furthermore, 11-hydroxy- Δ9-THC has been reported to be as psychoactive or even more psychoactive than the parent THCReference 400Reference 406Reference 478-Reference 480. Concentrations of both parent drug and metabolite peak between approximately 2 to 4 h after oral dosing, and decline over several daysReference 481.
Information from the dronabinol (Marinol®) product monograph suggests that single doses of 2.5 mg, 5 mg, and 10 mg of Δ9-THC in healthy volunteers result in mean plasma Cmax values of 11-hydroxy Δ9-THC of 1.19 ng/mL (range: 0.4 - 1.9 ng/mL), 2.23 ng/mL (range: 0.7 - 3.7 ng/mL), and 7.51 ng/mL (range: 2.25 - 12.8 ng/mL), respectivelyReference 227. Twice daily dosing of dronabinol (individual doses of 2.5 mg, 5 mg, 10 mg, b.i.d.) in healthy volunteers resulted in mean plasma Cmax values of 1.65 ng/mL (range: 0.9 - 2.4 ng/mL), 3.84 ng/mL (range: 1.5 - 6.1 ng/mL), and 7.95 ng/mL (range: 4.8 - 11.1 ng/mL) of 11-hydroxy Δ9-THC, respectivelyReference 227. Time to reach Cmax for 11-hydroxy Δ9-THC ranged from 30 min to 4 h, with a mean of approximately 2.5 hReference 227. As stated above, 11-hydroxy Δ9-THC has psychotomimetic properties equal to or greater than those of Δ9-THCReference 404Reference 406Reference 478-Reference 480Reference 482Reference 483.
2.2.4 Excretion
Δ9-THC and CBD levels in plasma decrease rapidly after cessation of smoking. Mean THC plasma concentrations are approximately 60% and 20% of peak plasma THC concentrations 15 and 30 min post-smokingReference 484, respectively, and are below 5 ng/mL THC 2 h after smoking, although mean serum THC concentrations may be slightly higher when smoking higher THC potency cigarettesReference 404. One study showed that CBD levels fall to below 5 ng/mL in the plasma about 2.5 h after smoking a 19 mg CBD cigaretteReference 410.
Following smoking, elimination of THC and its metabolites occurs via the feces (65%) and the urine (20%)Reference 78. Whole-body clearance of Δ9-THC and its hydroxy metabolite averages about 0.2 L/kg-h, but is highly variable due to the complexity of cannabinoid distributionReference 227. The psycho-inactive 11-nor-9-carboxy Δ9-THC is the primary acid metabolite of Δ9-THC excreted in urine and itReference 485 is the cannabinoid often screened for in forensic analysis of body fluidsReference 486Reference 487. A study that characterized cannabinoid elimination in blood from 30 male daily cannabis smokers during monitored sustained abstinence for up to 33 days on a closed residential unit found that low levels (approx. < 1 ng/mL) of both THC and its inactive metabolite 11-nor-9-carboxy THC were detected in blood up to one month after last smoking, which was reported by the authors as being four times longer than previously describedReference 459.
Following oral administration, THC and its metabolites are also excreted in both the feces and the urineReference 78Reference 462. Biliary excretion is the major route of elimination, with about half of a radiolabelled THC oral dose being recovered from the feces within 72 h in contrast to the 10 to 15% recovered from urineReference 462. Plasma clearance of CBD is similar to that of THC, ranging from 58 to 94 L/h (i.e. 960 - 1560 ml/min)Reference 400Reference 410. A large portion of administered CBD is excreted intact or as its glucuronideReference 463Reference 488Reference 489. Sixteen percent of an administered dose of CBD was recovered in the urine as intact or conjugated CBD within 72 h, while 33% of an administered dose of CBD was recovered mostly unchanged (accompanied by several mono-, di-hydroxylated and mono-carboxylic metabolites) in the feces within 72 hReference 410Reference 463.
The decline of Δ9-THC levels in plasma is multi-phasic, and the estimates of the terminal half-life of Δ9-THC in humans have progressively increased as analytical methods have become more sensitiveReference 446. While figures for the terminal elimination half-life of Δ9-THC appear to vary, it is probably safe to say that it averages at least four days and could be considerably longerReference 78. The variability in terminal half-life measurements are related to the dependence of this measure on assay sensitivity, as well as on the duration and timing of blood measurementsReference 490. Low levels of THC metabolites have been detected for more than five weeks in the urine and feces of cannabis usersReference 446. The degree of Δ9-THC consumption does not appear to influence the plasma half-life of Δ9-THCReference 401Reference 491.
Like THC, the decline of CBD levels is also multi-phasic, and the half-life of CBD in humans after smoking has been estimated at 27 - 35 h, and 2 - 5 days after oral administrationReference 401Reference 426Reference 464.
2.3 Pharmacokinetic-pharmacodynamic relationships
Much of the information on cannabinoid pharmacokinetic-pharmacodynamic relationships (mostly on Δ9-THC) is derived from studies of non-medical cannabis use rather than from studies looking at therapeutic use, but in either case, this relationship depends to some extent on the point in time at which observations are made following the administration of the cannabinoid. Furthermore, the temporal relationship between plasma concentrations of Δ9-THC and the associated clinical/therapeutic, psychotropic, cognitive and motor effects is not well established. But it is known that these effects often lag behind the plasma concentrations of Δ9-THC, and tolerance is known to develop to some of the effects but not to othersReference 128Reference 211Reference 490 (See Section 2.4 Tolerance and Dependence).
As mentioned above, the relationship between dose (and plasma concentration) versus response for possible therapeutic applications is ill-defined, except for some information obtained for oral dosing with dronabinol (synthetic Δ9-THC, marketed as Marinol® but no longer available in Canada), nabiximols (a botanical cannabis extract containing approximately equal concentrations of Δ9-THC and CBD as well as other cannabinoids, terpenoids and flavonoids, marketed as Sativex®), or nabilone (synthetic Δ9-THC analog marketed as Cesamet®) for their limited indicationsReference 227Reference 431Reference 492. More limited information is available for inhaled cannabisReference 58Reference 59. Interpretations of the pharmacokinetics of Δ9-THC are also complicated by the presence of active metabolites, particularly the potent psychoactive 11-hydroxy THC metabolite, which is found in higher concentration after oral administration than after inhalationReference 418Reference 477.
Target Δ9-THC plasma concentrations have been derived based on the subjective "high" response that may or may not be related to the potential therapeutic applications. Various pharmacodynamic models provide blood plasma concentration estimates in the range of 7 to 29 ng/mL Δ9-THC necessary for the production of a 50% maximal subjective "high" effectReference 490. Other studies suggest that Δ9-THC plasma concentrations associated with 50% of the maximum "high" effect range between 2 and 250 ng/mL Δ9-THC (median of 19 ng/mL; mean of 43 ng/mL Δ9-THC) for the smoked or intravenous routes, while for the oral route the values range between 1 and 8 ng/mL Δ9-THC (median and mean of 5 ng/mL Δ9-THC)Reference 137Reference 493. Notably, impairment of driving performance is seen with plasma concentrations between 7 and 10 ng/mL (whole blood, approximately 3 - 5 ng/mL) and this blood THC concentration has been compared to a blood-alcohol concentration (BAC) of 0.05% which itself is associated with driver impairmentReference 154.
Smoked cannabis
Simulation of multiple dosing with a 1% THC cigarette containing 9 mg Δ9-THC yielded a maximal "high" lasting approximately 45 min after initial dosing, declining to 50% of peak at about 100 min following smokingReference 211. A dosing interval of 1 h with this dose would give a "continuous high", and the recovery time after the last dose would be 150 min (i.e. 2.5 h). The peak Δ9-THC plasma concentration during this dosage is estimated at about 70 ng/mL.
One clinical study reported a peak increase in heart rate and perceived "good drug effect" within 7 min after test subjects smoked a 1 g cannabis cigarette containing either 1.8% or 3.9% THC (mean doses of Δ9-THC being 18 mg or 39 mg in the cigarette, respectively)Reference 149. Compared to the placebo, both doses yielded statistically significant differences in subjective and physiological measures; the higher dose was also significantly different from the lower dose for subjective effects, but not physiological effects such as an effect on heart rate. Pharmacokinetic-pharmacodynamic modelling of the concentration-effect relationship of Δ9-THC on CNS parameters and heart rate suggests that THC-evoked effects typically lag behind THC plasma concentration, with the effects lasting significantly longer than Δ9-THC plasma concentrationsReference 494. The equilibration half-life estimate for heart rate was approximately 7 min, but varied between 39 and 85 min for various CNS parametersReference 494. According to this model, the effects on the CNS developed more slowly and lasted longer than the effect on heart rate.
The psychomotor performance, subjective, and physiological effects associated with whole-blood Δ9-THC concentrations in heavy, chronic, cannabis smokers following an acute episode of cannabis smoking have been studiedReference 409. Subjects reported smoking a mean of one joint per day in the previous 14 days prior to the initiation of the study (range: 0.7 - 12 joints per day). During the study, subjects smoked one cannabis cigarette (mean weight 0.79 g) containing 6.8% THC, 0.25% CBD, and 0.21% CBN (w/w) yielding a total THC, CBD, and CBN content of 54, 2.0, and 1.7 mg of these cannabinoids per cigarette. Mean peak THC blood concentrations and peak Visual Analogue Scale (VAS) scores for different subjective measures occurred 15 min after starting smoking. According to the authors of the study, the pharmacodynamic-pharmacokinetic relationship displayed a counter-clockwise hysteresis (i.e. where for the same plasma concentration of a drug (e.g. THC), the pharmacological effect is greater at a later time point than at an earlier one) for all measured subjective effects (e.g. "good drug effect", "high", "stoned", "stimulated", "sedated", "anxious", and "restless"). This particular kind of relationship demonstrates a lack of correlation between blood concentrations of THC and observed effects, beginning immediately after the end of smoking and continuing during the initial distribution and elimination phases. All participants reported a peak subjective "high" between 66 and 85 on the VAS, with peak whole blood THC concentrations at the time of these responses ranging from 13 to 63 ng/mL. Following the start of cannabis smoking, heart rate increased significantly at the 30 min time point, diastolic blood pressure decreased significantly only from the 30 min to 1 h time point, and systolic blood pressure and respiratory rate were unaffected at any time.
A study that examined the acute subjective effects associated with smoked cannabis at three different doses (i.e. 29.3, 49.1 and 69.4 mg THC) reported that THC significantly increased feelings of "high", "dizziness", "impaired memory and concentration" as well as "down", "sedated" and "anxious" feelingsReference 495. In addition, the study also showed that higher doses of THC were associated with longer duration of subjective effects. Findings from the study showed that the time required to reach a maximal "high" rating was slightly delayed (11 - 16 min) compared to the time required to reach the peak THC serum concentration. The "high" rating declined after reaching the peak within the first 3.5 h post-dose. Scores on the VAS for "dizziness", "dry mouth", "palpitations", "impaired memory and concentration", "down", "sedated", and "anxious feelings" reached a maximum within the first 2 h post-dose and these effects were dose-dependent. With a dose of 29.3 mg THC in the cigarette (equivalent to, for example, a 300 mg joint containing 10% THC or 150 mg of a 20% THC joint), the maximal serum THC concentration was ~120 ng/mL and was associated with a 50% maximal "high". A dose of 49.1 mg THC in the cigarette (equivalent to, for example, a 500 mg joint containing 10% THC or a 250 mg joint containing 20% THC) was associated with a maximal serum THC concentration of 170 ng/mL and a 60% maximal "high". Finally, a THC dose of 69.4 mg of THC (equivalent to, for example, 700 mg of a 10% THC joint or 350 mg of a 20% THC joint) was associated with a serum THC concentration of 200 ng/mL and an 80% maximal "high". The THC-induced decrease in stimulation (i.e. sedation) and increase in anxiety lasted up to 8 h post-smoking. In fact, sedation was increased by almost six-fold compared to placebo. The low THC dose was associated with the highest ratings of "like the effects of the drug" and "want more of this drug".
Vapourized cannabis
Inhalation of vapourized cannabis (900 mg of 3.56% Δ9-THC; total available dose of 32 mg of Δ9-THC) resulted in mean plasma Δ9-THC levels of 126.1 ng/mL within 3 min after starting cannabis inhalation, rapidly declining to 33.7 ng/mL Δ9-THC at 10 min, and reaching 6.4 ng/mL Δ9-THC at 60 minReference 280. Peak Δ9-THC concentration (Cmax) was achieved at 3 min in all study participants. Maximal subjective "high" ratings occurred at 60 min following beginning of inhalation.
One clinical study reported that ad libitum vapourization of 500 mg cannabis containing a low-dose (2.9%) of THC (~14.5 mg THC), or high-dose (6.7%) of THC (~33.5 mg THC) was associated with median whole-blood Cmax values of 32.7 (low-dose) and 42.2 ng/mL (high-dose) THC, and median plasma Cmax values of 46.5 (low-dose) and 62.1 ng/mL (high-dose) THC at 10 min post-inhalationReference 206. Median whole-blood Cmax values for 11-hydroxy-THC were 2.8 (low-dose) and 5.0 ng/mL (high-dose) and median plasma Cmax values were 4.1 (low-dose) and 7 ng/mL (high-dose) at 10 - 11 min post-inhalation. Subjective effects were then measured at several time points and effects were correlated with concentrations of cannabinoids in oral fluid and blood. Blood THC was positively associated with "high", "good drug effect", "stimulated", "stoned", "anxious", and "restless" and with feelings of altered time, "slowed/slurred speech", "dizziness", and "dry mouth/throat". There were no significant differences between the effects seen with the low (2.9%) and the high (6.7%) dose of cannabis. Vapourized cannabis significantly increased measures of "stoned" and "sedated" immediately post-dose and lasted 3.3 h (or 4.3 h with the addition of alcohol). Feelings of "anxious" showed significant cannabis-dose effects through 1.4 h. Undesirable effects, including "feeling thirsty" and "dry mouth/throat", increased for the first 3.3 h post-dose. "Difficulty concentrating" and "altered sense of time" produced mixed effects over 2.3 h. Effects and time course of effects were similar between vapourized and smoked cannabis.
Another study measured 17 different psychoactive effects as a function of THC dose and time in vapourized cannabisReference 276. In this randomized, double-blind, placebo-controlled clinical study, patients inhaled a total of 8 to 12 puffs of vapourized cannabis containing either 0%, 2.9% or 6.7% THC (400 mg each). The 2.9% dose was associated with a Cmax of 68.5 ng/mL and the 6.7% dose was associated with a Cmax of 177.3 ng/mL. Plasma 11-hydroxy-THC Cmax for the 2.9% dose was 5.6 ng/mL and for the 6.7% dose was 12.8 ng/mL. The lower dose produced effects lower than that for the high dose and placebo effects were lower than both active doses for "any drug effect", "good drug effect", "high", "impaired", "stoned", "sedated" and "changes perceiving space". For "bad drug effect", "like the drug", "nauseous", "changes perceiving time", ratings with placebo were significantly lower than both active doses. The higher dose (6.7%) was associated with significantly higher ratings of "desires more", "hungry", "difficulty remembering things", "drunk", "confused", and "difficulty paying attention" compared with placebo, with only "drunk", "confused" and "difficulty paying attention" significantly different between the high and low dose. There was a clear dose-response effect for the majority of psychoactive effects.
Oral and oro-mucosal cannabinoids
The subjective and physiological effects after controlled administration of oro-mucosal nabiximols (Sativex®) or oral Δ9-THC have also been comparedReference 122. Increases in systolic blood pressure occurred with low (5 mg) and high (15 mg) oral doses of THC, as well as low (5.4 mg Δ9-THC and 5 mg CBD) and high (16.2 mg Δ9-THC and 15 mg CBD) oro-mucosal doses of nabiximols, with the effect peaking at around 3 h after administration. In contrast, diastolic blood pressure decreased between 4 and 8 h after dosing. Heart rate increased after all active treatments. A statistically significant increase in heart rate relative to placebo was observed after high-dose oral THC (15 mg Δ9-THC) and high-dose oro-mucosal nabiximols (16.2 mg Δ9-THC and 15 mg CBD), but the authors indicated that the increases appeared to be less clinically significant than those typically seen with smoked cannabis. High-dose oral THC (15 mg Δ9-THC) and high-dose oro-mucosal nabiximols (16.2 mg Δ9-THC and 15 mg CBD) were associated with significantly greater "good drug effects" compared to placebo, whereas low-dose oro-mucosal nabiximols (5.4 mg Δ9-THC and 5 mg CBD) was associated with significantly higher "good drug effects" compared to 5 mg THC. A subjective feeling of a "high" was reported to be significantly greater after 15 mg oral THC compared to placebo and to 5 mg oral THC. In contrast, neither the high nor the low doses of oro-mucosal nabiximols were reported to produce a statistically significant subjective "high" feeling. Study subjects reported being most "anxious" approximately 4 h after administration of 5 mg oral THC, 3 h after 15 mg oral THC, 5.5 h after low-dose nabiximols, and 4.5 h after high-dose oro-mucosal nabiximols. All active drug treatments induced significantly more anxiety compared to placebo. After 15 mg oral THC, the concentration of THC in plasma was observed to have a weak, but statistically significant, positive correlation with systolic and diastolic blood pressure, "good drug effect", and "high". After high-dose oro-mucosal nabiximols, positive correlations were also observed between plasma THC concentrations and "anxious", "good drug effect", "high", "stimulated", and M-scale (marijuana-scale) scores. Consistent with other studies, the authors of this study reported that linear correlations between plasma THC concentrations and physiological or subjective effects were weak. Lastly, although CBD did not appear to significantly modulate the effects of THC, the authors suggested it might have attenuated the degree of the subjective "high".
A dose run-up clinical study looking at the pharmacokinetic and pharmacodynamic profile of supratherapeutic oral doses of THC (i.e. 15 mg, 30 mg, 45 mg, 60 mg, 75 mg, 90 mg) in seven cannabis users reported that Cmax generally increased as a function of dose but varied considerably across subjects, especially at higher dosesReference 496. There was also substantial variability for Tmax both within and between subjects with an overall median of 3.3 h for both THC and 11-hydroxy-THC. THC dose-dependently elevated heart rate, and systolic blood pressure dropped at the lower dose (i.e. 30 mg) but increased at higher doses (i.e. 75 mg and 90 mg). No changes were noted for diastolic blood pressure. With regard to subjective responses, "any drug effect" and "thirsty"' ratings increased as a function of dose, however for effects such as "good drug effects", "high", "tired/sedated", "stoned", "forgetful" and "confused/difficulty concentrating" doses larger than 30 mg were not consistently associated with higher ratings.
2.4 Tolerance, dependence, and withdrawal symptoms
Tolerance
Tolerance, as defined by the Liaison Committee on Pain and Addiction (a joint committee with representatives from the American Pain Society, the American Academy of Pain Medicine, and the American Society of Addiction Medicine) is a state of adaptation in which exposure to the drug causes changes that result in a diminution of one or more of the drug's effects over timeReference 497.
Tolerance to the effects of cannabis or cannabinoids appears to result mostly from pharmacodynamic rather than pharmacokinetic mechanismsReference 328. Pre-clinical studies indicate that pharmacodynamic tolerance is mainly linked to changes in the availability of the cannabinoid receptors, principally the CB1 receptor, to signal. There are two independent but interrelated molecular mechanisms producing these changes: receptor desensitization (or uncoupling of the receptor from intracellular downstream signal transduction events), and receptor downregulation (resulting from the internalization and/or degradation of the receptor)Reference 498. Furthermore, within the brain, these adaptations appear to vary across different regions suggesting cellular- and tissue-specific mechanisms regulating desensitization/downregulationReference 328. Studies have reported that CB1 receptors in the caudate-putamen and its projection areas (e.g. globus pallidus and substantia nigra) show the least magnitude of CB1 receptor desensitization and downregulation, whereas CB1 receptors in the hippocampus exhibit the greatest magnitude of desensitization and downregulation in response to chronic THC exposureReference 499. CB1 receptors located in the striatum are also less susceptible to desensitization and downregulation relative to the hippocampusReference 499.
One clinical study showed that chronic cannabis use was associated with a global decrease in CB1 receptor availability in the brain with significant decreases in CB1 receptor availability in the temporal lobe, anterior and posterior cingulate cortices, and the nucleus accumbensReference 500. Study subjects were mostly male, had a mean age at onset of cannabis use of 16 years of age, a mean duration of cannabis use of 10 years, a mean amount of cannabis use of three joints per day, and 60% of the study subjects were considered heavy users (several times per day), 30% were moderate users (once per day to 3 - 4 times per week), and 10% used infrequently (two to three times per month or less). Furthermore, a couple of clinical studies have examined the time course of changes in the availability of CB1 receptors following chronic THC administration and abstinenceReference 334Reference 501. In the first study, heavy chronic daily cannabis smoking (average 10 joints/day for average of 12 years) was associated with reversible and regionally selective downregulation (20% decrease) of brain cortical (but not subcortical) cannabinoid CB1 receptorsReference 501. In the second study, cannabis dependence (with chronic, moderate daily cannabis smoking) was associated with CB1 receptor downregulation (i.e. ~15% decrease at baseline, not under intoxication or withdrawal) compared to healthy controlsReference 334. CB1 receptor downregulation began to reverse rapidly upon termination of cannabis use (within two days), and after 28 days of continuous monitored abstinence CB1 receptor availability was not statistically significantly different from that of healthy controls (although CB1 receptor availability never reached the levels seen with healthy controls). CB1 receptor availability was also negatively correlated with cannabis dependence and withdrawal symptoms.
The observed regional variations in cellular adaptations to THC in the brain may also generalize to other tissues or organs, explaining why tolerance develops to some of the effects of cannabis and cannabinoids but not to other effects. In animal models, the magnitude and time-course of tolerance appear to depend on the species, the cannabinoid ligand, the dose and duration of the treatment, and the measures employed to determine tolerance to cannabinoid treatmentReference 328.
Tolerance to most of the effects of cannabis and cannabinoids can develop after a few doses, and it also disappears rapidly following cessation of administrationReference 140. Tolerance has been reported to develop to the effects of cannabis on perception, psychoactivity, euphoria, cognitive impairment, anxiety, cortisol increase, mood, intraocular pressure (IOP), electroencephalogram (EEG), psychomotor performance, and nausea; some have shown tolerance to cardiovascular effects while others have notReference 324Reference 332Reference 333. There is also some evidence to suggest that tolerance can develop to the effects of cannabis on sleep (reviewed inReference 209). As mentioned above, the dynamics of tolerance vary with respect to the effect studied; tolerance to some effects develops more readily and rapidly than to othersReference 330Reference 331. However, tolerance to some cannabinoid-mediated therapeutic effects (i.e. spasticity, analgesia) does not appear to develop, at least in some patientsReference 216Reference 325Reference 327. According to one paper, in the clinical setting, tolerance to the effects of cannabis or cannabinoids can potentially be minimized by combining lower doses of cannabis or cannabinoids along with one or more additional therapeutic drugsReference 502.
One study reported that tolerance to some of the effects of cannabis, including tolerance to the "high", developed both when THC was administered orally (30 mg; q.i.d. for four days; total daily dose 120 mg)Reference 503 and when a roughly equivalent dose was given by smoking (3.1% THC cigarette; q.i.d. for four days)Reference 504. There was no diminution of the appetite-stimulating effect from either route of administration. In another study, the intensity of THC-induced acute subjective effect was reportedly decreased by up to 80% after 10 days of oral THC administration (10 - 30 mg THC every 3 - 4 h)Reference 505.
A clinical study that evaluated the effects of smoked cannabis on psychomotor function, working memory, risk-taking, subjective and physiological effects in occasional and frequent cannabis smokers following a controlled smoking regimen reported that when compared to frequent smokers, occasional smokers showed significantly more psychomotor impairment, more significant impairment of spatial working memory, significantly increased risk-taking and impulsivity, significantly higher scores for "high" ratings, for "stimulated" ratings, and more anxietyReference 181. Significantly higher scores were reported by occasional than frequent smokers for "difficulty concentrating", "altered sense of time", "feeling hungry", "feeling thirsty", "shakiness/tremulousness", and "dry mouth or throat". Compared with frequent smokers, occasional smokers had significantly increased heart rates relative to baseline and higher systolic and diastolic blood pressure just after dosing. These findings suggest that frequent cannabis users can develop some tolerance to some psychomotor impairments despite higher blood concentrations of THC. Occasional smokers also reported significantly longer and more intense subjective effects compared with frequent smokers who had higher THC concentrations suggesting tolerance can develop to the subjective effects.
A clinical study evaluated the development of tolerance to the effects of around-the-clock oral administration of THC (20 mg every 3.5 - 6 h) over six days, in 13 healthy male daily cannabis smokersReference 324. The morning THC dose increased intoxication ratings on day 2 but had less effects on days 4 (after administration of a cumulative 260 mg dose of THC) and 6, while THC lowered blood pressure and increased heart rate over the six-day period suggesting the development of tolerance to the subjective intoxicating effects of THC and the absence of tolerance to its cardiovascular effects. Tolerance to the subjective intoxicating effects of THC administered orally was manifested after a total exposure of 260 mg of THC over the course of four daysReference 324.
Another clinical study reported that while heavy chronic cannabis smokers demonstrated tolerance to some of the behaviourally-impairing effects of THC, these subjects did not exhibit cross-tolerance to the impairing effects of alcohol, and alcohol potentiated the impairing effects of THC on measures such as divided attentionReference 506.
An uncontrolled, open-label extension study of an initial five-week randomized trial of nabiximols in patients with MS and central neuropathic pain reported the absence of pharmacological tolerance (measured by a change in the mean daily dosage of nabiximols) to cannabinoid-induced analgesia, even after an almost two-year treatment period in a group of select patientsReference 327. Another long-term, open-label extension study of nabiximols in patients with spasticity caused by MS echoed these findings, also reporting the absence of pharmacological tolerance to the anti-spastic effects (measured by a change in the mean daily dosage of nabiximols) after almost one year of treatmentReference 325. A multi-centre, prospective, cohort, long-term safety study of patients using cannabis as part of their pain management regimen for chronic non-cancer pain reported small and non-significant increases in daily dose over a one-year study periodReference 216.
More recently, a double-blind, placebo-controlled, three-way cross-over clinical study with regular cannabis users suggested that tolerance may not develop towards some of the acute effects on neurocognitive functions despite regular cannabis useReference 415. One hundred and twenty-two subjects who regularly used cannabis (average duration of use: 7 years; range: 1 - 23 years), with an average rate of use of 44 use occasions (range: 2 - 100) over the course of the previous three months, participated in the study. Treatments consisted of vapourized placebo or 300 µg/kg THC (cannabis containing 11 - 12% THC). Acute administration of vapourized cannabis impaired performance across a wide range of neurocognitive domains: executive function, impulse control, attention and psychomotor function were significantly worse after cannabis compared to placebo. Frequency of cannabis use correlated significantly with change in subjective intoxication following cannabis administration and also correlated and interacted with changes in psychomotor performance meaning that subjective intoxication and psychomotor impairment following cannabis exposure decreased with increasing frequency of use, however the baseline for subjective intoxication and psychomotor impairment was already higher for frequent users compared to less frequent users (likely owing to already elevated THC body burden which can cause sufficient levels of intoxication and mild psychomotor impairment). The authors suggest that the neurocognitive functions of daily or near daily cannabis users can be substantially impaired from repeated cannabis use, during and beyond the initial phase of intoxication.
Pharmacokinetic tolerance (including changes in absorption, distribution, biotransformation and excretion) has also been documented to occur with repeated cannabinoid administration, but apparently occurs to a lesser degree than pharmacodynamic toleranceReference 507.
Dependence and withdrawal
Dependence can be divided into two independent, but in certain situations interrelated concepts: physical dependence and psychological dependence (i.e. addiction)Reference 497. Physical dependence, as defined by the Liaison Committee on Pain and Addiction, is a state of adaptation manifested by a drug-class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonistReference 497. Psychological dependence (i.e. addiction) is a primary, chronic, neurobiological disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations, and is characterized by behaviours that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and cravingReference 497. The ECS has been implicated in the acquisition and maintenance of drug taking behaviour, and in various physiological and behavioural processes associated with psychological dependence or addictionReference 2. In the former DSM-IV (diagnostic and statistical manual of mental disorders (fourth edition), the term 'dependence' was closely related to the concept of addiction which may or may not include physical dependence, and is characterized by use despite harm, and loss of control over useReference 508. There is evidence that cannabis dependence (physical and psychological) occurs, especially with chronic, heavy useReference 145Reference 190Reference 329. In the new DSM-5, the term "cannabis dependence" has been replaced with the concept of a "cannabis use disorder" (CUD) which can range in intensity from mild to moderate to severe with severity based on the number of symptom criteria endorsedReference 509. The DSM-5 defines a CUD as having the following diagnostic criteria: a problematic pattern of cannabis use leading to clinical significant impairment or distress, as manifested by at least two symptoms, occurring within a 12-month period. For a list of symptoms, please refer to the DSM-5 Reference 509.
Psychological dependence
Risk factors for transition from use to dependence have been identified and include being young, male, poor, having a low level of educational attainment, urban residence, early substance use onset, use of another psychoactive substance, and co-occurrence of a psychiatric disorderReference 510. Notably, the transition to cannabis dependence occurs considerably more quickly than the transition to nicotine or alcohol dependenceReference 510. It has been reported that after the first year of cannabis use onset, the probability of transition to dependence is almost 2%, while the lifetime prevalence of cannabis dependence among those who ever used cannabis is approximately 9%Reference 510. The prevalence of developing a CUD increases to between 33 and 50% among daily usersReference 511. More recent U.S. epidemiological data suggest that 12-month and lifetime prevalence of DSM-5 CUD was 2.5% and 6.3% respectively, and the corresponding DSM-IV 12-month and lifetime rates showed a substantial increase between 2001 - 2002 and 2012 - 2013 increasing from 12-month and lifetime rates of 1.5% and 8.5% respectively to 2.9% and 11.7% respectivelyReference 338. These increases in both 12-month and lifetime prevalence are thought to be driven by increases in the prevalence of cannabis users.
The National Epidemiological Survey on Alcohol and R elated Conditions (NESARC), a large U.S. national prospective study conducted among 34 653 respondents examining the association between cannabis use and risk of mental health and substance use disorders in the U.S. general adult population, reported that cannabis use (at Wave 1, 2001 - 2002) was associated with later development (at Wave 2, 2004 - 2005) of substance use disorders (i.e. any substance use disorder: OR = 6.2, 95% CI = 4.1 - 9.4; any alcohol use disorder: OR = 2.7, 95% CI = 1.9 - 3.8; any CUD: OR = 9.5, 95% CI = 6.4 - 14.1; any other drug use disorder: OR = 2.6, 95% CI = 1.6 - 4.4; and nicotine dependence: OR = 1.7; 95% CI = 1.2 - 2.4), but not any mood disorder or anxiety disorderReference 512. Higher frequency of cannabis use was associated with greater risk of disorder incidence and prevalence, supporting a dose-response association between cannabis use and risk of substance use disorders.
Another study using the U.S. NESARC data (2012 - 2013) found that the odds of 12-month and lifetime CUD were higher for men, Native Americans, unmarried individuals, those with low incomes, and young adults (e.g. among those 18 - 24 years of age compared to those over 45, OR = 7.2, 95% CI = 5.5 - 9.5)Reference 338. Furthermore, 12-month CUD was associated with other substance use disorders (OR = 6.0 - 9.3), affective/mood disorders (OR = 2.7 - 5.0), anxiety disorders (OR = 1.7 - 3.7), and personality disorders (OR = 3.8 - 5.0). Survey respondents with 12-month CUD differed significantly from others on all disability components of the survey, with disability increasing significantly, as cannabis disorder severity increased. Among participants with 12-month DSM-5 CUD, 61% had craving for cannabis, 32% had cannabis withdrawal symptoms, and 23% had both.
Comparing data between the NESARC 2001 - 2002 (Wave 1) and 2012 - 2013 (Wave 2), one study reported that the prevalence of cannabis use more than doubled between the two waves of the surveyReference 513. Furthermore, there was a large increase in CUD during this intervening time, with nearly 3 out of 10 cannabis users reporting a CUD in 2012 - 2013. Young adults were at highest risk of CUD in both survey waves (OR = 7.2 for ages 18 - 29; OR = 3.6 for ages 30 - 44) however, the relative increases in prevalence of CUD among adults aged 45 to 64 years and 65 years and older were much greater than the increases in young adults.
A retrospective study among a nationally representative sample of 6 935 Australian adults examining the initiation of cannabis use and transition to CUD found that the mean time from first use to the onset of CUD was 3.3 years (median time = 2 years), with 90% of cases manifesting within eight yearsReference 514. Younger age of initiation and other substance use were strong predictors of the transition from use to CUD. In fact, younger age of first cannabis use was associated with increased risk of transition to CUD, with each year older at first use associated with 11% lower odds of onset of CUD. Social phobia and panic disorder were also associated with transition from cannabis use to CUD. Male cannabis users had greater risk of CUD than female users, but among women, those with depression were more likely to develop a CUD. Early-onset of alcohol and daily cigarette smoking were each associated with marked increased risk of early initiation of cannabis use.
A handful of clinical studies have examined the differences between men and women with respect to development of dependence, withdrawal symptoms and relapseReference 515. See Section 2.5, Sex-dependent effects for additional information.
Physical dependence
Physical dependence is most often manifested in the appearance of withdrawal symptoms when use is abruptly halted or discontinued. Withdrawal symptoms associated with cessation of cannabis use (oral or smoked) appear within the first one to two days following discontinuation; peak effects typically occur between days 2 and 6 and most symptoms resolve within one to two weeksReference 516-Reference 518. The most common symptoms include craving, anger or aggression, irritability, anxiety, nightmares/strange dreams, insomnia/sleep difficulties, headache, restlessness, and decreased appetite or weight lossReference 190Reference 329Reference 342Reference 516Reference 517. Other symptoms appear to include depressed mood, chills, stomach pain, shakiness and sweatingReference 190Reference 329Reference 342Reference 517. Withdrawal symptoms are reported by up to one-third of regular users in the general population and by 50 - 95% in heavy users in treatment or in research studiesReference 519. Cannabis withdrawal symptoms appear to be moderately inheritable with both genetic and environmental factors at playReference 519. There are also emerging reports of increased physical dependence with highly potent cannabis extracts (e.g. concentrates such as butane hash oil and dabs) (OR = 1.2, p = 0.014)Reference 520Reference 521.
There are no approved pharmacotherapies for managing cannabis withdrawal symptomsReference 522. A range of medications have been explored including antidepressants (e.g. buproprion, nefadozone)Reference 523Reference 524, mood stabilizers (e.g. divalproex, lithium, lofexidine)Reference 525-Reference 527, and quetiapineReference 528 but only limited benefits have been observedReference 522. Zolpidem has also been explored as a potential pharmacotherapy to specifically target abstinence-induced disruptions in sleepReference 529Reference 530. However, agonist substitution therapy (e.g. dronabinol, nabilone, nabiximols) may be a more promising approachReference 522.
A pilot clinical study that measured the feasibility/effects of fixed and self-titrated dosages of nabiximols on craving and withdrawal among cannabis-dependent subjects found that high fixed dosages of nabiximols (i.e. up to 40 sprays per day or 108 mg THC and 100 mg CBD) were well tolerated and significantly reduced cannabis withdrawal symptoms during abstinence, but not craving, compared to placeboReference 339. Self-titrated doses were lower and showed limited efficacy compared to high fixed doses and subjects typically reported significantly lower ratings of "high" and shorter duration of "high" with nabiximols and placebo compared to smoking cannabis.
A randomized, double-blind, placebo-controlled, six-day, inpatient clinical study of nabiximols as an agonist replacement therapy for cannabis withdrawal symptoms reported that nabiximols treatment attenuated cannabis withdrawal symptoms and improved patient retention in treatmentReference 522. However, placebo was as effective as nabiximols in promoting long-term reductions in cannabis use at follow-up. Nabiximols treatment significantly reduced the overall severity of cannabis withdrawal symptoms relative to placebo including effects on irritability, depression and craving as well as a more limited effect on sleep disturbance, anxiety, appetite loss, physical symptoms and restlessness.
A placebo-controlled, within-subject, clinical study demonstrated that nabilone (6 - 8 mg daily) decreased cannabis withdrawal symptoms including abstinence-related irritability and disruptions in sleep and food intake in daily, non-treatment seeking cannabis smokersReference 531. It also decreased cannabis self-administration during abstinence in a laboratory model of relapse. While nabilone did not engender subjective ratings associated with abuse liability (i.e. drug liking, desire to take again), the high dose (8 mg) modestly decreased psychomotor task performance. A follow-up study found that nabilone (3 mg, b.i.d.) co-administered with zolpidem (12.5 mg) also ameliorated abstinence-induced disruptions in mood, sleep, and appetite, decreased cannabis smoking in the laboratory model of relapse, and did not affect cognitive performanceReference 529.
A double-blind, placebo-controlled, 11-week clinical trial testing lofexidine and dronabinol for the treatment of CUD reported no significant beneficial effect compared to placebo for promoting abstinence, reducing withdrawal symptoms, or retaining individuals in treatmentReference 532 in contrast to a previous study that showed efficacy of 40 mg dronabinol daily vs. placebo in alleviating withdrawal symptoms and improving treatment retention but not abstinenceReference 533.
Cannabidiol for cannabis and other drug dependence
A recent systematic review of the evidence of CBD as an intervention for addictive behaviours reported that to date, only 14 studies have been conducted, the majority in animals with only a handful in humansReference 341. The limited number of pre-clinical studies carried out to date suggest that CBD may have therapeutic potential for the treatment of opioid, cocaine and psychostimulant addiction, and some preliminary data suggest CBD may also be beneficial in cannabis and tobacco addiction in humansReference 341. The limited number of pre-clinical studies published thus far suggest CBD may have an impact on the intoxication and relapse phase of opioid addiction, while CBD does not appear to have an impact on the rewarding effects of stimulants (e.g. cocaine, amphetamine) but may affect relapseReference 341.
With respect to cannabis dependence, pre-clinical studies show that CBD is not reinforcing on its own, but its impact on cannabis-related dependence behaviour remains unclearReference 341. In one clinical study, a 19 year-old female with cannabis dependence exhibiting cannabis withdrawal symptoms upon cannabis cessation was administered up to 600 mg of CBD (range: 300 - 600 mg) over the course of an 11-day treatment period and CBD treatment was associated with a rapid decrease in withdrawal symptomsReference 341Reference 534. In another human study, cannabis with a higher CBD to THC ratio was associated with lower ratings of pleasantness for drug stimuli (explicit "liking"), but no group difference in "craving" or "stoned" ratings was notedReference 341Reference 535. However, a multi-site, double-blind, placebo-controlled study demonstrated that CBD (200 - 800 mg) had no effect on subjective ratings associated with cannabis abuse liabilityReference 536.
A randomized, double-blind, placebo-controlled clinical study of 24 tobacco smokers seeking treatment for tobacco dependence investigated the impact of CBD on nicotine addiction and found that inhalation of CBD (400 µg/inhalation), as needed, was associated with a significant reduction in the number of cigarettes smokedReference 341Reference 537.
A randomized, double-blind, crossover clinical study in 10 healthy volunteers examining the effects of CBD on the intoxication phase of alcohol addiction reported no differences in feelings of "drunk", "drugged", or "bad" between the alcohol only and the alcohol and CBD groupsReference 341Reference 538.
No pre-clinical studies exist on the use of CBD for hallucinogen-, sedative-, tobacco-, or alcohol-addictive behaviours and no human studies exist on the use of CBD for opioid-, psychostimulant-, hallucinogen-, or sedative-addictive behavioursReference 341.
2.5 Special populations
Pediatric/Adolescent
The ECS is present in early development, is critical for neurodevelopment and maintains expression in the brain throughout lifeReference 539. Furthermore, the ECS undergoes dynamic changes during adolescence with significant fluctuations in both the levels and locations of the CB1 receptor in the brain as well as changes in the levels of the endocannabinoids 2-AG and anandamideReference 539. The dynamic changes occurring in the ECS during adolescence also overlap with a significant period of neuronal plasticity that includes neuronal proliferation, rewiring and synaptogenesis, and dendritic pruning and myelination that occurs at the same timeReference 540. This period of significant neuroplasticity does not appear to be complete until at least the age of 25Reference 540. Thus, this neurodevelopmental time window is critical for ensuring proper neurobehavioural and cognitive development and is also influenced by external stimuli, both positive and negative (e.g. neurotoxic insults, trauma, chronic stress, drug abuse)Reference 540. Based on the available scientific evidence, youths are more susceptible to the adverse effects associated with cannabis use, especially chronic useReference 182Reference 541. Studies examining non-medical use of cannabis strongly suggest early onset (i.e. in adolescence and especially before age 15), regular and persistent cannabis use (of THC-predominant cannabis) is associated with a number of adverse effects on brain and behavioural development including CUD and addiction, other illicit drug use, compromised cognitive functioning and decreased IQ, deficits in attention, poorer educational attainment, suicidal ideation, suicide attempt, and increased risk of schizophrenia as well as an earlier onset of the latter diseaseReference 151Reference 542-Reference 552. Based on the current available evidence, it is unclear for how long some or all of the neurocognitive effects persist following cessation of use. Some investigators have found certain cognitive deficits to persist for up to one year or longer after cannabis cessation, while others have demonstrated a far shorter period of recovery (i.e. 28 days) for at least some of the evidenced deficitsReference 150Reference 151Reference 552-Reference 554. A recent literature review of observational and pre-clinical studies revealed consistent evidence of an association between adolescent cannabis use (frequent/heavy use) and persistent adverse neuropsychiatric outcomes in adulthood. Though the data from human studies do not establish causality solely from cannabis use, the pre-clinical studies in animals do indicate that adolescent exposure to cannabinoids can catalyze molecular processes leading to functional deficits in adulthood - deficits that are not found following adult exposure to cannabis. The authors note that definitive conclusions cannot be made yet as to whether cannabis use - on its own - negatively impacts the adolescent brain, and future research can help elucidate this relationship by integrating assessments of molecular, structural, and behavioral outcomesReference 555. Factors that may influence persistence of cognitive deficits can include age at onset of use, frequency and duration of use, co-morbidities, and use of other drugs (tobacco, alcohol, and other psychoactive drugs).
While adverse effects associated with THC-predominant cannabis use in youth have been well documented, far less is known about the adverse effects associated with CBD-predominant cannabis use. Nevertheless, as mentioned above, the ECS plays important roles in nervous system development in utero as well as during youth (see Section 7.4) and exposure to exogenous cannabinoids, especially at higher doses, on a daily basis and over a protracted period of time may alter the course of neurodevelopment (see Section 1.0 for additional information on the role of the ECS in the development of the nervous system).
Geriatric
There is evidence to suggest that like the changes seen with the ECS during development and adolescence, there are changes in the ECS associated with ageing. In rodents, there is a marked decline in the levels of CB1 mRNA and/or specific binding of CB1 agonists in the cerebellum, cortex, hippocampus and hypothalamus of older animalsReference 556. In addition, the coupling of CB1 receptors to G proteins is also reduced in specific brain areas in older animalsReference 556. Age-related changes in the expression of components of the ECS appear similar in rodents and humansReference 556. Disruption of CB receptors appears to enhance age-related decline of a number of tissues suggesting an important role for the ECS in the control of the ageing processReference 556.
In general, the elderly may be more sensitive to the effects of drugs acting on the CNSReference 557. A number of physiological factors may lie at the root of this increased sensitivity such as: (1) age-related changes in brain volume and number of neurons as well as alterations in neurotransmitter sensitivity which can all increase the pharmacological effects of a drug; (2) age-related changes in the pre- and post-synaptic levels of certain neurotransmitter receptors; (3) age-related changes in the sensitivity of receptors to neurotransmitters; and (4) changes in drug disposition in the elderly being generally associated with higher concentrations of psychotropic drugs in the CNS. There is very little information available on the effects of cannabis and cannabinoids in geriatric populations and based on current levels of evidence, no firm conclusions can be made with regard to the safety or efficacy of cannabinoid-based drugs in elderly patients (but see below for one of the few clinical studies of safety carried out specifically in geriatric populations)Reference 421Reference 557Reference 558. Furthermore, as cannabinoids are lipophilic, they may tend to accumulate to a greater extent in elderly individuals since such individuals are more likely to have an increase in adipose tissue, a decrease in lean body mass and total body water, and an increase in the volume of distribution of lipophilic drugsReference 557. Lastly, age-related changes in hepatic function such as a decrease in hepatic blood flow and slower hepatic metabolism can slow the elimination of lipophilic drugs and increase the likelihood of adverse effectsReference 557.
Clinical Studies
A randomized, double-blind, placebo-controlled, cross-over clinical trial that evaluated the pharmacokinetics of THC in 10 older patients with dementia (mean age 77 years) over a 12-week period reported that the median time to reach maximal concentration in the blood (Tmax) was between 1 and 2 h with THC pharmacokinetics increasing linearly with increasing dose but with wide inter-individual variationReference 421. Patients received 0.75 mg THC twice daily over the first six weeks and 1.5 mg THC twice daily over the second six-week period. The mean Cmax after the first 0.75 mg THC dose was 0.41 ng/mL and after the first 1.5 mg THC dose was 1.01 ng/mL. After the second dose of 0.75 mg THC or 1.5 mg THC, the Cmax was 0.50 and 0.98 ng/mL respectively.
Only one clinical study has thus far been carried out looking specifically at the safety of THC in an elderly population. This phase I, randomized, double-blind, double-dummy, placebo-controlled, cross-over trial of three single oral doses of Namisol®, a novel tablet form of THC (i.e. 3 mg, 5 mg, 6.5 mg THC)Reference 180 reported that, overall, the pharmacodynamic effects of THC in healthy older individuals were smaller than effects previously reported in young adults and that THC, at the doses tested, appeared to be well-tolerated by healthy older individualsReference 180. In this study, 12 adults aged 65 and older who were deemed to be healthy were included, and exclusion criteria included high falls risk, regular cannabis use, history of sensitivity to cannabis, drug and alcohol abuse, compromised cardiopulmonary function, and psychiatric comorbidities. The most commonly reported health problems were hypertension and hypercholesterolemia and subjects reported using an average of 2 medications (e.g. lipid-lowering drugs, aspirin, and beta-blockers). The most frequently reported adverse effects associated with THC were drowsiness (27%), dry mouth (11%), coordination disturbance (9%), headache (9%), difficulties concentrating (7%), blurred vision (5%), relaxation, euphoria and dizziness (5% each); nausea, dry eyes, malaise and visual hallucinations were all reported at a frequency of 2% in this trial. Adverse events first occurred within 20 min of dosing, with all adverse events occurring between 55 and 120 min after dosing and resolving completely within 3.5 h after dosing. There appeared to be a dose-dependent increase in the number of individuals reporting an increased number of adverse events with increasing doses of Namisol®. No moderate or serious adverse events were reported in this trial. While this clinical study adds important information regarding the safety and tolerability of THC in a healthy elderly population, additional studies are needed to evaluate the safety and tolerability of cannabis and cannabinoids in elderly populations having various co-morbidities.
Sex-dependent effects
In humans, sex-dependent differences have been often observed in the biological and behavioural effects of substances of abuse, including cannabisReference 559. In male animals, higher densities of CB1 receptors have been observed in almost all cerebral regions analyzed whereas in females a more efficient coupling of the CB1 receptor to downstream G-protein signaling has been observedReference 560. In humans, sex differences in CB1 receptor density have also been reported, with men having higher receptor density compared to womenReference 561. Sex-dependent differences have also been noted with respect to cannabinoid metabolism. Pre-clinical studies in females report increased metabolism of THC to 11-hydroxy-THC compared to males where THC was also biotransformed to at least three different, less active metabolitesReference 562. There is also evidence to suggest that effects of cannabinoids vary as a function of fluctuations in reproductive hormonesReference 515Reference 563. Together, these findings suggest that the neurobiological mechanism underlying the sex-dependent effects of cannabinoids may arise from sexual dimorphism in the ECS and THC metabolism, but also from the effects of fluctuations in hormone levels on the ECSReference 515Reference 563.
There is also evidence to suggest sex-dependent differences in subjective effects and development of dependence, withdrawal symptoms, relapse and incidence of mood disorders. Data combined from four double-blind, within-subject studies measuring the effects of smoked "active" cannabis (3.27 - 5.50% THC) against smoked "inactive" cannabis (0.0 % THC) showed that, when matched for cannabis use (i.e. near-daily), women reported higher ratings of abuse-related effects relative to men under "active" cannabis conditions but did not differ in ratings of intoxicationReference 515. These findings suggest that, at least among near-daily cannabis users, women may be more sensitive to the subjective effects of cannabis, especially effects related to cannabis abuse liability compared to men. Another study demonstrated dose-dependent sex differences in subjective responses to orally administered THCReference 564. In this study, women showed greater subjective effects at the lowest dose (5 mg), whereas men showed greater subjective responses at the highest (15 mg) dose. Together, these studies suggest that while women may be more sensitive to the subjective effects of THC at lower doses, they may develop tolerance to these effects at higher doses, which could, for example, have implications for the development of dependence. For example, while cannabis use among men is more prevalent and men appear to be more likely than women to become dependent on cannabis, women tend to have shorter intervals between the onset of use and regular use or development of dependence (commonly referred to as the "telescoping effect")Reference 565. In addition, women abstaining from cannabis use reported more withdrawal symptoms, with some being more severe, than those seen in men and which have been linked to relapseReference 566Reference 567. Women with CUD also present with higher rates of certain comorbid health problems such as mood and anxiety disordersReference 170Reference 568Reference 569.
3.0 Dosing
The College of Family Physicians of Canada, along with other provincial medical regulatory colleges, has issued a guidance document (in 2018) for authorizing the use of cannabis for medical purposes. Please consult these and any other official guidance documents, as applicable, for additional information regarding dosing and other matters associated with authorizing cannabis for medical purposes.
Cannabis has many variables that do not fit well with the typical medical model for drug prescribingReference 405. The complex pharmacology of cannabinoids, inter-individual (genetic) differences in cannabinoid receptor structure and function, inter-individual (genetic) differences in cannabinoid metabolism affecting cannabinoid bioavailability, prior exposure to and experience with cannabis/cannabinoids, pharmacological tolerance to cannabinoids, changes to cannabinoid receptor distribution/density and/or function as a consequence of a medical disorder, the variable potency of the cannabis plant material and varying amounts and ratios of different cannabinoids, and the different dosing regimens and routes of administration used in different research studies all contribute to the difficulty in reporting precise doses or establishing uniform dosing schedules for cannabis (and/or cannabinoids)Reference 405Reference 484.
While precise dosages have not been established, some "rough" dosing guidelines for smoked or vapourized cannabis have been published (see below). Besides smoking and vapourization, cannabis is known to be consumed in baked goods such as cookies or brownies, or drunk as teas or infusions. However, absorption of these products by the oral route is slow and erratic, varies with the ingested matrix (e.g. fat content), and the onset of effects is delayed with the effects lasting much longer compared to smoking (see Section 2.2); furthermore, dosages for orally administered products are even less well established than for smoking/vapourization, however, some preliminary data has emerged for dosing with cannabis oilsReference 137Reference 418Reference 422Reference 570Reference 571. Other forms of preparation reported in the lay literature include cannabis-based butters, candies, edibles, oils, compresses, creams, ointments, and tincturesReference 80Reference 572-Reference 575 but again, limited dosing information exists here with much of the information being anecdotal in nature.
Dosing remains highly individualized and relies largely on titrationReference 405. Patients with no prior experience with cannabis and initiating cannabis therapy for the first time are cautioned to begin at the very lowest dose and to stop therapy if unacceptable or undesirable side effects occur. Consumption of smoked/inhaled or oral cannabis should proceed slowly, waiting a minimum of 10 - 20 minutes between puffs or inhalations and waiting a very minimum of 30 minutes, but preferably 3 h, between bites of cannabis-based oral products (e.g. cookies, baked goods) to gauge for strength of effects or for possible overdosing. Subsequent dose escalation should be done slowly, once experience with the subjective effects is fully appreciated, to effect or tolerability. If intolerable adverse effects appear without significant benefit, dosing should be tapered and stopped. Tapering guidelines have not been published, but the existence of a withdrawal syndrome (see Section 2.4) suggests that tapering should be done slowly (i.e. over several days or weeks).
Minimal therapeutic dose and dosing ranges
Clinical studies of cannabis and cannabis-based products for therapeutic purposes are limited to studies carried out with dried cannabis that was smoked or vapourized and with synthetic or natural cannabis-based products that have received market authorization (i.e. dronabinol, nabilone, and nabiximols). With the possible exception of trials conducted with Epidiolex® (CBD-enriched oil) for epilepsyReference 576Reference 577 and one open-label pilot clinical trial of oral THC oil for symptoms associated with post-traumatic stress disorder (PTSD)Reference 571 there are no other clinical studies of fresh cannabis or cannabis oils for therapeutic purposes. As such, providing precise dosing guidelines for such products is not possible although existing sources of information can be used as a reference point (see below).
Prescription cannabinoids
Information obtained from the monograph for Marinol® (dronabinol; no longer available in Canada) indicates that a daily oral dose as low as 2.5 mg Δ9-THC is associated with a therapeutic effect (e.g. treatment of AIDS-related anorexia/cachexia). Naturally, dosing will vary according to the underlying disorder and the many other variables mentioned above. Dosing ranges for Marinol® (dronabinol) vary from 2.5 mg to 40 mg Δ9-THC/day (maximal tolerated daily human dose = 210 mg Δ9-THC/day)Reference 227. Average daily dose of dronabinol is 20 mg and maximal recommended daily dose is 40 mgReference 227. Doses less than 1 mg of THC per dosing session may further avoid incidence and risks of adverse effects. Dosing ranges for Cesamet® (nabilone) vary from 0.2 mg to 6 mg/dayReference 492Reference 578. Dosing ranges for Sativex® (nabiximols) vary from one spray (2.7 mg Δ9-THC and 2.5 mg CBD) to 16 sprays (43.2 mg Δ9-THC and 40 mg CBD) per dayReference 284Reference 431. Information from clinical studies with Epidiolex®, an oil-based extract of cannabis containing 98% CBD, suggests a daily dosing range between 5 and 20 mg/kg/dayReference 263Reference 576. For additional information on dosing, please see the Access to Cannabis for Medical Purposes Regulations - Daily Amount Fact Sheet (Dosage).
Survey and clinical data
Various surveys published in the peer-reviewed literature have suggested that the majority of people using smoked or orally ingested cannabis for medical purposes reported using between 10 and 20 g of cannabis per week or approximately 1 to 3 g of cannabis per dayReference 225Reference 405Reference 579.
An international, web-based, cross-sectional survey examining patients' experiences with different methods of cannabis intake reported that from among a group of 953 self-selected participants, from 31 countries, the vast majority preferred inhalation over other means of administration (e.g. teas, foods, prescription cannabinoid medications) for symptoms such as chronic pain, anxiety, loss of appetite, depression, and insomnia or sleeping disorder. Mean daily doses with smoked or vapourized cannabis were 3.0 g (median for smoked cannabis was 2 g per day; for vapourized cannabis it was 1.5 g per day)Reference 580. With foods/tinctures, mean daily dose was 3.4 g (median was 1.5 g per day), and with teas the mean daily dose was 2.4 g (median 1.5 g). Information regarding cannabinoid potencies of cannabis products (i.e. THC/CBD levels) was not available. Daily frequency of use for smoking was six times per day, whereas with vapourizing it was five times per day. Teas and food/tinctures were used on average twice per day. First onset of effects for smoking were noted on average around 7 min after start of smoking, 6.5 min after start of vapourizing, 29 min after ingestion of tea, and 46 min after ingestion of foods/tinctures. Other data suggests that those patients who use cannabis for medical purposes use up to one gram or less per day. For example, data from the Netherlands suggests the average daily dose of dried cannabis for medical purposes stood at 0.68 g per day (range: 0.65 - 0.82 g per day), whereas information obtained from the Israel medical cannabis program in 2016 suggests the average daily amount used by patients was slightly under 1.5 g (Health Canada personal communication). Canadian market data collected from licensed producers under the Access to Cannabis for Medical Purposes Regulations (ACMPRs) showed that, from April 2017 to March 2018, clients had been authorized by their healthcare practitioners to use, monthly, an average of 2.1-2.5 g/day of dried cannabis. However, since this data is collected per licensed producer, it does not include cases where clients split their authorization into two or more authorizations in order to register with more than one licensed producer at a time or personal production registrations with Health CanadaReference 581. There is no specific data on the average amount of oil authorized by healthcare practitioners since authorized amounts are always in g/day. To fulfill orders for oils, licensed producers equate oil to dried cannabis based on the formulation of their oil products. On average, licensed producers equate 1 g of dried cannabis to 6.6 g of oil. Using this average conversion factor, healthcare practitioners have authorized an equivalent average of 13.9-16.5 g/day of oil.
Satisfaction ratings for criteria such as onset of effects and ease of dose finding were reported to be higher for smoking and vapourizing (i.e. smoking/vapourizing favoured) over other means of administrationReference 580. However, prescription cannabinoid medications (e.g. dronabinol, nabilone, nabiximols) scored similarly to foods/tinctures and teas on satisfaction ratings related to daily dose needed, and ease of dose finding. Satisfaction ratings in terms of side-effects were higher for non-prescription unregulated cannabis products, with the inhaled route rated best, although the survey did not ask specific questions about the types of side effects. Satisfaction ratings were only slightly higher for orally ingested cannabis products for criteria such as duration of effects. Satisfaction ratings in terms of costs were slightly higher for smoking/vapourizing, teas, and foods/tinctures compared to prescription cannabinoid medications. Satisfaction ratings in terms of ease of preparation and intake were lowest for teas and foods/tinctures. The majority of survey participants had indicated having used cannabis products prior to onset of their medical condition.
A prospective, open-label, longitudinal study of patients with treatment resistant chronic pain reported that patients titrate their cannabis dose starting with one puff or one drop of cannabis oil per day, increasing in increments of one puff or one drop of oil per dose, three times per day until satisfactory pain relief was achieved or side effects appearedReference 582. THC concentrations in the smoked product ranged between 6 - 14 % THC and between 11 - 19 % in the oral oil formulations, with CBD concentrations between 0.2 - 3.8 % in the smoked product and 0.5 - 5.5 % in the oral oil formulation. Mean monthly prescribed amount of cannabis was 43 g or 1.4 g/day.
Data from randomized, double-blind, placebo-controlled clinical studies of smoked or vapourized cannabis used a daily dose of up to 3.2 grams of dried cannabis of varying potencies (range: 1 - 23 % THC; see Table 5).
Data from a pilot clinical trial with the Syqe Inhaler™ has shown that an inhaled (vapourized) dose of 3 mg THC (delivered from an amount as low as 15 mg of dried cannabis plant material at a potency of 20% THC; actual dose absorbed 1.5 mg THC) was associated with analgesic efficacy with minimal adverse effectsReference 58. In contrast to the gram amounts of cannabis used with smoked, vapourized, and oral routes of administration, the mean daily amounts for prescription cannabinoids such as dronabinol were 30 mg, for nabilone 4.4 mg, and for nabiximols 46 mg THC and 43 mg CBD (i.e. 17 sprays).
Taken together, data from patient surveys and clinical studies suggests that most patients use up to 3 g of dried cannabis per day for medical purposes, although much less (< 1 g/day) can be used with apparent efficacy and decreased incidence of side-effects.
Dosing and threshold of psychotropic effects
With respect to the relationship between dosing and psychotropic effects, it has been estimated that an inhaled dose of 0.045 - 0.1 mg/kg of THC (i.e. an individual inhaled dose of 3 - 6 mg THC) would be sufficient to reach the threshold for psychotropic effects, with an inhaled dose of 0.15 - 0.3 mg/kg THC (i.e. an individual inhaled dose of 10 - 20 mg THC) being sufficient to produce marked intoxicationReference 415Reference 583. Furthermore, it has been estimated that between one and three puffs of higher potency cannabis would be sufficient to produce significant psychoactive effectsReference 495. One study has shown that while cannabis smokers titrate their dose of THC by inhaling lower volumes of smoke when smoking "strong" joints (i.e. "skunk", > 15% THC), this did not fully compensate for the higher THC doses per joint when using "strong" cannabis and therefore users of more potent cannabis are exposed to greater quantities of THCReference 584. For oral administration, a dose of 0.15 - 0.3 mg/kg of THC (i.e. an individual oral dose of 10 - 20 mg THC) would be sufficient to reach the threshold for psychotropic effects and a dose of 0.45 - 0.6 mg/kg of THC (i.e. an individual oral dose of 30 - 40 mg of THC) would be sufficient to produce marked intoxicationReference 415Reference 583Reference 585.
Monitoring and clinical practice guidelines
The College of Family Physicians of Canada has published a guidance document describing a patient monitoring strategy/approach for physicians considering authorizing the use of marijuana for medical purposesReference 586. Other provincial bodies may also provide guidelines on monitoringReference 275. The College of Family Physicians of Canada has recently published a simplified guideline for prescribing medical cannabinoids in primary careReference 587.
Beaulieu et al. have elaborated recommendations for physicians with respect to the evaluation and management of patients that could be candidates for cannabis/cannabinoidsReference 275. The recommendations are as follows:
Table 2. Recommendations for the Evaluation and Management of Patients
- Take a medical history and perform a physical examination
- Assess symptoms to be treated, identify any active diagnoses, and ensure patients are under optimal management
- Assess psychological contributors and risk of addiction or substance abuse
- Document any history or current use of illicit or non-prescribed drugs, including cannabis and synthetic cannabinoids
- Determine the effect of previous use of cannabinoids for medical purposes
- Consider a urinary drug screening to assess current use of prescribed and non-prescribed medications
- Set goals for treatment with cannabis - e.g., pain reduction, increased functional abilities, improved sleep quality, increased quality of life, reduced use of other medications
- Develop a treatment plan incorporating these goals
- Discuss likely and possible side effects that might be experienced with cannabis/cannabinoid use
- Discuss the risks of addiction
- Develop a follow-up schedule to monitor the patient
- Determine whether the goals of treatment are being achieved and the appropriateness of the response
- Monitor for potential misuse or abuse (being aware of clinical features of cannabis dependence)
- Develop a treatment strategy, particularly for patients at risk
- Maintain an ongoing relationship with the patient
3.1 Smoking
According to the World Health Organization (WHO)Reference 588, a typical joint contains between 500 mg and 1.0 g of cannabis plant matter (average weight = 750 mg) which may vary in Δ9-THC content between 7.5 and 225 mg (i.e. typically between 1 and 30%; see Table 3), and in CBD content between 0 and 180 mg (i.e. between 0 and 24%). The majority of clinical trials with smoked cannabis for medical purposes have used joints of dried cannabis weighing between 800 and 900 mg. Estimates that are more recent suggest the mean weight of cannabis in a joint is 320 mgReference 589. The gram amount of cannabis plant material combusted in a "typical" puff has been estimated to range between 25 and 50 mg/puff, although amounts as high as 160 mg/puff have been notedReference 59Reference 143Reference 403Reference 583Reference 590.
The actual amount of Δ9-THC delivered in the smoke varies widely and has been estimated at 20 to 70%, the remainder being lost through combustion or side-stream smokeReference 405. Furthermore, the bioavailability of Δ9-THC (the fraction of Δ9-THC in the cigarette which reaches the bloodstream) from the smoking route is highly variable (2 - 56%) and influenced by the smoking topography (i.e. the number, duration, and spacing of puffs, hold time and inhalation volume)Reference 404. In addition, expectation of drug reward can also influence smoking dynamicsReference 591. Thus, the actual dose of Δ9-THC absorbed systemically when smoked is not easily quantified, but has been approximated to be around 25% of the total available amount of Δ9-THC in a cigaretteReference 141Reference 405Reference 592.
Relationship between a smoked/vapourized dose and an oral dose
Little reliable information exists regarding conversion of a "smoked dose" of THC to an equivalent oral dose. However, based solely on measures of bioavailability, multiplication of a "smoked dose" of Δ9-THC by a conversion factor of 2.5 (to correct for differences between the bioavailability of Δ9-THC through the smoked route (~25%) vs. the oral route (~10%), ~ three-fold difference between inhaled and oral routes) can yield an approximately equivalent oral dose of Δ9-THCReference 141Reference 583Reference 592. However, it is important to point out that these studies did not accurately measure the exact smoked dose of Δ9-THC that was delivered, and as such remains a very rough approximation. It is also important to emphasize that this "conversion factor" appears to relate mostly to psychoactive effects (e.g. euphoria, feeling mellow, feeling a good drug effect, feeling sedated, feeling stimulated, Addiction Research Center Inventory marijuana scale), psychomotor performance, and food intake and is based on a very small number of comparative pharmacology studiesReference 137Reference 592Reference 593. Further rigorous comparative pharmacology studies are required. In addition, no comparative studies have been done with vaping. In addition, this theoretical conversion factor may or may not apply for therapeutic effects. Indeed, it is important to highlight that two studies reported that individuals using cannabis for therapeutic purposes indicated they used approximately similar gram amounts of cannabis regardless of route of administrationReference 216Reference 580.
Plasma concentrations of Δ9-THC following smoking/vapourization and therapeutic efficacy
There are a small number of efficacy studies on the amounts of smoked/vapourized cannabis and plasma concentrations of Δ9-THC required for therapeutic effects (see Table 5 for a quick overview, and information throughout this document for more detailed information).
A Canadian dose-ranging study showed that a single inhalation of a 25 mg dose of smoked cannabis (Δ9-THC content 9.4%; total available dose of Δ9-THC = 2.35 mg) yielded a mean plasma Δ9-THC concentration of 45 ng/mL within 2 min after initiating smokingReference 59. The study reported improvements in sleep and pain relief in patients suffering from chronic neuropathic pain with minimal/mild psychoactive effects.
A single-dose, open-label, clinical trial of patients with neuropathic pain and using very low doses of inhaled THC reported a statistically significant improvement in neuropathic pain with minimal adverse effectsReference 58. In this clinical study, 10 patients suffering from neuropathic pain of any type were administered a vapourized dose of 3 mg of THC (available in the device; ~ 1.5 mg THC actually absorbed) resulting from vapourization of 15 mg of dried cannabis containing 20% THC. THC administration was associated with a statistically significant reduction in baseline VAS for pain intensity of 3.4 points (i.e. a 45% reduction in pain) within 20 min of inhalation, which returned to baseline within 90 min. THC was detected in blood within 1 min following inhalation and reached a maximum within 3 min at a mean THC concentration of 38 ng/ml and there were minimal/mild psychoactive effects.
A randomized controlled clinical trial of vapourized cannabis for the alleviation of pain and spasticity associated with spinal cord injury (SCI) and disease reported that median blood plasma concentrations of THC of 23 ng/mL (from vapourization of 46 mg of 2.9% low THC strength cannabis; estimated 1.3 mg THC inhaled) and 47 ng/mL (from vapourization of 56 mg of 6.7% higher strength cannabis; estimated 3.8 mg THC inhaled) were associated with an analgesic and anti-spastic responseReference 276 Many of the psychoactive effects showed a dose-dependency, with the low dose (2.9%) condition associated with lower intensity of psychoactive effects.
These above-mentioned studies suggest that, at least in the case of chronic neuropathic pain, psychoactive effects can be separated from therapeutic effects and that very low doses of THC may actually be sufficient to produce analgesia while keeping psychoactive effects to a minimum.
A review of U.S. state clinical trials on the use of smoked cannabis for the treatment of chemotherapy-induced nausea and vomiting (CINV) reported that plasma THC levels > 10 ng/mL were associated with the greatest suppression of nausea and vomiting but plasma levels between 5 and 10 ng/mL were also effectiveReference 296.
| % THC | mg THC per 750 mg dried plant materialTable 3 Footnote * ("average joint") |
|---|---|
| 1 | 7.5 |
| 2.5 | 18.75 |
| 5 | 37.5 |
| 10Table 3 Footnote † | 75Table 3 Footnote † |
| 15 | 112.5 |
| 20 | 150 |
| 30 | 225 |
Table 3 Footnotes
|
|
| Cannabinoid (Generic name) |
Brand/Registered name | Principal constituents/Source | Official status in Canada | Approved indications | Onset of effects (O) / Peak effects (P)/ Duration of action (D) | Route of administration | Availability on provincial/ territorial formulary | |
|---|---|---|---|---|---|---|---|---|
| Rx cannabinoids | DronabinolTable 4 Footnote † | Marinol®Table 4 Footnote †Reference 227 | Synthetic
Δ9-THC |
Approved (but no longer available in Canada-
see note)Table 4 Footnote † |
AIDS-related anorexia associated with weight loss;
Severe nausea and vomiting associated with cancer chemotherapy |
O: 30 - 60 min
P: 2 - 4 h D: Psychoactive effect: 4 - 6h Appetite stimulant effect: up to 24 h or longer |
Oral | MBTable 4 Footnote †; NBTable 4 Footnote †; NSTable 4 Footnote †; ONTable 4 Footnote †; PETable 4 Footnote †; QCTable 4 Footnote †; YTTable 4 Footnote † |
| Nabilone | Cesamet®Reference 492
RAN-Nabilone TEVA-Nabilone CO-Nabilone PMS-Nabilone ACT-Nabilone |
Synthetic
Δ9-THC analogue |
Marketed | Severe nausea and vomiting associated with cancer chemotherapy | O: 60 - 90 min
P: 3 - 4 h D: 8 - 12 h |
Oral | AB; BC; MB;
NB; NL; NS; NT; NU; ON; PE; QC; SK; YT. |
|
| Nabiximols
(THC+CBD and other minor cannabinoids, terpenoids, and flavonoids) |
Sativex®Reference 431 | Botanical
extract from established and well-characterized C. sativa strains |
MarketedTable 4 Footnote * | Table 4 Footnote * | O: 5 - 30 min
P: 1.5 - 4 h D: 12 - 24 h |
Oro-mucosal spray | NS | |
| Cannabidiol (CBD) | Epidiolex® | Botanical extract from established and well-characterized C. sativa strains | Being studied in clinical trials -
Not an approved product (as of March 2018) |
N/A | N/A | Oral | N/A | |
| Plant product | Cannabis
(smoked or vapourized) |
N/A | C. sativa (various) | Not an approved product | N/A | O: 5 min
P: 20 - 30 min D: 2 - 3 hReference 495Reference 594 |
Smoking or inhalation | N/A |
| Cannabis (oil for sublingual administration) | N/A | C. sativa (various) | Not an approved product | N/A | O: 5 - 30 min
P: 1.5 - 4 h D: 12 - 24 h [based on Sativex®Reference 431] |
Oral | N/A | |
| Cannabis
(oral edible) |
N/A | C. sativa
(various) |
Not an approved product | N/A | O: 30 - 90 min
P: 2 - 3 h D: 4 - 12 hReference 400 |
Oral | N/A | |
| Cannabis
(topical) |
N/A | C. sativa
(various) |
Not an approved product | N/A | N/A | Topical | N/A | |
Table 4 Footnotes
AB: Alberta; BC: British Columbia; MB: Manitoba; N/A: not applicable; NB: New Brunswick; NL: Newfoundland and Labrador; NS: Nova Scotia; NU: Nunavut; NT: Northwest Territories; ON: Ontario; PE: Prince Edward Island; QC: Quebec; Rx: prescription; SK: Saskatchewan; YT: Yukon |
||||||||
3.2 Oral
The pharmacokinetic information described in Section 2.2.1.3 reports the erratic and slow absorption of Δ9-THC from the oral route, and oral doses of THC are estimated from the information in the monograph for Marinol® (dronabinol, no longer available in Canada). A 10 mg b.i.d. dose of Marinol® (20 mg total Δ9-THC per day) yielded a mean peak plasma Δ9-THC concentration of 7.88 ng/mL (range: 3.33 - 12.42 ng/mL), with a bioavailability ranging between 10 and 20%Reference 227. By comparison, consumption of a chocolate cookie containing 20 mg Δ9-THC resulted in a mean peak plasma Δ9-THC concentration of 7.5 ng/mL (range: 4.4 - 11 ng/mL), with a bioavailability of 6%Reference 407. An 8 mg orally-administered THC tablet (Namisol®) yielded a mean plasma THC Cmax of 4 ng/mL and a similar mean plasma 11-hydroxy-THC Cmax Reference 595. Tea prepared from Cannabis flowering tops and leaves has been documented, but no data are available regarding efficacyReference 422.
Marinol
Although Marinol® (dronabinol) is no longer available for sale in Canada, the Marinol® product monograph suggests a mean of 5 mg Δ9-THC/day (range: 2.5 - 20 mg Δ9-THC/day) for AIDS-related anorexia associated with weight lossReference 227. A 2.5 mg dose may be administered before lunch, followed by a second 2.5 mg dose before supper. On the other hand, to reduce or prevent CINV, a dosage of 5 mg t.i.d. or q.i.d. is suggested. In either case, the dose should be carefully titrated to avoid the manifestation of adverse effects. Please consult the Marinol® drug product monograph for more detailed instructions.
Cesamet
The Cesamet® (nabilone) product monograph suggests administration of 1 to 2 mg of the drug, twice a day, with the first dose given the night before administration of the chemotherapeutic medicationReference 492. A 2 mg dose of nabilone gave a mean plasma concentration of 10 ng/mL nabilone, 1 to 2 h after administration. The second dose is usually administered 1 to 3 h before chemotherapy. If required, the administration of nabilone can be continued up to 24 h after the chemotherapeutic agent is given. The maximum recommended daily dose is 6 mg in divided doses. Dose adjustment (titration) may be required in order to attain the desired response, or to improve tolerability. More recent clinical trials report starting doses of nabilone of 0.5 mg at night for pain or insomnia in fibromyalgia, and for insomnia in PTSDReference 578Reference 596Reference 597. Please consult the Cesamet® drug product monograph for more detailed instructions.
Epidiolex
Data from an open-label clinical study of Epidiolex® for treatment-resistant childhood-onset epilepsy suggest that dosing with Epidiolex® (98 - 99% pure CBD oil) begin at a starting dose of 2 to 5 mg/kg per day divided in twice-daily dosing in addition to baseline antiepileptic drug regimen, then up-titrated by 2 to 5 mg/kg once a week until intolerance or a maximum dose of 25 mg/kg per day is reachedReference 262. In some specific situations, the study authors mention that an increase to a maximum dose of 50 mg/kg per day could be considered. In patients with drug- resistant seizures in the Dravet syndromeReference 576 or treatment-resistant Lennox-Gastaut syndromeReference 577, a dose of 20 mg/kg per day is efficacious and generally well tolerated.
Cannabis oil
Data from an open-label longitudinal study of cannabis oil for patients with treatment-resistant chronic non-cancer pain reported that patients titrated their cannabis oil dose starting with one drop of cannabis oil per day, increasing in increments of one drop of oil per dose, three times per day, until satisfactory analgesia was achieved or until side effects appearedReference 582. THC concentrations ranged from 11 - 19% and 0.5 - 5.5% CBD in cannabis oil in this study.
An open-label, pilot study of add-on oral THC (25 mg/ml in olive oil) for the treatment of symptoms associated with PTSD suggested dosing begin by placing 2.5 mg THC b.i.d. beneath the tongue (i.e. 0.1 mL of the oil solution) 1 h after waking up and 2 h before going to bedReference 571. Maximum daily dose was 5 mg b.i.d. (i.e. 0.2 mL b.i.d.), or a total 10 mg daily dose (i.e. 0.4 mL).
3.3 Oro-mucosal
Dosing with nabiximols (Sativex®) is described in the product monograph along with a titration method for proper treatment initiationReference 431. Briefly, dosing indications in the drug product monograph suggest that on the first day of treatment patients take one spray during the morning (anytime between waking and noon), and another in the afternoon/evening (anytime between 4 p.m. and bedtime). On subsequent days, the number of sprays can be increased by one spray per day, as needed and tolerated. A fifteen-minute time gap should be allowed between sprays. During the initial titration, sprays should be evenly spread out over the day. If at any time unacceptable adverse reactions such as dizziness or other CNS-type reactions develop, dosing should be suspended or reduced or the dosing schedule changed to increase the time intervals between doses. According to the drug product monograph, the average dose of nabiximols is five sprays per day (i.e. 13 mg Δ9-THC and 12.5 mg CBD) for patients with MS, whereas those patients with cancer pain tend to use an average of eight sprays per day (i.e. 21.6 mg Δ9-THC and 20 mg CBD). The majority of patients appear to require 12 sprays or less; dosage should be adjusted as needed and tolerated. Administration of four sprays to healthy volunteers (total 10.8 mg Δ9-THC and 10 mg CBD) was associated with a mean maximum plasma concentration varying between 4.90 and 6.14 ng/mL Δ9-THC and 2.50 to 3.02 ng/mL CBD depending whether the drug was administered under the tongue or inside the cheek. Please consult the Savitex® drug product monograph for more detailed information.
3.4 Vapourization
The Dutch Office of Medicinal Cannabis has published "rough" guidelines on the use of vapourizersReference 422. Although the amount of cannabis used per day needs to be determined on an individual basis, the initial dosage should be low and may be increased slowly as symptoms indicate. The amount of cannabis to be placed in the vapourizer may vary depending on the type of vapourizer used.
Studies using the Volcano® vapourizer have reported using up to 1 g of dried cannabis in the chamber, but 50 to 500 mg of plant material is typically usedReference 414; Δ9-THC concentrations up to 6.8% have been tested with the Volcano® vapourizerReference 402Reference 414. Subjects appeared to self-titrate their intake in accordance with the Δ9-THC content of the cannabisReference 402. Peak plasma Δ9-THC levels varied between 70 and 190 ng/mL depending on the strength of Δ9-THC. The levels of cannabinoids released into the vapour phase increased with the temperature of vapourizationReference 414. Vapourization temperature has typically been reported to be between 180 - 195 °CReference 422; higher temperatures (e.g. 230 °C) greatly increase the amounts of cannabinoids released, but also increase the amounts of by-productsReference 414.
One study reported the use of a uniform "cued" puffing procedure for vapourization with the Volcano® vapourizer: inhalation for five seconds, holding the breath for 10 seconds, and a 45-second pause before a repeat inhalationReference 280. Participants inhaled as much of the 900 mg dose of dried cannabis (3.56% THC; 32 mg THC) as they could tolerate. Vapourization temperature was set to 190 °C.
In another study, patients followed a similar "cued-puff" procedure and inhaled 4 puffs, followed by an additional round of between 4 and 8 puffs 2 h later for a total of between 8 and 12 puffs over a 2 h periodReference 598.
Another vapourization study also with the Volcano®, using the same cued-puff procedure, used 400 mg of dried cannabis of three variable strengths (1%, 4% and 7% THC or 4, 16 and 28 mg THC per dosing session)Reference 599. Vapourization temperature was 200 °C.
Lastly, a more recent set of studies again using the Volcano® vapourizer and the same "cued-puff" procedure, reported using 400 mg of dried cannabis with either 2.9% (12 mg THC) or 6.7% THC (27 mg THC), with a vapourizing temperature of 185 °CReference 276. Subjects inhaled 4 puffs at the beginning of the testing session, followed by an additional round of between 4 and 8 puffs 3 h later for a total of between 8 and 12 puffs over a 3 h period.
Data from a pilot clinical trial with the Syqe Inhaler™ has shown that an inhaled dose of as little as 3 mg THC (~1.5 mg THC absorbed, delivered from an amount as low as 15 mg of dried cannabis plant material at a potency of 20% THC) was associated with analgesic efficacy with minimal adverse effectsReference 58. THC was detected in the plasma within 1 min following inhalation and reached a maximum within 3 min at a mean THC concentration of 38 ng/ml.
4.0 Potential Therapeutic Uses
While there are countless anecdotal reports concerning the therapeutic uses of cannabis, clinical studies supporting the safety and efficacy of cannabis for therapeutic purposes in a variety of disorders are limited, but slowly increasing in number. Furthermore, the current level of evidence for the safety and efficacy of cannabis for medical purposes does not meet the requirements of the Food and Drugs Act and its Regulations except for those products that have received a notice of compliance and market authorization from Health Canada. With the exception of one small open-label, pilot clinical study of orally-administered THC in an olive oil solution for symptoms associated with PTSD and clinical trials of orally-administered CBD in an oil solution (Epidiolex®) for symptoms associated with childhood epilepsy (see section 4.6 Epilepsy), there are no well-controlled clinical studies on the use of other orally-administered cannabis products such as cannabis edibles (e.g. cookies, baked goods) or topicals for therapeutic purposes.
It has been repeatedly noted that the psychotropic side effects associated with the use of (psychoactive) cannabinoids have been found to limit their therapeutic utilityReference 23Reference 55Reference 57Reference 268Reference 600. Table 5 ("Published Positive, Randomized, Double-Blind, Placebo-Controlled, Clinical Trials on Smoked/Vapourized Cannabis and Associated Therapeutic Benefits") summarizes the information on published clinical trials that have been carried out thus far using smoked/vapourized cannabis and oil-based products.
A comprehensive review of 72 controlled clinical studies evaluating the therapeutic effects of cannabinoids (mainly orally administered THC, nabilone, nabiximols, or an oral extract of cannabis) up to the year 2005 reported that cannabinoids present an interesting therapeutic potential as anti-emetics, appetite stimulants in debilitating diseases (cancer and AIDS), analgesics, and in the treatment of MS, SCIs, Tourette's syndrome (TS), epilepsy, and glaucomaReference 601.
However, a more recent systematic review and meta-analysis of randomized clinical trials of cannabinoids (i.e. smoked cannabis, nabiximols, nabilone, dronabinol, CBD, THC, levonontradol, ajulemic acid) reported that most trials showed improvement in symptoms associated with cannabinoid use but the associations did not reach statistical significance in all trialsReference 179. Compared with placebo, cannabinoids were associated with a greater average number of patients showing a complete improvement in nausea and vomiting, reduction in pain, a greater average reduction in numerical rating scale pain assessment, and average reduction in the Ashworth spasticity scaleReference 179. There was also an increased risk of short-term adverse events with cannabinoids. Commonly reported adverse events included dizziness, dry mouth, fatigue, somnolence, euphoria, vomiting, disorientation, drowsiness, confusion, loss of balance and hallucinationsReference 179. Overall, the review and meta-analysis conducted using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach suggested that there was moderate-quality evidence to support the use of cannabinoids for the treatment of chronic neuropathic or cancer pain as well as MS-associated spasticity, but low-quality evidence to support use for CINV, weight gain in HIV infection, sleep disorders, and TSReference 179. The review and meta-analysis only included only one study with smoked cannabis and all other included clinical studies were with oral or oro-mucosal administration of cannabinoid-based medicines (e.g. nabiximols, nabilone, dronabinol).
The National Academy of Sciences, Engineering and Medicine (NASEM) has published a report on the health effects of cannabis and cannabinoidsReference 602. This comprehensive report includes information on the therapeutic effects of cannabis and the cannabinoids but also other health effects such as cancer, cardiometabolic risks, respiratory disease, immunity, injury and death, prenatal, perinatal and neonatal effects, psychosocial and mental health effects. It also discusses challenges and barriers in conducting cannabis research as well as recommendations to support and improve cannabis research. Much of the evidence included in the report came from systematic reviews and meta-analyses and selected high quality primary research. Evidence gathered from in vitro or in vivo animal studies was not included.
Dronabinol is the generic name for the oral form of synthetic Δ9-THC and is marketed in the U.S. as Marinol®. It was available for sale in Canada in capsules containing 2.5, 5, or 10 mg of the drug dissolved in sesame oil. It is indicated for the treatment of severe CINV in cancer patients, and for AIDS-related anorexia associated with weight lossReference 227. The drug is no longer sold in Canada (post-market discontinuation of the drug product as of February 2012; not for safety reasons). Please consult the Marinol® drug product monograph for more detailed information.
Nabilone is the generic name for an orally administered synthetic structural analogue of Δ9-THC, which is marketed in Canada as Cesamet® but also now available in generic forms (e.g. RAN-nabilone, PMS-nabilone, TEVA-nabilone, CO-nabilone, ACT-nabilone). It is available as capsules (0.25, 0.5, 1 mg) and is indicated for severe CINV in cancer patientsReference 492. Please consult the Cesamet® drug product monograph for more detailed instructions.
Nabiximols is the generic name for a whole-plant extract of two different, but standardized, strains of Cannabis sativa giving an oro-mucosal spray product containing approximately equivalent amounts of Δ9-THC (27 mg/mL) and CBD (25 mg/mL), and other cannabinoids, terpenoids, and flavonoids per 100 μl of dispensed spray. Nabiximols is marketed as Sativex® in Canada and has received a notice of compliance for use as an adjunctive treatment for the symptomatic relief of spasticity in adult patients with MS who have not responded adequately to other therapy, and who demonstrate meaningful improvement during an initial trial of therapy. It is also marketed (with conditions) as an adjunctive treatment for the symptomatic relief of neuropathic pain in adults with MS and (with conditions) as an adjunctive analgesic in adult patients with advanced cancer who experience moderate to severe pain during the highest tolerated dose of strong opioid therapy for persistent background painReference 431. Please consult the Sativex® drug product monograph for more detailed instructions.
Epidiolex® is the brand name for a whole-plant cannabis extract of a high CBD strain of Cannabis sativa and is an oral oil-based solution product containing > 98% CBD at a concentration of 100 mg/ml. Epidiolex® has received Orphan Drug Designation in the U.S. for the treatment of Lennox-Gastaut Syndrome, Dravet Syndrome and Tuberous Sclerosis Complex. At the time of writing this document Epidiolex® has not received a Notice of Compliance from Health Canada and is not marketed in Canada.
The existing scientific and clinical evidence for cannabis and certain cannabinoids in treating various symptoms associated with various medical conditions is summarized in the following sections beginning on the next page.
| Primary medical conditions and associated secondary end-points (if any) for which benefits were observed | Percent and dose of Δ9-THC (if known) | Trial duration; and number of patients/participants | Reference |
|---|---|---|---|
| HIV/AIDS-associated weight loss | One cannabis cigarette (~800 mg) containing 1.8% or 3.9% THC by weight, smoked once daily
(i.e. one dose per day) (~14 - 31 mg Δ9-THC /day) |
8 sessions total (3 sessions per week); 30 participants |
Reference 224 |
| HIV/AIDS-associated weight loss; disease-associated mood and insomnia | One cannabis cigarette (~800 mg) containing 2.0% or 3.9% THC by weight, smoked four times per day (i.e. four doses per day) (~64 - 125 mg of Δ9-THC /day) | 4 days total; 10 participants |
Reference 223 |
| Multiple sclerosis-associated pain and spasticity | One cannabis cigarette (~800 mg) containing 4% THC by weight, smoked once per day (i.e. one dose per day) (~32 mg Δ9-THC /day) | 3 days total; 30 patients |
Reference 278 |
| Central and peripheral chronic neuropathic pain (various etiologies) | One cannabis cigarette (~800 mg) containing either 3.5% or 7% THC by weight, smoked in bouts over a 3 h period (i.e. one dose per day) (daily dose of THC unavailable) | 1 day total; 38 patients |
Reference 222 |
| Chronic neuropathic pain from HIV-associated sensory neuropathy | One cannabis cigarette (~900 mg) containing 3.56% THC by weight, smoked three times daily (i.e. three doses per day) (~96 mg Δ9-THC /day) | 5 days total; 25 patients |
Reference 195 |
| HIV-associated chronic neuropathic pain refractory to other medications | One cannabis cigarette (~800 mg) containing between 1 and 8% THC by weight, smoked four times daily (i.e. four doses per day) (daily dose of THC unavailable) | 5 days total; 28 patients |
Reference 281 |
| Chronic post-traumatic or post-surgical neuropathic pain refractory to other medications and associated insomnia | One 25 mg dose of cannabis containing 9.4% THC by weight, smoked three times daily (i.e. three doses per day) (~7 mg Δ9-THC /day) | 5 days total; 21 patients | Reference 59 |
| Chronic pain of various etiologies (musculoskeletal, post-traumatic, arthritic, peripheral neuropathy, cancer, fibromyalgia, migraine, multiple sclerosis, sickle cell disease, thoracic outlet syndrome) | One 900 mg dose of vapourized cannabis containing 3.56% THC by weight administered three times per day (one dose the first day, three doses per day for next three days, one dose the last day) (~96 mg Δ9-THC /day) | 5 days total; 21 patients |
Reference 280 |
| Neuropathic pain of various etiologies (spinal cord injury, complex regional pain syndrome (CRPS) type I, causalgia-CRPS type II, diabetic neuropathy, multiple sclerosis, post-herpetic neuralgia, idiopathic peripheral neuropathy, brachial plexopathy, lumbosacral radiculopathy, and post-stroke neuropathy) | Inhalation of vapourized cannabis (800 mg) containing either a low (1.29% or 10.3 mg Δ9-THC) or a medium-dose of Δ9-THC (3.53% Δ9-THC or 28.2 mg Δ9-THC) | 3 sessions total; 39 patients |
Reference 598 |
| Crohn's disease | One cannabis cigarette (500 mg) containing 23% THC by weight, smoked twice daily (i.e. two doses per day) (23 mg Δ9-THC /day) | 8 weeks; 21 patients |
Reference 603 |
| Neuropathic pain of various etiologies | Inhalation of a single vapourized dose of 15 mg dried containing 20% Δ9-THC by weight (~3 mg Δ9-THC) | One session only; 10 patients |
Reference 58 |
| Diabetic peripheral neuropathy (i.e. diabetes mellitus type I and II) | Inhalation of single vapourized doses of dried cannabis (400 mg/dose) containing either low (1% Δ9-THC or 4 mg Δ9-THC), medium (4% Δ9-THC or 16 mg Δ9-THC) or high (7% Δ9-THC or 28 mg Δ9-THC) doses of Δ9-THC (four single dosing sessions; each separated by two weeks) | 4 sessions total; 16 patients |
Reference 599 |
| Neuropathic pain from spinal cord injury or disease | Inhalation of between 8 and 12 puffs from 400 mg of dried cannabis (2.9% and 6.7% THC) | 3 sessions total; 42 patients |
Reference 276 |
4.1 Palliative care
- The evidence thus far from some observational studies and clinical studies suggests that cannabis (limited evidence) and prescription cannabinoids (e.g. dronabinol, nabilone, or nabiximols) may be useful in alleviating a wide variety of single or co-occurring symptoms often encountered in the palliative care setting.
- These symptoms may include, but are not limited to, intractable nausea and vomiting associated with chemotherapy or radiotherapy, anorexia/cachexia, severe intractable pain, severe depressed mood and anxiety, and insomnia.
- A limited number of observational studies suggest that the use of cannabinoids for palliative care may also potentially be associated with a decrease in the number of some medications used by this patient population.
Among the goals of palliative care described by the WHO are relief from pain and other distressing symptoms, and the enhancement of quality of life (QoL)Reference 604. While integration of cannabis into mainstream medical use can be characterized as extremely cautious, its use appears to be gaining some ground in palliative care settings where the focus is on individual choice, patient autonomy, empowerment, comfort and especially QoLReference 605. Nevertheless, establishing the effectiveness of cannabis as a viable treatment option in a palliative care context requires a careful assessment of its effects in a wide range of conditions; such evidence is not yet abundant and further research is neededReference 606. Certain patient populations (e.g. the elderly or those suffering from pre-existing psychiatric disease) may also be more sensitive or susceptible to experiencing adverse psychotropic, cognitive, psychiatric or other effectsReference 607Reference 608.
Data from observational studies
A prospective, non-randomized, and unblinded observational case-series study assessing the effectiveness of adjuvant nabilone therapy in managing pain and symptoms experienced by 112 advanced cancer patients in a palliative care setting reported that those patients using nabilone had a lower rate of starting NSAIDs, tricyclic anti-depressants, gabapentin, dexamethasone, metoclopramide, and ondansetron and a greater tendency to discontinue these drugsReference 288. Patients were prescribed nabilone for pain relief (51%), for nausea (26%), and for anorexia (23%). Treated patients were started on 0.5 or 1 mg nabilone at bedtime during the first week and titrated upwards in increments of 0.5 or 1 mg thereafter. At follow-up, the majority of patients were on a 2 mg daily nabilone dose with a mean daily dose of 1.79 mg. The two primary outcomes of the study, pain and opioid use in the form of total morphine sulfate equivalents were reduced significantly in treated patients compared to untreated patients. Side effects from nabilone consisted mainly of dizziness, confusion, drowsiness, and dry mouth. Patients also demonstrated less tendency to initiate additional new medications and could reduce or discontinue baseline medications.
One observational study that examined over 100 self-reported cannabis-using patients in a cancer palliative care setting reported significant improvement in a variety of cancer and anti-cancer treatment-related symptoms including nausea, vomiting, mood disorders, fatigue, weight loss, anorexia, constipation, sexual function, sleep disorders, itching, and painReference 609. While the daily dose of cannabis remained constant throughout the study period, 43% of patients using pain medications reported a dose reduction and 1.7% reported a dose increase. In addition, 33% of cannabis-using patients reduced the dose of their anti-depression/anti-anxiety medications. No significant adverse effects were noted in those using cannabis, with the exception of a reported reduction in memory in about 20% to 40% of the study sample. The reported decrease in memory among a proportion of the study sample could be a function of cannabis use along with the use of other medications such as opioids, anti-depressants, or even vary with age. Improvements in symptom and distress scores were also noted. Limitations of the study included its observational nature, the lack of an appropriate control group, and the reliance on self-report.
Another observational study looking at the patterns of cannabis use among adult Israeli advanced cancer patients reported that of approximately 17,000 cancer patients monitored at a single Israeli healthcare institution, 279 patients were authorized to use cannabis for medical purposes; among these, the median age of patients was 60 years (range: 19 - 93 years) and the most common cancer diagnoses were lung (18%), ovarian (12%), breast (10%), colon (9%), and pancreatic (7.5%), and the majority (84%) of the patients had metastatic diseaseReference 237. The majority of patients (71%) were designated as active palliative, supportive (13%), and curative (6%). In most patients, cannabis was requested for multiple indications. The most common indication for which cannabis was prescribed was pain (76%), with anorexia (56%), generalized weakness (52%), and nausea (41%) also being common indications. Furthermore, 70% of patients reported improvement in pain control and general well-being, 60% reported improvement in appetite, 50% reported reduced nausea and vomiting, and 44% reported reduced anxiety with cannabis. Eighty-three percent of patients rated the overall efficacy of cannabis as being high. The most common route of administration (more than 90%) was smoking. While the majority of responders (62%) reported no adverse effects associated with the use of cannabis, the most commonly reported adverse effects were fatigue (20.3%) and dizziness (18.8%), while a minority of patients reported delusions (6%) and mood change (4.4%).
For information on the use of cannabis/cannabinoids for the control of nausea and vomiting please consult Section 4.3 of this document. For additional information on the use of cannabis/cannabinoids for anorexia/cachexia associated with HIV/AIDS infection or cancer, please consult Sections 4.4.1 and 4.4.2, respectively. For further information on the use of cannabis/cannabinoids for chronic pain syndromes (including cancer pain), please consult Sections 4.7.2.2 and 4.7.2.3. For further information on the use of cannabis/cannabinoids in the treatment of sleep disorders associated with chronic diseases please see Section 4.9.5.2, and please consult Section 4.9.9 for information on the use of cannabis/cannabinoids in oncology.
4.2 Quality of life
- The available clinical studies report mixed effects of cannabis and prescription cannabinoids on measures of quality of life (QoL) for a variety of different disorders.
A handful of clinical studies have used standardized QoL instruments to measure whether the use of cannabis or prescription cannabinoids (e.g. nabilone, dronabinol, or nabiximols) is associated with improvements in QoL. The evidence from these studies is summarized below.
Clinical studies with dronabinol
A randomized, double-blind, placebo-controlled, crossover trial of dronabinol (maximum dose of 10 mg Δ9-THC per day, for a total of three weeks) for the treatment of central neuropathic pain in patients suffering from MS reported statistically significant improvements in measures of QoL (36-Item Short Form Health Survey, SF-36; measures for bodily pain and mental health)Reference 610.
A two-centre, phase II, randomized, double-blind, placebo-controlled 22-day pilot study carried out in adult patients suffering from chemosensory alterations (i.e. changes in olfaction and gustation) and poor appetite associated with advanced cancer of various etiologies reported improved and enhanced chemosensory perception among patients treated with dronabinol (2.5 mg b.i.d.) compared to those receiving placeboReference 611. The majority (73%) of dronabinol-treated patients self-reported an increased overall appreciation of food compared to those receiving placebo (30%). While global scores on the Functional Assessment of Anorexia-Cachexia Therapy (FAACT) QoL instrument improved to a similar extent for dronabinol and placebo-treated groups, the FAACT sub-domain for anorexia-cachexia-related nutritional well-being improved with dronabinol compared to placebo. Statistically significant improvements were also noted for quality of sleep and relaxation with dronabinol treatment compared to placebo. According to the study authors, negative psychoactive effects were minimized by starting patients at a low dose (2.5 mg once a day for three days) followed by gradual dose escalation (up to a maximum of 7.5 mg dronabinol per day).
Clinical studies with cannabis extract
A multi-centre, phase III, randomized, double-blind, placebo-controlled, three-arm, parallel study in adult patients with advanced incurable cancer and suffering from cancer-related anorexia-cachexia syndrome concluded that neither cannabis extract (2.5 mg Δ9-THC, 1 mg CBD, for six weeks) nor THC (2.5 mg Δ9-THC b.i.d., for six weeks) provided any statistically significant benefit compared to placebo on measures of QoL (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, Core Module - EORTC QLQ-C30)Reference 315.
Clinical studies with nabilone
A randomized, double-blind, placebo-controlled trial of nabilone in patients suffering from fibromyalgia reported that adjuvant nabilone therapy (four weeks; maximum dose in the final week of treatment: 1 mg b.i.d.) was associated with a significant improvement in measures of QoL (VAS for pain, and the Fibromyalgia Impact Questionnaire)Reference 596.
An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel-assignment efficacy study of nabilone as an adjuvant in the treatment of long-standing diabetic peripheral neuropathic pain reported statistically significant improvements in measures of QoL (Composite EuroQoL five dimensions questionnaire, EQ-5D, Index Score) and overall patient status compared to placeboReference 612. Doses of nabilone ranged from 1 to 4 mg/day; treatment duration was five weeks.
A seven-week, randomized, placebo-controlled study comparing the effects of nabilone to placebo on QoL and side effects during radiotherapy for head and neck carcinomas reported that at the dosage used (0.5 - 2.0 mg/day titrated upwards over study duration), nabilone did not lengthen the time necessary for a 15% deterioration of QoL (measured on the EORTC QLQ-C30 and the EORTC QLQ-Head and Neck Module, H&N35, scales), and it was not better than placebo for relieving pain and nausea, or improving loss of appetite and weight, mood and sleepReference 613. There was also no statistically significant difference in the occurrence of adverse effects between the nabilone and placebo groups.
Clinical studies with nabiximols
A ten-week, prospective, randomized, double-blind, placebo-controlled trial assessing the safety and efficacy of nabiximols (Sativex®) as an adjunctive medication in the treatment of intractable diabetic peripheral neuropathy concluded that nabiximols failed to show statistically significant improvements in measures of QoL (EuroQOL, SF-36, and the McGill Pain and QoL Questionnaire)Reference 614.
A twelve-week, double-blind, randomized, placebo-controlled, parallel-group, enriched enrolment study of nabiximols as add-on therapy for patients with refractory spasticity concluded that there was no significant difference between active treatment and placebo on measures of QoL (EQ-5D Health State Index, EQ-5D Health Status VAS, SF-36)Reference 615.
A five-week, multi-centre, randomized, double-blind, placebo-controlled, parallel-group, graded-dose study evaluated the analgesic efficacy and safety of nabiximols in three dose ranges in opioid-treated cancer patients with poorly-controlled chronic painReference 284. The study reported the lack of any positive treatment effects on overall QoL in this study population even at the highest doses of nabiximols (11 - 16 sprays per day).
Clinical and observational studies with smoked cannabis
A randomized, double-blind, placebo-controlled, four-period, cross-over trial of smoked cannabis in the treatment of chronic neuropathic pain (chronic post-traumatic or post-surgical etiology) concluded that inhalation of smoked cannabis (25 mg of cannabis containing 2.5, 6.0, or 9.4% Δ9-THC, t.i.d. for five days) was not associated with a statistically significant difference compared to placebo on measures of QoL (EQ-5D Health Outcomes QoL instrument)Reference 59.
In contrast, a cross-sectional survey examining the benefits associated with cannabis use in patients with fibromyalgia reported a statistically significant benefit in the mental health component summary score of the SF-36 QoL questionnaire in patients who used cannabis compared to non-usersReference 184. However, no significant differences between cannabis and non-cannabis users were found in the other SF-36 domains, in the Fibromyalgia Impact Questionnaire, or the Pittsburgh Sleep Quality Index.
A preliminary observational, open-label, prospective, single-arm trial in a group of 13 patients suffering from Crohn's disease or ulcerative colitis reported that treatment with inhaled cannabis over a three-month period improved subjects' QoL, caused a statistically significant increase in subjects' weight, and improved the clinical disease activity index in patients with Crohn's diseaseReference 279. Patients reported a statistically significant improvement in their perception of their general health status, their ability to perform daily activities, and their ability to maintain a social life. Patients also reported a statistically significant reduction in physical pain as well as improvement in mental distress.
A recent systematic review and meta-analysis of 20 studies [11 randomized controlled trials (RCTs); 9 cohort/cross-sectional designs) examining the impact of a variety of cannabinoid-based products (herbal cannabis, nabiximols, nabilone, dronabinol, dexanabinol] on health-related quality of life (HRQoL) across multiple conditions reported no overall significant associations. The authors attributed the null findings to the heterogeneity of study characteristics, and the limitation in which HRQoL were secondary and not primary outcomes in most studies. However, the studies showing a positive relationship between cannabinoids and HRQoL were more likely to be from pain-related symptoms (neuropathic pain, multiple sclerosis, headaches, inflammatory bowel disease), while negative relationships were observed mostly in HIV patients who reported significant reductions in physical and mental HRQoLReference 616.
4.3 Chemotherapy-induced nausea and vomiting
- Pre-clinical studies show that certain cannabinoids (THC, CBD, THCV, CBDV) and cannabinoid acids (THCA and CBDA) suppress acute nausea and vomiting as well as anticipatory nausea.
- Clinical studies suggest that certain cannabinoids and cannabis (limited evidence) use may provide relief from chemotherapy-induced nausea and vomiting (CINV).
CINV is one of the most distressing and common adverse events associated with cancer treatmentReference 617. In the absence of effective anti-emetics, chemotherapy-associated nausea can be so severe that as many as 20% of patients opt to discontinue chemotherapeutic treatmentReference 618. Once a patient experiences nausea, it tends to persist throughout treatment and make subsequent episodes of nausea more severeReference 619. Post-treatment nausea is also associated with impaired patient functioning, increased anxiety, depression, and reduced QoL which can all negatively impact treatment adherence or even cause discontinuation of treatment entirelyReference 620.
While nausea typically occurs before vomiting, the two have distinct neural circuitries and can be separated behaviourallyReference 295. Furthermore, while the central mechanisms of vomiting are well-known, those responsible for nausea remain less well understoodReference 295. Nevertheless, scientific studies point to the insular cortex as the seat of sensations such as nausea and disgust, with other central regions (e.g. area postrema, parabrachial nucleus) as well as GI input also contributing to the generation of nauseaReference 295Reference 621.
Whereas chemotherapy-induced vomiting generally appears to be well-controlled with current first-line therapies/triple-combination therapies (e.g. 5-HT3 antagonists, neurokinin-1 antagonists, and corticosteroids), the associated acute, delayed, and especially anticipatory nausea remain more poorly controlled and the use of cannabis/cannabinoids may provide some measure of benefit in such casesReference 109Reference 297Reference 620. A significant proportion (25 - 59%) of patients undergoing chemotherapy experience anticipatory nausea during treatment and once it develops, it is refractory to standard treatment with 5-HT3 antagonistsReference 620. Non-specific anti-anxiety treatments (e.g. benzodiazepines) are used to treat anticipatory nausea but drawbacks include significant sedationReference 620.
It is important to note that excessive use of cannabis has been reported to paradoxically trigger a chronic cyclic vomiting syndrome (i.e. hyperemesis) (see Section 7.6.1 for further details on this syndrome).
Pre-clinical studies
Patient claims that smoked cannabis relieves CINV are widely recognized, and increasing evidence suggests a role for the ECS in the regulation of nausea and vomitingReference 109Reference 295Reference 620Reference 622-Reference 628. CB1 and CB2 receptors have been found in areas of the brainstem associated with emetogenic controlReference 629Reference 630, and results from animal studies suggest the anti-nausea and anti-emetic properties of certain cannabinoids (e.g. Δ9-THC, dronabinol, nabilone) are most likely related to their agonistic actions at centrally-located CB1 receptorsReference 99Reference 109Reference 631. Levels of 2-AG are increased in the visceral insular cortex during an acute episode of nausea in rats and localized blockade of 2-AG through targeted MAGL inhibition in the insular cortex reduces acute nauseaReference 294. Similarly, infusion of 2-AG into the insular cortex dose-dependently blocks anticipatory nausea, while infusion of anandamide was without effectReference 632. These findings suggest that 2-AG, but not anandamide, drives acute and anticipatory nausea. Elsewhere, elevation of endocannabinoids such as anandamide and 2-AG by inhibition of the endocannabinoid degradative enzymes FAAH and MAGL, has been shown to suppress acute and anticipatory nausea in animal modelsReference 295Reference 633 and localized infusion of a peripherally-restricted CB1 receptor agonist into the visceral insular cortex suppressed nausea-like behaviour in rats, whereas systemic administration had no effectReference 621.
An in vivo animal study and one small clinical study have also suggested Δ8-THC to be a more potent anti-emetic than Δ9-THCReference 99Reference 100. In addition to its actions at CB1 receptors, an in vitro study has also shown that Δ9-THC antagonizes the 5-HT3 receptorReference 634, a target of current standard anti-emetic drugs, raising the possibility that cannabinoids may exert their anti-emetic action through more than one mechanism. Other studies carried out in animal models of nausea and vomiting have shown that CBD (5 mg/kg, subcutaneous (s.c.)) suppressed chemical-induced vomiting (and nausea) through potential activation of somatodendritic 5-HT1A autoreceptors located in the dorsal raphe nucleusReference 627, while another study showed that the anti-nausea/vomiting effects of CBD could be reversed by pre-treatment with CBG (5 mg/kg, i.p.)Reference 628.
Cannabinoid acids and other cannabinoids
Additional work has revealed novel and important roles for cannabinoid acids (i.e. THCA, CBDA) in suppressing nausea and vomiting in animal modelsReference 116Reference 117Reference 622Reference 623Reference 625. In one study, when administered alone, a very low dose (0.5 µg/kg i.p.) of CBDA suppressed behaviour modelling acute nausea, and a subthreshold dose of CBDA (0.1 µg/kg i.p.), when administered along with ondansetron at a dose of 1 µg/kg produced an enhancement of the acute anti-nausea effectReference 625. In addition, the effective dose of CBDA that attenuated acute nausea was approximately 1 000 times lower than the effective dose for CBDReference 625. THCA at doses of 0.5 and 0.05 mg/kg (i.p.) reduced behaviours modelling acute nausea and vomiting, and at a dose of 0.05 mg/kg (i.p.) reduced behaviours modelling anticipatory nausea in animal models of acute and anticipatory nausea, and vomitingReference 623.
THCA has been shown to lack CB1 receptor activityReference 635 and administration of THCA was not associated with some of the classical animal behavioural signs of CB1 receptor agonists (i.e. hypothermia, catalepsy)Reference 623, supporting previous findings of lack of THCA-associated psychoactivity in animalsReference 636. THCA was also found to be at least 10 times more potent than THC in reducing acute and anticipatory nausea modelsReference 623.
Other work has shown that THC, CBDA, and the benzodiazepine chlordiazepoxide reduced behaviour modelling anticipatory nauseaReference 622. In this study, CBDA (0.001, 0.01, and 0.1 mg/kg i.p.) was shown to be between 5 and 500 times more potent than THC (0.5 mg/kg) in reducing anticipatory nausea and 20 times more potent than chlordiazepoxide (10 mg/kg). Treatment with CBDA was not associated with any effects on locomotor activity at any tested dose whereas chlordiazepoxide significantly reduced locomotor activity. Co-administration of subthreshold doses of CBDA (0.1 µg/kg i.p.) and THCA (5 µg/kg i.p.) reduced behaviour modelling anticipatory nausea, and pharmacological studies suggest the involvement of CB1 (for THCA) and 5-HT1A (for CBDA) receptors in the mechanism of suppression of anticipatory nausea. Further research is needed to resolve the conflicting evidence around the mechanism of action, if any, of THCA at the CB1 receptor. As for CBDA, a dose as low as 1 µg/kg (i.p.) potently suppressed anticipatory nausea in an animal model and compared to the doses of CBD needed for the same degree of effect (1 - 5 mg/kg i.p.), CBDA could be said to be between 1 000 and 5 000 times more potent than CBD in suppressing anticipatory nausea.
Additional animal studies have shown that administration of subthreshold doses of THC (0.01 and 0.1 mg/kg i.p.) and CBDA (0.01 and 0.1 µg/kg i.p.) reduced acute nausea, and higher doses of THC (1.0 and 10 mg/kg i.p.) or CBDA (1.0 and 10 µg/kg i.p.) alone also reduced acute nauseaReference 116. In contrast to the effect seen for acute nausea, combined subthreshold doses of THC and CBDA did not suppress anticipatory nausea in animalsReference 116. Higher doses of either THC (1.0 and 10 mg/kg i.p.) and/or CBDA (1.0 and 10 µg/kg i.p.) were effective in reducing anticipatory nausea. The higher dose of THC (10 mg/kg) was associated with hypoactivity, and this was not attenuated by CBDA.
A subsequent study examined the effects of combining CBD and THC, and CBDA and THC on acute nausea and vomitingReference 117. The study showed that 2.5 mg/kg CBD (i.p.), when combined with 1 mg/kg THC (i.p.), resulted in significant suppression of acute nausea and vomiting in an animal model and similarly, when 0.05 mg/kg (i.p.) CBDA was combined with 1 mg/kg THC, acute nausea and vomiting were significantly suppressed. Singular administration of either 2.5 mg/kg CBD, 1 mg/kg THC, or 0.05 mg/kg (i.p.) CBDA was not associated with any suppression of acute nausea and vomiting.
In addition to THC, CBD, THCA and CBDA, two other phytocannabinoids THCV and cannabidivarin (CBDV) have been studied, though to a far lesser extent, for their potential to alleviate nausea in animal modelsReference 620. THCV at a dose of 10 mg/kg (i.p.) and CBDV at a dose of 200 mg/kg (i.p.) have been shown to reduce acute nausea in rats, potentially through a CB1 receptor-independent mechanism, but nothing is known about their ability to suppress anticipatory nauseaReference 626.
Taken together, the findings from the above pre-clinical studies suggest that Δ9-THC, CBD, CBDA, and THCA can all suppress acute nausea and vomiting as well as anticipatory nausea to varying degrees, and with varying potencies and efficacies, whereas THCV and CBDV suppress acute nausea. Furthermore, certain subthreshold combinations of some of these cannabinoids can produce synergistic anti-nausea and vomiting effects compared to when used alone.
Clinical studies
The evidence for smoked cannabis and prescription cannabinoids such as nabilone (Cesamet®), dronabinol (Marinol®), (and levonantradol) in treating CINV has been reviewedReference 179Reference 210Reference 601Reference 637. One systematic review and meta-analysis of 28 randomized controlled trials (RCTs) (N = 2 454 participants) of cannabinoids using the GRADE approach reported a greater benefit of cannabinoids compared with both active comparators and placebo, but statistical significance was not reached in all of the studiesReference 179. The average number of patients showing a complete anti-nausea and vomiting response was greater with prescription cannabinoids (dronabinol or nabiximols) than placebo (OR = 3.82 [95% CI 1.55 - 9.42]).
While prescription cannabinoids present clear advantages over placebo in the control of CINV, the evidence from randomized clinical trials shows cannabinoids to be clinically only slightly better than conventional dopamine D2-receptor antagonist anti-emeticsReference 210Reference 637. In some cases, patients appeared to prefer the cannabinoids to these conventional therapies despite the increased incidence of adverse effects such as drowsiness, dizziness, dysphoria, depression, hallucinations, paranoia, and arterial hypotension. This may be explained in part by the notion that for certain patients a degree of sedation and euphoria may be perceived as beneficial during chemotherapy.
While no peer-reviewed clinical trials of smoked cannabis for the treatment of CINV exist, Musty and Rossi have published a review of U.S. state clinical trials on the subjectReference 296. Patients who smoked cannabis showed a 70 to 100% relief from nausea and vomiting, while those who used a Δ9-THC capsule experienced 76 to 88% relief. Plasma levels of > 10 ng/mL Δ9-THC were associated with the greatest suppression of nausea and vomiting, although levels ranging between 5 and 10 ng/mL were also effective. In all cases, patients were admitted only after they failed treatment with standard phenothiazine anti-emetics.
In one small open label trial with eight children with various blood cancers were administered Δ8-THC (18 mg/m2) two hours before the initiation of chemotherapy as well as every six hours for the next 24 hours showed that Δ8-THC successfully prevented vomiting and no delayed nausea or vomiting episodes were observed in the next two days following antineoplastic treatmentReference 100. Δ8-THC could also be administered at doses considerably higher than the doses of Δ9-THC generally administered to adult patients, with a lack of major side effects.
Few, if any, clinical trials directly comparing cannabinoids to newer anti-emetics such as 5-HT3 (Ondansetron, Granisetron) or NK-1 receptor antagonists have been reported to dateReference 617Reference 637. A small clinical trial comparing smoked cannabis (2.11% Δ9-THC, in doses of 8.4 mg or 16.9 mg Δ9-THC; 0.30% CBN; 0.05% CBD) to ondansetron (8 mg) in ipecac-induced nausea and vomiting in healthy volunteers showed that both doses of Δ9-THC reduced subjective ratings of queasiness and objective measures of vomiting; however, the effects were very modest compared to ondansetronReference 297. Furthermore, only cannabis produced changes in mood and subjective state. In another clinical study with a small sample size, ondansetron and dronabinol (2.5 mg Δ9-THC first day, 10 mg second day, 10 - 20 mg thereafter) provided equal relief of delayed CINV, and the combination of dronabinol and ondansetron did not provide added benefit beyond that observed with either agent aloneReference 638. However, two animal studies showed that low doses of Δ9-THC, when combined with low doses of the 5-HT3 receptor antagonists ondansetron or tropisetron, were more efficacious in reducing nausea and emesis frequency than when administered individuallyReference 639Reference 640. More research is required to determine if combination therapy provides added benefits above those observed with newer standard treatments.
A retrospective chart review of dronabinol use for CINV in an adolescent oncology population (i.e. leukemia, lymphoma, sarcoma, brain tumour) in a tertiary pediatric hospital reported that the majority of patients who received moderate or highly emetogenic chemotherapy and standard anti-emetogenic therapy (i.e. 5-HT3 receptor antagonist and corticosteroids) also received dronabinolReference 641. The most commonly prescribed dose of dronabinol in this study was 2.5 mg/m2 oral solution every 6 h (as needed), and the median number of dronabinol doses received per hospitalization was 3.5. Sixty percent of the pediatric patients in this study were reported to have had a "good" response to dronabinol. Limitations of this study include retrospective design, lack of a comparison group, lack of chemotherapy standardization, and lack of standardized anti-emetic regimens.
The use of cannabinoids (whether administered orally or by smoking cannabis) is currently considered a fourth-line adjunctive therapy in CINV when conventional anti-emetic therapies have failedReference 417Reference 642-Reference 646. Nabilone (Cesamet®) and dronabinol (Marinol®) are indicated for the management of severe nausea and vomiting associated with cancer chemotherapyReference 227Reference 492, however dronabinol is no longer available for sale on the Canadian market. Nabilone may be administered orally every 12 h at dosages ranging from 1 - 2 mg, whereas dronabinol may be administered every 6 - 8 h orally, rectally, or sub-lingually at doses ranging from 5 - 10 mgReference 311Reference 647.
4.4 Wasting syndrome (cachexia, e.g., from tissue injury by infection or tumour) and loss of appetite (anorexia) in AIDS and cancer patients, and anorexia nervosa
- The available evidence from human clinical studies suggests that cannabis (limited evidence) and dronabinol may increase appetite and caloric intake, and promote weight gain in patients with HIV/AIDS.
- However the evidence for dronabinol is mixed and effects modest for patients with cancer and weak for patients with anorexia nervosa.
The ability of acute cannabis exposure to increase appetite has been recognized anecdotally for many yearsReference 312. In addition, results from epidemiological studies suggest that people actively using cannabis have higher intakes of energy and nutrients than non-usersReference 648. Controlled laboratory studies with healthy subjects suggest acute exposure to cannabis, whether by inhalation or oral ingestion of Δ9-THC-containing capsules, correlates positively with an increase in food consumption, caloric intake, and body weightReference 312Reference 313. Studies showing a high concentration of CB1 receptors in brain areas associated with control of food intake and satiety lend further support to the link between cannabis consumption and appetite regulationReference 649-Reference 651. Furthermore, increasing evidence suggests a role for the ECS not only in modulating appetite, food palatability, and intake, but also in energy metabolism and the modulation of both lipid and glucose metabolism (reviewed inReference 19Reference 650-Reference 652).
4.4.1 To stimulate appetite and produce weight gain in AIDS patients
The ability of cannabis to stimulate appetite and food intake has been applied to clinical situations where weight gain is deemed beneficial such as in HIV-associated muscle wasting and weight loss.
A randomized, open-label, multi-center study to assess the safety and pharmacokinetics of dronabinol and megestrol acetate (an orexigenic), alone or in combination, found that only the high-dose megestrol acetate treatment alone (750 mg/day), but not dronabinol (2.5 mg b.i.d, 5 mg total Δ9-THC/day) alone or the combination of low-dose megestrol acetate (250 mg/day) and dronabinol (2.5 mg b.i.d, 5 mg total Δ9-THC/day), produced a significant increase in mean weight over 12 weeks of treatment in patients diagnosed with HIV-associated wasting syndromeReference 653. The lack of an observed clinical effect in this study could have been caused by too low a dose of dronabinol.
Despite the findings of the above-noted study, AIDS-related anorexia associated with weight loss was an approved indication in Canada for dronabinol (Marinol®) (no longer available in Canada). The Marinol® product monograph summarizes a six-week, randomized, double-blind, placebo controlled-trial in 139 patients, with the 72 patients in the treatment group initially receiving 2.5 mg dronabinol twice a day, then reducing the dose to 2.5 mg at bedtime due to side effects (feeling high, dizziness, confusion and somnolence)Reference 654. Over the treatment period, dronabinol significantly increased appetite, with a trend towards improved body-weight and mood and a decrease in nausea. At the end of the six-week period, patients were allowed to continue receiving dronabinol, during which appetite continued to improve. This secondary, open-label, 12 month follow-up study suggested that dronabinol was safe and effective for long-term use for the treatment of anorexia associated with weight loss in patients with AIDS. The use of higher doses of dronabinol (20 mg - 40 mg per day) has been reported both in the Marinol® product monographReference 227 as well as in the literatureReference 223Reference 224. However, caution should be exercised in escalating dosage because of the increased frequency of dose-related adverse effects.
A clinical study that used higher doses of dronabinol or smoked cannabis showed that acute administration of high doses of dronabinol (four to eight times the standard 2.5 mg Δ9-THC b.i.d. dose, or 10 - 20 mg Δ9-THC daily, three times per week for a total of eight sessions) and smoked cannabis (three puffs at 40 sec intervals; ~800 mg cigarettes containing 1.8 - 3.9% THC giving an estimated total daily amount of 14.4 mg - 31.2 mg THC in the cigarette, three times per week, over a total of eight study sessions) increased caloric intake in experienced HIV-positive cannabis smokers with clinically significant muscle mass lossReference 224. Another subsequent inpatient study employed even higher doses of dronabinol (20 - 40 mg total Δ9-THC daily, for four days) and smoked cannabis (~800 mg cannabis cigarettes containing 2.0 and 3.0% THC, administered four times per day, with an estimated 64 - 125 mg total Δ9-THC daily in the cigarette, over a total study period of four days)Reference 223. Both drugs produced substantial and comparable increases in food intake and body weight, as well as improvements in mood and sleepReference 223Reference 224. Others have shown that the cannabis-associated increase in body weight in this patient population appears to result from an increase in body fat rather than lean muscle massReference 655Reference 656.
A double-blind, cross-over, placebo-controlled pilot sub-study examining the effects of cannabis use on appetite hormones in HIV-infected adult men with HIV sensory neuropathy on combination anti-retroviral therapy (ART) found that compared to placebo, smoked cannabis (1 - 8% THC) was associated with significant increases in plasma levels of ghrelin (increase of 42% vs. decrease of 12% with placebo) and leptin (increase of 67% vs. 11.7% with placebo), and decreases in plasma levels of peptide YY (decrease of 14.2% vs. 23% increase with placebo)Reference 657. Higher THC levels were associated with greater increases in ghrelin showing a dose-response relationship, whereas higher THC levels were associated with smaller increases in leptin; no dose-response was observed for peptide YY.
A systematic review and meta-analysis of 28 RCTs (N = 2 454 participants) of cannabinoids (i.e. smoked cannabis, nabiximols, nabilone, dronabinol, CBD, THC, levonontradol, ajulemic acid) using the GRADE approach reported that there was some evidence that dronabinol was associated with an increase in weight when compared with placebo and that it may also be associated with increased appetite, greater percentage of body fat, reduced nausea, and improved functional status in patients with HIV/AIDSReference 179.
4.4.2 To stimulate appetite and produce weight gain in cancer patients
Anorexia is ranked as one of the more troublesome symptoms associated with cancer, with more than half of patients with advanced cancer experiencing a lack of appetite and/or weight lossReference 658Reference 659. While it is anecdotally known that smoking cannabis can stimulate appetite, the effects of smoking cannabis on appetite and weight gain in patients with cancer cachexia have not been studied. The results from clinical trials with oral Δ9-THC (dronabinol) or oral cannabis extract are mixed and the effects, if any, appear to be modest (reviewed inReference 314.
In two early studies, oral THC (dronabinol) improved appetite and food intake in some patients undergoing cancer chemotherapyReference 319Reference 320. An open-label study of dronabinol (2.5 mg Δ9-THC, two to three times daily, four to six weeks) in patients with unresectable or advanced cancer reported increases in appetite and food intake, but weight gain was only achieved in a few patientsReference 317Reference 318. Modest weight gain was obtained with a larger dosing regimen of dronabinol (5 mg t.i.d.), but the CNS side effects including dizziness and somnolence were limiting factorsReference 321. In contrast, a randomized, double-blind, placebo-controlled study involving cancer patients with related anorexia-cachexia syndrome failed to demonstrate any differences in patients' appetite across treatment categories (oral cannabis extract, Δ9-THC, or placebo)Reference 315. Furthermore, when compared to megestrol acetate, an orexigenic medication, dronabinol was significantly less efficacious in reported appetite improvement and weight gainReference 316.
A two-centre, phase II, randomized, double-blind, placebo-controlled, 22-day pilot study carried out in adult patients suffering from advanced cancer reported improved and enhanced chemosensory perception among patients treated with dronabinol (2.5 mg Δ9-THC b.i.d.) compared to those receiving placeboReference 611. The majority (73%) of dronabinol-treated patients self-reported an increased overall appreciation of food compared to those receiving placebo (30%). Similarly, the majority of dronabinol-treated patients (64%) reported increased appetite, whereas the majority of patients receiving placebo reported either decreased appetite (50%) or no change (20%). Total caloric intake per kilogram body weight did not differ significantly between treatment groups but did increase in both groups compared to baseline. Furthermore, compared to placebo, dronabinol-treated patients reported an increase in their protein intake as a proportion of total energy. According to the study authors, negative psychoactive effects were minimized by starting patients at a low dose (2.5 mg Δ9-THC once a day, for three days) followed by gradual dose escalation (up to a maximum of 7.5 mg dronabinol per day).
According to a review of the medical management of cancer cachexia, the current level of evidence for cannabinoids (e.g. dronabinol) in the treatment of this condition is lowReference 660. Cancer cachexia is not an approved indication for dronabinol in either Canada or the U.S.
4.4.3 Anorexia nervosa
The ECS has been implicated in appetite regulation and is suspected to play a role in eating disorders such as anorexia nervosaReference 650Reference 661. Increased peripheral ECS activity (i.e. increased plasma anandamide and increased CB1 mRNA expression in blood) has been found in patients with eating disordersReference 662. In spite of epidemiological and familial studies, which suggest a genetic basis for anorexia nervosa, genetic studies have thus far failed to agree on an association between genes coding for ECS proteins and the manifestation of anorexia nervosaReference 663Reference 664.
No studies have examined the effects of smoking cannabis on anorexia nervosa and limited information exists on the use of cannabinoids to treat anorexia nervosa. Furthermore, inter- and intra-species differences in animals with respect to anorexia nervosa-like behaviour have to some extent hampered pre-clinical research on the effects of Δ9-THC in this disorder.
One study in a mouse model of anorexia nervosa reported conflicting resultsReference 665, while another study in a rat model reported a significant attenuation in weight loss only at high doses of Δ9-THC (2.0 mg/kg/day Δ9-THC i.p.)Reference 666.
A small, randomized, crossover trial of oral Δ9-THC in female anorexic patients suggested that THC produced a weight gain equivalent to the active placebo (diazepam)Reference 323. Δ9-THC was administered in daily doses increasing from 7.5 mg (2.5 mg, t.i.d.) to a maximum of 30 mg (10 mg, t.i.d.), 90 min before meals, for a period of two weeks. Three of the eleven patients administered Δ9-THC also reported severe dysphoric reactions, withdrawing from the study.
Lastly, a four-week, prospective, double-blind, randomized, cross-over clinical study of 5 mg daily doses of dronabinol in 24 adult women with severe, chronic anorexia nervosa reported a small, yet significant increase in body mass index (BMI) compared to placeboReference 322.
4.5 Multiple sclerosis, amyotrophic lateral sclerosis, spinal cord injury and disease
- Evidence from pre-clinical studies suggests THC, CBD and nabiximols improve multiple sclerosis (MS) associated symptoms of tremor, spasticity and inflammation.
- The available evidence from clinical studies suggests cannabis (limited evidence) and certain cannabinoids (dronabinol, nabiximols, THC/CBD) are associated with some measure of improvement in symptoms encountered in MS and spinal cord injury (SCI) including spasticity, spasms, pain, sleep and symptoms of bladder dysfunction.
- Very limited evidence from pre-clinical studies suggests that certain cannabinoids modestly delay disease progression and prolong survival in animal models of amyotrophic lateral sclerosis (ALS), while the results from a very limited number of clinical studies are mixed.
MS is an (auto)immune-mediated, demyelinating and neurodegenerative chronic disease of the CNS that affects between 2 and 3 million people worldwide and is characterized by periods of relapsing and remitting neurological attacks and accumulating disability over many yearsReference 667Reference 668. Demyelination and axonal and neuronal loss within different neural pathways of the CNS lead to a variety of different cognitive, sensory and motor problems (e.g. pain and spasticity) that accumulate as the disease progressesReference 667. ALS is a progressive neurodegenerative disease caused by the selective damage of motor neurons in the spinal cord, brainstem, and motor cortexReference 669. Although most cases are sporadic, familial cases can occur in an autosomal recessive or dominant or dominant X-linked inheritance patternReference 670. The pathogenesis of ALS includes excitotoxic damage, chronic inflammation, oxidative stress, and protein aggregationReference 669.
One systematic review of the efficacy and safety of cannabinoids for the treatment of selected neurological disorders, including symptoms such as spasticity, central pain and painful spasms, urinary dysfunction, and tremor associated with, for example, MS suggested that, based on existing clinical trial data, cannabinoids were probably effective for reducing patient-reported and objective measures of spasticity, effective or probably effective for reducing central pain or painful spasms, probably effective for reducing the number of bladder voids/day, but probably ineffective for reducing bladder complaints and probably or possibly ineffective for reducing tremorReference 671.
In contrast to the findings of the above systematic review, a more recent systematic review and meta-analysis of 28 RCTs (N = 2 454 participants) of cannabinoids (i.e. smoked cannabis, nabiximols, nabilone, dronabinol, CBD, THC, levonontradol, ajulemic acid) using the GRADE approach reported that cannabinoids were associated with improvements in spasticity but that this failed to reach statistical significanceReference 179. Cannabinoids (nabiximols, dronabinol, and THC/CBD) were associated with a greater average improvement on the Ashworth scale for spasticity compared with placebo, although this did not reach statistical significance. Cannabinoids (nabilone and nabiximols) were also associated with a greater average improvement in spasticity assessed using numerical rating scales. The average number of patients who reported an improvement on a global impression of change score was also greater with nabiximols than placebo.
Differences between the findings from these two systematic reviews of cannabinoids for selected neurological disorders include differences in methodology, approach, and inclusion/exclusion criteria. Nevertheless, both systematic reviews suggest that cannabis/cannabinoids are associated with some measure of improvement in spasticity, spasms and pain in selected neurological disorders (e.g. MS, SCI/disease).
Below is a summary of the peer-reviewed evidence on the use of cannabis and cannabinoids in MS, ALS and SCI and disease.
4.5.1 Multiple sclerosis
A number of studies, both in patients suffering from MS and in animal models of the disease, suggest the disorder is associated with changes in endocannabinoid levels, although the findings are conflictingReference 667Reference 668Reference 672-Reference 675.
Pre-clinical studies
Pre-clinical studies across different animal species suggest cannabinoids improve the signs of motor dysfunction in experimental models of MS (reviewed inReference 667Reference 668Reference 676). Lyman was one of the first to report the effects of Δ9-THC in one such modelReference 677. In that study, affected animals treated with Δ9-THC either had no clinical signs of the disorder or showed mild clinical signs with delayed onset. The treated animals also typically had a marked reduction in CNS tissue inflammation compared to untreated animals. Subsequent studies in murine models of MS have supported and extended these findings demonstrating that Δ9-THC, but not CBD, ameliorated both tremor and spasticity and reduced the overall clinical severity of the diseaseReference 672Reference 678. Further work highlighted the importance of the CB1 receptor in controlling tremor, spasticity, and the neuro-inflammatory response. In contrast to findings with the CB1 receptor, the exact function of the CB2 receptor in MS remains somewhat unclear, although it is believed to play a role in regulating the neuro-inflammatory responseReference 678-Reference 680.
Two studies examined the potential therapeutic effects of three kinds of botanical-derived cannabis extracts on different mouse models of MS (i.e. Theiler's murine encephalomyelitis virus-induced demyelinating disease and the experimental autoimmune encephalitis)Reference 681Reference 682. Extracts used were a nabiximols-like extract, containing a 1:1 ratio of THC: CBD at 10 mg/kg for each phytocannabinoid, a THC-rich extract (5 mg/kg or 20 mg/kg) containing 67.1% THC, 0.3% CBD, 0.9% CBG, 0.9% CBC, and 1.9% other phytocannabinoids, or a CBD-rich extract (5 mg/kg or 20 mg/kg) containing 64.8% CBD, 2.3% THC, 1.1% CBG, 3.0% CBC, and 1.5% other phytocannabinoids. One of the studies reported that a 10-day treatment regimen with the nabiximols-like extract improved motor activity, reduced CNS infiltrates, microglial activity, axonal damage and restored myelin morphology and that the CBD-rich extract (5 mg/kg) alone appeared to alleviate the motor degeneration to a similar extent as the nabiximols-like extract, whereas the THC-rich extract (5 mg/kg) appeared to produce weaker effectsReference 681. The other study reported that treatment with the nabiximols-like extract (10 mg/kg) as well as the THC-rich extract (20 mg/kg), but not the CBD-rich extract (20 mg/kg), improved the neurological deficits typically observed with experimental autoimmune encephalitis in mice, as well as reduced the number and extent of cell aggregates present in the spinal cord; by contrast the CBD-rich extract appeared to only delay the onset of the disease without improving disease progression and reduced the cell infiltrates in the spinal cordReference 682. Taken together, the studies suggest that optimal therapeutic effects in these animal models of MS depend on a combination of THC, CBD and potentially other phytocannabinoids. Another study reported that daily topical treatment with a 1% CBD cream exerted neuroprotective effects against the experimental autoimmune encephalomyelitis model of MSReference 445. Treatment was associated with a diminished clinical disease score, attenuated paralysis of hind limbs, and improvements in histological scores (i.e. reduced demyelination, axonal loss, reduced inflammatory cell infiltration) and expression of pro-inflammatory cytokines.
Historical and survey data
In humans, published reports spanning 100 years suggest that people with spasticity (one of the symptoms associated with MS) may experience relief with cannabisReference 683. In the UK, 43% of patients with MS reported having experimented with cannabis at some point, and 68% of this population used it to alleviate the symptoms of MSReference 684. In Canada, the prevalence of medicinal use of cannabis among patients seeking treatment for MS, in the year 2000, was reported to be 16% in Alberta, with 43% of study respondents stating they had used cannabis at some point in their livesReference 226. Fourteen percent of people with MS surveyed in the year 2002 in Nova Scotia reported using cannabis for medical purposes, with 36% reporting ever having used cannabis for any purposeReference 225. MS patients reported using cannabis to manage symptoms such as spasticity and chronic pain as well as anxiety and/or depressionReference 225Reference 226. MS patients taking cannabis also reported improvements in sleep. Reputed dosages of smoked cannabis by these patients varied from a few puffs to 1 g or more at a timeReference 225.
Clinical studies with orally administered cannabinoid medications (cannabis extract, oral THC, nabiximols)
The results of randomized, placebo-controlled trials with orally administered cannabinoids for the treatment of muscle spasticity in MS are encouraging, but modest.
The large, multi-centre, randomized, placebo-controlled CAnnabis in Multiple Sclerosis (CAMS) study researching the effect of cannabinoids for the treatment of spasticity and other symptoms related to MS enrolled over 600 patientsReference 387. The primary outcome was change in overall spasticity scores measured using the Ashworth scale. The study did not show any statistically significant improvement in the (objective) Ashworth score in patients taking either an oral cannabis extract ((Cannador®) containing 2.5 mg Δ9-THC, 1.25 mg CBD, and < 5% other cannabinoids), or oral Δ9-THC, for 15 weeks. However, there was evidence of a significant treatment effect on subjective, patient-reported spasticity and pain, with improvement in spasticity using either orally administered cannabis extract (61%) (dosing: 5 - 25 mg Δ9-THC; 5 - 15 mg CBD/day; and < 5% other cannabinoids, adjusted to body weight and titrated according to side effects) or oral Δ9-THC (60%) (dosing: 10 - 25 mg Δ9-THC/day, adjusted to body weight and titrated according to side effects) compared to placebo (46%). Patients were concomitantly taking other medications to manage MS-associated symptoms. In contrast, a long-term (12 months), double-blind, follow-up to the CAMS study showed evidence of a small treatment effect of oral Δ9-THC (dosing: 5 - 25 mg Δ9-THC/day, adjusted to body weight and titrated according to side effects) on muscle spasticity measured by objective methods, whereas a subjective treatment effect on muscle spasticity was observed for both oral Δ9-THC and oral cannabis extract (Cannador®)Reference 685. Cannador® is not available in Canada at this time.
Other randomized clinical trials using standardized cannabis extract capsules (containing 2.5 mg Δ9-THC and 0.9 mg CBD per capsule)Reference 686 or nabiximols (Sativex®)Reference 432Reference 687Reference 688 reported similar results, in that improvements were only seen in patient self-reports of symptoms but not with objective measures (e.g. Ashworth scale). The reasons behind the apparent discrepancies between subjective and objective measures are not clear; however, a number of possible explanations may be found to account for the differences. For example, it is known that spasticity is a complex phenomenonReference 689 and is affected by patient symptoms, physical functioning, and psychological dispositionReference 685. Spasticity is also inherently difficult to measure, and has no single defining featureReference 688. In addition, the reliability and sensitivity of the Ashworth scale (for objectively measuring spasticity) has been called into questionReference 387Reference 688.
The efficacy, safety, and tolerability of a whole-plant cannabis extract administered in capsules (2.5 mg THC and 0.9 mg CBD/capsule) were studied in a fourteen-day, prospective, randomized, double-blind, placebo-controlled crossover clinical trial in patients with clinically stable MS-associated spasticity and an Ashworth score greater than 2Reference 686. Slightly more than half of the study subjects had a maintenance dose of 20 mg/day of THC or more (maximum of 30 mg THC/day). Patients were concomitantly taking anti-spasticity medications. Many study subjects had had previous experience with cannabis; a significant number of those who withdrew from the study upon starting treatment with the cannabis extract did not have previous experience with cannabis. While there were no statistically significant differences between active treatment with the cannabis extract and placebo, trends in favour of active treatment were observed for mobility, self-reported spasm frequency, and ability in getting to sleep. The cannabis extract was generally well tolerated with no serious adverse events during the study period. However, adverse events were slightly more frequent and more severe during the active treatment period.
Nabiximols
A six-week, multi-centre, randomized, double-blind, placebo-controlled, parallel-group clinical study of nabiximols (Sativex®) for the treatment of five primary symptoms associated with MS (spasticity, spasm frequency, bladder problems, tremor, and pain) reported mixed resultsReference 432. Patients had clinically confirmed, stable MS of any type, and were on a stable medication regimen. Approximately half of the study subjects in either the active or placebo groups had previous experience with cannabis, either non-medically or for medical purposes. While the global primary symptom score, which combined the scores for all five symptoms, was not significantly different between the active treatment group and the placebo group, patients taking cannabis extract showed statistically significant differences compared to placebo in subjective, but not objective measures of spasticity (i.e. Ashworth Score), in Guy's Neurological Disability Score, and in quality of sleep, but not in spasm frequency, pain, tremor, or bladder problems among other outcome measures. Patients self-titrated to an average daily maintenance dose of nabiximols of 40.5 mg THC and 37.5 mg CBD (i.e. ~15 sprays/day). Adverse effects associated with active treatment included dizziness, disturbance in attention, fatigue, disorientation, feeling drunk, and vertigo.
A long-term, open-label, follow-up clinical study of nabiximols (Sativex®) concluded that the beneficial effect observed in the study by Wade et al. 2004Reference 432 was maintained in patients who had initially benefited from the drugReference 687. The mean duration of study participation in subjects who entered the follow-up study was 434 days (range: 21 - 814 days). The average number of daily doses taken by the subjects remained constant or was slightly reduced over time. The average number of daily doses of nabiximols was 11, corresponding to a dose of 30 mg THC and 28 mg CBD/day. Long-term use of nabiximols in this patient population was associated with reductions in subjective measures of spasticity, spasm frequency, pain, and bladder problems. Dizziness, diarrhea, nausea, fatigue, headache, and somnolence were among the most frequently reported adverse effects associated with chronic nabiximols use in this study. A two-week withdrawal study, incorporated into the long-term follow-up study, suggested that cessation of nabiximols use was not associated with a consistent withdrawal syndrome but it was associated with withdrawal-type symptoms (e.g. interrupted sleep, hot/cold flushes, fatigue, low mood, decreased appetite, emotional lability, vivid dreams, intoxication) as well as re-emergence/worsening of some MS symptoms.
The efficacy, safety and tolerability of nabiximols in MS were investigated in a six-week, multi-centre, phase III, double-blind, randomized, parallel-group clinical study in patients with stable MS who had failed to gain adequate relief using standard therapeutic approachesReference 688. Patients had to have significant spasticity in at least two muscle groups, and an Ashworth score of 2 or more to be included in the study. A significant number of patients had previous experience with cannabis. Forty percent of subjects assigned treatment with nabiximols showed a ≥ 30% reduction in self-reported spasticity using an 11-point subjective numerical rating spasticity scale (sNRS) compared to subjects assigned to placebo (21.9%) (difference in favour of nabiximols = 18%; 95% CI = 4.73, 31.52; p = 0.014). Mean number of sprays per day was 9.4 (~25 mg THC and ~24 mg CBD). Subjects on placebo or nabiximols exhibited similar incidences of adverse effects, but adverse CNS effects were more common with the nabiximols group. The majority of adverse events were of mild or moderate severity (e.g. dizziness, fatigue, depressed mood, disorientation, dysgeusia, disturbance in attention, blurred vision).
An observational, prospective, multicenter, non-interventional, clinical practice study examined the safety and effectiveness of nabiximols in the treatment of symptoms associated with MS (i.e. the MObility improVEment in MS-induced spasticity study, MOVE 2)Reference 690. MS patients were followed over a three- to four-month period on outcomes, tolerability, QoL and treatment satisfaction. Prior to initiation on nabiximols, other anti-spastic medications were tried in 90% of study patients and the majority of the patients in the study (73%) were put on nabiximols. The mean number of nabiximols sprays/day was 6.9 (range: 1 - 12) reported at follow-up period 1, and 6.7 (range: 1 - 16) reported at follow-up period 2. Physician-based assessment of patients suggested a one-month course of treatment with nabiximols provided relief of resistant MS spasticity in the majority of patients who were administered the drug. After a one-month period, there was an initial response for spasticity detected in 42% of patients and a clinically relevant response for spasticity detected in 25% of these patients. At three-months' time, an initial response for spasticity was detected in 59% of patients and a clinically relevant response for spasticity detected in 40% of these patients. Scores in mean sleep disturbance decreased by 33% over a one-month treatment period in patients with an initial response, and by 40% in patients with a clinically relevant response. Scores on the combined modified Ashworth score (cMAS) decreased by 12% after one-month treatment in patients with an initial response and by 15% in patients with a clinically relevant response. Scores on the MSQoL-54 physical health composite scale and the mental health composite score showed statistically significant improvements over the three-month period in patients with an initial response and a clinically relevant response. After three-months' treatment with nabiximols, the mean EQ-5D-3L index value remained stable and a statistically significant reduction was observed in the percentages of patients considering muscle stiffness, restricted mobility, pain, and bladder disorders as most disturbing symptoms. Overall, at three-months' treatment time, almost 80% of the entire study population of patients on nabiximols was either "completely satisfied" or "satisfied" with the effectiveness of nabiximols. Most commonly observed adverse events with nabiximols were dizziness (4%), fatigue (2.5%), drowsiness (1.9%), nausea (1.9%), and dry mouth (1.2%).
A 12-month prolongation study of the MOVE 2 clinical trial to determine long-term effectiveness and safety of nabiximols in clinical practice reported that from among 52 patients enrolled in the study that were included in the effectiveness analysis, the mean spasticity numerical rating scale score decreased significantly from 6.0 points at baseline to 4.8 points after one month and remained at this level after the 12-month period, including in patients who were classified as "initial responders"Reference 691. At baseline, the mean sleep disturbance numerical rating scale (NRS) score was 5.1 points in the subsample of participants and after 12 months it decreased to 3.2 points; in patients with an initial response, scores dropped from 5.4 to 2.4, and in patients with a clinically relevant response mean sleep disturbance NRS scores decreased from 5.3 to 1.9 points. Furthermore, the mean values of the MSQoL-54 physical health composite score and the mean mental health composite score both showed improvements, but were not statistically significant. The EQ-5D-3L index value showed improvement over the 12-month period for those patients who showed an initial and clinically relevant response. Furthermore, at study end, fewer patients who showed an initial and clinically-relevant response considered the MS spasticity-related symptoms of muscle stiffness, pain, restricted mobility, fatigue, and bladder disorders as the most disturbing symptoms compared to baseline. From the patient's perspective, impairment of daily activities was significantly improved after 12-month treatment with nabiximols compared to baseline and fewer patients complained about daily impairment of activities and notably, the improvement was more prominent in responders than in the entire study group. The majority of patients did not report adverse events. Most commonly reported adverse events included GI disorders, psychiatric disorders, and nervous system disorders. Mean daily number of nabiximols sprays was 6.2 (range: 2 - 12) and at least one other anti-spastic drug was still prescribed in 28 patients (e.g. baclofen, tizanidine, tolperisone, or gabapentin).
A pilot, prospective, multicentre, non-interventional post-marketing surveillance study conducted to collect data on driving ability, tolerability and safety from 33 patients with MS starting nabiximols treatment reported that a four to six-week treatment period with nabiximols (average 5.1 sprays per day, or 13.7 mg THC and 12.8 mg CBD/day) was associated with a statistically significant improvement in self-rated spasticity and was also not associated with a statistically significant deterioration in patients' ability to drive, as measured in the laboratory using a battery of cognitive and psychomotor testsReference 692. However, less than half of the patients met the "fit to drive" criteria. In addition, 4 out of the 33 patients experienced a non-serious, mild or moderate adverse event associated with nabiximols treatment (e.g. dizziness and vertigo).
A non-randomized, non-placebo-controlled study quantitatively assessed the functional effects of nabiximols treatment on gait patterns in 20 patients with MSReference 693. Enrolled MS patients had an expanded disability status scale (EDSS) score of 5.3 at study start, were unresponsive to spasticity treatments, and were able to walk unaided for 6 min. Patients were treated with nabiximols for one month (average number of sprays per day = 5.6 or a daily dose of 15 mg THC and 14 mg CBD) and the study reported that nabiximols treatment was associated with statistically significant improvements in Gait Profile Score, speed, cadence and stride length.
A four-week, prospective, randomized, double-blind, placebo-controlled, crossover clinical study of 44 patients with progressive primary or secondary MS, with moderate to severe spasticity and inadequate response to anti-spasticity agents investigated nabiximols-induced changes in neurophysiological measures of spasticity in patients with lower limb MS-associated spasticity, as well as changes in spasticity and related functional parametersReference 694. At baseline, patients were concomitantly using glatiramer acetate, cyclophosphamide, azathioprine, fingolimod, natalizumab, interferon beta-1b, interferon beta-1a and methotrexate. Other medications included baclofen, eperisone, tizanidine, and benzodiazepines. Average daily dose of nabiximols was seven sprays per day or 18.9 mg THC and 17.5 mg CBD. The study reported no significant difference in the change from baseline to week 4 in the neurophysiological measure of spasticity (H/M ratio) with either nabiximols or placebo. Furthermore, no significant effect was found for all secondary neurophysiological measures. However, there was a statistically significant improvement in mean lower limb modified Ashworth scale score with nabiximols compared to placebo. There were no statistically significant differences for functional outcomes (timed 10 meter walk, 9-Hole Peg Test scores, pain NRS scores, sleep NRS scores, and Fatigue Severity Scale scores) between nabiximols and placebo. Most patients experienced an adverse event; the most commonly reported one was mild to moderate dizziness (21%), followed by lower limb weakness, vertigo, hypotension, hypertension, somnolence, and pharyngodynia. Most side effects were transient and appeared mostly during the titration phase or during increases in the number of sprays and resolved after reduction in the number of sprays. Limitations of the study included small sample size, short treatment period and relatively large number of study dropouts (14%) which limited the statistical power of the study.
A one-year, prospective, cohort study of 144 patients with moderate-to-severe MS spasticity and with evidence of inadequate response to traditional anti-spastic medications explored the efficacy, safety and tolerability of nabiximols at 4, 14, and 48 weeks and also assessed whether baseline demographic and clinical features could predict treatment responseReference 695. Patients were initially enrolled in a four-week "titration phase" to identify responders showing at least a 20% reduction in sNRS from baseline. Responders were then subsequently enrolled in the study. sNRS score dropped significantly in responders from 7.6 (baseline) to 5.2 at four weeks, with the mean number of daily sprays being 6.5 in responders vs. 7.7 in non-responders. sNRS score further improved in the responder group to a score of 5.0 (or a 30% clinically significant reduction in sNRS score) between 4 and 14 weeks' treatment. The cMAS was 4.0 at baseline in responders and significantly improved at four weeks' time and was persistently lower at 14 weeks' time compared to baseline. Nabiximols treatment was also associated with a significant improvement in the 10 min walking test after four weeks' treatment and improvement was maintained at 14 weeks compared to baseline. The ambulation index also showed a significant improvement in responders at 4 weeks and was maintained at 14 weeks despite an EDSS score that remained unchanged throughout the study period. Pain numerical rating score (pNRS) in responders showed a statistically significant decrease from 4.2 at baseline to 3.3 after 4 weeks' treatment and decreased further to 2.9 at 14 weeks. In responders who remained in the study at the 48-week follow-up, nabiximols efficacy was maintained with a spasticity score that remained statistically and clinically significantly lower than at baseline (i.e. 33% reduction) and the mean number of sprays taken daily was 6.2. Improvement in median cMAS was still evident, with a score of 3.0 at 48 weeks compared to 4.0 at baseline. The score on the pNRS was consistently lower at 48 weeks compared to baseline. No further improvement was noted for either the 10 min walking test or ambulation index. Eighty percent of patients in the study reported side effects, which appeared at a mean daily dose of 7.2 sprays (19.44 mg THC and 18 mg CBD). The most commonly reported side effects were confusion/ideomotor slowing (35%), dizziness (24%) and fatigue (20%). The majority of the reported side effects developed during the titration phase, were mild in intensity, and decreased with dosage adjustment. Nine percent of all patients enrolled in the study (responders and non-responders) discontinued treatment within 4 weeks of starting nabiximols because of side effects, while 9% of responders discontinued treatment for the same reason within 14 weeks of initiating treatment. One subject reported depersonalization two months after starting nabiximols while another subject developed depression. Lastly, demographic analysis suggested that patients with shorter disease duration and younger age tended to respond more favourably to nabiximols (i.e. "responders"). Study limitations included observational design, limited sample size, and lack of assessment of QoL and impairment in daily living.
CUPID and MUSEC clinical studies
The Cannabinoid Use in Progressive Inflammatory Brain Disease (CUPID) study was a randomized, double-blind, clinical investigation designed to measure whether orally administered Δ9-THC was able to slow the progression of MS. This three-year publicly-funded trial took place at the Peninsula Medical School in the U.K. and followed the earlier, one-year long, CAMS study. A total of 493 subjects with primary or secondary progressive, but not relapse-remitting, MS had been recruited from across the U.K. in 2006. The CUPID trial found no evidence to support an effect of Δ9-THC on MS progression, as measured by using either the EDSS or the MS Impact Scale 29 (MSIS-29). However, the authors concluded that there was some evidence to suggest a beneficial effect in participants who were at the lower end of the disability scale at the time of patient enrolment. Since the observed benefit only occurred in a small sub-group of patients, further studies would be required to more closely examine the reasons for this selective effectReference 696.
A double-blind, placebo-controlled, phase III clinical study (the MUltiple Sclerosis and Extract of Cannabis trial, MUSEC) published by the same group of researchers that conducted the CUPID trial, reported that a twelve-week treatment with an oral cannabis extract (Cannador®) (2.5 mg Δ9-THC and 0.9 mg CBD/capsule) was associated with a statistically significant relief in patient-reported muscle stiffness, muscle spasms, and body pain as well as a statistically significant improvement in sleep compared to placebo, in patients with stable MSReference 697. There were no statistically significant differences between cannabis extract and placebo on functional measures such as those examining the effect of spasticity on activities of daily living, ability to walk, or on social functioning. The majority of the patients using cannabis extract used total daily doses of 10, 15, or 25 mg of Δ9-THC with corresponding doses of 3.6, 5.4, and 9 mg of CBD. The majority of the study subjects were concomitantly using analgesics and anti-spasticity medications, but were excluded if they were using immunomodulatory medications (e.g. interferons). Active treatment with the extract was associated with an increase in the number of adverse events, but the majority of these were considered mild to moderate and did not persist beyond the study period. The highest number of adverse events were observed during the initial two-week titration period and appeared to decrease progressively over the course of the remaining treatment sessions. The most commonly observed adverse events were those associated with disturbances in CNS function (e.g. dizziness, disturbance in attention, balance disorder, somnolence, feeling abnormal, disorientation, confusion, and falls). Disturbances in GI function were the second most commonly occurring adverse events (e.g. nausea, dry mouth).
Clinical studies with smoked cannabis
There has only been one clinical study so far using smoked cannabis for symptoms associated with MSReference 278. The study, a double-blind, placebo-controlled, crossover clinical trial reported a statistically significant reduction in patient scores on the modified Ashworth scale for measuring spasticity after patients smoked cannabis once daily for three days (each cigarette contained 800 mg of 4% Δ9-THC; total available Δ9-THC dose of 32 mg per cigarette). Smoking cannabis was also associated with a statistically significant reduction in patient scores on the VAS for pain, although patients reportedly had low levels of pain to begin with. No differences between placebo and cannabis were observed in the timed-walk task, a measure of physical performance. Cognitive function, as assessed by the Paced Auditory Serial Addition Test, appeared to be significantly decreased immediately following administration of cannabis; however, the long-term clinical significance of this finding was not examined in this study. The majority of patients (70%) were on disease-modifying therapy (e.g. interferon β-1a, interferon β-1b, or glatiramer), and 60% were taking anti-spasticity agents (e.g. baclofen or tizanidine). Cannabis treatment was associated with a number of different, but commonly observed adverse effects including dizziness, headache, fatigue, nausea, feeling "too high", and throat irritation. Study limitations included the fact that the majority of patients had prior experience with cannabis, and that the study was unblinded since most of the patients were able to tell apart the placebo from the active treatment with cannabis.
Cannabis/cannabinoid tolerability in multiple sclerosis
Generally speaking, cannabis and orally administered prescription cannabinoids (e.g. dronabinol, nabilone, nabiximols, Cannador®) are reported to be well tolerated in patients with MSReference 686Reference 690-Reference 692Reference 694Reference 695Reference 698Reference 699. Clinical trials to date do not indicate serious adverse effects associated with the use of these prescription cannabinoid medications (or cannabis). However, there appears to be an increase in the number of non-serious adverse effects associated with the short-term use of cannabinoidsReference 4. The most commonly reported short-term physical adverse effects are dizziness, drowsiness, and dry mouthReference 387Reference 699.
Prolonged use of ingested or inhaled cannabis was associated with poorer performance on various cognitive domains (information processing speed, working memory, executive function, and visuospatial perception) in patients with MS according to one cross-sectional studyReference 233. Another cross-sectional study reported that while patients with MS who smoked cannabis daily are more cognitively impaired than non-users especially with respect to working memory, attention and information processing speed, no structural differences (lesion volume, global atrophy, diffusion tensor imaging [DTI] metrics) were discernible between users and non-usersReference 700. However, a follow-up study suggested that in the same cannabis-smoking patients, but not in the non-users, volume reductions in gray matter and white matter (in medial and lateral temporal regions, thalamus, basal ganglia, prefrontal cortex) were associated with the observed widespread cognitive deficitsReference 701.
In contrast, another study concluded that nabiximols treatment, in cannabis-naïve MS patients, was not associated with cognitive impairmentReference 699. However, the study did raise the possibility that higher dosages could precipitate changes in psychological disposition, especially in those patients with a prior history of psychosis. In any case, important information is generally lacking regarding the long-term adverse effects of chronic cannabinoid use in MS patients, and more generally in patients using for therapeutic purposes.
Bladder dysfunction associated with multiple sclerosis or spinal cord injury
Bladder dysfunction occurs in most patients suffering from MS or SCIReference 702. The most common complaints are increased urinary frequency, urgency, urge, and reflex incontinenceReference 703. cannabinoid receptors are expressed in human bladder detrusor and urotheliumReference 37Reference 38, and may help regulate detrusor tone and bladder contraction as well as affecting bladder nociceptive response pathways (reviewed inReference 38).
An early survey of MS patients regularly using cannabis for symptomatic relief of urinary problems reported that over half of these patients claimed improvement in urinary urgencyReference 538. A sixteen-week, open-label, pilot study of cannabis-based extracts (a course of nabiximols treatment followed by maintenance with 2.5 mg Δ9-THC only) for bladder dysfunction, in 15 patients with advanced MS, reported significant decreases in urinary urgency, number and volume of incontinence episodes, frequency, and nocturiaReference 704. Improvements were also noted in patient self-assessments of pain and quality of sleep. A subsequent RCT of 250 MS patients suggested a clinical effect of orally administered cannabinoids (2.5 mg Δ9-THC or 1.25 mg CBD with < 5% other cannabinoids per capsule, up to a maximum 25 mg/day) on incontinence episodesReference 702.
4.5.2 Amyotrophic lateral sclerosis
There is some pre-clinical evidence implicating the ECS in the progression of an ALS-like disease in mouse models of the disorder; under certain conditions, cannabinoids, or elevation of endocannabinoid levels through pharmacological inhibition or genetic ablation, have been reported to modestly delay disease progression and prolong survival in these animal models (reviewed inReference 705.
Anecdotal reports suggest decreased muscle cramps and fasciculations in ALS patients who smoked herbal cannabis or drank cannabis tea, with up to 10% of these patients using cannabis for symptom controlReference 706Reference 707.
Only two clinical trials of cannabis for the treatment of symptoms associated with ALS exist, and the results of the studies are mixed. In one four-week, randomized, double-blind, crossover pilot study of 19 ALS patients, doses of 2.5 to 10 mg per day of dronabinol (Δ9-THC) were associated with improvements in sleep and appetite, but not cramps or fasciculationsReference 708. In contrast, a shorter two-week study reported no improvement in these measures in ALS patients taking 10 mg of dronabinol per dayReference 707. In either case, dronabinol was well-tolerated with few reported side effects in this patient population at the tested dosages.
4.5.3 Spinal cord injury (or spinal cord disease)
Pre-clinical animal studies have shown the existence of an ECS in the spinal cord and a basal endocannabinoid tone in non-injured spinal cordsReference 709. While the role of the ECS in the intact spinal cord is only partially known, endocannabinoids modulate spinal cord analgesia as well as excitability, participating in the physiological control of reflexesReference 709. Pre-clinical animal studies suggest that SCI triggers changes in the activity of the ECS, with an acute spike in production of anandamide and 2-AG in the epicenter of the damaged areaReference 709. The spike in endocannabinoid levels, reflecting an active protective process induced by injury, returns to basal levels within a few days' post-injury; however 2-AG levels go through a subsequent secondary and more protracted rise in levels over a subsequent 28-day periodReference 709. Blocking both CB1 and CB2 receptors worsens SCI-associated damage, whereas stimulation of these two cannabinoid receptors appears to be protective and may also alleviate neuropathic pain associated with SCIReference 710-Reference 712. One pre-clinical study also reported a beneficial effect of CBD in restoring motor function and reducing extent of injury following SCI in a mouse modelReference 713. Subjective improvements have been anecdotally reported by SCI patients smoking cannabisReference 642Reference 714
However, despite the evidence from animal studies and anecdotal claims, limited clinical information exists regarding the use of cannabis and cannabinoids to treat symptoms associated with SCI such as pain, spasticity, muscle spasms, urinary incontinence, and difficulties sleeping. Double-blind, crossover, placebo-controlled studies of oral Δ9-THC and/or nabiximols suggested modest improvements in pain, spasticity, muscle spasms, and sleep quality in patients with SCIReference 642Reference 715Reference 716. More recently, a randomized, double-blind, placebo-controlled parallel study using a minimum of 15 to 20 mg Δ9-THC/day (mean daily doses of 31 mg Δ9-THC orally, or 43 mg Δ9-THC-hemisuccinate rectally) showed a statistically significant improvement in spasticity scores in patients with SCIReference 717 and a double-blind, placebo-controlled, crossover study using nabilone (0.5 mg b.i.d.) also showed an improvement in spasticity compared to placebo in patients with SCIReference 718.
A recent randomized, double-blind, placebo-controlled, cross-over clinical trial of vapourized cannabis showed analgesic and anti-spastic benefit for patients with SCI and diseaseReference 276. In this clinical trial, 42 patients (the majority of whom were currently using or had used cannabis) with neuropathic pain from SCI and disease were administered between 8 and 12 inhalations of cannabis placebo, or cannabis containing either low (2.9%) strength THC or high (6.7%) strength THC over an 8 h treatment session (400 mg dried cannabis material; vapourization temperature 185 ºC). While 400 mg of dried cannabis was placed in the vapourizer, only 45.9 mg (range: 29.9 - 83.8 mg) of the lower strength and 56.3 mg (range: 15.7 - 172.9 mg) of the higher strength cannabis was vapourized. These amounts and strengths suggest that on average between 1.3 and 3.8 mg of THC may have been inhaled (range: 0.86 - 11.6 mg THC). Median blood plasma concentrations of THC were 23 ng/mL (peak: 68.5 ng/mL) for the 2.9% strength and 47 ng/mL (peak: 177 ng/mL) for the 6.7% strength 3 h after an initial round of four inhalations and immediately after a second round of between four and eight additional inhalations. Pain intensity (primary outcome) decreased with increasing THC strength and was statistically significantly different from placebo for both strengths of THC after the first hour of exposure (round 1: 4 inhalations) and improved further compared to placebo after a second-round of inhalations (an additional 4 to 8 inhalations for a total of 8 to 12 inhalations overall). Pain relief showed a statistically significant difference between low and high strengths compared with placebo. The number of patients needed to treat (NNT) to achieve a 30% reduction in pain during the 8 h treatment session was 4 for the lower (2.9%) strength and 3 for the higher (6.7%) strength compared to placebo, whereas the NNT was 6 when comparing between the lower and higher strengths (but CIs were wide). By comparison, for neuropathic pain the NNT for pregabalin is 3.9 and for gabapentin, 3.8. Both strengths of cannabis provided statistically significant improvements on a variety of pain descriptors (i.e. sharpness, burning, aching, cold, sensitivity, unpleasantness, deep pain and superficial pain) but only the higher strength provided short-term relief of itching. No general effect was noted on allodynia. Only the lower strength (2.9%) was associated with a statistically significant decrease in spasticity and only 3 h after treatment initiation. Generally, there were no statistically significant differences between study medications on various measures of neuropsychological performance. Many of the psychoactive effects ("high", "good drug effect", "any drug effect", "impaired", "stoned", and "sedated") showed a dose dependency with greater effects with the higher dose compared to the lower dose and with both doses compared to placebo. The authors suggest that patients with SCI or disease who wish to avoid the psychomimetic effects while benefiting from the therapeutic effects consider using the lower dose (2.9%).
4.6 Epilepsy
- Anecdotal evidence suggests an anti-epileptic effect of cannabis (THC- and CBD-predominant strains).
- The available evidence from pre-clinical and limited clinical studies suggests certain cannabinoids (CBD) may have anti-epileptiform and anti-convulsive properties, whereas CB1R agonists (THC) may have either pro- or anti-epileptic properties.
- However, the clinical evidence for an anti-epileptic effect of cannabis is weaker, but emerging, and requires further study.
- Evidence from clinical studies with Epidiolex® (oral CBD) suggests efficacy and tolerability of Epidiolex® for drug-resistant seizures in treatment-resistant Dravet syndrome or Lennox-Gastaut syndrome.
- Evidence from observational studies suggests an association between CBD (in herbal and oil preparations) and a reduction in seizure frequency as well as an increase in quality of life among adolescents with rare and serious forms of drug-resistant epilepsy.
- Epidiolex® has received FDA approval (June 2018) for use in patients 2 years of age and older to treat treatment-resistant seizures associated with Dravet syndrome and Lennox-Gastaut syndrome.
Epilepsy is one of the most common neurological disorders with a worldwide prevalence of approximately 1%Reference 217Reference 719. It is not a singular disease entity, but a variety of disorders reflecting underlying brain dysfunction arising from many different causesReference 720. Epilepsy is characterized by recurrent, unprovoked seizures, which are transient occurrences of signs and symptoms caused by abnormal excessive or synchronous neuronal activity in the brainReference 720. Seizures can be of various types including genetic and occurring in childhood (e.g. Dravet Syndrome, Lennox-Gastaut), or acquired and occurring in adulthood (e.g. after severe head injury, stroke, or from a tumour)Reference 265. Co-morbidities associated with epilepsy include cognitive decline, depressive disorders, and schizophreniaReference 721.
Despite the availability of many anti-epileptic medications, close to 30% of patients with epilepsy remain refractory to conventional treatments leading them to search for other therapeutic modalities, such as cannabis (e.g. CBD-enriched cannabis oils)Reference 722.
The endocannabinoid system and epilepsy
The ECS is known to regulate cortical excitability, and endocannabinoids have been suggested to produce a stabilizing effect on the balance between excitatory and inhibitory neurotransmitters in the CNSReference 723.
Temporal lobe epilepsy, one of the most common kinds of epilepsy seen in adults, is associated with changes in the hippocampus where CB1 receptor expression is downregulated during the acute phase, shortly after the precipitating insult, but then upregulated in the chronic phase of the disorderReference 217Reference 265Reference 724Reference 725. Furthermore, it appears that the expression of the CB1 receptor on excitatory glutamatergic axon terminals, as well as the expression of DAGL, which is responsible for yielding the endocannabinoid 2-AG, are both downregulatedReference 265. In contrast, CB1 receptor expression on inhibitory GABAergic axon terminals appears to be upregulated. In addition, reduced levels of the endocannabinoid anandamide have been detected in the cerebrospinal fluid (CSF) of patients with untreated, newly diagnosed, temporal lobe epilepsyReference 726, whereas normally, anandamide is found in high concentrations in the hippocampus, a brain region known to be involved in epileptogenesis and seizure disordersReference 263. Taken together, these and other studies demonstrating changes in CB1 receptor and DAGL expression in the hippocampus and changes in anandamide levelsReference 727-Reference 729 suggest important and widespread changes in the functioning of the ECS in epilepsy. Since the ECS is generally thought to act as a neurotransmitter braking system, the reported dysregulation of the ECS in epilepsy may play a role in the generation and maintenance of epileptic seizuresReference 265. There is also some evidence to suggest that endocannabinoids promote the maintenance, but not the initiation, of epileptiform activity by activating CB1 receptors located on astrocytesReference 730.
Pre-clinical studies
In vitro and in vivo studies suggest certain phytocannabinoids (and endocannabinoids) can have anti-convulsive but also, in some cases, pro-convulsive rolesReference 263Reference 265Reference 266Reference 719Reference 721Reference 731-Reference 739.
CB1 receptors are located mainly pre-synaptically where they typically inhibit the release of classical neurotransmittersReference 740.The purported anti-epileptic effect of certain cannabinoids (e.g. THC) is thought to be mediated by CB1-receptor dependent pre-synaptic inhibition of glutamate releaseReference 265Reference 728Reference 741; on the other hand, epileptogenic effects may be triggered by pre-synaptic inhibition of GABA releaseReference 265Reference 736Reference 739Reference 742-Reference 744. CB1 receptor agonists (e.g. THC) therefore have the potential to trigger or suppress epileptiform activity depending upon which cannabinoid-sensitive pre-synaptic terminals are preferentially affected (i.e. glutamatergic or GABAergic)Reference 112Reference 266Reference 741. Because of the ability of CB1 receptor agonists such as THC to yield either pro- or anti-convulsant activities and because of the reported development of tolerance to their anti-convulsant effects, CB1 receptor agonists are thought to be unlikely to yield therapeutic benefit for patients with epilepsyReference 263Reference 266.
In contrast to the ambiguous situation with CB1 receptor agonists such as THC, phytocannabinoids such as CBD, CBDV, THCV, and CBN appear to mainly have anti-convulsant roles and may have more potential therapeutic value for the treatment of epilepsyReference 263Reference 266. A number of in vivo studies have demonstrated the anti-epileptic effects of CBD across different animal models of epilepsy (reviewed inReference 263). Early studies using various rat and mouse models of epilepsy reported that CBD was an effective anti-convulsant and its potency was significantly increased when combined with anti-epileptic drugs such as phenytoin and phenobarbital used to treat major seizuresReference 263Reference 745. In contrast, CBD reduced the anti-convulsant potencies of chlordiazepoxide, clonazepam, trimethadione, and ethosuximide used for minor seizuresReference 263Reference 745. ED50 doses for CBD in rats ranged from as low as 12 mg/kg (p.o.) to as high as 380 mg/kg (i.p.) in miceReference 263Reference 745Reference 746. Another study reported that CBD attenuated epileptiform activity in vitro in hippocampal slices and displayed anti-convulsant activity in vivo (100 mg/kg) in one rat model of epilepsy, attenuating seizure severity, tonic-clonic seizures and mortalityReference 735. A follow-up study by this same group examined the anti-convulsive effects of CBD in two other rat models of temporal lobe and partial epilepsyReference 733. CBD at doses of 1, 10, and 100 mg/kg significantly attenuated the percentage of animals displaying seizure events (temporal lobe epilepsy); however, there was no significant effect upon the mean number of seizure occurrences per animal or on seizure severity. In the model of partial seizure, CBD (1, 10, 100 mg/kg) decreased the percentage of animals that developed tonic-clonic seizures and was associated with decreased mortality rate (at 10 and 100 mg/kg), but had no effect on overall seizure severity. CBD was also reported to have some minor negative effects on motor function at a dose of 100 mg/kg, which was paradoxically attenuated when the dose was doubled (200 mg/kg)Reference 733.
The anti-convulsant effects of pure CBDV as well as botanical extracts containing CBDV (and significant amounts of CBD), with and without THC and THCV, have been investigated in a number of animal models of epilepsyReference 263Reference 719Reference 721Reference 747. CBDV (> 10 µM) was found to significantly attenuate epileptiform activity in vitro as well as having significant anti-convulsant effects in vivo (min. > 50 mg/kg i.p.) in different mouse models of epilepsyReference 747. A dose of 200 mg/kg (i.p.) of CBDV was associated with complete cessation of tonic convulsions in two models of epilepsy and attenuated seizure severity and mortality at a 200 mg/kg i.p. dose as well as significantly delaying seizure onset in a third epilepsy modelReference 747. Furthermore, co-administration of CBDV and the anti-epilepsy drugs valproate, ethosuximide, or phenobarbital was associated with significant anti-convulsant effectsReference 747. For example, co-administration of CBDV (200 mg/kg) with valproate (50 - 250 mg/kg) or ethosuximide (60 - 175 mg/kg) was associated with significant anti-convulsant effectsReference 747. Co-administration of 200 mg/kg CBDV and phenobarbital (10 - 40 mg/kg) was also associated with significant anti-convulsant effectsReference 747. CBDV did not appear to have any significant effects on motor performance at the tested doses and also appeared to be well-tolerated when co-administered with these anti-epileptic drugsReference 747. In mice and rats, CBDV showed significant anti-convulsive effects with doses ranging from 50 mg/kg to 400 mg/kg or moreReference 263Reference 719Reference 721. Furthermore, in vivo animal studies with two types of botanical extracts enriched in CBDV (47.4 - 57.8 %) and CBD (13.7 - 13.9%) with and without THC (1%) and THCV (2.5%) were studied for their anti-convulsive effects as well as their toxicitiesReference 721. The study found that both botanical extracts showed similar significant anti-convulsive actions in three different animal models of epilepsy and that the presence of THC/THCV at the doses administered in the extracts did not contribute to the anti-convulsive actionsReference 721. On the other hand, the presence of THC/THCV in the extract contributed to some observed adverse motor effectsReference 721. Lastly, CBDV was found to bind only weakly to the CB1 receptor, suggesting the anti-convulsant mechanism of action of CBDV is CB1-receptor independentReference 721.
In contrast with CBD and CBDV, the anti-convulsant effects of CBN have not been as well studied. In one study, CBN produced anti-convulsant effects with an ED50 of 18 mg/kgReference 263Reference 745.
Although in vitro studies show that THCV binds with relatively high affinity at CB1 receptorsReference 112Reference 748, THCV does not appear to be a potent CB1 receptor agonistReference 112Reference 263Reference 748. Instead, experimental studies suggest THCV acts more like a CB1 receptor antagonist and a potent CB2 receptor partial agonistReference 18Reference 112Reference 263Reference 748Reference 749. At higher doses however, THCV appears to have some agonist activity at the CB1 receptorReference 18. Furthermore, in vitro studies suggest THCV has some anti-epileptiform effects at micromolar concentrationsReference 112 and in vivo studies suggest THCV (0.25 mg/kg) has some limited anti-convulsant effects in one mouse model of epilepsyReference 112Reference 266.
There is little experimental evidence thus far for the anti-convulsant effects of CBG. While one in vitro study suggests anti-epileptiform activity for CBG, an in vivo study in rats suggests that in one model of epilepsy, CBG (at doses ranging from 50 - 200 mg/kg) does not have anti-convulsant effectsReference 263Reference 750.
Data from observational studies and patient surveys
According to some studies, about 20% of epilepsy patients are actively using cannabisReference 722Reference 731Reference 751Reference 752. A telephone survey of 136 patients of a Canadian tertiary care epilepsy centre revealed that 48% had used cannabis in their lifetime, 21% were active users, 13% were frequent users (one day per week or more), and 8.1% were heavy users (every other day or more)Reference 752. Three percent of subjects met the criteria for cannabis dependence. When asked about their personal experiences with cannabis use, 68% of respondents said their seizure severity improved, while 32% said there was no effect. With regard to seizure frequency, 54% claimed improvement, while 46% stated no effect. Eleven percent noted fewer side effects from medications when using cannabis, while 85% did not notice an effect. Forty-three percent of respondents stated medical reasons for cannabis use. The survey authors noted that cannabis use was associated with increased seizure frequency and longer duration of disease. While the reasons for these associations is not clear, it is possible that patients with more severe epilepsy are more prone to trying or using cannabis or that cannabis use is associated with worsening epilepsy.
Another study interviewed epilepsy outpatients at a tertiary epilepsy clinic in Germany. Out of 310 epilepsy patients that were interviewed, 28% said they had used cannabis in their lifetime while 63% had consumed cannabis after their epilepsy diagnosisReference 751. Almost 70% of epilepsy patients had partial epilepsy, a little over 20% had idiopathic generalized epilepsy, and approximately 10% were undetermined. Common reasons for cannabis use included curiosity, enjoyment and relaxation. The majority of patients (84%) who had started using cannabis after their epilepsy diagnosis did not observe any effect on their epilepsy, 5% had reported improvement in their seizures or symptoms associated with cannabis use, and 11% reported worsening of seizures associated with cannabis use.
A retrospective clinical chart review of 18 Canadian patients with epilepsy who were authorized to possess cannabis for medical purposes reported that 61% had focal epilepsy, with 39% having generalized epilepsyReference 753. Twenty-two percent had mesial temporal sclerosis, 17% had idiopathic epilepsy, 17% had epilepsy associated with a tumour, 11% had been diagnosed with Lennox-Gastaut, 11% had epilepsy associated with a congenital malformation, and 11% were classified as unknown. Psychiatric comorbidity was common (61%) with depression being the most frequent entity. Most patients had used an average of five anti-epileptic medications in the past. Eighty-nine percent of patients had a long history of cannabis use before obtaining an authorization to possess. Mode of administration was mainly by smoking (83%). Mean number of daily puffs was 4 and the estimated amount of cannabis consumed per day was 2 g. All patients that stopped cannabis use reported exacerbation of seizures associated with drug withdrawal. None reported status epilepticus as a complication. One hundred percent of patients reported improvement in seizure severity and seizure frequency. Eighty-nine percent of the patients reported no side effects, while all reported an improvement in mood disorders, and general well-being. Eighty-nine percent reported an improvement in sleep quality and appetite. Limitations of this study included its retrospective nature and bias associated with self-reporting, as well as the lack of a control group and its small sample size.
Treatment-resistant, childhood-onset epilepsy
The results from two parent surveys of children with treatment-resistant childhood epilepsy and who tried cannabis oils have been published and are summarized hereReference 215Reference 264. In one survey of 19 children, 13 had Dravet syndrome, 4 had Doose syndrome, 1 had Lennox-Gastaut and 1 had idiopathic early-onset epilepsyReference 264. Children ranged in age from 2 to 16 years. The parents reported that the children had a variety of different seizure types including focal, tonic-clonic, myoclonic, atonic, and infantile spasms. In virtually all cases, the study reported that the children had treatment-resistant epilepsy for more than three years before trying CBD-enriched cannabis. The children had tried an average of 12 other anti-epileptic medications before beginning CBD-enriched cannabis treatment. Dosages of CBD reported ranged from less than 0.5 mg/kg/day to 28.6 mg/kg/day, while dosages of THC were reported to range from 0 to 0.8 mg/kg/day. Duration of CBD-enriched cannabis use was reported to range from two weeks to over one year. Eighty-four percent of the parents that responded to the survey reported a reduction in their child's seizure frequency. Two parents reported a complete halt of seizures in their children after more than four months of treatment. Forty-two percent of the surveyed parents reported a greater than 80% reduction in seizure frequency, 16% reported a greater than 50% reduction in seizure frequency and the same proportion of parents reported a greater than 25% reduction as well as no reduction. Sixty-percent of parents reported weaning their child from another anti-epileptic medication after starting CBD-enriched cannabis treatment. Parent-reported beneficial effects included better mood (79%), increased alertness (74%), better sleep (68%), and decreased self-stimulation (32%), while adverse effects included drowsiness (37%), and fatigue (16%). Limitations of such a survey include the self-selection bias, lack of a control group, the inability to independently verify any of the parents' claims including information about dosing, as well as the small sample size and the under-representation of epilepsy types other than Dravet syndrome.
The results of a second parent surveyReference 215 have also been published. In this survey, 117 parents of children with treatment-resistant epilepsy responded. Forty-five percent of parents reported a child with infantile spasms and/or Lennox-Gastaut syndrome, while 13% reported severe myoclonic epilepsy of infancy (Dravet syndrome). Four percent reported myoclonic-astatic epilepsy (Doose syndrome) and 38% reported other types of epilepsy. Age range of children was 3 to 10 years and the median number of anti-epileptic medications tried and failed prior to trial of CBD-enriched cannabis preparations was eight. Median duration of CBD treatment was 6.8 months (range: 3.8 to 9.8 months). Median dosage of CBD in the preparations was 4.3 mg/kg/day (range: 2.9 to 7.5 mg/kg/day). The vast majority of respondents reported using CBD-enriched oil-based extracts, typically administered two to three times per day. The reported CBD to THC ratio in the oil preparations was at least 15:1. Eighty-five percent of respondents reported a reduction in seizure frequency, including 14% reporting complete seizure freedom while 9% reported no change and 4% reported an increase in seizure frequency. Eighty-six percent of respondents reported either an improvement or worsening within 14 days of starting treatment. Adverse effects associated with treatment included increased appetite (29.9%) and weight gain (29.1%). Interestingly, the median number of side effects reported during treatment was much lower than that reported before treatment. The reported decrease in the number of side effects during treatment was attributed to the claimed discontinuation of at least one anti-seizure medication during treatment. While overall, the prevalence of adverse effects was decreased during treatment with the cannabis preparations, the most often encountered adverse effects were drowsiness (12.8%), fatigue (9.4%), irritability (9.4%), and nausea (6.8%). Respondents reported improvement in sleep (53%), alertness (71%), and mood (63%). Again, as with the survey carried out by Porter et al., the survey by Hussain et al. 2015 carries the same limitations and the data must be interpreted with caution.
A retrospective chart review of 75 children and adolescents in Colorado who were given oral cannabis extracts for the treatment of refractory epilepsy reported that 57% of patients showed improvement in seizure control and 33% reported a > 50% reduction in seizuresReference 754. Average age was 7.3 years (range: 6 months to 18 years) when starting oral cannabis extract treatment. Four percent of the patients had Doose syndrome, 17% had Dravet syndrome, and 12% were diagnosed with Lennox-Gastaut syndrome. Among children with a specified syndrome, those with Lennox-Gastaut represented the greatest proportion of responders to oral cannabis extracts (89%), followed by those with Dravet syndrome (23%) and those with Doose syndrome appeared to respond the least (0%). When classified by seizure type, those with atonic seizures appeared to have the greatest response rate (44%), followed by those with focal (38%) and epileptic spasms (36%), generalized tonic-clonic (30%), absence (28%), myoclonic (20%), and tonic (17%)Reference 215. Reported improvements included an increase in alertness/behavior (33%), language (11%), motor skills (11%), and sleep (7%). Adverse events were reported in 44% of patients treated with an oral cannabis extract. Adverse effects associated with oral cannabis extract administration included worsening of seizures (13%), somnolence (12%), GI symptoms (11%), and irritability (5%). Surprisingly, there were no reported differences in response based on the strain or type of oral cannabis extract the patients were treated with (i.e. high CBD, CBD plus other oral cannabis extracts, THCA, and other oral cannabis extract types). The majority of patients used an oral cannabis extract with high CBD content with or without other oral cannabis extracts. Study limitations included small sample size, heterogeneity of products used, uncertain dosages of cannabinoids, inability to determine dose-response, and discrepancy in ratings of treatment benefit between families that had moved to Colorado for treatment vs. those that were state residents.
A retrospective, multicenter study examined the effect of CBD treatment for severe intractable epilepsy (i.e. acquired epilepsy, early epileptic encephalopathy with known genetic etiology, epileptic encephalopathy with unknown genetic etiology, congenital brain malformation, hypoxic ischemic encephalopathy, and other, with resistance to five to seven anti-epileptic medications, ketogenic diet and vagal nerve stimulation)Reference 213. The study examined the clinical records of clinic and phone call visits of children and adolescents (age range: 1 - 18) with refractory epilepsy being treated in four pediatric epilepsy centres in Israel. Seventy-four children and adolescents were included in the study and the reported daily dose of CBD (1 - 20 mg/kg/day) was administered over an average period of six months (minimum three months). Highest daily CBD dose was 270 mg/day. Eighty percent of the children included in the study used less than 10 mg/kg/day CBD with the remainder (20%) using more than 10 mg/kg/day CBD. The ratio of CBD to THC was 20: 1 and cannabinoids were dissolved in canola oil. Parents or older children reported any changes in seizure number. CBD treatment was associated with a reduction in seizure frequency as well as improved behaviour and alertness, improved language, improved communication and motor skills and improved sleep. Approximately half of the patients reported side effects with 18% reporting seizure aggravation, 22% reporting somnolence or fatigue and 7% reporting GI problems or irritability. Side effects led to withdrawal of cannabis oil extract in five patients. Limitations of the study include retrospective design, lack of a control group, no consistent rate of dosage elevation, reliance on parental report of effect on seizure frequency, short duration of the study and lack of long-term outcome, lack of EEG results, and no measurement of other drug levels.
Clinical studies
Note: Epidiolex® is the brand name for a whole-plant cannabis extract of a high CBD strain of Cannabis sativa and is an oral oil solution product containing > 98% CBD at a concentration of 100 mg/ml. Epidiolex® has received FDA approval (June 2018) for use in patients 2 and older to treat Dravet syndrome and Lennox-Gastaut syndrome. It has also received Orphan Drug Designation in the U.S. for the treatment of Lennox-Gastaut Syndrome, Dravet Syndrome and Tuberous Sclerosis Complex. At the time of writing of this publication, Epidiolex® has not received a Notice of Compliance from Health Canada, and is not marketed in Canada.
While there are many anecdotal accounts of dramatic improvements with cannabis-based products with high CBD to THC (e.g. 20 > 1) ratios, the available clinical evidence supporting the safety and efficacy of cannabis for epilepsy is relatively sparseReference 217Reference 266Reference 671. The available evidence from clinical studies is discussed below and summarized in a Cochrane reviewReference 217.
One randomized, placebo-controlled clinical study of nine individuals with uncontrolled temporal lobe epilepsy who had failed treatment with multiple anti-epileptic medications reported that two of the individuals that received daily doses of 200 mg of CBD for three months were seizure-free, one showed partial improvement and one did not show any improvementReference 217Reference 755. None of the placebo-treated patients showed any signs of improvement. No adverse effects were noted. Limitations of this study included lack of comparison between the CBD-treated group and the placebo-group for baseline seizure characteristics, small sample size, unclear methodology, possible lack of blinding, and lack of statistical analysis.
Another randomized, placebo-controlled clinical study of 15 epileptic patients suffering from uncontrolled temporal lobe epilepsy reported that daily treatment with doses of 200 to 300 mg of CBD (in combination with a variety of conventional anti-epileptic drugs) lasting 3 to 18 weeks was associated with seizure cessation in four (out of eight) patients treated with CBDReference 217Reference 756. One placebo-treated patient (out of seven) became seizure-free. Adverse reactions included somnolence. Limitations of this study included lack of comparison between the CBD-treated group and the placebo-group for baseline seizure characteristics, small sample size, unclear methodology, possible lack of blinding, and lack of statistical analysis.
One placebo-controlled clinical trial of 12 patients with frequent seizures who were not taking any anti-epileptic medications reported no statistically significant difference in seizure frequency between patients given daily doses of 200 to 300 mg of CBD for four weeks compared to placeboReference 217Reference 757. Reported adverse effects included drowsiness. Limitations of the study included small sample size, possible unblinding, lack of comparison between the CBD-treated group and the placebo-group for baseline seizure characteristics, and unclear methodology.
A randomized, double-blind, placebo-controlled, cross-over clinical study of 12 patients with incompletely controlled epilepsy reported that treatment with 100 mg of CBD, three times daily, for six months, appeared to be associated with a decrease in seizure frequency although seizure frequency was not well measured and no statistical analysis was performedReference 217Reference 758. CBD treatment also did not appear to be associated with any adverse behavioural changes. Limitations of this study included small sample size, lack of statistical analysis and lack of objective measurement of seizure frequency.
A Cochrane review of the clinical evidence for cannabinoid treatment for epilepsy reviewed the four clinical studies discussed aboveReference 755-Reference 758 and concluded that, based on their evaluation criteria, all of these reports were of low quality and no reliable conclusions could be drawn based on these studies regarding the efficacy of cannabinoids (CBD) as a treatment for epilepsy. However, a dose of 200 to 300 mg of CBD daily could be safely administered to small numbers of patients for short periods of time but the safety of long-term CBD treatment could not be reliably assessed in these studiesReference 217.
Treatment-resistant, childhood-onset epilepsy
A clinical study investigating differences in ECS components and in molecular targets associated with CBD action found an increase in expression levels of the voltage-dependent calcium channel α-1h subunit, in CB2 receptor gene expression, and a decrease in the expression of the serotonin transporter gene in lymphocytes isolated from Dravet Syndrome patientsReference 759.
A report from an expanded access investigational new drug (IND) trial of Epidiolex®, an oil-based cannabis extract containing 98% v/v CBD, examined the interaction between clobazam and Epidiolex® (CBD) during the treatment of refractory pediatric epilepsyReference 236. Thirteen subjects with refractory epilepsy were included in the study. Diagnoses included Dravet syndrome, Doose syndrome, cortical dysgenesis, isodicentric duplication chromosome 15q13, CDKL5 (Cyclin-Dependent Kinase-Like 5) mutation, Tuberous sclerosis complex, and lissencephaly. Seventy percent of the included patients had a > 50% decrease in seizures. Daily doses of Epidiolex® ranged from 5 mg/kg/day to a maximum of 25 mg/kg/day. The average daily dose of clobazam was 1 mg/kg/day with a range of 0.18 to 2.24 mg/kg/day. Co-administration of CBD and clobazam was associated with higher plasma levels of clobazam and its active metabolite n-desmethylclobazam and close monitoring of plasma levels of clobazam and n-desmethylclobazam is recommended as is dose adjustment of clobazam, as needed, to prevent overdose. Side effects were reported in 77% of the 13 study subjects and included drowsiness, ataxia, irritability, restless sleep, urinary retention, tremor and loss of appetite.
An expanded-access, prospective, open-label, 12-week clinical trial of Epidiolex® (98 - 99% CBD oil oral preparation, 100 mg/mL) in patients aged 1 to 30 years with severe, intractable, childhood-onset, treatment-resistant epilepsy (mainly Dravet and Lennox-Gastaut syndromes) examined whether addition of CBD to existing anti-epileptic treatment regimens would be safe, tolerated and efficaciousReference 262. Patients were started at a dose of CBD between 2 and 5 mg/kg/day divided into twice-daily dosing added to existing anti-epileptic treatments (i.e. ketogenic diet, clobazam, valproate), and slowly titrated upwards by 2 to 5 mg/kg once per week until intolerance or up to a maximum dose of 25 mg/kg per day (or up to a maximum of 50 mg/kg/day, depending on the study site). The maximum dose at the 12-week clinic visit was 41 mg/kg/day, and the mean CBD dose at 12 weeks was 23 mg/kg in the safety analysis group and in the efficacy analysis group. The median monthly frequency of motor seizures was 30 at baseline and 16 over the 12-week treatment period, and the median reduction in monthly motor seizures was 37%. The greatest reduction in seizures occurred in those patients with focal seizures (-55%) or atonic seizures (-54%), followed by tonic seizures (-37%), or tonic-clonic seizures (-16%). Combination therapy (CBD with clobazam or valproate) was associated with a greater reduction in seizures compared to patients not using clobazam or valproate. Adverse events were reported in 79% of the patients within the safety group. Adverse events in more than 5% of patients were somnolence (25%), decreased appetite (19%), diarrhea (19%), fatigue (13%), convulsions (11%), appetite changes (9%), status epilepticus (8%), lethargy (7%), changes in blood concentrations of concomitant anti-epileptic drugs (6%), gait disturbance and sedation. Most adverse events were mild or moderate and transient. Serious adverse events deemed possibly related to CBD use (10%) included status epilepticus (6%), diarrhea (2%), pneumonia (<1%), and weight loss (1%). Patients taking more than 15 mg/kg/day CBD were more likely to report diarrhea or related side-effects (e.g. weight loss). Three percent of the enrolled patients withdrew from the study, and reasons for study withdrawal included allergy to the sesame oil vehicle, hepatotoxicity, excessive somnolence and poor efficacy, GI intolerance, worsening seizures, and hyperammonemia. Major limitations of this study included open-label design and lack of an appropriate control group. In addition, the issue of a significant placebo response was noted by the authors to be of special significance in pediatric trials of cannabis-based treatments. The authors note that the placebo response in RCTs of add-on treatments in patients with epilepsy appears to be more significant in the pediatric population compared to adults (19% vs. 9.9 - 15%).
A randomized, double-blind, placebo-controlled trial was conducted to determine the efficacy and safety of Epidiolex® in treating drug-resistant seizures in the Dravet syndromeReference 576. After a 4-week baseline period, a total of 120 affected children and young adults (2.3 to 18.4 years old) were randomized (1:1) to receive either 20 mg/kg/day CBD oral solution or placebo, in addition to standard antiepileptic treatment, for 14 weeks (2 weeks of dose escalation and 12 weeks of dose maintenance). At the end of the treatment period there was a 10-day taper period (10% in dose reduction per day) followed by a 4-week follow-up period. The most common type of convulsive seizure was generalized tonic-clonic (78%) followed by secondarily generalized tonic-clonic seizures (21%). Nonconvulsive seizures were reported by 61% of the patients in the CBD group and 69% in the placebo group. Treatment with CBD decreased the median frequency of convulsive seizures per month (primary endpoint) from 12.4 (range: 3.9 to 1,717) to 5.9 (range: 0.0 to 2,159), while placebo had no effects (from 14.9 to 14.1). The adjusted median difference between the CBD and placebo groups in change in seizure frequency was -22.8 percentage points (95% CI = -41.1 to -5.4; p = 0.01). The effects of CBD on convulsive seizures were seen in the first month of the maintenance period. In the CBD group, 43% of the patients had at least a 50% reduction in the frequency of convulsive seizures compared to 27% in the placebo group (OR, 2.00; 95% CI = 0.93 to 4.30; p = 0.08). During the treatment period, 3 patients (5%) in the CBD group and no patients in the placebo group became seizure-free (p = 0.08). CBD decreased from 24.0 to 13.7 the median frequency of seizures per month (adjusted reduction 28.6%), while placebo decreased it from 41.5 to 31.1 (adjusted reduction 9.0%), for a significant adjusted median difference between groups of -19.2 percentage points (p = 0.03). There was no significant difference between groups for reduction in nonconvulsive seizures (p = 0.88). Common adverse events (>10% frequency) in the CBD group were somnolence (36%), diarrhea (31%), decreased appetite (28%), fatigue (20%), vomiting (15%), pyrexia (15%), lethargy (13%), upper respiratory tract infection (11%), and convulsion (11%). Most of them were mild or moderate in severity (84% in the CBD group) and considered related to the trial agent (75%). In the CBD group, 8 patients withdrew from the trial because of adverse events, compared with 1 in the placebo group. A total of 12 patients in the CBD group and 1 in the placebo group had elevated aminotransferase levels; they were all also taking valproate. Of the 9 patients who continued taking CBD (3 patients withdrew from the trial), enzyme levels returned to normal during the trial, suggesting transient metabolic stress on the liver. Differences in unpalability between the active treatment and placebo could have affected blinding in a small number of patients. The length of the trial did not allow for the assessment of the potential development of tolerance so additional data are needed to determine the long-term efficacy and safety of CBD for the Dravet syndromeReference 576.
A randomized, double-blind, placebo-controlled clinical trial was conducted to investigate the efficacy of Epidiolex® as add-on therapy for drop seizures in patients with treatment-resistant Lennox-Gastaut syndromeReference 577. After a 4-week baseline period, 171 eligible patients (aged 2-55 years) were randomized (1:1) to either receive 20 mg/kg CBD daily (n = 86) or placebo (n = 85) as 2 equivalent doses (morning and evening) for 14 weeks (2 weeks of dose escalation and 12 weeks of dose maintenance). The median percentage reduction in monthly drop seizure frequency from baseline (primary endpoint) was 43.9% [interquartile range (IQR) -69.6 to -1.9] in the CBD group and 21.8% (IQR -45.7 to 1.7) in the placebo group. The estimated median difference between the treatment groups was -17.21 (95% CI -30.32 to -4.09; p = 0.0135) during the 14-week treatment period. The treatment effect of CBD on the primary endpoint was established during the first 4 weeks of the maintenance period and was maintained during the full treatment period. In the CBD group, 38 patients (44%) had a reduction in drop seizure frequency of ≥50% from baseline during the treatment period compared with 20 patients (24%) in the placebo group (OR 2.57, 95% CI 1.33-4.97; p = 0.0043). There were 3 patients in the CBD group who were free of drop seizures throughout the 12-week maintenance period; their monthly frequency of drop seizures at baseline was in the lower range of 15.6 to 99.2. During the treatment period, CBD also significantly decreased the estimated median difference in the monthly frequency of total seizures [-21.1 (95% CI -33.3 to -9.4; p = 0.0005)] and non-drop seizures [-26.1 (95% CI -46.1 to -8.3; p = 0.0044)] compared to placebo. This suggested that add-on CBD may have broad spectrum effects on seizure reduction. Common adverse events (occurring in ≥10% of patients) in the CBD group were diarrhea (19%), somnolence (15%), pyrexia (13%), decreased appetite (13%) and vomiting (10%). Most of the adverse events were mild or moderate in severity (78% in the CBD group) and resolved by the end of the trial (61%). Adverse events led to study withdrawal in 12 patients (14%) in the CBD group and 1 (1%) patient in the placebo group. Of the 20 patients in the CBD group who had elevations in ALT or AST (>3 times upper limit of normal), irrespective of whether they were reported as adverse events, 16 were also taking valproate. The most common serious treatment-related adverse events (occurring in >3% of patients) were collectively reported in 4 patients in the CBD group and comprised increased ALT (n = 4), AST (n = 4) and γ-glutamyltransferase (n = 3) concentrations. No patients met standard criteria for drug-induced severe liver injury (Hy's law). Overall, this trial demonstrated that add-on CBD was efficacious for the treatment of patients with drop seizures associated with Lennox-Gastaut syndrome and was generally well tolerated. However, only a single dose of CBD was tested in this trial; dose-response effects will be assessed further in another study (GWPCARE3; ClinicalTrials.gov, number NCT02224560). Further assessment of the long-term efficacy and safety of CBD is being carried out in the ongoing open-label extension of this trial and will also be done using real-world data, once availableReference 577.
A double-blind, placebo-controlled clinical trial was conducted to determine the efficacy and safety of Epidiolex® (CBD) as an adjunct to conventional antiepileptic drugs to treat drop seizures in patients with Lennox-Gastaut syndrome, a severe developmental epileptic encephalopathyReference 760. A total of 225 patients (aged 2-55) with Lennox-Gastaut syndrome and ≥2 drop seizures per week during a 28-day baseline period were randomly assigned to receive 20 mg/kg CBD (n = 76), 10 mg/kg CBD (n = 73) or placebo (n = 76) as 2 equally divided doses daily for 14 weeks (2 weeks dose escalation followed by 12 weeks of maintenance). The median percent reduction from baseline in the frequency of drop seizures per 28 days during the treatment period (primary outcome) was 41.9% (p = 0.005), 37.2% (p = 0.002) and 17.2% in the 20 mg/kg CBD, 10 mg/kg CBD and placebo groups, respectively. During the treatment period, a total of 30 patients (39%) in the 20 mg/kg CBD group (OR 3.8; 95% CI 1.75-8.47; p < 0.001), 26 patients (36%) in the 10 mg/kg CBD group (OR 3.27; 95% CI 1.46-7.26; p = 0.003) and 11 patients (14%) in the placebo group had ≥50% reduction from their baseline in drop-seizure frequency. The percentage of patients who had ≥75% reduction from baseline in drop-seizure frequency was higher in the 20 mg/kg CBD group (25%) and the 10 mg/kg CBD group (11%) than in the placebo group (3%). No patients were free from drop seizures during the entire treatment period (day 1 onward); however, 5 patients (7%), 3 patients (4%) and 1 patient (1%) in the 20 mg/kg CBD, 10 mg/kg CBD and placebo groups, respectively, were free from drop seizures during the entire maintenance phase (day 15 onward). The median percent reduction from baseline in the frequency of all seizures per 28 days during the treatment period was 38.4% (p = 0.009), 36.4% (p = 0.002) and 18.5% in the 20 mg/kg CBD, 10 mg/kg CBD and placebo groups, respectively. Adverse events were reported in 72-94% of patients, the majority of which (89%) were considered mild or moderate in severity. The most common adverse events with CBD were somnolence (n = 14-25), decreased appetite (n = 11-21), and diarrhea (n = 7-12); these events occurred more frequently in the 20 mg/kg CBD group. Serious adverse events (n = 26 vs. n = 7) and trial withdrawal (n = 7 vs. n = 1) were more common in the CBD groups than in the placebo group. Serious adverse events considered related to CBD occurred in 7 patients (1 patient had multiple events) and included elevated aspartate aminotransferase concentration (n = 2), elevated alanine aminotransferase concentration (n = 1), elevatedγ-glutamyltransferase concentration (n = 1), somnolence (n = 1), increased seizures during weaning (n = 1), non-convulsive status epilepticus (n = 1), lethargy (n = 1), constipation (n = 1) and worsening chronic cholecystitis (n = 1). Maximum elevations in aspartate aminotransferase or alanine aminotransferase concentrations 3.2-12.2 times the upper limit of normal were the most common adverse events leading to trial withdrawal in the CBD groups (n = 5). Elevations in aminotransferase concentrations >3 times the upper limit of normal occurred more frequently in patients receiving 20 mg/kg CBD (n = 11) than in those receiving 10 mg/kg CBD (n = 3). In most of these cases (n = 11, 79%), patients were receiving valproate concomitantly. No patient met the criteria for severe drug-induced liver injury (DILI). The majority of these cases (n = 9) resolved after the dose of CBD was tapered, discontinued or the dose of another antiepileptic drug was reducedReference 760.
A recent systematic review of 36 studies (30 observational; 6 RCTs) regarding cannabinoids' impact as an adjunctive treatment in epileptic patients (mean age 16 years) suggested that pharmaceutical-grade CBD was more effective than placebo at reducing seizure frequency by 50%, achieving complete seizure freedom (RR 6.17, 95% CI 1.50 - 25.32), and improving quality of life (RR 1.73, 95% CI 1.33 - 2.26) compared to placebo. Adverse effects from pharmaceutical-grade CBD included drowsiness, fatigue, diarrhea, changes in appetite, and ataxia. These findings were specific to individuals with rare and serious forms of drug-resistant epilepsy; hence, the results cannot be generalized to adult/older population or to those with less severe epilepsy syndromesReference 761.
4.7 Pain
It is now well established that the ECS plays an important role in the modulation of nociceptive and pain states. Key in these roles is the specific positioning of the endocannabinoid signaling machinery at neuronal synapses in pain processing pathways at supraspinal, spinal, and peripheral levelsReference 24Reference 762-Reference 764.
Role of CB1 and CB2 receptors
The CB1 and CB2 receptors play important roles in nociception and pain. Structures involved in transmission and processing of nociceptive signals such as the nociceptors, the dorsal horn of the spinal cord, the thalamus, the periaqueductal grey matter, the amygdala and the rostroventromedial medulla show a moderate to high level of CB1 receptor expressionReference 765. In various animal models of chronic pain, both CB1 and CB2 receptor mRNA and protein levels in the CNS are upregulatedReference 765. Selective deletion of the CB1 receptor in mice appears to greatly attenuate the anti-nociceptive efficacy of cannabinoids in animal models of acute and chronic pain, suggesting an essential role for this receptor in modulating nociception and painReference 762Reference 766. At peripheral and central terminals of nociceptive sensory nerves, CB1 receptors gate the transduction of peripheral noxious stimuli into central neuronal pain signalsReference 762Reference 767, while in the spinal cord, CB1 receptors act to reduce or enhance propagation of pain signals to the brainReference 762Reference 768-Reference 770. At the neuronal circuit level, the end result of CB1 receptor activity can be either excitatory or inhibitory depending on the identity of the presynaptic cell and its location within the neural networkReference 762. In higher brain regions tasked with processing of nociceptive input such as the periaqueductal grey matter and the rostroventromedial medulla, the CB1 receptors can initiate descending inhibition or block descending facilitation to the spinal cord nociceptive circuitryReference 762Reference 771-Reference 776. Most importantly to the subject of pain, CB1 receptors are highly expressed in frontal-limbic pathways in the brain, which play a key role in the affective/emotional aspects of pain in humansReference 762Reference 772Reference 777. CB2 receptors appear to also play an important role in pain signaling, especially in the development of chronic pain states, by inhibiting the release of pro-inflammatory and pro-nociceptive mediators thereby attenuating the inflammatory and hyperalgesic responsesReference 762Reference 778. In this respect, the strategic localization of CB2 receptors on a variety of immune cells (macrophages, lymphocytes, and mast cells in the periphery), astrocytes and microglia in the CNS (i.e. the spinal cord) is essential to the roles of the CB2 receptors in modulating pain states.
Role of endocannabinoids, anandamide and 2-AG
Endocannabinoids such as anandamide and 2-AG have been shown to have analgesic or anti-nociceptive effects at peripheral, spinal, and central levels, mainly by virtue of their ability to stimulate the activity of the cannabinoid receptors, although other receptors (i.e. TRPV1) are also likely involvedReference 779. Peripheral inhibition of FAAH and MAGL enzymes (which hydrolyze anandamide and 2-AG respectively) and the resulting increase in the respective synaptic levels of anandamide and 2-AG has been shown to reduce nociception in animal models of acute and chronic painReference 762Reference 767Reference 780-Reference 791. Meanwhile, the arachidonoyl moiety of anandamide and 2-AG makes these endocannabinoids susceptible to metabolism by eicosanoid biosynthetic enzymes such as COXs, lipo-oxygenases (LOXs), and CYPs with the subsequent generation of known or potential pro -nociceptive prostamide endocannabinoid metabolitesReference 762Reference 792Reference 793. Therefore, the upregulation of COX-2 expression in chronic pain states may promote the additional production of these pro-nociceptive metabolites both peripherally and centrally thus contributing to nociception and painReference 765.
Considerations and caveats
Animal vs. human studies
Pre-clinical studies in animals predict that cannabinoids should relieve both acute and chronic pain in humans. However, results from both experimental models of pain in human volunteers and from clinical trials of patients suffering from pain instead suggest cannabinoids may be more effective for chronic rather than acute pain in humansReference 794-Reference 796. A number of possible explanations can exist to account for discrepancies in findings between animal studies and human clinical trials. Such explanations include interspecies differences, differences in experimental stimuli and protocols used in the studies, and differences in the outcomes measured in the studies. Data from animal pain models are mostly based on observations of behavioural changes, and cannabinoid doses sufficient to produce relevant anti-nociception in rodents are similar to those which cause other behavioural effects such as hypomotility and catatoniaReference 23Reference 797. This pharmacological overlap can make it difficult to distinguish between cannabinoid-associated anti-nociceptive effects and behavioural effectsReference 23Reference 797.
Experimental models of acute pain vs. chronic pain
Translation of research findings from human experimental models of pain (i.e. acute pain) to clinical (chronic) pain is also complex and not straightforwardReference 268. In contrast to acute pain, chronic pain is a complex condition that involves interaction between sensory, affective, and cognitive componentsReference 268. Furthermore, unlike acute pain, chronic pain is considered a disease and generally originates from prolonged acute pain that is not managed in a timely or effective mannerReference 798. Chronic pain also appears to involve distinct spatiotemporal neuronal mechanisms which differ from those recruited during acute, experimental painReference 799; chronic pain involves altered neural transmission and long-term plasticity changes in the peripheral and CNS which generate and maintain the chronic pain stateReference 798Reference 799. As such, it is difficult to compare studies of interventions for chronic pain with studies of experimentally-induced pain because of fundamental differences in the physiological state of the subjects, differences in the stimulus conditions and experimental protocols employed in the studies, and differences in the outcomes which are measuredReference 268.
Placebo effect
The placebo effect is another consideration to keep in mind when considering studies of cannabis/cannabinoids for the treatment of pain. The placebo effect, a psychobiological phenomenon, is perhaps more salient in disorders which have a more significant subjective or psychological component (e.g. pain, anxiety/depression), and may be somewhat less salient in diseases which have a more objective pathophysiological component (e.g. infectious diseases, cancer)Reference 800Reference 801. Of note, in one randomized, placebo-controlled clinical study of vapourized cannabis for painful diabetic neuropathy, the placebo effect was as high as 56% for euphoria and as high as 37.5% for somnolence out of a maximum 100% euphoria and 73.3% somnolence responses (observed with the highest THC dose condition at 7% THC)Reference 599. Emerging evidence also suggests an important role for the ECS in mediating placebo analgesiaReference 802-Reference 804. These findings highlight the complexities of studying the true analgesic potential of cannabinoids and underscore the importance of including a properly designed placebo control when studying the analgesic potential of cannabinoids.
Patient/study subject population
Many, if not most, of the clinical trials of cannabinoids for the treatment of pain (and even other disorders such as MS) have recruited patients or volunteers who have had prior exposure or experience with cannabis or cannabinoids. This has raised the issue of "unblinding" because any study subjects having prior experience with cannabis or cannabinoids would be more likely to be able to distinguish active treatment with these drugs from the placebo controlReference 612. Furthermore, a number of clinical trials of cannabis/cannabinoids for the treatment of pain (or other disorders) have also used an "open-phase" period which enriched for patients that responded favourably to the treatment and conversely, eliminated subjects who would have either responded poorly to cannabinoids or who would have had greater chances of experiencing adverse effectsReference 55. Therefore, the use of individuals with prior experience with cannabis or cannabinoids or the use of an "open-phase" period would increase the proportion of patients yielding results tending to overestimate some of the potential therapeutic benefits of cannabis/cannabinoids, while also tending to underestimate the extent or degree of adverse effects among the general patient populationReference 55Reference 612. There is also some evidence from pre-clinical and clinical studies that suggests sex-dependent effects on cannabinoid and cannabis-induced analgesia (see Section 2.5,Sex-dependent effects, for more information)Reference 563Reference 805-Reference 807.
Other considerations
It is also perhaps worth mentioning that a number of clinical studies suggest the presence of a relatively narrow therapeutic window for cannabis and prescription cannabinoids for the treatment of painReference 23Reference 55Reference 57Reference 797. The well-known psychotropic and somatic side-effects associated with the use of THC-enriched cannabis and cannabinoids (e.g. dronabinol, nabilone, nabiximols) are known to limit the general therapeutic utility of these drugs; it has therefore been suggested that it may be preferable to pursue therapies which focus on manipulation of the ECS (e.g. by inhibiting the endocannabinoid-degrading enzymes FAAH or MAGL), or to combine low doses of cannabinoids with low doses of other analgesics in order to achieve the desired therapeutic effects while minimizing the incidence, frequency, and severity of the adverse effectsReference 23Reference 57.
With the above considerations and caveats in mind, the sections below summarize the results of studies examining the analgesic potential of cannabis or cannabinoids in pre-clinical and clinical models of experimentally-induced acute pain, as well as in clinical studies of chronic pain.
4.7.1 Acute pain
- Pre-clinical studies suggest that certain cannabinoids can block the response to experimentally-induced acute pain in animal models.
- The results from clinical studies with smoked cannabis, oral THC, cannabis extract, and nabilone in experimentally-induced acute pain in healthy human volunteers are limited and mixed and suggest a dose-dependent effect in some cases, with lower doses of THC having an analgesic effect and higher doses having a hyperalgesic effect.
- Clinical studies of certain cannabinoids (nabilone, oral THC, levonontradol, AZD1940, GW842166) for post-operative pain suggest a lack of efficacy.
4.7.1.1 Experimentally-induced acute pain
Pre-clinical studies
Cannabinergic modulation of neuronal circuits in the brain and spinal cord can inhibit nociceptive processingReference 808-Reference 811 and a number of pre-clinical studies suggest that anandamide, THC, and certain synthetic cannabinoids block pain responses in different animal models of acute pain (reviewed inReference 23Reference 797).
Clinical studies with smoked cannabis
An early study by Hill of 26 healthy male cannabis smokers failed to demonstrate an analgesic effect of smoked cannabis (1.4% Δ9-THC, 12 mg Δ9-THC available in the cigarette) in response to transcutaneous electrical stimulationReference 812. The study did, however, report an increase in sensory and pain sensitivity to the applied stimulus. In contrast, Milstein showed that smoked cannabis (1.3% Δ9-THC, 7.5 mg Δ9-THC available in the cigarette) increased pain tolerance to a pressure stimulus in both healthy cannabis-naïve and cannabis-experienced subjects compared to placeboReference 813. Another study employing healthy cannabis smokers reported that smoking cannabis cigarettes (containing 3.55% Δ9-THC, or approximately 62 mg Δ9-THC available in the cigarette) was associated with a mild, dose-dependent, anti-nociceptive effect to a thermal heat stimulusReference 273. A more recent randomized, double-blind, placebo-controlled, crossover trial examined the effects of three different doses of smoked cannabis on intra-dermal capsaicin-induced pain and hyperalgesia in 15 healthy volunteersReference 268. Capsaicin was administered either 5 min or 45 min after smoking cannabis. Effects appeared to be dose- and time-dependent. No effect was observed 5 min after smoking, but analgesia was observed 45 min after smoking, and only with the medium dose of smoked cannabis (4% Δ9-THC); the low dose (2% Δ9-THC) had no effect whereas a high dose (8% Δ9-THC) was associated with significant hyperalgesia. This study identified a so-called "narrow therapeutic window"; a medium dose provided analgesic benefit, a high dose worsened the pain and was associated with additional adverse effects, and a low dose had no effect.
Clinical studies with oral THC and cannabis extract
A randomized, placebo-controlled, double-blind, crossover study of 12 healthy cannabis-naïve volunteers administered a single oral dose of 20 mg Δ9-THC reported a lack of a significant analgesic effect following exposure to a multi-model pain test battery (pressure, heat, cold, and transcutaneous electrical stimulation)Reference 272. In addition, significant hyperalgesia was observed in the heat pain test. Psychotropic and somatic side effects were common and included anxiety, perceptual changes, hallucinations, strange thoughts, ideas and mood, confusion and disorientation, euphoria, nausea, headache, and dizziness.
Another randomized, double-blind, active placebo-controlled, crossover study in 18 healthy female volunteers reported a lack of analgesia or anti-hyperalgesia with an oral cannabis extract containing 20 mg THC and 10 mg CBD (other plant cannabinoids were less than 5%) in two different experimental pain models (intra-dermal capsaicin or sunburn)Reference 267. Side effects (sedation, nausea, and dizziness) were frequently observed. Hyperalgesia was also observed at the highest dose as in the study conducted by Wallace (above)Reference 268.
Clinical studies with nabilone
A randomized, double-blind, placebo-controlled, crossover study of single oral doses of nabilone (0.5 mg or 1 mg) failed to show any analgesic effects during a tonic heat pain stimulusReference 814. However, an anti-hyperalgesic effect was observed at the highest administered dose, but only in female subjects. The authors noted a significant placebo effect and also suggested that the lack of analgesia could have been attributed to the single-dose administration of the cannabinoid; a gradual dose escalation could have potentially revealed an effect.
Similarly, a randomized, double-blind, placebo-controlled, crossover study in subjects receiving single oral doses of nabilone (1, 2, or 3 mg) failed to show any analgesic, or primary or secondary anti-hyperalgesic effects in response to capsaicin-induced pain in healthy male volunteersReference 600. Adverse effects of mild to moderate intensity were noted in the majority of subjects. Severe adverse reactions (e.g. dizziness, sedation, anxiety, agitation, euphoria, and perceptual and cognitive disturbances) were reported only at the highest administered dose (3 mg) in four subjects leading to their withdrawal from the study. Dose-dependent CNS effects were observed 1.5 to 6 h after dosing, reaching a maximum between 4 and 6 h after administration.
4.7.1.2 Post-operative pain
Despite the introduction of new standards, guidelines, and educational efforts, data indicate that post-operative pain continues to be under or poorly managed and many of the drugs commonly used in this setting either lack sufficient efficacy or cause unacceptable side effectsReference 270Reference 815. To date, there are eight published reports and a systematic review on the use of cannabinoids in post-operative painReference 269-Reference 271Reference 274Reference 816Reference 269-Reference 271Reference 274Reference 796Reference 816-Reference 819. The conclusions from the systematic review was that the studied cannabinoids (THC, nabilone, or an oral cannabis extract containing a 2: 1 ratio of THC to CBD, levonontradol, AZD1940, GW842166) were not ideally suited for the management of acute post-operative pain because they were either only moderately effectiveReference 270Reference 274, less effective than placeboReference 817, not different from placeboReference 271Reference 796Reference 816Reference 818Reference 271Reference 796, or even anti-analgesic at high dosesReference 269.
4.7.2 Chronic pain
Acute pain that is poorly managed can lead to chronic painReference 820Reference 821. In contrast to acute pain, chronic pain is typically considered a far more complex condition which involves physical, psychological, and psychosocial factors, and which contributes to a reduced QoLReference 822. The International Association for the Study of Pain defines pain as chronic if it persists beyond the normal tissue healing time of three to six monthsReference 823. Furthermore, chronic pain is associated with an abnormal state of responsiveness or increased gain of the nociceptive pathways in the CNS (referred to as "central sensitization"), as well as with alterations in cognitive functioningReference 823. The information below summarizes pre-clinical studies carried out in animal models of chronic pain, and clinical studies in human subjects administered an experimental stimulus mimicking chronic pain or in patients suffering from chronic pain of various etiologies.
4.7.2.1 Experimentally-induced inflammatory and chronic neuropathic pain
- Endocannabinoids, THC, CBD, nabilone and certain synthetic cannabinoids have all been identified as having an anti-nociceptive effect in animal models of chronic pain (inflammatory and neuropathic).
The anti-nociceptive efficacy of cannabinoids has been unequivocally demonstrated in several different animal models of inflammatory and neuropathic pain (reviewed inReference 765Reference 779Reference 824Reference 825}}). In addition, the findings from these studies suggest that modulation of the ECS through administration of specific cannabinoid receptor agonists, or by elevation of endocannabinoid levels, suppresses hyperalgesia and allodynia induced by diverse neuropathic states (reviewed inReference 765Reference 779Reference 825). As such, similar to the situation with acute pain, pre-clinical studies of chronic pain in animal models suggest that endocannabinoids (anandamide and 2-AG), THC, and several synthetic cannabinoids have beneficial effects in this pain state (reviewed inReference 23Reference 797Reference 825).
With respect to CBD, chronic oral administration of CBD effectively decreased hyperalgesia in a rat model of inflammatory painReference 826. One study suggested that a medium or a high dose of CBD attenuated tactile allodynia and thermal hypersensitivity in a mouse model of diabetic neuropathy, when administered early in the course of the disease; on the other hand, there was little, if any, restorative effect if CBD was administered at a later time pointReference 827. In contrast, the same study showed that nabilone was not as efficacious as CBD if administered early on, but appeared to have a small beneficial effect when administered later in the course of the disease. CBD also appeared to attenuate microgliosis in the ventral lumbar spinal cord, but only if administered early in the course of the disease, whereas nabilone had no effect. Xiong et al. (2012) reported that systemic and intrathecal administration of CBD potentiated glycine currents, through α3 glycine receptors, in dorsal horn neurons in rat spinal cord slices and also attenuated chronic inflammatory and neuropathic pain in vivoReference 828.
4.7.2.2 Neuropathic pain and chronic non-cancer pain in humans
- A few studies that have used experimental methods having predictive validity for pharmacotherapies used to alleviate chronic pain, have reported an analgesic effect of smoked cannabis.
- Furthermore, there is more consistent evidence of the efficacy of cannabinoids (smoked/vapourized cannabis, nabiximols, dronabinol) in treating chronic pain of various etiologies, especially in cases where conventional treatments have been tried and have failed.
Clinical studies with cannabinoids
A systematic review and meta-analysis of 28 RCTs (N = 2 454 participants) for chronic pain (including smoked cannabis, nabiximols, dronabinol) reported that there was moderate quality evidence of efficacy to support the use of cannabinoids to treat chronic pain of various etiologies mostly reducing central or peripheral neuropathic pain in individuals already receiving analgesic drugsReference 179. The working definition of chronic pain included neuropathic (central/peripheral), cancer pain, diabetic peripheral neuropathy, fibromyalgia, HIV-associated sensory neuropathy, refractory pain due to MS or other neurological condition, rheumatoid arthritis (RA), non-cancer pain (nociceptive/neuropathic), central pain, musculoskeletal pain and chemotherapy-induced pain. The average number of patients who reported a reduction in pain of at least 30% was greater with cannabinoids vs. placebo (OR = 1.41), although for smoked cannabis the effect was greater (OR = 3.43). Side effects appeared to be comparable to existing treatments and included dizziness/lightheadedness, nausea, fatigue, somnolence, euphoria, vomiting, disorientation, drowsiness, confusion, loss of balance, hallucinations, sedation, ataxia, a feeling of intoxication, xerostomia, dysgeusia, and hungerReference 172Reference 176Reference 829Reference 830. However, these adverse effects may be minimized by employing low doses of cannabinoids that are gradually escalated, as required.
The following summarizes the existing clinical information on the use of smoked/vapourized cannabis and cannabinoids (THC, nabilone, dronabinol and nabiximols) to treat neuropathic and chronic non-cancer pain.
Clinical studies with smoked or vapourized cannabis
A within-subject, randomized, placebo-controlled, double-dummy, double-blind clinical study compared the acute therapeutic analgesic potential of two potencies of smoked cannabis (1.98% and 3.56% THC, 800 mg cigarettes with 16 mg and 28 mg THC respectively) to two doses of dronabinol (10 and 20 mg) in response to an experimental pain stimulus (i.e. cold pressor test) that has predictive validity for pharmacotherapies used to treat chronic painReference 831. The study found that both cannabis and dronabinol produced analgesic effects in this model and there were also no significant differences between dronabinol and smoked cannabis in measures of pain sensitivity (i.e. latency to first feel pain). However, in terms of pain tolerance, low potency smoked cannabis (1.98% THC) and both low and high dronabinol doses increased the latency to report pain relative to placebo. Both strengths of cannabis and the high dronabinol dose (20 mg) decreased subjective ratings of pain intensity and bothersomeness of the cold-pressor test compared to placebo although these decreases were greater after cannabis relative to dronabinol. Both cannabis strengths and the high dronabinol dose increased subjective ratings of "high" and "good drug effect" relative to placebo, and both cannabis strengths (but not the low dronabinol dose) increased ratings of "stimulated" relative to placebo. Lastly, both strengths of cannabis and the high dronabinol dose increased ratings of "marijuana strength", "liking", and "willingness to take again". There did not appear to be any sex-dependent differences in terms of baseline pain measures, analgesic, subjective, or physiological effects across all cannabis or dronabinol conditions. Overall, dronabinol decreased pain sensitivity and increased pain tolerance and these effects peaked later and lasted longer compared to smoked cannabis, while smoked cannabis produced a greater attenuation of subjective ratings of pain intensity compared to dronabinol. Peak subjective ratings of dronabinol's drug effects occurred significantly earlier than decreases in pain sensitivity and increases in pain tolerance (60 min vs. 4 h). Limitations of this study include a potentially biased study population that consisted of daily cannabis users as well as the experimental nature of the pain stimulus in subjects not normally experiencing pain.
A retrospective analysis that compared the analgesic, subjective, and physiological effects of smoked cannabis (3.56 or 5.60% THC, 800 mg cigarettes with 28 mg and 45 mg THC respectively) in 21 men and 21 women under double-blind, placebo-controlled conditions showed that among men, cannabis significantly decreased pain sensitivity in the cold pressor test compared to placebo, while in women active cannabis failed to decrease pain sensitivity relative to placeboReference 807. Active cannabis increased pain tolerance in both men and women immediately after smoking as well as increased subjective ratings associated with abuse liability ("take again", "liking", "good drug effect"), drug strength, and "high" relative to placebo. Ratings of "high" varied as a function of sex, with men exhibiting elevated ratings throughout the session relative to women. Men also exhibited greater increases in heart rate after smoking cannabis compared to women. Study subjects smoked cannabis daily or near-daily, and smoked on average 7 to 10 cannabis cigarettes/day.
In a randomized, placebo-controlled study, a greater than 30% decrease in HIV-associated sensory neuropathic pain was reported in 52% of cannabis-experienced patients smoking cannabis cigarettes containing 3.56% Δ9-THC (32 mg total available Δ9-THC per cigarette), three times per day (96 mg total daily amount of Δ9-THC) for five days, compared to a 24% decrease in pain in the placebo groupReference 195. The NNT to observe a 30% reduction in pain compared to controls was 3.6 and was comparable to that reported for other analgesics in the treatment of chronic neuropathic pain. In the "experimentally-induced pain" portion of the study, smoked cannabis was not associated with a statistically significant difference in acute heat pain threshold compared to placebo. However, it did appear to reduce the area of heat and capsaicin-induced acute secondary hyperalgesia. Patients were taking other pain control medications during the trial such as opioids, gabapentin or other drugs. Adverse effects of smoked cannabis in this study included sedation, dizziness, confusion, anxiety, and disorientation.
In another randomized, double-blind, placebo-controlled, cross-over study of cannabis-experienced patients suffering from chronic neuropathic pain of various etiologies (complex regional pain syndrome (CRPS), central neuropathic pain from SCI or MS, or peripheral neuropathic pain from diabetes or nerve injury) reported that administration of either a low dose or a high dose of smoked cannabis (3.5% Δ9-THC, 19 mg total available Δ9-THC; or 7% Δ9-THC, 34 mg total available Δ9-THC) was associated with significant equianalgesic decreases in central and peripheral neuropathic painReference 222. No analgesic effect was observed in tests of experimentally-induced pain (tactile or heat stimuli) in these participants. Patients were taking other pain control medications during the trial such as opioids, anti-depressants, NSAIDs, or anti-convulsants. Adverse effects associated with the use of cannabis appeared to be dose-dependent and included feeling "high", sedation, confusion, and neurocognitive impairment. Cognitive changes appeared to be more pronounced with higher doses of Δ9-THC.
A phase II, double-blind, placebo-controlled, crossover clinical trial of smoked cannabis for HIV-associated refractory neuropathic pain reported a 30% decrease in HIV-associated, distal sensory predominant, polyneuropathic pain in 46% of patients smoking cannabis for five days (1 - 8% Δ9-THC, four times daily), compared to a decrease of 18% in the placebo groupReference 281. The NNT in this study was 3.5. Almost all of the subjects had prior experience with cannabis and were concomitantly taking other analgesics such as opioids, NSAIDs, anti-depressants or anti-convulsants. Adverse effects associated with the use of cannabis were reported to be frequent, with a trend for moderate or severe adverse effects during the active treatment phase compared to the placebo phase.
A randomized, double-blind, placebo-controlled, four-period, crossover clinical study of smoked cannabis for chronic neuropathic pain caused by trauma or surgery and refractory to conventional therapies reported that compared to placebo, a single smoked inhalation of 25 mg of cannabis containing 9.4% Δ9-THC (2.35 mg total available Δ9-THC per cigarette), three times per day (7.05 mg total Δ9-THC per day) for five days, was associated with a modest but statistically significant decrease in average daily pain intensityReference 59. In addition, there were statistically significant improvements in measures of sleep quality and anxiety with cannabis. The majority of subjects had previous experience with cannabis and most were concomitantly taking other analgesics such as opioids, anti-depressants, anti-convulsants, or NSAIDs. Adverse effects associated with the use of cannabis included headache, dry eyes, burning sensation in the upper airways (throat), dizziness, numbness, and cough.
A clinical study examined the effects of vapourized cannabis on the pharmacokinetics, subjective effects, pain ratings and safety of orally-administered opioids in patients suffering from chronic pain (musculoskeletal, post-traumatic, arthritic, peripheral neuropathy, cancer, fibromyalgia, MS, sickle cell disease, and thoracic outlet syndrome)Reference 280. The study reported that inhalation of vapourized cannabis (900 mg, 3.56% Δ9-THC), three times per day for five days, was associated with a statistically significant decrease in pain (-27%, CI = 9 - 46). Subjects were on stable doses of sustained-release morphine sulfate or oxycodone, and had prior experience with smoking cannabis. There was a statistically significant decrease in the Cmax of morphine sulfate, but not oxycodone, during cannabis exposure. No clinically significant adverse effects were noted, but all subjects reported experiencing a "high". The study design carried a number of limitations including small sample size, short duration, a non-randomized subject population, and the lack of a placebo.
A double-blind, placebo-controlled, crossover study of patients suffering from neuropathic pain of various etiologies (SCI, CRPS type I, causalgia-CRPS type II, diabetic neuropathy, MS, post-herpetic neuralgia, idiopathic peripheral neuropathy, brachial plexopathy, lumbosacral radiculopathy, and post-stroke neuropathy) reported that inhalation of vapourized cannabis (800 mg containing either a low dose of Δ9-THC (1.29% Δ9-THC; total available amount of Δ9-THC 10.3 mg) or a medium dose of Δ9-THC (3.53% Δ9-THC; total available amount of Δ9-THC 28.2 mg)) during three separate 6 h sessions was associated with a statistically significant reduction in pain intensityReference 598. Inhalation proceeded using a standardized protocol (i.e. the "Foltin procedure"): participants were verbally signaled to hold the vapourizer bag with one hand, put the vapourizer mouthpiece in their mouth, get ready, inhale (5 s), hold vapour in their lungs (10s), and finally exhale and wait before repeating the inhalation cycle (40s). Non-significant differences were observed between placebo and active treatments with respect to pain ratings at the 60 min time point following study session initiation. Following four cued inhalations of either dose of THC at the 60 min time point, a significant treatment effect was recorded 60 min later (i.e. at the 120 min time point following trial initiation). A second cued inhalation of vapourized cannabis, at the 180 min time point following trial initiation (four to eight puffs, flexible dosing, 2 h after first inhalation), was associated with continued analgesia lasting another 2 h. Both the 1.29% and 3.53% Δ9-THC doses were equianalgesic and significantly better in achieving analgesia than placebo. The NNT to achieve a 30% pain reduction was 3.2 for the low-dose vs. placebo, 2.9 for the medium-dose vs. placebo, and 25 for the medium- vs. the low-dose. The authors suggested that the NNT for active vs. placebo conditions is in the range of two commonly used anti-convulsants used to treat neuropathic pain (pregabalin, 3.9; gabapentin, 3.8). Using a Global Impression of Change rating scale, pain relief appeared to be maximal after the second dosing at 180 min, and dropped off between 1 and 2 h later. Both active doses had equal effects on ratings of pain "sharpness", while the low-dose was more effective than either the placebo or medium-dose for pain described as "burning" or "aching". All patients had prior experience with cannabis and were concomitantly taking other medications (opioids, anti-convulsants, anti-depressants, and NSAIDs). Cannabis treatment was associated with a small impairment of certain cognitive functions, with the greatest effects seen in domains of learning and memory. The study suffered from a number of drawbacks including a relatively small number of patients, a short study period, and the possibility of treatment unblinding.
A review of the use of smoked cannabis for the treatment of neuropathic pain suggested that the efficacy of smoked cannabis (NNT = 3.6, for a 30% reduction in pain) was comparable to that of traditional therapeutic agents (e.g. gabapentin, NNT = 3.8), slightly less than that observed with tricyclic antidepressants (NNT = 2.2), but better than lamotrigine (NNT = 5.4) and selective serotonin reuptake inhibitors (NNT = 6.7)Reference 832. The author reports that the concentrations of THC in the smoked cannabis ranged between 2 and 9% with an average concentration of 4% yielding good efficacy. Furthermore, the author suggests that cannabis may present a reasonable alternative or adjunctive treatment for patients with severe, refractory peripheral neuropathy who have tried other therapeutic avenues without satisfactory results. This review, along with another more recent reviewReference 275 provide a useful clinical algorithm for determining if a patient would be a candidate for treatment with cannabis for peripheral neuropathic pain (see Figure 3).
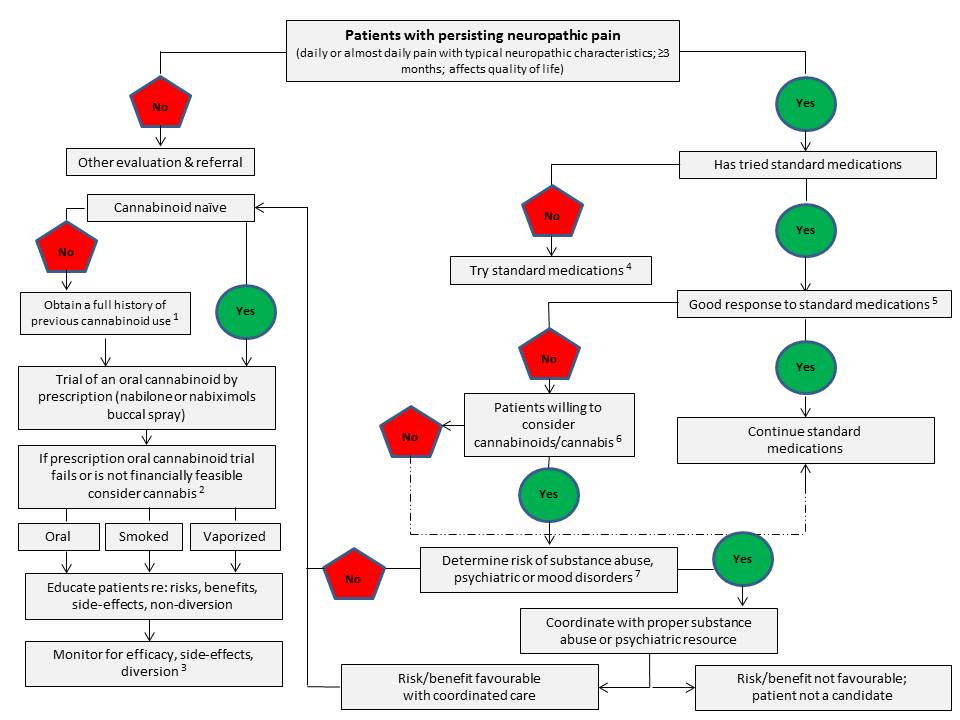
Figure 3 - Text description
Determine if the patient has persisting neuropathic pain. Screening criteria for persisting neuropathic pain include daily or almost daily pain with typical neuropathic characteristics; a duration of at least three months; and an impact on the patient's quality of life. If the patient does not meet the screening criteria for persisting neuropathic pain, conduct another evaluation and make a referral to another healthcare professional. If the patient does meet the screening criteria for persisting neuropathic pain, determine if the patient has tried standard medications. If no, try standard medications. (Note a: Standard medications include antidepressants, anticonvulsants, opioids, nonsteroidal anti-inflammatory drugs). If yes, determine if they have/had a good response to standard medications. (Note b: At least 30% reduction in pain intensity). If yes, continue standard medications. If no, determine if the patient is willing to consider cannabinoids or cannabis. (Note c: Consider past experience with cannabis or cannabinoids, potential for side effects or history of side effects, willingness to smoke/vaporize/ingest orally). If no, consider continuing standard medications. If yes, determine if there is a risk of substance abuse and psychiatric or mood disorders. (Note d: Determine substance abuse history; history of psychiatric or mood disorders. If yes or at high risk for substance abuse, proceed with caution and close observation (see Sections 2.4, 5.0, and 6.0); coordinate with substance abuse treatment programs. If there is a history or risk of psychiatric disease (schizophrenia) or bipolar disorder see Section 7.7.3 and consult with a psychiatric specialist before proceeding). If yes, coordinate with the proper substance abuse or psychiatric resource to determine if the risk/benefit is favourable with coordinated care. If it is not favourable, the patient is not a candidate for cannabinoids or cannabis. If it is favourable or if there is no risk of substance abuse and psychiatric or mood disorders, determine if the patient is cannabis or cannabinoid naïve. If no, obtain a full history of previous cannabinoid use. (Note e: Specific cannabinoid, dose, route of administration; symptoms treated and outcome; adverse effects). If the patient is cannabis or cannabinoid naïve or once the full history of previous cannabinoid use from a non-naïve patient has been obtained, try an oral cannabinoid by prescription, such as nabilone or nabiximols buccal spray. If the trial with the prescription oral cannabinoid fails or if it is not financially feasible, consider cannabis. (Note f: Discuss the fact that there are not yet clear guidelines regarding efficacy, doses and toxicity; raise awareness of oral and vaporized routes of cannabis administration; refer patient to Health Canada website and documents regarding access to cannabis product(s); follow the usual clinical guideline to start low and titrate dose slowly). Cannabis can be administered orally, by smoking or by vaporization. It is important to educate the patient regarding the risks, benefits, side effects and non-diversion of cannabis. Finally, monitor the patient to determine efficacy, side effects and diversion of cannabis and adjust treatment accordingly. (Note g: Efficacy should aim for at least 30% decrease in pain intensity).
Legend:
a Standard medications include antidepressants, anticonvulsants, opioids, nonsteroidal anti-inflammatory drugs.
b At least 30% reduction in pain intensity.
c Consider past experience with cannabis or cannabinoids, potential for side effects or history of side effects, willingness to smoke/vapourize/ingest orally.
d Determine substance abuse history; history of psychiatric or mood disorders. If yes or at high risk for substance abuse, proceed with caution and close observation (see Sections 2.4, 5.0, and 6.0); coordinate with substance abuse treatment programs. If there is a history or risk of psychiatric disease (schizophrenia) or bipolar disorder see Section 7.7.3 and consult with a psychiatric specialist before proceeding.
e Specific cannabinoid, dose, route of administration; symptoms treated and outcome; adverse effects.
f Discuss the fact that there are not yet clear guidelines regarding efficacy, doses and toxicity; raise awareness of oral and vapourized routes of cannabis administration; refer patient to Health Canada website and documents regarding access to cannabis product(s); follow the usual clinical guideline to start low and titrate dose slowly.
g Efficacy should aim for at least 30% decrease in pain intensity.
A single-dose, open-label, clinical trial of patients with neuropathic pain and using very low doses of THC (from vapourized cannabis) reported a statistically significant improvement in neuropathic pain with minimal adverse effectsReference 58. In this clinical study, 10 patients suffering from neuropathic pain of any type (SCI, CRPS, lumbosacral radiculopathy, pelvic neuropathic pain) of at least three months duration and on a stable analgesic regimen for at least 60 days (e.g. opioids, antidepressants, anticonvulsants, benzodiazepines, steroids, NSAIDs, cannabis) were administered a vapourized dose of 3 mg of THC (available in the device; ~ 1.5 mg THC actually delivered) resulting from vapourization of 15 mg of dried cannabis containing 20% THC. THC administration was associated with a statistically significant reduction in baseline VAS pain intensity of 3.4 points (i.e. a 45% reduction in pain) within 20 min of inhalation with a return to baseline within 90 min. Adverse effects were minimal but included lightheadedness for 10 min after inhalation which lasted approximately 30 min and then fully resolved. Subjects reported using between 2 and 40 g of cannabis per month (i.e. 0.067 g per day and 1.3 g per day). THC was detected in blood within 1 min following inhalation and reached a maximum within 3 min at a mean THC concentration of 38 ng/ml.
A Canadian multi-centre, prospective, cohort safety study of patients using cannabis as part of their pain management regimen for chronic non-cancer pain reported that cannabis use was not associated with an increase in the frequency of serious adverse events vs. controls, but was associated with an increase in the frequency of non-serious adverse eventsReference 216. In this study, 216 patients with chronic non-cancer pain (nociceptive, neuropathic, or both) using cannabis and 215 control patients with chronic pain with no cannabis use were followed for a period of one year and evaluated for frequency and type of adverse effects associated with the use of a standardized herbal cannabis product (CanniMed 12.5% THC, <0.5% CBD). A significant proportion of study subjects were taking opioids, anti-depressants or anti-convulsants. Almost one third of study subjects who reported smoking cannabis at least once reported consuming it exclusively by smoking, 44% reported smoking and oral ingestion, 14% reported vapourizing, smoking or ingesting cannabis orally, and slightly less than 4% reported only smoking or vapourizing. Secondary objectives were to examine the effects of cannabis use on pulmonary and neurocognitive function and to explore the effectiveness of cannabis for chronic non-cancer pain, including pain intensity and QoL. For the primary outcome, the total number of serious adverse events was similar between the cannabis group and the control group and none of the serious adverse events were considered to be either "certainly" or "very likely" related to the cannabis provided by the investigators. One serious adverse event (convulsion) was considered to be "probably/likely" related to the study cannabis. Patients in the cannabis-treatment group experienced a median of three events per subject (vs. a median of two events per subject among controls). The incidence rate of adverse events in the cannabis treatment group was 4.61 events/person-year and was significantly higher than in the control group where the incidence rate was 2.85 events/person-year. The most common adverse event categories in the cannabis-treatment group were nervous system (20%), GI (13.4%), and respiratory disorders (12.6%) and the rate of nervous system disorders, respiratory disorders, infections, and psychiatric disorders was significantly higher in the cannabis group than in the control group. Furthermore, mild (51%) and moderate (48%) events were more common than severe ones (10%) in the cannabis-treatment group. Somnolence (0.6%), amnesia (0.5%), cough (0.5%), nausea (0.5%), dizziness (0.4%), euphoric mood (0.4%), hyperhidrosis (0.2%), and paranoia (0.2%) were assessed as being "certainly/very likely" related to treatment with cannabis. Increasing the daily dose of cannabis was not associated with a higher risk of serious or non-serious adverse events, although the recommended maximum daily amount of cannabis was set at 5 g per day (the median daily cannabis dose was 2.5 grams per day). With respect to secondary outcomes, no difference in neurocognitive function was found between cannabis users and controls, after one year of treatment and after controlling for multiple potential confounders. No significant changes were noted in certain pulmonary function tests (Slow Vital Capacity, Functional Residual Capacity, Total Lung Capacity) over the course of the study period, although reductions were noted in residual volume, forced expiratory volume in one second (FEV1) and in the FEV1/forced vital capacity (FVC) ratio (0.78% decrease). No changes were observed in liver, renal or endocrine functions. In terms of efficacy for pain, compared to baseline, there was a significant reduction in average pain intensity in the cannabis-treatment group but not in the control (difference = 1.10). Notably, patients using cannabis had higher baseline pain and disability than controls. While there was a significant improvement from baseline pain intensity in both the control and cannabis-treatment groups, greater improvement of physical function was observed in the cannabis group vs. control. Lastly, the sensory component of pain and total symptom distress score (Edmonton Symptom Assessment System) as well as the total mood disturbance scale of the Profile of Mood States all showed improvement in the cannabis group vs. control. Limitations of the study included relatively small sample size and short follow-up time which prevented the identification of rare serious adverse events, a significant drop-out rate attributable to adverse events (especially among cannabis naïve and former users), perceived lack of efficacy, and/or dislike of the study product. The majority (66%) of individuals in the cannabis group was composed of experienced cannabis users and the authors of the study suggest that a higher rate of adverse events for cannabis may have been observed if only new cannabis users had been included. Therefore, the study findings regarding safety of cannabis use for chronic non-cancer pain cannot be generalized to patients who are cannabis naïve. Lastly, the study was not a RCT and allocation was not blinded, therefore improvements in secondary efficacy measures should be interpreted with caution.
A meta-analysis of randomized, double-blind, placebo-controlled trials of smoked/vapourized cannabis for neuropathic pain reported that inhaled cannabis resulted in short-term reductions in chronic neuropathic pain for one in every five to six patients treated (NNT = 5.6)Reference 833. Furthermore, the study results suggested that inhaled cannabis may be as potent as gabapentin (NNT = 5.9). In this study, one hundred and seventy-eight middle-aged participants with painful neuropathy of at least three months' duration were enrolled in the five North American RCTs examined - two RCTs recruited only HIV+ individuals with HIV-related chronic painful neuropathy, while the remaining three RCTs recruited patients with neuropathy secondary to trauma, SCI, diabetes mellitus and CRPS. No studies investigated outcomes beyond two weeks. Therapeutic effects appeared to increase with increasing THC content. Study withdrawals due to adverse effects were rare. Subjective side effects included mild anxiety, disorientation, difficulty concentrating, headache, dry eyes, burning sensation, dizziness, and numbness. Psychoactive effects (e.g. "feeling high") increased in frequency with increasing dose. Limitations of this study are mainly reflective of the limitations associated with the original studies (i.e. small number of available studies, small number of participants, shortcomings in allocation concealment, and attrition). The meta-analysis could not draw any conclusions regarding the long-term efficacy or safety of inhaled cannabis for chronic neuropathic pain, as the original studies did not extend past a maximum two-week period.
A randomized, double-blind, placebo-controlled, single-dose, cross over clinical trial of low, medium and high-dose vapourized cannabis in 16 patients with painful diabetic peripheral neuropathy measuring short-term efficacy and tolerability reported a statistically significant difference in spontaneous pain scores between doses and a statistically significant negative effect of the high dose on some neuropsychological measuresReference 599. Study participants had diabetes mellitus type I or II and had at least a six-month history of painful diabetic peripheral neuropathy. Subjects participated in four sessions, separated by two weeks and were exposed to placebo, low (1% THC, <1% CBD, 400 mg total plant material), medium (4% THC, <1% CBD, 400 mg total plant material) and high (7% THC, <1% CBD, 400 mg total plant material) doses of THC; actual doses of THC available for inhalation were estimated at 0, 4, 16, or 28 mg THC per dosing session. Baseline measurements of spontaneous pain, evoked pain and cognitive testing were performed. There was a reported statistically significant difference in spontaneous pain scores between doses, with the average pain intensity scores with the low, medium and high doses being significantly different from the placebo, and the average pain score with the high dose being significantly different from the average pain score in the medium, low dose and placebo; no statistically significant difference in average pain intensity was noted between the medium and low dose. There was a statistically significant reduction in mean evoked pain scores between the placebo and high dose, between the low and high dose, and between the medium and high dose of cannabis. On average, the lowest minimum pain score was achieved with the high dose (7% THC), and the highest minimum pain score was seen with the placebo dose. While results showed a statistically significant reduction in both spontaneous and evoked pain between doses, comparison of the proportions of participants who achieved at least 30% reduction in spontaneous and evoked pain scores was not statistically significant between the different doses. Performance on selected neurocognitive tests (attention/working memory) showed statistically significant differences between doses, with some impairments lasting up to 120 min post-administration. There was a dose-dependent effect in subjective "highness" score that dissipated after 4 h. Furthermore, the study findings suggested a correlation between subjective "highness" score and spontaneous pain score, with every 1-point increase in "highness" score associated with a pain score decrease of 0.32 points. Euphoria was noted in 100% of individuals at the highest dose (7% THC), and there was a statistically significant difference in euphoria between the high dose and placebo and the medium dose and placebo. Somnolence was noted in 73% of individuals at the high dose and was only statistically significant for the high dose vs. placebo. Interestingly, 56% of individuals reported euphoria with the placebo dose, suggesting a high expectancy rate. Limitations of the study included small sample size, underpowering, brief duration, limited neuropsychiatric testing, and potential unblinding.
A systematic review of RCTs examining cannabinoids (nabilone, oral mucosal cannabis spray, oral cannabis extract, smoked or vapourized cannabis, and FAAH inhibitors) in the treatment of chronic non-cancer pain was conducted according to Preferred Reporting Items for Sytematic Reviews and Meta-Analyses (PRISMA) guidelines on health care outcomes and showed that the majority of the trials demonstrated a significant analgesic effect as well as improvements in secondary outcomes (e.g. sleep, muscle stiffness, spasticity)Reference 176. Frequent adverse effects, likely caused by cannabis, included drowsiness, fatigue, dizziness, dry mouth, nausea and cognitive effects that were generally mild to moderate in severity and generally well tolerated. Serious adverse effects included urinary tract infection, head injury, and interstitial lung disease (oral cannabis extract), delirium (nabilone), and suicidal ideation and disorientation (oral mucosal cannabis spray). Limitations of the findings relate mainly to the short duration and small sample sizes of the included trials and the modest effect sizes. RCTs of longer duration and with a larger sample size are needed to confirm efficacy signals reported by the smaller "proof of concept" studies, and for longer term monitoring of patients to assess long-term safety.
Another systematic review of six RCTs (N = 226 patients) of smoked or vapourized cannabis for chronic non-cancer pain reported evidence for the use of low-dose cannabis in refractory neuropathic pain in conjunction with traditional analgesicsReference 172. Five out of the six included RCTs were considered high quality (using the Jadad scale). Two-hundred and twenty-six adults (mean age 45 to 50) with chronic neuropathic pain (HIV-associated neuropathy, post-traumatic neuropathy, mixed neuropathy) were included in the analysis. All included trials excluded patients with a history of psychotic disorders, previous history of cannabis abuse or dependence. Four of the five trials that allowed patients to continue using opioids, anticonvulsants, and anti-depressants reported that more than 50% of subjects used concomitant opioids. Dose of THC ranged from about 1% to 9.4% (by dry weight) with the total daily THC amount delivered ranging from 1.9 mg/day to a maximum of 34 mg/day. The two trials open to cannabis-naïve subjects reported dropouts or withdrawals associated with potential adverse effects of smoked cannabis (e.g. psychosis, persistent cough, feeling "high", dizziness, fatigue) with the remaining reasons for dropouts unrelated to adverse effects. All studies reported a statistically significant analgesic effect. Clinically meaningful analgesic effect (> 30% improvement in pain relief) was reported in only three of the included studies. Adverse effects included mainly neurologic or psychiatric events (e.g. headache, sedation, euphoria, dysphoria, poor concentration, attention and memory) and the incidence of these adverse effects appeared to increase in frequency with increasing dose of THC. The authors conclude that the short-term adverse cognitive effects reported in the included RCTs were similar to those experienced with opioids and suggest the same precautions used with opioids should be applied to cannabis. The authors suggest that low-dose THC (< 34 mg THC/day) is associated with an improvement in refractory neuropathic pain of moderate severity in adults using concurrent analgesics. Generalizability of the results in chronic non-cancer pain is limited by quality of the studies, small sample sizes, short duration, and dose and dose scheduling variability.
Clinical studies with orally administered prescription cannabinoids
Nabilone
An off-label, retrospective, descriptive study of 20 adult patients suffering from chronic non-cancer pain of various etiologies (post-operative or traumatic pain, reflex sympathetic dystrophy, arthritis, Crohn's disease, neuropathic pain, interstitial cystitis, HIV-associated myopathy, post-polio syndrome, idiopathic inguinal pain, and chronic headaches) reported subjective overall improvement and reduced pain intensity with nabilone as an adjunctive pain-relief therapyReference 822. Furthermore, beneficial effects on sleep and nausea were the main reasons for continuing use. Patients used between 1 and 2 mg of nabilone per day. Higher doses (3 - 4 mg/day) were associated with an increased incidence of adverse effects. These included dry mouth, headaches, nausea and vomiting, fatigue, cognitive impairment, dizziness, and drowsiness. Many patients were concomitantly taking other drugs such as NSAIDs, opioids, and various types of anti-depressants. Many of the subjects also reported having used cannabis in the past to manage symptoms. Limitations in study design included the lack of an appropriate control group and the small number of patients.
An enriched-enrolment, randomized-withdrawal, flexible-dose, double-blind, placebo-controlled, parallel-assignment efficacy study of nabilone as an adjuvant in the treatment of diabetic peripheral neuropathic pain reported a statistically significant decrease in pain compared to placebo, with 85% of the subjects in the nabilone group reporting a ≥ 30% reduction in pain from baseline to end point, and 31% of subjects in the nabilone group reporting a ≥ 50% reduction in pain from baseline to end pointReference 612. Subjects taking nabilone also reported statistically significant improvements in anxiety, sleep, QoL, and overall patient status. Doses of nabilone ranged from 1 to 4 mg/day. Most subjects were concomitantly taking a variety of pain medications including NSAIDs, opioids, anti-depressants, and anxiolytics. Adverse events associated with the nabilone intervention included dizziness, dry mouth, drowsiness, confusion, impaired memory, lethargy, euphoria, headache, and increased appetite although weight gain was not observed.
Dronabinol
A randomized, double-blind, placebo-controlled, crossover trial of patients suffering from MS-associated central neuropathic pain reported a decrease in central pain with 10 mg maximum daily doses of dronabinolReference 610. Dosing started with 2.5 mg dronabinol/day and employed gradual dose-escalation every other day; total trial duration was three weeks (range: 18 - 21 days). Pain medications, other than paracetamol, were not permitted during the trial. The NNT for 50% pain reduction was 3.5 (95% CI = 1.9 to 24.8). Fifty-four percent of patients had a ≥ 33% reduction in pain during dronabinol treatment compared with 21% of patients during placebo. The degree of pain reduction in this study was comparable to that seen with other drugs commonly used in the treatment of neuropathic pain conditions. There were no significant differences reported between the treatment group and placebo in thermal sensibility, tactile and pain detection, vibration sense, temporal summation, or mechanical or cold allodynia. However, there was a statistically significant increase in the pain pressure threshold in dronabinol-treated subjects. Self-reported adverse effects were common, especially during the first week of active treatment. These included lightheadedness, dizziness, drowsiness, headache, myalgia, muscle weakness, dry mouth, palpitations, and euphoria.
A phase I, randomized, single-dose, double-blind, placebo-controlled, crossover trial of 30 patients taking short- or long-acting opioids (68 mg oral morphine equivalents/day; range: 7.5 - 228 mg) for intractable, chronic non-cancer pain (of various etiologies) reported that both a 10 mg and 20 mg dose of dronabinol was associated with significant pain relief compared to placebo, although no difference in pain relief was observed between the two active treatmentsReference 287. Pain intensity and evoked pain were also significantly reduced in subjects who received the active treatments compared to placebo. Significant pain relief compared to baseline was also reported in an open-label, phase II extension to the initial phase I trial. Subjects were instructed in a stepwise dosage schedule beginning with a 5 mg/day dose, and titrating upwards to a maximum of 20 mg t.i.d. Significant side effects were observed in the majority of patients in the single-dose trial, were consistent with those observed in other clinical trials, and occurred more frequently in subjects receiving the highest dosage of the study medication. The authors reported that compared to the single-dose phase I trial, the frequency of self-reported side effects in the phase II open-label study decreased with continued use of dronabinol. Limitations in the design of the study included the small number of study subjects, the large number of subjects with a history of cannabis use, the lack of appropriate comparison groups, and the lack of an active placebo. Other limitations specific to the open-label phase II trial included the lack of a control group or crossover arm.
Nabiximols
Health Canada has approved Sativex® (with conditions) as an adjunct treatment for the symptomatic relief of neuropathic pain in MSReference 431.
A number of randomized, placebo-controlled, double-blind crossover and parallel studies have shown a significant reduction in central or peripheral neuropathic pain of various etiologies (e.g. brachial plexus avulsion, MS-related) following treatment with nabiximols (Sativex®)Reference 433Reference 834Reference 835. In all three studies, patients were concomitantly using other drugs to manage their pain (anti-epileptics, tricyclic anti-depressants, opioids, NSAIDs, selective serotonin reuptake inhibitors, benzodiazepines, skeletal muscle relaxants). The NNT for 30% pain reduction (deemed clinically significant) varied between 8 and 9, whereas the NNT for 50% pain reduction for central neuropathic pain was 3.7, and 8.5 for peripheral pain. In two of the three studies, the majority of subjects had prior experience with cannabis for therapeutic or non-medical purposesReference 834Reference 835. Furthermore, the majority of subjects allocated to the active treatment experienced minor to moderate adverse effects compared to the placebo group. These included nausea, vomiting, constipation, dizziness, intoxication, fatigue, and dry mouth among other effects.
According to the updated consensus statement and clinical guidelines on the pharmacological management of chronic neuropathic pain published by the Canadian Pain Society in 2014, cannabinoid-based therapies (e.g. dronabinol, nabiximols, smoked cannabis) are now considered to be third-line treatments (in 2007 they were considered fourth-line treatments) for neuropathic pain; mostly as adjuvant analgesics for pain conditions refractory to standard drugsReference 836Reference 837 (but also see Section 4.8.3 andReference 838 for updated clinical guidelines on the use of cannabinoids for the treatment of symptoms associated with fibromyalgia).
A nine-month (38-week) open-label, add-on extension study investigated the long-term efficacy, safety and tolerability of nabiximols in 380 patients (234 completed) with peripheral neuropathic pain associated with diabetes mellitus or allodynia and concomitantly using other analgesic therapyReference 839. One hundred and sixty-six patients had previously been taking nabiximols under a parent RCT (mean daily doses for allodynia, 8.9 sprays; mean daily doses for diabetic neuropathy, 9.5 sprays). Mean daily dose of nabiximols in the add-on extension trial was between six and eight pump actuations (16.2 mg THC and 15 mg CBD and 21.6 mg THC and 20 mg CBD) and no increase in pump actuations was noted over time suggesting the absence of tolerance to the study medication. Eleven percent of patients who had received nabiximols during the parent RCT study withdrew from the extension study due to adverse events while 27% of patients taking placebo during the parent study withdrew from the extension study due to adverse events. Thirteen percent of patients who had received nabiximols in the parent RCT withdrew because of lack of efficacy. Concomitant analgesic medication was used by 84% of patients. The most commonly used analgesic medications included anticonvulsants, tricyclic anti-depressants, opioids, and NSAIDs. Non-analgesic concomitant medications included 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors, angiotensin-converting enzyme (ACE) inhibitors, biguanides, and platelet aggregation inhibitors. The vast majority of patients had a history of previously trying and failing at least one analgesic for their peripheral neuropathic pain (i.e. anticonvulsants and NSAIDs). All patients showed an improvement in pNRS score over time, from an initial score of 6.9 at baseline in the parent RCTs to a score of 4.2 at the end of the nine-month open-label extension trial period. At least half of the patients reported a 30% clinically significant improvement in pain compared to parent RCT baseline, and a minimum of 30% of patients demonstrated a 50% improvement in pain over time. The maximum reduction in pain scores occurred between 14 and 26 weeks during the extension trial. Improvements in sleep quality NRS scores and EQ-5D health questionnaire outcomes were maintained into and over the course of the add-on extension study period. The most common all-cause adverse events reported by system organ class were nervous system disorders (44%), GI disorders (36%), general disorders and administration site conditions (24%), infections and infestations (23%), and psychiatric disorders (21%). The most common treatment-related adverse events were dizziness (19%), nausea (9%), dry mouth (8%), dysgeusia (7%), fatigue (7%), somnolence (7%), and feeling drunk (6%). The majority (74%) of treatment-related adverse events resolved without consequence by the end of the study period. However, adverse events that were reported to be continuing at study end included fatigue, dizziness, and insomnia. Eleven percent of patients experienced a serious adverse event during the study, with 1% experiencing a treatment-related adverse event. The serious adverse events that were considered to be treatment-related included nervous system disorders and psychiatric disorders: two patients experienced amnesia, and there was one event of paranoia and one suicide attempt. Eighteen percent of patients ceased study medication due to treatment-related adverse events. The majority of these events occurred within the first week of treatment.
4.7.2.3 Cancer pain
- The limited available clinical evidence with certain cannabinoids (dronabinol, nabiximols) suggests a modest analgesic effect of dronabinol and a modest and mixed analgesic effect of nabiximols on cancer pain.
Clinical studies with dronabinol
Two randomized, double-blind, placebo-controlled clinical studies suggested oral Δ9-THC (dronabinol) provided an analgesic benefit in patients suffering from moderate to severe continuous pain due to advanced cancer. The first study was a small dose-ranging study of 5, 10, 15, and 20 mg Δ9-THC, given in successive days, to 10 cancer patientsReference 840. Significant pain relief was found at the 15 and 20 mg dose levels, but at these higher doses patients were heavily sedated and mental clouding was common. A second, placebo-controlled study compared 10 and 20 mg oral Δ9-THC with 60 and 120 mg codeine in 36 patients with cancer painReference 285. While the lower and higher doses of THC were equianalgesic to the lower and higher doses of codeine, respectively, statistically significant differences in analgesia were only obtained between placebo and 20 mg Δ9-THC, and between placebo and 120 mg codeine. The 10 mg Δ9-THC dose was well tolerated, and despite its sedative effect appeared to have mild analgesic potential. The 20 mg Δ9-THC dose induced somnolence, dizziness, ataxia, and blurred vision. Extreme anxiety was also observed at the 20 mg dose in a number of patients.
Clinical studies with nabiximols
A randomized, double-blind, placebo-controlled, parallel-group clinical trial of patients suffering from intractable cancer pain (mixed, bone, neuropathic, visceral, somatic/incident) suggested that an orally administered THC: CBD extract (nabiximols), containing 2.7 mg of Δ9-THC and 2.5 mg CBD per dose, is an efficacious adjunctive treatment for such cancer-related pain which is not fully relieved by strong opioidsReference 138. Baseline daily median morphine equivalents ranged from 80 to 120 mg. Forty-three percent of patients (n = 60) taking the extract achieved a ≥ 30% improvement in their pain score, which was twice the number of patients who achieved this response in the THC only (n = 58) and placebo (n = 59) groups. Both the nabiximols and the THC medications were reported to be well tolerated in this patient population, and adverse events were reported to be similar to those seen in other clinical trials of nabiximols (e.g. somnolence, dizziness, and nausea).
This study was followed-up by an open-label extension study that evaluated the long-term safety and tolerability of nabiximols (as well as oro-mucosal THC spray) as an adjuvant pain treatment in patients with terminal cancer pain refractory to strong opioid analgesicsReference 283. Patients who had taken part in, fully complied with the study requirements of, had not experienced an unacceptable adverse event in the initial parent study, and that were expected to receive clinical benefit from nabiximols (with acceptable tolerability) were enrolled in the extension study. The most commonly reported (50%) pain type was mixed pain (nociceptive and neuropathic), followed by neuropathic pain (37%), and bone pain (28%). The median duration of treatment with nabiximols (n = 39 patients) was 25 days (range: 2 - 579 days) while the mean duration of treatment with oro-mucosal THC spray (n = 4 patients) was 151.5 days (range: 4 - 657 days). The average number of sprays/day for nabiximols during the last seven days of dosing was 5.4 vs. 14.5 for THC only. No dose escalation was noted in patients taking nabiximols beyond six months and up to one year following treatment initiation. Although the study was a non-comparative, open-label study with no formal hypothesis testing and mostly used descriptive statistics, a decrease from baseline in mean score on the Brief Pain Inventory Short-Form was observed for both "pain severity" and "worst pain" over the five weeks of treatment. However, the authors noted that the clinical investigators considered that their patients' pain control was sub-optimal. A negative change from baseline (i.e. indicating a worsening) was also reported in the physical functioning score on the EORTC QLQ-C30, although some improvements in scores for sleep and pain, between baseline and week five of treatment, were reported. Eight percent of the patients on nabiximols developed a serious nabiximols-associated adverse event. The most commonly reported adverse events for nabiximols were nausea/vomiting, dry mouth, dizziness, somnolence, and confusion.
In contrast to the above-mentioned studies using nabiximols, a randomized, double-blind, placebo-controlled, parallel group clinical trial of opioid-treated cancer patients with intractable chronic cancer pain (e.g. bone, mixed, neuropathic, somatic, visceral) reported no statistically significant difference between placebo and the nabiximols treatment group in the primary endpoint of 30% relief from baseline pain at study endReference 284. However, when using a continuous responder rate analysis as a secondary endpoint (i.e. comparing the proportion of active drug vs. placebo responders across the full spectrum of response from 0 to 100%), the study was able to report a statistically significant treatment effect in favour of nabiximols. Patients were taking median opioid equivalent doses ranging between 120 and 180 mg/day. Adverse events were dose-related, with only the highest dose group comparing unfavourably to placebo. The authors noted that the trial was a dose-ranging study, and that confirmatory studies are strongly warranted. The study design also did not permit the evaluation of a therapeutic index.
A randomized, placebo-controlled, cross-over pilot clinical trial of nabiximols for the alleviation of established chemotherapy-induced neuropathic pain reported no statistically-significant difference between the treatment and the placebo groups on a numerical rating scale for pain intensity (NRS-PI)Reference 282. The authors noted that five participants (responders) experienced a 2-point or greater drop in NRS-PI during treatment which was statistically significant compared to placebo. The mean dose of medication used in the treatment arm was eight sprays per day (range: 3 - 12) and 11 sprays in the placebo arm with most patients titrating to maximum dose in the placebo arm. Medication-related side effects were reported by the majority of participants and included fatigue, dry mouth, dizziness, nausea, headache, "fuzzy thinking" or "foggy brain", increased appetite and diarrhea. Ten participants continued into the extension phase of the trial and pain levels continued to decrease from a baseline of 6.9 to 5.0 at three months and 4.2 at six months. Average dose was 4.5 sprays per day (range: 2 - 10 sprays per day).
In Canada, nabiximols (Sativex®) is approved (with conditions) as an adjunctive analgesic in adults with advanced cancer who experience moderate to severe pain during the highest tolerated dose of strong opioid therapy for persistent background painReference 431. Current dosing recommendations for nabiximols suggest a maximum daily dose of 12 sprays (32.4 mg THC and 30 mg CBD) over a 24 h periodReference 122Reference 138Reference 431, although higher numbers of sprays/day have been used or documented in clinical studiesReference 284Reference 431. It should be noted that increases in the number of sprays/day were accompanied by increases in the incidence of adverse effects.
4.7.2.4 "Opioid-sparing" effects and cannabinoid-opioid synergy
- While pre-clinical and case studies suggest an "opioid-sparing" effect of certain cannabinoids, epidemiological and clinical studies with oral THC and nabiximols are mixed.
- Observational studies suggest an association between U.S. states with laws permitting access to cannabis (for medical and non-medical purposes) and lowered rates of prescribed opioids and opioid-associated mortality.
The "opioid-sparing" effect refers to the ability of a non-opioid medication (e.g. cannabis, THC) to confer adjunctive opioid analgesia with the use of a lower dose of the opioid, thereby decreasing opioid-associated side effects. While there are some pre-clinical data and data from case studies supporting such an effect for cannabinoids, this is less well-established in published clinical studies. Furthermore, there is some evidence from epidemiological/observational studies to suggest that individuals using opioids for chronic non-cancer pain may also use cannabis to manage distress from unmanaged pain, and that a certain portion of individuals using higher doses of opioids for chronic non-cancer pain may also have greater problems across a number of domains, including greater risk of a CUD.
The following information summarizes the results from pre-clinical, epidemiological and clinical studies investigating cannabinoid-opioid interactions and the potential "opioid-sparing effect" of cannabinoids.
Pre-clinical data
There is a fair amount of evidence to suggest a functional interaction between the cannabinoid and the opioid systems, although additional research is needed to understand precisely how the two systems communicate with one another. The evidence supporting a putative interaction between the cannabinoid and opioid systems comes from a number of observations. First, it is known that cannabinoids and opioids produce similar biological effects such as hypothermia, sedation, hypotension, inhibition of GI motility, inhibition of locomotor activity, and anti-nociceptionReference 841-Reference 843. Furthermore, neuroanatomical studies in animals have demonstrated overlapping tissue distribution of the cannabinoid and opioid receptors, with both receptor types found in nervous system tissues associated with the processing of painful stimuli, namely the periaqueductal gray, raphe nuclei, and central-medial thalamic nucleiReference 841-Reference 843. There is also some evidence that the CB1 and mu-opioid receptors can co-localize in some of the same neuronal sub-populations such as those located in the superficial dorsal horn of the spinal cordReference 841. This co-localization may play an important role in spinal-level modulation of peripheral nociceptive inputsReference 841. Both receptors also share similar signal transduction molecules and pathways, the activation of which generally results in the inhibition of neurotransmitter releaseReference 841Reference 843. The role of these receptors in inhibiting neurotransmitter release is further supported by their strategic localization on pre-synaptic membranesReference 841. Evidence from some pre-clinical studies also suggests that acute administration of cannabinoid receptor agonists can lead to endogenous opioid peptide release, and that chronic THC administration increases endogenous opioid precursor gene expression (e.g. preproenkephalin, prodynorphin, and proopiomelanocortin) in different spinal and supraspinal structures involved in the perception of painReference 841. A few studies have even demonstrated the existence of cannabinoid-opioid receptor heteromers, although the exact biological significance of such receptor heteromerization remains to be fully elucidatedReference 844Reference 845. Taken together, these findings suggest the existence of cross-talk between the cannabinoid and opioid systems. Furthermore, pre-clinical studies using a combination of different opioids (morphine, codeine) and cannabinoids (THC), at acute or sub-effective doses, have reported additive and even synergistic analgesic effectsReference 846-Reference 848Reference 848-Reference 851. A recent systematic review and meta-analysis of pre-clinical studies examining the strength of the existing evidence for the "opioid-sparing" effect of cannabinoids in the context of analgesia concluded that there was a significant opioid-sparing effect between morphine and THC when co-administered, although there was significant heterogeneity in the dataReference 852. Nevertheless, when compared to morphine administration alone, the median ED50 of morphine was 3.6 times lower when given in combination with THC. A significant "opioid-sparing" effect was also reported for THC when co-administered with codeine (ED50 9.5 times lower when THC combined with codeine vs. codeine alone).
Clinical case series and epidemiological data
A recent cross-sectional on-line survey of 2 897 participants from a databse of 67 422 medical cannabis patients in the state of California gathered data about the use of cannabis as a substitute for opioid and non-opioid-based pain medicationReference 853. The majority of the participant sample reported being able to decrease the amount of opioids they consumed when they also used cannabis. Limitations of this study included self-report and very low response rate (4%) and a biased sample population.
Analysis of patients case-series reported a reduction in opioid dose with cannabis use in the treatment of chronic non-cancer painReference 854. In one case, a 47-year-old woman with a 10-year history of chronic progressive MS with headache, multi-site joint pain, bladder spasm, and leg spasticity on a daily regimen of 75 mg of long-acting morphine, 24 mg tizanidine, and 150 mg sertraline at bedtime began also using cannabis at bedtime. Over the next six months, the patient began smoking two to four puffs of cannabis at bedtime on a regular basis and reported a reduction of morphine to 45 mg per day, tizanidine to 6 mg per day, and sertraline to between 100 and 150 mg at bedtime. The patient reported improvement in pain, spasticity, bladder spasm, and sleep. The patient also reported not experiencing any adverse effects other than feeling somewhat "high" if she smoked more than four puffs at a time. Another patient, a 35-year-old male with HIV, who experienced HIV-related painful peripheral neuropathy involving the lower limbs and hands and who was taking 360 mg of long-acting morphine per day with an additional 75 mg of morphine sulfate four times daily for breakthrough pain and gabapentin at 2 400 mg per day began using smoked cannabis in a dose of three to four puffs, three to four times per day. Over the next four months, the patient's dose of morphine decreased to 180 mg per day, and by nine months the patient discontinued the morphine followed by discontinuation of gabapentin. The patient also did not report any side effects associated with cannabis use. Lastly, a 44-year-old man with a six-year history of low back pain and left leg pain taking long-acting morphine at 150 mg per day and cyclobenzaprine 10 mg, t.i.d. with poor pain control began smoking cannabis, at a dose of several puffs to one joint, four to five times per day. After smoking cannabis on a regular basis for two weeks, the patient was able to decrease his morphine to 90 mg per day with a further reduction to 60 mg morphine per day and a reduction in cyclobenzaprine to 10 mg once daily with reported improvement in pain control. The authors of the case-series report that taken together, the three patients were able to reduce their opioid dose by 60 to 100% after starting the cannabis regimen. In addition, patients self-reported experiencing better pain control with the introduction of cannabis into their pain management strategy. All patients reported previous cannabis use before onset of morbidity.
A prospective, non-randomized, and unblinded observational case-series study assessing the effectiveness of adjuvant nabilone therapy in managing pain and symptoms experienced by 112 advanced cancer patients in a palliative care setting reported that those patients using nabilone had a lower rate of starting NSAIDs, tricyclic anti-depressants, gabapentin, dexamethasone, metoclopramide, and ondansetron and a greater tendency to discontinue these drugsReference 288. Patients were prescribed nabilone for pain relief (51%), for nausea (26%), and for anorexia (23%). Treated patients were started on 0.5 or 1 mg nabilone at bedtime during the first week and titrated upwards in increments of 0.5 or 1 mg thereafter. At follow-up, the majority of patients were on a 2 mg daily nabilone dose with a mean daily dose of 1.79 mg. The two primary outcomes of the study, pain and opioid use in the form of total morphine sulfate equivalents were reduced significantly in treated patients compared to untreated patients. Side effects from nabilone consisted mainly of dizziness, confusion, drowsiness, and dry mouth. Patients also demonstrated less tendency to initiate additional new medications and could reduce or discontinue baseline medications.
A time-series analysis that examined death certificate data over time (1999-2010) between U.S. states with medical cannabis programs and those without, to determine if there was an association between the presence of state medical cannabis laws and opioid analgesic overdose mortality rates, reported that age-adjusted opioid analgesic overdose death rate per 100 000 population in states that enacted medical cannabis laws was almost 25% lower than in states without such laws (95% CI = -37.5%, -9.5%)Reference 855. This association appeared to strengthen over time, with a decrease in the mean annual opioid overdose mortality rate of 19.9% in the first-year and a decrease in the mean annual opioid overdose mortality rate of 33.3% in year six after enactment of state medical cannabis laws. This study appears to suggest that medical cannabis laws are associated with reductions in opioid analgesic overdose mortality on a population level, however the mechanisms by which this appears to occur is unclear at this time and requires further investigation.
A time-series analysis that examined the association between Colorado's legalization of cannabis for non-medical purposes and opioid-related deaths (2000-2015) reported a 0.7 deaths/per month reduction in opioid-related deaths (b = -0.68; 95% CI = -1.34, -0.03). Specifically, there was a 6% decrease in opioid-related deaths two years following legalization of non-medical cannabis when compared to two control states (one allowing cannabis for medical purposes, one not allowing cannabis for medical or non-medical purposes). However, the authors note that the two-year follow-up window post-legalization is relatively short and further research involving longer follow-up periods (and examining additional states that have legalized cannabis for non-medical purposes) are needed to determine if these reductions are sustained or dissipate over timeReference 856.
Two recent observational studies using U.S health care data (Medicare and Medicaid) examined the difference in opioid prescription rates in U.S. states with and without legal access to cannabis. Bradford and colleaguesReference 857 longitudinally (2010-2015) found that states that implemented medical cannabis laws reported fewer daily doses of prescribed opioids (2.21 million/year) compared to states without medical cannabis laws. Parallel to this finding, Wen and HockenberryReference 858 cross-sectionally found that states with medical cannabis laws reported a 5.88% lower rate of prescribed opioids. This study further examined opioid prescribing patterns in states with laws regarding cannabis for non-medical purposes, and found that access to cannabis was also associated with reductions in opioid prescribing rates (i.e., 6.38% lower compared to states without non-medical cannabis legalization). Key limitations across these studies are the associative nature of the findings meaning that causality cannot be established, and the inability to determine if cannabis actually replaced or substituted for opioid use, as users potentially could have accessed and used opioids from other non-medical sources.
A cross-sectional retrospective survey of 244 patients accessing cannabis for medical purposes at a Michigan dispensary reported that the use of cannabis for medical purposes was associated with a significant decrease in opioid use, as well as a decrease in the number of other medications used and in the number of side effects associated with the use of other medications, as well as improvements in QoLReference 859. The majority (80%) of the study participants reported smoking cannabis daily. The mean decrease in self-reported opioid use among all study respondents was 64%. Furthermore, there was a statistically significant decrease in the number of other non-opioid medications (e.g. NSAIDs, disease-modifying anti-rheumatic drugs, anti-depressants, serotonin-norepinephrine reuptake inhibitors, and selective serotonin reuptake inhibitors) after cannabis use. Limitations of the study include potential recall bias, a self-selected population, self-report, and changes in the rates of physician prescription of opioids.
A prospective, open-label, single-arm, longitudinal study of 274 patients with treatment-resistant chronic pain (i.e. musculoskeletal widespread pain, peripheral neuropathic pain, radicular low back pain, cancer pain), examined the long-term effect of medicinal cannabis treatment on pain and functional outcomesReference 582. Intention-to-treat analysis was conducted on 206 patients who provided baseline data and 176 subjects completed the study and were included in the final analysis. Patients could use smoked cannabis, baked cookies or an olive oil extract as drops (up to a maximum equivalent of 20 g per month, but with the possibility of increasing this amount if warranted). Patients were instructed to titrate their cannabis dose starting with one cigarette puff (or one drop of cannabis oil) per day and increase by increments of one puff or drop per dose up to three times per day until satisfactory pain relief was achieved or side effects appeared. Subjects were instructed to refrain from driving for at least 6 h after consuming cannabis or longer if they felt disoriented or drowsy. THC concentrations in the smoked product were estimated at 6 - 14% THC and between 11 - 19% in the oral formulations, with the CBD concentrations between 0.2 - 3.8% in the smoked product and 0.5 - 5.5% in the oral formulations. Mean monthly-prescribed amount of cannabis at follow-up was 43 g (average of 1.4 g per day). Cannabis treatment was added to the existing analgesic regimen. Median daily dose among opioid users (in daily oral morphine sulfate equivalents) was 60 mg. At follow-up (mean of seven months from treatment start), pain symptom score improved from a median score of 83.3 to a median score of 75.0 (p < 0.001) on the Short-Form Treatment Outcomes in Pain Survey (S-TOPS) questionnaire with 66% of subjects reporting improvement, 8% reporting no change, and 26% reporting deterioration. In subgroup analyses, no differences were noted in the primary outcome between neuropathic and non-neuropathic pain, or between male and female patients. Improvements were also noted in Brief Pain Inventory (BPI) scores of pain severity and pain interference as well as with most social and emotional disability scores (i.e. S-TOPS scores for family-social disability, role-emotional disability, satisfaction with outcomes, sleep problem index). Opioid consumption at follow-up also decreased by 44%. The median (daily) oral morphine equivalent dose among subjects still receiving opioids at follow-up decreased from 60 mg to 45 mg but did not reach statistical significance. Nine subjects discontinued treatment due to mild to moderate adverse effects, mainly sedation, heaviness, nervousness, and difficulty concentrating. Two additional subjects discontinued treatment due to serious side effects: one because of elevated liver transaminases, and one elderly subject admitted to emergency care and hospitalized for confusion. Total rate of cannabis discontinuation was 5.3%. Study limitations included lack of a control group and open-label design, lack of frequent periodic assessment of all adverse events, and under-representation by women.
Findings from a two-year, prospective, cross-sectional, cohort study of 1 514 individuals prescribed pharmaceutical opioids for chronic non-cancer pain (the Pain and Opioids IN Treatment study) examined the extent to which cannabis is used by this groupReference 214. The study reported that one in six (16%) enrolled individuals had used cannabis for pain relief and 25%, reported they would have used it for pain relief if they had access. Among those using cannabis for pain, the average pain relief they reported from using cannabis was 70%, which was in contrast to the 50% average pain relief reported for opioid medications. Almost half (43%) had used cannabis for non-medical purposes at some time and 12% of the entire cohort met the criteria for an International Classification of Diseases (ICD) CUD in their lifetime. Those individuals reporting using cannabis for pain relief were on average younger and male, and were significantly more likely to have met criteria for a range of other licit and illicit substance use disorders and to meet criteria for moderate or severe depression and generalized anxiety. Individuals who had used cannabis for pain were more likely to have reported back and neck problems and had been living with pain for a significantly longer period compared to those not using cannabis for pain. Those who had used cannabis for pain reported greater pain severity, greater interference from and poorer coping with pain, and more days out of role in the past year compared to those who had not used. In addition, these individuals had been prescribed opioids for longer, were on higher opioid doses, were more likely to also have been prescribed benzodiazepines, and were more likely to be non-adherent with their opioid use. According to the authors, those individuals using cannabis for pain appeared to be a group with greater problems across a number of domains including psychological distress and substance use problems such that the use of cannabis for pain may reflect those characteristics. Alternatively, the authors suggest that the adjunctive use of cannabis for pain could reflect attempts by those individuals to manage distress, given the experience of greater interference from reported pain. Limitations of the study include potential for under-reporting, potential bias associated with self-reporting, and lack of information on amount of cannabis consumed and potency.
In support of the above findings, a study looking at the rates of CUD in a national sample of Veterans Health Administration patients (N = 1 316 464) with chronic non-cancer pain diagnoses and receiving opioid medications, suggested that greater numbers of prescription opioid fills were associated with greater likelihood of a diagnosis of a CUDReference 860. Patients prescribed opioids and diagnosed with a CUD were found to be significantly younger and more likely to be homeless. Those diagnosed with a CUD were also more likely to be diagnosed with hepatic disease and HIV, though less likely to be diagnosed with dementia and renal disease compared to those without a CUD. Patients diagnosed with a CUD were also more likely to be diagnosed with schizophrenia, other psychotic disorders, bipolar disorder, major depressive disorder, anxiety disorders, adjustment disorder, personality disorder, and dual diagnosis. Those with a CUD were also more likely to have been diagnosed with abuse or dependence of hallucinogens, cocaine, tobacco, amphetamine, opioids or alcohol. The authors conclude that the results of this study suggest that rather than cannabis functioning as an opioid substitute (i.e. CUD would be associated with less opioid use), these substances appear to complement each other as greater opioid medication use is associated with increased risk of CUD. Limitations of this study included a mostly homogenous population sample (male military veterans), and reliance on non-standardized semi-structured diagnostic interviews, raising the possibility that the actual prevalence of CUD in this patient population was under-estimated.
An epidemiological study using data gathered from wave 1 and 2 of NESARC (2001 - 2002 and 2004 - 2005) prospectively examined the association between cannabis use and incident non-medical prescription opioid use and disorder 3 years later, as well as whether cananbis use among adults with non-medical prescription opioid use was associated with subsequent decrease in non-medical opioid useReference 861. Cannabis use at wave 1 was associated with a significant increase in the odds of prevalent non-medical prescription opioid use during the follow-up period at wave 2 which persisted even after adjusting for confounders. This association was observed among adults without past-year cannabis use disorder and among adults with moderate or more severe pain at wave 1. Furthermore, among individuals without non-medical opioid use during the 12 months prior to the wave 1 interview, there was a significant association between cannabis use at wave 1 and incident non-medical opioid use during the follow-up period. Cannabis use also appeared to be associated with lower odds of decreasing levels of opioid use but decreases were markedly more common than increases in opioid use. After adjustment for other covariates, significant associations persisted between wave 1 cannabis use and prevalent and incident non-medical opioid use disorder at wave 2. Among adults with moderate or more severe pain at wave 1, cannabis use was associated with prevalent opioid use disorder in adjusted analyses. Despite the above findings, the great majority of adults who used cannabis did not go on to initiate or increase non-medical opioid use.
A preliminary, historical, small cohort study examined the association between enrollment in a medical cannabis program and prescription opioid useReference 862. Enrollment in a medical cannabis program was associated with a statistically significant higher odds of ceasing opioid prescriptions (OR = 17.27, CI = 1.89, 157.36), an OR = 5.12 of reducing daily opioid doses (CI = 1.56, 16.88). Improvements were noted in pain reduction, quality of life, social life, activity levels, and concentration with few side effects.
Data from clinical trials
A recent systematic review and meta-analysis of clinical studies examining the strength of the existing evidence for the "opioid-sparing" effect of cannabinoids in the context of analgesia concluded that there was an absence of randomized, well-controlled clinical studies that provide evidence of an "opioid-sparing" effect of cannabinoidsReference 852. Furthermore, the existing data from clinical trials looking at the "opioid-sparing" ability of cannabis are mixed. One double-blind, placebo-controlled, crossover clinical study of healthy human volunteers given low doses of THC, morphine, or a combination of the two drugs failed to find any differences between subjects' ratings of sensory responses to a painful thermal stimulusReference 863. However, the study did report that the combination of morphine and THC was associated with a decrease in the subjects' affective response to the painful thermal stimulus. The authors suggested that morphine and THC could combine to yield a synergistic analgesic response to the affective aspect of an experimentally-evoked pain stimulus.
A recent double-blind, placebo-controlled, within-subject clinical study examined if cannabis enhances the analgesic effects of (low dose) oxycodone and the impact of combining cannabis and oxycodone on abuse liability. Eighteen healthy 'current' cannabis smokers (at least 3 times/week; assessed by urine toxicology and self-report) were given oxycodone (0, 2.5, and 5.0 mg, P.O.) with smoked cannabis (0.0, 5.6% THC), and the analgesic effects were measured by the Cold-Pressor Test. Results revealed that oxycodone alone (5.0 mg) significantly increased pain threshold (F [1, 17] = 7.5, p ≤ 0.01) and tolerance (F [1, 17] = 5.4, p ≤ 0.05) compared to placebo (inactive cannabis and 0.0mg oxycodone). When administered with active cannabis, 5.0 mg oxycodone also increased pain tolerance compared to the placebo condition and active cannabis alone (F [1, 17] = 5.5, p ≤ 0.05). The combination of active cannabis and 2.5 mg oxycodone increased pain threshold and tolerance relative to the placebo condition (F [1, 17] = 5.9, p ≤ 0.05 and F [1, 17] = 6.5, p ≤ 0.05, respectively) and active cannabis alone (F [1, 17] = 5.2, p ≤ 0.05 and F [1, 17] = 5.5, p ≤ 0.05, respectively). In terms of abuse liability oxycodone did not increase subjective ratings of cannabis abuse or cannabis self-administration. However, a combination of oxycodone (2.5 mg) and cannabis yielded small but significant increases in oxycodone abuse liability (p ≤ 0.05). The researchers concluded that the findings demonstrate opioid-sparing effects of cannabis for analgesia that may be accompanied by increases in potential abuse liability pertaining to oxycodoneReference 864.
Another clinical studyReference 287 reported that patients suffering from chronic non-cancer pain and not responding to opioids experienced increased analgesia, decreased pain intensity, and decreased evoked pain when given either 10 or 20 mg dronabinol (for additional details see Section 4.7.2.2, under Clinical Studies With Orally Administered Prescription Cannabinoids).
In another study, it was reported that patients suffering from chronic pain of various etiologies, unrelieved by stable doses of opioids (extended release morphine or oxycodone), experienced a statistically significant improvement in pain relief (-27%, CI = 9 - 46) following inhalation of vapourized cannabis (900 mg, 3.56% THC, t.i.d. for five days)Reference 280 (for additional details see Section 4.7.2.2, under Clinical Studies With Smoked or Vapourized Cannabis). The findings from this study suggest that addition of cannabinoids (in this case inhaled vapourized cannabis) to existing opioid therapy for pain may serve to enhance opioid-associated analgesia.
In contrast, another study did not note a statistically significant decrease in the amounts of background or breakthrough opioid medications consumed by the majority of patients suffering from intractable cancer-related pain and taking either nabiximols or THCReference 138. Similarly, no statistically significant changes were observed in the amounts of background or breakthrough opioid doses taken by patients suffering from intractable cancer-related pain who were administered nabiximolsReference 284. However, the design of the latter study did not allow proper assessment of an "opioid-sparing effect" of nabiximols.
In summary, pre-clinical and case studies appear to support an "opioid-sparing" effect of THC but results from clinical and epidemiological studies are mixed. While "cannabinoid-opioid synergy" has been proposed as a way to significantly increase the analgesic effects of opioids while avoiding or minimizing tolerance to the effects of opioid analgesics and circumventing, or attenuating, the well-known undesirable side effects associated with the use of either cannabinoids or opioids, some of the evidence is mixed and requires further studyReference 841Reference 843.
4.7.2.5 Headache and migraine
- The evidence supporting using cannabis/certain cannabinoids to treat headache and migraine is very limited and mixed.
With regard to migraine, an endocannabinoid deficiency has been postulated to underlie the pathophysiology of this disorderReference 865; however, the evidence supporting this hypothesis is limited and mixed. Clinical studies suggest that the concentrations of anandamide are decreased in the CSF of migraineurs, while the levels of calcitonin-gene-related-peptide and nitric oxide (normally inhibited by anandamide and implicated in triggering migraine) are increasedReference 866Reference 867. In contrast, the activity of the anandamide-degrading enzyme FAAH is significantly decreased in chronic migraineurs compared to controlsReference 868.
While historical and anecdotal evidence suggest a role for cannabis in the treatment of headache and migraineReference 869, no controlled clinical studies of cannabis or prescription cannabinoids to treat headache or migraine have been carried out to dateReference 870Reference 871.
In one case-report, a patient suffering from pseudotumour cerebri and chronic headache reported significant pain relief after smoking cannabisReference 293. In another case-report, a patient complaining of cluster headaches refractory to multiple acute and preventive medications reported improvement with smoked cannabis or dronabinol (5 mg)Reference 291. However, these single-patient case-studies should be interpreted with caution.
A report indicated that cannabis use was very frequent among a population of French patients with episodic or chronic cluster headache, and of those patients who used cannabis to treat their headache, the majority reported variable, uncertain, or even negative effects of cannabis smoking on cluster headacheReference 290.
A retrospective chart review of 121 adults with a primary diagnosis of migraine headaches who were recommended migraine treatment or prophylaxis with cannabis for medical purposes by a physician from among two medical cannabis specialty clinics in Colorado reported that migraine headache frequency decreased from 10.4 to 4.6 headaches per month (p < 0.0001) with the use of cannabis for medical purposesReference 289. Forty percent of the patients reported positive effects with the most common effect being prevention of migraine headache, decreased frequency, and aborted migraine headache. Inhaled cannabis was reported as being more effective than oral ingestion. Negative effects were reported in 12% of patients, with edibles being associated with more negative effects (i.e. problems with timing and effect intensity).
It should also be noted that cannabis use has been associated with reversible cerebral vasoconstriction syndrome and severe headacheReference 292. In addition, headache is an often-observed adverse effect associated with the use of cannabis or prescription cannabinoid medicationsReference 59Reference 227Reference 431Reference 492Reference 688Reference 716, and headache is also one of the most frequently reported physical symptoms associated with cannabis withdrawalReference 872.
A recent review of the use of cannabis for headache disorders reported that there is insufficient evidence from well-controlled clinical trials to support the use of cannabis for headache, despite sufficient anecdotal and preliminary results as well as plausible neurobiological mechanisms to warrant clinical studiesReference 873.
4.8 Arthritides and musculoskeletal disorders
- The evidence from pre-clinical studies suggests stimulation of CB1 and CB2 receptors alleviates symptoms of osteoarthritis (OA), and THC and CBD alleviate symptoms of rheumatoid arthritis (RA).
- The evidence from clinical studies is very limited, with a modest effect of nabiximols for RA.
- There are no clinical studies of cannabis for fibromyalgia, and the limited clinical evidence with dronabinol and nabilone suggests a modest effect on decreasing pain and anxiety, and improving sleep.
- The role of cannabinoids in osteoporosis has only been investigated pre-clinically and is complex and conflicting.
The arthritides include a broad spectrum of different disorders (e.g. osteoarthritis (OA), rheumatoid arthritis (RA), ankylosing spondylitis, gout, and many others) all of which have in common the fact that they target or involve the joints. Scientific studies have demonstrated that joints, bone, and muscle all contain a working ECS, that some arthritides such as OA and RA are associated with changes in the functioning of the ECS, and that modulation of the ECS may help alleviate some of the symptoms associated with certain arthritidesReference 40-Reference 42Reference 778Reference 874-Reference 882. The section below summarizes the evidence for cannabis/cannabinoids in OA and RA. Also covered are musculoskeletal disorders such as fibromyalgia and osteoporosis.
Information from surveys
The 2011 Canadian Alcohol and Drug Use Monitoring Survey (CADUMS) indicated that a significant proportion of Canadians aged 15 and over who reported using cannabis for medical purposes reported using it for chronic pain associated with, for example, arthritisReference 883.
In addition, one study that explored the experiences of Australian individuals using cannabis for medical purposes reported that out of 128 participants in the survey, 35% said they used cannabis to treat symptoms associated with arthritisReference 884.
A self-administered survey of 947 individuals in the U.K. who reported ever having used cannabis for medical purposes revealed that 21% of the individuals surveyed said they had used cannabis for symptoms associated with arthritis. Seven percent of these individuals had been using cannabis continuously for a median of four yearsReference 579.
A survey of 628 Canadian individuals who self-reported using cannabis for medical purposes asked about individuals' use of cannabis for medical purposesReference 885. Approximately 15% of individuals reporting using cannabis for medical purposes used it to treat symptoms associated with arthritis pain, inflammation, insomnia, anxiety, depression, and spasms. Most reported preferring smoking (53%) compared to vapourizing or oral ingestion (both at 39%). The majority (47%) of individuals using cannabis for arthritis reported using cannabis four or more times per day and an equal proportion reported using at least 2 g per day or more; the median gram amount among those that used 2 g per day or more was approximately 4 g per day.
4.8.1 Osteoarthritis
Among the arthritides, OA is by far the most common type of arthritis and is the leading cause of disability in those over the age of 65 years in developed countriesReference 886. OA results from damage to the articular cartilage induced by a complex interplay of genetic, metabolic, biochemical and biomechanical factors followed by activation of inflammatory responses involving the interaction of the cartilage, subchondral bone and synovium resulting in further damage and degradation of the articular cartilage and subchondral bone, variable synovitis, and capsular thickeningReference 877Reference 878. The eventual outcomes are joint disability and severe painReference 877Reference 878. The pain associated with OA is generally inadequately or safely controlled with current analgesics, which has spurred the search for alternative therapeutic approachesReference 878. The disease affects both men and women, although it appears to occur more frequently in womenReference 877. In addition, OA most commonly affects people in middle age and the elderly, even though younger people may also be affected due to injury or overuseReference 877. The pain associated with OA includes both nociceptive and non-nociceptive components, as well as neuropathic and inflammatory components, and is associated with abnormally excitable pain pathways in the peripheral nervous system and the CNSReference 877. The pain and physical disability associated with OA are also accompanied by anxiety, depression and changes in cognition all of which have a negative impact on QoLReference 876. Neuroimaging studies have shown that several brain regions are involved in the processing of OA pain including bilateral activation of primary and secondary somatosensory cortices as well as the insular, cingulate, pre-frontal and orbito-frontal cortices, and the thalamus, as well as unilateral activation of the putamen and amygdalaReference 887Reference 888.
Pre-clinical studies
Animal models of OA suffer from a number of limitations such as differences in anatomy, functionality, dimensions, cartilage repair processes, and thickness in comparison with human jointsReference 877. In addition, the lesions that develop in animal models of OA correspond to those found in humans only in a particular stage of the diseaseReference 877. Furthermore, no animal model of OA completely reproduces the whole variety of signs and symptoms of human OA. Taken together, these factors all pose a number of significant challenges in translating findings obtained in animal models of OA to OA patients. Nevertheless, animal models of OA are useful in understanding the potential therapeutic effects of cannabis and cannabinoids.
There is increasing evidence that suggests an important role for the ECS in the pathophysiology of joint pain associated with OAReference 877. With regard to endocannabinoid tone, one animal study reported elevated levels of the endocannabinoids anandamide and 2-AG, and the "entourage" compounds PEA and OEA in the spinal cord of rats with experimentally-induced knee joint OAReference 889. While no changes were observed in the levels or the activities of the endocannabinoid catabolic enzymes FAAH or MAGL in the spinal cord of the affected rats, protein levels of the major enzymes responsible for endocannabinoid synthesis were reported to be significantly elevated in these animalsReference 889.
Both CB1 and CB2 receptors have been localized in knee joints confirming that local control of joint pain is achievable without the need to involve central cannabinoid receptorsReference 890Reference 891. Downregulation of CB1 and CB2 receptor gene expression was reported in the lumbar spinal cord of osteoarthritic mice, likely in response to an elevated endocannabinoid tone coming from the affected osteoarthritic jointsReference 892.
A study in rats reported that intra-articular injection of the CB1 receptor agonist arachidonyl-2-chloroethylamide in control animals was associated with a reduction in firing rate and suppression of nociceptive activity from pain fibers innervating the joints when the joints were subjected to either normal or noxious joint rotationReference 893. Furthermore, animals with osteoarthritic joints produced an augmented response to articular CB1 receptor activation. The anti-nociceptive effect was blocked by co-administration of a CB1 receptor antagonist in osteoarthritic joints, but not in control joints.
Local administration of URB597 (a FAAH inhibitor) by intra-arterial injection proximal to an osteoarthritic joint was associated with decreased mechanosensitivity of joint afferent fibres in two different rodent models of OAReference 894. Behavioural experiments carried out in OA rats suggested that treatment with the inhibitor also decreased joint pain measured by a decrease in hindlimb incapacitanceReference 894. In addition to an antinociceptive response to FAAH inhibition, URB597 has been shown to reduce leukocyte trafficking in the synovium indicating that endocannabinoids could have anti-inflammatory properties in jointsReference 880.
Systemic administration of a CB2 receptor agonist in a rat model of OA was associated with a dose-dependent reversal of decreased grip force in the affected limb, a proxy measure for painReference 895. The maximal analgesic efficacy was comparable to that seen with celecoxib in this animal model of OAReference 895.
In another animal study, the spinal lumbar CB2 receptor was shown to play a significant role in the modulation of osteoarthritic painReference 892. Furthermore, upregulation of CB2 receptor expression in the spinal lumbar cord was associated with attenuation of joint pain. In addition, lumbar spinal cord mu-opioid receptor expression was downregulated, while delta and kappa-opioid receptor expression was upregulated, suggesting functional interactions between the endocannabinoid and opioid systems. The decreased mu-opioid receptor expression and concomitant increase in kappa and delta opioid receptor expression could additionally contribute to the nociceptive component of the disease.
One animal study conducted in a rat model of OA reported that CB2 receptor mRNA levels were significantly increased in spinal cord of osteoarthritic ratsReference 896. Furthermore, selective stimulation of the CB2 receptor by systemic dosing with a synthetic cannabinoid receptor agonist was associated with significant attenuation of the development and maintenance of pain behaviour and spinal neuronal responses. Levels of pro-inflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF) α, and IL-10 were also significantly attenuated following treatment with the CB2 receptor agonist. Rats also did not appear to develop tolerance to the anti-nociceptive effects of the CB2 receptor agonist after multiple administrations of the drug. The study also showed a negative association between CB2 mRNA levels and chondropathy in post-mortem samples of human spinal cord.
An animal study of OA in mice reported that the condition was associated with significant increases in 2-AG levels in the prefrontal cortex, the area of the brain implicated in pain, cognitive and emotional processing, as well as in the plasmaReference 876. OA in this mouse model was also associated with increases in stress and anxiety-like behaviour in affected wild-type mice and in mice lacking CB1 receptor expression, but not in mice lacking CB2 receptor expression suggesting distinct roles for these two receptors in the pathophysiology of OA. Selective stimulation of CB1 and CB2 receptors was associated with improvements in mechanical allodynia. Lastly, patients with OA were shown to have significant increases in their plasma levels of 2-AG, but not anandamide, compared to healthy controls consistent with the findings obtained in the mouse model. Furthermore, expression of CB1 and CB2 receptors was upregulated in blood lymphocytes of these patients and significant positive correlations were noted between plasma levels of 2-AG, knee pain, and depression scores as well as significant negative correlations with SF-36 (QoL) and memory performance scores.
Further support for a role for the CB2 receptor in the pathophysiology of OA comes from a pre-clinical study in mice lacking CB2 receptor expressionReference 897. These mice developed significantly more severe OA compared with wild-type controls. Furthermore, treatment of wild-type mice with a CB2 receptor agonist was associated with partial protection from OA. In contrast, another study found that local delivery of a CB2 receptor agonist actually increased joint nociceptor activity and the resulting heightened pain response was thought to involve TRPV1 ion channelsReference 891.
A pre-clinical study in rats that investigated the effects of CBD on intravertebral disc degeneration showed that direct intradiscal injection of 120 nmol of CBD, but not lower doses of 30 or 60 nmol CBD, immediately after disc lesion significantly attenuated the extent of disc injury and the beneficial effect was maintained up to 15 days' post-injuryReference 898.
Clinical studies
There are no published clinical studies of cannabis for OA. In humans, one study found that the levels of the endocannabinoids anandamide and 2-AG in the synovial fluid of patients with OA were increased compared to non-inflamed normal controls, although the significance of these findings remains unclearReference 42.
One multi-centre, randomized, double-blind, double-dummy, placebo- and active-controlled crossover clinical trial of a FAAH inhibitor reported a lack of analgesic activity (Western Ontario and McMaster Universities pain score) in patients with OA of the kneeReference 899. In contrast, administration of naproxen in the study was associated with significant analgesia. Importantly, this clinical trial raised serious questions about the translatability of findings from animal studies to those conducted with humans since the FAAH inhibitor had shown efficacy in the animal model but not in humans. In addition, other issues of concern include the testing of the FAAH inhibitor on a heterogeneous population of OA patients and off-target effects (e.g. at TRPV1).
4.8.2 Rheumatoid arthritis
RA is a destructive, systemic, auto-immune inflammatory disease that affects a smaller, but not insignificant, proportion of the adult populationReference 886. It is characterized by chronic inflammatory infiltration of the synovium leading to progressive synovitis, and eventual cartilage and joint destruction, functional disability, significant pain, and systemic complications (e.g. cardiovascular, pulmonary, psychological, and skeletal disorders such as osteoporosis)Reference 879Reference 900Reference 901. As with OA, the ECS plays an important role in the pathophysiology of the disorder and manipulation of the ECS holds therapeutic promise.
Pre-clinical studies
A pre-clinical study in a rat model of RA reported that treatment with either THC or anandamide was associated with significant anti-nociception in the paw-pressure testReference 382. Another study in two different mouse models of RA (acute and chronic) reported that systemic administration (i.p.) of a range of doses of CBD (2.5 mg/kg, 5 mg/kg, 10 mg/kg, 20 mg/kg per day), after onset of acute arthritic symptoms, for a period of 10 days, was associated with the cessation of the progression of such symptomsReference 902. The daily 5 mg/kg i.p. dose was deemed to be the optimal dose for both acute (10 days) and chronic models (5-weeks) of arthritis. No obvious side effects were noted at any of the tested doses. Oral administration of 25 mg/kg of CBD for 10 days after onset of acute arthritic symptoms was associated with suppression of the progression of these symptoms, although the 50 mg/kg daily oral dose was almost equally effective. The 25 mg/kg daily oral dose was also effective in suppressing the progression of chronic arthritic symptoms when administered over a five-week period. Protective effects associated with exposure to CBD included the prevention of additional histological damage to arthritic hind-paw joints, suppression of TNF release from arthritic synovial cells, attenuation of lymph node cell proliferation, suppression of production of reactive oxygen intermediates and attenuation of lymphocyte proliferation.
The results from a study examining the anti-nociceptive effects of THC in a rat model of RA suggested that intraperitoneal administration of 4 mg/kg THC was associated with a significant decrease in the levels of spinal dynorphin, an increase in kappa-opioid receptor-mediated analgesia, and a decrease in NMDA-receptor-mediated hyperalgesiaReference 903. Another study by the same group and using the same animal model demonstrated that THC was equipotent and equiefficacious to morphine with regard to anti-nociception in the paw-pressure test, and that there was a synergistic anti-nociceptive interaction between THC and morphine in both arthritic and non-arthritic rats in the same paw-pressure testReference 384. A follow-up study again using the same animal model suggested an important role for the CB2 receptor in modulating the anti-nociceptive effects of THCReference 904.
Indeed, a number of additional studies have continued to support an important role for the CB2 receptor in RAReference 874Reference 879Reference 905. Tissue samples taken from human rheumatoid joints showed increased CB2 receptor expression compared to osteoarthritic joints, with expression of the CB2 receptor localized to the lining layer and interstitial sub-lining layer as well as follicle-like aggregatesReference 879Reference 905. Furthermore, CB2 receptor activation on fibroblast-like synoviocytes derived from rheumatoid joints was associated with inhibition of the production of a variety of inflammatory mediators seen in RA including IL-6, matrix metalloproteinase (MMP)-3, MMP-13, and chemokine (C-C motif) ligand (CCL) 2Reference 879Reference 905. CB2 receptor activation was also associated with dose-dependent amelioration of arthritis severity in a mouse model of RAReference 905. Selective stimulation of the CB2 receptor significantly decreased joint swelling, synovial inflammation, and joint destruction, as well as serum levels of anti-collagen II antibodies in a mouse model of RAReference 874. However, others have reported that stimulation of joint CB2 receptors causes synovial hyperaemia through a mechanism involving TRPV1 ion channelsReference 906. The vasodilator effect of these CB2 receptor agonists is attenuated in models of acute and chronic arthritis suggesting that CB2 receptors are downregulated in inflamed joints.
A recent pre-clinical study examined the efficacy of transdermal CBD for the reduction of inflammation and pain in a rat model of RAReference 907. In this study, gel preparations containing increasing doses of CBD (0.6, 3.1, 6.2, 62.3 mg/day) were applied to the dorsal skin surface for four consecutive days after induction of rheumatoid-like arthritis. Transdermal absorption resulted in dose-dependent increases in plasma concentrations of CBD. Four consecutive days of application resulted in mean plasma concentrations of 3.8 ng/mL, 17.5 ng/mL, 33.3 ng/mL, and 1 629.9 ng/mL, respectively. The three lower doses exhibited linear pharmacokinetic correlations, but not the highest dose. Furthermore, the 6.2 mg and the 62.3 mg gel doses of CBD significantly reduced joint swelling, limb posture scores as a rating of spontaneous pain, immune cell infiltration and thickening of the synovial membrane. The 6.2 mg dose of CBD optimally reduced swelling and synovial membrane thickness. CBD treatment was not associated with changes in exploratory behaviour suggesting the lack of psychoactive effects.
Clinical studies
In humans, one study found that the levels of the endocannabinoids anandamide and 2-AG in the synovial fluid of patients with RA were increased compared to non-inflamed normal controls, although the significance of these findings remains unclearReference 42.
There are no published clinical studies of cannabis for RA.
A preliminary clinical study assessing the effectiveness of nabiximols (Sativex®) for pain caused by RA reported a modest but statistically significant analgesic effect on movement and at rest, as well as improvement in quality of sleepReference 383. Administration of nabiximols was well tolerated and no significant toxicity was observed. The mean daily dose in the final treatment week was 5.4 pump actuations (equivalent to 14.6 mg THC and 13.5 mg CBD/day, treatment duration was three weeks). The differences observed were small and variable across the participants.
A Cochrane Collaboration review conducted in 2012 concluded that the evidence in support of the use of oro-mucosal cannabis (e.g. nabiximols) for the treatment of pain associated with RA is weak and given the significant side effect profile typically associated with the use of cannabinoids, the potential harms seem to outweigh any modest benefits achievedReference 900.
4.8.3 Fibromyalgia
Fibromyalgia is a disorder characterized by widespread pain (allodynia and hyperalgesia) and a constellation of other symptoms including sleep disorders, fatigue, and emotional or cognitive disturbancesReference 908. While the underlying pathophysiology of fibromyalgia remains unclear, disturbances in the recruitment or functioning of peripheral and central pain processing pathways and in the levels of several important neurotransmitters (serotonin, noradrenaline, dopamine, opioids, glutamate and substance P) have been noted in fibromyalgia patientsReference 909-Reference 912. Co-morbid depressive symptoms have also been associated with a more pronounced deficit in pain inhibition, as well as increased pain in fibromyalgia patientsReference 913.
Clinical studies with smoked or orally ingested cannabis
There are no clinical trials of smoked or ingested cannabis for the treatment of fibromyalgia. However, a cross-sectional survey of patients suffering from fibromyalgia found that the patients reported using cannabis (by smoking and/or eating) to alleviate pain, sleep disturbance, stiffness, mood disorders, anxiety, headaches, tiredness, morning tiredness, and digestive disturbances associated with fibromyalgiaReference 184. Subjects (mostly middle-aged women who did not respond to current treatment) reported statistically significant decreases in pain and stiffness, and statistically significant increases in relaxation and well-being 2 h after cannabis self-administration. Side effects included somnolence, dry mouth, sedation, dizziness, high, tachycardia, conjunctival irritation, and hypotension. The study suffered from a number of limitations including the study design, small sample size, variability in frequency and duration of cannabis use, and a biased subject population.
Clinical studies with prescription cannabinoid medications
There are relatively few properly controlled clinical studies examining the role of cannabinoids in the treatment of fibromyalgia. The available evidence is summarized below.
Dronabinol
A non-placebo controlled pilot study examining the effect of dronabinol monotherapy (2.5 - 15 mg Δ9-THC/day; with weekly increases of 2.5 mg Δ9-THC, up to a maximum of 15 mg THC/day) on experimentally-induced pain, axon reflex flare, and pain relief in patients suffering from fibromyalgia reported that a sub-population of such patients experienced significant pain relief (reduced pain perception) with 10 and 15 mg/day Δ9-THC, but no changes were observed in axon reflex flareReference 385. Touch-evoked allodynia and pinprick-induced hyperalgesia were also not significantly affected by Δ9-THC. Subjects who completed a three-month course of therapy (15 mg/day Δ9-THC) reported a > 50% decrease in pain. The study however suffered from low power due to the high rate of patient drop-out caused by intolerable side effects of the treatment.
A multi-center, retrospective study of patients suffering from fibromyalgia who were prescribed an average daily dose of 7.5 mg Δ9-THC, over an average treatment period of seven months, reported a significant decrease in pain score, a significant decrease in depression, and a reduction in the intake of concomitant pain-relief medications such as opioids, anti-depressants, anti-convulsants, and NSAIDs following treatment with Δ9-THCReference 386. It is important to note that the study had a number of considerable limitations (method of data collection, heterogeneous patient selection criteria, and high subject dropout rate) and as such, the results should be interpreted with caution.
Nabilone
A randomized, double-blind, placebo-controlled clinical trial of nabilone (1 mg b.i.d.) for the treatment of fibromyalgia showed statistically significant improvements in a subjective measure of pain relief and anxiety, as well as on scores on the fibromyalgia impact questionnaire, after four weeks of treatmentReference 596. However, no significant changes in the number of tender points or tender point pain thresholds were observed (note: the use of the "tender point" as a diagnostic criterion for fibromyalgia is no longer an absolute requirement)Reference 914. Patients were taking concomitant pain medications such as NSAIDs, opioids, anti-depressants, and muscle relaxants. Nabilone did not have any lasting benefit in subjects when treatment was discontinued.
A two-week randomized, double-blind, active-control, crossover clinical study of 29 patients suffering from fibromyalgia reported that nabilone (0.5 - 1.0 mg before bedtime) improved sleep in this patient populationReference 597.
The Canadian Clinical Guidelines for the Diagnosis and Management of Fibromyalgia Syndrome (endorsed by the Canadian Pain Society and the Canadian Rheumatology Association) indicate that with regards to possible treatments, a trial of a prescribed pharmacologic cannabinoid may be considered in a patient with fibromyalgia, particularly in the setting of important sleep disturbance (this recommendation was based on Level 3, Grade C evidence)Reference 838. For additional information regarding the use of cannabis/cannabinoids to alleviate sleep disorders or disturbances, please consult Section 4.9.5.2.
A Cochrane systematic review of the available evidence on the efficacy, safety and tolerability of cannabis products from randomized, double-blind, clinical trials of at least four week's duration for the treatment of fibromyalgia in adults reported that 1 mg nabilone at bedtime was not associated with high to moderate quality evidence for an outcome of efficacy (participant-reported pain relief of > 50%, and Patient Global Impression of Change much or very much improved), tolerability (withdrawal due to adverse events), and safety (serious adverse events)Reference 915. Low quality evidence was found for nabilone over placebo in pain relief and health-related quality of life, but not in fatigue, and nabilone over amitriptyline in improving sleep quality but not for pain and health-related quality of life. Non-serious adverse events associated with nabilone use included dizziness/drowsiness, dry mouth and vertigo and the incidence of non-serious adverse events with nabilone was higher compared with placebo or amitriptyline.
4.8.4 Muscular pain
Muscular pain affects a large share of the population and is a major clinical problemReference 916Reference 917. Findings from pre-clinical studies using two animal models of acute muscle pain suggest that both systemic (0.3 - 5 mg/kg i.p.) and local administration (0.0125 - 0.1 mg/kg i.m.) of THC is associated with a dose-dependent reduction in frequency of paw shaking and a reduction in time spent in nocifensive behaviour following a noxious muscular stimulusReference 916. Differences in the types of cannabinoid receptors engaged were observed according to the route of administration: systemic administration of THC was associated with engagement of CB1 and/or CB2 receptors, while local administration of THC in the paw was predominantly associated with engagement of CB2 receptorsReference 916. No human experimental or clinical studies exist with cannabinoids for muscular pain.
4.8.5 Osteoporosis
Osteoporosis is a disease characterized by reduced bone mineral density and an increased risk of fragility fracturesReference 918. It occurs when the normal cycle of bone remodelling is perturbed, leading to a net decrease in bone deposition and a net increase in bone resorptionReference 919.
Pre-clinical studies
CB1 and CB2 receptors have been detected in mouse osteoblasts and osteoclasts, although CB1 is expressed at very low levels compared to CB2Reference 20Reference 920Reference 921. In fact, it appears that CB1 receptors are expressed more abundantly in skeletal sympathetic nerve terminals in close proximity to osteoblastsReference 922. Besides the receptors, the endocannabinoids 2-AG and anandamide have been detected in mouse trabecular bone and in cultures of mouse osteoblasts and human osteoclastsReference 921Reference 923Reference 924. Taken together, these findings suggest the existence of a functional ECS in bone.
The role of the ECS in bone physiology has been investigated using mice carrying genetic deletions of either the CNR1 or CNR2 genes. The skeletal phenotypes of CB1 receptor knockout mice appear to vary depending on the gene targeting strategy used, the mouse strain, gender, time points at which the phenotypes were assessed, and the different experimental methodologies used to measure bone densityReference 20. In one CB1-deficient mouse strain, young female mice had normal trabecular bone with slight cortical expansion whereas young male mice had high bone massReference 920Reference 922. Loss of CB1 receptor function was associated with protection from ovariectomy-induced bone lossReference 920. In addition, antagonism of CB1 and CB2 receptors prevented ovariectomy-induced bone loss in vivoReference 920.
A subsequent study by the same group reported that CB1 knockout mice had increased peak bone mass but eventually developed age-related osteoporosisReference 918. The increased peak bone mass was attributed to a reduction in osteoclast formation and activity, with preservation of normal osteoblast activity. In contrast, age-related bone loss in the knockout mice appeared to be caused by preferential formation and accumulation of adipocytes at the expense of osteoblasts within the bone-marrow space, as well as decreased bone formationReference 918. In contrast to these studies, another study using a different gene targeting strategy and mouse strain reported that both male and female CB1 knockout mice exhibited low bone mass, increased numbers of osteoclasts, and a decrease in the rate of bone formationReference 922. The effects of ovariectomy in this mouse line were not examined, most likely because the baseline bone mass was too small to properly measure differences between mice subjected to ovariectomy and controls.
Another pre-clinical study in younger and older rats reported that blockade of CB1 receptor activity, by administration of rimonabant, had differential effects on glucocorticoid-induced cortical bone thickness and mean trabecular bone densityReference 925. In young rats, rimonabant attenuated the osteoporotic effects of chronic glucocorticoid treatment whereas in older rats, the opposite effect was noted. Furthermore, the findings from this study further support the idea that the CB1 receptor plays an age-related differential role in bone turnover processes.
In mice, activation of CB1 receptors by THC has been shown to significantly slow bone elongation and possibly overall body size, at least in female adolescent miceReference 926. The concentration of systemic THC administered in the mice (5 mg/kg/day) was reported to be similar to that described for human daily cannabis smokers.
A pre-clinical study in rats measuring the impact of cannabis smoke on bone healing around titanium implants reported that chronic exposure to cannabis smoke reduced cancellous bone healing around the implants by reducing bone filling and bone-to-implant contact inside the implant threadsReference 388. No such effect was observed for cortical bone.
The skeletal phenotypes of CB2 receptor knockout mice have also been investigated. Ofek reported that CB2-deficient mice display a low bone mass phenotype as well as age-related trabecular bone lossReference 927. These deficits were associated with increased numbers of osteoclasts and decreased numbers of osteoblast precursors. Furthermore, a selective CB2 receptor agonist was reported to increase osteoblast proliferation and activity and to decrease the formation of osteoclast-like cells in vitro, and administration of this agonist attenuated ovariectomy-induced bone loss in vivoReference 927. While a more recent study supported the finding of age-related bone loss, it failed to find any significant differences in peak bone mass between wild-type and knockout miceReference 928. Furthermore, in contrast to the study by OfekReference 927, selective stimulation of the CB2 receptor was associated with an increase in osteoblast differentiation and function rather than proliferation. Another study reported no differences in peak bone mass between CB2 receptor knockout mice and wild-type mice under normal conditionsReference 929. Age-related bone loss was not measured in this study. Genetic ablation of the CB2 receptor appeared to protect against ovariectomy-induced bone loss, an effect mimicked by administration of a CB2-selective antagonistReference 929. Conversely, results from in vitro studies suggested that CB2-selective agonists significantly increased osteoclast formation and osteoclast sizeReference 929. It may be relevant to note here that single nucleotide polymorphisms (SNPs) and SNP haplotypes located in the coding region of the CB2 receptor gene have also been associated with osteoporosis in humansReference 930-Reference 932.
4.9 Other diseases and symptoms
4.9.1 Movement disorders
The individual components of the ECS are particularly abundant in areas of the brain that control movement, such as the basal gangliaReference 933. Motor effects generally arise as a consequence of changes in ECS activity, with activation of the CB1 receptor typically resulting in inhibition of movementReference 933. A number of studies have reported changes in CB1 receptor levels and CB1 receptor activity in motor diseases such as Parkinson's disease (PD) and Huntington's disease (HD)Reference 934-Reference 937, and the findings from such studies suggest a complex link between the ECS and the pathophysiology of these and other neurological diseases.
A systematic review of the efficacy and safety of cannabinoids in movement disorders such as HD, PD, cervical dystonia and TS suggests that cannabinoids are either probably ineffective or of unknown efficacy and that the risks and benefits of cannabinoid treatment should be carefully weighedReference 671. In addition, comparative efficacy of cannabinoid vs. other therapies is unknown for these indicationsReference 671.
4.9.1.1 Dystonia
- Evidence from limited pre-clinical studies suggests that a synthetic CB1 and CB2 receptor agonist may alleviate dystonia-like symptoms, and CBD delays dystonia progression.
- Evidence from a limited number of case studies and small placebo-controlled or open-label clinical trials suggests improvement in symptoms of dystonia with inhaled cannabis, mixed effects of oral THC, improvement in symptoms of dystonia with oral CBD, and lack of effect of nabilone on symptoms of dystonia.
Dystonia involves overactivity of muscles required for normal movement, with extra force or activation of nearby but unnecessary muscles, and is often painful in addition to interfering with functionReference 938. Dystonia can be primary, including torticollis and blepharospasm/orofacial dyskinesias or dystonias (Meige syndrome) or part of another condition such as HD, and tardive dyskinesia after dopa-blocking drugsReference 938.
Pre-clinical data
A pre-clinical study in a hamster model of primary generalized dystonia reported a dose-dependent decrease in disease severity with administration of the synthetic CB1 and CB2 receptor agonist WIN 55,212-2Reference 939. However, anti-dystonic doses of the agonist were associated with severe side effects including depression of spontaneous locomotor activity and catalepsy. In addition, this CB receptor agonist increased the anti-dystonic effect of diazepamReference 939. A follow-up study by the same group confirmed the anti-dystonic efficacy of WIN 55,212-2 and also showed that CBD delayed the progression of dystonia, but only at a very high doseReference 940. A pre-clinical study of anti-psychotic-induced acute dystonia and tardive dyskinesia in monkeys showed that oral dyskinesia, but not dystonia, was dose-dependently reduced by the synthetic CB1 receptor agonist CP 55,940Reference 941.
Clinical data
While anecdotal reports suggest cannabis may alleviate symptoms associated with dystonia in humansReference 248, no properly controlled clinical studies of cannabis to treat dystonia have been published.
One case-study reported improvement in torticollis after smoking cannabisReference 942. Another case study reported improvement in a patient with central thalamic pain and right hemiplegic painful dystonia who smoked one joint in the morning once per week for three weeksReference 943. Following smoking, the patient reported complete pain relief and relief of paresthesia and marked improvement in dystonia with improved ability to write and take a few steps without support. Pain relief appeared to persist for up to 48 h after each episode of cannabis smoking. No tolerance to the effects of cannabis was noted and the patient discontinued opioid analgesic therapy. Another case report of a 25-year-old patient using cannabis for generalized dystonia secondary to Wilson's disease reported that smoking 3 or 4 g of cannabis per day was associated with significant improvement in his dystoniaReference 248. Physician observation supported the patient's claims: cannabis decreased the score on the Burke-Fahn-Marsden dystonia rating scale and the disability scale by 50% each. Therapeutic effects did not appear to persist beyond each 24 h period, requiring the patient to administer cannabis daily.
A placebo-controlled, single-dose trial with 5 mg of Δ9-THC administered orally to a musician with focal dystonia ("Musician's Dystonia") reported an improvement in motor control in the subject's affected hand, with tiredness and poor concentration cited as side effects associated with the use of Δ9-THCReference 250. The therapeutic effect persisted until 2 h after intake, with a progressive return to baseline values after 5 h.
An eight-week, phase IIa, cross-over, randomized, placebo-controlled trial of dronabinol (15 mg/day) in nine patients with cervical dystonia reported a lack of effect of dronabinol compared to placebo on any outcome measure (Toronto Western Spasmodic Torticollis Rating Scale - TWSTRS, VAS of pain, global impression of change)Reference 244. Most subjects experienced an adverse event, none of which was deemed serious. Adverse events with dronabinol included light-headedness, sleepiness, dry mouth, blurred vision, bitter-taste and vertigo, and were deemed mild.
Another case-study reported that dronabinol (2.5 mg, b.i.d. initially, then 5 mg, b.i.d.) was associated with improvement in dystonia in a patient with MS, paroxysmal dystonia, complex vocal tics, and cannabis dependence (minimum daily consumption of five cannabis joints) and who had previously reported symptom improvement after smoking cannabisReference 247. The patient also reported a significant reduction in cannabis craving, an improvement in quality of sleep, decreased vocalizations, decreased anxiety and decreased frequency of paroxysmal dystonia with dronabinol.
A six-week, open-label, pilot trial of five patients taking 100 to 600 mg/day of CBD reported modest dose-related improvements in dystonic movements in all study subjects, but a worsening of tremor and hypokinesia in two patients with co-existing PD administered doses of CBD > 300 mg/dayReference 261. Side effects of CBD were mild and included hypotension, dry mouth, psychomotor slowing, light-headedness, and sedation.
Results of a double-blind, randomized, placebo-controlled study of 15 patients taking a single 0.03 mg/kg dose of nabilone and not taking any other anti-dystonia medication showed no significant reduction in dystoniaReference 253.
4.9.1.2 Huntington's disease
- Evidence from pre-clinical studies reports mixed results with THC on Huntington's disease (HD)-like symptoms.
- Limited evidence from case studies and small clinical trials is mixed and suggests a lack of effect with CBD, nabilone and nabiximols, and a limited improvement in HD symptoms with smoked cannabis.
Pre-clinical and human experimental data
Results from studies carried out in animal models of HD as well as post-mortem studies carried out in deceased HD patients suggest that brain CB1 receptors, especially those found in the basal ganglia, are downregulated and/or desensitized as a result of the expression of the mutant Huntingtin protein, and that this occurs early in the course of the disease and prior to the appearance of overt clinical symptomsReference 934Reference 944-Reference 953. In vivo positron emission topography (PET) study of HD patients supports these findings, demonstrating profound decreases in CB1 receptor availability throughout the gray matter of the cerebrum, cerebellum, and brainstem of HD patients even in early stages of the diseaseReference 954. Additional pre-clinical and post-mortem studies in deceased HD patients indicate that the decrease in CB1 receptor levels appears to be accompanied by an increase in CB2 receptor levels in glial elements, astrocytes, and in reactive microglial cellsReference 949Reference 955. Thus, a significant amount of pre-clinical evidence and some limited clinical evidence suggests that changes in the ECS are tightly linked to the pathophysiology of HDReference 949Reference 952-Reference 954.
One pre-clinical study in a mouse model of HD reported no beneficial effects of Δ9-THC (10 mg/kg/day)Reference 956, while another study reported that Δ9-THC (2 mg/kg/day) was associated with decreased pathology and delayed onset of HD-like symptoms compared to untreated HD miceReference 951. Another pre-clinical animal study in a rat model of HD showed that CB2 receptor activation was associated with reduction in inflammatory markers associated with an HD-like phenotype and protection of striatal projection neuronsReference 957. A pre-clinical study has also reported that a restricted population of CB1 receptors selectively located on glutamatergic terminals in corticostriatal projections may play a potentially protective role in attenuating excitotoxic damage associated with excessive glutamate release in HD, raising the possibility that selective targeting of this receptor population may help attenuate neurodegeneration in patients with HDReference 958.
Clinical data
The results from single-patient case studies are mixed. In one study, daily doses of 1.5 mg nabilone increased choreatic movementsReference 256, while in another case improved mood and decreased chorea were noted in a patient who had smoked cannabis and who then continued on 1 mg nabilone b.i.d.Reference 959.
With regard to clinical studies, one double-blind, placebo-controlled, 15-week, crossover trial of 15 patients with HD taking 10 mg/kg/day of oral CBD did not report improvement in symptoms associated with HDReference 258. A randomized, double-blind, placebo-controlled, crossover pilot study found little or no beneficial effect of 1 or 2 mg nabilone over placebo in 37 patients with HDReference 245. However, nabilone was well tolerated in this patient population and did not appear to exacerbate chorea or HD-associated psychosis, although some adverse effects such as drowsiness and forgetfulness were noted. Patients were concomitantly taking other HD medications.
A more recently published 12-week, double-blind, randomized, placebo-controlled, cross-over, pilot trial examining the safety and tolerability of nabiximols in HD reported no significant differences on motor, cognitive, behavioural or functional outcomes associated with the use of nabiximols compared to placebo in 26 HD patients with the exception of an increased incidence of dizziness and reduced attention in the treatment groupReference 241. Limitations of the study include lack of power to determine if nabiximols is effective and safe in the long-term or if tested in larger populations. In addition, the authors suggest that the observed lack in efficacy may have been explained, at least in part, by treatment during the later stage of HD and that treatment at an earlier stage should be explored in future clinical studies.
4.9.1.3 Parkinson's disease
- The evidence from a limited number of pre-clinical, case, clinical and observational studies of certain cannabinoids for symptoms of Parkinson's disease (PD) is mixed.
- One case study of smoked cannabis suggests no effect while an observational study of smoked cannabis suggests improvement in symptoms.
- One small clinical study of nabilone suggests improvement in symptoms, while another clinical study of an oral cannabis extract (THC/CBD) and a clinical study with CBD suggest no improvement in symptoms.
A patient survey distributed among 630 patients attending a movement disorders clinic reported that out of the 339 respondents, 25% had used cannabis with 31% reporting benefit in rest tremor, 45% in bradykinesia, and 14% in dyskinesiaReference 960.
Pre-clinical and human experimental data
Endocannabinoid ligands, their synthesizing and degrading enzymes, and cannabinoid-activated receptors are highly abundant in the basal ganglia, the brain structures primarily affected in PDReference 933. Newly diagnosed PD patients and those undergoing PD medication washout were reported to have more than double the level of anandamide in their CSF compared to controls, and these results parallel those seen in animal models of PD where dopamine cell loss is accompanied by elevations in anandamide levelsReference 961. In animal models of PD, the levels of CB1 receptors appear to be downregulated during the early, pre-symptomatic stages of the disease, but during the intermediate and advanced phases of the disease there is an increase in CB1 receptor density and function and an increase in endocannabinoid levelsReference 961Reference 962. Together, these studies suggest a complex link between the pathophysiology of PD and changes in the ECS.
Results from some animal studies suggest cannabinoid receptor agonists induce hypokinesia and thus are reported to be unlikely as suitable first-line treatments for PDReference 933Reference 963. On the other hand, cannabinoid-induced hypokinesia could be useful in attenuating the dyskinesia observed in PD patients on long-term levodopa treatmentReference 963. Other animal studies suggest CB1 receptor antagonism (via treatment with rimonabant) partially attenuates hypokinesia associated with nigral cell death and promotes dopaminergic neuron survival in the substantia nigra pars compacta through an increase in astrocyte cell densityReference 964Reference 965. However, this beneficial effect of CB1 receptor antagonism could not be replicated in a small clinical studyReference 251. Given the current level of evidence for cannabinoids in the treatment of PD, it would appear that cannabinoid-based neuroprotective therapy for PD would need to be based on an adequate combination of selected compounds that confer antioxidant effects (e.g. through CB-receptor independent mechanisms) such as through activation of the nuclear PPAR receptor family, CB2 receptor activation and control of inflammation, and CB1 receptor antagonism to improve akinesia and reduce motor inhibitionReference 966. Combining a cannabinoid with anti-inflammatory and anti-oxidant properties (CBD) with a cannabinoid having mixed CB1 antagonist/CB2 agonist properties as well as anti-oxidant effects (THCV) may possibly hold some therapeutic potential, but much further research is requiredReference 966.
Clinical data
The results of clinical trials examining the role of cannabinoids (smoked cannabis, nabilone, CBD, rimonabant and a standardized oral cannabis extract) in the treatment of PD are mixed.
One case study involving five patients suffering from idiopathic PD found no improvement in tremor after a single episode of smoking cannabis (1 g cigarette containing 2.9% Δ9-THC, 29 mg total available Δ9-THC), whereas all subjects benefited from the administration of levodopa and apomorphineReference 259.
An open-label, observational study evaluated the clinical effect of smoked cannabis on motor and non-motor symptoms in 22 patients with PD who were using cannabis daily for at least two months with no major side effectsReference 242. Patients were asked to smoke their regular dose of cannabis (500 mg) and 30 min later, the motor and non-motor test batteries were administered and scores recorded by two clinicians. The mean total score on the motor Unified Parkinson's Disease Rating Scale (UPDRS) score improved significantly after cannabis exposure, from a score of 33 at baseline to a score of 23 after cannabis consumption (p < 0.001). Significant improvement was also noted in tremor, rigidity, bradykinesia, sleep and pain but none on posture. All patients were concomitantly taking other PD medications including levodopa, amantadine, rasagiline, selegiline, acetylcholinesterase inhibitor, and others. No serious adverse events were noted. Main self-reported adverse effects of long-term cannabis smoking were somnolence, drowsiness, palpitations, and bad taste. Study limitations included open-label design and short study period.
An exploratory, randomized, double-blind, placebo-controlled clinical study of antagonists to the neurokinin B, neurotensin and CB1 receptor (rimonabant) on the severity of motor symptoms and levodopa-induced dyskinesias after a single dose of levodopa in 24 patients with PD showed that at the dose used, all three drugs were well tolerated and could not improve Parkinsonian motor disabilityReference 251. Doses for neurokinin B, neurotensin and CB1 receptor antagonists were 180 mg, 200 mg, and 20 mg respectively. Each drug was administered once daily, 1 h before the administration of levodopa for 9 (neurokinin, neurotensin B) or 16 days (rimonabant).
A small randomized clinical trial of nabilone (0.03 mg/kg) in seven patients with PD found that nabilone reduced levodopa-induced dyskinesiaReference 254.
In contrast, a four-week, randomized, double-blind, crossover study demonstrated that an oral cannabis extract (2.5 mg Δ9-THC and 1.25 mg CBD per capsule, b.i.d.; maximum daily dose 0.25 mg/kg Δ9-THC) did not produce any pro- or anti-parkinsonian actionReference 249.
Lastly, an exploratory double-blind clinical trial of 21 patients with PD (without dementia or comorbid psychiatric conditions) assessed the motor and general symptoms score (UPDRS), functioning/well-being and QoL (39-item Parkinson Disease Questionnaire, PDQ-39) and possible neuroprotective effects (plasma brain-derived neurotrophic factor (BDNF) and proton magnetic resonance spectroscopy, H1-MRS) following treatment with placebo or CBD (75 mg or 300 mg/day) for six weeksReference 243. No statistically significant differences were observed between placebo and all CBD doses for UPDRS scores, plasma BDNF levels or H1-MRS measures. However, the 300 mg CBD dose was associated with a statistically significant difference in mean total scores from placebo in the PDQ-39 suggesting that the 300 mg daily CBD dose is associated with an improvement in QoL measures in PD patients with no psychiatric comorbidities.
4.9.1.4 Tourette's syndrome
- The limited evidence from small clinical studies suggests that oral THC improves certain symptoms of Tourette's syndrome (TS) (tics).
Anecdotal and case-reports have suggested amelioration of symptoms associated with TS when smoking cannabisReference 257Reference 260. In addition, a two-day, randomized, double-blind, placebo-controlled, crossover trial of single oral doses of Δ9-THC (5, 7.5, or 10 mg) in 12 adult patients with TS showed plasma concentration-related improvements in control of motor and vocal tics and obsessive-compulsive behaviour, with no serious side effects; although transient, mild side effects (e.g. headache, nausea, ataxia, fatigue, anxiety) were noted in five patientsReference 255. In contrast to healthy cannabis users, neither a 5 mg nor a 10 mg dose of Δ9-THC caused cognitive impairment in patients with TS. This study was followed up by a six-week, randomized, double-blind, placebo-controlled trial by the same research group. The authors reported a significant difference in tic reduction compared to placebo in some patients, and no detrimental effects on neuropsychological performance during or after treatment with 10 mg doses of Δ9-THCReference 252. The major limitations of all three clinical studies were their small sample size and their relatively short duration.
A Cochrane Collaboration Review examining the efficacy and safety of cannabinoids in treating tics, premonitory urges, and obsessive compulsive symptoms in patients with TS concluded that there was insufficient evidence to support the use of cannabinoids in treating tics and obsessive compulsive behaviour in persons suffering from TSReference 246.
However, a more recent systematic review and meta-analysis of 28 RCTs (N = 2 454 participants) of cannabinoids (i.e. smoked cannabis, nabiximols, nabilone, dronabinol, CBD, THC, levonontradol, ajulemic acid) using the GRADE approach concluded that based on two small placebo-controlled studies of orally-administered THC in capsule form in the treatment of symptoms associated with TS, oral THC may be associated with significant improvement in tic severity in patients with TSReference 179.
4.9.1.5 Spinocerebellar ataxias
There is emerging evidence of a role for the ECS in the pathophysiology of spinocerebellar ataxiasReference 967Reference 968. Post-mortem studies of cerebellar samples collected from deceased patients with hereditary autosomal dominant ataxias revealed significant increases in the protein expression levels of FAAH and MAGL in the Purkinje cells in the cerebellar granular layer, in neurons of the dentate nucleus, and in cerebellar white matter compared to controlsReference 968. In another study, the protein expression levels of the CB1 and CB2 receptors in these same areas of the cerebellum were also found to be significantly increased compared to controlsReference 967. These studies suggest an increase in the expression levels of a number of components of the ECS in cerebellar areas associated with hereditary autosomal dominant ataxias.
4.9.2 Glaucoma
- The limited evidence from small clinical studies suggests oral administration of THC reduces intra-ocular pressure (IOP) while oral administration of CBD may, in contrast, cause an increase in IOP.
Glaucoma is a multi-factorial disease characterized by the progressive degeneration of the optic nerve and the death of retinal ganglion cells ultimately leading to irreversible blindnessReference 969. Increased IOP has been implicated in the pathophysiology of glaucoma; however, inadequate blood supply to the optic nerve, oxidative damage, and apoptosis of retinal ganglion cells are also contributing factorsReference 390Reference 969-Reference 971. An ECS exists in a number of ocular tissues, and post-mortem studies have detected decreased levels of endocannabinoids in such tissues taken from deceased glaucoma patientsReference 972.
Ocular (as well as systemic) administration of cannabinoids typically lowers IOP by up to 30% (seeReference 390 for a full reference list). How cannabinoids reduce IOP is unclear, but several possible mechanisms have been proposed including reduction of capillary pressure, decreased aqueous humour production, and improved aqueous humour uveoscleral outflow and outflow facilityReference 973-Reference 977.
Results from a survey carried out among 1 516 glaucoma patients at tertiary glaucoma clinics in Toronto and Montreal suggested that approximately 13% of these patients claimed they used complementary and alternative medicines to treat glaucoma, and from among these patients 2.3% reported using cannabis to treat their glaucomaReference 978.
A well-controlled pilot clinical study of six patients with ocular hypertension or early primary open-angle glaucoma reported that single sub-lingual doses of 5 mg Δ9-THC (applied by means of an oro-mucosal spray) significantly but temporarily reduced IOP 2 h after administrationReference 389. A single sub-lingual dose of 20 mg CBD (co-administered with approx. 1 mg Δ9-THC) had no effect, while a single sub-lingual dose of 40 mg of CBD (co-administered with ~ 2 mg Δ9-THC) caused a significant transient increase in IOP 4 h after administration. A non-randomized, unmasked, uncontrolled clinical study reported some improvement in IOP after oral ingestion of Δ9-THC (2.5 or 5 mg q.i.d., up to a maximum of 20 mg/day; treatment duration range: 3 - 36 weeks) in patients with end-stage, open-angle glaucoma not responsive to standard medications or surgeryReference 391. Some patients appeared to develop tolerance to the IOP-lowering effects of Δ9-THC, and almost half discontinued treatment due to Δ9-THC-associated side effects (e.g. dizziness, dry mouth, sleepiness, depression, confusion). Aside from lowering IOP, cannabinoids such as Δ9-THC (and CBD) may also have neuroprotective effects which could also be useful in the management of glaucomaReference 390Reference 979-Reference 988.
In conclusion, while smoking or eating cannabis (or oral Δ9-THC) has been reported to reduce IOPReference 989-Reference 991, cannabinoid-based therapy appears to be limited by the short duration of cannabinoid action (3 - 4 h) and unwanted physical and psychotropic effects.
4.9.3 Asthma
- The limited evidence from pre-clinical and clinical studies on the effect of aerosolized THC on asthmatic symptoms is mixed.
- Inhalation of lung irritants generated from smoking/vapourizing cannabis may worsen asthmatic symptoms.
There is some historical and anecdotal evidence for cannabis as a treatment for asthmaReference 992. In terms of pre-clinical data, there is some evidence suggesting a role for the ECS in regulating bronchial smooth muscle toneReference 993 and studies with animals using classical and synthetic cannabinoids suggest a possible role for cannabinoid-based compounds in the treatment of asthmaReference 994-Reference 996.
Early clinical studies demonstrated significant decreases in airway resistance and increases in specific airway conductance in healthy, habitual cannabis smokers shortly after smoking cannabisReference 997Reference 998. This effect has been largely attributed to the bronchodilatory properties of Δ9-THCReference 999. However, for asthmatics, the benefits of smoking cannabis are likely to be minimal. While smoking cannabis appears to decrease bronchospasm, increase bronchodilatation, and modestly improve respiratory function in some asthmatics in the short-termReference 1000-Reference 1002, cannabis smoke contains noxious gases and particulates that irritate and damage the respiratory systemReference 999; hence, it is likely not a viable long-term therapy for asthma. A number of studies have also reported hypersensitivity reactions, including asthmatic attacks in response to inhalation of cannabis smokeReference 365Reference 366.
Importantly, therefore, alternate methods of Δ9-THC delivery by aerosol or oral administration have been studied. Doses of 100 and 200 µg of aerosolized Δ9-THC significantly improved ventilatory function in asthmatics and were generally well toleratedReference 1003Reference 1004. In another study, 5 to 20 mg of aerosolized Δ9-THC rapidly and effectively increased airway conductance in healthy subjects, but caused either bronchodilatation or bronchoconstriction in asthmaticsReference 1005. Oral administration of 10 mg Δ9-THC or 2 mg nabilone did not produce clinically significant bronchodilatation in patients with reversible airways obstructionReference 992Reference 1006Reference 1007.
4.9.4 Hypertension
CB1 receptors are expressed on various peripheral tissues including the heart and vasculature, and CB receptor agonists and endocannabinoids decrease arterial blood pressure and cardiac contractility (reviewed inReference 1008).
There are very few studies on the effects of cannabis or cannabinoids on hypertension. In one early study, inhalation of cannabis smoke from cigarettes containing 2.8% Δ9-THC caused a greater and longer-lasting decrease of arterial blood pressure in hypertensive subjects compared to normotensivesReference 1009. In one case-report, a woman with longstanding idiopathic intra-cranial hypertension reported improvement in her symptoms after smoking cannabis or after treatment with dronabinol (10 mg b.i.d. initially, then 5 mg b.i.d.).
There are no reports on the use of low-dose cannabinoids as supplementary therapy in hypertension.
4.9.5 Stress and psychiatric disorders
There are anecdotal and, in some cases, historical claims regarding the beneficial effects of cannabis and cannabinoids in the treatment of a variety of psychiatric disorders including anxiety, depression, sleep disorders, PTSD, and withdrawal symptoms associated with drug abuse/addiction. The following sections provide information gathered from the scientific and medical literature regarding the use of cannabis and cannabinoids in the treatment of such disorders.
The endocannabinoid system, stress and psychiatric disorders
Increasing evidence suggests an important role for the ECS in the regulation of stress, mood, and psychiatric disordersReference 167Reference 1010Reference 1011. Pharmacological or genetic disruption of endocannabinoid signaling in animals produces a neurobehavioural response that mimics the classical stress response including activation of the hypothalamic-pituitary-adrenal (HPA) axis, increased anxiety, suppressed feeding behaviour, reduced responsiveness to rewarding stimuli, hypervigilance and arousal, enhanced grooming behaviour and impaired cognitive flexibilityReference 167.
In animal models of acute stress, exposure to a variety of acute psychological stressors generally causes a rapid reduction in brain levels of anandamide which is accompanied by a number of behavioural and physiological responses including an increase in anxiety, increased activity of the HPA axis, a decrease in neurogenesis, decreased ability to extinguish fearful memories, and anhedonia, all of which are also hallmarks of mood disordersReference 167Reference 1011. Chronic stress also generally appears to produce reductions in anandamide similar to those seen with acute stressReference 167. However, in contrast to the situation with anandamide, acute and chronic stress cause a protracted increase in brain 2-AG levels that is preceded by increases in corticosterone resulting from increased HPA axis activityReference 167. Furthermore, elevations in brain 2-AG levels are associated with HPA axis response termination, HPA axis habituation, modulation of synaptic plasticity, decreased memory retrieval and a decrease in painReference 167. The ECS therefore appears to be both a target and a regulator of stress-induced activation of the HPA axisReference 167.
Endocannabinoids appear to reduce behavioural signs of anxiety, especially under stressful, aversive or otherwise challenging conditionsReference 167. Elevation of both 2-AG and anandamide signaling attenuates stress-induced anxiety, though apparently through different mechanismsReference 167Reference 1010. There is also increasing evidence pointing to a role for the ECS in facilitating the extinction of emotionally aversive memoriesReference 167Reference 1010. In humans, experimental studies employing pharmacological means of disrupting endocannabinoid signaling through the use of the CB1 receptor antagonist/inverse agonist rimonabant suggest that impairments in endocannabinoid signaling result in increased sensitivity to the effects of stress including anxiety and anhedoniaReference 167Reference 1010. Both depression and PTSD have been associated with reduced levels of circulating endocannabinoidsReference 167Reference 1010.
Taken together, the weight of the evidence suggests that the ECS functions as a homeostatic mechanism for buffering stress, inhibiting unnecessary HPA axis activation and promoting the recovery of the HPA axis once the stressful stimulus has passedReference 1010Reference 1011. Dysfunction of the ECS both increases sensitivity to stress and prolongs maladaptive responses to stress in the absence of any further stress stimulusReference 1010Reference 1011. Importantly, chronic stress appears to reduce the ability of the ECS to buffer stress effectively and can contribute to precipitation of psychopathology including anxiety and depressionReference 1010Reference 1011. Pharmacological interventions that function to raise endocannabinoid tone such as inhibition of the endocannabinoid degradative enzymes FAAH and MAGL appear to have anxiolytic and anti-depressive effects, at least in animal models of anxiety and depressionReference 167Reference 177Reference 1011. Emerging evidence suggests substrate-selective inhibition of COX-2 also increases brain endocannabinoid levels and may have anxiolytic effectsReference 167Reference 1012Reference 1013.
4.9.5.1 Anxiety and depression
- Evidence from pre-clinical and clinical studies suggests that THC exhibits biphasic effects on mood, with low doses of THC having anxiolytic and mood-elevating effects and high doses of THC having anxiogenic and mood-lowering effects.
- Limited evidence from a small number of clinical studies of THC-containing cannabis/certain prescription cannabinoids suggests that these drugs could improve symptoms of anxiety and depression in patients suffering from anxiety and/or depression secondary to certain chronic diseases (e.g. patients with HIV/AIDS, MS, and chronic neuropathic pain).
- Evidence from pre-clinical studies suggests that CBD exhibits anxiolytic effects in various animal models of anxiety, while limited evidence from clinical studies suggest CBD may have anxiolytic effects in an experimental model of social anxiety.
- Limited evidence from some observational studies also suggests that cannabis containing equal proportions of CBD and THC is associated with an attenuation of some perturbations in mood (anxiety/dejection) seen with THC-predominant cannabis in patients using cannabis for medical purposes.
As mentioned above, cannabis consumption, especially cannabis containing mainly THC, appears to dose-dependently affect anxiety behaviours, with low doses (of THC) being potentially anxiolytic and high doses (of THC) either ineffective or potentially anxiogenicReference 177. While acute consumption of higher doses of THC-predominant cannabis can, in some individuals and in certain novel or stressful environments, trigger significant anxiety which can resemble a panic attack, long-term cannabis users report reductions in anxiety, increased relaxation, and relief from tensionReference 191. One survey conducted among over 4 400 respondents suggested that those who consumed cannabis daily or weekly reported a decrease in depressed mood, and an increase in positive affect, compared to respondents who claimed they never consumed cannabisReference 1014. However, the study suffered from a number of serious drawbacks and should be interpreted with caution. Other epidemiological studies suggest the oppositeReference 1015Reference 1016. Daily users may also report anxiety reduction that may actually be relief of withdrawal symptoms associated with CUD. Furthermore, social anxiety disorder appears particularly related to CUD and according to at least one study, some people with social anxiety may come to rely on cannabis to help them cope in social situations, continuing to use cannabis despite experiencing negative consequences related to its use and thereby developing CUDReference 1017.
Pre-clinical studies
Pre-clinical (and clinical) evidence indicates important roles for the ECS in both anxiety and mood disorders. Results from animal studies suggest low doses of CB1 receptor agonists reduce anxiety-like behaviour and increase anti-depressant-like responsesReference 1018Reference 1019. CB1 receptor agonists appear to enhance central serotonergic and noradrenergic neurotransmission similar to the actions of anti-depressant medicationsReference 1020Reference 1021. On the other hand, high-level stimulation of the CB1 receptor, or administration of CB1 receptor antagonists, reverse this response and can also trigger depressive-like symptoms or depressionReference 189Reference 1020Reference 1022Reference 1023. Suppression of endocannabinoid signalling is sufficient to induce a depressive-like state both in animals and in humans (reviewed inReference 1024). Furthermore, basal serum concentrations of both anandamide and 2-AG have been found to be significantly reduced in women with major depressionReference 1025. These findings suggest proper endocannabinoid tone plays an important role in regulating mood.
Clinical and observational data for cannabis and THC
While the routine use of THC-predominant cannabis or prescription cannabinoid medications containing primarily THC (dronabinol) to treat primary anxiety or depression should be viewed with caution, and especially discouraged in patients with a history of psychotic disorders (see Section 7.7.3.2), limited clinical evidence indicates that these drugs may present alternative therapeutic avenues in patients suffering from anxiety or depression secondary to certain chronic diseases. For example, in a study of HIV+ patients who reported using cannabis to manage their symptoms, 93% cited an improvement in anxiety and 86% cited an improvement in depressionReference 1026. It is important to note that 47% of those surveyed reported deterioration in memory. In another within-subject, double-blind, placebo-controlled, clinical study of HIV+ cannabis smokers, high-dose dronabinol (5 mg q.i.d., for a total daily dose of 20 mg, for two days, followed by 10 mg q.i.d., for a total daily dose of 40 mg, for 14 days) was associated with an increase in self-reported "positive affect" (feeling "content"), but no change was observed in measures of anxiety or "negative affect"Reference 298. The dosage employed in this study was eight times the recommended starting dose for appetite stimulation (i.e. 2.5 mg b.i.d), and double the maximal daily recommended dose. Improved mood was also reported as a beneficial effect of cannabis consumption in patients suffering from MSReference 1027. Improvements in anxiety or depression were equally noted in a clinical study of patients suffering from chronic neuropathic pain who smoked cannabisReference 59. It may be interesting to note here that rimonabant, a CB1 receptor antagonist initially marketed as an anti-obesity medication, was withdrawn from the market because its use was associated with a significant incidence of anxiety, depression, and suicide, underscoring the role of the CB1 receptor in regulating moodReference 1023Reference 1028. For additional information on the association between cannabis and anxiety and depression please see Section 7.7.3.1 and between cannabis and suicide, please see Section 7.7.3.3.
Cannabidiol
Pre-clinical data
More than 30 pre-clinical studies have been carried out examining the anxiolytic effects of CBD in a variety of animal models of various types of anxiety disorders including generalized anxiety disorder, social anxiety disorder, panic disorder, obsessive-compulsive disorder and PTSDReference 171. In general, the findings from these pre-clinical studies support the anxiolytic effects of CBDReference 171. In addition, CBD also appears to have panicolytic and anti-compulsive effects and decreases autonomic arousal and conditioned fear expression. CBD also appears to enhance fear extinction, promote reconsolidation blockade, and prevent long-term anxiogenic effects of stressReference 171. While the exact anxiolytic mechanism of action of CBD is unclear, one proposed molecular target of CBD is the 5-HT1A receptorReference 171.
Clinical data
Findings from functional neuroimaging studies suggest differential cerebral blood flow effects associated with administration of CBD compared with those seen with placebo or THCReference 171. Single-photon emission computed tomography (SPECT) brain imaging studies showed that in contrast to placebo, CBD decreased regional cerebral blood flow in the limbic and paralimbic cortical areas, regions implicated in the pathophysiology of anxietyReference 1029. Furthermore, a randomized, double-blind, placebo-controlled study showed that 600 mg of CBD attenuated brain activity (blood oxygenation level-dependent response) in these cortical regions in response to anxiogenic stimuliReference 126. In contrast, 10 mg of Δ9-THC increased anxiety at baseline or in response to anxiogenic stimuli, and the brain regions affected by Δ9-THC differed from those affected by CBDReference 126. Although the precise mechanism by which CBD exerts its anxiolytic effects is not well established, it may act either by decreasing blood flow to brain regions associated with the processing of anxiety or fear-based stimuli (as mentioned above), or possibly through the modulation of serotonergic neurotransmissionReference 171Reference 1030Reference 1031
At least 10 clinical studies have examined the acute anxiolytic properties of CBDReference 171. Indeed, increasing evidence suggests pure CBD, at doses of several hundred milligrams (i.e. 300 - 600 mg, p.o.) may be effective in decreasing acute, experimentally-induced social anxiety in the clinic, although the extent to which CBD (at the relatively lower concentrations commonly found in THC-predominant cannabis) is able to achieve anxiolysis either in an experimental, or more importantly in a real-life setting remains uncertain. While clinical findings related to the anxiolytic effects of CBD are currently limited to acute experimental models of social anxietyReference 171, one observational study of 100 patients who self-reported using cannabis for medical purposes for conditions such as MS, chronic pain, nausea, cancer and psychological problems, reported that those who used cannabis with cannabinoid concentrations of 6% THC and 7.5% CBD (i.e. "low THC" condition) reported significantly less anxiety and dejection (i.e. feeling down, sad, depressed), but also reported less appetite stimulation, compared to those who reported using "high THC" (19% THC, <1% CBD) or "medium THC" (12% THC, <1% CBD) strainsReference 118.
4.9.5.2 Sleep disorders
- Human experimental data suggests cannabis and THC have a dose-dependent effect on sleep-low doses appear to decrease sleep onset latency and increase slow-wave sleep and total sleep time, while high doses appear to cause sleep disturbances.
- Limited evidence from clinical studies also suggests that certain cannabinoids (cannabis, nabilone, dronabinol, nabiximols) may improve sleep in patients with disturbances in sleep associated with certain chronic disease states.
Human experimental data
There is some evidence from experimental studies to suggest a role for the ECS in the regulation of sleep. Subjects deprived of sleep for a 24 h period had increased levels of OEA, a natural analogue of anandamide, in their CSF but not in serum, whereas levels of anandamide were unchangedReference 1032. Recent studies have shown daily variation in 2-AG concentrations that are amplified under sleep restrictionReference 1033. 2-AG levels appear lowest around midsleep and increase continually across the morning, peaking in the early to mid-afternoon with concentrations of 2-oleoylglycerol (2-OG), a structural analogue of 2-AG, following a similar patternReference 1034. In rats, both acute and sub-chronic administration of anandamide induces sleepReference 1035. Cannabis containing mainly THC, as well as Δ9-THC itself are known to have a number of effects on sleep in humans, which may be dose-dependent (i.e. low doses appearing beneficial on some measures of sleep, high doses causing sleep disturbances). In general, it appears that at low doses these substances (THC-predominant cannabis, THC) decrease sleep onset latency and are associated with greater ease in getting to sleep whereas the opposite is true at high doses; there is a consistent reduction in total rapid eye movement (REM) sleep and REM density (reviewed inReference 209Reference 340). Low doses of THC also increase beneficial slow-wave sleep (critical for learning, memory consolidation, and memory retrieval) and total sleep time, while high doses decrease slow-wave sleepReference 340. Furthermore, due to the long half-life of THC, sedative effects may typically persist into the day following administrationReference 209.
Data from withdrawal studies
Heavy cannabis users (mean number of joints smoked per week = 100) who abruptly discontinue cannabis use have been shown to exhibit changes in polysomnographic sleep measures, including lower total sleep times, less slow wave sleep, longer sleep onset, shorter REM latency, and worse sleep efficiency and continuity parameters compared to controlsReference 340Reference 1036. Trouble getting to sleep, nightmares and/or strange dreams, and night sweats were frequently cited symptoms associated with cannabis withdrawalReference 342. These sleep disturbances progress over the first two weeks of abstinenceReference 1037. Furthermore, sleep disturbances resulting from abrupt discontinuation of cannabis use may trigger users to relapseReference 403Reference 1037. The symptoms observed during abstinence from cannabis may alternatively reveal a pre-existing sleep disorder masked by the drug.
Clinical data
A systematic review and meta-analysis of 28 RCTs (N = 2 454 participants) of cannabinoids (i.e. smoked cannabis, nabiximols, nabilone, dronabinol, CBD, THC, levonontradol, ajulemic acid) using the GRADE approach reported that there was some evidence that cannabinoids may improve sleep (insomnia, sleep quality, sleep disturbance)Reference 179.
Indeed, a number of clinical studies point to a potential beneficial role for smoked cannabis or prescription cannabinoids (dronabinol, nabilone, nabiximols) in the treatment of sleep difficulties or disturbances associated with chronic pain (cancer pain, chronic non-cancer pain, diabetic peripheral neuropathy), HIV-associated anorexia-cachexia, MS, ALS, SCI, RA, fibromyalgia, inflammatory bowel disease (IBD), MS-associated bladder dysfunction, PTSD, chemosensory alterations and anorexia-cachexia associated with advanced cancerReference 59Reference 184Reference 185Reference 223-Reference 225Reference 298Reference 383Reference 578Reference 597Reference 611Reference 612Reference 642Reference 697Reference 704Reference 708Reference 715Reference 716Reference 822Reference 838. In most of these studies, the effect on sleep was measured as a secondary outcome.
Although presented elsewhere throughout the text in the relevant sections, brief summaries of a number of these studies are presented below.
Dronabinol
A four-week, randomized, double-blind, crossover pilot clinical study of 19 patients suffering from ALS taking 2.5 - 10 mg per day of dronabinol reported improvements in sleepReference 708. Two clinical studies reported that dronabinol (20 - 40 mg total Δ9-THC/day) and smoked cannabis (~800 mg cigarettes containing 2 or 3.9% THC, administered four times per day for four days, corresponding to an estimated daily amount of 64 - 125 mg of Δ9-THC consumed) produced improvements in mood and sleep in patients with HIV/AIDS-associated anorexia-cachexiaReference 223Reference 224. A clinical study of HIV+ cannabis smokers treated with dronabinol for 14 days (10 mg q.i.d., 40 mg daily) reported improvements in both objective and subjective measures of sleep, but only during the first eight days of the treatment regimenReference 298. A two-centre, phase II, randomized, double-blind, placebo-controlled, 22-day pilot clinical study carried out in adult patients suffering from chemosensory alterations and poor-appetite associated with advanced cancer of various etiologies reported statistically significant improvements in measures of quality of sleep and relaxation with dronabinol treatment (2.5 mg b.i.d.) compared to placeboReference 611. An open-label pilot study of add-on oral THC (25 mg/mL THC in olive oil; 2.5 mg THC b.i.d., maximal daily dose 10 mg THC) in patients with chronic PTSD reported improvement in sleep quality and frequency of nightmaresReference 571.
Nabilone
An off-label, retrospective, descriptive study of 20 adult patients suffering from chronic non-cancer pain of various etiologies (post-operative or traumatic pain, reflex sympathetic dystrophy, arthritis, Crohn's disease, neuropathic pain, interstitial cystitis, HIV-associated myopathy, post-polio syndrome, idiopathic inguinal pain, chronic headaches) reported beneficial effects of nabilone (1 - 2 mg/day) on sleepReference 822. An enriched-enrolment, randomized-withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone (1 - 4 mg/day), as an adjuvant in the treatment of diabetic peripheral neuropathic pain, reported statistically significant improvements in sleep and overall patient statusReference 612. A two-week, randomized, double-blind, active-control, crossover study of 29 patients suffering from fibromyalgia reported that nabilone (0.5 - 1.0 mg before bedtime) improved sleep in this patient populationReference 597. Two clinical studies looked at nabilone for sleep disturbances in PTSD. An open-label, non-placebo-controlled trial of nabilone for PTSD reported that nabilone treatment was associated with an improvement in sleep time, cessation or lessening of nightmare severity, and cessation of night sweatsReference 578. Dosing of nabilone was 0.5 mg, 1 h prior to bedtime; effective dose range was 0.2 mg to 4 mg nightly with all doses kept below 6 mg daily. A subsequent preliminary, randomized, double-blind, placebo-controlled cross-over clinical study of 10 Canadian male military personnel with PTSD who were not responsive to conventional treatment and who continued to experience trauma-related nightmares, received 0.5 mg nabilone or placebo and titrated to the effective dose (i.e. nightmare suppression) or to a maximum daily dose of 3 mg nabiloneReference 1038. Average dose achieved for nabilone was 2 mg/day. Treatment arms lasted for seven weeks each, with a two-week washout period in between. Half (50%) of the subjects reported a significant improvement in nightmare suppression on nabilone, while only 11% of subjects reported improvement with placebo.
Smoked cannabis
Surveys carried out among patients suffering from MS reported cannabis-associated improvements in sleep in this patient populationReference 225Reference 226. Reported dosages of smoked cannabis varied from a few puffs, to 1 g or more, at a timeReference 225. A cross-sectional survey of patients suffering from fibromyalgia reported that subjects claimed using cannabis (by smoking and/or eating) for a variety of symptoms associated with fibromyalgia, including sleep disturbanceReference 184. A cross-sectional survey of 291 patients with IBD (Crohn's disease or ulcerative colitis) reported that one of the reasons patients used cannabis was to improve sleepReference 185. A two-week, randomized, double-blind, placebo-controlled, cross-over study of patients suffering from chronic neuropathic pain reported that those who smoked 25 mg of cannabis containing 9.4% Δ9-THC, three times per day for five days (2.35 mg total available Δ9-THC per cigarette, or 7.05 mg total Δ9-THC per day), fell asleep more easily and more quickly, and experienced fewer periods of wakefulnessReference 59.
Orally administered prescription cannabinoid medications (Cannador and nabiximols)
A double-blind, placebo-controlled, phase III study, involving patients with stable MS (i.e. MUSEC study) reported that a 12-week treatment with an oral cannabis extract ("Cannador") (2.5 mg Δ9-THC and 0.9 mg CBD/dose) was associated with a statistically significant improvement in sleep compared to placeboReference 697. The majority of the patients using cannabis extract used total daily doses of 10, 15, or 25 mg of Δ9-THC with corresponding doses of 3.6, 5.4, and 9 mg of CBD. Results from double-blind, crossover, placebo-controlled clinical studies of oral Δ9-THC and/or Δ9-THC: CBD extract (nabiximols, marketed as Sativex®) suggested modest improvements in pain, spasticity, muscle spasms, and sleep quality in patients with SCIReference 642Reference 715Reference 716. A preliminary clinical study assessing the effectiveness of nabiximols in pain caused by RA reported a modest, but statistically significant, analgesic effect and consequent improvement in quality of sleepReference 383. The mean daily dose in the final treatment week was 5.4 pump actuations (equivalent to 14.6 mg Δ9-THC and 13.5 mg CBD). A sixteen-week, open-label pilot study of cannabis-based extracts (a course of nabiximols treatment followed by maintenance with 2.5 mg Δ9-THC only) for bladder dysfunction in 15 patients with advanced MS reported significant decreases in nocturia and improvement in patient self-assessment of sleep qualityReference 704.
The Canadian Guidelines for the Diagnosis and Management of Fibromyalgia Syndrome (endorsed by the Canadian Pain Society and the Canadian Rheumatology Association) recommend that with regards to possible treatments, a trial of a prescribed pharmacologic cannabinoid may be considered in a patient with fibromyalgia, particularly in the setting of important sleep disturbance (this recommendation was based on Level 3, Grade C evidence)Reference 838.
4.9.5.3 Post-traumatic stress disorder
- Pre-clinical and human experimental studies suggest a role for certain cannabinoids in alleviating post-traumatic stress disorder (PTSD)-like symptoms.
- However, while limited evidence from short-term clinical studies suggests a potential for oral THC and nabilone to decrease certain symptoms of PTSD, there are no long-term clinical studies for these preparations or any clinical studies of smoked/vapourized cannabis for PTSD.
- Limited evidence from observational studies suggests an association between herbal cannabis use and persistent/high levels of PTSD symptom severity over time.
- There is limited evidence to suggest an association between PTSD and CUD.
PTSD is a psychiatric disorder of significant prevalence and morbidityReference 1039. In the overall population, more than two thirds of individuals may experience a serious traumatic event at some point in their lifetimeReference 1039. PTSD refers to the development of a cluster of characteristic symptoms that follow exposure to an extreme traumatic stressor and which appears to involve aberrant memory processing and impaired adaptation to changed environmental conditionsReference 1040. Characteristic symptoms include persistent, intrusive recollections, or a re-experiencing of the original traumatic event (through dreams, nightmares, and dissociative flashbacks), numbing and avoidance, and increased arousalReference 578. Sleep disturbance also occurs in up to 90% of casesReference 1038. Patients with PTSD are also at risk for other psychological disorders, including but not limited to generalized anxiety disorder, major depressive disorder, and substance use disorder as well as physical problems including chronic pain, hypertension, and asthmaReference 1041. There appears to be a link between exposure to a traumatic event and cannabis use, especially in military veterans, and research suggests that individuals with PTSD may be particularly likely to use cannabis specifically to alleviate symptoms of PTSD and associated distressReference 1039Reference 1041Reference 1042. There is also evidence to suggest that particular symptoms and correlates of PTSD including anxiety, stress, insomnia and depression are among the most frequently cited reasons for cannabis useReference 1042. Despite much anecdotal evidence suggesting the benefits of cannabis use to treat PTSD, there is a lack of standardized large-scale controlled trials to make any firm conclusions regarding the efficacy or safety of cannabis for the treatment of PTSDReference 1043.
While affected individuals may use cannabis to cope with negative internal states, there is increasing evidence that these individuals may also experience more problematic cannabis use as well as heightened withdrawal and craving when not intoxicatedReference 1042. Indeed, compared to individuals who do not have PTSD, those who have PTSD (and especially those whose symptoms are severe) report significantly increased use of cannabis to cope and to sleep, increased severity of cannabis withdrawal, and experiences of craving related to compulsivity, emotionality, and anticipation, and these findings suggest the existence of a positive feedback loop between PTSD symptomatology and cannabis useReference 1042Reference 1044. In support of these findings, data from the National Comorbidity Study (NCS) has also shown that adults suffering from PTSD were three times more likely to have a diagnosis of cannabis dependence compared to those without PTSDReference 1045. In addition, an epidemiological study on the prevalence and correlates of DSM-5 CUD using data from the 2012 - 2013 wave of the NESARC-III study reported that past-year CUD was associated with PTSD (adjusted OR (AOR) = 4.3), and that lifetime CUD was also associated with PTSD (AOR = 3.8)Reference 338. Furthermore, the association between PTSD and past-year CUD increased with increasing severity of CUD (AOR = 2.1, 6.2, and 9.5 for mild, moderate, and severe CUD respectively). Furthermore, a study that examined the prevalence and correlates of 186 patients seeking the use of cannabis for medical purposes for the first time found that patients who screened positive for PTSD had higher percentages of lifetime prescription opioid, cocaine, prescription sedative, and street opioid use (55%, 38%, 41%, and 17% respectively), as well as a higher percentage of recent prescription sedative use (29%) than those patients who screened negative for PTSDReference 1046.
Role of the endocannabinoid system in PTSD
Increasing evidence suggests an important role for the ECS in PTSD. The ECS has been associated with the regulation of emotional states and cognitive processes, and neuroanatomical studies have detected the presence of ECS elements in a number of brain structures involved in learning and memory, and in structures which also play central roles in fear conditioning and response implicated in PTSD (reviewed inReference 1040). The ECS links stress exposure to changes in synaptic plasticity contributing to activation and feedback regulation of the HPA axis, and facilitates the activation of resilience factors during and/or after stress exposureReference 1047. It has been hypothesized that chronic stress creates a "hypocannabinergic state" that results in impaired fear extinction (as is seen in PTSD) and this state can be alleviated with CB1 receptor agonistsReference 1047. Fear-conditioning experiments in animals suggest a role for the amygdala-hippocampal-cortico-striatal circuit as a key brain circuit responsible for processing and storing fear-related memories and for coordinating fear-related behavioursReference 1048. Additional evidence in humans suggests that PTSD is characterized by over-activity or hyper-responsiveness of the amygdala, with deficient regulation of prefrontal cortical structures as well as abnormal hippocampal and basal ganglia functionsReference 1048. As similarities exist between the expression of fear and anxiety in humans suffering from phobias, PTSD, or other anxiety disorders, and the expression of conditioned fear in animals, the use of certain animal behavioural models to study PTSD is feasible and relevantReference 1040Reference 1049.
Pre-clinical data
There is evidence to suggest that the endocannabinoids, anandamide and 2-AG play important roles in the development and function of the PTSD neurocircuit, especially in stress responsesReference 1048. Impaired CB1 receptor function has been suggested as a potentially important etiological mechanism of PTSDReference 1048. Indeed, a number of pre-clinical studies demonstrate that deletion of the CB1 receptor or its inhibition by pharmacological antagonists prevent the extinction of aversive memories (i.e. learned inhibition of fear), a naturally adaptive processReference 1049-Reference 1052. Conversely, in some cases, CB1 receptor agonism or increased endocannabinoid-mediated neurotransmission (e.g. via inhibition of FAAH) appear to enhance extinction to some degreeReference 1049Reference 1052, but further research is required to clarify and substantiate this effect. Studies in animals also show that reduction of endocannabinoid levels (mainly 2-AG but also anandamide) via Dagla gene knockout is associated with increased anxiety, stress and fear responsesReference 1053. Taken together, the evidence from pre-clinical studies suggests a role for the ECS in the extinction of aversive memories and impairment of memory retrieval. Furthermore, the available evidence raises the possibility that manipulation of the ECS (via inhibition of FAAH, upregulation of DAGL, increased anandamide or 2-AG tone, or even perhaps via administration of CBD) can facilitate disruption of contextual fear memories as well as have anti-anxiogenic effectsReference 1039Reference 1054. These may represent potential therapeutic options for the treatment of diseases associated with inappropriate retention of aversive memories or inadequate responses to aversive situations, such as PTSD or phobiasReference 1050, although much additional research is needed.
Human experimental and clinical data
Studies in humans have shown that individuals with PTSD have lower circulating endocannabinoid concentrations and an upregulation of brain CB1 receptorsReference 1011Reference 1048Reference 1055-Reference 1057. In addition, there is evidence to suggest that humans (and mice) carrying a common variant of the FAAH gene (C385A; rs325520) conferring decreased FAAH protein stability and increased anandamide signaling showed decreased threat-related amygdala reactivity, increased reward-related ventral striatal reactivity, and enhanced fear extinctionReference 1058Reference 1059.
A double-blind, placebo-controlled, within-subject clinical study of 16 healthy volunteers looking at the effects of THC on amygdala reactivity to threat found that a 7.5 mg dose of dronabinol (vs. placebo) was associated with a significant reduction in amygdala reactivity to social signals of threat, but did not affect activity in primary visual and motor corticesReference 1060. These findings are consistent with evidence suggesting that, at least at low doses, THC may have an anxiolytic effect in central mechanisms of fear behaviours.
In one randomized, double-blind, placebo-controlled, between-subjects clinical study, 29 healthy volunteers (with many having minimal cannabis use) were administered either 7.5 mg dronabinol or placebo 2 h prior to extinction learning following a fear conditioning paradigmReference 1061. The study showed that pre-extinction administration of THC facilitated extinction of conditioned fear in healthy human subjects. Limitations of the study include the use of a healthy subject population (results may differ in other populations), and lack of generalizability of the results to a population of chronic cannabis users. The authors suggested that this study was the first in humans to demonstrate the feasibility of pharmacological enhancement of extinction learning, though they cautioned that additional development and clinical testing are warranted.
A follow-up study by the same group using functional magnetic resonance imaging (fMRI) in a randomized, double-blind, placebo-controlled, between-subjects study in 28 healthy volunteers (with many having minimal cannabis use) showed that study subjects who received 7.5 mg dronabinol (vs. placebo) showed decreased reactivity in the amygdala and increased activation of the ventromedial prefrontal cortex and the hippocampus to a previously extinguished conditioned stimulus during extinction memory recallReference 1062.
Another randomized, double-blind, placebo-controlled, between-subjects clinical study of 48 healthy participants found that CBD enhanced the consolidation of explicit fear extinction in humansReference 1063. In this study, participants were administered either 32 mg (a sub-anxiolytic dose) of inhaled CBD prior to extinction, 32 mg of CBDfollowing extinction, or placebo. CBD administered after extinction learning was associated with an attenuation of explicit fearful responding during recall and reinstatement. However, there was a trend for reduction in reinstatement in subjects administered CBD either before or after extinction. The authors suggest that the CBD-mediated attenuation of fearful responding was not likely due to an anxiolytic effect as there was no evidence of reduced anxiety following CBD administration. The authors also suggest that CBD may be a potential adjunct to extinction-based therapies for anxiety disorders and warrant further investigation.
A preliminary, open-label, pilot clinical study of add-on oral THC (25 mg/mL) in 10 patients with chronic PTSD and on stable medication (e.g. duloxetine, escitalopram, mirtazapine, buproprion, clonazepam, lorazepam) reported a statistically significant improvement in global symptom severity, sleep quality, frequency of nightmares and PTSD hyperarousal symptoms over the three-week study periodReference 571. Participants were instructed to begin dosing by placing 2.5 mg of THC b.i.d. (i.e. 0.1 mL of a 25 mg/mL olive oil solution containing THC) beneath the tongue, 1 h after waking up and 2 h before going to bed. Maximum daily dose was 5 mg THC b.i.d. (i.e. 0.2 mL b.i.d.), or a total 10 mg daily dose (i.e. 0.4 mL). A statistically significant decrease in symptom severity was observed in PTSD hyperarousal symptoms, clinical global impression scale (CGI-S), clinical global impression improvement (CGI-I), sleep quality, frequency of nightmares, and total Nightmare Effects Survey (NES) scores. Twenty percent of participants attained complete remission of nightmares by week 3. Adverse effects were reported in 40% of the subjects and consisted of dry mouth, headache, and dizziness. Limitations of this study included small sample size, open-label design and no placebo control as well as short follow-up period.
An open-label, non-placebo-controlled clinical trial of nabilone for PTSD was conducted in 47 non-military, civilian patients diagnosed with PTSD, having at least a two-year history of PTSD-related nightmares refractory to conventional therapies, a minimum of once weekly nightmares, and with no prior history of sensitivity to cannabinoids or evidence of psychotic reactionsReference 578. Patients did not discontinue any concomitant psychotropic medications, and were started on 0.5 mg nabilone, 1 h prior to bedtime. All doses were kept below 6 mg daily. The effective dose range varied between 0.2 mg and 4 mg nightly. Seventy-two percent of patients self-reported total cessation or lessening of severity of nightmares (treatment duration 4 - 12 months or longer). Other self-reported benefits included an improvement in sleep time, a reduction in daytime flashbacks, and cessation of night sweats. Reported side effects included light-headedness, amnesia, dizziness, and headache. No tolerance to nabilone was observed in this clinical trial.
A preliminary, randomized, double-blind, placebo-controlled cross-over clinical study of 10 Canadian male military personnel with PTSD who were not responsive to conventional treatment and who continued to experience trauma-related nightmares, received 0.5 mg nabilone or placebo and titrated to the effective dose (i.e. nightmare suppression) or to a maximum daily dose of 3 mg nabiloneReference 1038. Average daily dose achieved for nabilone was 2.0 mg/day. Treatment arms lasted for seven weeks each, with a two-week washout period in between. Score on the Global Impression of Severity of PTSD was 3.3 at screening (4 = extreme). The mean reduction in nightmares measured by the Clinician-Administered PTSD Scale (CAPS) for Recurring and Distressing Dream scores were -3.6 and -1.0 in the nabilone and placebo groups respectively (p = 0.03). Mean global improvement measured by the Clinical Global Impression of Change scale was statistically significant between the nabilone and placebo groups. Half (50%) of the subjects reported a significant improvement in nightmare suppression on nabilone, while only 11% of subjects reported improvement with placebo. Mean scores for the General Well-Being Questionnaire showed a difference from baseline of 20.8 and -0.4 for the nabilone and placebo groups respectively. Incidence rates of adverse events in the nabilone and placebo groups were approximately the same (50% vs. 60%, respectively). The most common adverse effects associated with nabilone treatment were dry mouth and headache. There were no serious adverse events or subject dropout. While the study findings are promising, the sample size was very small.
A recent systematic review found "insufficient evidence" around the benefits and harms of cannabis in treating PTSD among adults. Only five studies met inclusion criteria (pharmaceutical cannabinoids were excluded), two of which were systematic reviews that came to similar inconclusive conclusions with the current review, and three of which were observational studies, with two showing no association between cannabis use and PTSD outcomes, and one showing that cannabis use was longitudinally associated with more severe levels of PTSD symptoms compared to cannabis abstainers. The authors emphasized that evidence was too limited to draw any conclusions and clinical trials and more cohort-based studies are needed to determine the safety and efficacy of plant-based cannabis for PTSDReference 1064.
4.9.5.4 Alcohol and opioid withdrawal symptoms (drug withdrawal symptoms/drug substitution)
- Pre-clinical studies suggest CB1 receptor agonism (e.g. THC) may help increase the reinforcing properties of alcohol, increase alcohol consumption, and increase risk of relapse of alcohol use, as well as exacerbate alcohol withdrawal symptom severity.
- Pre-clinical studies suggest certain cannabinoids (e.g. THC) may alleviate opioid withdrawal symptoms.
- Evidence from observational studies suggests that cannabis use could help alleviate opioid withdrawal symptoms, but there is insufficient clinical evidence from which to draw any reliable conclusions.
There is increasing interest in the use of cannabis as a substitute for alcohol, opioids and other drugs, including illicit drugs, both in terms of decreasing drug withdrawal symptoms associated with abstinence from such drugs, but also in the context of decreasing some of the health risks associated with use of these drugs (e.g. opioid-associated morbidity and mortality). In the case of opioids, in vitro and in vivo studies have shown significant physiological and pharmacological overlap, cross-tolerance, mutual potentiation, and cross-talk between the endocannabinoid and the endogenous opioid systems (see Section 4.7.2.3)Reference 1065Reference 1066. In addition, both of these endogenous physiological mechanisms have been implicated in the mechanism of action of several other drugs with abuse and dependence potential such as ethanol, nicotine, and psychostimulantsReference 1065.
A survey that examined patterns of cannabis use and medical conditions and symptoms (Cannabis Access for Medical Purposes Survey, CAMPS) among 473 self-identified current users of cannabis for medical purposes reported that over 80% of respondents self-reported substituting cannabis for prescription drugs, over 51% for alcohol and over 32% for illicit substancesReference 1067. Median weekly amount of cannabis used was 14 g (or 2 g per day). The most commonly endorsed reasons for substitution were "less adverse side effects" and "better symptom management". Limitations of the study included self-report and lack of physician confirmation of medical conditions and extent of patient improvement (or lack thereof) as well as the potential for multiple responses from a single respondent and a biased sample population with an over-representation of individuals responding favorably to cannabis.
Alcohol
There is evidence to suggest complex functional interactions between ethanol and the ECS (reviewed inReference 1068). Acute and chronic administration of ethanol in animals is associated with brain region-specific changes in endocannabinoid levels (acute: increases/decreases in endocannabinoid levels; chronic: increases in endocannabinoid levels) and in the expression of ECS components (chronic: decreases in levels of CB1 receptor, and of FAAH)Reference 212. In human studies, acute administration of ethanol was associated with an increase in CB1 receptor availability, whereas chronic consumption of ethanol (i.e. in alcoholic patients) was associated with a significant reduction in CB1 receptor availability (20 - 30%) persisting at least two to four weeks into abstinenceReference 1069Reference 1070. Chronic alcohol consumption was also associated with decreased levels of FAAH, decreased CB1 receptor coupling to G proteins and decreased FAAH activityReference 212. CB1 receptor agonism as well as genetic deletion of FAAH, or its pharmacological inhibition, appears to mediate the reinforcing properties of ethanol, facilitates ethanol consumption, and enhances re-instatement of ethanol self-administration in animal modelsReference 1068. On the other hand, genetic ablation of CB1 receptor expression or its pharmacological inhibition (e.g. by rimonabant) generally results in decreased ethanol consumption in animal modelsReference 212. There is also some limited and mixed evidence gathered from animal studies that suggests the ECS may be involved in the modulation of alcohol withdrawal symptoms; with CB1 receptor agonism (e.g. by THC and nabilone) apparently exacerbating withdrawal severity and conversely, CB1 receptor antagonism either mitigating or worsening alcohol withdrawal symptomsReference 212Reference 1071-Reference 1074.
Opioids
Anecdotal information and findings from some animal studies suggest that cannabinoids (e.g. THC) might be useful in treating the symptoms associated with opioid withdrawalReference 843Reference 1075-Reference 1078, but there are no supporting clinical studies of efficacy in this regard. Nevertheless, the overlapping neuroanatomical distribution, convergent neurochemical mechanisms, and comparable functional neurobiological properties of the cannabinoid and opioid systems may help explain why cannabinoids could substitute for opioids to potentially alleviate withdrawal symptoms associated with opioid abstinenceReference 842. One literature review suggests that under certain circumstances, cannabis use can be associated with positive treatment prognosis among opioid-dependent cohortsReference 1066. Cannabis abuse and dependence were predictive of decreased heroin and cocaine use during treatment, and intermittent use of cannabis was associated with a lower percentage of positive opioid urine drug screens and improved medication compliance on naltrexone therapyReference 1066. A few qualitative studies have found that people who use heroin report that they are able to reduce their heroin use by using cannabisReference 1079Reference 1080. In one study looking at people who inject drugs (PWID), smoking cannabis was reported to reduce anxiety and craving experienced while transitioning away from daily heroin useReference 1079, while in another study, medical cannabis patients reported using cannabis to substitute or wean off prescription opioidsReference 1080. Another study found that street-recruited PWIDs who reported using cannabis used opioids (i.e. heroin) less frequentlyReference 1081. However, a study that investigated the use of smoked cannabis to alleviate symptoms of opioid withdrawal did not appear to find any effect of cannabis use on opioid-withdrawal symptomsReference 1082. In this study, 116 outpatient heroin and cocaine users (of whom 46 were also cannabis users) participating in a 10-week methadone-taper phase of a randomized clinical trial were assessed for self-rated opioid withdrawal symptoms. The study found that opioid withdrawal scores did not differ between users and non-cannabis users suggesting that smoked cannabis did not reduce opioid withdrawal symptoms in this patient population. Lastly, in a five-week, placebo-controlled, randomized, double-blind, safety study of dronabinol for the treatment of moderate-intensity opioid withdrawal symptoms in opioid-dependent adults, doses of 5 or 10 mg of dronabinol were well-tolerated, while doses of 20, 30 or 40 mg dronabinol produced sustained elevations in heart rate and anxiety/panic in some subjectsReference 1083.
4.9.5.5 Schizophrenia and psychosis
- Significant evidence from pre-clinical, clinical and epidemiological studies supports an association between cannabis (especially THC-predominant cannabis) and THC, and an increased risk of psychosis and schizophrenia.
- Emerging evidence from pre-clinical, clinical and epidemiological studies suggests CBD may attenuate THC-induced psychosis.
Schizophrenia is a chronic and devastating mental disorder which typically manifests in late adolescence or early adulthoodReference 1084. It is characterized by so-called positive symptoms, negative symptoms, and cognitive impairmentReference 1085. Positive symptoms include suspiciousness, paranoid and grandiose delusions, conceptual disorganization, fragmented thinking, and perceptual alterationsReference 1085. On the other hand, negative symptoms include blunted affect, emotional withdrawal, psychomotor retardation, lack of spontaneity and reduced rapportReference 1085. Cognitive deficits include deficits in verbal learning, short-term memory, working memory, executive function, abstract ability, decision-making, and attentionReference 1084Reference 1085. By comparison, psychotic-like episodes are characterized by derealisation, depersonalization, dissociation, hallucination, paranoia, impairment in concentration, and perceptual alterations and are typically of a transient and self-limited natureReference 1085.
Below is a discussion of the role of the ECS in schizophrenia and psychosis as well as a discussion of the role of THC and CBD in these disorders. While the evidence strongly suggests exposure to THC is detrimental to individuals who have a personal or family history of schizophrenia, the available evidence also suggests a potential anti-psychotic/anti-schizophrenic role for CBD, though additional research is required.
The endocannabinoid system and psychotic disorders
There is increasing evidence implicating the ECS in schizophrenia and psychosisReference 177Reference 1085Reference 1086. Findings from blood and CSF samples, and post-mortem, neuroimaging, and genetic studies lend strong support to the involvement of the ECS in schizophrenia and psychosisReference 177. For example, levels of anandamide were reported to be significantly elevated in the CSF and serum of patients with initial prodromal states of psychosisReference 1087. In addition, anandamide levels were also elevated in the CSF and serum of anti-psychotic-naïve patients with active schizophreniaReference 1088Reference 1089. Treatment of schizophrenic patients with dopamine D2 receptor antagonists (standard pharmacologic treatment for schizophrenia) also lowers anandamide levels to normalReference 1090Reference 1091. Post-mortem studies investigating CB1 receptor densities in the brains of deceased schizophrenic patients have also noted an upregulation of CB1 receptor levels in the dorsolateral pre-frontal cortex, anterior cingulate cortex, and posterior cingulate cortexReference 1092-Reference 1096, areas of the brain typically afflicted in schizophreniaReference 1086. Neuroimaging studies measuring in vivo CB1 receptor availability in schizophrenic patients also report a widespread increase in CB1 receptor levels in a number of other brain areas including the nucleus accumbens, insula, cingulate cortex, inferior frontal cortex, parietal cortex, mediotemporal lobe, and the ponsReference 1097Reference 1098. Genetic studies suggest that polymorphisms in a number of different genes such ascatechol-O-methytransferase (COMT),AKT Serine/Threonine Kinase 1 (AKT1),dopamine active transporter 1 (DAT1), cannabinoid receptor 1 (CNR1), and BDNF may increase individual vulnerability to psychosis and schizophrenia (see below and also Section 7.7.3.2) especially when interacting with environmental factors such as urbanicity, abuse/maltreatment/trauma, and cannabis or other substance useReference 1085.
Comorbidity of substance use disorders with psychotic disorders
Patients with severe mental illnesses such as schizophrenia are known to have high rates of substance use disorders, with cannabis being one of the substances most often used or misused by this populationReference 1099Reference 1100. Two competing hypotheses have tried to explain why patients with severe mental illnesses such as schizophrenia also have co-morbid substance abuse. The "self-medication" hypothesis, in the context of psychiatric disorders, posits that those who suffer from such disorders (e.g. patients with schizophrenia) consume cannabis in order to alleviate specific psychopathological symptoms or alternatively to diminish the side effects resulting from the use of medicationsReference 1100Reference 1101. For example, a recent review examining the reasons for cannabis use among individuals with psychotic disorders reported that the most common reasons for cannabis use in this population were related to the desire to improve mood and alleviate dysphoria, to relax and increase pleasure, to get "high", to decrease anxiety, to improve social life and to reduce boredomReference 1102. However, the authors note that despite the beneficial reasons and positive subjective effects claimed by individuals with psychotic disorders using cannabis, evidence suggests a deterioration in the positive symptoms of some patients and worse treatment adherence and clinical course with cannabis use. Further evidence against the "self-medication" hypothesis also comes from research suggesting that cessation of cannabis use in patients with schizophrenia is associated with an improvement in overall and cognitive functioning, as well as psychotic and depressive symptomsReference 1103. Indeed, a recent systematic review and meta-analysis showed that independent of stage of illness, continued cannabis use in patients with a pre-existing psychotic disorder was associated with a greater increase in relapse of psychosis compared to patients who never used or discontinued useReference 164. Continued use was also associated with longer hospital admissions. Furthermore, there was a greater effect of continued use over discontinued use on relapse, positive symptoms, and level of functioning, but not on negative symptoms. A subsequent observational study of patients 18 - 65 years of age with first-episode psychosis showed that former regular users of cannabis who stopped after the onset of psychosis had the most favourable illness course with regards to relapseReference 165. Continued high-frequency use (i.e. daily use) of high-potency (skunk-like) cannabis had the worst outcome (increased risk for subsequent relapse, more relapses, fewer months until relapse, and more intense psychiatric care). Another recent prospective cohort study reported that it is more likely than not that continued cannabis use after onset of psychosis is causally, and dose-dependently, associated with increased risk of relapse of psychosis resulting in psychiatric hospitalizationReference 166. While the "self-medication" hypothesis presents a compassionate, interesting, and attractive explanation to understand why schizophrenics have co-morbid substance abuse disorders, the evidence presented here as well as the lack of a relationship between early psychotic symptoms and an increased risk of later cannabis use have called the hypothesis into questionReference 1104-Reference 1106. On the other hand, the "addiction-vulnerability" hypothesis claims that substance abuse vulnerability and schizophrenic symptoms share a common neuropathologyReference 1105Reference 1107. In other words, this hypothesis rests on the idea that certain pathological alterations in brain structure and function will predispose certain individuals to developing both schizophrenia and substance abuse disorders.
Cannabis/THC and psychosis
There is much scientific evidence to suggest a robust positive association between cannabis use, especially THC-predominant cannabis, and the development of acute and persistent psychosis in some individuals, earlier onset of schizophrenia (especially in adolescents susceptible to psychotic disorders,Reference 187Reference 188Reference 196Reference 199Reference 202), as well as exacerbation of existing symptoms and a more complicated course of treatment in those who already suffer from schizophreniaReference 539Reference 1085Reference 1102Reference 1105Reference 1108Reference 1109. Despite these findings, the evidence suggests that cannabis is neither necessary nor sufficient to cause a persistent psychotic disorder; it appears instead that cannabis is but one factor that interacts with other factors to result in psychosisReference 183. Increasing evidence suggests that the link between cannabis and psychosis is further moderated by age at onset of use, childhood abuse (stressors), and genetic vulnerabilityReference 183.
Adolescence and young adulthood are critical developmental periods, and exposure to a variety of environmental stimuli, including cannabis, can adversely affect the proper course of neurobiological development and trigger the early onset of schizophrenia in those with a genetic vulnerabilityReference 539Reference 1085Reference 1109-Reference 1111. The period of brain maturation during adolescence spans from age 10 to 24 with continued synaptogenesis, myelogenesis, dendritic and synaptic pruning, volumetric growth, changes in receptor distribution, and programming of neurotrophic levels during this time, especially in the prefrontal cortex and the limbic systemReference 540Reference 1106. Adolescence is also the period of time where the brain's ECS undergoes dynamic changes including a spike in mRNA levels of the CB1 receptor, a steady increase in the level of anandamide, and a more pronounced decrease in the levels of 2-AGReference 539. The ECS is implicated in the myelination of various tracts and in neuroplasticity and synaptic functionReference 539. It is therefore conceivable that exogenously applied cannabinoids such as THC can perturb the fine balance of endocannabinoid levels and the proper functioning of the CB1 receptor resulting in a change in course of neurodevelopment during this period. In one case-control study with 280 people with a first episode of psychosis and 174 controls, patients reported using higher-potency cannabis containing high THC and low CBD compared to the controls who reported using cannabis containing equal amounts of THC and CBDReference 1112. Furthermore, daily use of high potency cannabis, containing high amounts of THC and low amounts of CBD, was associated with an earlier age of onset of psychosisReference 1113. Individuals who started using cannabis at age 15 or younger also had an earlier onset of psychosis than those who started after age 15Reference 1113.
Studies of animal models of schizophrenia report that chronic treatment of adolescent rats, but not adult rats, with a cannabinoid receptor agonist results in a schizophrenia-like phenotype that is accompanied by changes in basal neuronal activity in various brain structures including the nucleus accumbens, amygdala, caudate putamen, and the hippocampus (seeReference 1106Reference 1114Reference 1115).
Meanwhile, controlled clinical studies carried out in those with no history of psychotic disorders reported the manifestation of transient schizophrenia-like symptoms induced by the intravenous administration of Δ9-THCReference 201. These symptoms included transient positive psychotic symptoms, perceptual alterations, negative symptoms, euphoria, anxiety, and cognitive deficits in attention, working memory, and verbal recallReference 201. Likewise, intravenous administration of Δ9-THC in schizophrenics was associated with transient exacerbation of core psychotic symptomsReference 199. In summary, acute psychotomimetic symptoms associated with cannabis and/or THC-intoxication can include depersonalization, derealization, paranoia, ideas of reference, flight of ideas, pressured thought, disorganized thinking, persecutory delusions, grandiose delusions, auditory/visual hallucinations, and impairments in attention and memory (in about 20 - 50% of individuals)Reference 1085. These effects have been documented consistently with smoked cannabis, orally administered cannabis (5 - 20 mg THC) and intravenously administered THC (0.015 - 0.03 mg/kg)Reference 1085.
Genetic factors
A number of studies have investigated the influence of potential genetic factors in the development of psychosis and schizophrenia, and more specifically as a function of interaction with cannabis use. Some studies have focused on the role of genetic polymorphisms at the COMT geneReference 1116-Reference 1123, and others have focused on polymorphisms at the AKT1 geneReference 1124-Reference 1127. Taken together, the data from these studies strongly suggest that single-nucleotide polymorphisms at either the COMT or AKT1 genes interact with cannabis use to predict the age at onset, as well as the likelihood of developing psychosis or schizophrenia in vulnerable individuals. More recently, evidence has also emerged implicating polymorphisms at the CNR1, neuregulin 1 (NRG1) as well as the DAT1 gene and the BDNF gene and THC/cannabis use with onset of psychotomimetic effects as well as earlier age of onset of schizophreniaReference 1085Reference 1128-Reference 1130. Please consult Section 7.7.3.2 for additional information on the adverse psychiatric effects associated with the use of cannabis and psychoactive cannabinoids (such as THC), and the role of genetic predisposition on the risk of developing a psychotic disorder.
The findings presented above and in sections 7.7.3 and 7.7.3.2 suggest that cannabis use, especially THC-predominant cannabis, as well as exposure to Δ9-THC alone, would not be beneficial, and in fact would actually be harmful to those who may be suffering from psychotic disorders, or who may have a genetic predisposition or family history of psychosis or schizophrenia. In contrast, emerging evidence suggests CBD may protect against the psychosis-inducing effects of THC (see below).
Cannabidiol
In contrast to the harmful effects seen with THC and THC-predominant cannabis in psychosis and schizophrenia, there is some evidence from observational, and preliminary pre-clinical and clinical studies that suggests that CBD may protect against THC-induced psychosis and could even serve as a potential treatment for schizophrenia.
Observational studies
Two studies that analyzed cannabinoid levels in hair samples from 140 individuals found that those who had only THC in their hair exhibited greater positive symptoms with higher levels of hallucinations and delusions than those with both THC and CBD in their hair and those with no cannabinoidsReference 1131Reference 1132. On the other hand, another study of cannabis users failed to show any differences in the prevalence of psychotic-like symptoms between subjects who reported smoking cannabis containing "low" or "high" levels of CBD; however, the authors mention a number of confounding factors, including the lack of adjustment for alcohol consumption that could help explain this apparent inconsistency between studiesReference 535.
An internet-based, cross-sectional study of 1 877 individuals who had a consistent history of cannabis use reported that individuals who had consumed cannabis with a higher CBD to THC ratio reported experiencing fewer psychotic episodes; however, the authors noted that the observed effects were subtleReference 139. Furthermore, the study was hampered by a number of important methodological issues suggesting the conclusions should be interpreted with caution.
In one case-control study with 280 people with a first episode of psychosis and 174 controls, patients reported using higher-potency cannabis containing high THC and low CBD compared to the controls who reported using cannabis containing equal amounts of THC and CBDReference 1112. Furthermore, daily use of high potency cannabis, containing high THC and low CBD, was associated with an earlier age of onset of psychosis compared to non-cannabis usersReference 1113.
In a follow-up case-cohort study of 410 patients with first-episode psychosis and 370 population controls, daily use of "skunk-like" cannabis (very high THC, very low CBD), was associated with a more than five-fold increased risk of first-episode psychosis, whereas weekend use of "skunk-like" cannabis was associated with a nearly three-fold increased risk of first-episode psychosisReference 173. By contrast, the OR of a first-episode psychosis associated with the use of "skunk-like" cannabis less than once per week, or daily, weekend, or less-than-weekly use of lower potency cannabis was not statistically significant compared with never use of cannabisReference 173.
The above evidence suggests that the presence of THC and the absence of CBD in cannabis may increase the risk of experiencing psychotic reactions and also suggests a dose-response effect between THC and risk of first episode psychosis.
Pre-clinical and clinical studies
Consistent with these findings, a number of pre-clinical and clinical studies have suggested that CBD may in fact protect against the psychoactive and psychosis-inducing effects of THC and THC-predominant cannabis, and may also have therapeutic use in the treatment of individuals with psychosis and schizophreniaReference 133Reference 135Reference 1133-Reference 1142. One caveat to this is that in animal models it appears that pre-treatment with CBD 15 to 60 min prior to administration of THC, but not co-administration, is associated with increased blood and intracerebral levels of THC and THC-associated immobilityReference 123Reference 131. Furthermore, a higher ratio of CBD to THC also appears important in attenuating the psychoactive effects of THCReference 135Reference 1108Reference 1135.
Pre-clinical studies
Studies in certain rat and mouse models of psychosis suggest that CBD (at doses of 15 - 60 mg/kg or roughly equivalent human doses of 1.25 mg/kg to 10 mg/kg CBD) reduces psychotic-like behavioural effects in a manner comparable to that observed with atypical anti-psychotic drugsReference 1143Reference 1144.
Clinical studies with healthy volunteers
In perhaps one of the first clinical studies examining the effects of CBD on THC-induced psychoactivity, Karniol et al. administered placebo, THC (30 mg), CBD (15, 30 or 60 mg) or a combination of THC and CBD orally to 40 healthy male volunteers in a double-blind fashion and measured resulting subjective psychoactive effectsReference 135. Administration of 30 mg of THC resulted in strong psychological reactions (mainly anxiety), that in some cases reached a near-panic state, and significantly impaired performance on a time estimation task. Both of these effects were attenuated in a dose-dependent manner in the presence of increasing doses of CBD. A 2: 1 ratio of CBD to THC (60 mg: 30 mg) was most effective in attenuating the intensity of the psychoactive effects induced by THC in this study. CBD appeared to modify not only the intensity but also the quality of the psychoactive effects induced by THC.
In another study of 15 healthy volunteers, simultaneous inhalation of CBD (150 µg/kg) and THC (25µg/kg) attenuated the subjective euphoria associated with THC and showed a trend towards a decrease in THC-induced psychomotor impairmentReference 1134. No effect on THC-induced euphoria and psychomotor impairment was noted when the same dose of CBD was administered 30 minutes before THC.
In a double-blind, placebo-controlled clinical study, eight healthy volunteers were orally administered placebo, THC (0.5 mg/kg), CBD (1 mg/kg), or a mixture of THC (0.5 mg/kg) and CBD (1 mg/kg)Reference 133. Administration of THC alone was associated with a number of psychoactive effects, including depersonalization, disconnected thoughts, paranoid ideas and anxiety that were mostly blocked when CBD was co-administered with THC.
In another clinical study of nine healthy volunteers, a 200 mg oral dose of CBD was able to attenuate the impairment in binocular depth inversion (a model of impaired perception during psychotic states) induced by 1 mg of oral nabiloneReference 1141.
On the other hand, oral administration of a cannabis extract (containing 10 mg THC and 5.4 mg CBD), but not pure THC (10 mg THC), to 24 healthy volunteers in a placebo-controlled, double-blind clinical study was associated with decreased finger tapping frequency, a measure of motor disturbance related to schizophrenic symptomatology and severity of illnessReference 1135.
A pseudo-randomized, placebo-controlled, double-blind, within-subject clinical study showed that pre-treatment of healthy human subjects with CBD (5 mg i.v.), but not placebo, diminished the emergence of positive psychotic symptoms 30 min after i.v. administration of 1.25 mg of Δ9-THCReference 125.
In a randomized, double-blind, placebo-controlled clinical study of 48 healthy subjects that were administered placebo, THC (i.v. 1.5 mg) or CBD (p.o. 600 mg), CBD pre-treatment 3.5 h before THC administration attenuated THC-associated paranoia and impairment of episodic memory, but not working memoryReference 1136.
Taken together, the above findings suggest CBD, especially at ratios of 2 : 1 and when co-administered, can attenuate the acute psychotic and anxiogenic effects as well as certain aspects of cognitive impairment observed with administration of THC.
Clinical and case studies in patients with psychotic symptoms
One case report of a 19-year-old female schizophrenic patient treated with haloperidol and oral CBD reported that treatment with 1500 mg CBD daily for 26 days, but not with haloperidol, was associated with an attenuation of psychotic symptomsReference 1137. Another slightly larger case study by the same group reported a mild level of improvement in psychotic symptoms in one out of three treatment-resistant schizophrenic patients treated with 1280 mg oral CBD daily for four weeks; no adverse effects were notedReference 1139. In a clinical study, again by the same group, six patients with PD who also experienced psychotic symptoms were treated with 600 mg/day oral CBD for four weeksReference 1145. This treatment regimen was associated with a significant reduction in psychotic symptomatology without any adverse effects.
In a placebo-controlled, single-dose clinical study by Hallak et al. (2010), 28 schizophrenic patients were administered either placebo, 300 mg, or 600 mg CBD orally. While no improvements in psychotic symptomatology were noted, there were statistically significant improvements in attention with the placebo and the 300 mg CBD dose, but not the 600 mg dose of CBD where there appeared to be a potential worsening of attention possibly due to a sedative effect at the higher doseReference 1140.
A four-week, double-blind, parallel-group, randomized, active-controlled clinical trial comparing CBD (200 mg, q.i.d., up to a total daily amount of 800 mg) to amilsupride (a dopamine D2/D3 receptor antagonist used in the treatment of schizophrenia) reported that both drugs were associated with a significant clinical improvement in symptoms with no significant difference between the two treatmentsReference 1142. Treatment with CBD was well tolerated with significantly fewer side effects compared to those associated with anti-psychotic treatment (e.g. the presence of extra-pyramidal symptoms and increased prolactin release). In addition, CBD did not appear to significantly affect either hepatic or cardiac functions. CBD treatment, but not amilsupride, was also associated with an increase in serum levels of anandamide.
Taken together, the available evidence from a limited number of emerging observational, pre-clinical and clinical studies suggests that CBD may play a protective role against the manifestation of transient psychotic symptoms associated with exposure to THC or THC-predominant cannabis. CBD may also hold therapeutic promise in the treatment of individuals with psychotic symptoms or schizophrenia, though additional research is needed in this regard to confirm and substantiate this effect.
That being said, the extent to which CBD at the levels typically found in cannabis is able to ameliorate psychotic symptoms has not been firmly established and in fact, much of the cannabis consumed, whether for non-medical or medical purposes, typically contains relatively low levels of CBD and higher levels of THCReference 76Reference 1146. For example, the CBD content of street cannabis typically varies between 0.1 and 0.5%, although CBD levels of up to 8.8% (in hashish) have been notedReference 139. Therefore, as an example, a 1 g joint could contain between 1 mg (0.1%) and 88 mg (8.8%) of CBD-levels which are much lower than those usually administered in clinical trials (600 - 1500 mg/day)Reference 1147. Some strains of dried cannabis sold for medical purposes by Canadian producers licensed by Health Canada can contain as much as 24% CBD with little THC. Therefore, a 1 g joint of this strain of cannabis could contain up to 240 mg of CBD; still far lower a dose than that used in clinical trials of CBD for psychosis/schizophrenia. However, many licensed producers also sell cannabis strains with approximately equal concentrations of THC and CBD, and some with a 2: 1 CBD to THC ratio which has been reported as potentially helping to reduce the incidence of psychotic symptoms in individuals using cannabis. Though additional research is needed, patients who reported using cannabis with approximately equal levels of THC and CBD reported less perturbation of moodReference 118. Furthermore, licensed producers of cannabis for medical purposes are now also permitted to produce and sell cannabis oil, which can contain high levels of CBD (i.e. up to 24%).
In conclusion, consumption of cannabis that contains mainly THC as well as consumption of other psychoactive cannabinoids (e.g. dronabinol, nabilone) should be treated with considerable caution in patients with schizophrenia (or those at risk for psychosis) as these substances are believed to trigger psychotic episodes, lower the age of onset of symptoms, and contribute to a negative long-term prognosis in vulnerable individuals. Additionally, the therapeutic potential of CBD in the treatment of schizophrenia/psychosis, while promising, requires further research.
4.9.6 Alzheimer's disease and dementia
- Pre-clinical studies suggest that THC and CBD may protect against excitotoxicity, oxidative stress and inflammation in animal models of Alzheimer's disease (AD).
- Limited case, clinical and observational studies suggest that oral THC and nabilone are associated with improvement in a number of symptoms associated with AD (e.g. nocturnal motor activity, disturbed behaviour, sleep, agitation, resistiveness).
Dementia affects 36 million people worldwide where Alzheimer's disease (AD) accounts for 60 to 80% of these casesReference 557. While still a subject of some debate, a widely accepted theory underlying the pathophysiology of AD is that the deposition of amyloid-beta (Aβ) protein in specific brain regions leads to localized neuroinflammatory responses and accumulation of intra-cellular neurofibrillary tangles (composed of hyperphosphorylated tau protein); these events result in neuronal cell death with accompanying loss of functional synapses and changes in neurotransmitter levelsReference 1148. These pathological processes are thought to give rise to disease-associated symptoms such as memory deficits, and cognitive and motor impairmentsReference 1148.
The endocannabinoid system and Alzheimer's disease
There is some evidence to suggest an association between the ECS and the pathophysiology of ADReference 1148Reference 1149. One in vivo study reported elevation in the levels of the endocannabinoid 2-AG in response to intra-cerebral administration of Aβ1-42 peptide in animalsReference 1150. Another study using post-mortem brain samples from deceased AD patients showed that decreased anandamide levels were associated with increasing Aβ1-42 levels, but not with Aβ40 levels, amyloid plaque load, or tau protein phosphorylationReference 1151. Lastly, upregulation of CB2 receptors and FAAH (and FAAH activity) has been respectively observed in reactive microglia and astrocytes surrounding senile plaques in post-mortem brain tissues collected from AD patientsReference 1152.
Pre-clinical data
Pre-clinical studies suggest the ECS protects against excitotoxicity, oxidative stress, and inflammation - all key pathological events associated with the development of ADReference 1153.
Results from in silico and in vitro experiments suggest Δ9-THC could bind to and competitively inhibit acetylcholinesterase, which in the context of AD functions as a molecular chaperone and accelerates the formation of amyloid fibrils and forms stable complexes with AβReference 1154. In this way, Δ9-THC blocked the amyloidogenic effect of acetylcholinesterase, diminishing Aβ aggregationReference 1154. Other in vitro studies suggest that CBD may have neuroprotective, anti-oxidant, and anti-apoptotic effects, as well as the ability to prevent tau protein hyperphosphorylation in cellular models of ADReference 1155-Reference 1157. It has also been shown that endocannabinoids can prevent Aβ-induced lysosomal permeabilization and subsequent neuronal apoptosis in vitroReference 1153. An in vivo study reported that enhancement of endocannabinoid tone through inhibition of FAAH was associated with significant decreases in the amount of total amyloid precursor protein (APP), soluble Aβ1-40, and Aβ1-42 peptides and neuritic plaque density as well as decreased microgliosis and astrogliosis in a mouse model of ADReference 1158.
In vivo studies reported that CBD dose-dependently and significantly inhibited reactive gliosis and subsequent neuroinflammatory responses in Aβ-injected mice, at doses of 2.5 mg/kg/day and 10 mg/kg/day i.p., during a seven-day course of treatmentReference 1159. Another study using both in vitro and in vivo models of AD reported opposing roles for the CB1 and CB2 receptors in this context: CB1 receptor agonism and CB2 receptor antagonism were both associated with blunted Aβ-induced reactive astrogliosis and attenuation of neuroinflammatory marker expressionReference 1160.
Administration of non-psychoactive doses of THC-enriched botanical extract (67.1% THC, 0.3% CBD, 0.9% CBG, 0.9% CBC, and 1.9% other phytocannabinoids), a CBD-enriched botanical extract (64.8% CBD, 2.3% THC, 1.1% CBG, 3.0% CBC, and 1.5% other phytocannabinoids) or nabiximols (combination of THC and CBD, 2.7% THC and 2.5% CBD) for a period of five weeks at the early stages of the symptomatic phase blunted the memory impairment observed in AβPP/PS1 miceReference 1161. Furthermore, chronic exposure to THC-enriched botanical extract, but not CBD-enriched extract or nabiximols, resulted in reduced memory performance in wild-type mice compared to vehicle-treated littermates. While chronic treatment with THC, CBD or nabiximols did not significantly modify the total Aβ burden in the cortex or hippocampus of AβPP/PS1 mice, the combination of THC and CBD (nabiximols) reduced soluble Aβ1-42, but not Aβ1-40, protein levels suggesting a protective effect. THC, CBD or combination of both (nabiximols) was also associated with a reduction in astrogliosis associated with Aβ deposition and the combination of THC and CBD also significantly reduced microgliosis.
Clinical and observational data
There have been very few clinical studies of cannabis or cannabinoids for the treatment of AD. A 2009 Cochrane database systematic review of cannabinoids for the treatment of dementia concluded that there was insufficient clinical evidence to suggest that cannabinoids can be effective at improving disturbed behavior in dementia or in the treatment of other symptoms of dementiaReference 1162. For the moment, no firm conclusions can be drawn about the safety and efficacy of cannabinoid-based drugs in older individuals, which represent the population most likely to be affected by ADReference 557.
One double-blind, placebo-controlled, six-week, crossover study of 12 patients suffering from Alzheimer-type dementia reported that 5 mg of dronabinol (Δ9-THC) daily was associated with a decrease in disturbed behaviour and an increase in body weightReference 1163. However, adverse reactions such as fatigue, somnolence, and euphoria (presumably unwanted) were reported. One open-label pilot study of six patients suggested an evening dose of 2.5 mg dronabinol (Δ9-THC) reduced nocturnal motor activity and agitation in those who were severely dementedReference 1164. A placebo-controlled clinical study of 24 patients diagnosed with probable dementia of the Alzheimer-type with agitated behavior and given dronabinol (2.5 mg, b.i.d., for two weeks) showed reduced nocturnal motor activity compared to baseline with no reported incidence of adverse eventsReference 1165. In one case-report, a patient suffering from dementia of the Alzheimer-type who had been treated unsuccessfully with donepezil, memantine, gabapentin, trazodone, and citalopram was given nabilone (initially 0.5 mg at bedtime, and then twice per day) which provided immediate reduction in the severity of agitation and resistiveness and eventual improvement in various behavioural symptoms following six weeks of continuous treatmentReference 1166. A case-report of a 71-year old man with mixed vascular and frontotemporal dementia accompanied by sexual disinhibition reported failure to curb his behaviours despite trials with a variety of agents including sertraline, divalproex, trazodone, risperidone, and aripiprazoleReference 1167. Treatment with nabilone (0.5 mg every 8 h) resulted in significant improvement in behavioural symptoms, however sedation and lethargy were noted but only during the dose titration phase.
A retrospective chart review evaluated the data of 40 patients with dementia (13 with AD) who had been treated with dronabinol for an average of 17 days (range: 4 - 50 days) for behavioural or appetite disturbancesReference 421Reference 557Reference 1168. Administration of an average dronabinol dose of 7 mg/day was associated with significant improved scores on the Pittsburgh Agitation Scale and the Clinical Global Impression Scale, but not on the Global Assessment of Functioning ScaleReference 421Reference 557Reference 1168. Significant improvements were noted in sleep duration and percentage of food consumed during dronabinol treatment. Twenty-six adverse events were detected in the study and the most frequent events included sedation, delirium, urinary tract infection, and confusionReference 1168. While causality was not established, the adverse events did not lead to medication discontinuation.
It is unclear if the improvement in symptoms of AD associated with the use of psychoactive cannabinoids (THC, nabilone) are related to their non-specific sedative effects or to cannabinoid-specific mechanisms of action as some studies report sedation, somnolence, and fatigue while other reports suggests these adverse effects are transient and wear-off once the patient has passed the initial dose titration phase and has reached a stable dose of cannabinoid.
Nevertheless, it is also worth noting that one cross-sectional study reported that prolonged use of ingested or inhaled cannabis was associated with poorer performance on various cognitive domains (e.g. information processing speed, working memory, executive function, and visuospatial perception) in patients with MSReference 233. Similar adverse effects of cannabis/cannabinoids on cognition could potentially apply in the context of Alzheimer-type dementia.
4.9.7 Inflammation
The role of the ECS in inflammation is complex as this system has been implicated in both pro- and anti-inflammatory processesReference 1149. Endocannabinoids, such as anandamide and 2-AG, are known to be produced and released by activated immune cells and to act as immune cell chemoattractants promoting or directing the inflammatory responseReference 1169. On the other hand, cannabinoids can also suppress the production of pro-inflammatory cytokines and chemokines and thus may have therapeutic applications in diseases with an underlying inflammatory componentReference 1169Reference 1170. For information on other diseases with an inflammatory component such as the arthritides or IBD, please consult Sections 4.8 and 4.9.8.2, respectively, of this document.
4.9.7.1 Inflammatory skin diseases (dermatitis, psoriasis, pruritus)
- The results from pre-clinical, clinical and case studies on the role of certain cannabinoids in the modulation of inflammatory skin diseases are mixed.
- Some clinical and prospective case series studies suggest a protective role for certain cannabinoids (THC, CBD, HU-210), while others suggest a harmful role (cannabis, THC, CBN).
The skin possesses an ECSReference 43. CB1 and CB2 receptors are expressed in a number of skin cell types including epidermal keratinocytes, cutaneous nerves and nerve fibres, sebaceous cells, myoepithelial cells of eccrine sweat glands, sweat gland ducts, mast cells, and macrophagesReference 1171. The ECS and certain associated signaling pathways (e.g. PPARγ, TRPV1) appear to regulate the balance between keratinocyte proliferation, differentiation, and apoptosis; together, these systems may play a role in cutaneous homeostasis but also in diseases such as psoriasis, which is characterized by keratinocyte proliferation and inflammationReference 43Reference 1172-Reference 1174.
Pre-clinical and clinical studies
A pre-clinical study in mice with dinitrophenol fluorobenzene (DNFB)-induced allergic contact dermatitis reported that a topical solution containing 1 µM THC applied to the skin was associated with an attenuation of the inflammatory response that was independent of CB1/CB2 receptorsReference 1175. Another pre-clinical study reported that application of CBD (10 µM) to cultured human sebocytes and to a human skin organ culture inhibited the lipogenic ("pro-acne") actions of various compounds and suppressed sebocyte lipogenesis and proliferation while also exerting anti-inflammatory effects, raising the possibility that CBD may have the potential to act as an "anti-acne" therapyReference 1176. Another in vitro study showed that CBD and CBG (0.5 µM), but not CBDV, significantly reduced the expression of a number of genes expressed in differentiated human keratinocytes (i.e. keratins, involucrin, and transglutaminase) by increasing DNA methylation of the keratin 10 geneReference 1177. CBD also increased global DNA methylation levels raising the possibility that CBD can exert epigenetic control of skin differentiation and potentially pave the way towards new phytocannabinoid-based approaches to treating skin diseases, according to the authors of the study.
In clinical studies, experimentally-induced histamine-triggered pruritus was reduced by peripheral administration of the potent synthetic CB1/CB2 receptor agonist HU-210, and the accompanying increases in skin blood flow and neurogenic mediated flare responses were attenuatedReference 1178. In another clinical study, topically applied HU-210 significantly reduced the perception of localized pain in human subjects following locally restricted application of capsaicin to the skin, and reduced subsequent heat hyperalgesia and touch-evoked allodynia without any psychomimetic effectsReference 1179. More recently, three prospective case series reported on the use of a topical preparation of cannabis (prepared in sunflower oil) for pyoderma gangrenosumReference 1180. Between 0.5 and 1.0 mL of two different formulations of topical cannabis oils were used in the treatments (5 mg/mL THC and 6 mg/mL CBD; and 7 mg/mL THC and 9 mg/mL CBD), applied to the wound daily and up to 3 times daily, with additional application two to three times daily for breakthrough pain. Application of the topical cannabis oil preparation was associated with onset of analgesia within 5 minutes, with all cases demonstrating clinically significant reduction of pain greater than 30% and an accompanying statistically significant opioid-sparing effect.
A recent review of topical cannabinoids for inflammatory disorders and pain management concluded that despite promising data in rodent models, there are no rigorous studies confirming either safety or efficacy in humansReference 1181. With interventions that lead to active areas of wound healing, the application of topical cannabinoid products may increase the risk for contamination and infection unless the product is rigorously tested and approved for dermatological use.
There have also been some case-reports of contact urticaria following exposure to cannabis flowers, and extreme sensitization to Δ9-THC and CBN has also been observed in an animal model of contact dermatitisReference 1182Reference 1183 (and see Section 7.3 for additional information on hypersensitivity/allergy to cannabis).
Therefore, while it is possible that some cannabinoids (e.g. HU-210, CBD) may have therapeutic value in the treatment of certain inflammatory skin conditions (such as psoriasis, pruritus, dermatitis, and acne), it is also possible for some cannabinoids (cannabis, THC, CBD) to trigger adverse skin reactions. Much further research is required in this area.
4.9.8 Gastrointestinal system disorders (irritable bowel syndrome, inflammatory bowel disease, hepatitis, pancreatitis, metabolic syndrome/obesity)
Historical and anecdotal reports suggest that cannabis has been used to treat a variety of GI disorders (e.g. diarrhea, inflammation, and pain of GI origin)Reference 1184-Reference 1186.
The endocannabinoid system and gastrointestinal disorders
The expression of both the CB1 and CB2 receptors has been detected in the enteric nervous system of the GI tract (enteric neurons, nerve fibers and terminals), whereas the human colonic epithelium, colonic epithelial cells lines, and stomach parietal cells appear to only express the CB1 receptorReference 30Reference 31. CB2 receptor expression appears to be upregulated in sections of the colon in patients with IBDReference 33. While the expression and localization of endocannabinoid synthesizing enzymes have not been well determinedReference 33, studies in animals indicate that the endocannabinoid degradative enzymes FAAH and MAGL can be found in the enteric nervous system and other sites in the GI tractReference 33. For example, FAAH is expressed in the stomach and in the large and small intestines, and has also been localized to the cell bodies of the myenteric plexusReference 33. MAGL expression has been detected in the muscle and mucosal layers of the duodenum and the ileum, as well as in the proximal and distal colon, and in the nerve cell bodies and nerve fibers of the enteric nervous systemReference 1187. There also appears to be some regional variation in the levels of endocannabinoids in the gut; 2-AG appears to be more abundant in the ileum than the colon, whereas the opposite is true of anandamideReference 33. CB1 and CB2 receptors appear to be expressed in the pancreasReference 32, whereas the CB1, but not the CB2 receptor, is expressed in the liver under normal conditionsReference 34Reference 35.
Cannabinoids appear to have many functions in the digestive system including the inhibition of gastric acid production, GI motility, secretion and ion transport, and the attenuation of visceral sensation and inflammation (reviewed inReference 33). Perturbations in the levels of various components of the ECS have been noted in experimental animal models of GI disorders, as well as in clinical studies (reviewed inReference 33). The sections below summarize the information regarding the uses of cannabis and cannabinoids in the treatment of various disorders of the GI system.
4.9.8.1 Irritable bowel syndrome
- Pre-clinical studies in animal models of irritable bowel syndrome (IBS) suggest that certain synthetic cannabinoid receptor agonists inhibit colorectal distension-induced pain responses and slow GI transit.
- Experimental clinical studies with healthy volunteers reported dose- and sex-dependent effects on various measures of GI motility.
- Limited evidence from one small clinical study with dronabinol for symptoms of IBS suggests dronabinol may increase colonic compliance and decrease colonic motility index in female patients with diarrhea-predominant IBS (IBS-D) or with alternating pattern (alternating constipation/diarrhea) IBS (IBS-A), while another small clinical study with dronabinol suggests a lack of effect on gastric, small bowel or colonic transit.
Irritable bowel syndrome (IBS) is the most common functional GI disorder encountered in clinical medicineReference 1188. It is a spectrum of disorders characterized by the presence of chronic abdominal pain and/or discomfort and alterations in bowel habitsReference 1188Reference 1189. Symptom patterns can be divided into diarrhea predominant (IBS-D), constipation predominant (IBS-C), and an alternating pattern (alternating constipation/diarrhea) (IBS-A)Reference 1189Reference 1190. While the pathophysiology of IBS remains unclear, the disorder is thought to be caused by dysregulation of the 'brain-gut axis' in response to psychological or environmental stressors or to physical stressors such as infection or inflammation, and is characterized by altered gut motility and visceral hypersensitivityReference 1188. There is also some emerging evidence that suggests an association between genetic alterations in genes coding for certain ECS proteins (e.g. FAAH and CNR1) and the pathophysiology of IBSReference 1191-Reference 1193.
Pre-clinical data
A few pre-clinical studies in animal models of IBS have been carried out to date. Two studies have employed mechanically-induced colorectal distension to trigger an acute visceral pain response in rodents as a model of IBS-associated visceral hypersensitivity. One study in rats showed that intraperitoneal injection of different synthetic cannabinoid receptor agonists inhibited pain-related responses to experimentally-induced colorectal distension when administered prior to the experimental stimulusReference 1194. Intravenous administration of different synthetic cannabinoid receptor agonists also appeared to inhibit the overall pain-related responses to experimentally-induced colorectal distension in rats, as well as in mice, when administered after the experimental stimulusReference 1195. In another study, subcutaneous administration of CB1 or CB2-selective agonists was reported to reduce the enhanced small intestinal transit observed in a mouse model of post-inflammatory IBSReference 1196.
Clinical data with dronabinol
There are only a handful of clinical studies examining the effects of cannabinoids (dronabinol) in human experimental models of IBS and in patients with IBS.
One double-blind, randomized, placebo-controlled, parallel-group clinical study examined the effects of dronabinol on GI transit, gastric volume, satiation, and post-prandial symptoms in a group of healthy volunteersReference 1197. A 5 mg dose of dronabinol was associated with a significant delay in gastric emptying in female subjects, but not male subjects. No significant differences in either small bowel or colonic transit were observed between subjects administered dronabinol or placebo in any gender group. The 5 mg dose of dronabinol was used because a 7.5 mg dose caused intolerable side effects in more than half of the subjects. Adverse effects associated with the consumption of a 5 mg dose of dronabinol included dizziness/light-headedness, dry mouth, disturbed mental concentration, and nausea.
A subsequent double-blind, randomized, placebo-controlled, parallel-group clinical study investigated the effects of dronabinol on colonic sensory and motor functions of healthy human volunteersReference 1198. Administration of a 7.5 mg dose of dronabinol significantly increased colonic compliance, especially in females, and reduced pre- and post-prandial phasic colonic motility and pressure. Colonic compliance is defined as the change in distensibility of the colon in response to a change in applied intracolonic pressure and it is used as a measure of colonic viscoelastic properties and as an indicator of colonic motor/contractile activityReference 1198-Reference 1200. Decreased compliance is typically associated with urgency and diarrhea, while increased compliance is typically associated with constipationReference 1199Reference 1201. An increase in colonic compliance in this setting could indicate a return towards proper colonic function. In contrast to the results seen in the pre-clinical rodent studies, dronabinol increased the sensory rating of pain but did not affect the sensory rating of gas, or the thresholds for first sensation of either gas or pain during experimentally-induced random phasic distensionsReference 1198.
A double-blind, randomized, parallel-group clinical study investigated the effects of escalating doses of dronabinol on colonic sensory and motor functions in a population of mostly female patients diagnosed with IBS according to Rome III criteria (IBS-C, IBS-D, or IBS-A (i.e. alternating between diarrhea and constipation))Reference 1202. Only the highest dose of dronabinol tested (5 mg) was associated with a small, but statistically significant, increase in colonic compliance. Furthermore, the effect on colonic compliance appeared to be more pronounced in the IBS-D/A sub-group compared to IBS-C. No significant differences were observed on fasting or post-prandial colonic tone in response to dronabinol at any dose. However, the highest dose of dronabinol (5 mg) was associated with a significant reduction in the proximal left colon motility index, with a trend towards decreased colon motility indices. Treatment effects were significant on the proximal colon motility index in patients with IBS-D/A, but not in IBS-C, and only for the highest dose. Sensation thresholds and sensation scores for gas and pain during experimentally-induced ramp distensions did not differ significantly among the different treatment groups. The effects of genotype and dronabinol dose interaction on gas and pain sensation ratings, as well as on proximal fasting and distal fasting motility indices were also investigated. The results from these preliminary pharmacogenetic studies raise the possibility that the effects of dronabinol on colonic compliance and proximal colonic motility may be influenced by genetic variations in the FAAH and CNR1 genes, but further studies are required to substantiate this hypothesis.
A subsequent double-blind, randomized, placebo-controlled, parallel-group clinical study in a population of mostly female patients with IBS-D (Rome III criteria) further investigated gene-treatment interactions on colonic motility in this sub-set of IBS patientsReference 1203. Neither the 2.5 mg b.i.d. nor the 5 mg b.i.d. doses of dronabinol had any statistically significant effects on gastric, small bowel, or colonic transit. The effects on colonic transit were also examined as a function of genotype-by-treatment dose interaction. While treatment with dronabinol appeared to decrease colonic transit in subjects carrying the CNR1 rs806378 CT/TT polymorphism, these effects were not statistically significant. Adverse effects were reported not to differ significantly between treatment groups.
4.9.8.2 Inflammatory bowel diseases (Crohn's disease, ulcerative colitis)
- Pre-clinical studies in animal models of inflammatory bowel disease (IBD) suggest that certain cannabinoids (synthetic CB1 and CB2 receptor agonists, THC, CBD, CBG, CBC, whole plant cannabis extract) may limit intestinal inflammation and disease severity to varying degrees.
- Evidence from observational studies suggests that patients use cannabis to alleviate symptoms of IBD.
- A very limited number of small clinical studies with patients having IBD and having failed conventional treatments reported improvement in a number of IBD-associated symptoms with smoked cannabis.
IBDs include Crohn's disease and ulcerative colitisReference 1204. Crohn's disease is characterized by patchy trans-mural inflammation, which may affect any part of the GI tractReference 1205. Symptoms include abdominal pain, diarrhea and weight loss as well as systemic symptoms of malaise, anorexia, and/or feverReference 1205. Crohn's disease may cause intestinal obstruction due to strictures, fistulae, or abscessesReference 1205. Ulcerative colitis is characterized by diffuse mucosal inflammation limited to the colonReference 1205. Symptoms commonly include bloody diarrhea, colicky abdominal pain, urgency, or tenesmusReference 1205. Both diseases are associated with an equivalent increased risk of colonic carcinomaReference 1205.
The endocannabinoid system and inflammatory bowel diseases
ECS changes have been observed in the GI tracts of experimental animal models of IBD, as well as in those of IBD patientsReference 33Reference 1204. These changes include changes in the levels of endocannabinoids, cannabinoid receptors, and endocannabinoid synthesizing and degrading enzymesReference 30Reference 33Reference 1204Reference 1206-Reference 1208.
Pre-clinical data
Pre-clinical experiments in animal models of IBD suggest cannabinoids and endocannabinoids may limit intestinal inflammation and disease severity via activation of CB1 and CB2 receptorsReference 1209-Reference 1214.
Acute colitis
Mice bearing a genetic deletion of the CB1 receptor had a stronger colonic inflammatory responseReference 1209 following rectal administration of dinitrobenzene sulfonic acid (DNBS), an established method of inducing an acute colitis-like phenotype in miceReference 1215. In contrast to wild-type mice, histological examination of the colons of CB1 receptor knockout mice treated with DNBS revealed disruption of epithelial structure, with extensive hemorrhagic necrosis and neutrophil infiltration into the mucosa, and with acute inflammation extending into the sub-mucosa and muscle layerReference 1209. Pharmacological blockade of the CB1 receptor in wild-type mice produced similar effects accompanied by thickening of the bowel wall, inflammatory infiltrates, and an increase in lymphoid-follicle size associated with adherence to surrounding tissuesReference 1209. Furthermore, in contrast to CB1 receptor knockout mice, wild-type mice retained a significantly greater body weight following DNBS treatmentReference 1209. Treatment of wild-type mice with the potent synthetic CB1 and CB2 receptor agonist HU-210, prior to and after DNBS insult, significantly reduced the macroscopic colonic inflammatory responseReference 1209. Mice bearing a genetic deletion of the FAAH enzyme also displayed an attenuated inflammatory response to DNBS compared to wild-type littermatesReference 1209.
An analogous study found that CB1 and CB2 receptor knockout mice and CB1/CB2 receptor double knockout mice showed increased extent of colonic inflammation, increased loss of crypt architecture, increased hyperemia/edema, and an increased degree of infiltration of inflammatory cells compared to wild-type mice following trinitrobenzene sulfonic acid (TNBS)-induced acute colitisReference 1213. All three knockout strains exhibited severe transmural colitis, with severe loss of epithelium, thickening of the bowel wall, and inflammatory infiltrates compared to wild-type mice. Genetic deletion of either or both CB receptors in mice treated with TNBS was also associated with significantly increased mRNA levels of various pro-inflammatory cytokines compared to TNBS-treated wild-type mice.
TNBS-induced acute colitis in mice was associated with a significant upregulation of CB2 receptor mRNA levels in the proximal and distal colons of treated miceReference 1216. Intraperitoneal administration of CB2 receptor agonists, prior to and following TNBS-induced colitis, was associated with a reduction in the macroscopic damage score which is a linear scale measuring the extent of macroscopic damage to the colon and includes markers such as the presence or absence of hyperemia, ulceration, inflammation, adhesions, damage length, and diarrhea. Conversely, administration of a CB2 receptor antagonist aggravated TNBS-induced colitis.
In a different experimental mouse model of acute colitis, the CB1 receptor-selective agonist arachidonyl-2-chloroethylamide and the synthetic CB2 receptor-selective agonist JWH-133, when injected intraperitoneally prior to and after colonic insult, significantly reduced colon weight gain, colon shrinkage, colon inflammatory damage score, and diarrheaReference 1212.
Inhibition of the 2-AG degrading enzyme MAGL in mice by intraperitoneal administration of a MAGL inhibitor prior to induction of acute colitis by TNBS was associated with decreased macroscopic and histological colon alterations, as well as decreased colonic expression of pro-inflammatory cytokinesReference 1217. Inhibition of MAGL was also associated with a reduction in colitis-related systemic and central inflammation in the liver and the CNS. Co-administration of either CB1 or CB2 receptor-selective antagonists completely abolished the protective effect in the colon afforded by MAGL inhibition, and partially reversed the protective anti-inflammatory effects associated with MAGL inhibition in the liver.
Acute colitis and cannabidiol
Intraperitoneal injection of CBD (5 - 10 mg/kg) prior to DNBS-induced acute colitis was associated with a statistically significant attenuation of body weight loss caused by DNBSReference 1218. CBD also reduced the wet weight/colon length ratio of inflamed colonic tissue, a marker of the severity and extent of the inflammatory response. Furthermore, CBD (5 - 10 mg/kg) significantly reduced macroscopic damage associated with DNBS administration (mild edema, hyperemia, and small bowel adhesions) as well as microscopic damage (epithelium erosion, and mucosal and sub-mucosal infiltration of inflammatory cells with edema). Lastly, treatment with CBD significantly attenuated the observed increases in some biological markers associated with inflammation and oxidative stress, as well as attenuating the observed increases in the colonic levels of anandamide and 2-AG.
Another study reported that intraperitoneal (10 mg/kg) or intra-rectal (20 mg/kg) pre-treatment with CBD, again administered prior to induction of colitis by TNBS, caused a significant improvement of the colitis score and a decrease in the myeloperoxidase activity (a measure of neutrophil accumulation in colonic tissue)Reference 1219. No such differences were observed for orally administered CBD. Histological examination of colonic tissue further revealed decreased destruction of the epithelial lining, a reduction in colon thickness, and less infiltration of immunocytes compared to vehicle-treated mice. In contrast to the earlier studyReference 1218, no differences in body weight were observed between vehicle-treated and CBD-treated mice that had developed colitisReference 1219.
The effects of intraperitoneal injections of THC, CBD, and a combination of THC and CBD on TNBS-induced acute colitis in rats have been investigatedReference 1214. In one experiment, treatment with 10 mg/kg of THC alone, a combination of 5 mg/kg THC and 10 mg/kg CBD, a combination of 10 mg/kg THC and 10 mg/kg CBD, or sulfasalazine alone was associated with a statistically significant decrease in the macroscopic damage score. Myeloperoxidase activity, a measure of granulocyte infiltration, was significantly decreased in CBD-treated rats and in rats treated with 10 or 20 mg/kg THC, or 5 mg/kg THC and 10 mg/kg CBD. Treatment with 10 mg/kg CBD, 10 mg/kg THC, 10 mg/kg THC and 10 mg/kg CBD, or sulfasalazine alone was also associated with decreased disturbances in colonic motility resulting from TNBS-induced colitis.
A more recent study investigated the effects of a whole-plant cannabis extract with high CBD content on an experimental model of intestinal inflammationReference 1220. In this study, the authors showed that this extract, when given either intraperitoneally (at a dose of 30 mg/kg CBD) or by oral gavage (at a dose of 60 mg/kg CBD) following the manifestation of intestinal inflammation, decreased the extent of damage in the DNBS model of colitis. Furthermore, the extract, when administered at a starting dose of 1 mg/kg CBD (i.p.) and at 5 mg/kg (orally), dose-dependently reduced intestinal hypermotility in the croton oil model of intestinal hypermotility. However, while administration of pure CBD, at all doses tested, did not improve colitis, it did normalize croton oil-induced hypermotility both when given intraperitoneally and orally (at a dose of 5 mg/kg).
Acute colitis, cannabigerol and cannabichromene
A study that examined the effects of the non-psychotropic cannabinoid CBG on experimental IBD (i.e. colitis) reported that CBG at doses of 1 mg/kg i.p. (preventive) and 5 mg/kg i.p. (curative) administered either before (preventive) or after (curative) DNBS-induced acute colitis in mice significantly reduced the damaging effects of DNBS on colon weight/colon length ratioReference 1221. In follow-up studies, a 30 mg/kg curative dose of CBG was associated with reductions in the signs of colon injury, submucosal oedema, cell proliferation, intestinal permeability, myeloperoxidase activity (i.e. intestinal inflammation), superoxide dismutase activity, inducible nitric oxide synthase (iNOS) and COX-2 expression, reactive oxygen species production, and IL-1β, IL-10, interferon- γ (IFN-γ) levels observed in DNBS-treated inflamed colons.
Another study that examined the effects of another non-psychotropic cannabinoid, CBC, on experimental IBD (i.e. colitis) in mice reported that administration of CBC (1 mg/kg, i.p.) was associated with a significant reduction in the damaging effects of DNBS on colon weight/colon length ratio, as well as a significant reduction in intestinal permeability, myeloperoxidase activity, intestinal erosion, and cell proliferationReference 1222. In vitro studies further confirmed the anti-inflammatory effects of CBCReference 1222.
Chronic colitis
Intraperitoneal administration of the synthetic CB2 receptor-specific agonist JWH-133 significantly attenuated colitis-associated body weight loss, inflammation, leukocyte infiltration, and tissue damage in a mouse model of spontaneous chronic colitisReference 1223. This CB2 receptor specific agonist also reduced T-cell proliferation, increased T-cell apoptosis, and increased the numbers of mucosal and systemic mast cellsReference 1223.
Ileitis
Ileitis is characterized by disruption of the mucosa, infiltration of lymphocytes into the sub-mucosa, increased myeloperoxidase activity, and vascular permeabilityReference 1224. The effect of CBC on inflammation-induced hypermotility in a mouse model of intestinal ileitis has been studiedReference 1224. Administration of CBC (15 mg/kg i.p.) following croton oil-induced intestinal inflammation was associated with a decrease in the expression of CB1 and CB2 receptor mRNA in the jejunum, but not in the ileumReference 1224. CBC did not affect upper GI transit, colonic propulsion, or whole gut transit in untreated mice, but did reduce intestinal motility in croton oil-treated mice at 10 and 20 mg/kg i.p.Reference 1224. CBC also dose-dependently and significantly inhibited contractions induced by acetylcholine, as well as by electrical field stimulation, in vitro in ilea isolated from control mice and croton oil-treated miceReference 1224. The inhibitory effect of CBC appeared to be cannabinoid receptor-independentReference 1224.
Information from surveys
It has been estimated that between 10 and 12% of patients with IBD are active cannabis users, and surveys conducted in patients with IBD report that between 44 and 51% of patients with IBD have used cannabis at some point in their lifetimeReference 185Reference 372Reference 1225-Reference 1227. Furthermore, between 10 and 50% of IBD patients use cannabis for disease symptom control (i.e. for symptoms such as abdominal pain, nausea and diarrhea)Reference 185Reference 372Reference 1226Reference 1227.
Findings from a cross-sectional survey of 291 patients with IBD (Crohn's disease or ulcerative colitis) suggested that the vast majority of those patients reported using cannabis to relieve abdominal pain and to improve appetiteReference 185. In contrast to patients with Crohn's disease, a greater proportion of patients with ulcerative colitis reported using cannabis to improve diarrheal symptoms. In general, patients reported being more likely to use cannabis for symptom relief if they had a history of abdominal surgery, chronic analgesic use, alternative/complementary medicine use, and a lower SIBDQ (short IBD questionnaire) score. Both ulcerative colitis and Crohn's disease patients reported using cannabis to improve stress levels and sleep. The mean duration of cannabis use (current or previous) was seven years. The majority of cannabis users reported using once per month or less, but 16% reported using cannabis daily or several times per day. The vast majority (77%) of users reported smoking cannabis as a joint without tobacco, 18% of users smoked it with tobacco, 3% used a water pipe, and 1% reported oral ingestion. Approximately one-third of patients in this study reported significant side effects associated with the use of cannabis such as paranoia, anxiety, and palpitations. Other commonly reported side effects included feeling "high", dry mouth, drowsiness, memory loss, hallucinations, and depression.
A retrospective, observational study of 30 patients with Crohn's disease examined disease activity, use of medication, need for surgery, and hospitalization before and after cannabis useReference 372. The average duration of disease was 11 years (range: 1 - 41 years). Twenty patients suffered from inflammation of the terminal ileum, five had inflammation of the proximal ileum, and eight had Crohn's disease of the colon. The indication for cannabis was lack of response to conventional treatment in the majority of the patients, and chronic intractable pain in most of the other patients. Most patients smoked cannabis as joints (0.5 g cannabis/joint), a few inhaled the smoke through water, and one patient consumed cannabis orally. Of those who smoked cannabis, most smoked between one and three joints per day. One patient smoked seven joints per day. The average duration of cannabis use was two years (range: two months to nine years). All patients reported that consuming cannabis had a positive effect on their disease activity. The scores on the Harvey-Bradshaw index (an index of Crohn's disease activity) were significantly decreased following cannabis use, and the use of other medications (e.g. 5-ASA, corticosteroids, thiopurine, methotrexate, and TNF antagonist) also appeared to be significantly reduced following use of cannabis. The study was limited by design and small size.
A population-based analysis of cases from the National Health And Nutrition Examination Survey (NHANES) (2009-2010) of patients with ulcerative colitis or Crohn's disease vs. controls showed that subjects with IBD had a higher incidence of ever having used marijuana/hashish (i.e. 67% vs, 60%) as well as an earlier age of onset of the disease (i.e. 15.7 vs. 19.6 years)Reference 1227. Furthermore, IBD patients were less likely to have used marijuana or hashish daily, but they appeared to use more heavily when they did use (i.e. 65% with IBD used three or more joints per day vs. 81% without IBD that used two or fewer joints per day). Male sex and age over 40 appeared to predict marijuana/hashish use.
A prospective cohort survey study of 292 IBD patients examining the use of cannabis in IBD found that patients who reported using it for relief of symptoms associated with IBD (16%) reported using it to treat abdominal pain (90%), nausea and poor appetite (73% each), and diarrhea (42%)Reference 1226. The majority (61%) of cannabis-using patients in this survey reported smoking cannabis. Most cannabis-using patients also reported cannabis as being "very helpful" or "completely relieving" in treating the symptoms patients sought to relieve. Among past-users, the majority reported having used cannabis non-medically. Current cannabis users were younger than non-users, had lower SIBDQ scores, and were more likely to have chronic abdominal pain. Younger age, previous surgery, Crohn's disease and chronic abdominal pain predicted cannabis use for medical purposes. Current cannabis users were also more likely to be using narcotics to treat their abdominal pain than former users. Study limitations included possible patient recall bias, lack of objective measures of disease activity before and after cannabis use, and uncertainty around transposition of study findings to the broader IBD patient population.
A survey of 313 Canadian patients with IBD who reported using or not using cannabis for medical purposes examined the motives, patterns of use and subjective beneficial and adverse effects of patients who self-administered cannabis for medical purposesReference 1228. The findings suggested that 18% of patients surveyed reported using cannabis to treat symptoms associated with IBD. The majority of these reported using cannabis to reduce symptoms rather than for prophylactic use. The majority of cannabis-using patients reported smoking cannabis (95%), while only 9% reported oral ingestion and 5% by drinking. Among the cannabis-using patients, 91% said they felt cannabis helped with their IBD and these patients reported that cannabis helped with abdominal pain (84%), improvement of abdominal cramping (77%), improvement with joint pain (48%), and improvement in diarrhea (29%). Twenty percent of cannabis-using patients reported cannabis use allowed them to decrease the dose of their conventional IBD medications, 13% said they were altogether able to stop using their conventional IBD medications and 4% reported needing to increase their conventional IBD medications. However, it was also noted that prolonged cannabis use (for more than six months at a time), but not intermittent use, to treat IBD symptoms was a strong predictor of requiring surgery in patients with Crohn's disease (OR = 5.03, CI = 1.45 - 17.46), and it was also noted that the OR of prolonged cannabis use approached that of current tobacco smoking (OR = 5.71, 95% CI = 1.92 - 16.98). It was however unclear if the cannabis use preceded or followed the surgery, and as such no temporal associations between cannabis use and need for surgery could be established. Risk of hospitalization for IBD was not associated with cannabis use. Most of the cannabis-using patients experienced side effects associated with cannabis use including anxiety, increased appetite, dry mouth, drowsiness, and euphoria; intensities of effects were rated as mild. The majority (71%) of cannabis-using patients reported not needing to experience euphoria to obtain symptom improvement, while fewer patients (20%) claimed they needed "a high" for beneficial effect. Study limitations included possible referral bias, non-randomized sampling methodology, underestimation of true rate of cannabis use, and patient reporting bias.
Data from clinical studies
A double-blind, randomized, placebo-controlled, crossover clinical study examining the effects of 5 and 10 mg Δ9-THC in visceral sensitivity reported that Δ9-THC did not alter baseline rectal perception to experimentally-induced distension or sensory thresholds of discomfort after sigmoid stimulation compared to placebo, in either healthy controls or IBD patientsReference 1229. However, the authors did note a bias in the patient selection criteria, which could have explained the apparent lack of effect.
A preliminary, observational, open-label, prospective, single-arm clinical trial in a group of 13 patients suffering from Crohn's disease or ulcerative colitis reported that treatment with inhaled cannabis over a three month period improved subjects' QoL, caused a statistically significant increase in subjects' weight, and improved the clinical disease activity index in patients with Crohn's diseaseReference 279. Patients reported a statistically significant improvement in their perception of their general health status, their ability to perform daily activities, and their ability to maintain a social life. Patients also reported a statistically significant reduction in physical pain, as well as improvement in mental distress. No serious adverse events were noted. Study limitations included study design, subject selection bias, the lack of a proper control group and placebo, small number of subjects, and the inability to establish a dose-response effect.
An eight-week, randomized, double-blind, placebo-controlled pilot clinical study in a group of 21 patients suffering from Crohn's disease reported beneficial effects of smoking cannabis on disease severityReference 603. Patients smoked joints containing 0.5 g dried cannabis flowers containing 11.5 mg Δ9-THC (23% THC, < 0.5% CBD), twice daily, for eight weeks followed by a two week "washout" period. The primary objective of the study was the induction of remission, which was defined as a Crohn's disease activity index (CDAI) score of 150 or less after eight weeks of cannabis treatment. Secondary objectives were response rate (defined as a 100 point reduction in the CDAI), reduction of at least 0.5 mg/dl in C-reactive protein (CRP) levels, or improvement in QoL of at least 50 points as measured by SF-36. All patients were cannabis-naïve and had failed at least one form of medical treatment for the disease, including mesalamine, corticosteroids, thiopurines, methotrexate, or anti-TNF-α. Patients were concomitantly taking other medications during the study period (5-aminosalicylic acid (5-ASA), corticosteroids, purine analogue, methotrexate, opioids, and anti-TNF-α). Although 45% of patients in the study group achieved full remission (CDAI score £ 150) compared to 10% of patients in the placebo group, this difference was not statistically significant. Nevertheless, the response rate (CDAI reduction > 100 points) was 90% in the cannabis group and was significantly different from the placebo group. During the two-week washout period, the CDAI score returned to pre-study baseline levels suggesting that the beneficial effects of smoking cannabis were not maintained in the absence of treatment. Patients taking corticosteroids or opioids and assigned to the cannabis group were able to stop using the drugs during cannabis treatment. A statistically significant increase in QoL, measured using the SF-36 QoL instrument, was associated with cannabis treatment but not with placebo. Statistically significant improvements for pain, appetite and in patient satisfaction were reported with cannabis treatment but not with placebo. No significant changes were observed for CRP levels, liver or kidney function, or blood count parameters (e.g. hemoglobin levels, white blood cell count, and hematocrit) between the treatment and placebo groups, although the CRP levels in some individuals in both groups appeared to decrease by 0.5 mg/dl. According to the authors of the study, the reported improvements in disease activity appeared to be symptomatic, with no apparent objective evidence of reduction in inflammatory activity. Principal limitations of this study were the small sample size and a high probability of treatment unblinding. The authors reported the absence of any significant side effects associated with cannabis treatment. Furthermore, no withdrawal symptoms were reported during the two-week washout period.
Note: for sections 4.9.8.3, 4.9.8.4, and 4.9.8.5 below, no clinical studies examining the role of cannabis in the treatment of these disorders have been carried out to date.
4.9.8.3 Diseases of the liver (hepatitis, fibrosis, steatosis, ischemia-reperfusion injury, hepatic encephalopathy)
- Pre-clinical studies suggest CB1 receptor activation is detrimental in liver diseases (e.g. promotes steatosis, fibrosis); while CB2 receptor activation appears to have some beneficial effects.
- Furthermore, pre-clinical studies also suggest that CBD, THCV and ultra-low doses of THC may have some protective effects in hepatic ischemia-reperfusion injury and hepatic encephalopathy.
Mounting evidence suggests an important role for the ECS in the pathophysiology of a multitude of diseases affecting the liver with CB1 and CB2 receptors playing opposing roles: CB1 receptor activation is mostly harmful, whereas CB2 receptor activation is generally protectiveReference 35Reference 1230. CB1 receptors are expressed at low levels in the whole liver, hepatocytes, stellate cells, and hepatic vascular endothelial cells, but increased CB1 receptor expression has been detected in the context of diseases such as hepatocellular carcinoma and primary biliary cirrhosis (reviewed inReference 1231) as well as in alcohol-induced liver disease, non-alcoholic fatty liver disease (NAFLD), liver regeneration and fibrogenesisReference 1230. CB2 receptors are undetectable in normal liver but, like the CB1 receptors, they are upregulated in pathological conditions; these include NAFLD, liver fibrosis, regenerating liver, and hepatocellular carcinoma (reviewed inReference 1231). Increases in the concentrations of the endocannabinoids anandamide and 2-AG in the liver appear to vary depending on the pathophysiological condition in questionReference 35.
Steatosis and fibrosis
As mentioned above, CB1 and CB2 receptors appear to play opposing roles in the liver: activation of the CB1 receptors is implicated in the progression and worsening of alcoholic and metabolic steatosis, NAFLD, liver fibrogenesis, and circulatory failure associated with cirrhosis; stimulation of the CB2 receptors, in general, appears to confer beneficial effects in alcoholic fatty liver, hepatic inflammation, liver injury, liver regeneration, and fibrosis (reviewed inReference 35Reference 1230 and see alsoReference 373-Reference 375Reference 1232). Conversely, antagonism of the CB1 receptor appears to attenuate liver fibrosis in animal models by interfering with the production of several pro-fibrotic, pro-inflammatory, as well as anti-inflammatory mediators secreted in the liver during chronic liver injury and the wound healing processReference 373Reference 1233.
In vitro studies indicate that CBD may also play a protective role in attenuating liver fibrosis induced by acute liver injury or by chronic alcohol exposureReference 1234. CBD dose-dependently triggered the apoptosis of cultured, activated hepatic stellate cells isolated from the livers of rats chronically exposed to an ethanol dietReference 1234. The activation of hepatic stellate cells in response to liver injury is considered a key cellular event underlying hepatic fibrogenesisReference 1234. Furthermore, CBD dose-dependently promoted the selective apoptosis of activated hepatic stellate cells, but not control hepatic stellate cells or primary hepatocytes, by triggering an endoplasmic reticulum-associated cellular stress response leading to apoptosis; this effect was independent of CB receptor activationReference 1234.
Ischemia-reperfusion injury and hepatic encephalopathy
Ischemia-reperfusion injury is the main cause of both primary graft dysfunction (i.e. occurring in 10 - 30% of grafts) and primary non-function of liver allograft (i.e. occurring in 5% of grafts)Reference 1235. Pre-clinical studies indicate a protective role for CBD in hepatic ischemia/reperfusion injury, and hepatic encephalopathy, in mice and ratsReference 1236-Reference 1238.
Pre-treatment of mice with 3 or 10 mg/kg body weight CBD (i.p.), 2 h before induction of ischemia-reperfusion in liver, dose-dependently attenuated serum transaminase elevations at 2 and 6 h of reperfusion compared to vehicleReference 1236. CBD administered immediately following the induction of ischemia, or at 90 min of reperfusion, still attenuated hepatic injury measured at 6 h of reperfusion, though to a lesser extent than when administered prior to the induction of the ischemia-reperfusion injury. Pre-treatment with CBD also significantly reduced the signs of coagulation necrosis observed 24 h after ischemia-reperfusion, significantly attenuated hepatic cell apoptosis, significantly decreased the expression of pro-inflammatory chemokines and cytokines, attenuated neutrophil infiltration into the injury site, and decreased the expression of markers of tissue and cellular injury.
Similar beneficial findings in a rat model of ischemia-reperfusion injury were reported in a different study; however, CBD (5 mg/kg, i.v.) was administered after ischemia-reperfusion injuryReference 1237. CBD treatment resulted in significant reductions in serum transaminase levels, hepatic lipid peroxidation, and the attenuation of various markers of tissue or cellular injury associated with ischemia-reperfusion.
Administration of Δ8-THCV (3 or 10 mg/kg, i.p.) 2 h before induction of hepatic ischemia-reperfusion injury dose-dependently attenuated serum transaminase elevations at 2 and 6 h of reperfusion compared to vehicleReference 1239. Administration of Δ8-THCV post-ischemia attenuated, although to a lesser degree, the hepatic injury measured at 6 h of reperfusion. Pre-treatment with Δ8-THCV also significantly reduced the extent of coagulation necrosis in the liver, attenuated neutrophil infiltration, decreased the expression of hepatic pro-inflammatory chemokines and cytokines, reduced the hepatic levels of markers of oxidative stress, and decreased the extent of hepatocyte cell death following ischemia-reperfusion injury.
Intraperitoneal administration of CBD (5 mg/kg, i.p.) improved neurological, locomotor, and cognitive functions in a mouse model of fulminant hepatic encephalopathyReference 1238. CBD also attenuated the degree of astrogliosis, but did not affect the extent and severity of necrotic lesions in the liver. CBD partially restored whole brain 5-HT levels, as well as the levels of markers of liver function (ammonia, bilirubin, aspartate transaminase - AST, alanine transaminase - ALT) in affected mice.
Lastly, in contrast to high-dose THC (obtained with cannabis smoking or vapourizing), an ultra-low dose of THC (0.002 mg/kg) administered 2 h prior to induction of hepatic ischemia-reperfusion in mice was associated with a significant reduction in hepatic injury as well as significant attenuation of elevations in serum liver transaminases (ALT, AST), hepatic oxidative stress, and acute pro-inflammatory responses (e.g. elevations in TNF-α, IL-1 α, IL-10)Reference 1235.
4.9.8.4 Metabolic syndrome, obesity, diabetes
- Pre-clinical studies suggest acute CB1 receptor activation results in increased fat synthesis and storage while chronic CB1 receptor activation (or CB1 receptor antagonism) results in weight loss and improvement in a variety of metabolic indicators.
- Observational studies suggest an association between chronic cannabis use and an improved metabolic profile, while pre-clinical and very limited clinical evidence suggests a potential beneficial effect of THCV on glycemic control (in patients with type II diabetes).
The endocannabinoid system and energy metabolism
Increasing evidence suggests an important role for the ECS in the regulation of energy balance and metabolism since it exerts regulatory control on virtually every aspect related to search, intake, metabolism, and storage of caloriesReference 1240Reference 1241. Indeed, the ECS is expressed and functions in a variety of neuronal structures involved in regulating energy balance and metabolism such as the hypothalamus (which modulates energy balance and peripheral metabolism), the cortico-limbic structures (which modulate the hedonic aspects of food intake), and the brainstem (which coordinates central-peripheral communication)Reference 1240Reference 1241. Endocannabinoid tone also appears to be modulated by hormones and peptides including leptin, insulin, ghrelin, and corticosteroidsReference 19. Endocannabinoids, in turn, appear to modulate the release of neurotransmitters and neuropeptides such as opioids, serotonin, and GABA, which are known to play a role in regulating appetite mainly through central mechanismsReference 1242. Dysregulation of the ECS is associated with the development of metabolic syndrome and obesity, or conversely anorexia, but may also increase the risk of developing atherosclerosis and type-2 diabetesReference 12Reference 19Reference 1241Reference 1243.
Pre-clinical studies carried out in animal models of obesity and clinical studies performed in obese humans report increased endocannabinoid tone in adipose tissue, liver, pancreas, and in the hypothalamus compared to controlsReference 1244. Furthermore, studies have shown that plasma levels of anandamide and 2-AG play different roles in the regulation of eating behaviour; anandamide acts to start the intake of calories, while 2-AG appears responsible for maintaining the nutrient intake beyond physiological needsReference 1240.
As mentioned above, the regulation of energy balance by the ECS appears to occur both centrally (in the CNS, particularly in the hypothalamus) and peripherally (in multiple organs such as the white adipose tissue, skeletal muscle, pancreas, liver, and small intestine)Reference 12Reference 19Reference 1240Reference 1243Reference 1245. In general, overactivity of the ECS (e.g. CB1 receptor activation) is associated with increased nutrient intake (i.e. increased motivation for palatable food, increase in hedonic properties of palatable food, increased fat preference and intake, increased neural responses to sweet taste, increased odor sensitivity, increased food-seeking behaviour), enhanced energy storage (i.e. increased adipogenesis, decreased fatty acid oxidation, increased glucose uptake, increased insulin secretion, increased liver lipogenesis, decreased liver insulin clearance, decreased liver insulin-induced signaling), reduced energy expenditure (i.e. decreased white adipose tissue lipolysis, decreased mitochondrial biogenesis), and reduced thermogenesis (at the level of brown adipose tissue)Reference 19Reference 1240Reference 1241. Central and peripheral inhibition of CB1 receptor activity, and more generally of the ECS, is beneficial for the treatment of obesity and metabolic disordersReference 1240.
Pre-clinical data
THC and the role of the CB1 receptor
In pre-clinical in vitro studies, THC significantly inhibited basal and catecholamine-triggered lipolysis in a differentiated mouse adipocyte cell line in a concentration-dependent manner, and caused dose-dependent accumulation of lipid droplets in these cells whereas blockade of CB1 receptor activation was associated with the opposite effectReference 25Reference 1241Reference 1246-Reference 1252.
In mice, activation of the CB1 receptor resulted in increased de novo fatty acid synthesis in the liver and increased formation and storage of triglycerides in the adipose tissueReference 12Reference 1253-Reference 1255. In rats, central stimulation of the CB1 receptor was associated with the development of hepatic and adipose tissue insulin resistanceReference 1244. Mice lacking overall CB1 receptor gene expression were hypophagic and were leaner than wild-type mice regardless of diet, had lower plasma insulin levels, did not develop diet-induced insulin resistance or obesity, and had enhanced leptin sensitivityReference 656Reference 1252Reference 1253. In mice, targeted deletion of the CB1 receptor in the forebrain-projecting neurons in the hypothalamus and in the nucleus of the solitary tract, and partial deletion in sympathetic neurons were associated with a lean phenotype and resistance to diet-induced obesity (DIO) and increases in plasma levels of leptin, insulin, glucose, free fatty acids, and triglycerides; these effects resulted from an increase in lipid oxidation and thermogenesis as a consequence of enhanced sympathetic tone and a decrease in energy absorptionReference 1256. Similarly, partial targeted deletion of CNR1 in the adult mouse hypothalamus lead to a significant decrease in body weight gain triggered by an increase in energy expenditure, rather than a decrease in food intakeReference 1255.
Activation of CB1 receptors in hepatocytes favours lipid accumulation and causes liver steatosisReference 1253. Targeted deletion of CNR1 in mouse liver is associated with the development of DIO, but retention of glucose, insulin and leptin sensitivity and lipid indices; targeted hepatic re-expression of CNR1 in CNR1 knockout mice was associated with glucose intolerance and insulin resistance in response to a high-fat diet, but maintenance of proper body weightReference 1257Reference 1258.
Studies with CB1 receptpr antagonists/inverse agonists strongly suggest that antagonism/inverse agonism at the CB1 receptor is associated with reduced caloric intake, weight loss, improvement or reversal of hepatic steatosis, and restoration of insulin and glucose sensitivity and normal lipid indices in various animal models of DIOReference 656Reference 1259-Reference 1265. Clinical studies with the CB1 receptor antagonist rimonabant have strongly supported the data gathered from animal studiesReference 1266-Reference 1272. Muscle endocannabinoid levels and muscle CB1 receptor expression also appear to be altered by consumption of a high-fat diet, and in obesityReference 1241Reference 1273. Furthermore, activation of the ECS inhibits oxidative pathways and mitochondrial biogenesisReference 1241Reference 1274.
An animal study that investigated the effects of chronic THC administration on body weight gain and gut microbiota in mice reported that chronic daily treatment of DIO or lean mice with THC (dose = 2 mg/kg for three weeks and 4 mg/kg for one additional week) was associated with a reduction in weight and fat mass, as well as a reduction in energy intake in DIO mice, but not in lean miceReference 1275. Furthermore, the changes in gut microflora normally observed in DIO mice were prevented with the administration of THC. The change in body weight, fat mass, and daily energy intake appeared to be dose-dependent, with the 4 mg/kg dose being significantly more effective than the 2 mg/kg dose. DIO mice did not show any effect of THC over time on locomotor activity or altered gut transit at any of the tested doses of THC. In DIO mice, the high-fat diet led to an increase in the Firmicutes: Bacteroidetes ratio that was prevented by THC treatment. Furthermore, THC increased abundance of Akkermansia muciniphila spp. which has been implicated in controlling fat storage and adipose tissue metabolism leading to weight loss.
Taken together, the above findings suggest an important role for the CB1 receptor, both centrally and peripherally, in regulating energy balance; acute stimulation of the CB1 receptor promotes energy storage and lipogenesis, whereas CB1 receptor antagonism or chronic CB1 receptor agonism have the opposite effects. Consistent with some of these findings, acute administration of cannabis and prescription cannabinoids (dronabinol, nabilone) are known to increase appetite and body weight and have been used clinically to treat HIV/AIDS-associated anorexia-cachexia, and possibly also cancer-associated cachexia (see Sections 4.4.1 and 4.4.2, respectively).
Observational studies
In contrast to the effects of acute CB1 receptor agonism (e.g. acute THC exposure), studies examining the effects of chronic cannabis use on body weight and metabolic status in non-clinical populations have found the opposite effects.
One cross-sectional, case-control study that examined 30 cannabis smokers and 30 control subjects for any association between cannabis smoking and abdominal fat area, hepatic steatosis, insulin resistance, reduced β-cell function or dyslipidemia reported that chronic cannabis smoking was associated with a statistically significant lower total abdominal fat area and a lower subcutaneous abdominal fat area while no difference was noted for abdominal visceral fat areaReference 1276. However, chronic cannabis smokers showed a relative statistically significant increase in percentage of visceral fat compared to controls. Furthermore, chronic cannabis smoking was not associated with hepatic steatosis, insulin insensitivity, impaired pancreatic β-cell function or glucose intolerance. Median self-reported duration of cannabis use was 12 years (range: 2 - 38 years) and median number of joints smoked per day was 9.5 (range: 3 - 30). Percentage of visceral fat was not related to age, frequency or duration of cannabis use. Hepatic fat content was also not different between the cannabis and control groups and was not related to age, frequency, or duration of cannabis use. Fasting levels of glucose, insulin, total cholesterol, LDL cholesterol, triglycerides or free fatty acids were not different between control and cannabis users.
Other studies report that the prevalence of obesity appears to be significantly lower in cannabis users than in non-users, and the proportion of obese individuals also appeared to decrease with frequency of cannabis use according to a cross-sectional analysis of two U.S. epidemiological studiesReference 1277. In one study, the investigators examined data from the NESARC and the NCS-Replication (NCS-R) which are two face-to-face surveys of adults ages 18 years and older from the civilian non-institutionalized population residing in the United States. The NESARC counts 43 093 respondents (response rate 81%), while the NCS-R is an independent survey that counts 9 282 respondents (response rate 73%). The adjusted prevalence of obesity was 22% and 25% in participants who reported no cannabis use in the past 12 months in the NESARC and NCS-R respectivelyReference 1278. However, the adjusted prevalence of obesity was 14% and 17% in participants reporting the use of cannabis three days per week or more in the NESARC and the NCS-R respectivelyReference 1278. After adjusting for sex and age, as well as other drug use, the use of cannabis was associated with BMI differences in both samples.
Data from the NHANES III, (1988 - 1994), a cross-sectional survey of 10 896 adults, reported that current marijuana users had a lower age-adjusted prevalence of diabetes mellitus compared to non-marijuana using adults (OR = 0.42, 95% CI = 0.33 - 0.55)Reference 1279. Furthermore, the prevalence of elevated C-reactive protein was significantly higher among non-marijuana users (18.9%) than among past (13%), current light (16%), or heavy (9%) marijuana users. The lower odds of diabetes mellitus among marijuana users was statistically significant (OR = 0.36, 95% CI = 0.24 to 0.55). A meta-analysis of eight replication samples from large U.S. epidemiological studies, NHANES (2005 - 2012) and the National Survey on Drug Use and Health (NSDUH, 2005 - 2012), supported these findings, reporting that recently active cannabis smoking and diabetes mellitus are inversely associated, with an OR of 0.7 (95% CI = 0.6 - 0.8)Reference 1280.
Another study looking at 4 657 adult men and women from the NHANES (2005 - 2010) found that current marijuana use was statistically significantly associated with a smaller waist circumference, as well as 16% lower fasting insulin levels and 17% lower insulin resistance (homeostatic model assessment of insulin resistance, HOMA-IR)Reference 1281.
Another study that sought to determine the relationship between cannabis use, obesity, and insulin resistance based on data from 786 Inuit adults from the 2004 Nunavik Inuit Health Survey reported that cannabis use was highly prevalent in the study population (57%) and was statistically associated with a lower BMI, lower percentage fat mass, lower fasting insulin, and lower insulin resistance score (HOMA-IR)Reference 1282. In multivariate analysis, past-year cannabis use was associated with a 0.56 lower likelihood of obesity (95% CI = 0.37 - 0.84).
A review of cannabis use and cardiometabolic risk found a lower BMI and decreased fasting insulin, glucose, insulin resistance and prevalence of diabetes among current cannabis usersReference 1283.
Taken together, the above studies suggest an association between chronic cannabis use and an improved metabolic profile (i.e. lower BMI, lower fasting insulin, lower insulin resistance score, lower likelihood of obesity, lower prevalence of diabetes mellitus).
Role of the CB2 receptor
The CB2 receptor also appears to play an important role in energy balanceReference 1284. Pre-clinical studies in mice indicate that the CB2 receptor is expressed in epididymal adipose tissue in lean mice, and the levels of this receptor appear to increase in the non-parenchymal cell fractions of adipose tissue and liver in genetically obese mice or in wild-type mice fed a high-fat dietReference 1284. Furthermore, systemic administration of a CB2 receptor-selective agonist to lean or obese mice, or exposure of cultured fat pads to the same agonist, was associated with upregulation of a subset of genes linked to inflammation in the adipose tissue but not the liverReference 1284. Conversely, administration of a CB2-selective antagonist reduced inflammation both in adipose tissue and in liver of obese animals. Under a high-fat diet, mice lacking the CB2 receptor displayed a slower body weight progression and were more insulin sensitive than wild-type mice. CB2 knockout mice on a high-fat diet also exhibited minimal hepatic steatosis compared to wild-type mice. Mice deficient in CB2 receptor expression also exhibited increased food intake and body weight with age compared to wild-type miceReference 1285. The CB2 receptor knockout mice did not develop insulin resistance and showed enhanced insulin-stimulated glucose uptake in skeletal muscle.
Another study that examined the role of the CB2 receptor in obesity found that mice lacking CB2 receptor expression showed age-dependent obesity with hypertrophy of the visceral fat, immune cell polarization toward pro-inflammatory subpopulations in fat and liver, and hypertension as well as increased mortality despite normal blood glucoseReference 1286. These mice also developed stronger paw inflammation. These effects did not result from overeating or lack of physical activity. Conversely, CB2 receptor agonism in wild-type littermates fed a high-fat diet prevented diet-induced hypertension, and also reduced diet-induced pro-inflammatory immune responses but did not reduce weight gain. Taken together, these results suggest an important and complex role for the CB2 receptor in energy balance and obesity, and further studies are needed to better understand its role.
Other cannabinoids
Pure Δ9-THCV administered intraperitoneally (3 mg/kg, 10 mg/kg, or 30 mg/kg) in mice suppressed feeding and significantly reduced body weight gain, but this effect appeared to be blocked with a botanical extract containing both Δ9-THCV and Δ9-THCReference 113. Inclusion of CBD into the botanical extract, as a way of attenuating the proposed hyperphagic effects of THC in this study, resulted in a trend towards decreased food intake in treated mice, but the effect did not reach statistical significance.
In another study, chronic administration of 5 mg/kg and 12.5 mg/kg THCV in mice with DIO was associated with a statistically significant reduction of body fat mass but not total body weightReference 1287. THCV at the highest tested doses (5 and 12.5 mg/kg) also tended to increase energy expenditure. Additionally, THCV dose-dependently improved plasma fasting glucose and glucose tolerance following challenge and improved insulin sensitivity (i.e. fasting plasma insulin and insulin response). Administration of THCV was also associated with a reduction in liver triglyceride levels.
Lean and obese rats injected with a cannabis extract (on alternate days, for 28 days) containing a THC: CBN: CBD ratio of 1.0: 1.2: 0.4 (5 mg/kg Δ9-THC) exhibited a significant reduction in weight gain during the study period, but the cannabis extract treatment was not associated with any changes in either insulin or glucose levelsReference 1288.
A randomized, double-blind, placebo-controlled, parallel group pilot study investigated the efficacy and safety of CBD, THCV and combination treatment on glycemic and lipid parameters in patients with type II diabetesReference 1289. In this clinical study, 62 patients were randomized to five treatment arms: CBD (100 mg b.i.d.), THCV (5 mg b.i.d.), 1: 1 ratio of CBD and THCV (5 mg: 5 mg b.i.d.), 20: 1 ratio of CBD to THCV (100 mg: 5 mg b.i.d) or matched placebo for 13 weeks. Compared to placebo, THCV significantly decreased fasting plasma glucose and improved pancreatic β-cell function, improved adiponectin levels, and apolipoprotein A levels although plasma HDL levels were unaffected. Compared with baseline, CBD decreased resistin and increased glucose-dependent insulinotropic peptide and none of the combination treatments had a significant impact on end points. Furthermore, CBD and THCV appeared to be well tolerated. The majority of patients experienced adverse events that were mild to moderate in severity but the incidence of adverse events was similar between all treatment groups. Decreased appetite was the most commonly reported adverse event in all the groups except the 20: 1 CBD: THCV group. The authors suggest that THCV could represent a new therapeutic target in glycemic control in subjects with type II diabetes.
4.9.8.5 Diseases of the pancreas (diabetes, pancreatitis)
- Pre-clinical studies in experimental animal models of certain cannabinoids in the treatment of acute or chronic pancreatitis are limited and conflicting.
- Limited evidence from case studies suggests an association between acute episodes of heavy cannabis use and acute pancreatitis.
- Limited observational studies suggest an association between chronic cannabis use and lower incidence of diabetes mellitus.
- One small clinical study reported that orally administered THC did not alleviate abdominal pain associated with chronic pancreatitis.
Function of the endocannabinoid system in the pancreas
Although there appears to be a general lack of consensus as well as insufficient information regarding the exact expression, distribution, and function of the various ECS components in the pancreas among different species, the pancreas does appear to have at least some, and in certain cases many, of the individual elements of the ECSReference 1242Reference 1290Reference 1291.
Two studies using primary human islet cells suggest that the CB1 and CB2 receptors are expressed in these cells, and that stimulation of the CB1 receptor is associated with secretion of insulin and glucagon while stimulation of the CB2 receptor is associated with either increased or decreased insulin secretionReference 1242Reference 1290Reference 1292. More recently, the endocannabinoid 2-AG has been implicated in the regulation of both insulin and glucagon secretion in human pancreasReference 1291.
Intra-muscular administration of cannabis resin (containing 6.3% Δ9-THC, 3.2% CBD, and 1.9% CBN) at increasing doses (Δ9-THC at 2.5, 5.0, and 10 mg/kg) to dogs was associated with a progressive increase in plasma glucose levels which reached maximum values 90 min after administration, with a return to baseline values 180 min after administrationReference 1293. Injection of anandamide or a CB1 receptor-selective agonist in rats was associated with acute glucose intolerance, whereas administration of a CB1 receptor inverse agonist attenuated this effectReference 1294. In humans, intravenous injection of 6 mg of Δ9-THC to healthy, non-obese, male volunteers was associated with acute impairment of glucose tolerance in response to glucose challenge with no change in plasma insulin levelsReference 1295.
Survey data
A cross-sectional study of 10 896 adults, ages 20 to 59, who were participants in the NHANES III, a nationally representative sample of the U.S. population, reported that cannabis use was independently associated with a decreased prevalence of diabetes mellitus, and that cannabis users had lower odds of developing diabetes mellitus compared to non-usersReference 1279. The lowest prevalence of diabetes mellitus was seen in current, light cannabis users, but current heavy users and past users also had a lower prevalence of diabetes mellitus than non-cannabis users. Due to limitations in study methodology (e.g. cross-sectional nature of the study, self-report bias, and inconsistent sampling methodology) as well as the possibility of additional and uncontrolled confounding factors, the authors indicate that it is not yet possible to conclude that cannabis use does not lead to diabetes mellitus, nor that cannabis should be considered a treatment for this disorder.
Cannabis, the endocannabinoid system, and acute and chronic pancreatitis
Acute, heavy cannabis use has been linked to the development of acute pancreatitisReference 377-Reference 381. A recent systematic review of cannabis-induced acute pancreatitis suggests increased prevalence mainly amongst younger patients under 35 years of ageReference 381. Furthermore, subsequent causality analysis suggests that cannabis may be a possible risk factor for toxin-induced acute pancreatitis. Acute pancreatitis is a potentially lethal disorder involving inflammation, cell death, and complex neuroimmune interactions; the management of chronic pancreatitis remains clinically challenging with no definite cure and supportive measures are the only treatment availableReference 1296Reference 1297. Pancreatic tissue isolated from patients with acute pancreatitis has been reported to have a marked upregulation of CB1 and CB2 receptors in the acini and ducts as well as elevated levels of the endocannabinoid anandamide but not 2-AGReference 1296.
In a subsequent study, an increase in the expression levels of CB1 and CB2 receptors, and a decrease in the levels of endocannabinoids (anandamide and 2-AG) were noted in tissue samples isolated from patients suffering from chronic pancreatitis compared to pancreatic tissues isolated from healthy subjectsReference 1297. In addition, in contrast to the findings obtained for acute pancreatitisReference 1296, tissues isolated from patients with chronic pancreatitis appeared to have decreased levels of both anandamide and 2-AGReference 1297. Activation of CB1 and CB2 receptors in chronic pancreatitis-derived pancreatic stellate cells was also associated with the induction of a quiescent-cell phenotype as well as the downregulation of extracellular matrix protein production and inflammatory cytokine productionReference 1297.
Pre-clinical data and acute or chronic pancreatitis
There are only a handful of reports on the effects of cannabinoids in experimental animal models of acute or chronic pancreatitis, and the findings from these reports are conflicting.
Elevations in the plasma levels of anandamide have been noted in a rat model of severe acute pancreatitisReference 1298, and administration of the CB1 receptor antagonist AM251 after induction of pancreatitis appeared to improve the course of the diseaseReference 1298. In another study, administration of anandamide prior to induction of pancreatic damage further aggravated the usual course of the disease, whereas pre-treatment with the CB1 receptor antagonist AM251 prevented the development of cerulein-induced pancreatitis and when administered after injury also appeared to reverse cerulein-induced pancreatic damageReference 1299. Similarly, mice treated with the CB1 receptor antagonist rimonabant prior to cerulein-induced pancreatitis exhibited significantly decreased pancreatic damage as well as decreased production of inflammatory cytokinesReference 1300. Subcutaneous administration of a synthetic CB1 /CB2 receptor agonist, both prior to as well as after induction of acute pancreatitis in mice, attenuated the abdominal pain, inflammation, and tissue pathology associated with pancreatitisReference 1296. In contrast, a different study reported that pre-treatment of rats with a synthetic CB1/CB2 receptor agonist before induction of experimentally-induced pancreatitis attenuated the extent of tissue damage and the release of inflammatory cytokines, whereas administration of the same agonist after the induction of pancreatitis had the opposite effects and appeared to aggravate the course of the diseaseReference 1301. These contradictory findings may be due to differences in experimental methods, differences in timing of drug administration, differences in the types of agonists and antagonists that were used, differences in the route of administration, and differences in animal species.
Clinical data
A randomized, single dose, double-blinded, placebo-controlled, two-way, cross-over clinical study in 24 patients (sub-divided into daily opioid and non-opioid users) suffering from abdominal pain associated with chronic pancreatitis examined the analgesic efficacy, pharmacokinetics and tolerability of orally-administered 8 mg THC or active placebo (5 or 10 mg diazepam) in a double-dummy designReference 595. The study reported a lack of efficacy with THC in reducing chronic pain associated with chronic pancreatitis but good tolerance with only mild or moderate adverse events. No differences were noted between THC and diazepam in VAS measures of alertness, mood, and calmness but THC was associated with a significant increase in anxiety compared to diazepam. Heart rate was also significantly increased with THC compared to diazepam. Most frequent adverse events associated with THC were somnolence, dry mouth, dizziness and euphoric mood. No serious adverse events were noted. Pharmacokinetic parameters of THC were similar between opioid and non-opioid users and showed a delayed absorption and increased variability compared to healthy volunteers. Study limitations included small number of study subjects, short trial duration, single dose design, and low dosage of THC.
4.9.9 Anti-neoplastic properties
- Pre-clinical studies suggest that certain cannabinoids (THC, CBD, CBG, CBC, CBDA) often, but not always block growth of cancer cells in vitro and display a variety of anti-neoplastic effects in vivo, though typically at very high doses that would not be seen clinically.
- While limited evidence from one observational study suggests cancer patients use cannabis to alleviate symptoms associated with cancer (e.g. chemosensory alterations, weight loss, depression, pain), there has only been one limited clinical study in patients with glioblastoma multiforme, which reported that intra-tumoural injection of high doses of THC did not improve patient survival beyond that seen with conventional chemotherapeutic agents.
A number of studies have implicated the ECS in the pathophysiology of cancer. In general, endocannabinoids seem to have a protective effect against carcinogenesis, and proper regulation of local endocannabinoid tone is likely an important strategy in controlling the malignancy of different cancers - dysregulation of the ECS is associated with carcinogenesisReference 1302Reference 1303.
When compared with healthy tissues, the levels of endocannabinoids appear to be elevated in glioblastomas, meningiomas, pituitary adenomas, prostate and colon carcinomas, and endometrial sarcomasReference 1206Reference 1304-Reference 1308. Furthermore, the expression levels of cannabinoid receptors are also differentially regulated in normal versus malignant cells, with increased or decreased levels of these receptors varying with cancer type (reviewed inReference 1303). Such differences in the levels of endocannabinoids and in the patterns of expression levels of cannabinoid receptors across different cancer types reflect the complex role of the ECS in cancer and are likely to pose challenges to potential therapeutic approaches. Nonetheless, a large number of pre-clinical studies have shown that endocannabinoids, certain synthetic cannabinoid agonists, and some phytocannabinoids can inhibit tumour growth and progression of numerous types of cancers through various mechanisms including promotion of apoptosis, cell-cycle arrest/growth inhibition, and prevention of metastasis through inhibition of tumour invasion, migration, and neo-angiogenesis (reviewed inReference 1303Reference 1309).
In some in vitro studies, the anti-neoplastic effects of Δ9-THC appear to be biphasic: lower doses (under 100 nM) are considered pro-proliferative; higher doses (above 100 nM) are thought to be anti-proliferativeReference 1310, although many exceptions have been noted. Furthermore, cannabinoid concentrations above 100 nM, that is one to two orders of magnitude above the average affinity of these receptors for cannabinoids, are likely to produce off-target, cannabinoid receptor-independent effectsReference 1311. As a point of reference, single oral doses of dronabinol (Δ9-THC) of 2.5, 5, and 10 mg have been associated with mean peak Δ9-THC plasma concentrations of 0.65, 1.83, and 6.22 ng/mL, respectivelyReference 227. These concentrations correspond to concentrations of 2, 6, and 20 nM Δ9-THC. Doubling of these daily oral doses is associated with mean peak Δ9-THC plasma concentrations of 1.3, 2.9, and 7.9 ng/mL Δ9-THCReference 227, respectively, corresponding to 4, 9, and 30 nM Δ9-THC. Continuous dosing for seven days with 20 mg doses of dronabinol (total daily doses of 40 - 120 mg dronabinol) gave mean plasma Δ9-THC concentrations of ~20 ng/mL or 60 nMReference 420. Smoking a 1 g joint containing 12.5% Δ9-THC can be assumed, based on the literature, to yield peak plasma Δ9-THC concentrations between 50 and 100 ng/mL or more (see Section 3.1 Smoking, subsection Plasma concentrations of Δ9-THC following smoking). Such Δ9-THC plasma concentrations correspond to 160 and 320 nM Δ9-THC, respectively. Plasma concentrations of Δ9-THC are also known to vary widely across individuals, and diminish more rapidly when cannabis (or Δ9-THC) is smoked compared to when cannabis (or Δ9-THC) is ingested orally. With respect to doses expressed in mg/kg of body weight, a daily oral dose of 2.5 mg of dronabinol (Δ9-THC) can be estimated to correspond to a dose of approximately 0.04 mg/kg (assuming a body weight of 70 kg), whereas a daily oral dose of 40 mg of dronabinol would correspond to a dose of approximately 0.6 mg/kg of dronabinol. Smoking a 1 g joint containing 12.5% Δ9-THC would correspond to a hypothetical dose of 1.8 mg/kg Δ9-THC. These values represent estimative comparisons as the actual tissue concentrations of cannabinoids are likely to vary significantly both within and across individuals, among varying routes of administration and cell types; and micro-environments in vitro and in vivo are conceivably different.
The following paragraphs summarize the main findings from a number of pre-clinical in vitro and in vivo studies of cannabinoids in neoplastic diseases. Clinical data are presented at the end of this section.
Pre-clinical data
In vitro studies suggest that Δ9-THC decreases cell proliferation and increases cell death in human glioblastoma multiforme cell lines, with CB receptor activation accounting for only part of the observed effectsReference 1312. In the case of astrocytomas, higher concentrations were deemed to be clinically preferable because this would bypass CB receptor activation and induce apoptosis in all astrocytoma cell sub-populationsReference 1313. In the case of breast cancer, Δ9-THC reduced human breast cancer cell proliferation at concentrations of 4 to 10 μM, with more aggressive estrogen receptor-negative tumour cells being more sensitive to the effects of THCReference 1314. In apparent contrast, another study showed that Δ9-THC (50 μM in vitro or 50 mg/kg in vivo) enhanced breast cancer growth and metastasis even though the breast cancer cells did not express detectable levels of CB receptors suggesting a CB1 receptor-independent mechanism of actionReference 1315. Furthermore, Δ9-THC, CBD, and CBN all stimulated breast cancer cell proliferation at concentrations ranging from 5 to 20 μMReference 1316, but this effect appeared to depend, to some extent, on the hormonal milieu (with lower estrogen levels promoting, and higher estrogen levels inhibiting growth). On the other hand, cannabinoids such as CBG, CBC, CBDA, and THCA as well as cannabinoid-based extracts enriched in either Δ9-THC or CBD inhibited cell proliferation (in the micromolar range) in a number of different breast cancer cell linesReference 1317. In in vitro studies examining the role of cannabinoids in lung cancer, Δ9-THC (10 - 15 μM) attenuated growth factor-induced migration and invasion of non-small cell lung cancer cell linesReference 1318. In the case of colorectal cancer, Δ9-THC at concentrations of 2.5 μM and above (range: 7.5 - 12.5 μM) were associated with a decrease in colorectal cancer cell survival, whereas lower concentrations (100 nM - 1 μM) had no effectReference 1319. An in vitro study examining the role of THC in skin cancer reported that 5 and 10 µM THC had no effect on cell proliferation of HCmel12 or B16 skin cancer cellsReference 1320. Another in vitro study examining the anti-neoplastic effects of CBG on colon carcinogenesis found that CBG (3 - 30 µM) inhibited colon cancer cell viability but this effect was both time and environment-dependentReference 1321. Another study reported that a CBD botanical extract (66% CBD, 2.4% THC, 1.0% CBG, 0.9% CBDV, 0.3% CBDA, 0.1% CBN) as well as pure CBD (at concentrations of 1 - 5 µM) did not affect viability of colorectal cancer cells (DLD-1 and HCT116)Reference 1322. However, the CBD botanical extract and pure CBD exerted anti-proliferative effects on these colon cancer cells, but not on healthy cellsReference 1322. One other in vitro study assessed the anti-proliferative effect of CBD and THC used alone and in combination with radiotherapyReference 1323. In this study, treatment of two human and one murine glioma cell line with pure CBD, THC, or two cannabinoid botanical drug substances (BDS) enriched in either CBD or THC (CBD BDS = 64% CBD, 3.6% THC, 1.1% CBG, 5.2% CBC, 1.3% CBDV, 0.4% CBDA; or THC BDS = 65% THC, 0.4% CBD, 1.3% CBG, 1.8% CBC, 0.9% THCV, 0.4% THCA, 2% CBN, and 0.2% cannabitriol) was associated with a reduction in cell numbers in all three cell lines in a dose-dependent mannerReference 1323. A dose of approximately 10 µmol/L for all tested substances was associated with a 50% reduction in cell numbers (IC50). Combining pure THC and pure CBD was associated with a hyper-additive inhibitory effect on cell numbers. In additional experiments, pre-treatment of the three glioma cell lines with a combination of pure THC and CBD (10 µmol/L of each) along with irradiation was associated with a slowing of DNA double-strand break repair and a trend towards increased cell deathReference 1323. In another study, the anti-leukemic efficacy of THC was examined in several leukemia cell lines and native leukemia blasts cultured ex vivoReference 1324. THC produced significant and dose-dependent inhibition of cellular proliferation with an IC50 of 15 µM in a T-lymphoblastic leukemia cell line and with an IC50 of 18 µM in an acute myeloid leukemia cell line. Higher doses were associated with apoptosis that was CB1 and CB2 receptor-dependent. THC treatment of myeloid leukemia and lymphatic leukemia blasts cultured from patients and grown ex vivo was associated with a reduction in the number of viable cellsReference 1324.
Taken together, these and other in vitro studies suggest that cannabinoids often, but not always, exert growth-inhibiting actions on cultured cancer cells and can have complex biological effects in the context of malignancies. Differences in experimental conditions, cancer cell type, cell growth environment, CB-receptor expression, hormonal levels, and the existence of CB-receptor dependent and independent regulatory mechanisms all appear to affect the control of growth, proliferation, and invasion of cancer cells in response to cannabinoids. Furthermore, these findings also suggest that the effective inhibitory concentrations of Δ9-THC seen in vitro are significantly (i.e. one to four orders of magnitude) higher than the concentrations of Δ9-THC seen when it is taken clinically, depending on the route of administration.
A pre-clinical in vivo study in rats showed that intra-tumoural administration of Δ9-THC caused significant regression of intra-cranial malignant gliomas, and an accompanying increase in animal survival time without any neurotoxicity to healthy tissuesReference 1325. Furthermore, no substantial change was observed in certain behavioural measures suggesting that the effect of Δ9-THC was limited to diseased neural tissues. Other studies showed that peritumoural administration of 0.5 mg Δ9-THC/day, twice per week, for 90 days, significantly slowed focal breast tumour growth, blocked tumour generation, decreased total tumour burden, delayed the appearance of subsequent tumours, and impaired tumour vascularization in the ErbB2-positive metastatic breast cancer mouse modelReference 1326. Δ9-THC, at doses of 5 mg/kg/day, administered intraperitoneally or intra-tumourally, also dramatically decreased the growth and metastasis as well as the vascularization of xenografted non-small cell lung cancer cell lines in immunodeficient miceReference 1318. CBD (5 mg/kg) or CBD-rich extract (6.5 mg/kg) administered intra-tumourally or intraperitoneally, twice per week, to breast-cancer-cell-xenografted athymic mice significantly decreased both tumour volume and the number of metastatic nodulesReference 1317. Other investigators showed that intraperitoneal administration of CBD at 1 or 5 mg/kg/day significantly reduced the growth and metastasis of an aggressive breast cancer cell line in immune-competent miceReference 1327. Importantly, the primary tumour acquired resistance to the inhibitory properties of CBD by day 25 of treatment. An in vivo study that evaluated the anti-tumour efficacy of biodegradable polymeric microparticles allowing controlled release of THC (25 mg administered, 10 mg released) and CBD (27 mg administered, 11 mg released) into glioma xenografts showed a significant reduction in glioma growth. These doses are far higher than could be achieved by systemic administration of these cannabinoids and would also be associated with significant psychoactive effectsReference 1328. An in vivo study examining the anti-neoplastic effects of CBG on colon carcinogenesis found that CBG (3 and 10 mg/kg CBG) inhibited xenografted colon cancer cell growth by 45%Reference 1321. An in vivo study assessing the effect of a CBD botanical extract on colorectal cancer reported that a daily injection of the extract (5 mg/kg, i.p.) significantly lowered average tumour volume, but that effect was only maintained for seven days after which time no differences in tumour size were observed between the experimental and control groupsReference 1322. One study examined the effect of combining THC, CBD and radiotherapy in a mouse model of gliomaReference 1323. In this study, combining THC and CBD (100 µmol/L each) was associated with a reduction in tumour progression and further addition of irradiation to the combination cannabinoid treatment was associated with further reduction in tumour growthReference 1323. An in vivo study of the effects of THC in skin cancer reported that doses of 5 mg/kg THC/day (s.c.) significantly reduced the growth of HCmel12 melanomas but not B16 melanomasReference 1320. Furthermore, the anti-neoplastic effect was found to be CB receptor-dependent. Lastly, review of the in vivo anti-neoplastic activity of CBD reported that chronic systemic administration of CBD at doses in the range of 1 - 5 mg/kg was associated with anti-metastatic activity, while doses between 15 and 25 mg/kg of CBD administered systemically and 10 mg/kg CBD administered orally were needed to limit tumour progression in mouse xenograft model that more closely resemble primary tumour growthReference 1329. Furthermore, doses of THC and CBD of 4 mg/kg each delivered systemically and 100 mg/kg CBD delivered orally were reported to sensitize tumours to first line agents in mouse xenograft models that more closely resemble primary tumour growthReference 1329. Taken together, these studies suggest that cannabinoids such as Δ9-THC and CBD can, at least under a specific set of circumstances, have anti-neoplastic effects in various animal models of cancer at certain dose ranges.
Combining cannabinoids with other chemotherapeutic agents
Pre-clinical in vitro and in vivo studies investigating the effects of combining cannabinoids with frequently used chemotherapeutic agents have also been performed. One in vitro study showed that combining sub-maximal doses of Δ9-THC (0.75 μM) with cisplatin or doxorubicin reduced the viability of an astrocytoma cell line in a synergistic mannerReference 1330. Likewise, combining sub-maximal doses of Δ9-THC with temozolomide reduced the in vitro viability of several human glioma cell lines and primary cultures of glioma cells derived from human glioblastoma multiforme biopsiesReference 1331. Complementing these findings, an in vivo study showed that combined treatment with Δ9-THC (15 mg/kg/day) and temozolomide (5 mg/kg/day) reduced the growth of glioma tumour xenografts in mice in a synergistic mannerReference 1331. These studies suggest that cannabinoids might sensitize certain tumours to the anti-neoplastic action of conventional chemotherapeutic drugs.
Observational and clinical data
A non-randomized, cross-sectional survey and retrospective chart review of 15 patients (mostly male) with a history of head and neck cancer treated with radiotherapy or chemotherapy who had also used cannabis for medical purposes examined patient characteristics and stated reasons for use of cannabis for medical purposes to manage long-term head and neck cancer treatment-related morbiditiesReference 1332. The study revealed that most of the survey participants reported smoking cannabis while fewer reported ingestion, vapourization, and use of homemade concentrated oil. The majority of the patients reported using cannabis daily or more frequently. Cannabis was reported to provide benefit in altered sense, weight maintenance, depression, pain, appetite, dysphagia, xerostomia, muscle spasms, and sticky saliva as side effects of radiotherapy.
A case report of two children with septum pellucidum/forniceal pilocytic astrocytomas noted spontaneous regression of the tumours during the same period that cannabis was consumed via inhalation (reported frequency of three times per week to daily, strength and composition unknown)Reference 1333. The patients did not receive any adjuvant treatment following surgery and were followed-up post-operatively over the course of a number of years; regression of the tumours appeared to coincide with cannabis use that according to the authors raises the possibility that cannabis may have played a role in tumour regression.
There is only one report of a clinical study of Δ9-THC to treat cancerReference 1334. In this non-placebo controlled pilot study, nine patients with glioblastoma multiforme who had failed to respond to standard surgical and radiation therapy, had clear evidence of tumour progression, and had a minimum Karnofsky score of 60, were treated with 20 to 40 µg Δ9-THC intra-tumourally per day (with doses of up to 80 - 180 µg Δ9-THC per day). Median treatment duration was 15 days. Intra-tumoural administration of Δ9-THC appeared to be well tolerated and the effect of Δ9-THC on patient survival was similar to that observed in other studies using chemotherapeutic agents such as temozolomide or carmustineReference 1335Reference 1336. Administration of Δ9-THC reduced the expression of some molecular markers of glioblastoma multiforme progression in tumour specimens obtained from treated patientsReference 1330Reference 1334Reference 1337 and in vitro, Δ9-THC inhibited the proliferation and decreased the viability of tumour cells isolated from glioblastoma biopsies, most likely through a combination of cell-cycle arrest and apoptosisReference 1334Reference 1338. In addition, results from a separate in vitro study suggest that CBD enhanced the inhibitory effects of Δ9-THC on human glioblastoma cell proliferation and survivalReference 1338.
Despite the evidence presented in these and other studies, there is some concern regarding the use of Δ9-THC in anti-tumoural strategies, especially if it is administered systemically because of its high hydrophobicity, relatively low agonist potency, and its well-known psychoactive propertiesReference 1303Reference 1339Reference 1340. Much also remains to be known about the expression levels of the cannabinoid receptors in different cancers, the effects of different cannabinoids on different cancer cell types, the identification of factors that confer resistance to cannabinoid treatment, as well as the most efficient approaches for enhancing cannabinoid anti-tumoural activity whether alone or in combination with other therapiesReference 1317Reference 1339. Lastly, the apparent biphasic effect of cannabinoids further highlights the need for more comprehensive dose-response studiesReference 1341.
4.9.9.10 Emerging potential therapeutic uses
Atherosclerosis
There are a few pre-clinical reports which suggest that administration of a low dose of THC, a CB1 receptor antagonist, or a CB2 receptor agonist may reduce the progression of atherosclerosis in mouse models of the diseaseReference 1342-Reference 1344. Oral administration of THC (1 mg/kg/day) has been associated with significant inhibition of disease progression in the apolipoprotein E (ApoE) knockout mouse, a mouse model of atherosclerosisReference 1342. The beneficial effect of THC in this study was mediated by the CB2 receptor, likely through its inhibitory effects on immune system cells (macrophages and T-cells) located in or near atherosclerotic lesions. These findings were supported by another study that showed that intraperitoneal administration of a synthetic CB1/CB2 receptor agonist significantly reduced aortic plaque area in the ApoE knockout mouseReference 1344. Administration of the cannabinoid receptor agonist reduced macrophage adhesion and infiltration into the atherosclerotic plaque, as well as reducing the expression of vascular cellular adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and P-selectin in the aorta. Again, the observed beneficial effects appeared to result from activation of the CB2 receptor. A separate study confirmed the atheroprotective effects of selective CB2 receptor activation by demonstrating increased vascular leukocyte infiltration in atherosclerotic plaques in mice lacking both ApoE and CB2 receptors compared to ApoE knockout mice, and decreased atherosclerotic plaque formation and reduced vascular superoxide release in ApoE knockout mice treated with a CB2 receptor selective agonistReference 1345. In contrast to these findings, a different study showed that activation or deletion of the CB2 receptor did not modulate atherogenesis in the LDL receptor knockout mouse model of atherosclerosisReference 1346. Another study suggested that the CB2 receptor, while not affecting the size of atherosclerotic lesions in LDL receptor knockout mice, did increase lesional macrophage accumulation and smooth muscle cell infiltration, as well as reduce lesional apoptosis and alter the extra-cellular matrix of lesionsReference 1347. The findings from this study suggested that while the CB2 receptor did not play a significant role in the initial formation of atherosclerotic lesions, it did play a role in modulating the progression of the disease. On the other hand, activation of the CB1 receptor is associated with the release of reactive oxygen species and endothelial cell deathReference 1348, and CB1 receptor blockade by rimonabant in ApoE knockout mice was associated with a significant reduction in the relative size of aortic atherosclerotic lesionsReference 1343. In conclusion, it appears that in the case of atherosclerosis, the CB1 and CB2 receptors play opposing roles - the CB1 receptor appears to be atherogenic, whereas the CB2 receptor appears to be anti-atherogenicReference 1343Reference 1345Reference 1348-Reference 1350 although some uncertainty still remains regarding the exact role played by the CB2 receptorReference 1351. CBD has also been shown to potently inhibit the activity of the enzyme 15-lipoxygenase, which has been implicated in the pathophysiology of atherogenesisReference 1349Reference 1352. Further studies are needed in this area.
5.0 Precautions
The contraindications that apply to those considering using prescription cannabinoid-based therapies (such as nabilone (e.g. Cesamet®), nabiximols (e.g. Sativex®) or dronabinol (e.g. Marinol®, no longer available in Canada)) also apply to those considering using cannabis, especially THC-predominant cannabis. Healthcare professionals may also wish to consult the College of Family Physicians of Canada preliminary guidance document on authorizing dried cannabis for medical purposesReference 586 and the recent simplified guideline for prescribing medical cannabinoids in primary careReference 587.
The risk/benefit ratio of using cannabis (especially THC-predominant cannabis) should be carefully evaluated in patients with the following medical conditions because of individual variation in response and tolerance to its effects, as well as the difficulty in dosing noted in Section 3.0. Consult Figure 3 for additional guidance.
- Cannabis (especially cannabis administered by smoking or vapourization) containing primarily THC (and especially higher levels of THC with little if any CBD) should not be used in any person under the age of 25Reference 540Reference 1106, unless the benefit/risk ratio is considered by the physician to be favourable. The adverse effects of (THC-predominant) cannabis use on mental health are greater during development, particularly during adolescence (ages 10 to 24), than in adulthood with risks increasing with younger age, frequent use and THC potencyReference 151Reference 182Reference 198Reference 205Reference 541Reference 1116Reference 1120 (see Section 7.7.3). Emerging evidence suggests a statistically significant association between use of ultra-high potency cannabis concentrates such as BHO with higher levels of physical dependenceReference 520.
- Cannabis should not be used in any patient who has a history of hypersensitivity to any cannabinoid or to smoke (if cannabis will be smoked)Reference 365Reference 366Reference 393Reference 394Reference 1353Reference 1354 (see Section 7.3).
- Cannabis should not be used in patients with severe cardiovascular or cerebrovascular disease because of occasional hypotension, possible hypertension, syncope, tachycardia, myocardial infarction and strokeReference 141Reference 180Reference 353Reference 354Reference 1355-Reference 1357 (see Section 7.5).
- Smoked cannabis is generally not recommended in patients with respiratory disease (e.g. insufficiency such as asthma or chronic obstructive pulmonary disease)Reference 364Reference 365 (see Section 7.2).
- Cannabis should not be used in patients with severe liver or renal disease. In patients with ongoing chronic hepatitis C, daily cannabis use has been shown to be a predictor of steatosis severity in these individualsReference 34Reference 1358 (see Section 7.6.2).
- Cannabis containing primarily THC (with little if any CBD), and especially higher levels of THC, should not be used in patients with a personal history of psychiatric disorders (i.e. psychosis, schizophrenia, anxiety and mood disorders), or a familial history of schizophreniaReference 183Reference 1085 (see Section 7.7.3).
- Cannabis should be used with caution in patients with a history of substance abuse, including alcohol abuse, because such individuals may be more prone to abuse cannabis, which itself, is a frequently abused substanceReference 1078Reference 1359Reference 1360 (see Sections 2.4 and 4.9.5.4).
- Cannabis should be used with caution in patients receiving concomitant therapy with sedative-hypnotics or other psychoactive drugs because of the potential for additive or synergistic CNS depressant or psychoactive effectsReference 219-Reference 221 (also see Section 7.7). Cannabis may also exacerbate the CNS depressant effects of alcohol and increase the incidence of adverse effects and driving impairment (see Section 7.7.2). Patients should be advised of the negative effects of psychoactive cannabis/cannabinoids on memory, cognitive and psychomotor skills and to report any mental or behavioural changes that occur after using cannabisReference 233Reference 234.
- Cannabis is not recommended in women of childbearing age not on a reliable contraceptive, as well as those planning pregnancy, and those who are pregnant, or breastfeedingReference 61Reference 1361Reference 1362 (see Sections 6.0 and 7.4).
6.0 Warnings
Cannabis is one of the most widely abused illicit drugs, and can produce physical and psychological dependenceReference 145Reference 190Reference 329Reference 1363Reference 1364. The drug has complex effects in the CNS and can cause cognitive and memory impairment, changes in mood, altered perception, and decreased impulse control among many other effectsReference 235Reference 1365-Reference 1367.
Patients should be supervised when administration is initiated and should be monitored on a regular basis.
Dosing: In the case of smoked/vapourized cannabis, the dose required to achieve therapeutic effects and avoid adverse effects is difficult to estimate and is affected by the potency of the product, its processing, and by different smoking and vapourizing techniques. These techniques include depth of inhalation, duration of breath holding and the number and frequency of puffs, as well, as how much of the cigarette is smoked or how much plant material or vaping liquid is vapourized. Dosing should begin at lowest possible dose to maximize potential therapeutic effects and minimize risks of adverse effects. Smoking or vapourization should proceed slowly and cautiously in a gradual fashion (with sufficient time between puffs/inhalations to gauge effects - e.g. 30 min) and should cease if the patient begins to experience the following effects: disorientation, dizziness, ataxia, agitation, anxiety, tachycardia and orthostatic hypotension, depression, hallucinations, or psychosis. There is also insufficient information regarding oral dosing, but the patient should be made aware that the effects following oral administration only begin to be felt 30 min to 1 h or more after ingestion, and peak at 3 - 4 h, that consumption of cannabis-based products (e.g. cookies, baked goods) should proceed slowly, and that edibles should be consumed only in small amounts at a time with sufficient time between doses in order to gauge the effects and to prevent overdosingReference 227Reference 405 (see Section 3.0).
Psychosis: Anyone experiencing an acute psychotic reaction to cannabis or cannabinoids should promptly stop taking the drug and seek immediate medical attention. A psychotic reaction is defined as a loss of contact with reality characterized by one or more of the following: changes in thinking patterns (difficulty concentrating, memory loss, and/or disconnected thoughts), delusions (fixed false beliefs not anchored in reality), hallucinations (seeing, hearing, tasting, smelling or feeling something that does not exist in reality), changes in mood (intense bursts of emotion, absence of, or blunted emotions), very disorganized behaviour or speech, and thoughts of death and suicideReference 165Reference 173Reference 508Reference 1368 (see Section 7.7.3.2).
Occupational hazards: Patients using cannabis/cannabinoids should be warned not to drive or to perform hazardous tasks, such as operating heavy machinery, because impairment of mental alertness and physical coordination resulting from the use of cannabis or cannabinoids may significantly decrease their ability to perform such tasksReference 154Reference 155Reference 204Reference 229Reference 240Reference 495Reference 1369. Depending on the dose, the route of administration and the frequency of use, impairment can last for over 24 h after last use because of the long half-life of Δ9-THCReference 78Reference 152Reference 431Reference 1370Reference 1371. Furthermore, impairment can be exacerbated with co-consumption of other CNS depressants (e.g. benzodiazepines, barbiturates, opioids, anti-histamines, muscle relaxants, or ethanolReference 159Reference 219Reference 220Reference 227Reference 1372-Reference 1375 (see Section 7.7.2).
Pregnancy: Pre-clinical studies suggest that multiple components of the endocannabinoid system as well as endocannabinoid tone play a critical role in fertilization, oviductal transport, implantation, and fetal/placental development (reviewed inReference 1376-Reference 1378). In fact, CB1 and CB2 receptors are expressed (proteins) in rodent and human ovarian tissue, oviduct, uterus and testisReference 1378. These receptors are also detected (proteins) in oocytes at all stages of maturationReference 1378. Furtherrmore, CB1 receptor mRNA is expressed from the four-cell stage through the blastocyst stage, while CB2 receptor mRNA is expressed from the one-cell stage to the blastocyst stageReference 1379. High circulating levels of anandamide have been associated with an increased incidence of miscarriageReference 1380. In addition, there is a risk that maternal exposure to cannabis or cannabinoids could potentially adversely affect conception and/or maintenance of pregnancy. However, two recent systematic reviews and meta-analyses reported mixed conclusions about the harms to neonatal health with cannabis use in uteroReference 1361Reference 1362. Nevertheless, it may be prudent to avoid the use of cannabis during pregnancy as there is evidence of reduced neonatal birthweight and long-term developmental problems in children exposed to cannabis in uteroReference 1381-Reference 1384. THC readily crosses into the placentaReference 1384. CB1 receptors are expressed (proteins) in germ cells, from spermatogonia to spermatozoa, and Leydig cells, while CB2 receptors (proteins) are expressed in Sertoli cellsReference 1378.Men, especially those on the borderline of infertility and intending to start a family, are cautioned against using cannabis since exposure to cannabis or THC could potentially reduce the success rates of intended pregnanciesReference 396 (see Section 7.4).
Lactation: Cannabinoids are excreted in human milk and may be absorbed by the nursing babyReference 1385Reference 1386. Because of potential risks to the child, nursing mothers should not use cannabis.
6.1 Tolerance, dependence, and withdrawal symptoms
Tolerance, and psychological and physical dependence can occur with prolonged use of cannabisReference 181Reference 324Reference 329. Dependence develops slowly and appears more likely with higher, more frequent dosingReference 336Reference 337Reference 510Reference 512. Emerging evidence suggests use of ultra-high potency cannabis concentrates such as BHO is associated with greater levels of physical dependenceReference 520. See Section 2.4 for further information on tolerance, dependence, and withdrawal symptoms.
6.2 Drug interactions
Drug interactions involving cannabis and cannabinoids can be expected to vary considerably in their clinical significance given the wide variability in products, potencies, ratios of THC and CBD, doses, routes of administration, populations using cannabinoids and other factorsReference 468. However, some of the more clinically significant interactions may occur when cannabis is taken with other CNS depressant drugs such as sedative-hypnotics or alcoholReference 159Reference 219-Reference 221Reference 1373-Reference 1375Reference 1387Reference 1388. An overdose can occur if a patient is smoking/vapourizing cannabis and consuming orally administered cannabinoids, whether from prescription cannabinoid medications (e.g. dronabinol, nabilone), or from consumption of teas, baked goods or other productsReference 227Reference 431.
Xenobiotic-mediated inhibition or potentiation of cannabinoid metabolism
Δ9-THC is oxidized by the xenobiotic-metabolizing CYP mixed-function oxidases 2C9, 2C19, and 3A4 into approximately 80 metabolitesReference 78Reference 468,. Therefore substances that inhibit these CYP isoenzymes such as certain anti-depressants (e.g. fluoxetine, fluvoxamine, moclobemide, and nefazodone), proton pump inhibitors (e.g. cimetidine and omeprazole), macrolides (e.g.arithromycin, erythromycin, telithromycin, troleandomycin), anti-mycotics (e.g. itraconazole, fluconazole, ketoconazole, miconazole, voriconazole, posaconazole), calcium antagonists (e.g. diltiazem, verapamil), HIV protease inhibitors (e.g. ritonavir, indinavir, nelfinavir, saquinavir, telaprevir, atazanavir, boceprevir, lopinavir), amiodarone, conivaptan, sulfaphenazole, azamulin, ticlopidine, nootkatone, grapefruit juice, mibefradil, and isoniazid can potentially increase the bioavailability of Δ9-THC (and metabolites such as 11-hydroxy-THC) as well as the risk of experiencing THC- and 11-hydroxy-THC-related side effectsReference 422Reference 468Reference 470Reference 1389. Additive tachycardia, hypertension, and drowsiness have been reported with THC and concomitant consumption of tricyclic antidepressants such as amytryptiline, amoxapine, and desipramineReference 227. Additive hypertension, tachycardia, and possible cardiotoxicity have been reported with THC and concomitant consumption of sympathomimetic agents such as amphetamines and cocaineReference 227. Additive or supra-additive tachycardia and drowsiness have been reported with THC and concomitant consumption of atropine, scopolamine, antihistamines, or other anti-cholinergicsReference 227. Reversible hypomanic reaction has been reported with concomitant consumption of THC with disulfiramReference 227.
On the other hand, drugs that accelerate Δ9-THC metabolism via 2C9 and 3A4 isozymes such as rifampicin, carbamazepine, phenobarbital, phenytoin, primidone, rifabutin, troglitazone, avasimibe, and Saint John's Wort may conversely decrease the bioavailability of THC and CBD and hence their effectiveness if used in a therapeutic contextReference 422Reference 468Reference 470Reference 1389.
Like THC, CBD is also metabolized by CYP 2C19 and 3A4 but could also act as a potential substrate for CYP 1A1, 1A2, 2C9, 2D6, 2E1, and 3A5Reference 468. As such, the bioavailability of CBD could potentially be increased by many of the same substances listed for THC, as well as buproprion, paroxetine, quinidine, clomethiazole, diallyl, disulfide, diethyldithiocarbamate, and disulfiramReference 468.
CBN is metabolized by CYP 2C9 and 3A4 but could also act as a potential substrate for CYP 2C19Reference 468.
The Sativex® product monograph cautions against combining Sativex® with amitriptyline or fentanyl (or related opioids) which are metabolized by CYP 3A4 and 2C19Reference 431. One clinical study in healthy subjects that investigated the effects of rifampicin, ketoconazole, and omeprazole on the pharmacokinetics of THC and CBD delivered from Sativex® reported that co-administration of rifampicin with Sativex® is associated with slight decreases in the plasma levels of THC, CBD, and 11-hydroxy-THC, while co-administration of ketoconazole with Sativex® is associated with slight increases in plasma levels of THC, CBD, and significant increases in the plasma levels of the potent psychoactive metabolite 11-hydroxy-THC (i.e. more than three-fold)Reference 470. Co-administration of Sativex® with ketoconazole was also associated with an increase in the frequency of treatment-emergent adverse events primarily involving the nervous system. While no serious adverse effects were noted, there were increases in the incidence of somnolence, dizziness, euphoric mood, lethargy, anxiety, dysgeusia, and headache. No significant effects on plasma levels of THC, CBD or 11-hydroxy-THC were noted with omeprazole.
Cannabinoid-mediated regulation of drug metabolism and drug transport
While THC, CBD, and CBN are known to inhibit CYP isozymes such as CYP 1A1, 1A2, 1B1 and 2A6Reference 74Reference 468, smoke from cannabis may also induce CYP 1A1 and 1A2 to an extent similar to that seen with tobacco smoke with added effects when used in combination, most likely through the effects of polyaromatic hydrocarbons from burning plant material on the aromatic hydrocarbon receptorReference 1390. Induction of CYP 1A1/1A2 may result in decreased plasma levels of chlorpromazine and theophyllineReference 473Reference 1390-Reference 1393. Despite the potential for CYP induction from cannabis smoke, additional data from in vitro experiments suggests that Δ9-THC also has the potential to inhibit CYP isozymes 3A4, 3A5, 2C9, and 2C19, while CBD also has the potential to inhibit CYP 2C19, 3A4, and 3A5Reference 74Reference 431. THC, CBD or CBN as well as cannabis containing these cannabinoids may therefore increase the bioavailability of drugs metabolized by these enzymes. Such drugs include amitryptiline, phenacetin, phenytoin, theophylline, granisetron, dacarbazine, and flutamideReference 74.
There is also some evidence to suggest a potential interaction between CBD and phenytoin: both substances have closely related spatial conformation features, both act as anti-convulsants, CBD inhibits CYP 2C19, 3A4, 1A2, and 2A6 which metabolize phenytoin or phenytoin metabolites, and in addition, evidence from pre-clinical studies suggest CBD enhances the anticonvulsant effects of phenytoinReference 468Reference 745Reference 1394. As such, patients taking CBD and anti-convulsants such as phenytoin should be monitored for increased blood levels of phenytoin, and doses of phenytoin should be adjusted accordingly to avoid the potential for excess blood levels of phenytoin and a phenytoin overdose.
A clinical study in children with refractory epilepsy and taking CBD (Epidiolex®) (5 mg/kg/day up to a maximum of 25 mg/kg/day) and clobazam (mean daily dose = 1 mg/kg/day, range: 0.18 - 2.24 mg/kg/day) for seizure control reported a CBD-mediated elevation in plasma levels of clobazam and its metabolite, n-desmethylclobazamReference 236. Clobazam and n-desmethylclobazam are metabolized by CYP3A4 and 2C19 to varying degrees and CBD has been shown to inhibit both of these CYPs. Mean increase in clobazam levels was 60% at four weeks (but not deemed statistically significant) following treatment and a mean increase in n-desmethylclobazam levels of 500% at four weeks (deemed statistically significant). Nine out of 13 children showed a > 50% decrease in seizures, and side effects (increased seizure frequency, ataxia, restless sleep, tremor, drowsiness, irritability, loss of appetite, and urinary retention) were managed by a dose reduction in clobazam. The authors of the study recommend monitoring of clobazam and n-desmethylclobazam levels in the clinical care of patients concomitantly taking clobazam and CBD (Epidiolex®).
In addition, THC, carboxy-Δ9-THC, CBD, and CBN all stimulate, and in some cases even inhibit, the activity of the drug transporter P-glycoprotein in vitroReference 72. CBD may also potentially inhibit UDP-glucuronosyltransferases 1A9 and 2B7 and CBN may potentially inhibit UDP-glucuronosyltransferase 1A9Reference 468. This suggests a potential additional role for these cannabinoids in affecting the therapeutic drug efficacy and toxicity of co-administered drugsReference 72.
In light of the evidence, clinicians should therefore be aware of other medications that the patient is taking and carefully monitor patients using other drugs along with cannabis or cannabinoids.
Cannabinoid-opioid interaction
Patients taking fentanyl (or related opioids) and anti-psychotic medications (clozapine or olanzapine) may be at risk of experiencing adverse effects if co-consuming cannabis/cannabinoidsReference 471Reference 473Reference 474Reference 834Reference 1395.
In one study, subjects reported an increase in the intensity and duration of the "high" when oxycodone was combined with inhalation of vapourized THC-predominant cannabis; this effect was not observed when morphine was combined with inhalation of vapourized cannabisReference 280. Furthermore, in that study, inhalation of vapourized THC-predominant cannabis was associated with a statistically significant decrease in the Cmax of sustained-release morphine sulfate and the time to Cmax for morphine was also delayed, although the delay was not statistically significant. There were no changes in the AUC for morphine metabolites, or in the ratio of morphine metabolites to parent morphine. In contrast to the effects seen with morphine sulfate, inhalation of vapourized THC-predominant cannabis was not associated with any changes in oxycodone pharmacokinetics.
A double-blind, placebo-controlled, cross-over clinical study was carried out to determine the safety and pharmacokinetics of CBD co-administered with intravenous fentanylReference 1396. Seventeen healthy volunteers were recruited into the study and administered placebo, 400 or 800 mg oral CBD (10 - 15 mg/kg) followed by a single dose of either 0.5 or 1.0 µg/kg dose of intravenous fentanyl. No significant pharmacokinetic changes were noted with CBD and opioid co-administration at the doses tested. In addition, Systematic Assessment of Treatment Emergent Events (SAFTEE) data were similar between treatment groups without any respiratory depression or cardiovascular complications during any test session. Minor adverse events reported by subjects during and immediately after study sessions included: dizziness/drowsiness, itching or rash, headache, abdominal discomfort, nausea/vomiting, and diarrhea.
Evidence from pharmacogenetic studies
Pharmacogenetic studies have suggested that patients homozygous for the CYP2C9*3 allele appear to have impaired THC metabolism and may show greater intoxication than *1/*3 heterozygotes or *1/*1 homozygotesReference 465.
Data from clinical studies
A significant proportion of published clinical studies of cannabis or prescription cannabinoid medications have used patient populations that were taking concomitant medications for a variety of disorders such as neuropathic pain of various etiologiesReference 58Reference 59Reference 195Reference 216Reference 222Reference 280Reference 281Reference 287Reference 386Reference 433Reference 598Reference 598Reference 599Reference 612Reference 822Reference 834, cancer-related painReference 138Reference 283Reference 284, fibromyalgiaReference 184Reference 386Reference 596Reference 597, pain and spasticity associated with MSReference 278Reference 387Reference 432Reference 610Reference 686Reference 835, and symptoms associated with HD or PDReference 245Reference 254.
Examples of commonly-used medications seen in clinical trials of cannabis or prescription cannabinoid medications (e.g. dronabinol, nabilone and nabiximols) include NSAIDs (e.g. acetaminophen, COX-2 inhibitors), metamizol, topical steroids, muscle relaxants, short- and long-acting opioids (e.g. codeine, morphine, hydromorphone, oxycodone, oxycontin, tramadol, fentanyl, methadone), ketamine, anti-convulsants (e.g. gabapentin, pregabalin), anti-depressants (e.g. tricyclics, selective-serotonin re-uptake inhibitors, serotonin-norepinephrine re-uptake inhibitors, serotonin-antagonist re-uptake inhibitors), and anxiolytics.
According to the cited clinical studies, concomitant use of cannabis or prescription cannabinoid medications with other medications was reported to be well tolerated, and many of the observed adverse effects were those typically associated with the psychotropic effects of cannabis and cannabinoids (e.g. transient impairment of sensory and perceptual functions, abnormal thinking, disturbance in attention, dizziness, confusion, sedation, fatigue, euphoria, dysphoria, depression, paranoia, hallucinations, anxiety, headache, but also dry mouth, hypotension, tachycardia, throat irritation (with smoking) and gastrointestinal disorders (nausea)).
One study has reported that AIDS patients may be at an increased risk of experiencing adverse cardiovascular outcomes caused by interactions between cannabis and anti-retroviral drugs, such as ritonavir, which has itself been associated with adverse cardiovascular eventsReference 1397.
6.3 Drug screening tests
Because of the long half-life of elimination of cannabinoids and their metabolites, drug tests screening for cannabinoids can be positive for weeks after last cannabis/cannabinoid useReference 1398Reference 1399 depending on among other things, the sensitivities of the tests used, frequency of cannabis use and timing of testing.
7.0 Adverse effects
Reporting adverse reactions associated with the use of cannabis and cannabis products is important in gathering much needed information about the potential harms of cannabis and cannabis products for medical purposes. When reporting adverse reactions, please provide as much complete information as possible including the name of the licensed producer, the product brand name, the strain name, and the lot number of the product used in addition to all other information available for input in the adverse reaction reporting form. Providing Health Canada with as much complete information as possible about the adverse reaction will help Health Canada with any follow-ups or actions that may be required.
Healthcare practitioners and consumers are invited and encouraged to submit reports of all adverse effects associated with cannabis for medical purposes to Canada Vigilance in the following ways:
Report online, call toll-free at 1-866-234-2345, complete a Canada Vigilance Reporting Form and fax toll-free to 1-866-678-6789, or Mail to:
Canada Vigilance Program
Health Canada
Postal Locator 0701D
Ottawa, Ontario K1A 0K9
Postage paid labels, Canada Vigilance Reporting Form and the adverse reaction reporting guidelines are available on the MedEffect™ Canada Web site.
There is generally far more information available in the medical literature on the adverse effects associated with non-medical cannabis use than there is with therapeutic cannabis use. Accordingly, much of the information presented below regarding the adverse effects of cannabis use comes from studies carried out among non-medical users. Less information on the adverse effects associated with the use of cannabis for therapeutic purposes comes from clinical studies, mainly because of the small number of such studies that have been carried out to date. Furthermore, while there is some information on the short-term adverse effects associated with the use of cannabis for therapeutic purposes, much less information exists on the long-term consequences of cannabis use for therapeutic purposes because most of the available clinical studies were short-term.
A Canadian systematic review of the adverse effects of prescription cannabinoid medications concluded that the rate of non-serious adverse events was almost two-fold higher among those patients using prescription cannabinoid medications compared to controlsReference 1400. The most frequently cited adverse events associated with the use of prescription cannabinoid medications (e.g. dronabinol, nabilone, nabiximols) were nervous system disorders, psychiatric disorders, GI disorders, and vascular and cardiac disorders.
A multi-centre, prospective, cohort safety study of patients using cannabis as part of their pain management regimen for chronic non-cancer pain reported that cannabis use was not associated with an increase in the frequency of serious adverse events compared to controls, but was associated with an increase in the frequency of non-serious adverse eventsReference 216. In this study, 216 patients with chronic non-cancer pain (nociceptive, neuropathic, both) using cannabis and 215 control patients with chronic pain but no cannabis use were followed for a period of one year and evaluated for frequency and type of adverse effects associated with the use of a standardized herbal cannabis product (CanniMed 12.5% THC, <0.5% CBD). A significant proportion of study subjects were taking opioids, anti-depressants or anti-convulsants. Almost one third of study subjects consumed it exclusively by smoking, 44% by smoking and oral ingestion, 14% by vapourizing, smoking or ingesting cannabis orally, and slightly less than 4% reported only smoking or vapourizing. The most common adverse event categories in the cannabis-treatment group were nervous system (20%), GI (13.4%), and respiratory disorders (12.6%) and the rate of nervous system disorders, respiratory disorders, infections, and psychiatric disorders was significantly higher in the cannabis group than in the control group. Furthermore, mild (51%) and moderate (48%) events were more common than severe ones (10%) in the cannabis-treatment group. Somnolence (0.6%), amnesia (0.5%), cough (0.5%), nausea (0.5%), dizziness (0.4%), euphoric mood (0.4%), hyperhidrosis (0.2%), and paranoia (0.2%) were assessed as being "certainly/very likely" related to treatment with cannabis. Interestingly, increasing the daily dose of cannabis was not associated with a higher risk of serious or non-serious adverse events, although the total daily amount of cannabis allowed was set at 5 g per day (the median daily cannabis dose was 2.5 g per day).
An additional consideration in the evaluation of adverse effects associated with cannabis use is the concomitant use of tobacco and alcohol as well as other drugs, whether they are non-prescription, prescription, or illicit drugsReference 145Reference 1401-Reference 1404 (and also see Section 6.2).
7.1 Carcinogenesis and mutagenesis
- Evidence from pre-clinical studies suggests cannabis smoke contains many of the same carcinogens and mutagens as tobacco smoke and that cannabis smoke is as mutagenic and cytotoxic, if not more so, than tobacco smoke.
- However, limited and conflicting evidence from epidemiological studies has thus far been unable to find a robust and consistent association between cannabis use and various types of cancer, with the possible exception of a link between cannabis use and testicular cancer (i.e testicular germ cell tumours).
Pre-clinical studies
Qualitatively, cannabis smoke condensates have been shown to contain many of the same chemicals as tobacco smokeReference 84. Furthermore, a number of in vitro studies have provided strong evidence that smoke from burning cannabis is carcinogenic (reviewed inReference 140). The cytotoxic and mutagenic potential of cannabis smoke condensates were compared to their tobacco counterparts and in contrast to tobacco smoke condensates, those derived from cannabis smoke appeared to be more cytotoxic and mutagenic, while the opposite was true with respect to cytogenetic damageReference 82. In addition, for either cannabis or tobacco smoke, the particulate phase was substantially more cytotoxic than the gas phase. A follow-up global toxicogenomic analysis comparing tobacco and cannabis smoke condensates in vitro reported that tobacco smoke condensate exposure was associated with expression of genes involved in xenobiotic metabolism, oxidative stress, inflammation, and DNA damage responseReference 1405. Furthermore, these same pathways and functions were also significantly affected following exposure to cannabis smoke condensates suggesting that cannabis smoke condensates affect many of the same molecular processes and functions as tobacco smoke condensates, although some notable differences between cannabis and tobacco smoke condensates with regard to affected molecular pathways were notedReference 1405. Taken together, these studies suggest that cannabis smoke cannot be deemed "safer" than tobacco smoke. However, despite some persuasive in vitro data, the epidemiological evidence for a link between cannabis smoking and cancer remains mainly inconclusive because of conflicting results from a limited number of studies. Below is a summary of the evidence on cannabis use and cancer.
Epidemiological studies
One epidemiological study in relatively young clients of a health maintenance organization (HMO) found an increased incidence of prostate cancer in those men who smoked cannabis and other non-tobacco materialsReference 358. No other associations were found between cannabis use and other cancers; however, the study was limited by the demographics of the HMO clientele and the very low cannabis exposure threshold employed in the study to define "users".
A case-control study suggested that cannabis smoking may increase the risk of head and neck cancer (OR = 2.6; CI = 1.1 - 6.6), with a strong dose-response pattern compared to non-smoking controlsReference 359. However, the authors note a number of limitations with their study such as underreporting, inaccurate cannabis dose reporting, assay sensitivity, and low power.
A large population-based case-control study of 1 212 incident cancer cases and 1 040 cancer-free matched controls did not find a significant relationship between long-term cannabis smoking and cancers of the lung and upper aerodigestive tractReference 360.
However, a much smaller case-control study in young adults (≤ 55 years of age), examined 79 cases of lung cancer and 324 controls and reported that the risk of lung cancer increased by 8% (95% CI = 2 - 15%) for each "joint-year" (defined as the smoking of one joint per day for one year), after adjusting for cigarette smokingReference 361.
A population-based, longitudinal cohort study examined over 49 000 men aged 18 to 20 years old for cannabis use and other relevant health variables during military conscription in SwedenReference 1406. Participants were tracked over a 40-year period for incident lung cancer outcomes in nationwide linked medical registries. Analysis found that "heavy" cannabis smoking (but not "ever" use) was significantly associated with more than a two-fold risk (hazard ratio = 2.12, 95% CI = 1.08 - 4.14) of developing lung cancer over the 40-year follow-up period even after statistical adjustment for baseline tobacco use, alcohol use, respiratory conditions and socio-economic status. However, the vast majority of individuals reporting cannabis use also reported tobacco use and there was no clear evidence of a dose-response relationship between frequency of cannabis use and lung cancer outcomes. In addition, the study did not include a detailed assessment of use patterns of cannabis and tobacco preceding the baseline conscription process and it also did not have any information about tobacco and cannabis use after conscription.
A recent meta-analysis of 4 cohort studies and 30 case-control studies (11 studies on upper aerodigestive cancers, 6 studies on lung cancer, 3 studies on testicular germ cell tumours, 6 studies on childhood cancers, 1 study on all cancers, 1 study on anal cancer, 1 study on penile cancer, 2 studies on non-Hodgkin's lymphoma, 1 study on malignant primary glioma, 1 study on bladder cancer, and 1 study on Kaposi's sarcoma) examined the correlation between cannabis use and risk of various cancersReference 1407. The meta-analysis concluded that for head and neck cancer, the evidence was inconsistent but may be consistent with no association or with opposite directions of association depending on the subgroups of populations. For lung cancer, while the authors state that it was generally difficult to rule out residual confounding by tobacco use, they suggest that overall the studies available to date suggest no association with cannabis use though the authors are careful to point out that affirming no association is inherently difficult. In light of the multiple lines of evidence that suggest that smoking cannabis may be a risk factor for the development of cancer (e.g. presence of significant amounts of carcinogens in cannabis smoke, increased risks associated with cannabis-specific smoking topology, and pre-clinical and clinical evidence of pre-cancerous lesions) the lack of a clear association with cannabis use raises a number of interesting questions on the reasons behind the lack of an association including the potential anti-tumorigenic role of cannabinoids. Lastly, the meta-analysis concluded that the three case-control studies of testicular cancer reported similar findings with an increased risk observed for modest frequency and duration of use, while for cancers such as bladder cancer and childhood cancers the authors opine that there is insufficient data to make any firm conclusions on an association with cannabis use.
Despite the conflicting evidence surrounding the carcinogenic potential of cannabis smoke in humans, it is advisable to limit (or eliminate) the degree to which cannabis is smoked. Further well-controlled epidemiological studies are required to better establish whether there is causality between cannabis smoking and carcinogenesis in human populations.
Lastly, in the case of cancer patients, the potential risks of carcinogenesis and mutagenesis associated with smoking cannabis must be weighed against any potential therapeutic benefits for this patient population; routes of administration other than smoking (e.g. vapourization, oral administration) may warrant serious consideration. Because vapourization is a lower-temperature process compared with pyrolysis (i.e. smoking), vapourization appears to be associated with the formation of a smaller quantity of toxic by-products such as carbon monoxide, polycyclic aromatic hydrocarbons, and tar, as well as a more efficient extraction of Δ9-THC from the cannabis materialReference 402Reference 411-Reference 414. Taken together, these studies support that, owing to safety considerations, smoking should be avoided as a preferred route of cannabinoid administration and other modes of administration such as oral, oro-mucosal, vapourization or rectal administration should preferably be considered as these may be, in some respects, less harmful than smoking.
7.2 Respiratory tract
- Evidence from pre-clinical studies suggests that cannabis smoke contains many of the same respiratory irritants and toxins as tobacco smoke, and even greater quantities of some such substances.
- Case studies suggest that cannabis smoking is associated with a variety of histopathological changes in respiratory tissues, a variety of respiratory symptoms similar to those seen in tobacco smokers, and changes in certain lung functions with frequent, long-term use.
- The association between chronic heavy cannabis smoking (without tobacco) and chronic obstructive pulmonary disease, is unclear, but if there is one, is possibly small.
A review of the effects of regular cannabis smoking on the respiratory tract reported an increase in the prevalence of chronic cough and sputum production, wheezing, and shortness of breath and an increased incidence of acute bronchitic episodes or clinic visits for acute respiratory illnessReference 1408. However, at present, no conclusive positive associations can be drawn between cannabis smoking and incidence of lung or upper airway cancer, despite the presence of pro-carcinogenic compounds in cannabis smokeReference 1407Reference 1408 (and see Section 7.1). There have also been isolated case reports of pulmonary aspergillosis in immunocompromised patients smoking cannabis, reports of pulmonary tuberculosis in those smoking cannabis through contaminated water pipes, as well as reports of pneumothorax, pneumomediastinum, and lung bullae in heavy cannabis smokersReference 1408. Overall, the synthesis of the evidence suggests that the risks of pulmonary complications of regular cannabis smoking appear to be relatively smaller and lower than those associated with tobacco smoking, though this does not mean that cannabis smoking can be considered "safe" or safer than tobacco smoking. Furthermore, any risks associated with smoking cannabis should be weighed against any potential therapeutic effects of cannabis.
Below is a select summary of the literature on the effects of cannabis smoking on the respiratory tract.
Differences in the smoking techniques used by cannabis vs. tobacco smokers (i.e. larger puffs, deeper inhalation, and longer breath holding) are reported to result in three- or four-fold higher levels of tar, and five-fold higher levels of carbon monoxide being retained in the lungs during cannabis smoking compared to tobacco smokingReference 1408Reference 1409.
A systematic comparison of the mainstream smoke composition from cannabis (12.5% THC, < 0.5% CBD) and tobacco cigarettes (prepared in the same way and consumed in an identical manner), under two different sets of smoking conditions ("standard" and "extreme") has been reportedReference 84. The "standard" condition reflects typical tobacco cigarette smoking conditions, whereas the "extreme" condition approaches that typically seen in cannabis smoking. Ammonia in mainstream cannabis smoke was 20-fold greater than that found in tobacco smoke, and oxides of nitrogen and hydrogen cyanide were three to five times higher in cannabis smoke vs. tobacco smoke. Carbon monoxide was significantly lower in mainstream cannabis smoke, under both smoking conditions. Tar was statistically significantly higher in mainstream cannabis smoke but only under the "extreme" smoking condition.
Mucosal biopsy specimens taken from chronic cannabis smokers, who reported smoking only cannabis, showed a number of histopathological changes including basal cell hyperplasia, stratification, goblet cell hyperplasia, cell disorganization, inflammation, basement membrane thickening, and squamous cell metaplasiaReference 363Reference 1408. However, the study employed a small number of subjects and relied on the accuracy and integrity of the subjects' recall to establish smoking status as well as frequency and duration of smoking.
Epidemiological studies have found some changes in pulmonary function, especially in heavy cannabis smokers, including reduction of FEV1, an increase in airway resistance, and a decrease in airway conductanceReference 367-Reference 369. Heavy chronic cannabis smokers presented with symptoms of bronchitis, including wheezing, production of phlegm and chronic coughReference 145Reference 1410. All changes were most evident in heavy chronic users, defined as those who smoked more than three joints per day for 25 yearsReference 358Reference 1411, although evidence of measurable respiratory symptoms (e.g. decreased FEV1/FVC ratio) was also observed in young, cannabis-dependent individuals whose smoking behaviour was comparable to tobacco smokers consuming 1 to 10 cigarettes/dayReference 1412.
While the potential risk of developing chronic obstructive respiratory disease, with long-term cannabis use and/or dependence, has been claimed to be potentially as great as among tobacco usersReference 1412, a longitudinal study collecting repeated measurements of pulmonary function and smoking over a period of 20 years in a cohort of 5 115 men and women in four U.S. cities (i.e. the Coronary Artery Risk Development In Young Adults study, CARDIA) suggested a more complex picture. The study found a non-linear association between cannabis smoking and pulmonary functionReference 370. By comparison, tobacco smoking (current and lifetime) was linearly associated with lower FEV1 and FVC. Low levels of cumulative cannabis smoking were not associated with adverse effects on pulmonary function. Instead, at this level, cannabis smoking was associated with an increase in the FEV1 and FVC values. At up to seven "joint-years" (a "joint-year" defined as smoking one joint/day, 365 days/year) of lifetime exposure there was no evidence of decreased pulmonary function. However, heavy chronic cannabis smoking (> ~30 joint-years or > ~25 smoking episodes per month) was associated with an accelerated decline in pulmonary function (FEV1 but not FVC).
A cross-sectional observational study of 500 individuals in a general practice population (248 tobacco-only smoking individuals, 252 cannabis and tobacco smoking individuals) reported that individuals reporting smoking cannabis (and tobacco) self-reported more respiratory symptoms (i.e. expectoration of sputum, wheeze) than individuals only reporting smoking tobaccoReference 371. Most study participants who reported smoking cannabis said they smoked cannabis resin (in a joint along with tobacco), with a smaller group reporting smoking herbal cannabis. Each additional joint-year of cannabis use was associated with a small 0.3% increase (95% CI = 0.0 to 0.5) in prevalence of chronic obstructive pulmonary disease. Further research is needed to clarify the complex changes in lung function found in cannabis smokers, and to determine if there is a cause and effect relationship between cannabis smoking and the development of lung disease, especially chronic obstructive pulmonary disease.
Smoking cannabis may also increase the risk of developing respiratory infections in chronic usersReference 1413 through exposure to infectious organisms such as fungi and molds which can be found in the plant materialReference 1414, or alternatively by decreasing natural host defensesReference 1415. However, further epidemiological research is also required to establish a causal relationship between cannabis smoking and respiratory infections.
Vapourization of dried cannabis may be considered an alternative to smoking, although research is required to determine if there are any adverse effects associated with long-term vapourization on lung health/function. In addition, the picture has further evolved with the emergence of cannabis electronic cigarettes ("e-cigs" or "e-joints") containing THC and/or CBD in various solvent carriers such as propylene glycol, glycerol or bothReference 1416-Reference 1418. Despite being frequently advertised by manufacturers as a healthier alternative to smoking, there are many uncertainties about the impact of e-cigarettes on health and indoor air qualityReference 1419. Studies have reported that the aerosols generated from e-cigarettes can contain carcinogens such as formaldehyde, acetaldehyde and acrolein, especially when high voltage devices/settings are used, although even at normal operating settings the levels of formaldehyde, for example, may be elevated despite the absence of the so-called "dry hit" or "dry puff" characterized by an unpleasant taste that more experienced users can detectReference 1420. Various design and operating parameters have significant effects on emission levels of toxic compounds, including the choice of vapourizer and the battery power output, both of which determine the coil and vapour temperaturesReference 1421. Emissions are believed to be caused by the thermal degradation of propylene glycol and/or glycerolReference 1422Reference 1423 with the quantity of formaldehyde and other carbonyls increasing with increasing powerReference 1422-Reference 1424, and device temperatureReference 1425. Therefore, overly high temperatures and a prolonged contact of the heating coil with the e-liquid must be avoided to prevent the formation of toxic pyrolytic by-products, even though lower settings may also yield some of these toxic by-productsReference 1416. The extent of toxicant exposure that might occur during normal use of e-cigarettes is currently unclear, although one urinary biomarker study suggested that exposure to other reactive carbonyls (e.g. acrolein, crotonaldehyde) was significantly lower among vapers than among cigarette smokersReference 1426. Aside from inhaling the mentioned carcinogenic by-products, active vapers may also be inhaling relatively high concentrations of propylene glycol, glycerol, and aerosol particulatesReference 1419. Lastly, there have been a few case reports of exogenous lipoid pneumonia, eosinophilic pneumonitis, and subacute bronchial toxicity associated with vaping glycerol-based e-liquidsReference 1427-Reference 1429. For additional information on vapourization please consult Sections 1.1.1, 1.1.2, 2.2.1.2, 3.4, 4.7.2.2, 4.7.2.3, and Table 4.
7.3 Immune system
- Pre-clinical studies suggest certain cannabinoids have a variety of complex effects on immune system function (pro-/anti-inflammatory, stimulatory/inhibitory).
- The limited clinical and observational studies of the effects of cannabis on immune cell counts and effect on HIV viral load are mixed, as is the evidence around frequent cannabis use (i.e. daily/CUD) and adherence to ART.
- Limited but increasing evidence from case studies also suggests cannabis use is associated with allergic/hypersensitivity-type reactions.
Pre-clinical studies
Evidence from in vivo and in vitro studies suggests complex and apparently dichotomous roles for the ECS on immune system functionReference 26. First, CB1 and CB2 receptors are known to be expressed in various immunocytes (B cells, monocytes, neutrophils, T lymphocytes, macrophages, mast cells), with CB2 receptor expression generally being more abundant than CB1 receptor expression; the ratio of CB2 to CB1 receptor expression ranges between 10 and 100: 1, depending on the immune cell type in questionReference 26Reference 27. In addition, CB2 receptor expression is most abundant in B-cells, followed by natural killer cells, monocytes, neutrophils and lastly, T-cellsReference 1430. Second, immune cells also have the ability to synthesize, secrete, transport and catabolize endocannabinoidsReference 26. Third, while stimulation of the CB2 receptor appears to be generally associated with immunosuppressive effects, activation of the CB1 receptor appears to be associated with an opposing immunostimulatory effectReference 26. Fourth, whereas certain cannabinoids have been shown to modulate the release of pro- or anti-inflammatory cytokines, pro-inflammatory cytokines (such as TNF-α) have, in turn, been reported to affect the functioning of the ECS by upregulating the expression of both CB1 and CB2 receptor mRNA and protein levelsReference 27. Thus, there appears to be some level of cross-talk between the endocannabinoid and immune systems. Fifth, as is the case for some of its other effects, Δ9-THC appears to have a biphasic effect on immune system function. Low doses of Δ9-THC seem to have stimulatory or pro-inflammatory effects, while higher doses appear to have inhibitory or immunosuppressive effectsReference 392. Both Δ9-THC and CBD have been reported to modulate cell-mediated and humoural immunity, through CB receptor-dependent and CB receptor-independent mechanismsReference 392Reference 1431Reference 1432. Cannabinoids target various cellular signaling and transcriptional pathways resulting, in some instances, in the inhibition of pro-inflammatory cytokine release (e.g. IL-1β, IL-6, IFN-β), and/or stimulation of anti-inflammatory cytokine release (e.g. IL-4, IL-5, Il-10, IL-13)Reference 27Reference 392. CBD also appears to induce a shift in Th1/Th2 immunobalanceReference 1431.
While under certain circumstances, cannabinoids appear to have broad anti-inflammatory and immunosuppressive effects, which could be of benefit in pathological conditions having inflammatory characteristics, such effects may become problematic in the context of essential defensive responses to infectionsReference 26. For example, in vitro as well as in vivo experiments suggest cannabinoids (i.e. THC) have an impact on virus-host cell interactionsReference 1433. Cannabinoid treatment (i.e. THC) has been associated with increased viral replication of the herpes simplex virus-2, HIV-1, Kaposi's sarcoma-associated virus, influenza, and vesicular stomatitis virus, or has been associated with increases in surrogate measures of infection in these experimental models suggesting that at least some cannabinoids (THC) could have a detrimental effect with regard to viral infectionsReference 1430Reference 1434-Reference 1441. Another study has also shown that chronic THC exposure decreased the efficacy of the memory immune response to Candida albicans infection in a mouse modelReference 1442. However, in male rhesus macaques, chronic administration of THC (0.32 mg/kg b.i.d.) is associated with decreased early mortality from SIV infection, attenuation of plasma and CSF and gut viral load, decreased GI inflammatory responses, decreased viral replication, and modest retention of body massReference 1443-Reference 1445. However, similar protective effects were not observed in female macaquesReference 1446 suggesting a sex-dependent effect.
Thus, the available pre-clinical evidence suggests that cannabinoids may systematically influence viral infection through a number of mechanisms that include the regulation of host immunity and inflammatory responses, cell metabolism, the ability to enter the host cells, integrate into the host genome, replicate, and be released, as well as novel epigenomic and miRNA regulatory mechanismsReference 1434. Furthermore, the available information suggests that differences in the observed effects of cannabinoids on immune system function (i.e. immunosuppressive vs. immunostimulatory) may be explained by differences in the routes/methods of administration (smoked, oral, or other route), the length of exposure to the cannabinoid(s), the dose and type of cannabinoid used, and which receptors are preferentially targeted, and also by differences between species, the experimental protocols and outcome measures used, and in addition for clinical studies (see below), the health status/medical condition of the human subjectsReference 392.
Clinical studies
The effects of smoked cannabis/THC on the human immune system have been studied, albeit only to a limited degree and the evidence is mixed. While in vitro studies with human immune cells suggest that THC has immunosuppressive propertiesReference 1447-Reference 1453, data from clinical studies of smoked cannabis and psychoactive cannabinoids (oral THC, oral THC/CBD) do not appear to show an increased risk of infections or infestations in patients using smoked cannabis/cannabinoidsReference 216Reference 1400.
Cannabis and immune cell count
A major concern with immunocompromised individuals such as HIV-positive cannabis smokers, or patients smoking and undergoing cancer chemotherapy, is that they might be more vulnerable than other cannabis smokers to the immunosuppressive effects of cannabis or that they risk exposure to infectious organisms associated with cannabis plant materialReference 642. A group of studies has partially addressed the former concern.
In one study, HIV-positive patients on stable ART were randomized to smoked cannabis or oral dronabinol and showed no changes in CD4+ and CD8+ T-cell, B-cell, or NK cell counts and a number of other parameters, compared with placebo, over a 21-day study periodReference 1454. A longitudinal study of 481 HIV-infected men who used cannabis and who were followed over an average five-year period found that while cannabis use was generally associated with a higher CD4+ cell count in infected men and controls, no clinically meaningful associations, adverse or otherwise, between cannabis use and T-cell counts and percentages could be establishedReference 1455. Cannabis use was also not associated with an increased rate of progression to AIDS in HIV-infected individualsReference 1456. In another study, smoking cannabis was associated with lower plasma concentrations of the protease inhibitors indinavir and nelfinavir; whereas dronabinol or placebo had no effectReference 471. However, the decreased plasma levels of protease inhibitors were not associated with an elevated viral load, or changes in CD4+ or CD8+ cell countsReference 655. Furthermore, a retrospective, longitudinal, observational cohort study among ART-naïve illicit drug users reported that at least daily cannabis use was associated with lower plasma HIV-1 RNA viral load in the first year following seroconversionReference 1457. In another study, HIV positive cannabis users (light or moderate-to-heavy use) showed higher plasma CD4 counts and lower viral load than HIV positive non-cannabis users; the ART status of the subjects was not knownReference 1458. On the other hand, an observational study of 157 men who have sex with men found that cannabis use during sexual intercourse was significantly associated with higher likelihood of elevated seminal plasma HIV RNA viral load despite successful combined ARTReference 1459. In humans, smoking cannabis was also associated with poorer outcome in patients with chronic hepatitis CReference 1402Reference 1460.
Cannabis and anti-retroviral treatment adherence
One cross-sectional study examined the association between cannabis use status and adherence to ART as well as the association between cannabis use status, HIV symptoms, and side effects associated with ART among a sample of HIV-positive individualsReference 1461. The study reported that those subjects who had a CUD had a significantly lower adherence to treatment than those who reported using cannabis once or more per week, but less than daily or not at all. Those who had a CUD also had a higher viral load than those who used cannabis less than daily but at least once per week, as did those who did not use at all; absolute CD4 count was not significantly different between groups. Furthermore, those subjects with a CUD reported significantly more frequent and severe HIV symptoms and/or medication side effects than those who used cannabis less than daily (but at least once per week), or those who reported not using cannabis at all. One limitation to this study was its cross-sectional nature, precluding the ability to establish a cause-and-effect relationship.
On the other hand, a long-term, observational, prospective cohort survey study (the AIDS Care Cohort to evaluate Exposure to Survival Services, ACCESS) that examined the relationship between high-intensity cannabis use and adherence to ART among 523 HIV-positive illicit drug users reported that at least daily or more often than daily cannabis use was not associated with adherence to ARTReference 1462.
CBD and graft-versus-host disease
A phase II, non-randomized, uncontrolled, unblinded clinical study of the effects of CBD on the prevention of graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation reported that oral administration of CBD (300 mg/day) beginning seven days before transplantation and continuing for a period of 30 days post-transplantation was associated with a reduction in the incidence of acute GVHD when combined with standard GVHD prophylaxis (i.e. cyclosporine and methotrexate)Reference 1463. Furthermore, no Grade 3 or 4 toxicities were attributed to CBD treatment. Forty-eight adult patients were enrolled in this clinical trial, with 38 patients having acute leukemia or myelodysplastic syndrome and 35 patients given myeoablative conditioning. Limitations of the study included single-arm design, and retrospective comparison with historical control subjects. Nevetherless, the findings from this study suggest CBD may have significant immunosuppressive properties. Further research is needed.
Hypersensitivity/allergic reactions
There are increasing reports of hypersensitivity/allergic reactions to cannabisReference 365Reference 393Reference 394Reference 1353Reference 1354. Clinical symptoms of such reactions include sore throat, nasal congestion, rhinitis, conjunctivitis, pharyngitis, food allergy, eczema, contact urticaria, anaphylaxis, wheezing, dyspnea, palpebral angioedema and lacrimationReference 365Reference 393Reference 1353. In chronic and high dose users more severe manifestations of bronchitis and asthma with reduced vital capacity have been notedReference 1353. Furthermore, cannabis allergy has also been associated with cross-allergies to other plants such as wheat, tobacco, latex, nuts, and certain fruits and vegetables (e.g. tomato, cherry, tangerine, banana, citrus, grapefruit, pepper, fig, peach peel, apple, hops, grapes)Reference 365Reference 393Reference 394.
7.4 Reproductive and endocrine systems
- Pre-clinical evidence suggests certain cannabinoids can have negative effects on a variety of measures of reproductive health. Furthermore, limited evidence from human observational studies with cannabis appears to support evidence from some pre-clinical studies.
- Evidence from human observational studies also suggests a dose- and age-dependent association between cannabis use and testicular germ cell tumours.
- Pre-clinical evidence clearly suggests in utero exposure to certain cannabinoids is associated with a number of short and long-term harms to the developing offspring.
- However, evidence from human observational studies is complex and suggests that while confounding factors may account for associations between heavy cannabis use during pregnancy and adverse neonatal or perinatal effects, heavy cannabis use during pregnancy is associated with reduced neonatal birth weight.
Role of the endocannabinoid system in sexual physiology
The CB1 receptor is widely expressed in various brain structures such as the striatum, hippocampus, and the cerebellum, as well as the amygdala, the midbrain, and the cerebral cortex-brain structures involved in regulating different reproductive and sexual behaviours and endocrine functionsReference 397. For example, CB1 receptors within the striatum and cerebellum may regulate motor activity and function; CB1 receptors located within corticolimbic structures (e.g. pre-frontal cortex, amygdala and hippocampus) may regulate stress responsivity and emotional behaviour; CB1 receptors located within the dorsal raphe and ventral tegmental area may regulate genital reflexes, sexual motivation and inhibition; and lastly, CB1 receptors expressed within the hypothalamus and the pituitary gland may modulate endocrine effects through the HPA axis either directly by modulating the gonadotropin-releasing hormone or indirectly through other pathwaysReference 397Reference 1464.
CB1 receptor-mediated modulation of the HPA axis results in the suppression of luteinizing hormone, thyroid stimulating hormone, growth hormone, and prolactin release from the pituitary gland, while the effects on follicle stimulating hormone point to a probable suppression of releaseReference 395Reference 399Reference 1465Reference 1466. In animals, these effects are accompanied by changes in reproductive function and behaviour including anovulation, decreases in plasma testosterone levels, degenerative changes in spermatocytes and spermatids, and potential reduction in copulatory behaviourReference 1464Reference 1465. Aside from the roles of the cannabinoid receptors in the brain, the male and female reproductive systems also contain an ECS, and increasing experimental evidence suggests important roles for this ECS in regulating various reproductive functions such as folliculogenesis, spermatogenesis, ovulation, fertilization, oviductal transport, implantation, trophoblast survival, embryo development, pregnancy, and labour (reviewed inReference 39Reference 1376). Tight regulation of endocannabinoid signaling tone across multiple stages of early pregnancy appears critical for female reproductive successReference 1376.
Effects of cannabis on human sexual behaviour
There is a relative paucity of data with regard to the effects of cannabis or cannabinoids on human sexual behaviour. One review article has summarized the few available studies on the subjectReference 397. It concluded that in general, the effects of cannabis on sexual functioning and behaviour appear to be dose-dependent. For women, the available information suggests beneficial effects on sexual behaviour and functioning (e.g. reported increases in sensitivity to touch and in relaxation, and a corresponding increase in sexual responsiveness) at low to moderate doses, and potentially opposite responses at higher doses. For men, the available information suggests that cannabis intake at low to moderate doses may facilitate sexual desire and activity, but that at higher doses or with more frequent or chronic use it may inhibit sexual motivation as well as erectile function. Results obtained from animal studies appear to mirror some of these findings, although exceptions have been noted. Although the effects of cannabis on human sexual behaviour are still not well understood, some of its reported beneficial effects have been speculatively linked to its psychoactive properties (e.g. increase in tactile sensitivity/perception or slowing of temporal perception), and/or to a loss of inhibitions and an increased state of relaxation. In contrast, a recent cross-sectional epidemiological study among 28 176 women and 22 943 men reported that cannabis use frequency was associated with increased coital frequency in both women and menReference 398.
Effects on sperm and testicular health
The ECS has been implicated in spermatogenesis and production of testosteroneReference 1467-Reference 1471. Human spermatozoa have been shown to express functional CB1 and CB2 receptorsReference 1471. CB1 and CB2 receptors have been identified on the plasma membrane of human spermatozoa and the CB1 receptor has been further shown to be localized to the plasma membrane of the acrosomal region, although also to the midpiece, and the sperm tailReference 1471Reference 1472. The CB2 receptor on the other hand has been shown to be localized in the post-acrosomal region, midpiece and sperm tailReference 1471Reference 1473Reference 1474. In vitro studies have reported that activation of the CB1 receptor by anandamide can negatively affect human sperm motility, capacitation and the acrosome reactionReference 1471Reference 1472Reference 1474Reference 1475. Hyper- as well as hypo-activation of the CB2 receptor in male germ cells has been shown to disrupt the temporal dynamics of the spermatogenic cycleReference 1476. A cross-sectional study of 86 men presenting at an infertility clinic reported that seminal plasma anandamide levels were significantly lower in men with asthenozoospermia or oligoasthenoteratozoospermia compared with normozoospermic menReference 1471. In addition, levels of spermatozoal CB1 mRNA were significantly decreased in men with asthenozoospermia or oligoasthenoteratozoospermia compared with normozoospermic menReference 1471. These findings suggest an association between lower seminal plasma anandamide level and abnormal sperm motility. Furthermore, taken together, these findings suggest an important role for the ECS in sperm function and male reproductionReference 1477 and also raise the possibility that exposure to exogenous sources of cannabinoids (e.g. THC from cannabis) may affect sperm function. Cannabinoids are lipophilic and they can accumulate in membranes and testicular/epididymal fat from where they can be released slowly;this can affect spermatozoa and their functionReference 395.
THC
The effects of cannabis and Δ9-THC on human sperm have been investigated both in vivo and in vitroReference 395Reference 1478-Reference 1480. A significant decline in sperm count, concentration and motility, and an increase in abnormal sperm morphology were observed in men who smoked cannabis (8 - 20 cigarettes/day) for four weeksReference 1478. In an in vitro study, sperm motility and acrosome reactions were decreased in both the 90% and 45% sperm fractions, the 90% fraction being the one with the best fertilizing potential and the 45% fraction being a poorer sub-populationReference 1480. Decreased sperm motility was observed in both fractions in response to Δ9-THC concentrations, mimicking those attained non-medically (0.32 and 4.8 μM), and in the 45% fraction in response to Δ9-THC concentrations typically seen therapeutically (0.032 μM). Inhibition of the acrosome reaction was only observed at the highest Δ9-THC concentration tested (4.8 µM) in the 90% fraction, while the 45% fraction displayed decreased acrosome reactions at all three Δ9-THC concentrations tested. Such effects raise the possibility that cannabis (i.e. Δ9-THC) can impair crucial sperm functions and male fertility, especially in those males already on the borderline of infertilityReference 1480.
CBD
In young male mice, IP administration of CBD at dose levels of 10 or 25 mg/kg (57 mg or 142 mg/70 kg)Footnote i for 5 consecutive days did not adversely affect sperm morphologyReference 1481. In another study, female mice were exposed to a single oral dose of 50 mg/kg CBD (284 mg/70 kg)Footnote i on Day 12 of gestation or within 12 hours of parturition. Males whose mothers had received CBD on Day 1 postpartum had approximately 20% less spermatozoa. The percentage of successful impregnations by males whose mothers had received CBD was reduced compared to control. Testicular weight was also reduced in male mice exposed to CBD on Day 12 of gestationReference 1482. In another study, male offspring of female mice who received a single oral dose of 50 mg/kg CBD (284 mg/70 kg)Footnote i on gestational Day 18, had significantly increased testes and seminal vesicles weightsReference 1483. Maternal exposure to a single oral dose of 50 mg/kg CBD within 12 hours of parturition resulted in long-term alterations in neuroendocrine function in male and female offspring. In addition, in CBD-exposed males, testes weight was significantly reduced and testicular testosterone concentraton was reducedReference 1484.
Studies investigating the effects of cannabis consumption on testosterone levels in men have yielded conflicting resultsReference 397. While some investigators have found that acute or chronic cannabis consumption significantly lowered plasma testosterone levels in a dose-dependent manner, others have apparently failed to find similar effects, while a more recent study found an increase in testosterone levelsReference 396Reference 397. Differences in the reported effects of cannabis on testosterone levels among the various studies have been, in part, attributed to differences in the experimental protocols employedReference 397.
An epidemiological study examining the association between cannabis use and male reproductive hormones and semen quality among 1 215 healthy young men, 18 - 28 years of age, reported that regular cannabis smoking (> 1 / week) was associated with a 28% reduction (95% CI: -48, -1) in sperm concentration and a 29% reduction (95% CI: -46, -1) in sperm count after adjustment for confounders but was also associated with higher levels of testosteroneReference 396. Combined use of cannabis more than once per week with other non-medical drugs was associated with a 52% reduction (95% CI: -68, -27) in sperm concentration and 55% reduction in total sperm count (95% CI: -71, -31). The authors also noted higher testosterone levels in male cannabis smokers within the same range as cigarette smokers.
A systematic review and meta-analysis of studies examining cannabis exposure and risk of testicular cancer found that current, chronic and frequent cannabis use was associated with testicular germ cell tumours (TGCT) when compared to never-use of cannabisReference 362. Out of 149 records retrieved, only three case-control studiesReference 1485-Reference 1487 met the rigorous inclusion criteria for meta-analysis. The meta-analysis was inconclusive with respect to the association between ever-use of cannabis and the development of TGCT (pooled OR = 1.19, CI = 0.72 - 1.95) for ever-use compared to never-use. A similar finding was obtained with former use and TGCT (pooled OR = 1.54, CI = 0.84 - 2.85). In contrast, current use of cannabis increased the odds of development of TGCT by 62% (OR = 1.62, CI = 1.13 - 2.31). Furthermore, frequency of cannabis use was associated with TGCT development, with weekly (or greater) use nearly doubling the odds of TGCT development (OR = 1.92, CI = 1.35 - 2.72). In addition, there was evidence of an association between duration of cannabis use (> = 10 years vs. never-use) and TGCT development (OR = 1.50, CI = 1.08 - 2.09). There was also evidence of an association between cannabis use and non-seminoma development, with current use more than doubling the odds of tumour development (OR = 2.09, CI = 1.29 - 3.37). Those using cannabis on an at least weekly basis had 2.5 times greater odds of tumour development compared to those who never used. Those who had used cannabis for at least 10 years had nearly 2.5 times the odds of non-seminoma development compared to never-use. There was insufficient evidence to conclude a relationship between seminoma tumours and cannabis use. The authors of the study suggest that cannabis use before age 18 may increase the risk of developing non-seminoma TGCT (AOR = 2.80, CI = 1.60 - 5.10) compared to use after age 18 (AOR = 1.30, CI = 0.60 - 3.20).
Effects on foetal development and child/adolescent development
Foetal development
Cannabis is the substance most abused by pregnant women: in the U.S. its prevalence exceeds 10% among pregnant womenReference 1488. Women self-report using cannabis during pregnancy for its antiemetic properties, especially during the first trimesterReference 1489. Relatively little is known about the changes in cannabis pharmacokinetics during pregnancy and the maternal-fetal transfer and fetal pharmacokinetics of THCReference 1384. THC and its metabolites can be detected in meconium and infant urine (as an indicator of maternal cannabis use). THC readily crosses the placenta but may be actively transported out of the placentaReference 1384. Placental concentrations of THC have been reported to average 200 ng/g while the mean THC level in fetal remains was 119 ng/gReference 1384. Because the ECS is an evolutionarily conserved signaling network that has been shown to guide critical aspects of brain developmentReference 1488 and because THC has been shown to cross into the placenta, this has raised concern that cannabis use during pregnancy, and even during the perinatal period, can have deleterious effects on foetal development and potentially on child, adolescent and adult developmentReference 1384.
Pre-clinical studies
In vitro exposure to THC caused dose-dependent inhibition of embryonic development to blastocysts, but even at the highest concentration used (160 nM), there was never a complete arrest of embryonic development. THC was relatively less potent than the other synthetic cannabinoid agonists (CP 55,940, Win 55,212-2, and anandamide); the other cannabinoid agonists only required 0.7 to 14 nM to inhibit embryonic development. The developmental arrest primarily occurred between the four-cell and eight-cell stagesReference 1379.
In vitro, exposure to CBD at concentrations of 6.4 to 160 nM did not significantly alter embryonic developmentReference 1379Reference 1490. In addition, i n vitro exposure to 1 to 25 µM CBD did not affect the viability of stabilized nontumour cell lines (human keratinocytes, rat preadipocytes, and mouse monocyte macrophages). Viability of glial cells was also not affected by the treatment with CBD up to 50 µMReference 1490.
In utero exposure to THC or cannabinoids in rodents is associated with axonal bundle malformation prenatally; decreased birth weight neonatally; increased rearing and locomotor activity, hyperactivity, learning impairment, vocalization, and impaired synapse formation postnatally; altered open field performance, impaired consolidation of long-term memory and inhibited social interaction and play behaviour during adolescence; and memory impairment, reduced synaptic plasticity, cognitive impairment, altered social behaviour, and an anxiogenic-like profile in adulthoodReference 1381.
A study conducted in pregnant mice using a low dose of THC has been shown to alter the expression level of 35 proteins in the fetal cerebrumReference 62. Furthermore this study concretely identified a specific molecular target for THC in the developing CNS whose modifications can directly and permanently impair the wiring of neuronal networks during corticogenesis by enabling formation of ectopic neuronal filopodia and altering axonal morphology. Another in vitro study with retinal ganglion cell explants showed that CBD (300 nM) decreased neuronal growth cone size and filopodia number as well as total projection length and induced growth cone collapse and neurite retraction (i.e. chemo-repulsion) through the GPR55 receptorReference 63.
There is also some emerging evidence from pre-clinical studies that suggests the presence of multigenerational alterations in gene expression and neurotransmission in offspring following parental exposure to cannabinoidsReference 1491. Male and female rats exposed to THC were observed to produce offspring with decreased expression of cannabinoid, dopamine and glutamate receptors, reduced NMDA receptor binding, and enhanced long-term depression in the dorsal striatumReference 1491Reference 1492. Furthermore, THC exposure in mice has been shown to cause genome-wide changes in histone methylationReference 1491Reference 1493. Taken together, these findings raise the possibility that parental exposure to cannabinoids may confer multigenerational and potentially transgenerational effects on offspring gene expression, histone methylation, and neurotransmissionReference 1494.
Clinical studies
Results from human epidemiological studies examining short-term neonatal outcomes among women who smoked cannabis during pregnancy are equivocal for some effects; there have been some reports of reduced neonatal birth weight and lengthReference 1495-Reference 1498 or a slightly increased risk of sudden infant deathReference 1499, but other reports of no effectReference 1500-Reference 1502. However, a recent systematic review concluded that the most robust effect of cannabis was a reduced birth weightReference 1362. On the other hand, there appear to be some long-term effects on the development of children born to mothers who used cannabis heavily during pregnancy. Prenatal cannabis use has been associated with lower scores on language, memory and abstract/visual reasoning domains in children of preschool ageReference 1381Reference 1503-Reference 1505. In school-aged children, prenatal cannabis exposure was also associated with deficits in attention and presence of impulsivity and hyperactivityReference 1381Reference 1506-Reference 1508. Later, in children between 9 and 12 years of age, prenatal cannabis exposure was associated with decreased performance in executive functions (e.g. impaired working memory, inattention, impulsivity and inability to plan)Reference 1509Reference 1510 with these deficits also appearing in 13 to 16-year oldsReference 1511 and 18- to 22-year oldsReference 1512.
A prospective structural neuroimaging study in young children (ages 6 to 8) (i.e. the "Generation R" study) reported that while prenatal cannabis exposure was not associated with any significant differences in total brain volume, grey matter volume, white matter volume, or ventricular volume, prenatal cannabis use was associated with differences in cortical thicknessReference 1513. Compared with control subjects not exposed to cannabis, children who had prenatal cannabis exposure had thicker frontal cortices, whereas children prenatally exposed to tobacco exhibited cortical thinning mainly in the frontal and parietal cortices. Increased cortical thickness in cannabis-exposed children raise the possibility of decreased synaptic pruning and altered neurodevelopmental maturation in areas of the brain associated with higher-order cognitive functions.
An epidemiological study of 1 709 randomly selected high school students that investigated the association between parental CUD and risk for CUD among offspring reported higher risks of CUD among offspring with parental histories of CUD, hard drug disorders and antisocial personality disorderReference 1514. The hazard ratio for CUD was 1.93 (95% CI = 1.30 - 2.88) among offspring with parental histories of CUD, 1.96 (95% CI = 1.32 - 2.90) among offspring with parental histories of hard drug use disorders, and 1.73 (95% CI = 1.06 - 2.82) for the offspring of parents with antisocial personality disorder. The effect was particularly significant among female offspring with maternal CUD histories.
Evidence suggests that cannabinoids accumulate in the breast milk of mothers who smoke cannabis and are transferred to newborns through breastfeedingReference 1385Reference 1515. Indeed, the THC concentration of breast milk in humans may be up to eight-fold higher than that found in maternal bloodReference 1385Reference 1488. In a case-control studyReference 1516, exposure to cannabis from the mother's milk during the first month post-partum appeared to be associated with a decrease in infant motor development at one year of age.
A recent review on the risks of cannabis use in pregnancy indicated that more women are turning to cannabis for its antiemetic role in the first trimester, which represents the period of greatest risk for the detrimental effects of drugs to the fetus. However, though the evidence for the effects of cannabis on human prenatal development is currently limited, the authors state that the available research supports a cause for concern. The collective evidence highlights that women who used cannabis during pregnancy compared to women who did not use cannabis during pregnancy were more likely to: be anemic, have a lower birth weight infant, and require placements in neonatal intensive care. Other studies show links between fetal cannabis exposure and adverse long-term outcomes during the school years concerning impulse control, visual memory, and attention. The exact mechanisms behind these effects are understudied, but are theorized to result from cannabis' interference with nervous system development. The endocannabinoid system, - first detected around day 16 of human gestation, is thought to play an important role in neural circuitry and brain development by regulating neurogenesis and migration, the outgrowth of axons and dendrites, and axonal path findingReference 1517.
Effects on adolescent mental health
Adolescence is an important stage of behavioural maturation and brain development marked by significant neuroplasticity that leaves the brain open to influence by external factors such as drug useReference 551. Furthermore, the majority of psychiatric disorders first begin to make their appearance during late adolescence/early adulthood, including disorders such as drug abuse, drug dependence/addiction, anxiety, depression, bipolar disorder and schizophrenia/psychosisReference 1518Reference 1519. The broad and abundant expression of the CB1 receptor in neuronal circuits involved in dependence/addiction and psychiatric disorders suggest the possibility of an association between the ECS and the pathophysiology of these diseasesReference 551. During adolescence, the levels of the endocannabinoids anandamide and 2-AG fluctuate considerably across various brain regions such as the striatum and the prefrontal cortex, with the levels of 2-AG being reduced from early to late adolescence and the levels of anandamide appearing to continuously increase in the prefrontal cortex during the course of adolescenceReference 551. Growing evidence also suggests a differential effect of cannabis exposure (THC) on the human brain that varies according to age of exposure with some evidence suggesting the potential for long-lasting effects associated with early, chronic and long duration of useReference 182Reference 541Reference 551Reference 552. Also, see Sections 2.4, 4.9.5 and 7.7.3 for additional information.
7.5 Cardiovascular system
- Pre-clinical studies suggest that ultra-low doses of THC may be cardioprotective on experimentally-induced myocardial infarction.
- Evidence from case studies and observational studies suggests that acute and chronic smoking of cannabis is associated with harmful effects on vascular, cardiovascular and cerebrovascular health (e.g. myocardial infarction, strokes, arteritis) especially in middle-aged (and older) users.
- However, a recent systematic review suggests that evidence examining the effects of cannabis on cardiovascular health is inconsistent and insufficient.
While cannabis is known to cause peripheral vasodilatation, postural hypotension, and characteristic conjunctival reddening after smokingReference 1520, the most consistent acute physiological effect of smoking cannabis is dose-related tachycardiaReference 144Reference 346Reference 352. Tolerance to the cardiovascular effects (i.e. hypotension and tachycardia) with chronic use has been reported by some but not by othersReference 141Reference 181Reference 324Reference 1521Reference 1522. While cannabis-induced tachycardia is not usually considered dangerous for healthy young users, it may be dangerous to those already suffering from cardiac disorders or anginaReference 140Reference 1523. Inhalation of cannabis smoke reduces the amount of exercise required to cause an angina attack by 50%Reference 1524, and has been associated with a five-fold increased risk of myocardial infarction in the first hour following smokingReference 352. This increased risk may be caused by a Δ9-THC-related increase in cardiac output, myocardial oxygen demand, catecholamine levels, and carboxyhemoglobin as well as postural hypotensionReference 346Reference 347Reference 1525.
A review of drug reporting incidences to a French addictovigilance network, a spontaneous reporting system of serious drug abuse and dependence, over a four-year period (2006 to 2010) reported a doubling in the number of cardiovascular cannabis-related reportsReference 1357. While overall, the number of cardiovascular cannabis-related reports was small (i.e. 5 cases out of 468 cannabis-related reports in 2006 and 11 cases out of 309 cannabis-related reports in 2010), the increase over time was significant and cannabis-related cardiovascular reports represented almost 2% of all incidence reports for all drugs reported to the addictovigilance network. The authors suggest the low numbers likely represent a significant rate of under-reporting, as would be expected both for a typical spontaneous reporting pharmacovigilance program, and for an illicit drug. Patients were mostly men (86%) with an average age of 34 years, and almost half had a history of cardiac or vascular disease and risk factors. The majority of patients (60%) were also concomitant tobacco smokers. Of the 22 cardiac complications reported, 20 were for acute coronary symptoms and 2 were for heart rate disorders. There were also 10 reports for peripheral complications (lower limb or juvenile arteriopathies and Buerger-like diseases) and 3 for cerebral complications (acute cerebral angiopathy, transient cortical blindness, and spasm of cerebral artery). In nine cases, the event led to patient death.
Consistent with the findings of the above review, a number of case-reports of arteritis associated with long-standing, chronic, daily cannabis smoking have also been publishedReference 1526-Reference 1529. Case-reports have also suggested an association between chronic, daily cannabis smoking and multi-focal intracranial stenosisReference 1530 and strokeReference 356Reference 357. One case report described an incidence of hemorrhagic and ischemic stroke following high doses of cannabis (i.e. 4 g per day)Reference 1531. In this case, the 38 year-old patient had right-sided hemiplegia, motor aphasia, and impairment of consciousness and had a history of frequent alcohol consumption, tobacco smoking (18 pack-years) and cannabis use but no past history of hypertension or any other cardiac, neurological or vascular disease. The authors suggest that altered cerebral autoregulation and regional hypoperfusion may have played a role in the pathogenesis of cannabis-related ischemic stroke and cannabis-induced transient arterial hypertension, and that failure of cerebrovascular autoregulation may have played a role in cannabis-related hemorrhagic stroke.
A general population survey of over 7 500 individuals aged 20 to 64, examining the odds of lifetime stroke/transient ischemic attack (TIA) among participants who had reported smoking cannabis in the past year found that 2.1% had reported having a stroke/TIAReference 1356. After adjusting for age cohort, past-year cannabis users had 3.3 times the rate of stroke/TIA (95% CI = 1.8 - 6.3) with this figure diminishing slightly (incident rate ratio (IRR) = 2.3) after adjustment for covariates related to stroke such as tobacco smoking. The elevated risk of stroke/TIA was specific to individuals who used cannabis weekly or more often (IRR = 4.7, 95% CI = 2.1 - 10.7). Furthermore, cases were more common in the older age cohorts with an IRR of 4.9 in the 40 to 44 year-old group vs. the 20 to 24 year-old group and similarly an IRR of 18.1 in the 60 to 64 year-old age group vs. the 20 to 24 year-old age group.
One study has also reported that AIDS patients may be at an increased risk of experiencing adverse cardiovascular outcomes caused by interactions between cannabis and anti-retroviral drugs, such as ritonavir, which has itself been associated with adverse cardiovascular eventsReference 1397.
In contrast with the findings from the above studies with chronic cannabis use (THC), evidence has been obtained in a pre-clinical study that ultra-low doses of THC may be cardioprotectiveReference 1532. In this pre-clinical study, the authors report that pre-treatment of mice with an ultra-low dose of THC (0.002 mg/kg) 2 h and 48 h prior to induction of experimental myocardial infarction was associated with partial restoration of cardiac function, an effect that was not observed in mice treated only with a mixture of ethanol, cremophor and saline (1:1:18, respectively), the vehicle used for THC. In addition, pre-treatment with the ultra-low THC dose was associated with a statistically significant reduction in infarct size, significantly lower serum troponin T, reduction in tissue damage, and a decrease in the extent of tissue neutrophil infiltration. The study findings suggest that single application of an ultra-low dose of THC in mice provides a significant protection against an ischemic insult to the heart.
A recent systematic review of 24 studies (22 observational; 2 RCTs) suggests that evidence examining the effect of cannabis on cardiovascular health is inconsistent and insufficient. Based on the limited data, which was rated as poor to moderate quality with high risk of bias, there were no overall significant associations between cannabis use and adverse cardiovascular outcomes related to diabetes, dyslipidemia, acute myocardial infarction, stroke, or cardiovascular and all-cause mortality. Six studies did suggest a metabolic benefit from cannabis use, however, these studies were cross-sectional in nature and do not establish causality. The authors highlighted that data were from 'low risk' cohorts, and that including 'high risk' populations may have revealed different resultsReference 1533.
7.6 Gastrointestinal system and liver
- Evidence from case reports suggests chronic, heavy (THC-predominant) cannabis use is associated with an increased risk of cannabis hyperemesis syndrome (CHS).
- Limited evidence from observational studies suggests mixed findings between (THC-predominant) cannabis use and risk of liver fibrosis progression associated with hepatitis C infection.
7.6.1 Hyperemesis
There are an increasing number of case-reports being published regarding the CHS. CHS is a condition observed in people chronically using cannabis on a daily basis, often for years, and is characterized by severe, intractable episodes of nausea and cyclic vomiting accompanied by abdominal pain (typically epigastric or periumbilical); these symptoms seem to be relieved by compulsive hot water bathing or showeringReference 299-Reference 309. Cannabinoid hyperemesis appears to be triphasic with prodromal, hyperemetic and recovery phasesReference 1534. The prodomal phase includes nausea and abdominal discomfort, typically worse in the morning. During the hyperemetic phase severe volume depletion can occur accompanied by acute renal failure and electrolytic abnormalities. The recovery phase can last between a few days to months. The pathophysiology of CHS is not well understoodReference 307. Treatment of patients presenting with this syndrome has been reported to include cessation of cannabis use, rehydration, and psychological counsellingReference 305Reference 307. The efficacy of anti-emetics such as metoclopramide, ondansetron, prochlorperazine, and promethazine in relieving the symptoms of nausea and vomiting in patients with CHS appears to be of little valueReference 303Reference 305Reference 306Reference 309. One case-report suggests that lorazepam (1 mg i.v., followed by 1 mg tablets b.i.d.) may provide some benefit in alleviating the symptoms of CHS, at least in the short-termReference 1535.
Limited evidence from a number of case reports has suggested that topical application of capsaicin cream (0.075% to 0.25%) to the abdomen, or any part of the skin (e.g. back or chest), may help alleviate the symptoms associated with CHS within 30 to 45 min of application, with no secondary dermatologic effects, when other known therapeutic measures had failed, with the exception of haloperidolReference 1534Reference 1536Reference 1537.
7.6.2 Liver
A number of studies have strongly implicated the ECS in chronic liver diseaseReference 1538-Reference 1542. Studies in patients with chronic hepatitis C have found a significant association between daily cannabis smoking and moderate to severe fibrosisReference 1460, as well as cannabis smoking being a predictor of fibrosis progression and steatosis severityReference 1402. Steatosis is an independent predictor of fibrosis progression and an established factor of poor response to anti-viral therapyReference 1543. The authors of the cited studies recommend that patients with ongoing chronic hepatitis C be strongly advised to abstain from daily cannabis use. In contrast, a longitudinal cohort study reported that cannabis smoking was not associated with progression of liver disease, as measured with the AST-to-platelet ratio index (APRI) score, in individuals with HIV-Hepatitis C co-infectionReference 1544. While smoking cannabis did seem to accelerate progression to a clinical diagnosis of cirrhosis (hazard ratio = 1.33 per 10 joints/week; CI = 1.09 - 1.62), correcting for confounding factors appeared to attenuate this finding. Similarly, cannabis smoking was associated with a slightly increased risk of progression to clinically diagnosed cirrhosis and end-stage liver disease combined (hazard ratio: 1.13, CI = 1.01 - 1.28), but this effect was no longer significant when correcting for confounding factors. Differences in the conclusions between these studies may have been caused by differences in study methodology and also potentially by differences in degree of cannabis exposure (i.e. daily vs. weekly use). Another study showed that modest cannabis use (defined as anything less than daily use in this study) was associated with an increase in the duration of time that patients remained on ARTReference 376. This effect was postulated to contribute, at least in part, to an increase in the percentage of patients demonstrating a sustained virological response (i.e. the absence of detectable levels of hepatitis C virus RNA six months after completion of therapy).
7.7 Central nervous system
The most frequently reported adverse events encountered with (mainly psychoactive) cannabinoids involve the CNS. Commonly reported CNS events in controlled clinical trials with dronabinol (Marinol®, no longer available in Canada) and nabiximols (Sativex®) are intoxication-like reactions including drowsiness, dizziness, and transient impairment of sensory and perceptual functionsReference 227Reference 431. A "high" (easy laughing, elation, heightened awareness), which could be unwanted or unpleasant for some patients, was reported in 24% of the patients receiving Marinol® as an anti-emetic, and in 8% of patients receiving it as an appetite stimulantReference 227. Other adverse events occurring at a rate of > 1% for Marinol® include anxiety/nervousness, confusion, and depersonalizationReference 227. The rates of dizziness, euphoria, paranoia, somnolence, abnormal thinking ranged from 3 to 10%Reference 227. The rates of amnesia, ataxia, and hallucinations were > 10% when used as an anti-emetic at higher dosesReference 227. Dizziness is the most common intoxication effect with Sativex®, reported initially in 35% of patients titrating their dose; the reported incidence of this effect in long-term use is approximately 25%Reference 1545. All other intoxication-like effects are reported by less than 5% of users (with the exception of somnolence, 7%)Reference 1545. Other events reported for Sativex® include disorientation and dissociation. Many, if not all, of the above-noted CNS effects also occur with (THC-predominant) cannabis.
7.7.1 Cognition
- Evidence from clinical studies suggests acute (THC-predominant) cannabis use is associated with a number of acute cognitive effects.
- Evidence from observational studies suggests chronic cannabis use is associated with some cognitive and behavioural effects that may persist for varying lengths of time beyond the period of acute intoxication depending on a number of factors.
- Limited evidence from human clinical imaging studies suggests THC and CBD may exert opposing effects on neuropsychological/neurophysiological functioning.
- Evidence from mainly cross-sectional human clinical imaging studies suggests heavy, chronic cannabis use is associated with a number of structural changes in grey and white matter in different brain regions.
- Furthermore, early-onset use and use of high-potency, THC-predominant cannabis, has been associated with an increased risk of some brain structural changes and cognitive impairment.
The acute effects of cannabis use on cognition have been well studiedReference 150Reference 151Reference 182Reference 205Reference 541Reference 553. Acute exposure to cannabis (THC) impairs a number of cognitive faculties such as short-term memory, attention, concentration, executive functioning and visuoperception; CBD may protect from some of these impairmentsReference 150Reference 151Reference 182Reference 205Reference 541Reference 553Reference 1546-Reference 1548.
The long-term effects of cannabis exposure on cognition continue to be the subject of some debate. Some studies report a positive association between long-term cannabis consumption and cognitive deficitsReference 150Reference 151Reference 1549-Reference 1551, or suggest that some cognitive deficits persist after prolonged abstinence (especially when use is initiated during adolescence)Reference 150Reference 235Reference 552-Reference 554Reference 1547Reference 1552. However, other studies did not find an association between cannabis use and certain long-term cognitive declineReference 554Reference 1552Reference 1553. Methodological limitations, differences in types of cognitive measures investigated, and differences in length and frequency of exposure, age of onset at which use begins, and duration of abstinence as well as the presence of residual confounding factors and the absence of powerful effects have all contributed to difficulties in assessing the effects of chronic use, and may help explain the discrepancies among studies.
Nonetheless, studies generally suggest that chronic cannabis users may suffer varying degrees of cognitive impairment that have the potential to be long-lasting, especially if use begins earlier on in adolescence (< 16 years of age), is frequent (i.e. daily or near-daily), and persistent (i.e. over the course of years)Reference 147Reference 182Reference 205Reference 541Reference 552.
In patients with MS and using cannabis, one cross-sectional study showed that prolonged use of ingested or inhaled cannabis was associated with poorer performance on various cognitive domains (e.g. information processing speed, working memory, executive function, and visuospatial perception)Reference 233.
In a prospective longitudinal study investigating the association between persistent cannabis use and neuropsychological functioning in a birth cohort of 1 037 individuals followed over a period of 20 years, persistent cannabis use (i.e. CUD) beginning in adolescence was associated with statistically significant global neuropsychological decline across a number of domains of functioningReference 552. Furthermore, cessation of cannabis use, for a period of one year or more, did not appear to fully restore neuropsychological functioning among adolescent-onset persistent cannabis users. Correcting for a multitude of confounding factors did not appear to significantly diminish the effect.
However, another study that examined a shorter period of chronic use, more modest use, and in a slightly different age group found that cognitive deficits did not persist beyond the period of intoxicationReference 1553. In this longitudinal prospective cohort study of 2 235 teenagers (Avon Longitudinal Study of Parents And Children, ALSPAC), cannabis users appeared to have lower teenage IQ scores, and poorer educational performance compared to non-users. Furthermore, cannabis users also had higher rates of childhood behavioural problems, childhood depressive symptoms, other substance use (including cigarettes and alcohol) and maternal use of cannabis. However, after adjustment to account for group differences, cannabis use by age 15 did not predict either lower IQ scores at age 15 or poorer educational performance at age 16. The authors suggested that cannabis use at the modest levels used in this sample of teenagers was not by itself causally related to cognitive impairment but acknowledged that the short period of use (1 - 2 years), modest levels of use (≤1 week or less) and other factors does not rule out that chronic, frequent, and persistent cannabis use may have adverse effects on cognitive function.
A report that examined the associations between cannabis use and changes in intellectual performance in two longitudinal studies of adolescent twins discordant for cannabis use (n = 789 and n = 2 277) reported that those twins that had used cannabis had lower test scores compared to non-users and showed a significant decline in crystallized intelligence (i.e. verbal ability, general knowledge) between pre-adolescence and late adolescenceReference 1554. However, the report failed to find a dose-response relationship between frequency of use and change in IQ and cannabis-using twins did not show significantly greater IQ decline compared to their abstinent siblings. The limitations of this study included methodological challenges leading to an inability to properly measure a dose-response effect.
A recent longitudinal study that examined the adverse effects of cannabis on adolescent brain development reported that repeated heavy exposure to cannabis during adolescence could have a detrimental effect on resting functional connectivity, intelligence, and cognitive functionReference 1555. Compared to healthy controls, individuals with a diagnosis of CUD showed a decrease in functional connectivity in specific brain regions (i.e. the caudal anterior cingulate and dorsolateral and orbitofrontal cortices) over an 18-month study period. Greater cannabis use over the period between baseline and follow-up predicted low full-scale IQ and predicted lower cognitive function consistent with findings by Meier et al. (2012)Reference 552.
Data from structural and functional imaging studies
The ability of cannabis to affect a variety of cognitive processes both after acute and chronic exposure has inevitably raised questions regarding the structural and functional domains in the brain affected by short and long-term cannabis exposure. Two systematic reviews have been published looking at the acute and chronic effects of cannabis exposure on brain structure and functionReference 1556Reference 1557. In general, the findings from studies examining the effects of cannabis exposure on brain structure and function are mixed, mainly owing to the cross-sectional nature of the studies, the lack of consistent and extensive control for confounding variables and small sample sizesReference 1558. Findings from a number of such studies are summarized below.
Neurophysiological effects
In the first systematic review of 45 human and animal studies that examined the effects of acute exposure of cannabis on the brain, THC and CBD were found to exert opposing neurophysiological effects with the general exception of memory/verbal learning where CBD had no effectReference 1556. Acute administration of THC was consistently associated with increases in cerebral blood flow mainly in the prefrontal, insular, cerebellar, and anterior cingulate regions that are known to be enriched in CB1 receptors and which are responsible for directing a number of cognitive functions as well as playing important roles in the neurobiology of addiction. Subjective levels of intoxication, "feeling high", anxiety, altered time perception, depersonalization, dissociative experiences, and measures of confusion were correlated with increased global cerebral blood flow. Other brain areas where changes in cerebral blood flow were observed in response to THC administration included the basal ganglia, hippocampus/amygdala, thalamus, and all cerebral cortices. Abnormal brain activity has been observed following THC administration during the performance of tasks associated with memory, affective processing, attention, motor function, reward, as well as response inhibition, salience, and sensory processing. CBD appeared to modulate resting brain activity mainly in the limbic and paralimbic cortices, areas implicated in the pathophysiology of anxiety.
Structural effects
In the second systematic review of 43 studies, the findings suggest the existence of structural brain abnormalities (mainly in areas of the brain rich in CB1 receptors) and altered neural activity during resting state and under several different types of cognitive paradigms. In adolescents, the findings suggested structural and functional alterations that may appear soon after starting drug use and that could be related to genderReference 1557. In terms of structural abnormalities, the findings from available studies are heterogeneous with studies reporting either increases or decreases in gray matter volumes, however, the most consistently reported alterations were reduced hippocampal volume (reported to persist at least for several months after last use and associated with amount of cannabis used), as well as reduced amygdala, cerebellum and frontal cortex volume. Diffusion tensor imaging studies have found differences in white matter thickness in the corpus callosum as well as the frontal white matter fibre tract (increases or decreases) which according to the authors suggests that chronic cannabis exposure may alter white matter structural integrity either by affecting demyelination, causing axonal damage, or indirectly through delaying normal brain development. Functional imaging studies comparing activation in both adult and adolescent chronic cannabis users with healthy controls during the performance of different cognitive tasks suggest that chronic cannabis users use similar brain areas compared to healthy controls but demonstrate an altered pattern of brain activity. Despite this altered pattern of brain activity, the level of performance of the cannabis users on the cognitive tasks was generally within what the authors considered a normal range of test performance suggesting that the brains of chronic cannabis users engage in neuroadaptive behaviours, by, for example, recruiting other brain areas for tasks to maintain normal cognitive performance. However, while performance may not have been significantly altered in an artificial laboratory setting, the impact of these subtle brain alterations on real-life social and occupational tasks, especially in cognitively complex and demanding contexts, may be different. Limitations of this review include differences between the studies included in the review including methodological differences, socio-demographic differences and differences in gender, age of onset, lifetime use, and abstinence period before the acquisition of imaging data.
More recently, a retrospective study that examined brain morphology in a sample of adult and adolescent daily cannabis users and non-users reported that daily cannabis use was not associated with notable changes in gray matter volume or shape in a variety of brain areas including the nucleus accumbens, amygdala, hippocampus, and cerebellumReference 1559. Importantly, this study corrected for a number of confounding factors such as alcohol use and tobacco use that were not always corrected for in other studies. However, significant limitations of the study included a lack of information about the age of onset, history, and duration of exposure to cannabis (i.e. adult use was measured only over past two-month period and adolescent use was measured only over past three-month period), information about the potency or composition of the cannabis used, or socio-economic status. Other limitations of this study included its cross-sectional nature.
A study that investigated the association between cannabis potency, as well as frequency and age of first use, on the microstructural organization of the corpus callosum using diffusion tensory imaging tractography reported that frequent use of high-potency cannabis was associated with disturbed callosal microstructural organization in individuals with and without psychosisReference 1560. In this study, 56 individuals with a first-episode of psychosis (of which 37 were cannabis users) and 43 individuals without psychosis (of which 22 were cannabis users) were studied for evidence of structural differences in the corpus callosum, the largest white matter tract in the brain and containing a high abundance of CB1 receptors. High-potency cannabis users (patients and individuals without psychosis) showed significantly higher mean diffusivity in the corpus callosum (i.e. lower white matter tract density) than both low-potency users and never users. There was also a significant association between the frequency of use on total corpus callosum mean diffusivity with daily users having significantly higher mean diffusivity than both occasional and never users. Furthermore, daily users of high-potency cannabis had significantly higher mean diffusivity than daily low-potency users and those who never used or used weekly. Lastly, no statistically significant differences in corpus callosum mean diffusivity were noted between early onset and later onset users.
Another study examined the longitudinal changes in white matter microstructure after heavy cannabis use using diffusor tensor imagingReference 1561. In this study, 23 young adult regular cannabis users and 23 age, sex-, and IQ-matched non-cannabis using controls with limited substance use histories were entered into the study. Cannabis use began prior to 17 years of age. The study findings suggested that cannabis use was associated with deficits in structural white matter in a number of different brain regions. These effects on white matter integrity were dose-dependent suggesting that continued heavy cannabis use during adolescence and young adulthood was associated with more profound white matter deterioration and contributed to functional impairment (e.g. verbal learning).
A more recent study that examined associations between a number of key variables (i.e. age at onset of cannabis use, duration of use, frequency of use and dose) and changes in white matter integrity reported that increased cannabis use was associated with a decrease in white matter integrity in selected brain areasReference 1562. The study noted that changes (increases or decreases) in white matter integrity varied with age at onset of regular cannabis use, duration of use and current dose but not frequency of current use. Widespread changes in white matter integrity were noted within frontal, parietal and motor tracts with younger users having lower axial and radial diffusivity and older users having higher axial and radial diffusivity. Lower axial diffusivity is associated with reduced axonal volume while higher radial diffusivity is associated with reduced myelination; in other words, younger users showed decreased axonal volume, and increased myelination, while older users showed increased axonal volume, and decreased myelination. Importantly, previously unrecognized changes in white matter integrity associated with cannabis use were noted in older users (> 32 years of age). The authors suggest that exposure to lower potency cannabis during adolescence/early adulthood in combination with the effects of prolonged exposure to cannabis over many years results in disturbances in white matter integrity. Limitations of the study included its cross-sectional nature and a number of confounding factors including tobacco use, which was greater in the cannabis-using group vs. non-using group.
Another review of 31 studies examined the association between neuroanatomic alterations (especially in brain areas with high CB1 receptor density) and regular cannabis use (i.e. daily, near-daily use) as well as association with level of use (i.e. dose, duration, age at onset of use)Reference 1563. The study found the existence of neuroanatomic alterations in brain areas high in cannabinoid receptors (i.e. hippocampus, prefrontal cortex, amygdala, cerebellum), and greater dose and earlier age of onset were associated with these alterations. The majority of cannabis users started smoking cannabis between age 15 and 17 and duration of use varied greatly across examined studies (i.e. 2 years to 23 years of regular use). Lifetime episodes of cannabis use ranged from 402 to 5 625. Several, but not all, of the included studies controlled for the confounding effects of alcohol and tobacco. Abnormalities in cannabis users compared to controls were most consistently observed in the hippocampus followed by prefrontal regions with very high cannabinoid receptor densities (i.e. the lateral prefrontal cortex and the anterior cingulate cortex). Overall, the most consistent neuroanatomic alterations included: (1) volumetric reductions in all regions (except cerebellum and striatum where increases were observed), (2) higher gray matter densities in most regions (i.e. amygdala, prefrontal cortex, parietal cortex, striatum); (3) altered shape, sulcal-gyral anatomy; and (4) cortical thickness. Principally, areas with the highest densities of cannabinoid receptors most consistently saw neuroanatomic alterations. Cannabis dosage was most consistently associated with neuroanatomical alterations in the hippocampus and the prefrontal cortex, and less consistently with the amygdala, striatum, parahippocampal gyrus, insula and temporal pole. Age of onset of cannabis use was most consistently associated with prefrontal neuroanatomy, and less consistently with neuroanatomical alterations in the parahippocampal gyrus, temporal cortex, and global brain measures. Duration of regular use was most consistently associated with neuroanatomical alterations in the prefrontal cortex and the hippocampus but not the amygdala, the parahippocampal gyrus, the cerebellum and the striatum. Taken together, the studies reviewed in this literature review suggest that regular cannabis use is associated with neuroanatomic alterations in several brain regions with the most consistent changes seen in the hippocampus (reduced volume and gray matter density, altered shape), followed by changes in the amygdala and striatum, orbitofrontal cortex, parietal cortex, insular cortex, and cerebellum. Furthermore, some associations were found between higher cannabis dosage and hippocampal alterations and between earlier age of onset and alterations in the prefrontal cortex. The authors also mention preliminary evidence suggestive of a protective effect of CBD and toxic effect of THC in the hippocampus, cerebellum, prefrontal and lingual regions. In conclusion, early onset of use, duration of use, dose and relative ratio of THC to CBD were all associated with neuroanatomical alterations in various brain regions.
A recent review looked at the effects of cannabis use on brain structure and function from (mainly cross-sectional) imaging studiesReference 1564. The review made the following conclusions: (1) smaller hippocampal volumes in cannabis users relative to healthy controls has been one of the most consistently reported findings; (2) there is an inverse relationship between cannabis use and hippocampal volume; (3) dose and duration of cannabis use appear critical for effects of cannabis on hippocampal volume; (4) cannabis use is associated with smaller orbitofrontal cortex volumes; (5) early onset cannabis use interacts with adolescent developmental events leading to disruption of normal neurodevelopmental processes (e.g. pruning and plasticity); (6) pre-existing vulnerabilities interact with dose, duration, and onset of cannabis use to determine outcomes; (7) cannabis use is associated with less efficient and less mature white matter microstructure in the genu, rostrum, and splenium of the corpus callosum as well as the superior longitudinal fasciculus and arcuate fasciculus; (8) combined cannabis and alcohol use resulted in significantly greater alterations in white matter tracts (i.e. in the superior longitudinal fasciculus, right posterior thalamic radiations, right prefrontal thalamic fibres, right superior temporal gyrus, right inferior longitudinal fasciculus, and left posterior corona radiata); (9) early onset and more intense cannabis use during adolescence is linked to less brain activation, with users who started in later adolescence showing higher brain activation compared to earlier onset users; (10) cannabis use is associated with increased recruitment of additional brain regions not typically utilized to compensate for deficits in other regions.
A recent systematic review and meta-analysis of 69 cross-sectional studies (2152 cannabis users/6575 non-users) in adolescents and young adults (≤26 years of age) reported that frequent/heavy cannabis use was associated with a small effect size for reduced cognitive functioning relating to delayed memory, attention, and speed information processing (d, -0.25; 95% CI, -0.32 to -0.17). The effect size diminished, however, following 72 hours of abstinence, (d, -0.08; 95% CI, -0.22 to 0.07), suggesting that any acute cognitive impairment from cannabis use may be restored after three days of abstinence. No greater deficits were observed in adolescents compared to young adults. Key limitations of these findings are related to the cross-sectional design (causality not established) and unaccounted variables in analyses (e.g., previous duration of use, cognitive functioning prior to cannabis use)Reference 1565.
A recent literature review on THC potency supports that higher levels of potency, compared to lower levels, is associated with greater risk of cannabis use disorder, psychosis, acute cognitive impairment (especially in tasks that measured motor control and executive functioning), and structural changes of white matter in the corpus callosum. The authors recommend clinicians to not only ask and monitor patients' generic cannabis use frequency and duration, but also the specific concentrations of THC being used to better assess its adverse effects and risks. Within the context of prescribing cannabis for medical purposes, clinicians should weigh the potential risks of higher potency cannabis relative to its potential therapeutic effectsReference 1566.
7.7.2 Psychomotor performance and driving
- Evidence from experimental clinical studies suggests acute use of (THC-predominant) cannabis impairs a number of psychomotor and other cognitive skills needed to drive a motor vehicle.
- While chronic/frequent cannabis use may be associated with a degree of tolerance to some of the effects of cannabis in some individuals, chronic cannabis use can still pose risks to safe driving due, in part, to significant body burden of THC leading to a chronic level of psychomotor impairment.
- Evidence from clinical and epidemiological studies suggests a dose-response effect, with increasing doses of THC increasing the risk of motor vehicle crashes that can lead to injuries and death.
- Combining alcohol with cannabis (THC) is associated with an increased degree of impairment and increased risk of harm.
It is well known from studies carried out among non-medical cannabis users that exposure to THC-predominant cannabis and psychoactive cannabinoids impairs psychomotor performanceReference 140Reference 150Reference 238 and patients must be warned not to drive or operate complex machinery after acute consumption of smoked/vapourized or orally-ingested cannabis or consumption of psychoactive cannabinoid medications (e.g. dronabinol, nabilone, nabiximols) until a sufficient amount of time has elapsed to allow for safe driving. There is also now increasing evidence of chronic impairment associated with longer-term, frequent cannabis use (even with abstinence) that may also affect the ability to safely drive (Reference 150Reference 229Reference 692Reference 1567Reference 1568 and see below).
Evidence from human post-mortem studies shows that the brain can accumulate relatively high concentrations of THC and 11-hydroxy-THC, while the concentrations of these cannabinoids remain much lower in bloodReference 458. In this study, 12 paired post-mortem samples of blood and brain from individuals involved in fatal motor vehicle accidents were examined. In one case, THC concentration in the brain was 19.4 ng/g, while the blood concentration of THC was 4.4 ng/mL. In another case, brain THC concentration was 29.9 ng/g where the THC concentration in the blood was ≤ 0.2 ng/mLReference 458. Furthermore, examination of specific brain areas showed significant accumulation of THC and 11-hydroxy-THC in the substantia nigra, hippocampus, the occipital lobe, the striatum-putamen-pallidum, the frontal lobe, spinal cord and corpus callosum, the cortex and the white matterReference 458. These findings show that despite low to near undetectable blood levels of THC and 11-hydroxy-THC, these psychoactive cannabinoids can accumulate in a number of brain areas associated with thinking, decision-making/executive function, vision, memory and coordination and which play an important role in the safe operation of a motor vehicle.
Cannabis
A review article looking at the impairing psychomotor effects of cannabis on driving found that psychomotor testing performance is decreased for up to five to six hours after smoking cannabis, with the majority of impairment occurring in the first two hours after smoking, although others suggest a window of at least three to six hours after smokingReference 238. Given the variability in the data and the emergence of new studies with higher potency cannabis showing persistence of some psychoactive effects (e.g. sedation) up to eight hours after last inhalation, the authors of the study recommend that patients abstain from driving for a minimum of eight hours after achieving a subjective "high" from cannabis use, though the minimum waiting time may, for example, be longer in those that consumed cannabis orally as the onset of intoxication and psychomotor impairment is delayed compared to inhalation and lasts longer.
Clinical studies
Acute
Clinical studies have shown that acute cannabis administration (i.e. THC) affect areas of the brain involved in perception, attention, concentration, inhibitory/impulsivity control, executive control/decision-making, awareness, alertness, and coordination, all of which are required to safely operate a motor vehicle, although chronic cannabis users may develop tolerance to some, but not all, of the intoxicating/impairing effects associated with acute cannabis useReference 150Reference 238. Some of these effects may also persist beyond the period of acute intoxication, especially in chronic/frequent usersReference 150.
One clinical laboratory study reported that THC doses between 40 µg/kg and 300 µg/kg cause a dose-dependent reduction in performance on laboratory tasks measuring memory, divided and sustained attention, reaction time, tracking and motor functionReference 154.
Another clinical study evaluated the psychomotor and neurocognitive effects of acute exposure to smoked cannabis as a means to evaluate the acute effects of cannabis on skills needed to drive safely (i.e. accurately controlling a car and reacting quickly to events on the road)Reference 204. Domains examined included psychomotor function, working memory, risk taking, and subjective and physiological effects in frequent and occasional cannabis smokers following controlled smoking of a 6.8% THC cigarette (i.e. 54 mg total available THC in the cigarette) up to 22.5 h after smoking. Frequent smokers smoked on at least four occasions weekly, while occasional smokers smoked less than twice per week. Mean blood THC concentration at 0.5 h post-smoking was 32 ng/mL in frequent smokers and 17.4 ng/mL in occasional smokers. At six hours, frequent smokers had a blood THC concentration of 4.1 ng/mL while most subjects classified as occasional smokers had blood THC concentrations under 1.3 ng/mL. At 24 h, all occasional smokers' blood THC concentrations were below the limit of detection, while frequent smokers had a mean blood THC concentration of 2.9 ng/mL. Occasional smokers had significantly higher scores on measures of "high" and "stimulated" as well as more intense anxiety. Significantly higher scores were also reported by occasional users on measures of "difficulty concentrating" (at three hours) and "altered sense of time" (at three and four hours). The authors found that cannabis smoking significantly impaired psychomotor function up to 3.5 h after smoking a 6.8% THC cigarette. Cannabis smoking appeared to impair psychomotor function (tracking error, hits, false alarms and reaction time) to a greater degree in occasional smokers compared to frequent smokers, raising the possibility of tolerance to some of the impairing effects of cannabis in frequent smokers. Occasional smokers also reported significantly longer and more intense subjective effects compared with frequent smokers who had higher blood THC concentrations.
A case cross-over study that examined whether acute cannabis use leads to an increased collision risk among 860 drivers that presented to emergency departments in Toronto and Halifax with an injury from a traffic collision found that 11% of the presenting drivers (95% CI = 9.0 - 13.1) reported using cannabis before drivingReference 1569. Regression analysis that measured exposure with blood and self-report data found that cannabis use alone was associated with a four-fold increase (OR = 4.11; 95% CI = 1.98 - 8.52) in odds of a collision. Those individuals who used cannabis before driving were also more likely to be male (91%). Ethanol consumption was associated with an increase in the odds of a crash (OR = 3.89, 95% CI = 1.86 - 8.09).
A randomized, double-blind, placebo-controlled clinical study examined the acute effects of two different doses of THC (13 mg vs. 17 mg) on cognitive-motor skills (i.e. speed and accuracy), cognitive flexibility, decision-making ability, and time and distance estimation (i.e. from an approaching car) in regular cannabis usersReference 1369. Fourteen subjects that used cannabis on a daily basis for at least five years were recruited into the study. The 17 mg THC dose was associated with a significant increase in collisions against the walls in the virtual maze task, whereas the effects of both THC doses were also significant in some of the cognitive flexibility tests. A significant increase in a risk-taking task was also noted with the higher 17 mg THC dose. Effects of THC on subjective ratings of "satisfaction", "pleasure", "high", and "drug effect" were significantly increased in subjects on either the low (13 mg) or high (17 mg) THC dose compared to the placebo. These results appear to support a dose-response effect of THC on cognitive impairment affecting faculties required for safe operation of a motor vehicle. For reference purposes, a recent study estimated that the mean weight of cannabis in a joint is 300 mgReference 589. As such, a 300 mg joint with a potency of 4.3% THC would deliver 13 mg of THC whereas a 300 mg joint with a potency of 5.7% would deliver a 17 mg dose of THC.
A randomized, double-blind, placebo-controlled, crossover clinical study examined the acute effects of varying potencies of cannabis on ratings of a variety of subjective effects (i.e. intensity and duration of effects)Reference 495. One gram joints containing increasing doses of THC (i.e. 29 mg, 49 mg, and 69 mg) having respective THC potencies of 9.75%, 16%, or 23% THC in a group of regular non-medical cannabis users showed a strong effect of high-potency cannabis on ratings of subjective effects. Participants reported using an average of 7.7 joints per month in the past-year with an average duration of cannabis use of 7.7 years. Smoking of the low dose (29 mg THC) was associated with a mean serum THC Cmax of 120 ng/mL and a maximum "high" score of just under 60 on the VAS scale, smoking of the medium dose (49 mg THC) was associated with a mean serum THC Cmax of 160 ng/mL and was associated with a maximum "high" score of just over 60 on the VAS scale, whereas smoking of the highest dose (69 mg THC) was associated with a mean serum THC Cmax of 190 ng/mL and a maximum "high" rating of 80 on the VAS scale, further supporting a dose-response effect. While blood levels of THC declined rapidly and fell under 25 ng/mL within two hours post-dose at the highest dose, subjective ratings of "high" declined far more gradually and persisted for longer compared to the blood levels of THC. The scores on the VAS for dizziness, dry mouth, palpitations, impaired memory and concentration, down, sedated and anxious feelings reached maximum within the first two hours post-dose. THC dose effect was significant. At almost two hours after smoking the highest dose, participants who smoked the highest potency cannabis cigarette reported being much less alert, content and calm compared to those having smoked placebo. At four hours post-dose, scores of "feel a drug effect" rose correspondingly with increasing THC doses with significant differences between THC treatment conditions relative to placebo and between the high dose and the low dose. THC-induced decrease in stimulation and increase in anxiety lasted up to eight hours post-smoking. Overall, the study findings indicate that psychoactive and cognitive effects were most pronounced in the first two hours post-dose, although a significant increase in sedation was still measurable eight hours post-dose. The maximum rating of "high" was reached within minutes in all dose conditions, but was 1.4 times higher with the high THC dose (69 mg) than with the low dose (29 mg). The rating of dizziness doubled with the highest dose compared to the middle and low doses (29 and 49 mg THC) up to two hours post-smoking. Sedation was increased by almost six-fold with the highest THC dose (69 mg) compared to placebo. The subjective effects were felt as unpleasant with the middle and high THC doses, relative to the low dose (29 mg) which received the highest score on "like the drug" and "want more of the drug".
A double-blind, placebo-controlled, crossover study comparing the acute effects of a medium dose of dronabinol (20 mg) and of two cannabis milk decoctions, containing medium (16.5 mg) or high doses (45.7 mg) of THC, reported severe impairment on several performance skills required for safe drivingReference 1570. A "moderate" dose (21 mg of THC) was associated with impairments in motor and perceptual skills necessary for safe drivingReference 1571. In one study, performance impairment appeared to be less significant among heavy cannabis users compared to occasional users, potentially because of the development of tolerance or compensatory behaviourReference 221. It has been suggested that, unlike alcohol, cannabis users are aware of their level of intoxication and compensate by becoming hyper-cautious; in tasks such as driving, this kind of behaviour results in decreased speed, decreased frequency of overtaking, and an increase in following distanceReference 1572Reference 1573. Others disagree with this assertionReference 231Reference 1574.
A double-blind, placebo-controlled, randomized, three-way, crossover design study suggested that acute administration of dronabinol dose-dependently impaired driving performance in both occasional (defined as using a cannabinoid between 5 and 36 times per year) and heavy cannabis users (defined as using one to three joints per day, > 160 times per year)Reference 1575. However, the magnitude of the impairment appeared to be less in heavy users, possibly due to tolerance. The authors indicate that driving impairments after dronabinol were of clinical relevance and comparable to drivers operating their vehicles at a blood-alcohol (BAC) concentration of greater than 0.8 mg/mL (0.08 g%). Approximately 25% of the "heavy users" demonstrated impairment equivalent to, or worse than, that reported for drivers with a BAC of 0.5 mg/mL (0.05 g%). Driving impairments after dronabinol use were evident even though THC plasma concentrations were relatively low (varying between 2 and 10 ng/mL)Reference 230Reference 1575.
Chronic
There is also emerging evidence coming from studies with frequent, chronic non-medical cannabis users, having a high body load of THC, that report blood THC levels above 5 ng/mL (considered to be an "impairing" dose) for periods lasting several days after last THC exposureReference 229. These emerging findings raise the possibility of a persistent level of impairment that may last as long as three to seven days after last use in chronic, frequent (heavy) users of cannabis that may affect psychomotor skills needed for safe driving.
A clinical laboratory study that assessed the psychomotor function in chronic, daily cannabis smokers during three weeks of continuously monitored abstinence on a secure research unit found that performance on critical tracking and divided attention tasks was impaired even after three weeks' abstinenceReference 1567. In this study, 19 male, chronic, daily cannabis smokers who self-reported consuming 11 cannabis joints per day for the last 10 years (at least five days/week for six months prior to admission) were compared to a control group of occasional cannabis and/or MDMA users with regards to performance on two psychomotor tests: the critical tracking task which measures a subject's perceptual motor control and which has been demonstrated to be sensitive to the impairing effects of THC, and the other test being the divided attention task which assesses the ability of the individual to divide attention between two tasks performed simultaneously and which has also been demonstrated to be sensitive to the impairing effects of THC. Mean plasma THC and 11-hydroxy-THC levels on admission were 5.3 ng/mL and 2.1 ng/mL respectively while on day 8 after admission were 1.3 and 0.2 ng/mL respectively. Values for THC fell below 1 ng/mL on days 14 to 16. The findings showed that psychomotor performance (the critical tracking task and divided attention task) of chronic, daily cannabis smokers improved over three weeks of abstinence but remained significantly poorer than performance of the control group of occasional cannabis and MDMA users. The authors hypothesize that the observed persistent psychomotor impairments could have arisen from withdrawal effects, from residual THC concentrations in the blood as a result of heavy body burden of THC and release from peripheral stores, or lastly from the effects of cumulative lifetime intake reflecting persistent changes in psychomotor function in chronic cannabis smokers.
A study that characterized cannabinoid elimination in blood from 30 male daily chronic cannabis smokers during monitored sustained abstinence for up to 33 days on a closed residential unit found that both THC and its inactive metabolite 11-nor-9-carboxy-THC were detected in blood up to one month after last smoking, which was reported by the authors as being four times longer than previously describedReference 459. The study also reported that males had a shorter maximum detection window of 11-hydroxy-THC (72 h) compared with females (seven days). The vast majority of the participants were THC-positive on admission with a median concentration of 1.4 ng/mL of THC in the blood and the levels of THC decreased gradually over time.
A study examined the plasma cannabinoid detection windows for chronic frequent cannabis smokers and also attempted to determine if plasma concentrations of cannabinoids were correlated with psychomotor performance in critical tracking and divided attention tasksReference 229. Twenty-eight male participants who reported smoking an average of 10.6 joints per day (range: 1 - 30) for an average of 10.6 years (range: 4 - 28) and who abstained from cannabis smoking for a period of up to 30 days, had a baseline median range of blood THC on admission of 4.2 ng/mL. Blood THC concentrations significantly decreased 24 h after admission. Three days after admission, a significant number of participants had blood THC levels ≥ 5 ng/mL, while seven days after admission almost 30% of participants had blood THC levels greater than 2 ng/mL. One participant had a plasma THC concentration of ≥ 2 ng/mL for 18 consecutive days. THC was detected in some specimens as late as 30 days after admission (0.3 - 1.3 ng/mL). Years of prior cannabis use significantly correlated with THC concentration on admission. Tracking error was correlated with THC, 11-hydroxy-THC, and 11-nor-9-carboxy-THC at baseline and with 11-hydroxy-THC on day 8. No other outcome measures such as divided attention task or critical tracking task were significantly correlated with cannabinoid concentrations. Based on the findings of this study, the authors argue against the utility of detectable THC and 11-hydroxy-THC in the plasma of chronic frequent cannabis smokers as a reliable marker for recent cannabis use. Blood levels of 11-hydroxy-THC never appeared to exceed 2 ng/mL beyond 24 h, which the authors suggest could be a cut-off for recent cannabis use (within 24 h). The authors suggest that residual THC in plasma weeks after last smoking may be associated with impairment in frequent chronic cannabis smokers. Furthermore, they suggest that although partial tolerance may develop to some impairing effects of cannabis smoking among chronic frequent cannabis smokers, some residual impairment may limit the appropriate operation of a motor vehicle or mechanical equipment which could result in injury or in criminal litigation.
Epidemiological studies
A case-control study estimating accident risk for a variety of substances including alcohol, medicines, and illegal drugs found that the OR for accident risk for all the THC concentrations measured (1 to > 5 ng/mL) was statistically significantReference 1576. At whole-blood concentrations of ≥ 2 ng/mL THC, the risk of having an accident was significantly increased. One study found that the risk of responsibility for fatal traffic crashes while driving under the influence of cannabis (DUIC) increased with increasing blood concentrations of THC such that there was a significant dose-effect relationship between risk of responsibility for fatal traffic crashes and blood concentrations of THC. The study showed that the OR of having a fatal crash increased from 2.18, if blood concentrations ranged between 0 and 1 ng/mL of THC, to 4.72 if blood THC concentrations were ≥ 5 ng/mLReference 1577. The findings from this study further support the notion of a causal relationship between cannabis use and crashesReference 1577.
Another study suggested that drivers who were judged (by a police physician) as being impaired had higher blood THC concentrations than drivers judged not to be impaired (median: 2.5 ng/mL vs. 1.9 ng/mL)Reference 1578. Using a binary logistic regression model, the OR for being judged impaired appeared to increase with increasing drug concentrations from 2.9 ng/mL onwards. Serum THC concentrations between 2 and 5 ng/mL have been identified as a threshold above which THC-induced impairment of skills related to driving become apparentReference 154Reference 1576.
A meta-analysis of observational studies examining acute cannabis consumption and motor vehicle collision risk reported that DUIC was associated with a significantly increased risk of motor vehicle collisions compared with unimpaired driving, with an OR of 1.92 (95% CI = 1.35 - 2.73)Reference 230. Collision risk estimates were higher in case-control studies and studies of fatal collisions, than in culpability studies and studies of non-fatal collisions. It has been reported that individuals who drive within one hour of using cannabis are nearly twice as likely to be involved in motor vehicle accidents as those who do not consume cannabisReference 1571. For this meta-analysis, only observational studies with a control or comparison group, including cohort (historical prospective), case-control, and culpability designs were included, and experimental laboratory or simulator studies were excludedReference 230. Furthermore, only studies that assessed acute or recent cannabis use were examined. This meta-analysis supports the findings of other studies which suggest that cannabis use impairs the performance of the cognitive and motor tasks that are required for safe driving, thereby increasing the risk of collisionReference 230. Although driving simulator studies have reported a dose-response effect, in which elevated concentrations of THC were associated with increased crash risk, dose-response effects could not be established in this studyReference 230.
A systematic review and meta-analysis concluded that, after adjusting for study quality, cannabis use was associated with a seven-fold estimated risk of being involved in a fatal accident, benzodiazepine use was associated with a two-fold estimated risk of a fatal accident, and opiate use with a three-fold estimated risk of a fatal accidentReference 232. In contrast, cannabis use was associated with a 1.5-fold estimated risk of having an accident that only caused injury; benzodiazepine use was associated with a 0.71-fold estimated risk, whereas opiates were associated with a 21-fold estimated risk of having an accident that only caused injury.
Use for medical purposes and driving
A pilot, prospective, multicentre, non-interventional post-marketing surveillance study conducted to collect data on driving ability, tolerability and safety from 33 patients with MS starting nabiximols treatment reported that a four to six-week treatment period with nabiximols (average 5.1 sprays per day, or 13.7 mg THC and 12.8 mg CBD/day) was associated with a statistically significant improvement in self-rated spasticity and was also not associated with a statistically significant deterioration in patients' ability to drive, as measured in the laboratory using a battery of cognitive and psychomotor testsReference 692. However, less than half of the patients met the "fit to drive" criteria. In addition, 4 out of the 33 patients experienced a non-serious, mild or moderate adverse event associated with nabiximols treatment (e.g. dizziness and vertigo).
Cannabis and alcohol
Clinical studies
A within-subject, blinded, placebo-controlled driving simulator study examined the subjective feelings and driving abilities in 14 healthy students after smoking two different cannabis cigarettes of varying potencies (13 and 17 mg THC) or after alcohol intake (0.5 g/kg of body weight to a BAC of 0.05%)Reference 1579. All participants were low to moderate users of cannabis and alcohol, with a reported cannabis use of one to four times per month. While both alcohol and the lower dose of THC (13 mg) significantly increased reaction time compared to placebo, the magnitude of the effect was greater with the higher THC dose (17 mg). No residual effect on reaction time was found 24 h after smoking the highest THC dose. Compared to placebo, both the low (13 mg) and high (17 mg) THC doses significantly slowed average driving speed in a dose-dependent manner, while alcohol increased it. Both the low and high THC doses significantly increased lane position variability compared to placebo, while only the low THC dose significantly increased steering wheel deviations. Average speed, lane position variability and steering wheel deviation returned to baseline levels 24 h post-smoking. There also appeared to be a dose-dependent increase in the number of collisions with an apparent two-fold increase in the number of individuals having a collision with only a modest increase in the amount of THC administered (i.e. 4 mg or a 23% increase in THC). Subjective effects were also examined and the study found a significant increase in physical discomfort, physical effort, and lack of energy with the highest dose of THC compared to placebo, although the lowest dose also produced physical discomfort and effort. Although both the lowest THC dose (13 mg) and alcohol (0.05% BAC) appeared to produce driving impairment, there appeared to be differences in subjective effects between THC and alcohol.
A double-blind, counter-balanced, placebo-controlled driving simulator study reported that driving performance was more impaired in subjects who co-consumed alcohol and low or high doses of THC by smoking cannabis cigarettesReference 231. The level of THC detected in the blood was higher when cannabis was consumed along with alcohol than when consumed alone. It also appeared that regular cannabis users displayed more driving errors than non-regular cannabis users.
A double-blind, randomized, placebo-controlled, within-subject experimental driving simulator study with 18 subjects that self-reported using cannabis occasionally (≥ once in the last three months, ≥ three days/week) determined how blood THC concentrations were related to driving impairment with and without alcoholReference 228. The study found that vapourized cannabis (0.5 g dried vapourized cannabis with a THC concentration of either 2.9% THC, or 6.7% THC or 14.5 mg THC or 33.5 mg THC), when combined with alcohol (0.065% peak breath alcohol concentration), increased standard deviation of lateral position (SDLP) similar to 0.05 and 0.08% BAC. Furthermore, the effects of alcohol and cannabis on SDLP were additive rather than synergistic with 5 ng/mL THC and 0.05% BAC showing similar SDLP as 0.08% BAC alone.
A randomized, placebo-controlled, blinded clinical study that evaluated acute cannabinoid disposition in blood and plasma after controlled vapourized cannabis administration with and without low-dose oral alcohol administration found that low-dose oral alcohol administration significantly increased median maximum (Cmax) blood THC and 11-hydroxy-THC concentrationsReference 206. Nineteen healthy participants that self-reported consuming cannabis ≥ one time/three months but ≤ three days/week over the past three months (i.e. occasional use) completed all arms of the study. Vapourization of 0.5 g of dried cannabis flowers containing a low dose of THC (2.9% THC, 0.22% CBD) without any oral alcohol administration was associated with a median maximum blood (Cmax) THC level of 32.7 ng/mL, whereas vapourization of cannabis containing a high dose of THC (6.7% THC, 0.37% CBD) was associated with a median THC Cmax of 42.2 ng/mL. Under the same conditions, the median Cmax of 11-hydroxy-THC with the low THC dose was 2.8 ng/mL, whereas with the high THC dose the median Cmax of 11-hydroxy-THC was 5.0 ng/mL. Time to maximum THC and 11-hydroxy-THC blood levels was 10 min. Co-administration of an oral alcohol dose producing a breath alcohol concentration of 0.065% along with vapourization of the low THC dose was associated with median Cmax of THC of 35.3 ng/mL, whereas with the high THC dose the median THC Cmax was 67.5 ng/mL. With co-administration of alcohol, the median Cmax of 11-hydroxy-THC under the low THC dose was 3.7 ng/mL, whereas under the high THC dose the median Cmax of 11-hydroxy-THC was 6.0 ng/mL. These results suggest that co-consumption of alcohol with THC can result in significantly elevated concentrations of blood THC and 11-hydroxy-THC compared to THC alone that may contribute to increasing cognitive impairment which can compromise safe driving abilities. The authors of the study also suggest that vapourization of cannabis under the study conditions delivered THC in a similar manner to smoking and producing similar cannabinoid concentration profiles. Factors that affected vapourized THC delivery included heating temperature, number of balloon fillings, cannabis amount and blend, and length of time between volatilization and inhalation (i.e. possible adherence of THC to the balloon surface). Participants appeared to require less self-titration at the lower THC dose and more self-titration at the higher THC dose, which was reflected in greater blood THC variability under the high THC dose condition.
Epidemiological studies
A follow-up study investigated the effects of alcohol (0.05% BAC), THC (13 mg), and their combination on driving and non-driving tasks as well as the extent to which people are willing to drive based on their subjective sensations and their perceived effects of the drugsReference 220. Combining alcohol and THC resulted in a greater number of participants having collisions in a driving simulator task compared to alcohol or THC alone or placebo. Lane position variability increased significantly under the combined effects of alcohol and THC relative to the other treatments, which did not differ from each other. The combination of alcohol and THC caused a significantly greater sensation of "sedation" in comparison to all other treatments. Furthermore, the combination of THC and alcohol had significant and intense effects on particular dimensions of the Swedish Occupational Fatigue Inventory such as "lack of energy", "physical exertion", and "lack of motivation". Based on the study findings, the authors suggest that the subjects felt that the combination of alcohol and THC was the most potent treatment and had an additive effect on some of the subjective sensations compared to the effects of the two drugs in isolation. No residual effects of any treatment were observed 24 h after treatment.
A case-control study that examined driver crash data compiled by the U.S. National Center for Statistics and Analysis of the National Highway Traffic Safety Administration over a period of 17 years (1991 - 2008) found that the prevalence of THC and alcohol in car drivers aged 20 years and older involved in a fatal crash has increased approximately five-fold, from below 2% in 1991 to above 10% in 2008Reference 1372. Furthermore, the authors of the study reported that each 0.01 BAC unit increased the odds of an unsafe driving action, a proxy measure of crash responsibility, by approximately 9 to 11%. After adjusting for driver age, sex, alcohol, polydrug use, and previous driving record, drivers who were positive for THC alone had a 16% increased odds of an unsafe driving action. When alcohol and THC were combined, the odds of an unsafe driving action increased by approximately 8 to 10% for each 0.01 BAC unit increase over alcohol or THC alone. Drivers at typical BAC legal limits of 0.05 and 0.08 had greater odds of committing an unsafe driving action of 66% and 117% respectively when compared with sober, THC-free drivers. However, the authors suggest that when combined with THC these odds increased to 81% and 128% respectively. Furthermore, the THC and alcohol combination effect was most pronounced at the lowest levels of BAC. In other words, as BAC level increases, the impairing effects of alcohol dominate the THC-alcohol relationship. The authors concluded that drivers positive for both alcohol and THC had greater odds of making an error than drivers positive for either alcohol or cannabis alone.
Lastly, data from an annual repeated cross-sectional survey of Ontario adults that surveyed over 16 000 adults and recorded the incidence of self-reported collisions among drivers who reported driving under the influence of alcohol (DUIA) and DUIC found that drivers who reported neither a DUIA or a DUIC had the lowest prevalence of collisions (6.7%)Reference 1580. However, those reporting either a DUIA or a DUIC reported a significantly higher prevalence of collision involvement of 9.6%. The highest likelihood of collision involvement was found among drivers reporting both behaviours (30.5%). In other words, those who reported both a DUIC and a DUIA were more than three times as likely to be involved in a collision compared to those who reported one or the other behaviour (OR = 3.65, CI = 2.12 - 6.28).
7.7.3 Psychiatric effects
7.7.3.1 Anxiety, PTSD, depression and bipolar disorder
- Evidence from clinical studies suggests a dose-dependent, bi-phasic, effect of THC on anxiety and mood, where low doses of THC appear to have an anti-anxiety and mood-elevating effect whereas high doses of THC can produce anxiety and lower mood.
- Epidemiological studies suggest an association between (THC-predominant) cannabis use, especially chronic, heavy use and the onset of anxiety, depressive and bipolar disorders, and the persistence of symptoms related to PTSD, panic disorder, depressive disorder, and bipolar disorder.
- Preliminary evidence from surveys suggests an association between use of ultra-high-potency cannabis concentrate products (e.g. butane hash oil, BHO) and higher rates of self-reported anxiety and depression and other illicit drug as well as higher levels of physical dependence than with high-potency herbal cannabis.
Anxiety and depression
Epidemiological studies suggest a possible association between regular cannabis use and the development of anxiety and depression, however the available evidence of a link between cannabis use and anxiety/anxiety disorders and depression is more mixed and less consistent than that seen between cannabis use and psychosisReference 162. That being said, depression and anxiety disorders appear to be not only associated with cannabis dependence but are also predictive of whether individuals transition from use to dependenceReference 162. It appears that THC can exert bi-directional effects on anxiety and mood (i.e. anti-anxiety and mood-elevating effects at low doses, and anxiogenic and mood-lowering effects at higher doses), and ECS dysfunction, such as that caused by chronic, high-level activation of CB1 receptor signaling (or conversely CB1 receptor antagonism), especially during adolescence, can increase or exacerbate the risk for anxiety/anxiety disorders and depressionReference 1581Reference 1582. More recently, a few cross-sectional surveys have explored the effects of ultra-high potency cannabis concentrate products such as butane hash oil (BHO) on various psychiatric outcomes. In one study of 83 867 cannabis users, of which 5 922 reported using BHO, participants who reported a lifetime diagnosis of depression (OR = 1.15, p = 0.003), anxiety (OR = 1.72, p < 0.001) and other substance use (OR = 1.29, p < 0.001) were more likely to use BHO than only high potency herbal cannabisReference 1583. In addition, BHO users reported stronger negative effects and less positive effects with BHO compared to high potency herbal cannabis. In another study, more frequent BHO use was associated with higher levels of physical dependence (RR = 1.8, p < 0.001, adjusted RR = 1.2, p = 0.014), which remained significant even after adjustment for confoundersReference 520. While there was an association between BHO use and impaired control (RR = 1.3, p < 0.001), cannabis-related academic/occupational problems (RR = 1.5, p = 0.004), poor self-care (RR = 1.3, p = 0.002) and cannabis-related risk behaviour (RR = 1.2, p = 0.001), these associations did not persist after controlling for confounding factors.
Anxiety
Anecdotal claims of cannabis use to relieve anxiety have been postulated to actually result from a so-called "stress-misattribution hypothesis" which posits that cannabis users may potentially be misattributing symptoms of stress or tension to anxietyReference 1582Reference 1584. Under this hypothesis, affected individuals believe they are using cannabis to relieve symptoms of anxiety, reporting using cannabis to self-medicate, while in actuality experiencing not anxiety, but stress (i.e. tension, irritability, persistent symptoms of arousal) as well as, or instead of, symptoms of anxietyReference 1582Reference 1584.
The available evidence suggests a role for the ECS in modulating anxiety responses, under both basal non-aversive environmental conditions, but also under aversive or stressful environmental conditionsReference 177. Pharmacological enhancement of endocannabinoid signalling under aversive/stressful conditions in various animal models of anxiety either through inhibition of endocannabinoid degradation or through blockage of endocannabinoid re-uptake has been generally associated with anxiolysis mainly through a CB1 receptor-dependent mechanismReference 177.
Cannabis use, especially cannabis containing mainly THC, dose-dependently affects anxiety behaviours, with low doses generally being anxiolytic and high doses either ineffective or potentially anxiogenicReference 177. Indeed, consumption of THC-predominant cannabis has been shown to cause an acute and short-lasting episode of anxiety in approximately 20 - 30% of usersReference 1584, often resembling a panic attack; this is more often encountered in naïve cannabis users and those who consume higher doses of cannabis or THC (e.g. > 5 mg oral Δ9-THC), and also when cannabis is consumed in novel or stressful environmentsReference 189Reference 191. While clinical trials of cannabis, or oral Δ9-THC, to treat anxiety or depression show either a lack of improvement or worsening of these conditionsReference 1585-Reference 1588, there is some evidence that cannabis or cannabinoids may be useful in treating anxiety or depression secondary to other disorders (e.g. chronic pain, PTSD). In addition, while there is much pre-clinical evidence to suggest a role for CBD as an anxiolytic, there is less but emerging clinical evidence to suggest a potential role for CBD in alleviating social anxietyReference 171Reference 1589 and additional research is required. For more information on potential therapeutic uses of cannabis or cannabinoids, such as CBD, in the treatment of anxiety and depression, please consult Section 4.9.5.1.
Recent studies suggest the use of cannabis among individuals with anxiety disorders is associated with worsening of mental health-related functions. One study reported that mental health-related QoL was significantly lower among individuals with anxiety disorders who were also using cannabisReference 1590. Data for this study was gathered from the NESARC where face-to-face interviews were conducted with over 43 000 U.S. adults ages 18 and older from the civilian non-institutionalized population. Anxiety disorders included in this study referred to panic disorder, social anxiety disorder, specific phobia, and generalized anxiety disorder (GAD) in the last 12 months. "Regular cannabis use" was defined as use that was at least weekly and "occasional use" was defined as use that was less than weekly. Compared to non-users, both female and male regular cannabis users reported significantly more often that their emotional or physical problems interfered with social activities, they accomplished less because of emotional problems, and they performed work or other activities less carefully because of emotional problems. They also reported feeling peaceful and calm less often and tended to feel depressed more often. In contrast, few differences were found between occasional cannabis users and non-users in mental health-related QoL, although female occasional cannabis users reported feeling peaceful less of the time. Among males with anxiety disorders, occasional cannabis users reported feeling peaceful and calm more frequently than regular cannabis users, feeling depressed less often than regular cannabis users and feeling less interference with social activities compared to regular users. In contrast, regular cannabis use was associated with significantly poorer mental health-related QoL for both males and females compared to non-users. Regular cannabis use was also associated with significantly lower mean mental health scores among both males and females, and lower mean scores on subscales of social functioning among females and mental health, and role emotional subscales among males compared to occasional cannabis use. Linear regression analyses examining associations between levels of cannabis use and mental QoL showed significantly poorer mental QoL among regular, but not occasional cannabis users and was applicable to both females and males. The authors conclude that regular, but not occasional, cannabis use among individuals with anxiety disorders is associated with poorer mental health-related QoL and argue against the self-medication hypothesis.
A fifteen-year representative longitudinal cohort study among 1 943 individuals examining the association between adolescent cannabis use and common mental disorders into young adulthood reported no consistent associations between frequency of adolescent cannabis use and depression (i.e. major depressive episode) at age 29 yearsReference 1591. However, daily cannabis use was associated with a more than two-fold increased risk of anxiety disorder at age 29 (AOR = 2.5; 95% CI = 1.2 - 5.2), as was cannabis dependence (AOR = 2.2; 95% CI = 1.1 - 4.4). Among weekly and more than weekly (i.e. daily) adolescent cannabis users that continued daily cannabis use at 29 years, there was still a significant increased odds of anxiety disorder (AOR = 3.2; 95% CI = 1.1 - 9.2). Early, regular cannabis use in adolescence increased the risk of anxiety disorder at age 29, with slightly higher risks if regular use also occurred at 29 years.
A systematic literature review and meta-analysis, using data from 31 studies on samples drawn from 112 000 cases from the general population of 10 countries, quantitatively assessed the relationship between anxiety (i.e. anxiety diagnoses with or without comorbid depression according to DSM/ICD diagnostic criteria) and cannabis useReference 1592. The study reported a small positive association between anxiety and either cannabis use (OR = 1.24; 95% CI = 1.06 - 1.45; p = 0.006; N = 15 studies) or CUD (OR = 1.68; 95% CI = 1.23 - 2.31; p = 0.001; N = 13 studies), and between comorbid anxiety and depression and cannabis use (OR = 1.68; 95% CI = 1.17 - 2.40; p = 0.004; N = 5 studies). The positive association between anxiety and cannabis use (or CUD) was present in subgroups of studies with AORs for possible confounders and in studies with clinical diagnoses of anxiety. Cannabis use at baseline was also significantly associated with anxiety at follow-up in five studies (OR = 1.28; 95% CI = 1.06 - 1.54; p = 0.01). Individuals with various anxiety disorders and concurrent anxiety and depression were more likely to use cannabis or to have a CUD (i.e. dependence and/or abuse/harmful use) compared to those without anxiety disorders. The authors suggest that cannabis use could further exacerbate existing symptoms of anxiety depending on the genetic vulnerability, severity of anxiety symptoms, gender and age, among other factors. The findings are based on samples from the general population neither in treatment for anxiety nor for CUD.
A U.K. prospective, population-based cohort study (ALSPAC) of 4 561 individuals that investigated the associations between cannabis or cigarette use at age 16, and depression or anxiety at age 18, found weak evidence for an association between cannabis use and anxiety (unadjusted OR = 1.13, 95% CI = 0.98 - 1.31) that disappeared after fully adjusting for confounding factors (AOR = 0.96, 95% CI = 0.75 - 1.24)Reference 1593. Study limitations include (relatively) small sample size to detect a small effect, self-reported cannabis use, and assessment of outcomes by computerized interview.
A recent epidemiological study comparing data from two waves of the NESARC (2001 - 2002 and 2004 - 2005) and examining the relationship between cannabis use and risk of psychiatric disorders among 35 000 respondents, reported no association between past-year cannabis use and any anxiety disorder (OR = 0.9; 95% CI = 0.7 - 1.1)Reference 512. Limitations of the study include limited follow-up period (i.e. only three years), self-reported cannabis use, and limited categories of cannabis use frequency (i.e. no past-year cannabis use, some past-year cannabis use but less than one use episode per month, and greater than or equal to one use episode per month).
An epidemiological study providing the first nationally representative information on the prevalence and correlates of DSM-5 CUD using data from the 2012 - 2013 wave of the NESARC-III reported that past-year CUD was associated with any anxiety disorder (AOR = 2.8), and lifetime CUD was also associated with anxiety disorders (AOR = 2.9)Reference 338. Furthermore, the association between any anxiety disorder and past-year CUD increased with increasing severity of CUD (AOR = 2.2, 2.9, 4.4 for mild, moderate, and severe CUD respectively). The association between panic disorder/GAD and past-year CUD was particularly strong, with an AOR of 2.5, 2.8 and 6.6 (mild, moderate, severe CUD respectively) for panic disorder, and 3.0, 3.6, and 6.3 (mild, moderate, severe CUD respectively) for GAD.
Depression
Regarding depression, findings from pre-clinical studies suggest that reductions in ECS signaling are associated with depressive-like symptomsReference 177. Pharmacological manipulation of the ECS resulting in elevation of anandamide for example, has been associated with anti-depressant-like behaviour in animal models of chronic stressReference 177.
One review reported that the co-morbidity level between heavy or problematic cannabis use and depression, in surveys of the general population, exceeds what would be expected by chanceReference 1594. The authors also identified a modest association between early-onset regular or problematic use and later depression. However, limitations in the available research on cannabis and depression, including limitations in study design, as well as limitations in the ability to measure cannabis use, and limitations in the ability to measure depression were also highlighted.
A U.S. study of adults using longitudinal national survey data (n = 8 759) found that the odds of developing depression in past-year cannabis users was 1.4 times higher than the odds of non-users developing depressionReference 1595. However, after adjusting for group differences, the association was no longer significant. In a follow-up study, the same group looked at the relationship between cannabis use and depression among youth using a longitudinal cohort of 1 494 adolescents. Similar to the adult study, the results did not support the causal relationship between adolescent-onset cannabis use problems and early adult depressionReference 1596.
In contrast, another U.S. study based on the results of the 2001 - 2002 NESARC (n = 43 093) found that major depression was significantly associated with lifetime cannabis disorders and dependenceReference 1597. A subsequent analysis of the same data examining the association between cannabis use and health-related QoL among individuals with depressive disorders found that women with depressive disorders who used cannabis regularly reported poorer mental QoLReference 170. While the finding remained significant after adjusting for socio-demographic variables, it was not sustained after adjusting for comorbid anxiety disorders. Occasional cannabis use among women was not associated with lower QoL when compared with non-users. Little difference was noted among men with depressive disorders when comparing users to non-users.
A 2007 study using data from the NEtherlands MEntal Health Survey and Incidence Study (NEMESIS) found a modest increased risk of a first depressive episode (OR = 1.62; 1.06 - 2.48) associated with cannabis use, after controlling for strong confounding factorsReference 1598. Of greater significance in this study was the strong increased risk of bipolar disorder (OR = 4.98; 1.80 - 13.81) with cannabis use (see below for further information on cannabis and bipolar disorder). There was a dose-response relationship associated with the risk of 'any mood disorder' for almost daily and weekly users, but not for less frequent users.
A systematic review and meta-analysis of population-based longitudinal studies or case-control studies, nested within longitudinal designs, examined the association between cannabis use and the risk of psychotic or affective mental health outcomes (e.g. depression, suicidal thoughts, and anxiety)Reference 196. The overall AOR for depression outcomes associated with most frequent use of cannabis compared with non-users was 1.49 (95% CI = 1.15 - 1.94). With regards to suicidal ideation, the study reported significant heterogeneity in the data and was not able to conduct a meta-analysis and provide an overall AOR.
An integrative analysis of four Australasian cohorts (i.e. the Victorian Adolescent Health Cohort Study, the Personality and Total Health study, the Australian Temperament Project, and the Christchurch Health and Development Study) that studied the relationships between the use of cannabis and the development of symptoms of depression from mid-adolescence to adulthood among more than 6 900 participants reported a small to moderate association between weekly cannabis use and symptoms of depression compared to non-use of cannabis (0.3 - 0.5 SD)Reference 1599. After adjustment for confounding factors, the association between weekly cannabis use and symptoms of depression persisted, though it was slightly reduced (0.24 SD; 95% CI = 0.18 - 0.30). The strength of the association between cannabis use and depression also varied with age-the associations were strongest in mid-adolescence and reduced to generally weak and negligible effects in mature adulthood.
A longitudinal cohort study of 45 087 Swedish male conscripts examining the association between cannabis use and mental disorders reported no association between frequency of cannabis use and risk of depression even in subjects with the highest level of cannabis use (after adjustment for potential confounders)Reference 1600. However, the study did report a strong graded association between cannabis use and schizoaffective disorder, with heavy use conferring the greatest risk (OR = 7.5; 95% CI = 3.4 - 16.7) compared to those who had never used cannabis.
A fifteen-year representative longitudinal cohort study among 1 943 individuals examining the association between adolescent cannabis use and common mental disorders into young adulthood reported no consistent associations between frequency of adolescent cannabis use and depression (i.e. major depressive episode) at age 29 yearsReference 1591. However, daily cannabis use was associated with a more than two-fold increased risk of anxiety disorder at age 29 (AOR = 2.5; 95% CI = 1.2 - 5.2) as was cannabis dependence (AOR = 2.2; 95% CI = 1.1 - 4.4). Among weekly and more than weekly (i.e. daily) adolescent cannabis users that continued daily cannabis use at 29 years, there was still a significant increased odds of anxiety disorder (AOR = 3.2; 95% CI = 1.1 - 9.2). Early, regular cannabis use in adolescence increased the risk of anxiety disorder at age 29, with slightly higher risks if regular use also occurred at 29 years.
A systematic review and meta-analysis of 14 longitudinal studies examining the association between cannabis use and depression among a population of 76 058 subjects reported that the pooled OR for depression among individuals using cannabis compared with controls was 1.17 (95% CI = 1.05 - 1.30)Reference 1016. "Cannabis use" was defined as any cannabis use, monthly, or lifetime use on five occasions. Heavy cannabis use (defined as use meeting the DSM-IV criteria for CUD or alternatively at least weekly cannabis use), was associated with increased incidence of depression with a pooled OR = 1.62 (95% CI = 1.21 - 2.16). Depression was defined as including major depressive disorder, dysthymia, or depressive symptoms using validated clinical tools. The authors of the study concluded that cannabis use was associated with a modest increased risk of developing depressive disorders and that heavy cannabis use was associated with a stronger, but still moderate, increased risk for developing depression. Meta-regressions to detect any effect of age on the association between cannabis use and depression failed to show any effect, although the tests were underpowered because of the small number of studies included in the meta-analysis. The results of this systematic review and meta-analysis suggest the existence of a modest dose-dependent relationship between cannabis use and depressive symptoms. Limitations of the study included methodological and other limitations inherent in the primary studies included in the analysis.
A longitudinal study examined the influence of sub-clinical depressive symptoms on long-term functional and clinical outcomes in 64 first-episode psychosis patients who were cannabis users, and on the ability of patients to stop using cannabisReference 1601. The study reported that the presence of sub-clinical depressive symptoms in first-episode psychosis patients during five years of follow-up was associated with continued cannabis abuse (β = 4.45, 95% CI = 1.78 - 11.17, p = 0.001) and with worse functioning (β = -5.50, 95% CI = -9.02 - -0.33, p = 0.009). The authors suggest that sub-clinical depressive symptoms should be treated in first-episode psychosis patients to prevent the development of an unfavorable clinical and functional course, especially in cannabis users.
A U.K. prospective, population-based cohort study (ALSPAC) of 4 561 individuals that investigated the associations between cannabis or cigarette use at age 16 and depression or anxiety at age 18 found that both cannabis (unadjusted OR = 1.5, 95% CI = 1.26 - 1.80) and cigarette use (unadjusted OR = 1.37, 95% CI = 1.16 - 1.61) increased the odds of developing depression; adjustment for confounding factors attenuated these relationships though evidence of association persisted for cannabis use (AOR = 1.30, 95% CI = 0.98 - 1.72), implying a slightly greater than two-fold increase in risk of depression with the highest level of self-reported cannabis use (> 60 times) compared to never usersReference 1593. Study limitations include (relatively) small sample size to detect a small effect, self-reported cannabis use, and assessment of outcomes by computerized interview.
A recent epidemiological study comparing data from two waves of the NESARC (2001 - 2002 and 2004 - 2005) and examining the relationship between cannabis use and risk of psychiatric disorders reported no association between cannabis use and any mood disorder (OR = 1.1; 95% CI = 0.8 - 1.4)Reference 512. Limitations of the study include limited follow-up period (i.e. only three years), self-reported cannabis use, and limited categories of cannabis use frequency (i.e. no past-year cannabis use, some past-year cannabis use but less than one use episode per month, and greater than or equal to one use episode per month).
An epidemiological study providing the first nationally representative information on the prevalence and correlates of DSM-5 CUD using data from the 2012 - 2013 wave of the NESARC-III reported that past-year CUD was associated with major depressive disorder (AOR = 2.8) and lifetime CUD was also associated with major depressive disorder (AOR = 2.6)Reference 338. Furthermore, the association between major depressive disorder and past-year CUD increased with increasing severity of CUD (AOR = 2.2, 3.1, and 4.2 for mild, moderate, and severe CUD respectively).
Bipolar disorder
Bipolar I and II disorders have been reported to occur in approximately 1 - 3% and 3 - 5% of the population, respectivelyReference 1602. Bipolar disorders are also often complicated by co-occurring substance use disorders, which are associated with increased co-morbiditiesReference 1602. More specifically, cannabis is one of the most frequently abused illicit drugs in people diagnosed with bipolar disorderReference 193Reference 1603-Reference 1606. Lifetime cannabis use among bipolar patients appears to be around 70%, and approximately 30% of patients with bipolar disorder have a comorbidity of cannabis abuse or dependence - rates that exceed those observed in the total populationReference 1607. Cannabis use in bipolar disorder is also associated with poorer outcomes, increased symptom severity and poorer treatment complianceReference 1608. The current available evidence suggests there is a significant relationship between cannabis use and subsequent exacerbation and onset of mania symptoms and that cannabis may worsen the course of bipolar disorder by increasing the likelihood, severity or duration of manic phasesReference 1609. While cannabis use often precedes first manic episodes, cannabis use is hypothesized to be a potential cause, and a consequence of early bipolar disorderReference 1607.
Below is a summary of the studies that have examined the relationship between cannabis use and bipolar disorder, its effect on disease course, and its effect on treatment compliance.
One three-year, prospective study involving 4 815 subjects attempted to determine if baseline cannabis use increased the risk for development of manic symptoms, if the association between cannabis use and mania was independent of the emergence of psychotic symptoms, and if baseline mania predicted cannabis use at follow-upReference 1603. The authors found that cannabis use at baseline was associated with follow-up mania (OR = 5.32, 95% CI: 3.59, 7.89). After adjusting for confounding factors, the association persisted, although it was reduced (OR = 2.70, 95% CI: 1.54, 4.75). The risk of developing manic symptoms appeared to increase with increased baseline frequency of cannabis use. The effect size was largest for those who used cannabis three to four days per week, followed by those who used daily and one to two days per week, and lastly for those who used one to three days per month. The authors reported that manic symptoms at baseline did not predict cannabis use during follow-up. The authors concluded that cannabis use increased the risk of developing subsequent manic symptoms and that this effect was dose-dependent.
Another group of investigators conducted a five-year, prospective, cohort study examining three groups of patients: one where a CUD preceded the onset of bipolar disorder, another where bipolar disorder preceded a CUD, and one group with bipolar disorder onlyReference 1604. The authors found that cannabis use was associated with more time in affective (manic or mixed) episodes and with rapid cycling, but a causal relationship between cannabis use and bipolar disorder could not be established.
A separate prospective study which followed a group of type I bipolar patients over a 10-year period, beginning from the onset of illness, concluded that there was a strong association between cannabis use and manic/hypomanic episodes or symptoms, and that cannabis abuse preceded or coincided with, but did not follow, exacerbations of affective illnessReference 1610.
A two-year, prospective, observational study on the outcome of pharmacological treatment of mania (the European Mania in Bipolar Longitudinal Evaluation of Medication study, EMBLEM) followed 3 459 eligible in- and out-patients who were being treated for acute mania in bipolar disorder, assessing patients' current cannabis use as well as the influence of cannabis exposure on clinical and social treatment outcome measuresReference 193. The study concluded that during a one-year treatment period, patients using cannabis exhibited less treatment compliance and higher levels of overall illness severity, mania, and psychosis compared to non-users. Patients using cannabis also reported experiencing less satisfaction with life.
A preliminary study found that patients diagnosed with bipolar disorder with psychotic features were significantly more likely to carry a functional polymorphism in the promoter region of the 5-HT transporter gene and also have a diagnosis of cannabis abuse/dependence, compared to bipolar patients who did not exhibit psychotic symptomsReference 1606. Genetic studies have also raised the possibility of a link between allelic variants of the cannabinoid receptor gene (CNR1) and susceptibility to mood disordersReference 1611Reference 1612.
The influence of cannabis use on age at onset of both schizophrenia and bipolar disorder (with psychotic symptoms) has been studied using regression analysisReference 186. The authors of this study found that although cannabis and other substance use was more frequent in patients with schizophrenia than those diagnosed with bipolar disorder, cannabis use was nonetheless associated with a younger age at onset of both disorders. Cannabis use also preceded first hospitalization in the vast majority of cases (95.4%). Furthermore, the period of most intensive use ("several times per day") preceded first admission in 87.1% of the cases. In bipolar patients, cannabis use reduced age at onset by an average of nine years. In contrast, in schizophrenic patients, cannabis use reduced age at onset by an average of 1.5 years. No significant difference was noted in age at onset between male and female patients in either of the diagnostic groups.
Another study investigated which factors were associated with age at onset in bipolar disorder, and also examined the sequence of the onsets of excessive substance use and bipolar disorderReference 1613. A total of 151 patients with bipolar disorder (type I and II) receiving psychiatric treatment participated in the study. The authors found that when compared with alcohol use, excessive cannabis use (defined as either meeting DSM-IV criteria for substance use disorder, or weekly use of cannabis over a period of at least four years) was associated with an earlier age at onset in both primary and secondary bipolar disorder, even after adjusting for possible confoundersReference 1605. In addition, the mean age at onset of excessive cannabis use preceded the age at onset of bipolar disease; this was reversed in the alcohol group.
One study reported that when compared with controls, patients with bipolar disorder were almost seven times (95% CI: 5.41 - 8.52) more likely to report a lifetime history of cannabis useReference 1605. Furthermore, this association appeared to be gender-independent. Those patients who used cannabis after, or in tandem with, their onset of bipolar symptoms had a lower age at onset of the disorder (17.5 vs. 21.5 yrs). Furthermore, those who used cannabis prior to the onset of a bipolar disease episode were 1.75 times (95% CI: 1.05 - 2.91) more likely to report disability attributable to bipolar disorder.
On the other hand, a retrospective analysis of a large cohort of bipolar I subjects, with or without a history of a CUD, reported that bipolar patients with a CUD had similar age at onset as patients without such a substance use disorderReference 1614. However, patients with a CUD were more likely to have experienced psychosis at some time during the course of their illness compared to patients who never met the criteria for the disorder.
An epidemiological study using data from the 2001 - 2002 NESARC examined the relationship between bipolar disorder and CUD and reported that among approximately 2 000 individuals with a lifetime prevalence of bipolar disorder, the rates of CUD in the past 12 months were 7.2% (CI = 5.8 - 9.0) compared with 1.2% (CI = 1.1 - 1.3) in the general populationReference 1602. Furthermore, logistic regression analysis suggested that individuals with bipolar disorder and co-occurring CUD were at increased risk for nicotine dependence (AOR = 3.8), alcohol (AOR = 6.6) and drug (AOR = 11.9) use disorders as well as anti-social personality disorder (AOR = 2.8) compared to those without a CUD. Among individuals with bipolar disorder, the majority with CUD were male (62%) and young (18 - 29 years old) (70%). Furthermore, age-at-onset of bipolar disorder was earlier among individuals with co-occurring CUD, regardless of whether first episode was depressive or manic/hypomanic. No significant difference was found in lifetime rates of suicide attempts or suicidal ideation among individuals with and without a CUD. Among individuals with bipolar disorder and co-occurring CUD, 75% had a hypomanic, manic or depressive episode in the past 12 months. CUD was also associated with poorer physical QoL (i.e. arteriosclerosis). Limitations of the study included self-report methodology, lack of semi-structured interviews conducted by mental health professionals, and exclusion of the adolescent population that is most at-risk.
A cross-sectional observational study of 324 patients with bipolar disorder diagnosed through structured diagnostic interviews found evidence for a dose-response relationship between cannabis use and age at onset in bipolar disorder, which remained statistically significant after controlling for possible confounders (i.e. gender, family history, tobacco smoking, alcohol consumption, and other substance use disorders). However, the authors did not find an association between cannabis use and presenting polarity or presence of psychosisReference 1615. The findings show a decrease in age at onset of approximately three years in those who reported using cannabis > 10 times per month compared to never users or those who reported using < 10 times per month. Furthermore, those patients with a lifetime CUD (i.e. abuse or dependence) had the greatest decrease in age at onset of approximately five years compared to never users or those who used < 10 times per month. Patients with a depressive presenting polarity had a lower age at onset compared to those with (hypo) manic/mixed or mixed onsets, while age at onset decreased as level of cannabis use increased. The authors concluded that there is evidence for a dose-response relationship between cannabis use and earlier onset of bipolar disorder. They also suggest that there is a tendency for onset of bipolar disorder to be preceded by cannabis use suggesting that cannabis use may be a risk factor for precipitating bipolar disorder.
A three-year prospective follow-up survey exploring the association between cannabis use, major depressive disorder and bipolar disorder from the 2001 - 2002 NESARC found a crude association between weekly (i.e. 2.25 days of use per week and 1.88 joints per day) and daily/almost daily (i.e. 6.45 days of cannabis use per week and 3.45 joints per day) cannabis use and bipolar disorderReference 1616. However, this association no longer persisted after adjustment for confounding factors. The authors suggest that association between cannabis use and bipolar syndrome may be mediated by additional factors such as psychiatric and substance use disorders.
EMBLEM, a two-year prospective observational study in adults with manic/mixed episode of bipolar disorder, found that of 1 922 patients analyzed, previous cannabis users had the highest rates of remission (68.1%) and recovery (38.7%) and the lowest rates of recurrence (42.1%) and relapse (29.8%)Reference 1617. In contrast, current users had lower recovery and remission, higher recurrence, greater work impairment, and were more likely not to be living with a partner than never users. In addition, current cannabis users had a significantly higher rate of suicide attempts over the two-year follow-up compared with past and never users. These findings led the authors to conclude that bipolar patients who stop using cannabis during a manic/mixed episode have similar clinical and functional outcomes at two years compared to those who have never used cannabis, whereas patients who continue to use cannabis have a higher risk of recurrence and poorer functioning.
A study using experience sampling methodology (ESM), through diary entries, to track the temporal associations over a period of six days between cannabis, affect and bipolar disorder symptoms among 24 participants diagnosed with bipolar disorder type I or type II found that higher levels of positive affect increased the odds of using cannabis (OR = 1.25, CI = 1.06 - 1.47) and cannabis use was associated with subsequent increases in positive affect (but not negative affect), manic symptoms, and depressive symptomsReference 1608. On the other hand, neither negative affect, manic, nor depressive symptoms predicted the use of cannabis. The average number of joints used per day was 2.5, with the majority (54%) of respondents reporting using skunk-type (i.e. high potency) cannabis. The authors suggest that individuals with bipolar disorder are not using cannabis to self-medicate minor fluctuations in negative affect and bipolar symptoms. Limitations of the study include small sample size, self-report, lack of more granular information on cannabis potency, and limited evidence for validity of scales for mania and depression designed for the ESM study. The authors of the study emphasize that while some individuals perceive cannabis as a useful coping strategy in the management of bipolar disorder symptoms, the results of the study suggest cannabis is not being used to self-medicate changes in symptoms in the context of daily life and may actually be further complicating affective states.
A 24-month prospective, naturalistic, observational study using data gathered under the Bipolar Comprehensive Outcomes Study (BCOS) examined the impact of cannabis use in 239 patients with bipolar disorder type I and schizoaffective disorder-bipolar type and found that cannabis use was significantly associated with decreased likelihood of remission during the 24-month follow-up periodReference 1618. Subgroup analyses reported that cannabis use was significantly associated with lower remission rates on the Hamilton Depression Rating Scale in females and patients who were prescribed mood stabilizers. On the other hand, in males and patients prescribed olanzapine and/or a mood stabilizer, cannabis use was significantly associated with lower remission rates on the Young Mania Rating Scale. Remission rates appeared lowest in the group reporting concurrent cannabis and tobacco use, followed by the group reporting smoking only tobacco and the non-smoker group. Overall, the study authors suggest that cannabis use is associated with decreased likelihood of long-term remission in bipolar spectrum disorders with particular interaction effects of cannabis use and mood symptoms on gender and medication type.
An epidemiological study providing the first nationally representative information on the prevalence and correlates of DSM-5 CUD using data from the 2012 - 2013 wave of the NESARC-III reported that past-year CUD was associated with bipolar I disorder (AOR = 5.0) and lifetime CUD was also associated with bipolar I disorder (AOR = 3.8); past-year CUD was also associated with bipolar II disorder (AOR = 2.7) and lifetime CUD was also associated with bipolar II disorder (AOR = 2.8)Reference 338. Furthermore, only the association between bipolar I and past-year CUD increased with increasing severity of CUD (AOR = 3.4, 4.1, and 10.1 for mild, moderate, and severe CUD respectively).
A review article examining the state of the evidence regarding the use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts reported that cannabis use, in patients with bipolar disorder, is associated with increased risk of suicide attempts and with early age at onset of the disorder (reduced by between six and nine years)Reference 1619. Early age at onset is associated with greater number of rapid cycling episodes, mixed episodes, psychotic episodes, panic disorder, anxiety disorder, substance use disorder, major depression, worse response to lithium and suicidal behaviour. Limitations of the review article include the sparse literature on cannabis use, early age at onset of bipolar disorder and suicide attempts; the variable definition of early age at onset among the included studies; and methodological and other differences between the studies.
A recent systematic review of 12 cohort studies (2,588 individuals 'more exposed' to cannabis/9,371 'less exposed to cannabis') examined the longitudinal association between cannabis use and symptomatic outcomes among individuals living with a baseline anxiety or mood disorder. Relative to those less exposed to cannabis (including abstainers), the review provided consistent evidence that 'recent' cannabis use (within the last 6 months) was associated with negative symptomatic outcomes over time with respect to PTSD, panic disorder, bipolar disorder, and depressive disorder. Specifically, those using cannabis were more likely to report persistent symptoms over time and less likely to improve symptomatically from treatment (i.e., medication and/or psychotherapy). Some evidence further supported that reducing/stopping use was associated with more favourable outcomes. Overall, the study suggested that the available evidence does not support that cannabis can help long-term symptoms associated with anxiety and mood disorders, but rather, cannabis use may sustain symptoms longitudinally and prevent recovery efforts. However, the authors note that the findings should be interpreted with caution, considering the observational designs across studies (causality not established) and the biases associated with the samples (e.g., inpatients) and sources of cannabis consumed (i.e., unregulated sources with likely higher THC and minimal CBD concentrations)Reference 1620.
7.7.3.2 Schizophrenia and psychosis
- Evidence from clinical studies suggests that acute exposure to (THC-predominant) cannabis or THC is associated with dose-dependent, acute and transient behavioural and cognitive effects mimicking acute psychosis.
- Epidemiological studies suggest an association between (THC-predominant) cannabis use, especially early, chronic, and heavy use and psychosis and schizophrenia.
- The risk of schizophrenia associated with cannabis use is especially high in individuals who have a personal or family history of schizophrenia.
- Cannabis use is also associated with earlier onset of schizophrenia in vulnerable individuals and exacerbation of existing schizophrenic symptoms and worse clinical outcomes.
Acute psychotic reactions
THC-predominant cannabis and psychoactive cannabinoid (e.g. THC, nabilone, dronabinol, nabiximols) use have been linked to episodes of acute psychosis in both regular and drug-naïve usersReference 145Reference 182Reference 183Reference 200Reference 205Reference 541Reference 1085Reference 1621.
A clinical experimental study that involved intravenous administration of THC (paralleling peak blood levels of THC achieved non-medically by smoking) to healthy volunteers without a history of psychiatric disorders or current concomitant drug use showed that THC administration was associated with a variety of acute, transient behavioural and cognitive effects typically associated with an acute psychotic reactionReference 201. These effects included suspiciousness, paranoid and grandiose delusions, conceptual disorganization, and illusions. Depersonalization, derealization, distorted sensory perceptions, altered bodily perceptions, feelings of unreality, and extreme slowing of time were also reported. Furthermore, blunted affect, reduced rapport, lack of spontaneity, psychomotor retardation, and emotional withdrawal were observed.
Schizophrenia and psychosis
Schizophrenia is a chronic and devastating mental disorder that typically presents in late adolescence/early adulthoodReference 1084. Although the incidence of schizophrenia is relatively low at 10 - 22 per 100,000, its prevalence is relatively high (0.3 - 0.7 per 100) because of its chronic natureReference 1084.
Increasing evidence suggests an important role for the ECS in the pathophysiology of schizophrenia and psychosisReference 177Reference 1084Reference 1085 and also see Section 4.9.5.5 for more information. In addition, there is consensus across studies of a robust association between cannabis use and schizophrenia/psychosis. For example, a number of studies report that rates of cannabis use seem to be about twice as high among patients with psychosis than among controlsReference 1622. Furthermore, cannabis (and THC) has been shown to produce a full range of positive symptoms (e.g. suspiciousness, paranoid and grandiose delusions, hallucinations, conceptual disorganization, fragmented thinking, and perceptual alterations), negative symptoms (e.g. blunted affect, emotional withdrawal, psychomotor retardation, lack of spontaneity, and reduced rapport), and cognitive impairments (e.g. deficits in verbal learning, short-term memory, working memory, executive function, abstract ability, decision making, attention, and time perception abnormalities) in healthy volunteers that closely resemble the classical symptoms of schizophreniaReference 183Reference 1085.
The association between cannabis and psychosis fulfills many, but not all, of the standard criteria for causality such as temporal relationship, biological gradient, biological plausibility, coherence, consistency, and experimental evidenceReference 183Reference 1085. Furthermore, cannabis appears to be neither necessary nor sufficient to cause a persistent psychotic disorder such as schizophreniaReference 183Reference 1085. Rather, it appears that cannabis use is but a component cause that can, in concert with known and unknown factors, contribute to the overall risk of schizophreniaReference 183Reference 1085. For example, the link between cannabis and psychosis is moderated by factors such as age at onset of cannabis use, childhood abuse, and genetic vulnerabilityReference 183.
The weight of the evidence suggests the association between cannabis exposure and schizophrenia is modest but consistentReference 183. Furthermore, the bulk of the literature suggests that individuals with a family history of schizophrenia, individuals with prodromal symptoms, and individuals who have experienced discreet episodes of psychosis related to cannabis should be strongly discouraged from using (THC-predominant) cannabis and psychoactive cannabinoidsReference 183.
The following sections summarize some of the more salient literature regarding the association between cannabis use and schizophrenia and psychosis. Of note, the majority of studies have focused on cannabis use and positive symptoms, with far less attention being paid to the association between cannabis use and negative symptoms and cognitive deficits in schizophreniaReference 183.
A 15-year, prospective, longitudinal cohort study of over 45 000 male Swedish conscripts examining the association between cannabis use and risk of schizophrenia reported that the relative risk of schizophrenia among high consumers of cannabis (> 50 lifetime occasions) was 6.0 (95% CI = 4.0 - 8.9) compared with non-usersReference 203. The relative risk was 2.4 among the individuals that reported use of cannabis at least once compared with non-users (95% CI = 1.8 - 3.3). Furthermore, the relative risk was dose-dependent, increasing with increasing consumption level. Aside from cannabis consumption, diagnosis of other psychiatric disease other than schizophrenia at conscription, disturbed conditions of upbringing, solvent abuse and poor adjustment in school were all strongly associated with increased occurrence of schizophrenia. Adjustment for other confounders weakened the association between cannabis use and risk of schizophrenia, though the association persisted and was still statistically significant.
A three-year, prospective, longitudinal, population-based study of the prevalence, incidence, course and consequences of psychiatric disorders in the Dutch general population (NEMESIS) reported that baseline history of cannabis use increased the risk of a follow-up psychosis outcome for subjects with a lifetime absence of psychosis (AOR = 2.76, 95% CI 1.18 - 6.47) as well as increased the risk of severe level of psychotic symptoms (OR = 24.17, 95% CI = 5.44 - 107.46)Reference 202. In addition, there was a dose-response relation between exposure load and psychosis outcome. A strong additive interaction was found between cannabis use and established vulnerability to psychotic disorder (risk difference 54.7%) compared to those without an established vulnerability (risk difference 2.2%).
In a historical cohort study of over 50 000 male Swedish conscripts, self-reported use of cannabis in adolescence was associated with an increased risk of developing schizophrenia, and this risk was related to frequency of cannabis exposure (i.e. was dose-dependent according to frequency of use)Reference 1623. The AOR for lifetime cannabis use greater than 50 times was 6.7 among the group of individuals that reported using only cannabis.
The Dunedin Multidisciplinary Health and Development Study was a longitudinal, prospective cohort study of over 1 000 individuals followed from birth to age 26 that, among other goals, evaluated the effects of cannabis use on mental health outcomesReference 1624. The study evaluated the psychiatric health of individuals before drug use typically begins (at age 11) as well as at age 26, and also obtained information about drug use at ages 15 and 18 from individual self-reports. Linear regression analyses showed that individuals reporting cannabis use by age 15 and 18 had significantly more schizophrenia symptoms than controls at age 26, even after controlling for psychotic symptoms at age 11. Furthermore, the study reported that individuals who used cannabis at age 15, but not age 18, were more than four times as likely to have a diagnosis of schizophreniform disorder at age 26 than controls, however this effect was no longer significant after controlling for psychotic symptoms at age 11. Cannabis use by age 15 did not however predict depressive outcomes (i.e. depressive symptoms or depressive disorder) at age 26. The authors concluded that cannabis exposure among psychologically vulnerable adolescents, especially by age 15, should be strongly discouraged.
A review of five epidemiological studiesReference 202Reference 203Reference 1623-Reference 1625 by Arseneault et al. (2004) indicated that cannabis appears to be neither necessary nor sufficient to cause psychosis or schizophrenia but rather that it is only one factor in a larger constellation of contributing factorsReference 1622. On an individual level, cannabis use confers an overall two-fold increase in the relative risk for later schizophrenia (AOR = 2.34, CI 95% = 1.69 - 2.95), while at a population level, elimination of cannabis use would reduce the incidence of schizophrenia by approximately 8%, assuming the relationship is truly causalReference 1622.
A population-based, first-contact incidence study conducted in the Netherlands with 133 patients assessing the independent influences of gender and cannabis use on milestones of early course in schizophrenia reported that male patients were significantly younger than female patients at first social and/or occupational dysfunction, first psychotic episode, and first negative symptomsReference 1519. Cannabis-using patients were also significantly younger at these milestones than patients who did not use cannabis. Further analysis showed that cannabis use, but not gender, made an independent contribution to the prediction of age at first psychotic episode with male cannabis users on average almost seven years younger at onset of illness than male non-users.
The relationship between cannabis use and psychotic symptoms was also studied in a prospective cohort of 2 437 young people (ages 14 - 24 yrs) who had greater than average pre-disposition for psychosis, and who had first used cannabis during adolescenceReference 198. The study was part of the Early Developmental Stages of Psychopathology (EDSP) study in which data were collected on the prevalence, incidence, risk factors, comorbidity, and four-year course of mental disorders in a random regional representative population sample of adolescents and young adults. After adjustment for confounding factors, cannabis use at baseline was associated with an increase in the cumulative incidence of psychotic symptoms at follow-up four years later (AOR = 1.67, 95% CI = 1.13 - 2.46). The effect of cannabis use was much stronger in those individuals with any predisposition for psychosis at baseline (24% adjusted difference in risk, 95% CI = 7.9 - 39.7, p = 0.003) compared to those without (5.6%, 95% CI = 0.4 - 10.8, p = 0.003). The authors also found a dose-response relationship between frequency of cannabis use and the risk of psychosis. Near daily use of cannabis at baseline was associated with an AOR of more than 2 for any psychotic symptoms, while cannabis use less than once per month carried the same risk as no cannabis use. Lastly, predisposition for psychosis at baseline did not significantly predict cannabis use four years later (AOR = 1.42, 95% CI = 0.88 - 2.31). The authors conclude that any cannabis use at baseline moderately increases the risk of psychotic symptoms in young people but those individuals with a predisposition to psychosis have a far greater risk of developing psychotic symptoms as a result of cannabis use.
A 25-year longitudinal study of the health, development, and adjustment of a birth cohort of 1 265 New Zealand children (i.e. The Christchurch Health and Development Study) examining the association between cannabis use and mental health outcomes reported that daily users of cannabis had rates of psychotic symptoms that were between 1.6 and 1.8 times (p < 0.001) higher than non-users of cannabisReference 1626. Regression models indicated that cannabis use had a positive and significant effect on psychotic symptoms suggesting that increasing cannabis use was associated with increased symptom levels. Furthermore, according to the authors, the data suggest that it was unlikely that the development of psychotic symptoms led to increased cannabis use.
A systematic review and meta-analysis of population-based longitudinal studies or case-control studies, nested within longitudinal designs, that examined cannabis use and the risk of psychotic or affective mental health outcomes reported an increased risk of any psychotic outcome in individuals who had ever used cannabis compared with non-users (pooled AOR = 1.41, 95% CI = 1.20 - 1.65)Reference 196. This translated into an increase in risk of psychosis of about 40% in participants who had ever used cannabis. Furthermore, the findings appeared to show a dose-related effect, with greater risk to individuals who used cannabis most frequently (OR = 2.09, 95% CI = 1.54 - 2.84)Reference 186Reference 192Reference 196.
In one study, the relationship between age at onset of psychosis and other clinical characteristics in a sample of well-characterized patients diagnosed with bipolar disorder with psychosis, schizoaffective disorder, or schizophrenia, has been investigatedReference 192. The study concluded that lifetime cannabis abuse/dependence was associated with a significantly earlier age at onset of psychosis (3.1 years, 95% CI: 1.4 - 4.8). Furthermore, among those patients with lifetime cannabis abuse/dependence, the age at onset of cannabis abuse/dependence preceded the onset of psychotic illness by almost another three years. However, patients who had a lifetime cannabis abuse/dependence diagnosis and a lifetime alcohol abuse/dependence diagnosis had a significantly later age at onset of psychosis.
Another study looked at the influence of cannabis use on age at onset in both schizophrenia and bipolar disorder (with psychotic symptoms) using regression analysisReference 186. The authors of this study found that although cannabis and other substance use was more frequent in patients with schizophrenia than those diagnosed with bipolar disorder, cannabis use was nonetheless associated with a decrease in age at onset in both disorders. Cannabis use also preceded first hospitalization in the vast majority of cases (95.4%) and furthermore, the period of most intensive use ("several times per day") preceded first admission in 87.1% of the cases. In bipolar patients, cannabis use reduced age at onset of bipolar disorder by an average of nine years. In contrast, in schizophrenic patients, cannabis use reduced age at onset by an average of 1.5 years. No significant difference was noted in age at onset between male and female patients in either of the diagnostic groups.
A 35-year follow-up cohort study of 50 087 Swedish military conscripts examining the association between cannabis use and mental health outcomes found that the OR for psychotic outcomes among frequent cannabis users compared with non-users was 3.7 (95% CI = 2.3 - 5.8) for schizophrenia, 2.2 (95% CI = 1.0 - 4.7) for brief psychosis, and 2.0 (95% CI = 0.8 - 4.7) for other non-affective psychosesReference 1627. Furthermore, the risk of schizophrenia declined over the decades in moderate users but much less so in frequent users. Thus, the authors found a dose-dependent association between cannabis use and risk of schizophrenia. In addition, the presence of a brief psychosis did not increase the risk of later schizophrenia in cannabis users compared with those who did not use. According to the study authors, this suggested that cannabis does not seem to play a major role in the transition from brief psychotic episodes to schizophrenia. One of the main limitations of the Swedish conscript study was that data regarding use of cannabis was limited to the period before conscription.
A preliminary study that evaluated the effects of cannabis use on neurocognitive functions in 28 schizophrenia outpatients who met DSM-IV criteria for schizophrenia (age 18 - 45) reported a deficit in sustained attention and increased impulsivity in schizophrenia patients reporting heavy cannabis useReference 1628. However, it also appeared that heavy cannabis-using subjects generally had a higher level of functioning and did not differ from non-cannabis using schizophrenia patients in other tested functions, raising the possibility of higher pre-morbid functioning among cannabis-using schizophrenia patients. Since this study was cross-sectional, it is not possible to determine the causal relationship between neurocognitive functioning and heavy cannabis use among schizophrenia patients.
In one case-control study with 280 people with a first episode of psychosis and 174 healthy controls, patients reported using higher-potency cannabis containing high amounts of THC (16% THC) and low amounts of CBD ("skunk-like" cannabis) compared to the controls who reported using cannabis containing equal amounts of THC and CBDReference 1112. Furthermore, daily use of "skunk-like" cannabis was associated with an earlier age of onset of psychosis compared to non-cannabis usersReference 1113. In a follow-up case-cohort study by the same group of 410 patients with first-episode psychosis and 370 population controls, daily use of "skunk-like" cannabis was associated with a more than five-fold increased risk of first-episode psychosis, whereas use of "skunk-like" cannabis on weekends was associated with a nearly three-fold increased risk of first-episode psychosisReference 173. By contrast, the OR of a first-episode psychosis associated with the use of "skunk-like" cannabis less than once per week, use of hash every day, on weekends, and less than once per week was not statistically significant compared with never use of cannabisReference 173.
A prospective, population-based birth cohort study of 1 756 adolescents (ALSPAC) examined the relationship between cannabis, tobacco, and psychotic experiencesReference 1629. First, cigarette and cannabis use at age 16 were highly correlated. Next, cannabis use and cigarette use at age 16 were both associated, to a similar degree, with psychotic experiences at age 18 (OR = 1.48, 95% CI = 1.18 - 1.86; or alternatively a 3.2 fold increase in odds of psychotic experiences for those who used cannabis > 60 times). For cigarettes, the OR was 1.61 (95% CI = 1.31 - 1.98; or a 4.2-fold increase in odds in daily smokers vs. non-smokers). Adjustment for cigarette smoking frequency (AOR = 1.27, 95% CI = 0.91 - 1.76; or a 1.2-fold increase in risk in those who used cannabis most heavily compared to never users) or other illicit drug use (AOR = 1.25, 95% CI = 0.91 - 1.73), substantially attenuated the relationship between cannabis and psychotic experiences. The degree of attenuation was less when cannabis use was adjusted for in the cigarette-psychotic experience association (OR = 1.42, 95% CI = 1.05 - 1.92; or a 2.9-fold increase in risk in daily smokers compared to non-smokers). The study authors suggest that measurement of the risk of psychotic experiences associated with cannabis exposure is sensitive to confounding factors such as cigarette smoking, a behaviour which is highly correlated with cannabis use and which is difficult to tease out from cannabis use.
A longitudinal, case-control study in Sweden investigated the causal nature of the association between cannabis abuse and a future diagnosis of schizophrenia reported that within the general Swedish population, cannabis abuse was strongly associated with later schizophrenia (OR = 10.44, 95% CI = 8.99 - 12.11)Reference 1630. The association was substantially attenuated both by increasing temporal delay between cannabis abuse exposure and schizophrenia diagnosis and by controlling for increasing degrees of familial confounding. Fully controlling for familial confounders reduced the association between cannabis abuse and later schizophrenia (OR = 3.3 and 1.6 with three- and seven-year temporal delays respectively). Of note, opiate, sedative, cocaine/stimulant and hallucinogen abuse were also strongly associated with subsequent schizophrenia in the general population. Importantly, the authors of the study suggest that a large part of the cannabis abuse and schizophrenia association observed in the general population is not causal and results from confounding due to shared familial factors. Thus, shared genetic risk factors contribute substantially to the cannabis abuse and schizophrenia association. The authors also note that familial environmental factors also influence the co-occurrence of cannabis abuse and schizophrenia. Nonetheless, the authors of the study suggest that the findings of the study continue to support the hypothesis that cannabis abuse of sufficient severity has a significant causal impact on future risk for schizophrenia. Thus, it seems that risk of schizophrenia is subject to a number of influences including genetic predisposition, familial environment and severity of cannabis abuse.
A systematic review and meta-analysis of the literature investigating the association between the extent of cannabis consumption and psychosis-related outcomes found that higher levels of cannabis use were associated with increased risk for psychosis with an OR = 3.90 (95% CI = 2.84 - 5.34) for the risk of schizophrenia and other psychosis-related outcomes among the heaviest cannabis users compared to non-usersReference 1368.
An on-line, prospective study that recruited slightly more than 700 participants with the goal of investigating the existence of a longitudinal relationship between change in cannabis use and psychotic experiences reported that a reduction in cannabis use was associated with a reduced frequency of psychotic experiences at follow-up (β = -0.096, p = 0.01)Reference 1631. On the other hand, an increase in cannabis use was not significantly associated with the number of psychotic experiences at follow-up. While the decrease in cannabis use was associated with fewer positive symptoms at follow-up in the unadjusted model (β = -0.12, p = 0.002), this was not the case in the adjusted model (β = -0.06, p = 0.06). An increase in cannabis use was associated with a higher score in the community assessment of psychic experiences (CAPE) subscale of measures of positive symptoms (β = 0.07, p = 0.02) in the fully adjusted model, while no significant association was found between change in cannabis use and the "Negative" subscale. A decrease in cannabis use was predictive of a lower score at follow-up on the "Depressive" subscale but only in the unadjusted model. Given the findings, the authors suggest that cessation of cannabis use may be beneficial in reducing the risk of clinical psychosis, and especially the risk of positive symptoms, in the long term.
A recent systematic review and meta-analysis, that included 24 studies and over 16 000 participants, showed that independent of stage of illness, continued cannabis use in patients with a pre-existing psychotic disorder was associated with a greater increase in relapse of psychosis compared to patients who never used or discontinued useReference 164. Continued use was also associated with longer hospital admissions. Furthermore, there was a greater effect of continued use over discontinued use on relapse, positive symptoms, and level of functioning, but not on negative symptoms.
A subsequent observational study of 256 patients, 18 - 65 years of age, with first-episode psychosis showed that former regular users of cannabis who stopped using after the onset of psychosis had the most favourable illness course with regards to relapse, whereas continued high-frequency use (i.e. daily use) of high potency ("skunk-like") cannabis was associated with the worst outcomeReference 165. High-frequency, high-potency users had an OR = 3.28 (95% CI = 1.22 - 9.18) of a subsequent relapse, an OR = 1.77 (95% CI = 0.96 - 3.25) of more relapses, and an OR = 3.16 (95% CI = 1.26 - 8.09) of more intense psychiatric care after onset of psychosis as well as fewer months until a relapse occurred.
Another recent prospective cohort study of 220 patients with first-episode psychosis, 18 - 65 years of age, reported that there was an increase in the odds of experiencing a relapse of psychosis during periods of cannabis use relative to periods of no use (OR = 1.13; 95% CI = 1.03 - 2.24)Reference 166. The authors suggest that it is more likely than not that continued cannabis use after onset of psychosis is causally, and dose-dependently, associated with increased risk of relapse of psychosis resulting in psychiatric hospitalization.
Genetic factors
A number of studies have investigated the influence of potential genetic factors in the development of psychosis and schizophrenia, and more specifically as a function of interaction with cannabis use. Some studies have focused on the role of genetic polymorphisms at the COMT gene locusReference 1116-Reference 1120, while others have focused on polymorphisms at the AKT1 gene locusReference 1124-Reference 1127, the BDNF geneReference 1632, the DAT1 gene or the CNR1 gene lociReference 1633-Reference 1635.
Schizophrenia and the COMT gene
COMT regulates the breakdown of catecholamines, including neurotransmitters such as dopamine, epinephrine, and norepinephrineReference 1120. A missense mutation at codon 158 in the COMT gene, causing a substitution to the methionine (Met) at the positional valine (Val) (Val158Met), results in an enzyme with decreased activity and correspondingly slower dopamine catabolismReference 1636Reference 1637. Changes in dopaminergic tone and signaling are known to affect neurophysiological function, and these changes have been implicated in the pathophysiology of schizophreniaReference 1638. Although an earlier large-scale association study and meta-analysis failed to find a strong association between the Val158Met COMT polymorphism and vulnerability to schizophreniaReference 1639, later studies (below) appear to suggest an association.
Caspi et al.Reference 1116 followed an epidemiological birth cohort of 1 037 children longitudinally across the first three decades of life. They concluded that the COMT Val/Val homozygous genotype interacted with adolescent-onset cannabis use, but not adult-onset use, to predict the emergence of adult psychosis. Subsequent studies confirmed and extended these findingsReference 1117-Reference 1120Reference 1126. Carriers of the Val allele were most sensitive to Δ9-THC-induced psychotic experiences (especially if they scored highly on a psychosis liability assessment), and were also more sensitive to the Δ9-THC-induced memory and attention impairments compared to carriers of the Met alleleReference 1117. Homozygous carriers of the Val allele, but not subjects with the homozygous Met genotype, showed an increase in the incidence of hallucinations after cannabis exposure, but this was conditional on prior psychometric evidence of psychosis liabilityReference 1118. Those patients who were Val/Met heterozygous also appeared to be more sensitive to the effects of cannabis than Met homozygotes, but less sensitive than Val homozygotesReference 1118.
Another study suggested that cannabis use could reduce the (protective) delay effect of the COMT Met allele in influencing the age of onset of psychosisReference 1119. These findings were supported, and extended, by a subsequent study which showed that those who started using cannabis earlier had an earlier age at onset of psychiatric disorders, and that carriers of the Val homozygous genotype had an earlier age of onset of psychosis compared to Met carriersReference 1120. The authors of this study concluded that gene-environment interaction (i.e. the combination of the COMT Val to Met polymorphism and cannabis use) may modulate the emergence of psychosis in adolescentsReference 1120. In addition, evidence gathered from convergent functional genomic data implicates the COMT gene (as well as the CNR1 and 2 genes) in the pathophysiology of schizophreniaReference 1640.
Taken together, these studies also suggest the presence of a gene-dosage effect, with increasing disease risk among Val/Val homozygotes, moderate risk in Val/Met heterozygotes, and less risk among Met/Met homozygotes.
Schizophrenia and the AKT1 gene
Other studies have focused on the role of AKT1, a gene that encodes a protein kinase involved in the dopamine and cannabinoid receptor signaling cascades, in regulating cellular metabolism, cell stress, cell-cycle regulation, and apoptosis as well as regulating neuronal cell size and survivalReference 1124. In one study, the authors found evidence of a gene-environment interaction between a SNP in the AKT1 gene (rs2494732, C/C homozygous polymorphism) and cannabis useReference 1125. Individuals with the C/C homozygous polymorphism had an approximately two-fold increased risk of being diagnosed with a psychotic disorder after having used cannabis either daily or weeklyReference 1125. In contrast, C/T heterozygous individuals had only a slightly increased risk of developing cannabis-related psychosis compared to T/T homozygotes, which served as the controlsReference 1125. In another study by the same group, individuals with the rs2494732 C/C homozygous polymorphism exhibited a deficit in sustained attention, but not in verbal memory, even in the absence of current cannabis useReference 1124. A naturalistic study of 442 healthy, young cannabis users (308 males and 114 females) between 16 and 23 years of age examined associations between variations at the AKT1 gene locus and acute psychotic symptoms and cognitive function and level of THC in subjects' own cannabisReference 1127. The study found that variation at the AKT1 gene locus predicted acute psychotic response to cannabis along with cannabis dependence and baseline schizotypal symptoms. Furthermore, the study found that working memory following acute cannabis exposure was poorer in females compared to males.
Schizophrenia and the BDNF gene
One study found that cannabis use, before diagnosis of schizophrenia, was associated with a decrease in the age at onset of a psychotic disorder, decreasing the age at first hospital admission by almost three yearsReference 1632. Furthermore, a dose-dependent association between cannabis use and age at onset of psychotic symptoms was found, with an earlier onset of psychotic disorder in heavier users. A significant association between a younger age of first cannabis use and an earlier onset of psychotic disorder was also found, even after controlling for possible confounders. In this study, cannabis use independently predicted age at onset of a psychotic disorder in male patients, whereas in female patients cannabis use was only associated with age at onset of psychotic disorder in those who carried a Met allele mutation in the gene for BDNF. Female carriers of the mutant Met allele presented with psychotic symptoms seven years earlier than female patients who did not use cannabis and who had a BDNF Val/Val genotype.
In conclusion, given the evidence suggesting a strong genetic component in the modulation of psychosis, and especially psychosis or schizophrenia precipitated by cannabis use, the taking of a thorough patient medical history, especially one that includes a psychiatric history/evaluation, would be very valuable in determining whether cannabis/cannabinoids represent a sensible and viable therapeutic option.
A population-based study evaluated whether the association between cannabis use (by 16 years of age) and cortical maturation in adolescents is moderated by a polygenic risk score for schizophreniaReference 1110. In this study, three different population groups were examined: 1 024 adolescents of both sexes from the Canadian Saguenay Youth Study (SYS), 426 adolescents of both sexes from the IMAGEN study, and 504 male youth from the ALSPAC study. In total, 1 577 participants (aged 12 - 21 years) were studied. The findings of the study suggest a negative association between cannabis use in early adolescence and cortical thickness in male participants with a high polygenic risk score for schizophrenia. In the SYS and IMAGEN groups, higher risk scores were associated with a lower cortical thickness only in males who used cannabis. In the ALSPAC group, those individuals who used cannabis most frequently (≥ 61 occasions) had lower cortical thickness compared to those who never used cannabis and those with light use. The authors concluded that cannabis use in early adolescence moderates the association between the genetic risk for schizophrenia and cortical maturation among male individuals. Furthermore, the authors suggest that cannabis use might interfere with the maturation of the cerebral cortex in male adolescents at high risk for schizophrenia. Cannabis exposure may further accelerate the natural course of cortical thinning in male adolescents with a high polygenic risk score.
Identification of groups at high-risk
A number of studies have sought to identify subgroups of individuals who may be at particularly high risk of developing psychosis and schizophrenia associated with cannabis useReference 1109. Age of use, genetic susceptibility, family history, childhood trauma and strains of cannabis were all examined in a review by Gage et al. (2015)Reference 1109. Regarding age of use, the evidence suggests that earlier onset of cannabis use is associated with an increased risk of psychosis, schizophreniform disorder, or schizophrenia although it is not fully clear at the moment whether this is the result of a specific "window of vulnerability" in adolescence or rather the result of a longer period of cumulative use (i.e. those individuals who began using cannabis at an earlier age may have used cannabis on more occasions by the time the outcome measure was evaluated) or even a function of other confounding factors such as history of abuse or family socio-economic levelReference 1085Reference 1109Reference 1111.
A recent 15-year longitudinal study of 6534 adolescents from Finland suggested that cannabis use was associated with an increased risk in developing psychosis by age 30. The survey-based data found that individuals who tried cannabis at least five times or more were at the highest risk (HR = 6.5, 95% CI 3.0-13.9) of developing psychosis 15 years later, even after controlling for prodromal symptoms, poly-substance use, and parental psychosisReference 1641.
A recent 4-year longitudinal study monitoring individual-level data from 3720 Canadian adolescents found that 'frequent' (daily/near daily) cannabis use at baseline (age 13) predicted self-reported psychotic symptoms one year later (age 14). Participants completed an annual web-based survey for four years from age 13 to 16. In the subsequent time-point assessments following baseline, cannabis use predicted psychotic symptoms that year and one year later. For example, cannabis use at age 14 predicted psychotic symptoms at age 14 and one year later (age 15); cannabis use at age 15 predicted psychotic symptoms at age 15 and one year later (age 16). The overall findings imply that using cannabis often during the early teenage years may increase the risk in the development and persistence of psychotic symptoms. Key limitations in this study relate to the associative findings (causality not established) and not accounting for family history of psychosis in analysesReference 1642.
Regarding genetic susceptibility, a number of studies suggest an important role for a number of different genes in modulating the susceptibility to psychotic disorders in those who use cannabis (COMT, AKT1, BDNF, DAT1, NRG1, CNR1 and see previous section). While the evidence regarding the influence of the COMT gene has been called into question, other genes (AKT1, BDNF, DAT1, NRG1, CNR1) may still contribute to the risk of developing psychotic disorders associated with cannabis useReference 1085Reference 1109Reference 1111.
A few studies have found that childhood trauma when combined with cannabis use increases the absolute risk of psychosis to a greater degree than the sum of either risk factor aloneReference 1085Reference 1109Reference 1111. ORs of developing psychosis in adolescence where there is a history of abuse or trauma have been reported to be between 11.96 (95% CI = 2.10 - 68.22) and 20.9 (95% CI = 2.3 - 173.5)Reference 1085.
A positive family history of schizophrenia has also been linked to an increased risk of experiencing cannabis-induced psychotic disordersReference 1085. For example, one population-based cohort study of 2 276 309 individuals that sought to establish the rate ratios of cannabis-induced psychosis associated with predisposition to psychosis and other psychiatric disorders in a first-degree relative and compare them with the corresponding rate ratios for developing schizophrenia spectrum disorders reported that children with a mother with schizophrenia had a five-fold increased risk of developing schizophrenia and a 2.5-fold increased risk of developing cannabis-induced psychosisReference 1643.
Lastly, a number of studies have examined the association between use of different strains of cannabis and risk of psychosisReference 1085Reference 1109Reference 1111. Overall, the findings appear to suggest that strains with a higher THC to CBD content are associated with an increased risk of psychosis, although additional research is required to further substantiate these findingsReference 1109.
7.7.3.3 Suicidal ideation, attempts and mortality
- Evidence from epidemiological studies also suggests a dose-dependent effect between cannabis use and suicidality, especially in men.
Evidence from epidemiological studies suggests suicidal thoughts and behaviours (ideation, planning, attempt) are strongly related to substance use behaviours, including cannabis useReference 178. A number of epidemiological studies have found a statistically significant association between cannabis use, especially cannabis use that begins early and that is heavy (i.e. daily) and suicidalityReference 168Reference 169Reference 178Reference 1644. While the precise mechanism of action linking cannabis use, especially heavy use, with an increased risk of suicidality is not clear, evidence from clinical studies with rimonabant, a CB1 receptor antagonist, showed that rimonabant use was statistically significantly associated with an increased risk of suicidal ideation and attempt (OR = 1.9, 95% CI = 1.1 - 3.1)Reference 1645Reference 1646. Together, findings from epidemiological studies and clinical studies with rimonabant raise the possibility that downregulation of CB1 receptors achieved either through frequent heavy cannabis use (of THC-predominant/enriched cannabis) or administration of a CB1 receptor antagonist (e.g. rimonabant) may potentially trigger suicidality, especially in susceptible individuals.
One 30-year longitudinal cohort study (Christchurch Health and Development Study) of 1 265 children reported that, after controlling for personal and family characteristics, there remained a statistically significant correlation between suicidal ideation and at least monthly cannabis useReference 1644. In this study, regular cannabis use (i.e. at least several times per week to daily use) was estimated to significantly increase the risks of transitioning into suicidal thoughts for susceptible males but not females. Importantly, the study did not find a significant effect of suicidal ideation on the uptake of regular cannabis use (i.e. no reverse-causality).
A study using two community-based twin samples from the Australian Twin Registry composed of 9 583 individuals reported that all levels of cannabis use were associated with suicidal ideation, regardless of duration of use (OR = 1.28 - 2.00, p < 0.01)Reference 178. Cannabis use and endorsement of ≥ three symptoms were associated with unplanned (OR = 1.95 and 2.51 respectively, p < 0.05) but not planned, suicide attempts. Suicidal ideation, regardless of duration, showed a dose-dependent relationship with cannabis use, being more common in those reporting between three and six CUD symptoms (21 - 28%) compared to never users (6 - 12%), those with no symptoms (9 - 17%) and those with one to two CUD symptoms (13 - 21%). Importantly, associations persisted even after controlling for other psychiatric disorders and substance use. The study authors suggest the presence of overlapping genetic and environmental effects responsible for the co-variance between cannabis use and suicidal ideation.
A study using data drawn from waves 1 and 2 of NESARC reported that cannabis use was significantly associated with increased incidence of suicidality among men (AOR for any cannabis use = 1.91, 95% CI = 1.02 - 3.56), and particularly so with heavy use (i.e. daily/near-daily) (AOR = 4.28, 95% CI = 1.32 - 13.86), but not so among womenReference 168. Among women, baseline suicidality was associated with initiation of cannabis use among women (AOR = 2.34, 95% CI = 1.42 - 3.87), but not men. While the study reported a significant association between cannabis use and suicidal ideation, no association was found between cannabis use and suicide attempts.
A recent review and meta-analysis of the association between cannabis use and suicidality concluded that cannabis use, in particular heavy use (i.e. daily or near-daily) was associated with a modest effect on suicidalityReference 169. While the evidence of an association between acute cannabis use and imminent risk for suicidality was lacking, there was evidence to support that chronic cannabis use can predict suicidality. Pooled meta-analyses showed that any cannabis use was associated with increasing suicidal ideation (OR = 1.43, 95% CI = 1.13 - 1.83), and heavy cannabis use was associated with a higher risk of suicidal ideation (OR = 2.53, 95% CI = 1.00 - 6.39). Furthermore, pooled ORs estimate for any cannabis use and suicide attempt was 2.23 (95% CI = 1.24 - 4.00), and for any heavy cannabis use was 3.20 (95% CI = 1.72 - 5.94). Limitations of the meta-analysis include heterogeneity of the studies as well as publication bias.
7.7.3.4 Amotivational syndrome
- The available limited evidence for an association between cannabis use and an "amotivational syndrome" is mixed.
The term "amotivational syndrome" is generally used to qualify people who exhibit apathy, lack of motivation, social withdrawal, narrowing of interests, lethargy, impaired memory, impaired concentration, disturbed judgement, and impaired occupational achievementReference 1647.
Some investigators suggest that heavy, chronic use of cannabis is linked to the development of such a syndromeReference 1647; abstinence typically appears to result in resolution of symptomsReference 1365Reference 1648. However, other investigators have not found such a causal relationshipReference 1647Reference 1649. There is some speculation that earlier studies may have been confounded by a number of variables such as other substance abuse, poverty, or other psychiatric disorders that could lend alternate explanations to the so-called "amotivational syndrome"Reference 183.
8.0 Overdose/Toxicity
There has been no documented evidence of death exclusively attributable to cannabis overdose to dateReference 1650, most likely because of the sparse expression of CB1 receptors in the brainstem regions responsible for respiratory and cardiovascular controlReference 771. Using rodent LD50 values for oral administration, the equivalent lethal dose of THC in humans has been extrapolated to be >15,000 mgReference 1651Reference 1652. Using a cannabis sample that contains 20% THC as an example, someone would need to orally ingest 75,000 mg of cannabis to reach this amount, which is greater than the amount of cannabis a very heavy user would use in a day (1,025 mg, range 652-1,336 mg, based on European dataReference 1653). The margin of exposure for THC is >100 for individual exposure, population-based exposure calculated from prevalence data and population-based exposure calculated from sewage analysisReference 1654. Nevertheless, a cannabis and THC overdose can produce dose-dependent unwanted and potentially significant mental and physical effects, typically dizziness, sedation, intoxication (euphoria), cognitive impairment, transient impairment of sensory and perceptual functions, clumsiness, dry mouth, hypotension, or increased heart rateReference 227Reference 1655. These adverse effects are generally tolerable in healthy adults and not unlike those seen with other medicationsReference 140.
Acute psychological complications (e.g. panic attacks, severe anxiety, psychosis, paranoia, hallucinations, convulsions, hyperemesis etc.) that present to hospital Emergency Departments can be managed with conservative measures, such as reassurance in a quiet environment, and/or administration of benzodiazepines (5 to 10 mg diazepam p.o.) or i.v. fluids, if requiredReference 1656. As is stated in the case of overdose with Marinol®Reference 227, the signs and symptoms observed with smoked or ingested cannabis are an extension of the psychotomimetic and physiologic effects of THC. Individuals experiencing psychotic reactions should stop using cannabis or cannabinoids immediately and seek prompt medical/psychiatric attention. The Marinol® monograph suggests that in the case of a serious recent oral ingestion, these should be managed with gut decontaminationReference 227. In unconscious patients with a secure airway, activated charcoal should be instilled (30 to 100 g in adults, 1 to 2 g/kg in infants) via a nasogastric tubeReference 227. A saline cathartic or sorbitol may be added to the first dose of activated charcoalReference 227.
Differences in pharmacokinetics and pharmacodynamics between different routes of administration such as smoking/vapourization and oral ingestion confer different overdose risks. Inhalation is typically associated with a large and rapid increase in blood cannabinoid levels while oral ingestion is associated with a smaller and slower increase in blood cannabinoid levels (see Section 2.2.1 for more details). Consistent with these differences in pharmacokinetics, acute adverse effects associated with inhalation have a shorter onset of action as well as a shorter duration of action, while acute adverse effects associated with oral ingestion have a longer onset of action and a longer duration of action (see Sections 2.2.1.1 - 2.2.1.4 for more details). The sudden spike in higher blood levels of cannabinoids associated with inhalation could lead to an acute overdose episode if self-titration is not properly employed; one study has shown while that cannabis users titrate their dose of THC by inhaling lower volumes of smoke when smoking "strong" joints, this did not fully compensate for the higher THC doses per joint when using "strong" cannabis and therefore users of more potent cannabis are exposed to greater quantities of THCReference 584. On the other hand, the protracted onset of acute effects associated with oral ingestion can lead some individuals to consume more cannabis (and THC) than actually needed for a therapeutic effect in the belief that they have either not consumed enough or that an increased dose will lead to a faster onset of effects. These mistaken beliefs and actions could lead to an overdose. In one case series report from Colorado, five patients who were daily cannabis smokers and who reported using greater than 10 times the recommended dose of 10 mg of THC were admitted to psychiatric emergency services with edible cannabis-induced-psychosisReference 175. Symptoms reported included labile disorganized thinking, poor insight and judgement, hyperreligious delusions, flat affect, grandiose delusions, auditory and visual hallucinations, combative and agitated behaviour, paranoia, euphoria, rapid speech, flight of ideas, suicidal ideation, insomnia, depressed mood. In all of the cases, psychosis resolved within one to two days with treatment and all patients returned to their baseline, normal mental state. No further psychiatric treatment was recommended at discharge. Two patients had one previous episode of inhaled cannabis-induced psychosis. In one case, family history was positive for schizophrenia and bipolar disorder but uncertain for the other patients. Treatment consisted of intramuscular haloperidol and/or lorazepam/midazolam, oral olanzapine, seclusion/restraint, or oral risperidone. In one case report, a 19-year old man who overdosed on an edible cannabis product (i.e. a cannabis cookie) began reportedly exhibiting erratic speech and hostile behaviours within the first 2.5 h following consumption and died from bodily trauma resulting from a jump from a balcony approximately 3.5 h following consumption of the edibleReference 174.
THC
LD50 values for rats administered single oral doses of THC, or crude cannabis extract, are approximately 1,000 mg/kgReference 1657. Dogs and monkeys are able to tolerate significantly higher oral doses of THC, or cannabis extract, of 3,000 mg/kg (or greater in certain cases)Reference 1657. The estimated human lethal dose of intravenous THC is 30 mg/kg (2,100 mg/70 kg)Reference 227. Conversion of this dose to an average inhaled or oral dose suggests an average inhaled dose of 7,350 mg THC (range: 6,300 mg to 8,400 mg/70 kg) and an average oral dose of 31,500 mg (range: 21,000 mg to 42,000 mg/70 kg) THC, based on a conversion factor between three and six fold for intravenous to inhaled routes of administration, and between 10 and 20 fold for intravenous to oral routesReference 583Reference 1658. Significant CNS symptoms are observed with oral doses of 0.4 mg/kg (28 mg/70 kg) dronabinol (Marinol®). Signs and symptoms of severe intoxication with Marinol® include decreased motor coordination, lethargy, slurred speech and postural hypotensionReference 227.
CBD
LD50 values after single IV doses of CBD were 50 mg/kg (285 mg/70 kg)Footnote ii in miceReference 1659, 232 to 252 mg/kg (2,619 to 2,845 mg/70 kg)Footnote ii in ratsReference 431, and 212 mg/kg (4,787 mg/70 kg)Footnote ii in monkeysReference 1660. There were no deaths in rats and monkeys given daily oral doses of 25 to 300 mg/kg of CBD (282 mg to 6,774 mg/70 kg)Footnote ii for 90 daysReference 431. In human studies, CBD given once at oral doses of 15 to 160 mg, inhaled at a dose of 0.15 mg/kg (10.5 mg/70 kg)Footnote ii, or injected IV at doses of 5 to 30 mg did not produce adverse effects. In a case report, a teenager suffering from schizophrenia who received up to 1,500 mg/day of CBD had no adverse eventsReference 1490. In one study by Devinsky et al.Reference 262, the mean CBD dose at 12 weeks was 22.9 mg/kg (1,603 mg/70 kg)Footnote ii in patients with treatment-resistant epilepsy with 48 patients receiving up to 50 mg/kg/day (3,500 mg/70 kg)Footnote ii CBD escalated over a 12-week period. Adverse events were reported in 79% of patients, but most of them were mild or moderate and transient. Serious adverse events possibly related to CBD use were recorded in 20 patients (12%) and included status epilepticus, diarrhea, pneumonia, and weight loss. A post-hoc analysis showed that the CBD dose at week 12 was not correlated with the number of reported adverse events overallReference 262.
References
- Reference 1
-
Rodriguez de Fonseca F, del Arco I, Bermudez-Silva FJ, Bilbao A, Cippitelli A, Navarro M. The endocannabinoid system: Physiology and pharmacology. Alcohol Alcohol 2005 01;40(0735-0414; 0735-0414; 1):2-14.
- Reference 2
-
Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Ther 2011 12;132(1879-016; 0163-7258; 3):215-41.
- Reference 3
-
Maccarrone M, Gasperi V, Catani MV, Diep TA, Dainese E, Hansen HS, Avigliano L. The endocannabinoid system and its relevance for nutrition. Annu Rev Nutr 2010 08/21;30(1545-4312; 0199-9885):423-40.
- Reference 4
-
Aggarwal SK. Cannabinergic pain medicine: A concise clinical primer and survey of randomized-controlled trial results. Clin J Pain 2012 02/23;29(1536-5409; 0749-8047; 2):162-71.
- Reference 5
-
Hillard CJ. The endocannabinoid signaling system in the CNS: A primer. Int Rev Neurobiol 2015;125:1-47.
- Reference 6
-
Bradshaw HB, Walker JM. The expanding field of cannabimimetic and related lipid mediators. Br J Pharmacol 2005 02;144(0007-1188; 0007-1188; 4):459-65.
- Reference 7
-
De Petrocellis L, Di Marzo V. An introduction to the endocannabinoid system: From the early to the latest concepts. Best.Pract.Res.Clin.Endocrinol.Metab 2009 02;23(1878-1594; 1521-690; 1):1-15.
- Reference 8
-
De Petrocellis L, Di M,V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: Focus on G-protein-coupled receptors and transient receptor potential channels. J.Neuroimmune.Pharmacol. 2010 03;5(1557-1904; 1557-1890; 1):103-21.
- Reference 9
-
O'Sullivan SE, Kendall DA. Cannabinoid activation of peroxisome proliferator-activated receptors: Potential for modulation of inflammatory disease. Immunobiology 2010 08;215(1878-3279; 0171-2985; 8):611-6.
- Reference 10
-
Hansen HS. Palmitoylethanolamide and other anandamide congeners. proposed role in the diseased brain. Exp Neurol 2010 07;224(1090-2430; 0014-4886; 1):48-55.
- Reference 11
-
Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, Bisogno T, De PL, Di M,V, Mechoulam R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol 1998 07/17;353(0014-2999; 0014-2999; 1):23-31.
- Reference 12
-
Quarta C, Mazza R, Obici S, Pasquali R, Pagotto U. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends Mol Med 2011 09;17(1471-499; 1471-4914; 9):518-26.
- Reference 13
-
Battista N, Di TM, Bari M, Maccarrone M. The endocannabinoid system: An overview. Front Behav Neurosci 2012;6(1662-5153; 1662-5153):9.
- Reference 14
-
Horvath B, Mukhopadhyay P, Hasko G, Pacher P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am J Pathol 2012 02;180(1525-2191; 0002-9440; 2):432-42.
- Reference 15
-
Hermanson DJ, Marnett LJ. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev 2011 12;30(1573-7233; 0167-7659; 3-4):599-612.
- Reference 16
-
Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem 2008 Apr 4;283(14):9341-9.
- Reference 17
-
Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: Cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev 2011 10/12;111(1520-6890; 0009-2665; 10):5899-921.
- Reference 18
-
Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 2008 01;153(0007-1188; 0007-1188; 2):199-215.
- Reference 19
-
Di Marzo V, Piscitelli F, Mechoulam R. Cannabinoids and endocannabinoids in metabolic disorders with focus on diabetes. Handb Exp Pharmacol 2011(0171-2004; 0171-2004; 203):75-104.
- Reference 20
-
Bab I, Zimmer A. Cannabinoid receptors and the regulation of bone mass. Br J Pharmacol 2008 01;153(0007-1188; 0007-1188; 2):182-8.
- Reference 21
-
Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, et al. International union of pharmacology. XXVII. classification of cannabinoid receptors. Pharmacol Rev 2002 06;54(0031-6997; 0031-6997; 2):161-202.
- Reference 22
-
Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di M,V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, et al. International union of basic and clinical pharmacology. LXXIX. cannabinoid receptors and their ligands: Beyond CB and CB. Pharmacol Rev 2010 12;62(1521-0081; 0031-6997; 4):588-631.
- Reference 23
-
Kraft B. Is there any clinically relevant cannabinoid-induced analgesia? Pharmacology 2012;89(1423-0313; 0031-7012; 5-6):237-46.
- Reference 24
-
Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS.Neurol.Disord Drug Targets. 2009 12;8(1996-3181; 1871-5273; 6):403-21.
- Reference 25
-
Teixeira D, Pestana D, Faria A, Calhau C, Azevedo I, Monteiro R. Modulation of adipocyte biology by delta(9)-tetrahydrocannabinol. Obesity.(Silver.Spring) 2010 11;18(1930-739; 1930-7381; 11):2077-85.
- Reference 26
-
Greineisen WE, Turner H. Immunoactive effects of cannabinoids: Considerations for the therapeutic use of cannabinoid receptor agonists and antagonists. Int Immunopharmacol 2010 05;10(1878-1705; 1567-5769; 5):547-55.
- Reference 27
-
Jean-Gilles L, Gran B, Constantinescu CS. Interaction between cytokines, cannabinoids and the nervous system. Immunobiology 2010 08;215(1878-3279; 0171-2985; 8):606-10.
- Reference 28
-
Rice W, Shannon JM, Burton F, Fiedeldey D. Expression of a brain-type cannabinoid receptor (CB1) in alveolar type II cells in the lung: Regulation by hydrocortisone. Eur J Pharmacol 1997 05/30;327(0014-2999; 0014-2999; 2-3):227-32.
- Reference 29
-
Shmist YA, Goncharov I, Eichler M, Shneyvays V, Isaac A, Vogel Z, Shainberg A. Delta-9-tetrahydrocannabinol protects cardiac cells from hypoxia via CB2 receptor activation and nitric oxide production. Mol Cell Biochem 2006 02;283(0300-8177; 0300-8177; 1-2):75-83.
- Reference 30
-
Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, Ward S. Differential expression of cannabinoid receptors in the human colon: Cannabinoids promote epithelial wound healing. Gastroenterology 2005 08;129(0016-5085; 0016-5085; 2):437-53.
- Reference 31
-
Marquez L, Suarez J, Iglesias M, Bermudez-Silva FJ, Rodriguez de FF, Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS.One. 2009;4(1932-6203; 1932-6203; 9):e6893.
- Reference 32
-
Linari G, Agostini S, Amadoro G, Ciotti MT, Florenzano F, Improta G, Petrella C, Severini C, Broccardo M. Involvement of cannabinoid CB1- and CB2-receptors in the modulation of exocrine pancreatic secretion. Pharmacol Res 2009 03;59(1043-6618; 1043-6618; 3):207-14.
- Reference 33
-
Izzo AA, Sharkey KA. Cannabinoids and the gut: New developments and emerging concepts. Pharmacol Ther 2010 04;126(1879-016; 0163-7258; 1):21-38.
- Reference 34
-
Purohit V, Rapaka R, Shurtleff D. Role of cannabinoids in the development of fatty liver (steatosis). AAPS.J. 2010 06;12(1550-7416; 1550-7416; 2):233-7.
- Reference 35
-
Mallat A, Teixeira-Clerc F, Deveaux V, Manin S, Lotersztajn S. The endocannabinoid system as a key mediator during liver diseases: New insights and therapeutic openings. Br J Pharmacol 2011 08;163(1476-5381; 0007-1188; 7):1432-40.
- Reference 36
-
Jenkin KA, McAinch AJ, Grinfeld E, Hryciw DH. Role for cannabinoid receptors in human proximal tubular hypertrophy. Cell Physiol Biochem 2010;26(1421-9778; 1015-8987; 6):879-86.
- Reference 37
-
Gratzke C, Streng T, Park A, Christ G, Stief CG, Hedlund P, Andersson KE. Distribution and function of cannabinoid receptors 1 and 2 in the rat, monkey and human bladder. J Urol 2009 04;181(1527-3792; 0022-5347; 4):1939-48.
- Reference 38
-
Tyagi P, Tyagi V, Yoshimura N, Chancellor M. Functional role of cannabinoid receptors in urinary bladder. Indian J.Urol. 2010 01;26(1998-3824; 0970-1591; 1):26-35.
- Reference 39
-
Karasu T, Marczylo TH, Maccarrone M, Konje JC. The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Hum Reprod Update 2011 05;17(1460-2369; 1355-4786; 3):347-61.
- Reference 40
-
Idris AI, Ralston SH. Cannabinoids and bone: Friend or foe? Calcif Tissue Int 2010 10;87(1432-0827; 0171-967; 4):285-97.
- Reference 41
-
Watkins BA, Hutchins H, Li Y, Seifert MF. The endocannabinoid signaling system: A marriage of PUFA and musculoskeletal health. J Nutr Biochem 2010 12;21(1873-4847; 0955-2863; 12):1141-52.
- Reference 42
-
Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, Kendall DA, Scammell BE, Reeve AJ, Chapman V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res.Ther. 2008;10(1478-6362; 1478-6354; 2):R43.
- Reference 43
-
Biro T, Toth BI, Hasko G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: Novel perspectives and therapeutic opportunities. Trends Pharmacol Sci 2009 08;30(1873-3735; 0165-6147; 8):411-20.
- Reference 44
-
Mackie K. Signaling via CNS cannabinoid receptors. Mol Cell Endocrinol 2008 04/16;286(0303-7207; 0303-7207; 1-2):S60-5.
- Reference 45
-
Cabral GA. Marihuana and the immune system. In: G. G. Nahas, K. M. Sutin, D. J. Harvey, S. Agurell, editors. Marihuana and medicine. Totowa: Humana Press; 1999. ID: 2678; RP: NOT IN FILE.
- Reference 46
-
Cascio MG, Pertwee RG. Known pharmacological actions of nine non-psychotropic phytocannabinoids. In: R. G. Pertwee, editor. Handbook of cannabis. Oxforrd: Oxford University Press; 2015..
- Reference 47
-
Cascio MG, Zamberletti E, Marini P, Parolaro D, Pertwee RG. The phytocannabinoid, delta(9)-tetrahydrocannabivarin, can act through 5-HT(1)A receptors to produce antipsychotic effects. Br J Pharmacol 2015 Mar;172(5):1305-18.
- Reference 48
-
Pertwee RG, Cascio MG. Known pharmacological actions of delta-9-tetrahydrocannabinol and of four other chemical constituents of cannabis that activate cannabinoid receptors. In: R. G. Pertwee, editor. Handbook of cannabis. Oxford: Oxford University Press; 2015..
- Reference 49
-
De Petrocellis L, Ligresti A, Moriello AS, Allara M, Bisogno T, Petrosino S, Stott CG, Di Marzo V. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 2011 08;163(1476-5381; 0007-1188; 7):1479-94.
- Reference 50
-
Alger BE. Endocannabinoids: Getting the message across. Proc Natl Acad Sci U S A 2004 06/08;101(0027-8424; 0027-8424; 23):8512-3.
- Reference 51
-
Bisogno T. Endogenous cannabinoids: Structure and metabolism. J Neuroendocrinol 2008 05;20 Suppl 1(1365-2826; 0953-8194):1-9.
- Reference 52
-
Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: The hash realities. Nat Rev Neurosci 2007 Nov;8(11):885-95.
- Reference 53
-
Morgan CJ, Page E, Schaefer C, Chatten K, Manocha A, Gulati S, Curran HV, Brandner B, Leweke FM. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry 2013 May;202(5):381-2.
- Reference 54
-
Miller LK, Devi LA. The highs and lows of cannabinoid receptor expression in disease: Mechanisms and their therapeutic implications. Pharmacol Rev 2011 09;63(1521-0081; 0031-6997; 3):461-70.
- Reference 55
-
Martin-Sanchez E, Furukawa TA, Taylor J, Martin JL. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009 11;10(1526-4637; 1526-2375; 8):1353-68.
- Reference 56
-
Gowran A, Noonan J, Campbell VA. The multiplicity of action of cannabinoids: Implications for treating neurodegeneration. CNS.Neurosci.Ther. 2011 12;17(1755-5949; 1755-5930; 6):637-44.
- Reference 57
-
Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: Effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol Res 2012 11/02;67(1096-1186; 1043-6618; 1):94-109.
- Reference 58
-
Eisenberg E, Ogintz M, Almog S. The pharmacokinetics, efficacy, safety, and ease of use of a novel portable metered-dose cannabis inhaler in patients with chronic neuropathic pain: A phase 1a study. J Pain Palliat Care Pharmacother 2014 Sep;28(3):216-25.
- Reference 59
-
Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, Gamsa A, Bennett GJ, Collet JP. Smoked cannabis for chronic neuropathic pain: A randomized controlled trial. CMAJ 2010 08/30;182(1488-2329; 0820-3946; 14):E694-701.
- Reference 60
-
Gaffuri AL, Ladarre D, Lenkei Z. Type-1 cannabinoid receptor signaling in neuronal development. Pharmacology 2012;90(1-2):19-39.
- Reference 61
-
Zhou Y, Falenta K, Lalli G. Endocannabinoid signalling in neuronal migration. Int J Biochem Cell Biol 2014 Feb;47:104-8.
- Reference 62
-
Tortoriello G, Morris CV, Alpar A, Fuzik J, Shirran SL, Calvigioni D, Keimpema E, Botting CH, Reinecke K, Herdegen T, et al. Miswiring the brain: Delta9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J 2014 Apr 1;33(7):668-85.
- Reference 63
-
Cherif H, Argaw A, Cecyre B, Bouchard A, Gagnon J, Javadi P, Desgent S, Mackie K, Bouchard JF. Role of GPR55 during axon growth and target innervation. ENeuro 2015 Nov 9;2(5):10.1523/ENEURO.0011,15.2015. eCollection 2015 Sep-Oct.
- Reference 64
-
Russo EB, Hohmann AG. Role of cannabinoids in pain management. In: T. R. Deer, M. S. Leong, editors. Comprehensive treatment of chronic pain by medical, interventional, and behavioral approaches: The AMERICAN ACADEMY OF PAIN MEDICINE textbook on patient management. New York: Springer; 2012. ID: 2927; RP: NOT IN FILE.
- Reference 65
-
Guzman M. Cannabinoids: Potential anticancer agents. Nat Rev Cancer 2003 10;3(1474-175; 1474-175; 10):745-55.
- Reference 66
-
Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 2004 09;3(1474-1776; 1474-1776; 9):771-84.
- Reference 67
-
Bazzaz FA, Dusek D, Seigler DS, Haney AW. Photosynthesis and cannabinoid content of temperate and tropical populations of cannabis sativa.. Biochem Syst Ecol 1975;3(1):15-8.
- Reference 68
-
Radwan MM, ElSohly MA, El-Alfy AT, Ahmed SA, Slade D, Husni AS, Manly SP, Wilson L, Seale S, Cutler SJ, et al. Isolation and pharmacological evaluation of minor cannabinoids from high-potency cannabis sativa. J Nat Prod 2015 Jun 26;78(6):1271-6.
- Reference 69
-
Elsohly MA, Gul W. Constituents of cannabis sativa. In: R. G. Pertwee, editor. Handbook of cannabis. Oxford University Press; 2014..
- Reference 70
-
Elsohly MA, Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci 2005 12/22;78(0024-3205; 0024-3205; 5):539-48.
- Reference 71
-
Hanus L, Meyer S, Munoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: A unified inventory. Nat Prod Rep 2016;33(12):1357-92.
- Reference 72
-
Zhu HJ, Wang JS, Markowitz JS, Donovan JL, Gibson BB, Gefroh HA, Devane CL. Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J Pharmacol Exp Ther 2006 05;317(0022-3565; 0022-3565; 2):850-7.
- Reference 73
-
Balducci C, Nervegna G, Cecinato A. Evaluation of principal cannabinoids in airborne particulates. Anal Chim Acta 2009 05/08;641(1873-4324; 0003-2670; 1-2):89-94.
- Reference 74
-
Yamaori S, Kushihara M, Yamamoto I, Watanabe K. Characterization of major phytocannabinoids, cannabidiol and cannabinol, as isoform-selective and potent inhibitors of human CYP1 enzymes. Biochem Pharmacol 2010 06/01;79(1873-2968; 0006-2952; 11):1691-8.
- Reference 75
-
Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in cannabis (cannabaceae). Am J Bot 2004 06;91(0002-9122; 0002-9122; 6):966-75.
- Reference 76
-
Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, Elsohly MA. Potency trends of delta(9)-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008*. J Forensic Sci 2010 05/04;55(1556-4029; 0022-1198; 5):1209-17.
- Reference 77
-
Whittle BA, Guy GW. Development of cannabis-based medicines: Risk, benefit and serendipity. In: G. W. Guy, B. A. Whittle, P. J. Robson, editors. The medicinal uses of cannabis and cannabinoids. London: Pharmaceutical Press; 2004. ID: 2582; RP: NOT IN FILE.
- Reference 78
-
Huestis MA. Human cannabinoid pharmacokinetics. Chem.Biodivers. 2007 08;4(1612-1880; 1612-1872; 8):1770-804.
- Reference 79
-
Dussy FE, Hamberg C, Luginbuhl M, Schwerzmann T, Briellmann TA. Isolation of Delta9-THCA-A from hemp and analytical aspects concerning the determination of Delta9-THC in cannabis products. Forensic Sci Int 2005 04/20;149(0379-0738; 0379-0738; 1):3-10.
- Reference 80
-
Ashton CH. Pharmacology and effects of cannabis: A brief review. Br J Psychiatry 2001 02;178(0007-1250; 0007-1250):101-6.
- Reference 81
-
Fischedick J, Van der Kooy F, Verpoorte R. Cannabinoid receptor 1 binding activity and quantitative analysis of cannabis sativa L. smoke and vapor. Chem Pharm Bull 2010 02;58(1347-5223; 0009-2363; 2):201-7.
- Reference 82
-
Maertens RM, White PA, Rickert W, Levasseur G, Douglas GR, Bellier PV, McNamee JP, Thuppal V, Walker M, Desjardins S. The genotoxicity of mainstream and sidestream marijuana and tobacco smoke condensates. Chem Res Toxicol 2009 08;22(1520-5010; 0893-228; 8):1406-14.
- Reference 83
-
International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans: Tobacco smoke and involuntary smoking. 2004(83):81-3.
- Reference 84
-
Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, Desjardins S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two smoking conditions. Chem Res Toxicol 2008;21:494-502.
- Reference 85
-
Russo EB, McPartland JM. Cannabis is more than simply delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2003 02;165(0033-3158; 0033-3158; 4):431-2.
- Reference 86
-
Russo EB. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011 08;163(1476-5381; 0007-1188; 7):1344-64.
- Reference 87
-
Baker PB, Taylor BJ, Gough TA. The tetrahydrocannabinol and tetrahydrocannabinolic acid content of cannabis products. J Pharm Pharmacol 1981 06;33(0022-3573; 6):369-72.
- Reference 88
-
Decarboxylation of tetrahydrocannabinolic acid (THCA) to active THC [Internet]; c2016. Available from: http://eiha.org/media/2014/08/16-10-25-Decarboxylation-of-THCA-to-active-THC.pdf.
- Reference 89
-
Taschwer M, Schmid MG. Determination of the relative percentage distribution of THCA and delta(9)-THC in herbal cannabis seized in austria - impact of different storage temperatures on stability. Forensic Sci Int 2015 Sep;254:167-71.
- Reference 90
-
Wang M, Wang YH, Avula B, Radwan MM, Wanas AS, van Antwerp J, Parcher JF, ElSohly MA, Khan IA. Decarboxylation study of acidic cannabinoids: A novel approach using ultra-high-performance supercritical fluid Chromatography/Photodiode array-mass spectrometry. Cannabis Cannabinoid Res 2016 Dec 1;1(1):262-71.
- Reference 91
-
Garrett ER, Hunt CA. Physiochemical properties, solubility, and protein binding of delta9-tetrahydrocannabinol. J Pharm Sci 1974 07;63(0022-3549; 7):1056-64.
- Reference 92
-
Fairbairn JW, Liebmann JA, Rowan MG. The stability of cannabis and its preparations on storage. J Pharm Pharmacol 1976;28(1):1-7.
- Reference 93
-
Thomas BF, Parker VL, Caddell LW, Jones LV, Sabharwal SK, McDaniel AI, Keimowitz AR, Scheffler NM, Hart ED, Mitchell JM, et al. Composition and stability of a standard marihuana cigarette. In: C. G. Nahas, K. M. Sutin, D. J. Harvey, S. Agurell, editors. Marihuana and medicine. Totowa, New Jersey: Humana Press; 1999. ID: 2354; RP: NOT IN FILE.
- Reference 94
-
Turner CE, Hadley KW, Fetterman PS, Doorenbos NJ, Quimby MW, Waller C. Constituents of cannabis sativa L. IV. stability of cannabinoids in stored plant material. J Pharm Sci 1973 Oct;62(10):1601-5.
- Reference 95
-
Sevigny EL. Is today's marijuana more potent simply because it's fresher? Drug Test Anal 2013 Jan;5(1):62-7.
- Reference 96
-
Govaerts SJ, Hermans E, Lambert DM. Comparison of cannabinoid ligands affinities and efficacies in murine tissues and in transfected cells expressing human recombinant cannabinoid receptors. Eur J Pharm Sci 2004 11;23(0928-0987; 0928-0987; 3):233-43.
- Reference 97
-
Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem 2010;17(1875-533; 0929-8673; 14):1360-81.
- Reference 98
-
Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience 2001;106(0306-4522; 0306-4522; 1):1-4.
- Reference 99
-
Darmani NA, Janoyan JJ, Crim J, Ramirez J. Receptor mechanism and antiemetic activity of structurally-diverse cannabinoids against radiation-induced emesis in the least shrew. Eur J Pharmacol 2007 06/01;563(0014-2999; 0014-2999; 1-3):187-96.
- Reference 100
-
Abrahamov A, Abrahamov A, Mechoulam R. An efficient new cannabinoid antiemetic in pediatric oncology. Life Sci 1995;56(0024-3205; 0024-3205; 23-24):2097-102.
- Reference 101
-
Izzo AA, Borrelli F, Capasso R, Di M,V, Mechoulam R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 2009 10;30(1873-3735; 0165-6147; 10):515-27.
- Reference 102
-
Institute of Medicine. Cannabinoids and animal physiology. In: J. E. Joy, S. J. Watson, J. A. Benson, editors. Marijuana and medicine: Assessing the science base. Washington, DC: National Academy Press; 1999. ID: 2355; RP: NOT IN FILE.
- Reference 103
-
Musty RE. Natural cannabinoids: Interactions and effects. In: G. W. Guy, B. A. Whittle, P. J. Robson, editors. The medicinal uses of cannabis and cannabinoids. London: Pharmaceutical Press; 2004. ID: 2679; RP: NOT IN FILE.
- Reference 104
-
Ruhaak LR, Felth J, Karlsson PC, Rafter JJ, Verpoorte R, Bohlin L. Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from cannabis sativa. Biol Pharm Bull 2011;34(1347-5215; 0918-6158; 5):774-8.
- Reference 105
-
Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG. Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol 2010 01;159(1476-5381; 0007-1188; 1):129-41.
- Reference 106
-
Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 2015 Oct;172(20):4790-805.
- Reference 107
-
Brown AJ. Novel cannabinoid receptors. Br J Pharmacol 2007 11;152(0007-1188; 0007-1188; 5):567-75.
- Reference 108
-
McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and delta(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol 2015 Feb;172(3):737-53.
- Reference 109
-
Parker LA, Rock E, Limebeer C. Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol 2010 12/22;163(1476-5381; 0007-1188; 7):1411-22.
- Reference 110
-
Thomas A, Stevenson LA, Wease KN, Price MR, Baillie G, Ross RA, Pertwee RG. Evidence that the plant cannabinoid Delta9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br J Pharmacol 2005 12;146(0007-1188; 0007-1188; 7):917-26.
- Reference 111
-
Bolognini D, Costa B, Maione S, Comelli F, Marini P, Di M,V, Parolaro D, Ross RA, Gauson LA, Cascio MG, et al. The plant cannabinoid Delta9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br J Pharmacol 2010 06;160(1476-5381; 0007-1188; 3):677-87.
- Reference 112
-
Hill AJ, Weston SE, Jones NA, Smith I, Bevan SA, Williamson EM, Stephens GJ, Williams CM, Whalley BJ. Delta-tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia 2010 08;51(1528-1167; 0013-9580; 8):1522-32.
- Reference 113
-
Riedel G, Fadda P, McKillop-Smith S, Pertwee RG, Platt B, Robinson L. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br J Pharmacol 2009 04;156(1476-5381; 0007-1188; 7):1154-66.
- Reference 114
-
Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol 2005(0171-2004; 0171-2004; 168):1-51.
- Reference 115
-
Jacobs DS, Kohut SJ, Jiang S, Nikas SP, Makriyannis A, Bergman J. Acute and chronic effects of cannabidiol on delta(9)-tetrahydrocannabinol (delta(9)-THC)-induced disruption in stop signal task performance. Exp Clin Psychopharmacol 2016 Oct;24(5):320-30.
- Reference 116
-
Rock EM, Limebeer CL, Parker LA. Effect of combined doses of delta-tetrahydrocannabinol (THC) and cannabidiolic acid (CBDA) on acute and anticipatory nausea using rat (sprague- dawley) models of conditioned gaping. Psychopharmacology (Berl) 2015 Sep 18.
- Reference 117
-
Rock EM, Parker LA. Synergy between cannabidiol, cannabidiolic acid, and delta(9)-tetrahydrocannabinol in the regulation of emesis in the suncus murinus (house musk shrew). Behav Neurosci 2015 Jun;129(3):368-70.
- Reference 118
-
Brunt TM, van Genugten M, Honer-Snoeken K, van de Velde MJ, Niesink RJ. Therapeutic satisfaction and subjective effects of different strains of pharmaceutical-grade cannabis. J Clin Psychopharmacol 2014 Jun;34(3):344-9.
- Reference 119
-
Wright MJ,Jr, Vandewater SA, Taffe MA. Cannabidiol attenuates deficits of visuospatial associative memory induced by delta(9) tetrahydrocannabinol. Br J Pharmacol 2013 Dec;170(7):1365-73.
- Reference 120
-
Zuardi AW, Hallak JE, Crippa JA. Interaction between cannabidiol (CBD) and (9)-tetrahydrocannabinol (THC): Influence of administration interval and dose ratio between the cannabinoids. Psychopharmacology (Berl) 2012 01;219(1432-2072; 0033-3158; 1):247-9.
- Reference 121
-
Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem 2011 01;57(1530-8561; 0009-9147; 1):66-75.
- Reference 122
-
Karschner EL, Darwin WD, McMahon RP, Liu F, Wright S, Goodwin RS, Huestis MA. Subjective and physiological effects after controlled sativex and oral THC administration. Clin Pharmacol Ther 2011 03;89(1532-6535; 0009-9236; 3):400-7.
- Reference 123
-
Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, et al. Cannabidiol potentiates delta(9)-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl) 2011 Nov;218(2):443-57.
- Reference 124
-
Winton-Brown TT, Allen P, Bhattacharyya S, Borgwardt SJ, Fusar-Poli P, Crippa JA, Seal ML, Martin-Santos R, Ffytche D, Zuardi AW, et al. Modulation of auditory and visual processing by delta-9-tetrahydrocannabinol and cannabidiol: An FMRI study. Neuropsychopharmacology 2011 06;36(1740-634; 0006-3223; 7):1340-8.
- Reference 125
-
Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, O' Carroll CM, Seal M, Allen P, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010 02;35(1740-634; 0006-3223; 3):764-74.
- Reference 126
-
Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, Seal M, Surguladze SA, O'Carrol C, Atakan Z, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry 2009 01;66(1538-3636; 0003-990; 1):95-105.
- Reference 127
-
Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, Martin BR. Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology (Berl) 2006 06;186(0033-3158; 0033-3158; 2):226-34.
- Reference 128
-
Ilan AB, Gevins A, Coleman M, Elsohly MA, de WH. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol 2005 09;16(0955-8810; 0955-8810; 5-6):487-96.
- Reference 129
-
Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC versus standardized cannabis extract. Ther Drug Monit 2005 12;27(0163-4356; 0163-4356; 6):799-810.
- Reference 130
-
Fadda P, Robinson L, Fratta W, Pertwee RG, Riedel G. Differential effects of THC- or CBD-rich cannabis extracts on working memory in rats. Neuropharmacology 2004 12;47(0028-3908; 0028-3908; 8):1170-9.
- Reference 131
-
Reid MJ, Bornheim LM. Cannabinoid-induced alterations in brain disposition of drugs of abuse. Biochem Pharmacol 2001 Jun 1;61(11):1357-67.
- Reference 132
-
Zuardi AW, Karniol IG. Effects on variable-interval performance in rats of delta 9-tetrahydrocannabinol and cannabidiol, separately and in combination. Braz J Med Biol Res 1983 07;16(0100-879; 0100-879; 2):141-6.
- Reference 133
-
Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl) 1982;76(3):245-50.
- Reference 134
-
Zuardi AW, Finkelfarb E, Bueno OF, Musty RE, Karniol IG. Characteristics of the stimulus produced by the mixture of cannabidiol with delta 9-tetrahydrocannabinol. Arch Int Pharmacodyn Ther 1981 01;249(0003-9780; 0003-9780; 1):137-46.
- Reference 135
-
Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9 - tetrahydrocannabinol in man. Eur J Pharmacol 1974 09;28(0014-2999; 0014-2999; 1):172-7.
- Reference 136
-
Jones G, Pertwee RG. A metabolic interaction in vivo between cannabidiol and 1 -tetrahydrocannabinol. Br J Pharmacol 1972 06;45(0007-1188; 0007-1188; 2):375-7.
- Reference 137
-
Wachtel SR, Elsohly MA, Ross SA, Ambre J, de WH. Comparison of the subjective effects of delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002 06;161(0033-3158; 0033-3158; 4):331-9.
- Reference 138
-
Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010 02;39(1873-6513; 0885-3924; 2):167-79.
- Reference 139
-
Schubart CD, Sommer IE, van Gastel WA, Goetgebuer RL, Kahn RS, Boks MP. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr Res 2011 05/16;130(1573-2509; 1-3):216-21.
- Reference 140
-
Institute of Medicine. First, do no harm: Consequences of marijuana use and abuse. In: J. E. Joy, S. J. Watson, J. A. Benson, editors. Marijuana and medicine: Assessing the science base. Washington, DC: National Academy Press; 1999. ID: 2387; RP: NOT IN FILE.
- Reference 141
-
Zuurman L, Ippel AE, Moin E, van Gerven JM. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br J Clin Pharmacol 2009 01;67(1365-2125; 0306-5251; 1):5-21.
- Reference 142
-
Beaconsfield P, Ginsburg J, Rainsbury R. Marihuana smoking. cardiovascular effects in man and possible mechanisms. N Engl J Med 1972 08/03;287(0028-4793; 5):209-12.
- Reference 143
-
Perez-Reyes M. Marijuana smoking: Factors that influence the bioavailability of tetrahydrocannabinol. NIDA Res Monogr 1990;99(1046-9516):42-62.
- Reference 144
-
Aryana A, Williams MA. Marijuana as a trigger of cardiovascular events: Speculation or scientific certainty? Int J Cardiol 2007 05/31;118(1874-1754; 0167-5273; 2):141-4.
- Reference 145
-
Hall W, Solowij N. Adverse effects of cannabis. Lancet 1998 11/14;352(0140-6736; 9140):1611-6.
- Reference 146
-
Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol 1999 07;58(0301-0082; 4):315-48.
- Reference 147
-
Hollister LE. Health aspects of cannabis: Revisited. Int J Neuropsychopharmacol 1998 07;1(1461-1457; 1):71-80.
- Reference 148
-
Miller LL. Marihuana: Acute effects on human memory. In: C. G. Nahas, K. M. Sutin, D. J. Harvey, S. Agurell, editors. Marihuana and medicine. Totowa, New Jersey: Humana Press; 1999. ID: 2358; RP: NOT IN FILE.
- Reference 149
-
Hart CL, Ilan AB, Gevins A, Gunderson EW, Role K, Colley J, Foltin RW. Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacol Biochem Behav 2010 09;96(1873-5177; 0091-3057; 3):333-41.
- Reference 150
-
Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J.Addict.Med. 2011 03/01;5(1935-3227; 1932-0620; 1):1-8.
- Reference 151
-
Broyd SJ, van Hell HH, Beale C, Yucel M, Solowij N. Acute and chronic effects of cannabinoids on human cognition-A systematic review. Biol Psychiatry 2016 Apr 1;79(7):557-67.
- Reference 152
-
Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: Profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav 1990 11;37(0091-3057; 3):561-5.
- Reference 153
-
Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002 10;164(0033-3158; 0033-3158; 1):61-70.
- Reference 154
-
Ramaekers JG, Moeller MR, van RP, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Delta9-THC concentration in serum and oral fluid: Limits of impairment. Drug Alcohol Depend 2006 11/08;85(0376-8716; 0376-8716; 2):114-22.
- Reference 155
-
O'Kane CJ, Tutt DC, Bauer LA. Cannabis and driving: A new perspective. Emerg.Med.(Fremantle.) 2002 09;14(1035-6851; 3):296-303.
- Reference 156
-
Hansteen RW, Miller RD, Lonero L, Reid LD, Jones B. Effects of cannabis and alcohol on automobile driving and psychomotor tracking. Ann N Y Acad Sci 1976;282(0077-8923):240-56.
- Reference 157
-
Leirer VO, Yesavage JA, Morrow DG. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med 1991 03;62(0095-6562; 3):221-7.
- Reference 158
-
Smiley A. Marijuana: On-road and driving-simulator studies. In: H. Kalant, W. Corrigall, W. Hall, R. Smart, editors. The health effects of cannabis. Toronto, Canada: Centre of Addiction and Mental Health; 1999. ID: 2359; RP: NOT IN FILE.
- Reference 159
-
Kumar RN, Chambers WA, Pertwee RG. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia 2001 11;56(0003-2409; 0003-2409; 11):1059-68.
- Reference 160
-
British Medical Association. Therapeutic uses of cannabis. Amsterdam: Harwood Academic Publishers; 1997. ID: 2345; RP: NOT IN FILE.
- Reference 161
-
Hill MN, Froese LM, Morrish AC, Sun JC, Floresco SB. Alterations in behavioral flexibility by cannabinoid CB1 receptor agonists and antagonists. Psychopharmacology (Berl) 2006 08;187(0033-3158; 0033-3158; 2):245-59.
- Reference 162
-
Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJ, Parsons LH. Keep off the grass? cannabis, cognition and addiction. Nat Rev Neurosci 2016 May;17(5):293-306.
- Reference 163
-
Schoeler T, Kambeitz J, Behlke I, Murray R, Bhattacharyya S. The effects of cannabis on memory function in users with and without a psychotic disorder: Findings from a combined meta-analysis. Psychol Med 2016 Jan;46(1):177-88.
- Reference 164
-
Schoeler T, Monk A, Sami MB, Klamerus E, Foglia E, Brown R, Camuri G, Altamura AC, Murray R, Bhattacharyya S. Continued versus discontinued cannabis use in patients with psychosis: A systematic review and meta-analysis. Lancet Psychiatry 2016 Mar;3(3):215-25.
- Reference 165
-
Schoeler T, Petros N, Di Forti M, Klamerus E, Foglia E, Ajnakina O, Gayer-Anderson C, Colizzi M, Quattrone D, Behlke I, et al. Effects of continuation, frequency, and type of cannabis use on relapse in the first 2 years after onset of psychosis: An observational study. Lancet Psychiatry 2016 Aug 23.
- Reference 166
-
Schoeler T, Petros N, Di Forti M, Pingault JB, Klamerus E, Foglia E, Small A, Murray R, Bhattacharyya S. Association between continued cannabis use and risk of relapse in first-episode psychosis: A quasi-experimental investigation within an observational study. JAMA Psychiatry 2016 Sep 28.
- Reference 167
-
Morena M, Patel S, Bains JS, Hill MN. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 2016 Jan;41(1):80-102.
- Reference 168
-
Shalit N, Shoval G, Shlosberg D, Feingold D, Lev-Ran S. The association between cannabis use and suicidality among men and women: A population-based longitudinal study. J Affect Disord 2016 Nov 15;205:216-24.
- Reference 169
-
Borges G, Bagge CL, Orozco R. A literature review and meta-analyses of cannabis use and suicidality. J Affect Disord 2016 May;195:63-74.
- Reference 170
-
Aspis I, Feingold D, Weiser M, Rehm J, Shoval G, Lev-Ran S. Cannabis use and mental health-related quality of life among individuals with depressive disorders. Psychiatry Res 2015 Dec 15;230(2):341-9.
- Reference 171
-
Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 2015 Oct;12(4):825-36.
- Reference 172
-
Deshpande A, Mailis-Gagnon A, Zoheiry N, Lakha SF. Efficacy and adverse effects of medical marijuana for chronic noncancer pain: Systematic review of randomized controlled trials. Can Fam Physician 2015 Aug;61(8):e372-81.
- Reference 173
-
Di Forti M, Marconi A, Carra E, Fraietta S, Trotta A, Bonomo M, Bianconi F, Gardner-Sood P, O'Connor J, Russo M, et al. Proportion of patients in south london with first-episode psychosis attributable to use of high potency cannabis: A case-control study. Lancet Psychiatry 2015 Mar;2(3):233-8.
- Reference 174
-
Hancock-Allen JB, Barker L, VanDyke M, Holmes DB. Notes from the field: Death following ingestion of an edible marijuana product--colorado, march 2014. MMWR Morb Mortal Wkly Rep 2015 Jul 24;64(28):771-2.
- Reference 175
-
Hudak M, Severn D, Nordstrom K. Edible cannabis-induced psychosis: Intoxication and beyond. Am J Psychiatry 2015 Sep 1;172(9):911-2.
- Reference 176
-
Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: An updated systematic review of randomized controlled trials. J Neuroimmune Pharmacol 2015 Jun;10(2):293-301.
- Reference 177
-
Rubino T, Zamberletti E, Parolaro D. Endocannabinoids and mental disorders. Handb Exp Pharmacol 2015;231:261-83.
- Reference 178
-
Delforterie MJ, Lynskey MT, Huizink AC, Creemers HE, Grant JD, Few LR, Glowinski AL, Statham DJ, Trull TJ, Bucholz KK, et al. The relationship between cannabis involvement and suicidal thoughts and behaviors. Drug Alcohol Depend 2015 May 1;150:98-104.
- Reference 179
-
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015 Jun 23-30;313(24):2456-73.
- Reference 180
-
Ahmed AI, van den Elsen GA, Colbers A, van der Marck MA, Burger DM, Feuth TB, Rikkert MG, Kramers C. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: A randomized controlled trial. Eur Neuropsychopharmacol 2014 Sep;24(9):1475-82.
- Reference 181
-
Desrosiers NA, Himes SK, Scheidweiler KB, Concheiro-Guisan M, Gorelick DA, Huestis MA. Phase I and II cannabinoid disposition in blood and plasma of occasional and frequent smokers following controlled smoked cannabis. Clin Chem 2014 Apr;60(4):631-43.
- Reference 182
-
Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med 2014 Jun 5;370(23):2219-27.
- Reference 183
-
Wilkinson ST, Radhakrishnan R, D'Souza DC. Impact of cannabis use on the development of psychotic disorders. Curr Addict Rep 2014 Jun 1;1(2):115-28.
- Reference 184
-
Fiz J, Duran M, Capella D, Carbonell J, Farre M. Cannabis use in patients with fibromyalgia: Effect on symptoms relief and health-related quality of life. PLoS.One. 2011;6(1932-6203; 1932-6203; 4):e18440.
- Reference 185
-
Lal S, Prasad N, Ryan M, Tangri S, Silverberg MS, Gordon A, Steinhart H. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2011 10;23(1473-5687; 0954-691; 10):891-6.
- Reference 186
-
De Hert M, Wampers M, Jendricko T, Franic T, Vidovic D, De Vriendt N, Sweers K, Peuskens J, van WR. Effects of cannabis use on age at onset in schizophrenia and bipolar disorder. Schizophr Res 2011 03;126(1573-2509; 1-3):270-6.
- Reference 187
-
Korver N, Nieman DH, Becker HE, van de Fliert JR, Dingemans PH, de HL, Spiering M, Schmitz N, Linszen DH. Symptomatology and neuropsychological functioning in cannabis using subjects at ultra-high risk for developing psychosis and healthy controls. Aust N Z J Psychiatry 2010 03;44(1440-1614; 0004-8674; 3):230-6.
- Reference 188
-
Henquet C, van OJ, Kuepper R, Delespaul P, Smits M, Campo JA, Myin-Germeys I. Psychosis reactivity to cannabis use in daily life: An experience sampling study. Br J Psychiatry 2010 06;196(1472-1465; 0007-1250; 6):447-53.
- Reference 189
-
Moreira FA, Grieb M, Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: Focus on anxiety and depression. Best.Pract.Res.Clin.Endocrinol.Metab 2009 02;23(1532-1908; 1521-690; 1):133-44.
- Reference 190
-
Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: How close are we? CNS.Drugs 2009;23(1172-7047; 1172-7047; 7):543-53.
- Reference 191
-
Crippa JA, Zuardi AW, Martin-Santos R, Bhattacharyya S, Atakan Z, McGuire P, Fusar-Poli P. Cannabis and anxiety: A critical review of the evidence. Hum.Psychopharmacol. 2009 10;24(1099-1077; 0885-6222; 7):515-23.
- Reference 192
-
Ongur D, Lin L, Cohen BM. Clinical characteristics influencing age at onset in psychotic disorders. Compr Psychiatry 2009 01;50(1532-8384; 0010-440; 1):13-9.
- Reference 193
-
van Rossum I, Boomsma M, Tenback D, Reed C, van Os J. Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis. J Nerv Ment Dis 2009 01;197(1539-736; 0022-3018; 1):35-40.
- Reference 194
-
Zammit S, Moore TH, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Effects of cannabis use on outcomes of psychotic disorders: Systematic review. Br J Psychiatry 2008 11;193(1472-1465; 0007-1250; 5):357-63.
- Reference 195
-
Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology 2007 02/13;68(1526-632; 7):515-21.
- Reference 196
-
Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. Lancet 2007 07/28;370(1474-547; 9584):319-28.
- Reference 197
-
Boyce A, McArdle P. Long-term effects of cannabis. Pediatrics and Child Health 2007;18(1):37-41.
- Reference 198
-
Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen HU, van OJ. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ 2005 01/01;330(1468-5833; 7481):11-5.
- Reference 199
-
D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9-tetrahydrocannabinol effects in schizophrenia: Implications for cognition, psychosis, and addiction. Biol Psychiatry 2005 03/15;57(0006-3223; 0006-3223; 6):594-608.
- Reference 200
-
Favrat B, Menetrey A, Augsburger M, Rothuizen LE, Appenzeller M, Buclin T, Pin M, Mangin P, Giroud C. Two cases of "cannabis acute psychosis" following the administration of oral cannabis. BMC Psychiatry 2005;5(1471-244; 1471-244):17-22.
- Reference 201
-
D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology 2004 08;29(0893-133; 0006-3223; 8):1558-72.
- Reference 202
-
van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: A longitudinal population-based study. Am J Epidemiol 2002 08/15;156(0002-9262; 4):319-27.
- Reference 203
-
Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of swedish conscripts. Lancet 1987 Dec 26;2(8574):1483-6.
- Reference 204
-
Desrosiers NA, Ramaekers JG, Chauchard E, Gorelick DA, Huestis MA. Smoked cannabis' psychomotor and neurocognitive effects in occasional and frequent smokers. J Anal Toxicol 2015 May;39(4):251-61.
- Reference 205
-
Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction 2015 Jan;110(1):19-35.
- Reference 206
-
Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney G, Huestis MA. Controlled cannabis vaporizer administration: Blood and plasma cannabinoids with and without alcohol. Clin Chem 2015 Jun;61(6):850-69.
- Reference 207
-
Hunault CC, Mensinga TT, Bocker KB, Schipper CM, Kruidenier M, Leenders ME, De V,I, Meulenbelt J. Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC). Psychopharmacology (Berl) 2009 05;204(1432-2072; 0033-3158; 1):85-94.
- Reference 208
-
Corcoran CM, Kimhy D, Stanford A, Khan S, Walsh J, Thompson J, Schobel S, Harkavy-Friedman J, Goetz R, Colibazzi T, et al. Temporal association of cannabis use with symptoms in individuals at clinical high risk for psychosis. Schizophr Res 2008 12;106(0920-9964; 2-3):286-93.
- Reference 209
-
Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: Cocaine, ecstasy and marijuana. Sleep Med.Rev. 2008 10;12(1087-0792; 1087-0792; 5):381-9.
- Reference 210
-
Tramer MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: Quantitative systematic review. BMJ 2001 07/07;323(0959-8138; 7303):16-21.
- Reference 211
-
Harder S, Rietbrock S. Concentration-effect relationship of delta-9-tetrahydrocannabiol and prediction of psychotropic effects after smoking marijuana. Int J Clin Pharmacol Ther 1997 04;35(0946-1965; 4):155-9.
- Reference 212
-
Henderson-Redmond AN, Guindon J, Morgan DJ. Roles for the endocannabinoid system in ethanol-motivated behavior. Prog Neuropsychopharmacol Biol Psychiatry 2016 Feb 4;65:330-9.
- Reference 213
-
Tzadok M, Uliel-Siboni S, Linder I, Kramer U, Epstein O, Menascu S, Nissenkorn A, Yosef OB, Hyman E, Granot D, et al. CBD-enriched medical cannabis for intractable pediatric epilepsy: The current israeli experience. Seizure 2016 Feb;35:41-4.
- Reference 214
-
Degenhardt L, Lintzeris N, Campbell G, Bruno R, Cohen M, Farrell M, Hall WD. Experience of adjunctive cannabis use for chronic non-cancer pain: Findings from the pain and opioids IN treatment (POINT) study. Drug Alcohol Depend 2015 Feb 1;147:144-50.
- Reference 215
-
Hussain SA, Zhou R, Jacobson C, Weng J, Cheng E, Lay J, Hung P, Lerner JT, Sankar R. Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: A potential role for infantile spasms and lennox-gastaut syndrome. Epilepsy Behav 2015 Jun;47:138-41.
- Reference 216
-
Ware MA, Wang T, Shapiro S, Collet JP, COMPASS study team. Cannabis for the management of pain: Assessment of safety study (COMPASS). J Pain 2015 Dec;16(12):1233-42.
- Reference 217
-
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev 2014 Mar 5;3:CD009270.
- Reference 218
-
Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol 2011 03/23;72(1365-2125; 0306-5251; 5):735-44.
- Reference 219
-
Bramness JG, Khiabani HZ, Morland J. Impairment due to cannabis and ethanol: Clinical signs and additive effects. Addiction 2010 06;105(1360-0443; 0965-2140; 6):1080-7.
- Reference 220
-
Ronen A, Chassidim HS, Gershon P, Parmet Y, Rabinovich A, Bar-Hamburger R, Cassuto Y, Shinar D. The effect of alcohol, THC and their combination on perceived effects, willingness to drive and performance of driving and non-driving tasks. Accid Anal Prev 2010 11;42(1879-2057; 0001-4575; 6):1855-65.
- Reference 221
-
Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict 2009 05;18(1521-0391; 1055-0496; 3):185-93.
- Reference 222
-
Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, Fishman S. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J.Pain 2008 06;9(1526-5900; 1526-5900; 6):506-21.
- Reference 223
-
Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. Dronabinol and marijuana in HIV-positive marijuana smokers. caloric intake, mood, and sleep. J Acquir Immune Defic Syndr 2007 08/15;45(1525-4135; 1525-4135; 5):545-54.
- Reference 224
-
Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV(+) marijuana smokers: Acute effects on caloric intake and mood. Psychopharmacology (Berl) 2005 08;181(0033-3158; 1):170-8.
- Reference 225
-
Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology 2004 06/08;62(1526-632; 11):2098-100.
- Reference 226
-
Page SA, Verhoef MJ, Stebbins RA, Metz LM, Levy JC. Cannabis use as described by people with multiple sclerosis. Can J Neurol Sci 2003 08;30(0317-1671; 0317-1671; 3):201-5.
- Reference 227
-
Abbott Products Inc. Marinol product monograph. 2010.
- Reference 228
-
Hartman RL, Brown TL, Milavetz G, Spurgin A, Pierce RS, Gorelick DA, Gaffney G, Huestis MA. Cannabis effects on driving lateral control with and without alcohol. Drug Alcohol Depend 2015 Sep 1;154:25-37.
- Reference 229
-
Karschner EL, Swortwood MJ, Hirvonen J, Goodwin RS, Bosker WM, Ramaekers JG, Huestis MA. Extended plasma cannabinoid excretion in chronic frequent cannabis smokers during sustained abstinence and correlation with psychomotor performance. Drug Test Anal 2015 Jun 11.
- Reference 230
-
Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: Systematic review of observational studies and meta-analysis. BMJ 2012;344(1756-1833; 0959-535):e536.
- Reference 231
-
Downey LA, King R, Papafotiou K, Swann P, Ogden E, Boorman M, Stough C. The effects of cannabis and alcohol on simulated driving: Influences of dose and experience. Accid Anal Prev 2012 08/04;50(1879-2057; 0001-4575):879-86.
- Reference 232
-
Elvik R. Risk of road accident associated with the use of drugs: A systematic review and meta-analysis of evidence from epidemiological studies (in press). Accid Anal Prev 2012 07/09(1879-2057; 0001-4575).
- Reference 233
-
Honarmand K, Tierney MC, O'Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology 2011 03/29;76(1526-632; 0028-3878; 13):1153-60.
- Reference 234
-
Ilan AB, Smith ME, Gevins A. Effects of marijuana on neurophysiological signals of working and episodic memory. Psychopharmacology (Berl) 2004 11;176(0033-3158; 0033-3158; 2):214-22.
- Reference 235
-
Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 2002 03/06;287(0098-7484; 9):1123-31.
- Reference 236
-
Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015 Aug;56(8):1246-51.
- Reference 237
-
Waissengrin B, Urban D, Leshem Y, Garty M, Wolf I. Patterns of use of medical cannabis among israeli cancer patients: A single institution experience. J Pain Symptom Manage 2015 Feb;49(2):223-30.
- Reference 238
-
Neavyn MJ, Blohm E, Babu KM, Bird SB. Medical marijuana and driving: A review. J Med Toxicol 2014 Sep;10(3):269-79.
- Reference 239
-
Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, Lester H, Chisin R, Mechoulam R, Bar-Hamburger R, Freedman N, et al. Brain imaging study of the acute effects of Delta9-tetrahydrocannabinol (THC) on attention and motor coordination in regular users of marijuana. Psychopharmacology (Berl) 2008 Jan;196(1):119-31.
- Reference 240
-
Williamson EM, Evans FJ. Cannabinoids in clinical practice. Drugs 2000 12;60(0012-6667; 0012-6667; 6):1303-14.
- Reference 241
-
Lopez-Sendon Moreno JL, Garcia Caldentey J, Trigo Cubillo P, Ruiz Romero C, Garcia Ribas G, Alonso Arias MA, Garcia de Yebenes MJ, Tolon RM, Galve-Roperh I, Sagredo O, et al. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with sativex in huntington's disease. J Neurol 2016 Jul;263(7):1390-400.
- Reference 242
-
Lotan I, Treves TA, Roditi Y, Djaldetti R. Cannabis (medical marijuana) treatment for motor and non-motor symptoms of parkinson disease: An open-label observational study. Clin Neuropharmacol 2014 Mar-Apr;37(2):41-4.
- Reference 243
-
Chagas MH, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, dos Santos AC, Teixeira AL, Hallak JE, Crippa JA. Effects of cannabidiol in the treatment of patients with parkinson's disease: An exploratory double-blind trial. J Psychopharmacol 2014 Nov;28(11):1088-98.
- Reference 244
-
Zadikoff C, Wadia PM, Miyasaki J, Chen R, Lang AE, Fox SH. Cannabinoid, CB1 agonists in cervical dystonia: Failure in a phase IIa randomized controlled trial. Basal Ganglia 2011;1:91.
- Reference 245
-
Curtis A, Mitchell I, Patel S, Ives N, Rickards H. A pilot study using nabilone for symptomatic treatment in huntington's disease. Mov Disord 2009 11/15;24(1531-8257; 0885-3185; 15):2254-9.
- Reference 246
-
Curtis A, Clarke CE, Rickards HE. Cannabinoids for tourette's syndrome. Cochrane Database Syst Rev 2009(1469-493; 1361-6137; 4):CD006565.
- Reference 247
-
Deutsch SI, Rosse RB, Connor JM, Burket JA, Murphy ME, Fox FJ. Current status of cannabis treatment of multiple sclerosis with an illustrative case presentation of a patient with MS, complex vocal tics, paroxysmal dystonia, and marijuana dependence treated with dronabinol. CNS Spectr 2008 May;13(5):393-403.
- Reference 248
-
Uribe Roca MC, Micheli F, Viotti R. Cannabis sativa and dystonia secondary to wilson's disease. Mov Disord 2005 01;20(0885-3185; 0885-3185; 1):113-5.
- Reference 249
-
Carroll CB, Bain PG, Teare L, Liu X, Joint C, Wroath C, Parkin SG, Fox P, Wright D, Hobart J, et al. Cannabis for dyskinesia in parkinson disease: A randomized double-blind crossover study. Neurology 2004 10/12;63(1526-632; 7):1245-50.
- Reference 250
-
Jabusch HC, Schneider U, Altenmuller E. Delta9-tetrahydrocannabinol improves motor control in a patient with musician's dystonia. Mov Disord 2004 08;19(0885-3185; 0885-3185; 8):990-1.
- Reference 251
-
Mesnage V, Houeto JL, Bonnet AM, Clavier I, Arnulf I, Cattelin F, Le Fur G, Damier P, Welter ML, Agid Y. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and parkinson disease. Clin Neuropharmacol 2004 May-Jun;27(3):108-10.
- Reference 252
-
Muller-Vahl KR, Schneider U, Prevedel H, Theloe K, Kolbe H, Daldrup T, Emrich HM. Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in tourette syndrome: A 6-week randomized trial. J Clin Psychiatry 2003 04;64(0160-6689; 4):459-65.
- Reference 253
-
Fox SH, Kellett M, Moore AP, Crossman AR, Brotchie JM. Randomised, double-blind, placebo-controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Mov Disord 2002 01;17(0885-3185; 1):145-9.
- Reference 254
-
Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in parkinson's disease: A pilot study. Neurology 2001 12/11;57(0028-3878; 11):2108-11.
- Reference 255
-
Muller-Vahl KR, Koblenz A, Jobges M, Kolbe H, Emrich HM, Schneider U. Influence of treatment of tourette syndrome with delta9-tetrahydrocannabinol (delta9-THC) on neuropsychological performance. Pharmacopsychiatry 2001 01;34(0176-3679; 1):19-24.
- Reference 256
-
Muller-Vahl KR, Schneider U, Emrich HM. Nabilone increases choreatic movements in huntington's disease. Mov Disord 1999 11;14(0885-3185; 0885-3185; 6):1038-40.
- Reference 257
-
Hemming M, Yellowlees PM. Effective treatment of tourette's syndrome with marijuana. Journal of Psychopharmacology 1993;7(4):389-91.
- Reference 258
-
Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, Kennedy K, Schram K. Controlled clinical trial of cannabidiol in huntington's disease. Pharmacol Biochem Behav 1991 11;40(0091-3057; 3):701-8.
- Reference 259
-
Frankel JP, Hughes A, Lees AJ, Stern GM. Marijuana for parkinsonian tremor. J Neurol Neurosurg Psychiatr 1990 05;53(0022-3050; 5):436.
- Reference 260
-
Sandyk R, Awerbuch G. Marijuana and tourette's syndrome. J Clin Psychopharmacol 1988 12;8(0271-0749; 6):444-5.
- Reference 261
-
Consroe P, Sandyk R, Snider SR. Open label evaluation of cannabidiol in dystonic movement disorders. Int J Neurosci 1986 11;30(0020-7454; 4):277-82.
- Reference 262
-
Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, Miller I, Flamini R, Wilfong A, Filloux F, et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol 2016 Mar;15(3):270-8.
- Reference 263
-
dos Santos RG, Hallak JE, Leite JP, Zuardi AW, Crippa JA. Phytocannabinoids and epilepsy. J Clin Pharm Ther 2015 Apr;40(2):135-43.
- Reference 264
-
Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav 2013 Dec;29(3):574-7.
- Reference 265
-
Soltesz I, Alger BE, Kano M, Lee SH, Lovinger DM, Ohno-Shosaku T, Watanabe M. Weeding out bad waves: Towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci 2015 May;16(5):264-77.
- Reference 266
-
Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, Katz R, Di Marzo V, Jutras-Aswad D, Notcutt WG, et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014 Jun;55(6):791-802.
- Reference 267
-
Kraft B, Frickey NA, Kaufmann RM, Reif M, Frey R, Gustorff B, Kress HG. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology 2008 07;109(1528-1175; 0003-3022; 1):101-10.
- Reference 268
-
Wallace M, Schulteis G, Atkinson JH, Wolfson T, Lazzaretto D, Bentley H, Gouaux B, Abramson I. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology 2007 11;107(0003-3022; 0003-3022; 5):785-96.
- Reference 269
-
Beaulieu P. Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can J Anaesth 2006 08;53(0832-610; 8):769-75.
- Reference 270
-
Holdcroft A, Maze M, Dore C, Tebbs S, Thompson S. A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (cannador) for postoperative pain management. Anesthesiology 2006 05;104(0003-3022; 5):1040-6.
- Reference 271
-
Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain 2003 11;106(0304-3959; 1-2):169-72.
- Reference 272
-
Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain 2003 09;105(0304-3959; 1-2):79-88.
- Reference 273
-
Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend 2000 06/01;59(0376-8716; 3):261-75.
- Reference 274
-
Jain AK, Ryan JR, McMahon FG, Smith G. Evaluation of intramuscular levonantradol and placebo in acute postoperative pain. J Clin Pharmacol 1981 08;21(0091-2700; 8-9):320S-6S.
- Reference 275
-
Beaulieu P, Boulanger A, Desroches J, Clark AJ. Medical cannabis: Considerations for the anesthesiologist and pain physician. Can J Anaesth 2016 May;63(5):608-24.
- Reference 276
-
Wilsey B, Marcotte TD, Deutsch R, Zhao H, Prasad H, Phan A. An exploratory human laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease. J Pain 2016 Sep;17(9):982-1000.
- Reference 277
-
Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: A clinical review. JAMA 2015 Jun 23-30;313(24):2474-83.
- Reference 278
-
Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, Gouaux B. Smoked cannabis for spasticity in multiple sclerosis: A randomized, placebo-controlled trial. CMAJ 2012 07/10;184(1488-2329; 0820-3946; 10):1143-50.
- Reference 279
-
Lahat A, Lang A, Ben-Horin S. Impact of cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel disease patients: A pilot prospective study. Digestion 2012;85(1421-9867; 0012-2823; 1):1-8.
- Reference 280
-
Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther 2011 12;90(1532-6535; 0009-9236; 6):844-51.
- Reference 281
-
Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, Bentley H, Atkinson JH. Smoked medicinal cannabis for neuropathic pain in HIV: A randomized, crossover clinical trial. Neuropsychopharmacology 2009 02;34(1740-634; 0006-3223; 3):672-80.
- Reference 282
-
Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014 Jan;47(1):166-73.
- Reference 283
-
Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage 2012 11/07(1873-6513; 0885-3924).
- Reference 284
-
Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, McQuade R, Wright S, Fallon MT. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: A randomized, placebo-controlled, graded-dose trial. J.Pain 2012 05;13(1528-8447; 1526-5900; 5):438-49.
- Reference 285
-
Noyes R,Jr., Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther 1975 07;18(0009-9236; 1):84-9.
- Reference 286
-
Lynch ME, Young J, Clark AJ. A case series of patients using medicinal marihuana for management of chronic pain under the canadian marihuana medical access regulations. J Pain Symptom Manage 2006 11;32(0885-3924; 0885-3924; 5):497-501.
- Reference 287
-
Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, Jamison RN. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J.Pain 2008 03;9(1526-5900; 1526-5900; 3):254-64.
- Reference 288
-
Maida V, Ennis M, Irani S, Corbo M, Dolzhykov M. Adjunctive nabilone in cancer pain and symptom management: A prospective observational study using propensity scoring. J Support Oncol 2008 Mar;6(3):119-24.
- Reference 289
-
Rhyne DN, Anderson SL, Gedde M, Borgelt LM. Effects of medical marijuana on migraine headache frequency in an adult population. Pharmacotherapy 2016 Jan 9.
- Reference 290
-
Leroux E, Taifas I, Valade D, Donnet A, Chagnon M, Ducros A. Use of cannabis among 139 cluster headache sufferers. Cephalalgia 2012 11/29;33(1468-2982; 0333-1024; 3):208-13.
- Reference 291
-
Robbins MS, Tarshish S, Solomon S, Grosberg BM. Cluster attacks responsive to recreational cannabis and dronabinol. Headache 2009 06;49(1526-4610; 0017-8748; 6):914-6.
- Reference 292
-
Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain 2007 12;130(1460-2156; 0006-8950):3091-101.
- Reference 293
-
Evans RW, Ramadan NM. Are cannabis-based chemicals helpful in headache? Headache 2004 07;44(0017-8748; 0017-8748; 7):726-7.
- Reference 294
-
Sticht MA, Limebeer CL, Rafla BR, Abdullah RA, Poklis JL, Ho W, Niphakis MJ, Cravatt BF, Sharkey KA, Lichtman AH, et al. Endocannabinoid regulation of nausea is mediated by 2-arachidonoylglycerol (2-AG) in the rat visceral insular cortex. Neuropharmacology 2016 Mar;102:92-102.
- Reference 295
-
Parker LA, Rock EM, Sticht MA, Wills KL, Limebeer CL. Cannabinoids suppress acute and anticipatory nausea in preclinical rat models of conditioned gaping. Clin Pharmacol Ther 2015 Feb 17.
- Reference 296
-
Musty R, Rossi R. Effects of smoked cannabis and oral delta-9-tetrahydrocannabinol on nausea and emesis after cancer chemotherapy: A review of state clinical trials. J Cannabis Therapeutics 2001;1(1):29-42.
- Reference 297
-
Soderpalm AH, Schuster A, de WH. Antiemetic efficacy of smoked marijuana: Subjective and behavioral effects on nausea induced by syrup of ipecac. Pharmacol Biochem Behav 2001 07;69(0091-3057; 0091-3057; 3-4):343-50.
- Reference 298
-
Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, Vosburg SK, Haney M. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: A controlled laboratory study. Psychopharmacology (Berl) 2010 12;212(1432-2072; 0033-3158; 4):675-86.
- Reference 299
-
Sannarangappa V, Tan C. Cannabinoid hyperemesis. Intern Med J 2009 11;39(1445-5994; 1444-0903; 11):777-8.
- Reference 300
-
Donnino MW, Cocchi MN, Miller J, Fisher J. Cannabinoid hyperemesis: A case series. J Emerg Med 2011 04;40(0736-4679; 0736-4679; 4):e63-6.
- Reference 301
-
Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol 2010 05;24(0835-7900; 0835-7900; 5):284-5.
- Reference 302
-
Miller JB, Walsh M, Patel PA, Rogan M, Arnold C, Maloney M, Donnino M. Pediatric cannabinoid hyperemesis: Two cases. Pediatr Emerg Care 2010 12;26(1535-1815; 0749-5161; 12):919-20.
- Reference 303
-
Patterson DA, Smith E, Monahan M, Medvecz A, Hagerty B, Krijger L, Chauhan A, Walsh M. Cannabinoid hyperemesis and compulsive bathing: A case series and paradoxical pathophysiological explanation. J.Am.Board Fam.Med. 2010 11;23(1557-2625; 1557-2625; 6):790-3.
- Reference 304
-
Choung RS, Locke GR,III, Lee RM, Schleck CD, Zinsmeister AR, Talley NJ. Cyclic vomiting syndrome and functional vomiting in adults: Association with cannabinoid use in males. Neurogastroenterol Motil 2012 01;24(1365-2982; 1350-1925; 1):20,6, e1.
- Reference 305
-
Francis H. Emerging role of chronic cannabis usage and hyperemesis syndrome. South Med J 2011 09;104(1541-8243; 0038-4348; 9):665.
- Reference 306
-
Schmid SM, Lapaire O, Huang DJ, Jurgens FE, Guth U. Cannabinoid hyperemesis syndrome: An underreported entity causing nausea and vomiting of pregnancy. Arch Gynecol Obstet 2011 11;284(1432-0711; 0932-0067; 5):1095-7.
- Reference 307
-
Wallace EA, Andrews SE, Garmany CL, Jelley MJ. Cannabinoid hyperemesis syndrome: Literature review and proposed diagnosis and treatment algorithm. South Med J 2011 09;104(1541-8243; 0038-4348; 9):659-64.
- Reference 308
-
Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: A case series of 98 patients. Mayo Clin Proc 2012 02;87(1942-5546; 0025-6196; 2):114-9.
- Reference 309
-
Wild K, Wilson H. Cannabinoid hyperemesis. Emerg Med J 2012 01;29(1472-0213; 1472-0205; 1):67-9.
- Reference 310
-
Pisanti S, Malfitano AM, Grimaldi C, Santoro A, Gazzerro P, Laezza C, Bifulco M. Use of cannabinoid receptor agonists in cancer therapy as palliative and curative agents. Best.Pract.Res.Clin.Endocrinol.Metab 2009 02;23(1532-1908; 1521-690; 1):117-31.
- Reference 311
-
Sutton IR, Daeninck P. Cannabinoids in the management of intractable chemotherapy-induced nausea and vomiting and cancer-related pain. J.Support.Oncol. 2006 11;4(1544-6794; 1544-6794; 10):531-5.
- Reference 312
-
Mattes RD, Engelman K, Shaw LM, Elsohly MA. Cannabinoids and appetite stimulation. Pharmacol Biochem Behav 1994 09;49(0091-3057; 1):187-95.
- Reference 313
-
Foltin RW, Fischman MW, Byrne MF. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite 1988 08;11(0195-6663; 1):1-14.
- Reference 314
-
Reuter SE, Martin JH. Pharmacokinetics of cannabis in cancer cachexia-anorexia syndrome. Clin Pharmacokinet 2016 Feb 16.
- Reference 315
-
Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, Ko YD, Schnelle M, Reif M, Cerny T. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the cannabis-in-cachexia-study-group. J Clin Oncol 2006 07/20;24(1527-7755; 21):3394-400.
- Reference 316
-
Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, Pundaleeka S, Kardinal CG, Fitch TR, Krook JE, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: A north central cancer treatment group study. J Clin Oncol 2002 01/15;20(0732-183; 2):567-73.
- Reference 317
-
Nelson K, Walsh D, Deeter P, Sheehan F. A phase II study of delta-9-tetrahydrocannabinol for appetite stimulation in cancer-associated anorexia. J Palliat Care 1994;10(0825-8597; 1):14-8.
- Reference 318
-
Plasse TF, Gorter RW, Krasnow SH, Lane M, Shepard KV, Wadleigh RG. Recent clinical experience with dronabinol. Pharmacol Biochem Behav 1991 11;40(0091-3057; 3):695-700.
- Reference 319
-
Sallan SE, Cronin C, Zelen M, Zinberg NE. Antiemetics in patients receiving chemotherapy for cancer: A randomized comparison of delta-9-tetrahydrocannabinol and prochlorperazine. N Engl J Med 1980 01/17;302(0028-4793; 3):135-8.
- Reference 320
-
Ekert H, Waters KD, Jurk IH, Mobilia J, Loughnan P. Amelioration of cancer chemotherapy-induced nausea and vomiting by delta-9-tetrahydrocannabinol. Med J Aust 1979 12/15;2(0025-729; 12):657-9.
- Reference 321
-
Regelson W, Butler JR, Schulz J. Delta-9-tetrahydrocannabinol as an effective antidepressant and appetite-stimulating agent in advanced cancer patients. In: M. Braude, S. Szara, editors. The pharmacology of marihuana: A monograph of the national institute on drug abuse. New York: Raven Press; 1976. ID: 2375; RP: NOT IN FILE.
- Reference 322
-
Andries A, Frystyk J, Flyvbjerg A, Stoving RK. Dronabinol in severe, enduring anorexia nervosa: A randomized controlled trial. Int J Eat Disord 2014 Jan;47(1):18-23.
- Reference 323
-
Gross H, Ebert MH, Faden VB, Goldberg SC, Kaye WH, Caine ED, Hawks R, Zinberg N. A double-blind trial of delta 9-tetrahydrocannabinol in primary anorexia nervosa. J Clin Psychopharmacol 1983 06;3(0271-0749; 3):165-71.
- Reference 324
-
Gorelick DA, Goodwin RS, Schwilke E, Schwope DM, Darwin WD, Kelly DL, McMahon RP, Liu F, Ortemann-Renon C, Bonnet D, et al. Tolerance to effects of high-dose oral delta9-tetrahydrocannabinol and plasma cannabinoid concentrations in male daily cannabis smokers. J Anal Toxicol 2013 Jan-Feb;37(1):11-6.
- Reference 325
-
Serpell MG, Notcutt W, Collin C. Sativex long-term use: An open-label trial in patients with spasticity due to multiple sclerosis. J Neurol 2012 08/10;260(1432-1459; 0340-5354; 1):285-95.
- Reference 326
-
D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology 2008 09;33(1740-634; 0006-3223; 10):2505-16.
- Reference 327
-
Rog DJ, Nurmikko TJ, Young CA. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: An uncontrolled, open-label, 2-year extension trial. Clin Ther 2007 09;29(0149-2918; 0149-2918; 9):2068-79.
- Reference 328
-
Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: A review of studies in laboratory animals. Pharmacol Biochem Behav 2005 06;81(0091-3057; 0091-3057; 2):300-18.
- Reference 329
-
Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handb Exp Pharmacol 2005(0171-2004; 0171-2004; 168):691-717.
- Reference 330
-
De Vry J, Jentzsch KR, Kuhl E, Eckel G. Behavioral effects of cannabinoids show differential sensitivity to cannabinoid receptor blockade and tolerance development. Behav Pharmacol 2004 02;15(0955-8810; 0955-8810; 1):1-12.
- Reference 331
-
Pertwee R. Tolerance to and dependence on psychotropic cannabinoids. In: J. Pratt, editor. The biological bases of drug tolerance and dependence. London: Academic Press; 1991. ID: 2395; RP: NOT IN FILE.
- Reference 332
-
Compton DR, Dewey WL, Martin BR. Cannabis dependence and tolerance production. Adv Alcohol Subst Abuse 1990;9(0270-3106; 1-2):129-47.
- Reference 333
-
Jones RT, Benowitz N, Bachman J. Clinical studies of cannabis tolerance and dependence. Ann N Y Acad Sci 1976;282(0077-8923):221-39.
- Reference 334
-
D'Souza DC, Cortes-Briones JA, Ranganathan M, Thurnauer H, Creatura G, Surti T, Planeta B, Neumeister A, Pittman B, Normandin M, et al. Rapid changes in CB1 receptor availability in cannabis dependent males after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimaging 2016 Jan 1;1(1):60-7.
- Reference 335
-
Cooper ZD, Haney M. Cannabis reinforcement and dependence: Role of the cannabinoid CB1 receptor. Addict Biol 2008 06;13(1369-1600; 1355-6215; 2):188-95.
- Reference 336
-
Hall W, Solowij N. The adverse health and psychological consequences of cannabis dependence. In: R. A. Roffman, R. S. Stephens, editors. Cannabis dependence. Cambridge: Cambridge University Press; 2006. ID: 2645; RP: NOT IN FILE.
- Reference 337
-
Kalant H. Adverse effects of cannabis on health: An update of the literature since 1996. Prog Neuropsychopharmacol Biol Psychiatry 2004 08;28(0278-5846; 0278-5846; 5):849-63.
- Reference 338
-
Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, Jung J, Zhang H, Grant BF. Prevalence and correlates of DSM-5 cannabis use disorder, 2012-2013: Findings from the national epidemiologic survey on alcohol and related conditions-III. Am J Psychiatry 2016 Jun 1;173(6):588-99.
- Reference 339
-
Trigo JM, Lagzdins D, Rehm J, Selby P, Gamaleddin I, Fischer B, Barnes AJ, Huestis MA, Le Foll B. Effects of fixed or self-titrated dosages of sativex on cannabis withdrawal and cravings. Drug Alcohol Depend 2016 Apr 1;161:298-306.
- Reference 340
-
Garcia AN, Salloum IM. Polysomnographic sleep disturbances in nicotine, caffeine, alcohol, cocaine, opioid, and cannabis use: A focused review. Am J Addict 2015 Oct;24(7):590-8.
- Reference 341
-
Prud'homme M, Cata R, Jutras-Aswad D. Cannabidiol as an intervention for addictive behaviors: A systematic review of the evidence. Subst Abuse 2015 May 21;9:33-8.
- Reference 342
-
Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The cannabis withdrawal scale development: Patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend 2011 12/01;119(1879-0046; 0376-8716; 1-2):123-9.
- Reference 343
-
Renault PF, Schuster CR, Heinrich R, Freeman DX. Marihuana: Standardized smoke administration and dose effect curves on heart rate in humans. Science 1971 11/05;174(0036-8075; 0036-8075; 4009):589-91.
- Reference 344
-
Clark SC, Greene C, Karr GW, MacCannell KL, Milstein SL. Cardiovascular effects of marihuana in man. Can J Physiol Pharmacol 1974 06;52(0008-4212; 0008-4212; 3):706-19.
- Reference 345
-
O'Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, Ponto LB, Watkins GL, Hurtig RR, Hichwa RD. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology 2002 06;26(0893-133; 0006-3223; 6):802-16.
- Reference 346
-
Trouve R, Nahas G. Cardiovascular effects of marihuana and cannabinoids. In: C. G. Nahas, K. M. Sutin, D. J. Harvey, S. Agurell, editors. Marihuana and medicine. Totowa, New Jersey: Humana Press; 1999. ID: 2389; RP: NOT IN FILE.
- Reference 347
-
Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol 2002 11;42(0091-2700; 11):58S-63S.
- Reference 348
-
Hollister LE. Health aspects of cannabis. Pharmacol Rev 1986 03;38(0031-6997; 1):1-20.
- Reference 349
-
Miller RH, Dhingra RC, Kanakis C,Jr., Leon F, Rosen KM. The electrophysiological effects of delta-9-tetrahydrocannabinol (cannabis) on cardiac conduction in man. Am Heart J 1977 12;94(0002-8703; 0002-8703; 6):740-7.
- Reference 350
-
Lindsay AC, Foale RA, Warren O, Henry JA. Cannabis as a precipitant of cardiovascular emergencies. Int J Cardiol 2005 09/30;104(0167-5273; 0167-5273; 2):230-2.
- Reference 351
-
Beaconsfield P. Some cardiovascular effects of cannabis. Am Heart J 1974 02;87(0002-8703; 0002-8703; 2):143-6.
- Reference 352
-
Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation 2001 06/12;103(1524-4539; 23):2805-9.
- Reference 353
-
Mathew RJ, Wilson WH, Davis R. Postural syncope after marijuana: A transcranial doppler study of the hemodynamics. Pharmacol Biochem Behav 2003 05;75(0091-3057; 0091-3057; 2):309-18.
- Reference 354
-
Gorelick DA, Heishman SJ. Methods for clinical research involving cannabis administration. Methods Mol Med 2006;123(1543-1894; 1543-1894):235-53.
- Reference 355
-
Lundqvist T. Cognitive consequences of cannabis use: Comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav 2005 06;81(0091-3057; 2):319-30.
- Reference 356
-
Singh NN, Pan Y, Muengtaweeponsa S, Geller TJ, Cruz-Flores S. Cannabis-related stroke: Case series and review of literature. J.Stroke Cerebrovasc.Dis. 2012 10;21(1532-8511; 1052-3057; 7):555-60.
- Reference 357
-
Renard D, Taieb G, Gras-Combe G, Labauge P. Cannabis-related myocardial infarction and cardioembolic stroke. J.Stroke Cerebrovasc.Dis. 2012 01;21(1532-8511; 1052-3057; 1):82-3.
- Reference 358
-
Sidney S, Quesenberry Jr CP, Friedman GD, Tekawa IS. Marijuana use and cancer incidence (california, united states). Cancer Causes Control 1997;8:722-8.
- Reference 359
-
Zhang ZF, Morgenstern H, Spitz MR, Tashkin DP, Yu GP, Marshall JR, Hsu TC, Schantz SP. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev 1999 12;8(1055-9965; 12):1071-8.
- Reference 360
-
Hashibe M, Morgenstern H, Cui Y, Tashkin DP, Zhang ZF, Cozen W, Mack TM, Greenland S. Marijuana use and the risk of lung and upper aerodigestive tract cancers: Results of a population-based case-control study. Cancer Epidemiol Biomarkers Prev 2006 10;15(1055-9965; 10):1829-34.
- Reference 361
-
Aldington S, Harwood M, Cox B, Weatherall M, Beckert L, Hansell A, Pritchard A, Robinson G, Beasley R. Cannabis use and risk of lung cancer: A case-control study. Eur Respir J 2008 02;31(1399-3003; 0903-1936; 2):280-6.
- Reference 362
-
Gurney J, Shaw C, Stanley J, Signal V, Sarfati D. Cannabis exposure and risk of testicular cancer: A systematic review and meta-analysis. BMC Cancer 2015 Nov 11;15:897,015-1905-6.
- Reference 363
-
Fligiel SE, Roth MD, Kleerup EC, Barsky SH, Simmons MS, Tashkin DP. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest 1997 08;112(0012-3692; 0012-3692; 2):319-26.
- Reference 364
-
Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: A systematic review. Arch Intern Med 2007 02/12;167(0003-9926; 0003-9926; 3):221-8.
- Reference 365
-
Ocampo TL, Rans TS. Cannabis sativa: The unconventional "weed" allergen. Ann Allergy Asthma Immunol 2015 Mar;114(3):187-92.
- Reference 366
-
Tessmer A, Berlin N, Sussman G, Leader N, Chung EC, Beezhold D. Hypersensitivity reactions to marijuana. Ann Allergy Asthma Immunol 2012 Apr;108(4):282-4.
- Reference 367
-
Bloom JW, Kaltenborn WT, Paoletti P, Camilli A, Lebowitz MD. Respiratory effects of non-tobacco cigarettes. Br Med J (Clin Res Ed) 1987 12/12;295(0267-0623; 6612):1516-8.
- Reference 368
-
Tashkin DP, Coulson AH, Clark VA, Simmons M, Bourque LB, Duann S, Spivey GH, Gong H. Respiratory symptoms and lung function in habitual heavy smokers of marijuana alone, smokers of marijuana and tobacco, smokers of tobacco alone, and nonsmokers. Am Rev Respir Dis 1987 01;135(0003-0805; 1):209-16.
- Reference 369
-
Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons M, Tashkin DP. Airway inflammation in young marijuana and tobacco smokers. Am J Respir Crit Care Med 1998 03;157(1073-449; 3):928-37.
- Reference 370
-
Pletcher MJ, Vittinghoff E, Kalhan R, Richman J, Safford M, Sidney S, Lin F, Kertesz S. Association between marijuana exposure and pulmonary function over 20 years. JAMA 2012 01/11;307(1538-3598; 0098-7484; 2):173-81.
- Reference 371
-
Macleod J, Robertson R, Copeland L, McKenzie J, Elton R, Reid P. Cannabis, tobacco smoking, and lung function: A cross-sectional observational study in a general practice population. Br J Gen Pract 2015 Feb;65(631):e89-95.
- Reference 372
-
Naftali T, Lev LB, Yablecovitch D, Half E, Konikoff FM. Treatment of crohn's disease with cannabis: An observational study. Isr Med Assoc J 2011 08;13(1565-1088; 8):455-8.
- Reference 373
-
Patsenker E, Stoll M, Millonig G, Agaimy A, Wissniowski T, Schneider V, Mueller S, Brenneisen R, Seitz HK, Ocker M, et al. Cannabinoid receptor type I modulates alcohol-induced liver fibrosis. Mol Med 2011;17(1528-3658; 1076-1551; 11-12):1285-94.
- Reference 374
-
Trebicka J, Racz I, Siegmund SV, Cara E, Granzow M, Schierwagen R, Klein S, Wojtalla A, Hennenberg M, Huss S, et al. Role of cannabinoid receptors in alcoholic hepatic injury: Steatosis and fibrogenesis are increased in CB2 receptor-deficient mice and decreased in CB1 receptor knockouts. Liver Int. 2011 07;31(1478-3231; 1478-3223; 6):860-70.
- Reference 375
-
Reichenbach V, Ros J, Fernandez-Varo G, Casals G, Melgar-Lesmes P, Campos T, Makriyannis A, Morales-Ruiz M, Jimenez W. Prevention of fibrosis progression in CCl4-treated rats: Role of the hepatic endocannabinoid and apelin systems. J Pharmacol Exp Ther 2012 03;340(1521-0103; 0022-3565; 3):629-37.
- Reference 376
-
Sylvestre DL, Clements BJ, Malibu Y. Cannabis use improves retention and virological outcomes in patients treated for hepatitis C. Eur J Gastroenterol Hepatol 2006 10;18(0954-691; 0954-691; 10):1057-63.
- Reference 377
-
Grant P, Gandhi P. A case of cannabis-induced pancreatitis. JOP. 2004 01;5(1590-8577; 1590-8577; 1):41-3.
- Reference 378
-
Wargo KA, Geveden BN, McConnell VJ. Cannabinoid-induced pancreatitis: A case series. JOP. 2007;8(1590-8577; 1590-8577; 5):579-83.
- Reference 379
-
Bournet B, Buscail L. [Cannabis: A rare cause of acute pancreatitis]. Gastroenterol Clin Biol 2008 11;32(0399-8320; 0399-8320; 11):922-3.
- Reference 380
-
Belze O,Jr., Legras A, Ehrmann S, Garot D, Perrotin D. Cannabis-induced acute pancreatitis. Am J Emerg Med 2011 01;29(1532-8171; 0735-6757; 1):131-4.
- Reference 381
-
Barkin JA, Nemeth Z, Saluja AK, Barkin JS. Cannabis-induced acute pancreatitis: A systematic review. Pancreas 2017 Sep;46(8):1035-8.
- Reference 382
-
Smith FL, Fujimori K, Lowe J, Welch SP. Characterization of delta9-tetrahydrocannabinol and anandamide antinociception in nonarthritic and arthritic rats. Pharmacol Biochem Behav 1998 05;60(0091-3057; 0091-3057; 1):183-91.
- Reference 383
-
Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006 01;45(1462-0324; 1):50-2.
- Reference 384
-
Cox ML, Haller VL, Welch SP. Synergy between delta9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacol 2007 Jul 12;567(1-2):125-30.
- Reference 385
-
Schley M, Legler A, Skopp G, Schmelz M, Konrad C, Rukwied R. Delta-9-THC based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare, and pain relief. Curr Med Res Opin 2006 07;22(0300-7995; 0300-7995; 7):1269-76.
- Reference 386
-
Weber J, Schley M, Casutt M, Gerber H, Schuepfer G, Rukwied R, Schleinzer W, Ueberall M, Konrad C. Tetrahydrocannabinol (delta 9-THC) treatment in chronic central neuropathic pain and fibromyalgia patients: Results of a multicenter survey. Anesthesiol.Res.Pract. 2009;2009(1687-6970; 1687-6962; 2009):827290.
- Reference 387
-
Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, Thompson A. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomised placebo-controlled trial. Lancet 2003 11/08;362(1474-547; 9395):1517-26.
- Reference 388
-
Nogueira-Filho GR, Cadide T, Rosa BT, Neiva TG, Tunes R, Peruzzo D, Nociti FH,Jr., Cesar-Neto JB. Cannabis sativa smoke inhalation decreases bone filling around titanium implants: A histomorphometric study in rats. Implant Dent 2008 12;17(1538-2982; 1056-6163; 4):461-70.
- Reference 389
-
Tomida I, Azuara-Blanco A, House H, Flint M, Pertwee RG, Robson PJ. Effect of sublingual application of cannabinoids on intraocular pressure: A pilot study. J Glaucoma 2006 10;15(1057-0829; 5):349-53.
- Reference 390
-
Tomida I, Pertwee RG, zuara-Blanco A. Cannabinoids and glaucoma. Br J Ophthalmol 2004 05;88(0007-1161; 5):708-13.
- Reference 391
-
Flach AJ. Delta-9-tetrahydrocannabinol (THC) in the treatment of end-stage open-angle glaucoma. Trans Am Ophthalmol Soc 2002;100(0065-9533; 0065-9533):215-22.
- Reference 392
-
Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: An overview. Immunobiology 2010 08;215(1878-3279; 0171-2985; 8):588-97.
- Reference 393
-
Decuyper I, Ryckebosch H, Van Gasse AL, Sabato V, Faber M, Bridts CH, Ebo DG. Cannabis allergy: What do we know anno 2015. Arch Immunol Ther Exp (Warsz) 2015 Oct;63(5):327-32.
- Reference 394
-
Faber M, Van Gasse A, Sabato V, Hagendorens MM, Bridts CH, De Clerck LS, Ebo DG. Marihuana allergy: Beyond the joint. J Investig Allergol Clin Immunol 2015;25(1):70-2.
- Reference 395
-
du Plessis SS, Agarwal A, Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet 2015 Nov;32(11):1575-88.
- Reference 396
-
Gundersen TD, Jorgensen N, Andersson AM, Bang AK, Nordkap L, Skakkebaek NE, Priskorn L, Juul A, Jensen TK. Association between use of marijuana and male reproductive hormones and semen quality: A study among 1,215 healthy young men. Am J Epidemiol 2015 Sep 15;182(6):473-81.
- Reference 397
-
Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav 2010 06;58(1095-6867; 0018-506; 1):91-9.
- Reference 398
-
Sun AJ, Eisenberg ML. Association between marijuana use and sexual frequency in the united states: A population-based study. J Sex Med 2017 Nov;14(11):1342-7.
- Reference 399
-
Brents LK. Marijuana, the endocannabinoid system and the female reproductive system. Yale J Biol Med 2016 Jun 27;89(2):175-91.
- Reference 400
-
Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 2003;42(0312-5963; 4):327-60.
- Reference 401
-
Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, Hollister L. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev 1986 03;38(0031-6997; 1):21-43.
- Reference 402
-
Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: A pilot study. Clin Pharmacol Ther 2007 04/11;82(0009-9236; 5):572-8.
- Reference 403
-
McClure EA, Stitzer ML, Vandrey R. Characterizing smoking topography of cannabis in heavy users. Psychopharmacology (Berl) 2012 03;220(1432-2072; 0033-3158; 2):309-18.
- Reference 404
-
Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb Exp Pharmacol 2005(0171-2004; 168):657-90.
- Reference 405
-
Carter GT, Weydt P, Kyashna-Tocha M, Abrams DI. Medicinal cannabis: Rational guidelines for dosing. IDrugs. 2004 05;7(1369-7056; 5):464-70.
- Reference 406
-
Adams IB, Martin BR. Cannabis: Pharmacology and toxicology in animals and humans. Addiction 1996 Nov;91(11):1585-614.
- Reference 407
-
Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther 1980 09;28(0009-9236; 3):409-16.
- Reference 408
-
Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend 2009 08/01;103(1879-0046; 0376-8716; 3):107-13.
- Reference 409
-
Schwope DM, Bosker WM, Ramaekers JG, Gorelick DA, Huestis MA. Psychomotor performance, subjective and physiological effects and whole blood delta(9)-tetrahydrocannabinol concentrations in heavy, chronic cannabis smokers following acute smoked cannabis. J Anal Toxicol 2012 07;36(1945-2403; 0146-4760; 6):405-12.
- Reference 410
-
Ohlsson A, Lindgren JE, Andersson S, Agurell S, Gillespie H, Hollister LE. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed Environ Mass Spectrom 1986 Feb;13(2):77-83.
- Reference 411
-
Gieringer DH. Cannabis "vaporization". J Cannabis Therapeutics 2001;1(3):153-70.
- Reference 412
-
Gieringer D, St Laurent J, Goodrich S. Cannabis vaporizer combines efficient delivery of THC with effective suppression of pyrolytic compounds. J Cannabis Therapeutics 2004;4(1):7-27.
- Reference 413
-
Hazekamp A, Ruhaak R, Zuurman L, van GJ, Verpoorte R. Evaluation of a vaporizing device (volcano) for the pulmonary administration of tetrahydrocannabinol. J Pharm Sci 2006 06;95(0022-3549; 6):1308-17.
- Reference 414
-
Pomahacova B, Van der Kooy F, Verpoorte R. Cannabis smoke condensate III: The cannabinoid content of vaporised cannabis sativa. Inhal Toxicol 2009 11;21(1091-7691; 0895-8378; 13):1108-12.
- Reference 415
-
Ramaekers JG, van Wel JH, Spronk DB, Toennes SW, Kuypers KP, Theunissen EL, Verkes RJ. Cannabis and tolerance: Acute drug impairment as a function of cannabis use history. Sci Rep 2016 May 26;6:26843.
- Reference 416
-
Wilsey BL, Deutsch R, Samara E, Marcotte TD, Barnes AJ, Huestis MA, Le D. A preliminary evaluation of the relationship of cannabinoid blood concentrations with the analgesic response to vaporized cannabis. J Pain Res 2016 Aug 31;9:587-98.
- Reference 417
-
Walsh D, Nelson KA, Mahmoud FA. Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer 2003 03;11(0941-4355; 0941-4355; 3):137-43.
- Reference 418
-
Cone EJ, Johnson RE, Paul BD, Mell LD, Mitchell J. Marijuana-laced brownies: Behavioral effects, physiologic effects, and urinalysis in humans following ingestion. J Anal Toxicol 1988 07;12(0146-4760; 4):169-75.
- Reference 419
-
Iversen LL. The pharmacology of THC, the psychoactive ingredient in cannabis. In: The science of marijuana. New York, New York;: Oxford University Press; 2000. ID: 2348; RP: NOT IN FILE.
- Reference 420
-
Schwilke EW, Schwope DM, Karschner EL, Lowe RH, Darwin WD, Kelly DL, Goodwin RS, Gorelick DA, Huestis MA. Delta9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin Chem 2009 12;55(1530-8561; 0009-9147; 12):2180-9.
- Reference 421
-
Ahmed AI, van den Elsen GA, Colbers A, Kramers C, Burger DM, van der Marck MA, Olde Rikkert MG. Safety, pharmacodynamics, and pharmacokinetics of multiple oral doses of delta-9-tetrahydrocannabinol in older persons with dementia. Psychopharmacology (Berl) 2015 Jul;232(14):2587-95.
- Reference 422
-
Office of Medicinal Cannabis,The Netherlands Ministry of Health,Welfare and Sports. Medicinal cannabis, information for health care professionals. 2008.
- Reference 423
-
Agurell S, Carlsson S, Lindgren JE, Ohlsson A, Gillespie H, Hollister L. Interactions of delta 1-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. assay of cannabinol and cannabidiol by mass fragmentography. Experientia 1981 Oct 15;37(10):1090-2.
- Reference 424
-
Consroe P, Kennedy K, Schram K. Assay of plasma cannabidiol by capillary gas chromatography/ion trap mass spectroscopy following high-dose repeated daily oral administration in humans. Pharmacol Biochem Behav 1991 Nov;40(3):517-22.
- Reference 425
-
Nadulski T, Sporkert F, Schnelle M, Stadelmann AM, Roser P, Schefter T, Pragst F. Simultaneous and sensitive analysis of THC, 11-OH-THC, THC-COOH, CBD, and CBN by GC-MS in plasma after oral application of small doses of THC and cannabis extract. J Anal Toxicol 2005 Nov-Dec;29(8):782-9.
- Reference 426
-
Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel) 2012 May 21;5(5):529-52.
- Reference 427
-
Zgair A, Wong JC, Lee JB, Mistry J, Sivak O, Wasan KM, Hennig IM, Barrett DA, Constantinescu CS, Fischer PM, et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicine. Am J Transl Res 2016;8(8):3448-59.
- Reference 428
-
Merrick J, Lane B, Sebree T, Yaksh T, O'Neill C, Banks SL. Identification of psychoactive degradants of cannabidiol in simulated gastric and physiological fluid. Cannabis and Cannabinoid Research 2016;1(1):102-12.
- Reference 429
-
Watanabe K, Itokawa Y, Yamaori S, Funahashi T, Kimura T, Usami N, Yamamoto I. Conversion of cannabidiol to Δ9-tetrahydrocannabinol and related cannabinoids in artificial gastric juice, and their pharmacological effects in mice. Journal of Forensic Toxicology 2007;25(1):16-21.
- Reference 430
-
Newmeyer MN, Swortwood MJ, Barnes AJ, Abulseoud OA, Scheidweiler KB, Huestis MA. Free and glucuronide whole blood cannabinoids' pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: Identification of recent cannabis intake. Clinical Chemistry 2016;62(12):1579-92.
- Reference 431
-
Pharmaceuticals G. Sativex product monograph. 2010.
- Reference 432
-
Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler 2004 08;10(1352-4585; 4):434-41.
- Reference 433
-
Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: A randomised, double-blind, placebo-controlled clinical trial. Pain 2007 12/15;133(1872-6623; 0304-3959; 1-3):210-20.
- Reference 434
-
Al-Ghananeem AM, Malkawi AH, Crooks PA. Bioavailability of delta(9)-tetrahydrocannabinol following intranasal administration of a mucoadhesive gel spray delivery system in conscious rabbits. Drug Dev Ind Pharm 2011 Mar;37(3):329-34.
- Reference 435
-
Paudel KS, Hammell DC, Agu RU, Valiveti S, Stinchcomb AL. Cannabidiol bioavailability after nasal and transdermal application: Effect of permeation enhancers. Drug Dev Ind Pharm 2010 Sep;36(9):1088-97.
- Reference 436
-
Brenneisen R, Egli A, Elsohly MA, Henn V, Spiess Y. The effect of orally and rectally administered delta 9-tetrahydrocannabinol on spasticity: A pilot study with 2 patients. Int J Clin Pharmacol Ther 1996 10;34(0946-1965; 10):446-52.
- Reference 437
-
Mattes RD, Shaw LM, Edling-Owens J, Engelman K, Elsohly MA. Bypassing the first-pass effect for the therapeutic use of cannabinoids. Pharmacol Biochem Behav 1993 03;44(0091-3057; 3):745-7.
- Reference 438
-
Perlin E, Smith CG, Nichols AI, Almirez R, Flora KP, Cradock JC, Peck CC. Disposition and bioavailability of various formulations of tetrahydrocannabinol in the rhesus monkey. J Pharm Sci 1985 02;74(0022-3549; 0022-3549; 2):171-4.
- Reference 439
-
Elsohly MA, Little TL,Jr., Hikal A, Harland E, Stanford DF, Walker L. Rectal bioavailability of delta-9-tetrahydrocannabinol from various esters. Pharmacol Biochem Behav 1991 11;40(0091-3057; 0091-3057; 3):497-502.
- Reference 440
-
Elsohly MA, Stanford DF, Harland EC, Hikal AH, Walker LA, Little TL,Jr., Rider JN, Jones AB. Rectal bioavailability of delta-9-tetrahydrocannabinol from the hemisuccinate ester in monkeys. J Pharm Sci 1991 10;80(0022-3549; 0022-3549; 10):942-5.
- Reference 441
-
Valiveti S, Hammell DC, Earles DC, Stinchcomb AL. Transdermal delivery of the synthetic cannabinoid WIN 55,212-2: In vitro/in vivo correlation. Pharm Res 2004 07;21(0724-8741; 0724-8741; 7):1137-45.
- Reference 442
-
Valiveti S, Kiptoo PK, Hammell DC, Stinchcomb AL. Transdermal permeation of WIN 55,212-2 and CP 55,940 in human skin in vitro. Int J Pharm 2004 06/18;278(0378-5173; 0378-5173; 1):173-80.
- Reference 443
-
Stinchcomb AL, Valiveti S, Hammell DC, Ramsey DR. Human skin permeation of Delta8-tetrahydrocannabinol, cannabidiol and cannabinol. J Pharm Pharmacol 2004 03;56(0022-3573; 0022-3573; 3):291-7.
- Reference 444
-
Hammell DC, Zhang LP, Ma F, Abshire SM, McIlwrath SL, Stinchcomb AL, Westlund KN. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain 2016 Jul;20(6):936-48.
- Reference 445
-
Giacoppo S, Galuppo M, Pollastro F, Grassi G, Bramanti P, Mazzon E. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru 2015 Oct 21;23:48,015-0131-8.
- Reference 446
-
Harvey DJ. Absorption, distribution and biotransformation of the cannabinoids. In: C. G. Nahas, K. M. Sutin, D. J. Harvey, S. Agurell, editors. Marihuana and medicine. Totowa, New Jersey: Humana Press; 1999. ID: 2363; RP: NOT IN FILE.
- Reference 447
-
Johansson E, Agurell S, Hollister LE, Halldin MM. Prolonged apparent half-life of delta 1-tetrahydrocannabinol in plasma of chronic marijuana users. J Pharm Pharmacol 1988 05;40(0022-3573; 5):374-5.
- Reference 448
-
Widman M, Agurell S, Ehrnebo M, Jones G. Binding of (+)- and (minus)-delta-1-tetrahydrocannabinols and (minus)-7-hydroxy-delta-1-tetrahydrocannabinol to blood cells and plasma proteins in man. J Pharm Pharmacol 1974 11;26(0022-3573; 11):914-6.
- Reference 449
-
Garrett ER, Hunt CA. Pharmacokinetics of delta9-tetrahydrocannabinol in dogs. J Pharm Sci 1977 03;66(0022-3549; 3):395-407.
- Reference 450
-
Wahlqvist M, Nilsson IM, Sandberg F, Agurell S. Binding of delta-1-tetrahydrocannabinol to human plasma proteins. Biochem Pharmacol 1970 09;19(0006-2952; 9):2579-84.
- Reference 451
-
Widman M, Nilsson IM, Agurell S, Borg H, Granstrand B. Plasma protein binding of 7-hydroxy- 1-tetrahydrocannabinol: An active 1-tetrahydrocannabinol metabolite. J Pharm Pharmacol 1973 06;25(0022-3573; 6):453-7.
- Reference 452
-
Truitt EB,Jr. Biological disposition of tetrahydrocannabinols. Pharmacol Rev 1971 12;23(0031-6997; 4):273-8.
- Reference 453
-
Nahas GG. The pharmacokinetics of THC in fat and brain: Resulting functional responses to marihuana smoking. Hum.Psychopharmacol. 2001 04;16(1099-1077; 0885-6222; 3):247-55.
- Reference 454
-
Schou J, Prockop LD, Dahlstrom G, Rohde C. Penetration of delta-9-tetrahydrocannabinol and 11-OH-delta-9-tetrahydrocannabinol through the blood-brain barrier. Acta Pharmacol Toxicol 1977 07;41(0001-6683; 0001-6683; 1):33-8.
- Reference 455
-
Perez-Reyes M, Simmons J, Brine D, Davis KH, Wall ME. Rate of penetration of delta-9-tetrahydrocannabinol and 11-hydroxy-delta-9-tetrahydrocannabinol to the brain of mice. In: Nahas,G.G., Patton, D.M., editor. Marihuana: Chemistry, biochemistry, and cellular effects. New York: Springer Berlin Heidelberg; 1976..
- Reference 456
-
Grotenhermen F. Clinical pharmacokinetics of cannabinoids. In: E. B. Russo, F. Grotenhermen, editors. Handbook of cannabis therapeutics: From bench to bedside. New York: Routledge: Taylor and Francis Group; 2006..
- Reference 457
-
Pope HG,Jr., Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. J Clin Pharmacol 2002 11;42(0091-2700; 0091-2700; 11):41S-7S.
- Reference 458
-
Mura P, Kintz P, Dumestre V, Raul S, Hauet T. THC can be detected in brain while absent in blood. J Anal Toxicol 2005 Nov-Dec;29(8):842-3.
- Reference 459
-
Bergamaschi MM, Karschner EL, Goodwin RS, Scheidweiler KB, Hirvonen J, Queiroz RH, Huestis MA. Impact of prolonged cannabinoid excretion in chronic daily cannabis smokers' blood on per se drugged driving laws. Clin Chem 2013 Mar;59(3):519-26.
- Reference 460
-
Gunasekaran N, Long LE, Dawson BL, Hansen GH, Richardson DP, Li KM, Arnold JC, McGregor IS. Reintoxication: The release of fat-stored delta(9)-tetrahydrocannabinol (THC) into blood is enhanced by food deprivation or ACTH exposure. Br J Pharmacol 2009 11;158(1476-5381; 0007-1188; 5):1330-7.
- Reference 461
-
Lemberger L. Tetrahydrocannabinol metabolism in man. Drug Metab Dispos 1973 01;1(0090-9556; 0090-9556; 1):461-8.
- Reference 462
-
Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther 1983 09;34(0009-9236; 3):352-63.
- Reference 463
-
Ujvary I, Hanus L. Human metabolites of cannabidiol: A review on their formation, biological activity, and relevance in therapy. Cannabis and Cannabinoid Research 2016;1(1):90 - 101.
- Reference 464
-
Harvey DJ, Mechoulam R. Metabolites of cannabidiol identified in human urine. Xenobiotica 1990 Mar;20(3):303-20.
- Reference 465
-
Sachse-Seeboth C, Pfeil J, Sehrt D, Meineke I, Tzvetkov M, Bruns E, Poser W, Vormfelde SV, Brockmoller J. Interindividual variation in the pharmacokinetics of Delta9-tetrahydrocannabinol as related to genetic polymorphisms in CYP2C9. Clin Pharmacol Ther 2009 03;85(1532-6535; 0009-9236; 3):273-6.
- Reference 466
-
Oates JA. The science of drug therapy. In: L. L. Brunton, J. S. Lazo, K. L. Parker, editors. Goodman and gilman's the pharmacological basis of therapeutics. New York: McGraw-Hill; 2006. ID: 2616; RP: NOT IN FILE.
- Reference 467
-
Graham MJ, Lake BG. Induction of drug metabolism: Species differences and toxicological relevance. Toxicology 2008 12/30;254(0300-483; 0300-483; 3):184-91.
- Reference 468
-
Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: A systematic review. Drug Metab Rev 2014 Feb;46(1):86-95.
- Reference 469
-
Bornheim LM, Everhart ET, Li J, Correia MA. Characterization of cannabidiol-mediated cytochrome P450 inactivation. Biochem Pharmacol 1993 03/24;45(0006-2952; 6):1323-31.
- Reference 470
-
Stott C, White L, Wright S, Wilbraham D, Guy G. A phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of rifampicin, ketoconazole, and omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. Springerplus 2013 May 24;2(1):236,1801-2-236. Print 2013 Dec.
- Reference 471
-
Kosel BW, Aweeka FT, Benowitz NL, Shade SB, Hilton JF, Lizak PS, Abrams DI. The effects of cannabinoids on the pharmacokinetics of indinavir and nelfinavir. AIDS 2002 03/08;16(0269-9370; 4):543-50.
- Reference 472
-
Engels FK, de Jong FA, Sparreboom A, Mathot RA, Loos WJ, Kitzen JJ, de Bruijn P, Verweij J, Mathijssen RH. Medicinal cannabis does not influence the clinical pharmacokinetics of irinotecan and docetaxel. Oncologist 2007 Mar;12(3):291-300.
- Reference 473
-
Jusko WJ, Schentag JJ, Clark JH, Gardner M, Yurchak AM. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther 1978 10;24(0009-9236; 4):405-10.
- Reference 474
-
Zullino DF, Delessert D, Eap CB, Preisig M, Baumann P. Tobacco and cannabis smoking cessation can lead to intoxication with clozapine or olanzapine. Int Clin Psychopharmacol 2002 05;17(0268-1315; 3):141-3.
- Reference 475
-
Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol 1992 09;16(0146-4760; 5):276-82.
- Reference 476
-
Agurell S, Leander K. Stability, transfer and absorption of cannabinoid constituents of cannabis (hashish) during smoking. Acta Pharm Suec 1971 09;8(0001-6675; 4):391-402.
- Reference 477
-
Wall ME, Perez-Reyes M. The metabolism of delta 9-tetrahydrocannabinol and related cannabinoids in man. J Clin Pharmacol 1981 08;21(0091-2700; 8-9):178S-89S.
- Reference 478
-
Lemberger L, Martz R, Rodda B, Forney R, Rowe H. Comparative pharmacology of Delta9-tetrahydrocannabinol and its metabolite, 11-OH-Delta9-tetrahydrocannabinol. J Clin Invest 1973 Oct;52(10):2411-7.
- Reference 479
-
Hollister LE. Structure-activity relationships in man of cannabis constituents, and homologs and metabolites of delta9-tetrahydrocannabinol. Pharmacology 1974;11(1):3-11.
- Reference 480
-
Howlett AC. Regulation of adenylate cyclase in a cultured neuronal cell line by marijuana constituents, metabolites of delta-9-tetrahydrocannabinol, and synthetic analogs having psychoactivity. 1987;79(NIDA research monograph series).
- Reference 481
-
McGilveray IJ. Pharmacokinetics of cannabinoids. Pain Res.Manag. 2005;Autumn:15A-22A.
- Reference 482
-
Christensen HD, Freudenthal RI, Gidley JT, Rosenfeld R, Boegli G, Testino L, Brine DR, Pitt CG, Wall ME. Activity of delta8- and delta9-tetrahydrocannabinol and related compounds in the mouse. Science 1971 04/09;172(0036-8075; 979):165-7.
- Reference 483
-
Perez-Reyes M, Timmons MC, Lipton MA, Davis KH, Wall ME. Intravenous injection in man of 9 -tetrahydrocannabinol and 11-OH- 9 -tetrahydrocannabinol. Science 1972 08/18;177(0036-8075; 49):633-5.
- Reference 484
-
Huestis MA, Sampson AH, Holicky BJ, Henningfield JE, Cone EJ. Characterization of the absorption phase of marijuana smoking. Clin Pharmacol Ther 1992 07;52(0009-9236; 1):31-41.
- Reference 485
-
Huestis MA, Mitchell JM, Cone EJ. Urinary excretion profiles of 11-nor-9-carboxy-delta 9-tetrahydrocannabinol in humans after single smoked doses of marijuana. J Anal Toxicol 1996 10;20(0146-4760; 6):441-52.
- Reference 486
-
Martin BR, Cone EJ. Chemistry and pharmacology of cannabis. In: H. Kalant, W. Corrigall, W. Hall, R. Smart, editors. The health effects of cannabis. Toronto, Canada: Centre of Addiction and Mental Health; 1999. ID: 2367; RP: NOT IN FILE.
- Reference 487
-
Hawks RL. The constituents of cannabis and the disposition and metabolism of cannabinoids. NIDA Res Monogr 1982;42(1046-9516):125-37.
- Reference 488
-
Harvey DJ, Brown NK. Comparative in vitro metabolism of the cannabinoids. Pharmacol Biochem Behav 1991 Nov;40(3):533-40.
- Reference 489
-
Harvey DJ, Samara E, Mechoulam R. Comparative metabolism of cannabidiol in dog, rat and man. Pharmacol Biochem Behav 1991 Nov;40(3):523-32.
- Reference 490
-
Cone EJ, Huestis MA. Relating blood concentrations of tetrahydrocannabinol and metabolites to pharmacologic effects and time of marijuana usage. Ther Drug Monit 1993 12;15(0163-4356; 6):527-32.
- Reference 491
-
Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Comparison of cannabinoid pharmacokinetic properties in occasional and heavy users smoking a marijuana or placebo joint. J Anal Toxicol 2008 09;32(0146-4760; 0146-4760; 7):470-7.
- Reference 492
-
Canada V. Cesamet product monograph. 2009.
- Reference 493
-
Hollister LE, Gillespie HK, Ohlsson A, Lindgren JE, Wahlen A, Agurell S. Do plasma concentrations of delta 9-tetrahydrocannabinol reflect the degree of intoxication? J Clin Pharmacol 1981 08;21(0091-2700; 0091-2700; 8-9):171S-7S.
- Reference 494
-
Strougo A, Zuurman L, Roy C, Pinquier JL, van Gerven JM, Cohen AF, Schoemaker RC. Modelling of the concentration--effect relationship of THC on central nervous system parameters and heart rate -- insight into its mechanisms of action and a tool for clinical research and development of cannabinoids. J Psychopharmacol 2008 09;22(0269-8811; 0269-8811; 7):717-26.
- Reference 495
-
Hunault CC, Bocker KB, Stellato RK, Kenemans JL, de Vries I, Meulenbelt J. Acute subjective effects after smoking joints containing up to 69 mg Delta9-tetrahydrocannabinol in recreational users: A randomized, crossover clinical trial. Psychopharmacology (Berl) 2014 Dec;231(24):4723-33.
- Reference 496
-
Lile JA, Kelly TH, Charnigo RJ, Stinchcomb AL, Hays LR. Pharmacokinetic and pharmacodynamic profile of supratherapeutic oral doses of delta(9) -THC in cannabis users. J Clin Pharmacol 2013 Jul;53(7):680-90.
- Reference 497
-
Lynch ME, Watson CP. The pharmacotherapy of chronic pain: A review. Pain Res.Manag. 2006;11(1203-6765; 1203-6765; 1):11-38.
- Reference 498
-
Wu DF, Yang LQ, Goschke A, Stumm R, Brandenburg LO, Liang YJ, Hollt V, Koch T. Role of receptor internalization in the agonist-induced desensitization of cannabinoid type 1 receptors. J Neurochem 2008 02;104(1471-4159; 0022-3042; 4):1132-43.
- Reference 499
-
Lazenka MF, Selley DE, Sim-Selley LJ. Brain regional differences in CB1 receptor adaptation and regulation of transcription. Life Sci 2013 Mar 19;92(8-9):446-52.
- Reference 500
-
Ceccarini J, Kuepper R, Kemels D, van Os J, Henquet C, Van Laere K. 18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict Biol 2015 Mar;20(2):357-67.
- Reference 501
-
Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 2012 06;17(1476-5578; 1359-4184; 6):642-9.
- Reference 502
-
Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol 2009 02;156(1476-5381; 0007-1188; 3):397-411.
- Reference 503
-
Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology (Berl) 1999 02;141(0033-3158; 4):385-94.
- Reference 504
-
Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999 02;141(0033-3158; 0033-3158; 4):395-404.
- Reference 505
-
Jones RT, Benowitz NL, Herning RI. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol 1981 Aug-Sep;21(8-9 Suppl):143S-52S.
- Reference 506
-
Ramaekers JG, Theunissen EL, de Brouwer M, Toennes SW, Moeller MR, Kauert G. Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology (Berl) 2011 Mar;214(2):391-401.
- Reference 507
-
Maldonado R. Study of cannabinoid dependence in animals. Pharmacol Ther 2002 08;95(0163-7258; 0163-7258; 2):153-64.
- Reference 508
-
American Psychiatric Association. Substance-related disorders. In: American Psychiatric Association, editor. Diagnostic and statistical manual of mental disorders text revision (DSM-IV-TR). Washington, D.C.: American Psychiatric Association; 2000. ID: 2674; RP: NOT IN FILE.
- Reference 509
-
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 2013.
- Reference 510
-
Lopez-Quintero C, Perez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the national epidemiologic survey on alcohol and related conditions (NESARC). Drug Alcohol Depend 2011 May 1;115(1-2):120-30.
- Reference 511
-
van der Pol P, Liebregts N, de Graaf R, Korf DJ, van den Brink W, van Laar M. Predicting the transition from frequent cannabis use to cannabis dependence: A three-year prospective study. Drug Alcohol Depend 2013 Dec 1;133(2):352-9.
- Reference 512
-
Blanco C, Hasin DS, Wall MM, Florez-Salamanca L, Hoertel N, Wang S, Kerridge BT, Olfson M. Cannabis use and risk of psychiatric disorders: Prospective evidence from a US national longitudinal study. JAMA Psychiatry 2016 Apr;73(4):388-95.
- Reference 513
-
Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, et al. Prevalence of marijuana use disorders in the united states between 2001-2002 and 2012-2013. JAMA Psychiatry 2015 Dec;72(12):1235-42.
- Reference 514
-
Butterworth P, Slade T, Degenhardt L. Factors associated with the timing and onset of cannabis use and cannabis use disorder: Results from the 2007 australian national survey of mental health and well-being. Drug Alcohol Rev 2014 Sep;33(5):555-64.
- Reference 515
-
Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend 2014 Mar 1;136:85-91.
- Reference 516
-
Bonnet U, Specka M, Stratmann U, Ochwadt R, Scherbaum N. Abstinence phenomena of chronic cannabis-addicts prospectively monitored during controlled inpatient detoxification: Cannabis withdrawal syndrome and its correlation with delta-9-tetrahydrocannabinol and -metabolites in serum. Drug Alcohol Depend 2014 Oct 1;143:189-97.
- Reference 517
-
Lee D, Schroeder JR, Karschner EL, Goodwin RS, Hirvonen J, Gorelick DA, Huestis MA. Cannabis withdrawal in chronic, frequent cannabis smokers during sustained abstinence within a closed residential environment. Am J Addict 2014 May-Jun;23(3):234-42.
- Reference 518
-
Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry 2004 11;161(0002-953; 11):1967-77.
- Reference 519
-
Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology 2017 Aug 30.
- Reference 520
-
Meier MH. Associations between butane hash oil use and cannabis-related problems. Drug Alcohol Depend 2017 Oct 1;179:25-31.
- Reference 521
-
Loflin M, Earleywine M. A new method of cannabis ingestion: The dangers of dabs? Addict Behav 2014 Oct;39(10):1430-3.
- Reference 522
-
Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, Rivas GR, Holland RM, Muhleisen P, Norberg MM, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: A randomized clinical trial. JAMA Psychiatry 2014 Mar;71(3):281-91.
- Reference 523
-
Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology (Berl) 2001 May;155(2):171-9.
- Reference 524
-
Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology (Berl) 2003 Jan;165(2):157-65.
- Reference 525
-
Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: Effects of oral THC or divalproex. Neuropsychopharmacology 2004 Jan;29(1):158-70.
- Reference 526
-
Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2008 Mar;197(1):157-68.
- Reference 527
-
Johnston J, Lintzeris N, Allsop DJ, Suraev A, Booth J, Carson DS, Helliwell D, Winstock A, McGregor IS. Lithium carbonate in the management of cannabis withdrawal: A randomized placebo-controlled trial in an inpatient setting. Psychopharmacology (Berl) 2014 Dec;231(24):4623-36.
- Reference 528
-
Cooper ZD, Foltin RW, Hart CL, Vosburg SK, Comer SD, Haney M. A human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addict Biol 2013 Nov;18(6):993-1002.
- Reference 529
-
Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, Foltin RW, Haney M. Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology (Berl) 2016 Jul;233(13):2469-78.
- Reference 530
-
Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend 2011 Aug 1;117(1):38-44.
- Reference 531
-
Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology 2013 Jul;38(8):1557-65.
- Reference 532
-
Levin FR, Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, Nunes EV, Bisaga A, Dakwar E, Carpenter KM, et al. Dronabinol and lofexidine for cannabis use disorder: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 2016 Feb 1;159:53-60.
- Reference 533
-
Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 2011 Jul 1;116(1-3):142-50.
- Reference 534
-
Crippa JA, Hallak JE, Machado-de-Sousa JP, Queiroz RH, Bergamaschi M, Chagas MH, Zuardi AW. Cannabidiol for the treatment of cannabis withdrawal syndrome: A case report. J Clin Pharm Ther 2013 Apr;38(2):162-4.
- Reference 535
-
Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: Naturalistic study: Naturalistic study [corrected]. Br J Psychiatry 2010 10;197(1472-1465; 0007-1250; 4):285-90.
- Reference 536
-
Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, Gray KM, McRae-Clark A, Lofwall MR, Sparenborg S, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology 2016 Jul;41(8):1974-82.
- Reference 537
-
Morgan CJ, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: Preliminary findings. Addict Behav 2013 Sep;38(9):2433-6.
- Reference 538
-
Consroe P, Carlini EA, Zwicker AP, Lacerda LA. Interaction of cannabidiol and alcohol in humans. Psychopharmacology (Berl) 1979;66(1):45-50.
- Reference 539
-
Chadwick B, Miller ML, Hurd YL. Cannabis use during adolescent development: Susceptibility to psychiatric illness. Front Psychiatry 2013 Oct 14;4:129.
- Reference 540
-
Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, Sharma S. Maturation of the adolescent brain. Neuropsychiatr Dis Treat 2013;9:449-61.
- Reference 541
-
Hall W, Degenhardt L. The adverse health effects of chronic cannabis use. Drug Test Anal 2014 Jan-Feb;6(1-2):39-45.
- Reference 542
-
Pardini D, White HR, Xiong S, Bechtold J, Chung T, Loeber R, Hipwell A. Unfazed or dazed and confused: Does early adolescent marijuana use cause sustained impairments in attention and academic functioning? J Abnorm Child Psychol 2015 Oct;43(7):1203-17.
- Reference 543
-
Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction 2002 Sep;97(9):1123-35.
- Reference 544
-
Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol Soc 2012 Jul;18(4):678-88.
- Reference 545
-
Silins E, Horwood LJ, Patton GC, Fergusson DM, Olsson CA, Hutchinson DM, Spry E, Toumbourou JW, Degenhardt L, Swift W, et al. Young adult sequelae of adolescent cannabis use: An integrative analysis. Lancet Psychiatry 2014 Sep;1(4):286-93.
- Reference 546
-
Silins E, Fergusson DM, Patton GC, Horwood LJ, Olsson CA, Hutchinson DM, Degenhardt L, Tait RJ, Borschmann R, Coffey C, et al. Adolescent substance use and educational attainment: An integrative data analysis comparing cannabis and alcohol from three australasian cohorts. Drug Alcohol Depend 2015 Nov 1;156:90-6.
- Reference 547
-
Horwood LJ, Fergusson DM, Hayatbakhsh MR, Najman JM, Coffey C, Patton GC, Silins E, Hutchinson DM. Cannabis use and educational achievement: Findings from three australasian cohort studies. Drug Alcohol Depend 2010 Aug 1;110(3):247-53.
- Reference 548
-
Fergusson DM, Horwood LJ, Beautrais AL. Cannabis and educational achievement. Addiction 2003 Dec;98(12):1681-92.
- Reference 549
-
Lynskey MT, Coffey C, Degenhardt L, Carlin JB, Patton G. A longitudinal study of the effects of adolescent cannabis use on high school completion. Addiction 2003 May;98(5):685-92.
- Reference 550
-
Bray JW, Zarkin GA, Ringwalt C, Qi J. The relationship between marijuana initiation and dropping out of high school. Health Econ 2000 Jan;9(1):9-18.
- Reference 551
-
Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology 2014 Jan;76 Pt B:416-24.
- Reference 552
-
Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A 2012 Oct 2;109(40):E2657-64.
- Reference 553
-
Solowij N, Pesa N. Cannabis and cognition: Short- and long-term effects. In: D. Castle, R. M. Murray, Cyril Deepak D'Souza, editors. Marijuana and madness. Cambridge: Cambridge University Press; 2011..
- Reference 554
-
Pope HG,Jr., Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 2001 10;58(0003-990; 10):909-15.
- Reference 555
-
Levine A, Clemenza K, Rynn M, Lieberman J. Evidence for the risks and consequences of adolescent cannabis exposure. J Am Acad Child Adolesc Psychiatry 2017 Mar;56(3):214-25.
- Reference 556
-
Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 2015 Jan;16(1):30-42.
- Reference 557
-
Ahmed A, van der Marck MA, van den Elsen G, Olde Rikkert M. Cannabinoids in late-onset alzheimer's disease. Clin Pharmacol Ther 2015 Jun;97(6):597-606.
- Reference 558
-
van den Elsen GA, Ahmed AI, Lammers M, Kramers C, Verkes RJ, van der Marck MA, Rikkert MG. Efficacy and safety of medical cannabinoids in older subjects: A systematic review. Ageing Res Rev 2014 Mar;14:56-64.
- Reference 559
-
Fattore L, Fratta W. How important are sex differences in cannabinoid action? Br J Pharmacol 2010 Jun;160(3):544-8.
- Reference 560
-
Rubino T, Parolaro D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front Behav Neurosci 2011 Sep 28;5:64.
- Reference 561
-
Van Laere K, Goffin K, Casteels C, Dupont P, Mortelmans L, de Hoon J, Bormans G. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [(18)F]MK-9470 PET. Neuroimage 2008 Feb 15;39(4):1533-41.
- Reference 562
-
Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex difference in the oxidative metabolism of delta 9-tetrahydrocannabinol in the rat. Biochem Pharmacol 1991 Apr 15;41(8):1187-94.
- Reference 563
-
Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: A reflection of differences in the endocannabinoid system? Life Sci 2013 Mar 19;92(8-9):476-81.
- Reference 564
-
Fogel JS, Kelly TH, Westgate PM, Lile JA. Sex differences in the subjective effects of oral Delta9-THC in cannabis users. Pharmacol Biochem Behav 2016 Jan 15.
- Reference 565
-
Rubino T, Parolaro D. Sex-dependent vulnerability to cannabis abuse in adolescence. Front Psychiatry 2015 Apr 20;6:56.
- Reference 566
-
Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Sociodemographic characteristics of cannabis smokers and the experience of cannabis withdrawal. Am J Drug Alcohol Abuse 2010 Nov;36(6):311-9.
- Reference 567
-
Herrmann ES, Weerts EM, Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp Clin Psychopharmacol 2015 Dec;23(6):415-21.
- Reference 568
-
Agrawal A, Gardner CO, Prescott CA, Kendler KS. The differential impact of risk factors on illicit drug involvement in females. Soc Psychiatry Psychiatr Epidemiol 2005 Jun;40(6):454-66.
- Reference 569
-
Khan SS, Secades-Villa R, Okuda M, Wang S, Perez-Fuentes G, Kerridge BT, Blanco C. Gender differences in cannabis use disorders: Results from the national epidemiologic survey of alcohol and related conditions. Drug Alcohol Depend 2013 Jun 1;130(1-3):101-8.
- Reference 570
-
Hazekamp A, Bastola K, Rashidi H, Bender J, Verpoorte R. Cannabis tea revisited: A systematic evaluation of the cannabinoid composition of cannabis tea. J Ethnopharmacol 2007 08/15;113(0378-8741; 0378-8741; 1):85-90.
- Reference 571
-
Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, open-label, pilot study of add-on oral Delta9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin Drug Investig 2014 Aug;34(8):587-91.
- Reference 572
-
Pertwee RG. Tolerance to the effect of delta1-tetrahydrocannabinol on corticosterone levels in mouse plasma produced by repeated administration of cannabis extract or delta1-tetrahydrocannabinol. Br J Pharmacol 1974 07;51(0007-1188; 0007-1188; 3):391-7.
- Reference 573
-
Lozano I. The therapeutic uses of cannabis sativa (L.) in arabic medicine. J Cannabis Therapeutics 2001;1(1):63-70.
- Reference 574
-
Russo E. History of cannabis as a medicine. In: G. W. Guy, B. A. Whittle, P. J. Robson, editors. The medicinal uses of cannabis and cannabinoids. London: Pharmaceutical Press; 2004. ID: 2622; RP: NOT IN FILE.
- Reference 575
-
Russo EB. History of cannabis and its preparations in saga, science, and sobriquet. Chem.Biodivers. 2007 08;4(1612-1880; 1612-1872; 8):1614-48.
- Reference 576
-
Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA, Wright S, Cannabidiol in Dravet Syndrome Study Group. Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N Engl J Med 2017 May 25;376(21):2011-20.
- Reference 577
-
Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, Lyons PD, Taylor A, Roberts C, Sommerville K, et al. Cannabidiol in patients with seizures associated with lennox-gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018 Jan 25.
- Reference 578
-
Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS.Neurosci.Ther. 2009;15(1755-5949; 1755-5930; 1):84-8.
- Reference 579
-
Ware MA, Adams H, Guy GW. The medicinal use of cannabis in the UK: Results of a nationwide survey. Int J Clin Pract 2005 03;59(1368-5031; 3):291-5.
- Reference 580
-
Hazekamp A, Ware MA, Muller-Vahl KR, Abrams D, Grotenhermen F. The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. J Psychoactive Drugs 2013 Jul-Aug;45(3):199-210.
- Reference 581
-
Market data - Cannabis for Medical Purposes [Internet]; c09 July 2018. Available from: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/licensed-producers/market-data.html.
- Reference 582
-
Haroutounian S, Ratz Y, Ginosar Y, Furmanov K, Saifi F, Meidan R, Davidson E. The effect of medicinal cannabis on pain and quality of life outcomes in chronic pain: A prospective open-label study. Clin J Pain 2016 Feb 17.
- Reference 583
-
Grotenhermen F. Harm reduction associated with inhalation and oral administration of cannabis and THC. Journal of Cannabis Therapeutics 2001;1(3/4):133.
- Reference 584
-
van der Pol P, Liebregts N, Brunt T, van Amsterdam J, de Graaf R, Korf DJ, van den Brink W, van Laar M. Cross-sectional and prospective relation of cannabis potency, dosing and smoking behaviour with cannabis dependence: An ecological study. Addiction 2014 Jul;109(7):1101-9.
- Reference 585
-
Ramaekers JG, Berghaus G, van Laar MW, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use: An update. In: J. C. Verster, S. R. Pandi-Perumal, J. G. Ramaekers, J. J. de Gier, editors. Drugs, driving and traffic safety. 1st ed. Springer Science and Business Media; 2009..
- Reference 586
-
College of Family Physicians of Canada. Authorizing dried cannabis for chronic pain or anxiety: Preliminary guidance from the college of family physicians of canada. Mississauga, ON: College of Family Physicians of Canada; 2014.
- Reference 587
-
Allan GM, Ramji J, Perry D, Ton J, Beahm NP, Crisp N, Dockrill B, Dubin RE, Findlay T, Kirkwood J, et al. Simplified guideline for prescribing medical cannabinoids in primary care. Can Fam Physician 2018 Feb;64(2):111-20.
- Reference 588
-
Cannabis: a health perspective and research agenda [Internet]; c1997.
- Reference 589
-
Ridgeway G, Kilmer B. Bayesian interference for the distribution of grams of marijuana in a joint. Drug Alcohol Depend. 2016;165:175-80.
- Reference 590
-
Gray KM, Watson NL, Christie DK. Challenges in quantifying marijuana use. Am J Addict 2009 Mar-Apr;18(2):178-9.
- Reference 591
-
Cami J, Guerra D, Ugena B, Segura J, de la Torre R. Effect of subject expectancy on the THC intoxication and disposition from smoked hashish cigarettes. Pharmacol Biochem Behav 1991 09;40(0091-3057; 0091-3057; 1):115-9.
- Reference 592
-
Isbell H, Gorodetzsky CW, Jasinski D, Claussen U, von Spulak F, Korte F. Effects of (--)delta-9-trans-tetrahydrocannabinol in man. Psychopharmacologia 1967;11(2):184-8.
- Reference 593
-
Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral delta(9)-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2002 Dec;164(4):407-15.
- Reference 594
-
Barrus DG, Capogrossi KL, Cates SC, Gourdet CK, Peiper NC, Novak SP, Lefever TW, Wiley JL. Tasty THC: Promises and challenges of cannabis edibles. Methods Rep RTI Press 2016 Nov;2016.
- Reference 595
-
de Vries M, Van Rijckevorsel DC, Vissers KC, Wilder-Smith OH, Van Goor H. Single dose delta-9-tetrahydrocannabinol in chronic pancreatitis patients: Analgesic efficacy, pharmacokinetics and tolerability. Br J Clin Pharmacol 2016 Mar;81(3):525-37.
- Reference 596
-
Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J.Pain 2008 02;9(1526-5900; 1526-5900; 2):164-73.
- Reference 597
-
Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: Results of a randomized controlled trial. Anesth Analg 2010 02/01;110(1526-7598; 0003-2999; 2):604-10.
- Reference 598
-
Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J.Pain 2012 12/10;14(1528-8447; 1526-5900; 2):136-48.
- Reference 599
-
Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015 Jul;16(7):616-27.
- Reference 600
-
Kalliomaki J, Philipp A, Baxendale J, Annas P, Karlsten R, Segerdahl M. Lack of effect of central nervous system-active doses of nabilone on capsaicin-induced pain and hyperalgesia. Clin Exp Pharmacol Physiol 2012 04;39(1440-1681; 0305-1870; 4):336-42.
- Reference 601
-
Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol 2006 Apr 21;105(1-2):1-25.
- Reference 602
-
National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. Washington, D.C.: National Academies Press; 2017.
- Reference 603
-
Naftali T, Bar-Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with crohn's disease: A prospective placebo-controlled study. Clin Gastroenterol Hepatol 2013 Oct;11(10):1276,1280.e1.
- Reference 604
-
World Health Organization (WHO). WHO definition of palliative care. 2012.
- Reference 605
-
Green AJ, De-Vries K. Cannabis use in palliative care - an examination of the evidence and the implications for nurses. J Clin Nurs 2010 09;19(1365-2702; 0962-1067; 17-18):2454-62.
- Reference 606
-
Gardiner C, Ingleton C. Commentary on green AJ & de-vries K (2010) cannabis use in palliative care--an examination of the evidence and the implications for nurses. journal of clinical nursing 19, 2454-2462. J Clin Nurs 2010 11;19(1365-2702; 0962-1067; 21-22):3253-5.
- Reference 607
-
Glare P, Miller J, Nikolova T, Tickoo R. Treating nausea and vomiting in palliative care: A review. Clin.Interv.Aging 2011;6(1178-1998; 1176-9092):243-59.
- Reference 608
-
Fine PG. Treatment guidelines for the pharmacological management of pain in older persons. Pain Med. 2012 04;13 Suppl 2(1526-4637; 1526-2375):S57-66.
- Reference 609
-
Bar-Sela G, Vorobeichik M, Drawsheh S, Omer A, Goldberg V, Muller E. The medical necessity for medicinal cannabis: Prospective, observational study evaluating the treatment in cancer patients on supportive or palliative care. Evid Based Complement Alternat Med 2013;2013:510392.
- Reference 610
-
Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? randomised double blind placebo controlled crossover trial. BMJ 2004 07/31;329(1468-5833; 7460):253-60.
- Reference 611
-
Brisbois TD, de Kock IH, Watanabe SM, Mirhosseini M, Lamoureux DC, Chasen M, MacDonald N, Baracos VE, Wismer WV. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: Results of a randomized, double-blind, placebo-controlled pilot trial. Ann Oncol 2011 09;22(1569-8041; 0923-7534; 9):2086-93.
- Reference 612
-
Toth C, Mawani S, Brady S, Chan C, Liu C, Mehina E, Garven A, Bestard J, Korngut L. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain 2012 10;153(1872-6623; 0304-3959; 10):2073-82.
- Reference 613
-
Cote M, Trudel M, Wang C, Fortin A. Improving quality of life with nabilone during radiotherapy treatments for head and neck cancers: A randomized double-blind placebo-controlled trial. Ann Otol Rhinol Laryngol 2016 Apr;125(4):317-24.
- Reference 614
-
Selvarajah D, Gandhi R, Emery CJ, Tesfaye S. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (sativex) in painful diabetic neuropathy: Depression is a major confounding factor. Diabetes Care 2010 01;33(1935-5548; 0149-5992; 1):128-30.
- Reference 615
-
Novotna A, Mares J, Ratcliffe S, Novakova I, Vachova M, Zapletalova O, Gasperini C, Pozzilli C, Cefaro L, Comi G, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (sativex((R))), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 2011 09;18(1468-1331; 1351-5101; 9):1122-31.
- Reference 616
-
Goldenberg M, Reid MW, IsHak WW, Danovitch I. The impact of cannabis and cannabinoids for medical conditions on health-related quality of life: A systematic review and meta-analysis. Drug Alcohol Depend 2017 May 1;174:80-90.
- Reference 617
-
Navari RM. Pharmacological management of chemotherapy-induced nausea and vomiting: Focus on recent developments. Drugs 2009;69(0012-6667; 0012-6667; 5):515-33.
- Reference 618
-
Jordan K, Kasper C, Schmoll HJ. Chemotherapy-induced nausea and vomiting: Current and new standards in the antiemetic prophylaxis and treatment. Eur J Cancer 2005 Jan;41(2):199-205.
- Reference 619
-
Bovbjerg DH. The continuing problem of post chemotherapy nausea and vomiting: Contributions of classical conditioning. Auton Neurosci 2006 Oct 30;129(1-2):92-8.
- Reference 620
-
Rock EM, Limebeer CL, Parker LA. Anticipatory nausea in animal models: A review of potential novel therapeutic treatments. Exp Brain Res 2014 Aug;232(8):2511-34.
- Reference 621
-
Limebeer CL, Rock EM, Mechoulam R, Parker LA. The anti-nausea effects of CB1 agonists are mediated by an action at the visceral insular cortex. Br J Pharmacol 2012 Nov;167(5):1126-36.
- Reference 622
-
Rock EM, Limebeer CL, Navaratnam R, Sticht MA, Bonner N, Engeland K, Downey R, Morris H, Jackson M, Parker LA. A comparison of cannabidiolic acid with other treatments for anticipatory nausea using a rat model of contextually elicited conditioned gaping. Psychopharmacology (Berl) 2014 Aug;231(16):3207-15.
- Reference 623
-
Rock EM, Kopstick RL, Limebeer CL, Parker LA. Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in suncus murinus. Br J Pharmacol 2013 Oct;170(3):641-8.
- Reference 624
-
Rock EM, Parker LA. Suppression of lithium chloride-induced conditioned gaping (a model of nausea-induced behaviour) in rats (using the taste reactivity test) with metoclopramide is enhanced by cannabidiolic acid. Pharmacol Biochem Behav 2013 Oct;111:84-9.
- Reference 625
-
Rock EM, Parker LA. Effect of low doses of cannabidiolic acid and ondansetron on LiCl-induced conditioned gaping (a model of nausea-induced behaviour) in rats. Br J Pharmacol 2013 Jun;169(3):685-92.
- Reference 626
-
Rock EM, Sticht MA, Duncan M, Stott C, Parker LA. Evaluation of the potential of the phytocannabinoids, cannabidivarin (CBDV) and delta(9) -tetrahydrocannabivarin (THCV), to produce CB1 receptor inverse agonism symptoms of nausea in rats. Br J Pharmacol 2013 Oct;170(3):671-8.
- Reference 627
-
Rock EM, Bolognini D, Limebeer CL, Cascio MG, Anavi-Goffer S, Fletcher PJ, Mechoulam R, Pertwee RG, Parker LA. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br J Pharmacol 2012 04;165(1476-5381; 0007-1188; 8):2620-34.
- Reference 628
-
Rock EM, Goodwin JM, Limebeer CL, Breuer A, Pertwee RG, Mechoulam R, Parker LA. Interaction between non-psychotropic cannabinoids in marihuana: Effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews. Psychopharmacology (Berl) 2011 06;215(1432-2072; 0033-3158; 3):505-12.
- Reference 629
-
Hornby PJ. Central neurocircuitry associated with emesis. Am J Med 2001 12/03;111 Suppl 8A(0002-9343; 0002-9343):106S-12S.
- Reference 630
-
Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005 10/14;310(1095-9203; 0036-8075; 5746):329-32.
- Reference 631
-
Darmani NA. The cannabinoid CB1 receptor antagonist SR 141716A reverses the antiemetic and motor depressant actions of WIN 55, 212-2. Eur J Pharmacol 2001 10/26;430(0014-2999; 0014-2999; 1):49-58.
- Reference 632
-
Sticht MA, Limebeer CL, Rafla BR, Parker LA. Intra-visceral insular cortex 2-arachidonoylglycerol, but not N-arachidonoylethanolamide, suppresses acute nausea-induced conditioned gaping in rats. Neuroscience 2015 Feb 12;286:338-44.
- Reference 633
-
Limebeer CL, Abdullah RA, Rock EM, Imhof E, Wang K, Lichtman AH, Parker LA. Attenuation of anticipatory nausea in a rat model of contextually elicited conditioned gaping by enhancement of the endocannabinoid system. Psychopharmacology (Berl) 2014 Feb;231(3):603-12.
- Reference 634
-
Barann M, Molderings G, Bruss M, Bonisch H, Urban BW, Gothert M. Direct inhibition by cannabinoids of human 5-HT3A receptors: Probable involvement of an allosteric modulatory site. Br J Pharmacol 2002 11;137(0007-1188; 5):589-96.
- Reference 635
-
Ahmed SA, Ross SA, Slade D, Radwan MM, Zulfiqar F, Matsumoto RR, Xu YT, Viard E, Speth RC, Karamyan VT, et al. Cannabinoid ester constituents from high-potency cannabis sativa. J Nat Prod 2008 Apr;71(4):536-42.
- Reference 636
-
Grunfeld Y, Edery H. Psychopharmacological activity of the active constituents of hashish and some related cannabinoids. Psychopharmacologia 1969;14(3):200-10.
- Reference 637
-
Machado Rocha FC, Stefano SC, De Cassia HR, Rosa Oliveira LM, Da Silveira DX. Therapeutic use of cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: Systematic review and meta-analysis. Eur J Cancer Care (Engl) 2008 09;17(1365-2354; 0961-5423; 5):431-43.
- Reference 638
-
Meiri E, Jhangiani H, Vredenburgh JJ, Barbato LM, Carter FJ, Yang HM, Baranowski V. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin 2007 03;23(1473-4877; 0300-7995; 3):533-43.
- Reference 639
-
Kwiatkowska M, Parker LA, Burton P, Mechoulam R. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the suncus murinus (house musk shrew). Psychopharmacology (Berl) 2004 07;174(0033-3158; 0033-3158; 2):254-9.
- Reference 640
-
Wang Y, Ray AP, McClanahan BA, Darmani NA. The antiemetic interaction of Delta9-tetrahydrocannabinol when combined with tropisetron or dexamethasone in the least shrew. Pharmacol Biochem Behav 2009 01;91(0091-3057; 0091-3057; 3):367-73.
- Reference 641
-
Elder JJ, Knoderer HM. Characterization of dronabinol usage in a pediatric oncology population. J Pediatr Pharmacol Ther 2015 Nov-Dec;20(6):462-7.
- Reference 642
-
Institute of Medicine. The medical value of marijuana and related substances. In: J. E. Joy, S. J. Watson, J. A. Benson, editors. Marijuana and medicine: Assessing the science base. Washington, DC: National Academy Press; 1999. ID: 2372; RP: NOT IN FILE.
- Reference 643
-
Health Department of New South Wales,Australia. Working party on the use of cannabis for medical purposes.; 2000. Report nr 2:ID: 2373; RP: NOT IN FILE.
- Reference 644
-
Herrstedt J, Dombernowsky P. Anti-emetic therapy in cancer chemotherapy: Current status. Basic Clin.Pharmacol.Toxicol. 2007 09;101(1742-7835; 3):143-50.
- Reference 645
-
Canadian Pharmacists Association. Compendium of pharmaceuticals and specialties. Ottawa: Canadian Pharmacists Association; 2009. ID: 2537; RP: NOT IN FILE.
- Reference 646
-
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. 2010.
- Reference 647
-
Clary PL, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician 2009 06/15;79(0002-838; 0002-838; 12):1059-65.
- Reference 648
-
Smit E, Crespo CJ. Dietary intake and nutritional status of US adult marijuana users: Results from the third national health and nutrition examination survey. Public Health Nutr 2001 06;4(1368-9800; 3):781-6.
- Reference 649
-
Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci 2005 05;8(1097-6256; 1097-6256; 5):585-9.
- Reference 650
-
Matias I, Bisogno T, Di M,V. Endogenous cannabinoids in the brain and peripheral tissues: Regulation of their levels and control of food intake. Int.J.Obes.(Lond) 2006 04;30 Suppl 1(0307-0565):S7-S12.
- Reference 651
-
Tibirica E. The multiple functions of the endocannabinoid system: A focus on the regulation of food intake. Diabetol.Metab Syndr. 2010;2(1758-5996):5-10.
- Reference 652
-
Farrimond JA, Mercier MS, Whalley BJ, Williams CM. Cannabis sativa and the endogenous cannabinoid system: Therapeutic potential for appetite regulation. Phytother Res 2011 02;25(1099-1573; 0951-418; 2):170-88.
- Reference 653
-
Timpone JG, Wright DJ, Li N, Egorin MJ, Enama ME, Mayers J, Galetto G. The safety and pharmacokinetics of single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. the DATRI 004 study group. division of AIDS treatment research initiative. AIDS Res Hum Retroviruses 1997 03/01;13(0889-2229; 0889-2229; 4):305-15.
- Reference 654
-
Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 1995 02;10(0885-3924; 2):89-97.
- Reference 655
-
Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, et al. Short-term effects of cannabinoids in patients with HIV-1 infection: A randomized, placebo-controlled clinical trial. Ann Intern Med 2003 08/19;139(1539-3704; 4):258-66.
- Reference 656
-
Ravinet-Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 2004 04;28(0307-0565; 0307-0565; 4):640-8.
- Reference 657
-
Riggs PK, Vaida F, Rossi SS, Sorkin LS, Gouaux B, Grant I, Ellis RJ. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res 2012 Jan 11;1431:46-52.
- Reference 658
-
Tchekmedyian NS, Zahyna D, Halpert C, Heber D. Clinical aspects of nutrition in advanced cancer. Oncology 1992;49 Suppl 2(0030-2414; 0030-2414):3-7.
- Reference 659
-
Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: Relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer 2000 05;8(0941-4355; 0941-4355; 3):175-9.
- Reference 660
-
Mantovani G. Randomised phase III clinical trial of 5 different arms of treatment on 332 patients with cancer cachexia. Eur Rev Med Pharmacol Sci 2010 04;14(1128-3602; 1128-3602; 4):292-301.
- Reference 661
-
Monteleone P, Matias I, Martiadis V, De PL, Maj M, Di M,V. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology 2005 06;30(0893-133; 0006-3223; 6):1216-21.
- Reference 662
-
Monteleone P, Maj M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: Beyond the homeostatic control of food intake. Psychoneuroendocrinology 2013 Mar;38(3):312-30.
- Reference 663
-
Siegfried Z, Kanyas K, Latzer Y, Karni O, Bloch M, Lerer B, Berry EM. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: Differences between restricting and binging/purging subtypes. Am.J.Med.Genet.B Neuropsychiatr.Genet. 2004 02/15;125B(1552-4841; 1):126-30.
- Reference 664
-
Muller TD, Reichwald K, Bronner G, Kirschner J, Nguyen TT, Scherag A, Herzog W, Herpertz-Dahlmann B, Lichtner P, Meitinger T, et al. Lack of association of genetic variants in genes of the endocannabinoid system with anorexia nervosa. Child Adolesc.Psychiatry Ment.Health 2008;2(1753-2000; 1753-2000; 1):33-9.
- Reference 665
-
Lewis DY, Brett RR. Activity-based anorexia in C57/BL6 mice: Effects of the phytocannabinoid, Delta9-tetrahydrocannabinol (THC) and the anandamide analogue, OMDM-2. Eur Neuropsychopharmacol 2010 09;20(1873-7862; 0924-977; 9):622-31.
- Reference 666
-
Verty AN, Evetts MJ, Crouch GJ, McGregor IS, Stefanidis A, Oldfield BJ. The cannabinoid receptor agonist THC attenuates weight loss in a rodent model of activity-based anorexia. Neuropsychopharmacology 2011 06;36(1740-634; 0006-3223; 7):1349-58.
- Reference 667
-
Pryce G, Baker D. Endocannabinoids in multiple sclerosis and amyotrophic lateral sclerosis. Handb Exp Pharmacol 2015;231:213-31.
- Reference 668
-
Baker D, Pryce G, Jackson SJ, Bolton C, Giovannoni G. The biology that underpins the therapeutic potential of cannabis-based medicines for the control of spasticity in multiple sclerosis. Mult Scler Relat Disord 2012 Apr;1(2):64-75.
- Reference 669
-
Moreno-Martet M, Espejo-Porras F, Fernandez-Ruiz J, de Lago E. Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1(G93A) transgenic mice and evaluation of a sativex((R)) -like combination of phytocannabinoids: Interest for future therapies in amyotrophic lateral sclerosis. CNS Neurosci Ther 2014 Sep;20(9):809-15.
- Reference 670
-
Souza PVSd, Pinto WBVdR, Chieia MAT, Oliveira ASB. Clinical and genetic basis of familial amyotrophic lateral sclerosis. Arquivos De Neuro-Psiquiatria 2015;73(12):1026.
- Reference 671
-
Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the guideline development subcommittee of the american academy of neurology. Neurology 2014 Apr 29;82(17):1556-63.
- Reference 672
-
Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 2000 03/02;404(0028-0836; 0028-0836; 6773):84-7.
- Reference 673
-
Centonze D, Rossi S, Finazzi-Agro A, Bernardi G, Maccarrone M. The (endo)cannabinoid system in multiple sclerosis and amyotrophic lateral sclerosis. Int Rev Neurobiol 2007;82(0074-7742; 0074-7742):171-86.
- Reference 674
-
Di Filippo M, Pini LA, Pelliccioli GP, Calabresi P, Sarchielli P. Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J Neurol Neurosurg Psychiatr 2008 11;79(1468-330; 0022-3050; 11):1224-9.
- Reference 675
-
Jean-Gilles L, Feng S, Tench CR, Chapman V, Kendall DA, Barrett DA, Constantinescu CS. Plasma endocannabinoid levels in multiple sclerosis. J Neurol Sci 2009 12/15;287(1878-5883; 0022-510; 1-2):212-5.
- Reference 676
-
Pertwee RG. Cannabinoids and multiple sclerosis. Mol Neurobiol 2007 08;36(0893-7648; 0893-7648; 1):45-59.
- Reference 677
-
Lyman WD, Sonett JR, Brosnan CF, Elkin R, Bornstein MB. Delta 9-tetrahydrocannabinol: A novel treatment for experimental autoimmune encephalomyelitis. J Neuroimmunol 1989 06;23(0165-5728; 0165-5728; 1):73-81.
- Reference 678
-
Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, Ledent C, Cheng X, Carrier EJ, Mann MK, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med 2007 04;13(1078-8956; 1078-8956; 4):492-7.
- Reference 679
-
Pryce G, Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol 2007 02;150(0007-1188; 0007-1188; 4):519-25.
- Reference 680
-
Croxford JL, Pryce G, Jackson SJ, Ledent C, Giovannoni G, Pertwee RG, Yamamura T, Baker D. Cannabinoid-mediated neuroprotection, not immunosuppression, may be more relevant to multiple sclerosis. J Neuroimmunol 2008 01;193(0165-5728; 0165-5728; 1-2):120-9.
- Reference 681
-
Feliu A, Moreno-Martet M, Mecha M, Carrillo-Salinas FJ, de Lago E, Fernandez-Ruiz J, Guaza C. A sativex((R)) -like combination of phytocannabinoids as a disease-modifying therapy in a viral model of multiple sclerosis. Br J Pharmacol 2015 Jul;172(14):3579-95.
- Reference 682
-
Moreno-Martet M, Feliu A, Espejo-Porras F, Mecha M, Carrillo-Salinas FJ, Fernandez-Ruiz J, Guaza C, de Lago E. The disease-modifying effects of a sativex-like combination of phytocannabinoids in mice with experimental autoimmune encephalomyelitis are preferentially due to Delta9-tetrahydrocannabinol acting through CB1 receptors. Mult Scler Relat Disord 2015 Nov;4(6):505-11.
- Reference 683
-
Consroe P, Sandyk R. Therapeutic potential of cannabinoids in neurological disorders. In: R. Mechoulam, editor. Marijuana/Cannabinoids as therapeutic agents. Boca Raton, FL: CRC Press; 1986. ID: 2378; RP: NOT IN FILE.
- Reference 684
-
Chong MS, Wolff K, Wise K, Tanton C, Winstock A, Silber E. Cannabis use in patients with multiple sclerosis. Mult Scler 2006 10;12(1352-4585; 1352-4585; 5):646-51.
- Reference 685
-
Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, Reilly SM, Nunn AJ, Teare LJ, Fox PJ, Thompson AJ. Cannabinoids in multiple sclerosis (CAMS) study: Safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatr 2005 12;76(0022-3050; 12):1664-9.
- Reference 686
-
Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F, Gattlen B, Hagen U, Schnelle M, Reif M. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled, crossover study. Mult Scler 2004 08;10(1352-4585; 4):417-24.
- Reference 687
-
Wade DT, Makela PM, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler 2006 10;12(1352-4585; 5):639-45.
- Reference 688
-
Collin C, Davies P, Mutiboko IK, Ratcliffe S. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol 2007 03;14(1468-1331; 3):290-6.
- Reference 689
-
Hobart JC, Riazi A, Thompson AJ, Styles IM, Ingram W, Vickery PJ, Warner M, Fox PJ, Zajicek JP. Getting the measure of spasticity in multiple sclerosis: The multiple sclerosis spasticity scale (MSSS-88). Brain 2006 01;129(1460-2156; 0006-8950):224-34.
- Reference 690
-
Flachenecker P, Henze T, Zettl UK. Nabiximols (THC/CBD oromucosal spray, sativex(R)) in clinical practice--results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur Neurol 2014;71(5-6):271-9.
- Reference 691
-
Flachenecker P, Henze T, Zettl UK. Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur Neurol 2014;72(1-2):95-102.
- Reference 692
-
Freidel M, Tiel-Wilck K, Schreiber H, Prechtl A, Essner U, Lang M. Drug-resistant MS spasticity treatment with sativex((R)) add-on and driving ability. Acta Neurol Scand 2015 Jan;131(1):9-16.
- Reference 693
-
Coghe G, Pau M, Corona F, Frau J, Lorefice L, Fenu G, Spinicci G, Mamusa E, Musu L, Massole S, et al. Walking improvements with nabiximols in patients with multiple sclerosis. J Neurol 2015 Nov;262(11):2472-7.
- Reference 694
-
Leocani L, Nuara A, Houdayer E, Schiavetti I, Del Carro U, Amadio S, Straffi L, Rossi P, Martinelli V, Vila C, et al. Sativex((R)) and clinical-neurophysiological measures of spasticity in progressive multiple sclerosis. J Neurol 2015 Nov;262(11):2520-7.
- Reference 695
-
Ferre L, Nuara A, Pavan G, Radaelli M, Moiola L, Rodegher M, Colombo B, Keller Sarmiento IJ, Martinelli V, Leocani L, et al. Efficacy and safety of nabiximols (sativex((R))) on multiple sclerosis spasticity in a real-life italian monocentric study. Neurol Sci 2016 Feb;37(2):235-42.
- Reference 696
-
Ball S, Vickery J, Hobart J, Wright D, Green C, Shearer J, Nunn A, Cano MG, MacManus D, Miller D, et al. The cannabinoid use in progressive inflammatory brain disease (CUPID) trial: A randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Health Technol Assess 2015 Feb;19(12):vii,viii, xxv-xxxi, 1-187.
- Reference 697
-
Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG. MUltiple sclerosis and extract of cannabis: Results of the MUSEC trial. J Neurol Neurosurg Psychiatr 2012 11;83(1468-330; 0022-3050; 11):1125-32.
- Reference 698
-
Killestein J, Hoogervorst EL, Reif M, Kalkers NF, Van Loenen AC, Staats PG, Gorter RW, Uitdehaag BM, Polman CH. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002 05/14;58(0028-3878; 0028-3878; 9):1404-7.
- Reference 699
-
Aragona M, Onesti E, Tomassini V, Conte A, Gupta S, Gilio F, Pantano P, Pozzilli C, Inghilleri M. Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: A double-blind, placebo controlled, crossover study. Clin Neuropharmacol 2009 01;32(1537-162; 0362-5664; 1):41-7.
- Reference 700
-
Pavisian B, MacIntosh BJ, Szilagyi G, Staines RW, O'Connor P, Feinstein A. Effects of cannabis on cognition in patients with MS: A psychometric and MRI study. Neurology 2014 May 27;82(21):1879-87.
- Reference 701
-
Romero K, Pavisian B, Staines WR, Feinstein A. Multiple sclerosis, cannabis, and cognition: A structural MRI study. Neuroimage Clin 2015 Apr 9;8:140-7.
- Reference 702
-
Freeman RM, Adekanmi O, Waterfield MR, Waterfield AE, Wright D, Zajicek J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: A multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int Urogynecol J Pelvic Floor Dysfunct 2006 11;17(0937-3462; 0937-3462; 6):636-41.
- Reference 703
-
Sliwa JA, Bell HK, Mason KD, Gore RM, Nanninga J, Cohen B. Upper urinary tract abnormalities in multiple sclerosis patients with urinary symptoms. Arch Phys Med Rehabil 1996 03;77(0003-9993; 0003-9993; 3):247-51.
- Reference 704
-
Brady CM, DasGupta R, Dalton C, Wiseman OJ, Berkley KJ, Fowler CJ. An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult Scler 2004 08;10(1352-4585; 1352-4585; 4):425-33.
- Reference 705
-
Rossi S, Bernardi G, Centonze D. The endocannabinoid system in the inflammatory and neurodegenerative processes of multiple sclerosis and of amyotrophic lateral sclerosis. Exp Neurol 2010 07;224(1090-2430; 0014-4886; 1):92-102.
- Reference 706
-
Amtmann D, Weydt P, Johnson KL, Jensen MP, Carter GT. Survey of cannabis use in patients with amyotrophic lateral sclerosis. Am J Hosp Palliat Care 2004 03;21(1049-9091; 1049-9091; 2):95-104.
- Reference 707
-
Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: A randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatr 2010 05/24;81(1468-330; 0022-3050; 10):1135-40.
- Reference 708
-
Gelinas DF, Miller RG, Abood M. Pilot study of safety and tolerability of delta 9-THC (marinol) treatment for ALS. Amyotroph Lateral Scler Other Motor Neuron Disord 2002;3(2):23-4.
- Reference 709
-
Arevalo-Martin A, Molina-Holgado E, Garcia-Ovejero D. Cannabinoids to treat spinal cord injury. Prog Neuropsychopharmacol Biol Psychiatry 2016 Jan 4;64:190-9.
- Reference 710
-
Garcia-Ovejero D, Arevalo-Martin A, Petrosino S, Docagne F, Hagen C, Bisogno T, Watanabe M, Guaza C, Di M,V, Molina-Holgado E. The endocannabinoid system is modulated in response to spinal cord injury in rats. Neurobiol Dis 2009 01;33(1095-953; 0969-9961; 1):57-71.
- Reference 711
-
Hama A, Sagen J. Antinociceptive effect of cannabinoid agonist WIN 55,212-2 in rats with a spinal cord injury. Exp Neurol 2007 03;204(0014-4886; 0014-4886; 1):454-7.
- Reference 712
-
Hama A, Sagen J. Sustained antinociceptive effect of cannabinoid receptor agonist WIN 55,212-2 over time in rat model of neuropathic spinal cord injury pain. J Rehabil Res Dev 2009;46(1938-1352; 0748-7711; 1):135-43.
- Reference 713
-
Kwiatkoski M, Guimaraes FS, Del-Bel E. Cannabidiol-treated rats exhibited higher motor score after cryogenic spinal cord injury. Neurotox Res 2012 Apr;21(3):271-80.
- Reference 714
-
Malec J, Harvey RF, Cayner JJ. Cannabis effect on spasticity in spinal cord injury. Arch Phys Med Rehabil 1982 03;63(0003-9993; 0003-9993; 3):116-8.
- Reference 715
-
Maurer M, Henn V, Dittrich A, Hofmann A. Delta-9-tetrahydrocannabinol shows antispastic and analgesic effects in a single case double-blind trial. Eur Arch Psychiatry Clin Neurosci 1990;240(0940-1334; 0940-1334; 1):1-4.
- Reference 716
-
Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil 2003 02;17(0269-2155; 0269-2155; 1):21-9.
- Reference 717
-
Hagenbach U, Luz S, Ghafoor N, Berger JM, Grotenhermen F, Brenneisen R, Mader M. The treatment of spasticity with Delta9-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord 2007 08;45(1362-4393; 1362-4393; 8):551-62.
- Reference 718
-
Pooyania S, Ethans K, Szturm T, Casey A, Perry D. A randomized, double-blinded, crossover pilot study assessing the effect of nabilone on spasticity in persons with spinal cord injury. Arch Phys Med Rehabil 2010 05;91(1532-821; 0003-9993; 5):703-7.
- Reference 719
-
Amada N, Yamasaki Y, Williams CM, Whalley BJ. Cannabidivarin (CBDV) suppresses pentylenetetrazole (PTZ)-induced increases in epilepsy-related gene expression. PeerJ 2013 Nov 21;1:e214.
- Reference 720
-
Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J,Jr. Epileptic seizures and epilepsy: Definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia 2005 Apr;46(4):470-2.
- Reference 721
-
Hill TD, Cascio MG, Romano B, Duncan M, Pertwee RG, Williams CM, Whalley BJ, Hill AJ. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br J Pharmacol 2013 Oct;170(3):679-92.
- Reference 722
-
Detyniecki K, Hirsch L. Marijuana use in epilepsy: The myth and the reality. Curr Neurol Neurosci Rep 2015 Oct;15(10):65,015-0586-5.
- Reference 723
-
Vilela LR, Medeiros DC, Rezende GH, de Oliveira AC, Moraes MF, Moreira FA. Effects of cannabinoids and endocannabinoid hydrolysis inhibition on pentylenetetrazole-induced seizure and electroencephalographic activity in rats. Epilepsy Res 2013 May;104(3):195-202.
- Reference 724
-
Karlocai MR, Toth K, Watanabe M, Ledent C, Juhasz G, Freund TF, Magloczky Z. Redistribution of CB1 cannabinoid receptors in the acute and chronic phases of pilocarpine-induced epilepsy. PLoS One 2011;6(11):e27196.
- Reference 725
-
Magloczky Z, Toth K, Karlocai R, Nagy S, Eross L, Czirjak S, Vajda J, Rasonyi G, Kelemen A, Juhos V, et al. Dynamic changes of CB1-receptor expression in hippocampi of epileptic mice and humans. Epilepsia 2010 Jul;51 Suppl 3:115-20.
- Reference 726
-
Romigi A, Bari M, Placidi F, Marciani MG, Malaponti M, Torelli F, Izzi F, Prosperetti C, Zannino S, Corte F, et al. Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia 2010 05;51(1528-1167; 0013-9580; 5):768-72.
- Reference 727
-
Falenski KW, Blair RE, Sim-Selley LJ, Martin BR, DeLorenzo RJ. Status epilepticus causes a long-lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat pilocarpine model of acquired epilepsy. Neuroscience 2007 05/25;146(0306-4522; 0306-4522; 3):1232-44.
- Reference 728
-
Ludanyi A, Eross L, Czirjak S, Vajda J, Halasz P, Watanabe M, Palkovits M, Magloczky Z, Freund TF, Katona I. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci 2008 03/19;28(1529-2401; 0270-6474; 12):2976-90.
- Reference 729
-
Falenski KW, Carter DS, Harrison AJ, Martin BR, Blair RE, DeLorenzo RJ. Temporal characterization of changes in hippocampal cannabinoid CB(1) receptor expression following pilocarpine-induced status epilepticus. Brain Res 2009 03/25;1262(1872-6240; 0006-8993):64-72.
- Reference 730
-
Coiret G, Ster J, Grewe B, Wendling F, Helmchen F, Gerber U, Benquet P. Neuron to astrocyte communication via cannabinoid receptors is necessary for sustained epileptiform activity in rat hippocampus. PLoS One 2012;7(5):e37320.
- Reference 731
-
Szaflarski JP, Bebin EM. Cannabis, cannabidiol, and epilepsy--from receptors to clinical response. Epilepsy Behav 2014 Dec;41:277-82.
- Reference 732
-
Citraro R, Russo E, Ngomba RT, Nicoletti F, Scicchitano F, Whalley BJ, Calignano A, De Sarro G. CB1 agonists, locally applied to the cortico-thalamic circuit of rats with genetic absence epilepsy, reduce epileptic manifestations. Epilepsy Res 2013 Sep;106(1-2):74-82.
- Reference 733
-
Jones NA, Glyn SE, Akiyama S, Hill TD, Hill AJ, Weston SE, Burnett MD, Yamasaki Y, Stephens GJ, Whalley BJ, et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure 2012 Jun;21(5):344-52.
- Reference 734
-
Bhaskaran MD, Smith BN. Cannabinoid-mediated inhibition of recurrent excitatory circuitry in the dentate gyrus in a mouse model of temporal lobe epilepsy. PLoS One 2010 May 17;5(5):e10683.
- Reference 735
-
Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM, Whalley BJ, Stephens GJ. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther 2010 Feb;332(2):569-77.
- Reference 736
-
Shafaroodi H, Samini M, Moezi L, Homayoun H, Sadeghipour H, Tavakoli S, Hajrasouliha AR, Dehpour AR. The interaction of cannabinoids and opioids on pentylenetetrazole-induced seizure threshold in mice. Neuropharmacology 2004 09;47(0028-3908; 3):390-400.
- Reference 737
-
Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther 2003 10;307(0022-3565; 1):129-37.
- Reference 738
-
Clement AB, Hawkins EG, Lichtman AH, Cravatt BF. Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase. J Neurosci 2003 05/01;23(1529-2401; 9):3916-23.
- Reference 739
-
Wallace MJ, Martin BR, DeLorenzo RJ. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol 2002 10/11;452(0014-2999; 3):295-301.
- Reference 740
-
Alger BE. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog Neurobiol 2002 11;68(0301-0082; 4):247-86.
- Reference 741
-
Smith PF. Cannabinoids as potential anti-epileptic drugs. Curr Opin Investig Drugs 2005 07;6(1472-4472; 7):680-5.
- Reference 742
-
Mechoulam R, Lichtman AH. Neuroscience. stout guards of the central nervous system. Science 2003 10/03;302(1095-9203; 5642):65-7.
- Reference 743
-
Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci 2000 04/01;20(1529-2401; 7):2470-9.
- Reference 744
-
Nakatsuka T, Chen HX, Roper SN, Gu JG. Cannabinoid receptor-1 activation suppresses inhibitory synaptic activity in human dentate gyrus. Neuropharmacology 2003 07;45(0028-3908; 1):116-21.
- Reference 745
-
Consroe P, Wolkin A. Cannabidiol--antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J Pharmacol Exp Ther 1977 Apr;201(1):26-32.
- Reference 746
-
Consroe P, Benedito MA, Leite JR, Carlini EA, Mechoulam R. Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur J Pharmacol 1982 Sep 24;83(3-4):293-8.
- Reference 747
-
Hill AJ, Mercier MS, Hill TD, Glyn SE, Jones NA, Yamasaki Y, Futamura T, Duncan M, Stott CG, Stephens GJ, et al. Cannabidivarin is anticonvulsant in mouse and rat. Br J Pharmacol 2012 Dec;167(8):1629-42.
- Reference 748
-
Dennis I, Whalley BJ, Stephens GJ. Effects of Delta9-tetrahydrocannabivarin on [35S]GTPgammaS binding in mouse brain cerebellum and piriform cortex membranes. Br J Pharmacol 2008 Jul;154(6):1349-58.
- Reference 749
-
Ma YL, Weston SE, Whalley BJ, Stephens GJ. The phytocannabinoid delta(9)-tetrahydrocannabivarin modulates inhibitory neurotransmission in the cerebellum. Br J Pharmacol 2008 May;154(1):204-15.
- Reference 750
-
Hill AJ, Jones NA, Smith I, Hill CL, Williams CM, Stephens GJ, Whalley BJ. Voltage-gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci Lett 2014 Apr 30;566:269-74.
- Reference 751
-
Hamerle M, Ghaeni L, Kowski A, Weissinger F, Holtkamp M. Cannabis and other illicit drug use in epilepsy patients. Eur J Neurol 2014;21(1):167-70.
- Reference 752
-
Gross DW, Hamm J, Ashworth NL, Quigley D. Marijuana use and epilepsy: Prevalence in patients of a tertiary care epilepsy center. Neurology 2004 Jun 8;62(11):2095-7.
- Reference 753
-
Ladino LD, Hernandez-Ronquillo L, Tellez-Zenteno JF. Medicinal marijuana for epilepsy: A case series study. Can J Neurol Sci 2014 Nov;41(6):753-8.
- Reference 754
-
Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav 2015 Apr;45:49-52.
- Reference 755
-
Mechoulam R, Carlini EA. Toward drugs derived from cannabis. Naturwissenschaften 1978 Apr;65(4):174-9.
- Reference 756
-
Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, Sanvito WL, Lander N, Mechoulam R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 1980;21(0031-7012; 3):175-85.
- Reference 757
-
Ames FR, Cridland S. Anticonvulsant effect of cannabidiol. S Afr Med J 1986 01/04;69(0256-9574; 1):14.
- Reference 758
-
Trembly B, Sherman M. Double-blind clinical study of cannabidiol as a secondary anticonvulsant. Proceedings of the Marijuana '90 International Conference on Cannabis and Cannabinoids 1990:5.
- Reference 759
-
Rubio M, Valdeolivas S, Piscitelli F, Verde R, Satta V, Barroso E, Montolio M, Aras LM, Di Marzo V, Sagredo O, et al. Analysis of endocannabinoid signaling elements and related proteins in lymphocytes of patients with dravet syndrome. Pharmacol Res Perspect 2016 Mar 5;4(2):e00220.
- Reference 760
-
Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, Greenwood SM, Roberts C, Checketts D, VanLandingham KE, et al. Effect of cannabidiol on drop seizures in the lennox-gastaut syndrome. N Engl J Med 2018 May 17;378(20):1888-97.
- Reference 761
-
Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Nielsen S, Herkes GK, Farrell M, Degenhardt L. Evidence for cannabis and cannabinoids for epilepsy: A systematic review of controlled and observational evidence. J Neurol Neurosurg Psychiatry 2018 Jul;89(7):741-53.
- Reference 762
-
Woodhams SG, Sagar DR, Burston JJ, Chapman V. The role of the endocannabinoid system in pain. Handb Exp Pharmacol 2015;227:119-43.
- Reference 763
-
Zogopoulos P, Vasileiou I, Patsouris E, Theocharis SE. The role of endocannabinoids in pain modulation. Fundam Clin Pharmacol 2013 Feb;27(1):64-80.
- Reference 764
-
Hama AT, Sagen J. Cannabinoid receptor-mediated antinociception with acetaminophen drug combinations in rats with neuropathic spinal cord injury pain. Neuropharmacology 2010 03;58(1873-7064; 0028-3908; 4-5):758-66.
- Reference 765
-
Rani Sagar D, Burston JJ, Woodhams SG, Chapman V. Dynamic changes to the endocannabinoid system in models of chronic pain. Philos Trans R Soc Lond B Biol Sci 2012 Dec 5;367(1607):3300-11.
- Reference 766
-
Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 2007 07;10(1097-6256; 1097-6256; 7):870-9.
- Reference 767
-
Guindon J, Beaulieu P. Antihyperalgesic effects of local injections of anandamide, ibuprofen, rofecoxib and their combinations in a model of neuropathic pain. Neuropharmacology 2006 Jun;50(7):814-23.
- Reference 768
-
Woodhams SG, Wong A, Barrett DA, Bennett AJ, Chapman V, Alexander SP. Spinal administration of the monoacylglycerol lipase inhibitor JZL184 produces robust inhibitory effects on nociceptive processing and the development of central sensitization in the rat. Br J Pharmacol 2012 Dec;167(8):1609-19.
- Reference 769
-
Sagar DR, Jhaveri MD, Richardson D, Gray RA, de LE, Fernandez-Ruiz J, Barrett DA, Kendall DA, Chapman V. Endocannabinoid regulation of spinal nociceptive processing in a model of neuropathic pain. Eur J Neurosci 2010 04;31(1460-9568; 0953-816; 8):1414-22.
- Reference 770
-
Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schuttler J, et al. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 2009 Aug 7;325(5941):760-4.
- Reference 771
-
Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci 1991 Feb;11(2):563-83.
- Reference 772
-
Burns HD, Van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, Eng WS, Gibson R, Ryan C, Connolly B, Patel S, et al. 18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci U S A 2007 Jun 5;104(23):9800-5.
- Reference 773
-
Rea K, Roche M, Finn DP. Supraspinal modulation of pain by cannabinoids: The role of GABA and glutamate. Br J Pharmacol 2007 Nov;152(5):633-48.
- Reference 774
-
Petrosino S, Palazzo E, de Novellis V, Bisogno T, Rossi F, Maione S, Di Marzo V. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology 2007 Feb;52(2):415-22.
- Reference 775
-
Nadal X, La Porta C, Andreea Bura S, Maldonado R. Involvement of the opioid and cannabinoid systems in pain control: New insights from knockout studies. Eur J Pharmacol 2013 Sep 15;716(1-3):142-57.
- Reference 776
-
Martin WJ, Coffin PO, Attias E, Balinsky M, Tsou K, Walker JM. Anatomical basis for cannabinoid-induced antinociception as revealed by intracerebral microinjections. Brain Res 1999 Mar 20;822(1-2):237-42.
- Reference 777
-
Lee MC, Ploner M, Wiech K, Bingel U, Wanigasekera V, Brooks J, Menon DK, Tracey I. Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain 2013 Jan;154(1):124-34.
- Reference 778
-
Burston JJ, Woodhams SG. Endocannabinoid system and pain: An introduction. Proc Nutr Soc 2014 Feb;73(1):106-17.
- Reference 779
-
Starowicz K, Przewlocka B. Modulation of neuropathic-pain-related behaviour by the spinal endocannabinoid/endovanilloid system. Philos Trans R Soc Lond B Biol Sci 2012 Dec 5;367(1607):3286-99.
- Reference 780
-
Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, Tao Q, O'Neal ST, Walentiny DM, Wiley JL, et al. In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: Antinociceptive activity without cannabimimetic side effects. Br J Pharmacol 2014 Mar;171(6):1392-407.
- Reference 781
-
Desroches J, Charron S, Bouchard JF, Beaulieu P. Endocannabinoids decrease neuropathic pain-related behavior in mice through the activation of one or both peripheral CB(1) and CB(2) receptors. Neuropharmacology 2014 Feb;77:441-52.
- Reference 782
-
Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, Lichtman AH. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther 2013 Jun;345(3):492-501.
- Reference 783
-
Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol 2009 Jan;5(1):37-44.
- Reference 784
-
Kinsey SG, Long JZ, O'Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther 2009 Sep;330(3):902-10.
- Reference 785
-
Bisogno T, Burston JJ, Rai R, Allara M, Saha B, Mahadevan A, Razdan RK, Wiley JL, Di Marzo V. Synthesis and pharmacological activity of a potent inhibitor of the biosynthesis of the endocannabinoid 2-arachidonoylglycerol. ChemMedChem 2009 Jun;4(6):946-50.
- Reference 786
-
Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A, Tontini A, Mor M, Tarzia G, Calignano A, et al. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3'-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther 2007 Jul;322(1):236-42.
- Reference 787
-
Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, Breitenbucher JG, Chaplan SR, Webb M. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol 2006 May;148(1):102-13.
- Reference 788
-
Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): Effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 2005 Apr;313(1):352-8.
- Reference 789
-
Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, Cravatt BF. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: Evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther 2004 11;311(0022-3565; 2):441-8.
- Reference 790
-
Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 2003 Jan;9(1):76-81.
- Reference 791
-
Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol 2006 Feb;147(3):281-8.
- Reference 792
-
Turcotte C, Chouinard F, Lefebvre JS, Flamand N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol 2015 Jun;97(6):1049-70.
- Reference 793
-
Witkamp R, Meijerink J. The endocannabinoid system: An emerging key player in inflammation. Curr Opin Clin Nutr Metab Care 2014 Mar;17(2):130-8.
- Reference 794
-
Manzanares J, Julian M, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr.Neuropharmacol. 2006 07;4(1570-159; 1570-159; 3):239-57.
- Reference 795
-
Christie MJ, Mallet C. Endocannabinoids can open the pain gate. Sci.Signal. 2009;2(1937-9145; 88):pe57.
- Reference 796
-
Ostenfeld T, Price J, Albanese M, Bullman J, Guillard F, Meyer I, Leeson R, Costantin C, Ziviani L, Nocini PF, et al. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin J Pain 2011 05/02;27(1536-5409; 0749-8047; 8):668-76.
- Reference 797
-
Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs 2010 12/24;70(0012-6667; 0012-6667; 18):2409-38.
- Reference 798
-
Mirchandani A, Saleeb M, Sinatra R. Acute and chronic mechanisms of pain. In: N. Vadivelu, R. D. Urman, R. L. Hines, editors. Essentials of pain management. New York: Springer; 2011. ID: 3011; RP: NOT IN FILE.
- Reference 799
-
Seifert F, Maihofner C. Functional and structural imaging of pain-induced neuroplasticity. Curr.Opin.Anaesthesiol. 2011 10;24(1473-6500; 0952-7907; 5):515-23.
- Reference 800
-
Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci 2009 03;1156(1749-6632; 0077-8923):198-210.
- Reference 801
-
Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet 2010 02/20;375(1474-547; 0140-6736; 9715):686-95.
- Reference 802
-
Pecina M, Zubieta JK. Molecular mechanisms of placebo responses in humans. Mol Psychiatry 2015 Apr;20(4):416-23.
- Reference 803
-
Pecina M, Martinez-Jauand M, Hodgkinson C, Stohler CS, Goldman D, Zubieta JK. FAAH selectively influences placebo effects. Mol Psychiatry 2014 Mar;19(3):385-91.
- Reference 804
-
Benedetti F, Carlino E, Piedimonte A. Increasing uncertainty in CNS clinical trials: The role of placebo, nocebo, and hawthorne effects. Lancet Neurol 2016 Jun;15(7):736-47.
- Reference 805
-
Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol 2001 Oct 26;430(1):41-7.
- Reference 806
-
Craft RM, Wakley AA, Tsutsui KT, Laggart JD. Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by delta9-tetrahydrocannabinol and CP55,940 in the rat. J Pharmacol Exp Ther 2012 Mar;340(3):787-800.
- Reference 807
-
Cooper ZD, Haney M. Sex-dependent effects of cannabis-induced analgesia. Drug Alcohol Depend 2016 Oct 1;167:112-20.
- Reference 808
-
Martin BR, Compton DR, Semus SF, Lin S, Marciniak G, Grzybowska J, Charalambous A, Makriyannis A. Pharmacological evaluation of iodo and nitro analogs of delta 8-THC and delta 9-THC. Pharmacol Biochem Behav 1993 10;46(0091-3057; 0091-3057; 2):295-301.
- Reference 809
-
Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature 1998 09/24;395(0028-0836; 0028-0836; 6700):381-3.
- Reference 810
-
Finn DP, Jhaveri MD, Beckett SR, Roe CH, Kendall DA, Marsden CA, Chapman V. Effects of direct periaqueductal grey administration of a cannabinoid receptor agonist on nociceptive and aversive responses in rats. Neuropharmacology 2003 10;45(0028-3908; 0028-3908; 5):594-604.
- Reference 811
-
Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci 2004 11/03;24(1529-2401; 0270-6474; 44):9953-61.
- Reference 812
-
Hill SY, Schwin R, Goodwin DW, Powell BJ. Marihuana and pain. J Pharmacol Exp Ther 1974 02;188(0022-3565; 2):415-8.
- Reference 813
-
Milstein SL, MacCannell K, Karr G, Clark S. Marijuana-produced changes in pain tolerance. experienced and non-experienced subjects. Int Pharmacopsychiatry 1975;10(0020-8272; 3):177-82.
- Reference 814
-
Redmond WJ, Goffaux P, Potvin S, Marchand S. Analgesic and antihyperalgesic effects of nabilone on experimental heat pain. Curr Med Res Opin 2008 04;24(1473-4877; 0300-7995; 4):1017-24.
- Reference 815
-
Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet 2011 06/25;377(1474-547; 0140-6736; 9784):2215-25.
- Reference 816
-
Seeling W, Kneer L, Buchele B, Gschwend JE, Maier L, Nett C, Simmet T, Steffen P, Schneider M, Rockemann M. Delta(9)-tetrahydrocannabinol and the opioid receptor agonist piritramide do not act synergistically in postoperative pain. Anaesthesist 2006 Apr;55(4):391-400.
- Reference 817
-
Guillaud M, Legagneux F, Paulet C, Leoni J, Lassner J. Essai du levonontradol pour l'analgesie postoperatoire. Cahiers d'Anesthesiologie 1983;31:243-8.
- Reference 818
-
Kalliomaki J, Annas P, Huizar K, Clarke C, Zettergren A, Karlsten R, Segerdahl M. Evaluation of the analgesic efficacy and psychoactive effects of AZD1940, a novel peripherally acting cannabinoid agonist, in human capsaicin-induced pain and hyperalgesia. Clin Exp Pharmacol Physiol 2013 Mar;40(3):212-8.
- Reference 819
-
Stevens AJ, Higgins MD. A systematic review of the analgesic efficacy of cannabinoid medications in the management of acute pain. Acta Anaesthesiol Scand 2017 Mar;61(3):268-80.
- Reference 820
-
Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth 2010 12;105 Suppl 1(1471-6771; 0007-0912):i69-85.
- Reference 821
-
Fine PG, Burton AW, Passik SD. Transformation of acute cancer pain to chronic cancer pain syndromes. J.Support.Oncol. 2012 05;10(1544-6794; 1544-6794; 3):89-95.
- Reference 822
-
Berlach DM, Shir Y, Ware MA. Experience with the synthetic cannabinoid nabilone in chronic noncancer pain. Pain Med. 2006 01;7(1526-2375; 1526-2375; 1):25-9.
- Reference 823
-
de Vries M, van Rijckevorsel DC, Wilder-Smith OH, van Goor H. Dronabinol and chronic pain: Importance of mechanistic considerations. Expert Opin Pharmacother 2014 Aug;15(11):1525-34.
- Reference 824
-
Walker JM, Huang SM. Cannabinoid analgesia. Pharmacol Ther 2002 08;95(0163-7258; 0163-7258; 2):127-35.
- Reference 825
-
Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside. Neurotherapeutics. 2009 10;6(1878-7479; 1878-7479; 4):713-37.
- Reference 826
-
Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol 2007 02/05;556(0014-2999; 1-3):75-83.
- Reference 827
-
Toth CC, Jedrzejewski NM, Ellis CL, Frey WH. Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol.Pain 2010;6(1744-8069; 1744-8069):16.
- Reference 828
-
Xiong W, Cui T, Cheng K, Yang F, Chen SR, Willenbring D, Guan Y, Pan HL, Ren K, Xu Y, et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors. J Exp Med 2012 Jun 4;209(6):1121-34.
- Reference 829
-
Ashton JC, Milligan ED. Cannabinoids for the treatment of neuropathic pain: Clinical evidence. Curr Opin Investig Drugs 2008 01;9(1472-4472; 1472-4472; 1):65-75.
- Reference 830
-
Grotenhermen F. The toxicology of cannabis and cannabis prohibition. Chem.Biodivers. 2007 08;4(1612-1880; 1612-1872; 8):1744-69.
- Reference 831
-
Cooper ZD, Comer SD, Haney M. Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology 2013 Sep;38(10):1984-92.
- Reference 832
-
Grant I. Medicinal cannabis and painful sensory neuropathy. Virtual Mentor 2013 May 1;15(5):466-9.
- Reference 833
-
Andreae MH, Carter GM, Shaparin N, Suslov K, Ellis RJ, Ware MA, Abrams DI, Prasad H, Wilsey B, Indyk D, et al. Inhaled cannabis for chronic neuropathic pain: A meta-analysis of individual patient data. J Pain 2015 Dec;16(12):1221-32.
- Reference 834
-
Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: Results of a randomised controlled trial. Pain 2004 12;112(0304-3959; 0304-3959; 3):299-306.
- Reference 835
-
Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005 09/27;65(1526-632; 6):812-9.
- Reference 836
-
Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, Coderre T, Morley-Forster PK, Stinson J, Boulanger A, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the canadian pain society. Pain Res.Manag. 2007;12(1203-6765; 1):13-21.
- Reference 837
-
Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, Furlan A, Gilron I, Gordon A, Morley-Forster PK, et al. Pharmacological management of chronic neuropathic pain: Revised consensus statement from the canadian pain society. Pain Res Manag 2014 Nov-Dec;19(6):328-35.
- Reference 838
-
Fitzcharles MA, Ste-Marie PA, Goldenberg DL, Pereira JX, Abbey S, Choinier M, Ko G, Moulin D, Panopalis D, Proulx J, et al. 2012 canadian guidelines for the diagnosis and management of fibromyalgia syndrome. 2012.
- Reference 839
-
Hoggart B, Ratcliffe S, Ehler E, Simpson KH, Hovorka J, Lejcko J, Taylor L, Lauder H, Serpell M. A multicentre, open-label, follow-on study to assess the long-term maintenance of effect, tolerance and safety of THC/CBD oromucosal spray in the management of neuropathic pain. J Neurol 2015 Jan;262(1):27-40.
- Reference 840
-
Noyes R,Jr., Brunk SF, Baram DA, Canter A. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharmacol 1975 02;15(0091-2700; 2-3):139-43.
- Reference 841
-
Bushlin I, Rozenfeld R, Devi LA. Cannabinoid-opioid interactions during neuropathic pain and analgesia. Curr.Opin.Pharmacol. 2010 02;10(1471-4973; 1471-4892; 1):80-6.
- Reference 842
-
Desroches J, Beaulieu P. Opioids and cannabinoids interactions: Involvement in pain management. Curr Drug Targets 2010 04;11(1873-5592; 1389-4501; 4):462-73.
- Reference 843
-
Parolaro D, Rubino T, Vigano D, Massi P, Guidali C, Realini N. Cellular mechanisms underlying the interaction between cannabinoid and opioid system. Curr Drug Targets 2010 04;11(1873-5592; 1389-4501; 4):393-405.
- Reference 844
-
Rios C, Gomes I, Devi LA. Mu opioid and CB1 cannabinoid receptor interactions: Reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol 2006 06;148(0007-1188; 0007-1188; 4):387-95.
- Reference 845
-
Rozenfeld R, Bushlin I, Gomes I, Tzavaras N, Gupta A, Neves S, Battini L, Gusella GL, Lachmann A, Ma'ayan A, et al. Receptor heteromerization expands the repertoire of cannabinoid signaling in rodent neurons. PLoS.One. 2012;7(1932-6203; 1932-6203; 1):e29239.
- Reference 846
-
Welch SP, Stevens DL. Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. J Pharmacol Exp Ther 1992 07;262(0022-3565; 0022-3565; 1):10-8.
- Reference 847
-
Pugh G,Jr., Smith PB, Dombrowski DS, Welch SP. The role of endogenous opioids in enhancing the antinociception produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. J Pharmacol Exp Ther 1996 11;279(0022-3565; 0022-3565; 2):608-16.
- Reference 848
-
Smith FL, Cichewicz D, Martin ZL, Welch SP. The enhancement of morphine antinociception in mice by delta9-tetrahydrocannabinol. Pharmacol Biochem Behav 1998 06;60(0091-3057; 0091-3057; 2):559-66.
- Reference 849
-
Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci 2004 01/30;74(0024-3205; 0024-3205; 11):1317-24.
- Reference 850
-
Cichewicz DL, McCarthy EA. Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. J Pharmacol Exp Ther 2003 03;304(0022-3565; 0022-3565; 3):1010-5.
- Reference 851
-
Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol 2007 10/01;571(0014-2999; 0014-2999; 2-3):129-37.
- Reference 852
-
Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, Murnion B, Lintzeris N, Khor KE, Farrell M, Smith A, et al. Opioid-sparing effect of cannabinoids: A systematic review and meta-analysis. Neuropsychopharmacology 2017 Aug;42(9):1752-65.
- Reference 853
-
Reiman A, Welty M, Solomon P. Cannabis as a substitute for opioid-based pain medication: Patient self-report. Cannabis Cannabinoid Res 2017 Jun 1;2(1):160-6.
- Reference 854
-
Lynch ME, Clark AJ. Cannabis reduces opioid dose in the treatment of chronic non-cancer pain. J Pain Symptom Manage 2003 Jun;25(6):496-8.
- Reference 855
-
Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the united states, 1999-2010. JAMA Intern Med 2014 Oct;174(10):1668-73.
- Reference 856
-
Livingston MD, Barnett TE, Delcher C, Wagenaar AC. Recreational cannabis legalization and opioid-related deaths in colorado, 2000-2015. Am J Public Health 2017;107(11):1827-9.
- Reference 857
-
Bradford AC, Bradford WD, Abraham A, Adams GB. Association between US state medical cannabis laws and opioid prescribing in the medicare part D population. JAMA Internal Medicine 2018;178(5):667-72.
- Reference 858
-
Wen H, Hockenberry JM. Association of medical and adult-use marijuana laws with opioid prescribing for medicaid enrollees. JAMA Intern Med 2018 May 1;178(5):673-9.
- Reference 859
-
Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain 2016 Jun;17(6):739-44.
- Reference 860
-
Hefner K, Sofuoglu M, Rosenheck R. Concomitant cannabis abuse/dependence in patients treated with opioids for non-cancer pain. Am J Addict 2015 Sep;24(6):538-45.
- Reference 861
-
Olfson M, Wall MM, Liu SM, Blanco C. Cannabis use and risk of prescription opioid use disorder in the united states. Am J Psychiatry 2017 Sep 26:appiajp201717040413.
- Reference 862
-
Vigil JM, Stith SS, Adams IM, Reeve AP. Associations between medical cannabis and prescription opioid use in chronic pain patients: A preliminary cohort study. PLoS One 2017 Nov 16;12(11):e0187795.
- Reference 863
-
Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharmacol 2006 01/13;530(0014-2999; 1-2):54-8.
- Reference 864
-
Cooper ZD, Bedi G, Ramesh D, Balter R, Comer SD, Haney M. Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology 2018:1.
- Reference 865
-
Russo EB. Clinical endocannabinoid deficiency (CECD): Can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro.Endocrinol.Lett. 2004 02;25(0172-780; 0172-780; 1-2):31-9.
- Reference 866
-
Sarchielli P, Pini LA, Coppola F, Rossi C, Baldi A, Mancini ML, Calabresi P. Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology 2007 06;32(0893-133; 0006-3223; 6):1384-90.
- Reference 867
-
Villalon CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther 2009 12;124(1879-016; 0163-7258; 3):309-23.
- Reference 868
-
Cupini LM, Costa C, Sarchielli P, Bari M, Battista N, Eusebi P, Calabresi P, Maccarrone M. Degradation of endocannabinoids in chronic migraine and medication overuse headache. Neurobiol Dis 2008 05;30(1095-953; 0969-9961; 2):186-9.
- Reference 869
-
Greco R, Gasperi V, Maccarrone M, Tassorelli C. The endocannabinoid system and migraine. Exp Neurol 2010 07;224(1090-2430; 0014-4886; 1):85-91.
- Reference 870
-
Napchan U, Buse DC, Loder EW. The use of marijuana or synthetic cannabinoids for the treatment of headache. Headache 2011 03;51(1526-4610; 0017-8748; 3):502-5.
- Reference 871
-
McGeeney BE. Hallucinogens and cannabinoids for headache. Headache 2012 10;52 Suppl 2(1526-4610; 0017-8748):94-7.
- Reference 872
-
Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL, Gorelick DA. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend 2010 05/24;111(1879-0046; 0376-8716; 1-2):120-7.
- Reference 873
-
Lochte BC, Beletsky A, Samuel NK, Grant I. The use of cannabis for headache disorders. Cannabis Cannabinoid Res 2017 Apr 1;2(1):61-71.
- Reference 874
-
Gui H, Liu X, Liu LR, Su DF, Dai SM. Activation of cannabinoid receptor 2 attenuates synovitis and joint distruction in collagen-induced arthritis. Immunobiology 2015 Jun;220(6):817-22.
- Reference 875
-
Gui H, Tong Q, Qu W, Mao CM, Dai SM. The endocannabinoid system and its therapeutic implications in rheumatoid arthritis. Int Immunopharmacol 2015 May;26(1):86-91.
- Reference 876
-
La Porta C, Bura SA, Llorente-Onaindia J, Pastor A, Navarrete F, Garcia-Gutierrez MS, De la Torre R, Manzanares J, Monfort J, Maldonado R. Role of the endocannabinoid system in the emotional manifestations of osteoarthritis pain. Pain 2015 Oct;156(10):2001-12.
- Reference 877
-
La Porta C, Bura SA, Negrete R, Maldonado R. Involvement of the endocannabinoid system in osteoarthritis pain. Eur J Neurosci 2014 Feb;39(3):485-500.
- Reference 878
-
Rahman W, Dickenson AH. Emerging targets and therapeutic approaches for the treatment of osteoarthritis pain. Curr Opin Support Palliat Care 2015 Jun;9(2):124-30.
- Reference 879
-
Gui H, Liu X, Wang ZW, He DY, Su DF, Dai SM. Expression of cannabinoid receptor 2 and its inhibitory effects on synovial fibroblasts in rheumatoid arthritis. Rheumatology (Oxford) 2014 May;53(5):802-9.
- Reference 880
-
Krustev E, Reid A, McDougall JJ. Tapping into the endocannabinoid system to ameliorate acute inflammatory flares and associated pain in mouse knee joints. Arthritis Res Ther 2014 Sep 27;16(5):437,014-0437-9.
- Reference 881
-
Staunton CA, Mobasheri A, Barrett-Jolley R. High hopes for cannabinoid agonists in the treatment of rheumatic diseases. BMC Musculoskelet Disord 2014 Dec 4;15:410,2474-15-410.
- Reference 882
-
McDougall JJ, Linton P. Neurophysiology of arthritis pain. Curr Pain Headache Rep 2012 Dec;16(6):485-91.
- Reference 883
-
Canadian Alcohol and Drug Use Monitoring Survey (CADUMS) [Internet]: Health Canada; c2011 [cited 2015. Available from: http://hc-sc.gc.ca/hc-ps/drugs-drogues/stat/_2011/summary-sommaire-eng.php#a3.
- Reference 884
-
Swift W, Gates P, Dillon P. Survey of australians using cannabis for medical purposes. Harm Reduct J 2005 Oct 4;2:18.
- Reference 885
-
Walsh Z, Callaway R, Belle-Isle L, Capler R, Kay R, Lucas P, Holtzman S. Cannabis for therapeutic purposes: Patient characteristics, access, and reasons for use. Int J Drug Policy 2013 Nov;24(6):511-6.
- Reference 886
-
Dunkley L, Tattersall R. Osteoarthritis and the inflammatory arthritides. Surgery 2012;30(2):67-71.
- Reference 887
-
Baliki MN, Geha PY, Jabakhanji R, Harden N, Schnitzer TJ, Apkarian AV. A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain 2008 Oct 25;4:47,8069-4-47.
- Reference 888
-
Kulkarni B, Bentley DE, Elliott R, Julyan PJ, Boger E, Watson A, Boyle Y, El-Deredy W, Jones AK. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum 2007 Apr;56(4):1345-54.
- Reference 889
-
Sagar DR, Staniaszek LE, Okine BN, Woodhams S, Norris LM, Pearson RG, Garle MJ, Alexander SP, Bennett AJ, Barrett DA, et al. Tonic modulation of spinal hyperexcitability by the endocannabinoid receptor system in a rat model of osteoarthritis pain. Arthritis Rheum 2010 12;62(1529-0131; 0004-3591; 12):3666-76.
- Reference 890
-
McDougall JJ. Cannabinoids and pain control in the periphery. In: B. E. Cairns, editor. Peripheral receptor targets for analgesia: Novel approaches to pain management. Hoboken, New Jersey: John Wiley and Sons Inc.; 2009..
- Reference 891
-
Schuelert N, Zhang C, Mogg AJ, Broad LM, Hepburn DL, Nisenbaum ES, Johnson MP, McDougall JJ. Paradoxical effects of the cannabinoid CB2 receptor agonist GW405833 on rat osteoarthritic knee joint pain. Osteoarthritis Cartilage 2010 Nov;18(11):1536-43.
- Reference 892
-
La Porta C, Bura SA, Aracil-Fernandez A, Manzanares J, Maldonado R. Role of CB1 and CB2 cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate. Pain 2013 Jan;154(1):160-74.
- Reference 893
-
Schuelert N, McDougall JJ. Cannabinoid-mediated antinociception is enhanced in rat osteoarthritic knees. Arthritis Rheum 2008 01;58(0004-3591; 0004-3591; 1):145-53.
- Reference 894
-
Schuelert N, Johnson MP, Oskins JL, Jassal K, Chambers MG, McDougall JJ. Local application of the endocannabinoid hydrolysis inhibitor URB597 reduces nociception in spontaneous and chemically induced models of osteoarthritis. Pain 2011 05;152(1872-6623; 0304-3959; 5):975-81.
- Reference 895
-
Yao BB, Hsieh GC, Frost JM, Fan Y, Garrison TR, Daza AV, Grayson GK, Zhu CZ, Pai M, Chandran P, et al. In vitro and in vivo characterization of A-796260: A selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. Br J Pharmacol 2008 Jan;153(2):390-401.
- Reference 896
-
Burston JJ, Sagar DR, Shao P, Bai M, King E, Brailsford L, Turner JM, Hathway GJ, Bennett AJ, Walsh DA, et al. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PLoS One 2013 Nov 25;8(11):e80440.
- Reference 897
-
Sophocleous A, Borjesson AE, Salter DM, Ralston SH. The type 2 cannabinoid receptor regulates susceptibility to osteoarthritis in mice. Osteoarthritis Cartilage 2015 Sep;23(9):1586-94.
- Reference 898
-
Silveira JW, Issy AC, Castania VA, Salmon CE, Nogueira-Barbosa MH, Guimaraes FS, Defino HL, Del Bel E. Protective effects of cannabidiol on lesion-induced intervertebral disc degeneration. PLoS One 2014 Dec 17;9(12):e113161.
- Reference 899
-
Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 2012 Sep;153(9):1837-46.
- Reference 900
-
Richards BL, Whittle SL, Buchbinder R. Neuromodulators for pain management in rheumatoid arthritis. Cochrane Database Syst Rev 2012;1(1469-493; 1361-6137):CD008921.
- Reference 901
-
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011 12/08;365(1533-4406; 0028-4793; 23):2205-19.
- Reference 902
-
Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A 2000 Aug 15;97(17):9561-6.
- Reference 903
-
Cox ML, Welch SP. The antinociceptive effect of Delta9-tetrahydrocannabinol in the arthritic rat. Eur J Pharmacol 2004 Jun 16;493(1-3):65-74.
- Reference 904
-
Cox ML, Haller VL, Welch SP. The antinociceptive effect of Delta9-tetrahydrocannabinol in the arthritic rat involves the CB(2) cannabinoid receptor. Eur J Pharmacol 2007 Sep 10;570(1-3):50-6.
- Reference 905
-
Fukuda S, Kohsaka H, Takayasu A, Yokoyama W, Miyabe C, Miyabe Y, Harigai M, Miyasaka N, Nanki T. Cannabinoid receptor 2 as a potential therapeutic target in rheumatoid arthritis. BMC Musculoskelet Disord 2014 Aug 12;15:275,2474-15-275.
- Reference 906
-
McDougall JJ, Yu V, Thomson J. In vivo effects of CB2 receptor-selective cannabinoids on the vasculature of normal and arthritic rat knee joints. Br J Pharmacol 2008 Jan;153(2):358-66.
- Reference 907
-
Hammell DC, Zhang LP, Ma F, Abshire SM, McIlwrath SL, Stinchcomb AL, Westlund KN. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain 2016 Jul;20(6):936-48.
- Reference 908
-
Smith HS, Bracken D, Smith JM. Pharmacotherapy for fibromyalgia. Front Pharmacol. 2011;2(1663-9812):17.
- Reference 909
-
Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 2005 03;114(0304-3959; 0304-3959; 1-2):295-302.
- Reference 910
-
Clauw DJ, Arnold LM, McCarberg BH. The science of fibromyalgia. Mayo Clin Proc 2011 09;86(1942-5546; 0025-6196; 9):907-11.
- Reference 911
-
Normand E, Potvin S, Gaumond I, Cloutier G, Corbin JF, Marchand S. Pain inhibition is deficient in chronic widespread pain but normal in major depressive disorder. J Clin Psychiatry 2011 02;72(1555-2101; 0160-6689; 2):219-24.
- Reference 912
-
Becker S, Schweinhardt P. Dysfunctional neurotransmitter systems in fibromyalgia, their role in central stress circuitry and pharmacological actions on these systems. Pain Res.Treat. 2012;2012(2090-1550; 2090-1542):741746.
- Reference 913
-
de Souza JB, Potvin S, Goffaux P, Charest J, Marchand S. The deficit of pain inhibition in fibromyalgia is more pronounced in patients with comorbid depressive symptoms. Clin J Pain 2009 02;25(1536-5409; 0749-8047; 2):123-7.
- Reference 914
-
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The american college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res.(Hoboken.) 2010 05;62(2151-4658; 2151-464; 5):600-10.
- Reference 915
-
Walitt B, Klose P, Fitzcharles MA, Phillips T, Hauser W. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev 2016 Jul 18;7:CD011694.
- Reference 916
-
Bagues A, Martin MI, Sanchez-Robles EM. Involvement of central and peripheral cannabinoid receptors on antinociceptive effect of tetrahydrocannabinol in muscle pain. Eur J Pharmacol 2014 Dec 15;745:69-75.
- Reference 917
-
Sanchez Robles EM, Bagues Arias A, Martin Fontelles MI. Cannabinoids and muscular pain. effectiveness of the local administration in rat. Eur J Pain 2012 Sep;16(8):1116-27.
- Reference 918
-
Idris AI, Sophocleous A, Landao-Bassonga E, Canals M, Milligan G, Baker D, van't Hof RJ, Ralston SH. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab 2009 08;10(1932-7420; 1550-4131; 2):139-47.
- Reference 919
-
Bab I, Smoum R, Bradshaw H, Mechoulam R. Skeletal lipidomics: Regulation of bone metabolism by fatty acid amide family. Br J Pharmacol 2011 08;163(1476-5381; 0007-1188; 7):1441-6.
- Reference 920
-
Idris AI, van 't Hof RJ, Greig IR, Ridge SA, Baker D, Ross RA, Ralston SH. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med 2005 07;11(1078-8956; 1078-8956; 7):774-9.
- Reference 921
-
Whyte LS, Ford L, Ridge SA, Cameron GA, Rogers MJ, Ross RA. Cannabinoids and bone: Endocannabinoids modulate human osteoclast function in vitro. Br J Pharmacol 2012 04;165(1476-5381; 0007-1188; 8):2584-97.
- Reference 922
-
Tam J, Ofek O, Fride E, Ledent C, Gabet Y, Muller R, Zimmer A, Mackie K, Mechoulam R, Shohami E, et al. Involvement of neuronal cannabinoid receptor CB1 in regulation of bone mass and bone remodeling. Mol Pharmacol 2006 09;70(0026-895; 0026-895; 3):786-92.
- Reference 923
-
Tam J, Trembovler V, Di M,V, Petrosino S, Leo G, Alexandrovich A, Regev E, Casap N, Shteyer A, Ledent C, et al. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J 2008 01;22(1530-6860; 0892-6638; 1):285-94.
- Reference 924
-
Rossi F, Siniscalco D, Luongo L, De PL, Bellini G, Petrosino S, Torella M, Santoro C, Nobili B, Perrotta S, et al. The endovanilloid/endocannabinoid system in human osteoclasts: Possible involvement in bone formation and resorption. Bone 2009 03;44(1873-2763; 1873-2763; 3):476-84.
- Reference 925
-
Samir SM, Malek HA. Effect of cannabinoid receptors 1 modulation on osteoporosis in a rat model of different ages. J Physiol Pharmacol 2014 Oct;65(5):687-94.
- Reference 926
-
Wasserman E, Tam J, Mechoulam R, Zimmer A, Maor G, Bab I. CB1 cannabinoid receptors mediate endochondral skeletal growth attenuation by Delta9-tetrahydrocannabinol. Ann N Y Acad Sci 2015 Jan;1335:110-9.
- Reference 927
-
Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci U S A 2006 01/17;103(0027-8424; 0027-8424; 3):696-701.
- Reference 928
-
Sophocleous A, Landao-Bassonga E, van't Hof RJ, Idris AI, Ralston SH. The type 2 cannabinoid receptor regulates bone mass and ovariectomy-induced bone loss by affecting osteoblast differentiation and bone formation. Endocrinology 2011 06;152(1945-7170; 0013-7227; 6):2141-9.
- Reference 929
-
Idris AI, Sophocleous A, Landao-Bassonga E, van't Hof RJ, Ralston SH. Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology 2008 11;149(0013-7227; 0013-7227; 11):5619-26.
- Reference 930
-
Karsak M, Cohen-Solal M, Freudenberg J, Ostertag A, Morieux C, Kornak U, Essig J, Erxlebe E, Bab I, Kubisch C, et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet 2005 11/15;14(0964-6906; 0964-6906; 22):3389-96.
- Reference 931
-
Karsak M, Malkin I, Toliat MR, Kubisch C, Nurnberg P, Zimmer A, Livshits G. The cannabinoid receptor type 2 (CNR2) gene is associated with hand bone strength phenotypes in an ethnically homogeneous family sample. Hum Genet 2009 11;126(1432-1203; 0340-6717; 5):629-36.
- Reference 932
-
Huang QY, Li GH, Kung AW. Multiple osteoporosis susceptibility genes on chromosome 1p36 in chinese. Bone 2009 05;44(1873-2763; 1873-2763; 5):984-8.
- Reference 933
-
Fernandez-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol 2009 04;156(1476-5381; 0007-1188; 7):1029-40.
- Reference 934
-
Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in huntington's disease: A comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in huntington's disease. Neuroscience 2000;97(0306-4522; 0306-4522; 3):505-19.
- Reference 935
-
Romero J, Berrendero F, Perez-Rosado A, Manzanares J, Rojo A, Fernandez-Ruiz JJ, de Yebenes JG, Ramos JA. Unilateral 6-hydroxydopamine lesions of nigrostriatal dopaminergic neurons increased CB1 receptor mRNA levels in the caudate-putamen. Life Sci 2000;66(0024-3205; 0024-3205; 6):485-94.
- Reference 936
-
Lastres-Becker I, Fezza F, Cebeira M, Bisogno T, Ramos JA, Milone A, Fernandez-Ruiz J, Di M,V. Changes in endocannabinoid transmission in the basal ganglia in a rat model of huntington's disease. Neuroreport 2001 07/20;12(0959-4965; 0959-4965; 10):2125-9.
- Reference 937
-
Garcia-Arencibia M, Garcia C, Fernandez-Ruiz J. Cannabinoids and parkinson's disease. CNS.Neurol.Disord.Drug Targets. 2009 12;8(1996-3181; 1871-5273; 6):432-9.
- Reference 938
-
Koppel BS. Cannabis in the treatment of dystonia, dyskinesias, and tics. Neurotherapeutics 2015 Oct;12(4):788-92.
- Reference 939
-
Richter A, Loscher W. (+)-WIN 55,212-2, a novel cannabinoid receptor agonist, exerts antidystonic effects in mutant dystonic hamsters. Eur J Pharmacol 1994 11/03;264(0014-2999; 0014-2999; 3):371-7.
- Reference 940
-
Richter A, Loscher W. Effects of pharmacological manipulations of cannabinoid receptors on severity of dystonia in a genetic model of paroxysmal dyskinesia. Eur J Pharmacol 2002 11/15;454(0014-2999; 0014-2999; 2-3):145-51.
- Reference 941
-
Madsen MV, Peacock LP, Werge T, Andersen MB, Andreasen JT. Effects of cannabinoid CB(1) receptor agonism and antagonism on SKF81297-induced dyskinesia and haloperidol-induced dystonia in cebus apella monkeys. Neuropharmacology 2011 02;60(1873-7064; 0028-3908; 2-3):418-22.
- Reference 942
-
Marsden CD. Treatment of torsion dystonia In: A. Barbeau, editor. Disorders of movement, current status of modern therapy. Philadelphia, PA.: Lippincott; 1981..
- Reference 943
-
Chatterjee A, Almahrezi A, Ware M, Fitzcharles MA. A dramatic response to inhaled cannabis in a woman with central thalamic pain and dystonia. J Pain Symptom Manage 2002 Jul;24(1):4-6.
- Reference 944
-
Denovan-Wright EM, Robertson HA. Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic huntington's disease mice. Neuroscience 2000;98(0306-4522; 0306-4522; 4):705-13.
- Reference 945
-
Lastres-Becker I, Gomez M, de MR, Ramos JA, Fernandez-Ruiz J. Loss of cannabinoid CB(1) receptors in the basal ganglia in the late akinetic phase of rats with experimental huntington's disease. Neurotox.Res. 2002 11;4(1476-3524; 1029-8428; 7-8):601-8.
- Reference 946
-
Naver B, Stub C, Moller M, Fenger K, Hansen AK, Hasholt L, Sorensen SA. Molecular and behavioral analysis of the R6/1 huntington's disease transgenic mouse. Neuroscience 2003;122(0306-4522; 0306-4522; 4):1049-57.
- Reference 947
-
McCaw EA, Hu H, Gomez GT, Hebb AL, Kelly ME, Denovan-Wright EM. Structure, expression and regulation of the cannabinoid receptor gene (CB1) in huntington's disease transgenic mice. Eur J Biochem 2004 12;271(0014-2956; 0014-2956; 23-24):4909-20.
- Reference 948
-
Centonze D, Rossi S, Prosperetti C, Tscherter A, Bernardi G, Maccarrone M, Calabresi P. Abnormal sensitivity to cannabinoid receptor stimulation might contribute to altered gamma-aminobutyric acid transmission in the striatum of R6/2 huntington's disease mice. Biol Psychiatry 2005 06/15;57(0006-3223; 0006-3223; 12):1583-9.
- Reference 949
-
Pazos MR, Sagredo O, Fernandez-Ruiz J. The endocannabinoid system in huntington's disease. Curr Pharm Des 2008;14(1873-4286; 1381-6128; 23):2317-25.
- Reference 950
-
Dowie MJ, Bradshaw HB, Howard ML, Nicholson LF, Faull RL, Hannan AJ, Glass M. Altered CB1 receptor and endocannabinoid levels precede motor symptom onset in a transgenic mouse model of huntington's disease. Neuroscience 2009 09/29;163(1873-7544; 0306-4522; 1):456-65.
- Reference 951
-
Blazquez C, Chiarlone A, Sagredo O, Aguado T, Pazos MR, Resel E, Palazuelos J, Julien B, Salazar M, Borner C, et al. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in huntington's disease. Brain 2011 01;134(1460-2156; 0006-8950):119-36.
- Reference 952
-
Casteels C, Vandeputte C, Rangarajan JR, Dresselaers T, Riess O, Bormans G, Maes F, Himmelreich U, Nguyen H, Van LK. Metabolic and type 1 cannabinoid receptor imaging of a transgenic rat model in the early phase of huntington disease. Exp Neurol 2011 06;229(1090-2430; 0014-4886; 2):440-9.
- Reference 953
-
Mievis S, Blum D, Ledent C. Worsening of huntington disease phenotype in CB1 receptor knockout mice. Neurobiol Dis 2011 06;42(1095-953; 0969-9961; 3):524-9.
- Reference 954
-
Van Laere K., Casteels C, Dhollander I, Goffin K, Grachev I, Bormans G, Vandenberghe W. Widespread decrease of type 1 cannabinoid receptor availability in huntington disease in vivo. J Nucl Med 2010 09;51(1535-5667; 0161-5505; 9):1413-7.
- Reference 955
-
Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, Sagredo O, Benito C, Romero J, Azcoitia I, et al. Microglial CB2 cannabinoid receptors are neuroprotective in huntington's disease excitotoxicity. Brain 2009 11;132(1460-2156; 0006-8950):3152-64.
- Reference 956
-
Dowie MJ, Howard ML, Nicholson LF, Faull RL, Hannan AJ, Glass M. Behavioural and molecular consequences of chronic cannabinoid treatment in huntington's disease transgenic mice. Neuroscience 2010 09/29;170(1873-7544; 0306-4522; 1):324-36.
- Reference 957
-
Sagredo O, Gonzalez S, Aroyo I, Pazos MR, Benito C, Lastres-Becker I, Romero JP, Tolon RM, Mechoulam R, Brouillet E, et al. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: Relevance for huntington's disease. Glia 2009 Aug 15;57(11):1154-67.
- Reference 958
-
Chiarlone A, Bellocchio L, Blazquez C, Resel E, Soria-Gomez E, Cannich A, Ferrero JJ, Sagredo O, Benito C, Romero J, et al. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc Natl Acad Sci U S A 2014 Jun 3;111(22):8257-62.
- Reference 959
-
Curtis A, Rickards H. Nabilone could treat chorea and irritability in huntington's disease. J Neuropsychiatry Clin Neurosci 2006;18(0895-0172; 0895-0172; 4):553-4.
- Reference 960
-
Venderova K, Ruzicka E, Vorisek V, Visnovsky P. Survey on cannabis use in parkinson's disease: Subjective improvement of motor symptoms. Mov Disord 2004 Sep;19(9):1102-6.
- Reference 961
-
Pisani V, Moschella V, Bari M, Fezza F, Galati S, Bernardi G, Stanzione P, Pisani A, Maccarrone M. Dynamic changes of anandamide in the cerebrospinal fluid of parkinson's disease patients. Mov Disord 2010 05/15;25(1531-8257; 0885-3185; 7):920-4.
- Reference 962
-
Garcia-Arencibia M, Garcia C, Kurz A, Rodriguez-Navarro JA, Gispert-Sachez S, Mena MA, Auburger G, de Yebenes JG, Fernandez-Ruiz J. Cannabinoid CB1 receptors are early downregulated followed by a further upregulation in the basal ganglia of mice with deletion of specific park genes. J Neural Transm Suppl 2009(0303-6995; 0303-6995; 73):269-75.
- Reference 963
-
Papa SM. The cannabinoid system in parkinson's disease: Multiple targets to motor effects. Exp Neurol 2008 06;211(1090-2430; 0014-4886; 2):334-8.
- Reference 964
-
Gonzalez S, Scorticati C, Garcia-Arencibia M, de Miguel R, Ramos JA, Fernandez-Ruiz J. Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of parkinson's disease. Brain Res 2006 Feb 16;1073-1074:209-19.
- Reference 965
-
Cerri S, Levandis G, Ambrosi G, Montepeloso E, Antoninetti GF, Franco R, Lanciego JL, Baqi Y, Muller CE, Pinna A, et al. Neuroprotective potential of adenosine A2A and cannabinoid CB1 receptor antagonists in an animal model of parkinson disease. J Neuropathol Exp Neurol 2014 May;73(5):414-24.
- Reference 966
-
Fernandez-Ruiz J, Romero J, Ramos JA. Endocannabinoids and neurodegenerative disorders: Parkinson's disease, huntington's chorea, alzheimer's disease, and others. Handb Exp Pharmacol 2015;231:233-59.
- Reference 967
-
Rodriguez-Cueto C, Benito C, Fernandez-Ruiz J, Romero J, Hernandez-Galvez M, Gomez-Ruiz M. Changes in CB(1) and CB(2) receptors in the post-mortem cerebellum of humans affected by spinocerebellar ataxias. Br J Pharmacol 2014 Mar;171(6):1472-89.
- Reference 968
-
Rodriguez-Cueto C, Benito C, Romero J, Hernandez-Galvez M, Gomez-Ruiz M, Fernandez-Ruiz J. Endocannabinoid-hydrolysing enzymes in the post-mortem cerebellum of humans affected by hereditary autosomal dominant ataxias. Pathobiology 2014;81(3):149-59.
- Reference 969
-
Cheung W, Guo L, Cordeiro MF. Neuroprotection in glaucoma: Drug-based approaches. Optom Vis Sci 2008 06;85(1040-5488; 1040-5488; 6):406-16.
- Reference 970
-
Jarvinen T, Pate DW, Laine K. Cannabinoids in the treatment of glaucoma. Pharmacol Ther 2002 08;95(0163-7258; 2):203-20.
- Reference 971
-
Jampel H. American glaucoma society position statement: Marijuana and the treatment of glaucoma. J Glaucoma 2010 02;19(1536-481; 1057-0829; 2):75-6.
- Reference 972
-
Chen J, Matias I, Dinh T, Lu T, Venezia S, Nieves A, Woodward DF, Di M,V. Finding of endocannabinoids in human eye tissues: Implications for glaucoma. Biochem Biophys Res Commun 2005 05/20;330(0006-291; 0006-291; 4):1062-7.
- Reference 973
-
Porcella A, Casellas P, Gessa GL, Pani L. Cannabinoid receptor CB1 mRNA is highly expressed in the rat ciliary body: Implications for the antiglaucoma properties of marihuana. Brain Res Mol Brain Res 1998 07/15;58(0169-328; 1-2):240-5.
- Reference 974
-
Straiker AJ, Maguire G, Mackie K, Lindsey J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol Vis Sci 1999 09;40(0146-0404; 10):2442-8.
- Reference 975
-
Porcella A, Maxia C, Gessa GL, Pani L. The human eye expresses high levels of CB1 cannabinoid receptor mRNA and protein. Eur J Neurosci 2000 03;12(0953-816; 3):1123-7.
- Reference 976
-
Song ZH, Slowey CA. Involvement of cannabinoid receptors in the intraocular pressure-lowering effects of WIN55212-2. J Pharmacol Exp Ther 2000 01;292(0022-3565; 1):136-9.
- Reference 977
-
Porcella A, Maxia C, Gessa GL, Pani L. The synthetic cannabinoid WIN55212-2 decreases the intraocular pressure in human glaucoma resistant to conventional therapies. Eur J Neurosci 2001 01;13(0953-816; 2):409-12.
- Reference 978
-
Wan MJ, Daniel S, Kassam F, Mutti G, Butty Z, Kasner O, Trope GE, Buys YM. Survey of complementary and alternative medicine use in glaucoma patients. J Glaucoma 2012 02;21(1536-481; 1057-0829; 2):79-82.
- Reference 979
-
Yoles E, Belkin M, Schwartz M. HU-211, a nonpsychotropic cannabinoid, produces short- and long-term neuroprotection after optic nerve axotomy. J Neurotrauma 1996 01;13(0897-7151; 1):49-57.
- Reference 980
-
Shen M, Thayer SA. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol Pharmacol 1998 09;54(0026-895; 3):459-62.
- Reference 981
-
Levin LA. Direct and indirect approaches to neuroprotective therapy of glaucomatous optic neuropathy. Surv Ophthalmol 1999 06;43 Suppl 1(0039-6257):S98-101.
- Reference 982
-
Jin KL, Mao XO, Goldsmith PC, Greenberg DA. CB1 cannabinoid receptor induction in experimental stroke. Ann Neurol 2000 08;48(0364-5134; 2):257-61.
- Reference 983
-
Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 2001 10/04;413(0028-0836; 6855):527-31.
- Reference 984
-
Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C. Neuroprotective properties of cannabinoids against oxidative stress: Role of the cannabinoid receptor CB1. J Neurochem 2002 02;80(0022-3042; 3):448-56.
- Reference 985
-
Mechoulam R, Panikashvili D, Shohami E. Cannabinoids and brain injury: Therapeutic implications. Trends Mol Med 2002 02;8(1471-4914; 2):58-61.
- Reference 986
-
Braida D, Pegorini S, Arcidiacono MV, Consalez GG, Croci L, Sala M. Post-ischemic treatment with cannabidiol prevents electroencephalographic flattening, hyperlocomotion and neuronal injury in gerbils. Neurosci Lett 2003 07/31;346(0304-3940; 1-2):61-4.
- Reference 987
-
El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol 2006 01;168(0002-9440; 1):235-44.
- Reference 988
-
Gilbert GL, Kim HJ, Waataja JJ, Thayer SA. Delta9-tetrahydrocannabinol protects hippocampal neurons from excitotoxicity. Brain Res 2007 01/12;1128(0006-8993; 1):61-9.
- Reference 989
-
Hepler RS, Frank IR. Marihuana smoking and intraocular pressure. JAMA 1971 09/06;217(0098-7484; 0098-7484; 10):1392.
- Reference 990
-
Merritt JC, Crawford WJ, Alexander PC, Anduze AL, Gelbart SS. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology 1980 03;87(0161-6420; 0161-6420; 3):222-8.
- Reference 991
-
Zhan GL, Camras CB, Palmberg PF, Toris CB. Effects of marijuana on aqueous humor dynamics in a glaucoma patient. J Glaucoma 2005 04;14(1057-0829; 1057-0829; 2):175-7.
- Reference 992
-
Abboud RT, Sanders HD. Effect of oral administration of delta-tetrahydrocannabinol on airway mechanics in normal and asthmatic subjects. Chest 1976 10;70(0012-3692; 0012-3692; 4):480-5.
- Reference 993
-
Calignano A, Katona I, Desarnaud F, Giuffrida A, La RG, Mackie K, Freund TF, Piomelli D. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature 2000 11/02;408(0028-0836; 0028-0836; 6808):96-101.
- Reference 994
-
Jan TR, Farraj AK, Harkema JR, Kaminski NE. Attenuation of the ovalbumin-induced allergic airway response by cannabinoid treatment in A/J mice. Toxicol Appl Pharmacol 2003 04/01;188(0041-008; 0041-008; 1):24-35.
- Reference 995
-
Giannini L, Nistri S, Mastroianni R, Cinci L, Vannacci A, Mariottini C, Passani MB, Mannaioni PF, Bani D, Masini E. Activation of cannabinoid receptors prevents antigen-induced asthma-like reaction in guinea pigs. J Cell Mol Med 2008 12;12(1582-1838; 1582-1838; 6):2381-94.
- Reference 996
-
Fukuda H, Abe T, Yoshihara S. The cannabinoid receptor agonist WIN 55,212-2 inhibits antigen-induced plasma extravasation in guinea pig airways. Int Arch Allergy Immunol 2010;152(1423-0097; 1018-2438; 3):295-300.
- Reference 997
-
Vachon L, FitzGerald MX, Solliday NH, Gould IA, Gaensler EA. Single-dose effects of marihuana smoke. bronchial dynamics and respiratory-center sensitivity in normal subjects. N Engl J Med 1973 05/10;288(0028-4793; 0028-4793; 19):985-9.
- Reference 998
-
Tashkin DP, Shapiro BJ, Frank IM. Acute pulmonary physiologic effects of smoked marijuana and oral 9 -tetrahydrocannabinol in healthy young men. N Engl J Med 1973 08/16;289(0028-4793; 0028-4793; 7):336-41.
- Reference 999
-
Tashkin DP. Airway effects of marijuana, cocaine, and other inhaled illicit agents. Curr Opin Pulm Med 2001 03;7(1070-5287; 2):43-61.
- Reference 1000
-
Tashkin DP, Shapiro BJ, Frank IM. Acute effects of smoked marijuana and oral delta9-tetrahydrocannabinol on specific airway conductance in asthmatic subjects. Am Rev Respir Dis 1974 04;109(0003-0805; 0003-0805; 4):420-8.
- Reference 1001
-
Tashkin DP, Shapiro BJ, Lee YE, Harper CE. Effects of smoked marijuana in experimentally induced asthma. Am Rev Respir Dis 1975 09;112(0003-0805; 0003-0805; 3):377-86.
- Reference 1002
-
Gong H,Jr., Tashkin DP, Simmons MS, Calvarese B, Shapiro BJ. Acute and subacute bronchial effects of oral cannabinoids. Clin Pharmacol Ther 1984 01;35(0009-9236; 0009-9236; 1):26-32.
- Reference 1003
-
Williams SJ, Hartley JP, Graham JD. Bronchodilator effect of delta1-tetrahydrocannabinol administered by aerosol of asthmatic patients. Thorax 1976 12;31(0040-6376; 0040-6376; 6):720-3.
- Reference 1004
-
Hartley JP, Nogrady SG, Seaton A. Bronchodilator effect of delta1-tetrahydrocannabinol. Br J Clin Pharmacol 1978 06;5(0306-5251; 0306-5251; 6):523-5.
- Reference 1005
-
Tashkin DP, Reiss S, Shapiro BJ, Calvarese B, Olsen JL, Lodge JW. Bronchial effects of aerosolized delta 9-tetrahydrocannabinol in healthy and asthmatic subjects. Am Rev Respir Dis 1977 Jan;115(1):57-65.
- Reference 1006
-
Davies BH, Radcliffe S, Seaton A, Graham JD. A trial of oral delta-1-(trans)-tetrahydrocannabinol in reversible airways obstruction. Thorax 1975 02;30(0040-6376; 0040-6376; 1):80-5.
- Reference 1007
-
Gong H,Jr., Tashkin DP, Calvarese B. Comparison of bronchial effects of nabilone and terbutaline in healthy and asthmatic subjects. J Clin Pharmacol 1983 04;23(0091-2700; 0091-2700; 4):127-33.
- Reference 1008
-
Pacher P, Batkai S, Kunos G. Cardiovascular pharmacology of cannabinoids. Handb Exp Pharmacol 2005(0171-2004; 0171-2004; 168):599-625.
- Reference 1009
-
Crawford WJ, Merritt JC. Effects of tetrahydrocannabinol on arterial and intraocular hypertension. Int J Clin Pharmacol Biopharm 1979 05;17(0340-0026; 0340-0026; 5):191-6.
- Reference 1010
-
Hillard CJ. Stress regulates endocannabinoid-CB1 receptor signaling. Semin Immunol 2014 Oct;26(5):380-8.
- Reference 1011
-
Hill MN, Patel S. Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biol Mood Anxiety Disord 2013 Oct 22;3(1):19,5380-3-19.
- Reference 1012
-
Hermanson DJ, Hartley ND, Gamble-George J, Brown N, Shonesy BC, Kingsley PJ, Colbran RJ, Reese J, Marnett LJ, Patel S. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat Neurosci 2013 Sep;16(9):1291-8.
- Reference 1013
-
Hermanson DJ, Gamble-George JC, Marnett LJ, Patel S. Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol Sci 2014 Jul;35(7):358-67.
- Reference 1014
-
Denson TF, Earleywine M. Decreased depression in marijuana users. Addict Behav 2006 04;31(0306-4603; 0306-4603; 4):738-42.
- Reference 1015
-
Lev-Ran S, Le Foll B, McKenzie K, George TP, Rehm J. Cannabis use and cannabis use disorders among individuals with mental illness. Compr Psychiatry 2013 Aug;54(6):589-98.
- Reference 1016
-
Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: A systematic review and meta-analysis of longitudinal studies. Psychol Med 2014 Mar;44(4):797-810.
- Reference 1017
-
Buckner JD, Heimberg RG, Schneier FR, Liu SM, Wang S, Blanco C. The relationship between cannabis use disorders and social anxiety disorder in the national epidemiological study of alcohol and related conditions (NESARC). Drug Alcohol Depend 2012 Jul 1;124(1-2):128-34.
- Reference 1018
-
Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol 2005 09;16(0955-8810; 0955-8810; 5-6):315-31.
- Reference 1019
-
Moreira FA, Wotjak CT. Cannabinoids and anxiety. Curr.Top.Behav.Neurosci. 2010;2(1866-3370; 1866-3370):429-50.
- Reference 1020
-
Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci 2007 10/24;27(1529-2401; 0270-6474; 43):11700-11.
- Reference 1021
-
Bambico FR, Gobbi G. The cannabinoid CB1 receptor and the endocannabinoid anandamide: Possible antidepressant targets. Expert.Opin.Ther.Targets. 2008 11;12(1744-7631; 1472-8222; 11):1347-66.
- Reference 1022
-
Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol 2005 12;15(0924-977; 0924-977; 6):593-9.
- Reference 1023
-
Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet 2007 11/17;370(1474-547; 0140-6736; 9600):1706-13.
- Reference 1024
-
Gorzalka BB, Hill MN. Putative role of endocannabinoid signaling in the etiology of depression and actions of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry 2010 11/24;35(1878-4216; 0278-5846; 7):1575-85.
- Reference 1025
-
Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 2009 09;34(1873-3360; 0306-4530; 8):1257-62.
- Reference 1026
-
Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage 2005 04;29(0885-3924; 0885-3924; 4):358-67.
- Reference 1027
-
Page SA, Verhoef MJ. Medicinal marijuana use: Experiences of people with multiple sclerosis. Can Fam Physician 2006 01;52(0008-350; 0008-350):64-5.
- Reference 1028
-
Mitchell PB, Morris MJ. Depression and anxiety with rimonabant. Lancet 2007 11/17;370(1474-547; 0140-6736; 9600):1671-2.
- Reference 1029
-
Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, Azevedo-Marques PM, Hallak JE, McGuire PK, Filho BG. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 2004 02;29(0893-133; 0006-3223; 2):417-26.
- Reference 1030
-
Resstel LB, Tavares RF, Lisboa SF, Joca SR, Correa FM, Guimaraes FS. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br J Pharmacol 2009 01;156(1476-5381; 0007-1188; 1):181-8.
- Reference 1031
-
Gomes FV, Resstel LB, Guimaraes FS. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology (Berl) 2011 02;213(1432-2072; 0033-3158; 2-3):465-73.
- Reference 1032
-
Koethe D, Schreiber D, Giuffrida A, Mauss C, Faulhaber J, Heydenreich B, Hellmich M, Graf R, Klosterkotter J, Piomelli D, et al. Sleep deprivation increases oleoylethanolamide in human cerebrospinal fluid. J Neural Transm 2009 03;116(1435-1463; 0300-9564; 3):301-5.
- Reference 1033
-
Hanlon EC, Tasali E, Leproult R, Stuhr KL, Doncheck E, de Wit H, Hillard CJ, Van Cauter E. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep 2016 Mar 1;39(3):653-64.
- Reference 1034
-
Hanlon EC, Tasali E, Leproult R, Stuhr KL, Doncheck E, de Wit H, Hillard CJ, Van Cauter E. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J Clin Endocrinol Metab 2015 Jan;100(1):220-6.
- Reference 1035
-
Herrera-Solis A, Vasquez KG, Prospero-Garcia O. Acute and subchronic administration of anandamide or oleamide increases REM sleep in rats. Pharmacol Biochem Behav 2010 03;95(1873-5177; 0091-3057; 1):106-12.
- Reference 1036
-
Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Cadet JL, David PM, Verdejo-Garcia A, Benbrook AR. Sleep disturbance in heavy marijuana users. Sleep 2008 06;31(0161-8105; 0161-8105; 6):901-8.
- Reference 1037
-
Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Wang NY, Funderburk FR, Allen RP, David PM, Cadet JL. Polysomnogram changes in marijuana users who report sleep disturbances during prior abstinence. Sleep Med 2010 10;11(1878-5506; 1389-9457; 9):882-9.
- Reference 1038
-
Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 2015 Jan;51:585-8.
- Reference 1039
-
Trezza V, Campolongo P. The endocannabinoid system as a possible target to treat both the cognitive and emotional features of post-traumatic stress disorder (PTSD). Front Behav Neurosci 2013 Aug 9;7:100.
- Reference 1040
-
Lutz B. The endocannabinoid system and extinction learning. Mol Neurobiol 2007 08;36(0893-7648; 0893-7648; 1):92-101.
- Reference 1041
-
Betthauser K, Pilz J, Vollmer LE. Use and effects of cannabinoids in military veterans with posttraumatic stress disorder. Am J Health Syst Pharm 2015 Aug 1;72(15):1279-84.
- Reference 1042
-
Boden MT, Babson KA, Vujanovic AA, Short NA, Bonn-Miller MO. Posttraumatic stress disorder and cannabis use characteristics among military veterans with cannabis dependence. Am J Addict 2013 May-Jun;22(3):277-84.
- Reference 1043
-
Yarnell S. The use of medicinal marijuana for posttraumatic stress disorder: A review of the current literature. Prim Care Companion CNS Disord 2015 May 7;17(3):10.4088/PCC.15r01786. eCollection 2015.
- Reference 1044
-
Bonn-Miller MO, Babson KA, Vandrey R. Using cannabis to help you sleep: Heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend 2014 Mar 1;136:162-5.
- Reference 1045
-
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry 1995 Dec;52(12):1048-60.
- Reference 1046
-
Bohnert KM, Perron BE, Ashrafioun L, Kleinberg F, Jannausch M, Ilgen MA. Positive posttraumatic stress disorder screens among first-time medical cannabis patients: Prevalence and association with other substance use. Addict Behav 2014 Oct;39(10):1414-7.
- Reference 1047
-
Lutz B, Marsicano G, Maldonado R, Hillard CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci 2015 Dec;16(12):705-18.
- Reference 1048
-
Neumeister A, Seidel J, Ragen BJ, Pietrzak RH. Translational evidence for a role of endocannabinoids in the etiology and treatment of posttraumatic stress disorder. Psychoneuroendocrinology 2015 Jan;51:577-84.
- Reference 1049
-
Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 2006 11;188(0033-3158; 0033-3158; 4):641-9.
- Reference 1050
-
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002 08/01;418(0028-0836; 0028-0836; 6897):530-4.
- Reference 1051
-
Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the morris water maze. J Pharmacol Exp Ther 2002 06;301(0022-3565; 0022-3565; 3):915-24.
- Reference 1052
-
Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology 2005 03;30(0893-133; 0006-3223; 3):516-24.
- Reference 1053
-
Jenniches I, Ternes S, Albayram O, Otte DM, Bach K, Bindila L, Michel K, Lutz B, Bilkei-Gorzo A, Zimmer A. Anxiety, stress, and fear response in mice with reduced endocannabinoid levels. Biol Psychiatry 2016 May 15;79(10):858-68.
- Reference 1054
-
Stern CA, Gazarini L, Takahashi RN, Guimaraes FS, Bertoglio LJ. On disruption of fear memory by reconsolidation blockade: Evidence from cannabidiol treatment. Neuropsychopharmacology 2012 Aug;37(9):2132-42.
- Reference 1055
-
Pietrzak RH, Huang Y, Corsi-Travali S, Zheng MQ, Lin SF, Henry S, Potenza MN, Piomelli D, Carson RE, Neumeister A. Cannabinoid type 1 receptor availability in the amygdala mediates threat processing in trauma survivors. Neuropsychopharmacology 2014 Oct;39(11):2519-28.
- Reference 1056
-
Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, Hillard CJ, Yehuda R. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the world trade center attacks. Psychoneuroendocrinology 2013 Dec;38(12):2952-61.
- Reference 1057
-
Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, Potenza MN, Bailey CR, Lin SF, Najafzadeh S, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: A positron emission tomography study. Mol Psychiatry 2013 Sep;18(9):1034-40.
- Reference 1058
-
Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry 2009 Jul 1;66(1):9-16.
- Reference 1059
-
Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun 2015 Mar 3;6:6395.
- Reference 1060
-
Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci 2008 Mar 5;28(10):2313-9.
- Reference 1061
-
Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, Phan KL. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology 2013 Jan;64:396-402.
- Reference 1062
-
Rabinak CA, Angstadt M, Lyons M, Mori S, Milad MR, Liberzon I, Phan KL. Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiol Learn Mem 2014 Sep;113:125-34.
- Reference 1063
-
Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E, Curran HV, Morgan CJ. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl) 2013 Apr;226(4):781-92.
- Reference 1064
-
O'Neil ME, Nugent SM, Morasco BJ, Freeman M, Low A, Kondo K, Zakher B, Elven C, Motu'apuaka M, Paynter R, et al. Benefits and harms of plant-based cannabis for posttraumatic stress disorder: A systematic review. Ann Intern Med 2017 Sep 5;167(5):332-40.
- Reference 1065
-
Befort K. Interactions of the opioid and cannabinoid systems in reward: Insights from knockout studies. Front Pharmacol 2015 Feb 5;6:6.
- Reference 1066
-
Scavone JL, Sterling RC, Van Bockstaele EJ. Cannabinoid and opioid interactions: Implications for opiate dependence and withdrawal. Neuroscience 2013 Sep 17;248:637-54.
- Reference 1067
-
Lucas P, Walsh Z, Crosby K, Callaway R, Belle-Isle L, Kay R, Capler R, Holtzman S. Substituting cannabis for prescription drugs, alcohol and other substances among medical cannabis patients: The impact of contextual factors. Drug Alcohol Rev 2015 Sep 14.
- Reference 1068
-
Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: Implications for alcohol dependence and future directions for research. Alcohol 2012 05;46(1873-6823; 0741-8329; 3):185-204.
- Reference 1069
-
Hirvonen J, Zanotti-Fregonara P, Umhau JC, George DT, Rallis-Frutos D, Lyoo CH, Li CT, Hines CS, Sun H, Terry GE, et al. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry 2013 Aug;18(8):916-21.
- Reference 1070
-
Ceccarini J, Hompes T, Verhaeghen A, Casteels C, Peuskens H, Bormans G, Claes S, Van Laere K. Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J Neurosci 2014 Feb 19;34(8):2822-31.
- Reference 1071
-
Sprague GL, Craigmill AL. Effects of two cannabinoids upon abstinence signs in ethanol-dependent mice. Pharmacol Biochem Behav 1978 Jul;9(1):11-5.
- Reference 1072
-
Kralik PM, Ho BT, Matthews HR. Effect of delta-THC on ethanol withdrawal in mice. Experientia 1976 Jun 15;32(6):723-5.
- Reference 1073
-
Rubio M, Fernandez-Ruiz J, de Miguel R, Maestro B, Michael Walker J, Ramos JA. CB1 receptor blockade reduces the anxiogenic-like response and ameliorates the neurochemical imbalances associated with alcohol withdrawal in rats. Neuropharmacology 2008 May;54(6):976-88.
- Reference 1074
-
Rubio M, Villain H, Docagne F, Roussel BD, Ramos JA, Vivien D, Fernandez-Ruiz J, Ali C. Pharmacological activation/inhibition of the cannabinoid system affects alcohol withdrawal-induced neuronal hypersensitivity to excitotoxic insults. PLoS One 2011;6(8):e23690.
- Reference 1075
-
Deikel SM, Carder B. Attentuation of precipitated abstinence in methadone-dependent rats by delta9-THC. Psychopharmacol Commun 1976;2(0098-616; 0098-616; 1):61-5.
- Reference 1076
-
Vela G, Fuentes JA, Bonnin A, Fernandez-Ruiz J, Ruiz-Gayo M. Perinatal exposure to delta 9-tetrahydrocannabinol (delta 9-THC) leads to changes in opioid-related behavioral patterns in rats. Brain Res 1995 05/22;680(0006-8993; 0006-8993; 1-2):142-7.
- Reference 1077
-
Yamaguchi T, Hagiwara Y, Tanaka H, Sugiura T, Waku K, Shoyama Y, Watanabe S, Yamamoto T. Endogenous cannabinoid, 2-arachidonoylglycerol, attenuates naloxone-precipitated withdrawal signs in morphine-dependent mice. Brain Res 2001 08/03;909(0006-8993; 0006-8993; 1-2):121-6.
- Reference 1078
-
Reiman A. Cannabis as a substitute for alcohol and other drugs. Harm.Reduct.J. 2009;6(1477-7517; 1477-7517):35-9.
- Reference 1079
-
Wenger LD, Lopez AM, Comfort M, Kral AH. The phenomenon of low-frequency heroin injection among street-based urban poor: Drug user strategies and contexts of use. Int J Drug Policy 2014 May;25(3):471-9.
- Reference 1080
-
Peters DC. Patients and caregivers report using medical marijuana to decrease prescription narcotics use. Humboldt Journal of Social Relations 2013(35):24.
- Reference 1081
-
Kral AH, Wenger L, Novak SP, Chu D, Corsi KF, Coffa D, Shapiro B, Bluthenthal RN. Is cannabis use associated with less opioid use among people who inject drugs? Drug Alcohol Depend 2015 Aug 1;153:236-41.
- Reference 1082
-
Epstein DH, Preston KL. No evidence for reduction of opioid-withdrawal symptoms by cannabis smoking during a methadone dose taper. Am J Addict 2015 Jun;24(4):323-8.
- Reference 1083
-
Jicha CJ, Lofwall MR, Nuzzo PA, Babalonis S, Elayi SC, Walsh SL. Safety of oral dronabinol during opioid withdrawal in humans. Drug Alcohol Depend 2015 Dec 1;157:179-83.
- Reference 1084
-
Bossong MG, Jansma JM, Bhattacharyya S, Ramsey NF. Role of the endocannabinoid system in brain functions relevant for schizophrenia: An overview of human challenge studies with cannabis or 9-tetrahydrocannabinol (THC). Prog Neuropsychopharmacol Biol Psychiatry 2014 Jul 3;52:53-69.
- Reference 1085
-
Radhakrishnan R, Wilkinson ST, D'Souza DC. Gone to pot - A review of the association between cannabis and psychosis. Front Psychiatry 2014 May 22;5:54.
- Reference 1086
-
Fernandez-Espejo E, Viveros MP, Nunez L, Ellenbroek BA, Rodriguez de Fonseca F. Role of cannabis and endocannabinoids in the genesis of schizophrenia. Psychopharmacology (Berl) 2009 11;206(1432-2072; 0033-3158; 4):531-49.
- Reference 1087
-
Koethe D, Giuffrida A, Schreiber D, Hellmich M, Schultze-Lutter F, Ruhrmann S, Klosterkotter J, Piomelli D, Leweke FM. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br J Psychiatry 2009 04;194(1472-1465; 0007-1250; 4):371-2.
- Reference 1088
-
Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport 1999 06/03;10(0959-4965; 0959-4965; 8):1665-9.
- Reference 1089
-
De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003 08/19;2(1476-511; 1476-511):5.
- Reference 1090
-
Desfosses J, Stip E, Bentaleb LA, Lipp O, Chiasson JP, Furtos A, Venne K, Kouassi E, Potvin S. Plasma endocannabinoid alterations in individuals with substance use disorder are dependent on the "mirror effect" of schizophrenia. Front Psychiatry 2012 Sep 25;3:85.
- Reference 1091
-
Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, Klosterkotter J, Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology 2004 Nov;29(11):2108-14.
- Reference 1092
-
Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: Regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience 2001;103(1):9-15.
- Reference 1093
-
Dalton VS, Long LE, Weickert CS, Zavitsanou K. Paranoid schizophrenia is characterized by increased CB1 receptor binding in the dorsolateral prefrontal cortex. Neuropsychopharmacology 2011 Jul;36(8):1620-30.
- Reference 1094
-
Jenko KJ, Hirvonen J, Henter ID, Anderson KB, Zoghbi SS, Hyde TM, Deep-Soboslay A, Innis RB, Kleinman JE. Binding of a tritiated inverse agonist to cannabinoid CB1 receptors is increased in patients with schizophrenia. Schizophr Res 2012 Nov;141(2-3):185-8.
- Reference 1095
-
Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2004 Mar;28(2):355-60.
- Reference 1096
-
Newell KA, Deng C, Huang XF. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp Brain Res 2006 Jul;172(4):556-60.
- Reference 1097
-
Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, Ye W, Dannals RF, Ravert HT, Nandi A, et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage 2010 Oct 1;52(4):1505-13.
- Reference 1098
-
Ceccarini J, De Hert M, Van Winkel R, Peuskens J, Bormans G, Kranaster L, Enning F, Koethe D, Leweke FM, Van Laere K. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. Neuroimage 2013 Oct 1;79:304-12.
- Reference 1099
-
McCreadie RG. Use of drugs, alcohol and tobacco by people with schizophrenia: Case-control study. Br J Psychiatry 2002 10;181(0007-1250; 0007-1250):321-5.
- Reference 1100
-
Gregg L, Barrowclough C, Haddock G. Reasons for increased substance use in psychosis. Clin Psychol Rev 2007 05;27(0272-7358; 0272-7358; 4):494-510.
- Reference 1101
-
Khantzian EJ. The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence. Am J Psychiatry 1985 11;142(0002-953; 0002-953; 11):1259-64.
- Reference 1102
-
Perez LG, Santacana AM, Baquero DB, Perez-Sola V. Reasons and subjective effects of cannabis use among people with psychotic disorders: A systematic review. Actas. Esp. Psiquiatr 2014;42(2):83.
- Reference 1103
-
Rabin RA, Goodman MS, George TP, Barr MS. Neurobiology of comorbid substance use disorders in mental illness: A closer look at the underlying commonalities between cannabis and schizophrenia. Current Addiction Reports 2014;1:261.
- Reference 1104
-
Lembke A. Time to abandon the self-medication hypothesis in patients with psychiatric disorders. Am J Drug Alcohol Abuse 2012 11;38(1097-9891; 0095-2990; 6):524-9.
- Reference 1105
-
van Winkel R, Kuepper R. Epidemiological, neurobiological, and genetic clues to the mechanisms linking cannabis use to risk for nonaffective psychosis. Annu Rev Clin Psychol 2014;10:767-91.
- Reference 1106
-
Renard J, Krebs MO, Le Pen G, Jay TM. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front Neurosci 2014 Nov 10;8:361.
- Reference 1107
-
Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry 2001 07/15;50(0006-3223; 0006-3223; 2):71-83.
- Reference 1108
-
Iseger TA, Bossong MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res 2015 Mar;162(1-3):153-61.
- Reference 1109
-
Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: Epidemiologic evidence. Biol Psychiatry 2016 Apr 1;79(7):549-56.
- Reference 1110
-
French L, Gray C, Leonard G, Perron M, Pike GB, Richer L, Seguin JR, Veillette S, Evans CJ, Artiges E, et al. Early cannabis use, polygenic risk score for schizophrenia and brain maturation in adolescence. JAMA Psychiatry 2015 Oct 1;72(10):1002-11.
- Reference 1111
-
Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: Epidemiologic evidence. Biol Psychiatry 2015 Aug 12.
- Reference 1112
-
Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, Handley R, Luzi S, Russo M, Paparelli A, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry 2009 Dec;195(6):488-91.
- Reference 1113
-
Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, Marconi A, La Cascia C, Reis Marques T, Pariante C, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull 2014 Nov;40(6):1509-17.
- Reference 1114
-
Arnold JC, Boucher AA, Karl T. The yin and yang of cannabis-induced psychosis: The actions of delta(9)-tetrahydrocannabinol and cannabidiol in rodent models of schizophrenia. Curr Pharm Des 2012;18(32):5113-30.
- Reference 1115
-
Wegener N, Koch M. Neurobiology and systems physiology of the endocannabinoid system. Pharmacopsychiatry 2009 May;42 Suppl 1:S79-86.
- Reference 1116
-
Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: Longitudinal evidence of a gene X environment interaction. Biol Psychiatry 2005 05/15;57(0006-3223; 0006-3223; 10):1117-27.
- Reference 1117
-
Henquet C, Rosa A, Krabbendam L, Papiol S, Fananas L, Drukker M, Ramaekers JG, van OJ. An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 2006 12;31(0893-133; 0006-3223; 12):2748-57.
- Reference 1118
-
Henquet C, Rosa A, Delespaul P, Papiol S, Fananas L, van OJ, Myin-Germeys I. COMT ValMet moderation of cannabis-induced psychosis: A momentary assessment study of 'switching on' hallucinations in the flow of daily life. Acta Psychiatr Scand 2009 02;119(1600-0447; 0001-690; 2):156-60.
- Reference 1119
-
Pelayo-Teran JM, Perez-Iglesias R, Mata I, Carrasco-Marin E, Vazquez-Barquero JL, Crespo-Facorro B. Catechol-O-methyltransferase (COMT) Val158Met variations and cannabis use in first-episode non-affective psychosis: Clinical-onset implications. Psychiatry Res 2010 10/30;179(0165-1781; 0165-1781; 3):291-6.
- Reference 1120
-
Estrada G, Fatjo-Vilas M, Munoz MJ, Pulido G, Minano MJ, Toledo E, Illa JM, Martin M, Miralles ML, Miret S, et al. Cannabis use and age at onset of psychosis: Further evidence of interaction with COMT Val158Met polymorphism. Acta Psychiatr Scand 2011 06;123(1600-0447; 0001-690; 6):485-92.
- Reference 1121
-
Zammit S, Spurlock G, Williams H, Norton N, Williams N, O'Donovan MC, Owen MJ. Genotype effects of CHRNA7, CNR1 and COMT in schizophrenia: Interactions with tobacco and cannabis use. Br J Psychiatry 2007 Nov;191:402-7.
- Reference 1122
-
Zammit S, Owen MJ, Evans J, Heron J, Lewis G. Cannabis, COMT and psychotic experiences. Br J Psychiatry 2011 Nov;199(5):380-5.
- Reference 1123
-
Costas J, Sanjuan J, Ramos-Rios R, Paz E, Agra S, Tolosa A, Paramo M, Brenlla J, Arrojo M. Interaction between COMT haplotypes and cannabis in schizophrenia: A case-only study in two samples from spain. Schizophr Res 2011 Apr;127(1-3):22-7.
- Reference 1124
-
van Winkel R, van Beveren NJ, Simons C. AKT1 moderation of cannabis-induced cognitive alterations in psychotic disorder. Neuropsychopharmacology 2011 11;36(1740-634; 0006-3223; 12):2529-37.
- Reference 1125
-
van Winkel R. Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: Sibling analysis and proband follow-up. Arch Gen Psychiatry 2011 02;68(1538-3636; 0003-990; 2):148-57.
- Reference 1126
-
Di Forti M, Iyegbe C, Sallis H, Kolliakou A, Falcone MA, Paparelli A, Sirianni M, La Cascia C, Stilo SA, Marques TR, et al. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry 2012 11/15;72(1873-2402; 0006-3223; 10):811-6.
- Reference 1127
-
Morgan CJ, Freeman TP, Powell J, Curran HV. AKT1 genotype moderates the acute psychotomimetic effects of naturalistically smoked cannabis in young cannabis smokers. Transl Psychiatry 2016 Feb 16;6:e738.
- Reference 1128
-
Zhang P, Bian Y, Liu N, Tang Y, Pan C, Hu Y, Tang Z. The SNP rs1625579 in miR-137 gene and risk of schizophrenia in chinese population: A meta-analysis. Compr Psychiatry 2016 May;67:26-32.
- Reference 1129
-
Suarez-Pinilla P, Roiz-Santianez R, Ortiz-Garcia de la Foz V, Guest PC, Ayesa-Arriola R, Cordova-Palomera A, Tordesillas-Gutierrez D, Crespo-Facorro B. Brain structural and clinical changes after first episode psychosis: Focus on cannabinoid receptor 1 polymorphisms. Psychiatry Res 2015 Aug 30;233(2):112-9.
- Reference 1130
-
Cho Y, Ryu S, Huh I, Cho EY, Oh H, Lee YS, Lee WK, Park T, Kwon JS, Hong KS. Effects of genetic variations in NRG1 on cognitive domains in patients with schizophrenia and healthy individuals. Psychiatr Genet 2015 Aug;25(4):147-54.
- Reference 1131
-
Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry 2008 Apr;192(4):306-7.
- Reference 1132
-
Morgan CJ, Gardener C, Schafer G, Swan S, Demarchi C, Freeman TP, Warrington P, Rupasinghe I, Ramoutar A, Tan N, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med 2012 Feb;42(2):391-400.
- Reference 1133
-
Musty R. Cannabinoids and anxiety. In: R. Mechoulam, editor. Cannabinoids as therapeutics. Basel: Birkhaüser; 2005. ID: 2278; RP: NOT IN FILE.
- Reference 1134
-
Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB. Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin Pharmacol Ther 1976 Mar;19(3):300-9.
- Reference 1135
-
Roser P, Gallinat J, Weinberg G, Juckel G, Gorynia I, Stadelmann AM. Psychomotor performance in relation to acute oral administration of Delta9-tetrahydrocannabinol and standardized cannabis extract in healthy human subjects. Eur Arch Psychiatry Clin Neurosci 2009 Aug;259(5):284-92.
- Reference 1136
-
Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, Stone JM, Reichenberg A, Brenneisen R, Holt D, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol 2013 Jan;27(1):19-27.
- Reference 1137
-
Zuardi AW, Morais SL, Guimaraes FS, Mechoulam R. Antipsychotic effect of cannabidiol. J Clin Psychiatry 1995 Oct;56(10):485-6.
- Reference 1138
-
Zuardi AW, Crippa JA, Hallak JE, Moreira FA, Guimaraes FS. Cannabidiol, a cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res 2006 Apr;39(4):421-9.
- Reference 1139
-
Zuardi AW, Hallak JE, Dursun SM, Morais SL, Sanches RF, Musty RE, Crippa JA. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol 2006 Sep;20(5):683-6.
- Reference 1140
-
Hallak JE, Machado-de-Sousa JP, Crippa JA, Sanches RF, Trzesniak C, Chaves C, Bernardo SA, Regalo SC, Zuardi AW. Performance of schizophrenic patients in the stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr 2010 Mar;32(1):56-61.
- Reference 1141
-
Leweke FM, Schneider U, Radwan M, Schmidt E, Emrich HM. Different effects of nabilone and cannabidiol on binocular depth inversion in man. Pharmacol Biochem Behav 2000 May;66(1):175-81.
- Reference 1142
-
Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, Klosterkotter J, Hellmich M, Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2012 Mar 20;2:e94.
- Reference 1143
-
Zuardi AW, Rodrigues JA, Cunha JM. Effects of cannabidiol in animal models predictive of antipsychotic activity. Psychopharmacology (Berl) 1991;104(0033-3158; 0033-3158; 2):260-4.
- Reference 1144
-
Moreira FA, Guimaraes FS. Cannabidiol inhibits the hyperlocomotion induced by psychotomimetic drugs in mice. Eur J Pharmacol 2005 04/11;512(0014-2999; 0014-2999; 2-3):199-205.
- Reference 1145
-
Zuardi AW, Crippa JA, Hallak JE, Pinto JP, Chagas MH, Rodrigues GG, Dursun SM, Tumas V. Cannabidiol for the treatment of psychosis in parkinson's disease. J Psychopharmacol 2009 Nov;23(8):979-83.
- Reference 1146
-
[Internet]. Available from: https://canvasrx.com/.
- Reference 1147
-
Zuardi AW. Cannabidiol: From an inactive cannabinoid to a drug with wide spectrum of action. Rev.Bras.Psiquiatr. 2008 09;30(1516-4446; 1516-4446; 3):271-80.
- Reference 1148
-
Benito C, Nunez E, Pazos MR, Tolon RM, Romero J. The endocannabinoid system and alzheimer's disease. Mol Neurobiol 2007 08;36(0893-7648; 0893-7648; 1):75-81.
- Reference 1149
-
Koppel J, Davies P. Targeting the endocannabinoid system in alzheimer's disease. J Alzheimer's Dis 2008 11;15(1387-2877; 1387-2877; 3):495-504.
- Reference 1150
-
van der Stelt M, Mazzola C, Esposito G, Matias I, Petrosino S, De FD, Micale V, Steardo L, Drago F, Iuvone T, et al. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: Effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci 2006 06;63(1420-682; 1420-682; 12):1410-24.
- Reference 1151
-
Jung KM, Astarita G, Yasar S, Vasilevko V, Cribbs DH, Head E, Cotman CW, Piomelli D. An amyloid beta(42)-dependent deficit in anandamide mobilization is associated with cognitive dysfunction in alzheimer's disease. Neurobiol Aging 2011 05/03;33(1558-1497; 0197-4580; 8):1522-32.
- Reference 1152
-
Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in alzheimer's disease brains. J Neurosci 2003 Dec 3;23(35):11136-41.
- Reference 1153
-
Noonan J, Tanveer R, Klompas A, Gowran A, McKiernan J, Campbell VA. Endocannabinoids prevent beta-amyloid-mediated lysosomal destabilization in cultured neurons. J Biol Chem 2010 12/03;285(1083-351; 0021-9258; 49):38543-54.
- Reference 1154
-
Eubanks LM, Rogers CJ, Beuscher AE, Koob GF, Olson AJ, Dickerson TJ, Janda KD. A molecular link between the active component of marijuana and alzheimer's disease pathology. Mol.Pharm. 2006 11;3(1543-8384; 1543-8384; 6):773-7.
- Reference 1155
-
Iuvone T, Esposito G, Esposito R, Santamaria R, Di RM, Izzo AA. Neuroprotective effect of cannabidiol, a non-psychoactive component from cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J Neurochem 2004 04;89(0022-3042; 0022-3042; 1):134-41.
- Reference 1156
-
Esposito G, De FD, Carnuccio R, Izzo AA, Iuvone T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J.Mol.Med.(Berl) 2006 03;84(0946-2716; 0946-2716; 3):253-8.
- Reference 1157
-
Booz GW. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic Biol Med 2011 01/14;51(1873-4596; 0891-5849; 5):1054-61.
- Reference 1158
-
Vazquez C, Tolon RM, Grande MT, Caraza M, Moreno M, Koester EC, Villaescusa B, Ruiz-Valdepenas L, Fernandez-Sanchez FJ, Cravatt BF, et al. Endocannabinoid regulation of amyloid-induced neuroinflammation. Neurobiol Aging 2015 Nov;36(11):3008-19.
- Reference 1159
-
Esposito G, Scuderi C, Savani C, Steardo L,Jr., De FD, Cottone P, Iuvone T, Cuomo V, Steardo L. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br J Pharmacol 2007 08;151(0007-1188; 0007-1188; 8):1272-9.
- Reference 1160
-
Esposito G, Iuvone T, Savani C, Scuderi C, De FD, Papa M, Di M,V, Steardo L. Opposing control of cannabinoid receptor stimulation on amyloid-beta-induced reactive gliosis: In vitro and in vivo evidence. J Pharmacol Exp Ther 2007 09;322(0022-3565; 0022-3565; 3):1144-52.
- Reference 1161
-
Aso E, Sanchez-Pla A, Vegas-Lozano E, Maldonado R, Ferrer I. Cannabis-based medicine reduces multiple pathological processes in AbetaPP/PS1 mice. J Alzheimers Dis 2015;43(3):977-91.
- Reference 1162
-
Krishnan S, Cairns R, Howard R. Cannabinoids for the treatment of dementia. Cochrane Database Syst Rev 2009(1469-493; 1361-6137; 2):CD007204.
- Reference 1163
-
Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with alzheimer's disease. Int J Geriatr Psychiatry 1997 09;12(0885-6230; 9):913-9.
- Reference 1164
-
Walther S, Mahlberg R, Eichmann U, Kunz D. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology (Berl) 2006 05;185(0033-3158; 0033-3158; 4):524-8.
- Reference 1165
-
Mahlberg R, Walther S. Actigraphy in agitated patients with dementia. monitoring treatment outcomes. Z Gerontol Geriatr 2007 Jun;40(3):178-84.
- Reference 1166
-
Passmore MJ. The cannabinoid receptor agonist nabilone for the treatment of dementia-related agitation. Int J Geriatr Psychiatry 2008 01;23(0885-6230; 0885-6230; 1):116-7.
- Reference 1167
-
Zajac DM, Sikkema SR, Chandrasena R. Nabilone for the treatment of dementia-associated sexual disinhibition. Prim Care Companion CNS Disord 2015 Feb 19;17(1):10.4088/PCC.14l01695. eCollection 2015.
- Reference 1168
-
Woodward MR, Harper DG, Stolyar A, Forester BP, Ellison JM. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry 2014 Apr;22(4):415-9.
- Reference 1169
-
Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat.Rev.Immunol. 2005 05;5(1474-1733; 1474-1733; 5):400-11.
- Reference 1170
-
Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future.Med.Chem. 2009 10;1(1756-8927; 1756-8919; 7):1333-49.
- Reference 1171
-
Stander S, Schmelz M, Metze D, Luger T, Rukwied R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci 2005 06;38(0923-1811; 0923-1811; 3):177-88.
- Reference 1172
-
Toth BI, Dobrosi N, Dajnoki A, Czifra G, Olah A, Szollosi AG, Juhasz I, Sugawara K, Paus R, Biro T. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J Invest Dermatol 2011 05;131(1523-1747; 0022-202; 5):1095-104.
- Reference 1173
-
Maccarrone M, Di RM, Battista N, Gasperi V, Guerrieri P, Rossi A, Finazzi-Agro A. The endocannabinoid system in human keratinocytes. evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activation protein-1, and transglutaminase. J Biol Chem 2003 09/05;278(0021-9258; 0021-9258; 36):33896-903.
- Reference 1174
-
Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci 2007 02;45(0923-1811; 0923-1811; 2):87-92.
- Reference 1175
-
Gaffal E, Cron M, Glodde N, Tuting T. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy 2013 Aug;68(8):994-1000.
- Reference 1176
-
Olah A, Toth BI, Borbiro I, Sugawara K, Szollosi AG, Czifra G, Pal B, Ambrus L, Kloepper J, Camera E, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest 2014 Sep;124(9):3713-24.
- Reference 1177
-
Pucci M, Rapino C, Di Francesco A, Dainese E, D'Addario C, Maccarrone M. Epigenetic control of skin differentiation genes by phytocannabinoids. Br J Pharmacol 2013 Oct;170(3):581-91.
- Reference 1178
-
Dvorak M, Watkinson A, McGlone F, Rukwied R. Histamine induced responses are attenuated by a cannabinoid receptor agonist in human skin. Inflamm Res 2003 06;52(1023-3830; 1023-3830; 6):238-45.
- Reference 1179
-
Rukwied R, Watkinson A, McGlone F, Dvorak M. Cannabinoid agonists attenuate capsaicin-induced responses in human skin. Pain 2003 04;102(0304-3959; 0304-3959; 3):283-8.
- Reference 1180
-
Maida V, Corban J. Topical medical cannabis: A new treatment for wound pain-three cases of pyoderma gangrenosum. J Pain Symptom Manage 2017 Nov;54(5):732-6.
- Reference 1181
-
Hashim PW, Cohen JL, Pompei DT, Goldenberg G. Topical cannabinoids in dermatology. Cutis 2017 Jul;100(1):50-2.
- Reference 1182
-
Watson ES, Murphy JC, Turner CE. Allergenic properties of naturally occurring cannabinoids. J Pharm Sci 1983 08;72(0022-3549; 0022-3549; 8):954-5.
- Reference 1183
-
Williams C, Thompstone J, Wilkinson M. Work-related contact urticaria to cannabis sativa. Contact Derm 2008 01;58(1600-0536; 0105-1873; 1):62-3.
- Reference 1184
-
Mikuriya TH. Marijuana in medicine: Past, present and future. Calif Med 1969 01;110(0008-1264; 0008-1264; 1):34-40.
- Reference 1185
-
Kalant H. Medicinal use of cannabis: History and current status. Pain Res.Manag. 2001;6(1203-6765; 1203-6765; 2):80-91.
- Reference 1186
-
Zuardi AW. History of cannabis as a medicine: A review. Rev.Bras.Psiquiatr. 2006 06;28(1516-4446; 1516-4446; 2):153-7.
- Reference 1187
-
Duncan M, Thomas AD, Cluny NL, Patel A, Patel KD, Lutz B, Piomelli D, Alexander SP, Sharkey KA. Distribution and function of monoacylglycerol lipase in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 2008 12;295(0193-1857; 0193-1857; 6):G1255-65.
- Reference 1188
-
Kennedy PJ, Clarke G, Quigley EM, Groeger JA, Dinan TG, Cryan JF. Gut memories: Towards a cognitive neurobiology of irritable bowel syndrome. Neurosci Biobehav Rev 2012 01;36(1873-7528; 0149-7634; 1):310-40.
- Reference 1189
-
Storr MA, Yuce B, Andrews CN, Sharkey KA. The role of the endocannabinoid system in the pathophysiology and treatment of irritable bowel syndrome. Neurogastroenterol Motil 2008 08;20(1365-2982; 1350-1925; 8):857-68.
- Reference 1190
-
Yao X, Yang YS, Cui LH, Zhao KB, Zhang ZH, Peng LH, Guo X, Sun G, Shang J, Wang WF, et al. Subtypes of irritable bowel syndrome on rome III criteria: A multicenter study. J Gastroenterol Hepatol 2012 04;27(1440-1746; 0815-9319; 4):760-5.
- Reference 1191
-
Camilleri M, Carlson P, McKinzie S, Grudell A, Busciglio I, Burton D, Baxter K, Ryks M, Zinsmeister AR. Genetic variation in endocannabinoid metabolism, gastrointestinal motility, and sensation. Am J Physiol Gastrointest Liver Physiol 2008 01;294(0193-1857; 0193-1857; 1):G13-9.
- Reference 1192
-
Park JM, Choi MG, Cho YK, Lee IS, Kim SW, Choi KY, Chung IS. Cannabinoid receptor 1 gene polymorphism and irritable bowel syndrome in the korean population: A hypothesis-generating study. J Clin Gastroenterol 2011 01;45(1539-2031; 0192-0790; 1):45-9.
- Reference 1193
-
Camilleri M, Katzka DA. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. genetic epidemiology and pharmacogenetics in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012 05/15;302(1522-1547; 0193-1857; 10):G1075-84.
- Reference 1194
-
Sanson M, Bueno L, Fioramonti J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterol Motil 2006 10;18(1350-1925; 1350-1925; 10):949-56.
- Reference 1195
-
Brusberg M, Arvidsson S, Kang D, Larsson H, Lindstrom E, Martinez V. CB1 receptors mediate the analgesic effects of cannabinoids on colorectal distension-induced visceral pain in rodents. J Neurosci 2009 02/04;29(1529-2401; 0270-6474; 5):1554-64.
- Reference 1196
-
Kimball ES, Wallace NH, Schneider CR, D'Andrea MR, Hornby PJ. Small intestinal cannabinoid receptor changes following a single colonic insult with oil of mustard in mice. Front Pharmacol. 2010;1(1663-9812):132.
- Reference 1197
-
Esfandyari T, Camilleri M, Ferber I, Burton D, Baxter K, Zinsmeister AR. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: A randomized, placebo-controlled study. Neurogastroenterol Motil 2006 09;18(1350-1925; 1350-1925; 9):831-8.
- Reference 1198
-
Esfandyari T, Camilleri M, Busciglio I, Burton D, Baxter K, Zinsmeister AR. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: A randomized, placebo-controlled study. Am J Physiol Gastrointest Liver Physiol 2007 07;293(0193-1857; 0193-1857; 1):G137-45.
- Reference 1199
-
Rao SS, Singh S. Clinical utility of colonic and anorectal manometry in chronic constipation. J Clin Gastroenterol 2010 10;44(1539-2031; 0192-0790; 9):597-609.
- Reference 1200
-
Coulie B, Camilleri M, Bharucha AE, Sandborn WJ, Burton D. Colonic motility in chronic ulcerative proctosigmoiditis and the effects of nicotine on colonic motility in patients and healthy subjects. Aliment Pharmacol Ther 2001 05;15(0269-2813; 0269-2813; 5):653-63.
- Reference 1201
-
Lembo T, Wright RA, Bagby B, Decker C, Gordon S, Jhingran P, Carter E. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 2001 09;96(0002-9270; 0002-9270; 9):2662-70.
- Reference 1202
-
Wong BS, Camilleri M, Busciglio I, Carlson P, Szarka LA, Burton D, Zinsmeister AR. Pharmacogenetic trial of a cannabinoid agonist shows reduced fasting colonic motility in patients with nonconstipated irritable bowel syndrome. Gastroenterology 2011 11;141(1528-0012; 0016-5085; 5):1638-47.
- Reference 1203
-
Wong BS, Camilleri M, Eckert D, Carlson P, Ryks M, Burton D, Zinsmeister AR. Randomized pharmacodynamic and pharmacogenetic trial of dronabinol effects on colon transit in irritable bowel syndrome-diarrhea. Neurogastroenterol Motil 2012 04;24(1365-2982; 1350-1925; 4):358-e169.
- Reference 1204
-
Di Sabatino A, Battista N, Biancheri P, Rapino C, Rovedatti L, Astarita G, Vanoli A, Dainese E, Guerci M, Piomelli D, et al. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal.Immunol. 2011 09;4(1935-3456; 1933-0219; 5):574-83.
- Reference 1205
-
Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011 05;60(1468-3288; 0017-5749; 5):571-607.
- Reference 1206
-
Ligresti A, Bisogno T, Matias I, De PL, Cascio MG, Cosenza V, D'Argenio G, Scaglione G, Bifulco M, Sorrentini I, et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology 2003 09;125(0016-5085; 0016-5085; 3):677-87.
- Reference 1207
-
Guagnini F, Valenti M, Mukenge S, Matias I, Bianchetti A, Di PS, Ferla G, Di M,V, Croci T. Neural contractions in colonic strips from patients with diverticular disease: Role of endocannabinoids and substance P. Gut 2006 07;55(0017-5749; 0017-5749; 7):946-53.
- Reference 1208
-
D'Argenio G, Petrosino S, Gianfrani C, Valenti M, Scaglione G, Grandone I, Nigam S, Sorrentini I, Mazzarella G, Di M,V. Overactivity of the intestinal endocannabinoid system in celiac disease and in methotrexate-treated rats. J Mol Med 2007 05;85(0946-2716; 0946-2716; 5):523-30.
- Reference 1209
-
Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, Ferri GL, Sibaev A, Storr M, Lutz B. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest 2004 04;113(0021-9738; 0021-9738; 8):1202-9.
- Reference 1210
-
D'Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di M,V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J 2006 03;20(1530-6860; 0892-6638; 3):568-70.
- Reference 1211
-
Storr M, Emmerdinger D, Diegelmann J, Yuce B, Pfennig S, Ochsenkuhn T, Goke B, Lohse P, Brand S. The role of fatty acid hydrolase gene variants in inflammatory bowel disease. Aliment Pharmacol Ther 2009 03/01;29(1365-2036; 0269-2813; 5):542-51.
- Reference 1212
-
Kimball ES, Schneider CR, Wallace NH, Hornby PJ. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol 2006 08;291(0193-1857; 0193-1857; 2):G364-71.
- Reference 1213
-
Engel MA, Kellermann CA, Burnat G, Hahn EG, Rau T, Konturek PC. Mice lacking cannabinoid CB1-, CB2-receptors or both receptors show increased susceptibility to trinitrobenzene sulfonic acid (TNBS)-induced colitis. J Physiol Pharmacol 2010 02;61(1899-1505; 0867-5910; 1):89-97.
- Reference 1214
-
Jamontt JM, Molleman A, Pertwee RG, Parsons ME. The effects of delta-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br J Pharmacol 2010 06;160(1476-5381; 0007-1188; 3):712-23.
- Reference 1215
-
Di Paola R, Mazzon E, Patel NS, Genovese T, Muia C, Thiemermann C, De Sarro A, Cuzzocrea S. Beneficial effects of GW274150 treatment on the development of experimental colitis induced by dinitrobenzene sulfonic acid. Eur J Pharmacol 2005 01/10;507(0014-2999; 0014-2999; 1-3):281-9.
- Reference 1216
-
Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis 2009 11;15(1536-4844; 1078-0998; 11):1678-85.
- Reference 1217
-
Alhouayek M, Lambert DM, Delzenne NM, Cani PD, Muccioli GG. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J 2011 08;25(1530-6860; 0892-6638; 8):2711-21.
- Reference 1218
-
Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, Guadagno F, Petrosino S, Capasso F, Di M,V, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant cannabis sativa, is protective in a murine model of colitis. J.Mol.Med.(Berl) 2009 11;87(1432-1440; 0946-2716; 11):1111-21.
- Reference 1219
-
Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology 2012;89(1423-0313; 0031-7012; 3-4):149-55.
- Reference 1220
-
Pagano E, Capasso R, Piscitelli F, Romano B, Parisi OA, Finizio S, Lauritano A, Marzo VD, Izzo AA, Borrelli F. An orally active cannabis extract with high content in cannabidiol attenuates chemically-induced intestinal inflammation and hypermotility in the mouse. Front Pharmacol 2016 Oct 4;7:341.
- Reference 1221
-
Borrelli F, Fasolino I, Romano B, Capasso R, Maiello F, Coppola D, Orlando P, Battista G, Pagano E, Di Marzo V, et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol 2013 May 1;85(9):1306-16.
- Reference 1222
-
Romano B, Borrelli F, Fasolino I, Capasso R, Piscitelli F, Cascio M, Pertwee R, Coppola D, Vassallo L, Orlando P, et al. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br J Pharmacol 2013 May;169(1):213-29.
- Reference 1223
-
Singh UP, Singh NP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10(-/-) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol Appl Pharmacol 2012 01/15;258(1096-0333; 0041-008; 2):256-67.
- Reference 1224
-
Izzo AA, Capasso R, Aviello G, Borrelli F, Romano B, Piscitelli F, Gallo L, Capasso F, Orlando P, Di M,V. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from cannabis sativa, on inflammation-induced hypermotility in mice. Br J Pharmacol 2012 06;166(1476-5381; 0007-1188; 4):1444-60.
- Reference 1225
-
García-Planella E, Marín L, Domènech E, Bernal I, Mañosa M, Zabana Y, Gassull M. Use of complementary and alternative medicine and drug abuse in patients with inflammatory bowel disease. Medicina Clinica (Barc.) 2007;128(2):45.
- Reference 1226
-
Ravikoff Allegretti J, Courtwright A, Lucci M, Korzenik JR, Levine J. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis 2013 Dec;19(13):2809-14.
- Reference 1227
-
Weiss A, Friedenberg F. Patterns of cannabis use in patients with inflammatory bowel disease: A population based analysis. Drug Alcohol Depend 2015 Nov 1;156:84-9.
- Reference 1228
-
Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with crohn's disease. Inflamm Bowel Dis 2014 Mar;20(3):472-80.
- Reference 1229
-
Klooker TK, Leliefeld KE, van den Wijngaard RM, Boeckxstaens GE. The cannabinoid receptor agonist delta-9-tetrahydrocannabinol does not affect visceral sensitivity to rectal distension in healthy volunteers and IBS patients. Neurogastroenterol Motil 2011 01;23(1365-2982; 1350-1925; 1):30,5, e2.
- Reference 1230
-
Mallat A, Teixeira-Clerc F, Lotersztajn S. Cannabinoid signaling and liver therapeutics. J Hepatol 2013 Oct;59(4):891-6.
- Reference 1231
-
Tam J, Liu J, Mukhopadhyay B, Cinar R, Godlewski G, Kunos G. Endocannabinoids in liver disease. Hepatology 2011 01;53(1527-3350; 0270-9139; 1):346-55.
- Reference 1232
-
Teixeira-Clerc F, Belot MP, Manin S, Deveaux V, Cadoudal T, Chobert MN, Louvet A, Zimmer A, Tordjmann T, Mallat A, et al. Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration. Hepatology 2010 09;52(1527-3350; 0270-9139; 3):1046-59.
- Reference 1233
-
Giannone FA, Baldassarre M, Domenicali M, Zaccherini G, Trevisani F, Bernardi M, Caraceni P. Reversal of liver fibrosis by the antagonism of endocannabinoid CB1 receptor in a rat model of CCl(4)-induced advanced cirrhosis. Lab Invest 2012 03;92(1530-0307; 0023-6837; 3):384-95.
- Reference 1234
-
Lim MP, Devi LA, Rozenfeld R. Cannabidiol causes activated hepatic stellate cell death through a mechanism of endoplasmic reticulum stress-induced apoptosis. Cell Death.Dis. 2011;2(2041-4889):e170.
- Reference 1235
-
Hochhauser E, Lahat E, Sultan M, Pappo O, Waldman M, Sarne Y, Shainberg A, Gutman M, Safran M, Ari ZB. Ultra low dose delta 9-tetrahydrocannabinol protects mouse liver from ischemia reperfusion injury. Cell Physiol Biochem 2015;36(5):1971-81.
- Reference 1236
-
Mukhopadhyay P, Rajesh M, Horvath B, Batkai S, Park O, Tanchian G, Gao RY, Patel V, Wink DA, Liaudet L, et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med 2011 05/15;50(1873-4596; 0891-5849; 10):1368-81.
- Reference 1237
-
Fouad AA, Jresat I. Therapeutic potential of cannabidiol against ischemia/reperfusion liver injury in rats. Eur J Pharmacol 2011 11/16;670(1879-0712; 0014-2999; 1):216-23.
- Reference 1238
-
Avraham Y, Grigoriadis N, Poutahidis T, Vorobiev L, Magen I, Ilan Y, Mechoulam R, Berry E. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br J Pharmacol 2011 04;162(1476-5381; 0007-1188; 7):1650-8.
- Reference 1239
-
Batkai S, Mukhopadhyay P, Horvath B, Rajesh M, Gao RY, Mahadevan A, Amere M, Battista N, Lichtman AH, Gauson LA, et al. Delta8-tetrahydrocannabivarin prevents hepatic ischaemia/reperfusion injury by decreasing oxidative stress and inflammatory responses through cannabinoid CB2 receptors. Br J Pharmacol 2012 04;165(1476-5381; 0007-1188; 8):2450-61.
- Reference 1240
-
Mazier W, Saucisse N, Gatta-Cherifi B, Cota D. The endocannabinoid system: Pivotal orchestrator of obesity and metabolic disease. Trends Endocrinol Metab 2015 Oct;26(10):524-37.
- Reference 1241
-
Gatta-Cherifi B, Cota D. Endocannabinoids and metabolic disorders. Handb Exp Pharmacol 2015;231:367-91.
- Reference 1242
-
Li C, Jones PM, Persaud SJ. Role of the endocannabinoid system in food intake, energy homeostasis and regulation of the endocrine pancreas. Pharmacol Ther 2011 03;129(1879-016; 0163-7258; 3):307-20.
- Reference 1243
-
Silvestri C, Ligresti A, Di M,V. Peripheral effects of the endocannabinoid system in energy homeostasis: Adipose tissue, liver and skeletal muscle. Rev.Endocr.Metab Disord 2011 09;12(1573-2606; 1389-9155; 3):153-62.
- Reference 1244
-
O'Hare JD, Zielinski E, Cheng B, Scherer T, Buettner C. Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes 2011 04;60(1939-327; 0012-1797; 4):1055-62.
- Reference 1245
-
Engeli S. Central and peripheral cannabinoid receptors as therapeutic targets in the control of food intake and body weight. Handb Exp Pharmacol 2012(0171-2004; 0171-2004; 209):357-81.
- Reference 1246
-
Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrie P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 2003 Apr;63(4):908-14.
- Reference 1247
-
Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord 2003 Mar;27(3):289-301.
- Reference 1248
-
Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol 2005 Jul 11;517(3):174-81.
- Reference 1249
-
Matias I, Bisogno T, Di Marzo V. Endogenous cannabinoids in the brain and peripheral tissues: Regulation of their levels and control of food intake. Int J Obes (Lond) 2006 Apr;30 Suppl 1:S7-S12.
- Reference 1250
-
Matias I, Di Marzo V. Endocannabinoid synthesis and degradation, and their regulation in the framework of energy balance. J Endocrinol Invest 2006;29(3 Suppl):15-26.
- Reference 1251
-
Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab 2007 Jan-Feb;18(1):27-37.
- Reference 1252
-
Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 2003 Aug;112(3):423-31.
- Reference 1253
-
Osei-Hyiaman D, Depetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 2005 05;115(0021-9738; 0021-9738; 5):1298-305.
- Reference 1254
-
Pagano C, Pilon C, Calcagno A, Urbanet R, Rossato M, Milan G, Bianchi K, Rizzuto R, Bernante P, Federspil G, et al. The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab 2007 12;92(0021-972; 0021-972; 12):4810-9.
- Reference 1255
-
Cardinal P, Bellocchio L, Clark S, Cannich A, Klugmann M, Lutz B, Marsicano G, Cota D. Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology 2012 09;153(1945-7170; 0013-7227; 9):4136-43.
- Reference 1256
-
Quarta C, Bellocchio L, Mancini G, Mazza R, Cervino C, Braulke LJ, Fekete C, Latorre R, Nanni C, Bucci M, et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab 2010 04/07;11(1932-7420; 1550-4131; 4):273-85.
- Reference 1257
-
Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Batkai S, Marsicano G, Lutz B, Buettner C, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest 2008 09;118(0021-9738; 0021-9738; 9):3160-9.
- Reference 1258
-
Liu J, Zhou L, Xiong K, Godlewski G, Mukhopadhyay B, Tam J, Yin S, Gao P, Shan X, Pickel J, et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 2012 05;142(1528-0012; 0016-5085; 5):1218-28.
- Reference 1259
-
Ravinet TC, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 2003 02;284(0363-6119; 0363-6119; 2):R345-53.
- Reference 1260
-
Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O'Connor SE, Janiak P, Herbert JM. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab 2005 01;7(1462-8902; 1462-8902; 1):65-72.
- Reference 1261
-
Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le FG, Galiegue S, et al. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J 2005 09;19(1530-6860; 0892-6638; 11):1567-9.
- Reference 1262
-
Watanabe T, Kubota N, Ohsugi M, Kubota T, Takamoto I, Iwabu M, Awazawa M, Katsuyama H, Hasegawa C, Tokuyama K, et al. Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem 2009 01/16;284(0021-9258; 0021-9258; 3):1803-12.
- Reference 1263
-
Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes 2010 04;59(1939-327; 0012-1797; 4):926-34.
- Reference 1264
-
Crespillo A, Suarez J, Bermudez-Silva FJ, Rivera P, Vida M, Alonso M, Palomino A, Lucena MA, Serrano A, Perez-Martin M, et al. Expression of the cannabinoid system in muscle: Effects of a high-fat diet and CB1 receptor blockade. Biochem J 2011 01/01;433(1470-8728; 0264-6021; 1):175-85.
- Reference 1265
-
Bell-Anderson KS, Aouad L, Williams H, Sanz FR, Phuyal J, Larter CZ, Farrell GC, Caterson ID. Coordinated improvement in glucose tolerance, liver steatosis and obesity-associated inflammation by cannabinoid 1 receptor antagonism in fat aussie mice. Int.J.Obes.(Lond) 2011 12;35(1476-5497; 0307-0565; 12):1539-48.
- Reference 1266
-
Van Gaal L, Pi-Sunyer X, Despres JP, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: Pooled 1-year data from the rimonabant in obesity (RIO) program. Diabetes Care 2008 02;31 Suppl 2(1935-5548; 0149-5992):S229-40.
- Reference 1267
-
Van Gaal LF, Scheen AJ, Rissanen AM, Rossner S, Hanotin C, Ziegler O. Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: Two year results from the RIO-europe study. Eur Heart J 2008 07;29(1522-9645; 0195-668; 14):1761-71.
- Reference 1268
-
Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005 11/17;353(1533-4406; 0028-4793; 20):2121-34.
- Reference 1269
-
Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-north america: A randomized controlled trial. JAMA 2006 02/15;295(1538-3598; 0098-7484; 7):761-75.
- Reference 1270
-
Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: A randomised controlled study. Lancet 2006 11/11;368(1474-547; 0140-6736; 9548):1660-72.
- Reference 1271
-
Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-europe study. Lancet 2005 04/16;365(1474-547; 0140-6736; 9468):1389-97.
- Reference 1272
-
Despres JP, Ross R, Boka G, Almeras N, Lemieux I. Effect of rimonabant on the high-triglyceride/ low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: The ADAGIO-lipids trial. Arterioscler Thromb Vasc Biol 2009 03;29(1524-4636; 1079-5642; 3):416-23.
- Reference 1273
-
Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 2013 Apr 2;17(4):475-90.
- Reference 1274
-
Tedesco L, Valerio A, Dossena M, Cardile A, Ragni M, Pagano C, Pagotto U, Carruba MO, Vettor R, Nisoli E. Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: The role of eNOS, p38 MAPK, and AMPK pathways. Diabetes 2010 Nov;59(11):2826-36.
- Reference 1275
-
Cluny NL, Keenan CM, Reimer RA, Le Foll B, Sharkey KA. Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with Delta9-tetrahydrocannabinol. PLoS One 2015 Dec 3;10(12):e0144270.
- Reference 1276
-
Muniyappa R, Sable S, Ouwerkerk R, Mari A, Gharib AM, Walter M, Courville A, Hall G, Chen KY, Volkow ND, et al. Metabolic effects of chronic cannabis smoking. Diabetes Care 2013 Aug;36(8):2415-22.
- Reference 1277
-
Le Strat Y, Le Foll B. Obesity and cannabis use: Results from 2 representative national surveys. Am J Epidemiol 2011 Oct 15;174(8):929-33.
- Reference 1278
-
Le Foll B, Trigo JM, Sharkey KA, Le Strat Y. Cannabis and Delta9-tetrahydrocannabinol (THC) for weight loss? Med Hypotheses 2013 May;80(5):564-7.
- Reference 1279
-
Rajavashisth TB, Shaheen M, Norris KC, Pan D, Sinha SK, Ortega J, Friedman TC. Decreased prevalence of diabetes in marijuana users: Cross-sectional data from the national health and nutrition examination survey (NHANES) III. BMJ Open. 2012;2(2044-6055):e000494.
- Reference 1280
-
Alshaarawy O, Anthony JC. Cannabis smoking and diabetes mellitus: Results from meta-analysis with eight independent replication samples. Epidemiology 2015 Jul;26(4):597-600.
- Reference 1281
-
Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med 2013 Jul;126(7):583-9.
- Reference 1282
-
Ngueta G, Belanger RE, Laouan-Sidi EA, Lucas M. Cannabis use in relation to obesity and insulin resistance in the inuit population. Obesity (Silver Spring) 2015 Feb;23(2):290-5.
- Reference 1283
-
Vidot DC, Prado G, Hlaing WM, Arheart KL, Messiah SE. Emerging issues for our nation's health: The intersection of marijuana use and cardiometabolic disease risk. J Addict Dis 2014;33(1):1-8.
- Reference 1284
-
Deveaux V, Cadoudal T, Ichigotani Y, Teixeira-Clerc F, Louvet A, Manin S, Nhieu JT, Belot MP, Zimmer A, Even P, et al. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS.One. 2009;4(1932-6203; 1932-6203; 6):e5844.
- Reference 1285
-
Agudo J, Martin M, Roca C, Molas M, Bura AS, Zimmer A, Bosch F, Maldonado R. Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia 2010 12;53(1432-0428; 0012-186; 12):2629-40.
- Reference 1286
-
Schmitz K, Mangels N, Haussler A, Ferreiros N, Fleming I, Tegeder I. Pro-inflammatory obesity in aged cannabinoid-2 receptor-deficient mice. Int J Obes (Lond) 2016 Feb;40(2):366-79.
- Reference 1287
-
Wargent ET, Zaibi MS, Silvestri C, Hislop DC, Stocker CJ, Stott CG, Guy GW, Duncan M, Di Marzo V, Cawthorne MA. The cannabinoid delta(9)-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse models of obesity. Nutr Diabetes 2013 May 27;3:e68.
- Reference 1288
-
Levendal RA, Schumann D, Donath M, Frost CL. Cannabis exposure associated with weight reduction and beta-cell protection in an obese rat model. Phytomedicine 2012 05/15;19(1618-095; 0944-7113; 7):575-82.
- Reference 1289
-
Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, O'Sullivan SE, Tan GD. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care 2016 Oct;39(10):1777-86.
- Reference 1290
-
Li C, Bowe JE, Huang GC, Amiel SA, Jones PM, Persaud SJ. Cannabinoid receptor agonists and antagonists stimulate insulin secretion from isolated human islets of langerhans. Diabetes Obes Metab 2011 10;13(1463-1326; 1462-8902; 10):903-10.
- Reference 1291
-
Li C, Vilches-Flores A, Zhao M, Amiel SA, Jones PM, Persaud SJ. Expression and function of monoacylglycerol lipase in mouse beta-cells and human islets of langerhans. Cell Physiol Biochem 2012;30(1421-9778; 1015-8987; 2):347-58.
- Reference 1292
-
Bermudez-Silva FJ, Suarez J, Baixeras E, Cobo N, Bautista D, Cuesta-Munoz AL, Fuentes E, Juan-Pico P, Castro MJ, Milman G, et al. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia 2008 03;51(0012-186; 0012-186; 3):476-87.
- Reference 1293
-
De Pasquale A, Costa G, Trovato A. The influence of cannabis on glucoregulation. Bull Narc 1978 07;30(0007-523; 0007-523; 3):33-41.
- Reference 1294
-
Bermudez-Siva FJ, Serrano A, Diaz-Molina FJ, Sanchez V,I, Juan-Pico P, Nadal A, Fuentes E, Rodriguez de FF. Activation of cannabinoid CB1 receptors induces glucose intolerance in rats. Eur J Pharmacol 2006 02/15;531(0014-2999; 0014-2999; 1-3):282-4.
- Reference 1295
-
Hollister LE, Reaven GM. Delta-9-tetrahydrocannabinol and glucose tolerance. Clin Pharmacol Ther 1974 08;16(0009-9236; 0009-9236; 2):297-302.
- Reference 1296
-
Michalski CW, Laukert T, Sauliunaite D, Pacher P, Bergmann F, Agarwal N, Su Y, Giese T, Giese NA, Batkai S, et al. Cannabinoids ameliorate pain and reduce disease pathology in cerulein-induced acute pancreatitis. Gastroenterology 2007 05;132(0016-5085; 0016-5085; 5):1968-78.
- Reference 1297
-
Michalski CW, Maier M, Erkan M, Sauliunaite D, Bergmann F, Pacher P, Batkai S, Giese NA, Giese T, Friess H, et al. Cannabinoids reduce markers of inflammation and fibrosis in pancreatic stellate cells. PLoS.One. 2008;3(1932-6203; 1932-6203; 2):e1701.
- Reference 1298
-
Matsuda K, Mikami Y, Takeda K, Fukuyama S, Egawa S, Sunamura M, Maruyama I, Matsuno S. The cannabinoid 1 receptor antagonist, AM251, prolongs the survival of rats with severe acute pancreatitis. Tohoku J Exp Med 2005 10;207(0040-8727; 0040-8727; 2):99-107.
- Reference 1299
-
Dembinski A, Warzecha Z, Ceranowicz P, Dembinski M, Cieszkowski J, Pawlik WW, Konturek SJ, Tomaszewska R, Hladki W, Konturek PC. Cannabinoids in acute gastric damage and pancreatitis. J Physiol Pharmacol 2006 11;57 Suppl 5(1899-1505; 0867-5910):137-54.
- Reference 1300
-
Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, Swartz-Basile DA, Prather AD, Lillemoe KD. Cannabinoid receptor-1 blockade attenuates acute pancreatitis in obesity by an adiponectin mediated mechanism. J Gastrointest Surg 2009 05;13(1873-4626; 1091-255; 5):831-8.
- Reference 1301
-
Petrella C, Agostini S, Alema' GS, Casolini P, Carpino F, Giuli C, Improta G, Linari G, Petrozza V, Broccardo M. Cannabinoid agonist WIN55,212 in vitro inhibits interleukin-6 (IL-6) and monocyte chemo-attractant protein-1 (MCP-1) release by rat pancreatic acini and in vivo induces dual effects on the course of acute pancreatitis. Neurogastroenterol Motil 2010 11;22(1365-2982; 1350-1925; 11):1248,56, e323.
- Reference 1302
-
Velasco G, Hernandez-Tiedra S, Davila D, Lorente M. The use of cannabinoids as anticancer agents. Prog Neuropsychopharmacol Biol Psychiatry 2016 Jan 4;64:259-66.
- Reference 1303
-
Malfitano AM, Ciaglia E, Gangemi G, Gazzerro P, Laezza C, Bifulco M. Update on the endocannabinoid system as an anticancer target. Expert.Opin.Ther.Targets. 2011 03;15(1744-7631; 1472-8222; 3):297-308.
- Reference 1304
-
Pagotto U, Marsicano G, Fezza F, Theodoropoulou M, Grubler Y, Stalla J, Arzberger T, Milone A, Losa M, Di M,V, et al. Normal human pituitary gland and pituitary adenomas express cannabinoid receptor type 1 and synthesize endogenous cannabinoids: First evidence for a direct role of cannabinoids on hormone modulation at the human pituitary level. J Clin Endocrinol Metab 2001 06;86(0021-972; 0021-972; 6):2687-96.
- Reference 1305
-
Schmid PC, Wold LE, Krebsbach RJ, Berdyshev EV, Schmid HH. Anandamide and other N-acylethanolamines in human tumors. Lipids 2002 09;37(0024-4201; 0024-4201; 9):907-12.
- Reference 1306
-
Nithipatikom K, Endsley MP, Isbell MA, Falck JR, Iwamoto Y, Hillard CJ, Campbell WB. 2-arachidonoylglycerol: A novel inhibitor of androgen-independent prostate cancer cell invasion. Cancer Res 2004 12/15;64(0008-5472; 0008-5472; 24):8826-30.
- Reference 1307
-
Petersen G, Moesgaard B, Schmid PC, Schmid HH, Broholm H, Kosteljanetz M, Hansen HS. Endocannabinoid metabolism in human glioblastomas and meningiomas compared to human non-tumour brain tissue. J Neurochem 2005 04;93(0022-3042; 0022-3042; 2):299-309.
- Reference 1308
-
Cianchi F, Papucci L, Schiavone N, Lulli M, Magnelli L, Vinci MC, Messerini L, Manera C, Ronconi E, Romagnani P, et al. Cannabinoid receptor activation induces apoptosis through tumor necrosis factor alpha-mediated ceramide de novo synthesis in colon cancer cells. Clin Cancer Res 2008 12/01;14(1078-0432; 1078-0432; 23):7691-700.
- Reference 1309
-
Grimaldi C, Capasso A. The endocannabinoid system in the cancer therapy: An overview. Curr Med Chem 2011;18(1875-533; 0929-8673; 11):1575-83.
- Reference 1310
-
Pisanti S, Bifulco M. Endocannabinoid system modulation in cancer biology and therapy. Pharmacol Res 2009 08;60(1096-1186; 1043-6618; 2):107-16.
- Reference 1311
-
Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 2010 07;58(1098-1136; 0894-1491; 9):1017-30.
- Reference 1312
-
McAllister SD, Chan C, Taft RJ, Luu T, Abood ME, Moore DH, Aldape K, Yount G. Cannabinoids selectively inhibit proliferation and induce death of cultured human glioblastoma multiforme cells. J Neurooncol 2005 08;74(0167-594; 0167-594; 1):31-40.
- Reference 1313
-
Cudaback E, Marrs W, Moeller T, Stella N. The expression level of CB1 and CB2 receptors determines their efficacy at inducing apoptosis in astrocytomas. PLoS.One. 2010;5(1932-6203; 1932-6203; 1):e8702.
- Reference 1314
-
Caffarel MM, Sarrio D, Palacios J, Guzman M, Sanchez C. Delta9-tetrahydrocannabinol inhibits cell cycle progression in human breast cancer cells through Cdc2 regulation. Cancer Res 2006 07/01;66(0008-5472; 0008-5472; 13):6615-21.
- Reference 1315
-
McKallip RJ, Nagarkatti M, Nagarkatti PS. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol 2005 03/15;174(0022-1767; 0022-1767; 6):3281-9.
- Reference 1316
-
Takeda S, Yamaori S, Motoya E, Matsunaga T, Kimura T, Yamamoto I, Watanabe K. Delta(9)-tetrahydrocannabinol enhances MCF-7 cell proliferation via cannabinoid receptor-independent signaling. Toxicology 2008 03/12;245(0300-483; 0300-483; 1-2):141-6.
- Reference 1317
-
Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De PL, Laezza C, Portella G, Bifulco M, Di M,V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther 2006 09;318(0022-3565; 0022-3565; 3):1375-87.
- Reference 1318
-
Preet A, Ganju RK, Groopman JE. Delta9-tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene 2008 01/10;27(1476-5594; 0950-9232; 3):339-46.
- Reference 1319
-
Greenhough A, Patsos HA, Williams AC, Paraskeva C. The cannabinoid delta(9)-tetrahydrocannabinol inhibits RAS-MAPK and PI3K-AKT survival signalling and induces BAD-mediated apoptosis in colorectal cancer cells. Int J Cancer 2007 11/15;121(1097-0215; 0020-7136; 10):2172-80.
- Reference 1320
-
Glodde N, Jakobs M, Bald T, Tuting T, Gaffal E. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci 2015 Oct 1;138:35-40.
- Reference 1321
-
Borrelli F, Pagano E, Romano B, Panzera S, Maiello F, Coppola D, De Petrocellis L, Buono L, Orlando P, Izzo AA. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014 Dec;35(12):2787-97.
- Reference 1322
-
Romano B, Borrelli F, Pagano E, Cascio MG, Pertwee RG, Izzo AA. Inhibition of colon carcinogenesis by a standardized cannabis sativa extract with high content of cannabidiol. Phytomedicine 2014 Apr 15;21(5):631-9.
- Reference 1323
-
Scott KA, Dalgleish AG, Liu WM. The combination of cannabidiol and Delta9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol Cancer Ther 2014 Dec;13(12):2955-67.
- Reference 1324
-
Kampa-Schittenhelm KM, Salitzky O, Akmut F, Illing B, Kanz L, Salih HR, Schittenhelm MM. Dronabinol has preferential antileukemic activity in acute lymphoblastic and myeloid leukemia with lymphoid differentiation patterns. BMC Cancer 2016 Jan 16;16:25,015-2029-8.
- Reference 1325
-
Galve-Roperh I, Sanchez C, Cortes ML, Gomez dP, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med 2000 03;6(1078-8956; 1078-8956; 3):313-9.
- Reference 1326
-
Caffarel MM, Andradas C, Mira E, Perez-Gomez E, Cerutti C, Moreno-Bueno G, Flores JM, Garcia-Real I, Palacios J, Manes S, et al. Cannabinoids reduce ErbB2-driven breast cancer progression through akt inhibition. Mol.Cancer 2010;9(1476-4598; 1476-4598):196-206.
- Reference 1327
-
McAllister SD, Murase R, Christian RT, Lau D, Zielinski AJ, Allison J, Almanza C, Pakdel A, Lee J, Limbad C, et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat 2010 09/22;129(1573-7217; 0167-6806; 1):37-47.
- Reference 1328
-
Hernan Perez de la Ossa,D., Gil-Alegre ME, Ligresti A, Aberturas Mdel R, Molpeceres J, Torres AI, Di Marzo V. Preparation and characterization of delta(9)-tetrahydrocannabinol-loaded biodegradable polymeric microparticles and their antitumoral efficacy on cancer cell lines. J Drug Target 2013 Sep;21(8):710-8.
- Reference 1329
-
McAllister SD, Soroceanu L, Desprez PY. The antitumor activity of plant-derived non-psychoactive cannabinoids. J Neuroimmune Pharmacol 2015 Jun;10(2):255-67.
- Reference 1330
-
Carracedo A, Lorente M, Egia A, Blazquez C, Garcia S, Giroux V, Malicet C, Villuendas R, Gironella M, Gonzalez-Feria L, et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell 2006 04;9(1535-6108; 1535-6108; 4):301-12.
- Reference 1331
-
Torres S, Lorente M, Rodriguez-Fornes F, Hernandez-Tiedra S, Salazar M, Garcia-Taboada E, Barcia J, Guzman M, Velasco G. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol.Cancer Ther. 2011 01;10(1538-8514; 1535-7163; 1):90-103.
- Reference 1332
-
Elliott DA, Nabavizadeh N, Romer JL, Chen Y, Holland JM. Medical marijuana use in head and neck squamous cell carcinoma patients treated with radiotherapy. Support Care Cancer 2016 Aug;24(8):3517-24.
- Reference 1333
-
Foroughi M, Hendson G, Sargent MA, Steinbok P. Spontaneous regression of septum pellucidum/forniceal pilocytic astrocytomas--possible role of cannabis inhalation. Childs Nerv Syst 2011 Apr;27(4):671-9.
- Reference 1334
-
Guzman M, Duarte MJ, Blazquez C, Ravina J, Rosa MC, Galve-Roperh I, Sanchez C, Velasco G, Gonzalez-Feria L. A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer 2006 07/17;95(0007-0920; 0007-0920; 2):197-203.
- Reference 1335
-
Dinnes J, Cave C, Huang S, Milne R. A rapid and systematic review of the effectiveness of temozolomide for the treatment of recurrent malignant glioma. Br J Cancer 2002 02/12;86(0007-0920; 0007-0920; 4):501-5.
- Reference 1336
-
Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. the polymer-brain tumor treatment group. Lancet 1995 04/22;345(0140-6736; 0140-6736; 8956):1008-12.
- Reference 1337
-
Salazar M, Carracedo A, Salanueva IJ, Hernandez-Tiedra S, Lorente M, Egia A, Vazquez P, Blazquez C, Torres S, Garcia S, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest 2009 May;119(5):1359-72.
- Reference 1338
-
Marcu JP, Christian RT, Lau D, Zielinski AJ, Horowitz MP, Lee J, Pakdel A, Allison J, Limbad C, Moore DH, et al. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol.Cancer Ther. 2010 01;9(1538-8514; 1535-7163; 1):180-9.
- Reference 1339
-
Velasco G, Carracedo A, Blazquez C, Lorente M, Aguado T, Haro A, Sanchez C, Galve-Roperh I, Guzman M. Cannabinoids and gliomas. Mol Neurobiol 2007 08;36(0893-7648; 0893-7648; 1):60-7.
- Reference 1340
-
Parolaro D, Massi P. Cannabinoids as potential new therapy for the treatment of gliomas. Expert.Rev.Neurother. 2008 01;8(1744-8360; 1473-7175; 1):37-49.
- Reference 1341
-
Alexander A, Smith PF, Rosengren RJ. Cannabinoids in the treatment of cancer. Cancer Lett 2009 11/18;285(1872-7980; 0304-3835; 1):6-12.
- Reference 1342
-
Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005 04/07;434(1476-4687; 0028-0836; 7034):782-6.
- Reference 1343
-
Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, Nakayama M, Kaikita K, Obata T, Takeya M, et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation 2009 01/06;119(1524-4539; 0009-7322; 1):28-36.
- Reference 1344
-
Zhao Y, Yuan Z, Liu Y, Xue J, Tian Y, Liu W, Zhang W, Shen Y, Xu W, Liang X, et al. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol 2010 03;55(1533-4023; 0160-2446; 3):292-8.
- Reference 1345
-
Hoyer FF, Steinmetz M, Zimmer S, Becker A, Lutjohann D, Buchalla R, Zimmer A, Nickenig G. Atheroprotection via cannabinoid receptor-2 is mediated by circulating and vascular cells in vivo. J Mol Cell Cardiol 2011 12;51(1095-8584; 0022-2828; 6):1007-14.
- Reference 1346
-
Willecke F, Zeschky K, Ortiz RA, Colberg C, Auwarter V, Kneisel S, Hutter M, Lozhkin A, Hoppe N, Wolf D, et al. Cannabinoid receptor 2 signaling does not modulate atherogenesis in mice. PLoS.One. 2011;6(1932-6203; 1932-6203; 4):e19405.
- Reference 1347
-
Netherland CD, Pickle TG, Bales A, Thewke DP. Cannabinoid receptor type 2 (CB2) deficiency alters atherosclerotic lesion formation in hyperlipidemic ldlr-null mice. Atherosclerosis 2010 11;213(1879-1484; 0021-9150; 1):102-8.
- Reference 1348
-
Rajesh M, Mukhopadhyay P, Hasko G, Liaudet L, Mackie K, Pacher P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br J Pharmacol 2010 06;160(1476-5381; 0007-1188; 3):688-700.
- Reference 1349
-
Singla S, Sachdeva R, Mehta JL. Cannabinoids and atherosclerotic coronary heart disease. Clin Cardiol 2012 06;35(1932-8737; 0160-9289; 6):329-35.
- Reference 1350
-
Montecucco F, Di M,V. At the heart of the matter: The endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol Sci 2012 06;33(1873-3735; 0165-6147; 6):331-40.
- Reference 1351
-
Steffens S, Pacher P. Targeting cannabinoid receptor CB(2) in cardiovascular disorders: Promises and controversies. Br J Pharmacol 2012 09;167(1476-5381; 0007-1188; 2):313-23.
- Reference 1352
-
Takeda S, Usami N, Yamamoto I, Watanabe K. Cannabidiol-2',6'-dimethyl ether, a cannabidiol derivative, is a highly potent and selective 15-lipoxygenase inhibitor. Drug Metab Dispos 2009 08;37(1521-009; 0090-9556; 8):1733-7.
- Reference 1353
-
Nayak AP, Green BJ, Sussman G, Berlin N, Lata H, Chandra S, ElSohly MA, Hettick JM, Beezhold DH. Characterization of cannabis sativa allergens. Ann Allergy Asthma Immunol 2013 Jul;111(1):32-7.
- Reference 1354
-
Larramendi CH, Lopez-Matas MA, Ferrer A, Huertas AJ, Pagan JA, Navarro LA, Garcia-Abujeta JL, Andreu C, Carnes J. Prevalence of sensitization to cannabis sativa. lipid-transfer and thaumatin-like proteins are relevant allergens. Int Arch Allergy Immunol 2013;162(2):115-22.
- Reference 1355
-
Draz EI, Oreby MM, Elsheikh EA, Khedr LA, Atlam SA. Marijuana use in acute coronary syndromes. The American Journal of Drug and Alcohol Abuse 2016:1-7.
- Reference 1356
-
Hemachandra D, McKetin R, Cherbuin N, Anstey KJ. Heavy cannabis users at elevated risk of stroke: Evidence from a general population survey. Aust N Z J Public Health 2015 Nov 11.
- Reference 1357
-
Jouanjus E, Lapeyre-Mestre M, Micallef J, French Association of the Regional Abuse and Dependence Monitoring Centres (CEIP-A) Working Group on Cannabis Complications*. Cannabis use: Signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc 2014 Apr 23;3(2):e000638.
- Reference 1358
-
Hezode C, Zafrani ES, Roudot-Thoraval F, Costentin C, Hessami A, Bouvier-Alias M, Medkour F, Pawlostky JM, Lotersztajn S, Mallat A. Daily cannabis use: A novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology 2008 02;134(1528-0012; 0016-5085; 2):432-9.
- Reference 1359
-
Barrett SP, Darredeau C, Pihl RO. Patterns of simultaneous polysubstance use in drug using university students. Hum.Psychopharmacol. 2006 06;21(0885-6222; 0885-6222; 4):255-63.
- Reference 1360
-
Agrawal A, Lynskey MT. Does gender contribute to heterogeneity in criteria for cannabis abuse and dependence? results from the national epidemiological survey on alcohol and related conditions. Drug Alcohol Depend 2007 05/11;88(0376-8716; 0376-8716; 2-3):300-7.
- Reference 1361
-
Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal marijuana use and adverse neonatal outcomes: A systematic review and meta-analysis. Obstet Gynecol 2016 Oct;128(4):713-23.
- Reference 1362
-
Gunn JK, Rosales CB, Center KE, Nunez A, Gibson SJ, Christ C, Ehiri JE. Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-analysis. BMJ Open 2016 Apr 5;6(4):e009986,2015-009986.
- Reference 1363
-
Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr.Opin.Psychiatry 2006 05;19(0951-7367; 0951-7367; 3):233-8.
- Reference 1364
-
National Institute on Drug Abuse. NIDA InfoFacts: Marijuana. 2009.
- Reference 1365
-
Johns A. Psychiatric effects of cannabis. Br J Psychiatry 2001 02;178(0007-1250):116-22.
- Reference 1366
-
Mathew RJ, Wilson WH, Coleman RE, Turkington TG, DeGrado TR. Marijuana intoxication and brain activation in marijuana smokers. Life Sci 1997;60(0024-3205; 0024-3205; 23):2075-89.
- Reference 1367
-
Ramaekers JG, Kauert G, van RP, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology 2006 10;31(0893-133; 0006-3223; 10):2296-303.
- Reference 1368
-
Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the association between the level of cannabis use and risk of psychosis. Schizophr Bull 2016 Feb 15.
- Reference 1369
-
Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, Lester H, Chisin R, Sarne Y, Mechoulam R, Bar-Hamburger R, et al. A study investigating the acute dose-response effects of 13 mg and 17 mg delta 9- tetrahydrocannabinol on cognitive-motor skills, subjective and autonomic measures in regular users of marijuana. J Psychopharmacol 2008 Jun;22(4):441-51.
- Reference 1370
-
Kurzthaler I, Hummer M, Miller C, Sperner-Unterweger B, Gunther V, Wechdorn H, Battista HJ, Fleischhacker WW. Effect of cannabis use on cognitive functions and driving ability. J Clin Psychiatry 1999 06;60(0160-6689; 0160-6689; 6):395-9.
- Reference 1371
-
Reisfield GM, Wasan AD, Jamison RN. The prevalence and significance of cannabis use in patients prescribed chronic opioid therapy: A review of the extant literature. Pain Med. 2009 11;10(1526-4637; 1526-2375; 8):1434-41.
- Reference 1372
-
Dubois S, Mullen N, Weaver B, Bedard M. The combined effects of alcohol and cannabis on driving: Impact on crash risk. Forensic Sci Int 2015 Mar;248:94-100.
- Reference 1373
-
Ballard ME, de WH. Combined effects of acute, very-low-dose ethanol and delta(9)-tetrahydrocannabinol in healthy human volunteers. Pharmacol Biochem Behav 2011 02;97(1873-5177; 0091-3057; 4):627-31.
- Reference 1374
-
de Jong FA, Engels FK, Mathijssen RH, van ZL, Verweij J, Peters RP, Sparreboom A. Medicinal cannabis in oncology practice: Still a bridge too far? J Clin Oncol 2005 05/01;23(0732-183; 0732-183; 13):2886-91.
- Reference 1375
-
Ashton CH. Adverse effects of cannabis and cannabinoids. Br J Anaesth 1999 10;83(0007-0912; 0007-0912; 4):637-49.
- Reference 1376
-
Maccarrone M. Endocannabinoid signaling in female reproductive events: A potential therapeutic target? Expert Opin Ther Targets 2015;19(11):1423-7.
- Reference 1377
-
Battista N, Pasquariello N, Di TM, Maccarrone M. Interplay between endocannabinoids, steroids and cytokines in the control of human reproduction. J Neuroendocrinol 2008 05;20 Suppl 1(1365-2826; 0953-8194):82-9.
- Reference 1378
-
Correa F, Wolfson ML, Valchi P, Aisemberg J, Franchi AM. Endocannabinoid system and pregnancy. Reproduction 2016 Dec;152(6):R191-200.
- Reference 1379
-
Paria BC, Das SK, Dey SK. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc Natl Acad Sci U S A 1995 Oct 10;92(21):9460-4.
- Reference 1380
-
Habayeb OM, Taylor AH, Finney M, Evans MD, Konje JC. Plasma anandamide concentration and pregnancy outcome in women with threatened miscarriage. JAMA 2008 03/12;299(1538-3598; 0098-7484; 10):1135-6.
- Reference 1381
-
Calvigioni D, Hurd YL, Harkany T, Keimpema E. Neuronal substrates and functional consequences of prenatal cannabis exposure. Eur Child Adolesc Psychiatry 2014 Oct;23(10):931-41.
- Reference 1382
-
Fried PA. Conceptual issues in behavioral teratology and their application in determining long-term sequelae of prenatal marihuana exposure. J Child Psychol Psychiatry 2002 01;43(0021-9630; 1):81-102.
- Reference 1383
-
Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol 2002 May-Jun;24(3):309-20.
- Reference 1384
-
Grant KS, Petroff R, Isoherranen N, Stella N, Burbacher TM. Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol Ther 2017 Aug 25.
- Reference 1385
-
Perez-Reyes M, Wall ME. Presence of delta9-tetrahydrocannabinol in human milk. N Engl J Med 1982 09/23;307(0028-4793; 13):819-20.
- Reference 1386
-
Garry A, Rigourd V, Amirouche A, Fauroux V, Aubry S, Serreau R. Cannabis and breastfeeding. J.Toxicol. 2009;2009(1687-8205; 1687-8191):596149.
- Reference 1387
-
Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology (Berl) 1994 07;115(0033-3158; 3):340-9.
- Reference 1388
-
Lukas SE, Orozco S. Ethanol increases plasma delta(9)-tetrahydrocannabinol (THC) levels and subjective effects after marihuana smoking in human volunteers. Drug Alcohol Depend 2001 10/01;64(0376-8716; 2):143-9.
- Reference 1389
-
Spina E, Santoro V, D'Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: An update. Clin Ther 2008 07;30(0149-2918; 0149-2918; 7):1206-27.
- Reference 1390
-
Anderson GD, Chan LN. Pharmacokinetic drug interactions with tobacco, cannabinoids and smoking cessation products. Clin Pharmacokinet 2016 Nov;55(11):1353-68.
- Reference 1391
-
Jusko WJ, Gardner MJ, Mangione A, Schentag JJ, Koup JR, Vance JW. Factors affecting theophylline clearances: Age, tobacco, marijuana, cirrhosis, congestive heart failure, obesity, oral contraceptives, benzodiazepines, barbiturates, and ethanol. J Pharm Sci 1979 Nov;68(11):1358-66.
- Reference 1392
-
Gardner MJ, Tornatore KM, Jusko WJ, Kanarkowski R. Effects of tobacco smoking and oral contraceptive use on theophylline disposition. Br J Clin Pharmacol 1983 Sep;16(3):271-80.
- Reference 1393
-
Chetty M, Miller R, Moodley SV. Smoking and body weight influence the clearance of chlorpromazine. Eur J Clin Pharmacol 1994;46(6):523-6.
- Reference 1394
-
Tamir I, Mechoulam R, Meyer AY. Cannabidiol and phenytoin: A structural comparison. J Med Chem 1980 Feb;23(2):220-3.
- Reference 1395
-
Davison SN, Davison JS. Is there a legitimate role for the therapeutic use of cannabinoids for symptom management in chronic kidney disease? J Pain Symptom Manage 2011 04;41(1873-6513; 0885-3924; 4):768-78.
- Reference 1396
-
Manini AF, Yiannoulos G, Bergamaschi MM, Hernandez S, Olmedo R, Barnes AJ, Winkel G, Sinha R, Jutras-Aswad D, Huestis MA, et al. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med 2015 May-Jun;9(3):204-10.
- Reference 1397
-
Purnell JQ, Zambon A, Knopp RH, Pizzuti DJ, Achari R, Leonard JM, Locke C, Brunzell JD. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS 2000 01/07;14(0269-9370; 1):51-7.
- Reference 1398
-
Ellis GM,Jr., Mann MA, Judson BA, Schramm NT, Tashchian A. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clin Pharmacol Ther 1985 11;38(0009-9236; 0009-9236; 5):572-8.
- Reference 1399
-
Lowe RH, Abraham TT, Darwin WD, Herning R, Cadet JL, Huestis MA. Extended urinary Delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend 2009 11/01;105(1879-0046; 0376-8716; 1-2):24-32.
- Reference 1400
-
Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: A systematic review. CMAJ 2008 06/17;178(1488-2329; 0820-3946; 13):1669-78.
- Reference 1401
-
Amos A, Wiltshire S, Bostock Y, Haw S, McNeill A. 'You can't go without a fag...you need it for your hash'--a qualitative exploration of smoking, cannabis and young people. Addiction 2004 01;99(0965-2140; 0965-2140; 1):77-81.
- Reference 1402
-
Hezode C, Roudot-Thoraval F, Nguyen S, Grenard P, Julien B, Zafrani ES, Pawlotsky JM, Dhumeaux D, Lotersztajn S, Mallat A. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology 2005 07;42(0270-9139; 1):63-71.
- Reference 1403
-
Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend 2008 01/01;92(0376-8716; 0376-8716; 1-3):48-54.
- Reference 1404
-
Agrawal A, Scherrer JF, Lynskey MT, Sartor CE, Grant JD, Haber JR, Madden PA, Jacob T, Bucholz KK, Xian H. Patterns of use, sequence of onsets and correlates of tobacco and cannabis. Addict Behav 2011 12;36(1873-6327; 0306-4603; 12):1141-7.
- Reference 1405
-
Maertens RM, White PA, Williams A, Yauk CL. A global toxicogenomic analysis investigating the mechanistic differences between tobacco and marijuana smoke condensates in vitro. Toxicology 2013 Jun 7;308:60-73.
- Reference 1406
-
Callaghan RC, Allebeck P, Sidorchuk A. Marijuana use and risk of lung cancer: A 40-year cohort study. Cancer Causes Control 2013 Oct;24(10):1811-20.
- Reference 1407
-
Huang YH, Zhang ZF, Tashkin DP, Feng B, Straif K, Hashibe M. An epidemiologic review of marijuana and cancer: An update. Cancer Epidemiol Biomarkers Prev 2015 Jan;24(1):15-31.
- Reference 1408
-
Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc 2013 Jun;10(3):239-47.
- Reference 1409
-
Wu TC, Tashkin DP, Djahed B, Rose JE. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med 1988 02/11;318(0028-4793; 6):347-51.
- Reference 1410
-
Taylor DR, Fergusson DM, Milne BJ, Horwood LJ, Moffitt TE, Sears MR, Poulton R. A longitudinal study of the effects of tobacco and cannabis exposure on lung function in young adults. Addiction 2002 08;97(0965-2140; 8):1055-61.
- Reference 1411
-
Tashkin DP. Marihuana and the lung. In: C. G. Nahas, K. M. Sutin, D. J. Harvey, S. Agurell, editors. Marihuana and medicine. Totowa, New Jersey: Humana Press; 1999. ID: 2388; RP: NOT IN FILE.
- Reference 1412
-
Taylor DR, Poulton R, Moffitt TE, Ramankutty P, Sears MR. The respiratory effects of cannabis dependence in young adults. Addiction 2000 11;95(0965-2140; 0965-2140; 11):1669-77.
- Reference 1413
-
Denning DW, Follansbee SE, Scolaro M, Norris S, Edelstein H, Stevens DA. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med 1991 03/07;324(0028-4793; 10):654-62.
- Reference 1414
-
Moore BA, Augustson EM, Moser RP, Budney AJ. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med 2005 01;20(1525-1497; 0884-8734; 1):33-7.
- Reference 1415
-
Roth MD, Whittaker K, Salehi K, Tashkin DP, Baldwin GC. Mechanisms for impaired effector function in alveolar macrophages from marijuana and cocaine smokers. J Neuroimmunol 2004 02;147(0165-5728; 0165-5728; 1-2):82-6.
- Reference 1416
-
Giroud C, de Cesare M, Berthet A, Varlet V, Concha-Lozano N, Favrat B. E-cigarettes: A review of new trends in cannabis use. Int J Environ Res Public Health 2015 Aug 21;12(8):9988-10008.
- Reference 1417
-
Varlet V, Concha-Lozano N, Berthet A, Plateel G, Favrat B, De Cesare M, Lauer E, Augsburger M, Thomas A, Giroud C. Drug vaping applied to cannabis: Is "cannavaping" a therapeutic alternative to marijuana? Sci Rep 2016 May 26;6:25599.
- Reference 1418
-
Peace MR, Butler KE, Wolf CE, Poklis JL, Poklis A. Evaluation of two commercially available cannabidiol formulations for use in electronic cigarettes. Front Pharmacol 2016 Aug 29;7:279.
- Reference 1419
-
Geiss O, Bianchi I, Barahona F, Barrero-Moreno J. Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. Int J Hyg Environ Health 2015 Jan;218(1):169-80.
- Reference 1420
-
Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med 2015 Jan 22;372(4):392-4.
- Reference 1421
-
Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, Destaillats H. Emissions from electronic cigarettes: Key parameters affecting the release of harmful chemicals. Environ Sci Technol 2016 Sep 6;50(17):9644-51.
- Reference 1422
-
Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014 Oct;16(10):1319-26.
- Reference 1423
-
Gillman IG, Kistler KA, Stewart EW, Paolantonio AR. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol 2016 Mar;75:58-65.
- Reference 1424
-
Farsalinos KE, Voudris V, Poulas K. E-cigarettes generate high levels of aldehydes only in 'dry puff' conditions. Addiction 2015 Aug;110(8):1352-6.
- Reference 1425
-
Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health 2016 May;219(3):268-77.
- Reference 1426
-
Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BW, Vogel RI, Thompson E, Murphy SE, Hatsukami DK. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res 2015 Jun;17(6):704-9.
- Reference 1427
-
McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest 2012 Apr;141(4):1110-3.
- Reference 1428
-
Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med 2014 Jul;47(1):15-7.
- Reference 1429
-
Hureaux J, Drouet M, Urban T. A case report of subacute bronchial toxicity induced by an electronic cigarette. Thorax 2014 Jun;69(6):596-7.
- Reference 1430
-
Eisenstein TK, Meissler JJ. Effects of cannabinoids on T-cell function and resistance to infection. J Neuroimmune Pharmacol 2015 Jun;10(2):204-16.
- Reference 1431
-
LIU DZ, HU CM, HUANG CH, WEY SP, Jan TR. Cannabidiol attenuates delayed-type hypersensitivity reactions via suppressing T-cell and macrophage reactivity. Acta Pharmacol Sin 2010 12;31(1745-7254; 1671-4083; 12):1611-7.
- Reference 1432
-
Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, Vogel Z. Cannabinoids delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem 2010 01/15;285(1083-351; 0021-9258; 3):1616-26.
- Reference 1433
-
Reiss CS. Cannabinoids and viral infections. Pharmaceuticals (Basel) 2010;3(6):1873-86.
- Reference 1434
-
Tahamtan A, Tavakoli-Yaraki M, Rygiel TP, Mokhtari-Azad T, Salimi V. Effects of cannabinoids and their receptors on viral infections. J Med Virol 2016 Jan;88(1):1-12.
- Reference 1435
-
Mishkin EM, Cabral GA. Delta-9-tetrahydrocannabinol decreases host resistance to herpes simplex virus type 2 vaginal infection in the B6C3F1 mouse. J Gen Virol 1985 12;66 (Pt 12)(0022-1317; 0022-1317):2539-49.
- Reference 1436
-
Cabral GA, McNerney PJ, Mishkin EM. Delta-9-tetrahydrocannabinol enhances release of herpes simplex virus type 2. J Gen Virol 1986 09;67 (Pt 9)(0022-1317; 0022-1317):2017-22.
- Reference 1437
-
Cabral GA, Lockmuller JC, Mishkin EM. Delta 9-tetrahydrocannabinol decreases alpha/beta interferon response to herpes simplex virus type 2 in the B6C3F1 mouse. Proc Soc Exp Biol Med 1986 02;181(0037-9727; 0037-9727; 2):305-11.
- Reference 1438
-
Roth MD, Baldwin GC, Tashkin DP. Effects of delta-9-tetrahydrocannabinol on human immune function and host defense. Chem Phys Lipids 2002 12/31;121(0009-3084; 0009-3084; 1-2):229-39.
- Reference 1439
-
Buchweitz JP, Karmaus PW, Harkema JR, Williams KJ, Kaminski NE. Modulation of airway responses to influenza A/PR/8/34 by Delta9-tetrahydrocannabinol in C57BL/6 mice. J Pharmacol Exp Ther 2007 11;323(0022-3565; 0022-3565; 2):675-83.
- Reference 1440
-
Zhang X, Wang JF, Kunos G, Groopman JE. Cannabinoid modulation of kaposi's sarcoma-associated herpesvirus infection and transformation. Cancer Res 2007 08/01;67(0008-5472; 0008-5472; 15):7230-7.
- Reference 1441
-
Herrera RA, Oved JH, Reiss CS. Disruption of IFN-gamma- mediated antiviral activity in neurons: The role of cannabinoids. Viral Immunol 2008 06;21(0882-8245; 0882-8245; 2):141-52.
- Reference 1442
-
Blumstein GW, Parsa A, Park AK, McDowell BL, Arroyo-Mendoza M, Girguis M, Adler-Moore JP, Olson J, Buckley NE. Effect of delta-9-tetrahydrocannabinol on mouse resistance to systemic candida albicans infection. PLoS One 2014 Jul 24;9(7):e103288.
- Reference 1443
-
Molina PE, Amedee A, LeCapitaine NJ, Zabaleta J, Mohan M, Winsauer P, Vande Stouwe C. Cannabinoid neuroimmune modulation of SIV disease. J Neuroimmune Pharmacol 2011 Dec;6(4):516-27.
- Reference 1444
-
Molina PE, Winsauer P, Zhang P, Walker E, Birke L, Amedee A, Stouwe CV, Troxclair D, McGoey R, Varner K, et al. Cannabinoid administration attenuates the progression of simian immunodeficiency virus. AIDS Res Hum Retroviruses 2011 Jun;27(6):585-92.
- Reference 1445
-
Chandra LC, Kumar V, Torben W, Vande Stouwe C, Winsauer P, Amedee A, Molina PE, Mohan M. Chronic administration of Delta9-tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute simian immunodeficiency virus infection of rhesus macaques. J Virol 2015 Jan 15;89(2):1168-81.
- Reference 1446
-
Amedee AM, Nichols WA, LeCapitaine NJ, Stouwe CV, Birke LL, Lacour N, Winsauer PJ, Molina PE. Chronic delta(9)-tetrahydrocannabinol administration may not attenuate simian immunodeficiency virus disease progression in female rhesus macaques. AIDS Res Hum Retroviruses 2014 Dec;30(12):1216-25.
- Reference 1447
-
Morahan PS, Klykken PC, Smith SH, Harris LS, Munson AE. Effects of cannabinoids on host resistance to listeria monocytogenes and herpes simplex virus. Infect Immun 1979 Mar;23(3):670-4.
- Reference 1448
-
Shay AH, Choi R, Whittaker K, Salehi K, Kitchen CM, Tashkin DP, Roth MD, Baldwin GC. Impairment of antimicrobial activity and nitric oxide production in alveolar macrophages from smokers of marijuana and cocaine. J Infect Dis 2003 Feb 15;187(4):700-4.
- Reference 1449
-
Specter S, Lancz G. Effects of marijuana on human natural killer cell activity. Adv Exp Med Biol 1991;288:47-56.
- Reference 1450
-
Specter S, Lancz G, Goodfellow D. Suppression of human macrophage function in vitro by delta 9-tetrahydrocannabinol. J Leukoc Biol 1991 Nov;50(5):423-6.
- Reference 1451
-
Specter S, Lancz G, Westrich G, Friedman H. Delta-9-tetrahydrocannabinol augments murine retroviral induced immunosuppression and infection. Int J Immunopharmacol 1991;13(4):411-7.
- Reference 1452
-
Tindall B, Cooper DA, Donovan B, Barnes T, Philpot CR, Gold J, Penny R. The sydney AIDS project: Development of acquired immunodeficiency syndrome in a group of HIV seropositive homosexual men. Aust N Z J Med 1988 Feb;18(1):8-15.
- Reference 1453
-
Molina PE, Amedee AM, Winsauer P, Nelson S, Bagby G, Simon L. Behavioral, metabolic, and immune consequences of chronic alcohol or cannabinoids on HIV/AIDs: Studies in the non-human primate SIV model. J Neuroimmune Pharmacol 2015 Jun;10(2):217-32.
- Reference 1454
-
Bredt BM, Higuera-Alhino D, Shade SB, Hebert SJ, McCune JM, Abrams DI. Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients. J Clin Pharmacol 2002 11;42(0091-2700; 0091-2700; 11):82S-9S.
- Reference 1455
-
Chao C, Jacobson LP, Tashkin D, Martinez-Maza O, Roth MD, Margolick JB, Chmiel JS, Rinaldo C, Zhang ZF, Detels R. Recreational drug use and T lymphocyte subpopulations in HIV-uninfected and HIV-infected men. Drug Alcohol Depend 2008 04/01;94(0376-8716; 0376-8716; 1-3):165-71.
- Reference 1456
-
Di Franco MJ, Sheppard HW, Hunter DJ, Tosteson TD, Ascher MS. The lack of association of marijuana and other recreational drugs with progression to AIDS in the san francisco men's health study. Ann Epidemiol 1996 07;6(1047-2797; 1047-2797; 4):283-9.
- Reference 1457
-
Milloy MJ, Marshall B, Kerr T, Richardson L, Hogg R, Guillemi S, Montaner JS, Wood E. High-intensity cannabis use associated with lower plasma human immunodeficiency virus-1 RNA viral load among recently infected people who use injection drugs. Drug Alcohol Rev 2015 Mar;34(2):135-40.
- Reference 1458
-
Thames AD, Mahmood Z, Burggren AC, Karimian A, Kuhn TP. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care 2016 May;28(5):628-32.
- Reference 1459
-
Ghosn J, Leruez-Ville M, Blanche J, Delobelle A, Beaudoux C, Mascard L, Lecuyer H, Canestri A, Landman R, Zucman D, et al. HIV-1 DNA levels in peripheral blood mononuclear cells and cannabis use are associated with intermittent HIV shedding in semen of men who have sex with men on successful antiretroviral regimens. Clin Infect Dis 2014 Jun;58(12):1763-70.
- Reference 1460
-
Ishida JH, Peters MG, Jin C, Louie K, Tan V, Bacchetti P, Terrault NA. Influence of cannabis use on severity of hepatitis C disease. Clin.Gastroenterol.Hepatol. 2008 01;6(1542-7714; 1542-3565; 1):69-75.
- Reference 1461
-
Bonn-Miller MO, Oser ML, Bucossi MM, Trafton JA. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms (in press). J Behav Med 2012 10/07(1573-3521; 0160-7715).
- Reference 1462
-
Slawson G, Milloy MJ, Balneaves L, Simo A, Guillemi S, Hogg R, Montaner J, Wood E, Kerr T. High-intensity cannabis use and adherence to antiretroviral therapy among people who use illicit drugs in a canadian setting. AIDS Behav 2015 Jan;19(1):120-7.
- Reference 1463
-
Yeshurun M, Shpilberg O, Herscovici C, Shargian L, Dreyer J, Peck A, Israeli M, Levy-Assaraf M, Gruenewald T, Mechoulam R, et al. Cannabidiol for the prevention of graft-versus-host-disease after allogeneic hematopoietic cell transplantation: Results of a phase II study. Biol Blood Marrow Transplant 2015 Oct;21(10):1770-5.
- Reference 1464
-
Brown TT, Dobs AS. Endocrine effects of marijuana. J Clin Pharmacol 2002 11;42(0091-2700; 0091-2700; 11):90S-6S.
- Reference 1465
-
Sadeu JC, Hughes CL, Agarwal S, Foster WG. Alcohol, drugs, caffeine, tobacco, and environmental contaminant exposure: Reproductive health consequences and clinical implications. Crit Rev Toxicol 2010 08;40(1547-6898; 1040-8444; 7):633-52.
- Reference 1466
-
Rossato M, Pagano C, Vettor R. The cannabinoid system and male reproductive functions. J Neuroendocrinol 2008 05;20 Suppl 1(1365-2826; 0953-8194):90-3.
- Reference 1467
-
Sugiura T, Kondo S, Sukagawa A, Tonegawa T, Nakane S, Yamashita A, Waku K. Enzymatic synthesis of anandamide, an endogenous cannabinoid receptor ligand, through N-acylphosphatidylethanolamine pathway in testis: Involvement of ca(2+)-dependent transacylase and phosphodiesterase activities. Biochem Biophys Res Commun 1996 Jan 5;218(1):113-7.
- Reference 1468
-
Gye MC, Kang HH, Kang HJ. Expression of cannabinoid receptor 1 in mouse testes. Arch Androl 2005 May-Jun;51(3):247-55.
- Reference 1469
-
Cobellis G, Cacciola G, Scarpa D, Meccariello R, Chianese R, Franzoni MF, Mackie K, Pierantoni R, Fasano S. Endocannabinoid system in frog and rodent testis: Type-1 cannabinoid receptor and fatty acid amide hydrolase activity in male germ cells. Biol Reprod 2006 Jul;75(1):82-9.
- Reference 1470
-
Cacciola G, Chioccarelli T, Mackie K, Meccariello R, Ledent C, Fasano S, Pierantoni R, Cobellis G. Expression of type-1 cannabinoid receptor during rat postnatal testicular development: Possible involvement in adult leydig cell differentiation. Biol Reprod 2008 Oct;79(4):758-65.
- Reference 1471
-
Amoako AA, Marczylo TH, Marczylo EL, Elson J, Willets JM, Taylor AH, Konje JC. Anandamide modulates human sperm motility: Implications for men with asthenozoospermia and oligoasthenoteratozoospermia. Hum Reprod 2013 Aug;28(8):2058-66.
- Reference 1472
-
Rossato M, Ion Popa F, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocrinol Metab 2005 Feb;90(2):984-91.
- Reference 1473
-
Agirregoitia E, Carracedo A, Subiran N, Valdivia A, Agirregoitia N, Peralta L, Velasco G, Irazusta J. The CB(2) cannabinoid receptor regulates human sperm cell motility. Fertil Steril 2010 Mar 15;93(5):1378-87.
- Reference 1474
-
Aquila S, Guido C, Santoro A, Perrotta I, Laezza C, Bifulco M, Sebastiano A. Human sperm anatomy: Ultrastructural localization of the cannabinoid1 receptor and a potential role of anandamide in sperm survival and acrosome reaction. Anat Rec (Hoboken) 2010 Feb;293(2):298-309.
- Reference 1475
-
Aquila S, Guido C, Santoro A, Gazzerro P, Laezza C, Baffa MF, Ando S, Bifulco M. Rimonabant (SR141716) induces metabolism and acquisition of fertilizing ability in human sperm. Br J Pharmacol 2010 Feb;159(4):831-41.
- Reference 1476
-
Di Giacomo D, De Domenico E, Sette C, Geremia R, Grimaldi P. Type 2 cannabinoid receptor contributes to the physiological regulation of spermatogenesis. FASEB J 2016 Apr;30(4):1453-63.
- Reference 1477
-
Maccarrone M. Endocannabinoids and reproductive biology. Hum Reprod 2009 Jul;24(7):1771.
- Reference 1478
-
Hembree WC, Nahas GG, Zeidenberg P, Huang HFS. Changes in human spermatozoa associated with high-dose marihuana smoking. In: G. G. Nahas, K. M. Sutin, D. J. Harvey, S. Agurell, editors. Marihuana and medicine. Totowa: Humana Press; 1999. ID: 2665; RP: NOT IN FILE.
- Reference 1479
-
Hong CY, Chaput de Saintonge DM, Turner P, Fairbairn JW. Comparison of the inhibitory action of delta-9-tetrahydrocannabinol and petroleum spirit extract of herbal cannabis on human sperm motility. Hum Toxicol 1982 03;1(0144-5952; 0144-5952; 2):151-4.
- Reference 1480
-
Whan LB, West MC, McClure N, Lewis SE. Effects of delta-9-tetrahydrocannabinol, the primary psychoactive cannabinoid in marijuana, on human sperm function in vitro. Fertil Steril 2006 03;85(1556-5653; 3):653-60.
- Reference 1481
-
Zimmerman AM, Bruce WR, Zimmerman S. Effects of cannabinoids on sperm morphology. Pharmacology 1979;18(3):143-8.
- Reference 1482
-
Dalterio SL, deRooij DG. Maternal cannabinoid exposure. effects on spermatogenesis in male offspring. Int J Androl 1986 Aug;9(4):250-8.
- Reference 1483
-
Dalterio S, Steger R, Mayfield D, Bartke A. Early cannabinoid exposure influences neuroendocrine and reproductive functions in male mice: I. prenatal exposure. Pharmacol Biochem Behav 1984 Jan;20(1):107-13.
- Reference 1484
-
Dalterio S, Steger R, Mayfield D, Bartke A. Early cannabinoid exposure influences neuroendocrine and reproductive functions in mice: II. postnatal effects. Pharmacol Biochem Behav 1984 Jan;20(1):115-23.
- Reference 1485
-
Daling JR, Doody DR, Sun X, Trabert BL, Weiss NS, Chen C, Biggs ML, Starr JR, Dey SK, Schwartz SM. Association of marijuana use and the incidence of testicular germ cell tumors. Cancer 2009 Mar 15;115(6):1215-23.
- Reference 1486
-
Trabert B, Sigurdson AJ, Sweeney AM, Strom SS, McGlynn KA. Marijuana use and testicular germ cell tumors. Cancer 2011 Feb 15;117(4):848-53.
- Reference 1487
-
Lacson JC, Carroll JD, Tuazon E, Castelao EJ, Bernstein L, Cortessis VK. Population-based case-control study of recreational drug use and testis cancer risk confirms an association between marijuana use and nonseminoma risk. Cancer 2012 Nov 1;118(21):5374-83.
- Reference 1488
-
Alpar A, Di Marzo V, Harkany T. At the tip of an iceberg: Prenatal marijuana and its possible relation to neuropsychiatric outcome in the offspring. Biol Psychiatry 2016 Apr 1;79(7):e33-45.
- Reference 1489
-
Volkow ND, Compton WM, Wargo EM. The risks of marijuana use during pregnancy. Journal of American Medical Association 2016:E1-2.
- Reference 1490
-
Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a cannabis sativa constituent. Curr Drug Saf 2011 Sep 1;6(4):237-49.
- Reference 1491
-
Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog Biophys Mol Biol 2015 Jul;118(1-2):21-33.
- Reference 1492
-
Szutorisz H, DiNieri JA, Sweet E, Egervari G, Michaelides M, Carter JM, Ren Y, Miller ML, Blitzer RD, Hurd YL. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology 2014 May;39(6):1315-23.
- Reference 1493
-
Yang X, Hegde VL, Rao R, Zhang J, Nagarkatti PS, Nagarkatti M. Histone modifications are associated with Delta9-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. J Biol Chem 2014 Jul 4;289(27):18707-18.
- Reference 1494
-
Zumbrun EE, Sido JM, Nagarkatti PS, Nagarkatti M. Epigenetic regulation of immunological alterations following prenatal exposure to marijuana cannabinoids and its long term consequences in offspring. J Neuroimmune Pharmacol 2015 Jun;10(2):245-54.
- Reference 1495
-
Zuckerman B, Frank DA, Hingson R, Amaro H, Levenson SM, Kayne H, Parker S, Vinci R, Aboagye K, Fried LE, et al. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med 1989 03/23;320(0028-4793; 12):762-8.
- Reference 1496
-
Hurd YL, Wang X, Anderson V, Beck O, Minkoff H, Dow-Edwards D. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol Teratol 2005 03;27(0892-0362; 0892-0362; 2):221-9.
- Reference 1497
-
El-Marroun H, Tiemeier H, Steegers EA, Jaddoe VW, Hofman A, Verhulst FC, van den Brink W, Huizink AC. Intrauterine cannabis exposure affects fetal growth trajectories: The generation R study. J Am Acad Child Adolesc Psychiatry 2009 10/23;48(1527-5418; 0890-8567; 12):1173-81.
- Reference 1498
-
Gray TR, Eiden RD, Leonard KE, Connors GJ, Shisler S, Huestis MA. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clin Chem 2010 09;56(1530-8561; 0009-9147; 9):1442-50.
- Reference 1499
-
Scragg RK, Mitchell EA, Ford RP, Thompson JM, Taylor BJ, Stewart AW. Maternal cannabis use in the sudden death syndrome. Acta Paediatr 2001 01;90(0803-5253; 1):57-60.
- Reference 1500
-
Shiono PH, Klebanoff MA, Nugent RP, Cotch MF, Wilkins DG, Rollins DE, Carey JC, Behrman RE. The impact of cocaine and marijuana use on low birth weight and preterm birth: A multicenter study. Obstet Gynecol 1995 01;172(0002-9378; 1):19-27.
- Reference 1501
-
Fried PA, Watkinson B, Gray R. Growth from birth to early adolescence in offspring prenatally exposed to cigarettes and marijuana. Neurotoxicol Teratol 1999 09;21(0892-0362; 0892-0362; 5):513-25.
- Reference 1502
-
van Gelder MM, Reefhuis J, Caton AR, Werler MM, Druschel CM, Roeleveld N. Characteristics of pregnant illicit drug users and associations between cannabis use and perinatal outcome in a population-based study. Drug Alcohol Depend 2010 06/01;109(1879-0046; 0376-8716; 1-3):243-7.
- Reference 1503
-
Day NL, Richardson GA, Geva D, Robles N. Alcohol, marijuana, and tobacco: Effects of prenatal exposure on offspring growth and morphology at age six. Alcohol Clin Exp Res 1994 Aug;18(4):786-94.
- Reference 1504
-
Day NL, Richardson GA, Goldschmidt L, Robles N, Taylor PM, Stoffer DS, Cornelius MD, Geva D. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol 1994 Mar-Apr;16(2):169-75.
- Reference 1505
-
Fried PA, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Dev Behav Pediatr 1990 Apr;11(2):49-58.
- Reference 1506
-
Fried PA, O'Connell CM, Watkinson B. 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: Cognitive and language assessment. J Dev Behav Pediatr 1992 Dec;13(6):383-91.
- Reference 1507
-
Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol Teratol 1992 Sep-Oct;14(5):299-311.
- Reference 1508
-
Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: Effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol 1999 Mar-Apr;21(2):109-18.
- Reference 1509
-
Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 1998 May-Jun;20(3):293-306.
- Reference 1510
-
Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol 2002 05;24(0892-0362; 3):309-20.
- Reference 1511
-
Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 2003 Jul-Aug;25(4):427-36.
- Reference 1512
-
Smith AM, Fried PA, Hogan MJ, Cameron I. Effects of prenatal marijuana on visuospatial working memory: An fMRI study in young adults. Neurotoxicol Teratol 2006 Mar-Apr;28(2):286-95.
- Reference 1513
-
El Marroun H, Tiemeier H, Franken IH, Jaddoe VW, van der Lugt A, Verhulst FC, Lahey BB, White T. Prenatal cannabis and tobacco exposure in relation to brain morphology: A prospective neuroimaging study in young children. Biol Psychiatry 2015 Sep 1.
- Reference 1514
-
Kosty DB, Farmer RF, Seeley JR, Gau JM, Duncan SC, Lewinsohn PM. Parental transmission of risk for cannabis use disorders to offspring. Addiction 2015 Jul;110(7):1110-7.
- Reference 1515
-
Ahmad GR, Ahmad N. Passive consumption of marijuana through milk: A low level chronic exposure to delta-9-tetrahydrocannabinol(THC). J Toxicol Clin Toxicol 1990;28(0731-3810; 0731-3810; 2):255-60.
- Reference 1516
-
Astley SJ, Little RE. Maternal marijuana use during lactation and infant development at one year. Neurotoxicol Teratol 1990 03;12(0892-0362; 2):161-8.
- Reference 1517
-
Volkow ND, Compton WM, Wargo EM. The risks of marijuana use during pregnancy. JAMA 2017;317(2):129-30.
- Reference 1518
-
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 2005 Jun;62(6):593-602.
- Reference 1519
-
Veen ND, Selten JP, van der Tweel I, Feller WG, Hoek HW, Kahn RS. Cannabis use and age at onset of schizophrenia. Am J Psychiatry 2004 Mar;161(3):501-6.
- Reference 1520
-
Merritt JC, Cook CE, Davis KH. Orthostatic hypotension after delta 9-tetrahydrocannabinol marihuana inhalation. Ophthalmic Res 1982;14(0030-3747; 2):124-8.
- Reference 1521
-
Chesher G, Hall WD. Effects of cannabis on the cardiovascular and gastrointestinal systems. In: H. Kalant, W. Corrigal, W. D. Hall, R. Smart, editors. The health effects of cannabis. Toronto, Canada: Centre for Addiction and Mental Health; 1999..
- Reference 1522
-
Fisher BA, Ghuran A, Vadamalai V, Antonios TF. Cardiovascular complications induced by cannabis smoking: A case report and review of the literature. Emerg Med J 2005 Sep;22(9):679-80.
- Reference 1523
-
Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: Preventive strategies. Circulation 2011 07/19;124(1524-4539; 0009-7322; 3):346-54.
- Reference 1524
-
Aronow WS, Cassidy J. Effect of marihuana and placebo-marihuana smoking on angina pectoris. N Engl J Med 1974 06/11;291(0028-4793; 2):65-7.
- Reference 1525
-
Sidney S. Cardiovascular consequences of marijuana use. J Clin Pharmacol 2002 11;42(0091-2700; 11):64S-70S.
- Reference 1526
-
Cottencin O, Karila L, Lambert M, Arveiller C, Benyamina A, Boissonas A, Goudemand M, Reynaud M. Cannabis arteritis: Review of the literature. J.Addict.Med. 2010 12;4(1932-0620; 1932-0620; 4):191-6.
- Reference 1527
-
Noel B, Ruf I, Panizzon RG. Cannabis arteritis. J Am Acad Dermatol 2008 05;58(1097-6787; 0190-9622; 5):S65-7.
- Reference 1528
-
Combemale P, Consort T, Denis-Thelis L, Estival JL, Dupin M, Kanitakis J. Cannabis arteritis. Br J Dermatol 2005 01;152(0007-0963; 0007-0963; 1):166-9.
- Reference 1529
-
Disdier P, Granel B, Serratrice J, Constans J, Michon-Pasturel U, Hachulla E, Conri C, Devulder B, Swiader L, Piquet P, et al. Cannabis arteritis revisited--ten new case reports. Angiology 2001 01;52(0003-3197; 0003-3197; 1):1-5.
- Reference 1530
-
Wolff V, Lauer V, Rouyer O, Sellal F, Meyer N, Raul JS, Sabourdy C, Boujan F, Jahn C, Beaujeux R, et al. Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: A prospective study in 48 consecutive young patients. Stroke 2011 06;42(1524-4628; 0039-2499; 6):1778-80.
- Reference 1531
-
Ince B, Benbir G, Yuksel O, Koseoglu L, Uluduz D. Both hemorrhagic and ischemic stroke following high doses of cannabis consumption. Presse Med 2015 Jan;44(1):106-7.
- Reference 1532
-
Waldman M, Hochhauser E, Fishbein M, Aravot D, Shainberg A, Sarne Y. An ultra-low dose of tetrahydrocannabinol provides cardioprotection. Biochem Pharmacol 2013 Jun 1;85(11):1626-33.
- Reference 1533
-
Ravi D, Ghasemiesfe M, Korenstein D, Cascino T, Keyhani S. Associations between marijuana use and cardiovascular risk factors and outcomes: A systematic review. Ann Intern Med 2018.
- Reference 1534
-
Dezieck L, Hafez Z, Conicella A, Blohm E, O'Connor MJ, Schwarz ES, Mullins ME. Resolution of cannabis hyperemesis syndrome with topical capsaicin in the emergency department: A case series. Clin Toxicol (Phila) 2017 Sep;55(8):908-13.
- Reference 1535
-
Cox B, Chhabra A, Adler M, Simmons J, Randlett D. Cannabinoid hyperemesis syndrome: Case report of a paradoxical reaction with heavy marijuana use. Case.Report.Med. 2012;2012(1687-9635):757696.
- Reference 1536
-
Burillo-Putze G, Llorens P, Roman F. Use of capsaicin cream in cannabis hyperemesis syndrome. The Journal of Emergency Medicine 2017.
- Reference 1537
-
Pelissier F, Claudet I, Gandia-Mailly P, Benyamina A, Franchitto N. Use of capsaicin cream in cannabis hyperemesis syndrome. J Emerg Med 2017 Mar 9.
- Reference 1538
-
Batkai S, Jarai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med 2001 07;7(1078-8956; 1078-8956; 7):827-32.
- Reference 1539
-
Fernandez-Rodriguez CM, Romero J, Petros TJ, Bradshaw H, Gasalla JM, Gutierrez ML, Lledo JL, Santander C, Fernandez TP, Tomas E, et al. Circulating endogenous cannabinoid anandamide and portal, systemic and renal hemodynamics in cirrhosis. Liver Int. 2004 10;24(1478-3223; 1478-3223; 5):477-83.
- Reference 1540
-
Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A, Lotersztajn S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005 03;128(0016-5085; 0016-5085; 3):742-55.
- Reference 1541
-
Teixeira-Clerc F, Julien B, Grenard P, Tran Van NJ, Deveaux V, Li L, Serriere-Lanneau V, Ledent C, Mallat A, Lotersztajn S. CB1 cannabinoid receptor antagonism: A new strategy for the treatment of liver fibrosis. Nat Med 2006 06;12(1078-8956; 1078-8956; 6):671-6.
- Reference 1542
-
Siegmund SV, Schwabe RF. Endocannabinoids and liver disease. II. endocannabinoids in the pathogenesis and treatment of liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2008 02;294(0193-1857; 0193-1857; 2):G357-62.
- Reference 1543
-
Mallat A, Hezode C, Lotersztajn S. Environmental factors as disease accelerators during chronic hepatitis C. J Hepatol 2008 04;48(0168-8278; 0168-8278; 4):657-65.
- Reference 1544
-
Brunet L, Moodie EE, Rollet K, Cooper C, Walmsley S, Potter M, Klein MB, Canadian Co-infection Cohort Investigators. Marijuana smoking does not accelerate progression of liver disease in HIV-hepatitis C coinfection: A longitudinal cohort analysis. Clin Infect Dis 2013 Sep;57(5):663-70.
- Reference 1545
-
Guy GW, Stott CG. The development of sativex⌐- a natural cannabis-based medicine. In: R. Mechoulam, editor. Cannabinoids as therapeutics. Basel: Birkhäuser Verlag; 2005. ID: 2335; RP: NOT IN FILE.
- Reference 1546
-
Solowij N, Stephens R, Roffman RA, Babor T. Does marijuana use cause long-term cognitive deficits? JAMA 2002 05/22;287(0098-7484; 20):2653-4.
- Reference 1547
-
Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology 2002 11/12;59(0028-3878; 9):1337-43.
- Reference 1548
-
Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol 2008 04/16;286(0303-7207; 0303-7207; 1-2):S108-13.
- Reference 1549
-
Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM, Morris R, Krauskopf D, Satz P. Cognitive correlates of long-term cannabis use in costa rican men. Arch Gen Psychiatry 1996 11;53(0003-990; 11):1051-7.
- Reference 1550
-
Pope HG,Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug Alcohol Depend 2003 04/01;69(0376-8716; 3):303-10.
- Reference 1551
-
Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P. Neuropsychological deficits in long-term frequent cannabis users. Neurology 2006 03/14;66(1526-632; 5):737-9.
- Reference 1552
-
Lyketsos CG, Garrett E, Liang KY, Anthony JC. Cannabis use and cognitive decline in persons under 65 years of age. Am J Epidemiol 1999 05/01;149(0002-9262; 9):794-800.
- Reference 1553
-
Mokrysz C, Landy R, Gage SH, Munafo MR, Roiser JP, Curran HV. Are IQ and educational outcomes in teenagers related to their cannabis use? A prospective cohort study. J Psychopharmacol 2016 Feb;30(2):159-68.
- Reference 1554
-
Jackson NJ, Isen JD, Khoddam R, Irons D, Tuvblad C, Iacono WG, McGue M, Raine A, Baker LA. Impact of adolescent marijuana use on intelligence: Results from two longitudinal twin studies. Proc Natl Acad Sci U S A 2016 Feb 2;113(5):E500-8.
- Reference 1555
-
Camchong J, Lim KO, Kumra S. Adverse effects of cannabis on adolescent brain development: A longitudinal study. Cereb Cortex 2016 Feb 23.
- Reference 1556
-
Batalla A, Crippa JA, Busatto GF, Guimaraes FS, Zuardi AW, Valverde O, Atakan Z, McGuire PK, Bhattacharyya S, Martin-Santos R. Neuroimaging studies of acute effects of THC and CBD in humans and animals: A systematic review. Curr Pharm Des 2014;20(13):2168-85.
- Reference 1557
-
Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S, Torrens M, Pujol J, Farre M, Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: A systematic review of adolescent and adult findings. PLoS One 2013;8(2):e55821.
- Reference 1558
-
Lorenzetti V, Alonso-Lana S, Youssef GJ, Verdejo-Garcia A, Suo C, Cousijn J, Takagi M, Yucel M, Solowij N. Adolescent cannabis use: What is the evidence for functional brain alteration? Curr Pharm Des 2016 Aug 5.
- Reference 1559
-
Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J Neurosci 2015 Jan 28;35(4):1505-12.
- Reference 1560
-
Rigucci S, Marques TR, Di Forti M, Taylor H, Dell'Acqua F, Mondelli V, Bonaccorso S, Simmons A, David AS, Girardi P, et al. Effect of high-potency cannabis on corpus callosum microstructure. Psychol Med 2016 Mar;46(4):841-54.
- Reference 1561
-
Becker MP, Collins PF, Lim KO, Muetzel RL, Luciana M. Longitudinal changes in white matter microstructure after heavy cannabis use. Dev Cogn Neurosci 2015 Dec;16:23-35.
- Reference 1562
-
Jakabek D, Yucel M, Lorenzetti V, Solowij N. An MRI study of white matter tract integrity in regular cannabis users: Effects of cannabis use and age. Psychopharmacology (Berl) 2016 Aug 8.
- Reference 1563
-
Lorenzetti V, Solowij N, Yucel M. The role of cannabinoids in neuroanatomic alterations in cannabis users. Biol Psychiatry 2016 Apr 1;79(7):e17-31.
- Reference 1564
-
Brumback T, Castro N, Jacobus J, Tapert S. Effects of marijuana use on brain structure and function: Neuroimaging findings from a neurodevelopmental perspective. Int Rev Neurobiol 2016;129:33-65.
- Reference 1565
-
Scott JC, Slomiak ST, Jones JD, Rosen AF, Moore TM, Gur RC. Association of cannabis with cognitive functioning in adolescents and young adults: A systematic review and meta-analysis. JAMA Psychiatry 2018;75(6):585-95.
- Reference 1566
-
Pierre JM. Risks of increasingly potent cannabis: The joint effects of potency and frequency: As THC levels rise, the risk of psychosis, cognitive deficits, and structural brain changes increases. Current Psychiatry 2017;16(2):14-21.
- Reference 1567
-
Bosker WM, Karschner EL, Lee D, Goodwin RS, Hirvonen J, Innis RB, Theunissen EL, Kuypers KP, Huestis MA, Ramaekers JG. Psychomotor function in chronic daily cannabis smokers during sustained abstinence. PLoS One 2013;8(1):e53127.
- Reference 1568
-
Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem 2013 Mar;59(3):478-92.
- Reference 1569
-
Asbridge M, Mann R, Cusimano MD, Trayling C, Roerecke M, Tallon JM, Whipp A, Rehm J. Cannabis and traffic collision risk: Findings from a case-crossover study of injured drivers presenting to emergency departments. Int J Public Health 2014 Apr;59(2):395-404.
- Reference 1570
-
Menetrey A, Augsburger M, Favrat B, Pin MA, Rothuizen LE, Appenzeller M, Buclin T, Mangin P, Giroud C. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoids levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Delta9-THC. J Anal Toxicol 2005 07;29(0146-4760; 5):327-38.
- Reference 1571
-
Asbridge M, Poulin C, Donato A. Motor vehicle collision risk and driving under the influence of cannabis: Evidence from adolescents in atlantic canada. Accid Anal Prev 2005 11;37(0001-4575; 0001-4575; 6):1025-34.
- Reference 1572
-
Gieringer DH. Marijuana, driving, and accident safety. J Psychoactive Drugs 1988 01;20(0279-1072; 1):93-101.
- Reference 1573
-
Sexton BF, Tunbridge RJ, Brook-Carter N, Jackson PG, Wright K, Stark MM, Englehart K. The influence of cannabis on driving. Berkshire, UK: TRL Limited; 2007 2007/08. Report nr 477:ID: 2343; RP: NOT IN FILE; 23.
- Reference 1574
-
Moskowitz H. Marihuana and driving. Accid Anal Prev 1985 08;17(0001-4575; 4):323-45.
- Reference 1575
-
Bosker WM, Kuypers KP, Theunissen EL, Surinx A, Blankespoor RJ, Skopp G, Jeffery WK, Walls HC, van Leeuwen CJ, Ramaekers JG. Medicinal delta(9) -tetrahydrocannabinol (dronabinol) impairs on-the-road driving performance of occasional and heavy cannabis users but is not detected in standard field sobriety tests. Addiction 2012 10;107(1360-0443; 0965-2140; 10):1837-44.
- Reference 1576
-
Kuypers KP, Legrand SA, Ramaekers JG, Verstraete AG. A case-control study estimating accident risk for alcohol, medicines and illegal drugs. PLoS.One. 2012;7(1932-6203; 1932-6203; 8):e43496.
- Reference 1577
-
Laumon B, Gadegbeku B, Martin JL, Biecheler MB. Cannabis intoxication and fatal road crashes in france: Population based case-control study. BMJ 2005 12/10;331(1756-1833; 0959-535; 7529):1371.
- Reference 1578
-
Khiabani HZ, Bramness JG, Bjorneboe A, Morland J. Relationship between THC concentration in blood and impairment in apprehended drivers. Traffic.Inj.Prev. 2006 06;7(1538-9588; 1538-9588; 2):111-6.
- Reference 1579
-
Ronen A, Gershon P, Drobiner H, Rabinovich A, Bar-Hamburger R, Mechoulam R, Cassuto Y, Shinar D. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev 2008 May;40(3):926-34.
- Reference 1580
-
Sayer G, Ialomiteanu A, Stoduto G, Wickens CM, Mann RE, Le Foll B, Brands B. Increased collision risk among drivers who report driving after using alcohol and after using cannabis. Can J Public Health 2014 Feb 4;105(1):e92-3.
- Reference 1581
-
Volkow ND, Hampson AJ, Baler R. Don't worry, be happy: Endocannabinoids and cannabis at the intersection of stress and reward. Annu Rev Pharmacol Toxicol 2016 Sep 2.
- Reference 1582
-
Patel S, Hill MN, Hillard CJ. Effects of phytocannabinoids on anxiety, mood, and the endocrine system. In: R. G. Pertwee, editor. Handbook of cannabis. Oxford: Oxford University Press; 2014..
- Reference 1583
-
Chan GCK, Hall W, Freeman TP, Ferris J, Kelly AB, Winstock A. User characteristics and effect profile of butane hash oil: An extremely high-potency cannabis concentrate. Drug Alcohol Depend 2017 Sep 1;178:32-8.
- Reference 1584
-
Temple EC, Driver M, Brown RF. Cannabis use and anxiety: Is stress the missing piece of the puzzle? Front Psychiatry 2014 Nov 24;5:168.
- Reference 1585
-
Kotin J, Post RM, Goodwin FK. 9 -tetrahydrocannabinol in depressed patients. Arch Gen Psychiatry 1973 03;28(0003-990; 0003-990; 3):345-8.
- Reference 1586
-
Ablon SL, Goodwin FK. High frequency of dysphoric reactions to tetrahydrocannabinol among depressed patients. Am J Psychiatry 1974 04;131(0002-953; 0002-953; 4):448-53.
- Reference 1587
-
Glass RM, Uhlenhuth EH, Hartel FW, Schuster CR, Fischman MW. A single dose study of nabilone, a synthetic cannabinoid. Psychopharmacology (Berl) 1980;71(0033-3158; 0033-3158; 2):137-42.
- Reference 1588
-
Glass RM, Uhlenhuth EH, Hartel FW, Schuster CR, Fischman MW. Single-dose study of nabilone in anxious volunteers. J Clin Pharmacol 1981 08;21(0091-2700; 0091-2700; 8-9):383S-96S.
- Reference 1589
-
Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, Quevedo J, Roesler R, Schroder N, Nardi AE, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 2011 05;36(1740-634; 0006-3223; 6):1219-26.
- Reference 1590
-
Lev-Ran S, Le Foll B, McKenzie K, Rehm J. Cannabis use and mental health-related quality of life among individuals with anxiety disorders. J Anxiety Disord 2012 Dec;26(8):799-810.
- Reference 1591
-
Degenhardt L, Coffey C, Romaniuk H, Swift W, Carlin JB, Hall WD, Patton GC. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction 2013 Jan;108(1):124-33.
- Reference 1592
-
Kedzior KK, Laeber LT. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population--a meta-analysis of 31 studies. BMC Psychiatry 2014 May 10;14:136,244X-14-136.
- Reference 1593
-
Gage SH, Hickman M, Heron J, Munafo MR, Lewis G, Macleod J, Zammit S. Associations of cannabis and cigarette use with depression and anxiety at age 18: Findings from the avon longitudinal study of parents and children. PLoS One 2015 Apr 13;10(4):e0122896.
- Reference 1594
-
Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction 2003 11;98(0965-2140; 0965-2140; 11):1493-504.
- Reference 1595
-
Harder VS, Morral AR, Arkes J. Marijuana use and depression among adults: Testing for causal associations. Addiction 2006 10;101(0965-2140; 0965-2140; 10):1463-72.
- Reference 1596
-
Harder VS, Stuart EA, Anthony JC. Adolescent cannabis problems and young adult depression: Male-female stratified propensity score analyses. Am J Epidemiol 2008 09/15;168(1476-6256; 0002-9262; 6):592-601.
- Reference 1597
-
Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: Prevalence, correlates and co-morbidity. Psychol Med 2006 10;36(0033-2917; 0033-2917; 10):1447-60.
- Reference 1598
-
van Laar M, van Dorsselaer S, Monshouwer K, de Graaf R. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction 2007 08;102(0965-2140; 0965-2140; 8):1251-60.
- Reference 1599
-
Horwood LJ, Fergusson DM, Coffey C, Patton GC, Tait R, Smart D, Letcher P, Silins E, Hutchinson DM. Cannabis and depression: An integrative data analysis of four australasian cohorts. Drug Alcohol Depend 2012 Dec 1;126(3):369-78.
- Reference 1600
-
Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Allebeck P. Cannabis use and depression: A longitudinal study of a national cohort of swedish conscripts. BMC Psychiatry 2012 Aug 16;12:112,244X-12-112.
- Reference 1601
-
Gonzalez-Ortega I, Alberich S, Echeburua E, Aizpuru F, Millan E, Vieta E, Matute C, Gonzalez-Pinto A. Subclinical depressive symptoms and continued cannabis use: Predictors of negative outcomes in first episode psychosis. PLoS One 2015 Apr 15;10(4):e0123707.
- Reference 1602
-
Lev-Ran S, Le Foll B, McKenzie K, George TP, Rehm J. Bipolar disorder and co-occurring cannabis use disorders: Characteristics, co-morbidities and clinical correlates. Psychiatry Res 2013 Oct 30;209(3):459-65.
- Reference 1603
-
Henquet C, Krabbendam L, de GR, ten HM, van OJ. Cannabis use and expression of mania in the general population. J Affect Disord 2006 10;95(0165-0327; 0165-0327; 1-3):103-10.
- Reference 1604
-
Strakowski SM, DelBello MP, Fleck DE, Adler CM, Anthenelli RM, Keck PE,Jr., Arnold LM, Amicone J. Effects of co-occurring cannabis use disorders on the course of bipolar disorder after a first hospitalization for mania. Arch Gen Psychiatry 2007 01;64(0003-990; 0003-990; 1):57-64.
- Reference 1605
-
Agrawal A, Nurnberger JI,Jr., Lynskey MT. Cannabis involvement in individuals with bipolar disorder. Psychiatry Res 2011 02/28;185(0165-1781; 0165-1781; 3):459-61.
- Reference 1606
-
De Pradier M, Gorwood P, Beaufils B, Ades J, Dubertret C. Influence of the serotonin transporter gene polymorphism, cannabis and childhood sexual abuse on phenotype of bipolar disorder: A preliminary study. Eur Psychiatry 2010 10;25(1778-3585; 0924-9338; 6):323-7.
- Reference 1607
-
Bally N, Zullino D, Aubry JM. Cannabis use and first manic episode. J Affect Disord 2014 Aug;165:103-8.
- Reference 1608
-
Tyler E, Jones S, Black N, Carter LA, Barrowclough C. The relationship between bipolar disorder and cannabis use in daily life: An experience sampling study. PLoS One 2015 Mar 4;10(3):e0118916.
- Reference 1609
-
Gibbs M, Winsper C, Marwaha S, Gilbert E, Broome M, Singh SP. Cannabis use and mania symptoms: A systematic review and meta-analysis. J Affect Disord 2015 Jan 15;171:39-47.
- Reference 1610
-
Baethge C, Hennen J, Khalsa HM, Salvatore P, Tohen M, Baldessarini RJ. Sequencing of substance use and affective morbidity in 166 first-episode bipolar I disorder patients. Bipolar Disord 2008 09;10(1399-5618; 1398-5647; 6):738-41.
- Reference 1611
-
Martinez-Gras I, Hoenicka J, Ponce G, Rodriguez-Jimenez R, Jimenez-Arriero MA, Perez-Hernandez E, Ampuero I, Ramos-Atance JA, Palomo T, Rubio G. (AAT)n repeat in the cannabinoid receptor gene, CNR1: Association with schizophrenia in a spanish population. Eur Arch Psychiatry Clin Neurosci 2006 10;256(0940-1334; 0940-1334; 7):437-41.
- Reference 1612
-
Monteleone P, Bifulco M, Maina G, Tortorella A, Gazzerro P, Proto MC, Di FC, Monteleone F, Canestrelli B, Buonerba G, et al. Investigation of CNR1 and FAAH endocannabinoid gene polymorphisms in bipolar disorder and major depression. Pharmacol Res 2010 05;61(1096-1186; 1043-6618; 5):400-4.
- Reference 1613
-
Lagerberg TV, Sundet K, Aminoff SR, Berg AO, Ringen PA, Andreassen OA, Melle I. Excessive cannabis use is associated with earlier age at onset in bipolar disorder. Eur Arch Psychiatry Clin Neurosci 2011 09;261(1433-8491; 0940-1334; 6):397-405.
- Reference 1614
-
Braga RJ, Burdick KE, Derosse P, Malhotra AK. Cognitive and clinical outcomes associated with cannabis use in patients with bipolar I disorder. Psychiatry Res 2012 07/17;200(0165-1781; 0165-1781; 2-3):242-5.
- Reference 1615
-
Lagerberg TV, Kvitland LR, Aminoff SR, Aas M, Ringen PA, Andreassen OA, Melle I. Indications of a dose-response relationship between cannabis use and age at onset in bipolar disorder. Psychiatry Res 2014 Jan 30;215(1):101-4.
- Reference 1616
-
Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and mood disorders: A longitudinal study. J Affect Disord 2014 Oct 13;172C:211-8.
- Reference 1617
-
Zorrilla I, Aguado J, Haro JM, Barbeito S, Lopez Zurbano S, Ortiz A, Lopez P, Gonzalez-Pinto A. Cannabis and bipolar disorder: Does quitting cannabis use during manic/mixed episode improve clinical/functional outcomes? Acta Psychiatr Scand 2015 Feb;131(2):100-10.
- Reference 1618
-
Kim SW, Dodd S, Berk L, Kulkarni J, de Castella A, Fitzgerald PB, Kim JM, Yoon JS, Berk M. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig 2015 Jul;12(3):349-55.
- Reference 1619
-
Leite RT, Nogueira Sde O, do Nascimento JP, de Lima LS, da Nobrega TB, Virginio Mda S, Moreno LM, Sampaio BH, Souza FG. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plast 2015;2015:434127.
- Reference 1620
-
Mammen G, Rueda S, Roerecke M, Bonato S, Lev-Ran S, Rehm J. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: A systematic review of prospective studies. J Clin Psychiatry 2018 Jun 5;79(4):10.4088/JCP.17r11839.
- Reference 1621
-
Thornicroft G. Cannabis and psychosis. is there epidemiological evidence for an association? Br J Psychiatry 1990 07;157(0007-1250; 0007-1250):25-33.
- Reference 1622
-
Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: Examination of the evidence. Br J Psychiatry 2004 02;184(0007-1250):110-7.
- Reference 1623
-
Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in swedish conscripts of 1969: Historical cohort study. BMJ 2002 11/23;325(1468-5833; 7374):1199-203.
- Reference 1624
-
Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: Longitudinal prospective study. BMJ 2002 11/23;325(1468-5833; 7374):1212-3.
- Reference 1625
-
Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med 2003 Jan;33(1):15-21.
- Reference 1626
-
Fergusson DM, Horwood LJ, Ridder EM. Tests of causal linkages between cannabis use and psychotic symptoms. Addiction 2005 Mar;100(3):354-66.
- Reference 1627
-
Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol Med 2012 Jun;42(6):1321-8.
- Reference 1628
-
Lev-Ran S, Segev A, Braw Y, Levkovitz Y. Neurocognitive functions of heavy cannabis using schizophrenia patients. Eur Psychiatry 2012 Jul;27(5):365-8.
- Reference 1629
-
Gage SH, Hickman M, Heron J, Munafo MR, Lewis G, Macleod J, Zammit S. Associations of cannabis and cigarette use with psychotic experiences at age 18: Findings from the avon longitudinal study of parents and children. Psychol Med 2014 Dec;44(16):3435-44.
- Reference 1630
-
Giordano GN, Ohlsson H, Sundquist K, Sundquist J, Kendler KS. The association between cannabis abuse and subsequent schizophrenia: A swedish national co-relative control study. Psychol Med 2015 Jan;45(2):407-14.
- Reference 1631
-
van Gastel WA, Vreeker A, Schubart CD, MacCabe JH, Kahn RS, Boks MP. Change in cannabis use in the general population: A longitudinal study on the impact on psychotic experiences. Schizophr Res 2014 Aug;157(1-3):266-70.
- Reference 1632
-
Decoster J, van OJ, Kenis G, Henquet C, Peuskens J, De HM, van WR. Age at onset of psychotic disorder: Cannabis, BDNF Val66Met, and sex-specific models of gene-environment interaction. Am.J.Med.Genet.B Neuropsychiatr.Genet. 2011 04;156B(1552-485; 1552-4841; 3):363-9.
- Reference 1633
-
Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, Kambeitz J, Prata D, Williams S, Brammer M, Collier DA, McGuire PK. Preliminary report of biological basis of sensitivity to the effects of cannabis on psychosis: AKT1 and DAT1 genotype modulates the effects of delta-9-tetrahydrocannabinol on midbrain and striatal function. Mol Psychiatry 2012 Dec;17(12):1152-5.
- Reference 1634
-
Sherif M, Radhakrishnan R, D'Souza DC, Ranganathan M. Human laboratory studies on cannabinoids and psychosis. Biol Psychiatry 2016 Apr 1;79(7):526-38.
- Reference 1635
-
Krebs MO, Morvan Y, Jay T, Gaillard R, Kebir O. Psychotomimetic effects at initiation of cannabis use are associated with cannabinoid receptor 1 (CNR1) variants in healthy students. Mol Psychiatry 2014 Apr;19(4):402-3.
- Reference 1636
-
Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry 2006 07/15;60(0006-3223; 0006-3223; 2):141-51.
- Reference 1637
-
McIntosh AM, Baig BJ, Hall J, Job D, Whalley HC, Lymer GK, Moorhead TW, Owens DG, Miller P, Porteous D, et al. Relationship of catechol-O-methyltransferase variants to brain structure and function in a population at high risk of psychosis. Biol Psychiatry 2007 05/15;61(0006-3223; 0006-3223; 10):1127-34.
- Reference 1638
-
Miyake N, Thompson J, Skinbjerg M, Abi-Dargham A. Presynaptic dopamine in schizophrenia. CNS.Neurosci.Ther. 2011 04;17(1755-5949; 1755-5930; 2):104-9.
- Reference 1639
-
Fan JB, Zhang CS, Gu NF, Li XW, Sun WW, Wang HY, Feng GY, St CD, He L. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: A large-scale association study plus meta-analysis. Biol Psychiatry 2005 01/15;57(0006-3223; 0006-3223; 2):139-44.
- Reference 1640
-
Ayalew M, Le-Niculescu H, Levey DF, Jain N, Changala B, Patel SD, Winiger E, Breier A, Shekhar A, Amdur R, et al. Convergent functional genomics of schizophrenia: From comprehensive understanding to genetic risk prediction. Mol Psychiatry 2012 09;17(1476-5578; 1359-4184; 9):887-905.
- Reference 1641
-
Mustonen A, Niemelä S, Nordström T, Murray GK, Mäki P, Jääskeläinen E, Miettunen J. Adolescent cannabis use, baseline prodromal symptoms and the risk of psychosis. The British Journal of Psychiatry 2018;212(4):227-33.
- Reference 1642
-
Bourque J, Afzali MH, Conrod PJ. Association of cannabis use with adolescent psychotic symptoms. JAMA Psychiatry 2018.
- Reference 1643
-
Arendt M, Mortensen PB, Rosenberg R, Pedersen CB, Waltoft BL. Familial predisposition for psychiatric disorder: Comparison of subjects treated for cannabis-induced psychosis and schizophrenia. Arch Gen Psychiatry 2008 Nov;65(11):1269-74.
- Reference 1644
-
van Ours JC, Williams J, Fergusson D, Horwood LJ. Cannabis use and suicidal ideation. J Health Econ 2013 May;32(3):524-37.
- Reference 1645
-
Topol EJ, Bousser MG, Fox KA, Creager MA, Despres JP, Easton JD, Hamm CW, Montalescot G, Steg PG, Pearson TA, et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): A randomised, multicentre, placebo-controlled trial. Lancet 2010 Aug 14;376(9740):517-23.
- Reference 1646
-
Rimonabant Briefing Document: Endocrine and Metabolic Drugs Advisory Committee Meeting NDA 21-888 [Internet]; c2007 [cited 2016. Available from: http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4306b1-fda-backgrounder.pdf.
- Reference 1647
-
D'Souza DC, Sewell RA, Ranganathan M. Cannabis and psychosis/schizophrenia: Human studies. Eur Arch Psychiatry Clin Neurosci 2009 10;259(1433-8491; 0940-1334; 7):413-31.
- Reference 1648
-
Castle DJ, Solowij N. Acute and subacute psychomimetic effects of cannabis in humans. In: D. Castle, R. Murray, editors. Marijuana and madness. Cambridge: Cambridge University Press; 2004. ID: 2673; RP: NOT IN FILE.
- Reference 1649
-
Fridberg DJ, Vollmer JM, O'Donnell BF, Skosnik PD. Cannabis users differ from non-users on measures of personality and schizotypy. Psychiatry Res 2011 03/30;186(0165-1781; 0165-1781; 1):46-52.
- Reference 1650
-
Calabria B, Degenhardt L, Hall W, Lynskey M. Does cannabis use increase the risk of death? systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev 2010 May;29(3):318-30.
- Reference 1651
-
Gable RS. Comparison of acute lethal toxicity of commonly abused psychoactive substances. Addiction 2004 Jun;99(6):686-96.
- Reference 1652
-
World Health Organization (WHO). The health and social effects of nonmedical cannabis use..
- Reference 1653
-
van Laar M, Frijns T, Trautmann F, Lombi L. Part 1: Report I cannabis market: User types, availability and consumption estimates. In: F. Trautmann, B. Kilmer, P. Turnbull, editors. Further insights into aspects of the EU illicit drug markets. Publications Office of European Union; 2013..
- Reference 1654
-
Lachenmeier DW, Rehm J. Comparative risk assessment of alcohol, tobacco, cannabis and other illicit drugs using the margin of exposure approach. Sci Rep 2015 Jan 30;5:8126.
- Reference 1655
-
Robson P. Therapeutic aspects of cannabis and cannabinoids. Br J Psychiatry 2001 02;178(0007-1250):107-15.
- Reference 1656
-
Weinstein AM, Gorelick DA. Pharmacological treatment of cannabis dependence. Curr Pharm Des 2011;17(1873-4286; 1381-6128; 14):1351-8.
- Reference 1657
-
Thompson GR, Rosenkrantz H, Schaeppi UH, Braude MC. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicol Appl Pharmacol 1973 07;25(0041-008; 0041-008; 3):363-72.
- Reference 1658
-
Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers [Internet]Rockville, Maryland: U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research; c2005 [cited 2016 12/23]. Available from: http://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf.
- Reference 1659
-
Uliss DB, Dalzell HC, Handrick GR, Howes JF, Razdan RK. Hashish. importance of the phenolic hydroxyl group in tetrahydrocannabinols. J Med Chem 1975 Feb;18(2):213-5.
- Reference 1660
-
Rosenkrantz H, Fleischman RW, Grant RJ. Toxicity of short-term administration of cannabinoids to rhesus monkeys. Toxicol Appl Pharmacol 1981 Mar 30;58(1):118-31.
- Reference 1661
-
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016 Mar;7(2):27-31.
Footnotes
- Footnote 1
-
Human equivalent doses were calculated based on body surface area: animal doses in mg/kg were divided by 12.3 for mice1661
- Footnote 2
-
Human equivalent doses were calculated based on body surface area: animal doses in mg/kg were divided by 12.3 for mice, 6.2 for rats, and 3.1 for monkeys1661