Hazardous substance assessment - Acetic acid
Important note: Hazardous substance assessments are technical documents produced by Health Canada as educational and information resources for suppliers of hazardous products under the Hazardous Products Act (HPA) and its regulations. For more information on supplier roles and responsibilities, visit supplier responsibilities.
This hazardous substance assessment was conducted according to the Hazardous Products Regulations (HPR).
Identification
Chemical name:
Acetic acid
CAS #:
64-19-7
Chemical composition:
CH3COOH
Synonyms:
Methanecarboxylic acid; vinegar acid; Ethanoic acid monomer.
UN #:
2789 for glacial or over 80% acid by weightFootnote 1; 2790 for solution over 10% but not over 80% by weightFootnote 1.
Pictogram(s):
Figure 1.
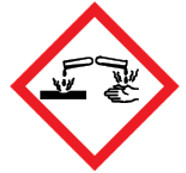
Figure 1 - Text Description
The symbol within the pictogram shows a container dripping liquid onto a piece of metal and another container dripping liquid onto a hand. This symbol indicates that hazardous products with this pictogram can:
- damage or destroy metal
- cause irreversible damage to the skin (for example, burns, blisters, scarring)
- produce tissue damage in the eye or vision loss that is irreversible or not fully reversible within 21 days
Figure 2.

Figure 2 - Text Description
The symbol within the pictogram is a flame with a line underneath it. This symbol indicates that hazardous products with this pictogram can ignite easily and burn rapidly if they are not stored and handled properly.
Figure 3.

Figure 3 - Text Description
The symbol within the pictogram is a human skull with two crossed bones behind it. The symbol indicates that hazardous products with this pictogram can cause death or poisoning.
WHMIS classification
Health hazards:
Acute Toxicity (Inhalation) – Category 3
Serious Eye Damage – Category 1
Physical hazards:
Flammable Liquids – Category 3
Health hazards
Acute Toxicity (Oral):
Does not meet criteria
An LD50 value of 3530 mg/kg (rat) has been reported Footnote 2.
The available data do not meet the classification criteria for Acute Toxicity (Oral).
Acute Toxicity (Dermal):
Does not meet criteria
An LD50 value of 1,060 mg/kg (rabbit) has been reportedFootnote 3; however, this datum is from a secondary source in which no study details are provided. This datum is, thus, insufficient for classification.
The available data do not meet the classification criteria for Acute Toxicity (Dermal).
Acute Toxicity (Inhalation – Gases):
Not applicable
Acetic acid is not a gas. The classification criteria for Acute Toxicity (Inhalation – Gases) do not apply to this substance.
Acute Toxicity (Inhalation – Vapours):
Category 3
Median lethal concentration (LC50): >8.5 mg/L, 4 h (male rat)Footnote 4.
In a study conducted similarly to the Organisation for Economic Co-operation and Development Test Guideline (OECD TG) 403, the LC50 was reported to be >8.5 to <9.9 mg/L in male rats when exposed to vapours for 4 hours (based on study summaryFootnote 4). The authors note corrosion to the respiratory tract.
The available data meet the classification criteria for Acute Toxicity (Inhalation - Vapours) – Category 3 [HPR 8.1.1(1)].
Acute Toxicity (Inhalation – Dusts and Mists):
No data available
Skin Corrosion / Irritation:
Category 1
Human: In a 4-hour human patch test, 10% acetic acid was slightly irritating to intact and abraded skin Footnote 4. Observations included weak to moderate erythema accompanied by edema and surface effects like glazing and scaling. No severe, irreversible skin damage was noted.
Animals: In studies conducted similarly to the OECD TG 404, application of 0.5 mL of 60%, 70% and 80% acetic acid to intact rabbit skin under semi-occlusive conditions for 4 hours resulted in eschar formation that persisted until day 14, with slight loosening around the edges of the scabFootnote 5Footnote 6Footnote 7. In another study, glacial acetic acid (equivalent to 95% acetic acid) caused complete destruction of the skin of guinea pigs after 24 hours of contact (based on study summaryFootnote 3). In another study conducted similarly to OECD TG 404, in which acetic acid was applied to the skin of rabbits at 20% and 25% for 4 hours, 20% acetic acid was moderately irritating (based on study summaryFootnote 4); however, the mean scores (24-48-72 hours) of <2.3/4 for both erythema and edema do not meet classification criteria for Category 2. At 25%, severe erythema, with a mean score of 3.92/4, and severe edema, with a mean score of 2.83/4, were observed. There were lesions and eschar formation in 3/3 animals, meeting the classification criteria for Category 1. Insufficient information was available for sub-categorization. In another study, a 4-hour exposure to 3.3% and 10% aqueous solutions of acetic acid to rabbit skin was not irritatingFootnote 8.
Purposely generated animal data demonstrate that acetic acid at a concentration greater than 20% is a skin-corrosive substance. Acetic acid at a concentration of ≤ 20% does not meet classification criteria for skin irritation.
The available data meet the classification criteria for Skin Corrosion – Category 1 [HPR 8.2.2(2)].
Serious Eye Damage / Eye Irritation:
Category 1
Human: Severe and permanent eye injuries have been reported in humans who mistakenly used acetic acid in lieu of prescribed eye dropsFootnote 9. Vinegar, which contains 4% to 10% of acetic acid, has been reported to cause pain, conjunctival hyperemia and occasionally permanent opacity of the human corneaFootnote 10.
Animals: In a study conducted similarly to OECD TG 405, administration of 0.1 mL of 3% and 10% acetic acid solutions in rabbit eyes resulted in corrosionFootnote 10. In another pre-guideline study in rabbits, an excess of a 1% acetic acid solution caused necrosis that was visible after staining, covering 3/4 of the cornea, and a more severe necrosis covering a smaller area of the cornea, with a dose as low as 0.005 mL Footnote 2. A 0.1 mL dose of 5% acetic acid produced pannus in 2/9 unwashed and in 3/9 washed eyesFootnote 11.
The available data meet the classification criteria for Serious Eye Damage – Category 1 [HPR 8.3.2(1)].
Respiratory Sensitization:
No data available
Skin Sensitization:
No data available
Germ Cell Mutagenicity:
Does not meet criteria
In Vivo: No data are available on acetic acid. A Mammalian Erythrocyte Micronucleus test was negative for acetic anhydride, the dehydration product of acetic acid, when tested in rats through inhalation (based on study summaryFootnote 4).
In Vitro: In an Ames test performed similarly to OECD TG 471, negative results were obtained in bacterial cells of Salmonella typhimurium, strains TA97, TA1535, TA100, TA1537, and TA98, with and without metabolic activationFootnote 12Footnote 13. In a chromosome aberration test performed similarly to OECD TG 473, negative results were obtained in Chinese hamster ovary cells with acetic acid, both in the presence and absence of metabolic activationFootnote 14. Acetic acid tested negative in a chromosome aberration test conducted similarly to OECD TG 473 in Chinese hamster lung fibroblast cells in the absence of metabolic activationFootnote 13.
The available data do not meet the classification criteria for Germ Cell Mutagenicity.
Carcinogenicity:
Does not meet criteria
An 8-month study in rats gavaged with 3% acetic acid did not find any tumour inductionFootnote 15.
A weekly skin application of acetic acid in mice at 667 µmol for 32 weeks did not result in a significant carcinogenic response (maximal tumor response of 0.73 papilloma/mouse). The animals were able to tolerate high doses when given sufficient time (1 week) to recover in between doses. Acetic acid was concluded to be a potential weak tumour promotor; however a clear correlation cannot be made between stimulated macromolecular synthesis or hyperplasia and tumor promotion when comparing phorbol esters (phorbol-12,13-ditetradecanoate, a weak tumour promoter) and acetic acidFootnote 16. Since there are other factors involved in tumour promotion, these data are insufficient for classification.
Acetic acid has not been reviewed by the International Agency for Research on Cancer (IARC), the National Toxicology Program (NTP), or the American Conference of Governmental Industrial Hygienists (ACGIH).
The available data do not meet the classification criteria for Carcinogenicity.
Reproductive Toxicity:
Does not meet criteria
A study designed similarly to EU Method B.31 (Prenatal Developmental Toxicity Study) was conducted in mice, rats and rabbits exposed to 0, 16, 74.3, 345 or 1600 mg/kg/day acetic acid (on gestational days 6-15 in mice and rats and on days 6-18 in rabbits) for 13 consecutive days. No significant signs of maternal toxicity or fetotoxicity were reported (based on study summariesFootnote 4Footnote 3.
The available data do not meet the classification criteria for Reproductive Toxicity.
Specific Target Organ Toxicity – Single Exposure:
Does not meet criteria
Oral Route of Exposure: In humans, 80-100% acetic acid has caused severe corrosive injury to the gastrointestinal and respiratory tractsFootnote 17Footnote 18Footnote 19Footnote 20Footnote 21. Side effects following the ingestion of industrial/commercial strengths of acetic acid include metabolic acidosis, leading to liver necrosis, hemolysis, myocardial infarction and kidney damage. Since acetic acid is a skin-corrosive substance, the effects may be secondary to its corrosive nature. Available data do not meet the classification criteria.
Dermal Route of Exposure: No data available
Inhalation Route of Exposure: Accidental inhalation of high concentrations of acetic acid in a workplace caused nose discomfort, including burning, irritation, or a runny nose Footnote 22. Other incidents of accidental exposure show patients experiencing throat irritation, airway hyperresponsiveness, shortness of breath, coughing, wheezing and reversible lung injury when accidentally exposed to glacial acetic acidFootnote 23Footnote 24. Inhalation exposure in mice and guinea pigs caused reversible upper respiratory tract irritationFootnote 25Footnote 26. Since acetic acid is a skin-corrosive substance, effects may be secondary to its corrosive nature.
The available data do not meet the classification criteria for Specific Target Organ Toxicity – Single Exposure.
Specific Target Organ Toxicity – Repeated Exposure:
Does not meet criteria
Oral Route of Exposure: In a non-good laboratory practice, non-guideline study, where the main purpose of the study was to report the possible preventive effects of dietary vinegar on blood pressure, hypertensive male rats were given a diet containing about 290 mg/kg vinegar for 8 weeks (based on study summaryFootnote 4). No adverse effects were observed. The test substance significantly lowered the blood pressure. Available data do not meet classification criteria.
Dermal Route of Exposure: No data available
Inhalation Route of Exposure: 5 workers were exposed to 82 and 265 ppm (cited as up to 0.200 and 0.650 mg/L) acetic acid during particular work phases for 7-12 yearsFootnote 27. Chronic bronchitis (asthmatic-like in 3 cases and emphysema in 1) was observed. At 5 ppm or 10 ppm exposures of acetic acid, no evidence of an irritative effect on pulmonary function, nasal swelling or nasal airway resistance was found in another human study (based on study summary Footnote 4). No animal data are available. There is not enough human evidence to meet classification criteria.
The available data do not meet the classification criteria for Specific Target Organ Toxicity – Repeated Exposure.
Aspiration Hazard:
No data available
No human data are available for acetic acid. This substance is not a liquid hydrocarbon.
Biohazardous Infectious Materials:
Not applicable
Acetic acid is not a microorganism, protein or nucleic acid.
Physical hazards
Explosives:
Not Evaluated*
* Explosives are excluded from the HPA and its regulations. Explosives are regulated under the Explosives Act. For more information, visit Natural Resources Canada.
Flammable Gases:
Not applicable
Acetic acid is not a gas. The classification criteria for Flammable Gases do not apply to this substance.
Aerosols:
Not evaluated
Classification of a hazardous product in the Aerosols hazard class is product dependent.
Oxidizing Gases:
Not applicable
Acetic acid is not a gas. The classification criteria for Oxidizing Gases do not apply to this substance.
Gases Under Pressure:
Not applicable
Acetic acid is not a gas. The classification criteria for Gases Under Pressure do not apply to this substance.
Flammable Liquids:
Category 3
Acetic acid has a flash point of 39 °C, closed cupFootnote 1.
The available data meet the classification criteria for Flammable Liquids – Category 3 [HPR 7.6.1(2)].
Flammable Solids:
Not applicable
Acetic acid is not a solid. The classification criteria for Flammable Solids do not apply to this substance.
Self-Reactive Substances and Mixtures:
Does not meet criteria
Acetic acid has an auto-ignition temperature of 465 °C Footnote 1. Self-Reactive Substances and Mixtures must have a self-accelerating decomposition temperature (SADT) of ≤75°C to meet the minimum classification in this hazard class.
The available data do not meet the classification criteria for Self-reactive Substances and Mixtures.
Pyrophoric Liquids:
Does not meet criteria
Acetic acid has an auto-ignition temperature of 465 °C Footnote 1. Pyrophoric Liquids react at room temperature.
The available data do not meet the classification criteria for Pyrophoric Liquids.
Pyrophoric Solids:
Not applicable
Acetic acid is not a solid. The classification criteria for Pyrophoric Solids do not apply to this substance.
Self-Heating Substances and Mixtures:
Does not meet criteria
Acetic acid has an auto-ignition temperature of 465 °CFootnote 1, which is well above the maximum spontaneous ignition temperature of 140 °C for classification.
The available data do not meet the classification criteria for Self-heating Substances and Mixtures.
Substances and Mixtures which, in Contact with Water, Emit Flammable Gases:
Not applicable
Acetic acid has a chemical structure that does not contain metals or metalloids, and is, therefore, excluded from classification [HPR 7.12.1(1)].
Oxidizing Liquids:
Not applicable
Paragraph 7.13.1(1)(b) of the HPR excludes from classification any organic liquid that contains oxygen, fluorine or chlorine if those elements are chemically bonded only to carbon or hydrogen. Acetic acid contains oxygen which is chemically bonded only to carbon and hydrogen.
Oxidizing Solids:
Not applicable
Acetic acid is not an organic peroxide. The classification criteria for Organic Peroxides do not apply to this substance.
Organic Peroxides:
Not applicable
Acetic acid is not an organic peroxide. The classification criteria for Organic Peroxides do not apply to this substance.
Corrosive to Metals:
No data available
No data are available to determine whether acetic acid meets the classification criteria for corrosiveness to metals; however, glacial acetic acid is recommended to be stored in aluminum and stainless steel vessels Footnote 1.
Combustible Dusts:
Not applicable
Acetic acid is not a solid. The classification criteria for Combustible Dusts do not apply to this substance.
Simple Asphyxiants:
Not applicable
Acetic acid is not a gas. The classification criteria for Simple Asphyxiants do not apply to this substance.
Chemicals Under Pressure:
Not applicable
Classification of a hazardous product in the Chemicals Under Pressure hazard class is product dependent.
Regulatory and other information
Regulatory information:
Hazardous substance assessments are prepared by Health Canada as educational and information resources. Under the HPA, suppliers of hazardous products must, upon the sale or importation of a hazardous product, provide a safety data sheet and label that meet the requirements set out in the HPR.
Other information:
The information and classifications contained in these hazardous substance assessments are based on publicly available sources, such as peer-reviewed literature or reports by international bodies. New information, including proprietary information, could have an impact on the classification of substances or hazardous products containing them. It is the responsibility of the supplier to ensure the accuracy, sufficiency, and reliability of their hazardous product classifications.
Last updated:
2024
Prepared by:
Workplace Hazardous Materials Bureau, Health Canada